Abstract
Previous brain imaging studies with chronic cocaine users (CU) using diffusion tensor imaging (DTI) mostly focused on fractional anisotropy to investigate white matter (WM) integrity. However, a quantitative interpretation of fractional anisotropy (FA) alterations is often impeded by the inherent limitations of the underlying tensor model. A more fine‐grained measure of WM alterations could be achieved by measuring fibre density (FD). This study investigates this novel DTI metric comparing 23 chronic CU and 32 healthy subjects. Quantitative hair analysis was used to determine intensity of cocaine and levamisole exposure—a cocaine adulterant with putative WM neurotoxicity. We first assessed the impact of cocaine use, levamisole exposure and alcohol use on group differences in WM integrity. Compared with healthy controls, all models revealed cortical reductions of FA and FD in CU. At the within‐patient group level, we found that alcohol use and levamisole exposure exhibited regionally different FA and FD alterations than cocaine use. We found mostly negative correlations of tract‐based WM associated with levamisole and weekly alcohol use. Specifically, levamisole exposure was linked with stronger WM reductions in the corpus callosum than alcohol use. Cocaine use duration correlated negatively with FA and FD in some regions. Yet, most of these correlations did not survive a correction for multiple testing. Our results suggest that chronic cocaine use, levamisole exposure and alcohol use were all linked to significant WM impairments in CU. We conclude that FD could be a sensitive marker to detect the impact of the use of multiple substances on WM integrity in cocaine but also other substance use disorders.
Keywords: cocaine, diffusion‐weighted imaging, levamisole
Our results suggest that chronic cocaine use, levamisole exposure and alcohol use were all linked to significant WM impairments in chronic cocaine users. We further conclude that FD could be a sensitive marker to detect the impact of the use of multiple substances on white matter integrity in cocaine users.
1. INTRODUCTION
Mortality risk is significantly elevated among individuals who reported lifetime use of cocaine based on a recent study including data from ~20.500 adults, yielding a hazard ratio of 1.27 (95% CI: 1.04–1.55). 1 Cocaine use is associated with several mechanisms of brain injury including ischaemic, haemorrhagic and metabolic changes. 2 , 3 , 4 , 5 In the last two decades, magnetic resonance imaging (MRI) techniques have provided insights into the complex structural brain abnormalities in cocaine users (CU). Chronic use of stimulants including cocaine is associated with both impairments in decision‐making 6 , 7 , 8 , 9 and structural abnormalities in brain grey and white matter (WM). 10 , 11 , 12 , 13 , 14 Diffusion tensor imaging (DTI) studies reported that chronic exposure to cocaine commonly results in reduced fractional anisotropy (FA), increased mean diffusivity (MD) or increased diffusivity in directions perpendicular (radial) to the prominent diffusion direction in parts of the corpus callosum (CC). 12 , 15 , 16 , 17 , 18 Less reliably, DTI signs of abnormal WM have also been seen in frontal and parietal brain regions. 15 , 19 , 20 , 21 , 22 , 23 , 24 Variations in the pattern of abnormal DTI values associated with chronic cocaine use or addiction is partly accounted for by comorbid alcohol consumption and by route of cocaine administration with smoking cocaine associated with lower FA and higher perpendicular diffusivity than intranasal administration. 16 Length of abstinence of cocaine use appears to be related with larger FA and smaller MD values in the CC, frontal, parietal, temporal and occipital lobes as well as the cerebellum. 19 In the study by Bell et al, cocaine abstinent patients (abstinence duration ranged between 5 days and 102 weeks) showed initially lower FA in the left anterior callosal fibres, left genu of the CC, right lateral superior longitudinal fasciculus (SLF), right callosal fibres and the superior corona radiata bilaterally when compared against non‐using controls. 25 Higher FA in the cocaine abstinent patients was observed in the splenium of the CC and in medial parts of the right SLF, a WM tract connecting frontal control areas to parietal regions. Differences between the cocaine abstinent groups were observed bilaterally in the inferior fronto‐occipital fasciculus (IFOF), right anterior thalamic radiation and ventral posterolateral nucleus of the thalamus as well as in the left superior corona radiata, SFL bilaterally, right cingulum and the WM of the right precentral gyrus.
The tensor model, and therefore FA measures, is inadequate to illustrate the underlying tissue structure in regions with complex fibre geometries and multiple fibre populations. 26 , 27 , 28 , 29 , 30 , 31 Voxels capturing these anatomical complexities occur frequently (60%–90% of all WM fibre voxels) throughout the brain WM due to limited spatial resolution and partial volume effects between adjacent tracts. 30 Although a reduction of FA is often thought to reflect axonal degeneration and demyelination, 32 , 33 , 34 , 35 complex fibre configurations may critically confound the interpretation of changes in tensor‐derived diffusion metrics and complicate a correct and quantitative interpretation of changes in DTI‐related parameters. 30 , 36 , 37 , 38 , 39 Yet, diffusion acquisition techniques and data analysis pipelines have been improved significantly by addressing these inherent problems of the tensor model. Higher order diffusion models based on acquisition schemes with high number of diffusion directions and new reconstruction methods as the constrained spherical deconvolution technique 40 were developed. These advances enable the resolution of multiple fibre directions within a single voxel and can improve the performance of fibre tractography significantly. 41 , 42 , 43 Additionally, there is a growing literature on removing tractography biases, which is a crucial prerequisite to derive absolute and quantitative measures from fibre tractograms. 44 , 45 However, the reliable extraction of quantitative measures from tractograms across different populations remains challenging. Recent developments in global top‐down tractography optimizations enable the estimation of fibre contributions and compartment fractions, 46 , 47 , 48 , 49 , 50 , 51 whereby all of these optimization methods have their own pitfalls; for a comprehensive overview, see literature. 52 Numerous models based on diffusion‐weighted data have been proposed to estimate parameters related to the restricted, intra‐axonal compartment, commonly referred to as fibre density (FD). 53 , 54 Smith et al 50 as well as Daducci and colleagues determined an optimal weight for each streamline, according to a biologically motivated forward model and the measured diffusion signal. 50 , 51 , 53 By assigning a weight of zero, false positive or implausible connections can be eliminated. The FD is calculated by multiplying each streamline contribution (fibre weight) by the streamline length. Previously, we have demonstrated that WM alterations were more prominent in FD compared with FA in patients with amyotrophic lateral sclerosis 55 and schizophrenia. 56 However, the potential advantages of the aforementioned innovations in basic neuroimaging methods have not been established for clinical neuroscience research of pathologies in substance use disorders such as chronic cocaine use. It is thus unknown whether microstructural WM fibre alterations can be quantified with the new FD measure.
Therefore, we applied a whole‐brain and regional tract‐based spatial statistics (TBSS) analysis approach 57 to test its potential in detecting microstructural alterations in FD between CU and healthy controls (HC). Based on the meta‐analysis of DTI‐based WM integrity studies, 12 we hypothesized to find lower FA and FD in CU in the CC as this region showed the highest effect size estimates across included studies. Moreover, two case reports of leukoencephalopathy in CU implicated a commonly added adulterant in street cocaine, levamisole, as a potential cause. 58 , 59 In fact, multifocal leukoencephalopathy has been shown previously in patients who received levamisole as a medication against ascariasis, recurrent aphthous ulcers and malignant melanoma and for adenocarcinoma of the colon. 60 , 61 , 62 , 63 Additionally, a dose‐dependent effect of levamisole exposure on neurocognitive functions and thinning of distinct cortical areas of CU has been shown recently. 64 Recently, we could additionally show that the use of levamisole‐adulterated cocaine is accompanied by an increased load of WM lesions. 65 Therefore, immune‐related neurotoxicity related to the cutting agent levamisole may contribute to cocaine‐associated brain injury. We thus examined if levamisole exposure is associated with WM integrity in CU. Further, we tested the impact of alcohol use on WM integrity, given that alcohol use disorders are highly prevalent chronic CU 66 and have a well‐established impact on WM microstructure. 67 , 68 Henceforth, by choosing a within‐patient group (see below) analytical approach, we aimed to disentangle the effects of cocaine, levamisole and alcohol use on local WM integrity in CU.
2. METHODS
2.1. Participants
We recruited chronic CU (n = 43) from inpatient and outpatient units of the Psychiatric University Hospital in Zurich and from affiliated institutions. 69 Age‐, sex‐ and education‐matched HC (n = 42) were recruited by online advertisements. For inclusion, CU had to report cocaine as primary substance of choice with a consumption level of >0.5 g per month and an abstinence duration of <6 months. Exclusion criteria for CU were the presence of DSM‐IV Axis I adult psychiatric disorders—except for cocaine, cannabis, nicotine, alcohol abuse/dependence, attention deficit hyperactivity disorder (ADHD) and a previous depressive episode. Controls were excluded if a current or previous DSM‐IV Axis I psychiatric disorder (except for nicotine dependence) or illegal substance use of >15 lifetime occasions or during the past 6 months (except for cannabis) was assessed. Before the testing session, participants were asked to abstain from illegal substances for at least 72 h and not to consume alcohol for 24 h. Urine samples were collected to verify self‐reports. When available, 6‐cm hair samples were cut from the occiput, enabling to estimate substance use during the last 6 months. Hair samples were analysed with liquid chromatography–tandem mass spectrometry. 70 Data from these 6‐month hair samples were used for (a) confirmation of regular cocaine use (cocaine hair concentration >500 pg/mg) 71 , 72 and (b) confirmation that cocaine is the primary used illicit substance and (c) for the quantification of levamisole. The ethics committee of the Canton of Zurich approved the study, which was in accordance with guidelines from the Helsinki declaration. All participants gave written informed consent.
