Abstract
Introduction
Graft-versus-host disease (GVHD) is a complication of haematopoietic stem cell transplantation (HSCT). GVHD may also develop following solid transplants or blood transfusions if white blood cells are transferred. GVHD affects multiple organs, including the oral tissues.
Objective
This pictorial review provides a background of GVHD to dental practitioners, describes the most common oral manifestations of GVHD and highlights the main treatment modifications needed to deliver dental care to patients with GVHD.
Methods
A narrative review enhanced with clinical photographs.
Results
Acute GVHD may manifest in the oral mucosa; however, it often develops immediately following HSCT when routine dental treatment is postponed. Chronic GVHD may manifest in the oral mucosa, the salivary glands and the musculoskeletal compartment. It may indirectly affect the teeth and the oral flora, putting the patient at risk for infections. Importantly, GVHD poses an increased risk for oral cancer.
Conclusion
GVHD has a wide range of oral manifestations, some of which may affect dental treatment.
Key words: Dry mouth, Graft-versus-host disease, Haematopoietic stem cell transplantation, Mucosal, Oral, Oral cancer
Introduction
Graft-versus-host disease (GVHD) is a complication of certain types of transplants in which the graft contains a large number of donor immune cells. GVHD is triggered by the reactivity of donor-derived immune cells against allogeneic recipient tissues. Typically, this process affects the skin, liver, eyes, gastrointestinal tract, lungs and joints. GVHD usually occurs following allogeneic haematopoietic stem cell transplantation (HSCT), although the condition may occur following a blood transfusion, intestine transplant or face transplant.
There are two categories of GVHD – acute and chronic – each with their own clinical presentation, timing relative to the transplant and pathogenesis. The old paradigm was that chronic GVHD (cGVHD) begins more than 100 days post-HSCT. This definition has been altered, and currently the diagnosis is made primarily based on clinical presentation, although chronologically, acute GVHD (aGVHD) often occurs earlier than cGVHD. In some patients, aGVHD and cGVHD occur concurrently, and this phenomenon is termed ‘overlap syndrome’. Late aGVHD occurs more than 100 days post-HSCT and is defined as signs and symptoms of aGVHD without cGVHD.1,2
The diagnosis of aGVHD is clinical and based on the development of signs in the skin (erythema, maculopapular rash), liver (hepatitis, jaundice) and gastrointestinal tract (nausea, vomiting, abdominal pain, diarrhoea, anorexia) within 100 days of HSCT in a patient not meeting the diagnostic criteria for cGVHD.3 One or more organs may be involved. Oral involvement in aGVHD is uncommon and non-specific, and may include erythema, with or without ulceration of the oral mucosa or lips.4 In the absence of a biopsy, the diagnosis of oral cGVHD relies on elimination of differential diagnoses and the coexistence of aGVHD in other organs. Considering the rarity of this entity in the oral tissues, it will not be discussed further in this review.
cGVHD is a systemic disease with a wide range of signs and symptoms that mimic autoimmune diseases, such as systemic lupus erythematosus, lichen planus, scleroderma, Sjögren syndrome, biliary cirrhosis and bronchiolitis obliterans syndrome. The oral tissues are commonly involved in cGVHD.
The objective of this review was to provide a background of GVHD to dental practitioners, describe the most common oral manifestations of GVHD and highlight the main treatment modifications needed to deliver dental care to patients with GVHD.
Systemic cGVHD
According to 2005 and 2014 consensus papers from The National Institutes of Health (NIH), the diagnosis of cGVHD is based on at least one diagnostic manifestation or at least one distinctive manifestation with a pertinent biopsy, laboratory test or other test that supports the diagnosis of cGVHD.3,5
Diagnostic manifestation refers to those signs that establish the presence of cGVHD without the need for further testing or evidence of other organ involvement. Distinctive manifestation of cGVHD refers to signs not usually found in aGVHD but insufficient in isolation to establish unequivocally a diagnosis of cGVHD. Additional testing may include pulmonary function tests, Schirmer's test or an evaluation by a specialist (such as an ophthalmologist or a gynaecologist), or radiographic imaging showing cGVHD. Table 1 lists the manifestations of cGVHD, classified as diagnostic and distinctive criteria for cGVHD. Likewise, there are common manifestations which refer to manifestations seen in both aGVHD and cGVHD. It is important to exclude infection and other causes that may confound or complicate the diagnosis of cGVHD.
Table 1.
Signs and symptoms of chronic graft-versus-host disease (cGVHD).3
| Site | Diagnostic criteria | Distinctive criteria | Other features or unclassified entities | Common manifestations |
|---|---|---|---|---|
| Skin | • Poikiloderma | • Depigmentation | • Sweat impairment | • Erythema |
| • Lichen planus-like features | • Papulosquamous lesions | • Ichthyosis | • Maculopapular rash | |
| • Sclerotic features | • Keratosis pilaris | • Pruritus | ||
| • Morphea-like features | • Hypopigmentation | |||
| • Lichen sclerosus-like features | • Hyperpigmentation | |||
| Nails | • Dystrophy | |||
| • Longitudinal ridging | ||||
| • Splitting or brittle features | ||||
| • Onycholysis | ||||
| • Pterygium unguis | ||||
| • Nail loss (usually symmetric, affects most nails) | ||||
| Scalp and body hair | • New onset of scarring or nonscarring scalp alopecia (after recovery from chemoradiotherapy) | • Thinning scalp hair, typically patchy, coarse or dull (not explained by endocrine or other causes) | ||
| • Loss of body hair• Scaling | • Premature grey hair | |||
| Mouth | • Lichen planus-like changes | • Xerostomia | • Gingivitis | |
| • Mucoceles | • Mucositis | |||
| • Mucosal atrophy | • Erythema | |||
| • Ulcers | • Pain | |||
| • Pseudomembranes | ||||
| Eyes | • New-onset dry, gritty or | • Photophobia | ||
| painful eyes | • Periorbital hyperpigmentation | |||
| • Cicatricial conjunctivitis• KCS• Confluent areas of punctate keratopathy | • Blepharitis (erythema of the eyelids with oedema) | |||
| Genitalia | • Lichen planus-like features | • Erosions | ||
| • Lichen sclerosus-like features | • Fissures | |||
| • Women: vaginal scarring or clitoral/labial agglutination | • Ulcers | |||
| • Men: phimosis or urethral/meatus scarring or stenosis | ||||
| GI tract | • Oesophageal webs | |||
| • Strictures or stenosis in the upper- to midthird of the oesophagus | • Exocrine pancreatic insufficiency | • Anorexia | ||
| • Nausea | ||||
| • Vomiting | ||||
| • Diarrhoea | ||||
| • Weight loss | ||||
| • Failure to thrive (infants and children) | ||||
| Liver | • Total bilirubin, alkaline phosphatase > 2 × upper normal limit | |||
| • ALT> 2 × upper normal limit | ||||
| Lung | • Bronchiolitis obliterans diagnosed with biopsy | • Air trapping and bronchieactasis on chest CT | • Cryptogenic organising pneumonia | |
| Muscles, fascia, joints | • Fasciitis | • Myositis or polymyositis | • Restrictive lung disease | |
| • Joint stiffness or contractures secondary to fasciitis or sclerosis | • Oedema | |||
| • Muscle cramps | ||||
| • Arthralgia or arthritis | ||||
| Haematopoietic and immune | • Thrombocytopenia | |||
| • Eosinophilia | ||||
| • Lymphopenia | ||||
| • Hypo- or hyper-gammaglobulinaemia | ||||
| • Autoantibodies (AIHA, ITP) | ||||
| • Raynaud's phenomenon | ||||
| • Pericardial or pleural effusions | ||||
| Other | • Ascites | |||
| • Peripheral neuropathy | ||||
| • Nephrotic syndrome | ||||
| • Myasthenia gravis | ||||
| • Cardiac conduction abnormality or cardiomyopathy |
AIHA, autoimmune haemolytic anaemia; ALT, alanine aminotransferase; CT, computed tomography; GI, gastrointestinal; ITP, idiopathic thrombocytopenic purpura; KCS, keratoconjunctivitis sicca.
