Abstract
Aims
Multimorbidity is common among heart failure (HF) patients and may attenuate guideline‐directed medical therapy (GDMT). Multimorbid patients are under‐represented in clinical trials; therefore, the effect of multimorbidity clustering on the prognosis of HF patients remains unknown. We evaluated the prevalence of multimorbidity clusters among consecutively registered hospitalized HF patients and assessed whether GDMT attenuated outcomes.
Methods and results
We examined 1924 hospitalized HF patients with reduced left ventricular ejection fraction (<50%) in a multicentre registry (West Tokyo HF Registry: WET‐HF). Ten comorbid conditions in the WET‐HF were abstracted: coronary artery disease, atrial fibrillation, stroke, anaemia, chronic obstructive pulmonary disease, renal dysfunction, obesity, hypertension, dyslipidaemia, and diabetes. Patients were divided into three groups (0–2: n = 451; 3–4: n = 787; and ≥5: n = 686) based on the number of comorbid conditions. The primary composite endpoint was all‐cause mortality and HF rehospitalization. The most prevalent comorbidities were renal dysfunction (67.9%), hypertension (66.0%), and anaemia (53.8%). Increased comorbidity was associated with increased adverse outcomes [3–4: hazard ratio (HR) 1.42, 95% confidence interval (CI) 1.13–1.77, P = 0.003; ≥5: HR 2.12, 95%CI 1.69–2.65, P < 0.001; and reference: 0–2] and lower GDMT prescription rate (0–2: 69.2%; 3–4: 57.7%; and ≥5: 57.6%). GDMT was associated with decreased adverse outcomes; this association was maintained even as the comorbidity burden increased but tended to weaken (0–2: HR 0.53, 95%CI 0.35–0.78; P = 0.001; 3–4: HR 0.82, 95%CI 0.65–1.04, P = 0.095; and ≥5: HR 0.81, 95%CI 0.65–1.00, P = 0.053; P for interaction = 0.156).
Conclusions
Comorbidity clusters were prevalent and associated with poorer outcomes. GDMT remained beneficial regardless of the comorbidity burden but tended to weaken with increasing comorbidity burden. Further research is required to optimize medical care in these patients.
Keywords: Multimorbidity, Comorbidity, Heart failure, Guideline‐directed medical therapy
Introduction
Heart failure (HF) is associated with significant morbidity and mortality and poses a tremendous burden on health care systems. 1 Guideline‐directed medical therapy (GDMT) using beta‐blockers and renin‐angiotensin system (RAS) inhibitors has been established as a first‐line treatment for HF with reduced ejection fraction (HFrEF). 2 , 3 In addition, novel disease‐modifying therapies (e.g. angiotensin receptor‐neprilysin inhibitor [ARNI] and sodium‐glucose cotransporter‐2 inhibitors [SGLT2is]) have shown incremental benefits for patients well tolerated with RAS inhibitors and beta‐blockers. 1 However, these findings are based on randomized clinical trials (RCTs) that enrolled relatively homogenous patient populations without the clustering of comorbid conditions. 4
Multimorbid conditions could conceivably alter the biological response to a trial therapy and/or the risk–benefit balance. For instance, GDMT is beneficial for comorbid conditions [e.g. RAS inhibitors for hypertension and chronic kidney diseases (CKD)]. Contrastingly, up‐titration of GDMT in patients with comorbid conditions could be challenging (e.g. RAS inhibitors in advanced CKD), 5 resulting in lower efficacy, poorer safety, and the occurrence of side effects. 4 Furthermore, both European and US clinical practice guidelines emphasize the evidence‐practice gap in optimization of pharmacological treatment in HFrEF patients with comorbidities. 2 , 3 Hence, a better understanding regarding the application of GDMT in multimorbid conditions in a real‐world setting is warranted.
Large‐scale registries that mandate consecutive patient enrolment reflect real‐world experience in HF management, and they are essential for the understanding of patient characteristics, care delivery, and outcomes of patients in clinical practice, providing insights into the beneficial effect of GDMT. Accordingly, the present study utilized the West Tokyo Heart Failure (WET‐HF) registry to investigate (i) the prevalence and prognostic impact of multimorbidity clustering, and (ii) whether the coexistence of comorbidities could affect the accomplishment of GDMT as well as its beneficial prognostic effect in Japanese HFrEF patients.
Methods
Study design and sample population
The details of the WET‐HF registry have been previously described. 6 This database is a prospective, multicentre cohort registry designed for the collection of data pertaining to the clinical backgrounds and outcomes of patients hospitalized with acute HF who fulfilled the Framingham criteria for HF 7 as the primary cause of admission. Before the launch of this registry, information regarding the objective of the present study, its social significance, and an abstract were provided to the University Hospital Medical Information Network of Japan for clinical trial registration (UMIN000001171).
The patient‐level data were collected from three university hospitals and three tertiary referral hospitals within the metropolitan Tokyo area. To obtain a robust assessment of patient care and outcomes, dedicated clinical research coordinators collected baseline data and outcomes from medical records as well as through interviews with treating physicians. Data were entered into an electronic data‐capturing system with a robust data query engine and system validations for data quality; outliers in the continuous variables or unexpected values in the categorical variables were selected by established criteria, and the originating institution was notified to verify the values. Moreover, the quality of the reporting was also verified by principal investigators (YS and SK) at least once annually; periodic queries were conducted to ensure quality. Patients who refused to participate in the study or presented with concurrent HF and acute coronary syndrome were excluded from registration. The study protocol was approved by the institutional review boards at each site, and research was conducted in accordance with the Declaration of Helsinki. Written or oral informed consent was obtained from each subject before the study.
