Abstract
Background
Encorafenib plus cetuximab with or without binimetinib showed increased objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) compared with chemotherapy plus anti-EGFR in previously treated patients with BRAF V600E-mutated (mut) metastatic colorectal cancer (mCRC). Although no formal comparison was planned, addition of binimetinib to encorafenib plus cetuximab did not provide significant efficacy advantage.
Patients and methods
This real-life study was aimed at evaluating safety, activity, and efficacy of encorafenib plus cetuximab with or without binimetinib in patients with BRAF V600E-mut mCRC treated at 21 Italian centers within a nominal use program launched in May 2019.
Results
Out of 133 patients included, 97 (73%) received encorafenib plus cetuximab (targeted doublet) and 36 (27%) the same therapy plus binimetinib (targeted triplet). Most patients had Eastern Cooperative Group Performance Status (ECOG-PS) of 0 or 1 (86%), right-sided primary tumor (69%), and synchronous disease (66%). Twenty (15%) tumors were DNA mismatch repair deficiency (dMMR)/microsatellite instability (MSI)-high. As many as 44 (34%) patients had received two or more prior lines of therapy, 122 (92%) were previously exposed to oxaliplatin, and 109 (82%) to anti-vascular endothelial growth factor (anti-VEGF). Most frequent adverse events were asthenia (62%) and anti-EGFR-related skin rash (52%). Any grade nausea (P = 0.03), vomiting (P = 0.04), and diarrhea (P = 0.07) were more frequent with the triplet therapy, while melanocytic nevi were less common (P = 0.06). Overall, ORR and disease control rate (DCR) were 23% and 69%, respectively, with numerically higher rates in the triplet group (ORR 31% versus 17%, P = 0.12; DCR 78% versus 65%, P = 0.23). Median PFS and OS were 4.5 and 7.2 months, respectively. Worse ECOG-PS, peritoneal metastases, and more than one prior treatment were independent poor prognostic factors for PFS and OS. Clonality of BRAF mutation measured as adjusted mutant allele fraction in tumor tissue was not associated with clinical outcome.
Conclusions
Our real-life data are consistent with those from the BEACON trial in terms of safety, activity, and efficacy. Patients in good general condition and not heavily pretreated are those more likely to derive benefit from the targeted treatment.
Key words: BRAF V600E mutation, BEACON trial, real-life studies, encorafenib, binimetinib, adjusted MAF
Highlights
-
•
Encorafenib plus cetuximab ± binimetinib is safe and effective for BRAF V600E mut mCRC even in the real-world setting.
-
•
Median OS is slightly shorter than in the BEACON trial, probably due to less selected patients in real life.
-
•
Patients deriving more benefit from targeted therapy are likely those in good general conditions and not heavily pretreated.
-
•
BRAF adjusted MAF is worth further investigation to better characterize the genomic heterogeneity of BRAF V600E mut mCRC.
Introduction
Patients with BRAF V600E-mutated (mut) metastatic colorectal cancer (mCRC) have poor prognosis and achieve limited benefit from standard treatments.1,2 The phase III BEACON study demonstrated significant benefit from encorafenib plus cetuximab with or without binimetinib compared with irinotecan-based chemotherapy plus anti-EGFR, in terms of objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) among 665 patients with pretreated BRAF V600E-mut mCRC.3,4 Although the study was not designed to formally compare the two experimental arms, at a post hoc descriptive analysis the addition of binimetinib did not seem to provide advantages in terms of PFS and OS, despite the increased ORR. Furthermore, it was associated with higher rates of adverse events (AEs), except for some BRAF inhibitor-specific toxicities that were less common in the triplet arm (i.e. melanocytic nevus, arthralgia).4
Based on these data, encorafenib plus cetuximab was approved by regulatory agencies worldwide for the treatment of patients with BRAF V600E-mut mCRC already exposed to at least one prior line of systemic therapy.5,6 In May 2019, a nominal use program was launched in Italy to offer this new target combination to previously treated BRAF V600E-mut patients. Both encorafenib and binimetinib were initially provided to be combined with cetuximab; however, after the updated results of the BEACON study, showing the limited clinical benefit added by binimetinib, only encorafenib has been supplied for subsequent patients since February 2020. Nonetheless, a recent analysis by Élez et al.7 performed on circulating tumor DNA collected at baseline from a small cohort of patients treated in the BEACON study showed that patients with higher BRAF mutant allele fraction (MAF) had worse prognosis, but could derive higher benefit from the addition of the MEK inhibitor.
Collecting clinical data from patients treated in the nominal use program at participating Italian Centers, we aimed at describing the safety and efficacy outcomes of encorafenib plus cetuximab with or without binimetinib in a real-life scenario. We also explored the potential prognostic and predictive roles of BRAF clonality measured as adjusted MAF (adjMAF) in tumor tissues.
Patients and methods
Data from patients included in the nominal use program at 21 participating Italian centers were retrospectively collected. Patients who received at least one dose of treatment, and with at least one disease reassessment after treatment start, or experiencing either clinical disease progression or death before the first disease evaluation were included in this analysis.
