Abstract
Introduction
HIV incidence remains high among African adolescent girls and young women (AGYW). The primary objective of this study is to assess pre‐exposure prophylaxis (PrEP) initiation, use, persistence and HIV acquisition among African AGYW offered PrEP in order to inform PrEP scale‐up.
Methods
POWER was a prospective implementation science evaluation of PrEP delivery for sexually active HIV‐negative AGYW ages 16–25 in family planning clinics in Kisumu, Kenya and youth and primary healthcare clinics in Cape Town and Johannesburg, South Africa. Follow‐up visits occurred at month 1 and quarterly for up to 36 months. PrEP users were defined based on the month 1 refill. PrEP persistence through month 6 was assessed using Kaplan–Meier survival analysis among AGYW with a month 1 visit, defining non‐persistence as an ≥15 day gap in PrEP availability for daily dosing. PrEP execution was evaluated in a subset with PrEP supply from the prior visit sufficient for daily dosing by measuring blood tenofovir diphosphate (TFV‐DP) levels.
Results
From June 2017 to September 2020, 2550 AGYW were enrolled (1000 in Kisumu, 787 in Cape Town and 763 in Johannesburg). Median age was 21 years, 66% had a sexual partner of unknown HIV status, and 29% had chlamydia and 10% gonorrhoea. Overall, 2397 (94%) initiated PrEP and 749 (31%) had a refill at 1 month. Of AGYW who could reach 6 months of post‐PrEP initiation follow‐up, 128/646 (20%) persisted with PrEP for 6 months and an additional 92/646 (14%) had a gap and restarted PrEP. TFV‐DP levels indicated that 47% (91/193) took an average of ≥4 doses/week. Sixteen HIV seroconversions were observed (incidence 2.2 per 100 person‐years, 95% CI 1.2, 3.5); 13 (81%) seroconverters either did not have PrEP dispensed in the study interval prior to seroconversion or TFV‐DP levels indicated <4 doses/week in the prior 6 weeks.
Conclusions
In this study of PrEP integration with primary care and reproductive health services for African AGYW, demand for PrEP was high. Although PrEP use decreased in the first months, an important fraction used PrEP through 6 months. Strategies are needed to simplify PrEP delivery, support adherence and offer long‐acting PrEP options to improve persistence and HIV protection.
Keywords: HIV, pre‐exposure prophylaxis, adherence, persistence, young women, Africa
1. INTRODUCTION
African adolescent girls and young women (AGYW) have had HIV incidence rates of 4% in recent HIV prevention research cohorts [1, 2, 3]. Oral tenofovir‐based pre‐exposure prophylaxis (PrEP) confers >90% protection against HIV when taken with high but not perfect adherence [4, 5, 6, 7]. Beginning in 2016 when Kenya and South Africa incorporated PrEP into HIV guidelines [8, 9, 10], PrEP access has increased for African AGYW.
Given the critical need for primary HIV prevention in this population, models of PrEP delivery are needed that reach AGYW and that foster PrEP use, specifically initiation, use (or execution) and persistence [11]. AGYW have trouble accessing reproductive healthcare due to transportation, long wait times, understaffed clinics and stigma from healthcare providers and community members about sexual activity among young, unmarried women [12, 13]. Family planning clinics, youth‐friendly clinics and mobile clinics provide sexually active youth with sexual and reproductive health (SRH) services, but little is known about whether AGYW will initiate and persist with PrEP in these settings.
The Prevention Options for Women Evaluation Research (POWER) study in Kenya and South Africa evaluated PrEP delivery models for African AGYW in diverse health settings, with the objective to assess PrEP initiation, use/execution and persistence when PrEP was offered as a routine service with minimal research procedures.
2. METHODS
2.1. Study population
From June 2017 through September 2020, AGYW in Kisumu, Kenya, and Cape Town and Johannesburg, South Africa were recruited (ClinicalTrials.gov NCT03490058) through peer outreach, social media and clinics. At enrolment, women were offered sexually transmitted infection (STI) testing and treatment, HIV testing, contraception and PrEP. Women were eligible if they were HIV negative by rapid tests, had vaginal intercourse in the prior 3 months and were interested in PrEP, PrEP initiation was not a requirement for enrolment. In Kisumu and Cape Town, women were eligible if they were between 16 and 25 years of age. In Johannesburg, if they were 18–25 years and urine pregnancy tests were negative.
The POWER study was implemented in facility‐based clinics and a mobile van providing reproductive health services to AGYW seeking family planning, SRH services and primary care, from which lessons about PrEP delivery could be adapted for programmatic scale up. Participating sites were family planning clinics in Kisumu (Kisumu Medical and Education Trust, KMET, and Jaramogi Oginga, Odinga Teaching and Referral Hospital, JOOTRH), the Tutu Teen Truck in townships near Cape Town [14], the Weltevreden public primary healthcare clinic in Cape Town and a youth‐friendly primary healthcare clinic (Ward 21) and public primary healthcare clinic (Jeppestown) in Johannesburg.
2.2. Study procedures
Women were counselled about risk reduction and HIV prevention and given the option to initiate PrEP. Women could decline PrEP or opt to stop PrEP at any time, and those who stopped PrEP could restart. Follow‐up occurred at 1 month after enrolment (or when PrEP was initiated, if after enrolment) and then quarterly for up to 36 months. Women were reminded of visits through text messages, WhatsApp messages or phone calls. Women were not provided travel reimbursement or incentives for participation and field‐based retention activities were not conducted, as programmatic HIV prevention settings do not provide compensation or active outreach.
Data collected at enrolment were limited to what is obtained in PrEP delivery programs, which did not include age of male partners. Enrolment data were used to calculate the modified VOICE HIV risk score with a maximum possible modified VOICE risk score of 8; a VOICE score of 5 or higher was associated with an annual HIV incidence of 6% in prior cohorts [15].
