Abstract
SARS-CoV-2 is the viral agent of COVID-19, a pandemic that surfaced in 2019. Although predominantly a respiratory ailment, patients with COVID-19 can have gastrointestinal (GI) and hepatobiliary manifestations. These manifestations are often mild and transient, but they can be severe and consequential. In the GI tract, ischemic enterocolitis is the most common and significant consequence of COVID-19. In the liver, the reported pathologic findings may often be related to consequences of severe systemic viral infection, but reports of hepatitis presumed to be due to SARS-CoV-2 suggest that direct viral infection of the liver may be a rare complication of COVID-19. In both the GI tract and liver, lingering symptoms of GI or hepatic injury after resolution of pulmonary infection may be part of the evolving spectrum of long COVID.
Keywords: COVID-19, Gastrointestinal, Colon, Liver, Pathology
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the viral agent responsible for coronavirus disease 2019 (COVID-19), a pandemic that surfaced in late 2019. Although largely a respiratory illness, COVID-19 can have extrapulmonary manifestations, including gastrointestinal (GI) and hepatobiliary manifestations. Most of these manifestations occur in the acute setting, particularly in patients with severe disease. Others occur later in the illness, sometimes even after recovery from COVID-19, and can be considered within the spectrum of what is increasingly recognized as “long-COVID,” or prolonged symptoms of the disease. COVID-19 may also occur in patients with preexisting GI or hepatic diseases, which has raised questions about the use of immunologic therapies in those patients, their risk of severe COVID-19, and poor outcomes. In addition, GI or hepatic complications of COVID-19 may not be directly related to the infection itself but may be caused by the various therapies that are used to prevent or combat the disease.
2. GI and hepatobiliary manifestations of COVID-19
2.1. Clinical findings in the GI tract
According to several studies, patients with COVID-19 often have GI manifestations. However, the exact proportion varies widely in studies that have addressed this question, ranging from 17 to 53%, with geographic differences possibly explaining some of these disparities [[1], [2], [3], [4]]. The most common symptoms these patients experience are anorexia, diarrhea, abdominal pain, nausea, and vomiting, although some authors do not include anorexia as a GI-specific symptom. In a meta-analysis of 60 studies including 4243 patients, GI symptoms occurred in 17.6% of patients, including 26.8% with anorexia, 12.5% with diarrhea, 10.2% with nausea/vomiting, and 9.2% with abdominal pain [5]. Usually, GI symptoms occur in patients who also have respiratory symptoms. In one study, GI symptoms preceded respiratory symptoms in 13% of cases, started concurrently in 44%, and followed other COVID-19 symptoms in 42% [3]. In some studies, 4–20% of patients present with GI symptoms only [4,6]. Those patients who manifest only GI symptoms may have a longer time interval between symptom onset and hospital admission [7].
Several studies have looked at whether GI symptoms are associated with more severe COVID-19 disease, and the results are mixed. For example, one study found that GI symptoms occurred in 17.1% of patients with severe disease, but in only 11.8% of patients without severe disease [5]. A recent paper that surveyed 2222 patients found that severe disease was more prevalent among patients who fell into symptom clusters that included GI symptoms [8]. A meta-analysis of 21 studies including 5285 patients found that abdominal pain was associated with a 2.8-fold increased risk of severe COVID-19, whereas the association between diarrhea and severe COVID-19 was regionally different [9]. Zheng and coinvestigators found that the risk of clinical deterioration was higher among patients with GI symptoms than those without [10]. However, these data are contradicted by studies that conclude the opposite; in one study, GI symptoms correlated with decreased levels of circulating cytokines, decreased disease severity, and decreased mortality [11]. A meta-analysis of 158 studies found no difference in intensive care unit admission rates between patients who experience GI symptoms and those who did not [12]. A recent study [13] of 1113 patients also did not find that GI symptoms were associated with more severe disease or worse outcome.
Although the majority of GI symptoms that patients with COVID-19 experience are mild, they can be severe. In one study, 2% of patients had bloody diarrhea and 18% had GI hemorrhage; a majority of the patients (63%) underwent radiologic imaging or endoscopic evaluation for these symptoms [3]. Bowel ischemia is a particularly consequential complication of COVID-19 infection. These patients infrequently have a history of prior thrombosis [14]. Patients present with worsening clinical course (such as increased vasopressor requirements and abdominal distention), increasing D-Dimer levels, and radiologic imaging indicative of ischemic bowel with bowel distention, intestinal pneumatosis, portal venous gas, and/or free abdominal air [[14], [15], [16]]. Rarely, patients with COVID-19 present with acute abdomen, with etiologies that include bowel obstruction, bowel perforation, acute appendicitis, acute acalculous cholecystitis, and acute pancreatitis [17,18].
Patients with digestive symptoms may have a longer duration between symptom onset and viral clearance, and are more likely to have a positive viral study in stool, even after viral clearance from the respiratory tract [5,19]. The presence of SARS-CoV-2 nucleic acid in feces from infected patients raises the possibility that the virus may be transmissible via the fecal-oral route, even after respiratory symptoms subside. However, it is uncertain whether virus shed in the feces is infectious. Viral RNA was detected in 29% of fecal samples in one study, and live virus was confirmed by electron microscopy in 2 patients [20]. In another study, viral RNA was detected in 53% of 73 hospitalized patients [21]. Live virus was isolated in 2 of 3 RNA-positive fecal samples [22]. However, in vitro studies have concluded that the virus is inactivated in the colon [23].
Endoscopy in the setting of COVID-19 is often done due to urgent indications, most often GI bleeding [24]. Examinations performed within 7 days of COVID-19 onset are more likely to show major findings [24]. Endoscopic findings in patients with COVID-19 and upper GI complaints include gastric, duodenal, or esophageal ulcers, various patterns of inflammation, erosions, ecchymoses, or edema [11,[24], [25], [26], [27]]. One case report describes diffuse small intestinal mucosal sloughing, reminiscent of graft-versus-host disease, detected by capsule endoscopy [28]. However, a significant number of upper GI tract examinations are normal (29.9% in one series) [24]. Colonoscopic examination in patients with COVID-19 has shown ischemic colopathy, nonspecific erythema, nonspecific colonic inflammation, and ulcerative inflammatory colitis [24,27]. Negative examinations are less common in the lower GI tract.
Importantly, not all of the findings on endoscopic examination are related to COVID-19. For example, some of the reported gastric ulcers prove to be malignancy or Helicobacter pylori, and some of the cases of ulcerating colitis are clinically felt to be flares of inflammatory bowel disease (IBD) [24]. Other endoscopic findings encountered in these patients that are not clearly related to COVID-19 include esophageal candidiasis, varices, polyps, bleeding angioectasias, and malignancies [24].
The vast majority of GI manifestations of COVID-19 resolve by 3 months after hospitalization [29]. However, long-term functional GI disorders (referred to as Disorders of Gut–Brain Interaction) are now being recognized, including postinfectious irritable bowel syndrome and dyspepsia. In one study of 280 patients with COVID-19 [30], 8.6% had chronic bowel dysfunction, 2.1% had dyspeptic symptoms, and 3.2% had both, 3 months after their acute illness. At 6 months follow-up, 5.3%, 2.1%, and 1.8% still had these symptoms, respectively. Another study [8] surveyed 108 subjects and found that 38 experienced functional dyspepsia and 26 experienced irritable bowel syndrome. Among subjects with at least one post–COVID-19 disorder of gut–brain interaction, 86% reported having had at least one GI symptom during their acute illness. This number increased to 92% among hospitalized patients.
2.2. Clinical findings in the hepatobiliary tract
In patients with COVID-19, abnormal liver biochemistries occur in 14–53% of patients, both at admission and during hospitalization [[31], [32], [33]]. Typically, patients have mild liver function test (LFT) elevations that recover without specific treatment [31,32]. The pooled rate for elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) is estimated at 20–22.5% and 14.6–20.1%, respectively [34,35]. Although less commonly reported, bilirubin, alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) are elevated in 6–21% of patients as well [[34], [35], [36], [37]].
The clinical literature shows that ALT and AST elevations are more common in severe COVID-19 compared to mild COVID-19 [38]. A retrospective observational cohort study reported that abnormal liver tests at admission and during hospitalization may also be associated with worse clinical outcomes [33]. Supportive evidence is provided by Zhang and colleagues, who reported that the majority of fatal COVID-19 cases (up to 78%) had clinical evidence of liver injury [39]. Given the central function of the liver in production of albumin, acute phase reactants, and coagulation factors, the high proportion of cases with liver injury suggests that hepatic dysfunction plays a critical role in multisystem organ dysfunction.
Rare case reports have described patients with COVID-19 who presented with acute liver failure. Some of these patients had or developed respiratory symptoms of acute respiratory distress syndrome with multiorgan dysfunction [40,41]. One woman with COVID-19 had elevated liver biochemistries at presentation, with ALT 697 IU/L, AST 1230 IU/L, and ALP 141 IU/L [41]; this patient developed respiratory symptoms 18 h after admission, with low oxygen saturation and bilateral interstitial opacities on chest imaging. Very rarely, patients with COVID-19 presenting with acute liver failure do not develop respiratory disease. Melquist and colleagues report a case of a young woman with systemic lupus erythematosus who presented with severe abdominal pain, nausea, and vomiting [42]. She progressed to fulminant liver failure with encephalopathy, without developing respiratory symptoms, and was treated with hydroxychloroquine and methylprednisolone for possible autoimmune etiology. Fiel and colleagues also describe a young, previously healthy woman who presented with nausea, vomiting, scleral icterus, and laboratory evidence of acute liver injury; as she continued to oxygenate well without radiographic evidence of lung disease, she was not treated for COVID-19 and showed gradual improvement with near normalization of liver biochemistries in the follow-up interval [43]. Determination of the etiology of acute liver failure for many patients with COVID-19 is challenging because of the possibility of drug injury or multiorgan failure in the setting of acute respiratory distress syndrome or sepsis. However, in one case, liver tissue demonstrated viral antigens by in situ hybridization (ISH) [42].
Rare reports describe patients with COVID-19 developing primary liver disease during their illness, such as autoimmune hepatitis (AIH) and primary biliary cirrhosis [44,45]. One case report describes a patient with COVID-19 with severe liver injury who developed secondary hemophagocytic lymphohistiocytosis, and in whom Wilson disease was unmasked [46].
Post–COVID-19 cholangiopathy is a form of secondary sclerosing cholangitis in critically ill patients (SSC–CIP), which is characterized by prolonged and marked cholestasis, generally lasting several months after recovery from the initial pulmonary manifestations [[47], [48], [49], [50]]. The majority of these patients suffered from particularly severe disease, with extensive hospital stays and subsequent rehabilitation. A retrospective study showed that 0.6% of patients with COVID-19 had persistent cholestatic injury, with ALP > 3× the upper limit of normal at a mean of 118 days after diagnosis, and persisted in some patients longer than a year [51]. Rarely, patients with persistent and severe cholestatic disease require liver transplantation [52]; however, a small case series suggests that most of these patients show gradual but continual normalization of liver biochemistries without additional intervention [50].
3. Mechanisms of GI and hepatobiliary injury
The mechanism of injury in the GI and hepatobiliary tracts is not entirely clear and is likely multifactorial. Potential mechanisms of COVID-19–associated injury include direct consequences of viral replication, systemic inflammatory and immune-mediated effects, vascular changes resulting in ischemia, drug-induced injury, and exacerbation of underlying disease.
3.1. Direct viral infection
Direct viral infection is one possible mechanism of SARS-CoV-2–related injury in the GI tract and liver. Viral entry into cells is mediated by binding to the angiotensin-converting enzyme 2 (ACE2) receptor on the cell surface followed by S-protein activation by transmembrane protease serine 2 (TMPRSS2). The relative expression of these proteins determines the ability of the virus to infect a particular cell type. The ACE2 receptor is highly expressed in GI epithelia, particularly the ileum, but also the duodenum, colorectum, and stomach [11,21,[53], [54], [55]]. Co-expression of ACE2 receptor and TMPRSS2 occurs in intestinal epithelial cells [23,56].
Several studies have documented viral particles in GI tissue, suggesting that direct infection of the GI tract mucosa may play a role in GI injury. Viral particles have been detected in the cytoplasm of gastric, duodenal, and rectal glandular cells by immunofluorescence [21]. In a study by Livanos and coauthors [11], viral nucleocapsid protein was detected by immunofluorescence in the small intestine in 11 of 12 patients, largely within MUC2-positive epithelial cells (presumably goblet cells) as well as in epithelial cells in crypts, although their presence did not correlate with histologic abnormalities. Viral antigens were patchy in the upper GI tract but diffuse in the ileum, possibly due to the increased number of goblet cells in the ileum. In addition, viral RNA is detectable by reverse transcriptase–polymerase chain reaction (RT-PCR) in biopsies from the esophagus, stomach, duodenum, and rectum [57]. By electron microscopy, viral particles have been detected in intestinal epithelial cells in the duodenum and ileum [11]. However, claims of viral particles detected by electron microscopy have been questioned as possibly being misinterpretations of viral mimics [[58], [59], [60]]. In vitro studies have demonstrated that enterocyte organoids can be infected by SARS-CoV-2 and may support its replication [23,61]. Together, these studies suggest that the SARS-CoV-2 virus can actively infect and replicate in the GI tract, causing direct organ dysfunction. Destruction of intestinal epithelial cells could result in changes in intestinal permeability, malabsorption, and altered intestinal secretion, and may explain diarrhea as well as other GI manifestations.
