Abstract
Background
Inorganic arsenic (iAs) is a ubiquitous metalloid and drinking water contaminant. Prenatal exposure is associated with birth outcomes across multiple studies. During metabolism, iAs is sequentially methylated to mono- and di-methylated arsenical species (MMAs and DMAs) to facilitate whole body clearance. Inefficient methylation (e.g., higher urinary % MMAs) is associated with increased risk of certain iAs-associated diseases. One-carbon metabolism factors influence iAs methylation, modifying toxicity in adults, and warrant further study during the prenatal period. The objective of this study was to evaluate folate, vitamin B12, and homocysteine as modifiers of the relationship between biomarkers of iAs methylation efficiency and birth outcomes.
Methods
Data from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort (2011–2012) with maternal urine and cord serum arsenic biomarkers and maternal serum folate, vitamin B12, and homocysteine concentrations were utilized. One-carbon metabolism factors were dichotomized using clinical cutoffs and median splits. Multivariable linear regression models were fit to evaluate associations between each biomarker and birth outcome overall and within levels of one-carbon metabolism factors. Likelihood ratio tests of full and reduced models were used to test the significance of statistical interactions on the additive scale (α = 0.10).
Results
Among urinary biomarkers, % U-MMAs was most strongly associated with birth weight (β = − 23.09, 95% CI: − 44.54, − 1.64). Larger, more negative mean differences in birth weight were observed among infants born to women who were B12 deficient (β = − 28.69, 95% CI: − 53.97, − 3.42) or experiencing hyperhomocysteinemia (β = − 63.29, 95% CI: − 154.77, 28.19). Generally, mean differences in birth weight were attenuated among infants born to mothers with higher serum concentrations of folate and vitamin B12 (or lower serum concentrations of homocysteine). Effect modification by vitamin B12 and homocysteine was significant on the additive scale for some associations. Results for gestational age were less compelling, with an approximate one-week mean difference associated with C-tAs (β = 0.87, 95% CI: 0, 1.74), but not meaningful otherwise.
Conclusions
Tissue distributions of iAs and its metabolites (e.g., % MMAs) may vary according to serum concentrations of folate, vitamin B12 and homocysteine during pregnancy. This represents a potential mechanism through which maternal diet may modify the harms of prenatal exposure to iAs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-022-00875-7.
Keywords: Inorganic arsenic, Birth weight, Gestational age, Effect modification, One-carbon metabolism
Background
Exposure to inorganic arsenic (iAs) is a substantial public health issue. As a result of its natural occurrence in soil and rock, globally, at least 200 million individuals are exposed at harmful levels in contaminated groundwater [1, 2]. In addition to contaminated drinking water, iAs exposure can also occur through diet (e.g., rice intake) [1]. Exposure to iAs is associated with adverse health outcomes [2] and is of particular concern for pregnant women due to associations with lower birth weight and preterm delivery [3–6]. Arsenic crosses the placenta and has been associated with molecular alterations in fetal cells and tissue [7–12]. Potential mechanisms linking prenatal iAs exposure to adverse birth outcomes may include generalized oxidative stress, epigenetic dysregulation of key genes involved in fetal growth, and altered function and expression of genes critical to proper placentation [7–10, 13]. As an example, higher iAs exposure is positively associated with cord blood expression of Soluble Fms-Like Tyrosine Kinase-1 (sFLT1), an antagonist of Vascular endothelial growth factor (VEGF) [10]. Impacts on the function and expression of genes related to placentation (e.g., sFLT1) and fetal growth (e.g., Potassium Voltage-Gated Channel Subfamily Q Member 1 (KCNQ1)) [10, 11] observed in relation to iAs exposure could also impair fetal development. Fortunately, modifiable factors influencing iAs metabolism may also be targets for public health intervention.
iAs is metabolized and eliminated from the body through a process that is influenced by micronutrients involved in one-carbon metabolism. This pathway is composed of the folate and methionine cycles and contributes to DNA synthesis, cellular growth, and proliferation [14]. During a critical step in the pathway, the methyl group of 5-methyl-tetrahydrofolate (5-methyl-THF) is transferred to homocysteine by methionine synthase in a reaction that utilizes vitamin B12 as a cofactor. This step generates methionine, an amino acid and component of several compounds [15]. Methionine is then activated to form S-adenosylmethionine (SAM), the primary methyl donor to iAs. Arsenic-3-methyltransferace (AS3MT) sequentially methylates iAs to mono- and dimethyl arsenical species (MMAs and DMAs), using SAM as a cofactor, to drive metabolism and tissue clearance. Inefficient methylation (e.g., higher urinary %MMAs) during pregnancy may indicate higher maternal and fetal exposure to trivalent MMAsIII, an arsenic species established as the most toxic form in most tissues, including placenta [8, 16, 17].
Each methylation reaction produces a methylated product and S-adenosylhomocysteine (SAH), which is hydrolyzed to homocysteine [15], a non-protein amino acid independently associated with pregnancy complications and intrauterine growth restriction [14]. Given the role of one-carbon metabolism in iAs metabolism, individual susceptibility to iAs toxicity is associated with serum concentrations of folate and vitamin B12 among adults [15, 18, 19]. Impacts during pregnancy, where one-carbon metabolism is already strained to support the demands of the developing fetus, are also likely and warrant further study. To this end, in the present study we explore maternal serum concentrations of one-carbon metabolism factors as potential modifiers of the relationship between biomarkers of methylation efficiency (e.g., % urinary MMAs) and birth outcomes.
We estimate associations between each biomarker and (1) birth weight and (2) gestational age overall and within levels of folate, vitamin B12, and homocysteine using data from the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort. Likelihood ratio tests of main effects models against full models incorporating interaction terms were also used to evaluate statistical interactions, or potential effect modification, on the additive scale. We hypothesized that greater bioavailability of folate and vitamin B12 would increase conversion of MMAs to DMAs, thereby attenuating negative associations between biomarkers and birth outcomes.
Methods
Study population
The Biomarkers of Exposure to ARsenic (BEAR) cohort comprises pregnant women who resided in Gómez Palacio, State of Durango, Mexico between August 2011 and March 2012 [6]. Arsenic contamination of drinking water is a global public health issue, and the State of Durango is characterized by especially high exposure risk [20]. Mothers were recruited for the study prior to delivery (usually within 24 hours of birth) at the General Hospital of Gómez Palacio, Mexico. Inclusion criteria consisted of: (a) a one-year minimum residence in the Gómez Palacio region, including urban locations of Gómez Palacio and Tlahualilo and their surrounding rural locations, (b) a confirmed, singleton, intrauterine pregnancy without complications (e.g., preeclampsia), and (c) a good overall health status (e.g., no indications of chronic or acute disease). In total, 221 women were approached for the study, and 93% (n = 206) provided informed consent for participation. Six of the women providing informed consent were excluded due to a confirmed twin pregnancy (n = 1) or sample collection failure (n = 5), with the final cohort composed of 200 mother-infant pairs.
Participants completed detailed questionnaires capturing information on demographic characteristics (e.g., time at residence and socioeconomic indicators) and sources of iAs exposure (e.g., sources of drinking, cooking and bathing water). Birth weight was measured at the time of delivery by the physician. Gestational age was estimated using the date of each mother’s last menstrual period. All study procedures were previously approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill and the Universidad Juárez del Estado de Durango, and each participant gave written, informed consent to participate and provide urine samples, drinking water samples, and umbilical cord blood.
Determination of total iAs and metabolites in urine and cord serum
To characterize maternal exposure to iAs, concentrations of iAs and its metabolites were assessed in spot urine samples. These samples were collected at the time of delivery, immediately transferred to cryovials, and stored in liquid nitrogen. Urine samples packed in dry ice were shipped to the University of North Carolina at Chapel Hill for further processing. To account for differences in water intake or differential hydration, the specific gravity (SG) of each urine sample was measured using a handheld refractometer (Reichert TX 400 #13740000; Reichert Inc., Depew, NY). Additionally, urine iAs (U-iAs) and metabolites (U-MMAs and U-DMAs) were measured using hydride generation atomic absorption spectroscopy (HG-AAS) with cryotrapping [21]. As applied, this method measured total U-iAs, total U-MMAs and total U-DMAs, without differentiating between trivalent (AsIII) and pentavalent (AsV) species. The limits of detection (LODs) for U-iAs, U-MMAs, and U-DMAs were 0.2, 0.1, and 0.1 μg/L, respectively. These LODs are based on the previously published instrumental LODs [21, 22] and consider volume and dilution of urine samples used for the HG-AAS analysis. Urine samples were adjusted for SG using the following formula: iAs x (mean SG in BEAR cohort-1)/(individual SG-1) [23]. Total urine arsenic (U-tAs) concentrations were estimated as the sum of SG-adjusted U-iAs, U-MMAs, and U-DMAs. Biomarkers of maternal iAs methylation efficiency were calculated as the percent of U-tAs (e.g., U-iAs/U-tAs × 100%).
