Abstract
Biomolecular condensates are membrane-less organelles enriched in specific proteins and nucleic acids, compartmentalized to perform biochemical functions. Such condensates are formed by the process of phase separation enabled by protein domains that allow for multivalent interactions. Chromosomal translocation-derived in-frame gene-fusions often result in proteins with non-native domain combinations and re-wire protein-protein interaction networks. Several recent studies have shown that for a subset of the fusion proteins, pathogenesis can be driven by the fusion protein’s ability to undergo phase transitions at non-physiologic cellular locations to form ectopic condensates. Here, we highlight how such ectopic phase transitions can alter biological processes and posit that dysfunction via protein phase separation at non-physiologic locations represents a generic route to oncogenic transformation.
Keywords: Liquid-liquid phase separation, oncofusion, biomolecular condensates, oncogenesis, prion-like domain
Oncofusion proteins and biomolecular condensation
Gene fusions represent one of the major categories of cancer driver mutations. While a subset of gene fusions joins non-coding regulatory regions from one of the fusion partners to the coding region of the other fusion partner (resulting in altered protein level), often though, in-frame fusions of coding regions are identified as the driver genomic aberration that is responsible for many cancers [1, 2]. How these fusion proteins elicit pathogenesis has been a question of immense interest, both in the context of understanding disease mechanisms and therapeutic interventions. Several molecular mechanisms for fusion protein-mediated pathogenesis have been identified over the years; examples include – the loss of functional domains (e.g., loss of SET domain in fused MLL genes abolish the proteins histone methyltransferase activity [3, 4]); recruitment of protein complexes at non-native locations (e.g., SSXRD domain fusion to SS18 in SS18-SSX fusions result in fusion protein-driven recruitment of chromatin remodelers to non-native genomic locations [5, 6]); fusion protein activity at ectopic locations (e.g., MAN2A1-FER fusion directs the fused protein to the Golgi apparatus resulting in FER tyrosine kinase activity at ectopic sub-cellular locations [7]). A number of recent studies, however, suggest a new paradigm-shifting molecular mechanism for fusion protein-driven oncogenesis where fusion proteins elicit their pathogenic effects via ectopic protein condensates formed through aberrant phase separation (PS) [8–15]. In this review, we summarize these results and discuss why and how ectopic phase separation is likely to be a generalizable biophysical framework for many in-frame fusion-driven cancer pathologies.
The basics of protein liquid-liquid phase separation (LLPS; biomolecular condensation)
Spatiotemporal separation of chemical processes is one of the salient features of living systems. It had been assumed that cells achieve such separation primarily by utilizing different membrane-bound sub-cellular organelles [16]. However, recent advances in cell biology reveal the crucial roles of many membrane-less organelles (MLOs) that form via phase separation [17–19]. As their name implies, these MLOs lack lipidic membranes that are characteristic of other sub-cellular organelles. Despite being membrane-less, these MLOs share certain features with their membrane-bound counterparts – (i) they can selectively enrich certain biomolecules and exclude others, (ii) they act as organizing sites of biological processes, and (iii) they can function as centers of biochemical reactions. We will not provide in-depth discussions on different MLOs and their functional roles here, instead, we refer our readers to excellent reviews that are already available elsewhere [20–23].
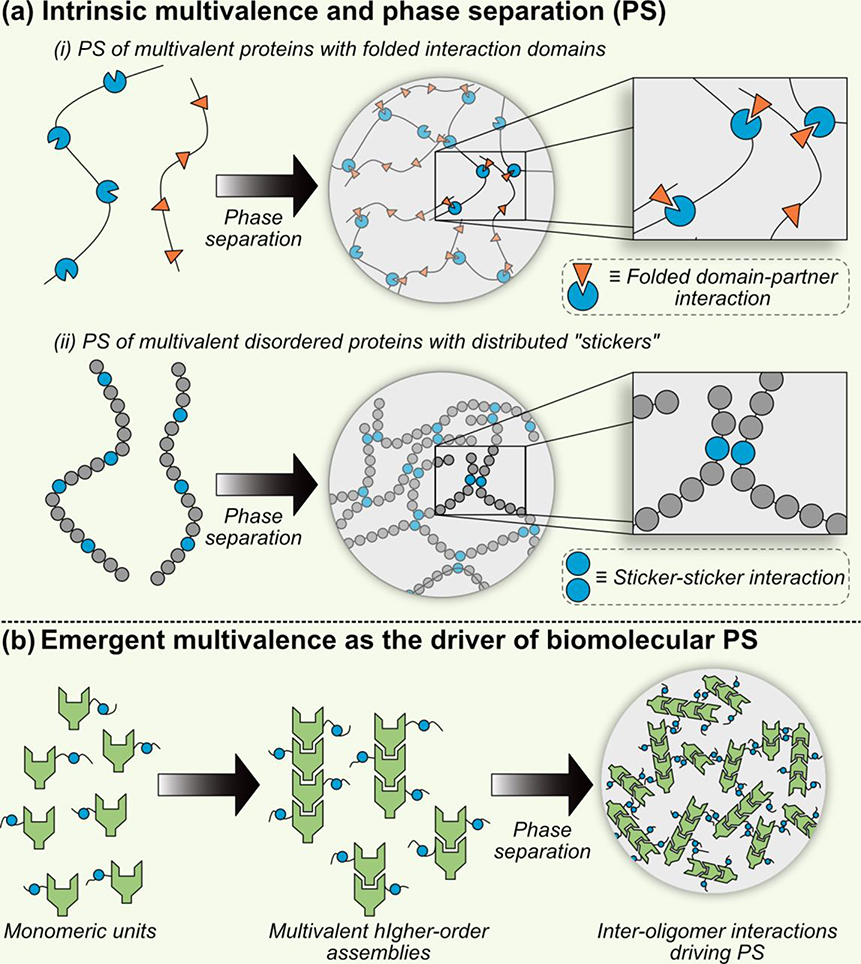
Phase separation is a physical process where polymers separate into phases of unequal densities (or concentrations) of the polymer under consideration. In cases where the separated phases behave as classical liquids, the process is termed as liquid-liquid phase separation. However, for better clarity and generality, we will refer this process as phase separation (PS) since emerging reports suggest that biomolecular condensates are best described as network fluids [24–26]. Phase separation has been extensively studied from a polymer physics perspective and is only realized to be highly relevant to cell biology in the last decade. Mechanistically, PS is often driven by intermolecular interactions that are multivalent (Fig. 1) [27]. Multivalent interactions provide necessary driving forces to physically link many molecules together and drive the PS process [28–30]. Within a cellular context and from the protein perspective, such multivalent associations can be driven by protein-protein (e.g., SH3-PRM systems), protein-nucleic acid (e.g., ribonucleoprotein-RNA systems) and in some cases through protein-ligand (e.g., Tau-heparin) interactions. The multivalent interaction-driving segment of the protein of interest can be either folded or disordered – for folded proteins, individual interaction domains often recognize sequence motifs and/or structural features that are present as repeats within their corresponding interaction partners; for disordered proteins, certain amino acids can act as “stickers” and mediate weak intermolecular interactions and are also present as repeat patterns. Below we briefly discuss these crucial molecular drivers of biomolecular PS.
Figure 1.
Molecular drivers of protein phase separation (PS). (a) PS driven by multivalent interactions encoded within a single chain (i.e., intrinsic multivalence). i. Proteins with multiple folded domains, where each folded domain can interact with corresponding binding partners. ii. Disordered proteins with distributed “stickers” that can initiate inter-chain interactions. Distributed folded domains in i and sticker residues in ii act as multivalent interaction module that drive PS. (b) Emergent multivalence and PS. Phase separation can also be driven by emergent properties of biomolecules. In this case, individual monomeric units lack the necessary multivalent characteristics to mediate PS. However, once the monomeric units assemble into higher order structures, they attain multivalent nature and therefore, can drive PS.
