Abstract
The roles of oxytocin (OT) and arginine-vasopressin (AVP) as crucial modulators of social cognition and related behaviours have been extensively addressed in the literature. The involvement of these neuropeptides in social cognition in ageing, however, and a potential mediating effect of basic cognitive capacities on this link, are not well understood. To fill these research gaps, this study assessed associations of plasma OT and AVP levels with dynamic emotion identification accuracy in generally healthy older men (aged 55–95 years) and probed the underlying roles of crystallized and fluid cognition in these associations. Higher plasma OT levels were associated with lower accuracy in dynamic emotion identification, with this negative relationship fully mediated by cognition. For plasma AVP levels, in contrast, there was no association with dynamic emotion identification accuracy. Integrated within existing theoretical accounts, results from this study advance understanding of the neuropeptide–social cognition link in ageing and support basic cognitive capacities as mediators in this association.
This article is part of the theme issue ‘Interplays between oxytocin and other neuromodulators in shaping complex social behaviours’.
Keywords: oxytocin, vasopressin, emotion identification, ageing, crystallized cognition, fluid cognition
1. Introduction
Oxytocin (OT) and arginine-vasopressin (AVP) are important modulators of social cognition and related behaviours [1–3]. Previous work, however, has almost exclusively focused on young subjects, and the role of these neuropeptides in social cognition in ageing is not well understood [4–7]. Also, processes underlying a neuropeptide–social cognition link in ageing are unknown, while basic cognition constitutes a promising candidate given its susceptibility to age-related changes. To fill these knowledge gaps, we determined associations of plasma OT and AVP levels with performance in dynamic emotion identification—a crucial social-cognitive skill—in a sample of generally healthy older men. We also, for the first time to our knowledge, tested crystallized and fluid cognitive capacities as mediators of the relationship between these neuropeptides and emotion identification skills in ageing.
OT and AVP are nonapeptide hormones in the neurohypophysial family, which are among the oldest neurohormones, evolved across various species over time [8,9], and have demonstrated critical importance for many physiological processes and behaviours [10–12]. Primarily produced by magnocellular neurons in the hypothalamus, they are secreted both peripherally into the blood and centrally throughout the brain [13,14]. Peripherally, they act on smooth muscles and are associated with the regulation of hydration [10,11]. Centrally, they target brain regions such as the amygdala, the striatum and the hippocampus, which are involved in social cognition [15].
OT and AVP have actions on both the peripheral nervous system (PNS) and the central nervous system (CNS). Their direct measurement in the CNS is invasive and difficult in humans, however, which has led researchers to use peripheral measurements of endogenous levels as a proxy for central processes. In particular, levels of endogenous OT and AVP can be quantified in blood plasma and then related to social processes and behaviour [16–19]. In addition, studies have administered exogenous OT and AVP intranasally to test their central mechanisms. Exogenous OT and AVP have been shown to temporarily increase neuropeptide levels in the CNS (i.e. measured in cerebrospinal fluid) and in the PNS (i.e. measured in blood plasma), supporting a central route of transport and neuromodulatory capacity of these neuropeptides ([20,21]; but see [22]).
Through research involving endogenous measurement and exogenous administration, OT and AVP have been associated with a wide array of complex social functions (see [23–27] for overviews). Some theories have focused on the social effects of OT (e.g. Social Salience Theory [28], approach/avoidance [29]). For example, it is possible that OT can increase the salience of social (as compared with non-social) cues through its effects on early attentional mechanisms [28]. Additionally, OT has been associated with increased affiliative prosocial behaviour [30,31], but AVP has been associated with aggressive and agonistic behaviour [32–34], and balance between the two neuropeptides is important for facilitating social cognition and related behaviour [11,35].
A critical component of social cognition is the ability to identify emotions conveyed through facial and/or vocal expressions in others (i.e. emotion identification) ([36]; see [37] and [38] for meta-analyses). In particular, some studies suggest that intranasal OT administration improves emotion identification [37–40], whereas intranasal AVP reduces performance [34,41,42]. Two meta-analyses found enhancement of emotion identification after intranasal OT (compared with placebo) administration, with some variation by emotion type (e.g. increased identification for happy and fearful emotions) and task paradigm (e.g. increased identification for happy emotions during short stimulus exposure and for fearful emotions during longer stimulus exposure; [37,38]). For AVP, in contrast, both Uzefovsky et al. [41] and Vadas et al. [42] found reduced ability to identify negative emotions. Of note, these previous studies exclusively examined young adults and yielded small effect sizes. Also, they used exogenous OT/AVP administration, and much less is known about the associations of endogenous OT/AVP with social cognition, and emotion identification specifically, and close to nothing about these associations in older adults.
Previous research has, however, examined the relationship between endogenous OT levels and social-cognitive abilities among clinical populations, such as in schizophrenia [43,44] and bipolar disorder [45]. For example, lower plasma OT levels were associated with lower metacognition (in schizophrenia [46]) and higher plasma OT was associated with increased perception of happy expressions (in women but not men, with and without schizophrenia [43]). By contrast, Spilka et al. [44] found that lower plasma OT was associated with decreased emotion identification accuracy in schizophrenia. In healthy populations, higher salivary plasma OT levels were associated with increased positive evaluation for happy expressions [47]. None of these previous studies, however, specifically addressed older adults.
A current entirely parallel robust line of research demonstrates age-related change in basic cognitive abilities [48,49]. Cognitive decline in ageing impacts the ability to process social-cognitive information. This literature differentiates crystallized (i.e. semantic knowledge acquired through past experiences) and fluid (i.e. processing and manipulation of new information) cognition [50–52], which both were associated with emotion identification performance ([53]; see [54] for a meta-analysis). In particular, greater crystallized cognition was found to correlate with better emotion identification [55,56]. Also, greater working memory capacity was related with more accurate emotion identification [57], but increased working memory load reduced emotion identification accuracy [58,59].
A mediating role of crystallized and fluid cognition in the link between OT/AVP and emotion identification, however, has yet to be determined. In fact, from the little that is known, higher plasma OT levels may be associated with faster processing speed ([60]; see also [61] and [62] for better performance on verbal memory in schizophrenia). By contrast, other studies in healthy adults using intranasal OT have found either no effect on cognition (Digit Span Task; [63]) or amnesic effects [64–66]. Further, consistent with a possible pattern of antagonistic effects of OT and AVP, in Plasencia et al. [60] lower plasma AVP levels were associated with higher processing speed. But there is other evidence that intranasal AVP increased short-term memory [64,67], consistent with pre-clinical work that exogenous AVP improved working memory and long-term spatial memory in a mouse model of Alzheimer's disease [68]. Thus, in brief, while there is some support for associations between OT/AVP and crystallized/fluid cognition, a connection to emotion identification in ageing has not been drawn yet—a research gap that the present study aimed to fill.
Taken together, here we determined the associations of plasma OT and AVP levels with dynamic emotion identification accuracy in a sample of older men (age range: 55–95 years). Further, for the first time, we probed the mediating role of crystallized and fluid cognition in this neuropeptide–social cognition link among older adults, to enhance understanding of basic cognitive processes in the effect of neuropeptides on social cognition.
2. Methods
(a) . Participants
The study sample included 104 generally healthy older men (92.3% White; 1.92% Hispanic/Latino) who were part of a larger clinical trial on the effects of OT on physical, cognitive and socioemotional ageing (OT Aging Study; NCT02069431) conducted in the Department of Psychology, the Institute on Aging, and the McKnight Brain Institute at the University of Florida. The larger project was unbalanced across the sexes, with fewer women than men, and previous research documents sex-dimorphic effects of OT [69–71], including in ageing [72,73]. Therefore, the current analysis comprised only older men. Also, only participants (N = 77) with complete data for all central variables for our respective analyses (plasma OT, plasma AVP, dynamic emotion identification accuracy, crystallized and fluid cognition, and age) were included here (results from the analysis on the full dataset using multiple imputations (N = 104) were comparable and are also reported below). Table 1 summarizes sample-descriptive information for demographics, health, mood, OT/AVP neuropeptide plasma levels, cognition, and dynamic emotion identification accuracy.
Table 1.
Sample-descriptive information. Age: in years. Education: self-reported number of years of formal education. Health: mental (Please rate your general physical health) and physical (Please rate your general mental health/mood) on a scale from 1 = poor to 10 = excellent. Mood: assessed via the Positive and Negative Affect Schedule (PANAS 20-item short version [74] plus six additional adjectives as suggested in [75], in general or how they felt on average, on a scale from 1 = very slightly or not at all to 5 = extremely. Neuropeptide plasma levels: oxytocin (OT) and arginine-vasopressin (AVP), in µg ml−1 quantified in blood plasma via enzyme immunoassay (EIA; Enzo Life Sciences). Cognition: assessed via the NIH Cognition Toolbox [50], crystallized and fluid (uncorrected composite scores; normative mean = 100, standard deviation = 15). Dynamic emotion identification accuracy: percentage correct across all emotions and modalities [76].
| measure | mean (s.d.) | median | range |
|---|---|---|---|
| demographics | |||
| age (years) | 71.61 (7.56) | 70.10 | 55.87–94.81 |
| education (years) | 16.75 (3.05) | 16 | 12–27 |
| health | |||
| mental health | 8.56 (1.16) | 9 | 5–10 |
| physical health | 8.09 (1.34) | 8 | 4–10 |
| mood | |||
| PANAS positive affect | 3.43 (0.61) | 3.46 | 1.54–4.69 |
| PANAS negative affect | 1.39 (0.40) | 1.23 | 1.00–2.77 |
| neuropeptide plasma levels | |||
| OT (µg ml−1) | 1.68 (0.70) | 1.56 | 0.53–5.12 |
| AVP (µg ml−1) | 1.84 (0.73) | 1.73 | 0.66–3.87 |
| cognition | |||
| NIH Toolbox crystallized cognition | 120.71 (12.06) | 120 | 95.00–167.00 |
| NIH Toolbox fluid cognition | 90.06 (9.56) | 90.89 | 63.00–116.03 |
| dynamic emotion identification | 0.63 (0.09) | 0.63 | 0.41–0.86 |
Participants were recruited between February 2016 and February 2020 via fliers and handouts around Alachua County, advertisements on websites for clinical trials (e.g. University of Florida Health Clinical Trials, clinicaltrials.gov), mailouts via laboratory- and university-internal participant registries, as well as via geolocator address purchases, newspaper advertisements and word-of-mouth. Participants underwent a phone pre-screening to determine general study eligibility. Inclusion criteria included the ability to provide consent, English-speaking, 55 years and older, and generally healthy (derived from a laboratory-internal health review that assessed any issues with vision and hearing, shortness of breath or chest tightness, feelings of fatigue, and psychiatric conditions such as anxiety, depression, inability to concentrate, or difficulty sleeping). General cognition was assessed via the Telephone Interview of Cognitive Status (TICS; [77]; cut-off, less than 30). Exclusion criteria included heavy drug or alcohol use, blood pressure greater than 180/100 mmHg, as well as urine osmolality greater than 1200 l with blood sodium levels less than 134 mEq l−1, history of inappropriate antidiuretic hormone secretion, and/or use of antidiuretic medication as OT and AVP are involved in water retention ([78]; see [79] for details regarding inclusion/exclusion).
