Abstract
An important role of modern forensic and clinical toxicologists is to monitor the adverse events of novel psychoactive substances (NPS). Following a prior review from 2013 to 2016, this critical literature review analyzes and evaluates published case reports for NPS from January 2017 through December 2020. The primary objective of this study is to assist in the assessment and interpretation of these cases as well as provide references for confirmation methods. Chemistry, pharmacology, adverse events and user profiles (e.g., polypharmacy) for NPS are provided including case history, clinical symptoms, autopsy findings and analytical results. Literature reviews were performed in PubMed and Google Scholar for publications using search terms such as NPS specific names, general terms (e.g., ‘designer drugs’ and ‘novel psychoactive substances’), drug classes (e.g., ‘designer stimulants’) and outcome-based terms (e.g., ‘overdose’ and ‘death’). Government and website drug surveillance databases and abstracts published by professional forensic science organizations were also searched. Toxicological data and detailed case information were extracted, tabulated, analyzed and organized by drug category. Case reports included overdose fatalities (378 cases), clinical treatment and hospitalization (771 cases) and driving under the influence of drugs (170 cases) for a total of 1,319 cases providing details of adverse events associated with NPS. Confirmed adverse events with associated toxidromes of more than 60 NPS were reported including synthetic cannabinoid, NPS stimulant, NPS hallucinogen, NPS benzodiazepine and NPS opioid cases. Fifty of these NPS were reported for the first time in January 2017 through December 2020 as compared to the previous 4 years surveyed. This study provides insight and context of case findings described in the literature and in digital government surveillance databases and websites during a recent 4-year period. This review will increase the awareness of adverse events associated with NPS use to better characterize international emerging drug threats.
Introduction
More than a decade after novel psychoactive substances (NPS) first appeared on the illicit drug market, the appeal of these drugs persists, requiring forensic investigations and constant efforts by crime laboratories, public health officials and law enforcement to stay current with rapidly changing trends. NPS—commonly described as novel, designer or synthetic drugs—are a global phenomenon. Efforts by manufacturers and distributors to circumvent scheduling laws and produce drugs with a possibly ambiguous legal status are driving the phenomenon. An additional demand is fueled by people who use drugs for a novel drug experience or nuanced drug high, with the added benefit of these drugs remaining undetectable in routine drug testing panels. In the 1980s, the term ‘designer drug’, coined by Gary Henderson, originally characterized heroin-like derivatives—such as the fentanyl analogs—but expanded with increasing 3,4-methylenedioxymethamphetamine (MDMA) popularity to encompass stimulants and hallucinogens, including many of the drugs described in Alexander Shulgin’s books ‘PiHKAL’ and ‘TiHKAL’ (1–3). Since the early 1980s, hundreds of NPS have been synthesized and introduced to the national and international drug markets. By 2005, the European Community adopted the term ‘new psychoactive substances’, defined as unscheduled ‘narcotic or psychotropic drugs…which may pose a threat to public health comparable to scheduled substances’ (4). The market continued to proliferate, with NPS emerging in almost every drug class for which there is a traditional therapeutic or illicit substance.
Herein, we have further refined the European Community definition of NPS as natural, synthetic or semisynthetic substances in pure form, mixture or preparation that can be categorized using at least one of the following criteria:
A substance that has been discovered or synthesized for the first time since the mid-2000s and is being ingested, regardless of degree of psychoactive effect (e.g., MDMB-4en-PINACA and N-pyrrolidino etonitazene);
A substance that was previously discovered, synthesized or reported (e.g., patents, literature and publications) but has been observed in the current illicit drug supply or identified in toxicological samples for the first time in more than 10 years (e.g., 2-methyl AP-237 and isotonitazene);
A substance that since the mid-2000s has been used in a novel way or differing manner from its originally intended use (i.e., different dosage form or amount to produce effects and different preparation) (e.g., loperamide and xylazine);
A substance that previously was not well described or studied but now presents significant challenges or threats due to an altered toxicological effect profile as a result of increased use or popularity (e.g., mitragynine) and/or
A substance that is not controlled by the United Nations drug conventions (1961 Single Convention on Narcotic Drugs or the 1971 Convention on Psychotropic Substances) but that may pose a public health threat comparable to that posed by substances listed in these conventions (e.g., quetiapine and O-desmethyl tramadol).
According to the United Nations Office on Drugs and Crime (UNODC) Early Warning Advisory (EWA) on NPS, 542 total NPS were reported in 2019 compared to 131 total NPS a decade earlier in 2009. However, trends related to new substances appearing over the last 5 years showed some stabilization (5). In the USA, the Drug Enforcement Administration’s (DEA’s) 2019 Annual Emerging Threat Report identified NPS in approximately 2% of the exhibits analyzed by DEA laboratories, and 17 new substances were first reported in 2019—or approximately one every 3 weeks (6). More rapid and comprehensive data from the Center for Forensic Science Research and Education’s (CFSRE’s) NPS Discovery show 24 new substances identified for the first time in the USA in 2020, with the majority being novel opioids, followed by new synthetic cannabinoids (SC) (7). Consistent with trends found in Europe, new identifications in the USA decreased in 2020 compared to previous years, which may be attributed to the COVID-19 pandemic and disruption in distribution (Figure 1).
Figure 1.
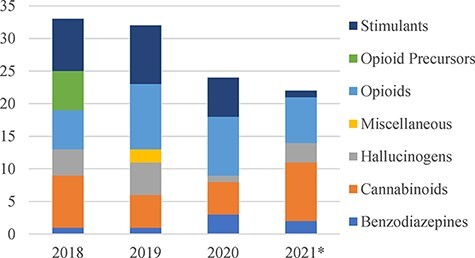
NPS reported per year by class in the U.S. (*data through November 2021 available from NPS Discovery).
This large number of new synthetic substances encompasses diverse chemical groups, many of which were pirated from medical journals, scientific literature or patent filings and clandestinely manufactured for illicit use (8). International and national scheduling actions proved effective in reducing the supply of NPS that has already emerged; however, new substances are still quickly introduced to the market as successors to newly scheduled drugs or classes (9, 10). Additional scheduling efforts have focused on precursor materials used in the synthesis of NPS, but this has not deterred overall production of new substances. Clandestine chemists have quickly moved to noncontrolled precursors and/or altered synthesis routes in response to controls on traditional precursors (5). The NPS supply chain involves an intricate web of research chemists, underground laboratories, large-scale manufacturers and global distribution networks. There appears to be relatively little NPS production in the USA with distribution focused on final processing, dilution/cutting and packaging of substances imported from China or other Asian or European countries (11). The globalization of the NPS problem, including sophisticated and highly networked transnational criminal organizations, is greatly facilitated by the ease of access via the web, where illicit substances can be anonymously purchased online with cryptocurrency and delivered to any destination in the world (12, 13). People who use drugs post on drug user forums and social media groups to discuss and debate the effects and merits of the latest NPS entering the market and drive demand. These digital forums also provide information related to effects, dosing recommendations, suggested routes of administration and other user-reported experiences.
The fast-paced NPS market requires clinicians, epidemiologists, drug treatment services, harm-reduction organizations, government regulators, prosecutors, law enforcement, analytical laboratories, researchers and other stakeholder groups to constantly monitor numerous data streams to stay abreast of emerging NPS, their proliferation, decline, health impacts and the analytical challenges that they pose. We previously published a comprehensive review of fatalities and adverse events linked to then-current NPS (from 2013 through 2016) associated with confirmed NPS ingestions, specifically involving NPS cannabinoids, stimulants, hallucinogens, benzodiazepines and opioids (14). These data were reported from emergency departments (EDs), medicolegal death investigations, impaired driving and other forensic casework. The goals of this review are to provide current data (i) for the most recent emerging drugs in this classification to assist forensic and clinical toxicologists and other previously mentioned stakeholders in their assessment and response and (ii) for analytical methods that can confirm the presence of NPS. The initial report is updated and focuses on NPS data reported during 2017 through 2020.
Methods
Literature reviews were performed in PubMed (National Center for Biotechnology Information, U.S. National Library of Medicine, Bethesda, MD) and Google Scholar (Google, Inc., Mountain View, CA) for publications dated from January 2017 through December 2020. Search terms included specific names of NPS identified in laboratory casework and published literature, as well as general terms (e.g., ‘designer drugs’, and ‘novel psychoactive substances’) and drug classes (e.g., ‘designer benzodiazepines’, ‘novel hallucinogens’ and ‘synthetic cannabinoids’), which were cross-referenced with outcome-based terms (e.g., ‘overdose’, ‘intoxication’, ‘death’ and ‘hospitalization’). In addition, government reports on websites discussing data for the years 2017 through 2020 were reviewed, including the National Forensic Laboratory Information System (NFLIS) (15), the European Monitoring Centre from Drugs and Drug Addiction (EMCDDA) (16) and NPS Discovery (17). Abstracts published by the Society of Forensic Toxicologists (18) and the American Academy of Forensic Sciences (19) from 2017 through 2020 were also investigated.
Only cases that include qualitative or quantitative toxicological confirmation of the NPS in an individual’s body fluids or tissues or the drug materials at the scene are included in the review. Drug identification could be in any biological matrix (e.g., blood, serum/plasma, urine, tissue and oral fluid). Toxicological data and detailed case information were extracted, tabulated and organized by drug category.
Tables were constructed for synthetic cannabinoid (SC), NPS stimulant, NPS hallucinogen, NPS benzodiazepine and NPS opioid cases. Data include case histories, clinical signs and symptoms, autopsy findings, NPS analytical results and qualitative and quantitative data for all identified drugs. Citations are included for all published case reports. Structures were obtained from various in-print and online resources, including standard reference material manufacturer websites, Scientific Working Group for the Analysis of Seized Drugs and ChemSpider (20, 21). All structures were verified by more than one source.
Synthetic Cannabinoids
Introduction
Approximately 280 new SC have entered the worldwide illicit drug market from January 2009 through January 2020 (22). Although many of these drugs persist at very low frequencies for years beyond their initial appearance, there is a consistent pattern of the drugs having a life cycle of 9–18 months. Once a new SC is identified and its prevalence increases, the DEA schedules the drug and a new SC typically already in the markets increases in prevalence to take its place. Only a few new SC have been introduced each year since 2017. The following sections provide a brief overview of SC chemistry, pharmacology, adverse effects and case reports published since 2017.
An ongoing challenge with SC is the inconsistency in naming conventions. SC are classified based on their structural attributes (i.e., head, core, tailing and linker); early examples include naphthoylindoles (JWH-018; Figure 2), phenylacetylindoles (JWH-250), tetramethylcyclopropylindoles (XLR-11; Figure 2) and indolecarboxylates (5 F-PB-22). Recently, indazole- and indole-carboxamides (e.g., AB-FUBINACA and MDMB-4en-PINACA) and gamma-carbolines (e.g., Cumyl-CH-MEGACLONE and Cumyl-PEGACLONE) have increased in popularity. Although structure-based terms are the norm, new drugs may be titled based on their structural similarity to older drugs that may have been named inconsistently or according to older naming conventions. The most commonly used and preferred naming convention for SC has been coined the modified Uchiyama system (23, 24). Today, most scientists working in this arena have defaulted to the naming that principal vendors of analytical standard reference materials have assigned; these vendors also use the modified Uchiyama system.
Figure 2.
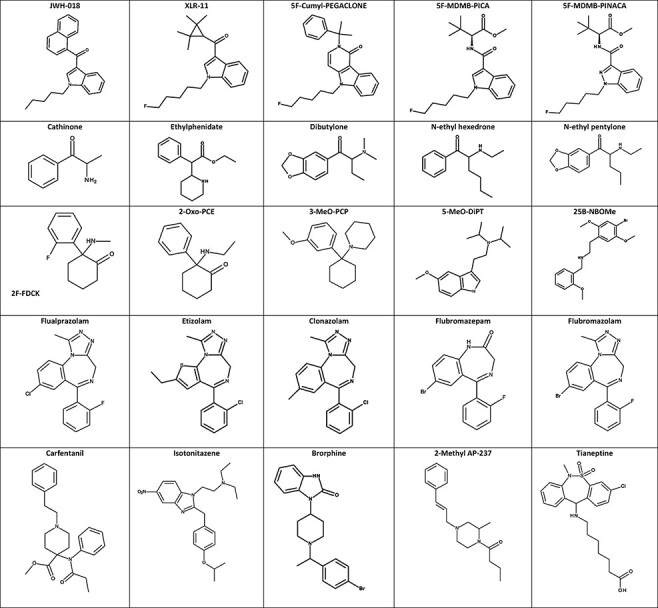
Structures of select NPS.
Pharmacology
Minor SC structural modifications do not generally produce major pharmacological changes. SC are sometimes regarded as ‘legal’ alternatives to ∆9-tetrahydrocannabinol (THC), the major psychoactive drug in Cannabis sativa, an agonist at the CB1 and CB2 cannabinoid receptors. CB1 agonists generally produce the sought-after euphoria and relaxation effects of cannabis, while CB2 agonists act on the immune system and offer novel mechanisms of action for potential pharmacotherapies. Receptor binding studies, functional assays and in vivo studies such as the mouse tetrad used to characterize SC pharmacology have been described in detail in previous publications (14, 25–28). SC were usually not studied in vivo when humans began recreational use; toxicologists and pharmacologists must rely on available in vitro or preclinical studies to suggest if new SC have pharmacological effects similar to THC. Comparing the binding affinity and activity of different SC is difficult because studies employ different evaluation methods (29). Generally, the newer indole- and indazole-carboxamides such as 5F-MDMB-PICA have been shown to be more potent than the original drugs such as JWH-018 and XLR-11. Furthermore, the pharmacokinetics and pharmacodynamics of SC vary compared to THC and each other. The paucity of animal and human studies leads to difficulty with interpreting toxicology results with respect to when an individual may have ingested an SC or predicting what specific effects an analyte may have, and toxicologists must rely on case studies as very few SC clinical studies are available. Nonetheless, new drugs have appeared each year during the period of study and evolved within broad general SC drug subclasses, such as those previously mentioned.
Table I (30–57) summarizes multiple reports describing SC adverse effects. Publications which included summaries of large numbers of cases or detailed tables of individual cases are not included in the table but rather summarized in the text below (58–62). Further, two recently published exhaustive review papers on SC include comprehensive case information not included here (63, 64).
Table I.
SC Receptor Agonists’ Case Histories with Clinical Symptoms, Autopsy Findings and Primary and Additional Drug Concentrations
| Drug | History | Clinical symptoms/Autopsy findings | Drug results (ng/mL, unless specified) | Reference |
|---|---|---|---|---|
| 5F-Cumyl-PEGACLONE | 37 y/o M found dead in apartment. Hx of mental health disorders and excessive cannabis use. | Brain and lung edema; bilateral cortical kidney bleeding, massive subacute stasis in liver and subcapsular hemorrhages and acute tubular kidney necrosis, TSS = 3 for 5F-Cumyl-PEGACLONE | Blood (central): 0.07 Blood (femoral): 0.45 9-OH-risperidone 18, trimipramine 250, +cinnarizine, +diphenhydramine Brain: 0.03 ng/g Urine: +5F-Cumyl-PEGACLONE metabolites (unspecified) |
(30) |
| 48 y/o F found dead at home. Hx of narcotic drugs and ‘spice’ use | Brain and pulmonary edema, acute blood stasis in liver and kidneys. COD acute heroin toxicity with contributory role of 5F-Cumyl-PEGACLONE (TSS = 1) | Blood (central): 0.21 Blood (femoral): 0.23 Morphine 297, 6-AM 20, codeine 21, oxazepam 450, alprazolam 10 and paroxetine <10 Urine: +5F-Cumyl-PEGACLONE metabolites (unspecified), 6-AM 12.3, dihydromorphine 29.7, noscapine 16, papaverine 27, +buprenorphine, +norbuprenorphine, +4F-MDMB-BINACA metabolites (unspecified), +AB-FUBINACA/FUB-AMB metabolites (unspecified) |
(30) | |
| 36 y/o M found near jail cell | Macroscopic organ examination unremarkable except for enlarged liver and spleen, TSS = 2 for 5F-Cumyl-PEGACLONE due to possible contributory role of pregabalin and temazepam | Blood (central): 0.22 Blood (femoral): 0.12 Pregabalin 6,000, temazepam 230, oxazepam 12, alprazolam 16, lorazepam <5 Urine: +5F-Cumyl-PEGACLONE metabolites (unspecified) |
(30) | |
| 33 y/o M found dead in apartment. Hx of ‘spice’ use | Asphyxiation signs: conjunctival petechiae and over-inflation/edema of lungs. Brain edema, massive blood stasis in heart and circulatory system, enlarged liver, TSS = 3 for 5F-Cumyl-PEGACLONE. COD: asphyxia due to SC induced vomiting and aspiration of partially digested gastric content | Blood (central): 0.35 Blood (femoral): 0.09 BZE 107, EME 11, +cocaine, +THC-COOH Urine: +5F-Cumyl-PEGACLONE metabolites (unspecified) |
(30) | |
| 5F-MDMB-PICA and 5Cl-ADAMANTYL-PINACA (5Cl-APINACA) | 67 y/o F went to ED 1.5–2 h after drinking yellow fluid from ‘winter melon tea’ bottle with upward eye rolling and brief limb jerking ∼45 min after ingestion. Drowsy with GCS = 6 by EMS | HR 111 bpm, RR 21/min, BP 125/52, Temp 36.3°C, SpO2 95% on 4 L/min nasal cannula. GCS = 15 ∼4.5 h after ingestion. Episodic hypotension during 87 h hospital stay | Urine: +5F-MDMB-PICA 3,3-dimethylbutanoic acid Seized drug: +5F-MDMB-PICA, +5CL-ADAMANTYL-PINACA |
(31) |
| 79 y/o F in bed, vomited 1×, became unconscious 1.5–2 h after drinking yellow fluid from ‘winter melon tea’ bottle. GCS = 7 by EMS. Transferred to ED | HR 67 bpm, RR 20/min, BP 113/71, Temp 37.0°C, SpO2 100% on room air. GCS = 15 ∼4 h after ingestion | Urine: +5F-MDMB-PICA 3,3-dimethylbutanoic acid Seized drug: +5 F-MDMB-PICA, +5CL-ADAMANTYL-PINACA |
(31) | |
| 5F-MDMB-PINACA (5F-ADB) | 47 y/o M with past hx of heroin and benzodiazepine use admitted to hospital for numbness and auditory hallucinations. Cardiovascular collapse on Day 2. Developed hypoxic ischemic encephalopathy and rhabdomyolysis. Developed pneumonia and died 11 days after admission | Macroscopic and microscopic examination of brain and lungs confirmed COD hypoxic ischemic encephalopathy and pneumonia following SC use | Antemortem urine (Day 4): +5F-MDMB-PINACA 3,3-dimethylbutanoic acid +7-amino-nitrazepam, +gabapentin |
(32) |
| 38 y/o M with hx of drug use arrived home looking pale. Went to bed and snored. Discovered cold and unresponsive 3 h later. Transferred to ED and pronounced dead | Enlarged heart, left ventricular hypertrophy, significant coronary atherosclerosis but no infarct. COD mixed drug toxicity with coronary artery disease | Blood and Urine: +5F-MDMB-PINACA 3,3-dimethylbutanoic acid +7-amino-nitrazepam, +amphetamine, +chloroquine, +codeine, +methadone, +methamphetamine, +nitrazepam, +nordiazepam |
(32) | |
| 8 patients (23–45 y/o; 7 M and 1 F) admitted to ED after IV heroin. Packets at scenes labeled ‘Santa Muerte’ (4), ‘50 Cal’ (4) and ‘Nick’ (1). All recovered following ED admission | 7 of 8 patients admitted to ED with classical opioid toxidrome, including CNS depression and pinpoint pupils. Following naloxone administration, patients exhibited severe agitation, combative behavior and anticholinergic and sympathomimetic toxidrome. Treated with benzodiazepines (8) and physostigmine (3) | Serum (N = 3): + +Cocaine (1), +heroin (3), +6-AM (3), +fentanyl (3), +THC (1), +alprazolam (1) Seized Drugs (N = 8): + +Heroin, +fentanyl |
(33) | |
| 49 y/o M with serious injury after jumping from second floor after drinking alcohol and smoking a herbal mixture. Died 6 days later | TSS = 3. COD polytrauma with leading craniocerebral injury | PM Blood: 0.2 Fentanyl 5.5, quetiapine 11.6, 7-OH-quetiapine 8.2, oxycodone 12.7, noroxycodone 2.7, oxymorphone 2.2 |
(34) | |
| 26 y/o M driving erratically | Unmotivated statements, changing moods, nonsensical statements, glassy eyes, sluggish, slow pupil response | Plasma: 0.19 +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA, 5OH-MDMB-PINACA 3,3-dimethylbutanoic acid, 4COOH-MDMB-PINACA, and others |
(34) | |
| 17 y/o F collapsed with foam on mouth and loss of consciousness after smoking a herbal mixture | N/A | Plasma: 0.11 | (34) | |
| 32 y/o M admitted to ED ∼15 h after taking what he believed to be ketamine | Dizziness, perception of rotating objects, incoordination, spasmodic rigidity, impaired gait, blepharospasm. Symptoms resolved ∼6 h and was discharged | Plasma: + | (35) | |
| 45 y/o M drove car into oncoming traffic and swerved into guard rail | Driver asleep with car running and lodged in guard rail, gear in reverse. Responsive to verbal stimuli, indicated ‘high’. Bilateral horizontal and vertical nystagmus, WAT unable to maintain heel-to-toe, lost count, raised arms; OLS put foot down, hands for balance; Arrested for operating vehicle while intoxicated | Blood: 26.4 +5F-MDMB-PINACA 3,3-dimethylbutanoic acid |
(36) | |
| 5F-MDMB-PINACA (5F-ADB) and 5F-MMB-PICA | 17 y/o M (n = 3) transported to ED after smoking ‘spice’ together | Psychomotor agitation, confusion, anxiety, psychosis, tachycardia, amnesia, temporary loss of consciousness | Urine: +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA | (37) |
| 14 y/o F transferred to ED after smoking ‘cherry bomb formula 6A’ and suffering seizure | Hypoactive, altered consciousness and headache | Urine: +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA, +5F-MMB-PICA 3-methylbutanoic acid Seized drug: +5F-MDMB-PINACA, +5F-MMB-PICA |
(37) | |
| 21 y/o M transferred to ED due psychomotor agitation and attempted suicide after smoking ‘spice’ | Agitation, altered language, bradypsychia, mydriasis and vomiting | Urine: +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA Herbal blend: +5F-MDMB-PINACA |
(37) | |
| 5F-MDMB-PINACA (5F-ADB) and MMB-FUBINACA (FUB-AMB) | 18 y/o M dead in bed after smoking three cigarettes containing ‘fake weed’ | Brain edema, acute respiratory distress syndrome, pulmonary edema, acute venous stasis in liver and spleen. COD acute respiratory failure due to acute intoxication with 5F-MDMB-PINACA and MMB-FUBINACA | Blood: 3.7 5F-MDMB-PINACA Seized drug: +5 F-MDMB-PINACA, +MMB-FUBINACA |
(38) |
| AB-CHMINACA | 29 y/o M discovered in cardiac arrest in office with three packages containing ‘leaves’ found nearby | Heavy lungs with severe alveolar effusions, air bubbles and hemorrhage, systemic hypoxia. COD AB-CHMINACA induced pulmonary edema resulting in systemic hypoxia | Blood: 7.6 ± 0.6 +AB-CHMINACA 3-methylbutanoic acid 56 ± 4, AB-CHMINACA 3-carboxyindazole 2.3 ± 0.1 +5F-MMB-PINACA (5F-AMB), +FUB-PB-22, +AB-FUBINACA. Seized Drug Packet 1: + AB-CHMINACA, +5F-MMB-PICA Seized Drug Packet 2: +5F-AB-PINACA Seized Drug Packet 3: +4-methyl-N-ethylpentedone |
(39) |
| AB-FUBINACA | 8 y/o M with known seizure disorder administered CBD oil purchased from online retailer. After 9 days seizure-free patient had >14 tonic-clonic episodes in 24 h | Intermittent agitation, delirium, depressed mental state, tachycardia, mydriasis. Returned to baseline and discharged in 2 days | Seized drug: + +CBD |
(40) |
| AB-FUBINACA and ADB-FUBINACA | 24 y/o M admitted to ED with acute confusion, agitation, visual hallucinations and palpitations after ingesting ‘2 drops’ of e-cigarette fluid ‘VapoFi’ mixed with fluid from unlabeled bottle believed to be ‘liquid cannabis’ | HR 169 bpm, RR 20/min, BP 163/93, Temp 36.3°C, SpO2 98% on room air. GCS 14/15. Supraventricular tachycardia, sinus tachycardia with multiple ventricular beats. Discharged after 22 h | ‘AB-FUBINACA’ Serum: 5.6 ‘ADB-FUBINACA’ Serum: 15.6 Seized drug: +AB-FUBINACA, +ADB-FUBINACA |
(41) |
| ADB-FUBINACA | 17 y/o M inhaled two puffs of ‘weed’ with friend. Both immediately began shivering uncontrollably and vomited. Assisted to home of friend to rest. Discovered unresponsive and cold to touch 6 h later. Transferred to ED and pronounced dead | At autopsy, internal macroscopic and microscopic examination of organs unremarkable. No injury or natural disease noted. COD: ADB-FUBINACA toxicity | Blood: 56 | (32) |
| 25 y/o M presented to ED with left severe hemiparesia, left hypesthesia, dysarthria and visual neglect after smoking ‘Freeze’ prior evening | Acute ischemic infarction of right middle cerebral artery, right cerebral edema | Urine: + +MDMB-CHMICA Seized drug: + |
(42) | |
| 38 y/o M inmate sent to medical center after 7-day hospitalization for abnormal behavior. Ovoid packets in GI tract (CT scan). 2 packages removed from the rectum, 2 by esophagogastroduodenoscopy and 22 by surgery on Days 7–8. 2 additional packages found in the rectum on Day 16 | HR 47 bpm, RR 12/min, BP 139/53, SpO2 95% on room air. Lethargic, unable to answer questions, shortness of breath, blank stare. Sinus bradycardia, hypoglycemia, hypotension, hypopnea, seizures following intubation. Intermittent hypertension, seizure activity and toxic encephalopathy until Day 16. After final two packets, mental status returned to baseline within 1 week. Discharged without neurological sequelae after 1 month |
Serum: 34 (Day 6); 17 (Day 8); +Diphenhydramine, +metoclopramide, +cocaine, +scopolamine, +midazolam Seized drug: + |
(43) | |
| AM-2201 | 18 y/o M stabbed F victim ∼20 times and injured 2 M witnesses after smoking ‘Mr. Green’ | Uncommunicative, calmly detached, sluggish motor movements, pupils slow to react to light, staggered gait and uncoordinated 1.5 h after incident. Psychiatric evaluation revealed abnormal personality and psychotic disorders tempore criminis, cannabis and ‘legal high’ addiction, and immature personality disorder. Psychiatric legal opinion: while performing charged acts individual totally or partially unable to appreciate nature and quality of acts and not in control of conduct due to mental defect | Blood: 0.48 THC-COOH 23 Seized drug: + |
(44) |
| MMB-FUBINACA (FUB-AMB) and PB-22 | 50 y/o M transferred to ED for altered mental status and chest pain after smoking ‘Scooby Snax Limited Edition Blueberry Potpourri’ | HR 52 bpm, RR 16/min, BP 87/52, Temp 36.7°C, SpO2 100% on 2 L nasal cannula. Somnolent, agitated with stimulation, combative. Intubated. Inferior wall myocardial infarction. Normal ECG and troponins on follow-up visits | Seized drug: +MMB-FUBINACA, +PB-22 | (45) |
| AMB-PINACA (MMB-PINACA) and ADB-PINACA | 29 y/o M transported by EMS for agitation and depressed consciousness after reported OD of heroin | Intubated; HR 75 bpm, RR 14 min, BP 152/0 (palpated), Temp 32.9°C, SpO2 100% on 100% inhaled O2, GCS 3T, ECG—Osborne waves and prolonged QRS (124 ms); discharged after 15 h | Serum: +AMB-PINACA, +ADB-PINACA |
(46) |
| 33 y/o M acting ‘bizarrely’, became unresponsive after tonic-clonic seizure after reported OD of heroin | EMS intubated. At ED HR 85 bpm, RR 14, BP 174/127, Temp 33.7°C, SpO2 100% on 100% inhaled O2, GCS 3T; normal ECG, EEG ‘mild bilateral cerebral dysfunction’; discharged after 24 h | Serum: +AMB-PINACA, +ADB-PINACA |
(46) | |
| 4-Cyano-Cumyl-BINACA | 29 y/o M transferred to ED by EMS after fall at home. 3-day hx of somnolence, weakness and vomiting | HR 105 bpm, BP 180/100, RR 22/min, Temp 39°C, SpO2 99% on room air. Altered mental state, combative, GCS 11. Nonoliguric kidney failure on Day 4. Mental status improved after dialysis on Days 5 and 6. Left AMA on Day 10 | Serum: 35.5 | (47) |
| Cumyl-PINACA | 36 y/o F customs inspector developed dry mouth, blurred vision, dizziness, balance disorder, weakness, numbness and palpitations 0.5 h after exposure to unknown viscous and sticky substance at the airport. Transferred to ED 6 h later | HR 105 bpm, BP 130/88; blurred vision, numbness, mydriasis, ataxia, somnolence, lethargy and confusion. 2 days post event reported amnesia and slowed perception | Blood: + Seized drug: + |
(48) |
| 22 y/o M customs inspector developed dizziness and weakness 0.5 h after exposure to unknown viscous and sticky substance at the airport. Transferred to ED 6 h later | HR 110 bpm, BP 145/95; Confused and mydriasis. Reported amnesia and slowed perception of time for 2 days | Blood: + Seized drug: + |
(48) | |
| 36 y/o M customs inspector developed blurred vision, dizziness, balance disorder, weakness and lethargy 0.5 h after exposure to unknown viscous and sticky substance at the airport. Transferred to ED 6 h later | HR 110 bpm, BP 130/80; somnolent and confused; mydriasis, ataxia; reported amnesia and slowed perception of time for 2 days | Blood: + Seized drug: + |
(48) | |
| JWH-122 | 18 y/o M with hx of extensive daily cannabis use admitted to addiction treatment unit with visual hallucinations after use of a ‘cannabis-like’ product | HR 88 bpm, RR 19/min, BP 130/80; mild hallucinations, blunted affect, anxiety and tension. Visual hallucinations and disturbances recurred following extensive cannabis intake for 4 years | Seized drug: + | (49) |
| MDMB-CHMICA | 9 patients presenting to ED. 23–62 y/o; 8 M and 1 F | Elevated plasma creatinine (6), dilated pupils (5), seizure (5), tachycardia (5), deep unconsciousness (5), respiratory depression (4), elevated blood and/or plasma lactate (4), agitation (3), delirium (3) and vomiting (3) | Serum: <1.3–86.4 (median = 18.6, mean = 24.5) +Diphenidine (1), +methylnaphthidate (1), +buprenorphine (2), +pregabalin (1), +5F-adamantyl-PINACA (1), +flubromazepam (1), +MMB-FUBINACA (1), +THJ-018 (1) |
(50) |
| 21 y/o M prison inmate presented to ED with hypercapnia | Bradycardia, 13 GCS. Administered naloxone, remained hypercapnic. ABG returned to normal 24 h post presentation | Blood: + +Mirtazapine/metabolites, +propranolol/metabolites (both prescribed) | (51) | |
| 23 y/o M prison inmate presented to ED after collapse and seizure | Administered naloxone and diazepam. Transferred to ED 70 min later. GCS 3, pulse 105 bpm, hypercapnic. Discharged ∼24 h after admission | Blood: + +Quetiapine/metabolite (prescribed), +promethazine/metabolite, +cocaine, +BZE, +levamisole |
(51) | |
| 43 y/o M prison inmate presented to ED after collapse and seizure | Administered naloxone and diazepam. At ED, GCS 3, pulse 114 bpm. 3 h post first episode, suffered tonic-clonic seizure. Released 24 h post admission | Blood: + +Olanzapine/metabolite (prescribed) |
(51) | |
| 5F-MDMB-PINACA (5F-ADB) and JWH-122 | 31 y/o M found dead in apartment. Postmortem interval ∼ 3 days. | ‘Tender’ coronary arteries, cerebral and pulmonary edema, cyanosis of internal organs | Blood: 0.57 JWH-122 12, diphenhydramine <10, doxylamine 83 Urine: 232 +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA, +JWH-122 metabolites (unspecified), doxylamine >500 |
(34) |
| MMB-FUBINACA (FUB-AMB) and EMB-FUBINACA (5 F-ADB) | 27 y/o M found dead in bed, hx of alcohol and ‘legal high’ use. COD: acute respiratory failure due to cardiotoxic effects of MMB-FUBINACA and EMB-FUBINACA | Congestion of internal organs; pulmonary edema, left-sided pleural adhesions | ‘MMB-FUBINACA’ Blood: ND Urine: 4.7 Urine (hydrolyzed): 8.2 ‘EMB-FUBINACA’ Blood: ND Urine: 0.2 Urine (hydrolyzed): 0.1 Blood: +Ethanol, +lorazepam, +haloperidol, +lidocaine |
(52) |
| MMB-FUBINACA (FUB-AMB) and 5F-MDMB-PINACA (5F-ADB) | 43 y/o F found dead in apartment following consumption of a herbal mixture. Estimated postmortem interval 3 days | Cerebral and pulmonary edema, hyperemia internal organs | Blood: 0.03 +5F-MDMB-PINACA 3,3-dimethylbutanoic acid, 5OH-MDMB-PINACA, 5OH-MDMB-PINACA 3,3-dimethylbutanoic acid, 4COOH-MDMB-PINACA and others, mirtazapine 7.5, +mirtazapine metabolites, lidocaine 43, 3-MeO-PCP 97 |
(34) |
| 5F-MMB-PINACA (5F-AMB) | 19 y/o M acting incoherently after sniffing/smoking unknown substance | BP 120/80; slow movements, slurred speech, disoriented, lethargic, hyporeflexia, uncoordinated, teeth grinding, fearful, loss of focus/memory, dry mucus membranes and tinnitus | Seized drug: + | (53) |
| NM-2201 | 25 y/o M presented to ED with agitation, double incontinence and incoordination in movement and speech 5 days after smoking ‘Black Mamba’ | Admission: GCS = 14, hyperactive agitation, left-sided incoordination, aphasia, generalized hypertonia, hyperreflexia, left-sided hemiparesis. Day 4: GCS 10, no visual tracking, elevated temp 38.7°C, diaphoresis, tachycardia, hypertension and agitation | Seized drug: + | (54) |
| O-2545 | 16 y/o M lost consciousness after smoking cannabis and ‘Bonsai’ | GCS E1M1V1, dilated pupils, unresponsive to light, tachycardia. Intubated due to persistent lack of consciousness and transferred to ICU. Discharged after overnight stay | Serum: + Urine: +THC-COOH |
(55) |
| UR-144 | 19 y/o F presented to ED in status epilepticus after using ‘space’ | Seizure activity 3 h, HR 138–150 bpm, RR 28/min, BP 90/60, Temp 37.1°C, severe biventricular failure; stress cardiomyopathy. Discharged on hospital Day 10 | Urine: + +UR-144 metabolites (unspecified) |
(56) |
| 27 y/o M with hx of polysubstance use became unresponsive within 1 h of inhaling ‘K2’. Transported to ED | HR 98 bpm, BP 144/84, Temp 36.2°C; Hypoxic on arrival, SpO2 100% on FiO2 0.5; Worsening bilateral alveolar infiltrates and diffuse alveolar hemorrhage on Day 2. Discharged 10 days post admission | Blood: +UR-144 5-hydroxypentyl (by ELISA) | (57) |
6-AM = 6-monoacetylmorphine, ABG = arterial blood gas, AMA = against medical advice; BP = blood pressure, BZE = benzoylecgonine, CBD = cannabidiol, CNS = central nervous system, CT = computed tomography, ECG = electrocardiogram, EEG = electroencephalogram, EME = ecgonine methyl ester, EMS = emergency medical services, F = female, GI = gastrointestinal, GCS = Glasgow Coma Score, HR = heart rate, hx = history, M = male, N/A = not available, OLS = one leg stand, PM = postmortem, RR = respiratory rate, SpO2 = oxygen saturation, Temp = temperature, THC-COOH = 11-nor-carboxy-delta-9-tetrahydrocannabinol, WAT = walk-and-turn, OD = overdose, ND = none detected, ICU = intensive care unit, ELISA = enzyme linked immunosorbent assay.
In general, individuals presented to the ED following use of or exposure to an herbal incense product that resulted in physiological or psychological distress. Commonly reported symptoms included agitation, tachycardia, lethargy, loss of consciousness and psychomotor impairment. Kleis et al. (60) published a 5F-MDMB-PICA (Figure 1) case series including five clinical cases, three fatalities and four driving under the influence of drugs (DUID) cases. 5F-MDMB-PICA serum concentrations in the clinical cases were <0.1–2.5 ng/mL. Three patients were aggressive, anxious or agitated, and one had a ‘subdued mood’; no mental/behavioral effects were reported in the fifth case. The four drivers had serum 5F-MDMB-PICA concentrations of 0.54–16 ng/mL. Another individual described as ‘aggressive’ also had a 1.6 ng/mL 4F-MDMB-BINACA serum concentration. All patients tested positive for other drugs including cannabinoids (N = 5), ethanol (N = 4), amphetamines (N = 2), benzodiazepines (N = 1), methadone (N = 1), doxylamine (N = 1) and cetirizine (N = 1).
While most clinical cases involved intentional product use, three unintentional exposure cases were reported. In one case, two patients reported to the ED approximately 1.5–2 h after drinking what they believed was winter melon tea (31). One patient experienced a seizure, was drowsy and was tachycardic at the time of presentation, and the other was unconscious with a low heart rate (67 beats per minute [bpm]) within the normal range. Both patients’ urine specimens were positive for 5F-MDMB-PICA 3,3-dimethylbutanoic acid, and the liquid they consumed tested positive for 5F-MDMB-PICA and 5Cl-ADAMANTYL-PINACA. An 8-year-old (y/o) male receiving zonisamide for seizures was given a commercially available cannabidiol (CBD) oil product by his parents despite not being diagnosed for Dravet or Lennox–Gastaut syndrome (40). The child’s neurologist approved treatment with this product that is not regulated or approved by the Food and Drug Administration. The first 9 days of CBD treatment were uneventful; however, later the boy experienced more than 14 tonic-clonic episodes in 24 h. CBD use was discontinued, and the patient was discharged after 2 days. Analysis of the CBD product indicated it contained both CBD and the indazole carboxamide SC AB-FUBINACA. Finally, Dobaja et al. (48) reported three customs inspectors who were occupationally exposed to the gamma-carboline SC Cumyl-PINACA. They were not wearing gloves and came in contact with a sticky substance during package examination. They immediately washed their hands but showed signs of an intoxicant exposure approximately 30 min after the exposure. They arrived at the ED 6 h later confused, lethargic, tachycardic and weak/numb. All symptoms resolved in 2 days, although the patients reported some lingering amnesia and slowed perception of time. Patient blood samples as well as the liquid the patients came in contact with were positive for Cumyl-PINACA.
