Abstract
Children with COVID-19 fare much better than adults but less is known about children with both COVID-19 and a cancer diagnosis in terms of clinical outcome and imaging. We describe our experience with a cohort of children with COVID-19 and cancer who have undergone medical imaging. We reviewed imaging and recorded clinical data and separated this group into two subgroups - hematologic and solid malignancies. Our observational data show that 1)children with hematologic malignancies may be at higher risk for complications, including death than, those with solid tumors, 2) that pulmonary imaging in the former group more often shows abnormalities and 3) that presence of pulmonary imaging abnormalities may portend an unfavorable outcome.
Keywords: COVID-19, cancer, Children, Imaging, Vaccination
1. Introduction
Ever since it was first reported in late 2019, much has been learned about the biology and clinical characteristics of the novel COVID-19 virus. Among the most widely recognized and striking features is the stark clinical differences between populations with differing risk categories. For example, children with COVID-19 are at a significantly lower risk for developing severe disease.1 Conversely, patients with cancer, in general, are at an elevated risk.2., 3., 4., 5. There have been relatively few published articles addressing the clinical course or outcomes of children with both COVID-19 and a cancer diagnosis.6., 7. The evidence to date suggests that this population tolerates the virus remarkably well. There have, however, been no articles in the English language literature specifically addressing imaging in children with COVID-19 and cancer. Here, we describe our experience imaging children with COVID-19 treated at a large cancer center and focus on imaging manifestations of lower respiratory illness.
Based on prior available published work, it has been shown that both adults and children with hematologic malignancies have worse clinical outcomes related to COVID-19 as compared with outcomes in patients with solid tumors.5., 6., 8., 9. With this knowledge, we separated our cohort into hematologic and solid tumor groups in order to confirm differences in morbidity and mortality related to COVID-19 in children and to determine whether these differences in severity are reflected on imaging studies. As a secondary aim, we searched for the presence of any imaging manifestations of possible COVID-19 related disease beyond the lungs. Having experienced multiple “waves” of COVID-19 resurgence with associated periodic increases in hospitalizations and fatalities - largely among unvaccinated individuals, this data which was derived prior to FDA Emergency Use Authorization of the first vaccine, may be instructive.
2. Methods
This retrospective review was approved by the local IRB and patient consent was waived. During the study period, all patients entering our facility were screened for COVID-19. This included RT-PCR for all patients who were admitted, undergoing sedation or receiving any myelosuppressive chemotherapy. The remaining patients entering the clinic were screened via a questioner and underwent RT-PCR if symptomatic or if there was a history of exposure to COVID-19. Those with documented COVID-19 positivity from at an outside facility were also included. Caregivers accompanying admitted patients or undergoing chemotherapy were all tested by RT-PCR.
A search of the Memorial Sloan Kettering database was conducted for patients less than 21 years of age with documented positive COVID-19 RT-PCR and had undergone one or more imaging studies between March and December of 2020. The electronic medical record (EMR) was reviewed for each patient and the following parameters were recorded: Patient age, date of COVID-19 RT-PCR test positivity, date of first negative COVID-19 PCR (if available), cancer type, treatment, administration of corticosteroids, presence of symptoms plausibly related to COVID-19 and, if present - the date of onset, and clinical outcome. Relevant symptoms included one or more of the following that were of new onset: cough, coryza, loss of smell/taste, fatigue, shortness of breath and fever or chills. One patient undergoing treatment for ovarian mucinous adenocarcinoma who presented with new onset abdominal pain and diarrhea was classified as asymptomatic from COIVD-19. The medical oncologic treatment status at the time of the COVID-19 diagnosis was also recorded and included: “off-treatment”, “none” or “active treatment”. For the hematologic malignancies, active treatment was further characterized by either “Induction” or “Maintenance” therapy, if known.