Eligibility criteria to enter the nominal use program reflected the Beacon Trial enrollment ones: patients ≥18 years old; diagnosed with histologically confirmed BRAF V600E mut mCRC; progressed to one or more lines of treatment, with evaluable disease per RECIST version 1.1; and with adequate bone marrow, renal, and liver functions.
All patients provided their written informed consent to the treatment.
In the triplet group, encorafenib and binimetinib were administered at a standard dose (300 mg daily and 45 mg twice daily, respectively), while cetuximab was administered weekly (400 mg per m2 as an initial dose, followed by 250 mg/m2 as subsequent doses), in 28-day cycles. In the doublet group, patients received encorafenib and cetuximab at the same schedule. Dose delays, modifications, and interruptions were adopted according to the recommendations of the BEACON study protocol.3
Disease assessment was performed every 8 weeks by contrast-enhanced computed tomography and responses were evaluated by RECIST version 1.1 criteria.
The following patients’ characteristics were collected before treatment start: age, Eastern Cooperative Group Performance Status (ECOG-PS), primary tumor location, number of metastatic sites, sites of metastases, prior resection of the primary tumor, prior lines of treatment, microsatellite instability (MSI), and time to metastases. MSI was tested by immunohistochemistry staining [DNA mismatch repair deficiency (dMMR)] and/or polymerase chain reaction (MSI-high), as per each center’s practice. BRAF V600E mutation was detected on primary tumors and/or metastatic tissue samples by next-generation sequencing (NGS), Sequenom MassARRAY using MALDI-TOF technology, real-time polymerase chain reaction (RT-PCR), and/or pyrosequencing, as per each center’s practice. For samples with available NGS results, BRAF MAF was defined as the percentage of mutant alleles within the totality of alleles in the samples. MAF was then adjusted (adjMAF) for the tumor cellularity determined in an hematoxylin and eosin-stained slide. adjMAFs <0.5 define subclonal events.8
AEs occurring during the treatment were collected and graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC-AE), version 4.03.
OS was calculated from the date of treatment start to death from any cause, with censoring at the date of last follow-up in living patients. PFS was calculated from the date of treatment start to disease progression or death, whichever occurred first. OS and PFS curves were estimated by the Kaplan–Meier method and compared by log-rank test. Hazard ratios (HRs) and 95% confidence interval (CI) were estimated by Cox proportional hazard model. The impact of patients’ baseline characteristics on PFS and OS was assessed at univariable analysis, and significant prognostic variables (P <0.10) were included in a multivariable model. The association between baseline characteristics and disease control rate (DCR) was performed by odds ratios (ORs) with corresponding 95% CI. Significant differences between the triplet and doublet groups in terms of AEs and tumor response were estimated by chi-square test, or Fisher’s exact test, as appropriate. Subgroup analyses of triplet versus doublet therapy for PFS and OS according to BRAF clonality were performed using an interaction test.
All statistical analyses were carried out using MedCalc Statistical Software version 19.4.1 (MedCalc Software Ltd, Ostend, Belgium) and SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study population
Among 133 patients treated at 21 Italian centers, 36 (27%) and 97 (73%) received triplet and doublet therapies, respectively. Patients’ baseline characteristics are summarized in Table 1. Overall, median age was 65 years, most patients (59%) were females, and had ECOG-PS of 0-1 (86%). Primary tumor was mainly located in the right colon (69%), and had been previously resected in 104 (78%) patients. As many as 88 (66%) patients had synchronous metastases and 53 (40%) with more than three organs involved, with liver (57%) and peritoneum (55%) being the most frequent sites. A total of 44 (33%) patients had already received two or more lines of therapy for the metastatic disease; oxaliplatin and anti-VEGF had been previously administered to most patients (92% and 82%, respectively). Forty-seven (35%) patients received subsequent lines of therapy after disease progression. Among 20 patients with dMMR/MSI-high tumors, 9 (45%) received immune checkpoint inhibitors (ICIs), 3 before and 6 after the study treatment.
Table 1.
