Abstract
Background
Person-centred care (PCC) is being internationally recognised as a critical attribute of high-quality healthcare. The International Alliance of Patients Organisations defines PCC as care that is focused and organised around people, rather than disease. Focusing on delivery, we aimed to review and evaluate the evidence from interventions that aimed to deliver PCC for people with serious physical illness and identify models of PCC interventions.
Methods
Systematic review of literature using Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We searched AMED, CINAHL, Cochrane Library, Embase, Medline, PsycINFO, using the following key concepts: patient/person-centred care, family centred care, family based care, individualised care, holistic care, serious illness, chronic illness, long-term conditions from inception to April 2022. Due to heterogeneity of interventions and populations studied, narrative synthesis was conducted. Study quality was appraised using the Joanna Briggs checklist.
Results
We screened n=6156 papers. Seventy-two papers (reporting n=55 different studies) were retained in the review. Most of these studies (n=47) were randomised controlled trials. Our search yielded two main types of interventions: (1) studies with self-management components and (2) technology-based interventions. We synthesised findings across these two models:
Self-management component: the interventions consisted of training of patients and/or caregivers or staff. Some studies reported that interventions had effect in reduction hospital admissions, improving quality of life and reducing costs of care.
Technology-based interventions: consisted of mobile phone, mobile app, tablet/computer and video. Although some interventions showed improvements for self-efficacy, hospitalisations and length of stay, quality of life did not improve across most studies.
Discussion
PCC interventions using self-management have some effects in reducing costs of care and improving quality of life. Technology-based interventions improves self-efficacy but has no effect on quality of life. However, very few studies used self-management and technology approaches. Further work is needed to identify how self-management and technology approaches can be used to manage serious illness.
PROSPERO registration number
CRD42018108302.
Keywords: health policy, quality in health care, clinical trials, health economics
Strengths and limitations of this study.
A study provides a systematic review of the evidence on the impact of person-centred interventions for serious physical illness in terms of outcomes and costs.
We used robust procedures for systematic reviewing and quality assessment of the studies included, in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines.
Most of the studies identified and included were conducted in high-income countries.
We conducted a narrative synthesis due to heterogeneity of the studies included (different disease population, different outcome measures and different trial end points).
Most of the studies included did not state the theoretical framework underpinning the person-centred interventions; however, many studies that reported the theoretical framework used the University of Gothenburg Centre for person-centred care of person-centred care and were conducted in Sweden across various clinical settings.
Introduction
WHO guidance emphasises person-centredness as a core component of healthcare professionals’ skills and quality healthcare.1 Integrated, person-centred care (PCC) is essential to achieving Universal Health Coverage (UHC).2 3 The core elements of PCC in health policy, medicine and nursing have been described as: patient participation and involvement, patient relationship with the healthcare professionals and context where care is delivered.4 The International Alliance of Patients’ Organisations defines PCC as ‘focused and organised around people, rather than disease’.5 PCC views individuals, families and communities as participants in health systems responsive to their needs.6
PCC aims to give meaningful assessment and equal weight to a patient’s subjective understanding of their illness, including their needs, concerns and expectations. This occurs, alongside a biomedical diagnosis; PCC also promote their equal participation in treatment decision-making and empowers them to take greater control of their own health and management of their condition.7
Our first systematic review identified and appraised the empirical evidence underpinning conceptualisations of ‘person-centredness’ for serious illness.8 Serious illness, as defined in that review, includes conditions that carry a high degree of clinical uncertainty, may require high care dependency because of decreased function, but may not be advanced.9 The review concluded that PCC (through valuing the social needs of patients, promoting quality of life and reform of health structures) will improve patients’ experience of interaction with healthcare systems.8 The review also concluded that primary data are needed that investigate the meaning and practice of PCC in a diverse diagnostic groups and settings.8
Re-engineering health systems to deliver PCC has particular relevance to low-income and middle-income countries (LMICs).6 10 Serious health-related suffering places a huge burden on health systems, with the greatest burden in LMICs. Projections from WHO mortality data estimate that LMICs face the largest proportional increase, largely due to ageing (155% increase in people with serious health-related suffering in the last year of life by 2060 to 5.14 million people).11 In such contexts, serious illness also places huge psychological, social, economic, physical and spiritual burdens on patients and (largely female) family caregivers.12–14 It carries a high risk of mortality, negatively impacts quality of life and daily function and is burdensome in symptoms, treatments and/or caregiver stress.15
PCC has great potential for patients, families, staff and the healthcare system in terms of engagement, enablement, management of symptoms and reduction in re-referrals, reducing readmission, frequent visits to primary care and/or emergency visits.16 Identification, refinement, adaptation and implementation of effective PCC interventions may thus help to achieve the WHO and UHC goals. However, no review to date has aimed to identify and synthesise the evidence for the outcomes and cost of PCC across serious physical illness. We aimed to review the evidence (in terms of outcomes and costs) for interventions that aim to deliver PCC to, or enhance person-centredness of care for, adults with serious physical illness.
Methods
Design
Systematic review of peer-reviewed literature drawing on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, with quality appraisal using the Joanna Briggs Institute Critical Appraisal checklist, and narrative synthesis of findings. A full protocol is registered with PROSPERO, CRD42018108302.17
Objectives
The objectives of this review were to (i) identify models of PCC interventions for adults with serious illness and how these were delivered; (ii) determine which outcomes have been measured as end points; (iii) appraise intervention effectiveness in terms of outcomes and costs, using narrative synthesis and (iv) evaluate the quality of the evidence.
Search strategy
The following databases were searched in January 2020: AMED, Assian, CINAHL, Cochrane Library, Embase, Medline, PsycINFO, Scopus and Web of Science. Key journals and reference lists from included studies and relevant review articles were hand searched. We conducted a search rerun limiting it from 2020 to April 2022 (online supplemental file 1).
bmjopen-2021-054386supp001.pdf (956.6KB, pdf)
The search strategy (table 1) was developed in consultation with an information specialist. We used the following key concepts, drawing on our prior review of the concepts and primary data underpinning PCC8: person/patient-centred care, family centred care, family based care, individualised care, holistic care. Data bases were searched from inception.
Table 1.
Search strategy
| Search strategy number | Key concepts | Key words |
| 1 | Patient centred Family centred Person centred Individualised Holistic |
Patient-centered care or patient-centred care or client-centred care or client-centered care or client-focused care or person-centred care or person-centered care or person-focused care or family-centred care or family-focused care or family-centered care or individuali?ed or holistic care or holistic health |
| 2 | Serious illness Chronic illness Long-term illness |
Chronic diseases or serious illness or chronic illness or long term conditions or long term illness |
Reference lists of identified papers and previous systematic reviews on PCC were hand searched.
Subject headings and word truncations were entered according to requirements of each database to map all potential keywords. Group 1 concepts were combined using the ‘OR’ function. Likewise group 2 concepts were combined using OR function. Finally, search strategies 1 and 2 were intersected using the ‘AND’ function.
Eligibility criteria
The inclusion and exclusion criteria are summarised in table 2.
Table 2.
Inclusion and exclusion criteria
| Inclusion | Exclusion | |
| Participants | All serious physical illness as defined by Kelly et al 2014; 2016: serious illness is a health condition that carries a high risk of mortality AND either negatively impacts a person’s daily function or quality of life, OR excessively strains their caregivers. Caregivers of patients with serious physical illness defined above. Healthcare professionals (doctors, nurses, social workers, etc) caring for patients with serious physical illness. |
Patients with conditions considered risk factors to develop serious illness such as hypertension. |
| Interventions | Any interventions delivered using a person-centred, or client-centred, or patient-centred, or family centred approach such as involving patients in decision-making about their care, setting goals and plans, patient being involved managing their own disease, interventions focused on the whole person, holistic approach. Interventions delivered in any format that is focused on the needs of the patients. | Any interventions delivered without patient involvement or decision making about their care or helping them take actions to support themselves. |
| Studies and comparator | Published intervention studies. Written in English language only. Evaluations using a comparator. The comparison group should either be usual care/standard care, or a comparator intervention. |
Unpublished studies, studies not written in English language, conference proceedings, conference abstracts, non-randomised trials. No comparison group. |
| Outcomes | Patient and family caregiver self-report outcomes, for example:
Formal and informal health service use. Costs of services. |
Outcomes not related to person-centred care (outcomes not focusing on physical, psychological social and spiritual aspects of care). |
Selection of studies, data collection and management
We report the search strategy process using the PRISMA flow chart.18 All references identified by the search strategy were exported to Endnote software and deduplicated. One reviewer (KBN) independently appraised all titles and abstracts against the inclusion and exclusion criteria. If the title and abstract was obviously irrelevant, the reference was excluded at this stage. Full-text retained references were obtained and appraised against inclusion and exclusion criteria, and if the decision was unclear this was discussed with a second reviewer (AC) and if necessary adjudicated by a third (RH).
Data extraction
KBN and AC extracted study data using methods described in the Cochrane handbook for systematic reviews of interventional studies.19 A standardised data extraction form was used to ensure consistency in the review.20 KBN extracted n=46 papers and AC extracted n=26 papers, then both authors peer-reviewed data extraction. Any queries were resolved through discussion. RH reviewed the final data extraction.
