Abstract
Objective
This study was undertaken to determine whether the vertical parasagittal approach or the lateral peri‐insular/peri‐Sylvian approach to hemispheric surgery is the superior technique in achieving long‐term seizure freedom.
Methods
We conducted a post hoc subgroup analysis of the HOPS (Hemispheric Surgery Outcome Prediction Scale) study, an international, multicenter, retrospective cohort study that identified predictors of seizure freedom through logistic regression modeling. Only patients undergoing vertical parasagittal, lateral peri‐insular/peri‐Sylvian, or lateral trans‐Sylvian hemispherotomy were included in this post hoc analysis. Differences in seizure freedom rates were assessed using a time‐to‐event method and calculated using the Kaplan–Meier survival method.
Results
Data for 672 participants across 23 centers were collected on the specific hemispherotomy approach. Of these, 72 (10.7%) underwent vertical parasagittal hemispherotomy and 600 (89.3%) underwent lateral peri‐insular/peri‐Sylvian or trans‐Sylvian hemispherotomy. Seizure freedom was obtained in 62.4% (95% confidence interval [CI] = 53.5%–70.2%) of the entire cohort at 10‐year follow‐up. Seizure freedom was 88.8% (95% CI = 78.9%–94.3%) at 1‐year follow‐up and persisted at 85.5% (95% CI = 74.7%–92.0%) across 5‐ and 10‐year follow‐up in the vertical subgroup. In contrast, seizure freedom decreased from 89.2% (95% CI = 86.3%–91.5%) at 1‐year to 72.1% (95% CI = 66.9%–76.7%) at 5‐year to 57.2% (95% CI = 46.6%–66.4%) at 10‐year follow‐up for the lateral subgroup. Log‐rank test found that vertical hemispherotomy was associated with durable seizure‐free progression compared to the lateral approach (p = .01). Patients undergoing the lateral hemispherotomy technique had a shorter time‐to‐seizure recurrence (hazard ratio = 2.56, 95% CI = 1.08–6.04, p = .03) and increased seizure recurrence odds (odds ratio = 3.67, 95% CI = 1.05–12.86, p = .04) compared to those undergoing the vertical hemispherotomy technique.
Significance
This pilot study demonstrated more durable seizure freedom of the vertical technique compared to lateral hemispherotomy techniques. Further studies, such as prospective expertise‐based observational studies or a randomized clinical trial, are required to determine whether a vertical approach to hemispheric surgery provides superior long‐term seizure outcomes.
Keywords: hemispherectomy, hemispherotomy, seizure outcomes, technique
Key Points.
Hemispherotomy is generally the first‐line approach over anatomic hemispherectomy, given concerns for perioperative complications and mortality
We performed a post hoc analysis of the HOPS study, which identified predictors of seizure freedom following hemispheric surgery
Functional hemispherotomy is safe and effective for appropriately selected children with medically intractable hemispheric epilepsy
Vertical hemispherotomy was independently associated with more durable seizure freedom than lateral peri‐insular/peri‐Sylvian or trans‐Sylvian hemispherotomy
The relationship between seizure freedom and vertical hemispherotomy was preserved after controlling for HOPS‐identified predictors of seizure freedom
1. INTRODUCTION
A subset of pediatric epilepsy patients suffer from epilepsy related to multilobar or hemispheric epileptogenic lesions. 1 Up to 40% of these patients are refractory to medical therapy, resulting in persistent disabling seizures, neurocognitive delay, and impaired quality of life. 2 Cerebral hemispherectomy, a technique involving disconnection or removal of the epileptic hemisphere, has been shown to result in excellent seizure freedom rates of 50%–80% of appropriately selected patients. 3 , 4
Since the inception of anatomic hemispherectomy 5 and refinement of Rasmussen's functional hemispherectomy, in which disconnection is performed with less cerebral tissue removal, 6 three major hemispherotomy techniques emerged in the 1990s, including the lateral peri‐Sylvian approach, 7 lateral peri‐insular approach, 8 and vertical parasagittal approach. 9 The peri‐Sylvian approach underwent a modification without opercular resection, termed the keyhole trans‐Sylvian approach. 10 In contemporary neurosurgery, hemispherotomy is generally the favorable first‐line approach, given concerns of blood loss, coagulopathy, high rates of hydrocephalus, superficial hemosiderosis, infection, and even mortality associated with anatomic hemispherectomy. 11 In addition to fewer complications, hemispherotomy may be associated with better seizure outcomes compared with anatomic hemispherctomy, 12 although a modern systematic review found no significant difference in seizure outcomes by different hemispheric procedures (i.e., anatomic or functional hemispherectomy, hemispherotomy, hemidecortication). 4
Surgical technique is an important modifiable factor that invariably impacts postoperative outcomes. Most of the modern variations of hemispherotomy may be broadly classified by the plane of approach: lateral versus vertical. Whereas the lateral approach frequently requires resection of the frontoparietal operculum, 8 the vertical approach requires resection of parasagittal cortex prior to accessing the corpus callosum and other white matter tracts for hemispheric disconnection. 9 In the largest and first notable vertical hemispherotomy series, Delalande et al. achieved 74% seizure freedom, 13 and a subsequent vertical series showed 92% seizure freedom. 14 Published lateral series are more numerous 15 , 16 , 17 , 18 , 19 and have demonstrated seizure freedom rates as high as 85% 18 to 90%. 17 A recent Italian study comparing lateral, vertical, and modified vertical approaches showed a higher rate of seizure freedom in vertical than lateral approaches (84% vs. 73%), although this did not reach statistical significance. 20 Because there are no high‐quality studies providing direct comparisons between techniques, the surgeon's general neurosurgical training and familiarity with a particular technique dictate which approach is used.
