PURPOSE
Ongoing supportive care using electronic health (eHealth) interventions has the potential to provide remote support and improve health outcomes for patients with breast cancer. This study aimed to evaluate the effectiveness of eHealth interventions on patient-reported outcomes (quality of life [QOL], self-efficacy, and mental or physical health) for patients during and after breast cancer treatment and patient-reported experience measures (acceptability and engagement).
METHODS
Systematic review with meta-analyses (random-effects model) of randomized controlled trials was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. Nine databases were searched using a prespecified search strategy. Patient-directed eHealth interventions for adult patients during or after active breast cancer treatment measuring QOL, self-efficacy, and mental (depressive, anxiety, and distress symptoms) or physical (physical activity, nutrition, and fatigue) health outcomes were included. Data from eligible full-text articles were independently extracted by six observers.
RESULTS
Thirty-two unique studies (4,790 patients) were included. All were health self-management interventions, and most were multicomponent (videos, forums, and electronic reminder systems) websites. Meta-analyses revealed a significant effect of eHealth interventions on QOL (standardized mean difference [SMD], 0.20 [95% CI, 0.03 to 0.36]), self-efficacy (SMD, 0.45 [95% CI, 0.24 to 0.65]), distress (SMD, –0.41 [95% CI,–0.63 to –0.20]), and fatigue (SMD, –0.37 [95% CI, –0.61 to –0.13]). Twenty-five studies (78.1%) measured patient-reported experience measures. Acceptability (n = 9) was high, with high ratings for satisfaction (range, 71%-100%), usefulness (range, 71%-95%), and ease-of-use (range, 73%-92%). Engagement (n = 25) decreased over time, but disease-focused information and interactive support were most engaging.
CONCLUSION
eHealth interventions may provide an acceptable and effective strategy for improving QOL, distress, self-efficacy, and fatigue among patients with breast cancer.
INTRODUCTION
Breast cancer is the most common cancer diagnosed among women worldwide.1 During active treatment (surgery, chemotherapy, and/or radiotherapy) and the years after (survivorship), many patients experience adverse side effects, including depression, anxiety, and fatigue, which can negatively affect quality of life (QOL).2-4 The resulting economic burden is high because of costs of procedures, hospital visits, and loss of productivity.5,6 Ongoing supportive care and health promotion during and after treatment may reduce this burden and improve QOL among survivors.4,7,8
CONTEXT
Key Objective
Are electronic health (eHealth) interventions (eg, websites and apps) effective for improving patient-reported outcomes among patients during and after breast cancer treatment? This systematic review with meta-analyses investigated the effectiveness, external validity, and patient-reported experience measures of eHealth interventions within the context of the Reach, Effectiveness, Adoption, Implementation and Maintenance framework.
Knowledge Generated
Our findings suggest that eHealth interventions had a broad reach and high uptake from a diverse (international and multilingual) sample of breast cancer survivors. Overall, eHealth interventions were effective for improving patient-reported outcomes (quality of life, self-efficacy, fatigue, and psychologic distress), and repeated contact with health professionals, interactive disease-specific features, and optional content may be key to effectiveness.
Relevance
eHealth interventions may provide an acceptable and feasible strategy to deliver continuity of health support to patients between medical appointments.
In-person support (eg, exercise programs and psychoeducation) during and after treatment can improve patient-reported health outcomes (PROs) such as anxiety, depression, physical activity, and QOL.9,10 Most programs promote self-management, which can improve self-efficacy11 and indirect benefits.9,10 Moreover, leading a healthy lifestyle can reduce risks of cancer recurrence and mortality.12,13 However, attending in-person visits can be difficult (location, cost, and work)5 and was exacerbated by cancer care closures during the global COVID-19 pandemic.14,15
Electronic health (eHealth) is an accessible strategy to deliver health information. eHealth platforms (eg, websites and videoconferencing) have proliferated during the COVID-19 pandemic.14,15 Telehealth replaced many in-person appointments16 and offers a scalable and flexible way to provide support, track PROs,16-19 and enable continuity of care between hospital visits.16 Importantly, eHealth strategies are well liked by patients in terms of acceptability and usefulness20,21 and can promote patient-centered medicine through codesign.22 There is growing systematic review evidence for eHealth interventions effectiveness for physical activity23,24 and PROs (QOL, stress, fatigue, and sleep)19,25,26 for patients with breast cancer. However, these often only include one health outcome and/or do not include meta-analyses or evaluations of barriers and enablers to implementation. A high-quality synthesis of randomized controlled trials (RCTs), evaluating numerous health outcomes during and after treatment and evaluating patient-reported experience measures (PREMs) such as acceptability, usefulness, and engagement of eHealth intervention features, is needed to inform future eHealth intervention development and attrition reduction.26 Therefore, this study aimed to investigate the effectiveness of eHealth interventions to improve PROs (QOL, self-efficacy, and mental [anxiety, depression, and distress] and physical [physical activity, nutrition, and fatigue] health) during and after breast cancer treatment. Implementation was also evaluated using the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM framework)27 plus PREMs to inform common features of effective interventions.
METHODS
A systematic review with meta-analyses was registered (The International Prospective Register of Systematic Reviews registration number CRD42019122689) and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines28,29 (Data Supplement 1, online only). Table 1 reports the study Population, Intervention, Comparator, Outcomes, and Settings inclusion criteria.
TABLE 1.
Study Inclusiona Criteria, Defined by the PICOS
Information Sources and Searches
Nine electronic databases (inception to present) were searched on October 21, 2019, and updated on June 27, 2021: PreMEDLINE, MEDLINE via OvidSP, Cochrane Central Registry of Controlled Trials, Embase via OvidSP, PsycINFO via OvidSP, Allied and Complementary Medicine, Scopus, Web of Science, and Cumulative Index to Nursing and Allied Health Literature via EBSCO. The search included truncations and synonyms of the following terms: breast neoplasms, breast cancer, breast tumor, mobile phone, smartphone, text-, electronic-, or multimedia-message, electronic mail, phone applications, computer, podcast, videos, internet, website, chatroom, message board, activity tracker, electronic health, mobile health, telemedicine, and electronic learning (Data Supplement 1).
Study Selection
Search results were exported to a citation management software program. Following removal of duplicates, titles and abstracts were independently reviewed by A.C.S. (all articles) and J.T.-K. and S.C.M.S. (each reviewed half) against inclusion criteria. Abstracts with unclear information were included for full-text review. Full-text articles were reviewed by A.C.S. (11 articles), R.R. (11 articles), N.H. (14 articles), J.T.–K. (10 articles), S.C.M.S. (11 articles), and Q.T. (11 articles). A.C.S. reviewed all articles to confirm inclusion or exclusion.
Data Collection Process
A prespecified electronic data extraction table following Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines28,29 and Cochrane Collaboration's Risk of Bias (RoB) tool30 was developed to extract (1) study information (author, year, country of origin, study design, and sample size); (2) participant information (demographics and medical history); (3) intervention and follow-up durations; (4) intervention and control group details; (5) primary and secondary outcomes; and (6) Cochrane RoB measures. A.C.S. pilot-tested the data extraction table with two articles (reviewed by R.R.). Data from full text articles were extracted by authors J.T.-K. (6 articles), S.C.M.S. (4 articles), R.R. (2 articles), A.C.S. (6 articles), N.H. (7 articles), and Q.T. (4 articles). A.C.S. reviewed all data for accuracy.
