Abstract
Known as a pivotal immunohemostatic response, immunothrombosis is activated to restrict the diffusion of pathogens. This beneficial intravascular defensive mechanism represents the close interaction between the immune and coagulation systems. However, its uncontrolled form can be life-threatening to patients with the critical coronavirus disease 2019 (COVID-19). Hyperinflammation and ensuing cytokine storm underlie the activation of the coagulation system, something which results in the provocation of more immune-inflammatory responses by the thrombotic mediators. This vicious cycle causes grave clinical complications and higher risks of mortality. Classified as an evolutionarily conserved family of the small non-coding RNAs, microRNAs (miRNAs) serve as the fine-tuners of genes expression and play a key role in balancing the pro/anticoagulant and pro-/anti-inflammatory factors maintaining homeostasis. Therefore, any deviation from their optimal expression levels or efficient functions can lead to severe complications. Despite their extensive effects on the molecules and processes involved in uncontrolled immunothrombosis, some genetic agents and uncontrolled immunothrombosis-induced interfering factors (e.g., miRNA-single nucleotide polymorphysms (miR-SNPs), the complement system components, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, and reactive oxygen species (ROS)) have apparently disrupted their expressions/functions. This review study aims to give an overview of the role of miRNAs in the context of uncontrolled immunothrombosis/thromboinflammation accompanied by some presumptive interfering factors affecting their expressions/functions in the critical COVID-19. Detecting, monitoring, and resolving these interfering agents mafy facilitate the design and development of the novel miRNAs-based therapeutic approaches to the reduction of complications incidence and mortality in patients with the critical COVID-19.
Keywords: SARS-CoV-2, COVID-19, Immunothrombosis, miRNAs, miRNAs-based therapy
1. Introduction
The coronavirus disease 2019 (COVID-19) is a multifaceted medical condition caused by the novel member of the Coronaviridae family named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Entering through angiotensin-converting enzyme 2 (ACE2)-mediated endocytosis or fusion, this virus can clinically create a heterogeneous infection. Nearly 20% of patients develop a critical condition. Despite the discovery and production of different vaccines worldwide, SARS-CoV-2 infections and COVID-19 critical forms are still being reported (especially in poor countries). Its mutation-prone nature (more than 50 mutations only caused by the Omicron variant) necessitates adopting the novel therapeutic methods by more precise diagnosis of COVID-19 molecular-immunogenetic mechanisms. In patients with the critical COVID-19, dysregulated immune reactions and high-level production of inflammatory mediators have been observed in the circulation and bronchoalveolar lavage fluid (BALF) causing a hyperinflammatory condition called the cytokine storm [1], [2], [3], [4], [5], [6], [7], [8]. At the same time, accomplished assessments have shown high rates of thrombotic events in these patients leading to a hypercoagulability situation and high mortality rates [1], [2], [3], [9], [10], [11], [12], [13]. In other words, the COVID-19 is a kind of endotheliopathy that causes hypercoagulability situation, exhausted fibrinolysis, and extensive vascular thrombosis associated greatly with a hyperinflammatory condition. According to the research evidence, there is a reciprocal correlation between the immune and coagulation systems that reinforce each other. In the critical COVID-19, the excessive immune responses have a key role in the incidence of uncontrolled immunothrombosis/thromboinflammation through the development of inflammation and induction of thrombotic factors (Fig. 1 ). Venous thromboembolism, ischemic stroke (IS), neurological disorders, acute coronary syndrome, and myocardial damage are among the grave clinical complications ensuing from the above-mentioned processes in patients with the critical COVID-19 [1], [2], [3], [10], [11], [12], [13], [14], [15], [16], [17], [18]. Normally, there is a balance between the pro/anticoagulant factors and pro-/anti-inflammatory ones that would maintain homeostasis [1]. Acting as post-transcriptional regulators of gene expression, microRNAs (miRNAs) are pivotal molecules for the well-balanced inflammatory and thrombotic processes. These non-coding RNAs mostly bind to the 3′-untranslated region (3′-UTR) of their target molecules and regulate their expressions [14], [19], [20], [21], [22]. Many studies have demonstrated the role of miRNAs in the pro-inflammatory pathways, activated in the severe/critical COVID-19, which we discussed in another paper [5]. As exemplified, an investigation into the mouse model of ovalbumin (OVA)/complete Freund's adjuvant (CFA)-induced neutrophilic asthma indicates a significant elevation of the nucleotide-binding domain (NOD)-like receptor protein 3 (NLRP3)-Inflammasome level and activity in miR-223−/− mice [23]. Moreover, a clinical study on patients with systemic lupus erythematosus (SLE) revealed the crucial role of miR-410 in targeting the signal transducer and activator of transcription 3 (STAT3) playing a key role in interleukin (IL)-6/IL-6 receptor (IL-6R) pathway [24]. Regarding the close correlation between pro-inflammatory and procoagulant processes as well as the bilateral role of miRNAs in their balance, any disruption to the expressions or efficient functions of these small RNAs can underlie the development of excessive inflammatory or thrombotic reactions consequently followed by the intensification of another system activation. Accordingly, this review study aims to discuss the role of miRNAs in the incidence of uncontrolled immunothrombosis/thromboinflammation accompanied by some interfering factors affecting their expressions/functions in the critical COVID-19 and to propose some miRNAs-based therapeutic approaches.
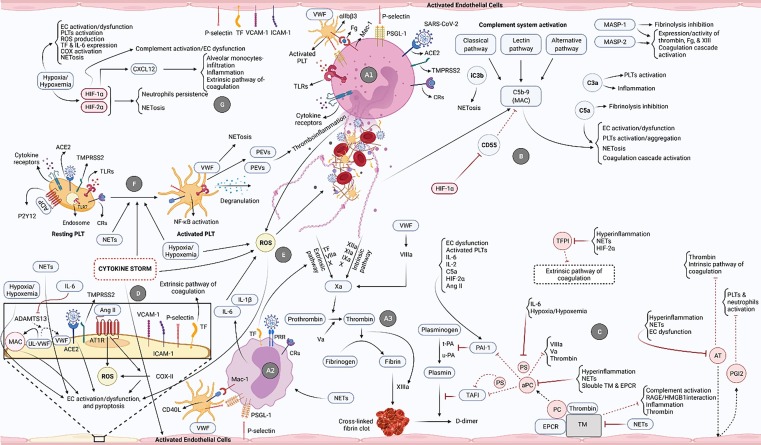
Fig. 1.
The most pivotal processes/pathways involved in the development of uncontrolled immunothrombosis in the critical COVID-19. A1) SARS-CoV-2/ACE2 interaction and ligation of different receptors of neutrophils provoke their activation and NETs formation. These cells then interact with PLTs and ECs and release NETs. A2) Ligation of diverse receptors of monocytes (e.g., pattern recognition receptors (PRRs) and CRs), their interplay with ECs and PLTs, and also released NETs cause the activation of monocytes followed by high-levels production of pro-inflammatory cytokines. A3) NETs are decorated with TF on the one hand and induce factor XII (FXII) on the other, respectively resulting in the activation of extrinsic and intrinsic coagulation pathways and formation of cross-linked fibrin clots. TF is also expressed on the monocytes and activates the extrinsic coagulation pathway. B) Activation of the complement system through all three pathways can lead to the formation of MAC, production of C3a, C5a, iC3b, etc. each of which directly and indirectly causes NETosis induction, inflammation development, and fibrinolysis inhibition. The MASP-1 and MASP-2 molecules also play key roles in the coagulation induction and fibrinolysis restraining. C) During the fibrinolysis process, plasminogen is converted into plasmin under the influence of t-PA and u-PA eventually leading to the breakdown of the cross-linked fibrin clots and generation of D-dimer. PAI-1 inhibits these plasminogen activators and is activated by several factors involved in uncontrolled immunothrombosis. Endogenous anticoagulants also play central roles provoking fibrinolysis and preventing the formation of the clots. In addition to the induction of some anti-inflammatory roles, TM forms a complex with thrombin and activates the PC (bound to the endothelial protein C receptor (EPCR)), whereas aPC inhibits the coagulation mediators in different ways. Another anticoagulant, PS, directly and indirectly (as an aPC's co-factor) participates in the fibrinolysis process. AT and TFPI also inhibit the coagulation pathways. In addition, AT restricts the NETosis through the induction of prostaglandin I2 (PGI2) production by the ECs. Involved in the uncontrolled immunothrombosis, multiple factors restrain the functions of anticoagulants resulting in inefficient fibrinolysis. D) SARS-CoV-2/ACE2 and Ang II/AT1R interactions, NETs, hypoxia/hypoxemia, and cytokine storm cause the activation/dysfunction and pyroptosis of ECs. The inhibition of ADAMTS13 by IL-6 also underlies the reduced conversion of ultra-large VWF (UL-VWF) into the normal size. This molecule then acts as a scaffold for the formation of MAC contributing to the ECs damage. The expression of TF and adhesion molecules by ECs is followed by the activation of the extrinsic coagulation pathway and NETosis. E) The high-levels production of ROS, induced by neutrophils, cytokine storm, hypoxia/hypoxemia, monocytes-released pro-inflammatory cytokines, etc., can provoke NETosis and ECs damage. F) SARS-CoV-2/ACE2 and endosomal TLR7/virus single-strand RNA (ssRNA) interactions, and also the ligation of different receptors of PLTs can cause their activation leading to the NETosis induction, PLT extracellular vesicles (PEVs) release, NF-κB pathway activation, and PLTs degranulation. The released NETs, cytokine storm, and hypoxia/hypoxemia are the other stimulants of PLTs activation. G) Hypoxia/hypoxemia stimulates different processes involved in the uncontrolled immunothrombosis and also mediates the production of HIF-1α and HIF-2α. These two factors perpetuate NETosis. HIF-1α also induces the activities of CXCL12 and the complement system. Therefore, it can proceed the ECs dysfunction leading to excessive NETosis, inflammation, and coagulation.
2. Interaction of the immune and coagulation systems in critical COVID-19
2.1. Uncontrolled immunothrombosis/thromboinflammation
The immune and coagulation systems cooperate to restrict the diffusion of pathogens. The term “immunothrombosis”, which is considered as an example of their interaction, refers to an effective endogenous process of the innate immune system induced by the pathogens and damaged cells. It can be considered a beneficial intravascular immune mechanism. However, its uncontrolled form can underpin the inflammation and immunologically-mediated microthrombi formation leading to thromboinflammation (Fig. 1). This physiological process is mostly mediated by the neutrophils and monocytes in which they release neutrophil extracellular traps (NETs) and express tissue factor (TF) on the one hand and restrain the endogenous anticoagulants on the other resulting in severe inflammation-induced coagulation [2], [3], [14], [25] (Fig. 1-A1-A3, 1C). In particular, the myeloperoxidase (MPO) of NETs provokes the monocytes toward the pro-inflammatory M1 phenotype contributing to the thromboinflammation and endothelial cells (ECs) damage [2], [26], [27] (Fig. 1-A2, 1-D, 1-E). The interaction of monocytes and platelets (PLTs), which has elevated in the COVID-19 patients, is another factor for more activation of the former and subsequently higher NETs formation [28], [29], [30] (Fig. 1-A2, 1-E). The broad infiltration of neutrophils into diverse organs and uncontrolled immunothrombosis associated with the disease exacerbation have been observed in patients with the critical COVID-19 [2], [3], [25], [31], [32]. Many immunologic-hemostatic processes participate in the incidence and development of the dysregulated form of immunothrombosis (Fig. 1), something which will be discussed in the following.
2.1.1. NETosis
Several in vitro and in vivo studies have reported that neutrophils release NETs in a process called “NETosis” following the stimulation by the inflammatory mediators or pathogens and also mitochondrial dysfunction. NETs are the structures that consist of cell-free DNA (cfDNA), histones, and granular as well as cytoplasmic proteins [3], [9], [14], [25], [33], [34], [35], [36], [37], [38], [39]. Moreover, multiple investigations have observed mitochondrial DNA in the NETs, such as the ones released by isolated neutrophils of patients with the systemic inflammatory response syndrome (SIRS) and also inflammatory-stimulated neutrophils of healthy donors. [40], [41], [42] (Fig. 1, Fig. 2). During the NETosis, neutrophil elastase (NE) and MPO translocate into the nucleus and proceed decondensation of DNA. Activities of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex (reactive oxygen species (ROS) production) and protein arginine deiminase 4 (PAD4) (histone citrullination) are the other important factors in the NETosis [14], [25], [33], [39], [43] (Fig. 2a-A1). In this regard, using the NADPH oxidase inhibitor can inhibit NETosis in the circulating neutrophils isolated from patients with coronary artery disease [43]. Furthermore, the inability to citrullinate the histones or form the NETs was observed in PAD4−/− mouse neutrophils [39]. Despite the beneficial roles of NETs (e.g., inducing type-I interferons (IFNs-I) and trapping the inflammatory mediators), their excessive formation is followed by vascular occlusion, hyperinflammation, and disease exacerbation [2], [3], [14], [25], [26], [33], [44], [45], [46], [47], [48]. In addition, NETs-derived thrombosis is mostly PLTs-dependent mediated by some adhesion molecules such as P-selectin and its receptor, called P-selectin glycoprotein ligand-1 (PSGL-1) (Fig. 1, Fig. 2). Consistently, high levels of neutrophils-PLTs aggregations, excessive NETs formation, and neutrophils activation markers have been reported in the critical COVID-19 [29], [30], [36], [49], [50]. In this context, a clinical study on patients with the severe and critical forms reported the augmented NETs in plasma, tracheal aspirate, and lung autopsies tissues. Other studies have found the vast infiltration of neutrophils along with the acute capillaritis and fibrin deposition in the lung autopsies of patients with the critical COVID-19. Their neutrophils were somewhat degenerated and trapped in fibers indicating the production of NETs [36], [51], [52]. These findings show that SARS-CoV-2, similar to the inflammatory mediators, can provoke the neutrophils toward the NETosis in the COVID-19 patients [36], [42], [53]. Most importantly, NETs can activate both extrinsic and intrinsic coagulation pathways [2], [14], [47], [54] (Fig. 1, Fig. 2, Fig. 1, Fig. 2). High levels of NETs and their components have been observed in the circulation of the patients with the critical COVID-19 positively associated with inflammation, endothelial dysfunction, and mortality [54], [55], [56], [57], [58]. The histones of NETs can dose-dependently increase thrombin generation and fibrin deposition through the inhibition of activated protein C (aPC) (known as an endogenous anticoagulant) and activation of PLTs [2], [59], [60], [61] (Fig. 1C, F). Moreover, several studies such as the ones conducted on golden Syrian hamsters with SARS-CoV-2 and patients with SLE reported that NETs damaged the ECs leading to the fibrin clots formation [47], [62], [63], [64], [65] (Fig. 1D). Another study also found that NETs acted as a scaffold and directly induced the activation of the complement system [33], [66], [67] (Figs. 1-A1, 1B).
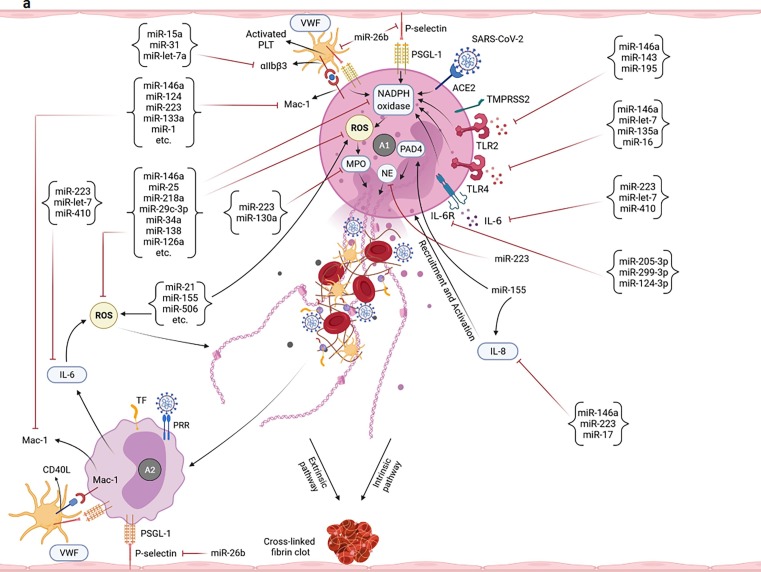
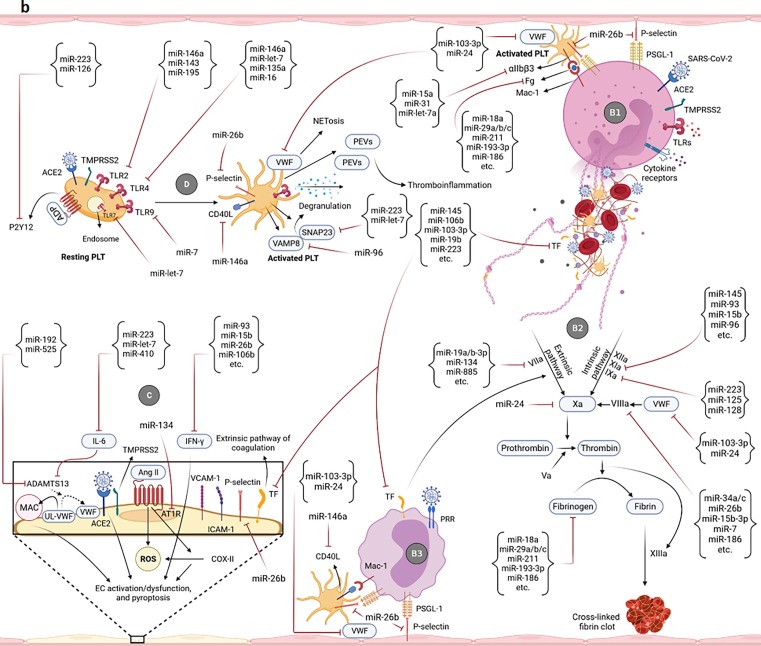
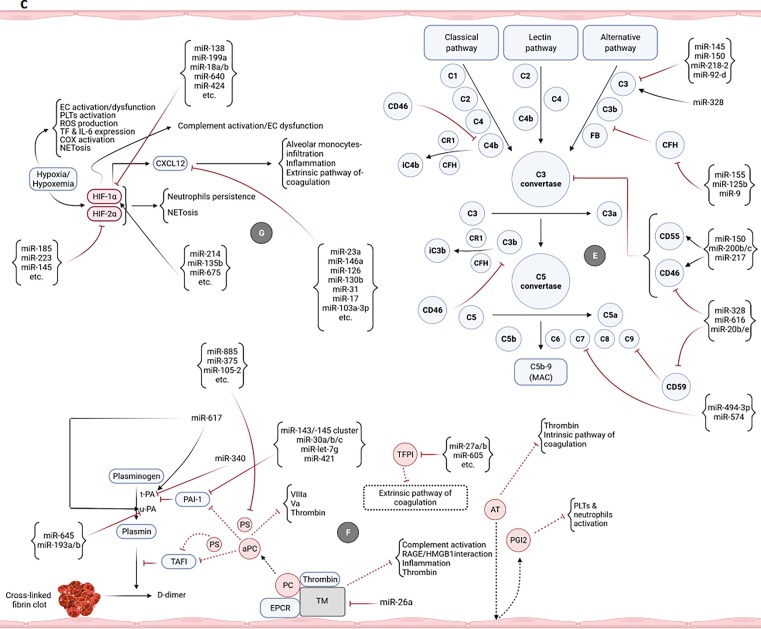
Fig. 2.
