Abstract
Background
Parkinson’s disease (PD) is genetically associated with the H1 haplotype of the MAPT 17q.21.31 locus, although the causal gene and variants underlying this association have not been identified.
Methods
To better understand the genetic contribution of this region to PD and to identify novel mechanisms conferring risk for the disease, we fine-mapped the 17q21.31 locus by constructing discrete haplotype blocks from genetic data. We used digital PCR to assess copy number variation associated with PD-associated blocks, and used human brain postmortem RNA-seq data to identify candidate genes that were then further investigated using in vitro models and human brain tissue.
Results
We identified three novel H1 sub-haplotype blocks across the 17q21.31 locus associated with PD risk. Protective sub-haplotypes were associated with increased LRRC37A/2 copy number and expression in human brain tissue. We found that LRRC37A/2 is a membrane-associated protein that plays a role in cellular migration, chemotaxis and astroglial inflammation. In human substantia nigra, LRRC37A/2 was primarily expressed in astrocytes, interacted directly with soluble α-synuclein, and co-localized with Lewy bodies in PD brain tissue.
Conclusion
These data indicate that a novel candidate gene, LRRC37A/2, contributes to the association between the 17q21.31 locus and PD via its interaction with α-synuclein and its effects on astrocytic function and inflammatory response. These data are the first to associate the genetic association at the 17q21.31 locus with PD pathology, and highlight the importance of variation at the 17q21.31 locus in the regulation of multiple genes other than MAPT and KANSL1, as well as its relevance to non-neuronal cell types.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13024-022-00551-x.
Keywords: 17q21.31, Parkinson’s disease, LRRC37A, Copy number variation, Astrocytes
Background
The MAPT 17q21.31 locus lies within a 1.5 Mb inversion region of high linkage disequilibrium (LD), conferring two distinct haplotypes; H1, which has a frequency of ~ 0.8 in European ancestry populations, and the less common, inverted H2 haplotype (frequency ~ 0.2), which is absent or lower frequency in East and South Asian populations (frequency 0—0.09) (Fig. 1A). The major haplotype, H1, has been genetically associated with increased risk for multiple neurodegenerative disorders, including APOE ɛ4-negative Alzheimer’s disease (AD) [1], corticobasal degeneration (CBD) [2], progressive supranuclear palsy (PSP) [3–5] and Parkinson’s disease (PD) [6–10].
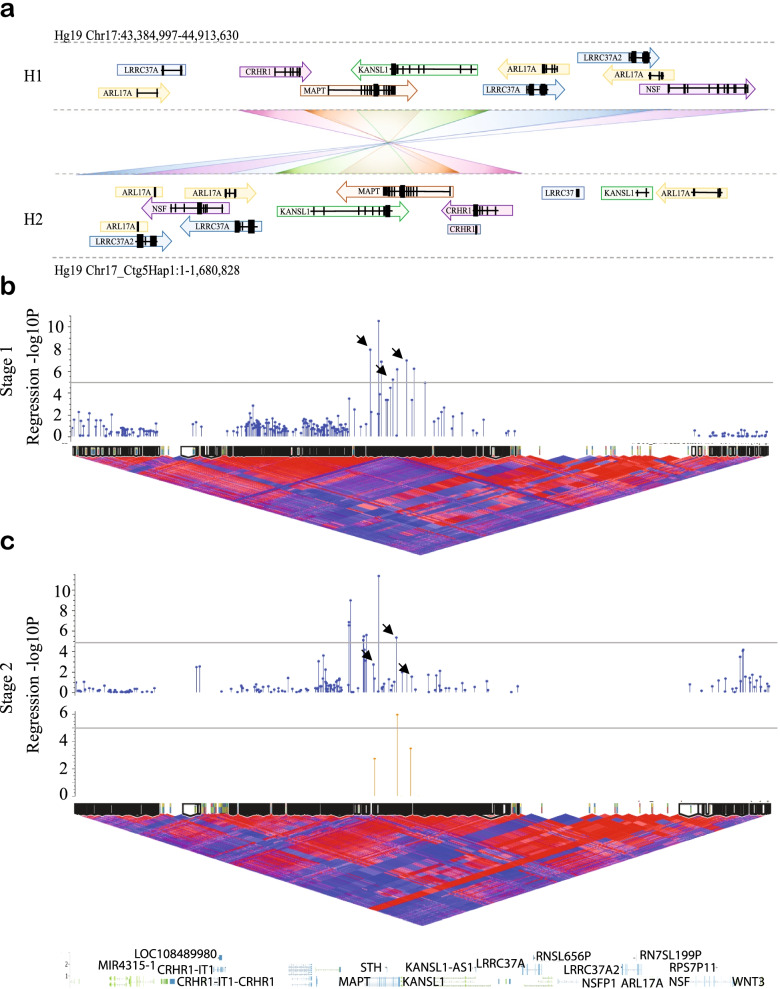
Fig. 1.
H1 sub-haplotypes within the MAPT 17q21.21 inversion region are associated with Parkinson’s disease risk. A. Structure of the 17q21.31 locus, which confers two distinct sub-haplotypes defined by gross structural inversion; H1 and H2. Direction of gene orientation in each haplotype is indicated by arrows. Each gene or partial gene is labeled with a distinct color and connected with a crossed rectangle between H1 and H2 to aid visualization of altered gene position between haplotypes. B-C. H1 sub-haplotype block association (-log10 p-value) with PD plotted above H1 homozygote D’ LD structure and sub-haplotype blocks generated from B. Stage 1 data and C. Stage 2 data, spanning Hg19 Chr17:43,384,997–44,913,630. C. Association (-log10 p-value) of blocks calculated in Stage 2 data (blue, top), and H1.1, H1.2 and H1.3 blocks as defined in Stage 1 applied to Stage 2 data (orange, bottom). In LD plots, red indicates high D’ and blue indicates low. Black arrows indicate similar blocks generated across Stage 1 and Stage 2 data. Grey lines indicate genome wide suggestive significance p-value of 1 × 10–5
PD is a movement disorder that commonly involves executive dysfunction and dementia [11, 12], but is classically characterized by bradykinesia, tremor, rigidity, postural instability, and numerous non-motor symptoms [13]. Neuropathologically, PD is an α-synucleinopathy defined by the presence of intraneuronal accumulation of α-synuclein in Lewy bodies throughout the substantia nigra, brainstem and forebrain [14, 15]. Despite the genetic association with the 17q21.31 MAPT/tau locus, aggregated tau is not a typical neuropathological feature of PD [15], although it is not rare for tauopathy to occur alongside α-synuclein inclusions in the substantia nigra [16, 17]. There is also no apparent association between PD and the H1c sub-haplotype [18, 19], which is strongly associated with risk for the primary tauopathies PSP and CBD [2, 3], indicating that different 17q21.31 locus variants and mechanisms may underlie the relative risk for each disease. Indeed, the 17q21.31 locus spans 1.5 Mb including multiple genes in addition to MAPT, and comprises several sub-haplotypes, defined by complex rearrangements and copy number variation in their distal regions [20, 21], the functional impact of which has not yet been explored. Indicative of this complexity, the 17q21.31 locus is genetically associated with many different phenotypes in addition to neurodegenerative diseases, the mechanisms for which remain largely unknown.
Given the specific loss of midbrain dopaminergic neurons in PD, this cell type has been the main focus of efforts to understand the mechanism of neuronal loss. Similarly, investigation of 17q21.31 variants is also often restricted to neurons, due to the high neuronal expression of MAPT. However, a causal role for astrocytes in PD pathogenesis has been recently proposed [22–25]. Indeed, many PD-associated genes are expressed in astrocytes and have known functional roles in astrocyte biology[24]. Furthermore, α-synuclein-positive aggregates have been identified in the cytoplasm of astrocytes in PD [26–28], indicating aberrant protein accumulation occurring as a result of either neuronal-glial transmission [22, 28, 29], or increased expression of endogenous α-synuclein in astrocytes themselves [25].
Although PD, like PSP and CBD, is associated with the H1 haplotype, the odds ratio for risk is substantially lower (1.5 vs 4.5) in PD [2–10]. Furthermore, in contrast to PSP and CBD, PD has not been associated with the H1c sub-haplotype [18, 19] and is not generally associated with the accumulation of tau. Together these observations suggest that the PD genetic association with the 17q21.31 locus may be driven by genes other than MAPT. Here, we report three sub-haplotype blocks associated with PD risk within the 17q21.31 H1 haplotype clade, with both protective and risk-associated sub-haplotypes within each block. We show that protective sub-haplotypes are associated with increased expression and copy number of LRRC37A/2, which we demonstrate is an astrocyte-enriched membrane-associated protein with a role in chemotaxis and the inflammatory response, and co-localizes with both soluble α-synuclein and Lewy bodies in human substantia nigra. These findings link the genetic association at the 17q21.31 locus with PD pathology, and support the hypothesis of astroglial dysfunction as a key contributing factor to PD disease pathogenesis.
Results
PD risk is associated with the 17q21.31 H1 haplotype
To confirm the genetic association of the 17q21.31 H1 haplotype with PD risk in our specific cohorts, we carried out a case–control association analysis across the region of interest (Figure S1A-E, Table S1) in two independent datasets (Stage 1 2,780 PD cases, 6,384 controls; Stage 2 2,699 cases, 2,230 controls; Table S2). The SNP with the strongest association in Stage 1 (rs17763050, p = 2.74 × 10–9) was in high LD with the known H1/H2 haplotype tag SNP rs8070723 (D’ = 0.98, Figure S1B-C, F). Both SNPs were associated with odds ratios (ORs) ~ 0.8 (95% CI ± 0.1; Figure S1B-C, Table S1), consistent with previously reported effect sizes6,8. Due to a smaller cohort size and consequent lack of power, the association with 17q21.31 was less prominent in Stage 2 data (Figure S1D-E, Table S1), and the top SNP did not tag the H2 haplotype. However, meta-analysis of both cohorts confirmed a significant association between PD risk and the 17q21.31 locus (Figure S1B-C, Table S1), with the H2 haplotype conferring protection (OR 0.82 (95% CI 0.76–0.89)) and the H1 haplotype therefore associated with increased risk.
As the major H1 haplotype was associated with increased risk for PD, we repeated the association analysis in H1 homozygotes alone in order to identify variants of H1 that may confer additional risk for PD (Figure S1G-J, Table S1). While association across the 17q21.31 locus was weaker in H1 homozygotes compared to the full data set, we observed a distinct signal spanning MAPT and KANSL1 in Stage 1 and Stage 2 analyses (Figure S1G-H). The Stage 1 top SNP, rs41543512, was associated with an OR of 1.21 (95% CI 1.10–1.32, p < 0.001; Figure S1I, Table S1), but did not reach statistical significance in Stage 2 data or meta-analysis (Table S1). The most significant SNP in the Stage 2 analysis (rs139217062) was not present in Stage 1 data, although the second most significant variant from Stage 2, rs16940711, was not significant in Stage 1 data or by meta-analysis (Table S1, Figure S1J). These data suggest that the H1 association with PD may be more complex than variation in individual SNPs.
