Abstract
Background
Synovial sepsis is a commonly occurring, potentially career‐ending or even life‐threatening orthopaedic emergency. Diagnosis of synovial sepsis is currently primarily based on synovial fluid analysis, which often leaves diagnostic ambiguity due to overlap of clinicopathological parameters between septic and aseptic inflammatory synovitis.
Objectives
To evaluate the reliability of lysozyme (LYS), myeloperoxidase (MPO) and elastase (ELT) as biomarkers for synovial sepsis in horses using a photometric assay to measure increased enzyme activity.
Study design
Prospective, single‐blinded, analytical, clinical study.
Methods
Equine synovial samples were assigned to one of three groups: (1) healthy controls (n = 10), (2) aseptic (n = 27) and (3) septic synovitis (n = 30). The enzyme activity assays (LYS, MPO and ELT) were compared with standard synovial fluid parameters and broad‐range bacterial 16S rDNA PCR.
Results
LYS and MPO activities were significantly different between septic synovial samples, and both aseptic and control samples (P < .001, LYS: confidence interval [CI]: 2.25‐3.41, resp., 2.21‐3.8, MPO: CI 0.752‐1.6, resp., 0.639‐1.81). LYS achieved a 100% sensitivity and 100% specificity in differentiating between septic and aseptic (cut‐off value 751.4) or control (cut‐off: 484.6) samples (P < .001). MPO reached 93.33% sensitivity, 100% specificity for distinguishing septic from control (cut‐off value: 0.1254) synovial samples and 93.33% sensitivity, 81.48% specificity for discriminating between septic and aseptic (cut‐off value: 0.1305) synovial samples (P < .001). ELT activity could not be measured in any synovial sample. Both the LYS and the MPO measurements showed a highly significant correlation with PCR (LYS r = .79, MPO r = .69), synovial leukocyte count (LYS r = .752, MPO r = .571), % neutrophils (LYS r = .751, MPO r = 0.663) and each other (r = .744, all P < .001).
Main limitations
Variation in horses’ signalment, affected synovial structures and synovial fluid freezing times may have affected the discriminative power of this study.
Conclusions
Increased MPO and LYS activities allow reliable, rapid diagnosis of synovial sepsis with high sensitivity and specificity.
Keywords: elastase, enzyme activity, horse, lysozyme, myeloperoxidase, septic arthritis, synovial infection
1. INTRODUCTION
Synovial sepsis is a commonly occurring, potentially life‐threatening (mortality: 10%–55%) or career‐ending (morbidity: 19%–50%) orthopaedic emergency of horses. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 Timely treatment is considered integral to a successful outcome and can achieve a return to performance rates as high as 81%. 11 , 12 Therefore, prompt diagnosis and therapy are essential to minimise the progressive detrimental effects of synovial infection and inflammation and prevent career‐ and life‐threatening sequelae of synovial sepsis. Positive bacterial culture results of synovial fluid and confirmation of intracellular bacteria within the neutrophils on gram stains are the gold standards to diagnose septic synovitis, but the sensitivity of either parameter is low, at only 31%–78.9% for bacterial culture and 25%–45% for gram stains of septic synovial fluid. 2 , 6 , 10 , 13 , 14 , 15 , 16 , 17 , 18 Furthermore, the turnaround time of bacterial culture, up to 4 days for a definitive diagnosis, is too long for emergency treatment decisions and would cause a potentially fatal delay. Therefore, the presumptive diagnosis of septic synovitis is typically based on clinical signs, such as lameness or a macroscopically visible opening into the synovial structure, cytological evidence of synovial fluid leucocytosis (5‐30 × 109 nucleated cells/L), neutrophilia (>80%–90% neutrophils) and increased synovial fluid total protein (>40 g/L). 2 , 5 , 6 , 7 , 8 , 19 However, the differential diagnosis between septic and nonseptic inflammatory synovitis is challenging due to the overlap of clinicopathological values. 6 , 18 , 20 Accordingly, clinicians often have to rely on ambiguous results to make the potentially life‐ and career‐relevant decision whether the horse requires synovial lavage with the associated anaesthetic and surgical risks and costs. Furthermore, determination of the synovial leukocyte count and neutrophil percentage requires laboratory equipment, cannot be carried out stall side and, thus, necessitates transport of the sample or the patient to a laboratory or hospital. This can cause significant delays and hurdles in the diagnosis and treatment of septic synovitis. Therefore, a test that can quickly provide reliable results with high sensitivity and specificity is urgently needed.
In response to synovial contamination, an immediate immune response is initiated, and inflammatory cells, predominantly neutrophils, are rapidly recruited to eliminate the invading pathogens. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 Coincident with phagocytosis of microorganisms, neutrophils produce reactive oxygen species, form extracellular traps and release antimicrobial granular enzymes to kill and degrade ingested microbes. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 Recently, increased levels of the granular enzymes lysozyme (LYS), myeloperoxidase (MPO) and elastase (ELT) in wound fluid have shown promise as biomarkers of wound infections in human patients, 35 , 36 , 37 , 38 , 39 , 40 with enhanced diagnostic sensitivity and specificity when evaluated in combination. 35 , 36 , 37 , 38 , 39 , 40 The three enzymatic biomarkers of neutrophil stimulation and degranulation have also been measured in synovial fluid. 41 , 42 , 43 , 44 While MPO showed promise as a marker for septic arthritis in horses, 41 , 42 , 43 , 44 evidence for the diagnostic value of the other two enzymes for the diagnosis of synovial sepsis is lacking. 41 , 42 , 43 , 44 Synovial LYS correlated well with synovial leukocyte levels and was established as a sensitive indicator of aseptic synovial inflammation, 41 , 42 , 43 , 44 but studies looking at its potential as a biomarker for synovial sepsis are missing. Equine synovial ELT, measured in its complexed and, hence, inhibited state, has demonstrated large variance between different joints and between aseptic and septic conditions. ELT enzyme activity has, however, not been compared with the leukocyte count, percentage neutrophils or total protein, which would reveal the potential of this marker compared with state‐of‐the‐art methods. 41 , 42 , 43 , 44
Using enzyme activity as a biomarker of synovial sepsis enables the development of a lateral‐flow‐based point‐of‐care diagnostic test, which would allow veterinarians to perform, analyse and act on test results stall side in a matter of minutes.
We hypothesise that LYS, MPO and ELT enzymatic activities are sensitive and specific markers of synovial sepsis. Therefore, this study aims to evaluate the diagnostic reliability of LYS, MPO and ELT activity to detect synovial sepsis in horses and differentiate septic from aseptic synovitis as the basis for developing a stall‐side diagnostic test.