All body and CNS medical imaging performed by the Department of Radiology (excluding dedicated extremity imaging) was recorded, including imaging studies performed within one month prior and three months after the date of COVID diagnosis. All imaging was reviewed by a pediatric radiologist with 10 years' experience (GGB) and selected findings were recorded. Findings were recorded if they met three criteria: 1) the finding was not clearly and directly related to the known underlying malignancy (i.e. change in size of known tumor or mass effect on an adjacent structure) 2) there is biologic plausibility that a finding could be related to the infection (i.e. pneumonia, effusions, bowel edema) and 3) the finding was not present on a study prior to the date of the COVID-19 PCR. These criteria were established to account for findings that may be of unclear etiology. For example, the etiology of findings such as vascular thrombi or colitis may be equivocal in a patient with both COVID-19 and a malignancy but these entities would not “clearly and directly be related to the known underlying malignancy” and are thus considered plausibly related to COVID-19.
Outcomes were classified in a binary fashion - either severe or not severe. Severe illness was defined as that requiring mechanical ventilation and/or resulted in patient demise.
3. Results
During the three-month study period, 48 pediatric patients were identified. Six patients were excluded as there was incomplete clinical history (mostly treated at another facility), leaving 42 patients included in the study. During the study period, 12 patients had brain imaging, 22 had nuclear medicine studies (including 16 PET and 7 MIBG studies, two patients underwent more than one study), 4 had CT scans of the abdomen (one patient underwent two studies), 5 had abdominal MRI (two patients underwent two scans), one patient underwent cardiac MR and two patients had breast ultrasound. No findings on any of these scans were thought to be plausible manifestations of COVID-19. One patient underwent an abdominal x-ray which revealed pneumoperitoneum and, at surgery, was found to have a perforated cecum which was considered to be related to treatment for multiply relapsed ALL (which included vincristine, PEG-asparaginase and doxorubicin) as opposed to having been related to COVID-19. 32 of the 42 patients underwent chest imaging (either x-ray and/or CT). Results of these are detailed below.
Of the 42 patients who underwent imaging, 12 (29%) had hematologic malignancies and 30 (71%) had solid tumors. Three of the patients with a hematologic malignancy (25%) were not on treatment, two of which completed treatment previously and one had not yet started as the oncologic diagnosis was concurrent with the diagnosis of COVID-19. Three patients with solid tumors (10%) were not on active treatment, one of which had not yet began treatment and the other two had disease which required only surgical resection. 8 of the 12 patients with hematologic malignancies and 11 of the 30 patients with solid tumors were receiving steroids during the period of COVID-19 infection.
No patients with solid tumors developed severe disease and 18 (60%) were asymptomatic. In the hematologic malignancy group, no patients were entirely asymptomatic but two (17%) were minimally symptomatic (fevers only). Among the patients with hematologic malignancies, 2 (17%) required mechanical ventilation (Fig. 1 ) and 2 (17%) patients died, yielding 4 (33%) that were considered to have severe disease. Three of the four patients with severe disease had received corticosteroids.
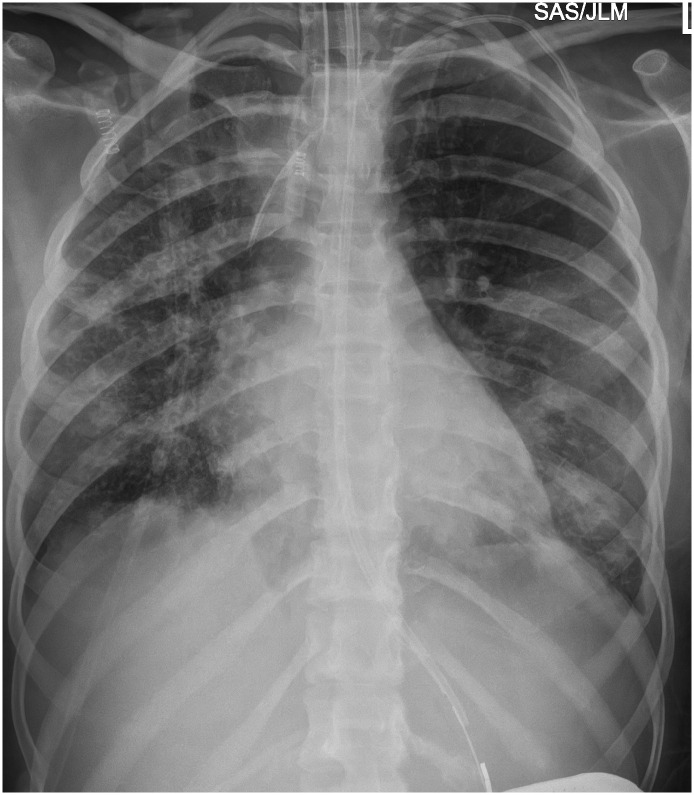
Fig. 1.
