Summary
The current COVID‐19 pandemic is caused by the SARS‐CoV‐2 coronavirus. The initial recognized symptoms were respiratory, sometimes culminating in severe respiratory distress requiring ventilation, and causing death in a percentage of those infected. As time has passed, other symptoms have been recognized. The initial reports of cutaneous manifestations were from Italian dermatologists, probably because Italy was the first European country to be heavily affected by the pandemic. The overall clinical presentation, course and outcome of SARS‐CoV‐2 infection in children differ from those in adults as do the cutaneous manifestations of childhood. In this review, we summarize the current knowledge on the cutaneous manifestations of COVID‐19 in children after thorough and critical review of articles published in the literature and from the personal experience of a large panel of paediatric dermatologists in Europe. In Part 1, we discuss one of the first and most widespread cutaneous manifestation of COVID‐19, chilblain‐like lesions. In Part 2, we review other manifestations, including erythema multiforme, urticaria and Kawasaki disease‐like inflammatory multisystemic syndrome, while in Part 3, we discuss the histological findings of COVID‐19 manifestations, and the testing and management of infected children, for both COVID‐19 and any other pre‐existing conditions.
Abstract
Click here for the corresponding questions to this CME article.
Introduction
Coronaviruses (CoVs) are enveloped, positive‐sense, single‐stranded RNA viruses with a nucleocapsid of helical symmetry, which can infect humans, animals and birds. Coronavirus infection in humans can cause a spectrum of conditions ranging from the seasonal common cold to deadly infections. Two highly pathogenic and transmissible coronavirus infections were previously recognized: the severe acute respiratory syndrome coronavirus (SARS‐CoV) and the Middle East respiratory syndrome coronavirus (MERS‐CoV).
The new SARS‐CoV‐2‐induced disease started in Wuhan City, China in December 2019, and soon spread around the world, being declared a pandemic on 11 March 2020. Initially presenting with respiratory symptoms, the disease was called Wuhan pneumonia in China. Later, involvement of other symptoms gained recognition. The initial reports of cutaneous manifestations in SARS‐CoV‐2 were from Italian dermatologists,1, 2 probably because Italy was the first European country to be heavily affected by the pandemic.
The overall clinical presentation, course and outcome of SARS‐CoV‐2 infection in children differ from those in adults. Similarly, the cutaneous manifestations of childhood COVID‐19 also differ from those of adults. While manifestations such as urticaria, maculopapular rash or vesicular rash can occur in people of all ages, certain manifestations such as chilblains, erythema multiforme (EM) and cutaneous manifestations of paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS) are more frequently seen in children and young patients.
In this review, we summarize the current knowledge on cutaneous manifestations of COVID‐19 in children after thorough and critical review of articles published in the literature and from the personal experience of a large panel of paediatric dermatologists in Europe.