(b) . Procedures
(i) . Study design
The study protocol was approved by the Institutional Review Board of the University of Florida. After written informed consent, participants took part in the larger clinical trial, which included one screening visit, followed by three pre-intervention visits, a four-week intranasal spray (OT or placebo) administration, and three post-intervention visits (intervention data not considered here; see [79] for details and procedural diagram, and [80] for an additional publication from the larger project). Participants were reimbursed for study participation. Only measures relevant to the current analysis at the pre-intervention phase are described below.
The present study used data from the screening visit and the second pre-intervention visit, which both lasted approximately 2 h. In the screening visit, participants provided demographic and health information as well as urine and blood samples (i.e. for screening purposes and for determination of neuropeptide plasma levels as described below), completed the NIH Toolbox Cognition Battery, and underwent a brief physical health examination with a licensed clinician. A few days later, during the second pre-intervention visit, participants performed first a face evaluation task, followed by the Dynamic Emotion Identification Task (described below), with identical task order for all participants.
(c) . Measures
(i) . Plasma oxytocin and arginine-vasopressin levels
A trained phlebotomist obtained blood samples. The large majority (76.3%) of the samples were collected between 8.45 am and 11.00 am. However, owing to scheduling logistics, 17.5% of the samples were completed between 11.00 am and 1.00 pm and 6.2% of the samples between 1.00 pm and 3.00 pm. The 10 ml EDTA vials were centrifuged at 2300 r.p.m. at a temperature of 4°C and a force of 1600g to aliquot the plasma, which was then stored in a freezer at −80°C until assayed. Highly sensitive enzyme immunoassay (EIA; Enzo Life Sciences) was used to measure OT and AVP concentrations in the blood plasma, as it has a minimal detection rate of 15.6 µg ml−1 for OT and 4.10 µg ml−1 for AVP. In addition, the EIA has minimal cross-reactivity for other neuropeptides. As directed by the immunoassay instructions, plasma was first diluted at a ratio of 1 : 8 for the OT and 1 : 2 for the AVP with the assay buffer to ensure reliability of the standard curve linear portion. To increase reliability within the assays, all samples were run at the same time. Coefficients of variance were less than 10 for OT and 8 for AVP in both inter- and intra-assays. Samples were not extracted as previous research indicates accurate measurement in human blood plasma with non-extracted samples [16,18], and two recent studies reported stronger relationships between behavioural outcomes and plasma levels of OT in unextracted samples (see [81,82]; see also [60] for more discussion). Table 1 summarizes plasma OT and AVP levels in the sample.
(ii) . Crystallized and fluid cognition
Participants completed the NIH Toolbox Cognition Battery [50,83], which comprises seven measures categorized into two cognitive domains: crystallized cognition (Picture Vocabulary Test, Oral Reading Recognition Test) and fluid cognition (Dimensional Change Card Sort Test, Flanker Inhibitory Control and Attention Test, Picture Sequence Memory Test, List Sorting Working Memory Test, Pattern Comparison Processing Speed Test). See electronic supplementary, table S1 for internal validity of the cognitive scores.
Table 1 summarizes the unadjusted scores for crystallized and fluid cognition in the sample. Given the racial/ethnic homogeneity of our sample and an age range from 55–95 years, which surpassed the upper limit of 85 years for age-standardized corrections provided by the NIH Toolbox Cognition Battery, we used the unadjusted crystallized and fluid cognition composite scores (i.e. not corrected for age, sex, education, or race/ethnicity). This approach and the range of uncorrected crystallized and fluid cognition scores in our sample are in line with previous literature using the NIH Toolbox Cognition Battery in older adults [84–86]. In our study, crystallized cognition scores might have been higher than the normative mean owing to the positive selectivity of the sample (i.e. given strict exclusion/inclusion criteria and high study demands).
(iii) . Dynamic emotion identification task
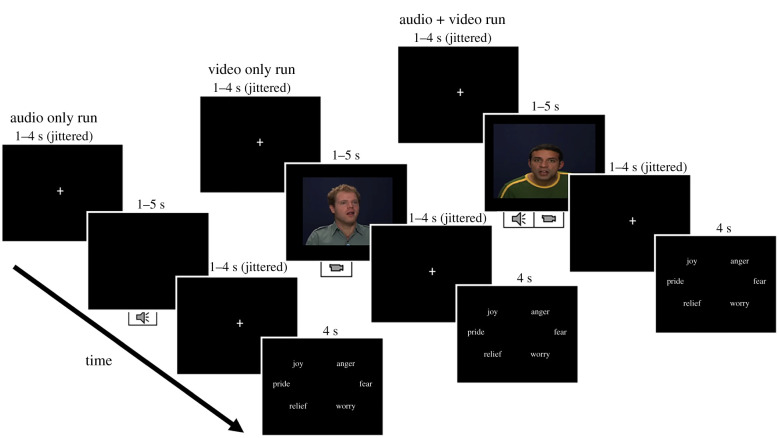
This task used stimuli from the Geneva Multimodal Emotional Portrayals (GEMEP) core stimuli set [76], from 10 different professional actors, all White (50% female), who displayed the emotions of joy, pride, relief, anger, fear and worry. Each of these emotions was visually and auditorily expressed via three modalities (audio, video, audio + video; figure 1). See electronic supplementary material, table S1 for internal validity of the emotion identification measures. The stimuli used in the present task were well-validated and used in other emotion-related research [87–89], including research with older adults to assess age-related differences in dynamic perceptual processing and emotion identification [90,91]. Stimuli were counterbalanced by age and sex of the actors, emotion type, and modality. All stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA) on a computer monitor.
Figure 1.
Overview and sample trials for each of the three experimental runs of the Dynamic Emotion Identification Task. Each trial presented a stimulus (audio, video or audio + video, depending on the run). Participants were instructed to identify the emotion displayed in each trial and select the corresponding button (joy, pride and relief on the left; anger, fear and worry on the right) when prompted via the response option screen. Each trial started with a jittered fixation cross, with a second jittered fixation between the stimulus presentation and the response option slide. (Online version in colour.)
Participants were asked to identify the emotion expressed via button presses on a Logitech game controller. Fixed for all participants, the right thumb was used to select one of the three negative emotions (anger, fear or worry) by pressing the right-side buttons, and the left thumb was used to select one of the three positive emotions (joy, pride or relief) by pressing the left-side buttons. Participants received task instructions and completed practice trials for familiarization.
Three experimental runs were administered. Each run contained one stimulus modality and had 36 trials, presenting one emotion at a time, for a total of 108 trials across the three runs. Each of the six emotions was presented six times during each run, for a total of 18 trials per emotion across the three runs. Both orders of run modality and order of emotion type within each run were counterbalanced across participants.
As shown in figure 1, each trial started with a jittered (1–4 s) fixation cross, followed by presentation of the stimulus (audio and/or video clip) that lasted for 1–5 s, followed by another jittered (1–4 s) fixation cross before the response options appeared. Responses were recorded while the response options were on the screen as well as during the subsequent fixation cross phase. Accuracy (‘correct’ versus ‘incorrect’ per trial) was recorded via E-Prime 2.0. Mean accuracy across all trials was computed for each participant to indicate a participant's ability to identify dynamic emotions. Table 1 summarizes dynamic emotion identification accuracy in the sample.
(d) . Analyses
First, we calculated bivariate correlations between our variables of interest (plasma OT, plasma AVP, crystallized cognition, fluid cognition, dynamic emotion identification accuracy, and age) using SPSS Statistics v. 26. Then we conducted mediation analyses using PROCESS v. 3.2 [92] in SPSS. In the main mediation model, plasma OT and AVP levels were the two predictors, crystallized and fluid cognition scores were the two mediators, and dynamic emotion identification accuracy was the outcome variable. Statistical significance of the indirect effects was determined by 95% confidence intervals (95% CI) using bootstrap with 10 000 samples.
Given the wide age range in our sample, we added chronological age as a covariate in all mediation models. We also performed a moderated mediation analysis considering two age groups among the older adults: a group of young-old individuals aged 55–74 years (n = 38) and a group of old-old individuals aged 75–95 years (n = 69; see [93–95] for a similar age categorization) to test for potential age-moderated effects on the relationship between plasma OT/AVP and dynamic emotion identification.
We furthermore conducted a control analysis with time of blood draw as a covariate (results from this control mediation analysis were consistent with those reported in the main text; see electronic supplementary material, table S2 for details). In addition, in an exploratory fashion, we conducted separate post hoc exploratory mediation analyses with plasma OT and AVP as predictors, crystallized and fluid cognition as mediators, and dynamic emotion identification accuracy for positive or negative emotions as the two outcome variables. Positive emotions included joy, pride and relief; negative emotions included anger, fear and worry.
Finally, as 26% of our original sample did not have complete data owing to technical issues, data quality, and attrition rates, we performed multiple imputations by chained equations (MICE [96]) in Stata [97] to permit examination of our effects in the full sample. After determining our data was missing completely at random (Little's MCAR test [98]; , p = 0.16), we used the variables plasma OT, plasma AVP, emotion identification accuracy, crystallized and fluid cognition, and age in 20 iterations. Results of the imputed dataset were consistent with results from the complete cases only dataset (see electronic supplementary material, table S3 for details).