The potentially devastating psychiatric effects of SC were highlighted by a 2017 report of an individual who stabbed multiple people, killing one, after using AM-2201 (44). The accused could not provide any explanation for his actions, indicating that he did not know why he attacked the individual who later succumbed to her injuries and that he injured the other two only because he was scared and trying to get away. No biological testing was performed, but the product tested positive for AM-2201. A psychiatric evaluation after the event determined that ‘while performing the acts as charged, the man was, due to mental defect, totally or partially unable to appreciate the nature and quality of his acts and be in control of his conduct’. The accused did not have any history of psychiatric disorders, and the authors opined that the psychotropic effects of AM-2201, in combination with the active component of cannabis, may have played a role in his actions.
Common signs and symptoms of SC use in drivers include driving erratically, failing to stay within the lane, crossing into oncoming traffic and being found asleep behind the wheel. In addition to the impaired driving cases that Table I summarizes, Kaneko et al. (58) reported on 96 DUID cases that occurred in 2012–2014, of which 93 involved SC. The 96 incidents were categorized as fatalities (N = 4), injuries (N = 51) or property damage (N = 41). Immediately following the collisions, drivers were described as having impaired consciousness (N = 73) and/or being excited/confused (N = 16). Seventy-three cases included at least one SC, and 20 involved an SC and a cathinone or the dissociative anesthetic diphenidine. SC identified in blood samples were AM-2232, 5F-PB-22, FUB-PB-22, NM-2201, AB-PINACA, NNE-1, 5F-AB-PINACA, AB-FUBINACA, 5F-MMB-PINACA (5F-AMB) and AB-CHMINACA. Kleis et al. reported four drivers in their case series on 5F-MDMB-PICA (60). Plant cannabinoids and alcohol were reported in one case in which the driver was involved in a hit-and-run crash, followed by a car chase. The driver was unable to stand upright, had slurred speech and exhibited erratic and confused behavior. The second driver was stopped after being observed leaving his lane, driving on the wrong side of the road and braking inappropriately. He was described as exuberantly happy upon a negative breath alcohol test but quickly became aggressive. The third driver was found sleeping in his stopped car (with the engine off) in the middle of an intersection. After being aroused, he was disoriented with a subdued mood. No driving behavior was reported on the final driver, and he was simply described as having fluttering eyelids and a tremor.
In addition to the nine deceased individuals summarized in Table I, there are three publications reporting deaths associated with SC use. In FL, the deaths of 54 prisoners involved a variety of SC, 37 cases had no other drug classes detected following a comprehensive drug screen. In cases in which other drugs were detected, only five contained drugs with significant abuse potential (59). Although blood and urine were initially tested in these early cases, no drugs were detected in blood leading to analysis of urine for SC metabolites in the later cases. 5F-MDMB-PINACA (5F-ADB; Figure 2), MMB-FUBINACA (FUB-AMB), 5F-MMB-PINACA (5F-AMB), MDMB-FUBINACA and/or AB-CHMINACA were detected in the samples. Morrow et al. (61) investigated the role of MMB-FUBINACA in nontraumatic deaths in Auckland, NZ. Following a review of all records in which ‘AMB-FUBINACA’ (synonyms MMB-FUBINACA and FUB-AMB) was referenced, 58 cases were identified that did not have trauma as the cause of death (COD). MMB-FUBINACA 3-methylbutanoic acid was identified in all 58 cases, with a mean blood concentration of 229 ng/mL (median = 140 ng/mL) in 41 cases where it was quantified. Only 15 cases had positive blood MMB-FUBINACA. MMB-FUBINACA toxicity was the primary COD in 42 cases, with 20 involving no other drugs or alcohol. Cumyl-PEGACLONE was reported in five of 472 deaths over an 18-month period in Australia’s Northern Territory (62). Postmortem blood concentrations of 0.73–3.0 ng/mL were reported. Authors employed the Toxicological Significance Score (TSS) system developed by Elliot et al. to categorize the role of Cumyl-PEGACLONE. This system evaluates the presence and concentration of an NPS along with other toxicological and pathological findings in a case and assigns the NPS a significance value from 1 (low) through 3 (high) (65). In 4 of 5 deaths, the TSS was determined to be ‘high’ (TSS = 3). In one case, the COD was determined to be acute drug toxicity (alcohol and Cumyl-PEGACLONE); three cases involved cardiac/coronary disease in the context of Cumyl-PEGACLONE (two with alcohol) use, and the final death involved positional asphyxia with Cumyl-PEGACLONE use and obesity. Coronary/cardiac disease, blood stasis, organ edema, hypoxia and the presence of other drugs are common in postmortem cases.
In general, the toxidrome associated with SC use is nonspecific and inconsistent. In addition, individual cases may have unusual and inexplicable findings due to concomitant drug use. This is illustrated by the mass casualty incident reported by Ershad et al. (Table I) (33). Six heroin users had effects not typically associated with opioid use, including agitation and tonic-clonic seizures. The heroin also contained fentanyl and 5F-MDMB-PINACA. In another case, the Centers for Disease Control and Prevention (CDC) became aware of SC users in Illinois showing signs and symptoms of anticoagulant exposure. During March–May 2018, 202 cases of suspected SC used with anticoagulant exposure were identified across nine states (66). Therefore, relying on investigative clues such as drug use history, examination of drug paraphernalia or products found with the patient and witness accounts is essential to determining if the case could involve SC. It is also important to remember that the content of herbal incense products, pills and potions purchased on the internet change rapidly, requiring laboratories to ensure assays are testing for the most current drugs.
Discussion
SC have significant physiological and neurological effects, with the potential for negative sequelae including death. However, the wide range of reported effects, comorbidities and co-ingested drugs complicates interpretation of the role that SC has in clinical, DUID and postmortem casework. Case reports involving the same drug may mention seemingly contradictory effects. For example, some patients exposed to ADB-FUBINACA presented with tachycardia and others with bradycardia. Some patients became agitated/anxious, and others were somnolent. Based on the cases reviewed, patients who are transported to the hospital for medical care typically recover without long-term negative effects. Most postmortem cases involve individuals who were discovered hours after the drug exposure, and autopsy findings are often similar to what is seen in central nervous system (CNS) depressant deaths, such as pulmonary edema. Table II provides a cumulative summary of SC’s effects on various anatomical systems based on the table included in Logan et al. (14).
Table II.
Summary of SC Receptor Agonists’ Toxicity Profiles
| Organ system | Symptoms and signs |
|---|---|
| CNS | Agitation, psychosis, irritability, seizures, sedation, coma, delirium, hallucinations, paranoia, anxiety, hypo/hyperreflexia and psychomotor impairment |
| Cardiovascular | Tachycardia, hypertension, acute coronary syndrome, arrhythmia, chest pain and myocardial infarction |
| Pulmonary | Respiratory depression and hypopnea |
| Other | Nausea, vomiting, fevers, mydriasis, blurred vision and acute kidney injury |
| Postmortem findings | Organ edema and congestion |
NPS Stimulants
Introduction
Currently, the most prevalent NPS category is novel stimulants worldwide (67). Many are derived from cathinone, a monoamine alkaloid present in the plant Catha edulis (Khat) (Figure 2). The methylated analogue of cathinone, methcathinone, was first synthesized in 1929 with reports of abuse as early as the 1990s (68). Since the early 2000s when 4-methylmethcathinone (mephedrone) and beta-keto amphetamines (methylenedioxypyrovalerone [MDPV]) emerged in the UK and Europe, novel stimulants proliferated and dominated the NPS market (68). By the late 2000s, novel drugs including methylone and alpha-pyrrolidinopentiophenone (α-PVP) had also increased in prevalence. By 2015, substituted methylenedioxyphenethyl-amines (e.g., ethylone and butylone) had increased in frequency in the USA and Europe. The availability of synthetic stimulants persists despite scheduling actions internationally and nationally.
The 2020 European Drug Report on Trends and Developments showed that cathinones accounted for about 36% of drug seizures, while another 3% of drug seizures were composed of phenethylamines in 2018 (67). In Europe, the number of synthetic cathinones identified in powders has significantly decreased in recent years (67). Following the appearance of methylone, structurally related analogues have appeared with varying degrees of popularity with recent substances including dibutylone, eutylone, pentylone, N-ethylpentylone (NEP) and N-ethylhexedrone. In the USA, the DEA’s emerging threat report for the third quarter of 2020 and the CFSRE’s fourth quarter 2020 trend report showed eutylone as the most frequently encountered cathinone in the USA (69, 70). Similarly, drugs structurally related to α-PVP, such as alpha-pyrrolidinoheptiophenone (PV8), alpha-pyrrolidinohexiophenone (α-PHP) and alpha-pyrrolidinoisohexanophenone (α-PiHP) have proliferated. Eutylone and NEP accounted for the highest percentages of reports for NPS in the phenethylamine category at 1.3% and 0.4%, respectively, as shown in the 2019 NFLIS annual report (71).
Methylphenidate (MPH) is widely prescribed for attention deficit hyperactivity disorder but also frequently misused for cognitive enhancement to improve memory and concentration, control anxiety and stimulate motivation and creativity. MPH requires a prescription; thus, illegal analogues emerged on the internet and darknet as cognitive enhancers for their nootropic and stimulant effects, and this resulted in deaths in some cases (72–75). Although few analytical methods for MPH analogs are available and many laboratories do not test for them, adverse effects were reported for structural derivatives, ethylphenidate (EPH) and 4-fluoromethylphenidate (4F-MPH).
Pharmacology
Synthetic cathinones’ interactions with dopamine transporters (DATs), serotonin transporters (SERTs) and norepinephrine transporters (NETs) were documented in in vitro (human cell lines) and preclinical models (76–80). Ring-substituted cathinones (such as methylone) act as DAT, SERT and NET substrates increasing dopamine, serotonin and norepinephrine release. The presence of a pyrrolidine ring, as in α-PVP, acts as a transport blocker (reuptake inhibitor) at DAT (77–80) increasing affinity and potency with increasing length of the α-carbon chain (79). Drugs with a higher DAT potency, including α-pyrrolidinophenones and 4-fluoroamphetamine (4-FA), exhibit stimulant properties similar to methamphetamine (79); cathinones with similar DAT and SERT potencies or a higher SERT potency have more empathogenic activity (e.g., ethylone) (80).
The onset of synthetic cathinone effects occurs within 30–45 min of administration, with desired effects lasting 1–3 h; undesirable effects can last for several days (81). Reported synthetic cathinone effects include increased energy, alertness, concentration and euphoria; effects are similar to those of amphetamine and cocaine (68, 76, 82, 83). Adverse physiological effects include cerebral edema, diaphoresis, hyperflexia, hypertension, hyperthermia, dilated pupils, tachycardia, myocardial infarction, seizures, bruxism, nausea and vomiting. Prominent adverse neuropsychiatric effects include agitation, aggression, hallucinations, paranoia, psychosis and serotonin syndrome. Hyperthermia, diaphoresis, tachycardia, agitation and hypertension are indicators of toxicity and overdose (82). Psychosis may be pronounced, with patients experiencing paranoia, hallucinations (primarily visual) and delusions. Toxic sequelae can include liver and kidney failure, rhabdomyolysis and development of increased pressure in a muscle compartment that can lead to muscle and nerve damage, blood flow abnormalities and ultimately death (‘compartment syndrome’) (68, 82). Table III provides a summary of the toxicity profiles related to synthetic stimulants. Additional information related to adverse effects associated with NPS stimulant use can be found in Table IV (84–94).
Table III.
NPS Stimulant Toxicity Profile
| Organ system | Symptoms and signs |
|---|---|
| CNS | Agitation, psychosis, delusions, aggression, irritability, paranoia, delirium, hallucinations, sedation, coma, abnormal behavior and altered fluctuating consciousness |
| Cardiovascular | Tachycardia, hypertension and palpitations |
| Pulmonary | Increased respiration rate |
| Other | Hypothermia, mydriasis, rhabdomyolysis, compartment syndrome and sweating |
| Postmortem findings | Organ edema and congestion |
Table IV.
NPS Stimulant Case Histories with Clinical Symptoms, Autopsy Findings and Primary and Additional Drug Concentrations
| Drug | History | Clinical symptoms/Autopsy findings | Drug results (ng/mL unless specified) | Reference |
|---|---|---|---|---|
| 4F-MPH | 26 y/o F admitted ED for severe psychomotor agitation, confusion, disorientation, confabulation, incoherent speech and crying after sniffing powder purchased on internet | Palpitations and mild tachycardia (100 bpm). BP, Temp and chemistries normal on admission. Tachycardia lasted 2 days until discharge | Blood: 32 Urine: 827 |
(84) |
| α-EAP and 4F-α-PVP | 28 y/o M unconscious at home, previous psychiatric hx and depression, also admitted to hospital 2 months earlier with loss of consciousness and aspiration pneumonia. During second admission admitted inhalation of ‘BON’S CRYSTAL’ | Time 1: ED GCS 4, SBP 117 mmHg, DBP 37 mmHg, HR 118 bpm, RR 14/min, 35°C, CT: diffuse bilateral ground glass opacity, diagnosed diffuse alveolar hemorrhage, admitted ICU and ventilated for 4 days, discharged on Day 7. Time 2: Unconscious, bilateral pulmonary infiltrate recurrence less serious than Time 1. Discharged on Day 2 |
Drug material confirmation: α-EAP and 4 F-αPVP | (85) |
| α-PHP | 39 y/o M took picture through bathroom window screaming ‘a ghost is there’, ran speaking incomprehensibly. Found covered in mud in rice field, transported to ED with hallucinations, delusion, fear, anxiety and restlessness | GCS 14, HR 101 bpm, SBP 131 bpm, DBP 68 mmHg, Temp 37°C, pO2 85%, biochemical exam revealed mild hepatic dysfunction, increased creatinine kinase and white blood cell counts | Serum: 175 (on admission) 64.6 (24 h), 43.6 (48 h), 27.0 (72 h), 15.7 (96 h), 13.4 (120 h), 6.75 (144 h), 2.98 (192 h), 1.79 (216 h) |
(86) |
| Dibutylone | 32 y/o F found deceased in bed; drug OD expected | N/A | Femoral blood: 383 Butylone 92.5, THC 2.8, THC-COOH 11 Urine: 3,100 Butylone 69.7 Vitreous: 250 Butylone 108 Liver: + +Butylone |
(87) |
| 46 y/o M found deceased in hotel room | N/A | Femoral blood: <10 Butylone 385, alprazolam 11, BZE 1,600, hydrocodone 400, oxycodone 12, tramadol 340 Urine: 16,500 Butylone 3,060 |
(87) | |
| 38 y/o M in fatal motor vehicle crash | N/A | Femoral blood: 61.5 Butylone: 6.55, ethanol 190 mg/dL, +4-FA, methylone 31, +dimethylone, ethylone <10 Urine: 2,140 Butylone 149, ethylone 269 |
(87) | |
| F unknown age suspected OD | N/A | Femoral blood: 1,400 Butylone 600 |
(87) | |
| 49 y/o M suspected bath salt use | N/A | Femoral blood: 10 +4-Chloro-alpha-PVP |
(87) | |
| M unknown age no hx | N/A | Central blood: 10 | (87) | |
| M unknown age no hx | N/A | Femoral blood: 13 Butylone 12 |
(87) | |
| M unknown age, alleged Molly use | N/A | Postmortem Blood: 14 | (87) | |
| F unknown age, suspected OD | N/A | Postmortem Blood: 40 Butylone 15.3, +p-FIBF |
(87) | |
| 37 y/o M with hx of recreational drug use. MDMA at electronic dance music festival. Ingested three capsules within 24 h of sample collection | N/A | Oral fluid: 123 Butylone 206, +dimethylone, +MDMA, +MDA |
(87) | |
| 22 y/o M with hx of recreational drug use MDMA at electronic dance music festival. Ingested two capsules within 24 h of sample collection | N/A | Oral fluid: 138 Butylone 291, +dimethylone, +MDMA, +MDA |
(87) | |
| Unknown sex, age and hx; at electronic dance music festival | N/A | Oral fluid: 1,926 Butylone 1,761, +dimethylone, +MDMA, +MDA |
(87) | |
| 29 y/o M with no hx; at electronic dance music festival | N/A | Oral fluid: + +Butylone |
(87) | |
| 18 y/o F with hx of recreational drug use, ingested two Molly within 24 h of sample collection at electronic dance music festival | N/A | Oral fluid: + +Butylone |
(87) | |
| 26 y/o M with unknown hx; at electronic dance music festival | N/A | Oral fluid: + +Butylone, +cocaine |
(87) | |
| EPH | 32 y/o M with unknown hx | N/A | Femoral blood: 110 Ritalinic acid 2140, +methadone, +EDDP, +Morphine, +Fentanyl, +MPH Urine: 987 Liver: 180 ng/g Pericardium fluid: 131 Stomach contents: 20.7 (200 mL) |
(72) |
| 38 y/o M with unknown hx | N/A | Femoral blood: 23 Ritalinic acid 943, +pregabalin, +fentanyl, +norfentanyl |
(72) | |
| 38 y/o M with unknown hx | N/A | Femoral blood: >2,000 +Tramadol, +acetaminophen, +morphine and metabolites, +6-AM, +acetone |
(72) | |
| 33 y/o F with unknown hx | N/A | Femoral blood: 1,900 +Methadone, +procyclidine, +propranolol, +morphine and metabolites, +diazepam, +temazepam and metabolites, +THC-COOH, +pregabalin, +methylthienylpropamine |
(72) | |
| 31 y/o F with unknown hx | N/A | Femoral blood: 1,200 +Ethanol, +morphine and metabolites, +diazepam and metabolites |
(72) | |
| 27 y/o M with unknown hx | N/A | Femoral blood: 760 +Ethanol, +diazepam and metabolite, +methylthienylpropamine |
(72) | |
| 37 y/o M with unknown hx | N/A | Femoral blood: 610 +Diazepam and metabolite, +mirtazapine |
(72) | |
| 31 y/o F with unknown hx | N/A | Femoral blood: 470 +Lignocaine, +methadone, +mirtazapine, +promethazine |
(72) | |
| 34 y/o M with unknown hx | N/A | Femoral blood: 410 +Ethanol, +methadone, +diazepam and metabolites, +THC-COOH |
(72) | |
| 38 y/o M with unknown hx | N/A | Femoral blood: 350 +α-Methyltryptamine, +etizolam, +diphenhydramine |
(72) | |
| 20 y/o M with unknown hx | N/A | Femoral blood: 320 +Fluoxetine and metabolite, +pregabalin, +zuclopenthixol, +morphine and metabolites, +etizolam, +pyrazolam, +2-MeO-diphenidine |
(72) | |
| 40 y/o M with unknown hx | N/A | Femoral blood: 250 +Methadone, +olanzapine, +diazepam and metabolites, +THC-COOH |
(72) | |
| 35 y/o M with unknown hx | N/A | Femoral blood: 140 +Methadone |
(72) | |
| 33 y/o F with unknown hx | N/A | Antemortem blood: 460 Femoral blood: 130 +Dihydrocodeine, +hydrocodone, +morphine, +nordiazepam, +ketamine, +acetaminophen, +alfentanil |
(72) | |
| 54 y/o M with unknown hx | N/A | Cardiac blood: 41 +Dihydrocodeine |
(72) | |
| 45 y/o M with unknown hx | N/A | Femoral blood: 40 +Diazepam and metabolites, +methadone, +morphine and metabolites, +6-AM |
(72) | |
| 44 y/o M with unknown hx | N/A | Femoral blood: 28 +Methadone, +diazepam and metabolites |
(72) | |
| 42 y/o M with unknown hx | N/A | Femoral blood: 15 +Ethanol, +dihydrocodeine, +morphine and metabolites, +diazepam and metabolites |
(72) | |
| 46 y/o F with unknown hx | N/A | Femoral blood: 10 +β-hydroxybutyrate, +mirtazapine, +codeine, +morphine, +diazepam and metabolite, +fluoxetine and metabolite, +acetaminophen |
(72) | |
| 25 y/o M with unknown hx | N/A | Femoral blood: 10 +Diazepam and metabolite, acetaminophen, +codeine and metabolites, +morphine and metabolites, +6-AM, +mirtazapine |
(72) | |
| 45 y/o M with unknown hx | N/A | Antemortem blood: 30 Femoral blood: 8 +Ethanol, +morphine and metabolites, +6-AM, +acetaminophen, +methadone, +nordiazepam Serum: 8 |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 2,180 | (72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 1,370 +BZE, +sertraline, +diphenhydramine |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 870 +Dothiepin, +methiopropamine, +ethanol |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 110 +Methadone, +EDDP, +zopiclone, +sertraline, +aripiprazole, +dehydroaripiprazole, +2-aminoindane, +ethanol |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 140 +Morphine, +codeine, + ketamine, +cocaine, +BZE, +venlafaxine, +O-desmethylvenlafaxine |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 30 +Methiopropamine, +5-APB/6-APB |
(72) | |
| M with unknown age and unknown hx | N/A | Femoral blood: 110 +Diazepam, +nordiazepam, +temazepam, +oxazepam, +morphine, +codeine |
(72) | |
| MPHP/4-MEAP | 39 y/o M with hx of drug addiction, found deceased in caravan | Multiple injection sites. Pulmonary and pericerebral edema and multivisceral congestion | Femoral blood: 47/1.6 THC 1.4, THC-COOH 6.6 Cardiac blood: 97/3.5 ethanol 50 mg/dL Urine: 2380/49,700 |
(88) |
| NEH | 21 y/o M with hx of drug and alcohol abuse taken to ED with aggression, disorientation and loss of consciousness. Sudden cardiac arrest 5.5 h after admission and unable to revive. Plastic bag with white powder found in underwear | At ED, hyperthermia and wide pupils that reacted to light. Tachycardia, tachypnea, blood pressure and anuria present. At autopsy, lung congestion, mild focal pulmonary edema, swelling and brain congestion, left ventricular hypertrophy and focal liver steatosis | Femoral blood: 145 Amphetamine 12, THC-COOH <5 |
(89) |
| 25 y/o M driver did not stop at road check. Stopped after a brief chase and officers noticed unnatural behavior. Driver appeared stimulated, cheerful with blurred speech. Driver admitted to taking mephedrone and 2 bags containing white powder | Subject exhibited clear speech, steady gait, normal pupils and normal pupillary reaction. Negative Romberg’s test and finger–nose test | Blood: 34 | (90) | |
| 27 y/o M involved in road crash resulting in death | N/A | Blood: 37 3-fluorophenmetrazine 9 |
(90) | |
| 18 y/o M DUID | N/A | Blood: 8 THC 1.6, THC-COOH 8.7 |
(90) | |
| NEP | 22 y/o M brought to ED with agitation and acute psychosis after ingesting instant coffee packet | Elevated temp, tachycardia, rhabdomyolysis and acute kidney and liver injuries | Urine: +N-ethylnorpentylone |
(91) |
| 22 y/o M admitted after ingesting instant coffee packet | 39.6°C Temp, delirium and agitation. Multiple organ failure | Urine: +N-ethylnorpentylone |
(91) | |
| 29 y/o M with hx of depression, admitted to ER agitated with visual hallucinations, delirium, mydriasis and nausea | Tachycardia 100 bpm, nonspecific ST interval alteration on ECG. Laboratory evaluation showed neutrophil leukocytosis and mild rhabdomyolysis | Urine: + *Urine positive 4 days postadmission |
(92) | |
| 31 y/o M with hx of cathinone use for 3 years and HIV, IV 0.2 g twice | Subject experienced bad trip and asthenia for 4 days | Blood: + Urine: + Powder: + |
(93) | |
| 36 y/o M with hx of cathinone use for 2 years, IV 0.2 g twice | Paranoia and auditory hallucinations for 4 days | Blood: + Urine: + Powder: + |
(93) | |
| 38 y/o M with hx of cathinone use last 3 years and HIV, IV injected 2 g drug | Dissociative effects, auditory hallucinations and paranoia for 5 days | Blood: + Urine: + Powder: + |
(93) | |
| 33 y/o M no hx of substance abuse and under pre-exposure prophylaxis with emtricitabine-tenofovir, insufflated drug with GHB | Delusional disorder and pharmacopsychosis for 2 days | Blood: + Urine: + +cocaine, +amphetamines Powder: + |
(93) | |
| 44 y/o M with hx of NPS use and HIV, oral ingestion | Delusional disorder | Blood: + Urine: + Powder: + |
(93) | |
| 32 y/o M attending rave party displayed psychomotor agitation and aggressiveness before fainting. Decedent died en route to hospital | At autopsy, facial swelling, cyanosis of extremities and yellow liquid coming from mouth and nostrils. Internal exam revealed generalized hemorrhage of pulmonary alveoli and abnormal liver size | Postmortem Blood: 170 | (94) | |
| 18 y/o M admitted to ED from rave party. Agitated and signs of several injuries | Tachycardia, mydriatic pupils, oscillation between psychomotor agitation and neurological depression | Serum: 7 Urine: + |
(94) | |
| 26 y/o F arrived at ED after report to poison control center. Found unconscious with sphincter release. Consumed ecstasy and cannabis at a party the night prior | At ED, subject confused, sleepy with tongue injuries from intentional biting, disconnected speech and visual hallucinations. Anterograde amnesia at discharge. Creatine kinase elevated | Urine: + +MDMA |
(94) | |
| 19 y/o attended rave and consumed alcohol and various drugs including 5 ecstasy tablets, 1 LSD blotter, 2 packs cigarettes and drank unknown amount of Catuaba | Subject conscious and oriented but agitated with palpitations and tachycardia (180 bpm). Creatine kinase elevated | Serum: 19 MDMA 54, ethanol 0.8 g/L Urine: + +MDMA, +caffeine, +cotinine |
(94) | |
| 35 y/o M with hx of alcohol and drugs over 2 days, found unconscious | Neurological depression GCS 5 and anisocoria. Elevated creatine kinase and lactase. At 6 h post admission, neurogenic shock and decerebration. 6 days postadmission vertebral artery dissection and cerebrovascular hemorrhage of brain stem. At 35 days, subject discharged in vegetative state with neurological damage to third cranial nerve | Serum: 149 Urine: + |
(94) | |
| 26 y/o M with hx of mental disorders admitted to psychiatry for differential diagnosis of drug misuse | Subject presented with psychosis, paranoia, sleeplessness and inconsistent speech. Elevated creatine kinase | Serum: 61 | (94) |
4F-α-PVP = 4-fluoro-alpha-pyrrolidinovalerophenone, DBP = diastolic blood pressure, EDDP = 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, EMS = emergency medical services, HIV = Human Immunodeficiency Virus, MDA = 3,4-Methylenedioxyamphetamine, pO2 = partial oxygen pressure, SBP = systolic blood pressure, GHB = gamma-hydroxybutyrate, 5-APB = 5-(2-Aminopropyl)benzofuran, 6-APB = 6-(2-Aminopropyl)benzofuran, GR = gram.
MPH works similarly to amphetamine in competing with catecholamines in the CNS to block DAT and NET and elevate synaptic monoamine concentrations (72, 73, 95). In vitro pharmacology documented more potent DAT than SERT inhibition by MPH analogues, suggesting predominately stimulant effects with high abuse liability, similar to amphetamine (73). 3,4-Dichloromethylphenidate inhibited NET 10-fold and DAT 2-fold more potently than MPH. NET (0.04–0.42 µM) and DAT (0.08–0.34 µM) inhibition potencies for 3,4-dichloroethylphenidate, ethylnaphthidate, 4F-MPH and 4-methylmethylphenidate were similar to those of MPH (73). Several MPH analogues inhibit NET at submicromolar concentrations, suggesting cardio- and psycho-stimulant properties.
MPH is almost completely absorbed when ingested orally, with peak plasma concentrations occurring 1–3 h after a standard oral dose that has a plasma half-life of 1.5–2.5 h (95). Adverse effects of MPH include pupil dilation, hair loss, depression, anorexia, headaches, decreases in libido, insomnia, restlessness, anxiety and hypersensitivity; anorexia and tachyarrhythmia are the most prevalent effects at high concentrations in blood (72). Abuse also produces paranoia, hallucinations, delusional disorder and euphoria. EPH is commonly insufflated, with an onset of action of 0–5 min and a duration of effects lasting 15–300 min (72). Adverse effects of EPH include tachycardia, hypertension, palpitations, endocarditis, fever, mydriasis, insomnia, irritability, paranoia, anxiety and delusional thoughts.
A comprehensive review during 2017–2020 was recently published, identifying 31 different synthetic cathinones in 75 reported fatal intoxications (96). NEP was found in 23 fatalities, with blood concentrations of 18.4–50,000 ng/mL and most commonly coadministered with other cathinones dibutylone, pentylone or butylone. In NEP-only cases, concentrations were 150–600 ng/mL. Case reports detailed aggression, confusion and cardiac arrest in several cases. 4-Chloromethcathinone (4-CMC) was the next most frequently reported cathinone analog associated with nine fatalities. 4-CMC blood concentrations were 0.9–1,870 ng/mL, but most cases included other drugs, frequently THC. N-Ethylhexylone (NEH) was confirmed in six fatalities (blood concentrations 7.2–285 ng/mL), always combined with other drugs. NEH has a short duration of effects, resulting in binge dosing. 3-Methylmethcathinone (3-MMC) was reported in five fatalities, with peripheral blood concentrations of 249–800 ng/mL. In these synthetic cathinone cases, polydrug consumption frequently included other synthetic cathinones, synthetic opioids (e.g., U-47700) or other stimulants (e.g., MDMA).
Ethylphenidate
EPH first gained attention in 2011 as a legal ethyl acetate MPH analogue; MPH is Schedule II in the USA (97). EPH can be formed by transesterification following MPH and ethanol coingestion, with a significant correlation between the area under the concentration versus time curve of MPH to EPH (98). EPH is a potent stimulant with high abuse potential including pleasurable stimulation, euphoria, cognitive enhancement, indecisiveness and anxiety (99). Physiological effects of elevated body temperature, increased heart rate and blood pressure, profuse sweating and muscle tension also were reported. Other drugs of abuse were frequently found in EPH fatalities (72) (Table IV). In these fatalities, EPH femoral blood concentrations were 8–2,180 ng/mL. In two cases, antemortem blood was collect with EPH concentrations of 30 and 460 ng/mL.
Dibutylone
Dibutylone is a substituted beta-keto-methylenedioxyamphetamine and one of the analogues in the popular series that includes methylone, ethylone and butylone. In the previously published review by Maida et al., dibutylone was a common finding in cases also positive for NEP, identified in 5 of 12 cases (96). Krotulski et al. reported 15 dibutylone cases, 9 postmortem with blood concentrations of 10–1,400 ng/mL and 3 recreational use with oral fluid concentrations of 123–1,926 ng/mL (87). In every dibutylone case, butylone was also found in blood from <10–600 ng/mL (Table IV). In vitro metabolism studies identified butylone as an N-demethylated dibutylone metabolite. Effects related to dibutylone ingestion were not reported in the case series; however, previous butylone reports included tachycardia, hypertension, rigid muscle tone, hypersalivation, mydriasis, hyperthermia, arrhythmias and altered behavior (100, 101). Dibutylone is expected to produce similar effects based on its structural similarity.
N-Ethylpentylone
NEP, first reported in 2014, became a frequently encountered NPS cathinone in the USA in 2016 (102). In an NEP case series of 31 reports by Krotulski et al., 26 were death investigations with blood NEP concentrations of 12–50,000 ng/mL (103). The other five intoxications involved hospitalizations with coingestion of other cathinones and severe psychiatric effects with delusion, auditory hallucinations, paranoia and dissociation persisting in some cases for days (93). Similarly, NEP was confirmed in subjects’ blood, serum and/or urine in a series of acute drug intoxications in Brazil—noting palpitations; tachycardia; agitation; aggression; coma and in one case, death—with blood concentrations of 7–170 ng/mL (94).
N-Ethylhexylone
NEH was the most frequently seized cathinone in 2017 and among the top three in 2018, according to the 2019 European Drug Markets Report (104). NEH is sold via the internet and reportedly has the strongest effects when snorted, although it is also administered by rectal, intravenous (IV), oral or smoked (i.e., e-cigarette) routes (89). Desired effects typically occur following 10–250 mg doses with an onset of effects for 2–25 min, but effects depend on the route of administration and drug tolerance. In an NEH fatality, the subject presented with hyperthermia, dilated pupils, tachycardia and elevated blood pressure (89). During the autopsy, congestion in the lungs and swelling and congestion in the brain were noted at a femoral blood concentration of 145 ng/mL. In three impaired driving cases, NEH concentrations were 8–34 ng/mL (90).
Other NPS stimulants
Other synthetic stimulants were identified less frequently, with three case reports from the α-pyrrolidinophenone derivative class. One involved α-PHP in a living individual who was acting irrationally and presenting with hallucinations, delusion, fear, anxiety and restlessness. At the ED, the patient’s Glasgow Coma Score (GCS) was 14, with serum concentrations of 175 ng/mL on admission to 1.8 ng/mL 216 h later (86). A second case involved an unconscious male found at home who was previously admitted to the ED 2 months prior because of loss of consciousness and aspiration pneumonia (85). At the first admission, his GCS was 4 with an elevated heart rate; alpha-ethylaminopentiophenone (α-EAP) and 4-fluoro-alpha-pyrrolidinovalerophenone were identified as the causative agents. In a third case, a male with a history of drug addiction had autopsy findings of pulmonary edema and multivisceral congestion (88). His femoral blood contained 47 ng/mL of 4-methyl-α-pyrrolidinohexiophenone (MPHP) and 1.6 ng/mL of 4-methyl-α-ethylaminopentiophenone (4-MEAP). In a nonfatal 4F-MPH case, a disoriented and confused individual with severe psychomotor agitation was admitted to the ED and had mild tachycardia lasting 2 days (84). 4F-MPH blood and urine concentrations were 32 and 827 ng/mL, respectively.
Discussion
NPS stimulants are among the most sought-after NPS classes due to the euphoria and altered metal state produced and the low perception of likelihood of adverse effects. Typical symptoms associated with synthetic stimulant use include elevated heart rate, increased blood pressure and dilated pupils (Table III). Individuals under the influence of these substances often experience hallucinations, paranoia, anxiety and/or aggression with the risk of excited delirium and life-threatening cardiovascular effects. In these cases, some effects such as asthenia, paranoia and hallucinations can persist for days after the initial onset of symptoms. Individuals with cardiac compromise are especially susceptible to toxicity and death.
Common autopsy findings in synthetic stimulant deaths included pulmonary edema and in some cases brain congestion (Table III). Scene investigation, paraphernalia recovery and/or subject self-report of ingested substances provided key data in these antemortem and postmortem cases that led to identifying the responsible NPS. Finding additional NPS stimulants along with other therapeutic and illicit substances was not uncommon in these cases.
Synthetic stimulant users often do not know what they are ingesting because these substances are often sold as ecstasy (i.e., MDMA). In one study comparing self-reporting drug ingestion to toxicological findings, 29.6% of users who believed they had ingested MDMA had consumed a novel stimulant (105). Given the persistence and prevalence of synthetic stimulants, laboratories should remain vigilant in monitoring these substances and pursue additional analytical testing in suspected cases of stimulant overdoses when traditional toxicology methods have negative findings. The continued emergence of novel synthetic stimulant drugs is expected given the historical popularity of these substances. Canceling electronic music events and closing dance clubs during the coronavirus disease 2019 pandemic in 2020 may have temporarily reduced the availability and popularity of the synthetic stimulant drug class.
NPS Hallucinogens
Introduction
Hallucinogens are naturally occurring or synthetic drugs that produce hallucinations, dissociation and out-of-body experiences. Novel hallucinogens are characterized as chemical derivatives and analogues of classical hallucinogens, such as the psychedelic lysergic acid diethylamide (LSD) or the dissociative drugs ketamine and phencyclidine (PCP). Novel hallucinogens are divided into six subcategories: PCP-like analogues, ketamine-like analogues, phenethylamine analogues, LSD-like analogues, tryptamine-like analogues and diarylethylamine analogues. Some novel synthetic drug classes were derived from legitimate pharmaceutical research, but others are experiments by illicit drug manufacturers or users seeking new or different experiences. Novel hallucinogen preparations include powders, pills, tablets, liquids and blotters (106). PCP-, ketamine- and LSD-like analogues are commonly ingested with the first two generally sold as solid materials and the last generally as a liquid or on blotters. Descriptions of these substances’ structures, syntheses and effects are frequently found in online forums and relevant popular publications, such as ‘PiHKAL’ (2) and ‘TiHKAL’ (3). The peer-reviewed literature and case reports of intoxications involving novel hallucinogens continue to grow. Table V (107–146) highlights adverse effects including hospitalization, death investigation and impaired driving cases—paired with toxicological findings and/or chemical confirmation from recent publications.
Table V.