Frontal chest radiograph shows multifocal airspace and reticular opacities in a patient with Acute Myelogenous Leukemia.
Chest imaging of three of the four patients with severe illness (all of whom had hematologic malignancies) yielded findings of multifocal airspace opacities consistent with pneumonia. The fourth patient passed away without documented chest imaging during the study period. This patient did have multifocal airspace opacities with clinical manifestations of pneumonia in early January 2020–12 weeks prior to his COVID-19 diagnosis. It is possible that this earlier episode was the manifestation of COVID-19 but occurred prior to testing availability. Among the seven patients with hematological malignancies and without severe disease, one showed multifocal airspace opacities, two had no chest imaging and the remainder had clear lungs (Fig. 1).
Of the 30 patients with solid tumors, 23 patients underwent lung imaging. Of these, no patients demonstrated multifocal airspace opacities. In contradistinction to the positive findings seen in the hematologic subgroup, the imaging findings in the solid tumor group were far less pronounced, most of whom had clear lungs with a distinct minority showing only atelectasis or Peribronchial thickening (Table 1, Table 2 ). One patient in this group required admission with imaging revealing only atelectasis. This patient had a history of comorbid hypertension.
Table 1.
Imaging data for patients with hematological cancers.
| Cancer type | Imaging result | Course | Treatment at time of COVID-19 Diagnosis | Steroid administration |
|---|---|---|---|---|
| ALL | No imaging during study period Coronavirus positive based on viral panel NOT specific for COVID-19 variant. Chest radiograph consistent with pneumonia. COVID-19 positive over one month later | Demise | Maintenance | ON steroids |
| ALL | No imaging | Fever to 103F, required supplemental O2 for hypoxia. | Maintenance | ON steroids |
| AML | Multifocal airspace opacities and pleural effusions. Reticular pattern bilateral | Demise | Maintenance | ON steroids |
| B-cell lymphoma | Multifocal airspace opacities | Ventilator | Induction | ON steroids |
| B-cell ALL | Clear | One febrile episode – otherwise asymptomatic | Induction | No steroids |
| Diffuse large B-cell lymphoma | Multifocal airspace opacities | |||
| Ventilator | Off treatment | No steroids | ||
| B-cell ALL | Clear | Symptomatic admitted and treated at outside hospital | Maintenance | On steroids |
| B-cell ALL | Clear | Fever; otherwise asymptomatic | Maintenance | NO steroids |
| ALL | Clear | Admitted to ICU but no ventilatory support needed | Maintenance | On steroids |
| ALL | Clear | Shortness of breath, night sweats and fevers (possibly from malignancy/possibly COVID-19 or combination) | None | On steroids |
| Histiocytic sarcoma | Infectious patchy opacities increased and then cleared | 102 fever for 2 days | Maintenance | No steroids |
| AML | No lung imaging | Fever and cough | Off treatment | On steroids |
Table 2.
