Abstract
Background and study aims Given the sizable number of patients with symptomatic gastroesophageal reflux disease (GERD) despite proton pump inhibitor (PPI) therapy, non-pharmacologic treatment has become increasingly utilized. The aim of this study was to analyze the cost-effectiveness of medical, endoscopic, and surgical treatment of GERD.
Patients and methods A deterministic Markov cohort model was constructed from the US healthcare payer’s perspective to evaluate the cost-effectiveness of three competing strategies: 1) omeprazole 20 mg twice daily; 2) transoral incisionless fundoplication (TIF 2.0); and 3) laparoscopic Nissen fundoplication [LNF]. Cost was reported in US dollars with health outcomes recorded in quality-adjusted life years (QALYs). Ten-year and lifetime time horizons were utilized with 3 % discount rate and half-cycle corrections applied. The main outcome was incremental cost-effectiveness ratio (ICER) with a willingness-to-pay threshold of $ 100,000 per QALY. Probabilistic sensitivity analyses were also performed.
Results In our base-case analysis, the average cost of TIF 2.0 was $ 13,978.63 versus $ 17,658.47 for LNF and $ 10,931.49 for PPI. Compared to the PPI strategy, TIF 2.0 was cost-effective with an incremental cost of $ 3,047 and incremental effectiveness of 0.29 QALYs, resulting in an ICER of $ 10,423.17 /QALY gained. LNF was strongly dominated by TIF 2.0. Over a lifetime horizon, TIF 2.0 remained the cost-effective strategy for patients with symptoms despite twice-daily 20-mg omeprazole. TIF 2.0 remained cost-effective after varying parameter inputs in deterministic and probabilistic sensitivity analyses and for scenario analyses in multiple age groups.
Conclusions Based upon this study, TIF 2.0 was cost-effective for patients with symptomatic GERD despite low-dose, twice-daily PPI.
Introduction
Gastroesophageal reflux disease (GERD) is a highly prevalent condition among patients presenting in the ambulatory setting and develops when gastric contents move retrograde from the stomach into the esophagus 1 2 . According to the Montreal consensus, GERD develops when this reflux of stomach contents results in troublesome symptoms or complications 3 4 5 . While some reflux is physiologic, symptoms of heartburn or regurgitation have been reported to occur in approximately one-fourth of patients in the United States annually 2 6 7 . Alongside this high prevalence, there exists a considerable cost of managing GERD, estimated to have a direct and indirect cost that accounts for $ 15 billion to $ 20 billion annually in the United States 8 . Within the last 5 years, expenditures for acid- suppressing drugs alone totaled approximately $ 60 billion 9 . Although pharmacotherapy with proton pump inhibitors (PPIs) is a universally accepted first-line strategy for the treatment of GERD, a significant number of individuals (ranging from 10 % to 40 % of patients) may fail to achieve complete resolution of symptoms 10 11 12 13 14 15 .
Given the significant impact of symptomatic reflux disease on quality of life (QoL), costs, and rising awareness of potential adverse effects from long-term PPI therapy, anti-reflux procedures including endoscopic or surgical options may be considered. Two strategies developed to reduce the short- and long-term sequelae of GERD include the endoscopic transoral incisionless fundoplication (TIF 2.0) procedure using the EsophyX device (Endogastric Solutions, Redmond, Washington, United States) and the traditional surgical approach involving laparoscopic Nissen fundoplication (LNF). These endoscopic and surgical strategies are generally reserved for patients who have persistent symptoms or develop adverse events (AEs) despite appropriate pharmacologic therapy 16 .
Yet despite these options being available for patients with refractory GERD, evaluation of these non-pharmacologic treatments – specifically LNF – as compared to standard PPI therapy has demonstrated mixed results 17 18 19 20 . Studies to examine the cost-effectiveness of these competing strategies also have been highly variable 21 22 23 . Given the sizable burden of GERD on the healthcare system, economic investigation as well as assessment of QoL is needed to improve patient outcomes. As such, the primary aim of this study was to analyze the cost-effectiveness of medical, endoscopic, and surgical treatment of refractory GERD.
Patients and methods
Markov model design
We developed a decision-analytic Markov cohort model to evaluate the cost-effectiveness of three competing strategies for the treatment of refractory GERD. These strategies are: 1) pharmacotherapy with omeprazole 20 mg twice daily; 2) endoscopic treatment with TIF 2.0; and 3) surgical treatment with LNF. This deterministic Markov model describes the natural course of GERD using Markov health states with transition and event probabilities 24 . The model simulated a patient’s transition across multiple health states including refractory reflux disease despite twice-daily omeprazole, well-controlled GERD on PPI treatment, resolution of GERD no longer requiring pharmacotherapy, and death. All patients in each strategy entered the model with health states corresponding to treatment-refractory GERD despite twice-daily omeprazole 20 mg. A summary figure of the transition-state model is shown in Fig. 1 .
Fig. 1 .
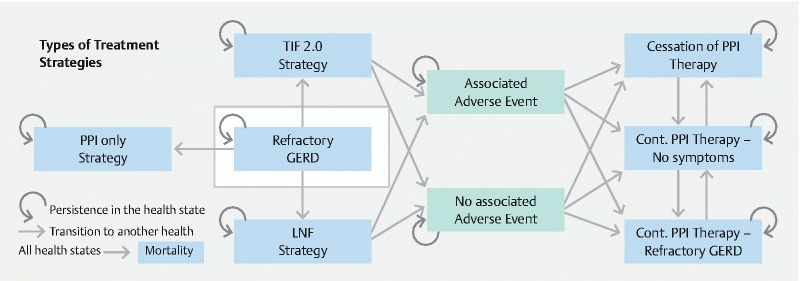
Markov state-transition diagram to evaluate the cost-effectiveness of PPI versus TIF 2.0 versus LNF for the treatment of refractory GERD.