We analysed the records of 4000 consecutive patients hospitalized for HF enrolled in the WET‐HF registry between January 2006 and December 2017 (Figure 1 A ). In the present analysis, 164 patients (4.1%) with in‐hospital death and 244 patients (6.1%) without recorded follow‐up information were excluded. Of the remaining 3592 patients who were stably discharged after index hospitalization, 2053 patients had left ventricular ejection fraction (LVEF) < 50%. Altogether, 129 patients (3.2%) with missing data on comorbidities were excluded, and the remaining 1924 patients (48.1%) were analysed as the HFrEF group in this study.
Figure 1.
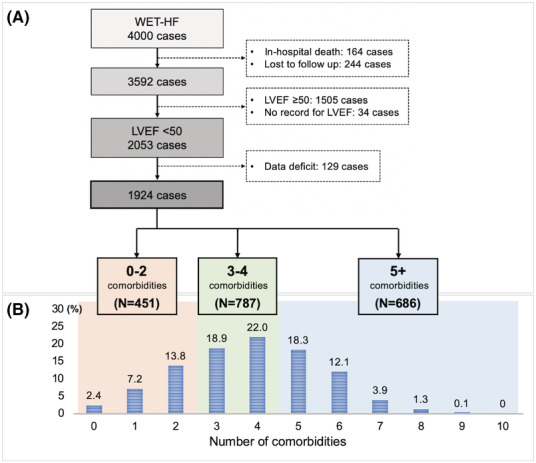
(A) Flowchart of patient selection. (B) Distribution according to the number of comorbidities. LVEF, left ventricular ejection fraction; WET‐HF, West Tokyo Heart Failure registry.
Definitions of clinical variables, comorbidities, and outcomes
As seen in Table 1 , the basic patient information retrieved included age, gender, LVEF, vital signs at discharge, body mass index (BMI) at discharge, New York Heart Association (NYHA) class at discharge, and therapeutic agents at discharge; these included beta‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, mineralocorticoid receptor antagonists (MRAs), or loop diuretics.
Table 1.
Baseline characteristics
| Variable | 0–2 comorbidities | 3–4 comorbidities | 5+ comorbidities | P value |
|---|---|---|---|---|
| n = 451 | n = 787 | n = 686 | ||
| Demographics and medical history | ||||
| Age (years) | 65 (52–76) | 75 (65–82) | 76 (68–82) | <0.001 |
| Male, n (%) | 288 (63.9) | 533 (67.7) | 517 (75.4) | <0.001 |
| Echocardiographic parameter | ||||
| LVEF (%) | 31 (23–40) | 35 (28–41) | 35 (28–41) | <0.001 |
| Comorbidities | ||||
| Obesity, n (%) | 43 (9.5) | 133 (16.9) | 173 (25.2) | <0.001 |
| Hypertension, n (%) | 133 (29.5) | 524 (66.6) | 612 (89.2) | <0.001 |
| Dyslipidaemia, n (%) | 38 (8.4) | 256 (32.5) | 511 (74.5) | <0.001 |
| Diabetes mellitus, n (%) | 45 (10.0) | 223 (28.3) | 459 (66.9) | <0.001 |
| COPD, n (%) | 2 (0.4) | 38 (4.8) | 40 (5.8) | <0.001 |
| Stroke/TIA, n (%) | 11 (2.4) | 63 (8.0) | 165 (24.1) | <0.001 |
| Anaemia, n (%) | 88 (19.5) | 436 (55.4) | 511 (74.5) | <0.001 |
| Renal dysfunction, n (%) | 149 (33.0) | 539 (68.5) | 619 (90.2) | <0.001 |
| Atrial fibrillation, n (%) | 125 (27.7) | 342 (43.5) | 332 (48.4) | <0.001 |
| Coronary artery disease, n (%) | 37 (8.2) | 230 (29.2) | 469 (68.4) | <0.001 |
| Vital signs and NYHA at discharge | ||||
| NYHA class III/IV, n (%) | 60 (13.4) | 158 (20.2) | 142 (20.8) | 0.003 |
| Systolic blood pressure (mmHg) | 102 (92–114) | 110 (100–121) | 110 (100–122) | <0.001 |
| Resting heart rate (b.p.m.) | 72 (64–82) | 70 (61–80) | 70 (62–80) | 0.078 |
| Medication or device therapy | ||||
| ACE inhibitors/ARBs, n (%) | 345 (76.5) | 516 (65.6) | 442 (64.4) | <0.001 |
| Beta‐blockers, n (%) | 396 (87.8) | 668 (84.9) | 594(86.6) | 0.331 |
| ≥50% of target dose a of beta‐blockers, n (%) | 136 (30.6) | 233 (30.2) | 203 (30.2) | 0.671 |
| GDMT, n (%) | 312 (69.2) | 454 (57.7) | 395 (57.6) | <0.001 |
| MRAs, n (%) | 216 (48.0) | 315 (40.1) | 263 (38.4) | 0.004 |
| Loop diuretics, n (%) | 334 (74.2) | 611 (77.6) | 538 (78.5) | 0.218 |
| ICD, n (%) | 47 (10.4) | 47 (6.0) | 52 (7.6) | 0.017 |
| CRT, n (%) | 25 (5.5) | 20 (2.5) | 32 (4.7) | 0.019 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; GDMT, guideline‐directed medical therapy; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; TIA, transient ischaemic attack.
Values are presented as median (interquartile range) or numbers and percentages (%). Obesity and renal dysfunction were defined as BMI ≥ 25 kg/m2 and eGFR < 60 mL/min/1.73 m2, respectively.
Proportion of patients treated with ≥50% of recommended beta‐blocker dose at discharge [Japanese Cardiology Society guideline recommendation; bisoprolol (5 mg) and carvedilol (20 mg)].