17q21.31 H1 sub-haplotype blocks spanning MAPT & KANSL1 are associated with PD risk
As SNP-based association analyses were unsuccessful in identifying significant H1 sub-haplotypic variants contributing to PD risk, we decided to leverage the presence of high linkage disequilibrium (LD) within this region to investigate sub-haplotype blocks. We calculated discrete sub-haplotype blocks spanning the 17q21.31 locus using the D’ measure of LD, and performed a logistic regression association analysis on each block (Fig. 1B-C, Table 1). This approach greatly improved the power to detect disease-associated H1 variants in both Stage 1 and Stage 2 data. In Stage 1, we observed a peak spanning MAPT and the first 5 exons of KANSL1 (Fig. 1B) that reached the genome-wide suggestive significance threshold of p = 1 × 10–5. Within this peak, three sub-haplotype blocks showed substantial overlap of SNPs in independently calculated blocks in Stage 2 data (Stage 1 blocks H1.1 (p = 1.73 × 10–6), H1.2 (p = 2.4 × 10–4) and H1.3 (p = 1.05 × 10–5); Fig. 1C, Table 1). We then applied the Stage 1-constructed blocks to Stage 2 data and observed replication of their association with PD risk (Fig. 1C, Table 1). Block H1.2 was highly significant in Stage 2 data (p = 1.12 × 10–9), while both blocks H1.1 and H1.3 were nominally significant (p < 0.002 and p < 0.0003, respectively).
Table 1.
17q21.31 H1 sub-haplotype blocks associated with PD susceptibility
| Stage 1 | Stage 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block | #Sub-haplotypes | #SNPs | Start | Stop | Gene | Location | p-value | -log10 p-value | FDR p-value | p-value | -log10 p-value | FDR p-value |
| H1.1 | 5 | 5 | 44,040,184 | 44,041,992 | MAPT | Intronic | 1.35E-08 | 7.87 | 1.73E-06 | 1.94E-03 | 2.71 | 4.50E-03 |
| H1.2 | 8 | 8 | 44,090,196 | 44,097,249 | MAPT | Intronic/Exonic | 6.58E-06 | 5.18 | 2.40E-04 | 1.12E-09 | 5.95 | 7.84E-06 |
| H1.3 | 7 | 9 | 44,119,987 | 44,131,305 | KANSL1 | Intronic | 1.23E-07 | 6.91 | 1.05E-05 | 2.39E-04 | 3.47 | 1.12E-03 |
In order to determine whether the sub-haplotype blocks we identified were merely recapitulating previously defined MAPT sub-haplotypes [30], we estimated the frequency of each MAPT sub-haplotype in Stage 1 data using tag SNPs rs1467967, rs242557, rs3785883, rs2471738 and rs7521. We then overlaid the frequency of these MAPT sub-haplotypes with the frequency of each sub-haplotype across blocks H1.1, H1.2 and H1.3 (Figure S1K). We did not see any association between previously defined MAPT sub-haplotypes and block sub-haplotypes, although H1c was enriched in, but not exclusive to, protective block sub-haplotypes H1.1c, H1.2b and H1.3b (Figure S1K). Furthermore, there was no significant association between any MAPT sub-haplotype and PD risk in these data (Table S3, Figure S1L), indicating that the sub-haplotype blocks we have identified detect variation across the 17q21.31 locus associated with PD risk that are independent of previously defined MAPT sub-haplotypes.
Blocks H1.1, H1.2 and H1.3 each consist of multiple SNPs in high LD (Figure S1M) that generate multiple sub-haplotypes (Figure S2A-F). As many SNPs within these blocks were imputed, we confirmed the existence and frequency of each block and sub-haplotype using whole genome sequence (WGS) data from AMP-PD (Table S4, amp-pd.org), with the exception of H1.1d. This sub-haplotype occurred at a higher frequency than identified in Stage 1 data (~ 0.2 vs 0.06–0.1), and at an equivalent frequency to Stage 2 data (~ 0.2). Further investigation indicated this discrepancy was a result of skewed frequency of the H1.1d tag SNP rs16940758 in the American (NIH) cohort in Stage 1 data, but occurred at a similar frequency to the WGS data in the other three contributing datasets, suggesting a possible technical or imputation error for this variant. All other variants of interest and sub-haplotypes occurred at a similar frequency across cohorts and WGS data (Table S4), indicating accuracy of imputation and sub-haplotype construction.
Each sub-haplotype was differentially associated with PD susceptibility (Figure S2A,C,E, Table 2), with each block containing both risk- and protective sub-haplotypes with ORs ranging from 0.37 (95% CI 0.32–0.42, p = 1.3 × 10–49, H1.1c) to 2.51 (95% CI 2.2–2.86, p = 2.4 × 10–45, H1.1e). Despite the presence of heterogeneity between Stages 1 and 2, the most frequently occurring sub-haplotypes were replicated in both analysis stages and by fixed effects meta-analysis (Table 2). Specifically, we identified two sub-haplotypes in block H1.1; H1.1b and H1.1e, which increased risk in both datasets when compared against the most common haplotype, with ORs ranging from 1.26–1.6 (fixed effects p < 0.001) and 1.45–2.51 (fixed effects p < 0.001), respectively (Figure S2A-B, Table 2). In the same block we also observed a protective sub-haplotype (H1.1c), associated with an OR ranging 0.37–0.96 (fixed effects p < 0.001; Figure S2A-B, Table 2). Blocks H1.2 and H1.3 encompassed multiple sub-haplotypes with frequencies < 0.1, and exhibited greater variability and heterogeneity across stages. However, we identified one risk-associated sub-haplotype in block H1.2 (H1.2c, OR = 1.12–1.31, fixed effects p < 0.01) and two protective sub-haplotypes in block H1.3 (H1.3b; OR = 0.95–0.43, fixed effects p < 0.001, H1.3 g; OR = 0.55–0.83, fixed effects p < 0.002; Table 2, Figure S2C-F). These data reflect the variability and complexity present across the 17q21.31 locus, and indicate that numerous H1 variants exist that are associated with differential levels of risk for PD.
Table 2.
H1 sub-haplotypes associated with PD susceptibility
| Stage 1 | Stage 2 | Meta | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block | Sub-haplotype ID | Sub-haplotype | Frequency (Case/Control) |
OR (95% CI) |
OR Fisher's exact psig | Frequency (Case/Control) |
OR (95% CI) |
OR Fisher's exact psig | RE OR (95% CI) | RE Heterogeneity (p) | RE psig | FE OR (95% CI) | FE Heterogeneity (p) | FE psig |
| H1.1 | H1.1a | ACTCT | 0.25 (0.26/0.24) | 1 | - | 0.21 (0.2/0.23) | 1 | - | 1 | - | - | 1 | - | - |
| H1.1b | ACTTG | 0.27 (0.38/0.22) |
1.6 (1.45–1.78) |
2.04E-19*** | 0.38 (0.39/0.36) |
1.26 (1.09–1.46) |
2.0E-03** |
1.43 (1.13–1.81) |
7.06 (0.0079) |
3.0E-03** |
1.48 (1.36–1.61) |
7.06 (0.0079) |
0*** | |
| H1.1c | GCCTG | 0.18 (0.09/0.23) |
0.37 (0.32–0.42) |
1.3E-49*** | 0.14 (0.13/0.16) |
0.96 (0.79–1.15) |
6.40E-01 |
0.59 (0.23–1.52) |
67.79 (< 0.0001) |
2.70E-01 |
0.51 (0.46–0.57) |
67.85 (< 0.0001) | 0*** | |
| H1.1d | ATCTG | 0.18 (0.06/0.24) |
0.22 (0.19–0.26) |
6.5E-92*** | 0.23 (0.24/0.21) |
1.29 (1.1–1.52) |
2.0E-03** |
0.54 (0.1–3.01) |
231.51 (< 0.0001) |
4.90E-01 |
0.49 (0.44–0.55) |
232.74 (< 0.0001) | 0*** | |
| H1.1e | ACCTG | 0.12 (0.21/0.08) |
2.51 (2.2–2.86) |
2.4E-45*** | 0.02 (0.02/0.02) |
1.45 (0.98–2.17) |
5.70E-02 |
1.97 (1.15–3.36) |
7.13 (0.0079) |
1.3E-02* |
2.37 (2.1–2.68) |
7.13 (0.0076) |
0*** | |
| H1.2 | H1.2a | TTTCGATG | 0.48 (0.44/0.5) | 1 | - | 0.49 (0.49/0.48) | 1 | - | 1 | - | - | 1 | - | - |
| H1.2b | TCTCGATG | 0.17 (0.18/0.16) |
1.27 (1.14–1.4) |
2.02E-05*** | 0.17 (0.17/0.18) |
0.93 (0.8–1.09) |
3.56E-01 |
1.09 (0.81–1.47) |
10.57 (0.001) |
5.70E-01 |
1.14 (1.04–1.24) |
10.58 (0.0011) |
3.0E-03** | |
| H1.2c | TTAAAATA | 0.15 (0.16/0.14) |
1.31 (1.16–1.47) |
4.52E-06*** | 0.19 (0.21/0.18) |
1.12 (0.97–1.3) |
1.21E-01 |
1.22 (1.05–1.42) |
2.65 (0.103) |
9.0E-03** |
1.23 (1.13–1.35) |
2.65 (0.1037) |
4.41E-06*** | |
| H1.2d | TTAAGATG | 0.07 (0.08/0.06) |
1.36 (1.16–1.59) |
1.2E-04*** | 0.04 (0.03/0.05) |
0.52 (0.38–0.69) |
3.46E-06*** |
0.84 (0.33–2.19) |
35.25 (< 0.0001) |
7.27E-01 |
1.07 (0.94–1.23) |
35.28 (< 0.0001) | 2.90E-01 | |
| H1.2e | CTTCGATG | 0.06 (0.06/0.06) |
1.17 (0.99–1.39) |
5.64E-02 | 0.08 (0.08/0.08) |
1.05 (0.85–1.3) |
6.76E-01 |
1.12 (0.99–1.28) |
0.72 (0.40) |
7.00E-02 |
1.12 (0.99–1.28) |
0.72 (0.397) |
7.00E-02 | |
| H1.2f | TTTCGGTG | 0.05 (0.05/0.05) |
1.16 (0.96–1.39) |
1.18E-01 | 0.02 (0.02/0.03) |
0.58 (0.41–0.83) |
7.1E-03** |
0.83 (0.42–1.64) |
12.41 (0.0004) |
6.00E-01 |
0.99 (0.84–1.16) |
12.41 (0.0004) |
9.10E-01 | |
| H1.2 g | TTAAAATG | 0.01 (0.01/0.01) |
1.1 (0.77–1.54) |
6.07E-01 | - | - | - | - | - | - | - | - | - | |
| H1.2 h | TTTCGACG | 0.01 (0.01/0.01) |
1.28 (0.89–1.84) |
1.64E-01 | - | - | - | - | - | - | - | - | - | |
| H1.3 | H1.3a | GACTGAGAT | 0.28 (0.32/0.27) | 1 | - | 0.32 (0.32/0.32) | 1 | - | 1 | - | - | 1 | - | - |
| H1.3b | CATTAGGGC | 0.18 (0.11/0.22) |
0.43 (0.38–0.49) |
1.66E-41*** | 0.18 (0.18/0.19) |
0.95 (0.81–1.11) |
5.22E-01 |
0.64 (0.29–1.44) |
58.53 (< 0.0001) |
2.80E-01 |
0.57 (0.52–0.63) |
58.57 (< 0.0001) | 0*** | |
| H1.3c | GATTGAGAT | 0.17 (0.21/0.16) |
1.11 (0.99–1.24) |
7.37E-02 | 0.16 (0.16/0.17) |
0.95 (0.80–1.12) |
5.33E-01 |
1.04 (0.89–1.21) |
2.24 (0.134) |
6.10E-01 |
1.06 (0.96–1.16) |
2.24 (0.1343) |
0.23 | |
| H1.3d | CTTTGGTGC | 0.15 (0.12/0.16) |
0.61 (0.54–0.7) |
1.58E-14*** | 0.2 (0.21/0.18) |
1.16 (0.99–1.36) |
5.81E-02 |
0.84 (0.45–1.56) |
35.27 (< 0.0001) |
5.80E-01 |
0.77 (0.7–0.85) |
35.28 (< 0.0001) | 2.96E-07*** | |
| H1.3e | CATTGGGGC | 0.09 (0.14/0.07) |
1.67 (1.45–1.92) |
2.79E-13*** | 0.02 (0.02/0.02) |
0.81 (0.54–1.21) |
2.83E-01 |
1.19 (0.58–2.42) |
12.01 (0.0004) |
6.30E-01 |
1.54 (1.35–1.75) |
12.01 (0.0005) |
4.57E-11*** | |
| H1.3f | CATTGGGGT | 0.08 (0.07/0.09) |
0.6 (0.51–0.70) |
9.34E-11*** | 0.09 (0.1/0.08) |
1.15 (0.93–1.42) |
2.00E-01 |
0.81 (0.44–1.5) |
21.37 (< 0.0001) |
5.10E-01 |
0.74 (0.66–0.84) |
21.38 (< 0.0001) | 3.59E-06*** | |
| H1.3 g | GATCGAGAT | 0.04 (0.04/0.04) |
0.83 (0.67–1.03) |
9.39E-02 | 0.03 (0.02/0.02) |
0.55 (0.38–0.78) |
5.58E-04*** |
0.7 (0.49–1.02) |
3.48 (0.062) |
6.60E-02 |
0.75 (0.63–0.9) |
3.48 (0.062) | 1.6E-03** | |
OR Odds ratio, CI Confidence interval, RE Random effects meta-analysis, FE Fixed effects meta-analysis. *p < 0.05, **p < 0.01, ***p < 0.001
