Abstract
Introduction
The impact of hepatorenal function on plasma biomarkers of neuropathology is unknown. Herein, we measured several plasma biomarkers in patients with cirrhosis.
Methods
Plasma phosphorylated tau (p‐tau181), neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), total tau (t‐tau), and ubiquitin carboxyl‐terminal hydrolase L1 (UCHL1) were measured in 135 adults with cirrhosis and 22 healthy controls using Simoa. Within cirrhosis, associations between biomarkers and hepatorenal function were explored using linear regression.
Results
p‐tau181, NfL, t‐tau, and UCHL1 were increased 2‐ to 4‐fold in cirrhosis, whereas GFAP was not increased. Within cirrhosis, creatinine moderately correlated with p‐tau181 (β = 0.75, P < .01), NfL (β = 0.32, P < .01), and t‐tau (β = 0.31, P < .01), but not GFAP (β = –0.01, P = .88) or UCHL1 (β = –0.05, P = .60), whereas albumin showed weak, inverse correlations: p‐tau181 (β = –0.18, P < .01), NfL (β = –0.22, P < .01), GFAP (β = –0.17, P < .05), t‐tau (β = –0.20, P = .02), and UCHL1 (β = –0.15, P = .09).
Conclusions
Elevated p‐tau181, NfL, and t‐tau in cirrhosis were associated with renal impairment and hypoalbuminemia, suggesting that hepatorenal function may be important when interpreting plasma biomarkers of neuropathology.
Keywords: Alzheimer's, dementia, cirrhosis, liver disease, neurodegeneration, plasma biomarkers
1. INTRODUCTION
Translation of biomarkers of neuropathology from cerebrospinal fluid (CSF) to plasma holds great promise for improving diagnosis and facilitating clinical trials in a host of neurodegenerative diseases, particularly Alzheimer's disease (AD). 1 Several CSF biomarkers have been successfully translated into plasma‐based assays, including phosphorylated tau (p‐tau181), neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), total tau (t‐tau), and ubiquitin carboxyl‐terminal hydrolase L1 (UCHL1).
Plasma p‐tau181 is specific for AD neuropathology and increased 3‐fold in AD. 2 , 3 NfL is a cytoskeletal protein that is elevated non‐specifically after neuronal injury in many diseases. 4 GFAP, a marker for astrogliosis associated with neuroinflammation, may be an early marker for patients who are at risk of neurodegenerative decline. 5 Finally, variable changes in plasma t‐tau and UCHL1 concentrations have been reported in both acute and chronic neurologic injury. 6 , 7 We selected these biomarkers as a diverse representation of neuropathology, with several approaching clinical use.
The relationship between peripheral plasma concentrations of these biomarkers and hepatorenal function is not well‐characterized; yet understanding these factors has been recognized as critical for successful advancement of these assays into clinic use. 8 In addition, recent work has shown plasma NfL and t‐tau can be elevated in the setting of renal dysfunction, 8 , 9 , 10 supporting our hypothesis that hepatorenal function may be important in interpreting these plasma biomarkers.
In this exploratory study, we examined the relationship between plasma biomarkers of neuropathology and several markers of hepatorenal function, using cirrhosis as a model of multi‐organ impairment.
2. METHODS
Participants with cirrhosis (N = 135) were chosen from the Functional Assessment in Liver Transplantation (FrAILT) study, and age‐ and sex‐matched healthy controls (N = 22) were selected from the Hillblom Healthy Aging study (see Supplementary Methods). A replication cohort of non‐matched controls was used to confirm the findings (N = 96). Within cirrhosis, a convenience sample of plasma specimens from two cohorts was selected: non‐alcoholic fatty liver disease (NAFLD, N = 99) and autoimmune hepatitis (AIH, N = 36). These two cohorts were chosen because of differences in etiology, with NAFLD more likely to have vascular risk factors that lead to renal impairment.
