Abstract
Context
Per- and polyfluoroalkyl substances (PFAS) and phthalates are 2 families of environmental endocrine disruptors that may be associated with areal lower bone mineral density (aBMD).
Objective
To examine associations between serum PFAS and urinary phthalate biomarker concentrations and their mixtures with aBMD Z-scores in adolescents.
Design, Patients, and Measures
We examined serial cross-sectional data from male (n = 453) and female (n = 395) 12- to 19-year-old participants in the 2011 through 2016 National Health and Nutrition Examination Survey with measures of serum PFAS, urinary phthalate metabolites, and dual-energy X-ray absorptiometry aBMD Z-scores (total body less head). In sex-specific models, we used linear regression to examine associations of individual PFAS and phthalate biomarkers with aBMD Z-scores, and Bayesian kernel machine regression to examine the association of the overall PFAS/phthalate biomarker mixture with aBMD Z-scores. We replicated the analysis, stratifying by race/ethnicity.
Results
Participants were (mean ± SD) 15 ± 2.1 years of age. In males, each doubling of serum perfluorooctanoate (PFOA), perfluorooctane sulfonate, urinary mono-isobutyl phthalate (MiBP), mono-n-butyl phthalate, and the overall PFAS/phthalate mixture was associated with a lower aBMD Z-score (eg, for PFOA: -0.24; 95% CI, -0.41 to -0.06). Serum PFOA and urinary MiBP were associated with higher aBMD Z-scores in females (eg, for PFOA: 0.09; 95% CI, -0.07 to 0.25). Findings did not differ by race/ethnicity.
Conclusions
Certain PFAS and phthalates may be associated with reduced bone mineral density in adolescent males. Bone mineral density tracks across the life course, so if replicated in longitudinal cohorts, this finding may have implications for lifelong skeletal health.
Keywords: bone mineral density, children, National Health and Nutrition Examination Survey, per- and polyfluoroalkyl substances, phthalates
Bone accrual primarily occurs during childhood and adolescence (1, 2); therefore, it is critically important to identify modifiable factors that negatively affect bone during this period to better promote bone health across the lifespan. The role of the synthetic chemical classes per- and polyfluoroalkyl substances (PFAS) and phthalates on bone health is of particular interest because of their high exposure prevalence in US children and adolescents (3) and ability to disrupt hormone-signaling pathways. PFAS impart water-, oil-, and dirt-resistant properties to diverse items, including nonstick cookware, clothing, and food packaging (4). Low-molecular-weight phthalates are primarily used in personal care products, whereas high-molecular-weight phthalates provide flexibility to plastics in building materials, food processing, and children’s toys (5). PFAS and phthalates may affect bone homeostasis through shared biological mechanisms. Both groups of chemicals activate peroxisome proliferator-activated receptor gamma, suppressing osteoblast formation (6, 7), and are androgen receptor antagonists that may inhibit androgen-mediated osteoblastogenesis (8, 9).
Previous epidemiological studies support associations of PFAS and phthalate biomarkers with lower areal bone mineral density (aBMD), but information is lacking for adolescents and on whether data are generalizable across race/ethnicities. Higher PFAS concentrations have been associated with lower aBMD Z-scores in 6- to 10-year-old children in a Boston-based cohort, in a small pilot study of 8- to 12-year-old obese children, and in adults in the National Health and Nutrition Examination Survey (NHANES) (10-12). Also, several studies have shown that postmenopausal females with higher urinary phthalate metabolite concentrations have lower aBMD (13-15). However, it is important to determine if prior findings on PFAS, phthalate metabolites, and bone health are generalizable to more racially and socioeconomically diverse populations because PFAS and phthalate exposure profiles differ by race, ethnicity, and socioeconomic status (16-18). There is also a gap in the literature regarding exposure vulnerability in adolescence, when bone accrual is at its peak.
Here, we address these gaps by describing associations between serum PFAS and urinary phthalate biomarker concentrations, and their complex mixtures, with aBMD Z-score in 12 to 19 year olds in the 2011 through 2016 NHANES. We hypothesize that adolescents with higher concentrations of serum PFAS and urinary phthalate biomarkers will have a lower aBMD Z-score.
Materials and Methods
Study Population
We combined data from 2011 and 2012, 2013 and 2014, and 2015 and 2016 NHANES, a serial cross-sectional survey of the noninstitutionalized US population. We selected these cycles because both PFAS and phthalate biomarkers have strong temporal trends (19, 20), making data from the most recent cycles most relevant. Also, previous cycles did not measure total body less head (TBLH) aBMD, a preferred skeletal site in children and adolescents because the skull is not responsive to environmental factors (21, 22). Participants completed in-person interviews, provided urine and blood samples, and underwent a physical examinations including dual-energy x-ray absorptiometry to measure aBMD. We restricted the study population to adolescents 12 to 19 years of age because serum PFAS was not measured in younger children and because adolescent (vs child) aBMD tracks more strongly with adult aBMD (23). Of 4027 participants between 12 and 19 years of age, 1015 had complete data on serum PFAS and urinary phthalate biomarker concentrations, and, of these, 896 also had data on aBMD. After excluding participants missing data on diet (n = 26) or other covariates (n = 22), 848 participants were included in this analysis. The Maine Medical Center institutional review board determined that this study was not human subjects research.
Serum PFAS and Urinary Phthalate Metabolite Measurements
During the examinations, NHANES technicians collected blood and urine samples. Serum and urine samples were stored at -20°C until shipped to the National Center for Environmental Health at the Centers for Disease Control and Prevention (CDC). CDC laboratory staff measured serum PFAS and urinary phthalate metabolite concentrations in a one-third random subsample of participants. CDC staff measured serum PFAS concentrations using online solid-phase extraction coupled to high-performance liquid chromatography-turbo ion spray ionization-tandem mass spectrometry, and quantified urinary phthalate metabolites using solid-phase extraction coupled with online high-performance liquid chromatography and tandem mass spectrometry (24, 25). NHANES analysts imputed values below the limit of detection (LOD) with (26).