PD-associated sub-haplotypes are associated with LRRC37A/2 gene expression in human brain
In order to elucidate the functional consequences of PD-associated H1 block sub-haplotypes we queried publicly available post-mortem human brain RNA-seq data from dorsolateral prefrontal cortex (PFC) and temporal cortex (TCX) from the AMP-AD and CommonMind consortia (Table S5). Despite the position of the H1 association peaks across MAPT and KANSL1, we did not observe any differences in the expression of either of these genes between any sub-haplotypes in any block (Fig. 2C, Figure S3A-C). The only genes within the 17q21.31 locus that had a significant association with H1 PD-associated sub-haplotypes were LRRC37A and its paralog LRRC37A2 (a.k.a LRRC37A/2; Fig. 2A-B, Figure S3D). We observed significantly increased LRRC37A/2 expression in protective sub-haplotypes, specifically in H1.1c and H1.3b (H1.1c ~ 4.7 fold, p < 0.001, H1.3b ~ fivefold, p < 0.001), as well as in sub-haplotypes whose effects were not replicated across PD data-sets; H1.2b and H1.3e (H1.2b ~ 5, p < 0.001, H1.3e ~ 2.9x, p < 0.01), but were protective in the Stage 2 PD analysis (Fig. 2A-B). qRT-PCR on postmortem prefrontal cortex from a small number of individuals supported the observation of increased LRRC37A/2 expression in these sub-haplotypes (Figure S3D).
Fig. 2.
PD-associated sub-haplotypes are associated with LRRC37A/2 expression and copy number A-C. Expression of A. LRRC37A, B. LRRC37A2 and C. MAPT in human brain tissue, measured by RNA-seq across three different cohorts, split by sub-haplotype in blocks H1.1, H1.2 and H1.3. D. Schematic of the regions of copy number variation in the 3’ distal end of the 17q21.31 locus, as defined by Boettger et al. 2012[20]. Black arrows indicate the location of dPCR probes for (left to right) beta, alpha, LRRC37A and gamma. E-G. Copy number of gamma and LRRC37A/2 regions in blocks E. H1.1, F. H1.2 and G. H1.3. H-I. Copy number of H. gamma and I. LRRC37A/2 regions between H1 and H2 homozygotes. All statistical comparisons are against the most common sub-haplotype. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001.
We observed a significant reduction in LRRC37A/2 expression in the PD risk-associated H1.1b sub-haplotype by qRT-PCR in human brain tissue (p < 0.01; Figure S3D), but not in the RNA-seq data. We also did not observe any difference in MAPT exons 2, 3 or 10 percent spliced in (PSI) values between sub-haplotypes (Figure S4A). Interestingly, LRRC37A/2 expression was significantly higher in the protective 17q21.31 H2 haplotype (Figure S4B), which is consistent with our observation of increased LRRC37A/2 expression in protective H1 sub-haplotypes. These data suggest that both the 17q21.31 H1/H2 and H1 sub-haplotype genetic associations with PD risk may be due to variable LRRC37A/2 expression.
Protective sub-haplotypes are associated with increased LRRC37A/2 copy number
The 17q21.31 locus is structurally complex and encompasses regions of copy number variation (CNV) at its distal ends [20, 21]. As sub-haplotype blocks within MAPT and KANSL1 were associated with altered LRRC37A/2 expression, we tested whether they also tagged structural variants [20] in individuals homozygous for sub-haplotypes of interest (Fig. 2D-H, Figure S5A-D). Using DNA derived from either blood or brain tissue (Table S5) we performed digital PCR (dPCR) for MAPT, alpha, beta and gamma regions (Fig. 2D) [20], as well as for LRRC37A/2 specifically (Fig. 2E-G, Figure S5A-D).
The majority of the structural variation in alpha and beta regions were found in the most common sub-haplotype for each block (H1.1a, H1.2a and H1.3a), with each subsequent sub-haplotype carrying fewer copies of these regions (Figure S5A-C). However, gamma and LRRC37A/2 CNVs varied by sub-haplotype within each block; those sub-haplotypes exhibiting increased LRRC37A/2 expression were also associated with significantly increased gamma (H1.1c R2 = 0.23, H1.2b R2 = 0.25, H1.3b R2 = 0.34, ~ 3–5 copies compared to 2–3 copies in controls, p < 0.05) and/or LRRC37A/2 copy number (H1.1c R2 = 0.06, H1.2b R2 = 0.08, H1.3b R2 = 0.09, ~ 8–11 copies compared to 5–10 copies in controls, p < 0.05; Fig. 2E-G). Interestingly, risk-associated sub-haplotype H1.1b was associated with increased gamma and LRRC37A/2 copy number (p < 0.01; Fig. 2E) but not LRRC37A/2 expression (Fig. 2A-B), indicating that additional factors likely contribute to LRRC37A/2 expression and PD risk in this locus, such as chromatin looping or variants within LRRC37A/2 itself. Consistent with the expression data, we also observed increased beta, gamma and LRRC37A/2 copy number in H2 homozygotes compared to H1 (Figure S5D). These data suggest that structural variation at the distal end of the 17q21.31 locus may underlie increased expression of LRRC37A/2 in protective H2 and H1 sub-haplotypes.
LRRC37A is a membrane-associated protein implicated in cellular migration, chemotaxis and inflammation
As PD sub-haplotypes converge on the expression and/or copy number of LRRC37A/2, we explored the likely function of this gene. We carried out RNA-seq analysis in HEK293T cells overexpressing LRRC37A/2 in order to mimic increased copy number and to elucidate a potential function for this gene. The number of significantly differentially expressed protein-coding genes (fold change ± 1.5, adjusted p < 0.05) in the context of LRRC37A/2 overexpression was minimal (28 upregulated, 21 downregulated), suggesting that LRRC37A/2 is unlikely to play a major regulatory role. In order to confirm that we were not observing spurious changes in gene expression due to gross overexpression in a cell culture model, we carried out a titration of LRRC37A/2 overexpression in HEK293T cells. We observed dose-dependent changes in the expression of genes that were significantly up or downregulated in our RNA-seq data (Figure S6A-D), confirming that the expression of these genes is likely to be altered by LRRC37A/2 expression.
Functional enrichment of gene ontology (GO) terms for significantly differentially expressed genes indicated a role for LRRC37A/2 at the cell membrane (Fig. 3A-B, Figure S6E-F), which we confirmed by western blot in HEK293T cells (Figure S6G). In addition, LRRC37A/2 overexpression also resulted in significant enrichment for cell communication (GO:0,007,154, p < 0.05) and neuroactive ligand-receptor interaction (KEGG:04,080, p < 0.05) pathways, as well as nominal enrichment for membrane-component-related pathways (Figure S6F).
Fig. 3.
LRRC37A/2 expression is associated with increased cellular migration, chemotaxis and inflammation. A-B. Significantly enriched A. Migration of cells and B. Chemotaxis pathways associated with LRRC37A/2 overexpression, derived from Ingenuity pathway analysis. Red genes indicate upregulation, green indicates downregulation C. qRT-PCR for LRRC37A expression in H1 and H2 homozygote iPSC-derived neurons and astrocytes. N = 3 in duplicate. ns = not significant, *p < 0.05. D. Western blots for LRRC37A/2 in cytosolic (C) and membrane (M) fractions from (top) iPSC-neurons and (bottom) iPSC-astrocytes. Cytosolic fractions were confirmed by labeling with an anti-HSP90 antibody, and membrane fractions were confirmed by labeling with an anti-Pan-Cadherin antibody, N = 6. E. Proportion of LRRC37A2-expressing astrocytes by 17q21.31 haplotype from human prefrontal cortex snuc-seq data [33]. Dot size = proportion of cells expressing LRRC37A2, depth of color = LRRC37A2 expression level. F. Representative immunofluorescence images of iPSC-derived astrocytes expressing either scrambled control or LRRC37A2 shRNA lentivirus constructs, labeled with antibodies against either IL-16 or IL-32. Scale bar = 10 µm. G. Quantification of mean grey intensity of astrocytes in immunofluorescence images represented in F. Each point represents the average of 12–16 individual images from an individual cell line. Four cell lines were analyzed. Points from the same iPSC line are connected between scrambled and LRRC37A2 shRNA conditions. Overall p-value indicates paired t-test of cell line image means. Asterisks denote significant between scrambled and LRRC37A2 conditions for each paired line. *p < 0.05, ***p < 0.001, ns = not significant. H. Wound confluence (%) of scratch wound repair over 50 h in scrambled control (dashed lines) and LRRC37A2 shRNA (solid lines) treated iPSC-derived astrocytes treated with either 200 ng/ml α-synuclein monomers (orange) or PBS (green). Statistical difference between groups over time was determined by ANCOVA and post-hoc Tukey tests. *p < 0.05, ***p < 0.001. N = 4. I. Representative images with quantification masks of iPSC-astrocytes at 0 h,16 h and 24 h following induction of a scratch wound in scrambled control (top) and LRRC37A2 shRNA (bottom) treated cells, with (right panels) and without (left panels) incubation with 200 ng/ml α-synuclein. Yellow masks indicate cell confluence, blue indicates the scratch wound area, and grey indicates cellular migration into the original wound area. Scale bar = 600 µm
Ingenuity pathway analysis (IPA) also suggested a role for LRRC37A/2 at the plasma membrane and in the extracellular space; specifically, these analyses indicated that increased LRRC37A/2 expression resulted in upregulated cellular movement pathways, such as increased migration of cells (p < 0.01, z score = 1.85; Fig. 3A) and upregulation of chemotaxis (p < 0.05, z score = 0.918; Fig. 3B). Several upregulated genes within these pathways are also essential in regulation of the inflammatory response; both IL17F and IL32 are pro-inflammatory cytokines that mediate the inflammatory response of astrocytes [31, 32], whereas IL16 acts as a chemoattractant for cells expressing CD4. These data therefore suggest that increased LRRC37A/2 expression may mediate astroglial inflammation and modify cellular migration in response to a stimulant, such as α-synuclein.
