Abstract
Background:
Few studies have assessed the relationship between multimorbidity patterns and mortality risk in the Chinese population. We aimed to identify multimorbidity patterns and examined the associations of multimorbidity patterns and the number of chronic diseases with the risk of mortality among Chinese middle-aged and older adults.
Methods:
We used data from the China Kadoorie Biobank and included 512,723 participants aged 30 to 79 years. Multimorbidity was defined as the presence of two or more of the 15 chronic diseases collected by self-report or physical examination at baseline. Multimorbidity patterns were identified using hierarchical cluster analysis. Cox regression was used to estimate the associations of multimorbidity patterns and the number of chronic diseases with all-cause and cause-specific mortality.
Results:
Overall, 15.8% of participants had multimorbidity. The prevalence of multimorbidity increased with age and was higher in urban than rural participants. Four multimorbidity patterns were identified, including cardiometabolic multimorbidity (diabetes, coronary heart disease, stroke, and hypertension), respiratory multimorbidity (tuberculosis, asthma, and chronic obstructive pulmonary disease), gastrointestinal and hepatorenal multimorbidity (gallstone disease, chronic kidney disease, cirrhosis, peptic ulcer, and cancer), and mental and arthritis multimorbidity (neurasthenia, psychiatric disorder, and rheumatoid arthritis). During a median of 10.8 years of follow-up, 49,371 deaths occurred. Compared with participants without multimorbidity, cardiometabolic multimorbidity (hazard ratios [HR] = 2.20, 95% confidence intervals [CI]: 2.14 − 2.26) and respiratory multimorbidity (HR = 2.13, 95% CI:1.97 − 2.31) demonstrated relatively higher risks of mortality, followed by gastrointestinal and hepatorenal multimorbidity (HR = 1.33, 95% CI:1.22 − 1.46). The mortality risk increased by 36% (HR = 1.36, 95% CI: 1.35 − 1.37) with every additional disease.
Conclusion:
Cardiometabolic multimorbidity and respiratory multimorbidity posed the highest threat on mortality risk and deserved particular attention in Chinese adults.
Keywords: Multimorbidity, Pattern, Mortality, Chinese
Introduction
Multimorbidity is often defined as the coexistence of two or more chronic diseases in an individual. Although the prevalence of multimorbidity increases with age, it was reported that more than half of people with multimorbidity were <65 years.[1] Multimorbidity leads to increased risk of functional decline,[2] polypharmacy,[3] disability[4] hospitalization,[5] and mortality,[6] posing a heavy medical burden on the health care system. The prevalence of multimorbidity increases with the number of single chronic diseases. However, some chronic diseases often do not cluster randomly. Chronic diseases that co-occur may share common underlying risk factors. Alternatively, one disease may arise as the consequence of another chronic condition or treatment.[7,8] Identification of multimorbidity patterns helps provide clues for prevention and treatment of disease as well as improvement of prognosis.
A systematic review of 14 studies conducted in Europe and the US has identified 97 disease patterns.[9] Despite heterogeneity in multimorbidity patterns across studies due to the diversity of the study population, diseases included, and statistical methods, three common multimorbidity patterns were observed, including cardiovascular and metabolic diseases, mental health problems, and musculoskeletal disorders. These findings may not be generalizable to the Chinese population, which has a different disease spectrum from that of the Western population. However, only a few studies have examined multimorbidity patterns in the Chinese population,[10–12] most of which were conducted among older adults.
Existing evidence has shown an increased risk of mortality with an increase in the number of diseases.[2,13,14] However, the single count of diseases may not reflect the impacts of disease severity or disease combinations on mortality risk.[15] Several studies, mainly from the Western population, have found that specific combinations of multimorbidity patterns may show different associations with risks of mortality.[6,8] However, no such evidence was available in the Chinese population.
This study aimed to identify multimorbidity patterns among 0.5 million Chinese middle-aged and older adults and to assess the associations of multimorbidity patterns and the number of chronic diseases with all-cause and cause-specific mortality.
Methods
Ethics approval
Ethics approval was obtained from the Oxford University Tropical Research Ethics Committee (Approval No. 025-04) and the Chinese Center for Disease Control and Prevention Ethical Review Committee (Approval No. 005/ 2004). All participants provided written informed consent.
Study design and participants
We used data from the China Kadoorie Biobank (CKB). Detailed information on the CKB study has been published elsewhere.[16] In brief, 512,725 participants aged 30 to 79 years were recruited from ten (five urban and five rural) study areas across China during 2004 to 2008. At baseline, an interviewer-administered laptop-based questionnaire survey was undertaken by trained health workers to collect information on sociodemographic factors, dietary and lifestyle factors, personal and family medical history. Physical measurements were undertaken for each participant, and a 10-mL blood sample was collected for long-term storage. Prebronchodilator forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were measured by trained staff following standard procedures.[17] In the present study, we excluded two participants with missing values for body mass index (BMI), leaving 512,723 participants in the final analysis.
Assessment of chronic disease status
At baseline survey, information about medical history was obtained through self-reported chronic diseases diagnosed by a doctor, supplemented by physical examination or blood test. In this study, we included 15 chronic diseases, namely, hypertension, diabetes, coronary heart disease, stroke or transient ischemic attack, tuberculosis, asthma, chronic obstructive pulmonary disease (COPD), gallstone diseases, peptic ulcer, cirrhosis/chronic hepatitis, chronic kidney disease, cancer, neurasthenia, psychiatric disorder, and rheumatoid arthritis.
Prevalent hypertension was defined as measured systolic blood pressure ≥140 mmHg, or measured diastolic blood pressure ≥90 mmHg, or self-reported doctor-diagnosed hypertension, or self-reported use of antihypertensive medication at baseline. Prevalent diabetes was defined as measured fasting blood glucose ≥7.0 mmol/L, measured non-fasting blood glucose ≥11.1 mmol/L, or self-reported doctor-diagnosed diabetes. Prevalent COPD was defined as the measured ratio of FEV1/FVC < 0.7 or self-reported doctor-diagnosed COPD at baseline. Past year major depression episode (MDE) and generalized anxiety disorder (GAD) at baseline were assessed using the Chinese version of the computerized Composite International Diagnostic Inventory-short form by face-to-face interviews.[18] A psychiatric disorder was defined as a self-reported doctor diagnosed psychiatric disorder, or MDE, or GAD. Participants presented with two or more of the 15 chronic diseases mentioned above were defined as having multimorbidity.
Assessment of covariates
The covariates, including sociodemographic characteristics (age, sex, and education level), lifestyle factors (smoking status; alcohol consumption; dietary intake of fresh fruits, vegetables, and red meat; and physical activity), were gathered by baseline questionnaire. Information on physical activity was obtained by asking participants about their usual type and duration of activities in occupational, commuting, domestic, and leisure-time related domains in the past 12 months.[19] Total physical activity level was calculated by multiplying the metabolic equivalent tasks (METs) value for each activity by hours spent on that activity per day and summing the MET-hours for all activities. Trained staff measured weight, height, and waist and hip circumference by using calibrated instruments. BMI was calculated as weight in kilograms divided by height in meters squared.
