Abstract
The phylogenetic diversity of the ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO, E.C. 4.1.1.39) large-subunit genes of deep-sea microorganisms was analyzed. Bulk genomic DNA was isolated from seven samples, including samples from the Mid-Atlantic Ridge and various deep-sea habitats around Japan. The kinds of samples were hydrothermal vent water and chimney fragment; reducing sediments from a bathyal seep, a hadal seep, and a presumed seep; and symbiont-bearing tissues of the vent mussel, Bathymodiolus sp., and the seep vestimentiferan tubeworm, Lamellibrachia sp. The RuBisCO genes that encode both form I and form II large subunits (cbbL and cbbM) were amplified by PCR from the seven deep-sea sample DNA populations, cloned, and sequenced. From each sample, 50 cbbL clones and 50 cbbM clones, if amplified, were recovered and sequenced to group them into operational taxonomic units (OTUs). A total of 29 OTUs were recorded from the 300 total cbbL clones, and a total of 24 OTUs were recorded from the 250 total cbbM clones. All the current OTUs have the characteristic RuBisCO amino acid motif sequences that exist in other RuBisCOs. The recorded OTUs were related to different RuBisCO groups of proteobacteria, cyanobacteria, and eukarya. The diversity of the RuBisCO genes may be correlated with certain characteristics of the microbial habitats. The RuBisCO sequences from the symbiont-bearing tissues showed a phylogenetic relationship with those from the ambient bacteria. Also, the RuBisCO sequences of known species of thiobacilli and those from widely distributed marine habitats were closely related to each other. This suggests that the Thiobacillus-related RuBisCO may be distributed globally and contribute to the primary production in the deep sea.
Phylogenetic information on deep-sea microorganisms that has been accumulated relates mainly to the 16S ribosomal DNA (rDNA) sequences (9, 33, 53). In understanding the microbial contribution to deep-sea primary production, the 16S rDNA-based phylogeny will be better complemented by knowledge of the genes encoding the enzymes relevant to carbon fixation. The genes encoding ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) represent such an enzyme that is involved in autotrophy. RuBisCO is the most abundant enzyme on the globe and has attracted much phylogenetic attention (5). RuBisCO catalyzes the assimilation of carbon dioxide to organic carbon via the Calvin-Benson cycle. The enzyme consists of large and small subunits (42). The site responsible for carbon fixation is in the large subunit (42). More than 20% of the amino acid residues in the large subunit are conserved among the higher plants (32). Generally, RuBisCO has two forms. Form I consists of large and small subunits (LnSn, typically L8S8), and form II contains only large subunits (Ln) with 25 to 30% amino acid sequence identity with those of form I (32). The large subunits of form I and form II are coded by genes designated cbbL and cbbM, respectively (37). Some organisms have two genes encoding the large subunit of form I, designated cbbL-1 and cbbL-2 (36). Some species that possess both forms I and II have three genes, which are cbbL-1, cbbL-2 encoding the large subunit of the form I, and cbbL-3 (= cbbM) (49, 78).
It is hypothesized that the common ancestor of RuBisCOs was similar to the form II enzyme (Ln), since this form is more adaptive to high CO2 concentrations, a condition which is presumed to have been present for the primitive Earth (25, 26, 70). The form I (LnSn) is believed to have evolved in response to the decline of CO2 and the emergence of oxygen as the Earth's atmosphere changed (40, 41, 63). The form I RuBisCOs are essentially found in two major forms, “green-like” and “red-like,” which show phylogenetic distance based on their amino acid compositions (76). The green-like RuBisCOs have two types, i.e., IA and IB, based on evolutionary relationships. Chloroplasts of terrestrial plants and green algae together with cyanobacteria carry type IB and are phylogenetically allied with type IA, which includes representatives of the alpha-, beta-, and gamma-proteobacteria which are greatly intermixed with regard to the relationships between their RuBisCOs (76). The red-like RuBisCOs have two types, IC and ID. Many nongreen algae carry type ID and are more closely related to the members of alpha- and beta-proteobacteria, which carry type IC. Two cyanobacteria, Prochlorococcus marinus and Synechococcus sp. strain WH7803, have RuBisCOs that are phylogenetically more closely related to the purple bacterial RuBisCOs type IA than the cyanobacterial RuBisCOs type IB (61, 75). Thus, form I is found predominantly in the photosynthetic organisms and aerobic chemolithoautotrophs. Organisms that fix CO2 anaerobically using RuBisCO, such as the purple nonsulfur photosynthetic bacterium Rhodospirillum rubrum, have form II (71). Chemoautotrophic endosymbionts of deep-sea mollusks usually bear form I, while the endosymbionts of vestimentiferans tubeworms and the epibionts of the vent polychaete Alvinellid and the vent shrimp Rimicaris exoculata have form II (55). A number of autotrophic bacteria, including some purple nonsulfur photosynthetic bacteria and thiobacilli, possess both forms (11, 17, 18). In the dual RuBisCO forms of purple nonsulfur bacteria, form I is more induced than form II under conditions of CO2 limitation (27). Some thiobacilli with the ability to respire nitrate under anaerobic conditions bear both RuBisCO forms (11). Therefore, it has been suggested that form II in these species of thiobacilli is synthesized under anaerobic conditions (11). Recently RuBisCO genes were isolated from anoxic archaea, and they form a group that is quite distinctly separated from the previous groups of known RuBisCO forms (39, 77). Thus, the RuBisCO forms occur in a very diverse group of prokaryotes, ranging from aerobic to anaerobic and from photoautotrophic to chemoautotrophic species. Hence, the diversity of deep-sea RuBisCO genes could provide a phylogenetic window to the diversity of autotrophic microbial communities.
Deep-sea hydrothermal vents and seeps are among the most productive habitats on the Earth (24). The large biomass typical for these sites depends on organic carbon fixed via chemosynthesis rather than photosynthesis (24). Energy for carbon fixation in chemosynthesis can be derived from the oxidation of diverse inorganic electron donors, such as H2, H2S, reduced iron, ammonia, and so on (14, 22, 35).
Phylogenetic diversity of deep-sea primary producers based on the genes of the functional protein, RuBisCO, has remained obscure. This study targets the construction of functional phylogenetic trees of deep-sea autotrophic microflora based on RuBisCO genes. This approach provides a new method to assess the diversity of microbial primary producers found in the spectacular deep-sea oases of hydrothermal vents and seeps.
MATERIALS AND METHODS
Sample collection.
A total of seven types of deep-sea samples were collected from five different areas, which included the Trans-Atlantic Geotraverse (TAG) hydrothermal mound in the Mid-Atlantic Ridge and the areas around Japan (Table 1). The samples included the hydrothermal water and chimney fragment; reducing sediment of a bathyal seep, a hadal seep, and a presumed seep; and symbiont-bearing tissues of the vent mussel Bathymodiolus sp. and the seep vestimentiferan tubeworm Lamellibrachia sp.
TABLE 1.
Samples used in this study
| Collection site | Habitat | Sample | Water depth (m) | No. (date) of dive | Manned submersible or unmanned ROVa | Instrument used for collection | Reference(s) |
|---|---|---|---|---|---|---|---|
| Kremlin site, TAG mound, Mid-Atlantic; 26°08.212′ N; 44°49.611′ W | Hydrothermal vent | Fragments of sulfide anhydrite chimney | 3,655 | 433 (May 1998) | Shinkai 6500 | Submersible's manipulator | 16 |
| Mid-Okinawa Trough; 27° 47.220′ N; 126°53.906′ E | Hydrothermal vent | Vent mussel, Bathymodiolus sp. | 1,035 | 416 (April 1999) | ROV Dolphin 3K | Submersible's manipulator | 34 |
| Hydrothermal vent water collected in vicinity of mussel colony | van Dorn water sampler | ||||||
| Sagami Trough; 35°59.9′ N; 139°13.6′ E | Bathyal seep | Tubeworm, Lamellibrachia sp. | 1,199 | 1,144 (October 1999) | Shinkai 2000 | Submersible's manipulator | 33, 59 |
| Sediment collected around tubeworm colony | Push-core sampler | ||||||
| Japan Trench; 40°02.85′ N; 144°16.50′ E | Hadal seep | Reducing sediment | 7,434 | 112 (April 1999) | ROV Kaiko | Push-core sampler | 15 |
| Northern Okushiri Ridge; 44°14′ N; 139°46.200′ E | Possible seep | Reducing sediment | 1,709 | 1,125 (August 1999) | Shinkai 2000 | Push-core sampler | 45 |
All submersibles were provided by JAMSTEC.
A fragment of a hydrothermal chimney was collected at the Kremlin site of the TAG hydrothermal mound in the Mid-Atlantic Ridge by the manned deep-sea submergence vehicle (DSV) Shinkai 6500 of the Japan Marine Science and Technology Center (JAMSTEC).
Hydrothermal water, i.e., a mixture of hydrothermal fluid and near-vent seawater, was collected at the Iheya vent site in the Mid-Okinawa Trough, southwestern Japan, using a Van Dorn sampler equipped on the remotely operated vehicle (ROV) Dolphin 3K (JAMSTEC). There was a possibility of contamination by ambient water, since the sampler was kept open until finishing the sample collection. However, the sampler was washed with the mixture of hydrothermal fluid and near-vent seawater that passed through the sampler during the presampling operation near the vent. Thus, we supposed that the influence of contamination was not visibly high. In fact, we did not detect by PCR the occurrence of RuBisCO genes in the ambient deep water.
Reducing sediments were collected from the known and presumed seeps: (i) a bathyal methane seep (1,199 m deep) at Sagami Trough, central Japan, collected by the DSV Shinkai 2000 (JAMSTEC); (ii) a hadal seep (7,434 m deep) in the Japan Trench, collected by the ROV Kaiko (JAMSTEC); and (iii) a presumed seep (1,709 m deep) at Northern Okushiri Ridge, northern Japan, collected by the DSV Shinkai 2000. This presumed seep was suggested on the basis of observation of bacterial mats and unidentified isopods and gastropods in association with fissures at this site, which was the epicenter of the Hokkaido Nansei-oki earthquake in July 1993 (45). All the sediment samples were taken by push-core samplers, and the microbiological samples were scooped from inside of the cores about 1 to 5 cm below the top.
Individuals specimens of the hydrothermal vent mussel, Bathymodiolus sp., were collected at the Iheya vent site in the Mid-Okinawa Trough by the ROV Dolphin 3K. Individual specimens of the seep vestimentiferan tubeworm, Lamellibrachia sp., were collected from the Sagami Trough by the DSV Shinkai 2000.
Thus, samples included in this study covered the survey of deep-sea RuBisCOs among a wide range of microbial habitats.
DNA extraction from water, chimney, and sediment samples.
The free-living microbial cells in the hydrothermal vent water sample were collected on board by filtering 2 liters of the hydrothermal vent water sample using the Sterivex-GS filter unit (pore size, 0.22 μm; Millipore Corp., Bedford, Mass.). After filtration, the filters were washed with SET buffer (20% sucrose, 50 mM EDTA, 50 mM Tris-HCl [pH 7.5]) and stored at −20°C until DNA extraction. Bulk DNA was extracted in the filter housing according to the method of Somerville et al. (65). No DNA contamination during the procedure was confirmed by the negative control with filter-sterilized, cell-free water.