Risk of Bias
The Cochrane Collaboration RoB tool was used to assess RoB of included studies.30 Domains for assessment included selection bias, including sequence generation and allocation sequence concealment, performance or detection bias via blinding of participants, personnel and outcome assessors, attrition bias via incomplete outcome data, and reporting bias via selective outcome reporting. Criteria for low, unclear, and high RoB within and across studies followed the Cochrane Handbook for Systematic Reviews of Interventions. RoB was independently assessed by authors J.T.-K. (6 articles), S.C.M.S. (4 articles), R.R. (2 articles), A.C.S. (12 articles), N.H. (7 articles), and Q.T. (4 articles). A.C.S. reviewed all RoB assessments to confirm accuracy.
RE-AIM Framework
The RE-AIM framework27 was used to evaluate potential broader impacts of eHealth interventions. Reach and representativeness was evaluated using the percentage of eligible patients enrolled in the study (n enrolled/n eligible × 100) and participant demographics (ethnicity, language, employment [part-/full-time], and education level). Efficacy was evaluated using the primary outcome's effect size (95% CI). Barriers to adoption were evaluated by who (personnel) recruited participants and where (setting). Implementation was evaluated by (1) adherence to intervention (eg, percentage of opened modules and completed intervention components), (2) percentage of dropouts of the most complex intervention (n postintervention follow-up/n baseline × 100), (3) intervention cost, and (4) author-reported plans to upscale or implement. Maintenance was evaluated by (1) time (months) results were maintained and (2) when intervention would become available (author-reported).
Statistical Methods
Primary and secondary outcome means and standard deviations (SDs) at postintervention follow-up for intervention and control groups were converted to standardized mean difference (SMD), using Hedges' G.31 Acknowledging differences across studies because of the varied population, length of intervention and length of follow-up, meta-analyses were performed fitting random-effects models32; restricted maximum-likelihood method was used to estimate and pool outcome SMDs (Hedges' G method) and 95% CI.
Quantitative heterogeneity was assessed by reporting the between study variance τ2, the 95% prediction intervals (which give an estimate where true effects are to be expected for 95% of similar studies that might be conducted in the future), and I2 (the proportion of variability attributable to heterogeneity rather than sampling error). We also conducted a formal test of homogeneity on the basis of Cochran's Q test (P < .1 considered statistically significant) and a series of univariable meta regressions, considered—as fixed-effect covariates—the following variables: population (patients; survivors; and patients and survivors), length of intervention (months), and follow-up (months). We also performed outlier detection and influence analyses using the leave-one-out method and reported Baujat plots to graphically display studies that overly contribute to the heterogeneity.
If studies included multiple intervention arms, only the most complex intervention was used in the meta-analyses, defined as having the largest number of intervention components. Several studies reported different measures of the same or multiple outcomes. To overcome multiplicity, the reductionist approach was used to select the most common measure used between studies for each outcome and separate meta-analyses, and forest plots were performed for each outcome.33 Outcome variables included in ≤ 2 studies were summarized in-text. For each outcome, small-study effects were evaluated using funnel plots and, when > 10 studies were included in the meta-analysis, regression-based Egger's test for funnel plot asymmetry was used. Statistical analyses were conducted according to the prespecified statistical analysis plan (The International Prospective Register of Systematic Reviews registration CRD42019122689) using RStudio version 1.3.1093.
RESULTS
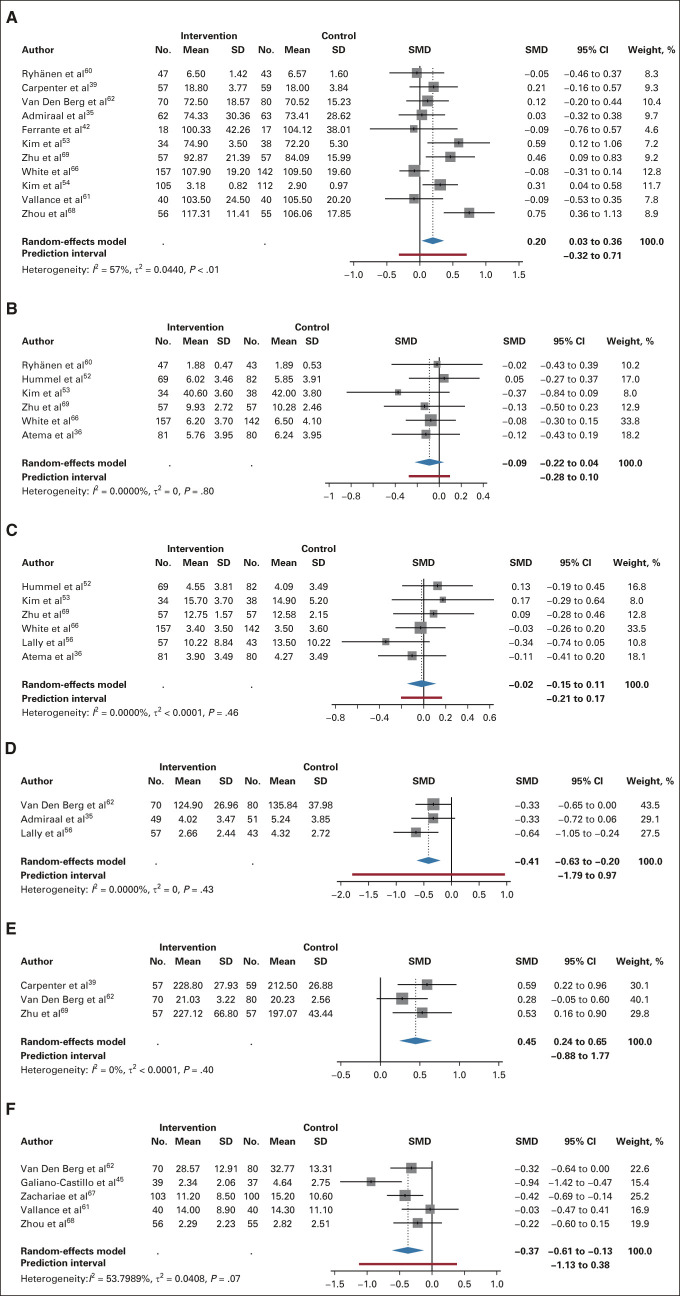
Thirty-six full-text articles34-69 with 32 unique studies were identified after removing duplicates and screening (Fig 1), representing 4,790 unique patients (Data Supplement 2, online only). Three studies49,50,55 used the same study population but only postintervention follow-up (6-month50) outcomes are reported. Moreover, participant recruitment for several studies overlapped.37,49,50,54,55 Only unique intervention groups are compared with control for each study. Ten34,38,40,43,44,48,58,59,64,65 studies were assessed to have high RoB (Data Supplement 1), plus one study was an outlier with high heterogeneity contribution to QOL45,46 (Data Supplement 1) and were therefore excluded from meta-analyses.
FIG 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 flow diagram of included studies for meta-analyses. eHealth, electronic health; RCT, randomized controlled trial.
