Abstract
Introduction:
Dyspnea is a complex symptom, which largely results from an imbalance between an afferent sensory stimulus and the corresponding efferent respiratory neuromuscular response. In addition, it is heavily influenced by the patient’s prior experiences and sociocultural factors.
Areas Covered:
The diagnostic approach to these patients requires a graded, systematic, and often multidisciplinary approach to determine what is the underlying pathophysiologic process. Utilization of objective data obtained through lab testing, imaging, and advanced testing, such as cardiopulmonary exercise testing, is often required to help identify underlying pathology contributing to a patient’s symptoms. This article will review dyspnea’s underlying pathophysiological mechanisms and standardized approaches to diagnoses. In the expert opinion section, we will discuss our own clinical approach to evaluating patients with persistent dyspnea.
Expert Opinion:
Unexplained dyspnea is a challenging diagnosis that occurs in patients with and without underlying cardiopulmonary diseases. It requires a systematic approach, which initially uses clinical evaluation in addition to standard imaging and clinical biomarkers. When diagnoses are not made during the initial evaluation, subsequent tests can include cardiopulmonary exercise test and methacholine challenge. To be certain of the correct diagnosis, It is imperative that the clinician determines dyspnea’s response to a particular therapeutic intervention.
Keywords: Dyspnea, CPET, deconditioning, dysfunctional breathing, exercise
1. Introduction
Dyspnea refers to unpleasant or uncomfortable respiratory sensations subjectively experienced by individuals [1]. It is a symptom frequently reported in large population studies of otherwise healthy individuals even after controlling for smoking [2]. In Norway, self-reported moderate or severe dyspnea was present in 5% and 13%, respectively, among nearly twenty thousand participants in a prospective cohort study [2]. In the general population, the prevalence of chronic breathlessness that limits exertion on a daily basis has been estimated 9%, and is more common in females, increased with age, and higher among those with less education or lower incomes [3]. Persistent dyspnea is one of the most common reasons why patients seek medical care in primary or subspecialty clinics [1]. It is a common and debilitating symptom that affects up to 50% of patients admitted to hospitals and as many as 25% of those seeking care in outpatient facilities [1]. According to the National Center for Health Statistics’ Vital and Health Statistics report in 2006, dyspnea-related encounters accounted for nearly 3 million visits outpatient visits per year [4]. Chronic dyspnea can be due to an underlying disease process, or it can be a symptom that occurs in the absence of any discernable organic etiology. Patients may also have persistent dyspnea despite optimal treatment of underlying pathologic processes resulting in chronic, persistent dyspnea, a condition referred to by many experts as Residual Exertional Dyspnea, which can be life altering for patients suffering from this disorder [5]
Regardless of its underlying etiology, dyspnea is associated with high medical resource utilization, increased mortality, and higher hospital costs. From 2008 to 2009 there were 8,400 dyspnea-related visits among Medicare and commercial plan beneficiaries with a prior history of coronary artery disease, which resulted in more than 58.5 and 45 million dollars in emergency room and hospitalization costs, respectively [6]. This large healthcare expenditure is likely driven by the diagnostic work up required to rule out serious, potentially life-threatening disease and by a lack of a standardized approach leading to unnecessary testing.
Dyspnea is a complex and frequently reported symptom. It is influenced by a patient’s culture, environment, individual perception, and past experiences. It may be the result of a dysfunctional breathing pattern or potentially herald an ominous diagnosis. The main objective of this review is to provide a practical overview on its evaluation and management, and to share our own diagnostic approach to patients with persistent breathlessness.
2. Pathobiology of dyspnea
While this is not intended to be a comprehensive review of the pathophysiology of dyspnea, it is important to highlight key concepts to understand diagnostic approaches. The control of breathing and its perception depend on a complex interplay of signals arising from within the central nervous system, both from the automatic centers in the brain stem and from the motor cortex (efferent), as well as from a variety of receptors in the upper airway, lungs, and chest wall (afferent) [7,8]. For most patients, dyspnea begins with a physiologic impairment that stimulates afferent receptors, which is perceived as an unpleasant sensation when this information is transmitted to the cerebral cortex. There are multiple afferent pathways sending information from mechanical, neurological, and metabolic sensors. These can be divided into central nervous system (medullary, cortical, and limbic), metabolic (carotid/aortic bodies, medullary chemoreceptors), and respiratory sensors (pulmonary stretch receptors, J-receptors, airway C-fibers, upper airway receptors, muscle spindles and tendon receptors, and chest wall joint receptors) etiologies[9]. Information from these sources is transmitted to and processed by the medullary respiratory center which sends efferent commands to the ventilatory muscles. During this process, a neurological copy of the command is simultaneously sent to the sensory cortex. This exchange between motor and sensory cortex is called a corollary discharge and is thought to be the mechanism by which conscious awareness of breathing effort occurs [10]. If the efferent ventilatory muscle response is appropriately responsive to the afferent input signals, respiration mostly occurs without conscious awareness. However, respiration can come into awareness at any moment either voluntarily or automatically if breathing needs to be attended to [10]. This process is also known as “gating” [11] and is the basis of monitoring essential physiological functions and adopting appropriate behavior [12]. When ventilation needs are physiologically increased, afferent stimuli direct the respiratory muscles to increase ventilation in tandem with the perception of dyspnea via inspiratory neural drive (IND). However, this process is hindered by mechanical limitations in some disease processes, resulting in an inability to translate these afferent inputs into efferent output. This disconnect results in a pathologic process known as neuromechanical dissociation, which causes increased IND and subsequently results in the perception of dyspnea to increase rapidly [5]. While this imbalance in respiratory afferent/efferent signals contributes to the feeling of dyspnea, its perception is ultimately influenced by culture, environment, and affective processing [13].