2.2. Psychopathological and neuropsychological assessment
A trained psychologist conducted a clinical interview to determine the presence of DSM‐IV Axis I diagnoses. 73 Subjective report of substance use including alcohol was assessed by the Interview for Psychotropic Drug Consumption. 74 Smoking habits were assessed by the use of the Fagerström Test for Nicotine Dependence. 75 The Barratt Impulsiveness Scale BIS‐11 76 and the ADHD self‐rating scale ADHD‐SR 77 to estimate trait impulsivity and ADHS symptomatology, respectively, were applied. In addition, we applied the Beck Depression Inventory (BDI).
2.3. MRI data acquisition
MRI data acquisition was performed on a 3T whole‐body MR scanner (Achieva, Philips Healthcare, Best, the Netherlands), equipped with 80 mT/m gradients and a 32‐channel receive head coil. Diffusion data were acquired using a diffusion‐weighted single‐shot spin‐echo echo‐planar imaging sequence with the following parameters: repetition time (TR): 6.64 s, echo time (TE): 53.6 ms, field of view (FOV): 240 × 240 mm2, 50 contiguous transversal slices, slice thickness: 2.5 mm, acquisition matrix: 96 × 96, SENSE factor: 2.5, partial Fourier encoding: 60%. The slices were positioned parallel to the anterior and posterior commissure defined on a T1‐weighted midline sagittal survey image. Diffusion acquisition was performed along 32 directions with a b‐value of 1000 s/mm2 and two signal averages. Additionally, four non‐diffusion‐weighted b = 0 s/mm2 scans were acquired resulting in a scan time of 8 min 31 s. For structural reference and anatomical priors for the tracking algorithm, T1‐weighted images were recorded using a three‐dimensional magnetization prepared rapid gradient‐echo (MP‐RAGE) sequence with 1‐mm isotropic resolution.
2.4. Diffusion data preprocessing
Before any preprocessing steps, quality control of all acquired diffusion data was assessed based on several criteria: First, diffusion tensor residuals were calculated for every acquired diffusion direction, and the nine slices in the whole diffusion dataset with the highest residuals were identified for visual inspection. Plots were generated depicting the 12 slices (four sagittal, four axial and four coronal directions) with the highest noise level. Second, mean signal intensity plots for every diffusion direction and the non‐diffusion‐weighted image were derived and plotted slice by slice in sagittal, axial and coronal directions. Artefacts, such as signal dropouts due to head motion, can easily be spotted on these plots. A trained MR physicist inspected the data for artefacts and rated the signal courses and fitting residuals of every subject on a Likert‐type scale. Preprocessing diffusion data followed a similar procedure previously described in our recent publication. 56 After denoising the raw data using the ‘dwidenoise function’ from the MRtrix3 software package, diffusion‐weighted data were first corrected for eddy‐current and motion‐induced distortions by registration the diffusion‐weighted images to the b0 image using the dwipreproc routine from MRtrix3 software package. This function makes use of the eddy tool implemented in FSL (FMRIB, Oxford, UK Version 6.0.0). 78 The Brain Extraction Tool (BET) from FSL was then applied to remove non‐brain tissue and estimate the inner and outer skull surfaces. Next, the diffusion data were corrected for susceptibility‐induced distortions using the bdp correction algorithm implemented in the BrainSuite software package (http://brainsuite.org). 79 Diffusion maps derived from the diffusion tensor, that is, FA, MD, radial diffusivity and axial diffusivity, were then calculated using the DTIFIT tool implemented in the FSL software package. In our study, we focus on FA and FD. Subsequently, constrained spherical deconvolution with recursive calibration of the response function 40 and fibre tractography was performed in MRtrix3 using the iFOD2 probabilistic tractography algorithm. 80 In order to apply biological tissue priors to the streamline generation, the ‘Anatomically‐Constrained Tractography’ option was selected (MRtrix3 tckgen ACT option). 45 Tractography seed points were determined dynamically according to the spherical‐deconvolution informed filtering of the tractogram model (MRtrix3 tckgen seed‐dynamic option for determining seed points based on the SIFT model). 49 Due to this dynamic seeding strategy within the whole WM, the distribution of streamlines is already approximating the apparent FD, and therefore, intrinsic tractography biases are reduced. In total, five million fibres were generated per subject. The resulting streamlines were optimized using the COMMIT framework 51 applying the parameters described elsewhere. 81 The derived intracellular compartment fraction of the COMMIT optimization corresponds to FD.
2.5. Intracranial volume
The intracranial volume (ICV) measure, sometimes referred to as total intracranial volume, refers to the estimated volume of the cranial cavity as outlined by the supratentorial dura matter or cerebral contour when dura is not clearly detectable. To extract the ICV for each subject, the following steps were conducted with FreeSurfer software (v5.3.0, http://surfer.nmr.mgh.harvard.edu/): motion correction, skull‐stripping 82 automated Talairach transformation, with subsequent segmentation of the WM, 29 , 83 correction of intensity variations due to magnetic field inhomogeneities and placement of grey/white and grey/cerebrospinal fluid borders based on intensity gradients. 84 , 85
2.6. Statistical analysis
To evaluate differences between the groups, voxel‐wise (whole‐brain) TBSS analysis based on a general linear model was performed using FSL's randomize tool 86 with 5000 permutations to correct for multiple comparisons (p < 0.05, corrected). All results included threshold‐free cluster enhancement (TFCE). 87 The TFCE correction method is somewhat similar to cluster‐based thresholding, but generally more robust and avoids the need for the arbitrary initial cluster‐forming threshold. Two contrasts were computed, testing for positive and negative differences of the FA and FD parameters between the HC and CU. To probe for the impact of cocaine, levamisole or alcohol on WM integrity, we computed three statistical models:
Model 1: Testing for the influence of cocaine use (pg/mg) on WM integrity. Here, we adjusted for age, sex, ADHD, nicotine (cigarettes per day), 3,4‐methylenedioxymethamphetamine (MDMA) (hair concentration, pg/mg), ICV, alcohol (weekly use; pure ethanol g per week) and levamisole (hair concentration, pg/mg).
Model 2: Testing for the influence of levamisole on WM integrity. Here, we adjusted for age, sex, ADHD, nicotine, MDMA, ICV, alcohol and cocaine use.
Model 3: Testing for the influence of alcohol on WM integrity. Here, we adjusted for age, sex, ADHD, nicotine, MDMA, ICV, levamisole and cocaine use.
Consistently, we included—apart from age, sex, ADHD and ICV—MDMA and nicotine in all models, as it has been shown that MDMA can alter GM and WM integrity, 88 whereas nicotine alters WM microstructure. 89 , 90
These models are likely to illustrate WM alterations by the particular substances compared with HC. Yet, as HC did not consume cocaine or levamisole, we could not enter all three variables (alcohol, cocaine and levamisole) in one statistical model to assess the strongest impact on WM integrity by a particular substance by this comparison. We therefore applied a within‐patient group analysis. Here, we first computed track‐specific (48 tracts; based on the JHU CBM‐DTI‐81 WM labels atlas 91 ) Spearman rank‐order correlations between FA and FD with substance‐specific values while controlling for the same covariates (apart from cocaine, levamisole and alcohol) as for the between‐group models (i.e. age, sex, ADHD, nicotine, MDMA and ICV). For significant (p < 0.05, uncorrected) correlations, we further compared the coefficients of the particular tracts between substances by means of the ‘corr_rtest’ function implemented in Matlab. By this analysis, we could assess which of the substances lead to the strongest WM impairment (e.g. seen as a significantly stronger negative correlation with FA and/or FD for a particular substance over the other substances).
3. RESULTS
3.1. Data quality
From the original sample (n = 85), seven (three CU and four HC) participants did not receive a DTI scan. 69 For these remaining participants (n = 38 HC and n = 40 CU), we had to exclude data from six HC and 17 CU, due to insufficient data quality caused by excessive head motion or the lack of information on cocaine and levamisole levels due to missing hair samples. Thus, the final included sample consists of 32 HC and 23 CU.
