Abstract
Background
The frequency of tumor cell dissemination in the peritoneal cavity is critically related to the progression of peritoneal metastases (PM). Recently, flow cytometry (FCM) has been successfully used to detect tumor cells in malignant effusions.
Methods
A total of 143 single cell suspensions derived from ascites or peritoneal lavages from patients with advanced gastric cancer (GC) were stained with monoclonal antibodies to CD45 and to CD326 as well as 4,6‐diamidino‐2‐phenylindole (DAPI) and FVS780. Using FCM, tumor‐leukocyte ratio (TLR) were calculated from CD45(−)CD326(+) tumor cell counts/ CD45(+)CD326(+) leukocyte counts in DAPI (+) FVS780(−) gated area. In 54 patients, the ratios of CD11b(+), CD4(+) and CD8(+) cells in CD45(+) leukocytes were evaluated in parallel.
Results
TLR of 69 patients with PM were significantly higher than those of 74 without PM (p < .001) and log(TLR) showed strong correlation with peritoneal cancer index scores in 51 PM (+) patients (r = 0.439). TLR in PM (+) patients also correlated with the ratio of CD11b (+) myeloid cells (r = 0.547), and correlated inversely with those of CD4(+) (r = −0.490) and CD8(+) T cells (r = −0.648). In PM (−) patients who underwent gastrectomy, TLR never exceeded 0.1% in patients with primary GC without serosal involvement (<T4). However, TLR in patients with T4 GC were significantly higher (p < .05) and peritoneal recurrence occurred in 6/8 patients whose TLR exceeded 0.1%.
Conclusion
TLR in peritoneal fluid reflects tumor burden and the immune environment in peritoneal cavity. Multicolor FCM may provide additional information which can be used for the treatment of the patients with PM.
Keywords: CD326, cytology, flow cytometry, intraperitoneal tumor cells, peritoneal metastasis
1. INTRODUCTION
Peritoneal metastases (PM) are a common site of recurrence in patients with abdominal malignancies especially in gastric and ovarian cancers (Isobe et al., 2011; Jemal et al., 2011; Prat & FIGO Committee on Gynecologic Oncology, 2015). PM are thought to be caused by free intraperitoneal free tumor cells, which have been exfoliated from the serosal surface of the primary tumor (Sodek et al., 2012; Thibault et al., 2014). Numerous studies have shown that the presence of tumor cells in peritoneal lavage (i.e., cytologic status) is the most important determinant to predict the development of peritoneal recurrences in patients with various types of abdominal malignancies (Bando et al., 1999; Bonenkamp et al., 1996; Hayes et al., 1999; Horattas et al., 1997; Tustumi et al., 2016; Vogel et al., 2000; Warshaw, 1991; Ziselman et al., 1984). Cytology status was determined by the morphological detection of tumor cells using hematoxylin/eosin or Papanicolaou staining as the gold standard. However, the results of cytologic diagnosis using conventional methods is varied among institutions or pathologists with a relatively high false negative rate (Leake, Cardoso, Seevaratnam, Lourenco, Helyer, Mahar, Rowsell, & Coburn, 2012; Motherby et al., 1999). Reverse transcription polymerase chain reaction method to detect tumor cell‐specific messenger RNA (mRNA), such as carcinoembryonic antigen or cytokeratin 19 or 20, has improved the sensitivity in detecting intraperitoneal tumor cells with a better correlation with peritoneal recurrence in gastric cancer (GC) (Dalal et al., 2008; Katsuragi et al., 2007; Kodera et al., 2002; Rossi Del Monte et al., 2012; Tustumi et al., 2016).However, the amplified mRNA may be derived from dead cells or phagocytes that have engulfed tumor cells, and can be released from hematopoietic cells in an inflammatory context. (Kowalewska et al., 2008), which may result in a false positive case. Immunostaining methods using specific mAbs to tumor specific antigens have been clinically introduced to increase sensitivity (Benevolo et al., 1998; Vogel et al., 1999). However, the use of this method has been limited because of the complexity of the staining procedure.
Flow cytometry (FCM) is an established method to detect the expression pattern of various antigens especially in cells of hematological origin and is widely used in the diagnosis of hematologic neoplasms (Craig & Foon, 2008). Recently, many investigators have applied FCM to detect disseminated tumor cells using mAb to EpCAM (CD326) which is widely overexpressed in a variety of human cancers (Baeuerle & Gires, 2007; Patriarca et al., 2012; Went et al., 2004), and found in effusion specimens obtained from the pleural or peritoneal cavities (Acosta et al., 2016; Davidson et al., 2007; Kitayama et al., 2014; Krishan et al., 2010; Pillai et al., 2013; Risberg et al., 2000; Szantho et al., 2018). The results in these studies suggest that CD326(+) tumor cells can be clearly distinguished from leukocytes which are positive for CD45, a pan‐leukocyte marker, and thus FCM is a useful method for the diagnosis of malignant effusions as an adjunct to cytology.
In a previous study, we used this method and further attempted to quantify the relative frequency of tumor cells by calculating the tumor‐leukocyte ratio (TLR) in each patient and showed that the TLRs are a biomarker to estimate peritoneal tumor burden in patients with PM from GC (Kitayama et al., 2015). In this method, however, the number of CD326(+) cells is very low relative to CD45(+) leukocytes in samples from patients with early stage PM and thus it is difficult to distinguish the real tumor cells from the cells with nonspecific staining. In this study, therefore, we additionally stained samples with nuclear‐specific dyes 4,6‐diamidino‐2‐phenylindole (DAPI) and calculated the TLRs in a DAPI (+) nucleated cell population in peritoneal lavage fluids as well as ascites obtained from patients newly diagnosed with advanced GC and assessed their clinical significance.
