Abstract
The role of the major estrogen estradiol (E2) on orofacial pain conditions remains controversial with studies reporting both a pronociceptive and antinociceptive role of E2. E2 modulation of peripheral serotonergic activity may be one mechanism underlying the female prevalence of orofacial pain disorders. We recently reported that female rats in proestrus and estrus exhibit greater serotonin (5HT)-evoked orofacial nocifensive behaviors compared to diestrus and males. Further coexpression of 5HT2A receptor mRNA in nociceptive trigeminal sensory neurons that express transient receptor potential vanilloid 1 ion channels (TRPV1) contribute to pain sensitization. E2 may exacerbate orofacial pain via 5HT-sensitive trigeminal nociceptors, but whether low or high E2 contributes to orofacial pain and by what mechanism remains unclear. We hypothesized that steady-state exposure to a proestrus level of E2 exacerbates 5HT-evoked orofacial nocifensive behaviors in female rats, we explored the transcriptome of E2-treated females, and we determined which E2 receptor contributes to sensitization of female trigeminal sensory neurons. We report that a diestrus level of E2 is protective against 5HT-evoked orofacial pain behaviors, which increase with increasing E2 concentrations, and E2 differentially alters several pain genes in the trigeminal ganglia. Further, E2 receptors co-expressed with 5HT2A and TRPV1 and enhanced capsaicin-evoked signaling in the trigeminal ganglia via ERα. Overall our data indicate that low, but not high, physiological levels of E2 protect against orofacial pain, and we provide evidence that ERα receptor activation, but not others, contribute to sensitization of nociceptive signaling in trigeminal sensory neurons.
Keywords: Estrogen, GPER, ERα, ERβ, Serotonin, Pain, 5HT2A receptor, Trigeminal Sensory Neurons
INTRODUCTION
Numerous pain conditions with similar prevalence in males and females during childhood are reported to be <2x higher in females after menarche [12; 25]. Pain conditions with higher prevalence in women largely include disorders that occur in the trigeminal sensory system. Given the variability in trigeminal pain conditions over childhood, during menarche and the child-bearing years, and at the onset of menopause, gonadal hormones estrogen, progesterone, and testosterone clearly contribute to sexually dimorphic pain mechanisms. Progesterone, testosterone, and their metabolites play a neuroprotective role and have anti-inflammatory and antinociceptive properties [4; 18; 28; 44; 113]. Estrogen (E2) exerts both pro- and antinociceptive effects on trigeminal pain. The effects of E2 are multifactorial and may depend on gonadal hormone levels (steady-state vs fluctuating), dosage or concentration used, the type of pain or pain model being examined, and which estrogen receptor (ER) is bound (classical nuclear receptors ERα and ERβ vs the membrane-bound G protein-coupled estrogen receptor GPER) [23; 40; 79].
One trigeminal pain mechanism that may be exacerbated by E2 is peripheral serotonergic modulation of trigeminal sensory neurons. Serotonin (5HT) is implicated in several pain disorders more prevalent in women [34; 37; 42]. While 5HT in the central nervous system is often antinociceptive, 5HT is a robust pronociceptive mediator in the periphery activating rat [64; 67; 85; 106; 109] and human nociceptors [33; 65; 108]. 5HT triggers pain by acting on excitatory 5HT receptors, such as 5HT2A, expressed on the transient receptor potential vanilloid 1 (TRPV1) population of nociceptors [53; 67; 85; 106; 121]. Recently, we reported 5HT evokes greater and longer-lasting pain behaviors in the female rat hindpaw [52] and vibrissal pad [54] during proestrus (peaking E2) and estrus (declining E2), and involving the 5HT2A receptor [52; 54] rather than the 5HT3 receptor [53]. Further, we reported that E2 enhances the nociceptive effects of 5HT on the TRPV1 population of trigeminal sensory neurons [54]. This is supported by our previous report that 5HT enhances release of calcitonin gene-related peptide (CGRP) from human dental pulp during the late luteal phase of the menstrual cycle, when E2 levels peak and rapidly decline [65].
While it is established that E2 modulates the serotonergic system and 5HT is pronociceptive at trigeminal nociceptors in a sexually dimorphic manner, whether low or high E2 contributes to orofacial pain and by what mechanism remains unclear. We hypothesized that steady-state exposure to a proestrus level of E2 exacerbates 5HT-evoked orofacial nocifensive behaviors in female rats, we explored the transcriptome of E2-treated females, and we determined which E2 receptor contributes to sensitization of female trigeminal sensory neurons. Here, we (1) manipulated E2 levels in adult female rats and measured 5HT-evoked nocifensive behavior, (2) examined the trigeminal ganglia transcriptome of hormone-manipulated female rats, (3) probed the TRPV1 population of female rat trigeminal sensory neurons for ERα, ERβ, GPER, and 5HT2A receptor mRNA, and (4) pharmacologically targeted the estrogen receptors in primary cultures of trigeminal sensory neurons to determine which estrogen receptor(s) are involved in serotonergic potentiation of nociceptive signaling.
METHODS
Subjects
A total of 3 adult male and 151 adult female Sprague–Dawley rats (200–300 g; Charles River Laboratories, Wilmington, MA) were used in the experiments. Rats were separated by sex and pair-housed in a 12:12-h light: dark cycle with ad libitum food and water access. All studies were approved by the Texas Woman’s University Institutional Animal Care and Use Committee and conform to federal guidelines and guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. This study was conducted in strict compliance with the Animal Welfare Act, implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals.
Vaginal cytology
Vaginal lavages were performed between 0900AM to 1100AM at 24-h intervals beginning 2 weeks (at least two consecutive cycles; 10 days) before testing to confirm that all female rats were cycling normally. Daily records were maintained on the stages of their cycle throughout experimental testing. Proestrus was identified as a predominance of nucleated epithelial cells and estrus was identified as a predominance of cornified epithelial cells. Diestrus 1 (or metestrus) was differentiated from diestrus 2 (or diestrus) by the presence of leukocytes [10; 66; 75]. When no significant differences were noted in behavior of diestrus 1 and diestrus 2 animals, these data were pooled and reported as such.
Ovariectomy
Female rats (n=135) were deeply gas anesthetized (3% induction; 2.5% maintenance) by inhalation of Isothesia (isoflurane, USP, Henry Schein Animal Health, Dublin, OH) and a single incision was made across the abdomen. The abdominal muscle was opened and the ovary bundles were ligated with 4-O silk sutures, excised, and removed, as previously described [117]. The fascia was closed with 5-O silk suture and the skin was closed with Vicryl sutures to prevent wicking. Rats were allowed 2 weeks for recovery and ovarian hormone dissipation as previously described (peak cycling E2 levels are at ~63 pg/mL vs. 2 weeks post-OVX levels at ~4 pg/mL) [105].
Drugs
β-estradiol 3-benzoate (E2; Sigma–Aldrich, St. Louis, MO) was dissolved in corn oil, stored as a 2 mg/mL solution at room temperature, and serial diluted to 2 μg/mL, 20 μg/mL, or 200 μg/mL on the day of use. Serotonin hydrochloride (5HT; Sigma–Aldrich) was dissolved in double-distilled water and diluted in 0.9% sterile saline or Hank’s balanced salt solution (HBSS) buffer immediately prior to each use. Capsaicin (CAP; Sigma–Aldrich) was dissolved in 100% ethanol in a fume hood and aliquots were stored at −20°C as 100 mM stocks. Capsaicin was freshly diluted in HBSS buffer prior to each use. β-Estradiol (E2; Sigma–Aldrich) was dissolved in 100% ethanol to create a 10 mM stock solution that was further diluted in HBSS buffer for a working solution of 50 nM. β-Estradiol 6-(O-carboxy-methyl) oxime: BSA (E2-BSA) was dissolved in HBSS to create a stock solution of 10 μM and was used at a final concentration of 50 nM. The estrogen receptor agonists/ antagonists, ICI 182,780, rel-1-[4-(6-bromo-1,3-benzodioxol-5-yl)-3aR,4S,5,9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone (G1), 1,3,5-Tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole (PPT), 2,3-Bis(4-hydroxyphenyl)propionitrile (DPN), Methyl-piperidino-pyrazole hydrate (MPP), and 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]-pyrimidin-3-yl]phenol (PHTPP) were dissolved in DMSO to prepare the stock solutions and further diluted in HBSS to final concentrations as listed in Table 1.
Table 1. Specifications and concentrations of the estrogen receptor (ER) agonists and antagonists applied to trigeminal ganglia neuron primary cultures.
E2-BSA: β-Estradiol 6-(O-carboxymethyl)oxime conjugated to bovine serum albumin (BSA); G-1: rel-1-[4-(6-bromo-1,3-benzodioxol-5-yl)-3aR,4S,5,9bS-tetrahydro-3H-cyclopenta[c]quinolin-8-yl]-ethanone; ICI 182,780: 7α,17β-[9-[(4,4,5,5,5 Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol; PPT: 1,3,5-Tris(4-hydroxyphenyl)-4-propyl-1H-pyrazole; DPN: 2,3-Bis(4-hydroxyphenyl)propionitrile; MPP: MPP Dihydrochloride; PHTPP: 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]-pyrimidin-3-yl]phenol.
| Pharmacologic | Target | Company | Cat. # | Concentration | Ref. |
|---|---|---|---|---|---|
| E2-BSA | Membrane-impermeable E2 | Sigma–Aldrich | E5630 | 50 nM | [95] |
| G-1 | GPER agonist | Cayman Chemical | 10008933 | 10 nM | [15; 93] |
| ICI 182,780 | ER antagonist; GPER agonist | Sigma–Aldrich | 5310420001 | 1 μM | [96] |
| PPT | ERα agonist | Sigma–Aldrich | H6036 | 100 nM | [21; 62; 96] |
| DPN | ERβ agonist | Sigma–Aldrich | H5915 | 15 nM | [21; 78; 96; 116] |
| MPP | ERα antagonist | Sigma–Aldrich | M7068 | 300 nM | [96; 107] |
| PHTPP | ERβ antagonist | Sigma–Aldrich | SML1355 | 1 μM | [21; 60] |
Silastic hormone capsule preparation and implantation
Capsules were prepared as previously described [73]. Briefly, Silastic ® tubing (0.058 in. ID x 0.077 in. OD (Dow Corning, Midland, Michigan) was cut into 1 cm capsules, sealed on one end with medical adhesive (Factor II, Inc., Lakeside, AZ), and allowed to dry overnight. E2 was weighed as 5%, 10%, or 20% of cholesterol and was mixed thoroughly to create a uniform powder. The hormone: cholesterol mixture was then packed into the capsule by tapping it gently. Once filled, the other end of the tubing was sealed and allowed to dry overnight. The capsules were then washed twice in 100% ethyl alcohol and immersed in 0.1M PBS for 48 hours. The capsules that sunk in 0.1M PBS were discarded and the floating capsules (indicating properly sealed ends) were used for implantation. The capsules were implanted at a final dose of 5% (physiological level comparable to diestrus; 15-20 pg/ml; one capsule), 20% (physiological level comparable to proestrus; 40-60 pg/ml; 2 capsules of 10%), 40% (supraphysiological level; 2 capsules of 20%) E2, or control (2 capsules of 100% cholesterol) [73]. The capsules were implanted subcutaneously into the back of ovariectomized female rats under anesthesia (2.5 - 3% isoflurane) using aseptic technique.
Orofacial Nocifensive Behavior Testing
Orofacial nocifensive behaviors were quantified as previously described [55]. Briefly, rats were placed in individual mirrored boxes and nocifensive behavior was recorded with a video camera for a 30-min period. The videos were manually quantified using iMovie software (Apple Inc., Mac OS) by counting the number of forelimb swipes directed at the injection site in 6 min bouts over a 30 min period and reported as a measure of spontaneous nocifensive behavior. Rat forelimb swipes have been characterized in the literature as spontaneous nocifensive behavior distinct from grooming and itch behaviors [48; 101; 104]. Data was collected by two independent observers blind to the experimental condition and their values were averaged. If there were substantial differences between the two observer’s counts (> 5 swipes), they were re-evaluated together to concur for final reporting.
Orofacial nocifensive behavior was tested in ovariectomized rats with E2 manipulation by either steady-state E2 treatment using silastic capsules (noted in above methodology section) or acute E2 injections (n = 8-9 per experimental treatment group). For the acute E2 group, ovariectomized females were gas anesthetized (2.5 - 3% isoflurane) and received a single subcutaneous injection (at the flank) of either 2 μg/mL E2 (physiological level comparable to diestrus; 15-20 pg/ml), 20 μg/mL E2 (physiological level comparable to proestrus; 40-60 pg/ml), 200 μg/mL E2 (supraphysiological level), or vehicle control (corn oil; 1 ml/kg per animal) [30; 86]. Though both experimental groups (injection vs capsule implantation) use different units, the resulting E2 released in the respective groups are physiologically comparable (2 μg/mL E2 and 5% E2 capsule will both release diestrus-level of hormone). One hour following acute E2 injections or 1 week following E2 capsule implantation, all rats received a single intradermal injection of 3 μg/50 μL 5HT into the right vibrissal pad (30-gauge needle). The number of forelimb swipes over the injected area were counted as described above as 5HT-evoked nocifensive behaviors (Timeline 1). Following completion of behavior testing, rats were rapidly decapitated and bilateral trigeminal ganglia were collected and stored at −80°C until further use.