3.2. Demographic and clinical values
Participants were mostly right‐handed (90.9%), and there was no group difference in handedness (chi‐square: p = 0.220). Groups did not differ regarding age, IQ, education and ICV (see Table 1). Although the original sample was matched for sex, after data quality‐related exclusions, the two groups slightly but non‐significantly differed in sex distribution. As expected, CU had significantly higher ADHD scores, alcohol intake, nicotine intake and MDMA hair concentration.
TABLE 1.
Demographics
| Measure | Group (HC = 32, CU = 23) | p‐value (t‐test/chi‐square) |
|---|---|---|
| Age (years) | HC: 31.43 (STD: 6.6) | 0.45 |
| CU: 32.91 (STD: 7.2) | ||
| Sex (female/male) | HC: 16/16 | 0.22 a |
| CU: 9/14 | ||
| Education (years) | HC: 10.5 (STD: 1.5) | 0.63 |
| CU: 10.4 (STD: 1.4) | ||
| Verbal IQ | HC: 108.6 (11.2) | 0.07 |
| CU: 102.5 (11.7) | ||
| Handedness | HC: 32 R, 0L | >0.05 a |
| CU: 20 R, 3L | ||
| ICV (mm3) | HC: 1592550 (STD: 142211) | 0.86 |
| CU: 1585372 (STD: 145853) | ||
| BDI score | HC: 2.3 (5) | 0.0016 |
| CU: 9.1 (9.8) | ||
| ADHD‐SR score | HC: 6.2 (STD: 5.9) | <0.001 |
| CU: 15.4 (STD: 7.9) | ||
| Alcohol (pure ethanol in g/week) | HC: 56.5 (STD: 45.7) | 0.0033 |
| CU: 253.9 (STD: 353.1) | ||
| Nicotine (cigarettes per day) | HC: 3.7 (STD: 5.2) | 0.04 |
| CU: 7.9 (STD: 9.1) | ||
| MDMA hair concentration (pg/mg) | HC: 0.99 (STD: 5.5) | 0.03 |
| CU: 322.2 (STD: 787.5) | ||
| Levamisole hair concentration (pg/mg) | HC: 0 | n.a. |
| CU: 4265.4 (STD: 6443.8) |
Note: Bold means p < 0.05.
Abbreviations: ADHD‐SR, Attention Deficit Hyperactivity Disorder Self‐Rating Scale; BDI, Beck Depression Scale; CU, cocaine users; HC, healthy controls; ICV, intracranial volume; L, left‐handed; MDMA, 3,4‐methylenedioxymethamphetamine; R, right‐handed.
Chi‐square test.
3.2.1. Whole‐brain between‐group analysis
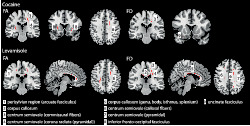
Model 1 (cocaine): The comparison between HC and CU revealed reduced FA in CU in the external/extreme capsule (IFOF), sagittal stratum (IFOF), centrum semiovale (callosal fibres, corona radiata, SLF I), internal capsule (posterior limb) and perisylvian region. For FD, reductions were seen in the external/extreme capsule (IFOF and uncinate fasciculus) and centrum semiovale (callosal fibres) (Figure 1, row on the top).
Model 2 (levamisole): We found lower FA in CU in the CC (callosal fibres running through the genu, body, isthmus and splenium), centrum semiovale (callosal fibres, corona radiata, SLF I) and perisylvian region. Similar results were obtained for FD (Figure 1, row on the bottom).
Model 3 (alcohol, Table 2): FA reductions in CU in the external/extreme capsule (IFOF), centrum semiovale (callosal fibres, corona radiata, SLF I) and perisylvian region. Similar results were obtained for FD.
FIGURE 1.
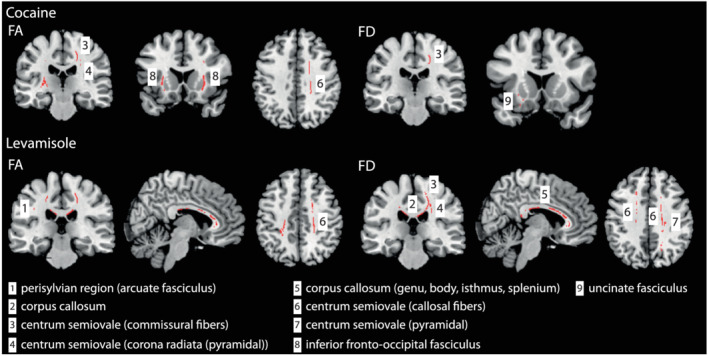
Between‐group differences for the cocaine and levamisole model. Cocaine use (top row) and levamisole exposure (bottom row) reduce FA and FD values in multiple WM tracts (for details, we refer to Table 2) comparing patients to controls. Results were adjusted for age, sex, ADHD, alcohol, cigarette use, MDMA, ICV, levamisole exposure (cocaine model) or cocaine use (levamisole model). All results are shown at p < 0.050 (corrected using threshold‐free cluster enhancement [TFCE] correction)
TABLE 2.
Summary of whole‐brain between group differences (HC > CU) for the three statistical models (cocaine, levamisole and alcohol)
| Model 1 (cocaine) | ||
|---|---|---|
| DTI measure: FA | ||
| Region | Fibre system | Hemisphere |
| External/extreme capsule | IFOF | Right and left |
| Sagittal stratum | IFOF | Left |
| Centrum semiovale |
‐ Callosal fibres ‐ Corona radiata (pyramidal tract) ‐ SLF (I) |
Right |
| Internal capsule (post. limb) | (Pyramidal tract) | Left |
| Perisylvian region | Arcuate fasciculus | Right |
| DTI measure: FD | ||
| Region | Fibre system | Hemisphere |
| External/extreme capsule |
‐ IFOF ‐ UF |
Left |
| Centrum semiovale | ‐ Callosal fibres | Right |
| Model 2 (levamisole) | ||
| DTI measure: FA | ||
| Region | Fibre system | Hemisphere |
| Corpus callosum | Callosal fibres running through the genu, body, isthmus and splenium | n.a. |
| Centrum semiovale |
‐ Callosal fibres ‐ Corona radiata (pyramidal tract) ‐ SLF (I) |
Right and left |
| Perisylvian region | Arcuate fasciculus | Left |
| DTI measure: FD | ||
| Region | Fibre system | Hemisphere |
| Corpus callosum | Callosal fibres running through the genu, body, isthmus and splenium | n.a. |
| Centrum semiovale |
‐ Callosal fibres ‐ Corona radiata (inclusive but not restricted to the pyramidal tract) ‐ SLF (I) |
Right and left |
| Perisylvian region | Arcuate fasciculus | Left |
| Model 3 (alcohol) | ||
| DTI measure: FA | ||
| Region | Fibre system | Hemisphere |
| External/extreme capsule | IFOF | Right and left |
| Centrum semiovale |
‐ Callosal fibres ‐ Corona radiata (pyramidal tract) ‐ SLF (I) |
Right and left |
| Perisylvian region | Arcuate fasciculus | Left |
| DTI measure: FD | ||
| Region | Fibre system | Hemisphere |
| Corpus callosum | Callosal fibres running through the isthmus and splenium | n.a. |
| Centrum semiovale |
‐ Callosal fibres ‐ Corona radiata (inclusive but not restricted to the pyramidal tract) ‐ SLF (I) |
Right and left |
| Perisylvian region | Arcuate fasciculus | Left |
Notes: All results are corrected for age, sex, ADHD, nicotine, MDMA, cocaine and intracranial volume. For Model 1, we additionally corrected for levamisole exposure and alcohol. For Model 2, we additionally corrected for cocaine and alcohol. For Model 3, we additionally corrected for cocaine and levamisole exposure. Differences are shown at p < 0.05 (corrected). Labelling is based on ICBM‐DTI‐81 WM labels atlas (48 WM tract labels).
Abbreviations: IFOF, inferior fronto‐occipital fasciculus; SLF, superior longitudinal fasciculus; UC, uncinate fasciculus.
All results are summarized in Table 2.
3.2.2. Within‐patient group analysis
The results are illustrated in Figure 2 and summarized in Table 3.
FIGURE 2.
