Abstract
While pharmacological treatment with methylphenidate (MPH) is a first line intervention for ADHD, its mechanisms of action have yet to be elucidated. We here seek to identify the white matter tracts that mediate MPH’s effect on beta oscillations. We implemented a double‐blind placebo‐controlled crossover design, where boys diagnosed with ADHD underwent behavioral and MEG measurements during a spatial attention task while on and off MPH. The results were compared with an age/IQ‐matched control group. Estimates of white matter tracts were obtained using diffusion tensor imaging (DTI). Via a stepwise model selection strategy, we identified the fiber tracts (regressors) significantly predicting values of the dependent variables of interest (i.e., oscillatory power, behavioral performance, and clinical symptoms): the anterior thalamic radiation (ATR), the superior longitudinal fasciculus (“parietal endings”) (SLFp), and superior longitudinal fasciculus (“temporal endings”) (SLFt). ADHD symptoms severity was associated with lower fractional anisotropy (FA) within the ATR. In addition, individuals with relatively higher FA in SLFp compared to SLFt, led to stronger behavioral effects of MPH in the form of faster and more accurate responses. Furthermore, the same parietotemporal FA gradient explained the effects of MPH on beta modulation: subjects with ADHD exhibiting higher FA in SLFp compared to SLFt also displayed greater effects of MPH on beta power during response preparation. Our data suggest that the behavioral deficits and aberrant oscillatory modulations observed in ADHD depend on a possibly detrimental structural connectivity imbalance within the SLF, caused by a diffusivity gradient in favor of parietal rather than temporal, fiber tracts.
Keywords: ADHD, methylphenidate, meg, oscillations
Short abstract
Impact Statement
We combine MEG and DTI data from ADHD children and matched controls, to uncover the link between structural and oscillatory features of attention and understand the mechanisms of action of stimulants (methylphenidate). We identify the main white matter tracts involved in ADHD symptomatology, and show that frontoparietal diffusivity predicts responsiveness to methylphenidate in the brain.
1. INTRODUCTION
The neural mechanisms underlying selective attention processes are contingent upon a complex interaction of brain networks. Prior studies using electro‐ and magneto‐encephalography (EEG/MEG) have provided insight on the diverse oscillatory patterns indexing distractor suppression and target processing. These oscillatory patterns are modulated in anticipation of visual stimuli, when participants prepare visual processing and responses by specifically allocating attentional resources in order to meet task demands (Herring et al., 2015; Tzagarakis et al., 2015; van Ede et al., 2012, 2017). One important function is the preparation of the motor action in response to a cued target (e.g., button press), which interacts with visual attentional allocation as well as working memory (Freek van Ede et al., 2019). The electrophysiological correlate underlying this behavioral component is reflected by a suppression of oscillations in sensorimotor areas. Specifically, modulation of brain activity in the beta band (15–30 Hz) has been associated with top‐down control of motor preparation, reflecting an interaction between cognitive and motor functions, which has been interpreted as increased excitability of task‐relevant brain areas (Berger et al., 2018; Doyle et al., 2005). This motivates the investigation of brain oscillations during attention tasks in relation to disorders associated with attentional problems.
Attention deficit‐hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by age‐inappropriate levels of inattention and hyperactivity‐impulsivity (American Psychiatric Association, 2013). Aberrant modulation of oscillatory activity has indeed been observed in individuals identified with ADHD, both in adults (ter Huurne et al., 2013, 2017, 2018) and pediatric populations (Arns et al., 2018; Lenartowicz et al., 2018; Vollebregt et al., 2016), with the latter representing the most prominent line of research, given the predominance of the disorder in early life (Polanczyk et al., 2010; Scahill & Schwab‐Stone, 2000). Weaker suppression of beta‐band activity (13–30 Hz) has been found in ADHD, prior to response preparation to a cued target (Mazaheri et al., 2014), reflecting a lack of appropriate motor planning. Furthermore, recent studies report on weakened beta modulation during working memory encoding in ADHD (Zammit & Muscat, 2019) consistent with the view that modulation of beta oscillations reflect cognitive functions (Engel & Fries, 2010; Richter et al., 2018).
Previously, we showed that beta depression in preparation to responses to a cued target is reduced in children with ADHD as compared to matched TD peers (Mazzetti et al., 2020). Additionally, in a double‐blind randomized placebo‐controlled design within the ADHD group we showed that MPH restores levels of beta depression in children with ADHD, by normalizing its values to the ones observed in the TD group. This effect might be caused by psychostimulants modulating the catecholamines' levels in the midbrain (Faraone, 2018). Specifically, MPH blocks the reuptake of norepinephrine and, particularly, dopamine at the synaptic cleft (Devilbiss et al., 2006; Hodgkins et al., 2012; Tang & Dafny, 2013) increasing their availability. A dopaminergic imbalance within networks mediated by the prefrontal cortex has indeed been proposed to underlie symptoms of attentional deficits and hyperactivity (Jarczok et al., 2019; Zhong et al., 2016). Consistently, the modulatory action of dopamine is alleged to mediate the interaction between frontoparietal and default mode attentional networks (Dang et al., 2012).
The link between beta oscillations and brain structural connectivity remains to be elucidated. This can be achieved by means of diffusion tensor imaging (DTI), which estimates the direction of diffusion of water molecules in the brain and is thought to reflect the underlying microstructural properties of white matter fiber tracts (Alexander et al., 2007; Chang et al., 2017). Among the DTI‐derived metrics is fractional anisotropy (FA), reflecting coherence of diffusion of water along the main tract direction (Assaf & Pasternak, 2008; Le Bihan et al., 2001) and, allegedly, the underlying tissue microstructure, such as integrity of myelin sheath, which impacts the overall mobility of water along axons (Beaulieu, 2002). DTI studies in ADHD have so far pointed to reduced white matter integrity across the brain (Lei et al., 2014; Onnink et al., 2015; van Ewijk et al., 2012). Diffusion in the angular bundle of the cingulum correlates with hyperactivity‐impulsivity scores in ADHD, suggesting the cingulum may play an important role in symptom severity and remission of (C. Damatac et al., 2020). Importantly, the superior longitudinal fasciculus, a white matter tract reported to be involved in sustaining spatial attention (de Schotten et al., 2011; Marshall et al., 2015), has been consistently implicated in relation to ADHD. In particular, lower integrity (FA) along this bundle has been associated with severity of ADHD symptoms and behavioral performance on cognitive tests (Konrad et al., 2010; Witt & Stevens, 2015; Wolfers et al., 2015). Taking advantage of the spatial resolution offered by MEG and the link to microstructural connectivity properties offered by DTI, this study aims to investigate the association between oscillatory and structural features in relation to attentional impairments in children with ADHD and typically developing (TD) children. We hence coupled the previously reported electrophysiological results (Mazzetti et al., 2020) with an analysis of white matter microstructural properties in the same subjects.
2. MATERIALS AND METHODS
A detailed explanation of participants' inclusion and exclusion criteria, attentional task, and study design has been previously described (Mazzetti et al., 2020) and can be found in Supplementary Materials.
2.1. Participants
The study included 27 children diagnosed with ADHD and 27 typically developing (TD) male children. A total of 10 children (9 ADHD) withdrew from the experiment after at least one session, due to unwillingness to proceed and/or excessive complications during testing. As a result, structural and diffusion‐weighted MRI scans and MEG data were acquired for a total of 49 (26 TD) and 46 participants (26 TD), respectively.
2.2. Experimental design
The study design is outlined in Figure 1. Overall, children in the ADHD group visited the lab three times, while children in the TD group twice. During the first intake‐session, participants from groups underwent behavioral and IQ testing. For the ADHD group, a further in‐depth intake was conducted to determine the medication dosage to be used during the task, based on operating procedures followed in prior studies (Linssen et al., 2014). Based on the screening, a standardized dosage was chosen (either 10 or 15 mg methylphenidate immediate release; IR‐MPH). Following the behavioral screenings, the MRI session took place for a duration of ~30mins.
FIGURE 1.
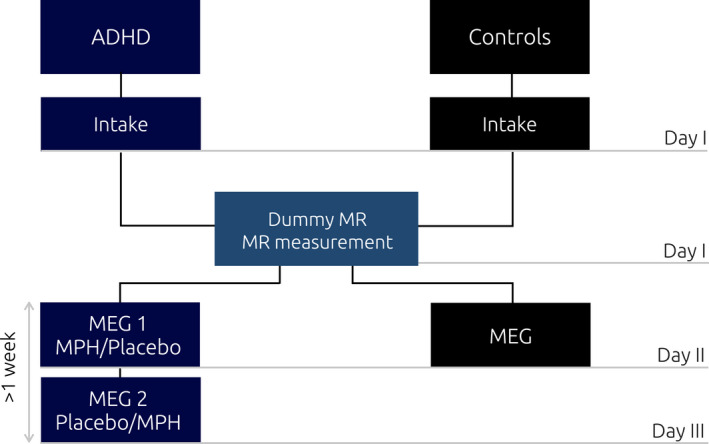
Experimental study design. aCase report form and behavioral test (line bisection task, WISCIII vocabulary and block design subscales, ADHD rating scale, CBCL); bpsychiatric intake (basic medical screening and dosage determination); cPolhemus digitizer; dmedication intake (1 hour prior to the beginning of experimental task); participants in the ADHD and controls group visited the laboratory three and two times, respectively. During the first visit (day I) the psychiatric assessment took place, which determined participants' suitability for the study. The same day, the dummy MR took place, followed by the actual MR scan. The second day (day II) both groups performed the attentional task while electromagnetic activity was recorded with the MEG. The ADHD group performed the task twice (day II and day III), once upon administration of MPH, and once upon administration of a placebo pill. This was done according to a randomized crossover design, half of the participants received the MPH on day II, while the other half on day III
During the second visit, both groups undertook the MEG testing. For the TD group, this constituted the last day of testing, while for the ADHD group, two MEG sessions were planned at two different visits, separated by at least 1‐week interval. The ADHD group performed the MEG task twice, that is, under two conditions (MPH and placebo), according to a double‐blind placebo controlled randomized design. Prior to each MEG session, a 24‐hour treatment suspension allowed to control for withdrawal symptoms related to drug administration (rebound effect) (Carlson & Kelly, 2003). MEG testing began 1 hour after medication intake, allowing to reach on average moderate plasma concentration (C max) of the drug along the experiment, which progressively increases and reaches its peak around the second hour post‐intake (Quinn et al., 2007). After completion of the MEG session, participants proceeded with their own regular stimulant.
