Abstract
Objectives
Antioxidants are common dietary compounds with multiple health benefits. This study aimed to identify the association between dietary antioxidant consumption and the incidence of type 2 diabetes (T2D) mellitus (defined using the Korean Diabetes Association criteria) in South Korean adults.
Design
Baseline and follow-up data from the Health Examinees (HEXA) study, a large-scale community-based genomic cohort study conducted in South Korea
Setting
A South Korean community.
Participants
A total of 20 594 participants, aged 40–79 years, who participated in the baseline and follow-up surveys of the HEXA study were included. After an average of 5 years of follow-up, there were 332 men and 360 women with T2D.
Results
Participants with the highest total flavonoid consumption (Q5) had a lower risk of T2D (men: HR 0.63; 95% CI 0.42 to 0.93; p value for trend=0.0169; and women: HR 0.54; 95% CI 0.438 to 0.78; p value for trend=0.0001) than those with the lowest consumption (Q1). Dietary total antioxidant capacity was significantly inversely associated with the development of T2D mellitus in women participants alone (HR 0.58; 95% CI 0.40 to 0.83; p value for trend=0.0004). Stratified analyses according to age and body mass index (BMI) showed that dietary total flavonoid consumption and total antioxidant capacity had a negative association with the development of T2D in women aged >52 years and women with BMI >25 kg/m2.
Conclusions
Dietary flavonoid consumption and total antioxidant capacity were associated with a lower risk of T2D in South Korean adults, especially in women aged >52 years and overweight. The findings of this study may provide reference data for the modification of dietary guidelines for South Koreans.
Keywords: diabetes & endocrinology, epidemiology, nutrition & dietetics, public health, statistics & research methods
Strengths and limitations of this study.
This study used a large-scale community-based genomic cohort study conducted in South Korea with 5 years of follow-up.
Stratified analyses were conducted to focus on one certain exposure.
Although this study reported a longitudinal relationship between antioxidant consumption and diabetes incidence, we could not assess the causality.
Dietary measurement errors were inevitable due to using self-reported Food Frequency Questionnaire.
Introduction
Diabetes is a metabolic disease characterised by high blood glucose levels, impaired glucose tolerance, impaired insulin secretion and insulin resistance.1 2 Currently, ≥400 million people are living with type 2 diabetes (T2D) mellitus worldwide.3 The prevalence of T2D mellitus increases with age. The 2018 Korean Diabetes Association diabetes fact sheet reported that the prevalence of T2D in the total population aged ≥65 years was 29.8%.4 It is projected that T2D will be the seventh leading cause of death by 2030.3 Given that T2D is accompanied by various serious complications, including cardiovascular disease, peripheral vascular disease, retinopathy, nephropathy, neuropathy and, recently, sarcopenia, its prevention and treatment are extremely important.3 5 Both genetic and environmental factors contribute to the development and progression of T2D mellitus. However, its growing prevalence is the result of result of changing dietary habits and lifestyles observed in modern societies.6
Dietary flavonoids, abundant in fruits and vegetables, are a group of naturally occurring polyphenolic compounds.7 8 There are seven subgroups of flavonoids: flavonols, flavanones, isoflavones, flavones, flavan-3-ols, anthocyanins and proanthocyanidins.9–11 Flavonoids are associated with various health-promoting effects, including anti-inflammatory, antihypertensive, antiobesity and antidiabetic.
Previous studies have reported that dietary antioxidants decreased oxidative stress, an important risk factor for T2D, played a key role as anti-inflammatory factors by blocking the nuclear factor kappa-light-chain-enhancer of the activated B (NF-κB) and mitogen-activated protein kinase (MAPK) cell signalling pathways. MAPK pathway was associated with the induction of proinflammatory genes and the promotion of Akt/protein kinase B, an insulin-signalling pathway.12–15 As a result, antioxidants improve insulin resistance, which is involved in the pathogenesis of T2D mellitus, by promoting the transportation of GLUT4 through the regulation of the insulin-signalling pathway.16 17
Previous studies have investigated the correlation between dietary antioxidants and obesity, dyslipidaemia and metabolic syndrome.18–21 Jafari Azad et al22 showed that a diet high in antioxidants had protective effects against the development of T2D in the Iranian population. However, few studies have been conducted to determine the association between dietary antioxidant intake and T2D in South Korean populations. In addition, whether there is a dose–response relationship between dietary antioxidants and T2D is unclear. It is pertinent to determine whether a relationship exists between dietary antioxidant intake and T2D in South Korean adults. Moreover, the effect of dietary antioxidants on T2D has not been investigated according to the flavonoid subclasses, antioxidant capacities of flavonoids or total antioxidant capacity (TAC), which is an index to indicate whole dietary antioxidant content.23 Therefore, we conducted this study to explore the association between dietary antioxidant and the incidence of T2D by analysing data from the Health Examinees (HEXA) study.
Methods
Patient and public involvement
Patients and the public were not involved in the design of the study.
Study population
This study was based on the baseline and follow-up data from the HEXA study, a large-scale community-based genomic cohort study conducted in South Korea. More specific details of the HEXA study design are described elsewhere.24 A total of 173 357 participants aged≥40 years were initially included in the baseline survey, which was conducted from 2003 to 2014; 65 642 of these participants were included in the follow-up survey, which was conducted from 2012 to 2016. At baseline, we excluded participants who had T2D mellitus or had no information on fasting plasma glucose or HbA1c levels (n=41 311) and those with a history of diseases closely related to T2D mellitus (ie, hyperlipidaemia, stroke, transient ischaemic attacks, angina pectoris and myocardial infarction) (n=3082). At follow-up, we excluded those with missing information on biomarkers for T2D mellitus (fasting plasma glucose, HbA1c) (n=11), those who had an implausible energy intake (<3349 or ≥16 743 kJ/day for men and <2093 or ≥14 650 kJ/day for women; n=63025) and those who were missing values for covariables such as drinking (n=9) and body mass index (BMI) (n=5). Ultimately, a total of 20 594 participants (6327 men and 14 267 women) were included in this study.
