Abstract
Background
Central line‐associated blood stream infections are accompanied by increased mortality and health care costs. The application of different types of dressings in infection control has not been fully investigated to date.
Aim
To assess the effects of two different dressing types on central line‐associated bloodstream infections.
Methods
A randomized, nonblinded, controlled trial was conducted. Central lines were randomly allocated to intervention (chlorhexidine gluconate transparent dressing, n = 259) and control groups (standard dressing, n = 215). The central line‐associated bloodstream infection rate was assessed.
Results
A statistically nonsignificant difference was noted in the overall central line‐associated bloodstream infection rates between the two groups. The frequency of dressing changes in the patients with the chlorhexidine gluconate transparent dressing was significantly lower than that in the patients with a standard dressing. The predominant type of infectious microorganisms isolated from the central line‐associated bloodstream infection episodes was Gram‐negative bacteria (57.2%). Gram‐positive bacteria and fungi were noted at lower percentages (28.5% and 14.3%, respectively).
Conclusion
The use of a chlorhexidine gluconate transparent dressing does not decrease the central line‐associated bloodstream infection rate, although it decreases the frequency of dressing changes so may save nursing time.
Keywords: central line‐associated bloodstream infections, chlorhexidine, critical care, dressing, nursing
SUMMARY STATEMENT
What is already known about this topic?
The use of a chlorhexidine gluconate transparent dressing has been reported to significantly reduce central line‐associated bloodstream infections or maintain low central line‐associated bloodstream infections in Western countries, but few studies have been carried out in Asian countries.
The chlorhexidine dressing does not decrease central line‐associated bloodstream infections due to the presence of Gram‐negative bacteria.
It has been reported that nurses prefer the chlorhexidine gluconate transparent dressing over the standard dressing.
What this paper adds?
Compared with the standard dressing, the chlorhexidine gluconate transparent dressing does not significantly decrease the central line‐associated bloodstream infection rate.
The predominant infectious microorganisms isolated from central line-associated bloodstream infections episodes were Gram-negative bacteria.
The cost of the chlorhexidine gluconate transparent dressing was significantly higher than that of the standard dressing, although the former could save nursing time due to the decreased frequency of dressing changes.
The implications of this paper are as follows:
The use of chlorhexidine gluconate transparent dressing does not offer any additional benefit in controlling infection.
Further studies are required to identify ways to control the colonization or infection of Gram‐negative bacteria in patients because Gram‐negative bacteria are the predominant infectious microorganisms that cause central line‐associated bloodstream infection.
The use of a chlorhexidine gluconate transparent dressing is proposed as an alternative choice that can reduce ICU nursing time in hospital settings and therefore should be considered by nursing managers for human resource cost control.
1. INTRODUCTION
Central lines are widely used in the management of critically ill patients and are required for special treatments, such as continuous renal replacement therapy and hemodynamic monitoring. Although central lines play a very important role in the management of critically ill patients, central line‐associated bloodstream infections (CLABSIs) are accompanied by increased morbidity, mortality, and health care costs (Maki, Kluger, & Crnich, 2006; Valles & Ferrer, 2009; Worth & McLaws, 2012).
According to the National Healthcare Safety Network (NHSN), central line placement occurred in 61.5% of patients in intensive care units (ICUs) at 65 hospitals in 2012, and the rate of associated bloodstream infections was 1.62 per 1000 central line days (Dudeck et al., 2013). Bion and colleagues reported a CLABSI rate of 1.48 per 1000 central line days among 215 ICUs in England (Bion et al., 2013). In addition, Leon conducted a study in Australia from 2009 to 2013 and reported a CLABSI rate of 1.26 per 1000 central line days (Worth, Spelman, Bull, Brett, & Richards, 2015). Unfortunately, developing countries exhibit a much higher CLABSI incidence, according to a study conducted by the International Nosocomial Infection Control Consortium (INICC) from January 2004 to December 2009 that included 422 ICUs from 36 countries. The pooled CLABSI rate in ICUs of developing countries has been reported at 6.8 per 1000 central line days (Rosenthal et al., 2012), and in four Beijing ICUs Shao and coworkers reported a CLABSI rate of 6.4 per 1000 central line days (Shao et al., 2014). Therefore, the prevention of CLABSIs in patients with central lines is vital and particularly relevant in the critical care field, as critically ill patients comprise a large proportion of the subjects requiring central lines.