2.3. The attention task
The task was presented as a child‐friendly adaptation of a Posner’s cueing paradigm for spatial orienting of attention, where a central cue (represented with a clown fish looking either at the left or at the right side of the screen) indicated the upcoming position of a target (a shark with an open mouth) in 80% of the trials (80–20% valid‐invalid trials). Participants were asked to indicate via button press the position of the target, while ignoring the distractor on the opposite screen side (shark with mouth closed) (see Figure 2 ).
FIGURE 2.
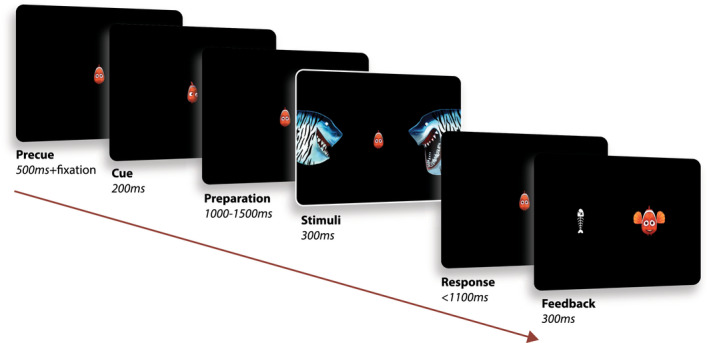
Attentional task. (Adapted from Mazzetti et al., 2020). The paradigm consisted in a child‐friendly adaptation of a Posner cueing paradigm for the study of spatial orienting of attention. Each trial (370 in total) began with the presentation of a fish in the middle of the screen, serving as fixation cross. An eye tracker ensured that the children kept proper fixation throughout the whole trial (as trials were stopped in case the subject performed a saccade). A cue was then presented for 200 ms, represented by the fish looking either at the left or right side of the screen (cue side equally distributed across trials). After a preparation interval jittered in the range 1100–1500 ms, the stimuli were then presented for 300 ms, on the two sides of the screen. The child was asked to respond, via button press, indicating the position of the target (shark with an open mouth), while ignoring the distractor on the other side (shark with mouth closed). A positive feedback (happy fish) was then presented if a correct answer was provided within the response interval (1100 ms). In case of wrong or no response, a negative feedback was presented (fish bone)
The experiment consisted of 370 trials, equally divided in 10 blocks, after which the participant was given the possibility to take a break and/or talk to the parents. Note that, for the ADHD group, the treatment order for the two MEG sessions was randomized across participants.
2.4. MEG data acquisition and analysis
Electromagnetic brain activity was recorded from the participants seated in a CTF 275‐sensor whole‐head MEG system with axial gradiometers (CTF MEG Systems, VSM MedTech Ltd.). Head position was monitored throughout the experiment via online head‐localization software, allowing, if necessary, readjustment of the participant’s position between blocks. Complete illustration of the steps followed for the analysis of power and beta oscillatory indices of interest, can be found in our previous article (Mazzetti et al., 2020) and are further described in the Supplementary Materials.
Importantly, in the above study, we report how the preparation to motor response to the cued target was accompanied by a desynchronization of beta oscillations in the MEG data at central sensors. For each subject, a Beta Preparation Index (PI[β]) was hence computed by considering the average beta band modulation over the sensors and time window of interest (f = 15–30 Hz, −1000 < t < 0 ms).
| (1) |
To estimate MPH modulatory effects exerted on beta preparation, we considered the difference in PI(β)) between MPH drug and placebo in the ADHD sample, referred to as ΔPI(β):
As corollary of Equation 1, the higher the ΔPI(β) value, the stronger the beta depression in response to MPH intake.
2.5. Structural data analysis
MRI and DTI data were acquired via a 3 T MAGNETOM Skyra MR scanner (Siemens AG, Healthcare Sector, Erlangen, Germany) with a product 32‐channel head coil. The protocol included a T1‐weighted MRI scan for anatomical reference and analysis and diffusion‐weighted MRI scans for performing fiber tractography.
Anatomical and DTI images were analyzed in FreeSurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/). The TRACULA toolbox (Tracts Constrained by Underlying Anatomy; Yendiki et al., 2011) was implemented for preprocessing of DWI images and for subsequent delineation of 18 major white matter tracts (eight bilateral and two interhemispheric): corticospinal tract (CST), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UNC), anterior thalamic radiations (ATR), cingulum‐cingulate gyrus bundle (CCG), cingulum‐angular bundle (CAB), superior longitudinal fasciculus‐parietal terminations (SLFP), superior longitudinal fasciculus‐temporal terminations (SLFT), and corpus callosum forceps major and minor (Fmaj, Fmin). TRACULA allows the automated reconstruction of major white matter pathways based on the global probabilistic approach described in (Jbabdi et al., 2007), and further extends it by incorporating anatomical knowledge in the prior probability function: each resulting segmented tract is the best fit not only given the observed diffusion data within each subject, but also given its similarity to the known tract anatomy in relation to gray matter segmentations from FreeSurfer.
The preprocessing steps implemented within TRACULA included: standard image distortion corrections (eddy current compensation, head motion correction), intra‐subject registration (individual DWI to individual T1), inter‐subject registration (individual T1 affine registration to the MNI template space), creation of cortical masks (parcellation via probabilistic information estimated from a manually labeled training set based on Desikan‐Killiany Atlas, Figure 3) and white‐matter masks (based on [44]), tensor fitting for extraction of tensor‐based measures, computation of anatomical priors for white‐matter pathways reconstruction (e.g., diffusion eigenvectors, eigenvalues, and FA for each voxel). Next, bedpostx was applied with a two‐fiber, ball‐and‐stick model to estimate the distributions of the diffusion parameters and create the input for probabilistic tractography (Behrens et al., 2007).
FIGURE 3.
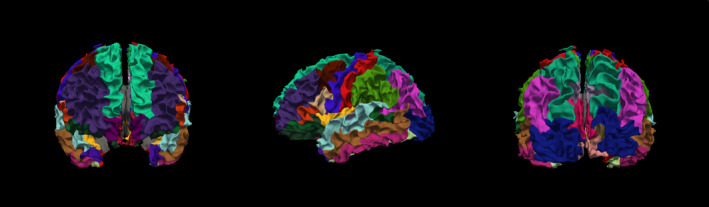
3D Rendering of cortical parcellation based on Desikan‐Killiany atlas in one sample TD subject. A cortical parcellation was generated prior to tract estimation, which are combined with prior distributions on the neighboring anatomical structures of each pathway and subcortical segmentation to constrain the tractography solutions (obviating the need for user interaction thus automating the process)
See Figure 4 for an example of TRACULA’s output in a single healthy TD participant, showing the posterior distribution for all the white matter pathways included in the segmentation pipeline. Each participant’s scan was then registered to standard MNI space for group‐level analyses. A pairwise correlation matrix for bilateral structures is presented in Figure 5, showing that no negative association was present between tracts’ FA values.
FIGURE 4.
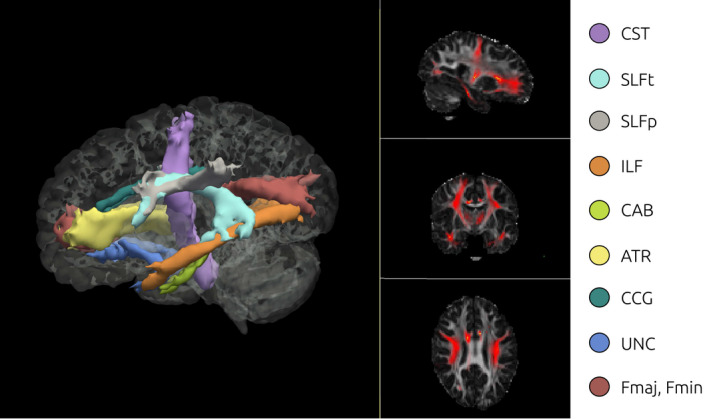
3D Isosurface rendering and 2D orthographic view of tract reconstructions in one sample subject obtained using TRACULA. Visualization of the probability distributions of all white‐matter pathways simultaneously overlaid on 4D brain mask. All 18 tracts are displayed at 20% of their maximum threshold
FIGURE 5.
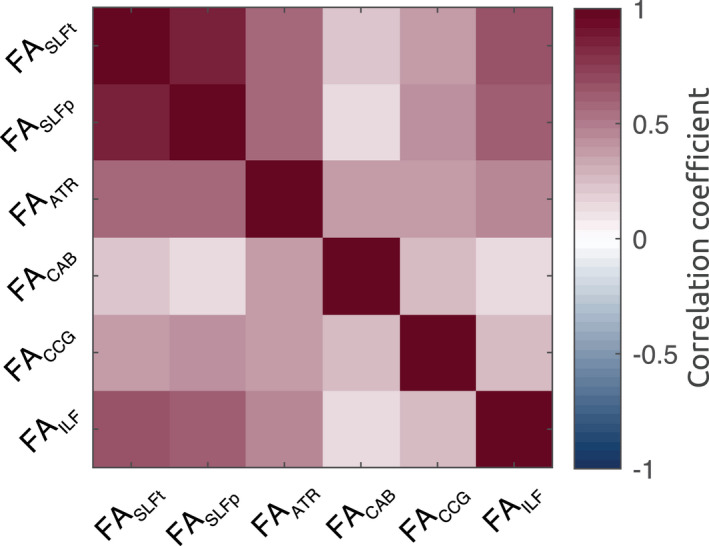
Correlation matrix of FA across bilateral white matter structures shows that no negative association subsists between segmented tracts
All statistical analyses were performed in MATLAB2019a. For all analyses, the main metric of interest was mean FA within the tracts.