Potential of miRNAs in targeting/regulation of key molecules involved in the development of uncontrolled immunothrombosis. a-A1) According to the research evidence, many miRNAs can target IL-8, ROS, and different receptors as well as enzymes participating in the NETosis process. Adhesion molecules-mediated neutrophils-PLTs and neutrophils-ECs interactions can also be affected by miRNAs. Some of these small RNAs also cause the production of ROS and induction of NETosis. The modulation of the NETosis process by miRNAs can indirectly affect some of the adverse outcomes resulting from their excessive production such as activation of monocytes, coagulation pathways, etc. a-A2) miRNAs can affect the interactions of monocytes with PLTs as well as ECs and control the immunothrombosis. The modulation of monocytes-released pro-inflammatory cytokines by miRNAs can also restrict the NETs formation. b-B1) Multiple miRNAs target the key molecules simultaneously involved in both NETosis and coagulation processes such as VWF, TF, Fg, and αIIbβ3. b-B2) miRNAs can also regulate the expression levels of molecules/factors with the key roles in extrinsic, intrinsic, and common pathways of coagulation leading to more control of thromboinflammation. b-B3) The PLTs-/ECs-monocytes interactions and TF on the surface of monocytes are targeted by miRNAs leading to the regulation of the coagulation cascade and immunothrombosis. b-C) Several miRNAs can affect the expression of molecules involved in the ECs activation/dysfunction and pyroptosis. Causing the ECs damage, ADAMTS13 is also targeted by some of these small RNAs. Targeting the TF on the ECs is followed by the limitation of the extrinsic coagulation pathway. b-D) The expression regulation of different receptors of PLTs with central roles in their activation is accomplished by miRNAs. Targeting some other molecules such as CD40L, VWF, P-selectin, SNAP23, and VAMP8 can also affect the activation and degranulation of PLTs leading to more restriction of uncontrolled immunothrombosis. c-E) Several miRNAs can target the complement factors and regulators. They negatively or positively regulate their expressions and affect the uncontrolled immunothrombosis. c-F) The negative and positive regulations of molecules involved in the fibrinolysis process are done by some miRNAs leading to the limitation of fibrin deposition and development of the uncontrolled immunothrombosis c-G) The expressions of hypoxia/hypoxemia-induced HIF-1α and HIF-2α are affected by several miRNAs. CXCL12 is also considered a target molecule of miRNAs affecting the uncontrolled immunothrombosis.
2.1.2. Complement system activation
As a humoral innate immune response, the activation of the complement system through all three classical, alternative, and lectin pathways can play a crucial role in the development of inflammation and coagulation [9], [10]. The enhanced activation of this system alongside the uncontrolled immunothrombosis was reported in the critical COVID-19. According to the research evidence, both SARS-CoV-2 proteins (e.g., nucleocapsid (N) and spike (S)) and immune complexes can activate the complement cascade in these patients. Considerably, the increased levels of soluble and deposited complement component (C) 5b-9 (membrane attack complex (MAC)), C4d (caused by C4b breakdown), and mannan-binding lectin serine protease (MASP)-2 have been observed in the lung microvasculature of the patients with the critical COVID-19. The evaluation of their purpuric skin lesions resulted in similar findings. Moreover, the elevated circular levels of C5a and C5b-9 along with the high expression of the C5a receptor (C5aR) on the surface of neutrophils and monocytes were found in the patients with the critical COVID-19. The increased expression of MASP-2 was also accompanied by the enhanced coagulation [54], [68], [69], [70], [71], [72]. The activated complement proteins play a central role in the uncontrolled immunothrombosis. They can directly (through neutrophils activation/recruitment) and indirectly (with the activation of TF, ECs, PLTs, and inflammation) provoke the NETosis [2], [3], [9], [14], [25], [33], [54], [73], [74], [75], [76], [77], [78], [79], [80] (Fig. 1B, F). The induction of PLTs activation/aggregation and expression of von Willebrand factor (VWF) by the activated ECs are pivotal in the development of NETosis, fibrin deposition, and dysregulated immunothrombosis [3], [10], [81], [82]. At the same time, in vitro and in vivo studies reported that the complement factors (e.g., C5a) could induce the expression of plasminogen activator inhibitor-1 (PAI-1) by different cells and inhibit fibrinolysis (Fig. 1C). In this regard, Shagdarsuren et al. reported the reduction of PAI-1 levels in the atherosclerosis-prone mice, treated with the C5aR antagonist. MASP-1 also activates thrombin activatable fibrinolysis inhibitor (TAFI) (known as a fibrinolysis inhibitor) [3], [83], [84], [85], [86] (Fig. 1B).
2.1.3. Exhausted fibrinolysis and inefficient endogenous anticoagulants
Following the increased formation of clots, the fibrinolysis process is activated whereby plasminogen is converted into the active plasmin under the influence of tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA). The active plasmin then breaks down the fibrin in the vasculature [87], [88], [89] (Fig. 1C). Being affected by different factors involved in the uncontrolled immunothrombosis (e.g., NETs and ECs activation/dysfunction), exhaustion/disruption of the fibrinolytic system occurs in patients with the critical COVID-19 leading to its inefficiency [2], [3], [9], [87], [90], [91] (Fig. 1C). Both hyperfibrinolysis (high levels of D-dimer) and hypofibrinolysis (plasmin consumption, its reduced fibrinolytic function, and high-level production of PAI-1) have been reported in these patients [3], [87], [88], [89], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102]. Moreover, hyperinflammation, NE, and hypoxia-inducible factor (HIF)-2α inhibit the tissue factor pathway inhibitor (TFPI), whereas MASP-1 activates TAFI resulting in the disrupted fibrinolysis [3], [85], [103], [104], [105] (Fig. 1B, C). The upregulated levels of D-dimer, t-PA, u-PA, soluble u-PA receptor (suPAR), and thrombin-anti-thrombin (AT) complexes have been reported in patients with the critical COVID-19 positively associated with the activation of neutrophils, NETs plasma levels, and mortality. Disruption to the functions of endogenous anticoagulants has also been observed in these patients. Based on the research evidence, activities and plasma levels of AT, PC, and protein S (PS) have been reduced in patients with the critical COVID-19 under the influence of the factors involved in the uncontrolled immunothrombosis and have negatively been associated with the disease exacerbation and mortality [2], [57], [87], [88], [98], [99], [100], [102], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116]. Furthermore, the increased soluble form (inefficient/attenuated form) of thrombomodulin (TM) (known as an anticoagulant and anti-inflammatory molecule) and its decreased tissue expression level were reported in patients with the critical COVID-19 accompanied by the disease severity [3], [97], [102], [105], [111], [117].
2.1.4. ECs activation and dysfunction
Activation, dysfunction, and pyroptosis of the ECs (known as important pathological events found in the critical COVID-19) can occur directly after the SARS-CoV-2 entrance and indirectly as a result of the immunologic-hemostatic responses (Fig. 1D). Regarding the crucial role of the endothelium in the maintenance of vascular homeostasis and immune responses, its dysfunction acts as a stimulator for uncontrolled immunothrombosis [2], [3], [9], [118], [119], [120]. Studies on the COVID-19 and severely septic patients have revealed that the interaction of the SARS-CoV-2-infected/activated ECs with neutrophils and monocytes can lead to the activation of the latter and higher levels of NETosis. The interplay between neutrophils and PLTs is another underlying factor in this process and ECs activation [53], [121] (Fig. 1, Fig. 2, Fig. 1, Fig. 2). As Nitric oxide (NO) is vital for the normal function of the ECs, the occurrence of oxidative stress and high-levels production of ROS (induced by the different factors involved in the uncontrolled immunothrombosis, and reducing NO bioavailability) are the most important mechanisms of the ECs dysfunction [26], [43], [118], [120], [122] (Fig. 1D, E). Accordingly, the decreased and increased levels of the NO and ROS have respectively been reported in the patients with the critical COVID-19 [5], [123], [124], [125]. Above all, ROS provoke NETosis [25], [43], [118] (Fig. 1E). NETs can directly activate the ECs and induce their apoptosis [14]. The excessive production of these molecules can also damage endothelium integrity and activate the complement system resulting in the ECs dysfunction [62], [66] (Fig. 1, Fig. 2, Fig. 1-D). According to in vitro and in vivo studies, angiotensin II (Ang II)/Ang II type 1 receptor (AT1R) axis and cytokine storm proceed the ECs dysfunction [2], [3], [9], [118], [120], [126], [127] (Fig. 1D). The elevated levels and activity of several markers related to the ECs activation/dysfunction and pyroptosis (e.g., VWF, P-selectin, intercellular adhesion molecule 1 (ICAM-I), vascular cell adhesion molecule 1 (VCAM-1), and caspase-1) were observed in the patients with the critical COVID-19 positively associated with the disease severity and mortality [102], [117], [119], [128], [129], [130], [131]. Known as a stimulator of coagulation, complement activation, and NETosis, the VWF plays a major role in the uncontrolled immunothrombosis [132], [133] (Fig. 1, Fig. 2, Fig. 1-D). Apparently, its increased expression is associated with the reduced activity of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) (known as a regulator of VWF multimers size) in the critical COVID-19. According to the research evidence, a high level of IL-6 (found in the critical COVID-19) inhibits the activity of this metalloproteinase [2], [9], [134], [135] (Fig. 1D). The negative correlation between NETs and ADAMTS13 levels was reported in the patients with the critical COVID-19 [57], [136].
2.1.5. PLTs activation
Apart from the key role of PLTs in the coagulation cascade, these cells have recently been considered the crucial and unique components of the immune system [137], [138], [139]. The immunologic functions of the PLTs are mediated by the pro-inflammatory molecules stored in their granules (degranulation) and/or newly synthesized (through the long-lived transcripts) [2], [9], [137], [140]. Moreover, several immune-inflammatory receptors such as toll-like receptors (TLRs) 1, 2, 4, 6, 7, 9, complement receptors (CRs), and cytokine receptors are expressed by the PLTs underlying their activation, launching the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway (in a non-genomic route), and release of the pro-inflammatory mediators. The ligation of non-immune receptors of the PLTs (e.g., P2Y12) also causes their activation. Most importantly, thrombocytes express the ACE2 and transmembrane protease, serine 2 (TMPRSS2) that can underpin the activation of these cells, PLTs-neutrophils interaction, and development of uncontrolled immunothrombosis [9], [141], [142], [143], [144], [145] (Fig. 1-F). In this connection, the increased expression levels of the markers related to the PLTs activation (e.g., P-selectin, VWF, and the cluster of differentiation 40 ligand (CD40L)) were reported in the critical COVID-19 [30], [49], [50], [102], [117], [146], [147]. Moreover, the activation of PLTs through TLR4 and TLR9 (with high expression levels in the critical COVID-19) is followed by their further interactions with neutrophils and NETosis [148], [149], [150]. According to different in vitro and in vivo studies, NETs induce pro-inflammatory effects through the activation of TLR2, 4, 9, and PLTs activation, in turn, leading to the higher expressions of these three TLRs [25], [141], [151], [152], [153]. The majority of molecules released by the activated PLTs (e.g., IL-1 beta (IL-1β), high mobility group box 1 (HMGB1), human β-defensin-1 (hBD-1), and complement factors) can also provoke NETosis. In this regard, it was observed that HMGB1−/− PLTs, isolated from the Ly5.1 C57BL/6 mice, failed to induce the generation of NETs [2], [3], [9], [33], [137], [154], [155] (Fig. 1F).
2.1.6. Hypoxia/hypoxemia
Hypoxia/hypoxemia is among the stimulant and supportive factors of uncontrolled immunothrombosis in the critical COVID-19 [2], [9]. Following the SARS-CoV-2 infection, extensive pulmonary infiltration of the neutrophils and other immune cells causes the local consumptive hypoxia, high-level production of ROS, and excessive NETosis. Moreover, the increased permeability of the endothelium is followed by the NETs leakage into the alveolar space, disruption to the gas exchange, and the incidence of hypoxemia [53]. Hypoxia/hypoxemia also acts as a predisposing factor in the promotion of inflammation and coagulation resulting in the further production of NETs [2], [3], [26], [27], [53] (Fig. 1E, G). Under hypoxic conditions, HIFs (e.g., HIF-1α and HIF-2α) are expressed by the immune cells and ECs. Although these two HIFs have been reported to be able to preclude the infection of the lung epithelial cells with the SARS-CoV-2, their inappropriate activities can be followed by the persistence of neutrophils, higher rates of the NETosis, and uncontrolled immunothrombosis [26], [156] (Fig. 1G). Consistently, the significantly elevated expression of the HIF-1α was reported in the COVID-19, especially in the critical form [157], [158]. The HIF-1α-induced C-X-C motif chemokine ligand 12 (CXCL12) also plays a pivotal role in the uncontrolled immunothrombosis by recruiting the leukocytes (Fig. 1G). In this context, the CXCL12-mediated transmigration of monocytes into the brain perivascular space and consequent neuroinflammation were observed in a study conducted on the spared nerve injury (SNI) mouse model [26], [159]. In a preclinical study on the C57BL/10 male mice, Pandya et al. have reported the inhibition of CD55 by HIF-1α. Therefore, this molecule can intensify the complement-mediated ECs damage [3], [27], [160] (Fig. 1B, G). According to in vitro and in vivo studies, HIF-2α causes the upregulated and downregulated expressions of the PAI-1 and TFPI, respectively. Therefore, it can take part in the uncontrolled immunothrombosis [26], [103], [161] (Fig. 1C).
3. miRNAs
The miRNAs are classified as an evolutionarily conserved family of the small non-coding RNAs with an average length of 22 nucleotides found in diverse forms such as intracellular (nuclear, cytoplasmic, and organelic), circular/extracellular (in the biofluids), and exosomal types. The transcription of the miRNAs genes (located in the exonic, intergenic, and intronic regions) is accomplished by the RNA polymerase II/III (RNA pol II/III) leading to the formation of primary stem-loop structures called primary miRNAs (Pri-miRNAs). The breakdown of these molecules by Drosha/DiGeorge critical region 8 (DGCR8) complex is followed by the generation of hairpin molecules called precursor miRNAs (Pre-miRNAs) transported into the cytoplasm by exportin-5 (XPO5). Afterwards, the Dicer/transactivation response element RNA-binding protein (TRBP) complex does more processing on them; therefore, mature miRNAs are produced. Finally, these mature strands are loaded on the argonaute (AGO) protein leading to the formation of miRNA-induced silencing complex (miRISC) which binds to the messenger RNAs (mRNAs). The binding sites of the miRNAs are mostly located in the 3′-UTR regions of their target mRNAs. However, research evidence has shown that they can also bind to the 5′-UTR region, gene promoters, and coding areas. Every single miRNA can regulate the expressions of several target mRNAs. At the same time, the expression of every single mRNA can be controlled by several miRNAs. This represents the functional extensiveness of the miRNAs. Although the interactions between the miRNAs and their targets usually cause the translation inhibition or instability/destruction of the latter, they can be followed by the induction of the gene expression under certain conditions. The miRNAs act as a post-transcriptional gene fine-tuner. However, the regulation of a gene’s expression or production of a protein can either arise from the direct interaction of a miRNA and the corresponding mRNA or targeting the mRNAs of the factors involved in the induction of a gene’s expression (e.g., transcription factors and receptors). In other words, the outcomes of the expression regulation of an mRNA by a miRNA can underlie the induction or repression of other genes on the transcription, post-transcription, translation, and post-translation levels depending on the role of the target mRNA (even if they are not the direct target of the considered miRNA). According to the research evidence, human miRNAs can, directly and indirectly, affect the expression of the molecules involved in the immunothrombosis [5], [21], [162], [163], [164], [165], [166], [167], [168]. Therefore, any disruption to their optimal expression levels or efficient functions can result in the incidence of an uncontrolled form of this process and thromboinflammation. Differential expressions of miRNAs have been reported in the COVID-19 patients [163], [169], [170], [171], [172], [173], [174], [175], [176], [177].
3.1. miRNAs affecting neutrophils/monocytes activities and NETosis
A wide spectrum of miRNAs can affect the activities of the neutrophils. For instance, miR-146a, miR-223, and miR-17 target the IL-8 and control their recruitment. However, miR-155 acts toward their increased infiltration and NETosis through the induction of the IL-8 and PAD4 genes [178], [179], [180] (Fig. 2a-A1). The miR-223 and miR-130a restrict the NETosis and preclude the development of uncontrolled immunothrombosis through the negative regulation of NE, MPO, and prevention of the perpetuated activity of neutrophils [180], [181], [182] (Fig. 2 a-A1). Receptors and adhesion molecules involved in the NETosis process are also among the target molecules of miRNAs. As exemplified, miR-15a, miR-31, and miR-let-7a reduce the expression/activation of the integrin αIIbβ3 (αIIbβ3) on the surface of the activated PLTs. The miR-26b targets the P-selectin, whereas miR-146a, miR-124, miR-223, miR-133a, miR-1, etc. target the macrophage-1 antigen (Mac-1) to limit neutrophils-/monocytes-PLTs interactions and regulate the NETs formation [138], [183], [184], [185], [186] (Fig. 2a-A1, 2a-A2). Moreover, targeting the TLR2 by miR-146a, miR-143, and miR-195, expression regulation of the TLR4 by miR-146a, miR-let-7, miR-135a, and miR-16, targeting the IL-6 by miR-223, miR-let-7, and miR-410, and expression regulation of the IL-6R by miR-205-3p, miR-299-3p, and miR-124-3p underlie the regulation of NETosis [5], [181], [183], [187], [188], [189] (Fig. 2a-A1, 2a-A2). The negative regulation of the expression/activation of the NADPH oxidases and the production of ROS by miR-146a, miR-25, miR-218a, miR-29c-3p, miR-34a, miR-138, miR-126a, etc. are among the other factors controlling the release of NETs [168], [183], [190], [191], [192]. However, the miR-21, miR-155, miR-506, etc. induce the ROS production and the resultant NETs formation [191] (Fig. 2a-A1, 2a-A2). In this connection, the disrupted expression of some above-mentioned miRNAs such as miR-223, miR-17, miR-130a, miR-15a, miR-31, miR-16, miR-124, miR-146a, miR-218, miR-126-3p (reduced), and miR-155 (elevated) were reported in the circulation of patients with the COVID-19 [170], [171], [173], [174], [175], [176], [193], [194], [195], [196], [197], [198], [199]. The downregulated level of miR-34a was also observed in the post-mortem lung biopsy of patients with severe forms of the disease [163]. All of the miRNAs affecting the other pathways/events (e.g., activation of PLTs and the complement system, in addition to hypoxia/hypoxemia) (Fig. 2b-D, 2c-E, 2c-G) also indirectly affect the neutrophils/monocytes activities and NETosis. Moreover, some miRNAs may interact with the genome of SARS-CoV-2 and regulate the expression of its genes [5], [200]. Therefore, they may have some indirect impacts on the activation of neutrophils/monocytes and the generation of NETs
3.2. miRNAs affecting coagulation cascade
The extrinsic, intrinsic, and common pathways of coagulation can be regulated by miRNAs. For instance, miR-145, miR-106b, miR-103-3p, miR-19b, miR-223, etc. target the TF, whereas miR-19a/b-3p, miR-134, miR-885, etc. target the factor VII (FVII) to control the extrinsic coagulation pathway [201], [202], [203] (Fig. 2b-B1-3, 2b-C). Furthermore, factor IX (FIX) is the target molecule of miR-223, miR-125, and miR-128. Studies have shown that several miRNAs such as miR-145, miR-93, miR-15b, miR-96, etc. can negatively regulate the intrinsic coagulation pathway by targeting the factor XI (FXI) [202], [203] (Fig. 2b-B2). The miR-24 and miR-103-3p through targeting the factor X (FX) and VWF, miR-34a/c, miR-26b, miR-15b-3p, miR-7, miR-186, etc. by targeting the factor VIII (FVIII), and miR-18a, miR-29a/b/c, miR-211, miR-193-3p, miR-186, etc. by targeting the fibrinogen (Fg) regulate the common coagulation pathway [202], [203], [204], [205] (Fig. 2b-B1-3, 2b-D). The non-optimal circular levels of some of these miRNAs such as miR-223, miR-93, miR-186, miR-18a (reduced), and miR-19a/b-3p as well as miR-15b (elevated) have been found in patients with the COVID-19 [170], [171], [174], [176], [197]. Decreased levels of miR-24 in ECs-derived extracellular vesicles (EVs) accompanied by more cerebrovascular complications have been reported in these patients [206]. The downregulation of miR-34a and miR-29b-3p was also reported in the post-mortem lung biopsy of patients with the severe COVID-19 [163]. In addition, the indirect effects of the miRNAs controlling the other pathways/events (e.g., neutrophils/monocytes activities, NETosis, ECs activation/dysfunction, and activation of PLTs as well as the complement system, fibrinolysis, and hypoxia/hypoxemia) (Fig. 2a, 2b-C, 2b-D, 2c-E-G) on the coagulation cascade cannot be ignored.