2. MATERIALS AND METHODS
2.1. Patients and samples
Samples of ascites or peritoneal lavages were recovered from 143 patients and analyzed in this study. Among them, 76 samples were obtained from the patients who underwent open abdominal surgery and 55 samples from patients who underwent staging laparoscopy for advanced gastric cancer at the Department of Gastrointestinal Surgery, Jichi Medical University between April 2016 and June 2020. In these patients, peritoneal lavage was performed using 500 ml of normal saline before operative manipulation and 250 ml samples of the lavage fluid were recovered. In the presence of ascites, 10–20 ml of ascites fluid was obtained just after entering abdominal cavity in laparotomy or laparoscopy. Among them, macroscopic PM (P1) was observed in 50 patients and microscopic PM was confirmed by cytology (P0CY1) in an additional seven patients, for whom peritoneal cancer index (PCI) scores were described according to the standardized PCI scoring system (Jacquet & Sugarbaker, 1996). In 12 patients with massive amount of malignant ascites, samples were obtained by direct paracentesis performed for palliation. Cytology status was evaluated by pathologists for all samples. This study was carried out in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Jichi Medical University (RIN A15‐163)
2.2. MAbs
Allophycocyanin‐conjugated mAb to CD326 (EpCAM) was purchased from Miltenyi Biotec (Auburn, CA). Fluorescein isothiocyanate‐conjugated mAb to CD45, Fc‐blocker, FVS780 and control mouse IgG1, were purchased from Becton‐Dickinson (San Jose, CA) and DAPI from ThermoFisher Scientific (Waltham, MA). Phycoerythrin‐conjugated mAb to CD4, allophycocyanin‐conjugated mAb to CD8 and BV605‐conjugated ant‐CD11b were purchased from BioLegend (San Diego, CA).
2.3. Cell processing and FCM
After the centrifugation of ascites or peritoneal lavage fluid at 1500 rpm for 10 min, the pellets were resuspended in phosphate buffered saline (PBS)+0.02% EDTA and overlaid on Ficoll–Hypaque solution (Pharmacia Biotech, Piscataway, NJ). After centrifugation at 3000 rpm for 10 min, the intermediate layer was washed twice with PBS+0.02% EDTA. During the procedure, most of the cell clusters were dissociated to form single cell suspensions. The cells (1 × 106) were suspended in 100 μl of PBS+0.02% EDTA and incubated with FVS780 to label dead cells. After washing with PBS, the cells were incubated with 10 μl of Fc‐blocker for 20 min and immunostained with fluorescein isothiocyanate‐conjugated mAb to CD45, phycoerythrin‐conjugated mAb to CD326 for 30 min at 4°C as per the manufacturers' recommendation. Then, the cells were treated with fixation buffer (Becton‐Dickinson, San Jose, CA) and DAPI was added at a final concentration of 0.1 μg/ml to detect nucleated cells. After washing with PBS, the cell suspension was applied to BD LSRFortessa™X‐20 (Becton‐Dickinson, San Jose, CA) and antigen expressions analyzed using Flow Jo™ software (Becton‐Dickinson). The TLR was calculated as CD326(+) cell counts/CD45(+) cell counts. In some experiments, the remnant cells were additionally stained with mAbs to CD45, CD4, CD8, and CD11b together with FVS780 and DAPI, and the ratios of cells positive for each antigen were calculated against CD45(+) whole leukocytes.
2.4. Statistics
Statistical analysis was performed using Graph Pad Prism 8. Statistical differences in clinical and pathological factors were evaluated with the Mann–Whitney U test. Correlation between cell densities were analyzed with Spearman's rank‐order correlation analysis. Survival rates were calculated using the Kaplan–Meier method and differences were evaluated with the log‐rank test. In all tests, the standard for a significant difference was set at p < .05.
3. RESULTS
3.1. Calculation of the TLR in peritoneal fluid from patients with GC
The profile of the peritoneal fluid samples analyzed with FCM is shown in Table 1. A total of 106–107 cells were generally recovered from 100 ml of peritoneal lavage fluid in patients who underwent surgery or 10 ml of malignant ascites of from patients with PM. The gating strategy for stained cells in a typical sample is shown in Figure 1 (upper panel). Nucleated cells and dead cells were clearly distinguished by DAPI and FVS780 staining, respectively. The expression of CD326 and CD45 were mutually exclusive in nucleated live cells detected in DAPI‐positive and FVS780‐negative area, and the numbers of CD45(−) CD326 (+) tumor cells and CD45(+) CD326(−) leukocytes were counted and the percentages of CD326 (+) tumor cells were calculated relative to CD45(+) leukocytes as the TLR. The total cell counts varied from 103 to 105 among samples and the calculated TLR were adopted as reliable values, only when more than 104 CD45(+) leukocytes were acquired.
TABLE 1.
Peritoneal fluid samples evaluated with flow cytometry
| Method of sample acquisition | Number | PM(−) | PM(+) | ||
|---|---|---|---|---|---|
| P0CY0 | P0CY1 | P1CY0 | P1CY1 | ||
| Laparotomy | 76 | 68 | 1 | 1 | 6 |
| Staging laparoscopy | 55 | 6 | 6 (2) | 12 (2) | 31 (10) |
| Paracentesis | 12 | 1 (1) | 11 (11) | ||
| Total | 143 | 74 | 7 | 14 | 48 |
Note: Data in parentheses are the number of ascitic fluid samples.
Abbreviations: CY, peritoneal cytology; P, macroscopic metastasis; PM, peritoneal metastasis.
FIGURE 1.
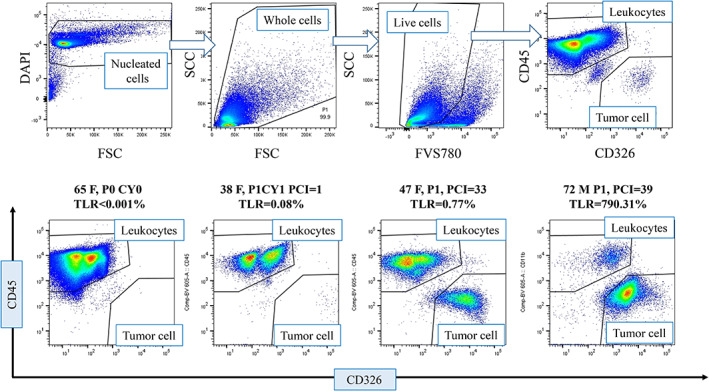
Examples of CD45(−) CD326(+) tumor cells and CD45(+) CD326(−) leukocytes in peritoneal fluid samples. The gating strategy in a representative sample (upper panel). Samples were immunostained as described in Section 2. Using Flow Jo software, DAPI (−) noncellular components were initially excluded and then FVS780(+) dead cells were excluded from all dots. Then, the single positive areas were gated in the live nucleated cell population and TLR was calculated as CD45(−) CD326(+) counts/CD45(+) CD326(−) counts. FACS profiles of a sample from a patient without PM (left figure of lower panel) and 3 from patients with PM (right figures of lower panel). DAPI, 4,6‐diamidino‐2‐phenylindole; FACS, fluorescence activated cell sorter; PM, peritoneal metastases; TLR, tumor‐leukocyte ratio [Color figure can be viewed at wileyonlinelibrary.com]
Peritoneal fluid samples usually contain many micro particles other than live cells which appear in the gated field of FCM plots. Therefore, in our previous method to calculate TLR (Kitayama et al., 2014), it is difficult to accurately distinguish the real tumor cells from non‐specific binding in cases of low TLR (Figure S1; upper panel). In contrast, in the new method, DAPI (−) debris or micro particles were removed from analysis and the percentages of nonspecific binding were much less than those examined by previous method, and thus TLR was directly calculated using the numbers of CD45(+) and CD326(+) dots (Figure S1; lower panel).