2.5.1. Model selection of white matter ROIs
In order to determine the relationship between mean FA along the white matter tracts segmented, as well as electrophysiological and behavioral measures, we implemented a general linear mixed model (mdl) specifying the FA values of the white matter structures as regressors for the prediction of each index of interest.
Consistent with the methods implemented in previous work (Mazzetti et al., 2019), and given the relative heterogeneity of current results in the field (Beare et al., 2017; Onnink et al., 2015), we implemented a data‐driven strategy aimed at selecting the optimal set of or regressors to be included in the model, via a stepwise model estimation strategy, commonly implemented in model selection (Fabozzi et al., 2014; Hastie et al., 2009).
We started by focusing on the behavioral performance, to constrain the optimal model, hence setting IESs as dependent variable. Next, we considered all possible combinations of 2 to 5 regressors, reflecting the FA values of the bilateral tracts segmented with TRACULA: SLFt, SLFp, ATR, CAB, CCG, and ILF (in addition to the model including all six regressors, referred to as the “full model”, i.e., mdl[0]). We hence separately considered the general linear mixed models (maximum likelihood estimation) derived from all possible combinations of n regressors, that is, including either 2, 3, 4, or 5 regressors (i.e., ROIs), by computing all possible unique permutations of n regressors from the subset defined. This resulted in a set of models for each of the four possibilities using 2, 3, 4, or 5 regressors. Next, for each of these subsets, we derived the model associated with the lowest Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), highest log likelihood (where the winning model would be the one associated with at least two highest criteria compared to the others in the same model subset). These values have been commonly used in model selection to identify the best predictor subsets for a statistical model (Ward, 2008). Upon selection, we ended up with the five “best” models, representative of each of the four model options described above.
In a final step, we identified the “winning model” among the four selected ones (based on the same criteria as the previous selection) and compared it with the following full model (mdl[0]) based on six regressors:
| (mdl0) |
where Group is a categorical variable describing the subjects' group (ADHD vs. TD).
We then tested how the winning model could account for behavioral performance (IES) (mdl[1]), ADHD symptoms (ADHD‐RS) (mdl[2]), beta modulation (PI(β)) (mdl[3]), and modulation of drug‐ related beta oscillatory response (ΔPI(β)) (mdl[4]). For each of the following derived models, beta coefficients and adjusted response plots were shown. The latter describing the response of the dependent variable of interest (e.g., IES) as a function of one predictor (e.g., FASLFT), while controlling the effects of all other predictors on both variables (i.e., all the other regressors in the model)).
3. RESULTS
While a total of 22 ADHD and 27 TD children underwent the MR session, only a smaller group of 18 ADHD and 26 TD successfully completed both MEG sessions (see Figure 2 for the task). In the following section, we report the results of the latter group, where we quantified the association between white matter tracts microstructure, behavioral performance, and MEG‐derived measures. The bigger sample was considered for the association between diffusion weighted imaging (DWI) results and ADHD symptoms score.
3.1. Behavioral performance
Behavioral performance in the ADHD group significantly improved following MPH administration (t[17] = 2.49, p = .023), as reflected by lower inverse efficiency scores (IESs: accuracy/reaction time). No significant difference in IES was found between TD and ADHD: the TD group did not perform better when compared to the ADHD group in the Placebo (t[42] = −.22, p = .827) nor in the MPH condition (t[42] = .77, p = .445).
3.2. Beta desynchronization in preparation to response to the cued target
As depicted in Figure 6, ADHD subjects in the placebo conditions exhibited lower overall beta depression, which was restored following MPH intake. This was observed as a diminished PI(β)s with values closer to those observed in the TD group (see Supplementary Methods and Materials). We will here focus on the association between beta modulation and white matter microstructural diffusion properties.
FIGURE 6.
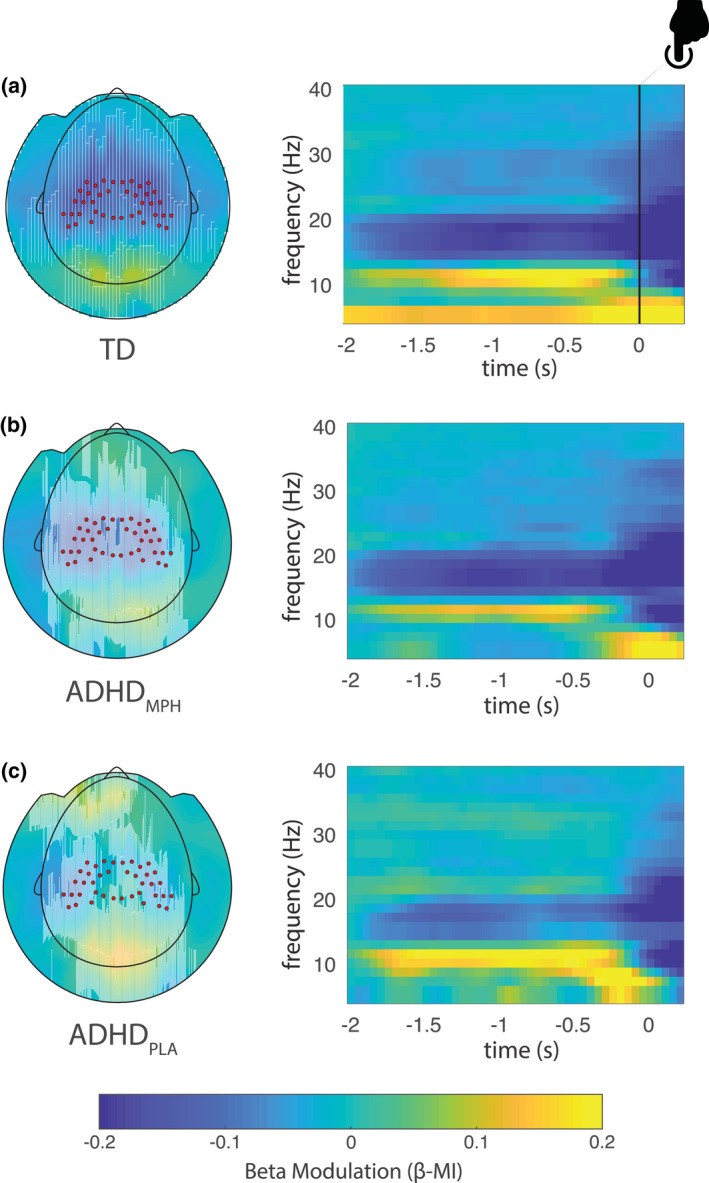
Beta modulation indices in the three conditions (adapted from (Mazzetti et al., 2020)). Topographic plot (left) and respective time frequency representations (TFRs) (right panel) of power modulation (β‐MI) for the typically developing group (TD) (a), ADHDMPH group (b), and ADHDPLA group (c). Red dots superimposed on the topographies denote sensors of interest as defined in Figure 4a. Notably, beta preparation is stronger in the TD group, while progressively decreases in the ADHDMPH group, being weakest in the ADHDPLA group
3.3. Definition of white matter structures of interest: Model building strategy
Following the step‐wise model selection criteria described in the Materials and Methods section, we identified a winning model with three regressors as the best fit (AIC = −67.34 and BIC = ‐53.09, Log Likelihood = 42.67, Adjusted R2 = .19) defined by the regressors FASLFt, FASLFp, and FAATR. A 3D rendering of the three white matters tracts selected (SLFT, SLFP, and ATR) is illustrated in Figure 7. The abovementioned regressors were then considered as predictors for the following general linear models of interest, predicting respectively IES (mdl1), ADHD‐RS (mdl2), PI(β) (mdl3), and ΔPI(β) (mdl4).
FIGURE 7.
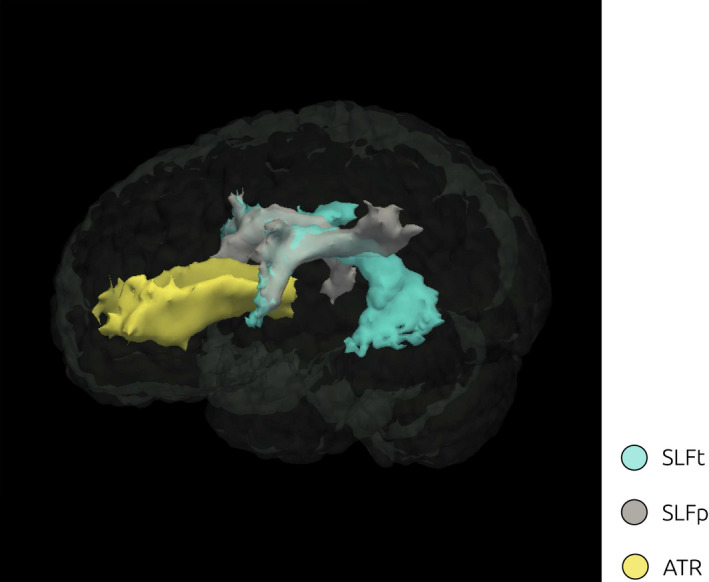
3D Rendering of white matter ROI. Visualization of the probability distributions of the ROIs identified as tracts of interest according to the model selection approach: SLFp, SLFt, and ATR
We first enquired whether FA along these tracts differed between groups. We hence used a two‐way unbalanced ANOVA with factors “tract” (ATR, SLFp, SLFt) and “group” (“TD”, “ADHD”) in relation to FA values. While the results yield a main effect of group (p = .009), showing a group difference in FA across all tracts, and tract (p = 4.8 × 10−13), reflecting a difference in FA between tracts, no significant interaction group by tract emerged (p = .453), indicating that the pattern of FA across tracts was similar across groups.
