Key Points
G-CSF is an effective long-term therapy for SCN with a wide range of effective doses requiring individualized dose titration.
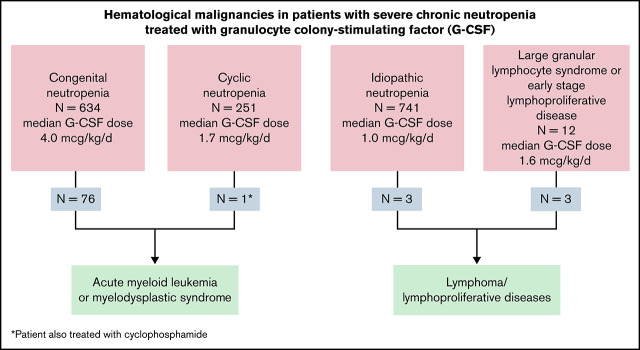
Eleven percent of patients with CN developed MDS/AML; patients with autoimmune/idiopathic neutropenia did not develop myeloid leukemia.
Visual Abstract
Abstract
Severe chronic neutropenia (SCN), defined as blood neutrophils <0.5 × 109/L for >3 months, is an uncommon hematological condition associated with recurrent and severe bacterial infections. After short-term clinical trials showed the benefits of granulocyte colony-stimulating factor (G-CSF) treatment for SCN, SCNIR (Severe Chronic Neutropenia International Registry) opened to determine the long-term benefits and safety of this treatment. This report summarizes findings from more than 16 000 patient-years of prospective observations for patients with congenital and acquired SCN. We observed that adverse outcomes depend on the underlying etiology. Myelodysplasia (MDS) and acute myeloid leukemia (AML) occur infrequently and largely in patients with congenital neutropenias. Having cyclic or chronic autoimmune/ idiopathic neutropenia portends a favorable prognosis. A few patients with idiopathic neutropenia evolve to develop lymphoid malignancies, but they do not appear to be at increased risk of myeloid malignancies, even with very long-term G-CSF therapy. Progression to systemic autoimmune diseases, bone marrow (BM) failure, aplastic anemia, or nonmyeloid malignancies are not expected consequences of SCN or treatment with G-CSF.
Introduction
Severe chronic neutropenia (SCN) is an uncommon hematological condition usually presenting with fever, otitis, respiratory infections, gingivitis, and/or cellulitis.1 Granulocyte colony-stimulating factor (G-CSF) was the first predictably effective treatment for SCN, approved by the US Food and Drug Administration (FDA) and the European Medicines Authority in 1994, based on a randomized controlled trial.2 The approval required longitudinal follow-up with safety observations leading to the opening of the SCNIR (Severe Chronic Neutropenia International Registry).3 Since 1994, the SCNIR has enrolled and prospectively followed children and adults with minimal criteria of ≥3 absolute neutrophil counts (ANCs) <0.5 × 109/L during a 3-month period. Follow-up was at least annually, recording significant health events and treatments. Patients were ineligible who had pancytopenia, aplastic anemia, myelodysplasia (MDS), myeloid or lymphoid malignancy, systemic autoimmune diseases, or neutropenia due to idiosyncratic reactions to drugs or cancer chemotherapy.
We previously reported on the risk of MDS and acute myeloid leukemia (AML) in patients with congenital neutropenia3-6 and longitudinal observations for other causes of SCN.7-11 For this report, we describe long-term outcomes for all US patients enrolled in SCNIR. Almost all of the patients received G-CSF, providing long-term data on the effectiveness and risks associated with this therapy.
Methods
This was a long-term prospective observational study of patients enrolled from May 1994 to June 2020. The original cohort was patients enrolled in the pivotal randomized trial.2 These patients were recruited by physicians at academic centers. With the opening of SCNIR, patients came from diverse sources (eg, adult and pediatric hematologists, primary care physicians, family members of enrolled patients, and self-referral). All potential enrollees were evaluated uniformly and offered enrollment if they qualified. The research was approved by the University of Washington Institutional Review Board; all participants signed written informed consent.
Enrollment criteria
Neutropenia.
ANC <0.5 × 109/L on ≥3 occasions over ≥3 months. Patients were grouped into 3 main categories. Cyclic neutropenia required 2 ANC cycles with a nadir of <0.2 × 109/L followed by increases usually to >1.0 × 109/L at approximately 3-week intervals. Congenital neutropenia was diagnosed based on early childhood-onset and/or genetic test results and ANC remaining <0.5 × 109/L beyond age 0.25 to 1.0 years. The autoimmune/idiopathic category included all others and was divided into subgroups based on the diagnosis of the referring physician and results of antineutrophil antibody testing performed through the office of the referring physician.
A bone marrow (BM) examination was originally required for enrollment. After about 10 years, the requirement for preenrollment BM report was dropped for patients with cyclic and autoimmune/idiopathic neutropenia because it was contrary to usual practice and because of the rarity of MDS or leukemia in these subgroups of patients. Referring physicians, however, were encouraged to obtain a marrow if, for any reason, it was clinically indicated.
Autoantibody testing.
At the time of referral, clinicians were encouraged to do antineutrophil antibody testing if the clinical diagnosis was autoimmune or idiopathic neutropenia, but these tests were not required. Because of the lack of specificity and availability of these tests, patients were enrolled as autoimmune neutropenia if this was the referring physician’s diagnosis.12 Other results for autoantibodies were retrieved from clinical records.
Exclusion criteria.
Diagnosis of drug-induced neutropenia (including chemotherapy), MDS, leukemia, aplastic anemia, HIV-associated neutropenia, rheumatoid arthritis, systemic lupus erythematosus, and other systemic autoimmune diseases.
Special groups.