The 10 comorbid conditions included in the analysis were coronary artery disease (CAD), atrial fibrillation, stroke or transient ischaemic attack, anaemia, chronic obstructive pulmonary disease (COPD), renal dysfunction, obesity, hypertension, dyslipidaemia, and diabetes mellitus. Among the clinically relevant comorbidities in HF based on international guidelines and prior analyses (Supporting Information, Table S1 ), we selected these 10 because of their data availability within the WET‐HF registry. The definition of comorbidities is as follows. Anaemia was defined according to the World Health Organization criteria [haemoglobin (at discharge) < 13 g/dL for men and <12 g/dL for women]. 8 Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease Study equation, and renal dysfunction was defined as ≥stage 3 kidney disease as set forth by the National Kidney Foundation (eGFR at discharge <60 mL/min/1.73 m2). 9 CAD was defined as the presence of myocardial ischemia/infarction‐associated cardiac dysfunction due to significant coronary stenosis/obstruction. Obesity was defined according to the BMI cut‐off recommended by the World Health Organization Western Pacific Region (BMI ≥ 25 kg/m2), because Japanese people are more likely to have metabolic disorders even with a BMI of 25–30 kg/m2. 10 Ascertaining the remaining comorbidities was based on physician/coordinator assessment from the medical history (any past or current diagnosis and/or treatment) at the time of baseline evaluation in the WET‐HF registry.
The HFrEF cohort was divided into three groups according to the tertiles of comorbidity burden (low: 0–2 comorbidities; medium: 3–4 comorbidities; and high: ≥5 comorbidities, respectively; Figure 1 B ). Patients taking both beta‐blockers and RAS inhibitors (angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers) were assigned to the GDMT group, 11 while patients taking either beta‐blockers or RAS inhibitors (not taking both) were assigned to the non‐GDMT group. MRAs were not included in GDMT, as the prescription rate of MRAs remains relatively low (22–29%) in the majority of HF registries. 12 , 13 Also excluded were ARNI and SGLT2is, as they were not approved in Japan at the time of this study. The primary outcome was the composite endpoint of all‐cause mortality and HF rehospitalization. All‐cause mortality, cardiac mortality, non‐cardiac mortality, and HF rehospitalization were similarly analysed as the secondary outcome, respectively. All deaths were reviewed and classified into cardiac or non‐cardiac death after referring to medical records. Central committee members reviewed the abstracted records and adjudicated the mode of death. Regarding HF rehospitalization, treating physicians at each participating hospital made decisions according to the usual standard of care.
Statistical analysis
With respect to descriptive statistics, all continuous variables are expressed as median with interquartile range (variables were non‐normally distributed), and categorical variables are expressed as numbers and percentages. For baseline characteristics, the three groups, separated by different comorbidity burdens, were compared using the Kruskal–Wallis test for continuous variables and the χ 2 test for categorical variables. Time‐to‐event curves were estimated, using the Kaplan–Meier method, to examine the impact of the comorbidity burden itself on composite endpoints or each component. We also verified the prognostic impact of GDMT based on the burden of comorbidities. A log‐rank test was performed to evaluate differences between those groups. The multivariate Cox proportional hazard model was used to evaluate the impact of multiple comorbidities on each endpoint among the patients with LVEF < 50%. The impact of GDMT on each endpoint was analysed separately regardless of comorbidity burden as well as with each comorbidity. Hazard ratios (HRs) and 95% confidence intervals (95% CI) were estimated, and P values were calculated from the Wald statistic. With reference to previous reports, 14 , 15 the HRs were adjusted for age, sex, NYHA class, systolic blood pressure at discharge, heart rate at discharge, LVEF, GDMT, and MRAs. We examined the evidence of differences in the estimated prognostic effect between the comorbidity groups by adding a multiplicative interaction between GDMT and comorbidity group.
We then performed sensitivity analysis to determine the impact of GDMT on each endpoint in patients with LVEF < 40%. We defined two additional exploratory subgroups of patients without high risk for clinical outcomes: (i) eGFR at discharge > 30 mL/min/1.73 m2 and (ii) Seattle Heart Failure Model (SHFM) expected 1 year survival rate > 80%. Furthermore, we analysed another subgroup of patients without applying the general exclusion criteria for clinical trials. To predict the 1 year survival rate, the SHFM scores were calculated based on the statistical model validating the use of the SHFM in Japanese patients hospitalized with HF. 16 For all statistical analyses, statistical significance was set at P < 0.05. Data analysis was performed using IBM SPSS Statistics for Mac, Version 26.0. Armonk, NY: IBM Corp.
Results
Patient characteristics
Of 1924 patients with HFrEF [median age: 74 (range: 63–81) years; male: 69.5%], 1739 patients (90.4%) had two or more comorbidities, and 35.7% had five or more comorbidities (Figure 1 B ). The three most common comorbidities were renal dysfunction, hypertension, and anaemia (67.9%, 66.0%, and 53.8%, respectively; Figure S1 ). COPD, stroke or transient ischaemic attack, and obesity were less prevalent (4.2%, 12.4%, and 18.1%, respectively). The distribution according to the number of comorbidities as well as each comorbidity were similar based on LVEF (<40 and 40–50%) (Figures S2 and S3).
The patients' characteristics according to comorbidity burden are presented in Table 1 . Patients with increased number of comorbidities were more frequently male of older age, with higher LVEF, NYHA classification, and a higher prevalence of CAD. GDMT prescription rate decreased as the number of comorbidities increased (0–2: 69.2%; 3–4: 57.7%; and ≥5: 57.6%). The prescription rate of beta‐blockers did not differ by comorbidity burden, and the GDMT prescription rate according to comorbidity burden is mainly attributable to lower prescription rates of RAS inhibitors. The prescription rate of MRAs was also lower in the group with a higher number of comorbidities.
Clinical outcomes
The median follow‐up period was 499 (range: 143–951) days. Kaplan–Meier estimates demonstrated, among the overall cohort, a higher crude rate of the composite endpoint of all‐cause mortality and HF rehospitalization; HF rehospitalization; and all‐cause, cardiac, and non‐cardiac mortality among patients with a higher number of comorbidities (Figures 2 and S4 ).
Figure 2.