This cost-effectiveness analysis was performed using the best estimates of all parameters and probabilities following the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine 25 . The analysis was conducted from the US healthcare payer’s perspective with a state-transition model utilizing a 10-year time horizon and cycle length of 12 months. Half-cycle corrections were applied and health effects and costs were discounted at 3 % per year, as per convention 26 . The main outcome measures were with a willingness-to-pay threshold of $ 100,000 per quality-adjusted life year (QALY) and net monetary benefit. Net monetary benefit uses the willingness-to-pay threshold to convert health gains into their monetary value as this provides more meaningful uncertainty intervals compared to the incremental cost-effectiveness ratio (ICER) approach. The cost-effective strategy is identified as that strategy with the highest ICER under a willingness-to-pay threshold of $ 100,000 per QALY. This Markov model was constructed using the decision-analytic software package TreeAge Pro 2020 (Healthcare Version) (TreeAge Software Inc., Williamstown, Massachusetts, United States).
Study population and model parameters
The hypothetical cohort consisted of patients aged 50 years who reported refractory GERD symptoms despite twice-daily PPI therapy. Refractory GERD in this study was defined as patients with inadequate or unsatisfactory symptomatic response to 12 weeks of twice-daily PPI therapy with a 20-mg dose of omeprazole, or equivalent 27 . This dosage was chosen as previous literature has demonstrated the magnitude of difference in efficacy between low- and maximal-dose omeprazole is insufficient to warrant routine twice-daily 40-mg use for GERD-associated symptoms 28 29 .
The TIF 2.0 procedure was approved by the US Food and Drug Administration for the treatment of GERD in 2007, and aims to create a full-thickness esophageal valve from inside the gastric body using serosa-to-serosa plications that include the muscle layers 30 31 32 . This endoscopic treatment strategy restores the dynamics of the angle of His and involves a 270-degree wrap, different compared to a LNF, which involves the creation of a complete 360-degree wrap with an anti-reflux valve created at the fundus of the stomach. All modeled patients undergoing endoscopic or surgical intervention had appropriate work-up to confirm GERD, including endoscopic evaluation as well as esophageal function testing with manometry and objective reflux monitoring. The cohort also met criteria to undergo both the TIF 2.0 and LNF procedures, including patients with a body mass index < 35 kg/m 2 , hiatal hernia < 2 cm, lack of grade C or D esophagitis per the Los Angeles classification, and no underlying esophageal motility disorders.
Model input parameters – including state-transition probabilities, health state utility weights, and costs – were obtained from published sources and are detailed in Table 1 33 . Disutility from AE was also accounted for and included procedure-associated AEs. To conduct probabilistic sensitivity analyses, we modeled parameter uncertainty using beta distributions for probabilities, gamma distributions for costs, and triangular distributions for utility weights. We modeled age-standardized background mortality from US life tables as published by the US Centers for Disease Control and Prevention 34 .
Table 1. Markov model inputs: medical versus endoscopic versus surgical therapies for refractory GERD.
| Probabilities | Estimates | Range (95 % CI) | Distribution | Supplemental references |
| Survive TIF procedure | 0.999 | 0.990 to 1.000 | Beta | 1 |
| Survive LNF procedure | 0.992 | 0.985 to 1.000 | Beta | 2–5 |
| Initial success rate, TIF | 0.990 | 0.970 to 1.000 | Beta | 6 |
| Initial success rate, LNF | 0.990 | 0.970 to 1.000 | Beta | 3, 7, 8 |
| Adverse event rate, TIF | 0.020 | 0.010 to 0.030 | Beta | 6 |
| Adverse event rate, LNF | 0.061 | 0.013 to 0.101 | Beta | 5, 9, 10 |
| Immediate PPI discontinuation rate, TIF | 0.890 | 0.820 to 0.950 | Beta | 6 |
| Immediate PPI discontinuation rate, LNF | 0.933 | 0.869 to 0.995 | Beta | 10, 11 |
| 1-year PPI discontinuation rate, TIF | 0.783 | 0.760 to 0.890 | Beta | 6, 12 |
| 1-year PPI discontinuation rate, LNF | 0.810 | 0.743 to 0.864 | Beta | 13, 14 |
| 2-year PPI discontinuation rate, TIF | 0.764 | 0.710 to 0.770 | Beta | 12, 15, 16 |
| 2-year PPI discontinuation rate, LNF | 0.760 | 0.706 to 0.805 | Beta | 17 |
| 3-year PPI discontinuation rate, TIF | 0.712 | 0.650 to 0.751 | Beta | 12, 16 |
| 3-year PPI discontinuation rate, LNF | 0.690 | 0.635 to 0.770 | Beta | 13, 18 |
| 5-year PPI discontinuation rate, TIF | 0.540 | 0.412 to 0.600 | Beta | 19, 20 |
| 5-year PPI discontinuation rate, LNF | 0.706 | 0.690 to 0.722 | Beta | 21 |
| 10-year PPI discontinuation rate, TIF | 0.417 | 0.330 to 0.510 | Beta | 20, 22 |
| 10-year PPI discontinuation rate, LNF | 0.589 | 0.570 to 0.608 | Beta | 21 |
| Costs (US $, 2019) | ||||
|
$ 761.00 | $ 570.75 to $ 951.25 | Gamma | 23 |
|
$ 230.00 | $ 172.50 to $ 287.50 | Gamma | 23 |
|
$ 588.00 | $ 441.00 to $ 697.50 | Gamma | 23 |
|
$ 1.54 | $ 0.39 to $ 1.93 | Gamma | 24 |
|
$ 4.39 | $ 1.10 to $ 5.49 | Gamma | 24 |
|
$ 2410.51 | $ 2035.65 to $ 2785.38 | Gamma | 25 |
|
$ 7314.62 | $ 6950.25 to $ 9499.96 | Gamma | 25 |
|
$ 3078.45 | $ 2955.08 to $ 3201.82 | Gamma | 25 |
|
$ 10393.11 | $ 12429.99 to $ 13832.44 | Gamma | 25 |
|
$ 5177.00 | $ 3882.75 to $ 6471.25 | Gamma | 23, 26 |
| Utilities (health states) | ||||
|
0.885 | 0.770 to 0.940 | Triangular | 3, 27, 28 |
|
0.998 | 0.980 to 1.000 | Triangular | 3, 29, 30 |
|
1.000 | – | – | – |
|
0.000 | – | – | – |
|
0.600 | 0.450 to 0.750 | Triangular | 3 |
|
0.500 | 0. 400 to 0.700 | Triangular | 3, 30 |
|
0.620 | 0. 550 to 0.800 | Triangular | 3, 30 |
|
0.620 | 0.500 to 0.750 | Triangular | 3, 30 |
GERD, gastroesophageal reflux disease; TIF, transoral incisionless fundoplication; LNF, laparoscopic Nissen fundoplication; CI, confidence interval; PPI, proton pump inhibitor.
