Summary
Background
The incidences of both paediatric obesity and autoimmune diseases have been increasing, but their relationship with one another is unclear.
Objective
To determine whether obesity or particular dietary patterns in school‐aged children are potential risk factors for autoimmune diseases during adolescence.
Methods
This matched case–control study included 525 children, followed up from a median age of 11.3 to 16.7 years. Of them, 105 children received primary autoimmune diagnoses (diabetes, thyroiditis, arthritis, or inflammatory bowel diseases) after baseline and generated the case group. Four children with matching age, sex, and residential area generated the control group of 420 children. At baseline, age‐ and sex‐specific body mass index categories were acquired and waist‐to‐height ratio (WHTR) was calculated. Central obesity was present when WHTR ≥0.5. Dietary patterns were analysed using a food frequency questionnaire (FFQ).
Results
School‐aged children with central obesity were 2.11 (OR, 95% CI 1.11–3.98) times more likely to develop autoimmune diseases before age of 19 years than those without central obesity. Being overweight was not related to the onset of these diseases (OR 1.60, 95% CI 0.89–2.87, nor were dietary patterns.
Conclusion
Central obesity in school‐aged children was related to the development of autoimmune diseases, while being overweight and dietary patterns were not.
Keywords: autoimmune thyroiditis (AIT), dietary patterns, eating habits, inflammatory bowel diseases (IBD), juvenile idiopathic arthritis (JIA), type 1 diabetes (DM)
1. INTRODUCTION
Autoimmune diseases are a group of complex immunological disorders, in which the immune system erroneously interprets normal tissues as foreign, generating unwanted attacks, and inflammations in different organs. This study focuses on four autoimmune diseases with partially to represent autoimmune diseases in individuals between the age of 11 and 19 years in general: type 1 diabetes (DM), autoimmune thyroiditis (AIT), juvenile idiopathic arthritis (JIA), and inflammatory bowel diseases (IBD). These diseases were chosen due to their potentially overlapping genetic pathways. In addition, their pathogeneses resemble each other, showing T‐cell organ infiltrations with intermittent inflammations, yet without specific triggering factors. Pancreatic T‐cell infiltration leads to depleted insulin secretion, hyperglycaemia, and eventually DM; thyroid gland infiltration leads to reduced thyroid hormone function and hypothyroidism seen in AIT; synovial membrane infiltration leads to joint inflammation and JIA; and gut mucosa infiltration leads to chronic autoimmune inflammations of the gastrointestinal tract seen in IBD. 1 , 2 , 3 , 4 , 5 , 6
In recent decades, the numbers of both autoimmune diseases and obesity have been increasing. 7 , 8 This has led researchers to wonder about their potential relationship, especially when obesity is seen as a continuous state of chronic low‐grade inflammation and has been associated with high disease activity in several autoimmune diseases. 9 , 10 While obesity may be related to the onset of hypothyroidism, 11 its role in the pathogenesis of DM, JIA, and IBD is still unclear.
‘Western type’ diet (with high fat, protein, sugar, and salt intake), when compared with more fibre‐rich diet, might be associated with inflammation‐related mechanisms in autoimmune diseases. 12 , 13 This finding is supported by previous studies, indicating an inflammatory role of a particular diet, and relating consumption of pro‐inflammatory foods with disease activities of autoimmune diseases. 14 , 15 , 16 Furthermore, long‐term dietary patterns have been shown to modify gut microbiota to a certain degree and, as a result, may contribute to the inflammation processes. 17 , 18 So far, most studies associating diet and autoimmune diseases have involved adults in a retrospective setting, or concerned the impact of diet on disease activities. Prospective studies to determine the relationship between dietary patterns, body composition, and the development of autoimmune diseases in children and adolescents have been deficient. This study aims to investigate whether dietary patterns (meal patterns, eating habits, and consumption frequencies of sugary products and fruits/vegetables), waist‐to‐height ratio (WTHR), and body mass index (BMI) in school‐aged children are risk factors for developing autoimmune diseases (DM, AIT, JIA, and IBD) in adolescence.
2. METHODS
2.1. Data sources and study population
The study population was derived from the Finnish Health in Teens (Fin‐HIT) cohort. 19 The Fin‐HIT cohort was assembled by school recruitment in vast, densely populated areas throughout Finland without specific exclusion criteria, comprising 11 407 school‐aged children. At baseline, when the children's median age was 11.3 years, questionnaire was used to obtain data concerning food consumption (food frequency questionnaire, FFQ) and meal patterns. In addition, weight, height, and waist circumference were objectively measured. After approximately 2.6 years from baseline, parents were asked to report their children's body measures, which were obtained at home with a provided measuring tape according to given instructions. These measures were available for 50% of the children in the cohort. 19
DM, AIT, JIA, and IBD (including Crohn's disease, ulcerative colitis, and IBD unclassified) diagnoses (from birth to 31 December 2018) were obtained by linking the participants' personal identifier number (presenting the date of birth and sex) with two national health registers—the Special Reimbursement Register and the Drug Purchase Register—maintained by the Finnish Social Insurance Institution. 20 The excellent coverage of these registers is described elsewhere. 21 Celiac disease has a known triggering factor (gluten) and requires no long‐term medical prescriptions, hence this diagnosis was not available in the registers we used.