An international, collaborative multi‐institutional study recently compiled the largest cohort of patients who underwent hemispheric surgery to date for the development of the Hemispheric Surgery Outcome Prediction Scale (HOPS). 21 The purpose of the HOPS study was to identify predictors of seizure freedom following hemispheric surgeries. Although it was not designed to answer the question of which technique (i.e., lateral vs. vertical) is superior, this dataset provides the best available data thus far to compare these two techniques across multiple centers internationally. In this study, we performed a post hoc analysis of the international, multicenter retrospective study of patients undergoing hemispherectomy. 21 The goal was to determine whether the vertical parasagittal or lateral peri‐insular/peri‐Sylvian hemispherotomy technique is associated with superior rates of long‐term seizure freedom.
2. MATERIALS AND METHODS
2.1. Original study
The design and results from the multicenter, international, retrospective cohort HOPS study have been previously reported in detail; it involved 32 pediatric epilepsy centers across 12 countries. All consecutive patients with drug‐resistant epilepsy who were younger than 19 years at the time of hemispherectomy, had at least one follow‐up following the first postoperative week, and had seizure outcome data were included in the study. Surgical techniques resulting in a functional or anatomical disconnection of one half of the brain, including anatomic hemispherectomies, hemidecortications, functional hemispherectomies, peri‐insular hemispherotomies, peri‐Sylvian hemispherotomies, trans‐Sylvian hemispherotomies, and open or endoscopically assisted parasagittal vertical hemispherotomies with the preoperative goal of seizure freedom were included. For patients who underwent a second hemispheric resection, only data from the first procedure were included. Patients with a planned subtotal hemispherectomy were excluded from the study.
Demographic, patient history, perioperative testing, and surgical variables were collected in the original study following a review of the literature and consultation of expert opinion, to develop the HOPS for presurgical prediction of seizure freedom. The outcome of interest was defined as the time to seizure recurrence or length of follow‐up for patients who were seizure‐free after surgery. All contributing centers participated in accordance with local research ethics, and the organizing center received institutional review board approval for the study.
2.2. Post hoc analysis
This post hoc subgroup analysis used the patient cohort from the HOPS study to assess the comparative efficacy of the vertical hemispherotomy and the lateral hemispherotomy. Only patients who underwent a vertical parasagittal, lateral peri‐insular, lateral peri‐Sylvian, or lateral trans‐Sylvian hemispherotomy were included in the post hoc efficacy analysis (Figure 1). Since the original HOPS study, 12 participants were incorporated into this study following the acquisition of adequate follow‐up data. Vertical parasagittal operations were placed in the vertical hemispherotomy cohort, whereas lateral peri‐insular and lateral peri‐Sylvian cases constituted the lateral hemispherotomy cohort. Patients who underwent other variants of the functional hemispherotomy (e.g., Rasmussen's hemispherectomy, hemidecortication) were excluded from the study. In particular, modern modifications of Rasmussen's hemispherectomy, such as the technique described at University of California, Los Angeles, were a priori not included, as the goal of the study was to compare hemispherotomy techniques with minimal tissue resection. 15 The clinical variables constituting the HOPS score (age at seizure onset, presence of generalized seizure semiology, presence of contralateral 18‐fluoro‐2‐deoxyglucose‐positron emission tomography [FDG‐PET] hypometabolism, etiologic substrate, and previous nonhemispheric resective surgery) were collected and used to calculate the HOPS score for each patient who was included. Outcome variables of interest included occurrence of first postoperative seizure and time to first postoperative seizure, or last follow‐up if the patient experienced no postoperative seizures. Final follow‐up was the time of seizure recurrence for patients who had an event (e.g., seizure recurrence after surgery).
FIGURE 1.
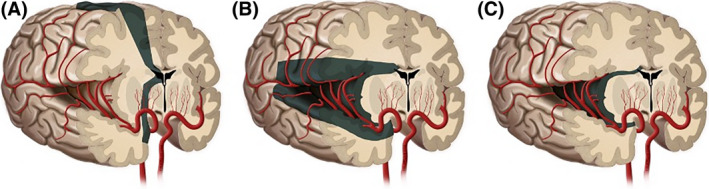
Illustration demonstrating hemispherotomy techniques. (A) Vertical parasagittal. (B) Lateral peri‐insular/peri‐Sylvian. (C) Lateral trans‐Sylvian
Baseline characteristics of the included participants were analyzed and presented using descriptive statistics. Continuous variables were presented using mean ± SD. Categorical variables were reported using frequency and percentages. Age at seizure onset was classified into three distinct categories (<3 months, 3 months–3.5 years, >3.5 years) and reported accordingly. The two surgical subgroups of interest (vertical vs. lateral) were compared for significant differences using the Mann–Whitney U‐test for continuous variables and the chi‐squared goodness of fit test for categorical variables.
Kaplan–Meier survival analysis with log‐rank test was performed to assess for differences in time to seizure recurrence between the two surgical subgroups. Multivariate mixed‐effects Cox and logistic regression models controlling for hemispherotomy technique and HOPS score were constructed, with the institution as a random‐effects variable. Missing data were addressed using the Markov chain Monte Carlo method of multiple imputation to generate 10 complete datasets for regression analysis. 22 Estimated parameters and standard errors were combined and determined by application of Rubin's rule. 23 Cox regression was performed to determine significant and independent predictors of time to first postoperative seizure, and logistic regression was done to identify covariates independently associated with seizure freedom. Only patients who had at least 1‐year follow‐up were included in the logistic regression analysis. Hazard ratios (HRs), odds ratios (ORs), p‐values, and 95% confidence intervals (CIs) were obtained for the regression analyses where appropriate. A two‐sided p‐value < .05 was used as the threshold for statistical significance in all analyses. All statistical analyses were performed in RStudio (v1.2.1335).