NPS Hallucinogens Case Histories with Clinical Symptoms, Autopsy Findings and Primary Drug and Additional Drug Concentrations
| Drug | History | Clinical symptoms/Autopsy findings | Drug results (ng/mL, unless specified) | Reference |
|---|---|---|---|---|
| 2C-E and Bromo-DragonFLY | 29 individuals (15 M, 14 F, 24–56 y/o) ingested an unknown drug; found lying on ground, shouting and screaming | Some unconscious and unresponsive; tachycardia, respiratory distress, pain, delusions, self-harm; some required sedation with benzodiazepines; all recovered within days | ‘2C-E’ Blood: <0.4 Urine: 1.5–183 ‘Bromo-DragonFLY’ Blood: 0.6–2 Urine: 1.6–35 |
(107) |
| 2-FDCK | 23 y/o M with hx of ketamine use; Insufflated what he believed was ketamine | Delirium and palpitation; BP 121/62, pulse 144 bpm, RR 20, SpO2 98% and Temp 36.3°C | Urine: 213 | (108) |
| 39 y/o M ingested instant coffee packet; admitted to ED and later to psychiatric ward with drug-induced acute psychosis and adjustment disorder | Delirium generalized weakness; BP 195/81, pulse 80 bpm, RR 20, SpO2 100% and Temp 35.6°C | Urine: 21.7 NEP 716 |
(108) | |
| 40 y/o M ingested instant coffee packet; admitted to ED and psychotic symptoms subsided 1 day later | Tactile hallucinations and vomiting; BP 132/79, pulse 134 bpm, RR 20, SpO2 93% and Temp 36.2°C | Urine: 104.8 Ketamine 121.9, norketamine 285.5, mephedrone 991.3 |
(108) | |
| 40 y/o M ingested instant coffee packet; admitted to ED and psychotic symptoms subsided 1 day later | Delirium and delusion of being monitored; BP 158/94, pulse 120 bpm, RR 20, SpO2 98% and Temp 38.4°C | Urine: 918.6 Eutylone 1007.9, mephedrone 991.3 |
(108) | |
| 22 y/o M ingested instant coffee packet; admitted to ED and discharged with consciousness recovery | Delirium, self-harm and agitation; BP 101/77, pulse 93 bpm, RR 19, SpO2 97% and Temp 36.3°C | Urine: 56.2 Eutylone 54.1, mephedrone 1,345 |
(108) | |
| Clinical case series 20 ketamine users (13 F and 7 M; 18–50 y/o) | Impaired consciousness (14), agitation (8), abnormal behavior (5), hallucination or delusion (4), loss of consciousness (4), dizziness (3), convulsion (2), hypertension (8), tachycardia (6), nausea or vomiting (3), hyperthermia (1), dyspnea (1) | Urine: + 2-Oxo-PCE (n = 9) DCK (n = 10) ketamine (n = 17) tiletamine (n = 2) |
(109) | |
| 6 DUID investigations | Suspected drug impairment | Blood: 0.005–0.48 mg/kg Hair: 0.007–0.034 ng/mg |
(110) | |
| 2-Oxo-PCE | 18 y/o M ingested instant coffee packet; admitted to ED and discharged with consciousness recovery. | Delirium and agitation; BP 111/88, pulse 140 bpm, RR 20, SpO2 100% and Temp 36.3°C; acute kidney injury, elevated creatinine kinase and lactic acidemia | Urine: 117.7 4-CEC 1950.2, eutylone 4,131.8, ethylone 1.8, methylone 119.6 |
(108) |
| 41 y/o F ingested instant coffee packet; admitted to ED and psychiatric ward with drug-induced acute psychosis and adjustment disorder | Delirium and suicide attempt by driving car into tree; BP 157/90, pulse 78 bpm, RR 17, SpO2 95% and Temp 37.5°C | Urine: 101.69 NEP 4545.61. mephedrone 91.54 |
(108) | |
| 52 y/o M found dead in bedroom; found with head hanging over bucket; drug paraphernalia at scene | Brain swelling (1410 g), moderate lung hyperhydration (420 g), inner organs filled with blood, urinary bladder moderately opened, tablets found in stomach and small bowel, acute respiratory infection, expanded right ventricle and atrium | Femoral blood: 375 DCK 7, venlafaxine 200, O-desmethylvenlafaxine 50, N-desmethylvenlafaxine 40 Heart blood: 2,159 Urine: 3468 Liver: 6,137 ng/g Bile: 3,290 Gastric contents: 3,086 |
(111) | |
| 56 clinical case series. | ‘25 cases 2-Oxo-PCE only’: impaired consciousness (21), confusion (15), abnormal behavior (11), agitation (5), aggressive behavior (6), loss of consciousness (5), convulsion (4), drowsiness (4), unstable emotion (2), psychotic symptoms (4), dizziness (1), nystagmus (1), unsteady gait (1), hypertension (20), tachycardia (10), hyperthermia (4) and rhabdomyolysis (2) | Urine: + Cocaine (n = 15), ketamine (n = 14), methamphetamine (n = 8), cannabis (n = 1) |
(112) | |
| 37 y/o M with hx of psoriasis; found collapsed at home | Hyperthermia (39.3°C), diabetic ketoacidosis and convulsion; BP 177/117, sinus tachycardia (116 bpm), GCS 8, UDS negative | Urine: + | (112) | |
| 25 y/o M found lying on ground; drowsy but agitated state; white powder in nose | Afebrile (37.1°C), GCS 9, sinus tachycardia 119 bpm, BP 143/96, UDS positive for morphine only | Urine: + | (112) | |
| 24 y/o M with no prior clinical hx found confused and wandering; white powder in nose | Afebrile (36°C), GCS 10, sinus rhythm (85 bpm), BP 146/98, UDS negative | Urine: + | (112) | |
| 3-HO-PCP | 56 y/o M with hx of myocardial infarction; admitted regular use of ‘Hexen’ (N-ethyl hexedrone) and recent 3-HO-PCP use | Hyperthermia (39.9°C), sinus tachycardia (150 bpm), reduced consciousness, ocular clonus and vertical nystagmus; GCS 6, RR 40, oxygen saturation 99%, HR 144 bpm, BP 145/93; developed rhabdomyolysis | Serum: + +3-HO-PCP metabolites, +N-ethyl hexedrone/metabolites, +clephedrone/metabolites Urine: + +3-HO-PCP metabolites, +3-MeO-PCP, +N-ethyl hexedrone/metabolites, +clephedrone/metabolites, +THC-COOH |
(113) |
| 19 y/o M hospitalized; ingestion of unknown pills | Altered state, delirious and euphoric; respiratory acidosis, right anisocoria, mydriatic pupils and hypothermia | Blood: 350 Ethanol 2 g/L Urine: 6109.2 |
(114) | |
| 21 y/o M hospitalized; nasal fracture; ingestion of unknown pills | Altered state; respiratory acidosis, right anisocoria, mydriatic pupils and hypothermia | Blood: 180.1 Ethanol 1.7 g/L Urine: 3,003.5 |
(114) | |
| 27 y/o M with hx of schizophrenia; found unconscious in car by police; reported feeling sedated and ‘loopy’; unable to remember events, denied drug use | Delusions, vital signs normal, borderline tachycardia | Urine: +(PCP screen) | (115) | |
| 2 fatal cases: 30 y/o F found murdered; 41 y/o F found dead at home | N/A | Blood: 63 and 498 Urine: 94 and 16,700 Hair: + |
(116) | |
| 19 y/o M with hx of drug use and depression; found awake at home; white powder and drug paraphernalia recovered | Tachycardic, hypertensive, tachypneic and catatonic; developed fever and lactic acidosis concomitant with psychomotor agitation and hallucinations; sinus rhythm, respiratory rate 22 bpm, pulse 130 bpm, BP 147/104, oxygen saturation 96%, GCS 11, pupils dilated | Blood: 400 ng/g | (117) | |
| 27 y/o M; found dead at home in bathtub; hx of drug use; regularly ordered hallucinogens online | Brain swelling, pulmonary edema and urine retention; burns on body from hot water | Blood: 380 ng/g | (117) | |
| 21 y/o M with suicidal intentions found unresponsive in room; hx of drug use | Brain edema, pulmonary edema, a patent foramen ovale and discrete coronary atherosclerosis | Blood: 180 ng/g Buprenorphine 2.2 ng/g, 5-MeO-MiPT 130 ng/g |
(117) | |
| 27 y/o M with psychiatric problems; found on chair in residence | Congestion of lungs and liver | Blood: 230 ng/g Methadone 440 ng/g, diazepam 530 ng/g, methamphetamine 40 ng/g, buprenorphine 0.2 ng/g |
(117) | |
| 29 y/o M with hx of bipolar disorder and drug use; found hanged in residence after leaving good-bye message; pills and drug paraphernalia at scene | Petechial hemorrhage of oral mucosa, brain edema, pulmonary congestion and edema, discrete coronary atherosclerosis and enlarged liver | Blood: 120 ng/g Flubromazolam 10 ng/g, +AB-FUBINACA, +THJ-018, buprenorphine 2 ng/g, methylphenidate 20 ng/g |
(117) | |
| 32 y/o M with hx of drug use; found dead in residence; drug materials and paraphernalia at scene | Fresh myocardial injury, congestion of internal organs and mucous membranes, pulmonary edema, brain and heart edema; incipient pneumonia and aspirated stomach content after vomiting | Blood: 60 ng/g Oxycodone 100 ng/g, amphetamine 130 ng/g, flubromazolam 9 ng/g, MT-45 110 ng/g, 4-MeO-PCP 30 ng/g, THJ-018, THC 1.1 ng/g |
(117) | |
| 27 y/o M with hx of drug use; found unresponsive in bed | Internal organ congestion, pulmonary edema, petechiae on heart surface | Blood: 50 ng/g Tramadol 60 ng/g, alprazolam 20 ng/g, fentanyl 25 ng/g, amphetamine 90 ng/g |
(117) | |
| 20 y/o F found dead in bed; reportedly taking tramadol and benzodiazepines | Pulmonary and cerebral edema and internal organ congestion | Blood: 80 ng/g Tramadol 13,000 ng/g |
(117) | |
| 40 y/o M with HIV admitted to hospital; went to bathroom to use drugs (0.2 mg IV); found lying on ground unconscious; patient survived; drug sample recovered for testing | RR 18, oxygen saturation 96%, sinus tachycardia 115 bpm and Temp 37.9°C | Drug product: + | (118) | |
| 17 y/o M purchased 3-MeO-PCP on internet; took 200 mg oral dose and admitted to ED; 7 days later returned to ED after insufflating 50 mg | BP 158/131, tachycardia (100 bpm), neurological manifestations; confusion, hypertonia, nystagmus and agitation; symptoms similar but less severe during second overdose | Blood: 17.1 Urine: 706.9 |
(119) | |
| 39 y/o F with hx of drug use; found dead at home after assault by partner | Parched horizontal ligature encircling neck; broad red area from intense subcutaneous hemorrhage; clear ligature abrasion; fractures with hemorrhage of superior horn of thyroid cartilage and right greater horns of hyoid bone; intense multi viscera congestion | Blood: 63 Urine: 94 Bile: 64 Hair: 731–893 pg/mg Ethanol 1.37 g/L, diazepam 157, cocaine 25 |
(120, 121) | |
| 41 y/o M found dead at home | Needle marks noted | Blood: 498 Urine: 16,700 |
(120) | |
| ∼ 35 y/o M with hx of cannabis and amphetamine use; found dead near lake with face in water; ingested ant poison drug prior to death | Temp 23.1°C, face, neck and eyes stowed; foam on mouth; small injuries on hands and legs | Blood: 152 Ethanol 1.2% Serum: 123 Amphetamine 85 Drug product: + |
(122) | |
| 36 y/o M found agitated on street | Confusion, aggressiveness, logorrhea, hypertonia, myosis, hypersalivation; BP 151/90, HR 100 bpm and Temp 37.3°C | Urine: + Ethanol 0.185 g/dL, +buprenorphine, +tramadol |
(123) | |
| 32 y/o M with hx of cannabis use; presented to ED for abdominal pain but painkillers inactive at high doses | BP 137/87, HR 83 bpm and Temp 36°C | Urine: + | (123) | |
| 37 y/o M admitted to ED after drug ingestion | Hypertensive (170/100), tachycardic (120), signs of psychosis and altered neurological status GCS 10–12; increased muscle tone with spastic leg postures; fluctuating state of consciousness | Serum: 49 Methamphetamine 121, amphetamine 10 |
(124) | |
| 40 y/o M with no hx drug use; referred to ED for cramps and deteriorating state of consciousness | Hypertensive (172/113), tachycardic (126), breathing spontaneously and normoglycemic; altered state of consciousness 12 GCS; did not obey commands; pupils isocoric and miotic | Serum: 66 Ethanol 1.02 g/L |
(124) | |
| 21 y/o M with hx of drug use; found naked and unresponsive; recently discharged from drug treatment; empty pill bottles recovered from person | MOD: accident; COD: acute intoxication due to polydrug consumption | Blood: 3,200 Ethanol 47 mg/dL, bupropion 1800, +delorazepam, +mitragynine, +paroxetine |
(125) | |
| 58 y/o M with hx of opiates and methamphetamine; significant health problems; found unresponsive in drug treatment facility | MOD: accident; COD: acute intoxication due to polydrug consumption | Blood: 630 +PCP (screen), methamphetamine 110 Urine: + +Amphetamine (screen) |
(125) | |
| 5-MeO-DiPT | 29 y/o M injected solution of 5-MeO-DiPT into the anus |
No abnormal signs/symptoms when presented to ED days later | Dried urine spot: 2.3 | (126) |
| 4 M suspected of using 5-MeO-DiPT | N/A | Urine: 0.3–0.8 | (126) | |
| 32 y/o M ingested LSD-like substance; found standing naked and without orientation in street; drugs recovered from residence | Agitated and aggressive; several abrasions on body and in bad state | Blood: 160 Methylphenidate 1.36, ritalinic acid 64.1, BZE 305 Urine: 3,380 + 5-MeO-MiPT metabolites, methylphenidate 671, ritalinic acid 2,230 Drug powder: + |
(127) | |
| 25B-NBOMe | 17 y/o M with hx of NPS use; ingested 25B-NBOMe tablets sublingually; friends monitored state for hours; found unresponsive by EMS and pronounced dead |
Hallucinogenic effects, convulsive episodes, various arrhythmias, led to cardiac arrest and death | Blood: 3.14 | (128) |
| 23 y/o M ingested substances labeled 25B-NBOMe 1 GR and 4-CMC 1 GR; found in residence and died in route to ED | Aggressive, agitated, moving anxiously, shouting, convulsions, heavy breathing and salivations | Blood: 66.5 4-CMC 2.14, +THC, THC-COOH 11, +ethanol |
(129) | |
| 23 y/o M ingested substances labeled 25B-NBOMe 1 GR and 4-CMC 1 GR; jumped out window of fifth floor and found dead | N/A |
Blood: 661 4-CMC 0.887, +THC, +THC-COOH, +ethanol |
(129) | |
| 24 y/o M ingested substances labeled 25B-NBOMe 1 GR and 4-CMC 1 GR; found in residence, transported to ED and lived |
Aggressive, agitated, moving anxiously, shouting | Blood: 38.4 +4-CMC, +THC, THC-COOH 13, +ethanol |
(129) | |
| 25C-NBOMe | 27 y/o M with re-admission to ED after ingestion of drugs ‘First admission’: found acting erratically, resisted arrest, was physically restrained and transported to ED ‘Second admission’: ∼ 3 weeks later; ingested tablets of 25I-NBOMe and transported to ED |
‘First admission’: aggressive, confrontational but alert; BP 139/90, HR 146, RR 28, Temp 36.6°C, oxygen saturation 98%; no evidence of trauma; tachycardia and agitated ‘Second admission’: agitated; BP 153/119, HR 120, RR 22, Temp 36.8°C and oxygen saturation 98% |
Blood: + | (130) |
| Teenage M attended party, drugs for sale; seen smoking cannabis; altered state of mind; taken home by friends; jumped in waterway and body later found downstream; plant material and blotters found on decedent | Death occurred by drowning | Peripheral blood: 2.8 25H-NBOMe 0.29, THC 15.5, THC-COOH 56 Central blood: 1.43 25H-NBOMe 0.13, THC 9.9, THC-COOH 8.5 Urine: 0.94 25H-NBOMe 0.14, THC-COOH 43 |
(131) | |
| 25I-NBOMe | 42 y/o M with no medical hx; severe headache ingested pediatric analgesic syrup replaced with ethanolic 25I-NBOMe ∼ 320 µg/mL developed restlessness | ‘Upon presentation’: vital signs unremarkable, excessively dilated pupils, strong sweating, disorientation and agitation. ‘30 min later’: severe agitation, screaming, coenesthesia, hallucinations; BP 127/97, HR 100; symptoms resolved in 6 h |
Serum: 4.2–34 25I-NBOH, 2C-I 2.1–12 Urine: 25I-NBOH 1.2, 2C-I 3.5–8.2 |
(132) |
| Two individuals insufflated white creamy powder | Impairment, altered behavior and visual hallucinations | Plasma: 37 and 72 Powder: + |
(133) | |
| Drug consumption | Forensic clinical evaluation of brain dysfunctions after cardiorespiratory arrest | Hair: 1.0–4.9 pg/mg | (134) | |
| 29 y/o M unconscious after instilling pink liquid in nose thought to be NBOMe(s) | Hypertonia, tremors, partial seizure, mydriasis, tachycardia, hypertension, hyperthermia, profuse sweating, serotonin syndrome; persistent cognitive and psychiatric abnormalities | Serum: 0.9 Urine: + + 25C-NBOMe metabolites Pink liquid: + |
(135) | |
| 17 y/o M ingested two lines of acid; found having multiple seizures and transported by EMS to ED | 2 tonic-clonic seizures, became unresponsive and apneic; BP 106/53, HR 140, Temp 38.9°C and oxygen saturation 98% | Serum: 3 2C-I 55 Urine: 10 2C-I 225 |
(136) | |
| 20 y/o with no medical hx ingested small dose of Alice in Wonderland had seizure episode during a party | Seizures, muscle swelling, diminished motor function; severe clinical toxicity and developed exceptionally massive rhabdomyolysis | Blood: 0.24 | (137) | |
| AL-LAD | 51 y/o M with hx of hyperlipidemia and vertebral artery stenosis; shot two teenagers and ignited a building; police placed individual in patrol car and he became pulseless; transported to ED in cardiac arrest | BP 73–123/54–90, HR 57–122, Temp 35.7–36.9°C, SpO2 98–100%, GCS 3, pupils 3 mm | Serum: + + lysergic acid Urine: + |
(138) |
| Diphenidine | 30 y/o M with hx of drug use found confused, agitated and unable to communicate; white powder labeled diphenidine 1 g recovered | Tachycardia (HR 160), tachypnea and miotic nonreactive pupils; agitation, disorientation and altered consciousness with GCS 9; Temp 38.0°C | Plasma: 308 Diclazepam 3.5, methylphenidate 3.0 Urine: 631 +methylphenidate, THC-COOH 120 Hair: 4,400 pg/mg Alpha-PVP 1,040 pg/mg, MDPV 120 pg/mg, methoxetamine 27 pg/mg, 4-FA 55 pg/mg, methylone Powder: + |
(139) |
| ∼ 25 y/o F; found dead in bed; NPS packages recovered | Slight abrasions on hands; no signs of natural disease; slight brain edema, pulmonary edema and organ congestion | Blood: 73 7-aminoflunitrazepam 86, 7-aminonimetazepam 27, chlorpheniramine 66 |
(140) | |
| 53 y/o M found dead in residence; ‘Heart Shot BLACK’ package with herbal material recovered and confirmed as 5F-ADB | No apparent specific injuries or internal findings | Blood: 12 ± 2.6 + Diphenidine metabolites, 5F-ADB 0.19 ± 0.04 and metabolites |
(141) | |
| DOC | Five nonfatal cases 18–23 y/o believed to ingest LSD | Severe clinical symptoms | Plasma: <18 Urine: 300–1,300 |
(142) |
| Two found dead after insufflating powder believed to be LSD; 4 years later, bodies exhumed for additional testing | No traumatic injury | Liver: 99 ng/g Spleen: 28 ng/g Bone: 14 ng/g Lung: 15 ng/g Hair: 32 pg/g |
(143) | |
| MXP | 21 y/o M with hx of bipolar disorder and drug use; patient reported use of 125–150 mg MXP purchased online; presented to ED; powder collected for testing | Agitation, aggressiveness, reported dissociative effects; Withdrawal after 7 days in hospital | Powder: + | (144) |
| 33 y/o M with hx of autism and treatment with methadone, loxapine and lorazepam; found agitated and presented to ED; small yellow pills collected from residence | Profuse sedation, hyperthermia, tachycardia (HR 140) and mydriasis; GCS 10; serotonin syndrome suspected | Urine: + + AMT Pills: + + AMT |
(145) | |
| MXPr | 26 y/o M with hx of drug use admitted to ED; drug recovered for analysis | Asymptomatic | Urine: + Drug product: + (95%) |
(146) |
5-MeO-DiPT = 5-methoxy-N,N,-diisopropyltrptamine, AMT = alpha-methyltryptamine, DCK = deschloroketamine, MOD = manner of death, UDS = urine drug screen.
During 2016–2018, ‘NBOMe’ drugs were the most commonly encountered novel hallucinogens (14); more recently (2017–2020), PCP- and ketamine-like analogues, including 3-methoxy-PCP (3-MeO-PCP), 2-fluorodeschloroketamine (2-FDCK) and N-ethyldeschloroketamine (2-Oxo-PCE, eticyclidone) are, in that order, the leading novel hallucinogens worldwide. Novel hallucinogen prevalence data are lacking for the USA; however, NPS Discovery toxicology testing of samples from across the country identified the most prevalent novel hallucinogen in 2018 as MeO-PCP and in 2020 as 2-FDCK (70). Among the United States Schedule I NBOMe(s), there were few 25B-NBOMe and 25C-NBOMe reports with the market shifting to 25I-NBOMe (147). Less frequently encountered novel hallucinogens included phenethylamine analogs 2C-E, Bromo-DragonFLY and DOC (2,5-dimethoxy-4-chloroamphetamine); the LSD-like analogue AL-LAD (6-allyl-6-nor-LSD); tryptamine-like analogues 5-MeO-MiPT and 5-methoxy-N,N,-diisopropyltrptamine (5-MeO-DiPT); diarylethylamine analogues diphenidine and methoxphenidine (MXP) and the ketamine-like analog methoxpropamine (MXPr).
Pharmacology
MeO-PCP, 2-FDCK and MXP bind to the glutamate N-methyl-D-aspartate receptor (NMDAR) (148–150). 3-MeO-PCP has the highest binding affinity compared to its 2- and 4-MeO-PCP isomers, with similar potency to PCP at the NMDAR (150). 3-MeO-PCP is metabolized via O-demethylation to 3-hydroxy-PCP (3-HO-PCP), which also is an emerging novel hallucinogen with even higher NMDAR affinity (120, 151). The concentration of 3-HO-PCP is thus an important aspect to consider when interpreting toxicological data from 3-MeO-PCP and 3-HO-PCP cases (152). 2-MXP, 3-MXP and 4-MXP are relatively selective NMDAR antagonists with IC50 of 56.5 ± 5.8, 30.3 ± 2.6 and 723.8 ± 69.9, respectively. These NMDAR antagonists exhibit a relatively similar potency order to MeO-PCP analogues (149). Diphenidine exhibited greater potency than 2-MXP and is thus less potent than PCP and ketamine (149). Anecdotal reports claim that 2-FDCK is more potent than ketamine; however, analytical data are not available.
NBOMe(s), like the classical hallucinogen LSD, are agonists of the serotonergic system (153–155). 25I-NBOMe is a potent full 5-HT2A receptor agonist, with a Ki of 0.044 ± 0.006 nM and an EC50 of 0.44 ± 0.07 nM (153); 25B-NBOMe and 25C-NBOMe are partial agonists, with Ki of 0.19 ± 0.01 nM (154) and 0.9 nM (155), respectively. The onset of effects of oral 25I-NBOMe in humans is 15–120 min, with a duration of action of 6–10 h (similar duration for 25B- and 25C-NBOMe).
Tryptamine-like analogues are also agonists at the SERT 5-HT2A receptors; however, these hallucinogens have lower binding affinity compared to LSD (156). 5-MeO-MiPT has moderate 5-HT2A receptor activity with a Ki of 0.163 ± 0.03 nM (156); 5-MeO-DiPT has a Ki of 0.560 nM and induced hallucinogenic effects in rodents (157, 158).
PCP-like analogs
3-MeO-PCP and 3-HO-PCP are methoxy- and hydroxy-substituted chemical derivatives of the traditional hallucinogen PCP, respectively, which can exist as positional isomers (e.g., 4-MeO-PCP and 4-HO-PCP). MeO-PCP derivatives were first synthesized in the 1960s and 1970s (159), but the first fatalities were reported in 2014 from Sweden (160). 3-MeO-PCP and 4-MeO-PCP are not explicitly controlled in the USA (147) but could be considered PCP analogs, federally controlled under Schedule II by the DEA.
During 2017–2020, nonfatal and fatal 3-MeO-PCP cases reported hospitalized individuals as delirious, confused and aggressive. Clinical signs and symptoms included hypertension, tachycardia, hypothermia, tachypnea, mydriasis and excessive sweating, with most individuals recovering from their overdose. Decedents were commonly found unresponsive with edema and lung, brain and heart congestion at autopsy. There was no distinction between quantitative toxicology concentrations for antemortem and postmortem cases. A nonfatal intoxication following 3-HO-PCP and NEH ingestion led to reduced consciousness, hyperthermia and tachycardia; a patient who was eventually discharged from the hospital also had rhabdomyolysis (113).
Ketamine-like analogues
Modified or substituted chemical derivatives of the traditional hallucinogen ketamine include 2-FDCK, which replaces the chlorine atom with fluorine, and 2-Oxo-PCE, which removes the chlorine atom and elongates the methyl chain to an ethyl chain on the secondary amine. 2-FDCK was first synthesized in 2009, but both ketamine-like analogues emerged on the world recreational drug market in 2016 (161), with 2-FDCK first identified in the USA in 2018 (162). In that same year, a cluster of 2-Oxo-PCE poisonings were reported (112). 2-FDCK and 2-Oxo-PCE are not explicitly controlled in the USA (147), although both were implicated in fatal and/or nonfatal overdoses. Individuals hospitalized following 2-FDCK intake experienced impaired consciousness, agitation, abnormal behavior, hallucinations and/or delusions, loss of consciousness, dizziness, convulsion, nausea and/or vomiting (109). Clinical signs and symptoms included hypertension, tachycardia, hyperthermia and dyspnea with individuals recovering and released from the hospital. 2-FDCK also was involved in six DUID investigations; however, limited information was available (110). No fatal cases involving 2-FDCK were reported.
Individuals hospitalized following 2-Oxo-PCE ingestion experienced impaired consciousness, confusion, abnormal behavior, agitation, aggressive behavior, loss of consciousness, convulsions, drowsiness, unstable emotion, psychotic symptoms, dizziness, nystagmus and unsteady gait (112). Clinical signs and symptoms included hypertension, tachycardia, hyperthermia and rhabdomyolysis, with a single reported fatal outcome (111). The decedent was found in his bedroom with his head hanging over a bucket. At autopsy, brain swelling and lung hyperhydration were noted, and tablets were found in the stomach and small bowel.
MXPr is a ketamine-like analogue with structural similarity to 2-Oxo-PCE but is structurally dissimilar from MXP and diphenidine. MXPr contains a 3-methoxy moiety and a propyl group on the amine. MXPr was reported in one nonfatal case in which the individual was reported to be asymptomatic after presentation to the hospital (146). Toxicology testing and chemical analysis confirmed MXPr ingestion.
Phenethylamine analogues
25I-NBOMe was the most commonly encountered phenethylamine-like novel hallucinogen during the period of review, with observed behaviors including agitation, aggression, seizures, euphoria, hallucinations and sweating (14). Hospital personnel reported tachycardia, hypertension, hyperthermia and mydriasis in patients. Similar case scenarios and clinical findings were reported for 25B-NBOMe and 25C-NBOMe.
A mass poisoning occurred when multiple individuals at a seminar ingested the same drug product containing 2C-E and Bromo-DragonFLY, yet there was considerable variability in the outcomes (107). Some individuals were found unconscious and unresponsive, with clinical signs and symptoms of tachycardia and respiratory distress. Some required sedation with benzodiazepines, but all recovered within days without any fatalities.
DOC intoxications and adverse effects were well characterized in ‘PiHKAL’ in 1991 (2, 14). Since 2017, five nonfatal and two fatal DOC intoxications were confirmed following ingestion of what was thought to be LSD (142). Also, DOC ingestion was confirmed in two exhumed bodies after their unexplained deaths followed insufflation of an ‘LSD’ powder (132).
LSD-like analogues
AL-LAD first emerged in Europe in 2015, and only one nonfatal LSD-like analogue intoxication was reported during 2017–2020 (138); however, comprehensive international data for this drug are not available (163). The individual acted erratically, shooting two teenagers and setting fire to a building. Police responded and apprehended the individual, who became unresponsive while in the patrol car and later had a cardiac arrest. Clinical signs and symptoms included tachycardia, hypotension, hypothermia and mydriasis.
Tryptamine-like analogs
5-MeO-MiPT and 5-MeO-DiPT share structural similarity with tryptamine but with the addition of a methoxy group on the indole and alkyl groups on the amine. 5-MeO-DiPT is a Schedule I substance in the USA, while 5-MeO-MiPT is not explicitly scheduled (147, 164). 5-MeO-DiPT intoxications are described in the literature, but details on case scenarios and clinical findings are lacking. In one case, no abnormal clinical signs or symptoms were reported (126). A naked individual was disoriented, agitated and aggressive with several abrasions after ingesting an LSD-like substance in another nonfatal intoxication (127). The biological samples and recovered drug material confirmed the presence of 5-MeO-MiPT intoxication. Positional isomers of these tryptamine-like analogues are also available; 4-MeO-MiPT was first documented in Europe in 2019 (16).
Diarylethylamine analogues
MXP is structurally related to diphenidine. Two nonfatal MXP cases since 2017 documented agitation, aggressiveness, dissociative effects and sedation in the patients (14). Clinical signs and symptoms for both included hyperthermia, tachycardia and mydriasis. In one case, serotonin syndrome was suspected, similar to findings that involved other novel hallucinogens and novel stimulants. Tachycardia, tachypnea and miosis were observed in two nonfatal and one fatal diphenidine intoxications; one individual experienced agitation, disorientation, altered consciousness and an inability to communicate (139). In the fatal case, abrasions were noted at autopsy, along with pulmonary and brain edema and organ congestion (140).
Discussion
Recently reported novel hallucinogens, documented adverse effects and toxicological confirmation of the responsible drug include PCP-like analogues (e.g., 3-MeO-PCP and 3-HO-PCP), ketamine-like analogues (e.g., 2-FDCK, 2-Oxo-PCE and MXPr), phenethylamine analogues (e.g., 25I-NBOMe, 25B-NBOMe, 25C-NBOMe, DOC, 2C-E and Bromo-DragonFLY), LSD-like analogues (e.g., AL-LAD), tryptamine-like analogues (e.g., 5-MeO-DiPT and 5-MeO-MiPT) and diarylethylamine analogs (e.g., MXP and diphenidine). Although these drugs are classified under one NPS class, adverse effects may be similar or can differ based on the specific novel hallucinogen ingested (Table VI). For example, intoxication with most substances resulted in agitation, aggressive behavior and/or delusions. 3-MeO-PCP was more commonly associated with hypothermia, whereas other novel hallucinogens produced hyperthermia. The most common physiological effect reported during hospitalization was tachycardia, a finding noted for all novel hallucinogens. Cases of excited delirium and serotonin syndrome were also reported.
Table VI.
Summary of Toxicity Profile of NPS Hallucinogen Drug Subclasses
| Organ system | PCP-like analogues | Ketamine analogues | Phenethylamine analogues |
|---|---|---|---|
| CNS | Delusions, confusion, aggression, psychomotor agitation, altered or fluctuating consciousness | Agitation, aggression, abnormal behavior, hallucination, delusions, impaired consciousness, loss of consciousness, unstable emotions, psychotic symptoms and unsteady gait | Agitation, aggression, seizures, euphoria, hallucinations and loss of consciousness |
| Cardiovascular | Tachycardia and hypertension | Tachycardia, hypertension and palpitations | Tachycardia, hypertension and cardiac arrest |
| Pulmonary | Tachypnea, respiratory acidosis | Dyspnea | Respiratory distress, heavy breathing and apnea |
| Other | Hypothermia, mydriasis, excessive sweating and vertical nystagmus | Hyperthermia, rhabdomyolysis, nystagmus, drowsiness, dizziness, convulsion, nausea, vomiting and self-harm | Hyperthermia, mydriasis, rhabdomyolysis, sweating, salivation, tremors and serotonin syndrome |
| Postmortem findings | Edema and congestion of lungs, brain and heart | Brain swelling and lung hyperhydration | Edema and congestion of lungs and brain |
There are no standard laboratory analytical procedures for the class of novel hallucinogens, and routine testing is generally lacking in the USA and internationally. This most likely results in underreporting of the prevalence for this NPS class. In a 2020 survey of laboratories performing DUID testing (n = 65), 83% test for PCP in blood (the same percentage as the 2016 survey); however, only 54% test for PCP in urine (down from 78%) (165, 166). Additionally, 69% (up from 50%) of laboratories routinely test for ketamine in blood, but only 40% tested for other ‘hallucinogens’. When asked what additional drugs should be added to the recommended scope for DUID testing, no laboratory suggested any novel hallucinogen drugs or subclasses. This could be because PCP was only the 20th most commonly encountered drug in DUID casework of survey respondents in 2020 (when ranking cumulative responses of the top 15 drugs from each respondent); the laboratories did not list any other hallucinogens, including ketamine, LSD or novel analogues. Survey results of the scope of analytical confirmation during death and DUID investigations are not currently available; however, PCP and ketamine are included in draft standards documents produced by the Academy Standards Board: ‘Standard for the Analytical Scope and Sensitivity of Forensic Toxicological Testing of Blood in Medicolegal Death Investigations’ and ‘Standard for the Analytical Scope and Sensitivity of Forensic Toxicological Testing of Blood in Impaired Driving Investigations’. As expected, no novel hallucinogens are included (167, 168). Although hallucinogens are not among the top drugs detected in DUID investigations, the above draft standards strongly suggest the need to test for novel hallucinogens in nonfatal intoxications, fatalities and DUID investigations based on case circumstances and investigative information.
With the current state of novel drug markets, laboratory personnel need to consider the challenges and limitations of testing for novel hallucinogens. Some immunoassay screening assays for traditional hallucinogens do cross-react with novel hallucinogens (e.g., specifically MeO-PCP derivatives with PCP kits) (169, 170). However, data for other subclasses regarding cross-reactivity are lacking, and laboratories should be aware that immunoassay screening will not detect most novel hallucinogens. Comprehensive toxicological screening by high-resolution mass spectrometry (HRMS) is a far superior approach for identifying novel hallucinogens when testing is requested. Novel hallucinogens and their metabolites identified in this review should be incorporated into screening applications, especially 3-MeO-PCP and 2-FDCK. Laboratories should also consider adding novel hallucinogens as they emerge. It is important for toxicologists to understand evolving NPS drug markets and to remain current with literature on novel hallucinogen intoxications and fatalities.
NPS Benzodiazepines
Introduction
The NPS market grew rapidly during 2008–2018, but new analogues in some subclasses such as SC slowed, while novel benzodiazepine analogues increased. Currently, the EMCDDA monitors 30 novel benzodiazepines, with 21 detected in 2015 or later (67, 171). In 2020, the EMCDDA reported 1.4 million tablets, 1.3 L of liquid and about 8 kg of powder of new benzodiazepine seizures. Novel benzodiazepines are typically distributed by pressing into tablet molds that appear similar to pharmaceutical alprazolam (Xanax®) or diazepam (Valium®) (172–176). This poses an additional health concern as individuals believe they are taking traditional benzodiazepines of known potency, dose and quality. Novel benzodiazepines are readily available online and marketed as ‘legal’ versions of authorized medications (67).
Pharmacology
As previously reported (14), the pharmacology of designer benzodiazepines is similar to that of traditional benzodiazepines. Benzodiazepines bind to gamma-aminobutyric acid A (GABAA) receptors that are ligand-gated chloride-selective ion channels activated by the inhibitory GABA neurotransmitter (177). Traditional benzodiazepines are classified as short- (1–12 h), intermediate- (12–40 h) or long (40–250 h)-acting drugs based on their half-lives (178). They are prescribed as anxiolytics, sedatives or anticonvulsants. Common side effects include drowsiness, lethargy, fatigue, dizziness, vertigo and loss of motor control. Long-term use of benzodiazepines can lead to tolerance, dependence and withdrawal (178). Novel benzodiazepines also are agonists at GABAA receptors, producing adverse effects such as amnesia, drowsiness and incoordination. Table VII provides a summary of the toxicological profile of novel benzodiazepines.
Table VII.
Summary of Toxicity Profile of NPS Benzodiazepines
| Organ system | Symptoms and signs |
|---|---|
| CNS | Amnesia, sedation, drowsiness, lethargy, slurred speech, incoordination, delayed comprehension and reaction time, dizziness, vertigo and coma |
| Cardiovascular | Bradycardia and hypotension |
| Pulmonary | Mild respiratory depression |
| Other | Bloodshot eyes |
Table VIII (179–187) provides, when available, detailed case histories, clinical symptoms, toxic effects, field sobriety testing, autopsy findings and biological data for cases involving NPS benzodiazepines.
Table VIII.