The pathogenesis of cGVHD is complex and multifactorial, involving simultaneous immune processes. For simplification, a recent review described a three-phase model6:
-
•
Phase 1: bacteria, fungi and their by-products penetrate the gastrointestinal epithelium. Tissue damaged as a result of the cytotoxic conditioning regimen produces cellular degradation products. The accumulation of these molecules causes inflammation, and activated T cells interact with dendritic cells and up-regulate cell degeneration and the formation of additional immunoreactive molecules.
-
•
Phase 2: the dendritic cells activate B cells and more T cells, causing the proliferation of specific types of helper-T cells.
-
•
Phase 3: cytokines trigger fibroblasts. This results in enhanced production of the extracellular matrix and sclerosis. B cells produce immunoglobulins that are deposited in various organs and contribute to organ inflammation and fibrosis.
The approach to prevent GVHD is through identification of the best matched donor, particularly regarding human leukocyte antigen (HLA), and family relationship. The incidence of GVHD is lower following transplants from related matched donors compared with unrelated donors. Additional factors that determine the risk for GVHD are: gender mismatch; source of stem cells (peripheral blood mobilised stem cells vs. stem cells harvested from bone marrow7); depletion of T cells from the graft; and the duration and type of immunosuppressive therapy.8 The immunosuppressive therapy administered to prevent GVHD usually includes calcineurin inhibitors, such as cyclosporine or tacrolimus, in combination with methotrexate, mycophenolate mofetil or sirolimus.
Post-HSCT, the patients are placed in a follow-up program, which is delivered by a multi-disciplinary team. This is essential in those with cGVHD, as the disease tends to fluctuate, affects many organs and is debilitating. Furthermore, the treatment itself may cause secondary complications that also need to be managed.
The treatment for cGVHD depends on the number of organs involved and on the severity of organ involvement. Moderate or severe symptoms, or involvement of several organs, are usually treated with systemic therapy, and steroids in moderate doses (e.g. prednisone 0.5–1 mg/kg/day) are the mainstay. In the past, GVHD was treated with azathioprine, while cyclosporine, tacrolimus and mycophenolate were used as second lines of therapy. Over the last decade, new interventions have been introduced. Currently, sirolimus, rituximab, imatinib, ibrutinib and ruxolitinib are more commonly used for treating cGVHD. Non-pharmacological modalities include extracorporeal photopheresis and phototherapy.
Ancillary therapies have an important role in symptom palliation, preventing secondary complications and maintaining quality of life. Each organ has a set of interventions that specifically address the symptoms associated with its cGVHD.
Oral mucosal cGVHD
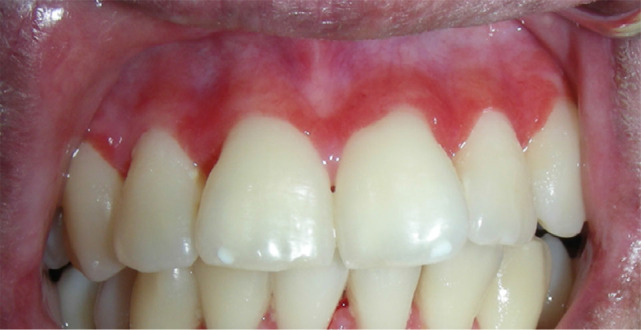
Clinically, mucosal lesions present as white striae, erythema and ulcers (Figure 1). There is a spectrum of mucosal damage. The spectrum ranges from erythema and erosive thinning of the mucosa at one end to loss of epithelial continuity and the formation of ulcers covered with yellowish fibrinous pseudomembranes at the other end. The buccal mucosa and sides of the tongue are the most common sites involved. The lesions may be associated with sensitivity or pain, especially during function and contact with acidic or spicy foods or beverages.9
Fig. 1.
Typical clinical presentation of chronic graft-versus-host disease (cGVHD) in the oral mucosae: (a) lichenoid lesions appear as white striations; (b) erythema, ulceration as well as subtle white striations.
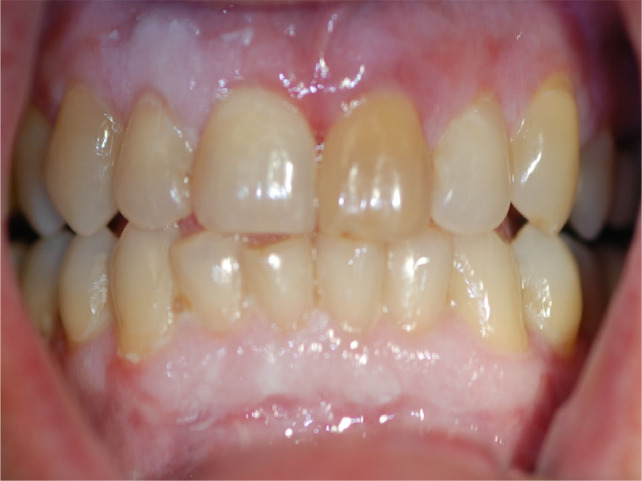
cGVHD in the gingival tissues manifests as desquamation and erythema, with or without white colour changes (Figure 2). Gingival sensitivity may restrict oral hygiene performance, which in turn causes dental plaque-induced gingivitis. The combined effect may be increased gingival bleeding.
Fig. 2.
Gingival involvement of chronic graft-versus-host disease (cGVHD).