3.4. Functional anisotropy of the superior longitudinal fasciculus relates to behavioral performance
We considered task performance in relation to the three identified tracts of the winning model. Beta coefficients and adjusted response plots (i.e., showing the response as a function of one predictor, averaged over the others) derived from the model are shown in Figure 8: SLFt and SLFp were associated with beta coefficients of opposite sign: while higher FA values in the SLFt were associated with a higher IES (p = 7 × 10−4), that is, worse performance (Figure 8b), a higher FA in the SLFp accounted for lower IES (p = .003), that is, better behavioral performance (Figure 8c). A significant interaction term furthermore emerged for FASLFt and FASLFp with Group (p = .002 and p = .023, associated with a negative and positive coefficient, respectively). A negative interaction term of FASLFt with Group indicates that the effect of FASLFt on IES for the TD group was relatively weaker than for the ADHD group. Vice versa, a positive interaction term of FASLFp with Group, denotes that the effect of FASLFp for the TD group was relatively stronger than for ADHD. On the other hand, the association between FAATR and behavioral performance did not reach statistical significance (p = .068).
| (mdl1) |
FIGURE 8.
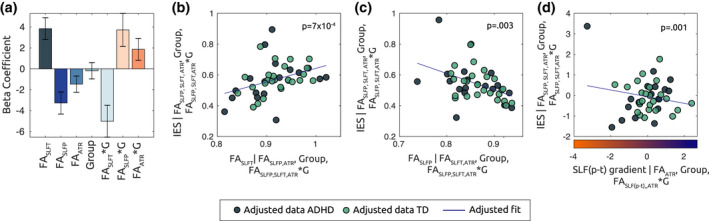
FA In SLFp and SLFt predict behavioral performance in the task. (a) The bar plot displays the beta coefficients associated with the linear mixed model mdl(1), where mean FA values within the identified tracts of interest are set as explanatory variables for behavioral performance, as indexed by the IES. Error bars indicate standard error of the mean. The adjusted response plots in (b) and (c) show, respectively, the behavioral performance (IES) as a function of the FASLFt and, FASLFp, while averaging over other regressors in the model in (a). (d) Adjusted response plot displaying the association between IES and parietotemporal gradient SLF (p–t), averaged over the residual regressors (mdl(1.a). Positive SLF (p–t) values indicate higher FA along parietal as compared to temporal endings within the SLF
Given the strong association between the two regressors FASLFt and FASLFp (r = .86, p = 2 × 10−4; see correlation matrix in Figure 5) and based on the high degree of overlap between the two reconstructed tracts (as they overlapped anteriorly; Figures 4 and 7) more analysis was required to determine how SLFp and SLFt related to performance. We hence computed a measure describing the parietal‐to‐temporal SLF imbalance, according to:
| (3) |
As a result, a given subject would display a specific degree of parietal‐to‐temporal diffusivity (i.e., imbalance) along the SLF tract: A higher value of the imbalance reflects a stronger diffusivity at parietal locations along the tract, and a lower value along the gradient reflected a stronger diffusivity at temporal locations.
We therefore considered a model specifically incorporating the parietotemporal imbalance:
| (mdl1a) |
Figure 8d shows the association between IES and FASLF(p–t). As a corollary of Equation (3), the negative significant partial association (p = .001) shows that subjects with a higher parietal than temporal FA along the SLF tract, were also the ones with a better behavioral performance. Also in this model, a significant interaction (p = .006) emerged between tract*Group, which reflected that the effect of FASLF(p–t) on IES was stronger for the TD group compared to the ADHD.
3.5. Fractional anisotropy of the ATR predicts ADHD symptoms
In order to assess whether the FA values of the white matter ROIs were related to symptoms (as measured by ADHD‐Rating Scale; ADHD‐RS), we considered the full sample of participants. We included symptoms across both the TD and the ADHD (placebo condition) group, hence embracing the notion that ADHD symptomatology derives from a “spectrum”, rather than from a dichotomous distinction with TD peers. The following model was then applied:
| (mdl2) |
The resulting model was significant (R2 = .22, p = .01, Figure 9a), and FAATR was associated with a significant partial coefficient (p = .007) showing a negative relationship with ADHD symptoms (Figure 9b,c): higher FA in the ATR predicted an overall lower ADHD symptomatology. FASLFp and FASLFt did not show a significant partial correlation with symptoms score (p = .494 and p = .309, respectively).
FIGURE 9.
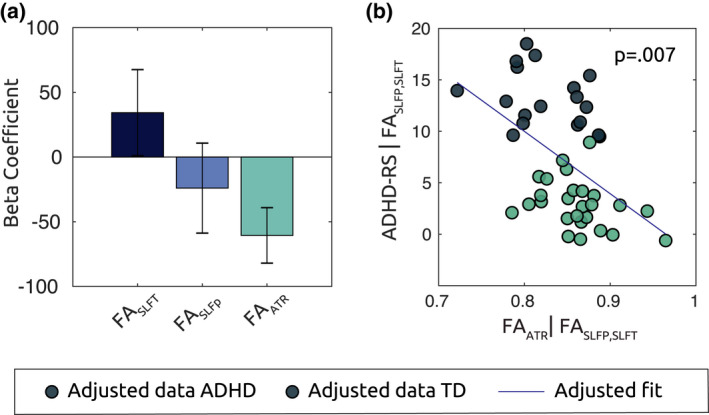
FA In ATR predicts ADHD symptoms severity in all subjects. (a) Bar plot shows beta coefficients associated with mdl(2), where mean FA values along the tracts of interest are defined as predictors for ADHD‐RS symptoms score in all subjects. FAATR is associated with a significant partial regression coefficient (p = .007). Scatter plot in (b) show the adjusted response plot of symptoms as a function of FAATR, controlling for the variance explained the other predictors. Lower diffusivity along the ATR corresponded to higher average ADHD symptomatology along the spectrum
3.6. Parietotemporal gradient along the SLF reflects methylphenidate’s effect on preparatory beta depression
Finally, we sought to investigate the relationship between FA values in the selected ROIs, and the patterns of MPH‐associated beta depression (PI(β)). As described in (Mazzetti et al., 2020), MPH intake normalized aberrant beta depression in ADHD, initially lower as compared to controls. In a first model, we aimed at identifying whether a combination of FA values in the selected ROIs accounted for the degree to which subjects were able to suppress their somatosensory beta power in preparation to a motor response (PI(β)). To this end, we considered PI(β)s for TD subjects and ADHD subjects in the placebo condition as dependent variable in the following model:
| (mdl3) |
The resulting overall model was not significant (p = .762), neither were the main nor the interaction effects in the regressors (FASLFt: p = .890; FASLFp: p = .985; FAATR: p = .754, FASLFt*Group: p = .934, FASLFp*Group: p = .689, FAATR*Group: p = .655).
Next, given the effects of MPH on modulations of the beta oscillations within the ADHD group, we enquired whether, instead, a linear combination of the ROIs' FA properties, could explain the changes in beta depression following medication intake (ΔPI(β)). We hence implemented the following model, consistently with the principles abovementioned:
| (mdl4) |
Here, we added the term mgMPH as a categorical variable to control for the dosage of MPH administered prior to the task (15/10 mg). The resulting model was significant with R2 = .83 and p = .003 (Figure 10a). Analyses of main effects showed a significant negative association between FASLFt and ΔPI(β) (b 1: p = .001) (Figure 10b), and a positive association between FASLFp and ΔPI(β) (b 2: p = 1 × 10−4) (Figure 10c), while no main effects for FAATR and mgMPH were found (p = .910 and p = .693, respectively).
FIGURE 10.
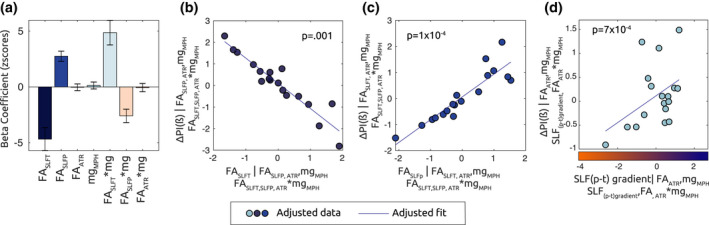
FA In SLFp and SLFt predict MPH effects on β depression in the ADHD group. (a) Bar plot shows beta regression coefficients associated with mdl(4), where mean FA values along the tracts of interest are defined as predictors for changes in beta modulation due to medication intake (ΔPI(β)). According to equation (2), a higher ΔPI (β) value for a given ADHD subject reflects bigger changes in depression of beta oscillations following medication intake. (b) Adjusted response plot showing ΔPI (β) as a function of FASLFt. A significant beta coefficient (p = .001) reveals that FASLFt predicted lower changes in β power depression due to MPH. (c) Adjusted response plot showing ΔPI(β) as a function of FASLFp. Higher FASLFp corresponded to stronger β depression changes following MPH intake (p = 1 × 10–4). (d) Adjusted response plot illustrating ΔPI (β) as a function of parietotemporal SLF gradient according to mdl(4.a). Higher SLF (p–t), for a given ADHD subject, reflected higher diffusivity at parietal endings, as compared to temporal endings, of the SLF. The significant positive relationship (p = 7 × 10–4) suggests that the gradient of parietotemporal diffusivity in the SLF is predictive of the effects of MPH on β depression
The main effects in the model denoted a bigger MPH effect for subjects displaying higher FA in the SLFp, while subjects displaying higher FA in the SLFt were the ones whose sensorimotor beta was less affected by MPH administration.
Although the model produced significant interaction terms between FASLFt and FASLFp with mgMPH (p = .001 and p = .002, respectively), we did not pursue any post hoc examination of such effect, given a very low ratio between 10/15 mg dosage in the sample (0.38) would not produce statistically reliable results.