Within the FrAILT cohort, examination included frailty testing (Liver Frailty Index [LFI]: http://liverfrailtyindex.ucsf.edu). 11 Number Connection Test (NCT), a modified version of the Trail‐Making Test (TMT) A, measured hepatic encephalopathy (HE) severity. 12 Montreal Cognitive Assessment (MoCA) was available for a subset of cirrhosis (N = 48) and all control participants. Laboratory data were collected within 3 months of evaluation. Plasma specimens were collected and processed as described previously, 3 with pre‐analytic variability between projects detailed in the Supplementary Methods. Participants provided written informed consent, and plasma samples were de‐identified.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed the literature using traditional sources, for example, PubMed. Several cerebrospinal fluid (CSF) biomarkers of neuropathology are now detectable in plasma, holding great promise for improving clinical practice. However, several recent publications describe non‐neurologic factors that influence plasma biomarker concentrations. The role of important drivers of peripheral physiology, however, including hepatic and renal function, has not been comprehensively examined.
Interpretation: Our findings suggest that hepatic and renal function affect concentrations of several plasma biomarkers of neuropathology, and that these factors must be considered when clinically interpreting the results.
Future Directions: These results need to be replicated in a neurodegenerative population, especially patients with chronic kidney disease or hypoalbuminemia. Further studies in the cirrhosis cohort will be directed at better understanding both the neurologic and non‐neurologic drivers of biomarker change in this population.
Biomarker concentrations were measured using Quanterix kits on the Simoa HD‐X platform (Supplementary Methods). Summary statistics utilized Mann‐Whitney U test, chi‐square, and two‐sample t‐tests. Linear regression estimated the strength of associations between the variables of interest and plasma biomarker concentrations (standardized β). Clinical measures were included based on prior evidence of association (age, sex, body mass index [BMI]), relationship to renal impairment (hypertension, diabetes), cirrhosis severity (components of Model for End‐Stage Liver Disease‐sodium [MELDNa]), or sequelae that may affect biomarker distribution or reflect neurologic injury (ascites, LFI, NCT).
3. RESULTS
3.1. Clinical characteristics
Participants with cirrhosis and controls were well matched on age and sex, whereas more controls were non‐Hispanic White (Table 1). Mean MELDNa in cirrhosis was 16 (SD 4), consistent with a transplant‐eligible stage of disease. Compared to AIH, NAFLD participants were older, had higher BMI, and had increased prevalence of hypertension and diabetes, consistent with known association with vascular risk factors. NAFLD also had worse renal function, lower total bilirubin, and more HE compared to AIH.
TABLE 1.
Clinical characteristics and plasma biomarkers in healthy controls and cirrhosis
| HC | Cirrhosis | NAFLD | AIH | p‐tau181 | NfL | GFAP | t‐tau | UCHL1 | |
|---|---|---|---|---|---|---|---|---|---|
| Clinical characteristics | (n = 22) | (n = 135) | (n = 99) | (n = 36) | Univariable association (β, P) Cirrhosis only | ||||
| Age, yearsa | 58 (11) | 59 (9) | 60 (8) * | 55 (12) * | −0.03 (.65) | .18 (.04) * | 0.14 (.11) | −0.04 (.65) | 0.04 (.65) |
| Sex, femaleb | 68% | 71% | 70% | 75% | −0.08 (.38) | 0.05 (.58) | −0.07 (.44) | 0.13 (.13) | −0.06 (.43) |
