Abstract
Objective
To evaluate the incidence of pulmonary metastases in the preoperative work‐up of patients with primary vulvar squamous cell carcinoma (SCC).
Design
Retrospective cohort study.
Setting
Tertiary referral centre.
Population
Patients treated for primary vulvar SCC from 2000 to 2018.
Methods
The pre‐operative chest imaging of 452 consecutively treated patients was documented with a minimal follow‐up period of 2 years.
Mean outcome measures
Incidence of pulmonary metastases, frequency of chest imaging and subsequent coincidental findings.
Results
In total, 80.8% of patients underwent pre‐operative chest imaging. Seven patients (1.9%), with a median tumour size of 80 mm, presented with pulmonary metastases. None of the patients with early stage disease and tumour size <40 mm who underwent radical local excision (RLE) with sentinel node (SN)‐procedure, was diagnosed with pulmonary metastasis. Chest imaging was performed by radiography (58.9%) and computerised tomography (CT) (41.1%). Coincidental findings were reported in 40.7% of patients who underwent CT, compared with 15.8% of patients undergoing radiography, resulting in additional diagnostics in 14.7 and 19.7% and being of limited consequence for outcome in 2.9 and 3.3%, respectively.
Conclusions
The incidence of pulmonary metastases in patients with primary vulvar SCC is extremely low, and none in patients with early stage disease undergoing the SN procedure. Chest imaging was performed in the majority of patients and was associated with frequent coincidental findings leading to clinically irrelevant diagnostic procedures. Therefore, we recommend omitting chest imaging in patients with early stage disease and tumours <40 mm, considering chest CT only in patients with large tumours and/or advanced stage disease.
Tweetable abstract
The incidence of pulmonary metastases is 1.9%, none in early stage disease planned for SN. Omitting chest imaging in this group is advised.
Keywords: Imaging, pre‐operative work‐up, pulmonary metastases, vulvar cancer
Tweetable abstract
The incidence of pulmonary metastases is 1.9%, none in early stage disease planned for SN. Omitting chest imaging in this group is advised.
Introduction
Vulvar cancer is a rare malignancy, accounting for 0.5% of all cancers in women. 1 , 2 The majority of vulvar cancers are squamous cell carcinomas (SCCs), related to lichen sclerosus or human papilloma virus (HPV)‐related high grade squamous intraepithelial lesions (HSIL). 3 Although vulvar SCC mostly affects women aged above 70 years, the incidence has increased in both the younger and the elderly population in the past decades, probably due to more prevalent HPV and overall increased life expectancy. 4
Standard treatment of vulvar SCC consists of surgery, comprising a radical local excision (RLE) of the primary tumour with either a sentinel lymph node (SLN) procedure or inguinofemoral lymphadenectomy (IFL), depending on the tumour size, focality and the suspicion of lymph node metastases (LNM).
Tumour spread in vulvar SCC can be either lymphatic or hematogenous. Whereas regional lymphatic spread to the groin is seen in 20–30% of patients, distant metastases are relatively rare, with a reported incidence of 3–8%. 5 Distant metastases are mainly observed in the lung and less frequently affect liver, bone or skin. The scarce data on pulmonary metastases estimate an incidence of 2–3%. Predictors for pulmonary metastasis are tumour size, depth of invasion (>1 mm) and nodal status. 5
The routine pre‐operative work‐up of patients with macro‐invasive vulvar SCC (depth of invasion >1 mm) includes groin evaluation by ultrasound, computerised tomography (CT) or magnetic resonance imaging (MRI). 2 To rule out pulmonary metastases, the Dutch, American and Canadian guidelines recommend a preoperative chest radiography. 6 , 7 , 8 The European Society of Gynaecological Oncology (ESGO) guideline recommends evaluation of the presence of distant metastases, but the method of choice is not specified. 9 In the case of (clinical suspicion of) nodal metastatic disease, however, a chest CT is recommended in addition to the abdominal CT for evaluation of the groin. 9
The overall sensitivity of chest radiography in detecting pulmonary metastases is reported to be around 30%, versus 75% when using CT. 10 , 11 , 12 The low sensitivity of chest radiography and low incidence of pulmonary metastases, call into question the efficacy of routine chest imaging in all patients with vulvar SCC, especially in those with early stage disease. Moreover, coincidental findings detected by routine chest imaging may lead to subsequent diagnostic procedures, that can delay primary surgical resection and increase health care costs without impact on outcome.