In the liver, single-cell transcriptome analysis from several studies (involving both human tissues and organoid cultures) has confirmed the presence of ACE2 receptor and TMPRSS2 in liver parenchymal cells and cholangiocytes [[62], [63], [64]]. Although ACE2 receptors are expressed on endothelial cells, sinusoidal endothelial cells appear to be negative [65]. Therefore, direct viral infection of the liver is possible.
In some autopsy series, attempts to detect virus in autopsy livers yielded negative results. For example, Fassan and colleagues [66] were unable to detect virus by ISH in 25 autopsy livers. However, others have had a measure of success. A combination of quantitative real-time PCR and ISH was used to detect viral RNA and replicative intermediates in 25% of the liver specimens in the study by Kaltschmidt and coinvestigators [67]. Viral protein was detected in hepatic stem cells, hepatocytes, and cholangiocytes. In the series by Lagana and colleagues, PCR for viral genes was positive in 55% of autopsy livers [68]. However, there was no correlation between PCR positivity and any of the histologic changes in their study. Immunohistochemistry (IHC) for SARS-CoV-2 highlighted portal macrophages in 5 of 17 cases in the study by Zhao and others [69]. By electron microscopy, viral particles were not identified in one series of 24 cases [70], but others reported virion-like particles in their cases [69]. In rare reports of patients with liver injury presumably due to COVID-19, some biopsies have demonstrated viral antigens by ISH [43,71] as well as electron microscopy [43], suggesting the SARS-CoV-2 can directly infect the liver.
In vitro studies have demonstrated that human bile duct organoids are susceptible to SARS-CoV-2 and support viral replication [72,73]. Furthermore, primary hepatocytes and cholangiocytes in culture and organoids infected with SARS-CoV-2 overexpress proinflammatory cytokines and down-regulate metabolic processes [64], raising the possibility that infection may alter the profile of proinflammatory or profibrogenic cytokines. In addition, there may be impairment of bile transport and bile acid signaling, in part from down-regulation of bile acid transporters and chloride channels [74,75].
3.2. Systemic cytokine release
A second proposed mechanism of GI and hepatobiliary injury involves inflammatory and immune mediators. COVID-19 disease has been associated with markedly increased levels of inflammatory markers and cytokines, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α), which may lead to an accumulation of immune cells in the GI tract [76] and induce hepatocellular cholestasis by down-regulating hepatobiliary uptake and excretory systems [77,78]. Some authors have suggested that immunosuppression is beneficial in blunting cytokine release, leading to improved outcomes [79].
Systemic cytokine release has been implicated in causing liver dysfunction in infections by other nonhepatotropic viruses, including previous coronaviruses such as severe acute respiratory syndrome coronavirus-1 (SARS-CoV-1) and the Middle East respiratory syndrome coronavirus (MERS-CoV) [80]. In influenza A, a viral-specific cytotoxic T-cell response regulated by Kupffer cells is known to cause a hepatitis, even in the absence of direct infection and viral antigens in the liver [81,82]. This inflammatory process related to nonhepatic sites of infection (usually respiratory viruses) is presumed to cause “collateral damage” via the infiltration of cytotoxic T cells [81], and likely plays a role in COVID-19 disease as well.
3.3. Hypoxic and thrombotic injury
A third proposed mechanism of GI and hepatobiliary injury involves hypoxic changes induced by respiratory failure, systemic coagulopathy, and right heart failure, all of which are complications of severe COVID-19 disease. COVID-19 respiratory failure requires intensive critical care management with invasive ventilatory intervention and vasoconstrictor therapy and may be accompanied by right ventricular dysfunction caused by high pulmonary vascular resistance [32]. In addition, viral infection of endothelial cells causes endothelial dysfunction, release of factor VIII, von Willebrand factor, and fibrinogen, resulting in thrombosis [17,83]. It has been well-described that patients with severe COVID-19 show widespread thromboses in large and small vessels and experience numerous ischemic complications, including acute pulmonary thrombotic events and acute limb ischemia [[84], [85], [86], [87]]. Similarly, the pathologic finding of vascular thrombosis in the GI tract and liver support the contention that hypercoagulability and thrombosis contribute to GI and liver injury.
4. Pathologic findings in the GI tract and liver
4.1. Pathology of the GI tract
Pathologic specimens in patients with COVID-19 are obtained to evaluate for a variety of GI complaints; most often, intestinal ischemia results in a biopsy or resection. Other indications include GI bleeding, diarrhea, and nausea/vomiting. However, patients who are positive for COVID-19 may also have GI diseases unrelated to the viral infection, and specimens may be procured related to those disorders, such as flares of IBD. In those cases, the biopsies or resections show features related to those diseases, and the pathology of those specimens will not be considered here. Also, some of the biopsies that are reported in the literature uncover issues such as neoplasia which are clearly independent of the viral infection [88]. In one study, biopsies obtained closer to the time of a COVID-19–positive nasopharyngeal swab were more likely to be associated with the viral illness itself [88]. Finally, many biopsies in these patients are histologically normal, despite the patient having GI complaints.
4.1.1. Ischemic enterocolitis in COVID-19
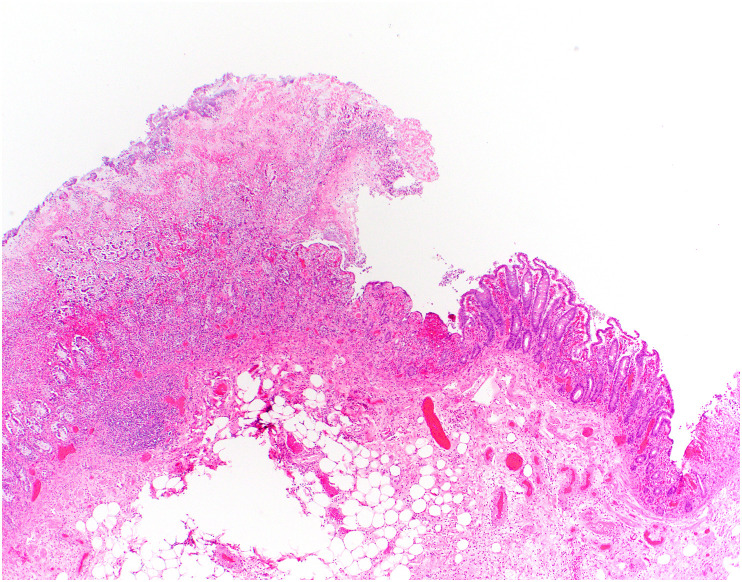
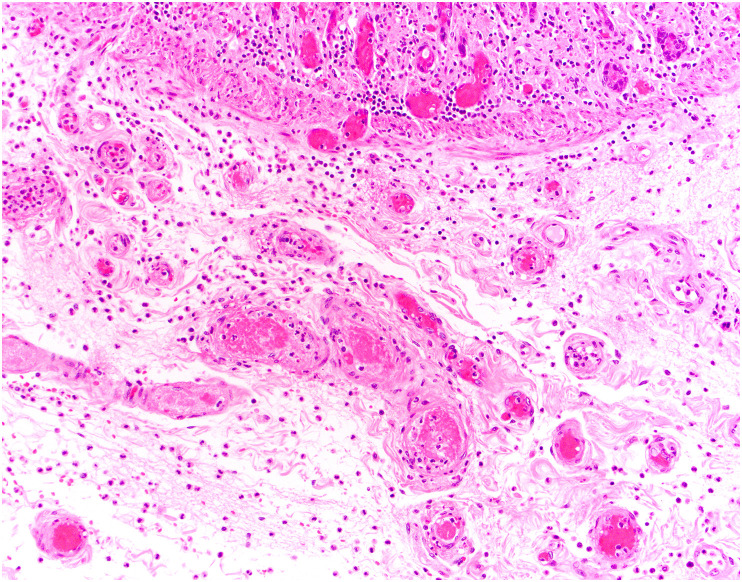
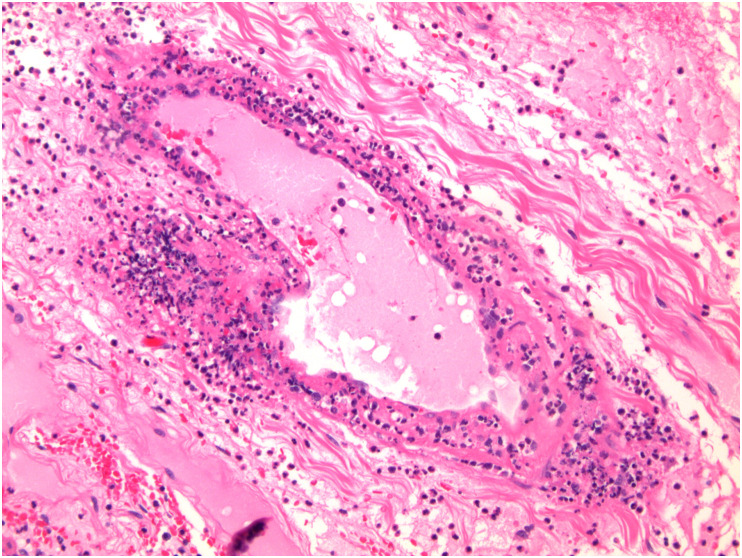
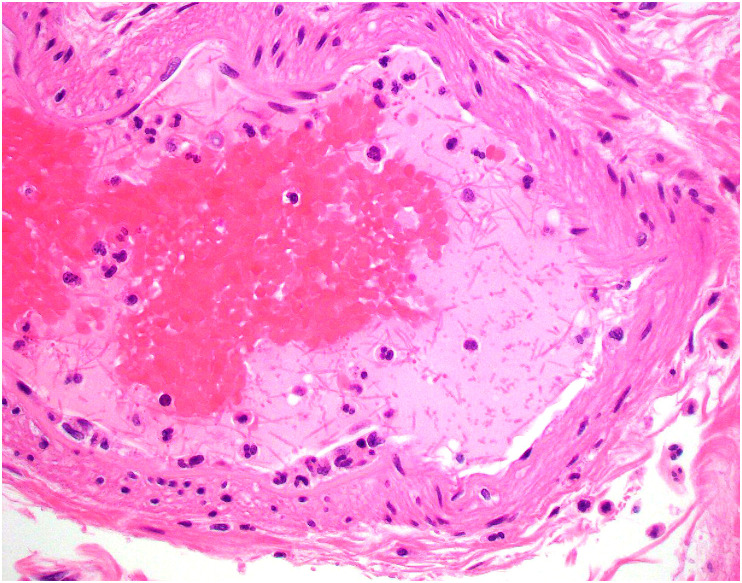
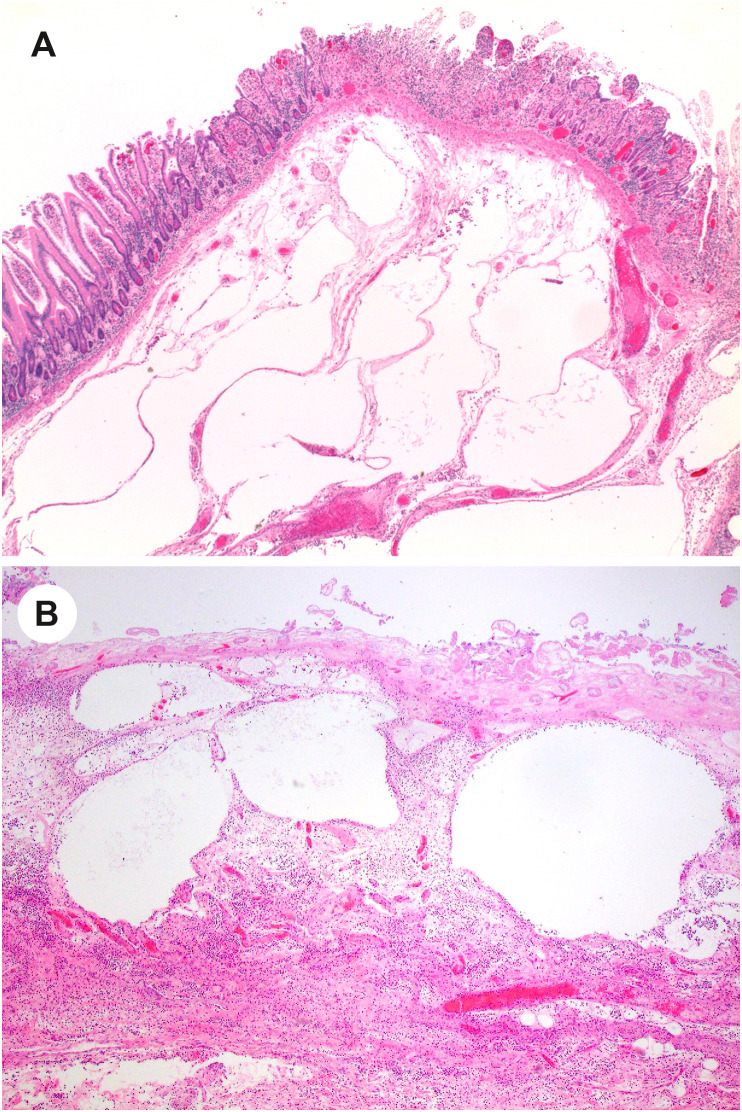
The most consistent pathologic findings in patients with COVID-19 relate to intestinal ischemia. In patients with ischemic bowel, resected specimens show well-described features of ischemic enteritis or colitis, including dilated segments of bowel with mucosal and mural necrosis, congestion, and hemorrhage [14,[88], [89], [90]]. Early in the pandemic, surgeons at Massachusetts General Hospital noted a peculiar yellow-tan discoloration of the serosa, with multiple discrete necrotic areas along the antimesenteric border of the small intestine [14,91,92]. However, a histologic correlate was not identified. Histologically, discrete areas of mucosal necrosis and occasional pseudomembranes are seen [14,88] (Fig. 1 ). A frequent finding in these bowels is thrombi in small mucosal and submucosal vessels, usually confined to the areas of mucosal necrosis [14,16,89,90] (Fig. 2 ). Some of the small vessels may show eosinophilic necrosis of the vessel wall with perivascular neutrophilic inflammation, confined to areas of necrosis [14,16] (Fig. 3 ). In addition, medium-sized veins may show fibrin strands admixed with luminal blood (Fig. 4 ). Pneumatosis intestinalis, characterized by large “empty” appearing spaces in a markedly edematous submucosa (Fig. 5 A- and B), is relatively common in COVID-19–related ischemia, although not specific for this etiology [14].