Alternative PPI costs shown in Supplementary Material.
Costs
This study was designed using costs incurred from the US healthcare system (i. e., payer’s) perspective. Each strategy included costs corresponding to a time horizon and varied based on health state. We also considered costs associated with medical treatments, procedures or surgeries, and procedure-associated AEs ( Table 1 ) . Costs of pharmacotherapy were derived from Micromedex Red Book and included a 40 % discount to the average wholesale price as per the ISPOR Task Force of Good Research Practices recommendation 35 36 . We derived the cost of endoscopic TIF 2.0 and surgical LNF procedures from national commercial Blue Health Intelligence data covering professional and facility claims for Current Procedural Terminology codes 43210 and 43280, respectively. To account for inflation, costs derived from previously published literature were converted to 2019 US dollars using the Consumer Price Index (Consumer Price Index United States Bureau of Labor Statistics) inflation calculator 37 .
Sensitivity analyses and additional modeling
One-way and probabilistic sensitivity analyses were also performed to explore the uncertainty around model input parameters 38 . For the probabilistic sensitivity analyses, we ran the model 100,000 times, each time with a parameter set drawn from the defined distributions 39 . This produces uncertainty intervals for the outcomes of interest, and from these, a cost-effectiveness acceptability curve for competing strategies using willingness-to-pay thresholds ranging from $ 0 to $ 100,000 per QALY gained. One-way sensitivity analyses also evaluated the effect of variations of the effectiveness and price of TIF 2.0 procedure in the model. We also performed scenario analyses for different PPI pharmacotherapies, for a lifetime horizon instead of 10 years, and for different cohort ages (from ages 18 to 79 years).
Results
Base case analysis
In the base-case analysis, the PPI-only strategy carried the lowest cost at $ 10,391, compared to an average cost of TIF 2.0 of $ 13,979 and $ 17,658 for LNF. Despite being the least costly strategy, continuing PPI therapy alone was associated with the lowest effectiveness (8.43 QALYs), followed by LNF (8.67 QALYs), and TIF 2.0 with the highest (8.73 QALYs) ( Table 2 ). At 10 years, 46.7 % of surviving patients in the TIF 2.0 strategy had discontinued all PPI therapy, and a further 35.3 % of patients had controlled GERD symptoms on PPI therapy. For the LNF group, a higher percentage of patients – 54.3 % – had discontinued omeprazole, with 28.6 % of patients having controlled symptoms on PPI therapy. A total of 12.1 % and 10.6 % of patients continued to report refractory GERD symptoms despite resuming PPI therapy at 10 years in the TIF 2.0 and LNF groups, respectively. These results for the TIF 2.0 and LNF strategies are compared to the assumed 100 % of surviving patients in the PPI-only therapy strategy still requiring a PPI for refractory GERD symptoms. Based on this analysis, LNF strategy was dominated by the TIF 2.0 strategy. Compared to the PPI-only strategy, TIF 2.0 was cost-effective with an incremental cost of $ 3,047 and incremental effectiveness of 0.29 QALYs, resulting in an ICER of $ 10,423 per QALY ( Fig. 2 ).
Table 2. Base case and probabilistic sensitivity analyses for PPI vs TIF 2.0 vs LNF.
