Abstract
Purpose
The present study investigated the long-lasting effect of prematurity, prenatal growth restriction, and associated factors on foveal and peripapillary choroidal thickness in adulthood.
Methods
The Gutenberg Prematurity Eye Study (GPES) is a retrospective cohort study with a prospective ophthalmologic examination in Germany. Foveal and peripapillary choroidal thicknesses were measured at different sectors using spectral-domain optical coherence tomography. Multivariable linear regression analyses were performed to determine associations among gestational age, birth weight percentile, retinopathy of prematurity (ROP) occurrence and treatment, and other perinatal factors, such as maternal smoking and others with foveal and global peripapillary choroidal thickness.
Results
A total of 735 eyes of 408 study participants were included (age = 28.4 ± 8.6 years, 229 women). Multivariable regression analyses revealed that foveal choroidal thinning was associated with maternal smoking during pregnancy (B = −38.1, 95% confidence interval [CI] = −65.5 to −10.7, P = 0.006), whereas other perinatal factors revealed no association. Global peripapillary choroidal thinning showed a trend to a lower birth weight percentile (B = 0.22, 95% CI = −0.01 to 0.45, P = 0.057). No correlation was observed between foveal and peripapillary choroidal thicknesses with visual acuity.
Conclusions
This study indicates that maternal cigarette smoking during pregnancy has adverse long-lasting effects on choroidal development in the fovea and low birth weight percentile as a surrogate marker for adverse perinatal growth might be linked with peripapillary choroidal thinning whereas prematurity showed no long-term effects.
Translational Relevance
Altered choroidal layer development caused by perinatal influence factors might be a risk factor for reduced visual function and predispose affected individuals to eye diseases in later life.
Keywords: birth weight, gestational age, retinopathy of prematurity, choroidal layer thickness
Introduction
Prematurity and fetal growth restriction affect ocular geometry in children,1–3 adolescents,4 and adults.5–9 Altered choroidal development has been reported in individuals born preterm and those with restricted fetal growth.10,11 Functions of the choroid include oxygen supply and nutrition of the outer retina, as well as regulation of eye growth, thus choroid alterations may result in lower visual functionality.11,12
The few studies evaluating the effects of prematurity and low birth weight on choroidal thickness (CT) at the posterior pole in childhood have yielded controversial findings, with some studies reporting no correlation between gestational age and subfoveal CT,13 whereas most studies found a an association between prematurity and/or birth weight and CT thinning in the macula.11,14–16 However, there is a lack of published data assessing the association of birth parameters with foveal and peripapillary choroidal thicknesses in adulthood. We previously reported that prematurity alone did not affect choroidal thickness in the peripapillary region, whereas fetal growth restriction was associated with choroid thinning10 in a cohort of individuals born preterm and term aged 4 to 10 years. However, it is unclear whether the effects of gestational age or birthweight have a long lasting impact on choroidal thickness in the foveal and peripapillary region in adulthood and whether retinopathy of prematurity (ROP) affects this association. This investigation analyzed foveal and peripapillary choroidal thickness in foveal and different peripapillary regions using spectral-domain optical coherence tomography (SD-OCT) in adulthood and assessed its relation to perinatal history.
Materials and Methods
Study Population
The Gutenberg Prematurity Eye Study (GPES) is a single-center cohort study at the University Medical Center of the Johannes Gutenberg University Mainz in Germany (UMCM). The participants included were individuals (i) born preterm or at term between 1969 and 2002 and (ii) were between 18 and 52 years of age at study enrollment, hence, was a retrospective cohort study with a prospective acquisition of follow-up data. For the GPES, every preterm newborn with a gestational age (GA) ≤ 32 weeks and every second randomly chosen preterm newborn with GA 33 to 36 weeks was contacted and invited to participate. From each month from 1969 to 2002, 6 (3 boys and 3 girls) randomly selected full-term subjects with a birth weight between the 10th and 90th percentile were also invited to serve as controls. Ophthalmic examinations were performed between 2019 and 2021, as reported earlier.7,17–21 The eligibility and effective recruitment flow diagram are shown in Supplementary Figure S1.
Written informed consent was obtained from all participants and the GPES complies with Good Clinical Practice (GCP), Good Epidemiological Practice (GEP), and the ethical principles of the Declaration of Helsinki. The study protocol and study documents were approved by the local ethics committee of the Medical Chamber of Rhineland-Palatinate, Germany (reference no. 2019-14161; original vote: 29.05.2019, latest update: 02.04.2020).