2.2.1. Laboratory testing
Rapid HIV testing was performed at PrEP initiation and follow‐up visits with reactive tests confirmed by local laboratories. HIV resistance testing on samples from seroconverters was performed using a commercial (ThermoFisher Scientific) or validated HIV‐1 population genotyping assay with a partial reverse transcriptase gene region [16]. Chlamydia trachomatis and Neisseria gonorrhoeae nucleic acid amplification testing was performed by local laboratories at enrolment and 6 months. At PrEP initiation, hepatitis B surface antigen and creatinine clearance was measured using the Cockcroft–Gault equation. Urine pregnancy testing was performed when clinically indicated in the Kenyan sites and at all PrEP dispensation visits in the South African sites, following national guidelines.
PrEP was withheld from women who had a reactive rapid HIV test, decreased creatinine clearance (<50 ml/minute in Kenya and <60 ml/minute in South Africa) and for South African participants who became pregnant, consistent with national guidelines at the time. Women could restart PrEP if confirmatory testing showed they were HIV negative, their estimated creatinine clearance returned to normal or after pregnancy. Women could take PrEP while breastfeeding.
Dried blood spots (DBS) for measurement of intracellular tenofovir diphosphate (TFV‐DP) were collected at enrolment and all follow‐up visits, but due to women's dislike of phlebotomy was changed to the 6 months visit after PrEP initiation when creatinine was measured to avoid an additional blood draw. Participants who were not comfortable with phlebotomy could decline DBS. DBS samples were obtained from HIV seroconverters at the first HIV‐positive visit. Intracellular TFV‐DP levels provide a measure of average PrEP adherence in the prior 6 weeks [17]. TFV‐DP levels were quantified at the University of Colorado, Denver, using an updated validated extraction procedure [18]. DBS thresholds were established from directly observed FTC‐TDF dosing; TFV‐DP levels of ≥1450 fmol/punch associated with seven doses per week, 800–1449 fmol/punch with 4–6 doses per week, 400–799 fmol/punch with 2–3 doses/per week and <400 for fmol/punch with <2 doses per week.
For seroconverters who did not have a DBS sample, plasma tenofovir levels were measured using a validated ultra‐performance liquid chromatographic, tandem mass spectrometry assay with linear values from 0.5 to 500 ng/ml, and lower limit of quantification of 0.5 ng/ml. Plasma TFV >40 ng/ml was correlated with 90% efficacy in the Partners PrEP Study [19].
2.3. Ethical review
The study was approved by ethics review committees at the Kenya Medical Research Institute, JOOTRH Independent Ethics Review Committee, University of Cape Town, University of Witwatersrand and University of Washington. Participants provided written informed consent in English or local languages. Parental consent was waived for participants ages 16–17 years in Kisumu and Cape Town.
2.4. Statistical analysis
The goal was to enrol 500 participants per site and up to 1000 participants per country, providing diversity by site and country with respect to PrEP delivery. Formal sample size calculations were not conducted. Descriptive analyses assessed participant demographic and behavioural characteristics by site. PrEP initiation was defined as receiving a bottle with 30 tablets at enrolment and PrEP use at 1 month as attending a visit 1 month post‐PrEP initiation and receiving a 90 day refill. PrEP persistence was assessed among women receiving a PrEP refill at 1 month; non‐persistence was defined as a gap of ≥15 days between the refill return date and the next PrEP dispensation (assuming daily dosing prior to the next PrEP dispensation). Six months is a typical period for evaluation of PrEP adherence and persistence in PrEP demonstration projects among African AGYW who have low risk perception and dynamic partnerships. PrEP persistence was defined as persistent use through 6 months and PrEP refill at the 6 month visit. The cumulative probability of PrEP persistence during the 6 months after PrEP initiation was modelled using the Kaplan–Meier estimator of the survival function. Analysis of the predictors of PrEP persistence beyond 6 months used multivariable Poisson regression, including variables significant at a p‐value<0.2 in the univariate analysis. The modified VOICE risk score was included as a categorical variable operationalized as 0–4, 5–6, 7–8 or missing.
PrEP availability was estimated for maximal coverage as the doses that could have been taken given the number of PrEP refills dispensed and the number of days of follow‐up. For PrEP execution, intracellular TFV‐DP was assessed from visits 3 or 6 months after enrolment. A subset of 11% samples were randomly selected from the two sites, which sent all DBS samples collected were tested, as well as all 33% samples sent from the third site.
All descriptive statistics and the Kaplan–Meier curve were generated in SAS 9.4 (SAS/STAT 14.1, Cary, NC, USA). Regression models for PrEP persistence were generated using R version 4.0.3.
3. RESULTS
3.1. Study participants
Between June 2017 and September 2020, 2550 AGYW were enrolled: 1000 from Kisumu, 787 from Cape Town and 763 from Johannesburg (Table 1). Their median age was 21 years (interquartile range, IQR 19–23), and all had a current partner, 66% of whom AGYW were reported to have unknown HIV status and 4% to be living with HIV. Seventy‐eight percent reported not knowing if their partner had other partners and 22% that their partner had other partners. Almost half (47%) were using contraception at enrolment, of whom 8% used oral contraception, 51% injectable contraception (depot medroxyprogesterone acetate or norethisterone enantate) and 27% a contraceptive implant. At enrolment, although only 7% reported STI symptoms, 29% were diagnosed with C. trachomatis and 10% with N. gonorrhoeae. Among those diagnosed with C. trachomatis or N. gonorrhoeae, STI symptoms were reported by 23% (179/770).
Table 1.