Bulk DNAs in the chimney fragment and sediment samples were extracted by the method of Porteuous et al. (54).
DNA extraction from symbiont tissues.
To extract genomic DNA from animal endosymbiont-bearing tissues, i.e., the gill of the vent mussel, Bathymodiolus sp., and the trophosome of the seep tubeworm, Lamellibrachia sp., the tissues were aseptically removed from the collected animals immediately after retrieval. The tissues were washed several times in prefiltrated autoclaved seawater. In order to remove contaminating epibiotic bacteria and free DNA, the tissues were suspended in TE buffer (per liter: 10 mM Tris-hydrochloride, 1 mM EDTA [pH 8]) incubated with lysozyme (1 mg ml−1) at room temperature for 30 min and further treated with DNase (10 μg ml−1) and MgCl2 (0.02 mM ml−1) at 37°C for 5 min. The treated tissues were washed several times with TE buffer with a higher concentration of EDTA (per liter: 50 mM Tris-hydrochloride, 50 mM EDTA [pH 8]) to remove any residual DNase and MgCl2. The cleaned tissues were kept at −80°C for the laboratory procedures.
Genomic DNA was extracted from 1 g of the clean, thawed endosymbiont tissues suspended in 1 ml of lysis buffer (per liter: 50 mM Tris-hydrochloride, 50 mM EDTA, 20 mM NaCl, 4 M urea [pH 8.0]), 500 μl of 5 M guanidine thiocyanate (Sigma), and 100 μl of proteinase K (20 mg ml−1) according to the method of Lippke et al. (38) with modifications. The solution was incubated at 60°C for 4 h. The crude lysate was centrifuged at 14,000 × g for 15 min at 4°C to precipitate the tissue remnants. The clear supernatant was transferred to a clean tube. DNA was purified from the supernatant using an EaZy Nucleic Acid isolation cycle pure kit (Omega Biotek catalogue no. D6493-02) according to the manufacturer's instructions. The purified DNA was subjected to electrophoresis on an 0.8% agarose gel, stained with 0.5 μg of ethidium bromide ml−1, and visualized by UV excitation. In addition to endosymbiont tissue DNA, animal DNA was also extracted from non-symbiont-containing tissue, such as vestimentum in the case of the tubeworm Lamellibrachia sp. and foot tissue in the case of the mussel Bathymodiolus sp., using the same protocol, to serve as a negative control for RuBisCO gene amplification.
RuBisCO oligonucleotide primers.
The RuBisCO oligonucleotide primers for the amplification of cbbL and cbbM genes were designed according to the amino-acid-conservative areas of the RuBisCO large subunit. The primer set for the amplification of the RuBisCO form I cbbL gene was designed from the sequence alignment data given for the cbbL genes of Anabaena sp. strain 7120, Synechococcus sp. strain PCC6301, and the deep-sea Alvinoconcha hessleri chemoautotrophic bacterial endosymbiont (6, 62, 69). The forward 20-mer primer (5′-GACTTCACCAAAGACGACGA-3′) corresponded to the nucleotide positions 595 to 615 of the Anabaena strain 7120 cbbL gene, and the reverse 20-mer primer (5′-TCGAACTTGATTTCTTTCCA-3′) corresponded to the complement of the nucleotide positions 1387 to 1405 of the same Anabaena 7120 cbbL gene. This primer set was used to amplify an approximately 800-bp segment of the cbbL gene.
The oligonucleotide primer set for the amplification of the RuBisCO form II cbbM gene was designed from multiple sequence alignment data for cbbM genes of the Riftia pachyptila endosymbiont and R. rubrum (46, 57). The forward 30-mer primer (5′-ATCATCAARCCSAARCTSGGCCTGCGTCCC-3′) corresponded to the nucleotide positions 663 to 693 of the R. pachyptila endosymbiont cbbM gene, and the reverse 30-mer primer (5′-MGAGGTGACSGCRCCGTGRCCRGCMCGRTG-3′) corresponded to the complement of the nucleotide positions 1033 to 1063 of the same RuBisCO cbbM gene. The amplification with this primer set would yield a 400-bp fragment from the cbbM gene.
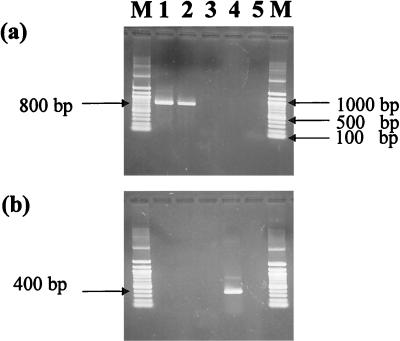
These primers were provided by the Funakoshi Company (Tokyo, Japan). The efficiency of designed primers for amplification of the expected target sizes was tested on the DNA of Synechococcus sp. strain PCC6301 (ATCC 27144), Thiobacillus ferrooxidans (ATCC 19859), Alcaligenes eutrophus (ATCC 29597), R. rubrum (ATCC 277), and Methanococcus jannaschii (ATCC 43067D), which represent varieties of RuBisCO types (Fig. 1).
FIG. 1.
Efficiency of cbbL and cbbM primers for amplification of RuBisCOs from different prokaryotic genomes. The expected size of the amplified fragment was 800 bp for RuBisCO cbbL (a) and 400 bp for cbbM (b). The amplified fragments were visualized by electrophoresis on a 1.5% agarose gel. Lanes: 1, Synechococcus sp. strain PCC6301 (ATCC 27144); 2, T. ferrooxidans (ATCC 19859); 3, A. eutrophus (ATCC 29597); 4, R. rubrum (ATCC 277); 5, M. jannaschii (ATCC 43067D); M, 100-bp DNA ladder marker (Biolabs). The cbbL and cbbM primer sets were specific for amplification of form I green-like and form II RuBisCOs, respectively.
Amplification, cloning, and sequencing of RuBisCO genes.
PCR amplifications of the RuBisCO genes from the purified genomic DNAs were carried out using the primer sets described above. The PCR mixture and PCR cycle conditions were set according to the method of Stein et al. (69) with modifications. For amplification of the cbbL gene, thermal cycling was initiated with denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 49°C for 1 min, and extension at 72°C for 3 min. The cbbM genes were amplified by initial denaturation at 94°C for 2 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 62°C for 1 min, and extension at 72°C for 3 min. In amplification of either form I or form II genes, the 30 cycles were followed by a final extension at 72°C for 15 min to allow 3′-A overhangs for the amplified PCR product to facilitate TA cloning. The PCR products were subjected to 1.5% agarose gel electrophoresis, stained with 0.5 μg of ethidium bromide ml−1, and visualized by UV excitation. The bands of the expected sizes (800 bp for form I and 400 bp for form II) were excised and eluted with a gel extraction kit (TOYOBO, Tokyo, Japan). The purified PCR products were TA cloned using the TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions. After the blue/white selection of the transformant colonies, the clone libraries were constructed and contained the inserts with the expected sizes. From each clone library, 50 clones were selected randomly and analyzed directly by DNA sequencing. In our case, the restriction fragment length polymorphism analysis was inefficient in grouping the clones. This was because the resolution of the restriction fragment length polymorphism analysis was not enough to divide the clones into groups.
The Topo plasmids were extracted from the randomly selected transformants using the alkaline miniprep method (1) and purified with the DNA microconcentrator filters (catalog no. 42416; Amicon). The sequence reaction was performed using the dye-terminator cycle-sequencing FS kit (Perkin-Elmer) with the T7 primers (60). DNA sequencing was carried out by an ABI model 373 automated DNA sequencer (Applied Biosystems, Perkin-Elmer).
Sequence analysis.
The RuBisCO form I insert (approximately 800-bp) sequences had a relatively comparable region of 500 bp and highly variable 3′ and 5′ regions of about 300 bp total. Thus, only the comparable 500-bp region from each sequenced cbbL insert was used for analysis. For sequence analysis of the RuBisCO form II cbbM gene, the 400 bp of the whole insert were used. The sequences used for analysis were compared with other sequences in the DNA Database Bank of Japan (DDBJ) for homologies using the program FASTA 3. Multiple alignments among the current sequences were performed using the multiple alignments program ClustalW (72). Sequences with more than 90% nucleotide identity showed 100% amino acid identity and were grouped into the same operational taxonomic unit (OTU) (19). Each OTU was represented by the clone having the highest-nucleotide-matching sequence with other clones within the same OTU. The nucleotide sequences of the OTUs were translated into amino acid sequences using the program Protein Engine (EBI). A phylogenetic tree was constructed based on deduced amino acid sequence alignment of current OTUs and those from the database using the neighbor-joining algorithm (58) by the software Tree View (50).
Nomenclature.
The sampled sites were abbreviated using the initials of the site names, as follows: TAG, Trans-Atlantic Geotraverse hydrothermal mound in Mid-Atlantic Ridge; MOT, Mid-Okinawa Trough; ST, Sagami Trough; JT, Japan Trench; and NOR, Northern Okushiri Ridge in the Japan Sea.
The sampled materials were abbreviated as follows: Chm, hydrothermal vent chimney fragments; Hvw, hydrothermal vent water; Sed, sediment; and Sym, endosymbiont.
For each sample type, two different clone libraries were constructed, one for the RuBisCO form I cbbL genes and the other for the RuBisCO form II cbbM genes. The number in the parentheses, such as (I) and (II), distinguished the cbbL and cbbM libraries, respectively. The clone libraries were named with the area abbreviation followed by the sample abbreviation and the form of RuBisCO; for example, TAG-Chm(I). In the case of the symbiont libraries, the area abbreviation indicated the source of the endosymbiont library, if it belonged to Bathymodiolus sp. [MOT-Sym(I)] or Lamellibrachia sp. [ST-Sym(II)]. The OTUs were arbitrarily numbered, and the OTU numbers were suffixed to the sample library codes, as in TAG-Chm (I)-1, for example.
Nucleotide sequence accession numbers.
The RuBisCO OTU sequences were registered in the DNA databases DDBJ, EBI, and GenBank under the accession numbers listed in Tables 2 and 3.
TABLE 2.