Chilblain‐like lesions
Classic chilblains (pernio) are defined as inflammatory skin lesions of the acral regions that persist for > 1 day.3 They are characterized by erythematous and oedematous macules, nodules and sometimes ulcerated plaques on the dorsal surface of fingers and toes. Skin lesions are triggered by cold, but may be rarely associated with connective tissue disease or malignant haematological disorders. Patients usually report pain and itching. Treatment is conservative as spontaneous resolution usually occurs. Lesions typically recur yearly during winter. Increased mean capillary diameter and increased apical capillary diameter have been described in nailfold capillaroscopy.4
From March 2020, a large number of reports of acral lesions resembling chilblains circulated on social media. Simultaneously, the relative search volumes provided by Google Trends demonstrated a worldwide increase in queries for ‘chilblains’, ‘fingers’, ‘toes’ and lesions associated with ‘coronavirus’ in France.5, 6 The first published paediatric case of possible SARS‐CoV‐2‐associated chilblain‐like lesions occurred in Italy, followed by reports of similar cases from Italy, Spain and the Middle East.2, 7, 8 Interestingly, chilblain‐like manifestations observed during the COVID‐19 pandemic differ from classic pernio by showing an equal sex distribution, absence of triggering factors, and involvement of the feet and sometimes the distal third of the legs. Patients with chilblains associated with COVID‐19 were mostly young, confined at home due to the spring lockdown and without previous history of pernio. However, clinical and laboratory data confirming the link are incomplete and contradictory.9
In a Spanish consensus, ‘pseudo‐chilblains’ were reported in 19% of 375 patients with skin manifestations of COVID‐19.10 Only 1 of the 71 cases had a previous history of pernio. SARS‐CoV‐2 infection was confirmed in 41% of the 71 cases and suspected in the remaining 59%. Interestingly, SARS‐CoV‐2 infection manifested with less severe disease in patients with pernio, and these acral skin manifestations were considered to be different from the acral ischaemic lesions described in adult patients who had severe pulmonary disease associated with hypercoagulability.11, 12
In a French retrospective study13 on 277 patients with COVID‐19, chilblain‐like lesions were the most frequent cutaneous manifestation in a mix of confirmed or suspected cases. A handful of series have been published in children and adolescents, with a male/female ratio of roughly 1 : 1 except for one series, which had a 2.8 : 1 ratio.14–18
Clinical manifestations
The lesions usually occur in children and adolescents in good health; they are rarely seen in children younger than 10 years of age.15 They appear on the feet in 74%–100% of the cases, but have also been described on the hands and fingers.15–17 The lesions are usually multiple, round and vary from a few millimetres to centimetres in size, affecting the entire toe with a clear demarcation at the metatarsophalangeal level (Fig. 1). Erythematous, violaceous or purpuric patches and swellings have been observed, which may appear infiltrative. The periungual and subungual skin is usually affected. In the subsequent evolution, the lesions may become vesiculobullous or present with dark‐purple or black crusts. The plantar region and the lateral aspect of the feet and the heels may also be involved, with coarse, ecchymotic and infiltrated lesions.
Figure 1.
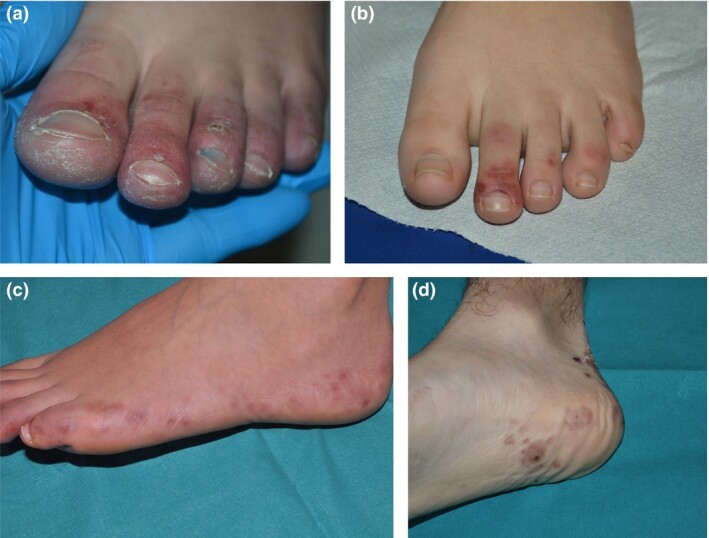
(a–d) The spectrum of acral ischaemic lesions in children in the setting of COVID‐19.