Baseline demographic and behavioural characteristics of POWER participants
| Kenya | South Africa | ||||||
|---|---|---|---|---|---|---|---|
| Kisumu | Cape Town | Johannesburg | |||||
| Characteristic | Total (n = 2550) | JOOTRH (n = 496) | KMET (n = 504) | TTT (n = 681) | Weltevreden (n = 106) | Jeppestown (n = 399) | Ward 21 (n = 364) |
| Age (years), median (IQR) | 21 (19–23) | 21 (19–23) | 21 (19–23) | 20 (18–22) | 19.5 (18–22) | 21 (20–23) | 21 (19–22.5) |
| Marital status a | |||||||
| Single, no partner | 40 (2%) | 4 (<1%) | 10 (2%) | 12 (2%) | 4 (4%) | 4 (1%) | 6 (2%) |
| Single, has partner | 2154 (85%) | 312 (63%) | 355 (70%) | 661 (97%) | 101 (95%) | 381 (96%) | 344 (95%) |
| Married; husband's only wife | 330 (13%) | 167 (34%) | 130 (26%) | 7 (1%) | 1 (<1%) | 12 (3%) | 13 (4%) |
| Sexual partners, past 3 months | |||||||
| Current N, median (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| HIV‐positive sex partner | 106 (4%) | 35 (7%) | 14 (3%) | 26 (4%) | 3 (3%) | 14 (4%) | 14 (4%) |
| Sex partner, unknown HIV status | 1672 (66%) | 302 (61%) | 305 (61%) | 555 (82%) | 90 (85%) | 227 (57%) | 193 (53%) |
| Knows or thinks partner has other partners | 549 (22%) | 90 (18%) | 178 (36%) | 134 (20%) | 14 (13%) | 68 (17%) | 65 (18%) |
| Condom use | |||||||
| Never | 689 (27%) | 219 (44%) | 166 (33%) | 137 (20%) | 28 (26%) | 97 (24%) | 42 (12%) |
| Sometimes | 1509 (59%) | 233 (47%) | 285 (57%) | 449 (66%) | 60 (57%) | 239 (60%) | 243 (67%) |
| Always | 341 (13%) | 42 (9%) | 49 (10%) | 91 (13%) | 18 (17%) | 62 (16%) | 79 (22%) |
| Any alcohol use in the past 3 months | 1056 (41%) | 38 (8%) | 69 (14%) | 514 (76%) | 78 (74%) | 173 (43%) | 184 (51%) |
| Drinks per week, median (IQR) | 3 (1–5) | 1 (1–1) | 1 (1–2.5) | 4 (2–5) | 5 (4–5) | 2 (1–5) | 1.5 (1–4) |
| STI diagnosis | |||||||
| Chlamydia trachomatis | 667 (29%) | 77 (16%) | 95 (19%) | 259 (41%) | 55 (52%) | 113 (35%) | 68 (27%) |
| Neisseria gonorrhoea | 221 (10%) | 26 (5%) | 35 (7%) | 100 (16%) | 21 (20%) | 25 (8%) | 14 (6%) |
| STI symptoms | 179 (7%) | 37 (7%) | 82 (16%) | 27 (4%) | 6 (6%) | 8 (2%) | 19 (5%) |
| HIV risk | |||||||
| Modified VOICE risk score b ; median (IQR) | 6 (5–7) | 6 (4–6) | 6 (4–6) | 7 (6–8) | 7 (7–8) | 6 (5–7) | 6 (5–7) |
| Contraceptive use | |||||||
| Any | 1208 (47%) | 181 (36%) | 248 (49%) | 324 (48%) | 56 (53%) | 192 (48%) | 207 (57%) |
| Oral | 92 (8%) | 4 (2%) | 8 (3%) | 11 (3%) | 2 (4%) | 37 (19%) | 30 (15%) |
| Injectable | 604 (51%) | 34 (19%) | 66 (27%) | 248 (77%) | 45 (80%) | 97 (51%) | 114 (58%) |
| Implant | 317 (27%) | 86 (48%) | 140 (57%) | 44 (14%) | 8 (14%) | 26 (14%) | 13 (7%) |
| IUD | 34 (3%) | 12 (7%) | 8 (3%) | 6 (2%) | 0 (0%) | 1 (<1%) | 7 (4%) |
| Condoms | 191 (16%) | 45 (25%) | 25 (10%) | 13 (4%) | 1 (2%) | 28 (15%) | 79 (40%) |
| Ever pregnant | 1213 (48%) | 238 (48%) | 291 (58%) | 221 (33%) | 29 (27%) | 276 (70%) | 158 (43%) |
| Currently pregnant | 162 (6%) | 129 (26%) | 31 (6%) | 2 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Laboratory screening for PrEP | |||||||
| Creatinine clearance ml/minute, median (IQR) | 135 (110–164) | 130 (111–154) | 97 (83–113) | 152 (128–179) | 152 (126–183) | 144 (122–176) | 143 (122–174) |
| Creatinine clearance >60 ml/minute | 2344 (99%) | 427 (100%) | 429 (97%) | 643 (100%) | 103 (100%) | 380 (100%) | 362 (100%) |
| Hepatitis B surface Ag positive | 14 (<1%) | 5 (1%) | 4 (<1%) | 3 (<1%) | 1 (<1%) | 0 | 1 (<1%) |
| Initiated PrEP c | 2397 (94%) | 440 (89%) | 459 (91%) | 659 (97%) | 103 (97%) | 379 (95%) | 357 (98%) |
<1% of women were in a polygamous marriage, widowed or divorced.
The modified VOICE risk score includes age<25; not married or living with partner; alcohol use in the past 3 months; partner does not provide financial/material support; and partner has other sex partners with a maximum score of 8.
2359 participants initiated PrEP at enrolment and 38 after enrolment.
3.2. PrEP initiation, use/execution and persistence
Over 99% of women were eligible for same day PrEP initiation based on an estimated creatinine clearance >60% (or >50% in Kenya) and <1% had a positive hepatitis B surface antigen. Of the 2550 women enrolled, 2397 (94%) initiated PrEP, 749 (31%) returned at 1 month and received a refill (Figure 1). Information is not available about the reported reasons for the 69% who did not return at 1 month. Of the 646 who returned at month 1 and had 6 or more months of follow‐up time to assess PrEP persistence over 6 months, 20% persisted with PrEP to 6 months without an ≥15 day gap in refills, and 14% restarted PrEP after a gap of ≥15 days (Figure 1).
Figure 1.