All data and analysis code can be found in the Open Science Framework repository: https://osf.io/xv38y/?view_only=3ca2fada765f42c3a5a0e30900108fc3.
3. Results
As shown in table 2, plasma OT and plasma AVP were both negatively correlated with crystallized cognition. Crystallized and fluid cognition were positively correlated, and both were positively correlated with dynamic emotion identification accuracy.
Table 2.
Bivariate correlations between the central study variables. Pearson's r values in bold indicate significance at p < 0.050.
| AVP | crystallized cognition | fluid cognition | dynamic emotion identification | age (years) | |
|---|---|---|---|---|---|
| OT | −0.016 (n = 95) | −0.260 (n = 95) | −0.167 (n = 95) | −0.203 (n = 83) | −0.121 (n = 99) |
| p = 0.879 | p = 0.011 | p = 0.106 | p = 0.065 | p = 0.234 | |
| AVP | −0.292 (n = 91) | −0.172 (n = 91) | −0.029 (n = 79) | −0.075 (n = 95) | |
| p = 0.005 | p = 0.103 | p = 0.800 | p = 0.470 | ||
| crystallized cognition | 0.359 (n = 100) | 0.388 (n = 83) | −0.053 (n = 100) | ||
| p = 0.001 | p = 0.001 | p = 0.601 | |||
| fluid cognition | 0.471 (n = 83) | −0.295 (n = 100) | |||
| p = 0.001 | p = 0.003 | ||||
| dynamic emotion identification | −0.287 (n = 85) | ||||
| p = 0.008 |
In the main mediation model, the total effect of plasma OT on dynamic emotion identification was significant (B = −0.034, s.e. = 0.015, t73 = −2.326, p = 0.023, partial R2 (R2p) = 0.069). However, the mediation analysis showed that the direct effect of plasma OT on dynamic emotion identification was not significant (B = −0.014, s.e. = 0.013, t71 = −1.091, p = 0.279, R2p = 0.016), while the indirect effects via both crystallized (B = −0.011, s.e. = 0.005, 95% CI [−0.022, −0.003]) and fluid (B = −0.008, s.e. = 0.006, 95% CI [−0.022, −0.001]) cognition were significant; these results indicated that the effect of plasma OT on dynamic emotion identification was fully mediated by crystallized and fluid cognition (B = −0.019, s.e. = 0.008, 95% CI [−0.039, −0.008]).
By contrast, the total effect of plasma AVP on dynamic emotion identification was not significant (B = 0.003, s.e. = 0.015, t73 = 0.188, p = 0.851, R2p = 0.001), nor was the direct effect (B = 0.022, s.e. = 0.014, t71 = 1.576, p = 0.120, R2p = 0.034). However, the indirect effect of plasma AVP on dynamic emotion identification via crystallized cognition was significant (B = −0.111, s.e. = 0.006, 95% CI [−0.023, −0.002]); but the indirect effect of plasma AVP on dynamic emotion identification was not significant for fluid cognition (B = −0.008, s.e. = 0.007, 95% CI [−0.023, 0.003]).
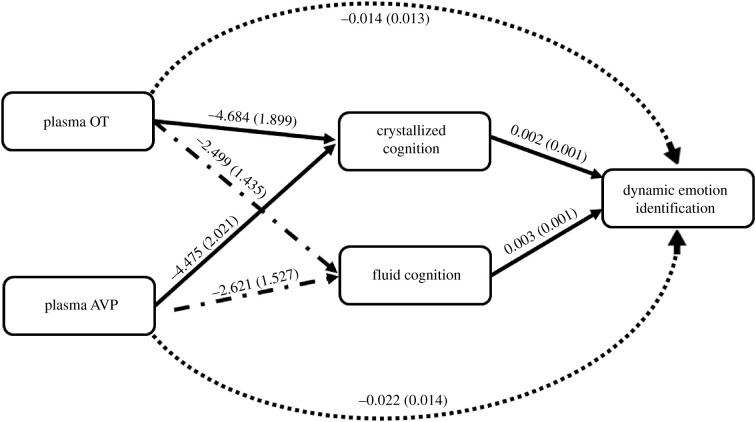
The effects of chronological age on fluid cognition (B = −0.428, s.e. = 0.142, t73 = −3.024, p = 0.003, R2p = 0.106) and on dynamic emotion identification (B = −0.003, s.e. = 0.001, t71 = −2.051, p = 0.044, R2p = 0.044) were significant, but the effect of chronological age on crystallized cognition was not significant (B = −0.048, s.e. = 0.187, t73 = 0.225, p = 0.799, R2p = 0.001). See figure 2 for coefficients of the paths in the mediation model. Further, the age moderation was not significant for the conditional indirect effects of OT (B = 0.009, s.e. = 0.011, 95% CI [−0.010,0.034]) or AVP (B = 0.011, s.e. = 0.012, 95% CI [−0.009, 0.041]), suggesting that the results were not age-dependent.
Figure 2.
Effects (B (s.e.
)) of plasma OT and plasma AVP levels on dynamic emotion identification accuracy with crystallized and fluid cognition scores as mediators (N = 77). Chronological age served as a covariate. Solid lines indicate p < 0.050; dash–dot lines indicate 0.050 < p < 0.100; fully dotted lines indicate p > 0.100.
Results from the exploratory mediation analyses with positive and negative emotions as outcome variables, finally, were largely consistent with results from the model with dynamic emotion identification accuracy averaged across all emotions; the only exceptions were a significant effect of fluid cognition on dynamic emotion identification for negative emotions (B = 0.004, s.e. = 0.001, t73 = 3.547, p = 0.001) and a non-significant mediation of the indirect effect of OT on dynamic emotion identification of positive emotions by fluid cognition (B = −0.005, s.e. = 0.005, 95% CI [−0.019, 0.002]; see electronic supplementary material, table S4 for details).
4. Discussion
The present study, for the first time to our knowledge, examined associations of plasma OT and AVP with dynamic emotion identification accuracy in generally healthy older men, and tested mediation of this neuropeptide–social cognition link via basic cognition. There are several key findings from this work: first, confirming previous results, greater age was associated with both lower fluid cognition [49] and less accurate dynamic emotion identification [55,57], and both crystallized and fluid cognition were positively associated with dynamic emotion identification accuracy [53,99,100]. This pattern of findings supports that both basic cognitive as well as social-cognitive skills are impacted by age-related processes and that basic cognitive abilities support more complex social-cognitive processes, such as dynamic emotion identification.
Second, and intriguingly, we observed a negative association between plasma OT and dynamic emotion identification in our sample of older men, with this OT–social cognition link fully mediated by both crystallized and fluid cognition. For plasma AVP, in contrast, there was no association with dynamic emotion identification, but there was a significant indirect effect via crystallized cognition. These novel findings will be discussed in more detail next.
Previous studies in healthy young adults support intranasal OT-enhanced emotion identification performance for static emotion expressions (see [37,38] for meta-analyses), with a comparable effect observed in older men ([101]; but see [102,103]). Going beyond these earlier studies by investigating associations between endogenous OT/AVP levels and dynamic emotion identification in older men, the present study, however, found a negative relationship between plasma OT levels and dynamic emotion identification accuracy, but no effect for plasma AVP levels.
These results suggest that the associations endogenous plasma OT has with emotion identification are not necessarily aligned with the performance enhancement effects observed after intranasal OT administration [37]. Divergence in these relationships could be due to several reasons, including variations in the brain mechanisms subserving endogenous versus exogenous OT function [104]. Acute OT administration has been shown to elevate OT levels [22,105] beyond natural levels [106], which has altered, and can enhance, performance on social-cognitive tasks (but see [107]). These enhancement effects, however, have been found in accordance with individual and contextual factors, such as among individuals low in social-cognitive proficiency ([108]; e.g. low dynamic emotion identification abilities among older adults [91]). There is also emerging evidence regarding the involvement of genetic variation in neuropeptide systems (e.g. OT receptor gene expression) in social-cognitive and behavioural outcomes [109–111]. Therefore, individual endogenous differences within the OT and AVP systems may further impact the link to social cognition. While the correlational approach taken in the present study does not allow confirmation of this speculation, future experimental research will be able to systematically address mechanistic questions regarding the specific neuromodulatory processes underlying the relationship between neuropeptides and social cognition in ageing (e.g. by sampling endogenous neuropeptide levels before as well as after exogenous neuropeptide administration, and over time; [5]).
Regarding the negative relationship between OT and dynamic emotion identification detected in this study, it is possible that endogenous OT serves a lesser role than anticipated in the distinguishing of dynamic, complex social stimuli. Multiple theories have been put forth over the years to help conceptualize and explain OT function in the processing of social stimuli and the execution of related behaviour (e.g. social salience, prosociality, approach/withdrawal; see [112] for a recent summary). Regarding emotion identification, much of the current literature relies on findings gathered from experimental tasks that use unimodal, static presentations of emotional faces [38,103]. The few studies that incorporated dynamic stimuli reported in these overviews all used dynamic morphed emotions and found no support for an overall OT-related improvement in emotion identification [40,69,113–115]. Few exceptions showed that OT improved dynamic emotion identification for happy [115], fearful [40,114] and sad [113] faces or for specific individuals (i.e. with certain genetic variations relating to OT sensitivity [69]).
The present study, in contrast, assessed endogenous neuropeptide involvement in the accuracy of identifying a combination of unimodal and multimodal dynamic emotions. Previous theories have suggested that exogenously elevated OT levels can promote the salience of socially relevant features and/or facilitate early-stage processing (in line with the Social Salience Hypothesis [28]). Based on the available literature [37,38], this effect may be particularly prominent for the processing of emotions in static faces. The use of dynamic stimuli, perhaps owing to the incorporation of contextually richer and naturalistic sensory cues, may lead to performance ceiling effects. Further support for this interpretation comes from OT studies with older adults that suggested OT-improved social cognition in contexts where minimal social information was available. In particular, Campbell et al. [101] found that OT enhanced emotion identification during a static emotion identification paradigm. By contrast, Horta et al. [103] found no behavioural OT effect in a dynamic emotion identification paradigm using morphed dynamic stimuli. Grainger et al. [116] also found no OT effect on emotion identification using static emotions but found that performance on a related social-cognitive construct (i.e. theory of mind) was increased for video vignettes with minimal contextual information. OT did not, however, alter theory of mind for video vignettes enhanced with additional contextual information through incorporation of paralinguistic cues. This possible interpretation of our effects should be systematically explored in future work, such as by comparing behaviour in response to both static and dynamic stimuli in one experimental paradigm.