3.3. miRNAs affecting ECs activation/dysfunction
The miRNAs can extensively affect the activation or dysfunction of ECs. As exemplified, miR-223, miR-let-7, and miR-410 by targeting the IL-6, miR-93, miR-15b, miR-26b, miR-106b, etc. through the expression regulation of the IFN gamma (IFN-γ), and miR-134 by targeting the AT1R participate in the regulation of their activation/dysfunction and pyroptosis. Moreover, the expression regulation of the P-selectin by miR-26b, and targeting the ADAMTS13 by miR-192 and miR-525 affect those processes [5], [180], [181], [187], [202], [207] (Fig. 2b-C). In this regard, the deregulated circular expression levels of some of the mentioned miRNAs such as miR-223, miR-93, miR-192, (reduced), and miR-15b (elevated) have been reported in patients with COVID-19 [176], [197], [198]. Moreover, the regulation of the other pathways/events (e.g., neutrophils/monocytes activities, NETosis, NADPH oxidases/ROS axis, coagulation cascade, the complement activation, and hypoxia/hypoxemia) (Fig. 2a, 2b-B2, 2c-E, 2c-G) by miRNAs can also affect the ECs activation/dysfunction and pyroptosis, indirectly. As mentioned earlier, some miRNAs may target the SARS-CoV-2 genome [5], [200]. Thus, they may affect the activation/dysfunction of ECs that express ACE2.
3.4. miRNAs affecting PLTs activation
The activation and degranulation of PLTs can be controlled by miRNAs. For instance, miR-146a, miR-143, miR-195, miR-let-7, miR-135a, miR-16, and miR-7 regulate the expression of the TLR2, 4, 7, 9. However, miR-146a can also target the CD40L on the surface of the PLTs [5], [183], [187], [188], [189], [208], [209] (Fig. 2b-B3, 2b-D). Their P2Y12 receptor is the target molecule of miR-223 and miR-126 [184], [210] (Fig. 2b-D). Moreover, targeting the synaptosomal-associated protein 23 (SNAP23) and vesicle-associated membrane protein 8 (VAMP8) (known as the vital molecules for the PLTs degranulation) by miR-223, miR-let-7, and miR-96, expression regulation of the P-selectin by miR-26b, and targeting the αIIbβ3 molecule by miR-15a, miR-31, and miR-let-7a can affect the PLTs activation and degranulation [138], [184], [185], [186], [187], [203], [210], [211], [212] (Fig. 2b-B1, 2b-B3, 2b-D). Among these miRNAs, the disrupted expression patterns of miR-146a, miR-16, miR-223, miR-126-3p, miR-15a, and miR-31 (reduced) have been found in the circulation of patients with the COVID-19 [174], [175], [176], [195], [196], [197], [198], [199]. It is remarkable that all of the miRNAs affecting the other pathways/events (e.g., neutrophils/monocytes activities, NETosis, coagulation cascade, and hypoxia/hypoxemia) (Fig. 2a, 2b-B2, 2c-G) can also indirectly impact the PLTs activation. The predicted miRNAs (targeting the genome of SARS-CoV-2) may be involved in the activation of these cells, as PLTs express ACE2 [5], [200].
3.5. miRNAs affecting complement system activation
Different complement components and regulators are among the target molecules of miRNAs. For instance, miR-145, miR-150, miR-218–2, and miR-92-d negatively regulate the C3 and miR-328 induces its deposition [213], [214], [215], [216]. The miR-494-3p and miR-574 also target the C7 [217] (Fig. 2c-E). Moreover, CD46 and CD59 are the target molecules of miR-328, miR-616, and miR-20b/e, and at the same time, miR-155, miR-125b, and miR-9 target another regulatory factor of this system called complement factor H (CFH) all resulting in the excessive complement activation [214], [218], [219], [220] (Fig. 2c-E). Several miRNAs such as miR-150, miR-200b/c, and miR-217 underpin the more regulated activity of the complement system through the positive regulation of CD46 and CD55 molecules [214], [221] (Fig. 2c-E). In this regard, the non-optimal circular expression levels of some of these miRNAs such as miR-150, miR-218, miR-494-3p, miR-20b (reduced), and miR-155 (elevated) have been observed in patients with the COVID-19 [173], [193], [196], [199], [222]. Furthermore, the indirect effects of the miRNAs on the other pathways/events (e.g., neutrophils/monocytes activities, NETosis, and hypoxia/hypoxemia) (Fig. 2a, 2c-G) involved in the activation of this system should be taken into consideration.
3.6. miRNAs affecting fibrinolysis process and endogenous anticoagulants
Several studies have reported that the activation of fibrinolysis process can be regulated by some miRNAs. For instance, miR-645 and miR-193a/b target the u-PA, whereas miR-340 controls the t-PA gene expression [223], [224], [225]. However, the miR-617 induces their both activations [226] (Fig. 2c-F). Multiple miRNAs such as miR-143/-145 cluster, miR-30a/b/c, miR-let-7 g, and miR-421 target the PAI-1 and regulate its expression [203], [227], [228] (Fig. 2c-F). Moreover, miR-27a/b, miR-605, etc. target the TFPI, miR-885, miR-375, miR-105–2, etc. regulate the expression of the PS, whereas miR-26a targets the TM to control the expression and function of the endogenous anticoagulants [202], [203], [229] (Fig. 2c-F). In this regard, the deregulated expression patterns of miR-340, miR-375 (reduced), and miR-27a/b (elevated) were found in patients with the COVID-19 [170], [196], [198], [222]. Considerably, all of the miRNAs affecting the other pathways/events (e.g., neutrophils/monocytes activities, NETosis, ECs dysfunction, the complement activation, and hypoxia/hypoxemia) can indirectly affect the fibrinolysis process and endogenous anticoagulants (Fig. 2a, 2b-C, 2c-E, 2c-G).
3.7. miRNAs affecting hypoxia/hypoxemia
Some miRNAs such as miR-138, miR-199a, miR-18a/b, miR-640, miR-424, etc. can negatively regulate the HIF-1α. However, miR-214, miR-135b, miR-675, etc. can induce its gene expression [212], [230], [231], [232]. The HIF-2α is a direct target of miR-185, miR-223, miR-145, etc. [232], [233], [234] (Fig. 2c-G). Moreover, miR-23a, miR-146a, miR-126, miR-130b, miR-31, miR-17, miR-103a-3p, etc. can inhibit the CXCL12 expression [183], [202], [235], [236], [237] (Fig. 2c-G). The non-optimal circular expression levels of miR-18a, miR-223, miR-146a, miR-126-3p, miR-31, miR-17 (reduced), and miR-199a (elevated) have been reported in patients with the COVID-19 [170], [171], [175], [176], [195], [196], [197], [198], [199]. Furthermore, all of the miRNAs affecting the other pathways/events (e.g., neutrophils and monocytes activities) (Fig. 2a) can also indirectly impact the hypoxia/hypoxemia.
4. miRNAs and interfering factors
Although miRNAs play crucial roles in controlling immunothrombosis process, clinical manifestations and paraclinical findings of patients with the critical COVID-19 indicate that the expressions and functions of these small RNAs have largely been disrupted [163], [169], [170], [171], [172], [173], [174], [175], [176], [177]. Various studies have demonstrated that several agents such as SARS-CoV-2-induced interfering factors can affect the expression levels and efficient functions of miRNAs which have been discussed in another paper [5]. According to in vitro and in vivo studies, the upregulated expressions of miRNAs are not always followed by their higher efficiency [238], [239]. Research evidence indicates that miRNAs regulate the expressions of their target molecules in a dose-dependent manner and display their best functions at optimal levels [240], [241]. The literature review revealed that several genetic agents and uncontrolled immunothrombosis-induced interfering factors could affect the expressions and functions of these non-coding RNAs (Table 1 ) which will be discussed in the following.
Table 1.
Some genetic agents and uncontrolled immunothrombosis-induced interfering factors effective on miRNAs functions/expressions that could be beneficial for the treatment of critical COVID-19.
| Interfering factor | Effect | Ref | Expected results |
|---|---|---|---|
| miR-SNPs | |||
| rs2431697 T allele | Expression reduction of miR-146a | [183], [246] | Increased expression of Mac-1, TLR2, TLR4, IL-8, CD40L, and CXCL12, Upregulated activity of NADPH oxidases and excessive production of ROS, Enhanced activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| rs2910164 CC genotype | [243] | ||
| rs57095329 A/G genotype | [244] | ||
| rs767649 A/T allele | Expression elevation of miR-155 | [245], [247] | Excessive production of ROS, IL-8, and PAD4, Reduced expression of CFH, Activation, dysfunction, and pyroptosis of ECs, Increased activation of neutrophils and monocytes, Higher complement activation, Enhanced NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| rs353292 CT/TT variant | Expression reduction of miR-143 | [248] | Increased expression of TLR2 and PAI-1, Upregulated activation of neutrophils, monocytes, and PLTs, Reduced/inefficient fibrinolysis, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| rs74693964 C/T variant | Expression reduction of miR-145 | [249] | Higher expression of TF, FXI, HIF-2α, C3, and PAI-1, Elevated complement activation, Increased neutrophils persistence and monocytes activation, Reduced/inefficient fibrinolysis, Upregulated NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| Complement system components | |||
| Sublytic doses of MAC | Expression elevation of miR-328 and miR-616 | [214] | Increased expression/deposition of C3, Inhibition of CD46 and CD59, Higher complement activation, Enhanced NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| C5a | Expression reduction of miR-193 and miR-133a | [250] | Increased production of Fg and Mac-1, Reduced u-PA and fibrinolysis regulation, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| Excessive expression of miR-26b | Deviation from optimal expression level and inefficient function of this miRNA, based on the manifestations of the critical COVID-19, Reduced regulation of P-selectin, FVIII, and IFN-γ expressions, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
||
| C3 | Expression reduction of miR-145 | [216] | Higher expression of TF, FXI, HIF-2α, C3, and PAI-1, Enhanced complement activation, Increased neutrophils persistence and monocytes activation, Reduced/inefficient fibrinolysis, Elevated NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| NADPH oxidases and ROS | |||
| ------- | Expression reduction of miR-29a/b, miR-29c-3p, miR-24–2, miR-145, miR-128, miR-let-7, miR-199a, miR-125b, and miR-126a | [192], [251], [252], [257], [258] | Increased expression/production of NADPH oxidases, ROS, Fg, VWF, TF, FIX, FX, FXI, HIF-1/2α, CXCL12, C3, PAI-1, IL-6, TLR4, TLR7, αIIbβ3, SNAP23, and P2Y12, Elevated neutrophils persistence and monocytes activation, Higher complement activation, Inefficient fibrinolysis, PLTs activation and degranulation, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| ------- | Expression elevation of miR-21, miR-146a, miR-9, and miR-143 | [251], [253], [254], [255], [256], [259] | Deviation from optimal expression levels and inefficient functions of these miRNAs, based on the manifestations of the critical COVID-19, Reduced regulation of Mac-1, TLR2, TLR4, IL-8, CD40L, CXCL12, and PAI-1 expressions, Upregulated activity of NADPH oxidases and excessive production of ROS, Decreased expression of CFH, Elevated activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Higher complement activation, Reduced/inefficient fibrinolysis, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| PAD enzymes and histone citrullination | |||
| ------- | Reduced expression of miR-let-7c-2, miR-29c, and miR-16 | [260], [261] | Upregulated expression of IL-6, TLR4, TLR7, SNAP23, and Fg, Enhanced activity of NADPH oxidases and excessive production of ROS, PLTs activation and degranulation, Activation, dysfunction, and pyroptosis of ECs, Increased neutrophils and monocytes activation, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| Hypoxia/hypoxemia | |||
| ------- | Expression elevation of miR-155, miR-21, miR-103, and miR-146a | [262], [264], [265], [266] | Deviation from optimal expression levels and inefficient functions of these miRNAs, based on the manifestations of the critical COVID-19, Upregulated activity of NADPH oxidases and excessive production of ROS, IL-8, and PAD4, Reduced expression of CFH, Decreased regulation of VWF, TF, CXCL12, Mac-1, TLR2, TLR4, IL-8, and CD40L expressions, Higher complement activation, Enhanced activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| ------- | Expression reduction of miR-150, miR-25, miR-15a, miR-205, and miR-34a | [267], [268], [269], [270], [271] | Elevated activity of NADPH oxidases and excessive production of ROS, αIIbβ3, IL-6R, and FVIII, Enhanced activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Higher complement activation, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| CXCL12 | Inefficient function of miR-31 | [237] | Increased expression of αIIbβ3 and CXCL12, Enhanced activation of neutrophils, monocytes, and PLTs, Upregulated NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| Coagulation and fibrinolysis mediators | |||
| Fibrin | Expression reduction of miR-146a | [272] | Increased expression of Mac-1, TLR2, TLR4, IL-8, CD40L, and CXCL12, Upregulated activity of NADPH oxidases and excessive production of ROS, Enhanced activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| PDGF-BB | Expression reduction of miR-145 | [273] | Higher expression of TF, FXI, HIF-2α, C3, and PAI-1, Elevated complement activation, Increased neutrophils persistence and monocytes activation, Reduced/inefficient fibrinolysis, Upregulated NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| aPC | Expression reduction of miR-199a, miR-29b, and miR-211 | [275] | Elevated expression of HIF-1α, Fg, and HIF-1α-induced CXCL12 Increased neutrophils persistence, Upregulated complement activation, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| t-PA | Expression reduction of miR-15a | [276] | Elevated expression of αIIbβ3, Enhanced activation of neutrophils, monocytes, and PLTs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
| Inefficient function of miR-146a | [272] | Increased expression of Mac-1, TLR2, TLR4, IL-8, CD40L, and CXCL12, Upregulated activity of NADPH oxidases and excessive production of ROS, Enhanced activation of neutrophils, monocytes, and PLTs, Activation, dysfunction, and pyroptosis of ECs, Increased NETosis, Severe inflammation and coagulation, Uncontrolled immunothrombosis |
|
Abbreviations: αIIbβ3, Integrin αIIbβ3; aPC, Activated protein C; C, Complement component; CD40L, Cluster of differentiation 40 ligand; CFH, Complement factor H; CXCL12, C-X-C motif chemokine ligand 12; ECs, Endothelial cells; Fg, Fibrinogen; FVIII, Factor VIII; FIX, Factor IX; FX, Factor X; FXI, Factor XI; HIF, Hypoxia-inducible factor; IFN-γ, Interferon gamma; IL, Interleukin; IL-6R, IL-6 receptor; Mac-1, Macrophage-1 antigen; MAC, Membrane attack complex; miRNAs, MicroRNAs; miR-SNPs, MicroRNA-single nucleotide polymorphisms; NADPH oxidase, Nicotinamide adenine dinucleotide phosphate oxidase; NET, Neutrophil extracellular trap; PAD, Protein arginine deiminase; PAI-1, Plasminogen Activator Inhibitor-1; PDGF-BB, Platelet-derived growth factor-BB; PLTs, Platelets; ROS, Reactive oxygen species; SNAP23, Synaptosomal-associated protein 23; TF, Tissue factor; TLR, Toll-like receptor; t-PA, Tissue plasminogen activator; u-PA, Urokinase plasminogen activator; VWF, Von Willebrand factor.
4.1. miRNA-single nucleotide polymorphisms
In fact, miRNA-single nucleotide polymorphisms (miR-SNPs) are the most rampant genetic variations in the human genome affecting the processing and expression of miRNAs (Table 1). As exemplified, the minor T allele of miR-SNP rs2431697 (located 16 kb upstream of pre-miR-146a) causes the reduced gene expression/production of mature miR-146a leading to the high-levels generation of pro-inflammatory mediators. In this regard, the in vitro and in vivo observations have revealed that the activation of neutrophils with this SNP is accompanied by more NETs formation consistent with the high plasma levels of NE found in the T allele carriers. The CC genotype of miR-SNP rs2910164 (located in the stem region opposite to mature miR-146a sequence) and the A/G genotype of miR-SNP rs57095329 (located in the promoter region of the miR-146a gene) are the two other SNPs in the miR-146a gene that can decrease its expression. The existence of the A/T allele of miR-SNP rs767649 (located in the regulation region of the miR-155 gene) can underlie the elevated transcriptional activity of this gene [183], [242], [243], [244], [245], [246], [247] (Table 1). Moreover, the reduced expressions of miR-143 and miR-145 have respectively been reported in the carriers of CT/TT variant of miR-SNP rs353292 (located 673 bp upstream from the start site) and C/T variant of miR-SNP rs74693964 (located 65 bp downstream of the miR-145 genomic region) [248], [249] (Table 1). Accordingly, the presence of the miR-SNPs in patients with the critical COVID-19 can be followed by the disrupted functions of these miRNAs (Table 1). The correlation between miR-SNPs and the incidence of various diseases (e.g., autoimmune disorders and cancers) has been proven. For instance, the downregulation of miR-146a, correlated with T allele of miR-SNP rs2431697, has been observed in patients with SLE, whereas higher expressions of miR-155 have been reported in patients with hepatocellular carcinoma (HCC) carrying the risk allele of miR-SNP rs767649. This can increase the risk of uncontrolled immunothrombosis in the COVID-19 patients with these comorbidities [246], [247].
4.2. Complement system components
According to the research evidence, sublytic (non-lytic) doses of MAC can affect the expression of miRNAs (Table 1). Although incomplete MAC complexes do not cause cell lysis, they activate many cellular inflammatory responses (e.g., NF-κB pathway) and affect the production of pro-inflammatory cytokines, adhesion molecules, and miRNAs. Accordingly, the prompt increased expressions of miR-328 and miR-616 have been reported following the cell stimulation with sublytic doses of MAC that can have specific effects on the expression of their target molecules and consequently different cellular processes [214] (Table 1). It has also been observed that C5a can change the expression of several miRNAs in the ECs. For instance, it significantly reduces the expression of miR-193 and miR-133a and underlies the increased expression of miR-26b [250] (Table 1). The C3-mediated downregulation of miR-145 has also been found [216] (Table 1).
4.3. NADPH oxidases and ROS
The reduced expressions of miR-29b, miR-24–2, miR-145, and miR-128, as well as the elevated expression of miR-21 have been observed under the influence of NADPH oxidases and ROS (Table 1). In fact, ROS can change the expression of these small regulatory RNAs in different ways such as affecting the enzymes involved in the miRNAs biogenesis, activities of transcription factors, and epigenetic modifications. Consistently, the ROS-induced enhanced activation of the NF-κB has been reported to result in the high-level production of miR-21, miR-146a, miR-9, and miR-143 in addition to the low-level expression of miR-let-7 (Table 1). Moreover, ROS reduce the generation of miR-199a and miR-125b through the induction of their genes hypermethylation [191], [251], [252], [253], [254], [255], [256], [257], [258], [259] (Table 1). Liu et al. reported that the NADPH oxidase 4 inhibitor provoked the expression of miR-29a and miR-29c-3p, and that the inhibition of NADPH oxidase 2 was followed by the induction of miR-126a expression [192] (Table 1).
4.4. PAD enzymes and histone citrullination
The PAD enzymes (e.g., PAD4 playing a key role in the NETosis) affect the chromatin organization and genes expression (e.g., miRNAs genes) by converting the arginine residues into the citrulline ones. According to the literature review, the expressions of miR-let-7c-2, miR-29c, and miR-16 are suppressed by the histone citrullination (Table 1), and the inhibition of PAD enzymes is accompanied by their expression restoration [260], [261].