Study Characteristics
Characteristics of included studies are summarized in Data Supplement 2. All studies were conducted in high-income countries, as defined by the 2020 World Bank gross national income per capita ≥ $12,696 US dollars.70 Fourteen (43.8%) studies recruited patients during treatment (patients),37,38,43,47-51,54,56,60,64,67-69 12 (37.5%) recruited patients after active treatment (survivors),34-36,41,42,44-46,52,57,61-63,65 five (15.6%) recruited patients and survivors,39,40,58,59,66 and one (3.1%) recruited patients with metastatic breast cancer.53 All participants were female (pooled mean age [pooled SD] 51.7 [8.9] years), with 26 studies (81.2%) having female sex as an inclusion criterion34-42,47,49-54,56-62,64,66-69 (Data Supplement 1). Most participants (3,644/4,790; 76.1%) were diagnosed with early stage (0-III) breast cancer,34,35,37-51,54,56-62,64-69 and 18 studies (56.2%)34-38,41,42,44-46,49,50,52,56,58,63,65-67 reported time since breast cancer diagnosis (pooled mean [pooled SD] 23.0 [17.0] months). Studies varied in their reporting of participants' medical history. The proportion of studies that reported that participants had a history of following medical treatments was: surgery 23/32 (71.9%) studies,34-36,39,41-46,51-53,56-58,60-62,64-69 chemotherapy (22/32; 68.8%),34,36,39,41-46,48,51-53,56,58,60-62,64-69 radiotherapy (19/32; 59.4%),34-36,39,41,42,44-46,51,52,56,58,60,62,64-68 endocrine therapy (15/32; 46.9%),34-36,41,42,44,51,52,60,62,64-67,69 and targeted therapy (9/32; 28.1%).34-36,41,42,52,60,65,66
Overview of eHealth Interventions
All interventions were multicomponent and promoted self-management. Most studies (24/32; 75.0%)34-38,41,42,45-47,49,50,52,54,56,60,62-67 used a website or web-based app. Five studies reported versions of the Comprehensive Health Enhancement Support System (CHESS) website.37,47,49,50,54,55 Seven interventions (21.9%) were mobile applications48,53,57,59,61,68,69 and two included a smartwatch.42,57,61 Interventions included interactive features such as videos (20/32; 62.5%37,41,42,45-47,49,50,54,56,60,62,64,65,67), peer-support via chatrooms (9/32, 28.1%37,38,47,49,50,54), instant messaging (8/32, 25.0%37,38,45-47,49,50,54,68,69), video (4/32, 12.5%34,44-46,65), or telephone consultations (13/32; 40.6%34-37,39,44-47,49,50,54-57,61) with health professionals. Some also included e-mails (12/32; 37.5%34,35,38,40-42,52,56,58,59,62,65,67,69) and text message reminders (3/32; 9.4%)38,42,59 to engage with websites. Twenty (62.5%) interventions included repeated (> 1) contact with researchers or health professionals34,36-40,42,44-46,49,50,52-57,59-61,65,68,69 and 7/32 (21.9%)37,42,44,49,50,54,57,61,65 provided participants with required technology. Six (18.8%) interventions were codesigned with patients.35,38,60,62,64,66 Intervention duration ranged from 3 weeks53 to 9 months.60,64 Most primarily focused on QOL37,43-47,49,50,55,57-61,68 or mental health (depressive symptoms, anxiety symptoms, and distress),38,43,48,56,62,65,66 and some on physical health (physical activity, fatigue, and nutrition)34,57,61 or self-efficacy.39,64,71 Process evaluations were collected in 25/32 (78.1%) studies (Data Supplement 1).34-36,38,39,41-50,52,53,56-59,61,62,64-67,69,71,72 Measurement details (questionnaires and domains) and data inclusions and exclusions for the meta-analyses are presented in Data Supplements 1 and 2.
Patient-Reported Outcomes
Quality of life.
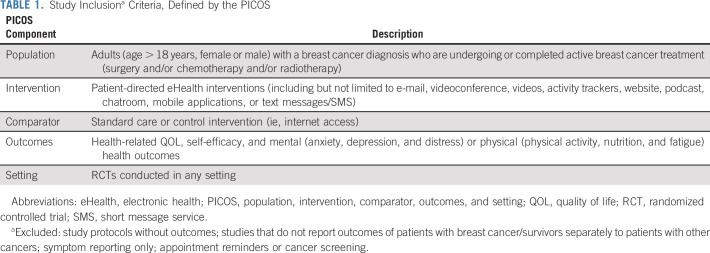
QOL was measured in 25/32 (78.1%) studies (Data Supplement 2),34-37,39-42,44-47,49-54,57-62,65,66,68,69 was the primary outcome in 12/25 (48.0%; Data Supplement 1), and 18/25 (72.0%) interventions included repeated health professional or researcher contact.34,36,37,39-42,44-47,49,50,52-54,57,59,61,65,68,69 Eight studies found a significant effect of their intervention (Data Supplement 1)34,45-47,53,54,59,68,69; all included repeated health care professional or researcher contact and 4/8 (50.0%) had QOL as the primary study outcome. Five health-related QOL measures, validated in patients with breast cancer, were used: European Organisation for the Research and Treatment of Cancer QOL Questionnaire C30,34,35,41-43,45,46,51,62,65,73 WHO QOL-BREF,53,54,74 Functional Assessment of Cancer Therapy—Breast,37,39,40,47,49,50,57,58,61,62,65,66,68,69 QOL Adult Cancer Survivors,42,75 and QOL Breast Cancer Patient Version.60,76 Higher scores reflected higher QOL. Two outliers were identified40,45,46 (one with high RoB40). The outlier with low RoB had a strong positive effect on QOL. It was the only tailored eHealth exercise program with individual supervision and repeated contact with researchers, and participant adherence rates were high (93.9%). After excluding outliers40,45,46 and studies with high RoB,34,40,44,58,59,65 a meta-analysis (n = 11) comparing intervention and control groups at the end of intervention demonstrated a SMD of 0.20 (95% CI, 0.03 to 0.36) increase in QOL favoring the intervention (Fig 2A; Data Supplement 1). Moderate heterogeneity was found between studies with τ2 of 0.04 and I2 = 57% (P < .01). Of those with a significant difference,53,54,68,69 all included personal contact via e-mail, telephone, or chat-room, and were multicomponent apps53,68,69 or websites.54 Patient type was a significant moderator for QOL, where studies that only included patients were more likely to result in higher QOL than studies including patients/survivors or only survivors (Data Supplement 1). Age, intervention period, and postintervention follow-up were not significant moderators.
FIG 2.
Forest plot of (A) quality of life, (B) anxiety symptoms, (C) depressive symptoms, (D) psychologic distress, (E) self-efficacy, and (F) fatigue outcomes for electronic health randomized controlled trials. SD, standard deviation; SMD, standardized mean difference.
Anxiety and depressive symptoms.
Anxiety36,38,48,52,53,60,64,66,69 and depressive symptoms36,38,48,52,53,56,64,66,69 were measured in nine studies (Data Supplements 1 and 2). Five (55.6%)36,38,52,53,69 interventions had repeated health professional or researcher contact; one38 found a significant effect but had high RoB (Data Supplement 1). Two anxiety and three depressive symptom measures were used: Hospital Anxiety Depression and Stress scale (anxiety or depression subscales),36,38,41,52,64,66 Beck's Depression Index,53 Center for Epidemiologic Studies Depression Scale,56 Spielberger State-Trait Anxiety Scale.53,60 All scales are reliable and valid measures of transient (state) anxiety or depressive symptoms in patients with breast cancer.77-80 Higher scores reflect higher anxiety or depressive symptoms. After excluding two studies with high RoB,38,64 a meta-analysis comparing anxiety (n = 6; Fig 2B) or depressive symptoms (n = 6; Fig 2C) between intervention and control groups at postintervention follow-up demonstrated no significant differences. Low heterogeneity was found between studies (anxiety symptoms: τ2 = 0, I2 = 0.0%, P = .80; depressive symptoms: τ2 = 0, I2 = 0.0%, P = .46). Patient type, age, intervention period, and postintervention follow-up were not significant moderators (Data Supplement 1).
Psychologic distress.
