Abstract
A variety of psychological and physical phenomena elicit variations in the diameter of pupil of the eye. Changes in pupil size are mediated by the relative activation of the sphincter pupillae muscle (decrease pupil diameter) and the dilator pupillae muscle (increase pupil diameter), innervated by the parasympathetic and sympathetic branches, respectively, of the autonomic nervous system. The current guidelines are intended to inform and guide psychophysiological research involving pupil measurement by (1) summarizing important aspects concerning the physiology of the pupil, (2) providing methodological and data-analytic guidelines and recommendations, and (3) briefly reviewing psychological phenomena that modulate pupillary reactivity. Because of the increased ease and tractability of pupil measurement, the goal of these guidelines is to promote accurate recording, analysis, and reporting of pupillary data in psychophysiological research.
Keywords: dilation, parasympathetic, pupil constriction, pupillography, pupillometry, sympathetic
1 |. INTRODUCTION
It has long been noted that a variety of psychological phenomena elicit changes in pupil diameter, beginning with observations by Archimedes and Galen that were followed up in 18th century treatises (e.g., Fontana, 1765). Interest in pupillary phenomena accelerated in the 19th century, with the reported observation that psychotic patients exhibit abnormal pupillary responses (Bach, 1908; Bumke, 1904), prompting a resurgence of psychological research in the 1960s by Hess, Kahneman, Beatty, and their colleagues in psychology, and Hakerem, Rubin, and Sutton in psychiatry (see Steinhauer & Hakerem, 1992, for review). In particular, a report of pupillary reactions during emotional processing (Hess & Polt, 1960) and problem-solving (Hess & Polt, 1964) spurred psychophysiological studies of pupillary dynamics of emotion and cognition. Whereas measuring the pupil was initially technically challenging, in recent years, both hardware and software advances have rendered pupillary measurement more tractable.
The primary goal of the current pupil measurement guidelines is to provide information important for conducting and reporting pupillary research in Psychophysiology and other psychologically oriented journals. An outstanding set of resources and summaries already exist in the lifelong work by Irene Loewenfeld (1993) whose two-volume tome summarizes a half-century of her own research, as well as a review of over 15,000 pertinent studies. A number of books devoted to pupillary research include those by Hess (1975), Janisse (1974, 1977), Alexandridis (1985), Zinn (1972). There are reviews of psychological factors (Goldwater, 1972; Granholm & Steinhauer, 2004; Mathôt, 2018; Tryon, 1975) as well as book chapters by Beatty and colleagues (Beatty, 1986; Beatty & Lucero-Wagoner, 2000); Kahneman’s book (1973) includes pupil change as a central measure of arousal and processing effort. A recently published standard in pupillography from a working group of the International Colloquium on the Pupil (Kelbsch et al., 2019) provides additional information, with many of those recommendations reiterated here.
Pupillary recording emanates from a tradition of medicine and physiology, with measures analogous to electroencephalography and electrocardiography. The first electronic scanning instrument for pupil recording, developed by Smith-Kline Corporation in collaboration with Otto Lowenstein and Irene Loewenfeld, was called a scanning pupillograph, and in most of medicine and physiology, the term pupillography is retained. Hess (1972) first introduced the term pupillometrics for studying the pupil in psychological research, a trend continued by Janisse (1977), and currently either term is appropriate.
The following sections (1) summarize important aspects concerning the physiology of the pupil, (2) discuss methodological, data-analytic, and reporting recommendations, and (3) briefly review psychological phenomena that modulate pupillary measures. A summary of available software related to pupillary analysis is also provided.
1.1 |. Physiology of the pupil
The pupil is a spherically shaped opening in the eye whose size is controlled by movements of the autonomically innervated iris musculature. Like many physiological measures, the activity of the pupil is bidirectional—it can change from smaller to larger (approximately 2–10 mm in humans) and vice versa, and its size is determined by the relative activation (and relaxation) of two sets of muscles: the sphincter pupillae (which actively decrease pupil size) and the dilator pupillae (which actively increase pupil size; see Figure 1). The interplay between these sets of smooth muscles, and their neural activation/inhibition, is responsible for both the observed baseline diameter and dynamic pupillary changes. The most apparent function of iris muscular control is to modulate the amount of light that enters the eye, reaching the retina. Under bright (photopic) illumination, the receptors in the central region of the retina are stimulated and pupil size decreases. One consequence of this constriction (contraction) is that the edge area (or pupillary margin) is decreased, reducing the dispersion of light, and sharpening the visual image. In addition to light, the pupil also constricts as the eye accommodates to near stimuli, and as near objects produce vergence (i.e., movement of the eyes toward the nose). Under conditions of darkness, dilation of the pupil increases the ability of the retina to detect dim light sources that stimulate the rod (scotopic) system. The following sections represent an integration of physiological and anatomical details as reported in sources including Alexandridis (1985), Loewenfeld (1958, 1993), Lowenstein and Loewenfeld (1962), Szabadi (2012, 2018), and Zinn (1972).
FIGURE 1.
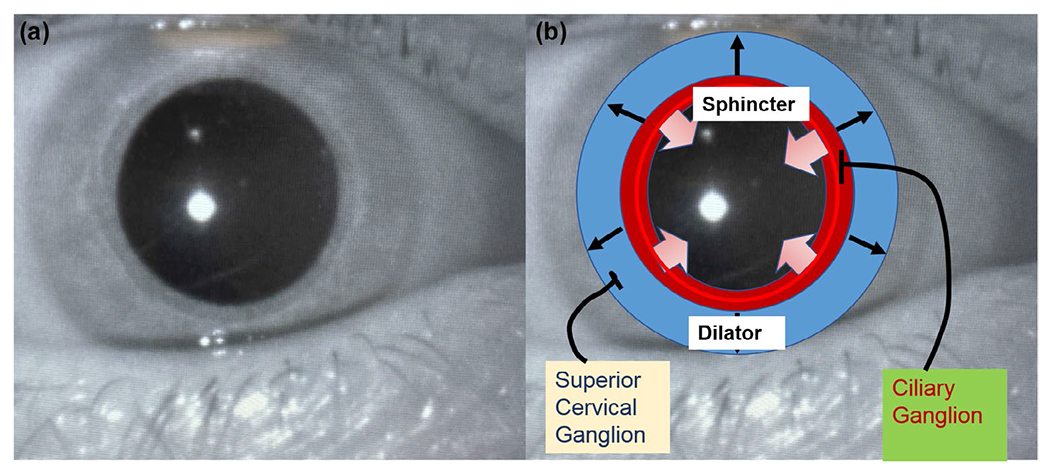
(a) Image of the eye. (b) Area of concentric sphincter muscles outlined in red, radial dilator muscles shown in blue
1.1.1 |. The iris
The iris of the eye, which lies on the lens, is comprised of muscles, nerve fibers, and blood vessels that are covered anteriorly by fluid and the cornea; the cornea magnifies the observed pupil by about an eighth (Loewenfeld, 1993). Different iris colors, produced by pigmentation in the iris tissue, have no significant effect on pupillary measures in psychophysiological studies, although there is slightly greater transmission of light through more lightly pigmented irises (e.g., blue), and uptake of topical pharmacological agents is slightly different for lighter, compared to darker, iris colors. No differences in the dark-adapted pupil diameter are related to eye color (Bradley et al., 2010).
Figure 1b illustrates the different topological arrangements and innervations of the iris sphincter and dilator muscles. The sphincter muscles are circular bands concentrated at the inner margins of the iris that are innervated by the parasympathetic nervous system. Activation of the sphincter results in pupil constriction, whereas inhibition of the sphincter prompts (relative) dilation; the term dilatation is also sometimes used. Muscarinic receptors in the sphincter muscles respond to acetylcholine released from presynaptic cholinergic neurons. Blocking of the muscarinic receptors by topical anticholinergic agents, such as tropicamide, results in relaxation of the sphincter, indirectly enlarging the pupil.
The dilator muscle, which directly enlarges the pupil, consists of radially oriented fibers that are innervated by the sympathetic nervous system, with alpha-adrenergic receptors that are stimulated by presynaptic release of noradrenaline from sympathetic pathways (but note that only the final preganglionic synapse releases noradrenaline). Each of the synapses emanates from the hypothalamus and involves acetylcholine transmission. Blocking of alpha-adrenergic receptors pharmacologically results in relaxation of the dilator muscles, decreasing pupil size. Centrally circulating drugs can have effects on the nervous system, as well as on the iris musculature. The pupil responds to and indexes activity throughout the brain in structures associated with both sympathetic and parasympathetic tone (Breeden et al., 2017), discussed in the next section.
1.1.2 |. Autonomic innervation
1.2.1 |. The parasympathetic pathway
Figure 2 depicts important afferent and efferent pathways involved in parasympathetic control. The motor center for the parasympathetic system is the Edinger–Westphal nucleus, a complex of cell groups found bilaterally near the oculomotor center of the midbrain, close to the superior colliculus. Edinger–Westphal neurons have a high intrinsic firing rate (Ichinohe & Shoumura, 2001; Zinn, 1972) and, even in darkness, there is some degree of minimal parasympathetic tone.
FIGURE 2.
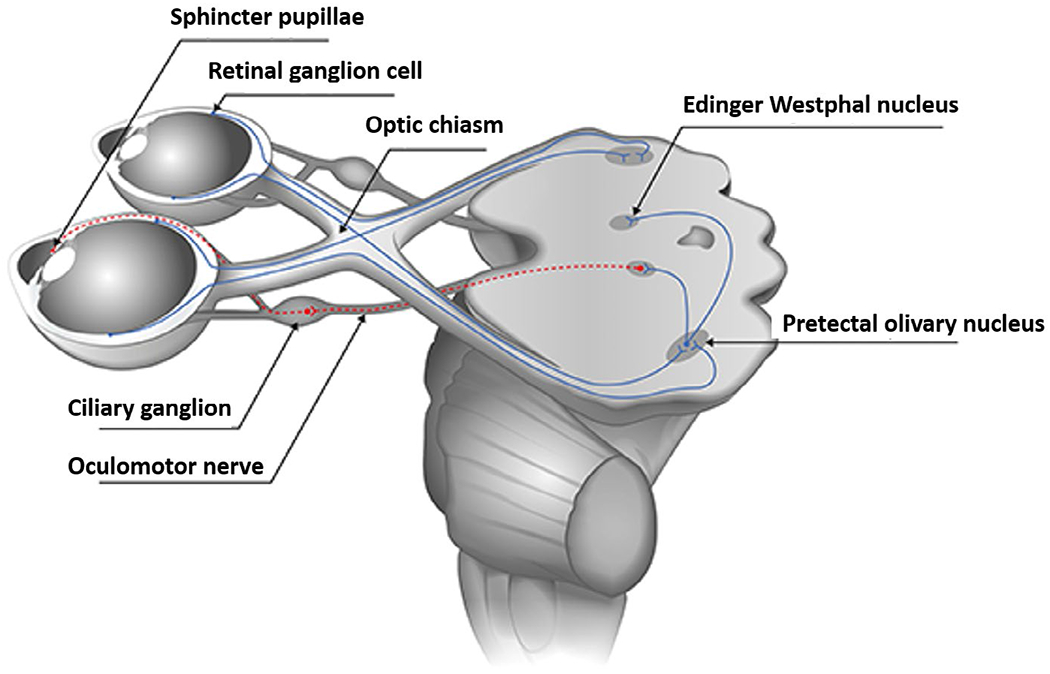
Schematic of the pupillary light reflex pathway (adapted from Kelbsch et al., 2019)
The afferent pathway for light-mediated reactions of the pupil involves excitation of the retina, with ganglion cells (the third layer of cells stimulated) forming the initial optic nerve’s fibers that terminate in the lateral geniculate nucleus of the thalamus. As many as 20% of these fibers are estimated to carry information for the pupillomotor system along the optic nerve (Zinn, 1972), with crossing of some fibers at the optic chiasma to form the optic tract. These fibers bypass the lateral geniculate nucleus, skirting the superior colliculi, and enter the bilateral pretectal nuclei of the mesencephalon, which communicate directly with each other. From the pretectal nuclei, postsynaptic neurons go either directly to the Edinger–Westphal nucleus, or bifurcate and pass through the posterior commissure to the opposite Edinger–Westphal nucleus. Because fibers cross both to and from the pretectal nuclei, each Edinger–Westphal nucleus receives retinal input from both eyes such that, in healthy individuals, the reflex elicited to light presented to one eye (“direct” response) results in a reflex of similar magnitude in the other eye (“consensual” response). Additional afferents related to accommodation and the near reflex include descending occipito-mesencephalic projections to the Edinger–Westphal nucleus.
The efferent pathway from the Edinger–Westphal nucleus to the pupil emerges via the oculomotor nerve (n. III), and involves a single synapse in the ciliary ganglion, from which a final short ciliary nerve stimulates the sphincter muscles. There are numerous inhibitory influences on the Edinger–Westphal nucleus, which decrease activity of the parasympathetic fibers of the oculomotor nerve, relaxing the sphincter and dilating the pupil. These include retino-mesencephalic fibers that produce “off responses” associated with the dark reaction (in which hypothalamic discharges travel through mesencephalic-reticular pathways to inhibit the Edinger–Westphal nuclei), as well as cortico-thalamo-hypothalamic pathways and ascending spino-reticular fibers that have direct inhibitory effects on the Edinger–Westphal nuclei (Lowenstein, 1955; Zinn 1972).
1.2.2 |. The sympathetic pathway
The integrative center for the pupillary sympathetic pathway is in the posterior region of the hypothalamus, with some contribution from lateral hypothalamic cells.
The specific afferents to the pupillary sympathetic center in the hypothalamus are numerous, yet not well delineated anatomically. Physiological studies show that both cortical and subcortical inputs are modulated through thalamic-hypothalamic pathways, including reticular activation, with pupillary motility reflecting activity throughout task-related networks (e.g., prefrontal and parietal engagement during cognitive and emotional tasks [Siegle et al., 2003, 2011]).
Despite evidence of multiple contributions to pupillary dilation, there has been a recent tendency to interpret pupil dilation as reflecting only the activity of the locus coeruleus (LC; e.g., Elman et al., 2017; Tsukahara & Engle, 2021; van der Linden et al., 2021; Yeung et al., 2021). The LC is a complex of neurons in the rostral pons of the brain, which is the primary site for the synthesis of noradrenaline (norepinephrine) and is closely associated with sympathetic arousal, having wide ranging effects throughout the brain and body. Both the hypothalamus and the nucleus paragigantocellularis (which controls sympathetically mediated heart rate and respiratory changes) stimulate the LC, and the LC efferently projects to the hypothalamus, thalamus, amygdala, cortex, and other brain regions mediating a host of sympathetically mediated reactions.
However, whereas in lower mammals there are known inhibitory pathways from the LC to the Edinger–Westphal nuclei, these direct pathways are not yet documented in humans, making it likely that sphincter muscle inhibition is mediated through additional thalamic and/or hypothalamic connections. Because of the widespread afferents to the sympathetic nervous system, the interpretation of sympathetically mediated pupillary changes as solely a direct index of LC activity is at odds with the many direct and indirect cortical and limbic afferents that contribute to pupillary changes. Data in the mouse model, for instance, document variability in the correspondence between pupil diameter and LC activity (Megemont et al., 2022). Reviews of LC activity in relation to pupillary dynamics appear in several sources (e.g., Aston-Jones & Cohen, 2005; Nieuwenhuis et al., 2011; Samuels & Szabadi, 2000a, 2000b; Szabadi, 2012, 2018).
Despite an incomplete understanding of afferents to the sympathetic nervous system, the efferent pathway from the posterior hypothalamus to the pupil is relatively well documented both anatomically and physiologically (Figure 3). First-order neurons descend through the brain stem, including the prerubral field. In the capsule of the red nucleus, some of the fibers cross the midline in the decussation of Forel (Zinn, 1972), and all fibers continue to the cervico-thoracic level of the spinal cord, synapsing at the gray matter ciliospinal center of Budge. Second-order neurons exit the spinal roots as part of the sympathetic chain, ending in the superior cervical ganglion, which innervates the head and neck; the neurotransmitter involved is acetylcholine. Third-order neurons terminate at the alpha-adrenergic receptors of the dilator muscles, where noradrenaline is released, resulting in pupil dilation.
FIGURE 3.
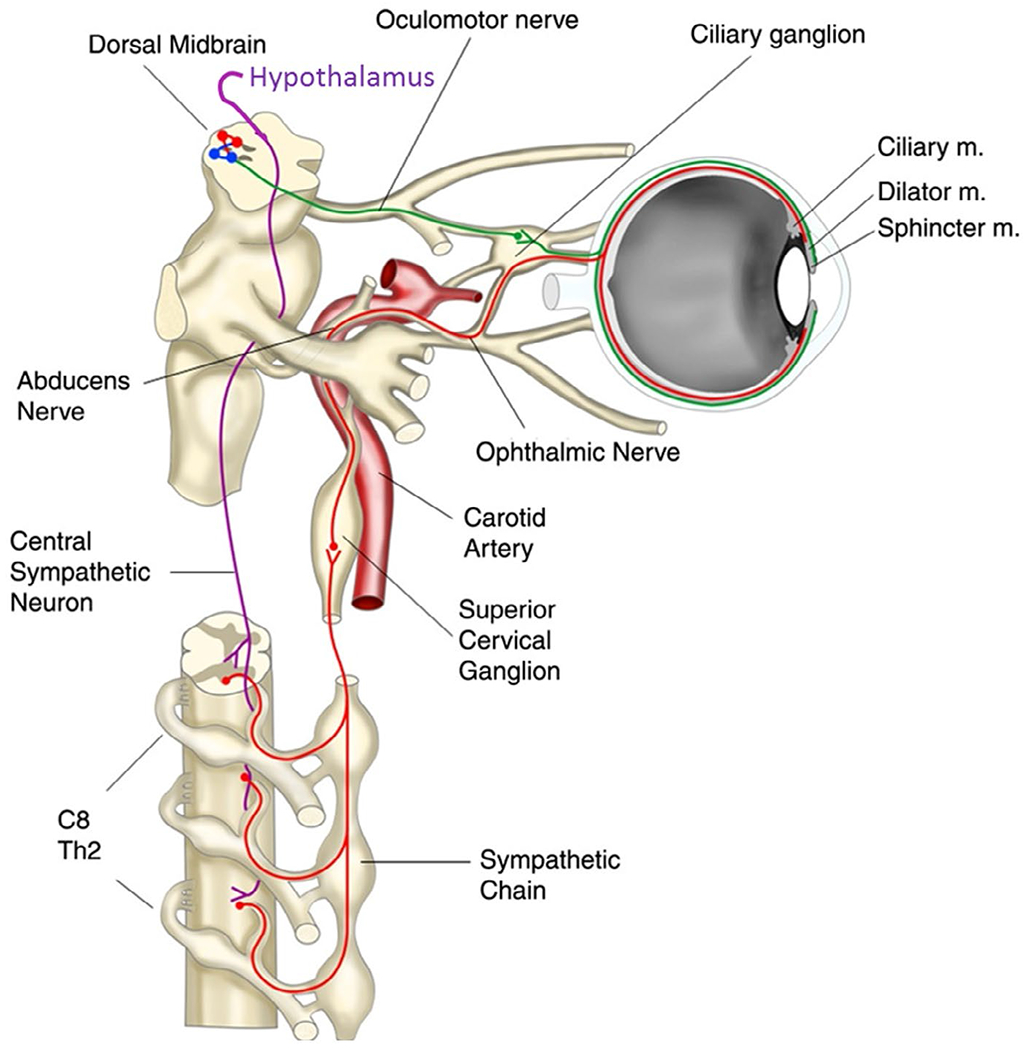
Sympathetic (magenta/red) and parasympathetic pathways (green) (adapted from Wilhelm, 2011)
1.2.3 |. Reciprocal relationship
Like many physiological systems, pupillary changes reflect dual influences of activation and/or inhibition of the parasympathetic and sympathetic nervous systems. When a light stimulus activates parasympathetic pathways that prompt direct pupil constriction, it also engages collaterals that inhibit sympathetic innervation to the dilator, thus allowing more effective constriction to occur. A similar effect occurs under conditions of sympathetic arousal, in which central inhibition of motor activity at the Edinger–Westphal nucleus reduces parasympathetic outflow to the sphincter muscles, contributing to the degree of pupil dilation. It has been suggested that dilation of the pupil during increasing workload, as well as slower tonic increases, may reflect primary parasympathetic inhibition, rather than only sympathetic dilation (Steinhauer et al., 2015; Steinhauer & Hakerem, 1992). In addition to central processes that selectively inhibit the opposing pathway, Loewenfeld (1993) has noted that the final sympathetic and parasympathetic motor fibers that stimulate the sphincter and dilator also have collateral projections that inhibit the antagonistic muscles.
1.2.4 |. Isolating sympathetic and parasympathetic influences on pupil diameter
Two general approaches have been noted for dissociating the contributions of sympathetic activation and parasympathetic inhibition to pupillary changes: manipulation of ambient light and temporary blockade of iris receptors by pharmacological agents (Steinhauer & Hakerem, 1992). Under normal room illumination, both the parasympathetic and sympathetic pathways are functional, whereas increasing ambient light increases the tonic activity of the parasympathetic system. Increasing light results in supranuclear inhibition of the Edinger–Westphal motor center, and subsequent relaxation of the sphincter muscle is more pronounced. In contrast, when room illumination is low or in complete darkness, there is less parasympathetic activity, and contributions of sympathetic activity are more prominent. A further consideration is that there is less overall variability in resting pupil diameter in darkness. Consequently, the signal-to-noise ratio for experimentally evoked changes in darkness is higher, making changes more detectable, especially when signal averaging is employed.
