Abstract
Background and Objective
We sought to review the latest developments in cortical visual prosthesis (CVP) systems and the significance of nanotechnology for the future. Over the past century, CVP systems have been researched and developed, resulting in various unique surgical and mechanical techniques. Research findings indicate that partial vision recovery is possible, with improvements in coarse target functions and performance in routine activities.
Methods
This review discusses the architecture and physiology of the visual cortex, the neuroplasticity of the blind brain, and the history of CVP development, and also provides an update on the CVP systems currently being examined in research and clinical trials. Due to advances in nanotechnology, it is possible to make CVPs that are smaller, more efficient, and more biocompatible than ever before.
Key Content and Findings
Currently, 3 CVPs have entered clinical trials, and several additional systems are undergoing preclinical reviews to determine the safety of the devices for chronic implantation. This development provides the first indication that the area of cortical vision restoration medication may be able to meaningfully benefit blind people. However, several significant technical and biological challenges need to be solved before the gap between artificial and natural eyesight can be reconciled. Rapid breakthroughs in nanotechnology have considerably increased its use in biological domains.
Conclusions
This paper summarizes the recent progress of CVP in recent years and its future development direction. It is forecasted that nanotechnology can provide better technical support for the development of CVP.
Keywords: Blindness, stimulation, visual cortex, electrode array, brain-computer interface
Introduction
The estimated number of people living with a visual impairment was around 285 million in 2010, and the projected number of legally blind individuals was approximately 39 million (1). Blindness and loss of vision are 2 of the most dreaded sensory impairments (2). Unfortunately, despite advances in modern medicine, millions of individuals worldwide must endure the difficulties associated with severe eyesight loss, which can have detrimental repercussions on their mental and physical health, including increased chronic disorders (3), accidents (4), social disengagement (5), depression (5,6), and death (7,8). From a socioeconomic standpoint, blindness has a detrimental effect on educational and vocational options, and carries health care expenses. Improvements in the daily lives of these individuals would not only benefit their quality of life, but could also significantly reduce their financial expenses (9). Dysfunction in the neural signal transduction connecting the retina to the visual brain is often the cause of vision loss. Vision restoration research is dedicated to assisting these individuals by developing therapies tailored to each indication, including gene therapy (10), stem cell therapy (11,12), optogenetics (13), vision restoration training, non-invasive stimulation (14), and vision prosthesis (15).
Over the last 3 decades, technology has improved to the point that electronic devices may now be implanted into the visual pathway to restore some sight. Visual neural prostheses are a technique for creating a visual experience by stimulating the visual signal directly. Once the retina is stimulated by light, visual information is translated into neural spike activity by photoreceptor cells. This activity is then conveyed to the lateral geniculate nucleus (LGN) of the thalamus via retinal ganglion cells and then to the primary visual cortex via thalamic synapses. Visual restoration is based on the premise that any section of the visual pathway may interface with a prosthesis that gathers visual field pictures through a camera, maps them properly to precise signals, and evokes the visual signal transduction accordingly. Several sites from the retina to the visual cortex are now used as targets for restoring vision (see Figure 1) (15,16).
Figure 1.
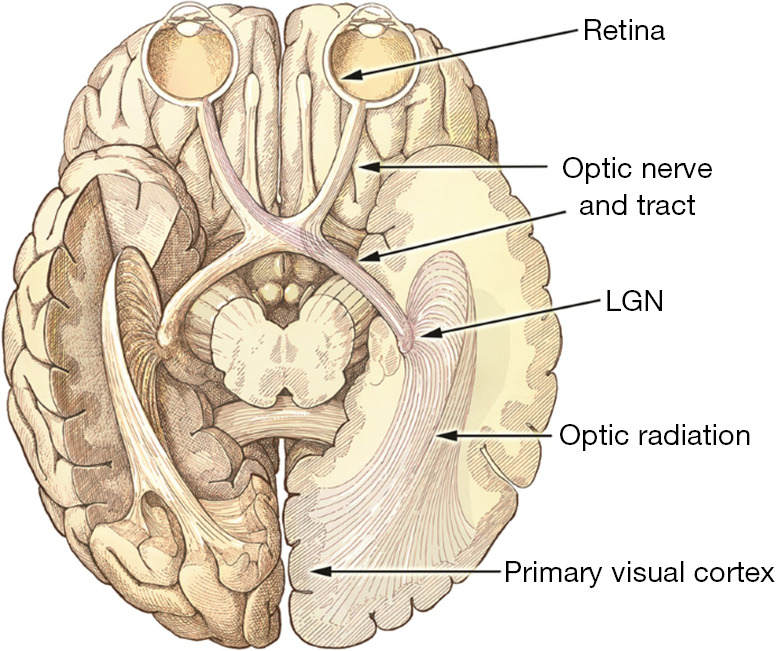
The ventral view of the human brain demonstrates the visual system’s development. Light normally enters the system through the eye, concentrates on the retina, and stimulates neurons. This activity originates in the retina, travels through the optic nerve bundle, crosses across, and terminates in the thalamic LGN. The crossover enables the visual hemisphere to concurrently interpret information from both eyes (the right hemisphere enters the left LGN and vice versa). The LGN then transfers signals to the main visual cortex (V1) and higher visual regions through optical radiation. This figure was reused from (15) under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0, http://creativecommons.org/licenses/by/4.0/). LGN, lateral geniculate nucleus.
Vision prostheses include retinal implants, optic nerve implants, LGN implants and visual cortex implants. This review will focus on visual cortex implants. Retinal prostheses are classified according to the location of the electrode array, i.e., preretinal subretinal and suprachiochoroid. The use of a retinal visual prosthesis requires an intact eyeball and functioning retinal ganglion cells and optic nerves. Therefore, any disease or injury that impairs these areas of the visual pathway will prevent the retinal approach. For patients with these conditions and severe vision loss, one option is to implant devices in other areas of the visual pathway, such as the optic nerve, LGN, or cortex. The retina and visual cortex have been the sites of choice for most visual prostheses using electrical stimulation. They are located at both extremes of the visual pathway and are more accessible surgically than deep brain structures such as the optic nerve and the LGN (17).
In people with retinitis pigmentosa or age-related macular degeneration, retinal prostheses are used to activate the inner retina, the remaining population of retinal ganglion cells, and/or bipolar cells (18). This strategy is theoretically favorable because stimulation occurs during the initial phases of visual perception, before any visual data processing by the brain (i.e., the LGN). The Second Sight Medical Product, referred to as Argus II, is the 1st retinal prosthesis to employ a retinal method in which microelectrode arrays are placed near nerve fibers on the retinal surface to activate the ganglion cells directly (19). Subjects’ performance on spatial motor tasks (20,21), motion detection (22), reading (23), and facial recognition are enhanced by Argus II.
Subretinal implants are an alternative to retinal prostheses. In subretinal implants, the electrode arrays are located on the outer surface of the retina and rely on the inner and middle layers of the retina’s normal processing. In Europe, Alpha IMS has been approved for commercial use as a subretinal implant (24). This product is capable of partially restoring light perception, which improves the daily quality of life of blind patients (25). Presently, the only commercially available visual prosthesis are retinal implants; however, such devices are only suitable for a limited proportion of the blind population (26). Some other researchers have tried the coordination of the active intraocular prosthesis with a cortical visual prosthesis (CVP) (27).