We a priori limited our analysis to PFAS and phthalate metabolites for which at least 60% of samples were > LOD, thus we included 5 serum PFAS—perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS), perfluorodecanoate (PFDA), perfluorohexane sulfonate, and perfluorononanoate—and 7 urinary phthalate metabolites—monocarboxyoctyl phthalate (MCOP), monocarboxy-isononyl phthalate (MCNP), mono(3-carboxypropyl) phthalate (MCPP), monobenzyl phthalate (MBzP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), and mono-isobutyl phthalate (MiBP). We also included ∑ di-2-ethylhexyl phthalate (∑DEHP), which we estimated as the molar sum of the DEHP metabolites, mono (2-ethylhexyl) phthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, mono(2-ethyl-5-oxohexyl) phthalate, and mono(2-ethyl-5-carboxypentyl phthalate (µmol/L) (19). We categorized ∑ DEHP, MCOP, MCNP, MCPP, and MBzP as high-molecular-weight urinary phthalate biomarkers, and MEP, MBP, and MiBP as low-molecular-weight urinary phthalate biomarkers. In 2011 and 2012, NHANES analysts calculated total serum PFOA and PFOS. For data from 2013 through 2016, we calculated PFOA as the sum of branch perfluorooctanoic acid isomers and n-perfluorooctanesulfonic acid, and calculated PFOS as the sum of n-perfluorooctanesulfonic acid and perfluoromethylheptanesulfonic acid isomers. These calculated sums are considered comparable to the total PFOS and PFOA as reported in 2011 and 2012 because the summed isomers of each chemical represent more than 95% of what was previously reported (25).
Bone Mineral Density
All eligible participants were invited to complete dual-energy x-ray absorptiometry scans, which were performed by a radiology technologist using a Hologic Discovery model A densitometer (27). The accuracy of the scans was ensured by regular quality control procedures and expert review of all scans. We used TBLH aBMD in our analysis because the International Society for Clinical Densitometry considers this skeletal site to be the most accurate and reliable for children and adolescents (21). We calculated aBMD Z-scores using published age-, sex-, height-, and race-specific reference curves (28).
Covariates
NHANES staff measured participants’ height with stadiometers using standardized protocols. Participants (or, if younger than 16 years, their proxy) reported their age, sex, race/ethnicity, and family size (29). NHANES calculates income to poverty ratio as the ratio of household income to the Department of Health and Human Services poverty threshold specific to their family size. Participants separately reported their moderate (eg, brisk walking, bicycling) and vigorous (eg, running, basketball) physical activity. We calculated hours per week of moderate or vigorous physical activity by multiplying the days per week spent engaged in an activity by daily minutes of activity, dividing by 60, and summing across moderate and vigorous activities. Participants were asked whether any household members smoked inside their home. CDC laboratory technicians measured cotinine in serum samples.
Participants also completed a 24-hour dietary recall, in which they reported the types and amounts of all foods consumed in the previous 24 hours (30). Reported foods were coded into food groups and nutrients using the US Department of Agriculture’s Food and Nutrient Database for Dietary Studies (31). We defined sugar-sweetened beverage intake using What We Eat in America food categories, a classification scheme of more than 150 unique foods and beverages (32), and categorized intake in cups (245 g). We categorized total dairy intake (cup equivalents) using the Food Patterns Equivalents Database, which converts dietary intake into the relatively broader US Department of Agriculture Food Patterns components (33).
Statistical Analysis
We examined distributions of serum PFAS and urinary phthalate metabolite concentrations and calculated Spearman correlations between individual biomarkers.
We first assessed linearity of associations using generalized additive models. Next, we used linear regression to examine single-chemical associations of serum PFAS and urinary phthalate biomarker concentrations with TBLH aBMD Z-scores. Because endocrine disruptor-health outcome associations, including for PFAS and aBMD (12, 34), often vary by sex, we present sex-specific findings for PFAS and phthalate biomarkers. We log-2 transformed serum PFAS and urinary phthalate biomarker concentrations to meet model assumptions and aid interpretation. We first ran models that were unadjusted for PFAS and models adjusted only for urinary creatinine for phthalates. We adjusted for urinary creatinine in phthalate models to improve precision (35). Next, we ran models additionally adjusted for covariates that we selected a priori based on previously described associations with PFAS and phthalate biomarkers and aBMD (19, 36-39): sociodemographics (age, race/ethnicity), physical activity, indoor smoking by a household member, total dairy intake, sugar-sweetened beverage intake, and temporal trend (ie, NHANES cycle [2011-2012, 2013-2014, 2015-2016]). Because effect estimates were similar between models with and without additional adjustment for income to poverty ratio and serum cotinine (data not shown), we excluded these variables from final models. Although our group and others have related additional food items to PFAS (eg, fish, meat/poultry) and phthalate biomarkers (eg, fish, baked goods, starchy vegetables) (40-43), we did not include them in our model because these foods are not strongly associated with aBMD (1). We did not adjust for body size and pubertal status because these variables may mediate the association between PFAS/phthalate biomarkers and aBMD (44-47). We adjusted for NHANES survey weights to account for bias that may have been introduced by oversampling (or undersampling) specific populations (eg, race/ethnicity) as part of the NHANES survey methodology (48). To facilitate comparison with Bayesian kernel machine regression (BKMR) mixture models that cannot incorporate survey analysis procedures, we also did not use survey analysis procedures to account for the complex sample design in single chemical models. Thus, like other NHANES studies using novel mixture methods (49), the findings from our study leverage the large sample size and racial/ethnic diversity of NHANES, but cannot be considered generalizable to the US population. Because serum PFAS and urinary phthalate biomarkers vary by race/ethnicity (17, 18), we stratified models by the most frequently sampled categories of race/ethnicity (non-Hispanic White, non-Hispanic Black, and Hispanic [Mexican American and other Hispanic]).
Because exposure to multiple PFAS and phthalates occurs simultaneously and both classes of chemicals may act through similar pathways to lower aBMD, we used BKMR to examine the association between the overall chemical mixture and aBMD Z-score. BKMR takes into account correlations and interactions between chemical biomarkers as well as potential nonlinearity of chemical biomarker–aBMD Z-score associations (50, 51). The BKMR model characterizes the association between individual chemical biomarkers and aBMD Z-score, holding other chemicals constant and also provides an overall mixture effect. We used a hierarchical variable selection method and posterior inclusion probabilities in the BKMR model to quantify the relative importance of each class of chemical (ie, PFAS, low-molecular-weight phthalate biomarkers, and high-molecular-weight phthalate biomarkers) and individual chemicals within each class to the overall mixture effect. We considered low-molecular-weight and high-molecular-weight phthalate biomarkers separately because their associations with health outcomes often vary (52). Within the BKMR model, we also assessed for chemical–chemical interactions by calculating the effect estimate for a single chemical (75th vs 25th percentile) on aBMD Z-scores under 2 scenarios, when all other chemicals were set to the 75th percentile and when all other chemicals were set to the 25th percentile, and comparing these 2 values. We modeled associations and 95% credible intervals using a Gaussian kernel function and Markov chain Monte Carlo algorithm with 10 000 iterations. Because we modeled serum PFAS and urinary phthalate biomarkers simultaneously, we used a common set of covariates, including age, race/ethnicity, physical activity, indoor smoking by a household member, total dairy intake, sugar-sweetened beverage intake, urinary creatinine, NHANES cycle, and NHANES weights.