To characterize fetal exposure to iAs, concentrations of iAs and its metabolites in cord serum (C-iAs, C-MMAs, and C-DMAs) were measured using hydride generation with cryotrapping coupled to inductively-coupled plasma mass spectrometry (HG-CT-ICP-MS), as described previously [24]. This technique provides sensitive LODs, and is thus suited for analysis of biological matrices with very low levels of arsenic species, like serum [25]. LODs for C-iAs, C-MMAs, and C-DMAs were 1.45, 0.06, and 0.12 pg/L, respectively. Additional details on LODs are provided in Laine et al. (2018) [5]. Total cord serum arsenic (C-tAs) concentrations were calculated as the sum of C-iAs, C-MMAs, and C-DMAs.
Determination of serum concentrations of folate, vitamin B12, and homocysteine
Folate, vitamin B12, and homocysteine were previously measured in maternal serum samples collected between admission to the hospital and the time of delivery [5]. All samples were stored at − 80 °C and shipped to Columbia University, New York, NY for the determination of serum concentrations. Maternal serum folate and vitamin B12 levels were quantified using radio protein-binding assay (SimulTRAC-S; MP Biomedicals, Orangeburg, NY, USA), and homocysteine levels were quantified via high performance liquid chromatography (HPLC) with fluorescence detection [26]. As described previously, and as in other iAs-exposed populations, B12 deficiency was defined at < 128 pM, folate deficiency was defined at < 9 nM, and hyperhomocysteinemia (high homocysteine for non-pregnant populations) was defined at > 10.4 nM [27, 28]. One-carbon metabolism factors were not measured in mothers (n = 7) with inefficient amounts of serum (< 200 μL) required for analyses.
Statistical analysis
Biomarker levels in maternal urine or infant cord serum below the LOD were adjusted using the following formula: LOD/ (√2) [29]. Descriptive statistics were calculated to describe select characteristics of the population. Relationships between variables were estimated using Spearman correlation tests. Correlation tests excluded mothers delivering preterm (n = 3) or those missing data on one-carbon metabolism factors (n = 7). Unadjusted and adjusted associations between biomarkers of iAs methylation efficiency (%) and first birth weight (grams) and then gestational age (weeks) were estimated overall and within levels of folate, vitamin B12, and homocysteine. Folate, vitamin B12, and homocysteine were dichotomized using clinical cut-offs (e.g., B12-deficient mothers vs. mothers with normal B12) and median splits. Using a directed acyclic graph approach, model covariates were selected for if they were: on an open backdoor path and/or (2) based on their a priori status as determinants of low birth weight [30] (see Additional file 1). The general model form was as follows:
where birth outcome is either birth weight or gestational age. X1 is the biomarker of iAs methylation efficiency (e.g., % MMAs). The β coefficient for Xi is the expected mean difference in birth weight or gestational age corresponding to a 1% increase in the respective biomarker. Zβ is a matrix of potential confounders. Model 1 included the following covariates: maternal age at delivery (years), smoking during pregnancy (0 = no, 1 = yes), and U-tAs (μg/L). Model 2 incorporated the same covariates and folate, vitamin B12, and homocysteine to account for the entire profile of one-carbon metabolism factors. Model 3 incorporated the same covariates as model 1 and excluded women reporting seafood intake within 7 days of providing samples to evaluate potential exposure misclassification resulting from additional sources of organic arsenic species in fish [31]. Models for birth weight excluded mothers delivering preterm (n = 3) to reduce confounding by gestational age [32], those missing data on smoking (n = 2), and those missing data on one-carbon metabolism factors (n = 7), resulting in the final inclusion of 188 mother-infant pairs in unadjusted and adjusted models (174 where C-tAs was evaluated). Models for gestational age excluded mothers missing data on smoking (n = 2) and one-carbon metabolism factors (n = 7), resulting in the final inclusion of 191 mother-infant pairs in unadjusted and adjusted models (177 where C-tAs was evaluated). The leave-one-out approach was also used in a sensitivity analysis to account for the dynamics of arsenic metabolism, as done in other studies [33, 34]. Results from models are presented as the mean difference (95% confidence interval (CI)) in birth weight or gestational age associated with a one-percent increase in each biomarker of iAs methylation efficiency.
Statistical interactions (representing potential effect modification) between biomarkers and one-carbon metabolism factors were also assessed on the additive scale using a likelihood ratio test (LRT) to test whether an interaction model fit the data better than a main effects model, with statistical significance defined at α < 0.10. The (1) interaction and (2) main effect model forms were as follows:
where X1 and X2 are the biomarker and one-carbon metabolism factor, respectively, and X3 represents the interaction between the two. P-values corresponding to each LRT are displayed alongside estimates from the corresponding stratified model.
Results
This study includes 200 mother-infant pairs (18–41 years old) participating in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort. Women were recruited in the State of Durango, Mexico, a region characterized by high exposure risk [20]. Select cohort demographics have previously been described [6]. In brief, nearly all (99.0%) women self-reported daily prenatal vitamin supplement intake and 21.7% reported recent seafood intake. Gestational ages ranged from 34 to 42 weeks and three infants were born preterm (< 37 completed weeks of gestation). Fifty-two percent of newborns were male, and the mean birth weight was 3339 g. The prevalence of low birth weight (< 2500 g) was minimal (n = 4), and the average birth weight among male and female newborns was 3453 (range of 2100 to 5120) grams and 3215 (range of 1800 to 4200) grams, respectively. The distributions of iAs, MMAs, DMAs, and total arsenic (tAs) measured in urine (U-) and cord serum (C-) are detailed in Table 1. The mean concentration of U-tAs was 37.54 μg/L, ranging from 4.33 to 319.74 μg/L. U-tAs concentrations were also highly correlated with drinking water concentrations of iAs [6]. Biomarkers of iAs exposure were below the limit of detection (LOD) for U-iAs (n = 7), U-MMAs (n = 4), C-iAs (n = 186), C-MMAs (n = 127), and C-DMAs (n = 54). Since fewer than 30% of samples contained measurements above the LOD for C-iAs and C-MMAs, only C-tAs was used to represent fetal exposure. The mean concentrations of vitamin B12, folate, and homocysteine were 130.97 pM, 40.93 nM, and 6.86 μM, respectively. Two women were deficient in folate (< 9 nM), 142 (74%) women were deficient in B12 (< 128 pM), and 14 (7%) women experienced hyperhomocysteinemia (> 10.4 μM).
Table 1.
