Abstract
Despite the high prevalence of sensory processing difficulties in children with autism spectrum disorder (ASD), little research has focused on the sex differences in sensory processing. Furthermore, there is a lack of knowledge on the female‐specific symptoms of ASD, contributing to later referral, diagnosis and intervention. In this study, we examined the sex differences in sensory processing symptoms in large cohorts of ASD children (N = 168; 26 females, 142 males) and typically developing (TD) children (N = 439; 209 females, 230 males). For this, we translated the sensory processing measure (SPM) and SPM – Preschool (SPM‐P) Home Forms to French. The SPM/SPM‐P are parent/caregiver questionnaires that assess typical behavioral responses to sensory stimuli. Overall, our results showed that the magnitude of the differences in sensory processing between males and females is larger in ASD children relative to TD children, with females showing more severe symptoms in Hearing, as well as Balance and Motion subscales. Additionally, linear discriminant analysis showed that the SPM/SPM‐P are good at discriminating TD children from ASD, children with higher accuracy rates for females than for males. These findings are discussed in light of the heterogeneity of sensory processing difficulties present in ASD. Overall, our results suggest that there seem to be female‐specific profiles in sensory processing difficulties in ASD. Implications of findings concerning sex differences in sensory processing and their potential for improving identification and diagnosis of ASD females are discussed.
Lay Summary
The present study examined sex differences in behavioral responses to sensory stimuli in children with autism spectrum disorder (ASD), and typically developing (TD) children. While there is a small trend for TD males to show more sensory processing atypicalities, female ASD children show significantly more atypical responses compared to their male counterparts. This has important implications for characterizing female autism profiles, and ultimately improving the chance for earlier detection, diagnosis and treatment.
Keywords: autism spectrum disorder (ASD), child development, sensory processing, sensory processing measure (SPM), sex differences
INTRODUCTION
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder (NDD), characterized by social communication difficulties and repetitive, stereotyped behaviors (American Psychiatric Association, 2013; M.‐C. Lai et al., 2014). ASD has an estimated prevalence rate of 1% in the general population (M.‐C. Lai et al., 2014; Lyall et al., 2017). This prevalence is higher in males in relation to females. Male to female ratios range between 4.3:1 in individuals with normal intellectual functioning and fall to 1.9:1 in ASD individuals with an Intellectual Quotient (IQ) below 70 (Fombonne, 2003). Large‐scale population‐based studies, however, have suggested that the prevalence of females in ASD cohorts may be higher, and high‐functioning females might be underdiagnosed for several reasons (M.‐C. Lai et al., 2015; Loomes et al., 2017). These include the female protective factor model (Jacquemont et al., 2014), suggesting that females diagnosed with ASD have an increased mutational burden. Related interpretations suggest that IQ has a preservation effect in high‐functioning ASD females, who develop compensation strategies such as camouflage (Bargiela et al., 2016; M.‐C. Lai et al., 2015). An additional explanation consists in the emerging notion of a female autism phenotype distinct from conventional, male‐based conceptualizations of the disorder (Bargiela et al., 2016). For instance, females with ASD are less likely to show externalizing behaviors and score lower on measures of stereotypical and repetitive behavior, being also more likely to show internalizing behaviors such as anxiety and depression (Mandy et al., 2012; Van Wijngaarden‐Cremers et al., 2014).
One area of ASD symptomatology which has suffered from the lack of studies on sex differences is the domain of sensory processing. Atypical behaviors in response to sensory stimuli are particularly prevalent in ASD, with over 90% of autistic individuals presenting an atypical sensory profile (Ben‐Sasson et al., 2019; Dellapiazza et al., 2018; Rogers & Ozonoff, 2005; Tomchek & Dunn, 2007). Sensory processing refers to the way we sense, perceive and respond to sensory stimuli present in the environment. This process is frequently referred to as a cascade that can be affected at different levels. It includes, for instance, how incoming information is detected, how it is processed and integrated in the brain and, finally, how the ensuing behavior or response is modulated (Balasco et al., 2019; Gliga et al., 2014; Macaluso & Driver, 2005; Miller et al., 2007; Thye et al., 2017). Sensory processing disorders (SPD) are diagnosed when an individual presents significant difficulties or atypicalities in detecting, modulating, interpreting or responding to sensory input (Miller et al., 2007). There are several methods for categorizing SPD. The most common is a scheme based on sensory modulation patterns: hypo‐responsiveness, which refers to delayed responses or unresponsiveness to sensory stimuli; hyper‐responsiveness, which is an exaggerated or even aversive reaction to sensory stimuli; and sensory seeking, which refers to unusual fascination with craving of sensory stimulation, often repetitive in nature (Ausderau et al., 2014; Baranek et al., 2013; Ben‐Sasson et al., 2019; Boyd et al., 2010; Miller et al., 2007; Tomchek & Dunn, 2007). One theoretical approach defines SPD as an umbrella term (Miller et al., 2007), which comprises three specific types of SPD: Sensory modulation disorder, based on modulation patterns of hyper/hypo‐responsiveness and seeking; sensory‐based motor disorder, based on praxis and postural disorders; and sensory discrimination disorder, based on sensory modalities such as visual, auditory, tactile, vestibular, proprioception and taste/smell.
A majority of the studies aiming to identify sensory subtypes based on empirical data have mostly used a sensory modulation approach (Ben‐Sasson et al., 2019; DeBoth & Reynolds, 2017). While this approach has proven to be useful in the scope of clinical evaluation and intervention (Ben‐Sasson et al., 2007; Miller Kuhaneck et al., 2010; Parham et al., 2007), sensory modulation patterns are more difficult to study in a research context. Over and under‐responses to sensory stimuli can have high levels of co‐occurrence (Ben‐Sasson et al., 2019; Dellapiazza et al., 2019), and they can also occur in different patterns across different modalities (Ausderau et al., 2014; Ausderau et al., 2016; DeBoth & Reynolds, 2017; A. E. Lane et al., 2011; A. E. Lane et al., 2014; Little et al., 2017a). For instance, modalities such as audition, taste–smell and touch seem to discriminate individuals with ASD from other clinical groups (Little et al., 2017b; McCormick et al., 2016; Osório et al., 2021; Schaaf & Lane, 2015; Tomchek & Dunn, 2007; Wiggins et al., 2009).In this context, using modality‐based approaches to the study of sensory systems could provide more information on how sensory modalities contribute to the different subtypes of sensory difficulties, and whether these are specific to one or more NDD (DeBoth & Reynolds, 2017).
Studies looking at sensory processing in ASD suggest that atypical sensory processing has cascading effects on developmental acquisitions (Baranek et al., 2018; Gliga et al., 2014; Glod et al., 2015; Ronconi et al., 2016), contributing to the emergence of a variety of clinical symptoms, such as delays in social and communication skills (Baker et al., 2008; Hilton et al., 2007; Hilton et al., 2010; Thye et al., 2017; Watson et al., 2011), maladaptive behaviors (Dellapiazza et al., 2019; Dellapiazza et al., 2018; A. E. Lane et al., 2010; Williams et al., 2018), repetitive behaviors (Boyd et al., 2010; Glod et al., 2019; Lidstone et al., 2014; Wigham et al., 2015), increased anxiety (S. A. Green & Ben‐Sasson, 2010; S. A. Green et al., 2012; S. J. Lane et al., 2012; Lidstone et al., 2014; Wigham et al., 2015) and sensorimotor coupling disorder (Mosconi et al., 2015).