Figure 4 illustrates pupil diameter changes in a digit span task conducted in light compared to darkness. Pupil dilation due to increasing memory load is greatest under moderate room illumination, with reduced dilation in darkness, suggesting the primary contribution is inhibition of the parasympathetic pathway. Similarly, pupil dilation elicited during an effortful or emotion task is larger in light than in darkness (Steinhauer et al., 2004; Widmann et al., 2018). Using a dark environment and auditory stimulation, an initial dilation beginning at ~500 ms was followed by more rapid dilation with a peak at ~1200 ms (Friedman et al., 1973; Hakerem, 1974). When measured in a brightly illuminated room, the latter peak is apparent, but a marked earlier (though less extensive) dilation also is seen (Johnston, 1979; Shiga & Ohkubo, 1979). Motor responses are also distinguished by an early dilation at ~600 ms. Steinhauer and Hakerem (1992) suggested that the early period of dilation (~300–600 ms) is related to inhibition of the parasympathetic system, whereas both late dilation (~1200–1400 ms) and sustained dilation involve sympathetic activation together with some degree of parasympathetic inhibition. The separation of early dilation associated with inhibition of the parasympathetic pathway, followed by a later sympathetically mediated dilation related to emotional arousal, was noted in recordings under different lighting conditions and reinforced by isolation of separate components by principal component analysis (PCA) in a study by Widmann et al. (2018; see Figure 5).
FIGURE 4.
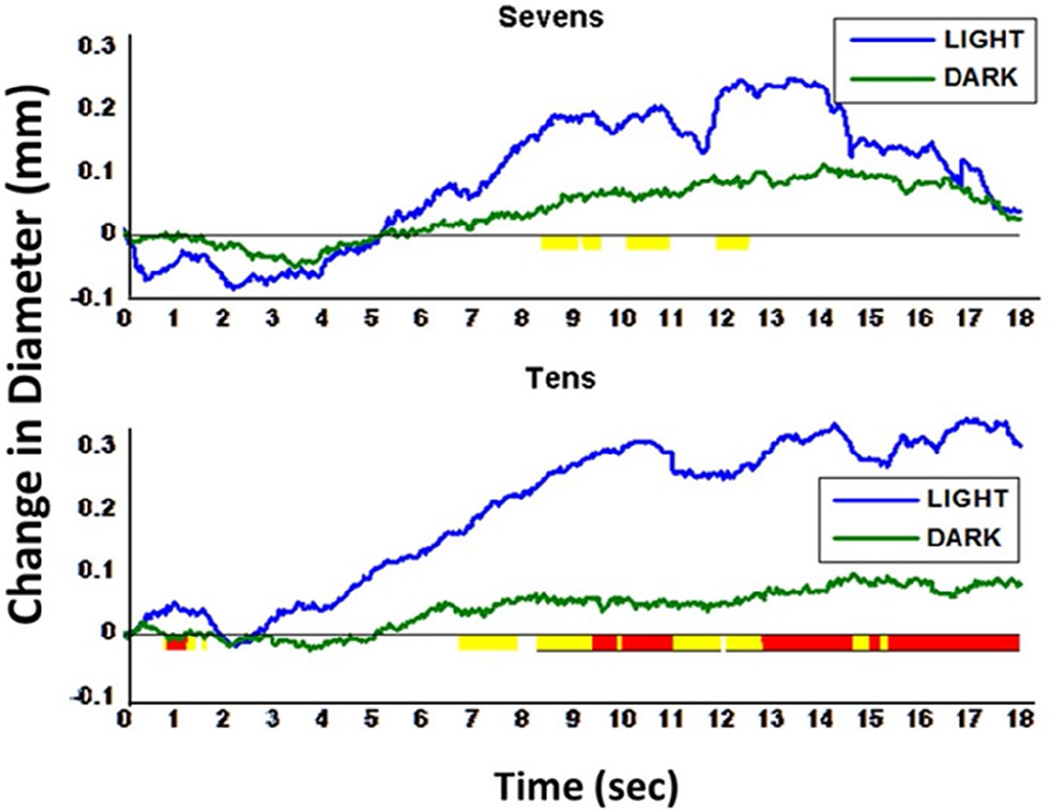
Pupillary dilation during digit span task (7 and 10 digits) under light and dark recording conditions. First digit was presented at 2 s. Increased dilation amplitude is observed for the pupil when recorded in light (blue) vs. darkness (green). Highlighting under the x-axis represents sample-wise significance, where yellow = p < .1 and red = p < .05. Underlined highlighted periods were long enough to infer significance using Guthrie and Buchwald’s (1991) Type I error correction technique (see Section 2.3.4)
FIGURE 5.
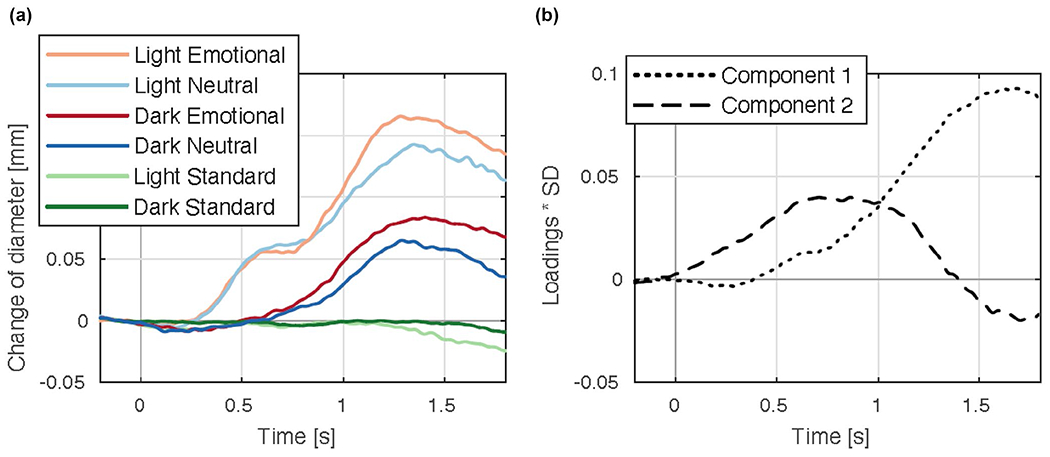
(a) Average pupillary waveforms when listening to novel emotional or neutral sounds in an auditory oddball task, recorded under conditions of light and darkness. (b) Two major principal components depicting factor loadings over time (adapted from Widmann et al., 2018)
A second approach to isolating sympathetic and parasympathetic contributions to pupillary changes involves transient administration of cholinergic or adrenergic antagonists to the iris by topical administration (drops) on the cornea. The use of alpha-adrenergic antagonists, such as thymoxamine or dapiprazole, which block dilator activity, measure specific action of the sphincter muscles. The use of tropicamide, which blocks the muscarinic receptor of the sphincter, allows action of the dilator muscles to be observed. When dilator action is blocked (thymoxamine) during transformation and memory load tasks, pupil dilation is unaffected (Matthews et al., 1991), indicating parasympathetic inhibition is the primary dilatory source in those experiments. Similar effects were observed using dapiprazole to block dilator action: pupil diameter increases with greater task demands (Steinhauer et al., 2004), and the reduction in the light reflex during a difficult task remains intact (Steinhauer et al., 2015). When tropicamide is used to block the sphincter muscles, no detectable light reflex is found, indicating successful sphincter blockade, although a remaining small but significant dilation is attributable to a minor sympathetic component. Reduction of the light reaction by stress (threat of shock) was also found to be mediated entirely by parasympathetic inhibition (Hou et al., 2007).
A single drop of 1% tropicamide (1% Mydriacyl) produces blockade of the dilator after ~25 min, and recovery is much faster than the higher dosages used in ophthalmological exams. Because artificial dilation of the pupil can lead to increased intraocular pressure in some individuals, screening for a “narrow angle” condition by an ophthalmologist or optometrist is essential. Unfortunately, neither thymoxamine or dapiprazole, or other equivalent alpha-adrenergic blocking agents are currently easily available. A more recent approach to isolating parasympathetic activity utilizes 2.5% phenylephrine to directly stimulate the alpha-adrenergic dilator receptors, thus enlarging the pupil, such that subsequent dilation during perceptual switching could be attributed to parasympathetic inhibition (Nakano et al., 2021).
2 |. METHODOLOGICAL CONSIDERATIONS
2.1 |. Equipment and data acquisition
Different methods for pupillary measurement have been employed across the centuries, with the first photographic methods, which allowed continuous measures of pupil change recorded on film, having the greatest impact. One drawback of the photographic method is the need for illumination, which itself affects pupil diameter. The development of infrared technology (Zworykin & Morton, 1936) provided the opportunity for continuous measurement of the pupil in darkness or in light conditions (Loewenfeld, 1993), as infrared illumination penetrates the somewhat cloudy cornea covering the pupil but does not directly stimulate photoreceptors. Infrared technology was first introduced in the electronic scanning pupillograph developed by Smith-Kline Laboratories in association with Lowenstein and Loewenfeld (1958) and remains the basis for current video-based pupillometers and eye-tracking systems.
The majority of video systems available for pupillary assessment were primarily developed to track the eye, rather than measure the pupil, by placing an infrared light source at an oblique angle to illuminate the eye, producing a detectable source with a center projection on the cornea. The location of this corneal reflection (described in screen coordinates) remains at the same location, regardless of rotation of the eye. As the eye moves, it is the change in the pupil screen coordinates from the corneal reflection center that are employed to define the distance and location of eye movements. Typically, a calibration procedure is used to correctly identify the location of eye movements on a display, but this calibration is solely related to the accuracy of eye tracking and does not need to be reported when pupillary data are the focus of an investigation. On the other hand, concurrent eye tracking is critical for many pupillary studies, as trials on which the eye inadvertently or incorrectly moves to a lighter or darker light source can affect pupillary measures.
When an infrared light source enters the pupil, no light is reflected back to a video detector. Circuitry was developed to identify the resulting black regions (originally of 512 lines for a TV) on a screen displaying the eye (see Figure 6). For example, if the pupil is enlarged to cover most of a screen (e.g., 500 lines for 10 mm), the resolution of the system is 50 lines per mm (0.02 mm of pupil diameter per line); output voltages or digital counts are proportional to the number of lines. Whereas early systems were sensitive only to vertical pupil diameter measurement, the next development additionally specified the brightness of the pixels on each line of the video display, which provides information regarding both horizontal and vertical pupil diameter. When partial eyelid closure obscures less than half of the pupil, a system that assesses the largest diameter in the horizontal plane is quite accurate. Current technology for measuring the pupil primarily involves video recording, with output of digital data to stored files, and both stand-alone eye-tracking systems, as well as integrated data presentation and data collection systems, are available commercially. Video cameras are typically located on a desk, but several devices are currently available for head-mounted cameras, allowing freer head movement.
FIGURE 6.
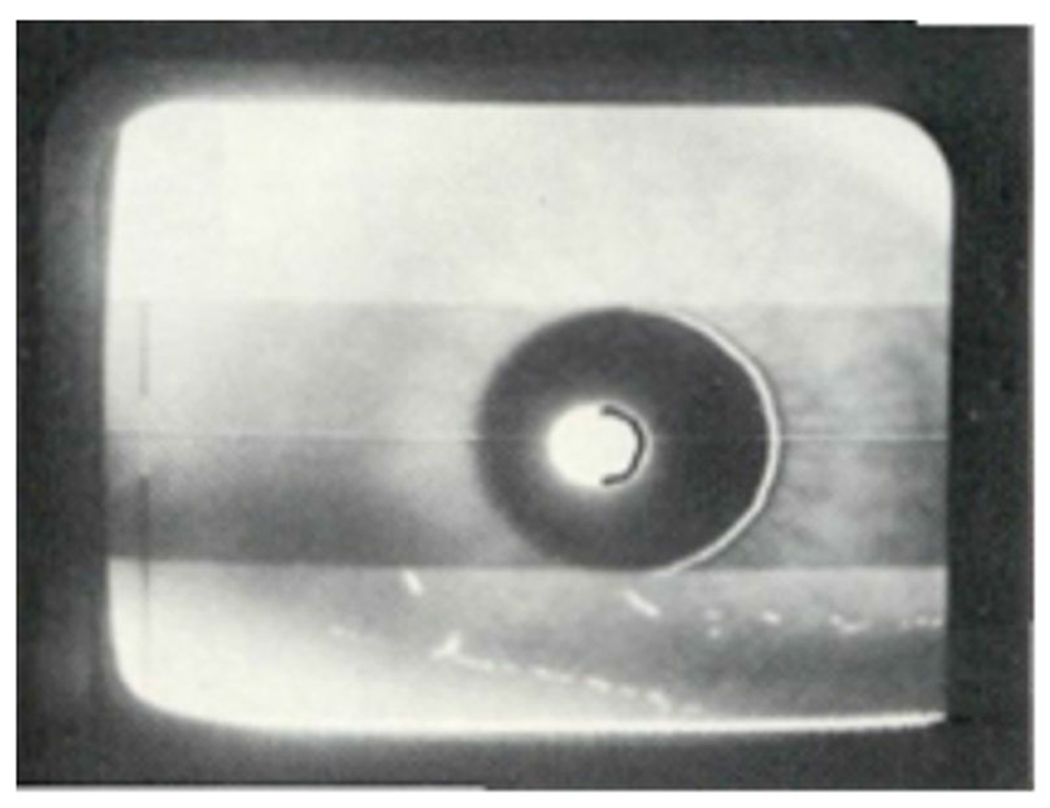
Video output using count of vertical scan lines. When measuring vertical pupil diameter, the center white spot is a reflection from an infrared light source, which is also measured when evaluating eye location for eye tracking
Because the pupil response is almost always consensual (i.e., both pupils react simultaneously, although see Cagiltay & Meneske Dalveren, 2019), either eye can be used to record pupillary responses, with the exception of recordings in individuals with neurological conditions associated with pupillary asymmetry (e.g., Horner’s syndrome). Nonetheless, reporting the eye from which the pupil is measured should be stated. Some systems allow dual-channel recording, which might prompt researchers to average pupil diameter over both eyes. To the extent there is noise in either channel; however, the output will be distorted, and it is recommended that one channel be selected for reporting, even in a dual-channel recording system.
2.1.1 |. Autofocusing and autotracking
When a camera is focused on an object, the image remains sharply in focus only for a small variation in distance (i.e., less than an inch). If the head is held in place (e.g., using a chin rest), the sharp eye image available for pupillary recording will show small and negligible variation. If the head makes movements that are closer or farther from the camera, however, the image of the eye can become blurred, degrading the signal. Many systems (all of which use optics to enlarge the eye image) include a feature called image autofocusing, which essentially changes the eye image by rotating the optics, enlarging or decreasing the size of the eye image—and the pupil. This has the effect of constantly changing the relative scaling of pupil diameter in indeterminate ways. Except for expensive systems that automatically recalibrate to the correct pupil diameter, autofocusing should not be enabled during pupil measurement. Though autofocusing is problematic for accurate pupillary measurement, the autotracking features of many systems, which rotate the camera in the vertical or horizontal plane when the eye moves from central fixation, are an advantage and should be employed.
2.1.2 |. Sampling rate
Sampling rates for pupillary and eye-tracking instruments are typically 50 or 60 Hz but can be as high as 2000 Hz in systems designed for high temporal resolution of eye movements. In such cases, it is reasonable to average the pupillary data across subsamples to provide an estimate of pupil size (e.g., for a 1500 Hz system, averaging every 25 samples results in an effective 60 Hz sampling rate). Although relatively rapid constriction changes can be seen to light onset, most of the changes in pupil diameter are slower, and frequency analysis shows little if any activity above 9 Hz. Thus, sampling rates as low as 20 Hz are capable of capturing the key frequency components of pupil change, although latency measures will be less accurate. There have been some reports of high-frequency pupillary oscillations (>30 Hz) related to cognitive processes, but these claims are neither clearly documented nor consistent with pupillary physiology. Higher frequency oscillations in the pupil signal are most likely associated with varying sensitivity of the recording instruments to edge detection, slight focus changes, and system noise, though there are claims that rapid oscillations inform cognitive processes (e.g., Marshall, 2002).
2.1.3 |. Calibration and conversion
The recommended standard unit for reporting pupil diameter is millimeters (mm). For psychophysiological studies, reporting to the nearest 0.01 mm is sufficient. Although some handheld or advanced systems report measurements directly in mm (usually accomplished through proprietary hardware/software), like other psychophysiological measurement systems, many laboratory eye-tracking instruments report pupil size in arbitrary digital units (AU) which do not allow direct cross-study comparison and require calibration and conversion to mm prior to experimental reporting. Calibration involves using a known (“artificial”) pupil, which some systems provide, or a commercially available handheld eye chart that includes pupils of varying diameters. To compute the scale factor necessary for converting pupil diameter from AU to mm, the calibration/artificial pupil is placed at the location (i.e., same distance from display) where the eye is typically measured, and its diameter recorded for a short interval. The resulting output file provides the diameter in arbitrary units (e.g., 200) of the artificial pupil (e.g., 4 mm), with the scale factor equal to AU/artificial pupil size (e.g., 200/4 = 50 AU per mm). The reciprocal 1/AU per mm provides the maximum possible experimental resolution of the system (1/50 equals 0.02 mm). Because the scaling factor is a linear computation, pupil diameter data can be scaled offline either before or after averaging. If there is an offset (zero signal is not zero mm), pupil diameter in mm can be computed as (AU-Offset)/scale factor.
The precision of recorded pupil diameter is a separate issue. Regardless of the range/resolution of AU values, this critical factor refers to the size of the pupil diameter change that the system can detect. If this information is not provided by the manufacturer, it can be determined using digital data from several pupillary recordings to compute the minimal variation in digital counts between measurements. For example, assume that a scaling factor of 200 digital points/mm has been determined following calibration with an artificial pupil. If there appear to be at least 5 AU difference between successive change points, accuracy is 5 pts/200 pts per mm or 0.025 mm. Accuracy to the nearest 0.01–0.05 mm is typical. Systems claiming accuracy to the nearest 0.001 mm are unrealistic and should be rounded to the nearest 0.005 or 0.01 mm for data analysis. In most cases, accuracy of eye movement assessments is not relevant to pupillary recordings and analysis, and eye-tracking systems that report accuracy of 0.05 degrees of movement do not necessarily provide relevant information regarding pupillary measurements, unless it is specifically stated by the manufacturer.
Some technical difficulties can arise when converting digitally recorded pupil diameter to mm. Several commercial systems designed primarily for eye tracking do not allow a standard calibration pupil to be recorded. In this case, there is no direct method for converting AU to mm (although proportional digital values consistent within each subject may be provided). These systems are not recommended when reporting pupil diameter data in Psychophysiology.
A separate complication relates to pupillary recordings using remote eye-tracking technology in the magnetic resonance imaging environment. These systems most often employ optics that enlarge and transmit the eye image to a camera that is necessarily located far from the scanner (and subject) and may not be able to be precisely calibrated. Most MRI studies must use some type of normalizing technique to scale the data (Siegle et al., 2003), including computing a proportion of maximum diameter or by normalizing the data (z-scores). A unique implementation placed an artificial pupil on the participant’s eyelid or goggle system to compute an individual scale factor (Johnstone et al., 2007; Urry et al., 2009). Currently, normalizing approaches are recommended for use only in MRI contexts, and require careful description in the Method.
2.1.4 |. Standardization
The distance from the camera and the magnification of optics affects the size of the eye image on the video field. The preferred method for recording the pupil is to determine the parameters that provide the best quality, magnification, and digital resolution. The first step is to determine the optimal distance from the eye to the camera from below or slightly to the side of the visual field to allow clear viewing of stimuli or fixation points. Then, the optical focus should be “locked” (not modified). As a best practice, establishing a standard AU:mm scale factor allows recalibration of the system should optics or locations be accidentally changed. When a new participant is tested and placed in position for pupil recording, the distance to the camera to provide a clear image should be determined by moving either the camera or the participant forward or backward to achieve the sharpest image—the optics should not be altered! This procedure provides the most consistent recording environment across participants and sessions. Checking the calibration values every few months has demonstrated high stability for pupillary recordings across several commercial systems. An alternative approach is to calibrate the pupil before each participant is placed in the apparatus, though this will most likely vary the accuracy of measurement across participants.
Participants are typically seated in a comfortable chair, and, in many studies, a chin or headrest is used to maintain the distance of the eye and head from the pupil recording device, as well as from visual stimuli and fixation points. Head-mounted (visor, eye-glass, or built-in to virtual reality headsets) or head-tracking devices are increasingly the norm and and do not require fixing head location, although local luminance effects will differ based on head and eye position (relative to the stimulus) in systems in which the display is external to the eye-camera. Thus, even with a head-mounted eye tracker, a chin or headrest is useful. In ideal conditions, the participant should be separate from the experimenter, either in a different room or separated in some way. During recordings, the device output (video, computer tracking) should be observed by the experimenter to monitor the quality of the eye image and signal. Many of the artifacts described below can be automatically identified in postprocessing (blinks, poor signal, w-waves) but some problems will only be noticed in real time, such as an extreme decrease in diameter or oscillations.
2.2 |. Data reduction
2.2.1 |. Blinks and artifact rejection
Common artifacts in the continuous pupillary signal are spontaneous or voluntary blinks that are typically short (~200 ms) but can last beyond a half second. Correcting pupillary data for blinks involves identifying blinks in the recording, determining the last good samples before the blink and first good sample after the blink, and applying linear interpolation during the blink period. Some researchers have employed more elaborate curve fitting for interpolation (Takeuchi et al., 2011), but this is not usually necessary. During complete closure of the eye, no signal can be recorded from the pupil, and, in many cases, the resulting output will be 0 AU, allowing blink detection. On trials where complete closure does not occur, one criterion for detecting a blink (in 50 or 60 Hz sampling systems) is when the change in diameter between two successive sample points is greater than 0.5 mm. This criterion can also be used to detect trials with spurious noise, as well as signals that fall to 0 or are out of range of possible pupil diameters (0, or >10 mm). If eye closure (or blink) occurs during the interval in which a discrete light stimulus is being presented, however, the trial should probably be excluded as not all the light has entered the retina. An example of corrected and uncorrected data is provided in Figure 7, in which blinks have been identified and then corrected; event markers are also noted in this sample (based on Siegle et al., 2008).