| Race/ethnicity | |||||||||
| Asian | 9% | 10% | 8% | 14% | – | – | – | – | – |
| Hispanic | 0% | 55% | 65% | 28% | – | – | – | – | – |
| Non‐Hispanic White | 91% | 30% | 21% | 56% | – | – | – | – | – |
| Otherd | 0% | 5% | 6% | 3% | – | – | – | – | – |
| MoCA | 28 (2) | 26 (3) | 26 (3) | 26 (3) | −0.05 (.76) | −0.03 (.82) | −0.07 (.63) | 0.16 (.27) | −0.06 (.70) |
| BMI, kg/m2,c | 25.5 (2.7) * | 31.6 (6.7) * | 33.7 (6.2) * | 25.8 (4.1) * | −0.07 (.39) | −0.02 (.79) | −0.12 (.16) | 0.04 (.69) | −0.12 (.17) |
| Hypertensionb | 36% | 45% | 55% * | 19% * | 0.05 (.61) | 0.11 (.21) | 0.08 (.33) | −0.01 (.91) | 0.03 (.69) |
| Diabetesb | 0% * | 50% * | 55% * | 19% * | .23 (<.01) * | .35 (<.01) * | 0.05 (.53) | 0.07 (.43) | −0.01 (.93) |
| CADb | 0% | 5% | 6% | 3% | 0.12 (.17) | 0.04 (.68) | 0.03 (.78) | 0.06 (.46) | −0.05 (.60) |
| Strokeb | 0% | 2% | 2% | 0% | 0.01 (.97) | .17 (<.05) * | −0.01 (.89) | 0.03 (.75) | −0.03 (.70) |
| Cirrhosis severity | (n = 135) | (n = 99) | (n = 36) | Univariable association (β,P) Cirrhosis only | |||||
|---|---|---|---|---|---|---|---|---|---|
| MELDNac | N/A | 16 (4) | 16 (5) | 16 (4) | .43 (<.01) * | 0.16 (.07) | 0.08 (.34) | .17 (<.05) * | 0.05 (.57) |
| Sodium, mEq/Lc | N/A | 136 (4) | 136 (3) | 135 (4) | −0.05 (.59) | −0.05 (.58) | −0.02 (.79) | 0.01 (.95) | −0.04 (.68) |
| Creatinine, mg/dLa | N/A | 1.2 (1.4) | 1.3 (1.6) * | .8 (.3) * | .75 (<.01) * | .32 (<.01) * | −0.01 (.88) | .31 (<.01) * | −0.05 (.60) |
| Total bilirubin, mg/dLa | N/A | 3.3 (2.7) | 3.0 (2.2) * | 4.1 (3.8) * | −0.06 (.48) | −0.16 (.06) | −0.03 (.75) | −0.09 (.29) | 0.02 (.85) |
| INRa | N/A | 1.5 (0.4) | 1.5 (0.4) | 1.4 (0.2) | 0.03 (.75) | −0.08 (.37) | 0.04 (.65) | −0.06 (.50) | 0.03 (.77) |
| Albumin, mg/dLc | N/A | 3.0 (0.6) | 3.0 (0.1) | 2.9 (0.1) | −.18 (.03) * | −.22 (.01) * | −.17 (<.05) * | −.20 (.02) * | −0.15 (.09) |
| Dialysisb | N/A | 4% | 6% | 0% | .58 (<.01) * | .31 (<.01) * | −0.06 (.50) | .17 (<.05) * | −0.05 (.56) |
| Ascitesb | N/A | 27% | 30% | 17% | 0.07 (.41) | 0.14 (0.10) | 0.12 (.17) | 0.08 (.36) | 0.02 (.82) |
| HEb | N/A | 54% | 60% * | 39% * | −0.05 (.59) | 0.06 (.57) | 0.04 (.72) | 0.00 (.99) | −0.04 (.69) |
| NCT, seca | N/A | 47 (22) | 49 (22) * | 42 (23) * | 0.04 (.67) | .17 (.05) * | 0.01 (.93) | −0.05 (.56) | −0.08 (.36) |
| LFI, scorec | N/A | 4.0 (0.7) | 4.1 (0.7) | 3.8 (0.8) | 0.13 (.13) | .43 (<.01) * | 0.00 (.99) | .22 (.01) * | −0.07 (.41) |
| Plasma biomarkers | (n = 22) | (n = 135) | (n = 99) | (n = 36) | Univariable association (β, P) Cirrhosis only | ||||
|---|---|---|---|---|---|---|---|---|---|
| PTau181, ng/mLa | 1.8 (0.9) * | 4.7 (4.5) * | 5.1 (4.6) * | 4.0 (4.2) * | – | .43 (<.01) * | .17 (.05) * | .43 (<.01) * | .18 (.03) * |
| NfL, ng/mLa | 13.2 (6.1) * | 39.6 (42) * | 43.8 (46.5) * | 28.2 (23.5) * | .43 (<.01) * | – | .19 (.03) * | .43 (<.01) * | 0.12 (.16) |
| GFAP, ng/mLa | 136 (89) | 220 (339) | 223 (349) | 210 (312) | .17 (.05) * | 0.19 (.03) * | – | .35 (<.01) * | .92 (<.01) * |
| Total tau, ng/mLa | 1.4 (.8) * | 7.6 (7.4) * | 8.0 (7.1) * | 6.5 (8.0) * | .43 (<.01) * | .43 (<.01) * | .35 (<.01) * | – | .41 (<.01) * |
| UCHL1, ng/mLa | 21 (12) * | 117 (311) * | 111 (311) | 133 (344) | .18 (.03) * | 0.12 (.16) | .92 (<.01) * | .41 (<.01) * | – |
Abbreviations: AIH, autoimmune hepatitis; BMI, body mass index; CAD, coronary artery disease; HE, hepatic encephalopathy; INR, international normalized ratio; LFI, liver frailty index; MELD, Model for End‐Stage Liver Disease; NAFLD, non‐alcoholic fatty liver disease.