3. RESULTS
3.1. Baseline characteristics
Of the 1269 participants, 672 (53.0%) underwent functional hemispherotomy and had adequate outcomes data, and were thus included for further investigation (Supplementary Table S1). There were 30 functional hemispherotomy patients initially excluded for not having sufficient outcomes data and 567 patients initially excluded for undergoing procedures not exclusively disconnective, of whom 198 (34.9%) underwent anatomical hemispherectomy, whereas the remaining 352 (62.1%) and 17 (3.0%) underwent Rasmussen‐style functional hemispherectomy and hemidecortication, respectively. Functional hemispherectomy was not included, as it is an older intermediary technique between anatomical hemispherectomy, in which the cerebral hemisphere is removed, and functional hemispherotomy, in which disconnection is performed with minimal tissue removal. 5 , 6 , 7 , 8 A Kaplan–Meier plot demonstrating the seizure freedom function in all hemispherotomy patients is shown in Figure 2. The probability of seizure freedom for the hemispherotomy cohort was 89.1% (95% CI = 86.4%–91.3%), 74.4% (95% CI = 69.9%–78.4%), and 62.4% (95% CI = 53.5%–70.2%) at 1, 5, and 10 years, respectively. Within the entire hemispherotomy population, 72 (10.7%) underwent a vertical parasagittal hemispherotomy, and 600 (89.3%) underwent a lateral hemispherotomy approach. The 72 vertical parasagittal hemispherotomy patients arose from four centers, with a median of 12 (range = 1–47) cases per center. The 600 lateral hemispherotomy patients arose from 21 centers, with a median of 15 (range = 1–129) cases per center.
FIGURE 2.
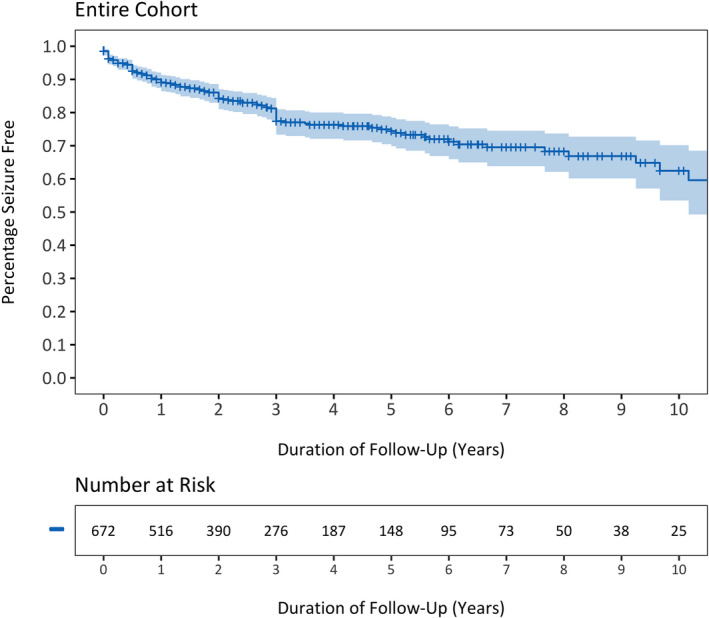
Kaplan–Meier curve depicting the seizure freedom function for the entire cohort of children undergoing lateral or vertical hemispherotomy for medically intractable epilepsy
Selected independent baseline characteristics for the two surgical subgroups of interest and their comparisons are detailed in Table 1. The mean HOPS score was 2.27 ± 1.05 and 2.35 ± 1.02, and the mean age at seizure onset was 1.89 ± 2.70 years and 2.34 ± 2.87 years, for the lateral and vertical hemispherotomy cohorts, respectively. There were no significant differences in mean HOPS score (p = .59), mean age at seizure onset (p = .17), and distribution of age at seizure onset (p = .35) between the two groups. Of the remaining predictive HOPS variables, compared to the lateral hemispherotomy group, the vertical hemispherotomy group had a significantly greater proportion of patients with a generalized seizure semiology (59.4% vs. 32.1%, p < .001) and bilateral interictal FDG‐PET hypometabolism (14.8% vs. 2.7%, p < .001). The two subgroups were otherwise comparable in their proportion of patients with a stroke etiology (p = .31) and history of nonhemispheric resective surgeries (p = .44) as well as their overall profile of specific nonstroke etiologies (p = .22). When comparing relevant non‐HOPS clinical variables, the vertical hemispherotomy group had a significantly greater proportion of patients with two or more seizure semiologies (74.5% vs. 47.4%, p < .001) and bilateral and synchronous interictal electroencephalographic (EEG) abnormalities (63.6% vs. 18.0%, p < .001). However, the proportion of patients with contralateral magnetic resonance imaging abnormalities was not significantly different (p = .92) between the two groups. Four centers contributed to the total 72 vertical procedures, of which two centers performed both vertical and lateral hemispherotomy techniques and two centers performed the vertical hemispherotomy technique exclusively.
TABLE 1.