Timeline 1.
Timeline illustrating the course of ovariectomy, hormone capsule implantation, and serotonin (5HT) injections for orofacial pain behavior testing experiments.
RNA Isolation and RNA sequencing (RNA-seq)
RNA was extracted from the trigeminal ganglia collected from the hormone treated rats following collection of 5HT-evoked pain behaviors using the Qiagen RN-easy kit protocol. Briefly, right trigeminal ganglia (n=3 per hormone treatment group as described above) was lysed using a Bead Mill Homogenizer (VWR, Radnor, PA). The lysate was passed through a gDNA spin column (Qiagen, Germantown, MD) to eliminate genomic DNA and flow-through was used for RNA isolation. A 1:1 70% molecular-grade ethanol was added to the flow-through and passed through a RNeasy spin column (Qiagen). The RNA collected on the membrane of the spin column was washed three times with provided buffer and the column was transferred to a sterile, RNase-free tube. RNA was eluted by adding 30-50 μL RNase-free water to the column and centrifuging for 1.5 min at 10,000 rpm. RNA was quantified using a Nanodrop™ spectrophotometer (ThermoFisher Scientific, Waltham, MA). RNA quality was confirmed by A260/280 ratio and only the samples that had a ratio of ≥2 and a concentration > 20 ng/μL were sent for sequencing to Novogene (sequencing lab at The University of California at Davis campus).
Novogene confirmed the RNA purity and integrity of the samples using a NanoPhotometer® spectrophotometer and the RNA Nano 6000 Assay Kit (Bioanalyzer 2100 system), respectively. Samples with RNA integrity number (RIN) > 8 were used for library preparation and sequencing on an Illumina platform. After sequencing, raw reads were cleaned to remove adapter reads and low-quality reads. The paired-end clean reads were then mapped to the rat reference genome using HISAT2 software and RPKM (Reads Per Kilobase of exon model per Million) was calculated to estimate the gene expression levels. Differential gene expression analysis of biological replicates was then performed using the DESeq2 R package and p values were adjusted using the Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with p value ≤0.05, FPKM > 1, and log2FoldChange ≥ 0.0 were considered as differentially expressed genes (DEGs) as per Novogene default thresholds and were compared against three databases specific to pain genes [Human Pain Genetics Database, Pain Research Forum - Pain Genes Database, and Database curated by Pokhilko et al.,2020 [92]]. Functional annotation of the differentially expressed genes and Gene Ontology (GO) graphs were performed using Blast2GO (v. 5.2.5) [38]. Morpheus (https://software.broadinstitute.org/morpheus) was used to generate the heat maps of differentially expressed genes. RNA-Seq data, including raw gene counts per gene, has been deposited to NCBI’s Gene Expression Omnibus [9; 32] and are accessible through GEA Series accession number GSE193773 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE193773).
In situ hybridization
Trigeminal ganglia were bilaterally extracted from intact males and cycling female rats (n= 3 per sex and stage of estrous cycle) and immediately frozen on dry ice. The tissues were stored at −80°C until processing. Bilateral ganglia were embedded in Tissue-Tek Optimal Cutting Temperature (O.C.T) compound (Sakura Finetek, Torrence, CA) and sectioned at 30 μm on a Leica Cryostat CM3050 (Leica Biosystems, Buffalo Grove, IL) set at −20°C. The slides were then stored at −80°C until further processing. For in situ hybridization, the RNAscope® Fluorescent Multiplex Assay was performed according to the manufacturer’s specifications (Advanced Cell Diagnostics bio-techne, Newark, CA) with optimizations performed for trigeminal ganglia tissue. Briefly, slides were fixed in 4% paraformaldehyde, sequentially dehydrated in 50% ethanol, 70% ethanol, and 100% ethanol, and stored at −20°C overnight. The next day, slides were air-dried and incubated at 60°C and a hydrophobic barrier was drawn using an ImmEdge Hydrophobic Barrier Pen (Vector Laboratories, Burlingame, CA). Following a hydrogen peroxide and protease IV treatment, the slides were treated with experimental probes against 5HT2A and TRPV1, positive control probes against ubiquitin C (UBC) or peptidyl-prolyl cis-trans isomerase B (PPIB), or negative control probes against targeting bacterial gene dihydrodipicolinate reductase (DapB) and incubated at 40°C for 2 hours. Slides were washed in buffer and amplification steps were performed with kit-provided AMP-1, AMP-2, and AMP-3, then slides were sequentially treated with OPAL fluorophores (OPAL 690 and OPAL 520; Akoya Biosciences, Marlborough, MA). Excess liquid was carefully drained from the slides and 1 - 2 drops of Prolong Gold antifade mounting medium (Fisher Scientific) with 4’,6-diamidino-2-phenylindole (DAPI) were added and slides were cover slipped and air-dried overnight at room temperature.
The slides were imaged using a Zeiss LSM 900 confocal microscope. Zeiss ZEN software was used to view and capture the images at 20X and 40X. Every 1:4 sections were imaged under the same gain as the control slides and 3–4 images were captured across the V1/V2 and V3 area of the left and right trigeminal ganglia section per animal. For quantification, cells expressing both 5HT2A and TRPV1 were manually counted and percent coexpression was calculated using the following formula:
Percent coexpression . Image manipulation was restricted to adjustments of brightness/contrast for the representative images presented.
Primary culture of rat trigeminal ganglia neurons
Trigeminal ganglia (n=4 rats per 24-well plate run in triplicate) were extracted from adult OVX female rats (~200 g) immediately following decapitation. Primary neuron cultures were prepared using previously described methods [67; 89]. Briefly, trigeminal ganglia were suspended in HBSS on ice and gently washed three times. After dissociation with collagenase (5%, Worthington Biochemical Corp, Lakewood, NJ) and trypsin (1%, Sigma–Aldrich) at 37 °C, the cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Waltham, MA) containing 10% fetal bovine serum, glutamine, penicillin-streptomycin, nerve growth factor (NGF, 100ng/ml; Harlan, Indianapolis, IN), and mitotic inhibitors (5-fluoro-2’-deoxyuridine and uridine). Cells were then lightly dissociated using a 20-gauge followed by a 23-gauge needle and then applied to 24-well poly-D-lysine-coated plates (Corning Inc., Corning, NY) and maintained in an incubator at 37 °C and 5% CO2.
Calcium Imaging
Trigeminal ganglia (n=4 rats per 96-well plate) were extracted from adult female rats (<200 g) immediately following decapitation and cultured as described above, except trypsin was replaced by dispase (2 mg/ml) as per calcium imaging recommendations [67]. Trigeminal ganglia primary cultures were maintained in an incubator for 9 days at 37 °C and 5% CO2. The assay was performed as per the Fluo-4 calcium imaging kit protocol (ThermoFisher Scientific, Waltham, MA). Briefly, culture media was aspirated from one column at a time (8 wells; A-H), each well was washed 1X with HBSS buffer, and was incubated with 100 μL Fluo-4 AM loading dye. The cells were then incubated at 37 °C and 5% CO2 for 30 minutes followed by 15 minutes at room temperature. The Fluo-4 was aspirated, cells were washed 1X with HBSS, and 100 μL of Neuro Background Suppressor (proprietary solution) was slowly pipetted into each well. Calcium imaging was recorded using BioTek Cytation 5 plate reader at the GFP optical filter. Baseline fluorescence reading was recorded for 30 sec followed by 50 μL dispense of either HBSS, 50 μM 5HT, 50 nM E2, or 5HT+E2. Responses were recorded for an additional 3 min. Once responses to each treatment were established, we then repeated the treatments and 50 μL of 30 nM CAP was dispensed and responses recorded over 2.5 min. The change in fluorescent intensity was calculated by subtracting average baseline read from the peak CAP response.
CGRP release assay
Trigeminal ganglia primary cultures were maintained for 5 days prior to running the CGRP release assay. The assay was performed using a protocol previously described [67]. Table 1 provides details on the drugs used in this experiment. Briefly, cultures were twice incubated for 15 minutes each with 300 μL HBSS. Superfusate was collected from the second incubation for measurement of the basal level of CGRP release prior to drug treatments. Cells were then treated for 15 minutes in either estrogen receptor agonists, antagonists, or vehicle followed by superfusate collection. Each superfusate collected was quantitated for CGRP levels by rat specific CGRP ELISA (Cayman Chemical, Ann Arbor, MI). All experiments were conducted in duplicate with n = 6 wells per treatment group for a total of approximately 12 wells per group.
For estrogen receptor agonist treatments, cells were pretreated row-wise with either E2-BSA (50 nM), G-1 (10 nM), ICI 182,780 (1 μM), PPT (100 nM), DPN (15 or 100 nM), or vehicle (HBSS or HBSS/DMSO). Superfusate was collected and cells were then stimulation for 15 minutes with 5HT (100 μM) in the continued presence of each drug, superfusate collected, and finally cells were stimulated with capsaicin (50 nM) in the continued presence of 5HT for 15 minutes and superfusate collected. For estrogen receptor antagonist treatments, cells were pretreated row-wise for 15 minutes with either MPP (300 nM), PHTPP (1 μM), or vehicle (HBSS or HBSS/DMSO). Superfusate was collected and cells were treated with E2 (50nM) for 15 minutes followed by superfusate collection. Cells were then stimulated with 5HT (100 μM) in the continued presence of E2 and each drug, superfusate collected, then stimulated with capsaicin (50 nM) followed by supernatant collection. The concentration of each drug was chosen based on previous reports on specificity (Table 1).
Data analysis
All data were analyzed and graphed with GraphPad Prism software version 9.2.0 (GraphPad, San Diego, CA). Orofacial nocifensive behavior data were expressed as mean ± standard error of the mean (SEM) number of forelimb swipes over a 30 min period (6 min bouts) and was analyzed by repeated measures two-way analysis of variance (ANOVA). For the in situ hybridization quantification, the mean percent coexpression were analyzed by unpaired t test comparing males with each stage of the estrous cycle with the a priori assumption that diestrus would not be significantly different than males. Calcium imaging data was reported as the change in Fluo-4 fluorescence intensity and groups were analyzed by unpaired t-test. CGRP release was reported as mean ± SEM percent of basal CGRP release and were analyzed by unpaired t-test or two-way ANOVA. The Grubb’s test (GraphPad Quick Calcs Online, the extreme studentized deviate method; [(mean-value) / standard deviation]) was used to exclude a single outlier within an experimental group if present. Further, 3 animals were removed from the study when environmental factors (significant noise in the animal facility) disrupted behavior testing. Dunnett’s test was used for behavior data and Bonferroni's correction was used for CGRP release assays to calculate a priori pairwise comparisons when significance was detected by ANOVA.
RESULTS
Steady-state, but not acute, diestrus level E2 treatment significantly reduced peripheral 5HT-evoked orofacial nocifensive behaviors in ovariectomized female rats
Our previous studies reported that 5HT evoked greater pain behaviors in female rats during proestrus and estrus [52; 55]. As E2 levels peak during proestrus but drop during estrus, it remained unclear whether E2 was acting in a pronociceptive or antinociceptive fashion. Here we performed steady-state and acute hormone manipulations in ovariectomized female rats to control the timing and concentration of E2 levels. We define acute exposure as a single subcutaneous injection of E2 given one hour before behavior testing and steady state exposure as continual exposure to the same level of E2 for one week. In the acute E2 exposure experiment, 5HT-evoked nocifensive behaviors were observed at the 7-12 minute time bout (Figure 1A; open bars). Acute exposure to physiological (gray bars) or supraphysiological (closed bars) E2 did not significantly alter 5HT-evoked nocifensive behaviors (Figure 1A) [p > 0.05]. In the steady-state E2 exposure experiment, 5HT-evoked nocifensive behaviors were observed at the 0-18 minute time bouts (Figure 1B; open bars). Steady-state exposure to physiological levels of E2 comparable to diestrus or proestrus significantly attenuated 5HT-evoked nocifensive behaviors [F (4, 112) = 8.459; p<0.0001] at the 0-6 minute time bout (Figure 1B; gray bars) [p ≤ 0.05]. 5HT-evoked nocifensive behaviors were unaltered by E2 at the 7-12 minute time bout [p > 0.05], but were again attenuated at the 13-18 minute time bout only in the females receiving diestrus-level E2 [p ≤ 0.01]. The highest 5HT-evoked nocifensive behaviors were observed in the animals that received the supraphysiological level E2 [p > 0.05] (Figure 1B; closed bars). These data concur with our previous studies that 5HT-evoked behavior peaks (20-30 swipes) within the first 18 minutes post-injection and can either be observed within the first 6-minute bout or can be split across multiple bouts during the first 18 minutes post-injection. Thus, and as orofacial nocifensive behavior is a spontaneous pain behavior test that is highly variable as compared to stimulus-evoked behavior testing, we have observed that peak in Figure 1B to be earlier as compared to Figure 1A. Overall, we report that steady-state diestrus level E2 significantly attenuates 5HT-evoked orofacial nocifensive pain behaviors.
Figure 1. Steady-state physiological levels of E2, but not acute E2 treatment, attenuates 5HT-evoked orofacial pain behaviors.
