Summary
Obesity is a chronic condition whose management is a critical challenge for physicians. The scientific community has increased its focus on the molecular mechanisms involved in obesity etiopathogenesis to better manage patients with obesity and its associated complications. The tight connection between adipose tissue and the immune system has been demonstrated to play a crucial role in inflammation, and melatonin is important for circadian rhythm regulation and metabolic homeostasis, in which it orchestrates several molecular mechanisms involved in obesity and associated inflammation. Melatonin also regulates innate and adaptive immunity; its antioxidant properties are linked to reduced predisposition to infection and weight gain in patients with obesity through the modulation of the immune response, which has a significant beneficial effect on inflammation and, consequently, on the metabolic state. Low melatonin levels have been linked to obesity, and melatonin supplementation can reduce body weight, improve metabolic profile, and ameliorate immune responses and pro‐inflammatory stimuli. The role of melatonin in obesity is mainly related to improved oxidative stress signaling, modulation of adipokine secretion, and a switching from white‐to‐brown adipose tissue phenotype and activity. Moreover, the role of melatonin in obesity modulation by controlling circadian rhythm has recently emerged as a pivotal mechanism for lipid and glucose metabolism dysfunction in adipose, muscle, and liver tissues. Melatonin may also regulate the immune system by acting directly on thymus morphology and activity as well as by modulating oxidative stress and inflammatory states during infections. The tight association between melatonin and immune response regulation is coordinated by Toll‐like receptors, which are rhythmically expressed during the day. Their expression may be strongly modulated by melatonin as their signaling is highly inhibited by melatonin. The current review summarizes studies of melatonin‐induced mechanisms involved in infection regulation, particularly the modulation of obesity‐associated inflammation and systemic complications.
Keywords: infections, melatonin, metaflammation, obesity
1. INTRODUCTION
Obesity is a public health problem with pandemic proportions, which has prompted the scientific community to better study the molecular mechanisms that can be effectively targeted by therapeutic strategies. 1 Obesity is characterized by a significant increase in adipose tissue caused by an imbalance in food intake and energy expenditure. It is a pathological condition that includes multifactorial aspects, including genetic and endocrine defects. 1 Moreover, obesity represents a crucial health problem both in developed and developing countries that is mainly linked to a general change in human lifestyle, associated with a sedentary lifestyle and availability of high‐energy refined foods. 1 , 2 The failure of the healthcare system in treating obesity during its early stages makes the management of patients with obesity a greater challenge because it requires the concomitant presence of several factors. These factors include the psychological predisposition of patients to ameliorate their physical illness and a multidisciplinary medical approach that can overload the healthcare system and dramatically impact economic and social costs. 1 , 3 , 4
Obesity has long been associated with increased morbidity and mortality caused by cardiovascular disease, 5 diabetes, 6 hyperlipidemia, 7 and complications from and predisposition to infectious diseases. 8 Complex modifications to adipose tissue arrangement, including immune cell infiltration and pro‐inflammatory cytokine secretion, impact predisposition to several infectious diseases. 8 Obesity is closely related to chronodisruption, which is characterized by the deregulation of physiological and behavioral central and peripheral circadian rhythms that contribute to obesity‐related metabolic impairment. Eating and sleeping time schedules can strongly influence the hunger‐satiety circuit and body weight gain, which are relevant synchronizers of the human biological clock 9 and in which melatonin plays an important role. Moreover, its intake has been suggested as a potential regulator of circadian rhythms, 10 the molecular mechanisms of which underlie the onset of obesity, 11 , 12 innate and adaptive immune responses, 13 and oxidative reactions during infections. 14 , 15 , 16 , 17
Here, we present a comprehensive review that discusses the main molecular mechanisms triggered by melatonin in the control of infections in an obesity setting, focusing on the regulation of chronic low‐grade inflammation (metaflammation) that occurs primarily through the antioxidant properties of melatonin and its ability to entrain the central and peripheral circadian rhythms.
2. SEARCH STRATEGY
The current review critically analyzes the basic and translational evidence of the role of melatonin in the regulation of infections during obesity by describing the available literature from 2000 to 2021. Particular attention has been given to the molecular mechanisms implicated in metaflammation and in the regulation of oxidative stress, of which melatonin is a strong modulator. The relevant literature was screened from PubMed (MEDLINE) using the search terms “obesity,” “inflammation,” “metaflammation,” “infections,” “circadian rhythm,” “melatonin,” “melatonin and circadian rhythm,” “melatonin and inflammation,” “melatonin and immune system,” “melatonin and infections,” “melatonin and glucose metabolism,” “melatonin and lipid metabolism,” “melatonin and aging,” “melatonin and thymus involution,” “melatonin and adipogenesis,” “melatonin and bone marrow adipogenesis,” and “melatonin and chemotherapy.”
3. THE LINK BETWEEN OBESITY, METAFLAMMATION, AND INFECTION
Adipose tissue is a metabolically active organ that is tightly interconnected with the immune system. Inflammation plays a pivotal role in the interaction between adipose tissue and the immune system. 18 Changes in adipose tissue architecture, including adipocyte hypertrophy and hypoxia and typical features of obesity, can induce pro‐inflammatory cytokine production that attracts immune cells at the local level in adipose tissue and triggers chronic low‐grade inflammation. 19 This chronic state of inflammation is mainly mediated by macrophages whose polarization from the anti‐inflammatory (M2) to pro‐inflammatory (M1) phenotype is important for the pathogenesis of metabolic disease. 19 , 20 Along with macrophages, lymphocytes (B and T cells) can infiltrate the hypertrophic adipose tissue and contribute to the increased production and release of pro‐inflammatory cytokines, including interleukin (IL)‐8, IL‐6, and tumor necrosis factor α (TNFα). 21 Studies on adipose tissue during obesity onset demonstrated that approximately two‐thirds of the stromal vascular fraction in adipose tissue is represented by immune cells, increasing the ability of adipose tissue to act as an immunological tissue and control systemic inflammation and metabolism. 18 , 22 In turn, the immune system may actively participate in several obesity‐linked disorders such as atherosclerosis and nonalcoholic steatosis. 22 More recently, the onset of infections has been found to be among the systemic complications associated with obesity. 23 Indeed, strong evidence indicates that patients with obesity with systemic metaflammation more frequently experience infections, contract more severe infections, and have poorer prognoses. 8
4. MELATONIN: SYNTHESIS AND GENERAL MECHANISMS OF ACTION
Melatonin, or 5‐methoxy‐N‐acetyltryptamine, is an indolamine secreted by the pineal gland during the dark phase of the circadian rhythm in both diurnal and nocturnal species, earning it the nickname “hormone of darkness.” 24 Melatonin has a half‐life of approximately 30 min and is produced from serotonin through two enzymatic steps: acetylation and transfer of a methyl group. Hence, serotonin and melatonin show opposite circadian secretions. 24 , 25 The main functions of melatonin are related to circadian rhythms and sleep–wake cycle control. 24 Moreover, melatonin is related to a wide range of physiological activities, such as memory, mood, anxiety control, blood pressure reduction, and osteoblast differentiation. 24 , 26 In addition, melatonin can regulate the secretion of gonadotrophin‐releasing hormone from the hypothalamic neurons, stimulate the secretion of progesterone from granulosa cells, and suppress the expression of estrogen receptors, 27 thus influencing the reproductive state. Exogenous melatonin is usually administered to treat disturbances of the sleep–wake cycle related to circadian rhythm disruption, such as jet lag, 28 and it has recently emerged as a supplement to conventional therapies for several diseases, including cancer. 29 The increasing use of melatonin in clinical practice is linked to its powerful anti‐inflammatory and analgesic effects. 30 All these processes are mediated by the specific binding of melatonin to G protein‐coupled receptors that are widely expressed in the central nervous system (CNS) and different peripheral organs; these receptors are divided into two types: melatonin receptor type 1a (MT1) and melatonin receptor type 1b (MT2). 24 , 27 , 31 MT1 is generally expressed in the skin and related to the inhibition of cyclic adenosine monophosphate (cAMP) formation, together with the inhibition of protein kinase A (PKA) and CREB phosphorylation. 24 , 27 , 31 MT1 can also increase the phosphorylation of mitogen‐activated protein kinase 1/2 (MAPK1/2), including the extracellular signal‐regulated kinase 1/2 (ERK1/2), as well as potassium conductance. Similarly, activation of the MT2 receptor leads to inhibition of both cAMP and cGMP formation, activation of protein kinase C (PKC) in the suprachiasmatic nuclei (SCN), and decreased calcium‐dependent dopamine release in the retina. 24 , 27 , 31
The activation of MT1 receptors leads to vasoconstriction, indicating that vascular tone is differently regulated by melatonin receptors, whereas the activation of MT2 receptors leads to vasodilation. 24 , 27 , 31 Moreover, melatonin interacts with intracellular proteins, such as calmodulin, calreticulin, and tubulin, suggesting melatonin may act as an antiproliferative agent in cancer. 32 Regarding immunomodulatory effects of melatonin, the expression and function of the retinoid‐related orphan nuclear hormone receptor (RZR/ROR) family was recently shown to be modulated by melatonin. 33 It has been demonstrated that IL‐2 and IL‐6, which are the main interleukins involved in immune and inflammatory responses, are produced following the activation of nuclear melatonin receptor RZR/ROR by human peripheral blood mononuclear cells (PBMCs). 34 Moreover, melatonin has also been widely used as an antioxidative agent and free radical scavenger because of its ability to stimulate several antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and glutathione reductase. 15 The antioxidant properties of melatonin are potentially useful in the prevention of inflammatory diseases, such as liver injury, 35 pulmonary, 36 cardiovascular diseases, 37 and cancer. 38
5. THE ROLE OF MELATONIN IN OBESITY
5.1. Melatonin: Implications of chronobiology on metabolic pathways
Melatonin is well known as one of the main mediators through which the central master clock coordinates several areas of the CNS and the peripheral organs, acting as an internal synchronizer, also named “internal zeitgeber” 24 (Figure 1). Melatonin production from the pineal gland is strictly controlled by the circadian rhythm and is chemically expressed in the dark. For this reason, melatonin production is always circumscribed to the night, regardless of the behavioral distribution of activity and the rest of mammalian species (diurnal, nocturnal, or crepuscular species). 24 In humans, the endogenous circadian clock regulates a wide range of physiological activities and is formed by a master clock residing in the suprachiasmatic nuclei of the hypothalamus, which receives information from external and internal inputs to synchronize the peripheral clocks with these inputs. 39 , 40 Master and peripheral clocks are composed of the same clock genes and proteins that oscillate at the single‐cell level and are finely regulated by transcriptional and translational feedback loops. 41 Specifically, two transcriptional activators, BMAL1 (brain and muscle aryl hydrocarbon receptor nuclear translocator [ARNT]‐like protein 1) and CLOCK (clock circadian regulator) form the BMAL1/CLOCK complex by heterodimerization in the cytoplasm that then translocates into the nucleus where it induces the gene expression of PERIOD (PER1/2/3) and CRYPTHOCROME (CRY1/2). 42 PER and CRY are two gene transcription factors for the PER/CRY complex by heterodimerization in the cytoplasm and also translocate into the nucleus to repress gene expression and the activity of the BMAL1/CLOCK complex, which is also able to repress PER/CRY protein expression and activity through proteasome‐mediated pathway activation. 42 In this way, the BMAL1/CLOCK and PER/CRY complexes make a negative feedback loop to control each other's function. Clock genes and proteins are rhythmically expressed during the day and cooperate to induce clock machinery function. 42 As major regulators of the master and peripheral clock, BMAL1/CLOCK can modulate the expression of several genes dislocated in various tissues, 43 mainly in the mouse liver, where Bmal1 has been demonstrated to bind 2000 target genes in a circadian‐dependent manner, with a peak of DNA binding occurring during the day. 44 Chromatin immunoprecipitation sequencing (ChIP‐seq) has been used to analyze mouse hepatic gene binding sites for clock genes, and we confirmed that Bmal1 binds genes during the middle of the day, whereas gene ontology analysis revealed a large predominance of gene expression regulated by Bmal1, particularly genes involved in metabolic pathways. 45 Moreover, the maximum occupancy of transcriptional activators by Bmal1/Clock genes occurs concomitantly with H3K9 acetylation, which is characteristic of an active promoter region. 45 In addition, in mouse intestine tissues, Bmal1 can regulate the circadian expression of three peroxisome proliferator‐activated receptor (PPAR) target lipid enzymes: acyl‐CoA oxidase (Aox), 3‐hydroxy‐3‐methylglutaryl coenzyme A (Hmg‐CoA) synthase and cellular retinol‐binding protein II (CrbpII) by directly acting on the promoter activities of these genes. 46 Conversely, Per and Cry bind mouse hepatic genes during the night concomitantly with Hk34 trimethylation, characteristic of an inactive promoter region. 45 Moreover, Per2‐deficient mice have been reported to show a strong reduction in total triacylglycerol and nonesterified fatty acids, resulting in dysregulated lipid metabolism. Per2 can inhibit PPARγ recruitment to target promoters, resulting in transcriptional activation. 47
FIGURE 1.