Distribution of cbbL OTUs within the clone libraries
| Area | OTU | Accession no. | % Nucleotide sequence divergencea | No. of clones
|
Frequency of OTU (%)b | |||
|---|---|---|---|---|---|---|---|---|
|
cbbL clone libraries
| ||||||||
| Hydrothermal vent water | Sediment | Chimney | Symbiont | |||||
| TAG Mound | TAG-Chm(I)-1 | AB038680 | 4.6 | 45 | 90 | |||
| TAG-Chm(I)-2 | AB038681 | 0 | 2 | 4 | ||||
| TAG-Chm(I)-3 | AB038682 | 0 | 1 | 2 | ||||
| TAG-Chm(I)-4 | AB038683 | 0 | 1 | 2 | ||||
| TAG-Chm(I)-5 | AB038684 | 0 | 1 | 2 | ||||
| Total | 50 | |||||||
| Mid-Okinawa Trough | MOT-Hvw(I)-1 | AB038636 | 2.2 | 5 | 10 | |||
| MOT-Hvw(I)-2 | AB038638 | 3.8 | 6 | 12 | ||||
| MOT-Hvw(I)-3 | AB038639 | 3.2 | 6 | 12 | ||||
| MOT-Hvw(I)-4 | AB038644 | 0 | 3 | 6 | ||||
| MOT-Hvw(I)-5 | AB038645 | 0 | 3 | 6 | ||||
| MOT-Hvw(I)-6 | AB038646 | 0 | 3 | 6 | ||||
| MOT-Hvw(I)-7 | AB038641 | 7.2 | 5 | 10 | ||||
| MOT-Hvw(I)-8 | AB038640 | 2.8 | 3 | 6 | ||||
| MOT-Hvw(I)-9 | AB038637 | 0 | 2 | 4 | ||||
| MOT-Hvw(I)-10 | AB038642 | 0 | 2 | 4 | ||||
| MOT-Hvw(I)-11 | AB038643 | 0 | 2 | 4 | ||||
| MOT-Hvw(I)-12 | AB038635 | 3.2 | 10 | 20 | ||||
| MOT-Sym(I)-1 | AB038634 | 4.4 | 50 | 100 | ||||
| Total | 50 | 50 | ||||||
| Sagami Trough | ST-Sed(I)-1 | AB038675 | 3.8 | 35 | 70 | |||
| ST-Sed(I)-2 | AB038676 | 0 | 5 | 10 | ||||
| ST-Sed(I)-3 | AB038679 | 0 | 1 | 2 | ||||
| ST-Sed(I)-4 | AB038677 | 1.6 | 6 | 12 | ||||
| ST-Sed(I)-5 | AB038678 | 1.4 | 3 | 6 | ||||
| Total | 50 | |||||||
| Japan Trench | JT-Sed(I)-1 | AB038633 | 3 | 38 | 76 | |||
| JT-Sed(I)-2 | AB038632 | 0.8 | 12 | 24 | ||||
| Total | 50 | |||||||
| Northern Okushiri Ridge | NOR-Sed(I)-1 | AB038687 | 4.6 | 20 | 40 | |||
| NOR-Sed(I)-2 | AB038685 | 8 | 19 | 38 | ||||
| NOR-Sed(I)-3 | AB038688 | 5 | 10 | 20 | ||||
| NOR-Sed(I)-4 | AB038686 | 0 | 1 | 2 | ||||
| Total | 50 | |||||||
% Nucleotide sequence divergence is the percentage of mismatched nucleotides between the clones within the OTU compared with the total length of analyzed nucleotide sequences (500 bp) (19). However, the clones within each OTU showed 100% amino acid identity.
Frequency of the OTU compared with the total number of clones analyzed in the clone library.
TABLE 3.
Distribution of cbbM OTUs within the clone libraries
| Area | OTU | Accession no. | % Nucleotide sequence divergencea | No. of clones
|
Frequency of OTU (%)b | |||
|---|---|---|---|---|---|---|---|---|
|
cbbM clone libraries
| ||||||||
| Hydrothermal vent water | Sediment | Chimney | Symbiont | |||||
| Mid-Okinawa Trough | MOT-Hvw(II)-1 | AB040510 | 9 | 46 | 92 | |||
| MOT-Hvw(II)-2 | AB040511 | 0 | 2 | 4 | ||||
| MOT-Hvw(II)-3 | AB040512 | 0 | 2 | 4 | ||||
| Total | 50 | |||||||
| Sagami Trough | ST-Sed(II)-1 | AB040504 | 1 | 35 | 70 | |||
| ST-Sed(II)-2 | AB040505 | 0 | 3 | 6 | ||||
| ST-Sed(II)-3 | AB040506 | 0.8 | 6 | 12 | ||||
| ST-Sed(II)-4 | AB040507 | 0 | 4 | 8 | ||||
| ST-Sed(II)-5 | AB040508 | 0 | 2 | 4 | ||||
| ST-Sym(II)-1 | AB032829 | 8.8 | 38 | 76 | ||||
| ST-Sym(II)-2 | AB040509 | 1 | 12 | 24 | ||||
| Total | 50 | 50 | ||||||
| Japan Trench | JT-Sed(II) -1 | AB040513 | 0 | 1 | 2 | |||
| JT-Sed(II)-2 | AB040514 | 0 | 1 | 2 | ||||
| JT-Sed(II)-3 | AB040515 | 5.2 | 30 | 60 | ||||
| JT-Sed(II)-4 | AB040516 | 0 | 1 | 2 | ||||
| JT-Sed(II)-5 | AB040517 | 3.85 | 9 | 18 | ||||
| JT-Sed(II)-6 | AB040518 | 6 | 8 | 16 | ||||
| Total | 50 | |||||||
| Northern Okushiri Ridge | NOR-Sed(II)-1 | AB040519 | 0 | 1 | 2 | |||
| NOR-Sed(II)-2 | AB040520 | 0 | 1 | 2 | ||||
| NOR-Sed(II)-3 | AB040521 | 0 | 1 | 2 | ||||
| NOR-Sed(II)-4 | AB040522 | 4.6 | 28 | 56 | ||||
| NOR-Sed(II)-5 | AB040523 | 4.8 | 9 | 18 | ||||
| NOR-Sed(II)-6 | AB040524 | 0 | 1 | 2 | ||||
| NOR-Sed(II)-7 | AB040525 | 2.2 | 8 | 16 | ||||
| NOR-Sed(II)-8 | AB040526 | 0 | 1 | 2 | ||||
| Total | 50 | |||||||
% Nucleotide sequence divergence is the percentage of mismatched nucleotides between the clones within the OTU compared with the total length of analyzed nucleotide sequences (400 bp) (19). However, the clones within each OTU showed 100% amino acid identity.
Frequency of the OTU compared with the total number of clones analyzed in the clone library.
RESULTS AND DISCUSSION
Efficiency of primers for RuBisCO gene amplifications.
Our initial approach was the amplification of RuBisCO genes from chemosynthetic bacteria, which are mainly responsible for carbon fixation in the deep-sea environment (3, 4, 12, 13, 23, 29). Since a wide range of chemosynthetic bacteria carry green-like form I and/or form II RuBisCOs (76), the RuBisCO primers were designed to amplify a wide range of green-like cbbL and cbbM genes but not to amplify red-like form I or archaea RuBisCO large-subunit genes (Fig. 1). The comparison of cbbL amino acid sequence data from a variety of species that carry green-like RuBisCOs, including Synechococcus sp. strain PCC6301, Synechococcus sp. strain WH7803, Anabaena sp. strain PCC7120, Chlamydomonas reinhardtii, T. ferrooxidans, Thiobacillus denitrificans, Hydrogenophilus thermoluteolus, and A. hessleri endosymbiont, and representatives of red-like form I, form II, and archaeal RuBisCOs from A. eutrophus; R. rubrum, Rhodobacter capsulatus, R. pachyptila endosymbiont, and T. denitrificans; and M. jannaschii, respectively, yielded a consensus for the regions from which the cbbL and cbbM primers were designed in form I green-like and form II RuBisCOs, respectively, but not in any other RuBisCOs.
Occurrence of a single RuBisCO gene in a symbiont-bearing tissue.
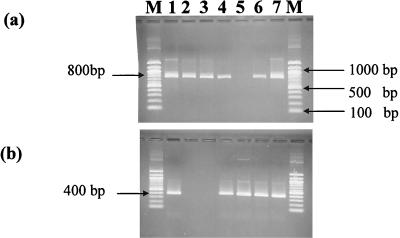
Both the genes encoding the large subunit of RuBisCO forms I and II (cbbL and cbbM) were amplified in most of the samples (Fig. 2). Exceptions to this biform were the samples of the vent mussel symbionts (MOT-Sym) and the seep vestimentiferan tubeworm symbionts (ST-Sym). MOT-Sym showed the amplification of only the RuBisCO form I gene (cbbL). Conversely, ST-Sym showed the amplification of only the RuBisCO form II gene (cbbM). This single-form occurrence may indicate the physiological adaptation of the endosymbionts to the habitat conditions (i.e., vent or seep) or to the host species (i.e., mussel or tubeworm).
FIG. 2.
The amplified 800-bp fragments of the large subunit for the RuBisCO form I gene (a) and 400-bp fragments of the RuBisCO form II gene (b). The fragments were amplified from the genomic DNA extracted from the collected samples and visualized by electrophoresis on a 1.5% agarose gel. Lanes: M, 100-bp DNA ladder marker (Biolabs); 1, MOT-Hvw; 2, MOT-Sym; 3, TAG-Chm; 4, ST-Sed; 5, ST-Sym; 6, JT-Sed; 7, NOR-Sed.
The reasons for the occurrence of a single RuBisCO gene in symbiont-bearing tissue are discussed from two viewpoints. The first associates the RuBisCO form distribution with the stable carbon isotope ratios of the organic matter assimilated by chemoautotrophic endosymbionts. Form I was detected in mollusk endosymbionts, which have a delta 13C value of −30‰, while form II was expressed in tubeworm endosymbionts, which have a delta 13C value of −11‰ (55). The isotopic difference may reflect the difference in the sources of CO2 derived from vent or seep or seawater, which in turn might select for a particular form of RuBisCO.
The second viewpoint is that the unequal distribution of the RuBisCO forms results from the chemical and kinetic properties of the forms (21). RuBisCO form I is adapted for aerobic conditions, while the form II is functional in anaerobic conditions (21). Corresponding to this observation, the mussel has endosymbionts located in the gills, which are external organs designed for efficient diffusive exchange of O2 and CO2 with seawater. This property of gills increases the chance of aerobic conditions that would make the RuBisCO form I enzyme more adaptive. In contrast, the vestimentiferan tubeworm endosymbionts are located in the trophosome, buried deep within the body of the animal, where the O2 concentration is tightly controlled by the host and the blood levels of CO2 are high, favoring the expression of form II (21, 68).
It should be noted that the chemoautotrophic symbiont of the vent mussel, Bathymodiolus thermophilus, has a psychrophilic nature, since the rate of thiosulfate-stimulated CO2 incorporation by this symbiont was maximum at 4°C and sometimes at 10°C, while CO2 incorporation was nonexistent or greatly diminished at 22°C (47). The discrepancy between the host thermophily and the symbiont psychrophily has not been fully elucidated.
We have also tried to amplify cbbL and cbbM from the endosymbionts of the seep giant clam Calyptogena soyoae, which has a developed gill and a reduced gut, collected from the same methane seep from which the tubeworm Lamellibrachia sp. was collected. However, neither form of RuBisCO genes was successfully amplified in this clam (data not shown). One possible explanation for this result is that this clam species may bear a methanotrophic symbiont, which may assimilate carbon via non-RuBisCO pathways. The occurrence of RuBisCO in the current tubeworm rather than in the clam may be correlated with the geochemical characters of this methane seep, where it has abundant methane in ambient water and sediment but has available hydrogen sulfide only in sediment (59). The seep-dwelling tubeworm Lamellibrachia sp. is known to incorporate hydrogen sulfide through its root buried in the sediment (28). These environmental characteristics may favor the occurrence of chemosynthetic symbionts, which carry RuBisCO in the tubeworm and may be methanotrophic symbionts in the clam gills, which have direct contact with the ambient methane-rich water.