The objective of our study is therefore to evaluate the incidence of pulmonary metastases in the pre‐operative work‐up of patients with primary vulvar SCC.
Methods
Patients
All consecutive patients treated for primary vulvar SCC ≥FIGO stage IB between 2000 and 2018 were included in the study. Treatment was performed at the Department of Gynaecological Oncology at the Radboud University Medical Centre, Nijmegen, the Netherlands, which is a tertiary referral centre for the treatment of vulvar cancer. Data were extracted from medical files including radiology and pathology reports. Patient characteristics such as age and body mass index (BMI), as well as clinical information regarding the tumour such as location (lateral or central), focality, size and FIGO stage (according to the 2009 classification 13 ) were obtained. FIGO stage IB and II were classified as early stage disease, advanced stage disease including FIGO IIIA–IVB. Histopathological characteristics of the tumour included tumour diameter, depth of invasion and differentiation grade. In patients not undergoing surgical treatment, the clinical FIGO stage and differentiation grade of the diagnostic punch biopsy were used for analysis.
Pre‐operative work‐up
During the time frame of our study, protocol dictated performance of a routine chest radiography as recommended by the national guideline. A chest CT was advised in patients with tumours >4 cm, multifocal tumours, clinically or histologically confirmed groin metastases or abnormalities at chest radiography. 14 , 15 (Figure S1).
To analyse adherence to previously described protocol and guidelines, the performance of chest imaging (yes/no) or reason for omittance, if retrievable, was registered for each individual patient. Exclusive performance of CT of the chest was recorded, not taking into account the abdominal CT used for staging, in which only the basal lung segments are displayed.
Chest radiographies were assessed by use of basic guidelines such as Felson’s principles of chest roentgenology. 16 Pulmonary nodules at chest CT were evaluated using the British Thoracic Society guideline for pulmonary nodules. 17
Results of the chest imaging, coincidental findings and the need for additional imaging or diagnostic procedures (positron emission tomography [PET]‐CT, biopsy, etc.) were documented, including the relevance of these findings for the patient’s prognosis.
Treatment and follow up
Surgical treatment was performed as previously described, unless patients had declined groin surgery after shared decision‐making. All data was collected and combined with histopathological data (date of surgery, procedure (RLE with SN/IFL/debulking of metastatic node), number of nodes removed, number of positive nodes). In patients receiving systemic or radiation therapy, the treatment mode (chemoradiation/radiotherapy) was registered.
After surgical treatment, patients visited the outpatient clinic for routine checks every 3 months, until 24 months postoperatively. Afterwards, there were follow‐up visits one or two times a year. The occurrence of a recurrence (local/regional, with/without distant metastases) and health status (alive/deceased) at the end of a follow‐up period of 2 years from date of surgery were recorded. In patients not undergoing surgical treatment, the date of diagnosis was used for calculation of the follow‐up time.
Outcome
Primary outcome was defined as the incidence of pulmonary metastases at chest imaging, being radiography and/or CT. Secondary outcomes were defined as the frequency of chest imaging, the incidence of coincidental findings (with or without significant impact on prognosis) and the need for additional diagnostic procedures.
Statistical analysis
Data were registered using Castor EDC, a web‐based database. Patients were given a study number and data were anonymised. Analyses were performed using IBM Statistical Package for the Social Sciences (SPSS) version 25.
The incidence of pulmonary metastases and the frequency of performing chest imaging were analysed using descriptive statistics. The Chi‐square, Fisher’s Exact and Mann–Whitney U‐test were used for the non‐parametric variables. The parametric variables were evaluated using independent t‐tests. P‐values <0.05 were considered significant and all tests were two‐sided.