Despite its prevalence and its impact on functioning, little has been said about sex‐differences in sensory processing in ASD. In a recent meta‐analysis on sensory symptoms in ASD, Ben‐Sasson et al. (2019) did not report sex as a significant moderator, likely due to the small number of ASD females included in most studies. Kumazaki et al. (2015) reported higher scores in female children on “taste, smell and touch response,” compared to their male counterparts using the childhood autism rating scale (CARS; Schopler et al., 1980). Lai and colleagues (2011) also found that female ASD adults had more lifetime sensory issues than IQ‐matched ASD males. They used the three sensory items from the Autism Diagnostic Interview–revised (ADI‐R) to create an “unusual sensory response” composite score (M.‐C. Lai et al., 2011, p. 3). The results from these studies spark an interest in further deciphering the sensory profiles of females by using more specific sensory instruments.
Assessing sensory processing is challenging due to individual heterogeneity and behavior that is often context‐dependent, which also evolves during development. In order to best capture behaviors that occur in response to sensory stimuli in various contexts, many studies have used parent or caregiver reports (Schaaf & Lane, 2015; Schauder & Bennetto, 2016). These reports have the advantage of providing easy access to information on observable behavior in response to sensory stimuli and, through the use of standardized scales, how typical the behavior is in relation to age‐matched samples from the general population (Dunn, 2014). Furthermore, caregiver reports have the ability to capture behaviors that occur over time and in different contexts, which are unlikely to appear in the time‐restricted context of a clinical evaluation or experimental study. They also allow for a quick collection of rich data with which group comparisons, factor analyses and individual‐centered statistical approaches are feasible (Uljarević et al., 2017).
The aim of this study is to study sex differences in behavioral responses to sensory stimuli in ASD children, compared to typically developing (TD) peers. For this purpose, we translated the sensory processing measure (SPM) and SPM ‐ Preschool (Miller Kuhaneck et al., 2010; Parham et al., 2007) questionnaires. Both instruments are widely used for standardized assessment of sensory symptoms (Burns et al., 2017; Yeung & Thomacos, 2020) in English‐speaking cohorts. The modality‐based structure of the SPM and SPM‐P is useful in the screening and profiling of sensory modalities that are affected across NDD.
METHOD
Participants
Two cohorts of participants were recruited (Table 1): a sample of TD children and a sample of children diagnosed with ASD. All participants were aged between 2 and 12 years 11 months. TD children were recruited from the general population via an online form. Inclusion criteria were the following: being enrolled in the regular school system and having no known NDD or learning disabilities. Children with ASD were all recruited in the Service des Troubles du Spect re de l'Autisme et apparentés at Lausanne University Hospital, Switzerland (STSA‐a). Trained psychologists and child psychiatrists established the diagnosis of ASD according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (American Psychiatric Association, 2013). This included a review of patients' medical and developmental history as well as assessment with the ADI‐R (Le Couteur et al., 2003), and the Autism Diagnosis Observation Scale‐2 (ADOS‐2; Lord et al., 2012). In both cohorts, parents or legal guardians responding to caregiver‐report questionnaires were required to be native or fluent French speakers. The study was reviewed and approved by the local Ethics committee and signed consent forms were obtained from participants or legal representatives prior to investigation.
TABLE 1.
Participant characteristics
| TD | ASD | Group differences | ||
|---|---|---|---|---|
| Mean age (SD) | Female | 6.40 (2.84) | 5.40 (2.61) | p = 0.086 |
| N | 209 | 26 | ||
| Male | 6.34 (3.05) | 5.20 (2.89) | p = 3.86e‐4 | |
| N | 230 | 142 | ||
| Total | 6.37 (2.95) | 5.23 (2.84) | p = 1.90e‐5 | |
| N | 439 | 168 | ||
| Mean NVIQ (SD) | Female | 111.47 (12.52) | 88.96 (20.27) | p = 5e‐6 |
| N | 32 | 23 | ||
| Male | 111.30 (11.94) | 87.79 (22.48) | p = 7.58e‐9 | |
| N | 37 | 125 | ||
| Total | 111.38 (12.12) | 87.97 (22.09) |
p = 1.78e‐1 4 |
|
| N | 69 | 148 | ||
| ADOS‐2 CSS: Total | Female | ‐ | 6.35 (1.85) | |
| N | 26 | |||
| Male | ‐ | 7.26 (1.81) | ||
| N | 138 | |||
| ADOS‐2 CSS: SA | Female | ‐ | 5.88 (2.03) | |
| N | 26 | |||
| Male | ‐ | 6.95 (1.99) | ||
| N | 138 | |||
| ADOS‐2 CSS: RRB | Female | ‐ | 7.27 (2.44) | |
| N | 26 | |||
| Male | ‐ | 7.70 (1.88) | ||
| N | 138 |
Measures
SPM and SPM‐P home forms
The SPM (Parham et al., 2007; Parham & Ecker, 2007) and the SPM – Preschool Home Forms (SPM‐P; Ecker & Parham, 2010; Miller Kuhaneck et al., 2010) are parent‐report questionnaires covering a range of behaviors and characteristics related to sensory processing, social participation and praxis. The SPM‐P is designed for children from 2 to 5 years old and the SPM for children from 5 to 12 years old.
SPM and SPM‐P consist of 75 items each. The SPM‐P items derive directly from those in the SPM, differing only on 18 age‐appropriate items. Ratings on each item are given on a four‐point Likert scale: 1 (Never), 2 (Sometimes), 3 (Often), and 4 (Always), according to the frequency of behaviors observed. The original instruments are norm‐referenced in English for a sample of American children and are comprised of seven normative subscales. These include the following sensory system scales: vision (VIS), hearing (HEA), touch (TOU), body awareness (BOD), and balance and motion (BAL). The last two subscales refer to internal sensory modalities –proprioception and vestibular system–, respectively. Two additional subscales – social participation (SOC) and planning and ideas (PLA) refer to higher integrative functioning –social functioning and praxis, respectively– that do not contribute to the total score. The total sensory system score (TOT) includes VIS, HEA, TOU, BOD, and BAL as well as a few additional items representing taste and smell that do not form a separate scale in the original instrument.
The publisher (Western Psychological Services [WPS]) and the author of the original English version of the questionnaires authorized the translation of the SPM and SPM‐P Home Forms. Two independent trained and bilingual psychologists translated the items of both forms into French. Both translations were then revised by two additional French native speakers, fluent in English, in order to reach semantic agreement on differing items. The final translated version was back‐translated to English by an independent translator. WPS reviewed the translation as well as back‐translation and final changes were made to the items in order to achieve the final authorized translated versions.
The age range for SPM‐P and SPM is 2–5 and 5–12 years old, respectively. The two instruments have an overlap of 1 year in administration. According to the manual's instructions, children aged between 5 years and 5 years 11 months were assessed with the SPM if they were attending school.
Nonverbal cognitive functioning
We used two different measures to assess global cognitive development (nonverbal intellectual quotient [NVIQ]) depending on the child's age and the ability to comply with the test. The Wechsler Preschool and Primary Scale of Intelligence, Fourth edition (Wechsler, 2012) and the Wechsler Intelligence Scale for Children, Fifth edition (Wechsler, 2014) were used to assess cognitive abilities in children from 2 years 6 months to 7 years 7 months, and from 6 to 16 years and 11 months, respectively. Both test batteries included verbal and non‐verbal subscales. For the purpose of this study, we used the NVIQ (M = 100; SD = 15) as the outcome measure of cognitive level. For younger children and those not being able to comply with the Wechsler scales, we used the Mullen Scales of Early Learning (MSEL; Mullen, 1995). The MSEL is a measure of cognitive ability and motor development in early childhood (from birth to 5 years 8 months). It comprises four subscales: Gross motor, Fine motor, Visual reception, Receptive language and Expressive language. For the purpose of this study, we used the Visual Reception (Standard Score; M = 100, SD = 15) as the outcome measure for global nonverbal abilities. In the ASD cohort, participants in the overlapping age ranges between instruments, the instrument most appropriate to the child's cognitive level was administered in the scope of their clinical evaluation. TD children were all assessed with the Wechsler scales.