NPS Benzodiazepine Case Histories with Clinical Symptoms, Autopsy Findings and Primary Drug and Additional Drug Concentrations
| Drug | History | Clinical symptoms/Field Sobriety Testing/Autopsy findings | Drug results (ng/mL unless specified) | Reference |
|---|---|---|---|---|
| Flualprazolam | 16 y/o M presented to ED after ingesting counterfeit Xanax tablet | Lethargy and slurred speech | Urine: 72.1 +cannabinoids |
(179) |
| 16 y/o M presented to ED after ingesting counterfeit Xanax tablet | CNS depression, slurred speech and mild respiratory depression; unresponsive to 0.4 mg IV naloxone | Blood: 14.6 Urine: 19.4 |
(179) | |
| 16 y/o F presented to ED after ingesting counterfeit Xanax tablet | Lethargy and slurred speech | Urine: 3.0 | (179) | |
| 23 Swedish postmortem cases | N/A | Femoral blood: Mean: 17.2 ng/g Median: 17 ng/g Range: 3–45 ng/g |
(180) | |
| 10 Finnish postmortem cases | N/A | Femoral blood: Mean: 25.0 ng/g Median: 22.5 ng/g Range: 6.6–68.0 ng/g |
(180) | |
| 167 US/Canadian postmortem cases | N/A | Blood: Mean: 20 ± 63 Median: 8.2 Range: 2–620 |
(181) | |
| 22 US DUID cases* *24 y/o M in MVC with bus |
*Standard Field Sobriety Test was uncoordinated, had delayed comprehension and reaction times and lethargic behavior | Blood: Mean: 22 (± 18) Median: 14 Range: 4.4–68 *13 |
(181) | |
| 30 y/o M IV drug user with suspected OD | COD attributed to mixed drug toxicity | NaF/KOx preserved blood: 2.8 Unpreserved blood: 3.0 pregabalin 12,000, +morphine, +codeine, +6-AM, +noscapine, +papaverine, +diazepam, +mirtazapine, +THC |
(182) | |
| 44 y/o M with hx of depression and possible heroin use discovered dead at home with drug paraphernalia: packet Xanax (alprazolam) tablets, empty pill dispenser, prescription medications and empty syringes | N/A | NaF/KOx preserved blood: 30.3 Unpreserved blood: 35.1 +methadone, +MDMA, +MDA, +pregabalin, +mirtazapine, +BZE, +diazepam, +morphine, +gabapentin |
(182) | |
| 40 y/o F with hx of mamba use (presumed SCRA), found deceased on floor of home | N/A | Unpreserved blood: 14.5 +methadone, +EDDP, +pregabalin, +mirtazapine, +4F MDMB-BINACA metabolites, +MDMB-4en-PINACA and metabolites |
(182) | |
| 37 y/o M found face down in mud | N/A | NaF/KOx preserved blood: 3.5 Unpreserved blood: 14.1 +methadone, +pregabalin, +etizolam, +MMB-PINACA metabolites, +carbamazepine, +THC-COOH |
(182) | |
| 40 y/o M with hx of alprazolam use, IV drug use and anxiety and depression, found lying on river embankment with rigor mortis. Bottle of methadone on him, 30 mL bottle nystatin in river and green herbal material and white pills in wallet | N/A | Unpreserved blood: 26.5 +diazepam, +nordiazepam, +methadone, +EDDP, +pregabalin |
(182) | |
| 51 y/o M with hx of depression and heroin use found deceased in his bathroom. Hepatitis C positive and in methadone program | N/A | NaF/KOx preserved blood: 3.1 +morphine, +codeine, +methadone, +EDDP, +alprazolam, +diazepam, +nordiazepam, +temazepam, +oxazepam, +sertraline, +pregabalin, +risperidone, +BZE |
(182) | |
| 57 y/o M with hx of chronic obstructive pulmonary disease, chest infections, heart problems and crack cocaine and heroin use | N/A | NaF/KOx preserved blood: 4.7 Unpreserved blood: 5.7 +cocaine, +BZE, +citalopram |
(182) | |
| 42 y/o F with hx of heroin abuse found unresponsive at home | N/A | NaF/KOx preserved blood: 15.1 +morphine, +codeine, +pregabalin |
(182) | |
| 42 y/o M with hx of IV drug use and self-harm found unconscious on pathway, CT scan: severe hypoxic brain injury | N/A | Antemortem citrate preserved blood: 8.5 +cocaine, +BZE, +mirtazapine, +nitrazepam, +alprazolam, +diazepam, +nordiazepam, +morphine, +codeine, +clonazepam, +pregabalin, +buprenorphine Serum: 4.9 |
(182) | |
| 123 DUID investigations, Sacramento County, CA | N/A | Blood: Mean: 25 Median: 18 Range: 5–154 |
(183) | |
| Ten antemortem cases | N/A | Blood: Range: 3.3–56 Median: 8.0 |
(184) | |
| Diclazepam | 334 antemortem cases | N/A | Blood: Range: 1.6–250 Median: 9.6 |
(184) |
| Diclazepam/Flubromazepam | 28 y/o M with hx of illicit drug use found deceased at home, believed he was taking benzos (etizolam) | N/A | Blood:
diclazepam: 70
flubromazepam: 10
methylamphetamine 290, amphetamine 150, U-47700 330, +lorazepam Urine: +etizolam |
(185) |
| Etizolam | 38 y/o M driving SUV in MVC | Observed having trouble maintaining speed and failing to come to smooth stop prior to MVC, slurred speech, bloodshot eyes, slow to respond and repeated questions/comments, failed field sobriety tests | Blood: 40 (∼2 h post incident) +Amphetamine |
(186) |
| 20 y/o F in noninjury 1-car collision after driving over median. Marijuana roaches in car | Officer observations: poor balance, failed field sobriety tests | Blood: 88 (∼1.75 h post incident) THC 11 ng/mL |
(186) | |
| 35 y/o M noninjury 1-car MVC | Officer observed: slurred speech, slow to respond, balance problems, droopy, bloodshot, glazed and watery eyes | Blood: 330 (∼3.5 h post incident) +methamphetamine, +amphetamine |
(186) | |
| 51 y/o M found deceased at home after consuming alcohol night prior, hx of alcohol dependence, chronic opioid use, anxiety, depression and hypertension | Nose and mouth had dark colored emesis | Cardiac blood: 29
ethanol 0.02, +methadone, +EDDP, +oxycodone, +oxymorphone, +norfentanyl, +mirtazapine Urine: 2 Vitreous humor: + |
(187) | |
| 29 y/o M with hx of alcohol abuse, hypertension anxiety and gastroesophageal reflux, found deceased at home, last known alive 3 days prior | Cardiomegaly and moderate pulmonary congestion/edema. No pill or pill fragments found in stomach | Cardiac blood: 45
fentanyl 6, ethanol 0.23, alprazolam 228, +alpha-hydroxyalprazolam, +chlordiazepoxide, +nordiazepam, +norfentanyl Urine: 13 Vitreous humor: + |
(187) | |
| 27 y/o F with hx of mental health issues found with suicide note and other medications on scene. Last known alive 1 week prior | Pulmonary anthracosis and drug material in stomach | Peripheral blood: 237
fentanyl 21, ethanol 0.12, alprazolam 282, cocaine 302, +BZE, +cocaethylene, +diphenhydramine, +methamphetamine, +nordiazepam, +norfentanyl Cardiac blood: 813 Vitreous humor: 2,921 |
(187) | |
| 34 y/o M with hx of illicit drug use found unresponsive on street, paramedics unable to revive. Syringe, spoon, tourniquet and alcohol located near decedent | Mild macrovesicular steatosis in liver, left ventricular hypertrophy and hepatoduodenal lymph adenopathy | Peripheral blood: 9
ethanol 0.23, 6-AM 11, morphine 185, +citalopram, +codeine, +diphenhydramine, +desalkylflurazepam, +nordiazepam Cardiac blood: <5 |
(187) | |
| 36 y/o M with hx of benzodiazepine and heroin abuse found lying in front of building, EMS unable to revive, pronounced dead at scene. Drug paraphernalia found on individual included white pills, syringe plunger, tourniquet and a cooker | Pulmonary edema and cerebral edema | Peripheral blood: 10
fentanyl 31, alprazolam 27, methamphetamine 1,212, +amphetamine, +EDDP, +methadone, +norfentanyl Urine: 8 |
(187) | |
| 30 y/o M with hx of heroin abuse found by girlfriend in hotel room snoring, EMS unable to revive. Small amount of green vegetable material, lighter, needle, cooker and bundles of possible illicit substances found at scene | Pulmonary edema and congestion and cerebral edema | Peripheral blood: 25
codeine 17, morphine 219, +6-AM, +alprazolam, +THC-COOH, +THC-OH, THC, +diphenhydramine Urine: 13 |
(187) | |
| 28 y/o M found deceased in car. Attended party a day prior and left by friend in car to ‘sleep it off’. No paraphernalia at scene. Family reported seeing him with pills of unknown content | Pulmonary edema and congestion | Peripheral blood: 15
alprazolam 179, +fentanyl, +7-aminoclonazepam, +acetaminophen, +alpha-hydroxyalprazolam, +chlorpheniramine, +diazepam, +methamphetamine, +nordiazepam Cardiac blood: 20 Urine: 20 |
(187) | |
| 30 y/o M found unresponsive in his room by housemate. Small bag with white substance, glass pipe, charred aluminum foil, lighter and plastic straw found at scene | Cardiomegaly, moderate pulmonary congestion/edema, and cerebral swelling | Peripheral blood: 6
fentanyl 5, cocaine 43, methamphetamine 246, +alprazolam, +amphetamine, +BZE, +cocaethylene, +carboxy-THC, +THC, +norfentanyl Urine: <5 |
(187) | |
| 61 y/o M found unresponsive during wellness check. Syringe and other prescription medications found at scene | Cardiomegaly, moderate pulmonary congestion/edema and cerebral swelling | Cardiac blood: 22
fentanyl 13, +THC-COOH, +gabapentin, +morphine, +norfentanyl Urine: 26 Vitreous humor: <5 |
(187) | |
| 30 y/o M with hx of cannabis, benzodiazepines and alcohol use, suicide ideations. Found unresponsive during wellness check, last known alive 1 day prior. Small bag with blue substance and scale with white powdery substance found on scene | N/A | Peripheral: 187
fentanyl 17, ethanol 0.02, flubromazolam 619, +alprazolam, +amphetamine, +delorazepam, +flualprazolam, +lorazepam, +methamphetamine, +norfentanyl Cardiac blood: 214 Urine: 64 Vitreous humor: 33 |
(187) | |
| 40 antemortem cases | N/A | Blood: Range: 15–300 Median: 54 |
(184) | |
| 49 y/o M found prone in bed | Femoral blood: 770 Cardiac blood: 2,820 |
(174) | ||
| Flubromazolam | 17 y/o M stopped due to failure to maintain lane position, driving below speed limit and almost collided with officer’s vehicle. Admitted taking Xanax | Officer’s observations: sluggish, slow to respond to questions, slurred speech, lethargic, balance issues and droopy bloodshot eyes. Failed sobriety tests | Blood: 17 (∼1.5 h post incident) THC 6.1 |
(186) |
| 18 y/o M driving sedan involved in MVC | Officer’s observations: slow to respond, repeated questions/comments, balance issues, slurred speech and bloodshot eyes. field sobriety tests could not be conducted due to injury | Blood: 18 (∼1.75 h post incident) THC 2.2 |
(186) | |
| 21 y/o M reported to highway patrol for erratic driving. Individual fell asleep in chair at hospital while waiting for blood draw | Officer’s observations: failed to maintain lane position and speed, improper lane changes, difficulty with motor control and inconsistent story. Failed field sobriety tests; DRE evaluation under the influence of cannabis | Blood: 19 (∼1 h post incident) BZE 348, THC 1.5 |
(186) | |
| 17 y/o F stopped by officer for failure to maintain lane position and driving in opposing lane | Officer’s observations: faint odor of alcohol, slurred speech, slow to respond to questions, failed field sobriety tests | Blood: 14 (∼2.75 h post incident) PBT (22 min post incident) 0.014 g% |
(186) | |
| 19 y/o F in noninjury MVC when she struck a legally parked vehicle | Officer’s observations: slurred speech, slow to response to questions and balance issues | Blood: 21 (∼2.5 h post incident) BZE 749, THC 11, +cocaine |
(186) | |
| 19 y/o M stopped by officer for failure to maintain lane position, found with drug paraphernalia, lit cannabis cigarette and counterfeit Xanax tablet | Officer observations: slurred speech, dexterity issues and poor balance | Blood: 7 (∼1.75 h post incident) Clonazepam 17, 7-aminoclonazepam 26, THC 27, +oxycodone |
(186) | |
| 22 y/o F driver in 2-car MVC, drove off roadway and overcorrected striking another vehicle | Officer observations: cannabis odor | Blood: 12 (∼1.5 h post incident) THC 2.9 |
(186) | |
| 35 y/o F stopped for failure to maintain lane position, almost driving into oncoming traffic, admitted smoking cannabis ∼30 min prior and cannabis in vehicle | Officer’s observations: cannabis odor and individual impaired, failed field sobriety tests | Blood: 31 (∼1.5 h post incident) THC 4.1 |
(186) | |
| 21 y/o M in 2-car MVC stated he consumed two bars of Xanax 3.5 h prior to collision and 1 g cocaine and acid/LSD | Officer’s observations: slurred speech, slow to respond to questions and balance issues | Blood: 8.2 (∼2.25 h post incident) BZE 356, THC 1 |
(186) | |
| 20 antemortem cases | N/A | Blood: Range: 4–36 Median: 5.6 |
(184) | |
| 5 antemortem cases | N/A | Blood: Range: 7–700 Median: 37 |
(184) | |
| Clonazolam | 22 antemortem cases | N/A | Blood: Range: 1.7–53 Median: 4.1 |
(184) |
| Phenazepam | 138 antemortem cases | N/A | Blood: Range: 1.8–850 Median: 22 |
(184) |
DRE = drug recognition expert, MVC = motor vehicle collision, PBT = preliminary breath test, SCRA = synthetic cannabinoid receptor agonist, SUV = sport utility vehicle, THC-OH = hydroxy-tetrahydrocannabinol.
Flualprazolam
Flualprazolam (Figure 2) is a triazolo-benzodiazepine patented in 1976 by Upjohn (188). Flualprazolam is a fluorinated analogue of alprazolam, but there is little information in the literature regarding its specific pharmacological effects because it was never legitimately marketed. Based on its structural similarity to traditional benzodiazepines, alprazolam and triazolam, and user reports, flualprazolam most likely has similar CNS depressant effects (189, 190). Flualprazolam was reported to be in circulation by the EMCDDA and DEA in 2018; as of the third quarter in 2020, flualprazolam was the most reported novel benzodiazepine in the USA. The first cases were identified in March 2018 by retrospective data mining of two biological specimens collected in Pennsylvania and Indiana (191). The rapid increase in positive cases worldwide led the World Health Organization (WHO), DEA and NPS Discovery to release reports and drug information sheets to inform clinicians and first responders about this new drug (183, 192–194).
In June 2019, six adolescents (five males and one female; 14–16 years old) in Oregon were admitted to the ED after ingesting what they believed were Xanax or alprazolam tablets (179). All displayed lethargy and slurred speech on admission. One developed mild respiratory depression, with a respiratory rate of 10 breaths per minute. All recovered within 6 h of displaying symptoms and were discharged. Urine samples from three adolescents contained 3–72.1 ng/mL flualprazolam, and one blood sample from another adolescent contained 14.6 ng/mL flualprazolam. A piece of a counterfeit tablet was also analyzed and contained about 2.75–3 mg flualprazolam but no alprazolam or other substances.
A collaborative effort reported flualprazolam concentrations in 23 postmortem cases from Sweden and 10 from Finland (180). The deaths (27 males and 6 females; 16–70 years old) occurred during February 2018–January 2019 in Sweden and June 2018–July 2019 in Finland. The median concentration and range were 18.0 ng/g and 3.0–68 ng/g, respectively, with no significant differences between countries of origin. In two cases, fatal poisoning from flualprazolam was listed as the COD and the only substance detected in femoral blood at 19 ng/g and 21 ng/g. In the other 20 cases, the most common additional substances detected were opioids, alcohol, pregabalin and antidepressants.
A 2020 study compiled quantitative flualprazolam concentrations for 197 cases (171 medicolegal death investigations, 22 DUID cases and 4 unclassified cases) submitted to NMS Labs (Willow Grove, PA) during August 2019–February 2020 (181). Demographic data were provided for 151 (113 males and 38 females) postmortem cases with a mean ± standard deviation of 35 ± 12, median of 34 and age range of 18–69 years. The cases occurred in 28 states in the USA and a single Canadian province. The most common other drug coadministrations were opioids including methadone, buprenorphine, oxycodone/oxymorphone, heroin (6-acetylmorphine [6-AM] and morphine), fentanyl (n = 106), carfentanil, tianeptine, mitragynine and isotonitazene. In addition to flualprazolam, etizolam was detected in 12 cases. There were 22 (20 males and 2 females) DUID cases submitted from Pennsylvania, Texas, Oregon, and Mississippi with a mean age of 27 years (17–51 years old) (181). Only flualprazolam at 13 ng/mL was identified in the blood of a 24-year-old male involved in a motor vehicle crash with a bus. He was uncoordinated, showed delayed comprehension and reaction times and demonstrated lethargic behavior during field sobriety testing. The most common substance found in addition to the flualprazolam in DUID cases was THC (n = 8), followed by ethanol (n = 4).
Flualprazolam was detected in 124 cases in Sacramento County, CA, during May 2018–August 2019, more than the traditional benzodiazepine alprazolam (183). Almost all flualprazolam cases were DUID investigations (n = 123). The flualprazolam concentrations ranged from 5 to 154 ng/mL, with a mean and median concentration of 18 ng/mL and 25 ng/mL, respectively.
Flualprazolam was reported at a low prevalence rate of only nine positive cases of 2,911 reported in the UK during April–December 2019 (182). The range of flualprazolam concentrations in the postmortem cases (n = 8) was 2.8–35.1 ng/mL in femoral blood. One of the previously mentioned positive cases had an antemortem serum concentration of 4.9 ng/mL and an antemortem citrate preserved blood concentration of 8.5 ng/mL. Flualprazolam concentrations in preserved and unpreserved blood were compared in four cases with all but one case having comparable concentrations.
Multiple Designer Benzodiazepines—clonazolam, diclazepam, etizolam, flualprazolam, flubromazepam, flubromazolam and phenazepam
In 2020, 33,700 cases that occurred in Norway during June 2016–September 2019 were evaluated for the presence of seven designer benzodiazepines: clonazolam, diclazepam, etizolam, flualprazolam (added to scope in December 2018), flubromazepam, flubromazolam and phenazepam (184). A novel benzodiazepine was identified in 575 cases or 1.7%; in 554 of these cases, subjects were living and were apprehended drivers or those arrested for other drug offenses. The most common age group was 30–34 years old, with 87% males. In 25 living subjects, the only impairing substance identified was one of the seven designer benzodiazepines, enabling comparison of biological sample benzodiazepine drug concentration to clinical tests of impairment (CTI) performed by physicians around the time of the incident. In 19 of the 25 single-drug cases, the CTI results showed mild, moderate or considerable impairment; no impairment was identified in the other six cases. Amphetamine, THC, clonazepam and methamphetamine were the most frequently detected drugs in conjunction with these novel benzodiazepines.
Diclazepam and flubromazepam
In January 2016, a 28 y/o male from Australia with a history of illicit drug use was found deceased at home after indicating to a friend that he was taking a ‘benzo’ he believed to be etizolam (185). Initial drug screening revealed only methamphetamine, amphetamine and trace levels of lorazepam. After an update to the library of the screening platform and a retrospective analysis of the data file months later, it was revealed that the sample also contained U-47700, diclazepam, flubromazepam and DOC.
There were also nine flubromazolam postmortem cases from the UK during April–December 2019 (182). All cases had additional toxicological findings including pregabalin, heroin, etizolam and SC (4F MDMB-BINACA and MDMB-4en-PINACA).
Etizolam
The concomitant intake of etizolam and an opioid in 10 decedents (9 males and 1 female; 27–61 years old) during 2017–2019 was investigated (187). Etizolam was quantified, where available, in peripheral and central blood, urine, vitreous humor and gastric fluid. The mean and median ages were 35.6 and 30 years, respectively. In general, the COD in these cases was listed as drug toxicity, but etizolam was never explicitly mentioned; in fact, only one case even referenced benzodiazepines being a contributing factor to death.
A 49 y/o German male was found dead with two plastic bags filled with a large number of white tablets stamped with XANAX (174). These tablets were identified as etizolam, which was also identified in the decedent’s stomach contents.
Etizolam and flubromazolam
In Wichita, KS, 12 DUID cases involved use of a designer benzodiazepine etizolam in three cases and flubromazolam in nine (186). Apart from one case, etizolam and/or flubromazolam were the only benzodiazepines, routine or novel, detected in blood samples. The most prevalent additional toxicology finding was THC. In all cases, arresting officers’ observations mentioned slurred speech, loss of balance and slow response to questions.
Clonazolam, delorazepam, diclazepam, flualprazolam and flubromazolam
In 2019, 33 NY samples collected during 2016–2018 were reanalyzed for the presence of the following novel benzodiazepines: 3-hydroxyphenazepam, clobazam, clonazolam, delorazepam, deschloroetizolam, diclazepam, flualprazolam, flubromazepam, flubromazolam, meclonazepam, nifoxipam and pyrazolam (195). It should be noted that there is a metabolic relation between diclazepam, lorazepam and delorazepam; however, it is not always possible to determine the parent drugs. Five of the aforementioned cases were positive for an additional novel benzodiazepine. The biological specimen for Case 1 was heart blood and Cases 2–5 were femoral blood. Case 1 was originally positive for lorazepam and etizolam, and the subsequent analysis also identified delorazepam (68 ng/mL), diclazepam (<1 ng/mL) and flubromazolam (40 ng/mL). Case 2 was positive for etizolam, and reanalysis identified delorazepam at 1.1 ng/mL. Case 3 initially tested positive for diazepam, nordiazepam, etizolam, lorazepam, oxazepam and temazepam, with delorazepam later identified at 68 ng/mL. Case 4 was positive for 7-aminoclonazepam and etizolam, with 1.1 ng/mL clonazepam later added. The initial analysis of Case 5 showed alprazolam, alpha-hydroxyalprazolam and lorazepam, but expanded testing identified 5.3 ng/mL delorazepam and 1.9 ng/mL flualprazolam.
Discussion
Novel benzodiazepines are a growing class of NPS that requires monitoring in forensic casework, especially DUID cases. Due to the mechanism of action of benzodiazepines, CNS depressant effects can be severe even when ingested alone, but coma and death occur rarely. Of all flualprazolam cases, only two listed toxicity associated with the drug with the manners of death being ‘unclear’ and accident (180). The greatest concern is when individuals co-ingest these drugs with other CNS depressants, causing synergistic effects that lead to unconsciousness, coma or death. In many of the cases discussed, a novel benzodiazepine was not the only impairing substance detected; individuals may have taken novel benzodiazepines to combat the effects of opioid withdrawal or the after effects of stimulant use or to increase the effects of other illicit substances (178, 196). Prescription and novel benzodiazepines do not generally result in death when taken alone and may not be emergency scheduled, but WHO recommended in December 2019 that flualprazolam and etizolam be classified as Schedule IV (197). The NPS market may be slowing, but the diversity of novel benzodiazepines in recent years is increasing, and novel benzodiazepines should be monitored in forensic toxicology analyses.
NPS Opioids
Introduction
Novel synthetic opioids (NSO) are agonists at opioid receptors and produce analgesia, euphoria, sedation and respiratory depression. In recent years, NSO have dominated the NPS market in the USA, leading to numerous hospitalizations, human performance cases and deaths (198–201). The rise and proliferation of NSO are an extension of the opioid epidemic, largely fueled in the USA by the increased use of illicit opioids (e.g., heroin and fentanyl) in response to pharmaceutical opioids (e.g., oxycodone) prescription (202–204).
The current wave of the US opioid epidemic began around 2013, with illicit fentanyl followed by the appearance of many fentanyl analogs (e.g., acetylfentanyl, butyrylfentanyl and 2-furanylfentanyl) and other novel opioids (e.g., AH-7921, MT-45 and U-47700) (205, 206). However, in response to core-structure scheduling actions enacted in 2018 by the US DEA to curb the spread of fentanyl analogues, there has been a shift toward novel and varied chemical subclasses of drugs with opioid activity. At present, NSO are commonly represented in six primary categories: fentanyl analogues, cyclohexylbenzamides, 2-benzylbenzimidazoles, benzimidazolones, cinnamylpiperazines and atypical opioid agonists (e.g., tianeptine); however, other NSO have emerged with varying degrees of prevalence including piperidylthiambutene, 2F-viminol and diphenpipenol (207–209). NSO have dominated the NPS market since the mid- to late-2010s, so there are multiple recent scientific reviews (64, 210–221). Hence, this review includes toxicologically confirmed case reports and/or those with chemical confirmations paired with reported adverse effects not described in other reviews (Table IX [222–246]).
Table IX.
NPS Opioids Case Histories with Clinical Symptoms, Autopsy Findings and Primary Drug and Additional Drug Concentrations
| Drug | History | Clinical symptoms/Autopsy findings | Drug results (ng/mL unless specified) | Reference |
|---|---|---|---|---|
| 3-Methylfentanyl | 80 y/o M died at hospital after 3-methylfentanyl OD | N/A | Antemortem blood: 1.1 | (222) |
| 30 y/o M found supine in bed with drug paraphernalia (blue wax baggies and capped syringe) nearby. Last known alive ∼24 h prior | Severe pulmonary and cerebral edema and urine retention. No injection sites observed | Blood: (±)-cis-3-MF 1.2, (±)-trans-3-MF 0.7 Morphine (free) 8.3, fentanyl 1.8, norfentanyl 0.2 Vitreous: 6-AM (free) 1.0 |
(223) | |
| 54 y/o M with hx of depression, anxiety and alcohol abuse | N/A | Blood: (±)-cis-3-MF 0.78, (±)-trans-3-MF 0.39 Fentanyl 0.54, norfentanyl 0.63, oxymorphone (free) 1.7, diazepam 850, nordiazepam 800, oxazepam 23, temazepam 27, alprazolam 55 |
(223) | |
| 36 y/o M found dead in residence with needle in arm | Autopsy findings included pulmonary edema | Blood: (±)-cis-3-MF 0.34, (±)-trans-3-MF 0.23 ethanol 188 mg/dL, +naloxone, diazepam 22, nordiazepam 69, chlordiazepoxide 110, morphine (free) 12, THC 0.7 Urine: +6-AM |
(223) | |
| 50 y/o M with remote hx of drug and alcohol abuse gurgling in sleep, stopped breathing. EMS unable to revive | Pulmonary and cerebral edema | Blood: (±)-cis-3-MF 1.34, (±)-trans-3-MF 0.69 Oxycodone (free) 6.2, BZE 56, 7-AMC 22, lamotrigine 4.6, quetiapine 710 Urine: +6-AM |
(223) | |
| 33 y/o M with hx of seizures, bipolar disorder and heroin abuse found dead with four stamp bags in pants | N/A | Blood: (±)-cis-3-MF 1.6, (±)-trans-3-MF 0.71 | (223) | |
| 22 y/o M found in girlfriend’s apartment hallway, transported by EMS to hospital, pronounced dead. Subject recently released from prison for driving without license and drug possession. Girlfriend stated he took two clonazepam and one oxycodone that night | Pulmonary and cerebral edema and urine retention. Injection sites observed | Blood: (±)-cis-3-MF 0.84, (±)-trans-3-MF 0.37 Oxycodone 62, oxymorphone (free) 1.1, 7-AMC 120, +naloxone |
(223) | |
| 22 y/o M unresponsive after insufflating what he believed was cocaine. Unmarked blue plastic baggies found by decedent’s feet consistent with heroin | Pulmonary and cerebral edema | Blood: (±)-cis-3-MF 0.18, (±)-trans-3-MF <0.10; ethanol 30 mg/dL, BZE 1100, +naloxone, THC 1.8 | (223) | |
| 46 y/o M with hx of heroin abuse, recently released from prison, found unresponsive in brother’s basement. EMS administered three doses of epinephrine, naloxone and intubated decedent. Two empty bags heroin found at scene | Pulmonary and cerebral edema | Blood: (±)-cis-3-MF 0.51, (±)-trans-3-MF 0.26 6-AM 33, morphine (free) 40, cocaine 100, cocaethylene 32, BZE 1600, oxycodone (free) 81, oxymorphone (free) 1.2, quetiapine 230 | (223) | |
| 55 y/o M with MVC, hx of chronic pain led to heroin and prescription opioids use. Found unresponsive at another residence. EMS administered 0.4 mg naloxone and started OD protocol. Unable to revive | Mild pulmonary edema | Blood: (±)-cis-3-MF 3.28, (±)-trans-3-MF 1.9 Cocaine 140, BZE 420, amitriptyline 420, nortriptyline 890, clonazepam 8.1, 7-AMC 180, +naloxone, ethanol 13 mg/dL |
(223) | |
| 26 y/o F with hx of opioid abuse and rehab. Witnessed insufflating white substance she believed was fentanyl. Boyfriend administered naloxone and second dose administered by police | Visceral congestion and mild pulmonary edema | Blood: (±)-cis-3-MF 0.33, (±)-trans-3-MF 0.25 Citalopram/escitalopram 370 | (223) | |
| 31 y/o M with hx of opioid addiction found unresponsive in bedroom with vomit around nose and mouth. Decedent insufflated drugs. 3 orange glassine baggies and straw found on desk | Pulmonary edema | Blood: (±)-cis-3-MF 0.35, (±)-trans-3-MF 0.19 | (223) | |
| 32 y/o M with hx of heroin abuse and outpatient rehab found unresponsive in kitchen with syringe in hand and three blue wax baggies and one clear baggie in shorts. Decedent’s spouse stated he relapsed | Pulmonary edema and IV injection site in antecubital fossa | Blood: (±)-cis-3-MF 0.71, (±)-trans-3-MF 0.38 Morphine (free) 14, fentanyl 1.5, norfentanyl 0.27 Urine: +6-AM |
(223) | |
| 32 y/o F with hx of heroin abuse currently in rehab found unresponsive after recently released from jail. Decedent found with zolpidem, quetiapine and lansoprazole, with syringe in package and plastic straw. Clear plastic bag with white powder found in bra at autopsy | Pulmonary edema, moderate cerebral edema and IV injection sites observed | Blood: (±)-cis-3-MF 0.74, (±)-trans-3-MF 0.69 p-FIBF 1.4, 4-ANPP 0.53, alprazolam 14, morphine (free) 14, quetiapine 370, zolpidem 17, THC 5, fentanyl 1.2, norfentanyl 0.2 Urine: +6-AM |
(223) | |
| 28 y/o M with hx of heroin abuse and rehab found dead in bathroom. Blue wax paper substance found on toilet seat and half straw near decedent | Pulmonary edema, moderate cerebral edema and urine retention | Blood: (±)-cis-3-MF 1.4, (±)-trans-3-MF 0.77; alprazolam 16, morphine (free) 25, dextro/levomethorphan 38, fentanyl 0.7 Urine: +6-AM |
(223) | |
| 36 y/o M with hx of asthma, heroin abuse and suicidal tendencies, recently released from prison, found unresponsive | Pulmonary and cerebral edema | Blood: (±)-cis-3-MF 0.38, (±)-trans-3-MF 0.19 | (223) | |
| 22 y/o M with hx of heroin abuse and rehab found unresponsive in bedroom. Unmarked baggie and syringe and empty blue paper wrapper from jean pocket found on scene. Decedent revived with naloxone twice previously | Pulmonary edema, moderate cerebral edema and IV injection sites observed | Blood: (±)-cis-3-MF 0.79, (±)-trans-3-MF 0.54 Alprazolam 6.5, morphine (free) 10, fentanyl 0.79, THC 1.0 |
(223) | |
| 30 y/o F with hx of drug abuse recently released from prison on drug charges, found unresponsive in room littered with syringes and empty blue and clear baggies. One baggie imprinted with word ‘OWL’ | Pulmonary edema. Injection sites observed on antecubital fossa, right hand and& neck | Blood: (±)-cis-3-MF 0.35, (±)-trans-3-MF 0.19 Clonazepam 7.7, 7-AMC 100, cocaine 160, BZE 1,100, morphine (free) 20, lamotrigine 0.21, dextro/levomethorphan 44 |
(223) | |
| Unknown gender and age DUID | N/A | Blood: (±)-cis-3-MF 0.21, (±)-trans-3-MF 0.13 +Caffeine |
(223) | |
| 29 y/o M DUID | N/A | Blood: (±)-cis-3-MF 0.79, (±)-trans-3-MF 0.19 Clonazepam 48, 7-AMC 64, cocaine 60, BZE 1700, fentanyl 1.0, norfentanyl 0.53 |
(223) | |
| Carfentanil | 33 y/o M with hx of ethanol, cannabis and cocaine use found bradypneic by EMS following insufflating white powder, admitted to hospital | RR 9 breaths/min, EMS 2 mg IV naloxone, assisted ventilation. At ED, another 2 mg IV naloxone without improvement. Subject comatose with pupillary miosis fixed at 2 mm, diaphoretic and warm. HR 113 bpm, BP 165/84 mmHg. Awoke 31 h post admission and discharged 3 days after admission | Venous blood: 22.4 (at admission), 1.0 (25.5 h post admission), 0.52 (31 h post admission; interpolated) Ethanol 99 mg/dL, norcarfentanil 3.95 (at admission), 0.93 (25.5 h post admission) Urine: +THC-COOH, +cocaine |
(224) |
| 41 y/o M insufflated white powder purported to be cocaine at private party. Comatose within minutes | Patient presented with myosis and bradypnea and subsequently hospitalized in ICU. Patient recovered and returned home after few days | Plasma: <0.01 Norcarfentanil <0.05 Urine (+1 h): 2.88 Norcarfentanil 8.8, cocaine 570, BZE 1,230, EME 367 |
(225) | |
| 48 y/o F with hx of depression and drug use, found unconscious at home after use of fentanyl purchased over internet. Empty boxes pregabalin and buprenorphine on scene. EMS medical care included sufentanil delivery. Admitted to hospital comatose | Patient miotic and naloxone administered without success. Hospitalized, intubated, ventilated and treated with lidocaine, etomidate and propofol. Recovery completed after 8 days in ICU and 12 days in internal medicine ward | Plasma: 32.4 Norcarfentanil 15.8, <0.04 fentanyl, sufentanil <0.05, buprenorphine 0.48, norbuprenorphine <0.5 Urine: 1,430 Norcarfentanil 1,820, fentanyl 1.7, sufentanil <0.05, buprenorphine 6.0, norbuprenorphine 19.4 |
(225) | |
| 41 y/o M with hx of opioids and benzodiazepines use found unconscious by family. When conscious, subject stated he took 0.025 mg (nasally) and 0.025 mg (IV) carfentanil with 0.5 g heroin 5 h prior | EMS found in respiratory depression (2 breaths/min), GCS 3. EMS administered three doses 0.4 mg naloxone. At ED subject lost consciousness again, given two more 0.4 mg naloxone and admitted to ICU with naloxone infusion | Urine: 457 +Norcarfentanil, +morphine-3-glucuronide, +codeine-6-glucuronide, +buprenorphine, +methadone, +benzodiazepines, +ethyl glucuronide, +ethyl sulfate |
(226) | |
| 16 y/o M found unconscious after taking unknown substance. Intubated, airlifted to hospital, regained consciousness after IV naloxone and flumazenil. White powder discovered in patient’s belongings was carfentanil. Patient was also prescribed atomoxetine | Patient hypotensive (71/58 mmHg), tachycardic (128 bpm), hypopneic and cyanotic (pO2 saturation 70%). Temp normal and normal pupils responded to light | Serum (1 h after use): 0.6 Norcarfentanil 0.2 Urine: 1.3 Norcarfentanil 0.5 |
(227) | |
| 31 y/o M with hx of heroin use. Last seen asking for money to buy drugs. Found deceased with drug paraphernalia, white powder and rock-like substance | Pulmonary edema, urinary retention, acute needle puncture marks, no significant natural disease. COD ruled carfentanil and ethanol toxicity | Blood: 0.13 Ethanol 226 mg/dL |
(228) | |
| 33 y/o F with hx of heroin use, last seen highly intoxicated while mixing heroin and fentanyl. Found deceased on mattress on her floor with drug paraphernalia nearby | Pulmonary edema and airway foam, urinary retention. No significant natural disease. COD ruled acute carfentanil and ethanol toxicity | Blood: 0.64 Ethanol 177 mg/dL, quinine 430 |
(228) | |
| 26 y/o F with hx of drug use found unresponsive after reportedly insufflating heroin. Drug paraphernalia on scene | Foam in airway, no significant natural disease. COD ruled carfentanil, methamphetamine, alprazolam and diazepam toxicity | Blood: 0.12 Methamphetamine 90, alprazolam 14, diazepam 57, nordiazepam 100 |
(228) | |
| 43 y/o F with hx of recent drug OD, found deceased with tourniquet on arm, drug paraphernalia and white powder present | Pulmonary edema, obesity, focally severe atherosclerosis of left circumflex artery. COD carfentanil, methamphetamine, alprazolam and opiate | Blood: 0.27 Morphine 30, methamphetamine 20, alprazolam 15 |
(228) | |
| 34 y/o F with hx of drug use, including heroin, found deceased in shed with syringes nearby | Drug bindle in axilla, track marks, pulmonary edema with foam, no significant natural disease. COD mixed drug toxicity (carfentanil, opiate and methamphetamine) | Blood: 0.44 Morphine 60, methamphetamine 180, BZE 150, clonazepam 2.7, 7-AMC 160 |
(228) | |
| 23 y/o M with hx of heroin use, found deceased slumped in bathroom with syringe in hand. Syringe analysis indicated heroin, fentanyl and methamphetamine | Pulmonary edema, acute needle puncture mark. COD ruled acute carfentanil toxicity | Blood: 0.14 Urine: 6-AM 6 |
(228) | |
| 43 y/o M with hx of opiate dependence from chronic pain, found deceased with syringe in antecubital fossa. Family denied hx of drug abuse; syringes reportedly for testosterone | Acute and chronic needle punctures; pulmonary edema with microscopy consistent with IV drug use; cardiomegaly and left ventricular hypertrophy. COD mixed carfentanil and mitragynine toxicity | Blood: 0.26 Alprazolam 27, mitragynine 160, citalopram 400 |
(228) | |
| 39 y/o M with hx of heroin dependence on methadone, found in alley and initially responded to naloxone. Pronounced dead at hospital | Pulmonary edema, anoxic brain injury. COD anoxic brain injury after resuscitated cardiopulmonary arrest due to carfentanil and cocaine toxicity | Blood: 0.19 Cocaine 30, BZE 580 |
(228) | |
| 23 y/o M with hx of heroin use, reportedly insufflated cocaine, found slumped in bathroom with spoon and a syringe in pants | Acute needle puncture, no significant natural disease. COD acute carfentanil toxicity | Blood: 0.16 | (228) | |
| 32 y/o M with hx of heroin dependence, found dead prone with drug paraphernalia present | Pulmonary edema, no significant natural disease. COD mixed carfentanil, fentanyl and methamphetamine toxicity | Blood: 0.12 Methamphetamine 410, amphetamine 80, 7-AMC 13 Liver: fentanyl 7.4 mcg/kg Urine: 6-AM 7 |
(228) | |
| 63 y/o M with hx of heroin use, reportedly insufflating heroin with someone else and both found unresponsive. Other person revived with naloxone | Pulmonary edema with mucus plugging. COD carfentanil and trazodone toxicity | Serum: 0.10 BZE 30, trazodone 0.21 mcg/mL |
(228) | |
| Cyclopropylfentanyl | 39 y/o M with hx of chronic pain treated with insufflated ground oxycodone. Discovered on couch in crouched position with small plastic bag with an unknown white powder | Autopsy findings included weak brain edema and blood congested lungs. Frothy fluid found in larynx and respiratory tract | Heart blood (at autopsy): 52.4 Femoral blood (collected 11 h post death): 15.7 Femoral blood (collected 29 h post death at autopsy): 19.8 Ethanol 127 mg/dL, codeine 480, diphenhydramine 1000, morphine 14, oxycodone 6, zolmitriptan 4.5 |
(229) |