Dietary assessment and estimation of antioxidant components
Dietary intake was assessed using the self-administered, 106-item Food Frequency Questionnaire (FFQ) developed for the Korean Genome Epidemiologic Study.24 Participants reported the frequencies and average portions of food or beverage items consumed during the last year before participating in the HEXA study. The reproducibility and validity of the FFQ have been assessed in a previous study using a reference method by collecting information on 12-day dietary records.26 The median correlation coefficient for all nutrients was 0.39 between the FFQ and 12-day dietary record, and the researchers concluded that the FFQ could be an acceptable tool for dietary assessment.26
In this study, we estimated the participants’ intake of antioxidant components using self-reported dietary data linked to the TAC database for common South Korean foods.11 27 Dietary TAC and intake of each flavonoid component were expressed as vitamin C equivalent antioxidant capacity (mg VCE/100 g). The intake of individual antioxidant components from a food item was calculated by multiplying the antioxidant component per gram of food item by the total weight in grams of daily intake of this food item. The daily intake of individual total dietary antioxidant components was calculated as the sum of the intake of each antioxidant component from all the food sources reported in the HEXA FFQ data (mg VCE/day). After summing all individual total dietary antioxidant components, we obtained the dietary TAC per person per day.
Total flavonoid intake was classified into seven categories: anthocyanidins (cyanidin, delphinidin, pelargonidin, malvidin, peonidin and petunidin), isoflavones (daidzein, genistein and glycitein), proanthocyanidins (proanthocyanidin dimer, proanthocyanidin trimer, proanthocyanidin-4-6mers, proanthocyanidin-7-10mers and proanthocyanidin polymers), flavonols (quercetin, kaempferol, myricetin and isorhamnetin), flavones (luteolin and apigenin), flavanones (eriodictyol, hesperetin and naringenin) and flavan-3-ols (catechin, epicatechin, epigallocatechin, theaflavin, theaflavin-3-gallate, theaflavin-3′-gallate and theaflavin-3-3-digallate).
Definition of T2D
T2D was determined in accordance with the definition provided by the Korean Diabetes Association.28 T2D was defined as a diagnosis by a physician, increased fasting plasma glucose level ≥6.99 mmol/L (126 mg/dL) or elevated HbA1c level ≥47.5 mmol/mol (6.5%).
Covariables
In the HEXA study, the sociodemographic information of each participant was collected using a questionnaire. Our covariables of interest included age, BMI, level of education (middle school or lower, high school, or college or higher) and health-related behaviours, such as smoking (current smoker, past smoker or non-smoker), alcohol consumption (never or current drinker), level of physical activity (inactive or active) and total energy intake (kJ/day). BMI was calculated as the quotient of the body weight (kg) and height (m) squared (kg/m2).29 Smoking status was categorised into three groups based on the participants’ responses to the question, ‘Have you smoked ≥20 packs (400 cigarettes) so far?’ Participants who answered ‘never’ were classified as ‘non-smokers’, those who answered ‘yes’ and were still smoking at the time of the survey were classified as ‘current smokers’ and those who answered ‘yes’ but had quit smoking at the time of the survey were classified as ‘past smokers’. Alcohol consumption was classified based on the responses to the question ‘Are you unable to drink or refuse to do so for religious or other reasons?’ in the HEXA survey. In the present analysis, we classified participants who replied ‘yes’ as ‘alcohol non-drinkers’; the rest were classified as ‘alcohol drinkers’. Regarding physical activity levels, participants were classified as ‘active’ if they reported that they engaged in exercises resulting in sweating for ≥30 min twice a week.30
Statistical analyses
All statistical analyses were sex stratified and performed using Statistical Analysis Systems software V.9.4 (SAS Institute). Statistical significance was set at p<0.05. Continuous variables were presented as means±SD, and the difference between them in the outcome groups was tested using a generalised linear model. The categorical variables were presented as numbers (percentages), and the difference between them in the outcome groups was tested using the χ2 test. A multivariable Cox proportional hazards regression model was used to estimate the HRs and 95% CIs for T2D after adjusting for categorical (educational level, current drinking status, current smoking status and physical activity) and continuous (age, BMI and energy intake) covariables. The lowest quintile (Q1) of TAC or flavonoid intake served as a reference group. The median value of each quintile group was modelled as a continuous variable in the Cox model to test the trend. We also estimated the HRs and 95% CIs for an SD increment in dietary TAC and flavonoid intake and conducted stratified analyses according to BMI, age, smoking status and alcohol consumption. The strata indices for continuous variables (BMI and age) were median value referred to a previous study.31
Results
A total of 20 594 individuals (aged 40–79 years) were included in this study. After an average of 5 years of follow-up, the incidence of T2D mellitus was 5.25% in men and 2.52% in women. The baseline general characteristics of participants according to quintiles of total flavonoid intake are shown in table 1. Among both men and women participants, the highest consumption group (Q5) included more non-smokers, more participants with higher educational levels and more participants who engaged in physical activity (all p value <0.05).