ADOS‐2 calibrated severity scores
Participants within our sample were assessed with different ADOS‐2 modules, according to their age and language level: 27 participants (one girl) were assessed with the Toddler Module, 57 participants (11 girls) were assessed with Module 1, 33 participants (five girls) were assessed with Module 2 and 47 participants (nine girls) were assessed with Module 3. In order to compare ADOS‐2 scores across all modules, we calculated total and domain calibrated severity scores (CSS) for all participants (Esler et al., 2015; Hus et al., 2014; Lord et al., 2012).
Statistical analyses
Crohnbach's alpha was calculated to examine the internal consistency of the French versions of SPM and SPM‐P. In order to test group and sex differences in sensory processing, we used the six subscales of the SPM/ SPM‐P instruments that represent sensory processing and that contribute to the total score (VIS, HEA, TOU, TAS, BOD, and BAL). Raw scores were converted to ratios to account for the different number of items in SPM and SPM‐P questionnaires. We subsequently generated Z‐scores from the TD group (M = 0, SD = 1).
All statistical tests were performed using R 3.6.2 (R Core Team, 2013). To determine to which extent SPM sensory subscales could predict whether a child belonged to the ASD or to the TD group, we used linear discrimination analysis (LDA) (P. E. Green, 1978). LDA is one of the most widely used classification algorithms and operates through the calculation of variance of several continuous variables within and between classes (Zhao et al., 1998).
In our analysis, LDA‐estimated membership in ASD or TD group derived from observed values in the six sensory subscales of SPM: VIS, HEA, TOU, TAS, BOD, and BAL. All variables were entered together, with the prior equivalent probability of belonging to a specific group. First, scatterplot matrices were generated for all sensory subscales. We then selected 75% (n = 456) of the dataset as the training set, and 25% (n = 151) was set as test dataset. Data selection was done randomly during data sorting. Commonly used evaluation measures were used to test the classification algorithms (Jain & Huang, 2004). A true positive (TP) is an ASD child estimated by the system as being ASD, whereas a false positive (FP) indicates that an ASD child is estimated as TD by the system. True negative (TN) corresponds to those who do not have ASD correctly predicted as TD, whereas false negative (FN) indicates that the children was misdiagnosed as not having ASD. We generated 100 iterations of the LDA with random selection of training/test data. For each iteration, we computed accuracy (the sum of TP and TN divided by the sum of TP, FP, FN, and TN), specificity (the proportion of TN that are correctly identified), and sensitivity (the proportion of TP that are correctly identified). Due to the low number of ASD females in the test dataset, no statistical comparison was conducted on sensitivity scores. Subsequently, standardized canonical function coefficients were given for each sensory subscale to compare their contribution to the linear discriminant factor.
In order to account for sex differences in the severity of autistic symptoms, we analyzed the ADOS‐2 CSS using non‐parametric statistics (Mann–Whitney U test). To explore the sex differences in ASD, we performed a multivariate analysis of variance (MANCOVA) to test for interaction effects between group and sex on the six SPM/SPM‐P subscales, controlling for age. When the group main effect was significant, we conducted post‐hoc analyses. We applied FDR correction to all post‐hoc comparisons to account for multiple testing (Benjamini & Hochberg, 1995) and adjusted p‐values are reported.
RESULTS
Table 1 presents the distribution of participants according to sex, group, mean age and NVIQ. Age, sex, and socio‐economic distribution of the TD and ASD samples are described in Table S1.
As expected, group‐wise χ 2 tests showed that there were significantly more male participants in the ASD group, (χ 2 1,M/F=142/26 = 80.10, p = 3.57e‐19) with a ratio of 5.4:1. There were no significant differences in sex ratio in the TD group, (χ 2 1,M/F=230/209 = 0.1.01, p = 0.316). The TD group is slightly older than the ASD group, with a mean difference of 1.14 years. There is no significant age difference between males and females in neither the TD nor the ASD group. As expected, the ASD group had significantly lower NVIQ than the TD group, t(215) = 8.237, p = 1.78e‐14). There were no sex differences in NVIQ in neither TD, t(67) = 0.058, p = 0.954, nor ASD, t(146) = 0.320, p = 0.817.
Descriptive statistics for ADOS‐2 CSS total and domain scores can be found in Table 1. We found no differences in overall CSS scores (U = 1282.0, p = 0.063), even though there is a slight trend for boys to have higher severity scores, this being mainly driven by differences in Social Affect CSS (U = 1295.0, p = 0.040). There were no differences in Restricted and Repetitive Behaviors CSS between girls and boys (U = 1681.0, p = 0.605).
Internal consistency of the SPM and SPM‐P instruments
Table 2 reports the internal consistency estimates for each instrument separately and for the combined sample. The internal consistency of the two questionnaires was above 0.70 when the two samples were combined, showing acceptable reliability estimates (Nunnally & Bernstein, 1994). However, the BOD and BAL subscales presented lower internal consistency estimates (just below or near 0.70) in the independent samples.
TABLE 2.
SPM‐P and SPM home French form internal consistency estimates (Crohnbach's alpha)
| Scale | No. of items | TD | ASD | Total |
|---|---|---|---|---|
| SPM‐P | N = 152 | N = 88 | N = 240 | |
| Social participation (SOC) | 8 | 0.84 | 0.86 | 0.90 |
| Vision (VIS) | 11 | 0.75 | 0.79 | 0.80 |
| Hearing (HEA) | 9 | 0.78 | 0.83 | 0.81 |
| Touch (TOU) | 14 | 0.76 | 0.80 | 0.81 |
| Body awareness (BOD) | 9 | 0.66 | 0.67 | 0.72 |
| Balance and motion (BAL) | 11 | 0.68 | 0.71 | 0.75 |
| Planning and ideas (PLA) | 9 | 0.81 | 0.78 | 0.85 |
| Total sensory systems (TOT) | 58 | 0.90 | 0.92 | 0.93 |
| SPM | N = 258 | N = 60 | N = 318 | |
| Social participation (SOC) | 8 | 0.88 | 0.84 | 0.91 |
| Vision (VIS) | 11 | 0.75 | 0.80 | 0.83 |
| Hearing (HEA) | 9 | 0.83 | 0.83 | 0.86 |
| Touch (TOU) | 14 | 0.75 | 0.83 | 0.84 |
| Body awareness (BOD) | 9 | 0.76 | 0.80 | 0.82 |
| Balance and motion (BAL) | 11 | 0.68 | 0.82 | 0.81 |
| Planning and ideas (PLA) | 9 | 0.82 | 0.80 | 0.87 |
| Total sensory systems (TOT) | 58 | 0.93 | 0.95 | 0.96 |
Abbreviations: SPM, sensory processing measure; SPM‐P, SPM – Preschool; TD, typically developing.
Group differences in sensory processing
Based on their scores in the six SPM/SPM‐P sensory subscales, the LDA was able to correctly estimate the membership of the children to the ASD or the TD group with an average accuracy of 80.48% (Figure 1). The accuracy was higher in females compared to males (89.3% vs. 75.2%, p < 0.001). Specificity scores showed that LDA correctly identified TD children belonging to the TD group in 93.4% of cases (96.5% for females, 90.9% for males). However, sensitivity scores (indicating a poorer performance) were considerably lower −48.5%–, when trying to identify ASD children. Most likely, this is due to the wide range of sensory processing profiles within the ASD group (Figure 2).
FIGURE 1.