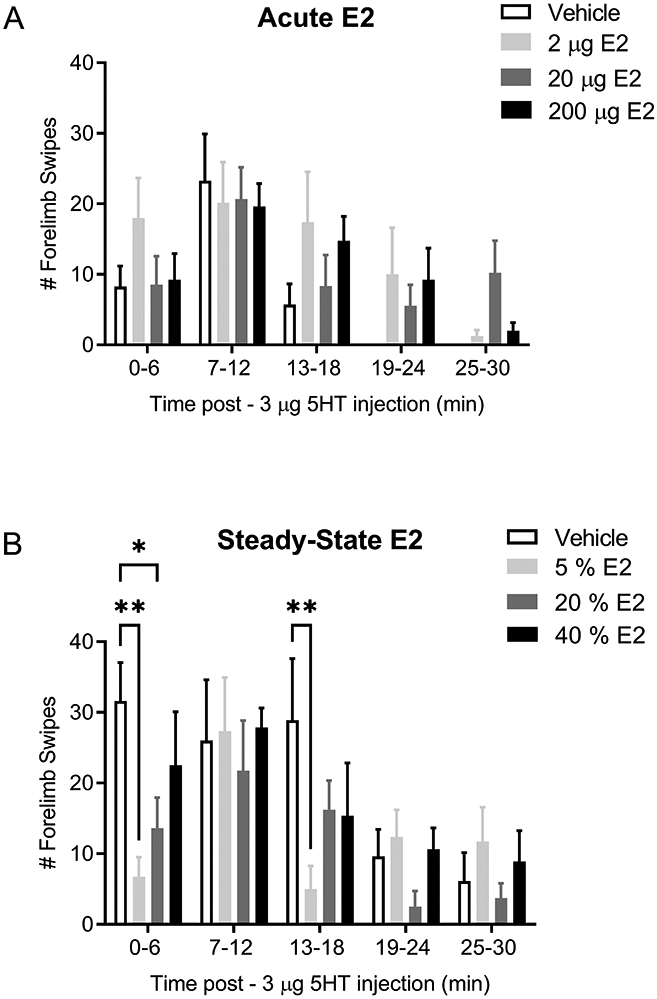
5HT-evoked orofacial pain behaviors were not significantly different in OVX females that received 2 μg E2 (light gray bars), 20 μg E2 (dark gray bars), or 200 μg E2 (closed bars) one hour prior to receiving 5HT injection as compared to vehicle control (open bars) (A). 5HT-evoked pain behaviors were significantly reduced in OVX females that were administered with steady-state physiological levels of 5% E2 (light gray bars) and 20% E2 capsules (dark grey bars) compared to vehicle control (open bars) at 0-6 min (B). Attenuation was also reported in the 5% E2 group at 13-18 min time bout. Attenuation was not observed in animals that received 40% E2 capsules (closed bars). *Denotes significant number of forelimb swipes compared to vehicle with significance in pairwise comparisons tested at p ≤ 0.05.
Several pain pathway related genes are altered in the trigeminal ganglia of E2-treated female rats
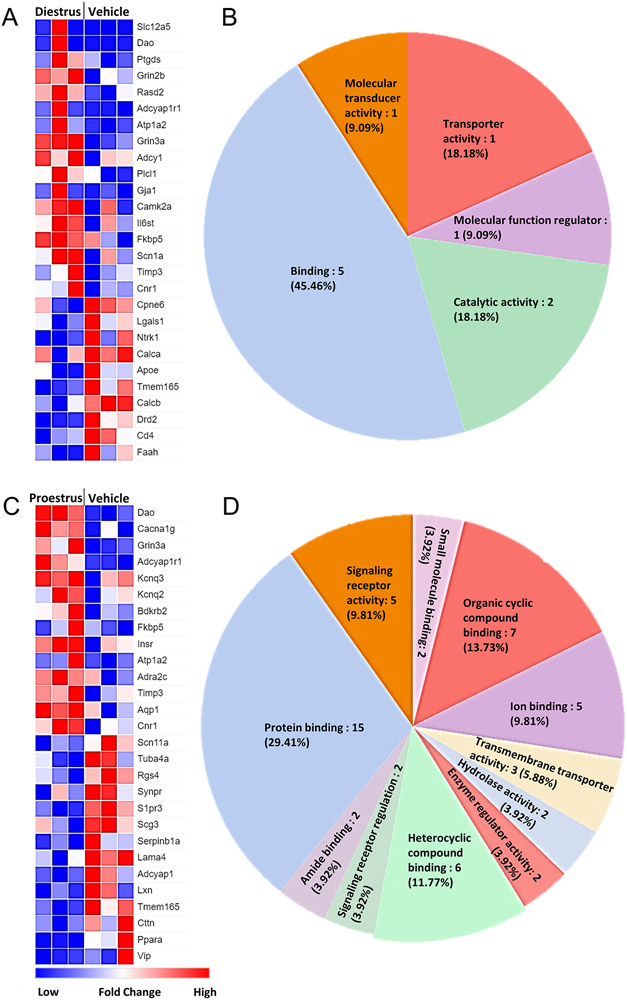
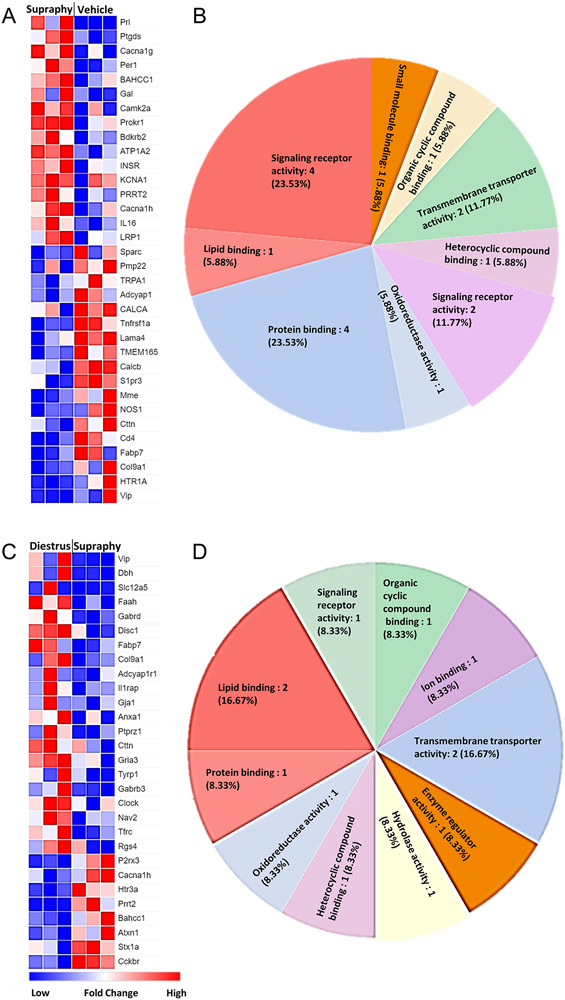
Since estrogen is a major hormone involved in multiple gene expression pathways in the cell, and we observed significant differences in the 5HT-evoked pain behaviors in the steady-state E2 experiment, we next determined the corresponding changes in trigeminal ganglia transcriptome of the E2-treated rats from the behavior experiments. Although the RNA preparation consists of 90-95% non-neuronal cells [77], several genes that directly or indirectly play a role in pain signaling were differentially expressed after E2 exposure. Expression of major genes involved in nociception and analgesia was significantly changed in steady-state diestrus (Figure 2A; 2B; Supplementary Figure 1A) and proestrus E2 group compared to vehicle (Figure 2C; 2D; Supplementary Figure 1B). Similarly, expression of major nociceptive genes was upregulated in the steady-state supraphysiological E2 group as compared to vehicle (Figure 3A; 3B; Supplementary Fig 2A) and steady-state diestrus E2 group (Figure 3C; 3D; Supplementary Fig 2B). The function of the DEGs in trigeminal pain signaling and modulation by E2 is listed in Table 2. Overall, gene expression related to pain signaling is altered in a manner dependent on estrogen dosage and exposure duration.
Figure 2. Several genes related to trigeminal pain processing are altered in the steady-state diestrus and proestrus E2 groups.
Differentially expressed genes (DEGs) in steady-state diestrus E2 group compared to vehicle (Veh; A) and molecular function analysis of the DEGs (B). Differentially expressed genes (DEGs) in steady-state proestrus E2 group compared to vehicle (Veh; C) and molecular function analysis of the DEGs (D). Blue = downregulation; red = upregulation. Only the genes that significantly changed (p<0.05) are shown.
Figure 3. Several genes related to trigeminal pain processing are altered in the steady-state supraphysiological E2 group.
Differentially expressed genes (DEGs) in steady-state supraphysiological (Supraphy) E2 group compared to vehicle (Veh; A) and molecular function analysis of the DEGs (B). Differentially expressed genes (DEGs) in steady-state supraphysiological E2 group compared to steady-state diestrus E2 (C) and molecular function analysis of the DEGs (D). Blue = downregulation; red = upregulation. Only the genes that significantly changed (p<0.05) are shown.
Table 2. Differentially expressed genes following E2 treatment.
List of known pain genes that were differentially expressed following steady-state treatment with diestrus-level E2 (Di), proestrus level E2 (Pro), supraphysiological-level E2 (Sp) E2, or vehicle (Veh) in ovariectomized female rats.
| Gene symbol |
Gene Name | Function | Comparison | Ref |
|---|---|---|---|---|
| Dao | Diamine oxidase | Scavenging extracellular histamine after mediator release | Pro > Veh Di > Veh |
[99] |
| Bdkrb2 | Bradykinin 2 receptor | Proinflammatory; pronociceptive | Pro > Veh Sp > Veh |
[95] |
| Cacna1g | Calcium channel subunit alpha 1G | Voltage-gated T-type calcium channel (Cav3.1) | Sp > Veh Pro > Veh |
[16] |
| Cacna1h | Calcium channel subunit alpha 1H | Voltage-gated T-type calcium channel (Cav3.2) | Sp > Veh Sp > Di |
[16] |
| Prrt2 | Prolein rich transmembrane protein | Negative regulator of Nav1.2/1.6 Complex disorders with migraine; type 4 familial hemiplegic migraine; in PRF pain genes |
Sp > Veh Sp > Di |
[35; 59] |
| Grin3a | NMDA receptor subunit 3a | Ionotropic glutamate receptor; involved in pain inhibition | Di > Veh Pro > Veh |
[81] |
| Gabrd | GABA receptor delta | Target for analgesia | Di > Sp | [70] |
| Adcyap1r1 | Pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 receptor (PAC1) | Involved in migraine Regulated by stress and E2 |
Pro > Veh Di > Veh Di > Sp |
[56; 128] |
| Adcyap1 | Pituitary adenylate cyclase-activating polypeptide 1 (PACAP) | Involved in migraine Neuropathic pain onset |
Pro < Veh Sp < Veh |
[29; 128] |
| Vip | Vasoactive intestinal polypeptide | Same superfamily as PACAP (glucagon/secretin); neuropathic pain maintenance | Pro < Veh Sp < Veh Sp < Di |
[29] |
| Prl | prolactin | Female-specific hyperalgesia Modulated by E2 |
Sp > Veh | [87; 115] |
| Calca | Calcitonin gene-related peptide alpha | Involved in migraine; female-specific responses in dura mater; prolactin increases release in female dura | Di < Veh Sp < Veh |
[5; 6; 128] |
| Calcb | Calcitonin gene-related peptide beta | Involved in migraine | Di < Veh Sp < Veh |
[128] |
| Fkbp5 | FK506 binding proteins | Co-chaperone of Hsp90 of steroid receptor complex (stabilizing and shuttling) Linked to stress disorders |
Pro > Veh Di > Veh |
[112; 125] |
| Atp1a2 | Na+/K+ pump subunit alpha 2 | Involved in familial migraine; E2 rescues age-related drop in ATPase; in PRF pain genes | Pro > Veh Di > Veh Sp > Veh |
[57; 59] |
| Timp3 | Metalloproteinase inhibitor 3 | Protects against cartilage degradation | Pro > Veh Di > Veh |
[61] |
| Cnr1 | Cannabinoid receptor 1 | Peripheral cannabinoid receptor; peripheral analgesia | Pro > Veh Di > Veh |
[123] |
| Faah | Fatty acid amide hydrolase | Inhibition reduces pain, linked to migraine; reduced FAAH leads to increased cannabinoid and 5HT activity; mutation leads to pain insensitivity; in PRF pain genes | Di < Veh Di > Sp |
[39; 82] |
| Rgs4 | Regulator of G protein signaling (RGS) 4 | Regulatory molecule that acts as a GTPase activating protein (GAP). Can deactivate Gi and Gq. Maintain chronic pain. | Pro < Veh Di > Sp |
[8] |
| S1pr3 | Sphingosine-1-phosphate receptor 3 | Acute mechanonociception via KCNQ2/3 (Pro > Veh) | Pro < Veh Sp < Veh |
[43] |
| Cttn | Cortactin | Actin rearrangement, regulates vascular permeability, works with S1P | Pro < Veh Sp < Veh |
[36] |
| Ptgds | Prostaglandin D2 synthase (PGD2) | Proinflammatory, produced by neuron, glia, immune cells Sex-dependent nociceptive signaling |
Di > Veh Sp > Veh |
[47; 111] |
| Insr | Insulin receptor | Insulin deficiency contributes to diabetic neuropathy | Pro > Veh Sp > Veh |
[127] |
| Lama4 | Laminin subunit alpha-4 | Heat pain sensitivity | Pro < Veh Sp < Veh |
[119] |
| Tmem165 | Transmembrane protein 165 | Intracellular calcium transporter | Pro < Veh Di < Veh |
[16] |
| Camk2a | Calcium/calmodulin dependent protein kinase II alpha | Serine/threonine protein kinase; loss contributes to switch from mechanoreception to pain | Di > Veh Sp > Veh |
[124] |
