Abstract
Background
Electrical muscle stimulation (EMS) is being evaluated as a possible alternative to exercise training to improve functional capacity in severely deconditioned patients with heart failure (HF). However, there is insufficient data on delayed effects of EMS starting early after decompensation. The aim of this study was to determine the impact of a short inpatient EMS intervention in severely deconditioned patients with HF on functional capacity and quality of life (QoL) over a follow-up period of 1 month.
Methods
This is a prospective randomised sham-controlled pilot study. 45 patients hospitalised for decompensated systolic HF (58% men, mean age 66.4±10.2 years) were randomised to EMS (n=22) or sham stimulation (n=23) of lower limbs starting within 3 days after admission. The intervention included 7–10 sessions lasting from 30 to 90 min. The 6-minute walking test distance (6-MWTD), Duke Activity Status Index (DASI) and Minnesota Living with Heart Failure Questionnaire (MLHFQ) were evaluated at baseline, discharge and after 1 month.
Results
All patients completed the programme with good EMS tolerance. 37 patients were included in the final analysis. At discharge, 6-MWTD improved from 206,1±61,3 to 299.5±91 m, DASI from 12.1±5.6 to 18.3±7.2 and MLHFQ from 55.6±8.5 to 34.2±9 with EMS compared with smaller improvements in the sham group (p<0.05 for all). One month after discharge, improvements in the EMS group remained significant for MLHFQ (p=0.004) and DASI (p=0.042) and statistically non-significant for 6-MWTD compared with the sham group.
Conclusions
Short-term in-hospital EMS leads to improvements in functional capacity and QoL in selected patients early after HF decompensation that are retained over 1 month after discharge and therefore may serve as initial intervention to improve physical capacity or as a bridge to further conventional exercise training. Larger studies are required to evaluate individual responses to an early initiation of EMS in decompensated HF as well as long-term effects.
Keywords: cardiac rehabilitation; heart failure, diastolic; heart failure, systolic
What is already known on this topic
Electrical muscle stimulation (EMS) is a promising alternative to physical exercise training in severely deconditioned patients with heart failure.
What this study adds
Short-term in-hospital EMS leads to improvements in functional capacity and quality of life in selected patients early after heart failure decompensation.
How this study might affect research, practice or policy
Early initiation of EMS may be used as initial intervention to improve physical capacity or as a bridge to further conventional exercise training in severely deconditioned patients with heart failure.
Introduction
Heart failure (HF) is prevalent worldwide and is associated with poor prognosis. Risk factors for morbidity and mortality include exercise intolerance and reduced functional capacity. Aerobic exercise training is recommended for stable patients with HF to improve functional capacity and symptoms and to reduce the risk of HF hospitalisations.1 However, exercise training capabilities are limited in many patients with HF due to markedly reduced cardiac output and peripheral muscle wasting. According to the literature, electrical muscle stimulation (EMS) has the potential to serve as an alternative or as initial approach before conventional training modalities in patients with HF with severely impaired exercise tolerance.2–16 However, in the majority of studies effects of EMS have been studied in outpatients with stable moderate HF, while data on its short-term use in advanced stages of HF or in the acute care setting are scarce. Deferred effects of inpatient EMS are missing, although the transition of a patient from hospital to outpatient care is the most vulnerable period which bears a high risk for re-hospitalisation.
The aim of this study was to evaluate the impact of in-hospital EMS started early after HF decompensation on functional capacity and quality of life (QoL) at discharge and 1 month after discharge by using 6-minute walking test distance (6-MWTD), Duke Activity Status Index (DASI) and Minnesota Living with Heart Failure Questionnaire results (MLHFQ) as primary endpoints.
Methods
This is a prospective randomised sham-controlled pilot trial designed to investigate the impact of early initiation of short-term EMS in patients with HF presenting with decompensated systolic HF on functional capacity and QoL with a follow-up period of 1 month.
Study population
We consecutively screened a cohort of 202 patients with reduced ejection fraction (EF ≤40%) admitted for acutely decompensated HF to the Cardiology department of Moscow City Hospital № 7 (currently City Clinical Hospital named after S.S.Yudin) over a period of 2 years. Inclusion criteria were: (1) age ≥18 years (2) hospitalisation for acute decompensation of systolic chronic heart failure (CHF). Exclusion criteria were: (1) acute major cardiovascular events (acute coronary syndrome, cardiogenic shock, acute stroke, pulmonary embolism) (2) uncontrolled life-threatening ventricular arrhythmias, (3) indications for urgent cardiovascular surgery including severe valvular heart disease (4) recent deep vein thrombosis (5) leg skin lesions (ulcers, maceration, eczema etc) (6) extensive peripheral oedema impeding effective myostimulation (7) inability to perform 6-minute walking test (6-MWT) or to complete the questionnaires due to cardiac or non-cardiac conditions.