Multivalent proteins with folded interaction domains:
These PS-drivers can be considered analogous to beads-on-a-string, where beads are folded interaction domains and strings are inter-domain connecting spacers (Fig. 1a, top panel). These beads can interact with their cognate binding partners via either sequence- or structure-specific interactions. Systematically characterized examples of such systems include polySH3-polyPRM, polySUMO-polySIM and polyUCUCU-PTB [29, 31]. In the polySH3-polyPRM system, SH3 (SRC homology 3 domain) is the folded domain that recognizes proline-rich motif (PRM) in a sequence-specific manner. When mixed together, polySH3 phase separates with polyPRM in a valence-dependent fashion – with higher valence resulting in facilitated intermolecular interactions and subsequent phase separation at a lower threshold concentration [29]. A similar mechanism is envisioned for the polySUMO-polySIM and polyUCUCU-PTB system as well [31].
Multivalent disordered proteins with repeated and distributed “stickers”:
This class of PS-drivers is perhaps more common and is abundantly present in many cellular MLOs. Mechanistically, disordered domain-mediated biomolecular phase separation can be described utilizing a stickers-and-spacers framework [27, 32, 33]. Here, “stickers” are individual amino acid residues that can interact with their corresponding partners through distinct weak interaction modes (e.g., charge-charge, cation-π, π-π, dipole-dipole) (Fig. 1a, bottom panel) [34]. Within the disordered domains that drive LLPS, these “stickers” are often present in repeat patterns, similar to the beads-on-a-string analogy presented before. Common sticker residues include amino acids with polar (G/Q/N/S), charged (R/K/D/E), and/or aromatic (Y/F) side groups [34]. These “stickers” can either interact with each other or mediate interaction with other biomolecules. Examples of sticker-sticker interactions in the context of biomolecular phase transitions are the Arg-Tyr interactions that are shown to be crucial to the LLPS of FET family proteins and other ribonucleoproteins [35]. On the other hand, charged sticker residues such as Arg/Lys can electrostatically interact with oppositely charged disordered biopolymers to mediate biomolecular condensation. A prime example of such electrostatic sticker-(biopolymer) interactions includes the association between Arg-rich disordered domain and RNA [25]. Tau-RNA and Tau-heparin interactions can also be considered an example of this category where associative phase separation is driven by the interaction between positively charged Lys residues (present within Tau’s disordered segment) and negative charges on RNA/heparin [36], respectively. The repeated and distributed patterning of the sticker residues within these disordered protein systems provide the necessary multivalency to drive their biomolecular condensation.
Phase separation mediated by “emergent multivalence” of supramolecular assemblies:
In addition to the aforementioned intrinsically multivalent systems, PS can also be driven by protein systems that are not multivalent on their own but can attain multivalency via higher-order assemblies. Such “emergent multivalence” is hierarchical in nature – first, individual units form complexes through oligomerization/clustering/micro-phase separation, and next, these formed complexes themselves drive liquid-liquid phase separation (Fig. 1b) [27]. A neat example of an emergent multivalent system is demonstrated by the PS of certain auxin response factor (ARF) transcription factor family members from the model dicot Arabidopsis thaliana [37]. ARF proteins are multi-domain proteins that contain a disordered prion-like middle region (MR) and a C-terminal folded oligomerization domain, PB1. PB1 domain mediates ARF19 oligomerization and these oligomers can then utilize their prion-like MR domains to drive ARF PS [37]. Mutation within the PB1 domain that disrupts ARF19 oligomerization abolishes the transcription factor’s ability to undergo phase separation suggesting that ARF19 transcription factor is unable to drive biomolecular condensation in its monomeric form. Rather, upon PB1-mediated oligomerization, ARF19 becomes multivalent and these “emergent multivalent assemblies” drive phase separation. This hierarchical process likely allows finer control of biomolecular phase separation within a cellular context [27].
It should be noted that while we present these three pathways as independent, in reality, the same biological system can incorporate aspects of all three pathways (especially considering the fact that most of the eukaryotic proteins harbor a combination of folded and disordered domains).
Phase separation at ectopic sites – A recurrent theme in multiple oncofusion-linked pathologies
Fusion proteins harbor domains from two distinct parent genes and combine functionalities that are otherwise spatially and/or temporally separated [38, 39]. A significant portion of these fusions gains non-native disordered domains [2, 39]. Since many disordered domains are drivers of biomolecular phase separation [34, 35, 40], it is not surprising oncofusion proteins, in general, have higher predicted phase separation propensity as compared to the human proteome [10]. Below we discuss three prime examples of oncofusions that result in non-native condensate formation at ectopic sub-cellular locations.
FET oncofusions – forming ectopic condensates on DNA
The FET oncofusions were first discovered in the early 1990s when researchers found a recurring and specific chromosomal translocation in patients with malignant soft-tissue cancers such as Ewing’s sarcoma. In FET oncofusions, disordered prion-like domains (PLDs) from respective RNA-binding proteins are fused to DNA-binding domains, causing these PLDs to behave as transactivation domains and induce aberrant gene expression [41, 42]. While most fusion oncogenes require secondary mutations for tumorigenesis, evidence for FET oncofusions suggests that they can be the sole drivers of oncogenicity [43, 44]. Moreover, the PLDs of the FET family proteins can be swapped between different FET oncofusion transcription factors, and in each case, it would lead to oncogenesis [45]. This evidence suggests a common mechanism for FET oncofusions-driven cellular transformation. However, the molecular level picture of FET fusion pathology was not clear.
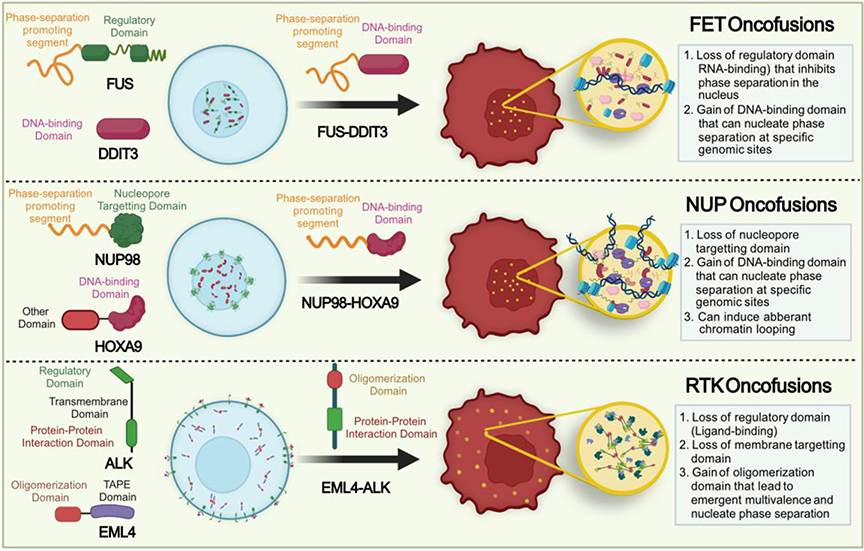
Mechanistically, PLDs have been shown to promote phase separation of the FET proteins and also mediate interactions between FET proteins and FET oncofusions [35, 46]. PLD-driven formation of distinct nuclear puncta was reported for FET oncofusions more than two decades ago [47]. The formation of these puncta was also shown to be temperature-dependent [48]. However, these observations were made prior to phase separation being recognized as a key driver for cellular MLO formation. More recently, multiple reports demonstrated that FET fusion proteins have the intrinsic capacity to form biomolecular condensates [11, 12, 49, 50]. In over-expression-based cell culture models, these ectopic FET condensates can recruit the transcriptional machinery including RNA Polymerase II, coactivators such as BRD4, and the chromatin remodeler mSWI/SNF (Fig. 2, top panel) [11, 12, 49, 51]. These data suggest that the FET oncofusions can form compartments capable of active transcription. In vitro work using single-molecule techniques showed that such compartments can recruit RNA polymerases and actively transcribe at specific DNA sites determined by the DNA-binding domain [11]. Therefore, within a cellular context, the PLD promotes phase separation of the fusion protein along with the recruitment of transcription machinery and the DBD determines the genes that are activated. It is therefore possible that ectopic condensation at non-physiological sites, leading to activation of oncogenes could be the molecular mechanism for tumorigenesis of proteins with a PLD-DBD architecture.