| Cd4 | CD4 molecule | Membrane glycoprotein of immune cells (T cells, B cells, macrophages) | Di < Veh Sp < Veh |
[74] |
| Bahcc1 | BAH domain and coiled-coil containing 1 | Unknown relevance to nociception, but was reported as a SNP in only females with TMD | Sp > Veh Sp > Di |
[98] |
| Fabp7 | Fatty acid binding protein 7 | Increased in DRG with nerve injury; related to regeneration and PPAR signaling | Sp < Veh Sp < Di |
[7; 27] |
| Col9a1 | Collagen type IX alpha chain | Mutation leads to painful stiff joints | Sp < Veh Sp < Di |
[19] |
| Disc1 | Disrupted in Schizophrenia (DISC1) scaffold protein | Deletion reduces thermal pain sensitivity | Di > Sp | [114] |
| Anxa1 | Annexin A1 | Anti-inflammatory; antinociceptive | Di > Sp | [90] |
| Clock | Circadian regulator | Dimerizes with bmal; target to reduce OA pain | Di > Sp | [26] |
| Nav2 | Neuron navigator, unc-53 homolog | Mutants have impaired sensory function; diminished TG and DRG sensory neurons | Di > Sp | [76] |
| P2rx3 | Purinergic receptor P2X3 | Orofacial pain (TMD) and burning mouth syndrome E2 reduces spinal P2X3 E2 attenuates P2X3 in DRG |
Sp > Di | [11; 69; 94; 102] |
| Cckbr | Cholecystokinin B receptor | Anti-opioid activity by heterodimerizing with MOR; E2 increases in MCF-7 cells | Sp > Di | [103] |
| Htr3A | 5HT3A receptor | Ionotropic, pain | Sp > Di | [31] |
| Per1 | Period circadian clock 1 | Altered in chronic migraine in male mice; alters chemokine release and mechanical hypersensitivity | Sp > Veh | [49; 83; 100] |
| Ptprz1 | protein tyrosine phosphatase receptor type Z1 | Alters responses to thermal and tactile stimuli | Di > Sp | [58] |
Estrogen receptor mRNA and 5HT2A receptor mRNA are observed in the TRPV1 population of trigeminal sensory neurons in female rats
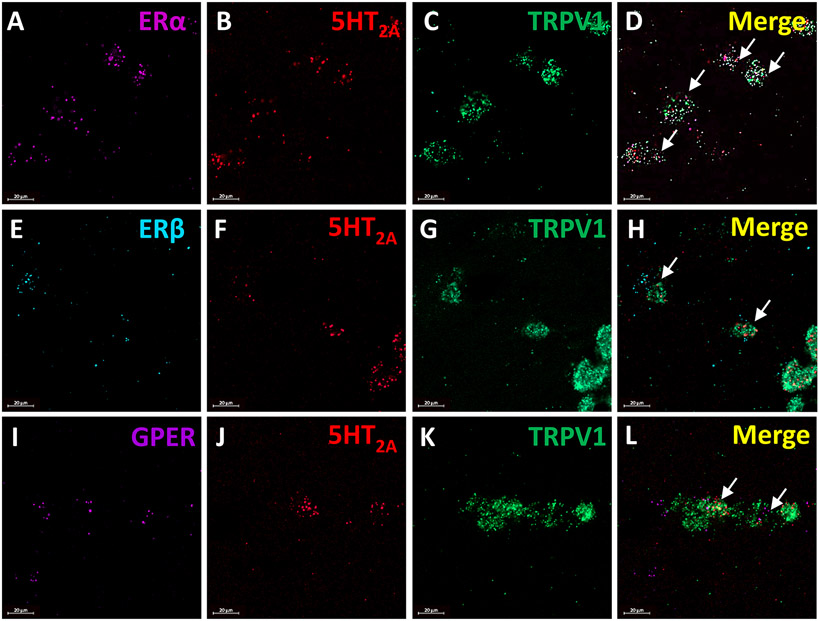
As our behavior data indicate that estrogen can modulate the effects of 5HT on orofacial pain behaviors, we next extracted the TGs of cycling female and male rats to determine whether estrogen receptors are localized to the 5HT-sensitive nociceptive (TRPV1+) population of sensory neurons. We report that ERα mRNA (Figure 4A), 5HT2A receptor mRNA (Figure 4B), and TRPV1 mRNA (Figure 4C) were expressed in the female rat trigeminal ganglia and were observed localized to the same small and medium diameter cells (Figure 4D). We also found ERß mRNA (Figure 4E), 5HT2A receptor mRNA (Figure 4F), and TRPV1 mRNA (Figure 4G) co-expressed by small and medium diameter cells (Figure 4H). GPER mRNA (Figure 4I), 5HT2A receptor mRNA (Figure 4J), and TRPV1 mRNA (Figure 4K) co-expressed by small and medium diameter cells (Figure 4L). Overall, in both males and females there was a subset of TRPV1 mRNA expressing cells that contained both 5HT2A receptor mRNA and estrogen receptors, notably ERα. In cells that express estrogen receptor, 5HT2A, and TRPV1 mRNA the approximate cell diameter was 19.3±2.2 μm in females. While males had less estrogen receptor mRNA expression in general, cells with co-expression were approximately 21.85±3.97 μm. Taken together, this data indicates that estrogen receptor mRNA is expressed in TRPV1 positive trigeminal ganglia neurons.
Figure 4. ERα, ERβ, and GPER mRNA along with 5HT2A mRNA coexpress with TRPV1 mRNA in the female rat trigeminal ganglia.
Panels A-D display representative images of the expression of ERα mRNA (violet; A) and 5HT2A mRNA (red; B) with TRPV1 ion channel mRNA (green; C) in the trigeminal ganglia (merge; D). Panels E-F display representative images of the expression of ERβ mRNA (blue; E) and 5HT2A mRNA (red; F) with TRPV1 ion channel mRNA (green; G) in the trigeminal ganglia (merge; H). Panels I-L depict representative images of the expression of GPER mRNA (purple; I) and 5HT2A mRNA (red; J) with TRPV1 ion channel mRNA (green; K) in the trigeminal ganglia (merge; L). Arrows indicate trigeminal ganglia cells co-expressing estrogen receptors and 5HT2A receptor mRNA with TRPV1 ion channel mRNA.
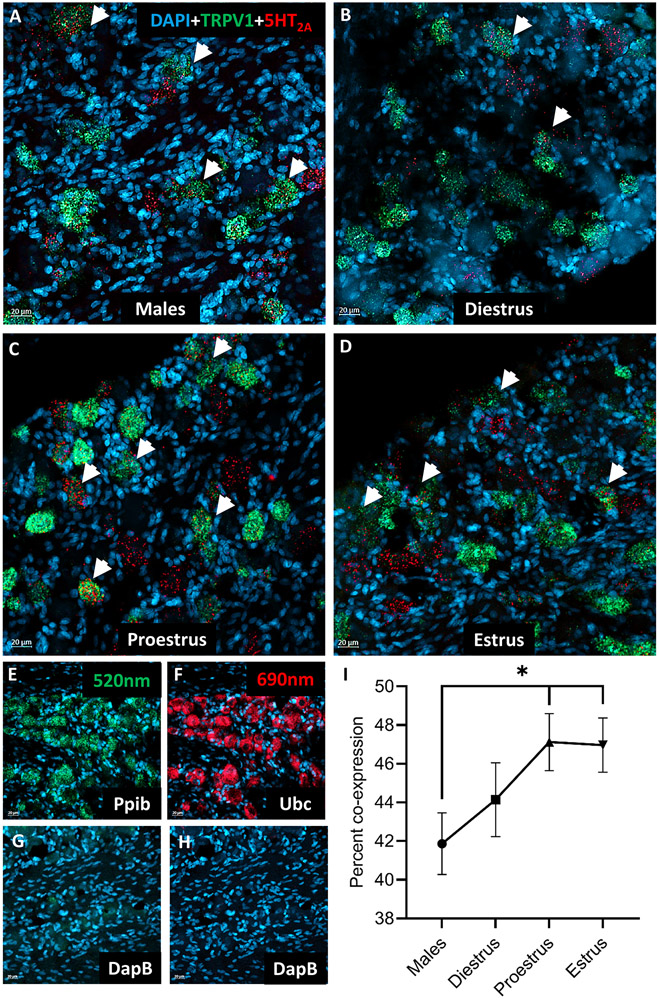
5HT2A receptor mRNA and TRPV1 mRNA coexpression is significantly higher in trigeminal sensory neurons of female rats during proestrus and estrus
Since our RNAseq data indicate that estrogen can alter trigeminal gene expression, we next quantified how many TRPV1 mRNA expressing trigeminal ganglia neurons also expressed 5HT2A mRNA and whether the amount of 5HT2A mRNA localized to the TRPV1 population displayed plasticity across the estrous cycle. TRPV1 mRNA (Figure 5A-D; green) and 5HT2A receptor mRNA (Figure 5A-D; red) were co-expressed in trigeminal ganglia cells (Figure 5A-D; arrowheads). Also shown are the positive controls for the probes in each channel (Figure 5E-F) and the negative controls (Figure 5G-H) in each channel. When quantified, we found that TGs that were extracted from females during estrus [p ≤ 0.05] or proestrus [p ≤ 0.05] contained significantly higher percent co-expression compared to males, while diestrus was comparable to males [p > 0.05]. In cells that express 5HT2A and TRPV1 mRNA the approximate cell diameter was 24.5±3.81 μm in females and 22.81±3.44 μm in males. Overall, during phases of hormonal fluctuation, co-expression of 5HT2A receptor mRNA and TRPV1 mRNA in trigeminal ganglia is significantly higher.
Figure 5. 5HT2A mRNA co-expression in the TRPV1 population of female trigeminal ganglia varies over the estrous cycle.
Representative images of the expression of TRPV1 ion channel mRNA (green) and 5HT2A receptor mRNA (red) with DAPI (blue) in the trigeminal ganglia of male (A) and cycling female rats across the stages of the estrous cycle [diestrus (B), proestrus (C), and estrus (D)]. Positive probe controls (E,F) and negative probe controls (G,H) for each channel are also presented. The percent co-expression of 5HT2A receptor mRNA and TRPV1 ion channel mRNA significantly varies across the stages of the estrous cycle and is higher than that observed in males (I). Arrowheads indicate trigeminal ganglia cells co-expressing TRPV1 ion channel mRNA and 5HT2A receptor mRNA. *Denotes a significant increase compared to males with significance in pairwise comparisons tested at p ≤ 0.05.
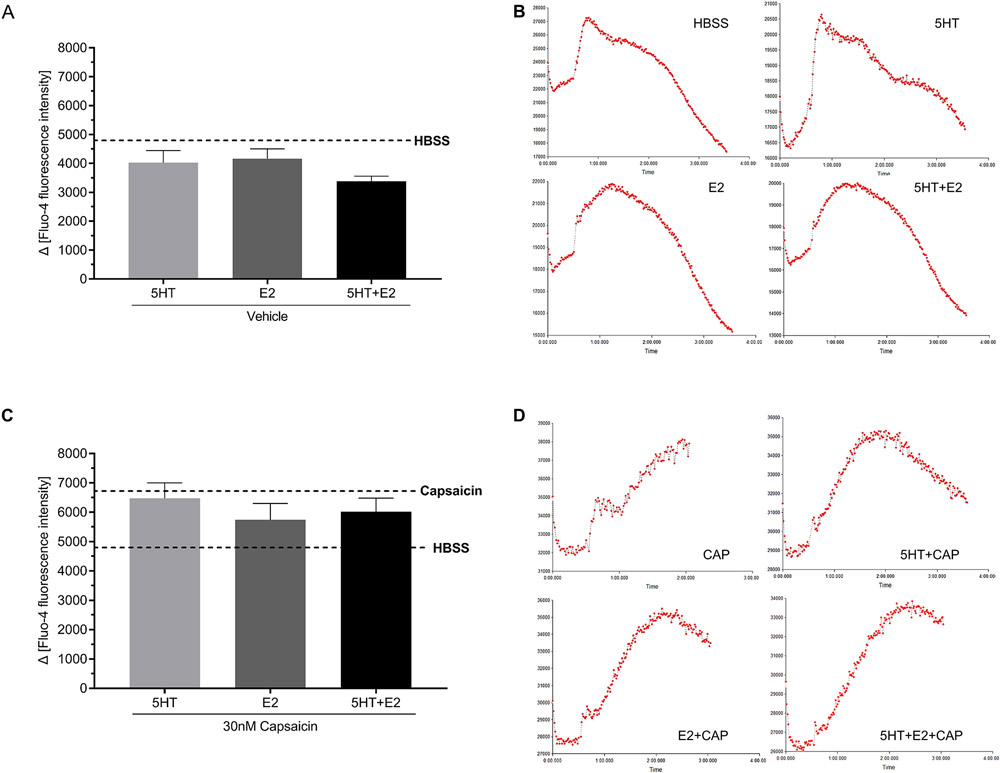
E2 and 5HT treatment does not enhance capsaicin-evoked calcium influx in female trigeminal ganglia neurons
As we have previously reported that E2 enhances serotonergic potentiation of capsaicin-evoked CGRP release [46] and 5HT enhances capsaicin-evoked calcium influx in male trigeminal ganglia neurons [56], we next tested whether 5HT can enhance capsaicin-evoked calcium influx in female trigeminal ganglia neurons and whether E2 further exacerbates calcium activity. Primary cultures of female trigeminal ganglia neurons were incubated in Fluo-4 and we observed that capsaicin evoked a significant and transient peak in calcium influx 0-120 seconds following stimulation as compared to HBSS only (Figure 6A; B; dotted lines) [p ≤ 0.05]. Neither 5HT (light grey bar) nor E2 (dark grey bar) alone or administered in combination (closed bar) altered calcium influx (Figure 6A) [p > 0.05]. A 3-minute 5HT, E2, or 5HT+E2 pretreatment prior to capsaicin stimulation did not alter the observed capsaicin-evoked calcium influx (Figure 6B) [p > 0.05]. Representative traces of calcium current per treatment are displayed in Figure 6C-H. Overall, we did not report any enhancement of calcium influx in female trigeminal ganglia following E2 and 5HT treatment.