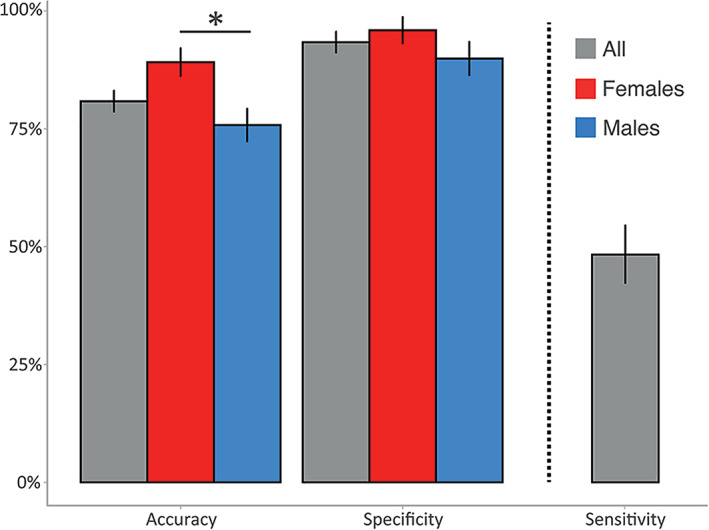
Linear discrimination analysis global accuracy and specificity for females (red), males (blue) and the combined sample (gray). Sensitivity scores were not split by sex due to the low number of autism spectrum disorder females in the testing dataset. Error bars represent the SD. *p < 0.001, after correction for multiple comparisons
FIGURE 2.
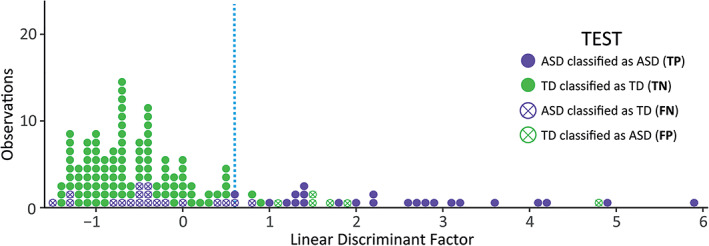
Dotplot displaying the number of individual observations (purple for autism spectrum disorder, green for typically developing) across the linear discriminant factor generated with the six sensory subscales. All dots correspond to the test subset (training subset is added in Figure S1). Individuals for which the linear discrimination analysis (LDA) wrongly estimated their membership to a group are displayed with a cross. LDA cutoff is displayed with a blue dotted line. TP, true positive; TN, true negative; FN, false negative; FP, false positive
Sex differences
The MANCOVA showed a significant main effect for group [V = 0.23, F(6, 597) = 30.252, p = 9.64e‐32]. ASD participants scored significantly higher than TD children on all SPM/SPM‐P subscales. The MANCOVA also showed a main effect for sex [V = 0.46, F(6, 597) = 4.767, p = 9.33e‐5], with girls scoring higher than boys in HEA (p = 0.012) and BAL (p = 0.025) subscales in both groups. The magnitude of this sex effect changed according to the group, as was proven by a significant interaction effect between group and sex on the SPM subscales [V = 0.29, F(6, 597) = 2.823, p = 0.010]. In line with the interaction plots (Figure 3), boys and girls in the TD group did not significantly differ on SPM/SPM‐P scores, although some subscales showed a small trend for higher scores in boys. However, in the ASD group, girls had generally higher scores, so the direction of the difference changed and the magnitude of the effect became significant in the subscales of Hearing (p = 0.001) and Balance and Motion (p = 0.035). Additionally, the Touch subscale showed a similar trend (p = 0.052). Regression analyses using the subset of participants with available NVIQ (TD = 69; ASD = 148; see Table 1) showed that NVIQ did not predict SPM/SPM‐P scores neither in the ASD group [= − 0.058, t(146) = −0.703, p = 0.483], nor in the TD group [= − 0.055, t(67) = 0.452, p = 0.653].
FIGURE 3.
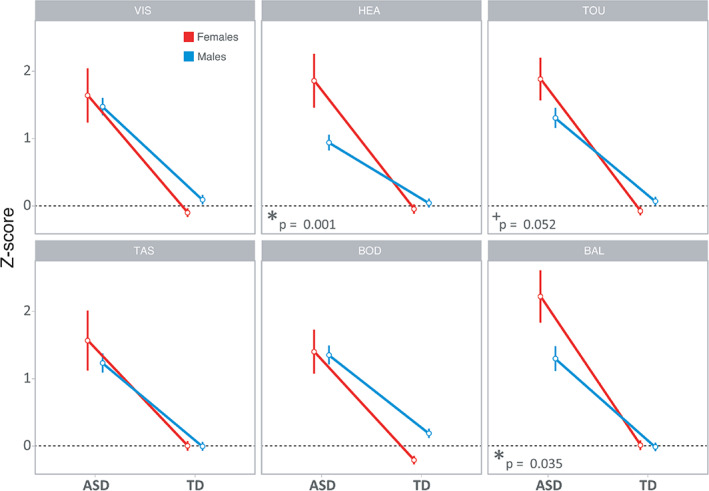
Normalized mean Z‐score of the six SPM sensory subscales separately for males (blue) and females (red) in each group. Error bars represent the standard error of the mean. *p < 0.05; +p = 0.05, after correction for multiple comparisons. SPM, sensory processing measure
DISCUSSION
In this study, we examined sex differences in behavioral responses to sensory stimuli in ASD children compared their TD counterparts. Overall, our results showed that the magnitude of the differences in sensory processing between males and females is larger in ASD children than in TD children. These differences are mainly driven by Hearing and Balance and Motion subscales, with a visible trend occurring in the Touch subscale. Including a TD sample was important, because it allowed us to account for sex differences existing in the normal population (M.‐C. Lai et al., 2015).
These results suggest that ASD females might be more likely to avoid or to be distressed by some auditory stimuli, to retreat from noisy environments or to be distracted by sounds that others do not notice. Differences in the Balance and Motion subscale suggest that females may have more difficulties with movement coordination and postural control. Previous research has been shown that ASD children have significant problems in maintaining postural control (Memari et al., 2014), with one study mentioning a small but significant gender effect (Minshew et al., 2004). Audition and postural control share a common neural pathway through the vestibulocochlear nerve (Squire et al., 2013). Additionally, auditory tasks have been shown to influence postural control (Riley et al., 2005). Further research should look into the specific and shared mechanisms underlying gender differences in Hearing and Balance in ASD children. Finally, females may have more difficulties in processing and responding to tactile stimuli, even though our results show only a slight trend. These results build on Kumazaki and colleagues' study (2015), which showed that female children (aged from 5 to 9 years) with high‐functioning ASD had significantly higher scores on CARS (Schopler et al., 1980) measures of “taste, smell and touch response” than their male counterparts.
Linear discriminant analyses conducted separately in males and females showed different accuracy rates. In the male sample, this was probably due to the overlap between TD children with higher sensory scores and ASD participants with lower sensory scores. These results are illustrative of the heterogeneity that characterizes sensory processing in ASD (DeBoth & Reynolds, 2017; Uljarević et al., 2016). It is very likely that the test sample contains ASD individuals with sensory processing scores in the lower ranges and are thus classified as TD. ASD children who do not demonstrate clinically significant or impairing sensory dysfunctions have been previously identified in mild sensory subtypes (Ausderau et al., 2016; DeBoth & Reynolds, 2017; A. E. Lane et al., 2011; A. E. Lane et al., 2014; A. E. Lane et al., 2010). In females, it seems that there is less overlapping in the higher end of the TD group and lower end of ASD, suggesting that ASD females have a more severe sensory profile, while TD females show very low levels of sensory symptoms. However, we acknowledge the need to interpret these results with caution, due to the reduced size of the female ASD sample.