Assessment of Pre-, Peri-, and Postnatal History
Medical data from UMCM medical records were collected for each participant including GA (weeks), birth weight (kg), percentile of birth weight for the respective GA, ROP (yes), stage of ROP, treatment of ROP with laser or cryotherapy, history of intraventricular hemorrhage, bronchopulmonary dysplasia, necrotizing enterocolitis, maternal placental insufficiency, maternal smoking during pregnancy, maternal preeclampsia, maternal diabetes, perinatal adverse events, and breastfeeding. Birth weight percentiles were calculated according to Voigt et al.22
Categorization
For descriptive analyses, study participants were divided into the following groups: full-term participants with a GA ≥ 37 weeks (group 1, control group); preterm participants with a GA between 33 and 36 weeks without ROP (group 2); preterm participants with a GA between 29 and 32 weeks without ROP (group 3); preterm participants with a GA ≤ 28 weeks without ROP (group 4); and preterm participants with a GA ≤ 32 weeks, ROP without ROP treatment (group 5), and with ROP treatment (group 6). If only one eye of a participant had previous ROP, the other eye that did not have ROP was excluded from the analysis, as reported earlier.7,17–21
Ophthalmological Examination
During the GPES examination, objective refraction and distance-corrected visual acuity of both eyes (ARK-1s; NIDEK, Oculus, Wetzlar, Germany) were examined, intraocular pressure was measured with a non-contact tonometer (NT 2000; Nidek Co./Japan), and ocular biometry was performed (LenStar 900; Haag Streit, Köniz, Switzerland).7,17–21
SD-OCT Examination
The macula was imaged with SD-OCT using a block scan 15 × 15 degrees in EDI-modus and 7.7 mm corneal curvature. Using a research software tool of the Heidelberg Eye Explorer Software (HEYEX, SPX; Heidelberg Engineering, Heidelberg, Germany), automatic segmentation of the choroidal thickness of the macula was performed. These measurements were recorded in microns. Choroidal thickness calculation in the fovea and perifoveal region was conducted using an Early Treatment Diabetic Retinopathy Study (ETDRS) grid with 1 mm, 2 mm, and 3 mm distance circles from the fovea. The deepest foveal depression of the total retina was assumed to be the foveal center of the choroid. Foveal choroidal thickness was defined as choroidal thickness in the central 1 mm zone and central foveal thickness as choroidal thickness in the foveal center. Each scan was checked by a board-certified ophthalmologist for decentration or layer segmentation errors, and eyes with such errors were excluded or, if possible, manually corrected.
SD-OCT of the peripapillary region was performed with a peripapillary scan of 12 degrees diameter centered on the optic disc, with eye-tracking and a standard corneal curvature of 7.7 mm and ametropia of 0 dpt. An automated choroidal thickness measurement was not provided by the software, so the manual alignment of the choroidal interfaces was performed by a masked investigator in the peripapillary two-dimensional view. The inner choroidal interface delineation was placed on Bruch's membrane to discriminate between the retinal pigment epithelium and the choroid. The outer choroidal interface delineation was placed between the choroid and the sclera. The peripapillary choroidal thickness was calculated using the Heidelberg Eye Explorer Software (HEYEX version 6.13.3.0) global and for each sector: superonasal, nasal, inferonasal, inferotemporal, temporal, superotemporal, as reported earlier.10 Adjustment to ocular magnification was performed considering corneal curvature and spherical equivalent.23 All SD-OCT measurements were conducted with non-mydriatic pupils and each scan underwent quality control by an experienced investigator. If decentration of the peripapillary or foveal scan or layer segmentation errors were detected, the scans were excluded. Furthermore, only high-quality images with a high signal strength of > 15 dB (range 0 = no signal to 40 = excellent signal) were included in this study. Thus, the present study only includes high-quality images with ideal centration, a high signal strength, and accurate automated delineation. Overall, the SD-OCT measurements in the present study were conducted in congruence to the OSCAR-IB recommendations for retinal OCT quality assessment.24
Covariates
Factors that may have affected macular and/or peripapillary choroidal thickness were selected based on clinical plausibility and chart review. This included sex, age, spherical equivalent, GA, birth weight, birth weight percentile, ROP, ROP treatment, perinatal adverse events, maternal placental insufficiency, maternal smoking during pregnancy, maternal preeclampsia, and breastfeeding. Perinatal adverse events were defined in congruence to the German query for quality control of the neonatal clinics as the occurrence of intraventricular hemorrhage (at least grade 3 or parenchymal hemorrhage) and/or the occurrence of necrotizing enterocolitis, and/or moderate or severe bronchopulmonary dysplasia.25
Statistical Analysis
The main outcome measures were the choroidal thickness in the foveal center and peripapillary global sector. The peripapillary (superonasal, nasal, inferonasal, inferotemporal, temporal, and superotemporal) and perifoveal sectors were examined for sensitivity analysis. Absolute and relative frequencies were calculated for dichotomous parameters, with mean and standard deviation calculated for approximately normally distributed data, otherwise median and interquartile range. Linear regression analysis with general estimating equations (GEEs) were applied to assess associations and account for correlations between corresponding eyes. Multivariable regression analyses (model 1) were conducted between the choroidal thickness of the fovea and the global peripapillary choroidal thickness with the key exposure measures, such as gestational age (weeks), birth weight (kilogram), birth weight percentile, ROP (yes), ROP treatment (yes), perinatal adverse events (yes), preeclampsia (yes), placental insufficiency (yes), smoking during pregnancy (yes), and breastfeeding (yes) after adjusting for age (years), sex (female), and spherical equivalent (diopter). A sensitivity analysis was performed, including height (cm) at study examination. Spearman correlations were computed to assess the association of global peripapillary choroidal thickness and foveal choroidal thickness with distant corrected visual acuity in univariate analyses. This is an explorative study and no adjustment for multiple testing was conducted. Consequently, P values should be regarded as a continuous parameter reflecting the level of evidence and are therefore reported exactly. Calculations were performed using commercial software (IBM SPSS 20.0; SPSS, Inc., Chicago, IL, USA).