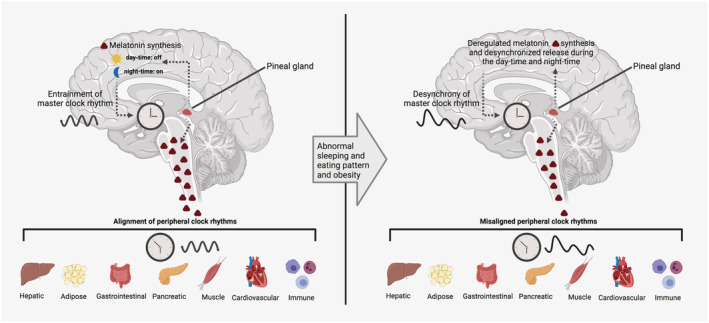
Melatonin regulation of peripheral clocks. All vertebrates synthesize and release melatonin through the pineal gland in a circadian‐dependent manner that has a classical peak during the night and is suppressed during the day. The nocturnal synthesis and release of melatonin are highly controlled by the master clock residing in the suprachiasmatic nucleus (SCN) through a fine molecular mechanism that is inhibited by light exposure. In turn, melatonin synchronizes peripheral clocks by acting at different levels and regulating several physiological functions. When melatonin synthesis and secretion are deregulated and/or desynchronized due to loss of master clock function, there is misalignment of peripheral clocks and a failure of physiological function control. Illustrations created with BioRender.com
Melatonin induces phase shifts and entrainment of the circadian clock by modulating clock gene expression and neuroplasticity. 24 , 48 , 49 Thereafter, nocturnal melatonin not only conveys temporal information to peripheral clocks in several organs but also harmonizes the central and peripheral clocks, improving circadian coordination. 50
Along with its synchronizing ability, melatonin is a key mediator between the circadian rhythm and lipid and carbohydrate metabolism at different tissue levels, such as skeletal muscle, adipose tissue, and the liver (Figure 2).
FIGURE 2.
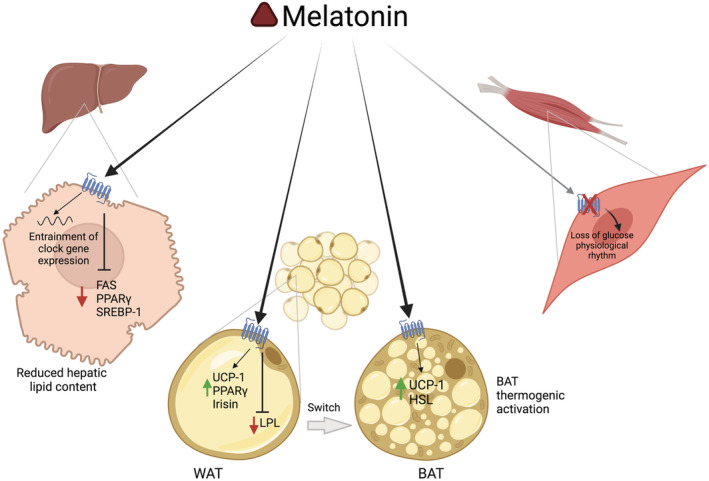
Molecular mechanisms induced by melatonin in different peripheral targets. Melatonin entrains the expression of clock genes in hepatocytes, reduces lipid accumulation, and decreases hepatic steatosis by downregulating Fas, Pparγ, and Srebp1 gene expression in the liver. The action of melatonin on white adipocytes induces a switch to the brown adipocyte phenotype, activating thermogenic action through stimulation of Ucp‐1, Irisin, and Pparγ gene expression. The lack of melatonin action in muscle cells induces a loss of the physiological rhythm of glucose. Illustrations created with BioRender.com
In particular, the effect of circadian rhythm disruption in skeletal muscle has been linked to dysregulation of glucose internalization, 51 insulin signaling, 52 and physical training. 53 MT1 and MT2 receptor knockout (KO) mice showed conserved Per2 and Bmal1 circadian expression but a loss of the blood glucose physiological rhythm. 54 Circadian rhythms play a fundamental role in lipid homeostasis, as reported in 10 healthy subjects with physiological cortisol and melatonin circulation levels consisting in cortisol nadir at 12:00 pm and melatonin zenith at 3:00 am. Cultured and synchronized myocytes with forskolin from the same subjects showed a superimposed circadian expression of lipid regulated by clock machinery and a diurnal lipid profile that was correlated with the transcription profiles of lipid key enzymes. 55 Moreover, Clock mutant mice retained melatonin diurnal oscillation but lost peripheral clock machinery components, particularly Per2, and had impaired glucose tolerance; however, these mice also had reduced plasma free fatty acids, increased plasma adiponectin, and improved insulin sensitivity. 56 Analysis of the main enzymes involved in lipid metabolism in epigonadal fat such as hormone‐sensitive lipase (hsl), adiponutrin, and desnutrin genes revealed that there was a difference in HSL expression in Clock mutant mice, as well as a loss of Per2 and a conserved Bmal1 genes expression rhythmicity. Conversely, adiponectin and desnutrin expression levels were maintained between mutant and wild type, but the circadian expression showed an anti‐phasic trend from 10:00 pm to 8:00 am. 56 Moreover, melatonin is synthesized and released during the dark hours of the night together with higher lipid adsorption, 57 suggesting a pivotal role of melatonin and the circadian system for the regulation of lipid homeostasis.
Patients with melatonin deficiency due to radiotherapy or surgical removal of the pineal gland who were administered 3 mg melatonin for 3 months displayed improved cholesterol and triglyceride blood levels, although body weight and liver fat remained unchanged. 58 These patients showed increased brown adipose tissue volume and activity, indicated by positron emission tomography–magnetic resonance imaging (MRI) measurement. 58 In animal models, melatonin was also reported to be important for the regulation of lipid levels and body fat composition. Male mice fed a high‐fat diet (HFD) and exposed to constant light for 24 h showed increased body weight and insulin resistance onset than mice in the control group, who were exposed for 12 h dark and 12 h light. 59 Moreover, the same mouse model exposed to constant light exhibited increased absorption and digestion of lipids. In addition, male obese, high fat and high caloric diet‐induced rats treated for 8 weeks with 10, 20, and 50 mg/kg body weight melatonin showed a significant increase in circulating irisin, a myokine that is particularly important in the switching of white adipose tissue into brown adipose tissue. 60 Furthermore, these doses of melatonin significantly reduced white adipocyte levels in inguinal depots and in the same district, as well as significantly increased Pparγ and uncoupling protein‐1 (Ucp‐1) gene expression levels that are associated with decreased lipoprotein (Lpl) transcriptional levels, thus confirming the browning transformation of white adipose tissue at the morphological and molecular level. 60 Similarly, male pinealectomized rats supplemented with melatonin showed higher physiological UCP‐1 gene and protein expression and Hsl gene expression levels in brown adipose tissue than in non‐treated rats. 61 Moreover, rat 62 and human 63 cultured adipocytes express MTs, and 1 nM melatonin was reported to regulate lipolysis isoproterenol‐induced when administered for 4 h. 62 Moreover, 1 nM melatonin combined with 5 nM insulin for 6 h is able to stimulate leptin secretion and gene expression f in cultured rat adipocytes. 64
The role of melatonin in the regulation of pluripotent mesenchymal stem cells (MSCs) in the bone marrow and adipose tissue has emerged. Human MSCs cultured in adipogenic‐inducing medium containing 10−4 M melatonin showed the downregulation of mRNA expression levels of markers of terminal adipocyte differentiation, such as LPL, adiponectin, and leptin and the upregulation of osteoblast differentiation markers, suggesting both anti‐adipogenic and anti‐osteoporotic effects. 65 Interestingly, the protective role of melatonin against bone marrow damage, particularly cadmium‐induced damage, has been demonstrated in vitro. 66 Human adipose MSCs chronically exposed to cadmium toxicity (e.g., 21 days of treatment with 0.5–2 μmol/L cadmium) showed a decrease in cellular lipid accumulation and attenuation of osteogenic response when cells were exposed to 50 nmol/L melatonin for 4 h compared with cells treated with cadmium and melatonin alone, respectively. 66 These results confirmed that melatonin inhibited the adipogenic differentiation of MSCs induced by cadmium exposure, leading to a preferential commitment of the precursor cell toward an osteogenic lineage. Similarly, the involvement of AMPK/β‐catenin signaling has been reported in C3H/10T1/2 mouse pluripotent stem cells. 67 Exposure to 100 μM melatonin for 3, 5, and 7 days promoted the osteogenic differentiation of C3H/10T1/2 cells and showed a synergistic effect when combined with bone morphogenetic protein 9 (BMP9). This effect was mediated by the phosphorylation of Smad1/5/8 and β‐catenin and by the activation of AMPKα proteins, which are the main factors involved in osteogenic cellular lineage development. 67
The anti‐lipogenic effect of melatonin in patients with nonalcoholic fatty liver disease (NAFLD), as well as the molecular mechanisms involved, such as the scavenger activity via stimulation of antioxidative enzymes has been investigated in in vivo human and animal models. 68 , 69 , 70 , 71 Conversely, a recent study comparing clock gene expression in PBMCs before and after a period of alcohol consumption in daytime and nighttime workers demonstrated significant differences in melatonin secretion and clock gene expression. 72 In the nighttime workers, alcohol consumption delayed physiological melatonin secretion, increased the total amount of PER1 expression, and decreased the total amount of CRY1 expression compared with daytime workers. 72 Similar results have been reported in obese mice exposed to constant light, fed with HFD and supplemented with 50 mg/kg body weight melatonin from 8:00 pm to 8:00 am. 59 After 10 weeks of melatonin exposure, obese mice showed decreased liver weight and hepatic lipid content levels, decreased circulating levels of glycemia, and ameliorated insulin sensitivity when compared with animals that did not receive melatonin treatment used as control group. 59
As demonstrated at the molecular level, constant light exposure can cause an anti‐circadian expression of clock genes in mouse liver tissues that restore the physiological pattern with melatonin supplementation. 59 The main proteins involved in cellular and tissue lipid accumulation, such as fatty acid synthase (FAS), cluster of differentiation 36 (CD36), PPARγ, and sterol regulatory element‐binding transcription factor‐1 (SREBP‐1), were upregulated in the liver of mice exposed to constant light, whereas they were decreased when the same mice were treated with 50 mg/kg body weight melatonin, suggesting a clear role for melatonin in the regulation of light/dark cycle and, consequently, lipid metabolism. 59 Figure 3 illustrates the effects of melatonin on the metabolic profiles and hepatic lipid homeostasis of mice exposed to constant light conditions.