More recently, several theoretical frameworks have been introduced that account for contextual and temporal variations in OT function. For example, the allostatic theory of OT argues that in addition to the widespread physiological functions this neuropeptide has in the body, the OT system is also involved in the adaptive process of sensing and responding to changes in the surrounding environment, which includes the processing of non-social and social cues [12]. Similarly, largely based on electrophysiological research on the modulatory impacts of OT on attention, the Tri-Phasic Model of OT (TRIO [112]) conceptualizes the temporal dynamics and social gating mechanisms of OT upon three levels of stimulus processing: (1) perception, (2) selection and (3) evaluation. According to this model, whereas OT facilitates early attention for processing indiscriminate characteristics in the perception phase, OT-related selective attention to social stimuli may occur in the later phases of stimulus processing. Another multi-stage framework attempts to integrate previous theories about OT and considers variations in the roles of OT across different components of social decision-making: from sensory input/perception to valuation and behavioural outputs. This framework, developed by Piva & Chang [117], and based on a collection of preclinical and human work, illustrates the involvement of OT in fine-tuning information that is gathered, processed, and assigned value to carry out socially based decisions. This theoretical account can be applied to the computational steps involved in emotion identification as a social decision-making process, which involves the perception and integration of various sensory cues, valuation, and behavioural selection from a pre-established set of emotions. Thus, OT may assume different modulatory roles in response to emotional stimuli (e.g. increasing salience, promoting approach/withdrawal), which may occur simultaneously (and/or concurrently) along a continuum of perception and behaviour [117]. Conceptualizing the social function of OT in this way may help account for mixed evidence regarding the impact of OT on the brain and behaviour during emotion identification ([37,38]; for examples in human ageing see [102,103,116]), including in the present study and for other social-cognitive tasks [107].
These aforementioned models of OT function can each account for the seemingly non-beneficial effects observed for OT in response to dynamic emotions as an example of contextual and/or temporal variation, but more systematic work is needed, especially with attention to the role of OT in social cognition across the adult lifespan [5]. Future research could specifically test this speculation by incorporating varied social contextual information (e.g. dynamic versus static stimuli, congruent versus incongruent cues, varying degrees of cue complexity) in experimental tasks to delineate the processes by which neuropeptides subserve the processing of cues encountered in real-world social interactions.
In this study, we also provide, for the first time to our knowledge, evidence that basic cognitive skills fully mediate the association between plasma OT and dynamic emotion identification. In particular, higher plasma OT levels were associated with lower levels of both crystallized and fluid cognition, while higher scores in these cognitive capacities were associated with more accurate dynamic emotion identification, in older men. The role of cognitive capacities underlying complex social-cognitive abilities has been well-documented, with both crystallized [55,56] and fluid [57] cognition identified as crucial components subserving emotion identification (see [54] for a meta-analysis). For example, fluid cognitive abilities may be key to processing multiple nonverbal cues conveyed by emotion expressions [118] and are involved in the dynamic adaptation of emotion perception during social interactions [57]. Similarly, crystallized cognitive abilities may be critical for the comprehension and ascribing meaning to perceived emotion expressions, based on previous knowledge [54,118,119]. Therefore, a deficit in these basic cognitive skills may be directly linked to challenges in more complex social-cognitive processes (but see [120,121]).
Ageing is characterized by declines in both basic cognitive (specifically fluid cognition [48,49]) and social-cognitive [122] skills, including emotion identification [99]. There are currently no effective pharmacological treatments to address age-related social-cognitive decline. Intranasal OT constitutes a promising candidate based on its effects on enhancing emotion identification in young adults [37,38] and has recently been discussed as a potential therapeutic agent for social-cognitive deficits in older adults [23,79,123]. In particular, exogenous OT has been found specifically beneficial for individuals low in social-cognitive abilities [124,125]. Leknes et al. [126], for example, reported greater OT-enhancement in sensitivity to emotional faces in participants with low baseline emotional sensitivity. Thus, exogenous OT may be useful for modulating processes impacted by age-related decline, including fluid cognition and emotion identification [54,118].
Our finding that both endogenous OT and AVP levels were negatively correlated with crystallized cognition (in addition to a negative correlation between plasma OT and fluid cognition) qualifies our current understanding of the relationship between neuropeptide plasma levels and social cognition in the context of ageing. This observed mediation of basic cognition on the neuropeptide–social cognition link has potential to spur future research questions on neuropeptide administration in ageing (e.g. Does increasing peripheral levels of OT via intranasal administration result in improved social-cognitive skills? Do pre-administration endogenous levels affect the impact of exogenously administered OT?).
Going beyond Plasencia et al. [60], which reported a positive association between plasma OT levels and processing speed (but not short-term memory) in both young and older adults, we assessed basic cognition more comprehensively in the present study via the NIH Toolbox Cognition Battery. This assessment battery comprises measures of attention, memory, and executive functioning, which have been found to negatively correlate with plasma OT levels in healthy adults [127] and patient populations (e.g. schizophrenia [46,62]). Building on results from the present study, a thorough examination is warranted for associations of both peripheral and central neuropeptide levels with a variety of cognitive processes (e.g. attention, executive function, decision-making) to specify respective interplays with social-cognitive capacities in ageing.
Of note, different from the observed effects pertaining to plasma OT, we found no associations between plasma AVP and dynamic emotion identification, though AVP plasma levels were negatively associated with crystallized cognition, similar to our findings with plasma OT. Largely in line with this null effect for the AVP–social cognition link, using intranasal administration, Thompson et al. [34] reported no effect of AVP on attention to neutral versus emotional faces. Other previous work using intranasal AVP, however, found differential effects on social-cognitive capacities by stimulus valence/emotion type as well as participant sex. For example, Uzefovsky et al. [41] reported that intranasal AVP, compared with placebo, reduced emotion identification for negative male faces, but they observed no effect for positive male or female faces, in a sample of young men. Vadas et al. [42] observed similar effects for angry faces in men with schizophrenia, while emotion identification decreased for sad faces but was enhanced for fearful faces after AVP administration in women with schizophrenia. Building on these previous findings, a systematic examination of the role of stimulus characteristics, such as valence and emotion type, as well as interindividual difference variables, such as sex, on the association between OT/AVP and social-cognitive function in ageing is warranted, by leveraging a larger sample that is well-powered to address these important qualifying questions.
It is also possible that the differences in the neuropeptide–social cognition link for OT versus AVP that we found in these data reflect age-related differences in these neuropeptide systems. For example, Campbell et al. [101] found intranasal OT administration improved emotion identification for older (but not young) men, while Uzefovsky et al. [41] showed that intranasal AVP decreased emotion identification in young men. These findings contrast with the present study, which observed a negative relationship between endogenous OT and emotion identification and no relationship between endogenous AVP and emotion identification in older men. Determination of specific differences in the neuromodulatory mechanisms behind both endogenous and exogenous OT and AVP and their respective impact on social cognition, especially in ageing, will continue to be an important topic for future research [128].
(a) . Limitations
Owing to logistics of the larger clinical trial (see [79]), blood samples and cognitive data were collected in a separate experimental session from the emotion identification data. There is evidence that endogenous OT and AVP do not show diurnal patterns [129,130], that peripheral plasma OT levels correlate with central endogenous levels [131], and that plasma OT levels remain stable and correlate over time [132,133], suggesting that one-time sampling of OT/AVP constitutes a representative measure of these neuropeptides. By contrast, recent work suggests that OT plasma levels vary across days [134] owing to fluctuations in sleep, physical activity, food intake, and hydration levels [105]. Thus, moving forward it will be necessary to specifically address temporal dynamics of neuropeptide action, including among older adults, and to determine differences between one-time versus repeated sampling in their representativeness of acute versus chronic neuropeptide levels and associated function in ageing.
Growing evidence suggests sex-dimorphism in OT and AVP systems and function [135–137]. In addition, there are sex differences in the effects of OT and AVP, specifically on social cognition [138], including emotion identification [43,139], and associated neurobiological processes ([3]; see also [140]). For example, Luo et al. [139] found that for women, but not men, intranasal OT decreased coupling of the amygdala, anterior cingulate, and inferior frontal gyrus when processing negative emotional faces. Rubin et al. [43] showed that for women with schizophrenia higher endogenous OT levels were associated with perceiving faces as happier, while no such association was observed in men with schizophrenia. Similarly supporting sex-dimorphic effects, Vadas et al. [42] reported less accurate identification of angry faces by men, while women became less accurate in identifying sad faces but more accurate in the identification of fearful faces after intranasal AVP administration. Furthermore, sex (in interaction with age) was found to modulate the relationship of OT and AVP with affiliative processes and cognition [60]. This emerging evidence combined supports the need for future research to systematically consider sex effects on the associations between plasma OT/AVP and dynamic emotion identification. The larger clinical trial that the present data came from included a sample of older women. This female sample (n = 48) was, however, much smaller than the male sample (n = 104),providing limited power for a comparison between the sexes. Future work would benefit from a thorough analysis of interactions of the OT/AVP systems with gonadal hormones, such as testosterone and oestrogen [141,142], implicated in the binding and receptor expression of neuropeptides [143–145], as underlying the neuropeptide sex-dimorphism in social cognition, including in ageing.
As noted above, measurement of central OT/AVP levels is highly invasive, requiring lumbar puncture to acquire cerebrospinal fluid. Therefore, in the present study, we used peripheral measures as a proxy for central neuropeptide levels. This approach is in line with other human research [22,128] and was based on evidence of strong correlations (r = 0.80) between peripheral and central OT in humans and non-human primates (using enzyme-linked immunoassays; [131]; see also [22,146] for meta-analyses). Measurement of central neuropeptide levels after OT administration in their relation to endogenous levels and associated effects on social cognition constitutes a promising future research avenue [5,79]. This work will also benefit from investigating neuropeptide receptors or gene expression of OT/AVP, associated with social behaviour and cognitive states [41,110,147,148]. For example, OT receptor gene expression has been linked to amygdala size [149] and neural activation during face processing [111] and has been associated with reduced functional brain connectivity in those with impaired social-cognitive abilities [147,150]. Additionally, OT receptor gene expression has been shown to have a unique role in social processes that are independent of the effects of plasma OT [151]. Further, factors such as early life experiences may alter the OT/AVP system long-term, rendering their assessment in future research warranted. For example, adults who had high levels of childhood adversity were found to have lower plasma OT, increased OT receptor gene methylation, and decreased response after OT administration [152], as well as altered brain activation after AVP administration [153]. Analyses of these separate but highly integrated components of the endogenous neuropeptide systems will be crucial in advancing knowledge of the role of neuropeptides in basic and social-cognitive skills.
