Abstract
Hypertension is the leading risk factor for global mortality and morbidity and those with hypertension are more likely to develop severe symptoms in cardiovascular and cerebrovascular system, which is closely related to abnormal renin-angiotensin system and elabela/apelin-apelin receptor (APJ) axis. The elabela/apelin-APJ axis exerts essential roles in regulating blood pressure levels, vascular tone, and cardiovascular dysfunction in hypertension by counterbalancing the action of the angiotensin II/angiotensin II type 1 receptor axis and enhancing the endothelial nitric oxide (NO) synthase/NO signaling. Furthermore, the elabela/apelin-APJ axis demonstrates beneficial effects in cardiovascular physiology and pathophysiology, including angiogenesis, cellular proliferation, fibrosis, apoptosis, oxidative stress, and cardiovascular remodeling and dysfunction during hypertension. More importantly, effects of the elabela/apelin-APJ axis on vascular tone may depend upon blood vessel type or various pathological conditions. Intriguingly, the broad distribution of elabela/apelin and alternative isoforms implicates its distinct functions in diverse cardiac and vascular cells and tissue types. Finally, both loss-of-function and gain-of-function approaches have defined critical roles of the elabela/apelin-APJ axis in reducing the development and severity of hypertensive diseases. Thus, targeting the elabela/apelin-APJ axis has emerged as a pre-warning biomarker and a novel therapeutic approach against progression of hypertension, and an increased understanding of cardiovascular actions of the elabela/apelin-APJ axis will help to develop effective interventions for hypertension. In this review, we focus on the physiology and biochemistry, diverse actions, and underlying mechanisms of the elabela/apelin-APJ axis, highlighting its role in hypertension and hypertensive cardiovascular injury and dysfunction, with a view to provide a prospective strategy for hypertensive disease therapy.
Keywords: Elabela, Apelin, APJ, Hypertension, Renin-angiotensin system
Introduction
Hypertension, a clinical syndrome characterized by increased systemic arterial blood pressure, has a severe impact on population health.[1] Hypertension is the primary risk factor for cardiovascular diseases, such as heart failure, atrial fibrillation, chronic kidney disease, and peripheral vascular disease.[1] Numerous studies that focused on the pathogenesis and clinical treatment of hypertension have been conducted recently. The apelin receptor (APJ) is involved in numerous processes within the cardiovascular system. Its agonists are considered as therapeutic agents to modulate cardiovascular homeostasis.[1] Previously, apelin was believed to be the only ligand for APJ, while a newly discovered endogenous peptidic ligand called elabela has been expected to become a biomarker and drug for cardiovascular disease.[2] Elabela, apelin, and APJ are widely expressed in human and rat cardiovascular system. The elabela/apelin-APJ axis exerts various important functions, including vascular tone regulation, heart contraction, angiogenesis, endoderm differentiation, and heart morphogenesis.[3] Therefore, a comprehensive understanding of the effects of elabela/ apelin-APJ axis on development and diseases of the cardiovascular system is critical. This review outlines recent advances with respect to the roles of elabela/apelin-APJ axis in the physiology and pathology of the cardiovascular system, as well as its possible functional mechanisms.
Physiology and Biochemistry of the Elabela/apelin-APJ Axis
APJ, a G-protein coupled receptor composed of an APLNR gene, was first discovered in 1993[4] and conserved in various species such as humans, monkeys, chimpanzees, rats, and mice.[5] APJ was initially conceived to be an orphan receptor, and does not bind to angiotensin II (Ang II), although it has 31% homology with angiotensin II type 1 receptor (AT1R). Apelin, a secreted protein extracted from a bovine stomach in 1998 and mapped to band q25–26.1 of chromosome X, was the first identified ligand for APJ. The apelin gene encodes a precursor peptide of 77 amino acids, which is cleaved into active fragments of 12–36 amino acids,[6] such as apelin-36, apelin-17, apelin-13, and pyroglutamate (pyr)-apelin-13 [Table 1].[7] All of the active fragments possess similar functions but display different tissue distribution, potency, and receptor binding affinity. Pyr1-apelin 13, with cyclized glutamine at the N-terminal residues and a longer half-period than apelin-13, is the predominant isoform in plasma and heart and functions as a potent cardiovascular protective factor.[8]
Table 1.
Amino acid sequence and distribution of apelin and elabela isoforms.
| Identity | Amino acid sequence | Expressed tissue |
| Apelin-77 Apelin-36 Apelin-17 Pyr-apelin-13 Apelin-13 | MNLRLCVQALLLLWLSLTAVCGGSLMPLPDGNGLEDGNHLVQRGSRNGP GPWQGGRRKFRRQRPRLSHKGPMPF LVQPRGSRNGPGPWQGGRRKFRRQRPRLSHKGPMPF FRRQRPRLSHKGPMPF ∗ERPRLSHKGPMPF RPRLSHKGPMPF | Vascular endothelium Central nervous system Adipose tissue Mammary gland Heart, lung, kidney Limbs, retina, liver, skin |
| Elabela-54 Elabela-32 Elabela-21 Elabela-11 | MRFQQFLFAFFIFIMSLLLISGQRPVNLTMRRKLRKHNCLQRRCMPLHSRVPFP QRPVNLTMRRKLRKHNCLQRRCMPLHSRVPFP RKHNCLQRRCMPLHSRVPFP MPLHSRVPFP | Pluripotent stem cells Heart, lung Placenta Kidney |
E represents pyroglutamate (Pyr).