| Base case analysis – low-dose omeprazole | PPI strategy | TIF strategy | LNF strategy |
| Cost (US) | $ 10,931.49 | $ 13,978.63 | $ 17,658.47 |
| Effectiveness (QALY) | 8.43 | 8.73 | 8.67 |
| Incremental cost-effectiveness (ICER) | – | $ 10,423.17 /QALY | Dominated |
| Net monetary benefit (NMB) | $ 832,487.85 | $ 858,674.99 | $ 849,824.33 |
| Probabilistic sensitivity analysis | PPI strategy | TIF strategy | LNF strategy |
| Cost (US) | $ 10,929.72 ± 2,831.77 | $ 13,979.95 ± 1,051.50 | $ 17,658.77 ± 807.35 |
| Effectiveness (QALY) | 8.43 ± 0.35 | 8.73 ± 0.03 | 8.67 ± 0.03 |
| Net monetary benefit (NMB) | $ 832,284.31 ± 34,744.38 | $ 858,678.24 ± 3,744.46 | $ 849,822.54 ± 3,573.07 |
| Lifetime time horizon | PPI strategy | TIF strategy | LNF strategy |
| Cost (US) | $ 35,915.83 | $ 27,799.08 | $ 28,789.17 |
| Effectiveness (QALY) | 27.71 | 29.93 | 29.92 |
| Incremental cost-effectiveness (ICER) | Dominated | – | Dominated |
| Net monetary benefit (NMB) | $ 2,735,169.67 | $ 2,965,359.61 | $ 2,962,914.28 |
| Maximum dose omeprazole – 10-year time horizon | PPI strategy | TIF strategy | LNF strategy |
| Cost (US) | $ 31,161.86 | $ 18,820.09 | $ 22,020.45 |
| Effectiveness (QALY) | 8.43 | 8.73 | 8.67 |
| Incremental cost-effectiveness (ICER) | Dominated | – | Dominated |
| Net monetary benefit (NMB) | $ 812,257.48 | $ 853,833.53 | $ 853,833.53 |
| Maximum dose omeprazole – lifetime time horizon | PPI strategy | TIF strategy | LNF strategy |
| Cost (US) | $ 102,383.44 | $ 58,217.34 | $ 53,750.17 |
| Effectiveness (QALY) | 27.71 | 29.93 | 29.92 |
| Incremental cost-effectiveness (ICER) | Dominated | $ 306,969.43 /QALY | – |
| Net monetary benefit (NMB) | $ 2,668,702.06 | $ 2,934,941.36 | $ 2,937,953.28 |
PPI, proton pump inhibitor; TIF, transoral incisionless fundoplication; LNF, laparoscopic Nissen fundoplication; QALY, quality-adjusted life year; NMB, net monetary benefit; ICER, incremental cost-effectiveness ratio.
Fig. 2 .
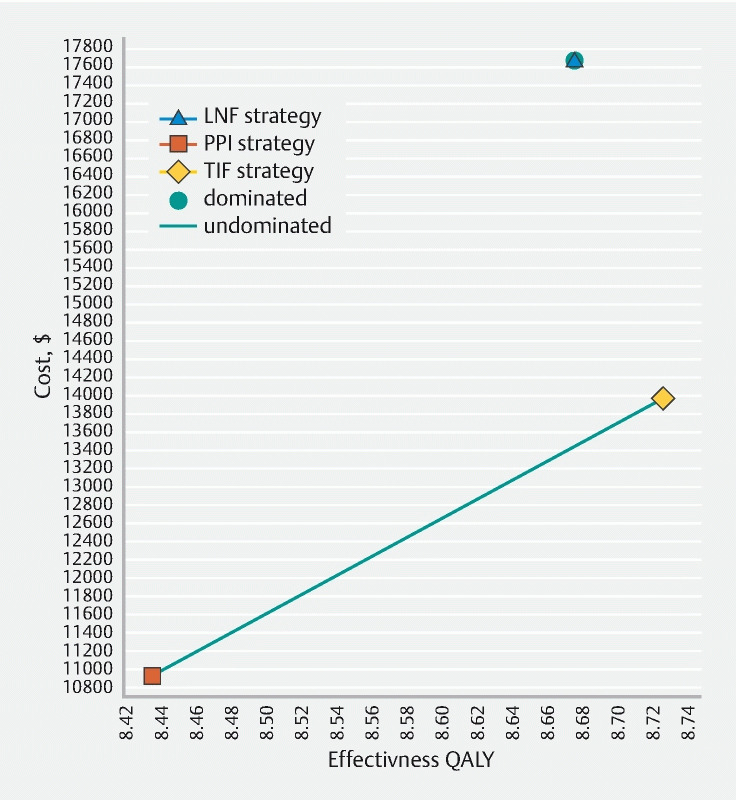
Cost-effectiveness plane comparing PPI versus TIF 2.0 versus LNF for refractory GERD at a willingness-to-pay threshold of $ 100,000 per QALY gained.
Probabilistic and one-way sensitivity analyses
Deterministic and probabilistic sensitivity analyses demonstrated that TIF 2.0 remained cost-effective across a broad set of parameter ranges at a willingness-to-pay threshold of $ 100,000 per QALY gained ( Table 2 ). Despite the presence of some overlap in the confidence intervals due to parameter uncertainties in these 100,000 iterations, the TIF 2.0 treatment strategy remained cost-effective ( Fig. 3 ). Comparison of TIF 2.0 to PPI therapy over each of the 100,000 probabilistic sensitivity iterations demonstrated that TIF 2.0 was cost-effective for the vast majority of iterations ( Supplemental Fig. 1 ). A cost-effectiveness acceptability curve ( Fig. 4 ) showed that TIF 2.0 was most likely to be the cost-effective strategy as long as willingness-to-pay was greater than $ 12,000 per QALY. Net monetary benefit also demonstrated TIF 2.0 to be cost-effective as compared to other strategies ( Supplemental Fig. 2 ).
Fig. 3.
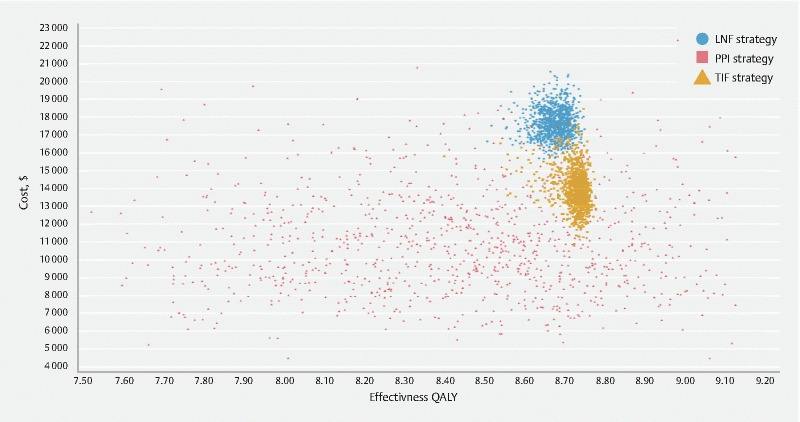
Cost-effectiveness scatterplot of probabilistic sensitivity analysis demonstrating the distribution of costs versus QALYs based on model parameter uncertainties.