Figure 2.
Ectopic condensation of oncofusion proteins. (top panel) In FET fusions, the N-terminal phase separation-prone (prion-like) disordered domain of FET family proteins is fused to the DNA binding domains of transcription factors. The fusion protein forms nuclear condensates and recruit transcriptional machinery to specific genomic loci [11, 12]. (middle panel) NUP98 fusions loss their nuclear pore targeting domain and often gain DNA-localization domains. This results in the formation of ectopic nuclear NUP fusion condensates [9, 10]. These condensates can act as neomporphic transcriptional activators, driving tumorigenesis. (bottom panel) Receptor tyrosine kinase (RTK) oncofusions lose their transmembrane domain and become cytoplasmic (vs. plasma membrane localization of wild-type RTKs). RTK fusions gain oligomerization domain, undergo LLPS and activate downstream signaling in a membrane-independent fashion [8, 69]. Template created with BioRender.com.
One recurring theme that has been observed in the FET family fusions is their abnormal interactions with chromatin remodelers [12, 45, 51, 52]. Engagement with chromatin remodelers was also observed for other PLD-containing transcriptional regulators, such as EBF1 and MN1 [53, 54]. Therefore, it is conceivable that PLD-containing fusion proteins can hijack the chromatin remodeler machinery to change the chromatin landscape, thereby acting like pioneer transcription factors to activate transcription de novo from otherwise silenced genomic loci (Fig. 2; top panel) [52]. Moreover, several subunits of the mSWI/SNF complex are enriched in PLDs that can further interact with other PLDs including those of FET proteins such as FUS through heterotypic PLD-PLD interactions [12]. This could be one of the mechanisms for enhanced mSWI/SNF recruitment to condensates formed by PLD-containing transcription factors. Simultaneously, the phase-separated FET oncofusion condensates can sequester away key factors of the transcriptional machinery and thereby reduce their actions at physiological sites. The mSWI/SNF complex suppresses the H3K27 methylation, but when FET oncofusions are expressed, the overall methylation levels of H3K27 are upregulated [45]. This supports a model where sequestration of mSWI/SNF into ectopic FET condensates can lead to loss of function.
Bioinformatics analysis of oncofusion proteins also identified many other oncogenic fusions with PLD-DBD architectures beyond the FET family [12]. Similar to the FET oncofusions, these PLD-containing fusions have been reported to interact with chromatin remodelers [55–58]. It would be interesting to see whether these oncofusion transcription factors also engage SWI/SNF complex members via heterotypic prion-prion interactions. Such an observation would generalize our model to oncofusion transcription factor-mediated pathologic transformation.
Nucleoporin oncofusions – from nuclear pore to on DNA condensates
Nucleoporin proteins are components of the Nuclear Pore Complex (NPC), a multi-subunit complex that acts as a gateway between the cytoplasm and the nucleus. The NPC allows for selective transport of molecules through the central pore and is composed of various nucleoporins enriched in intrinsically disordered regions (IDRs) with Phenylalanine-Glycine (FG) repeats that form phase-separated hydrogels within the transport channel of the complex [59].
Nucleoporin oncofusions include the N-terminal FG-repeat IDR of nucleoporin proteins fused to DNA-binding factors. Such fusion proteins no longer associate with the NPC but instead form ectopic phase-separated condensates at genomic sites as defined by the DNA binding partner to drive hematopoetic cell transformation [9, 10, 13]. There likely exists a very similar mechanism for oncogenesis of the FET and NUP fusions considering their IDRs are enriched in distributed aromatic residues which are known drivers of phase separation (Fig. 2; middle panel) [32] and their interactome data also show similar interaction partners [10]. Among NUP family, gene fusions of NUP98 are the most prevalent where the N-terminal of NUP98 is fused to more than 28 different proteins at its C-terminal in various hematopoietic malignancies [60]. Similar to IDRs of FET oncofusions, when overexpressed in cell culture models, NUP98 fusions phase separate and the NUP98 IDR of the fusion proteins can act as transactivation domains altering global gene expression patterns (Fig. 2, middle panel) [9, 10, 13]. Consistent with the phase separation model, replacement of the NUP IDR with FUS prion-like domain was sufficient to drive phase separation and oncogenic transformation, and replacement of phenylalanines of the NUP IDR with serines that prevent phase separation leads to loss of tumorigenic activity despite having a similar genome binding profile [9]. Moreover, interactome data of diverse NUP98 fusions show enrichment of proteins that tend to phase separate along with critical transcriptional machinery such as SWI/SNF, RNA polymerase II, transcription factors; and it was sufficient to use an artificial FG repeat to replace the NUP98 IDR to elicit phenotypes similar to the NUP98 fusion protein [10]. Such NUP oncofusion condensates can also induce aberrant chromatin looping, potentially leading to ‘super-enhancer’-like transcriptional activation [9]. These data clearly indicate that ectopic nuclear condensation of NUP fusions (i.e., mislocalization of NUP from NPC to nucleus) driven by the multivalent NUP IDRs is a major driver in the pathological transformation of cells.
RTK oncofusions – from the plasma membrane to cytoplasm
Signal transduction pathways are essential processes that convey information from the external surrounding to the cell interior. Signaling molecules such as cytokines, growth factors, and hormones interact with membrane-bound receptors on the surface of the cell initiating a biochemical cascade that can summon the cell to perform a myriad of functions in response to the stimuli. Receptor tyrosine kinases and G-protein coupled receptors are the most abundant cell surface receptors and their misregulation is commonly implicated in cancers [61, 62]. Signal transduction through receptor tyrosine kinases (RTKs) plays an important role in cellular proliferation and differentiation. Upon ligand binding, homo-oligomerization of the transmembrane receptor results in transphosphorylation of the intracellular domain of RTK and activation of the downstream signaling pathway. Mutations in RTKs or their overexpression leads to hyperactivation of the receptor leading to oncogenesis [63]. RTKs such as the fibroblast growth factor receptor 2 (FGFR2) as well as other membrane-associated receptors such as T-cell receptors are shown to undergo LLPS with downstream effectors to form signaling competent condensates on the inner surface of the cell membrane [64, 65].