Figure 6. Acute E2 does not alter serotonergic potentiation of capsaicin-evoked calcium influx.
Acute 5HT (light grey bars), E2 (dark grey bars), and the combination of 5HT+E2 (closed bars) induced similar calcium influx to HBSS (dotted line) (A). 30 nM capsaicin triggered significantly higher calcium influx as compared to HBSS (B), whereas acute pretreatment with 5HT (light grey bars), E2 (dark grey bars), and the combination of 5HT+E2 (closed bars) did not alter capsaicin-evoked calcium influx (B). Representative tracers are presented per treatment (D-H).
Membrane-bound estrogen receptors do not alter serotonergic potentiation of capsaicin-evoked CGRP release from trigeminal sensory neurons
As estrogen receptors are expressed on the 5HT-sensitive TRPV1 population of trigeminal sensory neurons and our previous study reported that E2 enhances serotonergic potentiation of capsaicin-evoked CGRP release from female trigeminal sensory neurons [55], our next series of experiments were designed to determine which E2 receptors are involved in the effects of E2 on 5HT-evoked pain. Pretreatment with 5HT (Figure 7A) or E2-BSA (Fig. 7B) alone did not significantly alter CGRP release comparable to vehicle [p > 0.05], while capsaicin did evoke significant CGRP release in these cells (Figure 7C) [p ≤ 0.05]. There was no effect of the membrane impermeable E2-BSA on 5HT and capsaicin-evoked CGRP release (Figure 7D) [p > 0.05]. Further, there was no effect of pretreatment with the GPER agonist G1 (Figure 8A) or the GPER agonist and classical estrogen receptor antagonist ICI 182,780 (Figure 8B) on 5HT- and capsaicin-evoked CGRP release [p > 0.05]. Thus, the drugs employed to target membrane estrogen receptors did not alter CGRP release.
Figure 7. E2-BSA does not increase serotonergic potentiation of capsaicin-evoked CGRP release.
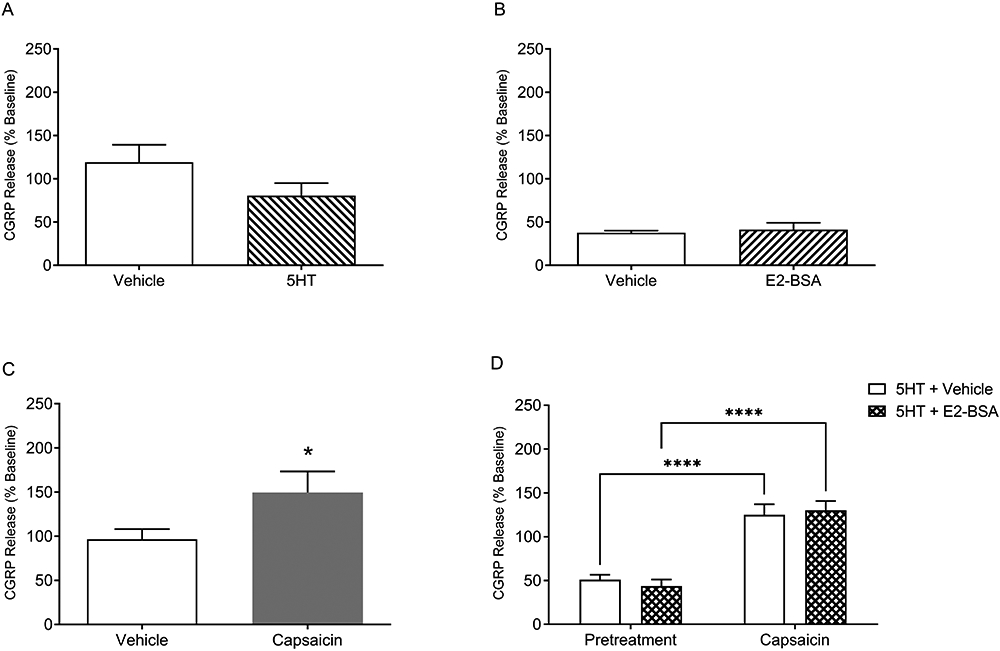
CGRP release was comparable to vehicle (open bar) with 5HT only (right diagonals) pretreatment (A) and E2-BSA only (left diagonals) pretreatment (B) as observed by percent change in baseline. Capsaicin (grey bar) treatment significantly increased CGRP release compared to vehicle as observed by percent change in baseline (C). E2-BSA+5HT (hatched bar) pretreatment did not significantly enhance capsaicin-evoked CGRP release as compared to the vehicle and 5HT only treatment (D). *Denotes a significant increase in CGRP release compared to vehicle with significance in pairwise comparisons tested at p ≤ 0.05.
Figure 8. Activating the membrane bound ER (GPER) does not increase serotonergic potentiation of capsaicin-evoked CGRP release.
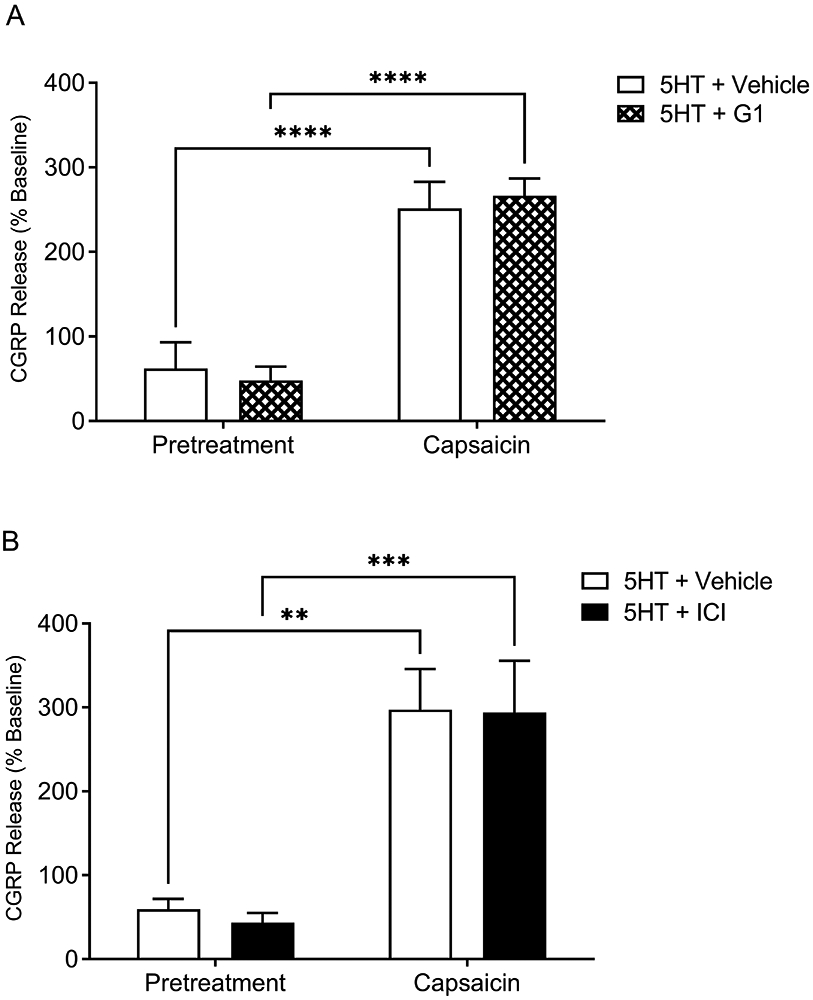
G1 (GPER agonist; hatched bar) pretreatment (A) and ICI 182,780 (GPER agonist / ER antagonist; closed bar) pretreatment (B) does not enhance capsaicin-evoked CGRP release compared to 5HT + Vehicle only (open bar) treatment. *Denotes a significant increase in CGRP release compared to vehicle with significance in pairwise comparisons tested at p ≤ 0.05.
Activation of ERα, but not ERβ, significantly enhances serotonergic potentiation of CGRP release from trigeminal sensory neurons
Since E2 can act on either membrane ERs (GPER) or the classic nuclear ERs (ERα and ERβ), we next tested if selectively targeting either ERα or ERβ would enhance CGRP release. Treatment with the ERα agonist PPT in the presence of 5HT significantly potentiated capsaicin-evoked CGRP release (closed bars) as compared to the 5HT and vehicle treatment (open bars) and compared to pretreatment prior to capsaicin stimulation (Figure 9A) [p ≤ 0.05]. While 5HT alone in an ovariectomized female rat does not significantly increase capsaicin-evoked CGRP release [54], selectively targeting the ERα receptor here resulted in significant potentiation of capsaicin-evoked CGRP release, which mirrored the effect of 17β-estradiol on serotonergic potentiation of capsaicin-evoked CGRP release [54]. Pretreatment with 15 nM ERβ agonist DPN did not potentiate capsaicin-evoked CGRP release (Figure 9B) [p > 0.05]. Of note, we initially applied 100 nM DPN which significantly enhanced capsaicin-evoked CGRP release similar to activating ERα (data not shown), however this dosage was reported to be nonspecific and have activity at ERα [78], so we switched to the 15 nM DPN concentration reported here. Pretreatment with the selective ERα antagonist MPP significantly attenuated 5HT potentiated CGRP release as compared to vehicle (Figure 9C) [p ≤ 0.05], while pretreatment with ERβ antagonist PHTPP did not attenuate 5HT potentiated CGRP release (Figure 9D) [p > 0.05]. In conclusion, the ERα receptor plays a key role in modulating the pronociceptive effects of 5HT on capsaicin-evoked CGRP release from female trigeminal neurons.
Figure 9. ERα plays a significant role in modulating serotonergic potentiation of capsaicin-evoked CGRP release.
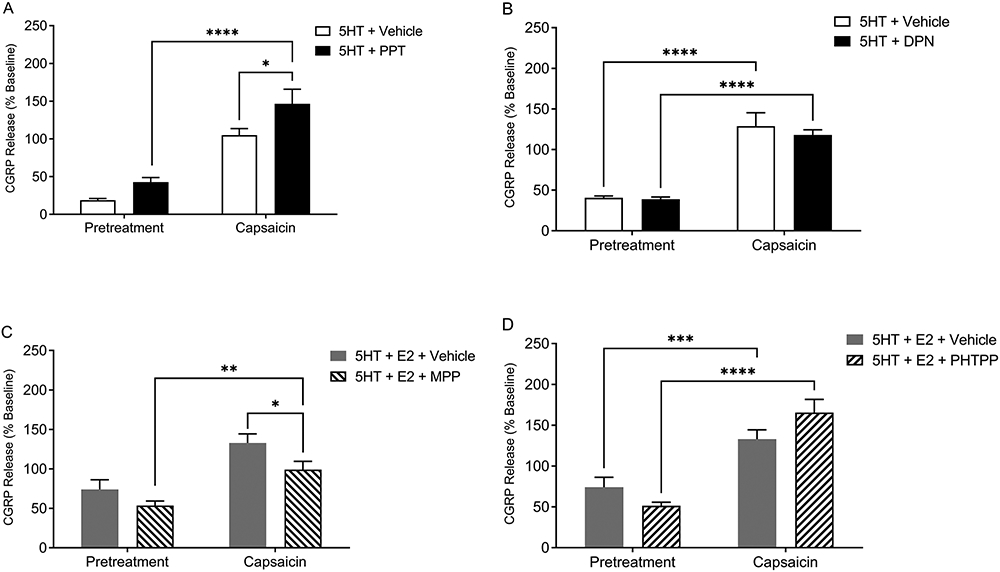
PPT (ERα agonist; closed bar) pretreatment (A), but not DPN (ERβ agonist; closed bar) pretreatment (B) significantly enhanced the serotonergic potentiation of capsaicin-evoked CGRP release as compared to 5HT+Vehicle control group (open bars). MPP+E2 (ERα antagonist; right diagonals) pretreatment (C), but not PHTPP+E2 (ERβ antagonist; left diagonals) pretreatment (D) significantly attenuated capsaicin-evoked CGRP release compared to 5HT + E2 (grey bar) treatment. *Denotes a significant increase in CGRP release compared to vehicle with significance in pairwise comparisons tested at p ≤ 0.05.