Optic nerve stimulation has also been demonstrated to induce phosphenes (28). Additionally, retinal prostheses are of little help to those who have visual loss resulting from severe retinal illness with neuronal loss, optic nerve damage, such as trauma, glaucoma, optic neuritis, or optic chiasmatic nerve injury. The LGN (29) and the visual cortex have been proven to be promising areas for electrical stimulation intervention in these individuals (30,31). However, because the LGN is a tiny region located deep in the brain, the number of electrodes and thus the number of probable phosphenes is limited. When the optic nerve is intact, it is a viable alternative to the retina, but electrode implantation is technically challenging, and visual percept resolution is not good enough (28,32). This limitation is likely due to the complexity in the axon arrangement with various regions for different visual field (33).
The stimulation of early visual cortex regions (V1, V2, and V3) produces optical illusions and is used to build cortical visual prostheses. This review seeks to shed light on the state of cortical prosthetics in vision restoration. We present a concise summary of the visual cortex anatomy and physiology, the adaptability of the blind brain, and the history of cortical visual prostheses. We then discuss the current state-of-the-art CVP systems and briefly outline recent scientific achievements in the design of devices now in development or clinical testing.
Using nano-bioelectonics, multiplexed, long-term, and deep brain recordings of neuronal activity with great spatial and temporal precision are conceivable (34). Nanostructures and nanomaterials are key components of nano-bioelectronic transistor-based sensors for monitoring, detecting, and triggering biological activity. In bioelectronic applications, nanostructures provide a number of advantages over larger structures. First, nanostructures have a high sensitivity due to their high surface-to-volume ratio. Further, the small size of nanostructures is comparable to that of biological building units, and as a result, they can merge smoothly with cells and tissues, enabling novel biological possibilities (35,36). In addition, nanoparticles have fewer metabolites, less side effects, and are not likely to cause immune rejection. Following advances in nanotechnology, it is now possible to create CVPs that are smaller, more efficient, and more biocompatible than ever before.
As a potential future research direction, the application of nanotechnology can help to increase the resolution of CVP. Due to the unique features of nanotechnology, it offers significant benefits for circumventing these constraints. We present the following article in accordance with the Narrative Review reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2858/rc).
Methods
The literature search was extensive in the PubMed database, with “cortical visual prosthesis” as the key term. The full texts of all potentially relevant articles were obtained, and relevant information was extracted. Basic visual cortex anatomy and physiology, history, types of CVP, and nanotechnology for CVP were reviewed (Table 1).
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | November 1, 2021 to January 20, 2022 |
| Databases and other sources searched | All from the PubMed database |
| Search terms used (including MeSH and free text search terms and filters) | Cortical visual prosthesis |
| Timeframe | June 1755 to December 2021 |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | English literatures including clinical trial, meta-analysis and review were collected for reviewing |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Xi Liu collected the literatures and extracted the relevant information. All the authors jointly discussed and selected the literatures to obtain the consensus of the review |
| Any additional considerations, if applicable | None |
Discussion
Basic visual cortex anatomy and physiology
Visual information is transmitted by the optic nerves, the 2nd pair of the cranial nerves. Photoreceptors (rod and cone cells) in the retina convert light to electrical impulses. After passing through the 2nd layer of bipolar cells, the signal reaches the retinal ganglion cells, the axons of which constitute the optic nerve. The ganglion cells’ axons are myelinated after leaving the back of the eyeball and exiting the orbit via the optic canal. When the optic nerve enters the middle cranial fossa, it transmits information from the left and right retinas and terminates at the optic chiasm. There, information from the nose and the retina’s temple flows through the opposing optic tract before skipping the pedunculus cerebri and terminating directly in the brain. The optic nerve does not connect to the brainstem nucleus; rather, it connects directly to the LGN (a portion of the thalamus), superior colliculus, parietal region, and hypothalamus. The geniculate nucleus transmits the majority of information from the optic tract to the visual brain. Conversely, certain neurons from optic nerve synapses are situated in regions implicated in reflex eye movements and circadian cycles. The occipital lobe is positioned near the back of the head (37) and plays a significant role in visual picture creation (38). The main task of the visual cortex is vision perception. The visual cortex is buried in the calcarine fissure on the occipital lobe’s medial surface (39-41), but it also extends into other regions, such as the lingual gyrus and the cuneus. Visual association cortices are located in the superior, middle, and inferior occipital gyri and are responsible for interpreting and adding meaning to visual data (42,43).
The Weber-Fechner laws are two related hypotheses in the field of psychophysics, known as Weber’s law and Fechner’s law. Both laws relate to human perception, more specifically the relation between the actual change in a physical stimulus and the perceived change. This includes stimuli to all senses: vision, hearing, taste, touch, and smell. Weber states that, “the minimum increase of stimulus which will produce a perceptible increase of sensation is proportional to the pre-existent stimulus”, while Fechner’s law is an inference from Weber’s law (with additional assumptions) which states that the intensity of our sensation increases as the logarithm of an increase in energy rather than as rapidly as the increase.
In previously sighted blind individuals, implantable neurostimulators that electrically activate the visual cortex create visual perceptions (44-46). Additionally, it has recently been established that visual experiences, referred to as phosphenes, may be induced in blind patients using neurostimulators licensed for long-term implantation (47,48). The electrical stimulation of the visual cortex in sighted and blind persons have demonstrated that the position and intensity of generated phosphenes could be regulated and modulated (48-50). Additionally, a recent study showed that the stimulation time could be fine-tuned to yield more complex visual experiences (47).
Individuals who have been blinded must develop new ways of life to compensate for their inability to see. As a consequence of these behavioral changes, the brain’s neurophysiology experiences a profound alteration (51). The brain’s adaptation capacity in the face of blindness is unknown. Numerous investigations have shown cross-modal plasticity in which the occipital cortex is recruited to process touch input (52). In both those born blind and those who become blind later in life, the occipital cortex is engaged during tactile tasks, such as Braille reading. The ability to read Braille is also compromised by the reversible disruption of occipital brain activity (e.g., by transcranial magnetic stimulation). Clinical trials have shown that touch processing requires occipital cortical processing. After suffering a bilateral occipital stroke, a congenitally blind patient was unable to read Braille (53,54).
Some individuals have a difficult time adjusting to being blind or visually handicapped. The occipital brains of these individuals seem to be unreconstructed or only partially repaired. This is a critical problem if the missing sensory modality may still be processed by areas of the brain that receive no sensory input. A late-blind patient reported experiencing unexpected visual hallucinations in his right visual field (55). In his left striate cortex, an arteriovenous anomaly was observed. This patient may be experiencing visual hallucinations because of the inappropriate cortical release or abnormal activation of neuronal ensembles in the regions affected by the occipital lesion. The successful development of new CVP treatments requires an understanding of the involved mechanisms for adaptive changes and the time course effect after sensory deprivation (56).
History of CVP
For several centuries, scientists have sought to restore vision to blind persons by activating the visual cortex, bypassing faulty eyes, and delivering information straight to higher visual centers (57). A French scientist named Charles Le Roy was interested in using electricity to treat ailments. To heal a blind patient, he devised a metal apparatus that he attached to the patient’s head and linked to a Leyden jar. Surprisingly, during electric shocks, the patient described seeing flashes of light (58). This was the first published demonstration of the visual cortex’s electrical excitability, and it spurred a wave of vision recovery efforts. In the early 20th century, neurosurgeons took advantage of the research opportunity provided by awake opened-skull patients to electrically stimulate their visual cortex, eliciting the impression of retinotopically arranged phosphenes (59). The electrical stimulation of the visual cortices was originally documented in 1918 in a research study, in which injured troops could perceive flashing light on the opposite half of their visual field following occipital lobe activation (60). In the 1920s and 1930s, the implantation of vision prosthesis devices in the visual cortex was developed. For example, a neurosurgeon, Foerster from Germany, stimulated the visual cortex by using electrodes, and found that the stimulated phosphenes moved when different areas were stimulated in the cortex (61).