Clinical characteristics of study population
| Characteristic | Lateral hemispherotomy, n = 600 | Vertical hemispherotomy, n = 72 | p |
|---|---|---|---|
| Centers, n | 21 | 4 | |
| HOPS score | 2.27 ± 1.05 | 2.35 ± 1.02 | .589 |
| Age at seizure onset, years | 1.89 ± 2.70 | 2.34 ± 2.87 | .171 |
| Age at seizure onset | |||
| <3 months | 187 (31.2%) | 16 (23.5%) | .345 |
| 3 months–3.5 years | 288 (48.0%) | 34 (50.0%) | |
| >3.5 years | 125 (20.8%) | 18 (26.5%) | |
| Generalized seizure semiology | |||
| Yes | 192 (32.1%) | 41 (59.4%) | <.001* |
| No | 407 (67.9%) | 28 (40.6%) | |
| Previous surgery | |||
| Yes | 92 (15.3%) | 8 (11.1%) | .438 |
| No | 508 (84.7%) | 64 (88.9%) | |
| Stroke etiology | |||
| Yes | 224 (37.5%) | 32 (44.4%) | .306 |
| No | 374 (62.5%) | 40 (55.6%) | |
| Epilepsy etiology | |||
| Porencephalic cyst/stroke | 224 (37.5%) | 32 (44.4%) | .220 |
| Malformations of cortical development | 114 (19.1%) | 14 (19.4%) | |
| Hemimegalencephaly | 92 (15.4%) | 4 (5.6%) | |
| Rasmussen's encephalitis | 67 (11.2%) | 6 (8.3%) | |
| Sturge–Weber syndrome | 26 (4.3%) | 5 (6.9%) | |
| Hemiconvulsion–hemiplegia syndrome | 7 (1.2%) | 0 (.0%) | |
| Other | 68 (11.4%) | 11 (15.3%) | |
| FDG‐PET scan | |||
| Bilateral hypometabolism | 16 (2.7%) | 9 (14.8%) | <.001* |
| Contralateral hypometabolism | 5 (.8%) | 0 (.0%) | |
| Ipsilateral hypometabolism | 244 (40.9%) | 50 (82.0%) | |
| Not scanned | 331 (55.5%) | 2 (3.3%) | |
| MRI scan | |||
| Contralateral lesion | 84 (14.2%) | 7 (12.7%) | .916 |
| Bilateral interictal EEG | |||
| Yes | 75 (18.0%) | 7 (63.6%) | <.001* |
| No | 342 (82.0%) | 4 (36.4%) | |
| Infantile spasms | |||
| Yes | 123 (28.5%) | 20 (36.4%) | .292 |
| No | 309 (71.5%) | 35 (63.6%) | |
| Number of seizure semiologies | |||
| One | 313 (52.2%) | 14 (25.5%) | <.001* |
| Two or more | 284 (47.4%) | 41 (74.5%) | |
| Seizure recurrence | |||
| Yes | 126 (21.0%) | 10 (13.9%) | .206 |
| No | 474 (79.0%) | 62 (86.1%) | |
Values are presented as number of patients (%) or mean ± SD (range).
Abbreviations: EEG, electroencephalogram; FDG‐PET, 18‐fluoro‐2‐deoxyglucose‐positron emission tomography; HOPS, Hemispheric Surgery Outcome Prediction Scale; MRI, magnetic resonance imaging.
*p < .05.
3.2. Outcomes analysis
A Kaplan–Meier plot demonstrating and comparing the seizure freedom functions in the lateral and vertical hemispherotomy strata is depicted in Figure 3. The probability of seizure freedom in the lateral hemispherotomy cohort at 1, 5, and 10 years was 89.2% (95% CI = 86.3%–91.5%), 72.1% (95% CI = 66.9%–76.7%), and 50.6% (95% CI = 46.6%–66.4%), whereas the probability of seizure freedom in the vertical hemispherotomy cohort was initially 88.8% (95% CI = 78.9%–94.3%) at 1 year and was then sustained at 85.5% (95% CI = 74.7%–92.0%) at 5 and 10 years. Log‐rank test showed a significant difference in time to first postoperative seizure when stratified by hemispherotomy technique, with the vertical hemispherotomy subgroup having a longer time to seizure recurrence (p = .01).
FIGURE 3.
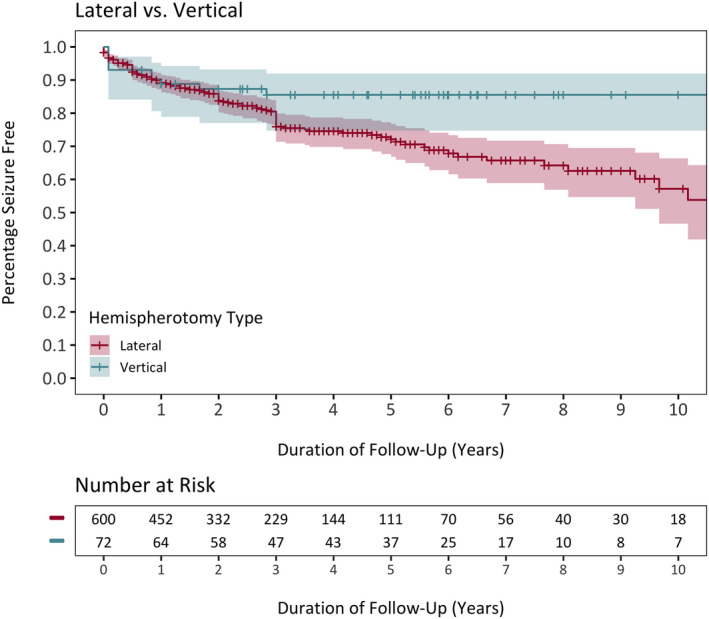
Comparison of Kaplan–Meier curves depicting the seizure freedom functions of vertical and lateral hemispherotomy cohorts
Results of the multivariate mixed‐effects Cox regression model correcting for the HOPS score are reported in Table 2. Hemispherotomy technique is significantly and independently associated with time to seizure recurrence, with the lateral approach conferring a faster progression to first postoperative seizure (HR = 2.56, p = .03). The HOPS score was also independently associated with time to first postoperative seizure, with higher scores leading to a faster time to seizure recurrence (HR = 1.43, p < .001). Results of the multivariate mixed‐effects logistic regression model for patients with at least 1‐year follow‐up and controlling for the HOPS score are depicted in Table 3. The findings followed a similar trend to the Cox regression analysis, with odds of seizure recurrence being significantly greater in patients who underwent lateral hemispherotomy compared to vertical hemispherotomy (OR = 3.67, p = .04).
TABLE 2.