LRRC37A/2 is expressed in astrocytes and impacts cellular migration and response to α-synuclein
Our pathway analysis indicated that LRRC37A/2 is associated with cellular signaling and pro-inflammatory pathways relevant to astroglial function. We therefore sought to confirm whether LRRC37A/2 was expressed and functional in these cells. As LRRC37A/2 expression and copy number was higher in 17q21.31 H2 haplotype carriers compared to H1, and due to difficulty in identifying multiple human iPSC lines with specific H1 sub-haplotypes, we compared the expression of LRRC37A/2 and genes altered by LRRC37A/2 expression in iPSC-derived neurons and astrocytes homozygous for either H1 or H2 haplotypes in order to identify the most relevant neural cell type (Fig. 3C, Figure S6H). While LRRC37A/2 was expressed in both cell types, we observed increased expression of LRRC37A/2 and associated genes in H2 astrocytes, but not in H2 neuronal cultures (Fig. 3C, Figure S6H), suggesting that H1/H2-associated LRRC37A/2 expression changes may be specifically impacting astroglial gene expression and function. We also confirmed that LRRC37A/2 was present in the plasma membrane in both iPSC-derived neurons and astrocytes by isolating cytosolic and membrane-associated proteins from each cell type and analyzing the resulting fractions by western blot (Fig. 3D). We further confirmed that 17q21.31 haplotype may be more relevant to astrocyte function by comparing LRRC37A/2 expression in single nuclei sequencing data (snuc-seq) from human prefrontal cortex [33] (Figure S6I). We found that LRRC37A2 was more highly expressed in all cell types compared to LRRC37A, indicating that this may be the driver behind any functional differences due to haplotype (Figure S6J). Furthermore, there was a clear H2 dose-dependent effect on the proportion of LRRC37A2-expressing astrocytes and level of expression (Fig. 3E) although due to the small population of astrocytes in this dataset (Figure S6I), the difference in LRRC37A2 expression between H1H1 and H2H2 astrocytes did not pass multiple test correction. In contrast, while there were fewer LRRC37A2-expressing cells in H1H1 cells compared to H1H2 and H2H2, there was no dose-dependent effect of the H2 haplotype on LRRC37A2 expression in either excitatory or inhibitory neuronal populations (Figure S6K).
To further validate the function of LRRC37A2 expression in astrocytes, we knocked down its expression in iPSC-derived astrocytes using lentiviral LRRC37A2 shRNA, which was confirmed by qRTPCR (Figure S7A). We then confirmed that genes that were upregulated in HEK293T cells in response to LRRC37A2 overexpression were reduced in astrocytes following LRRC37A2 knockdown (Figure S7A), thus validating the relevance of our HEK293T gene expression data. In addition, consistent with the RNA-seq data, we then demonstrated significantly reduced expression of cytokines IL-32 and IL-16 in LRRC37A2 knock-down astrocytes by immunofluorescence in 3/4 donor lines examined (Fig. 3F-G, Figure S7B-C). In order to determine whether LRRC37A2 expression was involved in cellular migration and response to stimulants, we conducted a scratch wound repair assay in the presence or absence of monomeric α-synuclein and measured the rate of wound repair over the course of 50 h (Fig. 3H-I). In the absence of α-synuclein, there was no significant difference in the rate of wound repair (% wound confluence) over time between LRRC37A2 shRNA and scrambled control groups (p > 0.05). Treatment with monomeric α-synuclein increased the rate of wound repair in scrambled control astrocytes (p = 0.05) indicating a responsiveness to α-synuclein as a stimulant. However, LRRC37A2 knock down astrocytes demonstrated no such response to α-synuclein exposure, and their rate of wound repair was significantly slower than compared to controls treated with α-synuclein (p < 0.001) (Fig. 3H-I). These data confirm a likely role for LRRC37A2 expression in disease-relevant astrocytic functions including inflammation, cellular migration and responsiveness to α-synuclein.
LRRC37A2 interacts with soluble and aggregated α-synuclein in human substantia nigra.
In order to assess whether LRRC37A/2 was expressed in mature astrocytes in human brain tissue, we carried out multiplex immunofluorescence staining in human substantia nigra from PD, PSP and aged controls (Fig. 4A). We found that in all cases, LRRC37A/2 co-localized with the astrocyte marker GFAP, but not the microglia marker IBA1 (Fig. 4A, Figure S7D). In contrast, α-synuclein positivity was observed only in PD substantia nigra, and hyperphosphorylated tau (labeled with AT8) was present only in PSP brain (Fig. 4A). Interestingly, in regions with Lewy body pathology there was reduced staining intensity of LRRC37A/2 in astrocytes, and colocalization of LRRC37A/2 with Lewy bodies (Fig. 4A). However, there was no association between tau AT8 positivity and LRRC37A/2 expression in PSP substantia nigra (Fig. 4A), indicating that LRRC37A/2 accumulation is specific to PD pathology. To validate the association of LRRC37A/2 with α-synuclein, we performed co-immunoprecipitation from control, PSP and PD substantia nigra tissue (Fig. 4B). We found that soluble α-synuclein bound LRRC37A/2 in all cases, whereas IgG alone did not (Fig. 4B), indicating that LRRC37A/2 and α-synuclein likely form a complex in human brain that becomes disordered in the context of PD pathogenesis. Furthermore, analysis of publicly available snuc-seq data from PD and control midbrain [34] indicated a trend towards reduced LRRC37A2 expression in PD astrocytes compared to controls (Figure S7E-F), consistent with a potentially protective effect of increased LRRC37A/2 expression. These data are not only the first to identify a role for LRRC37A/2 in astrocytes, but are the first to link the genetic association at the 17q21.31 locus with PD pathology.
Fig. 4.
LRRC37A/2 is expressed in astrocytes in the substantia nigra and is co-localized with α-synuclein A. Representative images from multiplex immunofluorescent staining of human control, PSP and PD substantia nigra sections with astrocyte marker GFAP, LRRC37A, α-synuclein and pathologically phosphorylated Tau (AT8). Scale bar = 100 µm, N = 4-5. B. Co-immunoprecipitation (Co-IP) of LRRC37A (top panel) with soluble α-synuclein (bottom panel) in substantia nigra from control (C), PSP and PD brain, N = 3. Whole protein lysates and IgG only controls were included for comparison
Discussion
By constructing discrete sub-haplotype blocks across the 17q21.31 H1 locus, we have identified multiple novel H1 sub-haplotypes associated with variable levels of PD risk, independent of the previously constructed sub-haplotypes across MAPT [30]. The association of some of these blocks with PD risk was inconsistent between stage 1 and stage 2 analyses, possibly due to genotyping and imputation error. We were able to identify blocks and sub-haplotypes with common protective effects across populations. The MAPT gene, encoding the microtubule associated protein tau, is central to this locus and is likely the causal gene for other neurodegenerative disorders genetically associated with the H1 haplotype, such as PSP and CBD [2, 3], given that they are characterized neuropathologically by tau hyperphosphorylation and accumulation. Recently, reduced expression of MAPT-AS1 has been identified in PD substantia nigra [38] and has been shown to have an inverse correlation with MAPT expression [39], suggesting a potential role for MAPT expression in PD pathogenesis. However, we did not observe any association between MAPT expression or splicing and 17q21.31 haplotype or sub-haplotypes in our analyses, indicating that any disease-associated MAPT regulation in PD brain may not be due to haplotype. Furthermore, while tau pathology can occur alongside α-synuclein inclusions in the substantia nigra [16, 17], it is not a typical neuropathological feature of PD [15]. The causal gene underlying the genetic association across the 17q21.31 locus with PD risk has therefore been unclear, although a recent study proposed that H1-associated variants in KANSL1 alter mitophagy [40]. In contrast, we did not find any effect of our PD-associated H1 sub-haplotypes on either KANSL1 expression or copy number, but did observe a consistent association with the expression of another gene in the locus, LRRC37A/2. However, these data do not rule out that KANSL1 variants may alter disease risk independently of LRRC37A/2 expression between the major haplotype clades and H1 sub-haplotypes.
We found that protective H1 sub-haplotypes were associated with increased expression of LRRC37A/2. This is consistent with increased expression of these genes in the protective H2 haplotype, suggesting that there is likely a shared mechanism of protection from PD between H2 and specific sub-haplotypes of H1. Furthermore, analysis of CNVs in the 3’ distal end of the 17q21.31 locus suggested that protective sub-haplotypes were tagging structural variants defined by increased gamma region [20] and LRRC37A/2 copy number, which likely underlies the increased expression of these genes. LRRC37A is a core duplicon on chromosome 17 [41] and is present at the inversion breakpoint of the 17q21.31 locus; it has been hypothesized that its propensity for CNVs is responsible for the evolutionary toggling of this region that resulted in the distinct H1 and H2 haplotypes [42]. Due to the complex structural variation surrounding LRRC37A and the presence of its paralog LRRC37A2, it is challenging to genotype or sequence this region of the genome. As such, our analyses have not been able to separate the contribution of each gene and have considered them together, however LRRC37A2 expression was consistently higher in both neurons and glial cells than LRRC37A and may therefore be the most relevant gene. As a consequence of the CNVs in this region, genotyping data across LRRC37A and LRRC37A2 is of low confidence and quality, and as such is excluded from GWAS analyses, as is visible in any association plot, and likely explains why the peak genetic signal for PD association was close to MAPT and KANSL1. The association between LRRC37A/2 variants and any disease or phenotype has therefore never been tested, and it is likely that additional variation within LRRC37A/2 itself is contributing to its altered expression and function that may also impact PD risk. Little is known about the function of LRRC37A/2, although it has been associated with an increased immune and inflammatory response [43], as well as with cellular migration and synapse formation [41]. Despite conducting our RNA-seq analysis of LRRC37A/2 overexpression in HEK293T cells, we observe enrichment of pathways consistent with these data; we found that increased LRRC37A/2 expression upregulated cellular migration and chemotaxis pathways, which are both essential mechanisms involved in wound healing and inflammation. Furthermore, we validated these gene expression changes and demonstrated a role for LRRC37A2 in astroglial migration in iPSC-derived astrocytes.