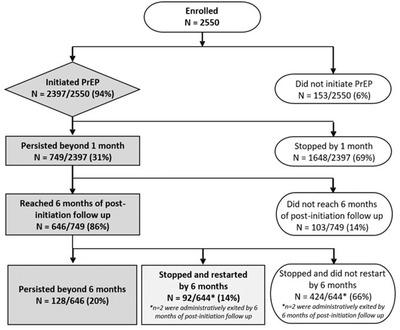
PrEP initiation and persistence beyond 6 months among women who initiated PrEP. The flow chart depicts PrEP initiation and persistence among the 2550 women who enrolled in the POWER cohort. PrEP persistence was assessed based on attendance of the 1 month visit for a PrEP refill, and persistence through 6 months among those reached at least 6 months of post‐PrEP initiation follow‐up time. PrEP non‐persistence was defined as a gap of ≥15 days between the refill return date and the next PrEP refill dispensation, assuming daily dosing prior to the next PrEP dispensation date. PrEP persistence beyond 6 months was defined as persistent use through 6 months and PrEP refill at the visit 6 month visit.
PrEP persistence beyond 6 months varied by site, ranging from 8% to 38% (Figure 2).
Figure 2.
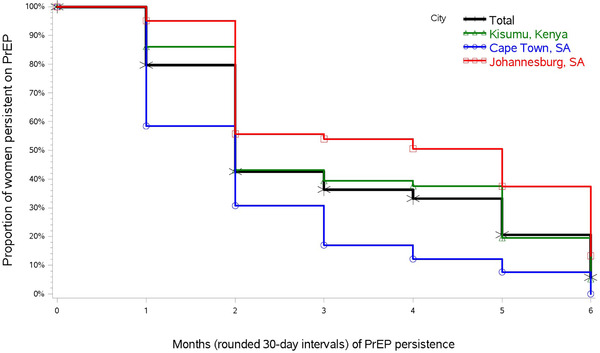
PrEP persistence among women who had PrEP persistence beyond 1 month. The cumulative probability of a PrEP non‐persistence failure event within 6 months of PrEP initiation was modeled using the Kaplan–Meier estimator of the survival function. The 749 women who had a PrEP refill at 1 month are included in the analysis. The vertical drop at each point indicates PrEP non‐persistence “events,” defined as a gap of ≥15 days between refills that would provide sufficient PrEP supply for daily dosing.
Older age, injectable contraception, intrauterine device or other contraceptive use, and reporting sometimes or always using condoms were significant baseline predictors of PrEP persistence beyond 1 month but not 6 months (Tables 2 and 3). The modified VOICE risk score was not predictive of PrEP persistence at 1 or 6 months.
Table 2.
Baseline predictors of PrEP persistence beyond 1 month
| N (%) or median (IQR) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| PrEP persisters | PrEP non‐persisters | Relative risk (95% CI) | p‐value | Relative risk a (95% CI) | p‐value | |
| N participants | 749 (31.3%) | 1648 (68.8%) | ||||
| Age (years) | 21 (19–23) | 20 (19–23) | 1.03 (1.01–1.06) | 0.007 | 1.04 (1.01–1.07) | 0.005 |
| Contraceptive use | ||||||
| None/condoms only | 378 (27.5%) | 999 (72.6%) | ref | 0.135 | ref | 0.038 |
| Oral | 31 (34.4%) | 59 (65.6%) | 1.25 (0.93–1.69) | <0.001 | 1.37 (1.02–1.84) | <0.001 |
| Injectable | 214 (37.0%) | 365 (63.0%) | 1.35 (1.17–1.54) | 0.022 | 1.37 (1.19–1.58) | 0.066 |
| Implant | 101 (33.9%) | 197 (66.1%) | 1.23 (1.03–1.48) | <0.001 | 1.19 (0.99–1.44) | <0.001 |
| IUD/other | 25 (48.1%) | 27 (51.9%) | 1.75 (1.30–2.35) | 0.135 | 1.65 (1.23–2.22) | 0.038 |
| Condom use, past 3 months | ||||||
| None | 179 (28.7%) | 444 (71.3%) | ref | 0.156 | ref | |
| Sometimes | 461 (31.9%) | 984 (68.1%) | 1.11 (0.96–1.28) | 0.203 | 1.22 (1.05–1.43) | 0.010 |
| Always | 105 (32.7%) | 216 (67.3%) | 1.14 (0.93–1.39) | 1.26 (1.02–1.55) | 0.031 | |
| Modified VOICE risk score a , b | ||||||
| 0–4 | 164 (35.5%) | 298 (64.5%) | ref | |||
| 5–6 | 273 (30.3%) | 629 (69.7%) | 0.85 (0.73–1.00) | 0.048 | 0.88 (0.75–1.05) | 0.149 |
| 7–8 | 305 (30.0%) | 709 (69.9%) | 0.85 (0.73–0.99) | 0.036 | 0.87 (0.73–1.05) | 0.144 |
Adjusted for site.
Maximum score on the modified VOICE risk score is eight.
Table 3.
Baseline predictors of PrEP persistence beyond 6 months, among women who persisted beyond 1 month
| N (%) or median (IQR) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| PrEP persisters | PrEP non‐persisters | Relative risk (95% CI) | p‐value | Relative risk a (95% CI) | p‐value | |
| N participants | 128 (19.8%) | 518 (80.2%) | ||||
| Age (years) | 21 (20–23) | 21 (19–23) | 1.05 (0.99–1.13) | 0.123 | 1.01 (0.94–1.09) | 0.729 |
| Contraceptive use | ||||||
| None/condoms only | 57 (17.5%) | 268 (82.5%) | ref | ref | ||
| Oral | 1 (44.4%) | 15 (55.6%) | 2.53 (1.56–4.12) | <0.001 | 1.84 (1.11–3.05) | 0.018 |
| Injectable | 39 (21.7%) | 141 (78.3%) | 1.24 (0.86–1.78) | 0.255 | 1.23 (0.86–1.74) | 0.260 |
| Implant | 13 (14.1%) | 79 (85.9%) | 0.81 (0.46–1.41) | 0.446 | 0.81 (0.46–1.41) | 0.455 |
| IUD/other | 7 (31.8%) | 15 (68.2%) | 1.81 (0.94–3.49) | 0.075 | 1.41 (0.74–2.68) | 0.290 |
| Condom use, past 3 months | ||||||
| None | 26 (16.6%) | 131 (83.4%) | ref | – | – | |
| Sometimes | 84 (20.9%) | 318 (79.1%) | 1.26 (0.85–1.88) | 0.254 | ||
| Always | 18 (21.4%) | 66 (78.6%) | 1.29 (0.75–2.22) | 0.349 | ||
| Modified VOICE risk score a , b | ||||||
| 0–4 | 33 (22.5%) | 114 (77.6%) | ref | ref | ||
| 5–6 | 57 (23.0%) | 191 (77.0%) | 1.02 (0.70–1.49) | 0.903 | 1.04 (0.72–1.51) | 0.818 |
| 7–8 | 38 (15.5%) | 207 (84.5%) | 0.69 (0.45–1.05) | 0.084 | 0.82 (0.54–1.26) | 0.369 |
Adjusted for site.