aMann‐Whitney.
bPearson's chi‐square test.
cTwo‐sample t‐test.
dBlack, African American, American Indian, Alaska Native.
*Bold if P ≤ .05).
3.2. Plasma biomarker concentrations in cirrhosis versus Controls
Compared to controls, mean biomarker concentrations for p‐tau181, NfL, t‐tau, and UCHL1 were 2‐ to 4‐fold higher in cirrhosis; and p‐tau181, NfL, and t‐tau were higher in NAFLD compared to AIH (Figure 1 , Table 1 ). To confirm these findings, a replication data set was generated for additional healthy controls (N = 96), and although this cohort was not age or sex matched (Table S1), the same degree of elevation was seen for p‐tau181, NfL, t‐tau, and UCHL1 (Figure S1).
FIGURE 1.
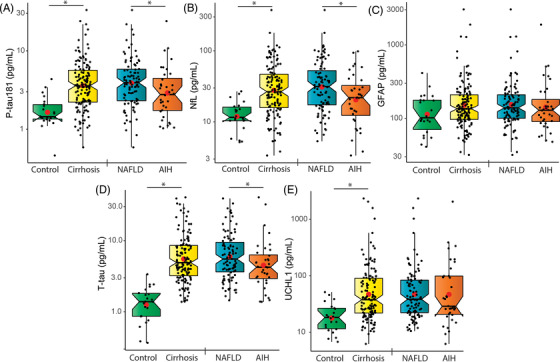
Comparison of plasma concentrations of (A) p‐tau181, (B) NfL, (C) GFAP, (D) t‐tau, and (E) UCHL1 between cognitively normal controls and cirrhosis, and between NAFLD and AIH. Note logarithmic y‐axis for display
3.3. Relationship between plasma biomarkers and hepatorenal function in cirrhosis
When univariable linear regression was used, creatinine showed a moderate positive association with three plasma biomarkers: p‐tau181, NfL, and t‐tau (Table 1 ), whereas no association was seen for GFAP or UCHL1. When stratifying creatinine below (N = 96) or above (N = 39) the upper limit of normal (ULN, reference range females: 0.55–1.02 mg/dL, males: 0.73–1.24 mg/dL), the association was weaker in cirrhosis patients with normal creatinine for p‐tau181 (normal Cr [β = 0.25, P = .02], Cr > ULN [β = 0.86, P < .01]), and present only at creatinine values >ULN for NfL (normal Cr [β = 0.17, P = .09], Cr > ULN [β = 0.35, P =.03]) and t‐tau (normal Cr [β = 0.04, P = .66], Cr > ULN [β = 0.45, P < .01]).
Albumin showed weak negative associations with all biomarkers, but UCHL1 did not reach statistical significance (β = –0.15, P = .09). No association was seen with sodium, total bilirubin, or international normalized ratio (INR). In addition, p‐tau181 was associated with diabetes, MELDNa, and dialysis; NfL with age, diabetes, stroke, dialysis, NCT, and LFI; and t‐tau with MELDNa, dialysis, and LFI.