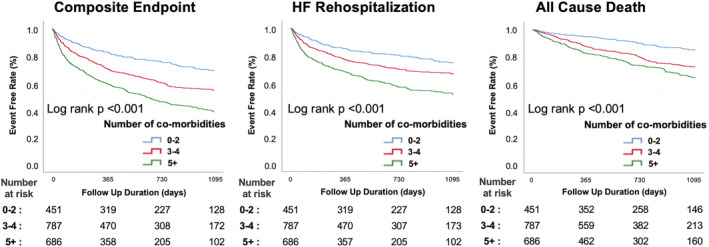
Kaplan–Meier cumulative event curves for the composite endpoint of all‐cause mortality and HF rehospitalization, HF rehospitalization, and all‐cause mortality, according to each comorbidity group. HF, heart failure.
After the adjustment using multivariate analysis, increased burden of comorbidities was an independent risk factor for the composite endpoint (3–4: HR 1.42, 95%CI 1.13–1.77, P = 0.003; ≥5: HR 2.12, 95%CI 1.69–2.65, P < 0.001, respectively, when compared with 0–2), HF rehospitalization (3–4: HR 1.31, 95%CI 1.01–1.69, P = 0.039; ≥5: HR 2.06, 95%CI 1.60–2.66, P < 0.001, respectively), and all‐cause mortality (3–4: HR 1.54, 95%CI 1.10–2.15, P = 0.012; ≥5: HR 1.97, 95%CI 1.41–2.75, P < 0.001, respectively) (Table S2). Multivariate analyses were used to evaluate the association of each comorbidity with the composite endpoint; diabetes mellitus (HR 1.25, 95%CI 1.07–1.46, P = 0.004), anaemia (HR 1.68, 95%CI 1.42–1.99, P < 0.001), renal dysfunction (HR 1.61, 95%CI 1.33–1.94, P < 0.001), atrial fibrillation (HR 1.18, 95%CI 1.02–1.37, P = 0.028), and CAD (HR 1.20, 95%CI 1.03–1.40, P = 0.021) were independent risk factors for the composite endpoint (Table S3).
Association with the implementation of guideline‐directed medical therapy
The incidence of composite endpoint, HF rehospitalization, and all‐cause mortality was significantly lower in patients with GDMT than in those without GDMT regardless of the comorbidity burdens, although the difference narrowed as the comorbidity burden increased (Figure 3 A–C ).
Figure 3.
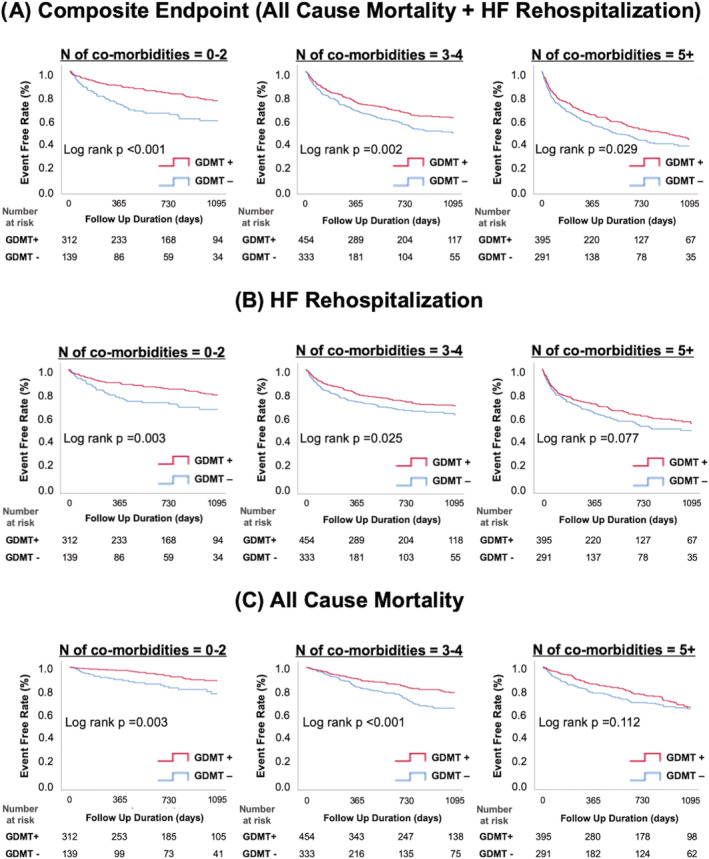
Kaplan–Meier cumulative event curves for (A) the composite endpoint of all‐cause mortality and HF rehospitalization, (B) HF rehospitalization, and (C) all‐cause mortality in each comorbidity group, divided into GDMT and non‐GDMT groups. GDMT, guideline‐directed medical therapy; HF, heart failure.
Furthermore, patients treated with GDMT were divided into groups according to the use of MRA. In all comorbidity groups, there was no significant difference in the composite endpoint regardless of MRA use (Figure S5 ).
Figure 4 shows the effect of GDMT for each subgroup assigned according to the comorbidity burden using multivariate analysis. Among the patients with fewer comorbidities (0–2 comorbidities), the use of GDMT was significantly associated with a lower rate of the composite endpoint (HR 0.53, 95%CI 0.35–0.78, P = 0.001), HF rehospitalization (HR 0.56, 95%CI 0.36–0.86, P = 0.008), and all‐cause mortality (HR 0.50, 95%CI 0.27–0.92, P = 0.025). GDMT remained beneficial regardless of the comorbidity burden in the composite endpoint (P for interaction = 0.156), HF rehospitalization (P for interaction = 0.356), or all‐cause mortality (P for interaction = 0.504). With increasing comorbidity burden, there was a trend towards a weak association between GDMT and lower composite outcomes, HF rehospitalization, and all‐cause mortality (3–4 comorbidities; HR 0.82, 95%CI 0.65–1.04, P = 0.095, HR 0.82, 95%CI 0.62–1.08, P = 0.153, HR 0.71, 95%CI 0.51–0.97, P = 0.034, respectively) (≥5 comorbidities; HR 0.81, 95%CI 0.65–1.00 P = 0.053, HR 0.82, 95%CI 0.64–1.05, P = 0.111, HR 0.78, 95%CI 0.58–1.06, P = 0.112, respectively).