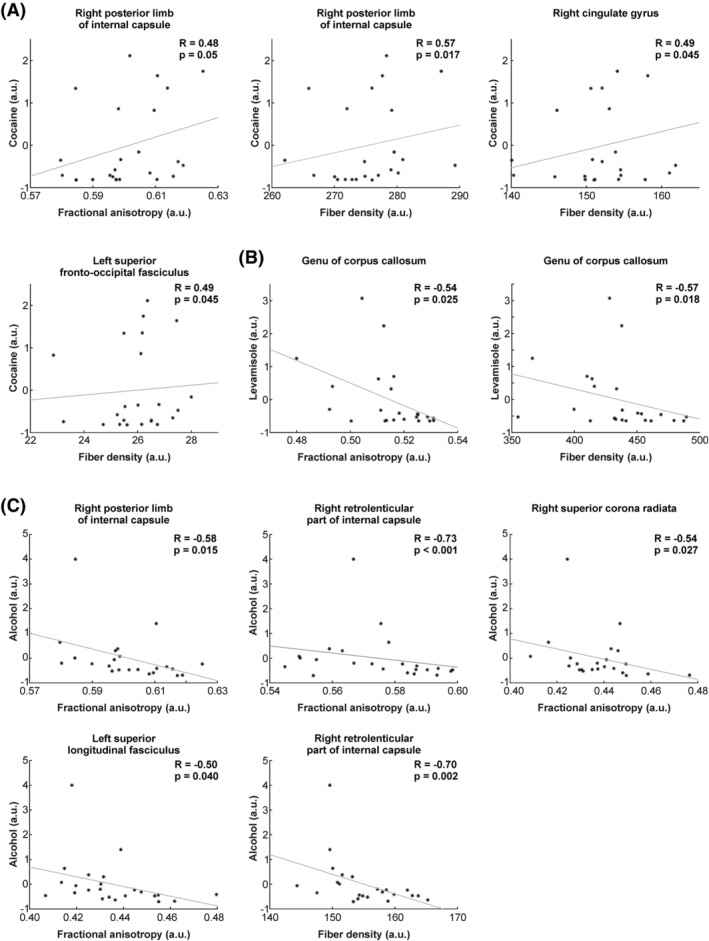
Relationship between different substances and indices of white matter integrity (i.e. FA and/or FD). (A) Relationship between indices of white matter integrity and cocaine exposure. (B) Relationship between indices of white matter integrity and levamisole exposure. (C) Relationship between indices of white matter integrity and alcohol consumption. For display purposes, the different individual values (i.e. cocaine, levamisole and alcohol) were normalized across patients. a.u., arbitrary units. The R and p‐values correspond to correlation analyses, including the six regressors as covariates (i.e. age, sex, ADHD, nicotine, MDMA and ICV)
TABLE 3.
Summary of significant correlations between FA and FD with cocaine, levamisole and alcohol
| Cocaine | |||||
|---|---|---|---|---|---|
| FA | |||||
| Between substance comparison | |||||
| Name | COC and FA | ALC and FA a | LEV and FA a | Comparison COC and ALC | Comparison COC and LEV |
| Right posterior limb of the internal capsule | R = 0.48 | R = −0.58 | R = 0.28 | p < 0.001 | 0.44 |
| 0.05 | 0.02 | 0.28 | |||
| FD | |||||
| Right posterior limb of internal capsule | R = 0.57 | R = −0.44 | R = 0.04 | p < 0.001 | 0.4 |
| 0.017 | 0.08 | 0.17 | |||
| Right cingulum (cingulate gyrus) | R = 0.49 | R = −0.12 | R = 0.25 | p < 0.037 | 0.4 |
| 0.045 | 0.65 | 0.35 | |||
| Left superior fronto‐occipital fasciculus | R = 0.49 | R = −0.09 | R = 0.30 | 0.048 | 0.5 |
| 0.045 | 0.75 | 0.23 | |||
| Levamisole | |||||
|---|---|---|---|---|---|
| FA | |||||
| Between substance comparison | |||||
| Name | LEV and FA | ALC and FA a | COC and FA a | Comparison LEV and ALC | Comparison LEV and COC |
| Genu of corpus callosum | R = −0.54 | R = 0.26 | R = −0.26 | 0.006 | 0.27 |
| 0.025 | 0.30 | 0.32 | |||
| FD | |||||
| Name | LEV and FD | ALC and FD* | COC and FD* | Comparison LEV and ALC | Comparison LEV and COC |
| Genu of corpus callosum | R = −0.57 | R = 0.25 | R = −0.31 | 0.005 | 0.30 |
| 0.018 | 0.33 | 0.23 | |||
| Alcohol | |||||
|---|---|---|---|---|---|
| FA | |||||
| Between substance comparison | |||||
| Name | ALC and FA | LEV and FA a | COC and FA a | Comparison ALC and LEV | Comparison ALC and COC |
| Right posterior limb of internal capsule | R = −0.58 | R = 0.28 | R = 0.48 | 0.003 | p < 0.001 |
| 0.015 | 0.28 | 0.05 | |||
| Right retrolenticular part of internal capsule | R = −0.73 | R = −0.05 | R = 0 | 0.006 | 0.004 |
| p < 0.001 | 0.85 | 1 | |||
| Right superior corona radiata | R = −0.53 | R = 0.09 | R = 0.22 | 0.029 | 0.009 |
| 0.027 | 0.72 | 0.39 | |||
| Left superior longitudinal fasciculus | R = −0.50 | R = −0.16 | R = −0.04 | 0.22 | 0.10 |
| 0.040 | 0.537 | 0.89 | |||
| FD | |||||
| Name | ALC and FD | LEV and FD a | COC and FD a | Comparison ALC and LEV | Comparison ALC and COC |
| Right retrolenticular part of internal capsule | R = −0.70 | R = 0.09 | R = 0.32 | 0.002 | p < 0.001 |
| 0.002 | 0.72 | 0.21 | |||
Note: The comparison of the correlation strength between two substances is listed in the last two columns of each table.
Abbreviations: ALC, alcohol; COC, cocaine; LEV, levamisole.
Correlations are reported for completeness.
Cocaine (pg/mg) showed a positive correlation of FA with WM of the right posterior limb of the internal capsule (r = 0.48, p = 0.050; Figure 2a), as well as a positive correlation with FD in posterior limb of the internal capsule (r = 0.57, p = 0.017), right cingulate gyrus (r = 0.49, p = 0.045) and in superior fronto‐occipital fasciculus (r = 0.49, p = 0.045; Figure 2a). For levamisole (pg/mg), negative correlations with FA (Figure 2b) were seen in the genu of the CC (r = −0.54, p = 0.025). In addition, FD negatively correlated with the genu of the CC (r = −0.57, p = 0.018).
Negative correlations between alcohol (pure ethanol in g/week) and FA (Figure 2c) were seen with the right posterior limb of the internal capsule (r = −0.58, p = 0.015), right retrolenticular part of the internal capsule (r = −0.73, p < 0.001), right superior corona radiata (r = −0.54, p = 0.027) and left SLF (r = −0.50, p = 0.040). The correlation of FA and right retrolenticular part of the internal capsule survives multiple comparison correction (p corrected = 0.05/49, i.e. 0.001). For FD, negative correlations with alcohol were observed for the right retrolenticular part of the internal capsule (r = −0.70, p = 0.002).
Next, we compared correlations strengths for significant WM clusters (summarized in Figures [Link], [Link], [Link]). For example, we found that FA (of the right posterior limb of internal capsule) was inversely correlated to alcohol consumption (Figure S2) and that this correlation significantly differed from the correlation of alcohol with levamisole or cocaine (all between‐substance correlation comparisons are summarized in Table 3).
As displayed in Figure 3, longer cocaine use (in years, normalized values in the figure) resulted in a significant positive correlation of FA with the right superior cerebellar peduncle (r = 0.51, p = 0.037) and a negative correlation of FD with the right cingulum hippocampus (r = −0.55, p = 0.022).
FIGURE 3.
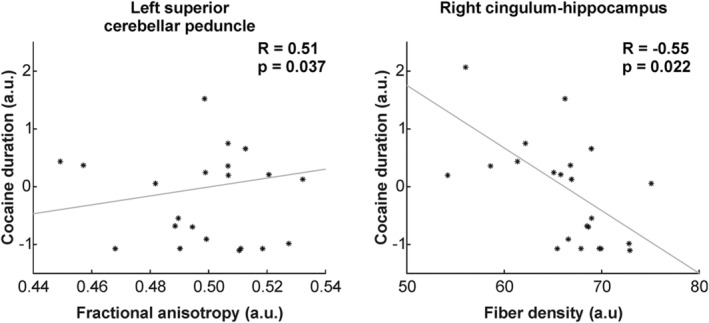
Relationship between the duration of cocaine consumption and indices of white matter integrity (i.e. FA and/or FD). A positive relationship between the duration of the cocaine consumption and the FA was observed for the left superior cerebellar peduncle. A negative correlation was seen with the right cingulum hippocampus. For display purposes, the individual duration of cocaine consumption were normalized across patients. a.u., arbitrary units. The R and p‐values correspond to correlation analyses, including the six regressors as covariates (i.e. age, sex, ADHD, nicotine, MDMA and ICV)
4. DISCUSSION
In this cross‐sectional DTI study, we found consistent WM alterations in CU compared with HC using FA and FD. Thus, apart from FA, FD appears as sensitive marker to identify WM changes specifically associated with cocaine, levamisole and alcohol in chronic CU. Some of these impairments were related to a particular substance, detected as significantly stronger negative correlations. Negative correlations were only observed for levamisole exposure and alcohol use. However, we demonstrated that at least a longer use of cocaine is related to greater WM impairments, which is, however, also inevitably associated with a longer levamisole exposure.