Fig. 1.
Ischemic enterocolitis in COVID-19. Low-power view of a colon specimen shows mucosal necrosis with a fibrinopurulent exudate (left) with a sharp transition to viable mucosa (right). The specimens also show congestion, submucosal edema, and epithelial injury characteristic of ischemic enterocolitis.
Fig. 2.
Fibrin thrombi in COVID-19–related ischemic enterocolitis.
Small vessels within the submucosa beneath necrotic mucosa shows fibrin thrombi in small vessels.
Fig. 3.
Perivascular neutrophils in the submucosa in COVID-19–related ischemic enterocolitis. Vessels within the submucosa may show changes reminiscent of vasculitis, with perivascular neutrophils and, in some cases, degeneration of the wall. (Courtesy of Dr. M. Lisa Zhang).
Fig. 4.
Fibrin strands in large submucosal vessels in COVID-19–related ischemic enterocolitis. In some cases of COVID-19–related ischemic bowel, large submucosal vessels show fibrin strands within stagnant blood. This may be a reflection of hypercoagulability.
Fig. 5.
Pneumatosis intestinalis in COVID-19–related ischemic enterocolitis. A and B, Two cases that demonstrate large “empty” spaces in the submucosa in ischemic enterocolitis that are compatible with pneumatosis intestinalis. (Courtesy of Dr. M. Lisa Zhang).
Biopsy specimens have also shown features of ischemia. Biopsies of the colon have been described as having typical features of ischemia, ranging from withered crypts, regenerative changes, and mildly active colitis to mucosal necrosis, hemorrhage, congestion, and microthrombi [16,24]. One report includes a description of a duodenal biopsy with ischemic changes characterized by patchy hemorrhage in the lamina propria, fibrin thrombi, and regenerative changes in the crypt epithelial cells [25]. Various methods to detect the virus or RNA, including IHC, ISH, and next generation sequencing (NGS), have been negative in most biopsies or resections of ischemic bowel [14,25,88], although rare cases have shown positive results either by RT-PCR or IHC [16,24].
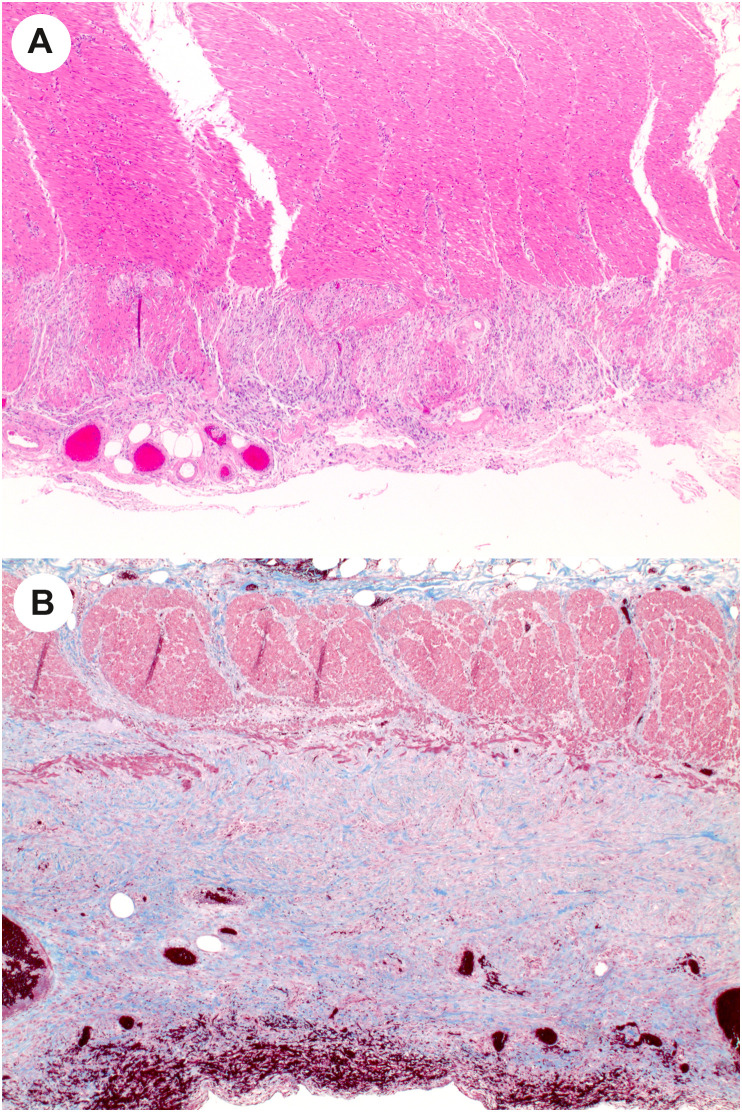
In the report by Zhang and colleagues [14], the authors describe 3 cases of ischemic bowel resections that had different features from those described earlier. In these 3 cases, the external layer of the muscularis propria showed changes ranging from necrosis, to replacement by a fibroblastic reaction, to fibrosis that can be highlighted on trichrome stain (Fig. 6 A- and B). These cases lacked the small vessel thrombi seen in the majority of their cases. The authors speculate that COVID-19 may, in selected individuals, lead to chronic ischemic-type changes. A report of strictures in the small bowel as a consequence of ischemia also suggests that bowel ischemia can have long-term consequences for these patients [93].
Fig. 6.
Muscularis propria attenuation in COVID-19–related ischemic enterocolitis. A, Rare cases of COVD-19 ischemic bowel show changes that are typically seen in chronic ischemic, such as attenuation of the external layer of the muscularis propria. B, Trichrome stain of a different case shows fibrous replacement of the external layer of the muscularis propria, as well as subserosal fibrosis. (Courtesy of Dr. M. Lisa Zhang).
4.1.2. GI mucosal injury in COVID-19
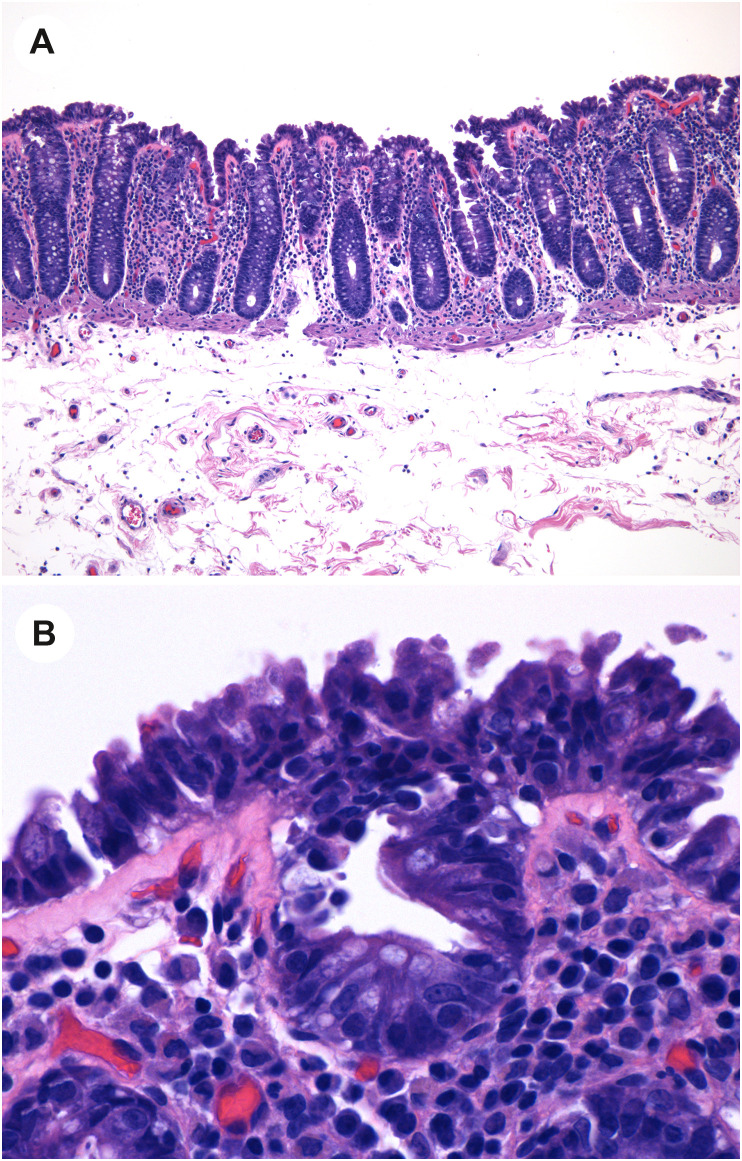
Other than ischemic changes, pathologic changes are relatively nonspecific in patients with COVID-19. For example, Xiao and colleagues described biopsy findings in a patient with COVID-19 [21]; they describe lymphocytes in the esophageal squamous epithelium and numerous lymphocytes and plasma cells in the stomach, duodenum, and rectum with edema. Another report includes a description of mild duodenal inflammation with lamina propria neutrophils and increased intraepithelial lymphocytes [11]. In the case mentioned earlier with small intestinal mucosal sloughing by capsule endoscopy, colonic biopsies showed edema, erosions, infiltration by lymphocytes, and epithelial cell apoptosis [28]. Finally, a report by Westerhoff and colleagues [88] describes ileal biopsies in a patient with COVID-19 that demonstrated active ileitis, villous lymphocytosis, increased goblet cells in the villi, and crypt hyperplasia. Neither ISH nor NGS was positive for SARS-CoV-2. Curiously, another of their cases had normal ileal mucosal biopsy findings but showed strong cytoplasmic and dotlike expression by ISH for SARS-CoV-2 RNA in the epithelial cells overlying a Peyer patch.
One report is noteworthy, as the authors describe cases of more prominent and widespread mucosal injury that may be due to COVID-19 infection. The mechanism of mucosal injury may be different in each of these cases and includes direct viral infection of the mucosal epithelial cells or systemic viral effects, including cytokine storms. In this report, Yantiss and colleagues [25] describe a patient with colonic angiodysplasia in whom the surrounding colonic mucosa showed epithelial cells on the mucosal surface forming small tufts, and the epithelial cells in the upper crypt had cytoplasmic blebs that projected into the lumen (Fig. 7 A- and B). The cells had mildly enlarged hyperchromatic nuclei. IHC for SARS-CoV-2 and ISH for spike protein-encoding RNA highlighted the areas of cytologic abnormality, and electron microscopy showed viral particles on the luminal surface (but not within the cells). This case suggests that SARS-CoV-2 can cause direct mucosal injury.
Fig. 7.