Maximum score on the modified VOICE risk score is 8.
Coverage calculations showed that 20% of women had sufficient PrEP supply to enable dosing ≥ 4 tablets per week over 6 months (data not shown). Among 1156 eligible for 3 and 6 month visits, 193 (16.7%) specimens were selected to measure intracellular TFV‐DP levels, which indicated that 19.0% had taken PrEP daily, 28.2% an average of 4–6 doses per week, 5.1% an average of 2–3 doses per week, 19.5% an average of 1 dose per week and 28.2% had TFV‐DP levels below the limit of quantification (BLQ, <31.25 fmol/punch; Table 4).
Table 4.
PrEP adherence based on intracellular tenofovir diphosphate levels at month 3 or 6
| Description | Total | JOOTRH and KMET Kisumu | Tutu Teen Truck Cape Town a | Ward 21 Johannesburg a |
|---|---|---|---|---|
| N samples tested (% of eligible visits b ) | 193 (16.7%) | 65 (11.3%) | 32 (11.1%) | 96 (32.9%) |
| DBS TFV‐DP fmol/punch Median (IQR) | 510 (16–1263) | BLQ c (16–341) | 770 (56–1404) | 1063 (218–1394) |
| Estimated average weekly PrEP use d | ||||
| 7 doses/week (≥1450 fmol/punch) | 19.0% | 10.8% | 25.0% | 21.9% |
| 4–6 doses/week (800–1449 fmol/punch) | 28.2% | 9.2% | 25.0% | 41.7% |
| 2–3 doses/week (400–799 fmol/punch) | 5.1% | 4.6% | 3.1% | 6.3% |
| <2 doses/week (31.25–399 fmol/punch) | 19.5% | 23.1% | 28.1% | 14.6% |
| None (BLQ c , <31.25 fmol/punch) | 28.2% | 52.3% | 18.8% | 15.6% |
The three cities included two facilities each, two of which (Jeppestown clinic in Johannesburg and Weltevreden clinic in Cape Town) were excluded in this table because they had few or no specimens available for testing.
Eligible visits for TFV‐DP assessment were month 3 and/or 6 visits, where the participant had PrEP supply from the prior visit sufficient for daily dosing, from 11% of visits from participants with PrEP supply estimated to be sufficient for daily dosing were randomly selected from the two sites, which sent all DBS samples collected, and all from the third site, which sent a subset of 33% DBS samples collected.
Below limit of quantification (<31.25 fmol/punch).
Thresholds for intracellular tenofovir diphosphate levels in red blood cells associated with average doses per week in the 6 weeks prior to the DBS sample were based on directly observed dosing studies in studies of MSM, which measured TFV‐DP levels
3.3. HIV and STI incidence
Sixteen post‐PrEP initiation seroconversions were observed over 741.8 person‐years of follow‐up for an HIV incidence of 2.2 per 100 person‐years (95% confidence interval [CI] 1.2, 3.5). Four seroconversions were detected at month 1, potentially indicating that they were infected at PrEP initiation (Table S1). Seven of 12 (58%) women who seroconverted after month 1 were known to not be taking PrEP at the time of seroconversion either because of missing the prior PrEP refill, declining PrEP or having previously discontinued PrEP. Thirteen seroconverters had DBS samples available for intracellular TFV‐DP levels, and two had plasma tenofovir tested because DBS was not available. All four who seroconverted to HIV at month 1 had detectable TFV‐DP, two at levels consistent with taking an average of 4 or more times per week. For the remaining nine who seroconverted after month 1 and had DBS available, TFV‐DP was undetectable for six, two had levels consistent with taking less than 4 times/week and one had levels consistent with taking an average of 4 or more times per week. Plasma tenofovir levels for two seroconverters for whom DBS were not available had results of 79.1 ng/ml and undetectable (<0.5 ng/ml), respectively.
Fourteen seroconverters had samples from which HIV could be amplified for testing for antiretroviral resistance, two had M184M/V mutations and both had high TFV‐DP levels at 1 month. Ten had no resistance mutations and three had NNRTI mutations.
The incidence of C. trachomatis and N. gonorrhoeae was 42.9/100 person‐years (95% CI 37.2, 49.2)—35.4/100 person‐years for C. trachomatis (95% CI 30.3, 41.2) and 13.0/100 person‐years for N. gonorrhoeae (95% CI 10,0, 16.7) of whom 42% of the women also had C. trachomatis and/or N. gonorrhoeae at enrolment.
4. DISCUSSION
PrEP was offered at SRH and primary healthcare clinics for AGYW in Kenya and South Africa. Initiation of PrEP was very high (94%) among these sexually active AGYW who were seeking health services, and who had multiple risk factors for HIV, including inconsistent condom use, a partner of unknown HIV status and a curable STI. The high rate of PrEP initiation indicates that AGYW were motivated even though only one‐third returned for a PrEP refill at 1 month. Of those who returned at 1 month, one‐third persisted with PrEP through 6 months or restarted PrEP within 6 months after a gap of ≥15 days between PrEP refills. The high frequency of early drop‐off could reflect the lack of reimbursement for transport and study participation, or other challenges with continuing PrEP. Site differences in PrEP persistence may reflect differences in PrEP delivery strategies and regional differences in PrEP rollout and awareness. Although there was high early drop‐off, a minority of women were able to persist with PrEP for 6 months.
