Abstract
Microbial community samples were collected from the anoxic zone of the Cariaco Basin at depths of 320, 500, and 1,310 m on a November 1996 cruise and were used to construct 16S ribosomal DNA libraries. Of 60 nonchimeric sequences in the 320-m library, 56 belonged to the ɛ subdivision of the Proteobacteria (ɛ-Proteobacteria) and 53 were closely related to ectosymbionts of Rimicaris exoculata and Alvinella pompejana, which are referred to here as epsilon symbiont relatives (ESR). The 500-m library contained sequences affiliated with the fibrobacteria, the Flexibacter-Cytophaga-Bacteroides division, the division Verrucomicrobia, the division Proteobacteria, and the OP3 candidate division. The Proteobacteria included members of the γ, δ, ɛ and new candidate subdivisions, and γ-proteobacterial sequences were dominant (25.6%) among the proteobacterial sequences. As in the 320-m library, the majority of the ɛ-proteobacteria belonged to the ESR group. The genus Fibrobacter and its relatives were the second largest group in the library (23.6%), followed by the δ-proteobacteria and the ɛ-proteobacteria. The 1,310-m library had the greatest diversity; 59 nonchimeric clones in the library contained 30 unique sequences belonging to the planctomycetes, the fibrobacteria, the Flexibacter-Cytophaga-Bacteroides division, the Proteobacteria, and the OP3 and OP8 candidate divisions. The proteobacteria included members of new candidate subdivisions and the β, γ, δ, and ɛ-subdivisions. ESR sequences were still present in the 1,310-m library but in a much lower proportion (8.5%). One archaeal sequence was present in the 500-m library (2% of all microorganisms in the library), and eight archaeal sequences were present in the 1,310-m library (13.6%). All archaeal sequences fell into two groups; two clones in the 1,310-m library belonged to the kingdom Crenarchaeota and the remaining sequences in both libraries belonged to the kingdom Euryarchaeota. The latter group appears to be related to the Eel-TA1f2 sequence, which belongs to an archaeon suggested to be able to oxidize methane anaerobically. Based on phylogenetic inferences and measurements of dark CO2 fixation, we hypothesized that (i) the ESR are autotrophic anaerobic sulfide oxidizers, (ii) sulfate reduction and fermentative metabolism may be carried out by a large number of bacteria in the 500- and 1,310-m libraries, and (iii) members of the Euryarchaeota found in relatively large numbers in the 1,310-m library may be involved in anaerobic methane oxidation. Overall, the composition of microbial communities from the Cariaco Basin resembles the compositions of communities from several anaerobic sediments, supporting the hypothesis that the Cariaco Basin water column is similar to anaerobic sediments.
The Cariaco Basin is the second largest of the world's anoxic pelagic systems and is the only large, truly marine, permanently anoxic basin (32). Because of the basin depth (1,400 m) and the restricted circulation caused by a sill at 90 to 140 m, the Cariaco Basin contains no oxygen below depths of about 240 to 320 m. Previous studies of the geochemistry of the Cariaco Basin have shown that the system is not in steady state, because the concentrations of silica, phosphate, sulfide, and methane have increased over the last 25 years (32, 36, 37, 51). However, Cariaco Basin waters are partially flushed from time to time, but the processes that trigger flushing and its frequency are still poorly understood. In the past, the major focus of research in the Cariaco Basin and similar systems has been on the transition zone (2, 12, 14, 16, 51). Chemoautotrophy in the transition zone has been previously reported for both the Black Sea (15, 38, 44) and the Cariaco Basin (40, 44, 45). The prokaryotic communities and the chemical environment of many permanently stratified basins have been investigated by traditional cultivation-dependent methods (31, 44). Tuttle and Jannasch (44) isolated several sulfide- and thiosulfate-oxidizing bacteria from the redox interfaces of the Cariaco Basin and the Black Sea. Clearly, this environment, like the ocean at large, is undersampled with respect to bacterial diversity because the culturability of marine bacteria is presently less than 0.1% (17).
Molecular biological approaches for studying microbial diversity have opened new perspectives for microbial ecology. Novel, cultivation-independent methods for studies of marine bacteria and archaea have revealed large numbers of unknown microorganisms, which appear to be largely unaffiliated with previous isolates from the same environment (5, 48). Most of the research in marine molecular ecology has been directed toward microbial populations of the water column (5, 6, 47, 48), marine sediments (10, 19, 30, 50), arctic ice (4), and salt marsh sediments (24, 33). Molecular ecological methods have also been used to study the distribution of bacterial populations in a fjord that is stratified and anoxic most of the time (Mariager Fjord, Denmark) (29, 41). However, comprehensive descriptions of microbial communities in anoxic water columns have not been reported previously.
This study focused on microbial communities in the anoxic zone of the Cariaco Basin, which has the advantage of being a relatively stable environment in which only the amount of substrate delivered from surface waters varies (40). Thus, analysis of 16S ribosomal DNA (rDNA) libraries obtained from one cruise has a high probability of being representative of the Cariaco Basin system in general. We postulate that the microbial community in the anoxic zone of the Cariaco Basin should resemble that of sediments more than that of oxic water columns. Using the 16S rDNA library approach, we examined vertical distributions of prokaryotic populations with depth for samples collected at the CARIACO time series station on 8 November 1996 (CAR25). Compositions of prokaryotic communities in the oxic water column have been more or less well studied (5, 6, 9, 47, 48), and many species appear to be cosmopolitan in distribution. Therefore, further study of surface waters in the Cariaco Basin seemed to be unwarranted, and previously published phylogenetic libraries were used in our comparisons.
The commonly observed distribution patterns of redox-sensitive elements and microbial abundance suggest that microorganisms in the Cariaco Basin (40) proliferate in response to gradients of specific sources of energy (reduced organic and inorganic compounds) and terminal electron acceptors (oxygen, nitrate, elemental sulfur, and oxidized metal). The identities and physiological capabilities of the prokaryotes in the layers are very poorly understood. A census of community diversity is the initial step towards the challenging goal of assigning specific microorganisms to particular biogeochemical processes.
MATERIALS AND METHODS
Water sampling and analyses.
Three water column samples were collected during a cruise on 8 November 1996 at a single sampling site located in the eastern basin of the Cariaco Basin system (10°30′N, 64°40′W) at depths of 320, 500, and 1,310 m. The samples were collected by using standard 8-liter Niskin bottles. For DNA extraction, 2 liters of seawater was filtered through Durapore membrane filters (diameter, 47 mm; pore size, 0.2 μm; Millipore). The membrane filters were then shredded and immediately immersed in 4.5 ml of autoclaved DNA extraction buffer (20 mM Tris-HCl [pH 7.5 to 8.2], 50 mM EDTA, 20 mM NaCl) and stored frozen (−20°C) until DNA was extracted. Complete depth profiles for physical properties, nutrients, sulfide, oxygen, bacterial counts, biomass, production, and dark CO2 fixation for this cruise have been published elsewhere (40).
DNA extraction and purification.
The procedure used for extraction of chromosomal DNA is a modification of a procedure described by Xu and Tabita (49). This procedure employs enzymatic and detergent-based lysis, which is a relatively gentle method that avoids excessive shearing of DNA, produces DNA of suitable quality for PCR, and reduces the risk of chimera formation during PCR. DNA was extracted from thawed membranes by adding 0.5 ml of 10% sodium dodecyl sulfate and 250 μl of a proteinase K solution (10 mg/ml), followed by incubation at 50°C for 10 min and freezing for 15 min. This freeze-thaw procedure was repeated two more times. The thawed mixture was extracted twice with water-saturated phenol (neutralized with 0.5 M Tris-HCl buffer, pH 7.8 to 8.5) and extracted once with a chloroform-isoamyl alcohol (24:1) solution. Nucleic acids were precipitated by adding 0.1 volume of 3 M sodium acetate (pH 5.2) and 2 volumes of chilled 95% (vol/vol) ethanol. The mixture was incubated at −80°C for at least 20 min and then centrifuged at 21,000 × g for 10 min. The DNA pellet was washed with 70% ethanol, left to dry in a desiccator for 10 to 15 min, and then dissolved in 100 μl of sterile high-performance liquid chromatography grade water (Sigma) and stored at −20°C.