Mucosal injury in COVID-19. A, Low-power view of colonic mucosa shows tufting of the superficial epithelium. B, High-power view shows that the enterocytes are injured with disarray, hyperchromatic disordered nuclei, epithelial tufting, and amphophilic cytoplasm. (Courtesy of Dr. Rhonda Yantiss).
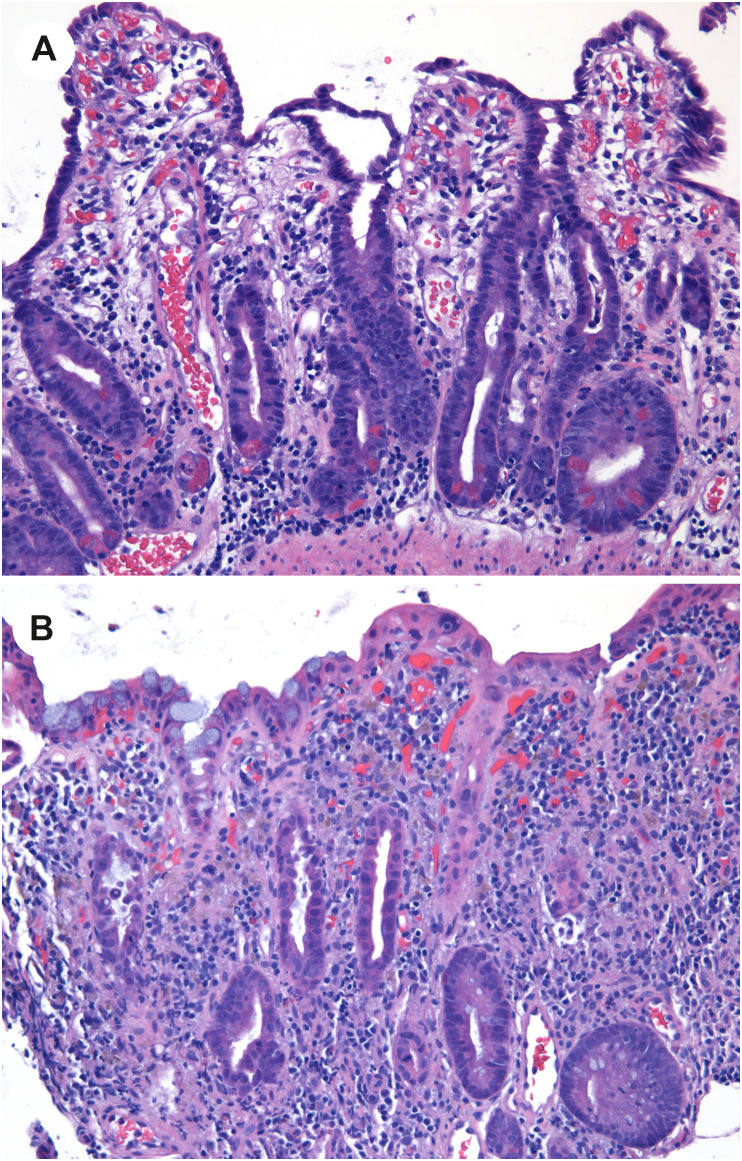
Another of their cases describes a patient whose duodenum, jejunum, ileum, and colon biopsies showed widespread mucosal injury [25]. In the duodenum and jejunum, the biopsies showed crypt hyperplasia, attenuated epithelial cells with decreased mucin, and nuclear enlargement (Fig. 8 A). In the same patient, ileal and colonic biopsies showed mucosal injury characterized by epithelial cell attenuation, reduced goblet cells, and in the colon, dilated crypts containing a small amount of necrotic cellular debris (Fig. 8B). IHC for SARS-CoV-2 spike protein and ISH for viral RNA were negative.
Fig. 8.
Widespread mucosal injury in COVID-19. A, Small intestinal biopsy in a patient with COVID-19 demonstrates villous blunting with cuboidalization and attenuation of the duodenal enterocytes, and crypt hyperplasia. B, A biopsy of the colon in the same patient shows cuboidal, disarrayed colonocytes with attenuated crypts and increased cellularity of the lamina propria. A few crypts (such as the one at left) show sloughed epithelial cells within the crypt. (Courtesy of Dr. Rhonda Yantiss).
4.2. Pathology of the hepatobiliary tract
The histologic features of COVID-19 in the liver have been described largely in autopsies of patients who have died from COVID-19. Rarely, hepatitis attributed to SARS-CoV-2 has been described. Finally, there is a body of literature on prolonged cholestatic injury after COVID-19 that is considered part of the spectrum of “long COVID.”
4.2.1. Liver autopsy findings in COVID-19
Descriptions of the liver in postmortem examination of patients with COVID-19 are almost entirely limited to patients who died of the pulmonary manifestations of the disease, rather than from liver injury specifically. These patients have a variety of histologic changes in the liver, some of which may be the result of organ failure, intubation, shock, and aggressive interventions. However, some of the reported changes appear to be related to specific systemic effects of SARS-CoV-2 infection, such as thrombosis. In some reports, preexisting liver disease in a minority of patients clouds the interpretation of the findings, particularly more pronounced inflammation or fibrosis in some of these liver specimens.
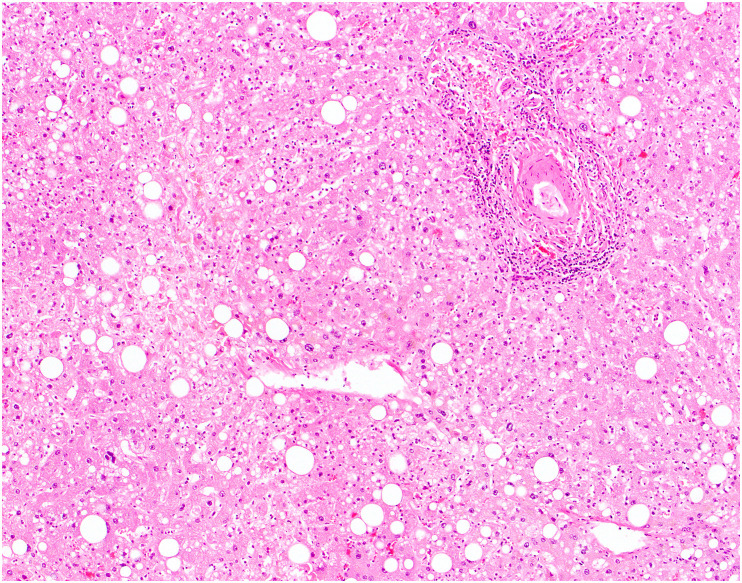
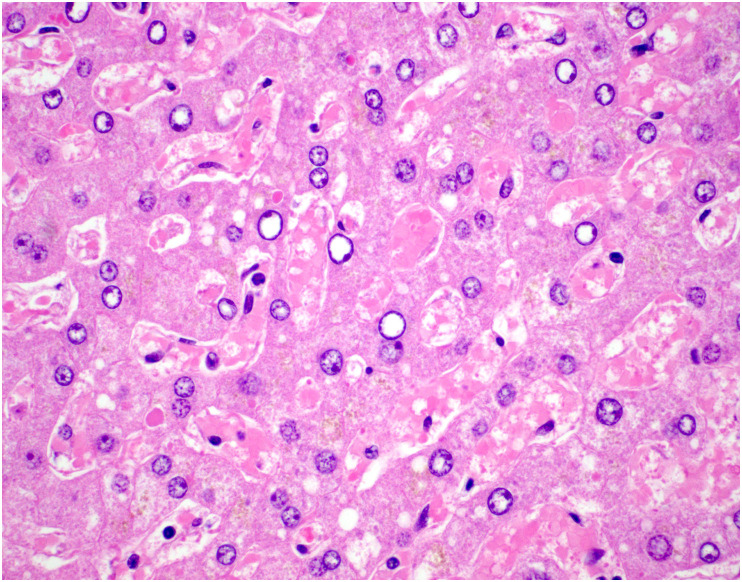
Grossly, the livers of patients who died of COVID-19 generally show varying degrees of steatosis (pale yellow appearance), congestion or nutmeg appearance, and ischemia [68,94]; uncommonly, they may show evidence of fibrosis with an indurated consistency and irregular surface [68,94]. Microscopically, the most frequently reported hepatic finding in autopsies of patients with COVID-19 is steatosis (Fig. 9 ). Steatosis is found in 50–83% of autopsy livers; the steatosis is usually mild in degree but can be moderate or marked, and both large and small droplet fat have been described [[68], [69], [70],95,96]. When zone has been recorded, most cases show panlobular distribution of steatosis, followed by zone 3 (or 2 and 3), and less commonly zone 1 [68]. Rarely, there is evidence of ballooning degeneration with Mallory-Denk bodies [68].
Fig. 9.
Steatosis in COVID-19. An autopsy from a patient who died of COVID-19 demonstrates patchy steatosis, including large droplet fat and scattered smaller droplets within hepatocytes, without strict zonal predilection.
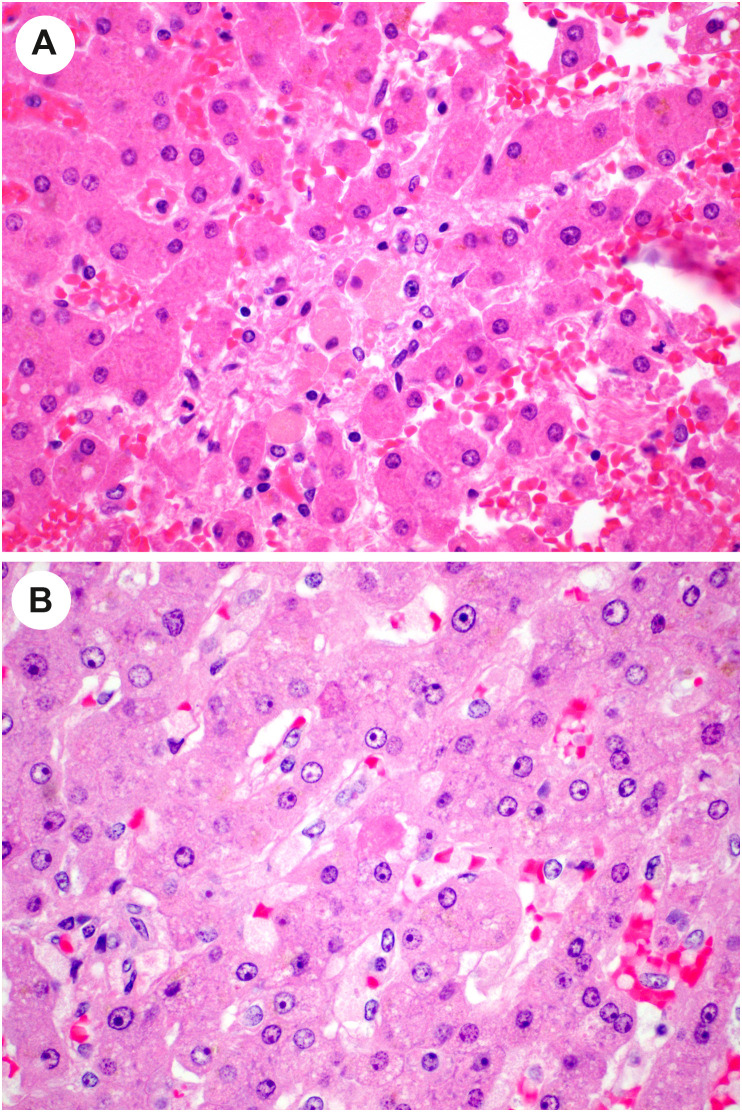
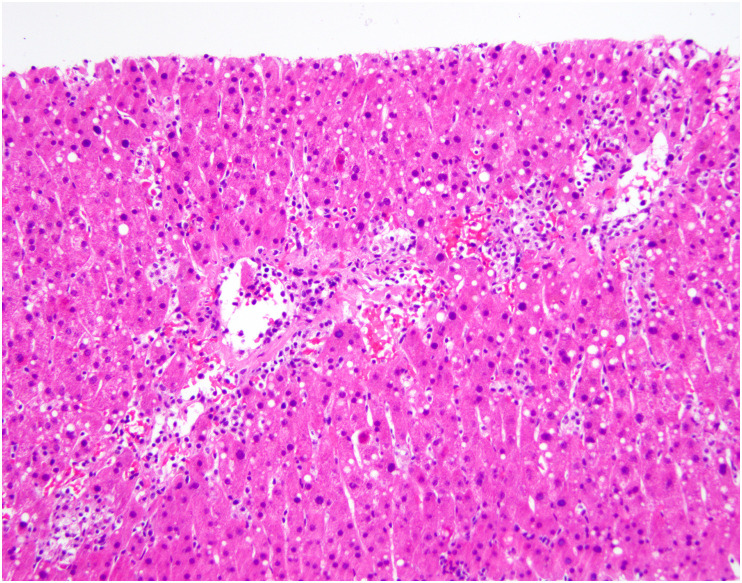
Lobular inflammation is usually mild but occasionally moderate or marked, with scattered necroinflammatory foci comprising lymphocytes and histiocytes, with occasional acidophil bodies [[68], [69], [70],94] (Fig. 10 A-B). One group reported slight to moderate swelling of the hepatocytes [70]. More dramatically, several authors report focal confluent necrosis or more extensive centrilobular ischemic-type necrosis, [66,[68], [69], [70],[94], [95], [96]] although this is likely the result of agonal events related to severe COVID-19 rather than the viral infection itself. Additional findings reported in a subset of cases include cholestasis (Fig. 11 ), ductular cholestasis suggestive of sepsis, ductular reaction, activation of Kupffer cells (Fig. 12 ), and evidence of hepatic regeneration with either mitotic activity or expansion of hepatic plates on reticulin stains [66,68,70,[94], [95], [96]]. Viral inclusions are not seen in these livers.