The results reported in our study contribute to a growing literature that aims to better understand the female clinical phenotype in ASD. An increasing body of empirical data stresses the importance of considering the female phenotype as being distinct from traditional conceptualizations of the disorder, which is mostly male‐driven (M.‐C. Lai et al., 2015). Although within our sample, ADOS‐2 total severity scores showed no differences between girls and boys, there was a trend for boys to show slightly higher severity in the Social Affect domain. This suggests that girls may show more sensory symptoms than boys with equivalent severity scores. Sensory issues could thus be a determinant aspect of the female phenotype of autism. Additionally, sensory deficits have been documented as early as in the sixth month of life of infants diagnosed later with ASD (Baranek et al., 2013; Estes et al., 2015). Including sensory processing in female‐oriented guidelines for ASD diagnosis could lead to an earlier and more accurate recognition of ASD in girls.
In the ASD sample tested in this study, males and females did not show differences in cognitive abilities, as measured by NVIQ. The male‐to‐female ratio in this sample is 5.46:1, which is consistent with the mean ratio found by Fombonne (2003) in samples with IQ over 70. Reductions in full‐scale IQ in females with ASD has been found to mediate the severity of some autistic symptoms, such as social communication and adaptive function, but not others, such as restricted interests (Frazier et al., 2014). For this reason, we tested for the relationship between NVIQ and sensory issues in the subset of participants with available NVIQ, finding no significant association. The lack of association between sensory processing and cognitive abilities has also been shown in other previous studies (Ben‐Sasson et al., 2019).
In order to test sex differences in the sensory domain in the two cohorts, we translated and tested the French versions of the SPM and the SPM‐P. The analysis of the internal consistency of each subscale in both groups showed acceptable reliability estimates, with similar patterns to those obtained in other studies (Hansen & Jirikowic, 2013; Miller Kuhaneck et al., 2010; Parham et al., 2007), including translated versions (Ahmad et al., 2020; Hadgu & Zeleke, 2017; C. Y. Y. Lai, Chung, et al., 2011). The linear discriminant analysis showed high accuracy for the SPM and SPM‐P sensory subscales that composed the total score, suggesting that it is a good tool for discriminating individuals with clinically relevant sensory issues from those without. These results build on previous studies using the SPM, which show a high level of agreement with other similar measures in the assessment of sensory processing (Sensory Profile; Dunn, 1999; Dunn, 2014) in children with NDDs, including Fetal Alcohol Spectrum Disorders (Hansen & Jirikowic, 2013) and ASD (C. Y. Y. Lai, Chung, et al., 2011), and TD children (Brown et al., 2010). Further analyses show high specificity scores but low sensitivity, suggesting that the discriminant function is better at correctly classifying TD individuals than ASD individuals. Overall, we believe that the French versions of the SPM and the SPM‐P are reliable and useful instruments to screen for behaviorally observed sensory atypicalities in children between 2 and 12 years old, using a modality‐based approach.
Whilst the SPM and SPM‐P are undoubtedly useful in clinical settings, the measurement of sensory symptoms is restricted to observable behavior (Schauder & Bennetto, 2016; Uljarević et al., 2017). Behavioral patterns that are observed by caregivers in everyday life should be combined with laboratory‐based observation by trained clinicians, thus preventing caregiver biases that can occur due to social expectations, but also to the caregivers' characteristics, including autistic traits (Warren et al., 2012). Additionally, laboratory‐based observation allows for more refined analyses of behaviors that caregivers are not trained to identify and interpret. Assessing sensory symptoms as they are observed in everyday life is the first step in screening the modalities that are most affected in clinical groups and subgroups. Subsequently, specific modalities can be targeted in experimental studies. In complement to previous studies that have been mostly based on modulation patterns, using a modality‐based approach to assess sensory processing symptoms can provide a finer‐grained picture of the sensory profiles and subtypes of clinical cohorts (DeBoth & Reynolds, 2017). This is all the more important in both sensory processing and sex‐specific studies, with findings that suggest that similar behavioral patterns can have different neurological correlates (Schauder & Bennetto, 2016). For instance, in females with ASD, sensory over‐responsivity was strongly associated with increased connectivity in the salience network and the prefrontal cortex, while in males it was associated with increased connectivity between salience and primary sensory networks, suggesting that underlying mechanisms for sensory over‐responsivity might be sex‐specific (Cummings et al., 2020). In order to advance in our understanding of how sensory processing difficulties arise and how they impact development and functioning, self‐report and behavioral measures should be allied to neural and psychophysiological methods (Schauder & Bennetto, 2016). This approach is in line with initiatives that emphasize the need to identify fundamental traits and then study underlying genetic, neural and cognitive mechanisms across typical development and clinical conditions, such as the research domain criteria (Cuthbert & Insel, 2013).
Limitations
Our study has several limitations. First, the reduced sample of girls with ASD in comparison to the number of boys compromises the statistical power of the analyses. Even if the male to female ratio is congruent with what has been found in other studies (Fombonne, 2003), future studies with a larger female samples are needed to corroborate our results and allow for a clearer definition of the female ASD sensory profile. Additionally, the SPM is a parent/caregiver rating questionnaire, which is subject to caregiver bias, including personal characteristics, such as autistic traits (Warren et al., 2012). This is all the more relevant in the case of sex, with respondents being potentially influenced by sex‐specific stereotypes and different expectations from girls' and boys' behaviors.
Conclusion
Overall, our results show that girls and boys with ASD present different sensory processing difficulties, in comparison with what is observed in their TD counterparts. This study contributes to the literature on sex‐specific presentation of symptoms in ASD and, more specifically, adds knowledge to the female sensory phenotype. Future directions include a refining the sensory profiles of female ASD children by using larger samples and assessing the sensory subtypes that occur within these. The assessment of sensory symptoms should also be combined with psychophysical and neurobiological assessments in order to understand the underlying mechanisms. Raising awareness to the symptom profile of ASD in girls will improve caregivers' and professionals' abilities to identify autistic symptoms in girls in earlier stages of their development. This will, in turn, increase their changes of receiving an ASD diagnosis and targeted intervention.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
This work was supported by the Hoffmann Foundation, Switzerland. The authors wish to acknowledge the contribution of the psychologists and psychiatrists at the Service des Troubles du Specter de l'Autisme et apparentés (Lausanne University Hospital) in the assessment and clinical diagnosis of the ASD cohort used in this study. Open Access Funding provided by Universite de Lausanne.