Table 1.
Baseline general characteristics of participants by total flavonoid consumption
| Antioxidant consumption | P value | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Men, n=6327 | ||||||
| Cases/person-years | 80/5103.00 | 74/5060.60 | 60/4995.40 | 71/5110.50 | 47/5182.60 | |
| Age (years) | 54.45±8.64 | 54.97±8.51 | 54.20±8.37 | 54.55±8.32 | 54.89±7.97 | 0.0758 |
| BMI (kg/m2) | 24.07±2.74 | 24.19±2.73 | 24.21±2.71 | 24.31±2.65 | 24.32±2.58 | 0.1348 |
| Smoking status (%) | <0.0001 | |||||
| Never | 357 (28.22) | 404 (31.91) | 395 (31.27) | 373 (29.56) | 426 (33.84) | |
| Past | 451 (35.65) | 516 (40.76) | 549 (43.47) | 586 (46.43) | 575 (45.67) | |
| Current | 457 (36.13) | 346 (27.33) | 319 (25.26) | 303 (24.01) | 258 (20.49) | |
| Educational level (%) | <0.0001 | |||||
| Under middle school | 382 (30.32) | 297 (23.52) | 244 (19.40) | 186 (14.77) | 135 (10.69) | |
| High school | 503 (39.92) | 521 (41.25) | 492 (39.11) | 504 (40.03) | 422 (33.41) | |
| College or above | 375 (29.76) | 445 (35.23) | 522 (41.49) | 569 (45.19) | 706 (55.90) | |
| Physical activity (%) | <0.0001 | |||||
| Inactive | 1070 (84.65) | 1024 (80.95) | 999 (79.03) | 976 (77.28) | 896 (70.94) | |
| Active | 194 (15.35) | 241 (19.05) | 265 (20.97) | 287 (22.72) | 367 (29.06) | |
| Current alcohol consumption (%) | 0.2578 | |||||
| No | 313 (24.74) | 337 (26.62) | 342 (27.04) | 340 (26.86) | 364 (28.77) | |
| Yes | 952 (75.26) | 929 (73.38) | 923 (72.96) | 926 (73.14) | 901 (71.23) | |
| Total energy intake (kcal/day) | 1624.56±401.00 | 1764.89±417.55 | 1842.23±431.57 | 1926.29±442.88 | 2110.85±499.16 | <0.0001 |
| Carbohydrate (E%) | 72.51±7.71 | 71.63±7.10 | 71.00±7.33 | 70.49±6.88 | 70.07±7.09 | <0.0001 |
| Protein (E%) | 12.99±2.41 | 13.33±2.12 | 13.52±2.25 | 13.79±2.26 | 13.97±2.37 | <0.0001 |
| Fat (E%) | 14.50±5.73 | 15.04±5.33 | 15.47±5.48 | 15.73±5.07 | 15.96±5.15 | <0.0001 |
| Dietary fibre intake (g/day) | 3.84±1.12 | 4.72±1.24 | 5.28±1.33 | 5.91±1.50 | 7.42±2.14 | <0.0001 |
| Total flavonoids (mg VCE/day) | 58.16±15.38 | 97.92±10.58 | 135.76±11.78 | 185.20±18.17 | 299.44±77.77 | <0.0001 |
| Anthocyanidins | 11.73±5.14 | 20.53±6.74 | 28.17±9.84 | 37.83±13.24 | 62.90±28.55 | <0.0001 |
| Isoflavones | 8.63±4.74 | 11.56±6.30 | 12.24±6.53 | 13.16±6.86 | 15.96±9.48 | <0.0001 |
| Proanthocyanidins | 23.59±9.01 | 41.24±10.74 | 59.17±14.68 | 80.74±21.27 | 135.10±47.83 | <0.0001 |
| Flavonols | 7.50±4.38 | 10.58±6.08 | 13.18±6.82 | 16.78±9.23 | 24.31±16.28 | <0.0001 |
| Flavones | 0.29±0.15 | 0.45±0.18 | 0.60±0.22 | 0.79±0.29 | 1.19±0.49 | <0.0001 |
| Flavanones | 1.80±1.52 | 3.20±1.96 | 4.56±2.74 | 6.01±3.76 | 10.29±6.33 | <0.0001 |
| Flavan-3-ols | 4.63±4.40 | 10.35±9.70 | 17.84±15.94 | 29.88±23.91 | 49.69±37.80 | <0.0001 |
| TAC (mg VCE/day) | 113.96±32.02 | 185.25±30.65 | 252.44±37.18 | 343.26±51.65 | 542.84±149.10 | <0.0001 |
| Women, n=14 267 | ||||||
| Cases/person-years | 89/11 021.20 | 82/11 303.30 | 76/11 322.20 | 59/11 526.80 | 54/11 650.80 | |
| Age (years) | 52.64±8.04 | 52.31±7.84 | 52.19±7.68 | 52.01±7.23 | 52.18±7.02 | 0.0832 |
| BMI (kg/m2) | 23.44±3.05 | 23.40±2.93 | 23.32±2.85 | 23.27±2.80 | 23.19±2.72 | 0.8879 |
| Smoking status (%) | <0.0001 | |||||
| Never | 2746 (96.32) | 2784 (97.72) | 2788 (97.82) | 2806 (98.39) | 2768 (97.23) | |
| Past | 31 (1.09) | 21 (0.74) | 24 (0.84) | 21 (0.74) | 35 (1.23) | |
| Current | 74 (2.60) | 44 (1.54) | 38 (1.33) | 25 (0.88) | 44 (1.55) | |
| Educational level (%) | <0.0001 | |||||
| Under middle school | 1150 (40.41) | 977 (34.33) | 901 (31.74) | 760 (26.73) | 625 (22.01) | |
| High school | 1236 (43.43) | 1305 (45.85) | 1297 (45.69) | 1368 (48.12) | 1341 (47.23) | |
| College or above | 460 (16.16) | 564 (19.82) | 641 (22.58) | 715 (25.15) | 873 (30.75) | |
| Physical activity (%) | <0.0001 | |||||
| Inactive | 2449 (85.96) | 2355 (82.63) | 2294 (80.46) | 2239 (78.48) | 2120 (74.49) | |
| Active | 400 (14.04) | 495 (17.37) | 557 (19.54) | 614 (21.52) | 726 (25.51) | |
| Current alcohol consumption (%) | 0.2756 | |||||
| No | 1900 (66.60) | 1967 (68.92) | 1932 (67.72) | 1928 (67.55) | 1966 (68.91) | |
| Yes | 953 (33.40) | 887 (31.08) | 921 (32.28) | 926 (32.45) | 887 (31.09) | |
| Total energy intake (kcal/day) | 1429.83±392.78 | 1577.31±420.56 | 1659.22±437.45 | 1761.14±460.69 | 1947.92±497.29 | <0.0001 |
| Carbohydrate (E%) | 71.87±8.06 | 71.25±7.93 | 70.67±7.63 | 70.30±7.36 | 69.97±7.30 | <0.0001 |
| Protein (E%) | 13.39±2.47 | 13.59±2.42 | 13.76±2.38 | 13.89±2.33 | 14.01±2.43 | <0.0001 |
| Fat (E%) | 14.74±6.03 | 15.16±5.91 | 15.57±5.65 | 15.81±5.45 | 16.02±5.33 | <0.0001 |
| Dietary fibre intake (g/day) | 3.80±1.11 | 4.61±1.21 | 5.13±1.30 | 5.97±1.52 | 7.38±2.10 | <0.0001 |
| Total flavonoids (mg VCE/day) | 71.44±17.73 | 116.97±11.49 | 159.39±13.42 | 213.52±19.24 | 333.98±94.24 | <0.0001 |
| Anthocyanidins | 15.24±6.21 | 25.89±8.12 | 35.35±11.02 | 46.82±14.12 | 74.77±32.62 | <0.0001 |
| Isoflavones | 8.63±4.48 | 10.67±5.74 | 11.70±6.42 | 13.28±7.25 | 15.09±9.07 | <0.0001 |
| Proanthocyanidins | 31.11±10.57 | 52.62±11.64 | 73.61±15.79 | 101.51±21.34 | 161.00±54.19 | <0.0001 |
| Flavonols | 8.09±4.63 | 11.18±5.92 | 13.54±7.50 | 16.94±9.30 | 24.05±16.19 | <0.0001 |
| Flavones | 0.35±0.18 | 0.55±0.23 | 0.71±0.28 | 0.89±0.34 | 1.31±0.62 | <0.0001 |
| Flavanones | 2.56±1.91 | 4.37±2.55 | 5.98±3.19 | 7.70±3.84 | 12.62±8.04 | <0.0001 |
| Flavan-3-ols | 5.45±4.85 | 11.68±10 | 18.51±14.74 | 26.38±20.18 | 45.13±36.98 | <0.0001 |
| TAC (mg VCE/day) | 135.61±36.42 | 216.26±31.08 | 289.3±38.71 | 380.21±51.07 | 587.77±176.53 | <0.0001 |
Values are presented as means±SDs or numbers (%). P values were calculated using a generalised linear model for continuous variables and χ2 test for categorical variables. P<0.05 is shown in bold.