FIGURE 7.
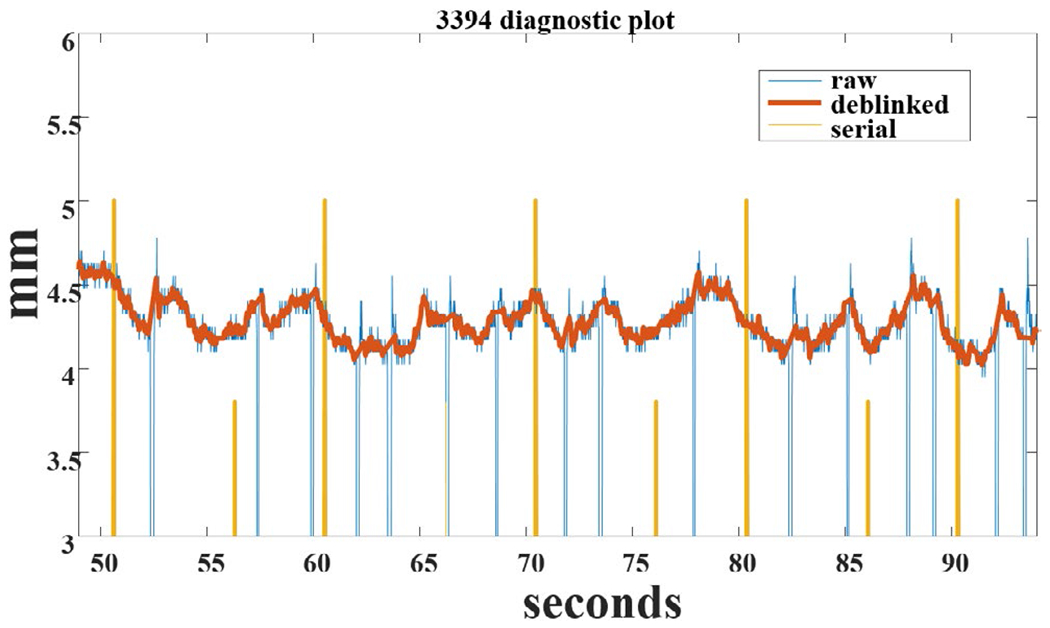
Zoomed segment of preprocessed pupillary data showing raw pupil horizontal diameter (blue), effect of smoothing and interpolation through blinks (red), and serial port markers showing trial onsets/events (yellow) (courtesy G. Siegle)
Many modern eye-tracking systems include automatic detection of blinks, setting the signal output to 0 during a blink and some researchers have published algorithms, MatLab code, or R code for automatic blink and artifact identification (Hershman et al., 2018, 2019; Mathôt et al., 2018; Sirois & Brisson, 2014). Spline interpolation has also been employed to replace missing data (Mathôt, 2013; Tsukahara, 2020). While many of the algorithms use data beginning as much as 200 ms prior to and 200 ms following the detected artifact interval, selecting just four sampling points prior to and after each artifact is usually sufficient to provide anchor points for interpolation. The method section should clearly describe the number and types of artifacts detected, and whether data were interpolated or rejected. The mean number of trials or data that were not utilized should also be reported.
There are several potential physiological artifacts that can occur: (a) An abrupt decrease of 1–2 mm in overall diameter (which may last for up to 30 s), and is attributed to bursts of midbrain activity which inhibit the Edinger–Westphal nucleus and are temporary physiological phenomena. (b) During recording of multiple light reactions, w-waves may occasionally appear (Loewenfeld, 1993) which are secondary constrictions observed after an initial constriction in the period during when re-dilation is expected. (c) Sleepiness-related oscillations (Wilhelm et al., 1998) are slow oscillations (<1 Hz) when the pupil is monitored in darkness (or in participants who are sleep deprived), originally called “fatigue waves” (Lowenstein et al., 1963), indexing spontaneous parasympathetic system activity. (d) A general decrease in overall pupil diameter across the experimental session, as participants lose alertness and/or become fatigued. Detrending can remove slow drift, but if conditions are randomized across trials, and a baseline computed from a brief prestimulus period, it is not necessary to detrend the data.
2.2.2 |. Parallax
When eye movements are allowed (or spontaneously occur), the shift in the optic axis can distort the shape of the pupil, affecting diameter measures, a phenomenon termed parallax (also called the pupillary foreshortening effect by Hayes & Petrov, 2016). A large rotation to the left or the right, for instance, will result in a smaller horizontal image and a large gaze shift upward or downward will similarly shrink vertical pupil size (Figure 8). An offline solution is to utilize the larger of the two diameters at each point in time, though this can lead to discontinuity in the signal. Rather, experimental controls should be employed to control shifts in the visual field and the execution of eye movements. In linguistic research, for instance, the position of a target picture in a visual-word paradigm can be randomized across trials (e.g., Schmidtke, 2014), such that stimulus presentation at each position does not differ between conditions. In addition, several procedures exist for correcting effects of gaze position on pupil size measurements (Gagl et al., 2011; Hayes & Petrov, 2016). In practice, if gaze shifts are small (less than ~10 degrees) and/or the number of trials large, effects due to parallax are minimal (Kooijman et al., 2021). In nonvisual contexts, on the other hand, large differences in gaze angle due to spontaneous scanning, or prompted by cognitive processing (e.g., raising eyes when thinking, remembering, imagining; Bakan & Shotland, 1969), may be more problematic, requiring monitoring of eye movements and possible correction for eye gaze shifts.
FIGURE 8.
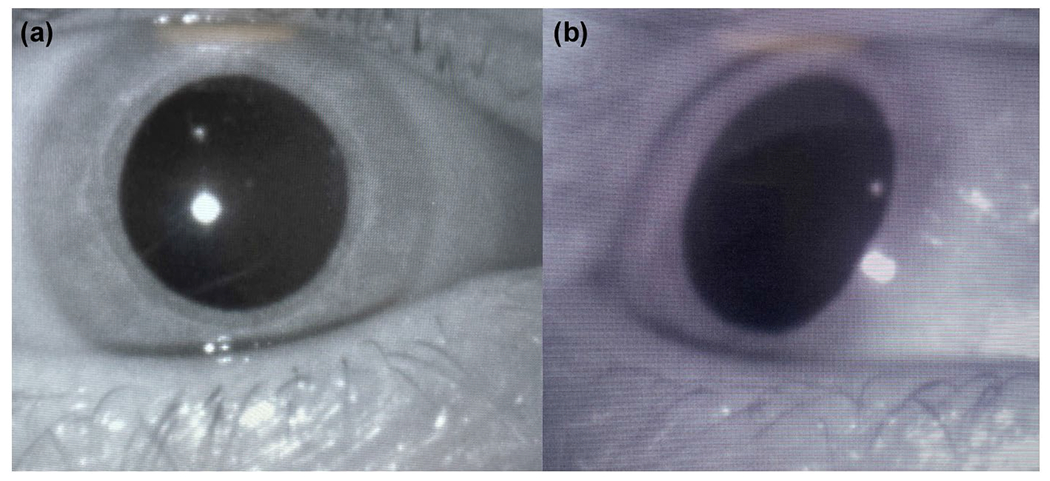
Parallax effect of lateral gaze. (a) The eye looking forward is circular. (b) The eye is rotated to the right, distorting the measurable horizontal diameter
2.2.3 |. Pupil diameter metrics
Although diameter, radius, or area of the pupil are reasonable representations of pupil size, diameter is the accepted reporting standard (Kelbsch et al., 2019). Logical arguments for using diameter exist as well: a small increase in the diameter of a small pupil will result in a proportionally larger area increase, compared to the same change in diameter size for a larger pupil, making comparisons difficult to interpret. Mean diameter in mm will sometimes be an appropriate measure such as when assessing differences across session or between different conditions in separate blocks (Friedman et al., 1973). In most psychophysiological studies, however, pupil motility is typically computed as a change from some baseline value, with Mathôt et al. (2018) and Reilly et al. (2019) concluding that subtraction of a baseline interval measure is associated with the most robust experimental effects. In addition, within typical recording limits, measures of absolute pupillary change tend to be linear (i.e., related to absolute change in mm), despite large differences in absolute pupil diameter between participants (e.g., Beatty, 1982; Beatty & Lucero-Wagoner, 2000). For trials with long intertrial intervals, a 1-s baseline is typically appropriate, with some studies have using shorter baselines ranging from 100 to 500 ms. The shortest recommended baseline period is 100 ms. When signal averaging across repeated trial conditions, the baseline can be computed from the average response curve.
Although pupil diameter changes, particularly in terms of the amplitude of the light reflex, are often expressed as proportional changes in ophthalmological and post-illumination pupil response (PIPR; see Sections 2.6 and 3.2.1) studies, these indices are less useful for psychophysiological experimentation. Proportional (synonyms: percent, ratio, nonlinear, divisive scaling) change scores introduce large variability across participants (Loewenfeld, 1993; Mathôt et al., 2018). For example, assuming a 0.5 mm dilation in a cognitive task for each of several participants with baseline pupil diameters of 3, 4, 5, or 6 mm, the computed percent changes are 16.6%, 12.5%, 10%, or 8.3%, respectively, which is much more variable than typically found for change scores across participants. There are very few conditions in which reporting of proportional change is optimal. If proportional indices best represent the data (e.g., as noted previously in the MRI context), mean baseline (initial) diameter should be reported in mm to allow interpretation and comparison across laboratories. For conditions that greatly affect the overall diameter or the amplitude of light reflexes (such as the post-illumination pupil response or reactivity to pharmacological agents), proportional change is sometimes reported, although, again, mm measurement of baseline pupil diameter and/or amplitude should be reported as well.
2.2.4 |. Signal averaging
Like many psychophysiological measures, pupil diameter changes can be stimulus-locked (to stimulus onset) or response-locked (to response onset), and signals are typically averaged across trials in a condition. The effect of signal averaging is to reduce the effects of sources of “noise” both in the physiological system and the recording apparatus, and results in increased amplitude resolution as well. Averaging across trials will produce some smoothing (decrease) of peak amplitude, as the exact peak shifts in time slightly across trials, but still represents the best estimate of peak amplitude and latency other than using single trial data. If there is latency jitter for the onset of the light reaction, averaging will delay the onset; a similar effect is produced by filtering (see Section 2.2.5).
Pupil diameter changes have a much higher signal-to-noise ratio than some psychophysiological measures (e.g., event-related brain potentials [ERPs]), and therefore typically require fewer trials to provide a good estimate of the average response. Woodmansee (1966) originally suggested a minimum of eight trials per condition, with five to 10 trials per condition usually sufficient, although more trials should be included if effects are expected to be small. In general, at least 15–20 trials are recommended for conditions where expected effects are on the order of several hundredths of a mm (e.g., feedback, probability manipulation), and at least five trials where expected effects are on the order of several tenths of a mm (e.g., digit span, working memory, emotion).
2.2.5 |. Smoothing
Raw pupil recordings or averages are often smoothed to remove small oscillatory and noise activity. Overly smooth pupil waveforms, however, tend to look artificial and should be avoided. For 50 or 60 Hz recordings, a 3–5 point moving average (or a similar digital filter) is usually sufficient. A second pass of the filter can further reduce noise. Any filtering procedure will slightly attenuate both peaks and troughs, although effects are negligible for most pupillary recordings. Table 1 lists the characteristics of one- and two-pass filters on common sampling rates (based on Ruchkin & Glaser, 1978).
TABLE 1.
Filter characteristics (−3 dB) for 3– and 5-point moving averages, using one or two passes. For example, for a 60 Hz system, applying a 3-point moving average once yields an effective 8.8 Hz filter
| Sampling frequency (Hz) | 3-point | 3-point | 5-point | 5-point |
|---|---|---|---|---|
| One-pass frequency (Hz) | Two-pass frequency (Hz) | One-pass frequency (Hz) | Two-pass frequency (Hz) | |
| 50 | 7.3 | 5.2 | 4.4 | 3.1 |
|
| ||||
| 60 | 8.8 | 6.2 | 5.3 | 3.7 |
|
| ||||
| 120 | 17.6 | 12.4 | 10.6 | 7.4 |
2.3 |. Data analysis
Pupil diameter measures are typically analyzed by extracting standard parameters (e.g., amplitude, latency, etc.) from pupillary waveforms for individual trials or by averaging across trials in a condition (which will not always result in the same effects). Alternatively, each point in the pupillary waveform average (across conditions) can be analyzed (with appropriate control of Type 1 error) or empirically defined parameters can be extracted (by trial or condition) from the waveforms.
2.3.1 |. Diameter change
Like many psychophysiological measures (e.g., ERPs), the change in pupil diameter can be indexed either by a peak change (constriction or dilation) or by an average change across a specific time interval. The selection of the appropriate time interval in which pupil diameter peaks or changes are measured will vary with specific experimental parameters. For example, as the number of digits increases in a digit span task, the time at which the maximum peak occurs lengthens, typically occurring slightly more than 1 s after the last digit is presented. Thus, in the digit span task, the pupil becomes smaller in the 2 s after the last digit, then a second peak is observed when a cue to report is provided. Each peak or interval may be of separate interest for analysis. For studies including a discrete significant event, such as feedback, peak diameter often occurs within 1–2 s following stimulus onset. In studies including more complex processing (memory recall, ordering of different set sizes, and mathematical operations), the peak diameter change may be even more delayed. In some experimental settings, especially if ambient light is high, there may also be an early peak of interest in the 400–900 ms range, though it will usually not be as distinct or large as the later peak, and this seems to be associated with central parasympathetic inhibition of the sphincter (Steinhauer & Hakerem, 1992; Widmann et al., 2018).
More generally, because specific stimulus and timing characteristics will affect the onset latency, duration, and peak latency of pupil dynamics, no standard time interval is appropriate for use in all studies; rather, as for other physiological measures, the duration of the appropriate interval should be determined based on the timing characteristics in the overall pupillary waveform. Including an illustration of the pupillary waveform(s) obtained in a study (rather than simply summary statistics) is therefore important support for the rationale of the time windows selected for peak scoring or interval averaging. Waveform data are most accurately presented averaged over participants (rather than illustrating a single participant or trial) and pupil diameter during the baseline period should be included in the plot. Reporting any measure of pupillary reactions (e.g., onset latency, rate of initial change, rate of redilation, etc.) will require similar assessment of timing characteristics in the pupillary waveforms in order to define appropriate intervals and durations for defining specific dependent measures.
2.3.2 |. Tonic diameter
Although diameter change is the most frequently reported pupillary measure, in some studies, absolute pupil diameter may be an appropriate measure, when, for instance, comparing overall diameter among different tasks, blocks, groups, or across an experimental session (e.g., Tsukahara et al., 2016). The paradigm may include a rest condition (e.g., at least 1 min), or an interval at the end of an adaptation period (e.g., 2 min) from which a baseline can be estimated. A definition of the baseline should be reported reflecting duration and/or number of points employed.
2.3.3 |. Light reflex measures
The light reflex is typically measured as the amplitude of initial pupil change, computed as the difference between the baseline diameter and the smallest or average diameter following stimulus onset in some time window (Figure 9). Other measures include differences in the rate of initial change or redilation, and a number of different latency measures have been proposed, including (a) latency to the initial constriction, which can be computed by determining the initial five points in which diameter change decreases, and assigning onset latency to the first point, (b) the latency of maximum velocity of constriction, (c) the latency of minimum diameter (the end of constriction), and (d) latency of redilation (recovered) 50% or 75% of diameter toward baseline. Initial redilation may reflect parasympathetic inhibition, while 50% and 75% may be related to sympathetically mediated dilation, especially in use of pictures. Curve fitting measures to determine latency to a second-order model preceded by a linear model are available (e.g., Bos, 1991; Lee et al., 1969).
FIGURE 9.
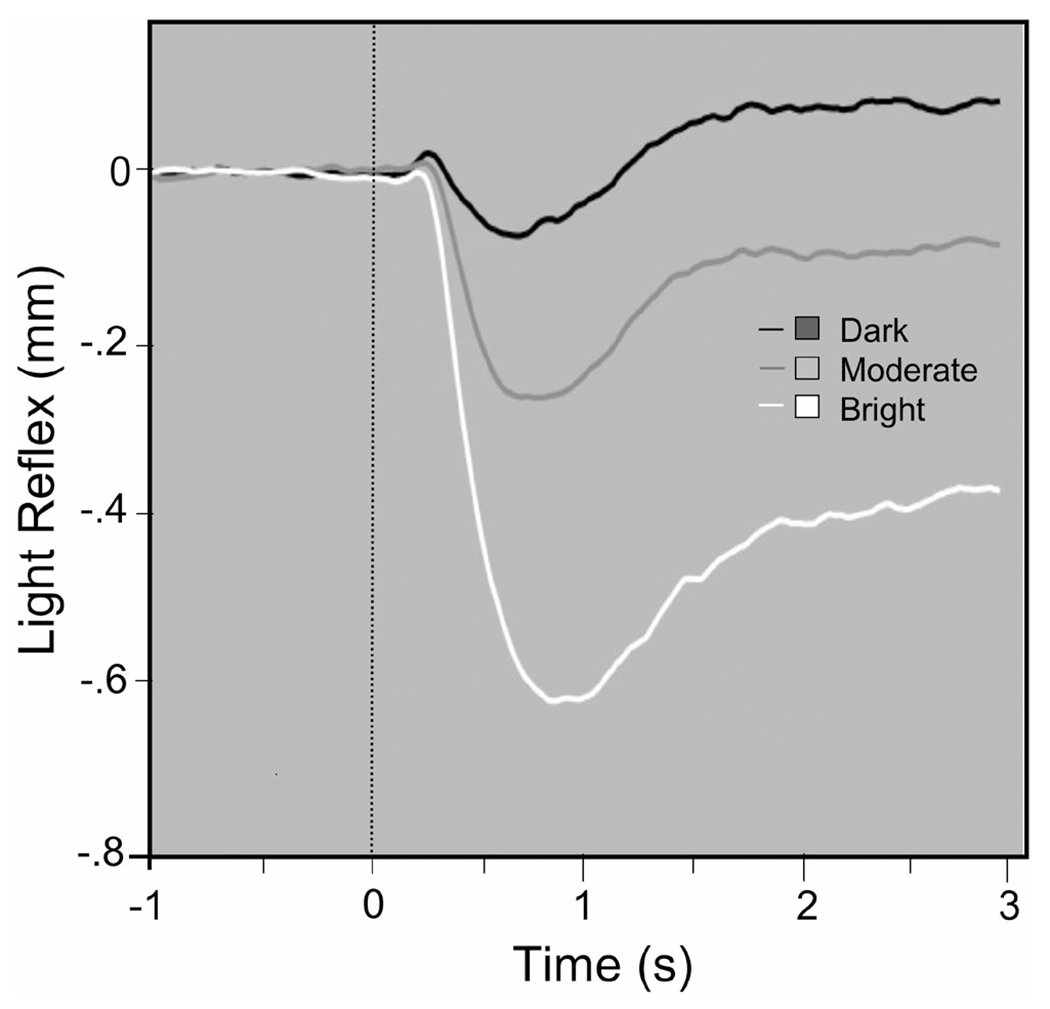
Pupil diameter waveforms when viewing bright, moderate, or dark stimuli illustrate changes in the amplitude of the light reflex (courtesy M. Bradley)
2.3.4 |. Waveform analyses
As with ERPs, pupillary data can be evaluated using massive univariate analyses that assess pupillary change at each time point sampled in the waveform, which is sometimes downsampled to a lower temporal resolution that preserves relevant waveform characteristics. If sustained reactivity (e.g., on the order of seconds) is of interest, downsampling to 1 or 2 Hz is common, although transient characteristics (e.g., peaks) will be missed. It is acceptable to compute both standard parameters (e.g., peak dilation) and univariate statistics at each point in a downsampled (condition-averaged) waveform.
There are several analytical approaches that minimize Type 1 error. The Guthrie and Buchwald (1991) technique assesses pupillary waveform data using Monte Carlo simulations to determine the minimum number of consecutive t tests that are unlikely to occur by chance at some waveform-wise level of significance (e.g., p = .10 or p = .05; see Figure 4). Its utility is in detecting long intervals of significance across highly autocorrelated pupillary waveforms (e.g., Aminihajibashi et al., 2020; Angulo-Chavira et al., 2017; Gotham et al., 2018; Kvamme et al., 2019; Lasaponara et al., 2019; Siegle et al., 2015). A different method for comparing differences across large numbers of measurements (e.g., waveforms) is described by Blair and Karniski (1993) (see software by Siegle et al. (2015) in Table 2, for implementation in MatLab) which is better for detecting short regions of large-effect differences but poorer for detecting longer regions with smaller effect sizes.
TABLE 2.
Software links (primarily MatLab and R) for pupil data analysis
Another inferential approach assesses differences among consecutive points in a pupillary waveform by computing the Bayes Factors for sets of data where there is an initial t test at each time point (Hershman et al., 2019, 2021). The Bayes factor (Kass & Raftery, 1995; Rouder et al., 2012) is a ratio of the marginal likelihood of the data supporting two competing hypotheses (usually a null and an alternative) and is calculated for data at each time point. In practice, the Bayes factor represents the relative likelihood of the data supporting one model, compared to the null hypothesis.
2.3.5 |. Empirical measures
The presence of large variations in the morphology of the pupillary waveforms may raise issues regarding the use of standard parameters when a feature is not present (van Rij et al., 2019). For instance, peaks may not occur, particularly in tasks with sequentially presented stimuli with a diffuse starting and endpoint, such as the detection of a target word in a speech stream. Thus, data reduction may involve grouping points in a pupillary waveform that strongly covary (e.g., PCA; Schluroff et al., 1986) or by extracting latent component waveforms (e.g., independent component analysis). Pupillary motility data have also been subjected to growth-curve analysis (McGarrigle et al., 2017), mixed-effect regression (Kuchinsky et al., 2013), generalized additive mixed modeling or nonlinear mixed-effects regression method (Lõo et al., 2016; van Rij, 2012), and Fourier/wavelet analyses (Jones et al., 2010). In theory, these analyses could be conducted on data that extend beyond task completion: Winn and Moore (2018), for instance, detected differences between participants with cochlear implants and normal-hearing listeners in speech processing that were only apparent following sentence offset. Note that differences in morphology, including latency of peak components, may be confounded in some of these types of analyses.