Chromosomal translocations have been found in lung and solid tumors where the RTK kinase domain is fused to other proteins that oligomerize. While RTKs are plasma membrane (PM) localized, the fusion proteins no longer localize to the membranes but instead are dispersed in the cytoplasm and form condensates [66, 67]. Multiple recent studies provided a mechanistic picture for the oncogenic properties of a subset of such fusion proteins [8, 14, 15]. Collectively, these studies report that EML4-ALK (an RTK oncofusion), where the microtubule protein EML4 is fused to the cytoplasmic domain of the RTK, ALK, forms cytoplasmic protein granules in patient-derived cell lines as well as overexpression-based cell culture models (Fig. 2, bottom panel). The phase separation is the result of fusion protein being able to form an emergent multivalent system which is dependent on both the oligomerization capability of the folded fusion partner and the phosphorylation of the kinase domain to sustain multivalent interactions with downstream effectors [8, 15]. The puncta/condensate formation was further dependent on the kinase activity of the fused ALK domain, as simple lower-order oligomerization of the fusion proteins lacking kinase activity led to a diffuse distribution. Enrichment of protein binding partners such as SOS1 and GRB2 through the phosphorylated residues was necessary for the multivalency, puncta formation, and consequently enhanced signaling activity. In a similar mechanism, NPM-ALK protein associated with anaplastic large cell lymphoma forms cytoplasmic granules containing mRNA but are distinct from stress granules or P-bodies and dependent on the kinase activity of ALK [68]. Another RTK oncofusion, CCCD6-RET showed a kinase-independent ability to form protein granules because the N-terminal coiled-coil domain of CCCD6 was sufficient to drive multivalent interactions. Inhibiting granule formation of CCCD6-RET led to decreased downstream signaling even in the presence of the fusion suggesting that the formation of ectopic condensates is necessary for oncogenesis, although the mechanism of their formation can vary among different RTK oncofusions [8]. Component-wise, RTK oncofusion condensates concentrate components of the RAS/MAPK pathway activators and deplete RAS pathway negative regulators such as phosphatases. It has been hypothesized that this enrichment of activators/depletion of negative regulators results in constitutively activated RAS pathway without the need for any ligand. Overall, these RTK fusions were able to initiate downstream cellular signaling within their ectopic cytoplasmic condensates in a membrane-independent fashion (unlike the wild-type RTKs where signal propagation is membrane-dependent). However, further investigations on other RTK fusions are required to generalize the proposed mode of oncogenesis.
A recent study also showed differences in material properties of condensates formed by different versions of the same RTK fusion (e.g., EML4-ALK). EML4-ALK V3 is more liquid-like compared to EML4-ALK V1 [15]. The EML4-ALK V1 harbors an additional TAPE domain compared to V3 which provides increased valence and more binding partners that reduce the mobility of the fusion protein making it solid-like. Such differences in material properties could be crucial determinants of signaling output and therefore, need to be considered when designing therapeutic agents against fusion proteins even if they are within the same family of oncofusions.
Pathology through ectopic condensation: How do ectopic condensates drive oncogenic transformations?
While our understandings on how MLOs function are rapidly evolving, current knowledge suggest that biomolecular condensates function through three distinct mechanisms: (i) they can recruit and locally enrich substrates of biomolecular reactions, resulting in enhanced reaction rates; (ii) they sequester/exclude signaling molecules, resulting in a fine-tuned regulation of downstream signaling processes; and (iii) they can act as organizing hubs for sub-cellular assemblies (further discussions on biomolecular condensates’ functional roles are provided in refs. [20, 30]). Not surprisingly, so far identified ectopic condensates of oncofusion proteins utilize some of these mechanisms to subvert physiologic cellular pathways and drive oncogenic transformations (Fig. 3). Below we try to provide a framework on the molecular mechanisms of these transformations.
Figure 3.
Distinct (non-exclusive) paths to pathology through ectopic condensation. Upon gene fusion, condensates can form at ectopic sub-cellular locations. (left path) These ectopic condensates can recruit functional cellular machineries, which can either result in gain of function at ectopic sites or loss of function at native sites. (middle path) Ectopic condensates can exclude certain biomolecules, while allowing enrichment/recruitment of others. (right path) Ectopic condensates can change their material states and drive pathologic outcomes. Template created with BioRender.com.
Ectopic condensates as recruitment hubs at non-native locations:
Physiologic biomolecular condensates can recruit and enrich many biomolecules for functional purposes [70]. Similarly, for fusion protein condensates, the domain that drives phase separation can also interact with many cellular client proteins and recruit them to ectopic cellular locations. This brings together two protein interaction networks that are otherwise distinct and typically not connected. This model is also supported by a large-scale bioinformatics analysis of fusion protein networks that revealed that oncofusions disproportionately connect proteins that are distant in protein-protein interaction network [38]. Upon recruitment to oncofusion ectopic condensates, these “client proteins” can become functional at not-native locations to drive oncogenesis (Fig. 3, left pathway). FUS-DDIT3 fusion protein (along with other FET oncofusion) condensates can be considered a prime example of such a phenomenon.
DDIT3 (alternate name: CHOP) is a transcription factor that can interact with its cognate genomic DNA binding sites in the cell nucleus [12, 71]. On the other hand, FUS is primarily an RNA-binding protein that can localize to both nuclear and cytoplasmic membrane-less bodies [72–76]. As discussed in the preceding section, in the FUS-DDIT3 fusion protein, the N-terminal prion-like disordered domain of FUS fuses to the DNA-binding domain of DDIT3 and drives the formation of nuclear condensates that potentially localize to the genomic DDIT3-binding sites [11, 12]. These ectopic condensates can then recruit many SWI/SNF complex proteins through prion-prion interactions. Thus, the fused prion-like disordered domain in the FUS oncofusion protein acts as a “client recruitment handle” in addition to its “PS-driver function”. Recruitment of a major chromatin remodeler complex to ectopic genomic loci by FUS oncofusion condensates results in broad chromatin remodeling, subsequent global changes in the cellular transcription profile, and ultimately oncogenic transformation. A similar mechanism is also operative for the EWSR1, where the PS-driver domain (EWSR1 prion-like domain) acts as a handle for ectopic recruitment of proteins/protein complexes and ultimately leads to cancerous transformation [52].
A variant of the “recruitment hub” model is likely to be operative for NUP98-oncofusion proteins. For NUP98 fusions, it was shown that fusion proteins can induce aberrant chromatin looping through phase separation [9]. These phase-separated bodies of NUP98 fusion proteins can then recruit gene regulatory proteins to induce leukemogenic gene expression and drive oncogenic transformation [10, 13]. Thus, for NUP98 oncofusions, ectopic physical reorganization of cellular substructures through phase separation, followed by the recruitment of regulatory proteins to these ectopic hubs can drive cellular leukemogenic reprogramming.
Neomorphic function through the selective exclusion of regulators from ectopic condensates:
An alternate mechanism for fusion protein condensates’ pathology is via selective exclusion of certain signaling regulators from the ectopic condensates while allowing others to localize. Cellular signaling typically involves a delicate balance between positive and negative regulators. Therefore, if a condensate allows partitioning of positive regulators and excludes negative regulators, then downstream signaling can be rapidly activated (Fig. 3, middle pathway). Physiologically, this mechanism was found to be operative in T-cell receptor (TCR) signaling [77], where major components of the TCR signaling pathway form submicron to micron-sized plasma membrane (PM)-bound clusters. Three critical TCR microcluster proteins (LAT along with its binding partners GRB2 and SOS1) form micrometer-sized condensates when reconstituted in vitro [65]. Subsequently, it was shown that in cellulo PM-associated LAT microclusters enrich kinases and exclude phosphatases to drive LAT phosphorylation, which subsequently promoted downstream biochemical reactions of the TCR signaling pathway [65].
Mirroring the physiologic TCR signaling activation through the selective exclusion of phosphatases from LCR condensates, (a subset of) RTK oncofusion condensates enriches RAS activating proteins and are deficient in the negative regulators of RAS signaling [8, 69]. This likely results in the activation of downstream RAS signaling through ectopic RTK fusions, a hypothesized mechanism discussed in the previous section. For these ectopic condensates, their pathologic outcomes are likely due to selective exclusion of a class of signaling regulators while allowing the localization of the opposite regulator class.