DISCUSSION
Pain conditions with higher prevalence in women largely include pain conditions that occur in the trigeminal sensory system, such as migraine, temporomandibular joint disorder (TMD) pain, orofacial varicella zoster-associated pain, and burning mouth syndrome. Women report increased severity and duration of trigeminal pain and often require higher doses of analgesics [28; 45; 84]. Underlying mechanisms have been postulated, including sex differences in pain modulation, contributions of sex chromosomes and genetics, modulation by gonadal and peptide hormones, and psychosocial factors [80; 84]. We elected to study the effects of E2 on peripheral 5HT neuromodulation of female trigeminal nociceptors, given that 5HT is implicated in several female-prevalent pain disorders within the trigeminal system [34; 37; 42]. We report that (1) a steady-state, but not acute, diestrus or proestrus level of E2 significantly attenuated 5HT-evoked orofacial nocifensive behaviors, (2) E2 modulated known pain genes in a time- and concentration-dependent manner, (3) nociceptive neurons expressing 5HT2A mRNA increased in numbers over the estrous cycle and co-expressed nuclear and membrane-bound ER mRNA, and (4) activation of ERα, but not ERβ or GPER, enhanced serotonergic potentiation of CGRP release which was reversed by ERα antagonism.
E2 can modulate the serotonergic system in several ways via estrogen response elements, protein-protein interactions, and via membrane estrogen-receptor mediated signaling cascades. E2 modulation of 5HT has been observed as (1) increased 5HT levels in the brain [41] and periphery with increasing E2 levels [1; 24], (2) increased expression of 5HT synthesizing enzymes [3; 13], (3) decreased expression of serotonin reuptake transporter (SERT) [14], (4) increased 5HT2A density and binding with hormone replacement therapy [97], and (5) higher capsaicin-evoked proinflammatory peptide and nocifensive behavior when E2 levels peak [68; 122]. ERα and ERβ are members of the nuclear receptor superfamily that bind estrogen response elements (EREs) in promoter regions [72]. ERα can synergistically act with transcription factor NF-κB to activate the 5HT1A promoter [120]. Membrane-bound GPERs activate downstream kinases, such as mitogen activated protein kinase (MAPK) and tyrosine kinases [2]. Studies have reported varying roles of ERs based on the pain model employed, agonist/antagonist used, and central vs. peripheral effects [20]. In a neuropathic pain model, ERβ selective agonists, but not ERα agonists, produced antiallodynic effects and alleviated hyperalgesia [71; 91]. Whereas in an inflammatory pain model, ERα increased the activation of NMDA receptors in the spinal cord [110] and activated the MAPK pathway [50], thus facilitating pronociceptive processing.
Evidently, the ER subtype activation, the resulting signaling cascade, and concentration of E2 play a vital role in determining the antinociceptive or pronociceptive effects of E2. Bradshaw et al., reported greater thermal hyperalgesia during proestrus following CFA hindpaw injection [17], whereas Clemente et al. reported that nociceptive responses to formalin injection were higher during diestrus [22]. We previously reported 5HT evoked pain behaviors in the hindpaw [52] and vibrissal pad [55] to be highest levels during proestrus and estrus. As we could not discern between the effects of high or low E2, in the present study we utilized ovariectomized female rats to control the E2 exposure time and concentration. When animals were injected with E2 for an acute period (1 hour), we observed 5HT-evoked nocifensive behaviors in agreement with our previous studies [55], however E2 did not significantly enhance serotonergic pain behaviors. When animals were exposed to steady-state levels of E2, diestrus-level E2 significantly attenuated 5HT-evoked nocifensive behavior. Proestrus-level E2 also attenuated 5HT-evoked nocifensive behaviors, to a lesser degree, but 5HT-evoked nocifensive behaviors were not attenuated by supraphysiological levels of E2. Together with our previous reports, these data indicate that (1) naturally fluctuating E2 levels enhance pain while steady low estradiol levels (such as during 2 days of diestrus) are protective against pain, (2) an acute injection of E2 is not sufficient to affect pain, and (3) supraphysiological levels contribute to or enhance pain.
The differential effects of E2 may be due to neural plasticity or differential gene expression. We found 5HT2A mRNA within the TRPV1 population of female trigeminal ganglia neurons increased as E2 levels increased over the estrous cycle. Though in situ hybridization data does not reflect the nerve innervation pattern in the vibrissal pad, it provides an anatomical substrate for the effects of E2 and 5HT within the trigeminal ganglia. Here, we focused our efforts on 5HT2A mRNA (although other 5HTRs may also be involved) as 5HT2A is a major excitatory G protein-coupled receptor previously reported in the TRPV1 population of trigeminal sensory neurons [67] that is capable of sensitizing TRPV1. In agreement, physiological levels of E2 are protective against 5HT-evoked pain, while increasing doses of E2 exacerbate 5HT-evoked pain. This is interesting as the literature has historically used supraphysiological E2 levels to test the effects of E2 on pain, while lower physiological levels may be antinociceptive.
With a steady-state supraphysiological dose of E2, we observed significantly higher bradykinin 2 receptor (Bdkrb2) and prolactin (Prl) expression; both are pronociceptive and proinflammatory. ERα activation enhances bradykinin signaling in female trigeminal sensory neurons [95] and prolactin is reported to play a role in female-specific hyperalgesia [87]. In support, various proteins involved in G protein signaling were upregulated in mice that received E2 replacement for 7 days [118]. Additionally, a prolein rich transmembrane protein (Prrt2) and a sodium potassium ATPase subunit Atp1a2, both linked to familial migraine [59], were significantly upregulated in response to supraphysiological E2 treatment. 5HT3 was upregulated with supraphysiological E2, which supports E2-5HT interaction in trigeminal pain disorders [13; 31]. It is important to note here that, while we observe 5HT3 receptor mRNA expression in female rodent trigeminal ganglia neurons [53; 67] , co-expression in the TRPV1 population is minimal and functional 5HT3 receptors have only been reported in medium-sized neurons [63; 88; 126] . This is supported by our recent study indicating that blocking the 5HT3 receptor in the vibrissal pad was not sufficient to reduce pain behaviors in female rats [53]. Several anti-inflammatory and analgesic genes [70; 81; 90] were upregulated in the steady-state diestrus-level E2 group, such as GABA receptor delta (Gabrd), NMDA receptor subunit 3a (Grin3a), and Annexin A1 (Anxa1), consistent with attenuation of 5HT-evoked pain behaviors. Of note, we also performed a more stringent analysis [46] using padj≤0.05 and four major DEGs remained: vasoactive intestinal peptide (VIP) and fatty acid binding protein 9 (Fabp7) were higher in the supraphysiological E2 treatment group compared to the diestrus-like group and a sodium/potassium ATPase pump subunit (ATP1A2) and a period circadian regulator (Per1) were higher in the supraphysiological E2 treatment group compared to the vehicle group. VIP is a major vasodilator and FABP7 disruption relays inflammation-associated analgesia [51]. Both ATP1A and Per have a reported role in migraine (Table 2). Overall, while both pro- and anti-inflammatory genes were differentially altered, we interpret these data as E2’s effects on inflammation depend on exposure and concentration which warrants our current research on the effects of E2 on inflammatory mediators involved in pain. Furthermore, these data provide the basis for future gene manipulation studies in mice to understand the role of specific pain genes on sex differences in trigeminal pain.
5HT enhances calcium influx in sensory neurons and we observed significant calcium influx in female trigeminal ganglia neurons treated with capsaicin. However, 5HT alone or in combination with E2 did not alter capsaicin-evoked calcium influx. Treatment was acute, however the modulatory effects of E2 and 5HT may require longer cell exposure, as supported by our behavior data, and may implicate differential membrane vs intracellular dynamics. We next measured peptidergic activity and looked for membrane-bound vs classical ER involvement, as our previous study observed that while 5HT alone did not significantly enhance capsaicin-evoked CGRP release in ovariectomized female rats, 5HT in the presence of E2 significantly potentiated capsaicin-evoked CGRP release [54]. The major difference between these techniques is that calcium influx is transient to a 3-minute E2+5HT exposure and the sensory cells are very sensitive to injector application, while the peptidergic assay is stable and has a 30-45-minute E2+5HT exposure. Indeed, we found that ERα agonism enhanced CGRP release, concurring with our previous study [46], and was reduced by ERα antagonism. Neither ERβ agonism or antagonism altered CGRP release. Pretreatment with membrane-impermeable E2 and GPER agonism did not mimic E2 enhancement of serotonergic potentiation of CGRP release, indicating potentiation is independent of membrane-bound ERs. Together these data indicate that ERα activation underlies the pronociceptive effects of E2 on trigeminal sensory neurons.
Overall, we present evidence that the E2 exposure and concentration determines whether E2 contributes to or attenuates 5HT-evoked orofacial pain and that activation of ERα, but not ERβ, is responsible for exacerbation of serotonergic potentiation of trigeminal pain signaling. Further mechanisms by which E2 modulates trigeminal sensory neurons include altering gene expression in the female trigeminal ganglia transcriptome and inducing neuroplasticity in 5HT2A receptors present on a subpopulation of nociceptive trigeminal ganglia neurons. These data contribute to the understanding of the complex and paradoxical role of E2 on orofacial pain and should be considered when treating trigeminal pain disorders in women following menarche, prior to menopause, and when electing estrogen replacement therapy.
Supplementary Material
Supplementary Figure 1. Biological processes of genes altered in the steady-state diestrus and proestrus E2 groups. Biological processes of differentially expressed genes (DEGs) in steady-state diestrus E2 group compared to vehicle (Veh; A) and in steady-state proestrus E2 group compared to vehicle (Veh; B). Only the genes that significantly changed (p<0.05) are shown.
Supplementary Figure 2. Biological processes of genes altered in the steady-state supraphysiological E2 group. Biological processes of differentially expressed genes (DEGs) in steady-state supraphysiological (Supraphy) E2 group compared to vehicle (Veh; A) and in steady-state supraphysiological E2 group compared to steady-state diestrus E2 (B). Only the genes that significantly changed (p<0.05) are shown.
ACKNOWLEDGMENTS
The authors would like to acknowledge Mr. Duane Baade for fabrication of the mirrored boxes for behavior testing, Rene Paulson, Ph.D. and DiAnna Hynds, Ph.D. for expert statistical consultation, and Stephanie Shiers, Ph.D. for expert guidance on RNAscope quantification. The authors would also like to acknowledge the technical assistance of Natalia Santos, Erica Rodriguez, Michael Paul Hunter, Soniya Sapkota, Anusha Adhikari, and Tia Tewari. The authors have no conflicts of interest to declare.
FUNDING
This research was supported by the National Institutes of Health NIDCR grant DE025970 awarded to DLA. This work was also funded, in part, by a Small Grant from the Texas Society for Microscopy awarded to SK, a Research Enhancement Program grant from TWU awarded to DLA, and a Center for Student Research grants from TWU awarded to SK.