Dyspnea is a multidimensional symptom that has been broadly divided into three categories a) work/effort, b) tightness, and c) air hunger. Each of these categories can occur in parallel and potentially have different underlying pathobiological processes [11]. “Air hunger” may derive from increased stimulation of brainstem respiratory centers by metabolic factors, such as hypercarbia or hypoxia, when the increased stimulation is not matched by an adequate ventilatory response. Whereas the sensation of “work/effort” is perceived by input from respiratory muscle afferents (velocity, shortening, pressure, and frequency). How this information matches the degree of effort for a given workload (exercise) or metabolic demand is processed by the awareness of outgoing voluntary motor command, i.e. corollary discharge [14]. The sensation of “work/effort” increases when the muscle load is increased due to derangements of ventilatory mechanics or when the muscles are weakened by fatigue [10]. Chest tightness, most commonly, arises from pulmonary afferents because of bronchoconstriction and not necessarily from increased work of breathing [13]. In asthmatic volunteers that were non-invasively mechanically ventilated and monitored during spontaneous breathing, the degree of chest tightness experienced was related to methacholine induced bronchoconstriction and was not related to the degree of hyperinflation or respiratory muscle activity [15]. This suggest that the tightness sensation primarily originates from airway receptors that can sense airway muscle contraction or airway irritation/inflammation.
3. Clinical evaluation
Conceptually, persistent dyspnea is not well defined. Acute dyspnea usually lasts over hours to days and is considered chronic when it lasts for longer periods of time [16]. Persistent dyspnea has been defined by some studies as being breathlessness lasting greater than four weeks and up to three months. Chronic, persistent dyspnea may be due to one organic cause, multiple etiologies occurring in concert with one another, or it may occur in the absence of a discernable organic cause. Whether it occurs in patients with a known underlying lung disease or not, the approach to persistent dyspnea is complex. The etiology may not be obvious and requires the integration of many layers of diagnostic information including clinical history, physical examination, physiological data, clinical biomarkers, and imaging studies to help arrive at a diagnosis. Given that persistent dyspnea is frequently multifactorial, a careful and systematic approach is required to differentiate a functional disorder from a more severe or even life-threatening condition. Unfortunately, published diagnostic algorithms are limited by single center driven experiences in small cohorts of patients and lack comparative effectiveness validation[17–19]. However, studies agree in recommending a tiered approach to evaluation and diagnosis with the overarching goal to identify the underlying corresponding pathophysiology. In most cases the primary problem is attributed to heart, lung, or neuromuscular abnormalities. In addition, anemia can cause subjective dyspnea through reduced oxygen carrying capacity in the blood. Although occult heart failure needs to be considered, it is an uncommon cause [1,20].
This review primarily focuses on patients in whom dyspnea is unexplained and has persisted for weeks to months, and frequently longer. Because the sensation of shortness of breath is subjective and heavily influenced by emotional, psychological, experiential, and contextual factors [1,21], it is imperative to have the patient describe what he or she means by having shortness of breath as these qualitative aspects can have diagnostic value. Breathing discomfort can be described in several ways depending on the underlying pathophysiology. For example, the sensation of increased effort or work of breathing is commonly associated with increased mechanical load, such as COPD and asthma, and in patients with neuromuscular weakness. Air hunger has been associated with stimulation of chemoreceptors by hypercapnia and/or hypoxemia with increased respiratory drive, whereas dyspnea that is associated with chest tightness more specifically indicates increased bronchial constriction [1,13].
Defining if the degree of breathing discomfort occurs differently in certain phases of the respiratory cycle can provide important diagnostic clues. For example, the inability or sensation to get air into the lungs can be suggestive of Paradoxical Vocal Fold Motion Disorder (PVFMD) or severely limited inspiratory capacity from lung hyperinflation and reduced inspiratory capacity [22,23]. Difficulty exhaling can, on the other hand, be highly suggestive of increased bronchial constriction, especially if it is associated with chest tightness. Recumbency and cardiopulmonary interactions during sleep can aggravate or exacerbate persistent dyspnea. Nocturnal dyspnea can be a manifestation of pulmonary vascular disease or heart failure, particularly when it occurs in the setting of orthopnea [24]. It may also be associated with severe obstructive airway disease and diaphragmatic weakness or other various neuromuscular disorders [25]. Other comorbidity factors like obesity and sleep apnea can be important contributors.
Physical examination is not sensitive and therefore the absence of physical findings cannot rule out conditions causing dyspnea but can be very useful for making specific diagnoses when abnormal exam findings are present. The clinical examination should look for evidence of lung sounds that indicate airway obstruction or parenchymal disease. Cardiac exam should evaluate for the presence of abnormal heart sounds with particular focus on identifying signs of heart failure, such as SIII, distended jugular veins, and peripheral edema. In addition to a careful cardiopulmonary exam, it is important to assess neuromuscular function. Point of care ultrasound can provide additional value in detecting abnormal lung parenchyma, pleural effusions, reduced cardiac function, or poor diaphragmatic excursion if any of these abnormalities are present [7,26].