Figure 4.
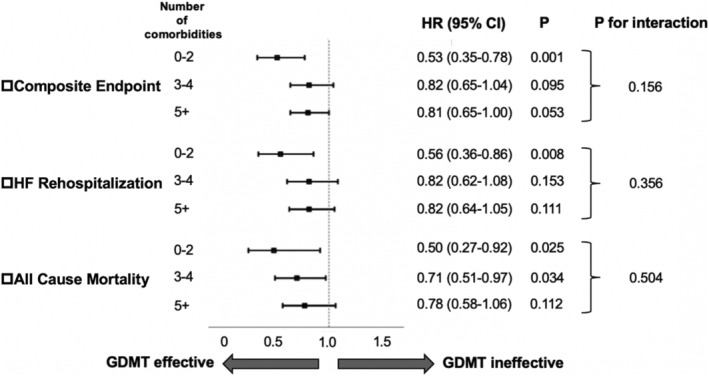
Adjusted hazard ratios for each endpoint in the subgroups with different comorbidity loads, comparing between GDMT group and non‐GDMT group. All hazard ratios were adjusted for age, sex, NYHA class, SBP, heart rate, LVEF, and MRAs. CI, confidence interval; HR, hazard ratio; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure.
Figure S6 illustrates the effect of GDMT on composite endpoints among subgroups defined according to the presence of each comorbidity using multivariate analysis. The association between GDMT and lower rate of the composite endpoint was consistent among each subgroup, except that this association was diminished in patients with anaemia.
In sensitivity analyses, the beneficial prognostic effects of GDMT were similarly maintained regardless of comorbidity burden, but there was a trend towards a weak association between GDMT and reduced adverse events with increasing comorbidity burden. Among patients with LVEF < 40% (n = 1331, Figure S7), the associations between GDMT and each outcome for each comorbidity group were similar. These associations were also similar among the selected subgroups of patients without high‐risk conditions; patients with eGFR ≥ 30 (n = 1581, Figure S8), SHFM expected 1 year survival rate ≥ 80% (n = 1824, Figure S9), and the subgroup of patients in which the general exclusion criteria for clinical trials (systolic blood pressure at discharge < 90 mmHg, heart rate at discharge < 50 b.p.m., eGFR < 30 mL/min/1.73 m2, and serum potassium level > 5.5 mEq/L) were not applied (n = 1354, Figure S10).
Discussion
The present study demonstrated that (i) the clustering of multimorbidity was common among hospitalized HFrEF patients; (ii) a greater burden of comorbidities was associated with increased composite endpoint of all‐cause mortality and HF rehospitalization, as well as lower GDMT prescription rate; and (iii) GDMT was consistently beneficial regardless of the comorbidity burden, but the association between GDMT and reduced risk of adverse events tended to weaken with increasing comorbidity burden.
Burden of comorbidity on the effect of guideline‐directed medical therapy
The impact of multiple comorbidities on the beneficial prognostic effect of GDMT has not been fully elucidated in clinical trials. There have been few post hoc analyses conducted with RCT data. 14 , 15 In the Study of Left Ventricular Dysfunction prevention and Study of Left Ventricular Dysfunction treatment trials, multiple comorbidities did not impact the effects of enalapril. In more recent Systolic Heart failure treatment with the IF inhibitor ivabradine Trial study, the increased comorbidity burden had no apparent effect on the size of the treatment effect of ivabradine. However, in clinical trial settings, the prevalence of individual comorbidities (e.g. CKD; 26.1–40.6%) 14 , 15 , 17 was lower compared with that in our real‐world setting. Therefore, the clustering of multimorbidity was less frequently seen in clinical trial settings (≥5 comorbidities: <5%). In comparison, clustering of multimorbidity was common in our registry [90.4% (≥2 comorbidities) and 35.7% (≥5 comorbidities)] that required consecutive patient enrolment. Moreover, with an ageing population in Japan, 18 there has been a rapid increase in the comorbidity burden over the past decade among HF patients in Japan. Given these specific characteristics, we had a unique opportunity to explore the impact of clustering of multimorbidity among a wide array of HF patients using our large‐scale registry of consecutively enrolled patients. To our knowledge, the impact of multiple comorbidities on the effectiveness of guideline‐based pharmacotherapy has not been evaluated in a registry‐based dataset. In contrast to the clinical trial populations, 14 , 15 we showed that the benefit of GDMT was continually maintained, but this association tended to weaken as the comorbidity burden increased in real‐world settings. The effect of GDMT among patients with HF with each comorbidity has been discussed previously (e.g. beta‐blockers in AF and RAS inhibitors in advanced CKD). 19 , 20 The precise mechanisms involved in the decreased benefit of GDMT in patients with comorbidities remain unclear, but there are several possibilities. One plausible mechanism for the reduced beneficial effect of GDMT among patients with increased comorbidity burdens could be changes in pharmacokinetics due to comorbid conditions (e.g. disease–drug interactions such as that seen with RAS inhibitors and renal dysfunction). 21 In previous findings, use of renin–angiotensin–aldosterone system inhibitors among recently hospitalized HF patients in real‐world settings was associated with increased hyperkalaemia‐associated morbidity and mortality; this was most likely due to an renin–angiotensin–aldosterone system inhibitor‐related interaction as it can be accentuated by other medications or coexisting conditions. 22 Another possibility may be the occurrence of therapeutic competition in cases when a medication for one disease inadvertently destabilizes another; this is seen with worsening of bronchospastic lung disease upon beta‐blocker treatment for HF 23 and worsening of HF upon treatment of diabetes with pioglitazone 24 and saxagliptin. 25 Furthermore, in the context of multimorbidity, the complex interactions among diseases and their medications could inadvertently induce detrimental effects; one comorbidity could often impact other comorbidities either directly (e.g. renal anaemia) or through adverse effects of their treatment (e.g. haemorrhagic anaemia due to antithrombotic therapy in CAD, stroke, and AF) with unpredictable worsening of HF. 4
Comorbid conditions in Japanese heart failure with reduced ejection fraction patients