Results
Between 2000 and 2018, 565 patients were diagnosed and treated for primary vulvar SCC. In total, 113 patients were excluded from the study because of FIGO stage IA disease (n = 28), undocumented diagnostic work‐up performed elsewhere (n = 25), palliative resection without preoperative chest imaging (n = 24), other histology than SCC (n = 18), non‐invasive disease at definitive pathology report (n = 7), concurrent malignancy (n = 6) or insufficient retrievable data (n = 5).
Finally, a total of 452 patients were included for analysis (Figure 1). Median age at diagnosis was 71 years, and the majority (59.1%) presented with early stage disease. Baseline characteristics are depicted in Table 1.
Figure 1.
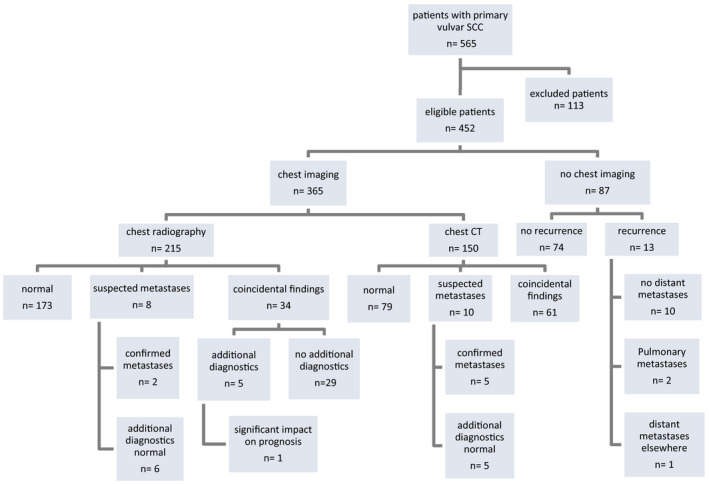
Radiological assessment of the chest and outcome, by primary mode of imaging, and follow up of patients without imaging.
Table 1.
Baseline characteristics of patients with and without chest imaging (n = 452)
|
Total (n = 452) |
Chest imaging (n = 365) |
No chest imaging (n = 87) | P‐value | |
|---|---|---|---|---|
| Age (median), years | 71 (34–95) | 72 (34–95) | 67 (38–87) | 0.151* |
| Surgical treatment (%) | ||||
| RLE | 431 (95.4%) | 344 (94.3%) | 87 (100%) | 0.020** |
| Treatment of groin | ||||
| SN | 200 (44.3%) | 150 (41.1%) | 50 (57.5%) | 0.006*** |
| LND | 194 (42.9%) | 166 (45.5%) | 28 (32.2%) | 0.034*** |
| Debulking metastatic node | 14 (3.1%) | 12 (3.3%) | 2 (2.3%) | 0.778** |
| None | 44 (9.7%) | 37 (10.1%) | 7 (8.0%) | 0.837*** |
| FIGO stage (%) | ||||
| IB | 247 (54.7%) | 180 (49.3%) | 67 (77.0%) | <0.001*** |
| II | 20 (4.4%) | 19 (5.2%) | 1 (1.2%) | 0.144** |
| IIIA | 97 (21.5%) | 85 (23.3%) | 12 (13.8%) | 0.053*** |
| IIIB | 19 (4.2%) | 17 (4.7%) | 2 (2.3%) | 0.550** |
| IIIC | 54 (11.9%) | 49 (13.4%) | 5 (5.7%) | 0.047*** |
| IVA | 6 (1.3%) | 6 (1.6%) | 0 (0.0%) | 0.601** |
| IVB | 9 (2.0%) | 9 (2.5%) | 0 (0.0%) | 0.217** |
| Focality (%) | ||||
| Unifocal | 385 (85.2%) | 308 (84.4%) | 77 (88.5%) | 0.326*** |
| Multifocal | 67 (14.8%) | 57 (15.6%) | 10 (11.5%) | |
| Tumour location (%) | ||||
| Central | 329 (72.8%) | 271 (74.2%) | 58 (66.7%) | 0.153*** |
| Lateral | 123 (27.2%) | 94 (25.8%) | 29 (33.3%) | |
| Pathology | ||||
| Tumour size (median), mm | 24.0 (0.7–130) | 25.0 (0.7–130) | 16.5 (1.0–52.0) | <0.001* |
| Depth of invasion (median), mm | 5.0 (0.9–40.0) | 5.0 (0.9–40.0) | 3.5 (1.1–35.0) | 0.001* |
| Grade (%) | ||||
| Well differentiated | 113 (25.0%) | 84 (23.0%) | 29 (33.3%) | 0.046*** |
| Moderately differentiated | 235 (52.0%) | 185 (50.7%) | 50 (57.5%) | 0.255*** |
| Poorly differentiated | 94 (20.8%) | 88 (24.1%) | 6 (6.9%) | <0.001*** |
| Unknown | 10 (2.2%) | 8 (2.2%) | 2 (2.3%) | 0.457** |
| Primary chemoradiation | 14 (3.1%) | 14 (3.8%) | 0 (0.0%) | 0.082** |
| Primary radiotherapy | 7 (1.5%) | 7 (1.9%) | 0 (0.0%) | 0.355** |
Mann–Whitney U‐test.