When the diagnosis is not obvious from the initial history and examination, there is general agreement that additional testing should be performed, including complete pulmonary function testing with pre- and post-bronchodilator spirometry, chest radiograph, laboratory testing (complete blood count, arterial gases, thyroid stimulating hormone [TSH], brain natriuretic peptide [BNP]), and evaluation of bronchial hyperresponsiveness with methacholine testing. If the diagnosis is not made at this stage, subsequent non-invasive testing [27] can include additional pulmonary testing with chest computed tomography (CT), ventilation perfusion scans, or even bronchoscopy (for example, to evaluate for dynamic airway collapse or stenosis) and additional cardiac testing (transthoracic echocardiogram (TTE), stress testing, nuclear cardiac imaging and catheterization). Cardiopulmonary exercise testing (CPET) is also proposed as part of the dyspnea diagnostic algorithm [17–19]. This test is an invaluable tool to understanding the underlying pathophysiology and narrowing the list of potential diagnoses as it can provide information not readily available with other testing modalities. For example, CPET can be used to measure operating lung volumes and identify abnormal breathing patterns that lead to ventilatory insufficiency during exercise. This in turn causes dissociation between IND and the physiologic response of the dynamic respiratory system, a mechanism that can significantly worsen the subjective feeling of dyspnea [28,29].
CPET’s findings can be broadly divided into a) “normal” physiological responses, b) oxygen delivery/utilization mismatch, c) mechanical ventilatory impairment, d) impaired pulmonary gas exchange/altered ventilatory control, e) cardiac limitations, f) obesity, and g) dysfunctional breathing/hyperventilation disorder [30]. This syndromic clustering, however, must be put into the clinical context with respect to history and prior diagnostic testing to decide what additional studies are needed next to make a specific diagnosis. Examples of specific pathologies in each syndromic category are listed in Table 1 (Adapted from [30]).
Table 1.
Cardiopulmonary Exercise Testing Patterns and Corresponding Potential Clinical Diagnosis
| Pattern | Differential Diagnosis |
|---|---|
| O2 Delivery/utilization mismatch | Chronic (systolic or diastolic) heart failure Pulmonary vascular disease Ischemic coronary disease Valvular Heart disease |
| Mechanical Ventilatory Impairment | COPD Interstitial lung disease Other persistent airflow obstructive disorders: asthma with airway remodeling, cystic fibrosis, bronchiectasis Chest wall disease Respiratory muscle dysfunction |
| Impaired gas exchange/altered ventilatory control. | Chronic (systolic or diastolic) heart failure Pulmonary vascular disease Lung V/Q mismatch disorders: COPD, interstitial lung disease |
Adopted from Cortozi Berton et al. (21)
Using a graded diagnostic approach that includes CPET has shown to yield a specific etiology for persistent unexplained dyspnea in 75 – 99% of patients [17–19]. As shown in Table 2, except for the Pratter et al study, most diagnoses made are non-cardiopulmonary and are primarily related to obesity, deconditioning and functional breathing disorders. This experience is mirrored in a larger, more recent study from the Brigham and Women’s Hospital Multidisciplinary Dyspnea/Exercise Intolerance Center, which diagnosed 36% of 864 patients with initial standard testing, and the remaining 64% with the use of CPET (Table 2) [31]. A considerable limitation is that a poor conditioning diagnosis by CPET does not necessarily rule out other underlying cardiac etiologies for dyspnea [19]. In addition, recent work by Neder et al has shown that CPET interpretation can be limited by over-reliance on rigid interpretative algorithms, under-recognition of limitations of the study modality, and misinterpretation of data obtained during the exam [32]. It is important to consider that the workup for persistent dyspnea is not completed until the patient’s response to therapy is subsequently determined.
Table 2.
Causes of unexplained and persistent dyspnea across different diagnostic algorithms.
| Del Paso et al (16) | Martinez et al (18) | Pratter et al (17) | Huang et al (22) | |
|---|---|---|---|---|
| Patients | N=77 | N=50 | N=123 | N=530 |
| Diagnosis made in n= | 63 | 49 | 122 | 530 |
| Respiratory | 36% | 32% | 53% | 17% |
| Asthma Intrathoracic focal obstruction of a large airway Extrathoracic upper airway obstruction | Hyperactive airways Interstitial lung disease Other lung diseases | Airflow obstruction Interstitial lung diseases Other lung diseases | Pulmonary hypertension | |
| Cardiovascular/circulatory | 14% | 10% | 16% | 18% |
| Coronary artery disease Arrhythmias Constrictive pericarditis | Ischemic Heart Disease Heart Failure with preserved ejection faction Atrial septal defect | Congestive heart failure/other cardiomyopathies Valvular heart disease Other | Heart failure with preserved ejection fraction | |
| Non-cardiopulmonary | 50% | 60% | 31% | 65% |
| Hyperventilation syndrome Poor conditioning Gastroesophageal Reflux disease Thyroid disease Unexplained | Obesity/deconditioning Psychogenic, normal Gastroesophageal Reflux disease | Obesity Psychogenic Deconditioning Others | Primary hyperventilation Dysautonomia Oxidative myopathy Others |
4. General Aspects of Persistent Dyspnea Evaluation and Management
Dyspnea is a complex symptom, not a specific condition [1], therefore reducing its burden requires a fundamental understanding of the causative mechanisms that can be targeted to improve it. This section does not intend to discuss the treatment of each potential condition in depth. It rather provides a general framework for patients who continue to experience symptoms despite optimal treatment of the primary disease or for patients whose diagnostic work up does not reveal an obvious cardiopulmonary cause of dyspnea. Below we discuss three frequently overlooked etiologies for persistent dyspnea in patients with otherwise negative cardiopulmonary workups. This is by no means an extensive list but it illustrates the type of conditions seen in patients often referred to subspecialty clinics for further evaluation and treatment.