We conducted statistical analyses using SAS EG, version 7.1 (SAS Institute, Inc.) and R (Core Team. 2018. R: A language and environment for statistical computing. Vienna Austria:R Foundation for Statistical Computing).
Results
Participants were (mean ± SD) 15 ± 2.1 years old and 47% female (Table 1). Twenty-seven percent of participants were non-Hispanic White, 23% were Mexican American, and 23% were non-Hispanic Black. Participants who were male, non-Hispanic Black, or reported more physical activity tended to have higher aBMD Z-scores. PFOS had the highest median concentration (3.2 ng/mL) of the serum PFAS, and MEP had the highest median concentration (34.5 ng/mL) of the urinary phthalate biomarkers (Table S1) (53). We observed a wide range of positive correlations between individual serum PFAS (eg, for PFDA and PFOS, correlation = 0.56; for PFDA and perfluorohexane sulfonate, correlation = 0.09) and individual urinary phthalate biomarkers (eg, for ∑ DEHP and MCNP, correlation = 0.84; for ∑ DEHP and MEP, correlation = 0.09); correlations between serum PFAS and urinary phthalate biomarkers were low (Figure S1) (53).
Table 1.
Unweighteda characteristics of 12- to 19-year-old participants (n = 848) in the 2011-2016 NHANES, overall and by total body less head aBMD Z-score quartiles
| aBMD Z-score quartilesb | |||||
|---|---|---|---|---|---|
| Total (N = 848) | Q1 (N = 212) | Q2 (N = 212) | Q3 (N = 212) | Q4 (N = 212) | |
| Mean ± SD or N (%) | |||||
| Age, y | 15 ± 2.1 | 15 ± 2.1 | 15 ± 2.2 | 15 ± 2.1 | 15 ± 2.0 |
| Female | 395 (47%) | 97 (46%) | 101 (48%) | 101 (48%) | 96 (45%) |
| Race/ethnicity | |||||
| Mexican American | 199 (23%) | 42 (20%) | 49 (23%) | 51 (24%) | 57 (27%) |
| Other Hispanic | 94 (11%) | 20 (9%) | 22 (10%) | 27 (13%) | 25 (12%) |
| Non-Hispanic White | 225 (27%) | 67 (32%) | 58 (27%) | 54 (25%) | 46 (22%) |
| Non-Hispanic Black | 198 (23%) | 47 (22%) | 51 (24%) | 52 (25%) | 48 (23%) |
| Non-Hispanic Asian | 89 (10%) | 27 (13%) | 21 (10%) | 21 (10%) | 20 (9%) |
| Other/multi-racial | 43 (5%) | 9 (4%) | 11 (5%) | 7 (3%) | 16 (8%) |
| Physical activity, hours per week | 5.9 ± 7.3 | 4.1 ± 5.5 | 5.4 ± 7.4 | 6.6 ± 7.6 | 7.3 ± 8.1 |
| Household member smokes indoors | 109 (13%) | 26 (12%) | 25 (12%) | 28 (13%) | 30 (14%) |
| Total dairy, cups | 1.8 ± 1.6 | 1.6 ± 1.3 | 1.9 ± 1.6 | 1.9 ± 1.6 | 1.9 ± 1.7 |
| Sugar-sweetened beverages, cups | 0.87 ± 1.5 | 0.85 (± 1.4) | 0.76 (± 1.2) | 1.0 (± 2.1) | 0.83 (± 1.3) |
| NHANES cycle | |||||
| 2011-2012 | 258 (30%) | 63 (30%) | 81 (38%) | 55 (26%) | 59 (28%) |
| 2013-2014 | 319 (38%) | 74 (35%) | 69 (33%) | 92 (43%) | 84 (40%) |
| 2015-2016 | 271 (32%) | 75 (35%) | 62 (29%) | 65 (31%) | 69 (33%) |
Abbreviations: aBMD, area bone mineral density; NHANES, National Health and Nutrition Examination Survey; Q, quartile.
aModels do not account for the complex sample design, and therefore results cannot be considered generalizable to the US population.
baBMD Z-score quartile minimum and maximum values: Q1: -4.01 to -1.12; Q2: 1.11 to -0.30; Q3: -0.30 to 0.39; Q4: 0.39 to 2.72.
Chemical Concentrations and aBMD Z-Scores in Males
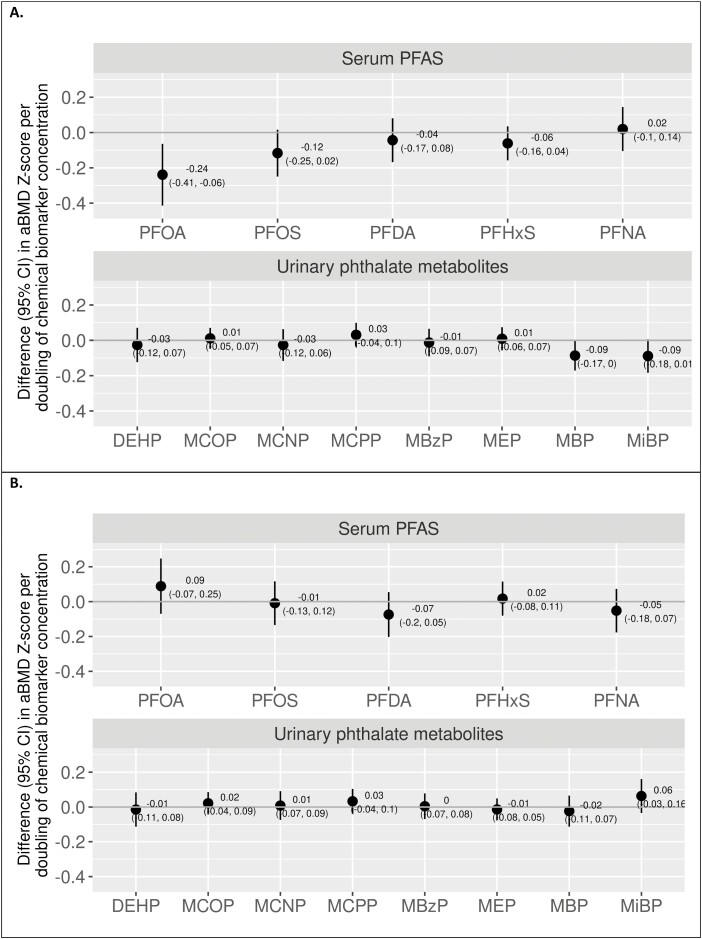
In multivariable single-chemical models in males, each biomarker doubling was associated with a lower aBMD Z-score for serum PFOA (difference in aBMD Z-score, -0.24; 95% CI, -0.41 to -0.06) and urinary MBP (-0.09; 95% CI, -0.17 to 0.00) (Fig. 1A). Serum PFOS and urinary MiBP were also associated with lower aBMD, but 95% CIs crossed the null. Other serum PFAS and urinary phthalate biomarkers were not associated with aBMD Z-scores. Findings from unadjusted models were generally similar (Table S2)(53).