Interestingly, investigators found differences in growth, development, and cardiac phenotypes between apelin knockout (KO) and APJ KO mice, implying that the existence of another endogenous ligand for APJ with biological action was earlier than that of apelin. Subsequently, two independent research teams identified a short-secreted peptide that binds to APJ and named it elabela/Toddler.9,10 The gene AK092578, which encodes the elabela protein, is a non-coding RNA located on chromosome 4.[8] The precursor peptide of elabela consists of 54 amino acids and is highly conserved in mammals. Elabela-54 is cleaved to generate a mature elabela-32 peptide with two conserved di-arginine motifs, resulting in further processing into elabela-2 or elabela-11 by furin-like endopeptidase [Table 1].[10] Different elabela isoforms may have different functions, of which elabela-32 is the most studied at present.
However, evidences indicate that apelin and elabela may not bind to APJ in the same way. Structure-activity relationship studies revealed that C-terminal moiety (Arg28, Val29, Pro30, Phe31, and Pro32) and His26 residues of elabela were most important for receptor binding,[11] whereas the key pharmacophores (Arg2, Pro3, Arg4, and Leu5) of apelin-13 are primarily located at the N-terminal. A regulatory role for elabela/apelin-APJ axis has been shown in cardiovascular physiology and pathophysiology, thus making it a potential target for cardiovascular drug discovery.
The Elabela/apelin-APJ Axis and Blood Pressure Regulation
To gain further insight into the relevance of elabela/apelin-APJ axis in blood pressure regulation, investigators have examined the expression of the axis in both patients with hypertension and animal models of hypertension. The results point to a critical role of elabela/apelin-APJ axis in regulating vascular tone during the development of hypertension, indicating predictive and therapeutic value of elabela/apelin-APJ axis in hypertension. Notably, a marked decrease in circulating elabela and apelin levels were observed in patients with essential hypertension and general hypertension [Table 2].12,13 In addition, elabela has been demonstrated to cause vasodilation in coronary arteries[14] to reverse vasopressor responses during pulmonary arterial hypertension and Ang II-induced hypertension [Table 2].15,16 Moreover, continued elabela treatment and elabela gene therapy effectively delayed elevation of blood pressure levels in hypertensive rats [Table 2].[17] Mechanistically, elabela-induced relaxation in isolated mouse aorta could not be abolished in the presence of nitric oxide (NO) synthase inhibitor (ng-nitro-l-arginine methyl ester [L-NAME]), suggesting a NO-independent mechanism in the relaxation response to elabela.[18] As a result, the specific mechanisms following elabela activation warrant detailed investigation in future studies in the context of hypertension.
Table 2.
Regulatory roles of the elabela/apelin-APJ axis in hypertension and related diseases.
| Experimental model/population | Experimental intervention | Effects | References |
| Patients with essential hypertension | – | ↓Circulating elabela levels | [12] |
| Ang II-induced hypertensive mice | Elabela | ↓Blood pressure levels | [16] |
| Improvement of cardiac dysfunction | |||
| High-salt diet-induced hypertensive rats | Elabela | ↓Blood pressure levels | [17] |
| Anesthetized normotensive animals | Apelin | ↓Blood pressure levels | [19] |
| Diabetic mice | Apelin | ↓Intrarenal arteries constriction | 24,25 |
| Hypertensive rats | Elabela-32 | ↓Blood pressure levels | [28] |
| ↓RAS activity | |||
| Hypertensive rats | – | ↓APJ mRNA and protein | 32,33 |
| Conscious sheep | Apelin | ↑Blood pressure levels | [38] |
| ↑Peripheral vascular resistance | |||
| Conscious rats | Apelin | ↑Blood pressure levels | [49] |
| Hypertensive rats with ADMA-damaged endothelial barrier | Apelin 13 | ↑Vasoconstriction | 39,40 |
| ICR mice | Apelin | ↑Blood pressure levels | [41] |
| Normotensive rats | Apelin | ↑Blood pressure levels | 45,46 |
| Rats’ aortic adventitial fibroblasts | Elabela | ↓Apoptosis | [51] |
| ↓Autophagy | |||
| ↓Vascular remodeling | |||
| Rats with pulmonary hypertension | Elabela | ↓Right ventricular systolic pressure levels | [52] |
| ↓Ventricular hypertrophy | |||
| ↓Pulmonary vascular remodeling | |||
| Elabela KO mice | Elabela | ↓Blood pressure levels | [54] |
| ↓Proteinuria | |||
| Patients with essential hypertension | – | ↓Circulating apelin levels | [55] |
| Cardiac dysfunction | |||
| Patients with aortic valve stenosis | – | ↓Apelin | [56] |
| ↑Pro-BNP | |||
| Patients with end-stage heart failure | Apelin-13 | Improvement of cardiac dysfunction | [57] |
| VSMCs | Apelin-13 | ↑VSMC cell cycle | 60,61 |
| ↑VSMC proliferation | |||
| ↑PI3K/AKT/ERK | |||
| ↑Jagged-1/Notch3 | |||
| PASMCs | Apelin | ↓Cellular proliferation | [62] |
| ↓Cellular migration | |||
| ↑PI3K/AKT/mTOR | |||
| ApoE KO mice | Apelin | ↑NO formation | [65] |
| ↓Ang II-induced atherosclerosis |
ADMA: Asymmetric dimethylarginine; AKT: Protein kinase B; Ang II: Angiotensin II; APJ: Apelin receptor; ERK: Extracellular signal-regulated kinase; ICR: Institute of Cancer Research; KO: Knock out; mTOR: Mammalian target of rapamycin; NO: Nitric oxide; PASMCs: Pulmonary arterial smooth muscle cells; PI3K: Phosphatidylinositol 3-kinase; Pro-BNP: Pro-brain natriuretic peptide; RAS: Renin-angiotensin system; VSMCs: Vascular smooth muscle cells.