4.1. Patients with persistent dyspnea with otherwise negative workups:
4.1.1. Dyspnea due to severe deconditioning
Severe deconditioning in otherwise adequately treated patients with chronic lung or cardiovascular disease can be an important cause of persistent dyspnea [33]. Indeed, respiratory, and peripheral muscle weakness are important independent predictors of breathlessness and exercise capacity [22,34]. Pulmonary rehabilitation has been shown to improve muscle strength, exercise capacity, quality of life, and to reduce the affective aspects of dyspnea [35,36]. Pulmonary rehabilitation is considered standard of care for patients with severe obstructive lung disease, including chronic obstructive pulmonary disease and asthma, as well as patients with pulmonary vascular disease and interstitial lung disease [36–40]. A trial of pulmonary rehabilitation is recommended in patients with severe chronic pulmonary diseases with persistent dyspnea, and in particularly those who are already maximally treated but are deconditioned based off of clinical suspicion or CPET result [41].
4.1.2. Dyspnea due to dysfunctional breathing
Dysfunctional breathing describes breathing disorders in which chronic changes in breathing pattern result in dyspnea and other symptoms in the absence of or of greater magnitude than explained by physiological respiratory or cardiac disease that is present [42]. These conditions may present alone, as a somatic manifestation of psychological conditions, or as a manifestation of underlying disease and in any individual there may be more than one of these factors at play [43]. The most important identifying criterion to identify dysfunctional breathing is the presence of breathlessness after potential pathology has been objectively ruled out or optimized by pharmacologic treatment [44]. Although there is no diagnostic gold standard for dysfunctional breathing, experts from the European Respiratory Society proposed a subclassification of this condition, including hyperventilation syndrome, periodic sigh breathing, thoracic dominant breathing, forced abdominal expiration, and thoraco-abdominal dissociation [42]. CPET can be useful in suggesting a diagnosis of dysfunctional breathing by detecting some of these erratic breathing patterns and abnormal ventilation responses to an increasing exercise load. While not necessarily diagnostic, their presence can suggest the possibility of dysfunctional breathing if they occur in an otherwise normal CPET [45]. Having a positive response to respiratory retraining and supportive management would affirm a functional breathing disorder as the etiology of dyspnea [46].
4.1.3. Dyspnea due to vocal cord dysfunction
Another common form of dysfunctional breathing causing persistent dyspnea is paradoxical vocal fold motion disorder (PVFMD), also known as vocal cord dysfunction or induced laryngeal obstruction [23]. PVFMD occurs when the vocal folds adduct during inspiration, giving the sensation of constricted breathing and shortness of breath. It usually occurs in response to specific triggers such as strong odors, dust, tobacco smoke, exercise, and stress. It is often misdiagnosed as asthma, which can cause significant comorbidities from unnecessary systemic steroid exposure [47]. Associated comorbidities such as rhinosinusitis and gastroesophageal reflux can worsen its severity. The diagnosis requires direct visualization of abnormal vocal fold movement via laryngoscopy. However, given its episodic nature, a normal laryngeal exam cannot rule out the diagnosis. To improve diagnostic accuracy, laryngoscopy can be done in conjunction with methacholine testing, exercising, or with a full CPET [48]. Standardized questionnaires can also help discriminate between symptomatic PVFMD and asthma [49]. A simple clinical predictive index using symptoms of throat tightness and dysphonia, absence of wheezing, and the presence of odors as a symptom trigger had good sensitivity (83%) and specificity (95%) for differentiating PVFMD from asthma [50]. This scoring index accurately made the diagnosis in 77.8% of subjects with laryngoscopy proven PVFMD [50]. In most cases, speech therapy and controlling comorbidities substantially improves this condition [41].
5. Expert opinion
In many cases, patients with persistent dyspnea have been evaluated and treated by other health professionals and yet they continue to experience burdensome symptoms. To maximize the possibility of making the right diagnosis and instituting adequate therapy requires a systematic, graded, and often multidisciplinary approach. This often begins by evaluating prior test results and weighing previous diagnostic considerations.
Doing a careful dyspnea assessment during the initial evaluation is key to developing an early differential diagnosis to help strategize diagnostic testing priority. Ultimately, the goal is to pair the patient’s symptom to an underlying pathophysiologic process [13]. As with any other symptom, it is important to understand onset, quality, duration, intensity, changes with physical activity or during rest, and mitigation or aggravating factors. Acute onset dyspnea can be associated with many serious acute pulmonary conditions including pulmonary embolism, large atelectasis or lung collapse, spontaneous pneumothorax, bronchoconstriction, flash pulmonary edema, or cardiovascular diseases such as myocardial ischemia, or may represent worsening of the primary underlying condition.
One of the most important aspects of the evaluation of persistent dyspnea is understanding a patient’s exercise tolerance and exertional symptoms and how this varies over time. Patients with more advanced obstructive airway diseases usually have some degree of day-to-day variation in dyspnea which becomes less variable as the disease severity progresses. In our experience, patients that have a predictable and constant level of dyspnea for a given effort threshold are either deconditioned, have an advanced cardiopulmonary disease, or have some degree of both. Persistent dyspnea that is inconsistently worsened by exertional activities or effort, is absent during sleep, and is at times also present at rest is highly suspicious for a functional breathing disorder [44].
An additional consideration should be made for patients presenting with persistent dyspnea following symptomatic infection with COVID-19 as patients in this population have an added diagnostic challenge due to the novel nature of the disease process. Patients dealing with long-term effects of COVID-19 are described as having post-acute COVID-19 syndrome (PCS) or “long-COVID” when symptoms persist for more than four weeks after recovery from acute illness [51]. Rates of persistent dyspnea in this patient group were found to be 39.5% in a recent pooled analysis of various observational studies [52]. It should be of note that symptoms of PCS have been observed in patients across the spectrum of severity of acute COVID and patients did not need to experience severe, acute illness to have persistent symptoms. In addition, patients do not need to have radiographically significant fibrotic pulmonary changes to experience breathlessness [51]. The mechanism of persistent dyspnea in patients with PCS is likely multifactorial and requires further investigation to help better elucidate the underlying mechanisms causing symptoms in these patients.