Occurrence of divergent RuBisCO genes in nonbiological samples.
The TAG hydrothermal chimney sample (TAG-Chm) showed no amplification of cbbM but showed cbbL gene diversity with 5 OTUs (Fig. 2 and Table 2). Another hydrothermal vent sample, i.e., hydrothermal vent water from the Mid-Okinawa Trough (MOT-Hvw), showed a low level of cbbM diversity, with 3 OTUs, and a high level of cbbL diversity, with 12 OTUs (Tables 2 and 3). The absence or low diversity of anoxic cbbM in the hydrothermal vent samples can likely be ascribed to that vigorous upwelling of anoxic vent fluid that causes the rapid mixture with oxic seawater and results in the microaerobic-to-aerobic nature of the near-vent condition (2, 31). This may favor the dominance of aerobic cbbL-bearing chemoautotrophs over non-cbbL-bearers in the near-vent habitat.
In contrast to the near-vent samples, the reducing sediment samples from the Japan Trench and the Northern Okushiri Ridge (JT-Sed and NOR-Sed) were characterized by a high level of cbbM diversity, with 6 and 8 OTUs, compared with the low level of cbbL diversity, with only 2 and 4 OTUs, respectively (Tables 2 and 3). The anoxic nature of these habitats may account for the divergence of the genes for anaerobic RuBisCO form II (15, 45).
Another sample of reducing sediment (ST-Sed) showed mid-range diversities for both cbbL and cbbM, with 5 OTUs recorded from each library. This leads to the idea that the Sagami Trough sediment may be microaerobic. This idea is supported by the fact that 16S rDNA sequences of ɛ-proteobacteria, to which many known microaerophiles belong, were recovered (33, 44).
The divergence of nucleotide sequences of clones within the OTU (Tables 2 and 3) is probably due to the nucleotide degeneracy that may yield the same amino acid. This is commonly known in enzyme sequence analysis (32).
Analysis of deep-sea RuBisCO sequences.
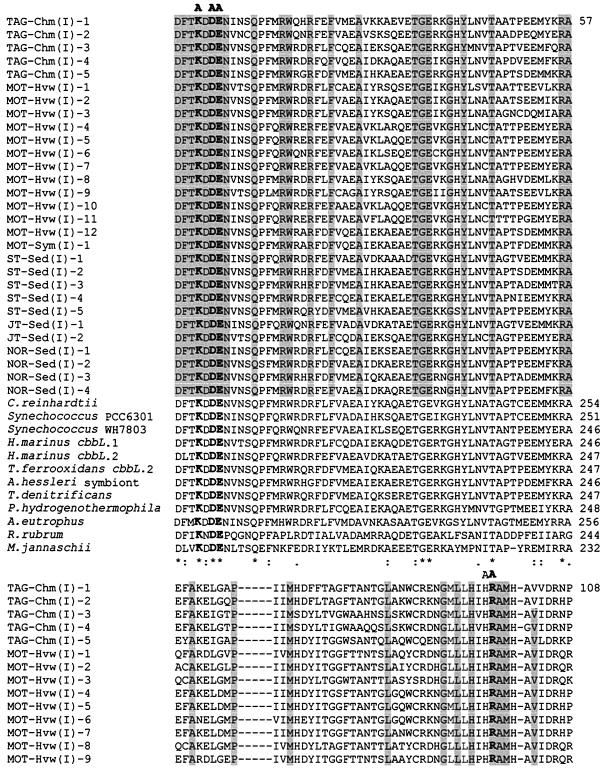
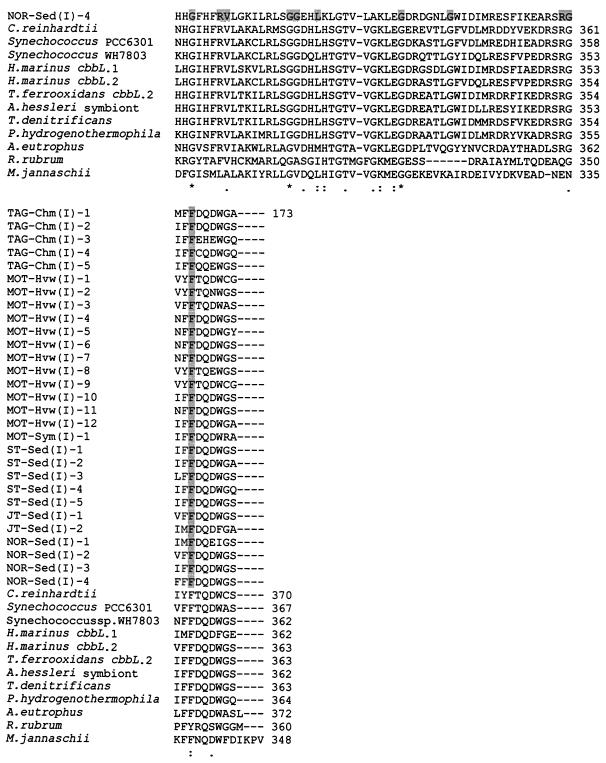
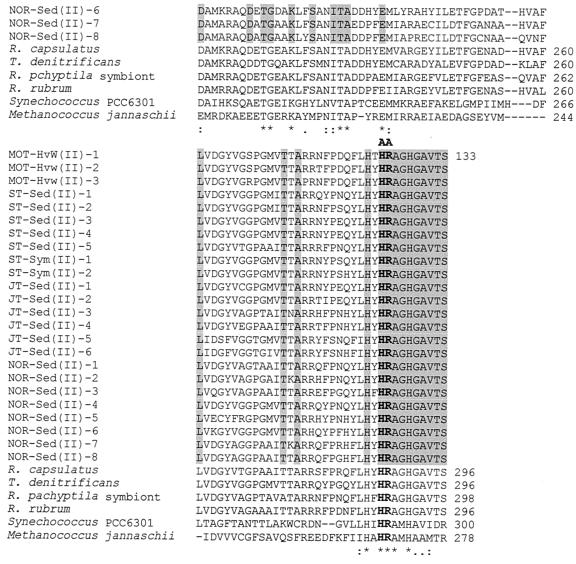
In order to analyze the current deep-sea RuBisCO genes, we have aligned the deduced amino acid sequences of the current OTUs with published sequences from several organisms (Fig. 3 and 4). The range of aligned cbbL and cbbM partial amino acid sequences corresponds to positions 195 to 367 and 170 to 300, respectively, of the RuBisCO large subunit of Synechococcus sp. strain PCC6301 (62). The positions of active and catalytic sites correspond to those of Synechococcus sp. strain PCC6301 (48, 62). All the current deep-sea OTUs possess the characteristic RuBisCO motif sequence, as in DFTKDDE for the cbbL group and GGDFIKNDE for the cbbM group (positions 192 to 201), except for MOT-Hvw(II)-2, in which the aspartic acid at position 195 was replaced by semiconserved substitution histidine, and NOR-Sed(II)-7 and -8, in which isoleucine-197 is replaced by a similar valine residue (Fig. 4). The aligned cbbL and cbbM corresponding amino acid sequences show several catalytic regions of nearly total conservation. Conserved regions include those surrounding the lysine residue at the consensus position 198 (Fig. 3 and 4), which has been identified in other RuBisCOs as the site of CO2 binding and carbamate formation during enzyme activation (42, 48). The other known active binding site residues, represented by the region flank Lys-172, His-291, Arg-292, His-324, Lys-331, and Leu-332 (32, 42, 48), were mostly conserved among the current OTUs, except in the cases of MOT-Hvw(I)-8 and NOR-Sed(I)-1, -2, -3, and -4, in which these amino acid residues were replaced by either conserved or dissimilar substitutions (Fig. 3). The mutagenesis studies of R. rubrum indicated that the substitution of lysine for His-324 and glutamic acid for Lys-331, such as in NOR-Sed(I)-4 and MOT-Hvw(I)-8, respectively, leads to inhibition of enzyme activity (20, 66). Not surprisingly, mutation of absolutely conserved catalytic residues has shown that they are indeed critical for catalysis, and even conservative substitutions result in a product that is nonfunctional or nearly so (32). It is not certain whether the OTUs MOT-Hvw(I)-8 and NOR-Sed(I)-1, -2, -3, and -4 may represent inactive RuBisCOs without confirmation by further studies. Inactive deep-sea RuBisCO was recorded previously in the mussel Bathymodiolus puteoserpentis (56).
FIG. 3.
Deduced amino acid partial sequence alignment of deep-sea cbbL OTUs with those from different types of form I and representatives of form II and archaeal RuBisCOs from R. rubrum and Methanococcus Jannaschii, respectively. Multiple sequence alignments were performed by using ClustalW (72). The accession numbers for each deduced large-subunit sequence that was used for comparison with current deduced cbbL OTUs are as follows: C. reinhardtii, J01399; Synechococcus sp. strain PCC6301, X03220; Synechococcus sp. strain WH7803, U46156; Hydrogenovibrio marinus cbbL.1, D43621; H. marinus cbbL.2, D43622; T. ferrooxidans cbbL.2, X70355; A. hessleri symbiont, M34536; T. denitrificans, L42940; Pseudomonas hydrogenothermophila, D30764; A. eutrophus, U20584; R. rubrum, X00286; M. jannaschii, U67564. The residue identities in all alignment sequences are marked with asterisks, conserved substitutions are marked with colons, and semiconserved substitutions are marked with periods (72). The shaded regions represent the identical amino acid residues in the current cbbL OTUs. Known active-site residues are labeled A (48, 62). Active-site residues that are identical in all sequences are in boldface type. The numbers of aligned cbbL amino acid positions of current OTUs and those of other species are at the right side.
FIG. 4.
Deduced amino acid partial sequence alignment of deep-sea cbbM OTUs with those from different types of form II and representative form I and archaeal RuBisCOs from Synechococcus sp. strain PCC6301 and M. jannaschii, respectively. Multiple sequence alignments were performed by using ClustalW (72). The accession numbers for all deduced large-subunit sequences that were used for comparison with current deduced cbbM OTUs are as follows: R. capsulatus, U23145; T. denitrificans, L37437; R. pachyptila endosymbiont, AF047688; R. rubrum, X00286; Synechococcus sp. strain PCC6301, X03220; M. jannaschii, U67564. The residue identities in all alignment sequences are marked with asterisks, conserved substitutions are marked with colons, and semiconserved substitutions are marked with periods (72). The shaded regions represent the identical amino acid residues in the current cbbL OTUs. Known active-site residues are labeled A (48, 62). Active-site residues that are identical in all sequences are in boldface type. The numbers of aligned cbbM amino acid positions of current OTUs and those of other species are at the right side.
Polyphyletic divergence of deep-sea RuBisCO genes.
A phylogenetic group based on 16S rDNA is usually displayed as a coherent group on a phylogram. In contrast, the phylograms of the RuBisCO large-subunit genes, cbbL and cbbM, are not similar to those based on 16S rDNA. The inconsistency between the RuBisCO gene distribution and the 16S rDNA-based affiliation among several groups of autotrophic proteobacteria, cyanobacteria, and green eukarya was previously ascribed to the multiple horizontal gene transfers of RuBisCO genes in different phylogenetic lineages (7, 52).