Results
Participant Characteristics
The present study included 735 eyes of 408 participants born preterm and full-term (age 28.4 +/− 8.6 years, 229 girls). A total of 241 eyes from 132 participants with a GA ≥ 37 weeks (group 1, control group), 250 eyes from 134 participants with a GA between 33 and 36 weeks without ROP (group 2), 144 eyes from 79 participants with a GA between 29 and 32 weeks without ROP (group 3), 29 eyes of 16 participants with GA ≤ 28 weeks without ROP (group 4), 57 eyes of 38 participants with GA between 24 and 32 weeks with ROP without treatment (group 5), and 14 eyes of 9 participants with GA between 24 and 32 and with postnatal treatment for ROP (group 6) were examined. Of the group treated for ROP, 5 participants (9 eyes) were treated with laser coagulation and 4 participants (5 eyes) were treated with cryocoagulation. The proportion of effective recruitment for each group is shown in Supplementary Figure S1. A total of 42 participants were excluded because SD-OCT measurement was not possible, scan quality was poor, and other parameters were missing to adjust for ocular enlargement (Table 1). Participants with successful choroidal thickness measurement of the present analyses had comparable age (28.2 ± 8.4 years with versus 30.1 ± 9.4 years without, P = 0.1) and sex (55.8% girls with versus 55.8% girls without, P = 1.0) but had slightly higher gestational age (34.4 ± 4.3 GA with versus 31.7 ± 4.9 GA without P < 0.001) compared to participants without successful choroidal thickness measurement.
Table 1.
Characteristics of the Study Sample (n = 408/735) of the Gutenberg Prematurity Eye Study (GPES) Stratified by Study Groups
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational Age | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| No ROP | No ROP | No ROP | ROP Without Treatment | ROP With Treatment | ||
| Participants (n)/eyes (n) | 132/241 | 134/250 | 79/144 | 16/29 | 38/57 | 9/14 |
| Sex, girls (%) | 76 (57.6%) | 80 (59.7%) | 40 (50.6%) | 8 (50.0%) | 21 (55.3%) | 4 (44.4%) |
| Age, y | 29.5 ± 9.1 | 29.4 ± 9.2 | 27.8 ± 7.9 | 23.9 ± 7.7 | 24.0 ± 4.0 | 26.0 ± 4.6 |
| Birth weight, g | 3422 ± 397 | 2079 ± 462 | 1556 ± 319 | 949 ± 184 | 1066 ± 384 | 814 ± 198 |
| Birth weight < 1500 g (yes) | 0 (0%) | 12 (9.0%) | 32 (40.5%) | 16 (100%) | 33 (86.8%) | 9 (100%) |
| Birth weight < 1000 g (yes) | 0 (0%) | 0 (0%) | 5 (6.3%) | 9 (56.3%) | 17 (44.7%) | 7 (77.8%) |
| Birth weight percentile | 48.5 ± 21.7 | 25.6 ± 24.3 | 44.9 ± 24.1 | 47.4 ± 22.6 | 38.3 ± 27.9 | 30.9 ± 26.1 |
| Gestational age (wk) | 39.3 ± 1.3 | 34.3 ± 1.0 | 30.7 ± 1.2 | 26.5 ± 1.6 | 28.0 ± 2.1 | 26.6 ± 2.2 |
| (Min–max) | (37–43) | (33–36) | (29–32) | (23–28) | (24–32) | (24–29) |
| ROP stage (1/2/3) | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 27/28/2 | 0/3/11 |
| Perinatal adverse events (yes)a | 1 (0.8%) | 3 (2.2%) | 3 (3.8%) | 2 (12.5%) | 11 (28.9%) | 7 (77.8%) |
| Intraventricular hemorrhage (yes)b | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (11.1%) |
| Bronchopulmonary dyplasy (yes)c | 1 (0.8%) | 0 (0%) | 2 (2.5%) | 0 (0%) | 9 (23.7%) | 3 (33.3%) |
| Necrotizing entercolitis (yes)d | 0 (0%) | 3 (2.2%) | 1 (1.3%) | 2 (12.5%) | 3 (7.9%) | 5 (55.6%) |
| Preeclampsia (yes) | 11 (8.3%) | 24 (17.9%) | 9 (11.4%) | 3 (18.8%) | 7 (18.4%) | 2 (22.2%) |
| Placental insufficiency (yes) | 2 (1.5%) | 16 (11.9%) | 2 (2.5%) | 0 (0%) | 2 (5.3%) | 0 (0%) |
| HELLP syndrome | 0 (0%) | 6 (4.5%) | 1 (1.3%) | 0 (0%) | 2 (5.3%) | 0 (0%) |
| Maternal smoking (yes)e | 6 (4.5%) | 8 (6.0%) | 7 (8.9%) | 1 (6.3%) | 4 (10.5%) | 1 (11.1%) |
| Gestational diabetes (yes) | 1 (0.8%) | 7 (5.2%) | 1 (1.3%) | 1 (6.3%) | 1 (2.6%) | 0 (0%) |
| Breastfeeding (yes) | 74 (56.1%) | 73 (54.5%) | 40 (50.6%) | 8 (50.0%) | 17 (44.7%) | 4 (44.4%) |
| Ocular parameters | ||||||
| Visual acuity (logMAR) OD | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.2) |