2. MATERIALS AND METHODS
2.1. Synovial samples
Synovial samples (approximately 1 mL) were obtained from three groups: (1) healthy controls, (2) horses with aseptic synovitis and (3) horses with septic synovitis. Healthy control samples were harvested post‐mortem under aseptic conditions from horses without clinical or macroscopic signs of joint disease, which had been subjected to euthanasia for reasons unrelated to this study (Tables 1 and S1).
TABLE 1.
The age (in years [y] and days [d]) of the included horses and the affected synovial structure are detailed for each of 3 groups (control, aseptic inflammatory and septic synovitis)
| Control | Aseptic | Septic | |
|---|---|---|---|
| Number of synovial structures | 10 | 27 | 30 |
| Number of horses | 10 | 23 | 22 |
| Age (median, range) | 15 y (4 y ‐ 25 y) | 11 y (3 y ‐ 20 y) | 8.5 y (12 d ‐ 26 y) |
| Synovial structure | |||
| Tarsocrural joint | 1 | 9 | 15 |
| Femoropatellar/Femorotibial joint | 1 | 6 | 7 |
| Metacarpo‐/Metatarsophalangeal joint | 3 | 3 | 1 |
| Antebrachio‐/Radiocarpal joint | 5 | 1 | 1 |
| Distal/Proximal interphalangeal joint | 3 | 1 | |
| Tarsometatarsal joint | 2 | ||
| Cubital joint | 1 | ||
| Digital flexor tendon sheath | 2 | 3 | |
| Tarsal sheath | 1 | 1 | |
Aseptic and septic synovial samples were collected during routine diagnostic synoviocentesis from equine cases presented to the hospital for any joint/tendon sheath disease. Septic synovitis was diagnosed based on a combination of history and clinical signs, such as lameness or a macroscopically visible opening into the synovial structure, as well as the results of synovial fluid analysis, including synovial WBC (≥20 × 109 nucleated cells/L), synovial neutrophil percentage (≥80%), presence of intracellular bacteria, bacterial culture and synovial total protein (≥40g/l) and blood serum amyloid A (SAA).
The diagnosis was confirmed (when synovial fluid quantities were sufficient) by broad‐range bacterial 16S ribosomal DNA gene polymerase chain reaction (PCR). Synovial samples for enzyme activity measurements and PCR were stored in plain sterile vials immediately after collection and frozen at −18°C until further analysis.
The person performing the enzyme assays was blinded to the diagnosis of the cases.
2.2. Lysozyme activity assay
LYS lysis activity was determined using a turbidimetric assay as previously described. 36 , 45 In brief, 290 μL of a 0.45 mg/mL peptidoglycan solution (from Micrococcus lysodeikticus, Sigma‐Aldrich) in sodium phosphate buffer (50 mM, pH 6.5) was incubated with 10 μL synovial fluid sample or reference enzyme solution (LYS from human neutrophils, Sigma‐Aldrich). The hydrolysis of peptidoglycan by LYS resulted in a loss of turbidity of the solution. Absorbance measurements were carried out at 450 nm for 10 minutes at 37°C using a microplate reader (Infinite M200 Pro, Tecan). A calibration curve with different LYS concentrations was performed to quantify the enzyme activities in the synovial samples.
2.3. MPO activity assay
MPO activity was determined with a guajacol‐based assay, as previously described. 37 Concisely, 10 μL of a synovial fluid sample or reference enzyme solution (MPO from human leukocytes, Szabo‐Scandic GmbH, Vienna, Austria) was added to 290 μL of reaction solution containing 87.4 mM guajacol in potassium phosphate buffer (100 mM, pH 7.0) and 0.35 mM H2O2 (Carl Roth). The MPO‐driven oxidation of guajacol and further formation of tetramers resulted in a colour change of the sample from colourless to red. This absorbance change at 470 nm was recorded over 60 seconds using a microplate reader (Infinite M200 Pro, Tecan). A calibration curve with different enzyme standards was used for the quantification of the MPO activities in the synovial samples.
2.4. Elastase activity assay
ELT activity was measured using the cleavage of N‐methoxysuccinyl‐ala‐ala‐pro‐val p‐nitroanilide (Szabo‐Scandic GmbH) dissolved in dimethyl sulphoxide and diluted with a sodium phosphate buffer (100 mM, pH 7.5) to a final concentration of 2 mM, as the chromogenic substrate. 35 Ten µL of a synovial fluid sample or reference enzyme solution (ELT from porcine pancreas, Szabo‐Scandic GmbH) was added to 90 µL of the substrate solution. The hydrolysis of the chromogenic substrate released 4‐nitroaniline, which changed the solution's colour to yellow. The absorbance was measured with a microplate reader (Infinite M200 Pro, Tecan) at 405 nm for 10 min at 37°C. The ELT activities were quantified using a calibration curve with different commercial enzyme activities.
2.5. PCR
DNA extraction and purification were performed under the cell culture lamina. All consumables were sterilised before use, and reagents were divided into aliquots to prevent exogenous DNA contamination. QIAamp DNA Blood Mini Kit (Qiagen, Germany) was used to isolate DNA. Before extraction, bacteria were pelleted from the synovial fluid samples according to the kit's protocol. The pellet was incubated overnight at 56°C with lysis buffer and proteinase K. The DNA was then extracted, according to the manufacturer's instructions, from the septic, aseptic and physiological samples at the same time. In addition, a negative control (DNA extraction from cultured equine tenocytes) was prepared for each independent DNA extraction to rule out contamination with exogenous amplifiable DNA at different stages of sample treatment. The presence of 16S DNA in each sample was evaluated by using the following primer pairs with PCR. The forward and reverse primer sequences were as follows: 5´‐CAGCTCGTGTCGTGAGATGT‐3’ and 5´‐AAGGGGCATGATGACTTGAC‐3’ respectively. The primer pairs were designed to amplify the highly conserved regions of the eubacterial 16S rRNA gene. The PCR reactions were performed in a total volume of 20 μL by using a Phusion High‐Fidelity PCR Kit (Thermo Fischer Scientific). The reaction mixture consisted of 1× GC Phusion Buffer, 200 μM dNTPs, 0.2 μM of each primer, 7% DMSO, 1 U Phusion DNA polymerase and 1 μL of sample DNA. The PCR was done with a Life ECO gene amplification instrument (BIOER, China). The reaction conditions for amplification were as follows: initial denaturation at 98°C for 30s, followed by 30 cycles of denaturation at 98°C for 10 s, primer annealing at 64°C for 30 s, extension 72°C for 30s and a final extension at 72°C for 5 min. PCR reactions were held at 4°C until visualisation. The amplicons were visualised with 1% agarose gel stained with ethidium bromide.
