Abstract
Coronavirus disease 2019 (COVID-19) can lead to an illness characterized by persistent symptoms which affect various organs and systems, known as long-COVID. This study aimed to assess the prevalence and clinical characteristics of long-COVID in children with immunodeficiency, in comparison to those without. A self-constructed questionnaire was created, which included questions regarding the child’s general health, the course of their COVID-19, their symptoms of long-COVID and its impact on their daily functioning, the diagnosis of multisystem inflammatory syndrome (MIS-C), and vaccination status. The questionnaire was completed by parents of 147 children — 70 children with a diagnosis of immunodeficiency (47.6%) and 77 who were immunocompetent (52.4%). Immunocompetent children were more significantly affected by long-COVID than those immunocompromised. Its prevalence in the first 12-week post-infection was 60.0% and 35.7% in these groups, respectively. Beyond this period, these percentages had dropped to 34.6% and 11.43%, respectively. Children who were immunocompetent reported more often symptoms of fatigue, reduced exercise tolerance, and difficulty concentrating. Meanwhile, there was a slight increase in complaints of gastrointestinal symptoms in immunocompromised patients. The risk of developing long-COVID increased with age and COVID-19 severity in both groups. Furthermore, the daily activities of immunocompetent children were limited more frequently (41.8%) than for those who were immunocompromised (25%).
Conclusions: Although immunocompromised children experienced long-COVID, its prevalence and impact on daily functioning were significantly lower than among immunocompetent children. However, as the pathomechanisms of long-COVID are not yet fully understood, it is not currently possible to fully explain these findings.
|
What is Known: • Long COVID is characterized by persistent symptoms following COVID-19, which can affect various tissues and organs, as well as mental health. • Due to the similar course of COVID-19 — mainly mild or asymptomatic — among children with and without immunodeficiency, the question arises, over whether the prevalence and severity of long-COVID is also similar in both groups. | |
|
What is New: • Immunocompromised children also suffer from long-COVID, but the prevalence is significantly lower than in the immunocompetent group of children. • The potential causes of less frequent and milder long-COVID in this group may be the milder course of COVID-19 and the state of reduced immunity protecting against neuroinflammation. |
Keywords: Long-COVID, SARS-CoV-2, Immunodeficiency, Pediatric population
Introduction
Our understanding of the course of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children has evolved over the last two and a half years of the current coronavirus pandemic. Initially, children were typically asymptomatic or had mild COVID-19 symptoms, and hospitalization rates were low and complications were rare [1]. Following the introduction of universal vaccinations in adults and adolescents, children under the age of 5 years became the primary victims of the disease, besides unvaccinated people. Indeed, they have been presenting with more severe symptoms and the hospitalization rate became slightly higher. Alongside this, two significant complications of COVID-19 have appeared, namely MIS-C and long-COVID [2]. While the former is a complication that occurs only in children and adolescents, the latter has affected all age groups and has only recently begun to attract the attention of pediatricians.
Long-COVID, otherwise known as post-COVID syndrome, is characterized by persistent symptoms following COVID-19. Such symptoms can affect the sensory, neurological, cardiovascular, and respiratory systems, as well as mental health. Since there are no strictly defined diagnostic criteria, researchers and physicians have attributed more than 200 symptoms to the syndrome. Some of the most common symptoms described are fatigue, dyspnea, cough, chest pain, headache, concentration difficulties, and sleep disturbances. According to guidelines published by the National Institute for Health and Care Experience (NICE), long-COVID can be divided into two categories [3]:
Ongoing symptomatic COVID-19: signs and symptoms of COVID-19 from 4 weeks up to 12-week post-infection.
Post-COVID-19 syndrome: signs and symptoms that continue or develop after SARS-CoV-2 infection, persist for more than 12 weeks from the onset of the disease and are not explained by alternative diagnoses. It usually manifests by a group of symptoms, which can fluctuate, change over time, and affect any part of the body.
The course of COVID-19 is mainly asymptomatic or oligosymptomatic in children and is thought to be similar among those immunocompetent and immunodeficient [4]. Therefore, the question arises over whether the prevalence and severity of long-COVID is also similar in both groups, or does immunodeficiency favor the development of the symptoms mentioned above.
In this regard, this study aimed to evaluate and compare the prevalence and clinical characteristics of long-COVID in immunocompetent and immunodeficient children, who were admitted to the COVID-19 subunit of the tertiary referral hospital in Warsaw, Poland.