| Visual acuity (logMAR) OS | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) | 0.2 (0.0; 0.5) |
| Spherical equivalent (diopter) OD | −0.93 ± 2.14 | −1.11 ± 1.91 | −0.65 ± 2.30 | 0.01 ± 1.48 | −1.13 ± 2.07 | −1.48 ± 3.47 |
| Spherical equivalent (diopter) OS | −1.29 ± 2.01 | −0.41 ± 2.53 | −0.65 ± 1.31 | −1.47 ± 4.68 | −0.60 ± 1.84 | 2.64 ± 4.6 |
| Axial length (mm) OD | 23.7 ± 1.2 | 23.7 ± 1.1 | 23.4 ± 1.0 | 23.0 ± 1.0 | 23.1 ± 1.2 | 22.2 ± 2.0 |
| Axial length (mm) OS | 23.7 ± 1.3 | 23.5 ± 1.1 | 23.5 ± 1.0 | 23.3 ± 1.2 | 23.4 ± 1.0 | 20.8 ± 1.1 |
| Intraocular pressure (mm Hg) OD | 15.3 ± 2.8 | 14.6 ± 3.0 | 14.8 ± 3.1 | 17.0 ± 3.7 | 15.3 ± 4.4 | 17.0 ± 3.3 |
| Intraocular pressure (mm Hg) OS | 16.0 ± 2.6 | 14.2 ± 2.9 | 16.3 ± 3.7 | 12.7 ± 0.6 | 15.6 ± 4.1 | 17.2 ± 4.4 |
G, gram; mm, millimeter; GA, gestational age; ROP, retinopathy of prematurity; y, years; n, number; OD, right eye; OS, left eye; HELLP syndrome, hemolysis, elevated liver enzymes, and low platelets.
aPerinatal adverse events were defined as the occurrence of intraventricular hemorrhage, b(at least grade 3 or parenchymal hemorrhage), cand/or the occurrence of necrotizing enterocolitis and/or, dmoderate or severe bronchopulmonary dysplasia, eMaternal smoking during pregnancy.
Descriptive Choroidal Thickness Parameters
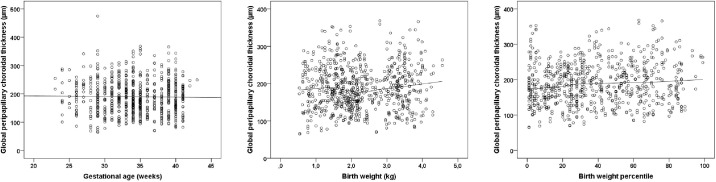
The choroidal thickness in the fovea and peripapillary choroidal thickness of the global measure as well as of the six sectors are presented in Table 2 and Supplementary Table S1. In extreme preterm individuals of groups four and five, descriptively increased and in ROP treated participants a descriptively decreased foveal choroidal thickness was observed. Peripapillary choroidal thickness showed no clear trend toward an alteration in choroidal thickness in relation to the gestational age. A tendency to a smaller peripapillary choroidal thickness in participants with a low birth weight percentile is shown in Figure 1.
Table 2.
Peripapillary and Foveal Choroidal Thickness (µm) in the Gutenberg Prematurity Eye Study (n = 408) Stratified by Gestational Age and ROP Status
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| Gestational Age | GA ≥ 37 | GA 33–36 | GA 29–32 | GA ≤ 28 | GA ≤ 32 | GA ≤ 32 |
| No ROP | No ROP | No ROP | ROP Without Treatment | ROP With Treatment | ||
| Participants/eyes (n) | 132/241 | 134/250 | 79/144 | 16/29 | 38/57 | 9/14 |
| Choroidal thickness macula (µm) | ||||||
| Right eye | ||||||
| Foveal center | 294.0 ± 68.1 | 282.9 ± 63.5 | 285.3 ± 67.7 | 339.6 ±45.3c | 316.9± 79.8 | 250.7 ± 53.2 |
| Fovea | 293.2 ± 67.4 | 283.1 ± 61.7 | 288.0 ± 67.4 | 339.0 ±41.4c | 313.4±78.4 | 247.7 ± 49.3 |
| Inner quadrantsa | 289.6 ± 64.2 | 281.7 ± 58.8 | 288.5 ± 65.5 | 338.3 ±41.2c | 308.5± 73.3 | 242.2 ± 50.4 |
| Outer quadrantsb | 284.3 ± 61.9 | 278.4 ± 55.9 | 284.9 ± 61.3 | 334.4± 40.1c | 294.6± 67.6 | 238.2 ± 42.7c |
| Left eye | ||||||
| Foveal center | 291.0 ± 67.0 | 285.1 ± 52.9 | 292.1 ± 54.8 | 266.3 ±64.7 | 310.5 ± 66.2 | 242.0 ± 52.1c |
| Fovea | 292.5 ± 66.2 | 285.7 ± 51.0 | 292.1 ± 51.2 | 268.0 ±59.1 | 310.2 ± 64.3 | 241.2 ± 51.3c |
| Inner quadrantsa | 290.6 ± 62.2 | 285.8 ± 48.2 | 290.6 ± 47.5 | 270.3± 53.1 | 307.9 ± 64.2 | 228.3 ± 49.3c |
| Outer quadrantsb | 284.4 ± 59.1 | 282.6 ± 45.3 | 284.8 ± 45.5 | 266.5± 53.0 | 302.3 ± 64.1 | 226.3 ± 48.5c |
| Peripapillary choroidal thickness (µm) | ||||||
| Right eye | ||||||
| Global | 189 ± 60 | 184 ± 55 | 192 ± 58 | 223 ± 68 | 186 ± 60 | 187 ± 71 |
| Superotemporal | 201 ± 67 | 194 ± 58 | 204 ± 63 | 239 ± 74 | 200 ± 64 | 201 ± 80 |
| Temporal | 198 ± 74 | 195 ± 66 | 203 ± 68 | 241 ± 65c | 205 ± 67 | 198 ± 79 |
| Inferotemporal | 165 ± 60 | 164 ± 60 | 172 ± 60 | 194 ± 67 | 169 ± 66 | 172 ± 60 |
| Inferonasal | 167 ± 56 | 162 ± 55 | 173 ± 59 | 196 ± 65 | 161 ± 60 | 171 ± 64 |