2.6. Data analysis
Statistical analyses were performed using Prism 8 software (GraphPad Prism version 8.4.1) and the R statistical programming language. 46 Values of P < .05 were considered to indicate statistical significance. Correlations between LYS and MPO activities, and the synovial leukocyte count, percentage of synovial neutrophils and PCR results were assessed by Spearman's correlation test. For both LYS as well as MPO, some measurements of control and aseptic samples were outside the dynamic range of the assay (below the detection limit or above the limit of reliable quantification, Figure S1). Within the dynamic range, the variance scaled approximately with the mean, such that we took the log after adding constants to achieve approximate normality (Figure S1). Since LYS measures were on average more than an order of magnitude higher than MPO measures, the constant added for LYS was 20 and that for MPO one. LYS (log(x + 20) transformed) and MPO (log(x + 1) transformed) measurements were compared between groups (control, aseptic and septic) using an ANOVA with Tukey's ‘Honest Significant Difference’ (HSD) multiple comparisons test. A Bartlett test was used to compare variances among groups. Residuals were tested for deviation from normality with a Shapiro‐Wilks test. We note that for such large sample sizes (up to 30 per group), deviations from the assumptions of linear models are expected, especially considering that some measures were outside the dynamic range. Note that linear models are quite robust to deviations from assumptions. Nevertheless, nonparametric Kruskal‐Wallis tests were also performed if the data did not meet the assumptions of the ANOVA. For a clinical application, however, where a decision needs to be made according to a single sample, nonparametric tests based on ranking are of limited use. Therefore, a receiver operating characteristic (ROC) curve plotting the true‐positive rate (sensitivity) against the false‐positive rate (1 – specificity) for all possible cut‐off values was used to determine the optimal cut‐off values for the LYS and MPO activities as well as for the percentage of synovial neutrophils and synovial leukocyte count using the Wilson‐Brown method to calculate confidence intervals. Note that these cut‐off values need to be validated and adjusted depending on further incoming clinical data.
3. RESULTS
3.1. Synovial samples
Sixty‐seven synovial samples obtained from 52 horses aged 12 days to 26 years were included in this study (Table 1). Based on routine diagnostic parameters (synovial leukocyte count and percentage of synovial neutrophils) and PCR, 30 samples (22 horses) were categorised as septic and 27 samples (24 horses, 4 of which are also included in the septic group and had been successfully treated) as aseptic (Table S1). The healthy control group included 10 samples (10 horses) from horses subjected to euthanasia for unrelated causes.
3.2. Lysozyme activity assay
Data were log‐transformed after 20 was added to each measurement (LYS measurement unit: U/mL). A Bartlett test of the residuals showed no significant deviations from variance homogeneity (Bartlett's K‐squared =5.39, df = 2, P‐value = .07). Residuals deviated significantly from normality (W = 0.95122, P = .01). A qq‐plot of the residuals indicates that these deviations are due to deviations at the low and high end of the dynamic range of the assay (Figure S1), while the values are close to their expectations along the quantile line in the middle of the region. Results from an ANOVA with Tukey's HSD multiple comparisons test showed that the measured LYS activity was significantly different (P < .001) between septic synovial samples and both aseptic (mean difference: 2.83, 95% confidence Interval [CI]: 2.25‐3.41) and control (mean difference 3.01, CI: 2.21‐3.8) samples (Figure 1; Table 2). A nonparametric Kruskal‐Wallis test of the overall hypothesis of equality of values among groups was significant (Kruskal‐Wallis chi‐squared = 49.239, df = 2, P < .001). A 100% sensitivity and 100% specificity were achieved for differentiating between septic and control synovial samples with a ROC‐calculated cut‐off of 484.5 U/mL and between septic and aseptic samples with a cut‐off of 751.4 U/mL (P < .001, area under the ROC curve [AUC]: 1, CI:1‐1, Table 3; Figure S2). All synovial samples classified as septic (30) were beyond the set cut‐off, all synovial samples classified as aseptic (27) or control (10) were below these respective values. Differentiation between control and aseptic samples only achieved a sensitivity of 66.67% and a specificity of 60.00% (likelihood ratio: 1.667) at a cut‐off of 67.07 (AUC: 0.6, CI: 0.4189‐0.7811, Tables 3 and S2; Figure S2).
FIGURE 1.
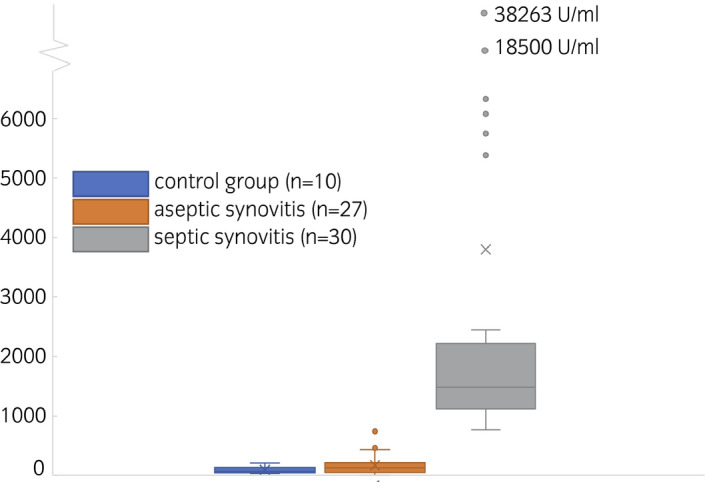
Boxplot of lysozyme activities of control group samples (blue, left), aseptic synovitis samples (orange, middle) and septic synovitis samples (grey, right). Means are indicated as x, medians as lines in the box. Whiskers are defined as max 1.5 times the interquartile range, outliers are visualised as points
TABLE 2.
ANOVA with Tukey's multiple comparisons test revealed significant differences between lysozyme (LYS) (log(x + 20)) and myeloperoxidase (MPO) (log(x + 1)) activities of septic synovial samples and both aseptic and control samples
| Comparative Groups | Mean Diff. | 95% CI | Adj. P‐value | |
|---|---|---|---|---|
| LYS | Control vs Septic | 3.01 | 2.21‐3.80 | <.001 |
| Control vs Aseptic | −0.177 | −0.985‐0.632 | .9 | |
| Septic vs Aseptic | 2.83 | 2.25‐3.41 | <.001 | |
| MPO | Control vs Septic | 1.225 | 0.639‐1.810 | <.001 |
| Control vs Aseptic | −0.047 | −0.640‐0.547 | >.9 | |
| Septic vs Aseptic | 1.178 | 0.752‐1.603 | <.001 |
Mean differences (diff.) are provided with the 95% confidence interval (CI) and the adjusted (adj.) P‐value.
TABLE 3.