Fig. 10.
Lobular injury in autopsy livers from patients with COVID-19. A, Focal lobular necrotic focus with pigmented macrophages and hepatocyte dropout. B, In this view, an acidophil body is noted (center) as well as increased histiocytes in sinusoids. (Courtesy of Dr. Stephen Lagana).
Fig. 11.
Cholestasis in autopsy liver from patient with COVID-19. High-power view shows enlarged reactive-appearing hepatocytes with feathery degeneration, and cytoplasmic pigment consistent with intracellular cholestasis. (Courtesy of Dr. Michael Torbenson).
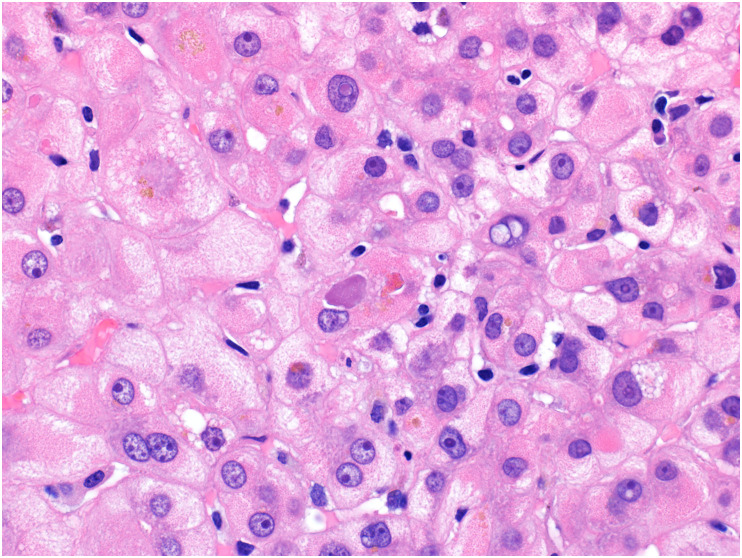
Fig. 12.
Kupffer cell hyperplasia in autopsy liver from patient with COVID-19. In this high-power view, the hepatic plates are somewhat disarrayed, and the sinusoids are expanded with numerous histiocytes and scattered inflammatory cells. Mild steatosis is also present.
Other groups have described portal and lobular inflammation in the liver at autopsy in patients with COVID-19 [68,70,94,95]. Portal inflammation has been reported in more than half of the autopsy cases [[68], [69], [70],94], comprising lymphocytes with infrequent plasma cells, eosinophils, or neutrophils [68]. Exceptionally, there is interface activity associated with the portal inflammation. One report describes increased numbers of histiocytes with vacuolated cytoplasm in the portal tracts of 5 cases, which were stained by IHC for SARS-CoV-2 [69]. Fibrosis is generally absent, but occasionally mild fibrosis is found; advanced fibrosis is an indication of preexisting chronic liver disease [66,69,70,94,95].
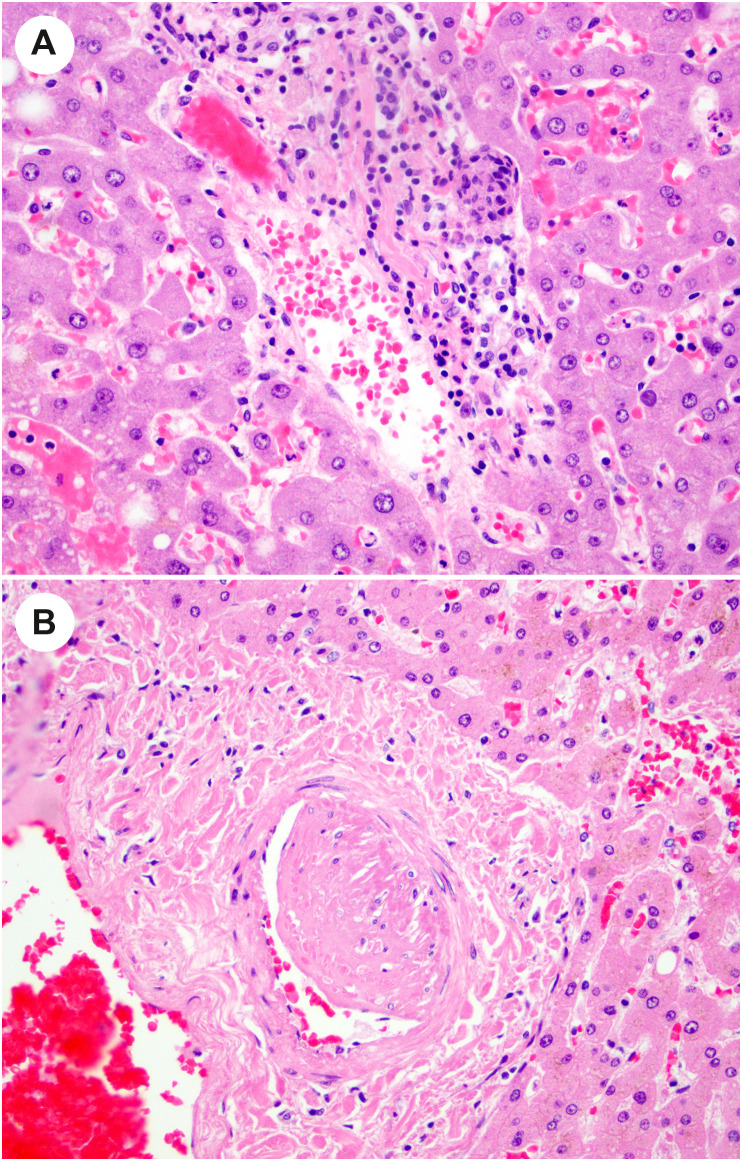
Vascular changes have been reported in autopsy descriptions of the liver in COVID-19. Sonzogni and coinvestigators describe numerous vascular alterations in a series of 48 autopsy liver specimens [97]. They report widened portal veins, some of which were herniated into the adjacent parenchyma or had fibrotic walls, thrombosed veins, thrombi in sinusoids (Fig. 13 ), and abnormal CD34 expression in affected areas of the lobules [97]. They also describe fragmentation of the smooth muscle layer of portal veins, and infiltration by inflammatory cells (Fig. 14 A). In the series by Lagana and colleagues, 6 of 40 cases had phlebosclerosis, reminiscent of veno-occlusive disease [68]. This group of investigators also reported muscular hypertrophy of the portal arteriole (always in association with venous phlebosclerosis) (Fig. 14B), fibrinoid necrosis with endothelial apoptosis, and sinusoidal microthrombi in 6 of 40 cases [68]. In addition, they describe 2 cases that had pale ovoid sinusoidal inclusions that were CD61 positive, suggestive of thrombotic bodies [68]. Sinusoidal platelet aggregates were also observed by Kaltschmidt and colleagues in 70% of 60 autopsy liver specimens [67]. In that series, microvascular thrombosis was observed primarily in nonhospitalized patients who had not received anticoagulation therapy (32%), as compared to hospitalized patients (3%) [67]. Others have observed platelet-fibrin thrombi in sinusoids in these liver [66,69] and portal vein thrombi [66]. Sinusoidal dilatation, portal vein dilatation, and congestion are other vascular changes noted in some reports [[66], [67], [68],70].
Fig. 13.
Thrombotic changes in the liver in patients who died of COVID-19. The lobule in this liver shows numerous fibrin thrombi within the sinusoids. (Courtesy of Dr. Stephen Lagana).
Fig. 14.
Vascular changes in autopsy livers in patients with COVID-19. A, In this portal tract, there is mild inflammation of the portal vein. B, A hypertrophied artery is present in this view of a portal tract. (Courtesy of Dr. Stephen Lagana).
Finally, in the series by Lagana and colleagues [68], granulomas were found in 3 cases, but the relevance of this finding is uncertain. One patient had fibrin ring granulomas, but had been exposed to various hepatotoxic medications; another had necrotizing granulomas forming grossly identifiable abscesses and structures suggestive of Schistosoma eggs, and the third patient had portal granulomas suggestive of primary biliary cholangitis but without clinical findings to support that diagnosis.
4.2.2. Liver biopsy findings in COVID-19
During the course of COVID-19, some patients have sufficiently elevated transaminases or alkaline phosphatase to raise concern for hepatic injury, and a liver biopsy may be performed to evaluate for hepatic injury. These patients may present with marked elevations of transaminases, fulminant hepatic failure, or cholestatic features with jaundice and pruritus.
In the series by Fassan and colleagues [66], three liver biopsies were performed in COVID-19 patients with significant elevations of LFTs. In that report, the biopsies showed similar features, including mild portal mononuclear infiltrate; preserved bile ducts; mild steatosis or microvesicular cytoplasmic vacuolization; mild lymphocytic lobular hepatitis with occasional acidophil bodies, pigmented macrophages, and Kupffer cell aggregates; and occasional mitotic figures [66]. In 1 of 2 biopsies tested, quantitative RT-PCR for SARS-CoV-2 was positive. In the report by Cai and others, a single patient underwent liver biopsy [98]. The biopsy demonstrated mild steatosis, and watery degeneration of the hepatocytes, which the authors attributed to ischemia or hypoxia. Otherwise, there were a few sinusoidal inflammatory cells.
More dramatic cases of marked lobular hepatitis suggest that SARS-CoV-2 can cause hepatitis. In one case report [42], a 35-year-old woman with COVID-19 presented with fulminant hepatic failure. A liver biopsy showed panlobular hepatitis with numerous histiocytes, zone 3 necrosis, mild steatosis, and hemophagocytosis. No ancillary techniques were available to confirm SARS-CoV-2 in the liver, but the clinical picture supported the diagnosis of SARS-CoV-2 hepatitis. In the series by Fiel and colleagues [43], liver biopsies were performed on 2 patients. One patient had a liver biopsy that demonstrated marked lobular disarray, inflammatory infiltrates, many acidophil bodies, ballooned hepatocytes, ceroid laden macrophages, and portal mild to moderate mixed inflammation comprising lymphocytes, plasma cells, eosinophils, and neutrophils; there was mild interface hepatitis and mild ductular reaction. The biopsy also showed centrilobular hepatocyte necrosis and dropout and mild endothelialitis. Bile ducts showed severe damage, with cytoplasmic eosinophilia and apoptotic cholangiocytes. CD61 IHC stain identified nonocclusive fibrin thrombi. ISH for SARS-CoV-2 was positive in rare cells but no cells were positive for the antisense strand probe, and therefore active viral replication could not be confirmed [43]. Electron microscopy demonstrated viral-like particles within sinusoidal endothelial cells.
Pirisi and colleagues describe the liver biopsy findings in 2 patients with COVID-19 who presented with jaundice and pruritus [99]. These patients’ liver biopsies showed canalicular bile, and scant lymphocytes and neutrophils within the sinusoids and in portal tracts. One patient had clear risk factors for cholestatic liver disease (consumption of gym supplements and a pathogenic mutation in ABCB4). The other patient had a mutation of 7-dehydrocholesterol reductase, which is involved in Lemli-Opitz syndrome. The authors report finding SARS-CoV-2 virion in cholangiocytes and hepatocytes [99]. However, as mentioned earlier, reports of virion detected by electron microscopy should be interpreted cautiously, as others have contended that subcellular structures are being misinterpreted as SARS-CoV-2 virion [59,60].
Rare cases of SARS-CoV-2 hepatitis were reported in liver transplant recipients. In the report by Fiel and colleagues [43], 1 patient was a liver transplant recipient. A biopsy of the allograft showed a mixed portal inflammatory infiltrate with occasional eosinophils, prominent bile duct injury with a few apoptotic cholangiocytes, and endothelialitis, suggestive of acute cellular rejection (ACR). The lobules showed lobular disarray with numerous acidophil bodies, foci of necrosis, and numerous mitotic figures. An immunostain for C4d highlighted a few endothelial cells lining portal and central venules, but a stain for CD61 failed to show fibrin thrombi. The lobular changes were interpreted as superimposed SARS-CoV-2 hepatitis. ISH for SARS-CoV-2 showed staining of rare cells, although the antisense strand probe was negative, as with their other case. Electron microscopy identified virus-like particles within hepatocytes. The patient's tacrolimus level was optimized, but otherwise he received no treatment for COVID-19, and his condition slowly resolved.