Patients with genetic disorders with neutropenia of varying severity (eg, Shwachman-Diamond syndrome [SDS], glycogen storage disease type 1b [GSD1b], Barth syndrome, Cohen syndrome, and Warts, Hypogammaglobulinemia, Infections, and Myelokathexis [WHIM] syndrome) were enrolled to improve the understanding of these disorders.
Treatment: G-CSF supply agreement.
Amgen, originally the exclusive supplier of G-CSF in the United States, provided filgrastim to US patients enrolled in SCNIR who provided annual follow-up data to build the safety profile for the new drug. The dose and schedule for G-CSF and other treatments were prescribed by the patient’s physician, not by SCNIR. Subsequently, other G-CSF suppliers were approved by the FDA; we included all available data on G-CSFs for all suppliers in this report.
Annual evaluations.
A dedicated staff member followed up on patients annually via e-mail, text, telephone, and letter with a standardized form for information on health, blood counts, infections, malignancies, and hospitalizations. Initially, SCNIR requested physicians to provide this information but shifted to asking patients to submit it to SCNIR, increasingly via internet communications. This increased patient and family understanding of neutropenia and increased the efficiency of reporting.
Oversight.
An Advisory Board of physicians from the United States, Australia, and Europe developed a handbook and website with forms, diagnosis and treatment information, and treatment recommendations.13 Over time, more than 700 US physicians participated by enrolling and following SCNIR patients.
Results
Enrollment
From 1994 to 2020, the Seattle SCNIR office received inquiries from 1799 physicians and 4544 patients or families about neutropenia and enrollment in the SCNIR; 1752 patients met the criteria and completed the registration in the following categories: congenital, 670 (38%); cyclic, 266 (15%); and autoimmune/idiopathic, 816 (47%). The overall mean follow-up was 9.3 years; 189 patients provided follow-up data for 15 to 20 years, 120 for 21 to 25 years, and 82 for >25 years; the longest participants came with data rolled over from the randomized trial. There are more than 16 000 patient-years of observational data, including those who died (1089 patient-years), resolved neutropenia (2073 patient-years, largely children), received hematopoietic stem cell transplants (HSCTs) (1629 patient-years, 483 of 1629 years from deceased patients), lost to follow-up (2028 patient-years), or withdrew from participation (645 patient-years) (see Table 1). Overall outcomes of transplantation, evolution to malignancies, and or death were known for approximately 80% of the enrolled patients; the percentages of lost-to-follow-up patients were: congenital, 18%; cyclic, 17%; autoimmune/idiopathic, 17%.
Table 1.
Demographics
| Congenital | Cyclic | Autoimmune/Idiopathic | |||||
|---|---|---|---|---|---|---|---|
| Age, yr | <18 | ≥18 | <18 | ≥18 | <18 | ≥18 | |
| Patients | 383 | 287 | 88 | 178 | 352 | 464 | |
| Age at last contact, yr | Median | 8.5 | 26.6 | 9.0 | 39.4 | 6.8 | 45.5 |
| Mean | 8.7 | 30.0 | 9.4 | 42.3 | 7.9 | 46.8 | |
| Range | (0.3-17.9) | (18.0-82.0) | (1.2-17.9) | (18.1-92.8) | (1.1-17.9) | (18.0-90.7) | |
| Years of clinical observation study | Median | 4.9 | 16.5 | 5.4 | 15.2 | 3.8 | 8.9 |
| Mean | 5.5 | 15.9 | 6.0 | 15.3 | 4.5 | 10.2 | |
| Range | (0-17.3) | (0-31.4) | (0-15.5) | (0.1-32.2) | (0-16.1) | (0-30.8) | |
| Years of observation by age | 2119 | 4552 | 526 | 2722 | 1582 | 4712 | |
| Total years of observation by diagnosis* | 6671 | 3248 | 6294 | ||||
Total years of observation by all diagnostic groups: 16 213.
Patients
Symptoms of cyclic and congenital neutropenia were usually present soon after birth, but the diagnosis often followed a series of acute febrile episodes because the infections resemble those of other young children. In older children and adults, the diagnosis was often made with routine blood counts when there were no symptoms of an infection at the time of the test. Table 2 shows ages at onset of symptoms, diagnosis, and the start of G-CSF.
Table 2.