In the Fin‐HIT cohort, 245 children received their primary diagnoses (DM, AIT, JIA, or IBD) by the end of follow‐up. Of them, girls were more commonly affected by AIT and JIA than boys, while AIT was more common in adolescence than in preschool age. 22 To limit the role of age, sex, and residential areas as profound confounders, a matched case–control design was chosen for this study. The case group consisted of 105 children with available FFQ, whose diagnoses were dated at least 1 month after recruitment. Of them, 34 (32.3%) developed DM, 39 (37.1%) AIT, 18 (17.1%) JIA, and 14 (13.3%) IBD during the follow‐up period. For each child in the case group, four children with matching age, sex, and residential area were selected from the same cohort by a person outside the study group, generating the control group of 420 children (Figure S1).
2.2. Anthropometric measures: Waist‐to‐height ratio (WHTR) and body mass index (BMI)
Based on the WHTR, the children were categorized as having a normal waist (WHTR <0.5) or being centrally obese (WHTR ≥0.5). 23 BMI (=weight [kg]/height [m]2) was categorized based on age and sex according to the International Obesity Task Force cutoffs as ‘underweight’ (comprised thinness grades II and III only, with standard deviation [SD] score equivalent <−1.88 for boys and <−1.79 for girls), ‘normal’ (SD score equivalent −1.88 to 1.31 for boys and −1.79 to 1.24 for girls), and ‘overweight’ (including both overweight and obese with SD score equivalent >1.31 for boys and >1.24 for girls). 24
2.3. Meal patterns, eating habits, sweet treat index (STI), plant consumption index (PCI)
Food consumption in the preceding month prior to baseline was analysed based on obtained FFQ data, which included 16 food items to make it simple enough for 9‐ to 12‐year‐old children to apprehend, yet sufficiently detailed to cover mandatory key indicators used to determine healthy and unhealthy dietary habits in children according to the Health Behaviour in School‐Aged Children study protocol. 25 These food items were: (1) fruits/berries, (2) fresh vegetables, (3) cooked vegetables, (4) sugary soft drinks. The additional food items for healthy eating behaviours were (5) dark grain bread, (6) milk or buttermilk, (7) fresh juice (no added sugar), and (8) water. For unhealthy eating behaviours, the additional food items were (9) pizza, (10) hamburger or hot dog, (11) biscuits/cookies, (12) ice cream, (13) chocolate/sweets, (14) sweet pastries, (15) salty snacks, and (16) sugary juice drinks. 26 The participants estimated their weekly consumption of these food items using a 7‐point scale ranging from 0 (not consumed) to 6 (consumed several times per day).
Eating habits and meal patterns at baseline have been assessed previously using FFQ and a questionnaire on the regularity of breakfast, lunch, and dinner during school days. 27 Children with unhealthy eating habits frequently consumed unhealthy food items such as pizza, hamburger/hot dog, sugary products, and salty snacks. The fruit and vegetable avoiders rarely consumed unhealthy food items, but they scarcely consumed fruits/berries, fresh juice, and fresh or cooked vegetables as well. Healthy eaters regularly consumed dark grain bread, milk, fruits/berries, fresh juice, and fresh grated or cooked vegetables, while they consumed unhealthy food items less often. Furthermore, children with regular meal patterns were those who had lunch and dinner every weekday, while the rest had irregular meal patterns. Children who ate breakfast every school day were classified as having a regular breakfast pattern, while the rest had an irregular breakfast pattern.
To estimate weekly consumption frequencies of sugary products and plants at baseline, FFQ answers were recoded into a times/week continuous variable: ‘not at all’ as 0, ‘less than once a week’ as 0.5 (assuming an average consumption of twice per month), ‘once a week’ as 1, ‘2–4 times a week’ as 3, ‘5–6 times a week’ as 5.5, ‘once a day’ as 7, and ‘several times a day’ as 14 times a week (assuming an average consumption of twice daily). The weekly consumption frequency of sugary products was evaluated using a sum variable referred to as a sweet treat index (STI). 28 It was calculated by adding up continuous weekly consumption frequencies of food and drink items representing sugary products: (1) chocolate/sweets, (2) biscuits/cookies, (3) ice cream, (4) sweet pastry, (5) sugary juice drinks, and (6) sugary soft drinks.
Plant consumption index (PCI)—a sum variable analogous to the STI, combining continuous weekly consumption frequencies of (1) fruits/berries, (2) fresh vegetables, and (3) cooked vegetables. It was used to estimate the weekly use of plants as a crude indicator for anti‐inflammatory food components. 14 , 29
2.4. Statistical methods
Each child who developed a primary autoimmune disease was compared with four matching controls. The data were presented with mean and standard deviation (SD); median and interquartile range (IQR); and numbers/proportion (%). Pearson's chi‐squared test, the independent samples t‐test, and the Kruskal–Wallis test were used as appropriate when comparing background information of case and control groups—to inquire how well the control group were matched, and to observe anthropometric characteristics (BMI SD score and WHTR) of both groups. Risk factor variables (central obesity, BMI categories, eating habits, meal patterns, STI, and PCI) were presented with odds ratio (OR) and 95% confidence interval (CI) using conditional logistic regression tests. A 5% statistical significance level was adopted. The software used was IBM SPSS Statistics 26.0.
2.5. Ethical considerations
The Fin‐HIT study protocol was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (decision number 169/13/03/00/10). The children and one of their guardians provided written informed consent.
3. RESULTS
Data on anthropometric measures and dietary patterns were collected approximately 3 years prior to established diagnoses. The matched case–control study population comprised 525 children (105 cases and 420 matching controls) with similar background characteristics (Table 1). The mean age of the study population was 11.3 years at baseline, and the children were followed‐up for a median of 5.1 (IQR 4.7–5.6) years. Approximately 58.1% of the study population were girls and 41.9% were boys. Of them, 28.7% were from the densely populated capital region. Moreover, anthropometric measures of the matched controls as one group well represented the measures of 11 162 children in the cohort, who did not develop autoimmune diseases.