5. Conclusion
Taken together, findings from this study inform the neuropeptide–social cognition link by demonstrating that basic crystallized and fluid cognitive capacities underlie the associations between endogenous plasma OT and dynamic emotion identification in healthy older men. These novel findings indicate that endogenous neuropeptides may have different neuromodulatory effects on social cognition from what has been previously observed with exogenous OT and AVP administration. Our findings support that the relationship between plasma OT and dynamic emotion identification neuropeptide–social cognitive association is mediated by basic cognitive skills in older men. Future work investigating moderations reflective of sex-dimorphisms, genetic variations, and differences in task stimulus characteristics will further advance understanding of the important link between neuropeptides and social-cognitive capacities across the adult lifespan and in ageing.
Acknowledgements
The authors would like to thank Eliany Perez, Desiree Lussier, Ian Frazier, Devon Weir, Bailey Hoffman and Sevilay Yumusak, along with the staff at the Social-Cognitive and Affective Development laboratory, the Institute on Aging, and the McKnight Brain Institute at the University of Florida, for their help with study logistics, participant recruitment and data collection. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Rebecca Polk, Email: r.polk@ufl.edu.
Marilyn Horta, Email: mhorta09@ufl.edu.
Natalie C. Ebner, Email: natalie.ebner@ufl.edu.
Ethics
The large project in which this study was embedded was approved by the University of Florida Institutional Review Board (IRB201300801) and registered as a clinical trial (NCT02069431). Written informed consent was obtained from all participants by trained research staff.
Data accessibility
All data and code are available on the Open Science Framework (OSF) repository: https://osf.io/xv38y/?view_only=3ca2fada765f42c3a5a0e30900108fc3.
The data are provided in the electronic supplementary material [154].
Authors' contributions
R.P.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, software, validation, visualization, writing—original draft, writing—review and editing; M.H.: conceptualization, data curation, investigation, methodology, project administration, software, validation, visualization, writing—original draft, writing—review and editing; T.L.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing—review and editing; E.P.: data curation, validation, writing—review and editing; M.O.: investigation, project administration, writing—review and editing; H.P.N.: data curation, investigation, methodology, validation, writing—review and editing; C.S.C.: data curation, investigation, methodology, resources, supervision, validation, writing—review and editing; N.C.E.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by: the University of Florida (UF) Claude D. Pepper Older Americans Independence Center (P30AG028740); the UF College of Liberal Arts and Sciences; the UF Department of Psychology; the UF Center for Cognitive Aging and Memory; the UF Jacquelin Goldman Research Grant; the UF Pain Research and Intervention Center of Excellence-Clinical and Translational Science Institute-Institute on Aging (ARG DTD 03–26–2008); the National Institute on Aging (R01AG059809); a National Institute on Aging Predoctoral Fellowship on Physical, Cognitive, and Mental Health in Social Context (T32AG020499); a National Institute on Aging Predoctoral Fellowship on Training in Non-Pharmacological Interventions for Cognition in Aging, MCI, and Alzheimer's Disease (T32AG020499); and the UF Substance Abuse Training Center in Public Health from the National Institute of Drug Abuse (T32DA035167). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Carter C, et al. 2020. Is oxytocin ‘nature's medicine’? Pharmacol. Rev. 72, 829-861. ( 10.1124/PR.120.019398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrichs M, von Dawans B, Domes G. 2009. Oxytocin, vasopressin, and human social behavior. Front. Neuroendocrinol. 30, 548-557. ( 10.1016/J.YFRNE.2009.05.005) [DOI] [PubMed] [Google Scholar]
- 3.Zink CF, Meyer-Lindenberg A. 2012. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm. Behav. 61, 400-409. ( 10.1016/J.YHBEH.2012.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebner NC, Maura GM, MacDonald K, Westberg L, Fischer H. 2013. Oxytocin and socioemotional aging: current knowledge and future trends. Front. Hum. Neurosci. 7, 487. ( 10.3389/fnhum.2013.00487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horta M, Pehlivanoglu D, Ebner NC. 2020. The role of intranasal oxytocin on social cognition: an integrative human lifespan approach. Curr. Behav. Neurosci. Rep. 7, 175-192. ( 10.1007/s40473-020-00214-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huffmeijer R, van IJzendoorn MH, Bakermans-Kranenburg MJ. 2013. Ageing and oxytocin: a call for extending human oxytocin research to ageing populations – a mini-review. Gerontology 59, 32-39. ( 10.1159/000341333) [DOI] [PubMed] [Google Scholar]
- 7.Sannino S, Chini B, Grinevich V. 2017. Lifespan oxytocin signaling: maturation, flexibility, and stability in newborn, adolescent, and aged brain. Dev. Neurobiol. 77, 158-168. ( 10.1002/dneu.22450) [DOI] [PubMed] [Google Scholar]
- 8.Gwee PC, Tay BH, Brenner S, Venkatesh B. 2009. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 9, 1-15. ( 10.1186/1471-2148-9-47/FIGURES/7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minakata H. 2010. Oxytocin/vasopressin and gonadotropin-releasing hormone from cephalopods to vertebrates. Ann. NY Acad. Sci. 1200, 33-42. ( 10.1111/J.1749-6632.2010.05569.X) [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. 2011. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 12, 524-538. ( 10.1038/nrn3044) [DOI] [PubMed] [Google Scholar]
- 11.Neumann ID, Landgraf R. 2012. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649-659. ( 10.1016/J.TINS.2012.08.004) [DOI] [PubMed] [Google Scholar]
- 12.Quintana DS, Guastella AJ. 2020. An allostatic theory of oxytocin. Trends Cogn. Sci. 24, 515-528. ( 10.1016/j.tics.2020.03.008) [DOI] [PubMed] [Google Scholar]
- 13.Gibbs DM. 1986. Vasopressin and oxytocin: hypothalamic modulators of the stress response: a review. Psychoneuroendocrinology 11, 131-139. ( 10.1016/0306-4530(86)90048-X) [DOI] [PubMed] [Google Scholar]
- 14.Purba JS, Hoogendijk WJG, Hofman MA, Swaab DF. 1996. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry 53, 137-143. ( 10.1001/archpsyc.1996.01830020055007) [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Lindenberg A. 2008. Impact of prosocial neuropeptides on human brain function. Prog. Brain Res. 170, 463-470. ( 10.1016/S0079-6123(08)00436-6) [DOI] [PubMed] [Google Scholar]
- 16.Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. 2007. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann. NY Acad. Sci. 1098, 312-322. ( 10.1196/annals.1384.006) [DOI] [PubMed] [Google Scholar]
- 17.Gouin JP, Carter CS, Pournajafi-Nazarloo H, Glaser R, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. 2010. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 35, 1082-1090. ( 10.1016/J.PSYNEUEN.2010.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. 2019. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology 107, 225-231. ( 10.1016/J.PSYNEUEN.2019.05.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polito AB, Goldstein DL, Sanchez L, Cool DR, Morris M. 2006. Urinary oxytocin as a non-invasive biomarker for neurohypophyseal hormone secretion. Peptides 27, 2877-2884. ( 10.1016/j.peptides.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 20.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. 2002. Sniffing neuropeptides: a transnasal approach to the human brain. Nat. Neurosci. 5, 514-516. ( 10.1038/nn0602-849) [DOI] [PubMed] [Google Scholar]
- 21.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. 2013. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38, 1985-1993. ( 10.1016/J.PSYNEUEN.2013.03.003) [DOI] [PubMed] [Google Scholar]
- 22.Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, Quintana DS. 2017. The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 78, 117-124. ( 10.1016/j.neuiorev.2017.04.017) [DOI] [PubMed] [Google Scholar]
- 23.Horta M, Kaylor K, Feifel D, Ebner NC. 2020. Chronic oxytocin administration as a tool for investigation and treatment: a cross-disciplinary systematic review. Neurosci. Biobehav. Rev. 108, 1-23. ( 10.1016/j.neubiorev.2019.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insel TR. 2010. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65, 768-779. ( 10.1016/J.NEURON.2010.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp AH, Guastella AJ. 2011. The role of oxytocin in human affect. Curr. Dir. Psychol. Sci. 20, 222-231. ( 10.1177/0963721411417547) [DOI] [Google Scholar]
- 26.Peled-Avron L, Abu-Akel A, Shamay-Tsoory S. 2020. Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neurosci. Biobehav. Rev. 114, 70-95. ( 10.1016/j.neuiorev.2020.04.023) [DOI] [PubMed] [Google Scholar]
- 27.Torres N, Martins D, Santos AJ, Prata D, Veríssimo M. 2018. How do hypothalamic nonapeptides shape youth's sociality? A systematic review on oxytocin, vasopressin and human socio-emotional development. Neurosci. Biobehav. Rev. 90, 309-331. ( 10.1016/J.NEUBIOREV.2018.05.004) [DOI] [PubMed] [Google Scholar]