4.5. Hypoxia/hypoxemia
Research evidence has shown that hypoxia/hypoxemia can affect the gene expression of molecules involved in the miRNAs biogenesis (e.g., Dicer) and also the ones participating in their functions (e.g., EIF2C4 encoding the AGO4). Accordingly, the upregulated expressions of miR-155, miR-21, miR-103, and miR-146a, as well as the reduced expressions of miR-150, miR-25, miR-15a, miR-205, and miR-34a were reported under the hypoxic condition in vitro and in vivo [231], [232], [262], [263], [264], [265], [266], [267], [268], [269], [270], [271] (Table 1). The miR-146a elevation was hypoxia level-dependent. The higher expression of CXCL12, induced by HIF-1α, was accompanied by the disrupted function of miR-31 [237] (Table 1).
4.6. Coagulation and fibrinolysis mediators
According to the research evidence, fibrin reduced the production of mature miR-146a in the ECs, and platelet-derived growth factor-BB (PDGF-BB), whose primary source was alpha-granules of the activated PLTs, suppressed the expression of miR-145 [272], [273], [274] (Table 1). Moreover, aPC had some reducing effects on the expression of miR-199a, miR-29b, and miR-211. The high levels of t-PA decreased the miR-15a expression levels and also caused the inefficient function of miR-146a [272], [275], [276] (Table 1).
5. Conclusion and therapeutic perspective
Representing the close interactions between the immune and coagulation systems, uncontrolled immunothrombosis/thromboinflammation is a life-threatening event leading to the administration of anticoagulant medicines in patients with the critical COVID-19. However, the efficiency of these medications is still open to dispute. Inadequate responses and also adverse effects such as the higher risks of severe hemorrhagic complications have been reported in some cases possibly resulting from the excessive hyperinflammation and disrupted functions of PLTs or coagulation factors [1], [2], [3], [88], [277], [278], [279], [280], [281], [282]. Moreover, preclinical and clinical studies respectively on relevant mouse models and patients with the critical COVID-19 reported the aggregated NETs-mediated vascular occlusion which is not resolved merely by anticoagulants [14], [53], [283]. In addition, NETs can trap the clots and impose some antifibrinolytic effects [284]. DNase and Ruxolitinib (a Janus kinase inhibitor) have respectively been utilized for the destruction of NETs and suppression of their formation [2], [283], [285]. However, they can remove the beneficial effects of NETs (e.g., provocation of the IFNs-I release). Moreover, a high level of human cathelicidin LL-37, which is produced by neutrophils and can stabilize the NETs against DNase, has been observed in the lung tissue and also in an embedded form inside the NETs of the COVID-19 patients and has led to the inefficiency of DNase [286], [287]. Cellular heparan sulfate (HS) has also been proposed as a co-receptor for the SARS-CoV-2/ACE2 interaction. Thus, exogenous heparin can affect viral attachment and infection [3], [288]. Heparin may also not work efficiently or even cause resistance in patients suffering from the critical COVID-19 with a low level of free AT and functional defects of ECs [87], [281]. Hence, the efficiency of anticoagulant medicines is yet to be completely confirmed in the critical COVID-19, and alternative therapeutic strategies are needed. As discussed earlier, miRNAs act as the fine-tuners of gene expression but not suppressors. Due to the extensive roles of these small RNAs in different cellular pathways, their optimal expression levels or efficient functions may underpin the homeostasis of the immune and coagulation systems rescuing the patients with the critical COVID-19. Above all, some of them (e.g., miR-146a and miR-155) regulate the expressions of several key molecules involved in uncontrolled immunothrombosis accentuating their roles in the regulation of this process (Fig. 2a-2c). However, multiple factors may disrupt their normal expressions and functions (Table 1). The adoption of some therapeutic strategies for resolving the interferer factors may pave the way for the optimal expression and efficient functions of miRNAs leading to the reduction of complications incidence and mortality in patients with the critical COVID-19. In this regard, clustered regularly interspaced short palindromic repeats (CRISPR)-based therapeutic approaches (e.g., CRISPR-associated protein 9 (Cas9)) can be beneficial. These are promising methods for a permanent modification of the genome. Most importantly, the novel designed web tool called SNP-CRISPR has improved the systematic study of the SNP variants and can be used for the elimination of negative effects of miR-SNPs on the expressions/functions of miRNAs [289]. Since the majority of these interfering agents result from the hyperinflammatory and hyperoxidant states, the utilization of anti-inflammatory and anti-oxidant drugs can be greatly advantageous in restraining uncontrolled immunothrombosis/thromboinflammation [3], [5]. Various studies have proven the brilliant anti-inflammatory and anti-oxidant effects of a wide spectrum of medications in the COVID-19 [290], [291], [292], [293]. In this regard, Al-kuraishy et al. reported that Metformin, with anti-oxidant properties, played a considerable role alleviating the severity of the COVID-19 and its complications (e.g., acute lung injury (ALI) and acute IS (AIS)) through the modulation of inflammatory reactions in patients with the type II diabetes mellitus (T2DM) comorbidity [291]. The sequential combination therapy with Colchicine and Doxycycline has also been followed by the restriction of inflammation and remission of the patients with the critical COVID-19 [292]. Another study has demonstrated that the administration of Allopurinol in patients with the critical COVID-19 can lead to a significant reduction of inflammatory factors and oxidative stress index [293]. The application of AMY-101 and Narsoplimab (C3 and MASP-2 inhibitors, respectively) has also been accompanied by attenuation of inflammation [294], [295]. Not only do these medications control the hyperinflammatory/hyperoxidant states but they may also recover the efficient functions and optimal expressions of miRNAs resulting in the limitation of uncontrolled immunothrombosis/thromboinflammation. Some other groups of drugs such as anti-heart failure ones, anti-histamines, leukotriene receptor antagonists (LTRAs), the phosphodiesterase (PDE) inhibitors, etc., with confirmed anti-inflammatory and anti-oxidant roles, can also be administrated for the amelioration of miRNAs performances [17], [18], [296], [297], [298], [299], [300], [301]. Produced in the body, some alarming agents can also be helpful. For instance, Neopterin plays a protective role against the ECs dysfunction and reduces the inflammatory pathways. The development of its agonists can be of great importance in retrieving the efficiency of the miRNAs [302]. The estrogen therapy, especially in women during the postmenopausal period, can also be prescribed due to its eminent anti-inflammatory roles [303]. Furthermore, as miRNAs display their best functions in the optimal levels [240], [241], their upregulated expressions are not always followed by their higher efficiency [238], [239]. Moreover, each miRNA can neutralize the functions of the other ones by affecting some target molecules (e.g., induction and inhibition of IL-8 by miR-155 and miR-146a, respectively). Therefore, the identification and application of the drugs affecting the miRNAs and bringing them to the optimal levels are more efficient than the administration of the ones merely affecting a special protein molecule [5], [21], [162]. The drugs β-D-mannuronic acid (M2000) and α-L-guluronic acid (G2013) are the two novel members of the non-steroidal anti-inflammatory drugs (NSAIDs) family whose anti-inflammatory and immunomodulatory properties have been proven in the cellular, animal, and human levels. Interestingly, they leave some regulatory effects on the expressions and functions of miRNAs (e.g., miR-146a and miR-155) [238], [239], [304], [305], [306], [307], [308], [309], [310], [311], [312], [313], [314], [315], [316], [317], [318], [319]. The modulation of the production and performance of these two miRNAs (and also the other miRNAs) may simultaneously alleviate the hyperinflammation and restrict the uncontrolled immunothrombosis/thromboinflammation. As the dysfunction of ECs is correlated with the activation of endothelial matrix metalloproteinase (MMP)-2 by MMP-9 (existing in NETs), the reduction of these MMPs by M2000 can be of great importance [62], [320]. Moreover, M2000 and G2013 have anti-oxidant and angioprotective properties reinforcing their positive effects on the limitation of uncontrolled immunothrombosis [321], [322], [323], [324]. In addition, the phase III clinical trial of drug G2013 (IRCT20080901001165N64) is being conducted on patients with moderate to severe symptoms of the COVID-19. The regulatory effects of Metformin, Colchicine, and Doxycycline on the expression levels of miRNAs have also been illustrated [325], [326], [327]. Moreover, the modulation of miRNAs content of EVs (e.g., exosomal ones), released by the cells with the key roles in the uncontrolled immunothrombosis (e.g., activated PLTs and ECs) through drug consumption or utilization of the non-immunogenic engineered exosomes, may limit the uncontrolled immunothrombosis/thromboinflammation in patients with the critical COVID-19 [162], [328].
A few studies have meticulously evaluated the roles of miRNAs in the critical COVID-19, especially in the uncontrolled immunothrombosis. Moreover, most studies have assessed the expression levels of miRNAs in the circulation of the patients. Their tissue as well as cellular expression amounts and functions have remained poorly identified. However, this review cast light on the pivotal roles of these small RNAs in the processes involved in the uncontrolled immunothrombosis and can act as a beneficial clue to design and conduct the future preclinical and clinical studies. In addition, the interfering factors, affecting the miRNAs expression/function, should be analyzed further in preclinical and clinical studies.
Funding
This review article received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Author contribution
SSM-J and MA collected the data following the raising the issue. SSM-J and MA then designed the figures and wrote/edited the manuscript in equal parts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
SSM-J and MA appreciate all of the friendly consultations and advice of Prof. Abbas Mirshafiey, Dept. of Immunology, School of public health, Tehran University of Medical Sciences (TUMS) to prepare the manuscript in the best way.
Data availability
No data was used for the research described in the article.
References
- 1.Leentjens J., van Haaps T.F., Wessels P.F., Schutgens R.E., Middeldorp S. COVID-19-associated coagulopathy and antithrombotic agents—lessons after 1 year. Lancet Haematol. 2021 doi: 10.1016/S2352-3026(21)00105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loo J., Spittle D.A., Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76(4):412–420. doi: 10.1136/thoraxjnl-2020-216243. [DOI] [PubMed] [Google Scholar]
- 4.Mirshafiey A., Aslani M., Mortazavi-Jahromi S.S. Possibilities for the Entrance of SARS-Cov-2 as an Archaeal Virus into the Ecosystem. Iranian J. Public Health. 2021:2608–2609. doi: 10.18502/ijph.v50i12.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslani M., Mortazavi-Jahromi S.S., Mirshafiey A. Cytokine storm in the pathophysiology of COVID-19: possible functional disturbances of miRNAs. Int. Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.108172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W., Li M., Luo G., Wu X., Su B., Zhao L., Zhang S., Chen X., Jia M., Zhu J. The inflammatory factors associated with disease severity to predict COVID-19 progression. J. Immunol. 2021;206(7):1597–1608. doi: 10.4049/jimmunol.2001327. [DOI] [PubMed] [Google Scholar]
- 7.Thwaites R.S., Sanchez Sevilla Uruchurtu A., Siggins M.K., Liew F., Russell C.D., Moore S.C., Fairfield C., Carter E., Abrams S., Short C.-E. Inflammatory profiles across the spectrum of disease reveal a distinct role for GM-CSF in severe COVID-19. Sci. Immunol. 2021;6(57):eabg9873. doi: 10.1126/sciimmunol.abg9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saris A., Reijnders T.D., Nossent E.J., Schuurman A.R., Verhoeff J., Van Asten S., Bontkes H., Blok S., Duitman J., Bogaard H.-J. Distinct cellular immune profiles in the airways and blood of critically ill patients with COVID-19. Thorax. 2021;76(10):1010–1019. doi: 10.1136/thoraxjnl-2020-216256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohansal Vajari M., Shirin M., Pourbagheri-Sigaroodi A., Akbari M.E., Abolghasemi H., Bashash D. COVID-19-related coagulopathy: A review of pathophysiology and pharmaceutical management. Cell Biol. Int. 2021 doi: 10.1002/cbin.11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher-Sandersjöö A., Bellander B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review, Thrombosis research. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Kuraishy H.M., Al-Gareeb A.I., Alkazmi L., Alexiou A., Batiha G.-E.-S. Levamisole Therapy in COVID-19. Viral Immunol. 2021;34(10):722–725. doi: 10.1089/vim.2021.0042. [DOI] [PubMed] [Google Scholar]
- 12.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., Kaptein F., van Paassen J., Stals M., Huisman M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C., Bouman C.C., Beenen L.F., Kootte R.S., Heijmans J. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ackermann M., Anders H.-J., Bilyy R., Bowlin G.L., Daniel C., De Lorenzo R., Egeblad M., Henneck T., Hidalgo A., Hoffmann M. Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ. 2021:1–15. doi: 10.1038/s41418-021-00805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edler C., Schröder A.S., Aepfelbacher M., Fitzek A., Heinemann A., Heinrich F., Klein A., Langenwalder F., Lütgehetmann M., Meißner K. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int. J. Legal Med. 2020;134(4):1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Kuraishy H.M., Al-Gareeb A.I., Qusti S., Alshammari E.M., Gyebi G.A., Batiha G.-E.-S. Covid-19-induced dysautonomia: a menace of sympathetic storm. ASN neuro. 2021;13 doi: 10.1177/17590914211057635. 17590914211057635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.H.M. Al-kuraishy, A.I. Al-Gareeb, M.S. Fageyinbo, G.E.-S. Batiha, Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19, Future Sci. OA (0) (2022) FSO797. [DOI] [PMC free article] [PubMed]
- 18.Onohuean H., Al-Kuraishy H.M., Al-Gareeb A.I., Qusti S., Alshammari E.M., Batiha G.-E.-S. Covid-19 and development of heart failure: mystery and truth. Naunyn-Schmiedeberg's Arch. Pharmacol. 2021;394(10):2013–2021. doi: 10.1007/s00210-021-02147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guterres A., de Azeredo Lima C.H., Miranda R.L., Gadelha M.R. What is the potential function of microRNAs as biomarkers and therapeutic targets in COVID-19? Infection, Genetics Evol. 2020;85 doi: 10.1016/j.meegid.2020.104417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Lei C., He Q., Pan Z., Xiao D., Tao Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Molecular cancer. 2018;17(1):64. doi: 10.1186/s12943-018-0765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortazavi-Jahromi S.S., Aslani M., Mirshafiey A. A comprehensive review on miR-146a molecular mechanisms in a wide spectrum of immune and non-immune inflammatory diseases. Immunol. Lett. 2020 doi: 10.1016/j.imlet.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 22.El-Nabi S.H., Elhiti M., El-Sheekh M. A new approach for COVID-19 treatment by micro-RNA. Med. Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu W., Wang Y., Ma Y., Yang J. MiR-223 plays a protecting role in neutrophilic asthmatic mice through the inhibition of NLRP3 inflammasome. Respir. Res. 2020;21(1):1–13. doi: 10.1186/s12931-020-01374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu D., Zhang N., Zhang X., Qin M., Dong Y., Jin L. MiR-410 down-regulates the expression of interleukin-10 by targeting STAT3 in the pathogenesis of systemic lupus erythematosus. Cell. Physiol. Biochem. 2016;39(1):303–315. doi: 10.1159/000445625. [DOI] [PubMed] [Google Scholar]
- 25.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Hussaniy H.A., Al-Harcan N.A.H., Alexiou A., Batiha G.-E.-S. Neutrophil Extracellular Traps (NETs) and Covid-19: A new frontiers for therapeutic modality. Int. Immunopharmacol. 2022;108516 doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing H., Chen X., Zhang S., Liu H., Zhang C., Du J., Li Y., Wu X., Li M., Xiang M. Neutrophil extracellular traps (NETs): the role of inflammation and coagulation in COVID-19. Am. J. Translational Res. 2021;13(8):8575. [PMC free article] [PubMed] [Google Scholar]
- 27.Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann. Hematol. 2020:1–7. doi: 10.1007/s00277-020-04138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansour A., Bachelot-Loza C., Nesseler N., Gaussem P., Gouin-Thibault I. P2Y12 inhibition beyond thrombosis: Effects on inflammation. Int. J. Mol. Sci. 2020;21(4):1391. doi: 10.3390/ijms21041391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Joncour A., Biard L., Vautier M., Bugaut H., Mekinian A., Maalouf G., Vieira M., Marcelin A.-G., Rosenzwajg M., Klatzmann D. Neutrophil–platelet and monocyte–platelet aggregates in COVID-19 patients. Thromb. Haemost. 2020;120(12):1733–1735. doi: 10.1055/s-0040-1718732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris G., Bortolasci C.C., Puri B.K., Olive L., Marx W., O'Neil A., Athan E., Carvalho A., Maes M., Walder K. Preventing the development of severe COVID-19 by modifying immunothrombosis. Life Sci. 2021;264 doi: 10.1016/j.lfs.2020.118617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 33.de Bont C.M., Boelens W.C., Pruijn G.J. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019;16(1):19–27. doi: 10.1038/s41423-018-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muraro S.P., De Souza G.F., Gallo S.W., Da Silva B.K., De Oliveira S.D., Vinolo M.A.R., Saraiva E.M., Porto B.N. Respiratory Syncytial Virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci. Rep. 2018;8(1):1–12. doi: 10.1038/s41598-018-32576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short K.R., von Köckritz-Blickwede M., Langereis J.D., Chew K.Y., Job E.R., Armitage C.W., Hatcher B., Fujihashi K., Reading P.C., Hermans P.W. Antibodies mediate formation of neutrophil extracellular traps in the middle ear and facilitate secondary pneumococcal otitis media. Infect. Immun. 2014;82(1):364–370. doi: 10.1128/IAI.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veras F.P., Pontelli M.C., Silva C.M., Toller-Kawahisa J.E., de Lima M., Nascimento D.C., Schneider A.H., Caetité D., Tavares L.A., Paiva I.M. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020;217(12):e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 38.Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P.R., Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P., Li M., Lindberg M.R., Kennett M.J., Xiong N., Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207(9):1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H.-U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009;16(11):1438–1444. doi: 10.1038/cdd.2009.96. [DOI] [PubMed] [Google Scholar]