Total flavonoid intake was the sum of anthocyanidins, isoflavones, proanthocyanidins, flavonols, flavones, flavanones and flavan-3-ols.
Total antioxidant capacity (TAC) was obtained by combining the individual antioxidant capacity of each antioxidant derived from every food item.
BMI, body mass index; VCE, vitamin C equivalents.
Table 2 shows the range of dietary antioxidant intake by quintiles. The associations between dietary antioxidant intake and the HRs of T2D mellitus are presented in table 3. All participants with the highest total dietary flavonoid intake (Q5) had a lower risk of developing T2D mellitus (men: HR 0.63; 95% CI 0.42 to 0.93; and women: HR 0.54; 95% CI 0.38 to 0.78; both p value for trend <0.05) than those with the lowest flavonoid intake (Q1). Consumption of more flavonols and proanthocyanidins had a protective effect against the development of T2D mellitus in men participants, and consumption of anthocyanidins, proanthocyanidins, flavonols, flavones and flavanones showed a protective effect against T2D mellitus in women participants (all p value for trend <0.05). After estimation of HRs according to quintiles of TAC, the Q5 group of women participants still showed a lower risk of T2D mellitus (HR 0.58; 95% CI 0.40 to 0.83; p value for trend=0.0004) than the Q1 group. However, although the TAC Q5 group of men participants did not show any significant association with T2D mellitus, they had an approximately 15% reduced risk of developing T2D mellitus for an SD increment in TAC (HR 0.85; 95% CI 0.75 to 0.96). After further adjustment for energy per cent from carbohydrate, fat, and protein and dietary fibre intake, the results remained largely unchanged (online supplemental tables S1 and S2).
Table 2.
Range of each dietary antioxidant intake by quintile
| Antioxidant consumption | |||||
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Men | |||||
| Total flavonoids (mg VCE/day) | 58.16 (13.07–80.30) | 97.92 (80.32–116.38) | 135.76 (116.38–156.75) | 185.20 (156.80–221.23) | 299.44 (221.46–1150.79) |
| Anthocyanidins | 9.69 (0.75–14.14) | 17.94 (14.14–21.97) | 26.58 (21.97–31.57) | 38.62 (31.57–46.86) | 68.33 (46.89–378.83) |
| Isoflavones | 5.07 (0.10–6.80) | 8.07 (6.80–9.35) | 10.78 (9.35–12.22) | 14.16 (12.22–16.57) | 23.48 (16.57–94.05) |
| Proanthocyanidins | 21.28 (1.43–31.14) | 39.10 (31.15–47.5) | 56.93 (47.52–67.33) | 81.94 (67.35–99.38) | 140.58 (99.41–723.02) |
| Flavonols | 5.25 (0.69–7.30) | 8.81 (7.30–10.32) | 11.89 (10.33–13.65) | 16.14 (13.65–19.27) | 30.25 (19.27–231.83) |
| Flavones | 0.23 (0.02–0.33) | 0.40 (0.33–0.48) | 0.57 (0.48–0.67) | 0.79 (0.67–0.93) | 1.32 (0.93–4.37) |
| Flavanones | 1.03 (0.00–1.77) | 2.43 (1.77–3.09) | 3.93 (3.09–4.89) | 6.16 (4.89–7.80) | 12.33 (7.80–62.77) |
| Flavan–3–ols | 2.98 (0.10–4.91) | 6.82 (4.92–8.87) | 11.79 (8.87–15.54) | 23.54 (15.56–41.05) | 67.26 (41.06–252.89) |
| TAC (mg VCE/day) | 111.21 (21.57–150.38) | 182.54 (150.45–215.60) | 251.21 (215.61–289.93) | 343.27 (289.98–406.09) | 549.53 (406.14–1811.29) |
| Women | |||||
| Total flavonoids (mg VCE/day) | 71.44 (7.28–96.65) | 116.97 (96.66–136.81) | 159.39 (136.81–183.19) | 213.52 (183.20–250.56) | 333.98 (250.59–1263.46) |
| Anthocyanidins | 12.85 (0.44–18.47) | 23.33 (18.48–28.19) | 34.01 (28.19–40.07) | 47.64 (40.07–56.64) | 80.24 (56.65–372.08) |
| Isoflavones | 4.83 (0.43–6.50) | 7.73 (6.50–8.96) | 10.31 (8.96–11.72) | 13.67 (11.72–16.02) | 22.83 (16.02–85.19) |
| Proanthocyanidins | 28.89 (2.21–41.00) | 50.74 (41.01–60.82) | 72.48 (60.83–84.94) | 101.70 (84.95–121.70) | 166.05 (121.73–633.15) |
| Flavonols | 5.72 (1.00–7.65) | 9.09 (7.65–10.55) | 12.11 (10.55–13.81) | 16.26 (13.81–19.38) | 30.62 (19.38–195.47) |
| Flavones | 0.28 (0.01–0.39) | 0.48 (0.39–0.57) | 0.67 (0.57–0.77) | 0.89 (0.77–1.05) | 1.50 (1.05–7.62) |
| Flavanones | 1.58 (0.00–2.58) | 3.44 (2.58–4.35) | 5.38 (4.35–6.55) | 7.95 (6.55–9.73) | 14.88 (9.73–132.99) |
| Flavan–3–ols | 3.82 (0.25–6.06) | 8.18 (6.06–10.41) | 13.17 (10.41–16.31) | 21.85 (16.31–30.72) | 60.14 (30.72–376.53) |
| TAC (mg VCE/day) | 132.65 (13.04–178.98) | 214.39 (178.99–248.73) | 287.62 (248.74–328.90) | 380.46 (328.94–442.53) | 594.04 (442.54–2240.46) |
Values were presented as mean (min–max).
TAC, total antioxidant capacity; VCE, vitamin C equivalents.
Table 3.