Unlike adult cases, in which 45% of the patients experienced COVID‐19 symptoms,19 children/adolescents are usually asymptomatic, although local pain and itch may occur (ranging from 9.4% to 57.8% of cases).8, 15–17
In four Spanish children with chilblains on the feet, of whom two also had involvement of the hands, complete clinical examination additionally revealed skin lesions consistent with EM, involving the hands, feet, forearms, elbows, arms, ankles, thighs, legs and ears.20 Skin target lesions of EM developing after chilblains were also reported in an Italian patient.21
Chilblain‐like manifestations in a series of 14 French patients presented similarities with lesions observed in type 1 interferonopathies such as Aicardi–Goutières syndrome and stimulator of interferon genes‐associated vasculopathy of infancy (SAVI).22
Dermoscopic features of COVID‐19 chilblains have been described in children and teenagers.23 Three main features were observed: a background area, globules and reticular network. The background area is present in all cases and is variously described as red, purple, brown or grey. Globules are seen in most cases, and are usually red to purple in colour. Finally, the grey–brown reticular network is seen in almost 30% of lesions, and is usually located peripherally within the background area. There are also anecdotal reports of nail splinter haemorrhages and dilated nail fold capillaries with loss of polarity and subcorneal haemorrhagic dots.
In one Italian study, 19 adolescents were studied with videocapillaroscopy.16 Capillary anomalies of both the fingers and the toes were described, even in cases where the skin lesions were limited to the feet only. Dilated capillaries were found with the same frequency on the fingers and toes, whereas pericapillary oedema and microhaemorrhages were more frequent on the toes. These findings could potentially suggest that COVID‐19 chilblains may be part of systemic involvement rather than induced by local factors. Moreover, these features appear more severe than those described in idiopathic chilblains, which do not have microhaemorrhages.4
Testing
SARS‐CoV‐2 testing by PCR of nasopharyngeal swabs has been negative in almost all children and adolescents with COVID‐related chilblains.24, 25 For children admitted to hospital under the suspicion of COVID‐19, PCR for SARS‐CoV‐2 has been reported positive in only 11% of the cases.26 In addition, chilblains may be a late manifestation when viral RNA is no longer detectable.27, 28
IgG and IgM antibodies against SARS‐CoV‐2 have been tested in a very limited number of cases. They were positive in only two of six serologically tested patients, both of them with positive RT‐PCR.9, 16, 17 Interestingly, in an Italian series of 19 adolescents, six were positive for IgA specific for the S1 domain of the spike protein and three were borderline for this antibody.16 Moreover, a family history of close contact with a symptomatic adult was reported for 47% of children in this series.
Treatment and outcome
All children and adolescents published thus far had a favourable outcome with spontaneous regression of the lesions and no complications. Rarely oral analgesics and antihistamines were administered.16–18 Oral gabapentin was used in one case for pain control.8 Steroids have been prescribed in cases with associated EM‐like eruption.20
Reported resolution times have ranged from 12 days10 to > 8 weeks.15 Some patients experienced new lesions during this time, but this did not affect the outcome.
SARS‐CoV‐2 infection pathogenesis and chilblain lesions
COVID‐19 severity ranges from asymptomatic to severe clinical manifestations, including lung, heart and kidney injuries, and hypoxic encephalopathy.29–31 If chilblains are related to COVID‐19 infection, some of the suggested pathogenic mechanisms are summarized below.