Fisher’s Exact test.
Chi‐square test.
Primary outcome
Pulmonary metastases
Seven patients (7/365; 1.9%) with primary vulvar SCC undergoing preoperative chest imaging presented with pulmonary metastases. In the majority of these patients (six of seven), a unifocal tumour was seen, with a median size of 80 mm (range 40–230 mm). Clinically or histologically confirmed groin metastases were found in six of seven patients (Table 2).
Table 2.
Overview of patients with lesions suspicious for pulmonary metastases at preoperative chest imaging (n = 7)
| Patient | Imaging | Histology obtained | Surgical treatment | FIGO stage | Focality | Tumour size (mm) | Depth of invasion (mm) | Groin metastasis | Follow up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CT | No | RLE, LND bilateral (palliative debulking for local control) | IVb | Unifocal | 79.0 | 14.0 | Bilateral (histology +) | Lymphangitis carcinomatosa (before start radiotherapy) 2 months later, deceased 1 month later |
| 2 | CT | No | None | IVb | Unifocal | 90.0 | Unknown | Right (physical exam + CT) | Early dementia, palliative radiotherapy. Deceased 4 months after diagnosis |
| 3 | Radiography, CT | Yes | RLE, LND bilateral (local control) | IVb | Unifocal | 40.0 | Unknown | Left (fixed nodes, histology +) | Deceased 4 months after surgical treatment |
| 4 | CT | No | None | IVb | Unifocal | 230.0 | Unknown | Bilateral (physical exam + CT) | Extensive locoregional spread and distant metastases. Deceased 1 month after diagnosis |
| 5 | Radiography, CT | No | RLE (palliative resection) | IVb | Unifocal | 100.00 | 10.0 | Right (physical exam + CT) | CT; atypical perifissural nodules and intrapulmonary lesions, probability of metastases 50%. No additional diagnostics at patient’s wish. Follow up by GP after 3 months |
| 6 | CT | No | RLE (palliative resection) | II (clinical) | Multifocal | 35.0 | 1.0 | Suspicious right (physical exam + ultrasound), but FNAC negative | CT: several perifissural nodules, probably lymph nodes with probability of metastases <25%, but poor quality images. Severe dementia, palliative resection, no additional diagnostics. Suspicion of local recurrence without chest imaging 7 months later, deceased 1 month later |
| 7 | CT | No | None | IVb | Unifocal | 80.0 | Unknown | Bilateral (physical exam + CT) | Palliative radiotherapy. Deceased 4 months later |
In only one patient was the presence of pulmonary metastases histologically confirmed; in the remaining, six diagnosis was solely based on chest imaging (Table 2).
When investigating the incidence of pulmonary metastases by treatment category, in none of the patients with early stage disease undergoing RLE with the SN procedure, was pulmonary metastases detected at pre‐operative chest imaging, compared with two in the LND group and five in the group of patients undergoing a debulking of a metastatic node or no groin surgery (Figure 2).
Figure 2.