Materials and methods
Procedure
A self-constructed questionnaire was used to conduct this study. This included questions about general health condition, diagnosis of MIS-C, COVID-19 vaccination status, the course of COVID-19, the presence of long-COVID symptoms, and how this impacted on the child’s daily functioning. The questionnaire is visible at the end of the article as a supplementary material.
Study group
The questionnaire was completed by parents of 147 children who had been admitted, at least three months prior, to the COVID-19 subunit in the Department of Pediatrics, Nutrition, and Metabolic Disorders, Children’s Memorial Health Institute in Warsaw, Poland. Participants were admitted at some point between the 1st of November 2020 and the 31st of January 2022. All participants had SARS-CoV-2 infection confirmed by nasopharyngeal swab, followed by reverse transcription polymerase chain reaction (RT-PCR) or rapid antigen test.
Division of the study group
Study participants were stratified into two separate cohorts, depending on whether or not they had an immunodeficiency. Children with immunodeficiency (n = 70) comprised 47.6% of the participants, while the control group (n = 77) comprised 52.4%. Inclusion in the immunodeficient cohort was dependent on the presence of tumors, liver or kidney transplantation, demyelinating diseases, ulcerative colitis treated with immunosuppressant’s, end-stage renal disease, and asplenia or primary immunodeficiency. Some of the participants displayed multiple of the inclusion criteria.
Statistical analysis
All data were analyzed using Microsoft Excel and Statistica 13 software. Quantitative data was evaluated using the Mann–Whitney U test when the distribution was significantly different from Gaussian distribution, which was determined in the Shapiro–Wilk test. Meanwhile, qualitative data was compared using the chi-square test. The probability value of p < 0.05 was considered to be statistically significant.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional Ethics Committee of the Children’s Memorial Health Institute in Warsaw, Poland (No. 41/KBE/2021).
Results
Characteristics of the group
Among immunocompromised children (37 males and 33 females), age ranged from 8 months to 17 years. This distribution was similar in the immunocompetent children (41 males and 36 females), ranging in age from 4 months to 17 years.
Within the immunodeficient cohort, the most common immunosuppressive factors were tumors during treatment, liver or kidney transplantation, ulcerative colitis, demyelinating diseases, and primary immunodeficiency.
None of the immunocompromised children suffered from allergic diseases, whereas the prevalence of these diseases in the control group was 28.6%. Immunization against COVID-19 was also significantly higher in immunocompetent children. Only one immunocompetent and no immunocompromised participant was diagnosed with MIS-C.
Detailed characteristics of the study group are presented in Table 1.
Table 1.
Characteristics of the study group
| ID ( +) n = 70 | ID (-) n = 77 | p | ||
|---|---|---|---|---|
| Age, y, median (range) | 7 (4–13) | 9 (4–13) | ||
| < 1 years old | 4 (5.7%) | 2 (2.6%) | 0.41 | |
| 1–5 years old | 24 (34.3%) | 22 (28.6%) | ||
| 6–10 years old | 16 (22.9%) | 17 (22.1%) | ||
