Abstract
Objectives:
After surgical repair, up to 70% of esophageal atresia (EA) patients suffer from gastroesophageal reflux disease (GERD). The ESPGHAN/NASPGHAN guidelines on management of gastrointestinal complications in EA patients were published in 2016. Yet, the implementation of recommendations on GERD management remains poor.
We aimed to assess GERD management in EA patients in more detail, to identify management inconsistencies, gaps in current knowledge, and future directions for research.
Methods:
A digital questionnaire on GERD management in EA patients was sent to all members of the ESPGHAN EA working group and members of the International network of esophageal atresia (INoEA).
Results:
Forty responses were received. Thirty-five (87.5%) clinicians routinely prescribed acid suppressive therapy for 1–24 (median 12) months. A fundoplication was considered by 90.0% of clinicians in case of refractory GERD with persistent symptoms despite maximal acid suppressive therapy and in 92.5% of clinicians in case of GERD with presence of esophagitis on EGD. Half of clinicians referred patients with recurrent strictures or dependence on transpyloric feeds. Up to 25.0% of clinicians also referred all long-gap EA patients for fundoplication, those with long-term need of acid suppressants, recurrent chest infections and feedings difficulties.
Conclusions:
Respondents’ opinions on the optimal duration for routine acid suppressive therapy and indications for fundoplication in EA patients varied widely. To improve evidence-based care for EA patients, future prospective multicenter outcome studies should compare different diagnostic and treatment regimes for GERD in patients with EA. Complications of therapy should be one of the main outcome measures in such trials.
What is Known
Up to 70% of esophageal atresia (EA) patients suffer from gastroesophageal reflux disease (GERD); up to 45% undergo a fundoplication.
In 2016, an international guideline for the post-operative management of EA patients was published, which included a section on GERD treatment.
Most statements in these guidelines were based on expert opinion rather than robust evidence.
What is New
GERD management in EA patients varies widely.
The majority of clinicians prescribe PPI routinely after birth, however the duration of therapy varies between 1–24 months.
Indications for fundoplication referral varied significantly between clinicians and no standard workup is performed before the operation.
Approximately 2.4 per 10,000 infants are born with esophageal atresia (EA) (1,2). Up to 70% of EA patients suffer from gastroesophageal reflux disease (GERD) (1,3–7), up to 45% of which require fundoplication (8).
In 2016, the international ESPGHAN/NASPGHAN guideline was published on gastrointestinal and nutritional complications. This included recommendations on surveillance-, diagnostic-, and treatment strategies for GERD (for an overview of recommendations, see file 1, Supplemental Digital Content, http://links.lww.com/MPG/C822) (8)).
Recently, clinical implementation of recommendations from this guideline was evaluated amongst clinicians involved in the care for EA patients (9). Recommendations for GERD screening with esophagogastroduodenoscopy (EGD) and routine prescription of acid suppressive therapy for GERD were implemented by most respondents. However, duration of this therapy was not surveyed in this study (9). Other recommendations were less well implemented in clinical practice: only half of the respondents implemented routine pH (with/without multichannel intraluminal impedance (MII)) monitoring and only a quarter applied the fundoplication indication recommendations. In addition, only 15% performed all the recommended tests prior to fundoplication (9).
Thus, despite the ESPGHAN/NASPGHAN guideline, management of GERD in EA patients appears to vary among clinicians involved in EA care. Reasons for variation may include unawareness of the guideline or conflicting recommendations between different guidelines (10). Additionally, the level of evidence for the guideline recommendations, which was ‘expert opinion’ in many, might also play a role in nonadherence (11).
Adherence to the guideline recommendations will enable better assessment of its efficacy, provide a starting point for comparative studies looking to optimize clinical care and most importantly, establish evidenced based care management for patients with EA.
To better understand the current management inconsistencies, we aimed to assess the awareness of the ESPGHAN guideline and to examine in detail the treatment of GERD in EA patients amongst an international sample of clinicians involved in the care for EA patients.
METHODS
A cross–sectional survey (see file 2, Supplemental Digital Content, http://links.lww.com/MPG/C822) was conducted to evaluate the management of GERD in pediatric EA patients amongst members of the ESPGHAN EA working group and members of the international network of esophageal atresia (INoEA). Members of these groups include clinicians, allied health professionals, and researchers interested in EA. INoEA also welcomes members of patient support groups. Due to European privacy legislation, authors were unable to select a subgroup of members for whom the questionnaire was relevant; thus, the survey was sent to all members of both groups. Members were asked to respond only if they were a clinician actively involved in GERD management of EA patients.