Osório, J. M. A. , Rodríguez‐Herreros, B. , Richetin, S. , Junod, V. , Romascano, D. , Pittet, V. , Chabane, N. , Jequier Gygax, M. , & Maillard, A. M. (2021). Sex differences in sensory processing in children with autism spectrum disorder. Autism Research, 14(11), 2412–2423. 10.1002/aur.2580
Funding information Hoffmann Foundation, Switzerland
REFERENCES
- Ahmad, N. M. , Kadar, M. , Chai, S. C. , Mohd Rasdi, H. F. , Razab, R. , & Dzalani Harun, N. A. (2020). Adaptation, validation and reliability testing of sensory processing measure home form Malay version for children with autism. Jurnal Sains Kesihatan Malaysia, 18(01), 37–45. 10.17576/jskm-2020-1801-06 [DOI] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders: DSM‐5 (5th ed.). American Psychiatric Association. [Google Scholar]
- Ausderau, K. K. , Furlong, M. , Sideris, J. , Bulluck, J. , Little, L. M. , Watson, L. R. , Boyd, B. A. , Belger, A. , Dickie, V. A. , & Baranek, G. T. (2014). Sensory subtypes in children with autism spectrum disorder: Latent profile transition analysis using a national survey of sensory features. Journal of Child Psychology and Psychiatry, 55(8), 935–944. 10.1111/jcpp.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausderau, K. K. , Sideris, J. , Little, L. M. , Furlong, M. , Bulluck, J. C. , & Baranek, G. T. (2016). Sensory subtypes and associated outcomes in children with autism spectrum disorders. Autism Research, 9(12), 1316–1327. 10.1002/aur.1626 [DOI] [PubMed] [Google Scholar]
- Baker, A. E. , Lane, A. E. , Angley, M. T. , & Young, R. L. (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. 10.1007/s10803-007-0459-0 [DOI] [PubMed] [Google Scholar]
- Balasco, L. , Provenzano, G. , & Bozzi, Y. (2019). Sensory abnormalities in autism Spectrum disorders: A focus on the tactile domain, from genetic mouse models to the clinic. Frontiers in Psychiatry, 10, 1016. 10.3389/fpsyt.2019.01016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek, G. T. , Watson, L. R. , Boyd, B. A. , Poe, M. D. , David, F. J. , & McGuire, L. (2013). Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology, 25(2), 307–320. 10.1017/S0954579412001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek, G. T. , Woynaroski, T. G. , Nowell, S. , Turner‐Brown, L. , DuBay, M. , Crais, E. R. , & Watson, L. R. (2018). Cascading effects of attention disengagement and sensory seeking on social symptoms in a community sample of infants at‐risk for a future diagnosis of autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 30–40. 10.1016/j.dcn.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargiela, S. , Steward, R. , & Mandy, W. (2016). The experiences of late‐diagnosed women with autism Spectrum conditions: An investigation of the female autism phenotype. Journal of Autism and Developmental Disorders, 46(10), 3281–3294. 10.1007/s10803-016-2872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Ben‐Sasson, A. , Cermak, S. A. , Orsmond, G. I. , Tager‐Flusberg, H. , Carter, A. S. , Kadlec, M. B. , & Dunn, W. (2007). Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. The American Journal of Occupational Therapy, 61(5), 584–592. 10.5014/ajot.61.5.584 [DOI] [PubMed] [Google Scholar]
- Ben‐Sasson, A. , Gal, E. , Fluss, R. , Katz‐Zetler, N. , & Cermak, S. A. (2019). Update of a meta‐analysis of sensory symptoms in ASD: A new decade of research. Journal of Autism and Developmental Disorders, 49(12), 4974–4996. 10.1007/s10803-019-04180-0 [DOI] [PubMed] [Google Scholar]
- Boyd, B. A. , Baranek, G. T. , Sideris, J. , Poe, M. D. , Watson, L. R. , Patten, E. , & Miller, H. (2010). Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research, 3(2), 78–87. 10.1002/aur.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T. , Morrison, I. C. , & Stagnitti, K. (2010). The reliability of two sensory processing scales used with school‐age children: Comparing the response consistency of mothers, fathers, and classroom teachers rating the same child. Journal of Occupational Therapy, Schools, & Early Intervention, 3(4), 331–347. 10.1080/19411243.2010.541775 [DOI] [Google Scholar]
- Burns, C. O. , Dixon, D. R. , Novack, M. , & Granpeesheh, D. (2017). A systematic review of assessments for sensory processing abnormalities in autism Spectrum disorder. Review Journal of Autism and Developmental Disorders, 4(3), 209–224. 10.1007/s40489-017-0109-1 [DOI] [Google Scholar]
- Cummings, K. K. , Lawrence, K. E. , Hernandez, L. M. , Wood, E. T. , Bookheimer, S. Y. , Dapretto, M. , & Green, S. A. (2020). Sex differences in salience network connectivity and its relationship to sensory over‐responsivity in youth with autism Spectrum disorder. Autism Research, 13(9), 1489–1500. 10.1002/aur.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, B. N. , & Insel, T. R. (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11, 126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoth, K. K. , & Reynolds, S. (2017). A systematic review of sensory‐based autism subtypes. Research in Autism Spectrum Disorders, 36, 44–56. 10.1016/j.rasd.2017.01.005 [DOI] [Google Scholar]
- Dellapiazza, F. , Michelon, C. , Oreve, M. J. , Robel, L. , Schoenberger, M. , Chatel, C. , Vesperini, S. , Maffre, T. , Schmidt, R. , Blanc, N. , Vernhet, C. , Picot, M.‐C. , Baghdadli, A. , & ELENA Study Group . (2019). The impact of atypical sensory processing on adaptive functioning and maladaptive behaviors in autism Spectrum disorder during childhood: Results from the ELENA cohort. Journal of Autism and Developmental Disorders, 50, 2142–2152. 10.1007/s10803-019-03970-w [DOI] [PubMed] [Google Scholar]
- Dellapiazza, F. , Vernhet, C. , Blanc, N. , Miot, S. , Schmidt, R. , & Baghdadli, A. (2018). Links between sensory processing, adaptive behaviours, and attention in children with autism spectrum disorder: A systematic review. Psychiatry Research, 270, 78–88. 10.1016/j.psychres.2018.09.023 [DOI] [PubMed] [Google Scholar]
- Dunn, W. (1999). Sensory profile user's manual. Psychological Corporation. [Google Scholar]
- Dunn, W. (2014). The sensory profile 2: User's manual. Pearson. [Google Scholar]
- Ecker, C. , & Parham, L. D. (2010). Sensory processing measure ‐ preschool (SPM‐P) home form. Western Psychological Services. [Google Scholar]
- Esler, A. N. , Bal, V. H. , Guthrie, W. , Wetherby, A. , Ellis Weismer, S. , & Lord, C. (2015). The autism diagnostic observation schedule, toddler module: Standardized severity scores. Journal of Autism and Developmental Disorders, 45(9), 2704–2720. 10.1007/s10803-015-2432-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes, A. , Zwaigenbaum, L. , Gu, H. , St John, T. , Paterson, S. , Elison, J. T. , Hazlett, H. , Botteron, K. , Dager, S. R. , Schultz, R. T. , Kostopoulos, P. , Evans, A. , Dawson, G. , Eliason, J. , Alvarez, S. , Piven, J. , & IBIS Network . (2015). Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Journal of Neurodevelopmental Disorders, 7(1), 24. 10.1186/s11689-015-9117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne, E. (2003). Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders, 33(4), 365–382. 10.1023/A:1025054610557 [DOI] [PubMed] [Google Scholar]
- Frazier, T. W. , Georgiades, S. , Bishop, S. L. , & Hardan, A. Y. (2014). Behavioral and cognitive characteristics of females and males with autism in the Simons simplex collection. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 329‐340 e321‐323. 10.1016/j.jaac.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga, T. , Jones, E. J. , Bedford, R. , Charman, T. , & Johnson, M. H. (2014). From early markers to neuro‐developmental mechanisms of autism. Developmental Review, 34(3), 189–207. 10.1016/j.dr.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glod, M. , Riby, D. M. , Honey, E. , & Rodgers, J. (2015). Psychological correlates of sensory processing patterns in individuals with autism Spectrum disorder: A systematic review. Review Journal of Autism and Developmental Disorders, 2(2), 199–221. 10.1007/s40489-015-0047-8 [DOI] [Google Scholar]
- Glod, M. , Riby, D. M. , & Rodgers, J. (2019). Short report: Relationships between sensory processing, repetitive behaviors, anxiety, and intolerance of uncertainty in autism spectrum disorder and Williams syndrome. Autism Research, 12(5), 759–765. 10.1002/aur.2096 [DOI] [PubMed] [Google Scholar]
- Green, P. E. (1978). Analyzing multivariate data. The Dryden Press. [Google Scholar]
- Green, S. A. , & Ben‐Sasson, A. (2010). Anxiety disorders and sensory over‐responsivity in children with autism spectrum disorders: Is there a causal relationship? Journal of Autism and Developmental Disorders, 40(12), 1495–1504. 10.1007/s10803-010-1007-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, S. A. , Ben‐Sasson, A. , Soto, T. W. , & Carter, A. S. (2012). Anxiety and sensory over‐responsivity in toddlers with autism spectrum disorders: Bidirectional effects across time. Journal of Autism and Developmental Disorders, 42(6), 1112–1119. 10.1007/s10803-011-1361-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadgu, H. , & Zeleke, S. (2017). Adaptation and validation of the home and school forms of the sensory processing measure‐preschool (SPM‐P) for use in Ethiopia. The Ethiopian Journal of Education, 37(2), 103–140. [Google Scholar]
- Hansen, K. D. , & Jirikowic, T. (2013). A comparison of the sensory profile and sensory processing measure home form for children with fetal alcohol spectrum disorders. Physical & Occupational Therapy in Pediatrics, 33(4), 440–452. 10.3109/01942638.2013.791914 [DOI] [PubMed] [Google Scholar]
- Hilton, C. L. , Graver, K. , & LaVesser, P. D. (2007). Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 1(2), 164–173. 10.1016/j.rasd.2006.10.002 [DOI] [Google Scholar]
- Hilton, C. L. , Harper, J. D. , Kueker, R. H. , Lang, A. R. , Abbacchi, A. M. , Todorov, A. , & LaVesser, P. D. (2010). Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(8), 937–945. 10.1007/s10803-010-0944-8 [DOI] [PubMed] [Google Scholar]
- Hus, V. , Gotham, K. , & Lord, C. (2014). Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders, 44(10), 2400–2412. 10.1007/s10803-012-1719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont, S. , Coe, B. P. , Hersch, M. , Duyzend, M. H. , Krumm, N. , Bergmann, S. , Beckmann, J. S. , Rosenfeld, J. A. , & Eichler, E. E. (2014). A higher mutational burden in females supports a "female protective model" in neurodevelopmental disorders. American Journal of Human Genetics, 94(3), 415–425. 10.1016/j.ajhg.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, A. , & Huang, J. (2004). Integrating independent components and linear discriminant analysis for gender classification. Paper presented at the Proceedings of the Sixth IEEE International Conference on Automatic Face and Gesture Recognition (FGR'04) .