Figure 1.
Single-chemical associations from unweighteda multivariableb linear regression models of individual serum PFAS and urinary phthalate biomarkers with total body less head aBMD Z-scores in 12- to 19-year-old males (A) and females (B), 2011-2016 National Health and Nutrition Examination Survey (males, N = 453; females, N = 395). aModels do not account for the complex sample design, and therefore results cannot be considered generalizable to the US population. bAdjusted for age, race/ethnicity, moderate/vigorous physical activity, indoor smoking by a household member, recent sugar-sweetened beverage consumption, recent total dairy consumption, NHANES weights, and NHANES cycle. Models for phthalates were additionally adjusted for urinary creatinine. Abbreviations: aBMD, areal bone mineral density; DEHP, di(2-ethylhexyl)phthalate; MBP, mono-n-butyl phthalate; MBzP, mono-benzyl phthalate; MCNP, mono(carboxynonyl) phthalate; MCOP, mono(carboxyoctyl) phthalate; MCPP, mono-(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MIBP, mono-isobutyl phthalate; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate.
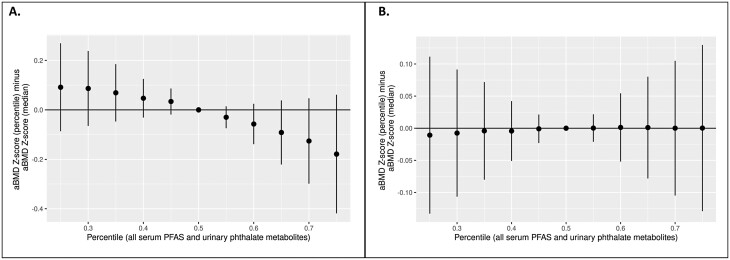
In line with the single-chemical models, in the BKMR mixture models for males, higher serum PFOA and PFOS and urinary MBP and MiBP were linearly associated with lower aBMD Z-scores, with the strongest associations for PFOA and MBP (Figure S2A) (53). ∑DEHP and MBzP were nonlinearly associated with aBMD Z-score, with males with the lowest and highest concentrations having the lowest aBMD Z-scores. The overall serum PFAS/urinary phthalate biomarker mixture was also associated with a lower aBMD Z-score, although 95% credible intervals crossed the null (Fig. 2A). Associations between individual serum PFAS or urinary phthalate biomarkers with aBMD Z-scores were not modified by other chemical biomarkers (data not shown). Posterior inclusion probabilities indicated that of the 3 chemical classes, high- and low-molecular-weight urinary phthalate biomarkers contributed the most to the overall fit of the model. PFOA had the highest contribution among the PFAS, ∑DEHP among the high-molecular-weight phthalate biomarkers, and MiBP among the low-molecular-weight phthalate biomarkers (Table 2), indicating that these chemicals are the primary drivers of the association with lower aBMD Z-score.
Figure 2.
Associations (95% credible intervals) from unweighteda Bayesian kernel machine regression models of the overall urinary phthalate metabolite and serum PFAS mixture with total body less head aBMD Z-scoreb in 12-19 year old males (A) and females (B), 2011-2016 National Health and Nutrition Examination Survey (males, n = 453; females, n = 395). aModels do not account for the complex sample design, and therefore results cannot be considered generalizable to the US population. bFrom BKMR models of the association between the overall urinary phthalate metabolite/serum PFAS mixture when all chemical biomarkers are set at a given quantile versus the median. Models are adjusted for age, race/ethnicity, moderate/vigorous physical activity, indoor smoking by a household member, recent sugar-sweetened beverage consumption, recent total dairy consumption, urinary creatinine concentrations, NHANES weights, and NHANES cycle. Abbreviation: aBMD, areal bone mineral density; NHANES, National Health and Nutrition Examination Survey; PFAS, per- and polyfluoroalkyl substances.
Table 2.
PIPs from Bayesian kernel machine regression modelsa of chemical mixtures and total body less head aBMD Z-scores in 12- to 19-year-old males (n = 453) and females (n = 395), 2011-2016 NHANES
| Biomarker groups | Group PIP | Individual biomarkers | Individual PIP | |
|---|---|---|---|---|
| Males | Serum PFAS | 0.65 | PFOA | 0.76 |
| PFOS | 0.12 | |||
| PFDA | 0.03 | |||
| PFHxS | 0.06 | |||
| PFNA | 0.03 | |||
| High-molecular-weight urinary phthalate biomarkers | 0.79 | ∑DEHPb | 0.65 | |
| MBzP | 0.22 | |||
| MCOP | 0.02 | |||
| MCNP | 0.06 | |||
| MCPP | 0.05 | |||
| Low-molecular-weight urinary phthalate biomarkers | 0.77 | MBP | 0.39 | |
| MiBP | 0.58 | |||
| MEP | 0.03 | |||
| Females | Serum PFAS | 0.33 | PFOA | 0.26 |
| PFOS | 0.10 | |||
| PFDA | 0.17 | |||
| PFHxS | 0.23 | |||
| PFNA | 0.24 | |||
| High-molecular-weight urinary phthalate biomarkers | 0.26 | ∑DEHPb | 0.19 | |
| MCOP | 0.23 | |||
| MCPP | 0.23 | |||
| MBzP | 0.19 | |||
| MCNP | 0.16 | |||
| Low-molecular-weight urinary phthalate biomarkers | 0.36 | MBP | 0.28 | |
| MiBP | 0.51 | |||
| MEP | 0.21 |
Abbreviations: aBMD, areal bone mineral density; BKMR, Bayesian kernel machine regression; DEHP, di(2-ethylhexyl)phthalate; MBzP, mono-benzyl phthalate; MCNP, mono(carboxynonyl) phthalate; MCOP, mono(carboxyoctyl) phthalate; MCPP, mono-(3-carboxypropyl) phthalate; MEP, mono-ethyl phthalate; MIBP, mono-isobutyl phthalate; NHANES, National Health and Nutrition Examination Survey; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoate; PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfonate; MBP, mono-n-butyl phthalate; PIP, posterior inclusion probability.