A first draft of the questionnaire was developed by UK and reviewed and adapted by all other authors. The final version was approved by all study-team members.
The survey was built in an online platform (Surveymonkey) and an invitation to fill out the questionnaire was sent out by email in 2020. Respondents were able to answer the question anonymously, yet, to enable comparison between different hospitals, participants could optionally provide the country/institution they worked in. As a means to optimize response rates, a reminder was sent 3 months after the initial invitation. The survey was open for responses for 4 months in total.
For analyses regarding hospital-broad management strategies, only the first respondent from each hospital was included. Respondents who did not mention where they worked were excluded (for these analyses only). Data regarding individual management strategies were analyzed in all respondents. This study was exempted from ethical review, as no individual patient data were included.
Data analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) for Windows, v 26.0 Armonk, NY: IBM Corp). Data were displayed as median and range, or as number and percentage. The chi-square test for trend was conducted to assess the difference between specializations of respondents with regard to the extent of guideline implementation. Chi-square test was used to compare outcomes per specific treatment strategy. A P value of <0.05 was considered statistically significant.
Results
Characteristics of Respondents
The survey was sent out to all members of INoEA and ESPGHAN EA WG (n = 372). These members consist of clinicians, allied health professionals, researchers and family support groups. Only clinicians involved in GERD management in EA patients were asked to answer the survey. Due to privacy legislation, authors are unaware how many of the 372 members were clinicians. A reliable response rate of the proportion of clinicians that had responded, could therefore not be calculated.
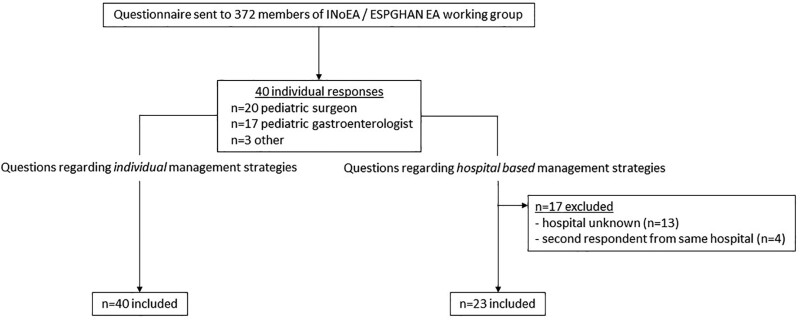
A total of 40 clinicians from 23 different hospitals (n = 13 did not mention where they work; 4 hospitals had 2 respondents) in at least 19 different countries (n = 2 respondents did not mention the country they work in) completed the questionnaire (see Fig. 1). Twenty-two (55.0%) worked in Europe, 5 (12.5%) in North America, seven (17.5%) in Oceania, 3 (7.5%) in Asia, and 1 (2.5%) in South America (see file 3, Supplemental Digital Content, http://links.lww.com/MPG/C822). Of respondents, 39 (97.5%) worked in a tertiary hospital and 1 (2.5%) in a secondary hospital.
FIGURE 1.
Flowchart of questionnaire responses.
The questionnaire was completed by 20 (50.0%) pediatric surgeons, 17 (42.5%) pediatric gastroenterologists, 2 (5.0%) pediatric pulmonologists, and 1 (2.5%) otolaryngologist. Thirteen surgeons (65.0%) and 7 gastroenterologists (41.2%) mentioned to have special interest in the EA population. Respondents saw a median of 30 (range 1–200; n = 2 responses missing) EA patients at their outpatient clinic and performed a median of 2 (range 0–20; (n = 10 responses missing) fundoplications per year in EA patients.
Fourteen respondents (35.0%) were member of the ESPGHAN EA working group and 26 (65.0%) were member of the international network of EA (INoEA). Nine (22.5%) were members of both groups. Most respondents (37/40, 92.5%) were aware of the international ESPGHAN/NASPGHAN EA guidelines. The 3 respondents unaware of the guideline were pediatric surgeons; 2/3 were INoEA members. None of which were a member of the ESPGHAN EA working group.