In the late 1960s, a cortical device was developed in a baboon model by Brindley (30,44,62). This early cortical device subsequently underwent clinical trials, and the results showed that phosphenes were elicited and constant in the visual field without penetrating electrodes (62). Dobelle examined 15 sighted patients undergoing surgery for different therapeutic reasons, and in doing so, amassed valuable data on electrode placement and the influence of electrode size and stimulation settings on perceptual quality (45). They next implanted a 64-electrode array into 2 blind persons and demonstrated the ability to simultaneously activate phantom vision representations of basic patterns, such as a square and a reverse letter “L” (63). In another experiment, 2 blind individuals were implanted with electrodes (64). They were also able to construct English letters in another experiment by scattering 6 electrodes and a light phantom around the visual area. They built a “cortical Braille” that could show random letters and synthetic words by sequentially and simultaneously stimulating these 6 electrodes. One of the individuals was 85% accurate in recognizing letters and was able to read brief phrases that had multiple words that were missing or mispronounced. Additionally, they created a portable version of the gadget that could be used with a video camera (65). Dobelle has made significant advances in this field (45,65). The outcomes of this trial were encouraging, but several psychological and medical side effects were observed (66).
After Dobelle’s experience, several researchers focused on the retina for implantation rather than the visual cortex. The Argus II by Second Sight Medical Products is the most popular retinal prosthesis. The development of cortical devices requires adequate engineering solutions and neurosurgical techniques. Recently, there has been renewed interest in considering the visual cortex as an implant site. An advantage of cortical devices over retinal devices is that patients with damage to the retina or early visual pathways remain candidates for cortical implantation. Currently, several groups from the United States of America (USA), Australia, Canada, and Spain are trying to develop novel devices for stimulating the visual cortex.
Types of CVP
Illinois Intracortical Visual Prosthesis (IIVP)
At the Illinois Institute of Technology, a device called the IIVP has been developed by the Laboratory of Neural Prosthetic Research. The team’s technical accomplishments include the development of a multichannel device (67), specialized surgical equipment (68), and ways to encode fictitious visual information input for the cortex (67). These devices will be implanted as a series of small 5-mm discs, each containing 16 electrodes, amounting to a total of 600–650 electrodes.
CORTIVIS
CORTIVIS is a European research collaboration made up of 6 university laboratories, 1 technical research organization, and 1 biomedical device company. Their headquarters are located in Alicante, Spain (17). Based on bio-inspiration, the CORTIVIS designed an artificial retina capturing the visual world that is intended to mimic the visual processing performed by the retina. The CORTIVIS study uses the Utah Electrode Array (UEA) (69), which comprises 100 electrodes measuring 1.0–1.5 mm in length. The design is implanted at the site of cortical layer 4c (the geniculate innervation target), causing the fewest possible neuronal injuries. An early study in monkeys demonstrated that electrical stimulation of implanted electrodes elicited visual perception (70), and early investigations in human epilepsy or brain tumor patients were undertaken after brain surgery. Promising results were obtained based on the safe implantation, high-quality visual cortex recordings, and induced perception of phosphenes (71). A novel technology dubbed “The High-Channel-Count Neuroprosthesis” was recently tested on monkeys with successful outcomes. In this trial, a total of 1,024 electrodes were implanted in the geniculate receiving layer of the primary visual cortex (V1) and in the ventral visual stream region V4. The monkeys implanted with these devices demonstrated the ability to distinguish basic shapes, movements, and letters (72).
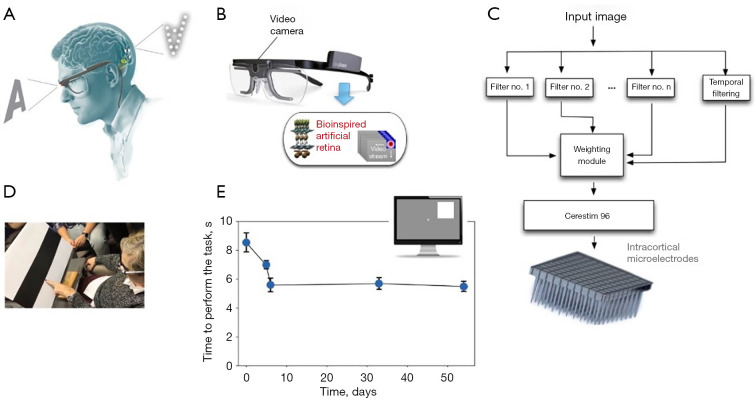
Michael (ClinicalTrials.gov identifier NCT02983370) implanted a 96-electrode intracortical microelectrode array in the visual cortex of a 57-year-old man who was completely blind for 6 months (73). Single-unit recordings were achievable, and phosphene-eliciting stimulation thresholds were within acceptable limits and remained consistent throughout the trial. Simple kinds of spatially patterned electrical stimulation elicited discriminable patterned percepts, enabling the blind person to distinguish object borders and identify various letters. Researchers also observed a learning mechanism that enabled the individual to distinguish stimulus patterns over several presentations (see Figure 2). The short-term outcomes in a single patient are promising. However, further research with more people and over a longer period needs to be conducted to determine whether intracortical microelectrodes may offer a limited but functional experience of vision to the blind.
Figure 2.
Bio-inspired artificial retina. (A) Schematic structure of CORTIVIS’s vision restoration idea. (B) The CORTIVIS image acquisition system. A video camera mounted to a spectacle frame captures the input visuals for later bio-inspired processing. (C) CORTIVIS signal processing module. (D) Using a bio-inspired artificial retina, a patient was able to discriminate between the boundary of the black and white bars. (E) Time needed to complete the item location job (4 potential sites) over a period of many days. This figure was reused with permission from (73).
Orion
The Orion™ cortical prosthesis is now being tested in clinical trials by Second Sight Medical Products (USA) (17). This device comprises a computer, a camera, and 60 surface electrodes that construct a subdural array that is linked to the medial occipital lobe. Once the video image has been analyzed, the information is wirelessly sent to the array. A pilot study on 1 blind individual established the device’s safety and basic operation. Additionally, 5 blind people have been recruited for ongoing research trials (ClinicalTrials.gov NCT03344848) that started in late 2017 and will last 5 years (16). Th initial findings revealed that patients were capable of seeing phosphenes (74).
Recent advances in biomedical engineering have accelerated the development of CVP devices. Individual electrode stimulation elicited phosphenes with locations consistent with the retinotopic map of the visual cortex. When many electrodes were triggered simultaneously, the degree to which the phosphenes blended into visible shapes was surprising. They often merged into larger phosphenes, making form identification difficult (75). However, due to the complexity of the brain, several researchers have examined and evaluated numerous methods for producing stimuli for visual perception. The researchers experimented with drawing shapes on the surface of the visual cortex using a dynamic series of electrode activations. In both sighted and blind patients, dynamic stimulation enabled the proper recognition of letter shapes predicted by the brain’s spatial map of the visual world. At a pace of up to 86 forms per minute, blind persons were able to recognize forms presented to them. These investigations demonstrate that a brain prosthesis may be capable of providing coherent sensations of visual shape (47).