Ectopic condensates and their material states – a potential route to pathogenesis:
Condensate material state refers to the physical properties that determine a condensate’s dynamics and can be quantified by measuring in-condensate macromolecular diffusion, viscosity, surface tension as well as viscoelasticity [78–80]. Mounting evidence suggests that condensate material states and dynamical properties are crucial to their biological functions [17], especially since condensates formed from different biomolecular systems have distinct material properties [25, 35, 81]. Furthermore, many biomolecular condensates change their material properties over time, a process that is known as “condensate aging (also maturation or hardening)” [17, 82, 83]. During this aging process, liquid-like condensates progressively become solid-like. The material state transition of biomolecular condensates (from liquid-like to solid-like) is often associated with many neurodegenerative pathologies, wherein condensates can act as sites or “hotbeds” of disease-associated protein aggregation [40, 84–86].
At the molecular level, many of the biomolecular condensates that have so far been reported to undergo aging are enriched with proteins that have prion-like domains [87–90]. In vitro studies clearly suggest that these prion-like domains are often sufficient to drive phase separation and prolonged incubation of their condensates lead to the formation of fibrillar aggregated assemblies [35, 40, 87, 91]. Further, mutations within the prion-like domains of these phase separating proteins significantly speed up the condensate liquid-to-solid transformation process [91–93]. These results clearly indicate a bifunctional role of prion-like domains in biomolecular phase separation, where PLDs can drive physiologic condensation as well as are responsible for pathologic condensate maturation.
Bioinformatics analysis of oncofusion proteins suggests that many recurrent cancer-linked fusions join a prion-like domain with other functional domains [12]. We posit that the fused prion-like domains will confer fusion proteins the ability to undergo PLD-mediated ectopic condensation. However, it remains to be seen whether these PLDs can also result in arrested ectopic fusion protein condensates and subsequently add another dimension to oncofusion-driven pathogenesis (see Outstanding Questions). While not directly related to fusion-mediated oncogenesis, altered condensate material states have already been implicated in multiple disease pathologies including HOXD13 mutation-associated hereditary synpolydactyly [94] and AKAP95 dysregulation-linked tumorigenesis [95].
Outstanding questions.
Do over-expression-based systems truly represent the cellular states of oncofusion proteins? Do oncofusion proteins form phase-separated condensates at their native endogenous expression levels?
How does fusion effect functions of individual domains? Is there an allosteric effect of the phase separating domain on the functional domain? For example, does fusion of prion-like domain affects DNA binding preferences and thermodynamics of (prion-like domain) – (DNA-binding domain) oncofusions?
Physiologic biomolecular condensates are spatiotemporally regulated by post-translational modifications (PTMs). Whether cells are able to regulate ectopic condensates through PTMs remains an open question.
Do ectopic condensates change their composition and/or material states spatiotemporally? If yes, how do these changes affect disease outcomes?
Do ectopic condensates primarily represent a gain-of-toxic function? Can these non-native condensates also result in the loss of physiologic function? Can the same oncofusion protein condensate drive oncogenesis through both gain of function and loss of function in a context-dependent manner?
How does cellular transcriptomic/proteomic state affect ectopic condensate-mediated pathology? Can the same ectopic condensate be highly pathogenic in one tissue (or genetic) background while remaining relatively benign in another background?
It is important to note that the three potential paths to pathology via ectopic condensates discussed here are not exclusive. That is, any oncofusion protein can utilize more than one of the paths shown in Figure 3. Further, instead of driving ectopic condensation, oncofusion proteins can also simply localize to native cellular condensates and perturb their physiologic functions to drive oncogenesis (Box 1).
Box 1: Oncofusions that perturb native biomolecular condensates.
DnaJB1-PKAcat oncofusions
G-coupled protein receptors (GPCRs) drive ligand-dependent signaling pathways such as the protein kinase A (PKA) pathway. The receptor activates the adenyl cyclase enzyme to convert ATP to cAMP, a second messenger that dissociates the catalytic subunit of PKA from its regulatory subunit to drive downstream signaling [61]. The regulatory subunit in the presence of cAMP undergoes LLPS-compartmentalizing both cAMP and the catalytic subunit at the site of activation [101]. This spatial constraint is necessary for the specificity of downstream signal transduction as cAMP is a small molecule that can otherwise diffuse freely and activate effectors of diverse GPCRs. However, the fusion protein DnaJB1-PKAcat formed by the translocation of the N-terminus of DnaJB1 to C-terminal PKAcat [102] was shown to disrupt the condensate formation through an unknown mechanism. This disruption leads to release of cAMP and its dispersion to other cytoplasmic effectors. A loss in the specificity of downstream signaling leads to activation of undesirable pathways that cause aberrant transcriptional programming and oncogenesis [101].
PML-RAR oncofusions
PML bodies are MLOs found in the nucleus that has been implicated in multiple functions including DNA damage response, transcriptional regulation, and tumor suppression. These nuclear bodies are believed to be formed through the process of LLPS with the PML protein acting as the scaffold essential for condensate formation [103]. The oncoproteins PML-RARα involves the fusion of domains of retinoic acid receptors (RAR) to the PML protein causing acute promyelocytic leukemia (APL) [104]. PML-RARα acts as a dominant-negative regulator disrupting the formation of PML bodies via sequestration of wild-type PML [105]. These disrupted PML bodies form microspeckles leading to dysregulation of cellular processes such as DNA strand repair [106].
BCR-ABL oncofusions
ABL is a tyrosine kinase predominantly found in the nucleus where it has DNA binding activity and is involved in DNA damage repair and apoptosis [107]. However, in many patients with chronic myelogenous leukemia (CML), the ABL domains are found juxtaposed with domains of BCR protein, and the fusion BCR-ABL is localized to the cytoplasm [108]. The fusions are found as cytoplasmic granules with liquid-like properties and kinase activity was necessary for the formation of functional granules that lead to oncogenesis [109]. The fusion proteins show constitutive kinase activity, activating pathways involved in cell proliferation, differentiation, adhesion, and cell survival [108]. These fusion proteins enrich within stress granules [109] and it is reasonable to believe that they can alter the composition and properties of stress granules.
Targeting ectopic condensates: Potential therapeutic approaches towards oncofusion-mediated cancer pathologies
The observation that many oncofusion proteins can form pathology-associated ectopic condensates opens up potential avenues of targeting these oncofusion-mediated diseases. Since phase-separated condensates have distinct solvation properties [96], it is possible that certain small molecules can be preferentially solvated into condensates primarily based on their physicochemical properties [97]. Consistent with this concept, a recent study reported that nuclear puncta/condensates can selectively partition many cancer therapeutics [98]. Therefore, future drug designing/screening approaches can aim to identify and optimize therapeutics that are preferentially recruited into oncofusion condensates to selectively block oncopathology driven by these ectopic condensates.