| 11–15 years old | 17 (24.3%) | 28 (36.4%) | ||
| 16–18 years old | 9 (12.9%) | 8 (10.4%) | ||
| Sex | ||||
| Female | 33 (47.1%) | 36 (46.8%) | 0.96 | |
| Male | 37 (52.9%) | 41 (53.2%) | ||
| BMI | ||||
| 17.2 (16.1–18.5) | 17.2 (15.9–19.6) | 0.88 | ||
| Severity of COVID-19 | ||||
| Asymptomatic | 32 (45.7%) | 10 (13.0%) | < 0.01 | |
| Mild | 27 (38.6%) | 37 (48.1%) | ||
| Moderate | 8 (11.4%) | 24 (31.2%) | ||
| Severe | 3 (4.3%) | 6 (7.8%) | ||
| MIS-C | ||||
| 0 (0.0%) | 1 (1.3%) | |||
| Immunodeficiency | ||||
| Tumors | 44 (62.9%) | N/A | ||
| Chemotherapy | 38 (54.3%) | |||
| Radiotherapy | 2 (2.9%) | |||
| Chemo- and radiotherapy | 4 (5.7%) | |||
| Liver transplantation + PTLD | 2 (2.9%) | |||
| Steroids + chemotherapy | ||||
| Liver transplantation | 8 (11.4%) | |||
| Steroids + other immunosuppressants | ||||
| Kidney transplantion | 7 (10.0%) | |||
| Steroids + other immunosuppressants | ||||
| Ulcerative colitis | 4 (5.7%) | |||
| Steroids + azathioprine | 3 (4.3%) | |||
| Steroids + vedolizumab | 1 (1.4%) | |||
| Multiple sclerosis | 2 (2.9%) | |||
| Steroids | 1 (1.4%) | |||
| Steroids + natalizumab | 1 (1.4%) | |||
| Devic’s disease | 1 (1.4%) | |||
| Immunoglobulins | ||||
| Primary immunodeficiency | 2 (2.9%) | |||
| Wiskott–Aldrich syndrome | 1 (1.4%) | |||
| DiGeorge syndrome | 1 (1.4%) | |||
| Allergic diseases | ||||
| 0 (0.0%) | 22 (28.6%) | < 0.01 | ||
| Vaccination against SARS-CoV-2 | ||||
| 3 (4.3%) | 20 (26.0%) | < 0.01 | ||
BMI, body mass index; COVID-19, coronavirus disease 2019; ID ( +),children with immunodeficiency; ID (-), children without immunodeficiency; MIS-C, multisystem inflammatory syndrome in children; PTLD, post-transplant lymphoproliferative disease
The course of SARS-CoV-2 infection
The course of SARS-CoV-2 infection among the immunocompromised cohort was mainly asymptomatic (45.7%) or mild (38.6%), with fever, cough, rhinitis, and fatigue as the most common symptoms. On the other hand, the immunocompetent cohort was more often symptomatic (87.0%) with a mild (48.1%) or moderate (31.2%) illness. The range of symptoms reported in both groups is presented in Table 2.
Table 2.
The range of symptoms of SARS-CoV-2 infection in the group of children with and without immunodeficiency
| ID ( +) n = 70 | ID (-) n = 77 | p | |||
|---|---|---|---|---|---|
| Lack of symptoms | 32 | 45.7% | 10 | 13.0% | 0.01 |
| Fever | 28 | 40.0% | 47 | 61.0% | 0.01 |
| Cough | 19 | 27.1% | 30 | 39.0% | 0.13 |
| Rhinitis | 18 | 25.7% | 35 | 45.5% | 0.01 |
| Dyspnea | 3 | 4.3% | 6 | 7.8% | 0.38 |
| Diarrhea | 3 | 4.3% | 10 | 13.0% | 0.06 |
| Vomiting | 6 | 8.6% | 10 | 13.0% | 0.38 |
| Fatigue | 13 | 18.6% | 33 | 42.9% | 0.01 |
| Headache | 6 | 8.6% | 24 | 31.2% | 0.01 |
| Muscle pain | 8 | 11.4% | 19 | 24.7% | 0.04 |
| Rash | 0 | 0.0% | 4 | 5.2% | 0.05 |
| Chest pain | 2 | 2.9% | 3 | 3.9% | 0.73 |
| Sore throat | 1 | 1.4% | 5 | 6.5% | 0.13 |
| Loss of taste and smell | 3 | 4.3% | 10 | 13.0% | 0.95 |
ID ( +), children with immunodeficiency; ID (-), children without immunodeficiency
Long-COVID
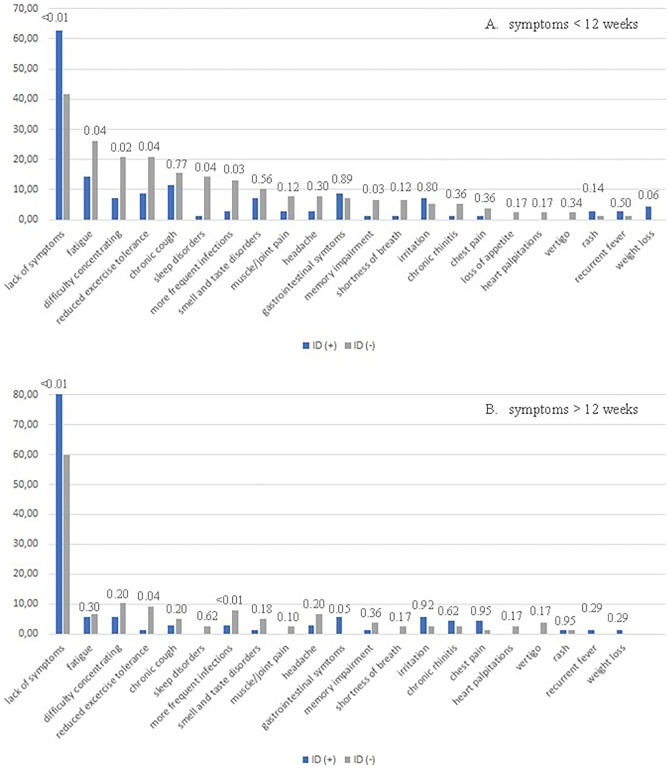
The prevalence of a range of long-COVID symptoms in both cohorts, divided into the period of time up to 12 weeks from the diagnosis of the infection and greater than 12 weeks, is presented in Fig. 1.