FIGURE 3.
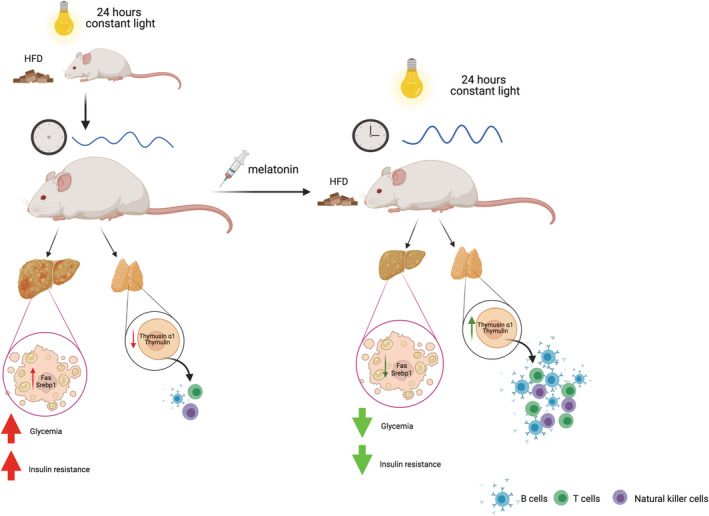
Metabolic and immune effects of melatonin in rodent models. High‐fat diet (HFD) mice exposed to constant light for 24 h showed dysregulated circadian rhythms accompanied by increased body weight. On the hepatic level, mice displayed upregulated levels of Fas and Srebp1 genes, which are associated with hepatomegaly and steatosis. These mice were also hyperglycemic and insulin resistant, and treatment with melatonin improved their metabolic profile by decreasing body weight and downregulating Fas and Srebp1 gene expression in the liver. Thus, hepatic steatosis decreased, and systemic insulin sensitivity and glycemia were improved. 59 Pinealectomized rats exposed to constant light showed decreased levels of thymosin α1 and thymulin, which are important factors secreted by the thymus involved in the maturation of immune cells (B cells, T cells, and natural killer cells). Interestingly, melatonin supplementation in this rat model promoted the secretion of thymosin α1 and thymulin, thus improving cell‐mediated immunity. 83 Illustrations created with BioRender.com
Melatonin supplementation improved the gut microbiota composition of HFD‐fed mice exposed to constant light by increasing the genera Roseburia and Eubacterium and reducing the ratio between Firmicutes and Bacteroidetes, which are butyrate‐producing bacteria. 59 Similarly, the anti‐obesogenic effects of melatonin on gut microbiota modulation have been demonstrated. 73 HFD‐fed mice treated daily for 2 weeks with 0.4 mg/ml melatonin showed improved visceral and subcutaneous adipose tissue accumulation when compared with those were not supplemented with melatonin used as control group. Similar to what was demonstrated in the control group, HFD mice supplemented with melatonin showed restored rhythmicity of clock genes that were otherwise lost as a result of HFD. 73 Fecal microbiota was explanted from the control, HFD, and HFD melatonin‐treated mice groups and transplanted into antibiotic‐treated mice at two different time points (8 am and 4 pm) to investigate the circadian response to HFD feeding. Although there was no significant difference detected in the dysmetabolism of HFD mice when melatonin was administered in the daytime or nighttime, a significant improvement in lipid content was revealed after circadian gut microbiota transplantation. In detail, transplantation in the control group at 4:00 pm significantly increased subcutaneous inguinal fat and circulating triglycerides compared with transplantation at 8:00 am. Moreover, transplantation in HFD melatonin‐treated mice groups showed decreased inguinal, perirenal, and periuterine fat depots when compared with control and HFD mice groups. 73 Moreover, no significant difference was observed in body weight gain after transplantation in the two different time points evaluated, and interestingly, the lipid profile of the receiving group was influenced by the time at which the microbiota were transplanted. 73 In particular, microbiota transplanted at 8:00 am in the HFD group increased serum triglyceride, cholesterol, and HDL concentrations in the receiving control group. However, this effect was slightly reversed when the control group received transplants from HFD melatonin‐treated mice at 8 am. Conversely, serum triglyceride levels decreased when microbiota from the HFD was transplanted into the receiving group at 4:00 pm. 73
These clinical and preclinical findings indicate that the role of melatonin in the control of adipose, muscle, and liver tissue fat accumulation, circulating lipid levels, and adipocyte differentiation is clearly linked to the expression of circadian rhythm components.
5.2. Melatonin: Implications of chronobiology on the immune system
The most important evidence of the relationship between immunity and cirrhosis is the rhythmic expression of Toll‐like receptors (TLRs) during the day. 74 , 75 TLR expression and function may be modulated by melatonin, 65 , 66 , 67 , 68 , 69 creating tight crosstalk between melatonin, circadian rhythm, and the immune system. TLRs are proteins expressed on the surface of many cells and within endosomes that play an important role in pathogen recognition and the consequent activation of the innate immune system. 76 In vivo and in vitro studies have demonstrated the circadian expression of TLRs and the effects of melatonin in the regulation of TLR expression, thus demonstrating a strong correlation between circadian rhythm and immunity. In particular, TLR9, which belongs to the group of endosomal receptors of the innate immune system and are capable of recognizing hypomethylated CpG sequences from DNA, showed circadian expression and functionality in in vivo studies in humans 74 and mice. 75 In humans, the daily variations in TLR9 responsiveness may modulate the efficacy of CpG oligodeoxynucleotide (ODN)‐adjuvanted immunization, 74 whereas in immunized mice, the maximum TLR9 responsiveness is Per2 expression‐related and, for this reason, correlated with maximum expression levels of Per2, which helped develop a stronger adaptive immunity 4 weeks after immunization, confirming the circadian regulation of this innate immune pathway. 75 Moreover, peritoneal macrophages isolated from circadian‐deficient Per2‐mutant mice and challenged with different pathogen‐associated molecular patterns (PAMPs) targeting multiple TLRs produced significantly lower amounts of TNF‐α and IL‐12 than macrophages isolated from wild‐type mice. 75 Similarly, an in vitro model of adherent splenocytes and splenic macrophages demonstrated that TLR3 protein levels fluctuated over the daily light–dark cycle and that mRNA levels of TLR2 and TLR6 exhibited rhythmic expression. 75
The inhibitory effect of melatonin on TLR action has been recently demonstrated in in vivo preclinical studies 77 , 78 and in vitro models. 79 , 80 , 81 In an in vivo animal model of ovarian cancer, it was reported that 200 μg/100 g body weight melatonin reduced TLR2 and TLR4 function by abolishing inflammatory status typical of ovarian cancer via myeloid differentiation factor 88 (MyD88) and TLR‐associated activator of interferon (TRIF) inhibition, 77 suggesting that melatonin can influence TLR expression by modulating the inflammatory responses observed in cancer. Moreover, a KO TLR2 mouse model demonstrated a feedback loop between TLR activation and melatonin synthesis and action. 78 Mice with allergic airway inflammation induced by intraperitoneal injection of ovalbumin showed increased TLR2 expression associated with increased activation of the nucleotide oligomerization domain‐like receptor family, pyrin domain containing‐3 (NLRP3) inflammasome and airway inflammation, which were significantly reduced in TLR2 KO mice. Additionally, wild‐type mice exhibited reduced melatonin biosynthesis, which was otherwise restored in the TLR2 KO mice, 78 demonstrating that the TLR2–NLRP3‐allergic airway inflammation circuit was responsible for decreased melatonin secretion. Similarly, in vitro experiments with macrophages demonstrated the inhibitory effects of melatonin on TLR3, 80 TLR4, 79 and TLR9 81 pro‐inflammatory effects through the prevention of the intracellular activation of pathways typically involved in cellular inflammatory responses, such as ERK1/2 and AKT phosphorylation, NF‐κB activation, and MyD88 transcription. 79 , 80 , 81
In addition to TLR expression, the role of melatonin in the regulation of immunity related to the circadian rhythm has also been studied in animal models. 82 , 83 In a rat model of arthritis, decreased levels of melatonin were detected, and ornithine decarboxylase activity (an index of lymph cell proliferation), which is a typical feature of inflammation of the submaxillary lymph node and spleen, was significantly increased, specifically with an acrophase in the afternoon or the morning in lymph nodes and spleen, respectively. 82 Surprisingly, daily injection of 100 μg melatonin increased the amplitude of ornithine decarboxylase activity, especially in old rats, suggesting not only the circadian role of melatonin in immune function regulation but also age dependency. 82 Moreover, pinealectomized rats continuously exposed to light showed decreased levels of thymosin α1 and thymulin proteins, which are secreted from the thymus and enhanced cell‐mediated immunity. 83 Moreover, exposure to 10 mg/kg body weight melatonin during the day caused an increase in the levels of thymosin α1 and thymulin in pinealectomized rats, demonstrating the role of melatonin in the regulation of immunity related to circadian rhythms. 83 Figure 3 summarizes the effects of melatonin on cell‐mediated immunity in pinealectomized rats exposed to constant light conditions.