PCR amplification.
Amplification of 16S rRNA genes from the samples was carried out by performing PCR by the procedure of Borneman et al. (3), with modifications. Either Taq DNA polymerase (Promega, Madison, Wis.) or Deep Vent DNA polymerase (New England Biolabs, Beverley, Mass.) was used. For amplification, each 50-μl reaction mixture contained the following components (final concentration or total amount): 1 μl of DNA (concentration, 100 to 200 ng/μl), 1× reaction buffer provided by each manufacturer, 2.5 mM MgCl2 for Taq polymerase or 7 mM MgSO4 for Deep Vent polymerase, 1 mM concentrations of deoxynucleoside triphosphates, 2.5 μM primer 530F, 2.5 μM primer 1494R, and 1.5 U of Taq polymerase or 1 U of Deep Vent polymerase. After all reagents were mixed and kept at 0°C, DNA was amplified by using a microprocessor-controlled heating block (Crocodile III; Appligene SA, Illkirch, France). Prior to amplification, DNA was denatured at 92°C for 5 min. Forty cycles of PCR were then performed at 92°C for 1 min (95°C for Deep Vent polymerase), 50°C for 30 s, and 72°C for 1 min, followed by 72°C for 3 min (3).
The universal primers used to amplify small-subunit ribosomal genes were as follows: 530F (5′-GCTCTAGAGCTGACTGACTGAGTGCCAGCMGCCGCGG-3′; position 530 to 546 [Escherichia coli numbering]) and 1494R (5′-GCTCTAGAGCTGACTGACTGAGGYTACCTTGTTACGACTT-3′; positions 1494 to 1523 [E. coli numbering]). Each primer contained an additional sequence (underlined) that has the stop codon TGA in all three reading frames. The sequence TCTAGA (restriction site of endonuclease XbaI) was introduced into the 5′ end of each primer. PCR products were purified by using a Wizard PCR Preps DNA purification system (Promega) according to the manufacturer's instructions.
The 530F and 1494R primers chosen for this research are universal primers which preferably amplify a 1-kb portion of the bacterial and archaeal 16S rRNA gene. The resulting PCR fragment contains a conserved region (nucleotides 531 to 785 [E. coli numbering], adjacent to the 530F primer binding sequence), hypervariable region V9, and a portion of region V8 (nucleotides 1253 to 1492 [E. coli numbering], adjacent to the 1494R primer binding sequence).
16S rDNA library construction.
In order to generate the 320-m 16S rDNA library, three different cloning strategies were used. If Deep Vent DNA polymerase was used for PCR amplification, blunt end ligation was carried out with pBluescript II SK(+) digested with restriction endonuclease EcoRV and PCR products. Thirty-eight clones in the library were generated by using this approach. Since Deep Vent DNA polymerase has a proof-reading activity, we made sure that inserts from the clones contained the complete primer sequences; i.e., the primer-template duplex was not proof read by Deep Vent DNA polymerase. For PCR products obtained from PCR performed with Taq polymerase, either cohesive end ligation was performed by digestion with restriction enzyme XbaI and ligation into the commercial vector pBluescript II SK(+)(Stratagene, La Jolla, Calif.) linearized with XbaI (four clones in the library) or PCR products were directly cloned into the pGEM-T vector (18 clones in the library). The four XbaI-generated clones in the library were chosen at random and not on the basis of the correct size (1 kb) of the insert. Each ligation mixture consisted of 7.5 μl of purified PCR product, 5 Weiss units of T4 ligase, 1× ligation buffer (Boehringer, Indianapolis, Ind.), and 25 ng of linear pBluescript II SK(+) or pGEM-T. Ligation mixtures were incubated at 16°C overnight. For the 500- and 1,310-m 16S rDNA libraries, only Taq DNA polymerase was used. Purified PCR products were ligated directly into the pGEM-T vector by using a 2× rapid ligation kit according to the instructions of the manufacturer (Promega). Ligation mixtures were used to transform competent cells of E. coli XL-1 Blue MRF′ [F′ (Tn10 proA+B+ lacIq ΔlacZM15) recA1 endA1 gyrA96 (Nalr) thi hsdR17 (rk− mk+) supE44 relA1 lac] by the procedure of Sambrook et al. (35) or by using commercially available high-efficiency competent cells of E. coli JM109 according to the suggestions of the manufacturer (Promega).
Template preparation and sequencing.
Plasmid DNA was isolated from randomly picked white colonies by a boiling method (35). Restriction fragment length polymorphism (RFLP) analysis of isolated plasmids was carried out by digestion with HinfI and HaeIII (New England Biolabs). DNA band patterns were visualized by electrophoresis in 2% Tris-borate-EDTA (TBE)–agarose gels (35). Single-stranded DNA was isolated from each clone representing an individual HinfI/HaeIII pattern according to Stratagene instructions and by using the VCM13 helper phage (Stratagene). Single- and double-stranded DNA sequencing was carried out with an ABI Prism BigDye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Foster City, Calif.). Plasmids which were pGEM-T-derivatives were sequenced with primer −21 M13 (5′-GTAAAACGACGGCCAGT-3′). Plasmids which were pBluescript II SK(+) derivatives were sequenced with primer KS (5′-GAGCTCCAGCTGCCATAG′-3). Sequences obtained with primers −21 M13 and KS were ∼500 bp long. In order to complete sequences of all or parts of the 1-kb fragment (amplified with primers 530F and 1494R), the following additional primers were used for sequencing: 16SF (5′-ACRGGATTAGATACCCVGG-3′; positions 781 to 799 [E. coli numbering]) and 16SR (5′-CCATTGTAVACAGGTGTDGCCC-3′; positions 1219 to 1241 [E. coli numbering]).
Preparation of phylogenetic trees and phylogenetic assignment.
Sequences retrieved from the Cariaco Basin 16S rDNA libraries were compared to the 16S rDNA sequences available in the 16S rRNA database by using the SEQUENCE_MATCH program from the Ribosomal Database Project (RDP) (20) and to sequences in the GenBank database by using the FASTA program (Wisconsin Package, version 9.1, of the Genetics Computer Group). The presence of chimeric sequences in the libraries was determined by using the CHECK_CHIMERA program from RDP.
For alignment, the rDNA clone sequences were separated into the following three groups: (i) sequences sequenced through a conserved region (adjacent to the primer 530F sequence) (group A), (ii) sequences sequenced through hypervariable region V9 and a portion of V8 (adjacent to the primer 1494R sequence) (group B), and (iii) sequences with the complete 1-kb sequence (group C). Sequences from each group were then aligned with their closest relatives by using the programs PILEUP (Genetics Computer Group) and ClustalW (J. Thompson and T. Gibson, European Molecular Biology Laboratory, Heidelberg, Germany) and representatives of the major phylogenetic taxa. Portions of the complete 1-kb sequences were also introduced into the alignments of the group A and B sequences. Trees were constructed by using two independent algorithms: distance analyses with Jukes-Cantor corrections (DNAdist) and FITCH from the PHYLIP package (J. Felsenstein, PHYLIP [Phylogeny Inference Package], version 3.57c) and maximum likelihood (DNAml program, also from PHYLIP). In order to determine confidence values for individual branches of each final tree, bootstrap analysis was applied to each tree generated by using the SEQBOOT and CONSENSE programs from the PHYLIP package.
Nucleotide sequence accession numbers.
The sequences of the Cariaco Basin rDNA clones have been deposited in the GenBank database under accession numbers AF224775 to AF224872.
RESULTS
Construction and analysis of 16S rDNA gene libraries.
DNA extracted from samples harvested from three anoxic depths in the Cariaco Basin was amenable to direct PCR amplification. The highest yield of chromosomal DNA was obtained with the 320-m sample, which agrees with the bacterial abundance trends measured for the cruise (40). Several independent sublibraries (independent repetitions of PCR and ligation reactions and transformations) were created for each depth. Clones of all sublibraries from a given depth were mixed to create three final libraries representing the three depths.