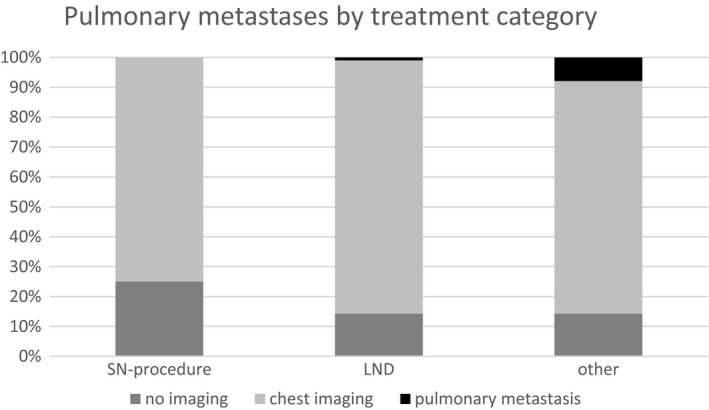
Incidence of pulmonary metastases in relation to primary surgical treatment approach (n = 452).
Secondary outcome
Chest imaging
In 365 of 452 patients (80.8%) pre‐operative radiological assessment of the chest was performed, compared with 87 of 452 patients (19.2%) in whom no chest imaging was done (Table 1, Figure 1).
Patients with chest imaging had a median age of 72 years and presented more often with advanced stage disease, compared with those patients without chest imaging. Mainly unifocal tumours with significantly larger tumour size (25.0 versus 16.5 mm) and more depth of invasion (5.0 versus 3.5 mm) were seen in the group of patients undergoing imaging.
A vast majority underwent RLE, combined with LND significantly more often than in the group of patients without chest imaging (45.5 versus 32.2%). A small portion of patients underwent primary (chemo)radiation.
A radiography was performed in 58.9% of patients (215/365) and a chest CT in 41.1% (150/365) (Figure 1). Among those patients with chest radiography, in 23 of 215 patients (10.7%) an additional chest CT was performed because of inconclusive findings or positive nodes at groin assessment.
Overall, the majority of patients with early stage disease underwent a chest radiography, whereas in patients with advanced stage disease, a CT was performed more often (data not shown).
With respect to the accuracy of chest imaging, in the group of patients primarily undergoing chest radiography, lesions suspicious for pulmonary metastases were seen in 8/215 patients (3.7%) (Figure 1). In only two of these eight patients, were pulmonary metastases confirmed, one by chest CT and one by chest CT and histology. In two of eight patients, an additional chest CT was performed which was also suspicious for pulmonary metastases, but this was eventually ruled out by histology in one patient and by PET‐CT in the other. In the four remaining patients with lesions suspicious for pulmonary metastases at chest radiography, the chest CT was normal.
In the group of patients primarily undergoing a CT of the chest, 10/150 patients (6.7%) with lesions suspicious for pulmonary metastases were identified. In 5/10, no additional diagnostics were performed because of convincing predictors of malignancy at chest CT (in 4 patients) or limited consequence for the course of treatment in a palliative setting (1 patient) (Figure 1). In 2/10 patients, pulmonary metastases were ruled out by negative histology, in 2/10 by a negative PET CT and in 1/10 by a secondary CT.
Pulmonary metastases were therefore identified in 25% of patients by chest radiography (2/8), compared with 50% of patients in which a chest CT was used (5/10).
Patients without chest imaging were younger and presented significantly more often with FIGO stage IB disease (Table 1). The reason for omitting chest imaging in 60/87 patients (69.0%) was unknown, in 21/87 (24.1%) unexpected macro‐invasive disease at pathological analysis after surgical resection (previous biopsy showing differentiated vulvar intraepithelial neoplasia [dVIN] or a micro‐invasive tumour [depth of invasion ≤1 mm]) and treatment in a palliative setting in 6/87 patients (6.9%).
When analysing the number of patients without chest imaging over time, no evident change was noted between the first and last years (Figure S2).
During the 2‐year follow up, no isolated pulmonary metastases were seen in any of the groups. In the group of patients with preoperative imaging of the chest, 85/365 patients (23.3%) developed a locoregional recurrence. In this group, six of 85 patients (7.1%) presented with pulmonary metastases and 20/85 with distant metastases elsewhere. In the group of patients without preoperative chest imaging, 13/87 (14.9%) of patients developed a locoregional recurrence, of which 2/87 (2.3%) had pulmonary metastases (Figure 1).