A well-studied approach to determining the underlying components contributing to waveform data is PCA (Donchin & Heffley, 1978), in which the average waveform for each condition and participant constitute the input matrix. This is most appropriate when there are no missing data conditions for any participants (Bradlow, 2002). If two underlying components have high covariance and contribute linearly to the waveform, they are not distinguishable. However, if they vary independently, the covariance is not linear, and PCA is able to distinguish separate components. Factors are extracted in the order of the amount of variance explained, and in pupillary studies, there are rarely more than three major factors. Each identified factor results in a specific loading at each time point, and, when plotted, the factor loadings themselves describe the form of the component as it varies over the temporal interval (but note that the sign of PCA loadings is arbitrary, and the raw data must be checked for correct interpretation—a negative loading may actually correspond to dilation). Factor scores can be computed for each participant and condition by multiplying the factor loading by the observed amplitude for a condition, and summing. Using PCA, Young et al. (1993) identified separate tonic and phasic aspects of the light reaction, and PCA has been employed to distinguish the components of dilation during cognitive tasks (Granholm & Verney, 2004; Granholm et al. 2009), emotional picture viewing (Bradley et al., 2017), auditory oddballs (Wetzel et al., 2016), and following stimulus offset (Siegle et al., 2001). Figure 5 (from Widmann et al., 2018) depicts average pupillary changes and the two major principal components that were derived.
Independent components analysis has also been used to identify the latent timeseries underlying pupillary waveforms (Jainta & Baccino, 2010). Such techniques may be particularly useful when analyzing the pupil in the context of group or individual differences.
2.4 |. Subject variables
2.4.1 |. Vision and pupil assessment
A number of basic eye assessments prior to experimentation are useful. A handheld Snellen chart can be used to get an estimate of visual acuity. A quick visual inspection of the eyes can be made to note whether pupils appear round and of apparent equal size (isochoric). Anisocoria (unequal pupil size) occurs in about 17% of the normal population, and if the difference is slight, selection of one or the other eye is arbitrary. Greatly unequal pupil sizes are often used as neurological indicators, and while they may have no clinical significance, these participants might be considered for exclusion. Abnormalities of the iris which appear as nonspheroidal pupils (e.g., key-hole pupils) are often difficult for pupillometer systems to measure accurately.
For motility, pupillary constriction can be noted when ambient lighting is increased. In addition, a test of accommodation provides a good indication of pupillary motility. This can be done while standing in front of the participant and asking the participant to focus on the experimenter’s finger, beginning at a distance and bringing it slowly toward them. The pupil should become smaller as the finger approaches, due to action of the accommodation and vergence pathways. Eye makeup, especially mascara, can interfere with accurate imaging of the pupil by camera-based systems so it is standard, in many laboratories, to require removal of eye makeup prior to assessments.
2.4.2 |. Ophthalmological and medical conditions
There are a number of ophthalmological conditions that interfere with pupillary motility, as well as potential surgical interventions, medical conditions, and neurological complications, making a general screen for health issues related to vision appropriate. For PIPR research, it should be reported whether participants were screened for retinal disease and diabetic retinopathy as such disorders impact the PIPR (Kelbsch et al., 2019).
Corrections for visual acuity are a separate matter, and there is no reason to exclude participants who use contact lenses, as these do not substantially change the observed size of the pupil or impact accurate recordings. The use of corrective glasses, however, can be problematic as reflections produced by the lenses can interfere with accurate measurement. One approach, if possible, is to place a corrective lens close to the eye rather than to use the participant’s own glasses.
2.4.3 |. Medication
A list of agents affecting pupil diameter can be found in Kelbsch et al. (2019, Table 2) and Loewenfeld (1993), with the following just a brief overview of key pharmacological effects relevant to psychophysiological research. Most people who have had a complete visual examination have experienced the temporary effect of tropicamide, which is dropped in the eye to block the muscarinic receptor from being stimulated, relaxing the sphincter and enlarging the pupil (this allows the retina to be examined without light closing the pupillary aperture). A major potential confound, especially in studies of psychiatric patients, is attributable to pharmacological effects on the iris musculature, as well as on the central innervating pathways. The most obvious effect is the blockade of the sphincter receptor by anticholinergic drugs (especially first-generation antipsychotic medications), as well as benztropine (Cogentin), which is used for a number of movement-related disorders, and neuroleptic-induced tardive dyskinesia, which, at sufficient doses, prevents the pupil from constricting and also reduces general pupillary motility. The anticholinergic effect of first-generation antipsychotics is typically expressed in comparison to chlorpromazine and benztropine equivalents for other anticholinergic agents (Ereshefsky & Richards, 1992; Foster, 1989; Sadock & Sadock, 2000). Chlorpromazine equivalents are available for the newer antipsychotics (Woods, 2003). Typically, these are used as covariates in patient studies (e.g., Granholm et al., 2007, 2017).
In addition to screening for anticholinergic medications, other systemic medications may affect pupil diameter. Common parasympathetic agonists are physostigmine, carbachol, and pilocarpine (glaucoma treatment), and parasympathetic antagonists include high-dose nicotine, atropine, scopolamine, and tropicamide. Similarly, there are known stimulating effects on the sympathetic system that include guanethidine, cocaine, ephedrine centrally, as well as effects of phenylephrine and adrenaline on the dilator muscle. Central blockade of the noradrenergic system includes alpha-methyl tyrosine, alpha-methyl dopa, debrisoquine, and 6-hydroxy dopamine (which destroys sympathetic nerve endings). Blockade of the dilator muscle is produced by a number of drugs including phenoxybenzamine, thymoxamine, and dapiprazole. The use of nonprescription or prescription drugs is problematic: cocaine, methamphetamine, LSD, and marijuana result in pupil dilation, while opioids such as oxycodone, heroin, and fentanyl lead to extreme miosis (constriction), often noted as pinpoint pupils.
2.4.4 |. Age and other subject variables
As in all psychophysiological studies, description of the sample should be reported, including ethnicity and gender. Although differences in the apparent pupil size between men and women have long been noted, empirical support is lacking. In a carefully conducted study, dark-adapted pupil diameter did not differ for female and male participants across a broad age range (Bradley et al., 2010), and a similar null effect of gender was found for pupil diameter under scotopic (dark) and photopic (light) conditions, which included both resting diameter and light reflex measures (Tekin et al., 2018).
There are two aspects related to inclusion of participants from varying ethnic backgrounds. The first is that representation of different ethnic groups should reflect the proportions in the local community and sample ethnicity should be reported. This is required in the United States for studies funded by the government, and is a key concept of Justice embodied in the Belmont Report and the American Psychological Association Ethical Principles and Code of Conduct. Historically, there has been inadequate reporting of participation by minorities in psychophysiological research (Chakraborty & Steinhauer, 2010). A separate issue, somewhat beyond the scope of these guidelines, is related to interpreting differences among ethnic groups. Differences in resting pupil diameter have been reported to vary with race/ethnicity (Kirkegaard & Nyborg, 2020), which may be related to differences in iris pigmentation, which might also affect differences in the amplitude of the light reflex(Kardon et al., 2013). When assessing pupillary reactivity as it varies with race or ethnicity during task performance, it is critical to consider contributing cultural factors (van der Wel & van Steenbergen, 2018; Verney et al., 2005).
Effects due to age are also important to consider. Based on studies involving over 1200 recordings, overall pupil diameter increased up to mid adolescence (in darkness, typically around 7–8 mm) and then decreased steadily by approximately 0.4 mm per decade (Loewenfeld, 1993; MacLachlan & Howland, 2002). Similarly, the amplitude of the pupillary light reaction decreases with age after late adolescence (Loewenfeld, 1993). However, because the range of dark-adapted diameters even within an age group can vary by as much as ±2 mm, resting diameter is not an accurate predictor of age. Although some changes are related to aging of the muscles and a decrease in the number of neurons in the LC, Loewenfeld (1993) notes that even into the 80s, a vigorous dilation response can be elicited. When considering age as a covariate, it is critical to assess whether there are age-related interactions with variables of interest such as cognitive load on pupillary reactivity (e.g., as in Silk et al., 2007).
Decreased dilation in a task context is a common finding when comparing older to younger people. During a sustained attention tracking task, pupil dilation in both young and older participants was associated with accurate tracking performance, although smaller diameter, more limited range, and reduced temporal variability were noted among the older participants (Zhao et al., 2019). In a study of arousal, in which alerting tones were presented, although older participants had smaller responses, alerting tones were more effective at increasing both speed and amplitude of dilation in older, compared to younger participants (He et al., 2020). Granholm et al. (1997, 2000) assessed the effects of age in patients with schizophrenic disorder, in which age was associated with a reduction in pupillary dilation during task processing. Morris et al. (1997) observed that, in a memory workload task, both schizophrenia and aging were associated with decreased dilation, which were separate and additive impairments.
2.4.5 |. Sleepiness and fatigue
In addition to the slow oscillations associated with sleep noted in Section 2.2.1, sleepiness is also associated with an overall decrease in tonic pupil diameter. The changes observed are the result of a decline in central nervous activation level predominantly triggered by the LC, so that increasing amplitude of oscillations can be used as an index of alertness.
Though recording of changes in diameter and oscillations were proposed as assessments of narcolepsy and drowsiness (Yoss et al., 1969, 1970), the development of better technology and automation permitted the development of a more standardized assessment, the pupillographic sleepiness test (PST; Wilhelm et al., 1998) involving 11 minutes of recording in darkness. This results in an objective measure of oscillatory activity under 0.8 Hz, the pupillary unrest index (PUI) which can be automatically computed and for which standards and criteria are available (Kelbsch et al., 2019; Lüdtke et al., 1998). Occupational sleepiness has been measured with the PUI as well as excessive daytime sleepiness in patients (Wilhelm et al., 2009). The PST is also validated in children and adolescents (von Lukowicz et al., 2021). Increased pupillary oscillations have been observed to negative emotional stimuli in sleep-deprived individuals (Franzen et al., 2009). Pupillary unrest has also been shown to index arousal symptoms in neurological disorders (Jain et al., 2011). For details of experimental conditions of the PST, standardized conduct, and analysis, see Kelbsch et al. (2019). Fatigue related to continued mental activity also has significant effects on pupillary motility (Bafna & Hansen, 2021).
2.4.6 |. Genetics
In a study of over 500 twin pairs, sensitivity to dilating agents showed intraclass correlations of 0.82 and 0.39 for monozygotic (MZ) and dizygotic (DZ) pairs, respectively, with heritability of 78%–80% (Hammond et al., 2000). In female twins, similar high heritability was shown for the light reaction, with intraclass correlations of 0.85 and 0.44 for MZ and DZ pairs, respectively (Ansari et al., 2021). More intriguing, perhaps, is the observation that the pupillographic record for individuals is highly individualistic, retaining overall shape similarities within individual subjects even as amplitude varies across different conditions (Hakerem, 1974).
2.5 |. Lighting and visual stimulation
Regardless of the modality of experimental stimulation, recording pupil diameter requires that the eyes are open, and the intensity of ambient lighting will affect both the resting level of pupil diameter (and the possible range of measured changes), as well as the relative contribution of parasympathetic or sympathetic nervous system activity. The law (or principle) of initial value (LIV; Wilder, 1957) posits that, for physiological data, the higher the measured baseline, the smaller the observed potential difference. The situation is somewhat more complex for pupil diameter, as both floor effects (pupil is as small as possible) and ceiling effects (pupil is as large possible) are possible. When illumination is high (prompting small initial pupil diameter), parasympathetic activity is also high, leading to greater (and also earlier) dilation associated primarily with inhibition of the parasympathetic pathway. In darkness (prompting larger pupil diameter), on the other hand, although some parasympathetic inhibition occurs, the major contribution to dilation will be sympathetic nervous system activity. Most data indicate that measuring pupillary change is optimal when baseline pupil diameter is in a midrange (e.g., 4–7 mm) associated with moderate illumination (Beatty, 1982, 1986; Mathôt et al., 2018; Reilly et al., 2019).
2.5.1 |. Light measurement
The lux (or lx) is the unit in the International System of Units (SI) for luminous emittance. It is a measure of the intensity of light as it strikes a surface and is the unit for measuring ambient lighting. Lux can be measured with an illuminance meter and indexes the number of lumens falling on a square meter (a foot-candle is the number of lumens falling on a square foot). The candela (cd), on the other hand, is the SI unit for luminous intensity, which indexes the luminous power detected by an eye looking at a light emitting surface from a particular angle or point of view. Luminance is a physical measure of intensity, while brightness refers to subjective intensity. The luminous intensity of a display is expressed in the SI measure candelas per meter squared, cd/m2 (though some systems record it as foot-lamberts ([ft-L]). Some light meters are capable of recording either lux or cd/m2, which are measured with separate sensors. For reporting purposes, both the light reaching the eye during experimental stimulation (e.g., monitors, displays, etc.) and ambient light can be reported in candelas per meter squared (cd/m2).
| Measurement of | Standard unit | Abbreviation | Measured with | |
|---|---|---|---|---|
| Illumination | Ambient and reflected light | lux | lx | Illuminance Meter |
| Luminance | Screen or light source | candela/meter2 | cd/m2 | Luminance Meter |
During experimental stimulation, stimuli (and baseline) lighting can be reported by placing the light meter sensor so that it is parallel to the eye (e.g., where the participant’s eye normally is positioned with respect to experimental stimulation) and by recording luminance intensity (cd/m2) as it varies with experimental conditions. Because modern computer (and phone) screens can be adjusted in multiple ways (e.g., through system settings, externals dials/buttons and software), care should be taken that system display and dial settings are identical for each participant, and that software control settings, if used, function properly to change screen illumination as desired.
2.5.2 |. Ambient light
Luminance of specific light sources (e.g., lamps, overhead lights, etc.) is typically much higher than that measured at the eye (as the light source may be in different locations). For example, a standing lamp (60 lux) located 3 m behind the participant will result in ambient light conditions of approximately 10 lux. Ambient light can be measured identically to monitors and displays, by placing the sensor parallel to the eye and recording luminance intensity (cd/m2) when the monitors and displays are turned off.
2.5.3 |. Dark adaptation
In darkness, the pupil will dilate in ~30 s and will be stable (large) after 2–3 min. This is not equivalent to full dark adaptation of the retina and visual sensitivity to light stimuli, which can take longer than 30 min. Five minutes of dark adaptation is the recommended minimal period to be used for psychophysiological experiments conducted in darkness.
2.5.4 |. Visual stimuli
For many psychophysiological experiments, the details of visual stimulation are left somewhat indeterminate, but for pupillary research, the size, color, and luminance of visual stimuli are essential variables that need to be specified in the method section to facilitate interpretability and replicability. It is critical to know the intensity of light stimuli reaching the pupil, both from specific light sources and, more typically in psychophysiological experiments, from displays or monitors. A change from a darker (such as a neutral prestimulus field) to a brighter display is likely to evoke a light reaction, and very often the amplitude of the light reaction can be greater than the response to psychological manipulations under study. The use of grayscale, rather than color, stimuli reduce the complexity of equating luminance factors across stimuli. Additional variables to report for visual stimulation are the solid angles subtended at the eye’s pupil, which depends on the size of visual stimuli on the display and the distance of the display (from the eye). As noted above, the luminance intensity of experimental stimuli (e.g., baseline, stimuli) should be measured and reported using a light luminance meter. For monochromatic color stimuli, the wavelength should be provided if possible.
Psychophysiological studies present a wide variety of visual stimuli in studies of perception, cognition, and emotion, ranging from fairly simple (e.g., gratings, Gabor patches, letter/number strings, shapes) to more complex displays (e.g., words, objects, photographs, movies, etc.). For modern studies presenting digitized visual stimuli, information regarding pixel intensity and pixel contrast, in addition to visual angle and luminance intensity, is important to report for cross-laboratory replication. Image or screen displays consist of an array (e.g., 1024 × 768) of pixels that vary in both color and depth. For an 8-bit grayscale stimulus, for example, pixel intensity will vary from 0 (black) to 256 (white), with intermediate pixel intensities representing shades of gray; specific pixel intensity values will vary with the color and depth of the image (e.g., 16-bit, 32-bit, etc.). Mean pixel intensity is computed as the average intensity across all pixels, whereas pixel contrast, describing the distribution of light and dark pixels, can be computed as the standard deviation of pixel intensities. Although reporting the mean pixel intensity and contrast of experimental (and baseline) stimulation is important for replicating stimulus conditions, it should be noted that the resulting luminance of the stimuli will depend upon visual display settings (e.g., its luminance and contrast), and will not vary with pixel contrast for stimuli equivalent in pixel intensity. On the other hand, the difference in luminance between stimuli that differ in pixel intensity (e.g., light, dark) will also depend on the display settings: Generally, as display luminance increases, the luminance difference between brighter and darker stimuli will increase in a nonlinear fashion.
When stimuli are presented foveally (e.g., typically 3–6 degrees of visual angle, with central fixation), controlling pixel intensity across experimental stimuli is usually sufficient as differences due to pixel contrast are minimal a when the entire stimulus is apprehended at each fixation. To reduce the possibility of maximal or minimal overall pupil diameter, black or white stimuli should be avoided, selecting instead midrange grayscales with contrast sufficient for easy perception. Relatedly, the stimulus surround should closely match the pixel intensity of the target stimulus, rather than using black or white backgrounds. Stimuli should be at least a few mm in diameter to reduce blurring for those who normally wear glasses. In addition, eye movements should be monitored to detect shifts in the visual field that may change luminance or result in distortion of the pupil (see Section 2.2.2), as both can affect the measured pupil size. In some cases, even if pupillary constriction due to the light reflex does not vary with condition, it can obscure experimental effects. In this case, some researchers have subtracted the mean pupil response across all conditions from each condition’s waveform to yield an apparent dilation, rather than relative constriction, response (e.g., Franzen et al., 2009).
For full-field stimulation, particularly in which eye movements are allowed (which also impacts potential parallax differences, see Section 2.2.2), pixel contrast can affect pupil diameter changes, primarily due to differential eye fixations on lighter, compared to darker, regions. As illustrated in Figure 10a, despite equivalent pixel intensity, stimuli with higher pixel contrast can prompt larger light reflexes than lower contrast versions (of the same stimulus), due to fixating brighter whites in higher contrast images. Moreover, as illustrated in Figure 10b, even two stimuli identical in pixel intensity and contrast can differ in the size and salience of relatively lighter and darker foreground regions, affecting pupil diameter as a function of the location of eye fixations. Figure 11a illustrates the strong linear relationship between the mean pixel intensity computed at each eye fixation and the amplitude of the light reflex for pictures equivalent in both pixel intensity and contrast. Moreover, the effects of pixel intensity at fixation on pupil diameter changes can occur past the initial light reflex, affecting the measured amplitude changes throughout the trial.
FIGURE 10.
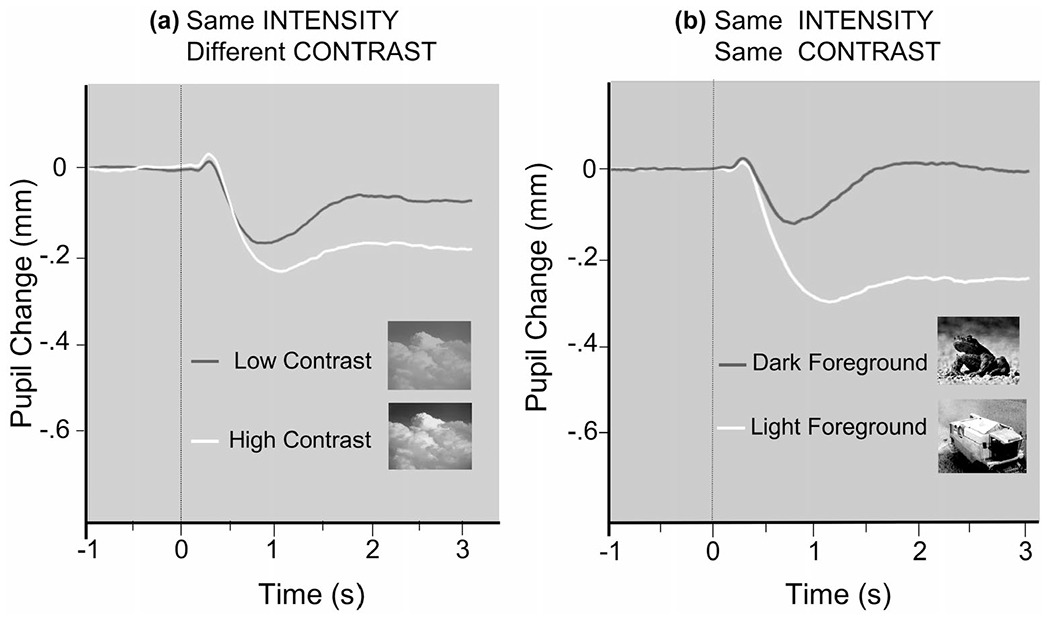
(a) For full-field stimuli that are equivalent in pixel intensity but different in pixel contrast, light reflex amplitude is greater for scenes that are high in contrast (brighter whites). (b) For stimuli that are equivalent in pixel intensity and contrast, light reflex amplitude is greater for scenes in which the foreground stimulus is brighter (courtesy M. Bradley)
FIGURE 11.