From the 320-, 500-, and 1,310-m libraries, 61, 56, and 65 colonies, respectively, with 1-kb inserts were randomly selected and screened by RFLP analysis. From these clones, 32, 45, and 59 unique RFLP band patterns, respectively, were identified. Further sequencing analysis confirmed that only 10, 32, and 44 clones, respectively, contained unique DNA sequences. Sequences which differed by less than 0.1% were consider identical. Thus, the 1,310-m library had the highest percentage of unique sequences among the three libraries, and the 320-m library had the lowest. The presence of chimeric sequences was determined by using the CHECK_CHIMERA program from RDP. The chimeric sequences identified (Table 1) were not included in further phylogenetic analyses. The 16S rDNA sequences are summarized in Table 1; sequences from the 320-, 500-, and 1,310-m libraries have the suffixes “a,” “b,” and “c,” respectively, in their designations.
TABLE 1.
16S rDNA sequences identified in anoxic water column of the Cariaco Basin in three clone libraries
| Phylogenetic affiliation | Representative clone (accession no.) | No. of clones in library
|
Closest relative
|
||||
|---|---|---|---|---|---|---|---|
| 320 m | 500 m | 1,310 m | Taxon | Accession no. | % Identity | ||
| Archaea | |||||||
| Crenarchaeota | Car132fc (AF224859) | 2 | Archaeon C20 | U71114 | 98.9 | ||
| Euryarchaeota | Car127c (AF224855) | 1 | Methanospirillum hungatei | M60880 | 74.5 | ||
| Car106b (AF224779) | 1 | Methanospirillum hungatei | M60880 | 74.9 | |||
| Car42fc (AF224831)a | 3 | Thermoplasma acidophilum | M38637 | 74.2 | |||
| Car68fc (AF224866) | 1 | Thermoplasma acidophilum | M38637 | 75.3 | |||
| Car154fc (AF224869) | 1 | Thermoplasma acidophilum | M38637 | 72.4 | |||
| Bacteria | |||||||
| Candidate division OP8 | Car60fc (AF224839) | 1 | OPB95 | AF027060 | 90.3 | ||
| Candidate division OP3 | Car11fa (AF224797) | 1 | OPB2 | AF027088 | 80.1 | ||
| Car136b (AF224787) | 2 | BD3-9 | AB015551 | 80.9 | |||
| Car30c (AF224825) | 1 | Koll 11 | AJ224540 | 82.6 | |||
| Car40rc (AF224830) | 1 | Geobacter metallireducens | L07834 | 81.2 | |||
| Car119rc (AF224848) | 1 | BD4-9 | AB015559 | 79.2 | |||
| Car121c (AF224850) | 1 | Methylobacterium sp. | D87985 | 79.1 | |||
| Car124rc (AF224853) | 1 | Clostridrium thermocellum | L09173 | 79.4 | |||
| Planctomycetes | Car65rc (AF224864) | 1 | BD2-3 | AB015533 | 85.9 | ||
| Car68rc (AF224865) | 1 | BD2-3 | AB015533 | 85.6 | |||
| Car122fc (AF224851) | 1 | BD2-3 | AB015533 | 85.2 | |||
| Fibrobacteria | Car104fb (AF224872)ab | 6 | SAR406 | U34043 | 89.4 | ||
| Car97fb (AF224822)b | 1 | SAR406 | U34043 | 86.1 | |||
| Car137rb (AF224788) | 1 | SAR406 | U34043 | 90.5 | |||
| Car53c (AF224775) | 2 | 1 | OCS307 | U41450 | 87.6 | ||
| Car129rc (AF224857)a | 2 | 2 | Clone pG-13 | AF001509 | 82.1 | ||
| Flexibacter-Cytophaga-Bacteroides | Car44rc (AF224833) | 1 | Bacteroides ovatus | X83952 | 92.2 | ||
| Car56rb (AF224809) | 1 | 1 | Sphingobacterium heparinum | M11657 | 98.5 | ||
| Car82fb (AF224811) | 1 | Flavobacterium balustinum | D14016 | 92.1 | |||
| Car114fc (AF224844) | 1 | Flavobacterium balustinum | D14016 | 99.2 | |||
| Car116rc (AF224845) | 1 | Acinetobacter sp. | Y15852 | 73 | |||
| Verrucomicrobia | Car98rb (AF224823) | 1 | Clone WCHB1-41 | AF050560 | 86.9 | ||
| Proteobacteria | |||||||
| Nonaffiliated proteobacteria | Car731c (AF224870) | 2 | A51p | X91474 | 88.2 | ||
| Car63a (AF224801) | 1 | Clone MUG16 | AB011308 | 73.2 | |||
| Car33fb (AF224808) | 2 | Lucina floridana symbiont | L25707 | 86.8 | |||
| β-Proteobacteria | Car117fc (AF224846) | 1 | Alcaligenes faecalis | AJ277669 | 97.5 | ||
| γ-Proteobacteria | Car16fa (AF224800) | 1 | Thiomicrospira sp. | L40811 | 86.7 | ||
| Car36rc (AF224827)a | 2 | Acyrthosiphon pisum symbiont | AB033779 | 93.0 | |||
| Car128fc (AF224856)c–e | 4 | 1 | Clone UN106 | D63592 | 99.4 | ||
| Car91fb (AF224817)c | 1 | Clone UN106 | D63592 | 99.7 | |||
| Car141fb (AF224791)d | 1 | Clone UN106 | D63592 | 99.3 | |||
| Car149fc (AF224862)e | 1 | Clone UN106 | D63592 | 99.7 | |||
| Car39fc (AF224829) | 1 | Citrobacter farmeri | AF025371 | 93.4 | |||
| Car70c (AF224867)a | 3 | 11 | PVB18 | U15114 | 99.1 | ||
| Car43fc (AF224832) | 1 | PVB18 | U15114 | 95.6 | |||
| Car140fb (AF224790) | 2 | PVB18 | U15114 | 95.5 | |||
| Car92rb (AF224818) | 1 | 1 | PVB18 | U15114 | 96.5 | ||
| Car123rc (AF224852) | 1 | Photobacterium phosphoreum | D25310 | 95.9 | |||
| δ-Proteobacteria | Car45fc (AF224834) | 1 | 2 | Clone SB-30 | AF029048 | 96.2 | |
| Car102rb (AF224777) | 1 | Clone SB-30 | AF029048 | 95.4 | |||
| Car148rc (AF224861) | 1 | Clone SB-30 | AF029048 | 88.4 | |||
| Car113fc (AF224843) | 1 | Clone SB-30 | AF029048 | 94.9 | |||
| Car99rb (AF224824) | 1 | Clone SB-30 | AF029048 | 91.4 | |||
| Car81fb (AF224810) | 1 | Clone A48u | X91471 | 90.0 | |||
| Car86rb (AF224813) | 1 | Clone NKB19 | AB013271 | 81.3 | |||
| Car93fb (AF224819) | 1 | Clone BD7-15 | AB015588 | 93.4 | |||
| Car95fb (AF224821) | 1 | Clone SB-21 | AF029045 | 93.9 | |||
| Car7ra (AF224796) | 1 | SAR276 | U65915 | 88.9 | |||
| Car150fc (AF224868) | 1 | RFLP25 | AF058007 | 95.7 | |||
| Car103rb (AF224871) | 2 | Bdellovibrio stolpii | AJ288899 | 90.5 | |||
| Car147fc (AF224860) | 1 | Strain S16 | Z69320 | 92.8 | |||
| Car37fc (AF224828) | 2 | Desulfobacter postgatei | M26633 | 83.6 | |||
| ɛ-Proteobacteria | Car153a (AF224805)a | 51 | Clone NKB12 | AB013264 | 94.6 | ||
| CarB13fa (AF224804) | 1 | Clone NKB12 | AB013264 | 96.7 | |||
| Car29rb (AF224806) | 3 | Clone NKB12 | AB013264 | 95.8 | |||
| Car55fb (AF224807) | 6 | Clone NKB12 | AB013264 | 95.6 | |||
| Car59rc (AF224838) | 4 | Clone NKB12 | AB013264 | 95.4 | |||
| Car125fc (AF224854) | 1 | Clone NKB12 | AB013264 | 95.6 | |||
| Car13a (AF224799) | 1 | Clone BD7-6 | AB013264 | 94.2 | |||
| Car2a (AF224795) | 1 | “Thiomicrospira denitrificans” | L40808 | 94.2 | |||
| Car107fb (AF224780) | 1 | “Thiomicrospira denitrificans” | L40808 | 91.7 | |||
| Car12fa (AF224798) | 2 | Arcobacter nitrofigilis | L14627 | 93.9 | |||
| Chimeras | 1 | 5 | 6 | ||||
| Total | 61 | 56 | 65 | ||||
Representative of a group of closely related sequences (>99% identity).