Fig. 4.
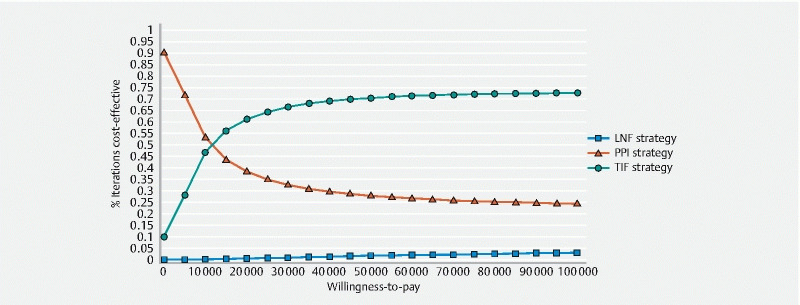
Cost-effectiveness acceptability curve comparing PPI versus TIF 2.0 versus LNF for refractory GERD at a willingness-to-pay threshold of $ 100,000.
Next, a one-way sensitivity analysis was performed to determine the threshold cost at which the TIF 2.0 strategy was no longer cost-effective. Assuming a constant facility claim cost of $ 7315 for the TIF 2.0 procedure, LNF becomes the cost-effective strategy if the professional claims cost for TIF 2.0 exceeds $ 11,725 (total cost of $ 19,040) ( Supplemental Fig. 3 ). Holding all other parameters at their respective base-case mean values, LNF became the preferred strategy if the initial success rate for TIF 2.0 was less than 92.56 % ( Supplemental Fig. 4 ).
Additional analyses
Additional analyses were performed to simulate different age groups. TIF 2.0 remained cost-effective for individuals from aged 18 to 79 years at a willingness-to-pay threshold less than $ 100,000 ( Supplemental Table 1 ). The surgical strategy was strongly dominated at all modeled ages. In addition, when extending the base-case time horizon to lifetime, TIF 2.0 remained the cost-effective strategy up to willingness-to-pay of $ 100,000 per QALY gained ( Table 2 ). At a lifetime time horizon, PPI and LNF were both strongly dominated by TIF 2.0.
To further improve generalizability, this Markov model was fit with various types of PPI medications (including costs associated with different and higher-dose PPI formulations) ( Supplemental Material ). Assuming patients had refractory symptoms despite a higher dose of twice-daily 40-mg omeprazole, TIF 2.0 remained a cost-effective strategy at a willingness-to-pay threshold of $ 100,000 per QALY gained over a 10-year time horizon ( Table 2 ). Due to the increased cost from the higher omeprazole dose, the PPI strategy became the most expensive strategy with TIF 2.0 being the least costly. However, when extending this time horizon to lifetime, LNF became the cost-effective strategy, assuming a willingness-to-pay threshold of $ 100,000 per QALY gained. TIF 2.0 was associated with an ICER of $ 306,969.43 /QALY, and thus was not cost-effective over a lifetime time horizon for patients with refractory symptoms to higher dose, twice-daily omeprazole. Based upon these data, a one-way sensitivity analysis was performed to determine the cost threshold for PPI medications that would make endoscopic therapy no longer cost-effective. Over a 10-year time horizon, TIF 2.0 remained cost-effective for all costs of PPI medications across all willingness-to-pay thresholds, due to decreased overall effectiveness of refractory GERD (i. e., reduced QALYs.) ( Supplemental Fig. 5 ).
Discussion
Using this model-based cost-effectiveness analysis, we demonstrated that endoscopic therapy with TIF 2.0 was cost-effective for treatment of GERD using a willingness-to-pay threshold of $ 100,000 per QALY gained. When compared to continued medical therapy with twice-daily omeprazole 20 mg and a surgical approach with LNF, TIF 2.0 appeared to be a cost-effective strategy over a 10-year time horizon. Extending this decision-analytic Markov state-transition cohort model to a lifelong horizon, TIF 2.0 remained a cost-effective treatment for patients on omeprazole 20 mg twice a day; however, for maximal-dose omeprazole, the LNF strategy was cost-effective at a willingness-to-pay threshold of less than $ 100,000 per QALY gained.
GERD is a highly prevalent condition, affecting approximately 15 % to 30 % of individuals in the United States 40 41 . Treatment with PPI should and will remain first-line for alleviation of reflux; however, given the number of individuals who remain symptomatic and those who wish to discontinue anti-reflux medications, alternative treatments including endoscopic and surgical options are needed. Although many patients with GERD who do not respond to PPI therapy may have reflux hypersensitivity or functional heartburn as the underlying etiology of symptoms, others with high reflux burden may benefit from non-pharmacologic therapy. Therefore, a thorough evaluation remains key to identifying optimal candidates for anti-reflux interventions, including performing a detailed history, careful endoscopic assessment, including measurement and identification of important landmarks such as Hill grade and hernia size, and esophageal function testing with manometry and objective reflux monitoring test 20 42 43 44 45 . It also remains important to underscore that long-term PPI therapy should be administered in the lowest effective dose possible, including on demand or intermittent therapy 16 .