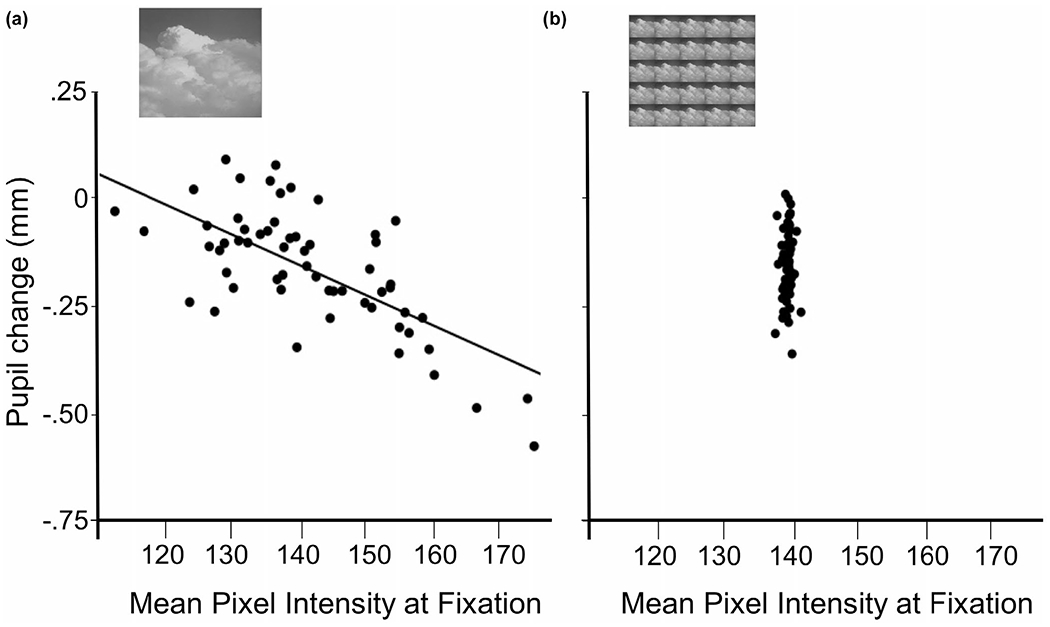
(a) Pupil diameter is linearly related to the pixel intensity (luminance) at fixation for scenes that are equivalent in pixel intensity and contrast (luminance) when full-field scenes are presented (inset) and eye movements allowed. (b) Pupil diameter is no longer linearly related to pixel intensity at fixation when scenes are presented in a matrix (5 × 5; inset) so that each fixation includes all luminance variations. In both (a) and (b) of Figure 11, each point is one scene and all scenes were pre-experimentally manipulated so that mean pixel intensity was 140 (8-bit grayscale stimulus) (courtesy M. Bradley)
Measures of stimulus pixel intensity and/or contrast alone, or their combination, do not provide a sufficient index of potential luminance differences on pupil diameter, as they do not take into account the relative salience of lighter and darker regions in stimulus comprehension, which is the primary factor influencing the position of eye fixations. One statistical solution to this problem is to compute the mean pixel intensity at each fixation (e.g., using a circular radius to approximate the luminance of the visual field at each fixation) and adjust pupil diameter data for differences in local luminance at fixation using analysis of covariance (e.g., Bradley et al., 2017, Experiment 1). The primary methodological solution is to present the stimulus foveally (with head and central fixation), rather than full field, so that each fixation includes the entire stimulus. Reducing stimulus size is not always optimal in many psychophysiological investigations, however, as details may be lost or difficult to perceive. A second methodological solution is to reduce the stimulus in size, but duplicate it (e.g., 25 times in a 5 × 5 array) which takes advantage of image redundancy to assist perception and comprehension (Bradley et al., 2017, Experiments 2 and 3). This procedure eliminates the differences in mean pixel intensity at each fixation (found even for stimuli equivalent in pixel intensity and contrast) and, as Figure 11b illustrates, also eliminates the relationship between pixel intensity at fixation and the amplitude of the light reflex. Presenting visual stimuli in this type of matrix is possible for static, but not dynamic, content (e.g., movies, videos, etc.) which may instead require statistical adjustment, foveal presentation, or some novel manipulation.
2.5.5 |. Baseline luminance
The selection of appropriate baseline (comparison) stimuli will vary with the specific goals of each investigation, although, as for experimental stimuli (and unlike other psychophysiological measures), black or white intertrial screens are rarely of use when measuring pupil diameter. For studies lacking visual stimulation (e.g., auditory, cognitive, etc.), an appropriate fixation point should be provided to minimize eye movements. In darkness or dim light, this can be accomplished by using a moderately illuminated red light source, such as an LED, which does not affect light adaptation. The best fixation stimulus is a composite of several close points in a diamond or triangular shape, since, after a few minutes using a single point fixation, there is likelihood of apparent movement—the autokinetic effect—which can increase eye movements.
For studies presenting visual stimuli, choosing the luminance of an appropriate baseline stimulus will primarily depend on whether the goal is to (1) attenuate the light reflex, or (2) measure the light reflex. In many experiments, the goal will primarily be to attenuate or reduce the light reflex in order to study the effects of cognition, attention, emotion, and other psychological factors on pupil response. If the experimental stimuli have identical pixel intensity and contrast on each trial, a constant baseline stimulus with the same properties will attenuate the light reflex. Moreover, equating the spatial extent (and/or spatial frequency) between baseline and experimental stimuli will additionally assist in attenuating the light reflex. Figure 12a (left) illustrates pupil diameter when the baseline stimulus is identical in pixel intensity and contrast (and luminance) with an upcoming (5 x 5 picture matrix) stimulus, and Figure 12a (right) illustrates the light reflex (change from baseline). Equating the luminance properties between baseline and experimental stimulation greatly attenuates initial constriction.
FIGURE 12.
Pupil diameter and the light reflex (change from baseline) as a function of baseline and experimental stimulation (5 × 5 matrix of pictures). (a) A constant baseline stimulus is equivalent in luminance to the experimental stimuli. (b) A variable baseline stimulus is equivalent in luminance to the experimental stimuli. (c) A constant baseline stimulus is different in luminance from experimental stimuli that vary across the study. (d) A variable baseline stimulus is different in luminance from experimental stimuli that vary across a study. The brighter the legend or lines, the brighter the baseline and/or experimental stimulus (courtesy M. Bradley)
Often, the experimental goal is to attenuate differences in the light reflex for experimental stimuli that naturally vary (or are experimentally manipulated to vary) in luminance. In this case, adapting pupil diameter by varying the baseline stimuli to match properties of the experimental stimuli will minimize the light reflex. Figure 12b (left) illustrates pupil diameter data from a study in which (5 x 5 picture matrix) stimuli varying in luminance were preceded by baseline stimuli that varied (trial by trial) but were equivalent in luminance to experimental stimuli, and Figure 12b (right) illustrates the resulting light reflex (change from baseline) following adaptation. Differences in the amplitude of the light reflex as a function of whether the experimental stimulus is bright or dark are completely eliminated by the appropriate adaptation period, although a small, equivalent, residual initial constriction remains.
When differences in light reflex amplitude are a focus of an investigation, experimental stimuli will necessarily vary in luminance. In many cases, a constant baseline stimulus that differs in luminance (e.g., pixel intensity and/or contrast) from the experimental stimuli will be sufficient to measure light reflex differences. Figure 12c (left) illustrates pupil diameter from a study in which a constant gray baseline image preceded either a darker or lighter stimulus (5x5 picture matrix), and Figure 12c (right) illustrates the resulting differences in the light reflex when viewing brighter, compared to darker, stimuli. If dynamic changes in the light reflex (e.g., from darker to lighter or lighter to darker) are of interest, a constant baseline stimulus is not useful. Rather, baseline stimuli that vary in luminance provide an index of light reflex reactivity, as illustrated in Figure 12d (left), prompting light reflexes of different amplitude (see Figure 12d, right). Determining the appropriate luminance properties for baseline stimulation will depend on the luminance properties of the experimental stimuli and may require pilot work to determine the optimal parameters.
2.6 |. Post-illumination pupil response (PIPR) considerations
Light stimulates both retinal rods and cones, but retinal ganglion cells containing melanopsin are another type of photosensitive cell found in the retina. Although pupillary light reflexes in the photopic range are the result of rod-, cone-, and melanopsin-driven responses, recent methods involving chromatic pupillometry yield the PIPR, a signature biomarker of human melanopsin function (Kelbsch et al., 2019). The PIPR assesses the level of the melanopsin-driven response in intrinsically photosensitive retinal ganglion cells (ipRGCs) projecting to the central nervous system. By measuring pupil constriction after a stimulus (post-illumination), an index of melanopsin-driven responses of the ipRGCs can be calculated (i.e., the PIPR), which relies on chromatic pupillometry as melanopsin cells are maximally sensitive to 480 nm blue light, while the PIPR is not elicited using narrow bandwidth red light.
2.6.1 |. Circadian rhythms
Because of daily variation in the PIPR (González-Menéndez et al., 2009; Hannibal et al., 2005; Sakamoto et al., 2004; Zele et al., 2011), it is important to control, methodologically or statistically, the time of testing as well as individual circadian time at testing (Roecklein et al., 2021). Diameter calculated at least 1.8 s poststimulus offset reflects human melanopsin function (Adhikari et al., 2015), and the average diameter from 10 to 30 s is most often reported. Reporting in both millimeters and percent of baseline diameter allows for comparison with prior reports and adjustment for individual differences in baseline pupil diameter, respectively. Interstimulus intervals of 90 to 120 s before subsequent stimuli should ensure full redilation between repeated stimuli.
2.6.2 |. PIPR stimulation
As Figure 13 illustrates, the pupil begins to constrict 200–500 ms after stimulus onset, with the shortest latency occurring after higher irradiances (Ellis, 1981). This is important because without pharmacological dilation of one eye, the pupil of the other eye would constrict during stimulus presentation if full-field stimuli are used. This means that the stimulus incident on the retina will decrease after about 200 ms in a manner that may differ across individuals.
FIGURE 13.
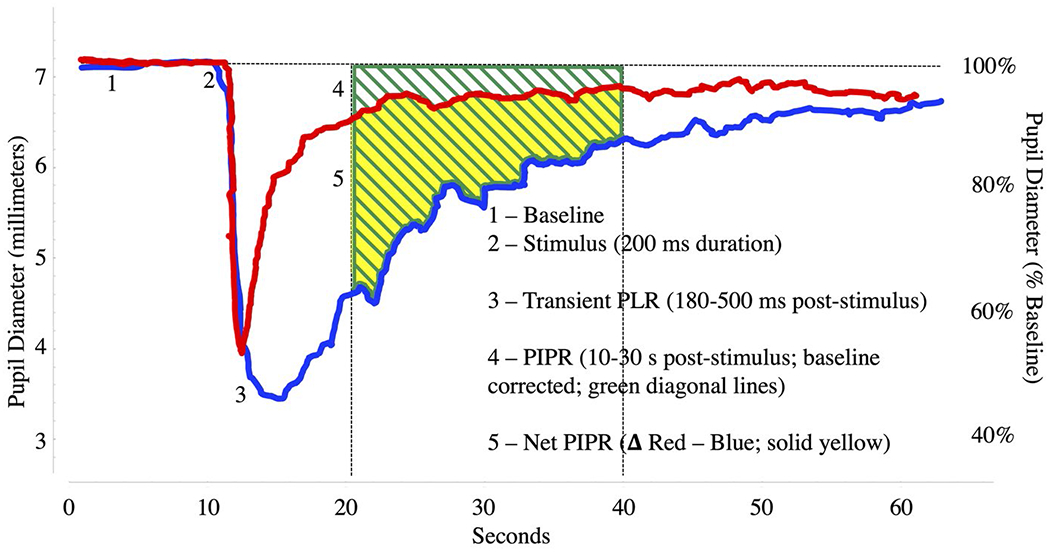
PIPR: Pupil light reflex (PLR) in response to 639 nm (red) and 463 nm (blue) narrow bandwidth stimuli of 200 ms duration, 14.5 log photons/cm2/s. The most commonly reported PIPR measure (4; AUC late; Adhikari et al., 2015) is indicated in yellow, along with other important PIPR metrics. Data reflect a single response to red and blue stimuli from a healthy control participant (courtesy K. Roecklein, P. Gamlin, P. Franzen, G. Siegle)
Stimuli shorter than 200 ms are concluded before this transient response begins, and do not therefore require mydriasis and contralateral recording. Alternatively, Maxwellian view stimuli presentation could be used, but this is not currently an option in commercially available pupillometers. Conveniently, the duration of the stimuli eliciting a maximal response are in the range of 200 ms–1 s (Adhikari et al., 2015). Irradiance approaching 13 log photons/cm2/s results in a half maximal response (Gamlin et al., 2007) and 14.5 log photons/cm2/s is relatively well tolerated by human research participants. Irradiance is reported as photon flux or irradiance rather than illuminance (photopic lux) as the sensitivity of the human visual system differs from the circadian system (An et al., 2009; Brainard et al., 2001; Gooley et al., 2010), and a “melanopic lux” represents the degree to which a given stimulus stimulates ipRGCs (Lucas et al., 2014). Ideal stimuli are monochromatic or narrow bandwidth stimuli approaching 482 nm peak irradiance (McDougal & Gamlin, 2010; Mure et al., 2009). A red or long wavelength stimulus is often included to provide a comparison response and a quantification of non-melanopsin-driven effects on the pupil such as mood-related autonomic arousal (Roecklein et al., 2021). Although sustained blue light can cause photochemical retinal injury (American Conference of Governmental Industrial Hygienists, 2010; American National Standards Institute, and Illuminating Engineering Society of North America, 2005; International Commission on Non-Ionizing Radiation Protection, 1997), the safe irradiance and duration levels are well above those described above for the PIPR (e.g., Roecklein, Wong, Ernecoff, et al., 2013), and published standards can be used to calculate and report the estimated threshold relative to their methods (Brainard et al., 2001, 2008; Glickman et al., 2006; West et al., 2011).
2.7 |. Abbreviations
A variety of amplitude and latency measures characterize pupil diameter reactivity. Consistent with the general guidelines for Psychophysiology, in which only very common acronyms are used, unique acronyms to describe various pupillary phenomena are generally not necessary. In the past, although PLR was sometimes used to refer to the “pupillary light reaction” or the “pupil light reflex,” the phrase “light reflex” is brief enough to state without requiring an acronym. Beatty (1982) used TEPR (task-evoked pupillary response) as a proxy for pupil dilation, but this has proven to be confusing and inconsistent in meaning, as dilation can be stimulus evoked, produced by missing stimuli, or as a light reaction, depending on stimulus properties. The term “PIPR” is standard in melanopsin studies to refer to the “post-illumination pupil response,” and PST (pupillographic sleepiness test) and PUI (pupillary unrest index) are considered standard abbreviations.
2.8 |. Available software/algorithms
There are several public sources that provide code or downloads for a variety of pupillary data analyses, with many using a MatLab or R platform. Some software may be limited to specific data collection devices and/or platforms. Software acquisition of video data for pupillary assessment has been developed by Zandi et al. (2021). Eye movement correction algorithms are presented by Gagl et al. (2011; see also Geller et al., 2020) and by Hayes and Petrov (2016). Identification of blinks and other artifacts has been addressed in multiple papers (Hershman et al., 2018, 2019; Kret & Sjak-Shie, 2019; Mathôt et al., 2018; Sirois & Brisson, 2014; Winn et al., 2018) as well as by Siegle, Steinhauer, and Granholm (see Siegle et al., 2003). Based on Sirois and Brisson (2014) code, Lemercier et al. (2014) provided an initial downloadable toolbox for pupil analysis. A more accessible platform is CHAP (Cohen and Hershman Analysis Pupil), an open source MatLab package developed by Hershman et al. (2019) that provides both parametric and Bayesian analyses, accepts raw (mm) or standardized (z score) data, and can be used with data from a number of different pupil and eye-tracking recording devices. Software to implement sample-by-sample waveform comparisons using the methods of Guthrie and Buchwald (1991) or of Blair and Karniski (1993) are described by Siegle et al. (2015). Table 2 provides links to available software.
3 |. PUPIL MODULATION IN PSYCHOPHYSIOLOGICAL STUDIES
3.1 |. Pupil constriction
3.1.1 |. Light reflex
The pupil responds reflexively to differences in luminance, and, as Figure 9 illustrates, pupil diameter decreases (constricts) with increasing stimulus brightness. Miosis refers to active constriction of the pupil; a miotic pupil is a small pupil. The prototypical stimulus for studying the light reflex is a light flash, and numerous early studies, described in Loewenfeld (1993), parametrically investigated the effects of luminance, duration, area of stimulation, timing, and color on the magnitude, latency, and duration of the flash-evoked light reflex (the reflexive blink to light flashes, which affect pupillary recording and analysis, is discussed in Section 2.5.4). The action of the pupil is subject to restrictions related to the latency of neural activity, as well as to iris motor movements overcoming musculature inertia. For bright, large field stimuli, minimum onset latency of the light reflex is about 200 ms, whereas less intense stimulation will typically extend onset latency. Generally, as flash intensity increases, onset latency is reduced, constriction amplitude is increased, and duration of constriction prolonged. Shorter, compared to longer duration, light flashes prompt smaller, faster pupillary changes, although these reactions also vary with timing and flash intensity, and, when measured in darkness, depend on the duration of dark adaptation. For flashes equivalent in luminance, stimulating a larger, compared to smaller, retinal area prompts greater constriction.
When a series of light flashes is presented, constriction is larger for initial compared to subsequent stimulation, as the pupil has typically not recovered to prestimulus (or dark adapted) diameter for later stimuli. As the frequency of flash stimulation increases, responses become integrated (i.e., individual light reactions not identifiable; occurs around 3 Hz), and the data are likely to only be interpretable through nonlinear analysis, rather than simple stimulus averaging. The effects of color on light reflex reactions are related to differences in apparent brightness, with variations similar to those found in color perception, and to stimulation of different photoreceptors, even if stimuli are matched for luminance.
Many of the pupillary effects that Loewenfeld originally called “psychosensory,” including those elicited during cognitive or affective processing, are primarily characterized by pupil dilation, but some also modulate light reflexes. Gavriysky (1991) found that simply requiring a button press to light flashes prompted less initial constriction and increased the onset latency of the resulting light reflex, and inhibition of light reflex amplitude is also found when light probes are delivered during a difficult (subtract 7), compared to a simple (add 1), mental arithmetic task (Steinhauer et al., 2000). The response to flickering light is also inhibited during presentation of multisensory stimuli (Yuan et al., 2021). Using pharmacological agents to selectively inactivate the sphincter or dilator muscles, Steinhauer et al. (2015) conclude that modulation of the light reflex during difficult cognitive processing is mediated by parasympathetic inhibition, which decreases sphincter constriction, increasing pupil diameter.
The effects of emotional processing on the light reflex have also been reported. Bitsios et al. (1996, 2004) presented light flashes as probes during the threat of shock, finding a “fear-inhibited light reflex,” in which initial constriction was inhibited when anticipating shock receipt, compared to rest or when anticipating a neutral sound. The light reflex is also reduced following presentation of photographic scenes depicting either pleasant or unpleasant, compared to neutral, content (Henderson et al., 2014, 2018), indicating that inhibition of the light reflex is not limited to aversive or fearful stimulation. Because the sympathetic nervous system, presumed to be active during emotional processing, both inhibits parasympathetic activity (reducing sphincter constriction), and stimulates the dilator muscle (increasing pupil diameter), the mechanism underlying reduced light reflex amplitude during emotional processing could reflect contributions from one or both pupillary muscles.
Studies using isoluminant, or near-isoluminant, simple stimuli such as visual gratings or Gabor patches have also reported differences in initial pupil constriction that vary with spatial frequency, contrast, and apparent motion (e.g., Barbur et al., 1992; Hu et al., 2019). For example, greater pupil constriction is found at the onset of apparent motion (Barbur et al., 1992) and for stimuli near the peak of the contrast sensitivity function (e.g., midrange frequencies; Hu et al., 2019). Moreover, pupil constriction is larger when attention (but not the eye) is directed toward midrange, compared to low, spatial frequency stimuli (Hu et al., 2019). Relatedly, when attention to a lighter or darker portion of the screen is cued in a Posner task (Mathôt et al., 2013), participants reporting the orientation of a peripherally presented Gabor patch showed less constriction (during redilation) for targets presented on the light side of the screen (in the absence of eye movements), suggesting some modulation of light reflex characteristics by luminance of the attended region.
3.1.2 |. Color and the post-illumination pupil response (PIPR)
Roecklein et al. (2021) describe research into the impact of environmental light levels on circadian, sleep, and mood disorders in addition to retinal diseases involving ganglion cells. Melanopsin underlies photoentrainment, the process by which the central circadian clock aligns with the solar day. A second melanopsin-mediated pathway directly modulates mood and alertness independently of the circadian though ipRGC projections to the habenula (Hattar et al., 2006).
Intracellular recording has confirmed that the PIPR is exclusively driven by melanopsin cells rather than rods and cones (Gamlin et al., 2007). M1-type melanopsin cells underlie both melanopsin contributions to pupil diameter and circadian photoentrainment (Schmidt et al., 2011). Beyond investigating the disorders of circadian photoentrainment, the PIPR can be used to study “melanopsin-related disorders” broadly (Gamlin et al., 2007), including glaucoma, diabetic retinopathy, and age-related macular degeneration. Clinical applications of the PIPR could extend from retinal diseases to disorders involving acute, direct, or circadian effects of light such as seasonal affective disorder (Park et al., 2011; Roecklein, Wong, Miller, et al., 2013; Wilhelm, 2010), symptoms of mania (Bullock et al., 2019), and delayed sleep-wake phase disorder (Abbott et al., 2021) as well as in chronobiology (e.g., Zele et al., 2011).
3.2 |. Pupil dilation
Loewenfeld (1958) emphasized that virtually every type of psychosensory stimulation, other than changes in illumination, elicits pupillary dilation, as do voluntary movements. Mydriasis, dilation, and dilatation all refer to enlargement of the pupil; a mydriatic pupil is a large pupil, and dilation or dilatation are used more frequently in psychological studies. Large pupils are sometimes considered attractive, perhaps because they imply interest, and active enlargement of the pupils has been noted as showing surprise in positive reactions (e.g., the “green shade” phenomenon in gambling, where players would wear a green shade to obscure dilation of the pupil from onlookers). However, the majority of pupil dilations observed in psychological experiments are modest in amplitude, typically in the range of 0.1–0.5 mm (Beatty, 1982), and, in many cases, consist of an initial dilation that increases rapidly to a peak (often around 1200–1400 ms) that may be sustained (Franzen et al., 2022; Siegle et al., 2001). The functional significance of pupil dilation, however, remains a source of conjecture and debate.