Concluding remarks
Gene fusions represent a major category of oncogenic genetic alterations [1]. In this review, we provided a summary of molecular mechanisms of pathogenesis for a subclass of oncofusions where the fusion protein gains the ability to undergo phase separation. We primarily presented the topic through the lens of “ectopic” phase separation of oncofusion proteins where biomolecular condensates at non-native sub-cellular locations can act as the drivers of oncogenic transformations. It should be noted that in addition to phase separation, other mechanisms of supramolecular assembly formation might also be operative for fusion protein-mediated oncogenesis [99, 100] – for example, for fusion transcription factors (e.g., FET fusions, NUP fusions), DNA binding to specific genomic loci through sequence-specific interactions might be sufficient to generate ectopic hubs that can drive the oncogenic program [97]. The role of ectopic hubs through sequence-specific interactions in the oncogenesis process might be more important than they currently appear based on the so-far reported in vitro and mostly over-expression-based in vivo data in the literature. Indeed, a recent study utilizing an endogenously tagged FET fusion protein showed that the oncofusion protein EWS-FLI1 does not form large condensates, rather remains relatively diffuse with small nuclear foci [50]. This observation would be consistent with an ectopic hub model, at least at the endogenous expression level of the tested cell line(s). Future endeavors should systematically characterize the differences between endogenous and overexpression-based systems to delineate the distinct yet plausibly overlapping roles of ectopic condensates and ectopic hubs in the oncogenesis process (see Outstanding Questions; also see box 2 for a brief discussion on ectopic condensates and ectopic hubs and their relevance to oncofusion transcription factors.)
Box 2: A continuum of membraneless assemblies – from nanoscopic hubs to mesoscopic condensates.
Figure I.
Dosage-dependent assembly states of phase separation-competent transcription factors. At low doses (i.e., pre-condensation regimes), proteins can form finite-sized clusters or hubs. These clusters can grow continuously in size as respective protein concentration increases. After reaching saturation concentration for phase separation (Csat), condensation ensues. A continuum of supramolecular assemblies may provide a unified view of aberrant transcriptional activity of oncofusion proteins through ectopic hub formation in conjunction with or without phase separation.
Many of the in vivo studies of biomolecular condensations utilize overexpression systems. To what extent the observations on biomolecular condensation from the overexpression-based system can be extended to native cells has been a subject of some debate [99, 100]. A recent study on FUS and other FET family proteins indicate that these proteins can form nanoscopic clusters at pre-condensation regimes [110]. For FET proteins, molecular forces that drive cluster formation at pre-condensation regimes are strongly coupled to sequence-encoded interactions that drive phase separation at post-condensation regimes. These observations suggest that hubs and condensates can be considered as two ends of a continuum – at low protein concentrations, phase separation-competent proteins can form reversible clusters (i.e., hubs); at high protein concentrations, these clusters can form mesoscopic condensates through phase separation. This model would imply that oncofusion transcription factors discussed in this review (i.e., FET and NUP oncofusions) are likely to form ectopic hubs at low protein dosage and can transition to ectopic phase-separated condensates when protein dosage is high enough (i.e., at concentration beyond the phase separation saturation concentration or Csat; Fig. I). We envision that both ectopic hubs and ectopic condensates could be relevant to oncofusion transcription factor-driven pathologies, in a patient- and disease state-dependent manner.
Irrespective of how they are assembled, it is likely that the formation of supramolecular membrane-less bodies at ectopic sites is a generic mechanism for many cancer pathogeneses. However, how cells respond to the initial ectopic condensates and how these condensates themselves are affected by cellular proteomic states remain open questions (see Outstanding Questions). Perhaps, during the early stages, cells have some sort of homeostasis mechanism that counters pathogenesis through ectopic assemblies. It is likely that protein post-translational modifications that are known to play key roles in the regulation of physiologic condensates, can also counteract pathologic ectopic condensates. This could explain why oncofusion pathogenesis is tissue-specific and may provide clues to designing therapeutic strategies that target these ectopic assemblies.
Highlights.
Phase separation has emerged as a crucial mechanism for the spatiotemporal regulation of functions within cells. Phase separation (PS) drives the formation of many membrane-less organelles (MLOs) that play indispensable roles in sub-cellular biochemistry.
Many protein domains can act as molecular drivers of biomolecular PS. When fused to non-phase separating proteins, they can confer phase separation capacities to these non-phase separating proteins.
Oncofusion proteins join domains from proteins that are otherwise distant in the protein-protein interaction network. Recent reports indicate that many oncofusion proteins gain the ability to undergo phase separation at non-native sub-cellular locations. These “ectopic condensates” of fusion proteins mediate pathologic outcomes through a variety of mechanisms.
Acknowledgments
We thank anonymous reviewers for critical reading of the manuscript and valuable comments. P.R.B acknowledges the National Institute of General Medical Sciences (R35 GM138186), National Institute of Aging (R21 AG064258 and R03 AG070510), National Center for Advancing Translational Sciences (R03 TR003387), Mae Stone Goode Trust, and the College of Arts and Sciences at the University at Buffalo, SUNY for financial support. The authors acknowledge all the other members of the Banerjee laboratory for stimulating discussions and helpful suggestions at the various stages of the manuscript preparation.
Glossary
- Gene-fusion
Combination of segments of two different genes, typically results in the formation of chimeric transcripts or deregulation of existing genes. Chromosomal translocation, interstitial deletion, or chromosomal inversion are the lead causes of gene fusions in cells
- In-frame gene-fusion
A gene-fusion in which the reading frame of the parent genes is maintained such that a chimeric protein product is formed. This chimeric protein has a combination of protein domains/sequence motifs from the parent proteins
- Membrane-less organelles (MLOs)
Subcellular compartments that lack a lipidic bilayer. Recent advances indicate that many of these MLOs form via phase separation of multivalent proteins
- FET family proteins
RNA-binding protein family consisting of three proteins FUS, EWSR1, and TAF15. All three proteins have a prion-like domain at the N-terminus and RNA-binding domain at the C-terminus and are involved in cellular processes such as transcription, DNA repair, and splicing among others
- Ewing’s sarcoma
A soft tissue cancer usually affecting children and young adults and often associated with chromosomal mismatches of #11 and #22 leading to gene fusions and fusion proteins
- Chromatin remodeler mSWI/SNF (BAF complex)
Multi-subunit complex that carries out ATP-dependent chromatin remodeling to regulate gene expression. They come in diverse forms – canonical BAF, poly-Bromo associated BAF, and non-canonical BAF depending on their subunit composition with distinct chromatin localization and tissue-specific expression and function
- DDIT3
Member of the C/EBP family of transcription factors usually expressed in response to a wide variety of cell stresses. Often found fused to FET family proteins in pediatric soft-tissue cancers
- Intrinsically disordered region (IDR)
Sequences/domains within a protein that lack a well-defined three-dimensional structure
- Prion-like domain (PLD)
A disordered protein domain enriched in polar amino acids- Q/N/S/Y and G and depleted of hydrophobic or charged residues. They are often found in RNA-binding proteins and are associated with neurodegenerative diseases associated with protein aggregation
- Signal transduction
Transmission of signals from a cell’s exterior through surface receptors and molecular pathways that amplify the signal all the way to transcriptional response to provide an appropriate physiological response. These are employed for cellular development and differentiation, stress response and apoptosis
- Condensate aging
A change in the material property of phase-separated condensates where the condensate dynamical properties are altered as a function of time. Typically assumed to be associated with protein aggregation disorder
Footnotes
Declaration of Interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mertens F et al. (2015) The emerging complexity of gene fusions in cancer. Nat Rev Cancer 15 (6), 371–81. [DOI] [PubMed] [Google Scholar]