Age at onset and G-CSF treatment
| Congenital | Cyclic | Autoimmune/Idiopathic | |||||
|---|---|---|---|---|---|---|---|
| Age, yr | <18 | ≥18 | <18 | ≥18 | <18 | ≥18 | |
| Age at presentation, yr | Median | 0.2 | 0.4 | 0.5 | 4.1 | 1.1 | 25.0 |
| Mean | 0.9 | 3.4 | 1.2 | 12.6 | 1.9 | 27.8 | |
| Range | (0-15.2) | (0-69.6) | (0-10.3) | (0-74.6) | (0-15.6) | (0-86.9) | |
| Age at diagnosis, yr | Median | 0.5 | 1.1 | 1.3 | 11.1 | 1.4 | 28.7 |
| Mean | 1.2 | 4.9 | 2.2 | 16.6 | 2.3 | 30.8 | |
| Range | (0-14.5) | (0-69.7) | (0-11.5) | (0-74.6) | (0-15.9) | (0-87.0) | |
| Age at start of G-CSF treatment, yr | Median | 0.9 | 7.4 | 1.6 | 21.4 | 1.7 | 33.3 |
| Mean | 2.2 | 11.1 | 2.6 | 24.4 | 2.8 | 35.1 | |
| Range | (0-15.2) | (0-74.3) | (0.03-10.8) | (0.1-74.6) | (0.02-16.1) | (0.1-87.5) | |
| Age at enrollment, yr | Median | 2.3 | 12.8 | 2.4 | 25.7 | 2.2 | 35.7 |
| Mean | 3.7 | 15.4 | 3.5 | 28.1 | 3.5 | 36.8 | |
| Range | (0.1-17.7) | (0.2-74.5) | (0.4-14.6) | (1.1-75.4) | (0.4-16.7) | (1.0-87.5) | |
Genetic testing
When the SCNIR opened, genetic diagnosis by DNA sequencing was just getting underway. Early SCNIR enrollees were critical for finding mutations in ELANE as the predominant cause of cyclic and congenital neutropenia.14,15 The congenital category enrolled in the US office of SCNIR now includes patients with mutations in ELANE (333), SBDS (26), TAZ (25), COH1 (19), CXCR4 (11), SLC37A4/G6PC (25), G6PC3 (4), WAS (5), TCIRG1 (21), CLBP (9), SRP54 (3), VPS45 (1), and HAX (1). Testing for these mutations was performed in many different laboratories. Some mutations (eg, JAGN1 and HAX1) were found rarely in the United States, although observed more frequently in European and Middle Eastern populations.14,16 Financial and insurance barriers limit testing, so the majority of US patients did not have genetic testing.17
Autoimmune and idiopathic neutropenia
Patients were reported with autoimmune neutropenia if this was the diagnosis assigned by the treating physician. Otherwise, these patients were enrolled as idiopathic neutropenia. Across all groups, we reviewed clinical testing for autoantibodies. The most frequent test was antineutrophil antibody testing. Overall, 335 patients were tested, of which 174 (52%) tested positive. There were positive tests in all groups: cyclic, 3/17 (18%); congenital, 22/88 (25%); and autoimmune/idiopathic 149/230, (65%). Other positive autoantibody results were antinuclear antibodies, 19/40 (47%); perinuclear antineutrophil cytoplasmic antibodies, 5/28 (18%); antineutrophil cytoplasmic antibodies, 2/28 (7%); proteinase 3, 2/11 (18%); and antidouble stranded DNA, 1/9 (11%). Eighty-three percent of the positive tests were in patients with autoimmune/idiopathic neutropenia, but testing was not systematic enough to allow the association of specific results with outcomes. Lymphocyte counts were significantly higher in childhood autoimmune/idiopathic neutropenia (<18 years) than in adults (≥18 years); P < .0001.
G-CSF treatment, neutrophil, and lymphocyte counts
Because the patients had ANCs <0.5 × 109/L and many had recurrent fevers and infections, most were treated with G-CSF for very long periods. Interruptions in G-CSF treatment predictably led to reversion of the ANC to its baseline for most patients and recurrence of the same symptoms and problems that led to treatment. The median ANCs with ranges at the time of enrollment in the SCNIR for each group, pediatric and adult, are shown in Tables 2 and 3. The high counts that extend the ranges probably reflected severe infections. G-CSF median treatment doses and responses are also presented in Tables 2 and 3. For patients on alternate-day or other than daily treatment, the doses were the calculated median daily dose.
Table 3.
| Congenital | Cyclic | Autoimmune/Idiopathic | |||||
|---|---|---|---|---|---|---|---|
| Age, yr | <18 | ≥18 | <18 | ≥18 | <18 | ≥18 | |
| Before G-CSF ANC | Median | 0.2 | 0.2 | 0.4 | 0.5 | 0.2 | 0.4 |
| Mean | 0.6 | 0.5 | 0.9 | 1.0 | 0.6 | 0.8 | |
| SEM | (±0.05) | (±0.05) | (±0.07) | (±0.07) | (±0.04) | (±0.04) | |
| Range | (0-22.5) | (0-33.4) | (0-30.1) | (0-36.5) | (0-34.6) | (0-38.5) | |
| Before G-CSF ALC | Median | 4.9 | 2.9 | 4.7 | 2.2 | 4.1 | 1.5 |
| Mean | 5.3 | 3.8 | 4.9 | 2.7 | 4.2 | 1.8 | |
| SEM | (±0.13) | (±0.15) | (±0.1 9) | (±0.13) | (±0.10) | (±0.05) | |
| Range | (0.04-20.6) | (0.01-18.7) | (0.4-14.6) | (0.03-28.2) | (0.18-16.0) | (0.04-54.6) | |
| G-CSF dosing (mcg/kg per day) | Median | 4.8 | 3.5 | 2.1 | 1.6 | 1.5 | 0.8 |
| Mean | 10.8 | 8.9 | 3.7 | 2.3 | 2.3 | 1.9 | |
| SEM | (±1.04) | (±1.12) | (±0.69) | (±0.15) | (±0.13) | (±0.15) | |
| Range | (0.03-536.9) | (0.01-283.5) | (0.1-48.9) | (0.01-33.7) | (0.01-40.0) | (0.01-42.9) | |
| After G-CSF ANC | Median | 1.3 | 1.5 | 1.9 | 2.5 | 2.2 | 2.1 |
| Mean | 2.6 | 3.3 | 4.0 | 5.2 | 3.9 | 3.7 | |
| SEM | (±0.12) | (±0.14) | (±0.36) | (±0.29) | (±0.16) | (±0.13) | |
| Range | (0-76.7) | (0-107.5) | (0-63.3) | (0-91.7) | (0-120.1) | (0-199.0) | |
| After G-CSF ALC | Median | 3.7 | 2.3 | 3.8 | 2.1 | 3.5 | 1.7 |
| Mean | 4.7 | 2.7 | 4.3 | 2.3 | 3.9 | 1.9 | |
| SEM | (±0.12) | (±0.09) | (±0.22) | (±0.05) | (±0.10) | (±0.05) | |
| Range | (0.0-76.2) | (0.0-49.8) | (0.01-45.7) | (0.0-44.5) | (0.0-39.3) | (0.0-74.0) | |
| G-CSF treatment, yr | Median | 4.3 | 17.9 | 4.5 | 17.1 | 2.0 | 9.2 |
| Mean | 5.3 | 16.9 | 5.4 | 16.3 | 3.2 | 10.7 | |
| SEM | (±0.22) | (±0.52) | (±0.48) | (±0.70) | (±0.17) | (±0.37) | |
| Range | (0-17.6) | (0-31.4) | (0-16.6) | (0-32.2) | (0-15.9) | (0-30.8) | |
ANC = neutrophils × 109/L.