All respondents from three countries (France (n = 3), Sweden (n = 2) and the Netherlands (n = 1)) reported the existence of previously established national EA guidelines. Existence of a national EA guideline was also reported by 1/6 Australian respondents, but others (5/6) from the same country were not aware of this national guideline.
MANAGEMENT OF GERD IN PEDIATRIC EA PATIENTS
Prescription of PPIs
Out of 40 respondents, 35 (87.5%) routinely prescribed acid suppressive therapy (median time 12 months, range 1–24 months). Of them, 2 were unaware of the ESPGHAN/NASPGHAN guideline. Fourteen (35.0%) prescribed acid suppressants for 12 months as recommended by the guideline. Six (15.0%) prescribed acid suppressants for a shorter time (<12 months), 12 (30.0%) for a longer time than recommended (>12 months) and 5 (12.5%) indicated that duration of therapy varied depending on the clinical presentation of the child.
Five respondents (12.5%; n = 4 surgeons, n = 1 gastroenterologist) did not routinely prescribe acid suppressants, with one of these respondents being unaware of the guideline.
Role of EGD/pH-MII in Diagnosis and Management of GERD
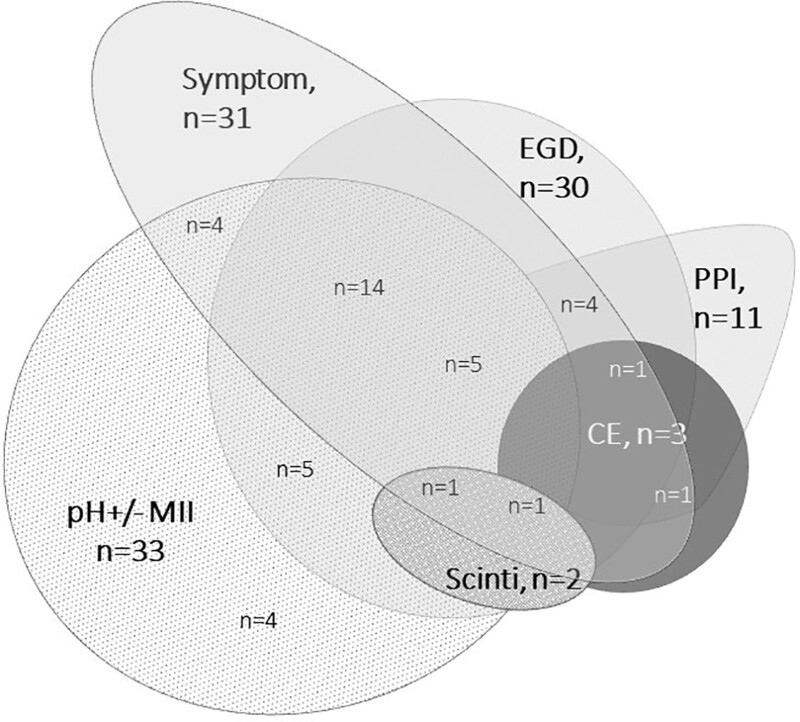
Twenty-six (65.0%) respondents performed a pH ± MII test and EGD with biopsies to diagnose/screen for GERD. Additional methods to diagnose GERD included symptom assessment (n = 30, 75.0%), a PPI trial (n = 10, 25.0%), EGD without biopsies (n = 1, 2.5%), contrast esophagogram (n = 3, 7.5%), and nuclear scintigraphy (n = 2, 5.0%). See Figure 2 for the different combinations of diagnostic tests used.
FIGURE 2.
Routinely performed investigations for GERD in EA patients. EGD = esophagogastroduodenoscopy with biopsies; CE = contrast esophagogram; pH ± MII = 24 hour pH-metry with/without impedance; scinti = nuclear scintigraphy; symptom = symptom assessment.
Fundoplication Indications
Indications for fundoplication included recurrent strictures (n = 23, 57.5%); GERD despite maximal acid suppressive therapy with presence of esophagitis on EGD (n = 37, 92.5%) or with persistent symptoms (n = 36, 90%). Half of respondents (n = 20, 50.0%) considered fundoplication if transpyloric feeds were needed and 31 (77.5%) in case of cyanotic spells thought to be GERD-related (n = 31, 78%). Other indications included need for long-term acid suppressive therapy (n = 10, 25%); recurrent respiratory tract infections (n = 8, 20%) and/or feeding difficulties (n = 6, 15%). Four respondents (10%) always routinely performed fundoplication in all long-gap EA patients, without performing objective assessment of GERD severity on medical management. Five (22.5%) considered fundoplication only in the clinical scenarios recommended by the guideline, without mentioning additional indications that are not supported by the guideline.