Fig. 1.
Prevalence of particular symptoms of long-COVID in a group of children with and without immunodeficiency, with a division into the periods of time up to (A) and over 12 weeks (B) from the diagnosis of the infection. The p-value is displayed above the graph’s bars
It is clear from the graph in Fig. 1 that immunocompetent children were more significantly affected by prolonged COVID-19 symptoms when compared to the immunocompromised cohort. This was evident within the first 12-week post-infection and also in the period after 12 weeks. The prevalence of ongoing symptomatic COVID-19 in the first 12-week post-infection, defined as the presence of at least one symptom, was 60.0% and 35.7% in these groups, respectively (p = 0.02). In the period beyond 12-week post-infection, the percentages dropped to 34.6% and 11.43%, respectively (p = 0.01).
During the first 12-week post-infection, the immunocompetent cohort reported more often symptoms of fatigue, reduced exercise tolerance, difficulty in concentrating, sleep disorders, and chronic coughing. In contrast, children in the immunocompromised cohort experienced gastrointestinal symptoms slightly more often. Beyond the 12-week post-infection period, the prevalence of almost all symptoms had significantly decreased. However, children in the immunocompetent cohort continued to display post-COVID-19 symptoms, including difficulty in concentrating, reduced exercise tolerance, fatigue, headache, and frequent infections. In turn, the children in the immunocompromised cohort continued to report the presence of fatigue, irritation, and gastrointestinal symptoms. Additionally, the parents of four immunocompromised children reported the persistence of SARS-CoV-2 in nasopharyngeal swabs, beyond 12-week post-infection.
During the first 12-week post-infection, the presence of long-COVID symptoms correlated with age. Indeed, the older the child, the greater the likelihood of developing symptoms. This was observed in all participants when considered as a whole and within the individual cohorts of immunocompromised and immunocompetent children. In the period beyond 12 weeks, there was no such relationship in any of the cohorts (Table 3).
Table 3.
Influence of age on the development of long-COVID symptoms in the whole study group and within the individual cohorts of immunocompromised and immunocompetent children
| Prevalence of long-COVID < 12-week post-infection in different age groups | ||||||
|---|---|---|---|---|---|---|
| ≤ 1 years old | 1–5 years old | 6–10 years old | 11–15 years old | 16–18 years old | p | |
| Whole study group | 1 (14.3%) | 11 (24.5%) | 15 (45.5%) | 26 (57.8%) | 10 (58.8%) | 0.01 |
| ID ( +) | 1 (25.0%) | 4 (16.7%) | 6 (37.5%) | 9 (52.9%) | 5 (55.6%) | 0.01 |
| ID (-) | 0 (0.0%) | 7 (38.9%) | 9 (52.9%) | 17 (60.7%) | 5 (62.5%) | 0.04 |
| Prevalence of long-COVID > 12-week post-infection in different age groups | ||||||
|---|---|---|---|---|---|---|
| ≤ 1 years old | 1–5 years old | 6–10 years old | 11–15 years old | 16–18 years old | p | |
| Whole study group | 1 (14.29%) | 8 (17.78%) | 6 (18.18%) | 14 (31.11%) | 3 (17.65%) | 0.24 |
| ID ( +) | 1 (25.0%) | 3 (12.5%) | 1 (6.25%) | 2 (11.8%) | 1 (11.1%) | 0.80 |
| ID (-) | 0 (0.0%) | 5 (22.7%) | 5 (29.4%) | 12 (42.9%) | 2 (25.0%) | 0.18 |
ID ( +), children with immunodeficiency; ID (-), children without immunodeficiency
Nonetheless, the relationship between the severity of COVID-19 symptoms and the presence of long-COVID, both up to 12 weeks and beyond 12 weeks, was significant. This was true for the study population as a whole, as well as for the immunodeficient cohort and the immunocompetent cohort (Table 4).
Table 4.