The aligned amino acid sequences were analyzed by maximum parsimony to generate the phylogenetic tree for cbbL and cbbM (Fig. 5). All the current OTUs diverge greatly from form I red-like and archaeal RuBisCOs. Most of the current cbbL and all cbbM OTUs were phylogenetically placed with different groups of autotrophic proteobacteria, which are abundant in deep-sea habitats (9, 29, 30, 33). These OTUs showed higher amino acid identities with those from the nearest neighbor species (tables 4 and 5). Most of the cbbL OTUs were located among green-like RuBisCO type IA (Fig. 5). Remarkably, MOT-Hvw(I)-1, -2, -3, -8, and -9 shared 85.2% (average) identity with the green alga C. reinhardtii (Eukarya), which carries green RuBisCO type IB (Table 4). TAG-Chm(I)-2 and MOT-Hvw(I)-4, -5, -6, -7, -10, and -11 displayed the highest amino acid identities (84, 90, 88, 94, 88, 90, and 89%, respectively) with the cyanobacterium Synechococcus sp. strain WH7803, which harbors green-like RuBisCO type IA (51, 75). There is a possibility of the flux of surface water phytoplankton to the deep sea, since they were recorded at depths of 3,100 and 4,465 m in the northeast Atlantic (73). The sinking of surface water phototrophs into the deep sea implies the possibility of genetic exchange between populations previously assumed to be genetically isolated, i.e., the autotrophs in the surface water and those in the deep sea (73). Moreover, close relatedness of the RuBisCO genes among deep-sea bacteria and cyanobacteria, as well as photosynthetic α-proteobacteria, was previously reported (57, 69). This phylogenetic similarity between the deep-sea chemoautotrophic OTUs and the RuBisCOs of photosynthetic organisms may indicate the lateral transfer of RuBisCO genes among deep-sea and surface water organisms.
FIG. 5.
Molecular phylogenetic tree based on the RuBisCO large-subunit amino acid sequences of the current OTUs and those of the nearest species from the database. Tree topography and evolutionary distance are given by the neighbor-joining method with Kimura distances. This tree is unrooted. Bootstrap values, calculated from 1,000 replicates, are indicated only at major nodes of the tree and are expressed as percentages. The letters in parentheses represent the expected classification from 16S rRNA or other studies: α, α-proteobacterium; β, β-proteobacterium; γ, γ-proteobacterium; C, cyanobacterium. Scale bar, 0.1 substitution per site.
TABLE 4.
Percentages of amino acid identity between the current deep-sea RuBisCO form I OTUs and the nearest neighbor species from the database
| OTU | % Identity with OTU:
|
|||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | ||
| 1 TAG-Chm(I)-1 | 78 | 68 | 71 | 77 | 64 | 69 | 65 | 75 | 74 | 74 | 76 | 66 | 63 | 77 | 75 | 76 | 75 | 78 | 77 | 71 | 71 | 78 | 80 | 74 | 72 | 76 | 71 | 72 | 69 | 78 | 71 | 80 | 81 | 78 | 81 | 69 | 54 | 28 | 37 | |
| 2 TAG-Chm(I)-2 | 69 | 70 | 70 | 66 | 70 | 70 | 78 | 77 | 81 | 78 | 69 | 66 | 81 | 80 | 72 | 71 | 81 | 72 | 69 | 72 | 73 | 83 | 75 | 71 | 75 | 69 | 74 | 71 | 84 | 73 | 83 | 75 | 76 | 76 | 69 | 53 | 32 | 36 | ||
| 3 TAG-Chm(I)-3 | 83 | 72 | 65 | 70 | 68 | 64 | 64 | 64 | 64 | 69 | 66 | 66 | 65 | 73 | 74 | 69 | 72 | 68 | 81 | 69 | 68 | 74 | 74 | 66 | 67 | 66 | 71 | 68 | 69 | 69 | 73 | 72 | 74 | 86 | 54 | 30 | 36 | |||
| 4 TAG-Chm(I)-4 | 72 | 67 | 72 | 69 | 65 | 65 | 65 | 64 | 69 | 68 | 66 | 65 | 72 | 73 | 72 | 72 | 68 | 81 | 71 | 71 | 72 | 73 | 71 | 66 | 67 | 74 | 68 | 68 | 72 | 74 | 72 | 73 | 84 | 58 | 34 | 39 | ||||
| 5 TAG-Chm(I)-5 | 66 | 72 | 71 | 70 | 69 | 69 | 69 | 69 | 66 | 71 | 71 | 89 | 88 | 75 | 90 | 80 | 72 | 77 | 73 | 74 | 75 | 74 | 80 | 72 | 72 | 72 | 71 | 74 | 88 | 84 | 86 | 73 | 53 | 31 | 37 | |||||
| 6 MOT-Hvw(I)-1 | 82 | 77 | 63 | 63 | 64 | 63 | 80 | 93 | 65 | 63 | 67 | 68 | 66 | 66 | 63 | 62 | 65 | 67 | 72 | 67 | 66 | 60 | 62 | 84 | 65 | 68 | 68 | 68 | 68 | 69 | 66 | 51 | 27 | 36 | ||||||
| 7 MOT-Hvw(I)-2 | 85 | 69 | 69 | 67 | 69 | 89 | 82 | 71 | 70 | 72 | 71 | 72 | 71 | 66 | 67 | 71 | 73 | 74 | 69 | 72 | 66 | 66 | 89 | 69 | 70 | 74 | 74 | 72 | 74 | 69 | 54 | 31 | 39 | |||||||
| 8 MOT-Hvw(I)-3 | 65 | 65 | 65 | 66 | 87 | 76 | 67 | 67 | 71 | 72 | 70 | 71 | 67 | 65 | 68 | 71 | 72 | 66 | 68 | 65 | 65 | 84 | 67 | 69 | 71 | 71 | 69 | 70 | 66 | 54 | 31 | 36 | ||||||||
| 9 MOT-Hvw(I)-4 | 95 | 89 | 94 | 66 | 62 | 95 | 95 | 71 | 71 | 79 | 70 | 66 | 68 | 71 | 78 | 72 | 66 | 74 | 66 | 68 | 68 | 90 | 69 | 80 | 74 | 75 | 75 | 66 | 56 | 31 | 36 | |||||||||
| 10 MOT-Hvw(I)-5 | 87 | 92 | 66 | 62 | 94 | 93 | 71 | 71 | 77 | 70 | 64 | 67 | 69 | 77 | 72 | 66 | 73 | 65 | 67 | 69 | 88 | 69 | 78 | 73 | 73 | 74 | 66 | 56 | 31 | 36 | ||||||||||
| 11 MOT-Hvw(I)-6 | 86 | 65 | 63 | 87 | 87 | 71 | 71 | 77 | 70 | 66 | 68 | 68 | 79 | 71 | 68 | 74 | 66 | 68 | 66 | 94 | 68 | 79 | 74 | 75 | 76 | 66 | 54 | 31 | 36 | |||||||||||
| 12 MOT-Hvw(I)-7 | 66 | 63 | 95 | 95 | 71 | 70 | 79 | 69 | 66 | 66 | 71 | 78 | 73 | 67 | 74 | 66 | 68 | 69 | 88 | 70 | 80 | 74 | 74 | 75 | 66 | 56 | 30 | 36 | ||||||||||||
| 13 MOT-Hvw(I)-8 | 79 | 68 | 68 | 69 | 68 | 74 | 69 | 65 | 64 | 68 | 74 | 72 | 69 | 69 | 65 | 64 | 86 | 67 | 69 | 74 | 69 | 69 | 71 | 66 | 51 | 31 | 36 | |||||||||||||
| 14 MOT-Hvw(I)-9 | 64 | 63 | 67 | 67 | 66 | 66 | 62 | 62 | 65 | 68 | 73 | 67 | 65 | 61 | 61 | 83 | 65 | 69 | 68 | 68 | 67 | 68 | 66 | 52 | 29 | 36 | ||||||||||||||
| 15 MOT-Hvw(I)-10 | 96 | 72 | 72 | 81 | 71 | 66 | 69 | 73 | 80 | 75 | 69 | 75 | 68 | 69 | 71 | 90 | 72 | 82 | 76 | 76 | 77 | 68 | 57 | 31 | 37 | |||||||||||||||
| 16 MOT-Hvw(I)-11 | 72 | 71 | 80 | 71 | 66 | 68 | 72 | 80 | 74 | 68 | 75 | 67 | 69 | 69 | 89 | 71 | 81 | 75 | 75 | 76 | 67 | 56 | 31 | 36 | ||||||||||||||||
| 17 MOT-Hvw(I)-12 | 96 | 76 | 93 | 81 | 75 | 77 | 75 | 80 | 77 | 76 | 83 | 75 | 73 | 74 | 77 | 76 | 89 | 88 | 88 | 77 | 54 | 31 | 39 | |||||||||||||||||
| 18 MOT-Sym(I)-1 | 75 | 93 | 83 | 75 | 77 | 74 | 80 | 78 | 75 | 83 | 75 | 74 | 73 | 76 | 75 | 89 | 89 | 88 | 78 | 55 | 30 | 39 | ||||||||||||||||||
| 19 ST-Sed(I)-1 | 77 | 70 | 71 | 74 | 94 | 75 | 72 | 80 | 71 | 74 | 75 | 81 | 73 | 95 | 78 | 77 | 78 | 72 | 60 | 32 | 37 | |||||||||||||||||||
| 20 ST-Sed(I)-2 | 87 | 74 | 78 | 75 | 79 | 76 | 77 | 86 | 75 | 74 | 72 | 76 | 77 | 89 | 88 | 87 | 76 | 54 | 30 | 37 | ||||||||||||||||||||
| 21 ST-Sed(I)-3 | 69 | 76 | 70 | 72 | 72 | 71 | 81 | 71 | 69 | 68 | 69 | 71 | 82 | 84 | 81 | 71 | 52 | 28 | 35 | |||||||||||||||||||||
| 22 ST-Sed(I)-4 | 68 | 70 | 74 | 74 | 68 | 69 | 68 | 68 | 71 | 71 | 71 | 76 | 74 | 77 | 83 | 54 | 29 | 39 | ||||||||||||||||||||||
| 23 ST-Sed(I)-5 | 74 | 74 | 75 | 75 | 76 | 71 | 72 | 71 | 69 | 74 | 84 | 81 | 83 | 68 | 54 | 32 | 36 | |||||||||||||||||||||||
| 24 JT-Sed(I)-1 | 74 | 71 | 80 | 71 | 73 | 74 | 83 | 72 | 96 | 77 | 77 | 77 | 71 | 59 | 33 | 37 | ||||||||||||||||||||||||
| 25 JT-Sed(I)-2 | 83 | 74 | 72 | 72 | 77 | 74 | 90 | 75 | 83 | 80 | 83 | 76 | 54 | 24 | 33 | |||||||||||||||||||||||||
| 26 NOR-Sed(I)-1 | 72 | 73 | 71 | 71 | 71 | 77 | 72 | 81 | 79 | 80 | 75 | 53 | 25 | 33 | ||||||||||||||||||||||||||
| 27 NOR-Sed(I)-2 | 72 | 79 | 72 | 78 | 72 | 83 | 80 | 79 | 81 | 69 | 54 | 28 | 37 | |||||||||||||||||||||||||||
| 28 NOR-Sed(I)-3 | 73 | 68 | 69 | 70 | 71 | 83 | 83 | 83 | 69 | 49 | 28 | 34 | ||||||||||||||||||||||||||||
| 29 NOR-Sed(I)-4 | 67 | 72 | 72 | 75 | 77 | 78 | 77 | 68 | 48 | 27 | 34 | |||||||||||||||||||||||||||||
| 30 Chlamydomonas reinhardtii | 69 | 72 | 75 | 74 | 73 | 74 | 72 | 56 | 31 | 39 | ||||||||||||||||||||||||||||||
| 31 Synechococcus sp. strain WH7803 | 72 | 83 | 77 | 77 | 79 | 69 | 57 | 30 | 37 | |||||||||||||||||||||||||||||||
| 32 Hydrogenovibrio marinus cbbL-1 | 74 | 78 | 74 | 77 | 72 | 54 | 25 | 34 | ||||||||||||||||||||||||||||||||
| 33 Hydrogenovibrio marinus cbbL-2 | 77 | 77 | 78 | 72 | 59 | 33 | 38 | |||||||||||||||||||||||||||||||||
| 34 Thiobacillus ferrooxidans cbbL-2 | 90 | 96 | 78 | 57 | 31 | 38 | ||||||||||||||||||||||||||||||||||
| 35 Alvinoconcha hessleri symbiont | 90 | 75 | 54 | 32 | 38 | |||||||||||||||||||||||||||||||||||
| 36 Thiobacillus denitrificans | 77 | 56 | 31 | 39 | ||||||||||||||||||||||||||||||||||||
| 37 Pseudomonas hydrogenothermophila | 59 | 31 | 40 | |||||||||||||||||||||||||||||||||||||
| 38 Alcaligenes eutrophusa | 32 | 36 | ||||||||||||||||||||||||||||||||||||||
| 39 Rhodospirillum rubruma | 26 | |||||||||||||||||||||||||||||||||||||||
| 40 Methanococcus jannaschiia | ||||||||||||||||||||||||||||||||||||||||
The species A. eutrophus, R. rubrum, and M. jannaschii, carrying form I red-like, form II, and archaeal RuBisCO, respectively, were used as out groups.
