Abstract
Background
Eosinophilic oesophagitis (EoE) is an increasingly common cause of dysphagia in both children and adults, as well as one of the most prevalent oesophageal diseases with a significant impact on physical health and quality of life. We have provided a single comprehensive guideline for both paediatric and adult gastroenterologists on current best practice for the evaluation and management of EoE.
Methods
The Oesophageal Section of the British Society of Gastroenterology was commissioned by the Clinical Standards Service Committee to develop these guidelines. The Guideline Development Group included adult and paediatric gastroenterologists, surgeons, dietitians, allergists, pathologists and patient representatives. The Population, Intervention, Comparator and Outcomes process was used to generate questions for a systematic review of the evidence. Published evidence was reviewed and updated to June 2021. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was used to assess the evidence and make recommendations. Two rounds of voting were held to assess the level of agreement and the strength of recommendations, with 80% consensus required for acceptance.
Results
Fifty-seven statements on EoE presentation, diagnosis, investigation, management and complications were produced with further statements created on areas for future research.
Conclusions
These comprehensive adult and paediatric guidelines of the British Society of Gastroenterology and British Society of Paediatric Gastroenterology, Hepatology and Nutrition are based on evidence and expert consensus from a multidisciplinary group of healthcare professionals, including patient advocates and patient support groups, to help clinicians with the management patients with EoE and its complications.
Keywords: DYSPHAGIA, DIET, ENDOSCOPY, OESOPHAGEAL DISEASE, PAEDIATRIC GASTROENTEROLOGY
Executive summary of recommendations
Definition
-
Eosinophilic oesophagitis is a condition characterised by symptoms of dysphagia and/or food impaction in adults, and feeding problems, abdominal pain and/or vomiting in children, with oesophageal histology showing a peak eosinophil count of ≥15 eosinophils/high power field (or ≥15 eosinophils/0.3 mm2 or >60 eosinophils/mm2, in the absence of other causes of oesophageal eosinophilia.
Grading of Recommendations, Assessment, Development and Evaluation (GRADE) of evidence: High.
Level of recommendation: Strong.
-
Eosinophilic oesophagitis is increasing in prevalence in both adults and children.
GRADE of evidence: High.
Level of recommendation: Not applicable.
-
There is seasonal variation in the symptoms of eosinophilic oesophagitis in many patients, which seems to be associated with higher pollen counts.
GRADE of evidence: Low.
Level of recommendation: Not applicable.
-
Eosinophilic oesophagitis is more common in men than women and in people of white ethnic origin compared with other ethnic groups. Having an affected first-degree relative increases the risk of eosinophilic oesophagitis. The incidence rises during adolescence and peaks in early adulthood.
GRADE of evidence: High.
Level of recommendation: Not applicable.
Clinical presentation
-
In adults, food bolus obstruction and dysphagia are strongly associated with a diagnosis of eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Not applicable.
-
In children, symptoms associated with a diagnosis of eosinophilic oesophagitis may be non-specific and vary with the age of the child.
GRADE of evidence: Moderate.
Level of recommendation: Not applicable.
-
All adults undergoing endoscopy should have oesophageal biopsies taken if they have endoscopic signs associated with eosinophilic oesophagitis, or symptoms of dysphagia or food bolus obstruction, with a normal looking oesophagus.
GRADE of evidence: High.
Level of recommendation: Strong.
-
All children undergoing endoscopy for upper gastrointestinal symptoms should have oesophageal biopsies taken to diagnose eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Endoscopy and biopsy to exclude eosinophilic oesophagitis should be undertaken in children with typical gastro-oesophageal reflux disease symptoms refractory to treatment with proton pump inhibitors.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Endoscopy and biopsies to exclude eosinophilic oesophagitis in adult patients with typical gastro-oesophageal reflux disease symptoms refractory to proton pump inhibitors is usually not indicated, given the low prevalence of eosinophilic oesophagitis in such patients, in the absence of clinical features associated with eosinophilic oesophagitis (eg, dysphagia or atopy).
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
In patients with food bolus obstruction, urgent referral to gastroenterology and an endoscopy on the next available endoscopy list, or as an immediate emergency is recommended, depending on clinical presentation.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Oesophageal biopsies should be taken at index endoscopy in patients with food bolus obstruction to diagnose eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
After spontaneous resolution of food bolus obstruction, patients should be booked for an endoscopy and outpatient review.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Maintenance therapy with topical steroid reduces the risk of recurrent food bolus obstruction.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
For an accurate diagnosis of eosinophilic oesophagitis, proton pump inhibitors should be withdrawn for at least 3 weeks prior to endoscopy and biopsy.
GRADE of evidence: Very low.
Level of recommendation: Strong.
-
In patients where a high index of suspicion exists for a diagnosis of eosinophilic oesophagitis but whose initial histology was not diagnostic, repeat endoscopy with adequate biopsies should be considered, if there were suggestive endoscopic features or typical symptoms of eosinophilic oesophagitis.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Diagnosing and treating eosinophilic oesophagitis effectively early in its natural history may prevent long-term complications of fibrosis and strictures requiring subsequent endoscopic intervention.
GRADE of evidence: Low.
Level of recommendation: Weak.
-
Eosinophilic oesophagitis that responds clinically and histologically to a proton pump inhibitor is the same disease as eosinophilic oesophagitis that fails to respond to a proton pump inhibitor.
GRADE of evidence: Low.
Level of recommendation: Weak.
-
Eosinophilic oesophagitis and gastro-oesophageal reflux disease are not mutually exclusive and can coexist in the same patient.
GRADE of evidence: High.
Level of recommendation: Not applicable.
-
Formal transition of care from paediatric to adult services may improve symptom control, concordance with therapy and reduce emergency presentations in eosinophilic oesophagitis.
GRADE of evidence: Very low.
Level of recommendation: Not applicable.
Investigation
-
At least six biopsies should be taken from different anatomical sites within the oesophagus for diagnosis and follow-up of eosinophilic oesophagitis.
Level of evidence: Moderate
Strength of recommendation: Strong.
-
Eosinophil density should be expressed as eosinophil counts per 0.3 mm2 (this equates to a conventional optical high power field) and the cut-off for a diagnosis should be ≥15 eosinophils per 0.3 mm2 in any biopsy specimen.
Level of evidence: Moderate.
Strength of recommendation: Strong.
-
Mucosal eosinophilia should be accompanied by other histological features of eosinophilic oesophagitis. These may include the presence of basal cell hyperplasia, oedema (spongiosis), eosinophil microabscesses, eosinophil layering, eosinophil degranulation and subepithelial sclerosis.
Level of evidence: Moderate.
Strength of recommendation: Strong.
-
In treated eosinophilic oesophagitis, histological response should be classified according to the eosinophil density. Remission is defined for clinical purposes as a maximum eosinophil count <15 eosinophils/0.3 mm2.
Level of evidence: Low.
Strength of recommendation: Strong.
-
Oesophageal physiological testing should be considered in patients with eosinophilic oesophagitis who have ongoing dysphagia, despite histological remission and the absence of fibrostenotic disease at endoscopy.
Level of evidence: Low
Strength of recommendation: Strong.
Management
-
After initiation of therapy (dietary or pharmacological treatment), endoscopy with biopsy while on treatment, is recommended to assess response, as symptoms may not always correlate with histological activity.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Elimination diets are effective in achieving clinico-histological remission in both adults and paediatric patients with eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
A six food elimination diet results in higher histological remission rates than two or four food elimination diets, but is associated with lower compliance and an increased number of endoscopies.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
When undertaking a dietary restriction therapy for eosinophilic oesophagitis, support from an experienced dietitian throughout both the elimination and reintroduction process is strongly recommended.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Combining elimination diets with pharmacological treatment is not routinely recommended but can be considered in cases of drug treatment failure.
GRADE of evidence: Very low.
Level of recommendation: Strong.
-
Allergy testing to foods (eg, skin prick, specific IgE and patch testing) is not recommended for choosing the type of dietary restriction therapy for eosinophilic oesophagitis.
GRADE of evidence: Low
Level of recommendation: Strong.
-
Exclusive elemental diets have a limited role in eosinophilic oesophagitis, with high efficacy but low compliance rates and should be reserved for patients refractory to other treatments.
GRADE of evidence: Low
Level of recommendation: Strong.
-
Proton pump inhibitor therapy is effective in inducing histological and clinical remission in patients with eosinophilic oesophagitis.
GRADE of evidence: Moderate
Level of recommendation: Strong.
-
Proton pump inhibitor therapy should be given two times per day for at least 8–12 weeks prior to assessment of histological response, while on treatment.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
In patients who achieve histological response, proton pump inhibitor therapy appears effective in maintaining remission.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Topical steroids are effective for inducing histological and clinical remission in eosinophilic oesophagitis.
GRADE of evidence: High.
Level of recommendation: Strong.
-
Clinical and histological relapse is high after withdrawal of topical steroid treatment, and following clinical review, maintenance treatment should be recommended.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Systemic steroids are not recommended in eosinophilic oesophagitis.
GRADE of evidence: High.
Level of recommendation: Strong.
-
Immunomodulators (eg, azathioprine, 6-mercaptopurine) are not recommended in the management of eosinophilic oesophagitis.
GRADE of evidence: Low.
Level of recommendation: Weak.
-
Monoclonal antibody therapies, such as anti-tumour necrosis factor (TNF) and anti-integrin therapies, that are typically used for inflammatory bowel disease are not recommended in the management of eosinophilic oesophagitis.
GRADE of evidence: Low.
Level of recommendation: Weak.
-
Novel biologics used in other allergic conditions (such as dupilumab, cendakimab and benralizumab) have shown promise in the treatment of eosinophilic oesophagitis.
GRADE of evidence: Low.
Level of recommendation: Weak.
-
Sodium cromoglycate, montelukast and anti-histamines are not recommended in the management of eosinophilic oesophagitis but may have a role in concomitant atopic disease.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
If symptoms recur while on treatment, we recommend repeating an endoscopy for assessment and to obtain further histology.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Patients with eosinophilic oesophagitis refractory to treatment and/or with significant concomitant atopic disease should be jointly managed by a gastroenterologist and specialist allergist to optimise treatment.
GRADE of evidence: Very low.
Level of recommendation: Weak.
Complications
-
Endoscopists can underestimate the frequency of strictures and narrow lumen oesophagus in eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Medical treatment with topical steroids is likely to reduce the development of strictures in eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Endoscopic dilatation is effective in improving symptoms in patients with fibrostenotic disease due to eosinophilic oesophagitis.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Endoscopic dilatation is safe in patients with eosinophilic oesophagitis and can be performed using either balloon or bougie dilators.
GRADE of evidence: High.
Level of recommendation: Strong.
-
Clinical outcomes of patients with stricture are better if therapeutic dilatation is combined with effective anti-inflammatory therapy with topical steroids.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Eosinophilic oesophagitis is the most common cause of spontaneous perforation of the oesophagus, and this can occur at any age from children to adults.
GRADE of evidence: High.
Level of recommendation: Not applicable.
-
In case of an eosinophilic oesophagitis perforation, a CT contrast study should be performed to assess the degree of extravasation.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
In case of a perforation in eosinophilic oesophagitis, if there is limited extravasation, the patient should be managed conservatively, with multidisciplinary input from gastroenterology, surgery and radiology specialists.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
The psychological impact of dietary therapy should be appreciated and discussed with patients with eosinophilic oesophagitis and their carers.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
Anxiety and depression in eosinophilic oesophagitis affects patients due to persistent symptoms and social restrictions and is alleviated by effective therapy.
GRADE of evidence: Low.
Level of recommendation: Strong.
-
If proton pump inhibitor therapy causes unwanted side effects (diarrhoea, gastrointestinal infections or magnesium deficiency), consider switching to alternative treatments such as diet or topical steroid.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Candida infection may occur in a small proportion of patients with eosinophilic oesophagitis treated with topical corticosteroids and should be managed by topical antifungals while continuing topical steroids.
GRADE of evidence: Moderate.
Level of recommendation: Strong.
-
Systemic side effects of topical steroids have not been documented during the long-term treatment of eosinophilic oesophagitis patients; continued monitoring of bone mineral density and adrenal suppression is recommended in children and adolescents.
GRADE of evidence: High.
Level of recommendation: Strong.
Future research
Research is needed into the cause and progression of eosinophilic oesophagitis, the course of the disease and into disease prevention.
Research is needed in non-endoscopic sampling techniques for disease diagnosis and follow-up of eosinophilic oesophagitis.
Research is needed into quantifying symptom severity in a standard manner that helps to guide therapy and record disease response.
Research is needed into patient education and shared decision-making in eosinophilic oesophagitis between patients and their doctors.
Research is needed to compare available drug therapies, including new biological drugs and or diets through randomised clinical trials.
Research is needed to understand the application of clinical guidelines in eosinophilic oesophagitis.
Introduction
Objectives
Eosinophilic oesophagitis (EoE) is a chronic inflammatory condition of the oesophagus which is increasingly being diagnosed in adults and children presenting with dysphagia or food bolus obstruction. The disease was first characterised as a clinical entity by Attwood and Straumann in two simultaneous publications in the early 1990s.1 2 The last decade has seen significant advances in the diagnosis and treatment of this condition with new drugs now either approved for clinical use or in phase 2/3 trials. The purpose of these guidelines is to provide a practical and evidence-based guide to the diagnosis, investigations and management of both adult and paediatric patients with EoE. These guidelines incorporate a review of published literature on EoE, subjected to the rigour of a Delphi consensus on specific statements derived from a PICO (Problem/population, Intervention, Comparator and Outcome) format using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology.
This guideline specifically aims:
To introduce new diagnostic criteria for EoE incorporating digital pathology and promote consistency in pathology reporting of oesophageal biopsies for EoE.
To review and standardise the diagnosis, treatment and follow-up of patients with EoE with special relevance to the National Health Service in the UK.
To summarise the optimal management of both children and adults with EoE, highlight gaps in our knowledge and set out future research priorities.
Guideline development process
The British Society of Gastroenterology (BSG) Oesophageal Section was commissioned in 2019 to write combined adult and paediatric guidelines on EoE, with a particular emphasis on practical guidance for clinicians diagnosing and treating this condition. While there are published European3 and American guidelines4 on EoE which are relevant to clinical practice in both adults and children in those geographical areas, there are no UK guidelines on EoE. Our aim was also to define the standards of care for diagnosis, treatment and management of complications of this condition in light of two significant changes to clinical practice: the introduction of digital pathology which has made the high-power field obsolete and necessitated the re-defining of the diagnostic criteria for EoE and the introduction of new drugs to manage this condition more effectively.
The guideline development group (GDG) included representatives of all relevant professional groups involved in the care of patients with EoE: adult and paediatric gastroenterologists (including representatives from the British Society of Paediatric Gastroenterology, Hepatology and Nutrition EoE working group), gastrointestinal surgeons, dietitians, allergists, patient representatives, patient support groups and gastrointestinal physiologists.
Methodology
The guidelines are relevant to both paediatric and adult patients with EoE and was developed according to GRADE methodology,5 in accordance with the principles of the Appraisal of Guidelines for Research and Evaluation, AGREE II tool6 (online supplemental table 2). The guidelines were commissioned by the Clinical Services Standards Committee (CSSC) of the BSG and the final document was approved at the CSSC and the Council of the BSG.
gutjnl-2022-327326supp003.pdf (385.2KB, pdf)
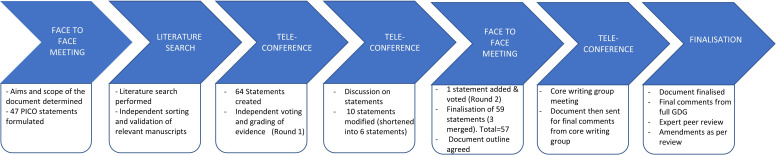
A GDG was constituted by inviting national experts in the field of adult and paediatric EoE based on clinical experience and previous research publications and chaired by AD. A comprehensive literature search was carried out in July 2019 and relevant papers collated on an electronic platform (Mendeley); this was updated in June 2021 and additional literature added to the platform (figure 1), with evaluation of full published papers only. The GDG met in 2019 to develop clinical questions structured by PICO development, to assimilate evidence and facilitate voting on draft statements and recommendations using a modified e-Delphi process. The GDG was also subdivided into seven sections and relevant literature reviewed by members of these sections. The GDG and any potential conflicts of interest for 12 months preceding the GDG formation were vetted and approved by the CSSC of the BSG.
Figure 1.
Flow chart of guideline development process. GDG, guideline development group; PICO, Population, Intervention, Comparator, Outcome.
Clinical areas that have been covered by the guideline were set by the GDG to include the following:
Definition and clinical epidemiology of EoE.
Clinical presentations, symptomatology, relation to gastro-oesophageal reflux disease, course of disease and access to care.
Investigations including blood tests, endoscopy, physiological tests and histology.
Treatment including treatment objectives, dietary and pharmacological management.
Complications and their management.
Future treatments and research priorities.
To achieve transparency and simplicity, the GRADE system classifies the quality of evidence in one of four levels—high, moderate, low and very low. Evidence based on randomised controlled trials begins as high quality evidence, but confidence in the evidence may be decreased for several reasons including: study limitations; inconsistency of results; indirectness of evidence; imprecision; and reporting bias. The GRADE system offers two grades of recommendations: ‘strong’ and ‘conditional/weak’. When the desirable effects of an intervention clearly outweigh the undesirable effects, or clearly do not, guideline panels offer strong recommendations. On the other hand, when the trade-offs are less certain—either because of low quality evidence or because evidence suggests that desirable and undesirable effects are closely balanced—conditional/weak recommendations are mandatory. In addition to the quality of the evidence, several other factors affect whether recommendations are strong or weak such as: uncertainty about the balance between desirable and undesirable effects, uncertainty or variability in values and preferences, and uncertainty about whether the intervention represents a wise use of resources. Where factual statements were made, for example, relating to the epidemiology of EoE, no strength of recommendation was made.
Statements derived from PICO questions and their GRADE strength of evidence and recommendations were subjected to two rounds of electronic Delphi voting to agree on the wording with >80% agreement required; any statements that did not reach the desired level of agreement after round 1 were modified and further voting undertaken until >80% agreement was attained (online supplemental file). Statements that could not be resolved after two rounds of voting were rejected.
gutjnl-2022-327326supp001.xlsx (25.8KB, xlsx)
The GDG met again in July 2021 to discuss any issues requiring further clarification and finalise the statements and supporting evidence (figure 1).
Dissemination and implementation of guidelines
The guidelines have been written to be of practical value for both adult and paediatric clinicians and to facilitate appropriate and timely diagnosis and treatment. The guidelines will be disseminated through publication and through presentation at national and regional meetings as well as through patient support groups. Wherever possible, we have tried to ensure that the implementation of these guidelines will not encounter resource barriers within a healthcare economy.
It is anticipated that these guidelines will need review and updating in 5 years to incorporate the rapid developments in this field.
Definition
Eosinophilic oesophagitis is characterised by symptoms of dysphagia and/or food impaction in adults, and feeding problems, abdominal pain and/or vomiting in children, with oesophageal histology showing a peak eosinophil count of ≥15 eosinophils/high power field (or ≥15 eosinophils/0.3 mm2 or >60 eosinophils/mm2.
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 100%.
EoE is now recognised to be a distinct clinicopathological entity characterised by a wide variety of oesophageal symptoms and feeding-related symptoms (particularly in children) with a severe impact on quality of life.7 It can be defined as a chronic, immune-mediated or antigen-mediated oesophageal disease characterised by symptoms related to oesophageal dysfunction and eosinophil-predominant mucosal inflammation.8 The current diagnostic criterion for oesophageal inflammation by eosinophils has been endoscopic biopsies showing a peak value of ≥15 eosinophils per high power field (hpf). This relates to when this condition was first described using analogue optical microscopes which had low power and high power magnification (typically 15× and 40×, respectively).1 2 With most laboratories now moving to digital optical microscopy, we propose that the definition of EoE be modified to incorporate this technology and the peak values of eosinophils should be expressed as ≥15 per 0.3 mm2. The recent updated International Consensus diagnostic criteria for EoE recommended that 15 eosinophils/hpf should be equivalent to 60 eosinophils per mm.2 9 10 However, the GDG were concerned that due to the patchy distribution of eosinophils in the oesophageal mucosa, it is more appropriate to count them per 0.3 mm2 and that there was insufficient evidence to change from the recognised threshold of 15 eosinophils per 0.3 mm2 (equivalent to 50 eosinophils per mm2).
Other conditions that can cause oesophageal eosinophilia, including eosinophilic gastrointestinal disease, connective tissue disorders, vasculitis, hypereosinophilic syndrome, Crohn’s disease and coeliac disease should be considered. These diagnostic criteria are applicable to all age groups and to patients with gastro-oesophageal reflux disease (GORD).
The GDG recommends that EoE should be diagnosed in patients with relevant oesophageal symptoms and a peak eosinophil count on oesophageal biopsy ≥15 per 0.3 mm2, after consideration of all causes of oesophageal eosinophilia.
Eosinophilic oesophagitis is increasing in prevalence in both adults and children
GRADE of evidence: High
Level of recommendation: Not applicable.
Level of agreement: 96%.