- 28.Shamay-Tsoory SG, Abu-Akel A. 2016. The social salience hypothesis of oxytocin. Biol. Psychiatry 79, 194-202. ( 10.1016/j.biopsych.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 29.Harari-Dahan O, Bernstein A. 2014. A general approach-avoidance hypothesis of oxytocin: accounting for social and non-social effects of oxytocin. Neurosci. Biobehav. Rev. 47, 506-519. ( 10.1016/J.NEUBIOREV.2014.10.007) [DOI] [PubMed] [Google Scholar]
- 30.Feldman R. 2012. Oxytocin and social affiliation in humans. Horm. Behav. 61, 380-391. ( 10.1016/j.yhbeh.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 31.Ross HE, Young LJ. 2009. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 30, 534-547. ( 10.1016/j.yfrne.2009.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albers HE. 2012. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm. Behav. 61, 283-292. ( 10.1016/J.YHBEH.2011.10.007) [DOI] [PubMed] [Google Scholar]
- 33.Heinrichs M, Domes G. 2008. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 170, 337-350. ( 10.1016/S0079-6123(08)00428-7) [DOI] [PubMed] [Google Scholar]
- 34.Thompson R, Gupta S, Miller K, Mills S, Orr S. 2004. The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology 29, 35-48. ( 10.1016/S0306-4530(02)00133-6) [DOI] [PubMed] [Google Scholar]
- 35.Benarroch EE. 2013. Oxytocin and vasopressin. Neurology 80, 1521-1528. ( 10.1212/WNL.0B013E31828CFB15) [DOI] [PubMed] [Google Scholar]
- 36.Mayer JD, Geher G. 1996. Emotional intelligence and the identification of emotion. Intelligence 22, 89-113. ( 10.1016/S0160-2896(96)90011-2) [DOI] [Google Scholar]
- 37.Leppanen J, Ng KW, Tchanturia K, Treasure J. 2017. Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neurosci. Biobehav. Rev. 78, 125-144. ( 10.1016/J.NEUBIOREV.2017.04.010) [DOI] [PubMed] [Google Scholar]
- 38.Shahrestani S, Kemp AH, Guastella AJ. 2013. The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: a meta-analysis. Neuropsychopharmacology 38, 1929-1936. ( 10.1038/npp.2013.86) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Averbeck BB. 2010. Oxytocin and the salience of social cues. Proc. Natl Acad. Sci. USA 107, 9033-9034. ( 10.1073/PNAS.1004892107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. 2010. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia 48, 179-184. ( 10.1016/J.NEUROPSYCHOLOGIA.2009.09.003) [DOI] [PubMed] [Google Scholar]
- 41.Uzefovsky F, Shalev I, Israel S, Knafo A, Ebstein RP. 2012. Vasopressin selectively impairs emotion recognition in men. Psychoneuroendocrinology 37, 576-580. ( 10.1016/J.PSYNEUEN.2011.07.018) [DOI] [PubMed] [Google Scholar]
- 42.Vadas L, et al. 2017. Sex-specific effect of intranasal vasopressin, but not oxytocin, on emotional recognition and perception in schizophrenia patients. Eur. Psychiatry 41(Suppl. 1), S387-S388. ( 10.1016/J.EURPSY.2017.02.430) [DOI] [Google Scholar]
- 43.Rubin LH, Carter CS, Drogos L, Jamadar R, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. 2011. Sex-specific associations between peripheral oxytocin and emotion perception in schizophrenia. Schizophr. Res. 130, 266-270. ( 10.1016/J.SCHRES.2011.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spilka MJ, Keller WR, Buchanan RW, Gold JM, Koenig JI, Strauss GP. 2022. Endogenous oxytocin levels are associated with facial emotion recognition but not gaze behavior in schizophrenia. Acta Psychiat. Scand. 145, 494-506. ( 10.1111/acps.13421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tas C, Brown EC, Onur E, Aydemir O, Brune M. 2016. The associations between endogenous oxytocin levels and emotion recognition in bipolar disorder. Bull. Clin. Psychopharmacol. 25, 19-26. ( 10.5455/BCP.20140514043545) [DOI] [Google Scholar]
- 46.Aydın O, Lysaker PH, Balıkçı K, Ünal-Aydın P, Esen-Danacı A. 2018. Associations of oxytocin and vasopressin plasma levels with neurocognitive, social cognitive and meta cognitive function in schizophrenia. Psychiatry Res. 270, 1010-1016. ( 10.1016/J.PSYCHRES.2018.03.048) [DOI] [PubMed] [Google Scholar]
- 47.Bhandari R, van der Veen R, Parsons CE, Young KS, Voorthuis A, Bakermans-Kranenburg MJ, Stein A, Kringelbach ML, van IJzendoorn MH. 2014. Effects of intranasal oxytocin administration on memory for infant cues: moderation by childhood emotional maltreatment. Social Neurosci. 9, 536-547. ( 10.1080/17470919.2014.932307) [DOI] [PubMed] [Google Scholar]
- 48.Harada CN, Natelson Love MC, Triebel KL. 2013. Normal cognitive aging. Clin. Geriatr. Med. 29, 737. ( 10.1016/J.CGER.2013.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salthouse TA. 2019. Trajectories of normal cognitive aging. Psychol. Aging 34, 17-24. ( 10.1037/PAG0000288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akshoomoff N, et al. 2013. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr. Soc. Res. Child Dev. 78, 119-132. ( 10.1111/MONO.12038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cattell RB. 1963. Theory of fluid and crystallized intelligence: a critical experiment. J. Educ. Psychol. 54, 1-22. ( 10.1037/H0046743) [DOI] [PubMed] [Google Scholar]
- 52.Horn JL, Cattell RB. 1966. Refinement and test of the theory of fluid and crystallized general intelligences. J. Educ. Psychol. 57, 253-270. ( 10.1037/H0023816) [DOI] [PubMed] [Google Scholar]
- 53.Suzuki A, Akiyama H. 2013. Cognitive aging explains age-related differences in face-based recognition of basic emotions except for anger and disgust. Aging Neuropsychol. Cogn. 20, 253-270. ( 10.1080/13825585.2012.692761) [DOI] [PubMed] [Google Scholar]
- 54.Schlegel K, Palese T, Mast MS, Rammsayer TH, Hall JA, Murphy NA. 2019. A meta-analysis of the relationship between emotion recognition ability and intelligence. Cogn. Emot. 34, 329-351. ( 10.1080/02699931.2019.1632801) [DOI] [PubMed] [Google Scholar]
- 55.Davis SK, Morningstar M, Qualter P. 2021. Ability EI predicts recognition of dynamic facial emotions, but not beyond the effects of crystallized IQ. Pers. Individ. Differ. 169, 109968. ( 10.1016/J.PAID.2020.109968) [DOI] [Google Scholar]
- 56.MacCann C, Joseph DL, Newman DA, Roberts RD. 2014. Emotional intelligence is a second-stratum factor of intelligence: evidence from hierarchical and bifactor models. Emotion 14, 358-374. ( 10.1037/A0034755) [DOI] [PubMed] [Google Scholar]
- 57.Lynn SK, Ibagon C, Bui E, Palitz SA, Simon NM, Barrett LF. 2016. Working memory capacity is associated with optimal adaptation of response bias to perceptual sensitivity in emotion perception. Emotion 16, 155-163. ( 10.1037/EMO0000111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips LH, Channon S, Tunstall M, Hedenstrom A, Lyons K. 2008. The role of working memory in decoding emotions. Emotion 8, 184. ( 10.1037/1528-3542.8.2.184) [DOI] [PubMed] [Google Scholar]
- 59.Salthouse TA, Pink JE. 2013. Why is working memory related to fluid intelligence? Psychon. Bull. Rev. 15, 364-371. ( 10.3758/PBR.15.2.364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plasencia G, Luedicke JM, Nazarloo HP, Carter CS, Ebner NC. 2019. Plasma oxytocin and vasopressin levels in young and older men and women: functional relationships with attachment and cognition. Psychoneuroendocrinology 110, 104419. ( 10.1016/j.psyneuen.2019.104419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feifel D, MacDonald K, Cobb P, Minassian A. 2012. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr. Res. 139, 207-210. ( 10.1016/J.SCHRES.2012.05.018) [DOI] [PubMed] [Google Scholar]
- 62.Strauss GP, Chapman HC, Keller WR, Koenig JI, Gold JM, Carpenter WT, Buchanan RW. 2019. Endogenous oxytocin levels are associated with impaired social cognition and neurocognition in schizophrenia. J. Psychiatr. Res. 112, 38-43. ( 10.1016/J.JPSYCHIRES.2019.02.017) [DOI] [PubMed] [Google Scholar]
- 63.Kapetaniou GE, Reinhard MA, Christian P, Jobst A, Tobler PN, Padberg F, Soutschek A. 2021. The role of oxytocin in delay of gratification and flexibility in non-social decision making. eLife 10, e1844. ( 10.7554/ELIFE.61844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fehm-Wolfsdorf G, Born J, Voigt KH, Fehm HL. 1984. Human memory and neurohypophyseal hormones: opposite effects of vasopressin and oxytocin. Psychoneuroendocrinology 9, 285-292. ( 10.1016/0306-4530(84)90007-6) [DOI] [PubMed] [Google Scholar]
- 65.Ferrier BM, Kennet DJ, Devlin MC. 1980. Influence of oxytocin on human memory processes. Life Sci. 27, 2311-2317. ( 10.1016/0024-3205(80)90499-3) [DOI] [PubMed] [Google Scholar]
- 66.Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, Hellhammer DH. 2004. Selective amnesic effects of oxytocin on human memory. Physiol. Behav. 83, 31-38. ( 10.1016/J.PHYSBEH.2004.07.020) [DOI] [PubMed] [Google Scholar]
- 67.Geng CH, Wang C, Yang J, Wang H, Ma RQ, Liu X, Wang CH. 2017. Arginine vasopressin improves the memory deficits in Han Chinese patients with first-episode schizophrenia. Peptides 97, 8-15. ( 10.1016/J.PEPTIDES.2017.09.002) [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, et al. 2020. AVP(4-8) improves cognitive behaviors and hippocampal synaptic plasticity in the APP/PS1 mouse model of Alzheimer's disease. Neurosci. Bull. 36, 254-262. ( 10.1007/S12264-019-00434-0/FIGURES/5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. 2016. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 10, 581-593. ( 10.1007/s11682-015-9411-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. 2015. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 9, 754-764. ( 10.1007/S11682-014-9333-9/FIGURES/3) [DOI] [PubMed] [Google Scholar]