Biogeography of the RuBisCO genes.
Several cbbL and cbbM OTUs from various geographic areas were closely related to those of sulfur-oxidizing thiobacilli. This was clearly observed with TAG-Chm(I)-1 and -5, MOT-Hvw(I)-12 and MOT-Sym(I)-1, ST-Sed(I)-2 and -5, and NOR-Sed(I)-1 and -3, which showed highest amino acid identities with the sulfur oxidizers T. ferrooxidans cbbL-2 and T. denitrificans (Table 4). Also, ST-Sed(II)-1, -2, -3, and -4, ST-Sym(II)-1 and -2, JT-Sed(II)-1 and -2, and NOR-Sed(II)-4, -5, and -6 displayed high amino acid identities with each other and the cbbM product of T. denitrificans (Table 5; Fig. 5). This close relationship between these cbbM OTUs suggests the habitat similarity of the known seeps at the Sagami Trough (off central Japan), the Japan Trench (off northeastern Japan), and a seep at the Northern Okushiri Ridge (off northwestern Japan), which has a dense microbial community similar to that of a known methane seep at the Sagami Trough based on fatty acid compositions (45).
TABLE 5.
The percentages of amino acid identity between the current deep-sea RuBisCO form II OTUs and the nearest neighbor species from the database
| OTU | % Identity with OTU:
|
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | |
| 1 MOT-Hvw(II)-1 | 85 | 81 | 76 | 77 | 73 | 75 | 75 | 76 | 72 | 74 | 75 | 71 | 71 | 70 | 70 | 72 | 72 | 71 | 76 | 72 | 73 | 66 | 66 | 76 | 75 | 72 | 70 | 31 | 30 |
| 2 MOT-Hvw(II)-2 | 73 | 68 | 70 | 66 | 66 | 67 | 68 | 63 | 66 | 69 | 63 | 64 | 63 | 63 | 63 | 64 | 61 | 69 | 65 | 67 | 61 | 60 | 69 | 68 | 65 | 64 | 29 | 27 | |
| 3 MOT-Hvw(II)-3 | 79 | 81 | 78 | 79 | 77 | 79 | 76 | 78 | 80 | 75 | 75 | 76 | 76 | 76 | 75 | 73 | 79 | 73 | 75 | 71 | 70 | 78 | 77 | 76 | 73 | 32 | 35 | ||
| 4 ST-Sed(II)-1 | 87 | 89 | 87 | 82 | 95 | 84 | 90 | 90 | 79 | 79 | 75 | 74 | 81 | 80 | 77 | 90 | 84 | 88 | 76 | 74 | 83 | 87 | 83 | 78 | 32 | 33 | |||
| 5 ST-Sed(II)-2 | 85 | 84 | 81 | 84 | 90 | 85 | 85 | 78 | 77 | 77 | 77 | 78 | 79 | 77 | 87 | 79 | 84 | 74 | 73 | 81 | 87 | 83 | 76 | 32 | 32 | ||||
| 6 ST-Sed(II)-3 | 93 | 78 | 87 | 87 | 95 | 94 | 75 | 75 | 72 | 71 | 75 | 76 | 75 | 87 | 78 | 83 | 70 | 69 | 76 | 83 | 81 | 74 | 35 | 34 | |||||
| 7 ST-Sed(II)-4 | 78 | 88 | 86 | 93 | 93 | 75 | 77 | 74 | 72 | 76 | 76 | 73 | 87 | 79 | 81 | 69 | 68 | 77 | 82 | 79 | 72 | 36 | 36 | ||||||
| 8 ST-Sed(II)-5 | 81 | 75 | 79 | 81 | 88 | 89 | 73 | 74 | 90 | 87 | 86 | 81 | 75 | 75 | 81 | 79 | 90 | 75 | 84 | 86 | 34 | 33 | |||||||
| 9 ST-Sym(II)-1 | 86 | 88 | 89 | 77 | 78 | 73 | 72 | 78 | 77 | 75 | 92 | 82 | 84 | 75 | 72 | 81 | 84 | 79 | 75 | 35 | 32 | ||||||||
| 10 ST-Sym(II)-2 | 87 | 87 | 73 | 75 | 71 | 71 | 72 | 73 | 71 | 84 | 77 | 81 | 69 | 67 | 74 | 87 | 75 | 69 | 35 | 34 | |||||||||
| 11 JT-Sed(II)-1 | 96 | 77 | 76 | 72 | 70 | 78 | 78 | 75 | 88 | 80 | 82 | 72 | 70 | 78 | 83 | 82 | 74 | 32 | 34 | ||||||||||
| 12 JT-Sed(II)-2 | 78 | 78 | 72 | 71 | 79 | 79 | 77 | 90 | 81 | 84 | 74 | 72 | 79 | 84 | 81 | 74 | 33 | 36 | |||||||||||
| 13 JT-Sed(II)-3 | 86 | 67 | 69 | 95 | 94 | 90 | 78 | 75 | 74 | 79 | 77 | 84 | 73 | 81 | 80 | 35 | 39 | ||||||||||||
| 14 JT-Sed(II)-4 | 72 | 74 | 86 | 84 | 81 | 78 | 74 | 75 | 78 | 76 | 89 | 73 | 77 | 80 | 35 | 31 | |||||||||||||
| 15 JT-Sed(II)-5 | 96 | 69 | 68 | 65 | 75 | 69 | 69 | 66 | 65 | 74 | 71 | 72 | 70 | 30 | 32 | ||||||||||||||
| 16 JT-Sed(II)-6 | 70 | 69 | 66 | 74 | 69 | 70 | 68 | 67 | 75 | 70 | 72 | 72 | 32 | 34 | |||||||||||||||
| 17 NOR-Sed(II)-1 | 94 | 92 | 79 | 73 | 74 | 79 | 78 | 85 | 75 | 81 | 81 | 35 | 39 | ||||||||||||||||
| 18 NOR-Sed(II)-2 | 90 | 78 | 75 | 74 | 78 | 75 | 83 | 75 | 81 | 78 | 35 | 38 | |||||||||||||||||
| 19 NOR-Sed(II)-3 | 75 | 71 | 72 | 75 | 75 | 82 | 74 | 79 | 79 | 35 | 39 | ||||||||||||||||||
| 20 NOR-Sed(II)-4 | 89 | 88 | 75 | 73 | 81 | 88 | 81 | 75 | 32 | 32 | |||||||||||||||||||
| 21 NOR-Sed(II)-5 | 81 | 69 | 68 | 75 | 79 | 74 | 69 | 30 | 32 | ||||||||||||||||||||
| 22 NOR-Sed(II)-6 | 72 | 70 | 78 | 84 | 76 | 71 | 32 | 33 | |||||||||||||||||||||
| 23 NOR-Sed(II)-7 | 93 | 80 | 70 | 75 | 78 | 33 | 35 | ||||||||||||||||||||||
| 24 NOR-Sed(II)-8 | 78 | 69 | 75 | 76 | 33 | 33 | |||||||||||||||||||||||
| 25 R. capsulatus | 76 | 80 | 84 | 35 | 33 | ||||||||||||||||||||||||
| 26 T. denitrificans | 75 | 69 | 32 | 32 | |||||||||||||||||||||||||
| 27 R. pachyptila endosymbiont | 81 | 37 | 37 | ||||||||||||||||||||||||||
| 28 R. rubrum | 38 | 32 | |||||||||||||||||||||||||||
| 29 Synechococcus strain PCC6301a | 39 | ||||||||||||||||||||||||||||
| 30 M. jannaschiia | |||||||||||||||||||||||||||||
The species Synechococcus strain PCC6301 and M. jannaschii, carrying form I and archaeal RuBisCO, respectively, were used as out groups.
The occurrence of cbbL and cbbM OTUs from various geographic areas related to thiobacilli suggests the global distribution of these sulfur oxidizers in the deep sea (23). High abundance of H2S over other reducing inorganic materials in the deep sea (29, 31, 59, 74) and dual possession of RuBisCO forms I and II (11, 36, 64) facilitate this global distribution of thiobacilli and make free-living and symbiotic thiobacilli the major primary producers in the deep-sea habitats (29, 30).
The genetic variation among endosymbiotic RuBisCOs and symbiont-ambient monophyly of RuBisCO genes.