Patients’ characteristics
| Characteristics | All patients (N = 133) | Enco/Cetux/Bini (N = 36) | Enco/Cetux (N = 97) |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 55 (41) | 17 (47) | 38 (39) |
| Female | 78 (59) | 19 (53) | 59 (61) |
| Age (years), median (range) | 65 (26-85) | 64 (29-80) | 68 (26-85) |
| ECOG-PS, n (%) | |||
| 0 | 54 (40) | 18 (50) | 36 (37) |
| 1 | 61 (46) | 13 (36) | 48 (50) |
| 2 | 18 (14) | 5 (14) | 13 (13) |
| Location of primary tumor, n (%) | |||
| Left colon | 30 (23) | 10 (27) | 20 (21) |
| Rectum | 11 (8) | 2 (6) | 9 (9) |
| Right colon | 92 (69) | 24 (67) | 68 (70) |
| Involvement of three or more organs, n (%) | 53 (40) | 12 (33) | 41 (42) |
| Liver metastases | 76 (57) | 25 (69) | 51 (53) |
| Peritoneal metastases | 73 (55) | 18 (50) | 55 (57) |
| Primary tumor resected, n (%) | 104 (78) | 30 (83) | 74 (76) |
| Prior lines of therapy, n (%) | |||
| 0 | 4 (3) | 0 (0) | 4 (4) |
| 1 | 85 (64) | 22 (61) | 63 (65) |
| 2 | 34 (26) | 9 (25) | 25 (26) |
| 3 | 5 (4) | 2 (6) | 3 (3) |
| ≥4 | 5 (4) | 3 (8) | 2 (2) |
| Prior oxaliplatin, n (%) | 122 (92) | 34 (94) | 88 (91) |
| Prior anti-VEGF, n (%) | 109 (82) | 32 (89) | 77 (79) |
| Prior anti-EGFR, n (%) | 10 (8) | 5 (14) | 5 (5) |
| MSI status, n (%) | |||
| MSI high | 20 (15) | 7 (19) | 13 (13) |
| MSS | 111 (83) | 27 (75) | 84 (87) |
| Unknown | 2 (2) | 2 (6) | 0 (0) |
| Baseline CEA, n (%) | |||
| >5 μg/L | 93 (70) | 27 (75) | 66 (68) |
| ≤5 μg/L | 26 (19) | 3 (8) | 23 (24) |
| Unknown | 14 (11) | 6 (17) | 8 (8) |
| Baseline LDH, n (%) | |||
| ≥250 U/L | 39 (30) | 13 (36) | 26 (27) |
| <250 U/L | 43 (32) | 8 (22) | 35 (36) |
| Unknown | 51 (38) | 15 (42) | 36 (37) |
| Treatment received, n (%) | |||
| Enco/Cetux/Bini | 36 (27) | 36 (100) | 0 |
| Enco/Cetux | 97 (73) | 0 | 97 (100) |
| Time to metastasis, n (%) | |||
| Synchronous | 88 (66) | 22 (61) | 66 (68) |
| Metachronous | 45 (34) | 14 (39) | 31 (32) |
| Subsequent lines, n (%) | 47 (35) | 16 (44) | 31 (32) |
Bini, binimetinib; CEA, carcinoembryonic antigen; Cetux, cetuximab; EGFR, endothelial growth factor receptor; ECOG-PS, Eastern Cooperative Group Performance status; LDH, lactate dehydrogenase; MSI, microsatellite instability; MSS, microsatellite stable; Enco, encorafenib; N, number; VEGF, vascular endothelial growth factor.
Numerically higher rates of males (47%), of patients with liver metastases (69%), and with lower disease burden (less than three sites involved in 67% of patients) were treated in the triplet group.
Safety
Overall, patients received a median of four cycles of therapy (range 1-19). A total of 27 (20%) patients were still on treatment at the time of the analysis, while 106 (80%) discontinued treatment, mainly because of disease progression (74%), and in a few cases due to AEs (4%), or other treatment-unrelated reasons (2%).
The median duration of exposure to treatment was 18 weeks in the whole population, 21 in the triplet group, and 19 in the doublet group.
To minimize hospital visits during the coronavirus disease 2019 (COVID-19) pandemic, 37 (28%) patients received a modified biweekly schedule of cetuximab (500 mg/m2 every 2 weeks) instead of the conventional weekly schedule, with no differences in the safety profile.
Cetuximab dose reduction was required in 5 (4%) patients because of AEs, while it was temporarily interrupted in 34 (26%) patients, due to AEs (13%) or to treatment-unrelated reasons (13%).
Encorafenib dose reduction was needed in 18 (14%) cases because of AEs, with a first dose reduction to 225 mg daily, and a second dose reduction to 150 mg daily in 10 (8%) and 8 (6%) cases, respectively. Encorafenib was temporarily interrupted in 47 (35%) patients, mainly (21%) because of AEs. No differences between triplet and doublet targeted therapies were reported in terms of dose reductions (P = 0.40) or temporary interruptions (P = 0.44).
AEs that occurred in the overall study population and in each treatment group are summarized in Table 2. Of 133 patients, 124 (93%) experienced at least one AE of any grade, 35 (97%) and 89 (92%) in the triplet and in the doublet groups, respectively (P = 0.44). Overall, the most common AEs were asthenia (62%), anti-EGFR skin rash (52%), nausea (36%), anemia (32%), and diarrhea (26%).
Table 2.
Adverse events
| Adverse events | Enco/Cetux/Bini (N = 36) |
Enco/Cetux (N = 97) |
P | Enco/Cetux/Bini (N = 36) |
Enco/Cetux (N = 97) |
P |
|---|---|---|---|---|---|---|
| Any grade, n (%) | Any grade, n (%) | Grade 3-4, n (%) | Grade 3-4, n (%) | |||
| Any adverse event | 35 (97) | 89 (92) | 0.44 | 12 (33) | 20 (21) | 0.20 |
| Asthenia | 23 (64) | 59 (61) | 0.90 | 3 (8) | 9 (9) | >0.99 |
| Anti-EGFR skin rash | 24 (66) | 45 (46) | 0.06 | 1 (3) | 2 (2) | >0.99 |
| Nausea | 19 (53) | 29 (30) | 0.03 | 3 (8) | 1 (1) | 0.06 |
| Diarrhea | 14 (39) | 21 (22) | 0.07 | 1 (3) | 2 (2) | >0.99 |
| Decreased appetite | 6 (17) | 28 (29) | 0.20 | 2 (6) | 3 (3) | 0.61 |
| Vomiting | 11 (31) | 13 (13) | 0.04 | 1 (3) | 1 (1) | 0.47 |
| Melanocytic nevus or skin lesions | 5 (14) | 29 (30) | 0.06 | 0 | 0 | — |
| Bowel obstruction | 2 (6) | 5 (5) | >0.99 | 1 (3) | 3 (3) | >0.99 |
| Anemia | 13 (36) | 30 (31) | 0.72 | 5 (14) | 5 (5) | 0.13 |
| Transaminase increase | 3 (8) | 12 (12) | 0.76 | 2 (6) | 0 | 0.07 |
| Creatinine increase | 5 (14) | 2 (2) | 0.02 | 0 | 1 (1) | >0.99 |
| Arthralgia | 3 (8) | 21 (22) | 0.13 | 0 | 2 (2) | >0.99 |
| Ocular toxicity | 0 | 1 (1) | >0.99 | 0 | 0 | — |
Bold/italic are statistically significant P values.