ALC = lymphocytes × 109/L.
Many patients came to the SCNIR with histories of treatment with diverse therapies, particularly in the early years of SCNIR, including prophylactic antibiotics and glucocorticosteroids. It is difficult to evaluate the effectiveness of these therapies and their combinations, but it appears that they offered little benefit and possible risks to these patients.
Table 3 shows ANC and absolute lymphocyte count (ALC) values for each patient category. Notably, the congenital group had the lowest pre-G-CSF neutrophil counts, and this difference was statistically significant compared with all groups (ANOVA, P = .006). The mean G-CSF dose was highest in the congenital group, though this was only statistically significant compared with the cyclic and autoimmune/idiopathic groups (ANOVA; P < .0001). On G-CSF, the mean and median ANC increased in all groups.
Lymphocyte counts were generally within normal limits, but adult patients with autoimmune/idiopathic neutropenia had lower ALCs than the other patient groups (t test; P < .0001). For patients with autoimmune/idiopathic neutropenia in SCNIR, none of the patients who developed a lymphoproliferative disorder had lymphocytosis or a diagnosis of large granular lymphocyte (LGL) leukemia at enrollment.
As previously reported, patients with congenital neutropenia often have moderate thrombocytosis, which reverts to normal on G-CSF.2,3 In many cases, the mild anemia associated with severe chronic neutropenia reverted toward normal if infections and chronic inflammation resolve.2,3
Fever, infections, and inflammation
Before G-CSF, there was a typical pattern of fever and infections. The most common events were fever, pharyngitis, otitis, bronchitis, and mouth ulcers. Oral hygiene was hard to maintain; chronic gingivitis was a common and persistent problem, particularly in patients with congenital neutropenia. Deep tissue infections (ie, pneumonia, abscesses in the liver, spleen, or other tissues) were uncommon. Cyclic neutropenia patients and others with very severe neutropenia were at risk of developing peritonitis and sepsis syndrome.
Patients whose neutrophil counts increased predictably had decreased incidence of fever and infection-related events.2 We believe this is the basis for patient acceptance of daily, alternate-day, or thrice weekly G-CSF treatment, despite injection discomfort and some bone pain. There was excellent adherence observed during a long period when G-CSF vials were returned and counted. Hospital records indicate that stays for infections decreased (before vs after G-CSF), and death from infections decreased. There were 36 recorded deaths from infections during the 16 213 patient-years of observation. These events were attributable to the failure to respond to G-CSF (18 patients), insufficient treatment (10 patients), or failure to maintain consistent treatment (8 patients). Deaths from infections were also complications of transplants or chemotherapy for MDS or leukemia (Table 6).
Table 6.
Overall outcomes for patients with severe chronic neutropenia
| Congenital | Cyclic | Autoimmune/Idiopathic | Total | |
|---|---|---|---|---|
| Patients | 670 | 266 | 816 | 1752 |
| AML/MDS | 76 (11.3%) | 1 (0.01%) | 0 | 77 |
| HSCT, n (%) | 127 (AML: 58; neutropenia: 68, CML: 1) (19.0%) |
4 (AML: 1; neutropenia: 3) (1.9%) |
6 (AML: 0; neutropenia: 5; CMML: 1) (1.0%) |
137 |
| Causes of death | 92 | 24 | 43 | 159 |
| Infection | 21 | 7 | 8 | 36 |
| HSCT, not AML related | 11 | 2 | 1 | 14 |
| AML with HSCT | 35 | 1 | 0 | 36 |
| AML without HSCT | 16 | 1 | 0 | 17 |
| Accident | 0 | 2 | 0 | 2 |
| LPDs | 3 | 0 | 5 | 8 |
| Cardiac | 0 | 2 | 5 | 7 |
| Unknown | 4 | 3 | 12 | 19 |
| Nonmyeloid malignancies (cancer) | 1 (renal) |
2 (1 colon, 1 hepatic) |
4 (2 colon, 1 hepatic, 1 lung) |
7 |
| Other | 1 | 4 | 8 | 13 |
CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; LPD, lymphoproliferative disease.
Outcomes: myeloid malignancies
Overall, MDS or AML has occurred in 77/1752 (4.4%) of the SCNIR-enrolled patients; 11.3% of the 670 congenital patients developed MDS/AML. Table 4 summarizes outcomes for leukemia treatments, dividing the events before and after 2000. This time point was chosen because HSCT after chemotherapy became the predominant mode of treatment around this time.18 The diagnoses and number of affected patients were severe congenital neutropenia (64; patients with or without known associate mutation), and others: SDS (5), GSD1b (3), WAS (2), CXCR4/WHIMs (1), HAX1 (1), and ELANE/cyclic neutropenia (1). There were no cases of AML/MDS in patients with autoimmune/idiopathic neutropenia. The median age at diagnosis of AML/MDS was 16.2 years (mean, 18.7 ± 1.6; standard error of the mean [SEM] [range, 0.40-70.6]); 76 of 77 had been treated with G-CSF, median dose was 7.7 mcg/kg per day (mean, 12.2 ± 1.3; SEM [range, 0.2-100]). The G-CSF dose was significantly higher in congenital patients evolving to MDS and AML; congenital mean G-CSF, 9.6 ± 0.84; SEM (range, 0.16-234.7); congenital patients developing MDS/AML mean G-CSF, 12.46 ± 1.4 SEM (range, 0.6-56.5); P < .0001. It is noteworthy that progression to AML/MDS was rare in patients with cyclic neutropenia; patients were generally treated with lower doses of G-CSF than the congenital group (see Table 3). In the observational cohort of 3248 patient-years, the 1 ELANE/cyclic patient who developed AML had also received long-term immunosuppressive treatment for an unrelated illness.8
Table 4.