In the term of symbiont RuBisCOs, the MOT-Sym(I)-1 of the mussel Bathymodiolus sp. displayed 89% amino acid identity with that of the hydrothermal vent gastropod A. hessleri endosymbiont from the Mariana Back-Arc Basin (69). The Bathymodiolus symbiont replaces a functionally dissimilar amino acid with that of the A. hessleri symbiont at positions 223, 224, 229, 237, 254, and 381 of the A. hessleri symbiont cbbL product (Fig. 3). From the seep worm symbionts (ST-Sym), 2 OTUs, ST-Sym(II)-1 and -2, were recorded and showed 86% amino acid identity to each other and 79 and 75% identity, respectively, with the hydrothermal vent Riftia pachyptila endosymbiont from the East Pacific Rise (57). It is not clear whether the ST-Sym(II) OTUs were derived from one single symbiont species or from two different species. While the endosymbionts of the vent tubeworm R. pachyptila consist of a single species with >90% homogeneity based on 16S rDNA sequences (10, 67), the seep worm may contain more than one endosymbiotic species (33, 43). To examine whether the endosymbiotic microflora of the seep worm is monospecific or di- or polyspecific, in situ identification and localization of the endosymbiotic cbbM bearers should be done by in situ hybridization. This work provides the basis for designing specific and nonspecific cbbM probes for in situ hybridization. Generally, these results carried implication regarding the genetic variation among endosymbiotic RuBisCOs of widely distributed gutless mollusks and tubeworm species. This genetic variation may be influenced by a variety of factors, including host genera, geographic locations, and bottom types.
Remarkably, MOT-Sym(I)-1 displayed the highest amino acid identity (96%) with the ambient free-living bacterium represented by MOT-Hvw(I)-12 (Table 4; Fig. 5). The same was true for ST-Sym(II)-1 and -2, which shared 95 and 90% identity with ST-Sed(II)-1 and -2, respectively (Table 5; Fig. 5). The 16S rDNA analysis suggested that the vestimentiferan symbiotic and ambient microfloras are closely related and formed monophyletic groups (8, 33). This monophyletic similarity of the symbiotic and ambient microfloras, based on 16S rDNA and on cbbL and cbbM, implies that the vent and seep animals acquire their symbionts through acquisition of free-living bacteria.
In conclusion, we propose that deep-sea microbial RuBisCO genes display a broad range of phylogenetic diversity. The distribution of the deep-sea RuBisCO genes cbbL and cbbM may correlate with certain characteristics of the microbial habitats. The phylogenetic relationship between symbiotic and ambient microflora was made apparent by the RuBisCO genes as well as by the standard 16S rDNA genes.
The limited knowledge of and low number of publications about deep-sea RuBisCO genes should be increased by more surveying of other types of RuBisCO genes, such as those corresponding to form I red-like and archaeal RuBisCOs in both free-living bacteria and endosymbionts in different deep-sea habitats. Moreover, endosymbiotic localization of the RuBisCO genes and the corresponding microbial species should be confirmed by other methods, such as simultaneous in situ hybridization of RuBisCO genes and 16S rDNA.
ACKNOWLEDGMENTS
We thank the operation teams of the DSVs Shinkai 2000 and Shinkai 6500 and the ROVs Dolphin 3K and Kaiko and the crew of the RVs Natsuhima, Yokosuka, and Kairei for their help in collecting the deep-sea samples. Our great thanks to the reviewers for their invaluable comments on the manuscript.
This work was partly supported by the Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan (no. 11833012), the Special Coordination Fund “Archaean Park Project” from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and the Collaborative Research Fund “Strategy for Life under Extreme Conditions” of the Graduate University for Advanced Studies, Japan.
REFERENCES
- 1.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K. Short protocols in molecular biology. New York, N.Y: Wiley; 1995. pp. 1–16. [Google Scholar]
- 2.Baker E, German C, Elderfield H. Hydrothermal plumes over spreading-center axes: global distributions and geological inferences. In: Humphris S, Zierenberg R, Mullineaux L, Thomson R, editors. Seafloor hydrothermal systems. Washington, D.C.: American Geophysical Union Press; 1995. pp. 47–71. [Google Scholar]
- 3.Cavanaugh C M. Symbiotic chemoautotrophic bacteria in marine invertebrates from sulfide-rich habitats. Nature. 1983;302:58. [Google Scholar]
- 4.Cavanaugh C M. Symbiosis of chemoautotrophic bacteria and marine invertebrates from hydrothermal vents and reducing sediment. Biol Soc Wash Bull. 1985;6:373–388. [Google Scholar]
- 5.Chase M W. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Ann Mo Bot Gard. 1993;80:528–580. [Google Scholar]
- 6.Curtis S E, Haselkorn R. Isolation and sequence of the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci USA. 1983;80:1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delwiche C F, Palmer J D. Rampant horizontal transfer and duplication of RuBisCO genes in eubacteria and plastids. Mol Biol Evol. 1996;13:873–882. doi: 10.1093/oxfordjournals.molbev.a025647. [DOI] [PubMed] [Google Scholar]
- 8.Di Meo C A, Wilbur A E, Holben W E, Feldman R A, Vrijenhoek R C, Cary S C. Genetic variation among endosymbionts of widely distributed vestimentiferan tubeworms. Appl Environ Microbiol. 2000;66:651–658. doi: 10.1128/aem.66.2.651-658.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Distel D L, Felbeck H, Cavanaugh C M. Evidence for phylogenetic congruence among sulfur-oxidizing chemoautotrophic bacterial endosymbionts and their bivalvia hosts. J Mol Evol. 1994;38:533–542. [Google Scholar]
- 10.Distel D L, Lane D J, Olsen G J, Giovannoni S J, Pace B, Pace N R, Stahl D A, Felbeck H. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16S rRNA sequences. J Bacteriol. 1988;170:2506–2510. doi: 10.1128/jb.170.6.2506-2510.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.English R S, Williams C A, Lorbach S C, Shively J M. Two forms of ribulose-1,5-bisphosphate carboxylase oxygenase from Thiobacillus denitrificans. FEMS Microbiol Lett. 1992;94:111–120. doi: 10.1016/0378-1097(92)90593-d. [DOI] [PubMed] [Google Scholar]
- 12.Felbeck H. Chemoautotrophic potential of the hydrothermal vent tubeworm Riftia pachyptila Jones (vestimentiferan) Science. 1981;213:336–338. doi: 10.1126/science.213.4505.336. [DOI] [PubMed] [Google Scholar]
- 13.Fisher C R. Chemoautotrophic and methanotrophic symbiosis in marine invertebrates. Rev Aquat Sci. 1990;2:399–436. [Google Scholar]
- 14.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 15.Fujikura K, Kojima S, Tamaki K, Maki Y, Hunt J, Okutani T. The deepest chemosynthesis-based community yet discovered from the hadal zone, 7326 m deep, in the Japan Trench. Mar Ecol Prog Ser. 1999;190:17–26. [Google Scholar]
- 16.Fujioka K, Kobayashi K, Kato K, Aoki M, Mitsuzawa K, Kinoshita M, Nishizawa A. Tide-related variability of TAG hydrothermal activity observed by deep-sea monitoring system and OBSH. Earth Planet Sci Lett. 1997;153:239–250. [Google Scholar]
- 17.Gibson J L, Tabita F R. Different molecular forms of d-ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas sphaeroides. J Biol Chem. 1977;252:943–949. [PubMed] [Google Scholar]
- 18.Gibson J L, Tabita F R. Isolation and preliminary characterization of two forms of ribulose 1,5-bisphosphate carboxylase from Rhodopseudomonas capsulatus. J Bacteriol. 1977;132:818–823. doi: 10.1128/jb.132.3.818-823.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godon J, Zumstein E, Dabert P, Habouzit F, Molletta R. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol. 1997;63:2802–2813. doi: 10.1128/aem.63.7.2802-2813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harpel R L, Larimer F W, Hartman F C. Functional analysis of the putative catalytic bases His-321 and Ser-368 of Rhodospirillum rubrum ribulose bisphosphate carboxylase/oxygenase by site-directed mutagenesis. J Biol Chem. 1991;266:24734–24740. [PubMed] [Google Scholar]
- 21.Haygood M G. The potential role of functional differences between RuBisCO forms in governing expression in chemoautotrophic symbiosis. Limnol Oceanogr. 1996;41:370–371. [Google Scholar]
- 22.Ingledew W J. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochim Biophys Acta. 1982;683:89–117. doi: 10.1016/0304-4173(82)90007-6. [DOI] [PubMed] [Google Scholar]
- 23.Jannasch H W. Chemosynthetically sustained ecosystems in the deep sea. In: Schlegel H G, Bowien B, editors. Autotrophic bacteria. Madison, Wis: Science Tech Publishers; 1989. pp. 147–166. [Google Scholar]
- 24.Jannasch H W, Nelson D C. Recent progress in the microbiology of hydrothermal vents. In: Klug M J, Reddy C A, editors. Current perspective in microbial ecology. Washington, D.C.: American Society for Microbiology; 1984. pp. 170–176. [Google Scholar]
- 25.Jordan D B, Ogren W L. Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature. 1981;291:513–515. [Google Scholar]
- 26.Jordan D B, Ogren W L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch Biochem Biophys. 1983;227:425–433. doi: 10.1016/0003-9861(83)90472-1. [DOI] [PubMed] [Google Scholar]
- 27.Jouanneau Y, Tabita F R. Independent regulation of synthesis of form I and form II ribulose bisphosphate carboxylase-oxygenase in Rhodopseudomonas sphaeroides. J Bacteriol. 1985;165:620–624. doi: 10.1128/jb.165.2.620-624.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julian D, Gail F, Wood E, Arp A, Fisher C. Roots as a site of hydrogen sulfide uptake in the hydrocarbon seep vestimentiferan Lamellibrachia sp. J Exp Biol. 1999;202:2245–2257. doi: 10.1242/jeb.202.17.2245. [DOI] [PubMed] [Google Scholar]
- 29.Karl D M. Bacterial production at deep-sea hydrothermal vents and cold seeps: evidence for chemosynthetic primary production. Symp Soc Gen Microbiol. 1987;41:319–360. [Google Scholar]
- 30.Karl D M, editor. Microbiology of deep-sea hydrothermal vents. Boca Raton, Fla: CRC Press, Inc.; 1995. Ecology of free-living, hydrothermal vent microbial communities; pp. 35–110. [Google Scholar]
- 31.Kataoka S, Ishibashi J, Yamanaka T, Chiba H. Topography and fluid of the Iheya geochemistry North Knoll seafloor hydrothermal system in the Okinawa Trough. Jpn Mar Sci Technol Center J Deep Sea Res. 2000;16:1–7. [Google Scholar]
- 32.Kellogg E A, Juliano N D. The structure and function of RuBisCO and their implications for systematic studies. Am J Bot. 1997;84:413–428. [PubMed] [Google Scholar]