- 2.Latysheva NS and Babu MM (2019) Molecular Signatures of Fusion Proteins in Cancer. ACS Pharmacol Transl Sci 2 (2), 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milne TA et al. (2005) Leukemogenic MLL fusion proteins bind across a broad region of the Hox a9 locus, promoting transcription and multiple histone modifications. Cancer Res 65 (24), 11367–74. [DOI] [PubMed] [Google Scholar]
- 4.Chan AKN and Chen CW (2019) Rewiring the Epigenetic Networks in MLL-Rearranged Leukemias: Epigenetic Dysregulation and Pharmacological Interventions. Front Cell Dev Biol 7, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banito A et al. (2018) The SS18-SSX Oncoprotein Hijacks KDM2B-PRC1.1 to Drive Synovial Sarcoma. Cancer Cell 34 (2), 346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulay G et al. (2021) The chromatin landscape of primary synovial sarcoma organoids is linked to specific epigenetic mechanisms and dependencies. Life Sci Alliance 4 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z-H et al. (2017) MAN2A1–FER Fusion Gene Is Expressed by Human Liver and Other Tumor Types and Has Oncogenic Activity in Mice. Gastroenterology 153 (4), 1120–1132.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tulpule A et al. (2021) Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Cell 184 (10), 2649–2664 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn JH et al. (2021) Phase separation drives aberrant chromatin looping and cancer development. Nature 595 (7868), 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terlecki-Zaniewicz S et al. (2021) Biomolecular condensation of NUP98 fusion proteins drives leukemogenic gene expression. Nat Struct Mol Biol 28 (2), 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo L et al. (2021) Loci-specific phase separation of FET fusion oncoproteins promotes gene transcription. Nat Commun 12 (1), 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis RB et al. (2021) FUS oncofusion protein condensates recruit mSWI/SNF chromatin remodeler via heterotypic interactions between prion-like domains. Protein Sci 30 (7), 1454–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandra B et al. (2021) Phase Separation mediates NUP98 Fusion Oncoprotein Leukemic Transformation. Cancer Discovery, candisc.0674.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Z et al. (2021) Phase separation of EML4-ALK in firing downstream signaling and promoting lung tumorigenesis. Cell Discov 7 (1), 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sampson J et al. (2021) Phase-separated foci of EML4-ALK facilitate signalling and depend upon an active kinase conformation. EMBO Rep 22 (12), e53693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Meer G et al. (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9 (2), 112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberti S and Hyman AA (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nature Reviews Molecular Cell Biology 22 (3), 196–213. [DOI] [PubMed] [Google Scholar]
- 18.Boeynaems S et al. (2018) Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 28 (6), 420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lafontaine DLJ et al. (2021) The nucleolus as a multiphase liquid condensate. Nature Reviews Molecular Cell Biology 22 (3), 165–182. [DOI] [PubMed] [Google Scholar]
- 20.Shin Y and Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357 (6357), eaaf4382. [DOI] [PubMed] [Google Scholar]
- 21.Mitrea DM and Kriwacki RW (2016) Phase separation in biology; functional organization of a higher order. Cell Commun Signal 14, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes E and Shorter J (2019) The molecular language of membraneless organelles. J Biol Chem 294 (18), 7115–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyman AA et al. (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- 24.Jawerth L et al. (2020) Protein condensates as aging Maxwell fluids. Science 370 (6522), 1317–1323. [DOI] [PubMed] [Google Scholar]
- 25.Alshareedah I et al. (2021) Programmable viscoelasticity in protein-RNA condensates with disordered sticker-spacer polypeptides. Nat Commun 12 (1), 6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh A et al. (2021) Shear relaxation governs fusion dynamics of biomolecular condensates. Nat Commun 12 (1), 5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi J-M et al. (2020) Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu Rev Biophys 49 (1), 107–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banjade S and Rosen MK (2014) Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 3, e04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li P et al. (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483 (7389), 336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hondele M et al. (2020) Membraneless organelles: phasing out of equilibrium. Emerging Topics in Life Sciences 4 (3), 343–354. [DOI] [PubMed] [Google Scholar]
- 31.Banani SF et al. (2016) Compositional Control of Phase-Separated Cellular Bodies. Cell 166 (3), 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin EW et al. (2020) Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 367 (6478), 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremer A et al. (2022) Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains. Nat Chem 14 (2), 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brangwynne CP et al. (2015) Polymer physics of intracellular phase transitions. Nat Phys 11 (11), 899–904. [Google Scholar]
- 35.Wang J et al. (2018) A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins. Cell 174 (3), 688–699 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y et al. (2019) Narrow equilibrium window for complex coacervation of tau and RNA under cellular conditions. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powers SK et al. (2019) Nucleo-cytoplasmic Partitioning of ARF Proteins Controls Auxin Responses in Arabidopsis thaliana. Mol Cell 76 (1), 177–190 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latysheva NS et al. (2016) Molecular Principles of Gene Fusion Mediated Rewiring of Protein Interaction Networks in Cancer. Mol Cell 63 (4), 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latysheva NS and Babu MM (2016) Discovering and understanding oncogenic gene fusions through data intensive computational approaches. Nucleic Acids Res 44 (10), 4487–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molliex A et al. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163 (1), 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertolotti A et al. (1999) The N-terminal domain of human TAFII68 displays transactivation and oncogenic properties. Oncogene 18 (56), 8000–10. [DOI] [PubMed] [Google Scholar]
- 42.Zinszner H et al. (1994) A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic transformation by CHOP. Genes Dev 8 (21), 2513–26. [DOI] [PubMed] [Google Scholar]
- 43.Riggi N et al. (2005) Development of Ewing’s sarcoma from primary bone marrow-derived mesenchymal progenitor cells. Cancer Res 65 (24), 11459–68. [DOI] [PubMed] [Google Scholar]
- 44.Riggi N et al. (2006) Expression of the FUS-CHOP fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res 66 (14), 7016–23. [DOI] [PubMed] [Google Scholar]
- 45.Linden M et al. (2019) FET family fusion oncoproteins target the SWI/SNF chromatin remodeling complex. EMBO Rep 20 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen C et al. (2013) A conserved N-terminal motif is required for complex formation between FUS, EWSR1, TAF15 and their oncogenic fusion proteins. FASEB J 27 (12), 4965–74. [DOI] [PubMed] [Google Scholar]
- 47.Thelin-Jarnum S et al. (2002) The myxoid liposarcoma specific TLS-CHOP fusion protein localizes to nuclear structures distinct from PML nuclear bodies. Int J Cancer 97 (4), 446–50. [DOI] [PubMed] [Google Scholar]
- 48.Goransson M et al. (2002) Temperature-dependent localization of TLS-CHOP to splicing factor compartments. Exp Cell Res 278 (2), 125–32. [DOI] [PubMed] [Google Scholar]
- 49.Owen I et al. (2021) The oncogenic transcription factor FUS-CHOP can undergo nuclear liquid-liquid phase separation. J Cell Sci 134 (17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chong S et al. (2021) Tuning levels of low-complexity domain interactions to modulate endogenous oncogenic transcription. bioRxiv, 2021.08.16.456551. [DOI] [PubMed] [Google Scholar]
- 51.Linden M et al. (2022) FET fusion oncoproteins interact with BRD4 and SWI/SNF chromatin remodeling complex subtypes in sarcoma. Mol Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boulay G et al. (2017) Cancer-Specific Retargeting of BAF Complexes by a Prion-like Domain. Cell 171 (1), 163–178 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y et al. (2020) A Prion-like Domain in Transcription Factor EBF1 Promotes Phase Separation and Enables B Cell Programming of Progenitor Chromatin. Immunity 53 (6), 1151–1167.e6. [DOI] [PubMed] [Google Scholar]