Car104fb and Car97fb exhibit 98.7% identity.
Car128fc and Car91fb exhibit 98.2% identity.
Car128fc and Car141fb exhibit 98.8% identity.
Car128fc and Car149fc exhibit 98.3% identity.
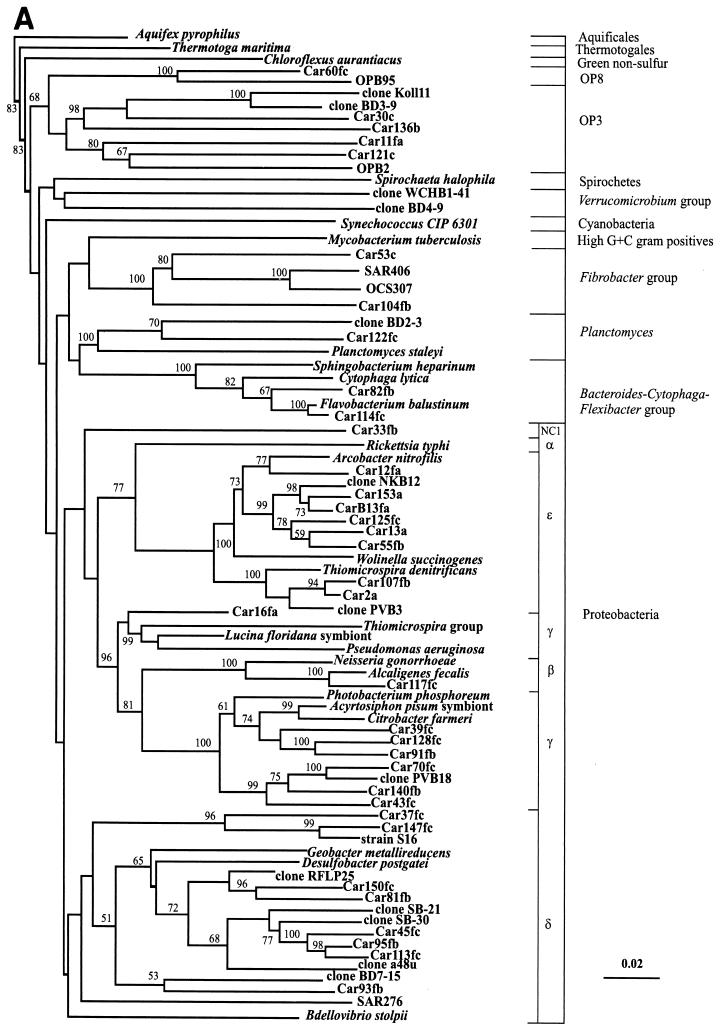
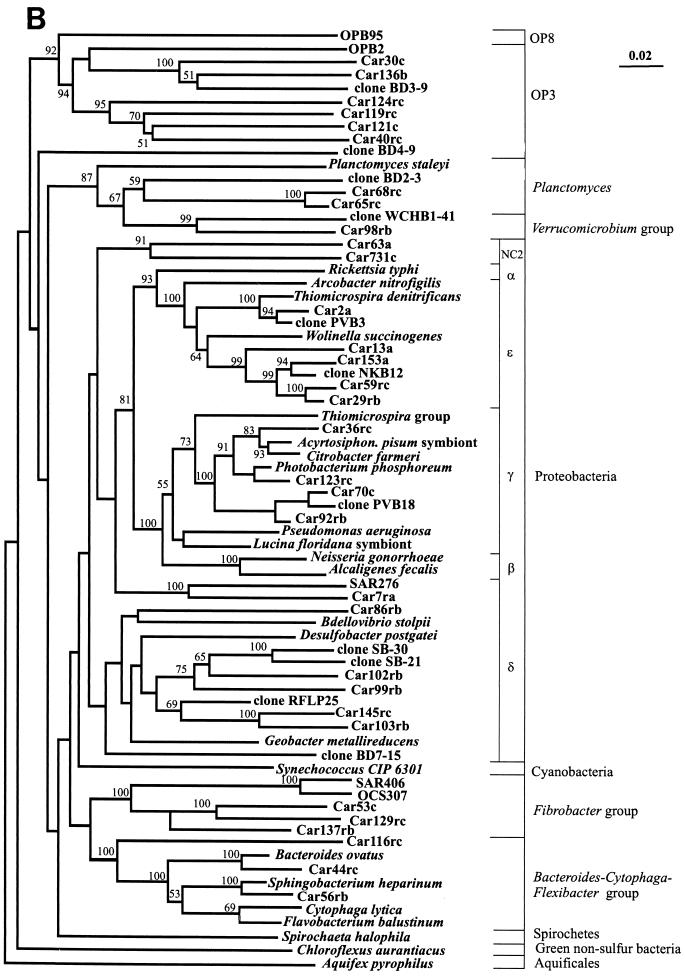
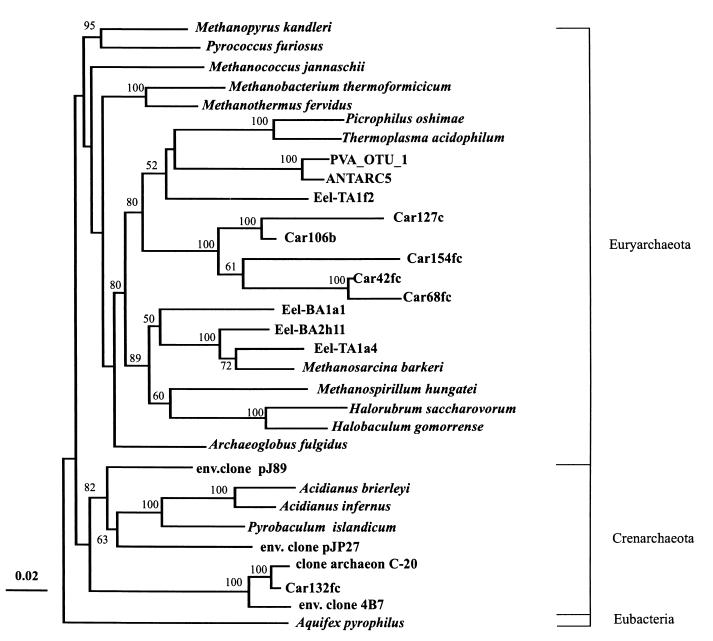
Close relatives of the nonchimeric sequences identified by searches in the RDP and GenBank databases are shown in Table 1. Since the 1-kb 16S rDNA fragments were not directionally cloned into pGEM-T and pBluescript II SK(+), the sequences were divided into two groups: sequences containing the more conserved regions (group A) and sequences containing more variable regions (group B). For several clones, the complete sequence of the 1-kb insert was determined (group C). In Table 1, group A clones are identified by the suffix “f” and group B clones are identified by the suffix “r”. Clones whose complete 1-kb inserts were sequenced (group C) have no “f” or “r” suffix. Three separate trees were constructed (see Materials and Methods) for each group of sequences by using two independent algorithms. Comparable portions of the group C sequences were also introduced into the alignments of the group A and B sequences. The positions of group C sequences were identical in all three trees, and therefore, trees containing only these sequences are not shown. Trees for the bacterial group A and C sequences and for the bacterial group B and C sequences are shown in Fig. 1A and B, respectively. Archaea and members of the ɛ subdivision of the division Proteobacteria (ɛ-proteobacteria) were placed on separate trees, and only trees for the group A and C sequences are shown in Fig. 2 and 3. Distance and maximum-likelihood algorithms generated essentially identical trees, and only trees constructed with distance-based methods are shown.
FIG. 1.