In one study, 5 blind and 15 sighted patients were stimulated by Orion™ electrodes that had been implanted in their visual cortex. In this research, the following 2 fixation methods were employed: (I) unimanual fixation, in which a single index finger was placed on a tactile fixation point; (II) bimanual fixation, in which the right index finger on left was overlaid on the tactile point. Additionally, by comparing absolute mapping (the stimulation of a single electrode) and relative mapping (the stimulation of 3 to 5 phosphenes), researchers found that bimanual fixation related relative mapping strategies resulted in the most precise estimates of phosphene organization (76). In conclusion, the above-mentioned techniques, together with a standard logarithmic model of visual cortex, may provide a practical way to improve the implementation of a CVP.
According to eye-position recordings synced with stimulation in patients implanted with the Orion CVP system, the location of cortical stimulus-evoked perception differs depending on the eye position at the time of stimulation. Even after years of blindness, eye motions are maintained to affect the location of cerebral stimulus-evoked light illusion perception. Future CVPs may include head movements that alter the camera’s whole field of vision, and movements that alter the camera’s field of interest within the vast field of view (77).
Intracortical Visual Prosthesis (ICVP) Project
In the ICVP, using 16 parylene-insulated iridium microelectrodes, a Wireless Floating Microelectrode Array (WFMA) was constructed and placed on the visual cortex surface, an integrated circuit microprocessor, and a microcoil with wireless power and activation. A video camera installed on eyeglasses or a headband communicates with the video processing unit, which translates pictures to a pattern that can be mapped to the array of electrodes. The signal is subsequently delivered to the head-mounted telemetry controller through the stimulation modules, which wirelessly distribute signals and power to each WFMA module. Over a 9.5-month period, the researchers implanted a WFMA into each of 6 rats’ left sciatic nerves and observed that a combination of wireless communication and a low-profile neural interface permitted very steady motor recruitment thresholds and fine motor control in the hind limb. The groundwork has been laid for the development of a visual brain prosthesis in the future (78). Clinical trials in humans are now ongoing (ClinicalTrials.gov Identifier: NCT04634383). The objective is to implant wireless electrical stimulation into blind patients’ visual brains to produce artificial vision, and investigate their responses to the electrical stimulation. WFMAs will be implanted in the brains of 5 patients. Next, electrical stimulation will be created to evoke visual impressions. Weekly testing will be conducted for a period of 1 to 3 years (79).
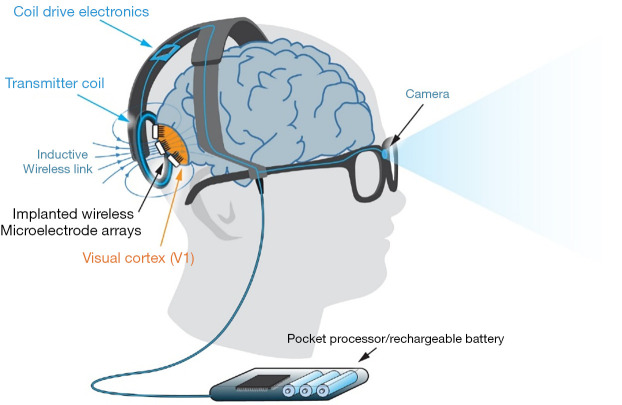
Gennaris
The Monash Vision Group (MVG) is a joint venture involving Monash University, Grey Innovation, MiniFAB, and Alfred Health in Australia. MVG is developing the “Gennaris” bionic vision system (see Figure 3) (80), a cortical vision prosthesis (17). The Gennaris consists of a headband outfitted with a camera, a visual processing device and software, a wireless transmitter, and a set of 9 mm × 9 mm brain tiles. The camera’s video is sent to the vision processor, which analyzes it and extracts the required information. The data are wirelessly sent to the circuitry embedded inside each implanted tile. Electrical pulses are generated from the data and used to stimulate the brain through the microelectrode array. The visual pattern is created using up to 473 light dots (phosphenes), providing crucial information about interior and outdoor environments, and identifying the presence of people and objects. MVG’s wireless transmission technology is integrated with the Gennaris headgear, which transmits data to up to 10 implants, each of which may stimulate 43 areas of the human visual cortex (81). Due to its direct link to the cortex, MVG’s technology has the potential to assist most patients who are absolutely blind (82). The formation of phosphenes has been validated in safety studies on experimental animals, and a histological investigation revealed little damage to the cortex following implantation, indicating that long-term stimulation is achievable without any deleterious effects (59,82). Numerous bionic eye projects are now in their infancy. Around 1 million electrodes are necessary for normal eyesight. Implants with 240 electrodes and peripheral electrodes are now being developed to expand the visual field of a person (83).
Figure 3.
The cortical vision prosthesis (Gennaris array). Images are captured by a camera implanted in a pair of glasses, which are then processed by an external pocket processor to produce stimulation protocols that are transferred to the implants via a transmitter coil. This inductive wireless connection transmits both power and data. This figure was reused with permission from (80).
The significance of nanotechnology for the future
Nanoparticles combine the characteristics of conventional solids (magnetic, optical, mechanical, and radiation) with the mobility of molecules, including the capacity to diffuse into living beings (84). This combination gives exciting possibilities for medicinal, industrial, and consumer product advancements. Nanotechnology has several uses in numerous medical sectors. First, relevant medications and viruses are first adsorbable onto nanoparticles for administration to reach the goal of precision medicine. Secondly, the creation of bioconjugated nanocrystals has accelerated the growth of medical imaging (85). Thirdly, the increased reactivity of nanoparticulate calcium-based biomaterials creates intriguing opportunities, particularly for dental and skeletal surgery. Fourth, due to the combination of molecular (mobility) and solid (e.g., reaction to electromagnetic fields) features of certain nanoparticles, magnetic nanoparticles have given birth to targeted drug delivery (magnetic accumulation of drug-loaded particles inside particular tissues). Magnetophoresis for increased uptake, cell sorting and manipulation, hyperthermia (external heating of particles within cancer tissue to destroy cells locally) and diagnostics are of significant interest (isolation of biomarkers from complex mixtures). Fifth, nanoparticulate antimicrobial agents have emerged fast and are capable of producing antibacterial effects under controlled circumstances and times (84).
Neurotechnology advancements over the last few decades have enabled a greater understanding of brain functions, such as motor control (86,87), speech processing, and synthesis (88). As a result of these discoveries, technical improvements in the field of brain-computer interfaces, such as partial mobility restoration (89) and speech decoding from cerebral activity (90) have been developed. For both neuroscientific and medicinal applications, it is crucial to integrate the coverage of large brain areas with a high sensor density (i.e., a large sensor count) (91). Nanomaterial technology may be used to enhance the amount of sensors, resulting in additional advancements in CVP. Nanostructures have several advantages over larger-scale structures in biosensing applications due to their high surface-to-volume ratio, which results in higher sensitivity, and their tiny size, which results in superior spatial resolution (single neuron resolution). The nanostructures’ modest size allows for the seamless integration of CVP with biological cells.