| 27 y/o M with hx of recreational drug use found deceased at home with 18 rectangular white pills marked ‘XANAX’ but identified as cyclopropylfentanyl | COD mixed drug intoxication | Blood: 29 Pregabalin 10 mcg/mL, morphine 49, amphetamine 365, MDMA 460, MDA 48, BZE 5.2, 7-AMC 250, buprenorphine 0.89, norbuprenorphine 6.2, THC 4.7 Urine: 610 |
(230) | |
| 25 y/o M developed nausea, profuse sweating and dyspnea 10 min after using a ‘fentanyl research chemical’ nasal spray from a local drug trafficker. He also used cannabis the same day. Symptoms progressed to coma, respiratory depression and admitted to hospital | Coma, miosis, RR 14/min. BP 127/70 mmHg and HR 65 bpm. Given 0.8 mg naloxone and breathing stabilized. Patient had decreased pO2 for 12 h, discharged after 1 day | Urine: 7 Cyclopropylnorfentanyl 220, THC-COOH 9.2, BZE 23, EME 9.2 |
(231) | |
| 25 y/o M with hx of oxycodone drug abuse after sports injury 5 years prior, found unresponsive in bed at home | Dry white foam purge on decedent’s mouth. COD alcohol, cocaine, oxycodone and cyclopropylfentanyl | Heart blood: 14 Oxycodone (free) 60, oxymorphone (free) 30, BZE 100 ng/mL, cocaethylene 70, ethanol 26 mg/dL |
(232) | |
| 24 y/o M found dead at home | Lung congestion with fluid and dark blood. Suspected COD polydrug intoxication | Blood: 20.4 +Heroin, +cocaine, +amphetamine (other stimulants), +therapeutic drugs |
(233) | |
| Furanylfentanyl | 53 y/o M with hx of heroin and other drugs use and depression found unresponsive at home, sitting on chair with head and chest collapsed on desk and butterfly needle inserted into left-hand vein. EMS noted rigor mortis and decedent was declared dead. White powder on scene was furanylfentanyl | Multivisceral congestion, pulmonary and cerebral edema, with no evidence of disease or physical injury. Petechia on lower limb surface. Recent needle punctures on back of hands and track marks on forearms | Femoral blood: 2.7 Cardiac blood: 11.8 Urine: 71.3 Bile: 7.7 Cerebrospinal fluid: 2.6 Gastric content: 40.1 ng/g |
(234) |
| 44 y/o M with hx of IV drug use found unresponsive at home. Attempted revival unsuccessful. Drug paraphernalia on scene, plastic bag containing white powder and two small plastic tubes containing colored liquid. White powder was furanylfentanyl and other crystalline powders contained MMMP. Two liquids for vaping contained glycerin, nicotine and furanylfentanyl | Coarse white froth in distal trachea and main bronchi and pulmonary edema and congestion | Blood: 1.6 MMMP 6.7, THC 10, THC-COOH 23, mirtazapine 200, paliperidone 30 quetiapine 200 |
(235) | |
| 19 y/o F insufflated a fentanyl powder called ‘China White’ and developed apnea. Found unresponsive, normotensive and bradypneic. Treated with supplemental oxygen and 3 mg IV naloxone, some mental status improvement. Powder identified as furanylfentanyl | In ED, lethargic, minimally responsive, cyanotic and coughing, acute respiratory distress and aspiration. Hypertensive 149/112 mmHg, tachycardic 143 bpm and hypoxic 80% pulse oximeter. Pupils 2 mm and reactive to light. Normal muscle tone, but acute lung injury due to aspiration. Extubated after 30 h and symptoms resolved | Serum: 3.6 Urine: 17.6 |
(236) | |
| 46 y/o F with hx of alcoholism, found dead in unsanitary apartment. Decomposition noted | No traumatic or preexisting lesions considered as COD | Cardiac blood: 14 U-47700 54, 4-ANPP 33, methadone 154, EDDP 27, methamphetamine 664, amphetamine 103, cocaine 12, BZE 840 |
(237) | |
| 30 y/o M with hx of drug and alcohol addiction found in father’s kitchen in cardiorespiratory arrest. Unable to revive. Recently released from jail and oxycodone dependent. Decedent had prescription for pregabalin, methylphenidate and clonazepam | Coronary artery presented moderate to severe atherosclerosis | Femoral blood: 0.89 U-47700 26, 4-ANPP 18, clonazepam 94, methylphenidate 15, pregabalin 5,571, THC 18, THC-COOH 32, THC-OH 3.3, naproxen 2,118, nortriptyline 14, diazepam 11 Cardiac blood: 2.4 U-47700 45 |
(237) | |
| beta-Hydroxyfentanyl | 22 y/o touched tongue to powdered ‘fentanyl’ to treat migraine headache, unresponsive and apneic. EMS gave 2 mg IM naloxone on arrival. Powder chemically verified as beta-hydroxyfentanyl | At ED, patient conscious, alert with tachycardia (141 bpm), hypertension (149/95 mmHg) and pO2 saturation 100%. Observed for 4 h | Serum: 6.5 +Alprazolam |
(236) |
| p-FIBF | 35 y/o M found dead in bathroom. Glass pipe with brown color contamination found at scene | COD circulatory failure associated with NPS | Blood: 74 Despropionyl p-fluorofentanyl 6.5, alpha-PiHP 5.6, THC-COOH 16 |
(238) |
| 29 y/o M with hx of drug use and rehab found dead with syringe in hand and empty bottles of steroids nearby. Decomposition noted | No traumatic or preexisting lesions noted during autopsy | Femoral blood: 27 Methoxyacetylfentanyl 14, cyclopropylfentanyl 0.10, 4-ANPP 9.7, alprazolam 12, quetiapine 58, gabapentin 15, +duloxetine, +U-47700, +acetylfentanyl, +despropionyl fluorofentanyl Cardiac blood: 31 Methoxyacetylfentanyl 70, cyclopropylfentanyl 0.15, 4-ANPP 3.1, +U-47700, +acetylfentanyl, +despropionyl fluorofentanyl, +N-methyl U-47931E |
(237) | |
| U-48800 | 41 y/o M with hx of cocaine and heroin use collapsed in hotel room bathroom. Decedent vomited all day and injected four bags of heroin. Stamp bags labeled ‘Stranger Danger’ with blue skull wearing hat and needle recovered at scene | Peripheral blood: 0.27 4-ANPP 0.4, cocaine 95, BZE 120, fentanyl 7.2, norfentanyl 11.3 |
(239) | |
| 46 y/o F found in supine position on bedroom floor with livor mortis. 13 stamped bags of ‘Stranger Danger’ with blue skull wearing hat recovered at scene | Peripheral blood: 5.3 4-ANPP 7, p-FIBF 0.13, cocaine 530, BZE 960, morphine 12, fentanyl 58, norfentanyl 8.3, acetylfentanyl 0.93 |
(239) | ||
| 58 y/o M with hx of IV heroin for past 5 days collapsed in front of mother. EMS transferred decedent to hospital, resuscitation efforts were unsuccessful | Peripheral blood: 2.8 Ethanol 203 mg/dL, 7-AMC 9, codeine 14, morphine 210, 6-AM 12, THC 0.62, +naloxone, +caffeine |
(239) | ||
| 39 y/o F with hx of drug abuse recently released from prison for drug charge. Text messages about illicit substances to ‘celebrate’. Drug paraphernalia found on scene included clean baggies with brown-white powder, spoon and loaded syringe | Peripheral blood: 6.2 4-ANPP 0.66, cocaine 140, BZE 3,300, morphine (free) 24, hydroxyzine 24, fentanyl 9.7, norfentanyl 1.7, acetylfentanyl 0.64, +caffeine, +cotinine, +levamisole |
(239) | ||
| 42 y/o M with hx of drug abuse last seen alive with withdrawal symptoms, including seizures and vomiting | Needle marks in antecubital fosse. Hypertensive cardiovascular disease | Peripheral blood: 1.9 Amphetamine 33, methamphetamine 61, +naloxone |
(239) | |
| 45 y/o M suspected OD | N/A | Femoral blood: 0.8 Cyclopropylfentanyl 2.5, ethanol 144 mg/dL, +cotinine |
(239) | |
| 33 y/o F with hx of heroin abuse, suspected OD | N/A | Iliac blood: 1.0 Cocaine 69, BZE 530, morphine 14, THC 7.3, dextromethorphan 26, fentanyl 7.5, norfentanyl 2.1, acetylfentanyl 0.18, 4-ANPP 0.13, U-47700 1.8, morphine 10, 6-AM 1.1, +caffeine, +levamisole, +naloxone |
(239) | |
| 36 y/o F unknown circumstances | N/A | Cardiac blood: 1.8 Methoxyacetylfentanyl 32, +4-ANPP, +caffeine |
(239) | |
| Isotonitazene | 27 y/o F with hx of heroin use found deceased in car with head in a bucket of water | N/A | Blood: 1.0 Fentanyl 5.7, norfentanyl 2.4, 4-ANPP 1.4, etizolam 6.2, diazepam 120, nordiazepam 210, oxazepam 22, THC 1.3, THC-COOH 64, +caffeine, +cotinine, +naloxone, +diphenhydramine, +quinine |
(9) |
| 27 y/o M with hx of heroin use found unresponsive at home with needles and substance believed to be heroin | Multiple track marks noted on body | Cardiac blood: 1.9 Etizolam 15, +caffeine, +acetaminophen, +diphenhydramine Vitreous: 0.1 Urine: 2.6 |
(9) | |
| 66 y/o M with hx of heroin use, hypertension and seizures found deceased in bed by family | Heart weighed 483 g with signs of stenosis. Right lung 832 g, left lung 667 g. Pulmonary edema, hypertensive, cardiovascular disease | Peripheral blood: 1.5 Fentanyl 2.9, norfentanyl 1.0, etizolam 13, levetiracetam 14 µg/mL, diphenhydramine 200, +cotinine, +quinine Urine: +morphine |
(9) | |
| 41 y/o F with hx of heroin use found unresponsive. 1.5 days prior visited ED at hospital for suspected drug OD and 5 days prior released from drug rehabilitation program. White powder found in pocket | N/A | Subclavian blood: 0.9 Fentanyl 5.8, norfentanyl 0.61, acetylfentanyl 0.69, 4-ANPP 1.6, morphine (free) 12, cocaine 89, BZE 800, +naloxone, +tramadol, +O-desmethyltramadol, +acetaminophen, +cotinine, +caffeine, +levamisole, +quinine Urine: 3.5 +Xylazine |
(9) | |
| 53 y/o M found unresponsive on back on floor of friend’s apartment. Showed friend white powdery substance night before | Pulmonary and cerebral edema, moderate atherosclerosis of left descending artery | Peripheral blood: 2.7 +4-ANPP, BZE 370, U-47700 0.34, +phenacetin, +levamisole, +diphenhydramine Urine: 4.0 |
(9) | |
| 27 y/o M found lifeless in cemetery | Linear puncture marks on right and left thighs. Pulmonary edema; right lung weighed 976 g and left 992 g. Kidneys red-brown and slightly granular | Peripheral blood: 1.8 Etizolam 30, THC-COOH 7.7, THC 1.2, diphenhydramine 190, +caffeine, +cotinine, +quinine +piperidylthiambutene Urine: 2.8 +piperidylthiambutene |
(9) | |
| 56 y/o F with hx of opiate use disorder, pain symptoms and previous suicide attempt by drug OD, found deceased slumped over toilet. Known suboxone user | Mild cardiomegaly (470 g) with focal histologic changes from hypertension. Pulmonary edema: right lung 930 g and left 800 g. Vascular congestion and kidney glomerulosclerosis and arteriolosclerosis | Iliac blood: 0.4 Flualprazolam 4.0, +naloxone, +hydroxyzine, +etizolam, +cotinine, +caffeine, +O-desmethylvenlafaxine, venlafaxine, norfluoxetine, +fluoxetine, +quetiapine |
(9) | |
| 41 y/o M with hx of heroin use and prior OD found deceased by hotel staff. White powdery substance found on scene | Red purge extruding from oral and nasal cavities. No foam cone noted. Distended bladder and organs congested | Subclavian blood: 1.7 Flualprazolam 6.4, +caffeine, +cotinine |
(9) | |
| Hx of depression and drug abuse, including fentanyl. White powder at scene chemically verified as isotonitazene | Blood organ congestion, gastric material in upper and lower respiratory tract, heavy lungs (lung weights 840 and 640 g) | Femoral blood: 2.28 Diazepam 29, nordiazepam 71, oxazepam 4.8, mefenamic acid <5.0 µg/mL, domperidone 6.0, acetaminophen 4.8 µg/mL Cardiac blood: 1.70 Urine: 1.88 Vitreous humor: 0.36 Liver: <0.05 ng/g Heart: 7.74 ng/g Brain: 18.6 ng/g |
(240) | |
| M with hx of isotonitazene use, including hospitalization for isotonitazene detoxication 2 months prior to death. White powder and pipe discovered at scene. Night of death decedent took lorazepam too | Blood organ congestion, gastric material in upper and lower respiratory tract, heavy lungs 1,260 and 900 g | Femoral blood: 0.59 Lorazepam 12, THC 56, THC-OH 1.8, THC-COOH 6.5, CBN 2.9 Cardiac blood: 1.13 Urine: 3.37 Vitreous humor: 0.12 Liver: <0.05 ng/g Heart: 2.17 ng/g Brain: 2.72 ng/g |
(240) | |
| Hx of depression | Blood organ congestion, gastric material in upper and lower respiratory tract, heavy lungs 610 and 580 g | Femoral blood: 0.74 Ethanol 57 mg/dL Cardiac blood: 0.70 Urine: 0.19 Vitreous humor: 0.65 Liver: <0.05 ng/g Brain: 4.45 ng/g |
(240) | |
| Brorphine | 24 y/o M with hx of opioid use, withdrawal symptoms in ED. Generalized pain in chest, abdomen and muscles, weakness, cramping, confusion and bradyphrenia. 1–2 months prior, started brorphine orally 4×/day and etizolam and prescribed sertraline and mirtazapine | Normal BP with tachycardia 114 bpm, O2 saturation 98% and Temp 37.3°C. Clammy and sweaty. Treatment with trazodone and nordiazepam | Serum: 69.4 +sertraline, +mirtazapine, +risperidone Serum (+60 h): 7.9 +trazodone, +nordiazepam |
(241) |
| 53 y/o M with hx of opioid use, suspected drug OD. ‘Heroin’ recovered at scene | N/A | Femoral blood: 10 Flualprazolam 50, +4-ANPP, +caffeine, +cotinine, +quinine, codeine 6.6, morphine 66, 6-AM 1.5, citalopram/escitalopram 76, fentanyl 3.4, norfentanyl, +isotonitazene Urine: 23 |
(10) | |
| 60 y/o M with unknown hx | N/A | Femoral blood: 0.9 +4-ANPP, +caffeine, methadone 160, EDDP 45, morphine 42, bupropion 18, hydroxybupropion 380, duloxetine 520, lamotrigine 6.8 µg/mL, gabapentin 15 µg/mL, fentanyl 14, norfentanyl 11, +flualprazolam Urine: 0.4 |
(10) | |
| 45 y/o M suspected drug OD | N/A | Femoral blood: 1.0 Flualprazolam 2.5, +4-ANPP, +caffeine, +cotinine, tramadol 33, THC 0.62, fentanyl 5.0 Urine: 1.9 |
(10) | |
| 57 y/o F suspected drug OD with drug paraphernalia at scene | N/A | Peripheral blood: + +4-ANPP, +caffeine, +cotinine, +naloxone, cocaine 110, BZE 1,300, THC 0.88, diphenhydramine 61, fentanyl 31, norfentanyl 5.5, acetyl fentanyl 0.13 |
(10) | |
| 42 y/o M suspected drug OD | N/A | Peripheral blood: 1.1 +4-ANPP, +caffeine, +cotinine, +naloxone, diphenhydramine 620, fentanyl 36, norfentanyl 1.4, morphine 110, 6-AM 7.3, +flualprazolam Urine: 3.3 |
(10) | |
| 60 y/o M suspected drug OD | N/A | Peripheral blood: 8.1 +Cotinine, sertraline 26, desmethylsertraline 110, verapamil 42, diphenhydramine 960, fentanyl 3.1, morphine 79, 6-AM 2.5, +flualprazolam Urine: 21 |
(10) | |
| 47 y/o M experienced sudden suspected drug OD death. Counterfeit pills identified as brorphine | N/A | Femoral blood: 2.5 +4-ANPP, +naloxone, oxycodone 22, sildenafil 35, n-desmethylsildenafil 10, fentanyl 16, norfentanyl 1.1 |
(10) | |
| 39 y/o M suspected drug OD | N/A | Peripheral blood: 6.7 +4-ANPP, +caffeine, +cotinine, +nicotine, alprazolam 14, tramadol 70, gabapentin 10 µg/mL, diphenhydramine 1,200, fentanyl 45, norfentanyl 2.1, codeine 6.5, morphine 290, hydromorphone 4.7, +flualprazolam Urine: 7.3 |
(10) | |
| 37 y/o F suspected drug OD | N/A | Peripheral blood: 0.7 Ethanol 138 mg/dL, +4-ANPP, +cotinine, +naloxone, THC-COOH 85, THC 18, diphenhydramine 110, fentanyl 22, +flualprazolam |
(10) | |
| 48 y/o M suspected drug OD | Puncture marks | Iliac blood: 0.1 Flualprazolam 5.4, +4-ANPP, +caffeine, +naloxone, morphine 8.0, diphenhydramine 190, fentanyl 4.7, norfentanyl 1.6, acetyl fentanyl 1.2, +clonazolam Hospital blood: 0.6 Urine: 0.2 |
(10) | |
| 47 y/o F suspected heroin OD | N/A | Femoral blood: 6.7 Flualprazolam 13, +4-ANPP, +cotinine, +naloxone, codeine 7.0, morphine 85, 6-AM 12, xylazine 170, amphetamine 55, methamphetamine 580, fentanyl 190, norfentanyl 5.4, acetyl fentanyl 0.15 Urine: 2.1 |
(10) | |
| 30 y/o F suspected drug OD. Died at hospital | N/A | Hospital blood: 0.3 +Caffeine, +naloxone, midazolam 20, amphetamine 110, methamphetamine 1900, MDA 9.8, MDMA 75, fentanyl 0.37, norfentanyl 0.97 Urine: 1.4 |
(10) | |
| 53 y/o M suspected drug OD | N/A | Hospital blood: Negative Flualprazolam 20, +4-ANPP, +caffeine, +naloxone, morphine 15, xylazine 30, fentanyl 19, norfentanyl 4.2 |
(10) | |
| 54 y/o F suspected drug OD | Pulmonary and cerebral edema noted at autopsy | Iliac blood: 0.1 EtOH 19 mg/dL, codeine 21, morphine 290, 6-AM 34, lamotrigine 0.60 µg/mL, topiramate 9,400, cyclobenzaprine 38, amphetamine 140, methamphetamine 730, fentanyl 17 |
(10) | |
| M unknown age suspected drug OD | N/A | Peripheral blood: 0.7 Ethanol 100 mg/dL, +4-ANPP, +caffeine, +cotinine, +naloxone, +nicotine, nordiazepam 130, chlordiazepoxide 66, lorazepam 9.4, THC 0.70, diphenhydramine 53, fentanyl 32, +flualprazolam |
(10) | |
| 51 y/o M suspected drug OD | N/A | Peripheral blood: 1.1 Ethanol 60 mg/dL, +4-ANPP, +caffeine, +cotinine, +naloxone, +nicotine, cocaine 71, BZE 1,600, diphenhydramine 98, fentanyl 9.3, norfentanyl 5.6, morphine 19, +flualprazolam Urine: 0.4 |
(10) | |
| 49 y/o F suspected drug OD | N/A | Peripheral blood: 3.8 +4-ANPP, +caffeine, +naloxone, diphenhydramine 260, fentanyl 21, norfentanyl 12, acetyl fentanyl 2.0, morphine 70, +flualprazolam Urine: 1.8 |
(10) | |
| 29 y/o M with hx of drug use, suspected drug OD. Illicit drugs found at scene | N/A | Peripheral blood: 1.1 Flualprazolam 3.6, +4-ANPP, +caffeine, +cotinine, +naloxone, +nicotine, +quinine, acetaminophen 16 µg/mL, 7-AMC 5.2, tramadol 70, diphenhydramine 490, amphetamine 10, methamphetamine 42, fentanyl 37, norfentanyl 1.3 Urine: 0.8 |
(10) | |
| 61 y/o F suspected drug OD | N/A | Peripheral blood: 0.4 Ethanol 57 mg/dL, +4-ANPP, +cotinine, +naloxone, +nicotine, alprazolam 65, BZE 330, morphine 33, 6-AM 2.3, gabapentin 9.9, fentanyl 21, norfentanyl 1.9 Urine: 0.2 |
(10) | |
| 61 y/o F with hx of obesity, tobacco use, hypertension, chronic obstructive pulmonary disease, schizophrenia, hyperlipidemia, found deceased at home following OD. Last seen alive 5 days prior, advanced decomposition | COD toxic effects of multiple substances, including brorphine and fentanyl | Cardiac blood: 2.0 Fentanyl 0.32, ethanol 27 mg/dL, chlorpromazine 82, gabapentin 6.8 mcg/mL |
(242) | |
| Tianeptine | 24 y/o M with 2-year hx of tianeptine and phenibut misuse, lethargic, slurred speech and found unresponsive. Transported to ED where he died 19 days after initial submission | Patient comatose with intact brainstem reflexes, intubated due to low respiratory rate. 2 days after admission, brain MRI indicated diffuse white matter damage characteristic of toxic leukoencephalopathy | Serum: 3,000 +Clonazepam, +7-AMC, +midazolam, +alpha-hydroxymidazolam, +THC, +THC-OH, +methylphenidate |
(243) |
| 36 y/o M IV tianeptine powder to ‘help him see into the future’. Became unresponsive, EMS called, naloxone administered and patient discharged 13 h after admission | Upon arrival at ED, excessive pupil constriction, sedation and RR 6/min noted | Urine: 2 Serum: Ethanol 133 mg/dL |
(244) | |
| 51 y/o M found deceased in hotel room with syringes beside bed | Cerebral and pulmonary edema, tracheal lumen edema, unilateral inguinal lesion to facilitate IV drug injection. Granulomatous inflammation foci and focal necrotic foci in lungs. Chronic liver inflammation and mild perivascular fibrosis of heart | Femoral blood: 4,401 Citalopram 288, +7-AMC |
(245) | |
| 38 y/o M with hx of drug abuse suddenly fainted and died in kitchen of his workplace. Syringes discovered in refrigerator. Tianeptine tablets located in pocket | Cerebral and pulmonary edema, tracheal lumen edema. Bilateral inguinal lesions for IV drug injections. Diffuse granulomatous inflammation loci as foreign body in lungs and chronic liver inflammation | Femoral blood: 538 +Nordiazepam |
(245) | |
| 28 y/o M with hx of alcohol, tobacco and illicit drug use, depression and anxiety, found lying on floor of home. Capsules and powders on scene, foil pouch labeled ‘Powder City Tianeptine 12.5 mg’ | Pulmonary edema, urinary retention, modest cardiomegaly and evidence of remote surgical closure of ductus arteriosus | Postmortem blood: 2,000 + 8-aminoclonazolam (presumptive) |
(246) | |
| 30 y/o M with hx of remote head injury found deceased in residence. Prescribed alprazolam for adjustment disorder, generalized anxiety disorder and paranoia. Drug paraphernalia at scene, included tianeptine powder ordered from internet | Recent puncture mark on right antecubital fossa. Hypertensive cardiovascular disease, pulmonary edema and pulmonary congestion | Postmortem blood: 8,400 Ethanol 12 mg/dL, alprazolam 33 |
(246) |
3-MF = 3-methyl fentanyl, 4-ANPP = N-phenethyl-4-piperidinone, 7-AMC = 7-aminoclonazolam, CBN = cannabinol, IM, intramuscular, MMMP = 2-methyl-4ʹ(methylthio)-2-morpholinopropiophenone, MRI = magnetic resonance imaging, THC-OH = 11-Hydroxy-delta-9-tetrahydrocannabinol.
NSO can be sold on the internet or the recreational drug market as powders, tablets or nasal sprays. These dosage formulations frequently contain multiple drugs (opioids and other drug classes), including new substances that can appear and disappear over a few months. NSO are also frequently found in preparations of other major drug classes, appearing in counterfeit pharmaceuticals, heroin and fentanyl packaged for street sale (215, 247–252). Further complicating the illicit opioid market is the presence of residual unreacted NSO precursor materials from illicit fentanyl manufacturing (205, 253, 254). Many recreational opioid users are unknowingly exposed to NSO, leading to an increased risk of adverse events including overdoses and deaths contributing to the public health crisis (255–258).
Pharmacology
Biomedical literature and pharmaceutical patents that result from research and transform into potential therapeutic analgesics have proven to be a source for pirating and repurposing research chemicals, especially opioids. The NSO subclass includes fentanyl analogs and chemically distinct nonfentanyl drugs, based on diverse drug scaffolds. These drugs may often retain µ-opioid receptor (MOR) agonism, conferring similar opioid effects. MOR activation results in a typical constellation of effects including sedation, analgesia, miosis, cough suppression, altered mental status, euphoria, decreased gastrointestinal motility and respiratory depression; the latter is the most significant concern for lethality in overdoses (259). Naloxone, a competitive MOR antagonist, successfully reverses overdose-related respiratory depressant effects for many NSO, but this has not been studied universally or systematically (260). Enhanced potency and/or prolonged duration of action may require multiple and/or higher naloxone doses and respiratory support over a sustained period to effect opioid symptom reversal (261).
Pharmacological studies can assist in determining an NSO’s potency, the potential public health threat it presents and the need for control and/or scheduling. In vitro studies can quantify the potency and efficacy of NSO in cells expressing opioid receptors, but in vivo studies are needed to investigate the analgesic and reinforcing effects of NSO in animal models (262). Potency and efficacy in animal models however do not necessarily reflect true potency or toxicity in humans. Caution must be used in estimating abuse potential or lethality in humans based on experimental values alone. More accurate estimates may be obtained by examining NSO concentrations in drug fatalities, especially when NSO are the only drugs present. However, practitioners should exercise caution when interpreting drug concentrations alone due to the unknowns surrounding tolerance. NSO analgesic potencies compared to fentanyl and heroin can vary widely, but in vitro binding affinity does not necessarily predict in vivo potency (259). Two of the most potent and dangerous fentanyl analogues, carfentanil and cis-3-methylfentanyl, have estimated relative analgesic potencies 10,000 and 6,000 times higher than morphine, respectively (31, 64). However, this does not mean that these two analogues are toxic by the same factors. For prior NSO, 2-furanylfentanyl has a higher affinity than fentanyl at the MOR, and acrylfentanyl, isobutyrylfentanyl and cyclopropylfentanyl have similar affinities (79). Structural similarity to a known opioid does not necessarily mean the NSO has sufficient pharmacological activity. For example, two supposed NSO, W-18 and benzylfentanyl, reached the illicit market in 2018, but they lacked significant pharmacological activity and did not gain any traction in the USA (263, 264).
Identifying the expanding number of NSO is challenging for many reasons including variability in location, dates of appearance, national and international proliferation and longevity among recreational markets. Some NSO, such as 2-furanylfentanyl, carfentanil and U-47700, are widely reported in a number of US states and other countries, but NSO are concentrated in one geographical area; for example, 3-methylfentanyl was popular only in PA and surrounding states (223, 265, 266). The popularity and life cycle of a drug on the recreational market are generally influenced by a number of factors; these factors include availability, distribution, potency, toxicity and information including anecdotal reports about adverse effects, shared among users by word of mouth or on internet forums and social media (267). Scheduling actions at the state or federal level also influence changes in supply, accompanied by a rapid market shift, which is demonstrated by the replacement of 2-furanylfentanyl with cyclopropylfentanyl and methoxyacetylfentanyl (268).
Fentanyl analogues
A variety of sites on the fentanyl core-structure backbone can be substituted to create numerous new fentanyl analogues. Common structural modifications include substitution to or on the phenethyl group, the piperidine ring, the aniline ring and/or the N-propionyl group. This can also result in drug manufacturers creating closely related structural isomers with similar chemical behaviors (e.g., butyrylfentanyl/isobutyrylfentanyl, cis-/trans-3-methylfentanyl and cyclopropylfentanyl/crotonylfentanyl), which then requires development of specialized analytical methods to separate and distinguish these isomers (223, 268–278).
Of the fentanyl analogues, acetylfentanyl was first to emerge on the recreational market in 2014. This analogue is less potent than fentanyl and did not last long in US markets on its own (279). Although acetylfentanyl is a known impurity in the pharmaceutical manufacturing of fentanyl (222), its continued presence at low levels in death cases is almost certainly an indication of fentanyl synthesis through the Janssen method or a similar process (280–282). Following the initial appearance of acetylfentanyl in 2014, additional analogues were encountered over the next 5 years including butyrylfentanyl; beta-hydroxythiofentanyl; 2-furanylfentanyl; carfentanil; ocfentanil; para-fluorobutyrylfentanyl; para-fluoroisobutyrylfentanyl; 4-methoxybutyrylfentanyl; 3-methylfentanyl; para-fluorofentanyl; ortho-fluorofentanyl; acrylfentanyl; tetrahydrofuranylfentanyl; cyclopropylfentanyl; methoxyacetylfentanyl; valerylfentanyl; 2,2-difluorofentanyl; 4-chloroisobutyrylfentanyl; para-hydroxy-butyrylfentanyl; benzoylfentanyl; 4-fluorofuranylfentanyl; cyclopentanoylfentanyl; beta-hydroxyfentanyl; chlorofentanyl and bromofentanyl (199, 235, 236, 253, 272, 283–306). Fentanyl analogues have been associated with adverse events in the USA and other countries (222, 232–234, 307–329).
The widespread availability of illicitly manufactured fentanyl and the spread of fentanyl analogs are responsible for the majority of opioid-related deaths through 2020 (330, 331). In particular, major spikes in mortality were attributed to the introduction of carfentanil into the illicit drug supply in Wayne County, MI, and Summit County, OH, during 2016–2017 (318, 328), as well as smaller outbreaks in other states including FL, PA and KY (326). The subsequent decline in US deaths in 2018 was postulated to carfentanil’s decreased availability, although the DEA’s core-structure scheduling ban of fentanyl analogues was most likely also a significant contributing factor for the overall decline in fentanyl analogue deaths after April 2018 (332, 333).
During 2015–2020, laboratories gradually developed and implemented new analytical methods to detect fentanyl analogues in toxicological casework, resulting in increased identification and reporting in peer-reviewed case reports, case series and epidemiological studies. During October 2016–April 2017, 2-furanylfentanyl (n = 1,228), carfentanil (n = 697), para-fluoroisobutyrylfentanyl/para-fluorobutyrylfentanyl (n = 563), U-47700 (n = 543) and acrylfentanyl (n = 266) were the most frequently reported NSO; average concentrations for 2-furanylfeantanyl, carfentanil and acrylfentanyl were less than 8 ng/mL, but concentrations ranged up to 760 ng/mL (272). Blood carfentanil concentrations were 0.2–9.3 ng/mL in nine postmortem cases from Lithuania (329). In 17 postmortem investigations from Michigan, the average blood carfentanil concentration was <1.2 ng/mL, but additional substances were detected in all cases (323). Postmortem femoral blood carfentanil concentrations were 0.01–0.54 ng/mL in 10 OH cases (222). During October 2016–April 2017, carfentanil concentrations were 0.10–14 ng/mL in 355 cases, with a mean of 0.57 ng/mL (334). In 29 postmortem femoral blood samples with only carfentanil detected, concentrations were 0.02–1.3 ng/mL; carfentanil concentrations in paired vitreous fluid samples were typically lower (335). Chesser et al. noted that NSO were more detectable in the brain than blood and the vitreous humor (336). Cyclopropylfentanyl femoral blood concentrations in four overdose deaths were 16.6–28.9 ng/mL, with a median of 23.7 ng/mL (271). Busardò et al. reported the average postmortem NSO blood concentrations for cyclopropylfentanyl (n = 8) at 7.8 ± 7.2 ng/mL, methoxyacetylfentanyl (n = 4) at 4.1 ± 2.3 ng/mL and furanylfentanyl (n = 1) at 3.6 ng/mL (269). Identifying multiple NSO in a single case is not uncommon; in fact, one case report mentioned that 17 different NSO had been detected (237). Findings in overdose cases related to fentanyl analogues include miosis, respiratory depression and coma, which is a common occurrence. At autopsy, common findings include pulmonary edema and congestion, cerebral edema and frothy fluid in the larynx and respiratory tract.
Cyclohexylbenzamides
U-47700 (trans-3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide), emerged in 2015–2016 as the first and most popular substance in the ‘U series’ and was placed under international control in 2017 (337). U-47700 is one of the first nonfentanyl NSO and was associated with numerous intoxications and fatalities. U-47700 is an N-substituted cyclohexylbenzamide investigated by Upjohn in the 1970s and 1980s as a potential therapeutic agent, but this NSO never advanced to human clinical trials (338, 339). Baumann et al. evaluated the medicinal chemistry, preclinical pharmacology, clandestine availability, methods for detection and forensic toxicology pharmacology of U-47700 and its analogs and postulated that the reason U-47700 was the only drug to gain traction in US illicit markets was because of its higher potency compared to other drugs in the series (340). A subsequent case series involving U-48800 was published (239).
2-Benzyl benzimidazole ‘Nitazenes’
Isotonitazene is structurally related to etonitazene, an opioid investigated for its analgesic properties but ultimately internationally controlled because of health threats (341, 342). Isotonitazene was first developed in 1957 and later emerged on the illicit opioid market in 2019, likely in response to fentanyl analogue core-structure scheduling (343). Isotonitazene’s potency and efficacy are comparable to fentanyl (344). Isotonitazene was first reported in Europe, Canada and the USA in 2019, followed by proliferation into 2020 (9, 240, 344–346). In the USA, the majority of cases centered around Midwestern states—including Illinois, Indiana, Michigan, Minnesota and Wisconsin (347). In June 2020, the DEA announced temporary scheduling of isotonitazene in Schedule 1 of the Controlled Substances Act (348). Reported postmortem blood concentrations of isotonitazene (0.4–9.5 ng/mL) are comparable to the potent fentanyl analogues carfentanil and 3-methylfentanyl. Pulmonary and cerebral edemas along with blood organ congestion were reported in autopsied cases.
Additional 2-benzyl benzimidazole opioid agonists include etonitazene, metonitazene, protonitazene, butonitazene, etodesnitazene and clonitazene (349–352). Lutz published a review of benzimidazole opioid agonists in 2012 and suggested that the abuse liability of etonitazene made it likely for other members of this class also to be used recreationally (353).
Benzimidazolones
Brorphine is a piperidine benzimidazolone, with slight structural similarities to fentanyl but outside the scope of fentanyl core-structure scheduling. Janssen Pharmaceuticals first developed this synthetic opioid subclass as a CNS depressant with morphine-like analgesic activity, but this is another example of a failed pharmaceutical candidate repurposed for recreational use (354). Brorphine was reported in Europe in early 2020 and emerged in the USA in June 2020 after the DEA enacted temporary scheduling of isotonitazene (241, 355). In vitro studies determined brorphine is a full MOR agonist with a higher potency than morphine (241, 356). Brorphine was confirmed in several deaths at concentrations <5 ng/mL in postmortem blood (10, 242) and was ultimately listed for temporary scheduling in the USA in December 2020 (357). In one case that did not result in death, the subject presented with a normal blood pressure but was tachycardic; observations indicated that the individual was also clammy and sweaty (241).
Cinnamylpiperazines
AP-237, 2-methyl-AP-237, AP-238 and para-methyl-AP-237 are cinnamylpiperazines that are structurally distinct from other synthetic opioid subclasses. AP-237 (bucinnazine) was developed in the 1960s for pain management among Chinese cancer patients, and 2-methyl-AP-237 was patented in the 1980s (202, 358–360). 2-Methyl-AP-237 has 68–156 times lower potency than fentanyl, which may account for the lack of popularity on the drug market (361). AP-237 is estimated to be 3.5–13 times less potent than 2-methyl-AP-237 (361, 362). para-Methyl-AP-237 was recently reported in the USA (363), but the low potencies of the cinnamylpiperazines may indicate a lack of interest within the NSO community.
Atypical opioid agonists
Substances that typically combine opioid and nonopioid mechanisms are considered atypical opioid agonists. Mitragynine was addressed in our previous NPS review and is still detected in toxicological casework; however, its role in adverse events continues to be debated. Tianeptine is another atypical opioid agonist with increasing popularity in the USA.
Tianeptine is legally prescribed in Europe, Asia and Latin America, but not in the USA. Tianeptine is pharmacologically classified as a modified tricyclic antidepressant and anxiolytic, but it is also a full agonist at MOR and the δ opioid receptor (364, 365). Therapeutic doses of tianeptine were investigated for major depression, depressed bipolar disorder, dysthymia and adjustment disorder (366). In the USA, tianeptine is sold online as a nootropic or cognitive enhancer. At high doses, tianeptine can produce euphoria. Other adverse effects include dependence, withdrawal and psychosis (367–370). Over the past 10–20 years, the CDC has reported an increase in National Poison Data System calls related to tianeptine because individuals have been self-administering it with little to no medical guidance and are unaware of the drug’s health risks (371–373).
Discussion
The infiltration of NSO into the drug market has exacerbated the ongoing opioid epidemic, posing significant risks to public health and safety. Individuals may be unintentionally exposed to NSO, which, depending on the drug, may have significantly higher potency compared to routinely encountered opioids. NSO are predominantly encountered with fentanyl and/or heroin, either in the drug material or in toxicology samples. This results in at least additive effects of CNS depression but complicates correlation and interpretation of drug use with those effects. Table X presents a summary of adverse effects by organ system.
Table X.
Summary of Toxicity Profile of NPS Opioids
| Organ system | Symptoms and signs |
|---|---|
| CNS | Analgesia, somnolence and sedation |
| Cardiovascular | Hypercarbia, acidosis and hypoxemia |
| Pulmonary | Hypoventilation that can progress to respiratory arrest |
| Other | Miosis; constipation; rapid onset of rigidity of muscles in the jaw, neck, chest wall and abdomen |
| Postmortem findings | Organ edema and congestion; urine retention; frothy watery fluid in airways |
Laboratories lack standardization for toxicological testing, and some laboratories may struggle to keep pace with the rapid emergence and changes related to NPS. Additionally, due to the potency of some NSO they may be detected in sub-nanogram concentrations and require higher analytical sensitivity. NSO pose a significant challenge due to their prevalence and propensity for adverse effects, including overdose and death caused by prolonged respiratory depression. NSO are commonly encountered with other traditional opioids; therefore, reporting of the commonly known drug (e.g., fentanyl) might be sufficient. Unless a toxicology laboratory maintains current testing methods and libraries, NSO are likely to be missed; these new drugs are typically tested for only when routine toxicology reporting is insufficient to explain scene/autopsy findings. Ultimately, the prevalence of NSO is certainly underestimated.
There have been successful efforts to stem the flow of NSO. For example, fentanyl core-structure scheduling reduced the spread of fentanyl analogues and overall cases involving fentanyl-related NSO. However, this also resulted in a shift toward more varied subclasses, further straining laboratories’ efforts and resources. Experience with NSO and NPS shows that an overall reduction in the emergence and use of these drugs is unlikely as long as there is user demand and a market or manner by which the drugs can be sold.
Limitations and Summary
This updated review reflects the fact that NPS markets have continued to evolve rapidly in the past 4 years since our initial 4-year review that ended in 2016. Data presented in this review consolidates reports of adverse events associated with the most prevalent NPS categories published between January 2017 and December 2020 and contained case-specific syndromic information, along with qualitative or quantitative toxicological confirmation. This information can assist forensic and clinical toxicologists in their assessment and interpretation of future cases involving these substances, as well as provide references to literature that may include analytical methods used for confirmation and typical concentrations of the drugs encountered in these cases. Reviews of the chemistry, pharmacology, selected adverse events and recommendations for testing for novel SC, stimulants, hallucinogens, benzodiazepines and opioids are provided. This includes case history, clinical symptoms and autopsy findings, where available. Each topic heading also includes the citation to the published case report.