4.1. HC versus patients: The role of cocaine use on WM integrity
A WM tract that showed consistently lower FA in CU across all examined models was the SLF‐I. The SLF‐I originates from the superior parietal lobe, which projects (ventrally) along the cingulate gyrus, to the superior frontal gyrus (dorsal spatial/motor network 92 ), terminating within the supplementary motor and premotor areas in the frontal lobe. This bundle plays a role in attention, response inhibition, proprioception and motor movement. 93 , 94 This finding is in line with previous behavioural data in rodents and humans showing specific impairments in response inhibition and flexible decision‐making in chronic CU. 21 , 95 , 96 In addition, the study by Lebel et al reported abnormal WM microstructure in adolescents with prenatal cocaine expose in the arcuate fasciculus, cingulum and CC. The arcuate fasciculus contains both long and short WM fibres that connect the frontal, parietal and temporal lobes. 97 It plays a key role in visuospatial processing and some aspects of language processing, such as prosody and semantics. 97 Here, we have not assessed tests related to language and visuospatial processing, but previous work has shown considerable impairments of chronic CU in visuospatial working memory and visuospatial paired associates learning 98 as well as in perception of speech, verbal fluency and other language functions. 99
Lower FA and FD values were also seen in the IFOF. The IFOF consists of two layers: The first one is superficial and antero‐superiorly directed, terminating in the inferior frontal gyrus. The second layer is deeper and comprises three portions: posterior, middle and anterior. 100 , 101 The posterior component ends in the middle frontal gyrus and dorsolateral prefrontal cortex. The middle component terminates in the middle frontal gyrus and lateral orbitofrontal cortex (OFC). The anterior one is directed to the OFC and frontal pole. Generally, the IFOF acts as a ‘multifunction’ bundle, with each anatomical subcomponent subserving different brain processing. The superficial layer and the posterior component of the deep layer, which connects the occipital extrastriate, temporo‐basal and inferior frontal cortices, might be relevant semantic processing. The middle component of the deep layer could play a role in multimodal sensory‐motor integration. Lastly, the anterior component of the deep layer might be involved in emotional and behavioural aspects (e.g. anxiety and depression). We cannot examine layer‐specific impairment by our DTI approach, but our results indicate the overall (mean) WM of the IFOF seem to be consistently disturbed in CU compared with HC.
The observed lower FA in the uncinate fasciculus in the CU is in line with two recent studies. One DTI study demonstrated lower FA in polysubstance users (including cocaine), compared with controls, in the body of the CC, anterior cingulate, uncinate fasciculus and retrolenticular part of the internal capsule. Duration of cocaine abstinence was positively correlated with FA in the uncinate fasciculus, posterior cingulate and fornix striatum. In the context of cocaine polysubstance use, chronicity of cocaine use was therefore likely to be associated with lower FA in the CC and chronicity of alcohol use with lower FA in the frontal‐striatal and frontal‐limbic tracts. Longer abstinence was associated with greater FA in frontal‐striatal and frontal‐limbic tracts. In a more recent study, it was shown that long‐term abstinence resulted in higher FA values (compared with current users) in the uncinate fasciculus as well as in the bilateral CC, SLF, inferior longitudinal fasciculus, uncinate fasciculus, left IFOF and the left ventral and dorsal medial frontal regions. 102
4.2. HC versus patients: The role of levamisole and alcohol on WM integrity in CU
The presence of higher levels of levamisole in hair and more weekly alcohol intake was related to stronger WM alterations (Model 3; Table 2), indicating that not only CU but also the cocaine‐adulterant levamisole and the additional use of alcohol impair WM microstructural integrity. Strikingly, association, commissural and projection fibres demonstrated WM impairments related to levamisole and alcohol. It has been shown that sex‐dependent alterations occur in the commissural tracts (callosal body), CC, SLF (association fibres), IFOF and uncinate fasciculus in children and adolescents with prenatal alcohol exposure. 103 In a ‘binge’ model of early prenatal alcohol exposure in sheep, Watari and co‐workers reported WM alterations in further WM regions, including the frontal gyral WM, temporal gyral WM, optic radiation, CC, septum pellucidum, fasciculus subcallosus and capsule externa. 104 Seigneurie et al reviewed the brain abnormalities that might underlie the risk towards alcohol dependence. 105 With respect to WM, the authors reported local WM volume deficits in the CC and in the right OFC and lower FA in the left IFOF and in the right optic radiation. However, in our study, we did not assess if our CU came from families with multiple cases of alcohol dependence or experienced prenatal alcohol exposure. We also did not record if intense alcohol consumption started prior to cocaine use or was intensified later during cocaine use episodes. A previous study found FA alterations in the optic radiation as well as in the different parts of the corona radiata, CC and capsule interna in chronic cocaine users. 15 However, authors conclude a number of variables within and between the cocaine and control groups could have biased the DTI results, including alcohol use, level of education, smoking status and (within the cocaine group) age of onset of cocaine use. In Model 3, we corrected for age, sex, ADHD, nicotine, MDMA, ICV, levamisole and cocaine use and still observed significant alterations in association, commissural and projection fibres. We conclude that our study is the first, to our knowledge, that identifies seen as alterations in FA and FD of alcohol in CU users. The CC showed alterations in FA and FD for Models 2 and 3. Related to Model 3, this finding is in line with our initial hypothesis, as it has been shown that impairments in CC (and other brain regions) can be reversed by alcohol abstinence. 19 , 25
A previous case report reported reduced FA and increased RD in a woman who developed multifocal inflammatory leukoencephalopathy after the treatment with levamisole for adenocarcinoma, 106 a result that is in line with our finding that levamisole contributes to WM integrity alterations in CU. Of note, none of our CU displayed the full picture of a multifocal leukoencephalopathy, as seen in levamisole‐medicated patients. 60 , 63 However, we have recently shown in an overlapping sample that levamisole‐exposed CU display more WM hyperintensities, 65 which are considered more subtle, chronic ischemic lesions in the WM caused by constriction and damage of subcortical small vessels. 107 Moreover, high levamisole exposure due to the use of contaminated cocaine was also going along with stronger impairment of executive functions and reduced cortical thickness in various region of the prefrontal cortex. 64 Consequently, the present data again confirm that levamisole—also as an adulterant of cocaine—might be neurotoxic specifically for WM structures.
4.3. Within‐patient group analysis
Surprisingly, we were not able to statistically support the hypothesis that higher cocaine hair concentrations also went along with lower FA or FD values. Yet, it seems that subjects with longer use of cocaine exhibited similar WM alterations as seen for levamisole exposure. This might relates to the change from cocaine to levamisole‐contaminated cocaine use along subjects' personal history of cocaine use. Furthermore, there was one WM region—the (superior) cerebellar peduncle—that contains the afferent and efferent tracts of the cerebellum, which showed a positive correlation of FA and cocaine use and duration. The tracts of this region connect the cerebellum with other parts of the central nervous system such as the pons, thalamus and prefrontal cortex. 108 The cerebellar peduncle supports refining motor movements or learning new motor skills, that is, a damage in this region most often results in imbalance and lack of proprioception. 109 , 110 , 111
Multifocal leukoencephalopathy associated with cocaine use, especially with the use of levamisole, leads to increasing abnormal MRI signal lesions with patchy restricted diffusion and heterogeneous enhancement deep in both hemispheres, including internal and external capsules, putamen, corona radiata and periventricular WM as well as in the cerebellar peduncle and midbrain, that is, the pons. 59 Yet, based on these results, we would have assumed a negative correlation between cerebral peduncle WM and cocaine use. However, a study by Todd et al demonstrated that abnormal (increased) substantia nigra morphology was linked to regular stimulant use, including cocaine. 112 As the substantia nigra is in close spatial proximity to the cerebral peduncle, it might be that the increase of FA is associated with abnormal morphology of the substantia nigra and surrounding tissue including the cerebral peduncle. Future studies are required that might examine the interaction of WM integrity of the cerebral peduncle and substantia nigra and cocaine use.