Following a careful history and clinical exam our diagnostic approach to patients with persistent dyspnea begins with noninvasive tests, which include full pulmonary function testing (lung volumes, DLCO, pre- and post-bronchodilation spirometry), blood chemistries, complete blood count, chest radiograph, and arterial blood gas. Our diagnostic approach to persistent dyspnea is detailed in Figure 1. If initial testing does not uncover the diagnosis, subsequent, more advanced testing modalities can include assessing for bronchial hyperresponsivess (BHR) with methacholine testing, TTE, and chest CT. Methacholine testing has reasonable diagnostic properties to evaluate if asthma is an underlying problem with an overall sensitivity of 77% and specificity of 96% [53]. Although it is recommended as an essential part of the unexplained dyspnea algorithm, its diagnostic properties in this specific patient population have not been studied. Resting TTE can evaluate for possible pulmonary hypertension by estimating pulmonary artery systolic pressures and right ventricular function. As a screening tool for pulmonary hypertension, TTE has a pooled sensitivity of 85%, a pooled specificity of 74%, and a positive likelihood ratio of 3.2 [54]. It also provides information regarding the size and function of the left ventricle and therefore can be a useful tool to determine if dyspnea is related to pulmonary hypertension, left ventricular dysfunction, or both [55]. In addition to providing information on the lung parenchyma, dynamic CT with an expiratory phase can detect smaller airway dysfunction resulting in air trapping seen as mosaicism on imaging [56]. In addition, it can provide diagnostic information for often difficult to diagnose airway disorders that can cause substantial dyspnea, such as excessive dynamic airway collapse in patients with tracheobronchomalacia or tracheal stenosis [57,58].
Figure 1. A practical algorithm to evaluating patients with persistent unexplained dyspnea.
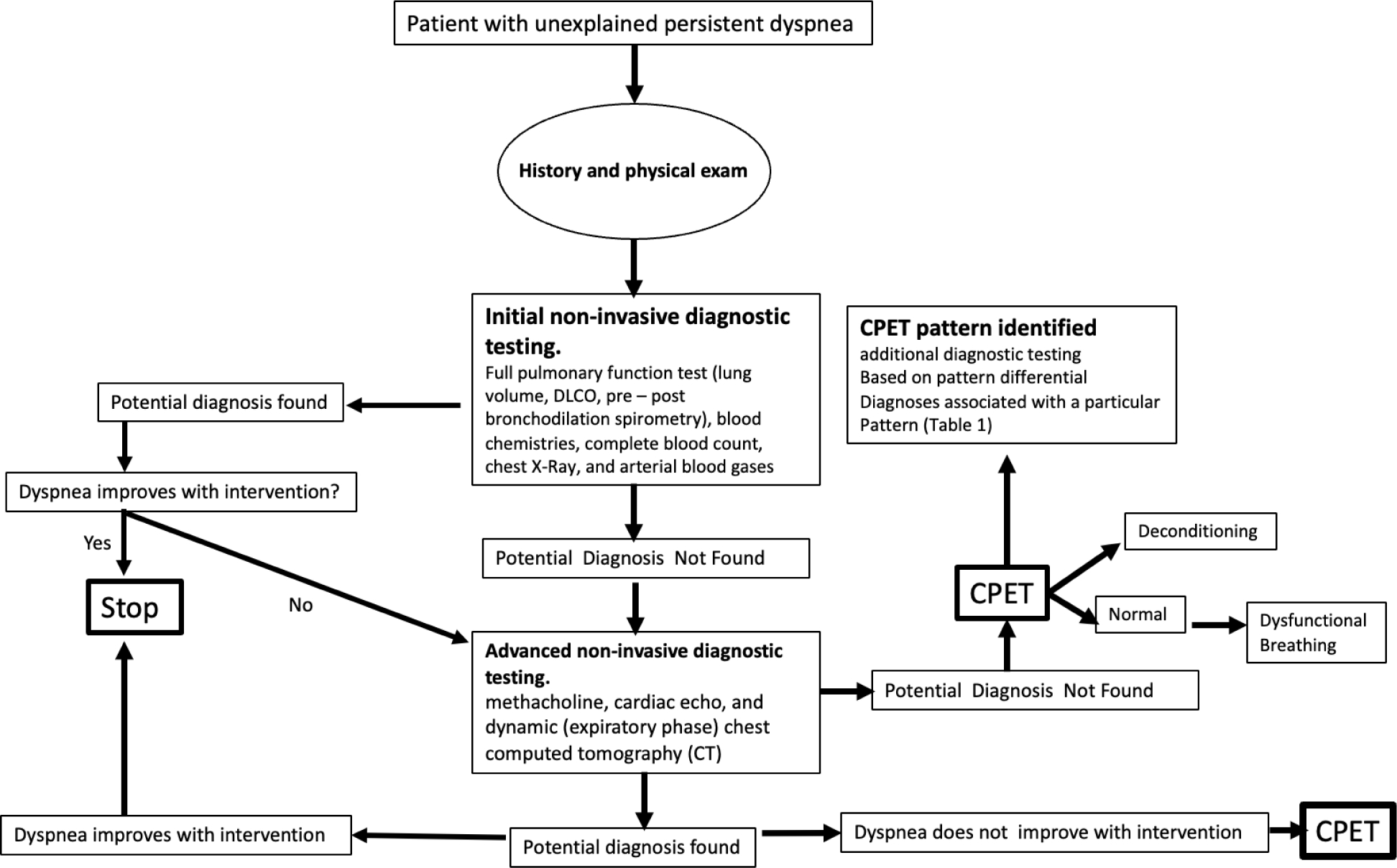
*DLCO: Diffusion capacity; CT: computed tomography; CPET: Cardiopulmonary exercise test
If the diagnosis is not apparent after noninvasive tests, we often proceed to CPET. In some institutions this test can be done with the addition of video laryngoscopy to evaluate for PVFMD as a potential source for exercise induced dyspnea. As mentioned earlier, CPET does not necessarily provide a specific diagnosis but rather integrates the physiological response data into syndromic categories (Table 1). Similar graded testing approaches to unexplained persistent dyspnea yield a diagnosis in 75% to more than 90% of patients. Patients remaining without a clear diagnosis that have a normal CPET can be reasonably assured that their cause of dyspnea is not related to a more serious cardiopulmonary process. Often, the reason for persistent dyspnea in these patients is associated with obesity and deconditioning or is associated with a breathing perception or functional disorder. However, it is important to remember that clinical judgment should guide the need for additional cardiac testing in some high-risk patients diagnosed with deconditioning by CPET.