Due to this study having minimal research procedures, we are limited in understanding factors associated with women not returning for their first PrEP refill. Predictors of PrEP use at 1 month indicate factors that could identify AGYW who need more support for persisting with PrEP, including younger age, lack of condom use and oral or injectable hormonal contraception use. The only significant predictor of PrEP persistence beyond both 1 and 6 months was oral contraceptive use at baseline. Oral contraceptive pills are not typically recommended for African AGYW due to high pregnancy rates of up to 16% [20]. Our finding suggests that experience in daily pill‐taking for contraception facilitated taking pills consistently for HIV prevention, and supports the evaluation of a dual prevention tablet with oral contraception and PrEP [21].
The moderate level of PrEP persistence may be indicative of PrEP use in programmatic delivery without reimbursement or active retention efforts, particularly among young women with dynamic sexual relationships and for whom medication adherence is typically low [22, 23], who often have challenges recognizing their risk for HIV or maintaining motivation to attend clinic or daily pill‐taking [24]. Qualitative findings from this study indicate that PrEP use was hampered by low community awareness, stigma and misconceptions about PrEP, daily pill‐taking was challenging for AGYW and that PrEP disclosure, social support, adolescent‐friendly health counselling and convenient access were key enablers for PrEP persistence [25]. PrEP demonstration projects in Kenya and South Africa have identified the need for an enabling environment to reduce stigma about sexuality and support AGYW PrEP users [26, 27].
Short gaps in PrEP refills in one‐fifth of the AGYW who continued PrEP beyond 6 months indicate the frequency of PrEP “pauses” when providers need to reassess women's risk and counsel about restarting PrEP. Proactive call‐back mechanisms and express visits may aid women in continuing both PrEP and contraception given low contraceptive continuation rates in African settings [28] and that contraceptive use was a predictor of PrEP persistence in this study. The MTN 034/REACH cross‐over study of oral PrEP and the dapirivine ring in African AGYW demonstrated that in the context of being provided options for adherence support strategies and monthly visits, almost all had high adherence over 18 months [29]. These strategies should be adapted and evaluated in programmatic settings.
In this study and recent PrEP studies in African AGYW [30, 31], >99% were eligible for same day PrEP initiation based on normal creatinine clearance and lack of active hepatitis B infection. Laboratory screening should not be a barrier to PrEP delivery for young women. Health service barriers, including phlebotomy for laboratory testing, limited clinic hours, stock outs of commodities, including contraceptives, staffing and fast track queues, need to be addressed to facilitate PrEP delivery. These factors could also be challenged with PrEP dispensation and persistence for longer‐acting agents, such as the dapivirine ring and injectable cabotegravir. Innovative, community‐based PrEP delivery strategies are needed to reduce barriers, including transportation costs and inconvenience of clinic visits.
Consistent condom use was low and prevalence of C. trachomatis and N. gonorrhoeae was high, among whom only a small minority reported symptoms with annual incidence almost as high as their prevalence. Syndromic STI management would miss treatment of these asymptomatic, curable STIs. Similarly, high rates of C. trachomatis and N. gonorrhoeae were observed in recent PrEP demonstration projects [30, 31, 32], highlighting the need for integration SRH services and PrEP delivery for African AGYW, including etiologic STI diagnosis, prompt treatment and expedited partner treatment [33].
Two women who were identified as HIV seroconverters 1 month after PrEP initiation had PrEP‐associated resistance, specifically M184M/V mutations conferring resistance to emtricitabine. Both participants could have been infected at enrolment and not detected by standard antibody tests during HIV seroconversion when viral loads are often high. PrEP‐associated resistance mutations during undiagnosed acute HIV infection are rare [6, 34] and are balanced by the benefits of preventing HIV infection in the great majority of users [35].
HIV incidence in this cohort was 2.2 per 100 woman‐years, which is a potential overestimate since several infections were identified in the first 1–3 months after PrEP initiation could represent baseline infections, which could not be retrospectively assessed with more sensitive assays because samples were not archived at enrolment. The HIV incidence is higher than recent demonstration projects of PrEP in African AGYW [30, 31]; in this cohort, women who acquired HIV had stopped PrEP or had low use based on their TFV‐DP levels. A control group was not included for ethical reasons of withholding a proven efficacious prevention method (PrEP) to women at risk. Thus, the HIV incidence cannot be compared to a contemporaneous cohort of women who were not offered PrEP, although risk behaviours and STI prevalence are similar to recent cohorts of African AGYW with an HIV incidence of 4% [1, 2, 3]. Proactive outreach may be useful in the first few weeks after PrEP initiation in order to assess women's risk perception and PrEP motivations, challenges with PrEP use to mitigate early drop‐off, and provide adherence support and visit reminders to women at higher risk. These strategies may improve continuation of longer‐acting PrEP formulations, as they are implemented.
This study has the strengths of evaluating PrEP uptake and persistence among African AGYW attending diverse facility‐based clinics and mobile clinical services with minimal research procedures and no compensation for travel and visits. Thus, the observed rates of PrEP uptake and persistence are indicative of what may be observed in programmatic PrEP delivery. The study has limited information about barriers to PrEP use, persistence and reasons for not returning or discontinuing PrEP, and HIV infection prior to PrEP initiation was not definable for four women because enrolment samples were not archived. Quantification of adherence among seroconverters was limited due to the single time point for DBS or plasma collection.
5. CONCLUSIONS
While the high PrEP initiation rates among African AGYW and feasibility of integrating PrEP into SRH and primary care are encouraging, the findings highlight the need for simpler delivery through fast track visits and community‐based refills to better support PrEP persistence. Differentiated PrEP support strategies are needed when delivering PrEP at scale for AGYW to reduce early drop‐off after PrEP initiation. While less user‐dependent and longer‐acting PrEP formulations for example dapivirine ring and injectable cabotegravir, provide preferable options for some women, they will also need to be integrated into primary care and SRH services with regular interactions for HIV testing and safety monitoring.