Phylogenetic relationships of partial 16S rDNA sequences from 56 unknown bacterial clones in the 320-m (designations with the suffix “a”), 500-m (designations with the suffix “b”), and 1,310-m (designations with the suffix “c”) Cariaco Basin libraries and 44 sequences from RDP and GenBank databases. Sequences from nucleotide 531 to nucleotide 1040 (A) and from nucleotide 952 to nucleotide 1480 (B) (E. coli 16S rRNA numbering) were aligned and placed in the trees. The sequences were aligned with ClustalW; distance matrices and phylogenetic trees were constructed by using the Jukes-Cantor and Fitch-Margoliash algorithms, respectively. Division level groupings are indicated on the right. New candidate subdivisions of proteobacteria are designated NC1 and NC2. Bar = 0.02 change per sequence position. The numbers at the nodes are bootstrap confidence values expressed as percentages of 100 bootstrap replications. Bootstrap values less than 50% are not shown.
FIG. 2.
Phylogenetic relationships of partial 16S rDNA sequences from 10 unknown ɛ-proteobacterial clones in the 320-m (designations with the suffix “a”), 500-m (designations with the suffix “b”), and 1,310-m (designations with the suffix “c”) Cariaco Basin libraries and 14 ɛ-proteobacterial sequences from RDP and GenBank databases. Sequences from nucleotide 531 to nucleotide 1040 (E. coli 16S rRNA numbering) were aligned and placed in the tree. The sequences were aligned with ClustalW; distance matrices and phylogenetic trees were constructed by using the Jukes-Cantor and Fitch-Margoliash algorithms, respectively. Bar = 0.01 change per sequence position. The numbers at the nodes are bootstrap confidence values expressed as percentages of 100 bootstrap replications. Bootstrap values less than 50% are not shown.
FIG. 3.
Phylogenetic relationships of partial 16S rDNA sequences from six unknown archaeal clones in the 500-m (designations with the suffix “b”) and 1,310-m (designations with the suffix “c”) Cariaco Basin libraries and 25 archaeal sequences from RDP and GenBank databases. Sequences from nucleotide 531 to nucleotide 1040 (E. coli 16S rRNA numbering) were aligned and placed in the tree. The sequences were aligned with ClustalW; distance matrices and phylogenetic trees were constructed by using the Jukes-Cantor and Fitch-Margoliash algorithms, respectively. Division level groupings are indicated on the right. Bar = 0.02 change per sequence position. The numbers at the nodes are bootstrap confidence values expressed as percentages of 100 bootstrap replications. Bootstrap values less than 50% are not shown.
In many cases, our phylogenetic placement of a sequence on a tree coincided with the placement of the sequence based on the closest relative found in databases. However, in a few cases, such as Car33fb, Car40rc, Car116rc, Car121rc, and Car124rc, the level of similarity with a close relative was low, and these sequences were affiliated based on their positions in the phylogenetic trees. In many cases (Car60fc, Car11fa, Car136b, Car30c, Car40rc, Car119rc, Car124rc, Car731c, Car63a, and Car33fb), Cariaco Basin clones were affiliated with candidate divisions and subdivisions, which made it impossible to hypothesize about their nearest cultivable relatives and their possible physiology. Surprisingly, several sequences in the Cariaco Basin 16S rDNA libraries were related to cultivable bacteria. 16S rDNA sequences of clones Car56rc, Car114fc, Car117fc, Car39fc, Car123rc, and Car2a appeared to be affiliated with the genera Sphingobacterium, Flavobacterium, Alcaligenes, Citrobacter, Photobacterium, and “Thiomicrospira,” respectively. Sequences of clones Car70c, Car43fc, Car140fb, and Car92rb were affiliated with Pseudoalteromonas and Moritella spp. In addition, several sequences retrieved from different environmental samples by other researchers exhibited relatively high levels of identity (up to 96%) with Cariaco Basin sequences.
Bacteria in the Cariaco Basin anoxic zone.
The majority (95%) of the sequences retrieved belonged to bacteria, and the division Proteobacteria was the dominant division. Bacterial sequences belonging to the following major divisions were identified: Cytophaga-Flexibacter-Bacteroides, fibrobacteria, planctomycetes, Verrucomicrobia, the OP8 and OP3 candidate divisions, and the β, δ, γ, and ɛ subdivisions of the Proteobacteria (Fig. 1A and 1B). Only three of the proteobacterial sequences (Car33fb, Car63a, and Car731c) could not be affiliated with any known proteobacterial subdivision. Therefore, these sequences appear to represent two new candidate subdivisions in the proteobacteria. Car33fb was tentatively assigned to the NC1 subdivision, and Car731c and Car63a were tentatively assigned to the NC2 subdivision. Only representatives of OP3 and the γ, δ, and ɛ subdivisions of the Proteobacteria were found at all three depths.
Bacteria in the 320-m library.
Of the 60 nonchimeric sequences in the 320-m 16S rDNA library, 56 (93.2%) belonged to the ɛ subdivision. One of the remaining four sequences belonged to each of the following groups: candidate division OP3 and the NC2, γ, and δ subdivisions of the Proteobacteria. Within the ɛ-proteobacteria, most of the sequences belonged to an as-yet-uncultured group first identified by Polz and Cavanaugh (28) as ectosymbionts of the shrimp Rimicaris exoculata (Fig. 2). Below, this group is referred to as the epsilon symbiont relatives (ESR). Three remaining ɛ-proteobacteria clustered with Arcobacter and “Thiomicrospira” spp. The only chimera found in the 320-m library also contained a portion of ESR 16S rDNA.
Bacteria in the 500-m library.
The 500-m library was more diverse, containing sequences from members of the fibrobacteria, the Flexibacter-Cytophaga-Bacteroides division, the Verrucomicrobia, the Proteobacteria, and the OP3 candidate division. The proteobacteria included representatives of the γ, δ, ɛ, and NC2 subdivisions, and γ-proteobacteria were dominant (25.6%). As in the 320-m library, the majority of the ɛ-proteobacteria appeared to be ESR. Fibrobacter and its relatives comprised the second largest group in the library (23.6%), followed by γ-proteobacteria, δ-proteobacteria, and ɛ-proteobacteria.
Bacteria in the 1,310-m library.
The 1,310-m library had the highest percentage of unique sequences; 59 nonchimeric clones in the library contained 38 unique sequences belonging to members of the planctomycetes, the fibrobacteria, the Flexibacter-Cytophaga-Bacteroides division, the Proteobacteria, and the OP3 and OP8 candidate divisions. The proteobacteria included representatives of the NC2, β, γ, δ, and ɛ subdivisions. ESR sequences were present in the 1,310-m library but comprised a much lower proportion of the clones (8.5%). Compared to the 500-m library, the 1,310-m library contained a lower proportion of fibrobacterial and δ-proteobacterial sequences. The largest group of bacteria at this depth was the γ-proteobacterial group, particularly Pseudoalteromonas/Moritella sp. strain Car70c. The 500- and 1,310-m libraries shared several identical sequences (including Car53c, Car56rb, Car92rb, and Car54fc), two groups of nearly identical sequences (represented by Car129rc and Car70c), and a group of closely related sequences (Car128fc, Car91fb, Car141fb, and Car149fc). ESR found in all three libraries were closely related to each other and did not comprise three different subgroups corresponding to three individual libraries.
Archaea in the anoxic zone of the Cariaco Basin.
No archaeal sequences were retrieved from the 320-m library. One archaeal sequence was present in the 500-m library (2% of all microorganisms in the library) and eight archaeal sequences were present in the 1,310-m library (13.6%). All archaeal sequences fell into two groups; one group was represented by two clones belonging to the kingdom Crenarchaeota, and the other included the remaining archaeal sequences in both libraries. The former group is closely related to the clone Archaeon C20 (Table 1 and Fig. 3) from Irish coastal waters, whose metabolism is unknown (22). The latter group is distantly related to cultured members of the kingdom Euryarchaeota. However, in the archaeal tree (Fig. 3) all sequences in this group appear to be related to the Eel-TA1f2 sequence retrieved from methane seep sediments in the Eel River basin (13). All Euryarchaeota sequences from the Cariaco Basin clustered together on the trees, forming a monophyletic clade confirmed by bootstrap analysis (Fig. 3).