The diagnostic evaluation for persistent dyspnea is only completed after evaluating the clinical response to a therapeutic intervention. In addition to instituting medical treatments for a specific diagnosis, there are some general approaches that can have clinical benefit regardless of the underlying disease process. For individuals with chronic lung disease, pulmonary rehabilitation effectively reduces shortness of breath while improving quality of life. For those not qualifying for pulmonary rehabilitation we usually refer to wellness programs that offer weight loss interventions and a tailored physical training program that includes muscle strengthening and gradual aerobic training. Respiratory retraining by speech therapy can be helpful for patients with breathing perception disorders, yet there is insufficient clinical data across published studies to determine its overall efficacy rate [59].
6. Conclusion
Persistent, unexplained dyspnea is frequently encountered in clinical practice. Finding a diagnosis or excluding serious underlying cardiopulmonary morbidities requires a clinically driven graded diagnostic algorithm. A systematic approach can make a diagnosis in most cases and a normal work up can rule out most serious cardiopulmonary etiologies for dyspnea. Clinicians need to remember that the ultimate test to confirm the underlying cause of dyspnea is to assess its response to a specific intervention.
Article Highlights.
Persistent dyspnea is a common and debilitating symptom that affects up to 50% of patients admitted to hospitals and as many as 25% of those seeking care in outpatient facilities
Dyspnea is a complex and frequently reported symptom. It is influenced by a patient’s culture, environment, individual perception, and past experiences. It may be the result of a dysfunctional breathing pattern or potentially herald an ominous diagnosis
When the diagnosis is not obvious from the initial history and examination, additional testing should include pulmonary function testing with pre- and post-bronchodilator spirometry, chest & cardiac imaging, laboratory testing (complete blood count, arterial gases, thyroid stimulating hormone [TSH], brain natriuretic peptide [BNP]), and evaluation of bronchial hyperresponsiveness with methacholine testing. If the diagnosis is not made at this stage, cardiopulmonary exercise testing (CPET), can be useful to understand the underlying pathophysiology and narrowing the list of potential diagnoses
Using a graded diagnostic approach that includes CPET has shown to yield a specific etiology for persistent unexplained dyspnea in 75 – 99% of patients
Physical deconditioning, dysfunctional breathing, paradoxical vocal fold motion, and obesity are frequent causes for persistent unexplained dyspnea in patients with or without underlying cardiopulmonary diseases.
Funding
This manuscript was funded by the National Institutes of Health, Heart and Lung Blood Institute: Grant # 1 R01 HL146542-01.
Footnotes
Declaration of Interest
F. Holguin is a member of the adjudication committee for the INSMED ASPEN Bronsocatib trial and is a member of the FDA’s Pulmonary and Allergy Drug Committee. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1. Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999. Jan;159(1):321–40. ** Major review of dysonea pathophysiology, classification and diagnostic approach
- 2.Frostad A, Soyseth V, Andersen A, et al. Respiratory symptoms as predictors of all-cause mortality in an urban community: a 30-year follow-up. J Intern Med. 2006. May;259(5):520–9. [DOI] [PubMed] [Google Scholar]
- 3.Currow DC, Plummer JL, Crockett A, et al. A community population survey of prevalence and severity of dyspnea in adults. J Pain Symptom Manage. 2009. Oct;38(4):533–45. [DOI] [PubMed] [Google Scholar]
- 4.Sondik EJ. Ambulatory Care Visits to Physician Offices, Hospital Outpatient Departments, and Emergency Departments: United States, 2001–02 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics; 2006. [cited 2021 08/30/2021]. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/viewer.html?pdfurl=https%3A%2F%2Fwww.cdc.gov%2Fnchs%2Fdata%2Fseries%2Fsr_13%2Fsr13_159.pdf&clen=4114578&chunk=true [Google Scholar]
- 5. Neder JA. Residual Exertional Dyspnea in Cardiopulmonary Disease. Ann Am Thorac Soc. 2020. Dec;17(12):1516–1525. ** Recent work highlighting the mechanisms for residual dyspnea in patients with cardiopulmonary disease
- 6.Bonafede M, Jing Y, Gdovin Bergeson J, et al. Impact of dyspnea on medical utilization and affiliated costs in patients with acute coronary syndrome. Hosp Pract (1995). 2011. Aug;39(3):16–22. [DOI] [PubMed] [Google Scholar]
- 7.Coccia CB, Palkowski GH, Schweitzer B, et al. Dyspnoea: Pathophysiology and a clinical approach. S Afr Med J. 2016. Jan;106(1):32–6. [DOI] [PubMed] [Google Scholar]
- 8. Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med. 1995. Dec 7;333(23):1547–53. ** Concise review on dyspnea’s pathophysiologic mechanisms
- 9.Scano G, Ambrosino N. Pathophysiology of dyspnea. Lung. 2002;180(3):131–48. [DOI] [PubMed] [Google Scholar]
- 10.Nishino T. Dyspnoea: underlying mechanisms and treatment. Br J Anaesth. 2011. Apr;106(4):463–74. [DOI] [PubMed] [Google Scholar]
- 11. Laviolette L, Laveneziana P, Faculty ERSRS. Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J. 2014. Jun;43(6):1750–62. ** Consensus report highlighting the multidementionality of dyspnea, in terms of mechanisms, evaluation and diagnostic workup.