Within these pathways we observe increased expression of pro-inflammatory cytokines IL17 and IL32, as well as the chemoattractant IL16, each of which are involved in the astrocytic inflammatory response [31, 32]. Neuroinflammation of the substantia nigra is considered a characteristic feature of PD in addition to neuronal loss [44, 45], and many genes associated with PD, such as GBA, LRRK2 and PINK1 are thought to have a role in the inflammatory response in astrocytes [24, 46]. Furthermore, the most significantly enriched pathway in our analysis, Neuroactive-ligand receptor interaction, is also involved in the inflammatory response and has previously been associated with PD; this pathway was significantly enriched in a functional assessment of PD GWAS signals [47], and is targeted by microRNAs that are upregulated in a Drosophila model of PD [48]. We also observe upregulation of TGFA, the infusion of which into the forebrain of a rat model of PD increased the proliferation of neuronal and glial progenitors and the production of dopaminergic neurons to the substantia nigra [49], indicating that this may be a protective growth factor against neuronal loss in PD. LRRC37A/2 overexpression in HEK293T cells was therefore able to recapitulate pathways associated with PD, despite being a cell type with limited relevance to PD pathogenesis.
As these expression data were indicative of pathways relevant to astrocyte biology, and we found that LRRC37A/2-associated gene expression changes were apparent in astrocytes but not neurons, we hypothesized that this was likely the most relevant cell type for LRRC37A/2 expression. Indeed, in human substantia nigra tissue we observe localization of LRRC37A/2 specifically in astrocytes. The contribution of astrocytic dysfunction to PD pathogenesis has gained attention in recent years, and is hypothesized to be a causal mechanism for the initiation and progression of PD [22–25]. Many genes associated with PD risk are expressed in astrocytes, the functions of which converge on the inflammatory response, lipid handling, mitochondrial health and lysosomal function [24]. Our finding that LRRC37A/2 is expressed in astrocytes and plays a role in the inflammatory response is therefore consistent with known pathogenic mechanisms of PD. Furthermore, we demonstrate that LRRC37A2 expression may play a role in the detection of extracellular monomeric α-synuclein by astrocytes, and consequently impacts migration and tissue repair. α-synuclein has previously been identified as a stimulant for astrocytes [50], inducing a reactive state [51]. This mechanism was impaired in the context of reduced LRRC37A2 expression, indicating that appropriate detection and response to stimulants by astrocytes is likely a protective mechanism against neuronal death.
However, the role of astroglial inflammation in PD is unclear [22]. As a further complication, in vitro studies of human astrocyte cultures indicate that α-synuclein induces the release of pro-inflammatory cytokines [22, 25], but these cells also release protective molecules such as GDNF in response to dopaminergic neuronal damage, and such trophic support may benefit neuronal survival [22]. In addition, the substantia nigra is considered to be particularly susceptible in PD as dopaminergic neurons in this region are surrounded by the lowest proportion of astrocytes in the brain [52]. Whether an inflammatory response in this context would be protective or exacerbate neuronal death is therefore unknown. As our data suggest increased LRRC37A/2 expression is protective and associated with increased expression of pro-inflammatory cytokines, astroglial inflammation in response to α-synuclein may therefore be protective.
Interestingly, we observe an interaction between LRRC37A/2 and soluble α-synuclein, as well as co-localization of LRRC37A/2 with Lewy bodies in PD substantia nigra. The function and mechanism of this interaction is untested, although it is likely that a complex is formed in astrocytes and propagated to neurons. iPSC-astrocytes have been reported as expressing low levels of endogenous α-synuclein, which is increased in cells derived from PD patients [25], and α-synuclein released from neuronal axon terminals is taken up by astrocytes [28], which can be further transferred to neurons [23, 29]. This raises the possibility that LRRC37A/2 may influence α-synuclein release, aggregation and/or propagation.
Conclusions
In conclusion, we have identified novel sub-haplotype variants of the 17q21.31 H1 clade significantly associated with protection against PD. While the genetic association across this locus is typically ascribed to MAPT or KANSL1, we find evidence for the involvement of a novel gene, LRRC37A/2, in PD risk. We propose that in a similar mechanism to other PD-associated genes, LRRC37A/2 is expressed in astrocytes and plays a role in the regulation of astroglial inflammation, specifically in the release of pro-inflammatory cytokines, chemotaxis and cellular migration. Importantly, we demonstrate that LRRC37A/2 interacts and co-localizes with α-synuclein and Lewy bodies, thus indicating a potential modifying role in the formation of PD pathology. These findings link the genetic association at the 17q21.31 H1 locus with PD pathology, and support the hypothesis of astroglial dysfunction as a key contributing factor to PD disease pathogenesis.
Methods
Genotype data treatment
Case and control data from several cohorts from the International Parkinson’s Disease Genetics Consortium (IPDGC; NIA, GER, FIN, NL, SP, McGill) [6, 9, 10] was kindly shared by Drs. Nalls, Singleton and Bandres-Ciga (NIH, Bethesda, MD; Tables S2, S6).
Pre-imputation QC
Each dataset was obtained with different QC filters already applied, and so were all subsequently passed through the same, more stringent QC pipeline to ensure consistency. Plink v1.9 [53] was used to perform quality control for all datasets. First, SNPs were filtered by a 98% call rate, and remaining SNPs with a MAF < 1% were excluded. Individuals with < 98% genotyping rate were then removed. To determine and correct for population stratification, principal components analysis was carried out in combination with Hapmap YRI, CEU and CHB populations [54] using EIGENSOFT [55]. Samples that did not cluster with the CEU European ancestry population were excluded. Identity by descent analysis was then conducted, and related individuals or potential sample duplicates (Z0 ≤ 0.8) were removed. We were unable to assess discordant sex information on data acquired from other sources, as the required information for this analysis was not provided to us. Variants that deviated from Hardy Weinberg equilibrium at a significance threshold < 1 × 10–4 were then removed. Chromosome 17 was then isolated and screened for strand mismatches.
Imputation and post-imputation QC
Filtered chromosome 17 data from each cohort was submitted individually to the Michigan imputation server [56] (https://imputationserver.sph.umich.edu) and imputed against the HRC r1.1 2016 panel using Eagle v2.3 phasing. Following imputation, SNPs with an r2 < 0.3 were removed, and remaining SNPs were filtered for a 99% call rate. Genotyping call rates for individuals were again filtered at 99%, and SNPs that deviated from Hardy–Weinberg equilibrium at a significance threshold < 1 × 10–6 were excluded. Individual cohorts were then merged, and finally filtered once more with a SNP call rate of 99%. Prior to analysis, variants were filtered to exclude SNPs with a MAF < 0.01.
Single SNP association analyses
Logistic regression association analyses using an additive model were carried out in SNP and Variation Suite v8.8.1 (SVS8) software (Golden Helix, Inc., Bozeman, MT, www.goldenhelix.com). As all potential covariate information was not available, the model was corrected using the first 10 principal components as calculated by SVS8. Associations were initially carried out on the entire cohort in order to confirm the 17q21.31 H1/H2 haplotype association. The data was then filtered for H1 homozygotes only, using tag SNP rs8070723 and the association analysis was repeated with the same parameters.
Meta-analysis of SNP effects across multiple datasets was carried out using the R package rmeta [57] using both Random Effects (DerSimonian-Laird) and Fixed Effects (Mantel–Haenszel) approaches. Calculation and visualization of linkage disequilibrium (LD) over large genomic ranges was carried out in SVS8 using both r2 and D’. Inspection of LD between individual SNPs of interest was carried out using Haploview [58].
Haplotype block construction and association
Haplotype blocks were constructed in SVS8 using the D’ measure of LD. Blocks were defined using guidelines as described by Gabriel et al. (2002) [59]. Each block contained a maximum of 15 markers within 160 kb of each other, with a D’ upper confidence bound ≥ 0.98 and a lower confidence bound ≥ 0.7. Haplotypes were estimated using an expectation–maximization (EM) algorithm with 50 iterations, and a convergence tolerance of 0.0001. Sub-haplotypes with a frequency < 0.01 were excluded from further analysis. Case–control association analyses were carried out per block using a logistic regression model. Odds ratios and associated Fisher’s exact p-values were calculated for each sub-haplotype within each block using the R package epitools [60].
MAPT sub-haplotypes were defined as previously described [30, 61]. Sub-haplotypes were estimated for each individual in Stage 1 data as described above, and case–control association analysis was conducted for association with each sub-haplotype as described above.
WGS data
A subset of processed whole genome sequencing data was obtained from the AMP-PD Parkinson’s Progression Markers Initiative (PPMI) (amp-pd.org/unified-cohorts/ppmi). Data were further processed using a publicly available, in-house WGS QC pipeline (https://github.com/ricardovialle/WGS-QC-Pipeline). Briefly, data were filtered to exclude variants with MAF < 1%, missingness > 5%, read depth < 200,000, mapping quality < 58.75 and > 61.25, and with an inbreeding coefficient < -0.8. Samples were excluded with a missingness > 10% and relatedness threshold > 0.125. This resulted in a final dataset of 3074 individuals. Sub-haplotypes were phased and estimated as described above, and frequencies for each H1.1, H1.2 and H1.3 sub-haplotype were determined.
Human brain expression analysis
Publicly available RNA-seq expression data from human postmortem dorsolateral prefrontal (PFC) and temporal (TCX) cortices (Table S5) and associated genotype data were obtained from Synapse (synapse.org; The Religious Orders Study and Memory and Aging Project (ROSMAP) syn3219045; MayoRNAseq syn5550404; CommonMind Consortium syn2759792). Genotype data for chromosome 17 underwent the same QC and imputation pipeline as described above. Data were stratified by 17q21.31 H1/H2 haplotype using the H2 tag SNP rs8070723. For sub-haplotype analysis, blocks previously defined in the PD analysis were applied to the genotype data and haplotypes were estimated in the same manner. Statistical analysis was carried out in R version 3.4.0. For analysis of MAPT splicing, percent spliced in (PSI) values were generated for exons 2, 3 and 10 using Mixture of Isoforms (MISO) [62]. Gene expression and PSI residuals were generated by linear regression using sex, age of death, post-mortem interval and RNA integrity score as covariates. The resulting residuals were then transformed into z-scores and combined across datasets. Statistical differences in gene expression between genotypes and sub-haplotypes were determined by linear regression applied to the adjusted and combined z-scores.
dPCR
Human genomic DNA and accompanying genotype data was kindly provided by Drs. Raj, Crary and Charney (Mount Sinai School of Medicine, NY) and by the Alzheimer’s Disease Research Center (ADRC; Table S5). Sub-haplotypes were called from these genotype data in the same manner as described above. To examine copy number variation in the 17q21.31 locus, digital PCR was carried out using the ThermoFisher QuantStudio 3D digital PCR chip system. Taqman dPCR probes for loci within the alpha, beta and gamma CNV regions [20], as well as within LRRC37A and MAPT were selected for analysis (Table S7).