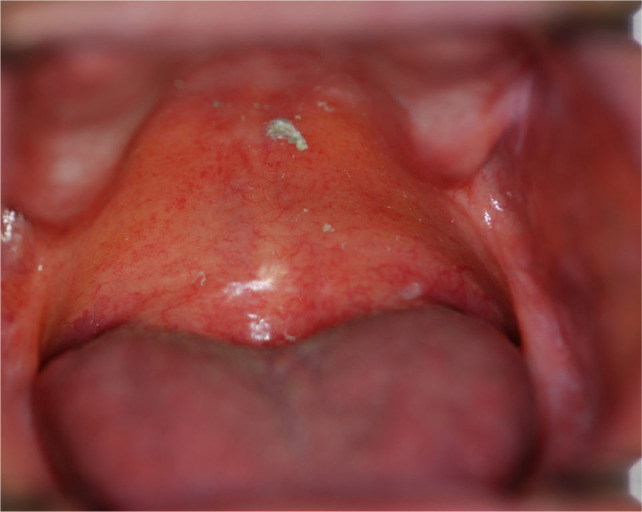
Erythema and ulcerations may present without typical white reticulation (Figure 3). According to the NIH consensus paper,3,5 a biopsy will be needed to assess these lesions. Unfortunately, there is no literature analysing the sensitivity of biopsy in a large sample of this type of oral manifestation. If the histopathology report does not confirm cGVHD, these lesions become a diagnostic challenge.
Fig. 3.
Chronic graft-versus-host disease (cGVHD) manifests as erythema and ulcerations without typical white reticulation.
White plaque (Figure 4) was removed from the list of diagnostic manifestations of oral cGVHD in the 2014 NIH consensus paper in order to ensure that malignant transformation is not misdiagnosed by presuming that it is cGVHD. To clarify, this change in the NIH consensus paper does not exclude a white plaque lesion from being diagnosed as cGVHD with histopathological confirmation.
Fig. 4.
White oral plaque in chronic graft-versus-host disease (cGVHD).
Salivary gland cGVHD
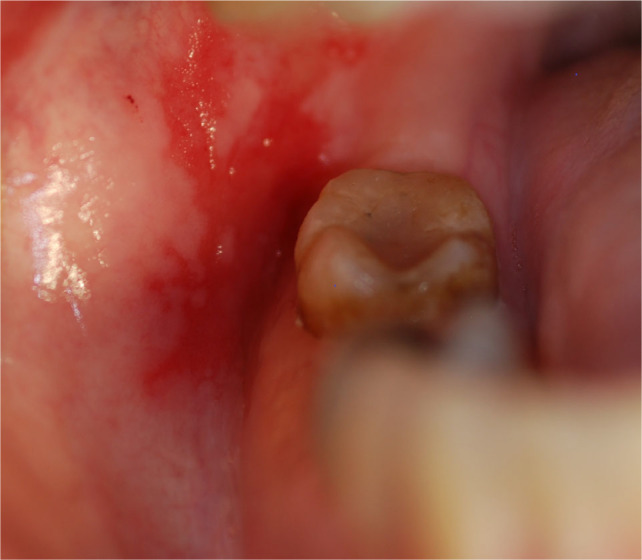
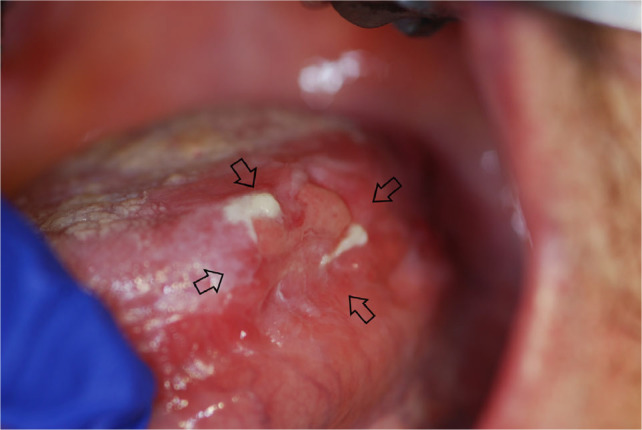
Multiple mucoceles are a hallmark of cGVHD; these commonly appear on the soft palate, on the lower labial and buccal vestibular mucosa and in other locations based on the anatomical distribution of the minor salivary glands (Figure 5). The lesions are usually not painful; however, patients describe them as annoying. Ruptured mucoceles may cause mucosal erosions and become sensitive. Unlike the mucoceles which often develop in otherwise healthy young individuals, mucoceles occurring in cGVHD are smaller, appear in clusters and are not preceded by local trauma.
Fig. 5.
Multiple superficial mucoceles in chronic graft-versus-host disease (cGVHD).
The major salivary glands are often affected by cGVHD, leading to dysfunction, lower saliva production and secretion (hyposalivation), dry mouth (xerostomia) and a change in the quality of the saliva. The saliva may have a greater mucoid fraction with less fluidity. There may be mucoid strings of saliva, dried deposits on the dorsum of the tongue, or ropy saliva in the floor of the mouth and lower vestibulum (Figure 6). Salivary gland dysfunction may lead to gland swelling and pain (Figure 7).
Fig. 6.
Dry mouth and soft deposits on the oral surface in chronic graft-versus-host disease (cGVHD).
Fig. 7.
Swelling of the parotid gland in chronic graft-versus-host disease (cGVHD).
Ranulae are large mucoceles deep within the tissue that do not rupture spontaneously. They are often located in the lower vestibulum or lower labial mucosa.
Musculoskeletal cGVHD
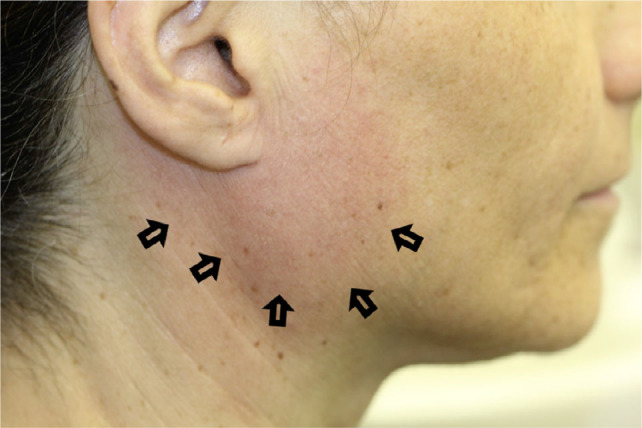
The sclerodermatous form of cGVHD manifests as limited mouth opening and loss of elasticity of the lips and tongue (Figure 8). This may have a great impact on oral function, oral hygiene, risk for infections from odontogenic origin and quality of life. Also, the ability to provide dental and oral treatment is compromised because of the restrictions in mouth opening.10 It was suggested that fibrotic pulp changes are caused by the sclerodermatous process of cGVHD.11
Fig. 8.
Restricted mouth opening because of sclerodermatous changes in chronic graft-versus-host disease (cGVHD) (the patient also had a history of radiotherapy to the neck).