Another case report was initially reported by Lagana and colleagues [100] and then subsequently reported with additional clinical information by Heinz and others [71]. The case is a 6-month-old girl who underwent a liver transplant and shortly afterward developed markedly elevated transaminases. A liver biopsy demonstrated a mixed portal inflammatory infiltrate composed of lymphocytes and eosinophils, lymphocytic cholangitis, and portal venulitis. Similar to the other case, the lobules demonstrated necroinflammatory foci with lymphocytes and acidophil bodies, either single or in clusters (Fig. 15 ). Kupffer cell prominence and central vein endothelialitis was noted [100]. Mild steatosis, predominantly macrovesicular, was present [100]. ISH for SARS-CoV-2 demonstrated positive staining in hepatocytes and a few inflammatory cells [71]. The biopsy was interpreted as ACR together with SARS-CoV-2 hepatitis. The patient failed to respond to mycophenolate and steroids, and ultimately began to improve when these immunosuppressants were weaned.
Fig. 15.
Hepatitis in a liver allograft in a patient with COVID-19. The lobule shows mononuclear inflammation with hepatocyte dropout, a few acidophil bodies, and small droplet steatosis in zone 3. Elsewhere, the portal changes suggested acute cellular rejection. (Courtesy of Dr. Stephen Lagana).
In the cases that occurred in patients who had received liver transplants, the portal histologic features suggest ACR while the lobular findings independently suggest SARS-CoV-2 hepatitis; alternatively, these cases may represent examples of ACR triggered by SARS-CoV-2 infection. Fiel and coauthors point out that the histologic findings of SARS-CoV-2 hepatitis in their transplant patient were similar to the biopsy findings in their nontransplant patient, in that both had portal mixed inflammation, duct injury, endothelialitis, and lobular hepatitis. Furthermore, they note that the clinical findings in the transplant patient were atypical for rejection. They propose that the portal and lobular histologic features in these cases are due solely to SARS-CoV-2 infection, rather than a combination of ACR and COVID-19 hepatitis [43]. Possibly, some of the histologic findings in SARS-CoV-2 hepatitis overlap with the classic features of ACR. A similar phenomenon has been described with other viral infections in allograft livers, such as CMV [101].
4.2.3. Post–COVID-19 cholangiopathy
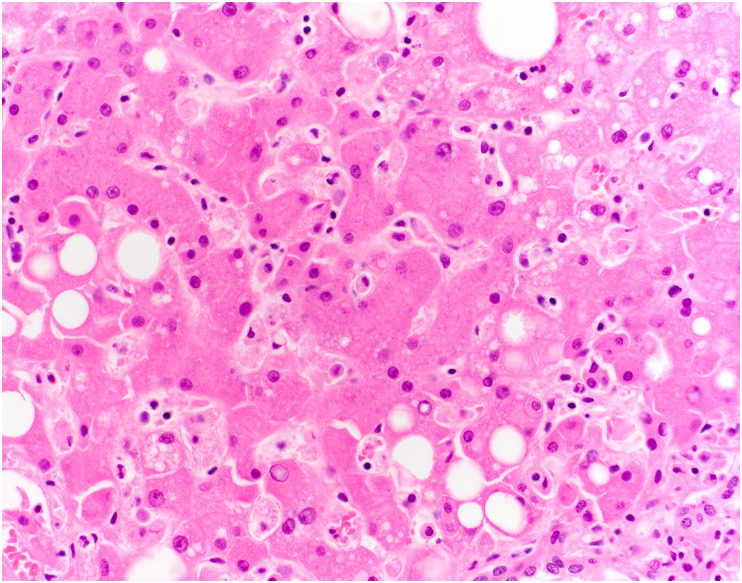
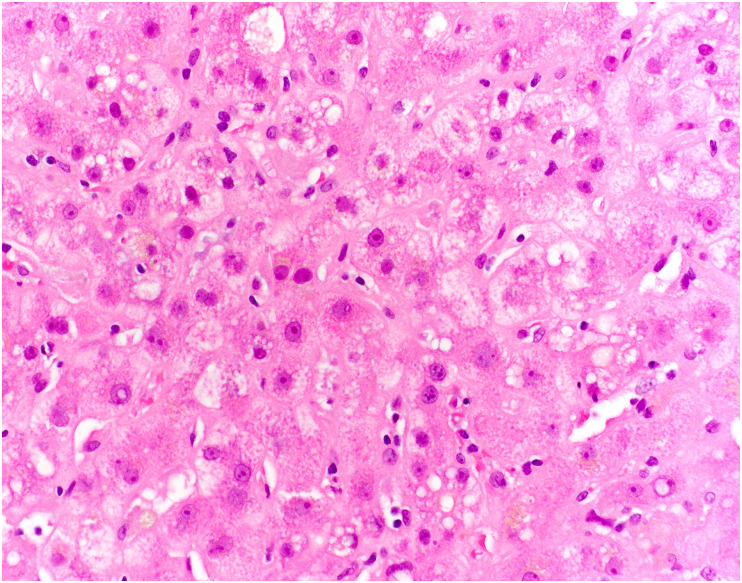
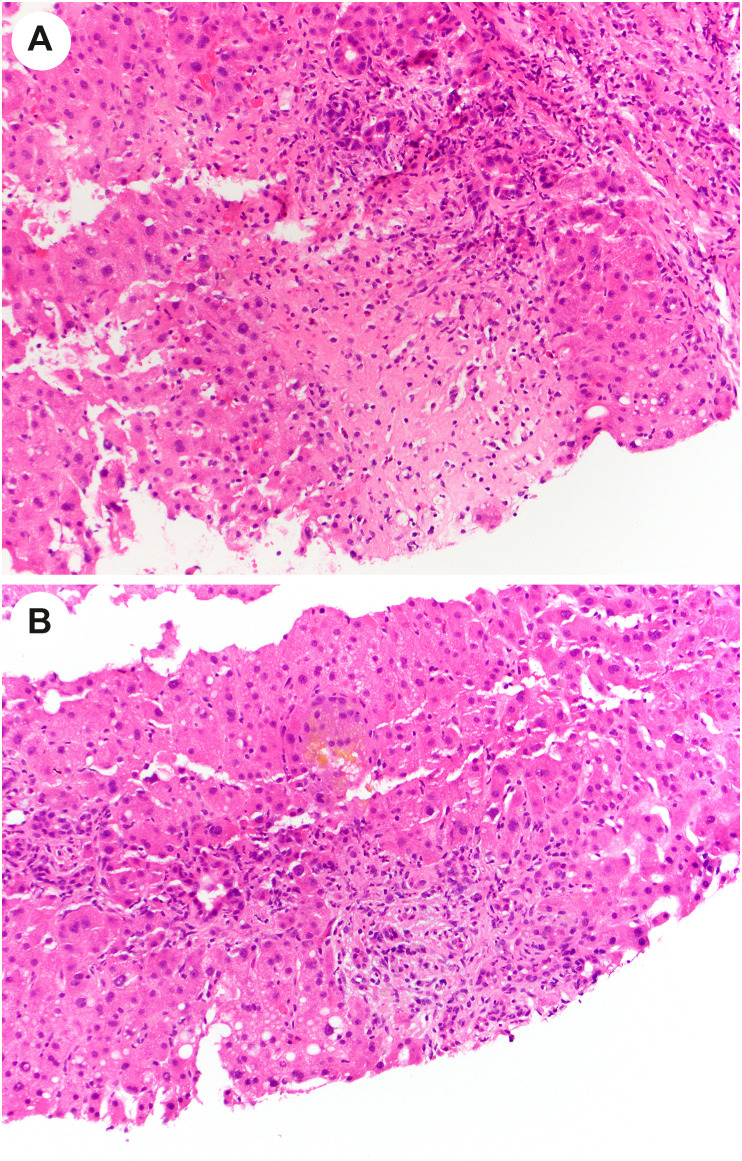
One of the long-term manifestations of COVID-19 is post–COVID-19 cholangiopathy, which is likely a form of secondary sclerosing cholangitis in critically ill patients [51]. Many of these patients have acute and/or chronic large duct obstruction on histology, without ductopenia [51]. One small case series [50] found that some patients showed histologic evidence of mild cholestatic injury, with a mixed portal inflammatory infiltrate, mild bile duct injury, canalicular cholestasis with cholestatic rosetting, and rare small bile infarcts (Fig. 16 ). Other patients showed marked biliary obstructive features, with extensive periportal edema, a neutrophilic portal inflammatory infiltrate, marked ductular reaction, and profound cholestasis with bile infarcts (Fig. 17 A and B). No cases showed evidence of SARS-CoV-2 by ISH. Rarely, patients with persistent cholestatic disease were treated with liver transplantation, and explanted livers showed histologic evidence of severe sclerosing cholangitis with hepatic abscesses, bile duct injury, and microarteriopathy with luminal obliteration [52]. One patient had focal features of sinusoidal obstructive syndrome (veno-occlusive disease) with centrilobular necrosis [49]. Although the mechanism of this process remains to be elucidated, the findings suggest an ischemic pathophysiology.
Fig. 16.
Post–COVID-19 cholangiopathy. In the lobule in this liver biopsy, the hepatocytes are enlarged, reactive appearing, and demonstrate feathery degeneration. There is also cytoplasmic pigment, consistent with intrahepatic cholestasis.
Fig. 17.
Portal obstructive type changes in post–COVID-19 cholangiopathy. A, A portal tract (upper center) shows inflammation and ductular reaction. Below the portal tract (center), there is an area of hepatocyte dropout suggestive of a bile infarct. B, In this portal tract, there is ductular reaction and periportal lobular cholestasis.
5. Preexisting GI and hepatic disease
The most important preexisting GI disorder in terms of COVID-19 is IBD. As the pandemic unfolded, there were concerns about whether patients with IBD were more likely to experience GI symptoms or, if they were taking steroids or immunomodulatory drugs, to develop severe COVID-19. In some studies, patients with IBD did not have an increased risk of GI symptoms, more severe COVID-19, greater risk of hospitalization, or mortality than controls without IBD [102,103]. Although studies suggest that thiopurines, steroids, and oral salicylate might confer increased risk of severe COVID-19 [104,105], the use of long-term biologics or immunomodulatory therapy does not appear to confer worse COVID-19 outcomes [103]. In fact, studies have suggested that tumor necrosis factor antagonists may reduce the severity of COVID-19 [106].
Given the prevalence of gastroesophageal reflux in the general population, many patients with COVID-19 are taking medications for reflux. This coincidence provided an opportunity to describe the risks or benefits of antireflux medications in COVID-19. Several studies, for example, report that patients with COVID-19 taking the histamine H2 receptor antagonist famotidine have improved clinical outcomes [[107], [108], [109]]. The effects of proton pump inhibitor (PPI) use and COVID-19 are uncertain. One study found that PPI use increased the risk of secondary infection in these patients [110], possibly due to the decreased gastric acidity leading to increased gastric microbiota and microaspiration, or due to immune effects of PPIs themselves. However, another study of 179 patients found that patients taking PPIs were less likely to be infected by SARS-CoV-2 or to develop COVID-19 [111].
In patients with COVID-19, the prevalence of preexisting liver disease is 2–11% [31,[112], [113], [114], [115]]. Chronic viral hepatitis and nonalcoholic fatty liver disease confer a higher risk of severe COVID-19 disease [116,117]. ACE2 receptor expression on hepatocytes has been shown to increase in fibrotic or cirrhotic conditions [118,119], possibly explaining worse disease in COVID-19 patients with decompensated cirrhosis [120]. Immunosuppressed liver transplant recipients are not known to experience more severe COVID-19 disease [121].
Although it is accepted that patients with NAFLD are at risk for more severe COVID-19 disease [122,123], whether the steatosis seen in the liver in patients with COVID-19 is due to the viral illness or preexisting NAFLD remains unclear. Some studies propose that the steatosis may be a consequence of dysregulated lipid metabolism and mitochondrial activity in the setting of a cytokine storm [74]. However, it should be acknowledged that a subset of these patients may have had preexisting NAFLD.
6. GI and hepatic injury secondary to COVID-19 therapies
Many of the drugs that are used to combat SARS-CoV-2 can cause GI or hepatic injury, and in some cases, it may be difficult to determine whether the GI or hepatic symptoms are related to the viral infection or its treatment. For example, lopinavir-ritonavir combination therapy, oseltamivir, remdesivir, and antimicrobials can cause diarrhea, nausea, and vomiting [18,124]. Some of the controversial agents used to prevent or treat COVID-19 can have toxic effects as well. For example, chloroquine and hydroxychloroquine have been reported to cause GI effects in about 20% of patients, including nausea, vomiting, diarrhea, and abdominal pain [125]. Ivermectin, a medication that generated significant controversy over whether it was effective to prevent severe COVID-19 or treat it, can have toxic effects, including “GI distress” [126].