The multimodal imaging study 113 emphasizes the tight link between WM alterations seen in several WM regions, including regions for which we found a negative correlation of FA/FD with elevated alcoholic intake (i.e. anterior corona radiata, body of the CC, cingulate gyrus, external capsule, fornix, IFOF, posterior corona radiata, retrolenticular limb of internal capsule and SLF). Specifically, Monning et al reported negative correlations of FA with the fMRI signal of regions of the frontoparietal and corticolimbic networks, which the authors interpreted as a loss of control over alcohol consumption. All our study participants were adults, but the onset of alcohol was likely during adolescence, which might affect even more WM structure than adult‐onset alcohol use as demonstrated recently. 114 All our results were corrected for age (as well as for sex, ADHD, nicotine, MDMA and ICV), which contribute differently on alcohol consumption on onset. Yet, nutrition might be another factor that might be considered when modelling human alcohol consumption and its link to WM alterations. 115
5. LIMITATIONS
Our study has some limitations. The sample size was relatively small specifically in the CU group. Unfortunately, our CU showed more agitation (i.e. more movement) in the scanner, likely explained by higher burden with ADHD symptoms, resulting in a strong dropout rate because of our strict imaging quality reasons. Moreover, we only used a cross‐sectional design, and future longitudinal studies will be necessary to test the long‐lasting impact of levamisole and alcohol on DTI metrics in CU. Hence, our results rely only on a correlation approach, and we could not examine the data in a causal manner. Another limitation is that we used a constrained spherical deconvolution algorithm for the tractography, for which the 32 acquired directions are few. The number of directions determines the L MAX, the maximum degree of harmonics that can be used; typically, L = 8 is used, and for this, at least 45 directions are needed. With 32 directions, we are limited to L = 6. In addition, most of our correlations do not survive a proper statistical correction and should therefore be taken as trends and interpreted with caution. Furthermore, the group comparison with the exclusion of specific covariates in the different models informs only partially informs about the true impact of each separate substance (i.e. cocaine, alcohol, levamisole and substances not considered in our models) on WM differences between controls and patients. Moreover, other factors, such as more pronounced affective psychopathology, may have impacted WM integrity results in our patients, warranting future investigation on the impact of such symptoms on WM integrity in CU.
6. CONCLUSION
Firstly, our results indicate that FD is a valid DTI marker that can detect (next to FA) structural alterations in CU. Secondly, cocaine‐, levamisole‐ and alcohol‐exposed CU demonstrated impaired WM structure. However, based on the correlation analysis comparing substances, alcohol and levamisole exposure were associated with stronger multiregional WM impairments than cocaine use itself. Thus, the reduction of levamisole in street cocaine should be a significant aim of current drug policymaking worldwide given that refraining from levamisole‐contaminated cocaine likely attenuates WM disease and related symptoms. Nevertheless, longer use of cocaine resulted in a similar reduction of WM integrity, even though these reductions occur in different WM regions.
FUNDING STATEMENT
Open access funding provided by Universitat Zurich. WOA Institution: Universitat Zurich. Blended DEAL: CSAL.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interest.
AUTHOR CONTRIBUTIONS
BQ and SH designed and conceptualized the study.SH performed data recording and data preprocessing. MM, PS, and LM analysed the imaging (DTI) data. WS helped with white matter labelling and data interpretation. MB supported the study by performing the hair sample analysis. ES provided additional funding. LM and BQ wrote the manuscript. All co‐authors read and corrected the original version of the manuscript. All authors approved the final version of the publication.
Supporting information
Figure S1. A positive relationship was observed for cocaine consumption and indices of white matter integrity (i.e. FA and/or FD) in several tracts, including right posterior limb of internal capsule, right cingulate gyrus and left superior fronto‐occipital fasciculus. This relationship between the cocaine consumption and indices of white matter integrity for these tracts is significantly stronger as compared alcohol consumption. For display purposes, the different individual consumption values (i.e. alcohol and cocaine) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
Figure S2. A negative relationship was observed for alcohol consumption and indices of white matter integrity (i.e. FA and/or FD) in several tracts, including right posterior limb of internal capsule, right retrolenticular part of internal capsule (survives multiple comparison correction) and right superior corona radiata. Importantly, this relationship between the alcohol consumption and indices of white matter integrity for these tracts is significantly stronger as compared to both levamisole and cocaine. For display purposes, the different individual consumption values (i.e. alcohol, levamisole, and cocaine) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
Figure S3. A negative relationship was observed for levamisole exposure and indices of white matter integrity (i.e. FA and/or FD) in the genu of CC. Importantly, this relationship between the levamisole exposure and indices of white matter integrity for these tracts is significantly stronger as compared to alcohol. For display purposes, the different individual consumption values (i.e. alcohol and levamisole) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
ACKNOWLEDGEMENTS
The study was supported by grants from the Swiss National Science Foundation (SNSF; grant nos. PP00P1‐123516/1 and PP00P1‐146326/1 and PP00P3_170683) and the Hartmann Müller Foundation (grant no. 1826) to BBQ as well as by a grant of the Fonds für wissenschaftliche Zwecke im Interesse der Heilung von psychischen Krankheiten (approval date: 16 November 2015) to SH who was additionally supported by research grants from the University of Zurich (grant nos. FK‐15‐038 and FK‐16‐042). The funders of the study did not influence the design and conduct of the study; the collection, management, analysis and interpretation of the data; the preparation, review and approval of the manuscript; and the decision to submit the manuscript for publication. Open Access Funding provided by Universitat Zurich.
Michels L, Moisa M, Stämpfli P, et al. The impact of levamisole and alcohol on white matter microstructure in adult chronic cocaine users. Addiction Biology. 2022;27 (3): e13149. doi: 10.1111/adb.13149
[Correction added on 8 April 2022, after first online publication: CSAL funding statement has been added.]
DATA AVAILABILITY STATEMENT
The data associated with this publication can be shared upon reasonable request.
REFERENCES
- 1. Walker ER, Pratt LA, Schoenborn CA, Druss BG. Excess mortality among people who report lifetime use of illegal drugs in the United States: a 20‐year follow‐up of a nationally representative survey. Drug Alcohol Depend. 2017;171:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Das G. Cardiovascular effects of cocaine abuse. Int J Clin Pharmacol Ther Toxicol. 1993;31(11):521‐528. [PubMed] [Google Scholar]
- 3. Kosten TR. Pharmacotherapy of cerebral ischemia in cocaine dependence. Drug Alcohol Depend. 1998;49(2):133‐144. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558‐2569. [DOI] [PubMed] [Google Scholar]
- 5. Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad Med J. 2007;83(980):389‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulka LM, Eisenegger C, Preller KH, et al. Altered social and non‐social decision‐making in recreational and dependent cocaine users. Psychol Med. 2014;44(5):1015‐1028. [DOI] [PubMed] [Google Scholar]
- 7. Hulka LM, Vonmoos M, Preller KH, et al. Changes in cocaine consumption are associated with fluctuations in self‐reported impulsivity and gambling decision‐making. Psychol Med. 2015;45(14):3097‐3110. [DOI] [PubMed] [Google Scholar]
- 8. Kluwe‐Schiavon B, Kexel A, Manenti G, et al. Sensitivity to gains during risky decision‐making differentiates chronic cocaine users from stimulant‐naïve controls. Behav Brain Res. 2020;379:112386. [DOI] [PubMed] [Google Scholar]
- 9. Kluwe‐Schiavon B, Viola TW, Sanvicente‐Vieira B, et al. Substance related disorders are associated with impaired valuation of delayed gratification and feedback processing: a multilevel meta‐analysis and meta‐regression. Neurosci Biobehav Rev. 2020;108:295‐307. [DOI] [PubMed] [Google Scholar]
- 10. Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta‐analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23(4):615‐624. [DOI] [PubMed] [Google Scholar]
- 11. Hirsiger S, Hänggi J, Germann J, et al. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. NeuroImage: Clinical. 2019;21:101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beard CL, Schmitz JM, Soder HE, et al. Regional differences in white matter integrity in stimulant use disorders: a meta‐analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 2019;201:29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lyoo IK, Streeter CC, Ahn KH, et al. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131(2):135‐145. [DOI] [PubMed] [Google Scholar]
- 14. Hall MG, Alhassoon OM, Stern MJ, et al. Gray matter abnormalities in cocaine versus methamphetamine‐dependent patients: a neuroimaging meta‐analysis. Am J Drug Alcohol Abuse. 2015;41(4):290‐299. [DOI] [PubMed] [Google Scholar]
- 15. Lane SD, Steinberg JL, Ma L, et al. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5(7):e11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma L, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104(3):262‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine‐dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610‐617. [DOI] [PubMed] [Google Scholar]
- 18. Moeller FG, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 19. Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35(7):1541‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. 2017;22(4):1048‐1056. [DOI] [PubMed] [Google Scholar]