Patients with MDS or AML diagnosed before 1 January 2000 or on and after 1 January 2000
| Before 01/01/2000 | After 01/01/2000 | Total | |
|---|---|---|---|
| Patients with MDS/AML | 29 | 48 | 77 |
| Patients with chemotherapy only, n (%) | 10 (34) | 8 (20) | 18 (23) |
| Living | 0 | 0 | 0 |
| Deceased | 9 (90) | 8 (100) | 17 (90) |
| Lost to follow-up | 1 (10) | 0 | 1 |
| Patients with chemotherapy and HSCT, n (%) | 19 (66) | 40 (83) | 59 (77) |
| Living | 5 (26) | 18 (45) | 23 (39) |
| Deceased | 14 (74) | 22 (55) | 36 (61) |
| Lost to follow-up | 0 | 0 | 0 |
Treatment for AML in SCN patients has evolved over the period of this study, with an increasing number of patients receiving HSCTs. Outcomes for AML/MDS patients receiving chemotherapy with HSCT before 2000 were poor, with 5/19 (26%) survivors (Table 4). Since 2000, survivorship has almost doubled, increasing to 18/40 (45%). European investigators have reported higher survival rates for transplantation after the development of leukemia as well as transplantation for patients at high risk of developing leukemia.19,20
Three patients had other hematological malignancies: chronic myeloid leukemia in a congenital neutropenia patient, HSCT (living); chronic lymphocytic leukemia in a cyclic neutropenia patient (living); and chronic myelomonocytic leukemia in an autoimmune/idiopathic neutropenia patient, HSCT (deceased).
Seven patients had reports of myelofibrosis by BM examinations. There were positive reports for 5 congenital and 2 autoimmune/idiopathic patients; the congenital patients were all from the early period of SCNIR (ie, before 2000). One of the 5 patients with congenital neutropenia (no known neutropenia-associated mutation) had 1 positive and 1 negative report of myelofibrosis before enrollment, developed massive splenomegaly, and died of sepsis at 2 years of age. The other 4 congenital neutropenia patients (all without known neutropenia-associated mutations) had ≥1 report of myelofibrosis on BM examination. Two of these patients had multiple BM reports showing inconsistent fibrosis; 1 had 3 positive reports. The other patient had 9 reports showing some evidence of fibrosis and 10 reports not noting fibrosis. The latter patient developed a T-cell lymphoproliferative disorder and died. Two of the 5 patients had a single positive report of myelofibrosis, and 1 had 6 negative reports. Both developed AML, 1 treated with HSCT, and both are now deceased. The 2 patients (enrolled at ages 41 and 42) with the diagnosis of chronic autoimmune/idiopathic neutropenia who developed myelofibrosis had negative BM examinations before G-CSF treatment; 1 patient had 3 reports, the other 1 report. One patient was treated with HSCT (living), and 1 patient died of sepsis about 1 year after developing myelofibrosis.
Outcomes: lymphoid malignancies
Fifteen of 1752 SCNIR patients developed lymphoid malignancies (Table 5). Twelve of 816 (1.5%) patients developing lymphoid malignancies occurred in patients with autoimmune/idiopathic neutropenia. These diagnoses at the time of enrollment were assigned by the referring physician; these patients were listed as idiopathic (11), autoimmune neutropenia (1), and congenital neutropenia (3). None of the patients had clinical characteristics diagnosis of LGL syndrome or LGL leukemia. The evolutions were primary to T-cell disorders (see supplemental Table 1). Three of the 15 patients had a positive test for antineutrophil antibodies. Among the 15 patients, the median age at evolution to a lymphoid malignancy was 46.9 years (range, 17.5-77.6). Eight had T-cell lymphoproliferative disease, 3 had non-Hodgkin lymphoma, and 1 had multiple myeloma. Twelve patients in the autoimmune/idiopathic group were deceased due to severe lymphoproliferative disease and its complications. Only 1 pediatric patient with autoimmune/idiopathic neutropenia developed a T-cell lymphoproliferative disorder. Table 5 summarizes these observations.
Table 5.
Evolution to LPDs
| Patients | Congenital | Cyclic | Autoimmune/ Idiopathic |
|---|---|---|---|
| Female/male | 305/365 | 145/121 | 573/243 |
| Total | 670 | 266 | 816 |
| Patients evolving to LPD | |||
| Patients evolving to LPD diagnosis | 3 | 0 | 12 |
| T-cell LPD | 2 | 0 | 8 |
| Non-Hodgkin lymphoma | 1 | 0 | 3 |
| Multiple myeloma | 0 | 0 | 1 |
LPD, lymphoproliferative disease.
It is important to note that none of the 816 patients in the autoimmune/idiopathic or lymphoproliferative primary diagnostic groups evolved to develop myeloid malignancies.
Other malignancies
Nonmyeloid and nonlymphoid malignancies were relatively rare, suggesting that severe chronic neutropenia does not predispose to other malignancies. For the group of 1752 SCNIR patients, other cancers have occurred mostly in older patients. They were dermatological malignancies (25; 7 before G-CSF, 18 on G-CSF), breast cancer (17; 9 before G-CSF, 8 on G-CSF), colon cancer (6; 1 before G-CSF, 5 on G-CSF), ovarian/endometrial cancer (3; 1 before G-CSF, 2 on G-CSF), hepatic cancer (2, both on G-CSF), renal cancer (1, on G-CSF), lung cancer (1, on G-CSF), prostate cancer (1, before G-CSF), sarcoma (1, on G-CSF), and thyroid cancer (1, on G-CSF). Although we have observed common nonmyeloid malignancies, there is no apparent predisposition to these malignancies in patients with a history of chronic neutropenia or as a result of their long-term treatment with G-CSF.