3.2.1 |. Conditioning
There is a nearly hundred-year background of studies of pupillary conditioning, including classical conditioning of autonomic responses, reported by Pavlov and Anrep (1927). In general, conditioning pupil dilation to unconditioned sensory stimuli, including sounds, touch, and pain, has been successful in both animals (most often cats) and humans (Finke et al., 2021), including infants (Brackbill & Fitzgerald, 1972). In contrast, attempts to classically condition the light reflex—that is, using a neutral stimulus to elicit a light-induced constriction—have failed to provide evidence of a conditioned light reaction, as Loewenfeld (1958) documented. One likely difficulty is that the light reaction is predominantly parasympathetically mediated, whereas sympathetically mediated unconditioned reactions are critical in classical conditioning paradigms. Kugelmass et al. (1969) found that when a light stimulus was omitted from the typical sound-light sequence, a dilation to the absence of the light was obtained, a phenomenon they referred to as paradoxical pupillary conditioning.
3.2.2 |. Orienting
Pavlov and Anrep (1927), later summarized and expanded by Sokolov (1963), considered the reliable pupil dilation found when presented with novel or startling stimuli, such as loud noises, sudden touch, or other unexpected sensory stimulation to be part of the complex of behavioral and autonomic responses involved in the orienting reaction. One hypothesis is that larger pupils allow low-light stimuli to be more detectable by rods, which benefits survival. As with other “orienting” reactions, repeated presentation results in a decrease or absence of the dilatory response (i.e., habituation).
3.2.3 |. Attention, arousal, and effort
A long tradition of research has established pupil dilation as an index of effort during explicit cognitive control (van der Wel & van Steenbergen, 2018). Early efforts broadly associated pupil dilation with heightened arousal, attention, or effort (Kahneman, 1973), and both greater cognitive load and stimulus uncertainty reliably increase baseline pupil diameter (Friedman et al., 1973; Steinhauer et al., 2000). Gradual changes in pupil diameter are also observed in tasks in which the workload is increased, as demonstrated in Figure 4 using a digit span task. Pupil diameter increases slowly as each additional number is added to the memory array, and as participants report the numbers, diameter gradually decreases (Granholm et al., 1997; Kahneman & Beatty, 1966). A number of studies have reported that increased load, demand, or effort is associated with increasing pupil dilation in working memory tasks such as the n-back (van der Wel & van Steenbergen, 2018). When memory storage is exceeded, there is a sudden decrease in pupil diameter, considered to index resource overload (Peavler, 1974; Poock, 1973).
Pupillary dilation is thus observed as processing demands increase, with dilation being bounded by the onset and offset of cognitive activity, as indexed by blinks (e.g., Siegle et al., 2008), and with fluctuation of pupillary motility at the task frequency reflecting performance on cognitive tasks (Jones et al., 2010), yielding a prospective index of response to cognitive training interventions (Siegle et al., 2014). When larger dilations are reported during difficult processing tasks for participants with better academic scores, however, it is difficult to determine whether it reflects greater resource allocation, or more effective processing ability (Ahern & Beatty, 1979. This issue also arises in associations of pupillary reactivity and assessment of intelligence (Dix & van der Meer, 2015; Verney et al., 2005). Some data suggest that higher frequency oscillations in pupil diameter may be associated with cognitive activity or workload as well (e.g., Bartells & Marshall, 2012; Marshall, 2002; Marshall et al., 2003).
Pupil dilation also increases as stimulus probability decreases (Friedman et al., 1973: Quiyuan et al., 1985; Steinhauer & Zubin, 1982, Figure 14), and there are slow pupillary increases related to anticipatory activity. Following a warning signal or initiating action, a slow increase in pupil diameter precedes the presentation of a subsequent stimulus (Friedman et al., 1973; Richer et al., 1983), similar to the contingent negative variation and stimulus preceding negativities identified in event-related brain potentials. Even for relatively long periods of anticipation, with unpredictable events up to at least 30 s, pupil diameter slowly increases (Jennings et al., 1998).
FIGURE 14.
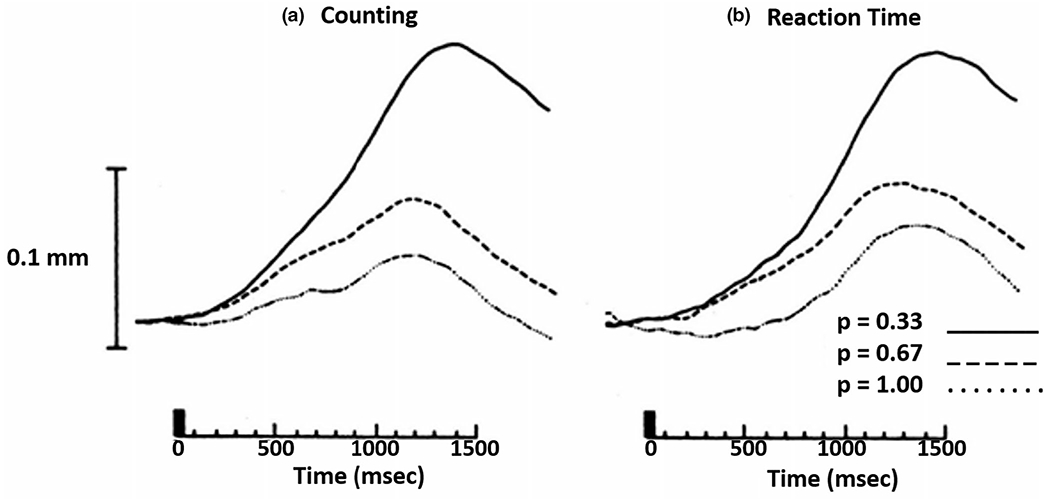
Increasing pupil dilation as event probability decreases for: (a) counting of rare tones (0.33 probability), and (b) RT to different tones (Steinhauer & Hakerem, 1992)
3.2.4 |. Emotion, stress, and pain
Hess (e.g., Hess & Polt, 1960) famously reported bidirectional effects of emotion on pupil change, reporting that the pupil dilated when viewing pleasant stimuli, but actively constricted (shut down) when viewing unpleasant content. This early research suffered from numerous methodological difficulties, including few stimuli, small subject sample sizes, and no statistical analysis. In more modern studies, pupil dilation is reliably enhanced when viewing emotional (pleasant or unpleasant), compared to neutral, scenes and covaries with changes in skin conductance activity, a sympathetically mediated response (Bradley et al., 2001, 2008). Other attempts to replicate Hess’ original findings continue to support the more recent finding of pupil dilation when viewing pleasant or unpleasant scenes, with no evidence for active constriction during aversive visual stimulation (e.g., de Winter et al., 2021).
Pupil dilation is also enhanced when imagining emotional, compared to neutral, events (Henderson et al., 2018), during active coping to avoid or escape aversive stimulation (Sege et al., 2020), during administration of painful stimuli (Chapman et al., 2014) and with increasing monetary incentives (Kahneman & Peavler, 1969; Dix & Li, 2020). Enhanced and sustained pupil dilation has also been observed during emotional word processing in adults with unipolar depression, compared to never-depressed individuals (Siegle et al., 2001, 2003) and individuals vulnerable to depression (Burkhouse et al., 2014; Steidtmann et al., 2010).
3.2.5 |. Reading and listening
Schmidtke (2017) distinguishes three broad areas in language research, in which pupillometry has been used that include auditory and orthographic language processing and speech production. Hochmann and Papeo (2014) studied phoneme-level processing in infants, finding that pupil dilation was larger for infrequent (deviant), compared to frequent, acoustic stimuli, useful as an index of perception and representation of acoustic stimuli in early infancy. In a second experiment, the authors observed larger pupil dilation for deviant speech sounds in 6- but not 3-month-old children, suggesting age-related differences in the ability to solve the so-called invariance problem for consonants coupled with different vowels (i.e., perceiving constant phoneme categories despite the lack of a constant relation between a phoneme and its acoustic property).
The effects of word frequency effects on pupil dilation have been reported in numerous studies. In a lexical decision task, low-frequency words were associated with larger pupil dilation, implying greater retrieval effort, compared to high-frequency words (Kuchinke et al., 2007). Similarly, Papesh and Goldinger (2012) observed frequency effects during a response preparation phase in a word production task, in which low-frequency words elicited greater pupil dilation suggesting speech planning is also more effortful for low-frequency stimuli. Pupillometry has also been used to probe other factors influencing semantic processing such as the impact of lexical competitors or neighborhood density (Schmidtke, 2014), cognate words in a second language (Guasch et al., 2016), and orthographic similarity (Geller et al., 2016).
At the sentence level, Demberg and Sayeed (2016) reported that pupil dilation is sensitive to grammatical and semantic violations as well as syntactic complexity. The authors conducted several experiments to verify that a novel measure of rapid small pupil dilations (using wavelet analysis, Index of Cognitive Activity) reliably index the linguistically induced cognitive load. Pupil dilation was elevated in sentences with low, compared to high, context(Winn, 2016). Similarly, conflicting prosody influences pupil dilation but only if no supporting (or even inconsistent) context information is provided (Engelhardt et al., 2010). Research into pragmatics shows that, for instance, indirect requests are associated with larger pupil size than plain statements (Tromp et al., 2016). In another study, in which participants listened to limericks, only rhythmic violations at the end of the last line but not semantic or syntactic violations resulted in larger pupil size (Scheepers et al., 2013).
Listening, as well as reading, effort, which is affected by characteristics of the speech signal and/or the listener, is also associated with pupil dilation. Environmental factors such as background noise (Kramer et al., 2013) enhance listening effort, prompting more pupil dilation. Similarly, in second language research, larger pupil diameter is found for nonnative, compared to native, speakers during sentence processing (Borghini & Hazan, 2018). Koelewijn et al. (2014) measured the mean pupil dilation throughout the presentation of spoken sentences to examine the effect of attention on the cognitive load while participants focused on either one or both of two different speakers. In this paradigm, the lengths of the sentences differed, and the overall processing load was assessed. Schmidtke (2014) examined the impact of word frequency and (phonological) neighborhood density on word retrieval effort in monolinguals and bilinguals using a visual world paradigm (self-paced response). In each trial, a carrier sentence together with a target word (presented aurally) had to be matched to one of four pictures. Peak pupil dilation and peak latency—aligned to the onset of the target word—was assessed and indicated a delayed pupil response for bilinguals compared to monolinguals together with a stronger effect of neighborhood density as well as word frequency, particularly for late bilinguals. This latter strong effect of word frequency in late bilinguals also showed up in the peak pupil dilation suggesting differences in retrieval effort (van Rijn et al., 2012) and therefore, probably in lexical access. Listening effort, as indexed by pupillary motility, is moderated by both task difficulty and associated rewards (Zhang et al., 2019), suggesting the utility of considering interactions of auditory, cognitive, and emotional pathways on pupillary reactivity.
3.2.6 |. Darkness and luminance decrease
In addition to changes in pupil diameter with increases in illumination, a number of pupillary responses occur to sudden periods of darkness. The “light-off” effect refers to pupil diameter changes following initial adaptation to cessation of light, in which there is a pupil dilation that is slower than the reflex constriction to the light source, and consists of two phases: an initial inhibition of parasympathetic activity which includes retinal “off” discharges (Loewenfeld, 1993), followed by the activation of sympathetic neurons that were inhibited in the light. The light-off effect is rarely encountered in psychophysiological studies. More common is the occurrence of a darkness reflex, which is a response to a pause—either darkness or dimming—followed by reinstantiation of the original light intensity. When longer than about a half second, there is a pupil dilation (from the absence of light), and, if the preadapting light is intense, a small dilation is followed by a light reflex. The darkness reflex is also likely to occur in response to blinks.
3.2.7 |. Sensory thresholds
Although there have been few studies of pupillary activity near sensory threshold detection levels (ranging from 33% to 67% detection), interesting findings have been reported. In one of the earliest studies to employ multiple trials and signal averaging techniques, Hakerem and Sutton (1966) examined threshold responses to brief light flashes. At threshold levels when an average light reaction was present, there was a clear dilation for trials on which the participants reported seeing the light, but no dilation occurred when no light detection was reported. A study of auditory tone detection by normal hearing and by hearing-impaired participants similarly reported dilation when a tone was heard, but not when the tone remained unreported (Mulla, 2019). Thus, dilation appears to be a sensitive indicator of individual perception, separate from sensory stimulation alone.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the recommendations and assistance of Barbara Wilhelm, Eric Granholm, Paul Gamlin, and Peter Franzen. This project was supported in part by the Department of Veterans Affairs Medical Research Service, National Institute of Mental Health (grants numbers MH055762, MH120431, MH096119, and MH096334), and German Research Foundation (Deutsche Forschungsgemeinshchaft, Project 390696704). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
To assist in both preparation of manuscripts and reviewing of papers, a checklist of relevant variables has been presented in Table 3.
TABLE 3.
Checklist for reporting pupil studies
| YES | NO | |
|---|---|---|
| Participants | ||
| Characteristics of participants, including age, gender, education, racial composition | ||
| Participants excluded from analyses | ||
| Screening for ophthalmological exclusions and pupillary motility | ||
| Screening for potential pharmacological interactions | ||
| Time of recording, season | ||
| Recording characteristics | ||
| Model of pupil or eye-tracking instrument and setup conditions Sampling rate (e.g., 50, 60, 120 Hz) | ||
| Resolution/precision of raw measurements (e.g., nearest 0.05 mm) | ||
| Initial output format—mm or arbitrary units | ||
| Calibration of pupil diameter in mm | ||
| Stimulus and timing parameters | ||
| Characteristics of auditory, tactile, or olfactory stimuli | ||
| Ambient light assessment: Luminance in cd/m2 | ||
| Visual stimuli: | ||
| Luminance in cd/m2 | ||
| Size of visual stimuli, distance from the eye, visual angle | ||
| Background or baseline stimulus field characteristics | ||
| Color, pixel intensity, and pixel contrast of baseline and experimental stimuli | ||
|
| ||
| Data reduction | ||
| Conversion of arbitrary units to mm | ||
| Detection of blinks and other artifacts | ||
| Type of interpolation | ||
| Filtering or smoothing methods | ||
| Mean number of trials per condition | ||
| Mean number or percent of trials included/excluded | ||
|
| ||
| Measurement procedures | ||
| Description of measures selected for analysis (e.g., peaks, averages, etc.), including rationale for time windows | ||
| Duration of prestimulus baseline | ||
|
| ||
| Results | ||
| Descriptive and inferential statistics | ||
| Rationale for analyses using %, ratios, or other transformations | ||
| Baseline values (in mm) for analyses using %, ratios, or other transformations | ||
| Statistical models and procedures described | ||
| Results reported with test statistics, in addition to p values | ||
| Appropriate adjustment for multiple comparisons | ||
|
| ||
| Figures | ||
| Waveform plots for important conditions (include tick marks for time, events; ordinate labeled in mm) | ||
Funding information
Department of Veterans Affairs Medical Research Service; German Research Foundation (Deutsche Forschungsgemeinshchaft), Grant/Award Number: Project 390696704; National Institute of Mental Health and Neurosciences, Grant/Award Number: MH055762, MH120431, MH096119 and MH096334
REFERENCES
- Abbott SM, Choi J, Wilson J, & Zee PC (2021). Melanopsin-dependent phototransduction is impaired in delayed sleep-wake phase disorder and sighted non-24-hour sleep-wake rhythm disorder. Sleep, 44(2), zsaa184. 10.1093/sleep/zsaa184 [DOI] [PubMed] [Google Scholar]
- Adhikari P, Zele AJ, & Feigl B (2015). The post-illumination pupil response (PIPR). Investigative Ophthalmology & Visual Science, 56(6), 3838–3849. 10.1167/iovs.14-16233 [DOI] [PubMed] [Google Scholar]
- Ahern S, & Beatty J (1979). Pupillary responses during information processing vary with scholastic aptitude test scores. Science, 205(4412), 1289–1292. 10.1126/science.472746 [DOI] [PubMed] [Google Scholar]
- Alexandridis E (1985). The Pupil (Die Pupille, translated by T. Telger). Springer. [Google Scholar]
- American Conference of Governmental Industrial Hygienists (2010). Nonionizing radiation and fields. In Documentation of the threshold limit values and biological exposure indices (pp. 135–147). American Conference of Governmental Industrial Hygienists. [Google Scholar]
- American National Standards Institute and Illuminating Engineering Society of North America. (2005). Recommended practice for photobiological safety for lamps and lamp systems—General requirements (pp. 1–28). American National Standards Institute and Illuminating Engineering Society of North America, RP 27.1–05. [Google Scholar]
- Aminihajibashi S, Hagen T, Laeng B, & Espeseth T (2020). Pupillary and behavioral markers of alerting and orienting: An individual difference approach. Brain and Cognition, 143, 105597. 10.1016/j.bandc.2020.105597 [DOI] [PubMed] [Google Scholar]
- An M, Huang J, Shimomura Y, & Katsuura T (2009). Time-of-day-dependent effects of monochromatic light exposure on human cognitive function. Journal of Physiological Anthropology, 28(5), 217–223. 10.2114/jpa2.28.217 [DOI] [PubMed] [Google Scholar]
- Angulo-Chavira AQ, García O, & Arias-Trejo N (2017). Pupil response and attention skills in Down syndrome. Research in Developmental Disabilities, 70, 40–49. 10.1016/j.ridd.2017.08.011 [DOI] [PubMed] [Google Scholar]
- Ansari AS, Vehof J, Hammond CJ, Bremner FD, & Williams KM (2021). Evidence that pupil size and reactivity are determined more by your parents than by your environment. Frontiers in Neurology, 12, 651755. 10.3389/fneur.2021.651755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Bach L (1908). Pupillenlehre. Anatomie, physiologie und pathologie. Methodik der untersuching. Karger. [Google Scholar]
- Bafna T, & Hansen JP (2021). Mental fatigue measurement using eye metrics: A systematic literature review. Psychophysiology, 58(6), e13828. 10.1111/psyp.13828 [DOI] [PubMed] [Google Scholar]
- Bakan P, & Shotland R (1969). Lateral eye movement, reading speed, and visual attention. Psychonomic Science, 15, 93–94. 10.3758/BF03336218 [DOI] [Google Scholar]
- Barbur JL, Harlow AJ, & Sahraie A (1992). Pupillary responses to stimulus structure, colour and movement. Ophthalmic & Physiological Optics, 12(2), 137–141. 10.1111/j.1475-1313.1992.tb00276.x [DOI] [PubMed] [Google Scholar]
- Bartells M, & Marshall SP (2012). Measuring cognitive workload across different eye tracking hardware platforms. In ETRA’12: Proceedings of the Symposium on Eye Tracking Research and Applications (pp. 161–164). 10.1145/2168556.2168582 [DOI] [Google Scholar]
- Beatty J (1982). Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin, 91(2), 276–292. 10.1037/0033-2909.91.2.276 [DOI] [PubMed] [Google Scholar]
- Beatty J (1986). The pupillary system. In Coles GH, Donchin E, & Porges SW (Eds.), Psychophysiology: Systems, process, and applications (pp. 43–50). Guilford Press. [Google Scholar]
- Beatty J, & Lucero-Wagoner B (2000). The pupillary system. In Cacioppo JT, Tassinary LG, & Berntson GG (Eds.), Handbook of psychophysiology (2nd ed., pp. 142–162). Cambridge University Press. [Google Scholar]
- Bitsios P, Szabadi E, & Bradshaw CM (1996). The inhibition of the pupillary light reflex by the threat of an electric shock: A potential laboratory model of human anxiety. Journal of Psychopharmacology, 10(4), 279–287. 10.1177/026988119601000404 [DOI] [PubMed] [Google Scholar]
- Bitsios P, Szabado E, & Bradshaw CM (2004). The fear-inhibited light reflex: importance of the anticipation of an aversive event. International Journal of Psychophysiology, 52, 87–95. 10.1016/j.ijpsycho.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Blair RC, & Karniski W (1993). An alternative method for significance testing of waveform difference potentials. Psychophysiology, 30(5), 518–524. 10.1111/j.1469-8986.1993.tb02075.x [DOI] [PubMed] [Google Scholar]
- Borghini G, & Hazan V (2018). Listening effort during sentence processing is increased for non-native listeners: A pupillometry study. Frontiers in Neuroscience, 12, 152. 10.3389/fnins.2018.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JE (1991). Detection of the pupil constriction latency. Medical & Biological Engineering & Computing, 29(5), 529–534. 10.1007/BF02442326 [DOI] [PubMed] [Google Scholar]
- Brackbill Y, & Fitzgerald HE (1972). Stereotype temporal conditioning in infants. Psychophysiology, 9(6), 569–577. 10.1111/j.1469-8986.1972.tb00766.x [DOI] [PubMed] [Google Scholar]
- Bradley JC, Bentley KC, Mughal AI, Bodhireddy H, Young RS, & Brown SM (2010). The effect of gender and iris color on the dark-adapted pupil diameter. Journal of Ocular Pharmacology and Therapeutics, 26(4), 335–340. 10.1089/jop.2010.0061 [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, & Lang PJ (2001). Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion, 1(3), 276–298. [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, & Lang PJ (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607. 10.1111/j.1469-8986.2008.00654.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Sapigao R, & Lang PJ (2017). Sympathetic ANS modulation of pupil diameter in emotional scene perception: Effects of hedonic content, brightness, and contrast. Psychophysiology, 54(10), 1419–1435. 10.1111/psyp.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradlow ET (2002). Exploring repeated measures data sets for key features using principal components analysis. International Journal of Research in Marketing, 19(2), 167–179. 10.1016/S0167-8116(02)00065-4 [DOI] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, & Rollag MD (2001). Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. The Journal of Neuroscience, 21(16), 6405–6412. 10.1523/JNEUROSCI.21-16-06405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Sliney D, Hanifin JP, Glickman G, Byrne B, Greeson JM, Jasser S, Gerner E, & Rollag MD (2008). Sensitivity of the human circadian system to short-wavelength (420-nm) light. Journal of Biological Rhythms, 23(5), 379–386. 10.1177/0748730408323089 [DOI] [PubMed] [Google Scholar]