Coincidental findings
In the group of patients with preoperative chest radiography, coincidental findings were seen in 34/215 patients (15.8%), of which one (2.9%) might be of consequence for the patient’s overall prognosis (severe emphysema and respiratory failure). In five of 34 patients (14.7%), additional diagnostics had to be performed; in two patients a CT, in one an abdominal ultrasound, in one a bone scan and in one follow up by chest radiography (the patient was claustrophobic and therefore unable to undergo a CT). In the remaining 29/34 patients, no additional diagnostics were performed and the findings were not expected to have an impact on the patient’s prognosis (Figure 1).
Coincidental findings were seen in 61/150 patients (40.7%) with a pre‐operative chest CT. Additional diagnostic procedures were performed in 12/61 patients (19.7%); a biopsy in 4/12 patients, a PET‐CT in 6/12, endobronchial ultrasound (EBUS) in 1/12 and a repeated chest radiography in the 1/12. In 2/61 patients (3.3%), these coincidental findings were expected to be of consequence for their prognosis (a bone metastases of a previous breast carcinoma at the thoracic spine in one, and a primary lung carcinoma in the other), in 37/61 patients the impact on their prognosis was unclear, and in the remaining 22/61 patients the coincidental findings were not considered to be significant.
Discussion
Main findings
In our study of 452 patients treated for primary vulvar SCC, the value of routine preoperative chest imaging in ruling out pulmonary metastasis was limited. At retrospective analysis adherence to current guidelines was good, with performance of preoperative chest imaging in 80.8% of patients. The incidence of pulmonary metastases, however, was extremely low (1.9%) and was mainly present in patients with large tumours and positive nodes. Coincidental findings at chest imaging were frequently seen and led to additional diagnostic procedures without having an impact on outcome.
Strengths and limitations
This is the first study to provide insight into the pre‐operative work‐up of patients with vulvar SCC with respect to chest imaging and adherence to (inter)national guidelines. A large cohort of patients was analysed over a follow‐up period of 2 years, with extensive data concerning the used modes of imaging. Inherent to the retrospective character of the study, some variables could not be identified from the patient files. As the incidence of pulmonary metastases is low, subgroup analysis could not be performed, limiting the translation of these findings. Furthermore, the used imaging techniques in the early study period are probably less sensitive than the currently used techniques (radiography with subtraction and chest CT with maximum intensity projection [MIP]), which could have distorted detection rates.
Interpretation
The overall incidence of pulmonary metastases of 1.9% detected in our study, is consistent with the incidence rate described in the scarce literature on this topic. Little is known about the mechanism of development of these metastases in patients with vulvar SCC. In the analysis of patterns of distant metastases at recurrence, Prieske et al. 5 described 385 patients with vulvar SCC, none of whom had distant metastases at first presentation. In total, 20 (5.1%) developed distant metastases during follow up, nine of which pulmonary, mostly occurring after several locoregional recurrences before first diagnosis of distant metastases.
Besides the low incidence of pulmonary metastases at primary diagnosis, the sensitivity of chest radiography in detecting pulmonary metastases as reported in the literature is low. 10 , 12 This is in line with our findings that pulmonary metastases were not detected by chest radiography alone, and were merely confirmed by chest CT. Although for proper determination of the sensitivity of chest radiography, subsequent CT scans should be performed, it is unlikely that pulmonary metastases were missed in the group of chest radiography alone during the 2‐year follow up.
Aside from the accuracy of detecting pulmonary metastases, the cost‐effectiveness of routine performance of chest radiography in all patients should be taken into account. Of all patients undergoing a pre‐operative chest radiography in 14.7%, additional diagnostic procedures were performed because of inconclusive or coincidental findings at chest radiography, which in only one patient (2.9%) might have had an impact on her prognosis. Using chest CT, coincidental findings were seen in nearly half of the patients; additional diagnostics were performed in 19.7% of these patients, leading to results expected to be of consequence for the patients’ prognosis in only 3.3%. These procedures might have delayed primary treatment and led to increased healthcare costs, as described in the majority of patients for whom there was no impact on outcome.