Forty-five patients were included in the study. Most common causes for non-inclusion were inability to perform 6-MWT due to severity of HF, refusal to participate in the study, impaired mental function, peripheral oedema and trophic skin changes.
The study protocol was conducted in accordance with the Declaration of Helsinki and the Consolidated Standards of Reporting Trials statement. Written informed consent was obtained from all the subjects and the protocol was approved by the local ethics committee. The patients could withdraw consent at any moment of the study. All patients received appropriate pharmacological therapy including loop diuretics, ACE inhibitors/angiotensin receptor blockers, beta-blockers and mineralocorticoid receptor antagonists according to the most recent European Heart Failure guidelines at the time of the study (2016). In all patients, doses of the beta-blockers were maintained at the highest possible levels and/or up-titrated to the maximum tolerated dose.
Randomisation and blinding
The design of the study was experimental measuring the effects of EMS on physical tolerance and QoL in a sample of patients with CHF with reduced EF in a randomised sham-controlled manner. Patients who met the inclusion criteria were assigned in a 1:1 ratio either to effective EMS of leg muscles (group of intervention, n=22) or sham electric stimulation (group of control, n=23) by using the random number generator (Analysis ToolPak, Excel, Microsoft). In order to eliminate possible biases, examiners and interpreters for echocardiography, 6-MWT and questionnaires were blinded to the group allocation. The patients were instructed not to share any information about EMS sessions with the examiners. In addition, the doctor applying EMS to the patients was blinded from further analysis of the results until study completion.
Patient assessment
On admission, all patients underwent standard clinical examination, laboratory testing and transthoracic echocardiography (TTE). Within 3 days after admission, patients completed the baseline MLHFQ, the DASI questionnaire and performed a 6-MWT. All assessments other than TTE were repeated at the time of discharge from the hospital and 1 month after discharge.
Assessment of physical tolerance
6-MWT was conducted following the American Thoracic Society guidelines.17 Daily activity was assessed by the DASI, which is a self-assessment 12-item questionnaire for estimating functional capacity representing common aspects of physical function such as personal care, ambulation, household tasks, sexual life and recreation with respective metabolic costs.18 Ability to perform major activities was assessed with the final score ranging between 0 and 58.2 points (higher scores indicating better functional capacity).
QoL assessment
QoL was assessed by using the MLHFQ, consisting of 21 items rated on a 6-point Likert psychometric scale, representing how HF affects the physical and emotional aspects of QoL from 0 (none) to 5 (very much).19 MLHFQ provides a total score ranging between 0 and 105, from best to worst QoL.
Muscle stimulation
EMS was performed using a Stimul-01 electro-myostimulator developed by the Institute of Biomedical Problems, Russian Academy of Sciences and Biofizpribor Company. The setting including the placing of the electrodes is shown in figure 1. The EMS stimulator was originally developed for the space station as a means of prevention of muscle wasting caused by microgravity. The device generates bipolar symmetrical rectangular electrical impulses lasting 1±0.05 ms with a frequency of 25±1 Hz in the cyclic mode (1±0.1 s of stimulation and 2±0.1 s of rest). We used wetted non-adhesive electrodes that are positioned on the skin and fixed by special straps over the upper and lower portions of the muscle groups providing stimulation and contraction of the muscle mass between the electrodes. Anterior and posterior femoral and tibial muscles of both legs were stimulated simultaneously.
Figure 1.