- 33.Kimura H, Higashide Y, Naganuma T. Enodosymbiotic and ambient microflora of vestimentiferan tubeworms. Jpn Mar Sci Technol Center J Deep Sea Res. 1999;15:25–33. [Google Scholar]
- 34.Kimura M, Uyeda S, Kato Y, Tanaka T, Yamano M, Gamo T, Sakai H, Kato S, Izawa E, Oomori T. Active hydrothermal mounds in the Okinawa Trough backarc basin, Japan. Tectonophysics. 1988;145:319–324. [Google Scholar]
- 35.Kuene J G, Tuovinen O H. The genera Thiobacillus and Thiomicrospira. In: Truper H G, Balows A, Schlegel H G, editors. The prokaryotes—a handbook on habitats, isolation, and identification of bacteria. I. New York, N.Y: Springer-Verlag; 1981. pp. 1023–1036. [Google Scholar]
- 36.Kusano T, Takeshima T, Inoue C, Sugawara K. Evidence for two sets of structural genes coding for ribulose bisphosphate carboxylase in Thiobacillus ferrooxidans. J Bacteriol. 1991;173:7313–7323. doi: 10.1128/jb.173.22.7313-7323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusian B, Bowien B. Organization and regulation of cbb CO2 genes in assimilation autotrophic bacteria. FEMS Microbiol Rev. 1997;21:135–155. doi: 10.1111/j.1574-6976.1997.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 38.Lippke J A, Strzempko M N, Raia F, Simon S L, French C K. Isolation of intact high-molecular-weight DNA by using guanidine isothiocyanate. Appl Environ Microbiol. 1987;53:2588–2589. doi: 10.1128/aem.53.10.2588-2589.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeda N, Kitano K, Fukui T, Ezaki S, Atomi H, Miki K, Imanaka T. Ribulose 1,5-bisphosphate carboxylase/oxygenase from the hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1 is composed solely of large subunits and forms a pentagonal structure. J Mol Biol. 1999;293:57–66. doi: 10.1006/jmbi.1999.3145. [DOI] [PubMed] [Google Scholar]
- 40.McFadden B A, Tabita F R. d-ribulose-1,5-diphosphate carboxylase and the evolution of autotrophy. BioSystems. 1974;6:93–112. doi: 10.1016/0303-2647(74)90002-1. [DOI] [PubMed] [Google Scholar]
- 41.McFadden B A, Torres-Ruiz J, Daniell H, Sarojini G. Interaction, functional relations and evolution of large and small subunits in RuBisCO from prokaryota and eukaryota. Philos Trans R Soc Lond Biol Sci. 1986;313:347–358. doi: 10.1098/rstb.1986.0042. [DOI] [PubMed] [Google Scholar]
- 42.Miziorko H M, Lorimer G H. Ribulose-1,5-bisphophate carboxylase-oxygenase. Annu Rev Biochem. 1983;52:507–535. doi: 10.1146/annurev.bi.52.070183.002451. [DOI] [PubMed] [Google Scholar]
- 43.Naganuma T, Naka J, Okayama Y, Minami A, Horikoshi K. Morphological diversity of the microbial population in a vestimentiferan tubeworm. J Mar Biotechnol. 1997;5:119–123. [Google Scholar]
- 44.Naganuma T, Kato C, Hirayama H, Moriyama N, Hashimoto J, Horikoshi K. Intracellular occurrence of epsilon proteobacteria 16S rDNA sequences in the vestimentiferan trophosome. J Oceanogr Jpn. 1997;53:193–197. [Google Scholar]
- 45.Naganuma T, Meisel C J, Wada H, Kato Y, Takeuchi A, Fujikura K, Naka J, Fujioka K. Sea-floor fissures, biological communities and sediment fatty acids of the Northern Okushiri Ridge, Japan Sea: implications for possible methane seepage. Island Arc. 1999;8:232–244. [Google Scholar]
- 46.Nargang F E, Mcintosh L, Somerville C. Nucleotide sequence of the ribulose bisphosphate carboxylase gene from Rhodospirillum rubrum. Mol Gen Genet. 1984;193:220–224. [Google Scholar]
- 47.Nelson D C, Hagen K D, Edwards D B. The gill symbiont of the hydrothermal vent mussel Bathymodiolus thermophilus is a psychrophilic, chemoautotrophic, sulfur bacterium. Mar Biol. 1995;121:487–495. [Google Scholar]
- 48.Newman J, Gutteridge S. The X-ray structure of Synechococcus PCC6301 ribulose-bisphosphate carboxylase/oxygenase-activated quaternary complex at 2.2-Å resolution. J Biol Chem. 1993;268:25876–25886. [PubMed] [Google Scholar]
- 49.Nishihara H, Yagushi T, Chung S Y, Suzuki K, Yanagi M, Yamasato K, Kodama T, Igarashi Y. Phylogenetic position of an obligatory chemoautotrophic, marine hydrogen-oxidizing bacterium, Hydrogenovibrio marinus, on the basis of 16S rRNA gene sequences and two form I RuBisCO gene sequences. Arch Microbiol. 1998;169:364–368. doi: 10.1007/s002030050584. [DOI] [PubMed] [Google Scholar]
- 50.Page R D M. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 51.Palenik B. Cyanobacterial community structure as seen from RNA polymerase gene sequence analysis. Appl Environ Microbiol. 1994;60:3212–3219. doi: 10.1128/aem.60.9.3212-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paoli G C, Soyer F, Shively J, Tabita F R. Rhodobacter capsulatus genes encoding form I ribulose-1,5-bisphosphate carboxylase/oxygenase (cbbLS) and neighbouring genes were acquired by horizontal gene transfer. Microbiology. 1998;144:219–227. doi: 10.1099/00221287-144-1-219. [DOI] [PubMed] [Google Scholar]
- 53.Polz M F, Cavanaugh C M. Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc Natl Acad Sci USA. 1995;92:7232–7236. doi: 10.1073/pnas.92.16.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porteuous L A, Armstrong J L, Sadler R J, Watrud L S. DNA extraction from sediment and cell suspensions. Curr Microbiol. 1994;29:301–307. [Google Scholar]
- 55.Robinson J, Cavanaugh C M. Expression of form I and form II RuBisCO in chemoautotrophic symbiosis: implications for the interpretation of stable carbon isotope values. Limnol Oceanogr. 1995;40:1496–1502. [Google Scholar]
- 56.Robinson J, Cavanaugh C M. Physiological and immunological evidence for two distinct C1-utilizing pathways in Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), endosymbiotic mussel from the Mid-Atlantic Ridge. Mar Biol. 1998;132:625–633. [Google Scholar]
- 57.Robinson J, Stein J, Cavanaugh C. Cloning and sequencing of a form II ribulose-bisphosphate carboxylase/oxygenase from the bacterial symbiont of the hydrothermal vent tubeworm Riftia pachyptila. J Bacteriol. 1998;180:1596–1599. doi: 10.1128/jb.180.6.1596-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saitou N, Nei M. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 59.Sakai H, Gamo T, Endow K, Ishibashi J, Ishizuka T, Yanagisawa F, Kusakabe M, Akagi T, Igarashi G, Ohta S. Geochemical study of the bathyal seep communities at the Hatsushima site, Sagami bay, central Japan. Geochem J. 1987;21:227–236. [Google Scholar]
- 60.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-termination inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada A, Kanai S, Maruyama T. Partial sequence of ribulose bisphosphate carboxylase/oxygenase and the phylogeny of Prochloron and Prochlorococcus (Prochlorales) J Mol Evol. 1995;40:671–677. doi: 10.1007/BF00160516. [DOI] [PubMed] [Google Scholar]
- 62.Shinozaki K, Yamada C, Takahata N, Sugiura M. Molecular cloning and sequence analysis of the cyanobacterial gene for the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci USA. 1983;80:4050–4054. doi: 10.1073/pnas.80.13.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shively J M, Devore W, Stratford L, Porter L, Medlin L, Stevens S E. Molecular evolution of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) FEMS Microbiol Lett. 1986;37:251–257. [Google Scholar]
- 64.Snead R M, Shively J M. d-ribulose-1,5-bisphosphate carboxylase from Thiobacillus neapolitanus. Curr Microbiol. 1978;1:309–314. [Google Scholar]
- 65.Somerville C, Knight I T, Straube W L, Colwell R. Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol. 1989;55:548–554. doi: 10.1128/aem.55.3.548-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soper T S, Mural R J, Larimer F W, Lee E H, Machanoff R, Hartman F C. Essentiality of Lys-329 of ribulose-1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum as demonstrated by site-directed mutagenesis. Protein Eng. 1988;2:39–44. doi: 10.1093/protein/2.1.39. [DOI] [PubMed] [Google Scholar]
- 67.Stahl D A, Lane D J, Olsen G J, Pace N R. Analysis of hydrothermal vent-associated symbionts by ribosomal RNA sequences. Science. 1984;224:409–411. doi: 10.1126/science.224.4647.409. [DOI] [PubMed] [Google Scholar]
- 68.Stein J, Felbeck H. Kinetic and physical properties of a recombinant RuBisCO from a chemoautotrophic endosymbiont. Mol Mar Biol Biotechnol. 1993;2:280–290. [PubMed] [Google Scholar]
- 69.Stein J, Haygood M, Felbeck H. Nucleotide sequence and expression of a deep-sea ribulose-1,5-bisphosphate carboxylase gene cloned from a chemoautotrophic bacterial endosymbiont. Proc Natl Acad Sci USA. 1990;87:8850–8854. doi: 10.1073/pnas.87.22.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tabita F R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol Rev. 1988;52:155–189. doi: 10.1128/mr.52.2.155-189.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabita F R. The biochemistry and metabolic regulation of carbon metabolism and CO2 fixation in purple bacteria. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Norwell, Mass: Kluwer; 1995. pp. 885–914. [Google Scholar]
- 72.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position specific gap penalties, and weight matrix choice. Nucleic Acids Res. 1989;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Turely C M, Mackie P J. Bacteria and cyanobacteria flux to the deep NE Atlantic on sedimenting particles. Deep-Sea Res Part I. 1995;42:1453–1474. [Google Scholar]
- 74.Von Damm K L. Controls on the chemistry in subseafloor hydrothermal fluids. In: Humphris S, Zierenberg R, Mullineaux S, Thomson R, editors. Seafloor hydrothermal systems. Washington, D.C.: American Geophysical Union Press; 1995. pp. 222–245. [Google Scholar]
- 75.Watson G M F, Tabita F R. Regulation, unique gene organization, and unusual primary structure of carbon fixation genes from a marine phycoerythrin-containing cyanobacterium. Plant Mol Biol. 1996;32:1103–1115. doi: 10.1007/BF00041394. [DOI] [PubMed] [Google Scholar]
- 76.Watson G M F, Tabita F R. Microbial ribulose 1,5-bisphosphate carboxylase/oxygenase: a molecule for phylogenetic and enzymological investigation. FEMS Microbiol Lett. 1997;146:13–22. doi: 10.1111/j.1574-6968.1997.tb10165.x. [DOI] [PubMed] [Google Scholar]
- 77.Watson G M F, Yu J, Tabita F R. Unusual ribulose 1,5-bisphosphate carboxylase/oxygenase of anoxic archaea. J Bacteriol. 1999;181:1569–1575. doi: 10.1128/jb.181.5.1569-1575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yagushi T, Chung S Y, Igarashi Y, Kodama T. Cloning and sequence of the L2 form of RuBisCO from a marine obligately autotrophic hydrogen-oxidizing bacterium, Hydrogenovibrio marinus strain MH-110. Biosci Biotech Biochem. 1994;58:1733–1737. doi: 10.1271/bbb.58.1733. [DOI] [PubMed] [Google Scholar]