Taken together, several studies have reported a central role for melatonin in the circadian regulation of metabolic and immune functions in human and animal models as well as in in vitro models, suggesting a pivotal therapeutic role for melatonin in the entrainment of metabolism and the immune system.
5.3. Melatonin: Implications of chronobiology on aging and immune system
Melatonin levels gradually decrease over the lifespan, 10 and this condition may be related to lowered sleep efficacy and circadian rhythm disruption. Diminished nocturnal melatonin secretion with associated disturbances in the sleep–wake rhythm has been reported in elderly healthy subjects. 84 Moreover, an in vivo study performed in a hamster model revealed that the expression of circadian clock machinery components was highly related to age, as demonstrated by the reduction of Bmal1, Clock, and Per1 gene expression in aged hamsters subjected to a light stimulus. 85 Moreover, old laboratory mice showed decreased Per2 gene expression in the SNC compared with young adult mice, regardless of light exposure. 86 The intricate role of melatonin in the regulation of clock gene expression and sleep–wake cycle modulation has been investigated in mouse models in which age has been correlated with increased daytime sleep and decreased nighttime wakefulness. 87 Moreover, old Wistar rats treated with 30 μg/kg body weight melatonin for 11 days showed restored circadian clock gene expression, which was otherwise altered by aging. 88 In 24‐month‐old rats, Bmal1, Cry1, and Cry2 gene expression in the SNC displayed an abolished daily rhythm whose synchronization was restored by melatonin supplementation, suggesting the role of melatonin in the balance of the sleep–wake cycle and circadian rhythm is significantly influenced by age. 88 Finally, because melatonin has potent antioxidant and free radical scavenger properties, it may play an important role in all age‐related disturbances, including sleep disturbances, thymus involution, and immune system dysfunction. 89
Age‐related disturbances are often associated with subcellular constituents, cells, and organ deterioration, which mainly result from increased toxic free radical action. 90 Therefore, to counterbalance the cell and tissue damage caused by free radicals, the body needs a piece of powerful machinery capable of eliminating these continuously produced reactive species. 90 Melatonin is an antioxidant capable of scavenging highly toxic hydroxyl radicals and stimulating the activity of a great number of antioxidative enzymes. 15 , 90 , 91 Particularly, a role in the restoration of age‐related thymus involution has been demonstrated in preclinical in vivo 92 , 93 and in vitro studies. 92 Thymus involution is a physiological process, particularly related to age, associated with immunosenescence that leads to a progressive reduction in thymus size and structure that can cause degeneration of the immune system and mainly affects T‐cell composition and efficiency. 94 In 22‐month‐old female mice and 7‐month‐old male mice, the daily exogenous supplementation of 60 μg melatonin for 60 and 40 consecutive days, respectively, increased thymus weight, thymocyte total number, and percentage at G2/S phases of the cell cycle, demonstrating the ability of melatonin to improve the replication rate of thymocytes and inhibit apoptotic cell death due to senescence and oxidative stress processes. 93 Restoration of the thymus has been reported to contribute to the immune response, indicating not only an improved thymus morphology but also improved thymus functionality. Melatonin supplementation in old mice induced a significant increase in natural killer cell activity compared with old untreated mice. 93 Similarly, cultured mouse thymocytes exposed to hydroxyl radicals (·OH) and subjected to apoptotic cell death were pretreated with 100 and 200 μM melatonin for 1 h to evaluate if melatonin participated in restoration of thymocyte proliferation. 92 The ·OH‐induced apoptotic cell death was almost completely abolished by 1 h of melatonin pretreatment through the significant reduction in caspase‐3 activity and DNA cleavage, as demonstrated with a terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay. 92
Considering these findings, melatonin not only functions in the regulation of circadian rhythm but also in age‐related immune dysfunction, such as thymus involution, by modulating oxidative processes associated with aging and loss of immune responses due to thymus regression. These findings further underline the strong scavenger capability of melatonin.
5.4. Melatonin: Insight into antioxidant molecular mechanisms
Alongside the well‐known role of melatonin as a pivotal regulator of the circadian rhythm, melatonin has recently emerged as a player in the control of body weight. Melatonin effects are mainly mediated by its antioxidant properties, 95 which are stronger than those of other indoles. 96 Indeed, several pathologies, including metabolic and immune dysfunctions, are tightly related to oxidative stress or even caused by chronic and long‐lasting oxidative stimuli. 95 The anti‐obesogenic actions of melatonin depend on several molecular mechanisms, as recently demonstrated by clinical 11 , 97 , 98 , 99 and preclinical data. 100 , 101 , 102 , 103 Surprisingly, a significant increase in melatonin serum levels between 6:00 pm and 8:00 am emerged when nondiabetic men with obesity were compared with lean men. 97 Moreover, nondiabetic men with obesity showed higher nocturnal plasma levels of melatonin than men with Type 2 diabetes mellitus (T2DM), indicating a higher amplitude of nocturnal melatonin production in nondiabetic subjects with obesity, which was mainly due to increased leptin levels that are capable of stimulating stronger sympathetic innervation and the pineal gland to secrete melatonin. 97 These findings demonstrated that nocturnal melatonin secretion was highly related to both body weight and insulin sensitivity, but specifically to leptin levels and action. 97
Although melatonin generally shows low toxicity and side effects, to avoid the risk of prolonged supra‐physiological diurnal melatonin levels, supplementation with exogenous melatonin should range from low doses of 0.3 mg 1 h before bedtime up to a maximum of 1 or 2 mg melatonin to best mimic its normal physiological levels. 104 Interestingly, subjects with obesity (five men and 10 women) supplemented with a high dose (10 mg/day) of melatonin and subjected to caloric restriction for 30 days showed significant weight loss, increased circulating levels of adiponectin and leptin, and decreased levels of resistin compared with subjects with obesity treated only with caloric restriction. 11 Additionally, the subjects with obesity supplemented with melatonin showed improved antioxidant defense machinery, as demonstrated by decreased levels of the oxidative stress biomarker malondialdehyde (MDA), demonstrating the positive effects of melatonin on body weight loss through the reduction of oxidative stress and adipokine regulation. 11 Women with obesity supplemented with melatonin at a high dose (6 mg/day) and subjected to a low‐calorie diet for 40 days showed only improved antioxidative stress defense but not an alteration in body weight and composition. 98 , 99 Similarly, a group of postmenopausal women with osteopenia who were treated with melatonin supplementation at different dosages (1 and 3 mg/day) for 12 months showed improved body composition and increased lean mass and upregulated levels of adiponectin compared with subjects treated with placebo. 99
Male patients with deficient melatonin secretion due to pineal gland surgical removal or radiotherapy damage in the pineal area showed improved volume and activity of brown adipose tissue when supplemented with 3 mg/day melatonin 30 min before sleep for 3 months, as demonstrated by integrated 18F‐fluorodeoxyglucose positron emission tomography magnetic resonance imaging (FDG‐PET/MRI) performed after cold exposure. 58 Similar results have been reported in experimental studies on animal models. Male obese Sprague–Dawley rats fed an HFD were treated with 30 mg/kg body weight melatonin for 3 weeks. 100 When melatonin was administered at zeitgeber time 11 lights‐out (the active phase of a nocturnal animal), the body weight of obese rats was significantly decreased compared with rats that did not receive melatonin used as control. Additionally, plasma glucose, triglycerides, and leptin levels decreased, confirming that melatonin may mediate body weight, lipid, and adipokine regulation. 100 Similarly, male obese C57BL/6 mice fed an HFD and treated with 1 mg/kg body weight melatonin during the dark phase for 10 weeks showed a significant decrease in food intake and body weight, accompanied by a significant reduction in fat depots in the inguinal, epididymal, and retroperitoneal zones. 12 Moreover, morphological analysis of inguinal region adipocytes demonstrated that obese mice treated with melatonin displayed a significant decrease in white adipocyte area and volume, which was associated with increased gene expression levels of CCAAT/enhancer‐binding protein α (C/EBPα), PPARγ, glucose transporter 4 (GLUT4), and adiponectin markers for mature adipocytes and insulin sensitivity. 12 In addition, genetically obese (ob/ob) mice, whose sex was not reported, that were exposed for 8 weeks to 100 mg/kg body weight melatonin showed a significant increase in adiponectin and a decrease in TNF‐α, resistin, and visfatin in adipose tissue deposits, thus indicating a reduction in adipose tissue inflammatory infiltration markers. 101
The antioxidative properties of melatonin are particularly important in the prevention of cardiovascular risk, mainly due to its protective effects on cardiomyocytes and heart features reported in mice. 102 , 103 Male ob/ob mouse models treated with 100 mg/kg body weight melatonin for 13 weeks showed ameliorated heart function and morphology through the restoration of sirtuin‐1 (SIRT‐1) protein expression and increased pAMPK/AMPK protein expression ratios that strongly contributed to decreased heart oxidative stress, typically observed in obese mice. 103 Similarly, melatonin may also reduce adipocyte hypertrophy and inversely regulate hydroxynonenal (4HNE), a product of cellular lipid peroxidation and adiponectin expression at the pericardium levels in male ob/ob mice models. 102
In vitro experiments revealed that melatonin may influence adipocyte differentiation by improving adipocyte functionality and switching them into brown adipocytes. In 3T3‐L1 preadipocytes 105 and bovine intramuscular preadipocytes (BIPs), 106 supplementation with 1 mM melatonin significantly stimulated C/EBPα gene expression and PPARγ gene and protein expression, two main regulators of terminal adipogenesis, thus showing melatonin involvement in white adipocyte differentiation. In addition, melatonin‐treated BIPs also formed smaller lipid droplets and abundantly expressed several genes and proteins associated with lipolysis, including HSL, adipocyte triglyceride lipase (ATGL), and perilipin‐1. 106 Melatonin exposure in BIPs reduced intracellular reactive oxygen species (ROS) levels by increasing the production and activities of superoxide dismutase 1 (SOD1) and glutathione peroxidase 4 (GPX4), which are responsible for destroying cellular free superoxide radicals. 106 Adipocytes explanted from the inguinal fat pads of Sprague–Dawley male rats during the mid‐light phase and were exposed to 1 nM melatonin to simulate a physiological dose and inhibited lipolysis via MT‐1 and MT‐2 receptors expressed by cultured cells. 62 According to in vivo results, the expression of UCP‐1, which is the main marker for adipose tissue browning, was also present at both the gene and protein levels in melatonin‐treated cells, confirming the role of melatonin in the induction of the white‐to‐brown adipose tissue shift and thus adipose tissue functionality. 107
Human adipose‐derived mesenchymal stem cells (AdMScs) exposed to 10 μM melatonin showed a higher proliferation rate and higher gene and protein expression levels of MT‐1 and MT‐2 receptors than untreated cells. 108 Contrary to what was observed in differentiated adipose tissue, in melatonin‐treated AdMScs, the expression levels of C/EBPα and lipid droplet accumulation was reduced when compared with untreated cells used as control, as demonstrated by Red Oil staining. 108 Surprisingly, when human mesenchymal stem cells (hMScs) were exposed to 10−8, 10−6 M, and 10−4 M melatonin, their differentiation lineage changed from adipogenic to osteogenic, which was associated with downregulated gene expression of PPARγ, leptin, LPL, and adiponectin, as well as unregulated markers of osteoblast differentiation such as osteopontin, osteocalcin and phosphatase alkaline after 10−4 M melatonin treatment. 65
Together, these findings demonstrate that melatonin can reduce adipogenesis and stimulate brown adipose tissue differentiation and that supplementation with high doses of melatonin can induce beneficial effects on adipose tissue functionality by stimulating brown adipose tissue activity and reducing total cholesterol and triglyceride levels.