The following variables were extracted: authors, year of publication, aims and objectives, setting and country, study design, selection of participants, sample characteristics, randomisation procedures, blinding of participants and outcome assessors, assessment of outcomes and measures used, description of the intervention and comparison group, intervention delivery, sample size, data analysis, loss to follow-up, findings for outcomes and costs and study conclusions (online supplemental file 2).
bmjopen-2021-054386supp002.pdf (713.5KB, pdf)
Assessment of methodological quality of the studies
We applied the Joanna Briggs Institute Critical Appraisal checklist for randomised and non-randomised trials to assess methodological quality of the studies.21 These are summarised in online supplemental file 3. This was conducted at individual study level. AC and KBN assessed each study independently, and thereafter discussed critical appraisal. Discrepancies in the assessment of quality between AC and KBN were resolved by discussion, and RH checked the critical appraisals of the papers.
bmjopen-2021-054386supp003.pdf (94.7KB, pdf)
Synthesis of the evidence
Due to heterogeneity of the studies, interventions, participants and outcomes, a meta-synthesis was not conducted. We performed narrative synthesis to synthesise the findings of the different studies using the Guidance on the Conduct of Narrative Synthesis in Systematic Reviews, which consists of four elements: (1) the role of theory in evidence synthesis, (2) developing a preliminary synthesis, (3) exploring relationships within and between studies and (4) assessing the robustness of the synthesis.22
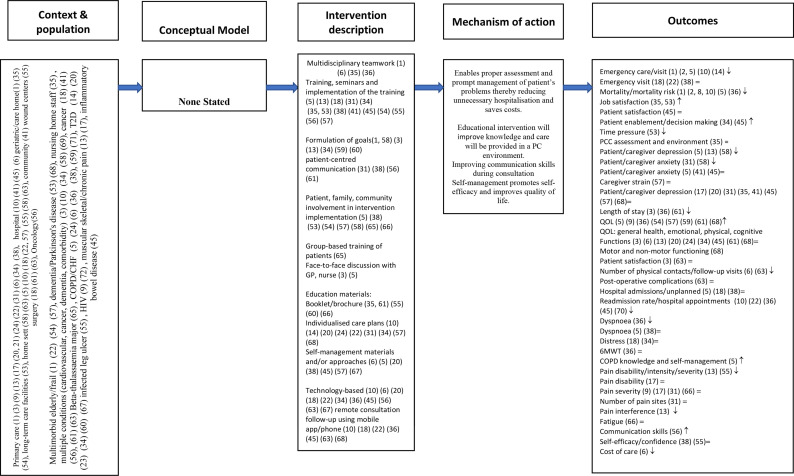
We developed two logic models (figures 1 and 2) to summarise the context, study population, to describe the intervention components, mechanism of action and outcomes. Figure 1 contains studies which reported a theory or conceptual framework which informed the development of the intervention. Figure 2 reports studies which did not state a theory or conceptual framework of the intervention.
Figure 1.
Logic model for interventions with a theoretical model. HbA1c, haemoglobin A1c; PCC, person-centred care; QOL, quality of life.
Figure 2.
Logic model for interventions without a theoretic model. 6MWT, six min walk test; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; GP, general practitioner; PCC, person-centred care; QOL, quality of life; T2D, type 2 diabetes.
A preliminary synthesis was undertaken in form of a thematic analysis involving listing and presenting results in tabular form. The results of the included studies were summarised in a narrative synthesis within a framework (participants, study aims, intervention description, usual care description, outcomes and measures used as presented in online supplemental file 2). For each study, the effects of the intervention on the outcomes tested is provided.
We explored relationships in the data, for example, similar study design use (randomised controlled trial (RCT) vs non-RCT), similar methods of randomisation, similar intervention components and mode of delivery and similar outcomes. We then made conclusions based on the robustness of the synthesis and the quality of evidence.
Patient and public involvement
Patient and public involvement was not conducted as part of this review.
Results
The PRISMA flow diagram (figure 3) presents the results of the search strategy. After deduplication, we screened n=5302 papers (title, abstract) and n=95 papers were retained for full-text screening. Of these, n=23 were excluded (reasons are reported in the flow chart) and n=72 papers (reporting 55 different studies) were retained in the review.
Figure 3.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Characteristics of the included studies
The n=56 studies included were conducted in 17 countries, the majority were high-income countries (n=13/17). Studies were conducted predominantly in Sweden n=16, the USA n=12, Canada n=4, Germany n=4, Australia n=3, Hong Kong=3, the UK=3 and Spain n=2. One study was conducted in each of the following countries: Brasil, Denmark, Iran, Kenya, The Netherlands, New Zealand, Norway, Singapore and Thailand. A further study was multicountry, conducted in Canada, Australia and the USA. Table 3 summarises number of studies conducted in each country.
Table 3.
Studies and countries
| Country | Number of studies | References |
| Sweden | 16 with 31 references/papers | 23–26 28–31 45–53 56 60 66 74–78 83 89 92 95 98 99 |
| USA | 12 with 13 references/papers | 27 32 33 35–37 61 62 70 79 82 90 91 |
| Canada | 4 | 38 67 71 90 |
| Germany | 4 | 39 68 84 85 |
| Australia | 3 with 4 references/papers | 34 69 72 90 |
| Hong Kong | 3 | 57 63 88 |
| UK | 3 | 40 41 58 |
| Spain | 2 | 42 64 |
| Brasil | 1 | 81 |
| Denmark | 1 | 54 |
| Iran | 1 | 86 |
| Kenya | 1 | 80 |
| The Netherlands | 1 | 43 |
| New Zealand | 1 | 65 |
| Norway | 1 | 73 |
| Singapore | 1 | 87 |
| Thailand | 1 | 59 |
| Australia, Canada and USA | 1 | 90 |
Study designs
Of the n=55 included studies, n=47 were RCTs, pre-test and post-test experimental/controlled before and after design,23–28 quasi-experimental study designs,29–32 a comparative study33 and a geographically controlled study.34 Of the n=47 RCTs, n=11 were clustered trials.35–44
Diagnostic groups
The interventions addressed the following diagnostic groups: n=12 heart failure,24 45–55 n=9 type 2 diabetes (T2D),34 37 38 41 56–59 n=8 chronic obstructive pulmonary disease (COPD),43 48 60–65 n=5 cancer,29 66–69 n=6 multimorbidity,27 39 70–73 n=3 fibromyalgia,42 74 75 n=3 rheumatoid arthritis,76–78 n=2 HIV,79 80 n=1 back pain,81 n=1 inflammatory bowel disease (IBD),40 n=1 osteoarthritis,82 n=1 stroke,83 n=1 chronic pain,35 n=1 dementia,84 n=1 Parkinson’s disease85 and n=1 beta-thalassaemia major.86
Intervention target and delivery
The interventions were nurse-led,33 36 61 65 69 76 80 84 87 nurse and physiotherapist-led,48 nurse, physician and social worker-led.27 45 71 73
The targets of the interventions were patient and caregiver dyads36 59 64 88 or delivered to both patients and healthcare professionals35 38 40 42 43 56 62 71 in T2D,38 56 chronic pain/fibromyalgia,35 42 COPD,43 62 IBD40 and multimorbidity71 populations. The interventions were technology-based involving a tablet computer or mobile phone37 38 40 57 63 64 69 87 or delivered to professionals such as doctors, nurses, social workers38 41 47 50 68 working with patients with heart failure,47 50 T2D38 41 and cancer.68.
Intervention components and delivery
Interventions delivered to healthcare professionals (nurses, doctors, physiotherapists) consisted of training, mentorship and support through lecturers, seminars and/or workshops in the philosophy and delivery of PCC,23 32 33 35 38 40 41 43 47–49 55 56 58 60 65 68 71 76 80 83 84 89 90 for example, clinical consultations using person-centred approach, person-centred communication and patient-centred self-management approach.29 37 40 41 56 60 64 65 68 70 71 Healthcare professionals then implemented what they learnt as they provided care to the patients and/or families.
Interventions delivered to patients and/or caregivers consisted of information provision, education and training.29–31 35 36 54 57–59 61–64 78 81 82 86 88 The interventions were either individualised and delivered face-to-face56 62 63 or delivered in groups.56 61 81 Educational materials, information leaflets, booklets, brochures were provided to participants.23 29 33 41 57 58 Some interventions delivered to patients focused on developing or creating a health plan. Participants identified or set aims or goals with targets to achieve and patients identified resources and tools to achieve the targets. Healthcare professionals worked with patients to achieve the targets and care was provided in line with patient needs and wants and what matters to them.24–28 30–32 34 35 38–40 45 47 48 55 60 62 65 66 70 72–74 77 87 The health plan was reviewed and revised when necessary.