Bibliography
- [1].Aloisi AM, Bonifazi M. Sex hormones, central nervous system and pain. Hormones and behavior 2006;50(1):1–7. [DOI] [PubMed] [Google Scholar]
- [2].Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, Katzenellenbogen J. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol Rev 2017;97(3):1045–1087. [DOI] [PubMed] [Google Scholar]
- [3].Asghari R, Lung MS, Pilowsky PM, Connor M. Sex differences in the expression of serotonin-synthesizing enzymes in mouse trigeminal ganglia. Neuroscience 2011;199:429–437. [DOI] [PubMed] [Google Scholar]
- [4].Averitt DL, Hornung RS, Murphy AZ. Role of Sex Hormones on Pain: Oxford University Press, 2019. [Google Scholar]
- [5].Avona A, Burgos-Vega C, Burton MD, Akopian AN, Price TJ, Dussor G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. The Journal of Neuroscience 2019;39(22):4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Avona A, Mason BN, Burgos-Vega C, Hovhannisyan AH, Belugin SN, Mecklenburg J, Goffin V, Wajahat N, Price TJ, Akopian AN, Dussor G. Meningeal CGRP-Prolactin Interaction Evokes Female-Specific Migraine Behavior. Ann Neurol 2021;89(6):1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Avraham O, Deng P-Y, Jones S, Kuruvilla R, Semenkovich CF, Klyachko VA, Cavalli V. Satellite glial cells promote regenerative growth in sensory neurons. Nature Communications 2020;11(1):4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Avrampou K, Pryce KD, Ramakrishnan A, Sakloth F, Gaspari S, Serafini RA, Mitsi V, Polizu C, Swartz C, Ligas B, Richards A, Shen L, Carr FB, Zachariou V. RGS4 Maintains Chronic Pain Symptoms in Rodent Models. J Neurosci 2019;39(42):8291–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res 2013;41(Database issue):D991–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 2005;146(4):1650–1673. [DOI] [PubMed] [Google Scholar]
- [11].Beneng K, Yilmaz Z, Yiangou Y, McParland H, Anand P, Renton T. Sensory purinergic receptor P2X3 is elevated in burning mouth syndrome. Int J Oral Maxillofac Surg 2010;39(8):815–819. [DOI] [PubMed] [Google Scholar]
- [12].Berkley KJ. Sex differences in pain. Behav Brain Sci 1997;20(3):371–380; discussion 435-513. [DOI] [PubMed] [Google Scholar]
- [13].Berman NEJ, Puri V, Chandrala S, Puri S, Macgregor R, Liverman CS, Klein RM. Serotonin in Trigeminal Ganglia of Female Rodents: Relevance to Menstrual Migraine. Headache: The Journal of Head and Face Pain 2006;46(8):1230–1245. [DOI] [PubMed] [Google Scholar]
- [14].Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biological Psychiatry 2000;47(6):562–576. [DOI] [PubMed] [Google Scholar]
- [15].Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol 2006;2(4):207–212. [DOI] [PubMed] [Google Scholar]
- [16].Bouron A Transcriptomic Profiling of Ca2+ Transport Systems During the Formation of the Cerebral Cortex in Mice. Cells 2020;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bradshaw H, Miller J, Ling Q, Malsnee K, Ruda MA. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain 2000;85(1-2):93–99. [DOI] [PubMed] [Google Scholar]
- [18].Calabrese D, Giatti S, Romano S, Porretta-Serapiglia C, Bianchi R, Milanese M, Bonanno G, Caruso D, Viviani B, Gardoni F. Diabetic neuropathic pain: a role for testosterone metabolites. J Endocrinol 2014;221(1):1–13. [DOI] [PubMed] [Google Scholar]
- [19].Carlsen S, Nandakumar KS, Holmdahl R. Type IX collagen deficiency enhances the binding of cartilage-specific antibodies and arthritis severity. Arthritis Research & Therapy 2006;8(4):R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen Q, Zhang W, Sadana N, Chen X. Estrogen receptors in pain modulation: cellular signaling. Biol Sex Differ 2021;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chhibber A, Woody SK, Karim Rumi MA, Soares MJ, Zhao L. Estrogen receptor β deficiency impairs BDNF–5-HT2A signaling in the hippocampus of female brain: A possible mechanism for menopausal depression. Psychoneuroendocrinology 2017;82:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Clemente JT, Parada CA, Veiga MC, Gear RW, Tambeli CH. Sexual dimorphism in the antinociception mediated by kappa opioid receptors in the rat temporomandibular joint. Neurosci Lett 2004;372(3):250–255. [DOI] [PubMed] [Google Scholar]
- [23].Craft RM. Modulation of pain by estrogens. Pain 2007;132 Suppl 1:S3–12. [DOI] [PubMed] [Google Scholar]
- [24].Csaba G, Kovács P, Pállinger É. Gender differences in the histamine and serotonin content of blood, peritoneal and thymic cells: a comparison with mast cells. Cell biology international 2003;27(4):387–389. [DOI] [PubMed] [Google Scholar]
- [25].Dao TT, LeResche L. Gender differences in pain. J Orofac Pain 2000;14(3):169–184; discussion 184-195. [PubMed] [Google Scholar]
- [26].Das V, Kc R, Li X, Varma D, Qiu S, Kroin JS, Forsyth CB, Keshavarzian A, van Wijnen AJ, Park TJ, Stein GS, I OS, Burris TP, Im HJ. Pharmacological targeting of the mammalian clock reveals a novel analgesic for osteoarthritis-induced pain. Gene 2018;655:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].De León M, Welcher AA, Nahin RH, Liu Y, Ruda MA, Shooter EM, Molina CA. Fatty acid binding protein is induced in neurons of the dorsal root ganglia after peripheral nerve injury. J Neurosci Res 1996;44(3):283–292. [DOI] [PubMed] [Google Scholar]
- [28].Delaruelle Z, Ivanova TA, Khan S, Negro A, Ornello R, Raffaelli B, Terrin A, Mitsikostas DD, Reuter U, European Headache Federation School of Advanced S. Male and female sex hormones in primary headaches. J Headache Pain 2018;19(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dickinson T, Fleetwood-Walker SM. VIP and PACAP: very important in pain? Trends in Pharmacological Sciences 1999;20(8):324–329. [DOI] [PubMed] [Google Scholar]
- [30].Diogenes A, Patwardhan AM, Jeske NA, Ruparel NB, Goffin V, Akopian AN, Hargreaves KM. Prolactin modulates TRPV1 in female rat trigeminal sensory neurons. J Neurosci 2006;26(31):8126–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Domocos D, Selescu T, Ceafalan LC, Iodi Carstens M, Carstens E, Babes A. Role of 5-HT1A and 5-HT3 receptors in serotonergic activation of sensory neurons in relation to itch and pain behavior in the rat. J Neurosci Res 2020;98(10):1999–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 2002;30(1):207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ernberg M, Hedenberg-Magnusson B, Kurita H, Kopp S. Effects of local serotonin administration on pain and microcirculation in the human masseter muscle. J Orofac Pain 2006;20(3):241–248. [PubMed] [Google Scholar]
- [34].Ernberg M, Lundeberg T, Kopp S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain 2000;84:339–346. [DOI] [PubMed] [Google Scholar]
- [35].Fruscione F, Valente P, Sterlini B, Romei A, Baldassari S, Fadda M, Prestigio C, Giansante G, Sartorelli J, Rossi P, Rubio A, Gambardella A, Nieus T, Broccoli V, Fassio A, Baldelli P, Corradi A, Zara F, Benfenati F. PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity. Brain 2018;141(4):1000–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].García Ponce A, Citalán Madrid AF, Vargas Robles H, Chánez Paredes S, Nava P, Betanzos A, Zarbock A, Rottner K, Vestweber D, Schnoor M. Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility. Scientific Reports 2016;6(1):29003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev 2017;97(2):553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, Nueda MJ, Robles M, Talón M, Dopazo J, Conesa A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Research 2008;36(10):3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Greco R, Demartini C, Zanaboni AM, Tumelero E, Reggiani A, Misto A, Piomelli D, Tassorelli C. FAAH inhibition as a preventive treatment for migraine: A pre-clinical study. Neurobiol Dis 2020;134:104624. [DOI] [PubMed] [Google Scholar]
- [40].Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ, Consensus Working Group of the Sex G, Pain SIGotI. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 2007;132 Suppl 1:S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache 2011;51(6):905–922. [DOI] [PubMed] [Google Scholar]
- [42].Gupta S, Nahas SJ, Peterlin BL. Chemical mediators of migraine: preclinical and clinical observations. Headache 2011;51(6):1029–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hill RZ, Hoffman BU, Morita T, Campos SM, Lumpkin EA, Brem RB, Bautista DM. The signaling lipid sphingosine 1-phosphate regulates mechanical pain. Elife 2018;7:e33285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hornung R, Benton W, Tongkhuya S, Uphouse L, Kramer P, Averitt D. Progesterone and Allopregnanolone Rapidly Attenuate Estrogen-Associated Mechanical Allodynia in Rats with Persistent Temporomandibular Joint Inflammation. Frontiers in Integrative Neuroscience 2020;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Isacson D, Bingefors K. Epidemiology of analgesic use: a gender perspective. Eur J Anaesthesiol Suppl 2002;26:5–15. [DOI] [PubMed] [Google Scholar]
- [46].Jafari M, Ansari-Pour N. Why, When and How to Adjust Your P Values? Cell J 2019;20(4):604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jang Y, Kim M, Hwang SW. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. Journal of Neuroinflammation 2020;17(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jansen-Olesen I, Christensen SLT, Olesen J. Migraine and Other Headaches. In Vivo Models for Drug Discovery 2014:231–260. [Google Scholar]
- [49].Jeong H, Moye LS, Southey BR, Hernandez AG, Dripps I, Romanova EV, Rubakhin SS, Sweedler JV, Pradhan AA, Rodriguez-Zas SL. Gene Network Dysregulation in the Trigeminal Ganglia and Nucleus Accumbens of a Model of Chronic Migraine-Associated Hyperalgesia. Frontiers in Systems Neuroscience 2018;12(63). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. PAIN 2011;152(5):1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaczocha M, Glaser ST, Maher T, Clavin B, Hamilton J, O'Rourke J, Rebecchi M, Puopolo M, Owada Y, Thanos PK. Fatty acid binding protein deletion suppresses inflammatory pain through endocannabinoid/N-acylethanolamine-dependent mechanisms. Molecular pain 2015;11:52–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kaur S, Benton WL, Tongkhuya SA, Lopez CMC, Uphouse L, Averitt DL. Sex Differences and Estrous Cycle Effects of Peripheral Serotonin-Evoked Rodent Pain Behaviors. Neuroscience 2018;384:87–100. [DOI] [PubMed] [Google Scholar]
- [53].Kaur S, Lopez-Ramirez A, Hickman TM, Hunter MP, Averitt DL. Expression of Serotonin Receptor Subtype 3A (5HT 3A ) on Rat Trigeminal Sensory Neurons. Texas Journal of Microscopy 2021;52(1). [PMC free article] [PubMed] [Google Scholar]
- [54].Kaur S, McDonald H, Tongkhuya S, Lopez CM, Ananth S, Hickman TM, Averitt DL. Estrogen exacerbates the nociceptive effects of peripheral serotonin on rat trigeminal sensory neurons. Neurobiology of Pain 2021:100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kaur S, McDonald H, Tongkhuya S, Lopez CM, Ananth S, Hickman TM, Averitt DL. Estrogen Exacerbates the Nociceptive Effects of Peripheral Serotonin on Rat Trigeminal Sensory Neurons. Neurobiology of Pain 2021b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].King SB, Toufexis DJ, Hammack SE. Pituitary adenylate cyclase activating polypeptide (PACAP), stress, and sex hormones. Stress 2017;20(5):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kumar P, Kale RK, Baquer NZ. Estradiol Modulates Membrane-Linked ATPases, Antioxidant Enzymes, Membrane Fluidity, Lipid Peroxidation, and Lipofuscin in Aged Rat Liver. Journal of Aging Research 2011;2011:580245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lafont D, Adage T, Gréco B, Zaratin P. A novel role for receptor like protein tyrosine phosphatase zeta in modulation of sensorimotor responses to noxious stimuli: Evidences from knockout mice studies. Behav Brain Res 2009;201(1):29–40. [DOI] [PubMed] [Google Scholar]
- [59].LaPaglia DM, Sapio MR, Burbelo PD, Thierry-Mieg J, Thierry-Mieg D, Raithel SJ, Ramsden CE, Iadarola MJ, Mannes AJ. RNA-Seq investigations of human post-mortem trigeminal ganglia. Cephalalgia : an international journal of headache 2018;38(5):912–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Li P, Xu Y, Gan Y, Wang L, Ouyang B, Zhang C, Luo L, Zhao C, Zhou Q. Estrogen Enhances Matrix Synthesis in Nucleus Pulposus Cell through the Estrogen Receptor β-p38 MAPK Pathway. Cellular Physiology and Biochemistry 2016;39(6):2216–2226. [DOI] [PubMed] [Google Scholar]
- [61].Li W, Wu M, Jiang S, Ding W, Luo Q, Shi J. Expression of ADAMTs-5 and TIMP-3 in the condylar cartilage of rats induced by experimentally created osteoarthritis. Arch Oral Biol 2014;59(5):524–529. [DOI] [PubMed] [Google Scholar]
- [62].Lin AH, Li RW, Ho EY, Leung GP, Leung SW, Vanhoutte PM, Man RY. Differential ligand binding affinities of human estrogen receptor-α isoforms. PLoS One 2013;8(4):e63199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lindquist KA, Belugin S, Hovhannisyan AH, Corey TM, Salmon A, Akopian AN. Identification of Trigeminal Sensory Neuronal Types Innervating Masseter Muscle. eNeuro 2021;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Loyd DR, Henry MA, Hargreaves KM. Serotonergic neuromodulation of peripheral nociceptors. Semin Cell Dev Biol 2013;24(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Loyd DR, Sun XX, Locke EE, Salas MM, Hargreaves KM. Sex differences in serotonin enhancement of capsaicin-evoked calcitonin gene-related peptide release from human dental pulp. Pain 2012;153(10):2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Loyd DR, Wang X, Murphy AZ. Sex Differences in μ-Opioid Receptor Expression in the Rat Midbrain Periaqueductal Gray Are Essential for Eliciting Sex Differences in Morphine Analgesia. The Journal of Neuroscience 2008;28(52):14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Loyd DR, Weiss G, Henry MA, Hargreaves KM. Serotonin increases the functional activity of capsaicin-sensitive rat trigeminal nociceptors via peripheral serotonin receptors. Pain 2011;152(10):2267–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lu Y-C, Chen C-W, Wang S-Y, Wu F-S. 17β-Estradiol mediates the sex difference in capsaicin-induced nociception in rats. Journal of Pharmacology and Experimental Therapeutics 2009;331(3):1104–1110. [DOI] [PubMed] [Google Scholar]