Imaging data for patients with non-hematological cancers.
| Cancer type | Imaging result | Course | Treatment at the time of COVID-19 diagnosis | Steroid administration |
|---|---|---|---|---|
| Wilms tumor | Peribronchial thickening | Cough, otherwise asymptomatic | Active treatment | On steroids |
| Neuroblastoma | Clear lungs | 3 days of flu like symptoms | Active treatment | No steroids |
| Embryonal rhabdomyosarcoma | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Leiomyosarcoma | Atelectasis | Hospitalized with cough, SOB, fever, respiratory distress | Active treatment | No steroids |
| Mixed germ cell tumor testis | Clear lungs | Slight SOB, fevers | None | No steroids |
| Calcifying nested stromal epithelial tumor | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Osteosarcoma | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Undifferentiated Sarcoma | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Osteosarcoma | Clear lungs | Fever, but otherwise asymptomatic | Active treatment | No steroids |
| Osteosarcoma | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Neuroblastoma | Clear lungs | Cough and body aches, low grade fever | Active treatment | No steroids |
| Astrocytoma | No lung imaging | Two days dry cough | Active treatment | NO steroids |
| Osteosarcoma | No lung imaging | Asymptomatic | Active treatment | No steroids |
| Osteosarcoma | Clear lungs (besides nodules on CT from known metastatic osteosarcoma) | Cough and fever | Active treatment | On steroids |
| Neuroblastoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Malignant peripheral nerve sheath tumor | No lung imaging | Flu like symptoms | Active treatment | ON steroids |
| Mixed germ cell tumor testis | No lung imaging | Asymptomatic | None | ON steroids |
| Osteosarcoma | Clear lungs | Asymptomatic | None | ON steroids |
| Synovial cell | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Epithelioid sarcoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Optic nerve glioma | No lung imaging | Asymptomatic | Active treatment | No steroids |
| Neuroblastoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Neuroblastoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Neuroblastoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Neuroblastoma | Clear lungs | One day of (after 10 days of covid+) cough, nasal congestion | Active treatment | No steroids |
| Pilocytic astrocytoma | No lung imaging | Chills/vomiting/fever | Active treatment | No steroids |
| Medulloblastoma | No lung imaging | Fever, otherwise asymptomatic | Active treatment | No steroids |
| Ewing sarcoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
| Wilms tumor | Clear lungs | Asymptomatic | Active treatment | On steroids |
| Ovarian mucinous cystadenocarcinoma | Clear lungs | Asymptomatic | Active treatment | No steroids |
4. Discussion
Consistent with prior work, our data suggest that children with hematologic malignancies are at much higher risk of acquiring severe disease from COVID-19 as compared with children with solid tumors. In parallel, we have shown that chest imaging is more likely to reveal multifocal pneumonia in the former group. In fact, although the cohort was small, multifocal pneumonia based on imaging was always associated with clinically severe disease in the hematologic group. Notably, patients with clear lungs on imaging but clinically symptomatic were represented in both groups.
Besides the variability in chemotherapy treatments, concurrent administration of glucocorticoids – often used in cancer treatments – present a confounding variable due to their known immunosuppressive role. Notably, three of the four patients (75%) with severe disease – all of whom were in the hematologic cancer group – had been given steroids prior to their COVID-19 diagnosis. However, the use of steroids is likely more a marker of having hematologic malignancy as 67% of all such patients received steroids whereas only 37% of patients with solid tumors did. Moreover, none of the 11 patients with solid tumors receiving steroid treatment had severe disease.
We did not find evidence of disease plausibly related to COVID-19 outside of the lungs. Such findings are well documented in others' work. For example, imaging manifestations of multifocal inflammatory syndrome [MIS] has been described elsewhere.10., 11., 12. As MIS is rare, our cohort was probably simply not large enough to capture such a case. However, it does appear from this data that MIS in children with cancer is not common.