Using the base-case results from this analysis, as well as the findings expanding to a lifetime time horizon, TIF 2.0 was cost-effective for patients with symptoms refractory to lower-dose omeprazole (20 mg twice daily) in the short-term; however, a surgical approach with LNF appeared to be cost-effective for patients with symptoms on maximal-dose PPI (40 mg twice daily) on modeling over a lifetime. Extrapolation of these data to a lifetime time horizon should be interpreted with some caution given the lack of long-term data with regard to TIF; however, these results appear to be consistent with previous literature demonstrating that more severe GERD may translate into reduced efficacy and a higher likelihood of restarting PPI medications post-TIF 2.0 46 47 . As such, we believe that TIF 2.0 may be better utilized in patients with lower reflux burden who experience continued symptoms despite treatment on lowerdose PPIs, and that LNF may be the optimal strategy for those with higher reflux burden with symptoms refractory to maximum-dose PPIs. Given the lack of a prospective, randomized, head-to-head comparison between TIF 2.0 and LNF, whether the severity of reflux burden and response to PPI at different dosing levels may distinguish effectiveness between the two interventions and be used as a marker to help procedural selection remains to be determined.
Previous literature comparing the outcomes of endoscopic and surgical anti-reflux treatment strategies have demonstrated variable results 21 22 23 . In a previous network meta-analysis by Richter and colleagues comparing LNF versus TIF 2.0, LNF demonstrated the greatest physiologic improvement associated with GERD on esophageal function testing based upon measurement of lower esophageal sphincter pressure and acid exposure; however, TIF 2.0 showed improved measures for health-related QoL (HRQoL) 48 . This may, in fact, account for some of the differences observed in our findings, as QALYs were noted to be higher for TIF 2.0 compared to LNF and PPI strategies, although more individuals who underwent LNF remained off PPI at 10 years. Prior studies have shown that LNF is often associated with more postoperative dysphagia and bloating during the early postsurgical period compared to Toupet fundoplication, which has a similar physiology to TIF 2.0 (i. e., 270-degree wrap) 49 . However, this difference dissipated over time with longer follow-up. Similar differences may be seen between TIF 2.0 and LNF patients that may explain the difference in overall HRQoL. TIF 2.0 patients may have higher QALYs due to a lower rate of dysphagia postoperatively. While this advantage over LNF may similarly decrease over time, the overall literature on TIF 2.0 has been limited to shorter follow-up and may not adequately address the change in longer-term quality-of-life differences between these two procedures.
This study has several limitations. First, inherent to cost-effective analyses are the assumptions made with regard to cost, equal access and availability of procedures, and the intrinsic heterogeneity of data abstracted to produce model estimates. Second, mathematical and statistical modeling, while designed to simulate a clinical setting, must be viewed in the context in which it is presented – with short-term follow-up data projected over a longer-term time horizon. In addition, individual patient characteristics are critically important in the treatment decision-making process – that is to say, not all patients may be eligible to undergo both TIF 2.0 or LNF procedures or patients may change the dose or frequency of PPI medications. In an effort to compare these non-pharmacologic treatment modalities in this cost-effectiveness analysis, this hypothetical patient cohort included only patients who were eligible to undergo either procedure. However, in clinical practice, patients with a large hiatal hernia may not be candidates for the TIF 2.0 procedure, and therefore, may benefit from a surgical approach. In addition, while this cohort included patients with documented refractory GERD on manometry and pH/impedance testing, it is also important to acknowledge these findings may not be generalizable to patients with functional heartburn or reflux hypersensitivity (a heterogenous group sometimes labeled inaccurately as having refractory GERD).
Furthermore, this cost-effectiveness analysis did not include additional procedures such as Stretta or LINX due to limited long-term literature at this time. Although data for TIF 2.0 are not yet available beyond 10 years, our base-case results including individuals with controlled GERD and resolved GERD (i. e., percentage of patients off and on PPI) in this model mirror published literature. While we attempted to account for GERD control and effectiveness on or off PPI medications, dose adjustments as well as potential sequalae from PPIs or GERD (including the development of Barrett’s esophagus) were not considered, although we acknowledge these may in large part shape the treatment decision for clinicians, perhaps considering endoscopic or surgical options even earlier for patients who remain at risk. Finally, the outcome measurement of GERD control may also be limited by the heterogeneity in instruments used to assess symptom management and severity across studies.
Despite these limitations, this study possesses several strengths. Although these results are based upon hypothetic Markov modeling, our results were validated using preexisting literature, real-world clinical data including costs of pharmacotherapy as well as procedure-associated costs (both facility and professional) based upon insurance claims data. In addition, despite long-term TIF 2.0 data, the base case of this analysis was based upon 10-year published literature, validated by current literature for both endoscopic and surgical treatment strategies. Perhaps most importantly, this cost-effectiveness analysis also included probabilistic and sensitivity analyses to account for uncertainty and help translate these results to clinical practice. In addition, one-way sensitivity analyses may propose cost thresholds for TIF 2.0 to remain a cost-effective strategy as well as suggest an initial success rate as a barometer for institutions or providers to measure procedural outcomes. Although more long-term data as well as head-to-head comparison studies are needed between endoscopic and surgical treatment strategies, economic investigation combined with objective and subjective measures of success are critically important.
Conclusions
In summary, TIF 2.0 was found to be cost-effective for patients with refractory GERD using the base-case analysis at a 10-year time horizon. One-way sensitivity and threshold analyses showed TIF 2.0 remained cost-effective up to a total procedural cost of $ 11,724.94 among patients on twice-daily 20-mg omeprazole. Expanding this base case to a lifetime time horizon, we found TIF 2.0 remained a cost-effective strategy; however, for patients refractory to maximum-dose PPI, LNF appeared to be cost-effective using a willingness-to-pay threshold of $ 100,000. While individual patient characteristics and factors outside the realm of this Markov model remain key to determine an appropriate treatment strategy, this cost-effectiveness analysis provides valuable insight and may assist in the shared decision-making process.