Implementation of the Central Line Bundle policy (hand hygiene, maximal barrier precaution, skin antisepsis with 2% chlorhexidine alcohol, optional catheter site selection, and daily evaluation and removal of unnecessary catheters) has been reported to significantly reduce the incidence of CLABSIs (Apisarnthanarak, Thongphubeth, Yuekyen, Warren, & Fraser, 2010; Sacks et al., 2014). In addition, CLABSI rates have been shown to decrease in the presence of an ICU care bundle policy and a monitoring compliance higher than and/or equal to 95% (≥95%) (Furuya et al., 2011).
However, Du reported the presence of several factors associated with CLABSIs, such as catheter dwelling time, catheter site, type of catheter, dressing type, and nursing care (Du, 2001). Thus, in addition to implementation of the Central Line Bundle policy, ICU nurses need to focus on other risk factors to prevent the incidence of CLABSIs. Although several types of dressings have been proposed recently, the optimal dressing that should be used in cases of infection has not yet been clarified.
Chlorhexidine‐containing devices, such as sponges and dressings, can help control CLABSIs by inhibiting the skin flora; they have previously been shown to exert beneficial effects on reducing CLABSI rates in Western countries (Karpanen et al., 2016; Pfaff, Heithaus, & Emanuelsen, 2012; Scheithauer et al., 2014; Timsit et al., 2012). Despite the standard infection control recommendations, CLABSIs are not satisfactorily controlled. Moreover, as chlorhexidine gluconate transparent dressing is mainly used in China, the aim of the present study was to assess the practicability and efficacy of chlorhexidine gluconate transparent dressing in reducing the rate of CLABSIs.
2. METHODS
2.1. Aims
The aim of the present study was to explore the efficacy and cost of chlorhexidine gluconate transparent dressings in decreasing CLABSI in critically ill patients.
2.2. Design
A single‐center, randomized, nonblinded controlled trial was conducted. It was not possible to blind group allocation due to the nature of the intervention and visibility of the dressing product.
2.3. Participants
A total of 474 central lines inserted for 304 patients from the Medical ICU (MICU) in a 2000‐bed teaching hospital in Beijing were investigated from January 2014 to September 2015. Central lines were randomly allocated to the intervention group (chlorhexidine gluconate transparent dressing, 259 lines with 1408 catheter days) or the control group (standard dressing, 215 lines with 1217 catheter days). Inclusion criteria were as follows: (a) patient age ≥ 18 years, (b) single central line catheterization by ICU doctors during a certain time period, and (c) patients with more than one central line inserted in sequence (new central line inserted after removal of the previous one) during the ICU stay. Patients with two or more concomitant central lines and those who were allergic to chlorhexidine were excluded from the study. Moreover, the patients with central lines that were not inserted by the ICU doctors were also excluded.
2.3.1. Sample size considerations
The CLABSI rate is expressed as the number of infections per 1000 catheter days. Therefore, the sample size was counted as catheter days. The sample size was calculated using Stata 15 software. The CLABSI rate of developing countries was 6.8 per 1000 central line days (Rosenthal et al., 2012), and we assumed that the CLABSI rate of CHG dressing was 4.2 per 1000 central line days. The effect size was set at 0.5 (two sided), α error was set at 0.05, and power was set at 0.8. A sample size of at least 1210 catheter days for each group was therefore required.
2.4. Randomization and blinding
Computer‐generated randomization was placed in a sealed envelope and used for participant allocation. On the day of central line insertion, a research nurse opened the sealed envelope and randomly allocated the patient to the intervention or control group. Since patients with more than one central line (new central line inserted only after removal of the previous line) during the ICU stay were also enrolled in the study, the same patient could be assigned to either the intervention or the control group. We did not use blinding in the present study due to the visibility of the dressing type following central line catheterization.
2.5. Intervention and control groups
The central lines were randomly allocated to the intervention group and control group. Participants in the control group received a standard dressing (IV3000, 10 cm × 12 cm, Smith & Nephew), while participants in the intervention group received a chlorhexidine gluconate transparent dressing (3M Tegaderm CHG, 8.5 cm × 11.5 cm). The nurses caring for both groups followed the Central Line Bundle police, with the same protocol for dressing changes.