Next, we mixed 10–104 MKN45, a human gastric cancer cell, in 105 cells derived from lavages of a patient without peritoneal metastasis and evaluated the TLR. The results showed a good correlation with the predicted values with relatively small error ranges (Figure S2).
3.2. TLR correlates with cytology status, presence of ascites, and PCI scores in patients with PM
The median (M) TLR in samples from peritoneal fluid of the patients with macroscopic PM (P1) or microscopic PM (CY1) was 0.429%. However, the TLRs were highly variable among the samples (0.004–1668%, n = 69) (Figure 1 lower panel, Figure 2a). In comparison, the TLR of the samples from patients without PM showed significantly lower values of TLR (M = 0.012%, 0–0.377%, p < .001, n = 74) (Figure 2a). Similarly, the median of the TLR of samples from patients with positive cytology was 0.539% (0.005–1668%, n = 55), which was significantly higher than those from patients with negative cytology samples (M = 0.015%, 0–0.815%, n = 88). (p < .001) (Figure 2b). Among 69 patients with PM, cytology was positive in 55 and negative in 14 patients, and TLR of patients with positive cytology was 0.539% (0.005–1669%) which was significantly higher than those from patients with negative cytology (M = 0.035%, 0.004–0.604%) (Figure 2c). The receiver operating characteristic (ROC) curve revealed that TLR correlates with microscopic as well as macroscopic PM with high accuracy (Figure S3a–c).
FIGURE 2.
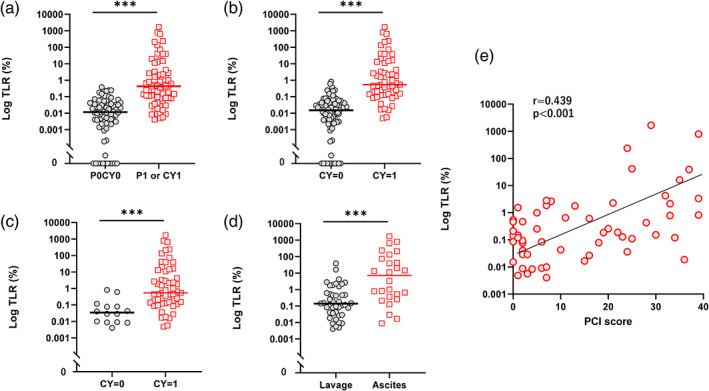
Tumor‐leukocyte ratios (TLR) in 143 patients with gastric cancer with or without peritoneal metastasis (PM). (a) TLR of 69 PM (+) and 74 PM (−) patients. Patients with PM [PM (+)] patients includes those with macroscopic tumor nodules (P1) and/or cytology positive (CY1) without macroscopic nodules, while patients without PM [PM (−)] patients are P0CY0. (b) TLR of 56 patients with positive cytology [CY (+)] patients and 87 with negative cytology [CY (−)]. (c) TLR of 55 CY1 and 14 CY0 patients among 69 patients with PM. (d) TLR of 26 samples from ascites and 43 samples from lavages in 69 patients with PM. (e) Correlation of peritoneal cancer index (PCI) scores and log values of TLR in 58 patients with PM. ***p < .001 with Mann–Whitney U test. Correlation was analyzed with Spearman's rank‐order correlation analysis [Color figure can be viewed at wileyonlinelibrary.com]
Samples were obtained from ascites in 26 patients and their TLR were much higher than those of other 43 samples from lavage samples in patients without ascites (M = 7.206%, 0.009–1668% vs. M = 0.139%, 0.006–39.00%, p < .001) (Figure 2d). Log(TLR) values correlated strongly with PCI scores in patients with PM (r = 0.439, p < .0001) (Figure 2e).
3.3. TLR correlates with the ratios of leukocyte populations in peritoneal fluid in PM (+) patients
In 54 peritoneal fluid samples which contained many cells, the ratios of CD4(+) and CD8(+) T cells as well as CD11b(+) myeloid cells to CD45(+) leukocytes populations were calculated in parallel (Figure 3). As shown in Figure 4a, the ratio of CD11b(+) myeloid cells to CD45(+) cells were markedly elevated in samples from patients with PM (M = 45.2%, 8.3–90.0%, n = 27 vs. M = 24.9%, 5.1–52.2% n = 27, p < .001). In contrast, the ratios of CD8(+) T cells to CD45(+) cells were significantly reduced in samples from patients with PM (M = 16.6%, 3.3–49.3%, n = 27 vs. M = 30.6%, 15.7–61.2%, n = 27, p < .001). The same trend was observed in CD4(+) T cells although the difference was not significant (M = 16.0%, 1.5–44.7%, n = 27 vs. M = 19.2%, 4.7‐40.6%, n = 27, p = .10). The ratios of CD11b (+) cells to CD45(+) cells showed a strong correlation with log(TLR) in 27 samples from patients with PM (r = 0.547, p = .0031), while the ratios of CD4(+) and CD8(+) T cells to CD45(+) cells showed contrast inverse correlations with TLR (CD4: r = −0.490, p = .0095, CD8: r = −0.648, p = .0003) (Figure 4b–d).