Nine studies measured psychologic distress (Data Supplement 2).34,35,38,41,43,44,56,62,65 Five34,38,43,56,62 found a significant intervention effect, with 4/5 (80%) having distress as the primary study outcome (Data Supplement 1). Four distress measures were used: Memorial Symptom Assessment Scale,38,41,43 Dutch Distress Thermometer,35,62 Distress Symptom Checklist,62,65 and Brief Symptom Inventory.34,44 All are reliable and valid measures of psychologic distress in patients with breast cancer81-83; higher scores reflect higher levels of distress. After excluding studies with high RoB,34,38,44,65 a meta-analysis (n = 3)34,35,38,56,62,65 comparing intervention and control groups at postintervention follow-up demonstrated a SMF of –0.41 (95% CI, –0.63 to –0.20) reduction in distress (Fig 2D). Low heterogeneity was found between studies (τ2 = 0, I2 = 0.0%, P = .43). Interventions with a significant improvement in psychologic distress were self-guided35,62 or health professional–supported (repeated contact via e-mail or telephone)56 multicomponent interactive websites with psychoeducation. Patient type, age, intervention period, and postintervention follow-up were not significant moderators (Data Supplement 1).
Self-efficacy.
Self-efficacy was measured in seven studies38-40,42,62,64,69 using validated scales and domains: Cancer Behavior Inventory (self-efficacy for coping with cancer),38-40,84 Stanford Inventory of Cancer Patient Adjustment,69,85 Self-Efficacy Scale,62 CHESS instrument (health self-efficacy),64 and Health Belief Survey (self-efficacy for physical activity and healthy eating42; Data Supplements 1 and 2). After removing two studies with high RoB,38,40,64 a meta-analysis was conducted for self-efficacy for coping with cancer (N = 3)39,62,69 comparing intervention to control at postintervention follow-up and found a 0.45 (0.24 to 0.65) increase in self-efficacy (Fig 2E). Low heterogeneity was found between the studies (τ2 < 0.001, I2 = 0%, P = .40). All three studies found a significant positive effect of the intervention on self-efficacy compared with usual care at follow-up, with 2/3 (66.7%) being the primary outcome. Interventions were multicomponent (videos, discussion groups, and e-mails) web- or app-based self-management programs promoting psychologic adjustment and health tracking during and after treatment. Two39,69 included repeated researcher or health professional contact and one provided automated weekly e-mails about new website content.62 Age, intervention period, and postintervention follow-up were not significant moderators (Data Supplement 1). Association between patient types could not be analyzed as there was one study in each patient type.
Fatigue.
Fatigue was measured in seven studies34,44-46,57,61,62,67,68 using validated measures: Checklist Individual Strength (Fatigue Severity Scale),34,62 Piper Fatigue Scale,45,46 Functional Assessment of Chronic Illness Therapy-Fatigue,57,61,67 Numerical Rating Scale (Fatigue subscale68; Data Supplements 1 and 2). Functional Assessment of Chronic Illness Therapy–Fatigue was reverse-scored; therefore, higher scores reflected higher levels of fatigue for all scales. After removing two studies with high RoB,34,44 a meta-analysis (n = 5) comparing intervention to control at postintervention follow-up found a –0.37 (–0.61 to –0.13) reduction in fatigue (Fig 2F). Moderate heterogeneity was found between studies (τ2 = 0.04, I2 = 54%, P = .07). Four studies reported significant improvements in fatigue at follow-up and three34,45,46,57,61 included repeated health professional or researcher contact. Four studies57,61,62,67,68 used multicomponent web-based psychoeducation, one included a website, mobile app, and smartwatch,57,61 and one included a web-based exercise program.46 Patient type, age, intervention period, and postintervention follow-up were not significant moderators (Data Supplement 1).
Other Outcomes
Three studies measured physical activity42,45,46,57,61; all were multicomponent websites and two included a smartwatch and repeated health professional or researcher contact42,57,61 (Data Supplement 2). The web-based exercise program45,46 and website plus smartwatch and mobile app57,61 found significant improvements in physical activity, but the interactive website with smartwatch and text message reminders (n = 37) did not.42 This study aimed to improve body mass index and nutrition42 but there was no difference between groups at follow-up.
Patient-Reported Experience Measures
Acceptability.
Nine studies (34.6%) evaluated participants' perceived intervention acceptability.34,35,39,42,45,46,53,56,58,65 Most participants found psychoeducational websites acceptable (satisfaction: 71%-100%34,35,39,45,46), useful (71%-95%35,42,45,46,56,72), easy to use (73%-92%42,56,72), and easy to understand (98%-100%45,46,56,72). One study reported that participants found written and video content more useful than psychoeducational activities (76%, 69%, and 49%, respectively),56,72 and participants of a web-based exercise program found videos valuable (mean rating: 3.8/4; 95%).45,46
Engagement.
Twenty-five studies (78.1%) evaluated participants' intervention engagement (logins, completed modules, and usage tracking).34-36,38,39,41-50,52,53,56-59,61,62,64-67,69,72 Participants' engagement was broad, completing 0%-100% modules. Most participants (61%-100%36,41,42,44,47,48,52,56,58,59,65-67,72) engaged with the intervention ≥ 1 time (login and opened module). However, nine studies reported engagement dropping over time41,42,47,48,52,56,58,65,67,72; 5/9 had repeated contact.42,47,52,56,65,72 For websites, participants engaged most with content about living with side effects, coping strategies,41,58 healthy living,41 advice,38 blogs,38 and discussion boards.58 One study found participant engagement with e-mails was consistently high; 35/37 participants (94.6%) engaged from baseline to 3 months.65 An avatar-based app game found quests, level-ups, and rewards most engaging.53
Risk of Bias
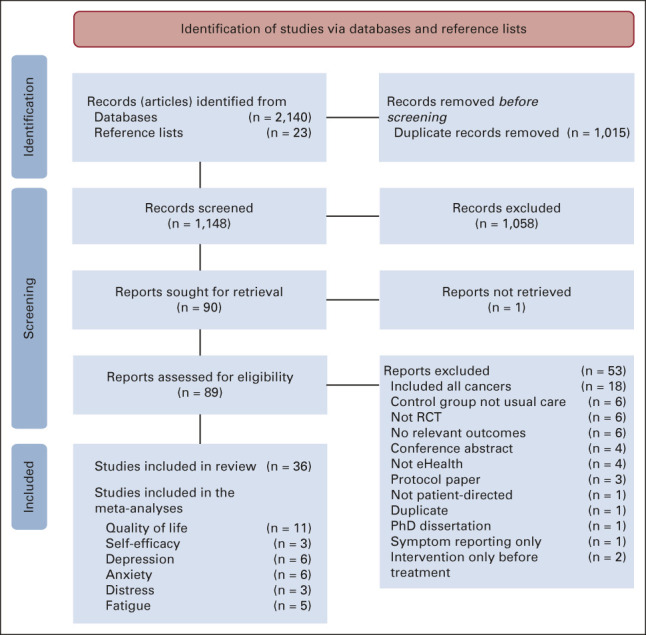
RoB within 29/32 (84.4%) studies was unclear35-37,39,41,42,47,48,51-54,56,57,60-63,66,67 or high34,38,40,43,44,49,50,58,59,64,65 because of lack blinding or issues with reporting attrition rates or study protocols (Fig 3; Data Supplement 1). Most studies adequately generated34-43,45,46,48-51,53,54,56-64,66-69 and concealed allocation.34-43,45-47,49-51,54,56,57,59-64,66-69 Patient blinding was not possible because of the nature of eHealth interventions and was not considered to increase RoB. However, 22 studies35-42,44,47-50,52-54,56,57,59-63,66 (68.8%) presented insufficient information to decide (unclear risk) regarding researcher and/or outcome assessor blinding, and four reported not blinding researchers (high risk).34,43,58,65 Twenty-four (75.0%) studies34-36,39,41-43,45-48,51-53,56-58,60-63,65-69 reported complete outcome data (low risk) and two had insufficient detail (unclear risk).37,54 In six studies,38,40,44,49,50,59,64 attrition was high or varied between groups, but comparisons or reasons for attrition were not provided. Finally, 20/32 (62.5%)35,37-42,44,47,48,50,51,54,56,58,60,64-69 did not reference a protocol or trial registration (unclear risk). No significant publication bias was found from assessing funnel plots except for distress and fatigue.
