Summary
Background
Increase in lipid levels associated with the treatment of inflammatory bowel disease (IBD) has previously been reported. However, it is unknown if this effect is similar for all IBD drug classes.
Aim
To precisely assess the effect of different IBD drug classes on lipid profiles
Methods
We performed a systematic literature search of randomised controlled trials and observational cohort studies that assessed lipid levels before and after induction (≤10 weeks) and maintenance (>10 weeks) of IBD treatment. Data of 11 studies (1663 patients) were pooled using random effects models. The influence of patient and disease characteristics on treatment effects on total cholesterol levels was analysed in 6 studies (1211 patients) for which individual data were available, using linear mixed models.
Results
A statistically significant increase in total cholesterol was observed after induction treatment with corticosteroids (+1.19 mmol/L, 95% confidence interval [CI95] +0.52 to +2.59), and tofacitinib (+0.66 mmol/L, CI95 +0.42 to +0.79), but not after anti−TNFα treatment (−0.11 mmol/L, CI95 −0.26 to +0.36 mmol/L). Similar differences were observed after maintenance treatment. Treatment effects were significantly related to age, but not with other factors. Lipid changes were inversely correlated with but not modified by CRP changes.
Conclusions
Increase in total cholesterol levels was strongest for corticosteroids followed by tofacitinib but was not observed for anti‐TNFα agents. Whether total cholesterol change associated with IBD treatment has an effect on cardiovascular risk requires further study.
Systematic review and meta‐analysis: effect of inflammatory bowel disease therapy on lipid levels

1. INTRODUCTION
Over the last 50 years, cardiovascular disease mortality has declined drastically due to improved primary and secondary prevention. However, this trend is decelerating as the prevalence of obesity and type 2 diabetes increase, and even early signs of reversal are shown in some populations. 1 Therefore, long‐term prognosis implies that cardiovascular disease will remain one of the most important causes of morbidity and mortality worldwide.
In analogy to other chronic inflammatory diseases, such as rheumatoid arthritis and psoriasis, inflammatory bowel disease (IBD) is associated with an increased risk of cardiovascular disease. 2 , 3 This elevated risk cannot be explained by traditional risk factors only, as the prevalence of obesity, hypertension and dyslipidaemia are similar or even lower in IBD patients compared to the general population. 4 , 5 Indeed, chronic systemic inflammation is considered an independent cardiovascular risk factor.
Counterintuitively, patients with an active chronic inflammatory disease have lower levels of total cholesterol, high‐density lipoprotein cholesterol (HDL‐c) and low‐density lipoprotein cholesterol (LDL‐c) compared to patients who are in disease remission and the general population. 6 This so‐called “lipid paradox” has been mirrored in other inflammatory states, amongst others rheumatoid arthritis and sepsis. 7 However although absolute lipid levels are lower, the properties of the lipid particles tend to be more pro‐atherogenic, with a shift towards relatively more small dense LDL‐c and pro‐inflammatory and pro‐thrombotic characteristics of HDL‐c associated with a higher risk of thrombus formation. 8 , 9 The severity of the underlying inflammation is associated with the magnitude of these lipid changes.
Randomised controlled trials and observational studies in patients with IBD, comprising Crohn's disease (CD) and ulcerative colitis (UC), demonstrated increases in lipid levels after therapy initiation. Recently, awareness of these changes was provoked by the results of the tofacitinib registration trials. After 8‐week induction therapy, a significant increase of total cholesterol, HDL‐c and LDL‐c levels was observed, which stabilised during maintenance therapy. 10 An inverse association was observed between the increase of lipid levels and C‐reactive protein (CRP) levels. 11 In addition to janus kinase inhibitors, lipid changes have been described in relation with other IBD therapies, such as corticosteroids and anti‐tumour necrosis factor‐alpha (TNFα) agents. 12 , 13 , 14
The translation of the study findings to clinical practice is challenging. For example, it is unknown if lipid levels are affected similarly by all classes of IBD therapy. Systematic analyses of available data might provide some guidance. Against this background, we performed a systematic review and meta‐analysis of randomised trials and observational studies of IBD treatment, with the main aim to precisely assess the effect of different drug classes on lipid profiles.
2. METHODS
2.1. Search strategy
This systematic review was performed according to the Cochrane Handbook for Systematic Reviews of Interventions 15 and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 16 We followed an a priori established protocol which is published at PROSPERO (CRD42019123338). In October 2018, a literature search was performed in electronic databases Embase, Medline Ovid, Web of Science, Cochrane CENTRAL and Google Scholar to identify published and unpublished literature. The electronic literature search (Table S1) was conducted by an expert librarian (WB); the final run was performed in December 2020. No time or language restrictions were applied. Abstracts from major gastroenterology conferences (Digestive Disease Week, European Crohn's and Colitis Organization, United European Gastroenterology Week, the American College of Gastroenterology, Digestive Disease Week and Crohn's and Colitis Foundation) were searched manually (2000‐2020). Reference lists were read to identify additional studies. Institutional review board approval was not required. De‐identified data were provided with data transfer agreements.