- 41.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22(2):146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., Barthwal M.K., Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS ONE. 2012;7(10):e48111. doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hofbauer T., Scherz T., Müller J., Heidari H., Staier N., Panzenböck A., Mangold A., Lang I.M. Arterial hypertension enhances neutrophil extracellular trap formation via an angiotensin-II-dependent pathway. Atherosclerosis. 2017;263:e67–e68. [Google Scholar]
- 44.Rosa B.A., Ahmed M., Singh D.K., Choreño-Parra J.A., Cole J., Jiménez-Álvarez L.A., Rodríguez-Reyna T.S., Singh B., Gonzalez O., Carrion R. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS-CoV-2 infection. Commun. Biol. 2021;4(1):1–14. doi: 10.1038/s42003-021-01829-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schauer C., Janko C., Munoz L.E., Zhao Y., Kienhöfer D., Frey B., Lell M., Manger B., Rech J., Naschberger E. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20(5):511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 46.Hahn J., Schauer C., Czegley C., Kling L., Petru L., Schmid B., Weidner D., Reinwald C., Biermann M.H., Blunder S. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33(1):1401–1414. doi: 10.1096/fj.201800752R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keragala C.B., Draxler D.F., McQuilten Z.K., Medcalf R.L. Haemostasis and innate immunity–a complementary relationship: a review of the intricate relationship between coagulation and complement pathways. Br. J. Haematol. 2018;180(6):782–798. doi: 10.1111/bjh.15062. [DOI] [PubMed] [Google Scholar]
- 48.J.-J. Lai, F.M. Cruz, K.L. Rock, Immune sensing of cell death through recognition of histone sequences by C-type lectin-receptor-2d causes inflammation and tissue injury, Immunity 52(1) (2020) 123-135. e6. [DOI] [PMC free article] [PubMed]
- 49.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., Cerotto V., Guglielmini G., Gori F., Malvestiti M. Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J. Infect. Dis. 2021;223(6):933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szturmowicz M., Demkow U. Neutrophil Extracellular Traps (NETs) in Severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. 2021;22(16):8854. doi: 10.3390/ijms22168854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C. Complement and tissue factor–enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020;130(11):6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M. Neutrophil extracellular traps in COVID-19. JCI insight. 2020;5(11) doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Middleton E.A., He X.-Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ng H., Havervall S., Rosell A., Aguilera K., Parv K., Von Meijenfeldt F.A., Lisman T., Mackman N., Thålin C., Phillipson M. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2021;41(2):988–994. doi: 10.1161/ATVBAHA.120.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925. doi: 10.1016/j.ebiom.2020.102925. Epub 2020/07/31. doi: 10.1016/j.ebiom. 2020.102925. PubMed PMID: 32745993, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Semeraro F., Ammollo C.T., Morrissey J.H., Dale G.L., Friese P., Esmon N.L., Esmon C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood, J. Am. Soc. Hematol. 2011;118(7):1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ammollo C.T., Semeraro F., Xu J., Esmon N., Esmon C. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J. Thromb. Haemost. 2011;9(9):1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. 2010;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015;74(7):1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pieterse E., Rother N., Garsen M., Hofstra J.M., Satchell S.C., Hoffmann M., Loeven M.A., Knaapen H.K., van der Heijden O.W., Berden J.H. Neutrophil extracellular traps drive endothelial-to-mesenchymal transition. Arterioscler. Thromb. Vasc. Biol. 2017;37(7):1371–1379. doi: 10.1161/ATVBAHA.117.309002. [DOI] [PubMed] [Google Scholar]
- 64.Becker K., Beythien G., de Buhr N., Stanelle-Bertram S., Tuku B., Kouassi N.M., Beck S., Zickler M., Allnoch L., Gabriel G. Vasculitis and neutrophil extracellular traps in lungs of golden Syrian hamsters with SARS-CoV-2. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.640842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H., Wang Q., Venugopal J., Wang J., Kleiman K., Guo C., Eitzman D.T. Obesity-induced endothelial dysfunction is prevented by neutrophil extracellular trap inhibition. Sci. Rep. 2018;8(1):1–7. doi: 10.1038/s41598-018-23256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H., Wang C., Zhao M.H., Chen M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 2015;181(3):518–527. doi: 10.1111/cei.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreiber A., Rousselle A., Becker J.U., Von Mässenhausen A., Linkermann A., Kettritz R. Necroptosis controls NET generation and mediates complement activation, endothelial damage, and autoimmune vasculitis. Proc. Natl. Acad. Sci. 2017;114(45):E9618–E9625. doi: 10.1073/pnas.1708247114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Trans. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., Panigada M., Aliberti S., Blasi F., Tedesco F. Complement activation in patients with COVID-19: a novel therapeutic target. J. Allergy Clin. Immunol. 2020;146(1):215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chauhan A.J., Wiffen L.J., Brown T.P. COVID-19: a collision of complement, coagulation and inflammatory pathways. J. Thromb. Haemost. 2020;18(9):2110–2117. doi: 10.1111/jth.14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali Y.M., Ferrari M., Lynch N.J., Yaseen S., Dudler T., Gragerov S., Demopulos G., Heeney J.L., Schwaeble W.J. Lectin pathway mediates complement activation by SARS-CoV-2 proteins. Front. Immunol. 2021;12:2645. doi: 10.3389/fimmu.2021.714511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarlhelt I., Nielsen S.K., Jahn C.X.H., Hansen C.B., Pérez-Alós L., Rosbjerg A., Bayarri-Olmos R., Skjoedt M.-O., Garred P. SARS-CoV-2 Antibodies Mediate Complement and Cellular Driven Inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.767981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritis K., Doumas M., Mastellos D., Micheli A., Giaglis S., Magotti P., Rafail S., Kartalis G., Sideras P., Lambris J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006;177(7):4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 74.Ikeda K., Nagasawa K., Horiuchi T., Tsuru T., Nishizaka H., Niho Y. C5a induces tissue factor activity on endothelial cells. Thromb. Haemost. 1997;77(02):394–398. [PubMed] [Google Scholar]
- 75.Landsem A., Fure H., Christiansen D., Nielsen E., Østerud B., Mollnes T., Brekke O. The key roles of complement and tissue factor in Escherichia coli-induced coagulation in human whole blood. Clin. Exp. Immunol. 2015;182(1):81–89. doi: 10.1111/cei.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Foreman K.E., Vaporciyan A.A., Bonish B.K., Jones M.L., Johnson K.J., Glovsky M.M., Eddy S.M., Ward P.A. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 1994;94(3):1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sauter R.J., Sauter M., Reis E.S., Emschermann F.N., Nording H., Ebenhöch S., Kraft P., Münzer P., Mauler M., Rheinlaender J. Functional relevance of the anaphylatoxin receptor C3aR for platelet function and arterial thrombus formation marks an intersection point between innate immunity and thrombosis. Circulation. 2018;138(16):1720–1735. doi: 10.1161/CIRCULATIONAHA.118.034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Subramaniam S., Jurk K., Hobohm L., Jäckel S., Saffarzadeh M., Schwierczek K., Wenzel P., Langer F., Reinhardt C., Ruf W. Distinct contributions of complement factors to platelet activation and fibrin formation in venous thrombus development. Blood, J. Am. Soc. Hematol. 2017;129(16):2291–2302. doi: 10.1182/blood-2016-11-749879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo R.-F., Ward P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 80.Krarup A., Wallis R., Presanis J.S., Gál P., Sim R.B. Simultaneous activation of complement and coagulation by MBL-associated serine protease 2. PLoS ONE. 2007;2(7):e623. doi: 10.1371/journal.pone.0000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.T. Wiedmer, C.T. Esmon, P.J. Sims, Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase, (1986). [PubMed]
- 82.Hattori R., Hamilton K., McEver R., Sims P. Complement proteins C5b–9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J. Biol. Chem. 1989;264(15):9053–9060. [PubMed] [Google Scholar]
- 83.Kastl S., Speidl W., Kaun C., Rega G., Assadian A., Weiss T., Valent P., Hagmueller G., Maurer G., Huber K. The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-κB activation. J. Thromb. Haemost. 2006;4(8):1790–1797. doi: 10.1111/j.1538-7836.2006.02046.x. [DOI] [PubMed] [Google Scholar]
- 84.Hess K., Ajjan R., Phoenix F., Dobó J., Gál P., Schroeder V. Effects of MASP-1 of the complement system on activation of coagulation factors and plasma clot formation. PLoS ONE. 2012;7(4):e35690. doi: 10.1371/journal.pone.0035690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dobó J., Schroeder V., Jenny L., Cervenak L., Závodszky P., Gál P. Multiple roles of complement MASP-1 at the interface of innate immune response and coagulation. Mol. Immunol. 2014;61(2):69–78. doi: 10.1016/j.molimm.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 86.Shagdarsuren E., Bidzhekov K., Mause S.F., Simsekyilmaz S., Polakowski T., Hawlisch H., Gessner J.E., Zernecke A., Weber C. C5a receptor targeting in neointima formation after arterial injury in atherosclerosis-prone mice. Circulation. 2010;122(10):1026–1036. doi: 10.1161/CIRCULATIONAHA.110.954370. [DOI] [PubMed] [Google Scholar]
- 87.Fang X.-Z., Wang Y.-X., Xu J.-Q., He Y.-J., Peng Z.-K., Shang Y. Immunothrombosis in Acute Respiratory Dysfunction of COVID-19. Front. Immunol. 2021;12:2028. doi: 10.3389/fimmu.2021.651545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zuo Y., Warnock M., Harbaugh A., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Knight J.S., Kanthi Y., Lawrence D.A. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nougier C., Benoit R., Simon M., Desmurs-Clavel H., Marcotte G., Argaud L., David J.S., Bonnet A., Negrier C., Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J. Thromb. Haemost. 2020;18(9):2215–2219. doi: 10.1111/jth.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bachler M., Bösch J., Stürzel D.P., Hell T., Giebl A., Ströhle M., Klein S.J., Schäfer V., Lehner G.F., Joannidis M. Impaired fibrinolysis in critically ill COVID-19 patients. Br. J. Anaesth. 2021;126(3):590–598. doi: 10.1016/j.bja.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onishi T., Shimonishi N., Takeyama M., Furukawa S., Ogiwara K., Nakajima Y., Kasahara K., Nishio K., Yoshimoto K., Inoue S. The balance of comprehensive coagulation and fibrinolytic potential is disrupted in patients with moderate to severe COVID-19. Int. J. Hematol. 2022:1–12. doi: 10.1007/s12185-022-03308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fei Y., Tang N., Liu H., Cao W. Coagulation DysfunctionA Hallmark in COVID-19. Arch. Pathol. Lab. Med. 2020;144(10):1223–1229. doi: 10.5858/arpa.2020-0324-SA. [DOI] [PubMed] [Google Scholar]
- 93.Wojta J., Huber K., Valent P. New aspects in thrombotic research: complement induced switch in mast cells from a profibrinolytic to a prothrombotic phenotype. Pathophysiol. Haemost. Thromb. 2003;33(5–6):438–441. doi: 10.1159/000083842. [DOI] [PubMed] [Google Scholar]
- 94.Brogren H., Wallmark K., Deinum J., Karlsson L., Jern S. Platelets retain high levels of active plasminogen activator inhibitor 1. PLoS ONE. 2011;6(11):e26762. doi: 10.1371/journal.pone.0026762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang S., Tanaka T., Inoue H., Ono C., Hashimoto S., Kioi Y., Matsumoto H., Matsuura H., Matsubara T., Shimizu K. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. 2020;117(36):22351–22356. doi: 10.1073/pnas.2010229117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.H.C. Kwaan, Coronavirus disease 2019: the role of the fibrinolytic system from transmission to organ injury and sequelae, Seminars in thrombosis and hemostasis, Thieme Medical Publishers, 2020, pp. 841-844. [DOI] [PMC free article] [PubMed]
- 97.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., De Laat B., Schiffman J.D., Yost C.C., Rondina M.T., Wolberg A.S. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler. Thromb. Vasc. Biol. 2021;41(1):401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.F.L. Wright, T.O. Vogler, E.E. Moore, H.B. Moore, M.V. Wohlauer, S. Urban, T.L. Nydam, P.K. Moore, R.C. McIntyre Jr, Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection, Journal of the American College of Surgeons 231(2) (2020) 193-203. e1. [DOI] [PMC free article] [PubMed]
- 99.Ibañez C., Perdomo J., Calvo A., Ferrando C., Reverter J., Tassies D., Blasi A. High D dimers and low global fibrinolysis coexist in COVID19 patients: what is going on in there? J. Thromb. Thrombolysis. 2021;51(2):308–312. doi: 10.1007/s11239-020-02226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D. Cabrera-Garcia, A. Miltiades, S. Parsons, K. Elisman, M.T. Mansouri, G. Wagener, N.L. Harrison, High levels of plasminogen activator inhibitor-1, tissue plasminogen activator and fibrinogen in patients with severe COVID-19, MedRxiv (2021) 2020.12. 29.20248869.
- 101.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goshua G., Pine A.B., Meizlish M.L., Chang C.-H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chaurasia S.N., Kushwaha G., Kulkarni P.P., Mallick R.L., Latheef N.A., Mishra J.K., Dash D. Platelet HIF-2α promotes thrombogenicity through PAI-1 synthesis and extracellular vesicle release. Haematologica. 2019;104(12):2482. doi: 10.3324/haematol.2019.217463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Massberg S., Grahl L., von Bruehl M.-L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A.B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16(8):887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 105.Francischetti I.M., Toomer K., Zhang Y., Jani J., Siddiqui Z., Brotman D.J., Hooper J.E., Kickler T.S. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobesh P.P., Trujillo T.C. Coagulopathy, venous thromboembolism, and anticoagulation in patients with COVID-19. Pharmacother.: J. Hum. Pharmacol. Drug Ther. 2020;40(11):1130–1151. doi: 10.1002/phar.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anaklı İ., Özcan P.E., Polat Ö., Orhun G., Alay G.H., Tuna V., Çeliksoy E., Kılıç M., Mercan M., Ali A. Prognostic value of antithrombin levels in COVID-19 patients and impact of fresh frozen plasma treatment: a retrospective study. Turkish J. Hematol. 2021;38(1):15. doi: 10.4274/tjh.galenos.2021.2020.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.G. Lippi, B.M. Henry, F. Sanchis-Gomar, Plasma antithrombin values are significantly decreased in coronavirus disease 2019 (COVID-19) patients with severe illness, Seminars in Thrombosis and Hemostasis, Thieme Medical Publishers, Inc., 2021, pp. 460-462. [DOI] [PubMed]
- 109.Gerotziafas G.T., Sergentanis T.N., Voiriot G., Lassel L., Papageorgiou C., Elabbadi A., Turpin M., Vandreden P., Papageorgiou L., Psaltopoulou T. Derivation and validation of a predictive score for disease worsening in patients with COVID-19. Thromb. Haemost. 2020;120(12):1680–1690. doi: 10.1055/s-0040-1716544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jin X., Duan Y., Bao T., Gu J., Chen Y., Li Y., Mao S., Chen Y., Xie W. The values of coagulation function in COVID-19 patients. PLoS ONE. 2020;15(10):e0241329. doi: 10.1371/journal.pone.0241329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mast A.E., Wolberg A.S., Gailani D., Garvin M.R., Alvarez C., Miller J.I., Aronow B., Jacobson D. SARS-CoV-2 suppresses anticoagulant and fibrinolytic gene expression in the lung. Elife. 2021;10:e64330. doi: 10.7554/eLife.64330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Enocsson H., Idoff C., Gustafsson A., Govender M., Hopkins F., Larsson M., Nilsdotter-Augustinsson Å., Sjöwall J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) Independently Predicts Severity and Length of Hospitalisation in Patients With COVID-19. Front. Med. 2021;8 doi: 10.3389/fmed.2021.791716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Velissaris D., Lagadinou M., Paraskevas T., Oikonomou E., Karamouzos V., Karteri S., Bousis D., Pantzaris N., Tsiotsios K., Marangos M. Evaluation of plasma soluble urokinase plasminogen activator receptor levels in patients with COVID-19 and non-COVID-19 pneumonia: an observational cohort study. J. Clin. Med. Res. 2021;13(9):474. doi: 10.14740/jocmr4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takeshita Y., Terada J., Hirasawa Y., Kinoshita T., Tajima H., Koshikawa K., Kinouchi T., Isaka Y., Shionoya Y., Fujikawa A. Elevated TAT in COVID-19 Patients with Normal D-Dimer as a Predictor of Severe Respiratory Failure: A Retrospective Analysis of 797 Patients. J. Clin. Med. 2021;11(1):134. doi: 10.3390/jcm11010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stanne T.M., Pedersen A., Gisslén M., Jern C. Low admission protein C levels are a risk factor for disease worsening and mortality in hospitalized patients with COVID-19. Thromb. Res. 2021;204:13–15. doi: 10.1016/j.thromres.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stoichitoiu L.E., Pinte L., Balea M.I., Nedelcu V., Badea C., Baicus C. Anticoagulant protein S in COVID-19: low activity, and associated with outcome. Rom J Intern Med. 2020;58(4):251–258. doi: 10.2478/rjim-2020-0024. [DOI] [PubMed] [Google Scholar]
- 117.J.A. Borovac, H.M. Van Beusekom, Thrombomodulin as an early marker of endotheliitis in COVID-19.