HRs of type 2 diabetes mellitus during follow-up according to antioxidant consumption divided into quintiles, where Q5 represents highest
| Antioxidant consumption | |||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P value for trend | HR for an SD increment | |
| Men | |||||||
| Total flavonoids | Ref | 0.98 (0.71, 1.34) | 0.90 (0.64, 1.26) | 0.91 (0.65, 1.27) | 0.63 (0.42, 0.93) | 0.0169 | 0.85 (0.75, 0.97) |
| Anthocyanidins | Ref | 0.79 (0.56, 1.11) | 0.99 (0.72, 1.36) | 0.86 (0.62, 1.20) | 0.71 (0.50, 1.03) | 0.1167 | 0.87 (0.77, 0.99) |
| Isoflavones | Ref | 1.45 (1.03, 2.06) | 1.12 (0.78, 1.60) | 1.19 (0.83, 1.71) | 1.36 (0.94, 1.97) | 0.3151 | 1.05 (0.94, 1.17) |
| Proanthocyanidins | Ref | 1.04 (0.76, 1.44) | 0.97 (0.70, 1.36) | 0.77 (0.54, 1.09) | 0.72 (0.50, 1.05) | 0.0247 | 0.88 (0.77, 0.99) |
| Flavonols | Ref | 1.49 (1.08, 2.05) | 0.90 (0.63, 1.30) | 0.97 (0.68, 1.38) | 0.82 (0.56, 1.19) | 0.0381 | 0.84 (0.73, 0.97) |
| Flavones | Ref | 1.01 (0.73, 1.41) | 1.01 (0.72, 1.41) | 0.84 (0.59, 1.20) | 0.85 (0.59, 1.22) | 0.2322 | 0.90 (0.80, 1.01) |
| Flavanones | Ref | 0.83 (0.59, 1.17) | 1.23 (0.89, 1.68) | 0.94 (0.67, 1.31) | 0.82 (0.57, 1.18) | 0.3313 | 0.94 (0.83, 1.06) |
| Flavan-3-ols | Ref | 0.89 (0.64, 1.24) | 1.12 (0.81, 1.55) | 0.79 (0.55, 1.12) | 0.75 (0.52, 1.08) | 0.0744 | 0.90 (0.79, 1.01) |
| TAC | Ref | 1.08 (0.78, 1.49) | 0.95 (0.68, 1.33) | 0.87 (0.62, 1.24) | 0.73 (0.50, 1.06) | 0.0448 | 0.85 (0.75, 0.96) |
| Women | |||||||
| Total flavonoids | Ref | 0.90 (0.66, 1.22) | 0.82 (0.60, 1.12) | 0.61 (0.44, 0.87) | 0.54 (0.38, 0.78) | 0.0001 | 0.80 (0.70, 0.90) |
| Anthocyanidins | Ref | 0.91 (0.68, 1.23) | 0.63 (0.45, 0.87) | 0.71 (0.52, 0.99) | 0.56 (0.39, 0.79) | 0.0006 | 0.85 (0.75, 0.97) |
| Isoflavones | Ref | 0.98 (0.71, 1.34) | 0.71 (0.51, 0.99) | 0.81 (0.58, 1.13) | 0.78 (0.56, 1.10) | 0.1353 | 0.92 (0.82, 1.04) |
| Proanthocyanidins | Ref | 0.90 (0.66, 1.23) | 1.04 (0.77, 1.41) | 0.66 (0.47, 0.93) | 0.50 (0.34, 0.72) | <0.0001 | 0.79 (0.70, 0.90) |
| Flavonols | Ref | 0.83 (0.61, 1.12) | 0.68 (0.49, 0.94) | 0.57 (0.41, 0.80) | 0.61 (0.43, 0.86) | 0.0040 | 0.88 (0.77, 1.00) |
| Flavones | Ref | 0.81 (0.60, 1.10) | 0.74 (0.54, 1.01) | 0.54 (0.38, 0.76) | 0.56 (0.39, 0.79) | 0.0003 | 0.81 (0.71, 0.93) |
| Flavanones | Ref | 0.73 (0.54, 0.98) | 0.56 (0.41, 0.77) | 0.57 (0.41, 0.78) | 0.54 (0.39, 0.76) | 0.0005 | 0.83 (0.73, 0.95) |
| Flavan-3-ols | Ref | 0.83 (0.60, 1.13) | 0.79 (0.57, 1.08) | 0.64 (0.45, 0.90) | 0.79 (0.57, 1.10) | 0.3681 | 0.93 (0.82, 1.05) |
| TAC | Ref | 0.82 (0.60, 1.11) | 0.86 (0.64, 1.17) | 0.51 (0.36, 0.73) | 0.58 (0.40, 0.83) | 0.0004 | 0.81 (0.71, 0.92) |
Results are presented as the HR for an SD increment in dietary antioxidant capacity using a Cox model.
The multivariable Cox proportional hazards regression model was adjusted for age, body mass index (BMI), educational level, physical activity, drinking status, smoking status and total energy intake.
Total flavonoid intake was the sum of anthocyanidins, isoflavones, proanthocyanidins, flavonols, flavones, flavanones and flavan-3-ols.
Total antioxidant capacity (TAC) was obtained by combining the individual antioxidant capacity of each antioxidant derived from every food item.
bmjopen-2022-065073supp001.pdf (76KB, pdf)
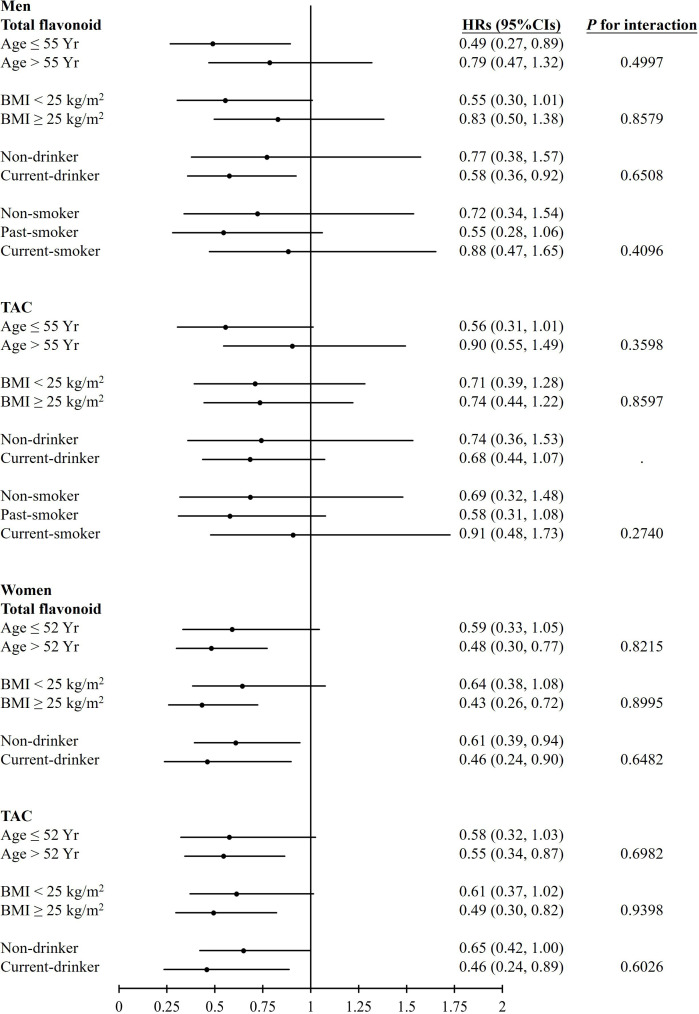
We also performed stratified analyses according to age, BMI, drinking status for both sexes and smoking status for men participants. Figure 1 shows the HRs of T2D mellitus in the Q5 and Q1 groups according to baseline age, baseline BMI and alcohol drinking status in the HEXA study. There was almost no significant association between T2D mellitus and dietary intake of antioxidant components in men participants. However, total flavonoid intake and dietary TAC showed a protective effect against the development of T2D mellitus in women participants who were aged >52 years, had a BMI ≥25 kg/m2 and regardless of alcohol consumption.