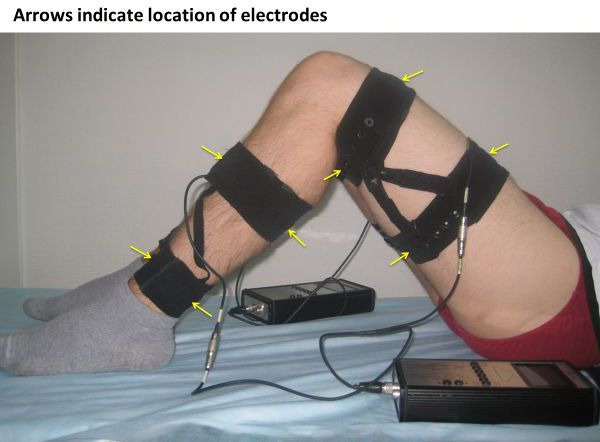
Position of the electrodes during electrical muscle stimulation
The intensity of stimulation in the EMS group was adjusted individually for each patient to achieve a visible muscle contraction followed by a gradual increase to attain maximum tolerable contraction. The stimulation intensity was adjusted at each session. In the sham group, the electrodes were positioned and fixed likewise and the stimulator switched on but minimal intensity was set not to cause any muscle contractions. Muscle stimulation procedures were performed 5 days a week. The duration of the first, second and third session was 30, 45 and 60 min in both groups with a maximum duration of the subsequent sessions of up to 90 min in the EMS group and 60 min in the sham group. Most patients were able to undergo 8 procedures in total before discharge with variations between 7 and 10 procedures. Average procedure time and the number of procedures did not differ significantly between groups (online supplemental figure 1).
openhrt-2022-001965supp001.pdf (78.3KB, pdf)
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences V.22 (IBM SPSS Statistics, USA). Sample size was calculated for detecting at least a 54 m difference in 6-MWTD and minimally important difference of 5 points for DASI and 10 points MLFHQ scores17–19 corresponding with changes in New York Heart Association class with a power of 80% and alpha risk of 5%. For this study, the sample size needed to achieve the calculated study power was 16 patients per group for 6-MWTD, 13 patients per group for DASI and 8 patients per group for MLHFQ.
Normality of distributions was tested with Shapiro-Wilk test. Continuous variables presented as mean value ±SD. T-test was used to compare the means of two groups. Categorical variables are shown as number and percentage and computed by using two-tailed Fischer’s exact test. Wilcoxon test was applied to determine the significance of difference in the linked samples. P value<0.05 was considered significant.
Results
All 45 patients completed the EMS or sham programme in the hospital. During the 1 month follow-up period, two patients assigned to EMS group dropped out of the study, one of them refused to attend the outpatient visits and one because of HF re-hospitalisation. Six patients assigned to the sham group did not complete the follow-up, three of them refused to attend and three had to be re-hospitalised with worsening of HF. There were no hospital readmissions during the 1 month of follow-up among patents which have been included in the final analysis.
The study flow chart is shown in figure 2.
Figure 2.
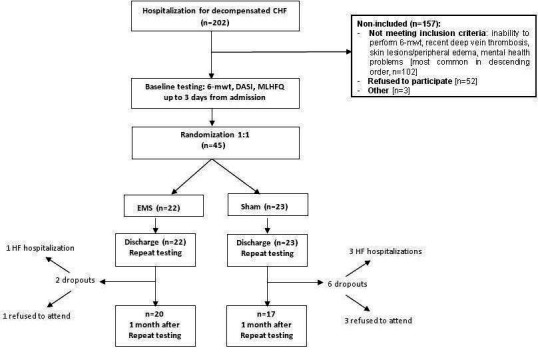
Study flow chart. CHF, chronic heart failure; DASI, Duke Activity Status Index; EMS, electrical muscle stimulation; HF, heart failure; MLHFQ, Minnesota Living with Heart Failure Questionnaire; 6-MWT, 6-minute walking test.
There was no statistically significant difference between the two groups in regard to age, sex or reason for HF, left ventricular ejection fraction (LVEF), NYHA functional class, HF medications and comorbidities. 6-MWTD, DASI and MLHFQ scores at baseline were comparable between groups (table 1).
Table 1.