Cell lines
Human induced pluripotent stem cells (iPSCs) were obtained from the Knight Alzheimer’s Disease Research Center at Washington University [63], the NIH Childhood-onset Schizophrenia study [64], the New York Stem Cell Foundation (NYSCF) and the New South Wales Brain Bank (NSWBB) (Table S8). The Icahn School of Medicine at Mount Sinai IRB reviewed the relevant operating protocols as well as this specific study and determined it was exempt from approval.
Cell culture
Unless otherwise specified, all cell culture materials were obtained from ThermoFisher Scientific. Human embryonic kidney cells (HEK293T) were cultured in Dulbecco's Modified Eagle Medium/F-12 with HEPES, supplemented with 10% fetal bovine serum (FBS) and 1% Penicillin–Streptomycin. Cells were passaged every 3–4 days using Trypsin–EDTA (0.25%). For LRRC37A/2 overexpression experiments, HEK293T cells were seeded at a density of 1.4 × 105 cells per well in 6-well plates and transfected with 0.5–2.5ug of LRRC37A plasmid (Origene) or empty vector control (Origene) using Lipofectamine 3000. Cells were harvested 48 h after transfection.
For qRTPCR, protein biochemistry and LRRC37A2 knockdown experiments, iPSC lines (Table S8) were maintained in complete StemFlex media supplemented with 1% penicillin/streptomycin on Matrigel (BD biosciences), and were differentiated to neural progenitor cells (NPCs) as previously described [65]. Forebrain neuron-enriched cultures and astrocyte cultures were differentiated from NPCs as previously described [65, 66]. Neuronal and astrocytic identity was confirmed by immunofluorescence for common neuronal and astrocytic markers (MAP2 (Abcam), Tuj1 (Cell Signaling Technologies), S100β (Sigma Aldrich) and EAAT1 (Abcam).
Genomic DNA was extracted using the DNeasy Blood and Tissue kit (Qiagen) and underwent genotyping with Taqman assays for H2 tag SNPs rs8070723 and rs1052553 in order to confirm 17q21.31 haplotype.
LRRC37A2 knockdown
iPSC-derived astrocytes from four donors were seeded at low density in a 24-well plate, and were infected with either LRRC37A2 shRNA viral particles (Santa Cruz) or scrambled control viral particles (Origene) with an MOI = 10 for 24 h. Infected cells were then washed with fresh media, then maintained and expanded as previously described [67]. LRRC37A2 knockdown was confirmed by qRT-PCR using commercially available Taqman probes, and was normalized to GAPDH expression.
Scratch wound assay
LRRC37A2 and scrambled control shRNA-treated astrocytes were seeded at high density into IncuCyte ImageLock plates coated with Matrigel (Corning). Upon reaching confluency, scratch wounds were applied to each well using the Incucyte WoundMaker tool, and cell culture media was replaced in order to remove dead cells and debris. Astrocytes were then either treated with 200 ng/ml monomeric recombinant α-synuclein (Abcam) or the equivalent volume of PBS. Cells were then imaged every hour for 50 h in the IncuCyte live cell imaging platform. Images were analyzed using the IncuCyte software with Scratch Wound module for automated detection of wound confluency and cell density.
Immunofluorescence and image analysis
iPSC-derived astrocytes were grown in 96-well plates and fixed with 10% Formalin (Sigma Aldrich) for 15 min at room temperature, followed by 3 × washes with PBS. Fixed cells were permeabilized with 0.1% Triton x-100 and blocked with 1% BSA in PBS for 30 min at room temperature. Cells were incubated with antibodies against either IL-16 or IL-32 (both Abcam) overnight at 4 °C, washed × 3 with PBS and incubated with secondary antibodies (AlexaFluor anti-rabbit 647, ThermoFisher Scientfic) at 1:100 for 2 h at room temperature. Cells were counterstained with DAPI for 10 min at room temperature, then washed × 3 with PBS. Labeled astrocytes underwent automated imaging on the ThermoFisher Cellnsight CX7 High Content Screening platform with a 40 × objective and widefield imaging. Exposure times for IL-16 and IL-32 were manually fixed across all fields and wells. Sixteen images per cell line were arbitrarily taken per condition. Image intensity analysis was carried out using ImageJ v1.53 s. A minimum grey intensity threshold was set to enable cell detection by positive pixels, and mean grey intensity was determined across positive pixels per field of view. qRT-PCR.
RNA was extracted from HEK293T cells, iPSC-derived neurons, astrocytes and human brain tissue using the RNeasy Mini kit (Qiagen) and reverse transcribed using the High-Capacity RNA-to-cDNA kit (ThermoFisher Scientific). Gene expression was measured by commercially available Taqman qRTPCR assays.
RNA-seq
RNA was prepared as described above. Library preparation with poly-A selection and sequencing with 150 base pair paired-end reads was carried out at Genewiz. Sequenced reads were trimmed for Illumina TruSeq adapters, and quantified for gene expression values in TPM (Transcripts Per Kilobase Million) using Salmon [68] guided by the GENCODE human transcriptome model (GRCh38 version 28, Ensembl 92). TPM data was imported into the R (version 3.5.1) programming environment for visualization and analysis, and differential expression of LRRC37A/2 overexpression compared to the control was analyzed using the moderated t-test implemented in limma [69]. Differentially expressed genes (DEGs) were defined by ± ≥ 1.5 expression fold change and adjusted p < 0.05. Gene set enrichment analysis was performed with the Broad Institute’s MSigDB annotations [70]. Analysis of GO enrichment terms was carried out using g:Profiler (https://biit.cs.ut.ee/gprofiler/gost) [71, 72] and visualized using Cytoscape v3.7.1 [73] with the EnrichmentMap [74] plugin. Additional pathway analyses were carried out using Ingenuity Pathway Analysis (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis) using genes with a fold change ± ≥ 1.
Protein biochemistry
Membrane and cytosolic proteins were isolated from HEK293T cells, iPSC-derived neurons and iPSC-derived astrocytes using the MEM-PER Plus Membrane Protein Extraction Kit (ThermoFisher Scientific), and protein concentrations were determined by bicinchoninic acid (BCA) assay (ThermoFisher Scientific). For western blotting, protein fractions were subject to SDS-PAGE electrophoresis through BOLT Bis–Tris gels (ThermoFisher Scientific) and were blotted onto nitrocellulose membranes. Membrane fractions were confirmed by labelling with an anti-pan-Cadherin antibody (Cell Signaling Technology), and cytosolic fractions were confirmed by labelling with anti-HSP90 (Cell Signaling Technology). Membranes were stripped using Restore plus western blot stripping buffer and re-probed with an anti-LRRC37A antibody (ThermoFisher Scientific).
Single nuclei sequencing data analysis
PD midbrain snuc-seq gene counts and cell metadata34 were downloaded from GEO (GSE157783). AD PFC snuc-seq filtered gene counts and cell metadata33 were downloaded from Synapse (syn18681734). Data underwent filtering and processing using Seurat v4.075,76. Briefly, for PD midbrain data, cells expressing fewer than 200 genes, with fewer than 2500 reads and with greater than 10% mitochondrial gene expression were removed. Count data was normalized using SCTransform77, while regressing out mitochondrial rate, number of expressed genes and the number of reads per cell. Principal components analysis was carried out using the top 3000 most variable genes, and data reduction was performed with UMAP78. Cell types were determined using the provided annotations on GEO. Astrocytes were then isolated and re-scaled. Differential gene expression between PD and control cells was carried out on the raw count data using the MAST model with percent mitochondrial gene expression as a covariate. For AD PFC data, cells with fewer than 2000 reads, expressing fewer than 200 genes and abnormally high ratios of counts mapped to mitochondrial genes relative to the total number of detected genes were removed. Count data was normalized using the SCANPY package79 and principal components analysis and data reduction was carried out as described above. Cell types were determined using the annotations provided in the cell metadata, and genotypes were determined using genotype data from the ROSMAP cohort as described above. Differential gene expression between haplotypes was carried out on the raw count data using the MAST model with pathological diagnosis, sex and age at death as covariates.
OPAL multiplex labelling
Formalin fixed paraffin embedded substantia nigra sections from human controls (N = 4), PSP (N = 5) and PD (N = 5) cases were acquired from the Mount Sinai Neuropathology Brain Bank, with neuropathological diagnosis being determined by Dr. John Crary. All post-mortem tissues were collected in accordance with the relevant guidelines and regulations at the Icahn School of Medicine at Mount Sinai. Multiplexed immunofluorescent staining was carried out on 4-6 µm sections using the Opal Polaris 7 color IHC detection kit (Akoya biosciences) according to manufacturer’s instructions. Briefly, slides were baked for 1 h at 65 °C, then deparaffinized with xylene and rehydrated with a graded series of ethanol concentrations. For epitope retrieval, slides were microwaved in AR buffer for 45 s at 100% power, followed by an additional 15 min at 20% power. After cooling, slides were blocked for 10 min in blocking buffer then incubated with the first primary antibody at room temperature for 30 min. Slides were rinsed three times in TBS-T, then incubated with the secondary polymer HRP for 1 h at room temperature. After additional washes, the first Opal fluorophore was incubated with the slides for 10 min at room temperature, followed by further washes in TBS-T. This process was repeated from the microwave treatment step for each additional primary antibody, followed by one final repetition of the microwave treatment to strip the primary-secondary antibody complex from the tissue. Once all primary antibodies had been introduced, slides were counterstained with DAPI for 5 min at room temperature, washed with TBS-T and coverslips were mounted using ProLong Diamond Antifade mounting reagent (ThermoFisher Scientific). Multispectral imaging was carried out using the Vectra Quantitative Pathology Imaging system, applying quantitative unmixing of fluorophores and removal of tissue autofluorescence. Images were visualized using the HALO image analysis platform (Indica Labs).
Co-Immunoprecipitation
Frozen substantia nigra tissue was selected from the same Control (N = 3), PSP (N = 3) and PD (N = 3) cases used for OPAL multiplex immunofluorescence. Protein lysates were generated using cell lysis buffer (NEB) and brief sonication on ice, followed by centrifugation to pellet insoluble material. Co-immunoprecipitation was carried out using the Dynabeads Protein G immunoprecipitation kit (ThermoFisher Scientific), with an anti-α-synuclein antibody (Abcam) as bait. Proteins bound to beads were eluted and assayed by western blot (as described above) and probed with an anti-LRRC37A antibody (ThermoFisher Scientific). Whole protein lysate and IgG only controls were run on the same blots.