Interventions were delivered either in nursing homes,23 primary care/outpatient care,35 38 39 62–64 66 71–73 76 77 80 surgical departments,29–31 67 69 inpatient facilities24–26 28 49 83 89 91 or in home and/or community settings.27 32 45 46 59 61 64 70 74 79 83 85 87
Some interventions involved using mobile technology,27 40 59 60 62 63 66 69 87 mobile app67 to contact patients at home. In some studies, patients in the intervention arm used either mobile-based or web-based eHealth tool pre-installed or downloaded it to use on their own mobile53 or a tablet computer to self-monitor blood glucose and blood pressure,57 or a web-based patient decision aid to populate their cardiometabolic and psychosocial profiles and general care priorities38 or to complete self-assessments using a computer touch screen and to develop a self-management action plan.37
Risk of bias studies included in the review
The majority of the studies (n=42) stated the method of randomisation, although this was not clearly stated in n=13 studies.34–37 40 42 45 54 58 64 65 67 83 Twenty-eight studies achieved allocation concealment, however n=19 did not clearly state allocation concealment.27 33 34 36 38 40 42 45 47 54 58 64 65 70 82 83 86 88 91 Blinding of participants was reported in only three studies.35 59 72 Blinding of outcome assessors was reported in n=21 studies,23 35 36 38 42 48 54 59 61 63 70 72 75–77 81 82 86–88 90 two studies stated that patients self-completed outcomes by post or through a web-based survey,60 64 while n=20 studies did not clearly state if outcome assessors were blinded. With respect to follow-up data collection, n=34 studies retained at least 80% participants to the final point of data collection. In n=19 studies, details were lacking regarding what constitutes usual care.23 24 27 34 36 39 42 46 48 54 64 65 69 77 79 84 86–88 The following studies included all participants including those who withdraw from the study in data analysis.40 42 47 48 59 62 63 68 71 86 92 93
Outcomes assessed
For patient outcomes quality of life was reported in n=22 studies.27 34 35 37–41 43 45 46 48 49 59 61–65 71 80 85 These studies were conducted in COPD,43 48 61–65 T2D,34 37 38 41 59 heart failure,45 46 chronically ill elderly,27 39 HIV,80 acute respiratory syndrome,49 chronic pain,35 Parkinson’s disease,85 IBD40 and multimorbidity71 populations.
General symptom burden was reported in n=4 studies in heart failure, chronically ill elderly, COPD and cancer.27 46 62 90 Fatigue symptom was reported in n=4 studies among patients with rheumatoid arthritis, COPD, stroke, chronic illnesses (elderly populations).48 70 77 94 Dyspnoea symptom was reported in n=3 COPD studies,48 61 63 while only one study reported data on sleep disturbance.70
Pain outcomes (severity/intensity, interference and disability) were reported in nine studies,33 35 42 70 78 80–82 95 among patients with chronic inflammatory arthritis,78 82 95 chronic pain, low back pain, infected chronic ulcers,35 81 HIV,80 multiple chronic diseases70 and fibromyalgia.42 Nine studies reported data on communication and satisfaction with treatment.35 39–41 48 67 78 83 95
Self-management and related outcomes were reported in the following studies: T2D self-management,59 COPD self-management and comorbidity,61 enablement,40 patient confidence in managing coronary heart disease and obtaining rheumatology care,33 47 95 self-efficacy,47 48 59 62 70–72 change from admission to discharge in the number of basic activities of daily living (ADLs) that the patient could perform independently,91 performance in activities,48 95 patient-reported health status and change in health activities27 71 and health education impact.72
The main psychosocial outcomes and concerns reported were psychiatric morbidity,80 psychological disturbance,42 95 concerns and well-being,41 anxiety and depression/mood,35 37 40 48 61 70 81 90 motor function,85 primary emotions,40 distress38 69–71 and decisional conflict.38
Caregiver outcomes assessed were depressive symptoms, caregiver strain, caregiver productivity loss,36 caregiver quality of life32 88 and caregiver burden.83 88 Other caregiver outcomes were informal care that is percentage-reported providing assistance with personal ADLs,83 participation in everyday occupations and life satisfaction.83
Healthcare professional outcomes included job strain,84 transition to palliative care, general communication, involvement of significant others,68 general practitioner (GP)’s knowledge about medication taken by the patient,39 and intention to engage in interprofessional shared decision making.38
Data on costs and healthcare utilisation
Six studies reported data on costs of healthcare utilisation,24 45 49 64 78 91 and four on number of hospital appointments.27 40 65 96 Two studies reported data on hospital admissions,27 65 and three studies reported length of hospital stay.62 91 96 Seven studies reported data on unplanned readmissions, emergency room attendance27 34 64 69 87 93 96 and four studies reported healthcare utilisation,27 39 72 93 and medications count (change in number of medications taken by the patient).39
Data on clinical outcomes
Clinical outcomes assessed were systolic and diastolic blood pressure,41 56 57 fasting blood sugar, haemoglobin A1c (HbA1c),37 56–59 body mass index, haemoglobin,41 56 lung function forced expiratory volume in 1 s/forced vital capacity ratio, exercise capacity,63 total cholesterol to high-density lipoprotein cholesterol ratio,37 serum ferritin, iron level, total iron binding capacity86 and mortality.61 63 87 93
Synthesis of the findings
We synthesised the findings using methods of narrative synthesis in systematic reviews.97 A narrative synthesis is presented based on the model which informed the intervention, interventions elements/components, mechanism of action, study population, study design (RCT or non-RCT) and outcomes.
Theoretical model/framework used by the study
The majority of the studies (n=34) did not report which theory or model informed the design or delivery of the interventions.27 32–43 54 57 58 63–65 67–69 72 73 79 81 82 84–88 90 91 One study was informed by the theory of Hernandez,56 three studies were developed and designed based on Bandura’s self-efficacy theory48 59 70 and another study used the person-centred palliative care model, Six S: self-image, self-determination, social relationships, symptom control, synthesis and surrender.45 46 One study reported the chronic care model and person-centred clinical method.71 PCC according to the University of Gothenburg Centre for Person-centred Care (GPCC) informed most of the studies conducted in Sweden.24–26 28–31 39 47 49–51 55 60 66 74–77 83 89 98 99
Mechanism of action of the interventions
For the GPCC model which involved three main parameters (initiation of partnership between the patient/caregiver and healthcare professional, implementing the partnership and documenting/safeguarding the partnership). This model was applied across different settings and populations. It also involved both patients and healthcare professionals in developing and designing the intervention and implementation.
PCC requires ongoing systematic engagement between the patient and healthcare professionals. Furthermore, it requires to be adapted to each patient population (cancer, HIV, COPD, T2D, etc) and context (primary care, outpatient, residential homes, emergency care, hospital, rehabilitation, etc). Care plans, goals of care discussed and revised as necessary continuously. Communication is also an important component in the GPCC model. Communication offered by the GPCC model gives patients (eg, inpatient setting) information and confidence about care processes and self-management of their own problems and concerns. This leads to understanding of the discharge processes and readiness and eagerness to return home which promotes self-efficacy. For the theory of Hernandez, self-efficacy and all other studies which did not state the theoretical framework, their mechanism of action were similar with the GPCC because they either had a self-management component or self-efficacy and were aimed at empowering the patient or caregiver or improving communication between the patient and the healthcare professional.
Interventions comprising a self-management component
Fifteen RCTs consisted of a self-management intervention or component. These were conducted in COPD,48 61 63 T2D,56 58 59 62 64 elderly with chronic conditions,27 70 72 cancer,66 IBD,40 multimorbidity36 71 populations. All the self-management interventions were educational and consisted of training of patients and/or caregivers27 36 48 59 63 64 66 70 72 or both healthcare professionals and patients/caregivers.36 40 56 58 Educational sessions were either group-based27 36 40 56 58 59 or individualised/face-to-face.61 63 Four of the 15 studies examined effects of the intervention on hospital admissions.27 61 63 64 Three studies showed positive benefits of self-management interventions in reducing hospital admissions. One of these four studies assessed mortality,61 another one length of stay in the hospital63 while one study assessed unplanned visits to the hospital.64 All studies reported positive benefits of the intervention in reducing mortality, length of hospital stay and unplanned visits. Six of the 15 studies assessed quality of life outcomes.36 40 59 61 63 66 In three studies, quality of life was assessed using the St George’s Respiratory Questionnaire36 61 63 and the results were significant. One study used the health-related quality of life measure and the results were non-significant, but significant on specific problems such as swallowing, social eating and feeding.66 Three studies reported non-significant results and assessed quality of life using the IBD questionnaire,40 the Thai Version short-form Health Survey59 and the Chronic Respiratory Disease Questionnaire.62 Hospital Anxiety and Depression Scale was used in three studies40 48 61 but only one reported significant findings61 and two reported non-significant findings.40 48 Self-efficacy was assessed in six studies48 59 62 70–72 with only one study reporting significant results.59 Knowledge on self-management was reported in two studies, T2D59 and COPD61 populations, with both studies reporting significant differences between the intervention and control groups.59 61
Technology-based interventions
Thirteen studies used technology. These were conducted among patients with T2D,37 38 57 59 cancer,66 67 69 COPD,60 63 64 chronic disease among elderly27 and IBD.40 Two of these studies were informed by the GPCC model60 66 and one was informed by Bandura’s model.59 The rest were not informed by a theoretical model. Most of these technology-based intervention studies used a telephone-based intervention.27 59 60 63 66 69 One study used a mobile app,67 web-based,38 four used tablet or computer technology37 38 57 64 and three used a video.38 40 The mechanism of action was similar across all these technology-based interventions. Patients were communicating using the phone or mobile app or tablet to ask for help if they have problems and concerns and healthcare professionals acted accordingly. This meant patient were involved in taking care of themselves and making decisions.