Ascertainment of mortality
The death information of cohort members during follow-up was mainly obtained from linkages with China's Disease Surveillance Points system[20] death registries and local residential records, supplemented by active confirmation by visiting community. The causes of death were coded by the International Classification of Diseases tenth revision (ICD-10). The primary outcome used in this study was death from all causes. The secondary outcomes were cause-specific deaths from cardiovascular diseases (CVD, ICD−10 codes: I20 − I25, I60 − I69), respiratory diseases (J00 − J99), cancer (C00 − C97), and other causes.
Statistical analysis
Baseline characteristics of all participants were presented as means or percentages by the number of chronic diseases (0, 1, 2, 3, and ≥4), adjusting for age, sex, and area, when appropriate. The linear trend test was performed by treating the number of chronic diseases as a continuous variable. The prevalence of participants with different numbers of chronic diseases (0, 1, 2, 3, and ≥4) among different age groups was plotted.
We employed hierarchical cluster analysis, a commonly used exploratory method, to classify participants into different multimorbidity patterns. Participants within the same cluster showed similar characteristics, whereas participants of different clusters were distinct from each other. Each chronic disease can only belong to one cluster. Yule's Q distance was used to measure the dissimilarity between chronic diseases. Wald's distance, which provides minimum variances by minimizing the sum of squares between the two clusters, was used to measure the distance between clusters. Dendrograms were plotted to visualize the aggregation of chronic diseases. The number of multimorbidity patterns extracted was balanced through dendrogram and clinical significance. Characteristics of participants across identified multimorbidity patterns were also presented, after adjusting for age, sex, and area, when appropriate. Considering the possible diversity of multimorbidity patterns across subgroups, we also plotted dendrograms by sex (men and women), baseline age (<60 years and ≥60 years), and region (urban area and rural area).
The observed/expected (O/E) ratios were used to identify multimorbidity dyads with the highest possibility to co-occur rather than by chance. The expected prevalence of multimorbidity pairs was calculated by multiplying the prevalence of these two diseases.[21] If the O/E ratio was >1, there exists a positive association for the disease pair. The higher the O/E ratio, the stronger the association.[22]
Person-years at risk were calculated from the baseline date to death, loss to follow-up, or December 31, 2017, whichever came first. Cox proportional hazards models, with age as the underlying timescale, were used to estimate the associations of number of chronic diseases, multimorbidity patterns, as well as multimorbidity dyads with all-cause and cause-specific mortality. The results were presented with hazard ratios (HRs) and 95% confidence intervals (CIs). The models were stratified by baseline age (5-year intervals), sex, and ten areas. Multivariable models were adjusted for baseline age, education level, smoking status, alcohol consumption, intake frequency of fresh fruits, vegetables, and red meat, physical activity, BMI, and waist circumference.
All analyses were carried out using Stata (version 15.0, StataCorp LP, College Station, TX, USA) and R version 4.0.4. The level of statistical significance was set at two-tailed P < 0.05.
Results
Characteristics of the study participants by number of chronic diseases
Of all participants included, the mean ± standard deviation of age was 52.0 ± 10.7 years; 59.0% (302,521/ 512,723) were women and 44.1% (226,192/512,723) lived in urban areas [Table 1]. The number (%) of participants with 0, 1, 2, 3, and ≥4 chronic diseases was 246,309 (48.0%), 185,330 (36.2%), 61,800 (12.1%), 14,997 (2.9%), and 4287 (0.8%), respectively. Participants with more chronic diseases were more likely to be older and live in urban areas. They also tended to be heavy drinkers, engage in lower levels of physical activity, and have higher values of BMI and waist circumference (P value for trend <0.05). Of the 15 types of chronic diseases, hypertension had the highest prevalence [35.2% (180,588/ 512,723)], followed by COPD [7.2% (37,057/512,723)], gallstone disease [6.0% (30,997/512,723)], and diabetes [5.9% (30,300/512,723)].
Table 1.
Baseline characteristics of participants by the number of chronic diseases
| Parameters | N of chronic diseases | P value for trend | |||||
| Total | 0 | 1 | 2 | 3 | ≥4 | ||
| Number (%) of participants | 512,723 | 246,309 (48.0) | 185,330 (36.1) | 61,800 (12.1) | 14,997 (2.9) | 4287 (0.8) | – |
| Socio-demographic factors | |||||||
| Age (years) | 52.0 (10.7) | 48.1 (9.6) | 54.1 (10.3) | 58.4 (9.8) | 61.1 (9.0) | 63.1 (8.3) | <0.001 |
| Women (%) | 59.0 | 60.9 | 57.1 | 56.9 | 59.2 | 62.4 | <0.001 |
| Urban area (%) | 44.1 | 43.0 | 42.0 | 49.0 | 59.4 | 73.4 | <0.001 |
| Middle school and higher (%) | 49.2 | 49.2 | 48.8 | 49.5 | 51.8 | 55.7 | <0.001 |
| Lifestyle factors | |||||||
| Male daily smoker∗ (%) | 67.7 | 68.1 | 67.2 | 67.5 | 67.1 | 69.1 | 0.016 |
| Female daily smoker∗ (%) | 2.8 | 2.8 | 2.9 | 2.8 | 3.1 | 3.0 | 0.094 |
| Male heavy drinker† (%) | 24.6 | 21.7 | 26.3 | 28.2 | 28.6 | 31.8 | <0.001 |
| Female heavy drinker† (%) | 1.5 | 1.4 | 1.6 | 1.7 | 1.7 | 1.7 | <0.001 |
| Eat fruits daily (%) | 18.8 | 19.0 | 18.5 | 18.8 | 19.5 | 20.1 | 0.795 |
| Eat vegetables daily (%) | 94.8 | 94.8 | 94.8 | 94.7 | 95.0 | 95.1 | 0.761 |
| Eat red meat 1−6 days/week (%) | 53.4 | 54.0 | 53.1 | 52.4 | 52.5 | 53.3 | <0.001 |
| Physical activity (MET h/day) | 21.1 | 21.7 | 21.1 | 19.4 | 18.4 | 17.8 | <0.001 |
| BMI (kg/m2) | 23.7 | 23.0 | 24.1 | 24.4 | 24.7 | 25.0 | <0.001 |
| Waist circumference (cm) | 80.3 | 78.5 | 81.4 | 82.7 | 83.7 | 84.5 | <0.001 |
| Prevalence of chronic diseases (%) | |||||||
| Hypertension | 35.2 | 0 | 63.0 | 77.6 | 83.1 | 86.0 | <0.001 |
| Diabetes | 5.9 | 0 | 4.6 | 23.0 | 35.7 | 45.3 | <0.001 |
| Coronary heart disease | 3.0 | 0 | 1.8 | 9.1 | 20.9 | 36.0 | <0.001 |
| Stroke or transient ischemic attack | 1.7 | 0 | 0.7 | 6.3 | 12.6 | 19.5 | <0.001 |
| Tuberculosis | 1.5 | 0 | 1.6 | 4.3 | 8.2 | 13.2 | <0.001 |
| Asthma | 0.5 | 0 | 0.4 | 1.8 | 5.3 | 11.8 | <0.001 |
| COPD | 7.2 | 0 | 8.5 | 22.9 | 34.5 | 44.7 | <0.001 |
| Gallstone disease | 6.0 | 0 | 6.8 | 19.4 | 32.6 | 47.6 | <0.001 |
| Peptic ulcer | 3.9 | 0 | 4.8 | 12.1 | 20.0 | 29.7 | <0.001 |
| Cirrhosis/chronic hepatitis | 1.2 | 0 | 1.4 | 4.0 | 8.0 | 14.5 | <0.001 |
| Chronic kidney disease | 1.5 | 0 | 1.5 | 4.6 | 8.8 | 17.2 | <0.001 |
| Cancer | 0.5 | 0 | 0.6 | 1.5 | 2.4 | 4.1 | <0.001 |
| Neurasthenia | 1.1 | 0 | 1.0 | 3.5 | 7.9 | 17.7 | <0.001 |
| Psychiatric disorder | 1.1 | 0 | 1.2 | 4.1 | 8.6 | 16.5 | <0.001 |
| Rheumatic arthritis | 2.1 | 0 | 2.1 | 6.5 | 12.5 | 23.2 | <0.001 |
Data are presented as n (%), mean (standard deviation), mean or percentage.