Figure 1.
HRs with 95% CIs for type 2 diabetes mellitus after comparison of antioxidant consumption in the Q5 and Q1 groups according to baseline age, baseline body mass index (BMI), alcohol consumption and smoking status in the Health Examinees study. TAC, total antioxidant capacity.
Discussion
In this study, we discovered that dietary total flavonoid consumption and TAC are both associated with a reduced risk of developing T2D mellitus. After further analysis stratified according to age and BMI, we found that dietary total flavonoid consumption and TAC had a protective effect against the development of T2D mellitus in women participants who were overweight or aged >52 years.
Oxidative stress, which is an imbalance between the production of reactive oxygen species (free radicals) and antioxidant defence mechanism, is a risk factor for T2D.17 Previous studies have shown that oxidative stress impairs the secretion of insulin by pancreatic beta cells and interferes with the insulin signalling pathway, thereby accelerating the development and progression of T2D by increasing insulin resistance.2 3 6 32 33 Oxidative stress can be regulated by antioxidants, which react with reactive oxygen species.34 The consumption of dietary flavonoids has been shown to be associated with lower incidences of T2D. Several previous studies have indicated that flavonoids decrease plasma glucose levels and improve lipid profile, insulin secretion and insulin resistance, factors which are implicated in the development of T2D.2 3 8 35 In a previous study, higher flavonol intake was associated with a 26% lower incidence of T2D.36 In addition, the authors observed a marginally significant inverse association between flavan-3-ol intake and the risk of T2D, but there was no association with anthocyanin intake.36 Knekt et al37 reported a marginally significant inverse association between the intake of the flavonols quercetin and myricetin, but not kaempferol, and the incidence of T2D in Finnish men and women. Quercetin, in particular, is known to decrease plasma glucose concentration, improve insulin concentration, preserve the integrity of pancreatic beta cells, alleviate T2D symptoms and reduce hepatic gene expression in streptozotocin-induced diabetic models.38 Flavan-3-ol and isoflavone intake is associated with a reduced risk of T2D and improved insulin resistance and serum insulin concentrations.39 Dietary flavone intake is negatively associated with systolic blood pressure, triglyceride level, triglyceride/high-density lipoprotein-cholesterol level and homeostatic model assessment of insulin resistance. Flavone intake may have some beneficial effects in the reduction of the prevalence of T2D in South Korean women.8 Consumption of foods rich in anthocyanins, particularly blueberries, apples and pears, is also inversely associated with the risk of T2D in the USA.40
A key potential mechanism for the protective effect of flavonoids against T2D is the protection of tissues from free oxygen radicals and lipid peroxidation through their antioxidant activity.41 In addition, anti-inflammatory functions, improvement of endothelial functions, reduction of blood cholesterol concentration and nicotinamide adenine dinucleotide phosphate oxidase activity are also associated with a reduced risk of T2D mellitus.41 Flavonoids are known to interact with molecular targets and affect NF-κB and MAPK signalling pathways.42 Furthermore, flavonoids modulate postprandial glucose levels by reducing the activities of digestive enzymes (α-amylase and α-glucosidase), decreasing the active transport of glucose across the intestinal brush border membrane and inhibiting glucose transporters.32 Antioxidant-rich fruits and vegetables contain relatively high fibre content, which can influence the beneficial effects of antioxidants against T2D.43 Furthermore, it has been reported that flavonoids inhibit α-glucosidase activity to alleviate hyperglycaemia.44 45
The effect of each type of flavonoid intake on the risk of T2D varies by sex. In this study, there was a correlation between anthocyanidin and proanthocyanidin intake and the risk of developing T2D in men. However, there was a greater correlation between the risk of T2D and intake of flavonoids, such as anthocyanidins, proanthocyanidins, flavonols, flavones and flavanones, in women than in men. These sex-specific results are often seen in other phytochemical-related studies. In a previous study conducted using 2008–2011 data from the Korea National Health and Nutrition Examination Survey, a high intake of flavonoids did not reduce the incidence of obesity and abdominal obesity in men but significantly reduced the obesity (18%) in women. In addition, high flavonoid intake was reported to reduce the incidence of abdominal obesity (19%) in that study.2 46 47 The variations in these results appear to be due to differences between the dietary intake patterns of South Korean men and women. Sex-specific dietary patterns have been reported in previous studies; namely men consume the recommended amount of vegetables more than women, whereas women consume the recommended amount of fruits more than men.48 In addition, women generally consume higher amounts of dietary antioxidants than men.2 49 Higher intake of dietary antioxidants can induce high plasma concentrations of antioxidants and more beneficial effects on preventing development of T2D. Furthermore, gonadal hormones (menopausal oestrogen and testosterone) have been implicated in sex-specific differences in glucose homeostasis.50 Healthy women have lower skeletal muscle mass, higher adipose tissue mass, more circulating free fatty acids and higher intramyocellular lipid content than men of the same age. These are all factors that could promote increased insulin resistance in women compared with men.50
Various factors, such as age and lifestyle, are known to contribute to the development and progression of T2D.17 The prevalence of T2D in South Koreans increases rapidly with age.4 Unhealthy lifestyle habits, such as smoking, excessive alcohol consumption and inactivity, are known to contribute to the development of diabetes.3 51 We found that men with T2D were older and more likely to be current drinkers and current smokers than those without diabetes. However, our stratified analysis showed that there was no correlation between these factors except for current alcohol consumption. On the other hand, women with T2D were significantly older and had significantly higher BMI than those without diabetes. Furthermore, the stratified analysis showed that antioxidant consumption was inversely related to the HR of T2D in older women (>52 years), women with a BMI >25 kg/m2, regardless of alcohol consumption. Although it is difficult to fully explain these variations in South Korean adults, these findings suggest that high antioxidant intake may be related to a decreased risk of T2D, especially in women with specific lifestyle habits.