2.6. Study outcome and definitions
The primary outcome was the CLABSI rate expressed as the number of infections per 1000 catheter days. The secondary outcomes were cost (dressing cost, antibiotic costs of CLABSIs, and frequency of dressing changes) and the predominant type of infectious microorganisms.
CLABSI was defined according to CDC/NHSN as bacteremia with a recognized pathogen when the organism isolated from blood cultures was not related to infection at another site. Alternatively, the organism could be a common skin contaminant from two or more blood cultures associated with signs and symptoms of infection (fever, chills, or hypotension) that could not be attributed to another site (O'Grady, Alexander, Burns, Dellinger,, & Garland, 2011).
The dressing cost was defined as the total price of the dressing used throughout the central line indwelling time, expressed as the frequency of dressing changes multiplied by the price of a single piece of dressing. The antibiotic cost of CLABSIs was defined as the total price of antibiotic medications used for the patient infected to treat the infection, expressed as the number of antibiotic medications multiplied by the price of one bottle of medicine. The frequency of dressing changes was defined as the total number of dressing changes during each central line duration. The predominant type of infectious microorganisms was defined as microorganisms isolated from CLABSI episodes.
2.7. Rigor
Every central line was monitored from insertion to catheter removal, discharge from the ICU, and/or death. A case‐record form was used to collect information, including demographics (age and sex), type of dressing, Acute Physiology and Chronic Health Evaluation (APACHE II), length of stay in the ICU, and central line data (reasons for catheterization, catheter site, catheter type, reasons for removal, catheter duration, and frequency of dressing changes during indwelling time, infected or not infected). Once a central line was suspected for CLABSI, the catheter was removed, and the blood culture data were monitored until a diagnosis of CLABSI could be determined by a physician of MICU or infectious disease department. Once a central line was diagnosed as CLABSI, the predominant infectious microorganisms and the cost of antibiotics were recorded until the infection was controlled or death or discharge occurred.
To avoid potential confounders, a CLABSI interventional package was implemented according to the recommendations of the Centers for Disease Control as well as the prevention guidelines in our unit that were in use since 2013. The care package included hand hygiene, maximal barrier precaution, skin antisepsis with 2% chlorhexidine alcohol, optional catheter site selection, and daily evaluation and removal of unnecessary catheters (Horan, Andrus, & Dudeck, 2008).
2.8. Ethical considerations
The institutional review board of the research hospital approved this study (ethical approval no. ZS‐925) (see Supplemental Fig. Ethical approval). The participants were well informed of the study aims, procedures, confidentiality, and anonymity of their data, and informed consent was obtained from each participant. Patient rights were fully considered at all times by the researchers. The participants were free to participate and/or withdraw at any time without their care being affected. This study alleviated patient discomfort and harm and provided a benefit to the participants.
2.9. Data analysis
Continuous variables were described as the mean (standard deviation, SD) and compared by Student's t test. Nonnormal distributions were represented as the median and interquartile range, and in cases of normal distribution, the Wilcoxon rank‐sum test was used. Categorical variables were assessed by chi‐square or Fisher's exact tests when the expected values were <10. Both Poisson distribution and Kaplan‐Meier curves were used to compare CLABSI incidence rates between the control and CHG groups. Ninety‐five percent confidence intervals (95% CIs) of odds ratios (ORs) were computed. A two‐tailed P value less than .05 (P < .05) was considered statistically significant. The statistical analyses were performed with IBM SPSS Statistics software version 21.0.
3. RESULTS
From January 2014 to September 2015, a total of 304 patients with 474 central lines met the inclusion criteria and were analyzed (intervention group, n = 259, and control group, n = 219) (Figure 1 ). These cases consisted of 14 CLABSIs in 2625 catheter days and exhibited a CLABSI rate of 5.68 per 1000 catheter days.
Figure 1.