Virus‐induced type I interferonopathy hypothesis
It has been shown that patients with severe COVID‐19 have an impaired interferon (IFN) type I response and increased tumour necrosis factor and interleukin production.32 One hypothesis is that patients with chilblains exhibit a strong IFN‐I response, thereby attenuating viral replication.33, 34 However, this early IFN‐I response could induce microangiopathic changes, producing a chilblain lupus erythematosus‐like eruption. Interestingly, chilblains are the most consistent feature of type I interferonopathies, and histological findings observed in COVID‐19 chilblains are similar to those in virus‐induced type I interferonopathy.14–16, 33 Furthermore, production of IFN‐α is higher in children and young adults.35
The innate response, particularly production of type I IFNs (IFN‐α and IFN‐β), constitutes the first line of defence against multiple viral infections.36 Chilblains are rarely seen in severe COVID‐19, and patients presenting with chilblains do not develop severe COVID‐19, indicating in part a good immune response,3 as in influenza virus infection.37
Thrombosis/coagulopathy hypothesis
Patients with SARS‐CoV‐2‐infection have an increased risk of thromboembolism, with increased D‐dimer levels,37 presence of fibrin thrombi within distended small vessels and capillaries in lung and heart seen on autopsy39, 40 and development of acral ischaemia.41 Thrombotic manifestations complicate disease in only a minority of patients with COVID‐19.42, 43 Microthrombi have been observed in chilblains,44 also with slightly increased D‐dimer level.15 No prothrombotic factor, such as cryoglobulinaemia or circulating lupus anticoagulant, has yet been found.16
Vasculitis hypothesis
Patients with neurological signs were found to have perfusion abnormalities in 100% of cases,31 and patients with COVID‐19 pneumonia have perfusion defects even without pulmonary emboli.45 The hypothesis of a specific microvascular pathology directly induced by SARS‐CoV‐2 has been proposed. Particular microvascular anomalies have been reported in lung biopsies, suggesting a direct viral effect on vessels.41, 45 Viral proteins were found by immunohistochemistry in endothelial cells and eccrine glands on skin biopsies from two patients with EM and chilblains20 and in seven with chilblains alone,44 but these findings need to be confirmed with larger studies. Endothelialitis or lymphocytic vasculitis are seen in COVID‐19 chilblains.44
Angiotensin‐converting enzyme (ACE)‐2 has been proposed as the membrane receptor of SARS‐CoV‐2.46 Endothelial cells have avery weak expression of ACE2 but pericytes have one of the highest expression levels of ACE2, thus making the pericyte a good candidate to explain microvascular inflammation and hypercoagulopathy in SARS‐CoV‐2 infection.45, 47, 48 Moreover, positive immunostaining for SARS‐CoV‐2 in epithelial cells of eccrine glands may be explained by the presence of ACE2 in these cells.49
Other factors
A change in habits during the pandemic and lockdown could also be responsible for COVID‐19 chilblains (e.g. lack of physical activity, walking barefoot at home, and physical and mental stress).24, 25 Although these factors may play a role, they have been inadequately studied. An increase in the frequency of chilblains has not been reported in other immobility or lockdown conditions such as immobile elderly people, children or adolescents rendered immobile by fractures or surgical operations, or young people under other stresses such as during examination periods.
Conclusion
Chilblain‐like lesions were reported worldwide during the first peak of the COVID‐19 pandemic. They affected patients with PCR‐demonstrated disease, those with suspected COVID‐19 and those for whom a link with SARS‐CoV‐2 was based only on the epidemiological context. A definite causal link between COVID‐19 and chilblain lesions has not been fully established, but appears likely.
Learning points.
Acral ischaemic lesions, similar to chilblains, have been reported worldwide during the COVID‐19 outbreak.
The lesions are more common in adolescents and young adults.
They most commonly involve the toes and feet, and less frequently fingers and hands.
They may appear as erythematous or violaceous swellings, dark purpuric macules or vesiculobullous lesions.
Most patients have an excellent general health status, with mild general symptoms or none.
Prominent vascular damage is the hallmark of COVID‐19‐related chilblains.
In most cases, nasopharyngeal PCR and blood serologies are negative.
Outcome is excellent, with recovery in all cases after 4–8 weeks.
The link between COVID‐19 and chilblains still remains to be proven, but seems likely.
Contributor Information
D. Andina, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Belloni‐Fortina, Pediatric Dermatology Unit Department of Medicine DIMED University of Padua Padua Italy
C. Bodemer, Department of Dermatology Hospital Necker Enfants MaladesParis Centre University Paris France
E. Bonifazi, Dermatologia Pediatrica Association Bari Italy
A. Chiriac, Nicolina Medical Center Iasi Romania
I. Colmenero, Department of Pathology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Diociaiuti, Dermatology Unit Bambino Gesù Children's HospitalIRCCS Rome Italy
M. El‐Hachem, Dermatology Unit Bambino Gesù Children's HospitalIRCCS Rome Italy
L. Fertitta, St Parascheva Infectious Diseases Hospital Iasi Romania
van D. Gysel, Department of Pediatrics O. L. Vrouw Hospital Aalst Belgium.