- Kumazaki, H. , Muramatsu, T. , Kosaka, H. , Fujisawa, T. X. , Iwata, K. , Tomoda, A. , Tsuchiya, K. , & Mimura, M. (2015). Sex differences in cognitive and symptom profiles in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 13‐14, 1–7. 10.1016/j.rasd.2014.12.011 [DOI] [Google Scholar]
- Lai, C. Y. Y. , Chung, J. C. C. , Chan, C. C. H. , & Li‐Tsang, C. W. P. (2011). Sensory processing measure‐HK Chinese version: Psychometric properties and pattern of response across environments. Research in Developmental Disabilities, 32(6), 2636–2643. 10.1016/j.ridd.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Lai, M.‐C. , Lombardo, M. V. , Auyeung, B. , Chakrabarti, B. , & Baron‐Cohen, S. (2015). Sex/gender differences and autism: Setting the scene for future research. Journal of the American Academy of Child and Adolescent Psychiatry, 54(1), 11–24. 10.1016/j.jaac.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M.‐C. , Lombardo, M. V. , & Baron‐Cohen, S. (2014). Autism. The Lancet, 383(9920), 896–910. 10.1016/s0140-6736(13)61539-1 [DOI] [PubMed] [Google Scholar]
- Lai, M.‐C. , Lombardo, M. V. , Pasco, G. , Ruigrok, A. N. , Wheelwright, S. J. , Sadek, S. A. , Chakrabarti, B. , MRC AIMS Consortium , & Baron‐Cohen, S. (2011). A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One, 6(6), e20835. 10.1371/journal.pone.0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, A. E. , Dennis, S. J. , & Geraghty, M. E. (2011). Brief report: Further evidence of sensory subtypes in autism. Journal of Autism and Developmental Disorders, 41(6), 826–831. 10.1007/s10803-010-1103-y [DOI] [PubMed] [Google Scholar]
- Lane, A. E. , Molloy, C. A. , & Bishop, S. L. (2014). Classification of children with autism spectrum disorder by sensory subtype: A case for sensory‐based phenotypes. Autism Research, 7(3), 322–333. 10.1002/aur.1368 [DOI] [PubMed] [Google Scholar]
- Lane, A. E. , Young, R. L. , Baker, A. E. , & Angley, M. T. (2010). Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorders, 40(1), 112–122. 10.1007/s10803-009-0840-2 [DOI] [PubMed] [Google Scholar]
- Lane, S. J. , Reynolds, S. , & Dumenci, L. (2012). Sensory overresponsivity and anxiety in typically developing children and children with autism and attention deficit hyperactivity disorder: Cause or coexistence? The American Journal of Occupational Therapy, 66(5), 595–603. 10.5014/ajot.2012.004523 [DOI] [PubMed] [Google Scholar]
- Le Couteur, A. , Lord, C. , & Rutter, M. (2003). Autism diagnostic interview‐revised (ADI‐R). Western Psychological Services. [Google Scholar]
- Lidstone, J. , Uljarević, M. , Sullivan, J. , Rodgers, J. , McConachie, H. , Freeston, M. , le Couteur, A. , Prior, M. , & Leekam, S. R. (2014). Relations among restricted and repetitive behaviors, anxiety and sensory features in children with autism spectrum disorders. Research in Autism Spectrum Disorders, 8(2), 82–92. 10.1016/j.rasd.2013.10.001 [DOI] [Google Scholar]
- Little, L. M. , Dean, E. , Tomchek, S. D. , & Dunn, W. (2017a). Classifying sensory profiles of children in the general population. Child: Care, Health and Development, 43(1), 81–88. 10.1111/cch.12391 [DOI] [PubMed] [Google Scholar]
- Little, L. M. , Dean, E. , Tomchek, S. D. , & Dunn, W. (2017b). Sensory processing patterns in autism, attention deficit hyperactivity disorder, and typical development. Physical & Occupational Therapy in Pediatrics, 38, 1–12. 10.1080/01942638.2017.1390809 [DOI] [PubMed] [Google Scholar]
- Loomes, R. , Hull, L. , & Mandy, W. P. L. (2017). What is the male‐to‐female ratio in autism Spectrum disorder? A systematic review and meta‐analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , DiLavore, P. , Risi, S. , Gotham, K. , & Bishop, S. (2012). Autism diagnostic observation schedule (2nd ed.). Western Psychological Services. [Google Scholar]
- Lyall, K. , Croen, L. , Daniels, J. , Fallin, M. D. , Ladd‐Acosta, C. , Lee, B. K. , Park, B. Y. , Snyder, N. W. , Schendel, D. , Volk, H. , Windham, G. C. , & Newschaffer, C. (2017). The changing epidemiology of autism Spectrum disorders. Annual Review of Public Health, 38, 81–102. 10.1146/annurev-publhealth-031816-044318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso, E. , & Driver, J. (2005). Multisensory spatial interactions: A window onto functional integration in the human brain. Trends in Neurosciences, 28(5), 264–271. 10.1016/j.tins.2005.03.008 [DOI] [PubMed] [Google Scholar]
- Mandy, W. , Chilvers, R. , Chowdhury, U. , Salter, G. , Seigal, A. , & Skuse, D. (2012). Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders, 42(7), 1304–1313. 10.1007/s10803-011-1356-0 [DOI] [PubMed] [Google Scholar]
- McCormick, C. , Hepburn, S. , Young, G. S. , & Rogers, S. J. (2016). Sensory symptoms in children with autism spectrum disorder, other developmental disorders and typical development: A longitudinal study. Autism, 20(5), 572–579. 10.1177/1362361315599755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memari, A. H. , Ghanouni, P. , Shayestehfar, M. , & Ghaheri, B. (2014). Postural control impairments in individuals with autism spectrum disorder: A critical review of current literature. Asian Journal of Sports Medicine, 5(3), e22963. 10.5812/asjsm.22963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Kuhaneck, H. , Ecker, C. , Parham, L. D. , Henry, D. A. , & Glennon, T. J. (2010). Sensory processing measure ‐ preschool (SPM‐P): Manual. Western Psychological Services. [Google Scholar]
- Miller, L. J. , Anzalone, M. E. , Lane, S. J. , Cermak, S. A. , & Osten, E. T. (2007). Concept evolution in sensory integration: A proposed nosology for diagnosis. The American Journal of Occupational Therapy, 61(2), 135–140. 10.5014/ajot.61.2.135 [DOI] [PubMed] [Google Scholar]
- Minshew, N. J. , Sung, K. , Jones, B. L. , & Furman, J. M. (2004). Underdevelopment of the postural control system in autism. Neurology, 63(11), 2056–2061. 10.1212/01.wnl.0000145771.98657.62 [DOI] [PubMed] [Google Scholar]
- Mosconi, M. W. , Mohanty, S. , Greene, R. K. , Cook, E. H. , Vaillancourt, D. E. , & Sweeney, J. A. (2015). Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in autism spectrum disorder. The Journal of Neuroscience, 35(5), 2015–2025. 10.1523/JNEUROSCI.2731-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen, E. M. (1995). Mullen scales of early learning. AGS Circle Pines. [Google Scholar]