Supplementary Information
Additional file 9. Supplementary table 2
Additional file 10. Supplementary table 3
Additional file 11. Supplementary table 4
Additional file 12. Supplementary table 5
Additional file 13. Supplementary table 6
Additional file 14. Supplementary table 7
Additional file 15. Supplementary table 8
Acknowledgements
We thank the Icahn School of Medicine at Mount Sinai Neuropathology Brain Bank CoRE for supplying post-mortem human brain tissues for analysis. We are grateful to the brank bank participants and their families for their contributions to research. Data used in the preparation of this article were obtained from the Accelerating Medicine Partnership® (AMP®) Parkinson’s Disease (AMP PD) Knowledge Platform. For up-to-date information on the study, visit https://www.amp-pd.org. The AMP® PD program is a public-private partnership managed by the Foundation for the National Institutes of Health and funded by the National Institute of Neurological Disorders and Stroke (NINDS) in partnership with the Aligning Science Across Parkinson's (ASAP) initiative; Celgene Corporation, a subsidiary of Bristol-Myers Squibb Company; GlaxoSmithKline plc (GSK); The Michael J. Fox Foundation for Parkinson's Research ; Pfizer Inc.; Sanofi US Services Inc.; and Verily Life Sciences. ACCELERATING MEDICINES PARTNERSHIP and AMP are registered service marks of the U.S. Department of Health and Human Services. Genome sequence data for the Lewy body dementia case-control cohort were generated at the Intramural Research Program of the U.S. National Institutes of Health. The study was supported in part by the National Institute on Aging (program #: 1ZIAAG000935) and the National Institute of Neurological Disorders and Stroke (program #: 1ZIANS003154). PPMI is sponsored by The Michael J. Fox Foundation for Parkinson’s Research and supported by a consortium of scientific partners: [list the full names of all of the PPMI funding partners found at https://www.ppmi-info.org/about-ppmi/who-we-are/study-sponsors]. The PPMI investigators have not participated in reviewing the data analysis or content of the manuscript. For up-to-date information on the study, visit www.ppmi-info.org. We are grateful to the New York Stem Cell Foundation for contributing iPSC lines for this study, as well as the New South Wales Brain Bank. Peripheral blood monon uclear cells (PBMCs) were obtained from the New South Wales Brain Bank at Neuroscience Research Australia, which is supported by the University of New South Wales and Neuroscience Research Australia. We thank the Icahn School of Medicine at Mount Sinai core for iPSC reprogramming services.
Abbreviations
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- CBD
Corticobasal degeneration
- PSP
Progressive supranuclear palsy
- dPCR
Digital polymerase chain reaction
- qRT-PCR
Quantitative reverse transcription polymerase chain reaction
- DNA
Deoxyribonucleic acid
- RNA-seq
Ribonucleic acid sequencing
- LD
Linkage disequilibrium
- Mb
Megabase
- OR
Odds ratio
- SNP
Single nucleotide polymorphism
- CI
Confidence interval
- CNV
Copy number variation
- GO
Gene ontology
- IPA
Ingenuity pathway analysis
- iPSC
Induced pluripotent stem cell
- GWAS
Genome-wide association study
- IgG
Immunoglobulin G
- AMP-AD
Accelerating Medicines Partnership Alzheimer’s disease
- PFC
Prefrontal cortex
- TCX
Temporal cortex
- MAPT
Microtubule associated protein tau
- LRRC37A/2
Leucine rich repeat containing 37A and 37A2
- KANSL1
KAT8 regulatory NSL complex subunit 1
- IL17F
Interleukin 17F
- IL32
Interleukin 32
- IL16
Interleukin 16
- CD4
Cluster of differentiation 4
- GFAP
Glial fibrillary acidic protein
- IBA1
Ionized calcium binding adaptor molecule 1
- GBA
Glucosylceramidase beta
- LRRK2
Leucine rich repeat kinase 2
- PINK1
PTEN induced kinase 1
- TGFA
Transforming growth factor alpha
- GDNF
Glial cell line-derived neurotrophic factor
- IPDGC
International parkinson’s disease genetics consortium
- NIA
National institute of aging
- GER
German
- FIN
Finnish
- NL
Netherlands
- SP
Spanish
- QC
Quality control
- MAF
Minor allele frequency
- YRI
Yoruba
- CEU
Northern Europeans from Utah
- CHB
Han Chinese in Beijing
- HRC
Haplotype reference consortium
- SVS
SNP variation suite
- EM
Expectation-maximization
- PSI
Percent spliced in
- MISO
Mixture of isoforms
- ADRC
Alzheimer’s disease research center
- IRB
Institutional review board
- FBS
Fetal bovine serum
- NPC
Neural progenitor cell
- TPM
Transcripts per million
- DEG
Differentially expressed gene
- BCA
Bicinchoninic acid
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- IHC
Immunohistochemistry
- AR
Antigen retrieval
- HRP
Horseradish peroxidase
- TBS-T
Tris-buffered saline with tween
Author contributions
Conceptualization: KRB, JFC, AMG. Methodology: KRB, JDC. Validation: KRB, DAP. Formal Analysis: KRB, DAP, YL, SB-C, ZG-O, PH, AS, SB, TP. Investigation: KRB, DAP, YL. Resources: AC, CMK, SJF, BHK, IP, YJP, AC, TR, JFC, AMG. Data Curation: KRB, YL, SB-C, ZG-O, PH, AS, SB, IPDGC, TP. Writing – Original Draft: KRB. Writing – Review and Editing: KRB, YL, AER, SB-C, ZG-O, CMK, IP, TP, TR, JFC, AMG. Visualization: KRB, TP. Supervision: AMG. Funding Acquisition: KRB, JFC, AMG. All authors read and approved the final manuscript.
Funding
This work was supported by funding from the BrightFocus Foundation (KRB), Association for Frontotemporal Degeneration (KRB) and CurePSP (KRB). The recruitment and clinical characterization of research participants at Washington University were supported by NIH P50 AG05681, P01 AG03991, and P01 AG026276. NIH AG062683 (J.TCW), Rainwater Charitable Organization (CMK), NIH AG046374 (CMK). The McGill cohort was supported by grants from the Michael J. Fox Foundation, the Canadian Consortium on Neurodegeneration in Aging (CCNA), the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) program, and Parkinson Canada. ZGO is supported by the Fonds de recherche du Québec—Santé (FRQS) Chercheurs-boursiers award given with Parkinson Quebec, and is a Parkinson Canada New Investigator awardee. The access to the McGill participants for this research has been made possible in part thanks to the Quebec Parkinson’s Network (http://rpq-qpn.ca/en/). NIH [R01AG054008, R01NS095252, R01AG060961, R01 AG060961] to JFC, the Rainwater Charitable Foundation/Tau Consortium (JFC), Genentech/Roche, and an Alexander Saint-Amand Scholarship (JFC). This work was supported in part by the Intramural Research Programs of the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Aging (NIA), and the National Institute of Environmental Health Sciences both part of the National Institutes of Health, Department of Health and Human Services: project numbers 1ZIA-NS003154, Z01-AG000949-02 and Z01-ES101986. BrightFocus Foundation,Association for Frontotemporal Degeneration,CurePSP,Rainwater Charitable Foundation,National Institutes of Health,Michael J. Fox Foundation for Parkinson's Research,Canadian Consortium on Neurodegeneration in Aging,Canada First Research Excellence Fund,Fonds de Recherche du Québec—Santé
Availability of data and materials
All aligned read counts and FASTQ files for LRRC37A-overexpressing HEK293T cells will be deposited to the Gene Expression Omnibus once the manuscript is accepted for publication.
Declarations
Declarations
Ethical Approval and Consent to Participate
Not applicable.
Consent for publication
All authors read and approved the final manuscript
Competing interests
AMG: Scientific advisory board (SAB) for Denali Therapeutics (2015–2018), SAB for Pfizer (2019), SAB for Genentech, consultant for GSK, AbbVie, Biogen and Eisai. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kathryn R. Bowles, Email: kathryn.bowles@mssm.edu
Derian A. Pugh, Email: derian.a.pugh@gmail.com
Yiyuan Liu, Email: yiyuan.liu@mssm.edu.
Tulsi Patel, Email: tulsi.patel2@mssm.edu.
Alan E. Renton, Email: alan.renton@mssm.edu
Sara Bandres-Ciga, Email: sarabandres@gmail.com.
Ziv Gan-Or, Email: ziv.gan-or@mcgill.ca.
Peter Heutink, Email: peter.heutink@dzne.de.
Ari Siitonen, Email: ari.siitonen@iki.fi.
Sarah Bertelsen, Email: sbertel2@gmail.com.
Jonathan D. Cherry, Email: jdcherry@bu.edu
Celeste M. Karch, Email: karchc@wustl.edu
Steven J. Frucht, Email: steven.frucht@nyumc.org
Brian H. Kopell, Email: brian.kopell@mountsinai.org
Inga Peter, Email: inga.peter@mssm.edu.
Y. J. Park, Email: youjeong.park@mssm.edu
Alexander Charney, Email: alexander.charney@mssm.edu.
Towfique Raj, Email: towfique.raj@mssm.edu.