The cut‐off values for lysozyme (LYS) and myeloperoxidase (MPO) activity measurements were calculated using the receiver operating characteristic curve (ROC) method
| Comparative groups | Cut‐off value | AUC (95% CI) | P‐value | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | |
|---|---|---|---|---|---|---|
| LYS | control ‐ septic | >484.6 |
1 (1‐1) |
<.0001 |
100 (88.65‐100) |
100 (72.25‐100) |
| aseptic ‐ septic | >751.4 |
1 (1‐1) |
<.0001 |
100 (88.65‐100) |
100 (87.54‐100) |
|
| control ‐ aseptic | >67.07 |
0.6 (0.4189‐0.7811) |
.4 |
66.67 (47.82‐81.36) |
60 (31.27‐83.18) |
|
| MPO | control ‐ septic | >0.1254 |
0.9783 (0.9371‐1) |
<.0001 |
93.33 (78.68‐98.82) |
100 (72.25‐100) |
| aseptic ‐ septic | >0.1305 |
0.9463 (0.8879‐1) |
<.0001 |
93.33 (78.68‐98.82) |
81.48 (63.3‐91.82) |
|
| control ‐ aseptic | >0.08700 |
0.6796 (0.5051‐0.8542) |
.0972 |
51.85 (33.99‐69.26) |
80 (49.02‐96.45) |
The cut‐off values are detailed with the corresponding area under the ROC curve (AUC), confidence intervals (CI), p‐values, sensitivity and specificity.
3.3. MPO activity assay
Data were log‐transformed after one was added to each measurement (MPO measurement unit: U/mL). A Bartlett test showed significant deviations from variance homogeneity among groups (Bartlett's K‐squared = 121.8, P < .001). Residuals also deviated significantly from normality (W = 0.77, P < .001). A qq‐plot of the original data indicates huge deviations from the assumptions of the normal distribution. A qq‐plot of the residuals produces a more nuanced picture, indicating that these deviations are due to big deviations at the low and high end of the dynamic range of the assay (Figure S1), while the residuals almost match the quantile line in the central region. Results from an ANOVA with Tukey's multiple comparisons test showed that the measured MPO activity was significantly different (P < .0001) between septic synovial samples and both aseptic (mean difference 1.178, CI: 0.752‐1.603) and control (mean difference 1.225, CI: 0.639‐1.81) samples (Figure 2; Table 2). A nonparametric Kruskal‐Wallis test of the overall hypothesis of equality of values among groups was significant (Kruskal‐Wallis chi‐squared = 42.63, df = 2, P < .001). A ROC‐calculated cut‐off of 0.1254 U/mL achieved a 93.33% sensitivity, and 100% specificity for discriminating between septic and control samples (AUC: 0.9783, CI:0.9371‐1) and a cut‐off of 0.1305 U/mL reached a 93.33% sensitivity and 81.48% specificity for differentiating between septic and aseptic (AUC: 0.9463, CI: 0.8879‐1) synovial samples (P < .001, Tables 3 and S2; Figures S2). Of the septic synovial samples, 28 (28/30) were above the set cut‐off, while 22 (22/27) of the aseptic and all control (10) synovial samples were below the respective cut‐off values. Differentiation between control and aseptic samples only achieved a sensitivity of 51.85% and specificity of 80.00% (likelihood ratio: 2.593) at a cut‐off of 0.087 (AUC: 0.6796, CI: 0.5051‐0.8542, Tables 3 and S2; Figure S2).
FIGURE 2.
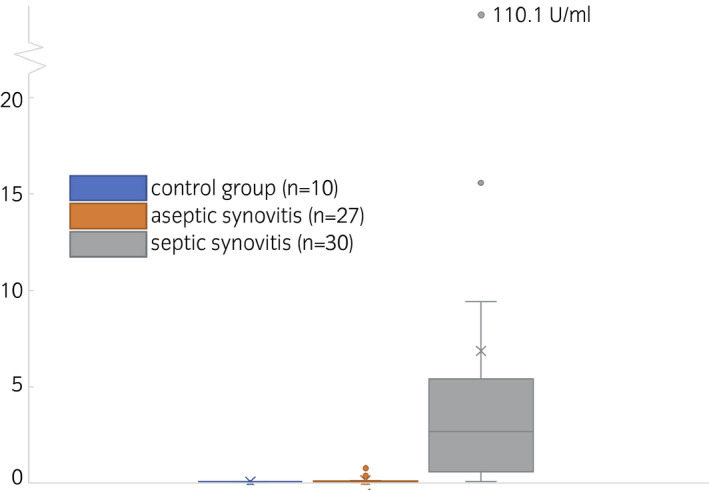
Boxplot of myeloperoxidase activities of control group samples (blue, left), aseptic synovitis samples (orange, middle) and septic synovitis samples (grey, right). Means are indicated as x, medians as lines in the box. Whiskers are defined as max 1.5 times the interquartile range, outliers are visualised as points
3.4. Elastase activity assay
ELT activity could not be measured in any synovial sample with the chosen method.
3.5. PCR
The 16S DNA PCR yielded negative results in all control samples and positive results in all septic samples but also in 7 of 27 aseptic synovial samples, although they showed no indication of sepsis clinically or in their synovial laboratory values (leukocyte count, percentage neutrophils and total protein). Two of the 7 PCR‐positive samples were derived from horses who have been successfully treated from septic synovitis in the past.
3.6. Synovial leukocyte count and percentage of synovial neutrophils
For the synovial leukocyte count, a ROC‐calculated cut‐off of 14500/μL reached a 65.52% sensitivity, and 100% specificity for differentiating between septic and aseptic (AUC: 0.9509, CI: 0.8857‐1) and for the percentage of synovial neutrophils, a cut‐off of 76% achieved a 96.55% sensitivity and 100% specificity for discriminating between septic and aseptic samples (AUC: 0.9854, CI: 0.9545‐1) and synovial samples (P < .001, Table 2; Figure S2).
3.7. Correlations
Both the LYS and the MPO measurements showed a statistically highly significant correlation with PCR (r = .79 and r = .69, respectively), synovial leukocyte count (r = .752 and r = .571 respectively), % neutrophils (r = 0.751 and r = .663 respectively) and each other (r = .744, P < .001, Table 4).
TABLE 4.
Spearman correlation (r) and corresponding P‐values are detailed for the correlation between PCR, synovial leukocyte count, percentage (%) synovial neutrophils, lysozyme (LYS) and myeloperoxidase (MPO) activity
| PCR | Synovial Leukocytes | % Neutrophils | LYS | MPO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | r | P | r | P | r | P | r | P | r | |
| PCR | 1.0000 | .02 | .4240 | .0040 | .4910 | <.001 | .7930 | <.001 | .6900 | |
| Synovial Leukocytes | .02 | .4240 | 1.0000 | .0000 | .7400 | <.001 | .7520 | <.001 | .5710 | |
| % Neutrophils | .004 | .4910 | <.001 | .7400 | 1.0000 | <.001 | .7510 | <.001 | .6630 | |
| LYS | <.001 | .7930 | <.001 | .7520 | .0000 | .7510 | 1.0000 | <.001 | .7440 | |
| MPO | <.001 | .6900 | <.001 | .5710 | .0000 | .6630 | <.001 | .7440 | 1.0000 | |
The LYS and the MPO measurements as well as the PCR showed a statistically highly significant correlation with synovial leukocyte count, % neutrophils and each other.