- 118.Fodor A., Tiperciuc B., Login C., Orasan O.H., Lazar A.L., Buchman C., Hanghicel P., Sitar-Taut A., Suharoschi R., Vulturar R. Endothelial dysfunction, inflammation, and oxidative stress in COVID-19—Mechanisms and therapeutic targets. Oxid. Med. Cell. Longevity. 2021;2021 doi: 10.1155/2021/8671713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nagashima S., Mendes M.C., Camargo Martins A.P., Borges N.H., Godoy T.M., Miggiolaro A.F.R.D.S., da Silva Dezidério F., Machado-Souza C., De Noronha L. Endothelial dysfunction and thrombosis in patients with COVID-19—brief report. Arterioscler. Thromb. Vasc. Biol. 2020;40(10):2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin Y., Ji W., Yang H., Chen S., Zhang W., Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduction Targeted Ther. 2020;5(1):1–13. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., McAvoy E., Sinclair G.D. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13(4):463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 122.Laforge M., Elbim C., Frère C., Hémadi M., Massaad C., Nuss P., Benoliel J.-J., Becker C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4):149. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dominic P., Ahmad J., Bhandari R., Pardue S., Solorzano J., Jaisingh K., Watts M., Bailey S.R., Orr A.W., Kevil C.G. Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doğan S., Bal T., Çabalak M., Dikmen N., Yaqoobi H., Ozcan O. Oxidative stress index can be a new marker related to disease severity in COVID-19. Turkish J. Biochem. 2021;46(4):349–357. [Google Scholar]
- 126.Desideri G., Bravi M.C., Tucci M., Croce G., Marinucci M.C., Santucci A., Alesse E., Ferri C. Angiotensin II inhibits endothelial cell motility through an AT1-dependent oxidant-sensitive decrement of nitric oxide availability. Arterioscler. Thromb. Vasc. Biol. 2003;23(7):1218–1223. doi: 10.1161/01.ATV.0000078521.51319.65. [DOI] [PubMed] [Google Scholar]
- 127.Ruhl L., Pink I., Kühne J.F., Beushausen K., Keil J., Christoph S., Sauer A., Boblitz L., Schmidt J., David S. Endothelial dysfunction contributes to severe COVID-19 in combination with dysregulated lymphocyte responses and cytokine networks. Signal Transduction Targeted Ther. 2021;6(1):1–15. doi: 10.1038/s41392-021-00819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong M., Jiang Y., Xia D., Xiong Y., Zheng Q., Chen F., Zou L., Xiao W., Zhu Y. Elevated expression of serum endothelial cell adhesion molecules in COVID-19 patients. J. Infect. Dis. 2020;222(6):894–898. doi: 10.1093/infdis/jiaa349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boknäs N., Laine C., Hillarp A., Macwan A.S., Gustafsson K.M., Lindahl T.L., Holmström M. Associations between hemostatic markers and mortality in COVID-19–Compounding effects of D-dimer, antithrombin and PAP complex. Thromb. Res. 2022;213:97–104. doi: 10.1016/j.thromres.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodrigues T.S., de Sá K.S., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., Gonçalves A.V., Perucello D.B., Andrade W.A., Castro R. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218(3) doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gragnano F., Sperlongano S., Golia E., Natale F., Bianchi R., Crisci M., Fimiani F., Pariggiano I., Diana V., Carbone A. The role of von Willebrand factor in vascular inflammation: from pathogenesis to targeted therapy. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/5620314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turner N.A., Moake J. Assembly and activation of alternative complement components on endothelial cell-anchored ultra-large von Willebrand factor links complement and hemostasis-thrombosis. PLoS ONE. 2013;8(3):e59372. doi: 10.1371/journal.pone.0059372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huisman A., Beun R., Sikma M., Westerink J., Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020 doi: 10.1111/ijlh.13244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martinelli N., Montagnana M., Pizzolo F., Friso S., Salvagno G.L., Forni G.L., Gianesin B., Morandi M., Lunardi C., Lippi G. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb. Res. 2020;193:170–172. doi: 10.1016/j.thromres.2020.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernández-Pérez M.P., Águila S., Reguilón-Gallego L., de los Reyes-García A.M., Miñano A., Bravo-Pérez C., de la Morena M.E., Corral J., García-Barberá N., Gómez-Verdú J.M. Neutrophil extracellular traps and von Willebrand factor are allies that negatively influence COVID-19 outcomes. Clin. Translational Med. 2021;11(1) doi: 10.1002/ctm2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ali R.A., Wuescher L.M., Worth R.G. Platelets: essential components of the immune system. Curr. Trends Immunol. 2015;16:65. [PMC free article] [PubMed] [Google Scholar]
- 138.Szilágyi B., Fejes Z., Póliska S., Pócsi M., Czimmerer Z., Patsalos A., Fenyvesi F., Rusznyák Á., Nagy G., Kerekes G. Reduced miR-26b expression in megakaryocytes and platelets contributes to elevated level of platelet activation status in sepsis. Int. J. Mol. Sci. 2020;21(3):866. doi: 10.3390/ijms21030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elzey B.D., Tian J., Jensen R.J., Swanson A.K., Lees J.R., Lentz S.R., Stein C.S., Nieswandt B., Wang Y., Davidson B.L. Platelet-mediated modulation of adaptive immunity: a communication link between innate and adaptive immune compartments. Immunity. 2003;19(1):9–19. doi: 10.1016/s1074-7613(03)00177-8. [DOI] [PubMed] [Google Scholar]
- 140.Booyse F.M., Rafelson M.E., Jr Stable messenger RNA in the synthesis of contractile protein in human platelets, Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein. Synthesis. 1967;145(1):188–190. doi: 10.1016/0005-2787(67)90673-9. [DOI] [PubMed] [Google Scholar]
- 141.Fard M.B., Fard S.B., Ramazi S., Atashi A., Eslamifar Z. Thrombosis in COVID-19 infection: Role of platelet activation-mediated immunity. Thrombosis J. 2021;19(1):1–11. doi: 10.1186/s12959-021-00311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kojok K., El-Kadiry A.-E.-H., Merhi Y. Role of NF-κB in platelet function. Int. J. Mol. Sci. 2019;20(17):4185. doi: 10.3390/ijms20174185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rivadeneyra L., Carestia A., Etulain J., Pozner R.G., Fondevila C., Negrotto S., Schattner M. Regulation of platelet responses triggered by Toll-like receptor 2 and 4 ligands is another non-genomic role of nuclear factor-kappaB. Thromb. Res. 2014;133(2):235–243. doi: 10.1016/j.thromres.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13(1):1–22. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Franciosi M.L.M., Lima M.D.M., Schetinger M.R.C., Cardoso A.M. Possible role of purinergic signaling in COVID-19. Mol. Cell. Biochem. 2021;476(8):2891–2898. doi: 10.1007/s11010-021-04130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hottz E.D., Azevedo-Quintanilha I.G., Palhinha L., Teixeira L., Barreto E.A., Pão C.R., Righy C., Franco S., Souza T.M., Kurtz P. Platelet activation and platelet-monocyte aggregate formation trigger tissue factor expression in patients with severe COVID-19. Blood. 2020;136(11):1330–1341. doi: 10.1182/blood.2020007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campo G., Contoli M., Fogagnolo A., Vieceli Dalla Sega F., Zucchetti O., Ronzoni L., Verri M., Fortini F., Pavasini R., Morandi L. Over time relationship between platelet reactivity, myocardial injury and mortality in patients with SARS-CoV-2-associated respiratory failure. Platelets. 2021;32(4):560–567. doi: 10.1080/09537104.2020.1852543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Panigrahi S., Ma Y., Hong L., Gao D., West X.Z., Salomon R.G., Byzova T.V., Podrez E.A. Engagement of platelet toll-like receptor 9 by novel endogenous ligands promotes platelet hyperreactivity and thrombosis. Circ. Res. 2013;112(1):103–112. doi: 10.1161/CIRCRESAHA.112.274241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stark R.J., Aghakasiri N., Rumbaut R.E. Platelet-derived Toll-like receptor 4 (Tlr-4) is sufficient to promote microvascular thrombosis in endotoxemia. PLoS ONE. 2012;7(7):e41254. doi: 10.1371/journal.pone.0041254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bezemer G.F., Garssen J. TLR9 and COVID-19: a multidisciplinary theory of a multifaceted therapeutic target. Front. Pharmacol. 2021;11:1958. doi: 10.3389/fphar.2020.601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dömer D., Walther T., Möller S., Behnen M., Laskay T. Neutrophil extracellular traps activate proinflammatory functions of human neutrophils. Front. Immunol. 2021;12:1190. doi: 10.3389/fimmu.2021.636954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsourouktsoglou T.-D., Warnatsch A., Ioannou M., Hoving D., Wang Q., Papayannopoulos V. Histones, DNA, and citrullination promote neutrophil extracellular trap inflammation by regulating the localization and activation of TLR4. Cell Rep. 2020;31(5) doi: 10.1016/j.celrep.2020.107602. [DOI] [PubMed] [Google Scholar]
- 153.Quillard T., Araújo H.A., Franck G., Shvartz E., Sukhova G., Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur. Heart J. 2015;36(22):1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kraemer B.F., Campbell R.A., Schwertz H., Cody M.J., Franks Z., Tolley N.D., Kahr W.H., Lindemann S., Seizer P., Yost C.C. Novel anti-bacterial activities of β-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011;7(11):e1002355. doi: 10.1371/journal.ppat.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maugeri N., Campana L., Gavina M., Covino C., De Metrio M., Panciroli C., Maiuri L., Maseri A., D'Angelo A., Bianchi M.E. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014;12(12):2074–2088. doi: 10.1111/jth.12710. [DOI] [PubMed] [Google Scholar]
- 156.Wing P.A., Keeley T.P., Zhuang X., Lee J.Y., Prange-Barczynska M., Tsukuda S., Morgan S.B., Harding A.C., Argles I.L., Kurlekar S. Hypoxic and pharmacological activation of HIF inhibits SARS-CoV-2 infection of lung epithelial cells. Cell reports. 2021;35(3) doi: 10.1016/j.celrep.2021.109020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tian M., Liu W., Li X., Zhao P., Shereen M.A., Zhu C., Huang S., Liu S., Yu X., Yue M. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduction Targeted Ther. 2021;6(1):1–13. doi: 10.1038/s41392-021-00726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Taniguchi-Ponciano K., Vadillo E., Mayani H., Gonzalez-Bonilla C.R., Torres J., Majluf A., Flores-Padilla G., Wacher-Rodarte N., Galan J.C., Ferat-Osorio E. Increased expression of hypoxia-induced factor 1α mRNA and its related genes in myeloid blood cells from critically ill COVID-19 patients. Ann. Med. 2021;53(1):197–207. doi: 10.1080/07853890.2020.1858234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mai C.-L., Tan Z., Xu Y.-N., Zhang J.-J., Huang Z.-H., Wang D., Zhang H., Gui W.-S., Zhang J., Lin Z.-J. CXCL12-mediated monocyte transmigration into brain perivascular space leads to neuroinflammation and memory deficit in neuropathic pain. Theranostics. 2021;11(3):1059. doi: 10.7150/thno.44364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pandya P.H., Fisher A.J., Mickler E.A., Temm C.J., Lipking K.P., Gracon A., Rothhaar K., Sandusky G.E., Murray M., Pollok K. Hypoxia-inducible factor-1α regulates CD55 in airway epithelium. Am. J. Respir. Cell Mol. Biol. 2016;55(6):889–898. doi: 10.1165/rcmb.2015-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qin Y., Zhang J., Babapoor-Farrokhran S., Applewhite B., Deshpande M., Megarity H., Flores-Bellver M., Aparicio-Domingo S., Ma T., Rui Y. PAI-1 is a vascular cell–specific HIF-2–dependent angiogenic factor that promotes retinal neovascularization in diabetic patients. Sci. Adv. 2022;8(9):eabm1896. doi: 10.1126/sciadv.abm1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aslani M., Mortazavi-Jahromi S.S., Mirshafiey A. Efficient Roles of miR-146a in Cellular and Molecular Mechanisms of Neuroinflammatory Disorders: An Effectual Review in Neuroimmunology. Immunol. Lett. 2021 doi: 10.1016/j.imlet.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 163.Centa A., Fonseca A.S., da Silva Ferreira S.G., Azevedo M.L.V., De Paula C.B.V., Nagashima S., Machado-Souza C., dos Santos Miggiolaro A.F.R., Pellegrino Baena C., De Noronha L. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am. J. Physiol.-Lung Cellular Mol. Physiol. 2021;320(3):L405–L412. doi: 10.1152/ajplung.00457.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nagalla S., Shaw C., Kong X., Kondkar A.A., Edelstein L.C., Ma L., Chen J., McKnight G.S., López J.A., Yang L. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood, J. Am. Soc. Hematol. 2011;117(19):5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li T.-R., Jia Y.-J., Ma C., Qiu W.-Y., Wang Q., Shao X.-Q., Lv R.-J. The role of the microRNA-146a/complement factor H/interleukin-1β-mediated inflammatory loop circuit in the perpetuate inflammation of chronic temporal lobe epilepsy. Dis. Models Mech. 2018;11(3):dmm031708. doi: 10.1242/dmm.031708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rodriguez-Rius A., Lopez S., Martinez-Perez A., Souto J.C., Soria J.M. Identification of a plasma MicroRNA profile associated with venous thrombosis. Arterioscler. Thromb. Vasc. Biol. 2020;40(5):1392–1399. doi: 10.1161/ATVBAHA.120.314092. [DOI] [PubMed] [Google Scholar]
- 167.Zapilko V., Fish R.J., Garcia A., Reny J.-L., Dunoyer-Geindre S., Lecompte T., Neerman-Arbez M., Fontana P. MicroRNA-126 is a regulator of platelet-supported thrombin generation. Platelets. 2020;31(6):746–755. doi: 10.1080/09537104.2020.1775804. [DOI] [PubMed] [Google Scholar]
- 168.Wan R.J., Li Y.H. MicroRNA-146a/NAPDH oxidase4 decreases reactive oxygen species generation and inflammation in a diabetic nephropathy model. Mol. Med. Rep. 2018;17(3):4759–4766. doi: 10.3892/mmr.2018.8407. [DOI] [PubMed] [Google Scholar]
- 169.Elemam N.M., Hasswan H., Aljaibeji H., Sharif-Askari N.S., Halwani R., Taneera J., Sulaiman N. Profiling Levels of Serum microRNAs and Soluble ACE2 in COVID-19 Patients. Life. 2022;12(4):575. doi: 10.3390/life12040575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li C., Hu X., Li L., Li J.h. Differential microRNA expression in the peripheral blood from human patients with COVID-19. J. Clin. Lab. Anal. 2020;34(10):e23590. doi: 10.1002/jcla.23590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fayyad-Kazan M., Makki R., Skafi N., El Homsi M., Hamade A., El Majzoub R., Hamade E., Fayyad-Kazan H., Badran B. Circulating miRNAs: Potential diagnostic role for coronavirus disease 2019 (COVID-19) Infection, Genetics Evol. 2021;94 doi: 10.1016/j.meegid.2021.105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Farr R.J., Rootes C.L., Rowntree L.C., Nguyen T.H., Hensen L., Kedzierski L., Cheng A.C., Kedzierska K., Au G.G., Marsh G.A. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog. 2021;17(7):e1009759. doi: 10.1371/journal.ppat.1009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Donyavi T., Bokharaei-Salim F., Baghi H.B., Khanaliha K., Janat-Makan M.A., Karimi B., Nahand J.S., Mirzaei H., Khatami A., Garshasbi S. Acute and post-acute phase of COVID-19: Analyzing expression patterns of miRNA-29a-3p, 146a–3p, 155–5p, and let-7b-3p in PBMC. Int. Immunopharmacol. 2021;97 doi: 10.1016/j.intimp.2021.107641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu J., Liu X., Shao J., Zhang Y., Lu R., Xue H., Xu Y., Wang L., Zhou H., Yu L. Expression of plasma IFN signaling-related miRNAs during acute SARS-CoV-2 infection and its association with RBD-IgG antibody response. Virology J. 2021;18(1):1–8. doi: 10.1186/s12985-021-01717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Keikha R., Hashemi-Shahri S.M., Jebali A. The relative expression of miR-31, miR-29, miR-126, and miR-17 and their mRNA targets in the serum of COVID-19 patients with different grades during hospitalization. Eur. J. Med. Res. 2021;26(1):1–8. doi: 10.1186/s40001-021-00544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Demiray A., Sarı T., Çalışkan A., Nar R., Aksoy L., Akbubak İ.H. Serum microRNA signature is capable of predictive and prognostic factor for SARS-CoV-2 virulence. Turkish J. Biochem. 2021;46(3):245–253. [Google Scholar]
- 177.Gutmann C., Khamina K., Theofilatos K., Diendorfer A.B., Burnap S.A., Nabeebaccus A., Fish M., McPhail M.J., O'Gallagher K., Schmidt L.E. Association of cardiometabolic microRNAs with COVID-19 severity and mortality. Cardiovasc. Res. 2022;118(2):461–474. doi: 10.1093/cvr/cvab338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oglesby I.K., Vencken S.F., Agrawal R., Gaughan K., Molloy K., Higgins G., McNally P., McElvaney N.G., Mall M.A., Greene C.M. miR-17 overexpression in cystic fibrosis airway epithelial cells decreases interleukin-8 production. Eur. Respir. J. 2015;46(5):1350–1360. doi: 10.1183/09031936.00163414. [DOI] [PubMed] [Google Scholar]
- 179.Hawez A., Al-Haidari A., Madhi R., Rahman M., Thorlacius H. MiR-155 regulates PAD4-dependent formation of neutrophil extracellular traps. Front. Immunol. 2019;10:2462. doi: 10.3389/fimmu.2019.02462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gantier M.P. The not-so-neutral role of microRNAs in neutrophil biology. J. Leukoc. Biol. 2013;94(4):575–583. doi: 10.1189/jlb.1012539. [DOI] [PubMed] [Google Scholar]
- 181.de Kerckhove M., Tanaka K., Umehara T., Okamoto M., Kanematsu S., Hayashi H., Yano H., Nishiura S., Tooyama S., Matsubayashi Y. Targeting miR-223 in neutrophils enhances the clearance of Staphylococcus aureus in infected wounds. EMBO Mol. Med. 2018;10(10):e9024. doi: 10.15252/emmm.201809024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pedersen C.C., Refsgaard J.C., Østergaard O., Jensen L.J., Heegaard N.H.H., Borregaard N., Cowland J.B. Impact of microRNA-130a on the neutrophil proteome. BMC Immunol. 2015;16(1):1–10. doi: 10.1186/s12865-015-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arroyo A.B., Fernández-Pérez M.P., Del Monte A., Águila S., Méndez R., Hernández-Antolín R., García-Barberá N., de Los Reyes-García A.M., González-Jiménez P., Arcas M.I. miR-146a is a pivotal regulator of neutrophil extracellular trap formation promoting thrombosis. Haematologica. 2021;106(6):1636. doi: 10.3324/haematol.2019.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Czajka P., Fitas A., Jakubik D., Eyileten C., Gasecka A., Wicik Z., Siller-Matula J.M., Filipiak K.J., Postula M. MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review. Front. Physiol. 2021;12:498. doi: 10.3389/fphys.2021.652579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen W., Harbeck M.C., Zhang W., Jacobson J.R. MicroRNA regulation of integrins. Translational Res. 2013;162(3):133–143. doi: 10.1016/j.trsl.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Müller D., Bosserhoff A.-K. Integrin β 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene. 2008;27(52):6698–6706. doi: 10.1038/onc.2008.282. [DOI] [PubMed] [Google Scholar]
- 187.Bayraktar R., Bertilaccio M.T.S., Calin G.A. The interaction between two worlds: microRNAs and Toll-like receptors. Front. Immunol. 2019;10:1053. doi: 10.3389/fimmu.2019.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Quinn E.M., Wang J.H., O’Callaghan G., Redmond H.P. MicroRNA-146a is upregulated by and negatively regulates TLR2 signaling. PLoS ONE. 2013;8(4):e62232. doi: 10.1371/journal.pone.0062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo H., Chen Y., Hu X., Qian G., Ge S., Zhang J. The regulation of Toll-like receptor 2 by miR-143 suppresses the invasion and migration of a subset of human colorectal carcinoma cells. Mol. Cancer. 2013;12(1):1–10. doi: 10.1186/1476-4598-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fu Y., Zhang Y., Wang Z., Wang L., Wei X., Zhang B., Wen Z., Fang H., Pang Q., Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am. J. Nephrol. 2010;32(6):581–589. doi: 10.1159/000322105. [DOI] [PubMed] [Google Scholar]
- 191.He J., Jiang B.-H. Interplay between reactive oxygen species and microRNAs in cancer. Curr. Pharmacol. Rep. 2016;2(2):82–90. doi: 10.1007/s40495-016-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu Z., Tuo Y.-H., Chen J.-W., Wang Q.-Y., Li S., Li M.-C., Dai G., Wang J.-S., Zhang Y.-L., Feng L. NADPH oxidase inhibitor regulates microRNAs with improved outcome after mechanical reperfusion. J. NeuroInterventional Surg. 2017;9(7):702–706. doi: 10.1136/neurintsurg-2016-012463. [DOI] [PubMed] [Google Scholar]
- 193.Haroun R.-A.-H., Osman W.H., Amin R.E., Hassan A.K., Abo-Shanab W.S., Eessa A.M. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology. 2022;54(1):104–110. doi: 10.1016/j.pathol.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martínez-Fleta P., Vera-Tomé P., Jiménez-Fernández M., Requena S., Roy-Vallejo E., Sanz-García A., Lozano-Prieto M., López-Sanz C., Vara A., Lancho-Sánchez Á. A Differential Signature of Circulating miRNAs and Cytokines Between COVID-19 and Community-Acquired Pneumonia Uncovers Novel Physiopathological Mechanisms of COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.815651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sabbatinelli J., Giuliani A., Matacchione G., Latini S., Laprovitera N., Pomponio G., Ferrarini A., Baroni S.S., Pavani M., Moretti M. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021;193 doi: 10.1016/j.mad.2020.111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Keikha R., Hashemi-Shahri S., Jebali A. The miRNA neuroinflammatory biomarkers in COVID-19 patients with different severity of illness. Neurología. 2021 doi: 10.1016/j.nrleng.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tang H., Gao Y., Li Z., Miao Y., Huang Z., Liu X., Xie L., Li H., Wen W., Zheng Y. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Translational Med. 2020;10(6):e200. doi: 10.1002/ctm2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.de Gonzalo-Calvo D., Benítez I.D., Pinilla L., Carratalá A., Moncusí-Moix A., Gort-Paniello C., Molinero M., González J., Torres G., Bernal M. Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Translational Res. 2021;236:147–159. doi: 10.1016/j.trsl.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.S. Srivastava, I. Garg, Y. Singh, R. Meena, A.A. Hembrom, N. Ghosh, B. Kumari, V. Kumar, M.R. Eslavath, S. Singh, Evaluation of altered miRNA expression pattern to predict COVID-19 severity, (2021). [DOI] [PMC free article] [PubMed]