The outcomes however varied across these studies. Self-efficacy was examined in two studies,59 60 with different population (COPD60 and T2D59) and they used different measures to assess self-efficacy, both studies reported significant improvement in self-efficacy. Quality of life was examined in five studies37 38 40 64 66 and they all used different measures. Only one study reported significant benefits of the intervention.66 Hospitalisations/Rehospitalisations, length of stay, unplanned visits were reported in four studies.27 60 63 64 All studies reported positive benefits of technology in reducing hospitalisations, length of stay and unplanned visits. Three of these studies were in COPD population,60 63 64 one in T2D population38 and another one study in the elderly population.27 Two studies reported data on knowledge of management of T2D.57 59 One study recruited participants with T2D and hypertension.57
However, only one study found that knowledge of T2D management was statistically significant between the intervention and control groups.59
One study reported data on patient assessment of chronic illness and found statistically significant differences between web-based decision aid intervention and usual care.38
Synthesis based on study design
Of the n=55 included studies, n=6 studies (n=10 papers) were non-RCT.23–26 28–32 73 Participants in these studies were elderly people with multimorbidity,73 total hip replacement,30 31 patients with cancer,29 chronic heart failure,24–26 28 patients approaching death and their family caregivers,32 healthcare professionals in nursing homes.23 Length of stay was assessed in heart failure, cancer and hip replacement studies and was significant in all studies.28–31 Quality of life was assessed in three studies,25 29 32 and two studies reported statistically significant differences between two groups,29 32 among patients with cancer29 and family caregivers of patients approaching death.32
For RCT design, n=12 studies did not clearly state the methods of randomisation. These were conducted in various populations: IBD,40 T2D,34 58 breast reconstruction,67 patients with stroke and their families,83 94 98 99 multimorbidity patients and their families,36 heart failure/COPD,45 46 65 chronic pain/musculoskeletal pain/fibromyalgia.35 42
Quality of life was assessed in seven studies and was statistically significant in three studies,36 45 46 but was statistically non-significant in four studies.35 40 65 94 Pain disability, intensity and interference was assessed in the chronic pain study and showed positive benefits in all outcomes,35 while the musculoskeletal pain (MSP)/fibromyalgia assessed pain intensity and number of tender points. Only number of tender points significantly reduced in the intervention compared with the control group.42 Healthcare utilisation was assessed in three studies.34 65 67 Emergency and elective admission rates significantly decreased in the intervention compared with the control group in T2D study,34 follow-up hospital visits significantly decreased in breast reconstruction study67 while hospital admissions were not statistically significant between two groups in COPD population.65 Caregiver outcomes: burden, mood/anxiety,94 depression and strain36 were not significantly different in both studies.
Thirty-nine RCTs clearly stated randomisation methods and these recruited participants from patient, family caregivers and healthcare professionals. The main patient population were COPD (n=6),43 48 60–63 T2D (n=6),37 38 41 56 57 59 multiple chronic conditions and/or elderly population n=7,55 64 69 71 75 78 92 arthritis n=4,30 36 52 61 cancer n=3,41 70 76 acute coronary syndrome n=6,25 33 34 39 40 96 HIV n=2 and Parkinson’s disease n=1.66 73 88
Quality of life, self-efficacy, health utilisation and costs of care were the main outcomes reported. Quality of life was assessed in n=16 studies, with six studies reporting statistically significant results. Quality of life was significant in a study among patients with chronic multiple conditions,72 COPD43 61 63 and HIV,79 80 but was not significant in T2D population,37 38 41 59 cancer,66 elderly with chronic conditions,39 acute coronary syndrome,49 89 COPD,62 multimorbidity71 and patients at end of life.90
Self-efficacy was assessed in nine studies,33 47 48 59 60 62 71 72 89 with only two reporting positive benefits of the intervention.47 59 Health utilisation was reported in 10 studies.27 39 60 61 63 69 72 79 87 91 Rehospitalisations significantly improved in COPD population and chronic multiple conditions,27 60 63 79 87 mortality also reduced in COPD and chronic multiple conditions.60 61 87
Healthcare use significantly reduced among the elderly with chronic conditions,39 length of hospital stay significantly reduced in one COPD study,63 but was non-significant in another COPD study,62 and among older people.91 Hospital admission/visit to emergency was not significant in COPD and cancer population.61 69 Healthcare use was not significant in chronic multiple conditions.72
Caregiver outcomes
Quality of life among caregivers and caregiver perceived burden significantly improved among family caregivers of older people in a geriatric practice.88 In a guided care intervention, quality of chronic Illness care, work productivity loss and absenteeism improved significantly for caregivers.36 However, depressive symptoms and caregiver strain were not significantly changed.36 In a cluster randomised controlled trial of a client-centred, ADL intervention for caregivers of people with stroke, caregiver burden, life satisfaction, perceived burden, mood, did not differ significantly.83
Healthcare professional outcomes
A training programme among oncologists resulted in significant changes in the following behavioural domains: transition to palliative care, general communication and involving significant others.68 A patient-centred communication intervention reported that GPs knowledge about medication taken by the patient was not significant.39 Job strain did not differ significantly between groups even though the intervention reported greater job satisfaction. Similarly, modified task and job analysis did not differ significantly, however time pressure did decrease significantly.84 Intention to engage in interprofessional shared decision making did not differ significantly in a Canadian trial.38
Costs of care and healthcare utilisation
A person-centred integrated intervention and a technology-based intervention for patients with heart failure reduced the costs of care in the Swedish and Spanish trials, a nurse-led rheumatology clinic versus rheumatologists-led clinic, and in acute coronary syndrome,24 45 49 64 78 however costs of services were not different among elderly admitted to a unit with acute illness.91
Hospital appointments decreased in the PC intervention compared with control in a multicentre cluster intervention for patients with IBD40 likewise in an interdisciplinary collaborative practice intervention hospital visits to see the physician reduced significantly.27 Patients in the individualised care plan intervention called out the ambulance more frequent than those who received usual care,65 even though the intervention group had more GP visits compared with control group (15.6 vs 11.6) in 12 months and the intervention group had more hospital admissions compared with the control group the differences were not statistically significant,65 healthcare utilisation was not significantly different between a clinician-led self-management trial and usual care.72 A quasi-experimental design also showed no significant differences on healthcare utilisation, hospitalisation, emergency department attendance.32
In an integrated practice unit and modified virtual ward model in Singapore, unplanned readmissions at 30, 90 and 180 days were significantly lower in the intervention group than the control group,87 emergency department attendance were significantly lower at 30, 90 and 180 days in the intervention.87 Likewise an interdisciplinary, collaborative practice intervention involving a primary care physician, a nurse and a social worker for community-dwelling seniors with chronic illnesses, showed significant changes in number of hospital admissions per patient per year, percentage of patients with one or more hospital readmissions within 60 days and mean number of visits to all physicians,27 fewer attendances at physical, occupational or speech therapy units39 compared with control group. However, change in percentage of patients with one or more visits to the emergency department, change in proportion of patients with one or more home care visits and change in number of patients with one or more nursing home placements and emergency visits were not significant.27 Similarly, in a centralised, nurse-delivered telephone-based service to improve care coordination and patient-reported outcomes after surgery for colorectal cancer unplanned readmission changes in emergency visits were non-significant.69
Mortality was significantly reduced in the community-based integrated care for frail patients with COPD.61 Mortality was significantly lower in an integrated practice unit and modified virtual ward model.87 A comprehensive care programme with multidisciplinary input for patients with COPD reported reduction in mortality rates compared with usual care.63 However, a team intervention for the multimorbid elderly reported that mortality risk at 3 and 6 months follow-up were all non-significant.93
A technology-based intervention of a home monitoring via mobile app on the number of in-person visits following ambulatory surgery showed that follow-up visits were significantly lower after surgery in the intervention compared with the control group,67 number of phone calls and emails made to the healthcare in 30 days after surgery were not significant.67 A person-centred communication intervention did not lead to change in number of medications taken by patient.39
In a Norwegian patient-centred team intervention number of emergency admissions, sum of emergency inpatient bed days, count of emergency re-admissions within 30 days of discharge, count of planned outpatient visits, count of emergency outpatient visits, mortality risk at 3 and 6 months follow-up were all non-significant.93
Clinical outcomes
Significant improvements were seen among patients with T2D and hypertension in systolic and diastolic blood pressure,57 likewise a patient-centred education programme among newly diagnosed patients with T2D, HbA1c was significant.58 Fasting blood sugar, HbA1c was not statistically different between the two groups.56 57 In a self-management trial in Sweden among patients with T2D, HbA1c was significant,56 but not significant in a Thai trial,59 and computer-based US trial.37 Furthermore, cholesterol levels were not different in a computer-based trial.37 Blood pressure (both systolic and diastolic) in a T2D trial,41 56 and haemoglobin were not significant.41 In a T2D UK trial body mass index was significant,41 but was not significant in a Swedish self-management trial for patients with T2D.56 An Iranian trial to test the effect of a holistic care programme on the reduction of iron overload in patients with beta-thalassaemia major change in serum ferritin at 3 months (mg/L), change in iron level at 3 months (μg/dL) were significant, but change in serum ferritin 1 year and 2 years postintervention, total iron binding capacity at 3 months, haemoglobin at 3 months were not significant.86
Discussion
Our review found a need for data on operationalising PCC in the delivery of care for patients with serious illness. Furthermore, findings show that PCC can be provided across all settings (hospitals: inpatient, outpatient, primary care, community settings and residential homes), but majorly in primary care. PCC can be achieved by involving patients, their families and healthcare professionals. PCC can also be provided using various approaches such as self-management interventions and technology-based interventions.