When first described over two decades ago, EoE was regarded as a rare disease. In the past decade there has been a rapid rise in its prevalence throughout the Western world, with mean estimates of 15/100 000 before 2007 and 63/100 000 since 2017.11 It is three times commoner in men, and is associated with atopic diseases such as allergic asthma, rhinitis and eczema. A meta-analysis of the incidence of EoE in population based studies across the world, calculated an overall pooled incident rate of 3.7/100 000/year (95% CI 1.7 to 6.5), higher in adults than in children.12 This is believed to be a true increase in the incidence of the disease and not simply an increase in endoscopic awareness and biopsy rates.12 The data for these reports are predominantly from high prevalence Western countries rather than from low prevalence Eastern countries.
There is seasonal variation in the symptoms of eosinophilic oesophagitis in many patients, which seems to be associated with higher pollen counts
GRADE of evidence: Low.
Level of recommendation: Not applicable
Level of agreement: 89%.
Because EoE is an allergic condition, its aetiopathogenesis has been attributed to environmental allergens such as aeroallergens and food allergens. There are links to EoE flares during pollen season and spring or summer seasons, associated with an increase in aeroallergen exposure.13–15 However, definite conclusions on the causal association of seasonality and aeroallergen exposure are difficult to establish due to the retrospective nature of most reports, and the lack of a mechanistic correlation of these associations with the immunobiology of EoE.
Eosinophilic oesophagitis is more common in men than women and in people of white ethnic origin compared with other ethnic groups. Having an affected first-degree relative increases the risk of eosinophilic oesophagitis. The incidence rises during adolescence and peaks in early adulthood
GRADE of evidence: High.
Level of recommendation: Not applicable.
Level of agreement: 100%.
The male predominance of EoE is well described, with a 3:1 preponderance and has been mainly described in white ethnic origin patients,16 although few studies have investigated other ethnic groups, making direct comparison more difficult. The peak incidence of EoE is seen in young adults and in the third and fourth decades of life. Studies of family history, twin concordance and heritability report a risk of 2% on the basis of results from a nuclear family based cohort of 914 probands with EoE and 63 twin probands.17 The prevalence of EoE is increased among first-degree family members, with one study demonstrating a 64-fold increased risk in brothers and 43-fold increased risk in fathers of index cases, while monozygotic twins had a 41% and dizygotic twins had a 22% prevalence of EoE, respectively. The mode of inheritance is complex and polygenic, with no autosomal dominant, recessive or X-linked patterns. Candidate gene studies and genome-wide association suggest association with genes that influence epithelial barrier function or T-helper cell-mediated immune responses. The magnitude of disease associated susceptibility for most of the genes reported in these analyses is modest (<2).
Clinical presentation
In adults, food bolus obstruction and dysphagia are strongly associated with a diagnosis of eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Not applicable.
Level of agreement: 95%.
Food bolus obstruction is a common presentation of EoE. In a retrospective study of 546 patients presenting with food bolus obstruction, in those who had oesophageal biopsies, 46% had histological evidence of EoE. EoE was also the strongest predictor of multiple presentations with bolus obstruction (OR 3.5 (95% CI 1.8 to 7.0)).18 A further retrospective study of 202 patients, who had endoscopy for foreign body impaction, reported that 26% of those who had oesophageal biopsies had EoE.19
Patients with EoE also commonly present with dysphagia. In a prospective series of 400 patients undergoing endoscopy for oesophageal symptoms, 7.3% had histological evidence of EoE. EoE was more common in men, patients aged under 50, patients with asthma and those with dysphagia and food impaction.20 A prospective study of 100 adult patients with non-obstructive dysphagia reported that 22% had EoE.21
Reflux symptoms and chest pain are less common in EoE but may be the presenting reports in some patients with EoE. A retrospective study of 353 patients with reflux symptoms reported that 7.7% of those biopsied at endoscopy had EoE.22 A retrospective review of 161 patients having endoscopy for non-cardiac chest pain reported that 6% had EoE.23
An insidious onset of symptoms and self-taught coping strategies such as food avoidance (eg, difficult to swallow textures such as bread and meat) and drinking large volumes of water with meals, can delay patients recognition and reporting of symptoms.
The GDG therefore recommend that EoE is strongly considered in all adult patients with dysphagia or food bolus obstruction.
In children, symptoms associated with a diagnosis of eosinophilic oesophagitis may be non-specific and vary with the age of the child
GRADE of evidence: Moderate.
Level of recommendation: Not applicable.
Level of agreement: 100%.
EoE presents with a wide range of different symptoms in children. Younger children are more likely to show non-specific symptoms whereas older children are more likely to present with specific symptoms of oesophageal dysfunction. In a multisite registry of 705 patients with EoE aged 6 months to 65 years, abdominal pain and vomiting were more common in children, while heartburn, chest pain, dysphagia and food impaction occurred infrequently in children and increased steadily with advancing age through childhood and into adulthood.24 In a retrospective case review of 620 children with EoE, children under 6 years were more likely to present with feeding difficulties (median age 2.8 years) or failure to thrive and vomiting (median age 5.1 years), whereas children over 6 years were more likely to present with abdominal pain (median age 9.0 years) or dysphagia and food impaction (median age 11.1 years).25 In a multinational European registry of 410 patients with EoE diagnosed under the age of 18, younger children were more likely to present with failure to thrive and diarrhoea (median age 6–7 years) and older children with abdominal pain and dysphagia (median age 10 years) or food bolus impaction (median age 12 years).26 A systematic review of EoE in patients of all ages reported that the most common symptoms in children were vomiting and abdominal pain, whereas the most common symptoms in adults were dysphagia, food impaction, heartburn and chest pain.27
The GDG therefore recommends that a diagnosis of EoE is considered in children of all ages with symptoms consistent to the age of the child.
All adults undergoing endoscopy should have oesophageal biopsies taken if they have endoscopic signs associated with eosinophilic oesophagitis, or symptoms of dysphagia or food bolus obstruction, with a normal looking oesophagus
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 85%.
While 7%–17% of patients with biopsy proven EoE may have a macroscopically normal appearance reported at endoscopy, specific findings of furrows, rings, white plaques, mucosal oedema, fragile mucosa, narrow calibre oesophagus and strictures are frequently observed endoscopically in patients with confirmed EoE. These features are subtle and require a degree of experience for their recognition. In a meta-analysis, the sensitivity of these endoscopic signs for diagnosing EoE was 15%–46% with a specificity of 90%–95% and positive predictive value of 51%–73%. At least one of these endoscopic findings was found in 93% of patients with EoE.28
The GDG therefore recommends that oesophageal biopsies are taken at the first presentation in all patients with dysphagia or food bolus obstruction and normal endoscopic appearance or with the above endoscopic signs associated with EoE.
All children undergoing endoscopy for upper gastrointestinal symptoms should have biopsies taken to diagnose eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 95%.
The macroscopic endoscopic appearances are not a reliable predictor of EoE, especially in children. In a single centre retrospective analysis of 189 paired biopsies samples and endoscopic scores in 115 children with EoE, macroscopic endoscopic scores (oedema, rings, exudates, furrows and strictures) correlated only moderately with either histological scores (r=0.61) or peak eosinophil counts (r=0.55).29 In a retrospective multicentre study of 84 children with EoE, mucosal granularity was seen in 42.8%, furrows in 25%, rings in 22.6% and exudates in 10.7%.30
Finally, a meta-analysis of 1015 patients with EoE reported that 21% of children with EoE had a macroscopically normal oesophagus.28
Due to the non-specific presenting symptoms of EoE, especially in younger children, and the fact that a significant proportion of children with EoE have a macroscopically normal oesophagus, the GDG recommends that all children with upper gastrointestinal symptoms sufficiently significant to warrant endoscopy should have biopsies taken to potentially diagnose EoE.
Endoscopy and biopsy to exclude eosinophilic oesophagitis should be undertaken in children with typical gastro-oesophageal reflux disease symptoms refractory to proton pump inhibitors
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
The presenting symptoms of EoE in children can be indistinguishable from GORD. In a retrospective study of 666 children with eosinophilic eosinophilia, 30% had been previously diagnosed with GORD.31 A retrospective study of 410 children with EoE reported that the most frequent indications for endoscopy were dysphagia (38%), gastro-oesophageal reflux (31.2%), bolus impaction (24.4%), chest pain (9.2%) and epigastric pain (8%).26 In this cohort, the median age at EoE diagnosis was 9 years, and although symptoms varied in different age groups, they were not unique for EoE. Proton pump inhibitor (PPI) treatment had previously failed in 70% of this group of children.
Symptoms of EoE can not only be indistinguishable from GORD, but there is also a substantial proportion of overlap between GORD and EoE with or without response to a PPI.32
The GDG recommends that children with persistent, typical GORD symptoms should undergo oesophago-gastro-duodenoscopy (OGD) with sufficient oesophageal biopsies, as they may represent children with clinical and histological features of EoE, in which PPI treatment has failed.
Endoscopy and biopsies to exclude eosinophilic oesophagitis in adult patients with typical gastro-oesophageal reflux disease symptoms refractory to proton pump inhibitors is usually not indicated, given the low prevalence of eosinophilic oesophagitis in such patients, in the absence of clinical features associated with eosinophilic oesophagitis (eg, dysphagia or atopy)
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 94%.
Two prospective case series of adult patients undergoing endoscopy and oesophageal biopsies for GORD symptoms refractory to PPI therapy report EoE is an uncommon finding. The reported prevalence was 0.8% and 4%, respectively.33 34 A further retrospective study of adults patients with heartburn and no response to one time per day PPI reported an EoE prevalence of 0.9%.35 When EoE was found, it was strongly associated with dysphagia (OR 12), younger age (OR 5) and atopy (OR 3).34
The GDG does not recommend endoscopy and oesophageal biopsies in patients with typical GORD symptoms refractory to PPIs, unless there are clinical features suggestive of EoE such as dysphagia and atopy.
In patients with food bolus obstruction, urgent referral to gastroenterology and an endoscopy on the next available endoscopy list, or as an immediate emergency is recommended, depending on clinical presentation
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 94%.
EoE is the most common cause of food bolus obstruction presenting to emergency departments and the incidence is increasing.18 36 Food bolus obstruction is the first presenting symptom in 30% of patients who are ultimately diagnosed with EoE.1 19 37 38 The only specific risk factor identified so far is the use of PPI as therapy for previously diagnosed EoE,39 but most episodes of food bolus obstruction occur in patients not previously diagnosed with EoE.
The key to initial management is reassurance and assessment of the risk of perforation, followed by urgent interventional endoscopy to remove the food bolus and take oesophageal biopsies.40 There is no evidence that conservative treatments such as fizzy drinks, baclofen, salbutamol or benzodiazepines are helpful in the management of this condition.41 There is no clear evidence for or against a bolus push or bolus extraction technique at endoscopy,37 42 but it is important to have anaesthetic support available for airway management if the airway could be compromised with adequate sedation. If a stricture is identified with macroscopic signs of EoE, then it is possible to perform an immediate dilatation, but in most cases (70%) there is no stricture once the bolus has been removed.42
The GDG recommends urgent referral of patients with food bolus obstruction to gastroenterology for endoscopic intervention to treat the food bolus and diagnose EoE if present.
Oesophageal biopsies should be taken at index endoscopy in patients with food bolus obstruction to diagnose eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
In patients presenting with food bolus obstruction, EoE is the most frequent diagnosis and found in up to 46% of patients.18 However, biopsies were not taken at endoscopy during the index presentation with food bolus obstruction in 73% of these patients. In a more recent study, 55% of patients with food bolus obstruction did not have biopsies taken at endoscopy during their first presentation and of those who were biopsied, insufficient biopsies to reliably exclude EoE were taken in 66% of patients.43 Finally, in patients presenting as an emergency with food bolus obstruction, if biopsies were not taken, 79% were lost to secondary care follow-up, missing the opportunity to diagnose EoE.44 Furthermore, in patients who have not had biopsies taken on their index endoscopy when presenting with a food-bolus obstruction, in those in whom a repeat endoscopy can be organised, there is a delay to diagnosis and follow-up of at least 2 years.38
Biopsy at the time of endoscopy for food bolus obstruction is not associated with an increased risk of oesophageal perforation. A retrospective study of 511 patients with EoE reported 10 perforations (2%), none of which were reported to be related to oesophageal biopsy.45 In a systematic review of 76 oesophageal perforations in patients of all ages with EoE, none were reported to be associated with oesophageal biopsy.46 There may be situations where it is considered unsafe to take biopsies after food bolus dis-impaction or removal, and in these circumstances it is essential that the patient is brought back for subsequent biopsy at a later date.
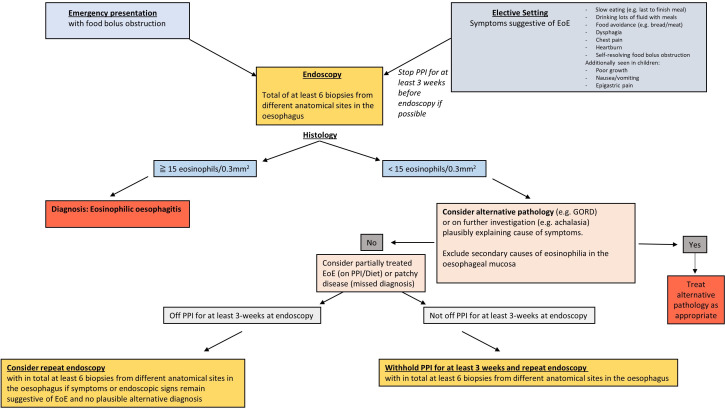
The GDG recommends that all patients have sufficient oesophageal biopsies taken at the time of endoscopy when presenting with food bolus obstruction, to avoid missing the opportunity to diagnose and treat EoE (figure 2).
Figure 2.
Eosinophilic oesophagitis diagnostic algorithm in emergency and elective settings. EoE, eosinophilic oesophagitis; GORD, gastro-oesophageal reflux disease; PPI, proton pump inhibitor.
After spontaneous resolution of food bolus obstruction, patients should be booked for an endoscopy and outpatient review
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 83%.
Since the most common benign cause of food bolus obstruction presenting to hospital is EoE, six oesophageal biopsies at two levels should be obtained at the index endoscopy. Dis-impaction of the food bolus alone and arranging elective repeat endoscopy to obtain diagnostic biopsies results in significant loss of patients to follow-up and failure to diagnose the underlying cause of food bolus obstruction, including EoE.18 19 47
The value of a planned outpatient review is to confirm the cause of the episode of food bolus obstruction, educate the patient and institute appropriate anti-inflammatory therapy for EoE if confirmed and this has not already been undertaken.
The GDG recommends that if food bolus obstruction spontaneously resolves or if it has not been possible to obtain sufficient diagnostic biopsies for EoE at index endoscopy, that elective endoscopy and outpatient review are arranged prior to discharge. It is expected that malignant causes of food bolus obstruction will be excluded before following this recommendation. Patients should be counselled on the importance of attending endoscopy and outpatient review before discharge.
Maintenance therapy with topical steroid reduces the risk of recurrent food bolus obstruction
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 94%.
The key to good management of food bolus obstruction is to appreciate that EoE is the most likely cause and to start therapy as early as possible to prevent recurrence and to improve quality of life.47 48 If endoscopic signs of EoE are clearly present and adequate biopsies have been taken then it is recommended that anti-inflammatory therapy is commenced. Failure to follow-up patients after food bolus obstruction and a lack of ongoing medical therapy is still a common problem19 42 and likely to result in further episodes of food bolus obstruction and unscheduled admissions. Data from an EoE cohort study of 206 patients show that swallowed topical corticosteroid treatment and oesophageal stricture were the only factors associated with recurrence of food bolus impaction on a multivariate analysis.49
For an accurate diagnosis of eosinophilic oesophagitis, proton pump inhibitors should be withdrawn for at least three weeks prior to endoscopy and biopsy
GRADE of evidence: Very low.
Level of recommendation: Strong.
Level of agreement: 89%
A meta-analysis of 33 studies of the response of patients with EOE to PPI reported a 51% pooled rate of entering histological remission, defined as an oesophageal eosinophil count below 15 per hpf on biopsy.50 These findings highlight an important issue for the diagnostic process in patients with potential EoE. Undertaking endoscopy and oesophageal biopsies to diagnose EoE in patients who are currently taking PPIs may prevent a definitive diagnosis of EoE, through suppression of oesophageal eosinophilia below the diagnostic level of 15 eosinophils per 0.3 mm2.
Unfortunately, there is lack of good quality data on patients with EoE, who had their diagnosis initially obscured by the impact of PPI therapy on their oesophageal biopsy eosinophil counts, to guide recommendations on a suitable time frame to withdraw PPIs for prior to endoscopy. Two patients with dysphagia have been described with no eosinophils or a maximum of 9 per hpf on their initial biopsies. Both patients’ EoE was only diagnosed following repeat biopsies with >15 eosinophils per hpf, after discontinuing PPIs for at least 3 weeks.51 Further data are clearly needed on this important issue for EoE diagnosis.
Since many patients will be referred for endoscopy for dysphagia on an urgent pathway, if PPIs have not been withdrawn for at least 3 weeks before endoscopy and EoE remains a possible diagnosis following initial endoscopy and biopsies, the GDG recommends repeating the endoscopy and biopsies, after at least 3 weeks off PPIs, to definitively exclude EoE. Whether PPIs have been discontinued and for how long should be clearly documented on the endoscopy report and histology request form, when biopsies are taken to diagnose EoE.
Although logistics are more challenging for symptomatic children referred for endoscopy as parents and general practitioners feel obliged to reduce symptoms, the GDG recommends withdrawal of PPI for 3 weeks before endoscopy to improve diagnostic accuracy and avoid the need for a repeat procedure (and a further general anaesthetic in younger children).
In patients where a high index of suspicion exists for a diagnosis of eosinophilic oesophagitis but whose initial histology was not diagnostic, repeat endoscopy with adequate biopsies should be considered, if there were suggestive endoscopic features or typical symptoms of eosinophilic oesophagitis
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 95%.
There is a lack of good quality data on the value of repeating endoscopy and oesophageal biopsies in patients with symptoms (dysphagia or food bolus obstruction in adults and older children) or endoscopic signs suggestive of EoE, but whose biopsies do not reveal diagnostic levels of eosinophils (≥15 per 0.3 mm2).
In the previous statement, the potential role of PPI therapy in obscuring a diagnosis of EoE on oesophageal biopsy was explained with the importance of considering repeating the endoscopy and biopsy under these circumstances. This advice should include ensuring there are no dietary exclusions that may mask results.
A retrospective case series described 59 patients with dysphagia without diagnostic histology for EoE (eosinophils <15 per hpf) on at least four mid-oesophageal biopsies. In a subgroup of 14 of these patients, who underwent repeat endoscopy and biopsy for persistent symptoms, 5 (36%) had diagnostic histology (eosinophils >15 per hpf) on their repeat biopsies.52
The GDG recommends considering repeating endoscopy and oesophageal biopsies in patients whose initial histology was not diagnostic, who had endoscopic signs suggestive of EoE or typical symptoms such as food bolus obstruction. However, further data are clearly needed to clarify which patients will benefit from this approach.
Diagnosing and treating eosinophilic oesophagitis effectively early in its natural history may prevent long-term complications of fibrosis and strictures requiring subsequent endoscopic intervention
GRADE of evidence: Low.
Level of recommendation: Weak.
Level of agreement: 80%.
Stricture formation is a major complication of untreated EoE and can also lead to food bolus obstruction. Oesophageal strictures frequently require endoscopic dilatation. There is some limited evidence that a delay in diagnosing EoE may lead to increased oesophageal fibrosis and subsequent stricturing. A retrospective study of 200 patients found that those with an interval between symptom onset and endoscopic diagnosis less than 2 years had an overall stricture rate of 17%, while those with a delay in diagnosis of more than 20 years had a stricture rate of 71%. Endoscopic features of fibrotic disease (such as endoscopic rings, strictures and crepe paper oesophagus) were reported in 47% of patients with a diagnostic delay of less than 2 years but 88% if the diagnostic delay was more than 20 years (p=0.02).53
A second study described similar findings in a retrospective review of 64 patients, confirming a longer diagnostic delay was associated with a narrower oesophageal lumen at endoscopy. Patients who had a luminal diameter more than 17 mm had a mean delay to diagnosis of 5 years compared with those who had a diameter of 10–16.9 mm (mean delay 11 years) and those with luminal diameter less than 10 mm (mean delay 15 years) (p=0.006 and p=0.002, respectively).54 Similarly in a large study of 721 patients from the Netherlands (including 117 children), delay in diagnosis was also shown to be associated with fibrostenotic disease (19% in those with a diagnostic delay <2 years to 52% with longer diagnostic delays).55
Smaller studies in children have shown that subepithelial fibrosis is reversible and tissue remodelling occurs following treatment with topical steroids or dietary therapy.56 57 It is therefore helpful to diagnose EoE at an early stage and ensure appropriate histological improvement as symptom improvement may only be partial and may not reflect ongoing inflammation with subsequent risk of fibrostenotic disease.
The GDG recommends the collection of more data on the natural history of fibrostenotic disease in EoE to establish in which patients long-term therapy is needed to alter the natural history.
Eosinophilic oesophagitis that responds clinically and histologically to a proton pump inhibitor is the same disease as eosinophilic oesophagitis that fails to respond to a proton pump inhibitor
GRADE of evidence: Low.