- 200.Chow J.-T.-S., Salmena L. Prediction and analysis of SARS-CoV-2-targeting MicroRNA in human lung epithelium. Genes. 2020;11(9):1002. doi: 10.3390/genes11091002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sahu A., Jha P.K., Prabhakar A., Singh H.D., Gupta N., Chatterjee T., Tyagi T., Sharma S., Kumari B., Singh S. MicroRNA-145 impedes thrombus formation via targeting tissue factor in venous thrombosis. EBioMedicine. 2017;26:175–186. doi: 10.1016/j.ebiom.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jankowska K.I., Sauna Z.E., Atreya C.D. Role of microRNAs in hemophilia and thrombosis in humans. Int. J. Mol. Sci. 2020;21(10):3598. doi: 10.3390/ijms21103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Teruel-Montoya R., Rosendaal F., Martinez C. MicroRNAs in hemostasis. J. Thromb. Haemost. 2015;13(2):170–181. doi: 10.1111/jth.12788. [DOI] [PubMed] [Google Scholar]
- 204.Jankowska K.I., McGill J., Pezeshkpoor B., Oldenburg J., Sauna Z.E., Atreya C.D. Further evidence that microRNAs can play a role in Hemophilia A disease manifestation: F8 gene downregulation by miR-19b-3p and miR-186-5p. Front. Cell Dev. Biol. 2020;8:669. doi: 10.3389/fcell.2020.00669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xiang Y., Cheng J., Wang D., Hu X., Xie Y., Stitham J., Atteya G., Du J., Tang W.H., Lee S.H. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood, J. Am. Soc. Hematol. 2015;125(22):3377–3387. doi: 10.1182/blood-2015-01-620278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gambardella J., Coppola A., Izzo R., Fiorentino G., Trimarco B., Santulli G. Role of endothelial miR-24 in COVID-19 cerebrovascular events. Crit. Care. 2021;25(1):1–3. doi: 10.1186/s13054-021-03731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhao L., Hua C., Li Y., Sun Q., Wu W. miR-525-5p inhibits ADAMTS13 and is correlated with Ischemia/reperfusion injury-induced neuronal cell death. Int. J. Clin. Exp. Med. 2015;8(10):18115. [PMC free article] [PubMed] [Google Scholar]
- 208.Xu L., Wen Z., Zhou Y., Liu Z., Li Q., Fei G., Luo J., Ren T. MicroRNA-7–regulated TLR9 signaling–enhanced growth and metastatic potential of human lung cancer cells by altering the phosphoinositide-3-kinase, regulatory subunit 3/Akt pathway. Mol. Biol. Cell. 2013;24(1):42–55. doi: 10.1091/mbc.E12-07-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen T., Li Z., Zhu W., Ge J., Zheng X., Pan X., Yan H., Zhu J. MicroRNA-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40L in oxLDL-stimulated dendritic cells. FEBS Lett. 2011;585(3):567–573. doi: 10.1016/j.febslet.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 210.Bijak M., Dzieciol M., Rywaniak J., Saluk J., Zielinska M. Platelets miRNA as a prediction marker of thrombotic episodes. Dis Markers. 2016;2872507 doi: 10.1155/2016/2872507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu Y.D., Zhuang X.P., Cai D.L., Cao C., Gu Q.S., Liu X.N., Zheng B.B., Guan B.J., Yu L., Li J.K. Let-7a regulates EV secretion and mitochondrial oxidative phosphorylation by targeting SNAP23 in colorectal cancer. J. Exp. Clin. Cancer Res. 2021;40(1):1–16. doi: 10.1186/s13046-020-01813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Su Y., Li Q., Zheng Z., Wei X., Hou P. Identification of genes, pathways and transcription factor-miRNA-target gene networks and experimental verification in venous thromboembolism. Sci. Rep. 2021;11(1):1–16. doi: 10.1038/s41598-021-95909-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang R., Zheng T., Cai X., Yu Y., Yu C., Guo L., Huang S., Zhu W., Zhu R., Yan Q. Genome-wide analyses of amphioxus microRNAs reveal an immune regulation via miR-92d targeting C3. J. Immunol. 2013;190(4):1491–1500. doi: 10.4049/jimmunol.1200801. [DOI] [PubMed] [Google Scholar]
- 214.Hillman Y., Mardamshina M., Pasmanik-Chor M., Ziporen L., Geiger T., Shomron N., Fishelson Z. MicroRNAs affect complement regulator expression and mitochondrial activity to modulate cell resistance to complement-dependent cytotoxicity. Cancer Immunol Res. 2019;7(12):1970–1983. doi: 10.1158/2326-6066.CIR-18-0818. [DOI] [PubMed] [Google Scholar]
- 215.Lu S.-Y., Fu C.-L., Liang L., Yang B., Shen W., Wang Q.-W., Chen Y., Chen Y.-F., Liu Y.-N., Zhu L. miR-218-2 regulates cognitive functions in the hippocampus through complement component 3–dependent modulation of synaptic vesicle release. Proc. Natl. Acad. Sci. 2021;118(14) doi: 10.1073/pnas.2021770118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen L., Fukuda N., Otsuki T., Tanaka S., Nakamura Y., Kobayashi H., Matsumoto T., Abe M. Increased complement 3 with suppression of miR-145 induces the synthetic phenotype in vascular smooth muscle cells from spontaneously hypertensive rats. J. Am. Heart Assoc. 2019;8(10):e012327. doi: 10.1161/JAHA.119.012327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Guo H., Yan Z., Hu Y., Huang X., Pan C. Complement C7 is specifically expressed in mesangial cells and is a potential diagnostic biomarker for diabetic nephropathy and is regulated by miR-494-3p and miR-574-5p. Diabetes, Metabolic Syndrome and Obesity: Targets Ther. 2021;14:3077. doi: 10.2147/DMSO.S311725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cui W., Zhang Y., Hu N., Shan C., Zhang S., Zhang W., Zhang X., Ye L. miRNA-520b and miR-520e sensitize breast cancer cells to complement attack via directly targeting 3′ UTR of CD46. Cancer Biol. Ther. 2010;10(3):232–241. doi: 10.4161/cbt.10.3.12277. [DOI] [PubMed] [Google Scholar]
- 219.Li Y.Y., Alexandrov P.N., Pogue A.I., Zhao Y., Bhattacharjee S., Lukiw W.J. miRNA-155 up-regulation and complement factor H (CFH) deficits in Down’s Syndrome. NeuroReport. 2012;23(3):168. doi: 10.1097/WNR.0b013e32834f4eb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lukiw W.J., Surjyadipta B., Dua P., Alexandrov P.N. Common micro RNAs (miRNAs) target complement factor H (CFH) regulation in Alzheimer’s disease (AD) and in age-related macular degeneration (AMD) Int. J. Biochem. Mol. Biol. 2012;3(1):105. [PMC free article] [PubMed] [Google Scholar]
- 221.Hillman Y., Mazkereth N., Farberov L., Shomron N., Fishelson Z. Regulation of complement-dependent cytotoxicity by MicroRNAs miR-200b, miR-200c, and miR-217. J. Immunol. 2016;196(12):5156–5165. doi: 10.4049/jimmunol.1502701. [DOI] [PubMed] [Google Scholar]
- 222.Akula S.M., Bolin P., Cook P.P. Cellular miR-150-5p may have a crucial role to play in the biology of SARS-CoV-2 infection by regulating nsp10 gene. RNA Biol. 2022;19(1):1–11. doi: 10.1080/15476286.2021.2010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Du Meng M.L., Han Y., Zhao D., Zhang X., Yang Y., Liu R. MicroRNA-645 targets urokinase plasminogen activator and decreases the invasive growth of MDA-MB-231 triple-negative breast cancer cells. OncoTargets Ther. 2018;11:7733. doi: 10.2147/OTT.S187221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Noh H., Hong S., Dong Z., Pan Z.K., Jing Q., Huang S. Impaired microRNA processing facilitates breast cancer cell invasion by upregulating urokinase-type plasminogen activator expression. Genes & cancer. 2011;2(2):140–150. doi: 10.1177/1947601911408888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yamashita D., Kondo T., Ohue S., Takahashi H., Ishikawa M., Matoba R., Suehiro S., Kohno S., Harada H., Tanaka J. miR340 suppresses the stem-like cell function of glioma-initiating cells by targeting tissue plasminogen activator. Cancer Res. 2015;75(6):1123–1133. doi: 10.1158/0008-5472.CAN-14-0938. [DOI] [PubMed] [Google Scholar]
- 226.Zhao C., Liu Z. MicroRNA 617 targeting SERPINE1 inhibited the progression of oral squamous cell carcinoma. Mol. Cell. Biol. 2021;41(6):e00565–e620. doi: 10.1128/MCB.00565-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Villadsen S., Bramsen J., Ostenfeld M., Wiklund E., Fristrup N., Gao S., Hansen T., Jensen T., Borre M., Ørntoft T. The miR-143/-145 cluster regulates plasminogen activator inhibitor-1 in bladder cancer. Br. J. Cancer. 2012;106(2):366–374. doi: 10.1038/bjc.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Park G., Son B., Kang J., Lee S., Jeon J., Kim J.-H., Yi G.-R., Youn H., Moon C., Nam S.Y. LDR-induced miR-30a and miR-30b target the PAI-1 pathway to control adverse effects of NSCLC radiotherapy. Mol. Ther. 2019;27(2):342–354. doi: 10.1016/j.ymthe.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu Y., Zhang M.H., Xue Y., Zhang T., Wu N., Guo W., Du X., Xu Y.L. Effect of microRNA-26a on vascular endothelial cell injury caused by lower extremity ischemia–reperfusion injury through the AMPK pathway by targeting PFKFB3. J. Cell. Physiol. 2019;234(3):2916–2928. doi: 10.1002/jcp.27108. [DOI] [PubMed] [Google Scholar]
- 230.Krutilina R., Sun W., Sethuraman A., Brown M., Seagroves T.N., Pfeffer L.M., Ignatova T., Fan M. MicroRNA-18a inhibits hypoxia-inducible factor 1α activity and lung metastasis in basal breast cancers. Breast Cancer Res. 2014;16(4):1–16. doi: 10.1186/bcr3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peng X., Gao H., Xu R., Wang H., Mei J., Liu C. The interplay between HIF-1α and noncoding RNAs in cancer. J. Exp. Clin. Cancer Res. 2020;39(1):1–19. doi: 10.1186/s13046-020-1535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Serocki M., Bartoszewska S., Janaszak-Jasiecka A., Ochocka R.J., Collawn J.F., Bartoszewski R. miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis. 2018;21(2):183–202. doi: 10.1007/s10456-018-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kinget L., Roussel E., Verbiest A., Albersen M., Rodríguez-Antona C., Graña-Castro O., Inglada-Pérez L., Zucman-Rossi J., Couchy G., Job S. MicroRNAs Targeting HIF-2α, VEGFR1 and/or VEGFR2 as potential predictive biomarkers for VEGFR tyrosine kinase and HIF-2α inhibitors in metastatic clear-cell renal cell carcinoma. Cancers. 2021;13(12):3099. doi: 10.3390/cancers13123099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang H., Pu J., Qi T., Qi M., Yang C., Li S., Huang K., Zheng L., Tong Q. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene. 2014;33(3):387–397. doi: 10.1038/onc.2012.574. [DOI] [PubMed] [Google Scholar]
- 235.Tian Y., Matsui S., Touma M., Wu Q., Sugimoto K. MicroRNA-342 inhibits tumor growth via targeting chemokine CXCL12 involved in macrophages recruitment/activation. Genes Cells. 2018;23(12):1009–1022. doi: 10.1111/gtc.12650. [DOI] [PubMed] [Google Scholar]
- 236.Arabanian L.S., Fierro F.A., Stölzel F., Heder C., Poitz D.M., Strasser R.H., Wobus M., Borhäuser M., Ferrer R.A., Platzbecker U. MicroRNA-23a mediates post-transcriptional regulation of CXCL12 in bone marrow stromal cells. Haematologica. 2014;99(6):997. doi: 10.3324/haematol.2013.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dai Y., Liu S., Xie X., Ding M., Zhou Q., Zhou X. MicroRNA-31 promotes chondrocyte proliferation by targeting C-X-C motif chemokine ligand 12. Mol. Med. Rep. 2019;19(3):2231–2237. doi: 10.3892/mmr.2019.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mortazavi-Jahromi S.S., Ahmadzadeh A., Rezaieyazdi Z., Aslani M., Omidian S., Mirshafiey A. The role of β-d-mannuronic acid, as a new non-steroidal anti-inflammatory drug on expression of miR-146a, IRAK1, TRAF6, NF-κB and pro-inflammatory cytokines following a clinical trial in rheumatoid arthritis patients. Immunopharmacol. Immunotoxicol. 2020;42(3):228–236. doi: 10.1080/08923973.2020.1742734. [DOI] [PubMed] [Google Scholar]
- 239.Mortazavi-Jahromi S.S., Jamshidi M.M., Farazmand A., Aghazadeh Z., Yousefi M., Mirshafiey A. Pharmacological effects of β-d-mannuronic acid (M2000) on miR-146a, IRAK1, TRAF6 and NF-κB gene expression, as target molecules in inflammatory reactions. Pharmacol. Rep. 2017;69(3):479–484. doi: 10.1016/j.pharep.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 240.Shu J., Xia Z., Li L., Liang E.T., Slipek N., Shen D., Foo J., Subramanian S., Steer C.J. Dose-dependent differential mRNA target selection and regulation by let-7a-7f and miR-17-92 cluster microRNAs. RNA Biol. 2012;9(10):1275–1287. doi: 10.4161/rna.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Miyashima S., Koi S., Hashimoto T., Nakajima K. Non-cell-autonomous microRNA165 acts in a dose-dependent manner to regulate multiple differentiation status in the Arabidopsis root. Development. 2011;138(11):2303–2313. doi: 10.1242/dev.060491. [DOI] [PubMed] [Google Scholar]
- 242.Águila S., Reyes-García A.M., Fernández-Pérez M.P., Reguilón-Gallego L., Zapata-Martínez L., Ruiz-Lorente I., Vicente V., González-Conejero R., Martínez C. MicroRNAs as New Regulators of Neutrophil Extracellular Trap Formation. Int. J. Mol. Sci. 2021;22(4):2116. doi: 10.3390/ijms22042116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Alipoor B., Ghaedi H., Meshkani R., Omrani M., Sharifi Z., Golmohammadi T. The rs2910164 variant is associated with reduced miR-146a expression but not cytokine levels in patients with type 2 diabetes. J. Endocrinol. Invest. 2018;41(5):557–566. doi: 10.1007/s40618-017-0766-z. [DOI] [PubMed] [Google Scholar]
- 244.Shao Y., Li J., Cai Y., Xie Y., Ma G., Li Y., Chen Y., Liu G., Zhao B., Cui L. The functional polymorphisms of miR-146a are associated with susceptibility to severe sepsis in the Chinese population. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/916202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Xie K., Ma H., Liang C., Wang C., Qin N., Shen W., Gu Y., Yan C., Zhang K., Dai N. A functional variant in miR-155 regulation region contributes to lung cancer risk and survival. Oncotarget. 2015;6(40):42781. doi: 10.18632/oncotarget.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Löfgren S.E., Frostegård J., Truedsson L., Pons-Estel B.A., D'Alfonso S., Witte T., Lauwerys B.R., Endreffy E., Kovács L., Vasconcelos C. Genetic association of miRNA-146a with systemic lupus erythematosus in Europeans through decreased expression of the gene. Genes Immun. 2012;13(3):268–274. doi: 10.1038/gene.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ji J., Xu M., Tu J., Zhao Z., Gao J., Chen M., Song J., Zhu H., Cheng X., Hui J. MiR-155 and its functional variant rs767649 contribute to the susceptibility and survival of hepatocellular carcinoma. Oncotarget. 2016;7(37):60303. doi: 10.18632/oncotarget.11206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yuan F., Sun R., Li L., Jin B., Wang Y., Liang Y., Che G., Gao L., Zhang L. A functional variant rs353292 in the flanking region of miR-143/145 contributes to the risk of colorectal cancer. Sci. Rep. 2016;6(1):1–7. doi: 10.1038/srep30195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang D., Liu Q., Ren Y., Zhang Y., Wang X., Liu B. Association analysis of miRNA-related genetic polymorphisms in miR-143/145 and KRAS with colorectal cancer susceptibility and survival. Biosci. Rep. 2021;41(4) doi: 10.1042/BSR20204136. BSR20204136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Eadon M.T., Jacob A., Cunningham P.N., Quigg R.J., Garcia J.G., Alexander J.J. Transcriptional profiling reveals that C 5a alters micro RNA in brain endothelial cells. Immunology. 2014;143(3):363–373. doi: 10.1111/imm.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Thulasingam S., Massilamany C., Gangaplara A., Dai H., Yarbaeva S., Subramaniam S., Riethoven J.-J., Eudy J., Lou M., Reddy J. miR-27b*, an oxidative stress-responsive microRNA modulates nuclear factor-kB pathway in RAW 264.7 cells. Mol. Cell. Biochem. 2011;352(1):181–188. doi: 10.1007/s11010-011-0752-2. [DOI] [PubMed] [Google Scholar]
- 252.Li Q., Liu X., Yin Y., Zheng J.-T., Jiang C.-F., Wang J., Shen H., Li C.-Y., Wang M., Liu L.-Z. Insulin regulates glucose consumption and lactate production through reactive oxygen species and pyruvate kinase M2. Oxid. Med. Cell. Longevity. 2014;2014 doi: 10.1155/2014/504953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pacifico F., Crescenzi E., Mellone S., Iannetti A., Porrino N., Liguoro D., Moscato F., Grieco M., Formisano S., Leonardi A. Nuclear factor-κB contributes to anaplastic thyroid carcinomas through up-regulation of miR-146a. J. Clin. Endocrinol. Metab. 2010;95(3):1421–1430. doi: 10.1210/jc.2009-1128. [DOI] [PubMed] [Google Scholar]
- 254.Bazzoni F., Rossato M., Fabbri M., Gaudiosi D., Mirolo M., Mori L., Tamassia N., Mantovani A., Cassatella M.A., Locati M. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc. Natl. Acad. Sci. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang X., Liu S., Hu T., Liu S., He Y., Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50(2):490–499. doi: 10.1002/hep.23008. [DOI] [PubMed] [Google Scholar]
- 256.Scisciani C., Vossio S., Guerrieri F., Schinzari V., De Iaco R., de Meo P.D.O., Cervello M., Montalto G., Pollicino T., Raimondo G. Transcriptional regulation of miR-224 upregulated in human HCCs by NFκB inflammatory pathways. J. Hepatol. 2012;56(4):855–861. doi: 10.1016/j.jhep.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 257.Iliopoulos D., Hirsch H.A., Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139(4):693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.He J., Xu Q., Jing Y., Agani F., Qian X., Carpenter R., Li Q., Wang X.R., Peiper S.S., Lu Z. Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep. 2012;13(12):1116–1122. doi: 10.1038/embor.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fierro-Fernández M., Busnadiego Ó., Sandoval P., Espinosa-Díez C., Blanco-Ruiz E., Rodríguez M., Pian H., Ramos R., López-Cabrera M., García-Bermejo M.L. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX 4 and TGFBR 2. EMBO Rep. 2015;16(10):1358–1377. doi: 10.15252/embr.201540750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.DeVore S.B., Young C.H., Li G., Sundararajan A., Ramaraj T., Mudge J., Schilkey F., Muth A., Thompson P.R., Cherrington B.D. Histone citrullination represses MicroRNA expression, resulting in increased oncogene mRNAs in somatolactotrope cells. Mol. Cell. Biol. 2018;38(19):e00084–e118. doi: 10.1128/MCB.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cui X., Witalison E.E., Chumanevich A.P., Chumanevich A.A., Poudyal D., Subramanian V., Schetter A.J., Harris C.C., Thompson P.R., Hofseth L.J. The induction of microRNA-16 in colon cancer cells by protein arginine deiminase inhibition causes a p53-dependent cell cycle arrest. PLoS ONE. 2013;8(1):e53791. doi: 10.1371/journal.pone.0053791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Spinello I., Quaranta M.T., Paolillo R., Pelosi E., Cerio A.M., Saulle E., Coco F.L., Testa U., Labbaye C. Differential hypoxic regulation of the microRNA-146a/CXCR4 pathway in normal and leukemic monocytic cells: impact on response to chemotherapy. Haematologica. 2015;100(9):1160. doi: 10.3324/haematol.2014.120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shen G., Li X., Jia Y.-F., Piazza G.A., Xi Y. Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol. Sin. 2013;34(3):336–341. doi: 10.1038/aps.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bruning U., Cerone L., Neufeld Z., Fitzpatrick S.F., Cheong A., Scholz C.C., Simpson D.A., Leonard M.O., Tambuwala M.M., Cummins E.P. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1α activity during prolonged hypoxia. Mol. Cell. Biol. 2011;31(19):4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Polytarchou C., Iliopoulos D., Hatziapostolou M., Kottakis F., Maroulakou I., Struhl K., Tsichlis P.N. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011;71(13):4720–4731. doi: 10.1158/0008-5472.CAN-11-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chen Z., Lai T.-C., Jan Y.-H., Lin F.-M., Wang W.-C., Xiao H., Wang Y.-T., Sun W., Cui X., Li Y.-S. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J. Clin. Investig. 2013;123(3):1057–1067. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu Q., Wang Y., Yang T., Wei W. Protective effects of miR-25 against hypoxia/reoxygenation-induced fibrosis and apoptosis of H9c2 cells. Int. J. Mol. Med. 2016;38(4):1225–1234. doi: 10.3892/ijmm.2016.2702. [DOI] [PubMed] [Google Scholar]
- 268.Jing X., Chen T., Huang C., Wang H., An L., Cheng Z., Zhang G. MiR-15a expression analysis in non-small cell lung cancer A549 cells under local hypoxia microenvironment. Eur. Rev. Med. Pharmacol. Sci. 2017;21(9):2069–2074. [PubMed] [Google Scholar]
- 269.Gandellini P., Giannoni E., Casamichele A., Taddei M.L., Callari M., Piovan C., Valdagni R., Pierotti M.A., Zaffaroni N., Chiarugi P. miR-205 hinders the malignant interplay between prostate cancer cells and associated fibroblasts. Antioxid. Redox Signal. 2014;20(7):1045–1059. doi: 10.1089/ars.2013.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Li H., Rokavec M., Jiang L., Horst D., Hermeking H. Antagonistic effects of p53 and HIF1A on microRNA-34a regulation of PPP1R11 and STAT3 and hypoxia-induced epithelial to mesenchymal transition in colorectal cancer cells. Gastroenterology. 2017;153(2):505–520. doi: 10.1053/j.gastro.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 271.Ikeda K.T., Hale P.T., Pauciulo M.W., Dasgupta N., Pastura P.A., Le Cras T.D., Pandey M.K., Nichols W.C. Hypoxia-induced pulmonary hypertension in different mouse strains: relation to transcriptome. Am. J. Respir. Cell Mol. Biol. 2019;60(1):106–116. doi: 10.1165/rcmb.2017-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Zhang L., Chopp M., Liu X., Teng H., Tang T., Kassis H., Zhang Z.G. Combination therapy with VELCADE and tPA is neuroprotective in aged rats after stroke and targets miRNA-146a and the toll-like receptor signaling pathway. Arterioscler. Thromb. Vasc. Biol. 2012;32(8):1856. doi: 10.1161/ATVBAHA.112.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cheng Y., Liu X., Yang J., Lin Y., Xu D.-Z., Lu Q., Deitch E.A., Huo Y., Delphin E.S., Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009;105(2):158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kardas G., Daszyńska-Kardas A., Marynowski M., Brząkalska O., Kuna P., Panek M. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front. Pharmacol. 2020;11:47. doi: 10.3389/fphar.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Moore C.C., McKillop I.H., Huynh T. MicroRNA expression following activated protein C treatment during septic shock. J. Surgical Res. 2013;182(1):116–126. doi: 10.1016/j.jss.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 276.Xiang W., Tian C., Lin J., Wu X., Pang G., Zhou L., Pan S., Deng Z. Plasma let-7i and miR-15a expression are associated with the effect of recombinant tissue plasminogen activator treatment in acute ischemic stroke patients. Thromb. Res. 2017;158:121–125. doi: 10.1016/j.thromres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 277.R. Zarychanski, A. Investigators, Therapeutic anticoagulation in critically ill patients with Covid-19–preliminary report, medRxiv (2021).