TABLE 1.
Characteristics of children who developed primary autoimmune diseases, their matching controls, and children in the Fin‐Hit cohort who did not develop autoimmune diseases
| Cases (N = 105) a | Matched controls (N = 420) b | p‐value | Children without diagnoses (N = 11 162) | p‐value | |
|---|---|---|---|---|---|
| Mean age, years ± SD | |||||
| At baseline | 11.3 ± 0.8 | 11.3 ± 0.8 | 0.988 c , f | 11.2 ± 0.8 | 0.025 c , g |
| Missing (%) | 0 | 0 | |||
| At the end of follow‐up | 16.8 ± 1.3 | 16.5 ± 1.2 | 0.126 c , f | 16.5 ± 1.5 | 0.069 c , g |
| Missing (%) | 0 | 0 | 419 (3.8) | ||
| Sex, n (%) | 0.979 d , f | 0.172 d , g | |||
| Boy | 44 (41.9) | 176 (41.9) | 5074 (47.6) | ||
| Girl | 61 (58.1) | 244 (58.1) | 5576 (52.4) | ||
| Missing | 0 | 0 | 512 (4.6) | ||
| Residential area, n (%) | 1.000 d , f | 0.162 d , g | |||
| Capital (South) | 30 (28.6) | 121 (28.8) | 3651 (32.8) | ||
| Inner South | 18 (17.1) | 69 (16.4) | 1232 (11.1) | ||
| West | 12 (11.4) | 50 (11.9) | 1128 (10.1) | ||
| East | 26 (24.8) | 106 (25.2) | 2373 (21.3) | ||
| North | 19 (18.1) | 74 (17.6) | 2737 (24.6) | ||
| Missing | 0 | 0 | 41 (0.4) | ||
| Available FFQ, n (%) | 105 (100.0) | 419 (99.8) | 10 214 (91.5) | ||
| Missing | 0 | 1 (0.2) | 948 (8.5) | ||
| Anthropometric measures at baseline: measured, n (%) | 103 (98.1) | 416 (99.0) | 10 420 (93.4) | ||
| BMI, median (IQR) | 17.7 (16.1–19.5) | 17.4 (15.9–19.2) | 0.447 e , f | 17.3 (15.8–19.2) | 0.269 e , g |
| Missing | 2 (1.9) | 0 | |||
| WHTR, mean ± SD | 0.44 ± 0.05 | 0.43 ± 0.04 | 0.101 c , f | 0.43 ± 0.05 | 0.070 c , g |
| Missing (%) | 2 (1.9) | 2 (0.5) | 742 (6.6) | ||
| Diagnosis, n (%) | |||||
| DM | 34 (32.4) | ||||
| AIT | 39 (37.1) | ||||
| JIA | 18 (17.1) | ||||
| IBD | 14 (13.3) | ||||
| Median age at diagnoses (IQR) | 13.8 (12.3–15.5) |
Of the 11 407 school‐aged children in the background cohort, 105 children with primary diagnosis (AIT, autoimmune thyroiditis; DM, type 1 diabetes mellitus; IBD, inflammatory bowel diseases; JIA, juvenile idiopathic arthritis) at least 1 month after baseline and available Food Frequency Questionnaire (FFQ) generated the case group. SD=Standard Deviation, IQR = Interquartile Range, BMI=Body Mass index (kg/m2), WHTR = Waist to height Ratio.
Four children with matching age, sex, and residential areas were chosen for each child in the case group, generating the control group.
Independent samples t‐test.
Pearson's chi‐square test.
Kruskall‐Wallis test.
Between case and controls.
Between case and all children without studied autoimmune diagnoses in the cohort (including the controls).
Central obesity was more prevalent in the 105 cases than in the 420 matched controls (16.2% vs. 9.8%) (Table 2). In fact, children with central obesity at baseline were 2.11 (95% CI 1.11–3.98) times more likely to develop an autoimmune disease during the follow‐up period than those without central obesity (Figure 1). This finding was not related to any particular diagnosis, hence supported the theory that central obesity could be a potential risk factor for autoimmune diseases in general. In addition, 19.0% of children in the case group were overweight compared with 14.3% in the control group. However, being overweight was not related to developing an autoimmune disease (OR 1.60, 95% CI 0.89–2.87). Approximately 2.6 years after baseline, 253 (48%) children (of which 52 developed an autoimmune disease) had body measures available. Of them, 92.8% remained in their previous WTHR category and 89.9% in their previous BMI category. No children in this study were underweight.
TABLE 2.