The prevalence on multiple comorbidities has predominantly been reported in observational studies conducted in Western countries. 26 The National Health and Nutrition Examination Survey reported that ~90% and 50% of HF patients had ≥2 and ≥5 comorbidities. 27 In another community cohort from southeastern Minnesota, ~80% and 35% of HFrEF patients had ≥2 and ≥5 comorbidities. 28 Although the frequency of clustering of multimorbidity is consistent with our study, there are differences in the distribution of each and in the individual comorbid conditions. For instance, compared with Western countries—where >60% of HFrEF patients have ischaemic aetiology 13 , 29 —in East Asia, there is a lower incidence of ischaemic cardiomyopathy (46.5% in ATTEND, 12 43.0% in KorAHF, 30 and 38.3% in our registries). In East Asian registries (including ours), the prevalence of obesity (BMI ≥ 30 kg/m2: 3.8%, mean BMI: 21.8–23.1 kg/m2) and COPD (4.2–10.7%) was less 30 , 31 compared with that in Western countries (BMI ≥ 30 kg/m2: 21–35%, mean BMI: 27.0–28.6 kg/m2) 13 , 32 (COPD: 22.0–31.9%). 13 , 29 The beneficial effect of medication could differ depending on the coexistence with obesity 33 or COPD. 34 Further studies using the multinational HF registry data will be needed to evaluate whether our findings are universal or unique to East Asia.
Perspective on future studies assessing clustering of multimorbidity
The increased number of comorbidities in HF patients has been associated with worse long‐term clinical outcomes, such as all‐cause mortality and HF hospitalization, regardless of setting (e.g. RCT/observational study and ambulatory/hospitalized patient) or consideration of LVEF classification, 13 , 14 and our study extends the notion to the Japanese patients, where both patient characteristics and distribution of comorbidities differ. 26 Notably, increased comorbidity burden was associated with well‐known prognostic factors (e.g. advanced age and increasing symptom severity), and further research is required to establish an ideal therapeutic approach for this vulnerable population. Furthermore, programmes dedicated to comorbidity prevention or reduction need to be developed. The current move towards multidisciplinary care is to develop disease management systems that span cardiology and non‐cardiology health care providers. 4 Further studies are needed to assess the efficacy of the integration of these preventive strategies into conventional HF management programmes. Elucidating whether individual comorbidities or particular clusters of comorbidities could affect the beneficial effect of GDMT could be important in future studies. Furthermore, machine learning could be useful in identifying phenogroups of HF by comorbidity with different responses to GDMT. Research involving such approaches could boost precision medicine by enabling tailored pharmacotherapy of HF based on differing comorbidities.
Study limitations
There are some limitations to this study. First, our study had an observational cohort design, and despite adjustment using various prognostic factors (e.g. age and NYHA functional class), unmeasured or unknown variables may have influenced the outcomes. Based on the data from this registry, we reported another group that is under‐represented in RCTs: HF patients for whom the beneficial effect of GDMT was diminished (older adults and those with advanced renal diseases). 11 , 21 The causality could not be demonstrated due to the nature of our study design (observational registry study). Pragmatic trials with cluster randomization, which are useful for addressing the real‐world effects of treatment due to their relaxed inclusion/exclusion criteria, may identify whether GDMT improves prognosis in HF patients in these groups that are under‐represented by RCTs. Secondary, standardized metrics of multiple comorbidities are still being refined, and the comorbidities in this study were extracted from the dataset of our registry; data on several comorbidities (e.g. cancer and cognitive impairment) were not taken into account. Furthermore, statistical tests with respect to the prognostic association of GDMT in each comorbidity burden subgroup were underpowered as the sample sizes were small. Third, we did not evaluate patient‐centred outcomes (e.g. quality of life and functional capacity). For HF patients with multiple comorbidities, HF may not necessarily be the most important health care concern; thus, the study may have benefited from additional patient‐centred assessments. 4 Fourth, our registry did not include patients treated with novel disease‐modifying pharmacotherapy for HF (i.e. ARNI and SGLT2is), as described previously. Fifth, our findings might not be applicable to other countries due to wide regional differences in the recommended target dose of GDMT as well as the rates of readmission and mortality in HF. Finally, we did not investigate the dose of RAS inhibitors and adverse renal events [i.e. renal replacement therapy (dialysis) and progression of CKD].
Conclusions
Multiple comorbidities were common in patients hospitalized with HFrEF in Japanese contemporary registries, and the increase in the comorbidity burden was associated with worse long‐term clinical outcomes. GDMT remained beneficial regardless of the comorbidity burden, but with increasing comorbidity burden, there was a trend towards a weak association between GDMT and reduced adverse outcomes.
Conflict of interest
Y.S. is affiliated with an endowed department that is supported by Nippon Shinyaku Co., Ltd., has received a research grant from the SECOM Science and Technology Foundation, and has received an honorarium from Otsuka Pharmaceutical Co., Ltd. S.K. has received an unrestricted research grant from the Department of Cardiology, Keio University School of Medicine, Bayer Pharmaceutical Co., Ltd., and Daiichi Sankyo Co., Ltd. S.T. has received a research grant from the Bayer Pharmaceutical Co., Ltd. The remaining authors have no conflicts of interest to disclose. There are no patents, products in development, or marketed products to declare.