- 21. Lebel C, Warner T, Colby J, et al. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatry Res. 2013;213(2):161‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Z, Santhanam P, Coles CD, et al. Prenatal cocaine exposure alters functional activation in the ventral prefrontal cortex and its structural connectivity with the amygdala. Psychiatry Res. 2013;213(1):47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lim KO, Wozniak JR, Mueller BA, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92(1–3):164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Son D, Wiers RW, Catena A, Perez‐Garcia M, Verdejo‐Garcia A. White matter disruptions in male cocaine polysubstance users: associations with severity of drug use and duration of abstinence. Drug Alcohol Depend. 2016;168:247‐254. [DOI] [PubMed] [Google Scholar]
- 25. Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine‐dependent individuals. Drug Alcohol Depend. 2011;114(2–3):159‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion‐tensor MRI. Magn Reson Med. 2001;45(5):770‐780. [DOI] [PubMed] [Google Scholar]
- 27. Alexander DC, Barker GJ, Arridge SR. Detection and modeling of non‐Gaussian apparent diffusion coefficient profiles in human brain data. Magn Reson Med. 2002;48(2):331‐340. [DOI] [PubMed] [Google Scholar]
- 28. Frank LR. Anisotropy in high angular resolution diffusion‐weighted MRI. Magn Reson Med. 2001;45(6):935‐939. [DOI] [PubMed] [Google Scholar]
- 29. Frank LR. Characterization of anisotropy in high angular resolution diffusion‐weighted MRI. Magn Reson Med. 2002;47(6):1083‐1099. [DOI] [PubMed] [Google Scholar]
- 30. Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34(11):2747‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. 2002;48(4):577‐582. [DOI] [PubMed] [Google Scholar]
- 32. Beaulieu C, Does MD, Snyder RE, Allen PS. Changes in water diffusion due to Wallerian degeneration in peripheral nerve. Magn Reson Med. 1996;36(4):627‐631. [DOI] [PubMed] [Google Scholar]
- 33. Ciccarelli O, Behrens TE, Altmann DR, et al. Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain. 2006;129(7):1859‐1871. [DOI] [PubMed] [Google Scholar]
- 34. Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria‐fornix in temporal lobe epilepsy. J Neurosci. 2010;30(3):996‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Madler B, Drabycz SA, Kolind SH, Whittall KP, MacKay AL. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T‐2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874‐888. [DOI] [PubMed] [Google Scholar]
- 36. Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi‐tissue constrained spherical deconvolution for improved analysis of multi‐shell diffusion MRI data. Neuroimage. 2014;103:411‐426. [DOI] [PubMed] [Google Scholar]
- 37. Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239‐254. [DOI] [PubMed] [Google Scholar]
- 38. Vos SB, Jones DK, Jeurissen B, Viergever MA, Leemans A. The influence of complex white matter architecture on the mean diffusivity in diffusion tensor MRI of the human brain. Neuroimage. 2012;59(3):2208‐2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wheeler‐Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61(5):1255‐1260. [DOI] [PubMed] [Google Scholar]
- 40. Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non‐negativity constrained super‐resolved spherical deconvolution. Neuroimage. 2007;35(4):1459‐1472. [DOI] [PubMed] [Google Scholar]
- 41. Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fillard P, Descoteaux M, Goh A, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage. 2011;56(1):220‐234. [DOI] [PubMed] [Google Scholar]
- 43. Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65(6):1532‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Girard G, Whittingstall K, Deriche R, Descoteaux M. Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage. 2014;98:266‐278. [DOI] [PubMed] [Google Scholar]
- 45. Smith RE, Tournier JD, Calamante F, Connelly A. Anatomically‐constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage. 2012;62(3):1924‐1938. [DOI] [PubMed] [Google Scholar]
- 46. Pestilli F, Yeatman JD, Rokem A, Kay KN, Wandell BA. Evaluation and statistical inference for human connectomes. Nat Methods. 2014;11(10):1058‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sherbondy AJ, Dougherty RF, Ananthanarayanan R, Modha DS, Wandell BA. Think global, act local; projectome estimation with BlueMatter. Med Image Comput Comput‐Assist Interv Miccai 2009, pt I, Proc. 2009;12:861‐868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sherbondy AJ, Rowe MC, Alexander DC. MicroTrack: an algorithm for concurrent projectome and microstructure estimation. Lect Notes Comput Sc. 2010;13:183‐190. [DOI] [PubMed] [Google Scholar]
- 49. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT: spherical‐deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298‐312. [DOI] [PubMed] [Google Scholar]
- 50. Smith RE, Tournier JD, Calamante F, Connelly A. SIFT2: enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage. 2015;119:338‐351. [DOI] [PubMed] [Google Scholar]
- 51. Daducci A, Dal Palu A, Lemkaddem A, Thiran JP. COMMIT: convex optimization modeling for microstructure informed tractography. IEEE Trans Med Imaging. 2015;34(1):246‐257. [DOI] [PubMed] [Google Scholar]
- 52. Daducci A, Dal Palu A, Descoteaux M, Thiran JP. Microstructure informed tractography: pitfalls and open challenges. Front Neurosci. 2016;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Calamante F, Smith RE, Tournier JD, Raffelt D, Connelly A. Quantification of voxel‐wise total fibre density: investigating the problems associated with track‐count mapping. Neuroimage. 2015;117:284‐293. [DOI] [PubMed] [Google Scholar]
- 54. Raffelt DA, Tournier JD, Smith RE, et al. Investigating white matter fibre density and morphology using fixel‐based analysis. Neuroimage. 2017;144(Pt A):58‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stampfli P, Sommer S, Czell D, et al. Investigation of neurodegenerative processes in amyotrophic lateral sclerosis using white matter fiber density. Clin Neuroradiol. 2019;29(3):493‐503. [DOI] [PubMed] [Google Scholar]
- 56. Stampfli P, Sommer S, Manoliu A, et al. Subtle white matter alterations in schizophrenia identified with a new measure of fiber density. Sci Rep‐Uk. 2019;9(1):4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage. 2006;31(4):1487‐1505. [DOI] [PubMed] [Google Scholar]
- 58. Vitt JR, Brown EG, Chow DS, Josephson SA. Confirmed case of levamisole‐associated multifocal inflammatory leukoencephalopathy in a cocaine user. J Neuroimmunol. 2017;305:128‐130. [DOI] [PubMed] [Google Scholar]
- 59. Vosoughi R, Schmidt BJ. Multifocal leukoencephalopathy in cocaine users: a report of two cases and review of the literature. BMC Neurol. 2015;15(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hook CC, Kimmel DW, Kvols LK, et al. Multifocal inflammatory leukoencephalopathy with 5‐fluorouracil and levamisole. Ann Neurol. 1992;31(3):262‐267. [DOI] [PubMed] [Google Scholar]
- 61. Liu HM, Hsieh WJ, Yang CC, Wu VC, Wu KD. Leukoencephalopathy induced by levamisole alone for the treatment of recurrent aphthous ulcers. Neurology. 2006;67(6):1065–1067. [DOI] [PubMed] [Google Scholar]
- 62. Wu VC, Huang JW, Lien HC, et al. Levamisole‐induced multifocal inflammatory leukoencephalopathy: clinical characteristics, outcome, and impact of treatment in 31 patients. Medicine (Baltimore). 2006;85(4):203–213. [DOI] [PubMed] [Google Scholar]
- 63. Xu N, Zhou W, Li S, Zhou G, Zhang N, Liang J. Clinical and MRI characteristics of levamisole‐induced leukoencephalopathy in 16 patients. J Neuroimaging. 2009;19(4):326‐331. [DOI] [PubMed] [Google Scholar]
- 64. Vonmoos M, Hirsiger S, Preller KH, et al. Cognitive and neuroanatomical impairments associated with chronic exposure to levamisole‐contaminated cocaine. Transl Psychiatry. 2018;8(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Conrad F, Hirsiger S, Winklhofer S, et al. Use of levamisole‐adulterated cocaine is associated with increased load of white matter lesions. J Psychiatry Neurosci. 2021. In Press;46(2):E281‐E291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Carroll KM, Rounsaville BJ, Bryant KJ. Alcoholism in treatment‐seeking cocaine abusers: clinical and prognostic significance. J Stud Alcohol. 1993;54(2):199‐208. [DOI] [PubMed] [Google Scholar]
- 67. Chumin EJ, Grecco GG, Dzemidzic M, et al. Alterations in white matter microstructure and connectivity in young adults with alcohol use disorder. Alcohol Clin Exp Res. 2019;43(6):1170‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chumin EJ, Goñi J, Halcomb ME, Durazzo TC, Dzemidzic M, Yoder KK. Differences in white matter microstructure and connectivity in nontreatment‐seeking individuals with alcohol use disorder. Alcohol Clin Exp Res. 2018;42(5):889‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hirsiger S, Hanggi J, Germann J, et al. Longitudinal changes in cocaine intake and cognition are linked to cortical thickness adaptations in cocaine users. Neuroimage Clin. 2019;21:101652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Scholz C, Cabalzar J, Kraemer T, Baumgartner MR. A comprehensive multi‐analyte method for hair analysis: substance‐specific quantification ranges and tool for task‐oriented data evaluation. J Anal Toxicol. 2020;45(7):701‐712. [DOI] [PubMed] [Google Scholar]
- 71. Bush DM. The U.S. mandatory guidelines for federal workplace drug testing programs: current status and future considerations. Forensic Sci Int. 2008;174(2–3):111‐119. [DOI] [PubMed] [Google Scholar]
- 72. Cooper GA, Kronstrand R, Kintz P, Society of Hair T . Society of hair testing guidelines for drug testing in hair. Forensic Sci Int. 2012;218(1–3):20‐24. [DOI] [PubMed] [Google Scholar]
- 73. APA . Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV. 4thed. American Psychiatric Association; 1994. [Google Scholar]
- 74. Quednow BB, Kuhn KU, Hoenig K, Maier W, Wagner M. Prepulse inhibition and habituation of acoustic startle response in male MDMA (‘ecstasy’) users, cannabis users, and healthy controls. Neuropsychopharmacology. 2004;29(5):982‐990. [DOI] [PubMed] [Google Scholar]
- 75. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119‐1127. [DOI] [PubMed] [Google Scholar]
- 76. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768‐774. [DOI] [PubMed] [Google Scholar]
- 77. Rosler M, Retz W, Retz‐Junginger P, et al. Instrumente zur Diagnostik der Aufmerksamkeitsdefizit‐/Hyperaktivitatsstorung (ADHS) im ErwachsenenalterSelbstbeurteilungsskala (ADHS‐SB) und Diagnosecheckliste (ADHS‐DC). Nervenarzt. 2004;75(9):888‐895. [DOI] [PubMed] [Google Scholar]
- 78. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782‐790. [DOI] [PubMed] [Google Scholar]
- 79. Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002;6(2):129‐142. [DOI] [PubMed] [Google Scholar]
- 80. Tournier JD, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. ISMRM, Stockholm; 2010.