5.5. Melatonin: Implications in chemotherapy responsiveness
Chemotherapy is one of the most used and effective treatments for cancer management but often shows high adverse reactions that severely limit its clinical applicability. However, a large body of evidence highlights the adjuvant role of melatonin in cancer treatment due to its antioxidant and immunoregulatory activities. 109 , 110 , 111 , 112 , 113 In particular, the concomitant administration of melatonin with chemotherapy has been associated with a better quality of life and increased free survival rate in patients with cancer. 114 , 115 Similarly, preclinical data from animal 116 , 117 and in vitro cancer models 118 , 119 , 120 have reported a therapeutic role for melatonin in the improvement of chemotherapy efficacy and in the reduction of chemotherapy‐associated side effects by decreasing ROS production, activating apoptotic pathways, and arresting the cell cycle in the G0/G1 phase or delaying the S phase. Interestingly, transgenic mice bearing mammary adenocarcinomas that were treated with cyclophosphamide, adriamycin, and 5‐fluorouracil (CAF) and adriamycin and docetaxel (AT) cytotoxic drug combinations during the morning and late afternoon were supplemented with 20 mg/L melatonin during the night 1 week before or 3 weeks after treatment or during both treatment periods. 121 After 14 and 21 days, all the treatment schedules had reduced tumor volume compared with controls, except when AT treatment was administered in the late afternoon without melatonin supplementation. This demonstrated a lower efficacy of AT compared with CAF if administered in the late afternoon. Surprisingly, the administration of AT in the late afternoon in combination with melatonin resulted in a more efficient chemotherapy schedule, indicating that melatonin improved AT chemotherapeutic action and the importance of chronobiology for chemotherapy treatment. 121
Taken together, this evidence highlights the efficacy of melatonin in enhancing chemotherapeutic effects in both in vivo and in vitro models, which supports the clinical data. In particular, melatonin effects seem to be mediated by antioxidative and antiproliferative/proapoptotic pathway activation in several cancer models.
5.6. Melatonin: Insight into anti‐inflammatory molecular mechanisms
Inflammation is a biological response in tissues and cells to harmful stimuli, such as pathogens, to protect the body from cellular injuries using the activation of immune cells, blood vessels, and cellular mediators. 122 In addition to its antioxidant properties, melatonin may also induce anti‐inflammatory actions, 123 and recent studies have suggested the important role of inflammasomes in the pathogenesis of different inflammatory diseases. 124 Inflammasomes are present in a variety of cells and activated by various endogenous or exogenous stimuli. Inflammasomes are intracellular multiprotein complexes that trigger inflammatory caspases, which in turn induce the secretion of pro‐inflammatory cytokines such as IL‐1β and IL‐18. To date, five different inflammasomes (nucleotide‐binding oligomerization domain‐like receptor pyrin domain‐containing [NLRP] 1–3) have been clearly identified: NLRP1, NLRP2, NLRP3, AIM2, and IPAF/NLRC4. 124 More recently, the role of melatonin in the modulation of inflammasomes has been reported, 125 , 126 , 127 particularly NLRP3, which is a multiprotein complex of the innate immune system that can be activated by microbial molecules, endogenous cytokines, or endogenous danger molecules, such as damage‐associated molecular patterns (DAMPs). 125
New therapeutic approaches aimed to reduce inflammatory processes without significantly altering physiological responses and with limited side effects are challenging for clinicians. 128 In this regard, melatonin, which has immunomodulatory properties and minimal toxicity, is well suited for this purpose. Scientific evidence for the role of melatonin in the immune response through the modulation inflammasome formation and action has been reported in the literature. Despite the diversity of pathological models used to evaluate this mechanism, they all focus on the inflammatory state on which melatonin acts. In a lipopolysaccharide (LPS)‐induced acute lung injury (ALI) mouse model, intratracheal administration of 30 mg/kg body weight melatonin 3 days before ALI induction improved histological changes in the lung caused by LPS stimulus, as well as lung density and aerated lung volume, and decreased the infiltration of macrophages and neutrophils and IL‐1β and caspase‐1 levels by inhibiting NLRP3 activation. 125 Similarly, it has been demonstrated that melatonin may delay intervertebral disc degeneration by inactivating the IL‐1β/NF‐κB‐NLRP3 positive feedback loop both in in vivo and in vitro models. 127 Rat models with intervertebral disc degeneration administered 30 mg/kg body weight melatonin for 3 weeks showed decreased levels of NLRP3, p20, IL‐1β, aggrecan, and collagen II levels when compared with rats that did not receive melatonin treatment, used as control group. Moreover, cultured rat nucleus pulposus cells treated with melatonin doses from 0.5 to 2 mM for 12–24–48 h showed decreased levels of NLRP3 and p20 protein expression compared with the untreated controls, thus demonstrating that the anti‐inflammatory properties of melatonin may be mediated by NLRP3 signaling. 127 Interestingly, in a chronic obstructive pulmonary disease rat model obtained by chronic exposure to cigarette smoke and LPS, 10 mg/kg/day melatonin improved lung function compared with untreated rats. 126 Moreover, lung tissues of rats treated with melatonin showed decreased NLRP3, cleaved caspase‐1, and ASC protein levels associated with higher SIRT‐1 protein expression, suggesting the airway anti‐inflammatory action of melatonin was SIRT‐1‐mediated. 126
These molecular data demonstrate that the anti‐inflammatory actions of melatonin are mainly mediated by the inhibition of NLRP3 inflammasome formation.
5.7. Melatonin, obesity, and the immune system: Human clinical evidence
Exposure to artificial light at night (ALAN) is associated with the disruption of the circadian system and has been shown to have detrimental effects on health. Exposure to ALAN is very frequent in industrialized countries because of shifts that require employees to workday and night shifts. Regarding overweight and obesity, there is growing evidence 129 , 130 of a relationship between night shift work or short sleep duration and indicators for adiposity, such as body mass index (BMI) or waist circumference (WC). Beyond the traditional determinants of obesity, such as energy intake and expenditure, mounting interest has been directed toward environmental factors, with recent evidence 131 , 132 for the association between exposure to ALAN and health outcomes. Although epidemiological studies exploring ALAN and excess adiposity are scarce, they are crucial to overcoming the hurdle of the use of sleep duration and night shift work as hypothetical proxies of ALAN exposure. Here, we summarize evidence from human studies that reported a link between ALAN and overweight/obesity conditions.
Only three cross‐sectional studies have examined outdoor ALAN captured by satellite sensors. Data from 8526 Korean participants in the KoGES study (47% men, mean age: 52.9 ± 9.0 years) highlighted that high ALAN exposure (i.e., living in bright areas) was significantly associated with obesity (definition based on BMI) after adjustment for confounding variables (odds ratio [OR] 1.20, 95% CI = 1.06–1.36, p = 0.003). 133 Similar findings were reported by Abay and Amare 134 in 33,586 Nigerian women (age 15–49 years), who showed a significant nonlinear relationship between satellite‐based night light intensity (above 50–75th, 75–90th, and above 90th percentiles of ALAN) and overweight and obesity occurrence using BMI classification, regardless of potential confounders. 134 The association between outdoor ALAN data from the US Defense database and obesity prevalence rates from a WHO countrywide database was investigated in an elegant study by Rybnikova et al. conducted in men and women from more than 80 countries. 135 ALAN was found to be a significant risk factor for obesity in women (B = 0.002–0.009, t = 2.739–2.877, p < 0.01) and men (B = 0.003–0.043, t = 1.972–2.658, p < 0.1). Thus, when other explanatory variables (i.e., country‐level development indicators) are included in the analysis, ALAN explained 72%–73% of worldwide overweight and obesity prevalence rates in women and 67%–68% in men. 135
In a Japanese study including 528 elderly men (47% men, mean age: 72.8 ± 6.5 years), ALAN exposure while sleeping was measured using a portable photometer. Body weight, BMI, and WC were significantly higher in the ALAN group (≥3 lx) than in the dim group (<3 lx). 136 Notably, ALAN exposure was associated with a higher OR for obesity (BMI: OR, 1.89; p = 0.02; abdominal obesity: OR, 1.62; p = 0.04) independent of demographic and socioeconomic variables. 136 Similar findings were reported by McFadden et al. in a large cohort (n = 113,343, mean age: 47.2 ± 13.6 years) of women living in the United Kingdom that used self‐reported lightness during bedtime to show an association with increased OR for overweight and obesity using both BMI and waist‐to‐hip ratios. 137 These observations are consistent with results from a cohort of 43,722 women from the Sister Study that involved subjects from the United States and Puerto Rico (mean age: 55.4 ± 8.9 years), which reported a positive association between self‐reported ALAN exposure while sleeping and higher prevalence ratios (PR) for obesity as identified using BMI (PR: 1.03, 95% CI 1.02–1.03) and visceral adiposity (WC, waist‐to‐hip ratio, and waist‐to‐height ratio). 138 After a 5.7‐year follow‐up, the prospective analysis revealed that sleeping with an ALAN source was associated with obesity (relative risk [RR]: 1.19, 95% CI: 1.06–1.34). 138 Another longitudinal study of 766 Japanese participants who were followed up over 21 months showed that both evening (≥100 lx) and nighttime (≥3 lx) exposures to objectively measured high light intensity were significantly associated with % waist‐to‐height gain and BMI augmentation in fully adjusted models. 136
Beyond the link between shift work and obesity, reports have also highlighted that the immune system may also be affected by shift work and can lead to an increased susceptibility to infections due to the disruption of adaptive and innate immune responses. 139 It has been reported that shift work affects the production rhythms of leukocytes, phagocytosis, and cytokine and their proliferative responses to antigens. 140 The increased number of lymphocytes and/or leukocytes in shift workers has been suggested to reflect increased inflammation, thus representing a favorable milieu for infections. Indeed, studies carried out in shift workers reported a higher incidence and severity of respiratory infections compared with non‐shift workers. 141 , 142 Night‐shift workers have also been reported to have a higher number of monocytes than non‐shift workers. However, there were no large differences in monocyte functionality and T‐cell proliferative responses to various stimuli between night‐shift and non‐shift workers. 139 In conclusion, shift workers have a higher risk of developing obesity and immune system disturbances than non‐shift workers. 139 Furthermore, the numbers of lymphocytes and/or leukocytes were higher in shift workers, although no significant differences were found in the numbers of other immune cells or the functional aspects of monocytes and T cells. 139 Changes in the immune system in addition to obesity could contribute to increased susceptibility to infections in shift workers. Thus, in humans, melatonin exerts protective effects against infections by indirectly improving the immune system by modifying the metabolism, inflammatory signals, and oxidative stress 143 , 144 , 145 (Figure 4).