aPIPs indicate the relative contribution of individual or group biomarkers to the overall fit of the BKMR model. Models are adjusted for age, race/ethnicity, physical activity, indoor smoking by a household member, urinary creatinine concentrations, NHANES weights, and NHANES cycle.
bMolar sum of mono (2-ethylhexyl) phthalate, mono(2-ethyl-5-hydroxyhexyl) phthalate, mono(2-ethyl-5-oxohexyl) phthalate, and mono(2-ethyl-5-carboxypentyl) phthalate.
Chemical Concentrations and aBMD Z-Score in Females
In females, serum PFOA and urinary MiBP were associated with higher aBMD Z-scores, but 95% CIs crossed the null (Fig. 1B). Other serum PFAS and urinary phthalate metabolites were not associated with aBMD Z-scores in single-chemical models. Mixture models were generally consistent with single-chemical models. Serum PFOA and urinary MiBP were positively associated with aBMD Z-score, and urinary MBP was associated with lower aBMD Z-score. There was no association between other individual serum PFAS, urinary phthalate metabolites (Figure S2B) (53), or the overall serum PFAS/urinary phthalate biomarker mixture (Fig. 2B) with aBMD Z-score in BKMR models. Individual chemical-aBMD Z-score associations were not modified by other serum PFAS or urinary phthalate biomarkers (data not shown).
Sensitivity Analyses
Effect estimates for were generally similar across race/ethnicity categories for both males and females in multivariable single-chemical models (Figure S3) (53) (eg, for PFOA, Hispanic males, -0.31 [95% CI, -0.69 to 0.07]; non-Hispanic White males, -0.31 [95% CI, -0.65 to 0.03]; non-Hispanic Black males, -0.26 [95% CI, -0.64 to 0.12]).
Discussion
In 2011 through 2016 NHANES, higher concentrations of several serum PFAS and urinary phthalate biomarkers, as well as the complex mixture of PFAS and phthalate biomarkers, were associated with lower aBMD Z-scores in adolescent males. In females, select serum PFAS and urinary phthalate biomarkers were associated with higher aBMD Z-scores, although 95% CIs crossed the null. Effect estimates were similar across different race/ethnicities.
Our findings align with previous studies that have shown prenatal (54, 55) and childhood (10, 11) PFAS concentrations are associated with lower aBMD in childhood. Our finding of an association of serum PFOS and PFOA with lower aBMD Z-scores in males is consistent with a recent study in the Health Outcomes and Measures of the Environment Study that reported an association between higher concentrations of prenatal PFOA with lower aBMD Z-scores at some skeletal sites in 12-year-old males, but not females (54). Our finding of a positive association between serum PFOA and aBMD in female adolescents, however, is in contrast to the Avon Longitudinal Study of Parents and Children, which found higher prenatal concentrations of several PFAS (including PFOS and PFOA) to be associated with lower aBMD in 17-year-old females (55). In the Boston-area Project Viva cohort, cross-sectional associations of higher PFAS with lower aBMD Z-scores in 8-year-old children did not differ by sex (10). Notably, the associations we observed were modestly stronger than the associations in males in Project Viva (change in TBLH aBMD Z-scores per doubling of PFOA: -0.26 [95% CI, -0.45 to -0.08] in NHANES vs -0.11 [95% CI, -0.23 to 0.00] in Project Viva) (10). The differences by sex and in the magnitude of the PFAS-aBMD association in NHANES vs Project Viva may reflect the timing of the aBMD measurement because peak bone accrual occurs somewhat earlier in adolescent females (~age 12 years) vs males (~14 years), and children in the Project Viva study had not yet initiated peak bone accrual. The field would benefit from longitudinal studies relating PFAS and phthalate metabolite concentrations to aBMD across childhood and adolescence to elucidate whether chemical-aBMD associations vary by age at aBMD measurement.
We found males with higher urinary MBP and MiBP to have lower aBMD Z-scores and females with higher urinary MiBP to have higher aBMD Z-scores. Positive associations between maternal phthalate biomarker concentrations and aBMD, particularly in females, were also reported in the Health Outcomes and Measures of the Environment Study (56). In the Generation R cohort, there was no association between maternal phthalate biomarker concentrations and bone mineral content in childhood (57). In contrast, previous cross-sectional studies consistently indicate that phthalate biomarkers, and particularly low-molecular-weight phthalate biomarkers, are associated with lower aBMD in postmenopausal females (13-15). In a cross-sectional analysis of the Women’s Health Initiative, those not using hormone replacement therapy with higher urinary concentrations of ∑ dibutyl phthalate (parent diester of MBP), MCPP, and ∑ diisobutyl phthalate (parent diester of MiBP) had lower total hip aBMD (14). In postmenopausal females, higher urinary MBP, MCPP, and MBzP were associated with lower total hip and femoral neck aBMD in the 2005 through 2008 NHANES (13), and higher concentrations of urinary MEP and ∑ low-molecular-weight phthalate metabolites were associated with lower total spine aBMD in the 2005 through 2010 NHANES (15). Associations between phthalate biomarkers and aBMD may be more evident later in life, when there is more variability in aBMD and low bone mass becomes prevalent.