Included former smokers who had quit smoking due to illness.
Heavy drinker refers to participants who drank >30 g/day pure alcohol for men or >15 g/day pure alcohol for women or those who had quit drinking. BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; MET: Metabolic equivalent tasks.
Overall, 15.8% (81,084/512,723) of participants had multimorbidity. The prevalence of multimorbidity increased steadily with age, with 6.4% (14,697/230,390) among participants aged <50 years, 17.0% (26,733/ 157,616) among 50 to 59 years, and 31.8% (39,654/ 124,717) among ≥60 years [Supplementary Figure 1]. The multimorbidity prevalence was higher in urban 18.7% (42,358/ 226,192) than rural participants 13.5% (38,726/ 286,531), and similar in men 16.3% (34,336/210,202) and women 15.5% (46,748/302,521).
Multimorbidity patterns
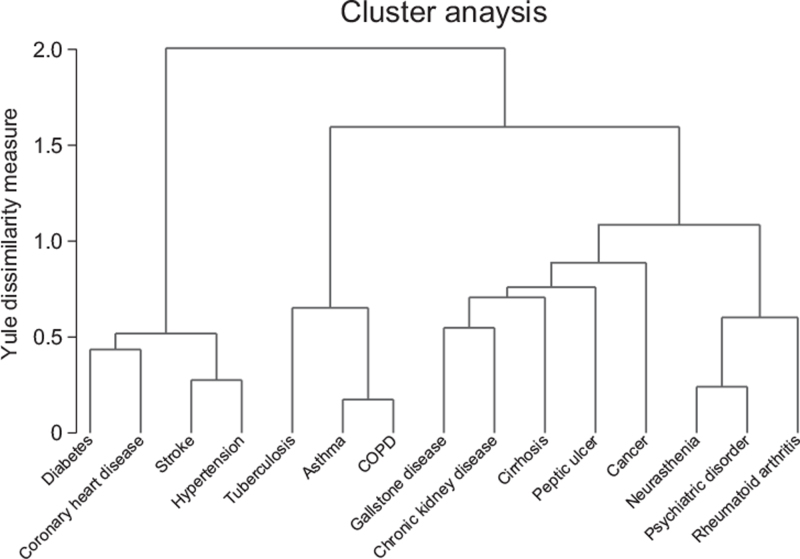
Among all participants, we identified four patterns of multimorbidity, namely, cardiometabolic multimorbidity [6.0% (30,868/512,723); diabetes, coronary heart disease, stroke, and hypertension], respiratory multimorbidity [0.4% (2120/512,723); tuberculosis, asthma, and COPD], gastrointestinal and hepatorenal multimorbidity [0.8% (4324/512,723); gallstone disease, chronic kidney disease, cirrhosis, peptic ulcer, and cancers], and mental and arthritis multimorbidity [0.2% (827/512,723); neurasthenia, psychiatric disorder, and rheumatoid arthritis) [Figure 1 and Table 2]. Besides, 1042 (0.2%) participants had more than two patterns of multimorbidity, 41,903 (8.2%) participants had no specific patterns of multimorbidity, and 431,639 (84.2%) participants did not have multimorbidity. Participants with mental and arthritis multimorbidity were more likely to be women [80.4% (665/827)], while participants with respiratory multimorbidity were more likely to be men [56.8% (1204/ 2120)]. Participants with cardiovascular multimorbidity had a lower level of physical activity and higher values of BMI and waist circumference [Table 2].
Figure 1.
Dendrograms of cluster analysis showing the distribution and aggregation of chronic diseases. COPD: Chronic obstructive pulmonary disease.
Table 2.