Select demographic and exposure characteristics for the Biomarkers of Exposure to ARsenic (BEAR) cohort (N = 200), 2011–2012
| Characteristic | n (% non-missing) or mean, median [range] |
|---|---|
| Maternal age at delivery (years) | 24, 23 [18–41] |
| Smoking statusa | |
| Nonsmokers | 185 (93.4) |
| Current smokers | 13 (6.6) |
| Prenatal vitamin daily intake | 197 (99.0) |
| Recent seafood intakeb | |
| No | 155 (78.3) |
| Yes | 43 (21.7) |
| Gestational age (weeks) | |
| All | 39, 40 [34–42] |
| < 37 | 3 (1.5) |
| ≥ 37 | 197 (98.5) |
| Newborn sex | |
| Male | 104 (52.0) |
| Female | 96 (48.0) |
| Birth weight (grams) | |
| All | 3339, 3355 [1800–5120] |
| Male | 3453, 3490 [2100–5120] |
| Female | 3215, 3150 [1800–4200] |
| Low birth weight (< 2500 g) | 4 (2.0) |
| Drinking water iAs (μg/L) | 24.6, 13.0 [<LODc–236.0] |
| Urinary biomarkers (SG-adjusted) | |
| U-tAs (μg/L) | 37.54, 23.32 [4.33, 319.74] |
| U-iAs (%) | 6.08, 5.25 [0.77–45.13] |
| U-MMAs (%) | 6.43, 6.02 [0.68–24.86] |
| U-DMAs (%) | 87.49, 88.48 [32.68–96.65] |
| U-iAs (μg/L)d | 2.1, 1.3 [0.14–23.0] |
| U-MMAs (μg/L)e | 2.3, 1.4 [0.12–18.2] |
| U-DMAs (μg/L) | 33.1, 20.6 [1.4–292.5] |
| Cord serum biomarkers | |
| C-tAs (pg/mL) | 1.35, 1.24 [1.15–4.0] |
| C-iAs (%) | 78.83, 82.86 [25.63–88.96] |
| C-MMAs (%) | 4.69, 3.68 [2.90–19.13] |
| C-DMAs (%) | 16.48, 13.45 [6.88–67.61] |
| C-iAs (pg/L)f | <LOD |
| C-MMAs (pg/L)g | <LOD |
| C-DMAs (pg/L)h | 0.26, 0.17 [<LOD-2.70] |
| One-carbon metabolism factorsi | |
| Folate (nM) | 40.93, 38.59 [7.06–171.46] |
| Folate deficient (< 9 nM) | 2 (1.0) |
| Homocysteine (μM) | 6.86, 6.37 [4.05–19.42] |
| Hyperhomocysteinemia (> 10.4 μM) | 14 (7.3) |
| B12 (pM) | 130.97, 117.17 [48.03–747.91] |
| B12 deficient (< 128 pM) | 142 (74.6) |
aMissing data on smoking status for 2 mothers
bMissing data on seafood consumption for 2 mothers
climit of detection (LOD) = 0.456 μg/L
dN = 7 below LOD
eN = 4 below LOD
fN = 186 below LOD
gN = 127 below LOD
hN = 54 below LOD
iN = 7 missing data on one-carbon metabolism factors
Generally, biomarkers of iAs methylation efficiency were strongly correlated with each other (Spearman correlation coefficient (ρ) > ±0.60), but the strength of other correlations was weak (ρ < ±0.40) (Fig. 1). Vitamin B12 was inversely correlated with U-tAs (ρ = − 0.25), while homocysteine was positively correlated (ρ = 0.24). Plasma folate was negatively correlated with homocysteine (ρ = − 0.23) and birth weight (ρ = − 0.15). Birth weight was also negatively correlated with % U-MMAs (ρ = − 0.14). Gestational age was correlated with birth weight (ρ = 0.17) and vitamin B12 (ρ = − 0.13).
Fig. 1.
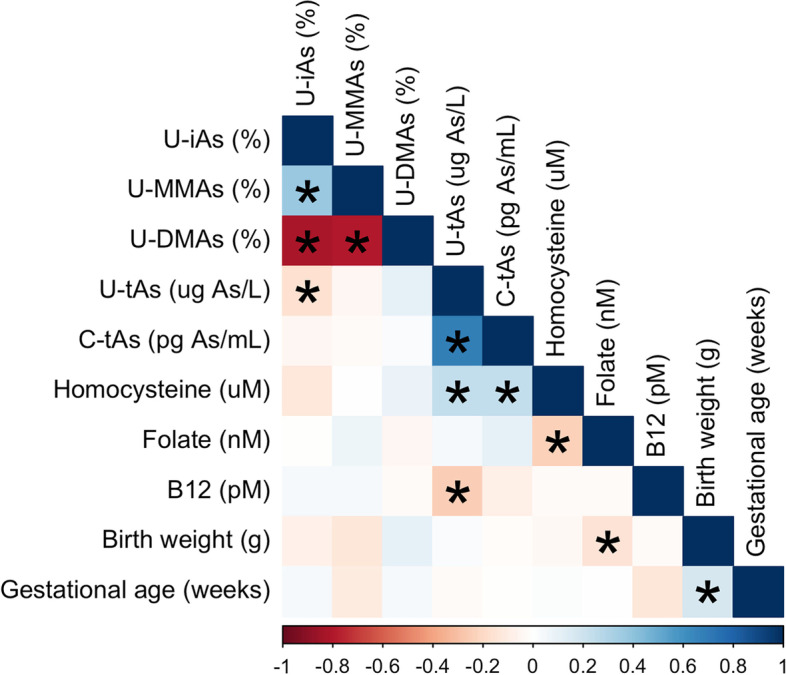
Correlations between biomarkers, one-carbon metabolism factors, and birth outcomes. Excluding preterm births (n = 3) and mothers missing data for one-carbon metabolism factors (n = 7). Significant (p < 0.05) Spearman correlations are indicated with an asterisk
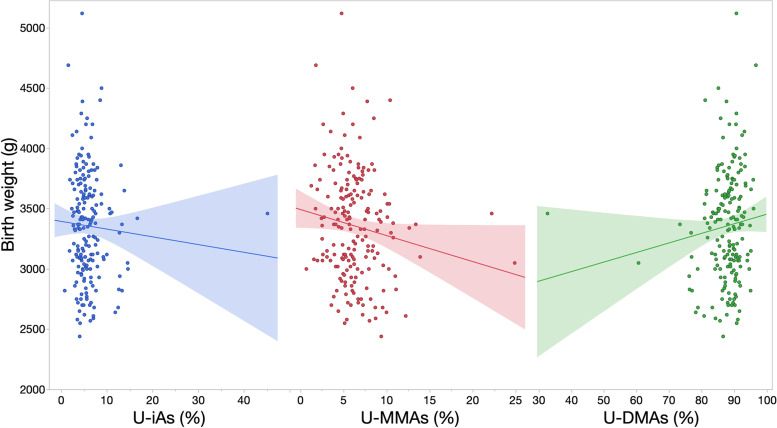
Associations (95% confidence intervals (CI)) between biomarkers of methylation efficiency and birth outcomes were first evaluated across all mother-infant pairs. Among urinary biomarkers, % U-MMAs was most strongly associated with birth weight in unadjusted and adjusted models (β = − 23.09, 95% CI: − 44.54, − 1.64) (Fig. 2, Table 2). Associations with C-tAs were greater in magnitude with wider confidence intervals (β = − 233.17, 95% CI: − 577.60, 111.26). Unadjusted associations were generally attenuated by the exclusion of potential outlying values (defined as values above Q3 + 1.5 x IQR or below Q1–1.5 x IQR) in the birth weight and biomarker distributions. Generally, adjustment for potential confounders, one-carbon metabolism factors, or excluding mothers reporting recent seafood intake attenuated associations between biomarkers and birth weight, with the exception of C-tAs, where β estimates became stronger and more negative. Using the leave-one-out approach, we included % U-MMAs as an additional covariate in the % U-DMAs model while adjusting for potential confounders in model 1. With this approach, the β estimate for % U-MMAs was strengthened (β = − 34.37, 95% CI: − 73.65, 4.91) and the β estimate for % U-DMAs shifted from positive to negative (β = − 6.66, 95% CI: − 26.06, 12.74) (see Additional file 2).
FIG. 2.
Unadjusted associations (standard error (SE)) between biomarkers and birth weight. Unadjusted linear regression models were fit to model the association (SE) between urinary biomarkers and birth weight, excluding infants born preterm (n = 3) and mothers missing data for one-carbon metabolism factors (n = 7)
Table 2.