Serum PFAS and urinary phthalate biomarkers may disrupt common pathways to affect aBMD. Both chemicals activate peroxisome proliferator-activated receptor-γ (58, 59), which impairs osteoblastogenesis (60). In addition, both chemicals induce hormonal changes that may disrupt bone homeostasis. PFAS and phthalates increase inflammatory cytokines (61, 62), lower IGF-1 (63, 64), antagonize androgen receptor pathways (8, 9), and lower testosterone levels in children and adults (64-66). Our finding of associations between higher levels of select chemicals and lower aBMD Z-score in males but not females may be related to differences in biological impact across chemicals. For example, in a large cross-sectional analysis of children in the C8 Health Project, males with higher biomarker concentrations of PFOA and PFOS, but not other PFAS, had lower testosterone (64). Likewise, MBP was weakly associated with lower testosterone in an analysis of 12- to 20-year-old males in the 2011 and 2012 NHANES. Notably, although urinary ∑ DEHP metabolites and MCOP have also been associated with lower testosterone in males (66), they were not associated with aBMD Z-score in males in our study. Because testosterone is a more important factor in bone formation in males compared with females (67), decreased testosterone could also explain the observed sex-specific effects on aBMD. Future studies should examine whether associations between ∑ DEHP metabolites and hormone concentrations follow a nonlinear dose-response such as we observed for the ∑ DEHP metabolites-aBMD Z-score association.
An important strength of our analysis was our use of sophisticated environmental mixture models to account for chemical coexposures. Consistent with single-chemical models, when we used BKMR to account for correlations and interactions between all of the measured chemicals, PFOA and MBP, and to a lesser extent, PFOS and MiBP, were associated with low aBMD Z-scores in males. Although ∑ DEHP and MBzP also had weak linear associations with aBMD Z-scores in males in single-chemical generalized additive and linear regression models, BKMR models revealed nonlinear associations, suggesting the importance of accounting for chemical coexposures. In females, we observed positive associations of serum PFOA and urinary MiBP with aBMD Z-score across both single-chemical and BKMR models, reinforcing our findings.
In the present study, we found that the chemical-aBMD associations were similar across non-Hispanic White, non-Hispanic Black, and Hispanic individuals. This is another important addition to the literature because chemical exposure profiles and bone health may vary by race/ethnicity. Prior studies have shown that non-Hispanic Black children have lower concentrations of several plasma PFAS (18) and higher urinary concentrations of low-molecular-weight phthalate metabolites (17). Also, non-Hispanic Black children have been shown to have higher aBMD (28). Future studies in larger populations would benefit by examining associations across a wider range of race/ethnicities.
Our study has several limitations. First, the cross-sectional design limits inference about the temporality of the association. Also, although a single measure of urinary phthalate metabolites has been shown to be moderately predictive of longer term (6-month) exposure (68-70), urinary phthalate metabolites have a short half-life (< 24 hours). Thus, whereas aBMD is a marker of lifetime bone accrual, a single measure of phthalate metabolites reflects exposure within the recent past, limiting our ability to see an association. The representativeness of a single biomarker is less of a concern for serum PFAS, which have half-lives of years (71), thus better reflecting longer term exposure in relation to aBMD. Second, prospective studies are needed to examine the extent to which pubertal status and body size mediate associations of PFAS and phthalate biomarkers with aBMD Z-scores. Finally, although we accounted for potential confounders including diet, physical activity, and environmental tobacco smoke, error in these measurements may have resulted in some residual confounding.
In conclusion, in a racially diverse population of 12 to 19 year olds, males with higher concentrations of PFOA, PFOS, MBP, and MiBP had lower aBMD Z-scores, whereas females with higher concentrations of PFOA and MiBP had higher aBMD Z-scores. Our findings have public health implications because reducing exposure to PFAS and phthalates may improve peak bone accrual in adolescent males and set the stage for improved bone health across the lifespan. Exposure to PFAS and phthalates can be reduced through avoidance of affected consumer products or, more equitably, through public policies requiring substitution of these chemicals with safer alternatives.
Glossary
Abbreviations
- aBMD
areal bone mineral density
- BKMR
Bayesian kernel machine regression
- CDC
Centers for Disease Control and Prevention
- DEHP
di(2-ethylhexyl)phthalate
- LOD
limit of detection
- MBP
mono-n-butyl phthalate
- MBzP
mono-benzyl phthalate
- MCNP
mono(carboxynonyl) phthalate
- MCOP
mono(carboxyoctyl) phthalate
- MCPP
mono-(3-carboxypropyl) phthalate
- MEP
mono-ethyl phthalate
- MIBP
mono-isobutyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- PFAS
per- and polyfluoroalkyl substances
- PFDA
perfluorodecanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctane sulfonate
- TBLH
total body less head
Contributor Information
Jenny L Carwile, Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME 04101, USA.
Shravanthi M Seshasayee, Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME 04101, USA.
Katherine A Ahrens, Muskie School of Public Service, University of Southern Maine, Portland, ME 04103, USA.
Russ Hauser, Department of Environmental Health and Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA 02115, USA.
Jeffrey B Driban, Division of Rheumatology, Allergy, and Immunology, Tufts Medical Center, Boston, MA 02111, USA.
Clifford J Rosen, Center for Clinical and Translational Research, Maine Medical Center Research Institute, Scarborough, ME 04074, USA.
Catherine M Gordon, Department of Pediatrics, Texas Children’s Hospital and Baylor College of Medicine, Houston, TX 77030, USA.
Abby F Fleisch, Center for Outcomes Research and Evaluation, Maine Medical Center Research Institute, Portland, ME 04101, USA; Pediatric Endocrinology and Diabetes, Maine Medical Center, Portland, ME 04102, USA.
Funding
This work was supported by the National Institutes of Health [R01ES030101].
Disclosures
The authors have nothing to disclose.
Data Availability
All datasets generated during and/or analyzed during the current study are publicly available and free of charge.