4. DISCUSSION
Synovial inflammation results in altered transsynovial permeability, predominant synoviocyte cell population and synoviocyte metabolism with corresponding changes in synovial fluid composition. 3 , 5 , 6 , 47 , 48 , 49 , 50 Synovial cellular changes precede both clinical symptoms and biochemical changes, and increases in synovial fluid leukocytes have been measured as early as 8 h following experimental inoculation of equine tarsocrural joints, depending on the size and species of the bacterial inoculum. 51 However, while neutrophilia (>80%) is one of the most consistent findings in septic synovial fluid 51 and a good indicator for the presence of synovial sepsis, its utility to monitor treatment success is limited as neutrophil percentage remained increased beyond the clinical threshold used for diagnosis of septic synovitis for 10 days after the elimination of an experimentally induced bacterial infection. 52 Hence, synovial fluid chemistry markers, like creatine kinase, lactate dehydrogenase, D‐lactate, MMP‐2, MMP‐9, SAA and glucose, have been evaluated in the past but do not provide sufficient sensitivity, specificity or predictive value for the diagnosis of the extent and type of synovitis and the evaluation of the treatment response. 16 , 18 , 42 , 53 , 54 , 55 , 56 , 57
The three antimicrobial proteins investigated in this study are expressed specifically in myeloid cells (macrophages and neutrophils) and play a major part in the innate host defence against microorganisms. 58 , 59 , 60 Using enzymes that mainly stem from neutrophils and are markers of neutrophil stimulation and degranulation for the detection of synovial sepsis offers the advantage of being based on the first‐line immune response to infection and measuring neutrophil activity rather than just cellular presence. 40 , 61 , 62 , 63 In noninfectious states, MPO plasma concentrations correlate with the total number of neutrophils in the blood, while LYS concentrations correlate with their turnover rate. 62 , 63 , 64 The small molecular weight (14‐15 kDa) protein LYS may function as a highly responsive biomarker of synovial inflammation and sepsis, as its half‐life in plasma is only 75 min, 61 and could, thus, serve as a reliable marker not just for initial diagnosis but also for monitoring of treatment success.
Indeed, the LYS assay used in this study achieved a 100% sensitivity and specificity for differentiating between septic and aseptic or healthy synovial fluid. Synovial fluid LYS levels were significantly higher in septic than in aseptic or healthy control samples, and there was no overlap in measurement values for the three groups. Similarly, the synovial MPO assay used in this study achieved a 93.33% sensitivity, 100% specificity for distinguishing septic from control synovial samples and 93.33% sensitivity, 81.48% specificity for discriminating between septic and aseptic synovial samples, confirming the previously demonstrated potential of MPO as infection marker. 61 Synovial fluid MPO levels were also significantly higher in septic than in aseptic or healthy control samples. In this study, ELT activity could not be detected in any synovial sample. Previous studies have measured the presence of complexed, inhibited ELT in synovia using ELISA, not enzymatic activity. 61 The apparent lack of active ELT in synovial fluid samples could be explained by the presence of proteinase inhibitors, such as alpha1‐antiproteinase and alpha2‐macroglobulin, in equine plasma and synovial fluid, which can interfere with ELT activity. 29 , 43 , 65 , 66 , 67 Accordingly, ELT catalytic activity, as required for the hydrolysis of N‐methoxysuccinyl ala‐ala‐pro‐val p‐nitroanilide in the fast enzyme assay, may be affected. 29 , 43 A similar inhibitory effect has also been observed for the poor immunocapture of ELT by the antibodies of the ELISA. 29 , 43 Therefore, the catalytic activity of ELT cannot be used as an infection marker.
The statistically highly significant correlation of both the LYS and the MPO measurements with PCR, synovial leukocyte count, percentage neutrophils and each other further supports their use as biomarkers for the diagnosis of septic and noninfectious synovitis. To date, diagnosis of septic synovitis relies on a combination of clinical examination and laboratory parameters, none of which provide a sufficiently reliable basis for clinical decisions to achieve gold standard status. Additionally, currently available diagnostics, such as synovial fluid cytology, require laboratory equipment and trained personnel and, thus, shipping of the sample or horse to a suitably equipped facility. The resulting hurdles for diagnosis of septic synovitis can have substantial clinical, welfare and economic ramifications. In contrast, LYS analysis not only achieved a 100% sensitivity and specificity for discriminating between septic and aseptic synovitis in this study, but it can also be used in a point‐of‐care test, allowing diagnosis of synovial sepsis stall side. We chose to measure LYS, MPO and ELT using enzyme activity assays rather than ELISAs as the necessary ingredients are more stable and much cheaper, the activity assays are easier and faster to perform and, hence, have greater potential for routine diagnosis. Moreover, measuring the activity of potential marker enzymes has the potential to show the progression of an infection, while ELISAs only assess the presence of a protein. In addition, enzyme activity assays can be transferred rather easily to, eg lateral flow devices, which can be used without any laboratory equipment and directly at the point of care, as colour changes or test lines are straightforward to interpret by the naked eye.
The diagnosis of synovial sepsis in this study was based on the clinical examination, degree of lameness, synovial leukocyte count, percentage of neutrophils and total protein and was confirmed by bacterial culture and the 16S PCR, which detects the specific 16S ribosomal‐DNA (16S rDNA) regions that are present in essentially all bacteria. Detection of bacterial 16S rDNA in synovial fluid samples is, therefore, indicative of active or recent synovial infection. 6 , 17 , 68 , 69 However, false‐positive results, as also seen in our study, may occur attributable to contamination during sampling and analysis, the presence of background DNA and the fact that PCR techniques detect DNA regardless of the viability of the source bacteria. 6 , 17 , 30 , 68 , 69 , 70 , 71 , 72 In this study, all 7 positive PCR results in nonseptic samples were found in the aseptic synovitis group, none in the control group. As in the aseptic synovitis group, only horses with sufficiently long follow‐up were included to ascertain the correct diagnosis of aseptic synovitis based on the clinical development of the horse (no increase in lameness or synovial effusion), a subclinical infection can be excluded as the cause for these positive PCR results. While two of the PCR‐positive samples in the aseptic group were obtained from horses that had recently been successfully treated for septic synovitis and, hence, might still have had residual bacterial DNA in the synovial fluid, the reason for the other positive results is unclear and must be assumed to be the result of contamination during sampling or aliquoting.