- 54.Riedel SS et al. (2021) Intrinsically disordered Meningioma-1 stabilizes the BAF complex to cause AML. Mol Cell 81 (11), 2332–2348 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunkel BD et al. (2021) Evidence of pioneer factor activity of an oncogenic fusion transcription factor. iScience 24 (8), 102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gryder BE et al. (2017) PAX3–FOXO1 Establishes Myogenic Super Enhancers and Confers BET Bromodomain Vulnerability. Cancer Discov 7 (8), 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagai M et al. (2001) Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci U S A 98 (7), 3843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laubscher D et al. (2021) BAF complexes drive proliferation and block myogenic differentiation in fusion-positive rhabdomyosarcoma. Nat Commun 12 (1), 6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt HB and Gorlich D (2015) Nup98 FG domains from diverse species spontaneously phase-separate into particles with nuclear pore-like permselectivity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gough SM et al. (2011) NUP98 gene fusions and hematopoietic malignancies: common themes and new biologic insights. Blood 118 (24), 6247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nieto Gutierrez A and McDonald PH (2018) GPCRs: Emerging anti-cancer drug targets. Cell Signal 41, 65–74. [DOI] [PubMed] [Google Scholar]
- 62.Du Z and Lovly CM (2018) Mechanisms of receptor tyrosine kinase activation in cancer. Mol Cancer 17 (1), 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saraon P et al. (2021) Receptor tyrosine kinases and cancer: oncogenic mechanisms and therapeutic approaches. Oncogene 40 (24), 4079–4093. [DOI] [PubMed] [Google Scholar]
- 64.Lin CC et al. (2022) Receptor tyrosine kinases regulate signal transduction through a liquid-liquid phase separated state. Mol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su X et al. (2016) Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352 (6285), 595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hrustanovic G et al. (2015) RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med 21 (9), 1038–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richards MW et al. (2015) Microtubule association of EML proteins and the EML4-ALK variant 3 oncoprotein require an N-terminal trimerization domain. Biochem J 467 (3), 529–36. [DOI] [PubMed] [Google Scholar]
- 68.Fawal M et al. (2011) Looking for the functions of RNA granules in ALK-transformed cells. Bioarchitecture 1 (2), 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuneo MJ and Mittag T (2021) Oncogenic signaling of RTK fusions becomes more granular. Mol Cell 81 (12), 2504–2506. [DOI] [PubMed] [Google Scholar]
- 70.Ditlev JA et al. (2018) Who’s In and Who’s Out—Compositional Control of Biomolecular Condensates. J Mol Biol 430 (23), 4666–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ron D and Habener JF (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6 (3), 439–53. [DOI] [PubMed] [Google Scholar]
- 72.Yang L et al. (2015) Subcellular localization and RNAs determine FUS architecture in different cellular compartments. Hum Mol Genet 24 (18), 5174–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sama RRK et al. (2013) FUS/TLS assembles into stress granules and is a prosurvival factor during hyperosmolar stress. J Cell Physiol 228 (11), 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marmor-Kollet H et al. (2020) Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis. Mol Cell 80 (5), 876–891 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hock E-M et al. (2018) Hypertonic Stress Causes Cytoplasmic Translocation of Neuronal, but Not Astrocytic, FUS due to Impaired Transportin Function. Cell Reports 24 (4), 987–1000.e7. [DOI] [PubMed] [Google Scholar]
- 76.Galganski L et al. (2017) Nuclear speckles: molecular organization, biological function and role in disease. Nucleic Acids Res 45 (18), 10350–10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao Q et al. (2021) Phase separation in immune signalling. Nature Reviews Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor NO et al. (2019) Quantifying Dynamics in Phase-Separated Condensates Using Fluorescence Recovery after Photobleaching. Biophys J 117 (7), 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alshareedah I et al. (2021) Quantifying viscosity and surface tension of multicomponent protein-nucleic acid condensates. Biophys J 120 (7), 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H et al. (2021) Surface tension and viscosity of protein condensates quantified by micropipette aspiration. Biophysical Reports 1 (1), 100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boeynaems S et al. (2019) Spontaneous driving forces give rise to protein-RNA condensates with coexisting phases and complex material properties. Proc Natl Acad Sci U S A 116 (16), 7889–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H et al. (2015) RNA Controls PolyQ Protein Phase Transitions. Mol Cell 60 (2), 220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aguzzi A and Altmeyer M (2016) Phase Separation: Linking Cellular Compartmentalization to Disease. Trends Cell Biol 26 (7), 547–58. [DOI] [PubMed] [Google Scholar]
- 84.Wolozin B (2014) Physiological protein aggregation run amuck: stress granules and the genesis of neurodegenerative disease. Discov Med 17 (91), 47–52. [PMC free article] [PubMed] [Google Scholar]
- 85.Babinchak WM and Surewicz WK (2020) Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation. J Mol Biol 432 (7), 1910–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsoi PS et al. (2021) Electrostatic modulation of hnRNPA1 low-complexity domain liquid-liquid phase separation and aggregation. Protein Sci 30 (7), 1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gotor NL et al. (2020) RNA-binding and prion domains: the Yin and Yang of phase separation. Nucleic Acids Res 48 (17), 9491–9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sprunger ML and Jackrel ME (2021) Prion-Like Proteins in Phase Separation and Their Link to Disease. Biomolecules 11 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Franzmann T and Alberti S (2018) Prion-like low-complexity sequences: Key regulators of protein solubility and phase behavior. J Biol Chem 294 (18), 7128–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hennig S et al. (2015) Prion-like domains in RNA binding proteins are essential for building subnuclear paraspeckles. J Cell Biol 210 (4), 529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel A et al. (2015) A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 162 (5), 1066–77. [DOI] [PubMed] [Google Scholar]
- 92.Murakami T et al. (2015) ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function. Neuron 88 (4), 678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mackenzie IR et al. (2017) TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron 95 (4), 808–816 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basu S et al. (2020) Unblending of Transcriptional Condensates in Human Repeat Expansion Disease. Cell 181 (5), 1062–1079.e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li W et al. (2020) Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat Cell Biol 22 (8), 960–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nott TJ et al. (2016) Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat Chem 8 (6), 569–75. [DOI] [PubMed] [Google Scholar]
- 97.Mittag T and Ansari AZ (2021) Fusion proteins form onco-condensates. Nat Struct Mol Biol 28 (7), 543–545. [DOI] [PubMed] [Google Scholar]
- 98.Klein IA et al. (2020) Partitioning of cancer therapeutics in nuclear condensates. Science 368 (6497), 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McSwiggen DT et al. (2019) Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Musacchio A (2022) On the role of phase separation in the biogenesis of membraneless compartments. EMBO J, e109952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang JZ et al. (2020) Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 182 (6), 1531–1544 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Honeyman JN et al. (2014) Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 343 (6174), 1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corpet A et al. (2020) PML nuclear bodies and chromatin dynamics: catch me if you can! Nucleic Acids Res 48 (21), 11890–11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kakizuka A et al. (1991) Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 66 (4), 663–74. [DOI] [PubMed] [Google Scholar]
- 105.Dyck JA et al. (1994) A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell 76 (2), 333–43. [DOI] [PubMed] [Google Scholar]
- 106.di Masi A et al. (2016) PML nuclear body disruption impairs DNA double-strand break sensing and repair in APL. Cell Death Dis 7, e2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang JY (2000) Regulation of cell death by the Abl tyrosine kinase. Oncogene 19 (49), 5643–50. [DOI] [PubMed] [Google Scholar]
- 108.Salesse S and Verfaillie CM (2002) BCR/ABL: from molecular mechanisms of leukemia induction to treatment of chronic myelogenous leukemia. Oncogene 21 (56), 8547–59. [DOI] [PubMed] [Google Scholar]
- 109.Kashiwagi S et al. (2019) Localization of BCR-ABL to Stress Granules Contributes to Its Oncogenic Function. Cell Struct Funct 44 (2), 195–204. [DOI] [PubMed] [Google Scholar]
- 110.Kar M et al. (2022) Phase separating RNA binding proteins form heterogeneous distributions of clusters in subsaturated solutions. bioRxiv, 2022.02.03.478969. [DOI] [PMC free article] [PubMed] [Google Scholar]