According to the same principle which led to mdl(1.a), we merged the two regressors FASLFp and FASLFt into the gradient denoted as FASLF(p–t), and considered an equivalent to mdl(4) as follows:
| (mdl4a) |
In Figure 10d we present an alternative and equivalent representation of the plot in Figure 10b,c, where the linear association between ΔPI(β) and FASLF(p–t) is presented. Here, we can observe a significant positive partial association (p = 7 × 10−4) which indicates that subjects with a higher parietal compared to temporal FA along the SLF tract, were also the ones whose beta depression was more affected (enhanced) by MPH.
3.7. Influence of head motion parameter estimates on FA values of interest
Linear regression models were fitted to the data in order to further evaluate the influence of residual head movement artifacts (translation and rotation) on FA values.
Three separate models were used to account for the effects of motion artifacts on FA of different tracts (SLFt, SLFp, and ATR), while translation and rotation estimates and their interaction with Group variable(ADHD/Controls) were specified as regressors, according to:
While linear regression models in relation to FASLFp and FASLFt were not significant (p = .325, p = .471, respectively), the model associated with FAATR reached significance (R2adjusted = .23; p = .027, Bonferroni corrected). When looking at individual partial regression coefficients, we identified a significant interaction between Group*translation in the control group (p = .015). These results indicate that higher head translation estimates were predictive of higher FA values of the ATR in the control group, but not in the ADHD group. On the other hand, when controlling for interaction with the group variable, head motion estimates parameters not associated with estimated FA values of SLFp and SLFt.
4. DISCUSSION
In the current study, we employed DTI to estimate microstructural properties of main bilateral fasciculi and explored their role in mediating behavior and beta power modulation associated with ADHD symptomatology. We showed that, in both groups, lower values of fractional anisotropy within the ATR were related to ADHD symptom severity and that parieto‐to‐temporal FA‐imbalance within the SLF accounted for behavioral performance in the attentional task. Importantly, in the ADHD group, the same SLF gradient was predictive of the effects of MPH on beta power modulation.
The ATR originates from anterior and medial nuclei of the thalamus and radiates along the anterior thalamic peduncle and the anterior limb of the internal capsule to reach the frontal cortex (George & Das, 2019; Jones, 2002). The thalamocortical feedback loop is crucial in conscious processing, and its role in attention is to provide a functional link between otherwise structurally segregated cortical areas, supporting different aspects of attentional selection, and working memory (Halassa & Kastner, 2017; Sherman, 2016). Prior morphological analyses have shown that ADHD is associated with altered shape and volume of thalamic nuclei, whose connections within several cortical regions, particularly frontal areas, seem aberrant in children with ADHD (Svatkova et al., 2016; Xia et al., 2012). On the other hand, the role of thalamus in the etiology of the disorder is controversial, given that case–control volumetric differences in this area were not confirmed in a recent meta‐analysis (Hoogman et al., 2017). The meta‐analysis did provide evidence for a different influence of age on thalamic volumes between clinical and nonclinical samples. The thalamus is a complex structure composed of a set of cytoarchitectonically segregated nuclei, each providing specific thalamocortical signals and relying on partially independent neural circuitry to mediate different cognitive functions. Hence, a more detailed investigations of thalamic involvement in the pathophysiology of ADHD should take into account and specific analysis of such diverse nuclei (Battistella et al., 2017), so far still absent in the ADHD literature. Arguably, currently the most convincing effects with regard to thalamus role in ADHD, are found in the context of connectivity studies, pointing to the importance of fronto‐striatal circuits and their disruption in association to the symptomatology (Cupertino et al., 2019). Here, we corroborated these findings by embracing a different approach: we investigated the association between thalamocortical diffusivity and clinically assessed symptoms on the basis of individual differences in FA values. In other words, our results speak to the importance of thalamocortical connections in mediating the interactions between attention and premotor functions which, if anomalous, can account for some of the behavioral symptoms associated with ADHD.
We postulate that the strong interconnections between the thalamus and striatal regions is one of the core variables to consider when aiming at identifying aberrant structural changes associated with ADHD. Reinforcing this finding, the TD group in our study displayed stronger anisotropic diffusivity along the ATR as compared to the ADHD group. This finding further highlights the role of the basal ganglia and their morphological and volumetric differences as potential predictors of the disorder (Aylward et al., 1996; Hoogman et al., 2017; Oldehinkel et al., 2016; Paclt et al., 2016; Qiu et al., 2009; Sethi et al., 2017; Von Rhein et al., 2016).
The second main and novel finding related the parieto‐to‐temporal FA‐imbalance within the SLF with behavioral performance in the attentional task (in both groups): a higher FA in the parietal‐SLF compared to the temporal‐SLF predicted faster and more accurate responses in both ADHD patients and controls. Furthermore, in the ADHD patients group, the same gradient explained the effects of MPH on beta modulation: individuals displaying higher FA in the parietal than the temporal SLF were also the ones whose beta power during response preparation increased more with MPH. FA along the SLF likely reflects the functions of the frontoparietal control network (FPCN). This network is one of the core anatomical components providing the basis of flexible attentional adaptations to different task demands (Dixon et al., 2018). While the above results linking FA imbalance within the SLF to behavior and beta oscillations are seemingly orthogonal to the previous finding relating FAATR to symptoms severity, the former are not independent from thalamo‐frontal influences. Indeed, the dopaminergic regulation of the prefrontal cortex and the striatum has been proposed to mediate the interaction between the different attentional systems (Dang et al., 2012; Jenni et al., 2017; Tomasi et al., 2016), some of which structurally rely on the SLF.
Dopaminergic availability is modulated by MPH (Arnsten, 2006; Devilbiss et al., 2006; Faraone, 2018), which blocks the reuptake of the neurotransmitter, hence allegedly increasing the functional interactions within attentional networks. Given these premises, it is not surprising that diffusivity along the superior longitudinal fasciculus reflects the effects of MPH: a stronger connectivity, as indexed by anisotropy along the tract, promotes communication between frontoparietal areas, which is further maximized by the stimulant’s action. Crucially, we found that an imbalance of FA in favor of parietal rather than temporal regions is associated with stronger effects of medication on beta modulation. Important nodes of the FPCN are found in dorsolateral prefrontal cortex, frontal eye fields, and intraparietal sulcus (Xuan et al., 2016), regions that are connected by the dorsal fibers of the SLF (Makris et al., 2005). Previous studies have already proposed that the mechanisms of action of stimulant medication in ADHD are strongly reflected by its activity on frontoparietal regions (Kowalczyk et al., 2019; Zammit & Muscat, 2019), whose under activation is one of the neural correlates of the disorder (Cai et al., 2019; Chiang et al., 2015; Rubia et al., 2011).
It is important to consider the link between dopaminergic coordination of attentional networks and increased beta modulation. Evidence from Parkinson’s Disease (PD) patients has provided a theoretical framework according to which dopamine levels within the basal ganglia‐cortical loop have a direct influence on beta oscillations (Jenkinson & Brown, 2011; Moran et al., 2011). Anomalous beta oscillations are highly correlated with PD pathology (Cole et al., 2017), with evidence from in vivo recordings and mathematical models suggesting they originate from dopamine degeneration in cortical projections to the striatum (McCarthy et al., 2011; Pavlides et al., 2015; Wang et al., 2018). Coupled with findings of increased striatal dopamine transporter (thus lower dopaminergic availability) in ADHD (Hesse et al., 2009; Jucaite et al., 2005; Krause et al., 2000; Tai et al., 2019), we here propose an attentional network which relies on the dopaminergic input from thalamo‐striatal regions to the prefrontal cortex, which in turn mediates the activity along the FPCN. Higher FA along frontoparietal tracts tends to reflect increased connectivity and is being investigated as an indirect tool to infer dopaminergic functions in the pathological brain (Lenfeldt et al., 2017; Lorio et al., 2019; Shimony et al., 2018). Relatively stronger parietal FA may thus underlying a facilitatory mechanisms for MPH action in the brain, as revealed by a stronger increase in preparatory beta desynchronization in children displaying higher parietal‐to‐temporal FA gradient.
Of important notice when dealing with regression analyses on brain neuroimaging data, is the potential presence of collinearity between variables, which is expressed in our data between FA in SLFt and SLFp. One way to address collinearity is to linearly transform the collinear variables (Frost, 2020; Tomaschek et al., 2018): in our results, we make use of a sum‐difference ratio transformation to merge the two collinear variables into one factor (referred to as “gradient” in the context of this study). By doing so, we do not only control for the collinearity effect, but are also able to offer a more intuitive overview on the relationship between diffusion measures and MEG measures, compared to multiple single tract analyses.
Although limited in its sample size, this study offers important new insights on the potential of multimodal imaging to investigate and identify the sources of attention performance in the brain: by coupling evidence from electrophysiological measures and information about white matter integrity, we are able to investigate the origin of aberrant brain activity observed in ADHD and improve our understanding of the mechanisms of action of stimulant medication. Furthermore, it might encourage future research on the issue, clarifying whether FA represents a predictor of MPH responsiveness or it is rather one of the neural targets of medication, as could potentially be inferred by longitudinal research.
ACKNOWLEDGMENTS
Open Access Funding provided by Universite de Geneve. [Correction added on 24 May 2022, after first online publication CRUI funding statement has been added.]
DISCLOSURES
Jan K Buitelaar has been in the past 3 years a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Medice, Takeda/Shire, Roche, and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, and royalties. The remaining authors declare no competing and financial interests.
AUTHOR CONTRIBUTIONS
Cecilia Mazzetti: Conceptualization; data curation; formal analysis; investigation; methodology; validation; visualization; writing – original draft; writing – review and editing. Christienne Gonzales Damatac: Methodology; software. Emma Sprooten: Supervision; writing – review and editing. Niels ter Huurne: Conceptualization; supervision. Jan K Buitelaar: Funding acquisition; supervision. Ole Jensen: Conceptualization; funding acquisition; resources; supervision; writing – review and editing.