| Nasal | 193 ± 62 | 187 ± 58 | 194 ± 59 | 220 ± 84 | 180 ± 64 | 181 ± 73 |
| Superonasal | 193 ± 60 | 187 ± 55 | 194 ± 61 | 233 ± 88 | 184 ± 68 | 191 ± 84 |
| Left eye | ||||||
| Global | 193 ± 58 | 187 ± 57 | 195 ± 58 | 201 ± 64 | 178 ± 52 | 187 ± 144 |
| Superotemporal | 203 ± 60 | 199 ± 62 | 209 ± 68 | 216 ± 62 | 187 ± 61 | 202 ± 147 |
| Temporal | 202 ± 67 | 196 ± 64 | 200 ± 69 | 211 ± 67 | 194 ± 59 | 202 ± 163 |
| Inferotemporal | 169 ± 58 | 164 ± 57 | 171 ± 62 | 172 ± 63 | 157 ± 54 | 180 ± 149 |
| Inferonasal | 168 ± 57 | 162 ± 60 | 170 ± 56 | 175 ± 60 | 144 ± 51c | 156 ± 116 |
| Nasal | 199 ± 63 | 193 ± 68 | 201 ± 58 | 206 ± 74 | 183 ± 58 | 182 ± 133 |
| Superonasal | 200 ± 63 | 198 ± 66 | 207 ± 65 | 211 ± 81 | 185 ± 59 | 195 ± 150 |
GA, gestational age; ROP, retinopathy of prematurity; µm, micrometer; OD, right eye; OS, left eye.
Linear regression analysis was applied to compare the different groups with the full-term control group (reference).
aMean value of the inner quadrants, bmean value of the outer quadrants, cStatistical difference (P < 0.05) compared to the full-term control group.
Figure 1.
Relationship of global peripapillary choroidal thickness with (A) gestational age, (B) birth weight, and (C) birth weight percentile in the Gutenberg Prematurity Eye Study (n = 408).
Linear Regression Analyses
The results of the multivariable linear regression analyses including adjustment for age, sex, and spherical equivalent are presented in Table 3. Maternal smoking during pregnancy was associated with decreased central foveal choroidal thickness (B = −38.1, 95% confidence interval [CI] = −65.5 to −10.7, P = 0.006), whereas the other perinatal parameters showed no association (Fig. 2). An increase in birth weight percentiles showed a trend with a thicker global peripapillary choroidal layer thickness (B = 0.22, 95% CI = −0.01 to 0.45, P = 0.057). Moreover, there was a trend toward a thinner choroidal thickness among participants with a maternal history of preeclampsia (B = −0.65, 95% CI = −1.36 to 0.05, P = 0.068). No correlation was observed between distant corrected visual acuity with central foveal (Spearman correlation coefficient rho = −0.03, P = 0.4) and peripapillary choroidal thickness (Spearman correlation coefficient rho = −0.05, P = 0.15).
Table 3.
Association Analysis of Global Peripapillary and Central Subfoveal Choroidal Layer Thickness With Different Perinatal Parameters (n = 408) for the Sample of the Gutenberg Prematurity Eye Study
| Model 1 | ||
|---|---|---|
| B [95% CI] | P Value | |
| Central subfoveal choroidal thickness [µm] | ||
| Gestational age (wk) | −0.03 (−1.88 to 1.81) | 0.9 |
| Birth weight (kilogram) | 1.12 (−7.32 to 9.56) | 0.8 |
| Birth weight percentile | 0.15 (−0.17 to 0.46) | 0.4 |
| ROP (yes) | 9.4 (−17.7 to 36.5) | 0.5 |
| ROP treatment (yes) | −37.1 (−104.4 to 30.3) | 0.3 |
| Perinatal adverse events (yes) | 7.45 (−35.4 to 50.5) | 0.7 |
| Preeclampsia (yes) | 5.37 (−13.2 to 23.9) | 0.6 |
| Placental insufficiency | −6.61 (−38.6 to 25.4) | 0.7 |
| Smoking during pregnancy (yes) | −38.1 (−65.5 to −10.7) | 0.006 |
| Breastfeeding (yes) | 5.63 (−9.38 to 20.6) | 0.1 |
| Global peripapillary choroidal thickness [µm] | ||
| Gestational age (wk) | 0.38 (−0.89 to 1.64) | 0.6 |
| Birth weight (kilogram) | 3.93 (−2.11 to 9.97) | 0.2 |
| Birth weight percentile | 0.22 (−0.01 to 0.45) | 0.057 |
| ROP (yes) | −13.96 (−33.02 to 5.11) | 0.15 |
| ROP treatment (yes) | −14.97 (−70.44 to 40.5) | 0.6 |
| Perinatal adverse events (yes) | −1.99 (−27.85 to 23.86) | 0.9 |
| Preeclampsia (yes) | −0.65 (−1.36 to 0.05) | 0.068 |
| Placental insufficiency | 6.55 (−21.68 to 34.77) | 0.7 |
| Smoking during pregnancy (yes) | 13.45 (−8.06 to 34.96) | 0.2 |
| Breastfeeding (yes) | 8.52 (−2.9 to 19.93) | 0.14 |
B, beta; CI, confidence interval; µm, micrometer.