Multivariate mixed‐effects Cox regression analysis for predictors of faster time to seizure recurrence, controlling for hemispherotomy technique (vertical and lateral) and HOPS score
| Variable | HR a | 95% CI | p |
|---|---|---|---|
| Hemispherotomy technique (relative to vertical) | |||
| Lateral | 2.56 | 1.08–6.04 | .034* |
| HOPS score | 1.43 | 1.17–1.75 | <.001* |
Abbreviations: CI, confidence interval; HOPS, Hemispheric Surgery Outcome Prediction Scale; HR, hazard ratio.
*p < .05.
HR > 1 indicates a faster time to seizure recurrence.
TABLE 3.
Multivariate mixed‐effects logistic regression analysis for predictors of seizure recurrence, controlling for hemispherotomy technique (vertical and lateral) and HOPS score
| Variable | OR a | 95% CI | p |
|---|---|---|---|
| Hemispherotomy technique (relative to vertical) | |||
| Lateral | 3.67 | 1.05–12.86 | .042* |
| HOPS score | 1.13 | .87–1.48 | .356 |
Abbreviations: CI, confidence interval; HOPS, Hemispheric Surgery Outcome Prediction Scale; OR, odds ratio.
*p < .05.
OR > 1 indicates greater odds of seizure recurrence.
4. DISCUSSION
Post hoc analysis of the multicenter HOPS study suggests a significantly greater durability of seizure freedom for children undergoing vertical compared with lateral hemispherotomy. The rate of seizure freedom between these groups is nearly identical at 1 year of follow‐up; however, seizure freedom was durable for the vertical group and significantly declined over time for the lateral group, most noticeably around 6 years postoperatively (Figure 3). This is consistent with our finding that vertical hemispherotomy is associated with a longer time to seizure recurrence via log‐rank test. Furthermore, the relationship between sustained seizure freedom and vertical hemispherotomy was preserved even after controlling for validated predictors of seizure freedom identified in the HOPS study through the HOPS score during multivariate regression, thereby increasing our confidence that differences in outcome are likely attributed to technique rather than patient variables known to confer differential seizure freedom.
Our findings contrast two studies directly comparing vertical and lateral techniques, which found favorable outcomes overall but no differences between groups. One of these studies was underpowered to detect differences in seizure outcome, 24 whereas the other was a national multicenter study comprising 90 patients across three main Italian epilepsy centers with a methodology similar to ours. 20 Our calculated rates of seizure freedom at the last follow‐up for vertical (86.1%) and lateral (79.0%) approaches were almost identical to the latter study, which had 84.2% and 73.1% seizure freedom in the vertical and lateral groups, respectively; however, the utilization of a modified interhemispheric approach by de Palma et al. yielded a much lower rate of seizure freedom for their vertical group overall and may explain the different conclusions between our findings. 20 For the vertical approach, our study included patients undergoing parasagittal technique only, which may be advantageous relative to modified vertical techniques. Compared with the original parasagittal approach, interhemispheric vertical modifications potentially require more lateral retraction for access to the temporal horn of the lateral ventricle, increase risk to the parasagittal draining veins, and increase risk to the healthy contralateral hemisphere. 25 With the recent development of endoscopic interhemispheric techniques, 26 , 27 the vertical approach has already become a heterogeneous group of interventions that warrant individual study given the possibility of different seizure outcomes. A possible explanation for previous comparative studies failing to find a difference between surgical techniques is that the cohorts were compared at a single, relatively short, timepoint (i.e., the time of final follow‐up). The data from the current study suggest the difference in efficacy between the vertical and lateral group is only apparent on long‐term follow‐up (i.e., over 6 years), and any study that compares seizure outcomes at conventional timepoints such as 1 or 2 years following surgery will also fail to capture this difference.
Given that complete disconnection is possible from both the vertical and lateral approaches, the reason for differing seizure outcomes is not immediately clear. One putative explanation is the higher likelihood of incomplete disconnection in the lateral hemispherotomy group. Given that seizure recurrence and the need for reoperation have been attributed to incomplete resection, 28 this may theoretically contribute to the greater durability of seizure freedom in patients undergoing vertical approaches. Insular epileptogenic onset is likely an underrepresented cause of refractory epilepsy in children, 29 , 30 and residual insular cortex has been identified as a cause of failure following hemispherectomy. 16 The intraoperative management of insular cortex varies considerably between lateral and vertical approaches. The vertical approach may provide a greater likelihood of complete disconnection of the insula and associated subcortical structures as they are undercut, whereas in the lateral approach, the insula is either left unresected (particularly in middle cerebral artery [MCA] stroke etiology cases), undercut, or resected piecemeal between the MCA branches. 15 , 17 , 19 In cases where the insula is resected in a piecemeal fashion, it is theoretically possible that residual insular cortex can remain inadvertently connected. However, this would certainly not explain the majority of recurrent cases. Incomplete frontobasal disconnection is another cause of seizure recurrence 31 and may have differed between groups. Some surgeons use the sphenoid ridge as guidance in the lateral approach, leaving the posterior third of the frontobasal connections unresected. 32 In contrast, the vertical approach more reliably allows for posterior disconnection to the foramen of Monroe. Additionally, the vertical approach entails a perithalamic cut that not only functionally disconnects the basal ganglia, 14 but also theoretically separates all potentially dysplastic neurons that failed to migrate from the subventricular zone to the cortex. 33 This may contribute significantly to surgical outcomes for congenital disorders including malformations of cortical development. Functional hemispherotomy is a complex procedure with intricate surgical steps required to successfully perform a complete hemispheric disconnection, a major predictor of seizure outcome. 28 , 34 Epilepsy program and surgical experience has been shown to play a significant role in obtaining a complete anatomical disconnection and obtaining higher likelihood of seizure freedom and better outcome. 35 , 36 , 37 Although this variable was not explicitly studied in this analysis, the learning curve could potentially explain the higher seizure recurrence rate in lateral than in vertical hemispherotomies.