References
- 1. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281-1386. doi: 10.1007/s00198-015-3440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gordon CM, Zemel BS, Wren TA, et al. The determinants of peak bone mass. J Pediatr. 2017;180:261-269. doi: 10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 3. CDC. Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/ [Google Scholar]
- 4. Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45(19):7954-7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 5. CHAP (Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives). Bethesda, MD: U.S. Consumer Product Safety Commission, Directorate for Health Sciences; 2014. Accessed September 4, 2020. https://www.cpsc.gov/s3fs-public/CHAP-REPORT-With-Appendices.pdf [Google Scholar]
- 6. Hurst CH, Waxman DJ. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297-308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto J, Yamane T, Oishi Y, Kobayashi-Hattori K. Perfluorooctanoic acid binds to peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation in 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 2015;79(4):636-639. doi: 10.1080/09168451.2014.991683. [DOI] [PubMed] [Google Scholar]
- 8. Kjeldsen LS, Bonefeld-Jørgensen EC. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int. 2013;20(11):8031-8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- 9. Engel A, Buhrke T, Imber F, et al. Agonistic and antagonistic effects of phthalates and their urinary metabolites on the steroid hormone receptors ERα, ERβ, and AR. Toxicol Lett. 2017;277:54-63. doi: 10.1016/j.toxlet.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 10. Cluett R, Seshasayee SM, Rokoff LB, et al. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: a cross-sectional study (Project Viva, United States). Environ Health Perspect. 2019;127(8):87006. doi: 10.1289/EHP4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khalil N, Ebert JR, Honda M, et al. Perfluoroalkyl substances, bone density, and cardio-metabolic risk factors in obese 8-12 year old children: a pilot study. Environ Res. 2018;160:314-321. doi: 10.1016/j.envres.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 12. Lin LY, Wen LL, Su TC, Chen PC, Lin CY. Negative association between serum perfluorooctane sulfate concentration and bone mineral density in US premenopausal women: NHANES, 2005-2008. J Clin Endocrinol Metab. 2014;99(6):2173-2180. doi: 10.1210/jc.2013-3409. [DOI] [PubMed] [Google Scholar]
- 13. Min KB, Min JY. Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. J Clin Endocrinol Metab. 2014;99(10):E1997-E2003. doi: 10.1210/jc.2014-2279. [DOI] [PubMed] [Google Scholar]
- 14. Reeves KW, Vieyra G, Grimes NP, et al. Urinary phthalate biomarkers and bone mineral density in postmenopausal women. J Clin Endocrinol Metab. 2021;106(7):e2567-e2569. doi: 10.1210/clinem/dgab189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeFlorio-Barker SA, Turyk ME. Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: a cross-sectional study of NHANES data 2005-2010. Int J Environ Health Res. 2016;26(3):326-345. doi: 10.1080/09603123.2015.1111312. [DOI] [PubMed] [Google Scholar]
- 16. Kingsley SL, Eliot MN, Kelsey KT, et al. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ Res. 2018;165:247-257. doi: 10.1016/j.envres.2018.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watkins DJ, Eliot M, Sathyanarayana S, et al. Variability and predictors of urinary concentrations of phthalate metabolites during early childhood. Environ Sci Technol. 2014;48(15):8881-8890. doi: 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harris MH, Rifas-Shiman SL, Calafat AM, et al. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6-10 year old American children. Environ Sci Technol. 2017;51(9):5193-5204. doi: 10.1021/acs.est.6b05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122(3):235-241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (January 2019). Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. https://www.cdc.gov/exposurereport/ [Google Scholar]
- 21. Gordon CM, Leonard MB, Zemel BS. 2013 pediatric position development conference: executive summary and reflections. J Clin Densitom. 2014;17(2):517219-517224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 22. Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17(2):225-242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Wu F, Winzenberg T, Jones G. Tracking of areal bone mineral density from age eight to young adulthood and factors associated with deviation from tracking: a 17-year prospective cohort study. J Bone Miner Res. 2018;33(5):832-839. doi: 10.1002/jbmr.3361. [DOI] [PubMed] [Google Scholar]
- 24. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Phthalates and Phthalate Alternative Metabolites Laboratory Procedure Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. Accessed May 13, 2021. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/PHTHTE_I_MET.pdf [Google Scholar]
- 25. Prevention CfDCa. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Perfluoroalkyl and Polyfluoroalkyl Substances Laboratory Procedure Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. Accessed May 13, 2021. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/PFAS_I.htm [Google Scholar]
- 26. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5(1):46-51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 27. Prevention CfDCa. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Body Composition Procedures Manual. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2017. Accessed May 13, 2021. https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Body_Composition_Procedures_Manual.pdf [Google Scholar]
- 28. Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160-3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). 2017. National Health and Nutrition Examination Survey Demographic Variables and Sample Weights (DEMO_I). Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2020. Accessed April 23, 2020. https://wwwn.cdc.gov/nchs/nhanes/2015-2016/DEMO_I.htm [Google Scholar]
- 30. U.S. Department of Agriculture. Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD). What We Eat in America, NHANES 2015-2016 Documentation: Dietary Interview - Individual Foods -- First Day (DR1IFF_I). 2018. Accessed April 23, 2020. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/DR1IFF_I.htm#Appendix_3
- 31. U.S. Department of Agriculture ARS. Food and Nutrient Database for Dietary Studies, 5.0. Beltsville Human Nutrition Research Center, Food Surveys Research Group (Beltsville, MD) and U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (Hyattsville, MD). May 23, 2019. Food Surveys Research Group Home Page, http://www.ars.usda.gov/ba/bhnrc/fsrg
- 32. U.S. Department of Agriculture, Agricultural Research Service, Beltsville Human Nutrition Research Center, Food Surveys Research Group, Beltsville, MD. Food Patterns Equivalents Database and Datasets 2011-2016. Accessed May 14, 2021. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/
- 33. Bowman S, Clemens J, Shimizu M, Friday J, Moshfegh A. Food Patterns Equivalents Database 2015-2016: Methodology and User Guide [Online]. http://www.ars.usda.gov/nea/bhnrc/fsrg
- 34. Khalil N, Chen A, Lee M, et al. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. population in NHANES 2009-2010. Environ Health Perspect. 2016;124(1):81-87. doi: 10.1289/ehp.1307909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192-200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boot AM, de Ridder MAJ, Pols HAP, Krenning EP, de Muinck Keizer-Schrama SMPF. Bone mineral density in children and adolescents: relation to puberty, calcium intake, and physical activity. J Clin Endocrinol Metab. 1997;82(1):57-62. doi: 10.1210/jcem.82.1.3665. [DOI] [PubMed] [Google Scholar]