Linear regression analysis using generalized estimating equations to control for correlations between right and left eyes. Model 1 tested the association between each parameter and choroidal thickness after adjusting for age (years), sex (girls), and spherical equivalent (diopter).
Figure 2.
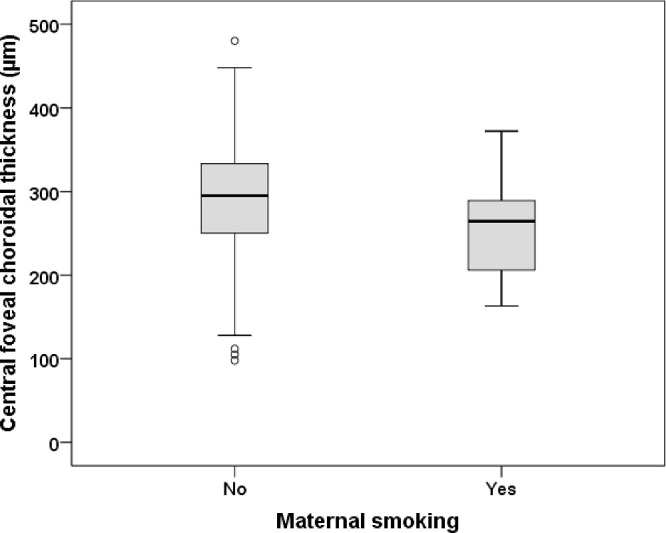
Central foveal choroidal layer thickness in participants with and without history of maternal smoking during pregnancy. Participants with maternal smoking during pregnancy revealed a thinner central foveal choroid.
Sensitivity Analysis
There was a significant association between increased adult height at study examination and peripapillary choroidal thickness (B = 0.93, 95% CI = 0.14 to 1.72, P = 0.021) but not with foveal choroidal thickness (B = 0.50, 95% CI = −0.55 to 1.55, P = 0.35).
Discussion
The present study provides new data in a cohort of 408 individuals born pre- and full-term that maternal cigarette smoking during pregnancy has adverse long-lasting effects on choroidal development in the fovea and low birth weight percentile as a surrogate marker for adverse perinatal growth might be linked to peripapillary choroidal thickness decrease, whereas prematurity and the postnatal occurrence of ROP and treatment showed no long-term effects.
No published data exist on peripapillary choroidal thickness for adults born preterm, while data on choroidal thickness in preterm children, mostly assessing the macular region, are limited and controversial, with prematurity previously linked to a thinning of the subfoveal and temporal choroidea in children.15,26 However, Acar et al. did not find an association between subfoveal choroidal thickness and prematurity,13 whereas the Copenhagen Child Cohort 2000 Eye study reported an association between being small for gestational age and subfoveal choroidal thinning.14 Several other studies found an association between prematurity and/or birth weight and CT thinning in the macula.11,14–16
These studies did not recruit adult subjects, nor did they assess the choroidal thickness of the peripapillary area. Our group has previously reported an associated reduction of peripapillary choroidal thickness in children born small for gestational age,10 but up-to-now, it is unclear whether this persists until adulthood. Interestingly, there was no association between peripapillary choroidal thickness and central foveal choroidal thickness with low gestational age, low birth weight, ROP manifestation, ROP treatment, and perinatal adverse events in the present study, thus prematurity alone does not explain alterations in foveal and peripapillary choroidal thickness. The development of peripapillary choroid in individuals born preterm and at term with perinatal growth restriction may in part be influenced by postnatal hyperoxia and subsequent choroidal involution.27 Furthermore, the optic nerve and its choriocapillaris form in a very early sensitive phase during embryonal development which might be influenced by adverse fetal growth during organ development probably leading to altered choroidal thickness in later life lasting until adulthood.28 Furthermore, the smaller central foveal choroidal thickness in adults with maternal history of cigarette smoking might be a first hint that toxic effects of maternal tobacco consumption may contribute to choroidal involution in fetal development in the macula.
There are inconclusive results in terms of the association between visual function and choroidal thickness thinning on the posterior pole in childhood. Whereas Wu and colleagues found a relationship between visual acuity and choroidal thickness in the fovea,11 others observed no correlation between foveal choroidal thickness and visual function.15 We found no association of visual acuity with peripapillary and central foveal choroidal thickness in the present study.