Other general operative considerations in favor of the vertical approach have been proposed, 14 including risk of injury to the contralateral hemisphere with the lateral approach, although this is rare in experienced hands. Intraoperative blood transfusion and the total duration of surgery have been shown to be less in vertical hemispherotomy. 24 Increased blood loss in lateral hemispherotomy is thought to be associated with the greater amount of tissue resected, with the exception of the keyhole technique. 19 The risk of hydrocephalus is thought to be higher in lateral hemispherotomy due to resection of the subarachnoid space of the Sylvian fissure, although the 2%–20% rate of hydrocephalus in lateral series 16 , 17 , 19 , 38 , 39 appears to be comparable with 3%–16% in vertical series. 13 , 14
It is important to note that there were several clinical differences between the two groups. Most notably, the vertical hemispherotomy group had a higher proportion of patients with factors known to be associated with poor seizure outcomes, including bilateral FDG‐PET abnormality 40 and generalized seizure semiology. 41 , 42 When comparing relevant non‐HOPS clinical variables, the vertical hemispherotomy group had a significantly greater proportion of patients with two or more seizure semiologies 43 and bilateral and synchronous interictal EEG abnormalities, 44 , 45 which are characteristics also associated with seizure recurrence. Nonetheless, vertical hemispherotomy remained significantly associated with more durable seizure freedom despite the attributes in favor of a better prognosis for the lateral subgroup. Overall, the apparent advantage of vertical hemispherotomy is not explained by or attributed to a more advantageous HOPS profile among the patient population.
Because fewer participants underwent vertical compared to lateral hemispherotomy, and those patients come from only four centers, our study is prone to sample bias. The risk of selection bias is further increased because patient selection by different epilepsy teams varies. Furthermore, the experience and ability to perform complete disconnections by individual surgeons may have varied unequally between the participating groups and could not be fully accounted for in this analysis, given that postoperative magnetic resonance imaging studies for collecting data on the completeness of each hemispherotomy were not initially acquired. This may have implications, particularly in peri‐insular regions, for why the durability of seizure freedom is greater for patients undergoing vertical hemispherotomy. Collectively, these variables may contribute to the distinct trend in durability of seizure outcomes seen in the vertical group compared with the lateral group (Figure 3). However, institution heterogeneity was still accounted for to the greatest extent possible through a mixed‐effects model, and the association between surgical technique and outcome endured even after doing so. This finding is consistent with previous single‐center studies that show sustained seizure freedom following vertical hemispherotomy. 13 , 14 Reasons for sustained durability are explored above, and further studies utilizing pre‐ and postoperative tractography, in addition to leveraging standard imaging, may reveal how incomplete disconnection contributes to seizure recurrence and how this varies by technique.
Other limitations include the utilization of data collected from the HOPS study, which was designed to detect preoperative variables associated with seizure freedom rather than differences between hemispherotomy techniques. There is one important disadvantage of the time‐to‐event Kaplan–Meier survival curve approach for seizure outcome used in this study; it will fail to account for the running down phenomenon, that is, gradual decline of seizures over several months or years until seizure freedom is achieved following surgery. In these situations, it is difficult to separate natural history from the effectiveness of surgery. To the extent that this phenomenon occurs, it would compromise the validity of both a TTE approach and a traditional categorical approach with a short duration of follow‐up. The absence of data regarding antiepileptic drug (AED) weaning is another limitation in comparing surgical techniques, as discontinuation of AEDs is the ultimate indicator of success following epilepsy surgery and is associated with improved cognition. 46 Complication data were not captured; they are needed to weigh the potential benefit of the different hemispherotomy techniques against their associated risks to better understand their overall role in the treatment of hemispheric epilepsy. The center effect was accounted for using a multivariate mixed‐effects model with the institution as a random‐effects variable; however, this model would not account for fundamental differences between centers in selecting surgical patients, hence the need for a well‐designed prospective comparative study.
Further studies are warranted to confirm or negate the findings of the current study, namely, that the vertical approach has superior durability compared to the lateral approach. Future studies designed to compare vertical versus lateral approaches may benefit from an expertise‐based trial rather than a randomized controlled trial (RCT). Although the lateral hemispherotomy approach is much more commonly performed than the vertical approach, the studies should seek to recruit a comparable number of participants undergoing each approach. Designing an RCT to assess surgical interventions can be limited by differential expertise bias, in which the following factors are unlikely to be equal: the number of participating surgeons with expertise in each procedure, the experience in each procedure of the same surgeon, and the technical difficulty of each technique being studied. 47 Similar to an RCT, this post hoc analysis may answer the question of which procedure is more effective in the real world. In contrast, an expertise‐based trial would allow us to avoid these limitations while answering the more pragmatic question of which technique is superior in the hands of experts who utilize a given approach. Considering the rarity of hemispherotomy procedures and the typical referral pattern to specialized centers for elective surgery, this study design would be especially well suited to address this clinical question.
CONFLICT OF INTEREST
G.W.M. is partly supported by the Davies/Crandall Endowed Chair for epilepsy research at University of California, Los Angeles. The views expressed in this article are not the official positions of any author's affiliated institution. P.L.P. has received research support from the National Institutes of Health (NIH), National Science Foundation (NSF), and Boston Healthcare Associates; has received royalty payments from Elsevier, Springer Publishing, and UpToDate; has served as a consultant for Agilis Biotherapeutics and GLG Health Care Council; and is an associate editor for the Journal of Child Neurology and on the editorial boards of Annals of Neurology, Epilepsia, Future Neurology, Music and Medicine, and Neurology. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
ACKNOWLEDGMENT
None.