FIGURE 3.
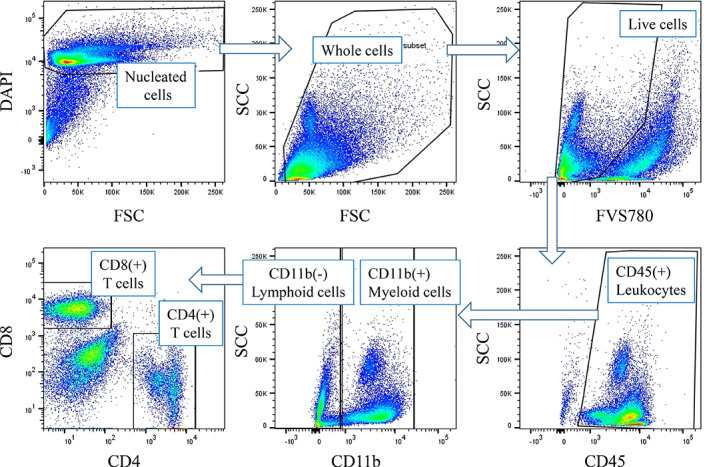
Gating strategy of leukocyte subpopulation in peritoneal fluids. The cells were immunostained as described with mAbs to CD11b, CD4, CD8, and CD45 together with DAPI and FVS780 as described in Section 2. Live and nucleated cells were selected in DAPI (+) and FVS780(−) gate as described in legend of Figure 1 and ratios of positive cells to CD45(+) cells were calculated. DAPI, 4,6‐diamidino‐2‐phenylindole [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
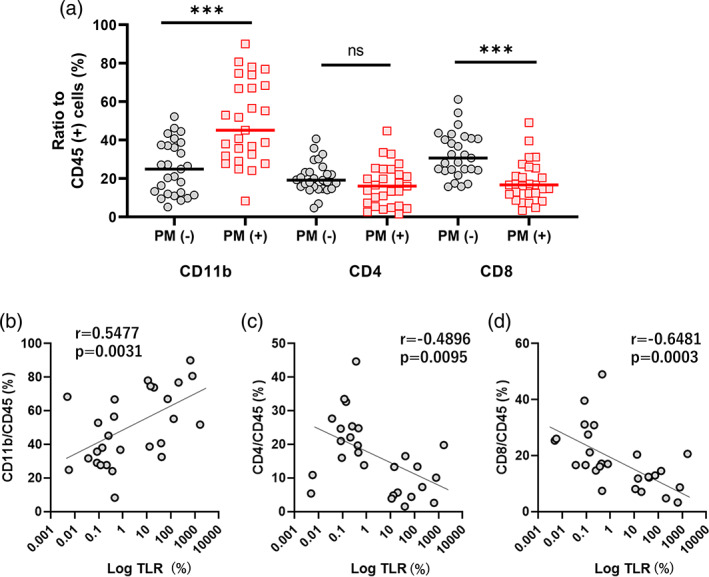
Leukocyte subpopulations in peritoneal fluids in PM (+) patients. The ratios of CD11b(+) myeloid cells as well as CD4(+) and CD8(+) T cells to CD45(+) pan‐leukocytes (a) and correlation with TLR (b). Intergroup differences and correlations were examined with Mann–Whitney U test and Spearman's rank‐order correlation analysis. ***p < .001. TLR, tumor‐leukocyte ratio [Color figure can be viewed at wileyonlinelibrary.com]
3.4. TLR correlates with peritoneal recurrence in patients of T4 GC without PM
As shown in Figure 2a, the TLR of 74 samples from patients without PM (P0CY0) were significantly less than those from patients with PM. We compared TLR in patients with primary tumors with serosal invasion (T4) and those without serosal exposure (<T4). In all samples from patients with <T4 GC, CD326‐reactive cells are less than 10 to more than 104 CD45(+) leukocytes and thus TLR were calculated less than 0.1% (M = 0.006%, 0–0.066%, n = 31). In comparison, TLR in patients with T4 tumors were M = 0.017% (0–0.248%, n = 43) which was significantly higher than those with <T4 tumors (p < .05) (Figure 5a).
FIGURE 5.
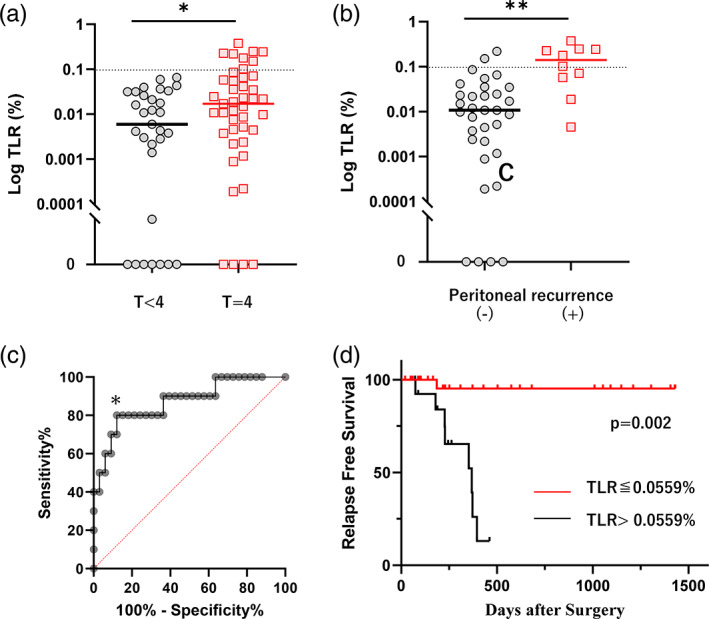
Tumor‐leukocyte ratios (TLR) are dependent on T‐stage in 74 patients without peritoneal metastases (PM) and correlates with postoperative peritoneal recurrence. (a) TLR in 31 patients with T < 4 and 43 with T4 GC. (b) TLR of 10 patients with peritoneal recurrence and 33 without recurrence. (c) ROC curve on the correlation between TLR and recurrence. *: Cutoff point of TLR was determined as 0.0559% by Youden Index with sensitivity, specificity, positive, and negative predictive values of 80.00%, 87.88%, 61.11%, and 94.11%, respectively. (d) Relapse free survival of the patients with T4 tumors after curative gastrectomy. **p < .01, ***p < .001 with Mann–Whitney U test. Survival was analyzed using the Kaplan–Meier method and p value was calculated with the log‐rank test. GC, gastric cancer; ROC, receiver operating characteristic [Color figure can be viewed at wileyonlinelibrary.com]
Among 43 patients who underwent gastrectomy for T4 tumors, peritoneal recurrence developed in 10 patients (23%) and their TLRs were significantly higher than those without peritoneal recurrence (M = 0.140%, 0.019–0.377%, n = 10 vs. M = 0.011%, 0–0.220%, n = 33, p < .01) (Figure 5b). ROC analysis showed TLR with cut off value of 0.0559% is a good predictor of recurrence with highest accuracy (Figure 5c, Figure S3d). When TLR exceeded 0.0559% in patients with T4 GC, 8/12 patients (67%) recurred on the peritoneal surface at a median of 7 months after surgery, and their relapse free survival (RFS) was significantly worse than that in patients with TLR < 0.0559% (Figure 5d). Multivariate Cox regression analyses revealed that TLR is an independent predictor for the RFS of these patients (Table 2). TLR also correlated with overall survival with marginal significance in multivariate analysis (Table 3).