Limitations of this approach in terms of assessing true prevalence or prevalence trends of NPS involvement in toxic emergencies resulting in death or hospitalization are severalfold. These include the testing capabilities of hospital and medical examiner/coroner toxicology laboratories performing the testing, since laboratory scopes will frequently lag behind the dates of emergence of the latest drugs. Intoxications from some NPS categories, notably the SC, are likely underdiagnosed and reported due to the low concentrations of the drugs, the fact that they are not detected in typical routine drug tests, lack of available immunoassay screening tests and lack of available standard reference materials (SRMs) for confirmatory analysis of the drugs and their metabolites. In addition, many hospitalized patients in toxic emergencies are treated symptomatically and released without even basic testing and with little follow-up as to the causative agent. This is particularly true for opioids, which represent the class of drugs responsible for the largest number of emergency room admissions. In these cases, after the subject has been treated with naloxone, responds and is released, there is no further toxicological investigation of the specific substances ingested. This is true also for benzodiazepines and other depressants where the subject is typically allowed to sleep off the effects of the drug. In addition, NPS benzodiazepines have highly variable cross-reactivity in immunoassays typically used in hospital emergency room (ER) toxicology panels and many likely go undetected (374, 375). In the stimulant category, many cathinone or phenethylamine NPS stimulants also do not cross-react on amphetamine or methamphetamine immunoassays. Furthermore, there can be additional factors, including available research funding for testing, geographic differences in drug trends, cases in teaching hospitals versus EDs and availability of medical toxicology consultants, among other reasons, that will influence whether cases are written up and submitted for publication. Many cases of intoxication were excluded from our review because of lack of appropriate toxicological confirmation or lack of case histories or were previously included in other review articles. In addition, not all case reports are indexed with search terms that would come up in our search, including spellings of chemical names, mentions of the drug class or category of NPS.
In short, the frequency of reports of intoxications indicted in our review should be used cautiously with a respect to estimation of comparative prevalence or public health impact of the various drug classes involved. In every case, these reports should be considered the tip of the iceberg in terms of the actual number of intoxications. The rates of identification of new substances, however, are important, and in aggregate, a good indicator of changes in drug markets over the review period. There is also some utility in evaluating the frequency of published reports between this period (2017–2020) and the previous reporting period covering 2013–2016. The greatest contribution of this survey of literature is in its spotlighting of symptomology, drug concentrations and diversity of members within specific drug classes.
Considering this review covering the period 2017–2020, together with our prior review covering 2013–2016, this work represents a collection of 8 years of published reports covering 600 citations cataloging adverse toxicological details for over 80 individual substances. In the present review, there were over 60 substances in the most prevalent classes including NPS benzodiazepines, SC, opioids, hallucinogens and stimulants. Again, the relevant prevalence of reporting may not reflect the prevalence of use and will be influenced by the testing capabilities of laboratories reporting.
Between 2017 and 2020, 63 substances were mentioned in qualifying reports. The types of case reports included overdose fatalities (378 cases), clinical treatment and hospitalization (771 cases) and DUID (170 cases). Figure 3 shows the overall number of cases by NPS class during these two 4-year periods and combined over the entire 8-year period covered by the two review cycles. Note that the NPS benzodiazepine category, which has the largest number of cases, is influenced by a few reviews with very large numbers of cases. Figure 4 depicts the overall frequency of NPS substances by drug class, reflecting the larger number of SC drugs, followed by hallucinogens, opioids, benzodiazepines and stimulants. This trend also reflects the general experience in terms of activity within drug classes in Europe reported by the EMCDDA. Generally, the most current 4-year period saw a 70% increase in new NPS over the prior 4-year period of 2013–2016. During 2017–2020, 50 new NPS not reported in the previous review article were identified in this assessment of the literature, representing cannabinoids (n = 19), hallucinogens (n = 11), stimulants (n = 10), opioids (n = 9) and benzodiazepines (n = 1).
Figure 3.
Total cases reported in the literature by NPS class.
Figure 4.
Overall frequency of NPS substances by drug class.
Synthetic cannabinoids
The introduction of new SC to illicit markets continues and while this NPS class has demonstrated far fewer unique substances compared to a decade ago, more new substances appeared in adverse event reports in 2017–2020 (n = 22) versus the period 2013–2016 (n = 11). SC are generally named using chemical structure conventions, such as the modified Uchiyama system, and are often based on names adopted by the vendors of analytical SRMs (23, 24). The pharmacokinetics and pharmacodynamics of SC vary compared to THC and each other and can yield contradictory effects (i.e., tachycardia versus bradycardia and agitation versus somnolence). Toxidromes and clinical effects of SC can be nonspecific and inconsistent; however, with unique sequelae, such as tonic-clonic seizures, respiratory depression and heightened agitation or sedation not resolved with naloxone observed in the context of drug use history, SC should be considered when opioid testing for legacy and NPS opioids is negative. SC are not routinely tested for and require special methods to detect them in biological samples. Testing of drug paraphernalia or drug products found with the patient can assist toxicologists in performing the appropriate toxicological testing to properly identify SC that may be involved.
Generally, the newer indole- and indazole-carboxamides (e.g., 5F-MDMB-PICA) are more potent than older SC, such as JWH-018 and XLR-11. During 2017–2020, the adverse events of 22 unique SC were reported in 60 cases: 43 clinical, 15 postmortem and 2 DUID. There were 19 instances of an additional SC confirmed (31.7%) with the reported SC, and another 19 instances of another drug identified (31.7%). Evidence of polydrug use in SC was substantially higher than in our previous review (2013–2016: 2 cases and 2017–2020: 44 cases). During 2013–2016 and 2017–2019, five SC were identified—AB-CHMINACA, AMB-FUBINACA (FUB-AMB) AB-PINACA, MDMB-CHMICA and UR-144 in both reviews.
NPS stimulants
The toxic profile of NPS stimulants includes CNS effects such as agitation, psychosis, delusions, aggression, irritability, paranoia, delirium, hallucinations, sedation, coma, abnormal behavior and altered fluctuating consciousness; cardiorespiratory effects such as tachycardia, hypertension, palpitations and increased respiration rate; and other symptomology such as hypothermia, mydriasis, rhabdomyolysis, compartment syndrome and sweating (most of these physiological findings are similar to traditional stimulants). Organ edema and congestion are often present in autopsy findings, as they are with traditional stimulants. During 2017–2020, a total of 10 NPS stimulants were reported; in DUID, clinical and fatal cases, this was an increase of six from only four new substances during the previous 4-year period. All 10 synthetic stimulants in the literature we reviewed were newly identified: 4-MEAP, 4F-MPH, α-EAP, 4F-αPVP, α-PHP, dibutylone, EPH, MPHP, NEH and NEP. Polydrug use for synthetic stimulants representing instances of subjects co-ingesting more than one synthetic stimulant appeared higher in recent reports than in the prior review (24 cases reported compared to 1). An even greater increase in the number of reported cases of synthetic stimulants combined with other drugs were reported during this same time. Opioid use with synthetic stimulants has also increased.
NPS hallucinogens
In this literature review, NPS hallucinogens represented the second largest number of novel substances in a class, after SC. It is likely, however, that due to limited testing for these diverse drug types, these reports may mask or underrepresent their true prevalence while their potential for severe psychiatric side effects may increase the likelihood of the cases being published. In addition, in many cases, these individuals were using multiple novel substances at the same time. NPS hallucinogens are largely analogues of traditional hallucinogens: PCP-like (e.g., 3-MeO-PCP and 3-HO-PCP); ketamine-like (e.g., 2-FDCK, 2-Oxo-PCE and MXPr); LSD-like (e.g., AL-LAD); tryptamine-like (e.g., 5-MeO-DiPT and 5-MeO-MiPT) and diarylethylamine-like (e.g., MXP and diphenidine). Although these drugs are analogues of traditional hallucinogens, immunoassay screening tests may not cross-react and confirmation tests must have the drugs indicated for proper identification. Common signs and symptoms of NPS hallucinogenic intoxication included agitation, aggressive behavior, delusions and tachycardia. Hypo- or hyperthermia, excited delirium, and serotonin syndrome were less frequently reported. In total, 14 NPS hallucinogens were reported in 151 cases during 2017–2020. In our prior review, three substances—25B-NBOMe, 25C-NBOMe and 25I-NBOMe—were previously mentioned and 11 novel hallucinogens were newly identified: 2-FDCK, 2-Oxo-PCE, 2C-E, 3-HO-PCP, 5-MeO-DiPT, AL-LAD, Bromo-DragonFLY, diphenidine, DOC, MXP and MXPr. Adverse events for NPS hallucinogens were detailed in 6 DUID, 123 clinical and 22 fatal cases. Moreover, novel multidrug use in individuals who use NPS hallucinogens was high and represented a diverse use profile—as noted in the top three drug classes (in order of highest concomitant use): other hallucinogens (64 cases showing at least 14 substances) were greater than stimulants (48 cases showing at least 12 substances), followed by cannabinoids (eight cases showing at least four substances). NPS hallucinogens were also confirmed in the presence of opioids, benzodiazepines, chlorpheniramine, antidepressants and ethanol.
NPS opioids
Opioid misuse in the USA reached epidemic proportions and continued to rise over three distinct phases beginning with pharmaceutical opioids (e.g., oxycodone and methadone) prescription in the early 1990s, followed by an increased use of illicit opioids (e.g., heroin and fentanyl) in the early 2010s and most recently the current phase of NSO, beginning in 2013 with the introduction of illicitly manufactured fentanyl (376). Illicit fentanyl is the major contributor to opioid death in the USA today. In response to the 2018 core-structure scheduling actions to address the proliferation of fentanyl analogues, divergent chemical subclasses of NSO have begun to emerge. During 2017–2020, there were 93 published case reports identified in our literature search of NSO: 9 clinical, 82 postmortem and 2 DUID. The number of NSO fatalities involving polydrug use also increased. Ninety percentage of the published cases (n = 84) had another drug confirmed in addition to the reported NSO. Nine new NSO were reported during 2017–2020 that had not been reported in our previous review—3-methylfentanyl, beta-hydroxyfentanyl, brorphine, carfentanil, cyclopropylfentanyl, isotonitazene, para-fluoroisobutyrylfentanyl (p-FIBF), tianeptine and U-48800.
NPS benzodiazepines
While NPS benzodiazepines have far fewer and less severe adverse effects than other NPS when ingested alone, coingestion with other CNS depressants or opioids results in synergistic effects that may lead to unconsciousness, coma or death. Toxidromes of NPS benzodiazepines are mostly CNS depressants in nature and indistinct from intoxication with medically prescribed benzodiazepines. The toxidrome includes amnesia, drowsiness, lethargy, slurred speech, incoordination, delayed comprehension and reaction time, dizziness, vertigo, sedation and coma. NPS benzodiazepines were the most frequently reported NPS class during 2013–2016 and 2017–2020, largely due to case series with large numbers of cases included from DUID or clinical populations. Often, the literature does not indicate if other drugs were identified as most of the cases were clinical, and comprehensive testing is generally not performed in a hospital setting. Flualprazolam was the only newly reported NPS benzodiazepine in published case reports during 2017 through 2020. Six NPS benzodiazepines that were reported during 2013–2016 and continued to be reported during 2017–2020 included clonazolam, diclazepam, etizolam, flubromazepam, flubromazolam and phenazepam.
Conclusions
The impact of emergent NPS continues to be a significant international public health threat, especially with respect to overdose fatalities. Opioid deaths continue to be the most impactful in relation to adverse events with 90% of the published case reports involving opioids resulting in fatalities, as opposed to clinical intoxications or DUID. Surveillance systems, such as NPS Discovery and NFLIS, indicate NPS classes for seized drugs are highest for stimulants, opioids and cannabinoids. Widespread awareness of a new substance in NPS markets typically happens after a mass poisoning or a series of deaths in a specific location. Lower frequency occurrences or events with less serious health outcomes are more likely to be overlooked. Routine approaches to analytical toxicology based on a tiered approach of immunoassay, coupled with targeted gas or liquid chromatography mass spectrometry screening are now more likely to fail to find the causative agent because the defined scope often does not include the most recently emerged NPS. With some drug classes, such as the typically neutral SC, widely used routine basic extraction chemistries are not capable of detecting these drugs.
For all these reasons, identifying the chemical cause of toxic incidents or deaths via traditional analytical approaches targeting common therapeutic or abused agents is not adequate for comprehensive detection in NPS markets. This realization is driving laboratories toward HRMS techniques such as time-of-flight mass spectrometry and more nontargeted analytical approaches, which while effective, add cost and complexity to analyses for detecting high-risk, low-frequency intoxication events. While this technology is increasingly available to some forensic toxicology laboratories, it creates an additional burden in terms of research and development, method validation and additional confirmations. It is less common for comprehensive testing to be performed to identify the causative agent in clinical intoxications because treatment is generally supportive and based on symptomology. In addition, drug testing results for nontraditional drugs (i.e., beyond traditional opiates, cocaine, amphetamines and benzodiazepines) are not directly used in patient care, so insurance companies typically will not reimburse for the cost of testing. Delays in identifying the presence of these NPS and making stakeholder communities in clinical medicine, medicolegal death investigation, harm reduction, rehabilitation and safety-sensitive drug testing programs aware of their presence have significant negative consequences for public health and public safety.
Challenges arise for forensic toxicologists in interpreting NPS results in general and even more so when multiple NPS and therapeutic or legacy drugs of abuse are present. Coingestion of NPS with other NPS or common drugs of abuse is common, but the interaction of the drugs is largely unstudied, leading to difficulties in formulating opinions based on polydrug toxicity and effects. Conclusions should generally be based on basic knowledge of general pharmacological effect, scene and circumstances, observation, autopsy findings or clinical assessments, field sobriety tests and other tools. Animal-based dose/response studies (EC50) or in vitro receptor efficacy or binding affinity profiles which are often lacking for NPS should be extrapolated with caution to humans. Polydrug combinations involving benzodiazepines and opioids are commonly encountered in forensic toxicology and are understood to be at least additive in nature. NPS or novel versions of these drugs are likely to cause increased toxicity when they are ingested in combination. NSO are often found along with fentanyl and/or each other, which will lead to a greater risk of overdose and death.
Recommendations
To better understand the public health and public safety threats from NPS, the USA needs to continue to support and expand ongoing efforts to develop a national rapid surveillance monitoring program that will provide real-time clinical and forensic toxicology data about emerging drugs and their known toxidromes and side effect profiles. Medical, forensic and treatment communities, policy makers, regulators and other stakeholders would benefit from more rapid access to this information. Today, new tools such as the CFSRE’s NPS Discovery program, the DEA’s Emerging Threat Reports, NFLIS and the UNODC EWA on NPS including the EWA Tox-Portal (377) are innovative approaches for collecting, analyzing and sharing data and real-time insights on toxicology and harm related to the use of NPS in the USA and worldwide. Such systems need to be monitored, managed and curated to ensure that the data are evidence-based, comprehensive and reported consistently, that the substances have been confirmed analytically and that there is a focus on rapid targeted dissemination of information to stakeholders within a timeframe that makes the information actionable in terms of epidemiological surveillance,
As noted in our prior review, toxicological testing of samples from individuals reporting to EDs and hospitals following NPS adverse events and ingestion would add greatly to our knowledge about the progression of toxicity in individuals harmed by these substances and the efficacy of treatments or countermeasures. The American College of Medical Toxicology has for a number of years run a program called the Toxic Investigators Consortium is now adding toxicological testing data to some of the cases where syndromic data are also collected, which will provide a great insight into the nuances and toxic profile of NPS (378). Similarly, as evidenced by the increased reporting during this review period of toxic effects, increased rates of testing by clinical and forensic laboratories are leading to more reports of adverse effects for more substances, improving our insight into this problem area.
The use of multiple substances by people who use drugs continues to rise. Polydrug-related deaths and case reports are increasing, and increased combined use of NPS opioids and NPS benzodiazepines as well as NPS stimulants and opioids is also evident from the reports reviewed herein. The more widespread use of a drug use evaluation system such as the TSS developed by Elliot et al. can further assist decision-makers in the inclusion of drug findings into death certificate data, making it easier to aggregate in national death statistics (65).
Finally, although toxicology laboratories have made significant progress with the increased availability of nontargeted HRMS screening, this approach needs to be applied more comprehensively to improve the detectability of NPS in clinical and forensic toxicology, a research need identified by the Organization of Scientific Area Committees for Forensic Science (379). Best practices for maintaining awareness of new and emerging substances and adding them to the scope of testing include maintaining a dialog with local/regional forensic chemistry laboratories to improve awareness about substances that are being seized and detected in the street drug supply; monitoring drug trends from domestic and international organizations and governments; reviewing data from poison centers and clinical populations; reviewing additions to state and federal drug schedules; and reviewing and acquiring SRM offered by SRM vendors to expand libraries and databases.
No single review of an area as diverse as the current NPS landscape can be comprehensive. As a bellwether of today’s patterns and preference of novel drug use, this review is limited by factors such as the search terms we used in our survey, the factors that drive academic publication of adverse drug effects and the testing abilities of laboratories that support both research and practice in clinical and forensic medicine. Collectively over this and our prior review of NPS adverse events between 2013 and 2016, greater than 1,700 published clinical and forensic cases are reported and provide some insight into the most prominent drugs and their documented harms.
Acknowledgments
Opinions or points of view expressed herein represent a consensus of the authors and do not necessarily represent the official position or policies of the US Department of Justice.
Contributor Information
Amanda L A Mohr, Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, 2300 Stratford Ave, Willow Grove, PA 19090, USA.
Barry K Logan, Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, 2300 Stratford Ave, Willow Grove, PA 19090, USA; NMS Labs, 200 Welsh Rd, Horsham, PA 19044, USA.
Melissa F Fogarty, Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, 2300 Stratford Ave, Willow Grove, PA 19090, USA.
Alex J Krotulski, Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, 2300 Stratford Ave, Willow Grove, PA 19090, USA.
Donna M Papsun, NMS Labs, 200 Welsh Rd, Horsham, PA 19044, USA.
Sherri L Kacinko, NMS Labs, 200 Welsh Rd, Horsham, PA 19044, USA.
Marilyn A Huestis, Center for Forensic Science Research and Education at the Fredric Rieders Family Foundation, 2300 Stratford Ave, Willow Grove, PA 19090, USA; Institute of Emerging Health Professions, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Jeri D Ropero-Miller, RTI International, Center for Forensic Sciences, 3040 East Cornwallis Rd, Research Triangle Park, NC 27709, USA.
Funding
This work was supported by the Forensic Technology Center of Excellence (Award 2016-MU-BX-K110), awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice.
References
- 1. Henderson G.L. (1988) Designer drugs: past history and future prospects. Journal of Forensic Sciences, 33, 569–575. [PubMed] [Google Scholar]
- 2. Shulgin A., Shulgin A. PiHKAL: A Chemical Love Story. Transform Press: Berkeley, CA, 1995. [Google Scholar]
- 3. Shulgin A.T., Shulgin A. TiHKAL: The Continuation. Transform Press: Berkeley, CA, 1997. [Google Scholar]
- 4. King L.A., Kicman A.T. (2011) A brief history of ‘new psychoactive substances’. Drug Testing and Analysis, 3, 401–403. [DOI] [PubMed] [Google Scholar]
- 5. Tettey J., Raithelhuber M., Crean C., Levissianos S., Soe T.N., Sim I., et al. (2020) Global Synthetic Drugs Assessment 2020, United Nations publication, Sales No. E.20.XI.9. [Google Scholar]
- 6. Drug Enforcement Administration . Special Testing and Research Laboratory emerging threat report 2019 Annual. https://ndews.umd.edu/sites/ndews.umd.edu/files/DEA-Emerging-Threat-Report-2019-Annual.pdf (Apr 6, 2020).
- 7. Monographs . (2019) NPS Discovery, May 24, 2019. https://www.npsdiscovery.org/reports/monographs/ (Feb 4, 2021).
- 8. Freye E., Levy J.V. Pharmacology and Abuse of Cocaine, Amphetamines, Ecstasy and Related Designer Drugs. Springer: Dordrecht, Netherlands, 2009. [Google Scholar]
- 9. Krotulski A.J., Papsun D.M., Kacinko S.L., Logan B.K. (2020) Isotonitazene quantitation and metabolite discovery in authentic forensic casework. Journal of Analytical Toxicology, 44, 521–530. [DOI] [PubMed] [Google Scholar]
- 10. Krotulski A.J., Papsun D.M., Noble C., Kacinko S.L., Logan B.K. (2020) Brorphine—Investigation and quantitation of a new potent synthetic opioid in forensic toxicology casework using liquid chromatography-mass spectrometry. Journal of Forensic Sciences, 66, 664–676. [DOI] [PubMed] [Google Scholar]
- 11. Drug Enforcement Administration, Strategic Intelligence Section . (2019) 2019 National Drug Threat Assessment. https://www.dea.gov/sites/default/files/2020-02/DIR-007-20%202019%20National%20Drug%20Threat%20Assessment%20-%20low%20res210.pdf (Feb 4, 2021).
- 12. Dargan P.I., Wood D.M. Novel Psychoactive Substances: Classification, Pharmacology and Toxicology. Academic Press: London, UK, 2013. [Google Scholar]
- 13. Drug Enforcement Administration . (2021) 2020 National Drug Threat Assessment. https://www.dea.gov/sites/default/files/2021-02/DIR-008-21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf (Oct 3, 2021).
- 14. Logan B.K., Mohr A.L.A., Friscia M., Krotulski A.J., Papsun D.M., Kacinko S.L., et al. (2017) Reports of adverse events associated with use of novel psychoactive substances, 2013-2016: a review. Journal of Analytical Toxicology, 41, 573–610. [DOI] [PubMed] [Google Scholar]
- 15. National Forensic Laboratory Information System (NFLIS) . https://www.deadiversion.usdoj.gov/nflis/ (Mar 6, 2022).
- 16. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) . http://www.emcdda.europa.eu/ (Mar 6, 2022).
- 17. NPS Discovery . https://www.npsdiscovery.org/ (Apr 27, 2021).
- 18. Society of Forensic Toxicologists, Inc. (SOFT) . http://www.soft-tox.org/ (Mar 6, 2022).
- 19. American Academy of Forensic Sciences (AAFS) . https://www.aafs.org/ (Mar 6, 2022).
- 20. Scientific Working Group for the Analysis of Seized Drugs (SWGDRUG) . http://swgdrug.org/ (Mar 6, 2022).
- 21. ChemSpider . http://www.chemspider.com/ (Mar 6, 2022).
- 22. Current NPS Threats Volume II . (2020) United Nations Office on Drugs and Crime. https://www.unodc.org/documents/scientific/Current_NPS_Threats_Volume_II_Web.pdf (Apr 6, 2021).
- 23. Uchiyama N., Matsuda S., Wakana D., Kikura-Hanajiri R., Goda Y. (2013) New cannabimimetic indazole derivatives, N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide (AB-PINACA) and N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (AB-FUBINACA) identified as designer drugs in illegal products. Forensic Toxicology, 31, 93–100. [Google Scholar]
- 24. Potts A.J., Cano C., Thomas S.H.L., Hill S.L. (2020) Synthetic cannabinoid receptor agonists: classification and nomenclature. Clinical Toxicology, 58, 82–98. [DOI] [PubMed] [Google Scholar]
- 25. Davidson C., Cao D., King T., Weiss S.T., Wongvisavakorn S., Ratprasert N., et al. (2021) A comparative analysis of kratom exposure cases in Thailand and the United States from 2010-2017. The American Journal of Drug and Alcohol Abuse, 47, 74–83. [DOI] [PubMed] [Google Scholar]
- 26. Gurney S.M.R., Scott K.S., Kacinko S.L., Presley B.C., Logan B.K. (2014) Pharmacology, toxicology, and adverse effects of synthetic cannabinoid drugs. Forensic Science Review, 26, 53–78. [PubMed] [Google Scholar]
- 27. White C.M. (2017) The pharmacologic and clinical effects of illicit synthetic cannabinoids. Journal of Clinical Pharmacology, 57, 297–304. [DOI] [PubMed] [Google Scholar]
- 28. Luethi D., Liechti M.E. (2020) Designer drugs: mechanism of action and adverse effects. Archives of Toxicology, 94, 1085–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walsh K.B., Andersen H.K. (2020) Molecular pharmacology of synthetic cannabinoids: delineating CB1 receptor-mediated cell signaling. International Journal of Molecular Sciences, 21, 6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giorgetti A., Mogler L., Halter S., Haschimi B., Alt A., Rentsch D., et al. (2020) Four cases of death involving the novel synthetic cannabinoid 5F-cumyl-PEGACLONE. Forensic Toxicology, 38, 314–326. [Google Scholar]
- 31. Kant A., Mong R., Tan H.H. (2020) Accidental ingestion of a novel psychoactive substance: a case report. Cureus, 12, e11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan S., Wu J., Lee B. (2019) Fatalities related to new psychoactive substances in Singapore-A case series. Forensic Science International, 304, 109892. [DOI] [PubMed] [Google Scholar]
- 33. Ershad M., Dela Cruz M., Mostafa A., Khalid M.M., Arnold R., Hamilton R. (2020) Heroin adulterated with the novel synthetic cannabinoid, 5F-MDMB-PINACA: a case series. Clinical Practice and Cases in Emergency Medicine, 4, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kraemer M., Fels H., Dame T., Musshoff F., Halter S., Mogler L., et al. (2019) Mono-/polyintoxication with 5F-ADB: a case series. Forensic Science International, 301, e29–e37. [DOI] [PubMed] [Google Scholar]
- 35. Zattera L., Errasti J., Supervía A. (2018) Intoxication by the synthetic cannabinoid 5-fluoro-ABD, acquired as ketamine. Medicina Clinica, 151, 168. [DOI] [PubMed] [Google Scholar]
- 36. McCain K.R., Jones J.O., Chilbert K.T., Patton A.L., James L.P., Moran J.H. (2018) Impaired driving associated with the synthetic cannabinoid 5F-ADB. Journal of Forensic Science & Criminology, 6. 10.15744/2348-9804.6.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barceló B., Pichini S., López-Corominas V., Gomila I., Yates C., Busardò F.P., et al. (2017) Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: a case series. Forensic Science International, 273, e10–e14. [DOI] [PubMed] [Google Scholar]
- 38. Ivanov I.D., Stoykova S., Ivanova E., Vlahova A., Burdzhiev N., Pantcheva I., et al. (2019) A case of 5F-ADB/FUB-AMB abuse: drug-induced or drug-related death? Forensic Science International, 297, 372–377. [DOI] [PubMed] [Google Scholar]
- 39. Maeda H., Kikura-Hanajiri R., Kawamura M., Nagashima E., Yoshida K.-I. (2018) AB-CHMINACA-induced sudden death from non-cardiogenic pulmonary edema. Clinical Toxicology (Philadelphia, Pa.), 56, 143–145. [DOI] [PubMed] [Google Scholar]
- 40. Rianprakaisang T., Gerona R., Hendrickson R.G. (2020) Commercial cannabidiol oil contaminated with the synthetic cannabinoid AB-FUBINACA given to a pediatric patient. Clinical Toxicology (Philadelphia, Pa.), 58, 215–216. [DOI] [PubMed] [Google Scholar]
- 41. Lam R.P.K., Tang M.H.Y., Leung S.C., Chong Y.K., Tsui M.S.H., Mak T.W.L. (2017) Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: a case report with quantitative analysis of serum drug concentrations. Clinical Toxicology (Philadelphia, Pa.), 55, 662–667. [DOI] [PubMed] [Google Scholar]
- 42. Moeller S., Lücke C., Struffert T., Schwarze B., Gerner S.T., Schwab S., et al. (2017) Ischemic stroke associated with the use of a synthetic cannabinoid (spice). Asian Journal of Psychiatry, 25, 127–130. [DOI] [PubMed] [Google Scholar]
- 43. Nacca N., Schult R., Loflin R., Weltler A., Gorodetsky R., Kacinko S., et al. (2018) Coma, seizures, atrioventricular block, and hypoglycemia in an ADB-FUBINACA body-packer. The Journal of Emergency Medicine, 55, 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rojek S., Kłys M., Maciów-Głąb M., Kula K. (2017) A new challenge in forensic toxicology exemplified by a case of murder under the influence of a synthetic cannabinoid - AM-2201. Legal Medicine (Tokyo, Japan), 27, 25–31. [DOI] [PubMed] [Google Scholar]
- 45. Hamilton R.J., Keyfes V., Banka S.S. (2017) Synthetic cannabinoid abuse resulting in ST-segment elevation myocardial infarction requiring percutaneous coronary intervention. The Journal of Emergency Medicine, 52, 496–498. [DOI] [PubMed] [Google Scholar]
- 46. Armenian P., Darracq M., Gevorkyan J., Clark S., Kaye B., Brandehoff N.P. (2018) Intoxication from the novel synthetic cannabinoids AB-PINACA and ADB-PINACA: a case series and review of the literature. Neuropharmacology, 134, 82–91. [DOI] [PubMed] [Google Scholar]
- 47. El Zahran T., Gerona R., Morgan B.W., Pomerleau A.C. (2019) A novel synthetic cannabinoid (cumyl-4-cyano-BINACA) resulting in hyperthermia, rhabdomyolysis, and renal failure in a 29-year-old patient: it’s not meningitis. Clinical Toxicology (Philadelphia, Pa.), 57, 421–422. [DOI] [PubMed] [Google Scholar]
- 48. Dobaja M., Grenc D., Kozelj G., Brvar M. (2017) Occupational transdermal poisoning with synthetic cannabinoid cumyl-PINACA. Clinical Toxicology (Philadelphia, Pa.), 55, 193–195. [DOI] [PubMed] [Google Scholar]
- 49. Coppola M., Mondola R. (2017) JWH-122 consumption adverse effects: a case of hallucinogen persisting perception disorder five-year follow-up. Journal of Psychoactive Drugs, 49, 262–265. [DOI] [PubMed] [Google Scholar]
- 50. Bäckberg M., Tworek L., Beck O., Helander A. (2017) Analytically confirmed intoxications involving MDMB-CHMICA from the STRIDA project. Journal of Medical Toxicology, 13, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyyappan C., Ford L., Vale A. (2017) Poisoning due to MDMB-CHMICA, a synthetic cannabinoid receptor agonist. Clinical Toxicology (Philadelphia, Pa.), 55, 151–152. [DOI] [PubMed] [Google Scholar]
- 52. Adamowicz P., Meissner E., Maślanka M. (2019) Fatal intoxication with new synthetic cannabinoids AMB-FUBINACA and EMB-FUBINACA. Clinical Toxicology (Philadelphia, Pa.), 57, 1103–1108. [DOI] [PubMed] [Google Scholar]
- 53. Sutlović D., Prkačin I., Vaiano F., Bertol E., Bratinčević M.V., Definis-Gojanović M. (2018) A case of synthetic cannabinoid poisoning in Croatia. Archives of Industrial Hygiene and Toxicology, 69, 186–190. [DOI] [PubMed] [Google Scholar]
- 54. Samra K., Boon I.S., Packer G., Jacob S. (2017) Lethal high: acute disseminated encephalomyelitis (ADEM) triggered by toxic effect of synthetic cannabinoid black mamba. BMJ Case Reports, 2017, bcr2016218431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Gorp F., Wejden L.C., Stienstra N.A., Kuck E.M., Haas L.E.M. (2017) Severe neurological symptoms following synthetic cannabinoid intoxication. The Netherlands Journal of Medicine, 75, 158–160. [PubMed] [Google Scholar]
- 56. Al Fawaz S., Al Deeb M., Huffman J.L., Al Kholaif N.A., Garlich F., Chuang R. (2019) A case of status epilepticus and transient stress cardiomyopathy associated with smoking the synthetic psychoactive cannabinoid, UR-144. The American Journal of Case Reports, 20, 1902–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Imtiaz M., Saha B., Sana Ullah U., Saha A. (2019) A case of acute life-threatening pulmonary hemorrhage from synthetic cannabinoid abuse. Case Reports in Pulmonology, 2019, 8137648.doi: 10.1155/2019/8137648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaneko S. (2017) Motor vehicle collisions caused by the ‘super-strength’ synthetic cannabinoids, MAM-2201, 5F-PB-22, 5F-AB-PINACA, 5F-AMB and 5F-ADB in Japan experienced from 2012 to 2014. Forensic Toxicology, 35, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hvozdovich J.A., Chronister C.W., Logan B.K., Goldberger B.A. (2020) Synthetic cannabinoid deaths in state of Florida prisoners. Journal of Analytical Toxicology, 44, 298–300. [DOI] [PubMed] [Google Scholar]
- 60. Kleis J., Germerott T., Halter S., Héroux V., Roehrich J., Schwarz C.S., et al. (2020) The synthetic cannabinoid 5F-MDMB-PICA: a case series. Forensic Science International, 314, 110410. [DOI] [PubMed] [Google Scholar]
- 61. Morrow P.L., Stables S., Kesha K., Tse R., Kappatos D., Pandey R., et al. (2020) An outbreak of deaths associated with AMB-FUBINACA in Auckland NZ. EClinicalMedicine, 25, 100460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tiemensma M., Rutherford J.D., Scott T., Karch S. (2021) Emergence of cumyl-PEGACLONE-related fatalities in the Northern Territory of Australia. Forensic Science, Medicine, and Pathology, 17, 3–9. [DOI] [PubMed] [Google Scholar]
- 63. Giorgetti A., Busardò F.P., Tittarelli R., Auwärter V., Giorgetti R. (2020) Post-mortem toxicology: a systematic review of death cases involving synthetic cannabinoid receptor agonists. Frontiers in Psychiatry, 11, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kraemer M., Boehmer A., Madea B., Maas A. (2019) Death cases involving certain new psychoactive substances: a review of the literature. Forensic Science International, 298, 186–267. [DOI] [PubMed] [Google Scholar]
- 65. Elliott S., Sedefov R., Evans-Brown M. (2018) Assessing the toxicological significance of new psychoactive substances in fatalities. Drug Testing and Analysis, 10, 120–126. [DOI] [PubMed] [Google Scholar]
- 66. Centers for Disease Control and Prevention (CDC) Health Alert Network (HAN) . (2018) Outbreak of Life-Threatening Coagulopathy Associated with Synthetic Cannabinoids Use. https://emergency.cdc.gov/han/han00410.asp (Mar 22, 2021).
- 67. European Monitoring Centre for Drugs and Drug Addiction . (2020) European Drug Report Trends and Developments 2020. Publications Office of the European Union, Luxembourg. https://www.emcdda.europa.eu/system/files/publications/13236/TDAT20001ENN_web.pdf (Mar 6, 2022). [Google Scholar]
- 68. German C.L., Fleckenstein A.E., Hanson G.R. (2014) Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sciences, 97, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Drug Enforcement Administration: Special Testing and Research Laboratory . (2020) DEA Emerging Threat Report Third Quarter 2020. https://cesar.umd.edu/sites/cesar.umd.edu/files/pubs/DEA-Emerging-Threat-Report-2020-Quarter-3.pdf (Jan 20, 2021).
- 70. Krotulski A.J., Mohr A., Logan B. (2020) Trend Report: Q3 2020 - NPS Stimulants & Hallucinogens in the United States. https://www.npsdiscovery.org/wp-content/uploads/2020/10/2020-Q3_NPS-Stimulants-and-Hallucinogens_Trend-Report.pdf (Mar 6, 2022).
- 71. U.S. Drug Enforcement Administration, Diversion Control Division . (2019) NFLIS-DRUG 2019 Annual Report. https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS-Drug-AR2019.pdf (Jan 20, 2021).
- 72. Carlier J., Giorgetti R., Varì M.R., Pirani F., Ricci G., Busardò F.P. (2019) Use of cognitive enhancers: methylphenidate and analogs. European Review for Medical and Pharmacological Sciences, 23, 3–15. [DOI] [PubMed] [Google Scholar]
- 73. Luethi D., Kaeser P.J., Brandt S.D., Krähenbühl S., Hoener M.C., Liechti M.E. (2018) Pharmacological profile of methylphenidate-based designer drugs. Neuropharmacology, 134, 133–140. [DOI] [PubMed] [Google Scholar]
- 74. Frati P., Kyriakou C., Del Rio A., Marinelli E., Vergallo G.M., Zaami S., et al. (2015) Smart drugs and synthetic androgens for cognitive and physical enhancement: revolving doors of cosmetic neurology. Current Neuropharmacology, 13, 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zaami S., Tagliabracci A., Berretta P., Busardò F.P., Marinelli E. (2020) Use of methylphenidate analogues as cognitive enhancers: the prelude to cosmetic neurology and an ethical issue. Frontiers in Psychiatry, 10, 1006.doi: 10.3389/fpsyt.2019.01006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Marusich J.A., Antonazzo K.R., Wiley J.L., Blough B.E., Partilla J.S., Baumann M.H. (2014) Pharmacology of novel synthetic stimulants structurally related to the ‘bath salts’ constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology, 87, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Baumann M.H., Solis E., Watterson L.R., Marusich J.A., Fantegrossi W.E., Wiley J.L. (2014) Baths salts, spice, and related designer drugs: the science behind the headlines. Journal of Neuroscience, 34, 15150–15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Simmler L., Buser T., Donzelli M., Schramm Y., Dieu L.-H., Huwyler J., et al. (2013) Pharmacological characterization of designer cathinones in vitro. British Journal of Pharmacology, 168, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eshleman A.J., Wolfrum K.M., Reed J.F., Kim S.O., Swanson T., Johnson R.A., et al. (2017) Structure-activity relationships of substituted cathinones, with transporter binding, uptake and release. Journal of Pharmacology and Experimental Therapeutics, 360, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Weaver M.F., Hopper J.A., Gunderson E.W. (2015) Designer drugs 2015: assessment and management. Addiction Science & Clinical Practice, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Marinetti L.J., Antonides H.M. (2013) Analysis of synthetic cathinones commonly found in bath salts in human performance and postmortem toxicology: method development, drug distribution and interpretation of results. Journal of Analytical Toxicology, 37, 135–146. [DOI] [PubMed] [Google Scholar]
- 82. Miotto K., Striebel J., Cho A.K., Wang C. (2013) Clinical and pharmacological aspects of bath salt use: a review of the literature and case reports. Drug and Alcohol Dependence, 132, 1–12. [DOI] [PubMed] [Google Scholar]
- 83. Mas-Morey P., Visser M.H.M., Winkelmolen L., Touw D.J. (2013) Clinical toxicology and management of intoxications with synthetic cathinones (‘bath salts’). Journal of Pharmacy Practice, 26, 353–357. [DOI] [PubMed] [Google Scholar]
- 84. Papa P., Valli A., Di Tuccio M., Frison G., Zancanaro F., Buscaglia E., et al. (2019) Analytically confirmed intoxication by 4-fluoromethylphenidate, an analog of methylphenidate. Journal of Analytical Toxicology, 43, e1–e7. [DOI] [PubMed] [Google Scholar]
- 85. Nitta M., Tamakawa T., Kamimura N., Honda T., Endoh H. (2020) A case of diffuse alveolar hemorrhage following synthetic cathinone inhalation. World Journal of Emergency Medicine, 11, 182–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fujita Y., Mita T., Usui K., Kamijo Y., Kikuchi S., Onodera M., et al. (2018) Toxicokinetics of the synthetic cathinone α-pyrrolidinohexanophenone. Journal of Analytical Toxicology, 42, e1–e5. [DOI] [PubMed] [Google Scholar]
- 87. Krotulski A.J., Mohr A.L.A., Papsun D.M., Logan B.K. (2018) Dibutylone (bk-DMBDB): intoxications, quantitative confirmations and metabolism in authentic biological specimens. Journal of Analytical Toxicology, 42, 437–445. [DOI] [PubMed] [Google Scholar]
- 88. Lelievre B., Richeval C., Coulon A., Iwanikow D., Brofferio M., Deguigne M., et al. (2019) Case report of a drug-related death following the use of cathinone derivatives (MPHP and 4-MEAP). Toxicologie Analytique et Clinique, 31, S43. [Google Scholar]
- 89. Domagalska E., Banaszkiewicz L., Woźniak M.K., Kata M., Szpiech B., Kaliszan M. (2020) Fatal N-Ethylhexedrone Intoxication. Journal of Analytical Toxicology, 45, e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mikołajczyk A., Adamowicz P., Tokarczyk B., Sekuła K., Gieroń J., Wrzesień W., et al. (2017) Determination of N-ethylhexedrone, a new cathinone derivative, in blood collected from drivers – analysis of three cases. Problems in Forensic Sciences, 109, 53–63. [Google Scholar]
- 91. Ling D.-A., Weng T.-I., Chen J.-Y., Chen G.-Y., Hwa H.-L., Fang C.-C. (2020) Clinical manifestation and quantitative urinary analysis of N-ethylnorpentylone abuse in patients to the emergency department. Clinical Toxicology (Philadelphia, Pa.), 58, 935–937. [DOI] [PubMed] [Google Scholar]
- 92. Gallo M., Giampreti A., Butera R., Eleftheriou G., Faraoni L., Bedussi F., et al. (2018) Acute psychosis after repeated use of N-ethyl pentylone (Ephylone) sold as cocaine. Clinical Toxicology (Philadelphia, Pa.), 56, 912–1092. [Google Scholar]
- 93. Serre A., Vuillot O., Eiden C., Gambier J., Berger A., Mathieu O., et al. (2019) Acute psychiatric disorders related to fake cathinone: ephylone. Journal of Analytical Toxicology, 43, e1–e2. [DOI] [PubMed] [Google Scholar]
- 94. Costa J.L., Cunha K.F., Lanaro R., Cunha R.L., Walther D., Baumann M.H. (2019) Analytical quantification, intoxication case series, and pharmacological mechanism of action for N-ethylnorpentylone (N-ethylpentylone or ephylone). Drug Testing and Analysis, 11, 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Challman T.D., Lipsky J.J. (2000) Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings, 75, 711–721. [DOI] [PubMed] [Google Scholar]
- 96. La Maida N., Di Trana A., Giorgetti R., Tagliabracci A., Busardò F.P., Huestis M.A. (2021) A review of synthetic cathinone-related fatalities from 2017 to 2020. Therapeutic Drug Monitoring, 43, 52–68. [DOI] [PubMed] [Google Scholar]
- 97. Casale J.F., Hays P.A. (2011) Ethylphenidate: an analytical profile. Microgram Journal, 8, 58–61. [Google Scholar]
- 98. Markowitz J.S., DeVane C.L., Boulton D.W., Nahas Z., Risch S.C., Diamond F., et al. (2000) Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol. Drug Metabolism and Disposition, 28, 620–624. [PubMed] [Google Scholar]
- 99. Soussan C., Kjellgren A. (2017) ‘Chasing the High’ – experiences of ethylphenidate as described on international internet forums. Substance Abuse: Research and Treatment, 9, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rojek S., Kłys M., Strona M., Maciów M., Kula K. (2012) ‘Legal highs’—toxicity in the clinical and medico-legal aspect as exemplified by suicide with bk-MBDB administration. Forensic Science International, 222, e1–6. [DOI] [PubMed] [Google Scholar]
- 101. Zsedényi C.K., Zachar G., Csillag A., Ádám Á. (2014) Effect of synthetic cathinones: mephedrone, butylone and 3,4 methylene-dioxypyrovalerone (MDPV) on social separation induced distress vocalization, vigilance and postural control of young domestic chicks. Neuroscience Letters, 580, 88–93. [DOI] [PubMed] [Google Scholar]
- 102. Expert Committee on Drug Dependence: World Health Organization . (2018) Critical Review Report: N-Ethylnorpentylone. https://www.who.int/medicines/access/controlled-substances/N-Ethylnorpentylone.pdf (Jan 20, 2021).