Associations (95% CI) between biomarkers and birth weight (grams) and gestational age (weeks) overall
| Unadjusted β (95% CI) | Model 1a β (95% CI) | Model 2b β (95% CI) | Model 3c β (95% CI) | |
|---|---|---|---|---|
| Birth weight (grams) | ||||
| % U-iAs | −6.11 (− 22.80, 10.58) | −4.61 (− 21.21, 11.98) | −5.55 (− 22.28, 11.19) | − 3.65 (− 21.25, 13.96) |
| % U-MMAs | −20.84 (− 42.65, 0.97) | −23.09 (− 44.54, − 1.64) | − 22.92 (−44.50, − 1.35) | − 21.07 (−45.24, 3.11) |
| % U-DMAs | 7.62 (− 3.16, 18.40) | 7.56 (− 3.11, 18.22) | 7.91 (− 2.82, 18.65) | 6.42 (− 5.21, 18.05) |
| C-tAs (pg As/mL) | −51.47 (− 225.84, 122.90) | − 233.17 (− 577.60, 111.26) | −238.72 (− 585.25, 107.82) | − 385.54 (− 892.83, 121.76) |
| Gestational age (weeks) | ||||
| % U-iAs | 0.01 (−0.04, 0.05) | 0 (− 0.04, 0.05) | 0.01 (− 0.04, 0.05) | 0 (− 0.05, 0.05) |
| % U-MMAs | − 0.03 (− 0.09, 0.02) | −0.04 (− 0.10, 0.02) | −0.04 (− 0.10, 0.02) | −0.03 (− 0.10, 0.03) |
| % U-DMAs | 0.01 (− 0.02, 0.03) | 0.01 (− 0.02, 0.04) | 0.01 (− 0.02, 0.04) | 0.01 (− 0.02, 0.04) |
| C-tAs (pg As/mL) | −0.06 (− 0.54, 0.41) | 0.87 (0, 1.74) | 0.86 (− 0.01, 1.74) | 0.90 (− 0.31, 2.12) |
aModel 1 adjusts for smoking status during pregnancy, maternal age, and U-tAs
bModel 2 adjusts for smoking status during pregnancy, maternal age, U-tAs, B12, folate, and homocysteine
cModel 3 adjusts for smoking status during pregnancy, maternal age, and U-tAs and excludes mothers reporting recent seafood intake
Mean differences in gestational age were less than 0.5-days for urinary biomarkers and in the unadjusted model for C-tAs (Table 2). Neither adjustment for covariates nor exclusion of mothers reporting recent seafood intake had a substantial influence on estimates for urinary biomarkers. In contrast, each one-unit increase in C-tAs was associated with a nearly +1-week mean difference in gestational age after adjustment for covariates (β = 0.87, 95% CI: 0, 1.74) and exclusion of mothers reporting recent seafood intake (β = 0.90, 95% CI: − 0.31, 2.12).
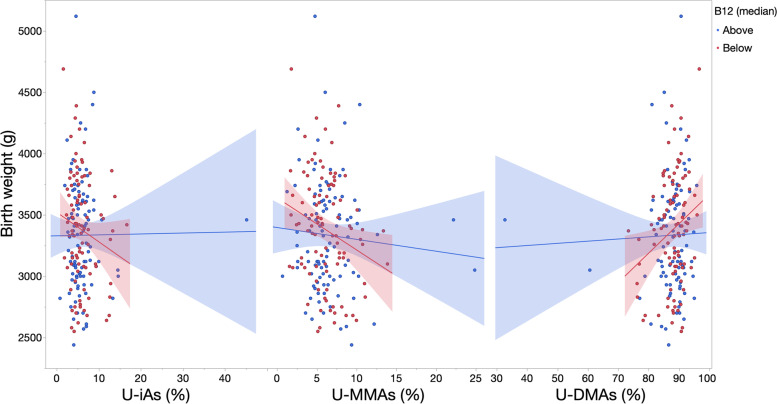
Adjusted models were next stratified by levels of one-carbon metabolism factors to evaluate heterogeneity of effects (Table 3). The negative associations between arsenic variables, including % U-iAs, % U-MMAs and C-tAs, and birth weight were stronger among women with vitamin B12 and folate below the median, as compared to those falling within a healthier range (Figs. 3 and 4). An opposite trend was observed in relation to % U-DMAs, where the negative association between % U-DMAs and birth weight was more positive in relation to lower levels of folate or vitamin B12. As an example shown in Table 3, each one-percent increase in U-MMAs was associated with a − 14.57 g (95% CI: − 57.98, 28.85) mean difference in birth weight among infants born to women with normal B12 levels versus a − 28.69 g (95% CI: − 53.97, − 3.42) mean difference in infants born to B12-deficient mothers.
Table 3.
Associations (95% CI) between biomarkers and birth weight (grams) within levels of one-carbon metabolism factors
| Biomarker | Level | Model 1a β (95% CI) | p | Model 2b β (95% CI) | p | Model 3c β (95% CI) | p |
|---|---|---|---|---|---|---|---|
| % U-iAs | Normal B12 | 2.07 (−22.72, 26.85) | 4.31 (− 20.01, 28.64) | 1.08 (−26.06, 28.22) | |||
| B12 deficient | −16.81 (− 42, 8.37) | 0.19 | − 18.27 (− 43.57, 7.03) | 0.15 | − 16.10 (− 43.96, 11.77) | 0.30 | |
| Normal homocysteine | −3.12 (− 20.05, 13.81) | − 3.29 (− 20.25, 13.66) | − 2.73 (− 20.69, 15.22) | ||||
| Hyperhomocysteinemia | −79.06 (− 196.3, 38.19) | 0.16 | − 81.20 (− 215.70, 53.31) | 0.15 | − 59.58 (− 161.44, 42.27) | 0.31 | |
| B12 > median | 1.99 (− 18.07, 22.04) | 3.61 (− 16.06, 23.28) | 0.10 (− 21.56, 21.77) | ||||
| B12 < =median | − 22.79 (− 54.67, 9.09) | 0.18 | − 24.60 (− 56.30, 7.11) | 0.15 | −13.66 (− 48.67, 21.35) | 0.47 | |
| Folate >median | 0.83 (− 17.05, 18.71) | 0.81 (− 17.25, 18.86) | −1.27 (− 20.05, 17.51) | ||||
| Folate <=median | −17.57 (− 53.40, 18.26) | 0.37 | − 17.58 (− 53.55, 18.39) | 0.38 | − 8.90 (− 49.20, 31.40) | 0.71 | |
| Homocysteine <median | 2.07 (− 16.33, 20.46) | 1.44 (− 16.93, 19.81) | 3.20 (− 16.47, 22.87) | ||||
| Homocysteine > = median | − 27.96 (− 62.33, 6.41) | 0.07 | −31.19 (− 64.67, 2.28) | 0.07 | − 28.63 (− 67.80, 10.54) | 0.12 | |
| % U-MMAs | Normal B12 | − 14.57 (− 57.98, 28.85) | − 6.43 (− 49.81, 36.95) | −17.65 (− 68.03, 32.73) | |||
| B12 deficient | −28.69 (− 53.97, − 3.42) | 0.47 | − 28.37 (− 53.63, − 3.10) | 0.45 | −24.52 (− 53.14, 4.09) | 0.71 | |
| Normal homocysteine | −21.71 (− 44.14, 0.72) | − 21.53 (− 44.08, 1.03) | − 21.91 (− 47.24, 3.42) | ||||
| Hyperhomocysteinemia | −63.29 (− 154.77, 28.19) | 0.41 | −90.66 (− 193.61, 12.30) | 0.39 | −35.73 (− 119.84, 48.38) | 0.76 | |
| B12 > median | − 10.51 (− 38.16, 17.14) | −5.30 (− 32.72, 22.12) | −11.86 (− 43.30, 19.57) | ||||
| B12 < =median | − 46.23 (− 82.26, − 10.19) | 0.12 | − 42.57 (− 78.65, − 6.49) | 0.12 | − 41.54 (− 84.48, 1.40) | 0.22 | |
| Folate >median | −18.46 (− 43.09, 6.18) | −17.69 (−42.41, 7.03) | −20.58 (− 47.45, 6.29) | ||||
| Folate <=median | −23.45 (− 65.74, 18.84) | 0.88 | −18.87 (− 62.27, 24.53) | 0.94 | − 14.34 (− 65.27, 36.59) | 0.82 | |
| Homocysteine <median | −8.40 (− 38.64, 21.84) | −9.69 (− 39.89, 20.52) | −11.77 (− 47.49, 23.94) | ||||
| Homocysteine > = median | −33.76 (− 64.90, − 2.61) | 0.21 | − 28.99 (− 59.88, 1.91) | 0.23 | −28.20 (−62.70, 6.29) | 0.51 | |