Investigations Prior to Fundoplication
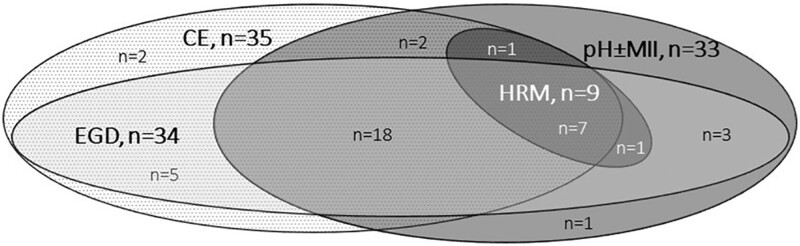
Twenty-five (62.5%) respondents performed a contrast esophagogram, EGD with biopsies and pH±MII (n = 13 pH-MII; n = 12 pH-only) prior to fundoplication. The other 15 (37.5%) respondents performed one or two preoperative tests. Seven (17.5%) performed an HRM/HRIM in addition to the three tests recommended by the guidelines. See Figure 3 for different combinations of diagnostic tests performed.
FIGURE 3.
Routinely performed investigations before fundoplication in EA patients. CE = contrast esophagogram; EGD = esophagogastroduodenoscopy with biopsies; pH ± MII = 24 hour pH-metry with/without impedance.
Differences in GERD Management by Gastroenterologists and Surgeons
Overall, management of GERD did not significantly differ between gastroenterologists and surgeons, nor between general gastroenterologists and surgeons with and without a declared special interest in managing EA patients (See file 4, Supplemental Digital Content, http://links.lww.com/MPG/C822).
However, when looking at separate components of GERD management, a PPI trial as a diagnostic test was significantly more often used by surgeons compared to gastroenterologists (P = 0.016) whereas need for long-term transpyloric feeds was more frequently considered as an indication for fundoplication by gastroenterologists than surgeons (P = 0.005).
Differences in GERD management between ESPGHAN- and non-ESPGHAN members
EGD with biopsies for GERD workup was significantly more often performed by ESPGHAN members (n = 14) compared to non-ESPGHAN members (n = 26; P = 0.015). Apart from this, guideline recommendations were equally followed by ESPGHAN members vs non-ESPGHAN members.
Differences in GERD Management within the Same Hospital
In this survey, four hospitals were represented by two respondents. Even within the same hospital, there was a lack of consistency among clinicians regarding tests used for GERD diagnosis, duration of routine PPI prescription and indications for fundoplication. Additionally, not all clinicians were aware of the existence of their local guideline, or international ESPGHAN/NASPGHAN guideline.
Care of EA Patients post Initial Repair
In 22/23different hospitals, a median of 12 (range 2–30) surgical repairs per year were performed. In 1/23 centers, primary surgical repairs of EA were no longer performed due to centralization of care, but follow-up did still take place.
Standardized follow-up protocols were available in 17/23 (74%) hospitals. In 13/23 (56.5%) hospitals, the pediatric surgeon was primarily responsible for the follow-up of EA patients, whereas in 7/23 (30.4%) hospitals a multidisciplinary team was responsible and in 3/23 (13.0%) the pediatric gastroenterologist.
Amongst the 23 different hospitals, 20 (87%) had a multidisciplinary EA team (see Table 1 for all disciplines involved in the teams). In 11/20 (55.5%) hospitals, a pediatric gastroenterologist, pediatric pulmonologist, and otolaryngologist were part of the team.
TABLE 1.
Specialties involved in multidisciplinary care for EA patients in the 23 hospitals represented in this survey
| Specialization | Number (%) |
|---|---|
| Pediatric surgeon | 19 (95%) |
| Pediatric gastroenterologist | 18 (90%) |
| Pediatric pulmonologist | 17 (85%) |
| Dietician | 16 (80%) |
| Pediatric otolaryngologist | 11 (55%) |
| Clinical geneticist | 11 (55%) |
| Speech pathologist | 11 (55%) |
| Psychologist | 11 (55%) |
| Social worker | 11 (55%) |
| Physiotherapist | 6 (30%) |
| Nurse coordinator | 6 (30%) |
| Other* | 4 (20%) |
*One radiologist, 1 neonatologist, 1 pediatric dentist, 1 research coordinator.