John F. Crary, Email: john.crary@mountsinai.org
A. M. Goate, Email: alison.goate@mssm.edu
References
- 1.Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert J-C, Chung J, Naj AC. a Novel Alzheimer Disease Locus Located Near the Gene Encoding Tau Protein. 2016;21:108–17. [DOI] [PMC free article] [PubMed]
- 2.Kouri N, Ross OA, Dombroski B, et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015;6:1–7. doi: 10.1038/ncomms8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Höglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JA, Chen Z, Won H, et al. Joint genome-wide association study of progressive supranuclear palsy identifies novel susceptibility loci and genetic correlation to neurodegenerative diseases. Mol Neurodegener. 2018;13:1–11. doi: 10.1186/s13024-018-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastor P, Ezquerra M, Perez JC, et al. Novel Haplotypes in 17q21 Are Associated with Progressive Supranuclear Palsy. Ann Neurol. 2004;56:249–258. doi: 10.1002/ana.20178. [DOI] [PubMed] [Google Scholar]
- 6.Bandrés-ciga S, Ryan T, Javier F, et al. Genome-wide assessment of Parkinson ’ s disease in a Southern Spanish population. Neurobiol Aging. 2016;45(213):e3–213.e9. doi: 10.1016/j.neurobiolaging.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desikan RS, Schork AJ, Wang Y, et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry 2015;20(12):1588–95. [DOI] [PMC free article] [PubMed]
- 8.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandres-Ciga S, Ahmed S, Sabir MS, et al. The genetic architecture of Parkinson disease in Spain: characterizing population-specific risk, differential haplotype structures, and providing etiologic insight. Mov Disord. 2019 doi: 10.1101/609016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalls MA, Blauwendraat C, Vallerga CL, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease : a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–102. [DOI] [PMC free article] [PubMed]
- 11.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67:1605–1612. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Zaccai J, Brayne C. A Systematic Review of Prevalence Studies of Dementia in Parkinson ’ s Disease. Mov Disord. 2005;20:1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- 13.Massano J, Bhatia KP. Clinical Approach to Parkinson’s Disease: Features, Diagnosis, and Principles of Management. Cold Spring Harb Perspect Med. 2012;2(6):1–15. [DOI] [PMC free article] [PubMed]
- 14.Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. alpha-Synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 15.Dickson DW. Neuropathology of Parkinson Disease. Parkinsonism Relat Disord. 2018;46:1–11. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Gao F, Wang D, et al. Tau Pathology in Parkinson ’ sDisease. Front Neurol. 2018;9:1–7. doi: 10.3389/fneur.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espay AJ, Vizcarra JA, Marsili L, et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019;92:329–337. doi: 10.1212/WNL.0000000000006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabetian CP, Hutter CM, Factor S a, et al. Association Analysis of MAPT H1 Haplotype and Subhaplotypes in Parkinson’s Disease. Ann Neurol. 2007;62:137–44. doi: 10.1002/ana.21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandrovcova J, Pittman AM, Malzer E, et al. Association of MAPT haplotype-tagging SNPs with sporadic Parkinson’s disease. Neurobiol Aging. 2009;30:1477–1482. doi: 10.1016/j.neurobiolaging.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Boettger LM, Handsaker RE, Zody MC, McCarroll SA. Structural haplotypes and recent evolution of the human 17q21.31 region. Nat Genet. 2012;44:881–5. [DOI] [PMC free article] [PubMed]
- 21.Steinberg KM, Antonacci F, Sudmant PH, et al. Structural diversity and African origin of the 17q21.31 inversion polymorphism. Nat Publ Gr. 2012;44:872–80. doi: 10.1038/ng.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brück D, Wenning GK, Stefanova N, Fellner L. Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis. 2016;85:262–274. doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.di Domenico A, Carola G, Calatayud C, et al. Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem Cell Reports. 2019;12:213–229. doi: 10.1016/j.stemcr.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth HDE, Hirst WD, Wade-Martins R. The Role of Astrocyte Dysfunction in Parkinson’s Disease Pathogenesis. Trends Neurosci. 2017;40:358–370. doi: 10.1016/j.tins.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonninen TM, Hämäläinen RH, Koskuvi M, et al. Metabolic alterations in Parkinson’s disease astrocytes. Sci Rep 2020; 10. DOI:10.1038/s41598-020-71329-8. [DOI] [PMC free article] [PubMed]
- 26.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo Yh, Takahashi H. NACP alphasynuclein-positive filamentous inclusions. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/PL00007400. [DOI] [PubMed] [Google Scholar]
- 27.Song YJC, Halliday GM, Holton JL, et al. Degeneration in different parkinsonian syndromes relates to astrocyte type and astrocyte protein expression. J Neuropathol Exp Neurol. 2009;68:1073–1083. doi: 10.1097/NEN.0b013e3181b66f1b. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Sastre M, Del Tredici K. Development of α-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Suk JE, Patrick C, et al. Direct transfer of α-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittman A, Myers A, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42:837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothhammer V, Quintana FJ. Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol. 2015;37:625–638. doi: 10.1007/s00281-015-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y, Zhu Y. Important role of the IL-32 inflammatory network in the host response against viral infection. Viruses. 2015;7:3116–3129. doi: 10.3390/v7062762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathys H, Davila-velderrain J, Peng Z, et al. Single-cell transcriptomic analysis of Alzheimer ’ s disease. Nature. 2019;570:332–337. doi: 10.1038/s41586-019-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smajic S, Prada-Medina CA, Landoulsi Z, et al. Single-cell sequencing of the human midbrain reveals glial activation and a neuronal state specific to Parkinson’s disease. Brain. 2021 doi: 10.1101/2020.09.28.20202812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly MP, Paschou P, Grigorenko E, et al. The Distribution and Most Recent Common Ancestor of the 17q21 Inversion in Humans. Am J Hum Genet. 2010;86:161–171. doi: 10.1016/j.ajhg.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Juan P, Moreno S, de Rojas I, et al. The MAPT H1 Haplotype Is a Risk Factor for Alzheimer’s Disease in APOE ε4 Non-carriers. Front Aging Neurosci. 2019;11:1–9. doi: 10.3389/fnagi.2019.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gayán J, Galan JJ, González-Pérez A, et al. Genetic Structure of the Spanish Population. BMC Genomics 2010; 11. DOI:10.1186/1471-2164-11-326. [DOI] [PMC free article] [PubMed]
- 38.Coupland KG, Kim WS, Halliday GM, Hallupp M, Dobson-Stone C, Kwok JBJ. Role of the long non-coding RNA MAPT-AS1 in regulation of microtubule associated protein tau (MAPT) expression in Parkinson’s disease. PLoS ONE. 2016;11:1–14. doi: 10.1371/journal.pone.0157924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simone R, Javad F, Emmett W, et al. MIR-NATs repress MAPT translation and aid proteostasis in neurodegeneration. Nature. 2021;594:117–123. doi: 10.1038/s41586-021-03556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soutar MPM, Melandri D, Annuario E, et al. Regulation of mitophagy by the NSL complex underlies genetic risk for Parkinson’s disease at Chr16q11.2 and on the MAPT H1 allele. bioRxiv 2020. DOI:10.1101/2020.01.06.896241. [DOI] [PMC free article] [PubMed]
- 41.Giannuzzi G, Siswara P, Malig M, et al. Evolutionary dynamism of the primate LRRC37 gene family. Genome Res. 2013;23:46–59. doi: 10.1101/gr.138842.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zody MC, Jiang Z, Fung H-C, et al. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat Genet. 2008;40:1076–83. doi: 10.1038/ng.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falola MI, Wiener HW, Wineinger NE, et al. Genomic Copy Number Variants: Evidence for Association with Antibody Response to Anthrax Vaccine Adsorbed. PLoS One 2013; 8. DOI:10.1371/journal.pone.0064813. [DOI] [PMC free article] [PubMed]
- 44.Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 45.Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:1–12. doi: 10.1186/1742-2094-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Sloan SA, Clarke LE, et al. Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang A, Martin ER, Vance JM, Cai X. Detecting genetic interactions in pathway-based genome-wide association studies. Genet Epidemiol. 2014;38:300–309. doi: 10.1002/gepi.21803. [DOI] [PubMed] [Google Scholar]
- 48.Kong Y, Liang X, Liu L, et al. High throughput sequencing identifies MicroRNAs mediating α-synuclein toxicity by targeting neuroactive-ligand receptor interaction pathway in early stage of Drosophila Parkinson’s disease model. PLoS ONE. 2015;10:1–24. doi: 10.1371/journal.pone.0137432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallon J, Reid S, Kinyamu R, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee HJ, Kim C, Lee SJ. Alpha-synuclein stimulation of astrocytes: Potential role for neuroinflammation and neuroprotection. Oxid Med Cell Longev. 2010;3:283–287. doi: 10.4161/oxim.3.4.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Yang T, Liang M, Xie J, Song N. Astrocyte dysfunction in Parkinson’s disease: from the perspectives of transmitted α-synuclein and genetic modulation. Transl Neurodegener. 2021;10:1–17. doi: 10.1186/s40035-020-00225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mena MA, García De Yébenes J. Glial cells as players in parkinsonism: The ‘good,’ the ‘bad,’ and the ‘mysterious’ glia. Neuroscientist. 2008;14:544–60. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 53.Purcell S, Neale B, Todd-Brown K, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Consortium TIH The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 55.Patterson N, Price AL, Reich D. Population structure and eigenanalysis. PLoS Genet. 2006;2:2074–2093. doi: 10.1371/journal.pgen.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das S, Forer L, Schonherr S, et al. Next-generation genotype imputation service and methods Sayantan. 2016;48:1284–1287. doi: 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lumley T. rmeta: Meta-Analysis. R package version 3.0. 2018; : https://CRAN.R-project.org/package=rmeta.
- 58.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 59.Gabriel SB, DeFelice M, Rotimi C, et al. The structure of haplotype blocks in the human genome. Science. 2002;296(80-):2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 60.Aragon TJ. epitools: Epidemiology Tools. R package version 0.5–10.1; 2020. https://CRAN.R-project.org/package=epitools.
- 61.Heckman MG, Brennan RR, Labbé C, et al. Association of MAPT Subhaplotypes With Risk of Progressive Supranuclear Palsy and Severity of Tau Pathology. JAMA Neurol. 2019;76(6):1–8. [DOI] [PMC free article] [PubMed]
- 62.Katz Y, Wang ET, Airoldi EM, Burge CB. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods. 2010;7:1009–1015. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karch CM, Kao AW, Karydas A, et al. A Comprehensive Resource for Induced Pluripotent Stem Cells from Patients with Primary Tauopathies. Stem cell reports. 2019;13:1–17. doi: 10.1016/j.stemcr.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffman GE, Hartley BJ, Flaherty E, et al. Transcriptional signatures of schizophrenia in hiPSC-derived NPCs and neurons are concordant with post-mortem adult brains. Nat Commun 2017; 8. DOI:10.1038/s41467-017-02330-5. [DOI] [PMC free article] [PubMed]
- 65.Bowles KR, Julia TCW, Qian L, Jadow BM, Goate AM. Reduced variability of neural progenitor cells and improved purity of neuronal cultures using magnetic activated cell sorting. PLoS ONE. 2019;14:1–18. doi: 10.1371/journal.pone.0213374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tcw J, Wang M, Pimenova AA, et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 2017;9:600–614. doi: 10.1016/j.stemcr.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tcw J, Wang M, Pimenova AA, et al. An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports. 2017;9:600–614. doi: 10.1016/j.stemcr.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon: fast and bias-aware quantification of transcript expression using dual-phase inference. Nat Methods. 2017;14:417. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian A, Subramanian A, Tamayo P, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reimand J, Kull M, Peterson H, Hansen J, Vilo J. G:Profiler-a web-based toolset for functional profiling of gene lists from large-scale experiments. Nucleic Acids Res. 2007;35:1–8. doi: 10.1093/nar/gkm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raudvere U, Kolberg L, Kuzmin I, et al. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019;47:1–8. [DOI] [PMC free article] [PubMed]
- 73.Shannon P, Markiel A, Ozier O, et al. Cytoscape : A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003;13(11):2498–504. [DOI] [PMC free article] [PubMed]
- 74.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: A network-based method for gene-set enrichment visualization and interpretation. PLoS One 2010; 5. DOI:10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed]
- 75.Stuart T, Butler A, Hoffman P, et al. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions , technologies , and species. Nat Biotechnol 2018; 36. DOI:10.1038/nbt.4096. [DOI] [PMC free article] [PubMed]
- 77.Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:1–15. doi: 10.1186/s13059-019-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McInnes L, Healy J, Melville J. UMAP: Uniform manifold approximation and projection for dimension reduction. arXiv:1802.03426;2018.
- 79.Wolf F, Angerer P, Theis F. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19. 10.1111/1462-2920.13787. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 9. Supplementary table 2
Additional file 10. Supplementary table 3
Additional file 11. Supplementary table 4
Additional file 12. Supplementary table 5
Additional file 13. Supplementary table 6
Additional file 14. Supplementary table 7
Additional file 15. Supplementary table 8
Data Availability Statement
All aligned read counts and FASTQ files for LRRC37A-overexpressing HEK293T cells will be deposited to the Gene Expression Omnibus once the manuscript is accepted for publication.