Most of the studies included in the review were conducted in high-income countries predominantly in Sweden and the USA, and most of the studies using technology were conducted in high-income countries. Most participants in these studies had heart failure, T2D, COPD, cancer and arthritis. The core component of the intervention included workshop training of healthcare professionals on communication skills, training patients and families on self-assessment, identifying their problems and concerns, creating action plans based on the problems, identifying resources to self-management of the problems and evaluating the care. These components are in line with a systematic review of effective elements in a patient-centred and multimorbidity care.100 The main outcomes reported across most studies were quality of life, healthcare utilisation and self-efficacy.
Some studies found effectiveness of PCC interventions in improving quality of life, self-efficacy, health utilisation and reducing costs of care. However, some studies reported no significant differences between PCC interventions and usual care on those outcomes.
Most studies which used person-centred self-management approaches and technology demonstrated positive benefits of the interventions in reducing hospital admissions, length of stay and unplanned visits. This finding concurs with a review of self-management interventions in respiratory and cardiovascular illness which reported that self-management support interventions reduces healthcare utilisation without compromising patient health outcomes.101 However, self-efficacy outcomes were mostly significant in technology-based interventions, but not significant across most studies which used self-management approaches. Studies reported conflicting results on quality-of-life outcomes. Three of the six studies which used self-management approaches reported statistically significant results while only one of the six technology-based interventions reported statistically significant findings. It seems that involving a person in decision making enables them to manage their own disease through technology which leads to reduced hospital visits and length of hospital admission. Our results concur with a previous scoping review that reported positive benefits of information and communication technology PCC interventions on five main chronic diseases (diabetes, cardiovascular, chronic respiratory, stroke and cancer).102
In terms of synthesis based on study design, most non-RCT reported significantly improved quality of life and reduced length of hospital stay. For RCT, of the 20 studies that reported data on quality-of-life outcomes, 9 of them reported significant results, however some of these studies did not clearly state the method of randomisation. Our findings are in line with a previous review of palliative care interventions for patients with incurable illness, which concluded that quality-of-life outcomes favoured palliative care interventions.103
Most of the RCTs demonstrated positive effects on the interventions in reducing re/hospitalisation, and improving health utilisation, however self-efficacy was non-significant across most RCTs.
Very few studies delivered the intervention to healthcare professionals (n=4) and caregivers (n=3). Quality of life improved and perceived burden significantly reduced in two caregiver studies. Our findings concur with a review of caregiving intervention in cancer population.104 105
However, psychosocial outcomes remained unchanged in our review. This is contrary to a review of multicomponent and psycho-educational interventions designed to support caregivers in their role such as training, education and skill which found positive benefits in reducing depression and burden of caregiving.106 Our data are also at odds with findings among family caregivers in oncology populations which showed improved emotional support.104
Studies among healthcare professionals showed positive benefits on time pressure and communication skills, but no differences were reported on knowledge and job strain outcomes. No study reported data on implementation science outcomes among healthcare professionals. The methodological quality of these studies was poor due small sample sizes, unclear randomisation methods and allocation concealment, therefore studies that reported data on caregivers and healthcare professional outcomes are inconclusive.
Only two studies from this review demonstrated that person-centred interventions were effective in reducing pain outcomes, with five studies showing that interventions had no effect on pain and physical symptoms such as fatigue, shortness of breath in COPD and heart disease populations. However, a previous review on self-initiated interventions among patients with cancer with peripheral neuropathy showed that strategies were beneficial in reducing symptoms and concerns.107
Patient communication and satisfaction with PCC interventions was significant in three of the six studies that reported data on this outcome. Our findings agree with a systematic review on effectiveness of communication-related quality improvement interventions for patients with advanced and serious illness which reported significant improvements on patients’ satisfaction with care.103 108
This review has shown that PCC interventions reduced costs of care in heart failure, COPD, acute coronary syndrome and rheumatology populations. This is in line with a meta-analysis on the economics of palliative care for adults with serious illness admitted to a facility that reported lower costs of palliative care consultations than usual care.109 Previous studies have reported that integrated palliative care (breathless support service) reduces costs in patients with cancer and their families.110 However, the same intervention resulted in extra mean costs of £799 in non-malignant conditions and their families,111 therefore we can attribute the differences due to diagnosis or type of serious illness.
In our review, of the six studies that reported data on costs, five reported that PCC resulted in reduction of costs of care.24 45 49 64 78 All these studies were conducted in primary care or home setting and two of these recruited both patients and family members as study participants.45 64 The disease conditions were CHF,24 45 64 acute coronary syndrome49 and rheumatoid arthritis.78 The majority of these studies were conducted in Sweden informed by the GPCC model of care,24 45 49 78 while one was conducted in Spain.64
The intervention comprised routines for establishment of a partnership between patients and/or families and healthcare professionals (who received training on how to provide PCC, developing a health plan with the patients and/or families. The health plan also contained agreed goals,24 45 49 64 78 these interventions were integrated in primary care. In PCC interventions informed by GPCC, healthcare professionals acquire knowledge and skills to practice PCC. Presumably this reduces hospital attendance, thereby saving time and costs travelling to the health facility. However, these are not clearly stated in the studies so we can only speculate. The only study which reported non-significant differences between the intervention and control on costs of care was among elderly people admitted to a hospital unit with acute illness.91 This study differs from the other studies in terms of setting, and it has a heterogenous group of patients with CHF, cancer, dementia, chronic lung disease, cardiovascular disease and it is not clear which model informed the intervention.
Some studies included in this review showed significant improvements in both clinical and psychosocial outcomes, while some showed no improvements in either of them. For example, among patients with beta-thalassaemia major, significant results were reported on clinical outcomes such as serum ferritin (mg/L) and iron levels (μg/dL) including change in physical activity: 6 min walk test,86 a technology-based trial of a person-centred tablet computer-based self-monitoring system for chronic disease (T2D and/or hypertension)57 reported significant improvement on systolic and diastolic blood pressure but did not show significant differences on fasting blood sugar levels and patient’s knowledge of T2D and hypertension. In HIV population, a Kenyan trial showed no differences between groups on the primary outcome of pain, but showed significant differences between groups on psychiatric morbidity and quality of life80 and another study showed no significant differences on both clinical and psychosocial outcomes in T2D population.37
Strengths and limitations
It is interesting to note that the majority of the studies (n=31) achieved relative complete follow-up, that is at least 80% of the participants were followed up at trial end points. This is encouraging considering that is it challenging to follow-up participants with serious illnesses. We used robust procedures for systematic reviewing and quality assessment of the studies included, in line with PRISMA reporting guidelines, however we did not use a checklist for health economic outcome studies. We only used the Joanna Briggs Institute Critical Appraisal checklist for randomised controlled studies. Furthermore, Grading of Recommendations, Assessment, Development and Evaluations was not used for the quality of evidence for each outcome.112 Most of the studies included did not state the theoretical framework underpinning the person-centred interventions. However, many studies that reported the theoretical framework used the GPCC and were conducted in Sweden across various clinical settings. Most of the studies identified and included were conducted in high-income countries.
Meta-analysis was not possible in this review due to heterogeneity of studies. Studies were from different patient populations, different trial designs (parallel trials or clustered trials), different sample sizes, different interventions and dimensions, different outcomes and measures used, different follow-up periods and intervals and interventions delivered in different settings. Some interventions targeted healthcare professionals and outcomes assessed among patients and healthcare professionals. Some interventions targeted patients and family dyads and captured data from both patients and their families, while some interventions targeted patients only, and family caregivers only.
Furthermore, interventions were delivered or led by different groups of professionals such as nurses, physiotherapists, physicians, social workers.
Due to nature of the interventions, it was difficult to blind study participants and those delivering the intervention, however three studies blinded study participants and two studies blinded those who delivered the intervention. It is challenging to design double-blinded or triple-blinded complex person-centred interventions. However, it is possible to blind outcome assessors. In this review, n=21 studies blinded outcomes assessors and 2 studies used postal questionnaires or web-based survey.
Some studies clearly stated the PCC model which informed the intervention while some studies did not state the PCC model. We still included studies that did not state the PCC model after critically reading through the text to understand important concepts and elements of PCC such as holistic care, coordinated physical health and supportive services, person-focused care, multidisciplinary team approach, involvement of patient and family and emphasise on person and family outcomes, respectful care and responsive to individual patient preferences, needs and values to guide all clinical decisions.113 114 It is possible that through this process, we might have missed some papers.
Conclusions, implications for policy, practice and research
There is some evidence that PCC interventions using self-management have some effects in reducing health utilisation, costs of care and improving quality of life.
Technology-based interventions also reduces healthcare utilisation and improves self-efficacy but appears to have less effect on quality of life. However, very few studies used self-management and technology approaches. Further work is needed to identify how self-management and technology PCC approaches can be used in serious illness across different disease conditions and settings. The majority of studies clearly defined what constituted usual care or the comparator. This shows that it is possible to design and deliver a PCC intervention in different care settings where this is currently not being practised.
PCC can be designed and evaluated using robust study designs, and can be delivered in primary, secondary and tertiary care including home settings and residential homes. Institutions should therefore consider implementing PCC interventions using locally available resources.
PCC interventions can target patients, their families or healthcare professionals. PCC research has mainly focused in high-income countries, more research needs to be done in LMICs. Further work is required to consider designing and evaluating PCC interventions at community level targeting community health workers and family members. Few studies (6/55) examined costs of PCC interventions. Health service researchers should consider incorporating costs of PCC or health economic outcomes when designing and evaluating complex PCC interventions.