Intraperitoneal injection of apelin has been implicated in downregulation of both systolic and diastolic pressure levels in anesthetized normotensive animals [Table 2].[19] Shortly thereafter, intravenous administration of apelin was shown to cause hypotensive effects[20] and venodilation.[21] Apelin administration increased NO production in hypertensive mice and rats.22,23 The blood pressure-lowering effects of apelin were blocked by L-NAME. The studies revealed that apelin inhibited the calcification of vascular smooth muscle cells (VSMCs)[24] and abrogated abnormal Ang II-induced contraction in intra-renal arteries from diabetic mice through increasing phosphorylation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/endothelial nitric oxide synthase (eNOS) signaling [Figure 1 and Table 2].[25] These findings suggested that apelin promotes vasodilation via a NO-dependent mechanism. Moreover, apelin promotes vasodilation via a prostanoid-dependent mechanism, as apelin administration promotes vasodilation in human mammary arteries, and this vasodilation can be abolished by cyclooxygenase inhibitor.26,27
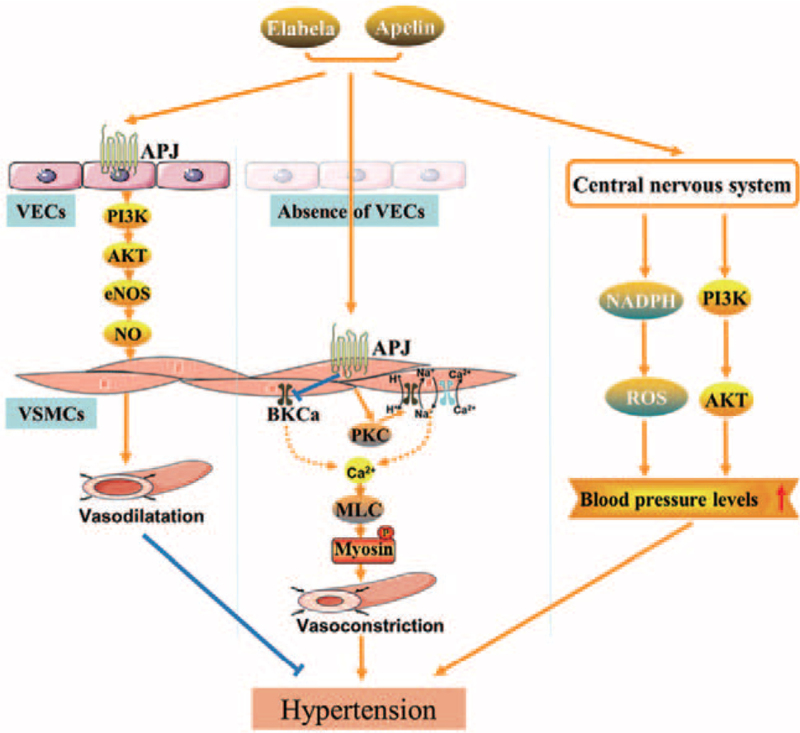
Figure 1.
Underlying mechanisms of elabela/apelln-APJ axis in regulating the blood pressure levels in the peripheral and the central nervous system. Both elabela and apelin activate endothelial APJ producing vasodilatation by pathways including the PI3K/AKT/eNOS signaling. Moreover, the elabela/apelin-APJ axis induces vasoconstriction in the absence of endothelium through the PKC, Na+–Ca2+ exchange, and BKCa-dependent pathways. Finally, in the central nervous system, activation of the elabela/apelin-APJ axis promotes superoxide formation by enhancing the PI3K/Akt phosphorylated signaling, thereby contributing to blood pressure elevation. AKT: Protein kinase B; APJ: Apelin receptor; BKCa: Large-conductance Ca2+-activated K+ channel; eNOS: Endothelial nitric oxide synthase; MLC: Myosin light chain; NADPH: Nicotinamide adenine dinucleotide phosphate; NO: Nitric oxide; PI3K: Phosphatidylinositol 3-kinase; PKC: Protein kinase C; ROS: Reactive oxidative species; VECs: Vascular endothelial cells; VSMCs: Vascular smooth muscle cells.
The renin-angiotensin system (RAS) is a key pathway in the development and progression of hypertension. It has been reported that administration of exogenous elabela-32 significantly lowered systolic blood pressure levels in hypertensive rats, accompanied with a reduction of Ang II and prorenin/renin excretion, supporting the antagonistic interaction between elabela and intrarenal RAS [Table 2 and Figure 2].[28] Apelin-induced vasodilation and depressor responses are preserved during RAS activation both in healthy humans and in patients with heart failure.[3]
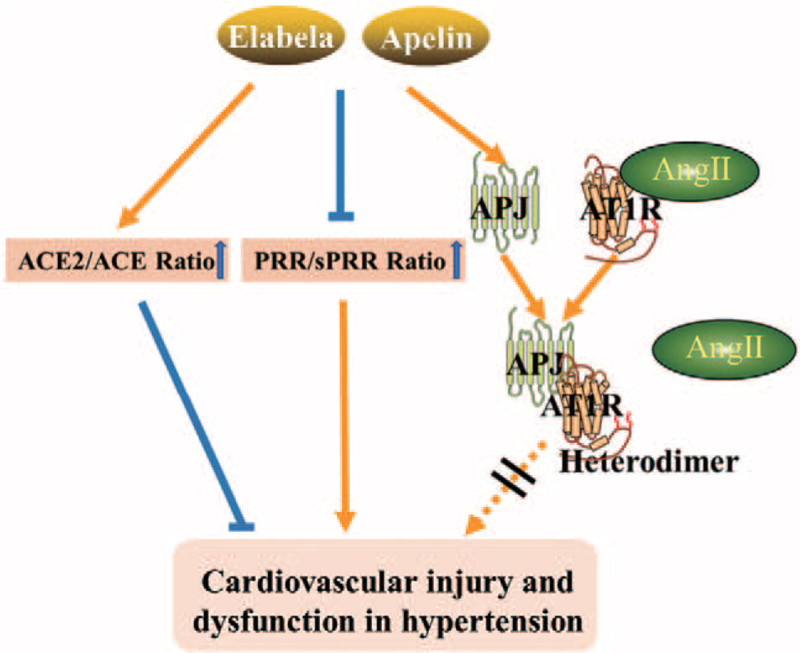
Figure 2.