- 12. Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol. 2009. May 30;167(1):72–86. * Explains the bases for the corollary discharge mechanism
- 13. Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012. Feb 15;185(4):435–52. ** Updated dyspnea review from a concensus expert panel
- 14.Moosavi SH, Banzett RB, Butler JP. Time course of air hunger mirrors the biphasic ventilatory response to hypoxia. J Appl Physiol (1985). 2004. Dec;97(6):2098–103. [DOI] [PubMed] [Google Scholar]
- 15. Binks AP, Moosavi SH, Banzett RB, et al. “Tightness” sensation of asthma does not arise from the work of breathing. Am J Respir Crit Care Med. 2002. Jan 1;165(1):78–82. * Highlights bronchospasm as a unique dyspnea dimention
- 16.Wahls SA. Causes and evaluation of chronic dyspnea. Am Fam Physician. 2012. Jul 15;86(2):173–82. [PubMed] [Google Scholar]
- 17. DePaso WJ, Winterbauer RH, Lusk JA, et al. Chronic dyspnea unexplained by history, physical examination, chest roentgenogram, and spirometry. Analysis of a seven-year experience. Chest. 1991. Nov;100(5):1293–9. ** Diagnostic alorithm for patients with unexplained dyspnea.
- 18. Pratter MR, Abouzgheib W, Akers S, et al. An algorithmic approach to chronic dyspnea. Respir Med. 2011. Jul;105(7):1014–21. ** Diagnostic alorithm for patients with unexplained dyspnea.
- 19. Martinez FJ, Stanopoulos I, Acero R, et al. Graded comprehensive cardiopulmonary exercise testing in the evaluation of dyspnea unexplained by routine evaluation. Chest. 1994. Jan;105(1):168–74. ** Diagnostic alorithm for patients with unexplained dyspnea. Also discusses CPET use and limitations on its interpretation.
- 20.Ramalho SHR, Santos M, Claggett B, et al. Association of Undifferentiated Dyspnea in Late Life With Cardiovascular and Noncardiovascular Dysfunction: A Cross-sectional Analysis From the ARIC Study. JAMA Netw Open. 2019. Jun 5;2(6):e195321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massart A, Hunt DP. Management of Refractory Breathlessness: a Review for General Internists. J Gen Intern Med. 2021. Apr;36(4):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Donnell DE, James MD, Milne KM, et al. The Pathophysiology of Dyspnea and Exercise Intolerance in Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2019. Jun;40(2):343–366. ** excellent review of dyspnea mechanisms in COPD
- 23.Matrka L. Paradoxic vocal fold movement disorder. Otolaryngol Clin North Am. 2014. Feb;47(1):135–46. [DOI] [PubMed] [Google Scholar]
- 24.Mukerji V. Dyspnea, Orthopnea, and Paroxysmal Nocturnal Dyspnea. In: rd, Walker HK, Hall WD, et al. , editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: 1990. [PubMed] [Google Scholar]
- 25.Perez T. [Neuromuscular disorders - assessment of the respiratory muscles]. Rev Neurol (Paris). 2006. Apr;162(4):437–44. [DOI] [PubMed] [Google Scholar]
- 26.Buhumaid RE, St-Cyr Bourque J, Shokoohi H, et al. Integrating point-of-care ultrasound in the ED evaluation of patients presenting with chest pain and shortness of breath. Am J Emerg Med. 2019. Feb;37(2):298–303. [DOI] [PubMed] [Google Scholar]
- 27.Oelsner EC, Lima JA, Kawut SM, et al. Noninvasive tests for the diagnostic evaluation of dyspnea among outpatients: the Multi-Ethnic Study of Atherosclerosis lung study. Am J Med. 2015. Feb;128(2):171–180 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell DE, Milne KM, Vincent SG, et al. Unraveling the Causes of Unexplained Dyspnea: The Value of Exercise Testing. Clin Chest Med. 2019. Jun;40(2):471–499. [DOI] [PubMed] [Google Scholar]
- 29. Neder JA, Berton DC, Marillier M, et al. The role of evaluating inspiratory constraints and ventilatory inefficiency in the investigation of dyspnea of unclear etiology. Respir Med. 2019. Oct - Nov;158:6–13. ** Excellent review on inspiratory constraints and ventialtor inefficiency as a dyspnea mechanism
- 30.Berton DC, Mendes NBS, Olivo-Neto P, et al. Pulmonology approach in the investigation of chronic unexplained dyspnea. J Bras Pneumol. 2021;47(1):e20200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W, Resch S, Oliveira RK, et al. Invasive cardiopulmonary exercise testing in the evaluation of unexplained dyspnea: Insights from a multidisciplinary dyspnea center. Eur J Prev Cardiol. 2017. Jul;24(11):1190–1199. [DOI] [PubMed] [Google Scholar]
- 32. Neder JA, Phillips DB, Marillier M, et al. Clinical Interpretation of Cardiopulmonary Exercise Testing: Current Pitfalls and Limitations. Front Physiol. 2021;12:552000. ** Excellent comprehensive review on CPET
- 33. O’Donnell DE, McGuire M, Samis L, et al. The impact of exercise reconditioning on breathlessness in severe chronic airflow limitation. Am J Respir Crit Care Med. 1995. Dec;152(6 Pt 1):2005–13. * Importance of exercise training and rehabiliation of dyspnea symptom