Role of Esophageal Manometry prior to Fundoplication
Of the 23 hospitals represented in this survey, 12 currently perform high resolution (impedance) manometries. Only 5 of the centers performed HRM in all EA patients prior to fundoplication.
DISCUSSION
Our results show that GERD management in EA patients varies widely amongst an international cohort of mainly pediatric surgeons and pediatric gastroenterologists working at tertiary hospitals and often with a special interest in this patient group. This lack of agreement amongst clinicians shows the need for multicenter prospective outcome trials that may provide evidence to support the right diagnostic and therapeutic regimes in these children. In our study, we identified 2 major inconsistencies in GERD management: the timeframe for routine prescription of PPIs varied between 1 and 24 months and indications to perform fundoplication differed greatly amongst clinicians.
To date, some other studies have evaluated management of EA patients as well. They also demonstrate that management strategies varied greatly between centers (12–15). However, most of these studies were published before the international guideline became available, and the majority of these studies only evaluated perioperative management strategies. Some of these studies evaluated the use of acid suppressive therapy. These studies reported varying times of PPI description, in line with our results (9,12,14).
Lack of sufficient scientific data may be the cause for variation in both length of PPI prescription and indication for fundoplication after EA repair. The ESPGHAN/NASPGHAN guideline recommends prescribing PPIs during the first year of life. This timeframe is based on expert opinion rather than scientific evidence (8). In the first weeks after surgery, PPI are thought to be necessary for protection of the surgical anastomosis. In addition, it is well known that GERD is common in infants with EA. Esophageal acid exposure may be a factor contributing to anastomotic stricture formation, although evidence for the latter is conflicting (16–19). Starting routine PPI therapy is therefore recommended and our respondents generally adhere to this recommendation.
The prevalence of GERD is known to decrease with age in EA patients. A longitudinal follow–up study in 100 EA patients reported that 64% had GERD at 18 months, decreasing to 23% at a median age of 65 months as measured with pH-testing off-PPI (20). Furthermore, an increasing number of side effects of prolonged PPI has been found, including increased respiratory infections, bacterial overgrowth (21,22), asthma (23), bone fractures and micronutrient deficiencies (24,25). It is also hypothesized that acid suppressants may increase the risk for food allergies and eosinophilic esophagitis (EoE) (26). Therefore, it is important to quantify the optimal duration for routine PPI treatment, in order to achieve maximal prevention of GERD-related complications whilst minimizing potential complications associated with prolonged PPI use. As a first step toward assessing this, a retrospective multicenter study should be carried out, evaluating the long-term results of different PPI subscription times (i.e. occurrence of GERD-related complications and PPI-related side effects and complications). Based on results of such study, a randomized prospective study in which EA patients receive routine prescription of PPIs for different durations could shed more light on the true incidence of GERD-related complications as well as presence of adverse effects related to PPIs (16–19).
Another inconsistency amongst respondents was the indication to perform a fundoplication in EA patients (27–31). To date, trials assessing fundoplication outcomes in different clinical situations in EA patients are lacking. However, it is well known that a significant number of these patients experience symptom recurrence post fundoplication. These studies are however small and often retrospective and not all patients had objectified GERD using EGD and/or pH-MII pre fundoplication. A large international prospective study of fundoplication outcomes in a well-characterized cohort of EA patients with GERD, objectively confirmed with EGD and pH-MII testing, will help overcome this gap. Additionally, the role of HRIM as a predictive tool for post-fundoplication dysphagia and swallowing/aspiration needs to be further evaluated. Specific pressure-flow metrics in HRIM appear to predict risk for post-fundoplication dysphagia in the older neurologically normal pediatric GERD population (32); however, relevance of these findings in EA patients with major motility disorders is unknown. The lack of clear evidence that supports the performance of HRIM in EA patients is also reflected in our survey, where only 5 respondents performed HRIM routinely prior to fundoplication. A small study on 16 pediatric EA patients and 13 controls showed marked dysmotility and abnormal bolus transport in EA patients, however the measured parameters using Pressure Flow Analysis did not correlate with symptoms of dysphagia (32).