Fallah A, Lewis E, Ibrahim GM, Kola O, Tseng C‐H, Harris WB, et al. Comparison of the real‐world effectiveness of vertical versus lateral functional hemispherotomy techniques for pediatric drug‐resistant epilepsy: A post hoc analysis of the HOPS study. Epilepsia. 2021;62:2707–2718. 10.1111/epi.17021
REFERENCES
- 1. Perry MS, Duchowny M. Surgical management of intractable childhood epilepsy: curative and palliative procedures. Semin Pediatr Neurol. 2011;18:195–202. [DOI] [PubMed] [Google Scholar]
- 2. Bailet LL, Turk WR. The impact of childhood epilepsy on neurocognitive and behavioral performance: a prospective longitudinal study. Epilepsia. 2000;41:426–31. [DOI] [PubMed] [Google Scholar]
- 3. Moosa AN, Gupta A, Jehi L, Marashly A, Cosmo G, Lachhwani D, et al. Longitudinal seizure outcome and prognostic predictors after hemispherectomy in 170 children. Neurology. 2013;15(80):253–60. [DOI] [PubMed] [Google Scholar]
- 4. Griessenauer CJ, Salam S, Hendrix P, Patel DM, Tubbs RS, Blount JP, et al. Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr. 2015;15:34–44. [DOI] [PubMed] [Google Scholar]
- 5. McKenzie K. The present status of a patient who had right cerebral hemisphere removed. JAMA. 1938;111:168. [Google Scholar]
- 6. Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10(2):71–8. [DOI] [PubMed] [Google Scholar]
- 7. Schramm J, Behrens E, Entzian W. Hemispherical deafferentation: an alternative to functional hemispherectomy. Neurosurgery. 1995;36:509–15; discussion 515–6. [DOI] [PubMed] [Google Scholar]
- 8. Villemure JG, Mascott CR. Peri‐insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995;37:975–81. [DOI] [PubMed] [Google Scholar]
- 9. Delalande O. Hemispherotomy: a new procedure for central disconnection. Epilepsia. 1992;33:99–100. [Google Scholar]
- 10. Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001;49(4):891–900; discussion 900–1. [DOI] [PubMed] [Google Scholar]
- 11. Peacock WJ, Chugani H, Shewmon DA, Shields WD. Classical hemispherectomy for the control of intractable seizures in children with infantile hemiplegia. J Epilepsy. 1990;3(Suppl):183–5. [Google Scholar]
- 12. Tuxhorn I, Holthausen H. Paediatric epilepsy syndromes and their surgical treatment. London, UK: John Libbey and Co; 1997. [Google Scholar]
- 13. Delalande O, Bulteau C, Dellatolas G, Fohlen M, Jalin C, Buret V, et al. Vertical parasagittal hemispherotomy: surgical procedures and clinical long‐term outcomes in a population of 83 children. Neurosurgery. 2007;60(2 Suppl 1):ONS19–32; discussion ONS32. [DOI] [PubMed] [Google Scholar]
- 14. Dorfer C, Czech T, Dressler A, Gröppel G, Mühlebner‐Fahrngruber A, Novak K, et al. Vertical perithalamic hemispherotomy: a single‐center experience in 40 pediatric patients with epilepsy. Epilepsia. 2013;54(11):1905–12. [DOI] [PubMed] [Google Scholar]
- 15. Cook SW, Nguyen ST, Hu B, Yudovin S, Shields WD, Vinters HV, et al. Cerebral hemispherectomy in pediatric patients with epilepsy: comparison of three techniques by pathological substrate in 115 patients. J Neurosurg. 2004;100(2):125–41. [DOI] [PubMed] [Google Scholar]
- 16. Cats EA, Kho KH, Van Nieuwenhuizen O, Van Veelen CW, Gosselaar PH, Van Rijen PC. Seizure freedom after functional hemispherectomy and a possible role for the insular cortex: the Dutch experience. J Neurosurg. 2007;107(4 Suppl):275–80. [DOI] [PubMed] [Google Scholar]
- 17. Villemure JG, Daniel RT. Peri‐insular hemispherotomy in paediatric epilepsy. Childs Nerv Syst. 2006;22(8):967–81. [DOI] [PubMed] [Google Scholar]
- 18. Kwan A, Ng WH, Otsubo H, Ochi A, Snead OC 3rd, Tamber MS, et al. Hemispherectomy for the control of intractable epilepsy in childhood: comparison of 2 surgical techniques in a single institution. Neurosurgery. 2010;67(2 Suppl Operative):429–36. [DOI] [PubMed] [Google Scholar]
- 19. Schramm J, Kuczaty S, Sassen R, Elger CE, von Lehe M. Pediatric functional hemispherectomy: outcome in 92 patients. Acta Neurochir. 2012;154(11):2017–28. [DOI] [PubMed] [Google Scholar]
- 20. de Palma L, Pietrafusa N, Gozzo F, Barba C, Carfi‐Pavia G, Cossu M, et al. Outcome after hemispherotomy in patients with intractable epilepsy: comparison of techniques in the Italian experience. Epilepsy Behav. 2019;93:22–8. [DOI] [PubMed] [Google Scholar]
- 21. Fallah A, Weil AG, Lewis EC, Ibrahim GM, Kola O, Tseng CH, et al. Hemispherectomy outcome prediction scale: development and validation of a seizure freedom prediction tool. Epilepsia. 2021;62(5):1064–73. [DOI] [PubMed] [Google Scholar]
- 22. van Buuren S, Groothuis‐Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 23. Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 24. Iwasaki M, Uematsu M, Osawa S, Shimoda Y, Jin K, Nakasato N, et al. Interhemispheric vertical hemispherotomy: a single center experience. Pediatr Neurosurg. 2015;50(5):295–300. [DOI] [PubMed] [Google Scholar]
- 25. Dorfer C, Khalaveh F, Dorfmüller G, Czech T. Surgical anatomy of vertical perithalamic hemispherotomy. Oper Neurosurg (Hagerstown). 2020;18(5):511–7. [DOI] [PubMed] [Google Scholar]