Footnotes
Competing interests Thomas R. McCarty has no conflicts to disclose. Pichamol Jirapinyo has the following disclosures: Apollo Endosurgery – Research Support, Fractyl – Research Support, GI Dynamics – Research Support, Endogastric Solutions – Consultant. Lyndon P. James has no conflicts to disclose. Sanchit Gupta has no conflicts to disclose. Walter W. Chan has the following disclosures: Ironwood – scientific advisory board. Christopher C. Thompson has the following disclosures: Apollo Endosurgery – Consultant/Research Support (Consulting fees/Institutional Research Grants), Aspire Bariatrics – Research Support (Institutional Research Grant), BlueFlame Healthcare Venture Fund – General Partner, Boston Scientific – Consultant (Consulting fees), Covidien/Medtronic – Consultant (Consulting Fees), EnVision Endoscopy (Board Member), Fractyl – Consultant/Advisory Board Member (Consulting Fees), GI Dynamics – Consultant (Consulting Fees)/ Research Support (Institutional Research Grant), GI Windows – Ownership interest, Olympus/Spiration – Consultant (Consulting Fees)/Research Support (Equipment Loans), Spatz – Research Support (Institutional Research Grant), USGI Medical – Consultant (Consulting Fees)/Advisory Board Member (Consulting fees)/Research Support (Research Grant).
Supplementary material :
References
- 1.Zagari R M, Fuccio L, Wallander M A et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrettʼs oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–1359. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Dubois D, Coulie B et al. Prevalence and socioeconomic impact of upper gastrointestinal disorders in the United States: results of the US Upper Gastrointestinal Study. Clin Gastroenterol Hepatol. 2005;3:543–552. doi: 10.1016/s1542-3565(05)00153-9. [DOI] [PubMed] [Google Scholar]
- 3.Flook N, Jones R, Vakil N. Approach to gastroesophageal reflux disease in primary care: Putting the Montreal definition into practice. Can Fam Physician. 2008;54:701–705. [PMC free article] [PubMed] [Google Scholar]
- 4.Pace F, Bazzoli F, Fiocca R et al. The Italian validation of the Montreal Global definition and classification of gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2009;21:394–408. doi: 10.1097/MEG.0b013e32830a70e2. [DOI] [PubMed] [Google Scholar]
- 5.Vakil N, van Zanten S V, Kahrilas P.The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus Am J Gastroenterol 20061011900–1920.; quiz 43 [DOI] [PubMed] [Google Scholar]
- 6.Gomez J E. Typical and atypical presentations of gastroesophageal reflux disease and its management. Bol Asoc Med P R. 2004;96:264–269. [PubMed] [Google Scholar]
- 7.Richter J E. Typical and atypical presentations of gastroesophageal reflux disease. The role of esophageal testing in diagnosis and management. Gastroenterol Clin North Am. 1996;25:75–102. doi: 10.1016/s0889-8553(05)70366-6. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen N J, Hansen R A, Morgan D R et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol. 2006;101:2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 9.Peery A F, Crockett S D, Murphy C C et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254–272 e11. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gawron A J, Bell R, Abu Dayyeh BK et al. Surgical and endoscopic management options for patients with GERD based on proton pump inhibitor symptom response: recommendations from an expert U. S. panel. Gastrointest Endosc. 2020;92:78–87 e2. doi: 10.1016/j.gie.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Serag H, Becher A, Jones R. Systematic review: persistent reflux symptoms on proton pump inhibitor therapy in primary care and community studies. Aliment Pharmacol Ther. 2010;32:720–737. doi: 10.1111/j.1365-2036.2010.04406.x. [DOI] [PubMed] [Google Scholar]
- 12.Crawley J A, Schmitt C M. How satisfied are chronic heartburn sufferers with their prescription medications? Results of the patient unmet needs study. J Clin Outcomes Manag. 2000;7:29. [Google Scholar]
- 13.The Gallup Organization I . Princeton, NJ: The Gallup Organization; 2000. The 2000 Gallup study of consumersʼ use of stomach relief products. [Google Scholar]
- 14.Fass R. Proton-pump inhibitor therapy in patients with gastro-oesophageal reflux disease: putative mechanisms of failure. Drugs. 2007;67:1521–1530. doi: 10.2165/00003495-200767110-00001. [DOI] [PubMed] [Google Scholar]
- 15.Lu M, Malladi V, Agha A et al. Failures in a proton pump inhibitor therapeutic substitution program: lessons learned. Dig Dis Sci. 2007;52:2813–2820. doi: 10.1007/s10620-007-9811-7. [DOI] [PubMed] [Google Scholar]
- 16.Katz P O, Gerson L B, Vela M F.Guidelines for the diagnosis and management of gastroesophageal reflux disease Am J Gastroenterol 2013108308–328.; quiz 29 [DOI] [PubMed] [Google Scholar]
- 17.Galmiche J P, Hatlebakk J, Attwood S et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969–1977. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 18.Grant A M, Cotton S C, Boachie C et al. Minimal access surgery compared with medical management for gastro-oesophageal reflux disease: five year follow-up of a randomised controlled trial (REFLUX) BMJ. 2013;346:f1908. doi: 10.1136/bmj.f1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg S K, Gurusamy K S. Laparoscopic fundoplication surgery versus medical management for gastro-oesophageal reflux disease (GORD) in adults. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD003243.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spechler S J, Hunter J G, Jones K M et al. Randomized Trial of medical versus surgical treatment for refractory heartburn. N Engl J Med. 2019;381:1513–1523. doi: 10.1056/NEJMoa1811424. [DOI] [PubMed] [Google Scholar]