Influence of the severity of COVID-19 on the development of long-COVID symptoms in the whole study group and within the individual cohorts of immunocompromised and immunocompetent children
| Severity of COVID-19 and the prevalence of long-COVID < 12-week post-infection | |||||
|---|---|---|---|---|---|
| Asymptomatic | Mild | Moderate | Severe | p | |
| Whole study group | 8 (19.1%) | 30 (46.9%) | 22 (68.8%) | 7 (77.8%) | < 0.01 |
| ID ( +) | 5 (15.6%) | 12 (44.4%) | 5 (62.5%) | 2 (66.7%) | 0.01 |
| ID (-) | 3 (30.0%) | 18 (48.7%) | 17 (70.9%) | 5 (83.3%) | 0.02 |
| Severity of COVID-19 and the prevalence of long-COVID > 12-week post-infection | |||||
|---|---|---|---|---|---|
| Asymptomatic | Mild | Moderate | Severe | p | |
| Whole study group | 3 (7.1%) | 10 (15.6%) | 13 (40.6%) | 6 (66.7%) | < 0.01 |
| ID ( +) | 2 (6.25%) | 2 (7.4%) | 3 (37.5%) | 2 (66.7%) | 0.02 |
| ID (-) | 1 (10.0%) | 8 (21.6%) | 10 (41.7%) | 4 (66.7% | 0.01 |
ID ( +), children with immunodeficiency; ID (-), children without immunodeficiency
However, no correlation was found between allergic diseases and the symptoms of long-COVID in the control group, which was the only one presenting allergies, either up to 12 weeks or beyond 12-week post-infection (Fig. 2).
Fig. 2.
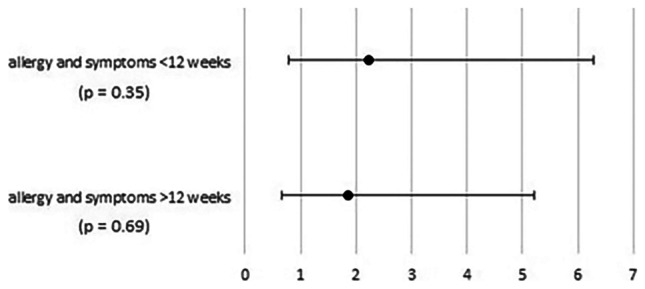
Odds ratios and associated 95% confidence intervals for the impact of allergic diseases on the development of long-COVID symptoms in the immunocompetent cohort, both up to 12 weeks and beyond 12-week post-infection
According to the parents’ of the participants, the presence of long-COVID symptoms limited the daily activities of immunocompetent children more frequently (41.8%) than it did for the immunocompromised children (25.0%; p = 0.03).
Discussion
Initial reports of long-COVID were described in Italian adults during August of 2020 [5], with the first five children suffering from this syndrome identified by Ludvigsson in Sweden in November of 2020 [6]. According to his observations of these five children, the most common symptoms were fatigue, dyspnea, palpitations, chest pain, headache, concentration difficulties, and muscle weakness. Following the publication of this information, Ludvigsson was contacted by parents of a further 35 Swedish children with similar complications [7], and similar reports began to quickly emerge from other parts of the world. The second such report of these symptoms in children were described by Buensenso et al., from Rome. This report described 123 children whose parents had been surveyed around 5 months after diagnosis of COVID-19. Symptoms were reported to persist in 58% of the children, the most common of which were insomnia, fatigue, nasal congestion, headache, muscle pain, and difficulty concentrating [8]. Subsequent studies reported similar symptoms, although the prevalence of long-COVID varied widely (4–66%) [9]. A larger prospective controlled study, which included a cohort of 1734 children who had tested positive for SARS-CoV-2, was published in August of 2021. The authors, Molteni et al., observed that children most often displayed symptoms of fatigue, headache, and anosmia. However, they did not report impaired concentration, anxiety, or memory disturbances. Moreover, they estimated the prevalence of post-COVID symptoms to be only 4.4% on day 28 following a positive test and 1.8% on day 56. Nonetheless, this percentage was significantly higher than in the control group, while the authors also suggested that older children (> 12 years of age) experienced a higher incidence of symptoms than younger [10].