- 103. Krotulski A.J., Papsun D.M., De Martinis B.S., Mohr A.L.A., Logan B.K. (2018) N-ethyl pentylone (ephylone) intoxications: quantitative confirmation and metabolite identification in authentic human biological specimens. Journal of Analytical Toxicology, 42, 467–475. [DOI] [PubMed] [Google Scholar]
- 104. European Monitoring Centre for Drugs and Drug Addiction and Europol . (2019) EU Drug Markets Report 2019. Publications Office of European Union, Luxembourg. https://www.emcdda.europa.eu/publications/joint-publications/eu-drug-markets-report-2019_en (Mar 6, 2022). [Google Scholar]
- 105. Krotulski A.J., Mohr A.L.A., Fogarty M.F., Logan B.K. (2018) The detection of novel stimulants in oral fluid from users reporting ecstasy, Molly and MDMA ingestion. Journal of Analytical Toxicology, 42, 544–553. [DOI] [PubMed] [Google Scholar]
- 106. NIDA . (2019) Hallucinogens DrugFacts. https://www.drugabuse.gov/publications/drugfacts/hallucinogens (Dec 20, 2020).
- 107. Iwersen-Bergmann S., Lehmann S., Heinemann A., Schröder C., Müller A., et al. (2019) Mass poisoning with NPS: 2C-E and Bromo-DragonFly. International Journal of Legal Medicine, 133, 123–129. [DOI] [PubMed] [Google Scholar]
- 108. Weng T.-I., Chin L.W., Chen L.-Y., Chen J.-Y., Chen G.-Y., Fang C.-C. (2021) Clinical characteristics of patients admitted to emergency department for the use of ketamine analogues with or without other new psychoactive substances. Clinical Toxicology, 59, 528–531. [DOI] [PubMed] [Google Scholar]
- 109. Tang M.H.Y., Li T.C., Lai C.K., Chong Y.K., Ching C.K., Mak T.W.L. (2020) Emergence of new psychoactive substance 2-fluorodeschloroketamine: toxicology and urinary analysis in a cluster of patients exposed to ketamine and multiple analogues. Forensic Science International, 312, 110327. [DOI] [PubMed] [Google Scholar]
- 110. Davidsen A.B., Mardal M., Holm N.B., Andreasen A.K., Johansen S.S., Noble C., et al. (2020) Ketamine analogues: comparative toxicokinetic in vitro–in vivo extrapolation and quantification of 2-fluorodeschloroketamine in forensic blood and hair samples. Journal of Pharmaceutical and Biomedical Analysis, 180, 113049. [DOI] [PubMed] [Google Scholar]
- 111. Theofel N., Möller P., Vejmelka E., Kastner K., Roscher S., Scholtis S., et al. (2019) A fatal case involving N-ethyldeschloroketamine (2-Oxo-PCE) and venlafaxine. Journal of Analytical Toxicology, 43, e2–e6. [DOI] [PubMed] [Google Scholar]
- 112. Tang M.H.Y., Chong Y.K., Chan C.Y., Ching C.K., Lai C.K., Li Y.K., et al. (2018) Cluster of acute poisonings associated with an emerging ketamine analogue, 2-oxo-PCE. Forensic Science International, 290, 238–243. [DOI] [PubMed] [Google Scholar]
- 113. Dunlop L.C., Wood D., Archer J., Hudson S., Dargan P. (2020) Severe toxicity to the new psychoactive substances 3-hydroxyphencyclidine and N-ethylhexedrone: an analytically confirmed case report. Journal of Medical Toxicology, 16, 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bertol E., Pascali J., Palumbo D., Catalani V., Di Milia M.G., Fioravanti A., et al. (2017) 3-MeO-PCP intoxication in two young men: first in vivo detection in Italy. Forensic Science International, 274, 7–12. [DOI] [PubMed] [Google Scholar]
- 115. Chang B.N., Smith M.P. (2017) A case of unusual drug screening results. Clinical Chemistry, 63, 958–961. [DOI] [PubMed] [Google Scholar]
- 116. Grossenbacher F., Cazaubon Y., Feliu C., Ameline A., Kintz P., Passouant O., et al. (2019) About 5 cases with 3 MeO-PCP including 2 deaths and 3 non-fatal cases seen in France in 2018. Toxicologie Analytique Et Clinique, 31, 332–336. [Google Scholar]
- 117. Johansson A., Lindstedt D., Roman M., Thelander G., Nielsen E.I., Lennborn U., et al. (2017) A non-fatal intoxication and seven deaths involving the dissociative drug 3-MeO-PCP. Forensic Science International, 275, 76–82. [DOI] [PubMed] [Google Scholar]
- 118. van den Bersselaar L.R., van der Hoeven J.G., de Jong B. (2021) Feeling death, being alive: 4-methylethcathinone/pentedrone addiction and 3-methoxyphencyclidine intoxication. Addictive Disorders & Their Treatment, 20, 69–73. [Google Scholar]
- 119. Berar A., Allain J.-S., Allard S., Lefevre C., Baert A., Morel I., et al. (2019) Intoxication with 3-MeO-PCP alone. Medicine, 98, e18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ameline A., Greney H., Monassier L., Raul J., Kintz P. (2019) Metabolites to parent 3-MeO-PCP ratio in human urine collected in two fatal cases. Journal of Analytical Toxicology, 43, 321–324. [DOI] [PubMed] [Google Scholar]
- 121. Kintz P., Ameline A., Walch A., Farrugia A., Raul J.-S. (2019) Murdered while under the influence of 3-MeO-PCP. International Journal of Legal Medicine, 133, 475–478. [DOI] [PubMed] [Google Scholar]
- 122. de Jong L.A.A., Olyslager E.J.H., Duijst W.L.J.M. (2019) The risk of emerging new psychoactive substances: the first fatal 3-MeO-PCP intoxication in The Netherlands. Journal of Forensic and Legal Medicine, 65, 101–104. [DOI] [PubMed] [Google Scholar]
- 123. Grossenbacher F., Cazaubon Y., Colot P.-E., Passouant O., Marty H., Djerada Z., et al. (2019) Three clinical cases-reports in France with 3-MeO-PCP in 2018. The ‘Angel Dust’, baby it is here! Toxicologie Analytique Et Clinique, 31, S40–S41. [Google Scholar]
- 124. Zidkova M., Hlozek T., Balik M., Kopecky O., Tesinsky P., Svanda J., et al. (2017) Two cases of non-fatal intoxication with a novel street hallucinogen: 3-methoxy-phencyclidine. Journal of Analytical Toxicology, 41, 350–354. [DOI] [PubMed] [Google Scholar]
- 125. Mitchell-Mata C., Thomas B., Peterson B., Couper F. (2017) Two fatal intoxications involving 3-methoxyphencyclidine. Journal of Analytical Toxicology, 41, 503–507. [DOI] [PubMed] [Google Scholar]
- 126. Yan X., Yuan S., Yu Z., Zhao Y., Zhang S., Wu H., et al. (2020) Development of an LC-MS/MS method for determining 5-MeO-DIPT in dried urine spots and application to forensic cases. Journal of Forensic and Legal Medicine, 72, 101963. [DOI] [PubMed] [Google Scholar]
- 127. Grafinger K.E., Hädener M., König S., Weinmann W. (2018) Study of the in vitro and in vivo metabolism of the tryptamine 5-MeO-MiPT using human liver microsomes and real case samples. Drug Testing and Analysis, 10, 562–574. [DOI] [PubMed] [Google Scholar]
- 128. Al-Imam A. (2018) 25b-NBOMe: a case report of sudden death and insightful view of Google Trends data. Iranian Journal of Psychiatry and Behavioral Sciences, 12, e9870. [Google Scholar]
- 129. Wiergowski M., Aszyk J., Kaliszan M., Wilczewska K., Anand J.S., Kot-Wasik A., et al. (2017) Identification of novel psychoactive substances 25B-NBOMe and 4-CMC in biological material using HPLC-Q-TOF-MS and their quantification in blood using UPLC–MS/MS in case of severe intoxications. Journal of Chromatography B, 1041–1042, 1–10. [DOI] [PubMed] [Google Scholar]
- 130. Zygowiec J., Solomon S., Jaworski A., Bloome M., Gotlib A. (2017) 25C-NBOMe Ingestion. Clinical Practice and Cases in Emergency Medicine, 1, 295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morini L., Bernini M., Vezzoli S., Restori M., Moretti M., Crenna S., et al. (2017) Death after 25C-NBOMe and 25H-NBOMe consumption. Forensic Science International, 279, e1–e6. [DOI] [PubMed] [Google Scholar]
- 132. Hermanns-Clausen M., Angerer V., Kithinji J., Grumann C., Auwärter V. (2017) Bad trip due to 25I-NBOMe: a case report from the EU project SPICE II plus. Clinical Toxicology, 55, 922–924. [DOI] [PubMed] [Google Scholar]
- 133. Ameline A., Kintz P., Blettner C., Bayle É., Raul J.-S. (2017) Identification of 25I-NBOMe in two intoxications cases with severe hallucinations. Toxicologie Analytique Et Clinique, 29, 117–122. [Google Scholar]
- 134. Ameline A., Farrugia A., Raul J.-S., Kintz P. (2017) Retrospective demonstration of 25I-NBOMe acute poisoning using hair analysis. Current Pharmaceutical Biotechnology, 18, 786–790. [DOI] [PubMed] [Google Scholar]
- 135. Richeval C., Boucher A., Humbert L., Phanithavong M., Wiart J.-F., Moulsma M., et al. (2017) Retrospective identification of 25I-NBOMe metabolites in an intoxication case. Toxicologie Analytique Et Clinique, 29, 71–81. [Google Scholar]
- 136. Thornton S.L., Hoehn S., Gerona R.R. (2018) Seizures, systemic inflammatory response, and rhabdomyolysis associated with laboratory-confirmed 2C-I and 25-I exposure. Pediatric Emergency Care, 34, e181. [DOI] [PubMed] [Google Scholar]
- 137. Waldman W., Kała M., Lechowicz W., Gil D., Anand J.S. (2018) Severe clinical toxicity caused by 25I-NBOMe confirmed analytically using LC-MS-MS method. Acta Biochimica Polonica, 65, 567–571. [DOI] [PubMed] [Google Scholar]
- 138. Blumenberg A., Hendrickson R.G. (2020) A letter reporting a case of fatal ventricular dysrhythmia associated with the LSD analog AL-LAD. Clinical Toxicology, 58, 143–145. [DOI] [PubMed] [Google Scholar]
- 139. Gerace E., Bovetto E., Corcia D.D., Vincenti M., Salomone A. (2017) A case of nonfatal intoxication associated with the recreational use of diphenidine. Journal of Forensic Sciences, 62, 1107–1111. [DOI] [PubMed] [Google Scholar]
- 140. Kinoshita H., Tanaka N., Takakura A., Abe H., Kumihashi M., Shibayama T., et al. (2017) An autopsy case of death by combined use of benzodiazepines and diphenidine. Soudni Lekarstvi, 62, 40–43. [PubMed] [Google Scholar]
- 141. Kusano M., Zaitsu K., Taki K., Hisatsune K., Nakajima J., Moriyasu T., et al. (2018) Fatal intoxication by 5F-ADB and diphenidine: detection, quantification, and investigation of their main metabolic pathways in humans by LC/MS/MS and LC/Q-TOFMS. Drug Testing and Analysis, 10, 284–293. [DOI] [PubMed] [Google Scholar]
- 142. Abbara C., Ferec S., Leroux G., Bretaudeau-Deguigne M., Lelievre B., Boels D., et al. (2017) 2,5-Dimethoxy-4-chloroamphetamine, a LSD-like designer drug: clinical and analytical documentation of non-fatal exposure in five patients. Toxicologie Analytique Et Clinique, 29, 82–89. [Google Scholar]
- 143. Aknouche F., Ameline A., Kernalleguen A., Arbouche N., Maruejouls C., Kintz P. (2020) Toxicological investigations in a death involving 2,5-dimethoxy-4-chloamphetamine (DOC) performed on an exhumed body. Journal of Analytical Toxicology, 45, e1–e7. [DOI] [PubMed] [Google Scholar]
- 144. Champeau W., Eiden C., Gambier J., Peyriere H. (2017) Methoxphenidine use disorder: first case notified to the French Addictovigilance Network. Journal of Clinical Psychopharmacology, 37, 376–377. [DOI] [PubMed] [Google Scholar]
- 145. Chrétien B., Bourgine J., Hamel Sénécal L., Bretaudeau-Deguigne M., Boels D., Lelong-Boulouard V., et al. (2018) Severe serotonin syndrome in an autistic new psychoactive substance user after consumption of pills containing methoxphenidine and α-methyltryptamine. Journal of Clinical Psychopharmacology, 38, 94–96. [DOI] [PubMed] [Google Scholar]
- 146. Goncalves R., Castaing N., Loquet A., Richeval C., Molimard M., Titier K. (2020) Identification and analytical characterization of recently emerged methoxpropamine (MXPr) in product and human urine samples. Toxicologie Analytique Et Clinique, 32, 249–250. [Google Scholar]
- 147. Drug Enforcement Administration . (2016) Controlled Substances. 2016. https://www.deadiversion.usdoj.gov/schedules/orangebook/c_cs_alpha.pdf (Nov 12, 2016).
- 148. Roth B.L., Gibbons S., Arunotayanun W., Huang X.-P., Setola V., Treble R., et al. (2013) The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate NMDA receptor. PLoS One, 8, e59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wallach J., Kang H., Colestock T., Morris H., Bortolotto Z.A., Collingridge G.L., et al. (2016) Pharmacological investigations of the dissociative ‘legal highs’ diphenidine, methoxphenidine and analogues. PLoS One, 11, e0157021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Morris H., Wallach J. (2014) From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs: PCP to MXE. Drug Testing and Analysis, 6, 614–632. [DOI] [PubMed] [Google Scholar]
- 151. Davidsen A.B., Mardal M., Johansen S.S., Dalsgaard P.W., Linnet K. (2020) In vitro and in vivo metabolism and detection of 3-HO-PCP, a synthetic phencyclidine, in human samples and pooled human hepatocytes using high resolution mass spectrometry. Drug Testing and Analysis, 12, 987–993. [DOI] [PubMed] [Google Scholar]
- 152. Kamenka J.M., Chiche B., Goudal R., Geneste P., Vignon J., Vincent J.P., et al. (1982) Chemical synthesis and molecular pharmacology of hydroxylated 1-(1-phenylcyclohexyl)piperidine derivatives. Journal of Medicinal Chemistry, 25, 431–435. [DOI] [PubMed] [Google Scholar]
- 153. World Health Organization: Expert Committee on Drug Dependence . (2014) 25I-NBOMe: Critical Review Report. http://www.who.int/medicines/areas/quality_safety/4_19_review.pdf (Nov 12, 2016).
- 154. World Health Organization: Expert Committee on Drug Dependence . (2014) 25B-NBOMe: Critical Review Report. http://www.who.int/medicines/areas/quality_safety/4_17_review.pdf (Nov 12, 2016).
- 155. World Health Organization: Expert Committee on Drug Dependence . (2014) 25C-NBOMe: Critical Review Report. https://legal-high-inhaltsstoffe.de/sites/default/files/uploads/25c-nbome.pdf (Nov 12, 2016).
- 156. Rickli A., Moning O.D., Hoener M.C., Liechti M.E. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. European Neuropsychopharmacology, 26, 1327–1337. [DOI] [PubMed] [Google Scholar]
- 157. Sogawa C., Sogawa N., Tagawa J., Fujino A., Ohyama K., Asanuma M., et al. (2007) 5-Methoxy-N,N-diisopropyltryptamine (Foxy), a selective and high affinity inhibitor of serotonin transporter. Toxicology Letters, 170, 75–82. [DOI] [PubMed] [Google Scholar]
- 158. Fantegrossi W.E., Harrington A.W., Kiessel C.L., Eckler J.R., Rabin R.A., Winter J.C., et al. (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacology, Biochemistry, and Behavior, 83, 122–129. [DOI] [PubMed] [Google Scholar]
- 159. Bäckberg M., Beck O., Helander A. (2015) Phencyclidine analog use in Sweden—intoxication cases involving 3-MeO-PCP and 4-MeO-PCP from the STRIDA project. Clinical Toxicology, 53, 856–864. [DOI] [PubMed] [Google Scholar]
- 160. EMCDDA . (2014) Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. http://www.emcdda.europa.eu/attachements.cfm/att_240380_EN_TDAN15001ENN.pdf (Nov 14, 2016).
- 161. European Monitoring Centre for Drugs and Drug Addiction . (2016) EMCDDA–Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA. https://www.emcdda.europa.eu/system/files/publications/4724/TDAN17001ENN_PDFWEB.pdf (Mar 6, 2022).
- 162. Krotulski A.J., Logan B. (2018) 2F-Deschloroketamine. https://www.forensicscienceeducation.org/wp-content/uploads/2018/09/2F-Deschloroketamine_090418_ToxicologyAnalyticalReport.pdf (Dec 20, 2020).
- 163. European Monitoring Centre for Drugs and Drug Addiction . (2015) EMCDDA–Europol 2015 Annual Report on the implementation of Council Decision 2005/387/JHA. https://www.emcdda.europa.eu/system/files/publications/2880/TDAS16001ENN.pdf (Mar 6, 2022).
- 164. Drug Enforcement Administration . (2020) 5-METHOXY-N,N-DIISOPROPYLTRYPTAMINE (Street Names: Foxy, or Foxy methoxy). https://www.deadiversion.usdoj.gov/drug_chem_info/5meodipt.pdf (Mar 6, 2022).
- 165. D’Orazio A.L., Scott K.S., Mohr A.L.A., Logan B.K. (2016) 2016 Survey: Updates for Recommendations for Drug Testing in DUID &Traffic Fatality Investigations. https://www.forensicscienceeducation.org/wp-content/uploads/2016/04/Full-Survey-Report.pdf (Nov 16, 2016).
- 166. D’Orazio A.L., Mohr A.L.A., Logan B.K. (2020) 2020 Survey: Updates for Recommendations for Drug Testing in DUID & Traffic Fatality Investigations. https://www.forensicscienceeducation.org/wp-content/uploads/2020/07/Survey-Report-Final.pdf (Dec 21, 2020).
- 167. AAFS Standards Board . (2021) Standard for the analytical scope and sensitivity of forensic toxicological testing of blood in medicolegal death Investigations. 2021. http://www.asbstandardsboard.org/wp-content/uploads/2021/08/119_Std_e1.pdf (Dec 21, 2020).
- 168. AAFS Standards Board . (2021) Standard for the Analytical Scope and Sensitivity of Forensic Toxicological Testing of Blood in Impaired Driving Investigations. 2021. http://www.asbstandardsboard.org/wp-content/uploads/2021/08/119_Std_e1.pdf (Dec 21, 2020).
- 169. Bakota E., Arndt C., Romoser A.A., Wilson S.K. (2016) Fatal intoxication involving 3-MeO-PCP: a case report and validated method. Journal of Analytical Toxicology, 40, 504–510. [DOI] [PubMed] [Google Scholar]
- 170. McIntyre I.M., Trochta A., Gary R.D., Storey A., Corneal J., Schaber B. (2015) A fatality related to two novel hallucinogenic compounds: 4-methoxyphencyclidine and 4-hydroxy-N-methyl-N-ethyltryptamine. Journal of Analytical Toxicology, 39, 751–755. [DOI] [PubMed] [Google Scholar]
- 171. European Monitoring Centre for Drugs and Drug Addiction . (2020) European Drug Report 2020: Key Issues. Publications Office of European Union, Luxembourg. https://www.emcdda.europa.eu/system/files/publications/13238/TD0420439ENN.pdf (Mar 6, 2022). [Google Scholar]
- 172. Taylor P. (2020) New substance found in counterfeit Xanax in Canada. October 5, 2020. https://www.securingindustry.com/pharmaceuticals/new-substance-found-in-counterfeit-xanax-in-canada/s40/a12376/ (Dec 16, 2020).
- 173. Blakey K. (2020) Designer Benzodiazepines: What’s In Fake ‘Xanax’? November 2020. https://novelpsychoactivesubstances.eu/npsonline/portfolio-items/designer-benzodiazepines-whats-in-fake-xanax-k-blakey-a-thompson-a-griffiths/ (Nov 18, 2020).
- 174. Kolbe V., Rentsch D., Boy D., Schmidt B., Kegler R., Büttner A. (2020) The adulterated XANAX pill: a fatal intoxication with etizolam and caffeine. International Journal of Legal Medicine, 134, 1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. McKenna J. (2020) Flualprazolam: new psychoactive substance sends six Oregon teens to ER. Physician’s Weekly, 2020. https://www.physiciansweekly.com/flualprazolam-new-psychoactive-substance-sends-six-oregon-teens-to-er/ (Nov 25, 2020).
- 176. Fake Valium is killing those who buy pills online, charity warns . (2019) The Guardian, February 3, 2019. http://www.theguardian.com/society/2019/feb/03/fake-valium-increase-drugs-deaths (Mar 7, 2021).
- 177. Manchester K.R., Lomas E.C., Waters L., Dempsey F.C., Maskell P.D. (2018) The emergence of new psychoactive substance (NPS) benzodiazepines: a review. Drug Testing and Analysis, 10, 392–393. [DOI] [PubMed] [Google Scholar]
- 178. Griffin C.E., Kaye A.M., Bueno F.R., Kaye A.D. (2013) Benzodiazepine pharmacology and central nervous system–mediated effects. The Ochsner Journal, 13, 214–223. [PMC free article] [PubMed] [Google Scholar]
- 179. Blumenberg A., Hughes A., Reckers A., Ellison R., Gerona R. (2020) Flualprazolam: report of an outbreak of a new psychoactive substance in adolescents. Pediatrics, 146, e20192953. 10.1542/peds.2019-2953. [DOI] [PubMed] [Google Scholar]
- 180. Kriikku P., Rasanen I., Ojanperä I., Thelander G., Kronstrand R., Vikingsson S. (2020) Femoral blood concentrations of flualprazolam in 33 postmortem cases. Forensic Science International, 307, 110101. [DOI] [PubMed] [Google Scholar]
- 181. Papsun D.M., Krotulski A.J., Homan J., Temporal K.D.H., Logan B.K. (2021) Flualprazolam blood concentrations in 197 forensic investigation cases. Journal of Analytical Toxicology 45, 226–232. [DOI] [PubMed] [Google Scholar]
- 182. Rice K., Hikin L., Lawson A., Smith P.R., Morley S. (2021) Quantification of flualprazolam in blood by LC–MS-MS: a case series of nine deaths. Journal of Analytical Toxicology, 45, 410–416. [DOI] [PubMed] [Google Scholar]
- 183. Papsun D.M., Triebold C. Emerging drug: flualprazolam. Toxtalk (2019), 43, 25. [Google Scholar]
- 184. Heide G., Høiseth G., Middelkoop G., Øiestad Å.M.L. (2020) Blood concentrations of designer benzodiazepines: relation to impairment and findings in forensic cases. Journal of Analytical Toxicology, 44, 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Partridge E., Trobbiani S., Stockham P., Charlwood C., Kostakis C. (2018) A case study involving U-47700, diclazepam and flubromazepam—application of retrospective analysis of HRMS data. Journal of Analytical Toxicology, 42, 655–660. [DOI] [PubMed] [Google Scholar]
- 186. Rohrig T.P., Osawa K.A., Baird T.R., Youso K.B. (2021) Driving impairment cases involving etizolam and flubromazolam. Journal of Analytical Toxicology, 45, 93–98. [DOI] [PubMed] [Google Scholar]
- 187. Gevorkyan J., Kinyua J., Pearring S., Rodda L.N. (2021) A case series of etizolam in opioid-related deaths. Journal of Analytical Toxicology, 45, e4–e17. [DOI] [PubMed] [Google Scholar]
- 188. Hester J.B. Jr (1976) 6-Phenyl-4H-S-Triazolo[4,3-a][1,4] Benzodiazepines. Oct 19, 1976. [DOI] [PubMed]
- 189. Zawilska J.B., Wojcieszak J. (2019) An expanding world of new psychoactive substances—designer benzodiazepines. NeuroToxicology, 73, 8–16. [DOI] [PubMed] [Google Scholar]
- 190. Ntoupa P.-S.A., Papoutsis I.I., Dona A.A., Spiliopoulou C.A., Athanaselis S.A. (2021) A fluorine turns a medicinal benzodiazepine into NPS: the case of flualprazolam. Forensic Toxicology, 39, 368–376. [Google Scholar]
- 191. Krotulski A.J., Logan B.K. (2019) Flualprazolam. https://www.npsdiscovery.org/wp-content/uploads/2019/06/Flualprazolam_062519_ToxicologyAnalyticalReport.pdf (Nov 23, 2020).
- 192. World Health Organization . (2019) Critical review report: flualprazolam. 42nd Expert Committee on Drug Dependence.
- 193. Krotulski A.J., Papsun D.M., Homan J., Nelson L.S., Logan B.K. (2019) Flualprazolam: Potent Benzodiazepine Identified Among Death and Impaired Driving Cases in the U.S. https://www.npsdiscovery.org/wp-content/uploads/2019/12/Public-Alert_Flualprazolam_NPS-Discovery_120519.pdf (Mar 7, 2021).
- 194. Drug Enforcement Administration . (2020) Flualprazolam. Drug and Chemical Evaluation Section, Office of Diversion Control. https://www.deadiversion.usdoj.gov/drug_chem_info/flualp.pdf (Mar 6, 2022).
- 195. Mei V., Concheiro M., Pardi J., Cooper G. (2019) Validation of an LC-MS/MS method for the quantification of 13 designer benzodiazepines in blood. Journal of Analytical Toxicology, 43, 688–695. [DOI] [PubMed] [Google Scholar]
- 196. European Monitoring Centre for Drugs and Drug Addiction . (2018) The Misuse of Benzodiazepines Among High-Risk Opioid Users in Europe. https://www.emcdda.europa.eu/system/files/publications/2733/Misuse%20of%20benzos_POD2015.pdf (Nov 23, 2020).
- 197. News: December . 2019 – WHO: World Health Organization Recommends 12 NPS for Scheduling. https://www.unodc.org/LSS/Announcement/Details/021820a0-8746-42a4-9ee3-47ce50b30ca3 (Mar 7, 2021).
- 198. Helander A., Bäckberg M. (2017) New Psychoactive Substances (NPS) - the Hydra monster of recreational drugs. Clinical Toxicology (Philadelphia, Pa.), 55, 1–3. [DOI] [PubMed] [Google Scholar]
- 199. Helander A., Bäckberg M., Signell P., Beck O. (2017) Intoxications involving acrylfentanyl and other novel designer fentanyls - results from the Swedish STRIDA project. Clinical Toxicology (Philadelphia, PA), 55, 589–599. [DOI] [PubMed] [Google Scholar]
- 200. Sofalvi S., Schueler H.E., Lavins E.S., Kaspar C.K., Brooker I.T., Mazzola C.D., et al. (2017) An LC-MS-MS method for the analysis of carfentanil, 3-methylfentanyl, 2-furanyl fentanyl, acetyl fentanyl, fentanyl and norfentanyl in postmortem and impaired-driving cases. Journal of Analytical Toxicology, 41, 473–483. [DOI] [PubMed] [Google Scholar]
- 201. Tiscione N.B., Alford I. (2018) Carfentanil in impaired driving cases and the importance of drug seizure data. Journal of Analytical Toxicology, 42, 476–484. [DOI] [PubMed] [Google Scholar]
- 202. Ciccarone D. (2019) The triple wave epidemic: supply and demand drivers of the US opioid overdose crisis. International Journal of Drug Policy, 71, 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jones C.M., Bekheet F., Park J.N., Alexander G.C. (2020) The evolving overdose epidemic: synthetic opioids and rising stimulant-related harms. Epidemiologic Reviews, 42, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. (2018) Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR. Morbidity and Mortality Weekly Report, 67, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. UNODC Opioid Strategy . (2020) Global SMART Update: The Growing Complexity of the Opioid Crisis. United Nations Office on Drugs and Crime. https://www.unodc.org/documents/scientific/Global_SMART-2020-Vol_24_web.pdf (Dec 6, 2020).
- 206. Jannetto P.J., Helander A., Garg U., Janis G.C., Goldberger B., Ketha H. (2019) The fentanyl epidemic and evolution of fentanyl analogs in the United States and the European Union. Clinical Chemistry, 65, 242–253. [DOI] [PubMed] [Google Scholar]
- 207. Krotulski A.J., Fogarty M.F., Logan B.K. (2019) Piperidylthiambutene. NMS Labs. https://www.npsdiscovery.org/wp-content/uploads/2019/09/Piperidylthiambutene_091819_NMSLabs_Report.pdf (Feb 13, 2021).
- 208. Krotulski A.J., Fogarty M.F., Logan B.K. (2019) 2F-Viminol. NMS Labs. https://www.npsdiscovery.org/wp-content/uploads/2019/12/2F-Viminol_120419_NMSLabs_Report.pdf (Feb 13, 2021).