Supporting information
Supinfo
Mazzetti, C., Gonzales Damatac, C., Sprooten, E., ter Huurne, N., Buitelaar, J. K., & Jensen, O. (2022). Dorsal‐to‐ventral imbalance in the superior longitudinal fasciculus mediates methylphenidate’s effect on beta oscillations in ADHD. Psychophysiology, 59, e14008. 10.1111/psyp.14008
Funding informationThe authors gratefully acknowledge the support of the Marie‐Curie ITN grant ChildBrain (grant number 641652)
REFERENCES
- Alexander, A. L. , Lee, J. E. , Lazar, M. , & Field, A. S. (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (5th ed.) (A. P. Association & A. P. A. D.‐5 T. Force [eds.]). American Psychiatric Association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Arns, M. , Vollebregt, M. A. , Palmer, D. , Spooner, C. , Gordon, E. , Kohn, M. , Clarke, S. , Elliott, G. R. , & Buitelaar, J. K. (2018). Electroencephalographic biomarkers as predictors of methylphenidate response in attention‐deficit/hyperactivity disorder. European Neuropsychopharmacology, 28(8), 881–891. 10.1016/j.euroneuro.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Arnsten, A. F. T. (2006). Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology, 31(11), 2376–2383. 10.1038/sj.npp.1301164 [DOI] [PubMed] [Google Scholar]
- Assaf, Y. , & Pasternak, O. (2008). Diffusion tensor imaging (DTI)‐based white matter mapping in brain research: A review. Journal of Molecular Neuroscience, 34(1), 51–61. 10.1007/s12031-007-0029-0 [DOI] [PubMed] [Google Scholar]
- Aylward, E. H. , Reiss, A. L. , Reader, M. J. , Singer, H. S. , Brown, J. E. , & Denckla, M. B. (1996). Basal ganglia volumes in children with attention‐deficit hyperactivity disorder. Journal of Child Neurology, 11(2), 112–115. 10.1177/088307389601100210 [DOI] [PubMed] [Google Scholar]
- Battistella, G. , Najdenovska, E. , Maeder, P. , Ghazaleh, N. , Daducci, A. , Thiran, J. P. , Jacquemont, S. , Tuleasca, C. , Levivier, M. , Bach Cuadra, M. , & Fornari, E. (2017). Robust thalamic nuclei segmentation method based on local diffusion magnetic resonance properties. Brain Structure and Function, 222(5), 2203–2216. 10.1007/s00429-016-1336-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare, R. , Adamson, C. , Bellgrove, M. A. , Vilgis, V. , Vance, A. , Seal, M. L. , & Silk, T. J. (2017). Altered structural connectivity in ADHD: A network based analysis. Brain Imaging and Behavior, 11(3), 846–858. 10.1007/s11682-016-9559-9 [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system—a technical review. NMR in Biomedicine, 15(7–8), 435–455. 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- Behrens, T. E. J. , Berg, H. J. , Jbabdi, S. , Rushworth, M. F. S. , & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage, 34(1), 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, C. , Müller‐Godeffroy, J. , Marx, I. , Reis, O. , Buchmann, J. , & Dück, A. (2018). Methylphenidate promotes the interaction between motor cortex facilitation and attention in healthy adults: A combined study using event‐related potentials and transcranial magnetic stimulation. Brain and Behavior, 8(12), 1–15. 10.1002/brb3.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, W. , Griffiths, K. , Korgaonkar, M. S. , Williams, L. M. , & Menon, V. (2019). Inhibition‐related modulation of salience and frontoparietal networks predicts cognitive control ability and inattention symptoms in children with ADHD. Molecular Psychiatry, 26, 4016–4025. 10.1038/s41380-019-0564-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, G. A. , & Kelly, K. L. (2003). Stimulant rebound: How common is it and what does it mean? Journal of Child and Adolescent Psychopharmacology, 13(2), 137–142. 10.1089/104454603322163853 [DOI] [PubMed] [Google Scholar]
- Chang, E. H. , Argyelan, M. , Aggarwal, M. , Chandon, T. S. S. , Karlsgodt, K. H. , Mori, S. , & Malhotra, A. K. (2017). The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two‐photon microscopy of CLARITY intact brains. Neuroimage, 147(June 2016), 253–261. 10.1016/j.neuroimage.2016.11.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, H. L. , Chen, Y. J. , Lo, Y. C. , Tseng, W. Y. I. , & Gau, S. S. F. (2015). Altered white matter tract property related to impaired focused attention, sustained attention, cognitive impulsivity and vigilance in attention‐deficit/hyperactivity disorder. Journal of Psychiatry and Neuroscience, 40(5), 325–335. 10.1503/jpn.140106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, S. R. , van der Meij, R. , Peterson, E. J. , de Hemptinne, C. , Starr, P. A. , & Voytek, B. (2017). Nonsinusoidal beta oscillations reflect cortical pathophysiology in parkinson’s disease. Journal of Neuroscience, 37(18), 4830–4840. 10.1523/JNEUROSCI.2208-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupertino, R. B. , Soheili‐Nezhad, S. , Grevet, E. H. , Bandeira, C. E. , Picon, F. A. , Tavares, M. E. , Naaijen, J. , van Rooij, D. , Akkermans, S. , Vitola, E. S. , Zwiers, M. P. , Hoekstra, P. J. , Breda, V. , Oosterlaan, J. , Hartman, C. A. , Beckmann, C. F. , Buitelaar, J. K. , Franke, B. , Bau, C. H. D. , & Sprooten, E. (2019). Reduced fronto‐striatal volume in attention‐deficit/hyperactivity disorder in two cohorts across the lifespan. NeuroImage. Clinical. BioRxiv, i(51), 790204. 28, 102403. 10.1101/790204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damatac, C. , Chauvin, R. , Zwiers, M. , van Rooij, D. , Akkermans, S. , Naaijen, J. , Hoekstra, P. , Hartman, C. , Oosterlaan, J. , Franke, B. , Buitelaar, J. , Beckmann, C. , & Sprooten, E. (2020). White matter microstructure in attention‐deficit/hyperactivity disorder: A systematic tractography study in 654 individuals. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1101/787713 [DOI] [PubMed] [Google Scholar]
- Dang, L. C. , O’Neil, J. P. , & Jagust, W. J. (2012). Dopamine supports coupling of attention‐related networks. Journal of Neuroscience, 32(28), 9582–9587. 10.1523/JNEUROSCI.0909-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schotten, M. T. , Dell’Acqua, F. , Forkel, S. J. , Simmons, A. , Vergani, F. , Murphy, D. G. M. , & Catani, M. (2011). A lateralized brain network for visuospatial attention. Nature Neuroscience, 14(10), 1245–1246. 10.1038/nn.2905 [DOI] [PubMed] [Google Scholar]
- Devilbiss, D. M. , Kelley, A. E. , Schmeichel, B. , Andrzejewski, M. E. , Berridge, C. W. , Hamilton, C. , Arnsten, A. F. T. , & Spencer, R. C. (2006). Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biological Psychiatry, 60(10), 1111–1120. 10.1016/j.biopsych.2006.04.022 [DOI] [PubMed] [Google Scholar]
- Dixon, M. L. , De La Vega, A. , Mills, C. , Andrews‐Hanna, J. , Spreng, R. N. , Cole, M. W. , & Christoff, K. (2018). Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proceedings of the National Academy of Sciences of the United States of America, 115(7), E1598–E1607. 10.1073/pnas.1715766115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, L. M. F. , Yarrow, K. , & Brown, P. (2005). Lateralization of event‐related beta desynchronization in the EEG during pre‐cued reaction time tasks. Clinical Neurophysiology, 116(8), 1879–1888. 10.1016/j.clinph.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Engel, A. K. , & Fries, P. (2010). Beta‐band oscillations–signalling the status quo? Current Opinion in Neurobiology, 20(2), 156–165. 10.1016/j.conb.2010.02.015 [DOI] [PubMed] [Google Scholar]
- Fabozzi, F. J. , Focardi, S. M. , Rachev, S. T. , & Arshanapalli, B. G. (2014). Appendix E: Model selection criterion: AIC and BIC. The Basics of Financial Econometrics, 41(1979), 399–403. 10.1002/9781118856406.app5 [DOI] [Google Scholar]
- Faraone, S. V. (2018). The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention‐deficit/hyperactivity disorder and other psychiatric comorbidities. Neuroscience and Biobehavioral Reviews, 87(January), 255–270. 10.1016/j.neubiorev.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, J. (2020). Regression analysis: An intuitive guide for using and interpreting linear models. James D. Frost, Statistics by Jim Publishing. https://books.google.ch/books?id=1UPzzQEACAAJ [Google Scholar]
- George, K. , & Das, J. M. (2019). Neuroanatomy, thalamocortical radiations. StatPearls Publishing; January. https://www.ncbi.nlm.nih.gov/books/NBK546699/ [PubMed] [Google Scholar]
- Halassa, M. M. , & Kastner, S. (2017). Thalamic functions in distributed cognitive control. Nature Neuroscience, 20(12), 1669–1679. 10.1038/s41593-017-0020-1 [DOI] [PubMed] [Google Scholar]
- Hastie, T. , Tibshirani, R. , & Friedman, J. (2009). The elements of statistical learning. Springer Series in Statistics, Springer New York. 10.1007/978-0-387-84858-7 [DOI] [Google Scholar]
- Herring, J. D. , Thut, G. , Jensen, O. , & Bergmann, T. O. (2015). Attention modulates TMS‐locked alpha oscillations in the visual cortex. Journal of Neuroscience, 35(43), 14435–14447. 10.1523/JNEUROSCI.1833-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse, S. , Ballaschke, O. , Barthel, H. , & Sabri, O. (2009). Dopamine transporter imaging in adult patients with attention‐deficit/hyperactivity disorder. Psychiatry Research—Neuroimaging, 171(2), 120–128. 10.1016/j.pscychresns.2008.01.002 [DOI] [PubMed] [Google Scholar]