Baseline clinical and demographic characteristics of study patients and EMS parameters
| Patients’ characteristics | EMS (n=22) | Sham (n=23) | P value |
| Age (years) | 64.5±11.0 | 68.9±9.0 | ns |
| Male, n (%) | 15 (68.2) | 11 (47.8) | ns |
| Ischaemic CHF, n (%) | 14 (63.6) | 16 (69.5) | ns |
| Post MI | 10 (45.5%) | 11 (47.8%) | ns |
| h/o revascularisation (CABG/PCI) | 8 (36.4%) | 7 (30.4%) | ns |
| Arterial hypertension, n (%) | 19 (86.4) | 21 (91.3) | ns |
| Permanent atrial fibrillation n (%) | 9 (41) | 7 (30.4) | ns |
| Valvular heart disease, n (%) | 4 (18.1) | 3 (13.0) | ns |
| Diabetes mellitus, n (%) | 5 (22.7) | 7 (30.4) | ns |
| LVEF, % | 32.3±3.5 | 30.8±6.1 | ns |
| Average length of hospital stay, days | 11.7±2.7 | 11.9±2.4 | ns |
| NYHA class, n (%) (at the time of enrolment) III IV |
17 (77.2) 5 (22.7) |
19 (82.6) 4 (17.4) |
ns |
| Loop diuretics, n (%) | 22 (100) | 23 (100) | ns |
| ACEi/ARBs, n (%) | 20 (90.9) | 22 (95.6) | ns |
| Beta-blockers, n (%) | 20 (90.9) | 19 (82.6) | ns |
| MRAs, n (%) | 19 (86.4) | 18 (78.3) | ns |
| Digoxin, n (%) | 7 (31.8) | 6 (26.1) | ns |
| Statins, n (%) | 19 (86.4) | 18 (78.3) | ns |
| CRT-P/CRT-D, n (%) | 1 (4.5) | 2 (8.7) | ns |
| ICD, n (%) | 1 (4.5) | 1 (4.3) | ns |
ACEi, ACE inhibitors; ARBs, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CHF, chronic heart failure; CRT-P/D, cardiac resynchronisation therapy (pacing/defibrillator); EMS, neuromuscular electrical stimulation; h/o, history of; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRAs, mineralocorticoid/aldosterone receptor antagonists; ns, non-significant; NYHA, New York Heart Association; PCI, percutaneous coronary interventions.
Safety and tolerability
No significant acute changes of heart rate (Δ±20 b.p.m) and blood pressure (Δ±20 mm Hg) during EMS procedures were observed (thresholds according to Ref. 21). There were no adverse events requiring discontinuation of EMS procedures. Four patients reported mild muscle pain after the first sessions that resolved spontaneously. Two patients reported some mild-to-moderate general discomfort (most likely due to anxiety) without any alterations of vital signs throughout all EMS sessions which did not resolve with decreasing the stimulation intensity. However, none of the patients did discontinue the course of stimulation.
Functional capacity and QoL
At discharge, all measures of functional capacity and QoL improved significantly in the EMS group compared with the baseline values (table 2). In the sham group, the QoL, which was defined by a lower score on the MLHFQ, also improved significantly, while DASI and 6-MWTD demonstrated only a trend to improvement without reaching statistical significance (table 2). Patients who underwent EMS had significantly better scores of MLHFQ and DASI, as well as 6-MWTD compared with those in the sham group (figure 3).
Table 2.
Changes in quality of life and functional status
| Allocation | Parameter | Baseline | Discharge | 1 month |
| EMS | ||||
| MLHFQ score | 55.63±8.53 | 34.18±9.02* | 37.77±7.25* | |
| DASI score | 12.11±5.61 | 18.29±7.17* | 17.51±4.59* | |
| 6-MWTD, m | 206.09±61.29 | 299.5±91.05* | 284.63±83.17* | |
| Sham | ||||
| MLHFQ score | 56.47±7.11 | 48.73±8.09* | 46.39±8.01* | |
| DASI score | 11.60±3.75 | 13.37±4.32 | 14.39±4.11* | |
| 6-MWTD, m | 211.39±51.62 | 236.82±54.73 | 248.43±69.79* |
*P<0.05—for difference between baseline and after procedures inside the groups.
DASI, Duke Activity Status Index; EMS, neuromuscular electrical stimulation; m, metres; MLHFQ, Minnesota Living with Heart Failure Questionnaire; 6-MWTD, 6-minute walking test distance.
Figure 3.
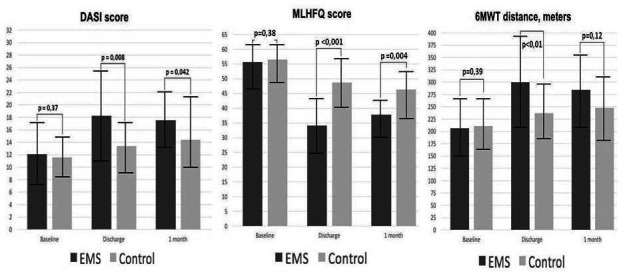
Comparison of MLHFQ, DASI and 6-minute walking test distance between groups at baseline, discharge and 1 month of follow-up. Higher scores for MLHFQ indicate lesser quality of life. EMS, electrical muscle stimulation; DASI, Duke Activity Status Index; MLHFQ, Minnesota Living with Heart Failure Questionnaire; 6-MWT, 6-minute walking test.