| % U-DMAs | Normal B12 | 1.25 (−15.84, 18.34) | −1.10 (− 18, 15.80) | 1.95 (−17.05, 20.94) | |||
| B12 deficient | 15.72 (0.92, 30.52) | 0.12 | 16.11 (1.30, 30.91) | 0.11 | 14.53 (−2.34, 31.41) | 0.23 | |
| Normal homocysteine | 6.45 (−4.53, 17.42) | 6.45 (−4.55, 17.45) | 6.04 (−5.92, 18) | ||||
| Hyperhomocysteinemia | 47.81 (−9.56, 105.17) | 0.16 | 57.96 (−5.51, 121.43) | 0.15 | 31.83 (−20.20, 83.86) | 0.38 | |
| B12 > median | 1.44 (−11.42, 14.31) | −0.37 (−13.05, 12.32) | 2.35 (−11.82, 16.52) | ||||
| B12 < =median | 24.85 (4.27, 45.42) | 0.05 | 24.22 (3.80, 44.64) | 0.05 | 20.13 (−4.31, 44.57) | 0.18 | |
| Folate >median | 3.59 (−7.90, 15.08) | 3.48 (−8.10, 15.06) | 4.70 (−7.52, 16.92) | ||||
| Folate <=median | 15.25 (−8.53, 39.04) | 0.40 | 13.78 (−10.32, 37.89) | 0.42 | 8.91 (−19.51, 37.33) | 0.76 | |
| Homocysteine <median | 0.50 (−12.34, 13.34) | 1.04 (−11.79, 13.86) | 0.18 (−13.96, 14.33) | ||||
| Homocysteine > = median | 19.8 (1.47, 38.12) | 0.05 | 18.86 (0.95, 36.77) | 0.05 | 18.63 (−2.23, 39.49) | 0.13 | |
| C-tAs | Normal B12 | −91.24 (− 1287.92, 1105.43) | −20.88 (− 1195.59, 1153.83) | −304.12 (− 1645.15, 1036.91) | |||
| B12 deficient | − 243.29 (−598.57, 111.98) | 0.40 | − 230.77 (− 586.09, 124.55) | 0.38 | − 359.19 (− 932.36, 213.97) | 0.41 | |
| Normal homocysteine | − 231.68 (− 582.59, 119.24) | − 239.46 (− 592.17, 113.25) | − 382.58 (− 906.63, 141.46) | ||||
| Hyperhomocysteinemia | −202.33 (− 2782.91, 2378.25) | 0.67 | −215.25 (− 3567.24, 3136.73) | 0.63 | − 475.41 (− 2951.40, 2000.58) | 0.54 | |
| B12 > median | −254.95 (− 1069.99, 560.09) | − 218.68 (− 1018.69, 581.33) | − 265.88 (− 1216.77, 685.01) | ||||
| B12 < =median | − 204.70 (− 594.89, 185.49) | 0.92 | −183.57 (− 571.92, 204.78) | 0.81 | − 425.17 (− 1116.72, 266.38) | 0.86 | |
| Folate >median | −238.02 (− 606.24, 130.21) | −210.04 (− 580.34, 160.25) | −313.47 (− 1004.61, 377.68) | ||||
| Folate <=median | −231.13 (− 987.04, 524.78) | 0.40 | −210.99 (− 970.89, 548.9) | 0.42 | −475.40 (− 1354.61, 403.81) | 0.67 | |
| Homocysteine <median | − 291.31 (−1004.29, 421.68) | −195.95 (− 915.66, 523.77) | −72.96 (− 967.59, 821.68) | ||||
| Homocysteine > = median | − 360.07 (− 816.94, 96.80) | 0.08 | − 422.54 (− 866.64, 21.56) | 0.07 | − 597.58 (− 1327.28, 132.12) | 0.88 |
p-values represent a likelihood ratio test comparing the main effects model to a full model including an interaction term
aModel 1 adjusts for smoking status during pregnancy, maternal age, and U-tAs
bModel 2 adjusts for smoking status during pregnancy, maternal age, U-tAs, and additional one-carbon metabolism factors
cModel 3 adjusts for smoking status during pregnancy, maternal age, and U-tAs and excludes mothers reporting recent seafood intake
Fig. 3.
Unadjusted associations (SE) between biomarkers and birth weight within levels of vitamin B12. Unadjusted linear regression models were fit to model the association (SE) between urinary biomarkers and birth weight for each level of vitamin B12 (greater or less than/equal to the median), excluding infants born preterm (n = 3) and mothers missing data for one-carbon metabolism factors (n = 7)
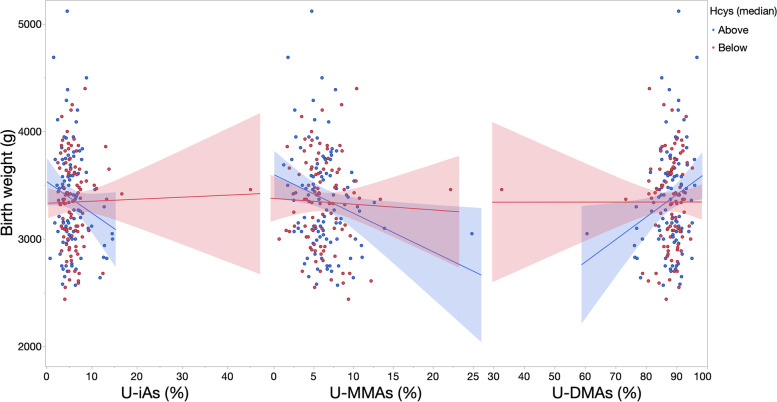
Fig. 4.
Unadjusted associations (SE) between biomarkers and birth weight within levels of homocysteine. Unadjusted linear regression models were fit to model the association (SE) between urinary biomarkers and birth weight for each level of homocysteine (less or greater than/equal to the median), excluding infants born preterm (n = 3) and mothers missing data for one-carbon metabolism factors (n = 7)
These trends were conserved in relation to homocysteine, where the negative association between biomarkers and birth weight was stronger among infants born to mothers with hyperhomocysteinemia or serum concentrations equal to or greater than the median. As an example, shown in Table 3, each one-percent increase in U-MMAs was associated with a − 21.71 g (95% CI: − 44.14, 0.72) mean difference in birth weight among infants born to women with normal levels homocysteine versus a − 63.29 g (95% CI: − 154.77, 28.19) mean difference among infants born to mothers with hyperhomocysteinemia. Also seen in Table 3, the negative association between biomarkers and birth weight was strongest, yet most imprecise, in relation to C-tAs. Neither adjustment for additional one-carbon metabolism factors nor exclusion of mothers reporting seafood intake had a substantial influence on trends in estimates. Effect modification was significant (likelihood ratio test (LRT) p-value < 0.10) on the additive scale for homocysteine (in relation to % U-iAs, % U-DMAs, and C-tAs) and vitamin B12 (in relation to % U-DMAs).
Models evaluating gestational age were also stratified by levels of one-carbon metabolism factors (Table 3). No meaningful associations between urinary arsenic variables and gestational age were observed. However, as seen in Table 3, the association between C-tAs and gestational age ranged from − 0.45-weeks to + 2.87-weeks, albeit with wider confidence intervals. Generally, the positive association between C-tAs and gestational age was stronger among infants born to mothers with higher serum levels of vitamin B12. This trend was conserved in relation to homocysteine. Effect modification was significant on the additive scale for folate (in relation to % U-iAs, % U-MMAs, and % U-DMAs) despite associations not being meaningful Table 4.
Table 4.