Strengths and limitations
The main strength of this study was that it was conducted using a large-scale community-based genomic cohort study with 5 years of follow-up on average. Stratified analyses were conducted in the current study to focus on one certain exposure. This study had some limitations. First, although this study reported a longitudinal relationship between dietary antioxidant consumption and T2D incidence, we did not assess the causality. Second, we obtained dietary information and information on the intake of antioxidant components using self-reported FFQ; thus, dietary measurement errors were inevitable. However, the 106-item FFQ has been previously verified.26 In addition, further studies are needed to measure the flavonoid concentration to verify the data. Third, we did not quantify the amount of alcohol consumption and smoking. Nevertheless, we found no association between smoking status and T2D after stratification analyses. Dietary antioxidants showed a protective effect against the development of T2D only in women who were non-drinkers.
Conclusions
The findings of this large-scale prospective cohort study suggest that dietary antioxidant consumption is associated with a lower risk of T2D in South Korean adults. The findings of this study can serve as a reference or guide for the modification of food intake recommendations in dietary guideline policies in South Korea. However, further studies are needed to validate the results of this study.
Supplementary Material
Acknowledgments
This study was performed using data from the HEXA study, which was supported by the National Genome Research Institute, Korea Centers for Disease Control and Prevention.
Footnotes
L-JT and SBH contributed equally.
Contributors: SS supervised the project. SS contributed to the conceptualisation or design of this study. SJ and HJ contributed to establishing the antioxidants database. L-JT conducted the formal analysis. SS verified and validated the outcomes. L-JT and SBH cowrote the first draft of the manuscript. SJ and HJ reviewed and revised the article critically. All authors approved the final version of the article for publication. SS and L-JT had full access to all the data in the study and take full responsibility for the integrity of the data and accuracy of the data analysis. SS is acting as guarantor.
Funding: This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (Ministry of Science and ICT, MSIT) (No 2022R1F1A1074279). This research was supported by the Chung-Ang University Young Scientist Scholarship (CAYSS) in 2021.
Disclaimer: The study sponsor/funder was not involved in the design of the study; the collection, analysis and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study are available from the National Genome Research Institute, Korea Centers for Disease Control and Prevention. However, restrictions apply to the availability of these data, which were used under licence for this study, and as such are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Genome Research Institute, Korea Centers for Disease Control and Prevention.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Institutional Review Board (IRB) of the Ethics Committee of the Korean Genome and Epidemiology Study of the Korea National Institute of Health (IRB: No. E-1503-103-657). Participants gave informed consent to participate in the study before taking part.
References
- 1.Bacanli M, Dilsiz SA, Başaran N, et al. Effects of phytochemicals against diabetes. Adv Food Nutr Res 2019;89:209–38. 10.1016/bs.afnr.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Yeon J-Y, Bae YJ, Kim E-Y, et al. Association between flavonoid intake and diabetes risk among the Koreans. Clin Chim Acta 2015;439:225–30. 10.1016/j.cca.2014.10.042 [DOI] [PubMed] [Google Scholar]
- 3.Quansah D, Ha K, Jun S, et al. Associations of dietary antioxidants and risk of type 2 diabetes: data from the 2007–2012 Korea National health and nutrition examination survey. Molecules 2017;22:1664. 10.3390/molecules22101664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BY, Won JC, Lee JH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J 2019;43:487–94. 10.4093/dmj.2019.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Yu TY. Diabetes and sarcopenia. J Korean Diabetes 2017;18:239–47. 10.4093/jkd.2017.18.4.239 [DOI] [Google Scholar]
- 6.Burton-Freeman B, Brzeziński M, Park E, et al. A selective role of dietary anthocyanins and flavan-3-ols in reducing the risk of type 2 diabetes mellitus: a review of recent evidence. Nutrients 2019;11:841. 10.3390/nu11040841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma G, Prakash D, Gupta C. Phytochemicals of nutraceutical importance: do they defend against diseases. In: Prakash D, Sharma G, eds. Phytochemicals of nutraceutical importance. Nosworthy Way: CABI, 2014: 1–24. [Google Scholar]
- 8.Oh JS, Kim H, Vijayakumar A, et al. Association of dietary flavonoid intake with prevalence of type 2 diabetes mellitus and cardiovascular disease risk factors in Korean women aged ≥30 years. J Nutr Sci Vitaminol 2017;63:51–8. 10.3177/jnsv.63.51 [DOI] [PubMed] [Google Scholar]
- 9.Kawser Hossain M, Abdal Dayem A, Han J, et al. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int J Mol Sci 2016;17:569. 10.3390/ijms17040569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci 2016;5:e47. 10.1017/jns.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun S, Shin S, Joung H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br J Nutr 2016;115:480–9. 10.1017/S0007114515004006 [DOI] [PubMed] [Google Scholar]
- 12.Jafari Azad B, Yaseri M, Daneshzad E, et al. Interaction between Apo A-II -265T>C polymorphism and dietary total antioxidant capacity on some anthropometric indices and serum lipid profile in patients with type 2 diabetes mellitus. J Nutr Sci 2021;10:e9. 10.1017/jns.2020.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi S, Xin Y, Guo Y, et al. Ampelopsin reduces endotoxic inflammation via repressing ROS-mediated activation of PI3K/Akt/NF-κB signaling pathways. Int Immunopharmacol 2012;12:278–87. 10.1016/j.intimp.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev 2016;2016:5276130. 10.1155/2016/5276130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran V, Saravanan R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum Exp Toxicol 2015;34:884–93. 10.1177/0960327114561663 [DOI] [PubMed] [Google Scholar]
- 16.Li S-S, Wu J, Chen L-G, et al. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in Lotus (Nelumbo nucifera). PLoS One 2014;9:e108860. 10.1371/journal.pone.0108860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halim M, Halim A. The effects of inflammation, aging and oxidative stress on the pathogenesis of diabetes mellitus (type 2 diabetes). Diabetes Metab Syndr 2019;13:1165–72. 10.1016/j.dsx.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 18.Kim S-A, Kim J, Jun S, et al. Association between dietary flavonoid intake and obesity among adults in Korea. Appl Physiol Nutr Metab 2020;45:203–12. 10.1139/apnm-2019-0211 [DOI] [PubMed] [Google Scholar]