The value of routine preoperative chest imaging has been a subject of debate in several other fields of gynaecological oncology. In endometrial cancer, the overall incidence of pulmonary metastases is also low (1.0%) and is specifically seen in high‐risk subtypes, leading to the suggestion of Amkreutz et al. 18 that the probability of detecting these metastases during diagnostic work‐up is low and that chest imaging could be omitted in patients with low‐risk endometrial cancer. In cervical cancer as well, routine pulmonary screening by chest radiography did not seem to be of value in the work‐up of early stage disease, as stated by Hoogendam et al. 19 Only in advanced stage disease imaging of the chest, is use of PET/CT advised.
As five of seven patients with pulmonary metastases in our study presented with a tumour ≥80 mm, selection of patients in whom chest imaging should be performed based on this cut‐off point would be a possibility. Two of seven patients with pulmonary metastases (28.6%), however, would then be missed. Considering that in none of the patients with early stage disease undergoing RLE with the SN procedure were pulmonary metastases present at pre‐operative chest imaging, and in none of the patients were isolated pulmonary metastases seen at the 2‐year follow up, omitting chest imaging in patients with early stage disease and tumours <40 mm seems to be a safer and more logical step. In our study, this would account for 44.3% of the cohort (200 patients), resulting in the saving of the costs of routine imaging. Performance of a validation study using a large dataset from a national cancer registry would be recommended to confirm this hypothesis.
Conclusion
In our retrospective cohort study, the incidence of pulmonary metastases is low, and there were none in patients with early stage disease undergoing an RLE with the SN procedure. A vast majority of patients underwent pre‐operative chest imaging in which substantial coincidental findings were seen, resulting in unnecessary additional diagnostic procedures. Therefore, we recommend omitting chest imaging in patients with early stage disease and tumours <40 mm. In patients with advanced stage disease or large tumours, preoperative imaging using chest CT (combined with abdominal CT for groin staging) could be considered. Validation of this hypothesis in a large cancer registry is recommended.
Disclosure of interests
None declared. Completed disclosure of interest forms are available to view online as supporting information.
Contribution to authorship
N. Pleunis; conceptualisation, planning, resources, carrying out, analysing, writing original draft. A. W. Pouwer; carrying out, resources, writing review and editing. M. J. Ploegmakers; resources, writing review and editing. J. A. de Hullu; conceptualisation, planning, resources, writing review and editing. J. M. A. Pijnenborg; conceptualsation, planning, resources, writing review and editing.
Details of ethical approval
The study was reviewed by the ethics committee of the Radboud University Medical Centre and approval was obtained (date 6 March 2017, file number 2017‐3191).
Funding
The study was not funded.
Supporting information
Figure S1. Diagnostic routing of patients with vulvar SCC ≥ FIGO‐stage IB.
Figure S2. Overview of performance of chest imaging by year of diagnosis, excluding patients with palliative treatment.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Pleunis N, Pouwer AW, Ploegmakers MJ, de Hullu JA, Pijnenborg JMA. Low incidence of pulmonary metastases in vulvar cancer patients: limited value of routine chest imaging based on a cohort study. BJOG 2022;129:769–776.