4. DISCUSSION
In this cross‐sectional retrospective study, plasma biomarkers of neuropathology were measured in 135 participants with cirrhosis and compared to 22 age‐ and sex‐matched cognitively normal controls. Concentrations of p‐tau181, NfL, t‐tau, and UCHL1 were markedly elevated, roughly 2‐ to 4‐fold, in comparison to controls, a magnitude reported previously in neurodegenerative disease. 2 , 3 These elevations were confirmed in a non‐matched replication cohort of controls. Within cirrhosis, three biomarkers were associated with renal function, especially p‐tau181, and to a lesser extent NfL and t‐tau, particularly at creatinine concentrations above the ULN, which is consistent with prior reports, 9 whereas GFAP and UCHL1 showed no dependence on renal clearance. Further support was found for an association with renal function for p‐tau181, NfL, and t‐tau, as higher biomarker concentrations were seen in NAFLD, which had higher creatinine compared to AIH. Finally, albumin demonstrated a weak inverse association with all biomarkers, although UCHL1 was not statistically significant.
Our findings complement prior work showing that several non‐neurologic factors such as age, BMI, chronic kidney disease, and diabetes can differentially impact peripheral levels of plasma biomarkers. 8 , 10 , 13 , 14 Using cirrhosis as a model with multi‐system involvement, we add to this work by showing the differential dependence on renal clearance and circulating albumin for five biomarkers, suggesting that hepatorenal function should be considered, especially for p‐tau181, NfL, and t‐tau. Although these findings need replication, we recommend cautious interpretation of plasma p‐tau181 in patients with chronic kidney disease or hypoalbuminemia to avoid misattributing elevations caused by hepatorenal dysfunction to AD.
It is notable that no association was found with other measures of cirrhosis severity, including sodium, total bilirubin, or INR. Specifically, the lack of relationship to bilirubin or INR suggests that associations with albumin are not driven by impaired hepatic protein clearance or synthesis, but by albumin‐biomarker binding interactions in the plasma matrix. In addition, although we did not replicate the association of NfL and t‐tau with BMI, 10 patients with cirrhosis have pathologic changes in fluid distribution (e.g., ascites), which makes BMI an unreliable marker of blood volume. Finally, the association of NfL and t‐tau with LFI, a measure of frailty in cirrhosis, may be explained by the increased prevalence of peripheral neuropathy in cirrhosis populations.
Our findings raise important questions about the relationship between cirrhosis and biomarkers of neuropathology. We have shown that hepatorenal function can impact these biomarkers, but cirrhosis is also known to cause a spectrum of neurologic changes, ranging from encephalopathy to coma. Although we observed no relationship to MoCA for any biomarker, MoCA was decreased in cirrhosis compared to controls, suggesting that cognitive changes are present. We speculate that more comprehensive cognitive testing may reveal associations with biomarkers of neuropathology in cirrhosis, perhaps after controlling for hepatorenal function. We also found that NfL showed a weak correlation to NCT, a measure of HE severity, hinting at its potential utility as a marker for HE if peripheral confounds can be controlled.
We acknowledge limitations of our analysis: chiefly, important differences in pre‐analytic processing (e.g., collection tube) limit the interpretation of differences between controls and cirrhosis, 15 as does lack of cirrhosis severity labs in controls. An additional uncontrolled confound includes lack of fasting. Traditional tools for assessing neurologic involvement, such as comprehensive cognitive testing, brain imaging, or CSF studies, were not available in this retrospective cirrhosis cohort, partly because lumbar puncture is unsafe in this population due to clotting dysfunction. Finally, although replication of several known associations supports the extrapolation of our results, repeating these studies in larger studies of healthy controls and neurodegenerative disease is critical.
Future work could use these biomarkers to probe the extent that cirrhosis causes neuropathologic changes, potentially mediated through vascular changes in the brain or possibly even increased risk of Alzheimer's pathology triggered by inflammatory mechanisms, and prospective studies could include comprehensive cognitive testing and independent measures of neuropathology, for example, magnetic resonance imaging (MRI) and positron emission tomography (PET).
In conclusion, we found that participants with cirrhosis had higher concentrations of p‐tau181, NfL, t‐tau, and UCHL1 compared to controls. Changes were differentially associated with hepatorenal function, with p‐tau181, NfL, and t‐tau associating with renal function. Consideration of these factors may be critical for clinical interpretation and successful translation of these plasma biomarkers into real‐world clinical use.