Because we take care of immunocompromised children in our department on a daily basis who, based on our observations, suffer from COVID-19 surprisingly mildly and at the same time develop post-COVID complications much less frequently, we decided to investigate the phenomenon of long-COVID in this particular group. Especially that, according to our current knowledge, similar studies have not been conducted yet. On the one hand, it could bring us closer to the answer why this group is spared from COVID-19, and on the other hand, it could explain the so far little-known pathomechanism of long-COVID. Unfortunately, the prevalence of symptoms in the immunodeficient children cannot be compared as no similar studies are available in the literature. Therefore, we can only compare our control group, in which the prevalence of long-COVID was relatively high (60.0%) and increased with age. Moreover, the most frequently reported symptoms described by us were consistent with those obtained by previous researcher. Like others, we did not find a relationship between long-COVID and the presence of allergic diseases. However, we have demonstrated that the syndrome affects immunocompromised children less often. Why immunodeficient children are spared from the development of long-COVID is unclear, though mechanisms underlying this complication can be investigated. Although the underlying cause of long-COVID has yet to be established, there are several possible theories that do not exclude one another [11, 12].
One of the possible causes may be the higher frequency of asymptomatic SARS-CoV-2 infection in this group. Although it has been suggested that the severity of COVID-19 does not correlate with the presence or severity of long-COVID [13, 14], it is currently postulated that this relationship is dependent on the type of symptoms. Organ or tissue damage caused by SARS-CoV-2, such as fibrosis and scarring, as well as inflammation, may lead to long-term consequences [11], such as severe pneumonia, chronic cough, or breathing difficulties [15]. Based on a 14-year-old girl with long-COVID symptoms and lung dysfunction, Buonsenso et al. were the first to prove that COVID-19, through ongoing inflammation, can lead to lung perfusion deficits with accompanying microvascular and endothelial damage. The consequences of this condition may be severe respiratory, cardiovascular, and general symptoms [16]. This would explain the presence of chronic respiratory symptoms and persistent declines in blood oxygen saturation in several of our patients after severe COVID-19 pneumonia requiring intensive treatment. However, this pathomechanism does not explain the presence of the neurocognitive symptoms of long-COVID.
As for the symptoms that affect the central nervous system, such as concentration and sleep disorders, fatigue, and irritability, the pathomechanisms seem to be at least partially understood. Recent reports have suggested that the spike protein of the virus can cross the blood–brain barrier and cause perivascular inflammation [17, 18]. Moreover, molecular mimicry between the spike protein and human proteins contributes to autoimmunity and activates toll-like receptors (TLRs), leading to the release of inflammatory cytokines [19]. Various researchers have linked these phenomena to neuroinflammation that may damage brain blood vessels and brain cells, contributing to long-COVID [20–22]. Additionally, mast cells may also contribute, as they have been shown to be activated by SARS-CoV-2 and release numerous chemokines and cytokines that may promote inflammatory symptoms [11, 23]. Therefore, we propose that the state of reduced immunity may constitute a protective factor against this inflammatory reaction and, thus, protect against the symptoms of long-COVID.
Another possible pathomechanism of long-COVID is the persistence of SARS-CoV-2 reservoirs in specific tissues, with some patients infected with the virus not able to clear it for a long period. Therefore, they are characterized by viral ribonucleic acid (RNA) and proteins in nasopharyngeal swabs and samples of feces [24, 25]. Moreover, immunosuppression seems to facilitate SARS-CoV-2 persistence [11, 26, 27], which leads to mutations in the virus that confer resistance to a typical class of neutralizing antibodies. It is possible that SARS-CoV-2 evades the immune response and thus causes persistent symptoms [27]. Among several of our immunocompromised patients, we observed the persistence of SARS-CoV-2 for up to 3 months; however, this did not lead to the symptoms of long-COVID. Nevertheless, it was a significant inconvenience, as parents reported that it hindered the treatment of underlying diseases.
Another reason for the persistence of post-COVID symptoms is the reactivation of previously harbored pathogens, due to dysregulation of the host immune response by SARS-CoV-2. It is well-known that during their lifetime, people accumulate persistent viruses in their bodies, mainly herpesviruses. When the host is in a state of good health these pathogens do not cause any symptoms, however, they can reactivate under conditions of reduced immunity or stress. It has been shown that SARS-CoV-2 can dysregulate the immune response that keeps these viruses latent, a process regulated by host interferons, leading to a change in their gene expression or protein production that generate persistent symptoms [11, 28]. Various researchers have reported the reactivation of Epstein-Barr virus (EBV), varicella-zoster virus (VZV), and human herpesviruses 6 and 7, during COVID-19 [29–31]. These viruses can infect new organs or tissues, generate new symptoms, or induce neuroinflammatory processes in the central nervous system [11]. It appears that this reactivation should be better promoted in children with immunodeficiency. However, we observe a more intense immune response to the viral infection among immunocompetent children, which may play a significant role in its interaction with herpesviruses. Further research is needed to clarify this issue.