FIG 3.
Cochrane risk of bias scores (%low, unclear, and high risk) across bias domains (selective reporting, incomplete outcome data, blinding, allocation concealment, and random sequence generation) for the 18 included breast cancer electronic health studies. RoB, risk of bias.
RE-AIM Framework
The results are presented in Data Supplement 2. Twenty-eight (87.5%) studies reported reach, with 16/28 (57.1%) reporting 71.9%-92.5% of eligible patients enrolled.41-43,45-48,51-53,56,57,59,61,62,66,68,69 Ten (69.2%) studies reported 11 ethnicities (Data Supplement 1). Thirty-one studies reported participants' main language, resulting in 10 unique languages (Dutch, English, Norwegian, Mandarin, Swedish, Spanish, Japanese, Korean, Finnish, and Danish). Non-English speakers were excluded in 10/32 (31.2%) studies37,39,42,44,47,49,50,54,56,57,61,66 (United States and Australia). Twenty-two (68.8%) studies reported employment status; 16.7%-80.3% of participants were employed part- or full-time. Education levels were reported in 29/32 (90.6%) studies, with 20/32 (62.5%)34,37-40,42,44-46,48-52,54,56,57,59,61,64,66,67 reporting > 50% of participants had some university education or higher.
Efficacy (effect size [95% CI] of primary outcome) was reported in 15/32 (46.8%) studies34-36,39,43,45-47,52,54,56,58,59,62,67,69 (Cohen's d or eta-squared); three studies had a large effect size,34,45,46,67 five medium,36,39,52,56,69 and seven small.35,43,47,54,58,59,62 For adoption barriers, health professionals or researchers conducted recruitment for all studies and 22/32 (68.8%) recruited participants in-person (hospital and cancer center). For implementation, intervention adherence ranged from 29%-100% of participants completing all scheduled components.34-36,38,39,41-50,52,53,56-59,61,62,64-67,69 Dropouts of the most complex intervention ranged from 1.8% to 37.5%, with 16/32 (46.9%) having ≤ 10% dropouts. Cost was reported in three studies, including a free website and app42,48 and paid app ($77 US dollars/6 months).59 Three studies42,51,59 reported plans to upscale, with the interventions already publicly available. Fourteen (43.8%) studies reported maintenance of results; 6/12 (50.0%) sustaining results for 1.5-12 months.36,39,41,45-47,59,62,67 Four studies reported if the intervention would become available, with three publicly available42,51,59 and one unlikely to become available because of capacity required.45,46
DISCUSSION
The current systematic review with meta-analyses and RE-AIM framework revealed that eHealth interventions had broad reach, with high uptake among diverse (international and multilingual) patients with breast cancer and a significant positive impact on PROs QOL, health self-efficacy, psychologic distress, and fatigue compared with control postintervention but not anxiety or depressive symptoms. The moderator analysis revealed improved QOL for patients compared with survivors. Intervention dropouts were low and PREMs revealed eHealth interventions were acceptable, useful, and easy to use, but attrition was common. Few studies reported maintenance of the results, intervention cost, or plans to upscale, and the RoB assessment highlighted variation in blinding procedures.
This review revealed that many interventions with a significant improvement in PROs (self-efficacy, QOL, distress, and fatigue) included repeated health professional or researcher contact. Moreover, improvements in QOL occurred during treatment, when patients interact regularly with their health care team.86,87 All interventions were multicomponent, and studies did not specify which component affected behavior change. However, PREMs revealed participants were most engaged with supportive features such as e-mails, telephone, chat functions, text messages,46 and health reminders.53 For example, the CHESS website was associated with improved social support by improving participants' information and emotional-social competence, therefore increasing emotional functioning and QOL.55 This is consistent with behavior change theories such as Social Cognitive Theory88 and Control Theory89 that posit providing encouragement, identifying barriers, and setting and reviewing behavioral goals support behavior change. Moreover, one video-based support group found participants who received peer support rated the intervention significantly higher than those who did not.65 Similarly, a systematic review of reviews found that eHealth interventions were effective for improving perceived support in patients with various cancers.90 However, the current systematic review revealed a paucity of studies reporting costs of staff time or plans to scale up, which mirrors RE-AIM findings of a multicomponent adult obesity behavior-change intervention.91 Other systematic reviews found eHealth interventions cost-effective across specialty areas (pulmonary, ophthalmology, cardiovascular, and public health),92,93 especially for people in rural areas. This review revealed that incorporating optional low-cost support features such as e-mails, text messages, or chat functions with peers or health professionals may be beneficial, but economic evaluations are needed.
eHealth interventions did not improve anxiety and depressive symptoms. This result may be due to a floor effect, whereby participants' baseline anxiety and depressive symptom scores were within a healthy range. The incidence of anxiety and depression among patients with breast cancer ranges between 18%-33%94 and 9%-66%,94,95 respectively. Studies within this review did not recruit anxious or depressed patients. In primary care, some evidence suggests that eHealth interventions can decrease anxiety and depressive symptoms96 and there is growing evidence of benefits in cancer care.97 However, more research is needed to evaluate the effectiveness in patients with breast cancer with anxiety and depression.
Participants within the current systematic review found multicomponent eHealth interventions acceptable, useful, and easy to use, with few dropouts, but engagement reduced over time. This aligns with the Technology Acceptance Model,98 which posits that user acceptance, usefulness, and ease of use are critical to technology usage. Technology user attrition is common99 and attributed to a lack of perceived benefit and difficult-to-use interventions. Preprototype user acceptance testing98 or codesign has potential to improve delivery and engagement,100 but this review found that few interventions were codesigned. Overall, this review found that participants engaged most with information regarding side effects, healthy living,41 general advice,38 and interactive features (blog posts,38 e-mail contact,65 and incentives53). Other research found that participants were more likely to remain engaged if they enjoyed the intervention, found it useful, easy to use,99 easy to understand, and trustworthy.101 There is contradictory evidence that eHealth intervention personalization improves engagement or efficacy.102-105 However, differentiating between end-user and researcher-chosen personalization may be critical. For example, a recent review103 found that participants preferred interventions with interactive features that could be turned on/off. Gamification, incentives, and rewards may also improve engagement via extrinsic motivation.106 Future studies should consider using the TAM to codesign eHealth interventions with end users, and analyzing end-user personalization on engagement and health outcomes.
Although the current review summarized international RCTs targeting various PROs, there are limitations. First, all studies were conducted in high-income countries, with younger, highly educated women, and this may mean those with lower eHealth literacy were not included.107 Therefore, the results may not be generalizable to low- or middle-income countries or women of older age or less education. Second, RCTs recruiting patients with various cancers and summarized combined results were not included because it was not possible to determine results specific to breast cancer. Non-RCT designs (eg, adaptive trials)108 were also not included. Third, planned moderator analyses between sexes could not be conducted because all participants were female; most studies excluded men but this is a growing population with minimal support.109 Finally, the RoB assessment highlighted that most studies did not clearly report blinding procedures or protocols. Importantly, studies with high RoB were excluded from meta-analyses, which improved precision of the treatment effect and the reliability of pooled effects.
In conclusion, this systematic review with meta-analyses revealed that eHealth interventions had a significant positive impact on QOL, self-efficacy, distress, and fatigue at follow-up compared with usual care. Most interventions were multicomponent, web-based, health self-management programs. On the basis of patient preferences, future eHealth interventions should consider including practical disease- and health-management information via videos and written material, social support opportunities, and optional communication features. Importantly, interventions codesigned with end users may improve engagement.
ACKNOWLEDGMENT
The authors thank Caroline Wu for her constant support as a research manager. They also thank University of Sydney Librarian Tess Aitkin for her guidance and support with the search strategy.