Unlike conventional approaches based on electrical stimulation via metal electrodes, recent advances in materials and nanoengineering have enabled novel means of neuronal interaction. Carbon nanotubes (CNTs) (92), nanocrystalline diamonds (NCDs) (93), and silicon nanowires (Si NWs) (94) have garnered attention as potential options for CVP development. Through their distinctive surface shape and charge injection methods, these materials increase the electrochemical characteristics and mechanical connection of neural electrodes. Additionally, nanomaterials have been proposed for optically activating neurons (95) and stimulating the light-insensitive retina.
CNTs
CNTs have various benefits over metal stimulating electrodes. For example, when a capacitive charge transfer mechanism is combined with a large surface area with electrochemical materials, high charge injection capacity, high specific capacitance, and low interfacial impedance are achieved (92). CNTs also act as a scaffold for neuronal development and attachment, and a mechanical process of neurite entanglement has been proposed to assist this high neuron-CNT affinity (96). To improve biocompatibility, CNTs may be simply modified with a variety of bioactive compounds (e.g., polymers, peptides, and proteins) (97). CNTs were first proposed as a substrate for neural development by Mattson et al. (98). Since then, substantial research has shown that CNTs are suitable for supporting neuronal development and neurite branching, electrically interacting with neurons, and can even be used for brain implants (92). The mechanical compatibility of flexible CNT MEAs constructed entirely of CNT embedded in different polymeric supports (e.g., parylene, medical tape, and polydimethylsiloxane) has also been investigated. David-Pur et al. used an unique manufacturing process to construct flexible CNT arrays, which involved growing loosely bonded high-density CNT patterns on a silicon dioxide substrate and then peeling them off to expose a flexible framework (92). When nitrogen or trimethyl boron is added to a NCD, it becomes electrically conductive.
NCDs
NCDs have also been suggested as a material for neuroelectrodes in recent years (99). When nitrogen or trimethyl boron is added to a NCD, it becomes electrically conductive (100). Both the biocompatibility and usability of nitrogen and boron doped diamond (BDD) coatings for neuronal stimulation are encouraging (101). Nitrogen doped ultra-NCD electrodes were electrochemically activated or coated with platinum (Pt) or electrodeposited iridium oxide film (EIROF) to increase the double layer capacitance and decrease the impedance (100). To produce comparable findings, BDD electrodes were supplemented with NCD deposition onto vertically aligned CNTs as a template interlayer (102).
Bendali et al. recently claimed that the resolution of cortical visual implants could be improved by adopting a BDD/protein electrode covering that particularly repels glial cell adherence and proliferation while facilitating neuronal connection (101). CVPs are usually in contact with the surface glial layer, which may affect the effective resolution of the device. Neuronal electrodes usually end up in contact with the surface sealed glial layer rather than with the neuron, leading to a progressive loss of function. The scientists proposed a design in which a protein-coated base stimulates glial cell proliferation, pushing them away from an uncoated penetrating electrode tip (101).
Optoelectronic Si NWs
Ha et al. are developing a hybrid optoelectrical retinal prosthesis using Si NWs as the light sensors (94). The system includes an inductive telemetry connection, a demodulator pulsed by stimulation, a series capacitor with charge-balance, and a Si NW electrode array placed under the retinal. The system requires external power, which is supplied by a single wireless inductive connection. Each NW is designed to behave as an electrode capable of penetrating the retina. The electrode array are linked to the stimulator and an adjacent ground electrode by 2 wires. When voltage bias is introduced, the device creates a current pulse that couples with the intensity transmitted by the light stimulated NWs. This hybrid optoelectronic system enables the control of spatial (incidence light) and temporal (electrical bias) stimulation, and tunable gain at lower light intensity thresholds. Compared to previous systems for electrical retinal activation, this concept has the distinct advantage of requiring virtually no extra gear to increase resolution beyond the electrode array density.
Conclusions
Restoring eyesight is a difficult but necessary objective. The increase in blind people’s quality of life would be significant, and both the physical problems and the financial constraints associated with their visual loss would be alleviated. However, there are a number of limitations to visual prosthesis that must be overcome before functional vision may be restored.
With regard to electrode placement, intracortical electrodes have advantages over subdural electrodes. Subdural electrodes need milliampere-range currents and stimulate populations of neurons over millimeters of cortex, hence limiting their resolution. In addition, the stimulation of nearby subdural electrodes may produce interference, resulting in the production of a single, big phosphene rather than multiple tiny ones. Using depth electrodes evoked phosphenes by stimulating tiny groups of neurons situated within a few hundred micrometers of the electrode tip with median stimulation currents of 23 to 50 mA. Consequently, intracortical electrodes cause smaller and more precise phosphene percepts; nevertheless, a comprehensive comparison of phosphenes produced by subdural and intracortical stimulation has not yet been conducted (72).
Each hemisphere of the human primary visual cortex measures 25 to 30 square centimeters, and future implants should cover a large enough visual field area with a sufficient density of phosphenes to produce interpretable perception. Additionally, there is a need to create high channel count wireless technologies and to develop durable, biocompatible electrodes that minimize the risk of gliosis, tissue damage, and encapsulation (103).
There are numerous advantages to stimulating the V1 area for vision restoration; however, the engineering constraints of the implants indicate that the stimulation of the V1 area is not sufficient to restore visual sense with a resolution sufficient to live a proper daily life for blind individuals. A combination of temporally and spatially coherent electrical stimulation targeting distinct areas is a promising strategy for increasing resolution. Implants in V1, V2, and V3 increase visual resolution by creating more phosphenes over the visual field, enable safe insertion distances, and ensure intracortical electrodes are targeted in foveal locations.
Presently, the surgical risks outweigh the minimal benefits of invasive prosthesis. Major neurosurgical procedures are inherently dangerous and may result in major complications, such as infection, inflammation, and neurodegeneration, and other neurological problems. Another factor limiting their use is that they are not suitable for those who have been blind from birth. Their efficacy is conditional on the presence of a fully developed visual system with an acquired visual repertoire. The amount of phosphenes can be raised in a presenting image to increase the probable resolution of an image; however even the most powerful neurotechnology-based CVP would still have resolution orders of magnitude lower than a computer display. For example, optogenetics (104), a more precise stimulation technology, may eventually replace electrical stimulation as a way of speeding progress in the future. The link between artificial neuronal activity and perception remains primitive, and we must increase our knowledge of this relationship before we can artificially generate a veridical picture of the visual world in the mind’s eye using any approach for activating neurons. At least 5 teams are actively undertaking CVP investigations worldwide, many of which are already conducting clinical trials with promising early findings.