Associations of central obesity and being overweight in school‐aged children with the onset of paediatric autoimmune diseases (DM, AIT, JIA, and IBD) a
| Baseline anthropometric measures | Cases a | Controls b | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| Autoimmune diseases | Central obesity, n (%) | N = 105 | N = 420 | ||
| No (WHTR<0.5) | 86 (81.9) | 373 (88.8) | Reference | Reference | |
| Yes (WHTR ≥0.5) | 17 (16.2) | 41 (9.8) | 1.93 (1.04–3.57) | 2.11 (1.11–3.98) | |
| Missing | 2 (1.9) | 6 (1.4) | |||
| BMI categories, n (%) | |||||
| Normal weight | 83 (79.0) | 356 (84.8) | Reference | Reference | |
| Overweight | 20 (19.0) | 60 (14.3) | 1.51 (0.86–2.67) | 1.60 (0.89–2.87) | |
| Missing | 2 (1.9) | 4 (1.0) | |||
| DM | Central obesity, n (%) | N = 34 | N = 136 | ||
| No (WHTR<0.5) | 26 (76.5) | 122 (89.7) | Reference | Reference | |
| Yes (WHTR ≥0.5) | 6 (17.6) | 12 (8.8) | 2.97 (0.97–9.05) | 3.20 (0.97–10.5) | |
| Missing | 2 (5.9) | 2 (1.5) | |||
| BMI categories, n (%) | |||||
| Normal weight | 28 (82.4) | 113 (83.1) | Reference | Reference | |
| Overweight | 4 (11.7) | 22 (16.2) | 0.80 (0.24–2.71) | 0.86 (0.24–3.04) | |
| Missing | 2 (5.9) | 1 (0.7) | |||
| AIT | Central obesity, n (%) | N = 39 | N = 156 | ||
| No (WHTR<0.5) | 34 (87.2) | 139 (89.1) | Reference | Reference | |
| Yes (WHTR ≥0.5) | 5 (12.8) | 16 (10.3) | 1.28 (0.44–3.70) | 1.36 (0.45–4.10) | |
| Missing | 0 | 1 (0.6) | |||
| BMI categories, n (%) | |||||
| Normal weight | 30 (76.9) | 133 (85.3) | Reference | Reference | |
| Overweight | 9 (23.1) | 23 (14.7) | 1.66 (0.73–2.80) | 1.70 (0.72–4.02) | |
| Missing | 0 | 0 | |||
| JIA | Central obesity, n (%) | N = 18 | N = 72 | ||
| No (WHTR <0.5) | 15 (83.3) | 61 (84.7) | Reference | Reference | |
| Yes (WHTR ≥0.5) | 3 (16.7) | 8 (11.1) | 1.61 (0.38–6.81) | 1.55 (0.28–8.63) | |
| Missing | 0 | 3 (4.2) | |||
| BMI categories, n (%) | |||||
| Normal weight | 14 (77.8) | 60 (83.3) | Reference | Reference | |
| Overweight | 4 (22.2) | 9 (12.5) | 2.01 (0.54–7.51) | 1.76 (0.36–8.59) | |
| Missing | 2 (11.1) | 3 (4.2) | |||
| IBD | Central obesity, n (%) | N = 14 | N = 56 | ||
| No (WHTR<0.5) | 11 (78.6) | 51 (91.1) | Reference | Reference | |
| Yes (WHTR ≥0.5) | 3 (21.4) | 5 (8.9) | 2.90 (0.56–15.0) | 2.55 (0.45–14.6) | |
| Missing | 0 | 0 | |||
| BMI categories, n (%) | |||||
| Normal weight | 11 (78.6) | 50 (89.3) | Reference | Reference | |
| Overweight | 3 (21.4) | 6 (10.7) | 1.66 (0.73–2.80) | 2.16 (0.34–13.7) | |
| Missing | 0 | 0 | |||
Data were collected approximately 2 years prior to diagnosis. Median age at the time of the diagnosis was 13.75 (IQR 12.25–15.54).Of the 11 407 school‐aged children in the background cohort, 105 children who obtained primary diagnosis (AIT, autoimmune thyroiditis; DM, type 1 diabetes mellitus; IBD, inflammatory bowel diseases; JIA, juvenile idiopathic arthritis) at least 1 month after baseline and had available Food Frequency Questionnaire generated the case group. OR, odds ratio; CI, confidence interval; WHTR, waist to height ratio; BMI, body mass index, weight (kg)/height2 (m2). Categorization was based on IOTF cut‐offs. 24 No children were underweight in this study.
Each child in the case group were compared with four children in the control group with matching age, sex, and residential area using conditional logistic regression test. Adjusted OR considered breakfast pattern, meal pattern, and eating habits.
FIGURE 1.
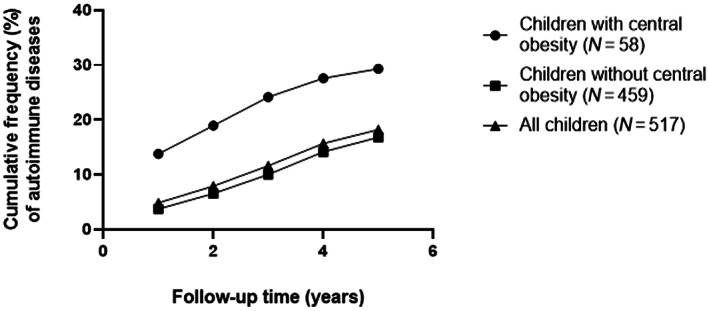
Cumulative frequency (%) of autoimmune diseases (DM, AIT, JIA, and IBD) in children with central obesity (WHTR ≥0.5) at baseline, in children without central obesity (WHTR <0.5), and in all children in this study. Finding was shown until median follow‐up time of 5.1 years (IQR 4.8–5.6). AIT, autoimmune thyroiditis; DM, type 1 diabetes mellitus; IBD, inflammatory bowel diseases; IQR, interquartile range; JIA, juvenile idiopathic arthritis; WHTR, waist to height ratio
Weekly consumption frequencies of the 16 food items individually were not related to the development of autoimmune diseases when adjusted with all other 15 food items and central obesity (Figure 2 and Table S1). Meal patterns, eating habits, SCI, and PCI were not associated with the development of autoimmune diseases either (Table 3). The food consumption characteristics, the PCI, and the STI of the 420 matched controls well represented 11 162 children in the total cohort who did not develop studied autoimmune diseases (Figure S2).
FIGURE 2.