Gian Luca Di Tanna
Honoraria: Amgen
Kerry A. Sherman
Research Funding: MedConsent (Inst)
Elisabeth Elder
Honoraria: Roche, MSD Oncology
Consulting or Advisory Role: MSD Oncology
No other potential conflicts of interest were reported.
SUPPORT
No specific funding was received for this study. However, A.C.S. is funded by the Australian Government Research Training Program Scholarship and University of Sydney Supplementary Postgraduate Research Scholarship in Breast Cancer. S.R.P. is funded by a National Health and Medical Research Council Early Career Fellowship (APP1157438) and National Heart Foundation Postdoctoral Fellowship (HF102164). K.K.H. is funded by a National Health and Medical Research Council Investigator Grant Emerging Leader Fellowship (APP1196724). J.R. is funded by a National Health and Medical Research Council Investigator Grant No. (GNT2007946).
AUTHOR CONTRIBUTIONS
Conception and design: Anna C. Singleton, Julie Redfern, Stephanie R. Partridge
Administrative support: Rebecca Raeside
Collection and assembly of data: Anna C. Singleton, Rebecca Raeside, Karice K. Hyun, Stephanie R. Partridge, Nashid Hafiz, Qiang Tu, Justin Tat-Ko, Stephanie Che Mun Sum
Data analysis and interpretation: Karice K. Hyun, Gian Luca Di Tanna, Anna C. Singleton, Julie Redfern, Stephanie R. Partridge, Kerry A. Sherman, Elisabeth Elder
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Electronic Health Interventions for Patients With Breast Cancer: Systematic Review and Meta-Analyses
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Gian Luca Di Tanna
Honoraria: Amgen
Kerry A. Sherman
Research Funding: MedConsent (Inst)
Elisabeth Elder
Honoraria: Roche, MSD Oncology
Consulting or Advisory Role: MSD Oncology
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Fiszer C, Dolbeault S, Sultan S, et al. : Prevalence, intensity, and predictors of the supportive care needs of women diagnosed with breast cancer: A systematic review. Psychooncology 23:361-374, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Ganz PA, Yip CH, Gralow JR, et al. : Supportive care after curative treatment for breast cancer (survivorship care): Resource allocations in low- and middle-income countries. A Breast Health Global Initiative 2013 consensus statement. Breast 22:606-615, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Mols F, Vingerhoets AJJM, Coebergh JW, et al. : Quality of life among long-term breast cancer survivors: A systematic review. Eur J Cancer 41:2613-2619, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Miedema B, Easley J: Barriers to rehabilitative care for young breast cancer survivors: A qualitative understanding. Support Care Cancer 20:1193-1201, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Legood R, dos-Santos-Silva I, et al. : Global treatment costs of breast cancer by stage: A systematic review. PLoS One 13:e0207993, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nekhlyudov L, Mollica MA, Jacobsen PB, et al. : Developing a quality of cancer survivorship care framework: Implications for clinical care, research, and policy. J Natl Cancer Inst 111:1120-1130, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mead KH, Raskin S, Willis A, et al. : Identifying patients' priorities for quality survivorship: Conceptualizing a patient-centered approach to survivorship care. J Cancer Surviv 14:939-958, 2020 [DOI] [PubMed] [Google Scholar]
- 9.McNeely ML, Campbell KL, Rowe BH, et al. : Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can Med Assoc J 175:34-41, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews H, Grunfeld EA, Turner A: The efficacy of interventions to improve psychosocial outcomes following surgical treatment for breast cancer: A systematic review and meta-analysis. Psychooncology 26:593-607, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Xu A, Wang Y, Wu X: Effectiveness of e-health based self-management to improve cancer-related fatigue, self-efficacy and quality of life in cancer patients: Systematic review and meta-analysis. J Adv Nurs 75:3434-3447, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Falagas ME, Zarkadoulia EA, Ioannidou EN, et al. : The effect of psychosocial factors on breast cancer outcome: A systematic review. Breast Cancer Res 9:R44, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitz AE, Baumgartner RN, Baumgartner KB, et al. : Healthy lifestyle impact on breast cancer-specific and all-cause mortality. Breast Cancer Res Treat 167:171-181, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helm EE, Kempski KA, Galantino MLA: Effect of disrupted rehabilitation services on distress and quality of life in breast cancer survivors during the COVID-19 pandemic. Rehabil Oncol 38:153-158, 2020 [Google Scholar]
- 15.Rittberg R, Mann A, Desautels D, et al. : Canadian cancer centre response to COVID-19 pandemic: A national and provincial response. Curr Oncol 28:233-251, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekhlyudov L, Duijts S, Hudson SV, et al. : Addressing the needs of cancer survivors during the COVID-19 pandemic. J Cancer Surviv 14:601-606, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis J, Ray P, Liaw ST: Recent worldwide developments in eHealth and mHealth to more effectively manage cancer and other chronic diseases—A systematic review. Yearb Med Inform 93-108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, West BT, Barton DL, et al. : Acceptance and use of eHealth/mHealth applications for self-management among cancer survivors. Stud Health Technol Inform 245:131-135, 2017 [PMC free article] [PubMed] [Google Scholar]
- 19.Triberti S, Savioni L, Sebri V, et al. : eHealth for improving quality of life in breast cancer patients: A systematic review. Cancer Treat Rev 74:1-14, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Gorini A, Mazzocco K, Triberti S, et al. : A P5 approach to m-health: Design suggestions for advanced mobile health technology. Front Psychol 9:2066, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson BA, Lindgren BR, Blaes AH, et al. : The new normal? Patient satisfaction and usability of telemedicine in breast cancer care. Ann Surg Oncol 28:5668-5676, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eyles H, Jull A, Dobson R, et al. : Co-design of mHealth delivered interventions: A systematic review to assess key methods and processes. Curr Nutr Rep 5:160-167, 2016 [Google Scholar]
- 23.Dorri S, Asadi F, Olfatbakhsh A, et al. : A Systematic Review of Electronic Health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer 27:25-46, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Singleton A, Raeside R, Partridge SR, et al. : Co-designing a lifestyle-focused text message intervention for women after breast cancer treatment: Mixed methods study. J Med Internet Res 23:e27076, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Liu Y, Jiang J, et al. : Effect of telehealth interventions on quality of life in cancer survivors: A systematic review and meta-analysis of randomized controlled trials. Int J Nurs Stud 122:103970, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Tokgöz P, Dockweiler C. Telemedicine in rehabilitation aftercare for women with breast cancer—A systematic literature review [in German]. Rehabilitation (Stuttg) 61:17-24, 2022 [DOI] [PubMed] [Google Scholar]
- 27.Glasgow RE, Vogt TM, Boles SM: Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am J Public Health 89:1322-1327, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM, et al. : The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372:n71, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 8:336-341, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thomas J, Chandler J, et al. : Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ, John Wiley & Sons, 2019 [Google Scholar]
- 31.Durlak JA: How to select, calculate, and interpret effect sizes. J Pediatr Psychol 34:917-928, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Borenstein M, Hedges LV, Higgins JPT, et al. : A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97-111, 2010 [DOI] [PubMed] [Google Scholar]