DISCUSSION
Investigations of the compositions of microbial communities are important steps in understanding the role of bacterial and archaeal populations in biogeochemical processes. Due to poor culturability of natural bacteria, particularly anaerobic bacteria, we used molecular approaches based on rDNA sequences to investigate microbial community structure in the anoxic Cariaco Basin. However, we caution that the 16S rDNA library strategy for sequence retrieval contains several sources of potential bias. A bias caused by the reannealing kinetics of product molecules can skew gene frequencies when PCR product concentrations exceed threshold values (39). Another important potential bias is that organisms belonging to the domain Archaea have been found to have only one or a few gene copies of the 16S rRNA gene, while members of the domain Bacteria can have from one to seven or more copies, which may bias amplification towards the Bacteria (26). Therefore, the frequency of rDNA clones should be regarded as qualitative information on community composition. Nonetheless, 16S rDNA libraries have provided valuable qualitative descriptions of microbial diversity that allow comparisons between communities in different environments (39).
We created 16S rDNA libraries for three microbial community samples collected at depths of 320, 500, and 1,310 m. Oxygen measurements (40) have shown that all three samples were collected from the anoxic zone of Cariaco Basin. The composition of the 320-m library was markedly different from the compositions of the other two libraries. Theoretically, the differences in composition may have arisen from differences in the PCR and/or cloning strategies used in the construction of the three libraries. Unlike the 500- and 1,310-m libraries, the 320-m library was constructed by using three cloning strategies. Thirty-eight clones in this library were generated by blunt-end cloning of DeepVent polymerase-generated PCR products, and 18 clones were generated by cloning Taq polymerase-generated PCR products into pGEM-T. In contrast, all clones in the 500- and 1,310-m libraries were generated by using only the latter cloning technique. However, we observed that the compositions of the two 320-m sublibraries were identical, indicating that use of either of the two cloning strategies does not introduce artifacts during library construction. Since the diversity of the 320-m library is so low, the 18-clone sublibrary alone appears to be large enough to claim that the microbial community at this depth is substantially different from the communities in the 500- and 1,310-m samples.
The difference between the 320-m sample and the two other samples may be explained by different chemical conditions at the three depths. Nitrogen cycling and particularly iron and manganese cycling may be important for bacterial populations at 320 m since some oxidized forms of these elements may be available (40). Hastings and Emerson (12) have shown that concentration of elemental sulfur peaks immediately above the O2-H2S interface, suggesting that S0 may also be available as an oxidant below this interface. Furthermore, several sources may supply energy to communities at 320 m; these include sedimenting organic detritus from above, H2S from below to fuel chemoautotrophy, and organic by-products from chemoautotrophy (40). Thus, the presence of several electron acceptors and donors is likely to be the reason why the 320-m microbial community is so different from the communities at the two other depths. The chemical conditions at 500 and 1,310 m appear to be very similar; the H2S concentration only increases from 23 to 70 μM and the concentrations of terminal electron acceptors (SO42− and CO2) remain fairly constant. Indeed, there is some similarity in the compositions of the microbial communities at these depths. In both libraries γ-, δ-, and ɛ-proteobacteria are present. However, the number of δ- and ɛ- proteobacterial clones decreases with depth whereas the proportion of γ-proteobacterial clones increases. The major differences between the 500- and 1,310-m libraries are that (i) fibrobacterial sequences are dominant in the 500-m library and they almost disappear at 1,310 m and (ii) the number of archaeal sequences in the libraries substantially increases with depth.
Overall, the diversity of 16S rDNA sequences in the 1,310-m sample is not typical of a water column but rather resembles that of a sediment or soil (10, 18, 19, 30, 50). One possible explanation is that the 1,310-m sample area is approximately 60 m off the bottom and motile sediment bacteria migrate up in the water column. Another possible explanation is that seismic activity or mass wasting on the basin's walls causes turbidity flows and thereby resuspends sediments and associated microorganisms (42). Resuspended bacteria and small particles may remain in the water column long after the sediment settles because of their negligible settling velocities.
The compositions of microbial communities from the deep Cariaco Basin resemble the compositions of microbial communities from several anoxic sediments. For example, Cariaco Basin sequences are related to sequences from deepwater sediments collected around the Japanese islands (19). Members of the OP3 candidate division, Car136r and BD3-9, are found in Cariaco Basin water and Japanese island sediments, respectively. Sequences belonging to members of the planctomycetes division in the Cariaco Basin libraries (Car65rc, Car68rc, and Car122fc) are similar to the sequence of clone BD2-3 from Suruga Bay sediments (19). The Cariaco Basin δ-proteobacterial sequence Car86rb is 93.5% similar to the sequence of clone BD7-15 from the Calyptogena community in the Japan Trench (18). ESR closely related to the Cariaco Basin ESR have been found at other locations, including the sediments around the Japanese islands (18, 19). Thus, our results support the hypothesis that the deep Cariaco Basin water column is similar to anoxic sediment, but vertical distribution gradients vary over tens of meters rather than millimeters to centimeters.
ɛ-Proteobacteria.
ESR bacteria belonging to the ɛ-proteobacterial subdivision are found in all three Cariaco Basin libraries. The closest relatives of the ESR are groups of bacteria found in the Sulfur River (1) and deepwater sediments (18, 19). Cariaco Basin, Sulfur River, and deepwater sediment sequences exhibit 94 to 96% identity for bases 530 to 1494 (E. coli numbering) of 16S rRNA. Other close relatives include clone SB-17 from a sulfate-reducing consortium (27) and ectosymbionts of R. exoculata (28) and Alvinella pompejana (11). Our phylogenetic analysis showed that all these sequences comprise a distinct taxonomic group, which is more related to the Thiovulum-Campylobacter group than to the Helicobacter-Wolinella group (Fig. 3).
Little is known about the biology of ectosymbionts and their relatives. The R. exoculata and A. pompejana symbionts, as well as Thiovulum spp., are apparently aerobic autotrophic sulfide oxidizers. The ectosymbiont of R. exoculata forms a mat at a Mid-Atlantic Ridge hydrothermal vent site, colonizes shrimp bodies, and is a dominant bacterium in this environment (28). The environment of Sulfur River is conducive to sulfide oxidation, suggesting that local ESR are also involved in sulfide oxidation. Several cultured ɛ-proteobacteria are able to oxidize sulfide anaerobically (8, 25, 43). Thus, we hypothesize that ESR are sulfide-oxidizing bacteria. Their presence in the 320-m library also suggests that they are responsible for dark CO2 fixation observed at this depth (40). Several potential electron acceptors may be available at 320 m for ESR. Although oxygen is not present at this depth, these organisms may respire nitrate or manganese or iron oxides. ESR were also found in the 500- and 1,310-m libraries. If they were anaerobic sulfide oxidizers, they would not have any electron acceptors available at these depths. Consistent with the absence of electron acceptors, no dark CO2 fixation was detected at depths below 350 m during the November 1996 cruise (40). One possible explanation is that the ESR at 500 and 1,310 m are transported from 320 m by sinking organic debris and are not members of the local active microbial community.
Another interesting possibility is that ESR in the Cariaco Basin may be ectosymbionts of larger eukaryotic organisms. Zubkov et al. (52) reported that in the Black Sea one group of ciliates (species belonging to the order Scuticociliatida) populated the upper layer of the H2S zone and that a significant proportion of them possessed ectosymbiotic bacteria. In the Cariaco Basin during the November 1996 cruise, elevated concentrations of flagellated and ciliated protozoans were evident both in the oxygenated upper 200 m and in the anoxic water of the transition zone (Taylor, unpublished data). The depth distributions of both groups of protozoans mirrored the profiles obtained for total bacterial numbers and productivity. The dark CO2 uptake peak (330 m) coincided with elevated numbers of flagellates but not ciliates (data not shown). No large ciliates or ciliates with ectosymbionts have been documented for the Cariaco Basin yet.