One month after discharge, improvements in the EMS group remained significant for MLHFQ (p=0.004) and DASI (p=0.042) and statistically non-significant for 6-MWTD compared with smaller improvements in the sham group.
There was no meaningful difference in NYHA class and guideline based medical therapy between groups at 1 month after discharge.
Discussion
The main and new finding of this study is that short-term in-hospital EMS for patients early after HF decompensation by the time of discharge was able to generate beneficial effects on exercise tolerance, functional capacity and QoL of these patients that are retained over a follow-up period of 1 month. Our finding supports the concept of using EMS early after acute HF decompensation as a bridge to physical exercise training during periods of severe muscular deconditioning.
Previous studies related to EMS in HF mostly included outpatients in stable medical state.16 We enrolled patients at the latest 3 days after admission for HF decompensation. There are only limited data on the impact of EMS in patients hospitalised with advanced or acutely decompensated HF and its feasibility is still under investigation.20–24 However conventional training modalities are limited in these severely deconditioned patients. Moreover, prolonged immobilisation additionally contributes to skeletal muscle weakness in patients hospitalised for HF.25 A recent meta-analysis by Liu et al illustrates that EMS in critical patients prevents intensive care unit-acquired muscle weakness, shortens the length of stay in the hospital and may improve the ability to perform daily activities by the time of discharge.26 Thus, one can assume that similar effects of EMS can be achieved shortly after HF decompensation. High rates of hospital readmission for patients with HF reflect particular vulnerability of patients with HF early after discharge and may indicate low compliance to treatment including non-pharmacological measures such as physical activity.27–29 Through initial fitness improvement, EMS offers a bridge to conventional exercise or can be regarded as a substitute for physical training during the hospital-to-home transition period. Even more important, early initiation of EMS may prevent additional muscle wasting during hospitalisation in patients with HF with high prevalence of sarcopenia and frailty and may also increase their acceptance of recommendations for cardiac rehabilitation.30–32
In our study, even a short-term EMS course was sufficient to increase 6-MWTD and to improve QoL corresponding with previous reports.20–22 Immediate beneficial effects of EMS are observed even after a single session which might partly be explained by peripheral vasodilation and better muscle perfusion.22 33 Likewise, healthy subjects demonstrate changes in inflammatory cytokines similar to the effects of active exercise after only 30 min of EMS.34 To our surprise, there was no significant difference in 6-MWTD between the two groups in our study after 1 month. It may be argued that patients from the sham group had to increase their level of daily activity after discharge anyway bringing them closer to the patients from the EMS group in terms of physical tolerance. Another explanation my be that individual differences between patients have generally an important impact on results in studies with small sample sizes.
The impact of a particular EMS stimulation protocol on outcome is largely unknown. Various stimulation parameters and protocols have been used previously in mainly small studies. Most of the protocols involved prolonged EMS courses of up to 5–8 weeks.2 Unfortunately, no reliable data are available comparing different methods of EMS application. Short-term and long-term effects of EMS may vary depending on the regimen and the duration of the course. Nuhr showed that chronic low frequency stimulation induced a shift in the muscle fibre type from fast glycolytic to slow oxidative fibres.10 Small earlier trials found that high frequency EMS produced improvements in muscle strength and metabolic measures of exercise capacity in highly selected patients and found that higher frequency stimulation normally used for muscle strengthening was not suitable to produce a sustained increase in oxygen uptake. Instead, very low frequencies were preferable probably because of lesser fatigue of the type I oxidative muscle fibres, even in severely deconditioned patients with HF.35 Finally, we know at least from one study, that there was no beneficial effect by adding EMS to conventional exercise training in HF.36 37 Our study shows beneficial effects of low-intensity stimulation on exercise capacity, whereas evaluation of changes in strength was not part of this investigation. In our protocol, low-intensity EMS allowed simultaneous stimulation of both anterior and posterior muscle groups for a longer period of time. In comparison to previous studies, our protocol allowed to involve a larger number of muscle groups during a session leading to simultaneous effective contraction of anterior muscles as opposed to contracting posterior muscle groups and vice versa. EMS regimen with counteraction of opposite muscle groups stretched against each other markedly improves the benefits of the procedures. Focus on a larger amount of stimulated muscle groups is known to increase efficacy of EMS regardless of the duration of the procedures.38 This may at least partially explain why even eight EMS sessions in average were enough to provide beneficial effects in our study.