Funding
The West Tokyo Heart Failure Registry was supported by a grant from the Japan Agency for Medical Research and Development (S.K. 201439013C), Grants‐in‐Aid for Scientific Research (T.Y. JPSS KAKENHI, 23591062 and 26461088; T.K. 17K09526 and 20K08408; and A.G. 21K08087), a Grant‐in‐Aid for Young Scientists (Y.S. JPSS KAKENHI, 18K15860), a Grant‐in‐Aid for Clinical Research from the Japanese Circulation Society (Y.S. 2019), a Grant‐in‐Aid from the Japanese Ministry of Health, Labor and Welfare (S.K. H29‐Refractory Disease‐034), a Health Labour Science Research Grant (S.K. 14528506), and Sakakibara Clinical Research Grant for the Promotion of Science (T.Y. 2012–2019).
Supporting information
Figure S1. Distribution of each co‐morbidity.
Figure S2. Distribution according to the number of co‐morbidities (LVEF<40 vs. 40 ≤ LVEF<50).
Figure S3. Distribution of each co‐morbidity (LVEF<40 vs. 40 ≤ LVEF<50).
Figure S4 Kaplan–Meier cumulative event curves for cardiac and non‐cardiac mortality, according to each co‐morbidity group.
Figure S5. Kaplan–Meier cumulative event curves for the composite endpoint of all‐cause mortality and HF rehospitalization in each co‐morbidity group, divided into GDMT with and without MRA, and the others.
Figure S6. Effect of each comorbidity on GDMT.
Figure S7. In a cohort restricted to patients with LVEF <40%, adjusted hazard ratios for each endpoint in subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S8. In a cohort excluding patients with eGFR <30, adjusted hazard ratios for each endpoint in the subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S9. In a cohort excluding patients with <80% of 1 year survival rate predicted by Seattle Heart Failure Model, adjusted hazard ratios for each endpoint in the subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S10. Adjusted hazard ratios for each endpoint in subgroups with different co‐morbidity burdens to compare the GDMT and non‐GDMT groups, in a cohort in which the four general exclusion criteria for clinical trials (SBP at discharge <90 mmHg; heart rate at discharge <50 bpm; eGFR <30 mL/min/1.73 m2; and serum potassium level >5.5 mEq/l) were not applied.
Table S1. Co‐morbidities of heart failure discussed in guidelines and previous papers.
Table S2. Impact of different co‐morbidity loads on each endpoint.
Table S3. The association between each comorbidity and primary outcome (1924 cases, LVEF <50%).
Takeuchi, S. , Kohno, T. , Goda, A. , Shiraishi, Y. , Kawana, M. , Saji, M. , Nagatomo, Y. , Nishihata, Y. , Takei, M. , Nakano, S. , Soejima, K. , Kohsaka, S. , Yoshikawa, T. , and West Tokyo Heart Failure Registry Investigators (2022) Multimorbidity, guideline‐directed medical therapies, and associated outcomes among hospitalized heart failure patients. ESC Heart Failure, 9: 2500–2510. 10.1002/ehf2.13954.
References
- 1. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020; 324: 488–504. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members , Document reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 4. Forman DE, Maurer MS, Boyd C, Brindis R, Salive ME, Horne FM, Bell SP, Fulmer T, Reuben DB, Zieman S, Rich MW. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018; 71: 2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group , de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen ML, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen JC, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 6. Takei M, Kohsaka S, Shiraishi Y, Goda A, Izumi Y, Yagawa M, Mizuno A, Sawano M, Inohara T, Kohno T, Fukuda K, Yoshikawa T. Effect of estimated plasma volume reduction on renal function for acute heart failure differs between patients with preserved and reduced ejection fraction. Circ Heart Fail. 2015; 8: 527–532. [DOI] [PubMed] [Google Scholar]
- 7. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 8. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968; 405: 5–37. [PubMed] [Google Scholar]
- 9. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139: 137–147. [DOI] [PubMed] [Google Scholar]
- 10. Ota T, Takamura T, Hirai N, Kobayashi K. Preobesity in World Health Organization classification involves the metabolic syndrome in Japanese. Diabetes Care. 2002; 25: 1252–1253. [DOI] [PubMed] [Google Scholar]
- 11. Akita K, Kohno T, Kohsaka S, Shiraishi Y, Nagatomo Y, Izumi Y, Goda A, Mizuno A, Sawano M, Inohara T, Fukuda K, Yoshikawa T. Current use of guideline‐based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event‐free survival. Int J Cardiol. 2017; 235: 162–168. [DOI] [PubMed] [Google Scholar]
- 12. Kajimoto K, Minami Y, Sato N, Kasanuki H. Investigators of the acute decompensated heart failure syndromes R. Etiology of heart failure and outcomes in patients hospitalized for acute decompensated heart failure with preserved or reduced ejection fraction. Am J Cardiol. 2016; 118: 1881–1887. [DOI] [PubMed] [Google Scholar]
- 13. Iorio A, Senni M, Barbati G, Greene SJ, Poli S, Zambon E, di Nora C, Cioffi G, Tarantini L, Gavazzi A, Sinagra G, di Lenarda A. Prevalence and prognostic impact of non‐cardiac co‐morbidities in heart failure outpatients with preserved and reduced ejection fraction: a community‐based study. Eur J Heart Fail. 2018; 20: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 14. Bohm M, Robertson M, Ford I, Borer JS, Komajda M, Kindermann I, Böhm M, Maack C, Lainscak M, Swedberg K, Tavazzi L. Influence of cardiovascular and noncardiovascular co‐morbidities on outcomes and treatment effect of heart rate reduction with ivabradine in stable heart failure (from the SHIFT trial). Am J Cardiol. 2015; 116: 1890–1897. [DOI] [PubMed] [Google Scholar]
- 15. Bohm M, Pogue J, Kindermann I, Poss J, Koon T, Yusuf S. Effect of comorbidities on outcomes and angiotensin converting enzyme inhibitor effects in patients with predominantly left ventricular dysfunction and heart failure. Eur J Heart Fail. 2014; 16: 325–333. [DOI] [PubMed] [Google Scholar]
- 16. Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, Nagatomo Y, Sujino Y, Fukuoka R, Sawano M, Kohno T, Fukuda K, Anzai T, Shadman R, Dardas T, Levy WC, Yoshikawa T. Validation and recalibration of Seattle heart failure model in Japanese acute heart failure patients. J Card Fail. 2019; 25: 561–567. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 18. United Nations, Department of Economic and Social Affairs, Population Division (2019). World population prospects 2019, online edition. https://population.un.org/wpp/Publications/. Accessed 12 December 2020.