The sequences Car2a and Car107fb came from bacteria closely related to “Thiomicrospira denitrificans.” “T. denitrificans” is a strictly anaerobic, autotrophic, sulfide-oxidizing denitrifier (25, 43). A bacterium with this metabolism would be well adapted to the Cariaco Basin environment just below the O2-H2S interface. Nitrate and nitrite typically penetrate 10 to 20 m deeper than O2 in the Cariaco Basin water column, so potentially sulfide can be oxidized at the expense of nitrate by chemoautotrophic bacteria.
Two sequences in the 320-m library belonged to members of the genus Arcobacter. The genera Arcobacter and Campylobacter are closely related and are both microaerophiles (21). Although some Campylobacter spp. are able to use elemental sulfur as an electron acceptor, the ability of Arcobacter species to respire elemental sulfur has never been studied; however, this genus has other respiratory pathways similar to those of the genus Campylobacter (dimethyl sulfoxide, succinate, and nitrate pathways).
Microbial communities in the Cariaco Basin at depths of 500 and 1,310 m.
Many sequences in the 500- and 1,310-m libraries belong to members of candidate divisions or subdivisions or show low levels of similarity with their closest sequenced relatives. This makes any inferences concerning their possible metabolism or role in the microbial community impossible. Other sequences came from bacteria which could be phylogenetically affiliated rather accurately, but these sequences occurred at low frequencies in the libraries and are likely to be insignificant for Cariaco Basin ecosystem functioning. Of the sequences in the 500-m library, the SAR406-OSC307-related sequences comprise the largest group. SAR406 and OCS307 sequences were initially identified as cosmopolitan sequences found in oxic pelagic systems (9). The Cariaco Basin relatives of SAR406 and OCS307 are relatively distantly related to each other and to cultured Fibrobacter representatives, which are strictly anaerobic fermentative bacteria. Therefore, it is impossible to make any metabolic inferences about them. γ-Proteobacterial and δ-proteobacterial sequences comprise two other large groups found in the 500-m library. Based on levels of identity to their closest relatives, it is reasonable to speculate that at least some of them belong to fermentative bacteria and sulfate reducers, respectively. Close association of these two physiological groups is ecologically advantageous; fermenters can supply sulfate reducers with electron donors, and in turn sulfate reducers can remove inhibiting products of fermentation, such as molecular hydrogen and formate.
There were fewer sulfate reducers and fermenters in the 1,310-m library than in the 500-m library. Another relatively large group of sequences in the 1,310-m library belongs to the Euryarchaeota. Although these sequences are distantly related to sequences retrieved from a methane seep in the Eel River basin (13), they cluster together on our trees. Hinrichs et al. (13) have suggested that unusual Eel River Euryarchaeota are able to oxidize methane anaerobically. The Cariaco Basin is rich in methane (37), and therefore, this type of metabolism might be expected in this environment.
Sequences related to operational taxonomic group PVB18 (23) were present in large numbers in both the 500- and 1,310-m libraries. PVB18, which initially was discovered as a member of the mat community of the underwater volcano Mount Pele (23), and its Cariaco Basin relatives are closely affiliated with Pseudoalteromonas haloplanktis and Moritella marina and are more distantly related to Shewanella spp. and Ferrimonas spp. (34). Despite the fact that the P. haloplanktis and M. marina 16S rRNA sequences exhibit 98% identity (34; unpublished data), these organisms were classified in two different genera (7). The majority of bacteria belonging to the genus Pseudoalteromonas are strictly nonfermentative aerobes (8), whereas M. marina is a facultative fermenter (46). Thus, we can only speculate that the Cariaco Basin PVB18 relatives possess fermentative metabolism.
Further research is required to corroborate our hypothesis about the role of ESR in sulfur cycling in the Cariaco Basin. We are planning to amplify ribulose-1,5-bisphosphate carboxylase genes from the 320-m DNA sample and to verify that they are indeed linked to the 16S rRNA gene(s) of ESR. Direct measurements of sulfur cycling also must be made to test the hypothesis that there is coupling of sulfur to metal (iron and manganese) cycles in the Cariaco Basin.
ACKNOWLEDGMENTS
We are grateful to the captain and crew of the B/O Hermano Gines and to the staff of the Estación de Investigaciones Marinas de Margarita (Fundación la Salle de Ciencias Naturales, Punta de Piedras, Edo. Nueva Esparta, Venezuela) for their assistance in this study.
This research was supported in part by grants OCE-941570, OCE-9711318, and OCE-9730278 (to M.I.S. and G.T.T.).
Footnotes
Contribution no. 1184 from Marine Sciences Research Center, State University of New York at Stony Brook.
REFERENCES
- 1.Angert E, Northup D, Reysenbach A-L, Peek A S, Goebel B M, Pace N R. Molecular phylogenetic analysis of a bacterial community in Sulphur River, Parker Cave, Kentucky. Am Mineral. 1998;83:1583–1592. [Google Scholar]
- 2.Belyaev V I, Sovga E E, Lyubartseva S P. Modeling the hydrogen-sulfide zone of the Black Sea. Ecol Model. 1997;96:51–59. [Google Scholar]
- 3.Borneman J, Skroch P W, O'Sullivan K M, Palus J A, Rumjanek N G, Jansen J L, Nienhuis J, Triplett E W. Molecular microbial diversity of an agricultural soil in Wisconsin. Appl Environ Microbiol. 1996;62:1935–1943. doi: 10.1128/aem.62.6.1935-1943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman J P, McCammon S A, Brown M V, Nichols D S, McMeekin T A. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl Environ Microbiol. 1997;63:3068–3078. doi: 10.1128/aem.63.8.3068-3078.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field K G, Gordon D, Wright T, Rappe M, Urback E, Vergin K, Giovannoni S J. Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine planktonic bacteria. Appl Environ Microbiol. 1997;63:63–70. doi: 10.1128/aem.63.1.63-70.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gauthier G, Gauthier M, Christen R. Phylogenetic analysis of the genera Alteromonas, Shewanella, and Moritella using genes coding for small-subunit ribosomal rRNA sequences and division of the genus Alteromonas into two genera, Alteromonas (emended) and Pseudoalteromonas gen. nov., and proposal of twelve new species combinations. Int J Syst Bacteriol. 1995;45:755–761. doi: 10.1099/00207713-45-4-755. [DOI] [PubMed] [Google Scholar]
- 8.Gevertz D, Telang A J, Voordouw G, Jenneman G E. Isolation and characterization of strains CVO and FWKO B, two novel nitrate-reducing, sulfide-oxidizing bacteria isolated from oil field brine. Appl Environ Microbiol. 2000;66:2491–2501. doi: 10.1128/aem.66.6.2491-2501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon D A, Giovannoni S J. Detection of stratified microbial population related to Chlorobium and Fibrobacter species in the Atlantic and Pacific oceans. Appl Environ Microbiol. 1996;62:1171–1177. doi: 10.1128/aem.62.4.1171-1177.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray J P, Herwig R P. Phylogenetic analysis of the bacterial communities in marine sediments. Appl Environ Microbiol. 1996;62:4049–4059. doi: 10.1128/aem.62.11.4049-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad A, Camacho F, Durand P, Cary S C. Phylogenetic characterization of the epibiotic bacteria associated with the hydrothermal vent polychaete Alvinella pompejana. Appl Environ Microbiol. 1995;61:1679–1687. doi: 10.1128/aem.61.5.1679-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hastings D, Emerson S. Sulfate reduction in the presence of low oxygen levels in the water column of the Cariaco Trench. Limnol Oceanogr. 1988;33:391–396. [Google Scholar]