Level of recommendation: Weak.
Level of agreement: 100%.
Historically, a diagnosis of EoE required a failure to respond to a trial of a PPI or normal 24-hour pH monitoring as a diagnostic criterion, in an attempt to exclude GORD as a cause for the eosinophilia.58 However, it was subsequently recognised that EoE, in the absence of features of GORD, could also respond to PPIs. The term PPI responsive oesophageal eosinophilia was then proposed for patients with >15 eosinophils per hpf on oesophageal biopsies, who responded clinically and histologically to a PPI.59
However, consistent data have subsequently shown that EoE in both adults and children that responds to a PPI and EoE that does not respond to a PPI are otherwise indistinguishable and the same disease clinically, endoscopically, on 24-hour pH monitoring, histologically, immunologically and have the same molecular characteristics.9 60–64
The GDG recommends that following the consideration of alternative local and systemic causes of oesophageal eosinophilia (see table 1), all patients with ≥15 eosinophils per 0.3 mm2 at biopsy should be regarded as having EoE. It is also possible that more than one cause of oesophageal eosinophilia can coexist in patients (eg, EoE and GORD). PPI therapy may form part of subsequent therapy for EoE but PPI response should not be used to characterise a subgroup of patients with EoE or to exclude EoE.
Table 1.
Conditions other than eosinophilic oesophagitis associated with oesophageal eosinophilia
| Condition | |
| Primary | Gastro-oesophageal reflux disease |
| Achalasia | |
| Eosinophilic gastroenteritis or colitis with eosinophilic oesophagitis | |
| Infection (fungal or viral) | |
| Pill oesophagitis | |
| Secondary | Hyper-eosinophilic syndrome |
| Drug hypersensitivity reactions | |
| Connective Tissue diseases |
Eosinophilia in the oesophagus is rare and data for an eosinophil density of ≥15/0.3 mm2 for causes other than eosinophilic oesophagitis is limited.
Eosinophilic oesophagitis and gastro-oesophageal reflux disease are not mutually exclusive and can coexist in the same patient
GRADE of evidence: High.
Level of recommendation: Not applicable.
Level of agreement: 100%.
GORD and EoE are the most common oesophageal diseases worldwide. The worldwide population prevalence of frequent GORD symptoms is estimated to be 9283 per 100 000 population.65 In a systematic review and meta-analysis, the pooled prevalence of EoE was 34.4 cases per 100 000 population, and was higher for adults (42.2 per 100 000) than for children (34 per 100 000).11 Even if there was no interaction between the two conditions, it would be expected therefore that GORD would occur by coincidence in at least 10% of patients with EoE.
Limited data from historical case series of patients with EoE suggests a high prevalence of excess total acid exposure on 24-hour pH monitoring in 56% of patients.66 67
There are a number of possible interactions between GORD and EoE. The association of the two conditions may simply be coincidental and more common than expected by chance, due to shared risk factors for the two conditions. Alternatively, EoE may add to or cause GORD via delayed acid clearance following gastro-oesophageal reflux, due to the effects of EOE tissue remodelling and subepithelial fibrosis on oesophageal peristalsis68 or through increased oesophageal mucosal permeability, due to cytotoxic substances released by eosinophils in EoE.69 Finally, it is possible that increased mucosal permeability due to GORD predisposes to the development of EoE, through allowing food allergens to penetrate the oesophageal mucosa and stimulate an inflammatory response or through reflux-induced expression of eosinophil chemoattractants.70
The GDG recommends that clinicians consider the possibility of patients with EoE also having GORD when endoscopic or clinical features suggest this.
Formal transition of care from paediatric to adult services may improve symptom control, concordance with therapy and reduce emergency presentations in eosinophilic oesophagitis
GRADE of evidence: Very low.
Level of recommendation: Not applicable.
Level of agreement: 94%.
The exact proportion of paediatric patients with EoE reaching adulthood who require ongoing therapy and chronic disease management is not well defined. In one study of 58 patients with EoE with a mean follow-up of 8 years, 81% of young adults had symptomatic improvement of their dysphagia compared with childhood. Two-thirds of the patients did not use steroids or PPIs in the 12 months prior to assessment.71 In a cross-sectional single centre study of children with EoE followed up as young adults, 37% reported dysphagia, 49% were on PPI therapy and 76% followed allergy-directed diets.72
The transition process needs to address adherence to evidence-based therapy, social and psychological functioning and the importance of follow-up in adult-orientated health systems.3 Lack of knowledge about EoE and treatment has been attributed to loss of concordance and follow-up. Non-concordance with therapy and emergency presentations have been described after leaving paediatric care.73
A case summary of four adolescents with EoE illustrated the value of introducing a questionnaire to assess knowledge and readiness of patients with EoE for successful transition, individual patient and family circumstances and the involvement of a multidisciplinary team for children with EoE, comprising gastroenterologists, dietitians, allergists and psychologists.73
In Europe, differences in paediatric and adult practice have been reported regarding EoE diagnostic procedures and treatment74 75 in a survey involving 465 paediatric and 743 adult gastroenterologists. Although topical steroids were the preferred second-line therapy, paediatric gastroenterologists opted more frequently for elimination diets (48%) than adult gastroenterologists (14%). While 89% of paediatric gastroenterologists had read guidelines on EoE, only 58% of adult gastroenterologists had done so.74 These differences may negatively impact on the outcome of transition from paediatric to adult care.
Taken together, structured, long-term data on the outcomes of transition of children with EoE into adulthood are lacking, and the GDG recommends more studies to establish if formal, structured transition to adult services will significantly improve the outcomes for patients and reduce emergency presentations in adult care.
Investigations
At least six biopsies should be taken from different anatomical sites within the oesophagus for diagnosis and follow-up of eosinophilic oesophagitis
Level of evidence: Moderate.
Strength of recommendation: Strong.
Level of agreement: 100%.
Although macroscopic endoscopic appearances can suggest EoE, some of these endoscopic changes can also be seen in other conditions (eg, post-radiotherapy strictures), or can be confused with other pathology (eg, candidial oesophagitis). In a significant proportion of patients, EoE can be missed either because the mucosa appears normal or the changes are too subtle and not easily recognised without prior clinical suspicion. Regardless of endoscopic appearance, histology is required to secure the diagnosis. The distribution of the eosinophilic infiltrate is often patchy, and this can often make the diagnosis more difficult to confirm.59 76 To improve the likelihood of sampling the correct site and securing a diagnosis, multiple biopsies are required from different sites within the oesophagus. The current body of evidence is not uniformly clear with regard to the minimum or maximum number of biopsies required, nor which sites are the most appropriate for samples to be taken. Between 2004 and 2019, 16 retrospective, prospective and case studies described the association between numbers of biopsies and oesophageal sites in the diagnosis of EoE, with the numbers of patients included in each study ranging from 30 to 213. Eleven published studies suggested taking biopsies from two sites, the proximal and distal oesophagus,9 59 76–84 two studies also suggested including the mid oesophagus,85 86 while others were unclear. Similarly, the number of biopsies suggested is also variable; however, all suggest obtaining multiple biopsies with recommendations ranging between four and six, with six biopsies equating to a 97%–100% chance of making a positive diagnosis.
The GDG recommends that six biopsies should be taken from at least two different sites in the oesophagus. Further, in order to maximise the chances of making an EoE diagnosis, there should be a combination of targeted biopsies from visible areas of mucosal surface abnormality (eg, white spots, furrows) and non-targeted biopsies among the six biopsies.
Eosinophil density should be expressed as eosinophil counts per 0.3 mm2 (this equates to a high power field) and the cut-off for a diagnosis should be >15 eosinophils per 0.3mm2 in any biopsy specimen
Level of evidence: Moderate.
Strength of recommendation: Strong.
Level of agreement: 100%.
Eosinophilic oesophagitis is a clinicopathological diagnosis and therefore clinical, endoscopic and histological features should be taken into account to arrive at a diagnosis. In order to differentiate EoE from other inflammatory conditions (such as GORD which typically has an eosinophil count <5 eosinophils per hpf) a histological threshold of 15 eosinophils hpf has conventionally been widely accepted as confirmatory of a diagnosis of EoE, with a sensitivity of 100% and a specificity of 96%.59 76 Histologically, the current situation is suboptimal in that in modern microscopes, there can be up to a twofold variation in the area considered to be within a ‘high power field’, an issue which will be resolved with reporting from digitally scanned images. The literature is predominantly based on the highest (peak) eosinophil density within a hpf (standardised at 0.3 mm2 in more recent publications).59 76 Thus counting larger areas of squamous epithelium may well not be reliable.
The GDG therefore recommends that the highest eosinophil density to define the eosinophil concentration should be within a 0.3 mm2 area and this figure should be expressed as the highest (peak) eosinophil density per 0.3 mm2.
Mucosal eosinophilia should be accompanied by other histological features of eosinophilic oesophagitis. These include the presence of basal cell hyperplasia, oedema (spongiosis), eosinophil microabscesses, eosinophil layering, eosinophil degranulation and subepithelial sclerosis
Level of evidence: Moderate.
Strength of recommendation: Strong.
Level of agreement: 100%.
In EoE there may be a marked variation in the eosinophil density, both between patients at presentation and within individual patients at different time points in the course of their disease. There may also be an overlap in eosinophil counts with other conditions, such as GORD.59 76 It is therefore important that, beyond simple eosinophil density, other histological features should be considered to support the diagnosis of EoE. These include the presence of basal cell hyperplasia, oedema (spongiosis), eosinophil microabscesses, eosinophil layering, eosinophil degranulation and subepithelial sclerosis, which should all be taken into account when considering the diagnosis.59 87 Indeed such biomarkers of disease activity have been described using the Eosinophilic Esophagitis Histology Scoring System87 as well as the Eosinophilic Esophagitis Histology Remission Score (EoEHRS), which are validated histological scoring systems in EoE research trials but not yet widely implemented in routine clinical practice.88 It should be noted that the subepithelial layer is assessed in less than half of patients when biopsies are taken using standard biopsy forceps.89
The GDG recommends that the histological description in the diagnosis of EoE should not only define the peak eosinophil count within the defined field of vision, but other concomitant histological features that lend support to the diagnosis should also be included.
In treated eosinophilic oesophagitis, histological response should be classified according to the eosinophil density. Remission is defined for clinical purposes as a maximum/peak eosinophil count <15 eosinophils/0.3 mm2
Level of evidence: Low.
Strength of recommendation: Strong.
Level of agreement: 100%.
In the management of EoE it is useful to have an objective assessment of response to treatment, both in the management of individual patients and to allow for the comparison of different treatment regimens. Assessment of response may be complicated by variations in eosinophil density over both time and anatomical location in the oesophagus.59 76 There currently appears to be little consensus on the criteria for histological remission, but going forward it would be very useful to establish such criteria.90 It is suggested that a histological remission following treatment be defined as peak eosinophil density <15 eosinophils per 0.3 mm2 and a deep/complete remission be defined as peak eosinophil density <5 eosinophils per 0.3 mm2.59 91–93
The GDG recommends that further research should be undertaken with the aim of establishing histological criteria to define remission; however, currently we suggest a peak eosinophil count less than 5 eosinophils per 0.3 mm2 as being in keeping with deep/complete remission and between 5 and 15 eosinophils per 0.3 mm2 is in keeping with histological remission.
Oesophageal physiological testing should be considered in patients with eosinophilic oesophagitis who have ongoing dysphagia despite histological remission and the absence of fibrostenotic disease at endoscopy
Level of evidence: Low
Strength of recommendation: Strong
Level of agreement: 95%.
The functional pathophysiology of symptoms in EoE is often overlooked, with the emphasis primarily on histology and endoscopic appearances. Although EoE can be associated with strictures and narrowing because of fibrosis, there are many patients with EoE who despite being in histological remission with no evidence of fibrostenotic disease at endoscopy remain symptomatic.94 95 High-resolution manometry (HRM) assesses oesophageal body and lower oesophageal sphincter function using pressure sensors to define peristalsis and bolus clearance,96 and is a reasonable next step in the assessment pathway of these patients who continue to have oesophageal symptoms despite apparently adequate treatment of oesophageal eosinophilia.
Studies examining oesophageal motility patterns in EoE report a variety of motility patterns, ranging from non-specific and normal,97 to hypotensive and ineffective motility98–100 as well as obstructive features including an achalasia-type picture.101–105 Dysmotility seems to correlate with disease severity, longevity and symptoms,100 particularly for obstruction.101 104 Oesophageal wall thickness also seems to correlate with the degree of contractile vigour, and in turn, symptoms.106 Oesophageal planimetry (EndoFLIP, Medtronic) may also be useful in assessing oesophageal compliance and although currently an experimental tool, may come into routine clinical practice in the future.
A possible limitation of these studies however is that the HRM studies were undertaken using only small volume water swallows, which does not usually reproduce the symptoms that patients with EoE get when they eat solid food; it also does not represent normal eating behaviour. However the correlation of HRM metrics with symptoms in patients is not fully established.107 EoE often includes solid food dysphagia and assessment of the cause of symptoms may be limited if HRM is undertaken only with small volume water swallows. This might also explain the variability in manometry patterns described in the literature. Studies have demonstrated an increased diagnostic yield of motility disorders as a result of the inclusion of solids during HRM in unselected patients referred for investigation of oesophageal motility,108 particularly when there is functional obstruction.109 This technique has now been included as a standard in the most recent iteration of the Chicago Classification of motility disorders.110
The GDG recommends that full evaluation of persistent, refractory dysphagia in patients with EoE with apparently normal endoscopic findings should include oesophageal physiological testing and barium swallow studies where appropriate, as described in the BSG guidelines on oesophageal manometry and reflux monitoring111 and the Chicago classification.96 Solid swallows during HRM should be considered in order to replicate the presenting symptoms of EoE.
Management
After initiation of therapy (dietary or pharmacological treatment), endoscopy with biopsy while ‘on treatment’ is recommended to assess response, as symptoms may not always correlate with histological activity
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
The aim of treatment in EoE is to induce long-term clinical and histological remission; using an analogy from inflammatory bowel disease (IBD), it is therefore important to assess for mucosal healing after initiating therapy. Besides clinical response with improvement in symptoms of dysphagia, retrosternal discomfort or vomiting/regurgitation, it is essential to check for histological remission by endoscopy and biopsy, after a defined time period on treatment—usually between 8 and 12 weeks after commencing treatment and depending on local access times for endoscopy. Non-invasive testing techniques such as Cytosponge (Medtronic, USA)112 and string test113 114 have not been validated in sufficiently large studies to potentially replace endoscopic biopsies in EoE, although they have been shown to be promising in small studies.
Clinical remission in EoE is difficult to define as there are no validated symptom questionnaires to assess clinical response to treatment. In adult patients, available assessment tools include the Eosinophilic Esophagitis symptom Assessment Index,115 but generally speaking the improvement in objective endoscopic and histological parameters are more consistent than subjective symptomatic assessment.116 Furthermore, a meta-analysis of 1202 patients found only a moderate association between symptomatic and histological response with high heterogeneity, with 41% of patients reporting a symptomatic response in the absence of a histological response.117
In children, the Paediatric Eosinophilic Esophagitis Symptom Score (V.2.0) has been validated for different paediatric ages and parent proxy-reported symptoms.118 119 In a single-centre study, moderate association was reported for symptoms, histological activity in the upper oesophagus and gene transcripts linked to EoE as markers of oesophageal activity.119
The criteria for histological and endoscopic improvement after treatment are being investigated as research priorities to be core outcome metrics, but at present a histological threshold of <15 eosinophils/mm3 is the only accepted response criterion and not clinical improvement, as symptom improvement is often only partial, with ongoing inflammation thus being left untreated.
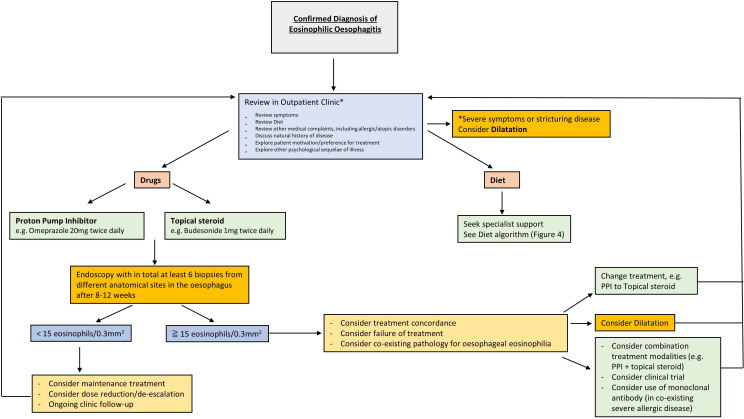
The GDG recommends histological assessment as the best criterion for response after initiating dietary or pharmacological treatment in EoE.120 This should be undertaken between 8 and 12 weeks (figure 3).
Figure 3.
Eosinophilic oesophagitis management algorithm in adults and children. PPI, proton pump inhibitor.
Elimination diets are effective in achieving clinico-histological remission in both adults and children with eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 89%.
The link between diet and EoE was first identified in 1995 when 10 children with oesophageal eosinophilia and gastro-oesophageal reflux had histological and clinical improvements on amino-acid based (elemental) feed and relapsed with food reintroduction.121
Due to the challenges associated with this dietary approach, the six food elimination diet (SFED) was devised, consisting of empiric avoidance of six common food allergens: cow’s milk, wheat, egg, soy, peanuts/tree nuts, fish and seafood. The first reported study in a paediatric EoE population using SFED described clinical and histological remission in 74%.122
On meta-analysis,123 the SFED was shown to have a histological response rate of 72.1%, with results consistent across both adults and children. Elemental diets had a response rate of 90.8% and allergy-test directed diets of 45.5%. However the quality of the studies included in the meta-analysis was not assessed and many were observational with no randomised controlled trials. A further meta-regression of the SFED124 showed histological remission in 69% and symptom improvement in 87.3%. Again, the quality of the studies was not assessed. SFEDs are difficult to introduce into routine clinical practice due to the high level of commitment required by patients and the need for multiple follow-up endoscopies, as well as the fact that on reintroduction the majority of responders to the SFED diet actually have only one or two foods that trigger their symptoms. Therefore, simpler dietary elimination strategies such as four-food elimination diet and two-food elimination diet (FFED and TFED) have been developed and shown to be effective in 40%–50% of the patients. Most patients with an identified dietary trigger respond to cow’s milk and wheat elimination.
In a retrospective study of 337 children from Europe, the most common causative allergens identified were milk (42%), egg (21.5%), wheat (10.9%), peanut (9.9%) and soy (8.4%). The most successful two-food combinations were assigned to milk and wheat in 37%, or milk and egg in 33%.125 A one or two food elimination diet stepping up to a more restrictive four food elimination has been proposed as a clinically effective strategy for dietary management of EoE. Subsequent prospective observational studies have concluded that elimination diets are effective.120 126–128
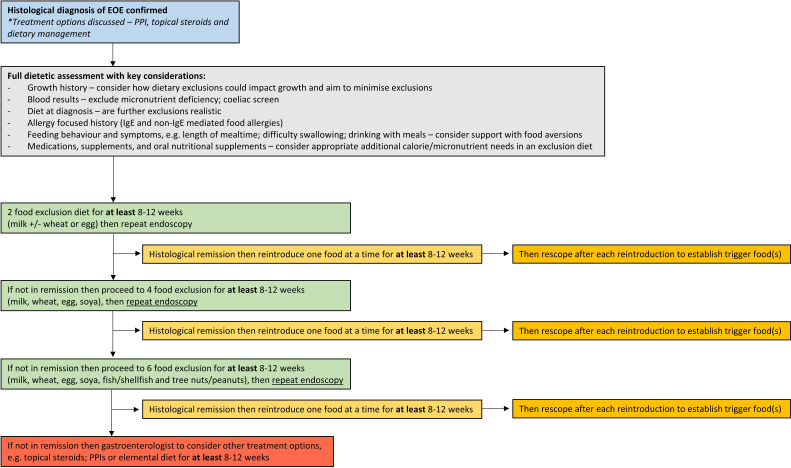
The GDG recommends that if dietary treatments are considered for EoE, they should only be carried out under the supervision of an experienced dietitian, and commenced with a TFED, stepping up to more restrictive diets, with appropriate endoscopic and histological assessments between 8 and 12 weeks later (figure 4).
Figure 4.
Dietary management of eosinophilic oesophagitis in children (grey) and adults. EoE, eosinophilic oesophagitis; PPI, proton pump inhibitor.
The six food elimination diet results in higher histological remission rates than two or four food elimination diets, but is associated with lower compliance and an increased number of endoscopies
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
A multicentre prospective study of the ‘step-up’ approach to dietary management128 showed clinico-histological remission in 43% for two food (milk and wheat), 60% for four food (milk, wheat, egg and legumes) and 79% for SFED. This also estimated that the approach resulted in a reduction of endoscopy use by 20% compared with the ‘step-down’ approach of the traditional SFED.
Additionally of the 74 of participants who did not initially respond to the TFED, 20 (28%) were unwilling to step up to the FFED. Out of the 44 who did not respond to the FFED, 17 (39%) were unwilling to step up to SFED. This may be due to the restrictiveness of avoiding so many food groups or reduced motivation following multiple failed attempts. A pragmatic approach is to give patients the right information in order to make an informed choice weighing up the chance of success versus the restrictiveness and motivation needed to step up to further restrictions.