Bini, binimetinib; Cetux, cetuximab; EGFR, endothelial growth factor receptor; Enco, encorafenib; N, number; —, test not done.
Nausea (53% versus 30%; P = 0.03), vomiting (31% versus 13%; P = 0.04), and creatinine increase (14% versus 2%; P = 0.02) occurred more frequently in the triplet group. Not significant increase of anti-EGFR skin rash (66% versus 46%; P = 0.06) and diarrhea (39% versus 22%; P = 0.07) was reported in patients treated with the triplet combination. By contrast, melanocytic nevi (14% versus 30%; P = 0.06) and arthralgia (8% versus 22%; P = 0.13) were less common in this treatment subgroup.
In the overall population, 32 (24%) patients experienced at least one grade 3-4 AEs, with no differences between the two treatment groups (33% triplet and 21% doublet; P = 0.20). Globally, most common G3-4 AEs were asthenia (9%) and anemia (8%; Table 2). One patient suffered from ocular toxicity, specifically G2 anterior uveitis, in the doublet arm (1% versus none in triplet; P = 1.00).
Activity and efficacy
Thirty (23%) patients achieved objective response, and 61 (46%) showed disease stabilization as best response, with an overall DCR of 69%. ORRs were numerically higher in the triplet group compared with doublet (31% versus 17%; P = 0.12), as well as DCRs (78% versus 65%, P = 0.23).
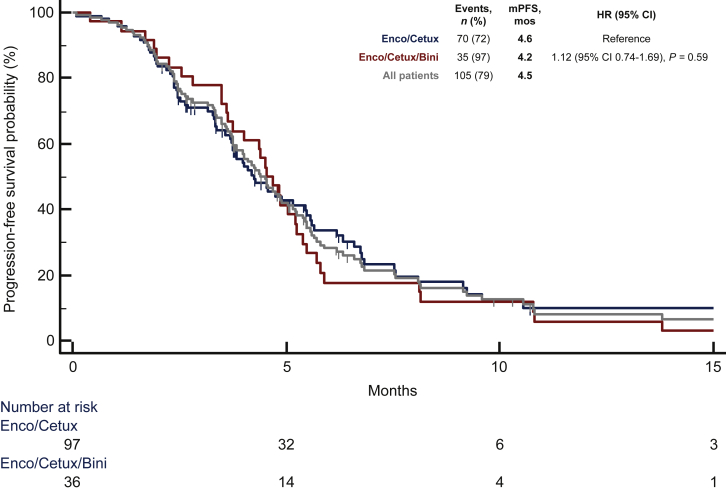
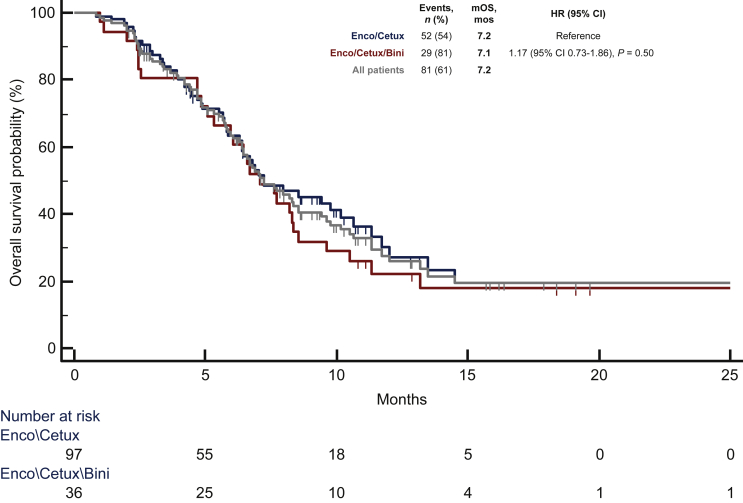
At a median follow-up of 10.8 months, 105 (79%) progression events were registered, 35 (97%) in the triplet group and 70 (72%) in the doublet group. Overall, median PFS was 4.5 months, without significant differences between treatment subgroups [4.2 months with the triplet versus 4.6 with the doublet; HR 1.12 (95% CI 0.74-1.69); P = 0.59; Figure 1]. As many as 81 (61%) deaths were recorded during the study follow-up, 29 (81%) in the triplet group and 52 (54%) in the doublet group. Overall, median OS was 7.2 months, and it was similar in the two subgroups [7.1 months for the triplet, and 7.2 for the doublet; HR 1.17 (95% CI 0.73-1.86); P = 0.50; Figure 2].