- 34.Farooqi MAM, Killian K, Satia I. The impact of muscle strength on exercise capacity and symptoms. ERJ Open Res. 2020. Oct;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadell K, Webb KA, Preston ME, et al. Impact of pulmonary rehabilitation on the major dimensions of dyspnea in COPD. COPD. 2013. Aug;10(4):425–35. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015. Feb 23(2):CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zampogna E, Paneroni M, Cherubino F, et al. Effectiveness of a Pulmonary Rehabilitation Program on Persistent Asthma Stratified for Severity. Respir Care. 2019. Dec;64(12):1523–1530. [DOI] [PubMed] [Google Scholar]
- 38.Nopp S, Klok FA, Moik F, et al. Outpatient Pulmonary Rehabilitation in Patients with Persisting Symptoms after Pulmonary Embolism. J Clin Med. 2020. Jun 10;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozemek C, Berry MJ, Arena R. A Review of Exercise Interventions in Pulmonary Arterial Hypertension and Recommendations for Rehabilitation Programing. J Cardiopulm Rehabil Prev. 2019. May;39(3):138–145. [DOI] [PubMed] [Google Scholar]
- 40.Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res. 2018. Sep 20;19(1):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013. Mar;123(3):727–31. [DOI] [PubMed] [Google Scholar]
- 42. Boulding R, Stacey R, Niven R, et al. Dysfunctional breathing: a review of the literature and proposal for classification. Eur Respir Rev. 2016. Sep;25(141):287–94. ** Excellent review on the clinical evaluation and classification of dysfunctional breathing
- 43.Barker N, Everard ML. Getting to grips with ‘dysfunctional breathing’. Paediatr Respir Rev. 2015. Jan;16(1):53–61. [DOI] [PubMed] [Google Scholar]
- 44.Niggemann B. How to diagnose psychogenic and functional breathing disorders in children and adolescents. Pediatr Allergy Immunol. 2010. Sep;21(6):895–9. [DOI] [PubMed] [Google Scholar]
- 45.Ionescu MF, Mani-Babu S, Degani-Costa LH, et al. Cardiopulmonary Exercise Testing in the Assessment of Dysfunctional Breathing. Front Physiol. 2020;11:620955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas M, McKinley RK, Freeman E, et al. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax. 2003. Feb;58(2):110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newman KB, Mason UG 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Respir Crit Care Med. 1995. Oct;152(4 Pt 1):1382–6. [DOI] [PubMed] [Google Scholar]
- 48.Olin JT, Clary MS, Fan EM, et al. Continuous laryngoscopy quantitates laryngeal behaviour in exercise and recovery. Eur Respir J. 2016. Oct;48(4):1192–1200. [DOI] [PubMed] [Google Scholar]
- 49.Ye J, Nouraie M, Holguin F, et al. The Ability of Patient-Symptom Questionnaires to Differentiate PVFMD From Asthma. J Voice. 2017. May;31(3):382 e1–382 e8. [DOI] [PubMed] [Google Scholar]
- 50.Traister RS, Fajt ML, Landsittel D, et al. A novel scoring system to distinguish vocal cord dysfunction from asthma. J Allergy Clin Immunol Pract. 2014. Jan-Feb;2(1):65–9. [DOI] [PubMed] [Google Scholar]
- 51.Crook H, Raza S, Nowell J, et al. Long covid-mechanisms, risk factors, and management. BMJ. 2021. Jul 26;374:n1648. [DOI] [PubMed] [Google Scholar]
- 52.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2021. Aug 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumino K, Sugar EA, Irvin CG, et al. Methacholine challenge test: diagnostic characteristics in asthmatic patients receiving controller medications. J Allergy Clin Immunol. 2012. Jul;130(1):69–75 e6. [DOI] [PubMed] [Google Scholar]
- 54.Ni JR, Yan PJ, Liu SD, et al. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: a systematic review and meta-analysis. BMJ Open. 2019. Dec 22;9(12):e033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hillis GS, Bloomfield P. Basic transthoracic echocardiography. BMJ. 2005. Jun 18;330(7505):1432–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kligerman SJ, Henry T, Lin CT, et al. Mosaic Attenuation: Etiology, Methods of Differentiation, and Pitfalls. Radiographics. 2015. Sep-Oct;35(5):1360–80. [DOI] [PubMed] [Google Scholar]
- 57.Heyer CM, Nuesslein TG, Jung D, et al. Tracheobronchial anomalies and stenoses: detection with low-dose multidetector CT with virtual tracheobronchoscopy--comparison with flexible tracheobronchoscopy. Radiology. 2007. Feb;242(2):542–9. [DOI] [PubMed] [Google Scholar]
- 58.Hammond K, Ghori UK, Musani AI. Tracheobronchomalacia and Excessive Dynamic Airway Collapse. Clin Chest Med. 2018. Mar;39(1):223–228. [DOI] [PubMed] [Google Scholar]
- 59.Barker NJ, Jones M, O’Connell NE, et al. Breathing exercises for dysfunctional breathing/hyperventilation syndrome in children. Cochrane Database Syst Rev. 2013. Dec 18(12):CD010376. [DOI] [PMC free article] [PubMed] [Google Scholar]