A. Hernández‐Martín, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
T. Hubiche, Department of Dermatology Université Côte d'Azur Nice France
C. Luca, Nicolina Medical Center Iasi Romania
L. Martos‐Cabrera, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain
A. Maruani, Department of Dermatology Unit of Pediatric Dermatology University of ToursSPHERE‐INSERM1246CHRU Tours Tours France
F. Mazzotta, Dermatologia Pediatrica Association Bari Italy
A. D. Akkaya, Department of Dermatology Ulus Liv Hospital Istanbul Turkey
M. Casals, Department of Dermatology Hospital Universitari de Sabadell Barcelona Spain
J. Ferrando, Department of Dermatology Hospital Clìnic Barcelona Spain
R. Grimalt, Faculty of Medicine and Health Sciences Universitat Internacional de Catalunya Barcelona Spain
I. Grozdev, Department of Dermatology Children’s University Hospital Queen Fabiola Brussels Belgium
V. Kinsler, Department of Paediatric Dermatology Great Ormond Street Hospital for Children NHS Foundation Trust London UK
M. A. Morren, Pediatric Dermatology Unit Department of Pediatrics and Dermato‐Venereology University Hospital Lausanne and University of Lausanne Lausanne Switzerland
M. Munisami, Department of Dermatology and Sexually Transmitted Diseases Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) Puducherry India
A. Nanda, As'ad Al‐Hamad Dermatology Center Kuwait City Kuwait
M. P. Novoa, Department of Dermatology Hospital San Jose Bogota Colombia
H. Ott, Division of Pediatric Dermatology Children's Hospital Auf der Bult Hannover Germany
S. Pasmans, Erasmus MC University Medical Center RotterdamSophia Children's Hospital Rotterdam The Netherlands
C. Salavastru, Department of Paediatric Dermatology Colentina Clinical HospitalCarol Davila University of Medicine and Pharmacy Bucharest Romania
V. Zawar, Department of Dermatology Dr Vasantrao Pawar Medical College Nashik India
A. Torrelo, Department of Dermatology Hospital Infantil Universitario Niño Jesús Madrid Spain.
CPD questions
Learning objective
To gain up‐to‐date knowledge about the features, development and treatment of COVID‐19‐related chilblains in children.
Question 1
Which of the following statements about chilblains in the setting of COVID‐19 is true?
(a) Lesions predominate on the fingers.
(b) Severe itch and pain are associated in most cases.
(c) Chilblains often precede the other systemic symptoms and signs.
(d) More than 90% of patients had systemic symptoms of COVID‐19.
(e) Chilblains are much more common in adolescents and young adults than in young children.
Question 2
Which of the following statements about chilblains in patients with COVID‐19 is true?
(a) Most cases occurred in patients with household contacts with proven COVID‐19.
(b) Most patients have been positive when tested for nasopharyngeal PCR.
(c) Most patients have had positive results for serological SARS‐CoV‐2 tests.
(d) Chilblains have been reported only in patients with negative PCR.
(e) The incidence of COVID‐19‐related chilblains paralleled the incidence of cases during the COVID‐19 outbreak.
Question 3
What is the expected evolution of COVID‐19‐related chilblains?
(a) Evolution to thromboembolism in 20% of cases.
(b) Digital necrosis and amputations in 25% of cases.
(c) Rapid spontaneous resolution in < 5 days.
(d) Spontaneous resolution in < 10 weeks, without sequelae.
(e) Chronic course for months.
Question 4
What is the most consistent anomaly in laboratory tests for patients with COVID‐19‐related chilblains?
(a) Positive lupus anticoagulant.
(b) Elevated D‐dimer.
(c) Elevated cryoglobulins.
(d) Decreased fibrinogen levels.
(e) There are usually no abnormal laboratory results.
Question 5
Which of the following is the recommended treatment for COVID‐19‐related chilblains?
(a) Oral corticosteroids.
(b) Subcutaneous heparin.
(c) Oral antihistamines.