Interaction between the elabela/apelin-APJ axis and RAS. On one hand, the elabela/apelin-APJ axis counterbalances the activation of RAS by regulating the ratio of ACE2/ACE and PRR/sPRR. On the other hand, the elabela/apelin-APJ axis prevents the binding of Ang-II to AT1R by promoting the formation of heterodimer between APJ and AT1R, contributing to the improvement of cardiovascular injury and dysfunction during hypertension. ACE: Angiotensin-converting enzyme; ACE2: Angiotensin-converting enzyme 2; Ang II: Angiotensin II; APJ: Apelin receptor; AT1R: Angiotensin II type 1 receptor; PRR: (Pro)renin receptor; RAS: Renin-angiotensin system; sPRR: Soluble (pro)renin receptor.
Furthermore, activation of the elabela/apelin-APJ axis has an antagonistic effect toward AT1R-mediated responses, either by forming heterodimers with the receptor or by increasing NO-dependent signaling [Figure 2].29,30 APJ is often co-expressed with AT1R and functions as an endogenous counter-regulator in the blood vessel wall.30,31 Both mRNA and protein levels of APJ were reduced in heart, kidney, and aorta of hypertensive rats [Table 2].32,33 APJ can form heterodimers with bradyki-nin receptors, neurotensin receptor-1, and κ-opioid receptors,30,34,35,36 all of which are involved in cardiovascular regulation and all of whose ligands are sensitive to angiotensin-converting enzyme 2 (ACE2) proteolysis,[37] implying that homodimers-oligomers of APJ could possibly mediate different signaling events in comparison to APJ monomers.
Most available evidences indicate that apelin plays a significant role in lowering blood pressure in hypertensive animal models. Nonetheless, several studies have reported the effect of systemic administration of apelin on blood pressure elevation. For instance, a brief initial decrease in arterial pressure was observed after intravenous administration of apelin in conscious sheep, followed by increased arterial pressure and peripheral vascular resistance [Table 2].[38] The biphasic hemodynamic response is possibly related to the use of high-dose apelin, and to the compensatory response caused by the rapid decrease in blood pressure. Apelin may also cause concentration-dependent vasoconstriction in isolated caudal arteries when endothelial cells are damaged by asymmetric dimethylarginine [Table 2].39,40 In L-NAME-treated mice, apelin has been found to induce elevated levels of systolic blood pressure by activating APJ on VSMCs, while endothelial cells are dysfunctional.40,41 Based on the vascular bed and underlying conditions, apelin may cause either vasodilation or vasoconstriction.[27] These dual actions of apelin are attributed to the presence of APJ in both the endothelial and smooth muscle cell layers of blood vessel walls and the integrity of endothelial cells.40,42,43 Notably, apelin-mediated vasoconstriction was significantly elevated in the presence of an α1-adrenergic receptor (α1-AR) agonist while it was significantly decreased in APJ-overexpression but α1-AR-deficient mice.[44] These results indicate that the interaction between APJ and α1-AR is an important and complex mechanism contributing to vasoconstriction. However, the molecular mechanisms underlying apelin-induced vasoconstriction are poorly understood, and the downstream signaling of heterodimerization between APJ and α1-AR merits further investigation.
In addition, apelin may act on the central nervous system to regulate peripheral vascular function. The increased expression of apelin gene was found in rostral ventrolateral medulla of spontaneously hypertensive rats. Subsequently, overexpression of apelin in rostral ventrolateral medulla of normotensive rats was demonstrated to increase mean arterial blood pressure through nicotinamide adenine dinucleotide phosphate oxidase-dependent superoxide formation [Table 2].45,46 Central apelin promotes vasoconstriction through increasing myosin light chain phosphorylation via Gαi/o-dependent activation of protein kinase C and Na+–Ca2+ exchange-dependent pathways [Figure 1].[47] Vasopressor responses to apelin have been observed following injection of the peptide into cardiovascular regulatory regions of the brain.48,49 In conscious rats, intracerebroventricular and intravenous administration of apelin caused a dose-dependent increase in mean arterial pressure, but the peripheral effects of apelin were relatively weak compared to its central effects, and central regulatory mechanisms of blood pressure are mainly related to the neurohormonal mechanism and sympathetic nervous system [Table 2].[49] The reason for these discrepancies of apelin in blood pressure regulation is unclear. However, possible explanations are as follows. The pressor response to intravenous injection of apelin may be related to the direct effect of apelin on the heart or peripheral sympathetic nervous system. Another possible explanation may be related to experiment conditions: as the injection is implemented in the context of no anesthesia, the blood pressure levels of rats will definitely increase under stress.