An approach to dietary management of EoE has been summarised in figure 4.
The gradual increase in remission rates has to be carefully balanced with the patients health-related quality of life (related to wider restrictions), potential nutritional deficits, eating behaviour and mid to longer-term adherence to dietetic, diagnostic and therapeutic plans.129
When undertaking a dietary restriction therapy for eosinophilic oesophagitis, support from an experienced dietitian throughout the elimination and reintroduction process is strongly recommended
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
Elimination diets for EoE present risks of nutritional adequacy, feeding difficulties in young children, impaired growth in children and weight loss in adults.130
Elimination diets are in their very nature restrictive and in EoE usually involve cutting out one or more staple food groups such as milk or wheat. Dairy products are a good source of calcium and also provide protein, phosphorus, vitamin B12 and vitamin D. Wheat provides iron, fibre and B vitamins. Therefore, eliminating these foods has the potential for nutritional deficiencies. This risk is likely to be greater when other dietary restrictions are in place, for instance concomitant food allergies or lifestyle choices (eg, vegan/plant-based diets). A dietetic consultation for EoE involves not only education on accurately eliminating foods but advice on replacing food groups and achieving nutritional adequacy.
A systematic review evaluating vitamin deficiencies in paediatric EoE identified only five studies, the majority of which were rated as poor quality.131 The results suggested suboptimal vitamin D levels in this group, although any link with diet was not clear.
One of the included studies was a prospective evaluation of 53 children with GORD or EoE.132 Although serum nutritional markers were normal in both groups, 3-day food diaries showed suboptimal dietary calcium and vitamin D intakes in those with EoE. This study also found that the children had higher scores for abnormal feeding behaviours compared with historical healthy controls. Feeding difficulties in children with EoE have also been reported in other studies.133 134 Dietitians play a key role in the assessment management of feeding difficulties and therefore input is strongly recommended for any dietary elimination.
A systematic review of nutrient intake and growth in children with multiple IgE mediated food allergies identified six relevant studies.135 The findings were that there was a higher risk of growth impairment and possible higher risk of nutrient deficiencies, although results were inconsistent. One study did find that children who had had no dietary counselling were more likely to be deficient in calcium and vitamin D.
Another study evaluated 100 children with cow’s milk allergy before and after dietary elimination.136 Both growth markers and biochemical parameters were affected compared with healthy controls and the difference was more pronounced with earlier onset. In atopic dermatitis, 225 children and adults completed 3-day food diaries to assess nutritional adequacy.137 An association was found between a higher number of food allergies and more nutritional deficiencies.
Given the complexity of food elimination diets, help from an experienced dietitian is important to structure the eating plans, and to avoid vitamin deficiencies consequent on these complex dietary exclusion regimes. Implementing forms of elimination diet in children depends on induction and surveillance of a paediatric dietitian embedded in an individual treatment plan to ensure that the nutritional, psychosocial and developmental needs of the child are addressed.
A single centre UK study that included a dedicated dietitian reported high levels of efficacy with elimination diets in a retrospective service evaluation.138 This approach is supported by the American Academy of Allergy, Asthma and Immunology recommendation that ‘a referral to a registered dietitian for any individual prescribed an elimination diet for EoE’.139 Therefore, support from an experienced dietitian is recommended to mitigate these risks as well as to help the patient to succeed on the diet. This is particularly relevant for children and in transition from paediatric to adult care.
The GDG recommends that all paediatric patients and most adult patients embarking on dietary management of EoE should be managed by a multiprofessional team of an experienced clinician, a specialist dietitian and for selected patients an allergist.
Combining elimination diets with pharmacological treatment is not routinely recommended but can be considered in cases of treatment failure
GRADE of evidence: Very low.
Level of recommendation: Strong.
Level of agreement: 94%.
In a small paediatric retrospective study, histological remission rates for swallowed steroids versus allergy-test directed diets versus both therapies were 50%, 60% and 80%, respectively.140 Similar results were seen for symptomatic remission, although only 70% had repeat oesophageal biopsies.
A 3-month trial of swallowed steroids and a TFED (milk and soy) was compared with a further 3 months of just the elimination diet in a retrospective cohort study of 29 children.141 Median eosinophil counts decreased from 51 to 2 per hpf (p<0.001) with combined treatment and increased to 31 with diet alone (p=0.07). Dysphagia improved with both approaches.
A similar study in 23 adults reported that combination therapy with topical corticosteroids and an elimination diet improved overall symptoms in 88%.142 However, peak eosinophil counts did not significantly fall (reduced from mean 54 to 36 (p=0.12)) and apart from a reduction in endoscopic furrows from 84% to 55% (p=0.02), there were no significant reductions in other endoscopic features. Of note, previous failure to respond to monotherapy was documented in 90% of the patients.
The GDG recommends commencing treatment in patients with EoE with a single modality therapy of either diet or pharmacotherapy; for most patients this will be pharmacotherapy which is easier to implement than dietary restriction that requires motivation, multiple endoscopies and support from a specialist dietitian.143 Combination therapy of drugs and diet should be reserved for selected patients who fail monotherapy and have access to a multiprofessional team including a dietitian to follow them up and monitor response carefully (figure 3).
Allergy testing to foods (skin prick, specific IgE, patch testing) is not recommended for choosing the type of dietary restriction therapy for eosinophilic oesophagitis
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
Concomitant atopic disease such as rhinitis, asthma and eczema are common in patients with EoE.24 144–146 A recent systematic review of 21 studies including over 50 000 adult and paediatric patients matched to healthy controls reported a higher prevalence of allergic rhinitis in patients with EoE compared with eczema or asthma.146 However, EoE does not appear to be an IgE-mediated disease. One study looking at the efficacy of an antibody against IgE (omalizumab) compared with placebo over 16 weeks147 did not find any benefit, but histological analysis of the oesophageal biopsies showed an increase in IgG-4 in the lamina propria of patients with EoE compared with controls; however the role of IgG-4 in the pathogenesis of EoE remains uncertain. Food-specific IgE/IgG antibodies have been detected in oesophageal samples but the relevance to underlying pathophysiology and management of EoE is unclear.148 149 Targeted dietary elimination on the basis of IgE testing is no more effective than empirical dietary elimination.123 150 Similarly, patch testing (for delayed, non-IgE-mediated allergy) has failed to demonstrate any therapeutic benefit.151 Allergy testing is therefore not recommended in guiding dietary elimination.
Meta-analysis of published studies suggests that allergy test directed diets have the lowest histological remission rates.123 However, unlike the other elimination diets in the analysis, there was considerable heterogeneity between studies, attributable to varying methods of allergy testing (skin prick, specific IgE, patch testing) as well as their interpretation. In addition, EoE is considered to be primarily a non-IgE-mediated condition152 and therefore testing for food triggers using IgE-based methods is unlikely to be accurate
The GDG does not recommend food specific antibody testing (IgE or IgG-4) in patients with EoE or antibody-directed dietary elimination for the management of EoE.
Exclusive elemental diets have a limited role in eosinophilic oesophagitis, with high efficacy but low concordance rates and should be reserved for patients refractory to other treatments
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
Exclusive elemental diets using amino-acid based feeds have been found to be highly effective in inducing remission123 127 but they are expensive, have high withdrawal rates and weight loss and non-concordance are common.127 153 In paediatric studies many were fed via enteral feeding tubes and in UK clinical practice exclusive elemental diets are rarely used, as they are not sustainable in the long term.
A retrospective study comparing the outcomes of elemental versus SFED in children examined five children who failed SFED.122 Of these, four responded (three fully and one partially), and one refused treatment. No other evidence was found for the use of elemental diet following failure of other treatments.
An exclusive elemental diet may be considered as a second-line or third-line therapy after a failure of the food elimination diet.154
Amino acid feeds may also be beneficial as a supplement to an elimination diet,155 particularly when weight loss occurs or extensive foods are avoided.
Polymeric or partially hydrolysed (semi-elemental) feeds are not suitable for EoE as they contain intact or partially intact milk proteins, with milk being the most commonly reported EoE dietary trigger. The goal of the elemental diet is to remove all food antigens from the diet with a synthetic amino-acid based feed.
The GDG recommends the use of elemental diets only for selected patients, after a careful multiprofessional discussion, in the setting of treatment refractoriness to all conventional treatments.
Proton pump inhibitor therapy is effective in inducing histological and clinical remission in patients with eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
First-line treatment of EoE with PPI monotherapy is widely practiced,156 though evidence for its efficacy has historically been limited to data from observational studies with small numbers of patients. Early studies used widely varying diagnostic criteria and definitions of response, not least because of the previously held but now abandoned distinction between PPI responsive disease and ‘true’ EoE.
A recent prospective observational study of 231 children157 reported a 27.7% clinicopathological response rate after PPI treatment and identified endoscopic features predictive of treatment failure including endoscopic features (visible oedema (OR 2.04); linear furrows (OR 2.14); proximal eosinophilic infiltrate (OR 3.26)).
A systematic review and meta-analysis of 33 studies (predominantly small, retrospective case-series) with a pooled patient population of 431 adults and 188 children reported partial clinical and histological response rates of 60.8% and 50.5%, respectively.50
Comparison of studies is difficult due to heterogeneity, with different PPI doses used, variable symptom response rates and differences in the terminology of histological remission used (eg, <5 eosinophils per hpf versus <15 eosinophils per hpf).
A more recent systematic review and meta-analysis of 11 heterogenous randomised controlled trials158 with a pooled patient population of 456 adults and children with EoE ranked PPI treatment as more effective than placebo, systemic steroid and biological treatment (anti-interleukin-5 antibody) but less effective than topical budesonide preparations.
A recent cross-sectional study of 534 adults and 76 children using data collected from a European registry156 reported histological response in 48.8% and clinical response in 71.0% of the patients. Additionally, the study demonstrated more favourable rates of response in patients with an inflammatory phenotype when compared with fibrotic disease.
In the majority of studies, PPI was taken for 8 weeks and then repeat biopsies were taken to assess histological response. In a prospective study, 57 children who had responded to an initial 8-week course of PPI treatment (omeprazole 1 mg/kg two times per day (up to 40 mg two times per day were given maintenance treatment for 12 months in form of esomeprazole 1 mg/kg/day, maximum dose 40 mg/day).159 70.1% exhibited long-term histological remission over 12 months. Long-term data on relapse rates on PPI are however lacking.
The GDG considers PPI treatment to be effective in patients with EoE based on a combination of symptom response and histological remission.
Proton pump inhibitor therapy should be given two times per day for at least 8–12 weeks prior to assessment of histological response while on treatment
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
The majority of published studies include 8 weeks of treatment with PPI followed by assessment of response by means of repeat endoscopy and oesophageal biopsies. Agents and doses varied widely, though regimes typically included a minimum dose of omeprazole 40 mg daily or equivalent and this is likely to reflect current widespread clinical practice. Omeprazole is the only PPI to that has been assessed for the treatment of EoE.
A meta-analysis in 201550 described a non-significant trend towards increased efficacy for two times per day dosing compared with a one time per day dose.
A significant difference in clinicopathological response rates was reported in patients with EoE prescribed high-dose PPI, for example, omeprazole 20 mg two times per day have been reported as being higher (50.8%), than in those given standard or low-dose regimes (35.8%).156 This study included an analysis of the effects of treatment duration for inducing remission. Treatment of 8–10 weeks conferred a response rate of 50.4%. Longer treatment duration (>10–12 weeks) was associated with a greater rate of response (65.2%) though the effect was observed to diminish in patients treated longer than this (44.1% response rate) which the authors speculate might be related to treatment concordance.
While PPI therapy is not licensed for use in EoE, its use in certain situations as highlighted above has been shown to be effective. In such circumstances, it is also essential that patients and their general practitioners are made clear of the reasons for the prescription of PPI, that is, for the management of EoE, rather than as a GORD treatment. Dose reduction to lower doses is not indicated, especially in primary care.
The GDG therefore recommends that if using PPI therapy to manage EoE, that omeprazole at a dose of 20 mg two times per day is used with a clear explanation of its indication in correspondence with the primary care team.
In patients who achieve histological response, proton pump inhibitor therapy appears effective in maintaining remission
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 89%.
There are limited published data and no prospective randomised trials to define appropriate long-term maintenance strategies in patients with EoE who have responded to PPI treatment.
In a prospective study, 57 children who had responded to an initial 8-week course of PPI treatment (omeprazole 1 mg/kg two times per day (up to 40 mg two times per day)) were given maintenance treatment for 12 months. Long-term histological remission was exhibited by 70.1%.159
A retrospective cohort study of 75 PPI responsive patients observed sustained histological remission in 73% and clinical remission in 100% on maintenance PPI therapy for at least 12 months.160 Sixteen of the patients discontinued treatment after at least 12 months and of these, 14 (87.5%) suffered symptom recurrence and all exhibited histological recurrence.
A subsequent study of 40 PPI responsive patients with EoE reported similar results with 81% in long-term clinicopathological remission.161
The use of maintenance PPI treatment in 172 of the 630 PPI treated patients in a registry study156 reported that of those with complete clinical and histological follow-up data (n=103), 69.9% exhibited sustained clinicopathological remission. In line with other, smaller series, the effectiveness of PPIs in maintaining disease remission was closely related to the degree of response seen with initial treatment.
There are no published studies of maintenance treatment of more than 12 months duration, but because of the high risk of relapse on stopping therapy, the GDG suggest that maintenance PPI therapy can be considered as a long-term treatment in patients with EoE who are in clinical and histological remission.
Topical steroids are effective for inducing histological and clinical remission in eosinophilic oesophagitis
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 100%.
Topical steroids have been shown to be highly effective in inducing remission in EoE. At least 11 randomised controlled trials, summarised in meta-analysis and systematic reviews, demonstrate that topical steroid therapy is effective. A large meta-analysis162 showed histological remission with an OR of 13.66 (95% CI 2.65 to 70.34).
However, variability in agents, dosing, delivery systems, length of treatment and end points in the studies hampers comparison among studies. A commonly use dose for swallowed fluticasone is 880 mcg (four puffs) two times per day, and in a placebo controlled trial histological response was achieved in 62% versus 0% on placebo.163 However the difference in clinical remission rates was not significant (57% vs 33%).
A study comparing viscous budesonide and fluticasone showed histological remission was significantly higher with the oral viscous budesonide group (64% vs 27%).164 A subsequent randomised controlled trial of budesonide slurry and fluticasone showed equivalence for the two therapies with 100% vs 94.7%, for histological remission.165
The impact on clinical remission has been less clear until a recent study of budesonide orodispersible tablet 1 mg two times per day.48 This landmark study demonstrated clinicopathological remission (eosinophil counts <5/hpf, with dysphagia and odynophagia scores <2) in 57.6% at week 6 versus 0% on placebo, and in 84% during an open label extension to 12 weeks.
No direct comparison has been conducted between fluticasone and budesonide in children. In a single-centre prospective study, 90% of 20 children went into remission after 12 weeks using oral viscous budesonide.166
Regarding the induction dosage of the oral viscous preparation of budesonide in children, most centres use an induction dosage of budesonide 1 mg/day if less than 150 cm or 2 mg/day if greater than 150 cm either given as a single dose or divided in two doses per day. In a single centre series and multicentre series of older children, higher doses of 4 mg/day (for children greater than 150 cm or children older than 11 years) have been used for patients not responding to the standard dosage of 2 mg/day. So far there is no consensus among paediatric gastroenterologists about timing, dosage and de-escalation of the induction treatment beyond 12–24 weeks of induction treatment, but results from placebo-controlled randomised studies are expected to provide more evidence.
In adults, the GDG support the use of orodispersible budesonide over other swallowed steroid formulations in the induction treatment of EoE given its regulatory approval in both the UK and the Europe. For adolescents, the use of orodispersible budesonide may be beneficial but currently requires approval from local authorities. In children, the GDG support the use of oral viscous budesonide in age-appropriate viscous formulations and volume in the induction treatment of EoE.
Clinical and histological relapse is high after withdrawal of topical steroid treatment, and following clinical review, maintenance treatment should be recommended
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 94%.
A phase-3 double blind randomised placebo controlled trial comparing maintenance treatment with either 0.5 mg two times per day or 1.0 mg two times per day orodispersible budesonide with placebo over 48 weeks showed persistent remission in 73.5% and 75% patients in the treatment arms compared with 4.4% in the placebo arm, respectively.167 The benefit of orodispersible budesonide was seen across all groups of patients with EoE, and the median time to relapse was 87 days in the placebo group compared with >350 days in both treatment groups. This study reported greater efficacy than all previous studies on maintenance therapy using swallowed inhaled topical steroids such as fluticasone or budesonide slurry. In the adult budesonide slurry study only 35.7% of the patients maintained histological remission after 1 year, with three-quarters of them experiencing symptomatic relapse despite treatment.168 This treatment is safe with no serious adverse events and only minor adverse effects of Candida albicans infection in up to 22% of he patients, which did not have any impact on daily life activities and did not need the study medication to be stopped.
Following a prospective, double-blind, placebo controlled study involving 30 paediatric patients treated with either placebo or 2 mg two times per day oral viscous budesonide (OVB) for 12 weeks, an open label extension study of 24 weeks providing either 2 mg OVB one time per day or 1.5–2.0 mg two times per day demonstrated sustained histological and endoscopic remission (defined as <6 eosinophils/hpf) in 49% of OVB patients (and <15 eosinophils/hpf in 58%) previously treated with placebo and 23% (<6 eosinophils/hpf), or 28% (<15 eosinophils/hpf) of those previously treated with OVB.169
Based on this initial data of maintenance of remission with orodispersible budesonide treatment over a 12-month period, the GDG recommends the use of this formulation over others for the maintenance of remission of EoE in adults with the option for adolescents subject to local approval (figure 3). For children, OVB is the recommended preparation, with maintenance dosage tailored according to individual response and surveillance of potential side effects.
Systemic steroids are not recommended in EoE
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 100%.
In a randomised controlled trial of 80 children, patients were randomised to either prednisolone (1 mg/kg two times per day) or swallowed fluticasone (220 mg or 440 mg four times per day according to age) for 12 weeks.170 Histological remission was similar at week 4 (94%), but adverse events were more common in the prednisolone group (40%: hyperphagia, weight gain, cushingoid appearances) versus the fluticasone group (15%: all oesophageal candidiasis only).
The use of systemic steroids to treat EoE in children was reported in 5.3% of patients in a retrospective register.143 In 20 of these 22 patients, the indication was stricturing disease. At follow-up endoscopy, 95% showed resolution of the strictures, 67% had normal eosinophili counts per hpf. While all patients improved clinically, 75% (15/20) were reported as asymptomatic. Only transient, minor side effects of systemic steroids, including hyperphagia in 50%, weight gain in 25%, hyperactivity and acne were reported. All patients continued with standard EoE treatments. Patients were followed-up for a mean of 66 months (IQR 24–77.5, range 9–249). Three patients, who had only partially responded to systemic steroids, underwent oesophageal dilation.171
There are no data on the use of systemic steroids for the management of EoE in adults.
The GDG therefore does not recommend the use of systemic steroids in the management of EoE in either paediatric or adult patients in non-stricturing disease.
Immunomodulators (azathioprine, mercaptopurine) are not recommended in management of eosinophilic oesophagitis
GRADE of evidence: Low.
Level of recommendation: Weak.
Level of agreement: 89%.
There is insufficient evidence to recommend immunomodulators in the treatment of EoE. In a case series of three patients published in 2007,172 who were all steroid dependent, immunomodulators induced clinical and histological remission but the patients relapsed on cessation of treatment. There are very few studies with low quality evidence on the use of immunomodulators in the management of refractory EoE and these agents are therefore not recommended.
Monoclonal antibody therapies, such as anti-TNF and anti-integrin therapies, that are typically used for inflammatory bowel disease are not recommended in the management of eosinophilic oesophagitis
GRADE of evidence: Low.
Level of recommendation: Weak.
Level of agreement: 100%.
Given that EoE and IBD are both chronic, immune-mediated disorders of the gastrointestinal tract, there has been interest regarding the co-existence of these two conditions.
A prospective cohort analysis from the Truven MarketScan database (2009–2016) of 134 013 536 individuals reported that compared with individuals without either diagnosis, the risk of EoE was higher in patients with Crohn’s disease (incidence rate ratio (IRR)=5.4, p<0.01) and ulcerative colitis (IRR=3.5, p<0.01).173
It has therefore been postulated that using biological medications, commonly used in the management of IBD may have a positive impact on the symptoms and histological profile of patients with EoE.
Infliximab is a chimeric IgG1 monoclonal antibody that inhibits tumour necrosis factor (TNF)-α and has been a cornerstone of the management of both ulcerative colitis and Crohn’s disease, as well as in other immune disorders such as rheumatoid arthritis.174 An open label study investigated the use of infliximab at a dose of 5 mg/kg in patients with severe corticosteroid dependent EoE.175 In this clinicopathological study, there was little improvement in symptom score pre-infliximab and 4 weeks after the second infusion.