FIGURE 4.
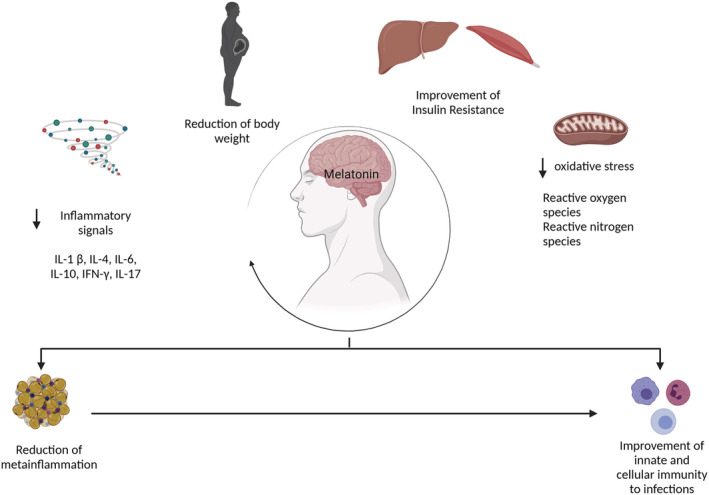
Possible mechanistic routes in which melatonin affects the infection process. Melatonin can reduce body weight and thus contributes to the reduction of visceral adipose tissue, which is the main source of inflammatory signals. In addition, melatonin has been reported to have antioxidant properties that reduce both reactive oxygen species (ROS) and nitrogen species. The reduction of low‐grade inflammation, along with antioxidant effects, contributes to an improved insulin resistance at both hepatic and muscle sites. All these mechanisms result in an improvement in both innate and cellular immunity to infections. Illustrations created with BioRender.com
6. CONCLUSIONS
In conclusion, a review of the scientific literature of the molecular mechanisms of melatonin in the regulation of body weight and fat composition as well as the immune responses in obesity revealed that melatonin anti‐inflammatory and antioxidant actions can influence not only body weight and energy metabolism but also immune responses to infections. The close correlation between circadian rhythms and immunity has been demonstrated in several in vivo and in vitro models and highlights the pivotal role of melatonin as an entrainer of both metabolic and immune functions, which can be further improved by the direct effect of melatonin on the metaflammation state of obese conditions.
This review touches on a very innovative topic that is poorly investigated. Nevertheless, substantial analysis of the literature has pointed to different experimental models of how melatonin is related to obesity and immunity. The strength of this review is the collective analysis of the molecular mechanisms underlying the predisposition to infection in patients with obesity and points to the importance to consider these mechanisms as targets of future therapeutic strategies. Unfortunately, the lack of specific studies evaluating the role of melatonin concomitantly with the regulation of chronodisruption and infections in obesity conditions implies a limitation that still requires addressing in the field.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The study was funded by PRIN from Ministry of University and Research Grants 2020NCKXBR (to A.C.), entitled “Suscettibilità alle malattie infettive nell'obesità: una valutazione endocrina, traslazionale e sociologica (SIDERALE).” Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
Pivonello C, Negri M, Patalano R, et al. The role of melatonin in the molecular mechanisms underlying metaflammation and infections in obesity: A narrative review. Obesity Reviews. 2022;23(3):e13390. doi: 10.1111/obr.13390
Funding information PRIN from Ministry of University and Research Grants, Grant/Award Number: 2020NCKXBR
REFERENCES
- 1. Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288‐298. [DOI] [PubMed] [Google Scholar]
- 2. Kwaifa IK, Bahari H, Yong YK, Noor SM. Endothelial dysfunction in obesity‐induced inflammation: molecular mechanisms and clinical implications. Biomolecules. 2020;10(2):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montesi L, el Ghoch M, Brodosi L, et al. Long‐term weight loss maintenance for obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016;9:37‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patalano R, de Luca V, Vogt J, et al. An innovative approach to designing digital health solutions addressing the unmet needs of obese patients in Europe. Int J Environ Res Public Health. 2021;18(2):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr. 2019;11(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Goblan AS, Al‐Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Muscogiuri G, Pugliese G, Laudisio D, et al. The impact of obesity on immune response to infection: plausible mechanisms and outcomes. Obes Rev. 2021;22(6):e13216. [DOI] [PubMed] [Google Scholar]
- 9. Pickel L, Sung HK. Feeding rhythms and the circadian regulation of metabolism. Front Nutr. 2020;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. 2019;20(1):49‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szewczyk‐Golec K, Rajewski P, Gackowski M, et al. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie‐restricted diet. Oxid Med Cell Longev. 2017;2017:8494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Farias T, Cruz MM, de Sa R, et al. Melatonin supplementation decreases hypertrophic obesity and inflammation induced by high‐fat diet in mice. Front Endocrinol (Lausanne). 2019;10:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srinivasan V, Maestroni GJ, Cardinali DP, et al. Melatonin, immune function and aging. Immun Ageing. 2005;2(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pivonello C, Negri M, Pivonello R, Colao A. How may obesity‐induced oxidative stress affect the outcome of COVID‐19 vaccines? Lesson learned from the infection. Stress. 2021;1(2):119‐122. [Google Scholar]
- 15. Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. A review. J Biomed Sci. 2000;7(6):444‐458. [DOI] [PubMed] [Google Scholar]
- 16. Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat Endocr Metab Immune Drug Discov. 2012;6(1):30‐39. [DOI] [PubMed] [Google Scholar]
- 17. Bahrampour Juybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S. Melatonin potentials against viral infections including COVID‐19: current evidence and new findings. Virus Res. 2020;287:198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wensveen FM, Valentic S, Sestan M, et al. Interactions between adipose tissue and the immune system in health and malnutrition. Semin Immunol. 2015;27(5):322‐333. [DOI] [PubMed] [Google Scholar]
- 19. Gustafson B, Gogg S, Hedjazifar S, Jenndahl L, Hammarstedt A, Smith U. Inflammation and impaired adipogenesis in hypertrophic obesity in man. Am J Physiol Endocrinol Metab. 2009;297(5):E999‐E1003. [DOI] [PubMed] [Google Scholar]
- 20. Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta‐inflammation. Transl Res. 2018;191:29‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185(3):1836‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring). 2015;23(3):512‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond). 2013;37(3):333‐340. [DOI] [PubMed] [Google Scholar]
- 24. Cipolla‐Neto J, Amaral FGD. Melatonin as a hormone: new physiological and clinical insights. Endocr Rev. 2018;39(6):990‐1028. [DOI] [PubMed] [Google Scholar]
- 25. Klein DC. Serotonin N‐acetyltransferase. A personal historical perspective. Adv Exp Med Biol. 1999;460:5‐16. [PubMed] [Google Scholar]
- 26. Emet M, Ozcan H, Ozel L, Yayla M, Halici Z, Hacimuftuoglu A. A review of melatonin, its receptors and drugs. Eur J Med. 2016;48(2):135‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubocovich ML, Rivera‐Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8(4):d1093‐d1108. [DOI] [PubMed] [Google Scholar]
- 28. Buscemi N, Vandermeer B, Hooton N, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis. BMJ. 2006;332(7538):385‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez‐Barcelo EJ, Mediavilla MD, Tan DX, Reiter RJ. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17(19):2070‐2095. [DOI] [PubMed] [Google Scholar]
- 30. Carrascal L, Nunez‐Abades P, Ayala A, Cano M. Role of melatonin in the inflammatory process and its therapeutic potential. Curr Pharm des. 2018;24(14):1563‐1588. [DOI] [PubMed] [Google Scholar]
- 31. Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein‐coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Han JH, Chang IH, Myung SC, et al. A novel pathway underlying the inhibitory effects of melatonin on isolated rat urinary bladder contraction. Korean J Physiol Pharmacol. 2012;16(1):37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lardone PJ, Guerrero JM, Fernandez‐Santos JM, et al. Melatonin synthesized by T lymphocytes as a ligand of the retinoic acid‐related orphan receptor. J Pineal Res. 2011;51(4):454‐462. [DOI] [PubMed] [Google Scholar]
- 34. Garcia‐Maurino S, Gonzalez‐Haba MG, Calvo JR, et al. Melatonin enhances IL‐2, IL‐6, and IFN‐gamma production by human circulating CD4+ cells: a possible nuclear receptor‐mediated mechanism involving T helper type 1 lymphocytes and monocytes. J Immunol. 1997;159(2):574‐581. [PubMed] [Google Scholar]
- 35. Zhang JJ, Meng X, Li Y, et al. Effects of melatonin on liver injuries and diseases. Int J Mol Sci. 2017;18(4):673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Matos Cavalcante AG, de Bruin PF, de Bruin VM, et al. Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized, double‐blind, placebo‐controlled study. J Pineal Res. 2012;53(3):238‐244. [DOI] [PubMed] [Google Scholar]
- 37. Sun H, Gusdon AM, Qu S. Effects of melatonin on cardiovascular diseases: progress in the past year. Curr Opin Lipidol. 2016;27(4):408‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chuffa LGA, Reiter RJ, Lupi LA. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38(10):945‐952. [DOI] [PubMed] [Google Scholar]
- 39. Bollinger T, Schibler U. Circadian rhythms ‐ from genes to physiology and disease. Swiss Med Wkly. 2014;144:w13984. [DOI] [PubMed] [Google Scholar]
- 40. Refinetti R. Comparison of light, food, and temperature as environmental synchronizers of the circadian rhythm of activity in mice. J Physiol Sci. 2015;65(4):359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buhr ED, Takahashi JS. Molecular components of the mammalian circadian clock. Handb Exp Pharmacol. 2013;217:3‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cox KH, Takahashi JS. Circadian clock genes and the transcriptional architecture of the clock mechanism. J Mol Endocrinol. 2019;63(4):R93‐R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome‐wide and phase‐specific DNA‐binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9(2):e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Inoue I, Shinoda Y, Ikeda M, et al. CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator‐activated receptor (PPAR) response element. J Atheroscler Thromb. 2005;12(3):169‐174. [DOI] [PubMed] [Google Scholar]