Limitations
We recognise several limitations in our study. We enrolled a relatively small number of patients with a limited follow-up time, which may reduce the power of the study. However, according to most recent evidence this is a common limitation for all studies with EMS in patients with HF of similar disease severity with a crude number of 10–15 patients for each group on average.2 24 39 Thus, our results provide some additional information to the knowledge gap. The high percentage of non-inclusion due to common real-life causes in a relatively large cohort of screened patients with HF does not eliminate certain selection bias. Larger studies are required to evaluate individual responses to an early initiation of EMS in decompensated HF as well as long-term effects. Although it was a randomised study, response to HF treatment could vary between individuals with potential impact on the level of improvements of functional status and QoL. Levels of brain natriuretic peptide (BNP) were not investigated based on limited real-life facilities of the city community hospital where the study was conducted. However, according to the most recent guidelines BNP is still not required for diagnosing of heart failure with reduced ejection fraction (HFrEF) in case of low LVEF and obvious clinical signs and symptoms of HF. Only a relatively small percentage of our patients had implanted devices mainly due to economic limitations. At the time of the study, neither sacubitril/valsartan, nor SGLT2 inhibitors were widely implemented in the patients with HFrEF. Fully blinding was not possible due to differences in subjective perception of effective EMS and sham procedures by the patients. Physical tolerance was not evaluated with more precise methods such as cardiopulmonary exercise testing because of the disease severity in these patients. Activity of daily living after discharge was measured only by DASI score without using more definitive modalities like accelerometry.
Conclusion
Short-term in-hospital EMS early after HF decompensation has beneficial immediate effects on functional capacity and QoL that are maintained at 1 month after discharge. In selected patients with HF, EMS may be considered as initial intervention for patients who are unable to exercise, either as a substitute or as a bridge to conventional exercise training. Further studies are needed to explore the full potential of early EMS under these circumstances.
Footnotes
Twitter: @hugo saner
Contributors: All coauthors contributed to the conception or design, acquisition, analysis and interpretation of the results. Three authors (MP, HS and IG) drafted the manuscript. All coauthors critically revised the manuscript and gave final approval. MP is responsible for the overall content as the guarantor
Funding: The study was supported by the Ministry of Science and Higher Education of the Russian Federation under agreement № 075-15-2022-298 from 18 April 2022 about the grant in the form of subsidy from the federal budget to provide government support for the creation and development of a world-class research center, the “Pavlov Center for Integrative Physiology to Medicine, High-tech Healthcare and Stress Tolerance Technologies”.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available from the first author upon reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by Ethical Committee of the Sechenov First Moscow State Medical University. Participants gave informed consent to participate in the study before taking part.
References
- 1.McDonagh TA, Metra M, Adamo M. Esc scientific document group, 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. European Heart Journal, 21 Sep 2021;42:372. 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 2.Gomes Neto M, Oliveira FA, Reis HFCD, et al. Effects of neuromuscular electrical stimulation on physiologic and functional measurements in patients with heart failure: a systematic review with meta-analysis. J Cardiopulm Rehabil Prev 2016;36:157–66. 10.1097/HCR.0000000000000151 [DOI] [PubMed] [Google Scholar]
- 3.Karavidas A, Arapi SM, Pyrgakis V, et al. Functional electrical stimulation of lower limbs in patients with chronic heart failure. Heart Fail Rev 2010;15:563–79. 10.1007/s10741-010-9171-9 [DOI] [PubMed] [Google Scholar]