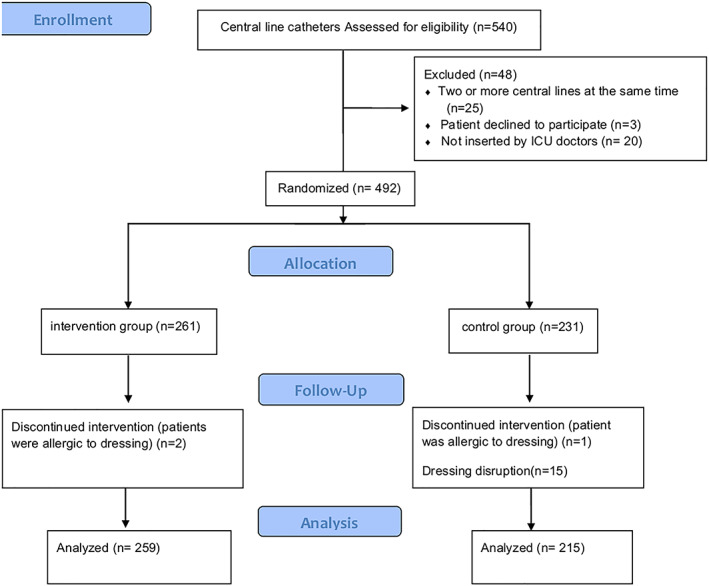
Flow diagram
3.1. General clinical features of the groups
The demographic and clinical characteristics of all patients are summarized in Table 1. The clinical characteristics of the central lines in the two groups are shown in Table 2. The groups did not differ significantly with regard to the clinical characteristics of the central lines.
Table 1.
Patient characteristics
| Variable | Results |
|---|---|
| Male, n (%) | 204 (67.1%) |
| Age, mean (SD) | 57.53 (18.85) |
| APACHE II score, mean (SD) | 22.64 (7.055) |
| ICU day, median (range) | 13 (7, 23) |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, Intensive Care Unit.
Table 2.
Clinical characteristics of the central lines inserted in the two groups
| Variable | Intervention Group | Control Group | χ 2 /z | P |
|---|---|---|---|---|
| Reason for catheterization, n (%) | 5.043 | .283 | ||
| Hemodynamic monitor | 37 (14.3) | 28 (13.0) | ||
| Infection of previous line suspected | 43 (16.6) | 29 (13.5) | ||
| Special drug infusion | 117 (45.2) | 96 (44.7) | ||
| Continuous renal replacement therapy | 49 (18.9) | 56 (26.0) | ||
| Previous line blocked | 13 (5.0) | 6 (2.8) | ||
| Type of central line, n (%) | 1.736 | .629 | ||
| Swan‐Ganz catheter | 29 (11.2) | 20 (9.3) | ||
| Triple lumen line (thin) | 165 (63.7) | 130 (60.5) | ||
| Triple lumen line (thick) | 55 (21.2) | 55 (25.6) | ||
| Others | 10 (3.9) | 10 (4.6) | ||
| Catheterization site, n (%) | 0.343 | .842 | ||
| Internal jugular vein | 135 (52.1) | 107 (49.8) | ||
| Subclavian vein | 8 (3.1) | 6 (2.8) | ||
| Femoral vein | 116 (44.8) | 102 (47.4) | ||
| Reason for removal, n (%) | 8.581 | .072 | ||
| Suspected infection | 64 (24.7) | 30 (14.0) | ||
| Not required | 81 (31.3) | 76 (35.3) | ||
| Death | 36 (13.9) | 34 (15.8) | ||
| Line blockage | 18 (16.9) | 18 (8.4) | ||
| Patient discharge | 60 (23.2) | 57 (26.5) | ||
| Catheter days, median (range) | 4 (3, 7) | 5 (3, 7) | −0.724 | .469 |
Data are presented as n (%) or median (range). Intervention group, CHG dressing; control group, IV3000 dressing.
3.2. Efficacy of CHG dressing in decreasing CLABSI
The mean CLABSI rates in the CHG and control groups were 5.68 per 1000 catheter days (eight CLABSIs in 1408 catheter days) and 4.93 per 1000 catheter days (six CLABSIs in 1217 catheter days), respectively, indicating no statistically significant differences between the two groups according to both Poisson distribution (P = .256) and Kaplan‐Meier curves (log rank test, χ 2 = 0.028, P = .868). With regard to the indwelling time of the central lines that remained for longer than 9 days in both groups, the incidence of CLABSI in the CHG group was not significantly lower than that of the control group (Figure 2).
Figure 2.

Kaplan‐Meier curves of the two groups
3.3. Predominant infectious microorganisms and cost of the two groups
The predominant infectious microorganisms isolated from CLABSI episodes were Gram‐negative bacteria (57.2%), followed by Gram‐positive bacteria (28.5%) and fungi (14.3%) (Figure 3). The differences in predominant infectious microorganisms between the two groups were not statistically significant as assessed by Fisher's exact test (Fisher value = 0.977, P = .776).