Drug-induced liver injury (DILI) is an important issue in this patient population as well. A retrospective cohort study showed that patients with preexisting chronic liver disease were at high risk for DILI with antiviral therapy [127]. In vitro pharmacokinetic studies have shown that some antiviral drugs inhibit key liver transporters, including ABCB11/BSEP, SLC47A1/MATE1, SLC22A1/OCT1, and SLC01B3/OATP1B3 [128]. Although no reports of liver toxicity from remdesivir have been published, some literature describes that occasional COVID-19 patients treated with lopinavir and ritonavir develop elevated AST, ALT, and total bilirubin [32,129]. Hydroxychloroquine has been frequently used in severe COVID-19 disease but has been implicated in cases of hepatotoxicity, with a higher risk in critically ill patients [130]. Usually, LFTs are mildly elevated and normalize with the cessation of hydroxychloroquine; however, liver biochemistries can be markedly elevated, and fulminant hepatic failure has been reported [131]. Ketamine is commonly used as a peri-intubation sedative and has rarely caused secondary sclerosing cholangitis. In one published case, administration of ketamine resulted in an abrupt increase in alkaline phosphatase and radiographic findings of intrahepatic biliary dilatation and beading, with biochemical normalizing after cessation of ketamine; histology in this patient was nonspecific with a ductular reaction and lobular inflammation [132].
Some of the drugs used to treat COVID-19 may cause indirect injury by predisposing these patients to opportunistic infections. For example, case reports describe CMV colitis in patients with COVID-19 treated with the anti–IL-6 inhibitor tocilizumab [133,134]. In addition, the use of broad-spectrum antibiotics in these patients may predispose them to Clostridium difficile colitis.
Vaccine-related AIH-like liver injury has also been described. Several case reports detail patients who present with an acute AIH-like hepatitis after mRNA vaccination [[135], [136], [137], [138]]. These patients were aged between 35 and 80 years, with liver-specific symptoms that presented between 4 and 35 days after the first dose or 7 days after the second dose. Liver biopsies in these patients showed classic AIH morphology, with prominent interface hepatitis, marked lobular inflammation, and, occasionally, centrilobular necrosis [135,136]. One patient with primary sclerosing cholangitis was diagnosed with de novo AIH after vaccination [139]. All patients were responsive to immunosuppressive therapy. It is possible that the SARS-CoV-2 mRNA vaccine may disturb self-tolerance and trigger autoimmune responses through cross-reactivity with host cells; however, it is also possible that these patients had subclinical AIH that was unmasked by the vaccine or coincidentally presented after vaccination.
7. Summary
SARS-CoV-2 is the viral agent responsible for COVID-19, a predominantly respiratory illness in which a significant number of patients experience GI and hepatic manifestations, particularly those with severe disease. Although most of these GI and hepatic manifestations are mild, occasionally they cause significant morbidity or mortality in these patients. Preexisting GI or hepatic disease may affect the disease course of COVID-19, and may alter the outcome of the viral illness. As we begin to understand more about the long-term effects of COVID-19, the long-term GI and hepatic manifestations of this viral illness will become increasingly defined.
Acknowledgments
We would like to acknowledge Drs. Stephen Lagana, Rhonda Yantiss, M. Lisa Zhang, and Michael Torbensen for their figure contributions.
Footnotes
Competing interests: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Zhang H., Liao Y.-S., Gong J., et al. Clinical characteristics of coronavirus disease (COVID-19) patients with gastrointestinal symptoms: a report of 164 cases. Dig Liver Dis. 2020;52:1076–1079. doi: 10.1016/j.dld.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajifathalian K., Krisko T., Mehta A., et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159:1137–1140. doi: 10.1053/j.gastro.2020.05.010. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmunzer B.J., Spitzer R.L., Foster L.D., et al. Digestive manifestations in patients hospitalized with coronavirus disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355–1365. doi: 10.1016/j.cgh.2020.09.041. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao T.-T., Zhang G.-Q., Pellegrini E., et al. COVID-19 and its effects on the digestive system. World J Gastroenterol. 2021;27:3502–3515. doi: 10.3748/wjg.v27.i24.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung K.S., Hung I.F.N., Chan P.P.Y., et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan L., Mu M., Yang P., et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakhli R.E., Shanker A., Sarosiek I., et al. Gastrointestinal symptoms and the severity of COVID-19: disorders of gut–brain interaction are an outcome. Neurogastroent Motil. 2022 doi: 10.1111/nmo.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng W., Qi K., Ye M., et al. Gastrointestinal symptoms are associated with severity of coronavirus disease 2019: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;34:168–176. doi: 10.1097/meg.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 10.Zheng T., Yang C., Wang H., et al. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J Med Virol. 2020;92:2735–2741. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livanos A.E., Jha D., Cossarini F., et al. Intestinal host response to SARS-CoV-2 infection and COVID-19 outcomes in patients with gastrointestinal symptoms. Gastroenterology. 2021;160:2435–2450. doi: 10.1053/j.gastro.2021.02.056. e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shehab M., Alrashed F., Shuaibi S., Alajmi D., Barkun A. Gastroenterological and hepatic manifestations of patients with COVID-19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta-analysis. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabesh E., Soheilipour M., Sami R., et al. Gastrointestinal manifestations in patients with coronavirus disease-2019 (COVID-19): impact on clinical outcomes. J Res Medical Sci Official J Isfahan Univ Medical Sci. 2022;27:32. doi: 10.4103/jrms.jrms_641_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M.L., Jacobsen F., Pepe-Mooney B.J. Clinicopathological findings in patients with COVID-19-associated ischaemic enterocolitis. Histopathology. 2021;79:1004–1017. doi: 10.1111/his.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhayana R., Som A., Li M.D., et al. Abdominal imaging findings in COVID-19: preliminary observations. Radiology. 2020;297 doi: 10.1148/radiol.2020201908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui M., Wang Q., Xin A.W., et al. Vascular thrombosis and vasculitis in the gastrointestinal tract are associated with poor prognosis in patients with COVID-19 - PubMed. Int J Clin Exp Pathol. 2021;14:1069–1079. [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S., Parikh C., Verma D., et al. Bowel ischemia in COVID-19: a systematic review. Int J Clin Pract. 2021;75 doi: 10.1111/ijcp.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt R.H., East J.E., Lanas A., et al. COVID-19 and gastrointestinal disease: implications for the gastroenterologist. Dig Dis Basel Switz. 2020;39:1–21. doi: 10.1159/000512152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han C., Duan C., Zhang S., et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao F., Sun J., Xu Y., et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26:1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zang R., Castro M.F.G., McCune B.T., et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanella G., Capurso G., Burti C., et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: an international multicentre study. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yantiss R.K., Qin L., He B., et al. Intestinal abnormalities in patients with SARS-CoV-2 infection. Am J Surg Pathol. 2022;46:89–96. doi: 10.1097/pas.0000000000001755. [DOI] [PubMed] [Google Scholar]
- 26.Massironi S., Viganò C., Dioscoridi L., et al. Endoscopic findings in patients infected with 2019 novel coronavirus in Lombardy, Italy. Clin Gastroenterol Hepatol. 2020;18:2375–2377. doi: 10.1016/j.cgh.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie X., Sheng L., Han C., et al. Features of capsule endoscopy in COVID-19 patients with a six-month follow-up: a prospective observational study. J Med Virol. 2022;94:246–252. doi: 10.1002/jmv.27308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamakawa T., Ishigami K., Takizawa A., et al. Extensive mucosal sloughing of the small intestine and colon in a patient with severe COVID-19. Den Open. 2022;2:e42. doi: 10.1002/deo2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizvi A., Patel Z., Liu Y., et al. Gastrointestinal sequelae three and six months after hospitalization for coronavirus disease 2019. Clin Gastroenterol Hepatol. 2021;19:2438–2440. doi: 10.1016/j.cgh.2021.06.046. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghoshal U.C., Ghoshal U., Rahman M.M., et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: a case–control study. J Gastroenterol Hepatol. 2022;37:489–498. doi: 10.1111/jgh.15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/s2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hundt M.A., Deng Y., Ciarleglio M.M., Nathanson M.H., Lim J.K. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72:1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Therapeut. 2020;52 doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parasa S., Desai M., Chandrasekar V.T., et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar -M.P., Mishra S., Jha D.K., et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711–722. doi: 10.1007/s12072-020-10071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav D.K., Singh A., Zhang Q., et al. Involvement of liver in COVID-19: systematic review and meta-analysis. Gut. 2021;70:807–809. doi: 10.1136/gutjnl-2020-322072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.-J., Ni Z.-Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B., Zhou X., Qiu Y., et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15 doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber S., Mayerle J., Irlbeck M., Gerbes A.L. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365–1367. doi: 10.1136/gutjnl-2020-321350. [DOI] [PubMed] [Google Scholar]
- 41.Wander P., Epstein M., Bernstein D. COVID-19 presenting as acute hepatitis. Am J Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melquist S., Estepp K., Aleksandrovich Y., et al. COVID-19 presenting as fulminant hepatic failure. Medicine. 2020;99 doi: 10.1097/md.0000000000022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiel M.I., Jamal S.M.E., Paniz-Mondolfi A., et al. Findings of hepatic severe acute respiratory syndrome coronavirus-2 infection. Cell Mol Gastroenterol Hepatol. 2021;11:763–770. doi: 10.1016/j.jcmgh.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong J.K., Chopra S., Kahn J.A., Kim B., Khemichian S. Autoimmune hepatitis triggered by COVID-19. Intern Med J. 2021;51:1182–1183. doi: 10.1111/imj.15420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartoli A., Gitto S., Sighinolfi P., Cursaro C., Andreone P. Primary biliary cholangitis associated with SARS-CoV2 infection. J Hepatol. 2021;74:1245–1246. doi: 10.1016/j.jhep.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lubnow M., Schmidt B., Fleck M., et al. Secondary hemophagocytic lymphohistiocytosis and severe liver injury induced by hepatic SARS-CoV-2 infection unmasking Wilson's disease: balancing immunosuppression. Int J Infect Dis. 2021;103:624–627. doi: 10.1016/j.ijid.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groff D., Sun A., Ssentongo A.E., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edwards K., Allison M., Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roth N.C., Kim A., Vitkovski T., et al. Post–COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 50.Shih A.R., Hatipoglu D., Wilechansky R., et al. Persistent cholestatic injury and secondary sclerosing cholangitis in COVID-19 patients. Arch Pathol Lab Med. 2022 doi: 10.5858/arpa.2021-0605-sa. Online. [DOI] [PubMed] [Google Scholar]