Secondary complications of oral cGVHD
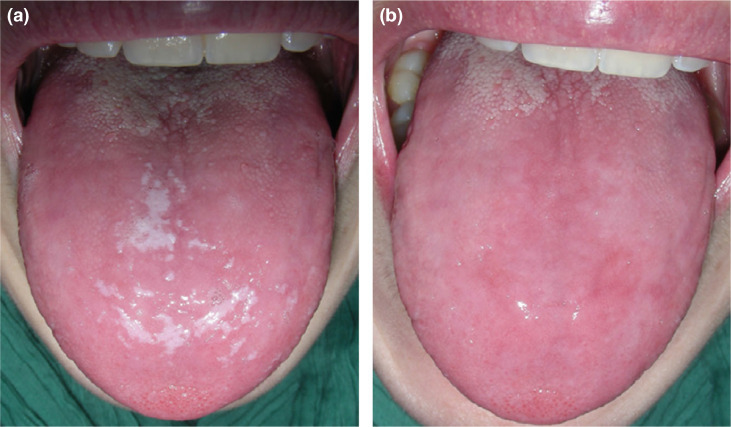
Oral candidiasis may arise concomitantly with oral cGVHD as a result of dry mouth, immunosuppression, treatment with systemic or topical corticosteroids and occasional antibiotic use (Figure 9). Oral candidiasis may present as white removable plaques, erythematous patches, angular cheilitis, rhomboid glossitis or, to a much lesser extent, as white non-removable plaques.
Fig. 9.
Oral candidiasis in chronic graft-versus-host disease (cGVHD); (a) White pseudomembranous candidiasis (oral thrush); (b) The same cGVHD patient after removing of the creamy white lesions by gentle scraping leaving behind an underlying erythematous mucosal surface.
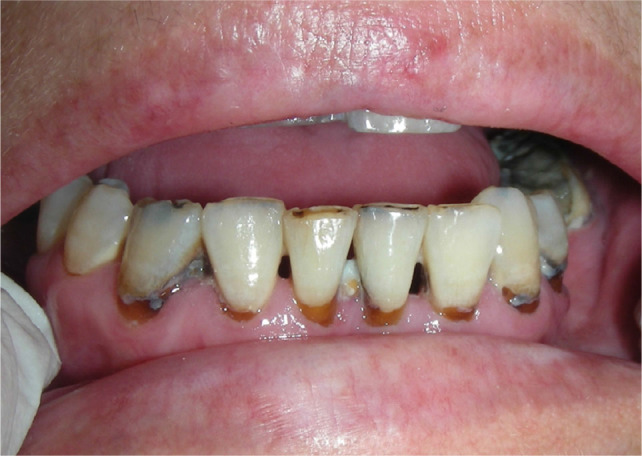
Rampant caries may develop in patients with hyposalivation, such as in those with cGVHD. This type of dental decay often involves the proximal and cervical surfaces. It may also involve atypical sites, such as the incisal edge (Figure 10).
Fig. 10.
Rampant dental caries secondary to hyposalivation in chronic graft-versus-host disease (cGVHD).
Patients post-HSCT are at higher risk of developing a second primary cancer, in particular oral cancer. cGVHD lesions can undergo malignant transformation. The most common oral cancer reported is squamous cell carcinoma (Figure 11). Furthermore, long-term follow-up of these patients also suggests an increased risk for recurrence of oral carcinomas.12
Fig. 11.
Oral squamous cell carcinoma in chronic graft-versus-host disease (cGVHD) on the base of the tongue.
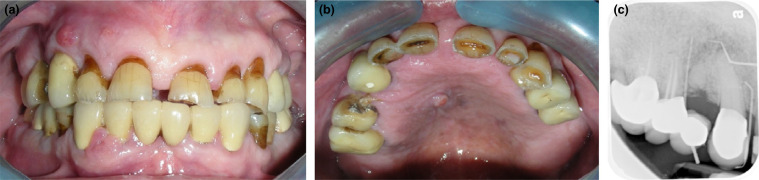
Benign lesions, including pyogenic granuloma and verruciform xanthoma, have been reported post-HSCT in patients with cGVHD (Figure 12). It is assumed that the chronic inflammation triggers an excessive tissue response that forms papillary epithelial hyperplasia and foamy macrophages in connective tissue.13
Fig. 12.
Medication (bisphosphonate)-related osteonecrosis of the jaws in a patient post-haematopoietic stem cell transplantation (HSCT); (a) Front - fold-to-fold, and (b) palatal views, showing 3 sinus tracts without visible bone exposure, and (c) tracing periapical radiograph with gutta percha points demonstrating the involved necrotic bone.
Associated oral lesions
Involuntary mouth clenching is infrequently reported in patients post-HSCT. It is unclear if this is part of the cGVHD, an adverse effect of the steroids used to treat the cGVHD or from a co-existing condition. Clenching occurs numerous times throughout the day, with very strong biting forces that may injure adjacent surfaces, such as the tongue.
Taste changes have been reported in patients post-HSCT and these usually return to normal a year after HSCT. The association between taste change and cGVHD is controversial.14,15
Osteonecrosis of the jaw may develop in patients post-HSCT. The long-term use of high-dose steroids results in osteoporosis, which is treated with alendronate, a risk factor for osteonecrosis of the jaw. Additionally, some multiple myeloma patients undergo allogeneic HSCT. Given that the treatment for multiple myeloma includes intravenous bisphosphonates, by the time such patients develop GVHD, they are already at a higher risk for medication-related osteonecrosis of the jaw (Figure 12).
In the last decade, targeted therapy has become an important treatment for cGVHD. Accordingly, oral complications associated with these medications may be observed in patients with cGVHD. Sirolimus was reported to induce oral ulcerations (Figure 13).16 The elliptical lesions usually have a central yellowish fibrinous membrane and an erythematous periphery; larger lesions have a more irregular shape. Ibrutinib may induce mucositis which has been described as aphthous-like lesions.17
Fig. 13.
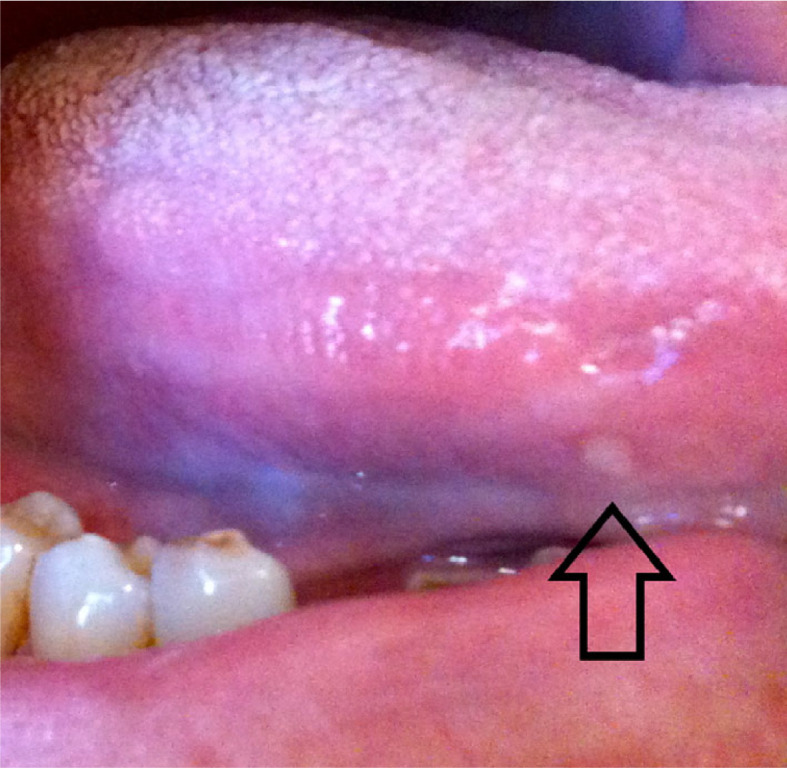
Stomatitis in a patient treated with targeted therapy; the patient had multiple simultaneous lesions.