Baseline characteristics of participants by multimorbidity patterns
| Parameters | Total | With no multimorbidity | Cardiometabolic multimorbidity | Respiratory multimorbidity | Gastrointestinal and hepatorenal multimorbidity | Mental and arthritis multimorbidity | With ≥2 patterns of multimorbidity | With no specific patterns of multimorbidity |
| Number (%) of participants | 512,723 | 431,639 (84.2) | 30,868 (6.0) | 2120 (0.4) | 4324 (0.8) | 827 (0.2) | 1042 (0.2) | 41,903 (8.2) |
| Socio-demographic factors | ||||||||
| Age (years) | 52.0 (10.7) | 50.7 (10.3) | 61.0 (8.8) | 59.6 (10.4) | 54.2 (10.1) | 53.2 (9.8) | 62.8 (8.8) | 58.3 (9.9) |
| Women (%) | 59.0 | 59.3 | 58.6 | 43.2 | 60.5 | 80.4 | 62.5 | 56.8 |
| Urban area (%) | 44.1 | 42.6 | 60.0 | 50.3 | 52.1 | 57.1 | 77.4 | 45.9 |
| Middle school and higher (%) | 49.2 | 49.0 | 49.9 | 50.3 | 53.2 | 54.5 | 56.6 | 50.0 |
| Lifestyle factors | ||||||||
| Male daily smoker∗ (%) | 67.7 | 67.7 | 66.4 | 69.1 | 67.4 | 62.4 | 73.0 | 68.2 |
| Female daily smoker∗ %) | 2.8 | 2.8 | 2.5 | 3.6 | 3.5 | 2.8 | 2.6 | 3.0 |
| Male heavy drinker† (%) | 24.6 | 23.9 | 30.7 | 24.7 | 25.3 | 24.4 | 31.6 | 26.7 |
| Female heavy drinker† (%) | 1.5 | 1.5 | 1.5 | 1.7 | 1.9 | 2.8 | 0.7 | 1.7 |
| Eat fruits daily (%) | 18.8 | 18.8 | 17.8 | 19.3 | 22.0 | 20.5 | 20.6 | 19.7 |
| Eat vegetables daily (%) | 94.8 | 94.8 | 95.1 | 94.3 | 94.7 | 91.7 | 95.1 | 94.5 |
| Eat red meat 1 − 6 days/week (%) | 53.4 | 53.6 | 52.7 | 52.4 | 51.2 | 51.1 | 51.7 | 52.7 |
| Physical activity (MET h/day) | 21.1 | 21.4 | 18.3 | 18.6 | 19.8 | 20.1 | 17.9 | 19.9 |
| BMI (kg/m2) | 23.7 | 23.5 | 25.3 | 22.5 | 23.4 | 23.2 | 24.7 | 24.0 |
| Waist circumference (cm) | 80.3 | 79.8 | 85.4 | 78.0 | 79.8 | 79.1 | 84.1 | 81.3 |
| Prevalence of chronic diseases (%) | ||||||||
| Hypertension | 35.2 | 28.2 | 94.5 | 28.1 | 26.7 | 27.5 | 77.7 | 69.5 |
| Diabetes | 5.9 | 2.0 | 58.5 | 2.5 | 3.1 | 4.6 | 43.1 | 5.9 |
| Coronary heart disease | 3.0 | 0.8 | 20.1 | 2.0 | 3.2 | 5.1 | 31.1 | 3.4 |
| Stroke or transient ischemic attack | 1.7 | 0.3 | 16.5 | 0.4 | 0.4 | 0.8 | 12.9 | 0.8 |
| Tuberculosis | 1.5 | 0.7 | 1.5 | 47.2 | 1.9 | 1.4 | 11.8 | 5.9 |
| Asthma | 0.5 | 0.2 | 0.5 | 55.5 | 0.4 | 1.0 | 18.8 | 1.8 |
| COPD | 7.2 | 3.8 | 7.3 | 97.9 | 7.8 | 10.7 | 34.4 | 31.0 |
| Gallstone disease | 6.0 | 3.0 | 8.2 | 6.8 | 75.5 | 10.7 | 54.2 | 25.4 |
| Peptic ulcer | 3.9 | 2.2 | 3.2 | 5.3 | 59.6 | 9.6 | 35.4 | 15.2 |
| Cirrhosis/chronic hepatitis | 1.2 | 0.6 | 1.2 | 1.8 | 25.2 | 2.3 | 18.9 | 5.0 |
| Chronic kidney disease | 1.5 | 0.7 | 2.0 | 1.5 | 22.5 | 2.4 | 23.2 | 5.8 |
| Cancer | 0.5 | 0.3 | 0.5 | 0.5 | 8.3 | 0.4 | 5.4 | 1.9 |
| Neurasthenia | 1.1 | 0.5 | 1.4 | 1.8 | 2.6 | 73.4 | 16.1 | 5.6 |
| Psychiatric disorder | 1.1 | 0.5 | 1.9 | 1.9 | 2.2 | 59.9 | 17.0 | 6.7 |
| Rheumatic arthritis | 2.1 | 0.9 | 2.7 | 3.1 | 3.8 | 56.5 | 17.3 | 11.1 |
Data are presented as n (%), mean (standard deviation), mean or percentage.
Included former smokers who had quit smoking due to illness.
Heavy drinker refers to participants who drank >30 g/day pure alcohol for men or >15 g/day pure alcohol for women or those previous heavy drinkers who had quit drinking. BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; MET: Metabolic equivalent tasks.
The patterns of multimorbidity demonstrated some variations across subgroups [Supplementary Figures 2–4]. The patterns in men, rural residents, and those aged <60 years mirrored those in the overall population. Whereas for women, urban residents, and participants aged ≥60 years, cardiovascular multimorbidity remained stable, but the other three patterns were not exactly the same as those in the overall participants. Asthma usually co-occurred with COPD, while tuberculosis usually co-occurred with cirrhosis. Other digestive and hepatorenal diseases are often clustered with mental diseases and arthritis.
Among all the multimorbidity dyads, the multimorbidity pairs with the highest O/E ratios were psychiatric disorder and neurasthenia (O/E ratio = 8.00), followed by asthma and COPD (O/E ratio = 6.00) [Table 3].
Table 3.