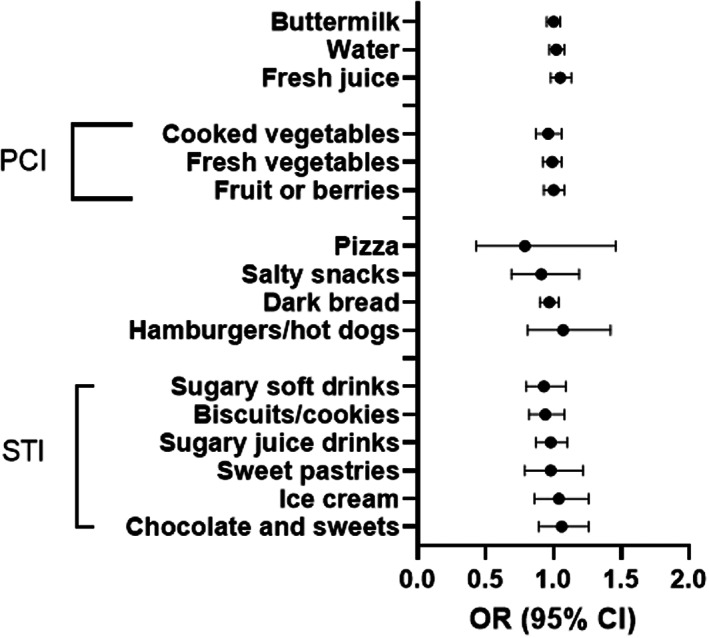
Relationship between food items based on food frequency questionnaire (FFQ) and the onset of autoimmune diseases (DM, AIT, JIA, and IBD)a. a Autoimmune diseases in this study are: AIT, autoimmune thyroiditis; DM, type 1 diabetes mellitus; IBD, inflammatory bowel diseases; JIA, juvenile idiopathic arthritis; PCI, plant consumption index; STI, sweet treat index. Case group = 105 adolescents who later developed autoimmune diseasesa. Four children with matching age, sex, and residential areas were chosen for each child in the case group, generating the control group of 420 children. Data were collected approximately 2 years prior to diagnosis. Median age at the time of the diagnosis was 13.8 (IQR 12.3–15.5). Each child in the control group were compared with four matching children in the control group using conditional logistic regression (survival cox regression): adjusted OR considered each of the 16 food items and central obesity. p > 0.05 for all food items
TABLE 3.
Relationships between breakfast pattern, meal pattern, eating habits, sweet treat index (STI), plant consumption index (PCI), and the onset of autoimmune diseases (DM, AIT, JIA, and IBD) a
| Cases a (N = 105) | Controls b (N = 420) | Odds ratio (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Breakfast pattern, n (%) | ||||
| Regular | 85 (81.0) | 333 (79.3) | Reference | Reference |
| Irregular | 20 (19.0) | 77 (18.3) | 1.01 (0.59–1.72) | 0.75 (0.41–1.37) c |
| Missing | 0 | 10 (2.4) | ||
| Meal pattern, n (%) | ||||
| Regular | 72 (68.6) | 304 (72.4) | Reference | Reference |
| Irregular | 33 (31.4) | 107 (25.5) | 1.32 (0.82–2.13) | 1.20 (0.71–2.02) c |
| Missing | 0 | 9 (2.1) | ||
| Eating habits (%) | ||||
| Healthy eater | 42 (40.0) | 168 (40.0) | Reference | Reference |
| Fruit and vegetable avoider | 44 (41.9) | 183 (43.6) | 0.97 (0.60–1.55) | 1.07 (0.54–2.12) c |
| Unhealthy eater | 14 (13.3) | 54 (12.9) | 1.04 (0.53–2.05) | 0.98 (0.60–1.59) c |
| Missing | 5 (4.8) | 15 (3.6) | ||
| Sweet Treat Index times/week, median (IQR) | 6.3 (4.0–11.5) | 7.0 (4.0–11.5) | 0.99 (0.95–1.02) | 0.99 (0.95–1.02) d |
| Missing (%) | 19 (18.1) | 35 (8.3) | ||
| Plant Consumption Index times/week, median (IQR) | 10.0 (5.5–15.5) | 11.5 (7.0–18.0) | 0.98 (0.96–1.01) | 0.99 (0.96–1.02) d |
| Missing (%) | 0 | 1 (0.2) | ||
Data were collected approximately 2 years prior to diagnosis. Median age at the time of the diagnosis was 13.75 (IQR 12.25–15.54). Of the 11 407 school‐aged children in the background cohort, 105 children with primary diagnosis (AIT, autoimmune thyroiditis; DM, type 1 diabetes mellitus; IBD, inflammatory bowel diseases; JIA, juvenile idiopathic arthritis) at least 1 month after baseline and available Food Frequency Questionnaire generated the case group. STI, the frequency of sugary product consumptions per week; PCI, the frequency of fruit and vegetables consumptions per week.
Each child in the case group were compared with four children in the control group with matching age, sex, and residential area using conditional logistic regression test. Adjusted analysis included breakfast pattern, meal pattern, and central obesity.
Eating habit as covariates.
STI and PCI as covariates.
4. DISCUSSION
Data on anthropometric measures and dietary patterns of children who developed autoimmune diseases were compared with the data of controls with matching age‐, sex‐, and residential area (as potentially profound confounders). The control group eligibly represented all children in the cohort, who did not develop autoimmune disease of interest. Hence, our result using matched case–control design could be extended to cover larger population. We showed that the odds of developing a primary autoimmune disease during adolescence (<19 years) was doubled in school‐aged children with central obesity. This finding was not associated with any particular autoimmune diagnosis nor was it supported by BMI‐based overweight. Furthermore, we observed no significant associations between dietary patterns and the likelihood of developing an autoimmune disease.