Scoring
NIH scales
In 2006, the NIH cGVHD Task Force published a proposed system to grade oral cGVHD severity.5 This system was initially proposed for research purposes; however, over the years it was adopted in the clinical setting. Alongside the general cGVHD grading are organ-specific scales.
The 2006 oral scale included a 12 field matrix that combined four types of oral manifestations, for which three severity levels were defined (Figure 14 top).5 Each level of severity received a proportional score, which were summed to give an overall score. The oral manifestations were lichenoid, erythema, ulceration and mucoceles. The definition of the severity of the lichenoid and ulcerative lesions was based on the relative surface area involved, whereas the definition of the erythema was based on the severity of the red colour and the surface area involved. The definition of the severity of mucoceles was based on the number of mucoceles in the soft palate. The maximum score was 15. The scale referred to symptoms of pain, dry mouth and sensitivity (irritation while eating food that is usually well tolerated).
Fig. 14.
The 2006 (top) and 2014 (bottom) National Institutes of Health's oral scale for grading the severity of chronic graft-versus-host disease (cGVHD).3,5
Several validation studies identified the strengths and weaknesses of the scale.18, 19, 20 In 2014, the NIH cGVHD Task Force revised the oral cGVHD scale.21 After revision, only three oral manifestations (lichenoid, erythema and ulceration) remained (Figure 14 bottom), with a maximum score of 12. Some of the changes are linked to the validation studies, whereas others were new additions. This new scale has not been validated. Pain and dry mouth were removed from the standard questionnaire in the new scale.
Importantly, removal of ‘mucocele’ from the matrix reflected the fact that there was no clinical correlation between the erythema/ulceration/lichenoid scores and the mucocele score. This may be explained by a pathogenesis for mucoceles that is not related to mucosal damage. The change to the scale in no way claims that mucoceles are not a manifestation of cGVHD. Likewise, removal of the ‘pain’ assessment from the oral cGVHD questionnaire was explained by the presence of this question in a general cGVHD assessment questionnaire. The focus of the NIH questionnaire is the standard questions required in a transplant clinic. It is clear that hospital dentistry or specialist oral medicine clinics will add oral tissue-specific questions, such as dry mouth and oral pain.
Other scales
In the early 1990s, a proposed scale for oral cGVHD combined, into a total score, the type of oral manifestations with the size of areas involved. The severity of the manifestations atrophy, pseudomembrane, erythema, hyperkeratosis, lichenoid, ulceration and oedema were scored. Erythema, atrophy, hyperkeratosis, lichenoid and oedema were scored on a scale of 0–3, where ‘0’ was normal, ‘1’ was mild, ‘2’ was moderate and ’3’ was severe. Ulceration and pseudomembrane were scored based on the estimated surface area involved, where ‘0’ was none, ‘1’ was 0–1 cm2, ‘2’ was 1–2 cm2 and ‘3’ was >2 cm2. Scores for these variables were recorded for each of the following surfaces: lips (upper and lower); labial mucosa (upper and lower); buccal mucosa (right and left); tongue (dorsal, lateral and ventral); floor of mouth; palate (hard, soft); and gingivae. The total matrix included 91 fields and provided a map of the lesions, their type and their severity.22 This scale also referred to symptoms of pain and dry mouth.
Additional cGVHD-specific scales were reported in the literature before publication of the NIH scale.23,24 Likewise, scales for lichenoid conditions were used to assess patients with cGVHD.25,26
Treatments for oral cGVHD
Mucosal cGVHD
The first treatment goal is to control the level of activity of the oral cGVHD and the associated symptoms. The treatment for cGVHD is systemic (see ‘General’ section). However, when the oral cGVHD is resistant to systemic treatment or when the oral tissues are the only organ involved, topical treatment has an important role.
Several topical agents have been studied for treatment of oral cGVHD. The ideal topical agent should have high potency and low bioavailability, providing a strong local affect and limiting the risk of systemic adverse effects.
While steroids are the mainstay for oral cGVHD, in immunosuppressed patients (particularly in those with a dry mouth) oral candidiasis may develop. Therefore, a preventive course of topical antifungal treatment should be considered concomitantly with topical steroids.
The second treatment goal is early detection of malignant changes, for which patients with oral cGVHD are at high risk. Screening twice yearly is recommended by the NIH.27
Steroids
Budesonide 0.03%–0.06% mouthwash has been reported, in numerous studies, to be effective for treatment of oral cGVHD.24,28, 29, 30, 31 One study comparing budesonide mouthwash with dexamethasone mouthwash found that budesonide was superior in the treatment of severe oral cGVHD.32,33 Dexamethasone 0.01% mouthrinse has been used to treat oral cGVHD.32,34 Historically, dexamethasone 0.04% elixir was available commercially. Nowadays, a similar rinse may be compounded, with optional adjustments to the solvent in order to avoid alcohol-induced irritation, and possibly higher concentrations of dexamethasone (reviewed in Correa & Schubert).35 Dexamethasone is used frequently for the treatment of oral lichen planus, but there is little research on its use in oral cGVHD.
Other topical steroids suggested in the literature as expert opinions include triamcinolone, fluocinonide, clobetasol, betamethasone and prednisolone.35, 36, 37, 38, 39, 40 The selection of a particular topical steroid is based on the size of the area involved, the severity of the lesions, the cost and patient preference.41
When the cGVHD affects a large area, a solution will be preferred, whereas when the affected sites are small or localised, a gel or cream is preferable. Exceptions to this concept are sites where access is difficult (e.g., the back of the tongue), sites that are very mobile (i.e., the gel will not adhere) or sites where there is heavy saliva flow (e.g., the floor of the mouth).42
When the severity of the cGVHD is high, a more potent agent is indicated, or a combination of topical agents may be used.43 Another option for severe or localised resistant lesions is an intralesional steroid injection, where triamcinolone is usually used. A list of topical agents for the management of cGVHD, together with the appropriate concentrations and dosing protocols, is included in the NIH 2014 consensus paper (Table 2).27
Table 2.