Observed and expected prevalence of chronic disease pairs and association with mortality
| Parameters | No. of disease pairs | Observed prevalence (%) | Expected prevalence | Ratio O/E | Deaths | Mortality (deaths/ 1000 PYs) | HR (95% CI) |
| Cardiometabolic multimorbidity | |||||||
| Diabetes + hypertension | 18,220 | 3.55 | 2.08 | 1.71 | 4686 | 25.9 | 2.32 (2.24 − 2.39) |
| Chronic heart disease + hypertension | 9574 | 1.87 | 1.06 | 1.76 | 2567 | 26.8 | 1.82 (1.74 − 1.90) |
| Stroke + hypertension | 6814 | 1.33 | 0.61 | 2.18 | 2614 | 42.0 | 2.65 (2.54 − 2.76) |
| Diabetes + chronic heart disease | 2699 | 0.53 | 0.18 | 2.94 | 870 | 33.4 | 2.42 (2.25 − 2.60) |
| Diabetes + stroke | 1537 | 0.30 | 0.10 | 3.00 | 689 | 51.6 | 3.41 (3.15 − 3.68) |
| Chronic heart disease + stroke | 1227 | 0.24 | 0.05 | 4.80 | 489 | 43.9 | 2.63 (2.39 − 2.88) |
| Respiratory multimorbidity | |||||||
| Tuberculosis + COPD | 1240 | 0.24 | 0.11 | 2.18 | 430 | 36.3 | 1.98 (1.80 − 2.18) |
| Asthma + COPD | 1240 | 0.24 | 0.04 | 6.00 | 377 | 30.7 | 2.36 (2.13 − 2.62) |
| Tuberculosis + asthma | 121 | 0.02 | 0.01 | 2.00 | 44 | 38.0 | 2.61 (1.94 − 3.51) |
| Gastrointestinal and hepatorenal multimorbidity | |||||||
| Gallstone disease + peptic ulcer | 2247 | 0.44 | 0.24 | 1.83 | 218 | 9.0 | 0.99 (0.87 − 1.13) |
| Gallstone disease + kidney disease | 1082 | 0.21 | 0.09 | 2.33 | 121 | 10.5 | 1.38 (1.15 − 1.65) |
| Gallstone disease + cirrhosis | 733 | 0.14 | 0.07 | 2.00 | 144 | 19.1 | 2.17 (1.84 − 2.56) |
| Kidney disease + peptic ulcer | 523 | 0.10 | 0.06 | 1.67 | 61 | 10.9 | 1.42 (1.10 − 1.83) |
| Peptic ulcer + cirrhosis | 362 | 0.07 | 0.05 | 1.40 | 79 | 22.0 | 2.26 (1.81 − 2.83) |
| Gallstone disease + cancers | 213 | 0.04 | 0.03 | 1.33 | 63 | 31.2 | 3.22 (2.52 − 4.13) |
| Kidney disease + cirrhosis | 169 | 0.03 | 0.02 | 1.50 | 31 | 17.5 | 2.02 (1.42 − 2.87) |
| Peptic ulcer + cancers | 143 | 0.03 | 0.02 | 1.50 | 44 | 32.8 | 2.60 (1.93 − 3.49) |
| Cirrhosis + cancers | 54 | 0.01 | 0.01 | 1.00 | 24 | 51.7 | 5.09 (3.40 − 7.61) |
| Kidney disease + cancers | 51 | 0.01 | 0.01 | 1.00 | 13 | 27.5 | 2.14 (1.24 − 3.70) |
| Mental and arthritis multimorbidity | |||||||
| Neurasthenia + rheumatic arthritis | 449 | 0.09 | 0.02 | 4.50 | 51 | 10.2 | 1.18 (0.89 − 1.55) |
| Psychiatric disorder + neurasthenia | 416 | 0.08 | 0.01 | 8.00 | 34 | 7.3 | 1.38 (0.98 − 1.93) |
| Psychiatric disorder + rheumatic arthritis | 249 | 0.05 | 0.02 | 2.50 | 33 | 11.7 | 1.92 (1.36 − 2.70) |
All models were stratified by age, sex, and area. A multivariable model was adjusted for age (years), education level (no formal school, primary school, middle school, high school, college, or university or higher), smoking status (non-smoker; former smoker who had stopped for reasons other than illness; current smoker or former smoker who had stopped for illness with 1 − 14,15 − 24, or ≥25 cigarettes/day), alcohol consumption (not weekly drinker, ex-regular drinker; weekly but not a daily drinker; daily drinker with 1 − 14 g, 15 − 29 g, 30 − 59 g, or ≥60 g pure alcohol/day), intake frequency of fresh fruits, vegetables, and red meat (days/week, values were assigned according to the midpoint value of intake frequency: never or rarely = 0, monthly = 0.5, 1 − 3 days/week = 2, 4 − 6 days/week = 5, or daily = 7), physical activity (MET h/day), BMI (kg/m2), and waist circumference (cm). BMI: Body mass index; COPD: Chronic obstructive pulmonary disease; CI: Confidence intervals; HR: Hazard ratios; MET: Metabolic equivalent tasks; O/E: Observed/ expected; PY: Person year.
Association of multimorbidity patterns with mortality
Over a median of 10.8 years (total person years: 5,551,974) of follow-up, 49,371 deaths occurred, including 18,421 deaths from CVD, 4652 deaths from respiratory diseases, 15,750 deaths from cancer, and 10,548 deaths from other causes. For disease pairs, compared with participants with no multimorbidity, participants with co-occurrence of cirrhosis and cancer had the highest risk of mortality (HR = 5.09, 95% CI: 3.40 − 7.61), followed by those with diabetes and stroke (HR = 3.41, 95% CI: 3.15–3.68) [Table 3].
Further analyses by the patterns we derived showed that, compared with participants with no multimorbidity, cardiometabolic multimorbidity (HR = 2.20, 95% CI: 2.14–2.26) and respiratory multimorbidity (HR = 2.13, 95% CI: 1.97–2.31) had relatively higher risks of mortality, followed by gastrointestinal and hepatorenal multimorbidity (HR = 1.33, 95% CI: 1.22 − 1.46). No statistically significant increased mortality risk was found for participants with mental and arthritis multimorbidity (HR = 1.18, 95% CI: 0.93 − 1.50), possibly due to the small number of deaths [Table 4]. Participants with two or more patterns of multimorbidity had <ieq/>-fold increased risk of mortality (HR = 2.21, 95% CI: 1.98 − 2.48), whereas participants of no specific pattern of multimorbidity had 46% higher risk (HR = 1.46, 95% CI: 1.42 − 1.50). In the analyses of cause-specific mortality, participants with cardiometabolic multimorbidity had the highest risk of death from CVD, followed by those with respiratory multimorbidity [Table 5]. For death from respiratory diseases, participants with respiratory multimorbidity had the highest risk, followed by those with cardiometabolic multimorbidity. For deaths from cancer, participants with gastrointestinal and hepatorenal multimorbidity had the highest risk.
Table 4.
Association of patterns of multimorbidity and number of chronic diseases with mortality
| Parameters | Deaths | Mortality (deaths/1000 PYs) | HR (95% CI) |
| Patterns of multimorbidity | |||
| With no multimorbidity | 31,854 | 6.7 | 1.00 |
| Cardiometabolic multimorbidity | 8330 | 27.3 | 2.20 (2.14 − 2.26) |
| Respiratory multimorbidity | 656 | 31.6 | 2.13 (1.97 − 2.31) |
| Gastrointestinal and hepatorenal multimorbidity | 501 | 10.9 | 1.33 (1.22 − 1.46) |
| Mental and arthritis multimorbidity | 67 | 7.2 | 1.18 (0.93 − 1.50) |
| With ≥2 patterns of multimorbidity | 309 | 30.2 | 2.21 (1.98 − 2.48) |
| With no specific patterns of multimorbidity | 7654 | 17.4 | 1.46 (1.42 − 1.50) |
| Number of chronic diseases | |||
| 0 | 11,117 | 4.1 | 1.00 |
| 1 | 20,737 | 10.4 | 1.59 (1.55 − 1.63) |
| 2 | 12,284 | 19.2 | 2.25 (2.19 − 2.31) |
| 3 | 3871 | 25.8 | 2.69 (2.59 − 2.80) |
| ≥4 | 1362 | 32.6 | 3.19 (3.01 − 3.38) |
| Any | 49,371 | 8.9 | 1.36 (1.35 − 1.37) |
All models were stratified by age, sex, and area. Multivariable models were adjusted for the same covariates as those in Table 3. CI: Confidence intervals; HR: Hazard ratios; PY: Person year.