- Nunnally, J. C. , & Bernstein, I. H. (1994). Psychometric theory. McGraw‐Hill, Inc. [Google Scholar]
- Osório, J. M. A. , Romascano, D. , Rodríguez‐Herreros, B. , Junod, V. , Habbeger, A. , Pain, A. , Richetin, S. , Yu, P. , Isidor, B. , van Maldergem, L. , Pons, L. , Manificat, S. , Chabane, N. , Gygax, M. J. , & Maillard, A. M. (2021). Touch and olfaction/tast differentiate children carrying a 16p11.2 deletion from children with ASD in early development. Molecular Autism, 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham, L. D. , & Ecker, C. (2007). Sensory processing measure (SPM) home form. Western Psychological Services. [Google Scholar]
- Parham, L. D. , Ecker, C. , Miller Kuhaneck, H. , Henry, D. A. , & Glennon, T. J. (2007). Sensory processing measure (SPM): Manual. Western Psychological Services. [Google Scholar]
- R Core Team . (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing. [Google Scholar]
- Riley, M. A. , Baker, A. A. , Schmit, J. M. , & Weaver, E. (2005). Effects of visual and auditory short‐term memory tasks on the spatiotemporal dynamics and variability of postural sway. Journal of Motor Behavior, 37(4), 311–324. 10.3200/JMBR.37.4.311-324 [DOI] [PubMed] [Google Scholar]
- Rogers, S. J. , & Ozonoff, S. (2005). Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, 46(12), 1255–1268. 10.1111/j.1469-7610.2005.01431.x [DOI] [PubMed] [Google Scholar]
- Ronconi, L. , Molteni, M. , & Casartelli, L. (2016). Building blocks of others' understanding: A perspective shift in investigating social‐communicative deficit in autism. Frontiers in Human Neuroscience, 10, 144. 10.3389/fnhum.2016.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf, R. C. , & Lane, A. E. (2015). Toward a best‐practice protocol for assessment of sensory features in ASD. Journal of Autism and Developmental Disorders, 45(5), 1380–1395. 10.1007/s10803-014-2299-z [DOI] [PubMed] [Google Scholar]
- Schauder, K. B. , & Bennetto, L. (2016). Toward an interdisciplinary understanding of sensory dysfunction in autism Spectrum disorder: An integration of the neural and symptom literatures. Frontiers in Neuroscience, 10, 268. 10.3389/fnins.2016.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler, E. , Reichler, R. J. , DeVellis, R. F. , & Daly, K. (1980). Toward objective classification of childhood autism: Childhood autism rating scale (CARS). Journal of Autism and Developmental Disorders, 10, 91–103. 10.1007/BF02408436 [DOI] [PubMed] [Google Scholar]
- Squire, L. R. , Berg, D. , Bloom, F. E. , Du Lac, S. , Ghosh, A. , & Spitzer, N. C. (2013). Fundamental neuroscience (4th ed.). Elsevier. [Google Scholar]
- Thye, M. D. , Bednarz, H. M. , Herringshaw, A. J. , Sartin, E. B. , & Kana, R. K. (2017). The impact of atypical sensory processing on social impairments in autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 151–167. 10.1016/j.dcn.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchek, S. D. , & Dunn, W. (2007). Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy, 61, 190–200. [DOI] [PubMed] [Google Scholar]
- Uljarević, M. , Baranek, G. T. , Vivanti, G. , Hedley, D. , Hudry, K. , & Lane, A. E. (2017). Heterogeneity of sensory features in autism spectrum disorder: Challenges and perspectives for future research. Autism Research, 10(5), 703–710. 10.1002/aur.1747 [DOI] [PubMed] [Google Scholar]
- Uljarević, M. , Lane, A. E. , Kelly, A. , & Leekam, S. (2016). Sensory subtypes and anxiety in older children and adolescents with autism spectrum disorder. Autism Research, 9(10), 1073–1078. 10.1002/aur.1602 [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden‐Cremers, P. J. , van Eeten, E. , Groen, W. B. , Van Deurzen, P. A. , Oosterling, I. J. , & Van der Gaag, R. J. (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta‐analysis. Journal of Autism and Developmental Disorders, 44(3), 627–635. 10.1007/s10803-013-1913-9 [DOI] [PubMed] [Google Scholar]
- Warren, Z. , Vehorn, A. , Dohrmann, E. , Nicholson, A. , Sutcliffe, J. S. , & Veenstra‐Vanderweele, J. (2012). Accuracy of phenotyping children with autism based on parent report: What specifically do we gain phenotyping "rapidly"? Autism Research, 5(1), 31–38. 10.1002/aur.230 [DOI] [PubMed] [Google Scholar]
- Watson, L. R. , Patten, E. , Baranek, G. T. , Poe, M. , Boyd, B. A. , Freuler, A. , & Lorenzi, J. (2011). Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research, 54(6), 1562–1576. 10.1044/1092-4388(2011/10-0029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2012). WPPSI‐IV: Echelle d'intelligence de Wechsler pour la période pré‐scolaire et primaire: Quatrième édition. ECPA, Les Editions du Centre de Psychologie Appliquée. [Google Scholar]
- Wechsler, D. (2014). WISC‐V: Echelle d'intelligence de Wechsler pour enfants et adolescents: Cinquième édition. ECPA, Les Editions du Centre de Psychologie Appliquée. [Google Scholar]
- Wiggins, L. D. , Robins, D. L. , Bakeman, R. , & Adamson, L. B. (2009). Brief report: Sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of Autism and Developmental Disorders, 39(7), 1087–1091. 10.1007/s10803-009-0711-x [DOI] [PubMed] [Google Scholar]
- Wigham, S. , Rodgers, J. , South, M. , McConachie, H. , & Freeston, M. (2015). The interplay between sensory processing abnormalities, intolerance of uncertainty, anxiety and restricted and repetitive behaviours in autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(4), 943–952. 10.1007/s10803-014-2248-x [DOI] [PubMed] [Google Scholar]
- Williams, K. L. , Kirby, A. V. , Watson, L. R. , Sideris, J. , Bulluck, J. , & Baranek, G. T. (2018). Sensory features as predictors of adaptive behaviors: A comparative longitudinal study of children with autism spectrum disorder and other developmental disabilities. Research in Developmental Disabilities, 81, 103–112. 10.1016/j.ridd.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, L. H. J. , & Thomacos, N. (2020). Assessments of sensory processing in infants and children with autism spectrum disorder between 0–12 years old: A scoping review. Research in Autism Spectrum Disorders, 72, 101517. 10.1016/j.rasd.2020.101517 [DOI] [Google Scholar]
- Zhao, W. , Chellappa, R. , & Nandhakumar, N. (1998). Empirical performance analysis of linear discriminant classifiers. Paper presented at the IEEE Conference Computer Vision Pattern Recognition .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