- 71.Rilling JK, et al. 2014. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology 39, 237-248. ( 10.1016/J.PSYNEUEN.2013.09.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebner NC, Horta M, Lin T, Feifel D, Fischer H, Cohen RA. 2015. Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. 7, 175. ( 10.3389/fnagi.2015.00175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA. 2016. Oxytocin's effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology 69, 50-59. ( 10.1016/j.psyneuen.2016.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Social Psychol. 54, 1063-1070. ( 10.1037/0022-3514.54.6.1063) [DOI] [PubMed] [Google Scholar]
- 75.Röcke C, Li SC, Smith J. 2009. Intraindividual variability in positive and negative affect over 45 days: do older adults fluctuate less than young adults? Psychol. Aging 24, 863. ( 10.1037/A0016276) [DOI] [PubMed] [Google Scholar]
- 76.Bänziger T, Mortillaro M, Scherer KR. 2012. Introducing the Geneva Multimodal expression corpus for experimental research on emotion perception. Emotion 12, 1161-1179. ( 10.1037/A0025827) [DOI] [PubMed] [Google Scholar]
- 77.Brandt J, Spencer M, Folstein M. 1988. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1, 111-117. [Google Scholar]
- 78.McCann SM, Antunes-Rodrigues J, Jankowski M, Gutkowska J. 2002. Oxytocin, vasopressin and atrial natriuretic peptide control body fluid homeostasis by action on their receptors in brain, cardiovascular system and kidney. Prog. Brain Res. 139, 309-328. ( 10.1016/S0079-6123(02)39027-7) [DOI] [PubMed] [Google Scholar]
- 79.Rung JM, et al. 2021. Safety and tolerability of chronic intranasal oxytocin in older men: results from a randomized controlled trial. Psychopharmacology (Berl.) 238, 2405-2418. ( 10.1007/S00213-021-05862-3/FIGURES/2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdes-Hernandez PA, et al. 2021. Chronic oxytocin administration in older men modulates functional connectivity during animacy perception. Aging Brain 1, 100023. ( 10.1016/J.NBAS.2021.100023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu C, Hammock EAD, Joiner TE. 2020. Unextracted plasma oxytocin levels decrease following in-laboratory social exclusion in young adults with a suicide attempt history. J. Psychiatr. Res. 121, 173-181. ( 10.1016/J.JPSYCHIRES.2019.11.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saxbe D, Khaled M, Horton KT, Mendez AJ. 2019. Maternal prenatal plasma oxytocin is positively associated with prenatal psychological symptoms, but method of immunoassay extraction may affect results. Biol. Psychol. 147, 107718. ( 10.1016/J.BIOPSYCHO.2019.107718) [DOI] [PubMed] [Google Scholar]
- 83.Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, Wagster MV. 2010. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 9, 138-139. ( 10.1016/S1474-4422(09)70335-7) [DOI] [PubMed] [Google Scholar]
- 84.Hausman HK, et al. 2020. The role of resting-state network functional connectivity in cognitive aging. Front. Aging Neurosci. 12, 177. ( 10.3389/FNAGI.2020.00177/BIBTEX) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scott EP, Sorrell A, Benitez A. 2019. Psychometric properties of the NIH Toolbox Cognition Battery in healthy older adults: reliability, validity, and agreement with standard neuropsychological tests. J. Int. Neuropsychol. Soc. 25, 857-867. ( 10.1017/S1355617719000614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Snitz BE, et al. 2020. Associations between NIH Toolbox Cognition Battery and in vivo brain amyloid and tau pathology in non-demented older adults. Alzheimer's Dement. 12, e12018. ( 10.1002/DAD2.12018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dael N, Goudbeek M, Scherer KR. 2013. Perceived gesture dynamics in nonverbal expression of emotion. Perception 42, 642-657. ( 10.1068/p7364) [DOI] [PubMed] [Google Scholar]
- 88.Schlegel K, Grandjean D, Scherer KR. 2012. Emotion recognition: unidimensional ability or a set of modality- and emotion-specific skills? Pers. Individ. Differ. 53, 16-21. ( 10.1016/J.PAID.2012.01.026) [DOI] [Google Scholar]
- 89.Shuman V, Clark-Polner E, Meuleman B, Sander D, Scherer KR. 2017. Emotion perception from a componential perspective. Cogn. Emot. 31, 47-56. ( 10.1080/02699931.2015.1075964/SUPPL_FILE/PCEM_A_1075964_SM4894.DOCX) [DOI] [PubMed] [Google Scholar]
- 90.Abo Foul Y, Eitan R, Mortillaro M, Aviezer H. 2022. Perceiving dynamic emotions expressed simultaneously in the face and body minimizes perceptual differences between young and older adults. J. Gerontol. B 77, 84-93. ( 10.1093/GERONB/GBAB064) [DOI] [PubMed] [Google Scholar]
- 91.Cortes DS, Tornberg C, Bänziger T, Elfenbein HA, Fischer H, Laukka P. 2021. Effects of aging on emotion recognition from dynamic multimodal expressions and vocalizations. Scient. Rep. 11, 2647. ( 10.1038/s41598-021-82135-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayes AF. 2018. Introduction to mediation, moderation, and conditional process analysis, 2nd edn. New York, NY: Guilford Press. [Google Scholar]
- 93.Ebner NC, et al. 2020. Uncovering susceptibility risk to online deception in aging. J. Gerontol. B 75, 522-533. ( 10.1093/geronb/gby036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Small BJ, Dixon RA, McArdle JJ. 2011. Tracking cognition–health changes from 55 to 95 years of age. J. Gerontol. B, 66(Suppl. 1), i153-i161. ( 10.1093/GERONB/GBQ093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuan Y, Li J, Zhang N, Fu P, Jing Z, Yu C, Zhao D, Hao W, Zhou C. 2021. Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. BMC Psychiatry 21, 54. ( 10.1186/S12888-021-03059-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. 2011. Multiple imputation by chained equations: what is it and how does it work? Int. J. Methods Psychiatr. Res. 20, 40. ( 10.1002/MPR.329) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.StataCorp. 2019. Stata statistical software: release 16. College Station, TX: StataCorp.
- 98.Little RJA. 1988. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 83, 1198-1202. ( 10.1080/01621459.1988.10478722) [DOI] [Google Scholar]
- 99.Hayes GS, Mclennan SN, Henry JD, Phillips LH, Terrett G, Rendell PG, Pelly RM, Labuschagne I. 2020. Task characteristics influence facial emotion recognition age-effects: a meta-analytic review. Psychol. Aging 35, 295-315. ( 10.1037/PAG0000441) [DOI] [PubMed] [Google Scholar]
- 100.Ruffman T, Henry JD, Livingstone V, Phillips LH. 2008. A meta-analytic review of emotion recognition and aging: implications for neuropsychological models of aging. Neurosci. Biobehav. Rev. 32, 863-881. ( 10.1016/J.NEUBIOREV.2008.01.001) [DOI] [PubMed] [Google Scholar]
- 101.Campbell A, Ruffman T, Murray JE, Glue P. 2014. Oxytocin improves emotion recognition for older males. Neurobiol. Aging 35, 2246-2248. ( 10.1016/j.neurobiolaging.2014.04.021) [DOI] [PubMed] [Google Scholar]
- 102.Grainger SA, Henry JD, Steinvik HR, Vanman EJ, Rendell PG, Labuschagne I. 2018. Intranasal oxytocin does not reduce age-related difficulties in social cognition. Horm. Behav. 99, 25-34. ( 10.1016/j.yhbeh.2018.01.009) [DOI] [PubMed] [Google Scholar]
- 103.Horta M, Ziaei M, Lin T, Porges EC, Fischer H, Feifel D, Spreng RN, Ebner NC. 2019. Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiol. Aging 78, 42-51. ( 10.1016/J.NEUROBIOLAGING.2019.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borland JM, Rilling JK, Frantz KJ, Albers HE. 2018. Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology 44, 97-110. ( 10.1038/s41386-018-0129-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Quintana DS, Westlye LT, Smerud KT, Mahmoud RA, Andreassen OA, Djupesland PG. 2018. Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm. Behav. 102, 85-92. ( 10.1016/j.yhbeh.2018.05.004) [DOI] [PubMed] [Google Scholar]
- 106.Gossen A, Hahn A, Westphal L, Prinz S, Schultz RT, Gründer G, Spreckelmeyer KN. 2012. Oxytocin plasma concentrations after single intranasal oxytocin administration – a study in healthy men. Neuropeptides 46, 211-215. ( 10.1016/J.NPEP.2012.07.001) [DOI] [PubMed] [Google Scholar]
- 107.Tabak BA, Teed AR, Castle E, Dutcher JM, Meyer ML, Bryan R, Irwin MR, Lieberman MD, Eisenberger NI. 2019. Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: evidence from a randomized controlled trial. Psychoneuroendocrinology 107, 124-132. ( 10.1016/j.psyneuen.2019.04.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bartz JA, Nitschke JP, Krol SA, Tellier PP. 2019. Oxytocin selectively improves empathic accuracy: a replication in men and novel insights in women. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 1042-1048. ( 10.1016/j.bpsc.2019.01.014) [DOI] [PubMed] [Google Scholar]
- 109.Audunsdottir K, Quintana D. 2021. Oxytocin's dynamic role across the lifespan. PsyArXiv. ( 10.31234/OSF.IO/GPE7F) [DOI]
- 110.Quintana DS, Rokicki J, van der Meer D, Alnæs D, Kaufmann T, Córdova-Palomera A, Dieset I, Andreassen OA, Westlye LT. 2019. Oxytocin pathway gene networks in the human brain. Nat. Commun. 10, 668. ( 10.1038/s41467-019-08503-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Westberg L, Henningsson S, Zettergren A, Svärd J, Hovey D, Lin T, Ebner NC, Fischer H. 2016. Variation in the oxytocin receptor gene is associated with face recognition and its neural correlates. Front. Behav. Neurosci. 10, 178. ( 10.3389/FNBEH.2016.00178/BIBTEX) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pehlivanoglu D, Myers E, Ebner NC. 2020. Tri-Phasic Model of Oxytocin (TRIO): a systematic conceptual review of oxytocin-related ERP research. Biol. Psychol. 154, 107917. ( 10.1016/j.biopsycho.2020.107917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kirkpatrick M, Lee R, Wardle MC, Jacob S, De Wit H. 2014. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacoly 39, 1654–1663. ( 10.1038/npp.2014.12) [DOI] [PMC free article] [PubMed]