Our study showed that central obesity was a potential risk factor for autoimmune diseases during adolescence in general (represented by DM, AIT, JIA, and IBD), but BMI‐based overweight by itself was not. This could be explained by the fact, that BMI measure does not consider the shape of the body nor the location of the fat, to be precise. On the contrary, central obesity predominantly corresponds with the amount of adipose tissue around the waist. Intriguingly, adipose tissue itself has been described to have a pro‐inflammatory effect, and its accumulation may contribute to a chronic low‐grade inflammation. 30 Furthermore, a correlation between waist circumference and increased T‐cells activity in the adipose tissue have been discovered. 31 Since autoimmune diseases in our study (DM, AIT, JIA, and IBD) are also presented with tissue T‐cells infiltration, we speculated that the increased activation of adipose tissue T‐lymphocytes might have induced autoimmunity in other tissues as well—contributing to the development of autoimmune diseases. More studies on the mechanism of this connection are warranted.
Previous studies have linked central obesity to a higher risk of positive thyroid peroxidase antibodies and AIT. 11 In children, central obesity was not associated with JIA's disease activity, 32 nor was it linked to the progression of DM in children diagnosed with positive autoantibodies. 33 In contrast, being overweight was related to more severe IBD. 34 Even though these studies were not contradictory to ours, none of them related central obesity to the development of autoimmune diseases in previously healthy children. In this way our study was unique.
We did not find any relationship between dietary patterns e.g. meal patterns, eating habits, consumption frequencies of sugary products and/or plants, and development of autoimmune diseases, but what about previous studies? A Swedish register‐based case–control study involving children aged 7–14 years with matching age, sex, and residential area has shown that elevated energy intake and higher intake of carbohydrates, especially of disaccharides and sucrose, increased DM risk. 35 However, this study did not present the association between the patients' high energy intake and central obesity/overweight, which could be a confounding factor. Moreover, data on dietary intakes 1 year prior to diagnosis were recalled using an extensive quantitative FFQ within an interview by a dietitian. Our dietary data were collected prior to diagnosis, reflecting food consumption within 1 month preceding baseline recruitment—therefore, had less recall bias compared with data collected after diagnosis.
We are unaware of previous paediatric studies linking dietary patterns with the development of AIT or JIA. Low vegetable consumption and high sugar intake might be related to ulcerative colitis but not to Crohn's disease in adults, and diet with pro‐inflammatory characteristics have been proposed to increase the risk for ulcerative colitis in adult patients. 17 , 36 The number of paediatric IBD in our study was small, so we combined Crohn's disease and ulcerative colitis, and we did not notice the association between diet and the development of IBD.
So far, no convincing results of the relationship between particular nutrient intake and the development of autoimmune diseases have been presented, even though the role of high protein intake and an imbalance of omega‐3/6 fatty acid intake have been investigated. 37 , 38 , 39 Even though our study focused on dietary patterns in general rather than on particular nutrient intake, it did not affirm the association between diet and development of autoimmune diseases either. Nevertheless, diet might still be indirectly associated with autoimmune diseases through promoting or preventing central obesity. Previous studies have shown that regular intake of fruit/vegetables may prevent overweight and central obesity in children and adolescents. 40 , 41 In our study, plant consumption did not differ between children who developed autoimmune diseases and their matching controls, when measured in school‐aged children prior to the onset of autoimmune diseases. Therefore, the mechanisms on how fruit/vegetable consumption frequencies might indirectly prevent autoimmune diseases by preventing central obesity or potentially by acting as anti‐inflammatory agents requires further studies.
Our study had several strengths. The Fin‐HIT cohort as a source population had only small variations in socioeconomic status. 28 The derived control population were further matched based on age, sex, and residential area. This would naturally limit the numbers of potential confounding factors, and even small differences would be important. In addition, our present study is the first case–control study to investigate whether central obesity, overweight, and dietary patterns in school‐aged children are related to the development of common paediatric autoimmune diseases (DM, AIT, JIA, and IBD) in a prospective setting. Weight, height, and waist circumference were objectively measured at baseline before the onset of autoimmune diseases instead of being self‐reported. In addition, our data were not influenced by potential nutritional intervention that are sometimes practiced after receiving diagnosis. Moreover, the control group represented well the entire Fin‐HIT cohort as it had similar age, anthropometric measures, and food consumptions based on the FFQ. This means that our findings could be generalized to entail the whole cohort—or possibly even the regions in Finland that are represented by the cohort.
Since there were no children with underweight in this study, we could not analyse the effect of being underweight on the development of autoimmune diseases. We also did not have confirmed data on parental autoimmune diagnoses nor on the children's genetic predisposing factors that might act as confounders. These were our limitations. On the other hand, the parental autoimmune diseases could also be related to the central obesity of the parents, due to unhealthy lifestyle of the whole family, for example. In this case, the role of the children's genetic background concerning autoimmune diseases might be intertwined with the role of familial (lifestyle and genetic) central obesity, making it difficult to address separately.
Another limitation concerns the rigid nature of our data. Even though our study observed the development of autoimmune diseases in a prospective cohort, the questionnaire, and anthropometric measures were static. Hence, we were unable to assess potential changes over time, which might occur both in food consumption patterns and in anthropometric measures (especially during puberty and growth spurts) just before the onset of the studied autoimmune diseases. However, although dietary data were collected only once at baseline, it is plausible to assume only minor changes in dietary patterns throughout childhood as suggested by previous longitudinal studies. 42 , 43 , 44 Since follow‐up time in our study was relatively short (approximately 5 years), the changes in eating behaviour would likely be even less than in those studies with longer follow‐up time. Furthermore, based on body measures 2–3 years after baseline, 90% of the children stayed in their previous BMI and/or WTHR categories, suggesting that these variables are relatively stable in adolescents during a short‐term follow‐up. Thus, we did not change our conclusion. We also suggest that central obesity might act as an inflammatory trigger. Once present, it may induce a chronic inflammatory process that continues despite of potential longitudinal fluctuation in central adiposity. This hypothesis warrants further studies.