Topical preparations for oral chronic graft-versus-host-disease (cGVHD).*
| Preparation | Family | Drug | Concentration | Dosage | Available commercially in the USA |
|---|---|---|---|---|---|
| Solutions | Steroids | Dexamethasone | 0.1 mg/mL (0.01%) | 10 mL, 3–6 times daily | Yes |
| Dexamethasone | 0.4 mg/mL (0.04%)† | 10 mL, 3–6 times daily | No | ||
| Prednisolone | 3 mg/mL (0.3%) | 5 mL, 3–6 times daily | Yes | ||
| Budesonide | 0.3 mg/mL (0.03%) | 10 mL, 2–4 times daily | No | ||
| Clobetasol | 0.5 mg/mL (0.05%) | 5 mL, 3 times daily | No | ||
| Triamcinolone | 1 mg/mL (0.1%) | 10 times daily | Yes | ||
| Triamcinolone | 10 mg/mL (1%) | 5 mL, 3–6 times daily | No | ||
| Betamethasone† | 0.02 mg/mL (0.002%) | 10 mL, 3–4 times daily | No | ||
| Calcineurin inhibitor | Tacrolimus (oral solution) | 0.1 mg/mL (0.01%) | 5 mL, 3 times daily | No | |
| Creams/gels/ointments | Steroids | Clobetasol cream/gel | 0.05% | Twice daily | Yes |
| Triamcinolone cream† | 0.1% | Twice daily | Yes | ||
| Triamcinolone cream† | 0.5% | Twice daily | Yes | ||
| Halobetasol cream† | 0.05% | Twice daily | Yes | ||
| Betamethasone cream/ointment/gel† | 0.05% | Twice daily | Yes | ||
| Betamethasone cream/ointment† | 0.1% | Twice daily | Yes | ||
| Betamethasone ointment† | 0.05% | Twice daily | Yes | ||
| Fluocinonide gel | 0.05% | Twice daily | Yes | ||
| Other immunomodulators | Tacrolimus ointment | 0.1% | Three times daily | Yes | |
| Intralesional injection | Steroid | Triamcinolone† | 40 mg/mL, 0.5 mL/cm2 | 1 -3 sessions, 2 weeks apart | Yes |
Based on the 2014 National Institutes of Health (NIH) consensus paper.27
Preparations that are in use for oral vesicullobulous diseases and were suggested in the literature specifically for oral cGVHD.
Non-steroidal agents and other therapies
Tacrolimus was suggested as a topical treatment for oral cGVHD in several case reports.44, 45, 46, 47
Palliation of oral cGVHD-associated pain was achieved with a CO2 laser.48 Although the sample size was small, the pain relief was immediate and the drop in the pain score was drastic. Intraoral phototherapy using PUVA or UVB has also been reported to be effective in small case series.49, 50, 51
A novel treatment approach using a topical platelet-rich gel reportedly reduced pain and accelerated healing.52
Salivary gland cGVHD
The treatment approach to hyposalivation is based on artificially increasing oral moisture. The minimally invasive options include synthetic moistening agents, chewing sugarless sour-sweet candy (gustatory stimulation) or mechanical stimulation (parotid massage). Pharmacological stimulation may be achieved with cholinergic agonists, such as pilocarpine (5–10 mg, three or four times daily) and cevimeline (15–30 mg, two or three times daily).53 Electrical stimulation has also been studied in patients with oral cGVHD.54
An important component in the management of dry mouth is prevention of secondary complications. Frequent application of high-concentration fluoride may reduce the risk for dental caries.
Musculoskeletal cGVHD
The limitation of mouth opening is progressive. Therefore, patients with cGVHD should be directed to perform physical therapy to increase the elasticity of the peri-oral tissues, as soon as limited motion is noticed. The exercises should be performed daily. Cessation of physical therapy results in regression.
Implications for dental practice
Hospital dental departments collaborate with the transplant team and establish protocols for dental treatment, allowing information flow between the dental team and the transplant team and access to shared medical records. Dental clinics in the community are advised to consult with the transplant team before the dental examination to discuss diagnoses, medications, allergies and surgical history, as well as recent laboratory tests.
Usually, in patients with cGVHD, the stem cells are engrafted and blood cell counts are within, or close to, the normal range. However, deviations from the normal range may occur. Therefore, it is advised to assess the blood cell counts of patients post-HSCT periodically and before any invasive dental procedures.
If the white and red blood lineages and platelet count are within the normal range, there are no restrictions on dental treatments. If the patient is neutropenic (white blood cell count < 1 × 109 cells/L), only emergency dental procedures should be performed and the patient will probably need antibiotic prophylaxis. If the patient is anaemic (haemoglobin < 8 g/dL), the dentist should be aware of the increased risk for orthostatic hypotension and take measures to prevent falls. There is no uniform platelet count cut-off that identifies a risk for bleeding. When the platelet count is < 80 × 109 cells/L, the dentist should prepare local haemostatic agents. The commercially available haemostatic agents vary between countries. It seems that the most potent haemostatic agents are tranexamic acid and aminoca-proic acid. Thrombin- and collagen-based commercially available agents are designed to fit surgical sites and are also useful. A platelet count of < 20 × 109 cells/L is a contraindication for routine dental care; however, severe bleeding may also occur with higher platelet counts. Therefore, if the patient is thrombocytopenic, the dentist should balance the risk for bleeding versus the benefit of the dental treatment. If surgery is necessary, a platelet transfusion may be needed.
As cGVHD may affect the liver, the coagulation may be affected; therefore, prothrombin time (and the derived measure, international normalized ratio) and partial thromboplastin time should be assessed before invasive procedures, and the dentist should time the dental treatment accordingly. Likewise, if liver function tests are abnormal, the dentist should adjust the dose of medications that are metabolised in the liver. Useful online tools are available and offer clinicians information about recommended dose adjustments.
The oral manifestations of cGVHD (i.e. cGVHD in the salivary glands, oral mucosa, gingivae and musculoskeletal tissues) directly affect dental treatment. The effect of cGVHD on the dental treatment will be explained in regards to each of these oral tissue.
Considering the hyposalivation and higher risk for dental caries in cGVHD, patients with cGVHD are advised to maintain meticulous oral hygiene. The reader is referred to the ‘Basic Oral Care’ recommendations from the Multinational Association of Supportive Care in Cancer.55 Briefly, the dentist should educate the patient about the importance of oral hygiene in light of their medical condition, and offer high-concentration fluoride applications. The high-concentration fluoride can be applied daily in the form of a toothpaste (which may require a prescription), in the form of a gel applied in custom-made trays, or delivered periodically in the dental office in the form of gel in a tray or varnish applied with an applicator.
For patients with mucosal cGVHD or desquamative gingivitis, the tissues may be sensitive to the acidity of the toothpaste. A neutral-pH toothpaste may limit the sensitivity. Also, some patients may be sensitive to the mint flavour, and therefore other flavours should be offered. Children's toothpaste may be more tolerable; however, the lower fluoride concentration in such formulations may not be sufficient to prevent dental caries. Ultra-soft toothbrushes and single-tufted brushes may be helpful for patients with desquamative gingivitis because they may be less traumatic to the gums.
In patients with sclerodermatous cGVHD, the mouth opening may be limited, restricting access to perform oral hygiene effectively and increasing the risk of rampant dental caries as well as gingival and periodontal diseases. Such patients should be encouraged to practice meticulous oral hygiene and have frequent dental check-ups. In patients with severely limited mouth opening, dental treatment may be compromised, and extractions may be the only option. Consequently, the treatment may require monitoring in an operating room and possibly general anaesthesia to relax the maxillofacial muscles and secure the airway. In severe cases, palliative incisions in the corners of the lips may be needed.