COMPETING INTERESTS
PLA reports grants from NIH, during the conduct of the study; grants and consulting fees from Gilead Sciences and advisory board fees from Merck, ViiV outside the submitted work; JMB reports grants from NIH during the conduct of the study; personal fees from Gilead Sciences, Janssen, Merck, outside the submitted work; CC reports grants from NIH and has received advisory board fees from Merck and Gilead Sciences outside the submitted work; DD reports grants from NIH; RH has received funding from NIH and Children's Investment Fund Foundation and consulting fees from World Health Organization outside the submitted work; JEH has received advisory board fees from Merck outside the submitted work; GOM received grants from CDC and NIH; UMP received grants from NIH, IPM and received personal fees from Merck outside the submitted work; ER received personal fees from Gilead Science outside the submitted work; and AvdS received personal fees from Merck outside the submitted work.
AUTHORS’ CONTRIBUTIONS
CC, JMB and RJ contributed to funding acquisition. CC, JMB, DD, EB, SDM, LGB, GOM, AvdS, UMP, PLA, JEH and RH designed the study. RJ, VO, ER, DT, FM, GB, SM and JFM were responsible for project administration. LK and LW analysed the data. CC wrote the original draft. JMB, DD, LGB, EB, SDM, SM, GB, RJ, VO, ER, DT, FM, JFM, GB, GOM, AvdS, UMP, PLA, JEH and RH contributed to writing, reviewing and editing the manuscript.
FUNDING
This study was funded by the United States Agency for International Development, Cooperative Agreement AID‐OAA‐A‐15‐00034.
DISCLAIMER
Gilead Sciences donated tenofovir disoproxil fumarate‐emtricitabine to the study.
Supporting information
Table S1. Summary of post‐PrEP seroconversions with PrEP refill history, initial HIV‐1 viral load and resistance mutations.
ACKNOWLEDGEMENTS
We acknowledge the contributions of the study participants and the POWER study teams.
Kisumu, Kenya: Josephine Odoyo, Hilda Machafu, Mary‐Josephine Osore, Ethel Osome, Fredrick Omondi, Ben Kwach, David Ang'awa, Bernard Nyerere, Merceline Awuor, Annabel Dola, John Bosco Tsetso, Sherine Odek, Sylvia Mugalla, Vincent Momanyi, Peris Otieno, Brenda Misiko, Joel Odondi, Calvin Obuya, Boblief Otieno, Eric Seda, Alfred Obiero, Rachel Mwakisha, Petronilla Njenga, KMET staff and JOOTRH staff.
CapeTown, South Africa: Dorothy Zakariya, Lindile Nonjinge, Robin Julies, Pamela Fuzile, Thando Xeketwana, Lwazi Thami, Nkosiyabo Futshane, Desmond Raqa, Nomvuyiseko Mkatshana, Yolanda Mpanda and Weltevreden clinic staff.
Johannesburg, South Africa : Thapelo Tlou, Kefilwe Kgabo, Samukelo Mbele, Phumzile Nyamane, Lerato Lunika, Sanele Gumede, Melt Ndlovu, Nomhle Khoza and Jeppestown clinic staff.
Clinical Trial Number: NCT03490058.
Contributor Information
Connie L. Celum, Email: ccelum@uw.edu.
the POWER Study Team:
Josephine Odoyo, Hilda Machafu, Mary‐Josephine Osore, Ethel Osome, Fredrick Omondi, Ben Kwach, David Ang'awa, Bernard Nyerere, Merceline Awuor, Annabel Dola, John Bosco Tsetso, Sherine Odek, Sylvia Mugalla, Vincent Momanyi, Peris Otieno, Brenda Misiko, Joel Odondi, Calvin Obuya, Boblief Otieno, Eric Seda, Alfred Obiero, Rachel Mwakisha, Petronilla Njenga, KMET staff, JOOTRH staff, Dorothy Zakariya, Lindile Nonjinge, Robin Julies, Pamela Fuzile, Thando Xeketwana, Lwazi Thami, Nkosiyabo Futshane, Desmond Raqa, Nomvuyiseko Mkatshana, Yolanda Mpanda, Weltevreden clinic staff, Thapelo Tlou, Kefilwe Kgabo, Samukelo Mbele, Phumzile Nyamane, Lerato Lunika, Sanele Gumede, Melt Ndlovu, Nomhle Khoza, and Jeppestown clinic staff
DATA AVAILABILITY STATEMENT
Data may be available upon request.