CONFLICTS OF INTEREST
A.L.B. receives research support from the NIH, the Rainwater Charitable Foundation, the Association for Frontotemporal Degeneration, the Bluefield Project to Cure Frontotemporal Dementia, the Alzheimer's Drug Discovery Foundation, and the Alzheimer's Association. He has served as a consultant for Alector, AGTC, Arkuda, Arvinas, AZTherapies, GSK, Oligomerix, Ono, Regeneron, Roche, Samumed, Stealth, Third Rock, Transposon, and Wave, and received research support from Biogen, Eisai, and Regeneron. The remaining authors have no actual or potential conflicts to disclose, including commercial affiliations or financial arrangements. The funding agencies providing financial support played no role in the analysis of the data or the preparation of this manuscript.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENTS
This study was funded by National Institutes of Health (NIH) TL1TR001871‐05 (Berry), NIH K23AG059888 (Rojas), NIH 2RF1AG032289 (Kramer), and NIH R01AG038791 (Boxer) and grants from the UC Cures Alzheimer's Disease Program (Boxer), Rainwater Charitable Foundation (Boxer), Bluefield Project (Boxer), L. Hillblom Foundation (Possin), NIH R01AG059183 (Lai), NIH R21AG067554 (Lai), NIH P30DK026743 (Lai), American Gastroenterological Association Pilot Award (Lai), NIH K23AG073514 (VandeVrede), American Academy of Neurology, the Alzheimer's Association, and the American Brain Foundation (VandeVrede).
Berry K, Asken BM, Grab JD, et al. Hepatic and renal function impact concentrations of plasma biomarkers of neuropathology. Alzheimer's Dement. 2022;14:e12321. 10.1002/dad2.12321
Jennifer C. Lai and Lawren VandeVrede are co‐senior authors.
Clinical Trial Registry Website: https://clinicaltrials.gov Trial Number: NCT03228290.
REFERENCES
- 1. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21(1):6777. [DOI] [PubMed] [Google Scholar]
- 2. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379–386. [DOI] [PubMed] [Google Scholar]
- 3. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bridel C, van Wieringen WN, Zetterberg H, et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta‐analysis. JAMA Neurol. 2019;76(9):1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang KK, Yang Z, Sarkis G, Torres I, Raghavan V. Ubiquitin C‐terminal hydrolase‐L1 (UCH‐L1) as a therapeutic and diagnostic target in neurodegeneration, neurotrauma and neuro‐injuries. Expert Opin Ther Targets. 2017;21(6):627–638. [DOI] [PubMed] [Google Scholar]
- 7. Rubenstein R, Chang B, Yue JK, et al. Comparing plasma phospho tau, total tau, and phospho tau‐total tau ratio as acute and chronic traumatic brain injury biomarkers. JAMA Neurol. 2017;74(9):1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akamine S, Marutani N, Kanayama D, et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep. 2020;10(1):20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246–257. [DOI] [PubMed] [Google Scholar]
- 10. Syrjanen JA, Campbell MR, Algeciras‐Schimnich A, et al. Associations of amyloid and neurodegeneration plasma biomarkers with comorbidities. Alzheimers Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kardashian A, Ge J, McCulloch CE, et al. Identifying an optimal liver frailty index cutoff to predict waitlist mortality in liver transplant candidates. Hepatology. 2021;73(3):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry K, Duarte‐Rojo A, Grab JD, et al. Cognitive impairment and physical frailty in patients with cirrhosis. Hepatol Commun. 2022;6(1):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khalil M, Pirpamer L, Hofer E, et al., Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manouchehrinia A, Piehl F, Hillert J, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann Clin Transl Neurol. 2020;7(1):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verberk IMW, Misdorp EO, Koelewijn J, et al. Characterization of pre‐analytical sample handling effects on a panel of Alzheimer's disease‐related blood‐based biomarkers: Results from the Standardization of Alzheimer's Blood Biomarkers (SABB) working group. Alzheimers Dement. 2021. doi:10.1002/alz.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