Limitations of the study include the diverse ages (2 weeks‐26 years) of the included horses, the variable aetiology of septic arthritis and the broad range of affected structures. We chose to include horses of all ages and synovial sepsis of all aetiologies and structures, as we aimed to validate the test for clinical use and the variety of our study samples reflect the patient population presenting to our hospital for the diagnosis and treatment of possible septic synovitis. As expected, we did not find differences in enzyme assay results between different age groups, aetiologies or affected synovial structures. While long‐term sequelae, such as osteoarthritis or tendon adhesions, and the corresponding prognosis for return to function may differ between septic arthritis, tenosynovitis and bursitis, the diagnosis of septic synovitis is not based on structure‐specific markers (eg markers of cartilage or tendon‐specific catabolism) but on markers of septic synovitis, which have been established analogously in the literature for all synovial structures (joints, tendon sheaths and bursae 5 ). However, further studies including larger numbers of animals of different ages and septic synovitis of various aetiologies and anatomical structures are indicated to further validate and refine the cut‐off values for the enzyme activity assays in various clinical settings.
In conclusion, considering the clear‐cut results (highly significant with both sensitivity and specificity 100%) and clinical relevance, the LYS enzyme activity assay can be used as a reliable marker enzyme for the diagnosis of septic and noninfectious synovitis. The MPO enzyme activity assay can be used to confirm the diagnosis of septic synovitis, as it discriminates nearly as well. LYS and MPO activity assays can easily be transferred to a point‐of‐care diagnostic kit to provide a reliable and quick stall‐side diagnosis of synovial sepsis and allow immediate therapeutic intervention.
CONFLICT OF INTERESTS
E. Sigl is a co‐founder of Qualizyme Diagnostics GmbH & Co KG. C. Gamerith is an employee of this company, which applied for a patent for the biomarker assays.
AUTHOR CONTRIBUTIONS
R. Haralambus contributed to study design and execution, data analysis and interpretation, and manuscript preparation. A. Florczyk, S. Gültekin, S. Brandt and M. Schnierer contributed to study execution. E. Sigl contributed to study design, and data analysis and interpretation. C. Vogl contributed to data analysis and interpretation, and manuscript preparation. C. Gamerith contributed to study execution, data analysis and interpretation, and manuscript preparation. F. Jenner contributed to study design, data analysis and interpretation, and manuscript preparation. All authors have read and approved the manuscript.
ETHICAL ANIMAL RESEARCH
Research ethics committee oversight not currently required by this journal: procedures were performed as part of clinical investigations or, with consent, post‐mortem.
INFORMED CONSENT
Owners gave informed consent for their animals’ inclusion in the study.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/evj.13459.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Haralambus R, Florczyk A, Sigl E, et al. Detection of synovial sepsis in horses using enzymes as biomarkers. Equine Vet J. 2022;54:513–522. 10.1111/evj.13459
Clemens Gamerith and Florien Jenner shared last author.
Funding information
The study was financed by the Austrian Research Promotion Agency (grant number: 853013) and the Styrian business promotion agency (grant number: 1000054206).
DATA ACCESSIBILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tulamo R, Bramlage LR, Gabel AA. Sequential clinical and synovial fluid changes associated with acute infectious arthritis in the horse. Equine Vet J. 1989;21:325–31. [DOI] [PubMed] [Google Scholar]
- 2. Schneider RK, Bramlage LR, Moore RM, Mecklenburg LM, Kohn CW, Gabel AA. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. 1992;24:436–42. [DOI] [PubMed] [Google Scholar]
- 3. Spiers S, May SA, Harrison LJ, Bennett D, Edwards GB. Proteolytic enzymes in equine joints with infectious arthritis. Equine Vet J. 1994;26:48–50. [DOI] [PubMed] [Google Scholar]
- 4. Wright I, Smith MRW, Humphrey DJ, Eaton‐Evans T, Hillyer MH. Endoscopic surgery in the treatment of contaminated and infected synovial cavities. Equine Vet J. 2003;35:613–9. [DOI] [PubMed] [Google Scholar]
- 5. Morton AJ. Diagnosis and treatment of septic arthritis. Vet Clin North Am: Equine Pract. 2005;21:627–49. [DOI] [PubMed] [Google Scholar]
- 6. Steel CM. Equine synovial fluid analysis. Vet Clin North Am: Equine Pract. 2008;24:437–54. [DOI] [PubMed] [Google Scholar]
- 7. Milner PI, Bardell DA, Warner L, Packer MJ, Senior JM, Singer ER, et al. Factors associated with survival to hospital discharge following endoscopic treatment for synovial sepsis in 214 horses. Equine Vet J. 2014;46:701–5. [DOI] [PubMed] [Google Scholar]
- 8. Auer JA, Stick JA. Equine Surgery (5th edn). Elsevier Health Sciences; 2018. [Google Scholar]
- 9. Bryant HA, Dixon JJ, Weller R, Bolt DM. Use of positive contrast radiography to identify synovial involvement in horses with traumatic limb wounds. Equine Vet J. 2019;51:20–3. [DOI] [PubMed] [Google Scholar]
- 10. Gilbertie JM, Schnabel LV, Stefanovski D, Kelly DJ, Jacob ME, Schaer TP. Gram‐negative multi‐drug resistant bacteria influence survival to discharge for horses with septic synovial structures: 206 Cases (2010–2015). Vet Microbiol. 2018;226:64–73. [DOI] [PubMed] [Google Scholar]
- 11. Crosby DE, Labens R, Hughes KJ, Nielsen S, Hilbert BJ. Factors associated with survival and return to function following synovial infections in horses. ‐ PubMed ‐ NCBI. Front Vet Sci. 2019;6:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Orsini JA. Meta‐analysis of clinical factors affecting synovial structure infections and prognosis. J Equine Vet Sci. 2017;55:105–14. [Google Scholar]
- 13. Madison JB, Sommer M, Spencer PA. Relations among synovial membrane histopathologic findings, synovial fluid cytologic findings, and bacterial culture results in horses with suspected infectious arthritis: 64 cases (1979–1987). J Am Vet Med Assoc. 1991;198:1655–61. [PubMed] [Google Scholar]