Associations (95% CI) between arsenic biomarkers and gestational age (weeks) within levels of one-carbon metabolism factors
| Biomarker | Level | Model 1a β (95% CI) | p | Model 2b β (95% CI) | p | Model 3c β (95% CI) N = 145 | p |
|---|---|---|---|---|---|---|---|
| % U-iAs | Normal B12 | 0 (− 0.05, 0.05) | 0 (− 0.05, 0.05) | − 0.01 (− 0.06, 0.05) | |||
| B12 deficient | 0.01 (− 0.06, 0.08) | 0.87 | 0.01 (− 0.06, 0.08) | 0.93 | 0.01 (− 0.07, 0.09) | 0.97 | |
| Normal homocysteine | 0 (− 0.04, 0.05) | 0.02 (− 0.03, 0.07) | 0 (− 0.05, 0.05) | ||||
| Hyperhomocysteinemia | − 0.02 (− 0.19, 0.15) | 0.92 | − 0.02 (− 0.10, 0.06) | 0.92 | − 0.02 (− 0.26, 0.22) | 0.90 | |
| B12 > median | 0.02 (− 0.04, 0.07) | 0.03 (− 0.02, 0.08) | 0.01 (− 0.04, 0.07) | ||||
| B12 < =median | − 0.02 (− 0.10, 0.06) | 0.36 | − 0.06 (− 0.15, 0.04) | 0.39 | 0 (− 0.09, 0.09) | 0.65 | |
| Folate >median | 0.02 (− 0.03, 0.07) | 0 (− 0.04, 0.05) | 0.02 (− 0.03, 0.07) | ||||
| Folate <=median | − 0.05 (− 0.15, 0.04) | 0.09 | − 0.01 (− 0.20, 0.19) | 0.07 | − 0.05 (− 0.15, 0.06) | 0.21 | |
| Homocysteine <median | 0.01 (− 0.05, 0.06) | 0.01 (− 0.05, 0.06) | 0 (− 0.06, 0.05) | ||||
| Homocysteine > = median | − 0.01 (− 0.10, 0.07) | 0.63 | − 0.01 (− 0.10, 0.08) | 0.67 | 0.01 (− 0.09, 0.11) | 0.96 | |
| % U-MMAs | Normal B12 | −0.01 (− 0.11, 0.08) | − 0.02 (− 0.11, 0.08) | − 0.03 (− 0.13, 0.07) | |||
| B12 deficient | −0.06 (− 0.13, 0.02) | 0.35 | − 0.06 (− 0.13, 0.01) | 0.36 | −0.04 (− 0.12, 0.04) | 0.72 | |
| Normal homocysteine | −0.04 (− 0.10, 0.02) | 0.01 (− 0.07, 0.08) | − 0.04 (− 0.11, 0.03) | ||||
| Hyperhomocysteinemia | 0.01 (− 0.13, 0.15) | 0.67 | −0.09 (− 0.18, 0) | 0.57 | 0.03 (− 0.16, 0.21) | 0.66 | |
| B12 > median | 0 (−0.07, 0.08) | 0.01 (− 0.06, 0.08) | 0 (−0.08, 0.08) | ||||
| B12 < =median | −0.09 (− 0.17, 0) | 0.10 | − 0.11 (− 0.22, 0) | 0.08 | −0.05 (− 0.16, 0.06) | 0.43 | |
| Folate >median | 0 (−0.07, 0.07) | −0.04 (− 0.11, 0.02) | 0.01 (− 0.07, 0.08) | ||||
| Folate <=median | −0.1 (− 0.21, 0) | 0.08 | 0 (− 0.16, 0.17) | 0.04 | − 0.12 (− 0.24, 0.01) | 0.11 | |
| Homocysteine <median | −0.06 (− 0.15, 0.03) | −0.06 (− 0.15, 0.03) | −0.07 (− 0.17, 0.03) | ||||
| Homocysteine > = median | −0.02 (− 0.10, 0.06) | 0.40 | −0.02 (− 0.10, 0.06) | 0.44 | 0.01 (− 0.08, 0.10) | 0.25 | |
| % U-DMAs | Normal B12 | 0 (−0.03, 0.04) | 0 (−0.03, 0.04) | 0.01 (−0.03, 0.05) | |||
| B12 deficient | 0.02 (−0.03, 0.06) | 0.41 | 0.02 (−0.03, 0.06) | 0.44 | 0.01 (−0.04, 0.06) | 0.70 | |
| Normal homocysteine | 0.01 (−0.02, 0.04) | −0.01 (− 0.04, 0.02) | 0.01 (− 0.02, 0.04) | ||||
| Hyperhomocysteinemia | 0 (−0.09, 0.09) | 0.90 | 0.04 (−0.02, 0.09) | 0.84 | −0.01 (− 0.13, 0.12) | 0.89 | |
| B12 > median | −0.01 (− 0.04, 0.03) | − 0.01 (− 0.04, 0.02) | 0 (− 0.04, 0.03) | ||||
| B12 < =median | 0.04 (− 0.02, 0.09) | 0.11 | 0.06 (0, 0.12) | 0.11 | 0.02 (−0.05, 0.08) | 0.42 | |
| Folate >median | −0.01 (− 0.04, 0.02) | 0.01 (− 0.02, 0.04) | − 0.01 (− 0.04, 0.02) | ||||
| Folate <=median | 0.06 (0, 0.12) | 0.03 | 0 (−0.10, 0.11) | 0.01 | 0.06 (−0.01, 0.13) | 0.07 | |
| Homocysteine <median | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.05) | 0.01 (−0.03, 0.05) | ||||
| Homocysteine > = median | 0.01 (−0.04, 0.06) | 0.91 | 0.01 (−0.04, 0.06) | 0.89 | −0.01 (− 0.06, 0.05) | 0.70 | |
| C-tAs | Normal B12 | 1.53 (−0.01, 3.08) | 1.50 (−0.04, 3.04) | 1.46 (−0.06, 2.98) | |||
| B12 deficient | 0.39 (−0.63, 1.41) | 0.10 | 0.38 (−0.65, 1.41) | 0.11 | 0.18 (−1.47, 1.83) | 0.06 | |
| Normal homocysteine | 0.94 (0.03, 1.85) | 1.91 (0.44, 3.38) | 0.99 (−0.28, 2.26) | ||||
| Hyperhomocysteinemia | −0.37 (−3.33, 2.60) | 0.77 | 0.28 (−0.74, 1.31) | 0.83 | −0.97 (−4.34, 2.40) | 0.63 | |
| B12 > median | 2.16 (0.70, 3.62) | 0.18 (−0.84, 1.20) | 1.90 (0.38, 3.43) | ||||
| B12 < =median | 0.30 (−0.71, 1.31) | 0.14 | 2.41 (0.87, 3.95) | 0.11 | 0.10 (−1.76, 1.95) | 0.07 | |
| Folate >median | 0.15 (−0.86, 1.16) | 0.96 (0.05, 1.88) | −0.45 (−2.32, 1.41) | ||||
| Folate <=median | 2.38 (0.87, 3.88) | 0.21 | −0.42 (−4.25, 3.41) | 0.20 | 2.34 (0.74, 3.93) | 0.86 | |
| Homocysteine <median | 2.26 (0.54, 3.98) | 2.32 (0.57, 4.07) | 2.87 (0.91, 4.83) | ||||
| Homocysteine > = median | 0.48 (−0.66, 1.62) | 0.10 | 0.53 (−0.63, 1.68) | 0.09 | −0.37 (−2.16, 1.42) | 0.18 |
p-values represent a likelihood ratio test comparing the main effects model to a full model including an interaction term
NS Not significant
aModel 1 adjusts for smoking status during pregnancy, maternal age, and U-tAs
bModel 2 adjusts for smoking status during pregnancy, maternal age, U-tAs, and additional one-carbon metabolism factors
cModel 3 adjusts for smoking status during pregnancy, maternal age, and U-tAs and excludes mothers reporting recent seafood intake
Discussion
One-carbon metabolism factors modify iAs toxicity and the severity of iAs-associated health outcomes among adults [19, 35], but are understudied during the prenatal period [15]. To this end, we tested effect modification of associations between biomarkers of iAs methylation efficiency and birth outcomes by folate, vitamin B12, and homocysteine in a cohort of mother-infant pairs residing in Durango, Mexico. This region is characterized by high levels of arsenic in drinking water [20], ranging up to 236 μg/L. The average concentration of 37.54 U-tAs (μg/L) measured in the BEAR cohort was an order of magnitude higher than those measured in US pregnancy cohorts (range: 1.96–28.75 μg/L) [36, 37], approaching those observed in highly exposed populations in Bangladesh (range: 5.1–325 μg/L) [38, 39]. Effect modification by vitamin B12 and homocysteine was significant on the additive scale for some associations with birth weight. Using stratified models, we found that the negative association between arsenic biomarkers and birth weight was stronger among infants born to mothers with lower levels of vitamin B12 or folate. Findings for gestational age were less compelling, as meaningful differences were only observed with respect to C-tAs. These findings highlight a potential role for maternal diet in modifying iAs-associated lower birth weight.