- Hodgkins, P. , Shaw, M. , Coghill, D. , & Hechtman, L. (2012). Amfetamine and methylphenidate medications for attention‐deficit/ hyperactivity disorder: Complementary treatment options. European Child and Adolescent Psychiatry, 21(9), 477–492. 10.1007/s00787-012-0286-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman, M. , Bralten, J. , Hibar, D. P. , Mennes, M. , Zwiers, M. P. , Schweren, L. S. J. , van Hulzen, K. J. E. , Medland, S. E. , Shumskaya, E. , Jahanshad, N. , de Zeeuw, P. , Szekely, E. , Sudre, G. , Wolfers, T. , Onnink, A. M. H. , Dammers, J. T. , Mostert, J. C. , Vives‐Gilabert, Y. , Kohls, G. , … Franke, B. (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: A cross‐sectional mega‐analysis. The Lancet Psychiatry, 4(4), 310–319. 10.1016/S2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok, T. A. , Haase, R. , Bluschke, A. , Thiemann, U. , & Bender, S. (2019). Bereitschaftspotential and lateralized readiness potential in children with attention deficit hyperactivity disorder: Altered motor system activation and effects of methylphenidate. European Neuropsychopharmacology, 29(8), 960–970. 10.1016/j.euroneuro.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Jbabdi, S. , Woolrich, M. W. , Andersson, J. L. R. , & Behrens, T. E. J. (2007). A Bayesian framework for global tractography. Neuroimage, 37(1), 116–129. 10.1016/j.neuroimage.2007.04.039 [DOI] [PubMed] [Google Scholar]
- Jenkinson, N. , & Brown, P. (2011). New insights into the relationship between dopamine, beta oscillations and motor function. Trends in Neurosciences, 34(12), 611–618. 10.1016/j.tins.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Jenni, N. L. , Larkin, J. D. , & Floresco, S. B. (2017). Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. Journal of Neuroscience, 37(26), 6200–6213. 10.1523/JNEUROSCI.0030-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, E. G. (2002). Thalamic circuitry and thalamocortical synchrony. Philosophical Transactions of the Royal Society B: Biological Sciences, 357(1428), 1659–1673. 10.1098/rstb.2002.1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucaite, A. , Fernell, E. , Halldin, C. , Forssberg, H. , & Farde, L. (2005). Reduced midbrain dopamine transporter binding in male adolescents with attention‐deficit/hyperactivity disorder: Association between striatal dopamine markers and motor hyperactivity. Biological Psychiatry, 57(3), 229–238. 10.1016/j.biopsych.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Konrad, A. , Dielentheis, T. F. , El Masri, D. , Bayerl, M. , Fehr, C. , Gesierich, T. , Vucurevic, G. , Stoeter, P. , & Winterer, G. (2010). Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. European Journal of Neuroscience, 31(5), 912–919. 10.1111/j.1460-9568.2010.07110.x [DOI] [PubMed] [Google Scholar]
- Kowalczyk, O. S. , Cubillo, A. I. , Smith, A. , Barrett, N. , Giampietro, V. , Brammer, M. , Simmons, A. , & Rubia, K. (2019). Methylphenidate and atomoxetine normalise fronto‐parietal underactivation during sustained attention in ADHD adolescents. European Neuropsychopharmacology, 29(10), 1102–1116. 10.1016/j.euroneuro.2019.07.139 [DOI] [PubMed] [Google Scholar]
- Krause, K. H. , Dresel, S. H. , Krause, J. , Kung, H. F. , & Tatsch, K. (2000). Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: Effects of methylphenidate as measured by single photon emission computed tomography. Neuroscience Letters, 285(2), 107–110. 10.1016/S0304-3940(00)01040-5 [DOI] [PubMed] [Google Scholar]
- Le Bihan, D. , Mangin, J.‐F. , Poupon, C. , Clark, C. A. , Pappata, S. , Molko, N. , & Chabriat, H. (2001). Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging, 13(4), 534–546. 10.1002/jmri.1076 [DOI] [PubMed] [Google Scholar]
- Lei, D. , Ma, J. , Du, X. , Shen, G. , Jin, X. , & Gong, Q. (2014). Microstructural abnormalities in the combined and inattentive subtypes of attention deficit hyperactivity disorder: A diffusion tensor imaging study. Scientific Reports, 4, 6875. 10.1038/srep06875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz, A. , Mazaheri, A. , Jensen, O. , & Loo, S. K. (2018). Aberrant modulation of brain oscillatory activity and attentional impairment in attention‐deficit/hyperactivity disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(1), 19–29. 10.1016/j.bpsc.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfeldt, N. , Eriksson, J. , Åström, B. , Forsgren, L. , & Mo, S. J. (2017). Fractional anisotropy and mean diffusion as measures of dopaminergic function in Parkinson’s disease: Challenging results. Journal of Parkinson’s Disease, 7(1), 129–142. 10.3233/JPD-161011 [DOI] [PubMed] [Google Scholar]
- Linssen, A. M. W. , Sambeth, A. , Vuurman, E. F. P. M. , & Riedel, W. J. (2014). Cognitive effects of methylphenidate in healthy volunteers: A review of single dose studies. International Journal of Neuropsychopharmacology, 17(6), 961–977. 10.1017/S1461145713001594 [DOI] [PubMed] [Google Scholar]
- Lorio, S. , Sambataro, F. , Bertolino, A. , Draganski, B. , & Dukart, J. (2019). The combination of DAT‐SPECT, structural and diffusion MRI predicts clinical progression in Parkinson’s disease. Frontiers in Aging Neuroscience, 11(March), 1–13. 10.3389/fnagi.2019.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris, N. , Kennedy, D. N. , McInerney, S. , Sorensen, A. G. , Wang, R. , Caviness, V. S. , & Pandya, D. N. (2005). Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cerebral Cortex, 15(6), 854–869. 10.1093/cercor/bhh186 [DOI] [PubMed] [Google Scholar]
- Marshall, T. R. , Bergmann, T. O. , & Jensen, O. (2015). Frontoparietal structural connectivity mediates the top‐down control of neuronal synchronization associated with selective attention. PLoS Biology, 13(10), 1–17. 10.1371/journal.pbio.1002272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri, A. , Fassbender, C. , Coffey‐Corina, S. , Hartanto, T. A. , Schweitzer, J. B. , & Mangun, G. R. (2014). Differential oscillatory electroencephalogram between attention‐deficit/ hyperactivity disorder subtypes and typically developing adolescents. Biological Psychiatry, 76(5), 422–429. 10.1016/j.biopsych.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetti, C. , Staudigl, T. , Marshall, T. R. , Zumer, J. M. , Fallon, S. J. , & Jensen, O. (2019). Hemispheric asymmetry of globus pallidus relates to alpha modulation in reward‐related attentional tasks. The Journal of Neuroscience, 610–619, 9221–9236. 10.1523/JNEUROSCI.0610-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzetti, C. , ter Huurne, N. , Buitelaar, J. K. , & Jensen, O. (2020). Methylphenidate normalizes aberrant beta oscillations and reduces alpha power during retention in children with ADHD. BioRxiv, March 13, 2020.990929. 10.1101/2020.03.13.990929 [DOI] [Google Scholar]
- McCarthy, M. M. , Moore‐Kochlacs, C. , Gu, X. , Boyden, E. S. , Han, X. , & Kopell, N. (2011). Striatal origin of the pathologic beta oscillations in Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11620–11625. 10.1073/pnas.1107748108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, R. J. , Mallet, N. , Litvak, V. , Dolan, R. J. , Magill, P. J. , Friston, K. J. , & Brown, P. (2011). Alterations in brain connectivity underlying beta oscillations in parkinsonism. PLoS Computational Biology, 7(8), e1002124. 10.1371/journal.pcbi.1002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel, M. , Beckmann, C. F. , Pruim, R. H. R. , Van Oort, E. S. B. , Franke, B. , Hartman, C. A. , Hoekstra, P. J. , Oosterlaan, J. , Heslenfeld, D. , Buitelaar, J. K. , & Mennes, M. (2016). Attention‐deficit/hyperactivity disorder symptoms coincide with altered striatal connectivity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 1(4), 353–363. 10.1016/j.bpsc.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnink, A. M. H. , Zwiers, M. P. , Hoogman, M. , Mostert, J. C. , Dammers, J. , Kan, C. C. , Vasquez, A. A. , Schene, A. H. , Buitelaar, J. , & Franke, B. (2015). Deviant white matter structure in adults with attention‐deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 63, 14–22. 10.1016/j.pnpbp.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paclt, I. , Přibilová, N. , Kollárová, P. , Kohoutová, M. , Dezortová, M. , Hájek, M. , & Csemy, L. (2016). Reverse asymmetry and changes in brain structural volume of the basal ganglia in ADHD, developmental changes and the impact of stimulant medications. Neuroendocrinology Letters, 37(1), 29–32. [PubMed] [Google Scholar]
- Pavlides, A. , Hogan, S. J. , & Bogacz, R. (2015). Computational models describing possible mechanisms for generation of excessive Beta oscillations in Parkinson’s disease. PLoS Computational Biology, 11(12), 1–29. 10.1371/journal.pcbi.1004609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk, G. , Caspi, A. , Houts, R. , Kollins, S. H. , Rohde, L. A. , & Moffitt, T. E. (2010). Implications of extending the ADHD age‐of‐onset criterion to age 12: Results from a prospectively studied birth cohort. Journal of the American Academy of Child & Adolescent Psychiatry, 49(3), 210–216. 10.1016/j.jaac.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Qiu, A. , Crocetti, D. , Adler, M. , Mahone, E. M. , Denckla, M. B. , Miller, M. I. , & Mostofsky, S. H. (2009). Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. American Journal of Psychiatry, 166(1), 74–82. 10.1176/appi.ajp.2008.08030426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, D. , Bode, T. , Reiz, J. L. , Donnelly, G. A. E. , & Darke, A. C. (2007). Single‐dose pharmacokinetics of multilayer‐release methylphenidate and immediate‐release methylphenidate in children with attention‐deficit/hyperactivity disorder. Journal of Clinical Pharmacology, 47(6), 760–766. 10.1177/0091270007299759 [DOI] [PubMed] [Google Scholar]