Table 5.
Association of patterns of multimorbidity with cause-specific mortality
| Parameters | Deaths | Mortality (deaths/1000 PYs) | HR (95% CI) |
| Death from cardiovascular disease | |||
| With no multimorbidity | 10,894 | 2.3 | 1.00 |
| Cardiometabolic multimorbidity | 4458 | 14.6 | 2.98 (2.87 − 3.10) |
| Respiratory multimorbidity | 141 | 6.8 | 1.34 (1.13 − 1.58) |
| Gastrointestinal and hepatorenal multimorbidity | 89 | 1.9 | 0.73 (0.59 − 0.90) |
| Mental and arthritis multimorbidity | 23 | 2.5 | 1.29 (0.86 − 1.95) |
| With ≥2 patterns of multimorbidity | 129 | 12.6 | 2.47 (2.08 − 2.95) |
| With no specific patterns of multimorbidity | 2687 | 6.1 | 1.45 (1.39 − 1.52) |
| Death from respiratory diseases | |||
| With no multimorbidity | 2565 | 0.5 | 1.00 |
| Cardiometabolic multimorbidity | 545 | 1.8 | 1.87 (1.70 − 2.06) |
| Respiratory multimorbidity | 261 | 12.6 | 7.16 (6.28 − 8.16) |
| Gastrointestinal and hepatorenal multimorbidity | 44 | 1.0 | 1.21 (0.89 − 1.63) |
| Mental and arthritis multimorbidity | 6 | 0.6 | 1.47 (0.66 − 3.27) |
| With ≥2 patterns of multimorbidity | 46 | 4.5 | 3.38 (2.52 − 4.55) |
| With no specific patterns of multimorbidity | 1185 | 2.7 | 2.23 (2.08 − 2.39) |
| Death from cancer | |||
| With no multimorbidity | 11,498 | 2.4 | 1.00 |
| Cardiometabolic multimorbidity | 1513 | 5.0 | 1.12 (1.06 − 1.18) |
| Respiratory multimorbidity | 146 | 7.0 | 1.34 (1.14 − 1.58) |
| Gastrointestinal and hepatorenal multimorbidity | 247 | 5.4 | 1.74 (1.53 − 1.98) |
| Mental and arthritis multimorbidity | 19 | 2.0 | 0.83 (0.53 − 1.31) |
| With ≥2 patterns of multimorbidity | 73 | 7.1 | 1.44 (1.15 − 1.82) |
| With no specific patterns of multimorbidity | 2254 | 5.1 | 1.23 (1.17 − 1.29) |
| Death from other causes | |||
| With no multimorbidity | 6897 | 1.5 | 1.00 |
| Cardiometabolic multimorbidity | 1814 | 6.0 | 2.71 (2.56 − 2.87) |
| Respiratory multimorbidity | 108 | 5.2 | 1.84 (1.52 − 2.22) |
| Gastrointestinal and hepatorenal multimorbidity | 121 | 2.6 | 1.57 (1.31 − 1.88) |
| Mental and arthritis multimorbidity | 19 | 2.0 | 1.52 (0.97 − 2.38) |
| With ≥2 patterns of multimorbidity | 61 | 6.0 | 2.51 (1.95 − 3.24) |
| With no specific patterns of multimorbidity | 1528 | 3.5 | 1.48 (1.39 − 1.56) |
All models were stratified by age, sex, and area. Multivariable models were adjusted for the same covariates as those in Table 3. CI: Confidence intervals; HR: Hazard ratios; PY: Person year.
Association of number of chronic diseases with mortality
We observed a graded increased risk of the number of chronic diseases with all-cause mortality. The mortality risk increased by 36% (HR = 1.36, 95% CI: 1.35 − 1.37) with every additional disease [Table 4]. Compared with participants without any aforementioned chronic diseases, the multivariable-adjusted HRs for those with 1, 2, 3, and ≥4 diseases were 1.59 (1.55 − 1.63), 2.25 (2.19 − 2.31), 2.69 (2.59 − 2.80), and 3.19 (3.01 − 3.38), respectively.
Discussion
In our cohort of 0.5 million Chinese adults, 15.8% of participants had multimorbidity. The prevalence of multimorbidity increased with age and was higher in urban than rural participants. We identified four multimorbidity patterns, including cardiometabolic multimorbidity, respiratory multimorbidity, gastrointestinal and hepatorenal multimorbidity, and mental and arthritis multimorbidity. Of the four multimorbidity patterns identified, cardiome-tabolic multimorbidity was the most common pattern in the current population. Individuals with cardiometabolic and respiratory multimorbidity had the highest risk of mortality.
Comparison with other studies
Previous studies found that multimorbidity prevalence varied from 6.4% to 76.5% among Chinese people aged 60 and older in the communities.[23,24] Comparing multimorbidity prevalence with other studies was difficult due to the inconsistency of participants’ characteristics, the number and types of chronic diseases considered, and the cut-off used to define multimorbidity. Our study showed that the prevalence of multimorbidity increased with age, with the prevalence of 6.4% in <50 years, 17.0% in 50 to 59 years, and 31.8% in ≥60 years. Although older adults had a higher prevalence of multimorbidity, in line with previous studies, multimorbidity was not a privilege for older adults.[1,25] In line with findings from The China Health and Retirement Survey (CHARLS),[10] we also found that urban participants had a higher multimorbidity prevalence than rural participants. This urban-rural difference may be explained by the high prevalence of chronic diseases or the high detection rate owing to better access to medical care services among urban residents. Some studies showed that women had higher prevalence of multimorbidity than men.[10,26] However, we did not find significant gender differences for multimorbidity prevalence.