Seventy-four percent of mothers in the BEAR cohort were deficient in vitamin B12, a clinical condition independently associated with maternal and neonatal outcomes, including impaired fetal growth [40]. The relationship between vitamin B12 intake and the distribution of iAs and metabolites has varied between studies (as reviewed in [15, 41]), but we previously identified higher mean concentrations of U-tAs in B12-deficient mothers in the BEAR cohort [5]. Higher levels of MMAs in maternal urine were negatively associated with birth weight and placenta weight in this cohort [6]. Since MMAsIII is established as the most toxic arsenic species in most tissues including placenta [8, 16, 17], it is notable that we identified stronger negative associations between % U-MMAs and birth weight among infants born to women with lower B12 levels. Among infants born to mothers with B12 levels below the median, a 10% increase in % U-MMAs was associated with a mean difference in birth weight exceeding that associated with smoking 6 to 10 cigarettes daily during pregnancy [42]. Group differences were generally more pronounced between strata of vitamin B12, as compared to between strata of folate. According to the “methylfolate trap” hypothesis, B12 deficiency prevents a) the remethylation of homocysteine to methionine, thereby reducing SAM synthesis, and b) the metabolism of 5-methyl-THF to THF. This causes 5-methyl-THF, homocysteine and SAH to accumulate in cells and presents as a functional folate deficiency [40]. Given the high prevalence of mothers with B12 deficiency, this functional folate deficiency may explain why group differences were more pronounced between strata of vitamin B12. Also, overt B12 deficiency was highly prevalent, whereas few participants appeared to be overtly folate deficient, even in the low folate strata. Nonetheless, higher levels of folate were protective against iAs-associated lower birth weight in this study, as seen in prior studies conducted in pregnant populations using single [43] and multi-nutrient [44] approaches.
The present study is among the first to examine homocysteine in relation to iAs-associated lower birth weight. We observed stronger and more negative associations between % U-iAs, % U-MMAs and C-tAs and birth weight in higher maternal homocysteine strata. Homocysteine is a metabolite of one-carbon metabolism that is independently associated with preeclampsia, intrauterine growth retardation, and other adverse pregnancy outcomes [45, 46]. Prior research in adult populations exposed to iAs highlight a relationship between higher levels of homocysteine and lower % DMAs [47, 48], reflecting incomplete methylation of iAs to DMAs. It is also speculated that homocysteine competitively inhibits transport of other amino acids to impact syncytiotrophoblast metabolism and function, as well as the supply of nutrients to the fetus [14].
Associations between C-tAs and birth outcomes were larger and more imprecise, as compared to urinary biomarkers. Generally, the association between C-tAs and gestational age was more positive among infants born to mothers with higher vitamin B12 or lower homocysteine levels, but less consistent for associations with birth weight. Larger, more imprecise estimates likely reflect the narrow range of C-tAs (1.15 to 4.0 pg As/mL). Inference is further complicated by the use of left-censored data, given the number of samples below the LOD for cord serum arsenicals.
This study is not without limitations. Given its size, the BEAR cohort is underpowered to detect statistical interactions. Moreover, stratified analyses required even smaller sample sizes, decreasing precision. Second, one-carbon metabolism factors were assessed at the end of pregnancy and maternal plasma levels are altered over the course of pregnancy in order to support fetal growth [14]. Early pregnancy may be a more critical window of susceptibility given the association between iAs exposure and the expression of genes related to placentation and imprinting [10, 11]. Given the cross-sectional design of the study, there is also a potential for reverse causation, where iAs may hinder one-carbon metabolism. Additionally, protein intake and maternal body mass index (BMI) are important potential confounders not measured in this study. We previously identified a positive association between BMI and % U-DMAs among adults [49], and have also identified a relationship between pre-pregnancy BMI and the expression of genes underlying nutrient metabolism in the placenta [50]. Despite 74% of mothers in the BEAR cohort being deficient in vitamin B12, nearly all reported daily prenatal vitamin intake. This disparity may reflect recall bias or the consistent decrease in serum vitamin B12 that occurs throughout pregnancy as it is transported across the placenta to the developing fetus [51–53]. Future studies should utilize longitudinal data with repeated urinary measures and capture potential sources of arsenic exposure (e.g., drinking water and/or diet). Finally, we evaluate biomarkers of prenatal iAs methylation individually, but mixtures can exert joint effects that differ from individual species. Multi-nutrient deficiencies are also prevalent in undernourished populations [54], limiting the generalizability of our findings to other iAs-exposed populations, especially those in regions characterized by food insecurity.
Conclusions
In a cross-sectional study, we found that associations between % U-MMAs and birth weight were attenuated by elevated levels of vitamin B12 and folate in maternal serum. These trends were also observed using strata defined by clinical cut-offs. Differences in rates of conversion of MMAs to DMAs may occur according to levels of one-carbon metabolism factors. This study may have public health implications as it positions B vitamins as potential modifiers of the harms of exposure to iAs during pregnancy, but validation is needed given the limitations of our study design. In most arsenic-endemic regions, folic acid fortification is not mandated [19]. Vitamin B12 deficiency is also prevalent in regions characterized by iAs-contamination of drinking water [40]. Future studies should measure arsenic biomarkers, micronutrients, and fetal growth parameters in early pregnancy and across each trimester to capture the dynamic relationship between one-carbon metabolism, fetal development, and iAs exposure that may not be observed in this study. The incorporation of unmeasured potential confounders (e.g., additional micronutrients, energy and protein intake, and BMI) and social factors will also be important given the reality of interactions between environmental and structural factors. Though we demonstrate potential protection against the harms of prenatal arsenic exposure by B vitamins, efforts supporting remediation in drinking water should remain priority.
Supplementary Information
Additional file 1: Figure S1. Simplified directed acyclic graph (DAG).
Additional file 2: Table S1. Results from models fit using the leave-one-out approach.
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number F31ES033925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- iAs
Inorganic arsenic
- MMAs
Monomethylated arsenic
- DMAs
Dimethylated arsenic
- LRT
Likelihood ratio test
- SAM
S-adenosylmethionine
- 5-methyl-THF
5-methyl-tetrahydrofolate
- SAH
S-adenosylhomocysteine
- BEAR
Biomarkers of Exposure to ARsenic
- SG
Specific gravity
- U-tAs
Total urinary arsenic
- U-iAs
Urinary inorganic arsenic
- U-MMAs
Urinary monomethylated arsenic
- U-DMAs
Urinary dimethylated arsenic
- C-tAs
Total cord serum arsenic
- C-iAs
Cord serum inorganic arsenic
- C-MMAs
Cord serum monomethylated arsenic
- C-DMAs
Cord serum dimethylated arsenic
- HG-CT-ICP-MS
Hydride generation with cryotrapping coupled to inductively coupled plasma mass spectrometry
- LOD
Limit of detection
- CI
Confidence interval
- sFLT1
Soluble Fms-Like Tyrosine Kinase-1
- VEGF
Vascular endothelial growth factor
- KCNQ1
Potassium Voltage-Gated Channel Subfamily Q Member 1
- DAG
Directed acyclic graph
Authors’ contributions
JC and PB conceptualized, analyzed, and interpreted the data. JC completed data curation, preparation of the original draft, and data visualization. MS, RF, MG, MA, and GG carried out study recruitment and sample processing. All authors read and approved the final manuscript.