In addition, apelin inhibits NO-induced activation of large-conductance Ca2+-activated K+ channel (BKCa) channels in cerebral artery smooth muscle cells, which may be another mechanism leading to vasoconstriction [Figure 1].43,50 In intracerebroventricular administration, elabela binds and activates APJ, resulting in arginine vasopressin release and enhanced PI3K-AKT phosphorylation, consequently raising blood pressure, which may further cause cardiovascular remodeling [Figure 1].[1]
The Elabela/apelin-APJ Axis and Hypertensive Cardiovascular Injury
Consistent with its critical roles in blood pressure regulation, the elabela/apelin-APJ axis exhibits potent effects in the development and severity of hypertensive diseases. Endogenous elabela levels were reduced in hypertensive patients associated with hypertension-related vascular damage.[12] Intriguingly, in our previously published works, we revealed the Ang II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts, leading to vascular injury and remodeling, which could be dramatically reversed by pretreatment with elabela.16,51,52 We also found that hypertensive patients with heart failure with higher plasma elabela levels had a better major adverse cardiac event-free survival than those with lower plasma elabela levels, implying that decreased plasma elabela levels in hypertensive patients were closely associated with left ventricular systolic dysfunction.[53] In a rat model of pulmonary hypertension, administration of exogenous elabela improves right ventricular systolic pressure levels and pulmonary vascular remodeling.[52] Elabela deficiency promotes pre-eclampsia and cardiovascular malformations while exogenous elabela alleviates pre-eclampsia symptoms in elabela-deficient mice [Table 2].[54]
The role of apelin in the pathophysiology of hypertensive disease has also received considerable attention. Circulating apelin levels are reduced in essential hypertensive patients, and decreased apelin levels are independently associated with more profound cardiac dysfunction.[55] Meanwhile, apelin levels were downregulated in patients with aortic valve stenosis in comparison to controls.[56] Apelin has been revealed to restore declining heart function, which is consistent with the beneficial therapeutic effects of apelin [Table 2].41,57
In addition to causing a depressor response during renovascular hypertension via APJ-kappa-opioid receptor heterodimers,58,59 apelin-13 also remarkably accelerated the cell cycle process of VSMCs, and promoted the transition from G0/G1 to S phase during mitosis and stimulated VSMC proliferation via PI3K/AKT/extracellu-lar signal-regulated kinase[60] and Jagged-1/Notch3[61] signaling pathways. In contrast, apelin treatment reduces pulmonary VSMCs proliferation in hypoxic conditions via PI3K/AKT/mammalian target of rapamycin signaling pathways [Table 2].[62] Likewise, downregulation of apelin signaling during pulmonary hypertension is correlated with hyper-proliferation of pulmonary endothelial cells and smooth muscle cells.[63] Apelin attenuated Ang II-induced contractions in pulmonary arteries from normoxic animals, but not in arteries from animals exposed to chronic hypoxia, implying the changes of the APJ in the signal transduction downstream.[64]
Of note, apelin increases NO formation to quench superoxide-induced changes in the vascular wall, which eliminate Ang II-induced atherosclerosis in ApoE KO mice [Table 2].[65] The apelin-APJ axis increased ACE2 promoter activity in vitro and upregulated ACE2 expression in failing hearts in vivo, which could increase conversion of Ang II to Angiotensin 1–7.[66] In contrast to apelin, the elabela-APJ axis protects from pressure-overload-induced heart failure, possibly by suppressing ACE rather than ACE2 [Figure 2].[16]
Activation of the elabela/apelin-APJ axis exhibited beneficial effects in the context of endothelial dysfunction such as end-stage heart failure, atherosclerosis, and obesity.67,68 The elabela/apelin-APJ axis may be a mediator for atherosclerosis,[69] as the apelin gene was upregulated in atherosclerosis plaques.[56] Collectively, the elabela/apelin-APJ axis has multiple protective functions in hypertensive conditions through its involvement in regulation of VSMCs and endothelial cells; the regulation is complex and might change during different pathologic conditions. Therefore, the means to precisely modulate the axis so as to develop it in a beneficial direction needs to be determined.
Conclusion and Perspective
Hypertension has been recognized as a main risk factor for worldwide mortality. There is a continuing need for potent medications to reduce mortality and improve patients’ adherence to the hypertension treatment. Administration of apelin or elabela typically results in marked reductions in systolic/diastolic blood pressure levels and increases in blood flow. The elabela/apelin-APJ axis exerts essential roles in regulating vascular tone by counterbalancing the action of the Ang II/AT1R axis and activating the eNOS/NO signaling. Furthermore, The elabela/apelin-APJ axis demonstrates beneficial effects in vascular physiology and pathophysiology, such as angiogenesis, cellular proliferation, fibrosis, apoptosis, oxidative stress, and cardiovascular remodeling and dysfunction. Intriguingly, the effects of the elabela/apelin-APJ axis on vascular tone may depend upon blood vessel type or various pathological conditions. More importantly, the broad distribution of elabela/apelin and alternative isoforms implicated its distinct functions in diverse cardiac and vascular cells and tissue types.