- 209. Cannaert A., Hulpia F., Risseeuw M., Van Uytfanghe K., Deconinck E., Van Calenbergh S., et al. (2021) Report on a new opioid NPS: chemical and in vitro functional characterization of a structural isomer of the MT-45 derivative diphenpipenol. Journal of Analytical Toxicology, 45, 134–140. [DOI] [PubMed] [Google Scholar]
- 210. Brunetti P., Pirani F., Carlier J., Giorgetti R., Busardò F.P., Lo Faro A.F. (2021) A 2017–2019 update on acute intoxications and fatalities from illicit fentanyl and analogs. Journal of Analytical Toxicology, 45, 537–554. [DOI] [PubMed] [Google Scholar]
- 211. Concheiro M., Chesser R., Pardi J., Cooper G. (2018) Postmortem toxicology of new synthetic opioids. Frontiers in Pharmacology, 9, 1210.doi: 10.3389/fphar.2018.01210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Drummer O.H. (2018) Fatalities caused by novel opioids: a review. Forensic Sciences Research, 4, 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Tabarra I., Soares S., Rosado T., Gonçalves J., Luís Â., Malaca S., et al. (2019) Novel synthetic opioids – toxicological aspects and analysis. Forensic Sciences Research, 4, 111–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Solimini R., Pichini S., Pacifici R., Busardò F.P., Giorgetti R. (2018) Pharmacotoxicology of non-fentanyl derived new synthetic opioids. Frontiers in Pharmacology, 9, 654.doi: 10.3389/fphar.2018.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Pichini S., Solimini R., Berretta P., Pacifici R., Busardò F.P. (2018) Acute intoxications and fatalities from illicit fentanyl and analogues: an update. Therapeutic Drug Monitoring, 40, 38–51. [DOI] [PubMed] [Google Scholar]
- 216. Zawilska J.B. (2017) An expanding world of novel psychoactive substances: opioids. Frontiers in Psychiatry, 8, 110.doi: 10.3389/fpsyt.2017.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Armenian P., Vo K.T., Barr-Walker J., Lynch K.L. (2018) Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology, 134, 121–132. [DOI] [PubMed] [Google Scholar]
- 218. Giorgetti A., Centola C., Giorgetti R. (2017) Fentanyl novel derivative-related deaths. Human Psychopharmacology: Clinical and Experimental, 32, e2605. [DOI] [PubMed] [Google Scholar]
- 219. Suzuki J., El-Haddad S. (2017) A review: fentanyl and non-pharmaceutical fentanyls. Drug and Alcohol Dependence, 171, 107–116. [DOI] [PubMed] [Google Scholar]
- 220. Salle S., Bodeau S., Dhersin A., Ferdonnet M., Goncalves R., Lenski M., et al. (2019) Novel synthetic opioids: a review of the literature. Toxicologie Analytique Et Clinique, 31, 298–316. [Google Scholar]
- 221. Frisoni P., Bacchio E., Bilel S., Talarico A., Gaudio R.M., Barbieri M., et al. (2018) Novel synthetic opioids: the pathologist’s point of view. Brain Sciences, 8, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Schueler H.E. (2017) Emerging Synthetic Fentanyl Analogs. Academic Forensic Pathology, 7, 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Fogarty M.F., Papsun D.M., Logan B.K. (2018) Analysis of cis- and trans-3-methylfentanyl by liquid chromatography–high resolution mass spectrometry and findings in forensic toxicology casework. Drug Testing and Analysis, 10, 1474–1482. [DOI] [PubMed] [Google Scholar]
- 224. Uddayasankar U., Lee C., Oleschuk C., Eschun G., Ariano R.E. (2018) The pharmacokinetics and pharmacodynamics of carfentanil after recreational exposure: a case report. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 38, e41–e45. [DOI] [PubMed] [Google Scholar]
- 225. Allibe N., Fouilhe Sam-Lai N., Willeman T., Jourdil J.-F., Bartoli M., Mallaret M., et al. (2019) Norcarfentanil: carfentanil misuse or remifentanil treatment? Forensic Toxicology, 37, 488–495. [Google Scholar]
- 226. Franzén L., Beck O., Helander A. (2019) An analytically confirmed non-fatal intoxication by carfentanil in Sweden. Clinical Toxicology (Philadelphia, PA), 57, 372–374. [DOI] [PubMed] [Google Scholar]
- 227. Müller S., Nussbaumer S., Plitzko G., Ludwig R., Weinmann W., Krähenbühl S., et al. (2018) Recreational use of carfentanil - a case report with laboratory confirmation. Clinical Toxicology (Philadelphia, Pa.), 56, 151–152. [DOI] [PubMed] [Google Scholar]
- 228. Wilcoxon R.M., Middleton O.L., Meyers S.E., Kloss J., Love S.A. (2018) The elephant in the room: outbreak of carfentanil deaths in Minnesota and the importance of multiagency collaboration. Academic Forensic Pathology, 8, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Brockbals L., Staeheli S.N., Gentile S., Schlaepfer M., Bissig C., Bolliger S.A., et al. (2019) Fatal poisoning involving cyclopropylfentanyl — Investigation of time-dependent postmortem redistribution. Forensic Science International, 294, 80–85. [DOI] [PubMed] [Google Scholar]
- 230. Brede W.R., Krabseth H.-M., Michelsen L.S., Aarset H., Jamt J.-P., Slørdal L. (2019) A wolf in sheep’s clothing. Journal of Analytical Toxicology, 43, e7–e8. [DOI] [PubMed] [Google Scholar]
- 231. Wilde M., Sommer M.J., Auwärter V., Hermanns-Clausen M. (2020) Acute severe intoxication with cyclopropylfentanyl, a novel synthetic opioid. Toxicology Letters, 320, 109–112. [DOI] [PubMed] [Google Scholar]
- 232. Castellino C., Van Cleve D., Cabrera R. (2021) Two cyclopropyl fentanyl case studies in Los Angeles. Journal of Analytical Toxicology, 45, 105–109. [DOI] [PubMed] [Google Scholar]
- 233. Matey J.M., García-Ruíz C., Montalvo G., Gómez-Soro J.C., Gutierrez-Delicado D., Rodríguez-Gallardo J., et al. (2020) Ultraviolet-visible and high-resolution mass spectrometry for the identification of cyclopropyl-fentanyl in the first fatal case in Spain. Journal of Analytical Toxicology, 44, 927–935. [DOI] [PubMed] [Google Scholar]
- 234. Freni F., Pezzella S., Vignali C., Moretti M., Cisini S., Rossetti C., et al. (2019) A case report on potential postmortem redistribution of furanyl fentanyl and 4-ANPP. Forensic Science International, 304, 109915. [DOI] [PubMed] [Google Scholar]
- 235. Nash C., Butzbach D., Stockham P., Scott T., Abroe G., Painter B., et al. (2019) A fatality involving furanylfentanyl and MMMP, with presumptive identification of three MMMP metabolites in urine. Journal of Analytical Toxicology, 43, 291–298. [DOI] [PubMed] [Google Scholar]
- 236. Hendrickson R.G., Akpunonu P., Hughes A.R., Gerona R. (2019) Highly potent fentanyl analogs: apnea from exposure to small quantities of β-hydroxyfentanyl and furanylfentanyl. Clinical Toxicology (Philadelphia, PA), 57, 813–815. [DOI] [PubMed] [Google Scholar]
- 237. Garneau B., Desharnais B., Beauchamp-Doré A., Lavallée C., Mireault P., Lajeunesse A. (2020) Challenges related to three cases of fatal intoxication to multiple novel synthetic opioids. Journal of Analytical Toxicology, 44, 86–91. [DOI] [PubMed] [Google Scholar]
- 238. Adamowicz P., Bakhmut Z., Mikolajczyk A. (2020) Screening procedure for 38 fentanyl analogues and five other new opioids in whole blood by liquid chromatography-tandem mass spectrometry. Journal of Applied Toxicology, 40, 1033–1046. [DOI] [PubMed] [Google Scholar]
- 239. Fogarty M.F., Mohr A.L.A., Papsun D.M., Logan B.K. (2022) Analysis of the illicit opioid U-48800 and related compounds by LC–MS-MS and case series of fatalities involving U-48800. Journal of Analytical Toxicology, 46, 17–24. [DOI] [PubMed] [Google Scholar]
- 240. Mueller F., Bogdal C., Pfeiffer B., Andrello L., Ceschi A., Thomas A., et al. (2021) Isotonitazene: fatal intoxication in three cases involving this unreported novel psychoactive substance in Switzerland. Forensic Science International, 320, 110686. [DOI] [PubMed] [Google Scholar]
- 241. Verougstraete N., Vandeputte M.M., Lyphout C., Cannaert A., Hulpia F., Van Calenbergh S., et al. (2021) First report on brorphine: the next opioid on the deadly new psychoactive substances’ horizon? Journal of Analytical Toxicology, 44, 937–946. [DOI] [PubMed] [Google Scholar]
- 242. Vohra V., King A.M., Jacobs E., Aaron C. (2021) Death associated with brorphine, an emerging novel synthetic opioid. Clinical Toxicology (Philadelphia, Pa.), 59, 851–852.doi: 10.1080/15563650.2021.1879111 [DOI] [PubMed] [Google Scholar]
- 243. Goodnough R., Li K., Fouladkou F., Lynch K.L., Shah M., Smollin C.G., et al. (2018) Notes from the field: toxic leukoencephalopathy associated with tianeptine misuse - California, 2017. MMWR. Morbidity and Mortality Weekly Report, 67, 769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Dempsey S.K., Poklis J.L., Sweat K., Cumpston K., Wolf C.E. (2017) Acute toxicity from intravenous use of the tricyclic antidepressant tianeptine. Journal of Analytical Toxicology, 41, 547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Ketenci H.Ç., Çakır E., Beyhun N.E., Boz H., Okumuş H., Cingöz G., et al. (2018) death due to tianeptine injection through inguinal incision: two case reports. The Bulletin of Legal Medicine, 23, 123–128. [Google Scholar]
- 246. Bakota E.L., Samms W.C., Gray T.R., Oleske D.A., Hines M.O. (2018) Case reports of fatalities involving tianeptine in the United States. Journal of Analytical Toxicology, 42, 503–509. [DOI] [PubMed] [Google Scholar]
- 247. Ciccarone D., Ondocsin J., Mars S.G. (2017) Heroin uncertainties: exploring users’ perceptions of fentanyl-adulterated and -substituted ‘heroin’. The International Journal on Drug Policy, 46, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Klar S.A., Brodkin E., Gibson E., Padhi S., Predy C., Green C., et al. (2016) Furanyl-fentanyl overdose events caused by smoking contaminated crack cocaine — British Columbia, Canada, July 15–18, 2016. Health Promotion and Chronic Disease Prevention in Canada: Research, Policy and Practice, 36, 200–201. [Google Scholar]
- 249. Fogger S.A. (2019) Methamphetamine use: a new wave in the opioid crisis? Journal of Addictions Nursing, 30, 219–223. [DOI] [PubMed] [Google Scholar]
- 250. Han Y., Yan W., Zheng Y., Khan M.Z., Yuan K., Lu L. (2019) The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Translational Psychiatry, 9, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. LaRue L., Twillman R.K., Dawson E., Whitley P., Frasco M.A., Huskey A., et al. (2019) Rate of fentanyl positivity among urine drug test results positive for cocaine or methamphetamine. JAMA Network Open, 2, e192851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. DiSalvo P., Cooper G., Tsao J., Romeo M., Laskowski L.K., Chesney G., et al. (2021) Fentanyl-contaminated cocaine outbreak with laboratory confirmation in New York City in 2019. The American Journal of Emergency Medicine, 40, 103–105. [DOI] [PubMed] [Google Scholar]
- 253. UNODC Research . (2017) Global SMART update 2017. Fentanyl and its analogues - 50 years on. United Nations Office on Drugs and Crime, Vienna. https://www.drugsandalcohol.ie/26968/ (Dec 23, 2020). [Google Scholar]
- 254. Avedschmidt S., Schmidt C., Isenschmid D., Kesha K., Moons D., Gupta A. (2019) Acetyl fentanyl: trends and concentrations in metro Detroit. Journal of Forensic Sciences, 64, 149–153. [DOI] [PubMed] [Google Scholar]
- 255. Lovrecic B., Lovrecic M., Gabrovec B., Carli M., Pacini M., Maremmani A.G.I., et al. (2019) Non-medical use of novel synthetic opioids: a new challenge to public health. International Journal of Environmental Research and Public Health, 16, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Mars S.G., Rosenblum D., Ciccarone D. (2019) Illicit fentanyls in the opioid street market: desired or imposed? Addiction (Abingdon, England), 114, 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Goggin M.M., Gozum S.D., Miller A., Janis G.C. (2018) Anodyne by design; measuring the prevalence of esoteric designer opioids in pain management patients. Journal of Analytical Toxicology, 42, 384–391. [DOI] [PubMed] [Google Scholar]
- 258. Daniulaityte R., Carlson R.R., Juhascik M.P., Strayer K.E., Sizemore I.E., (2019) Street fentanyl use: experiences, preferences, and concordance between self-reports and urine toxicology. The International Journal on Drug Policy, 71, 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Baumann M.H., Majumdar S., Le Rouzic V., Hunkele A., Uprety R., Huang X.P., et al. (2018) Pharmacological characterization of novel synthetic opioids (NSO) found in the recreational drug marketplace. Neuropharmacology, 134, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Krotulski A.J., Chapman B.P., Marks S.J., Ontiveros S.T., Devin-Holcombe K., Fogarty M.F., et al. (2021) Sentanyl: a comparison of blood fentanyl concentrations and naloxone dosing after non-fatal overdose. Clinical Toxicology (Philadelphia, PA), 60, 197–204. [DOI] [PubMed] [Google Scholar]
- 261. Raheemullah A., Andruska N. (2019) Fentanyl analogue overdose: key lessons in management in the synthetic opioid age. Journal of Opioid Management, 15, 428–432. [DOI] [PubMed] [Google Scholar]
- 262. Baumann M.H., Pasternak G.W. (2018) Novel synthetic opioids and overdose deaths: tip of the iceberg? Neuropsychopharmacology, 43, 216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Huang X.-P., Che T., Mangano T.J., Le Rouzic V., Pan Y.-X., Majumdar S., et al. (2017) Fentanyl-related designer drugs W-18 and W-15 lack appreciable opioid activity in vitro and in vivo. JCI Insight, 2, e97222.doi: 10.1172/jci.insight.97222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Chen Z.R., Irvine R.J., Somogyi A.A., Bochner F. (1991) Mu receptor binding of some commonly used opioids and their metabolites. Life Sciences, 48, 2165–2171. [DOI] [PubMed] [Google Scholar]
- 265. NFLIS Drug Special Maps Release . Tracking Fentanyl and Fentanyl-Related Substances Reported by NFLIS-Drug by State 2016-2017. https://www.ncjrs.gov/App/Publications/abstract.aspx?ID=277928 (Dec 1, 2020).
- 266. US Department of Justice Drug Enforcement Administration Diversion Control Division . (2020) Special NFLIS-Drug Maps Release: Tracking Fentanyl and Fentanyl-Related Compounds Reported in NFLIS-Drug, by State: 2018–2019.
- 267. Moeller K., Svensson B. (2021) ‘Shop Until You Drop’: valuing fentanyl analogs on a Swedish internet forum. Journal of Drug Issues, 51, 181–195. [Google Scholar]
- 268. Fogarty M.F., Papsun D.M., Logan B.K. (2018) Analysis of fentanyl and 18 novel fentanyl analogs and metabolites by LC–MS-MS, and report of fatalities associated with methoxyacetylfentanyl and cyclopropylfentanyl. Journal of Analytical Toxicology, 42, 592–604. [DOI] [PubMed] [Google Scholar]
- 269. Busardò F.P., Carlier J., Giorgetti R., Tagliabracci A., Pacifici R., Gottardi M., et al. (2019) Ultra-high-performance liquid chromatography-tandem mass spectrometry assay for quantifying fentanyl and 22 analogs and metabolites in whole blood, urine, and hair. Frontiers in Chemistry, 7, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Lee J., Krotulski A.J., Fogarty M.F., Papsun D.M., Logan B.K. (2019) Chromatographic separation of the isobaric compounds cyclopropylfentanyl, crotonylfentanyl, methacrylfentanyl, and para-methylacrylfentanyl for specific confirmation by LC-MS/MS. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1118–1119, 164–170. [DOI] [PubMed] [Google Scholar]
- 271. Maher S., Elliott S.P., George S. (2018) The analytical challenges of cyclopropylfentanyl and crotonylfentanyl: an approach for toxicological analysis. Drug Testing and Analysis, 10, 1483–1487. [DOI] [PubMed] [Google Scholar]
- 272. Moody M.T., Diaz S., Shah P., Papsun D., Logan B.K. (2018) Analysis of fentanyl analogs and novel synthetic opioids in blood, serum/ plasma, and urine in forensic casework. Drug Testing and Analysis, 10, 1358–1367. [DOI] [PubMed] [Google Scholar]
- 273. Larabi I.A., Martin M., Etting I., Pfau G., Edel Y., Alvarez J.C. (2020) Development and validation of liquid chromatography-tandem mass spectrometry targeted screening of 16 fentanyl analogs and U-47700 in hair: application to 137 authentic samples. Drug Testing and Analysis, 12, 1298–1308. [DOI] [PubMed] [Google Scholar]
- 274. Mallette J.R., Casale J.F., Hays P.A. (2019) Characterization and differentiation of cyclopropylfentanyl from E-crotonylfentanyl, Z-crotonylfentanyl, and 3-butenylfentanyl. Science & Justice, 59, 67–74. [DOI] [PubMed] [Google Scholar]
- 275. Strayer K.E., Antonides H.M., Juhascik M.P., Daniulaityte R., Sizemore I.E., (2018) LC-MS/MS-based method for the multiplex detection of 24 fentanyl analogues and metabolites in whole blood at sub ng mL-1 concentrations. ACS Omega, 3, 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sofalvi S., Lavins E.S., Brooker I.T., Kaspar C.K., Kucmanic J., Mazzola C.D., et al. (2019) Unique structural/stereo-isomer and isobar analysis of novel fentanyl analogues in postmortem and DUID whole blood by UHPLC–MS-MS. Journal of Analytical Toxicology, 43, 673–687. [DOI] [PubMed] [Google Scholar]
- 277. Qin N., Xiang P., Shen B., Zhuo X., Shi Y., Song F. (2019) Application of a validated UHPLC-MS/MS method for 28 fentanyl-analogue and novel synthetic opioids in whole blood in authentic forensic cases. Journal of Chromatography B, 1124, 82–99. [DOI] [PubMed] [Google Scholar]
- 278. Zhang Y., Sheng Z., Hua Z., Liang C., Cai Z., Wang R., et al. (2020) Simultaneous separation and determination of 32 fentanyl-related substances, including seven sets of isomeric fentanyl analogues, by ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. Journal of Separation Science, 43, 3735–3747. [DOI] [PubMed] [Google Scholar]
- 279. Hassanien S.H., Bassman J.R., Naccarato C.M.P., Twarozynski J.J., Traynor J.R., Iula D.M., et al. (2020) In vitro pharmacology of fentanyl analogs at the human mu opioid receptor and their spectroscopic analysis. Drug Testing and Analysis, 12, 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Dwyer J.B., Janssen J., Luckasevic T.M., Williams K.E. (2018) Report of increasing overdose deaths that include acetyl fentanyl in multiple counties of the southwestern region of the commonwealth of Pennsylvania in 2015–2016. Journal of Forensic Sciences, 63, 195–200. [DOI] [PubMed] [Google Scholar]
- 281. Moss D.M., Brown D.H., Douglas B.J. (2019) An acetyl fentanyl death in Western Australia. Australian Journal of Forensic Sciences, 51, 73–77. [Google Scholar]
- 282. Vandeputte M.M., Krotulski A.J., Hulpia F., Van Calenbergh S., Stove C.P. (2022) Phenethyl-4-ANPP: a marginally active byproduct suggesting a switch in illicit fentanyl synthesis routes. Journal of Analytical Toxicology, 46, 350–357.doi: 10.1093/jat/bkab032 [DOI] [PubMed] [Google Scholar]
- 283. Krotulski A.J., Walton S.E., Fogarty M.F., Mohr A.L.A., Logan B.K. (2020) Bromofentanyl. NPS Discovery at CFSRE. https://www.npsdiscovery.org/wp-content/uploads/2020/12/Bromofentanyl_121720_CFSRE-Toxicology_Report.pdf (Feb 13, 2021).
- 284. Krotulski A.J., Logan B.K. (2019) FluoroFuranylfentanyl. Center for Forensic Science Research & Education. https://www.npsdiscovery.org/wp-content/uploads/2019/05/FluoroFuranylfentanyl_012319_ToxicologyAnalyticalReport.pdf (Feb 13, 2021).
- 285. Brandt S.D. (2018) Critical Review Report: p-Fluoro-butyrylfentanyl. World Health Organisation. http://www.who.int/medicines/access/controlled-substances/Parafluorobutyrfentanyl.pdf (Dec 5, 2020). [Google Scholar]
- 286. WHO (2019) Expert Committee on Drug Dependence: forty-first report. Geneva: World Health Organization; (WHO Technical Report Series, No. 1018). Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/bitstream/handle/10665/325073/9789241210270-eng.pdf?ua=1 (Mar 6, 2022).
- 287. Expert Committee on Drug Dependence . (2017) Critical Review Report: Acrylolyfentanyl. World Health Organization. https://www.who.int/medicines/access/controlled-substances/CriticalReview_Acrylolyfentanyl.pdf?ua=1 (Dec 6, 2020). [Google Scholar]
- 288. Expert Committee on Drug Dependence . (2017) Critical Review Report: Tetrahydrofuranylfentanyl (THF-F). World Health Organization. https://www.who.int/medicines/access/controlled-substances/CriticalReview_THFF.pdf (Dec 6, 2020). [Google Scholar]
- 289. Expert Committee on Drug Dependence . (2017) Critical_Review Report: Carfentanil. World Health Organization. https://www.who.int/medicines/access/controlled-substances/Critical_Review_Carfentanil.pdf (Dec 6, 2020). [Google Scholar]
- 290. Expert Committee on Drug Dependence . (2017) Critical Review Report: Furanyl Fentanyl. World Health Organization. https://www.who.int/medicines/access/controlled-substances/CriticalReview_FuranylFentanyl.pdf?ua=1 (Dec 6, 2020). [Google Scholar]
- 291. Breindahl T., Kimergård A., Andreasen M.F., Pedersen D.S. (2017) Identification of a new psychoactive substance in seized material: the synthetic opioid N-phenyl-N-[1-(2-phenethyl)piperidin-4-yl]prop-2-enamide (Acrylfentanyl). Drug Testing and Analysis, 9, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Degreef M., Blanckaert P., Berry E.M., van Nuijs A.L.N., Maudens K.E. (2019) Determination of ocfentanil and W-18 in a suspicious heroin-like powder in Belgium. Forensic Toxicology, 37, 474–479. [Google Scholar]
- 293. Liu C., Li T., Han Y., Hua Z., Jia W., Qian Z. (2018) The identification and analytical characterization of 2,2′-difluorofentanyl. Drug Testing and Analysis, 10, 774–780. [DOI] [PubMed] [Google Scholar]
- 294. Oldenhof S., Ten Pierick A., Bruinsma J., Eustace S., Hulshof J., van den Berg J., et al. (2020) Identification of a novel fentanyl analogue: p-Hydroxy-butyrylfentanyl. Drug Testing and Analysis, 12, 152–155. [DOI] [PubMed] [Google Scholar]
- 295. Richeval C., Baillieux M., Pawlak G., Phanithavong M., Wiart J., Humbert L., et al. (2019) Benzoylfentanyl and parafluorobutyrfentanyl: some analytical and metabolism data. Toxicologie Analytique Et Clinique, 31, 258–267. [Google Scholar]
- 296. Vincenti F., Pagano F., Montesano C., Sciubba F., Di Cocco M.E., Gregori A., et al. (2020) Multi-analytical characterization of 4-fluoro-furanyl fentanyl in a drug seizure. Forensic Chemistry, 21, 100283. [Google Scholar]
- 297. Rab E., Flanagan R.J., Hudson S. (2019) Detection of fentanyl and fentanyl analogues in biological samples using liquid chromatography-high resolution mass spectrometry. Forensic Science International, 300, 13–18. [DOI] [PubMed] [Google Scholar]
- 298. Gampfer T.M., Wagmann L., Richter M.J., Fischmann S., Westphal F., Meyer M.R. (2020) Toxicokinetic studies and analytical toxicology of the new synthetic opioids cyclopentanoyl-fentanyl and tetrahydrofuranoyl-fentanyl. Journal of Analytical Toxicology, 44, 449–460. [DOI] [PubMed] [Google Scholar]
- 299. Krotulski A.J., Papsun D.M., Friscia M., Swartz J.L., Holsey B.D., Logan B.K. (2018) Fatality following ingestion of tetrahydrofuranylfentanyl, U-49900 and methoxy-phencyclidine. Journal of Analytical Toxicology, 42, e27–e32. [DOI] [PubMed] [Google Scholar]
- 300. Shanks K.G., Behonick G.S. (2017) Detection of carfentanil by LC–MS-MS and reports of associated fatalities in the USA. Journal of Analytical Toxicology, 41, 466–472. [DOI] [PubMed] [Google Scholar]
- 301. Guerrieri D., Rapp E., Roman M., Druid H., Kronstrand R. (2017) Postmortem and toxicological findings in a series of furanylfentanyl-related deaths. Journal of Analytical Toxicology, 41, 242–249. [DOI] [PubMed] [Google Scholar]
- 302. Guerrieri D., Rapp E., Roman M., Thelander G., Kronstrand R. (2017) Acrylfentanyl: another new psychoactive drug with fatal consequences. Forensic Science International, 277, e21–e29. [DOI] [PubMed] [Google Scholar]
- 303. Butler D.C., Shanks K., Behonick G.S., Smith D., Presnell S.E., Tormos L.M. (2018) Three cases of fatal acrylfentanyl toxicity in the United States and a review of literature. Journal of Analytical Toxicology, 42, e6–e11. [DOI] [PubMed] [Google Scholar]
- 304. Helland A., Brede W.R., Michelsen L.S., Gundersen P.O.M., Aarset H., Skjølås J.E., et al. (2017) Two hospitalizations and one death after exposure to ortho-fluorofentanyl. Journal of Analytical Toxicology, 41, 708–709. [DOI] [PubMed] [Google Scholar]
- 305. Martucci H.F.H., Ingle E.A., Hunter M.D., Rodda L.N. (2018) Distribution of furanyl fentanyl and 4-ANPP in an accidental acute death: a case report. Forensic Science International, 283, e13–e17. [DOI] [PubMed] [Google Scholar]
- 306. Park J.N., Rashidi E., Foti K., Zoorob M., Sherman S., Alexander G.C. (2021) Fentanyl and fentanyl analogs in the illicit stimulant supply: results from U.S. drug seizure data, 2011–2016. Drug and Alcohol Dependence, 218, 108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. O’Donnell J.K., Halpin J., Mattson C., Goldberger B., Gladden R. (2017) Deaths involving fentanyl, fentanyl analogs, and U-47700 — 10 states, July–December 2016. MMWR. Morbidity and Mortality Weekly Report, 66, 1197–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Colon-Berezin C., Nolan M.L., Blachman-Forshay J., Paone D. (2019) Overdose deaths involving fentanyl and fentanyl analogs — New York City, 2000–2017. MMWR. Morbidity and Mortality Weekly Report, 68, 37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Butler D.C., Batalis N.I. (2017) Opioid-associated deaths in South Carolina, 2013-2016: a retrospective review. Academic Forensic Pathology, 7, 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Dai Z., Abate M.A., Smith G.S., Kraner J.C., Mock A.R. (2019) Fentanyl and fentanyl-analog involvement in drug-related deaths. Drug and Alcohol Dependence, 196, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Daniulaityte R., Juhascik M.P., Strayer K.E., Sizemore I.E., Zatreh M., Nahhas R.W., et al. (2019) Trends in fentanyl and fentanyl analogue-related overdose deaths – Montgomery County, Ohio, 2015–2017. Drug and Alcohol Dependence, 198, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Daniulaityte R., Juhascik M.P., Strayer K.E., Sizemore I.E., Harshbarger K.E., Antonides H.M., et al. (2017) Overdose deaths related to fentanyl and its analogs - Ohio, January-February 2017. MMWR. Morbidity and Mortality Weekly Report, 66, 904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Peterson B.L., Schreiber S., Fumo N., Lerner E.B. (2019) Opioid deaths in Milwaukee County, Wisconsin 2013-2017: the primacy of heroin and fentanyl. Journal of Forensic Sciences, 64, 144–148. [DOI] [PubMed] [Google Scholar]
- 314. Serinelli S., White S., Arunkumar P., Wang D., Gitto L. (2019) The outbreak of fentanyl-related deaths in Cook County, Illinois, between October 2015 and December 2017: a retrospective study and a comparison with previous data. Journal of Forensic Sciences, 64, 1735–1742. [DOI] [PubMed] [Google Scholar]
- 315. Zibbell J.E., Aldridge A., Cauchon D., DeFiore-Hymer J., Conway K. (2019) Association of law enforcement seizures of heroin, fentanyl, and carfentanil with opioid overdose deaths in Ohio, 2014-2017. JAMA, 2, e1914666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Fagiola M., Hahn T., Avella J. (2019) Five postmortem case reports with qualitative analysis of cyclopropylfentanyl by LC–MS-MS. Journal of Analytical Toxicology, 43, e1–e6. [DOI] [PubMed] [Google Scholar]
- 317. Claridge H., Williams B.D., Copeland C.S. (2020) A deadly trend in fentanyl fatalities (England, 1998–2017). British Journal of Clinical Pharmacology, 86, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. King A., Foley D., Arfken C., Aaron C., Sung L., Hlavaty L. (2019) Carfentanil-associated mortality in Wayne County, Michigan, 2015-2017. American Journal of Public Health, 109, 300–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Delcher C., Wang Y., Vega R.S., Halpin J., Gladden R.M., O’Donnell J.K., et al. (2020) Carfentanil outbreak — Florida, 2016–2017. MMWR. Morbidity and Mortality Weekly Report, 69, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Massey J., Kilkenny M., Batdorf S., Sanders S.K., Ellison D., Halpin J., et al. (2017) Opioid overdose outbreak — West Virginia, August 2016. MMWR. Morbidity and Mortality Weekly Report, 66, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Papsun D., Hawes A., Mohr A.L.A., Friscia M., Logan B.K. (2017) Case series of novel illicit opioid-related deaths. Academic Forensic Pathology, 7, 477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Vohra V., Hodgman M., Marraffa J., Barba K., Stoppacher R. (2020) Fentanyl- and fentanyl analog-related deaths across five counties in Central New York between 2013 and 2017. Clinical Toxicology (Philadelphia, Pa.), 58, 112–116. [DOI] [PubMed] [Google Scholar]
- 323. Corsi N., Dragovic L. (2019) Fatal overdoses involving carfentanil: a case series. Journal of Forensic Science and Medicine, 5, 147. [Google Scholar]
- 324. Simonsen K.W., Kriikku P., Thelander G., Edvardsen H.M.E., Thordardottir S., Andersen C.U., et al. (2020) Fatal poisoning in drug addicts in the Nordic countries in 2017. Forensic Science International, 313, 110343. [DOI] [PubMed] [Google Scholar]
- 325. Kahl J.H., Gonyea J., Humphrey S.M., Hime G.W., Boland D.M. (2018) Quantitative analysis of fentanyl and six fentanyl analogs in postmortem specimens by UHPLC–MS-MS. Journal of Analytical Toxicology, 42, 570–580. [DOI] [PubMed] [Google Scholar]
- 326. Elliott S.P., Hernandez Lopez E. (2018) A series of deaths involving carfentanil in the UK and associated post-mortem blood concentrations. Journal of Analytical Toxicology, 42, e41–e45. [DOI] [PubMed] [Google Scholar]
- 327. Mayer S., Boyd J., Collins A., Kennedy M.C., Fairbairn N., McNeil R. (2018) Characterizing fentanyl-related overdoses and implications for overdose response: findings from a rapid ethnographic study in Vancouver, Canada. Drug and Alcohol Dependence, 193, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Waite K., Deeken A., Perch S., Kohler L.J. (2017) Carfentanil and current opioid trends in Summit County, Ohio. Academic Forensic Pathology, 7, 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Fomin D., Baranauskaite V., Usaviciene E., Sumkovskaja A., Laima S., Jasulaitis A., et al. (2018) Human deaths from drug overdoses with carfentanyl involvement—new rising problem in forensic medicine. Medicine, 97, e13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Pardo B., Taylor J., Caulkins J.P., Kilmer B., Reuter P., Stein B.D. (2019) Understanding America’s Surge in Fentanyl and Other Synthetic Opioids. RAND Corporation, Santa Monica, CA. https://www.rand.org/pubs/research_briefs/RB10091.html (Dec 1, 2020). [Google Scholar]
- 331. Zoorob M. (2019) Fentanyl shock: the changing geography of overdose in the United States. International Journal of Drug Policy, 70, 40–46. [DOI] [PubMed] [Google Scholar]
- 332. Drug Enforcement Administration . (2018) Schedules of controlled substances: temporary placement of fentanyl-related substances in Schedule I. Temporary amendment; temporary scheduling order. Federal Register, 83, 5188–5192. [PubMed] [Google Scholar]
- 333. Weedn V.W., Elizabeth Zaney M., McCord B., Lurie I., Baker A. (2021) Fentanyl-related substance scheduling as an effective drug control strategy. Journal of Forensic Sciences, 66, 1186–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Papsun D., Isenschmid D., Logan B.K. (2017) Observed carfentanil concentrations in 355 blood specimens from forensic investigations. Journal of Analytical Toxicology, 41, 777–778. [DOI] [PubMed] [Google Scholar]
- 335. Chatterton C.N., Handy R.P., Shoemaker G.K., Scott-Ham M. (2020) The distribution and redistribution of carfentanil in post mortem samples. Forensic Science International, 309, 110215. [DOI] [PubMed] [Google Scholar]
- 336. Chesser R. (2018) Analysis of synthetic opioids in postmortem blood, vitreous humor, and brain tissue. CUNY Academic Works, 2018. https://academicworks.cuny.edu/jj_etds/88 (Mar 6, 2022).
- 337. Sharma K.K., Hales T.G., Rao V.J., NicDaeid N., McKenzie C. (2019) The search for the ‘next’ euphoric non-fentanil novel synthetic opioids on the illicit drugs market: current status and horizon scanning. Forensic Toxicology, 37, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Cheney B.V., Szmuszkovicz J., Lahti R.A., Zichi D.A. (1985) Factors affecting binding of trans-N-[2-(methylamino)cyclohexyl]benzamides at the primary morphine receptor. Journal of Medicinal Chemistry, 28, 1853–1864. [DOI] [PubMed] [Google Scholar]
- 339. Szmuszkovicz J., Von Voigtlander P.F. (1982) Benzeneacetamide amines: structurally novel non-mu opioids. Journal of Medicinal Chemistry, 25, 1125–1126. [DOI] [PubMed] [Google Scholar]
- 340. Baumann M.H., Tocco G., Papsun D.M., Mohr A.L., Fogarty M.F., Krotulski A.J. (2020) U-47700 and its analogs: non-fentanyl synthetic opioids impacting the recreational drug market. Brain Sciences, 10, 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Carroll F.I., Lewin A.H., Mascarella S.W., Seltzman H.H., Reddy P.A. (2020) Designer drugs: a medicinal chemistry perspective (II). Annals of the New York Academy of Sciences, 1248, 18–38. [DOI] [PubMed] [Google Scholar]
- 342. Moolten M.S., Fishman J.B., Chen J.C., Carlson K.R. (1993) Etonitazene: an opioid selective for the mu receptor types. Life Sciences, 52, PL199–203. [DOI] [PubMed] [Google Scholar]
- 343. Hunger A., Kebrle J., Rossi A., Hoffmann K. (1957) Synthese basisch substituierter, analgetisch wirksamer Benzimidazol-Derivate. Experientia, 13, 400–401. [DOI] [PubMed] [Google Scholar]
- 344. Blanckaert P., Cannaert A., Uytfanghe K.V., Hulpia F., Deconinck E., Calenbergh S.V., et al. (2020) Report on a novel emerging class of highly potent benzimidazole NPS opioids: chemical and in vitro functional characterization of isotonitazene. Drug Testing and Analysis, 12, 422–430. [DOI] [PubMed] [Google Scholar]
- 345. European Monitoring Centre for Drugs and Drug Addiction . (2020) EMCDDA initial report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1-ethanamine (isotonitazene). Publications Office of European Union, Luxembourg. https://www.emcdda.europa.eu/system/files/publications/13028/EMCDDA-Initial-report_Isotonitazene.pdf (Mar 6, 2022). [Google Scholar]
- 346. Krotulski A.J., Papsun D.M., Fogarty M.F., Nelson L., Logan B.K. (2019) Public Health Alert: Isotonitazene. NPS Discovery. https://www.npsdiscovery.org/wp-content/uploads/2019/11/Public-Alert_Isotonitazene_NPS-Discovery_111919-1.pdf (Jan 10, 2021).
- 347. Shover C.L., Falasinnu T.O., Freedman R.B., Humphreys K. (2021) Emerging characteristics of isotonitazene-involved overdose deaths: a case-control study. Journal of Addiction Medicine, 15, 429–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Drug Enforcement Administration . (2020) Temporary placement of isotonitazene in Schedule I. Federal Register, 85, 36819–36823. [Google Scholar]
- 349. Zagorski C.M., Myslinski J.M., Hill L.G. (2020) Isotonitazene as a contaminant of concern in the illegal opioid supply: a practical synthesis and cost perspective. International Journal of Drug Policy, 86, 102939. [DOI] [PubMed] [Google Scholar]
- 350. Siczek M., Zawadzki M., Siczek M., Chłopaś-Konowałek A., Szpot P. (2020) Etazene (N,N-diethyl-2-{[(4-ethoxyphenyl)methyl]-1H-benzimidazol-1-yl}-ethan-1-amine (dihydrochloride)): a novel benzimidazole opioid NPS identified in seized material: crystal structure and spectroscopic characterization. Forensic Toxicology, 39, 146–155. [Google Scholar]
- 351. Krotulski A.J., Shuda S., Fogarty M.F., Decker S. (2020) Metonitazene. NMS Labs. https://www.npsdiscovery.org/wp-content/uploads/2020/07/Metonitazene_073020_NMSLabs_Report.pdf (Feb 13, 2021).
- 352. Vandeputte M.M., Uytfanghe K.V., Layle N., St. Germaine D., Iula D.M., Stove C. (2021) Synthesis, chemical characterization, and μ-opioid receptor activity assessment of the emerging group of ‘nitazene’ 2-benzylbenzimidazole synthetic opioids. ACS Chemical Neuroscience, 12, 1241–1251. [DOI] [PubMed] [Google Scholar]
- 353. Lutz P. (2012) Benzimidazole and its derivatives—from fungicides to designer drugs. A new occupational and environmental hazards. Medycyna Pracy, 63, 505–513. [PubMed] [Google Scholar]
- 354. Adamson D.W., Green A.F. (1950) A new series of analgesics. Nature, 165, 122. [DOI] [PubMed] [Google Scholar]
- 355. Krotulski A.J., Papsun D.M., Noble C., Kacinko S.L., Nelson L., Logan B.K. (2020) Public Health Alert: Brorphine. NPS Discovery. https://www.npsdiscovery.org/wp-content/uploads/2020/07/Public-Alert_Brorphine_NPS-Discovery_072720.pdf (Jan 10, 2021).
- 356. Kennedy N.M., Schmid C.L., Ross N.C., Lovell K.M., Yue Z., Chen Y.T., et al. (2018) Optimization of a series of mu opioid receptor (MOR) agonists with high G protein signaling bias. Journal of Medicinal Chemistry, 61, 8895–8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Drug Enforcement Administration . (2020) Temporary placement of brorphine in Schedule I. Federal Register, 85, 78047–78050. [Google Scholar]
- 358. Irikura T., Nishino K., Ito N., Ito M., Ohkubo H. (1970) Studies on analgesic agents. VI. Analgesic effect of 1-butyryl-4-cinnamylpiperazine hydrochloride. Japanese Journal of Pharmacology, 20, 287–293. [DOI] [PubMed] [Google Scholar]
- 359. Yu S.-Y., Wang J.-J., Huang Y.-G., Hu B., Wang K., Li P.P., et al. (2017) Managing pain in patients with cancer: the Chinese good pain management experience. Journal of Global Oncology, 3, 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Furlan D. (1985) US Patent for Methyl-piperazino derivatives with analgesic activity Patent. December 31, 1985. https://patents.justia.com/patent/4562191 (Jan 10, 2021).
- 361. Vandeputte M.M., Cannaert A., Stove C.P. (2020) In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances. Archives of Toxicology, 94, 3819–3830. [DOI] [PubMed] [Google Scholar]
- 362. Krotulski A.J., Fogarty M.F., Logan B.K. (2019) AP-237. NMS Labs. https://www.npsdiscovery.org/wp-content/uploads/2019/09/AP-237_091619_NMSLabs_Report.pdf (Feb 2, 2021).
- 363. Krotulski A.J., Fogarty M.F., Logan B.K. (2020) Para-Methyl-AP-237. NMS Labs. https://www.npsdiscovery.org/wp-content/uploads/2020/04/para-Methyl-AP-237_041320_NMSLabs_Report.pdf (Feb 2, 2021).
- 364. Gassaway M.M., Rives M.-L., Kruegel A.C., Javitch J.A., Sames D. (2014) The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Translational Psychiatry, 4, e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Brink C.B., Harvey B.H., Brand L. (2006) Tianeptine: a novel atypical antidepressant that may provide new insights into the biomolecular basis of depression. Recent Patents on CNS Drug Discovery, 1, 29–41. [DOI] [PubMed] [Google Scholar]
- 366. Wagstaff A.J., Ormrod D., Spencer C.M. (2001) Tianeptine: a review of its use in depressive disorders. CNS Drugs, 15, 231–259. [DOI] [PubMed] [Google Scholar]
- 367. Springer J., Cubała W.J. (2018) Tianeptine abuse and dependence in psychiatric patients: a review of 18 case reports in the literature. Journal of Psychoactive Drugs, 50, 275–280. [DOI] [PubMed] [Google Scholar]
- 368. Nabil S., Guan N.C., Rashid R.A. (2018) Tianeptine dependence: a case report. Malaysian Journal of Psychiatry, 27, 47–51. [Google Scholar]
- 369. Karim A., Ioannou C. (2020) Tianeptine abuse leading to an episode of psychosis: a case report and literature review. Journal of Psychiatric Practice, 26, 146–148. [DOI] [PubMed] [Google Scholar]
- 370. Lauhan R., Hsu A., Alam A., Beizai K. (2018) Tianeptine abuse and dependence: case report and literature review. Psychosomatics, 59, 547–553. [DOI] [PubMed] [Google Scholar]
- 371. El Zahran T., Schier J., Glidden E., Kieszak S., Law R., Bottei E., et al. (2018) Characteristics of tianeptine exposures reported to the National Poison Data System - United States, 2000-2017. MMWR. Morbidity and Mortality Weekly Report, 67, 815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Marraffa J.M., Stork C.M., Hoffman R.S., Su M.K. (2018) Poison control center experience with tianeptine: an unregulated pharmaceutical product with potential for abuse. Clinical Toxicology (Philadelphia, PA), 56, 1155–1158. [DOI] [PubMed] [Google Scholar]
- 373. Rushton W., Whitworth B., Brown J., Kurz M., Rivera J. (2021) Characteristics of tianeptine effects reported to a poison control center: a growing threat to public health. Clinical Toxicology (Philadelphia, Pa.), 59, 152–157. [DOI] [PubMed] [Google Scholar]
- 374. O’Connor L.C., Torrance H.J., McKeown D.A. (2016) ELISA detection of phenazepam, etizolam, pyrazolam, flubromazepam, diclazepam and delorazepam in blood using Immunalysis® benzodiazepine kit. Journal of Analytical Toxicology, 40, 159–161. [DOI] [PubMed] [Google Scholar]
- 375. Pettersson Bergstrand M., Helander A., Hansson T., Beck O. (2017) Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays. Drug Testing and Analysis, 9, 640–645. [DOI] [PubMed] [Google Scholar]
- 376. Understanding the Epidemic . (2021) CDC’s Response to the Opioid Overdose Epidemic | CDC October 2, 2021. https://www.cdc.gov/opioids/basics/epidemic.html (Oct 3, 2021).
- 377. EWA/Tox - Login . https://www.unodc.org/tox/#/login (Oct 3, 2021).
- 378. Spyres M.B., Farrugia L.A., Kang A.M., Aldy K., Calello D.P., Campleman S.L., et al. (2020) The Toxicology Investigators Consortium Case Registry-the 2019 Annual Report. Journal of Medical Toxicology: Official Journal of the American College of Medical Toxicology, 16, 361–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Emerging Drugs of Abuse and Therapeutic Agent . (2015) OSAC Research Needs Assessment. October 2015. https://www.nist.gov/system/files/documents/forensics/osac/OSAC-Research-Needs-TOX_Emerging-Drugs.pdf (Nov 22, 2021).