Figure 3.

Infectious microorganisms obtained from 14 CLABSIs
The average dressing cost for central lines in the chlorhexidine dressing group ($31.8 [15.8, 47.7]) was significantly higher than that for central lines in the standard dressing group ($5.6 [3.8, 7.5], P < .001). The average cost required for the use of antibiotics due to CLABSI in patients with the chlorhexidine dressing ($387 [304, 456]) was not significantly lower than that in patients with the standard dressing ($540[348, 827]; z = 1.575, P = .141). The average frequency of dressing changes for each central line with the chlorhexidine dressing (2 [1, 3]) was significantly lower (z = −9.099, P < .001) than that with the standard dressing (3 [2, 4]).
4. DISCUSSION
The present study demonstrated that the CLABSI incidence in patients with the chlorhexidine dressing (5.68 per 1000 catheter days) was not significantly higher than that in patients with the IV3000 dressing (4.93 per 1000 catheter days). This finding was inconsistent with the results of other studies demonstrating that a chlorhexidine dressing could decrease the CLABSI rate. Scheithauer et al. (2014) reported a CLABSI incidence of 1.51 per 1000 catheter days following the use of a chlorhexidine dressing, which was significantly lower than the rate obtained with a normal dressing (5.87 per 1000 catheter days) in that study. In addition, Pfaff et al. (2012) demonstrated that a chlorhexidine dressing could maintain the CLABSI rate to a value as low as 0.051 per 1000 catheter days. Furthermore, Timsit et al. (2012) reported that a chlorhexidine dressing not only decreased the CLABSI rate but also reduced catheter bacterial colonization. In agreement with these studies, Karpanen et al. (2016) suggested that compared with a standard dressing, a chlorhexidine dressing significantly decreased bacterial colonization of central lines. However, Roberts and Cheung (1998) found that bacterial colonization of the central line lumen and patient skin following the use of a chlorhexidine dressing was not significantly different from that observed with a normal dressing.
The discrepancies between the current findings and previous reports could be explained by the following. Several risk factors have been associated with CLABSIs that are relevant to the processes involved—from insertion to removal of the central line—such as the type of catheter and the insertion site, hand hygiene, and maximal barrier precaution. Dressing is one contributing factor, but it might not be the most important factor. Scheithauer et al. (2014) indicated that a chlorhexidine dressing did not decrease CLABSIs due to Gram‐negative bacteria, but in the present study, 50% of CLABSIs in the CHG group were caused by Gram‐negative bacteria. In addition, dressings protect the catheterization site of central lines from infection caused by microorganisms and ensure the survival of the central line. However, as demonstrated by a meta‐analysis (Safdar et al., 2014), the type of dressing, such as the chlorhexidine‐containing type, did not help control infection in cases in which CLABSI was a result of central line lumen microorganism infection. The present study did not explore the type of infection with regard to the 14 cases of CLABSIs. Contributing factors such as central line lumen infection or insertion site were not examined, which may account for the differences noted compared with other studies.
In this study, the predominant infectious microorganisms isolated from CLABSI episodes were Gram‐negative bacteria, notably Acinetobacter baumannii, which was in agreement with the findings of a previous study (Bukhari et al., 2014). However, it was also reported (Nemoto et al., 2015) that Gram‐positive bacteria were the main infectious microorganisms in CLABSI episodes. In the present study, 45.9% of the central lines were inserted through the femoral vein, and femoral vein insertion is reportedly one of the risk factors for infection by Gram‐negative bacteria (Mermel et al., 2009). Moreover, in the medical center where these data were collected, chlorhexidine solution was used for disinfection purposes both prior to catheter insertion and during catheter maintenance, which could aid in the inhibition and selective killing of Gram‐positive bacteria. This practice may explain why the predominant infectious microorganism that was noted in the present study was Gram‐negative bacteria.