In line with other studies,[13,14] we found a dose-response relationship between the number of chronic diseases and mortality. A meta-analysis of 26 studies, mainly conducted in European and US populations and older adults aged ≥65 years, showed that the HRs (95% CIs) were 1.73 (1.41 − 2.13) and 2.72 (1.81 − 4.08) for participants with ≥2 and ≥3 diseases, respectively, when compared with those without multimorbidity.[14] A report of UK Biobank, with 0.5 million participants aged 37 to 73 years, showed that compared with participants without long-term conditions, the 7-year mortality risk (HRs [95%CIs]) were 1.46 (1.38 − 1.54), 1.77 (1.68 − 1.87), 2.14 (2.01 − 2.28), and 2.79 (2.61 − 2.98) for those with 1, 2, 3, and 4 long-term conditions, respectively.[13]
We extracted four types of multimorbidity patterns. Consistent with previous studies, we observed a little variation across sub-populations by age, sex, and region.[10,26] Most of the multimorbidity patterns found in our study have been documented in previous studies. The cardiometabolic multimorbidity, characterized by coronary heart disease, diabetes, hypertension, and stroke, was probably one of the most common patterns identified by researchers,[9,12,27,28] and was also the most stable pattern across different subgroups in our study. We found that participants with cardiometabolic multimorbidity had the highest risk of mortality (HR = 2.20); consistently, all disease pairs within this pattern had HRs ranging from 1.82 to 3.41. Results from the Emerging Risk Factors Collaboration of 91 cohorts showed that any combination of cardiometabolic multimorbidity had multiplicative mortality risk, and the HRs for participants with 1, 2, and 3 cardiometabolic diseases were about 2, 4, and 8, respectively.[29] The longitudinal studies of the National Health Interview Survey in America and the Swedish National Study on Aging and Care also showed, when compared with other patterns, cardiometabolic patterns posed the highest threat on mortality.[8,28]
Few previous studies have observed respiratory multimorbidity. The Global Burden of Disease Study 2019 showed that COPD, behind ischemic heart disease and stroke, was the third most common cause of death in China.[30] Tuberculosis and asthma also pose a high medical burden in the Chinese population.[31,32] Our study found that the respiratory multimorbidity, including COPD, tuberculosis, and asthma, carried a similar higher mortality risk to cardiometabolic multimorbidity. A study of National Health Interview Survey among US older adults identified a similar “respiratory condition” class (including emphysema/COPD, asthma, and arthritis) which had a relatively high mortality risk (HR = 2.14; 95% CI: 1.87 − 2.46), following the “complex cardiome-tabolic” group (HR = 5.30; 95% CI: 4.52 − 6.22).[28]
The mental and arthritis multimorbidity mainly included neurasthenia, psychiatric disorder, and arthritis, and sometimes co-occurred with digestive diseases. Previous literature also reported similar patterns like “mental-articular pattern,”[33] “mental-musculoskeletal pattern,”[4] or “mental and somatic multimorbidity.”[34] A study in the US reported that neuropsychiatric class had about two fold increased mortality risk.[6] We did not find a statistically significant association between mental and arthritis multimorbidity and mortality, but we found that dyad of psychiatric disorder and rheumatic arthritis had a 92% increased risk of mortality.
The gastrointestinal and hepatorenal multimorbidity, characterized by diseases of the digestive system and cancer, was less reported in previous studies. The CHARLS study, similar to ours, identified two distinct patterns of “stomach-arthritis cluster” and “hepatorenal cluster.”[10] The coexistence of digestive diseases may be due to the high prevalence of gastrointestinal diseases, liver diseases, and kidney diseases in China.[30] The common pathways may include unhealthy dietary habits, other immunology factors, and biological mechanisms.[35] Alternatively, one disease may be the consequence of the other. We demonstrated that, overall, gastrointestinal and hepatorenal multimorbidity carried a 33% increased risk of mortality than those without multimorbidity. While the dyads, including cirrhosis or cancer, had a relatively high mortality risk, with the HRs ranging from 2.1 to 5.1. However, there were no comparable studies.
Strength and Limitations
The strengths of the present study included its prospective design, large sample size, long-time follow-up, and a broad range of multi-system and prevalent chronic diseases. To our knowledge, this is by far the first study in China to comprehensively examine the association of both the number of chronic diseases and the patterns of multimorbidity with mortality in middle-aged and older adults. Some limitations need to be mentioned. First, we used baseline disease information, which may change during follow-up. However, a previous study of our research team showed that the multimorbidity patterns at baseline and the second resurvey were relatively consistent.[36] Participants enrolled at baseline were more likely to be survivors since those with high-fatality diseases may have died before the survey. Therefore, the current patterns identified possibly reflect multimorbidity patterns among long-term survivors. Second, disease status information was mainly from self-report, which may cause under-reporting of undiagnosed conditions. However, this bias was likely to be non-differential, and thus drove the associations toward the null. Third, although the baseline survey inquired about common diseases of multi-systems, the number of diseases included was still limited, which may affect the results of the analyses.
Conclusions
In our prospective study of 0.5 million Chinese adults, we identified four multimorbidity patterns, namely, cardio-metabolic multimorbidity, respiratory multimorbidity, gastrointestinal and hepatorenal multimorbidity, and mental and arthritis multimorbidity. Cardiometabolic multimorbidity was the predominant pattern among Chinese adults. Cardiometabolic multimorbidity and respiratory multimorbidity carried the highest mortality risk and deserved particular attention. Our study highlighted the importance of preventing the occurrence of multimorbidity, especially by targeting shared risk factors, managing patients with multiple chronic diseases, and reinforcing the need to develop clinical guidelines focusing on multimorbidity. Future studies are warranted to explore the possible mechanisms of co-occurring multiple chronic diseases.
Acknowledgements
The most important acknowledgment is to the participants in the study and the members of the survey teams in each of the ten regional centers, as well as to the project development and management teams based at Beijing, Oxford, and the ten regional centers.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81941018). The CKB baseline survey and the first re-survey were supported by a grant from the Kadoorie Charitable Foundation in Hong Kong, China. The long-term follow-up is supported by grants from the UK Wellcome Trust (Nos. 212946/Z/18/Z, 202922/Z/16/Z, 104085/Z/14/Z, and 088158/Z/09/Z), grants from the National Key R&D Program of China (Nos. 2016YFC0900500 and 2016YFC1303904), National Natural Science Foundation of China (No. 81390540), and Chinese Ministry of Science and Technology (No. 2011BAI09B01).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Fan J, Sun Z, Yu C, Guo Y, Pei P, Yang L, Chen Y, Du H, Sun D, Pang Y, Zhang J, Gilbert S, Avery D, Chen J, Chen Z, Lyu J, Li L. Multimorbidity patterns and association with mortality in 0.5 million Chinese adults. Chin Med J 2022;135:648–657. doi: 10.1097/ CM9.0000000000001985
Supplemental digital content is available for this article.