We have relatively narrow data on food consumption. Even though the FFQ we used provided a fast and easy way to categorize children based on consumption frequencies of indicatory food items, it provided only rough estimations of dietary choices. For example, we were not able to assess specific food ingredients, portion sizes, nergy intake, or other nutrient intakes which were required in calculating different dietary inflammatory scores and indices in previous studies. 14 , 29 We are also aware of the possibility of underreporting in answering questionnaires, particularly concerning questions that were related to unhealthy lifestyles. Yet, it is safe to assume that this tendency would be similar in both case and control groups.
In conclusion, central obesity in school‐aged children increased the likelihood of developing autoimmune diseases (represented by DM, AIT, JIA, and IBD) in adolescence. Therefore, paying attention to the relative waist circumference of school‐aged children during check‐ups is necessary—especially concerning children from families with a strong history of autoimmune diseases. Even though dietary patterns were not associated with the onset of autoimmune diseases in this study, we still recommend healthy eating habit with high fruit and vegetable consumption to avoid central obesity as a relatively simple way to try to restrict the potential development of autoimmune diseases in adolescents.
CONFLICT OF INTEREST
There are no potential conflicts of interest to disclose.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
Laura Räisänen conceptualized and designed the study, carried out the initial data analyses, interpreted data, searched the literatures, wrote the initial manuscript, generated figures, and tables, and revised the manuscript.
Sohvi Lommi conceptualized and designed the study, helped with data analyses, searched the literatures, reviewed the manuscript, and critically reviewed the manuscript for important intellectual content.
Elina Engberg conceptualized and designed the study, helped with data interpretation, and reviewed the manuscript.
Kaija‐Leena Kolho conceptualized, designed, and supervised the study, helped with data interpretation, and critically reviewed the manuscript for important intellectual content.
Heli Viljakainen participated in data collection, conceptualized, designed, supervised the study, helped with data interpretation, and critically reviewed the manuscript for intellectual content.
The study was supported by Folkhälsan's research fund and the Päivikki Sakari Sohlberg Foundation. In addition, Laura Räisänen received Tampere University Hospital's research grant. Kaija‐Leena Kolho received Helsinki University Grant and a Grant from Pediatric Research Foundation.
Räisänen L, Lommi S, Engberg E, Kolho K‐L, Viljakainen H. Central obesity in school‐aged children increases the likelihood of developing paediatric autoimmune diseases. Pediatric Obesity. 2022;17(3):e12857. doi: 10.1111/ijpo.12857
Funding information Folkhälsan Research Fund; Päivikki and Sakari Sohlberg Foundation; Tampere University Hospital Research Fund
Contributor Information
Laura Räisänen, Email: laura.raisanen@tuni.fi.
Heli Viljakainen, Email: heli.viljakainen@helsinki.fi.
REFERENCES
- 1. Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60:1739‐1753. [DOI] [PubMed] [Google Scholar]
- 2. Richard‐Miceli C, Criswell LA. Emerging patterns of genetic overlap across autoimmune disorders. Genome Med. 2012;4(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paschou SA, Papadopoulou‐Marketou N, Chrousos G, et al. On type 1 diabetes mellitus pathogenesis. Endocr Connect. 2017;7:R38‐R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonelli A, Ferrari SM, Corrado A, et al. Autoimmune thyroid disorders. Autoimmun Rev. 2014;14:174‐180. [DOI] [PubMed] [Google Scholar]
- 5. Martini A, Prakken B, Albani S, et al. Juvenile idiopathic arthritis. Lancet. 2011;377:2138. [DOI] [PubMed] [Google Scholar]
- 6. Giuffrida P, Corazza GR, Di Sabatino A. Old and new lymphocyte players in inflammatory bowel disease. Dig Dis Sci. 2017;63:277‐288. [DOI] [PubMed] [Google Scholar]
- 7. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2016;3:151‐155. [Google Scholar]
- 9. Versini M, Jeandel P‐, Rosenthal E, et al. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13(9):981–1000. [DOI] [PubMed] [Google Scholar]
- 10. Winer D, Luck H, Tsai S, et al. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23:413‐426. [DOI] [PubMed] [Google Scholar]
- 11. Song R, Wang B, Yao Q, et al. The impact of obesity on thyroid autoimmunity and dysfunction: a systematic review and meta‐analysis. Front Immunol. 2019;10:2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaubeck M, Haller D. Reciprocal interaction of diet and microbiome in inflammatory bowel diseases. Curr Opin Gastroenterol. 2015;31:464‐470. [DOI] [PubMed] [Google Scholar]
- 13. Manzel A, Muller DN, Hafler DA, et al. Role of “western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2013;14:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature‐derived, population‐based dietary inflammatory index. Public Health Nutr. 2014;17:1689‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsumoto Y, Shivappa N, Sugioka Y, et al. Change in dietary inflammatory index score is associated with control of long‐term rheumatoid arthritis disease activity in a Japanese cohort: the TOMORROW study. Arthritis Res Ther. 2021;23(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamers CR, Roos D, Nicole M, Witteman BJM. The association between inflammatory potential of diet and disease activity: results from a cross‐sectional study in patients with inflammatory bowel disease. BMC Gastroenterol. 2020;20:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Racine A, Carbonnel F, Chan SSM, et al. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm Bowel Dis. 2016;22:345‐354. [DOI] [PubMed] [Google Scholar]
- 18. Bolte LA, Vich Vila A, Imhann F, et al. Long‐term dietary patterns are associated with pro‐inflammatory and anti‐inflammatory features of the gut microbiome. Gut. 2021;70:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Figueiredo RA d O, Simola‐Ström S, Rounge TB, et al. Cohort profile: the Finnish Health in Teens (fin‐HIT) study: a population‐based study. Int J Epidemiol. 2019;48:23‐24h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niemelä H. Social Security in Finland, Helsinki, Finland: Social Insurance Institution (KELA), Finnish Centre for Pensions (ETK), Finnish Pension Alliance (TELA), and Finnish Ministry of Social Affairs and Health . 2006.