Figure 1.
Median progression-free survival (mPFS) for overall population, doublet, and triplet.
Bini, binimetinib; Cetux, cetuximab; CI, confidence interval; Enco, encorafenib; HR, hazard ratio; mos, months; mPFS, median progression-free survival; n, number.
Figure 2.
Median overall survival for overall population, doublet, and triplet.
Bini, binimetinib; Cetux, cetuximab; CI, confidence interval; Enco, encorafenib; HR, hazard ratio; mOS, median overall survival; mos, months; mPFS, median progression-free survival; n, number.
Among patients with proficient MMR/microsatellite stable (pMMR/MSS) tumors (N = 111), 27 (25%) achieved objective response, and 47 (42%) disease stabilization, with an overall DCR of 67%. ORR (33% versus 21%; P = 0.21) and DCR (81% versus 62%; P = 0.07) were numerically higher for the triplet group compared with the doublet group. Median PFS was 4.6 months, without significant differences between doublet and triplet targeted therapies [4.2 versus 4.8 months; HR 1.06 (95% CI 0.67-1.69); P = 0.79]. Median OS was 7.0 months, and it was similar between treatment groups [7.0 months for doublet versus 7.1 for triplet; HR 1.25 (95% CI 0.75-2.07); P = 0.37].
Age <70 years (P = 0.06), ECOG-PS ≥1 (P = 0.03), higher disease burden (P = 0.05), liver metastases (P = 0.05), and peritoneal metastases (P = 0.02) were associated with a significantly higher risk of disease progression at the univariate analyses. The association of ECOG-PS ≥1 (P = 0.02) and peritoneal metastases (P = 0.04) with worst PFS was confirmed in the multivariable model (Table 3). ECOG-PS ≥1 (P < 0.001), right-sided primary tumor (P = 0.04), three or more metastatic sites (P = 0.02), liver (P = 0.03) and peritoneal metastases (P = 0.05), unresected primary tumor (P = 0.07), two or more prior lines of therapy (P = 0.03), and pMMR/MSS status (P = 0.04) were significantly associated with worse OS in the univariate analyses. Among them, worse ECOG-PS (P = 0.002), peritoneal metastases (P = 0.01), and two or more prior lines of therapy (P = 0.01) confirmed their independent association with poor prognosis at the multivariable model (Table 3).
Table 3.
Univariate and multivariate analyses for progression-free survival and overall survival
| Characteristics | All patients (n = 133), n (%) | Progression-free survival |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
||||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | ||
| Age (years) | |||||||||
| <70 | 83 (62) | 1 | 0.06 | 1 | 0.12 | 1 | 0.21 | — | — |
| ≥70 | 50 (38) | 0.67 (0.44-1.02) | 0.71 (0.47-1.09) | 0.73 (0.45-1.19) | — | ||||
| ECOG-PS | |||||||||
| 0 | 54 (40) | 1 | 0.03 | 1 | 0.02 | 1 | <0.001 | 1 | 0.002 |
| 1-2 | 79 (60) | 1.54 (1.04-2.29) | 1.63 (1.09-2.45) | 2.37 (1.45-3.85) | 2.40 (1.40-4.11) | ||||
| Location of primary tumor | |||||||||
| Right colon | 92 (69) | 1 | 0.49 | — | - | 1 | 0.04 | 1 | 0.37 |
| Left colon or rectum | 41 (31) | 0.86 (0.57-1.31) | — | 0.62 (0.39-0.98) | 0.80 (0.49-1.30) | ||||
| Number of metastatic sites | |||||||||
| Less than three organs | 80 (60) | 1 | 0.05 | 1 | 0.63 | 1 | 0.02 | 1 | >0.99 |
| Three or more organs | 53 (40) | 1.47 (1.00-2.17) | 1.11 (0.72-1.72) | 1.74 (1.11-2.73) | 1.00 (0.60-1.68) | ||||
| Liver metastases | |||||||||
| No | 57 (43) | 1 | 0.05 | 1 | 0.21 | 1 | 0.03 | 1 | 0.30 |
| Yes | 76 (57) | 1.51 (1.01-2.25) | 1.32 (0.86-2.00) | 1.67 (1.05-2.66) | 1.33 (0.78-2.27) | ||||
| Peritoneal metastases | |||||||||
| No | 60 (45) | 1 | 0.02 | 1 | 0.04 | 1 | 0.05 | 1 | 0.01 |
| Yes | 73 (55) | 1.57 (1.06-2.32) | 1.59 (1.03-2.46) | 1.57 (1.00-2.47) | 1.98 (1.19-3.30) | ||||
| Primary tumor resected | |||||||||
| Yes | 104 (78) | 1 | 0.20 | — | — | 1 | 0.07 | 1 | 0.28 |
| No | 29 (22) | 1.35 (0.85-2.15) | — | 1.64 (0.98-2.75) | 1.38 (0.78-2.46) | ||||
| Prior lines of therapy | |||||||||
| 0-1 | 89 (67) | 1 | 0.31 | — | — | 1 | 0.03 | 1 | 0.01 |
| ≥2 | 44 (33) | 1.23 (0.82-1.84) | — | 1.63 (1.05-2.56) | 1.83 (1.13-2.96) | ||||
| MSI status | |||||||||
| MSS | 111 (83) | 1 | 0.85 | — | 1 | 0.04 | 1 | 0.08 | |
| MSI high | 20 (15) | 0.95 (0.57-1.61) | — | 0.48 (0.24-0.96) | 0.50 (0.23-1.08) | ||||
| Time to metastasis | |||||||||
| Metachronous | 45 (34) | 1 | 0.13 | — | — | 1 | 0.35 | — | — |
| Synchronous | 88 (66) | 1.37 (0.91-2.06) | — | 1.25 (0.78-2.03) | — | ||||
Bold/italic are statistically significant P values.