- 51.Faruqui S., Okoli F.C., Olsen S.K., et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 52.Durazo F.A., Nicholas A.A., Mahaffey J.J., et al. Post–COVID-19 cholangiopathy—a new indication for liver transplantation: a case report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med-Prc. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H., Kang Z., Gong H., et al. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 56.Dong M., Zhang J., Ma X., et al. ACE2, TMPRSS2 distribution and extrapulmonary organ injury in patients with COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin L., Jiang X., Zhang Z., et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 58.Stahl K., Bräsen J.H., Hoeper M.M., David S. Absence of SARS-CoV-2 RNA in COVID-19-associated intestinal endothelialitis. Intensive Care Med. 2021;47:359–360. doi: 10.1007/s00134-020-06326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldsmith C.S., Miller S.E., Martines R.B., Bullock H.A., Zaki S.R. Electron microscopy of SARS-CoV-2: a challenging task. Lancet Lond Engl. 2020;395:e99. doi: 10.1016/s0140-6736(20)31188-0. e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bullock H.A., Goldsmith C.S., Zaki S.R., Martines R.B., Miller S.E. Difficulties in differentiating coronaviruses from subcellular structures in human tissues by electron microscopy. Emerg Infect Dis. 2021;27:1023–1031. doi: 10.3201/eid2704.204337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamers M.M., Beumer J., van der Vaart J., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pirola C.J., Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40 doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Bioph Res Co. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L., Han Y., Nilsson-Payant B.E., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136. doi: 10.1016/j.stem.2020.06.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., Goor H van. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fassan M., Mescoli C., Sbaraglia M., et al. Liver histopathology in COVID-19 patients: a mono-Institutional series of liver biopsies and autopsy specimens. Pathol Res Pract. 2021;221:153451. doi: 10.1016/j.prp.2021.153451. 153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaltschmidt B., Fitzek A.D.E., Schaedler J., et al. Hepatic vasculopathy and regenerative responses of the liver in fatal cases of COVID-19. Clin Gastroenterol Hepatol. 2021;19:1726–1729. doi: 10.1016/j.cgh.2021.01.044. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagana S.M., Kudose S., Iuga A.C., et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020:1–9. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao C.L., Rapkiewicz A., Maghsoodi-Deerwester M., et al. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19) Hum Pathol. 2021;109:59–68. doi: 10.1016/j.humpath.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chu H., Peng L., Hu L., et al. Liver Histopathological analysis of 24 postmortem findings of patients with COVID-19 in China. Front Med. 2021;8 doi: 10.3389/fmed.2021.749318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heinz N., Griesemer A., Kinney J., et al. A case of an Infant with SARS-CoV-2 hepatitis early after liver transplantation. Pediatr Transplant. 2020 doi: 10.1111/petr.13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lui V.C.-H., Hui K.P.-Y., Babu R.O., et al. Human liver organoid derived intra-hepatic bile duct cells support SARS-CoV-2 infection and replication. Sci Rep-UK. 2022;12:5375. doi: 10.1038/s41598-022-09306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y., Ren X., Lu J., et al. Mechanistic insight of SARS-CoV-2 infection using human hepatobiliary organoids. Gut. 2022 doi: 10.1136/gutjnl-2021-326617. gutjnl-2021-326617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Liu S., Liu H., et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807–816. doi: 10.1016/j.jhep.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao B., Ni C., Gao R., et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Calitri C., Fumi I., Ignaccolo M.G., et al. Gastrointestinal involvement in paediatric COVID-19 — from pathogenesis to clinical management: a comprehensive review. World J Gastroenterol. 2021;27:3303–3316. doi: 10.3748/wjg.v27.i23.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geier A., Fickert P., Trauner M. Mechanisms of Disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastr. 2006;3:574–585. doi: 10.1038/ncpgasthep0602. [DOI] [PubMed] [Google Scholar]
- 78.Horvatits T., Drolz A., Trauner M., Fuhrmann V. Liver injury and failure in critical illness. Hepatology. 2019;70:2204–2215. doi: 10.1002/hep.30824. [DOI] [PubMed] [Google Scholar]
- 79.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polakos N.K., Cornejo J.C., Murray D.A., et al. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169–1178. doi: 10.2353/ajpath.2006.050875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belz G.T., Altman J.D., Doherty P.C. Characteristics of virus-specific CD8+ T cells in the liver during the control and resolution phases of influenza pneumonia. Proc Natl Acad Sci USA. 1998;95:13812–13817. doi: 10.1073/pnas.95.23.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parry A.H., Wani A.H., Yaseen M. Acute mesenteric ischemia in severe coronavirus-19 (COVID-19): possible mechanisms and diagnostic pathway. Acad Radiol. 2020;27:1190. doi: 10.1016/j.acra.2020.05.016. 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/s2213-2600(20)30076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153 doi: 10.1093/ajcp/aqaa062. aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellosta R., Luzzani L., Natalini G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Erdinc B., Sahni S., Gotlieb V. Hematological manifestations and complications of COVID-19. Adv Clin Exp Med. 2021;30:101–107. doi: 10.17219/acem/130604. [DOI] [PubMed] [Google Scholar]
- 88.Westerhoff M., Jones D., Hrycaj S.M., et al. Gastrointestinal pathology in samples from coronavirus disease 2019 (COVID-19)–Positive patients. Arch Pathol Lab Med. 2021;145:1062–1068. doi: 10.5858/arpa.2021-0137-sa. [DOI] [PubMed] [Google Scholar]
- 89.Amarapurkar A.D., Vichare P., Pandya N., Deshpande S. Haemorrhagic enteritis and COVID-19: causality or coincidence. J Clin Pathol. 2020;73:686. doi: 10.1136/jclinpath-2020-206743. 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiu C., Sarwal A., Mon A., Tan Y., Shah V. Gastrointestinal: COVID-19 related ischemic bowel disease. J Gastroenterol Hepatol. 2021;36:850. doi: 10.1111/jgh.15254. 850. [DOI] [PubMed] [Google Scholar]
- 91.Gartland R.M., Velmahos G.C. Bowel necrosis in the setting of COVID-19. J Gastrointest Surg. 2020;24:2888–2889. doi: 10.1007/s11605-020-04632-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hwabejire J.O., Kaafarani H.M.A., Mashbari H., et al. Bowel ischemia in COVID-19 infection: one-year surgical experience. Am Surg. 2021;87:1893–1900. doi: 10.1177/00031348211038571. [DOI] [PubMed] [Google Scholar]
- 93.Pang J.H.Q., Tang J.H., Eugene-Fan B., Lee C.L., Low J.K. A peculiar case of small bowel stricture in a COVID-19 patient with congenital adhesion band and superior mesenteric vein thrombosis. Ann Vasc Surg. 2020;70:286–289. doi: 10.1016/j.avsg.2020.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmit G., Lelotte J., Vanhaebost J., Horsmans Y., Bockstal M.V., Baldin P. The liver in COVID-19-related death: protagonist or innocent bystander? Pathobiology. 2021;88:88–94. doi: 10.1159/000512008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chornenkyy Y., Mejia-Bautista M., Brucal M., et al. Liver pathology and SARS-CoV-2 detection in formalin-fixed tissue of patients with COVID-19a single-institution experience. Am J Clin Pathol. 2021;155 doi: 10.1093/ajcp/aqab009. aqab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beigmohammadi M.T., Jahanbin B., Safaei M., et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol. 2021;29:135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sonzogni A., Previtali G., Seghezzi M., et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110–2116. doi: 10.1111/liv.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Q., Huang D., Yu H., et al. Characteristics of liver tests in COVID-19 patients. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pirisi M., Rigamonti C., D'Alfonso S., et al. Liver infection and COVID-19: the electron microscopy proof and revision of the literature. Eur Rev Med Pharmaco. 2021;25:2146–2151. doi: 10.26355/eurrev_202102_25120. [DOI] [PubMed] [Google Scholar]
- 100.Lagana S.M., Michele S.D., Lee M.J., et al. COVID-19–associated hepatitis complicating recent living donor liver transplantation. Arch Pathol Lab Med. 2020;144:929–932. doi: 10.5858/arpa.2020-0186-sa. [DOI] [PubMed] [Google Scholar]
- 101.Shih A.R., Naini B.V., Westerhoff M.M., Alpert M.L., Masia M.R., Misdraji M.J. Cytomegalovirus hepatitis in allograft livers may show histologic features of acute cellular rejection. Arch Pathol Lab Med. 2022 doi: 10.5858/arpa.2021-0551-OA. In press. [DOI] [PubMed] [Google Scholar]
- 102.Maconi G., Bosetti C., Monti A.D., et al. Risk of COVID 19 in patients with inflammatory bowel diseases compared to a control population. Dig Liver Dis. 2021;53:263–270. doi: 10.1016/j.dld.2020.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh S., Khan A., Chowdhry M., Bilal M., Kochhar G.S., Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:1575–1578. doi: 10.1053/j.gastro.2020.06.003. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brenner E.J., Ungaro R.C., Gearry R.B., et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Gastroenterology. 2020;159:481–491. doi: 10.1053/j.gastro.2020.05.032. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ungaro R.C., Brenner E.J., Gearry R.B., et al. Effect of IBD medications on COVID-19 outcomes: results from an international registry. Gut. 2021;70:725–732. doi: 10.1136/gutjnl-2020-322539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rizzello F., Calabrese C., Salice M., et al. COVID-19 in IBD: the experience of a single tertiary IBD center. Dig Liver Dis. 2021;53:271–276. doi: 10.1016/j.dld.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Janowitz T., Gablenz E., Pattinson D., et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. 2020;69:1592–1597. doi: 10.1136/gutjnl-2020-321852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Freedberg D.E., Conigliaro J., Wang T.C., et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159:1129–1131. doi: 10.1053/j.gastro.2020.05.053. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mather J.F., Seip R.L., McKay R.G. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am J Gastroenterol. 2020;115 doi: 10.14309/ajg.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luxenburger H., Sturm L., Biever P., et al. Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID-19: coincidence or underestimated risk factor? J Intern Med. 2020;289 doi: 10.1111/joim.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Blanc F., Waechter C., Vogel T., et al. Therapeutic prevention of COVID-19 in elderly: a case–control study. Geroscience. 2021;43:2333–2343. doi: 10.1007/s11357-021-00397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu X.-W., Wu X.-X., Jiang X.-G., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen T., Dai Z., Mo P., et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. Journals Gerontology Ser. 2020;75 doi: 10.1093/gerona/glaa089. glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen L., Huang S., Yang J., et al. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507. doi: 10.1111/jvh.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ji D., Qin E., Xu J., et al. Implication of non-alcoholic fatty liver diseases (NAFLD) in patients with COVID-19: a preliminary analysis. J Hepatol. 2020;73:451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paizis G., Tikellis C., Cooper M.E., et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790. doi: 10.1136/gut.2004.062398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang M., Li X., Meng Y., et al. Upregulation of angiotensin-converting enzyme (ACE) 2 in hepatic fibrosis by ACE inhibitors. Clin Exp Pharmacol. 2010;37:e1–e6. doi: 10.1111/j.1440-1681.2009.05302.x. [DOI] [PubMed] [Google Scholar]
- 120.Xiao Y., Pan H., She Q., Wang F., Chen M. Prevention of SARS-CoV-2 infection in patients with decompensated cirrhosis. Lancet Gastroenterol Hepatol. 2020;5:528–529. doi: 10.1016/s2468-1253(20)30080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transplant. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 122.Mahamid M., Nseir W., Khoury T., et al. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2021;33 doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen V.L., Hawa F., Berinstein J.A., et al. Hepatic steatosis is associated with increased disease severity and liver injury in coronavirus disease-19. Dig Dis Sci. 2021;66:3192–3198. doi: 10.1007/s10620-020-06618-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohamed D.Z., Ghoneim M.E.-S., Abu-Risha S.E.-S., Abdelsalam R.A., Farag M.A. Gastrointestinal and hepatic diseases during the COVID-19 pandemic: manifestations, mechanism and management. World J Gastroenterol. 2021;27:4504–4535. doi: 10.3748/wjg.v27.i28.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doyno C., Sobieraj D.M., Baker W.L. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin Toxicol. 2020;59:12–23. doi: 10.1080/15563650.2020.1817479. [DOI] [PubMed] [Google Scholar]
- 126.Temple C., Hoang R., Hendrickson R.G. Toxic effects from Ivermectin use associated with prevention and treatment of COVID-19. N Engl J Med. 2021;385:2197–2198. doi: 10.1056/nejmc2114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruan X., Lu X., Wang K., et al. Liver injury after antiviral treatment of critically ill patients with COVID-19: a single-centered retrospective cohort study. Ann Palliat Med. 2021:32. doi: 10.21037/apm-20-1581. 32. [DOI] [PubMed] [Google Scholar]
- 128.Ambrus C., É Bakos, Sarkadi B., Özvegy-Laczka C., Telbisz Á. Interactions of anti-COVID-19 drug candidates with hepatic transporters may cause liver toxicity and affect pharmacokinetics. Sci Rep-UK. 2021;11 doi: 10.1038/s41598-021-97160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao B., Wang Y., Wen D., et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Falcão M.B., Cavalcanti LP. de G., Filho N.M.F., Brito CAA de. Case report: hepatotoxicity associated with the use of hydroxychloroquine in a patient with COVID-19. Am J Trop Med Hyg. 2020;102:1214–1216. doi: 10.4269/ajtmh.20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Makin A.J., Wendon J., Fitt S., Portmann B.C., Williams R. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569. doi: 10.1136/gut.35.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Knooihuizen S.A.I., Aday A., Lee W.M. Ketamine-induced sclerosing cholangitis (KISC) in a critically ill patient with COVID-19. Hepatology. 2021 doi: 10.1002/hep.31650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khatib M.Y., Shaik K.S., Ahmed A.A., et al. Tocilizumab-induced cytomegalovirus colitis in a patient with COVID-19. Clin Case Reports. 2021;9:148–152. doi: 10.1002/ccr3.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carll W.C., Rady M.Y., Salomao M.A., Patel B., Singh V.P., Sen A. Cytomegalovirus haemorrhagic enterocolitis associated with severe infection with COVID-19. BMJ Open Gastroenterol. 2021;8 doi: 10.1136/bmjgast-2020-000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Garrido I., Lopes S., Simões M.S., et al. Autoimmune hepatitis after COVID-19 vaccine – more than a coincidence. J Autoimmun. 2021;125:102741. doi: 10.1016/j.jaut.2021.102741. 102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination: a rare adverse effect? Hepatology. 2022;75:489–490. doi: 10.1002/hep.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bril F., Diffalha S.A., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) Vaccine: causality or casualty? J Hepatol. 2021;75:222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou T., Fronhoffs F., Dold L., Strassburg C.P., Weismüller T.J. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis – should we be more vigilant? J Hepatol. 2021;76:218–220. doi: 10.1016/j.jhep.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]