Other adverse outcomes
There were few other hematological complications in the 1752 patients. It is noteworthy that 4 patients in the autoimmune/idiopathic group developed systemic lupus erythematosus. Immune thrombocytopenic purpura occurred in patients with congenital (2), cyclic (2), and autoimmune/idiopathic (1) neutropenia. One patient developed glomerulonephritis associated with chronic hepatitis. Seven other patients developed chronic hepatitis (subtype information not available).
Mortality
As expected, infections were the most common cause of death, usually pneumonia, bacteremia, or sepsis syndrome, usually a consequence of severe neutropenia from failure to administer G-CSF, lack of response to G-CSF, or as a complication of HSCT (Table 6). Patients with cyclic neutropenia remained at risk of death from infections. Unfortunately, as noted above, these deaths and severe infections were too often attributable to inconsistencies with G-CSF treatment. Overall, autoimmune/idiopathic neutropenia patients have a superior prognosis.
Discussion
SCN is an unusual hematological condition with many causes, both hereditary and acquired.1,21 Its pathophysiology is complex1,14; the common complications are recurrent and severe infections because of inadequate delivery of neutrophils from the BM to the blood and tissues.21 Most of these disorders result from the ineffective production of neutrophils.22 The hereditary disorders result in cell loss in the developmental pathway from the promyelocyte to the mature peripheral blood neutrophil.14 The acquired disorders are most commonly considered autoimmune/idiopathic diseases, with autoantibodies or lymphocyte-derived cytokines impairing neutrophil production at the latter stages of their development.23
This report focuses on long-term outcomes for patients with SCN treated with G-CSF, the dominant therapy for this condition for the last 30 years. G-CSF therapy increases neutrophil counts for almost all SCN patients. The breadth of this effect is attributable to this cytokine’s capacity to stimulate neutrophil production, accelerate neutrophil development, suppress neutrophil apoptosis, and cause the near-immediate release of mature neutrophils from the BM into the blood.24-26 Used preventively in SCN, G-CSF therapy decreases episodes of febrile neutropenia, antibiotic use, and infections.2 The clinical benefit is enormous: because of fewer febrile episodes, G-CSF promotes well-being and increases productivity at work and in school.27,28 There are now many reports on the effectiveness of this therapy.29,30
The SCNIR began as a safety study of the long-term effects of a then-novel biological therapy.3 It steadily accrued records for sufficient numbers of patients to report on patient safety and adverse outcomes.4-8 From the beginning, the SCNIR was concerned about the risk of leukemia, especially since most of the diseases being treated were known to be associated, though rarely, with leukemic transformation.14 For ELANE-associated neutropenia, there are specific mutations associated with more severe outcomes.7,31 The mechanisms whereby certain mutations increase the risk of leukemic transformation are gradually emerging.32,33 High doses of G-CSF may be a risk factor for leukemic transformation, as we and others have reported.34 However, the patients who require the largest doses of G-CSF to achieve a neutrophil count >1.0 × 109/L are the patients who appear to be at the greatest risk.
Long-term SCNIR follow-up indicates that the risk for severe hematological outcomes (eg, myeloid malignancies) resides almost exclusively in patients with congenital neutropenia. In most cases, these develop several years after the start of G-CSF. We have not observed myeloid malignancies in the populations we classified as autoimmune/idiopathic neutropenia, although others have reported natural killer (NK) cell expansion, hairy cell leukemia, and MDS in these patients.35 In this longitudinal study, we observed a few autoimmune/idiopathic patients who developed a lymphoproliferative disorder. However, the diagnostic workup was not uniform, but none had elevated lymphocyte counts or other testing to indicate LGL syndrome or LGL leukemia (Table 6).36-39 Our estimate of the frequency of a lymphoid malignancy in autoimmune/idiopathic neutropenia (12/816 [1.5%]) was higher than that for lymphoproliferative malignancies in patients with systemic lupus erythematosus or celiac disease,40,41 but lower than primary immune deficiency disorders.42 Our findings, however, are consistent with those of Fattizzo and colleagues, who reported that 5 out of 76 patients with autoimmune/idiopathic neutropenia developed NK cell expansion and lymphoproliferative disorders.35 Based on increasing evidence for T-cell–mediated suppression of myeloid cell development in both idiopathic neutropenia and LGL syndrome, we believe adult idiopathic neutropenia and LGL syndrome may be overlapping conditions. For patients with adult-onset idiopathic neutropenia, we recommend that clinicians characterize BM and blood lymphocyte populations by fluorescence-activated cell sorting analysis, examine BM for clusters of lymphoid cells, and do gene rearrangement studies in challenging patients.
Our observations also underscore the differences in the conditions called “autoimmune neutropenia” in children and adults.9,43 The autoimmune/idiopathic children had higher lymphocyte count than autoimmune/idiopathic adults and are far more likely than adults to have spontaneous remissions. We have not observed a worsening of neutropenia in children or adults with autoimmune neutropenia with G-CSF treatment. On the other hand, we have not recorded a spontaneous remission in an adult patient (ie, ≥18 years at onset) with the diagnosis of autoimmune or idiopathic neutropenia. Another important observation is the infrequency of severe adverse events associated with long-term G-CSF treatment. When we began SCNIR, it was unknown if this potent growth factor would alter growth or predispose malignancies. We have not observed either. In the first few weeks of G-CSF treatment, bone pain, myalgias, and headaches were common, but these adverse effects largely disappeared with longer-term treatment, especially for patients on daily or alternate-day therapy.