TABLE 2.
Univariate and multivariate analysis on the correlation between clinicopathological variables and relapse free survival (RFS) of the patients who underwent curative surgery for gastric cancer with macroscopic serosal exposure (T4)
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | (71≤/<70) | 2.65 | 0.792–8.867 | .114 | 1.094 | 0.261–4.579 | .901 |
| Gender | (F/M) | 0.866 | 0.273–2.750 | .807 | |||
| Macroscopic type | (type IV/nontype IV) | 3.393 | 1.316–14.660 | .016 | 5.447 | 1.478–20.080 | .011 |
| Operation | (Total gastrectomy/distal gastrectomy) | 0.783 | 0.252–2.429 | .672 | |||
| Pathological type | (undifferentiated/differentiated) | 1.352 | 0.358–5.109 | .657 | |||
| Pathological T | (t4/t3) | 1.209 | 0.262–5.586 | .808 | |||
| Pathological N | (n2–3/n0–1) | 3.278 | 0.423–25.420 | .256 | |||
| TLR | (0.0559%>/<0.0559%) | 17.31 | 3.538–77.200 | .0004 | 19.87 | 3.073–128.500 | .002 |
Abbreviations: CI, confidence interval; HR, hazard ratio; RFS, relapse free survival; TLR, tumor‐leukocyte ratio.
TABLE 3.
Univariate and multivariate analysis on the correlation between clinicopathological variables and overall survival of the patients who underwent curative surgery for gastric cancer with macroscopic serosal exposure (T4)
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age | (71≤/<70) | 5.753 | 1.232–28.860 | .026 | 3.922 | 0.7896–19.480 | .095 |
| Gender | (Female/male) | 1.003 | 0.303–3.222 | .996 | |||
| Macroscopic Type | (Type IV/nontype IV) | 2.225 | 0.454–10.900 | .278 | |||
| Operation | (Total gastrectomy/distal gastrectomy) | 0.338 | 0.089–1.238 | .111 | 0.486 | 0.118–1.994 | .316 |
| Pathological type | (Undifferentiated/differentiated) | 0.451 | 0.137–1.487 | .201 | |||
| Pathological T | (t4/t3) | 0.905 | 0.232–5.519 | .885 | |||
| Pathological N | (n2–3/n0–1) | 3.569 | 0.454–28.500 | .226 | |||
| TLR | (0.0559%>/<0.0559%) | 4.576 | 1.257–16.660 | .0210 | 3.622 | 0.993–13.210 | .051 |
Abbreviations: CI, confidence interval; HR, hazard ratio; TLR, tumor‐leukocyte ratio.
4. DISCUSSION
Although cytological detection of tumor cells in peritoneal lavages fluid is the most important prognostic factor for the development of peritoneal recurrence in patients with GC (Bando et al., 1999; Bonenkamp et al., 1996), the results are largely dependent on the processing of samples and interpretation by pathologists, which sometimes result in confusion in the clinical evaluation of outcome of patients with positive/negative cytology (Leake, Cardoso, Seevaratnam, Lourenco, Helyer, Mahar, Rowsell, & Coburn, 2012; Lorenzen et al., 2010). Although molecular detection of tumor cell‐specific mRNA showed better correlation to peritoneal recurrence in patients with GC, the clinical issue of false‐positive cases remains to be addressed (Dalal et al., 2008; Katsuragi et al., 2007; Kodera et al., 2002; Rossi Del Monte et al., 2012).
Recently, FCM has been successfully used to detect tumor cells expressing CD326 (EpCAM) antigen in malignant effusions (Acosta et al., 2016; Davidson et al., 2007; Krishan et al., 2010; Pillai et al., 2013; Risberg et al., 2000; Szantho et al., 2018). Using this method, we tried to classify the patients with PM from GC by the number of tumor cells disseminated in peritoneal fluids (Kitayama et al., 2014). As reported previously, peritoneal cells were immunostained with mAbs to CD326 and CD45, a pan‐leukocyte marker, and the frequency of CD45(−) CD326(+) tumor cells was calculated to obtain the TLR using CD45(+) CD326(−) leukocytes as an internal control. In this method, most of the tumor cell clusters are dissociated to single cells during the washing procedure in EDTA containing buffer, and immunostaining is performed in cell suspensions. Moreover, dead cells were discriminated with 7AAD, a dead cell specific marker, which makes it possible to quantify the relative frequency of tumor cells in the peritoneal cavity. However, peritoneal fluid samples usually contain many small particles other than real cells which often bind antibodies in a non‐specific manner which hampers the accurate count of tumor cells as well as leukocytes. Therefore, we also stained cells with isotype control for anti‐CD326 mAb and calculated TLR as a ratio of CD326 (+) tumor cells to leukocytes—those of isotype control (+) cells to leukocytes (Figure S1, upper panel). However, since CD326(+) cell counts are usually very low in the early stages of PM (TLR = 0.01–1.0%), it is a critical issue for the measurement of accurate TLR, especially in lavage samples. In this study, therefore, we additionally stained the samples with DAPI which can distinguish the real cells from other micro‐particle components with a UV laser. This enables the acquisition of a much larger number (104–105 order) of real cells gated in the FCM profile and give a more accurate and reproducible values for TLR (Figure S1, lower panel).