- 19. Hein AM, Scialla JJ, Edmonston D, Cooper LB, DeVore AD, Mentz RJ. Medical management of heart failure with reduced ejection fraction in patients with advanced renal disease. JACC Heart Fail. 2019; 7: 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GYH, Coats AJS, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014; 384: 2235–2243. [DOI] [PubMed] [Google Scholar]
- 21. Higuchi S, Kohsaka S, Shiraishi Y, Katsuki T, Nagatomo Y, Mizuno A, Sujino Y, Kohno T, Goda A, Yoshikawa T. Association of renin‐angiotensin system inhibitors with long‐term outcomes in patients with systolic heart failure and moderate‐to‐severe kidney function impairment. Eur J Intern Med. 2019; 62: 58–66. [DOI] [PubMed] [Google Scholar]
- 22. Sarwar CM, Papadimitriou L, Pitt B, Pina I, Zannad F, Anker SD, Piña I, Gheorghiade M, Butler J. Hyperkalemia in heart failure. J Am Coll Cardiol. 2016; 68: 1575–1589. [DOI] [PubMed] [Google Scholar]
- 23. Morales DR, Jackson C, Lipworth BJ, Donnan PT, Guthrie B. Adverse respiratory effect of acute beta‐blocker exposure in asthma: a systematic review and meta‐analysis of randomized controlled trials. Chest. 2014; 145: 779–786. [DOI] [PubMed] [Google Scholar]
- 24. Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta‐analysis of randomised clinical trials. Lancet. 2007; 370: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 25. Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013; 369: 1317–1326. [DOI] [PubMed] [Google Scholar]
- 26. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 27. Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011; 124: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chamberlain AM, St Sauver JL, Gerber Y, Manemann SM, Boyd CM, Dunlay SM, Rocca WA, Finney Rutten LJ, Jiang R, Weston SA, Roger VL. Multimorbidity in heart failure: a community perspective. Am J Med. 2015; 128: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kapoor JR, Kapoor R, Ju C, Heidenreich PA, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. JACC Heart Fail. 2016; 4: 464–472. [DOI] [PubMed] [Google Scholar]
- 30. Kang J, Park JJ, Cho YJ, Oh IY, Park HA, Lee SE, Kim MS, Cho HJ, Lee HY, Choi JO, Hwang KK, Kim KH, Yoo BS, Kang SM, Baek SH, Jeon ES, Kim JJ, Cho MC, Chae SC, Oh BH, Choi DJ. Predictors and prognostic value of worsening renal function during admission in HFpEF versus HFrEF: data from the KorAHF (Korean acute heart failure) registry. J Am Heart Assoc. 2018; 7: e007910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nochioka K, Shiba N, Kohno H, Miura M, Shimokawa H. Both high and low body mass indexes are prognostic risks in Japanese patients with chronic heart failure: implications from the CHART study. J Card Fail. 2010; 16: 880–887. [DOI] [PubMed] [Google Scholar]
- 32. Powell‐Wiley TM, Ngwa J, Kebede S, Lu D, Schulte PJ, Bhatt DL, Yancy C, Fonarow GC, Albert MA. Impact of body mass index on heart failure by race/ethnicity from the get with the guidelines‐heart failure (GWTG‐HF) registry. JACC Heart Fail. 2018; 6: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elkholey K, Papadimitriou L, Butler J, Thadani U, Stavrakis S. Effect of obesity on response to spironolactone in patients with heart failure with preserved ejection fraction. Am J Cardiol. 2021; 146: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang YL, Xiang ZJ, Yang JH, Wang WJ, Xu ZC, Xiang RL. Association of beta‐blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta‐analysis. Eur Heart J. 2020; 41: 4415–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of each co‐morbidity.
Figure S2. Distribution according to the number of co‐morbidities (LVEF<40 vs. 40 ≤ LVEF<50).
Figure S3. Distribution of each co‐morbidity (LVEF<40 vs. 40 ≤ LVEF<50).
Figure S4 Kaplan–Meier cumulative event curves for cardiac and non‐cardiac mortality, according to each co‐morbidity group.
Figure S5. Kaplan–Meier cumulative event curves for the composite endpoint of all‐cause mortality and HF rehospitalization in each co‐morbidity group, divided into GDMT with and without MRA, and the others.
Figure S6. Effect of each comorbidity on GDMT.
Figure S7. In a cohort restricted to patients with LVEF <40%, adjusted hazard ratios for each endpoint in subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S8. In a cohort excluding patients with eGFR <30, adjusted hazard ratios for each endpoint in the subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S9. In a cohort excluding patients with <80% of 1 year survival rate predicted by Seattle Heart Failure Model, adjusted hazard ratios for each endpoint in the subgroups with different co‐morbidity loads, comparing between GDMT group and non‐GDMT group.
Figure S10. Adjusted hazard ratios for each endpoint in subgroups with different co‐morbidity burdens to compare the GDMT and non‐GDMT groups, in a cohort in which the four general exclusion criteria for clinical trials (SBP at discharge <90 mmHg; heart rate at discharge <50 bpm; eGFR <30 mL/min/1.73 m2; and serum potassium level >5.5 mEq/l) were not applied.
Table S1. Co‐morbidities of heart failure discussed in guidelines and previous papers.
Table S2. Impact of different co‐morbidity loads on each endpoint.
Table S3. The association between each comorbidity and primary outcome (1924 cases, LVEF <50%).