We recognize the limitation of this study, including the incomplete definition of the immunological status of patients with autoimmune/idiopathic neutropenia, lack of genetic analyses for all patients, and lack of standardization of the timing and reading of BM examinations. We have observed that over time BM examinations have become less frequent in the United States, probably driven by patient preference and costs. We believe that more support is needed for genetic analyses and interpretations. Nevertheless, the SCNIR has demonstrated the value of a registry to collect and longitudinally analyze data to describe clinical outcomes for patients with rare diseases causing severe chronic neutropenia.44
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors acknowledge the dedicated support of the SCNIR staff, the voluntary support of the many physicians and nurses who have cared for the patients, and the extraordinary cooperation of the patients and their families.
This work was supported in part by a National Institutes of Health, National Institutes of Allergy and Infectious Diseases Grant (R24AI049393), Amgen and the Amgen Foundation (Thousand Oaks, CA), The Ella Jewell Foundation, and the National Neutropenia Network of patients and families.
Authorship
Contribution: D.C.D. was the primary author of the study; A.A.B. conducted data collection and analysis; J.A.S. organized and analyzed data on autoimmune/idiopathic neutropenia; J.A.C. assisted in collecting and analyzing data on HSCT; D.C.L. led studies on genetic diagnosis; M.A.B. provided clinical data and advice; and P.E.N. assisted in authorship and provided clinical data.
Conflict-of-interest disclosure: D.C.D. reports research funding from X4 Pharma, Amgen Inc, and Emendo Bio; consultancy for Amgen, X4 Pharma, and Emendo; and equity ownership in Athelas, Inc. P.E.N. reports consultancy and honoraria from X4 Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: David C. Dale, University of Washington, Box 356422, 1959 NE Pacific St, Rm 522, Seattle, WA 98195-6422; e-mail: dcdale@uw.edu.
References
- 1.Dale DC, Welte K. Neutropenia. In: Kausahnsky K, Lichtman MA, Prchal JT, Levi MM, Burn LJ, eds. Williams Hematology, 10th ed. New York, NY: McGraw Hill; 2021:1021-1036 [Google Scholar]
- 2.Dale DC, Bonilla MA, Davis MW, et al. A randomized controlled phase III trial of recombinant human granulocyte colony-stimulating factor (filgrastim) for treatment of severe chronic neutropenia. Blood. 1993;81(10):2496-2502. [PMC free article] [PubMed] [Google Scholar]
- 3.Dale DC, Cottle TE, Fier CJ, et al. Severe chronic neutropenia: treatment and follow-up of patients in the Severe Chronic Neutropenia International Registry. Am J Hematol. 2003;72(2):82-93. [DOI] [PubMed] [Google Scholar]
- 4.Freedman MH, Bonilla MA, Fier C, et al. Myelodysplasia syndrome and acute myeloid leukemia in patients with congenital neutropenia receiving G-CSF therapy. Blood. 2000;96(2):429-436. [PubMed] [Google Scholar]
- 5.Rosenberg PS, Alter BP, Link DC, et al. Neutrophil elastase mutations and risk of leukaemia in severe congenital neutropenia. Br J Haematol. 2008;140(2):210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg PS, Zeidler C, Bolyard AA, et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150(2):196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makaryan V, Zeidler C, Bolyard AA, et al. The diversity of mutations and clinical outcomes for ELANE-associated neutropenia. Curr Opin Hematol. 2015;22(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale DC, Bolyard A, Marrero T, et al. Long-term effects of G-CSF therapy in cyclic neutropenia. N Engl J Med. 2017;377(23):2290-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dale DC, Bolyard AA. An update on the diagnosis and treatment of chronic idiopathic neutropenia. Curr Opin Hematol. 2017;24(1):46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newburger PE; American Society of Hematology Educational Program . Autoimmune and other acquired neutropenias. Hematology (Am Soc Hematol Educ Program). 2016;2016(1):38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dale DC, Bolyard AA, Marrero T, et al. Neutropenia in glycogen storage disease Ib: outcomes for patients treated with granulocyte colony-stimulating factor. Curr Opin Hematol. 2019;26(1):16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boxer LA, Bolyard AA, Schwinzer B, et al. Antineutrophil antibodies lead to mistaken identity in severe congenital neutropenia. [abstract] Blood. 2005;106(11):385. [Google Scholar]
- 13.Dale DC, Bolyard AA. Severe chronic neutropenia international registry patient handbook. Available at: https://www.scnir-neutropenia.uw.edu/ [DOI] [PMC free article] [PubMed]
- 14.Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primers. 2017;3(3):17032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale DC, Person RE, Bolyard AA, et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000; 96(7):2317-2322. [PubMed] [Google Scholar]
- 16.Yılmaz Karapınar D, Patıroğlu T, Metin A, et al. Homozygous c.130-131 ins A (pW44X) mutation in the HAX1 gene as the most common cause of congenital neutropenia in Turkey: report from the Turkish Severe Congenital Neutropenia Registry. Pediatr Blood Cancer. 2019;66(10):e27923. [DOI] [PubMed] [Google Scholar]
- 17.Furutani E, Newburger PE, Shimamura A. Neutropenia in the age of genetic testing: advances and challenges. Am J Hematol. 2019;94(3):384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferry C, Ouachée M, Leblanc T, et al. Hematopoietic stem cell transplantation in severe congenital neutropenia: experience of the French SCN register. Bone Marrow Transplant. 2005;35(1):45-50. [DOI] [PubMed] [Google Scholar]