- 21.Funk L M, Zhang J Y, Drosdeck J M et al. Long-term cost-effectiveness of medical, endoscopic and surgical management of gastroesophageal reflux disease. Surgery. 2015;157:126–136. doi: 10.1016/j.surg.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 22.Arguedas M R, Heudebert G R, Klapow J C et al. Re-examination of the cost-effectiveness of surgical versus medical therapy in patients with gastroesophageal reflux disease: the value of long-term data collection. Am J Gastroenterol. 2004;99:1023–1028. doi: 10.1111/j.1572-0241.2004.30891.x. [DOI] [PubMed] [Google Scholar]
- 23.Epstein D, Bojke L, Sculpher M J et al. Laparoscopic fundoplication compared with medical management for gastro-oesophageal reflux disease: cost effectiveness study. BMJ. 2009;339:b2576. doi: 10.1136/bmj.b2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siebert U, Alagoz O, Bayoumi A M et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Med Decis Making. 2012;32:690–700. doi: 10.1177/0272989X12455463. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein M C, Siegel J E, Gold M R et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 26.Carias C, Chesson H W, Grosse S D et al. Recommendations of the Second Panel on Cost Effectiveness in Health and Medicine: A Reference, Not a Rule Book. Am J Prev Med. 2018;54:600–602. doi: 10.1016/j.amepre.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340–1354. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 28.Bate C M, Booth S N, Crowe J P et al. Does 40 mg omeprazole daily offer additional benefit over 20 mg daily in patients requiring more than 4 weeks of treatment for symptomatic reflux oesophagitis? Aliment Pharmacol Ther. 1993;7:501–507. doi: 10.1111/j.1365-2036.1993.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 29.Cho Y K, Choi M G, Bak Y T et al. Efficacy of S-pantoprazole 20 mg compared with pantoprazole 40 mg in the treatment of reflux esophagitis: a randomized, double-blind comparative trial. Dig Dis Sci. 2012;57:3189–3194. doi: 10.1007/s10620-012-2297-y. [DOI] [PubMed] [Google Scholar]
- 30.Cadiere G B, Buset M, Muls V et al. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg. 2008;32:1676–1688. doi: 10.1007/s00268-008-9594-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadiere G B, Rajan A, Rqibate M et al. Endoluminal fundoplication (ELF) -- evolution of EsophyX, a new surgical device for transoral surgery. Minim Invasive Ther Allied Technol. 2006;15:348–355. doi: 10.1080/13645700601040024. [DOI] [PubMed] [Google Scholar]
- 32.McCarty T R, Itidiare M, Njei B et al. Efficacy of transoral incisionless fundoplication for refractory gastroesophageal reflux disease: a systematic review and meta-analysis. Endoscopy. 2018;50:708–725. doi: 10.1055/a-0576-6589. [DOI] [PubMed] [Google Scholar]
- 33.Cost-Effectiveness Analysis (CEA) Registry Center for the Evaluation of Value and Risk in Health. Tufts Medical CenterAvailable at:https://cevr.tuftsmedicalcenter.org/databases/cea-registry
- 34.Arias E, Xu J.United States Life Tables, 2017. National Vital Statistics Reports. 68;7Available at:https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_07-508.pdf [PubMed]
- 35.Hay J W, Smeeding J, Carroll N V et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report -- Part I. Value Health. 2010;13:3–7. doi: 10.1111/j.1524-4733.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 36.Red Book - Healthcare Drug Pricing Resource. Micromedex 2.0. Truven Healthn Analytics, Inc. 2016Available at:https://www.micromedexsolutions.com/
- 37.CPI Consumer Price IndexAvailable at:https://www.bls.gov/cpi/data.htm
- 38.Doubilet P, Begg C B, Weinstein M C et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 39.Claxton K, Sculpher M, McCabe C et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14:339–347. doi: 10.1002/hec.985. [DOI] [PubMed] [Google Scholar]
- 40.Locke G R, 3rd, Talley N J, Fett S L et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 41.El-Serag H B, Sweet S, Winchester C C et al. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–880. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ip S, Tatsioni A, Conant A.Predictors of clinical outcomes following fundoplication for gastroesophageal reflux disease remain insufficiently defined: a systematic review Am J Gastroenterol 2009104752–758.; quiz 9 [DOI] [PubMed] [Google Scholar]
- 43.Morgenthal C B, Lin E, Shane M D et al. Who will fail laparoscopic Nissen fundoplication? Preoperative prediction of long-term outcomes. Surg Endosc. 2007;21:1978–1984. doi: 10.1007/s00464-007-9490-7. [DOI] [PubMed] [Google Scholar]
- 44.Chan W W, Haroian L R, Gyawali C P. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–2949. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 45.Patel A, Sayuk G S, Kushnir V M et al. GERD phenotypes from pH-impedance monitoring predict symptomatic outcomes on prospective evaluation. Neurogastroenterol Motil. 2016;28:513–521. doi: 10.1111/nmo.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell R C, Mavrelis P G, Barnes W E et al. A prospective multicenter registry of patients with chronic gastroesophageal reflux disease receiving transoral incisionless fundoplication. J Am Coll Surg. 2012;215:794–809. doi: 10.1016/j.jamcollsurg.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Trad K S, Barnes W E, Simoni G et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO Randomized Clinical Trial. Surg Innov. 2015;22:26–40. doi: 10.1177/1553350614526788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter J E, Kumar A, Lipka S et al. Efficacy of laparoscopic Nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. 2018;154:1298–1308 e7. doi: 10.1053/j.gastro.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 49.Du X, Hu Z, Yan C et al. A meta-analysis of long follow-up outcomes of laparoscopic Nissen (total) versus Toupet (270 degrees ) fundoplication for gastro-esophageal reflux disease based on randomized controlled trials in adults. BMC Gastroenterol. 2016;16:88. doi: 10.1186/s12876-016-0502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