Furthermore, no improvement was seen in the endoscopic assessment of oesophageal appearances or eosinophil count. While this experience was limited to only three patients, other histological parameters suggest differences in disease mechanisms as an explanation for these observations. TNF-α levels were reduced in all patients after treatment, but this did not correlate with eotaxin-3 expression, which did not change at all with two patients, and were seen to be increased in the third. Thus, given current knowledge of the mechanism of disease in EoE, combined with these data, TNF-α blockade is unlikely to have any clinical impact on EoE.
The anti-α4β7 integrin, vedolizumab has also been studied in the management of EoE. There have been descriptions in small cohorts of patients with eosinophilic gastroenteritis who have responded to vedolizumab.176 There have also been case reports describing concomitant EoE and Crohn’s disease that appeared to respond to vedolizumab.177 While it has been suggested that inhibition of α4β7 integrin may reduce eosinophil migration and survival in the oesophagus, there have yet to be any larger studies demonstrating this and no high-quality data on vedolizumab’s role in EoE treatment.
The GDG therefore suggest that outside of the indications for biological use in confirmed IBD, there is insufficient evidence to recommend the use of these agents for EoE alone.
Novel biologics used in other allergic conditions (such as dupilumab, cendakimab and benralizumab) have shown promise for the treatment of eosinophilic oesophagitis
GRADE of evidence: Low.
Level of recommendation: Weak.
Level of agreement: 88%.
The anti-interleukin (IL)4 receptor monoclonal antibody dupilumab has been used in the management of chronic allergic diseases such as eczema,178 and asthma.179 Mechanistically, targeting the IL-4 and IL-13 pathway has been considered a potentially useful strategy in managing patients with EoE. In a phase 2 randomised trial of adult patients with active EoE, the effects of dupilumab were investigated in 23 patients and compared with 24 placebo controls.180 The group receiving weekly injections of dupilumab (300 mg for 12 weeks) were found to have a significant reduction in their symptoms of dysphagia at week 10 using the Straumann Dysphagia Instrument patient-reported outcome score (mean reduction of 3.0 compared with 1.3 in the placebo group, p=0.0304). Furthermore, the peak mean eosinophil count per hpf was reduced by 86.8 eosinophils (107.1%) compared with placebo, (p<0.001). Endoscopic parameters also improved with the treatment group (both EREF score) and oesophageal distensibility, at week 12 compared with placebo (p=0.006 and p<0.001, respectively). While some side effects were described in the treatment group, these were mild and generally well tolerated (35% had erythema at the injection site, and 17% had nasopharyngitis, compared with placebo, 8% and 4%, respectively, with no serious adverse events reported).
The anti-IL-13 monoclonal antibody cendakimab (RPC4046) has also been studied in phase 2 trials.181 In 99 adults in a 16-week trial, there was a significant reduction in mean eosinophil count and dysphagia scores. This was also true in steroid refractory cases (n=47). A phase-3 multicentre study is underway (CC-93538) to evaluate its use for induction and maintenance treatment.
Other targets such as the IL-5 pathway may be important in the management of EoE. The anti-IL5 agent, mepolizumab was studied in a randomised, placebo controlled double blind trial182 and found to show a 54% reduction in oesophageal eosinophil count compared with controls (5%), p=0.03, although symptom improvement was not demonstrated.
The anti-IL5 receptor monoclonal antibody, benralizumab has also been demonstrated to be efficacious for the treatment of eosinophilic asthma183 and thus a potential candidate for patient management. There have been cases reported of EoE in patients with eosinophilic asthma in whom benralizumab was prescribed for the asthma but also resulted in complete resolution of dysphagia symptoms and histological evidence of deep remission.184 Benralizumab use in patients with hypereosinophilic disorder in a randomised, double blind, placebo controlled phase-2 study resulted in significant lowering of eosinophil count in both serum as well as tissue185 with 74% of patients having sustained response at 48 weeks, and no adverse events limiting treatment.
The phase 3 trial MESSINA, evaluating the use of benralizumab in patients with EoE will add further to the understanding of whether IL-5 inhibition can help both symptomatic and histological response at week 24.
In the absence of randomised controlled trial data in EoE alone, the GDG cannot currently recommend the use of dupilumab, cendakimab or benralizumab, but they may be treatment options in patients with coexisting allergic diseases. Pending the results of further studies, these drugs do show promise as future treatment options (figure 3).
Sodium cromoglycate, montelukast and antihistamines are not recommended in the management of eosinophilic oesophagitis but may have a role in concomitant atopic disease
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 94%.
With the overlap between EoE and allergic disorders, medications commonly used in atopic conditions, such as mast cell stabilising agents, and leukotriene antagonists have been considered as potential therapeutic targets in EoE.
While, laboratory studies have demonstrated a reduction in immunological response following the use of sodium cromoglycate,186 these findings have not been demonstrated in the clinical setting. Liacouras et al, described the use of 4 weeks of sodium cromoglycate in 14 children, which resulted in no improvement in symptom or histological profiles.187
Leukotriene antagonists such as montelukast have been investigated in a randomised, placebo-controlled, double blind trial188 to evaluate maintenance of remission induced by steroid. In a study of 20 mg/day of montelukast, 40% of the treatment group and 23.8% of the control group were in remission after 26 weeks, with the OR for remission with montelukast of 0.48 (95% CI 0.10 to 2.16, p=0.33).
There is therefore no convincing evidence for the use of these agents, or antihistamines in the management of EoE, but they are licenced and useful treatment options for other atopic illness.
The GDG therefore does not recommend the use of these drugs in the primary management of the symptoms of EoE, but recognise their role in co-existing allergic disease.
If symptoms recur while on treatment, we recommend repeating an endoscopy for assessment and to obtain further histology
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 94%.
There are numerous causes for dysphagia symptoms in patients on treatment with EoE, including refractory inflammatory disease, previously undetected fibrostenotic disease, as well as potential sequelae of treatment such as the presence of oesophageal candidiasis.48 167
The GDG recommend repeat assessment in any patient with symptom recurrence to obtain histological assessment of the eosinophil count/0.3 mm2, as well as to rule out complications of disease or treatment.
Patients with eosinophilic oesophagitis refractory to treatment and/or with significant concomitant atopic disease should be jointly managed by a gastroenterologist and specialist allergy clinic to optimise treatment.
Level of Evidence: Very low.
Strength of recommendation: Weak.
Level of agreement: 88%.
Concomitant atopic disease is common in patients with EoE. While there is no high quality evidence that EoE responds to treatment of concomitant atopic disease, it is good clinical practice to optimise the management of atopic disease in patients with significant symptoms.
As about half of children with EoE have other atopic diseases, and in some on dietetic restrictions for IgE-mediated food allergies, joint allergy clinics with an allergist and dietician are suitable to establish individualised plans to implement elimination diets required for food allergies, ensure that nutritional demands are met and discuss individual preference and feasibilities of treatment options for EoE, in order to explore the options to treat by dietetical or pharmacological means underlying non-IgE mediated immunological pathways in EoE.
Complications
Endoscopists can underestimate the frequency of strictures and narrow lumen oesophagus in eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 89%.
The presenting phenotype of EoE may be inflammatory or fibrotic. It is commoner for younger patients to present with inflammation and then progress to strictures, whereas in older children or adults established strictures are more common at presentation.53 189 A delay in the diagnosis of EoE increases the risk of stricture formation in a time-dependent manner. One of the difficulties of managing EoE is the unpredictability of future fibrotic remodelling. Some patients seem not to progress while others progress at varying speed making decisions on maintenance therapy and complication prevention difficult to individualise.
At least 10% of patients with EoE develop strictures and an additional number have a narrow calibre oesophagus,53 55 often difficult to predict from the presenting phenotype or from biopsy.190 Oesophageal narrowing is usually difficult to detect by simple endoscopy191 and may be more apparent on barium study or by EndoFLIP distensibility testing.192 193 The most common presenting site of a stricture is the distal oesophagus but strictures can occur anywhere along the length of the oesophagus.194
Medical treatment with topical steroids is likely to reduce the development of strictures in eosinophilic oesophagitis
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 83%.
EoE is a chronic condition that may progress to fibrostenotic disease if an effective anti-inflammatory is not prescribed.195 196 Recurrence of stricturing may occur within a year after dilatation unless an effective maintenance anti-inflammatory therapy is used.167 197 198 The underlying fibrosis in children was significantly reduced in 54 patients with EoE from 92% at baseline to 39% after 24 months of maintenance swallowed fluticasone.197
Endoscopic dilatation is effective in improving symptoms in patients with fibrostenotic disease
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
EoE can lead to fibrostenotic change due to persistent oesophageal inflammation over time. This can present as a narrow calibre oesophagus (<13 mm) and strictures. The majority of data relating to dilatation procedures in EOE has been obtained from adult studies. Paediatric data are more scarce, and has been limited to case series, but has been shown to be safe and effective when performed.199 200
Only one randomised controlled trial201 has been published comparing dilatation and medical therapy (fluticasone topical steroid). Twenty-nine patients were randomised to dilatation and 21 to fluticasone. Importantly not all patients had stricturing disease and hence there was no difference in symptom improvement with dilatation.
The majority of efficacy data on dilatation is therefore from retrospective single centre studies. A meta-analysis of nine studies202 found clinical improvement documented in 75% of patients (95% CI 59% to 93%, I2=86%).
Since all studies are retrospective, they will be affected by some reporting bias. Not all studies reported the calibre at follow-up, with most reporting dysphagia scores. The BSG dilatation guidelines203 suggest a target diameter up to 16 mm is a satisfactory end point, as evidence suggests no improvement of symptoms beyond this point.
Longer time between dilatations has been observed in those who receive topical steroid treatment, given that endoscopic dilatation has no effect on inflammation.
Intra-lesional triamcinolone may also be beneficial and has been shown as offering potential to increase the diameter size reached when performing dilatation in patients with EoE, although it does not appear to affect number of dilatations required.194
For technical details on the methods of performing dilatation, we would recommend referral to the UK guidelines on dilatation in clinical practice.203
Endoscopic dilatation is safe in patients with eosinophilic oesophagitis and can be performed using either balloon or bougie dilators
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 94%.
The indication for dilatation in EoE is the presence of a stricture and symptoms despite effective anti-inflammatory therapy such as topical steroids.204 Pain is a common occurrence both during and after the procedure and patients should be warned about this.205 In the circumstances of a tight oesophageal stricture impairing swallowing and nutritional intake, dilatation may be instituted before a medical anti-inflammatory therapy has been commenced. Deep mucosal tears are fairly common following dilatation (9%)206 and are associated with pain during and after endoscopy.
The risks of perforation during endoscopy or dilatation of EoE strictures are no higher than that seen in other benign conditions. Among 671 dilatations in EoE, one perforation was reported but there were frequent mucosal tears.207 Following the publication of international guidelines59 and with improved understanding of the approach to stricture dilatation,203 the frequency of reported perforation is now very low at 0.38% in a meta-analysis of 1820 adult and paediatric EoE dilatations,208 and no more that is seen in other benign conditions of the oesophagus such as peptic stricture, and lower than is seen in achalasia and malignancy.209 Dilatation is equally effective and safe in adults and in children, with no differences in outcome between bougie or through the scope balloon dilators.208–210 New techniques such as bougie caps have also been used and found to be a technically feasible and safe option.211
Clinical outcomes of patients with stricture are better if therapeutic dilatation is combined with effective anti-inflammatory therapy such as topical steroids
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
The quality of dysphagia improvement after dilatation therapy is good and lasts up to 1 year.205 212 The degree and duration of symptom improvement relies on the resolution of remodelling in the oesophageal wall and this is best treated with topical steroids in addition to dilatation.213 The use of dilatation may be the primary treatment for EoE214 but in that circumstance effective anti-inflammatory therapy should be commenced immediately after the dilatation.
Use of systemic steroids in children was reported in 20 children with stricturing disease out of a retrospective cohort of 410 children in Europe. Eighteen had presented with dysphagia or food bolus obstruction and 76% had worsened while on PPI treatment. After systemic steroid treatment, at follow-up endoscopy 95% showed resolution of the strictures and 67% had normal eosinophils/hpf counts. While all patients improved clinically, 75% (15/20) were reported as asymptomatic. Only transient, minor side effects of systemic steroids, including hyperphagia (10/20), weight gain (5/20), hyperactivity (2/20) and acne (1/20) were reported. All patients continued with standard EoE treatments. Patients were followed-up for a mean of 66 months (IQR 24–77.5, range 9–249). Three patients, who had only partially responded to systemic steroids, underwent oesophageal dilation.171
In paediatric patients with EoE, moderate-to-severe oesophageal strictures may respond safely and rapidly to short courses of systemic steroids bridged to standard EoE treatments.
The GDG recommend use of anti-inflammatory medication in combination with dilatation therapy for both children and adults.
Eosinophilic oesophagitis is the most common cause of spontaneous perforation of the oesophagus, and this can occur at any age from children to adults
GRADE of evidence: High.
Level of recommendation: Not applicable.
Level of agreement: 100%.
EoE is now the most common cause of spontaneous perforation of the oesophagus, and this can occur at any age from children to adults.13 27 36 215 In a meta-analysis of EoE presentation,46 the authors identified 76 adults with perforated oesophagus. In a paediatric study,215 five children in France were identified with a perforated oesophagus with EoE, which when added to the four case reports of children with perforation and the meta-analysis previously46 give a total of 85 published cases.
Perforation is usually spontaneous and when it occurs after instrumentation it is more commonly associated with the use of rigid endoscopic procedures36 (2 of 10 rigid endoscopies compared with 0 of 241 with flexible endoscopy), or the push technique of bolus obstruction (five patients).46 The remainder of perforations were spontaneous. It is important to understand the differences between an EoE perforation from one due to Boerhaave’s syndrome because their treatment and their outcome differ significantly.216 Boerhaave’s syndrome is a large full thickness tear in lower third of the distal oesophagus with massive contamination of right thorax, which often requires surgical intervention, and has a high mortality at >50%. In EoE, perforation usually occurs at the time of a food bolus obstruction.36 217 218 This is usually multiple and generally small, partial tears or dissection of tissue planes,46 219 with limited extravasation, mostly air and liquid and not associated with a large amount of food products in the mediastinum or in the thoracic cavity. Surgery is required in a minority (30%) and mortality is not reported.46 Most resolve with conservative treatment of placement of a nasogastric tube, intravenous fluids and prophylactic antibiotics.
In case of an eosinophilic oesophagitis perforation, a CT contrast study should be performed to assess the degree of extravasation
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 89%.
A CT contrast study should be performed to assess the degree of extravasation, which is usually limited to within 2–3 cms of the lumen and if there is limited extravasation, the perforation should be managed conservatively. Longer tears may require endoscopic or surgical intervention.59 220
In case of a perforation in eosinophilic oesophagitis, if there is limited extravasation, the patient should be managed conservatively, with multidisciplinary input from gastroenterology, surgery and radiology specialists
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 100%.
An EoE perforation should be managed by a multidisciplinary team including a gastrointestinal surgeon, gastroenterologist, radiologist and dietitian. Since previously released guidelines59 the involvement of multiple disciplines in the management of EoE perforation has become standardised.217 Decision-making by multiple disciplines is important221 222 because of the implications of radiology interpretation, the possible need for repeated endoscopic drainage, the decisions on nutrition and the timing of other more invasive interventions such as stents, thoracic cavity drainage or surgical intervention to the oesophagus.223
Prior to multidisciplinary team management, outcomes of EoE perforation were relatively poor. The evolution of new options in the management of a perforated oesophagus has identified the need for careful decision-making in the early stages based on the extent of extra luminal and thoracic cavity involvement.224
Where there is a limited (<3 cms) cavity or extravasation, the cavity should be drained by an endoscopically placed drain, with or without suction.223 225
Endoscopic vacuum therapy may be indicated in this situation, as good results are obtained in anastomotic leaks and perforations in children but no specific data are available on perforations in EoE.
It is recognised that endoscopic assessment and intervention play a major role in the early phases of managing spontaneous perforation of the oesophagus.226
Any cavities should be drained via nasal tube and reassessed daily in the initial stages. The use of stents is indicated when the tears are slow to heal, or there is a significant extramural cavity, adequately drained.46 227 228
The GDG recommends that the management of extensive leaks should be individualised and discussed in a multidisciplinary team. Stents and drains require expertise and experience in handling this complex circumstance.
The psychological impact of dietary therapy should be appreciated and discussed with patients with eosinophilic oesophagitis and their carers
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 95%.
The psychological impact of dietary therapy is significant229 and should be understood and discussed with patients with EoE and their carers because social exclusion is common in this setting, due to both the physical difficulties of eating in public and the restriction on dietary content in an exclusion diet. This may be compounded by the observation that psychiatric morbidities and psychiatric medication use are more common in patients with EoE, particularly older women.230
Using a validated EoE tool for children and parents, quality of life scores were worse for those children treated with dietary restrictions than for those with no restrictions (patient self-report: 61.6 vs 74.3 (p<0.01); parent proxy-report: 65.5 vs 74.7 (p<0.01])231 confirming the psychological burden that exclusion diets may have on some patients.
Anxiety and depression in eosinophilic oesophagitis affects patients due to persistent symptoms and social restrictions and is alleviated by effective therapy
GRADE of evidence: Low.
Level of recommendation: Strong.
Level of agreement: 100%.
Anxiety and depression in EoE affects patients due to persistent symptoms, social restrictions and this is alleviated by effective therapy.232
Higher mental health support needs has been reported in children with EoE.233 Contributing factors were identified as significant dietary restrictions, repeat endoscopies and percutaneous endoscopic gastrostomy (PEG) feeding. Sixty-nine per cent of the children reported social difficulties, 41% anxiety, 33% sleep difficulty, 28% depression, 26% school problems and anxiety and depression were reported to increase with age.129
If Proton pump inhibitor therapy causes unwanted side effects (diarrhoea, gastrointestinal infections or magnesium deficiency), then consider switching to alternative treatments such as diet or topical steroid
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 94%.
The literature on PPI side effects is extensive and often describes associations without proof of casual association.234 For that reason we recommend switching away from PPI therapy only if the side effects are clearly caused by the PPI. This is relatively certain in cases of diarrhoea as this resolves within days of stopping the PPI. In cases of gastrointestinal infections such as Campylobacter jejuni a course of anti-microbial therapy may be necessary as well as stopping the PPI. In cases of magnesium deficiency the relationship may be multifactorial and involve, for example, diuretic medication as well as PPI and individualised decision-making is needed.
Candida infection may occur in a small proportion of patients with eosinophilic oesophagitis treated with topical corticosteroids and should be managed by topical antifungals while continuing topical steroids
GRADE of evidence: Moderate.
Level of recommendation: Strong.
Level of agreement: 89%.
Histologically confirmed symptomatic oropharyngeal or oesophageal candidiasis was seen in 5 patients out of 136 (3.6%) treated with 0.5–1.0 mg two times per day orodispersible budesonide tablets for over a year (induction 12 weeks + maintenance therapy 48 weeks). The infection resolved with oral nystatin suspension in all cases and did not require stopping the oral topical steroid therapy.167 Data on topical steroids in children (swallowed inhaled or viscous) are currently limited, and results of prospective trials are still pending.
Systemic side effects of topical steroids have not been documented during the long- term treatment of patients with eosinophilic oesophagitis; continued monitoring of bone mineral density and adrenal suppression is recommended in children and adolescents
GRADE of evidence: High.
Level of recommendation: Strong.
Level of agreement: 94%.
In a follow-up study of patients with EoE treated with budesonide orodispersible tablets for more than 1 year the mean serum cortisol levels were similar (1 mg or 0.5 mg two times per day 12.1 and 11.3 ug/dL) to those on placebo (10.1 ug/dL).167 In that study a decrease in morning cortisol below the lower limit of normal (6.2 ug/dL) was observed in four patients (3%) but this had no clinical effects. Until further data are available for children and adolescents, the GDG recommends that monitoring of adrenal gland suppression and, for long-term higher dosage, bone mineral density for these younger patients on long-term topical steroid therapy is considered, especially in children also receiving topical steroids for other conditions such as asthma who are exposed to a cumulative effect.
Future research
The future direction of research in EoE requires a knowledge of the gaps in understanding and not necessarily in areas where we already have much knowledge. Hence the literature that might support the future direction of research may be lacking.
Research is needed into the cause and progression of eosinophilic oesophagitis, the course of the disease and into disease prevention
First, there is more to learn about the disease itself, the risk factors for acquiring the condition and the underlying cause or causes. Theories that include early life events (birth mode, formula feeding, exposure to antibiotics and to PPI medication), and food antigen reactions, and genetic susceptibility are very popular but they do not explain why the condition started to be reported only in the 1980s, nor do they explain the huge rise in the incidence of the condition since then. Understanding the geographical and racial variations might provide some clues but the root cause analysis is currently very incomplete.235 The discovery of anoctamin-1 as a key driver of oesophageal epithelial proliferation in EoE236 needs confirmation in humans and translation into an effective drug target. The study of the biology of progression from the EoE inflammatory phenotype to a fibrotic one has begun to yield results but none ready for clinical application.237–239
Currently there is no knowledge that would help prevent or avoid EoE. Given its increasing frequency, and the increasing burden both in quality of life and in economic terms there is a great need to develop prevention strategies.