The frequency of dressing changes in patients with the chlorhexidine dressing was significantly lower than that in patients with the standard dressing. It has been reported that nurses preferred chlorhexidine dressing over the standard dressing type; in addition, it exhibits better flexibility and adherence than a standard dressing and can prevent unplanned catheter extraction to some extent (Maryniak, 2009; Olson & Heilman, 2008; Rupp et al., 2008). Moreover, chlorhexidine dressings can be changed every 7 days without the risk of infection instead of 3 days, which is the average time used for conventional dressing (Timsit et al., 2009). The aforementioned characteristics could explain the decreased frequency of dressing changes and the reason why chlorhexidine dressings are a more popular choice among nurses. Most importantly, a decreased frequency of dressing changes can save nursing time and may be helpful in saving human resource costs.
A limited number of studies have reported the impact of the economic costs of different dressings required for the control of CLABSI. The present study found that the dressing cost required for the CHG group was significantly higher than that for the control group, as the price of a CHG dressing ($15.80) is nearly 10 times higher than that of the IV3000 dressing ($1.80) in China. Although central lines with CHG dressings are changed less frequently, the cost of the dressing is considerably higher. Moreover, the study demonstrated that the antibiotic costs required for the treatment of CLABSIs in patients who received the chlorhexidine dressing were not significantly lower than those in patients who received the standard dressing, perhaps because the types of antibiotic medications used were similar between the two groups.
4.1. Limitations
The present study has several limitations. For example, although it was conducted prospectively over a period of 21 months, the sample size was limited, and all patients were from a single medical center. Therefore, the current results may not represent the actual situation in China. In addition, 45 of the 304 patients enrolled received more than one central line in sequence during their ICU stay, and the central lines of the same patient could have been randomly distributed into either the CHG or control groups. These factors might have affected our results. Future studies are warranted to identify ways to control the colonization or infection of Gram‐negative bacteria in critically ill patients with central lines, which would potentially decrease the incidence of CLABSIs. Moreover, future studies should address the efficacy of including only one catheter per patient and assess the potential inhibition of CLABSIs that are not caused by microbial colonization through the lumen.
5. CONCLUSION
This study confirmed that Gram‐negative bacteria were the predominant infectious microorganisms that caused CLABSI. The use of chlorhexidine gluconate transparent dressing does not offer any additional advantage in controlling infection since it does not significantly decrease the CLABSI rates in ICU patients compared with a standard dressing. However, it is considered an optimal choice that can save ICU nursing time by significantly decreasing the frequency of dressing changes in hospital settings. The chlorhexidine gluconate transparent dressing should be considered by nursing managers for controlling human resources costs, especially in cases where there is a low number of nurses in hospitals or medical centers.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORSHIP STATEMENT
Kunrong Yu conceived the study. Kunrong Yu, Yanling Meng, and Yanwei Zhao were responsible for data management and study design. Meishan Lu and Zheng Li were responsible for data analysis. all authors drafted and revised the manuscript.
Supporting information
Data S1
ACKNOWLEDGEMENTS
We express our sincere appreciation to Professor Bin Du and all staff at the MICU, Peking Union Medical College Hospital.
Yu K, Lu M, Meng Y, Zhao Y, Li Z. Chlorhexidine gluconate transparent dressing does not decrease central line‐associated bloodstream infection in critically ill patients: A randomized controlled trial. Int J Nurs Pract. 2019;25:e12776. 10.1111/ijn.12776
Kunrong Yu, Yanling Meng, and Yanwei Zhao contributed equally to this work.