Although elabela/apelin–APJ axis plays critical roles in blood pressure regulation, the underlying mechanisms have not been fully elucidated. A growing number of studies are devoted to shed new light on the regulatory roles of the elabela/apelin–APJ axis in vascular physiology and pathophysiology. At present, many clinical trials are evaluating the potential benefits of elabela/apelin analogs and novel APJ agonists in treating various cardiovascular disorders. Therefore, novel and innovative pharmacological approaches and anti-hypertensive drugs are expected to be developed to achieve long-term blood pressure reduction, improve hypertensive organ damage in people with hypertension, and thus reduce mortality and health care costs worldwide. Thus, targeting the elabela/apelin–APJ axis has emerged as a promising approach against progression of hypertension and hypertensive cardiovascular dysfunction.
Funding
This study was supported by the grants from the General Program and the National Major Research Plan Training Program of the National Natural Science Foundation of China (No. 81770253 and No. 91849111) and the Shanghai Sailing Program (No. 20YF1444100).
Conflicts of interest
None.
Footnotes
How to cite this article: Song J, Tang J, Zhang Z, Liu Y, Zhong J. Targeting the elabela/apelin-apelin receptor axis as a novel therapeutic approach for hypertension. Chin Med J 2022;135:1019–1026. doi: 10.1097/CM9.0000000000001766
Jiawei Song and Jianqiong Tang contributed equally to this work.
References
- 1.Xu C. The Elabela in hypertension, cardiovascular disease, renal disease, and preeclampsia: an update. J Hypertens 2021; 39:12–22. doi: 10.1097/HJH.0000000000002591. [DOI] [PubMed] [Google Scholar]
- 2.Yang P, Maguire JJ, Davenport AP. Apelin, Elabela/Toddler, and biased agonists as novel therapeutic agents in the cardiovascular system. Trends Pharmacol Sci 2015; 36:560–567. doi: 10.1016/j. tips.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mughal A, O’Rourke ST. Vascular effects of apelin: mechanisms and therapeutic potential. Pharmacol Ther 2018; 190:139–147. doi: 10.1016/j.pharmthera.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993; 136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 5.Pitkin SL, Maguire JJ, Kuc RE, Davenport AP. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol 2010; 160:1785–1795. doi: 10.1111/J.1476-5381.2010. 00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaleyeva LM, Shaltout HA, Varagic J. Apelin-13 in blood pressure regulation and cardiovascular disease. Curr Opin Nephrol Hypertens 2016; 25:396–403. doi: 10.1097/MNH.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 7.Fischer C. A patent review of apelin receptor (APJR) modulators (2014–2019). Expert Opin Ther Pat 2020; 30:251–261. doi: 10.1080/13543776.2020.1731473. [DOI] [PubMed] [Google Scholar]
- 8.Zhen EY, Higgs RE, Gutierrez JA. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal Biochem 2013; 442:1–9. doi: 10.1016/j.ab.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell 2013; 27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science 2014; 343:1248636.doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murza A, Sainsily X, Coquerel D, Cote J, Marx P, Besserer-Offroy E, et al. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J Med Chem 2016; 59:2962–2972. doi: 10.1021/acs. jmedchem.5b01549. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Yang X, Ouyang S, He J, Yu B, Lin X, et al. Declined circulating Elabela levels in patients with essential hypertension and its association with impaired vascular function: a preliminary study. Clin Exp Hypertens 2020; 42:239–243. doi: 10.1080/10641963.2019.1619756. [DOI] [PubMed] [Google Scholar]
- 13.Xie H, Luo G, Zheng Y, Hu D, Peng F, Xie L. Lowered circulating apelin is significantly associated with an increased risk for hypertension: a meta-analysis. Clin Exp Hypertens 2017; 39:435–440. doi: 10.1080/10641963.2016.1267199. [DOI] [PubMed] [Google Scholar]
- 14.Perjes A, Kilpio T, Ulvila J, Magga J, Alakoski T, Szabo Z, et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic Res Cardiol 2016; 111:2.doi: 10.1007/s00395-015-0521-6. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Kuc RE, Brame AL, Dyson A, Singer M, Glen RC, et al. [Pyr1]Apelin-13(1-12) is a biologically active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr1]Apelin-13. Front Neurosci 2017; 11:92.doi: 10.3389/fnins.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato T, Sato C, Kadowaki A, Watanabe H, Ho L, Ishida J, et al. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc Res 2017; 113:760–769. doi: 10.1093/cvr/cvx061. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber CA, Holditch SJ, Generous A, Ikeda Y. Sustained ELABELA gene therapy in high-salt diet-induced hypertensive rats. Curr Gene Ther 2017; 16:349–360. doi: 10.2174/1566523217666161121111906. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Yu D, Wang M, Wang Q, Kouznetsova J, Yang R, et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep 2015; 5:8170.doi: 10.1038/srep08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem 2000; 74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 20.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem 2001; 77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng X, Cheng XS, Pang CCY. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol 2003; 470:171–175. doi: 10.1016/s0014-2999 (03)01821-1. [DOI] [PubMed] [Google Scholar]