Rapid advances in nanotechnology have significantly boosted its use in biological fields. Due to their enhanced electrochemical activity, nanostructured materials are readily applicable to energy devices, and their incorporation into scalable and human-compatible implanted brain interfaces has the potential to significantly improve the performance of clinical and research electrodes. Nanometer-sized electrodes have the potential to be used in CVPs to increase the quality of the visual signal. Nanotechnology advancements enable the development of CVPs that are smaller, more efficient, and more biocompatible than ever before.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program of China (Grant No. 2017YFA0701304) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. KYCX_2076).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-2858/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-2858/coif). XL reports that this work was supported by Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. KYCX_2076). CS, HG, XL, PC, and XD report that this work was supported by the National Key Research and Development Program of China (Grant No. 2017YFA0701304). The other authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol 2012;96:614-8. 10.1136/bjophthalmol-2011-300539 [DOI] [PubMed] [Google Scholar]
- 2.Chader GJ, Weiland J, Humayun MS. Artificial vision: needs, functioning, and testing of a retinal electronic prosthesis. Prog Brain Res 2009;175:317-32. 10.1016/S0079-6123(09)17522-2 [DOI] [PubMed] [Google Scholar]
- 3.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: implications for health and functioning. Am J Public Health 2004;94:823-9. 10.2105/AJPH.94.5.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivers RQ, Norton R, Cumming RG, et al. Visual impairment and risk of hip fracture. Am J Epidemiol 2000;152:633-9. 10.1093/aje/152.7.633 [DOI] [PubMed] [Google Scholar]
- 5.Jones GC, Rovner BW, Crews JE, et al. Effects of depressive symptoms on health behavior practices among older adults with vision loss. Rehabil Psychol 2009;54:164-72. 10.1037/a0015910 [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Bullard KM, Cotch MF, et al. Association between depression and functional vision loss in persons 20 years of age or older in the United States, NHANES 2005-2008. JAMA Ophthalmol 2013;131:573-81. 10.1001/jamaophthalmol.2013.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng DD, Christ SL, Lam BL, et al. Visual acuity and increased mortality: the role of allostatic load and functional status. Invest Ophthalmol Vis Sci 2014;55:5144-50. 10.1167/iovs.14-14202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EE, Egleston BL, West SK, et al. Visual acuity change and mortality in older adults. Invest Ophthalmol Vis Sci 2005;46:4040-5. 10.1167/iovs.05-0687 [DOI] [PubMed] [Google Scholar]
- 9.Frick KD, Gower EW, Kempen JH, et al. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 2007;125:544-50. 10.1001/archopht.125.4.544 [DOI] [PubMed] [Google Scholar]
- 10.Amato A, Arrigo A, Aragona E, et al. Gene Therapy in Inherited Retinal Diseases: An Update on Current State of the Art. Front Med (Lausanne) 2021;8:750586. 10.3389/fmed.2021.750586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stern JH, Tian Y, Funderburgh J, et al. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018;22:834-49. 10.1016/j.stem.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He XY, Zhao CJ, Xu H, et al. Synaptic repair and vision restoration in advanced degenerating eyes by transplantation of retinal progenitor cells. Stem Cell Reports 2021;16:1805-17. 10.1016/j.stemcr.2021.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AR, Gilbert F. Restoring vision using optogenetics without being blind to the risks. Graefes Arch Clin Exp Ophthalmol 2022;260:41-5. 10.1007/s00417-021-05477-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong J, Dong P, Kedar S, et al. Three-dimensional shape analysis of peripapillary retinal pigment epithelium-basement membrane layer based on OCT radial images. Nanotechnol Rev 2021;10:453-64. 10.1515/ntrev-2021-0035 [DOI] [Google Scholar]
- 15.Mirochnik RM, Pezaris JS. Contemporary approaches to visual prostheses. Mil Med Res 2019;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niketeghad S, Pouratian N. Brain Machine Interfaces for Vision Restoration: The Current State of Cortical Visual Prosthetics. Neurotherapeutics 2019;16:134-43. 10.1007/s13311-018-0660-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen PJ, Ayton LN. Development and Experimental Basis for the Future of Prosthetic Vision. In: Chang A, Mieler WF, Ohji M. Macular Surgery. Singapore: Springer, 2020:449-62. [Google Scholar]
- 18.Margalit E, Maia M, Weiland JD, et al. Retinal prosthesis for the blind. Surv Ophthalmol 2002;47:335-56. 10.1016/S0039-6257(02)00311-9 [DOI] [PubMed] [Google Scholar]
- 19.Luo YH, da Cruz L. The Argus(®) II Retinal Prosthesis System. Prog Retin Eye Res 2016;50:89-107. [DOI] [PubMed]
- 20.Ahuja AK, Dorn JD, Caspi A, et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol 2011;95:539-43. [DOI] [PMC free article] [PubMed]
- 21.Luo YH, Zhong JJ, da Cruz L. The use of Argus® II retinal prosthesis by blind subjects to achieve localisation and prehension of objects in 3-dimensional space. Graefes Arch Clin Exp Ophthalmol 2015;253:1907-14. [DOI] [PubMed]
- 22.Dorn JD, Ahuja AK, Caspi A, et al. The Detection of Motion by Blind Subjects With the Epiretinal 60-Electrode (Argus II) Retinal Prosthesis. JAMA Ophthalmol 2013;131:183-9. [DOI] [PMC free article] [PubMed]
- 23.da Cruz L, Coley BF, Dorn J, et al. The Argus II epiretinal prosthesis system allows letter and word reading and long-term function in patients with profound vision loss. Br J Ophthalmol 2013;97:632-6. [DOI] [PMC free article] [PubMed]
- 24.Edwards TL, Cottriall CL, Xue K, et al. Assessment of the Electronic Retinal Implant Alpha AMS in Restoring Vision to Blind Patients with End-Stage Retinitis Pigmentosa. Ophthalmology 2018;125:432-43. 10.1016/j.ophtha.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stingl K, Bartz-Schmidt KU, Besch D, et al. Artificial vision with wirelessly powered subretinal electronic implant alpha-IMS. Proc Biol Sci 2013;280:20130077. 10.1098/rspb.2013.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haim M. Epidemiology of retinitis pigmentosa in Denmark. Acta Ophthalmol Scand Suppl 2002;(233):1-34. 10.1046/j.1395-3907.2002.00001.x [DOI] [PubMed] [Google Scholar]
- 27.Shim S, Seo K, Kim SJ. A preliminary implementation of an active intraocular prosthesis as a new image acquisition device for a cortical visual prosthesis. J Artif Organs 2020;23:262-9. 10.1007/s10047-020-01168-x [DOI] [PubMed] [Google Scholar]
- 28.Veraart C, Raftopoulos C, Mortimer JT, et al. Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 1998;813:181-6. 10.1016/S0006-8993(98)00977-9 [DOI] [PubMed] [Google Scholar]
- 29.Pezaris JS, Reid RC. Demonstration of artificial visual percepts generated through thalamic microstimulation. Proc Natl Acad Sci U S A 2007;104:7670-5. 10.1073/pnas.0608563104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brindley GS, Lewin WS. The visual sensations produced by electrical stimulation of the medial occipital cortex. J Physiol 1968;194:54-P. [PubMed]