TLR calculated by this modified method correlated strongly with the presence of PM, especially with positive cytology (CY1), and vary greatly from less than 0.1% to more than 1000%, which is consistent with the results of the previous study (Kitayama et al., 2015). In this study, we also found that the log(TLR) has a strong correlation with PCI scores in patients with PM. In general, staging laparoscopy is used for the evaluation of the degree of PM since metastatic nodules on the peritoneal surface are often too small to be accurately measured by imaging studies (Leake, Cardoso, Seevaratnam, Lourenco, Helyer, Mahar, Law, & Coburn, 2012; Wang & Chen, 2011). TLR reflects the progression of peritoneal lesions and thus can be clinically used as a biomarker to estimate the peritoneal tumor burden without the need for invasive laparoscopy.
TLR also well correlated with the composition of immune cells in the peritoneal cavity. In patients with PM, the ratio of CD11b (+) myeloid cells was significantly increased, while that of CD8(+) T cells reduced. There is little information about changes of immune cell populations in the human peritoneal cavity with pathological conditions. However, the number of CD8(+) T cells has been reported to be reduced in ascites of the patients with PM from ovarian cancer (Fossati et al., 2015; Giuntoli 2nd et al., 2009). The results in this study are consistent with their results and suggest that markedly increased TLR in patients with highly advanced PM is associated with disruption of T cell mediated immunity in the peritoneal cavity. Monocyte/macrophages are known to be the main myeloid cell population in the peritoneal cavity. Previous studies on the peritoneal fluid from patients with endometriosis (Miller et al., 2020) or patients with PM from GC (Yamaguchi et al., 2016) have shown that peritoneal macrophages tend to be polarized to the M2‐phenotype with immunosuppressive properties. In some cases, in our series, we confirmed that the majority of the CD11b (+) cells were positive for CD14 and highly express a M2 marker, CD163 (data not shown). From these results, the number of CD11b (+) macrophages is increased in peritoneal fluid from patients with PM are believed to suppress the local immunity and play a supportive role for the progression of peritoneal lesions.
Another important finding is that TLR is an excellent predictor of peritoneal recurrence in patients who underwent gastrectomy with curative intent due to the absence of PM. In this series, if the primary tumor was not exposed to the serosal surface (T < 4), TLR never exceeded 0.1% with no peritoneal recurrence (max = 0.066%). In these patients, the positive test for CD326 is supposed to be the result of nonspecific staining or caused by the contamination of epithelial cells in the course of sample acquisition. However, if the primary tumor was exposed to the serosal surface (T4), TLR often had high values. In fact, TLR exceeded 0.1% in 8/43 (19%) patients with T4 GC and peritoneal recurrence developed in 6/8 (75%) patients. In patients whose TLR were more than 0.0559%, 8 (67%) patients developed peritoneal recurrence. In these cases, a significant number of tumor cells are believed to exist in the peritoneal cavity even though there was a diagnosis of CY0 with conventional cytology study. Therefore, the patients with TLR higher than these values should be considered at a high risk for developing peritoneal recurrence. Analysis of a larger number of peritoneal lavage specimens is needed to establish a reliable threshold value of TLR to define patients at high risk of peritoneal recurrence.
In summary, the results of this study demonstrate a method to quantify tumor cells in the peritoneal cavity of patients with advanced GC using FCM. This modified method is simple, rapid, and the resulting TLR are objective and well correlate with clinical features. Recent studies have shown that EpCAM (CD326) antigen is downregulated in some circulating tumor cells, which might also be observed in peritoneal tumor cells (Konigsberg et al., 2011; Lieto et al., 2015). However, recent fluorescence activated cell sorter (FACS) methodology has been remarkably improved by the introduction of multicolor analysis (Maciorowski et al., 2017), and it may be possible to detect the expression of a number of antigens in single cell suspensions. Immunophenotyping of intraperitoneal cells using multicolor FCM may provide useful information for the treatment of PM in the future.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
This study was supported by Japan Society for the Promotion of Science (17H04286) and by Keirin Race Fund from JKA Foundation in flow cytometric analysis using LSRFortessa. We thank Ms. H. Hayakawa, J. Shinohara, and I. Nieda for their technical and clerical assistance.