REFERENCES
- Apisarnthanarak, A. , Thongphubeth, K. , Yuekyen, C. , Warren, D. K. , & Fraser, V. J. (2010). Effectiveness of a catheter‐associated bloodstream infection bundle in a Thai tertiary care center: A 3‐year study. American Journal of Infection Control, 38(6), 449–455. 10.1016/j.ajic.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Bion, J. , Richardson, A. , Hibbert, P. , Beer, J. , Abrusci, T. , McCutcheon, M. , … Harrison, D. (2013). ‘Matching Michigan': A 2‐year stepped interventional programme to minimise central venous catheter‐blood stream infections in intensive care units in England. BMJ Quality and Safety, 22(2), 110–123. 10.1136/bmjqs-2012-001325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari, S. , Banjar, A. , Baghdadi, S. , Baltow, B. , Ashshi, A. , & Hussain, W. (2014). Central line associated blood stream infection rate after intervention and comparing outcome with national healthcare safety network and international nosocomial infection control consortium data. Annals of Medical and Health Sciences Research, 4(5), 682–686. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/25328774, 10.4103/2141-9248.141499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, B. (2001). Catheter‐related infection. Chinese Journal for Clinicians, 7, 9–11. 10.3969/j.issn.1008-1089.2001.07.005 [DOI] [Google Scholar]
- Dudeck, M. A. , Weiner, L. M. , Allen‐Bridson, K. , Malpiedi, P. J. , Peterson, K. D. , Pollock, D. A. , … Edwards, J. R. (2013). National Healthcare Safety Network (NHSN) report, data summary for 2012, device‐associated module. American Journal of Infection Control, 41(12), 1148–1166. 10.1016/j.ajic.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya, E. Y. , Dick, A. , Perencevich, E. N. , Pogorzelska, M. , Goldmann, D. , & Stone, P. W. (2011). Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS ONE, 6(1), e15452. 10.1371/journal.pone.0015452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan, T. C. , Andrus, M. , & Dudeck, M. A. (2008). CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control, 36(5), 309–332. Retrieved from. http://europepmc.org/abstract/MED/18538699, 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- Karpanen, T. J. , Casey, A. L. , Whitehouse, T. , Nightingale, P. , Das, I. , & Elliott, T. S. (2016). Clinical evaluation of a chlorhexidine intravascular catheter gel dressing on short‐term central venous catheters. American Journal of Infection Control, 44(1), 54–60. 10.1016/j.ajic.2015.08.022 [DOI] [PubMed] [Google Scholar]
- Maki, D. G. , Kluger, D. M. , & Crnich, C. J. (2006). The risk of bloodstream infection in adults with different intravascular devices: A systematic review of 200 published prospective studies. Mayo Clinic Proceedings, 81(9), 1159–1171. 10.4065/81.9.1159 [DOI] [PubMed] [Google Scholar]
- Maryniak, K. (2009). Clinical performance and nursing satisfaction of a transparent chlorhexidine gluconate IV securement dressing with peripherally inserted central catheters. Journal of the Association for Vascular Access, 14(4), 200–203. Retrieved from. http://www.sciencedirect.com/science/article/pii/S1552885509700741, 10.2309/java.14-4-5 [DOI] [Google Scholar]
- Mermel, L. A. , Allon, M. , Bouza, E. , Craven, D. E. , Flynn, P. , O'Grady, N. P. , … Warren, D. K. (2009). Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 49(1), 1–45. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/19489710, 10.1086/599376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto, T. , Kunishima, H. , Shimizu, G. , Hirose, M. , Yamasaki, Y. , Nishisako, H. , … Matsuda, T. (2015). Factors predicting the cause and prognosis of central line‐associated bloodstream infections. Journal of Infection and Chemotherapy, 21(2), 118–122. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/25483264, 10.1016/j.jiac.2014.10.010 [DOI] [PubMed] [Google Scholar]
- O'Grady, N. P. , Alexander, M. , Burns, L. A. , Dellinger, E. P. , Garland, J. , Heard, S. O. , … Healthcare infection control practices advisory, C (2011). Guidelines for the prevention of intravascular catheter‐related infections. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 52(9), e162–e193. Retrieved from. http://europepmc.org/abstract/MED/21460264, http://europepmc.org/articles/PMC3106269?pdf=render, http://europepmc.org/articles/PMC3106269, http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=EBI&pubmedid=21460264, http://www.pubmedcentral.nih.gov/picrender.fcgi?tool=EBI&pubmedid=21460264&action=stream&blobtype=pdf, 10.1093/cid/cir257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, C. , & Heilman, J. M. (2008). Clinical performance of a new transparent chlorhexidine gluconate central venous catheter dressing. Journal of the Association for Vascular Access, 13(1), 13–19. Retrieved from. http://www.sciencedirect.com/science/article/pii/S1552885508701272, 10.2309/java.13-1-4 [DOI] [Google Scholar]