- 278.A.H. Talasaz, P. Sadeghipour, H. Kakavand, M. Aghakouchakzadeh, B.W. Van Tassell, E. Kordzadeh-Kermani, A. Gheymati, H. Ariannrjad, S.H. Hosseini, S. Jamalkhani, Antithrombotic therapy in COVID-19: systematic summary of ongoing or completed randomized trials, medRxiv (2021).
- 279.Ohn M.H., Ng J.R., Ohn K.M., Luen N.P. Double-edged sword effect of anticoagulant in COVID-19 infection. BMJ Case Reports CP. 2021;14(3):e241955. doi: 10.1136/bcr-2021-241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Thomas W., White D., Cox-Morton S., MacDonald S., Besser M. Heparin failure and COVID-19: should we explore other anticoagulants? An observational report regarding in-vitro recovery of anticoagulant action in COVID-19 patients in intensive care. Thromb. Res. 2020;195:226. doi: 10.1016/j.thromres.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.White D., MacDonald S., Bull T., Hayman M., de Monteverde-Robb R., Sapsford D., Lavinio A., Varley J., Johnston A., Besser M. Heparin resistance in COVID-19 patients in the intensive care unit. J. Thromb. Thrombolysis. 2020;50(2):287–291. doi: 10.1007/s11239-020-02145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nadeem R., Thomas S.J., Fathima Z., Palathinkal A.S., Alkilani Y.E., Dejan E.A., mohammad ismail Darwish I., Alsubousi A.A., Backour A.M., Kandeel H. Pattern of anticoagulation prescription for patients with Covid-19 acute respiratory distress syndrome admitted to ICU. Does it impact outcome? Heart Lung. 2021;50(1):1–5. doi: 10.1016/j.hrtlng.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jiménez-Alcázar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y.J., Long A.T., Bilyy R., Krenn V., Renné C., Renné T. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358(6367):1202–1206. doi: 10.1126/science.aam8897. [DOI] [PubMed] [Google Scholar]
- 284.Ducroux C., Di Meglio L., Loyau S., Delbosc S., Boisseau W., Deschildre C., Ben Maacha M., Blanc R., Redjem H., Ciccio G. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49(3):754–757. doi: 10.1161/STROKEAHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 285.Fisher J., Mohanty T., Karlsson C., Khademi S.H., Malmström E., Frigyesi A., Nordenfelt P., Malmstrom J., Linder A. Proteome profiling of recombinant DNase therapy in reducing NETs and aiding recovery in COVID-19 patients. Mol. Cell. Proteomics. 2021;100113 doi: 10.1016/j.mcpro.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.N. de Buhr, A.C. Parplys, M. Schroeder, T. Henneck, B. Schaumburg, S. Stanelle-Bertram, D. Jarczak, A. Nierhaus, J. Hiller, S. Peine, Impaired Degradation of Neutrophil Extracellular Traps: A Possible Severity Factor of Elderly Male COVID-19 Patients, J. Innate Immunity 1-16. [DOI] [PMC free article] [PubMed]
- 287.Neumann A., Völlger L., Berends E.T., Molhoek E.M., Stapels D.A., Midon M., Friães A., Pingoud A., Rooijakkers S.H., Gallo R.L. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J. Innate Immun. 2014;6(6):860–868. doi: 10.1159/000363699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.T.M. Clausen, D.R. Sandoval, C.B. Spliid, J. Pihl, H.R. Perrett, C.D. Painter, A. Narayanan, S.A. Majowicz, E.M. Kwong, R.N. McVicar, SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2, Cell 183(4) (2020) 1043-1057. e15. [DOI] [PMC free article] [PubMed]
- 289.Chen C.-L., Rodiger J., Chung V., Viswanatha R., Mohr S.E., Hu Y., Perrimon N. SNP-CRISPR: a web tool for SNP-specific genome editing, G3: Genes. Genomes, Genetics. 2020;10(2):489–494. doi: 10.1534/g3.119.400904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Al-kuraishy H., Al-Gareeb A.I., Guerreiro S.G., Cruz-Martins N., Batiha G.-E.-S. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front. Cardiovasc. Med. 2021;8:335. doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Al-Kuraishy H.M., Al-Gareeb A.I., Alblihed M., Cruz-Martins N., Batiha G.-E.-S. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: the anti-inflammatory role of metformin. Front. Med. 2021;8:110. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Al-Kuraishy H.M., Al-Gareeb A.I., Qusty N., Cruz-Martins N., Batiha G.-E.-S. Sequential doxycycline and colchicine combination therapy in Covid-19: The salutary effects. Pulm. Pharmacol. Ther. 2021;67 doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Al-Kuraishy H.M., Al-Gareeb A.I., Al-Niemi M.S., Aljowaie R.M., Almutairi S.M., Alexiou A., Batiha G.-E.-S. The Prospective Effect of Allopurinol on the Oxidative Stress Index and Endothelial Dysfunction in Covid-19. Inflammation. 2022:1–17. doi: 10.1007/s10753-022-01648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Rambaldi A., Gritti G., Micò M.C., Frigeni M., Borleri G., Salvi A., Landi F., Pavoni C., Sonzogni A., Gianatti A. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiology. 2020;225(6) doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Al-Kuraishy H.M., Al-Rubiey H.F., Al-Buhadily A.K., Al-Gareeb A.I. Anti-histamines and Covid-19: Hype or Hope. J. Pakistan Med. Assoc. 2021;71(12):S144–S148. [PubMed] [Google Scholar]
- 297.Al-Kuraishy H.M., Al-Gareeb A.I., Almulaiky Y.Q., Cruz-Martins N., Batiha G.-E.-S. Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: The enigmatic entity. Eur. J. Pharmacol. 2021;904 doi: 10.1016/j.ejphar.2021.174196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Lugnier C., Al-Kuraishy H.M., Rousseau E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem. Pharmacol. 2021;185 doi: 10.1016/j.bcp.2021.114431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Al-Kuraishy H.M., Al-Gareeb A.I., Alzahrani K.J., Alexiou A., Batiha G.-E.-S. Niclosamide for Covid-19: bridging the gap. Mol. Biol. Rep. 2021;48(12):8195–8202. doi: 10.1007/s11033-021-06770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Al-Kuraishy H.M., Al-Gareeb A.I., Alqarni M., Cruz-Martins N., El-Saber Batiha G. Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front. Pharmacol. 2021;12:136. doi: 10.3389/fphar.2021.642822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Al-Kuraishy H.M., Hussien N.R., Al-Niemi M.S., Al-Gareeb A.I. Colchicine in the management of Covid-19: With or lieu of evidence. J. Pakistan Med. Assoc. 2021:S127–S132. [PubMed] [Google Scholar]
- 302.Al-Kuraishy H.M., Al-Gareeb A.I., Alzahrani K.J., Cruz-Martins N., Batiha G.-E.-S. The potential role of neopterin in Covid-19: a new perspective. Mol. Cell. Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Al-Kuraishy H.M., Al-Gareeb A.I., Faidah H., Al-Maiahy T.J., Cruz-Martins N., Batiha G.-E.-S. The looming effects of estrogen in Covid-19: a rocky rollout. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.649128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mirshafiey A., Taeb M., Mortazavi-Jahromi S.S., Jafarnezhad-Ansariha F., Rehm B.H., Esposito E., Cuzzocrea S., Matsuo H. Introduction of β-d-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property based on COX-1/COX-2 activity and gene expression. Pharmacol. Rep. 2017;69(5):1067–1072. doi: 10.1016/j.pharep.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 305.Mirshafiey A., Mortazavi-Jahromi S.S., Taeb M., Cuzzocrea S., Esposito E. Evaluation of the effect of α-L-guluronic acid (G2013) on COX-1, COX-2 activity and gene expression for introducing this drug as a novel NSAID with immunomodulatory property. Recent Pat. Inflammation Allergy Drug Discovery. 2018;12(2):162–168. doi: 10.2174/1872213X12666180607121809. [DOI] [PubMed] [Google Scholar]
- 306.M. Aslani, A. Ahmadzadeh, Z. Aghazadeh, M. Zaki-Dizaji, L. Sharifi, M. Hosseini, A. Mirshafiey, Influence of β-D-mannuronic acid, as a new member of non-steroidal anti-inflammatory drugs family, on expression pattern of chemokines and their receptors in rheumatoid arthritis, Current drug discovery technologies (2019). [DOI] [PubMed]
- 307.Aslani M., Ahmadzadeh A., Rezaieyazdi Z., Mortazavi-Jahromi S.S., Barati A., Hosseini M., Mirshafiey A. The situation of chemokine ligands and receptors gene expression, following the oral administration of drug mannuronic acid in rheumatoid arthritis patients, recent patents on inflammation & allergy. Drug Discovery. 2020;14(1):69–77. doi: 10.2174/1872213X13666191114111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Mortazavi Jahromi S.S., Jamshidi M.M., Yousefi M., Navabi S.S., Motamed N., Zavareh F.T., Mirshafiey A. Inhibitory effect of G2013 molecule as a novel immunomodulatory agent, on miR-155 gene expression in HEK-Blue hTLR4 cell line. Eur. J. Inflamm. 2016;14(2):86–92. [Google Scholar]
- 309.Mortazavi-Jahromi S.S., Aslani M., Omidian S., Ahmadzadeh A., Rezaieyazdi Z., Mirshafiey A. Immunopharmacological effect of β-d-mannuronic acid (M2000), as a new immunosuppressive drug, on gene expression of miR-155 and its target molecules (SOCS1, SHIP1) in a clinical trial on rheumatoid arthritis patients. Drug Dev. Res. 2020;81(3):295–304. doi: 10.1002/ddr.21619. [DOI] [PubMed] [Google Scholar]
- 310.Gaafar N., Aslani M., Aghazadeh Z., Mortazavi-Jahromi S., Razavi A., Mirshafiey A. Effects of mannuronic acid (M2000) on gene expression profile of signal transducer and activator of transcription proteins (STATs) in rheumatoid arthritis patients. Reumatismo. 2020;72(2):93–102. doi: 10.4081/reumatismo.2020.1235. [DOI] [PubMed] [Google Scholar]
- 311.Mortazavi-Jahromi S.S., Nazeri S., Jafarnezhad-Ansariha F., Oraei M., Mirshafiey A. Assessment of immunological profile in ankylosing spondylitis patients following a clinical trial with guluronic acid (G2013), as a new NSAID with immunomodulatory properties. Immunol. Res. 2019;67(1):108–115. doi: 10.1007/s12026-018-9042-3. [DOI] [PubMed] [Google Scholar]
- 312.Rezaieyazdi Z., Farooqi A., Soleymani-Salehabadi H., Ahmadzadeh A., Aslani M., Omidian S., Sadoughi A., Vahidi Z., Khodashahi M., Zamurrad S. International multicenter randomized, placebo-controlled phase III clinical trial of β-d-mannuronic acid in rheumatoid arthritis patients. Inflammopharmacology. 2019;27(5):911–921. doi: 10.1007/s10787-018-00557-2. [DOI] [PubMed] [Google Scholar]
- 313.Mortazavi-Jahromi S.S., Alizadeh S., Javanbakht M.H., Mirshafiey A. Anti-diabetic effect of β-D-mannuronic acid (M2000) as a novel NSAID with immunosuppressive property on insulin production, blood glucose, and inflammatory markers in the experimental diabetes model. Arch. Physiol. Biochem. 2019;125(5):435–440. doi: 10.1080/13813455.2018.1481094. [DOI] [PubMed] [Google Scholar]
- 314.Ahmadi H., Jamshidi A.R., Gharibdoost F., Mahmoudi M., Rastkari N., Mostafaei S., Fattahi M.J., Vojdanian M., Cuzzocrea S., Rehm B.H. A phase I/II randomized, controlled, clinical trial for assessment of the efficacy and safety of β-D-mannuronic acid in rheumatoid arthritis patients. Inflammopharmacology. 2018;26(3):737–745. doi: 10.1007/s10787-018-0475-z. [DOI] [PubMed] [Google Scholar]
- 315.Mortazavi-Jahromi S.S., Farazmand A., Motamed N., Navabi S.S., Mirshafiey A. Effects of guluronic acid (G2013) on SHIP1, SOCS1 induction and related molecules in TLR4 signaling pathway. Int. Immunopharmacol. 2018;55:323–329. doi: 10.1016/j.intimp.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 316.Fattahi M.J., Ahmadi H., Jafarnezhad-Ansariha F., Mortazavi-Jahromi S.S., Rehm B.H., Cuzzocrea S., Matsuo H., Mirshafiey A. Oral administration effects of β-D-mannuronic acid (M2000) on Th17 and regulatory T cells in patients with ankylosing spondylitis. Biomed. Pharmacother. 2018;100:495–500. doi: 10.1016/j.biopha.2018.02.059. [DOI] [PubMed] [Google Scholar]
- 317.Jahanbakhshi M., Babaloo Z., Mortazavi-Jahromi S.S., Shokri M.M., Ahmadi H., Mirshafiey A. Modification of sexual hormones in rheumatoid arthritis patients by M2000 (β-d-mannuronic acid) as a Novel NSAID with immunosuppressive property, Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune. Endocrine & Metabolic Disorders) 2018;18(5):530–536. doi: 10.2174/1871530318666180418111354. [DOI] [PubMed] [Google Scholar]
- 318.Mirshafiey A., Cuzzocrea S., Rehm B., Matsuo H. M2000: a revolution in pharmacology. Med. Sci. Monit. 2005;11(8):PI53-PI63. [PubMed] [Google Scholar]
- 319.Omidian S., Aghazadeh Z., Ahmadzadeh A., Aslani M., Hosseini M., Abbasi S., Mirshafiey A. Evaluating mannuronic acid effect on gene expression profile of inflammatory mediators in rheumatoid arthritis patients. Iranian J. Allergy, Asthma Immunol. 2021:1–13. doi: 10.18502/ijaai.v21i1.8612. [DOI] [PubMed] [Google Scholar]
- 320.Gaafar N.A., Aslani M., Aghazadeh Z., Razavi A., Mirshafiey A. The oral administration effect of drug mannuronic acid (M2000) on gene expression of matrix and tissue inhibitor of metalloproteinases in rheumatoid arthritis patients. Curr. Drug Discov. Technol. 2020;17(5):704–710. doi: 10.2174/1570163816666190620113320. [DOI] [PubMed] [Google Scholar]
- 321.S.S. Mortazavi-Jahromi, S. Alizadeh, M.H. Javanbakht, A. Mirshafiey, Anti-Diabetic and Angio-Protective Effect of Guluronic Acid (G2013) as a New Nonsteroidal Anti-Inflammatory Drug in the Experimental Model of Diabetes, Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 20(3) (2020) 446-452. [DOI] [PubMed]
- 322.Taeb M., Jafarzadeh A., Mortazavi-Jahromi S.S., Zainodini N., Mirzaei M.R., Jafarnezhad-Ansariha F., Aghazadeh Z., Mirshafiey A. Effect of β-D-mannuronic acid (M2000) on oxidative stress enzymes’ gene using healthy donor peripheral blood mononuclear cells for evaluating the anti-aging property. Curr. Drug Discov. Technol. 2019;16(3):265–271. doi: 10.2174/1570163815666180515122834. [DOI] [PubMed] [Google Scholar]
- 323.Taeb M., Mortazavi-Jahromi S.S., Jafarzadeh A., Mirzaei M.R., Mirshafiey A. An in vitro evaluation of anti-aging effect of guluronic acid (G2013) based on enzymatic oxidative stress gene expression using healthy individuals PBMCs. Biomed. Pharmacother. 2017;90:262–267. doi: 10.1016/j.biopha.2017.03.066. [DOI] [PubMed] [Google Scholar]
- 324.Mortazavi-Jahromi S.S., Alizadeh S., Javanbakht M.H., Mirshafiey A. Cardioprotective effect of β-d-mannuronic acid (M2000) as a novel NSAID on gene expression of oxLDL scavenger receptors in the experimental diabetic model. Immunopharmacol. Immunotoxicol. 2018;40(4):284–289. doi: 10.1080/08923973.2018.1455209. [DOI] [PubMed] [Google Scholar]
- 325.Demirsoy İ.H., Ertural D.Y., Balci Ş., Çınkır Ü., Sezer K., Tamer L., Aras N. Profiles of circulating miRNAs following metformin treatment in patients with type 2 diabetes. J. Medical Biochem. 2018;37(4):499–506. doi: 10.2478/jomb-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Barraclough J.Y., Joglekar M.V., Januszewski A.S., Martínez G., Celermajer D.S., Keech A.C., Hardikar A.A., Patel S. A MicroRNA signature in acute coronary syndrome patients and modulation by colchicine. J. Cardiovasc. Pharmacol. Therapeutics. 2020;25(5):444–455. doi: 10.1177/1074248420922793. [DOI] [PubMed] [Google Scholar]
- 327.Zhang K., Zhao L., Ma Z., Wang W., Li X., Zhang Y., Yuan M., Liang X., Li G. Doxycycline attenuates atrial remodeling by interfering with microRNA-21 and downstream phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K) signaling pathway. Medical Science Monitor: Int. Medical J. Experimental Clin. Res. 2018;24:5580. doi: 10.12659/MSM.909800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Tran P.H., Xiang D., Tran T.T., Yin W., Zhang Y., Kong L., Chen K., Sun M., Li Y., Hou Y. Exosomes and nanoengineering: a match made for precision therapeutics. Adv. Mater. 2020;32(18):1904040. doi: 10.1002/adma.201904040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.