- 13.Hinrichs K-U, Hayes J M, Sylva S P, Brewer P G, DeLong E F. Methane-consuming archaebacteria in marine sediments. Nature. 1999;398:302–305. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs L, Emerson S, Huested S S. Trace metal geochemistry in the Cariaco Trench. Deep Sea Res. 1987;34:965–981. [Google Scholar]
- 15.Jørgensen B B, Fossing H, Wirsen C O, Jannasch H G. Sulfide oxidation in the anoxic Black Sea chemocline. Deep Sea Res. 1991;38:S1083–S1113. [Google Scholar]
- 16.Karl D M, LaRock P A, Shultz D J. Adenosine triphosphate and organic carbon in the Cariaco Trench. Deep Sea Res. 1977;24:105–113. [Google Scholar]
- 17.Kogure K U, Simidu U, Taga N. Distribution of viable marine bacteria in neritic seawater around Japan. Can J Microbiol. 1980;26:318–323. doi: 10.1139/m80-052. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Guenzennec J, Nichols P, Henry P, Yanagibayashi M, Kato C. Microbial diversity in Nankai Trough sediments at a depth of 3,843 m. J Oceanogr. 1999;55:635–642. [Google Scholar]
- 19.Li L, Kato C, Horikoshi K. Bacterial diversity in deep-sea sediments from different depths. Biodivers Conserv. 1999;8:659–677. [Google Scholar]
- 20.Maidak B L, Cole J R, Parker C T, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCling C R, Patriquin D G, Davis R E. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. Int J Syst Bacteriol. 1983;33:605–612. [Google Scholar]
- 22.McInerney J O, Mullarkey M, Wernecke M E, Powell R. Phylogenetic analysis of group I marine archaeal rRNA sequences emphasizes the hidden diversity within the primary group Archaea. Proc R Soc Lond B Biol Sci. 1997;264:1663–1669. [Google Scholar]
- 23.Moyer C L, Dobbs F C, Karl D. Phylogenetic diversity of the bacterial community from a microbial mat at an active, hydrothermal vent system Loihi Seamount, Hawaii. Appl Environ Microbiol. 1995;61:1555–1562. doi: 10.1128/aem.61.4.1555-1562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munson M A, Nedwell D B, Embley T M. Phylogenetic diversity of Archaea in sediment samples from coastal salt marsh. Appl Environ Microbiol. 1997;63:4729–4733. doi: 10.1128/aem.63.12.4729-4733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent sample by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen K, Hallbeck L, Arlinger J, Erlandson A, Jahromi N. Investigation of the potential for microbial contamination of deep aquifers during drilling using 16S rRNA gene sequencing and culturing methods. J Microbiol Methods. 1997;30:179–192. [Google Scholar]
- 27.Phelps C D, Kerkhof L J, Young L Y. Molecular characterization of a sulfate-reducing consortium which mineralizes benzene. FEMS Microbiol Ecol. 1998;27:269–279. [Google Scholar]
- 28.Polz M F, Cavanaugh C M. Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc Natl Acad Sci USA. 1995;92:7232–7236. doi: 10.1073/pnas.92.16.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsing N B, Fossing H, Ferdelman T G, Andersen F, Thamdrup B. Distribution of bacterial populations in a stratified fjord (Mariager Fjord, Denmark) quantified by in situ hybridization and related to chemical gradients in the water column. Appl Environ Microbiol. 1996;62:1391–1404. doi: 10.1128/aem.62.4.1391-1404.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravenschlag K, Sahm K, Pernthaler J, Amann R. High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol. 1999;65:3982–3989. doi: 10.1128/aem.65.9.3982-3989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repeta D J, Simpson D J, Jørgensen B B, Jannasch H W. Evidence for oxygenic photosynthesis from the distribution of bacteriochlorophylls in the Black Sea. Nature. 1989;342:69–72. doi: 10.1038/342069a0. [DOI] [PubMed] [Google Scholar]
- 32.Richards F A. The Cariaco Basin (Trench) Oceanogr Mar Biol Annu Rev. 1975;13:11–67. [Google Scholar]
- 33.Rooney-Varga J N, Devereux R, Evans R S, Hines M E. Seasonal changes in the relative abundance of uncultivated sulfate-reducing bacteria in a salt marsh sediment and in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol. 1997;63:3895–3901. doi: 10.1128/aem.63.10.3895-3901.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosselló-Mora R A, Ludwig W, Kampfer P, Amann R, Schleifer K H. Ferrimonas balearica gen. nov. spec. nov., a new marine facultative Fe(III)-reducing bacterium. Syst Appl Microbiol. 1995;18:196–202. [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Scranton M I, Sayles F L, Bacon M P, Brewer P G. Temporal changes in the hydrography and chemistry of the Cariaco Trench. Deep Sea Res. 1987;34:945–963. [Google Scholar]
- 37.Scranton M I. Temporal variations in the methane content of the Cariaco Trench. Deep Sea Res. 1988;35:1511–1523. [Google Scholar]
- 38.Sorokin Y I, Sorokin P Y, Audeev V A, Sorokin D Y, Ilchenko S V. Biomass, production and activity of bacteria in the Black Sea with special reference to chemosynthesis and the sulfur cycle. Hydrobiologia. 1995;308:61–76. [Google Scholar]
- 39.Suzuki M, Rappé M, Giovannoni S. Kinetic bias in estimates of coastal picoplankton community structure obtained by measurements of small-subunit rRNA gene PCR amplicon length heterogeneity. Appl Environ Microbiol. 1998;64:4522–4529. doi: 10.1128/aem.64.11.4522-4529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor G T, Iabichella M, Ho T-Y, Scranton M I, Thunell R C, Muller-Karger F, Varela R. Chemoautotrophy in the redox transition zone of the Cariaco Basin: a significant midwater source of organic carbon production. Limnol Oceanogr. 2001;46:146–163. [Google Scholar]
- 41.Teske A, Wawer C, Muyzer G, Ramsing N B. Distribution of sulfate-reducing bacteria in a stratified fjord (Mariager Fjord, Denmark) as evaluated by most-probable-number counts and denaturing gradient gel electrophoresis of PCR-amplified ribosomal DNA fragments. Appl Environ Microbiol. 1996;62:1405–1415. doi: 10.1128/aem.62.4.1405-1415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thunell R, Tappa E, Varela R, Llano M, Astor Y, Muller-Karger F, Bohrer R. Increased marine sediment suspension and fluxes following an earthquake. Nature. 1999;398:233–236. [Google Scholar]
- 43.Timmer-ten Hoor A. A new type of thiosulfate-oxidizing, nitrate-reducing microorganism: Thiomicrospira denitrificans sp. nov. Neth J Sea Res. 1975;9:343–351. [Google Scholar]
- 44.Tuttle J H, Jannasch H W. Sulfide- and thiosulfate-oxidizing bacteria in anoxic marine basins. Mar Biol. 1973;20:64–70. [Google Scholar]
- 45.Tuttle J H, Jannasch H W. Microbial dark assimilation of CO2 in the Cariaco Trench. Limnol Oceanogr. 1977;24:746–753. [Google Scholar]
- 46.Urakawa H, Kita-Tsukamoto K, Steven S E, Ohwada K, Colwell R R. A proposal to transfer Vibrio marinus (Russell 1891) to a new genus Moritella gen. nov. as Moritella marina com. nov. FEMS Microbiol Lett. 1998;165:373–378. doi: 10.1111/j.1574-6968.1998.tb13173.x. [DOI] [PubMed] [Google Scholar]
- 47.Wise M G, McArthur J V, Shimkets L J. Bacterial diversity of a Carolina bay as determined by 16S rRNA gene analysis: conformation of novel taxa. Appl Environ Microbiol. 1997;63:1505–1514. doi: 10.1128/aem.63.4.1505-1514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright T D, Vergin K L, Boyd P W, Giovannoni S J. A novel δ-subdivision proteobacterial lineage from the lower ocean surface layer. Appl Environ Microbiol. 1997;63:1441–1448. doi: 10.1128/aem.63.4.1441-1448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H H, Tabita F R. Ribulose-1,5-bisphosphate carboxylase/oxygenase gene expression and diversity of Lake Erie planktonic microorganisms. Appl Environ Microbiol. 1996;62:1913–1921. doi: 10.1128/aem.62.6.1913-1921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanagibayashi M, Nogi Y, Li L, Kato C. Changes in the microbial community in Japan Trench sediment from a depth of 6292 m during cultivation without decompression. FEMS Microbiol Lett. 1999;170:271–279. doi: 10.1111/j.1574-6968.1999.tb13384.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J Z, Millero F J. The chemistry of the anoxic waters in the Cariaco Trench. Deep Sea Res. 1993;40:1023–1041. [Google Scholar]
- 52.Zubkov M V, Sazhin A F, Flint M V. The microplankton organisms at the oxic-anoxic interface in the pelagic Black Sea. FEMS Microbiol Ecol. 1992;101:245–250. [Google Scholar]