- 19.Fioredda F, Iacobelli S, van Biezen A, et al. ; Severe Aplastic Anemia the Inborn Error, and the Pediatric Disease Working Parties of the European Society for Blood and Bone Marrow Transplantation (EBMT) and Stem Cell Transplant for Immunodeficiencies in Europe (SCETIDE) . Stem cell transplantation in severe congenital neutropenia: an analysis from the European Society for Blood and Marrow Transplantation. Blood. 2015; 126(16):1885-1892, quiz 1970. [DOI] [PubMed] [Google Scholar]
- 20.Rotulo GA, Beaupain B, Rialland F, et al. HSCT may lower leukemia risk in ELANE neutropenia: a before-after study from the French Severe Congenital Neutropenia Registry. Bone Marrow Transplant. 2020;55(8):1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale DC, Guerry D IV, Wewerka JR, Bull JM, Chusid MJ. Chronic neutropenia. Medicine (Baltimore). 1979;58(2):128-144. [DOI] [PubMed] [Google Scholar]
- 22.Price TH, Lee MY, Dale DC, Finch CA. Neutrophil kinetics in chronic neutropenia. Blood. 1979;54(3):581-594. [PubMed] [Google Scholar]
- 23.Mastrodemou S, Stalika E, Vardi A, et al. Cytotoxic T cells in chronic idiopathic neutropenia express restricted antigen receptors. Leuk Lymphoma. 2017;58(12):2926-2933. [DOI] [PubMed] [Google Scholar]
- 24.Chatta GS, Price TH, Allen RC, Dale DC. Effects of in vivo recombinant methionyl human granulocyte colony-stimulating factor on the neutrophil response and peripheral blood colony-forming cells in healthy young and elderly adult volunteers. Blood. 1994;84(9):2923-2929. [PubMed] [Google Scholar]
- 25.Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood. 1996;88(1):335-340. [PubMed] [Google Scholar]
- 26.Pedersen CC, Borup R, Fischer-Nielsen A, et al. Changes in gene expression during G-CSF-induced emergency granulopoiesis in humans. J Immunol. 2016;197(5):1989-1999. [DOI] [PubMed] [Google Scholar]
- 27.Jones E, Bolyard AA, Dale DC. Quality of life of patients with severe chronic neutropenia receiving long-term treatment with granulocyte colony-stimulating factor. JAMA. 1993;270(9):1132-1133. [DOI] [PubMed] [Google Scholar]
- 28.Michniacki TF, Merz LE, McCaffery H, Connelly JA, Walkovich K. Quality of life and patient-reported outcomes in chronic severe neutropenia conditions. Int J Hematol. 2021;113(5):735-743. [DOI] [PubMed] [Google Scholar]
- 29.Sicre de Fontbrune F, Moignet A, Beaupain B, et al. ; French Severe Chronic Neutropenia Registry . Severe chronic primary neutropenia in adults: report on a series of 108 patients. Blood. 2015;126(14):1643-1650. [DOI] [PubMed] [Google Scholar]
- 30.Wan C, Yu H-H, Lu M-Y, et al. Clinical manifestations and outcomes of pediatric chronic neutropenia. J Formos Med Assoc. 2012;111(4): 220-227. [DOI] [PubMed] [Google Scholar]
- 31.Garg B, Mehta HM, Wang B, Kamel R, Horwitz MS, Corey SJ. Inducible expression of a disease-associated ELANE mutation impairs granulocytic differentiation, without eliciting an unfolded protein response. J Biol Chem. 2020;295(21):7492-7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link DC. Mechanisms of leukemic transformation in congenital neutropenia. Curr Opin Hematol. 2019;26(1):34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dannenmann B, Klimiankou M, Oswald B, et al. iPSC modeling of stage-specific leukemogenesis reveals BAALC as a key oncogene in severe congenital neutropenia. Cell Stem Cell. 2021;28(5):906-922.e6. [DOI] [PubMed] [Google Scholar]
- 34.Donadieu J, Leblanc T, Bader Meunier B, et al. ; French Severe Chronic Neutropenia Study Group; Experience of the French Severe Chronic Neutropenia Study Group . Analysis of risk factors for myelodysplasias, leukemias and death from infection among patients with congenital neutropenia. Haematologica. 2005;90(1):45-53. [PubMed] [Google Scholar]
- 35.Fattizzo B, Zaninoni A, Consonni D, et al. Is chronic neutropenia always a benign disease? Evidences from a 5-year prospective study. Eur J Intern Med. 2015;26(8):611-615. [DOI] [PubMed] [Google Scholar]
- 36.Gazitt T, Loughran TP Jr. Chronic neutropenia in LGL leukemia and rheumatoid arthritis. Hematology (Am Soc Hematol Educ Program). 2017;2017(1):181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wlodarski MW, Nearman Z, Jiang Y, Lichtin A, Maciejewski JP. Clonal predominance of CD8(+) T cells in patients with unexplained neutropenia. Exp Hematol. 2008;36(3):293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savola P, Brück O, Olson T, et al. Somatic STAT3 mutations in Felty syndrome: an implication for a common pathogenesis with large granular lymphocyte leukemia. Haematologica. 2018;103(2):304-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajala HLM, Porkka K, Maciejewski JP, Loughran TP Jr, Mustjoki S. Uncovering the pathogenesis of large granular lymphocytic leukemia-novel STAT3 and STAT5b mutations. Ann Med. 2014;46(3):114-122. [DOI] [PubMed] [Google Scholar]
- 40.Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther. 2018;20(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Gils T, Nijeboer P, Overbeek LI, et al. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: consequences for follow-up: celiac disease, lymphoma and GI carcinoma. United European Gastroenterol J. 2018;6(10):1485-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riaz IB, Faridi W, Patnaik MM, Abraham RS. A systematic review on predisposition to lymphoid (B and T cell) neoplasias in patients with primary immunodeficiencies and immune dysregulatory disorders (inborn errors of immunity). Front Immunol. 2019;10:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fioredda F, Rotulo GA, Farruggia P, et al. Late-onset and long-lasting autoimmune neutropenia: an analysis from the Italian Neutropenia Registry. Blood Adv. 2020;4(22):5644-5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dale DC, Bolyard AA, Steele LA, Zeidler C, Welte K; Severe Chronic Neutropenia International Registry . Registries for study of nonmalignant hematological diseases: the example of the Severe Chronic Neutropenia International Registry. Curr Opin Hematol. 2020;27(1):18-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.