Bini, binimetinib; Cetux, cetuximab; CI, confidence interval; ECOG-PS, Eastern Cooperative Group Performance Status; Enco, encorafenib; HR, hazard ratio; MSI, microsatellite instability; MSS, microsatellite stable; N, number.
ECOG-PS ≥1 was the only baseline characteristic significantly associated with lower chance of achieving disease control [OR 0.28 (95% CI 0.12-0.65); P = 0.003; Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100506].
BRAF mutant allele fraction and treatment outcome
BRAF mutation was assessed by NGS in 53 (40%) cases. The calculated adjMAF was ≥0.5 (clonal event) and <0.5 (subclonal event) in 21 (40%) and 32 (60%) cases, respectively, with a median value of 0.44 (IQR 0.30-0.75). Distribution different than normal reflects a subclonal event for BRAF mutation.8 No significant differences were reported between groups in terms of PFS [4.2 months for subclonal and 5.7 for clonal; HR 0.81 (95% CI 0.44-1.50); P = 0.50] and OS [6.9 months for subclonal and 9.8 for clonal; HR 0.70 (95% CI 0.35-1.43); P = 0.33; Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100506]. No significant interaction effect between the treatment arm and clonality in terms of PFS (P = 0.76) or OS (P = 0.56) was observed (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100506).
Discussion
BRAF V600E mutation recently turned from an awfully negative prognostic marker into a positive predictor of benefit from a targeted strategy.1 Based on the results of the BEACON study, regulatory agencies approved the combination of the BRAF inhibitor encorafenib with the anti-EGFR antibody cetuximab as a new treatment option for this poor prognosis subgroup, with modest benefit from all previously available regimens.1, 2, 3, 4, 5, 6 The phase II SWOG S1406 trial confirmed the efficacy of the combination of a BRAF inhibitor (vemurafenib) with an anti-EGFR (cetuximab), thus corroborating the efficacy of the combination strategy in overcoming the intrinsic resistance of BRAF V600E mCRC to the use of BRAF inhibitors as single agents.1,9
Results of pivotal randomized trials are the essential starting point to bring new treatment options into the daily clinical practice. At the same time, real-life data may provide useful safety and efficacy information about the adoption of these new agents in a less clinically selected population, thus helping to optimize the treatment management and to properly position the new option in the therapeutic route of impacted patients.
To this end, we collected data from a real-life population treated with encorafenib plus cetuximab with or without binimetinib in the frame of a nominal use program at 21 participating Italian centers.
Our population shared similar characteristics with patients enrolled in the BEACON study, except for a higher percentage of patients with ECOG-PS of 2 (14%) that were not eligible in the randomized study, and a higher percentage of patients previously exposed to more than two lines of therapy (8%) that were <1% in the BEACON trial.3,4
Safety results in our cohort were highly consistent with those previously reported3,4: AEs were mostly G1/2 and most frequent G3 or 4 events were diarrhea, asthenia, anemia, and nausea. The triplet combination was associated with an increased overall rate of AEs, but the addition of binimetinib was also able to mitigate some encorafenib-related toxicities, such as arthralgia and the occurrence of melanocytic nevi.