Supplementary Material
Footnotes
Twitter: @KennedyNkhoma6, @RHardingCSI
Contributors: KBN planned, conducted searches and submitted the manuscript. KBN and AC extracted data. KBN and AC assessed quality of the included studies and compared assessments. RH reviewed data extraction and quality appraisal. AG, RP, IP, LF, LG and SV contributed to design and interpretation. All authors approved the manuscript. KBN is the guarantor.
Funding: This work is funded by the National Institute of Health Research (NIHR) Global Health Research Unit on Health System Strengthening in sub-Saharan Africa, King's College London (GHRU 16/136/54) using UK aid from the UK Government to support global health research.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data for this review are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.WHO . Person-Centred health care: a policy framework. Geneva: WHO; 2007. [Google Scholar]
- 2.Gillian Le RM, Bestall J, Ensor T. Leeds University of Leeds; 2014.
- 3.WHO . Universal health coverage: moving towards better health action framework for the Western Pacific region WHO; 2016. [Google Scholar]
- 4.Kitson A, Marshall A, Bassett K, et al. What are the core elements of patient-centred care? A narrative review and synthesis of the literature from health policy, medicine and nursing. J Adv Nurs 2013;69:4–15. 10.1111/j.1365-2648.2012.06064.x [DOI] [PubMed] [Google Scholar]
- 5.IhiousdffIP-CHRnepP, 2007
- 6.WHO . Who global strategy on people-centred and integrated health services; 2015.
- 7.Wilberforce M, Challis D, Davies L, et al. Person-centredness in the care of older adults: a systematic review of questionnaire-based scales and their measurement properties. BMC Geriatr 2016;16:63. 10.1186/s12877-016-0229-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giusti A, Nkhoma K, Petrus R, et al. The empirical evidence underpinning the concept and practice of person-centred care for serious illness: a systematic review. BMJ Glob Health 2020;5. 10.1136/bmjgh-2020-003330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley AS, Covinsky KE, Gorges RJ, et al. Identifying older adults with serious illness: a critical step toward improving the value of health care. Health Serv Res 2017;52:113–31. 10.1111/1475-6773.12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO . People-centred care in low and middle income countries; 2010.
- 11.Sleeman KE, de Brito M, Etkind S, et al. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health 2019;7:e883–92. 10.1016/S2214-109X(19)30172-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding R, Leam C. Clinical notes for informal carers in palliative care: recommendations from a random patient file audit. Palliat Med 2005;19:639–42. 10.1191/0269216305pm1092oa [DOI] [PubMed] [Google Scholar]
- 13.Moens K, Higginson IJ, Harding R, et al. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage 2014;48:660–77. 10.1016/j.jpainsymman.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 14.Streid J, Harding R, Agupio G, et al. Stressors and resources of caregivers of patients with incurable progressive illness in sub-Saharan Africa. Qual Health Res 2014;24:317–28. 10.1177/1049732314523682 [DOI] [PubMed] [Google Scholar]
- 15.Kelley AS. Defining "serious illness". J Palliat Med 2014;17:985. 10.1089/jpm.2014.0164 [DOI] [PubMed] [Google Scholar]
- 16.Little P, Everitt H, Williamson I, et al. Observational study of effect of patient centredness and positive approach on outcomes of general practice consultations. BMJ 2001;323:908–11. 10.1136/bmj.323.7318.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nkhoma K, Giusti A, Petrus R. A systematic review investigating the effectiveness of person-centred interventions in serious physical illness 2018. https://www.crd.york.ac.uk/prospero/#recordDetails [DOI] [PMC free article] [PubMed]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Green S. Cochrane handbook for systematic reviews of interventions 4.2. 5 [updated May 2005]The cochrane library, 2005. [Google Scholar]
- 20.Centre for Review and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care, 2009. Available: http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf
- 21.JBICACf R. CTAa, docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_, 2020 RCTpAJ. critical appraisal checklist for randomised controlled trials, 2016. Available: https://jbi.global/critical-appraisal-tools
- 22.Popay J, Roberts HM, Sowden A. Guidance on the conduct of narrative synthesis in sytematic reviews. Institute for health research. Available: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiK6fuyk9fgAhVDtnEKHV9-AUgQFjAAegQIBxAC&url=http%3A%2F%2Fciteseerx.ist.psu.edu%2Fviewdoc%2Fdownload%3Fdoi%3D10.1.1.178.3100%26rep%3Drep1%26type%3Dpdf&usg=AOvVaw3xMoGRunApJPo0_YYk1hqo.Guidanceontheconductofnarrativesynthesisinsytematicreviews
- 23.Bökberg C, Behm L, Wallerstedt B, et al. Evaluation of person-centeredness in nursing homes after a palliative care intervention: pre- and post-test experimental design. BMC Palliat Care 2019;18:44. 10.1186/s12904-019-0431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson E, Ekman I, Swedberg K, et al. Person-centred care for patients with chronic heart failure - a cost-utility analysis. Eur J Cardiovasc Nurs 2016;15:276–84. 10.1177/1474515114567035 [DOI] [PubMed] [Google Scholar]
- 25.Ekman I, Wolf A, Olsson L-E, et al. Effects of person-centred care in patients with chronic heart failure: the PCC-HF study. Eur Heart J 2012;33:1112–9. 10.1093/eurheartj/ehr306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudas K, Olsson L-E, Wolf A, et al. Uncertainty in illness among patients with chronic heart failure is less in person-centred care than in usual care. Eur J Cardiovasc Nurs 2013;12:521–8. 10.1177/1474515112472270 [DOI] [PubMed] [Google Scholar]
- 27.Sommers LS, Marton KI, Barbaccia JC, et al. Physician, nurse, and social worker collaboration in primary care for chronically ill seniors. Arch Intern Med 2000;160:1825–33. 10.1001/archinte.160.12.1825 [DOI] [PubMed] [Google Scholar]
- 28.Ulin K, Olsson L-E, Wolf A, et al. Person-centred care - An approach that improves the discharge process. Eur J Cardiovasc Nurs 2016;15:e19–26. 10.1177/1474515115569945 [DOI] [PubMed] [Google Scholar]
- 29.Öhlén J, Sawatzky R, Pettersson M, et al. Preparedness for colorectal cancer surgery and recovery through a person-centred information and communication intervention – a quasi-experimental longitudinal design. PLoS One 2019;14:e0225816. 10.1371/journal.pone.0225816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsson L-E, Karlsson J, Berg U, et al. Person-Centred care compared with standardized care for patients undergoing total hip arthroplasty—a quasi-experimental study. J Orthop Surg Res 2014;9:95. 10.1186/s13018-014-0095-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson L-E, Hansson E, Ekman I. Evaluation of person-centred care after hip replacement-a controlled before and after study on the effects of fear of movement and self-efficacy compared to standard care. BMC Nurs 2016;15:53. 10.1186/s12912-016-0173-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britt HR, JaKa MM, Fernstrom KM, et al. Quasi-Experimental evaluation of lifecourse on utilization and patient and caregiver quality of life and experience. Am J Hosp Palliat Care 2019;36:408–16. 10.1177/1049909118817740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelechi TJ, Mueller M, Spencer C, et al. The effect of a nurse-directed intervention to reduce pain and improve behavioral and physical outcomes in patients with critically colonized/infected chronic leg ulcers. J Wound Ostomy Continence Nurs 2014;41:111–21. 10.1097/WON.0000000000000009 [DOI] [PubMed] [Google Scholar]
- 34.Mills PD, Harvey PW, COAG coordinated care trial . Beyond community-based diabetes management and the COAG coordinated care trial. Aust J Rural Health 2003;11:131–7. [PubMed] [Google Scholar]
- 35.Dobscha SK, Corson K, Perrin NA, et al. Collaborative care for chronic pain in primary care: a cluster randomized trial. JAMA 2009;301:1242–52. 10.1001/jama.2009.377 [DOI] [PubMed] [Google Scholar]
- 36.Wolff JL, Giovannetti ER, Boyd CM, et al. Effects of guided care on family caregivers. Gerontologist 2010;50:459–70. 10.1093/geront/gnp124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasgow RE, Nutting PA, King DK, et al. Randomized effectiveness trial of a computer-assisted intervention to improve diabetes care. Diabetes Care 2005;28:33–9. 10.2337/diacare.28.1.33 [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Choi D, Bruno BA, et al. Impact of MyDiabetesPlan, a web-based patient decision aid on decisional conflict, diabetes distress, quality of life, and chronic illness care in patients with diabetes: cluster randomized controlled trial. J Med Internet Res 2020;22:e16984. 10.2196/16984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schäfer I, Kaduszkiewicz H, Mellert C, et al. Narrative medicine-based intervention in primary care to reduce polypharmacy: results from the cluster-randomised controlled trial MultiCare agenda. BMJ Open 2018;8:e017653. 10.1136/bmjopen-2017-017653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy A, Nelson E, Reeves D, et al. A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self-help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technol Assess 2003;7:1–113. 10.3310/hta7280 [DOI] [PubMed] [Google Scholar]
- 41.Kinmonth AL, Woodcock A, Griffin S, et al. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The diabetes care from diagnosis research team. BMJ 1998;317:1202–8. 10.1136/bmj.317.7167.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alamo MM, Moral RR, Pérula de Torres LA. Evaluation of a patient-centred approach in generalized musculoskeletal chronic pain/fibromyalgia patients in primary care. Patient Educ Couns 2002;48:23–31. 10.1016/s0738-3991(02)00095-2 [DOI] [PubMed] [Google Scholar]