Research is needed into non-endoscopic sampling techniques for disease diagnosis and follow-up of eosinophilic oesophagitis
The major limitation in diagnosing EoE is the need for endoscopy and biopsy. There are also limitations in random biopsy sampling, due to the patchy nature of the disease, although advances in endoscopic imaging may potentially overcome this.
Methods of detection that avoid the need for endoscopy and biopsy include string tests, the Cytosponge and other novel technologies. These are in the early stages of development and are not fully characterised and calibrated in relation to the normal range.
It is very unlikely that a blood test will be an accurate diagnostic marker of EoE because of the pathophysiology of the condition as an IgG4 local mucosal immune reactivity. Research is concentrating on alternative ways to assess the cells in the lining of the oesophagus. This issue has been a critical problem during the COVID-19 pandemic which has brought into focus the potential value of the non-endoscopic methods of the Cytosponge and string tests.114 However, not enough research has been published on their use for them to be recommended in a current guideline. The GDG also recognise that other means of assessment of patients with EoE are being evaluated such as the use of transnasal endoscopy which may be advantageous in reducing patient discomfort where multiple attendances are anticipated to take biopsies and assess for histological remission (such as in food elimination diet).
Research is needed into quantifying symptom severity in a standard way that helps to guide therapy and record disease response
A symptom severity questionnaire needs to be developed that will facilitate structured semi-quantitative comparison of symptoms before and after therapy. Existing questionnaires have only been taken up in research practice or drug therapy trials.240
Paediatric validated questionnaires have been developed to measure health-related quality of life7 in EoE, which have been used in children and parents by proxy. These can be obtained in different languages and for different cultures.
A clear decision tool to aid initial therapy is needed. This may be in the form of a patient’s symptom severity questionnaire, on its own or combined with other observations such as the severity of endoscopic appearance or the presence of fibrosis.117 Discrimination of the suitability of the different treatment options to the individual patient would be greatly aided by such a tool. While there have been early examples of this,231 241 242 more research is needed.
Research is needed into patient education and shared decision-making in eosinophilic oesophagitis between patients and their doctors
It is of paramount importance to develop balanced patient information so that decision-making on therapy is based on a full understanding of the implications of each approach—diet and drug types to patients’ symptoms and quality of life. Currently much of the information patients glean is from uncontrolled internet sources and can be heavily biased based on the limited experience of the authors. Patient organisations have an important role to provide accessible patient information on EoE and its management. Medical organisations should work with patient support organisations such as the Eos Network in developing patient educational material (EosNetwork.org). Also the combination of medical and patient input into the direction of research, such as that of Consortium of Eosinophilic Gastrointestinal disease Researchers (CEGIR),243 is a clear example of good practice in research. Due to the availability of global internet connectivity, there is value in ensuring that these organisations have a global perspective.
In our current circumstances a health economic assessment of different strategies of therapy for EoE is essential for good decision-making and use of heath resources. National Institute for Health and Care Excellence (NICE) has recently published guidance on drug therapy of EoE which has established the value of using dedicated topical steroid therapy. This currently only addresses induction therapy, but hopefully will in the near future examine maintenance therapy. There is no comparative study of the cost effectiveness of dietary, and other drug strategies relative to the use of dedicated topical steroids.
Research is needed to compare available drug therapies, including new biological drugs and/or diets in randomised clinical trials
New drug developments such as the introduction of the biological agents need to be assessed, both in terms of effectiveness and cost-effectiveness. Some circumstances, such as multiple atopic conditions coinciding may favour their use especially if the symptoms are severe. There may be age cohorts that benefit more than others, such as young or adolescent patients where aggressive therapies may make a bigger difference to long-term quality of life, and where the lack of needing concordance with a daily tablet regime could give greater reliability with disease response.
The issues of concordance and long-term viability of dietary regimes needs support and research to ensure that not all patients end up requiring a drug therapy if an alternative effective diet can be identified and maintained.244
Work on the role of biological therapy and novel targets in EoE is underway, although at an early stage currently. In addition to phase 3 trials for dupilumab, NCT03633617 (adults) and NCT04394351 (paediatrics), cendakimab NCT04753697, and benralizumab (adults) NCT04543409180 245–247 which are underway. There are also novel drugs and drug targets such as losartan, kallikrein and Siglec-8.248 It is important that the medical community are able to work with the pharmaceutical industry to ensure that these trials are performed and completed to a high standard so that the scientific outcomes add to our knowledge of EoE disease management. With better understanding of the pathophysiology of the disease, we may also be able to identify phenotypes for more personalised treatment.
Research is needed to understand the application of clinical guidelines in eosinophilic oesophagitis
As a final recommendation, we consider that research is needed to ensure that guidelines have true clinical applicability, are acceptable to clinicians and patients. Methods of dissemination and uptake should be researched and validated. Until now guidelines have been produced but it is common to hear that practice does not necessarily follow guidelines and it is important to understand why.249
Other research needs have been summarised in table 2.
Table 2.
Research needs in eosinophilic oesophagitis
| Area | Research need | Example |
| Natural history | Better understanding of onset of disease and natural history of EoE in both treated and untreated individuals | Long-term follow-up data in treated patients |
| Cause of EoE | Understand better the pathophysiological mechanisms underlying cause of disease | Better understanding of genetic predisposition and disease triggers |
| Clinical presentation | Validated symptom questionnaires for disease monitoring | Use of EEsAI and HRQoL |
| Diagnosis | Clearer definition of histological disease diagnosis | Assess value of mast cell activity, fibroblast activity and density of submucosal fibrosis |
| Less invasive methods of disease detection | Cytosponge or string test, transnasal endoscopy | |
| Potential of systemic biomarkers of disease | Blood messenger RNA levels of CD101 and CD274 expressing eosinophils | |
| Assessment of the timing and value of stopping a PPI before a diagnostic biopsy | Current advice on 3 weeks gap needs further study | |
| The relevance of allergy testing in EoE | Value of testing for treatment of symptoms in other organs | |
| Management | Comparisons of therapeutic strategies and which one to use as first line options | Using standardised symptom questionnaires to distinguish optimum initial therapy |
| Role of novel biologics in the management algorithm | Use of drugs such as dupilimab, benralizumab and cendakimab in both steroid naïve and steroid unresponsive patients | |
| Prevention of EoE | Long-term outcomes of each therapeutic option with comparison of efficacy and side effects | Follow-up data of patients on therapy |
| Prognosis | The value of achieving deep remission versus remission and threshold levels of their definition | Value of suppressing eosinophil count <5 eosinophils/0.3 mm2 compared with <15 eosinophils/0.3 mm2 |
EEsAI, Eosinophilic Esophagitis symptom Assessment Index; EoE, eosinophilic oesophagitis; HRQoL, health-related quality of life; PPI, proton pump inhibitor.
gutjnl-2022-327326supp002.pdf (531.1KB, pdf)
Acknowledgments
We are grateful to the Clinical Standards and Services Committee of the British Society of Gastroenterology for commissioning these guidelines, and to the following stakeholders and societies who have provided valuable review and comment during the guideline development process: British Society of Gastroenterology Oesophageal Section, British Society of Paediatric Gastroenterology, Hepatology and Nutrition, EOS Network. We also thank Professor Arjan Bredenoord (Academic Medical Center, Amsterdam) and Professor Glenn Furuta (University of Colorado School of Medicine) for providing expert external peer review, advice, guidance and feedback.
Footnotes
Twitter: @anjan_dhar6, @LiverpoolAndrew, @CarolLRead
Contributors: AD, HNH, SEA, NJT undertook the literature search and initial sorting of manuscripts. Subsequent review of manuscripts was undertaken by Core Writing Group leads: AD (Definition and Epidemiology), NJT (Clinical presentation, symptoms and access to care), MRN (Investigations-Histology), RS (Investigations-non-histology), JMD (Treatment), SEA (Complications and Future research), MKHA (Paediatric sections). PICO Statements and guideline statements were developed within these working groups by all members. All members contributed to discussion and voting (organised by HNH). All core writing group leads contributed to manuscript writing within their group with AD and HNH completing all other sections of the manuscript. All other authors fulfil the ICMJE recommendations for authorship with appropriate involvement at all stages of the guideline development process, and drafting of the manuscript. AD and HNH contributed equally to this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: See Supplementary Table 1.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Attwood SE, Smyrk TC, Demeester TR, et al. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 1993;38:109–16. 10.1007/BF01296781 [DOI] [PubMed] [Google Scholar]
- 2. Straumann A, Spichtin HP, Bernoulli R. [Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings]. Schweiz Med Wochenschr 1994;124:1419–29. [PubMed] [Google Scholar]
- 3. Lucendo AJ, Molina-Infante J, Arias Ángel, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J 2017;5:335–58. 10.1177/2050640616689525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirano I, Chan ES, Rank MA, et al. AGA Institute and the joint Task force on Allergy-Immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology 2020;158:1776–86. 10.1053/j.gastro.2020.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brouwers MC, Kho ME, Browman GP, et al. Agree II: advancing Guideline development, reporting and evaluation in health care. Can Med Assoc J 2010;182:E839–42. 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucendo AJ, Arias-González L, Molina-Infante J, et al. Systematic review: health-related quality of life in children and adults with eosinophilic oesophagitis-instruments for measurement and determinant factors. Aliment Pharmacol Ther 2017;46:401–9. 10.1111/apt.14194 [DOI] [PubMed] [Google Scholar]
- 8. Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med 2015;373:1640–8. 10.1056/NEJMra1502863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellon ES, Liacouras CA, Molina-Infante J, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the agree conference. Gastroenterology 2018;155:1022–33. 10.1053/j.gastro.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma C, Schoepfer AM, Safroneeva E. Development of a core outcome set for therapeutic studies in eosinophilic esophagitis (COREOS): an international multidisciplinary consensus. Gastroenterology 2021;161. 10.1053/j.gastro.2021.04.080 [DOI] [PubMed] [Google Scholar]
- 11. Navarro P, Arias Ángel, Arias-González L, et al. Systematic review with meta-analysis: the growing incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2019;49:1116–25. 10.1111/apt.15231 [DOI] [PubMed] [Google Scholar]
- 12. Arias Á, Pérez-Martínez I, Tenías JM, et al. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther 2016;43:3–15. 10.1111/apt.13441 [DOI] [PubMed] [Google Scholar]
- 13. Dellon ES, Hirano I. Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology 2018;154:319–32. 10.1053/j.gastro.2017.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prasad GA, Alexander JA, Schleck CD, et al. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol 2009;7:1055–61. 10.1016/j.cgh.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moawad FJ, Veerappan GR, Lake JM, et al. Correlation between eosinophilic oesophagitis and aeroallergens. Aliment Pharmacol Ther 2010;31:509–15. 10.1111/j.1365-2036.2009.04199.x [DOI] [PubMed] [Google Scholar]
- 16. Kapel RC, Miller JK, Torres C, et al. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 2008;134:1316–21. 10.1053/j.gastro.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 17. Alexander ES, Martin LJ, Collins MH, et al. Twin and family studies reveal strong environmental and weaker genetic cues explaining heritability of eosinophilic esophagitis. J Allergy Clin Immunol 2014;134:e1. 10.1016/j.jaci.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperry SLW, Crockett SD, Miller CB, et al. Esophageal foreign-body impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest Endosc 2011;74:985–91. 10.1016/j.gie.2011.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ntuli Y, Bough I, Wilson M. Recognising eosinophilic oesophagitis as a cause of food bolus obstruction. Frontline Gastroenterol 2020;11:11–15. 10.1136/flgastro-2019-101176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veerappan GR, Perry JL, Duncan TJ, et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clinical Gastroenterology and Hepatology 2009;7:420–6. 10.1016/j.cgh.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 21. Ricker J, McNear S, Cassidy T, et al. Routine screening for eosinophilic esophagitis in patients presenting with dysphagia. Therap Adv Gastroenterol 2011;4:27–35. 10.1177/1756283X10384172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salem SB, Kushner Y, Marcus V, et al. The potential impact of contemporary developments in the management of patients with gastroesophageal reflux disease undergoing an initial gastroscopy. Can J Gastroenterol 2009;23:99–104. 10.1155/2009/859271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Achem SR, Almansa C, Krishna M, et al. Oesophageal eosinophilic infiltration in patients with noncardiac chest pain. Aliment Pharmacol Ther 2011;33:1194–201. 10.1111/j.1365-2036.2011.04652.x [DOI] [PubMed] [Google Scholar]
- 24. Chehade M, Jones SM, Pesek RD, et al. Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population from the Consortium for Food Allergy Research. J Allergy Clin Immunol 2018;6:1534–44. 10.1016/j.jaip.2018.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spergel JM, Brown-Whitehorn TF, Beausoleil JL, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr 2009;48:30–6. 10.1097/MPG.0b013e3181788282 [DOI] [PubMed] [Google Scholar]
- 26. Hoofien A, et al. Pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr 2019;68:552–8. [DOI] [PubMed] [Google Scholar]
- 27. Shaheen NJ, Mukkada V, Eichinger CS, et al. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus 2018;31. 10.1093/dote/doy015. [Epub ahead of print: 01 Aug 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim HP, Vance RB, Shaheen NJ, et al. The prevalence and diagnostic utility of endoscopic features of eosinophilic esophagitis: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:e5. 10.1016/j.cgh.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiremath G, Correa H, Acra S, et al. Correlation of endoscopic signs and mucosal alterations in children with eosinophilic esophagitis. Gastrointest Endosc 2020;91:785–94. 10.1016/j.gie.2019.11.031 [DOI] [PubMed] [Google Scholar]
- 30. Iwanczak B, Janczyk W, Ryzko J, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci 2011;56:151–7. 10.2478/v10039-011-0038-7 [DOI] [PubMed] [Google Scholar]
- 31. DeBrosse CW, Collins MH, Buckmeier Butz BK, et al. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982-1999. J Allergy Clin Immunol 2010;126:112–9. 10.1016/j.jaci.2010.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eluri S, Dellon ES. Proton pump inhibitor-responsive oesophageal eosinophilia and eosinophilic oesophagitis: more similarities than differences. Curr Opin Gastroenterol 2015;31:309–15. 10.1097/MOG.0000000000000185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sá CCde, Kishi HS, Silva-Werneck AL, et al. Eosinophilic esophagitis in patients with typical gastroesophageal reflux disease symptoms refractory to proton pump inhibitor. Clinics 2011;66:557–61. 10.1590/s1807-59322011000400006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. García-Compeán D, González González JA, Marrufo García CA, et al. Prevalence of eosinophilic esophagitis in patients with refractory gastroesophageal reflux disease symptoms: a prospective study. Dig Liver Dis 2011;43:204–8. 10.1016/j.dld.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 35. Poh CH, Gasiorowska A, Navarro-Rodriguez T, et al. Upper GI tract findings in patients with heartburn in whom proton pump inhibitor treatment failed versus those not receiving antireflux treatment. Gastrointest Endosc 2010;71:28–34. 10.1016/j.gie.2009.08.024 [DOI] [PubMed] [Google Scholar]
- 36. Straumann A, Bussmann C, Zuber M, et al. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin Gastroenterol Hepatol 2008;6:598–600. 10.1016/j.cgh.2008.02.003 [DOI] [PubMed] [Google Scholar]
- 37. Kerlin P, Jones D, Remedios M, et al. Prevalence of eosinophilic esophagitis in adults with food bolus obstruction of the esophagus. J Clin Gastroenterol 2007;41:356–61. 10.1097/01.mcg.0000225590.08825.77 [DOI] [PubMed] [Google Scholar]
- 38. Lenz CJ, Leggett C, Katzka DA, et al. Food impaction: etiology over 35 years and association with eosinophilic esophagitis. Diseases of the Esophagus 2019;32. 10.1093/dote/doy093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hiremath GS, Hameed F, Pacheco A, et al. Esophageal food impaction and eosinophilic esophagitis: a retrospective study, systematic review, and meta-analysis. Dig Dis Sci 2015;60:3181–93. 10.1007/s10620-015-3723-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kassim T, Gapp J, Walters RW, et al. Immediate dilation in esophageal food impaction is safe and effective but performed infrequently: observations from a large Midwest US cohort. Dis Esophagus 2020;33. 10.1093/dote/doz084. [Epub ahead of print: 16 Jan 2020]. [DOI] [PubMed] [Google Scholar]
- 41. Lee WH, Grover Z, Borland M, et al. Medical Disimpaction for children with organic esophageal foreign body in the era of eosinophilic esophagitis. Pediatr Emerg Care 2018;Publish Ahead of Print. 10.1097/PEC.0000000000001673 [DOI] [PubMed] [Google Scholar]
- 42. Marashi Nia SF, Aghaie Meybodi M, Sutton R, et al. Outcome, complication and follow-up of patients with esophageal foreign body impaction: an academic institute’s 15 years of experience. Diseases of the Esophagus 2020;33. 10.1093/dote/doz103 [DOI] [PubMed] [Google Scholar]
- 43. Cook D, Zala A, Bollipo S, et al. Oesophageal food bolus obstruction and eosinophilic oesophagitis. Intern Med J 2019;49:1032–4. 10.1111/imj.14389 [DOI] [PubMed] [Google Scholar]
- 44. Chang JW, Olson S, Kim JY, et al. Loss to follow-up after food impaction among patients with and without eosinophilic esophagitis. Dis Esophagus 2020;32:doz056. 10.1093/dote/doz056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolf WA, Piazza NA, Gebhart JH, et al. Association between body mass index and clinical and endoscopic features of eosinophilic esophagitis. Dig Dis Sci 2017;62:143–9. 10.1007/s10620-016-4357-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arias-González L, Rey-Iborra E, Ruiz-Ponce M, et al. Esophageal perforation in eosinophilic esophagitis: a systematic review on clinical presentation, management and outcomes. Dig Liver Dis 2020;52:245–52. 10.1016/j.dld.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 47. Schupack DA, Lenz CJ, Geno DM, et al. The evolution of treatment and complications of esophageal food impaction. United European Gastroenterol J 2019;7:548–56. 10.1177/2050640619836052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lucendo AJ, Miehlke S, Schlag C, et al. Efficacy of budesonide Orodispersible tablets as induction therapy for eosinophilic esophagitis in a randomized placebo-controlled trial. Gastroenterology 2019;157:74–86. 10.1053/j.gastro.2019.03.025 [DOI] [PubMed] [Google Scholar]
- 49. Kuchen T, Straumann A, Safroneeva E, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy 2014;69:1248–54. 10.1111/all.12455 [DOI] [PubMed] [Google Scholar]
- 50. Lucendo AJ, Arias Ángel, Molina-Infante J. Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016;14:13–22. 10.1016/j.cgh.2015.07.041 [DOI] [PubMed] [Google Scholar]
- 51. Odiase E, Schwartz A, Souza RF, et al. New eosinophilic esophagitis concepts call for change in proton pump inhibitor management before diagnostic endoscopy. Gastroenterology 2018;154:e3. 10.1053/j.gastro.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ravi K, Talley NJ, Smyrk TC, et al. Low grade esophageal eosinophilia in adults: an unrecognized part of the spectrum of eosinophilic esophagitis? Dig Dis Sci 2011;56:1981–6. 10.1007/s10620-011-1594-1 [DOI] [PubMed] [Google Scholar]