- 14. Robinson CS, Timofte D, Singer ER, Rimmington L, Rubio‐Martínez LM. Prevalence and antimicrobial susceptibility of bacterial isolates from horses with synovial sepsis: a cross‐sectional study of 95 cases. Vet J. 2016;216:117–21. [DOI] [PubMed] [Google Scholar]
- 15. Dumoulin M, Pille F, van den Abeele A, Boyen F, Boussauw B, Oosterlinck M, et al. Use of blood culture medium enrichment for synovial fluid culture in horses: a comparison of different culture methods. Equine Vet J. 2010;42:541–6. [DOI] [PubMed] [Google Scholar]
- 16. Faraj AA, Omonbude OD, Godwin P. Gram staining in the diagnosis of acute septic arthritis. Acta Orthop Belg. 2002;68:388–91. [PubMed] [Google Scholar]
- 17. Pille F, Martens A, Schouls LM, Peelman L, Gasthuys F, Schot CS, et al. Detection of bacterial DNA in synovial fluid from horses with infectious synovitis. ‐ PubMed ‐ NCBI. Res Vet Sci. 2004;77:189–95. [DOI] [PubMed] [Google Scholar]
- 18. Stack JD, Cousty M, Steele E, Handel I, Lechartier A, Vinardell T, et al. Comparison of serum amyloid a measurements in equine synovial fluid with routine diagnostic methods to detect synovial infection in a clinical environment. Front Vet Sci. 2019;6:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shmerling RH, Delbanco TL, Tosteson AN, Trentham DE. Synovial fluid tests. What should be ordered? J Am Med Assoc. 1990;264:1009–14. [PubMed] [Google Scholar]
- 20. Cousty M, Stack JD, Tricaud C, David F. Effect of arthroscopic lavage and repeated intra‐articular administrations of antibiotic in adult horses and foals with septic arthritis. Vet Surg. 2017;46:1008–16. [DOI] [PubMed] [Google Scholar]
- 21. Bainton DF, Ullyot JL, Farquhar MG. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971;134:907–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selsted ME, Martinez RJ. Lysozyme: primary bactericidin in human plasma serum active against Bacillus subtilis. Infect Immun. 1978;20:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rice WG, Ganz T, Kinkade JJ, Selsted ME, Lehrer RI, Parmley RT. Defensin‐rich dense granules of human neutrophils. Blood. 1987;70:757–65. [PubMed] [Google Scholar]
- 24. Flodgaard H, Østergaard E, Bayne S, Svendsen A, Thomsen J, Engels M, et al. Covalent structure of two novel neutrophile leucocyte‐derived proteins of porcine and human origin. Eur J Biochem. 1991;197:535–47. [DOI] [PubMed] [Google Scholar]
- 25. Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arnljots K, Sørensen O, Lollike K, Borregaard N. Timing, targeting and sorting of azurophil granule proteins in human myeloid cells. Leukemia. 1998;12:1789–95. [DOI] [PubMed] [Google Scholar]
- 27. Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus‐induced arthritis and sepsis. Arthritis Rheum. 2000;43:2276–82. [DOI] [PubMed] [Google Scholar]
- 28. Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–27. [DOI] [PubMed] [Google Scholar]
- 29. Ceusters JD, Serteyn DA, Minguet G, de la Rebière de Pouyade G, Romainville J, Deby‐Dupont GP, et al. An in vitro whole blood model to test the effects of different stimuli conditions on the release of myeloperoxidase and elastase by equine neutrophils. Vet Immunol Immunopathol. 2012;150:221–7. [DOI] [PubMed] [Google Scholar]
- 30. Boff D, Crijns H, Teixeira MM, Amaral FA, Proost P. Neutrophils: beneficial and harmful cells in septic arthritis. IJMS. 2018;19:468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bruhn O, Grötzinger J, Cascorbi I, Jung S. Antimicrobial peptides and proteins of the horse‐insights into a well‐armed organism. Vet Res. 2011;42:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teng T‐S, Ji A‐L, Ji X‐Y, Li Y‐Z. Neutrophils and Immunity: from bactericidal action to being conquered. J Immunol Res. 2017;2017:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Ann Rev Pathol. 2014;9:181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. [DOI] [PubMed] [Google Scholar]
- 35. Hasmann A, Gewessler U, Hulla E, Schneider KP, Binder B, Francesko A, et al. Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp Dermatol. 2011;20:508–13. [DOI] [PubMed] [Google Scholar]
- 36. Hasmann A, Wehrschuetz‐Sigl E, Kanzler G, Gewessler U, Hulla E, Schneider KP, et al. Novel peptidoglycan‐based diagnostic devices for detection of wound infection. Diagn Microbiol Infect Dis. 2011;71:12–23. [DOI] [PubMed] [Google Scholar]
- 37. Hasmann A, Wehrschuetz‐Sigl E, Marold A, Wiesbauer H, Schoeftner R, Gewessler U, et al. Analysis of myeloperoxidase activity in wound fluids as a marker of infection. Ann Clin Biochem. 2013;50:245–54. [DOI] [PubMed] [Google Scholar]
- 38. Heinzle A, Papen‐Botterhuis NE, Schiffer D, Schneider KP, Binder B, Schintler M, et al. Novel protease‐based diagnostic devices for detection of wound infection. Wound repair and regeneration. 2013;21:482–9. [DOI] [PubMed] [Google Scholar]
- 39. Schiffer D, Blokhuis‐Arkes M, van der Palen J, Sigl E, Heinzle A, Guebitz GM. Assessment of infection in chronic wounds based on the activities of elastase, lysozyme and myeloperoxidase. Br J Dermatol. 2015;173:1529–31. [DOI] [PubMed] [Google Scholar]
- 40. Blokhuis‐Arkes MHE, Haalboom M, van der Palen J, Heinzle A, Sigl E, Guebitz G, et al. Rapid enzyme analysis as a diagnostic tool for wound infection: Comparison between clinical judgment, microbiological analysis, and enzyme analysis. Wound repair and regeneration. 2015;23:345–52. [DOI] [PubMed] [Google Scholar]
- 41. Torbeck RL, Prieur DJ. Plasma and synovial fluid lysozyme activity in horses with experimental cartilage defects. Am J Vet Res. 1979;40:1531–6. [PubMed] [Google Scholar]
- 42. Fietz S, Bondzio A, Moschos A, Hertsch B, Einspanier R. Measurement of equine myeloperoxidase (MPO) activity in synovial fluid by a modified MPO assay and evaluation of joint diseases – An initial case study. Res Vet Sci. 2008;84:347–53. [DOI] [PubMed] [Google Scholar]
- 43. Dagleish MP, Wakeman KD, McDiarmid AM. A preliminary evaluation of the use of equine neutrophil elastase 2A concentration in synovial fluid as a marker for joint inflammation in horses. Equine Vet J. 2003;35:623–6. [DOI] [PubMed] [Google Scholar]
- 44. Wauters J, Franck T, Pille F, Martens A, Demeyere K, Sys S, et al. Flow cytometric detection of myeloperoxidase in horse neutrophils: a novel technique in equine diagnostic research. Vet Immunol Immunopathol. 2011;144:417–22. [DOI] [PubMed] [Google Scholar]