- [69].Lu Y, Jiang Q, Yu L, Lu ZY, Meng SP, Su D, Burnstock G, Ma B. 17β-estradiol rapidly attenuates P2X3 receptor-mediated peripheral pain signal transduction via ERα and GPR30. Endocrinology 2013;154(7):2421–2433. [DOI] [PubMed] [Google Scholar]
- [70].Luo Y, Kusay AS, Jiang T, Chebib M, Balle T. Delta-containing GABAA receptors in pain management: Promising targets for novel analgesics. Neuropharmacology 2021;195:108675. [DOI] [PubMed] [Google Scholar]
- [71].Ma J-N, McFarland K, Olsson R, Burstein ES. Estrogen Receptor Beta Selective Agonists as Agents to Treat Chemotherapeutic-Induced Neuropathic Pain. ACS Chem Neurosci 2016;7(9):1180–1187. [DOI] [PubMed] [Google Scholar]
- [72].Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell 1995;83(6):835–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mannino CA, South SM, Inturrisi CE, Quinones-Jenab V. Pharmacokinetics and effects of 17beta-estradiol and progesterone implants in ovariectomized rats. J Pain 2005;6(12):809–816. [DOI] [PubMed] [Google Scholar]
- [74].Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. Journal of Biomedicine and Biotechnology 2011;2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp 2012(67):e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].McNeill EM, Roos KP, Moechars D, Clagett-Dame M. Nav2 is necessary for cranial nerve development and blood pressure regulation. Neural Development 2010;5(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Messlinger K, Russo AF. Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 2018;39(13):1661–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 2001;44(24):4230–4251. [DOI] [PubMed] [Google Scholar]
- [79].Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci 2012;13(12):859–866. [DOI] [PubMed] [Google Scholar]
- [80].Mogil JS, Bailey AL. Sex and gender differences in pain and analgesia. Prog Brain Res 2010;186:141–157. [DOI] [PubMed] [Google Scholar]
- [81].Mohamad O, Song M, Wei L, Yu SP. Regulatory roles of the NMDA receptor GluN3A subunit in locomotion, pain perception and cognitive functions in adult mice. J Physiol 2013;591(1):149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Moreira FA. Serotonin, the Prefrontal Cortex, and the Antidepressant-Like Effect of Cannabinoids. The Journal of Neuroscience 2007;27(49):13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Morioka N, Saeki M, Sugimoto T, Higuchi T, Zhang FF, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. Downregulation of the spinal dorsal horn clock gene Per1 expression leads to mechanical hypersensitivity via c-jun N-terminal kinase and CCL2 production in mice. Mol Cell Neurosci 2016;72:72–83. [DOI] [PubMed] [Google Scholar]
- [84].Nasser SA, Afify EA. Sex differences in pain and opioid mediated antinociception: Modulatory role of gonadal hormones. Life Sciences 2019;237:116926. [DOI] [PubMed] [Google Scholar]
- [85].Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol 2006;576(Pt 3):809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Otto C, Kantner I, Nubbemeyer R, Schkoldow J, Fuchs I, Krahl E, Vonk R, Schüler C, Fritzemeier K-H, Erben RG. Estradiol Release Kinetics Determine Tissue Response in Ovariectomized Rats. Endocrinology 2012;153(4):1725–1733. [DOI] [PubMed] [Google Scholar]
- [87].Patil M, Belugin S, Mecklenburg J, Wangzhou A, Paige C, Barba-Escobedo PA, Boyd JT, Goffin V, Grattan D, Boehm U, Dussor G, Price TJ, Akopian AN. Prolactin Regulates Pain Responses via a Female-Selective Nociceptor-Specific Mechanism. iScience 2019;20:449–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Patil MJ, Hovhannisyan AH, Akopian AN. Characteristics of sensory neuronal groups in CGRP-cre-ER reporter mice: Comparison to Nav1.8-cre, TRPV1-cre and TRPV1-GFP mouse lines. PLoS One 2018;13(6):e0198601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-Induced Functional Competence and Trafficking of the δ-Opioid Receptor in Trigeminal Nociceptors. The Journal of Neuroscience 2005;25(39):8825–8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Pei L, Zhang J, Zhao F, Su T, Wei H, Tian J, Li M, Shi J. Annexin 1 exerts anti-nociceptive effects after peripheral inflammatory pain through formyl-peptide-receptor-like 1 in rat dorsal root ganglion. British Journal of Anaesthesia 2011;107(6):948–958. [DOI] [PubMed] [Google Scholar]
- [91].Piu F, Cheevers C, Hyldtoft L, Gardell LR, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Schiffer HH, Donello JE, Olsson R, Gil DW, Brann MR. Broad modulation of neuropathic pain states by a selective estrogen receptor beta agonist. European Journal of Pharmacology 2008;590(1):423–429. [DOI] [PubMed] [Google Scholar]
- [92].Pokhilko A, Nash A, Cader MZ. Common transcriptional signatures of neuropathic pain. PAIN 2020;161(7):1542–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005;307(5715):1625–1630. [DOI] [PubMed] [Google Scholar]
- [94].Rosyidi RM, Priyanto B, Wardhana DPW, Prihastomo KT, Hikmi S, Turchan A, Rozikin. P2X3 receptor expression in dorsal horn of spinal cord and pain threshold after estrogen therapy for prevention therapy in neuropathic pain. Annals of Medicine and Surgery 2020;60:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rowan MP, Berg KA, Milam SB, Jeske NA, Roberts JL, Hargreaves KM, Clarke WP. 17β-Estradiol Rapidly Enhances Bradykinin Signaling in Primary Sensory Neurons In Vitro and In Vivo. Journal of Pharmacology and Experimental Therapeutics 2010;335(1):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Rowan MP, Berg KA, Roberts JL, Hargreaves KM, Clarke WP. Activation of estrogen receptor alpha enhances bradykinin signaling in peripheral sensory neurons of female rats. J Pharmacol Exp Ther 2014;349(3):526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Rybaczyk LA, Bashaw MJ, Pathak DR, Moody SM, Gilders RM, Holzschu DL. An overlooked connection: serotonergic mediation of estrogen-related physiology and pathology. BMC women's health 2005;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sanders AE, Jain D, Sofer T, Kerr KF, Laurie CC, Shaffer JR, Marazita ML, Kaste LM, Slade GD, Fillingim RB, Ohrbach R, Maixner W, Kocher T, Bernhardt O, Teumer A, Schwahn C, Sipila K, Lahdesmaki R, Mannikko M, Pesonen P, Jarvelin M, Rizzatti-Barbosa CM, Meloto CB, Ribeiro-Dasilva M, Diatchenko L, Serrano P, Smith SB. GWAS Identifies New Loci for Painful Temporomandibular Disorder: Hispanic Community Health Study/Study of Latinos. J Dent Res 2017;96(3):277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Schwelberger H Histamine: Biology and Medical Aspects, edited by Falus A: Budapest: SpringMed Publishing, 2004. [Google Scholar]
- [100].Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res 2018;96(6):1002–1020. [DOI] [PubMed] [Google Scholar]
- [101].Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 2008;139(3):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Shinoda M, Ozaki N, Asai H, Nagamine K, Sugiura Y. Changes in P2X3 receptor expression in the trigeminal ganglion following monoarthritis of the temporomandibular joint in rats. Pain 2005;116(1):42–51. [DOI] [PubMed] [Google Scholar]
- [103].Singleton DW, Feng Y, Chen Y, Busch SJ, Lee AV, Puga A, Khan SA. Bisphenol-A and estradiol exert novel gene regulation in human MCF-7 derived breast cancer cells. Molecular and Cellular Endocrinology 2004;221(1):47–55. [DOI] [PubMed] [Google Scholar]
- [104].Spradley JM, Davoodi A, Carstens MI, Carstens E. Effects of acute stressors on itch- and pain-related behaviors in rats. Pain 2012;153(9):1890–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ström JO, Theodorsson E, Theodorsson A. Order of magnitude differences between methods for maintaining physiological 17β-oestradiol concentrations in ovariectomized rats. Scandinavian Journal of Clinical and Laboratory Investigation 2008;68(8):814–822. [DOI] [PubMed] [Google Scholar]
- [106].Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci 2004;24(43):9521–9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists Selective for Estrogen Receptor α. Endocrinology 2002;143(3):941–947. [DOI] [PubMed] [Google Scholar]
- [108].Sung D, Dong X, Ernberg M, Kumar U, Cairns BE. Serotonin (5-HT) excites rat masticatory muscle afferent fibers through activation of peripheral 5-HT3 receptors. Pain 2008;134(1-2):41–50. [DOI] [PubMed] [Google Scholar]
- [109].Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience 1992;48(2):485–490. [DOI] [PubMed] [Google Scholar]
- [110].Tang B, Ji Y, Traub RJ. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain 2008;137(3):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tavares-Ferreira D, Ray PR, Sankaranarayanan I, Mejia GL, Wangzhou A, Shiers S, Uttarkar R, Megat S, Barragan-Iglesias P, Dussor G, Akopian AN, Price TJ. Sex Differences in Nociceptor Translatomes Contribute to Divergent Prostaglandin Signaling in Male and Female Mice. Biological Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tong M, Jiang Y. FK506-Binding Proteins and Their Diverse Functions. Curr Mol Pharmacol 2015;9(1):48–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Vasiadi M, Kempuraj D, Boucher W, Kalogeromitros D, Theoharides TC. Progesterone inhibits mast cell secretion. Int J Immunopathol Pharmacol 2006;19(4):787–794. [DOI] [PubMed] [Google Scholar]
- [114].Walsh J, Tighe O, Lai D, Harvey R, Karayiorgou M, Gogos J, Waddington J, O'Tuathaigh C. Disruption of thermal nociceptive behaviour in mice mutant for the schizophrenia-associated genes NRG1, COMT and DISC1. Brain research 2010;1348:114–119. [DOI] [PubMed] [Google Scholar]
- [115].Watters JJ, Chun TY, Kim YN, Bertics PJ, Gorski J. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol Endocrinol 2000;14(11):1872–1881. [DOI] [PubMed] [Google Scholar]
- [116].Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-β agonist diarylpropionitrile: biological activities of R-and S-enantiomers on behavior and hormonal response to stress. Endocrinology 2009;150(4):1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and Behavior 2004;45(3):201–208. [DOI] [PubMed] [Google Scholar]
- [118].Wiesenfeld-Hallin Z, Xu XJ, Langel U, Bedecs K, Hökfelt T, Bartfai T. Galanin-mediated control of pain: enhanced role after nerve injury. Proceedings of the National Academy of Sciences of the United States of America 1992;89(8):3334–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Williams FMK, Scollen S, Cao D, Memari Y, Hyde CL, Zhang B, Sidders B, Ziemek D, Shi Y, Harris J, Harrow I, Dougherty B, Malarstig A, McEwen R, Stephens JC, Patel K, Menni C, Shin S-Y, Hodgkiss D, Surdulescu G, He W, Jin X, McMahon SB, Soranzo N, John S, Wang J, Spector TD. Genes contributing to pain sensitivity in the normal population: an exome sequencing study. PLoS Genet 2012;8(12):e1003095–e1003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wissink S, van der Burg B, Katzenellenbogen BS, van der Saag PT. Synergistic Activation of the Serotonin-1A Receptor by Nuclear Factor-κB and Estrogen. Molecular Endocrinology 2001;15(4):543–552. [DOI] [PubMed] [Google Scholar]
- [121].Wu P, Arris D, Grayson M, Hung C-N, Ruparel S. Characterization of sensory neuronal subtypes innervating mouse tongue. PLOS ONE 2018;13(11):e0207069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yamagata K, Sugimura M, Yoshida M, Sekine S, Kawano A, Oyamaguchi A, Maegawa H, Niwa H. Estrogens Exacerbate Nociceptive Pain via Up-Regulation of TRPV1 and ANO1 in Trigeminal Primary Neurons of Female Rats. Endocrinology 2016;157(11):4309–4317. [DOI] [PubMed] [Google Scholar]
- [123].Yamamoto T, Mulpuri Y, Izraylev M, Li Q, Simonian M, Kramme C, Schmidt BL, Seltzman HH, Spigelman I. Selective targeting of peripheral cannabinoid receptors prevents behavioral symptoms and sensitization of trigeminal neurons in mouse models of migraine and medication overuse headache. Pain 2021;162(8):2246–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yu H, Pan B, Weyer A, Wu H-E, Meng J, Fischer G, Vilceanu D, Light AR, Stucky C, Rice FL, Hudmon A, Hogan Q. CaMKII Controls Whether Touch Is Painful. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015;35(42):14086–14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene–Stress–Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology 2016;41(1):261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 2002;22(3):1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhai X, Sun C, Rong P, Li S, McCabe MF, Wang X, Mao J, Wang S. A Correlative Relationship Between Chronic Pain and Insulin Resistance in Zucker Fatty Rats: Role of Downregulation of Insulin Receptors. The Journal of Pain 2016;17(4):404–413. [DOI] [PubMed] [Google Scholar]
- [128].Zhang Q, Han X, Wu H, Zhang M, Hu G, Dong Z, Yu S. Dynamic changes in CGRP, PACAP, and PACAP receptors in the trigeminovascular system of a novel repetitive electrical stimulation rat model: Relevant to migraine. Mol Pain 2019;15:1744806918820452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Biological processes of genes altered in the steady-state diestrus and proestrus E2 groups. Biological processes of differentially expressed genes (DEGs) in steady-state diestrus E2 group compared to vehicle (Veh; A) and in steady-state proestrus E2 group compared to vehicle (Veh; B). Only the genes that significantly changed (p<0.05) are shown.
Supplementary Figure 2. Biological processes of genes altered in the steady-state supraphysiological E2 group. Biological processes of differentially expressed genes (DEGs) in steady-state supraphysiological (Supraphy) E2 group compared to vehicle (Veh; A) and in steady-state supraphysiological E2 group compared to steady-state diestrus E2 (B). Only the genes that significantly changed (p<0.05) are shown.