- 22.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 2001; 99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang ZZ, Wang W, Jin HY, Chen X, Cheng YW, Xu YL, et al. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension 2017; 70:1165–1175. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 24.Zhong JC, Yu XY, Huang Y, Yung LM, Lau CW, Lin SG. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res 2007; 74:388–395. doi: 10.1016/j.cardiores.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhong JC, Huang Y, Yung LM, Lau CW, Leung FP, Wong WT, et al. The novel peptide apelin regulates intrarenal artery tone in diabetic mice. Regul Pept 2007; 144:109–114. doi: 10.1016/j.regpep.2007. 06.010. [DOI] [PubMed] [Google Scholar]
- 26.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 2009; 54:598–604. doi: 10.1161/HYPERTENSIO-NAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 27.Rikitake Y. The apelin/APJ system in the regulation of vascular tone: friend or foe? J Biochem 2021; 169:383–386. doi: 10.1093/jb/mvaa129. [DOI] [PubMed] [Google Scholar]
- 28.Xu C, Wang F, Chen Y, Xie S, Sng D, Reversade B, et al. ELABELA antagonizes intrarenal renin-angiotensin system to lower blood pressure and protects against renal injury. Am J Physiol Renal Physiol 2020; 318:F1122–F1135. doi: 10.1152/ajprenal.00606.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X, Iida S, Yoshikawa A, Senbonmatsu R, Imanaka K, Maruyama K, et al. Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens Res 2011; 34:701–706. doi: 10.1038/hr.2011.19. [DOI] [PubMed] [Google Scholar]
- 30.Siddiquee K, Hampton J, McAnally D, May L, Smith L. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric transinhibition. Br J Pharmacol 2013; 168:1104–1117. doi: 10.1111/j.1476-5381.2012.02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mughal A, Sun C, O’Rourke ST. Activation of large conductance, calcium-activated potassium channels by nitric oxide mediates apelin-induced relaxation of isolated rat coronary arteries. J Pharmacol Exp Ther 2018; 366:265–273. doi: 10.1124/jpet.118.248682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najafipour H, Hekmat AS, Nekooian AA, Esmaeili-Mahani S. Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. Regul Pept 2012; 178:43–50. doi: 10.1016/j. regpep.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Najafipour H, Vakili A, Shahouzehi B, Hekmat AS, Masoomi Y, Hajahmadi MY, et al. Investigation of changes in apelin receptor mRNA and protein expression in the myocardium and aorta of rats with two-kidney, one-clip (2K1C) Goldblatt hypertension. J Physiol Biochem 2015; 71:165–175. doi: 10.1007/s13105-015-0394-z. [DOI] [PubMed] [Google Scholar]
- 34.Bai B, Cai X, Jiang Y, Karteris E, Chen J. Heterodimerization of apelin receptor and neurotensin receptor 1 induces phosphorylation of ERK (1/2) and cell proliferation via Galphaq-mediated mechanism. J Cell Mol Med 2014; 18:2071–2081. doi: 10.1111/jcmm.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai B, Liu L, Zhang N, Wang C, Jiang Y, Chen J. Heterodimerization of human apelin and bradykinin 1 receptors: novel signal transduction characteristics. Cell Signal 2014; 26:1549–1559. doi: 10.1016/j. cellsig.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Chen J, Bai B, Du H, Liu Y, Liu H. Heterodimerization of human apelin and kappa opioid receptors: roles in signal transduction. Cell Signal 2012; 24:991–1001. doi: 10.1016/j.cell-sig.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 2002; 277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 38.Charles CJ, Rademaker MT, Richards AM. Apelin-13 induces a biphasic haemodynamic response and hormonal activation in normal conscious sheep. J Endocrinol 2006; 189:701–710. doi: 10.1677/joe.1.06804. [DOI] [PubMed] [Google Scholar]
- 39.Wang LY, Zhang DL, Zheng JF, Zhang Y, Zhang QD, Liu WH. Apelin-13 passes through the ADMA-damaged endothelial barrier and acts on vascular smooth muscle cells. Peptides 2011; 32:2436–2443. doi: 10.1016/j.peptides.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Han X, Zhang DL, Yin DX, Zhang QD, Liu WH. Apelin-13 deteriorates hypertension in rats after damage of the vascular endothelium by ADMA. Can J Physiol Pharmacol 2013; 91:708–714. doi: 10.1139/cjpp-2013-0046. [DOI] [PubMed] [Google Scholar]
- 41.Nagano K, Ishida J, Unno M, Matsukura T, Fukamizu A. Apelin elevates blood pressure in ICR mice with L-NAME-induced endothelial dysfunction. Mol Med Rep 2013; 7:1371–1375. doi: 10.3892/mmr.2013.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr (1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol 2001; 132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mughal A, Sun C, O’Rourke ST. Apelin reduces nitric oxide-induced relaxation of cerebral arteries by inhibiting activation of large-conductance, calcium-activated K channels. J Cardiovasc Pharmacol 2018; 71:223–232. doi: 10.1097/FJC.0000000000000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rozenfeld R, Devi LA. Exploring a role for heteromerization in GPCR signalling specificity. Biochem J 2011; 433:11–18. doi: 10.1042/BJ20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao F, Modgil A, Zhang Q, Pingili A, Singh N, O’Rourke ST, et al. Pressor effect of apelin-13 in the rostral ventrolateral medulla: role of NAD (P)H oxidase-derived superoxide. J Pharmacol Exp Ther 2011; 336:372–380. doi: 10.1124/jpet.110.174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Yao F, Raizada MK, O’Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res 2009; 104:1421–1428. doi: 10.1161/CIRCRESAHA.108.192302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hashimoto T, Kihara M, Ishida J, Imai N, Yoshida S, Toya Y, et al. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2006; 26:1267–1272. doi: 10.1161/01.ATV.0000218841.39828.91. [DOI] [PubMed] [Google Scholar]