- 53. Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 2013;145:1230–2. 10.1053/j.gastro.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 54. Lipka S, Kumar A, Richter JE. Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J Clin Gastroenterol 2016;50:134–40. 10.1097/MCG.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 55. Warners MJ, Oude Nijhuis RAB, de Wijkerslooth LRH, et al. The natural course of eosinophilic esophagitis and long-term consequences of undiagnosed disease in a large cohort. Am J Gastroenterol 2018;113:836–44. 10.1038/s41395-018-0052-5 [DOI] [PubMed] [Google Scholar]
- 56. Lieberman JA, Morotti RA, Konstantinou GN, et al. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy 2012;67:1299–307. 10.1111/j.1398-9995.2012.02881.x [DOI] [PubMed] [Google Scholar]
- 57. Rajan J, Newbury RO, Anilkumar A, et al. Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol 2016;137:e8. 10.1016/j.jaci.2015.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 2007;133:1342–63. 10.1053/j.gastro.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 59. Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:2–3. 10.1016/j.jaci.2011.02.040 [DOI] [PubMed] [Google Scholar]
- 60. Dellon ES, Speck O, Woodward K, et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am J Gastroenterol 2013;108:1854–60. 10.1038/ajg.2013.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Francis DL, Foxx-Orenstein A, Arora AS, et al. Results of ambulatory pH monitoring do not reliably predict response to therapy in patients with eosinophilic oesophagitis. Aliment Pharmacol Ther 2012;35:300–7. 10.1111/j.1365-2036.2011.04922.x [DOI] [PubMed] [Google Scholar]
- 62. Moawad FJ, Schoepfer AM, Safroneeva E, et al. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther 2014;39:603–8. 10.1111/apt.12636 [DOI] [PubMed] [Google Scholar]
- 63. Moawad FJ, Wells JM, Johnson RL, et al. Comparison of eotaxin-3 biomarker in patients with eosinophilic oesophagitis, proton pump inhibitor-responsive oesophageal eosinophilia and gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2015;42:231–8. 10.1111/apt.13258 [DOI] [PubMed] [Google Scholar]
- 64. Wen T, Dellon ES, Moawad FJ, et al. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J Allergy Clin Immunol 2015;135:187–97. 10.1016/j.jaci.2014.08.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. The global, regional, and national burden of gastro-oesophageal reflux disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. lancet. Gastroenterol. Hepatol 2020;5:561–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Remedios M, Campbell C, Jones DM, et al. Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006;63:3–12. 10.1016/j.gie.2005.07.049 [DOI] [PubMed] [Google Scholar]
- 67. Peterson KA, Thomas KL, Hilden K, et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig Dis Sci 2010;55:1313–9. 10.1007/s10620-009-0859-4 [DOI] [PubMed] [Google Scholar]
- 68. Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol 2012;303:G1175–87. 10.1152/ajpgi.00313.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Furuta GT, Nieuwenhuis EES, Karhausen J, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol 2005;289:G890–7. 10.1152/ajpgi.00015.2005 [DOI] [PubMed] [Google Scholar]
- 70. Souza RF, Huo X, Mittal V, et al. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 2009;137:1776–84. 10.1053/j.gastro.2009.07.055 [DOI] [PubMed] [Google Scholar]
- 71. Bohm M, Jacobs JW, Gupta A, et al. Most children with eosinophilic esophagitis have a favorable outcome as young adults. Dis Esophagus 2017;30:1–6. 10.1111/dote.12454 [DOI] [PubMed] [Google Scholar]
- 72. Menard-Katcher P, Marks KL, Liacouras CA, et al. The natural history of eosinophilic oesophagitis in the transition from childhood to adulthood. Aliment Pharmacol Ther 2013;37:114–21. 10.1111/apt.12119 [DOI] [PubMed] [Google Scholar]
- 73. Dellon ES, Jones PD, Martin NB, et al. Health-care transition from pediatric to adult-focused gastroenterology in patients with eosinophilic esophagitis. Dis Esophagus 2013;26:7–13. 10.1111/j.1442-2050.2011.01315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tourlamain G, Garcia-Puig R, Gutiérrez-Junquera C, et al. Differences in management of eosinophilic esophagitis in Europe: an assessment of current practice. J Pediatr Gastroenterol Nutr 2020;71:83–90. 10.1097/MPG.0000000000002672 [DOI] [PubMed] [Google Scholar]
- 75. Zifman E, Banai H, Shamir R, et al. Practice differences in the diagnosis and management of eosinophilic esophagitis among adult and pediatric Gastroenterologists in Israel. J Pediatr Gastroenterol Nutr 2018;67:34–9. 10.1097/MPG.0000000000001909 [DOI] [PubMed] [Google Scholar]
- 76. Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc 2006;64:313–9. 10.1016/j.gie.2006.04.037 [DOI] [PubMed] [Google Scholar]
- 77. Nielsen JA, Lager DJ, Lewin M, et al. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol 2014;109:515–20. 10.1038/ajg.2013.463 [DOI] [PubMed] [Google Scholar]
- 78. Straumann A, Spichtin H-P, Bucher KA, et al. Eosinophilic esophagitis: red on microscopy, white on endoscopy. Digestion 2004;70:109–16. 10.1159/000080934 [DOI] [PubMed] [Google Scholar]
- 79. Parfitt JR, Gregor JC, Suskin NG, et al. Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients. Mod Pathol 2006;19:90–6. 10.1038/modpathol.3800498 [DOI] [PubMed] [Google Scholar]
- 80. Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol 2007;102:2300–13. 10.1111/j.1572-0241.2007.01396.x [DOI] [PubMed] [Google Scholar]
- 81. Shah A, Kagalwalla AF, Gonsalves N, et al. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol 2009;104:716–21. 10.1038/ajg.2008.117 [DOI] [PubMed] [Google Scholar]
- 82. Dellon ES, Gonsalves N, Hirano I, et al. Acg clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013;108:679–92. 10.1038/ajg.2013.71 [DOI] [PubMed] [Google Scholar]
- 83. Yantiss RK, Greenson JK, Spechler S. American registry of pathology expert opinions: evaluating patients with eosinophilic esophagitis: practice points for endoscopists and pathologists. Ann Diagn Pathol 2019;43:151418. 10.1016/j.anndiagpath.2019.151418 [DOI] [PubMed] [Google Scholar]
- 84. Yantiss RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory disorders of the gastrointestinal tract. Am J Gastroenterol 2009;104:774–83. 10.1038/ajg.2008.108 [DOI] [PubMed] [Google Scholar]
- 85. Dellon ES, Speck O, Woodward K, et al. Distribution and variability of esophageal eosinophilia in patients undergoing upper endoscopy. Mod Pathol 2015;28:383–90. 10.1038/modpathol.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vanstapel A, Vanuytsel T, De Hertogh G. Eosinophilic peak counts in eosinophilic esophagitis : a retrospective study. Acta Gastroenterol Belg 2019;82:243–50. [PubMed] [Google Scholar]
- 87. Collins MH, Martin LJ, Alexander ES, et al. Newly developed and validated eosinophilic esophagitis histology scoring system and evidence that it outperforms peak eosinophil count for disease diagnosis and monitoring. Dis Esophagus 2017;30:1–8. 10.1111/dote.12470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Collins MH, Martin LJ, Wen T, et al. Eosinophilic esophagitis histology remission score: significant relations to measures of disease activity and symptoms. J Pediatr Gastroenterol Nutr 2020;70:598–603. 10.1097/MPG.0000000000002637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bussmann C, Schoepfer AM, Safroneeva E, et al. Comparison of different biopsy forceps models for tissue sampling in eosinophilic esophagitis. Endoscopy 2016;48:1069–75. 10.1055/s-0042-117274 [DOI] [PubMed] [Google Scholar]
- 90. Eke R, Li T, White A, et al. Systematic review of histological remission criteria in eosinophilic esophagitis. JGH Open 2018;2:158–65. 10.1002/jgh3.12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Albert D, Heifert TA, Min SB, et al. Comparisons of fluticasone to budesonide in the treatment of eosinophilic esophagitis. Dig Dis Sci 2016;61:1996–2001. 10.1007/s10620-016-4110-9 [DOI] [PubMed] [Google Scholar]
- 92. Straumann A, Katzka DA. Diagnosis and treatment of eosinophilic esophagitis. Gastroenterology 2018;154:346–59. 10.1053/j.gastro.2017.05.066 [DOI] [PubMed] [Google Scholar]
- 93. Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. The role of proton pump inhibitors in the management of pediatric eosinophilic esophagitis. Front Pediatr 2018;6:119. 10.3389/fped.2018.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Heer J, Miehlke S, Rösch T, et al. Histologic and clinical effects of different topical corticosteroids for eosinophilic esophagitis: lessons from an updated meta-analysis of placebo-controlled randomized trials. Digestion 2021;102:377–85. 10.1159/000507571 [DOI] [PubMed] [Google Scholar]
- 95. Miehlke S, Lucendo AJ, Straumann A, et al. Orodispersible budesonide tablets for the treatment of eosinophilic esophagitis: a review of the latest evidence. Therap Adv Gastroenterol 2020;13:1756284820927282. 10.1177/1756284820927282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high‐resolution manometry: Chicago classification version 4.0 © . Neurogastroenterology & Motility 2021;33:e14058. 10.1111/nmo.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. von Arnim U, Kandulski A, Weigt J, et al. Correlation of high-resolution manometric findings with symptoms of dysphagia and endoscopic features in adults with eosinophilic esophagitis. Dig Dis 2017;35:472–7. 10.1159/000458407 [DOI] [PubMed] [Google Scholar]
- 98. Savarino EV, Tolone S, Bartolo O, et al. The GerdQ questionnaire and high resolution manometry support the hypothesis that proton pump inhibitor-responsive oesophageal eosinophilia is a GERD-related phenomenon. Aliment Pharmacol Ther 2016;44:522–30. 10.1111/apt.13718 [DOI] [PubMed] [Google Scholar]
- 99. Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil 2011;23:e111. 10.1111/j.1365-2982.2010.01633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Rhijn BD, Oors JM, Smout AJPM, et al. Prevalence of esophageal motility abnormalities increases with longer disease duration in adult patients with eosinophilic esophagitis. Neurogastroenterol Motil 2014;26:1349–55. 10.1111/nmo.12400 [DOI] [PubMed] [Google Scholar]
- 101. Colizzo JM, Clayton SB, Richter JE. Intrabolus pressure on high-resolution manometry distinguishes fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. Dis Esophagus 2016;29:551–7. 10.1111/dote.12360 [DOI] [PubMed] [Google Scholar]
- 102. Nennstiel S, Bajbouj M, Becker V, et al. High-resolution manometry in patients with eosinophilic esophagitis under topical steroid therapy-a prospective observational study (HIMEOS-study). Neurogastroenterol Motil 2016;28:599–607. 10.1111/nmo.12753 [DOI] [PubMed] [Google Scholar]
- 103. Weiss AH, Iorio N, Schey R. Esophageal motility in eosinophilic esophagitis. Rev Gastroenterol Mex 2015;80:205–13. 10.1016/j.rgmx.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 104. Martín Martín L, Santander C, Lopez Martín MC, et al. Esophageal motor abnormalities in eosinophilic esophagitis identified by high-resolution manometry. J Gastroenterol Hepatol 2011;26:1447–50. 10.1111/j.1440-1746.2011.06770.x [DOI] [PubMed] [Google Scholar]
- 105. Ghisa M, Laserra G, Marabotto E, et al. Achalasia and obstructive motor disorders are not uncommon in patients with eosinophilic esophagitis. Clinical Gastroenterology and Hepatology 2021;19:1554–63. 10.1016/j.cgh.2020.07.056 [DOI] [PubMed] [Google Scholar]
- 106. Muroi K, Kakushima N, Furukawa K, et al. Subjective symptoms in patients with eosinophilic esophagitis are related to esophageal wall thickness and esophageal body pressure. Dig Dis Sci 2021;66:2291–300. 10.1007/s10620-020-06527-5 [DOI] [PubMed] [Google Scholar]
- 107. Xiao Y, Kahrilas PJ, Nicodème F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol 2014;109:521–6. 10.1038/ajg.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ang D, Misselwitz B, Hollenstein M, et al. Diagnostic yield of high-resolution manometry with a solid test meal for clinically relevant, symptomatic oesophageal motility disorders: serial diagnostic study. Lancet Gastroenterol Hepatol 2017;2:654–61. 10.1016/S2468-1253(17)30148-6 [DOI] [PubMed] [Google Scholar]
- 109. Sanagapalli S, McGuire J, Leong RW, et al. The clinical relevance of manometric esophagogastric junction outflow obstruction can be determined using rapid drink challenge and solid swallows. Am J Gastroenterol 2021;116:280–8. 10.14309/ajg.0000000000000988 [DOI] [PubMed] [Google Scholar]
- 110. Fox MR, Sweis R, Yadlapati R, et al. Chicago classification version 4.0 © technical review: Update on standard high‐resolution manometry protocol for the assessment of esophageal motility. Neurogastroenterology & Motility 2021;33:e14120. 10.1111/nmo.14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Trudgill NJ, Sifrim D, Sweis R, et al. British Society of gastroenterology guidelines for oesophageal manometry and oesophageal reflux monitoring. Gut 2019;68:1731–50. 10.1136/gutjnl-2018-318115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Katzka DA, Smyrk TC, Alexander JA, et al. Accuracy and safety of the Cytosponge for assessing histologic activity in eosinophilic esophagitis: a two-center study. Am J Gastroenterol 2017;112:1538–44. 10.1038/ajg.2017.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Furuta GT, Kagalwalla AF, Lee JJ, et al. The oesophageal string test: a novel, minimally invasive method measures mucosal inflammation in eosinophilic oesophagitis. Gut 2013;62:1395–405. 10.1136/gutjnl-2012-303171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ackerman SJ, Kagalwalla AF, Hirano I, et al. One-Hour esophageal string test: a Nonendoscopic minimally invasive test that accurately detects disease activity in eosinophilic esophagitis. Am J Gastroenterol 2019;114:1614–25. 10.14309/ajg.0000000000000371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Safroneeva E, Coslovsky M, Kuehni CE, et al. Eosinophilic oesophagitis: relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther 2015;42:1000–10. 10.1111/apt.13370 [DOI] [PubMed] [Google Scholar]
- 116. Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 2016;150:e4. 10.1053/j.gastro.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Warners MJ, Hindryckx P, Levesque BG, et al. Systematic review: disease activity indices in eosinophilic esophagitis. Am J Gastroenterol 2017;112:1658–69. 10.1038/ajg.2017.363 [DOI] [PubMed] [Google Scholar]
- 118. Franciosi JP, Hommel KA, DeBrosse CW, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol 2011;11:126. 10.1186/1471-230X-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Martin LJ, Franciosi JP, Collins MH, et al. Pediatric eosinophilic esophagitis symptom scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J Allergy Clin Immunol 2015;135:1519–28. 10.1016/j.jaci.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kagalwalla AF, Wechsler JB, Amsden K, et al. Efficacy of a 4-Food elimination diet for children with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2017;15:e7. 10.1016/j.cgh.2017.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 1995;109:1503–12. 10.1016/0016-5085(95)90637-1 [DOI] [PubMed] [Google Scholar]
- 122. Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol 2006;4:1097–102. 10.1016/j.cgh.2006.05.026 [DOI] [PubMed] [Google Scholar]
- 123. Arias A, González-Cervera J, Tenias JM, et al. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 2014;146:1639–48. 10.1053/j.gastro.2014.02.006 [DOI] [PubMed] [Google Scholar]
- 124. Cotton CC, Erim D, Eluri S, et al. Cost utility analysis of topical steroids compared with dietary elimination for treatment of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2017;15:841–9. 10.1016/j.cgh.2016.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hoofien A, Dias JA, Malamisura M, et al. Pediatric eosinophilic esophagitis: results of the European retrospective pediatric eosinophilic esophagitis registry (RetroPEER). J Pediatr Gastroenterol Nutr 2018. 10.1097/MPG.0000000000002215 [DOI] [PubMed] [Google Scholar]
- 126. Molina-Infante J, Arias A, Barrio J, et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J Allergy Clin Immunol 2014;134:e1. 10.1016/j.jaci.2014.07.023 [DOI] [PubMed] [Google Scholar]
- 127. Warners MJ, Vlieg-Boerstra BJ, Verheij J, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Aliment Pharmacol Ther 2017;45:777–87. 10.1111/apt.13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Molina-Infante J, Arias Ángel, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: the 2-4-6 study. J Allergy Clin Immunol 2018;141:1365–72. 10.1016/j.jaci.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 129. Harris RF, Menard-Katcher C, Atkins D, et al. Psychosocial dysfunction in children and adolescents with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2013;57:500–5. 10.1097/MPG.0b013e31829ce5ad [DOI] [PubMed] [Google Scholar]
- 130. Cianferoni A, Shuker M, Brown-Whitehorn T, et al. Food avoidance strategies in eosinophilic oesophagitis. Clin Exp Allergy 2019;49:269-284. 10.1111/cea.13360 [DOI] [PubMed] [Google Scholar]
- 131. Fissinger A, Mages KC, Solomon AB. Vitamin deficiencies in pediatric eosinophilic esophagitis: a systematic review. Pediatr Allergy Immunol 2020;31:835–40. 10.1111/pai.13297 [DOI] [PubMed] [Google Scholar]
- 132. Mehta P, Furuta GT, Brennan T, et al. Nutritional state and feeding behaviors of children with eosinophilic esophagitis and gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr 2018;66:603–8. 10.1097/MPG.0000000000001741 [DOI] [PubMed] [Google Scholar]
- 133. Mukkada VA, Haas A, Maune NC, et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics 2010;126:e672–7. 10.1542/peds.2009-2227 [DOI] [PubMed] [Google Scholar]
- 134. Menard-Katcher C, Henry M, Furuta GT, et al. Significance of feeding dysfunction in eosinophilic esophagitis. World J Gastroenterol 2014;20:11019–22. 10.3748/wjg.v20.i31.11019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sova C, Feuling MB, Baumler M, et al. Systematic review of nutrient intake and growth in children with multiple IgE-mediated food allergies. Nutr Clin Pract 2013;28:669–75. 10.1177/0884533613505870 [DOI] [PubMed] [Google Scholar]
- 136. Isolauri E, Sütas Y, Salo MK, et al. Elimination diet in cow's milk allergy: risk for impaired growth in young children. J Pediatr 1998;132:1004–9. 10.1016/s0022-3476(98)70399-3 [DOI] [PubMed] [Google Scholar]
- 137. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract 2013;7:488–94. 10.4162/nrp.2013.7.6.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Hunter H, Pupinyte K, Wong T, et al. Multidisciplinary approach to the management of adult eosinophilic oesophagitis in the United Kingdom. Clin Exp Allergy 2018;48:1752–6. 10.1111/cea.13279 [DOI] [PubMed] [Google Scholar]
- 139. Groetch M, Venter C, Skypala I, et al. Dietary therapy and nutrition management of eosinophilic esophagitis: a work group report of the American Academy of allergy, asthma, and immunology. J Allergy Clin Immunol 2017;5:312–24. 10.1016/j.jaip.2016.12.026 [DOI] [PubMed] [Google Scholar]
- 140. Constantine G, Seth N, Chokshi N, et al. Combination steroid and test-based food elimination for eosinophilic esophagitis: a retrospective analysis. J Pediatr Gastroenterol Nutr 2017;64:933–8. 10.1097/MPG.0000000000001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Reed CC, Safta AM, Qasem S, et al. Combined and alternating topical steroids and food elimination diet for the treatment of eosinophilic esophagitis. Dig Dis Sci 2018;63:2381–8. 10.1007/s10620-018-4931-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Reed CC, Tappata M, Eluri S, et al. Combination therapy with elimination diet and corticosteroids is effective for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:2800–2. 10.1016/j.cgh.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hoofien A, Dias JA, Malamisura M, et al. Pediatric eosinophilic esophagitis: results of the European retrospective pediatric eosinophilic esophagitis registry (RetroPEER). J Pediatr Gastroenterol Nutr 2019;68:552–8. 10.1097/MPG.0000000000002215 [DOI] [PubMed] [Google Scholar]
- 144. Olson AA, Evans MD, Johansson MW, et al. Role of food and aeroallergen sensitization in eosinophilic esophagitis in adults. Ann Allergy Asthma Immunol 2016;117:e2. 10.1016/j.anai.2016.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Simon D, Marti H, Heer P, et al. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. J Allergy Clin Immunol 2005;115:1090–2. 10.1016/j.jaci.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 146. González-Cervera J, Arias Ángel, Redondo-González O, et al. Association between atopic manifestations and eosinophilic esophagitis: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2017;118:e2. 10.1016/j.anai.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 147. Clayton F, Fang JC, Gleich GJ, et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 2014;147:602–9. 10.1053/j.gastro.2014.05.036 [DOI] [PubMed] [Google Scholar]
- 148. Peterson K, Lin E, Saffari H, et al. Food-specific antibodies in oesophageal secretions: association with trigger foods in eosinophilic oesophagitis. Aliment Pharmacol Ther 2020;52:997–1007. 10.1111/apt.15879 [DOI] [PubMed] [Google Scholar]
- 149. Ramaswamy AT, No JS, Anderson L, et al. Esophageal IgE, IgG4, and mucosal eosinophilia in individuals with dysphagia. Int Forum Allergy Rhinol 2019;9:870–5. 10.1002/alr.22339 [DOI] [PubMed] [Google Scholar]
- 150. Rank MA, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA Institute and the joint Task force on allergy-immunology practice parameters. Ann. allergy, asthma Immunol Off. Publ. Am. Coll. Allergy, Asthma, Immunol 2020;124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol 2012;130:e5. 10.1016/j.jaci.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 152. Simon D, Cianferoni A, Spergel JM, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy 2016;71:611–20. 10.1111/all.12846 [DOI] [PubMed] [Google Scholar]
- 153. Peterson KA, Byrne KR, Vinson LA, et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am J Gastroenterol 2013;108:759–66. 10.1038/ajg.2012.468 [DOI] [PubMed] [Google Scholar]