- 33.López-López JA, Page MJ, Lipsey MW, et al. : Dealing with effect size multiplicity in systematic reviews and meta-analyses. Res Synth Methods 9:336-351, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Abrahams HJG, Gielissen MFM, Donders RRT, et al. : The efficacy of internet-based cognitive behavioral therapy for severely fatigued survivors of breast cancer compared with care as usual: A randomized controlled trial. Cancer 123:3825-3834, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Admiraal JM, van der Velden AWG, Geerling JI, et al. : Web-based tailored psychoeducation for breast cancer patients at the onset of the survivorship phase: A multicenter randomized controlled trial. J Pain Symptom Manag 54:466-475, 2017 [DOI] [PubMed] [Google Scholar]
- 36.Atema V, van Leeuwen M, Kieffer JM, et al. : Efficacy of internet-based cognitive behavioral therapy for treatment-induced menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol 37:809-822, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Baker TB, Hawkins R, Pingree S, et al. : Optimizing eHealth breast cancer interventions: Which types of eHealth services are effective? Transl Behav Med 1:134-145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borosund E, Cvancarova M, Moore SM, et al. : Comparing effects in regular practice of e-communication and web-based self-management support among breast cancer patients: Preliminary results from a randomized controlled trial. J Med Internet Res 16:e295, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter KM, Stoner SA, Schmitz K, et al. : An online stress management workbook for breast cancer. J Behav Med 37:458-468, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chee W, Lee Y, Im E-O, et al. : A culturally tailored Internet cancer support group for Asian American breast cancer survivors: A randomized controlled pilot intervention study. J Telemed Telecare 23:618-626, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang SY, Wang YL, Lu WH, et al. : Long-term effectiveness of an E-based survivorship care plan for breast cancer survivors: A quasi-experimental study. Patient Educ Couns 103:549-555, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Ferrante JM, Devine KA, Bator A, et al. : Feasibility and potential efficacy of commercial mHealth/eHealth tools for weight loss in African American breast cancer survivors: Pilot randomized controlled trial. Transl Behav Med 10:938-948, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fjell M, Langius-Eklof A, Nilsson M, et al. : Reduced symptom burden with the support of an interactive app during neoadjuvant chemotherapy for breast cancer—A randomized controlled trial. Breast 51:85-93, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freeman LW, White R, Ratcliff CG, et al. : A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: Reducing symptoms and barriers to care. Psychooncology 24:910-918, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galiano-Castillo N, Arroyo-Morales M, Lozano-Lozano M, et al. : Effect of an internet-based telehealth system on functional capacity and cognition in breast cancer survivors: A secondary analysis of a randomized controlled trial. Support Care Cancer 25:3551-3559, 2017 [DOI] [PubMed] [Google Scholar]
- 46.Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, et al. : Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 122:3166-3174, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Gustafson DH, Hawkins RP, McTavish F, et al. : Internet-based interactive support for cancer patients: Are integrated systems better? J Commun 58:238-257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Handa S, Okuyama H, Yamamoto H, et al. : Effectiveness of a smartphone application as a support tool for patients undergoing breast cancer chemotherapy: A randomized controlled trial. Clin Breast Cancer 20:201-208, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Hawkins RP, Pingree S, Shaw B, et al. : Mediating processes of two communication interventions for breast cancer patients. Patient Educ Couns 81:S48-S53, 2010. (suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawkins RP, Pingree S, Baker TB, et al. : Integrating eHealth with human services for breast cancer patients. Transl Behav Med 1:146-154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou IC, Lin HY, Shen SH, et al. : Quality of life of women after a first diagnosis of breast cancer using a self-management support mHealth app in Taiwan: Randomized controlled trial. JMIR MHealth UHealth 8:e17084, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hummel SB, van Lankveld JJDM, Oldenburg HSA, et al. : Efficacy of internet-based cognitive behavioral therapy in improving sexual functioning of breast cancer survivors: Results of a randomized controlled trial. J Clin Oncol 35:1328-1340, 2017 [DOI] [PubMed] [Google Scholar]
- 53.Kim HJ, Kim SM, Shin H, et al. : A mobile game for patients with breast cancer for chemotherapy self-management and quality-of-life improvement: Randomized controlled trial. J Med Internet Res 20:e273, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim SC, Shaw BR, Shah DV, et al. : Interactivity, presence, and targeted patient care: Mapping e-health intervention effects over time for cancer patients with depression. Health Commun 34:162-171, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim SC, Hawkins RP, Shah DV, et al. : Understanding how e-health interventions meet psychosocial needs of breast cancer patients: The pathways of influence on quality of life and cancer concerns. PsychoOncology 29:1704-1712, 2020 [DOI] [PubMed] [Google Scholar]
- 56.Lally RM, Kupzyk KA, Bellavia G, et al. : CaringGuidance[TM] after breast cancer diagnosis eHealth psychoeducational intervention to reduce early post-diagnosis distress. Support Care Cancer 28:2163-2174, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lynch BM, Nguyen NH, Moore MM, et al. : A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: The ACTIVATE Trial. Cancer 125:2846-2855, 2019 [DOI] [PubMed] [Google Scholar]
- 58.Owen JE, Klapow JC, Roth DL, et al. : Randomized pilot of a self-guided internet coping group for women with early-stage breast cancer. Ann Behav Med 30:54-64, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Rosen KD, Paniagua SM, Kazanis W, et al. : Quality of life among women diagnosed with breast cancer: A randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psychooncology 27:2023-2030, 2018 [DOI] [PubMed] [Google Scholar]
- 60.Ryhänen AM, Rankinen S, Siekkinen M, et al. : The impact of an empowering Internet-based Breast Cancer Patient Pathway program on breast cancer patients' clinical outcomes: A randomised controlled trial. J Clin Nurs 22:1016-1025, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Vallance JK, Nguyen NH, Moore MM, et al. : Effects of the ACTIVity and TEchnology (ACTIVATE) intervention on health-related quality of life and fatigue outcomes in breast cancer survivors. Psychooncology 29:204-211, 2020 [DOI] [PubMed] [Google Scholar]
- 62.Van Den Berg SW, Gielissen MFM, Custers JAE, et al. : BREATH: Web-based self-management for psychological adjustment after primary breast cancer-results of a multicenter randomized controlled trial. J Clin Oncol 33:2763-2771, 2015 [DOI] [PubMed] [Google Scholar]
- 63.van der Hout A, van Uden-Kraan CF, Holtmaat K, et al. : Role of eHealth application oncokompas in supporting self-management of symptoms and health-related quality of life in cancer survivors: A randomised, controlled trial. Lancet Oncol 21:80-94, 2020 [DOI] [PubMed] [Google Scholar]
- 64.Ventura F, Sawatzky R, Ohlen J, et al. : Challenges of evaluating a computer-based educational programme for women diagnosed with early-stage breast cancer: A randomised controlled trial. Eur J Cancer Care (Engl) 26, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Visser A, Prins JB, Jansen L, et al. : Group medical consultations (GMCs) and tablet-based online support group sessions in the follow-up of breast cancer: A multicenter randomized controlled trial. Breast 40:181-188, 2018 [DOI] [PubMed] [Google Scholar]
- 66.White V, Farrelly A, Pitcher M, et al. : Does access to an information-based, breast cancer specific website help to reduce distress in young women with breast cancer? Results from a randomised trial. Eur J Cancer Care 27:e12897, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Zachariae R, Amidi A, Damholdt MF, et al. : Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: A randomized controlled trial. J Natl Cancer Inst 110:880-887, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou K, Wang W, Zhao W, et al. : Benefits of a WeChat-based multimodal nursing program on early rehabilitation in postoperative women with breast cancer: A clinical randomized controlled trial. Int J Nurs Stud 106:103565, 2020 [DOI] [PubMed] [Google Scholar]