- 81. Sommer S, Kozerke S, Seifritz E, Staempfli P. Fiber up‐sampling and quality assessment of tractograms ‐ towards quantitative brain connectivity. Brain Behav. 2017;7(1):e00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060‐1075. [DOI] [PubMed] [Google Scholar]
- 83. Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11‐22. [DOI] [PubMed] [Google Scholar]
- 84. Dale AM, Fischl B, Sereno MI. Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179‐194. [DOI] [PubMed] [Google Scholar]
- 85. Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162‐176. [DOI] [PubMed] [Google Scholar]
- 86. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92:381‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Smith SM, Nichols TE. Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83‐98. [DOI] [PubMed] [Google Scholar]
- 88. Daumann J, Koester P, Becker B, et al. Medial prefrontal gray matter volume reductions in users of amphetamine‐type stimulants revealed by combined tract‐based spatial statistics and voxel‐based morphometry. Neuroimage. 2011;54(2):794‐801. [DOI] [PubMed] [Google Scholar]
- 89. Huang H, Zhang Y, Cheng J, Wang W, Wen M. Evaluating the changes of white matter microstructures in tobacco addicts based on diffusion tensor imaging. Med Sci Monit. 2020;26:e919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yu D, Yuan K, Zhang B, et al. White matter integrity in young smokers: a tract‐based spatial statistics study. Addict Biol. 2016;21(3):679‐687. [DOI] [PubMed] [Google Scholar]
- 91. Mori S, Wakana S, van Zijl PCM, Nagae‐Poetscher LM. MRI Atlas of Human White Matter. Firsted. The Netherlands Elsevier; 2005. [Google Scholar]
- 92. Parlatini V, Radua J, Dell'Acqua F, et al. Functional segregation and integration within fronto‐parietal networks. Neuroimage. 2017;146:367‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122(2):250‐261. [DOI] [PubMed] [Google Scholar]
- 94. Schmahmann JD, Smith EE, Eichler FS, Filley CM. Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci. 2008;1142(1):266‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Narayana PA, Herrera JJ, Bockhorst KH, et al. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221(3):220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tang VM, Lang DJ, Giesbrecht CJ, et al. White matter deficits assessed by diffusion tensor imaging and cognitive dysfunction in psychostimulant users with comorbid human immunodeficiency virus infection. BMC Res Notes. 2015;8(1):515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44(8):1105‐1132. [DOI] [PubMed] [Google Scholar]
- 98. Vonmoos M, Hulka LM, Preller KH, et al. Cognitive dysfunctions in recreational and dependent cocaine users: role of attention‐deficit hyperactivity disorder, craving and early age at onset. Br J Psychiatry. 2013;203(1):35‐43. [DOI] [PubMed] [Google Scholar]
- 99. Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189‐204. [DOI] [PubMed] [Google Scholar]
- 100. Hau J, Sarubbo S, Perchey G, et al. Cortical terminations of the inferior fronto‐occipital and uncinate fasciculi: anatomical stem‐based virtual dissection. Front Neuroanat. 2016;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sarubbo S, de Benedictis A, Maldonado IL, Basso G, Duffau H. Frontal terminations for the inferior fronto‐occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi‐component bundle. Brain Struct Funct. 2013;218(1):21‐37. [DOI] [PubMed] [Google Scholar]
- 102. He Q, Li D, Turel O, Bechara A, Hser YI. White matter integrity alternations associated with cocaine dependence and long‐term abstinence: Preliminary findings. Behav Brain Res. 2020;379:112388. [DOI] [PubMed] [Google Scholar]
- 103. Uban KA, Herting MM, Wozniak JR, Sowell ER, CIFASD . Sex differences in associations between white matter microstructure and gonadal hormones in children and adolescents with prenatal alcohol exposure. Psychoneuroendocrinology. 2017;83:111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Watari H, Born DE, Gleason CA. Effects of first trimester binge alcohol exposure on developing white matter in fetal sheep. Pediatr Res. 2006;59(4):560‐564. [DOI] [PubMed] [Google Scholar]
- 105. Seigneurie AS, Guerin Langlois C, Limosin F. Cognitive vulnerability to alcohol dependence: related neuroanatomic endophenotypes. Encephale. 2013;39(5):320‐325. [DOI] [PubMed] [Google Scholar]
- 106. Kim YW, Hwang YH, Kang DH, et al. The diagnostic role of diffusion tensor imaging in multifocal inflammatory leukoencephalopathy. Int J Neurosci. 2014;124(5):383‐386. [DOI] [PubMed] [Google Scholar]
- 107. Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age‐related white matter changes. Acta Neuropathol. 2011;122(2):171‐185. [DOI] [PubMed] [Google Scholar]
- 108. Ji Q, Edwards A, Glass JO, Brinkman TM, Patay Z, Reddick WE. Measurement of projections between dentate nucleus and contralateral frontal cortex in human brain via diffusion tensor tractography. Cerebellum. 2019;18(4):761‐769. [DOI] [PubMed] [Google Scholar]
- 109. Hanaie R, Mohri I, Kagitani‐Shimono K, et al. Altered microstructural connectivity of the superior cerebellar peduncle is related to motor dysfunction in children with autistic spectrum disorders. Cerebellum. 2013;12(5):645‐656. [DOI] [PubMed] [Google Scholar]
- 110. Liang Z, Zeng J, Zhang C, et al. Progression of pathological changes in the middle cerebellar peduncle by diffusion tensor imaging correlates with lesser motor gains after pontine infarction. Neurorehabil Neural Repair. 2009;23(7):692‐698. [DOI] [PubMed] [Google Scholar]
- 111. Soulard J, Huber C, Baillieul S, et al. Motor tract integrity predicts walking recovery: a diffusion MRI study in subacute stroke. Neurology. 2020;94(6):e583‐e593. [DOI] [PubMed] [Google Scholar]
- 112. Todd G, Noyes C, Flavel SC, et al. Illicit stimulant use is associated with abnormal substantia nigra morphology in humans. PLoS One. 2013;8(2):e56438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Monnig MA, Thayer RE, Caprihan A, et al. White matter integrity is associated with alcohol cue reactivity in heavy drinkers. Brain Behav. 2014;4(2):158‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhao Q, Sullivan EV, Honnorat N, et al. Association of heavy drinking with deviant fiber tract development in frontal brain systems in adolescents. JAMA Psychiat. 2021;78(4):407‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aldusary N, Traber GL, Freund P, et al. Abnormal connectivity and brain structure in patients with visual snow. Front Hum Neurosci. 2020;14:582031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A positive relationship was observed for cocaine consumption and indices of white matter integrity (i.e. FA and/or FD) in several tracts, including right posterior limb of internal capsule, right cingulate gyrus and left superior fronto‐occipital fasciculus. This relationship between the cocaine consumption and indices of white matter integrity for these tracts is significantly stronger as compared alcohol consumption. For display purposes, the different individual consumption values (i.e. alcohol and cocaine) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
Figure S2. A negative relationship was observed for alcohol consumption and indices of white matter integrity (i.e. FA and/or FD) in several tracts, including right posterior limb of internal capsule, right retrolenticular part of internal capsule (survives multiple comparison correction) and right superior corona radiata. Importantly, this relationship between the alcohol consumption and indices of white matter integrity for these tracts is significantly stronger as compared to both levamisole and cocaine. For display purposes, the different individual consumption values (i.e. alcohol, levamisole, and cocaine) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
Figure S3. A negative relationship was observed for levamisole exposure and indices of white matter integrity (i.e. FA and/or FD) in the genu of CC. Importantly, this relationship between the levamisole exposure and indices of white matter integrity for these tracts is significantly stronger as compared to alcohol. For display purposes, the different individual consumption values (i.e. alcohol and levamisole) were normalized across patients. a.u. – arbitrary units. The R and p values correspond to correlation analyses, including the six regressors as covariates (i.e., age, sex, ADHD, nicotine, MDMA, and ICV).
Data Availability Statement
The data associated with this publication can be shared upon reasonable request.