In comparison to the duration of PPI therapy and indications for fundoplication, respondents to our survey showed more agreement regarding the use of diagnostic tests for GERD. Approximately two-thirds of respondents performs EGD and pH-MII both for regular GERD screening (65.0%) as well as for preoperative screening prior to fundoplication (62.5%). This could be due to the fact that a higher level of evidence is available for these statements (according to the GRADE classification system) (3,8,33–38).
Available literature shows that symptoms in EA patients do not correlate with GERD findings on EGD (4,5,38–40) and more importantly that routine endoscopies in asymptomatic or mildly symptomatic EA patients can detect abnormalities, including reflux esophagitis, EoE, and strictures in up to 30% of cases (39–41). In line with these studies, preliminary prospective data show that routine screening at the age of 12 months as recommended by the guideline shows abnormal findings including strictures, EoE and erosive esophagitis in more than a third of patients (40). These data provide evidence to support the recommended surveillance screening tests even in asymptomatic children. Further studies are needed to find the optimal time-window for such tests.
This study has some limitations. First, most respondents worked in high income countries and none of the respondents worked in the African continent. INoEA and ESPGHAN EA WG welcome members from all over the world; however, many of the members work in Europe or the United States of America. Results from countries other than those surveyed could be different. Second, the results of our questionnaire summarize a general picture of GERD management in EA patients, yet specific management strategies for different age groups, different clinical backgrounds, and for asymptomatic vs symptomatic patients were not surveyed and remain unclear. Third, this survey was completed by clinicians with expertise in EA management, mainly working at tertiary hospitals. This may therefore not reflect knowledge in smaller centers. Nevertheless, most clinicians would agree that EA is a rare disease which needs management in specialized reference centers with a multidisciplinary team. Finally, a response bias, which is inherent to survey studies, may be present. We attempted to reduce response bias, by sending out a reminder for this survey.
This is the first international study to thoroughly evaluate current management strategies for GERD in EA patients amongst a cohort of clinicians who are involved in the care for EA patients. We collected data from clinicians from at least 19 different countries, thereby providing an overview of GERD management in EA patients in tertiary centers.
Given the significant heterogeneity in the management of GERD and referral pathways for antireflux surgery in the EA cohort, we feel that large multicenter studies retrospectively evaluating outcomes of current PPI and fundoplication strategies are a first necessary step. This may lead to prospective studies specifically looking at the role and timing of PPI therapy and screening pH-impedance and EGD. In addition, more research is needed to provide clinicians with an evidence-based preoperative strategy for selecting those patients that may benefit from fundoplication.
CONCLUSIONS
Whilst GERD and its complications are common in EA patients, the management of GERD in terms of PPI therapy and selecting patients for fundoplication varies greatly in this cohort. To improve patient outcomes, it is necessary to investigate the optimal duration for routine treatment with PPI post initial repair in order to minimize GERD-related complications as well as PPI-related side effects. Prospective studies assessing fundoplication outcomes in EA patients as well as any potential role for motility assessments to guide patient selection are needed.
Supplementary Material
Footnotes
Sources of Funding: This work was supported by an ESPGHAN Networking Grant
Drs van Wijk and Krishnan contributed equally to this article.
On behalf of The European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) esophageal atresia working group and International Network of Esophageal Atresia members.