- 43.Slok AHM, Kotz D, van Breukelen G, et al. Effectiveness of the assessment of burden of COPD (ABC) tool on health-related quality of life in patients with COPD: a cluster randomised controlled trial in primary and hospital care. BMJ Open 2016;6:e011519. 10.1136/bmjopen-2016-011519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dambha-Miller H, Cooper AJM, Simmons RK, et al. Patient-Centred care, health behaviours and cardiovascular risk factor levels in people with recently diagnosed type 2 diabetes: 5-year follow-up of the ADDITION-Plus trial cohort. BMJ Open 2016;6:e008931. 10.1136/bmjopen-2015-008931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sahlen K-G, Boman K, Brännström M. A cost-effectiveness study of person-centered integrated heart failure and palliative home care: based on a randomized controlled trial. Palliat Med 2016;30:296–302. 10.1177/0269216315618544 [DOI] [PubMed] [Google Scholar]
- 46.Brännström M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. prefer: a randomized controlled study. Eur J Heart Fail 2014;16:1142–51. 10.1002/ejhf.151 [DOI] [PubMed] [Google Scholar]
- 47.Fors A, Swedberg K, Ulin K, et al. Effects of person-centred care after an event of acute coronary syndrome: two-year follow-up of a randomised controlled trial. Int J Cardiol 2017;249:42–7. 10.1016/j.ijcard.2017.08.069 [DOI] [PubMed] [Google Scholar]
- 48.Zakrisson A-B, Arne M, Hasselgren M, et al. A complex intervention of self-management for patients with COPD or CHF in primary care improved performance and satisfaction with regard to own selected activities; a longitudinal follow-up. J Adv Nurs 2019;75:175–86. 10.1111/jan.13899 [DOI] [PubMed] [Google Scholar]
- 49.Pirhonen L, Bolin K, Olofsson EH, et al. Person-Centred care in patients with acute coronary syndrome: cost-effectiveness analysis alongside a randomised controlled trial. Pharmacoecon Open 2019;3:495–504. 10.1007/s41669-019-0126-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fors A, Taft C, Ulin K, et al. Person-Centred care improves self-efficacy to control symptoms after acute coronary syndrome: a randomized controlled trial. Eur J Cardiovasc Nurs 2016;15:186–94. 10.1177/1474515115623437 [DOI] [PubMed] [Google Scholar]
- 51.Fors A, Gyllensten H, Swedberg K, et al. Effectiveness of person-centred care after acute coronary syndrome in relation to educational level: subgroup analysis of a two-armed randomised controlled trial. Int J Cardiol 2016;221:957–62. 10.1016/j.ijcard.2016.07.060 [DOI] [PubMed] [Google Scholar]
- 52.Fors A, Ekman I, Taft C, et al. Person-centred care after acute coronary syndrome, from hospital to primary care - A randomised controlled trial. Int J Cardiol 2015;187:693–9. 10.1016/j.ijcard.2015.03.336 [DOI] [PubMed] [Google Scholar]
- 53.Wolf A, Fors A, Ulin K, et al. An eHealth diary and Symptom-Tracking tool combined with Person-Centered care for improving self-efficacy after a diagnosis of acute coronary syndrome: a substudy of a randomized controlled trial. J Med Internet Res 2016;18:e40. 10.2196/jmir.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kikkenborg Berg S, Støier L, Moons P, et al. Emotions and health: findings from a randomized clinical trial on psychoeducational nursing to patients with implantable cardioverter defibrillator. J Cardiovasc Nurs 2015;30:197–204. 10.1097/JCN.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 55.Fors A, Ekman I, Ulin K, et al. P625Person-centred care is effective after an event of acute coronary syndrome; particularly in patients with low educational level - two-year follow-up of a randomised controlled trial. Eur Heart J 2017;38:116. 10.1093/eurheartj/ehx501.P62528158566 [DOI] [Google Scholar]
- 56.Jutterström L, Hörnsten Å, Sandström H, et al. Nurse-Led patient-centered self-management support improves HbA1c in patients with type 2 diabetes-A randomized study. Patient Educ Couns 2016;99:1821–9. 10.1016/j.pec.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 57.Or C, Tao D. A 3-month randomized controlled pilot trial of a patient-centered, computer-based self-monitoring system for the care of type 2 diabetes mellitus and hypertension. J Med Syst 2016;40:81. 10.1007/s10916-016-0437-1 [DOI] [PubMed] [Google Scholar]
- 58.Windrum P, García-Goñi M, Coad H. The impact of patient-centered versus Didactic education programs in chronic patients by severity: the case of type 2 diabetes mellitus. Value Health 2016;19:353–62. 10.1016/j.jval.2016.01.014 [DOI] [PubMed] [Google Scholar]
- 59.Wichit N, Mnatzaganian G, Courtney M, et al. Randomized controlled trial of a family-oriented self-management program to improve self-efficacy, glycemic control and quality of life among Thai individuals with type 2 diabetes. Diabetes Res Clin Pract 2017;123:37–48. 10.1016/j.diabres.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 60.Fors A, Blanck E, Ali L. Person-Centred telephone-support is effective in patients with chronic obstructive pulmonary disease and/or chronic heart failure-six-month follow-up of a randomized controlled trial. European Journal of Heart Failure 2018;20:194. 10.1371/journal.pone.0203031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernández C, Alonso A, Garcia-Aymerich J, et al. Effectiveness of community-based integrated care in frail COPD patients: a randomised controlled trial. NPJ Prim Care Respir Med 2015;25:15022. 10.1038/npjpcrm.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thom DH, Willard-Grace R, Tsao S, et al. Randomized controlled trial of health coaching for vulnerable patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2018;15:1159–68. 10.1513/AnnalsATS.201806-365OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko FWS, Cheung NK, Rainer TH, et al. Comprehensive care programme for patients with chronic obstructive pulmonary disease: a randomised controlled trial. Thorax 2017;72:122–8. 10.1136/thoraxjnl-2016-208396 [DOI] [PubMed] [Google Scholar]
- 64.de Batlle J, Massip M, Vargiu E, et al. Implementing mobile Health-Enabled integrated care for complex chronic patients: intervention effectiveness and cost-effectiveness study. JMIR Mhealth Uhealth 2021;9:e22135. 10.2196/22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin IR, McNamara D, Sutherland FR, et al. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis 2004;1:191–5. 10.1191/1479972304cd047oa [DOI] [PubMed] [Google Scholar]
- 66.Hansson E, Carlström E, Olsson L-E, et al. Can a person-centred-care intervention improve health-related quality of life in patients with head and neck cancer? A randomized, controlled study. BMC Nurs 2017;16:9. 10.1186/s12912-017-0206-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstrong KA, Coyte PC, Brown M, et al. Effect of home monitoring via mobile APP on the number of In-Person visits following ambulatory surgery: a randomized clinical trial. JAMA Surg 2017;152:622–7. 10.1001/jamasurg.2017.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goelz T, Wuensch A, Stubenrauch S, et al. Specific training program improves oncologists' palliative care communication skills in a randomized controlled trial. J Clin Oncol 2011;29:3402–7. 10.1200/JCO.2010.31.6372 [DOI] [PubMed] [Google Scholar]
- 69.Young JM, Butow PN, Walsh J, et al. Multicenter randomized trial of centralized nurse-led telephone-based care coordination to improve outcomes after surgical resection for colorectal cancer: the connect intervention. J Clin Oncol 2013;31:3585–91. 10.1200/JCO.2012.48.1036 [DOI] [PubMed] [Google Scholar]
- 70.Mielenz TJ, Tracy M, Jia H, et al. Creation of the Person-Centered wellness home in older adults. Innov Aging 2020;4. 10.1093/geroni/igaa005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fortin M, Stewart M, Ngangue P, et al. Scaling up patient-centered interdisciplinary care for multimorbidity: a pragmatic mixed-methods randomized controlled trial. Ann Fam Med 2021;19:126–34. 10.1370/afm.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed RL, Roeger L, Howard S, et al. A self-management support program for older Australians with multiple chronic conditions: a randomised controlled trial. Med J Aust 2018;208:69–74. 10.5694/mja17.00127 [DOI] [PubMed] [Google Scholar]
- 73.Berntsen GKR, Dalbakk M, Hurley JS, et al. Person-Centred, integrated and PRO-active care for multi-morbid elderly with advanced care needs: a propensity score-matched controlled trial. BMC Health Serv Res 2019;19:682. 10.1186/s12913-019-4397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsson A, Palstam A, Löfgren M, et al. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia--a randomized controlled trial. Arthritis Res Ther 2015;17:161. 10.1186/s13075-015-0679-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ericsson A, Palstam A, Larsson A, et al. Resistance exercise improves physical fatigue in women with fibromyalgia: a randomized controlled trial. Arthritis Res Ther 2016;18:176. 10.1186/s13075-016-1073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergsten U, Almehed K, Baigi A, et al. A randomized study comparing regular care with a nurse-led clinic based on tight disease activity control and person-centred care in patients with rheumatoid arthritis with moderate/high disease activity: a 6-month evaluation. Musculoskeletal Care 2019;17:215–25. 10.1002/msc.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feldthusen C, Dean E, Forsblad-d'Elia H, et al. Effects of Person-Centered physical therapy on Fatigue-Related variables in persons with rheumatoid arthritis: a randomized controlled trial. Arch Phys Med Rehabil 2016;97:26–36. 10.1016/j.apmr.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 78.Larsson I, Fridlund B, Arvidsson B, et al. A nurse-led rheumatology clinic versus rheumatologist-led clinic in monitoring of patients with chronic inflammatory arthritis undergoing biological therapy: a cost comparison study in a randomised controlled trial. BMC Musculoskelet Disord 2015;16:817. 10.1186/s12891-015-0817-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gustafson DH, Hawkins RP, Boberg EW, et al. The use and impact of a computer-based support system for people living with AIDS and HIV infection. Proc Annu Symp Comput Appl Med Care 1994:604–8. [PMC free article] [PubMed] [Google Scholar]