About 28% of patients received a modified biweekly cetuximab schedule due to the COVID-19 pandemic, with no differences in the safety profile. Although no specific data are currently available with regard to the combination of the biweekly administration of cetuximab with binimetinib, the Food and Drug Administration (FDA) recently approved this schedule for the use of cetuximab monotherapy and in combination with chemotherapy. This was based on the evidence of comparable pharmacokinetic exposure, safety, efficacy, and survival outcomes between the two regimens.10 Moreover, in the ongoing BREAKWATER study, patients with BRAF V600E-mut mCRC receive biweekly cetuximab in association with encorafenib with or without chemotherapy.11
ORR and PFS results were similar to those reported in the BEACON study.3,4 To properly interpret findings from our real-world experience, and in particular the comparisons between the doublet and triplet targeted therapies, it should be acknowledged that patients were not randomized but longitudinally assigned to one of the two treatments according to a temporal criterion. The triplet was associated with numerically higher ORR, with no differences in terms of PFS and OS, thus supporting the use of encorafenib plus cetuximab as a standard option in this setting, but also making the identification of a subgroup of patients that may achieve more benefit from the addition of binimetinib an intriguing issue considering that achieving tumor shrinkage is frequently needed in these patients to prevent the onset of disease-related symptoms. To this purpose, we aimed at verifying whether patients with higher BRAF V600E MAF in their tumor tissues were more likely to derive benefit from the addition of the MEK inhibitor as suggested in a recent subgroup analysis of the BEACON trial.7 Indeed, the allele fraction of a mutated driver oncogene can be considered a surrogate biomarker of tumor burden and aggressiveness, with possible prognostic and predictive meaning.12,13 This could be especially relevant when considering the high degree of heterogeneity of BRAF V600E-mut mCRC, where BRAF V600E mutation occurs as a subclonal rather than as a clonal event,14, 15, 16 and where the clinical phenotype of the disease is heterogeneous too.17 Nevertheless, consistent with other studies conducted on CRC archival tissue,8 our ancillary analysis was not able to demonstrate a prognostic or predictive impact of BRAF adjMAF. adjMAF was chosen instead of MAF to mitigate the bias related to the heterogeneity in tumor cellularity and ploidy, and to derive an indirect measure of mutation clonality.8
As major limitations of this finding, it should be acknowledged that the adjMAF was available only for a small subgroup of patients included in this analysis and that NGS performed on archival tissue specimens collected at the time of the diagnosis might not properly reflect tumor molecular characteristics after one or more lines of treatment as tumors dynamically evolve under the pressure exerted by administered drugs.14, 15, 16, 17, 18, 19
Compared with patients enrolled in the BEACON study,3,4 those treated in our cohort reported a shorter (∼2 months less) median OS, probably as a consequence of the higher percentage of patients with poorer general conditions, and more heavily pretreated, as previously discussed.
Finally, we tried to understand which clinical characteristics independently affect the prognosis of patients with BRAF V600E-mut mCRC when treated with encorafenib with cetuximab with or without binimetinib. ECOG-PS ≥1, presence of peritoneal metastases, and two or more prior lines of therapy were independent poor prognostic factors for PFS and/or OS. As expected, these findings suggest that the targeted strategy should be offered as soon as possible to patients with BRAF V600E-mut mCRC in their therapeutic route, that is, after progression to the first line of therapy, while waiting for results of trials investigating the upfront use of this option.12,20 The association of dMMR/MSI-high status with longer OS at the univariate analysis was not confirmed in the multivariable model and was likely due to the effect of ICIs administered after the targeted therapy to half of these patients. Indeed, the impressive efficacy of ICIs in dMMR/MSI-high tumors is independent of BRAF mutational status, thus making the choice of immunotherapy a preferable upfront option also among patients with BRAF V600E-mut and dMMR/MSI-high tumors.21,22
Conclusions
Our study confirms the safety and efficacy data of encorafenib plus cetuximab with or without binimetinib reported in the BEACON trial in a real-life multicentric cohort of patients with pretreated BRAF V600E-mut mCRC. Patients in good general conditions and not heavily pretreated are more likely to derive benefit from the treatment, thus the use of the targeted strategy should not be delayed to later lines of treatment.
Acknowledgements
The authors are grateful to patients and their families and to the investigators from the participating Italian centers.
Funding
Partially funded by the Italian Ministry of Health grant “Ricerca Finalizzata Giovani Ricercatori” [grant number GR-2019-12368903].
Disclosure
MS reports being on the advisory board and speakers’ bureau for MERCK, MSD, Sanofi, Amgen, BMS, EISAI, and Servier. SL reports being on the speakers’ bureau for Amgen, Merck, Roche, Lilly, Bristol-Myers Squibb, Pierre-Fabre, GSK, and Servier; playing a consulting or advisory role for Amgen, MSD, Merck Serono, Lilly, Astra Zeneca, Incyte, Daiichi-Sankyo, Bristol-Myers Squibb, and Servier; research funding to institution from Bayer, Merck, Amgen, Roche, Lilly, AstraZeneca, and Bristol-Myers Squibb. CC reports honoraria from Amgen, Bayer, Merck, Roche, and Servier; performing a consulting or advisory role for Amgen, Bayer, MSD, and Roche; being on the speakers’ bureau for Servier; receiving/received research funding from Bayer, Merck, and Servier; travel, accommodations, and expenses from Roche and Servier. All other authors have declared no conflicts of interest.
Supplementary data
Median progression-free survival (A) and median overall survival (B) for patients with available BRAF adjMAF. Legend: N, number; mPFS, median progression-free survival; mOS, median overall survival; Mos, months; HR, hazard ratio; CI, confidence interval; adjMAF, adjusted mutant allele fraction. adjMAF < 0.5 defines subclonal event.
Forest plot of treatment effect on progression-free survival and overall survival according BRAF adjMAF. Legend: N, number; HR, hazard ratio; CI, confidence interval; Enco, encorafenib; Cetux, cetuximab; Bini, binimetinib; adjMAF, adjusted mutant allele fraction. adjMAF < 0.5 defines subclonal event.