- 45. Shugar D. The measurement of lysozyme activity and the ultra‐violet inactivation of lysozyme. Biochimica et Biophysica Acta (BBA) ‐ Gene Regulatory Mechanisms. 1952;8:302–9. [DOI] [PubMed] [Google Scholar]
- 46. R Core Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. https://www.R‐project.org [Google Scholar]
- 47. Persson L. On the synovia in horses. A clinical and experimental study. Acta Vet Scand Suppl. 1971;35:3–77. [PubMed] [Google Scholar]
- 48. Tew WP, Hotchkiss RN. Synovial fluid analysis and equine joint disorders. J Equine Vet Sci. 1981;1:163–70. [Google Scholar]
- 49. van Pelt RW. Interpretation of synovial fluid findings in the horse. J Am Vet Med Assoc. 1974;165:91–5. [PubMed] [Google Scholar]
- 50. McCarty WJ, Cheng JC, Hansen BC, Yamaguchi T, Firestein GS, Masuda K, et al. The biophysical mechanisms of altered hyaluronan concentration in synovial fluid after anterior cruciate ligament transection. Arthritis Rheumatol. 2012;64:3993–4003. https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/22933328/?tool=EBI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tulamo RM, Bramlage LR, Gabel AA. The influence of corticosteroids on sequential clinical and synovial fluid parameters in joints with acute infectious arthritis in the horse. Equine Vet J. 1989;21:332–7. [DOI] [PubMed] [Google Scholar]
- 52. Koziy RV, Yoshimura S, Dickinson R, Rybicka JM, Moshynskyy I, Ngeleka M, et al. Use of standard diagnostic techniques to determine eradication of infection in experimental equine septic arthritis. Can J Vet Res. 2019;83:24–33. [PMC free article] [PubMed] [Google Scholar]
- 53. Yancik SA, McIlwraith CW, Wagner AE, Trotter GW. Evaluation of creatine kinase and lactate dehydrogenase activities in clinically normal and abnormal equine joints. Am J Vet Res. 1987;48:463–6. [PubMed] [Google Scholar]
- 54. Fietz S, Einspanier R, Hoppner S, Hertsch B, Bondzio A. Determination of MMP‐2 and ‐9 activities in synovial fluid of horses with osteoarthritic and arthritic joint diseases using gelatin zymography and immunocapture activity assays. Equine Vet J. 2008;40:266–71. [DOI] [PubMed] [Google Scholar]
- 55. Kidd JA, Barr ARS, Tarlton JF. Use of matrix metalloproteinases 2 and 9 and white blood cell counts in monitoring the treatment and predicting the survival of horses with septic arthritis. Vet Rec. 2007;161:329–34. [DOI] [PubMed] [Google Scholar]
- 56. Ludwig EK, Brandon Wiese R, Graham MR, Tyler AJ, Settlage JM, Werre SR, et al. Serum and synovial fluid serum amyloid a response in equine models of synovitis and septic arthritis. Vet Surg. 2016;45:859–67. [DOI] [PubMed] [Google Scholar]
- 57. Robinson CS, Singer ER, Piviani M, Rubio‐Martínez LM. Are serum amyloid A or D‐lactate useful to diagnose synovial contamination or sepsis in horses? Vet Rec. 2017;181:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cross M, Mangelsdorf I, Wedel A, Renkawitz R. Mouse lysozyme M gene: isolation, characterisation, and expression studies. Proc Natl Acad Sci USA. 1988;85:6232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000;96:719–26. [PubMed] [Google Scholar]
- 60. Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: a new player in autoimmunity. Cell Immunol. 2017;317:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hansen NE, Karle H, Andersen V. Lysozyme turnover in the rat. J Clin Invest. 1971;50:1473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hansen NE, Malmquist J, Thorell J. Plasma Myeloperoxidase And Lactoferrin Measured By Radioimmunoassay: Relations To Neutrophil Kinetics. Acta Medica Scandinavica. 1975;198:437–43. [DOI] [PubMed] [Google Scholar]
- 63. Hansen NE, Karle H, Andersen V, Malmquist J, Hoff GE. Neutrophilic granulocytes in acute bacterial infection. Sequential studies on lysozyme, myeloperoxidase and lactoferrin. Clin Exp Immunol. 1976;26:463–8. [PMC free article] [PubMed] [Google Scholar]
- 64. Salciccia A, Grulke S, de la Rebière de Pouyade G, Franck T, Detilleux J, Serteyn D, et al. Assessment of systemic inflammation by time‐trends of blood granulocyte count and plasma myeloperoxidase and elastase concentrations following colic surgery in horses. J Vet Emerg Critical Care. 2016;26:541–8. [DOI] [PubMed] [Google Scholar]
- 65. de la Rebière de Pouyade G, Riggs LM, Moore JN, Franck T, Deby‐Dupont G, Hurley DJ, et al. Equine neutrophil elastase in plasma, laminar tissue, and skin of horses administered black walnut heartwood extract. Vet Immunol Immunopathol. 2010;135:181–7. [DOI] [PubMed] [Google Scholar]
- 66. Wilkinson DJ, del Carmen Arques M, Huesa C, Rowan AD. Serine proteinases in the turnover of the cartilage extracellular matrix in the joint: implications for therapeutics. Br J Pharmacol. 2019;176:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baici A, Salgam P, Cohen G, Fehr K, Böni A. Action of collagenase and elastase from human polymorphonuclear leukocytes on human articular cartilage. Rheumatol Int. 1982;2:11–6. [DOI] [PubMed] [Google Scholar]
- 68. Crabill MR, Cohen ND, Martin LJ, Simpson RB, Burney N. Detection of bacteria in equine synovial fluid by use of the polymerase chain reaction. Vet surg: VS: the official journal of the American College of Veterinary Surgeons. 1996;25:195–8. [DOI] [PubMed] [Google Scholar]
- 69. Elmas CR, Koenig JB, Bienzle D, Cribb NC, Cernicchiaro N, Coté NM, et al. Evaluation of a broad range real‐time polymerase chain reaction (RT‐PCR) assay for the diagnosis of septic synovitis in horses. Can J Vet Res. 2013;77:211–7. [PMC free article] [PubMed] [Google Scholar]
- 70. Canvin JM, Goutcher SC, Hagig M, Gemmell CG, Sturrock RD. Persistence of Staphylococcus aureus as detected by polymerase chain reaction in the synovial fluid of a patient with septic arthritis. Br J Rheumatol. 1997;36:203–6. [DOI] [PubMed] [Google Scholar]
- 71. Fenollar F, Roux V, Stein A, Drancourt M, Raoult D. Analysis of 525 samples to determine the usefulness of PCR amplification and sequencing of the 16S rRNA gene for diagnosis of bone and joint infections. J Clin Microbiol. 2006;44:1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhandal J, Hayashi K, Kim S‐Y, Klein M, Wong A, Toupadakis CA, et al. Detection of bacterial DNA by PCR in dogs with stifle pathology. Vet Surg. 2013;68:814–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