- 37. Göckener B, Weber T, Rüdel H, Bücking M, Kolossa-Gehring M. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ Int. 2020;145:106123. doi: 10.1016/j.envint.2020.106123. [DOI] [PubMed] [Google Scholar]
- 38. Blum M, Harris SS, Must A, Phillips SM, Rand WM, Dawson-Hughes B. Household tobacco smoke exposure is negatively associated with premenopausal bone mass. Osteoporos Int. 2002;13(8):663-668. doi: 10.1007/s001980200090. [DOI] [PubMed] [Google Scholar]
- 39. Ahn H, Park YK. Sugar-sweetened beverage consumption and bone health: a systematic review and meta-analysis. Nutr J. 2021;20(1):41. doi: 10.1186/s12937-021-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin P-ID, Cardenas A, Hauser R, et al. Dietary characteristics associated with plasma concentrations of per- and polyfluoroalkyl substances among adults with pre-diabetes: cross-sectional results from the Diabetes Prevention Program Trial. Environ Int. 2020;137:105217-105217. doi: 10.1016/j.envint.2019.105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carwile JL, Seshasayee SM, Ahrens KA, Hauser R, Chavarro JE, Fleisch AF. Dietary correlates of urinary phthalate metabolite concentrations in 6-19 year old children and adolescents. Environ Res. 2021;204(Pt B):112083. doi: 10.1016/j.envres.2021.112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trasande L, Sathyanarayana S, Jo Messito M, R SG, Attina TM, Mendelsohn A. Phthalates and the diets of U.S. children and adolescents. Environ Res. 2013;126:84-90. doi: 10.1016/j.envres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 43. Seshasayee SM, Rifas-Shiman SL, Chavarro JE, et al. Dietary patterns and PFAS plasma concentrations in childhood: Project Viva, USA. Environ Int. 2021;151:106415. doi: 10.1016/j.envint.2021.106415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Carwile JL, Seshasayee SM, Aris IM, et al. Prospective associations of mid-childhood plasma per- and polyfluoroalkyl substances and pubertal timing. Environ Int. 2021;156:106729. doi: 10.1016/j.envint.2021.106729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gilsanz V, Chalfant J, Kalkwarf H, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158(1):100-5, 105.e1, 105.e1-2. doi: 10.1016/j.jpeds.2010.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lopez-Espinosa MJ, Fletcher T, Armstrong B, et al. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with age of puberty among children living near a chemical plant. Environ Sci Technol. 2011;45(19):8160-8166. doi: 10.1021/es1038694. [DOI] [PubMed] [Google Scholar]
- 47. Domazet SL, Grøntved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: the European Youth Heart Study. Diabetes Care. 2016;39(10):1745-1751. doi: 10.2337/dc16-0269. [DOI] [PubMed] [Google Scholar]
- 48. Ahrens KA, Cole SR, Westreich D, Platt RW, Schisterman E, FA. cautionary note about estimating effects of secondary exposures in cohort studies. Am J Epidemiol. 2015;181(3):198-203. doi: 10.1093/aje/kwu276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li M-C, Mínguez-Alarcón L, Bellavia A, et al. Serum beta-carotene modifies the association between phthalate mixtures and insulin resistance: the National Health and Nutrition Examination Survey 2003-2006. Environ Res. 2019;178:108729-108729. doi: 10.1016/j.envres.2019.108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health. 2018;17(1):67. doi: 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493-508. doi: 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eales J, Bethel A, Galloway T, et al. Human health impacts of exposure to phthalate plasticizers: an overview of reviews. Environ Int. 2021;158:106903. doi: 10.1016/j.envint.2021.106903. [DOI] [PubMed] [Google Scholar]
- 53. Carwile JL, Seshasayee SM, Ahrens KA, et al. Associations of PFAS and Phthalate Biomarker Concentrations with Bone Mineral Density in Adolescents: 2011-2016 NHANES. Online resource. figshare. Updated 10 March 2022. 10.6084/m9.figshare.18427205.v4 [DOI]
- 54. Buckley JP, Kuiper JR, Lanphear BP, et al. Associations of maternal serum perfluoroalkyl substances concentrations with early adolescent bone mineral content and density: the Health Outcomes and Measures of the Environment (HOME) Study. Environ Health Perspect. 2021;129(9):97011. doi: 10.1289/EHP9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jeddy Z, Tobias JH, Taylor EV, Northstone K, Flanders WD, Hartman TJ. Prenatal concentrations of perfluoroalkyl substances and bone health in British girls at age 17. Arch Osteoporos. 2018;13(1):84. doi: 10.1007/s11657-018-0498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuiper JR, Braun JM, Calafat AM, et al. Associations of pregnancy phthalate concentrations and their mixture with early adolescent bone mineral content and density: the Health Outcomes and Measures of the Environment (HOME) study. Bone. 2022;154:116251. doi: 10.1016/j.bone.2021.116251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van Zwol-Janssens C, Trasande L, Asimakopoulos AG, et al. Fetal exposure to bisphenols and phthalates and childhood bone mass: a population-based prospective cohort study. Environ Res. 2020;186:109602. doi: 10.1016/j.envres.2020.109602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bility MT, Thompson JT, McKee RH, et al. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82(1):170-182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- 59. Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol Sci. 2006;92(2):476-489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- 60. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885-896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 61. Campioli E, Martinez-Arguelles DB, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diabetes. 2014;4(5):e115. doi: 10.1038/nutd.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh TS, Lee S, Kim HH, Choi JK, Kim SH. Perfluorooctanoic acid induces mast cell-mediated allergic inflammation by the release of histamine and inflammatory mediators. Toxicol Lett. 2012;210(1):64-70. doi: 10.1016/j.toxlet.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 63. Boas M, Frederiksen H, Feldt-Rasmussen U, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118(10):1458-1464. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6-9 years of age: a cross-sectional analysis within the C8 Health Project. Environ Health Perspect. 2016;124(8):1269-1275. doi: 10.1289/ehp.1509869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woodward MJ, Obsekov V, Jacobson MH, Kahn LG, Trasande L. Phthalates and sex steroid hormones among men from NHANES, 2013-2016. J Clin Endocrinol Metab. 2020;105(4):e1225-e1234. doi: 10.1210/clinem/dgaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meeker JD, Ferguson KK. Urinary phthalate metabolites are associated with decreased serum testosterone in men, women, and children from NHANES 2011-2012. J Clin Endocrinol Metab. 2014;99(11):4346-4352. doi: 10.1210/jc.2014-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Soyka LA, Fairfield WP, Klibanski A. Hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85(11):3951-3963. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 68. Braun JM, Smith KW, Williams PL, et al. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect. 2012;120(5):739-745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734-1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teitelbaum SL, Britton JA, Calafat AM, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257-269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 71. Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate,perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298-1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated during and/or analyzed during the current study are publicly available and free of charge.