- Richter, C. G. , Coppola, R. , & Bressler, S. L. (2018). Top‐down beta oscillatory signaling conveys behavioral context in early visual cortex. Scientific Reports, 8(1), 1–12. 10.1038/s41598-018-25267-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia, K. , Halari, R. , Cubillo, A. , Smith, A. B. , Mohammad, A. M. , Brammer, M. , & Taylor, E. (2011). Methylphenidate normalizes fronto‐striatal underactivation during interference inhibition in medication‐nave boys with attention‐deficit hyperactivity disorder. Neuropsychopharmacology, 36(8), 1575–1586. 10.1038/npp.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill, L. , & Schwab‐Stone, M. (2000). Epidemiology of Adhd in school‐age children. Child and Adolescent Psychiatric Clinics of North America, 9(3), 541–555. 10.1016/S1056-4993(18)30106-8 [DOI] [PubMed] [Google Scholar]
- Sethi, A. , Evelyn‐Rahr, E. , Dowell, N. , Jain, S. , Voon, V. , Critchley, H. D. , Harrison, N. A. , & Cercignani, M. (2017). Magnetization transfer imaging identifies basal ganglia abnormalities in adult ADHD that are invisible to conventional T1 weighted voxel‐based morphometry. Neuroimage: Clinical, 15(March), 8–14. 10.1016/j.nicl.2017.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. M. (2016). Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience, 19(4), 533–541. 10.1038/nn.4269 [DOI] [PubMed] [Google Scholar]
- Shimony, J. S. , Rutlin, J. , Karimi, M. , Tian, L. , Snyder, A. Z. , Loftin, S. K. , Norris, S. A. , & Perlmutter, J. S. (2018). Validation of diffusion tensor imaging measures of nigrostriatal neurons in macaques. PLoS One, 13(9), e0202201. 10.1371/journal.pone.0202201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatkova, A. , Nestrasil, I. , Rudser, K. , Goldenring Fine, J. , Bledsoe, J. , & Semrud‐Clikeman, M. (2016). Unique white matter microstructural patterns in ADHD presentations—A diffusion tensor imaging study. Human Brain Mapping, 37(9), 3323–3336. 10.1002/hbm.23243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, Y. C. , Chi, M. H. , Chu, C. L. , Chiu, N. T. , Yao, W. J. , Chen, P. S. , & Yang, Y. K. (2019). Availability of striatal dopamine transporter in healthy individuals with and without a family history of ADHD. Journal of Attention Disorders, 23(7), 665–670. 10.1177/1087054716654570 [DOI] [PubMed] [Google Scholar]
- Tang, B. , & Dafny, N. (2013). Dorsal raphe neuronal activities are modulated by methylphenidate. Journal of Neural Transmission, 120(5), 721–731. 10.1007/s00702-012-0917-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Huurne, N. , Lozano‐Soldevilla, D. , Onnink, M. , Kan, C. , Buitelaar, J. , & Jensen, O. (2017). Diminished modulation of preparatory sensorimotor mu rhythm predicts attention‐deficit/hyperactivity disorder severity. Psychological Medicine, 47(11), 1947–1956. 10.1017/S0033291717000332 [DOI] [PubMed] [Google Scholar]
- ter Huurne, N. , Onnink, M. , Kan, C. , Buitelaar, J. , & Jensen, O. (2018). Diminished modulation of preparatory sensorimotor mu rhythm predicts attention‐deficit / hyperactivity disorder severity. Psychological Medicine, 2017, 1947–1956. 10.1017/S0033291717000332 [DOI] [PubMed] [Google Scholar]
- ter Huurne, N. , Onnink, M. , Kan, C. , Franke, B. , Buitelaar, J. , & Jensen, O. (2013). Behavioral consequences of aberrant alpha lateralization in attention‐deficit/hyperactivity disorder. Biological Psychiatry, 74(3), 227–233. 10.1016/j.biopsych.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Tomaschek, F. , Hendrix, P. , & Baayen, R. H. (2018). Strategies for addressing collinearity in multivariate linguistic data. Journal of Phonetics, 71, 249–267. 10.1016/j.wocn.2018.09.004 [DOI] [Google Scholar]
- Tomasi, D. , Wang, G. J. , & Volkow, N. D. (2016). Association between striatal dopamine D2/D3 receptors and brain activation during visual attention: Effects of sleep deprivation. Translational Psychiatry, 6(5), e828. 10.1038/tp.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis, C. , West, S. , & Pellizzer, G. (2015). Brain oscillatory activity during motor preparation: Effect of directional uncertainty on beta, but not alpha, frequency band. Frontiers in Neuroscience, 9(JUN), 1–13. 10.3389/fnins.2015.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , de Lange, F. P. , & Maris, E. (2012). Attentional cues affect accuracy and reaction time via different cognitive and neural processes. Journal of Neuroscience, 32(30), 10408–10412. 10.1523/JNEUROSCI.1337-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , Chekroud, S. R. , Stokes, M. G. , & Nobre, A. C. (2019). Concurrent visual and motor selection during visual working memory guided action. Nature Neuroscience, 22(3), 477–483. 10.1038/s41593-018-0335-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede, F. , Niklaus, M. , & Nobre, A. C. (2017). Temporal expectations guide dynamic prioritization in visual working memory through attenuated α oscillations. Journal of Neuroscience, 37(2), 437–445. 10.1523/JNEUROSCI.2272-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk, H. , Heslenfeld, D. J. , Zwiers, M. P. , Buitelaar, J. K. , & Oosterlaan, J. (2012). Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta‐analysis. Neuroscience and Biobehavioral Reviews, 36(4), 1093–1106. 10.1016/j.neubiorev.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Vollebregt, M. A. , Zumer, J. M. , ter Huurne, N. , Buitelaar, J. K. , & Jensen, O. (2016). Posterior alpha oscillations reflect attentional problems in boys with attention deficit hyperactivity disorder. Clinical Neurophysiology, 127(5), 2182–2191. 10.1016/j.clinph.2016.01.021 [DOI] [PubMed] [Google Scholar]
- Von Rhein, D. , Oldehinkel, M. , Beckmann, C. F. , Oosterlaan, J. , Heslenfeld, D. , Hartman, C. A. , Hoekstra, P. J. , Franke, B. , Cools, R. , Buitelaar, J. K. , & Mennes, M. (2016). Aberrant local striatal functional connectivity in attention‐deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(6), 697–705. 10.1111/jcpp.12529 [DOI] [PubMed] [Google Scholar]
- Wang, D. D. , de Hemptinne, C. , Miocinovic, S. , Ostrem, J. L. , Galifianakis, N. B. , Luciano, M. S. , & Starr, P. A. (2018). Pallidal deep‐brain stimulation disrupts pallidal beta oscillations and coherence with primary motor cortex in Parkinson’s disease. Journal of Neuroscience, 38(19), 4556–4568. 10.1523/JNEUROSCI.0431-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E. J. (2008). A review and comparison of four commonly used Bayesian and maximum likelihood model selection tools. Ecological Modelling, 211(1–2), 1–10. 10.1016/j.ecolmodel.2007.10.030 [DOI] [Google Scholar]
- Witt, S. T. , & Stevens, M. C. (2015). Relationship between white matter microstructure abnormalities and ADHD symptomatology in adolescents. Psychiatry Research—Neuroimaging, 232(2), 168–174. 10.1016/j.pscychresns.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers, T. , Onnink, A. M. H. , Zwiers, M. P. , Arias‐Vasquez, A. , Hoogman, M. , Mostert, J. C. , Kan, C. C. , Slaats‐Willemse, D. , Buitelaar, J. K. , & Franke, B. (2015). Lower white matter microstructure in the superior longitudinal fasciculus is associated with increased response time variability in adults with attentiondeficit/hyperactivity disorder. Journal of Psychiatry and Neuroscience, 40(5), 344–351. 10.1503/jpn.140154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, S. , Li, X. , Kimball, A. E. , Kelly, M. S. , Lesser, I. , & Branch, C. (2012). Thalamic shape and connectivity abnormalities in children with attention‐ deficit/hyperactivity disorder. Psychiatry Research—Neuroimaging, 204(2–3), 161–167. 10.1016/j.pscychresns.2012.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan, B. , Mackie, M. A. , Spagna, A. , Wu, T. , Tian, Y. , Hof, P. R. , & Fan, J. (2016). The activation of interactive attentional networks. Neuroimage, 129, 308–319. 10.1016/j.neuroimage.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki, A. , Panneck, P. , Srinivasan, P. , Stevens, A. , Zöllei, L. , Augustinack, J. , Wang, R. , Salat, D. , Ehrlich, S. , Behrens, T. , Jbabdi, S. , Gollub, R. , & Fischl, B. (2011). Automated probabilistic reconstruction of white‐matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics, 5(October), 1–12. 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit, N. , & Muscat, R. (2019). Beta band oscillatory deficits during working memory encoding in adolescents with attention‐deficit hyperactive disorder. European Journal of Neuroscience, February, 50(5), 2905–2920. 10.1111/ejn.14398 [DOI] [PubMed] [Google Scholar]
- Zhong, P. , Liu, W. , & Yan, Z. (2016). Aberrant regulation of synchronous network activity by the attention‐deficit/hyperactivity disorder‐associated human dopamine D4 receptor variant D4.7 in the prefrontal cortex. Journal of Physiology, 594(1), 135–147. 10.1113/JP271317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