Funding
This work was supported by the National Institute of Environmental Health Sciences [grant numbers F31ES033925, P42ES031007, T32ES007018, R01ES019315] and the Royster Society of Fellows.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All study procedures were approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill and the Universidad Juárez del Estado de Durango, and each participant gave written, informed consent to participate and provide biological samples.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Arsenic - Fact sheet. 2018. [Google Scholar]
- 2.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 3.Milton AH, et al. A review of the effects of chronic arsenic exposure on adverse pregnancy outcomes. Int J Environ Res Public Health. 2017;14(6):1–31. doi: 10.3390/ijerph14060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quansah R, et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect. 2015;123(5):412–421. doi: 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laine JE, et al. Maternal one carbon metabolism and arsenic methylation in a pregnancy cohort in Mexico. J Expo Sci Environ Epidemiol. 2018;28(5):505–514. doi: 10.1038/s41370-018-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laine JE, et al. Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect. 2015;123(2):186–192. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119(2):258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meakin CJ, et al. Inorganic arsenic as an endocrine disruptor: modulation of the glucocorticoid receptor pathway in placental cells via CpG methylation. Chem Res Toxicol. 2019;32(3):493–499. doi: 10.1021/acs.chemrestox.8b00352. [DOI] [PubMed] [Google Scholar]
- 9.Rager JE, et al. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014;55(3):196–208. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remy S, et al. Expression of the sFLT1 gene in cord blood cells is associated to maternal arsenic exposure and decreased birth weight. Plos One. 2014;9(3):e92677. doi: 10.1371/journal.pone.0092677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rojas D, et al. Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicol Sci. 2015;143(1):97–106. doi: 10.1093/toxsci/kfu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fry RC, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. Plos Genet. 2007;3(11):e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kile ML, et al. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics. 2014;9(5):774–782. doi: 10.4161/epi.28153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalhan SC. One carbon metabolism in pregnancy: Impact on maternal, fetal and neonatal health. Mol Cell Endocrinol. 2016;435:48–60. doi: 10.1016/j.mce.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abuawad A, et al. Nutrition, one-carbon metabolism and arsenic methylation. Toxicology. 2021;457:152803. doi: 10.1016/j.tox.2021.152803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrick JS, et al. Monomethylarsonous acid (MMA (III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163(2):203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- 17.Shankar S, Shanker U, Shikha Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. ScientificWorldJ. 2014;2014:304524. doi: 10.1155/2014/304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilsner JR, et al. Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environ Health Perspect. 2009;117(2):254–260. doi: 10.1289/ehp.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozack AK, Saxena R, Gamble MV. Nutritional influences on one-carbon metabolism: effects on arsenic methylation and toxicity. Annu Rev Nutr. 2018;38:401–429. doi: 10.1146/annurev-nutr-082117-051757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alarcon-Herrera MT, et al. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci Total Environ. 2020;698:134168. doi: 10.1016/j.scitotenv.2019.134168. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Zavala A, et al. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer) J Anal At Spectrom. 2008;23:342–351. doi: 10.1039/B706144G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Zavala A, et al. Analysis of arsenical metabolites in biological samples. Curr Protoc Toxicol. 2009;42:4 33 1–4 33 17. doi: 10.1002/0471140856.tx0433s42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nermell B, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106(2):212–218. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Matousek T, et al. Direct speciation analysis of arsenic in whole blood and blood plasma at low exposure levels by hydride generation-cryotrapping-inductively coupled plasma mass spectrometry. Anal Chem. 2017;89(18):9633–9637. doi: 10.1021/acs.analchem.7b01868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilschefski SC, Baxter MR. Inductively coupled plasma mass spectrometry: introduction to analytical aspects. Clin Biochem Rev. 2019;40(3):115–133. doi: 10.33176/AACB-19-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer CM, Huff DL, Gunter EW. Rapid and accurate HPLC assay for plasma total homocysteine and cysteine in a clinical laboratory setting. Clin Chem. 1999;45(2):290–2. doi: 10.1093/clinchem/45.2.290. [DOI] [PubMed] [Google Scholar]
- 27.Howe CG, et al. Folate and cobalamin modify associations between S-adenosylmethionine and methylated arsenic metabolites in arsenic-exposed Bangladeshi adults. J Nutr. 2014;144(5):690–697. doi: 10.3945/jn.113.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238–S244. doi: 10.1177/15648265080292S129. [DOI] [PubMed] [Google Scholar]
- 29.Gardner RM, et al. Arsenic methylation efficiency increases during the first trimester of pregnancy independent of folate status. Reprod Toxicol. 2011;31(2):210–218. doi: 10.1016/j.reprotox.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- 31.Navas-Acien A, et al. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res. 2011;111(1):110–118. doi: 10.1016/j.envres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001;30(6):1233–1241. doi: 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- 33.Spratlen MJ, et al. The association of arsenic exposure and arsenic metabolism with the metabolic syndrome and its individual components: prospective evidence from the strong heart family study. Am J Epidemiol. 2018;187(8):1598–1612. doi: 10.1093/aje/kwy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo CC, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care. 2015;38(4):620–627. doi: 10.2337/dc14-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vahter ME. Interactions between arsenic-induced toxicity and nutrition in early life. J Nutr. 2007;137(12):2798–2804. doi: 10.1093/jn/137.12.2798. [DOI] [PubMed] [Google Scholar]
- 36.Howe CG, et al. Arsenic and birth outcomes in a predominately lower income Hispanic pregnancy cohort in Los Angeles. Environ Res. 2020;184:109294. doi: 10.1016/j.envres.2020.109294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih YH, Scannell Bryan M, Argos M. Association between prenatal arsenic exposure, birth outcomes, and pregnancy complications: an observational study within the National Children’s Study cohort. Environ Res. 2020;183:109182. doi: 10.1016/j.envres.2020.109182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S, et al. Determinants of arsenic methylation efficiency and urinary arsenic level in pregnant women in Bangladesh. Environ Health. 2019;18(1):94. doi: 10.1186/s12940-019-0530-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gliga AR, et al. Prenatal arsenic exposure is associated with increased plasma IGFBP3 concentrations in 9-year-old children partly via changes in DNA methylation. Arch Toxicol. 2018;92(8):2487–2500. doi: 10.1007/s00204-018-2239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and Perinatal Health. Adv Nutr. 2015;6(5):552–563. doi: 10.3945/an.115.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sijko M, Kozlowska L. Influence of dietary compounds on arsenic metabolism and toxicity. Part II-Human Studies. Toxics. 2021;9(10):1–23. [DOI] [PMC free article] [PubMed]
- 42.Kataoka MC, et al. Smoking during pregnancy and harm reduction in birth weight: a cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):67. doi: 10.1186/s12884-018-1694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, et al. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Environ Health Perspect. 2008;116(3):315–321. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, et al. Maternal blood cadmium, lead and arsenic levels, nutrient combinations, and offspring birthweight. BMC Public Health. 2017;17(1):354. doi: 10.1186/s12889-017-4225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaiday AN, et al. Effect of homocysteine on pregnancy: a systematic review. Chem Biol Interact. 2018;293:70–76. doi: 10.1016/j.cbi.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Murphy MM, Fernandez-Ballart JD. Homocysteine in pregnancy. Adv Clin Chem. 2011;53:105–137. doi: 10.1016/B978-0-12-385855-9.00005-9. [DOI] [PubMed] [Google Scholar]
- 47.Niedzwiecki MM, et al. Serum homocysteine, arsenic methylation, and arsenic-induced skin lesion incidence in Bangladesh: a one-carbon metabolism candidate gene study. Environ Int. 2018;113:133–142. doi: 10.1016/j.envint.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gamble MV, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113(12):1683–1688. doi: 10.1289/ehp.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bommarito PA, et al. One-carbon metabolism nutrient intake and the association between body mass index and urinary arsenic metabolites in adults in the Chihuahua cohort. Environ Int. 2019;123:292–300. doi: 10.1016/j.envint.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark J, et al. Pre-pregnancy BMI-associated miRNA and mRNA expression signatures in the placenta highlight a sexually-dimorphic response to maternal underweight status. Sci Rep. 2021;11(1):15743. doi: 10.1038/s41598-021-95051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Behere RV, et al. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: a systematic review and implications for policy in India. Front Endocrinol (Lausanne) 2021;12:619176. doi: 10.3389/fendo.2021.619176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bae S, et al. Vitamin B-12 status differs among pregnant, lactating, and control women with equivalent nutrient intakes. J Nutr. 2015;145(7):1507–1514. doi: 10.3945/jn.115.210757. [DOI] [PubMed] [Google Scholar]
- 53.Sukumar N, et al. Prevalence of vitamin B-12 insufficiency during pregnancy and its effect on offspring birth weight: a systematic review and meta-analysis. Am J Clin Nutr. 2016;103(5):1232–1251. doi: 10.3945/ajcn.115.123083. [DOI] [PubMed] [Google Scholar]
- 54.Bailey RL, West KP, Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Simplified directed acyclic graph (DAG).
Additional file 2: Table S1. Results from models fit using the leave-one-out approach.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.