- 80.Lowther K, Selman L, Simms V, et al. Nurse-Led palliative care for HIV-positive patients taking antiretroviral therapy in Kenya: a randomised controlled trial. Lancet HIV 2015;2:e328–34. 10.1016/S2352-3018(15)00111-3 [DOI] [PubMed] [Google Scholar]
- 81.Machado LAC, Azevedo DC, Capanema MB, et al. Client-centered therapy vs exercise therapy for chronic low back pain: a pilot randomized controlled trial in Brazil. Pain Med 2007;8:251–8. 10.1111/j.1526-4637.2006.00225.x [DOI] [PubMed] [Google Scholar]
- 82.Murphy SL, Lyden AK, Smith DM, et al. Effects of a tailored activity pacing intervention on pain and fatigue for adults with osteoarthritis. Am J Occup Ther 2010;64:869–76. 10.5014/ajot.2010.09198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bertilsson A-S, Eriksson G, Ekstam L, et al. A cluster randomized controlled trial of a client-centred, activities of daily living intervention for people with stroke: one year follow-up of caregivers. Clin Rehabil 2016;30:765–75. 10.1177/0269215515603780 [DOI] [PubMed] [Google Scholar]
- 84.Berendonk C, Kaspar R, Bär M, et al. Improving quality of work life for care providers by fostering the emotional well-being of persons with dementia: a cluster-randomized trial of a nursing intervention in German long-term care settings. Dementia 2019;18:1286–309. 10.1177/1471301217698837 [DOI] [PubMed] [Google Scholar]
- 85.Eggers C, Dano R, Schill J, et al. Patient-Centered integrated healthcare improves quality of life in Parkinson's disease patients: a randomized controlled trial. J Neurol 2018;265:764–73. 10.1007/s00415-018-8761-7 [DOI] [PubMed] [Google Scholar]
- 86.Arian M, Memarian R, Oghazian MB, et al. The effect of a holistic care program on the reduction of iron overload in patients with beta-thalassemia major: a randomized clinical trial. Iran Red Crescent Med J 2018;20:e60820. 10.5812/ircmj.60820 [DOI] [Google Scholar]
- 87.Low LL, Tan SY, Ng MJM, et al. Applying the integrated practice unit concept to a modified virtual ward model of care for patients at highest risk of readmission: a randomized controlled trial. PLoS One 2017;12:e0168757. 10.1371/journal.pone.0168757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu DSF,. Effects of a health and social collaborative case management model on health outcomes of family caregivers of frail older adults: preliminary data from a pilot randomized controlled trial. J Am Geriatr Soc 2016;64:2144–8. 10.1111/jgs.14259 [DOI] [PubMed] [Google Scholar]
- 89.Pirhonen L, Olofsson EH, Fors A, et al. Effects of person-centred care on health outcomes-A randomized controlled trial in patients with acute coronary syndrome. Health Policy 2017;121:169–79. 10.1016/j.healthpol.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 90.Chochinov HM, Kristjanson LJ, Breitbart W, et al. Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomised controlled trial. Lancet Oncol 2011;12:753–62. 10.1016/S1470-2045(11)70153-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Landefeld CS, Palmer RM, Kresevic DM, et al. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med 1995;332:1338–44. 10.1056/NEJM199505183322006 [DOI] [PubMed] [Google Scholar]
- 92.Larsson I, Fridlund B, Arvidsson B, et al. A nurse-led rheumatology clinic versus rheumatologist-led clinic in monitoring of patients with chronic inflammatory arthritis undergoing biological therapy: a cost comparison study in a randomised controlled trial. BMC Musculoskelet Disord 2015;16:354. 10.1186/s12891-015-0817-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berntsen G, Høyem A, Lettrem I, et al. A person-centered integrated care quality framework, based on a qualitative study of patients' evaluation of care in light of chronic care ideals. BMC Health Serv Res 2018;18:479. 10.1186/s12913-018-3246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedman A, Eriksson G, von Koch L, et al. Five-Year follow-up of a cluster-randomized controlled trial of a client-centred activities of daily living intervention for people with stroke. Clin Rehabil 2019;33:262–76. 10.1177/0269215518809791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Larsson I, Fridlund B, Arvidsson B. Treatment outcomes from a nurse-led rheumatology clinic in monitoring of anti-TNF therapy-a randomised controlled trial. Arthritis and Rheumatism 2012;10:667. [Google Scholar]
- 96.Bergmo TS, Berntsen GK, Dalbakk M, et al. The effectiveness and cost effectiveness of the patient-centred team (PACT) model: study protocol of a prospective matched control before-and-after study. BMC Geriatr 2015;15:133. 10.1186/s12877-015-0133-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Popay J, Roberts HM, Sowden A, et al. Guidance on the conduct of narrative synthesis in sytematic reviews. Institute for health research, 2006. Available: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=2ahUKEwiK6fuyk9fgAhVDtnEKHV9-AUgQFjAAegQIBxAC&url=http%3A%2F%2Fciteseerx.ist.psu.edu%2Fviewdoc%2Fdownload%3Fdoi%3D10.1.1.178.3100%26rep%3Drep1%26type%3Dpdf&usg=AOvVaw3xMoGRunApJPo0_YYk1hqo
- 98.Bertilsson A-S, Ranner M, von Koch L, et al. A client-centred ADL intervention: three-month follow-up of a randomized controlled trial. Scand J Occup Ther 2014;21:377–91. 10.3109/11038128.2014.880126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guidetti S, Ranner M, Tham K, et al. A "client-centred activities of daily living" intervention for persons with stroke: One-year follow-up of a randomized controlled trial. J Rehabil Med 2015;47:605–11. 10.2340/16501977-1981 [DOI] [PubMed] [Google Scholar]
- 100.Poitras M-E, Maltais M-E, Bestard-Denommé L, et al. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv Res 2018;18:446. 10.1186/s12913-018-3213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Panagioti M, Richardson G, Small N, et al. Self-Management support interventions to reduce health care utilisation without compromising outcomes: a systematic review and meta-analysis. BMC Health Serv Res 2014;14:356. 10.1186/1472-6963-14-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wildevuur SE, Simonse LWL. Information and communication technology-enabled person-centered care for the "big five" chronic conditions: scoping review. J Med Internet Res 2015;17:e77. 10.2196/jmir.3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol 2011;9:87–94. 10.1016/j.suponc.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 104.Ferrell B, Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin 2017;67:318–25. 10.3322/caac.21396 [DOI] [PubMed] [Google Scholar]
- 105.Northouse LL, Katapodi MC, Song L. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. A cancer Journal for clinicians. 2010;60:317–39. 10.3322/caac.20081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parker D, Mills S, Abbey J. Effectiveness of interventions that assist caregivers to support people with dementia living in the community: a systematic review. Int J Evid Based Healthc 2008;6:137–72. 10.1097/01258363-200806000-00002 [DOI] [PubMed] [Google Scholar]
- 107.Ogle T, Alexander K, Miaskowski C, et al. Systematic review of the effectiveness of self-initiated interventions to decrease pain and sensory disturbances associated with peripheral neuropathy. J Cancer Surviv 2020;14:444–63. 10.1007/s11764-020-00861-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fawole OA, Dy SM, Wilson RF, et al. A systematic review of communication quality improvement interventions for patients with advanced and serious illness. J Gen Intern Med 2013;28:570–7. 10.1007/s11606-012-2204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.May P, Normand C, Cassel JB, et al. Economics of palliative care for hospitalized adults with serious illness: a meta-analysis. JAMA Intern Med 2018;178:820–9. 10.1001/jamainternmed.2018.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Farquhar MC, Prevost AT, McCrone P, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? findings of a mixed-method randomised controlled trial. BMC Med 2014;12:194. 10.1186/s12916-014-0194-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Farquhar MC, Prevost AT, McCrone P, et al. The clinical and cost effectiveness of a breathlessness intervention service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials 2016;17:185. 10.1186/s13063-016-1304-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guyatt GH, Oxman AD, Vist GE, et al. Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kogan AC, Wilber K, Mosqueda L. Person-Centered care for older adults with chronic conditions and functional impairment: a systematic literature review. J Am Geriatr Soc 2016;64:e1–7. 10.1111/jgs.13873 [DOI] [PubMed] [Google Scholar]
- 114.American Geriatrics Society Expert Panel on Person-Centered Care . Person-Centered care: a definition and essential elements. J Am Geriatr Soc 2016;64:15–18. 10.1111/jgs.13866 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-054386supp001.pdf (956.6KB, pdf)
bmjopen-2021-054386supp002.pdf (713.5KB, pdf)
bmjopen-2021-054386supp003.pdf (94.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data for this review are available on reasonable request.