Takahashi K, Kurashina K, Saito S, et al. Flow cytometry‐based analysis of tumor‐leukocyte ratios in peritoneal fluid from patients with advanced gastric cancer. Cytometry. 2021;100:666–675. 10.1002/cyto.b.21978
Funding information Japan Society for the Promotion of Science, Grant/Award Number: 17H04286; Keirin Race Fund from JKA Foundation
REFERENCES
- Acosta, M. , Pereira, J. , & Arroz, M. (2016). Screening of carcinoma metastasis by flow cytometry: A study of 238 cases. Cytometry. Part B, Clinical Cytometry, 90, 289–294. [DOI] [PubMed] [Google Scholar]
- Baeuerle, P. A. , & Gires, O. (2007). EpCAM (CD326) finding its role in cancer. British Journal of Cancer, 96, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bando, E. , Yonemura, Y. , Takeshita, Y. , Taniguchi, K. , Yasui, T. , Yoshimitsu, Y. , Fushida, S. , Fujimura, T. , Nishimura, G. , & Miwa, K. (1999). Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. American Journal of Surgery, 178, 256–262. [DOI] [PubMed] [Google Scholar]
- Benevolo, M. , Mottolese, M. , Cosimelli, M. , Tedesco, M. , Giannarelli, D. , Vasselli, S. , Carlini, M. , Garofalo, A. , & Natali, P. G. (1998). Diagnostic and prognostic value of peritoneal immunocytology in gastric cancer. Journal of Clinical Oncology, 16, 3406–3411. [DOI] [PubMed] [Google Scholar]
- Bonenkamp, J. J. , Songun, I. , Hermans, J. , & van de Velde, C. J. (1996). Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. The British Journal of Surgery, 83, 672–674. [DOI] [PubMed] [Google Scholar]
- Craig, F. E. , & Foon, K. A. (2008). Flow cytometric immunophenotyping for hematologic neoplasms. Blood, 111, 3941–3967. [DOI] [PubMed] [Google Scholar]
- Dalal, K. M. , Woo, Y. , Kelly, K. , Galanis, C. , Gonen, M. , Fong, Y. , & Coit, D. G. (2008). Detection of micrometastases in peritoneal washings of gastric cancer patients by the reverse transcriptase polymerase chain reaction. Gastric Cancer, 11, 206–213. [DOI] [PubMed] [Google Scholar]
- Davidson, B. , Dong, H. P. , Holth, A. , Berner, A. , & Risberg, B. (2007). Flow cytometric immunophenotyping of cancer cells in effusion specimens: Diagnostic and research applications. Diagnostic Cytopathology, 35, 568–578. [DOI] [PubMed] [Google Scholar]
- Fossati, M. , Buzzonetti, A. , Monego, G. , Catzola, V. , Scambia, G. , Fattorossi, A. , & Battaglia, A. (2015). Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti‐EpCAMxanti‐CD3). Gynecologic Oncology, 138, 343–351. [DOI] [PubMed] [Google Scholar]
- Giuntoli, R. L., 2nd , Webb, T. J. , Zoso, A. , Rogers, O. , Diaz‐Montes, T. P. , Bristow, R. E. , & Oelke, M. (2009). Ovarian cancer‐associated ascites demonstrates altered immune environment: Implications for antitumor immunity. Anticancer Research, 29, 2875–2884. [PubMed] [Google Scholar]
- Hayes, N. , Wayman, J. , Wadehra, V. , Scott, D. J. , Raimes, S. A. , & Griffin, S. M. (1999). Peritoneal cytology in the surgical evaluation of gastric carcinoma. British Journal of Cancer, 79, 520–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horattas, M. C. , Evasovich, M. R. , & Topham, N. (1997). Colorectal carcinoma and the relationship of peritoneal cytology. American Journal of Surgery, 174, 334–337. [DOI] [PubMed] [Google Scholar]
- Isobe, Y. , Nashimoto, A. , Akazawa, K. , Oda, I. , Hayashi, K. , Miyashiro, I. , Katai, H. , Tsujitani, S. , Kodera, Y. , Seto, Y. , & Kaminishi, M. (2011). Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer, 14, 301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet, P. , & Sugarbaker, P. H. (1996). Peritoneal‐plasma barrier. Cancer Treatment and Research, 82, 53–63. [DOI] [PubMed] [Google Scholar]
- Jemal, A. , Bray, F. , Center, M. M. , Ferlay, J. , Ward, E. , & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61, 69–90. [DOI] [PubMed] [Google Scholar]
- Katsuragi, K. , Yashiro, M. , Sawada, T. , Osaka, H. , Ohira, M. , & Hirakawa, K. (2007). Prognostic impact of PCR‐based identification of isolated tumour cells in the peritoneal lavage fluid of gastric cancer patients who underwent a curative R0 resection. British Journal of Cancer, 97, 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, J. , Emoto, S. , Yamaguchi, H. , Ishigami, H. , Kamei, T. , Yamashita, H. , Seto, Y. , Matsuzaki, K. , & Watanabe, T. (2014). Flow cytometric quantification of intraperitoneal free tumor cells in patients with peritoneal metastasis. Cytometry. Part B, Clinical Cytometry, 86, 56–62. [DOI] [PubMed] [Google Scholar]
- Kitayama, J. , Emoto, S. , Yamaguchi, H. , Ishigami, H. , Onoyama, H. , Yamashita, H. , Seto, Y. , Matsuzaki, K. , & Watanabe, T. (2015). Flow cytometric quantification of intraperitoneal free tumor cells is a useful biomarker in gastric cancer patients with peritoneal metastasis. Annals of Surgical Oncology, 22, 2336–2342. [DOI] [PubMed] [Google Scholar]
- Kodera, Y. , Nakanishi, H. , Ito, S. , Yamamura, Y. , Kanemitsu, Y. , Shimizu, Y. , Hirai, T. , Yasui, K. , Kato, T. , & Tatematsu, M. (2002). Quantitative detection of disseminated free cancer cells in peritoneal washes with real‐time reverse transcriptase‐polymerase chain reaction: A sensitive predictor of outcome for patients with gastric carcinoma. Annals of Surgery, 235, 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg, R. , Obermayr, E. , Bises, G. , Pfeiler, G. , Gneist, M. , Wrba, F. , de Santis, M. , Zeillinger, R. , Hudec, M. , & Dittrich, C. (2011). Detection of EpCAM positive and negative circulating tumor cells in metastatic breast cancer patients. Acta Oncologica, 50, 700–710. [DOI] [PubMed] [Google Scholar]
- Kowalewska, M. , Chechlinska, M. , & Nowak, R. (2008). Carcinoembryonic antigen and cytokeratin 20 in peritoneal cells of cancer patients: Are we aware of what we are detecting by mRNA examination? British Journal of Cancer, 98, 512–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan, A. , Ganjei‐Azar, P. , Hamelik, R. , Sharma, D. , Reis, I. , & Nadji, M. (2010). Flow immunocytochemistry of marker expression in cells from body cavity fluids. Cytometry. Part A, 77, 132–143. [DOI] [PubMed] [Google Scholar]