References
- 1.Grothey A., Fakih M., Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959–967. doi: 10.1016/j.annonc.2021.03.206. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E., Cervantes A., Nordlinger B., Arnold D., ESMO Guidelines Working Group Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S., Grothey A., Yaeger R., et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 4.Tabernero J., Grothey A., Cutsem E.V., et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E–mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol. 2021;39:273–384. doi: 10.1200/JCO.20.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. BRAFTOVI® (encorafenib) Summary of Product Characteristics. Silver Spring, MD: FDA. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210496s006lbl.pdfhttps://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-combination-cetuximab-metastatic-colorectal-cancer-braf-v600e-mutation Available at.
- 6.European Medicines Agency . European Medicines Agency; London UK: 2018. BRAFTOVI® (encorafenib) Summary of Product Characteristics.https://www.ema.europa.eu/en/medicines/human/EPAR/braftovi Available at. [Google Scholar]
- 7.Élez E., Ros J., Martini G., et al. LBA-3 Integrated analysis of cell-free DNA (cfDNA) BRAF mutant allele fraction (MAF) and whole exome sequencing in BRAFV600E metastatic colorectal cancer (mCRC) treated with BRAF-antiEGFR +/- MEK inhibitors. Ann Oncol. 2021;32:S226–S227. [Google Scholar]
- 8.Dienstmann R., Elez E., Argiles G., et al. Analysis of mutant allele fractions in driver genes in colorectal cancer – biological and clinical insights. Mol Oncol. 2017;11:1263–1272. doi: 10.1002/1878-0261.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopetz S., Guthrie K.A., Morris V.K., et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) J Clin Oncol. 2021;39:285–294. doi: 10.1200/JCO.20.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh A.R., Gonzalez-Gugel E., Smolyakova N., et al. Efficacy and safety of cetuximab dosing (biweekly vs weekly) in patients with KRAS wild-type metastatic colorectal cancer: a meta-analysis. Oncologist. 2022;27:371–379. doi: 10.1093/oncolo/oyab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopetz S., Grothey A., Yaeger R., et al. BREAKWATER: randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC) J Clin Oncol. 2021;39 TPS3619-TPS3619. [Google Scholar]
- 12.Elez E., Chianese C., Sanz-García E., et al. Impact of circulating tumor DNA mutant allele fraction on prognosis in RAS-mutant metastatic colorectal cancer. Mol Oncol. 2019;13:1827–1835. doi: 10.1002/1878-0261.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loree J.M., Dowers A., Tu D., et al. Expanded low allele frequency RAS and BRAF V600E testing in metastatic colorectal cancer as predictive biomarkers for cetuximab in the randomized CO.17 Trial. Clin Cancer Res. 2021;27:52–59. doi: 10.1158/1078-0432.CCR-20-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Normanno N., Rachiglio A.M., Lambiase M., et al. Heterogeneity of KRAS, NRAS, BRAF and PIK3CA mutations in metastatic colorectal cancer and potential effects on therapy in the CAPRI GOIM trial. Ann Oncol. 2015;26:1710–1714. doi: 10.1093/annonc/mdv176. [DOI] [PubMed] [Google Scholar]
- 15.Molinari C., Marisi G., Passardi A., et al. Heterogeneity in colorectal cancer: a challenge for personalized medicine? Int J Mol Sci. 2018;19:3733. doi: 10.3390/ijms19123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angerilli V., Fontana E., Lonardi S., et al. Intratumor morphologic and transcriptomic heterogeneity in V600EBRAF-mutated metastatic colorectal adenocarcinomas. ESMO Open. 2021;6(4):100211. doi: 10.1016/j.esmoop.2021.100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loupakis F., Intini R., Cremolini C., et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: the ‘BRAF BeCool’ study. Eur J Cancer. 2019 Sep;118:121–130. doi: 10.1016/j.ejca.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Cremolini C., Rossini D., Dell’Aquila E., et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–350. doi: 10.1001/jamaoncol.2018.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antoniotti C., Pietrantonio F., Corallo S., et al. Circulating tumor DNA analysis in colorectal cancer: from dream to reality. JCO Precis Oncol. 2019;3:1–14. doi: 10.1200/PO.18.00397. [DOI] [PubMed] [Google Scholar]
- 20.Van Cutsem E., Taieb J., Yaeger R., et al. O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer. Ann Oncol. 2021;32:S222. doi: 10.1200/JCO.22.01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taieb J., Lapeyre-Prost A., Laurent Puig P., et al. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br J Cancer. 2019;121:434–442. doi: 10.1038/s41416-019-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.André T., Shiu K.-K., Kim T.W., et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. N Engl J Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Median progression-free survival (A) and median overall survival (B) for patients with available BRAF adjMAF. Legend: N, number; mPFS, median progression-free survival; mOS, median overall survival; Mos, months; HR, hazard ratio; CI, confidence interval; adjMAF, adjusted mutant allele fraction. adjMAF < 0.5 defines subclonal event.
Forest plot of treatment effect on progression-free survival and overall survival according BRAF adjMAF. Legend: N, number; HR, hazard ratio; CI, confidence interval; Enco, encorafenib; Cetux, cetuximab; Bini, binimetinib; adjMAF, adjusted mutant allele fraction. adjMAF < 0.5 defines subclonal event.