- 47. Grimaldi B, Bellet MM, Katada S, et al. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010;12(5):509‐520. 10.1016/j.cmet.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52(2):139‐166. [DOI] [PubMed] [Google Scholar]
- 49. Hiragaki S, Baba K, Coulson E, Kunst S, Spessert R, Tosini G. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS ONE. 2014;9(9):e106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Med Rev. 2005;9(1):5‐9. [DOI] [PubMed] [Google Scholar]
- 51. Hodge BA, Wen Y, Riley LA, et al. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet Muscle. 2015;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Negri M, Pivonello C, Simeoli C, et al. Cortisol circadian rhythm and insulin resistance in muscle: effect of dosing and timing of hydrocortisone exposure on insulin sensitivity in synchronized muscle cells. Neuroendocrinology. 2021;111(10):1005‐1028. 10.1159/000512685 [DOI] [PubMed] [Google Scholar]
- 53. Aoyama S, Shibata S. The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci. 2017;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Owino S, Contreras‐Alcantara S, Baba K, Tosini G. Melatonin signaling controls the daily rhythm in blood glucose levels independent of peripheral clocks. PLoS ONE. 2016;11(1):e0148214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Loizides‐Mangold U, Perrin L, Vandereycken B, et al. Lipidomics reveals diurnal lipid oscillations in human skeletal muscle persisting in cellular myotubes cultured in vitro. Proc Natl Acad Sci U S a. 2017;114(41):E8565‐E8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kennaway DJ, Owens JA, Voultsios A, Wight N. Adipokines and adipocyte function in clock mutant mice that retain melatonin rhythmicity. Obesity (Silver Spring). 2012;20(2):295‐305. [DOI] [PubMed] [Google Scholar]
- 57. Bonmati‐Carrion MA, Arguelles‐Prieto R, Martinez‐Madrid MJ, et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15(12):23448‐23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Halpern B, Mancini MC, Bueno C, et al. Melatonin increases brown adipose tissue volume and activity in patients with melatonin deficiency: a proof‐of‐concept study. Diabetes. 2019;68(5):947‐952. [DOI] [PubMed] [Google Scholar]
- 59. Hong F, Pan S, Xu P, et al. Melatonin orchestrates lipid homeostasis through the hepatointestinal circadian clock and microbiota during constant light exposure. Cell. 2020;9(2):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tung YT, Chiang PC, Chen YL, Chien YW. Effects of melatonin on lipid metabolism and circulating irisin in Sprague‐Dawley rats with diet‐induced obesity. Molecules. 2020;25(15):3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Souza CAP, Gallo CC, de Camargo LS, et al. Melatonin multiple effects on brown adipose tissue molecular machinery. J Pineal Res. 2019;66(2):e12549. [DOI] [PubMed] [Google Scholar]
- 62. Zalatan F, Krause JA, Blask DE. Inhibition of isoproterenol‐induced lipolysis in rat inguinal adipocytes in vitro by physiological melatonin via a receptor‐mediated mechanism. Endocrinology. 2001;142(9):3783‐3790. [DOI] [PubMed] [Google Scholar]
- 63. Brydon L, Petit L, Delagrange P, Strosberg AD, Jockers R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology. 2001;142(10):4264‐4271. [DOI] [PubMed] [Google Scholar]
- 64. Alonso‐Vale MI, Andreotti S, Peres SB, et al. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Endocrinol Metab. 2005;288(4):E805‐E812. [DOI] [PubMed] [Google Scholar]
- 65. Zhang L, Su P, Xu C, et al. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J Pineal Res. 2010;49(4):364‐372. 10.1111/j.1600-079x.2010.00803.x [DOI] [PubMed] [Google Scholar]
- 66. Knani L, Bartolini D, Kechiche S, et al. Melatonin prevents cadmium‐induced bone damage: first evidence on an improved osteogenic/adipogenic differentiation balance of mesenchymal stem cells as underlying mechanism. J Pineal Res. 2019;67(3):e12597. [DOI] [PubMed] [Google Scholar]
- 67. Jiang T, Xia C, Chen X, et al. Melatonin promotes the BMP9‐induced osteogenic differentiation of mesenchymal stem cells by activating the AMPK/beta‐catenin signalling pathway. Stem Cell Res Ther. 2019;10(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pan M, Song YL, Xu JM, Gan HZ. Melatonin ameliorates nonalcoholic fatty liver induced by high‐fat diet in rats. J Pineal Res. 2006;41(1):79‐84. [DOI] [PubMed] [Google Scholar]
- 69. Gonciarz M, Gonciarz Z, Bielanski W, et al. The effects of long‐term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: a pilot study. J Physiol Pharmacol. 2012;63(1):35‐40. [PubMed] [Google Scholar]
- 70. Celinski K, Konturek PC, Slomka M, et al. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non‐alcoholic fatty liver disease‐‐14 months follow up. J Physiol Pharmacol. 2014;65(1):75‐82. [PubMed] [Google Scholar]
- 71. Pakravan H, Ahmadian M, Fani A, et al. The effects of melatonin in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Adv Biomed Res. 2017;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sato K, Meng F, Francis H, et al. Melatonin and circadian rhythms in liver diseases: functional roles and potential therapies. J Pineal Res. 2020;68(3):e12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yin J, Li Y, Han H, et al. Administration of exogenous melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high‐fat diet. mSystems. 2020;5(3):e00002‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martinez‐Garcia EA, Zavala‐Cerna MG, Lujano‐Benitez AV, et al. Potential chronotherapeutic optimization of antimalarials in systemic lupus erythematosus: is toll‐like receptor 9 expression dependent on the circadian cycle in humans? Front Immunol. 2018;9:1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Silver AC, Buckley SM, Hughes ME, Hastings AK, Nitabach MN, Fikrig E. Daily oscillations in expression and responsiveness of toll‐like receptors in splenic immune cells. Heliyon. 2018;4(3):e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vijay K. Toll‐like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chuffa LG, Fioruci‐Fontanelli BA, Mendes LO, et al. Melatonin attenuates the TLR4‐mediated inflammatory response through MyD88‐ and TRIF‐dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer. 2015;15(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu HM, Zhao CC, Xie QM, Xu J, Fei GH. TLR2‐melatonin feedback loop regulates the activation of NLRP3 inflammasome in murine allergic airway inflammation. Front Immunol. 2020;11:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Arranz A, Androulidaki A, Zacharioudaki V, et al. Vasoactive intestinal peptide suppresses toll‐like receptor 4 expression in macrophages via Akt1 reducing their responsiveness to lipopolysaccharide. Mol Immunol. 2008;45(10):2970‐2980. [DOI] [PubMed] [Google Scholar]
- 80. Huang SH, Cao XJ, Wei W. Melatonin decreases TLR3‐mediated inflammatory factor expression via inhibition of NF‐kappa B activation in respiratory syncytial virus‐infected RAW264.7 macrophages. J Pineal Res. 2008;45(1):93‐100. [DOI] [PubMed] [Google Scholar]
- 81. Xu X, Wang G, Ai L, Shi J, Zhang J, Chen YX. Melatonin suppresses TLR9‐triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci Rep. 2018;8(1):15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cardinali DP, Brusco LI, Garcia Bonacho M, Esquifino AI. Effect of melatonin on 24‐hour rhythms of ornithine decarboxylase activity and norepinephrine and acetylcholine synthesis in submaxillary lymph nodes and spleen of young and aged rats. Neuroendocrinology. 1998;67(5):349‐362. 10.1159/000054333 [DOI] [PubMed] [Google Scholar]
- 83. Molinero P, Soutto M, Benot S, Hmadcha A, Guerrero JM. Melatonin is responsible for the nocturnal increase observed in serum and thymus of thymosin alpha1 and thymulin concentrations: observations in rats and humans. J Neuroimmunol. 2000;103(2):180‐188. [DOI] [PubMed] [Google Scholar]
- 84. Mishima K, Okawa M, Shimizu T, Hishikawa Y. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. J Clin Endocrinol Metab. 2001;86(1):129‐134. [DOI] [PubMed] [Google Scholar]
- 85. Kolker DE, Fukuyama H, Huang DS, Takahashi JS, Horton TH, Turek FW. Aging alters circadian and light‐induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18(2):159‐169. [DOI] [PubMed] [Google Scholar]
- 86. Weinert H, Weinert D, Schurov I, Maywood ES, Hastings MH. Impaired expression of the mPer2 circadian clock gene in the suprachiasmatic nuclei of aging mice. Chronobiol Int. 2001;18(3):559‐565. [DOI] [PubMed] [Google Scholar]
- 87. Paulose JK, Wang C, O'Hara BF, Cassone VM. The effects of aging on sleep parameters in a healthy, melatonin‐competent mouse model. Nat Sci Sleep. 2019;11:113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mattam U, Jagota A. Differential role of melatonin in restoration of age‐induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats. Biogerontology. 2014;15(3):257‐268. [DOI] [PubMed] [Google Scholar]
- 89. Karasek M. Melatonin, human aging, and age‐related diseases. Exp Gerontol. 2004;39(11–12):1723‐1729. [DOI] [PubMed] [Google Scholar]
- 90. Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56(3):359‐384. [DOI] [PubMed] [Google Scholar]
- 91. Srinivasan V, Spence DW, Pandi‐Perumal SR, et al. Melatonin in mitochondrial dysfunction and related disorders. Int J Alzheimers Dis. 2011;2011:326320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tian YM, Li PP, Jiang XF, Zhang GY, Dai YR. Rejuvenation of degenerative thymus by oral melatonin administration and the antagonistic action of melatonin against hydroxyl radical‐induced apoptosis of cultured thymocytes in mice. J Pineal Res. 2001;31(3):214‐221. [DOI] [PubMed] [Google Scholar]
- 93. Tian YM, Zhang GY, Dai YR. Melatonin rejuvenates degenerated thymus and redresses peripheral immune functions in aged mice. Immunol Lett. 2003;88(2):101‐104. [DOI] [PubMed] [Google Scholar]
- 94. Gui J, Mustachio LM, Su DM, Craig RW. Thymus size and age‐related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis. 2012;3(3):280‐290. [PMC free article] [PubMed] [Google Scholar]
- 95. Karaaslan C, Suzen S. Antioxidant properties of melatonin and its potential action in diseases. Curr Top Med Chem. 2015;15(9):894‐903. [DOI] [PubMed] [Google Scholar]