Data availability
Data are available on request from the authors.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Koh W‐J, Greer BE, Abu‐Rustum NR, Campos SM, Cho KR, Chon HS, et al. Vulvar cancer, Version 1.2017, NCCN clinical practice guidelines in oncology. JNCCN 2017;15:92–120. [DOI] [PubMed] [Google Scholar]
- 3. Pleunis N, Schuurman M, Van Rossum M, Bulten J, Massuger L, De Hullu J, et al. Rare vulvar malignancies; incidence, treatment and survival in the Netherlands. Gynecol Oncol 2016;142:440–5. [DOI] [PubMed] [Google Scholar]
- 4. Schuurman M, van den Einden L, Massuger L, Kiemeney L, van der Aa M, de Hullu J. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur J Cancer. 2013;49:3872–80. [DOI] [PubMed] [Google Scholar]
- 5. Prieske K, Haeringer N, Grimm D, Trillsch F, Eulenburg C, Burandt E, et al. Patterns of distant metastases in vulvar cancer. Gynecol Oncol 2016;142:427–34. [DOI] [PubMed] [Google Scholar]
- 6. WOG . National Guideline vulvar carcinoma. [https://www.oncoline.nl/vulvacarcinoom2011]. Accessed 2 May 2011. [Google Scholar]
- 7. American Cancer Society . Vulvar cancer ‐ Imaging tests. [https://www.cancer.org/cancer/vulvar‐cancer/detection‐diagnosis‐staging/how‐diagnosed.html2018]. Accessed 21 July 2020. [Google Scholar]
- 8. Canadian Cancer Society . Vulvar cancer – diagnosis. [https://www.cancer.ca/en/cancer‐information/cancer‐type/vulvar/diagnosis/?region=qc2019]. Accessed 1 January 2019. [Google Scholar]
- 9. Oonk M, Planchamp F, Baldwin P, Bidzinski M, Brännström M, Landoni F, et al. European Society of Gynaecological Oncology guidelines for the management of patients with vulvar cancer. Int J Gynecol Cancer. 2017;27:832–7. [DOI] [PubMed] [Google Scholar]
- 10. Canvasser NE, Stouder K, Lay AH, Gahan JC, Lotan Y, Margulis V, et al. The usefulness of chest X‐rays for T1a renal cell carcinoma surveillance. J Urol 2016;196:321–6. [DOI] [PubMed] [Google Scholar]
- 11. Arunachalam PS, Putnam G, Jennings P, Messersmith R, Robson AK. Role of computerized tomography (CT) scan of the chest in patients with newly diagnosed head and neck cancers. Clin Otolaryngol Allied Sci 2002;27:409–11. [DOI] [PubMed] [Google Scholar]
- 12. Lazzaron AR, Vieira MV, Damin DC. Should preoperative chest computed tomography be performed in all patients with colorectal cancer? Colorectal Dis 2015;17:184–90. [DOI] [PubMed] [Google Scholar]
- 13. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- 14. Missrie I, Hochhegger B, Zanon M, Capobianco J, César de Macedo Neto A, Maciel RP, et al. Small low‐risk pulmonary nodules on chest digital radiography: can we predict whether the nodule is benign? Clin Radiol 2018;73:902–6. [DOI] [PubMed] [Google Scholar]
- 15. Takashima S, Sone S, Li F, Maruyama Y, Hasegawa M, Kadoya M. Indeterminate solitary pulmonary nodules revealed at population‐based CT screening of the lung: using first follow‐up diagnostic CT to differentiate benign and malignant lesions. AJR Am J Roentgenol 2003;180:1255–63. [DOI] [PubMed] [Google Scholar]
- 16. Goodman L. Felson's Principles of Chest Roentgenology. A Programmed Text. Amsterdam: Elsevier; 2020, 288 p. [Google Scholar]
- 17. British Thoracic Society . BTS guidelines for the investigation and management of pulmonary nodules. [https://www.brit‐thoracic.org.uk/document‐library/guidelines/pulmonary‐nodules/bts‐guidelines‐for‐the‐investigation‐and‐management‐of‐pulmonary‐nodules.2015] [DOI] [PubMed] [Google Scholar]
- 18. Amkreutz LCM, Mertens HJMM, Nurseta T, Engelen MJA, Bergmans M, Nolting E, et al. The value of imaging of the lungs in the diagnostic workup of patients with endometrial cancer. Gynecol Oncol 2013;131:147–50. [DOI] [PubMed] [Google Scholar]
- 19. Hoogendam JP, Zweemer RP, Verkooijen HM, de Jong PA, van den Bosch MAAJ, Verheijen RHM, et al. No value for routine chest radiography in the work‐up of early stage cervical cancer patients. PLoS One 2015;10:e0131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Diagnostic routing of patients with vulvar SCC ≥ FIGO‐stage IB.
Figure S2. Overview of performance of chest imaging by year of diagnosis, excluding patients with palliative treatment.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Data Availability Statement
Data are available on request from the authors.