The dentist should ask the patient about current or past use of bisphosphonates. If the patient has used, or is currently using, these medications, the protocols for preventing medication-related osteonecrosis of the jaws should be followed.56
Long-term steroid use may suppress adrenal gland function and the patient may not be able to respond appropriately to stress. In cases when extreme stress is anticipated, steroid supplementation may be needed before dental treatment. The current concept is that dental treatment under general anaesthesia requires steroid supplementation. Importantly, the combination of stressful factors, such as sleep deprivation, severe dental pain, the need for extensive oral surgery and anxiety, should be considered. There is no literature that makes specific recommendations about steroid supplementation dosages based on the type or extent of the oral surgical procedures, and therefore clinical judgement is needed.
Summary
cGVHD is a systemic disease affecting several types of oral tissues, and demonstrates variable presentations. Topical treatments have an important role in controlling oral symptoms and improving quality of life. The implications of cGVHD should be considered during oral evaluation and dental treatment. The higher risk for oral cancer in this patient population requires routine clinical evaluation and occasional biopsies. Collaboration with the transplant team and oral medicine specialists provides the support needed to deliver the dental care safely.
Conflict of interest
This paper is not funded. SE is a consultant to Falk Pharma GmbH.
REFERENCES
- 1.Lee SJ. Classification systems for chronic graft-versus-host disease. Blood. 2017;129(1):30–37. doi: 10.1182/blood-2016-07-686642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora M, Nagaraj S, Witte J, et al. New classification of chronic GVHD: added clarity from the consensus diagnoses. Bone Marrow Transpl. 2009;43(2):149–153. doi: 10.1038/bmt.2008.305. [DOI] [PubMed] [Google Scholar]
- 3.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transpl. 2015;21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ion D, Stevenson K, Woo SB, et al. Characterization of oral involvement in acute graft-versus-host disease. Biol Blood Marrow Transpl. 2014;20(11):1717–1721. doi: 10.1016/j.bbmt.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 5.Pavletic SZ, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. Response Criteria Working Group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow. Transplantation. 2006;12(3):252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 7.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elad S, Zadik Y, Yarom N. Oral Complications of Nonsurgical Cancer Therapies. Atlas Oral Maxillofac Surg Clinics North Am. 2017;25(2):133–147. doi: 10.1016/j.cxom.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Zadik Y. Restricted mouth opening in chronic graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(2):201–202. doi: 10.1016/j.oooo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 11.Gomes CB, Treister NS, Miller B, et al. Pulp obliteration in a patient with sclerodermatous chronic graft-versus-host disease. J Endodontics. 2016;42(4):678–680. doi: 10.1016/j.joen.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Elad S, Zadik Y, Zeevi I, et al. Oral cancer in patients after hematopoietic stem-cell transplantation: long-term follow-up suggests an increased risk for recurrence. Transplantation. 2010;90(11):1243–1244. doi: 10.1097/TP.0b013e3181f9caaa. [DOI] [PubMed] [Google Scholar]
- 13.Shahrabi Farahani S, Treister NS, Khan Z, et al. Oral verruciform xanthoma associated with chronic graft-versus-host disease: a report of five cases and a review of the literature. Head Neck Pathol. 2011;5(2):193–198. doi: 10.1007/s12105-011-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, Konuma T, Miwa Y, et al. A cross-sectional study on late taste disorders in survivors of allogeneic hematopoietic cell transplantation. Ann Hematol. 2017;96(11):1841–1847. doi: 10.1007/s00277-017-3087-6. [DOI] [PubMed] [Google Scholar]
- 15.Boer CC, Correa ME, Miranda EC, et al. Taste disorders and oral evaluation in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45(4):705–711. doi: 10.1038/bmt.2009.237. [DOI] [PubMed] [Google Scholar]
- 16.Mahe E, Morelon E, Lechaton S, et al. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation. 2005;79(4):476–482. doi: 10.1097/01.tp.0000151630.25127.3a. [DOI] [PubMed] [Google Scholar]
- 17.Vigarios E, Beylot-Barry M, Jegou MH, et al. Dose-limiting stomatitis associated with ibrutinib therapy: a case series. Br J Haematol. 2019;185(4):784–788. doi: 10.1111/bjh.15620. [DOI] [PubMed] [Google Scholar]
- 18.Elad S, Zeevi I, Or R, et al. Validation of the National Institutes of Health (NIH) scale for oral chronic graft-versus-host disease (cGVHD) Biol Blood Marrow Transpl. 2010;16(1):62–69. doi: 10.1016/j.bbmt.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Treister NS, Cook EF, Jr, Antin J, et al. Clinical evaluation of oral chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2008;14(1):110–115. doi: 10.1016/j.bbmt.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Fassil H, Bassim CW, Mays J, et al. Oral chronic graft-vs.-host disease characterization using the NIH scale. J Dent Res. 2012;91(7):45s–51s. doi: 10.1177/0022034512450881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Wolff D, Kitko C, et al. Measuring therapeutic response in chronic graft-versus-host disease. national institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group Report. Biol Blood Marrow Transpl. 2015;21(6):984–999. doi: 10.1016/j.bbmt.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert MM, Williams BE, Lloid ME, et al. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer. 1992;69(10):2469–2477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 23.Escudier M, Ahmed N, Shirlaw P, et al. A scoring system for mucosal disease severity with special reference to oral lichen planus. Br J Dermatol. 2007;157(4):765–770. doi: 10.1111/j.1365-2133.2007.08106.x. [DOI] [PubMed] [Google Scholar]
- 24.Elad S, Or R, Garfunkel AA, et al. Budesonide: a novel treatment for oral chronic graft versus host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):308–311. doi: 10.1067/moe.2003.23. [DOI] [PubMed] [Google Scholar]
- 25.Chainani-Wu N, Silverman S, Jr, Reingold A, et al. Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(1):51–58. doi: 10.1016/j.tripleo.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Piboonniyom SO, Treister N, Pitiphat W, et al. Scoring system for monitoring oral lichenoid lesions: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(6):696–703. doi: 10.1016/j.tripleo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter PA, Kitko CL, Elad S, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transpl. 2015;21(7):1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sari I, Altuntas F, Kocyigit I, et al. The effect of budesonide mouthwash on oral chronic graft versus host disease. Am J Hematol. 2007;82(5):349–356. doi: 10.1002/ajh.20814. [DOI] [PubMed] [Google Scholar]