- 26. Sood S, Marupudi NI, Asano E, Haridas A, Ham SD. Endoscopic corpus callosotomy and hemispherotomy. J Neurosurg Pediatr. 2015;16:681–6. [DOI] [PubMed] [Google Scholar]
- 27. Chandra PS, Kurwale N, Garg A, Dwivedi R, Malviya SV, Tripathi M. Endoscopy‐assisted interhemispheric transcallosal hemispherotomy: preliminary description of a novel technique. Neurosurgery. 2015;76(4):485–94; discussion 494–5. [DOI] [PubMed] [Google Scholar]
- 28. Volpon Santos M, Teixeira TL, Ioriatti ES, Thome U, de Andrade P, Hamad A, et al. Risk factors and results of hemispherotomy reoperations in children. Neurosurg Focus. 2020;48(4):E5. [DOI] [PubMed] [Google Scholar]
- 29. Weil AG, Fallah A, Lewis EC, Bhatia S. Medically resistant pediatric insular‐opercular/perisylvian epilepsy. Part 1: Invasive monitoring using the parasagittal transinsular apex depth electrode. J Neurosurg Pediatr. 2016;18(5):511–22. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen DK, Nguyen DB, Malak R, Leroux JM, Carmant L, Saint‐Hilaire JM, et al. Revisiting the role of the insula in refractory partial epilepsy. Epilepsia. 2009;50(3):510–20. [DOI] [PubMed] [Google Scholar]
- 31. Chen S, Guan Y, Liu C, Du X, Zhang Y, Chen S, et al. Treatment for patients with recurrent intractable epilepsy after primary hemispherectomy. Epilepsy Res. 2018;139:137–42. [DOI] [PubMed] [Google Scholar]
- 32. Lew SM, Koop JI, Mueller WM, Matthews AE, Mallonee JC. Fifty consecutive hemispherectomies: outcomes, evolution of technique, complications, and lessons learned. Neurosurgery. 2014;74(2):182–94; discussion 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doetsch F, Caillé I, Lim DA, García‐Verdugo JM, Alvarez‐Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–16. [DOI] [PubMed] [Google Scholar]
- 34. Bartoli A, El Hassani Y, Jenny B, Momjian S, Korff CM, Seeck M, et al. What to do in failed hemispherotomy? Our clinical series and review of the literature. Neurosurg Rev. 2018;41(1):125–32. [DOI] [PubMed] [Google Scholar]
- 35. Kurwale NS, Patil SB, Jagtap SA, Joshi A, Nilegaonkar S, Bapat D, et al. Failed hemispherotomy: insights from our early experience in 40 patients. World Neurosurg. 2021;146:e685–90. [DOI] [PubMed] [Google Scholar]
- 36. Bouthillier A, Weil AG, Martineau L, Létourneau‐Guillon L, Nguyen DK. Operculoinsular cortectomy for refractory epilepsy. Part 2: Is it safe? J Neurosurg. 2019;20:1–11. [DOI] [PubMed] [Google Scholar]
- 37. Heller AC, Padilla RV, Mamelak AN. Complications of epilepsy surgery in the first 8 years after neurosurgical training. Surg Neurol. 2009;71(6):631–7; discussion 637. [DOI] [PubMed] [Google Scholar]
- 38. Lew SM, Matthews AE, Hartman AL, Haranhalli N, Post‐Hemispherectomy Hydrocephalus Workgroup . Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia. 2013;54(2):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weil AG, Fallah A, Wang S, Ibrahim GM, Elkaim LM, Jayakar P, et al. Functional hemispherectomy: can preoperative imaging predict outcome? J Neurosurg. 2020;25(6):567–73. [DOI] [PubMed] [Google Scholar]
- 40. Traub‐Weidinger T, Weidinger P, Gröppel G, Karanikas G, Wadsak W, Kasprian G, et al. Presurgical evaluation of pediatric epilepsy patients prior to hemispherotomy: the prognostic value of 18F‐FDG PET. J Neurosurg Pediatr. 2016;25(6):683–8. [DOI] [PubMed] [Google Scholar]
- 41. Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Shinnar S, et al. Predicting long‐term seizure outcome after resective epilepsy surgery: the multicenter study. Neurology. 2005;65(6):912–8. [DOI] [PubMed] [Google Scholar]
- 42. Englot DJ, Breshears JD, Sun PP, Chang EF, Auguste KI. Seizure outcomes after resective surgery for extra‐temporal lobe epilepsy in pediatric patients. J Neurosurg Pediatr. 2013;12(2):126–33. [DOI] [PubMed] [Google Scholar]
- 43. Sarkis RA, Jehi L, Bingaman W, Najm IM. Seizure worsening and its predictors after epilepsy surgery. Epilepsia. 2012;53(10):1731–8. [DOI] [PubMed] [Google Scholar]
- 44. Carmant L, Kramer U, Riviello JJ, Helmers SL, Mikati MA, Madsen JR, et al. EEG prior to hemispherectomy: correlation with outcome and pathology. Electroencephalogr Clin Neurophysiol. 1995;94(4):265–70. [DOI] [PubMed] [Google Scholar]
- 45. Hu WH, Zhang C, Zhang K, Shao XQ, Zhang JG. Hemispheric surgery for refractory epilepsy: a systematic review and meta‐analysis with emphasis on seizure predictors and outcomes. J Neurosurg. 2016;124(4):952–61. [DOI] [PubMed] [Google Scholar]
- 46. Lamberink HJ, Boshuisen K, Otte WM, Geleijns K, Braun KPJ, TimeToStop Study Group . Individualized prediction of seizure relapse and outcomes following antiepileptic drug withdrawal after pediatric epilepsy surgery. Epilepsia. 2018;59(3):e28–33. [DOI] [PubMed] [Google Scholar]
- 47. Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330(7482):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