- 69.Zhu J, Ebert L, Liu X, et al. : Mobile breast cancer e-support program for Chinese women with breast cancer undergoing chemotherapy (part 2): Multicenter randomized controlled trial. JMIR Mhealth Uhealth 6:e104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bank TW: Classifying countries by income. 2019. https://datatopicsworldbankorg/world-development-indicators/stories/the-classification-of-countries-by-incomehtml
- 71.Zhu H, Chen X, Yang J, et al. : Mobile breast cancer e-support program for Chinese women with breast cancer undergoing chemotherapy (part 3): Secondary data analysis. JMIR MHealth UHealth 8:e18896, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lally RM, Kupzyk K, Gallo S, et al. : Use of an unguided, web-based distress self-management program after breast cancer diagnosis: Sub-analysis of caringguidance pilot study. J Med Internet Res 22:e19734, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan ML, Idris DB, Teo LW, et al. : Validation of EORTC QLQ-C30 and QLQ-BR23 questionnaires in the measurement of quality of life of breast cancer patients in Singapore. Asia Pac J Oncol Nurs 1:22-32, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van Esch L, Den Oudsten BL, De Vries J: The World Health Organization quality of life instrument-short form (WHOQOL-BREF) in women with breast problems. Int J Clin Health Psychol 11:5-22, 2011 [Google Scholar]
- 75.Avis NE, Ip E, Foley KL: Evaluation of the Quality of Life in Adult Cancer Survivors (QLACS) scale for long-term cancer survivors in a sample of breast cancer survivors. Health Qual Life Outcomes 4:92, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ferrell BR, Dow KH, Grant M: Measurement of the quality of life in cancer survivors. Qual Life Res 4:523-531, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Love AW, Kissane DW, Bloch S, et al. : Diagnostic efficiency of the hospital anxiety and depression scale in women with early stage breast cancer. Aust N Z J Psychiatry 36:246-250, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Bener A, Alsulaiman R, Doodson L, et al. : Comparison of reliability and validity of the breast cancer depression anxiety stress scales (DASS-21) with the Beck Depression Inventory-(BDI-II) and Hospital Anxiety and Depression Scale (HADS). Int J Behav Res Psychol 4:197-203, 2016 [Google Scholar]
- 79.Hann D, Winter K, Jacobsen P: Measurement of depressive symptoms in cancer patients: Evaluation of the center for epidemiological studies depression scale (CES-D). J Psychosom Res 46:437-443, 1999 [DOI] [PubMed] [Google Scholar]
- 80.Spielberger CD, Gorsuch RL, Lushene R, et al. : State-trait anxiety inventory for adults: Manual, instrument and scoring guide consulting. Palo Alto, CA, Psychologists Press Mind Garden, 1983 [Google Scholar]
- 81.Chang VT, Hwang SS, Feuerman M, et al. : The memorial symptom assessment scale short form (MSAS-SF). Cancer 89:1162-1171, 2000 [DOI] [PubMed] [Google Scholar]
- 82.Zwahlen D, Hagenbuch N, Carley MI, et al. : Screening cancer patients' families with the distress thermometer (DT): A validation study. Psychooncology 17:959-966, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Derogatis LR: Symptom checklist-90-revised, Brief Symptom Inventory, and BSI-18, in Handbook of Psychological Assessment in Primary Care Settings (ed 2). New York, NY, Routledge/Taylor & Francis Group, 2017, pp 599-629 [Google Scholar]
- 84.Merluzzi TV, Martinez Sanchez MA: Assessment of self-efficacy and coping with cancer: Development and validation of the Cancer Behavior Inventory. Health Psychol 16:163-170, 1997 [DOI] [PubMed] [Google Scholar]
- 85.Cunningham AJ, Lockwood GA, Cunningham JA: A relationship between perceived self-efficacy and quality of life in cancer patients. Patient Educ Couns 17:71-78, 1991 [DOI] [PubMed] [Google Scholar]
- 86.Khatcheressian JL, Wolff AC, Smith TJ, et al. : American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24:5091-5097, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Senkus E, Kyriakides S, Ohno S, et al. : Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v8-v30, 2015. (suppl 5) [DOI] [PubMed] [Google Scholar]
- 88.Bandura A: Health promotion from the perspective of social cognitive theory. Psychol Health 13:623-649, 1998 [Google Scholar]
- 89.Carver CS, Scheier MF: Control theory: A useful conceptual framework for personality–social, clinical, and health psychology. Psychol Bull 92:111-135, 1982 [PubMed] [Google Scholar]
- 90.Slev VN, Mistiaen P, Pasman HRW, et al. : Effects of eHealth for patients and informal caregivers confronted with cancer: A meta-review. Int J Med Inform 87:54-67, 2016 [DOI] [PubMed] [Google Scholar]
- 91.Compernolle S, De Cocker K, Lakerveld J, et al. : A RE-AIM evaluation of evidence-based multi-level interventions to improve obesity-related behaviours in adults: A systematic review (the SPOTLIGHT project). Int J Behav Nutr Phys Act 11:147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Delgoshaei B, Mobinizadeh M, Mojdekar R, et al. : Telemedicine: A systematic review of economic evaluations. Med J Islam Repub Iran 31:113, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rinaldi G, Hijazi A, Haghparast-Bidgoli H: Cost and cost-effectiveness of mHealth interventions for the prevention and control of type 2 diabetes mellitus: A systematic review. Diabetes Res Clin Pract 162:108084, 2020 [DOI] [PubMed] [Google Scholar]
- 94.Maass SWMC, Roorda C, Berendsen AJ, et al. : The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 82:100-108, 2015 [DOI] [PubMed] [Google Scholar]
- 95.Pilevarzadeh M, Amirshahi M, Afsargharehbagh R, et al. : Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res Treat 176:519-533, 2019 [DOI] [PubMed] [Google Scholar]
- 96.Deady M, Choi I, Calvo RA, et al. : eHealth interventions for the prevention of depression and anxiety in the general population: A systematic review and meta-analysis. BMC Psychiatry 17:310, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Penedo FJ, Oswald LB, Kronenfeld JP, et al. : The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol 21:e240-e251, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis FD, Venkatesh V: Toward preprototype user acceptance testing of new information systems: Implications for software project management. IEEE Trans Eng Manag 51:31-46, 2004 [Google Scholar]
- 99.Eysenbach G: The law of attrition. J Med Internet Res 7:e11, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bombard Y, Baker GR, Orlando E, et al. : Engaging patients to improve quality of care: A systematic review. Implement Sci 13:98, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardiker NR, Grant MJ: Factors that influence public engagement with eHealth: A literature review. Int J Med Inform 80:1-12, 2011 [DOI] [PubMed] [Google Scholar]
- 102.Conway N, Webster C, Smith B, et al. : eHealth and the use of individually tailored information: A systematic review. Health Informatics J 23:218-233, 2016 [DOI] [PubMed] [Google Scholar]
- 103.Carter DD, Robinson K, Forbes J, et al. : Experiences of mobile health in promoting physical activity: A qualitative systematic review and meta-ethnography. PLoS One 13:e0208759, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Armanasco AA, Miller YD, Fjeldsoe BS, et al. : Preventive health behavior change text message interventions: A meta-analysis. Am J Prev Med 52:391-402, 2017 [DOI] [PubMed] [Google Scholar]
- 105.Head KJ, Noar SM, Iannarino NT, et al. : Efficacy of text messaging-based interventions for health promotion: A meta-analysis. Soc Sci Med 97:41-48, 2013 [DOI] [PubMed] [Google Scholar]
- 106.Sardi L, Idri A, Fernández-Alemán JL: A systematic review of gamification in e-Health. J Biomed Inform 71:31-48, 2017 [DOI] [PubMed] [Google Scholar]
- 107.Moon Z, Zuchowski M, Moss-Morris R, et al. : Disparities in access to mobile devices and e-health literacy among breast cancer survivors. Support Care Cancer 30:117-126, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahajan R, Gupta K: Adaptive design clinical trials: Methodology, challenges and prospect. Indian J Pharmacol 42:201-207, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fentiman IS, Fourquet A, Hortobagyi GN: Male breast cancer. Lancet 367:595-604, 2006 [DOI] [PubMed] [Google Scholar]