- 21. Furu K, Wettermark B, Andersen M, et al. The Nordic countries as a cohort for Pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106:86‐94. [DOI] [PubMed] [Google Scholar]
- 22. Räisänen L, Viljakainen H, Sarkkola C, et al. Perinatal risk factors for pediatric onset type 1 diabetes, autoimmune thyroiditis, juvenile idiopathic arthritis, and inflammatory bowel diseases. Eur J Pediatr. 2021;180(7):2115‐2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCarthy HD, Ashwell M. A study of central fatness using waist‐to‐height ratios in UKchildren and adolescents over two decades supports the simple message—‘keep your waist circumference to less than half your height’. Int J Obes (Lond). 2006;30:988‐992. [DOI] [PubMed] [Google Scholar]
- 24. Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut‐offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284‐294. [DOI] [PubMed] [Google Scholar]
- 25. Roberts C, Freeman J, Samdal O, et al. The Health Behaviour in School‐aged Children (HBSC) study: methodological developments and current tensions. Int J Public Health. 2009;54(Suppl 2):140‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vereecken CA, Maes L. A Belgian study on the reliability and relative validity of the Health Behaviour in School‐Aged Children food‐frequency questionnaire. Public Health Nutr. 2003;6:581‐588. [DOI] [PubMed] [Google Scholar]
- 27. de Oliveira Figueiredo RA, Viljakainen J, Viljakainen H, et al. Identifying eating habits in Finnish children: a cross‐sectional study. BMC Public Health. 2019;19:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lommi S, Figueiredo RA d O, Tuorila H, et al. Frequent use of selected sugary products associates with thinness, but not overweight during preadolescence: a cross‐sectional study. Br J Nutr. 2020;124:631‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Byrd DA, Judd SE, Flanders WD, et al. Development and validation of novel dietary and lifestyle inflammation scores. J Nutr. 2019;149:2206‐2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mancuso P. The role of adipokines in chronic inflammation. Immunotargets Ther. 2016;5:47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Travers RL, Motta AC, Betts JA, et al. The impact of adiposity on adipose tissue‐resident lymphocyte activation in humans. IJO. 2015;39:762‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelajo CF, Lopez‐Benitez JM, Miller LC. Obesity and disease activity in juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2012;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cedillo M, Libman IM, Arena VC, et al. Obesity, islet cell autoimmunity, and cardiovascular risk factors in youth at onset of type 1 autoimmune diabetes. J Clin Endocrinol Metab. 2015;100:E82‐E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yerushalmy‐Feler A, Galai T, Moran‐Lev H, et al. BMI in the lower and upper quartiles at diagnosis and at 1‐year follow‐up is significantly associated with higher risk of disease exacerbation in pediatric inflammatory bowel disease. Eur J Pediatr. 2020;180(1):21‐29. [DOI] [PubMed] [Google Scholar]
- 35. Pundziute‐Lyckå A, Persson L, Cedermark G, et al. Diet, growth, and the risk for type 1 diabetes in childhood: a matched case‐referent study. Diabetes Care. 2004;27:2784‐2789. [DOI] [PubMed] [Google Scholar]
- 36. Shivappa N, Hébert JR, Rashvand S, et al. Inflammatory potential of diet and risk of ulcerative colitis in a case‐control study from Iran. Nutr Cancer. 2016;68:404‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashvand S, Somi MH, Rashidkhani B, et al. Dietary protein intakes and risk of ulcerative colitis. MJRI. 2015;29:253. [PMC free article] [PubMed] [Google Scholar]
- 38. Schreiner P, Martinho‐Grueber M, Studerus D, et al. Nutrition in inflammatory bowel disease. Digestion. 2020;101:120‐135. [DOI] [PubMed] [Google Scholar]
- 39. Scaioli E, Liverani E, Belluzzi A. The imbalance between n‐6/n‐3 polyunsaturated fatty acids and inflammatory bowel disease: a comprehensive review and future therapeutic perspectives. Int J Mol Sci. 2017;18:2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthews VL, Wien M, Sabaté J. The risk of child and adolescent overweight is related to types of food consumed. Nutr J. 2011;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bradlee ML, Singer MR, Qureshi MM, et al. Food group intake and central obesity among children and adolescents in the third National Health and nutrition examination survey (NHANES III). Public Health Nutr. 2010;13:797‐805. [DOI] [PubMed] [Google Scholar]
- 42. Mikkilä V, Räsänen L, Raitakari OT, et al. Consistent dietary patterns identified from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. Br J Nutr. 2005;93:923‐931. [DOI] [PubMed] [Google Scholar]
- 43. Movassagh E, Baxter‐Jones A, Kontulainen S, et al. Tracking dietary patterns over 20 years from childhood through adolescence into young adulthood: the Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients. 2017;9:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lommi S, Engberg E, Tuorila H, et al. Sex‐and weight‐specific changes in the frequency of sweet treat consumption during early adolescence: a longitudinal study. BJN. 2021;1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