2.2. Study selection
Two reviewers (JS and RP) independently scanned titles and abstracts to determine eligibility. Articles were selected if they met inclusion criteria: (a) randomised controlled trials (RCT) or observational cohort studies, (b) population consisted of IBD patients, including ulcerative colitis (UC) and Crohn's disease (CD), (c) intervention with IBD drug therapy, including 5‐aminosalicylic acids, steroids, immunosuppressants, biologicals, Janus Kinase/Signal Transducer and Activator of Transcription (JAK‐STAT) inhibitors and calcineurin inhibitors, (d) serum levels of total cholesterol, HDL‐c, LDL‐c and/or triglycerides were measured before and after initiation of aforementioned therapy, (e) minimum sample size of 20 participants. Exclusion criteria were (a) case reports, in vitro and animal studies, and (b) studies that solely investigated the effect of dietary supplements, probiotics, parenteral nutrition, or gastrointestinal surgery. There were no restrictions regarding intensity, frequency or timing of the intervention.
2.3. Data extraction and quality assessment
Data extraction and quality assessment were performed in duplicate by the two authors individually. Collected data consisted of: (a) first author's name, (b) year of publication, (c) study design, (d) type of intervention, (e) number of participants in treatment groups, (f) treatment duration, (g) concomitant medication use at baseline, (h) age, (i) sex, (j) IBD diagnosis, (k) disease duration, (l) disease extent, (m) body mass index (BMI) and (n) serum levels of total cholesterol, HDL‐c, LDL‐c, triglycerides and CRP at baseline and during follow‐up. Authors were approached by email if they were willing to share individual datasets, or alternatively, complete data request forms with instructions for analysis prior to data transfer. Assessment of quality was performed using Cochrane Collaboration Tool for RCTs and Newcastle Ottawa Scale for observational cohort studies. 17 , 18
2.4. Outcome
The primary outcome was serum total cholesterol change after induction and maintenance treatment. Secondary outcomes were changes in HDL‐c, LDL‐c and triglycerides. Assuming that the plateau phase observed after tofacitinib induction therapy applies to other drug classes, lipid changes from baseline were analysed after induction (≤10 weeks) and maintenance treatment (first visit after >10 weeks). 19
2.5. Data synthesis and analysis
All our meta‐analyses are performed on log2 transformed lipid data to obtain normal distributions. If necessary, mg/dL were converted to mmol/L using 38.67 as convergence factor for total cholesterol, HDL‐c and LDL‐c, and 88.5 for triglycerides, respectively. For each drug class, study‐specific mean changes (follow‐up minus baseline) in lipid levels were pooled using random‐effect models, while applying the method of Fleming and DeMets (1996). 20 Effect sizes were expressed as pooled mean difference (MD) on the Log2 scale, along with 95% confidence intervals (CI95), and visualised by forest plots. For ease of understanding, effect sizes are also presented on the linear scale (in mmol/L), using the pooled mean baseline value as reference. The degree of heterogeneity between studies was analysed by I2 statistics and expressed in percentage of variation (>50% substantial). 21 Risk of publication bias was explored using visual inspection of funnel plot asymmetry and objectively judged using the Egger's regression test, provided that sufficient studies were eligible in line with recommendations. 22
When available, mean differences between baseline and follow‐up values, standard deviations (SD) and standard errors (SE) were directly determined using individual patient data (calculated ourselves or obtained from investigators). Mean differences based on aggregated data were calculated as the difference between the reported mean baseline and follow‐up values. The SD of the mean difference was then estimated as
with r being the Pearson correlation coefficient between the baseline and follow‐up measurement, which was estimated using the available individual patient data of the drug class concerned.
To analyse possible (modifying) effects of patient and disease characteristics on drug‐induced total cholesterol changes, individual patient data were analysed using linear mixed‐effect models, assuming a random intercept between studies, as well as between subjects within studies, and a fixed slope for predictor variables. 23 We considered age, sex, BMI, CRP, IBD subtype, disease duration and steroid use at baseline as potential influencing factors (Table S2).
A two‐sided P‐value <0.05 was considered statistically significant for all statistical tests. Data analyses were performed in SAS® 9.4 Software (M7 2020).