(d) Oral gabapentin.
(e) No treatment is necessary in most cases.
Instructions for answering questions
This learning activity is freely available online at http://www.wileyhealthlearning.com/ced
Users are encouraged to
Read the article in print or online, paying particular attention to the learning points and any author conflict of interest disclosures.
Reflect on the article.
Register or login online at http://www.wileyhealthlearning.com/ced and answer the CPD questions.
Complete the required evaluation component of the activity.
Once the test is passed, you will receive a certificate and the learning activity can be added to your RCP CPD diary as a self‐certified entry.
This activity will be available for CPD credit for 2 years following its publication date. At that time, it will be reviewed and potentially updated and extended for an additional period.
References
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol 2020; 34: e212–13. [DOI] [PubMed] [Google Scholar]
- Mazzotta F, Troccoli T. Acute acro‐ischemia in the child at the time of COVID‐19. Eur J Pediat Dermatol 2020; 30: 71–4. [Google Scholar]
- Cappel JA, Wetter DA. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc 2014; 89: 207–15. [DOI] [PubMed] [Google Scholar]
- Ozmen M, Kurtoglu V, Can G et al. The capillaroscopic findings in idiopathic pernio: is it a microvascular disease? Mod Rheumatol 2013; 23: 897–903. [DOI] [PubMed] [Google Scholar]
- Hughes M, Rogers S, Lepri G et al. Further evidence that chilblains are a cutaneous manifestation of COVID‐19 infection. Br J Dermatol 2020; 183: 596–8. 10.1111/bjd.19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger N, Scrivener JN. The use of google trends for acral symptoms during COVID‐19 outbreak in France. J Eur Acad Dermatol Venereol 2020; 34: e358–60. [DOI] [PubMed] [Google Scholar]
- Alramthan A, Aldaraji W. Two cases of COVID‐19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol 2020; 45: 746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaní J, Baselga E, Mitjà O et al. Chilblain and acral purpuric lesions in Spain during covid confinement: retrospective analysis of 12 cases. Actas Dermosifiliogr 2020; 111: 426–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; 34: e29–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 2020; 183: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cao W, Xiao M et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi 2020; 41: E006. [DOI] [PubMed] [Google Scholar]
- Li T, Lu H, Zhang W. Clinical observation and management of COVID‐19 patients. Emerg Microbes Infect 2020; 9: 687–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Masson A, Bouaziz JD, Sulimovic L et al. Chilblains are a common cutaneous finding during the COVID‐19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol 2020; 83: 667–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna C, Monzani NA, Rocchi A et al. Chilblain‐like lesions in children following suspected COVID‐19 infection. Pediatr Dermatol 2020; 37: 437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andina D, Noguera‐Morel L, Bascuas‐Arribas M. et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol 2020; 37: 406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hachem M, Diociaiuti A, Concato C et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Lara G, Linares‐González L, Ródenas‐Herranz T, Ruiz‐Villaverde R. Chilblain‐like lesions in pediatrics dermatological outpatients during the COVID‐19 outbreak. Dermatol Ther 2020; e13516. 10.1111/dth.13516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S, Barbagallo T, Frasin LA et al. Acral cutaneous lesions in the time of COVID‐19. J Eur Acad Dermatol Venereol 2020; 34: e346–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EE, McMahon DE, Lipoff JB et al. Pernio‐like skin lesions associated with COVID‐19: a case series of 318 patients from 8 countries. J Am Acad Dermatol 2020; 83: 486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelo A, Andina D, Santonja C et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol 2020; 37: 442–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotta F, Troccoli T. Erythema multiforme in time of COVID‐19. Eur J Pediatr Dermatol 2020; 2020: 88–9. [Google Scholar]