- Leake, P. A. , Cardoso, R. , Seevaratnam, R. , Lourenco, L. , Helyer, L. , Mahar, A. , Law, C. , & Coburn, N. G. (2012). A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative‐intent resection of gastric cancer. Gastric Cancer, 15(Suppl. 1), S38–S47. [DOI] [PubMed] [Google Scholar]
- Leake, P. A. , Cardoso, R. , Seevaratnam, R. , Lourenco, L. , Helyer, L. , Mahar, A. , Rowsell, C. , & Coburn, N. G. (2012). A systematic review of the accuracy and utility of peritoneal cytology in patients with gastric cancer. Gastric Cancer, 15(Suppl. 1), S27–S37. [DOI] [PubMed] [Google Scholar]
- Lieto, E. , Galizia, G. , Orditura, M. , Romano, C. , Zamboli, A. , Castellano, P. , Mabilia, A. , Auricchio, A. , DEV, F. , & Gemei, M. (2015). CD26‐positive/CD326‐negative circulating cancer cells as prognostic markers for colorectal cancer recurrence. Oncology Letters, 9, 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen, S. , Panzram, B. , Rosenberg, R. , Nekarda, H. , Becker, K. , Schenk, U. , Hofler, H. , Siewert, J. R. , Jager, D. , & Ott, K. (2010). Prognostic significance of free peritoneal tumor cells in the peritoneal cavity before and after neoadjuvant chemotherapy in patients with gastric carcinoma undergoing potentially curative resection. Annals of Surgical Oncology, 17, 2733–2739. [DOI] [PubMed] [Google Scholar]
- Maciorowski, Z. , Chattopadhyay, P. K. , & Jain, P. (2017). Basic multicolor flow cytometry. Current Protocols in Immunology, 117, 5.4.1–5.4.38. [DOI] [PubMed] [Google Scholar]
- Miller, J. E. , Ahn, S. H. , Marks, R. M. , Monsanto, S. P. , Fazleabas, A. T. , Koti, M. , & Tayade, C. (2020). IL‐17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Frontiers in Immunology, 11, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motherby, H. , Nadjari, B. , Friegel, P. , Kohaus, J. , Ramp, U. , & Bocking, A. (1999). Diagnostic accuracy of effusion cytology. Diagnostic Cytopathology, 20, 350–357. [DOI] [PubMed] [Google Scholar]
- Patriarca, C. , Macchi, R. M. , Marschner, A. K. , & Mellstedt, H. (2012). Epithelial cell adhesion molecule expression (CD326) in cancer: A short review. Cancer Treatment Reviews, 38, 68–75. [DOI] [PubMed] [Google Scholar]
- Pillai, V. , Cibas, E. S. , & Dorfman, D. M. (2013). A simplified flow cytometric immunophenotyping procedure for the diagnosis of effusions caused by epithelial malignancies. American Journal of Clinical Pathology, 139, 672–681. [DOI] [PubMed] [Google Scholar]
- Prat, J. , & FIGO Committee on Gynecologic Oncology . (2015). Staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication of guidelines from the International Federation of Gynecology and Obstetrics (FIGO). Obstetrics and Gynecology, 126, 171–174. [DOI] [PubMed] [Google Scholar]
- Risberg, B. , Davidson, B. , Dong, H. P. , Nesland, J. M. , & Berner, A. (2000). Flow cytometric immunophenotyping of serous effusions and peritoneal washings: Comparison with immunocytochemistry and morphological findings. Journal of Clinical Pathology, 53, 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi Del Monte, S. , Ranieri, D. , Mazzetta, F. , Kazemi Nava, A. , Raffa, S. , Torrisi, M. R. , & Ziparo, V. (2012). Free peritoneal tumor cells detection in gastric and colorectal cancer patients. Journal of Surgical Oncology, 106, 17–23. [DOI] [PubMed] [Google Scholar]
- Sodek, K. L. , Murphy, K. J. , Brown, T. J. , & Ringuette, M. J. (2012). Cell‐cell and cell‐matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Reviews, 31, 397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szantho, E. , Karai, B. , Ivady, G. , Bedekovics, J. , Szegedi, I. , Petras, M. , Ujj, G. , Ujfalusi, A. , Kiss, C. , Kappelmayer, J. , & Hevessy, Z. (2018). Comparative analysis of multicolor flow cytometry and immunohistochemistry for the detection of disseminated tumor cells. Applied Immunohistochemistry & Molecular Morphology, 26, 305–315. [DOI] [PubMed] [Google Scholar]
- Thibault, B. , Castells, M. , Delord, J. P. , & Couderc, B. (2014). Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Reviews, 33, 17–39. [DOI] [PubMed] [Google Scholar]
- Tustumi, F. , Bernardo, W. M. , Dias, A. R. , Ramos, M. F. , Cecconello, I. , Zilberstein, B. , & Ribeiro‐Junior, U. (2016). Detection value of free cancer cells in peritoneal washing in gastric cancer: A systematic review and meta‐analysis. Clinics, 71, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, P. , Ruschoff, J. , Kummel, S. , Zirngibl, H. , Hofstadter, F. , Hohenberger, W. , & Jauch, K. W. (1999). Immunocytology improves prognostic impact of peritoneal tumour cell detection compared to conventional cytology in gastric cancer. European Journal of Surgical Oncology, 25, 515–519. [DOI] [PubMed] [Google Scholar]
- Vogel, P. , Ruschoff, J. , Kummel, S. , Zirngibl, H. , Hofstadter, F. , Hohenberger, W. , & Jauch, K. W. (2000). Prognostic value of microscopic peritoneal dissemination: Comparison between colon and gastric cancer. Diseases of the Colon and Rectum, 43, 92–100. [DOI] [PubMed] [Google Scholar]
- Wang, Z. , & Chen, J. Q. (2011). Imaging in assessing hepatic and peritoneal metastases of gastric cancer: A systematic review. BMC Gastroenterology, 11, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshaw, A. L. (1991). Implications of peritoneal cytology for staging of early pancreatic cancer. American Journal of Surgery, 161, 26–29. [DOI] [PubMed] [Google Scholar]
- Went, P. T. , Lugli, A. , Meier, S. , Bundi, M. , Mirlacher, M. , Sauter, G. , & Dirnhofer, S. (2004). Frequent EpCam protein expression in human carcinomas. Human Pathology, 35, 122–128. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, T. , Fushida, S. , Yamamoto, Y. , Tsukada, T. , Kinoshita, J. , Oyama, K. , Miyashita, T. , Tajima, H. , Ninomiya, I. , Munesue, S. , Harashima, A. , Harada, S. , Yamamoto, H. , & Ohta, T. (2016). Tumor‐associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer, 19, 1052–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziselman, E. M. , Harkavy, S. E. , Hogan, M. , West, W. , & Atkinson, B. (1984). Peritoneal washing cytology. Uses and diagnostic criteria in gynecologic neoplasms. Acta Cytologica, 28, 105–110. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