- 31.Rosenfeld JV, Wong YT. Neurobionics and the brain-computer interface: current applications and future horizons. Med J Aust 2017;206:363-8. 10.5694/mja16.01011 [DOI] [PubMed] [Google Scholar]
- 32.Nishida K, Sakaguchi H, Kamei M, et al. Visual Sensation by Electrical Stimulation Using a New Direct Optic Nerve Electrode Device. Brain Stimul 2015;8:678-81. 10.1016/j.brs.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 33.Naito J. Retinogeniculate projection fibers in the monkey optic nerve: a demonstration of the fiber pathways by retrograde axonal transport of WGA-HRP. J Comp Neurol 1989;284:174-86. 10.1002/cne.902840203 [DOI] [PubMed] [Google Scholar]
- 34.Angle MR, Cui B, Melosh NA. Nanotechnology and neurophysiology. Curr Opin Neurobiol 2015;32:132-40. 10.1016/j.conb.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 35.Gruner G. Carbon nanotube transistors for biosensing applications. Anal Bioanal Chem 2006;384:322-35. 10.1007/s00216-005-3400-4 [DOI] [PubMed] [Google Scholar]
- 36.El-Atab N, Shaikh SF, Hussain MM. Nano-scale transistors for interfacing with brain: design criteria, progress and prospect. Nanotechnology 2019;30:442001. 10.1088/1361-6528/ab3534 [DOI] [PubMed] [Google Scholar]
- 37.Georgiev DD. Quantum information and consciousness: a gentle introduction. Boca Raton: CRC Press, 2017. [Google Scholar]
- 38.Glickstein M. The discovery of the visual cortex. Sci Am 1988;259:118-27. 10.1038/scientificamerican0988-118 [DOI] [PubMed] [Google Scholar]
- 39.Tootell RB, Hadjikhani NK, Vanduffel W, et al. Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci U S A 1998;95:811-7. 10.1073/pnas.95.3.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bridge H. Mapping the visual brain: how and why. Eye (Lond) 2011;25:291-6. 10.1038/eye.2010.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawachi J. Brodmann Areas 17, 18, and 19 in the Human Brain: An Overview. Brain Nerve 2017;69:397-410. [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Fukuyama H, Honda M, et al. Essential role of the right superior parietal cortex in Japanese kana mirror reading: An fMRI study. Brain 2000;123:790-9. 10.1093/brain/123.4.790 [DOI] [PubMed] [Google Scholar]
- 43.Georgiev DD, Georgieva I, Gong Z, et al. Virtual Reality for Neurorehabilitation and Cognitive Enhancement. Brain Sci 2021;11:221. 10.3390/brainsci11020221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol 1968;196:479-93. 10.1113/jphysiol.1968.sp008519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobelle WH, Mladejovsky MG. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J Physiol 1974;243:553-76. 10.1113/jphysiol.1974.sp010766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt EM, Bak MJ, Hambrecht FT, et al. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain 1996;119:507-22. 10.1093/brain/119.2.507 [DOI] [PubMed] [Google Scholar]
- 47.Beauchamp MS, Oswalt D, Sun P, et al. Dynamic Stimulation of Visual Cortex Produces Form Vision in Sighted and Blind Humans. Cell 2020;181:774-83.e5. 10.1016/j.cell.2020.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niketeghad S, Muralidharan A, Patel U, et al. Phosphene perceptions and safety of chronic visual cortex stimulation in a blind subject. J Neurosurg 2019;132:2000-7. 10.3171/2019.3.JNS182774 [DOI] [PubMed] [Google Scholar]
- 49.Murphey DK, Maunsell JH, Beauchamp MS, et al. Perceiving electrical stimulation of identified human visual areas. Proc Natl Acad Sci U S A 2009;106:5389-93. 10.1073/pnas.0804998106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winawer J, Parvizi J. Linking Electrical Stimulation of Human Primary Visual Cortex, Size of Affected Cortical Area, Neuronal Responses, and Subjective Experience. Neuron 2016;92:1213-9. 10.1016/j.neuron.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci 2004;27:649-77. 10.1146/annurev.neuro.27.070203.144220 [DOI] [PubMed] [Google Scholar]
- 52.Burton H, Snyder AZ, Conturo TE, et al. Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol 2002;87:589-607. 10.1152/jn.00285.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamilton R, Keenan JP, Catala M, et al. Alexia for Braille following bilateral occipital stroke in an early blind woman. Neuroreport 2000;11:237-40. 10.1097/00001756-200002070-00003 [DOI] [PubMed] [Google Scholar]
- 54.Merabet L, Thut G, Murray B, et al. Feeling by sight or seeing by touch? Neuron 2004;42:173-9. 10.1016/S0896-6273(04)00147-3 [DOI] [PubMed] [Google Scholar]
- 55.Alfaro A, Climent R, Vilanova H, et al. Plasticidad transmodal en sujectos ciegos: Es posible la actividad visual en una corteza deaferentizada. Rev Esp Neurol 2003;37:1093. [Google Scholar]
- 56.Fernández E, Pelayo F, Romero S, et al. Development of a cortical visual neuroprosthesis for the blind: the relevance of neuroplasticity. J Neural Eng 2005;2:R1-12. 10.1088/1741-2560/2/4/R01 [DOI] [PubMed] [Google Scholar]
- 57.Bosking WH, Beauchamp MS, Yoshor D. Electrical Stimulation of Visual Cortex: Relevance for the Development of Visual Cortical Prosthetics. Annu Rev Vis Sci 2017;3:141-66. 10.1146/annurev-vision-111815-114525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LeRoy C. Où l'on rend compte de quelques tentatives que l'on a faites pour guérir plusieurs maladies par l'électricité. Hist Acad Roy Sciences Memoires Math Phys 1755;60:87-95. [Google Scholar]
- 59.Ptito M, Bleau M, Djerourou I, et al. Brain-Machine Interfaces to Assist the Blind. Front Hum Neurosci 2021;15:638887. 10.3389/fnhum.2021.638887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Löwenstein K, Borchardt M. Symptomatologie und elektrische Reizung bei einer Schußverletzung des Hinterhauptlappens. Deutsche Zeitschrift für Nervenheilkunde 1918;58:264-92. 10.1007/BF01629694 [DOI] [Google Scholar]
- 61.Foerster O. Contributions to the pathophysiology of the visual pathway and visual sphere. J Psychol Neurol 1929;39:435-63. [Google Scholar]
- 62.Brindley GS, Donaldson PE, Falconer MA, et al. The extent of the region of occipital cortex that when stimulated gives phosphenes fixed in the visual field. J Physiol 1972;225:57P-8P. [PubMed] [Google Scholar]
- 63.Dobelle WH, Mladejovsky MG, Girvin JP. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 1974;183:440-4. 10.1126/science.183.4123.440 [DOI] [PubMed] [Google Scholar]
- 64.Dobelle WH, Mladejovsky MG, Evans JR, et al. "Braille" reading by a blind volunteer by visual cortex stimulation. Nature 1976;259:111-2. 10.1038/259111a0 [DOI] [PubMed] [Google Scholar]
- 65.Dobelle WH. Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO J 2000;46:3-9. 10.1097/00002480-200001000-00002 [DOI] [PubMed] [Google Scholar]
- 66.Lane FJ. Methods and results from interviews of eleven recipients of a visual cortex implant: An analysis of their experiences. In: The Eye and the Chip: World Congress on Artificial Vision. Detroit, 2012. [Google Scholar]
- 67.Srivastava NR, Troyk PR. Some solutions to technical hurdles for developing a practical intracortical visual prosthesis device. Conf Proc IEEE Eng Med Biol Soc 2006;2006:2936-9. 10.1109/IEMBS.2006.259987 [DOI] [PubMed] [Google Scholar]
- 68.Tawakol O, Bredeson SD, Troyk PR. Preparation of a neural electrode implantation device for in-vivo surgical use. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:4507-10. 10.1109/EMBC.2016.7591729 [DOI] [PubMed] [Google Scholar]