- Bouaziz JD, Duong T, Jachiet M et al. Vascular skin symptoms in COVID‐19: a French observational study. J Eur Acad Dermatol Venereol 2020; 34. 10.1111/jdv.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Andina D, Noguera‐Morel L et al. Dermoscopy features of COVID‐19‐related chilblains in children and adolescents. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A, Peeters C, Verroken A et al. Evaluation of chilblains as a manifestation of the covid‐19 pandemic. JAMA Dermatol 2020; 156: 998–1003. 10.1001/jamadermatol.2020.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca‐Ginés J, Torres‐Navarro I, Sánchez‐Arráez J et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID‐19 pandemic. JAMA Dermatol 2020; 156: 992. 10.1001/jamadermatol.2020.2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagarro A, Epalza C, Santos M. et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr 2019; 2020: e201346. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez‐Valle A, Fernandez‐Nieto D, Diaz‐Guimaraens B et al. Acro‐ischemia in hospitalized COVID‐19 patients. J Eur Acad Dermatol Venereol 2020. 10.1111/jdv.16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Xu SB, Lin YX et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020; 133: 1039–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of Covid‐19 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PubMed] [Google Scholar]
- Lescure FX, Bouadma L, Nguyen D et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis 2020; 20: 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J, Kremer S, Merdji H et al. Neurologic in severe SARS‐CoV‐2 Infection. N Engl J Med 2020; 382: 2268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj J, Yatim N, Barnabei L et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid‐19 patients. Science 2020; 369: 718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro CM, Mulvey JJ, Laurence J et al. The differing pathophysiologies that underlie COVID‐19‐associated perniosis and thrombotic retiform purpura: a case series. British Journal of Dermatology. 2020. 10.1111/bjd.19415. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolivras A, Dehavay F, Delplace D et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep 2020; 6: 489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ju J, Weyand CM, Goronzy JJ. Age‐associated failure to adjust type I IFN receptor signaling thresholds after T cell activation. J Immunol 2015; 195: 865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, Fehr AR, Zheng J et al. IFN‐I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest 2019; 129: 3625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Mayer‐Barber K, Sher A et al. Type I interferons in infectious disease. Nat Rev Immunol 2015; 15: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannis D, Ziogas I, Gianni P. Coagulation disorders in coronavirus infected patients: COVID‐19, SARS‐CoV‐1, MERS‐CoV and lesson from the past. J Clin Virol 2020; 127: 104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SE, Akmatbekov A, Harbert JL et al. Pulmonary and cardiac pathology in African American patients with COVID‐19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8: 681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Verleden SE, Kuehnel M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID‐19. N Engl J Med 2020; 383: 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao M, Zhang S et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med 2020; 2020: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Samkari H, Karp Leaf RS, Dzik WH et al. COVID and coagulation: bleeding and thrombotic manifestation of SARS‐CoV infection. Blood 2020; 136: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardot‐Leccia N, Hubiche T, Dellamonica J et al. Pericyte alteration sheds light on micro‐vasculopathy in COVID‐19 infection. Intensive Care Med 2020; 46: 1777–8. 10.1007/s00134-020-06147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenero I, Santonja C, Alonso‐Riano M et al. SARS‐COV‐2 endothelial infection causes COVID‐19 chilblains: histopathological, immunohistochemical and ultrastructural study of 7 paediatric patients. Br J Dermatol 2020; 183: 729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M, Som A, Mendoza DP et al. Hypoxaemia related to COVID‐19: vascular and perfusion abnormalities on dual‐energy CT. Lancet Infect Dis 2020. 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta F, Matera MG, Cazzola M, Bianco A. Severe respiratory SARS‐CoV2 infection: does ACE2 receptor matter? Respir Med 2020; 168: 105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler CGK, Allon SJ, Nyquist SK et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020; 181: 1016–35. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Mäe MA, Sun Y et al. Pericyte‐specific vascular expression of SARS‐CoV‐2 receptor ACE2 – implications for microvascular inflammation and hypercoagulopathy in COVID‐19 patients. Med Hypotheses 2020; 144: 110015.32592919 [Google Scholar]
- Hamming I, Timens W, Bulthuis MLC et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