Strengths and Limitations
The current study findings are derived from a single study center in a hospital-based cohort, leading to limitations in terms of generalizability. Furthermore, there may be a bias in contact data availability and consent to participate but the effective recruitment efficacy proportion was relatively high (see Supplementary Fig. S1). In addition, individuals born extremely preterm are particularly at an increased risk for reduced visual acuity and nystagmus, thus they cannot adequately fixate during SD-OCT imaging. Another limitation is the small number of participants in the group with extreme prematurity without ROP (n = 16) and in the group with ROP requiring treatment (n = 9). This has to be considered when interpreting study results. Furthermore, the number of participants with bronchopulmonary disease was rather small and thus potentially limiting the associations between perinatal adverse events respective bronchopulmonary disease requiring oxygen support and choroidal thickness measurement.
A strength of the present analysis is the ocular examination, including SD-OCT imaging of the oldest and largest cohort of adults born preterm with and without ROP in the medical literature. Further strengths are the review of the participants’ medical charts and their mothers for a detailed overview of the perinatal history enabling statistical models, including several perinatal parameters potentially affecting choroidal thickness in the long term. Strict standardized operating procedures to ensure high data quality and masked investigators for participants’ birth characteristics were incorporated to reduce the risk of bias.
Conclusion
Prematurity and ROP do not affect choroidal thickness in the peripapillary and foveal region in adulthood, although there was a tendency for peripapillary choroidal thinning with a decrease in birth weight percentile, indicating that perinatal growth restriction might lead to life-long thinner peripapillary choroidal thickness. Furthermore, maternal smoking during pregnancy seems to have long-lasting effects leading to foveal choroidal thinning in adulthood.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Funding (Stufe I) of the University Medical Center of Johannes Gutenberg-University Mainz. The present study was supported by the Ernst- und Berta-Grimmke Stiftung and the Else Kröner-Fresenius-Stiftung. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Schuster AK holds the professorship for ophthalmic healthcare research endowed by “Stiftung Auge” and sponsored by “Deutsche Ophthalmologische Gesellschaft” and “Berufsverband der Augenärzte Deutschlands e.V.”
Authors' contributions: Conceived and designed the study: A.F. and AKS. Analyzed the data: A.F., K.S., S.D.G., S.G., E.M., M.S.U., and A.K.S. Wrote the first draft of the manuscript: A.F., K.S., and S.D.G. Critically revised the manuscript: A.F., K.S., S.D.G., S.G., E.M., M.S.U., B.S., N.P., and A.K.S. All authors read and approved the final manuscript. This study contains parts of the thesis of Kai Schulze.
Access to data, Responsibility, and Analysis: A.F. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analyses were performed by A.F.
The analysis presents clinical data of a cohort. This project constitutes a major scientific effort with high methodological standards and detailed guidelines for analysis and publication to ensure scientific analyses are on the highest level. Therefore, data are not made available for the scientific community outside the established and controlled workflows and algorithms. To meet the general idea of verification and reproducibility of scientific findings, we offer access to data at the local database upon request at any time. Interested researchers make their requests to the coordinating PI of the Gutenberg Prematurity Eye Study (GPES) (Achim Fieß; achim.fiess@unimedizin-mainz.de). More detailed contact information is available at the homepages of the UM (www.unimedizin-mainz.de).
The whole study team thanks all participants who took part in this study, and the whole GPES, which includes an enthusiastic team to explore perinatal factors on long-term eye development.
Disclosure: N. Pfeiffer, Novartis (F), Ivantis (F), Santen (F), Thea (F), Boehringer Ingelheim Deutschland GmbH & Co. KG (F), Alcon (F), Sanoculis (F); A.K. Schuster, Allergan (F), Bayer (F), Heidelberg Engineering (F), PlusOptix (F), Norvartis (F); A. Fieß, None; K. Schulze, None; S.D. Grabitz, None; S. Gißler, None; E. Mildenberger, None; M.S. Urschitz, None; B. Stoffelns, None
References
- 1. Fieß A, Kolb-Keerl R, Knuf M, et al.. Axial Length and Anterior Segment Alterations in Former Preterm Infants and Full-Term Neonates Analyzed With Scheimpflug Imaging. Cornea. 2017; 36: 821–827. [DOI] [PubMed] [Google Scholar]
- 2. Fieß A, Schuster AK, Kolb-Keerl R, et al.. Corneal Aberrations in Former Preterm Infants: Results From The Wiesbaden Prematurity Study. Invest Ophthalmol Vis Sci. 2017; 58: 6374–6378. [DOI] [PubMed] [Google Scholar]