- Pfaff, B. , Heithaus, T. , & Emanuelsen, M. (2012). Use of a 1‐piece chlorhexidine gluconate transparent dressing on critically ill patients. Critical Care Nurse, 32(4), 35–40. 10.4037/ccn2012956 [DOI] [PubMed] [Google Scholar]
- Roberts, B. , & Cheung, D. (1998). Biopatch—A new concept in antimicrobial dressings for invasive devices. Australian critical care: official journal of the Confederation of Australian Critical Care Nurses, 11(1), 16–19. Retrieved from. http://europepmc.org/abstract/MED/9708081, 10.1016/s1036-7314(98)70426-6 [DOI] [PubMed] [Google Scholar]
- Rosenthal, V. D. , Bijie, H. , Maki, D. G. , Mehta, Y. , Apisarnthanarak, A. , Medeiros, E. A. , … Jayatilleke, K. (2012). International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004‐2009. American Journal of Infection Control, 40(5), 396–407. 10.1016/j.ajic.2011.05.020 [DOI] [PubMed] [Google Scholar]
- Rupp, M. E. , Cavalieri, J. , Delaney, K. , Lundgren, K. , Stammers, L. , & Beach, S. (2008). Prospective, randomized, controlled trial assessing the clinical performance of a transparent chlorhexidine gel pad intravascular catheter dressing. Retrieved from Orlando: Society for Healthcare Epidemiology of America (SHEA). [Google Scholar]
- Sacks, G. D. , Diggs, B. S. , Hadjizacharia, P. , Green, D. , Salim, A. , & Malinoski, D. J. (2014). Reducing the rate of catheter‐associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. American Journal of Surgery, 207(6), 817–823. 10.1016/j.amjsurg.2013.08.041 [DOI] [PubMed] [Google Scholar]
- Safdar, N. , O'Horo, J. C. , Ghufran, A. , Bearden, A. , Didier, M. E. , Chateau, D. , & Maki, D. G. (2014). Chlorhexidine‐impregnated dressing for prevention of catheter‐related bloodstream infection: a meta‐analysis*. Critical Care Medicine, 42(7), 1703–1713. Retrieved from. http://europepmc.org/abstract/MED/24674924, http://europepmc.org/articles/PMC4258905?pdf=render, http://europepmc.org/articles/PMC4258905, 10.1097/CCM.0000000000000319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer, S. , Lewalter, K. , Schroder, J. , Koch, A. , Hafner, H. , Krizanovic, V. , … Lemmen, S. W. (2014). Reduction of central venous line‐associated bloodstream infection rates by using a chlorhexidine‐containing dressing. Infection, 42(1), 155–159. 10.1007/s15010-013-0519-7 [DOI] [PubMed] [Google Scholar]
- Shao, W. B. , Wang, L. , Cai, M. , Li, Y. M. , Wang, Z. F. , Liu, J. Y. , … Sun, X. H. (2014). Investigation of the compliance of preventive measures of catheter‐related bloodstream infections and evaluation of the practices. China Medical Herald, 25, 99–101. [Google Scholar]
- Timsit, J. F. , Mimoz, O. , Mourvillier, B. , Souweine, B. , Garrouste‐Orgeas, M. , Alfandari, S. , … Lucet, J. C. (2012). Randomized controlled trial of chlorhexidine dressing and highly adhesive dressing for preventing catheter‐related infections in critically ill adults. American Journal of Respiratory and Critical Care Medicine, 186(12), 1272–1278. 10.1164/rccm.201206-1038OC [DOI] [PubMed] [Google Scholar]
- Timsit, J. F. , Schwebel, C. , Bouadma, L. , Geffroy, A. , Garrouste‐Orgeas, M. , Pease, S. , … Lucet, J. C. (2009). Chlorhexidine‐impregnated sponges and less frequent dressing changes for prevention of catheter‐related infections in critically ill adults: A randomized controlled trial. JAMA, 301(12), 1231–1241. 10.1001/jama.2009.376 [DOI] [PubMed] [Google Scholar]
- Valles, J. , & Ferrer, R. (2009). Bloodstream infection in the ICU. Infectious Disease Clinics of North America, 23(3), 557–569. 10.1016/j.idc.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Worth, L. J. , & McLaws, M. L. (2012). Is it possible to achieve a target of zero central line associated bloodstream infections? Current Opinion in Infectious Diseases, 25(6), 650–657. 10.1097/QCO.0b013e32835a0d1a [DOI] [PubMed] [Google Scholar]
- Worth, L. J. , Spelman, T. , Bull, A. L. , Brett, J. A. , & Richards, M. J. (2015). Central line‐associated bloodstream infections in Australian intensive care units: Time‐trends in infection rates, etiology, and antimicrobial resistance using a comprehensive Victorian surveillance program, 2009‐2013. American Journal of Infection Control, 43(8), 848–852. 10.1016/j.ajic.2015.03.036 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