REFERENCES
- 1. ECHO Trial Consortium . HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open‐label trial. Lancet. 2019;394(10195):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baeten JM, Palanee‐Phillips T, Brown ER, Schwartz K, Soto‐Torres LE, Govender V, et al. Use of a vaginal ring containing dapivirine for HIV‐1 prevention in women. N Engl J Med. 2016;375(22):2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNAIDS . Women and HIV: a spotlight on adolescent girls and young women. Geneva, Switzerland. 2019. Available from: https://www.unaids.org/en/resources/documents/2019/women‐and‐hiv. Accessed July 5, 2022. [Google Scholar]
- 4. Molina JM, Charreau I, Spire B, Cotte L, Chas J, Capitant C, et al. Efficacy, safety, and effect on sexual behaviour of on‐demand pre‐exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV. 2017;4(9):e402–10. [DOI] [PubMed] [Google Scholar]
- 5. McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre‐exposure prophylaxis to prevent the acquisition of HIV‐1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open‐label randomised trial. Lancet. 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanscom B, Janes HE, Guarino PD, Huang Y, Brown ER, Chen YQ, et al. Preventing HIV‐1 infection in women using oral pre‐exposure prophylaxis: a meta‐analysis of current evidence. J Acquir Immune Defic Syndr. 2016;73:606–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministry of Health . Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya 2016. Nairobi, Kenya: National AIDS & STI Control Programme; 2016. [Google Scholar]
- 9. Masyuko S, Mukui I, Njathi O, Kimani M, Oluoch P, Wamicwe J, et al. Pre‐exposure prophylaxis rollout in a national public sector program: the Kenyan case study. Sex Health. 2018;15(6):578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bekker LG, Rebe K, Venter F, Maartens G, Moorhouse M, Conradie F, et al. Southern African guidelines on the safe use of pre‐exposure prophylaxis in persons at risk of acquiring HIV‐1 infection. South Afr J HIV Med. 2016;17(1):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Straten A, Montgomery ET, Hartmann M, Minnis A. Methodological lessons from clinical trials and the future of microbicide research. Curr HIV/AIDS Rep. 2013;10(1):89–102. [DOI] [PubMed] [Google Scholar]
- 12. Nkosi B, Seeley J, Ngwenya N, McHunu SL, Gumede D, Ferguson J, et al. Exploring adolescents and young people's candidacy for utilising health services in a rural district, South Africa. BMC Health Serv Res. 2019;19(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wood K, Jewkes R. Blood blockages and scolding nurses: barriers to adolescent contraceptive use in South Africa. Reprod Health Matters. 2006;14(27):109–18. [DOI] [PubMed] [Google Scholar]
- 14. Rousseau E, Bekker LG, Julies RF, Celum C, Morton J, Johnson R, et al. A community‐based mobile clinic model delivering PrEP for HIV prevention to adolescent girls and young women in Cape Town, South Africa. BMC Health Serv Res. 2021;21(1):888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balkus JE, Brown E, Palanee T, Nair G, Gafoor Z, Zhang J, et al. An empiric HIV risk scoring tool to predict HIV‐1 acquisition in African women. J Acquir Immune Defic Syndr. 2016;72(3):333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oral abstracts of the 11th IAS Conference on HIV Science, 18–21 July 2021. J Int AIDS Soc. 2021;24 Suppl 4(Suppl 4):e25755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castillo‐Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yager J, Castillo‐Mancilla J, Ibrahim ME, Brooks KM, McHugh C, Morrow M, et al. Intracellular tenofovir‐diphosphate and emtricitabine‐triphosphate in dried blood spots following tenofovir alafenamide: the TAF‐DBS Study. J Acquir Immune Defic Syndr. 2020;84(3):323–30. [DOI] [PubMed] [Google Scholar]
- 19. Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr. 2014;66(3):340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reid SE, Dai JY, Wang J, Sichalwe BN, Akpomiemie G, Cowan FM, et al. Pregnancy, contraceptive use, and HIV acquisition in HPTN 039: relevance for HIV prevention trials among African women. J Acquir Immune Defic Syndr. 2010;53(5):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Friedland BA, Mathur S, Haddad LB. The promise of the dual prevention pill: a framework for development and introduction. Front Reprod Health. 2021;3:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stoner MCD, Rucinski KB, Giovenco D, Gill K, Morton JF, Bekker LG, et al. Trajectories of PrEP adherence among young women aged 16 to 25 in Cape Town, South Africa. AIDS Behav. 2021;25(7):2046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Celum CL, Delany‐Moretlwe S, Baeten JM, van der Straten A, Hosek S, Bukusi EA, et al. HIV pre‐exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc. 2019;22(Suppl 4):e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O'Rourke S, Hartmann M, Myers L, Lawrence N, Gill K, Morton JF, et al. The PrEP journey: understanding how internal drivers and external circumstances impact the PrEP trajectory of adolescent girls and young women in Cape Town, South Africa. AIDS Behav. 2021;25(7):2154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rousseau E, Katz AWK, O'Rourke S, Bekker LG, Delany‐Moretlwe S, Bukusi E, et al. Adolescent girls and young women's PrEP‐user journey during an implementation science study in South Africa and Kenya. PLoS One. 2021;16(10):e0258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakasone SE, Chimbindi N, Mthiyane N, Nkosi B, Zuma T, Baisley K, et al. “They have this not care ‐ don't care attitude:” a mixed methods study evaluating community readiness for oral PrEP in adolescent girls and young women in a rural area of South Africa. AIDS Res Ther. 2020;17(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Were D, Musau A, Mugambi M, Plotkin M, Kabue M, Manguro G, et al. An implementation model for scaling up oral pre‐exposure prophylaxis in Kenya: Jilinde project. Gates Open Res. 2021;5:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smit JA, Beksinska ME. Hormonal contraceptive continuation and switching in South Africa: implications for evaluating the association of injectable hormonal contraceptive use and HIV. J Acquir Immune Defic Syndr. 2013;62(3):363–5. [DOI] [PubMed] [Google Scholar]
- 29. Ngure K, Nair G, Szydlo D, Brown E, Akello C, Macdonald P, et al. Choice and adherence to dapivirine ring or oral PrEP by young African women in REACH. Conference on Retroviruses and Opportunistic Infections (CROI); 14–24, February Virtual 2022.
- 30. Celum CL, Gill K, Morton JF, Stein G, Myers L, Thomas KK, et al. Incentives conditioned on tenofovir levels to support PrEP adherence among young South African women: a randomized trial. J Int AIDS Soc. 2020;23(11):e25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye BJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Delany‐Moretlwe S, Mgodi N, Bekker L‐G, Baeten J, Pathak S, Donnell D, et al. O10.3 High curable STI prevalence and incidence among young African women initiating PrEP in HPTN 082. Sex Transm Infect. 2019;95(Suppl 1):A60–1. [Google Scholar]
- 33. Ong JJ, Baggaley RC, Wi TE, Tucker JD, Fu H, Smith MK, et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta‐analysis. JAMA Netw Open. 2019;2(12):e1917134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riddel J, Amico KR, Mayer KH. HIV preexposure prophylaxis: a review. JAMA. 2018;319(12):1261–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of post‐PrEP seroconversions with PrEP refill history, initial HIV‐1 viral load and resistance mutations.
Data Availability Statement
Data may be available upon request.