- Breeden AL, Siegle GJ, Norr ME, Gordon EM, & Vaidya CJ (2017). Coupling between spontaneous pupillary fluctuations and brain activity relates to inattentiveness. European Journal of Neuroscience, 45(2), 260–266. 10.1111/ejn.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock B, McGlashan EM, Burns AC, Lu BS, & Cain SW (2019). Traits related to bipolar disorder are associated with an increased post-illumination pupil response. Psychiatry Research, 278, 35–41. 10.1016/j.psychres.2019.05.025 [DOI] [PubMed] [Google Scholar]
- Bumke O (1904). Die pupillenstörungen, dei geistes – und nervenkrankheiten (physiologie und pathologie der irisbewegungen). Fischer. [Google Scholar]
- Burkhouse KL, Siegle GJ, & Gibb BE (2014). Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(9), 1009–1016. 10.1111/jcpp.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagiltay NE, & Menekse Dalveren GG (2019). Are left- and right-eye pupil sizes always equal? Journal of Eye Movement Research, 12(2), 10.16910/jemr.12.2.1. 10.16910/jemr.12.2.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty BH, & Steinhauer SR (2010). Reporting of minority participation rates and racial differences in schizophrenia and psychophysiological research: Improving but still not adequate. Schizophrenia Research, 123(1), 90–91. 10.1016/j.schres.2010.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CR, Bradshaw DH, Donaldson GW, Jacobson RC, & Nakamura Y (2014). Central noradrenergic mechanisms and the acute stress response during painful stimulation. Journal of Psychopharmacology, 28(12), 1135–1142. 10.1177/0269881114543718 [DOI] [PubMed] [Google Scholar]
- de Winter J, Petermeijer SM, Kooijman L, & Dodou D (2021). Replicating five pupillometry studies of Eckhard Hess. International Journal of Psychophysiology, 165, 145–205. 10.1016/j.ijpsycho.2021.03.003 [DOI] [PubMed] [Google Scholar]
- Demberg V, & Sayeed A (2016). The frequency of rapid pupil dilations as a measure of linguistic processing difficulty. PLoS ONE, 11(1), e0146194. 10.1371/journal.pone.0146194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix A, & Li SC (2020). Incentive motivation improves numerosity discrimination: Insights from pupillometry combined with drift-diffusion modelling. Scientific Reports, 10(1), 2608. 10.1038/s41598-020-59415-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix A, & van der Meer E (2015). Arithmetic and algebraic problem solving and resource allocation: the distinct impact of fluid and numerical intelligence. Psychophysiology, 52(4), 544–554. 10.1111/psyp.12367 [DOI] [PubMed] [Google Scholar]
- Donchin E & Heffley E,F (1978). Multivariate analysis of event-related potential data: A tutorial review. In Otto DA (Ed.), Multidisciplinary perspectives in event-related brain potential research. US Environmental Protection Agency, U.S. Govt. Printing Office, (pp. 555–572). EPA-600/9-71-043. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=9101KFDJ.txt [Google Scholar]
- Ellis CJ (1981). The pupillary light reflex in normal subjects. The British Journal of Ophthalmology, 65(11), 754–759. 10.1136/bjo.65.11.754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Panizzon MS, Hagler DJ Jr., Eyler LT, Granholm EL, Fennema-Notestine C, Lyons MJ, McEvoy LK, Franz CE, Dale AM, & Kremen WS (2017). Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex, 97, 60–69. 10.1016/j.cortex.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt PE, Ferreira F, & Patsenko EG (2010). Pupillometry reveals processing load during spoken language comprehension. Quarterly Journal of Experimental Psychology, 63(4), 639–645. 10.1080/17470210903469864 [DOI] [PubMed] [Google Scholar]
- Ereshefsky L, & Richards A (1992). Psychosis. In Young LY & Koda-Kimble MA (Eds.), Applied Therapeutics: The Clinical Use of Drugs (pp. 1189–1230). Applied Therapeutics, Inc. [Google Scholar]
- Finke JB, Roesmann K, Stalder T, & Klucken T (2021). Pupil dilation as an index of Pavlovian conditioning. A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, S0149-7634(21)00387-0. Advance online publication. 10.1016/j.neubiorev.2021.09.005 [DOI] [PubMed] [Google Scholar]
- Fontana DF (1765). Dei moti dell’iride. Lucca: Jacopo Giusti for V. Landi (Italian; cited by Loewenfeld, 1958, 1963). https://openlibrary.org/works/OL17980470W/Dei_moti_dell%27iride [Google Scholar]
- Foster P (1989). Neuroleptic equivalence. The Pharmaceutical Journal, 290, 431–432. [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, & Siegle GJ (2009). Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology, 80(3), 300–305. 10.1016/j.biopsycho.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen L, Cabugao A, Grohmann B, Elalouf K, & Johnson AP (2022). Individual pupil size changes as a robust indicator of cognitive familiarity differences. PloS one, 17(1), e0262753. 10.1371/journal.pone.0262753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Hakerem G, Sutton S, & Fleiss JL (1973). Effect of stimulus uncertainty on the pupillary dilation response and the vertex evoked potential. Electroencephalography and Clinical Neurophysiology, 34(5), 475–484. 10.1016/0013-4694(73)90065-5 [DOI] [PubMed] [Google Scholar]
- Gagl B, Hawelka S, & Hutzler F (2011). Systematic influence of gaze position on pupil size measurement: analysis and correction. Behavior Research Methods, 43(4), 1171–1181. 10.3758/s13428-011-0109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, & Dacey DM (2007). Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Research, 47(7), 946–954. 10.1016/j.visres.2006.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavriysky VS (1991). Human pupillary light reflex and reaction time at different intensity of light stimulation (a simple motor reaction to modify the human pupillogram). International Journal of Psychophysiology, 11(3), 261–268. 10.1016/0167-8760(91)90020-x [DOI] [PubMed] [Google Scholar]
- Geller J, Still ML, & Morris AL (2016). Eyes wide open: Pupil size as a proxy for inhibition in the masked-priming paradigm. Memory & Cognition, 44(4), 554–564. 10.3758/s13421-015-0577-4 [DOI] [PubMed] [Google Scholar]
- Geller J, Winn MB, Mahr T, & Mirman D (2020). GazeR: A package for processing gaze position and pupil size data. Behavior Research Methods, 52(5), 2232–2255. 10.3758/s13428-020-01374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman G, Byrne B, Pineda C, Hauck WW, & Brainard GC (2006). Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biological Psychiatry, 59(6), 502–507. 10.1016/j.biopsych.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Goldwater BC (1972). Psychological significance of pupillary movements. Psychological Bulletin, 77(5), 340–355. 10.1037/h0032456 [DOI] [PubMed] [Google Scholar]
- González-Menéndez I, Contreras F, Cernuda-Cernuda R, & García-Fernández JM (2009). Daily rhythm of melanopsin-expressing cells in the mouse retina. Frontiers in Cellular Neuroscience, 3, 3. 10.3389/neuro.03.003.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, & Lockley SW (2010). Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Science Translational Medicine, 2(31), 31ra33. 10.1126/scitranslmed.3000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham KO, Siegle GJ, Han GT, Tomarken AJ, Crist RN, Simon DM, & Bodfish JW (2018). Pupil response to social-emotional material is associated with rumination and depressive symptoms in adults with autism spectrum disorder. PloS One, 13(8), e0200340. 10.1371/journal.pone.0200340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Fish SC, & Verney SP (2009). Pupillometric measures of attentional allocation to target and mask processing on the backward masking task in schizophrenia. Psychophysiology, 46(3), 510–520. 10.1111/j.1469-8986.2009.00805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm E, Morris S, Asarnow RF, Chock D, & Jeste DV (2000). Accelerated age-related decline in processing resources in schizophrenia: Evidence from pupillary responses recorded during the span of apprehension task. Journal of the International Neuropsychological Society: JINS, 6(1), 30–43. 10.1017/s1355617700611049 [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris SK, Sarkin AJ, Asarnow RF, & Jeste DV (1997). Pupillary responses index overload of working memory resources in schizophrenia. Journal of Abnormal Psychology, 106(3), 458–467. 10.1037//0021-843x.106.3.458 [DOI] [PubMed] [Google Scholar]
- Granholm E, & Steinhauer SR (2004). Pupillometric measures of cognitive and emotional processes. International Journal of Psychophysiology, 52(1), 1–6. 10.1016/j.ijpsycho.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Granholm E, & Verney SP (2004). Pupillary responses and attentional allocation problems on the backward masking task in schizophrenia. International Journal of Psychophysiology, 52(1), 37–51. 10.1016/j.ijpsycho.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Granholm E, Verney SP, Perivoliotis D, & Miura T (2007). Effortful cognitive resource allocation and negative symptom severity in chronic schizophrenia. Schizophrenia Bulletin, 33(3), 831–842. 10.1093/schbul/sbl040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm EL, Panizzon MS, Elman JA, Jak AJ, Hauger RL, Bondi MW, Lyons MJ, Franz CE, & Kremen WS (2017). Pupillary responses as a biomarker of early risk for Alzheimer’s Disease. Journal of Alzheimer’s Disease, 56(4), 1419–1428. 10.3233/JAD-161078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch M, Ferr ÉP, & Haro J (2016). Pupil dilation is sensitive to the cognate status of words: Further evidence for non-selectivity in bilingual lexical access. Bilingualism: Language and Cognition, 20(1), 49–54. 10.1017/S1366728916000651 [DOI] [Google Scholar]
- Guthrie D, & Buchwald JS (1991). Significance testing of difference potentials. Psychophysiology, 28(2), 240–244. 10.1111/j.1469-8986.1991.tb00417.x [DOI] [PubMed] [Google Scholar]
- Hakerem G (1974). Conceptual stimuli, pupillary dilation and evoked cortical potentials: A review of recent advances. In Janisse M-P (Ed.), Pupillary dynamics and behavior (pp. 135–158). Plenum Press. [Google Scholar]
- Hakerem G, & Sutton S (1966). Pupillary response at visual threshold. Nature, 212(5061), 485–486. 10.1038/212485a0 [DOI] [PubMed] [Google Scholar]
- Hammond CJ, Snieder H, Spector TD, & Gilbert CE (2000). Factors affecting pupil size after dilatation: The Twin Eye Study. The British Journal of Ophthalmology, 84(10), 1173–1176. 10.1136/bjo.84.10.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Georg B, Hindersson P, & Fahrenkrug J (2005). Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. Journal of Molecular Neuroscience, MN, 27(2), 147–155. 10.1385/JMN:27:2:147 [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, & Berson DM (2006). Central projections of melanopsin-expressing retinal ganglion cells in the mouse. The Journal of Comparative Neurology, 497(3), 326–349. 10.1002/cne.20970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TR, & Petrov AA (2016). Mapping and correcting the influence of gaze position on pupil size measurements. Behavior Research Methods, 48(2), 510–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Heindel WC, Nassar MR, Siefert EM, & Festa EK (2020). Age-related changes in the functional integrity of the phasic alerting system: A pupillometric investigation. Neurobiology of Aging, 91, 136–147. 10.1016/j.neurobiolaging.2020.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RR, Bradley MM, & Lang PJ (2014). Modulation of the initial light reflex during affective picture viewing. Psychophysiology, 51(9), 815–818. 10.1111/psyp.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RR, Bradley MM, & Lang PJ (2018). Emotional imagery and pupil diameter. Psychophysiology, 55, e13050. 10.1111/psyp.13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman R, Henik A, & Cohen N (2018). A novel blink detection method based on pupillometry noise. Behavior Research Methods, 50(1), 107–114. 10.3758/s13428-017-1008-1 [DOI] [PubMed] [Google Scholar]
- Hershman R, Henik A, & Cohen N (2019). CHAP: Open-source software for processing and analyzing pupillometry data. Behavior Research Methods, 51(3), 1059–1074. 10.3758/s13428-018-01190-1 [DOI] [PubMed] [Google Scholar]
- Hershman R, Levin Y, Tzelgov J, & Henik A (2021). Neutral stimuli and pupillometric task conflict. Psychological Research, 85(3), 1084–1092. 10.1007/s00426-020-01311-6 [DOI] [PubMed] [Google Scholar]
- Hess EH (1972). Pupillometrics: A method of studying mental, emotional, and sensory processes. In Greenfield NS & Sternbach RA (Eds.), Handbook of Psychophysiology (pp. 491–531). Holt, Rinehart & Winston. [Google Scholar]
- Hess EH (1975). The tell-tale eye: How your eyes reveal hidden thoughts and emotions. Van Nostrand Reinhold. [Google Scholar]
- Hess EH, & Polt JM (1960). Pupil size as related to interest value of visual stimuli. Science, 132(3423), 349–350. 10.1126/science.132.3423.349 [DOI] [PubMed] [Google Scholar]
- Hess EH, & Polt JM (1964). Pupil size in relation to mental activity during simple problem-solving. Science, 143(3611), 1190–1192. 10.1126/science.143.3611.1190 [DOI] [PubMed] [Google Scholar]
- Hochmann J-R, & Papeo L (2014). The invariance problem in infancy: A pupillometry study. Psychological Science, 25(11), 2038–2046. 10.1177/0956797614547918 [DOI] [PubMed] [Google Scholar]
- Hou RH, Samuels ER, Langley RW, Szabadi E, & Bradshaw CM (2007). Arousal and the pupil: Why diazepam-induced sedation is not accompanied by miosis. Psychopharmacology, 195(1), 41–59. 10.1007/s00213-007-0884-y [DOI] [PubMed] [Google Scholar]
- Hu X, Hisakata R, & Kaneko H (2019). Effects of spatial frequency and attention on pupillary response. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 36(10), 1699–1708. 10.1364/JOSAA.36.001699 [DOI] [PubMed] [Google Scholar]
- Ichinohe N, & Shoumura K (2001). Marked miosis caused by deafferenting the oculomotor nuclear complex in the cat. Autonomic Neuroscience: Basic & Clinical, 94(1-2), 42–45. 10.1016/S1566-0702(01)00342-3 [DOI] [PubMed] [Google Scholar]
- International Commission on Non-Ionizing Radiation Protection. (1997). Guidelines on limits of exposure to broad-band incoherent optical radiation (0.38 to 3 microM). Health Physics, 73(3), 539–554. [PubMed] [Google Scholar]
- Jain S, Siegle GJ, Gu C, Moore CG, Ivanco LS, Studenski S, Greenamyre JT, & Steinhauer SR (2011). Pupillary unrest correlates with arousal symptoms and motor signs in Parkinson disease. Movement Disorders, 26(7), 1344–1347. 10.1002/mds.23628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jainta S, & Baccino T (2010). Analyzing the pupil response due to increased cognitive demand: an independent component analysis study. International Journal of Psychophysiology, 77(1), 1–7. 10.1016/j.ijpsycho.2010.03.008 [DOI] [PubMed] [Google Scholar]
- Janisse M-P (Ed.). (1974). Pupillary dynamics and behavior. Plenum Press. [Google Scholar]
- Janisse M-P (1977). Pupillometry: The psychology of the pupillary response. Hemisphere Publishing. [Google Scholar]
- Jennings JR, van der Molen MW, & Steinhauer SR (1998). Preparing the heart, eye, and brain: Foreperiod length effects in a nonaging paradigm. Psychophysiology, 35(1), 90–98. [PubMed] [Google Scholar]
- Johnston VS (1979). Stimuli with biological significance. In Begleiter H (Ed.), Evoked brain potentials and behavior (pp. 1–12). Plenum Press. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, & Davidson RJ (2007). Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27(33), 8877–8884. 10.1523/jneurosci.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NP, Siegle GJ, Muelly ER, Haggerty A, & Ghinassi F (2010). Poor performance on cognitive tasks in depression: Doing too much or not enough? Cognitive, Affective & Behavioral Neuroscience, 10(1), 129–140. 10.3758/CABN.10.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D (1973). Attention and effort. Prentice-Hall. [Google Scholar]
- Kahneman D, & Beatty J (1966). Pupil diameter and load on memory. Science, 154(3756), 1583–1585. 10.1126/science.154.3756.1583 [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Peavler WS (1969). Incentive effects and pupillary changes in association learning. Journal of Experimental Psychology, 79(2), 312–318. 10.1037/h0026912 [DOI] [PubMed] [Google Scholar]
- Kardon RH, Hong S, & Kawasaki A (2013). Entrance pupil size predicts retinal illumination in darkly pigmented eyes, but not lightly pigmented eyes. Investigative Ophthalmology & Visual Science, 54(8), 5559–5567. 10.1167/iovs.13-12319 [DOI] [PubMed] [Google Scholar]
- Kass RE, & Raftery AE (1995). Bayes factors. Journal of the American Statistical Association, 90, 773–795. 10.1080/01621459.1995.10476572 [DOI] [Google Scholar]
- Kelbsch C, Strasser T, Chen Y, Feigl B, Gamlin PD, Kardon R, Peters T, Roecklein KA, Steinhauer SR, Szabadi E, Zele AJ, Wilhelm H, & Wilhelm BJ (2019). Standards in pupillography. Frontiers in Neurology, 10, 129. 10.3389/fneur.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkegaard EOW, & Nyborg H (2020). Pupil size and intelligence: A large-scale replication study. The Mankind Quarterly, 60(4), 1–15. 10.46469/mq.2020.60.4.5 [DOI] [Google Scholar]
- Koelewijn T, Shinn-Cunningham BG, Zekveld AA, & Kramer SE (2014). The pupil response is sensitive to divided attention during speech processing. Hearing Research, 312, 114–120. 10.1016/j.heares.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman L, Dodou D, Jansen ST, Themans TS, Russell J, Petermeijer SM, Doorman J, Hablé JH, Neubert DS, Vos M, & de Winter J (2021). Is accommodation a confounder in pupillometry research? Biological Psychology, 160, 108046. 10.1016/j.biopsycho.2021.108046 [DOI] [PubMed] [Google Scholar]
- Kramer SE, Lorens A, Coninx F, Zekveld AA, Piotrowska A, & Skarzynski H (2013). Processing load during listening: The influence of task characteristics on the pupil response. Language and Cognitive Processes, 28(4), 426–442. 10.1080/01690965.2011.642267 [DOI] [Google Scholar]
- Kret ME, & Sjak-Shie EE (2019). Preprocessing pupil size data: Guidelines and code. Behavior Research Methods, 51(3), 1336–1342. 10.3758/s13428-018-1075-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke L, Võ ML, Hofmann M, & Jacobs AM (2007). Pupillary responses during lexical decisions vary with word frequency but not emotional valence. International Journal of Psychophysiology, 65(2), 132–140. 10.1016/j.ijpsycho.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Kuchinsky SE, Ahlstrom JB, Vaden KI Jr., Cute SL, Humes LE, Dubno JR, & Eckert MA (2013). Pupil size varies with word listening and response selection difficulty in older adults with hearing loss. Psychophysiology, 50(1), 23–34. 10.1111/j.1469-8986.2012.01477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelmass S, Hakerem G, & Mantgiaris L (1969). A paradoxical conditioning effect in the human pupil. The Journal of General Psychology, 80(1), 115–127. 10.1080/00221309.1969.9711278 [DOI] [PubMed] [Google Scholar]
- Kvamme TL, Pedersen MU, Overgaard M, Rømer Thomsen K, & Voon V (2019). Pupillary reactivity to alcohol cues as a predictive biomarker of alcohol relapse following treatment in a pilot study. Psychopharmacology, 236(4), 1233–1243. 10.1007/s00213-018-5131-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaponara S, Fortunato G, Dragone A, Pellegrino M, Marson F, Silvetti M, Pinto M, D’Onofrio M, & Doricchi F (2019). Expectancy modulates pupil size both during endogenous orienting and during re-orienting of spatial attention: A study with isoluminant stimuli. The European Journal of Neuroscience, 50(5), 2893–2904. 10.1111/ejn.14391 [DOI] [PubMed] [Google Scholar]
- Lee RE, Cohen GH, & Boynton RM (1969). Latency variation in human pupil contraction due to stimulus luminance and/or adaptation level. Journal of the Optical Society of America, 59(1), 97–103. 10.1364/josa.59.000097 [DOI] [PubMed] [Google Scholar]
- Lemercier A, Guillot G, Courcoux P, Garrel C, Baccino T, & Schlich P (2014). Pupillometry of taste: Methodological guide—From acquisition to data processing—And toolbox for MATLAB. The Quantitative Methods for Psychology, 10(2), 179–195. 10.20982/tqmp.10.2.p179 [DOI] [Google Scholar]
- Loewenfeld IE (1958). Mechanisms of reflex dilatation of the pupil; historical review and experimental analysis. Documenta Ophthalmologica. Advances in Ophthalmology, 12, 185–448. 10.1007/BF00913471 [DOI] [PubMed] [Google Scholar]
- Loewenfeld IE (1993). The pupil: Anatomy, physiology, and clinical applications. Iowa State University Press. [Google Scholar]
- Lõo K, van Rij J, Järvikivi J, & Baayen H (2016). Individual differences in pupil dilation during naming task. In Papafragou (Ed.), Proceedings of the 38th Annual Conference of the Cognitive Science Society Austin, TX (pp. 550–555). [Google Scholar]
- Lowenstein O (1955). Pupillary reflex shapes and topical clinical diagnosis. Neurology, 5(9), 631–644. 10.1212/wnl.5.9.631 [DOI] [PubMed] [Google Scholar]
- Lowenstein O, Feinberg R, & Loewenfeld IE (1963). Pupillary movements during acute and chronic fatigue. Investigative Ophthalmology & Visual Science, 2, 138–157. [Google Scholar]
- Lowenstein O, & Loewenfeld IE (1958). Electronic pupillography; A new instrument and some clinical applications. A.M.A. Archives of Ophthalmology, 59(3), 352–363. [PubMed] [Google Scholar]
- Lowenstein O, Loewenfeld IE, 1962. The pupil. In: Davson. (Ed.), The eye (Vol. 3, pp. 231–267). Academic Press. [Google Scholar]
- Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockley SW, O’Hagan JB, Price LL, Provencio I, Skene DJ, & Brainard GC (2014). Measuring and using light in the melanopsin age. Trends in Neurosciences, 37(1), 1–9. 10.1016/j.tins.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdtke H, Wilhelm B, Adler M, Schaeffel F, & Wilhelm H (1998). Mathematical procedures in data recording and processing of pupillary fatigue waves. Vision Research, 38, 2889–2896. 10.1016/S0042-6989(98)00081-9 [DOI] [PubMed] [Google Scholar]
- MacLachlan C, & Howland HC (2002). Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthalmic & Physiological Optics, 22(3), 175–182. 10.1046/j.1475-1313.2002.00023.x [DOI] [PubMed] [Google Scholar]
- Marshall SP (2002). The index of cognitive activity: Measuring cognitive workload. In Proceedings of the IEEE 7th Conference on Human Factors and Power Plants, 2002 (pp. 7–7). https://ieeexplore.ieee.org/abstract/document/1042860 [Google Scholar]
- Marshall SP, Pleydell-Pearce CW, & Dickson BT (2003). Integrating psychophysiological measures of cognitive workload and eye movements to detect strategy shifts. In Proceedings of the 36th Annual Hawaii International Conference on System Sciences, 2003 (p. 6). https://ieeexplore.ieee.org/abstract/document/1174298 [Google Scholar]
- Mathôt S (2013). A simple way to reconstruct pupil size during eye blinks. figshare. 10.6084/m9.figshare.688001.v1 [DOI] [Google Scholar]
- Mathôt S (2018). Pupillometry: Psychology, physiology, and function. Journal of Cognition, 1(1), 16. 10.5334/joc.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S, Fabius J, Van Heusden E, & Van der Stigchel S (2018). Safe and sensible preprocessing and baseline correction of pupil-size data. Behavior Research Methods, 50(1), 94–106. 10.3758/s13428-017-1007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt S, van der Linden L, Grainger J, & Vitu F (2013). The pupillary light response reveals the focus of covert visual attention. PLoS ONE, 8(10), e78168. 10.1371/journal.pone.0078168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Middleton W, Gilmartin B, & Bullimore MA (1991). Pupillary diameter and cognitive load. Journal of Psychophysiology, 5(3), 265–271. [Google Scholar]
- McDougal DH, & Gamlin PD (2010). The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Research, 50(1), 72–87. 10.1016/j.visres.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarrigle R, Dawes P, Stewart AJ, Kuchinsky SE, & Munro KJ (2017). Pupillometry reveals changes in physiological arousal during a sustained listening task. Psychophysiology, 54(2), 193–203. 10.1111/psyp.12772 [DOI] [PubMed] [Google Scholar]
- Megemont M, McBurney-Lin J, & Yang H (2022). Pupil diameter is not an accurate real-time readout of locus coeruleus activity. eLife, 11, e70510. 10.7554/eLife.70510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SK, Granholm E, Sarkin AJ, & Jeste DV (1997). Effects of schizophrenia and aging on pupillographic measures of working memory. Schizophrenia Research, 27(2-3), 119–128. 10.1016/S0920-9964(97)00065-0 [DOI] [PubMed] [Google Scholar]
- Mulla R (2019). Loudness perception at and near elevated threshold: Is soft still soft? (Doctoral dissertation). University of Pittsburgh. [Google Scholar]
- Mure LS, Cornut PL, Rieux C, Drouyer E, Denis P, Gronfier C, & Cooper HM (2009). Melanopsin bistability: A fly’s eye technology in the human retina. PLoS ONE, 4(6), e5991. 10.1371/journal.pone.0005991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ichiki A, & Fujikado T (2021). Pupil constriction via the parasympathetic pathway precedes perceptual switch of ambiguous stimuli. International Journal of Psychophysiology, 167, 15–21. 10.1016/j.ijpsycho.2021.06.006 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, De Geus EJ, & Aston-Jones G (2011). The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology, 48(2), 162–175. 10.1111/j.1469-8986.2010.01057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papesh MH, & Goldinger SD (2012). Pupil-BLAH-metry: Cognitive effort in speech planning reflected by pupil dilation. Attention, Perception & Psychophysics, 74(4), 754–765. 10.3758/s13414-011-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, & Hood DC (2011). Toward a clinical protocol for assessing rod, cone, and melanopsin contributions to the human pupil response. Investigative Ophthalmology & Visual Science, 52(9), 6624–6635. 10.1167/iovs.11-7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP, & Anrep GV (1927). Conditioned reflexes; an investigation of the physiological activity of the cerebral cortex. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavler WS (1974). Pupil size, information overload, and performance differences. Psychophysiology, 11(5), 559–566. 10.1111/j.1469-8986.1974.tb01114.x [DOI] [PubMed] [Google Scholar]
- Poock GK (1973). Information processing vs pupil diameter. Perceptual and Motor Skills, 37(3), 1000–1002. 10.2466/pms.1973.37.3.1000 [DOI] [PubMed] [Google Scholar]
- Qiyuan J, Richer F, Wagoner BL, & Beatty J (1985). The pupil and stimulus probability. Psychophysiology, 22(5), 530–534. 10.1111/j.1469-8986.1985.tb01645.x [DOI] [PubMed] [Google Scholar]
- Reilly J, Kelly A, Kim SH, Jett S, & Zuckerman B (2019). The human task-evoked pupillary response function is linear: Implications for baseline response scaling in pupillometry. Behavior Research Methods, 51(2), 865–878. 10.3758/s13428-018-1134-4 [DOI] [PubMed] [Google Scholar]
- Richer F, Silverman C, & Beatty J (1983). Response selection and initiation in speeded reactions: A pupillometric analysis. Journal of Experimental Psychology Human Perception and Performance, 9(3), 360–370. 10.1037//0096-1523.9.3.360 [DOI] [PubMed] [Google Scholar]
- Roecklein KA, Franzen PL, Wescott DL, Hasler BP, Miller MA, Donofry SD, DuPont CM, Gratzmiller SM, Drexler SP, Wood-Vasey WM, & Gamlin PD (2021). Melanopsin-driven pupil response in summer and winter in unipolar seasonal affective disorder. Journal of Affective Disorders, 291, 93–101. 10.1016/j.jad.2021.04.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein KA, Wong PM, Ernecoff NC, Miller MA, Donofry SD, Kamarck ML, Wood-Vasey WM, & Franzen PL (2013). The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Research, 210(1), 150–158. 10.1016/j.psychres.2013.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, & Brainard GC (2013). Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neuroscience and Biobehavioral Reviews, 37(3), 229–239. 10.1016/j.neubiorev.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouder JN, Morey RD, Speckman PL, & Province JM (2012). Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology, 56, 356–374. 10.1016/J.JMP.2012.08.001 [DOI] [Google Scholar]
- Ruchkin DS, & Glaser EM (1978). Multivariate analysis of event-related potential data: A tutorial review. In Otto DA (Ed.), Multidisciplinary perspectives in event-related brain potential research (pp. 579–581). US. Environmental Protection Agency, U.S. Govt. Printing Office. EPA-600/9-71-043. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=9101KFDJ.txt [Google Scholar]
- Sadock BJ, & Sadock VA (Eds.). (2000). Kaplan & Sadock’s comprehensive textbook of psychiatry (7th ed.). Lippincott Williams and Wilkins Publishers. [Google Scholar]
- Sakamoto K, Liu C, & Tosini G (2004). Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. The Journal of Neuroscience, 24(43), 9693–9697. 10.1523/JNEUROSCI.2556-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, & Szabadi E (2000a). Functional neuroanatomy of the locus coeruleus: Its role in the regulation of arousal and autonomic function. Part I: Principles of functional organisation. Current Neuropsychopharmacology, 6, 235–253. 10.2174/157015908785777229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, & Szabadi E (2000b). Functional neuroanatomy of the locus coeruleus: Its role in the regulation of arousal and autonomic function. Part II: Physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Current Neuropsychopharmacology, 6, 254–285. 10.2174/157015908785777193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers C, Mohr S, Fischer MH, & Roberts AM (2013). Listening to Limericks: A pupillometry investigation of perceivers’ expectancy. PLoS ONE, 8(9), e74986. 10.1371/journal.pone.0074986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluroff M, Zimmermann TE, Freeman RB, Hofmeister K, Lorscheid T, & Weber A (1986). Pupillary responses to syntactic ambiguity of sentences. Brain and Language, 27(2), 322–344. 10.1016/0093-934X(86)90023-4 [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, & Hattar S (2011). Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends in Neurosciences, 34(11), 572–580. 10.1016/j.tins.2011.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J (2014). Second language experience modulates word retrieval effort in bilinguals: Evidence from pupillometry. Frontiers in Psychology, 5, 137. 10.3389/fpsyg.2014.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke J (2017). Pupillometry in linguistic research. Studies in Second Language Acquisition, 40(03), 529–549. 10.1017/s0272263117000195 [DOI] [Google Scholar]
- Sege CT, Bradley MM, & Lang PJ (2020). Motivated action: Pupil diameter during active coping. Biological Psychology, 153, 107885. 10.1016/j.biopsycho.2020.107885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga N, & Ohkubo Y (1979). Pupillary responses to auditory stimuli—A study about the change of the pattern of the pupillary reflex dilation. Tohoku Psychologica Folia, 38, 57–65. [Google Scholar]
- Siegle GJ, D’Andrea W, Jones N, Hallquist MN, Stepp SD, Fortunato A, Morse JQ, & Pilkonis PA (2015). Prolonged physiological reactivity and loss: Association of pupillary reactivity with negative thinking and feelings. International Journal of Psychophysiology, 98(2 Pt 2), 310–320. 10.1016/j.ijpsycho.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, & Matt GE (2001). Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry, 49(7), 624–636. 10.1016/s0006-3223(00)01024-6 [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ichikawa N, & Steinhauer S (2008). Blink before and after you think: Blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology, 45(5), 679–687. 10.1111/j.1469-8986.2008.00681.x [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Price RB, Jones NP, Ghinassi F, & Thase ME (2014). You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clinical Psychological Science, 2(4), 455–471. 10.1177/2167702614536160 [DOI] [Google Scholar]
- Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, & Thase ME (2011). Remission prognosis for cognitive therapy for recurrent depression using the pupil: Utility and neural correlates. Biological Psychiatry, 69(8), 726–733. 10.1016/j.biopsych.2010.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, & Carter CS (2003). Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. NeuroImage, 20(1), 114–124. 10.1016/s1053-8119(03)00298-2 [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, & Siegle GJ (2007). Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. The American journal of Psychiatry, 164(12), 1873–1880. 10.1176/appi.ajp.2007.06111816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois S, & Brisson J (2014). Pupillometry. Wiley Interdisciplinary Reviews: Cognitive Science, 5(6), 679–692. 10.1002/wcs.1323 [DOI] [PubMed] [Google Scholar]
- Sokolov YN (1963). Perception and the conditioned reflex. Translated by S. W. Waydenfeld. Pergamon Press. [Google Scholar]
- Steidtmann D, Ingram RE, & Siegle GJ (2010). Pupil response to negative emotional information in individuals at-risk for depression. Cognition and Emotion, 24(3), 480–496. 10.1080/02699930902738897 [DOI] [Google Scholar]
- Steinhauer SR, Condray R, & Kasparek A (2000). Cognitive modulation of midbrain function: Task-induced reduction of the pupillary light reflex. International Journal of Psychophysiology, 39(1), 21–30. 10.1016/s0167-8760(00)00119-7 [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Condray R, & Pless ML (2015). Pharmacological isolation of cognitive components influencing the pupillary light reflex. Journal of Ophthalmology, 2015, 179542. 10.1155/2015/179542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer SR, & Hakerem G (1992). The pupillary response in cognitive psychophysiology and schizophrenia. Annals of the New York Academy of Sciences, 658, 182–204. 10.1111/j.1749-6632.1992.tb22845.x [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, & Pless M (2004). Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology, 52(1), 77–86. 10.1016/j.ijpsycho.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, & Zubin J (1982). Vulnerability to schizophrenia: Information processing in the pupil and event-related potential. In Usdin E & Hanin I (Eds.), Biological markers in psychiatry and neurology (pp. 371–385). Pergamon Press. [Google Scholar]
- Szabadi E (2012). Modulation of physiological reflexes by pain: role of the locus coeruleus. Frontiers in Integrative Neuroscience, 6, 94. 10.3389/fnint.2012.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E (2018). Functional organization of the sympathetic pathways controlling the pupil: Light-inhibited and light-stimulated pathways. Frontiers in Neurology, 9, 1069. 10.3389/fneur.2018.01069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Puntous T, Tuladhar A, Yoshimoto S, & Shirama A (2011). Estimation of mental effort in learning visual search by measuring pupil response. PloS One, 6(7), e21973. 10.1371/journal.pone.0021973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin K, Sekeroglu MA, Kiziltoprak H, Doguizi S, Inanc M, & Yilmazbas P (2018). Static and dynamic pupillometry data of healthy individuals. Clinical & Experimental Optometry, 101(5), 659–665. 10.1111/cxo.12659 [DOI] [PubMed] [Google Scholar]
- Tromp J, Hagoort P, & Meyer AS (2016). Pupillometry reveals increased pupil size during indirect request comprehension. Quarterly Journal of Experimental Psychology (2006), 69(6), 1093–1108 10.1080/17470218.2015.1065282. [DOI] [PubMed] [Google Scholar]
- Tryon WW (1975). Pupillometry: A survey of sources of variation. Psychophysiology, 12(1), 90–93. 10.1111/j.1469-8986.1975.tb03068.x [DOI] [PubMed] [Google Scholar]
- Tsukahara JS (2020). Pupillometry: An R package to preprocess pupil data (v0.5.0). 10.5281/zenodo.4464854 [DOI] [Google Scholar]
- Tsukahara JS, & Engle RW (2021). Fluid intelligence and the locus coeruleus-norepinephrine system. Proceedings of the National Academy of Sciences of the United States of America, 118(46), e2110630118. 10.1073/pnas.2110630118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara JS, Harrison TL, & Engle RW (2016). The relationship between baseline pupil size and intelligence. Cognitive Psychology, 91, 109–123. 10.1016/j.cogpsych.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, & Davidson RJ (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage, 47(3), 852–863. 10.1016/j.neuroimage.2009.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden D, Tops M, & Bakker AB (2021). The neuroscience of the flow state: Involvement of the locus coeruleus norepinephrine system. Frontiers in Psychology, 12, 645498. 10.3389/fpsyg.2021.645498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wel P, & van Steenbergen H (2018). Pupil dilation as an index of effort in cognitive control tasks: A review. Psychonomic Bulletin & Review, 25(6), 2005–2015. 10.3758/s13423-018-1432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rij J (2012). Pronoun processing: Computational, behavioral, and psychophysiological studies in children and adults (Doctoral thesis). University of Groningen. [Google Scholar]
- van Rij J, Hendriks P, van Rijn H, Baayen RH, & Wood SN (2019). Analyzing the time course of pupillometric data. Trends in Hearing, 23, 2331216519832483. 10.1177/2331216519832483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn H, Dalenberg JR, Borst JP, & Sprenger SA (2012). Pupil dilation co-varies with memory strength of individual traces in a delayed response paired-associate task. PLoS ONE, 7(12), e51134. 10.1371/journal.pone.0051134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney SP, Granholm E, Marshall SP, Malcarne VL, & Saccuzzo DP (2005). Culture-fair cognitive ability assessment: Information processing and psychophysiological approaches. Assessment, 12(3), 303–319. 10.1177/1073191105276674 [DOI] [PubMed] [Google Scholar]
- von Lukowicz H, Poets CF, Peters T, Wilhelm B, Schlarb A, & Urschitz MS (2021). Validity of the pupillographic sleepiness test for the diagnosis of daytime sleepiness in children and adolescents and its relationship to sleepiness-associated outcomes. Sleep Medicine, 83, 145–150. 10.1016/j.sleep.2021.04.030 [DOI] [PubMed] [Google Scholar]
- West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, Maida J, Bowen C, Sliney DH, Rollag MD, Hanifin JP, & Brainard GC (2011). Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. Journal of Applied Physiology, 110(3), 619–626. 10.1152/japplphysiol.01413.2009 [DOI] [PubMed] [Google Scholar]
- Wetzel N, Buttelmann D, Schieler A, & Widmann A (2016). Infant and adult pupil dilation in response to unexpected sounds. Developmental Psychobiology, 58(3), 382–392. 10.1002/dev.21377 [DOI] [PubMed] [Google Scholar]
- Widmann A, Schröger E, & Wetzel N (2018). Emotion lies in the eye of the listener: Emotional arousal to novel sounds is reflected in the sympathetic contribution to the pupil dilation response and the P3. Biological Psychology, 133, 10–17. 10.1016/j.biopsycho.2018.01.010 [DOI] [PubMed] [Google Scholar]
- Wilder J (1957). The law of initial value in neurology and psychiatry; facts and problems. The Journal of Nervous and Mental Disease, 125(1), 73–86. 10.1097/00005053-195701000-00009 [DOI] [PubMed] [Google Scholar]
- Wilhelm B, Wilhelm H, Lüdtke H, Streicher P, & Adler M (1998). Pupillographic assessment of sleepiness in sleep-deprived healthy subjects. Sleep, 21(3), 258–265. [PubMed] [Google Scholar]
- Wilhelm BJ (2010). Das Auge der Inneren Uhr—Pupillenforschung in neuem Licht [The eye of the inner clock—Pupil research in a new light]. Klinische Monatsblatter fur Augenheilkunde, 227(11), 840–844. 10.1055/s-0029-1245658 [DOI] [PubMed] [Google Scholar]
- Wilhelm BJ, Widmann A, Durst W, Heine C, & Otto G (2009). Objective and quantitative analysis of daytime sleepiness in physicians after night duties. International Journal of Psychophysiology, 72(3), 307–313. 10.1016/j.ijpsycho.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Wilhelm H (2011). Disorders of the pupil. In Handbook of clinical neurology (Vol. 102, pp. 427–466). 10.1016/B978-0-444-52903-9.00022-4 [DOI] [PubMed] [Google Scholar]
- Winn MB (2016). Rapid release from listening effort resulting from semantic context, and effects of spectral degradation and cochlear implants. Trends in Hearing, 20, 2331216516669723. 10.1177/2331216516669723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MB, & Moore AN (2018). Pupillometry reveals that context benefit in speech perception can be disrupted by later-occurring sounds, especially in listeners with cochlear implants. Trends in Hearing, 22, 2331216518808962. 10.1177/2331216518808962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MB, Wendt D, Koelewijn T, & Kuchinsky SE (2018). Best practices and advice for using pupillometry to measure listening effort: An introduction for those who want to get started. Trends in Hearing, 22, 11–32. 10.1177/2331216518800869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodmansee JJ (1966). Methodological problems in pupillographic experiments. Proceedings, 74th Annual Convention. American Psychological Association, 1, 133–134. [Google Scholar]
- Woods SW (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry, 64(6), 663–667. 10.4088/jcp.v64n0607 [DOI] [PubMed] [Google Scholar]
- Yeung MK, Lee TL, Han Y, & Chan AS (2021). Prefrontal activation and pupil dilation during n-back task performance: A combined fNIRS and pupillometry study. Neuropsychologia, 159, 107954. 10.1016/j.neuropsychologia.2021.107954 [DOI] [PubMed] [Google Scholar]
- Yoss RE, Moyer NJ, & Hollenhorst RW (1970). Pupil size and spontaneous pupillary waves associated with alertness, drowsiness, and sleep. Neurology, 20(6), 545–554. 10.1212/wnl.20.6.545 [DOI] [PubMed] [Google Scholar]
- Yoss RE, Moyer NJ, & Ogle KN (1969). The pupillogram and narcolepsy. A method to measure decreased levels of wakefulness. Neurology, 19(10), 921–928. 10.1212/wnl.19.10.921 [DOI] [PubMed] [Google Scholar]
- Young RS, Han BC, & Wu PY (1993). Transient and sustained components of the pupillary responses evoked by luminance and color. Vision Research, 33(4), 437–446. 10.1016/0042-6989(93)90251-q [DOI] [PubMed] [Google Scholar]
- Yuan X, Cheng Y, & Jiang Y (2021). Multisensory signals inhibit pupillary light reflex: Evidence from pupil oscillation. Psychophysiology, 58(8), e13848. 10.1111/psyp.13848 [DOI] [PubMed] [Google Scholar]
- Zandi B, Lode M, Herzog A, Sakas G, & Khanh TQ (2021). PupilEXT: Flexible open-source platform for high-resolution pupillometry in vision research. Frontiers in Neuroscience, 15, 676220. 10.3389/fnins.2021.676220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zele AJ, Feigl B, Smith SS, & Markwell EL (2011). The circadian response of intrinsically photosensitive retinal ganglion cells. PloS ONE, 6(3), e17860. 10.1371/journal.pone.0017860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Siegle GJ, McNeil MR, Pratt SR, & Palmer C (2019). The role of reward and task demand in value-based strategic allocation of auditory comprehension effort. Hearing Research, 381, 107775. 10.1016/j.heares.2019.107775 [DOI] [PubMed] [Google Scholar]
- Zhao S, Bury G, Milne A, & Chait M (2019). Pupillometry as an objective measure of sustained attention in young and older listeners. Trends in Hearing, 23, 2331216519887815. 10.1177/2331216519887815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn KM (1972). The pupil. Charles C. Thomas. [Google Scholar]
- Zworykin VK, & Morton GA (1936). The electron telescope. Electronics, 9, 10–13. [Google Scholar]