- 19.Kim S-A, Joung H, Shin S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr J 2019;18:37. 10.1186/s12937-019-0459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Ham J-O, Lee B-K. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 2015;31:111–8. 10.1016/j.nut.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 21.Kim S, Song Y, Lee JE, et al. Total antioxidant capacity from dietary supplement decreases the likelihood of having metabolic syndrome in Korean adults. Nutrients 2017;9:1055. 10.3390/nu9101055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jafari Azad B, Yaseri M, Daneshzad E, et al. Interaction between Apo A-II -265T > C polymorphism and dietary total antioxidant capacity on some oxidative stress and inflammatory markers in patients with type 2 diabetes mellitus. Br J Nutr 2021:1–17. 10.1017/S0007114521002993 [DOI] [PubMed] [Google Scholar]
- 23.Daneshzad E, Keshavarz S-A, Qorbani M, et al. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: a cross-sectional study among diabetic women. Clin Nutr ESPEN 2020;37:187–94. 10.1016/j.clnesp.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 24.Health Examinees Study Group . The health Examinees (HEXA) study: rationale, study design and baseline characteristics. Asian Pac J Cancer Prev 2015;16:1591–7. 10.7314/apjcp.2015.16.4.1591 [DOI] [PubMed] [Google Scholar]
- 25.Shin S, Lim J, Lee H-W, et al. Association between the prevalence of metabolic syndrome and coffee consumption among Korean adults: results from the health Examinees study. Appl Physiol Nutr Metab 2019;44:1371–8. 10.1139/apnm-2018-0880 [DOI] [PubMed] [Google Scholar]
- 26.Ahn Y, Kwon E, Shim JE, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007;61:1435–41. 10.1038/sj.ejcn.1602657 [DOI] [PubMed] [Google Scholar]
- 27.Jun S, Chun OK, Joung H. Estimation of dietary total antioxidant capacity of Korean adults. Eur J Nutr 2018;57:1615–25. 10.1007/s00394-017-1447-6 [DOI] [PubMed] [Google Scholar]
- 28.Ko S-H, Kim S-R, Kim D-J, et al. 2011 clinical practice guidelines for type 2 diabetes in Korea. Diabetes Metab J 2011;35:431–6. 10.4093/dmj.2011.35.5.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korea Disease Control and Prevention Agency . Obesity, 2021. Available: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do [Accessed 06 Jan 2021].
- 30.Shin S, Lee H-W, Kim CE, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the health Examinees study. Nutrients 2017;9:687. 10.3390/nu9070687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratjen I, Schafmayer C, Enderle J, et al. Health-Related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Cancer 2018;18:1156. 10.1186/s12885-018-5075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Mangelinckx S, Adams A, et al. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat Prod Commun 2015;10:1934578X1501000–200. 10.1177/1934578X1501000140 [DOI] [PubMed] [Google Scholar]
- 33.Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci 2016;23:87. 10.1186/s12929-016-0303-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ham D, Jun S, Kang M, et al. Consumption of Korean foods with high flavonoid contents reduces the likelihood of having elevated C-reactive protein levels: data from the 2015–2017 Korea National health and nutrition examination survey. Nutrients 2019;11:2370. 10.3390/nu11102370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y-J, Zhan J, Liu X-L, et al. Dietary flavonoids intake and risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Clin Nutr 2014;33:59–63. 10.1016/j.clnu.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 36.Jacques PF, Cassidy A, Rogers G, et al. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. 10.3945/jn.113.177212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knekt P, Kumpulainen J, Järvinen R, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560–8. 10.1093/ajcn/76.3.560 [DOI] [PubMed] [Google Scholar]
- 38.Dhanya R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed Pharmacother 2022;146:112560. 10.1016/j.biopha.2021.112560 [DOI] [PubMed] [Google Scholar]
- 39.Curtis PJ, Sampson M, Potter J, et al. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35:226–32. 10.2337/dc11-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiland H, Slavin J. Systematic review of pears and health. Nutr Today 2015;50:301–5. 10.1097/NT.0000000000000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain T, Tan B, Murtaza G, et al. Flavonoids and type 2 diabetes: evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol Res 2020;152:104629. 10.1016/j.phrs.2020.104629 [DOI] [PubMed] [Google Scholar]
- 42.Mansuri ML, Parihar P, Solanki I, et al. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr 2014;9:400. 10.1007/s12263-014-0400-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P-Y, Fang J-C, Gao Z-H, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. J Diabetes Investig 2016;7:56–69. 10.1111/jdi.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YQ, Zhou FC, Gao F, et al. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J Agric Food Chem 2009;57:11463–8. 10.1021/jf903083h [DOI] [PubMed] [Google Scholar]
- 45.Liu S, Li D, Huang B, et al. Inhibition of pancreatic lipase, α-glucosidase, α-amylase, and hypolipidemic effects of the total flavonoids from Nelumbo nucifera leaves. J Ethnopharmacol 2013;149:263–9. 10.1016/j.jep.2013.06.034 [DOI] [PubMed] [Google Scholar]
- 46.Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. 10.3945/ajcn.111.028894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Im J, Kim M, Park K. Association between the phytochemical index and lower prevalence of Obesity/Abdominal obesity in Korean adults. Nutrients 2020;12:2312. 10.3390/nu12082312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee H-S, Cho Y-H, Park J, et al. Dietary intake of phytonutrients in relation to fruit and vegetable consumption in Korea. J Acad Nutr Diet 2013;113:1194–9. 10.1016/j.jand.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 49.Kim YJ, Park MY, Chang N, et al. Intake and major sources of dietary flavonoid in Korean adults: Korean National health and nutrition examination survey 2010-2012. Asia Pac J Clin Nutr 2015;24:456–63. 10.6133/apjcn.2015.24.3.04 [DOI] [PubMed] [Google Scholar]
- 50.Mauvais-Jarvis F. Gender differences in glucose homeostasis and diabetes. Physiol Behav 2018;187:20–3. 10.1016/j.physbeh.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Diabetes Association, Bantle JP, Wylie-Rosett J, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American diabetes association. Diabetes Care 2008;31 Suppl 1:S61–78. 10.2337/dc08-S061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-065073supp001.pdf (76KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data that support the findings of this study are available from the National Genome Research Institute, Korea Centers for Disease Control and Prevention. However, restrictions apply to the availability of these data, which were used under licence for this study, and as such are not publicly available. Data are however available from the authors upon reasonable request and with permission of the National Genome Research Institute, Korea Centers for Disease Control and Prevention.