- 48.Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci 2002; 101:32–38. doi: 10.1016/s1566-0702 (02)00178-9. [DOI] [PubMed] [Google Scholar]
- 49.Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regul Pept 2005; 125:55–59. doi: 10.1016/j. regpep.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 50.Modgil A, Guo L, O’Rourke ST, Sun C. Apelin-13 inhibits large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle cells via a PI3-kinase dependent mechanism. PLoS One 2013; 8:e83051.doi: 10.1371/journal.pone.0083051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song JJ, Yang M, Liu Y, Song JW, Wang J, Chi HJ, et al. MicroRNA-122 aggravates angiotensin II-mediated apoptosis and autophagy imbalance in rat aortic adventitial fibroblasts via the modulation of SIRT6-elabela-ACE2 signaling. Eur J Pharmacol 2020; 883:173374.doi: 10.1016/j.ejphar.2020.173374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, et al. Elabela/Toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 2017; 135:1160–1173. doi: 10.1161/CIRCULATIONAHA.16.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Z, Zhao L, Martin S, Zhang Y, Dong Y, Zhong JC, et al. Lower plasma elabela levels in hypertensive patients with heart failure predict the occurrence of major adverse cardiac events: a preliminary study. Front Cardiovasc Med 2021; 8:638468.doi: 10.3389/fcvm.2021.638468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ho L, van Dijk M, Chye STJ, Messerschmidt DM, Chng SC, Ong S, et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017; 357:707–713. doi: 10.1126/science.aam6607. [DOI] [PubMed] [Google Scholar]
- 55.Przewlocka-Kosmala M, Kotwica T, Mysiak A, Kosmala W. Reduced circulating apelin in essential hypertension and its association with cardiac dysfunction. J Hypertens 2011; 29:971–979. doi: 10.1097/HJH.0b013e328344da76. [DOI] [PubMed] [Google Scholar]
- 56.Kucukosmanoglu M, Sahin S, Urgun OD, Yildirim A, Kilic S, Sen O, et al. The impact of transcatheter aortic valve implantation (TAVI) on serum apelin levels in patients with aortic valvular stenosis. Braz J Cardiovasc Surg 2020; 36:372–378. doi: 10.21470/1678-9741-2020-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koguchi W, Kobayashi N, Takeshima H, Ishikawa M, Sugiyama F, Ishimitsu T. Cardioprotective effect of apelin-13 on cardiac performance and remodeling in end-stage heart failure. Circ J 2012; 76:137–144. doi: 10.1253/circj.cj-11-0689. [DOI] [PubMed] [Google Scholar]
- 58.Yeganeh-Hajahmadi M, Najafipour H, Rostamzadeh F. The differential effects of low and high doses of apelin through opioid receptors on the blood pressure of rats with renovascular hypertension. Hypertens Res 2017; 40:732–737. doi: 10.1038/hr.2017.28. [DOI] [PubMed] [Google Scholar]
- 59.Rostamzadeh F, Najafipour H, Yeganeh-Hajahmadi M, Joukar S. Opioid receptors mediate inotropic and depressor effects of apelin in rats with 2K1C-induced chronic renovascular hypertension. Clin Exp Pharmacol Physiol 2018; 45:187–197. doi: 10.1111/1440-1681.12860. [DOI] [PubMed] [Google Scholar]
- 60.Liu C, Su T, Li F, Li L, Qin X, Pan W, et al. PI3K/Akt signaling transduction pathway is involved in rat vascular smooth muscle cell proliferation induced by apelin-13. Acta Biochim Biophys Sin (Shanghai) 2010; 42:396–402. doi: 10.1093/abbs/gmq035. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Li L, Xie F, Zhang Z, Guo Y, Tang G, et al. Jagged-1/Notch3 signaling transduction pathway is involved in apelin-13-induced vascular smooth muscle cells proliferation. Acta Biochim Biophys Sin (Shanghai) 2013; 45:875–881. doi: 10.1093/abbs/gmt085. [DOI] [PubMed] [Google Scholar]
- 62.Zhang H, Gong Y, Wang Z, Jiang L, Chen R, Fan X, et al. Apelin inhibits the proliferation and migration of rat PASMCs via the activation of PI3K/Akt/mTOR signal and the inhibition of autophagy under hypoxia. J Cell Mol Med 2014; 18:542–553. doi: 10.1111/jcmm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med 2013; 19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersen CU, Markvardsen LH, Hilberg O, Simonsen U. Pulmonary apelin levels and effects in rats with hypoxic pulmonary hypertension. Respir Med 2009; 103:1663–1671. doi: 10.1016/j.rmed.2009. 05.011. [DOI] [PubMed] [Google Scholar]
- 65.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest 2008; 118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sato T, Suzuki T, Watanabe H, Kadowaki A, Fukamizu A, Liu PP, et al. Apelin is a positive regulator of ACE2 in failing hearts. J Clin Invest 2013; 123:5203–5211. doi: 10.1172/JCI69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomolka RS, Cudnoch-Jedrzejewska A, Czarzasta K, Szczepanska-Sadowska E. Reduction of pressor response to stress by centrally acting apelin in spontaneously hypertensive rats. J Basic Clin Physiol Pharmacol 2015; 26:233–236. doi: 10.1515/jbcpp-2014-0066. [DOI] [PubMed] [Google Scholar]
- 68.Schinzari F, Veneziani A, Mores N, Barini A, Di Daniele N, Cardillo C, et al. Beneficial effects of apelin on vascular function in patients with central obesity. Hypertension 2017; 69:942–949. doi: 10.1161/HYPERTENSIONAHA.116.08916. [DOI] [PubMed] [Google Scholar]
- 69.Hashimoto T, Kihara M, Imai N, Yoshida S, Shimoyamada H, Yasuzaki H, et al. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol 2007; 171:1705–1712. doi: 10.2353/ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]