- 154. Peterson KA, Boynton KK. Which patients with eosinophilic esophagitis (EoE) should receive elemental diets versus other therapies? Curr Gastroenterol Rep 2014;16:364. 10.1007/s11894-013-0364-y [DOI] [PubMed] [Google Scholar]
- 155. Lucendo AJ, Arias Ángel, González-Cervera J, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J Allergy Clin Immunol 2013;131:797–804. 10.1016/j.jaci.2012.12.664 [DOI] [PubMed] [Google Scholar]
- 156. Laserna-Mendieta EJ, Casabona S, Guagnozzi D, et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: results from the EoE connect registry. Aliment Pharmacol Ther 2020;52:798–807. 10.1111/apt.15957 [DOI] [PubMed] [Google Scholar]
- 157. Vieira GG, Ribeiro LBM, Truppel SK, et al. Endoscopic and histological characteristics in patients with eosinophilic esophagitis responsive and non-responsive to proton pump inhibitors. J Pediatr 2020;96:638–43. 10.1016/j.jped.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tomizawa Y, Melek J, Komaki Y, et al. Efficacy of pharmacologic therapy for eosinophilic esophagitis: a systematic review and network meta-analysis. J Clin Gastroenterol 2018;52:596–606. 10.1097/MCG.0000000000000878 [DOI] [PubMed] [Google Scholar]
- 159. Gutiérrez-Junquera C, Fernández-Fernández S, Cilleruelo ML, et al. Long-term treatment with proton pump inhibitors is effective in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2018;67:210–6. 10.1097/MPG.0000000000001952 [DOI] [PubMed] [Google Scholar]
- 160. Molina-Infante J, Rodriguez-Sanchez J, Martinek J, et al. Long-Term loss of response in proton pump Inhibitor-Responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and Rhinoconjunctivitis. Am J Gastroenterol 2015;110:1567–75. 10.1038/ajg.2015.314 [DOI] [PubMed] [Google Scholar]
- 161. Gómez-Torrijos E, García-Rodríguez R, Castro-Jiménez A, et al. The efficacy of step-down therapy in adult patients with proton pump inhibitor-responsive oesophageal eosinophilia. Aliment Pharmacol Ther 2016;43:534–40. 10.1111/apt.13496 [DOI] [PubMed] [Google Scholar]
- 162. Sawas T, Dhalla S, Sayyar M, et al. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther 2015;41:797–806. 10.1111/apt.13147 [DOI] [PubMed] [Google Scholar]
- 163. Alexander JA, Jung KW, Arora AS, et al. Swallowed fluticasone improves histologic but not symptomatic response of adults with eosinophilic esophagitis. Clinical Gastroenterology and Hepatology 2012;10:e1:742–9. 10.1016/j.cgh.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 164. Dellon ES, Sheikh A, Speck O, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 2012;143:e1. 10.1053/j.gastro.2012.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Dellon ES, Woosley JT, Arrington A, et al. Efficacy of budesonide vs fluticasone for initial treatment of eosinophilic esophagitis in a randomized controlled trial. Gastroenterology 2019;157:65–73. 10.1053/j.gastro.2019.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Oliva S, Rossetti D, Papoff P, et al. A 12-week maintenance therapy with a new prepared viscous budesonide in pediatric eosinophilic esophagitis. Dig Dis Sci 2019;64:1571–8. 10.1007/s10620-018-5449-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Straumann A, Lucendo AJ, Miehlke S, et al. Budesonide Orodispersible tablets maintain remission in a randomized, placebo-controlled trial of patients with eosinophilic esophagitis. Gastroenterology 2020;159:1672–85. 10.1053/j.gastro.2020.07.039 [DOI] [PubMed] [Google Scholar]
- 168. Straumann A, Conus S, Degen L, et al. Long-Term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clinical Gastroenterology and Hepatology 2011;9:e1:400–9. 10.1016/j.cgh.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 169. Dellon ES, Katzka DA, Collins MH, et al. Safety and efficacy of budesonide oral suspension maintenance therapy in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 2019;17:666–73. 10.1016/j.cgh.2018.05.051 [DOI] [PubMed] [Google Scholar]
- 170. Schaefer ET, Fitzgerald JF, Molleston JP, et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin Gastroenterol Hepatol 2008;6:165–73. 10.1016/j.cgh.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 171. Hoofien A, Rea F, Espinheira MdoC, et al. Systemic steroids have a role in treating esophageal strictures in pediatric eosinophilic esophagitis. Dig Liver Dis 2021;53:324–8. 10.1016/j.dld.2020.11.025 [DOI] [PubMed] [Google Scholar]
- 172. Netzer P, Gschossmann JM, Straumann A, et al. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur J Gastroenterol Hepatol 2007;19:865–9. 10.1097/MEG.0b013e32825a6ab4 [DOI] [PubMed] [Google Scholar]
- 173. Limketkai BN, Shah SC, Hirano I, et al. Epidemiology and implications of concurrent diagnosis of eosinophilic oesophagitis and IBD based on a prospective population-based analysis. Gut 2019;68:2152–60. 10.1136/gutjnl-2018-318074 [DOI] [PubMed] [Google Scholar]
- 174. Lamb CA, Kennedy NA, Raine T, et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Straumann A, Bussmann C, Conus S, et al. Anti-TNF-Alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J Allergy Clin Immunol 2008;122:425–7. 10.1016/j.jaci.2008.06.012 [DOI] [PubMed] [Google Scholar]
- 176. Kim HP, Reed CC, Herfarth HH, et al. Vedolizumab treatment may reduce steroid burden and improve histology in patients with eosinophilic gastroenteritis. Clin Gastroenterol Hepatol 2018;16:1992–4. 10.1016/j.cgh.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Nhu QM, Chiao H, Moawad FJ, et al. The Anti-α4β7 integrin therapeutic antibody for inflammatory bowel disease, Vedolizumab, ameliorates eosinophilic esophagitis: a novel clinical observation. Am J Gastroenterol 2018;113:1261–3. 10.1038/s41395-018-0145-1 [DOI] [PubMed] [Google Scholar]
- 178. Wang F-P, Tang X-J, Wei C-Q, et al. Dupilumab treatment in moderate-to-severe atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci 2018;90:190–8. 10.1016/j.jdermsci.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 179. Castro M, Rabe KF, Corren J, et al. Dupilumab improves lung function in patients with uncontrolled, moderate-to-severe asthma. ERJ Open Res 2020;6. 10.1183/23120541.00204-2019. [Epub ahead of print: 27 01 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of Dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020;158:111–22. 10.1053/j.gastro.2019.09.042 [DOI] [PubMed] [Google Scholar]
- 181. Hirano I, Collins MH, Assouline-Dayan Y, et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology 2019;156:592-603.e10. 10.1053/j.gastro.2018.10.051 [DOI] [PubMed] [Google Scholar]
- 182. Straumann A, Conus S, Grzonka P, et al. Anti-Interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 2010;59:21–30. 10.1136/gut.2009.178558 [DOI] [PubMed] [Google Scholar]
- 183. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016;388:2115–27. 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 184. Schneider A, Rubinstein A. BENRALIZUMAB intended for eosinophilic asthma leads to complete resolution of eosinophilic EOSPHAGITIS. Annals of Allergy, Asthma & Immunology 2018;121:S127. 10.1016/j.anai.2018.09.422 [DOI] [Google Scholar]
- 185. Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N Engl J Med 2019;380:1336–46. 10.1056/NEJMoa1812185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Silva FMdeCE, de Oliveira EE, Ambrósio MGE, et al. Disodium cromoglycate treatment reduces TH2 immune response and immunohistopathological features in a murine model of Eosinophilic Esophagitis. Int Immunopharmacol 2020;83:106422. 10.1016/j.intimp.2020.106422 [DOI] [PubMed] [Google Scholar]
- 187. Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol 2005;3:1198–206. 10.1016/s1542-3565(05)00885-2 [DOI] [PubMed] [Google Scholar]
- 188. Alexander JA, Ravi K, Enders FT, et al. Montelukast does not maintain symptom remission after topical steroid therapy for eosinophilic esophagitis. Clinical Gastroenterology and Hepatology 2017;15:e2:214–21. 10.1016/j.cgh.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 189. Menard-Katcher C, Furuta GT, Kramer RE. Dilation of pediatric eosinophilic esophagitis: adverse events and short-term outcomes. J Pediatr Gastroenterol Nutr 2017;64:701–6. 10.1097/MPG.0000000000001336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kasagi Y, Dods K, Wang JX, et al. Fibrostenotic eosinophilic esophagitis might reflect epithelial lysyl oxidase induction by fibroblast-derived TNF-α. J Allergy Clin Immunol 2019;144:171-182. 10.1016/j.jaci.2018.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther 2014;40:1333–40. 10.1111/apt.12977 [DOI] [PubMed] [Google Scholar]
- 192. Lin Z, Kahrilas PJ, Xiao Y, et al. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therap Adv Gastroenterol 2013;6:97–107. 10.1177/1756283X12470017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Desprez C, Roman S, Leroi AM, et al. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP ®) in the gastrointestinal tract: A systematic review. Neurogastroenterology & Motility 2020;32:e13980. 10.1111/nmo.13980 [DOI] [PubMed] [Google Scholar]
- 194. Eluri S, Tappata M, Huang KZ, et al. Distal esophagus is the most commonly involved site for strictures in patients with eosinophilic esophagitis. Dis Esophagus 2020;33. 10.1093/dote/doz088. [Epub ahead of print: 05 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Straumann A. The natural history and complications of eosinophilic esophagitis. Gastrointest Endosc Clin N Am 2008;18:99-118; ix. 10.1016/j.giec.2007.09.009 [DOI] [PubMed] [Google Scholar]
- 196. Dellon ES, Kim HP, Sperry SLW, et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79:e4. 10.1016/j.gie.2013.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Andreae DA, Hanna MG, Magid MS, et al. Swallowed fluticasone propionate is an effective long-term maintenance therapy for children with eosinophilic esophagitis. Am J Gastroenterol 2016;111:1187–97. 10.1038/ajg.2016.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Schupack DA, Ravi K, Geno DM, et al. Effect of maintenance therapy for eosinophilic esophagitis on need for recurrent dilation. Dig Dis Sci 2021;66:503–10. 10.1007/s10620-020-06192-8 [DOI] [PubMed] [Google Scholar]
- 199. Al-Hussaini A. Savary dilation is safe and effective treatment for esophageal narrowing related to pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr 2016;63:474–80. 10.1097/MPG.0000000000001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Chan LJ, Tan L, Dhaliwal J, et al. Treatment outcomes for eosinophilic esophagitis in children with esophageal atresia. Dis Esophagus 2016;29:563–71. 10.1111/dote.12368 [DOI] [PubMed] [Google Scholar]
- 201. Kavitt RT, Ates F, Slaughter JC, et al. Randomized controlled trial comparing esophageal dilation to NO dilation among adults with esophageal eosinophilia and dysphagia. Dis Esophagus 2016;29:983–91. 10.1111/dote.12398 [DOI] [PubMed] [Google Scholar]
- 202. Moawad FJ, Cheatham JG, DeZee KJ. Meta-analysis: the safety and efficacy of dilation in eosinophilic oesophagitis. Aliment Pharmacol Ther 2013;38:713–20. 10.1111/apt.12438 [DOI] [PubMed] [Google Scholar]
- 203. Sami SS, Haboubi HN, Ang Y, et al. UK guidelines on oesophageal dilatation in clinical practice. Gut 2018;67:1000–23. 10.1136/gutjnl-2017-315414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Schoepfer AM, Gschossmann J, Scheurer U, et al. Esophageal strictures in adult eosinophilic esophagitis: dilation is an effective and safe alternative after failure of topical corticosteroids. Endoscopy 2008;40:161–4. 10.1055/s-2007-995345 [DOI] [PubMed] [Google Scholar]
- 205. Bohm ME, Richter JE. Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2011;33:748–57. 10.1111/j.1365-2036.2011.04593.x [DOI] [PubMed] [Google Scholar]
- 206. Jung KW, Gundersen N, Kopacova J, et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc 2011;73:15–21. 10.1016/j.gie.2010.09.036 [DOI] [PubMed] [Google Scholar]
- 207. Jacobs JW, Spechler SJ, Spechler, SSpechler S. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig Dis Sci 2010;55:1512–5. 10.1007/s10620-010-1165-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Moawad FJ, Molina-Infante J, Lucendo AJ, et al. Systematic review with meta-analysis: endoscopic dilation is highly effective and safe in children and adults with eosinophilic oesophagitis. Aliment Pharmacol Ther 2017;46:96–105. 10.1111/apt.14123 [DOI] [PubMed] [Google Scholar]
- 209. Lucendo AJ, Molina-Infante J. Esophageal dilation in eosinophilic esophagitis: risks, benefits, and when to do it. Curr Opin Gastroenterol 2018;34:226–32. 10.1097/MOG.0000000000000442 [DOI] [PubMed] [Google Scholar]
- 210. Mark JA, Anderson BT, Pan Z, et al. Comparative analysis of adverse events after esophageal balloon and bougie dilations in children. J Pediatr Gastroenterol Nutr 2019;68:630–4. 10.1097/MPG.0000000000002237 [DOI] [PubMed] [Google Scholar]
- 211. Schoepfer AM, Henchoz S, Biedermann L, et al. Technical feasibility, clinical effectiveness, and safety of esophageal stricture dilation using a novel endoscopic attachment cap in adults with eosinophilic esophagitis. Gastrointest Endosc 2021;94:912–9. 10.1016/j.gie.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 212. Kim JP, Weingart G, Hiramoto B, et al. Clinical outcomes of adults with eosinophilic esophagitis with severe stricture. Gastrointest Endosc 2020;92:44–53. 10.1016/j.gie.2020.01.015 [DOI] [PubMed] [Google Scholar]
- 213. Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 2010;65:109–16. 10.1111/j.1398-9995.2009.02142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Katzka DA. Esophageal dilation as the primary treatment for eosinophilic esophagitis. Gastroenterol Hepatol 2019;15:320–2. [PMC free article] [PubMed] [Google Scholar]
- 215. Donnet C, Destombe S, Lachaux A, et al. Esophageal perforation in eosinophilic esophagitis: five cases in children. Endosc Int Open 2020;8 :E830–3. vol.. 10.1055/a-0914-2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. AIDEM HP, BAKER LD. The Boerhaave syndrome. JAMA 1964;187:57. 10.1001/jama.1964.03060140063021 [DOI] [PubMed] [Google Scholar]
- 217. Runge TM, Eluri S, Cotton CC, et al. Causes and outcomes of esophageal perforation in eosinophilic esophagitis. J Clin Gastroenterol 2017;51:805–13. 10.1097/MCG.0000000000000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Sengupta N, Tapper EB, Corban C, et al. The clinical predictors of aetiology and complications among 173 patients presenting to the emergency department with oesophageal food bolus impaction from 2004-2014. Aliment Pharmacol Ther 2015;42:91–8. 10.1111/apt.13237 [DOI] [PubMed] [Google Scholar]
- 219. Fianchi F, De Matteis G, Cianci R, et al. Acute intramucosal dissection in eosinophilic esophagitis. Clin J Gastroenterol 2019;12:525–9. 10.1007/s12328-019-00990-y [DOI] [PubMed] [Google Scholar]
- 220. Søreide JA, Viste A. Esophageal perforation: diagnostic work-up and clinical decision-making in the first 24 hours. Scand J Trauma Resusc Emerg Med 2011;19:66. 10.1186/1757-7241-19-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ben-David K, Behrns K, Hochwald S, et al. Esophageal perforation management using a multidisciplinary minimally invasive treatment algorithm. J Am Coll Surg 2014;218:768–74. 10.1016/j.jamcollsurg.2013.12.033 [DOI] [PubMed] [Google Scholar]
- 222. Ben-David K, Lopes J, Hochwald S, et al. Minimally invasive treatment of esophageal perforation using a multidisciplinary treatment algorithm: a case series. Endoscopy 2011;43:160–2. 10.1055/s-0030-1256094 [DOI] [PubMed] [Google Scholar]
- 223. Vermeulen BD, van der Leeden B, Ali JT, et al. Early diagnosis is associated with improved clinical outcomes in benign esophageal perforation: an individual patient data meta-analysis. Surg Endosc 2021;35:3492–505. 10.1007/s00464-020-07806-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Markar SR, Mackenzie H, Wiggins T, et al. Management and outcomes of esophageal perforation: a national study of 2,564 patients in England. Am J Gastroenterol 2015;110:1559–66. 10.1038/ajg.2015.304 [DOI] [PubMed] [Google Scholar]
- 225. Spaander MCW, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: European Society of gastrointestinal endoscopy (ESGE) clinical guideline. Endoscopy 2016;48:939–48. 10.1055/s-0042-114210 [DOI] [PubMed] [Google Scholar]
- 226. Kuppusamy MK, Felisky C, Kozarek RA, et al. Impact of endoscopic assessment and treatment on operative and non-operative management of acute oesophageal perforation. Br J Surg 2011;98:818–24. 10.1002/bjs.7437 [DOI] [PubMed] [Google Scholar]
- 227. Larsson H, Attwood S. Eosinophilic esophagitis (EoE); a disease that must not be neglected - implications of esophageal rupture and its management. BMC Gastroenterol 2020;20:185. 10.1186/s12876-020-01330-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Gubler C, Bauerfeind P. Self-expandable stents for benign esophageal leakages and perforations: long-term single-center experience. Scand J Gastroenterol 2014;49:23–9. 10.3109/00365521.2013.850735 [DOI] [PubMed] [Google Scholar]
- 229. Votto M, Castagnoli R, De Filippo M, et al. Behavioral issues and quality of life in children with eosinophilic esophagitis. Minerva Pediatr 2020;72:424–32. 10.23736/S0026-4946.20.05913-7 [DOI] [PubMed] [Google Scholar]
- 230. Reed CC, Corder SR, Kim E, et al. Psychiatric comorbidities and psychiatric medication use are highly prevalent in patients with eosinophilic esophagitis and associate with clinical presentation. Am J Gastroenterol 2020;115:853–8. 10.14309/ajg.0000000000000597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Aceves SS, King E, Collins MH, et al. Alignment of parent- and child-reported outcomes and histology in eosinophilic esophagitis across multiple CEGIR sites. J Allergy Clin Immunol 2018;142:e1:130–8. 10.1016/j.jaci.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Taft TH, Guadagnoli L, Edlynn E. Anxiety and depression in eosinophilic esophagitis: a scoping review and recommendations for future research. J Asthma Allergy 2019;12:389–99. 10.2147/JAA.S193045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Lynch MK, Avis KT, Dimmitt RA, et al. Topical review: eosinophilic esophagitis in children: implications for health-related quality of life and potential avenues for future research. J Pediatr Psychol 2015;40:727–32. 10.1093/jpepsy/jsv032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Dhar A, Maw F, Dallal HJ, et al. Side effects of drug treatments for gastro-oesophageal reflux disease: current controversies. Frontline Gastroenterol 2022;13:flgastro-2019-101386. 10.1136/flgastro-2019-101386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Moawad FJ, Dellon ES, Achem SR, et al. Effects of race and sex on features of eosinophilic esophagitis. Clin Gastroenterol Hepatol 2016;14:23–30. 10.1016/j.cgh.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 236. Vanoni S, Zeng C, Marella S, et al. Identification of anoctamin 1 (ANO1) as a key driver of esophageal epithelial proliferation in eosinophilic esophagitis. J Allergy Clin Immunol 2020;145:239-254.e2. 10.1016/j.jaci.2019.07.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Armbruster-Lee J, Cavender CP, Lieberman JA, et al. Understanding fibrosis in eosinophilic esophagitis: are we there yet? J Leukoc Biol 2018;104:31–40. 10.1002/JLB.5MR1017-395R [DOI] [PubMed] [Google Scholar]
- 238. Muir AB, Wang JX, Nakagawa H. Epithelial-Stromal crosstalk and fibrosis in eosinophilic esophagitis. J Gastroenterol 2019;54:10–18. 10.1007/s00535-018-1498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Manresa MC, Chiang AWT, Kurten RC, et al. Increased production of light by T cells in eosinophilic esophagitis promotes differentiation of esophageal fibroblasts toward an inflammatory phenotype. Gastroenterology 2020;159:1778–92. 10.1053/j.gastro.2020.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 2014;147:1255–66. 10.1053/j.gastro.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Franciosi JP, Hommel KA, Greenberg AB, et al. Development of the pediatric quality of life Inventory™ eosinophilic esophagitis module items: qualitative methods. BMC Gastroenterol 2012;12:135. 10.1186/1471-230X-12-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Franciosi JP, Hommel KA, Bendo CB, et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J Pediatr Gastroenterol Nutr 2013;57:57–66. 10.1097/MPG.0b013e31828f1fd2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Aceves S, Collins MH, Rothenberg ME, et al. Advancing patient care through the Consortium of eosinophilic gastrointestinal disease researchers (CEGIR). J Allergy Clin Immunol 2020;145:28–37. 10.1016/j.jaci.2019.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Wang R, Hirano I, Doerfler B, et al. Assessing adherence and barriers to long-term elimination diet therapy in adults with eosinophilic esophagitis. Dig Dis Sci 2018;63:1756–62. 10.1007/s10620-018-5045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Hirano I, et al. Efficacy of Dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2019. [DOI] [PubMed] [Google Scholar]
- 246. Gann PH, Deaton RJ, McMahon N, et al. An anti-IL-13 antibody reverses epithelial-mesenchymal transition biomarkers in eosinophilic esophagitis: phase 2 trial results. J Allergy Clin Immunol 2020;146:367–76. 10.1016/j.jaci.2020.03.045 [DOI] [PubMed] [Google Scholar]
- 247. Olivieri B, Tinazzi E, Caminati M, et al. Biologics for the treatment of allergic conditions: eosinophil disorders. Immunol Allergy Clin North Am 2020;40:649–65. 10.1016/j.iac.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 248. Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight 2019;4. 10.1172/jci.insight.126219. [Epub ahead of print: 03 10 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Chang JW, Saini SD, Mellinger JL, et al. Management of eosinophilic esophagitis is often discordant with guidelines and not patient-centered: results of a survey of Gastroenterologists. Dis Esophagus 2019;32. 10.1093/dote/doy133 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2022-327326supp003.pdf (385.2KB, pdf)
gutjnl-2022-327326supp001.xlsx (25.8KB, xlsx)
gutjnl-2022-327326supp002.pdf (531.1KB, pdf)