- 4.Banerjee P, Caulfield B, Crowe L, et al. Prolonged electrical muscle stimulation exercise improves strength, peak VO2, and exercise capacity in patients with stable chronic heart failure. J Card Fail 2009;15:319–26. 10.1016/j.cardfail.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Kadoglou NP, Mandila C, Karavidas A, et al. Effect of functional electrical stimulation on cardiovascular outcomes in patients with chronic heart failure. Eur J Prev Cardiol 2017;24:833–9. 10.1177/2047487316687428 [DOI] [PubMed] [Google Scholar]
- 6.Karavidas A, Driva M, Parissis JT, et al. Functional electrical stimulation of peripheral muscles improves endothelial function and clinical and emotional status in heart failure patients with preserved left ventricular ejection fraction. Am Heart J 2013;166:760–7. 10.1016/j.ahj.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 7.Quittan M, Wiesinger GF, Sturm B, et al. Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: a single-blind, randomized, controlled trial. Am J Phys Med Rehabil 2001;80:206–14. 10.1097/00002060-200103000-00011 [DOI] [PubMed] [Google Scholar]
- 8.Vaquero AF, Chicharro JL, Gil L, et al. Effects of muscle electrical stimulation on peak VO2 in cardiac transplant patients. Int J Sports Med 1998;19:317–22. 10.1055/s-2007-971924 [DOI] [PubMed] [Google Scholar]
- 9.Maillefert JF, Eicher JC, Walker P, et al. Effects of low-frequency electrical stimulation of quadriceps and calf muscles in patients with chronic heart failure. J Cardiopulm Rehabil 1998;18:277–82. 10.1097/00008483-199807000-00004 [DOI] [PubMed] [Google Scholar]
- 10.J. Nuhr M, et al. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J 2004;25:136–43. 10.1016/j.ehj.2003.09.027 [DOI] [PubMed] [Google Scholar]
- 11.Dobsák P, Nováková M, Siegelová J, et al. Low-frequency electrical stimulation increases muscle strength and improves blood supply in patients with chronic heart failure. Circ J 2006;70:75–82. 10.1253/circj.70.75 [DOI] [PubMed] [Google Scholar]
- 12.Harris S, LeMaitre JP, Mackenzie G, et al. A randomised study of home-based electrical stimulation of the legs and conventional bicycle exercise training for patients with chronic heart failure. Eur Heart J 2003;24:871–8. 10.1016/S0195-668X(02)00822-9 [DOI] [PubMed] [Google Scholar]
- 13.Deley G, Kervio G, Verges B, et al. Comparison of low-frequency electrical myostimulation and conventional aerobic exercise training in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 2005;12:226–33. 10.1097/01.hjr.0000166455.23346.a5 [DOI] [PubMed] [Google Scholar]
- 14.Karavidas A, Parissis J, Arapi S, et al. Effects of functional electrical stimulation on quality of life and emotional stress in patients with chronic heart failure secondary to ischaemic or idiopathic dilated cardiomyopathy: a randomised, placebo-controlled trial. Eur J Heart Fail 2008;10:709–13. 10.1016/j.ejheart.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 15.Parissis J, Karavidas A, Farmakis D, et al. Efficacy and safety of functional electrical stimulation of lower limb muscles in elderly patients with chronic heart failure: a pilot study. Eur J Prev Cardiol 2015;22:831–6. 10.1177/2047487314540546 [DOI] [PubMed] [Google Scholar]
- 16.Sbruzzi G, Ribeiro RA, Schaan BD, et al. Functional electrical stimulation in the treatment of patients with chronic heart failure: a meta-analysis of randomized controlled trials. Eur J Cardiovasc Prev Rehabil 2010;17:254–60. 10.1097/HJR.0b013e328339b5a2 [DOI] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 18.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke activity status index). Am J Cardiol 1989;64:651–4. 10.1016/0002-9149(89)90496-7 [DOI] [PubMed] [Google Scholar]
- 19.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota living with heart failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. pimobendan multicenter research group. Am Heart J 1992;124:1017–25. 10.1016/0002-8703(92)90986-6 [DOI] [PubMed] [Google Scholar]
- 20.Kondo T, Yamada S, Tanimura D, et al. Neuromuscular electrical stimulation is feasible in patients with acute heart failure. ESC Heart Fail 2019;6:975–82. 10.1002/ehf2.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ennis S, McGregor G, Hamborg T, et al. Randomised feasibility trial into the effects of low-frequency electrical muscle stimulation in advanced heart failure patients. BMJ Open 2017;7:a016148. 10.1136/bmjopen-2017-016148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groehs RV, Antunes-Correa LM, Nobre TS, et al. Muscle electrical stimulation improves neurovascular control and exercise tolerance in hospitalised advanced heart failure patients. Eur J Prev Cardiol 2016;23:1599–608. 10.1177/2047487316654025 [DOI] [PubMed] [Google Scholar]