- 114.Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, Domes G. 2012. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology 37, 475-481. ( 10.1016/J.PSYNEUEN.2011.07.015) [DOI] [PubMed] [Google Scholar]
- 115.Marsh AA, Yu HH, Pine DS, Blair RJR. 2010. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl.) 209, 225-232. ( 10.1007/S00213-010-1780-4) [DOI] [PubMed] [Google Scholar]
- 116.Grainger SA, Henry JD, Steinvik HR, Vanman EJ. 2019. Intranasal oxytocin does not alter initial perceptions of facial trustworthiness in younger or older adults. J. Psychopharmacol. 33, 250-254. ( 10.1177/0269881118806303) [DOI] [PubMed] [Google Scholar]
- 117.Piva M, Chang SWC. 2018. An integrated framework for the role of oxytocin in multistage social decision-making. Am. J. Primatol. 80, 10. ( 10.1002/AJP.22735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davitz J. 1964. The communication of emotional meaning. Oxford, UK: McGraw Hill. [Google Scholar]
- 119.Rosip JC, Hall JA. 2004. Knowledge of nonverbal cues, gender, and nonverbal decoding accuracy. J. Nonverbal Behav. 28, 267-286. ( 10.1007/S10919-004-4159-6) [DOI] [Google Scholar]
- 120.Phillips LH, MacLean RDJ, Allen R. 2002. Age and the understanding of emotions: neuropsychological and sociocognitive perspectives. J. Gerontol. B Psychol. Sci. Social Sci. 57, P526-P530. ( 10.1093/geronb/57.6.P526) [DOI] [PubMed] [Google Scholar]
- 121.Sullivan S, Ruffman T. 2004. Emotion recognition deficits in the elderly. Int. J. Neurosci. 114, 403-432. ( 10.1080/00207450490270901) [DOI] [PubMed] [Google Scholar]
- 122.Fernandes C, Barbosa F, Martins IP, Marques-Teixeira J. 2021. Aging and social cognition: a comprehensive review of the literature. Psychol. Neurosci. 14, 1-15. ( 10.1037/PNE0000251) [DOI] [Google Scholar]
- 123.Finger EC, et al. 2015. Oxytocin for frontotemporal dementia. Neurology 84, 174-181. ( 10.1212/WNL.0000000000001133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, Lydon JE. 2010. Effects of oxytocin on recollections of maternal care and closeness. Proc. Natl Acad. Sci. USA 107, 21 371-21 375. ( 10.1073/PNAS.1012669107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luminet O, Grynberg D, Ruzette N, Mikolajczak M. 2011. Personality-dependent effects of oxytocin: greater social benefits for high alexithymia scorers. Biol. Psychol. 87, 401-406. ( 10.1016/J.BIOPSYCHO.2011.05.005) [DOI] [PubMed] [Google Scholar]
- 126.Leknes S, Wessberg J, Ellingsen DM, Chelnokova O, Olausson H, Laeng B. 2013. Oxytocin enhances pupil dilation and sensitivity to ‘hidden’ emotional expressions. Social Cogn. Affect. Neurosci. 8, 741-749. ( 10.1093/SCAN/NSS062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tops M, Buisman-Pijlman FTA, Boksem MAS, Wijers AA, Korf J. 2012. Cortisol-induced increases of plasma oxytocin levels predict decreased immediate free recall of unpleasant words. Front. Psychiatry 3, 43. ( 10.3389/FPSYT.2012.00043/BIBTEX) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Crockford C, Deschner T, Ziegler TE, Wittig RM. 2014. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front. Behav. Neurosci. 8, 68. ( 10.3389/FNBEH.2014.00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Barreca T, Franceschini R, Siani C, Messina V, Francaviglia N, Perria C, Rolandi E. 1988. Diurnal pattern of plasma and cerebrospinal-fluid vasopressin levels in hydrocephalic patients: absence of a circadian rhythm and of a correlation between plasma. Horm. Res. Paediatr. 30, 28-31. [DOI] [PubMed] [Google Scholar]
- 130.Kagerbauer SM, Debus JM, Martin J, Gempt J, Jungwirth B, Hapfelmeier A, Podtschaske AH. 2019. Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides 78, 101977. ( 10.1016/J.NPEP.2019.101977) [DOI] [PubMed] [Google Scholar]
- 131.Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel JR, Sirigu A. 2017. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Scient. Rep. 7, 17222. ( 10.1038/s41598-017-17674-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bendix M, Uvnäs-Moberg K, Petersson M, Gustavsson P, Svanborg P, Åsberg M, Jokinen J. 2015. Plasma oxytocin and personality traits in psychiatric outpatients. Psychoneuroendocrinology 57, 102-110. ( 10.1016/J.PSYNEUEN.2015.04.003) [DOI] [PubMed] [Google Scholar]
- 133.Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, Feldman R. 2013. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology 38, 694-701. ( 10.1016/J.PSYNEUEN.2012.08.011) [DOI] [PubMed] [Google Scholar]
- 134.Martins D, Gabay AS, Mehta M, Paloyelis Y. 2020. Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. eLife 9, e62456. ( 10.7554/ELIFE.62456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Vries GJ. 2008. Sex differences in vasopressin and oxytocin innervation of the brain. Prog. Brain Res. 170, 17-27. ( 10.1016/S0079-6123(08)00402-0) [DOI] [PubMed] [Google Scholar]
- 136.Dumais KM, Veenema AH. 2016. Vasopressin and oxytocin receptor systems in the brain: sex differences and sex-specific regulation of social behavior. Front. Neuroendocrinol. 40, 1-23. ( 10.1016/J.YFRNE.2015.04.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.MacDonald KS. 2012. Sex, receptors, and attachment: a review of individual factors influencing response to oxytocin. Front. Neurosci. 6, 194. ( 10.3389/FNINS.2012.00194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu Q, Lai J, Du Y, Huang T, Prukpitikul P, Xu Y, Hu S. 2019. Sexual dimorphism of oxytocin and vasopressin in social cognition and behavior. Psychol. Res. Behav. Manag. 12, 337. ( 10.2147/PRBM.S192951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo L, et al. 2017. Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage 162, 127-137. ( 10.1016/J.NEUROIMAGE.2017.08.079) [DOI] [PubMed] [Google Scholar]
- 140.Quintana DS, Lischke A, Grace S, Scheele D, Ma Y, Becker B. 2021. Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research. Mol. Psychiatry 26, 80-91. ( 10.1038/S41380-020-00864-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. 2011. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 126, 97. ( 10.1037/A0026464) [DOI] [PubMed] [Google Scholar]
- 142.Schneider E, Müller LE, Ditzen B, Herpertz SC, Bertsch K. 2021. Oxytocin and social anxiety: interactions with sex hormones. Psychoneuroendocrinology 128, 105224. ( 10.1016/J.PSYNEUEN.2021.105224) [DOI] [PubMed] [Google Scholar]
- 143.Dellovade TL, Zhu YS, Pfaff DW. 1999. Thyroid hormones and estrogen affect oxytocin gene expression in hypothalamic neurons. J. Neuroendocrinol. 11, 1-10. ( 10.1046/J.1365-2826.1999.00250.X) [DOI] [PubMed] [Google Scholar]
- 144.Tribollet E, Audigier S, Dubois-Dauphin M, Dreifuss JJ. 1990. Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats. An autoradiographical study. Brain Res. 511, 129-140. ( 10.1016/0006-8993(90)90232-Z) [DOI] [PubMed] [Google Scholar]
- 145.Young L, Wang Z, Donaldson R, Rissman E. 1998. Estrogen receptor α is essential for induction of oxytocin receptor by estrogen. Neuroreport 9, 933-936. [DOI] [PubMed] [Google Scholar]
- 146.Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. 2013. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 25, 668-673. ( 10.1111/jne.12038) [DOI] [PubMed] [Google Scholar]
- 147.Moerkerke M, Bonte ML, Daniels N, Chubar V, Alaerts K, Steyaert J, Boets B. 2021. Oxytocin receptor gene (OXTR) DNA methylation is associated with autism and related social traits – a systematic review. Res. Autism Spectr. Disord. 85, 101785. ( 10.1016/J.RASD.2021.101785) [DOI] [Google Scholar]
- 148.Wilczyński KM, Siwiec A, Janas-Kozik M. 2019. Systematic review of literature on single-nucleotide polymorphisms within the oxytocin and vasopressin receptor genes in the development of social cognition dysfunctions in individuals suffering from autism spectrum disorder. Front. Psychiatry 10, 380. ( 10.3389/FPSYT.2019.00380/BIBTEX) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Inoue H, et al. 2010. Association between the oxytocin receptor gene and amygdalar volume in healthy adults. Biol. Psychiatry 68, 1066-1072. ( 10.1016/J.BIOPSYCH.2010.07.019) [DOI] [PubMed] [Google Scholar]
- 150.Yamasue H. 2013. Function and structure in social brain regions can link oxytocin-receptor genes with autistic social behavior. Brain Dev. 35, 111-118. ( 10.1016/J.BRAINDEV.2012.08.010) [DOI] [PubMed] [Google Scholar]
- 151.Ebner NC, et al. 2019. Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int. J. Psychophysiol. 136, 22-32. ( 10.1016/J.IJPSYCHO.2018.01.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ellis BJ, Horn AJ, Carter CS, van IJzendoorn MH, Bakermans-Kranenburg MJ. 2021. Developmental programming of oxytocin through variation in early-life stress: four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 86, 101985. ( 10.1016/J.CPR.2021.101985) [DOI] [PubMed] [Google Scholar]
- 153.Thijssen S, Van’t Veer AE, Witteman J, Meijer WM, van IJzendoorn MH, Bakermans-Kranenburg MJ. 2018. Effects of vasopressin on neural processing of infant crying in expectant fathers. Horm. Behav. 103, 19-27. ( 10.1016/J.YHBEH.2018.05.014) [DOI] [PubMed] [Google Scholar]
- 154.Polk R, Horta M, Lin T, Porges E, Ojeda M, Nazarloo HP, Carter CS, Ebner NC. 2022. Evaluating the neuropeptide–social cognition link in ageing: the mediating role of basic cognitive skills. Figshare. ( 10.6084/m9.figshare.c.6017062) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and code are available on the Open Science Framework (OSF) repository: https://osf.io/xv38y/?view_only=3ca2fada765f42c3a5a0e30900108fc3.
The data are provided in the electronic supplementary material [154].