There are several limitations to our study. The sample size is small, and the dataset may be underpowered for detection of additional correlations between imaging, symptomatology and outcome. An additional limitation includes bias toward hospitalized patients as these patients all required COVID-19 testing (regardless of symptomatology), potentially skewing the data toward patients who were more ill. Many clinic outpatients were also tested though many may have postponed visits during the peak of the crisis. Another limitation was lack of imaging available for several patients. In fact, one patient who had Acute Lymphoblastic Leukemia and passed away did show multifocal pneumonia on chest imaging however we did not include this in the statistics because the imaging was done more than one month before COVID-19 PCR positivity. Interestingly, this patient was shown to have coronavirus on a viral panel at that time, but this was before awareness of community spread (January 2020) and thus before availability of the COVID-19 specific RT-PCR. However, inclusion of this patient would serve to have only strengthened the evidence supporting the notion of more profound disease seen on imaging in line with worse clinical status and outcomes in the hematologic malignant group.
The apparent more pronounced clinical and radiographic manifestations of COVID-19 in the hematologic disease cohort can be reasonably suspected to be due to the disease itself as leukocytes are directly involved by the malignant process. However, although the implied causation is likely, caution is warranted given the confounding effects of differing therapies between patients with hematologic malignancies and those with solid tumors, as a group. A study of older adults with Chronic Lymphocytic Leukemia (CLL), however, does offer some evidence that a hematologic malignancy directly renders a patient highly susceptible to serious disease from COVID-19. CLL is unique in that many patients have indolent disease and may not be initially treated which offers a subgroup to study morbidity in a treatment naïve cohort. Interestingly, Mato, et al., have shown that such patients are at high risk of serious disease and death, regardless of treatment status, suggesting it is not the treatment itself that introduces the risk.13 Their data cannot be necessarily extrapolated to the pediatric population where CLL is a very rare occurrence, but their data does support the notion of a causative link.
Our data showing differences in susceptibility to severe disease between hematologic and solid malignancies is in line with prior reports. Based on this data, it appears that multifocal pneumonia on imaging is more frequent in and portends a poorer outcome in children with both a diagnosis of hematologic cancer and COVID-19.
Funding source
Supported by NIH Core Grant (P30 CA008748).
Declaration of competing interest
None.
References
- 1.Wald E.R., Schmit K.M., Gusland D.Y. A pediatric infectious disease perspective on COVID-19. Clin Infect Dis. 2021;72(9):1660–1666. doi: 10.1093/cid/ciaa1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan,China. Ann Oncol. 2020;31(7):894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salunke A.A., Nandy K., Pathak S.K., Shah J., Kamani M., Kotakotta V., et al. Impact of COVID-19 in cancer patients on severity of disease and fatal outcomes: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1431–1437. doi: 10.1016/j.dsx.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdal G.S., Polat O., Erdem G.U., Korkusuz R., Hindilerden F., Yilmaz M., et al. The mortality rate of COVID-19 was high in cancer patients: a retrospective single-center study. Int J Clin Oncol. 2021;26(5):826–834. doi: 10.1007/s10147-021-01863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulad F., Kamboj M., Bouvier N., Mauguen A., Kung A.L. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6(9):1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hrusak O., Kalina T., Wolf J., Balduzzi A., Provenzi M., Rizzari C., et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Cancer. 2020;132:11–16. doi: 10.1016/j.ejca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yigenoglu T.N., Ata N., Altuntas F., Bascı S., Dal M.S., Korkmaz S., et al. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93(2):1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumfield E., Levin T.L., Kurian J., Lee E.Y., Liszewski M.C. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19) Am J Roentgenol. 2020;216(2):507–517. doi: 10.2214/AJR.20.24032. [DOI] [PubMed] [Google Scholar]
- 11.Feldstein L.R., Tenforde M.W., Friedman K.G., Newhams M., Rose E.B., Dapul H., et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–1087. doi: 10.1001/jama.2021.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am J Emerg Med. 2021;39(253):e1–e2. doi: 10.1016/j.ajem.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]