Although this paper is produced by the ESPGHAN EA working group it does not necessarily represent ESPGHAN policy and is not endorsed by ESPGHAN. The authors have no financial relationships relevant to this article to disclose.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Pedersen RN, Calzolari E, Husby S, et al. Oesophageal atresia: prevalence, prenatal diagnosis and associated anomalies in 23 European regions. Arch Dis Child. 2012;97:227–32. [DOI] [PubMed] [Google Scholar]
- 2.Nassar N, Leoncini E, Amar E, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. 2012;94:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catalano P, Di Pace MR, Caruso AM, et al. Gastroesophageal reflux in young children treated for esophageal atresia: evaluation with pH-multichannel intraluminal impedance. J Pediatr Gastroenterol Nutr. 2011;52:686–90. [DOI] [PubMed] [Google Scholar]
- 4.Castilloux J, Soglio DB-D, Faure C, et al. Endoscopic assessment of children with esophageal atresia: lack of relationship of esophagitis and esophageal metaplasia to symptomatology. Can J Gastroenterol. 2010;24:312–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koivusalo A, Pakarinen MP, Rintala RJ, et al. The cumulative incidence of significant gastrooesophageal reflux in patients with oesophageal atresia with a distal fistula—a systematic clinical, pH-metric, and endoscopic follow-up study. J Pediatr Surg. 2007;42:370–4. [DOI] [PubMed] [Google Scholar]
- 6.Taylor ACF, Breen KJ, Auldist A, et al. Gastroesophageal reflux and related pathology in adults who were born with esophageal atresia: a long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:702–6. [DOI] [PubMed] [Google Scholar]
- 7.Burjonrappa SC, Youssef S, St-Vil D, et al. What is the incidence of Barrett’s and gastric metaplasia in esophageal atresia/tracheoesophageal fistula (EA/TEF) patients? Eur J Pediatr Surg. 2011;2:25–9. [DOI] [PubMed] [Google Scholar]
- 8.Krishnan U, Mousa H, Dall’Oglio L, et al. ESPGHAN-NASPGHAN Guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr. 2016;63:550–70. [DOI] [PubMed] [Google Scholar]
- 9.O’Donnell JEM, Purcell M, Mousa H, et al. Clinician knowledge of societal guidelines on management of gastrointestinal complications in Esophageal Atresia. J Pediatr Gastroenterol Nutr. 2021;72:232–8. [DOI] [PubMed] [Google Scholar]
- 10.Dingemann C, Eaton S, Aksnes G, et al. ERNICA consensus conference on the management of patients with esophageal atresia and tracheoesophageal fistula: follow-up and framework. Eur J Pediatr Surg. 2020;30:475–82. [DOI] [PubMed] [Google Scholar]
- 11.Jin YH, Tan LM, Khan KS, et al. Determinants of successful guideline implementation: a national cross-sectional survey. BMC Med Inform Decis Mak. 2021;21:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shawyer AC, Pemberton J, Flageole H, et al. Post-operative management of esophageal atresia-tracheoesophageal fistula and gastroesophageal reflux: a Canadian Association of Pediatric Surgeons annual meeting survey. J Pediatr Surg. 2014;49:716–9. [DOI] [PubMed] [Google Scholar]
- 13.Zani A, Eaton S, Hoellwarth ME, et al. International survey on the management of esophageal atresia. Eur J Pediatr Surg. 2014;24:3–8. [DOI] [PubMed] [Google Scholar]
- 14.Lal D, Miyano G, Juang D, et al. Current patterns of practice and technique in the repair of esophageal atresia and tracheoesophageal fistua: an IPEG survey. J Laparoendosc Adv Surg Tech A. 2013;23:635–8. [DOI] [PubMed] [Google Scholar]
- 15.Lal DR, Gadepalli SK, Downard CD, et al. Perioperative management and outcomes of esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2017;52:1245–51. [DOI] [PubMed] [Google Scholar]
- 16.Donoso F, Lilja HE. Risk factors for anastomotic strictures after Esophageal Atresia repair: prophylactic proton pump inhibitors do not reduce the incidence of strictures. Eur J Pediatr Surg. 2017;27:50–5. [DOI] [PubMed] [Google Scholar]
- 17.Stenström P, Anderberg M, Börjesson A, et al. Prolonged use of proton pump inhibitors as stricture prophylaxis in infants with reconstructed esophageal atresia. Eur J Pediatr Surg. 2017;27:192–5. [DOI] [PubMed] [Google Scholar]
- 18.Righini Grunder F, Petit LM, Ezri J, et al. Should proton pump inhibitors be systematically prescribed in patients with esophageal atresia after surgical repair? J Pediatr Gastroenterol Nutr. 2019;69:45–51. [DOI] [PubMed] [Google Scholar]