- 69.Normann RA, Fernandez E. Clinical applications of penetrating neural interfaces and Utah Electrode Array technologies. J Neural Eng 2016;13:061003. 10.1088/1741-2560/13/6/061003 [DOI] [PubMed] [Google Scholar]
- 70.Normann RA, Greger B, House P, et al. Toward the development of a cortically based visual neuroprosthesis. J Neural Eng 2009;6:035001. 10.1088/1741-2560/6/3/035001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez E, Alfaro A, Toledano R, et al. Perceptions elicited by electrical stimulation of human visual cortex. Invest Ophthalmol Vis Sci 2015;56:777. [Google Scholar]
- 72.Chen X, Wang F, Fernandez E, et al. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science 2020;370:1191-6. 10.1126/science.abd7435 [DOI] [PubMed] [Google Scholar]
- 73.Fernández E, Alfaro A, Soto-Sánchez C, et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J Clin Invest 2021;131:e151331. 10.1172/JCI151331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barry MP, Salas MA, Patel U, et al. Video-mode percepts are smaller than sums of single-electrode phosphenes with the Orion® visual cortical prosthesis. Invest Ophthalmol Vis Sci 2020;61:927. [Google Scholar]
- 75.Roelfsema PR. Writing to the mind’s eye of the blind. Cell 2020;181:758-9. 10.1016/j.cell.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 76.Oswalt D, Bosking W, Sun P, et al. Multi-electrode stimulation evokes consistent spatial patterns of phosphenes and improves phosphene mapping in blind subjects. Brain Stimul 2021;14:1356-72. 10.1016/j.brs.2021.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caspi A, Barry MP, Patel UK, et al. Eye movements and the perceived location of phosphenes generated by intracranial primary visual cortex stimulation in the blind. Brain Stimul 2021;14:851-60. 10.1016/j.brs.2021.04.019 [DOI] [PubMed] [Google Scholar]
- 78.Frederick RA, Troyk PR, Cogan SF. Wireless microelectrode arrays for selective and chronically stable peripheral nerve stimulation for hindlimb movement. J Neural Eng 2021. doi: . 10.1088/1741-2552/ac2bb8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanie-Jahromi F, Azizi A, Shariat S, et al. Effect of Electrical Stimulation on Ocular Cells: A Means for Improving Ocular Tissue Engineering and Treatments of Eye Diseases. Biomed Res Int 2021;2021:6548554. 10.1155/2021/6548554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong YT, Feleppa T, Mohan A, et al. CMOS stimulating chips capable of wirelessly driving 473 electrodes for a cortical vision prosthesis. J Neural Eng 2019;16:026025. 10.1088/1741-2552/ab021b [DOI] [PubMed] [Google Scholar]
- 81.Lowery AJ, Rosenfeld JV, Lewis PM, et al. Restoration of vision using wireless cortical implants: The Monash Vision Group project. Annu Int Conf IEEE Eng Med Biol Soc 2015;2015:1041-4. 10.1109/EMBC.2015.7318543 [DOI] [PubMed] [Google Scholar]
- 82.Rosenfeld JV, Wong YT, Yan E, et al. Tissue response to a chronically implantable wireless intracortical visual prosthesis (Gennaris array). J Neural Eng 2020;17:046001. 10.1088/1741-2552/ab9e1c [DOI] [PubMed] [Google Scholar]
- 83.Suvvari TK, Madhu MT, Nagendra S. Bionic eye: An iconic innovation. TNOA J Ophthalmic Sci Res 2021;59:52. 10.4103/tjosr.tjosr_168_20 [DOI] [Google Scholar]
- 84.Stark WJ. Nanoparticles in biological systems. Angew Chem Int Ed Engl 2011;50:1242-58. 10.1002/anie.200906684 [DOI] [PubMed] [Google Scholar]
- 85.Selvan ST, Patra PK, Ang CY, et al. Synthesis of silica-coated semiconductor and magnetic quantum dots and their use in the imaging of live cells. Angew Chem Int Ed Engl 2007;46:2448-52. 10.1002/anie.200604245 [DOI] [PubMed] [Google Scholar]
- 86.Jeannerod M. Motor cognition: What actions tell the self. Oxford: OUP Oxford, 2006. [Google Scholar]
- 87.Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci 1988;8:2928-37. 10.1523/JNEUROSCI.08-08-02928.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galantucci B, Fowler CA, Turvey MT. The motor theory of speech perception reviewed. Psychon Bull Rev 2006;13:361-77. 10.3758/BF03193857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Capogrosso M, Milekovic T, Borton D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016;539:284-8. 10.1038/nature20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anumanchipalli GK, Chartier J, Chang EF. Speech synthesis from neural decoding of spoken sentences. Nature 2019;568:493-8. 10.1038/s41586-019-1119-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stringer C, Pachitariu M, Steinmetz N, et al. Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019;364:255. 10.1126/science.aav7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.David-Pur M, Bareket-Keren L, Beit-Yaakov G, et al. All-carbon-nanotube flexible multi-electrode array for neuronal recording and stimulation. Biomed Microdevices 2014;16:43-53. 10.1007/s10544-013-9804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hadjinicolaou AE, Leung RT, Garrett DJ, et al. Electrical stimulation of retinal ganglion cells with diamond and the development of an all diamond retinal prosthesis. Biomaterials 2012;33:5812-20. 10.1016/j.biomaterials.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 94.Ha S, Khraiche ML, Akinin A, et al. Towards high-resolution retinal prostheses with direct optical addressing and inductive telemetry. J Neural Eng 2016;13:056008. 10.1088/1741-2560/13/5/056008 [DOI] [PubMed] [Google Scholar]
- 95.Bareket-Keren L, Hanein Y. Novel interfaces for light directed neuronal stimulation: advances and challenges. Int J Nanomedicine 2014;9 Suppl 1:65-83. 10.2147/IJN.S51193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Voge CM, Stegemann JP. Carbon nanotubes in neural interfacing applications. J Neural Eng 2011;8:011001. 10.1088/1741-2560/8/1/011001 [DOI] [PubMed] [Google Scholar]
- 97.Bottini M, Rosato N, Bottini N. PEG-modified carbon nanotubes in biomedicine: current status and challenges ahead. Biomacromolecules 2011;12:3381-93. 10.1021/bm201020h [DOI] [PubMed] [Google Scholar]
- 98.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci 2000;14:175-82. 10.1385/JMN:14:3:175 [DOI] [PubMed] [Google Scholar]
- 99.Ahnood A, Meffin H, Garrett DJ, et al. Diamond Devices for High Acuity Prosthetic Vision. Adv Biosyst 2017;1:e1600003. 10.1002/adbi.201600003 [DOI] [PubMed] [Google Scholar]
- 100.Garrett DJ, Ganesan K, Stacey A, et al. Ultra-nanocrystalline diamond electrodes: optimization towards neural stimulation applications. J Neural Eng 2012;9:016002. 10.1088/1741-2560/9/1/016002 [DOI] [PubMed] [Google Scholar]
- 101.Bendali A, Agnès C, Meffert S, et al. Distinctive glial and neuronal interfacing on nanocrystalline diamond. PLoS One 2014;9:e92562. 10.1371/journal.pone.0092562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piret G, Hébert C, Mazellier JP, et al. 3D-nanostructured boron-doped diamond for microelectrode array neural interfacing. Biomaterials 2015;53:173-83. 10.1016/j.biomaterials.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 103.Chen SC, Suaning GJ, Morley JW, et al. Simulating prosthetic vision: II. Measuring functional capacity. Vision Res 2009;49:2329-43. 10.1016/j.visres.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 104.Ortiz-Rios M, Agayby B, Balezeau F, et al. Optogenetic stimulation of primate V1 reveals local laminar and large-scale cortical networks related to perceptual phosphenes. bioRxiv 2021. doi: . 10.1101/2021.06.01.446505 [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as