- 23.Forestieri P, Bolzan DW, Santos VB, et al. Neuromuscular electrical stimulation improves exercise tolerance in patients with advanced heart failure on continuous intravenous inotropic support use-randomized controlled trial. Clin Rehabil 2018;32:66–74. 10.1177/0269215517715762 [DOI] [PubMed] [Google Scholar]
- 24.de Araújo CJS, Gonçalves FS, Bittencourt HS, et al. Effects of neuromuscular electrostimulation in patients with heart failure admitted to ward. J Cardiothorac Surg 2012;7:124. 10.1186/1749-8090-7-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehn TA, Munkvik M, Lunde PK, et al. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease-specific myopathy or a result of deconditioning? Heart Fail Rev 2012;17:421–36. 10.1007/s10741-011-9289-4 [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Luo J, Zhou J, et al. Intervention effect of neuromuscular electrical stimulation on ICU acquired weakness: a meta-analysis. Int J Nurs Sci 2020;7:228–37. 10.1016/j.ijnss.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Wal MHL, van Veldhuisen DJ, Veeger NJGM, et al. Compliance with non-pharmacological recommendations and outcome in heart failure patients. Eur Heart J 2010;31:1486–93. 10.1093/eurheartj/ehq091 [DOI] [PubMed] [Google Scholar]
- 28.Nieminen MS, Dickstein K, Fonseca C, et al. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int J Cardiol 2015;191:256–64. 10.1016/j.ijcard.2015.04.235 [DOI] [PubMed] [Google Scholar]
- 29.Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the study Group on exercise training in heart failure of the heart failure association of the European Society of cardiology. Eur J Heart Fail 2012;14:451–8. 10.1093/eurjhf/hfs048 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Zhang J, Ni W, et al. Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail 2021;8:1007–17. 10.1002/ehf2.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smart NA, Dieberg G, Giallauria F. Functional electrical stimulation for chronic heart failure: a meta-analysis. Int J Cardiol 2013;167:80–6. 10.1016/j.ijcard.2011.12.019 [DOI] [PubMed] [Google Scholar]
- 32.Karavidas A, Parissis JT, Matzaraki V, et al. Functional electrical stimulation is more effective in severe symptomatic heart failure patients and improves their adherence to rehabilitation programs. J Card Fail 2010;16:244–9. 10.1016/j.cardfail.2009.10.023 [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Masuda T, Kamiya K, et al. A single session of neuromuscular electrical stimulation enhances vascular endothelial function and peripheral blood circulation in patients with acute myocardial infarction. Int Heart J 2016;57:676–81. 10.1536/ihj.15-493 [DOI] [PubMed] [Google Scholar]
- 34.Truong AD, Kho ME, Brower RG, et al. Effects of neuromuscular electrical stimulation on cytokines in peripheral blood for healthy participants: a prospective, single-blinded study. Clin Physiol Funct Imaging 2017;37:255–62. 10.1111/cpf.12290 [DOI] [PubMed] [Google Scholar]
- 35.Minogue CM, Caulfield BM, Reilly RB. What are the electrical stimulation design parameters for maximum VO2 aimed at cardio-pulmonary rehabilitation? Annu Int Conf IEEE Eng Med Biol Soc 2007:31. 10.1109/IEMBS.2007.4352818 [DOI] [PubMed] [Google Scholar]
- 36.Iliou MC, Vergès-Patois B, Pavy B, et al. Effects of combined exercise training and electromyostimulation treatments in chronic heart failure: a prospective multicentre study. Eur J Prev Cardiol 2017;24:1274–82. 10.1177/2047487317712601 [DOI] [PubMed] [Google Scholar]
- 37.Adams V. Is it beneficial to add electromyostimulation to conventional exercise training in heart failure? Eur J Prev Cardiol 2017;24:1594–5. 10.1177/2047487317717822 [DOI] [PubMed] [Google Scholar]
- 38.Saitoh M, Dos Santos MR, Anker M, et al. Neuromuscular electrical stimulation for muscle wasting in heart failure patients. Int J Cardiol 2016;225:200–5. 10.1016/j.ijcard.2016.09.127 [DOI] [PubMed] [Google Scholar]
- 39.Palau P, Domínguez E, López L, et al. Inspiratory muscle training and functional electrical stimulation for treatment of heart failure with preserved ejection fraction: the TRAINING-HF trial. Rev Esp Cardiol 2019;72:288–97. 10.1016/j.rec.2018.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-001965supp001.pdf (78.3KB, pdf)
Data Availability Statement
Data are available from the first author upon reasonable request.