- 96. Rios P, Cardoso R, Morra D, et al. Comparative effectiveness and safety of pharmacological and non‐pharmacological interventions for insomnia: an overview of reviews. Syst Rev. 2019;8(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mantele S, Otway DT, Middleton B, et al. Daily rhythms of plasma melatonin, but not plasma leptin or leptin mRNA, vary between lean, obese and type 2 diabetic men. PLoS ONE. 2012;7(5):e37123. 10.1371/journal.pone.0037123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mesri Alamdari N, Mahdavi R, Roshanravan N, Lotfi Yaghin N, Ostadrahimi AR, Faramarzi E. A double‐blind, placebo‐controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res. 2015;47(7):504‐508. [DOI] [PubMed] [Google Scholar]
- 99. Amstrup AK, Sikjaer T, Pedersen SB, Heickendorff L, Mosekilde L, Rejnmark L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: a randomized placebo‐controlled trial. Clin Endocrinol (Oxf). 2016;84(3):342‐347. [DOI] [PubMed] [Google Scholar]
- 100. Prunet‐Marcassus B, Desbazeille M, Bros A, et al. Melatonin reduces body weight gain in Sprague Dawley rats with diet‐induced obesity. Endocrinology. 2003;144(12):5347‐5352. [DOI] [PubMed] [Google Scholar]
- 101. Favero G, Stacchiotti A, Castrezzati S, et al. Melatonin reduces obesity and restores adipokine patterns and metabolism in obese (ob/ob) mice. Nutr Res. 2015;35(10):891‐900. [DOI] [PubMed] [Google Scholar]
- 102. Stacchiotti A, Favero G, Giugno L, Golic I, Korac A, Rezzani R. Melatonin efficacy in obese leptin‐deficient mice heart. Nutrients. 2017;9(12):1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Favero G, Franco C, Stacchiotti A, Rodella LF, Rezzani R. Sirtuin1 role in the melatonin protective effects against obesity‐related heart injury. Front Physiol. 2020;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Vural EM, van Munster BC, de Rooij SE. Optimal dosages for melatonin supplementation therapy in older adults: a systematic review of current literature. Drugs Aging. 2014;31(6):441‐451. [DOI] [PubMed] [Google Scholar]
- 105. González A, Alvarez‐García V, Martinez‐Campa C, Alonso‐González C, Cos S. Melatonin promotes differentiation of 3T3‐L1 fibroblasts. J Pineal Res. 2012;52(1):12‐20. 10.1111/j.1600-079x.2011.00911.x [DOI] [PubMed] [Google Scholar]
- 106. Yang W, Tang K, Wang Y, Zhang Y, Zan L. Melatonin promotes triacylglycerol accumulation via MT2 receptor during differentiation in bovine intramuscular preadipocytes. Sci Rep. 2017;7(1):15080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kato H, Tanaka G, Masuda S, et al. Melatonin promotes adipogenesis and mitochondrial biogenesis in 3T3‐L1 preadipocytes. J Pineal Res. 2015;59(2):267‐275. [DOI] [PubMed] [Google Scholar]
- 108. Heo JS, Pyo S, Lim JY, et al. Biological effects of melatonin on human adiposederived mesenchymal stem cells. Int J Mol Med. 2019;44(6):2234‐2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kim BC, Shon BS, Ryoo YW, Kim SP, Lee KS. Melatonin reduces X‐ray irradiation‐induced oxidative damages in cultured human skin fibroblasts. J Dermatol Sci. 2001;26(3):194‐200. [DOI] [PubMed] [Google Scholar]
- 110. Casado‐Zapico S, Rodriguez‐Blanco J, Garcia‐Santos G, et al. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res. 2010;48(1):72‐80. [DOI] [PubMed] [Google Scholar]
- 111. Nishimura J, Saegusa Y, Dewa Y, et al. Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress‐mediated hepatocellular tumor promotion of oxfendazole in rats. Arch Toxicol. 2010;84(2):143‐153. [DOI] [PubMed] [Google Scholar]
- 112. Ma Z, Xu L, Liu D, et al. Utilizing melatonin to alleviate side effects of chemotherapy: a potentially good partner for treating cancer with ageing. Oxid Med Cell Longev. 2020;2020:6841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Mehrzadi S, Pourhanifeh MH, Mirzaei A, Moradian F, Hosseinzadeh A. An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 2021;21(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wang YM, Jin BZ, Ai F, et al. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta‐analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69(5):1213‐1220. [DOI] [PubMed] [Google Scholar]
- 115. Sookprasert A, Johns NP, Phunmanee A, et al. Melatonin in patients with cancer receiving chemotherapy: a randomized, double‐blind, placebo‐controlled trial. Anticancer Res. 2014;34(12):7327‐7337. [PubMed] [Google Scholar]
- 116. Wang TH, Hsueh C, Chen CC, et al. Melatonin inhibits the progression of hepatocellular carcinoma through microRNA Let7i‐3p mediated RAF1 reduction. Int J Mol Sci. 2018;19(9):2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Huang J, Shan W, Li N, et al. Melatonin provides protection against cisplatin‐induced ovarian damage and loss of fertility in mice. Reprod Biomed Online. 2021;42(3):505‐519. [DOI] [PubMed] [Google Scholar]
- 118. Cos S, Blask DE, Lemus‐Wilson A, Hill AB. Effects of melatonin on the cell cycle kinetics and “estrogen‐rescue” of MCF‐7 human breast cancer cells in culture. J Pineal Res. 1991;10(1):36‐42. [DOI] [PubMed] [Google Scholar]
- 119. Uguz AC, Cig B, Espino J, et al. Melatonin potentiates chemotherapy‐induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53(1):91‐98. [DOI] [PubMed] [Google Scholar]
- 120. Pariente R, Bejarano I, Rodriguez AB, Pariente JA, Espino J. Melatonin increases the effect of 5‐fluorouracil‐based chemotherapy in human colorectal adenocarcinoma cells in vitro. Mol Cell Biochem. 2018;440(1–2):43‐51. 10.1007/s11010-017-3154-2 [DOI] [PubMed] [Google Scholar]
- 121. Panchenko AV, Tyndyk ML, Maydin MA, et al. Melatonin administered before or after a cytotoxic drug increases mammary cancer stabilization rates in HER2/Neu mice. Chemotherapy. 2020;65(1–2):42‐50. [DOI] [PubMed] [Google Scholar]
- 122. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65(12 Pt 2):S140‐S146. [DOI] [PubMed] [Google Scholar]
- 123. Favero G, Franceschetti L, Bonomini F, et al. Melatonin as an anti‐inflammatory agent modulating inflammasome activation. Int J Endocrinol. 2017;2017:1835195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. de Zoete MR, Palm NW, Zhu S, Flavell RA. Inflammasomes. Cold Spring Harb Perspect Biol. 2014;6(12):a016287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60(4):405‐414. [DOI] [PubMed] [Google Scholar]
- 126. Peng Z, Zhang W, Qiao J, He B. Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL‐1beta in rats with COPD. Int Immunopharmacol. 2018;62:23‐28. [DOI] [PubMed] [Google Scholar]
- 127. Chen F, Jiang G, Liu H, et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL‐1beta/NF‐kappaB‐NLRP3 inflammasome positive feedback loop. Bone Res. 2020;8(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hall CJ, Wicker SM, Chien AT, et al. Repositioning drugs for inflammatory disease ‐ fishing for new anti‐inflammatory agents. Dis Model Mech. 2014;7(9):1069‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang S, Wang H, Wang Y, Yu M, Yuan J. Association of rotating night shift work with body fat percentage and fat mass index among female steelworkers in north China. Int J Environ Res Public Health. 2021;18(12):6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Garfield V. The association between body mass index (BMI) and sleep duration: where are we after nearly two decades of epidemiological research? Int J Environ Res Public Health. 2019;16(22):4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J. Effects of artificial light at night on human health: a literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int. 2015;32(9):1294‐1310. [DOI] [PubMed] [Google Scholar]
- 132. Touitou Y, Reinberg A, Touitou D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017;173:94‐106. [DOI] [PubMed] [Google Scholar]
- 133. Koo YS, Song JY, Joo EY, et al. Outdoor artificial light at night, obesity, and sleep health: cross‐sectional analysis in the KoGES study. Chronobiol Int. 2016;33(3):301‐314. [DOI] [PubMed] [Google Scholar]
- 134. Abay KA, Amare M. Night light intensity and women's body weight: evidence from Nigeria. Econ Hum Biol. 2018;31:238‐248. [DOI] [PubMed] [Google Scholar]
- 135. Rybnikova NA, Haim A, Portnov BA. Does artificial light‐at‐night exposure contribute to the worldwide obesity pandemic? Int J Obes (Lond). 2016;40(5):815‐823. [DOI] [PubMed] [Google Scholar]
- 136. Obayashi K, Saeki K, Iwamoto J, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross‐sectional analysis of the HEIJO‐KYO study. J Clin Endocrinol Metab. 2013;98(1):337‐344. [DOI] [PubMed] [Google Scholar]
- 137. McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: cross‐sectional analyses of over 100,000 women in the breakthrough generations study. Am J Epidemiol. 2014;180(3):245‐250. [DOI] [PubMed] [Google Scholar]
- 138. Park YM, White AJ, Jackson CL, et al. Association of exposure to artificial light at night while sleeping with risk of obesity in women. JAMA Intern Med. 2019;179(8):1061‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Loef B, Nanlohy NM, Jacobi RHJ, et al. Immunological effects of shift work in healthcare workers. Sci Rep. 2019;9(1):18220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Labrecque N, Cermakian N. Circadian clocks in the immune system. J Biol Rhythms. 2015;30(4):277‐290. [DOI] [PubMed] [Google Scholar]
- 141. Boden K, Brasche S, Straube E, Bischof W. Specific risk factors for contracting Q fever: lessons from the outbreak Jena. Int J Hyg Environ Health. 2014;217(1):110‐115. [DOI] [PubMed] [Google Scholar]
- 142. Mohren DC, Jansen NW, Kant IJ, et al. Prevalence of common infections among employees in different work schedules. J Occup Environ Med. 2002;44(11):1003‐1011. [DOI] [PubMed] [Google Scholar]
- 143. Radogna F, Diederich M, Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80(12):1844‐1852. [DOI] [PubMed] [Google Scholar]
- 144. Poeggeler B, Reiter RJ, Hardeland R, Tan DX, Barlow‐Walden LR. Melatonin and structurally‐related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 1996;2(3):179‐184. [DOI] [PubMed] [Google Scholar]
- 145. Cipolla‐Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371‐381. [DOI] [PubMed] [Google Scholar]