- 3. Fieß A, Janz J, Schuster AK, et al.. Macular morphology in former preterm and full-term infants aged 4 to 10 years. Graefes Arch Clin Exp Ophthalmol = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2017; 255: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 4. Fieß A, Schuster AK, Pfeiffer N, Nickels S.. Association of birth weight with corneal power in early adolescence: Results from the National Health and Nutrition Examination Survey (NHANES) 1999-2008. PLoS One. 2017; 12: e0186723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fieß A, Schuster AK, Nickels S, et al.. Association of Low Birth Weight With Altered Corneal Geometry and Axial Length in Adulthood in the German Gutenberg Health Study. JAMA Ophthalmol. 2019; 137: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fieß A, Nickels S, Schulz A, et al.. The relationship of ocular geometry with refractive error in normal and low birth weight adults. J Optom. 2020; 14: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fieß A, Nauen H, Mildenberger E, et al.. Ocular geometry in adults born extremely, very and moderately preterm with and without retinopathy of prematurity: results from the Gutenberg Prematurity Eye Study. Br J Ophthalmol, https://doi.org/10.1136/bjopthalmol-2021-320907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fieß A, Nickels S, Urschitz MS, et al.. Association of Birth Weight with Peripapillary Retinal Nerve Fiber Layer Thickness in Adulthood—Results from a Population-Based Study. Invest Ophthalmol Vis Sci. 2020; 61: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fieß A, Wagner FM, Urschitz MS, et al.. Association of Birth Weight With Foveolar Thickness in Adulthood: Results From a Population-Based Study. Invest Ophthalmol Vis Sci. 2021; 62: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fieß A, Christian L, Kolb-Keerl R, et al.. Peripapillary Choroidal Thickness in Former Preterm and Full-Term Infants Aged From 4 to 10 Years. Invest Ophthalmol Vis Sci. 2016; 57: 6548–6553. [DOI] [PubMed] [Google Scholar]
- 11. Wu WC, Shih CP, Wang NK, et al.. Choroidal thickness in patients with a history of retinopathy of prematurity. JAMA Ophthalmol. 2013; 131: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 12. Nickla DL, Wallman J.. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acar DE, Acar U, Tunay ZO, Arman A, Goksuluk D.. Retinal choroidal and retinal nerve fiber layer thickness in former preterm and full-term infants aged 4 to 8 years. Intl Ophthalmol. 2021; 41: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 14. Li XQ, Munkholm A, Larsen M, Munch IC.. Choroidal thickness in relation to birth parameters in 11- to 12-year-old children: the Copenhagen Child Cohort 2000 Eye Study. Invest Ophthalmol Vis Sci. 2015; 56: 617–624. [DOI] [PubMed] [Google Scholar]
- 15. Anderson MF, Ramasamy B, Lythgoe DT, Clark D.. Choroidal thickness in regressed retinopathy of prematurity. Eye. 2014; 28: 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tiryaki Demir S, Bas EK, Karapapak M, et al.. Effect of Prematurity on Foveal Development in Early School-Age Children. Am J Ophthalmol. 2020; 219: 177–185. [DOI] [PubMed] [Google Scholar]
- 17. Fieß A, Gißler S, Mildenberger E, et al.. Anterior Chamber Angle in Adults Born Extremely, Very, and Moderately Preterm with and without Retinopathy of Prematurity-Results of the Gutenberg Prematurity Eye Study. Children (Basel, Switzerland). 2022; 9: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fieß A, Gißler S, Mildenberger E, et al.. Optic nerve head morphology in adults born extreme, very and moderate preterm with and without retinopathy of prematurity: Results from the Gutenberg Prematurity Eye Study. Am J Ophthalmol. 4, 10.1016/j.apo.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 19. Fieß A, Fauer A, Mildenberger E, et al.. Refractive error, accommodation and lens opacification in adults born preterm and full-term: Results from the Gutenberg Prematurity Eye Study (GPES). Acta Ophthalmol, 10.1111/aos.15116. [DOI] [PubMed] [Google Scholar]
- 20. Fieß A, Gißler S, Fauer A, et al.. Short report on retinal vessel metrics and arterial blood pressure in adult individuals born preterm with and without retinopathy of prematurity: results from the Gutenberg Prematurity Eye Study. Acta Ophthalmologica, 10.1111/aos.15132. [DOI] [PubMed] [Google Scholar]
- 21. Fieß A, Hufschmidt-Merizian C, Gißler S, et al.. Dry Eye Parameters and Lid Geometry in Adults Born Extremely, Very, and Moderately Preterm with and without ROP: Results from the Gutenberg Prematurity Eye Study. J Clin Med. 2022; 11: 2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voigt M, Rochow N, Schneider KT, et al.. New percentile values for the anthropometric dimensions of singleton neonates: analysis of perinatal survey data of 2007-2011 from all 16 states of Germany. Zeitschrift fur Geburtshilfe und Neonatologie. 2014; 218: 210–217. [DOI] [PubMed] [Google Scholar]
- 23. Garway-Heath DF, Rudnicka AR, Lowe T, Foster PJ, Fitzke FW, Hitchings RA.. Measurement of optic disc size: equivalence of methods to correct for ocular magnification. Br J Ophthalmol. 1998; 82: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tewarie P, Balk L, Costello F, et al.. The OSCAR-IB Consensus Criteria for Retinal OCT Quality Assessment. PLoS One. 2012; 7: e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. IQTIG - Institut für Qualitätssicherung und Transparenz im Gesundheitswesen; Geburtshilfe - Bundesauswertung zum Erfassungsjahr 2017 - Qualitätsindikatoren; online abrufbar unter: “ https://iqtig.org/downloads/auswertung/2017/16n1gebh/QSKH_16n1-GEBH_2017_BUAW_V02_2018-08-01.pdf.” Accessed February 8, 2019.
- 26. Park KA, Oh SY.. Analysis of spectral-domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Sci. 2012; 53: 7201–7207. [DOI] [PubMed] [Google Scholar]
- 27. Shao Z, Dorfman AL, Seshadri S, et al.. Choroidal involution is a key component of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 6238–6248. [DOI] [PubMed] [Google Scholar]
- 28. Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Intl J Develop Biol. 2004; 48: 1045–1058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.