Our study has some limitations. The biggest one is that the questionnaire used was self-constructed and that it was based on the subjective assessment of long-COVID symptoms. However, no standardized and especially objective tool for the diagnosis of this complication has been created so far. The second limitation is the small size of the study group. Therefore, further studies on a larger group are necessary to reliably determine the prevalence and characteristics of long-COVID among immunocompromised children.
Conclusions
Immunocompromised children suffer from prolonged COVID-19 symptoms, but the prevalence was significantly lower than in the immunocompetent group of children. Indeed, they often presented with fatigue, chronic cough, and gastrointestinal symptoms, yet most of the symptoms subsided within 3 months and rarely affected the daily functioning of immunocompromised patients. The risk of developing long-COVID in this group increased with age and with the severity of symptoms of SARS-CoV-2 infection.
The potential causes of less frequent and milder long-COVID among immunocompromised children may be the milder course of COVID-19, which does not lead to tissue damage, and the state of reduced immunity protecting against neuroinflammation. Nevertheless, a tendency for the persistence of SARS-CoV-2 reservoirs in this group and reactivation of the latent herpesviruses should promote the development of post-COVID symptoms.
Based on current scientific data, and due to the fact that the pathomechanism of long-COVID is not fully understood, it is impossible to fully explain the lower prevalence and milder course of this complication among immunocompromised children. Therefore, this issue requires further research.
Abbreviations
- BMI
Body mass index
- COVID-19
Coronavirus disease 2019
- EBV
Epstein-Barr virus
- ID ( +)
Children with immunodeficiency
- ID (-)
Children without immunodeficiency
- MIS-C
Multisystem inflammatory syndrome in children
- NICE
National Institute for Health and Care Experience
- PTLD
Post-transplant lymphoproliferative disease
- RNA
Ribonucleic acid
- RT-PCR
Reverse transcription polymerase chain reaction
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- TLRs
Toll-like receptors
- VZV
Varicella-zoster virus
Authors' contributions
KK conceptualized and designed this study, collected the data, performed the initial analyses, and drafted the initial manuscript. PB and JK conceptualized and designed this study, reviewed and revised the manuscript. All authors had full access to all the data in this study, and they accept responsibility to submit it for publication.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional Ethics Committee of the Children's Memorial Health Institute in Warsaw, Poland (No. 41/KBE/2021).
Consent to participate
Informed consent was obtained from the parents of all participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.NICE 2021 (2021) COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE Guideline 188. NICE. Available at: www.nice.org.uk/ng188 [PubMed]
- 4.Kuczborska K, Książyk J (2021) Prevalence and course of SARS-CoV-2 infection among immunocompromised children hospitalised in the Tertiary Referral Hospital in Poland. J Clin Med 10(19):4556. 10.3390/jcm10194556 [DOI] [PMC free article] [PubMed]
- 5.Carfì A, Bernabei R, Landi F (2020) Gemelli against COVID-19 post-acute care study group. Persistent symptoms in patients after acute COVID-19. JAMA 324(6):603–605. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed]
- 6.Ludvigsson JF. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021;110(3):914–921. doi: 10.1111/apa.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludvigsson JF. Reporting suspicions of long COVID in children is justified during this global emergency. Acta Paediatr. 2021;110(4):1373. doi: 10.1111/apa.15762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buonsenso D, Munblit D, De Rose C, Sinatti D, Ricchiuto A, Carfi A, Valentini P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110(7):2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmermann P, Pittet LF, Curtis N (2021) How common is Long COVID in children and adolescents? Pediatr Infect Dis J 40(12): e482-e487. 10.1097/INF.0000000000003328 [DOI] [PMC free article] [PubMed]
- 10.Molteni E, Sudre CH, Canas LS et al (2021) Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2 [published correction appears in Lancet Child Adolesc Health. 2021 31 August]. Lancet Child Adolesc Health 5(10):708–718. 10.1016/S2352-4642(21)00198-X [DOI] [PMC free article] [PubMed]