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380:37–43. doi: 10.1016/s0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Wei MY, Mukamal KJ. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am J Epidemiol 2018; 187:103–112. doi: 10.1093/aje/kwx198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarnall AJ, Sayer AA, Clegg A, Rockwood K, Parker S, Hindle JV. New horizons in multimorbidity in older adults. Age Ageing 2017; 46:882–888. doi: 10.1093/ageing/afx150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Almaraz A, Manrique-Espinoza B, Ávila-Funes JA, Chatterji S, Naidoo N, Kowal P, et al. Disability, quality of life and all-cause mortality in older Mexican adults: association with multimorbidity and frailty. BMC Geriatr 2018; 18:236.doi: 10.1186/s12877-018-0928-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akugizibwe R, Calderón-Larrañaga A, Roso-Llorach A, Onder G, Marengoni A, Zucchelli A, et al. Multimorbidity patterns and unplanned hospitalisation in a cohort of older adults. J Clin Med 2020; 9:4001.doi: 10.3390/jcm9124001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen QD, Wu C, Odden MC, Kim DH. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2019; 74:1265–1270. doi: 10.1093/gerona/gly205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Zhang J, Chen S, Weissman S, Olatosi B, Li X. Comorbidity patterns among people living with HIV: a hierarchical clustering approach through integrated electronic health records data in South Carolina. AIDS Care 2021; 33:594–606. doi: 10.1080/09540121.2020.1844864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vetrano DL, Roso-Llorach A, Fernández S, Guisado-Clavero M, Violán C, Onder G, et al. Twelve-year clinical trajectories of multimorbidity in a population of older adults. Nat Commun 2020; 11:3223.doi: 10.1038/s41467-020-16780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prados-Torres A, Calderón-Larrañaga A, Hancco-Saavedra J, Poblador-Plou B, van den Akker M. Multimorbidity patterns: a systematic review. J Clin Epidemiol 2014; 67:254–266. doi: 10.1016/j.jclinepi.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Yao SS, Cao GY, Han L, Chen ZS, Huang ZT, Gong P, et al. Prevalence and patterns of multimorbidity in a Nationally Representative Sample of Older Chinese: results from the China Health and Retirement Longitudinal Study. J Gerontol A Biol Sci Med Sci 2020; 75:1974–1980. doi: 10.1093/gerona/glz185. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Peng W, Li M, Li X, Yang T, Li C, et al. Association between multimorbidity patterns and disability among older people covered by long-term care insurance in Shanghai, China. BMC Public Health 2021; 21:418.doi: 10.1186/s12889-021-10463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J, Chao J, Chen W, Xu H, Wu Z, Chen H, et al. Multimorbidity in the community-dwelling elderly in urban China. Arch Gerontol Geriatr 2017; 68:62–67. doi: 10.1016/j.archger.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Jani BD, Hanlon P, Nicholl BI, McQueenie R, Gallacher KI, Lee D, et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med 2019; 17:74.doi: 10.1186/s12916-019-1305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2016; 67:130–138. doi: 10.1016/j.archger.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Stirland LE, González-Saavedra L, Mullin DS, Ritchie CW, Muniz-Terrera G, Russ TC. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ 2020; 368:m160.doi: 10.1136/bmj. m160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Z, Chen J, Collins R, Guo Y, Peto R, Wu F, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol 2011; 40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Zhu L, Wei Y, Lv J, Guo Y, Bian Z, et al. Association between adiposity measures and COPD risk in Chinese adults. Eur Respir J 2020; 55:1901899.doi: 10.1183/13993003.01899-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mezuk B, Chen Y, Yu C, Guo Y, Bian Z, Collins R, et al. Depression, anxiety, and prevalent diabetes in the Chinese population: findings from the China Kadoorie Biobank of 0.5 million people. J Psychosom Res 2013; 75:511–517. doi: 10.1016/j.jpsychores.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H, Bennett D, Li L, Whitlock G, Guo Y, Collins R, et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: the China Kadoorie Biobank study. Am J Clin Nutr 2013; 97:487–496. doi: 10.3945/ajcn.112.046854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Hu J, Rao KQ, Ma J, Rao C, Lopez AD. Mortality registration and surveillance in China: history, current situation and challenges. Popul Health Metr 2005; 3:3.doi: 10.1186/1478-7954-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc 2009; 57:225–230. doi: 10.1111/J.1532-5415.2008. 02109.x. [DOI] [PubMed] [Google Scholar]
- 22.Wong A, Boshuizen HC, Schellevis FG, Kommer GJ, Polder JJ. Longitudinal administrative data can be used to examineh multi-morbidity, provided false discoveries are controlled for. J Clin Epidemiol 2011; 64:1109–1117. doi: 10.1016/j.jclinepi.2010. 12.011. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Huang J, Lv Y, Li G, Peng X. Status of prevalence study on multimorbidity of chronic disease in China: systematic review. Geriatr Gerontol Int 2015; 15:1–10. doi: 10.1111/ggi.12340. [DOI] [PubMed] [Google Scholar]
- 24.Wang HHX, Wang JJ, Wong SYS, Wong MCS, Li FJ, Wang PX, et al. Epidemiology of multimorbidity in China and implications for the healthcare system: cross-sectional survey among 162,464 community household residents in southern China. BMC Med 2014; 12:188.doi: 10.1186/s12916-014-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor AW, Price K, Gill TK, Adams R, Pilkington R, Carrangis N, et al. Multimorbidity: not just an older person's issue. Results from an Australian biomedical study. BMC Public Health 2010; 10:718.doi: 10.1186/1471-2458-10-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abad-Díez JM, Calderón-Larrañaga A, Poncel-Falcó A, Poblador-Plou B, Calderón-Meza JM, Sicras-Mainar A, et al. Age and gender differences in the prevalence and patterns of multimorbidity in the older population. BMC Geriatr 2014; 14:75.doi: 10.1186/14712318-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioakeim-Skoufa I, Poblador-Plou B, Carmona-Pírez J, Díez-Man-glano J, Navickas R, Gimeno-Feliu LA, et al. Multimorbidity patterns in the general population: results from the EpiChron cohort study. Int J Environ Res Public Health 2020; 17:4242.doi: 10.3390/ijerph17124242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng DD, Loewenstein DA, Christ SL, Feaster DJ, Lam BL, McCollister KE, et al. Multimorbidity patterns and their relationship to mortality in the US older adult population. PLoS One 2021; 16:e0245053.doi: 10.1371/journal.pone.0245053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Angelantonio E, Kaptoge S, Wormser D, Willeit P, Butterworth AS, Bansal N, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015; 314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020; 396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet 2014; 383:2057–2064. doi: 10.1016/s0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 32.Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet 2019; 394:407–418. doi: 10.1016/s0140-6736(19)31147-x. [DOI] [PubMed] [Google Scholar]
- 33.Garin N, Koyanagi A, Chatterji S, Tyrovolas S, Olaya B, Leonardi M, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci 2016; 71:205–214. doi: 10.1093/gerona/glv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäfer I, von Leitner EC, Schön G, Koller D, Hansen H, Kolonko T, et al. Multimorbidity patterns in the elderly: a new approach of disease clustering identifies complex interrelations between chronic conditions. PLoS One 2010; 5:e15941.doi: 10.1371/journal.pone. 0015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corley DA, Schuppan D. Food, the immune system, and the gastrointestinal tract. Gastroenterology 2015; 148:1083–1086. doi: 10.1053/j.gastro.2015.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun ZJ, Fan JN, Yu CQ, Guo Yu, Bian Z, Pei P, et al. Prevalence, patterns and long-term changes of multimorbidity in adults from 10 regions of China (in Chinese). Chin J Epidemiol 2021; 42:755–762. doi: 10.3760/cma.j.cn112338-20200305-00259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.