- 19.Tambucci R, Isoldi S, Angelino G, et al. Evaluation of gastroesophageal reflux disease 1 year after esophageal atresia repair: paradigms lost from a single snapshot? J Pediatr. 2021;228:155–163.e1. [DOI] [PubMed] [Google Scholar]
- 20.Flatrès C, Aumar M, Ley D, et al. Prevalence of acid gastroesophageal reflux disease in infants with esophageal atresia/tracheoesophageal fistula. Pediatr Res. 2021;91:977–83. [DOI] [PubMed] [Google Scholar]
- 21.Canani RB, Cirillo P, Roggero P, et al. Therapy with gastric acidity inhibitors increases the risk of acute gastroenteritis and community-acquired pneumonia in children. Pediatrics. 2006;117:e817–20. [DOI] [PubMed] [Google Scholar]
- 22.Tighe MP, Afzal NA, Bevan A, et al. Current pharmacological management of gastro-esophageal reflux in children: an evidence-based systematic review. Paediatr Drugs. 2009;11:185–202. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Wintzell V, Ludvigsson JF, et al. Association between proton pump inhibitor use and risk of asthma in children. JAMA Pediatr. 2021;175:3941–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–15. [DOI] [PubMed] [Google Scholar]
- 25.Orel R, Benninga MA, Broekaert IJ, et al. Drugs in focus: proton pump inhibitors. J Pediatr Gastroenterol Nutr. 2021;72:645–53. [DOI] [PubMed] [Google Scholar]
- 26.Pali-Schöll I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy. 2011;66:469–77. [DOI] [PubMed] [Google Scholar]
- 27.Rintala RJ. Fundoplication in patients with esophageal atresia: patient selection, indications, and outcomes. Front Pediatr. 2017;5:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito C, Langer JC, Schaarschmidt K, et al. Laparoscopic antireflux procedures in the management of gastroesophageal reflux following esophageal atresia repair. J Pediatr Gastroenterol Nutr. 2005;40:349–51. [DOI] [PubMed] [Google Scholar]
- 29.Curci MR, Dibbins AW. Problems associated with a nissen fundoplication following tracheoesophageal fistula and Esophageal Atresia repair. JAMA Surgery. 1988;123:618–20. [DOI] [PubMed] [Google Scholar]
- 30.Kwiatek MA, Kahrilas PJ, Soper NJ, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies J, Hughes J, Leach S, et al. Prevalence of malnutrition and feeding difficulties in children with esophageal atresia. J Pediatr Gastroenterol Nutr. 2017;64:e100–5. [DOI] [PubMed] [Google Scholar]
- 32.Courbette O, Omari T, Aspirot A, et al. Characterization of Esophageal motility in children with operated esophageal atresia using high-resolution impedance manometry and pressure flow analysis. J Pediatr Gastroenterol Nutr. 2020;71:304–9. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mousa HM, Rosen R, Woodley FW, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2011;52:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frohlich T, Otto S, Weber P, et al. Combined Esophageal multichannel intraluminal impedance and pH monitoring after repair of esophageal atresia. J Pediatr Gastroenterol Nutr. 2008;47:443–9. [DOI] [PubMed] [Google Scholar]
- 36.van Wijk M, Knuppe F, Omari T, et al. Evaluation of gastroesophageal function and mechanisms underlying gastroesophageal reflux in infants and adults born with esophageal atresia. J Pediatr Surge. 2013;48:2496–505. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen RN, Markøw S, Kruse-Andersen S, et al. Esophageal Atresia: gastroesophageal functional follow-up in 5–15 year old children. J Pediatr Surg. 2013;48:2487–95. [DOI] [PubMed] [Google Scholar]
- 38.Shah R, Varjavandi V, Krishnan U, et al. Predictive factors for complications in children with esophageal atresia and tracheoesophageal fistula. Dis Esophagus. 2015;28:216–23. [DOI] [PubMed] [Google Scholar]
- 39.Schalamon J, Lindahl H, Saarikoski H, et al. Endoscopic follow-up in esophageal atresia-for how long is it necessary? J Pediatr Surg. 2003;38:702–4. [DOI] [PubMed] [Google Scholar]
- 40.van Lennep M, Krishnan U, Benninga MA, et al. Clinical implications of esophagogastroduodenoscopy, pH-(impedance) and high resolution manometry in esophageal atresia patients according to international guidelines (oral presentation at Pediatric Neurogastroenterology & Motility conference, Adelaide). Pediatric Neurogastroenterology & Motility. 2021. Available at: https://www.frontiersin.org/books/1st_World_Congress_on_Pediatric_Neurogastroenterology_and_Motility/4688. [Google Scholar]
- 41.Schneider A, Gottrand F, Bellaiche M, et al. Prevalence of barrett esophagus in adolescents and young adults with Esophageal Atresia. Annal Surg. 2016;264:1004100–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.