- 11.Proal AD, VanElzakker MB (2021) Long COVID or Post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol 12:698169. 10.3389/fmicb.2021.698169 [DOI] [PMC free article] [PubMed]
- 12.Lledó GM, Sellares J, Brotons C et al (2022) Post-acute COVID-19 syndrome: a new tsunami requiring a universal case definition. Clin Microbiol Infect 28(3):315–318. 10.1016/j.cmi.2021.11.015 [DOI] [PMC free article] [PubMed]
- 13.Townsend L, Dyer AH, Jones K et al (2020) Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 15(11):e0240784. 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed]
- 14.Bell ML, Catalfamo CJ, Farland LV, Ernst KC, Jacobs ET, Klimentidis YC, Jehn M, Pogreba-Brown K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS ONE. 2021;16(8):e0254347. doi: 10.1371/journal.pone.0254347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai DK, Sharma P, Kumar R (2021) Post covid 19 pulmonary fibrosis. Is it real threat? Indian J Tuberc 68(3):330–333. 10.1016/j.ijtb.2020.11.003 [DOI] [PMC free article] [PubMed]
- 16.Buonsenso D, Di Giuda D, Sigfrid L et al (2021) Evidence of lung perfusion defects and ongoing inflammation in an adolescent with post-acute sequelae of SARS-CoV-2 infection. Lancet Child Adolesc Health 5(9):677–680. 10.1016/S2352-4642(21)00196-6 [DOI] [PMC free article] [PubMed]
- 17.Zhang L, Zhou L, Bao L et al (2021) SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther 6(1):337. 10.1038/s41392-021-00719-9 [DOI] [PMC free article] [PubMed]
- 18.Welcome MO, Mastorakis NE. Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology. 2021;29(4):939–963. doi: 10.1007/s10787-021-00806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onofrio L, Caraglia M, Facchini G, Margherita V, De Placido S, Buonerba C (2020) Toll-like receptors and COVID-19: a two-faced story with an exciting ending. Future Sci OA 6(8):FSO605. 10.2144/fsoa-2020-0091 [DOI] [PMC free article] [PubMed]
- 20.Karnik M, Beeraka NM, Uthaiah CA, Nataraj SM, Bettadapura ADS, Aliev G, Madhunapantula SV. A review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol Neurobiol. 2021;58(9):4535–4563. doi: 10.1007/s12035-021-02399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harvey WT, Carabelli AM, Jackson B et al (2021) SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19(7):409–424. 10.1038/s41579-021-00573-0 [DOI] [PMC free article] [PubMed]
- 22.Theoharides TC (2022) Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome?. Mol Neurobiol 59(3):1850–1861. 10.1007/s12035-021-02696-0 [DOI] [PMC free article] [PubMed]
- 23.Afrin LB, Weinstock LB, Molderings GJ (2020) COVID-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis 100:327–332. 10.1016/j.ijid.2020.09.016 [DOI] [PMC free article] [PubMed]
- 24.Sun J, Xiao J, Sun R et al (2020) Prolonged persistence of SARS-CoV-2 RNA in body fluids. Emerg Infect Dis 26(8):1834–1838. 10.3201/eid2608.201097 [DOI] [PMC free article] [PubMed]
- 25.Vibholm LK, Nielsen SSF, Pahus MH et al (2021) SARS-CoV-2 persistence is associated with antigen-specific CD8 T-cell responses. EBioMedicine 64:103230. 10.1016/j.ebiom.2021.103230 [DOI] [PMC free article] [PubMed]
- 26.Choi B, Choudhary MC, Regan J et al (2020) Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 383(23):2291–2293. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed]
- 27.Clark SA, Clark LE, Pan J et al (2021) SARS-CoV-2 evolution in an immunocompromised host reveals shared neutralisation escape mechanisms. Cell 184(10):2605–2617. 10.1016/j.cell.2021.03.027 [DOI] [PMC free article] [PubMed]
- 28.Acharya D, Liu G, Gack MU (2020) Dysregulation of type I interferon responses in COVID-19. Nat Rev Immunol 20(7):397–398. 10.1038/s41577-020-0346-x [DOI] [PMC free article] [PubMed]
- 29.Drago F, Ciccarese G, Rebora A, Parodi A (2021) Human herpesvirus-6, -7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J Med Virol 93(4):1850–1851. 10.1002/jmv.26549 [DOI] [PMC free article] [PubMed]
- 30.Xu R, Zhou Y, Cai L, Wang L, Han J, Yang X, Chen J, Chen J, Ma C, Shen L. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br J Dermatol. 2020;183(6):1145–1147. doi: 10.1111/bjd.19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T, Song J, Liu H, Zheng H, Chen C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci Rep. 2021;11(1):10902. doi: 10.1038/s41598-021-90351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Not applicable.