- 29.Utsman RA, Epstein JB, Elad S. Budesonide for local therapy of complex oral mucosal immune-mediated inflammatory diseases: case reports. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(1):e11–e17. doi: 10.1016/j.tripleo.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Elad S, Zeevi I, Finke J, et al. Improvement in oral chronic graft-versus-host disease with the administration of effervescent tablets of topical budesonide-an open, randomized, multicenter study. Biol Blood Marrow Transpl. 2012;18(1):134–140. doi: 10.1016/j.bbmt.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Bertelli L, Di Nardo G, Zama D, et al. A new formulation of an old drug: a potential new therapy in the management of oral cGvHD. J Pediatr Hematol Oncol. 2016;38(8):e295–e297. doi: 10.1097/MPH.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 32.Park AR, La HO, Cho BS, et al. Comparison of budesonide and dexamethasone for local treatment of oral chronic graft-versus-host disease. Am J Health Syst Pharm. 2013;70(16):1383–1391. doi: 10.2146/ajhp120567. [DOI] [PubMed] [Google Scholar]
- 33.Zadik Y, Nakdimon I, Meyerowitz C, et al. Topical budesonide for severe oral chronic graft-versus-host disease. Am J Health Syst Pharm. 2014;71(3):181–182. doi: 10.2146/ajhp130520. [DOI] [PubMed] [Google Scholar]
- 34.Wolff D, Anders V, Corio R, et al. Oral PUVA and topical steroids for treatment of oral manifestations of chronic graft-vs.-host disease. Photodermatol Photoimmunol Photomed. 2004;20(4):184–190. doi: 10.1111/j.1600-0781.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 35.Schubert MM, Correa ME. Oral graft-versus-host disease. Dent Clin North Am. 2008;52(1):79–109. doi: 10.1016/j.cden.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Franca CM, Domingues-Martins M, Volpe A, et al. Severe oral manifestations of chronic graft-vs.-host disease. J Am Dent Assoc. 2001;132(8):1124–1127. doi: 10.14219/jada.archive.2001.0338. [DOI] [PubMed] [Google Scholar]
- 37.Couriel D, Carpenter PA, Cutler C, et al. Ancillary therapy and supportive care of chronic graft-versus-host disease: national institutes of health consensus development project on criteria for clinical trials in chronic Graft-versus-host disease: V. Ancillary Therapy and Supportive Care Working Group Report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow. Transplantation. 2006;12(4):375–396. doi: 10.1016/j.bbmt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Treister N, Duncan C, Cutler C, et al. How we treat oral chronic graft-versus-host disease. Blood. 2012;120(17):3407–3418. doi: 10.1182/blood-2012-05-393389. [DOI] [PubMed] [Google Scholar]
- 39.Mays JW, Fassil H, Edwards DA, et al. Oral chronic graft-versus-host disease: current pathogenesis, therapy, and research. Oral Dis. 2013;19(4):327–346. doi: 10.1111/odi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoopler ET. Management of oral chronic graft-versus-host disease. J Canadian Dent Assoc. 2013;79:d37. [PubMed] [Google Scholar]
- 41.Elad S, Zinchuk K, Li S, et al. Economic and Practical Considerations in the Treatment of Oral Mucosal Chronic Graft-Versus-Host Disease. Biol Blood Marrow Transpl. 2018;24(8):1748–1753. doi: 10.1016/j.bbmt.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Zadik Y, Elad S, Shapira A, et al. Treatment of oral mucosal manifestations of chronic graft-versus-host disease: dexamethasone vs. budesonide. Expert Opin Pharmacother. 2017;18(3):235–242. doi: 10.1080/14656566.2017.1282464. [DOI] [PubMed] [Google Scholar]
- 43.Treister N, Li S, Kim H, et al. An Open-Label Phase II Randomized Trial of Topical Dexamethasone and Tacrolimus Solutions for the Treatment of Oral Chronic Graft-versus-Host Disease. Biol Blood Marrow Transpl. 2016;22(11):2084–2091. doi: 10.1016/j.bbmt.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Conrotto D, Broccoletti R, Carcieri P, et al. Topical tacrolimus and periodontal therapy in the management of a case of oral chronic GVHD characterized by specific gingival localization. Case Rep Dentist. 2014;2014:1–3. doi: 10.1155/2014/127219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fricain JC, Sibaud V, Swetyenga N, et al. Long-term efficacy of topical tacrolimus on oral lesions of chronic graft-versus-host disease. Br J Dermatol. 2007;156(3):588–590. doi: 10.1111/j.1365-2133.2006.07679.x. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez AR, Sheridan PJ, Rogers RS. Successful treatment of oral lichen planus-like chronic graft-versus-host disease with topical tacrolimus: a case report. J Periodontol. 2004;75(4):613–619. doi: 10.1902/jop.2004.75.4.613. [DOI] [PubMed] [Google Scholar]
- 47.Brown RS, Edwards D, Walsh-Chocolaad T, et al. Topical tacrolimus with custom trays in the treatment of severe oral chronic graft-versus-host disease refractory to a potent topical steroid therapy: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(4):e26–e30. doi: 10.1016/j.oooo.2012.07.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elad S, Or R, Shapira MY, et al. CO2 laser in oral graft-versus-host disease: a pilot study. Bone Marrow Transplant. 2003;32(10):1031–1034. doi: 10.1038/sj.bmt.1704272. [DOI] [PubMed] [Google Scholar]
- 49.Vogelsang GB, Wolff D, Altomonte V, et al. Treatment of chronic graft-versus-host disease with ultraviolet irradiation and psoralen (PUVA) Bone Marrow Transplant. 1996;17(6):1061–1067. [PubMed] [Google Scholar]
- 50.Elad S, Garfunkel AA, Enk CD, et al. Ultraviolet B irradiation: a new therapeutic concept for the management of oral manifestations of graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(4):444–450. doi: 10.1016/s1079-2104(99)70059-4. [DOI] [PubMed] [Google Scholar]
- 51.Treister N, Li S, Lerman MA, et al. Narrow-band UVB phototherapy for management of oral chronic graft-versus-host disease. Photodermatol Photoimmunol Photomed. 2015;31(2):75–82. doi: 10.1111/phpp.12141. [DOI] [PubMed] [Google Scholar]
- 52.Bojanic I, Mravak Stipetic M, Pulanic D, et al. Autologous blood as a source of platelet gel for the effective and safe treatment of oral chronic graft-versus-host disease. Transfusion. 2018;58(6):1494–1499. doi: 10.1111/trf.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elad S, Jensen SB, Raber-Durlacher JE, et al. Clinical approach in the management of oral chronic graft-versus-host disease (cGVHD) in a series of specialized medical centers. Support Care Cancer. 2015;23(6):1615–1622. doi: 10.1007/s00520-014-2503-x. [DOI] [PubMed] [Google Scholar]
- 54.Zadik Y, Zeevi I, Luboshitz-Shon N, et al. Safety and efficacy of an intra-oral electrostimulator for the relief of dry mouth in patients with chronic graft versus host disease: Case series. Medicina Oral Patologia Oral y Cirugia Bucal. 2014;19(3):e212–e219. doi: 10.4317/medoral.19429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elad S, Raber-Durlacher JE, Brennan MT, et al. Basic oral carefor hematology-oncology patients and hematopoietic stem cell transplantation recipients: a position paper from the joint task force of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) and the European Society for Blood and Marrow Transplantation (EBMT) Support Care Cancer. 2015;23(1):223–236. doi: 10.1007/s00520-014-2378-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruggiero SL, Dodson TB, Fantasia J, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]