3. RESULTS
3.1. Eligible studies
The study selection process is shown in Figure 1. The systematic search identified 2561 citations. After title and abstract evaluation, 2504 were excluded not meeting inclusion criteria. Of the 57 articles selected for full‐text search and review, 31 were excluded because of various reasons. One review article yielded one extra study, resulting in the inclusion of 27 articles for qualitative synthesis. Agreement between reviewers for trial eligibility was good (κ statistic 0.74). We successfully contacted 11 authors: from 8 studies individual patient data were shared, 9 , 10 , 13 , 14 , 24 , 25 , 26 , 27 from three studies aggregated data were provided. 28 , 29 , 30
FIGURE 1.
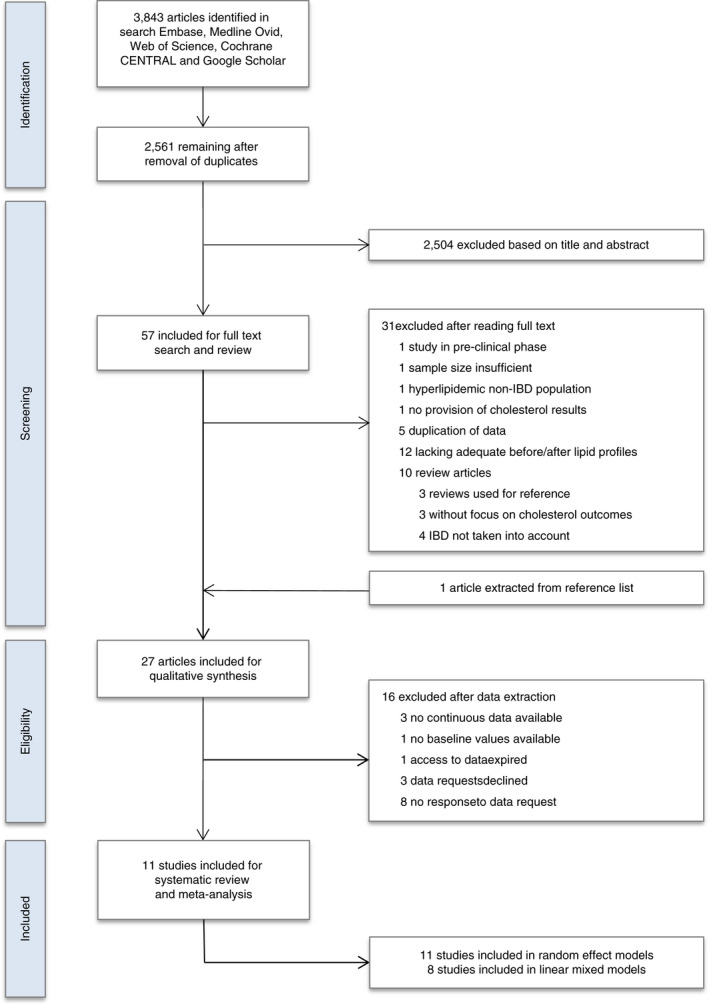
Flow diagram of study selection procedure for systematic review and meta‐analysis
3.2. Description of studies
Characteristics of included studies are summarised in Table 1. Included studies were published between 2012 and 2020. Of the 11 included studies, eight were observational cohort studies 9 , 13 , 14 , 24 , 25 , 27 , 28 , 29 and three RCT (two phase 2 and one phase 3). 10 , 26 , 30 Percentage of IBD patients (n = 1663) diagnosed with CD and UC were 15% (n = 250) and 85% (n = 1413), respectively. All studies provided data on sex and age; nine studies on BMI and disease duration; and seven on concomitant steroid use. All individual patient datasets contained information on CRP levels at baseline and during follow‐up. The intervention period ranged between 8 weeks 9 and 3 years. 14 None of the studies stated hyperlipidaemia or the use of lipid‐lowering drugs as exclusion criteria.
TABLE 1.
Baseline characteristics of included studies in systematic review and meta‐analysis
| Drug | Author (year of publication) | Study design | Population size, n | Therapy duration, weeks | Men (%) | Age, years (SD) | BMI, kg/m2 (SD) | IBD diagnosis (%) | Disease duration, years (SD) | Steroid use at baseline (%) | Disease extent (%) | CRP, mg/L (IQR) | Total cholesterol, mmol/L (SD) | HDL‐c, mmol/L (SD) | LDL‐c, mmol/L (SD) | Triglycerides, mmol/L (SD) | Time points, weeks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticosteroids | Farkas (2014) 9 | OS | 19 | 12 | 13 (68.4) | 36.4 (±14.8) | 23.2 (±4.7) |
CD 6 (31.6) UC 13 (6.8) |
5.7 (±8.8) | NA |
E2: 5 (38.5) E3: 8 (61.5) L1: 3 (50) L2 3 (50) L3: ‐ |
9.4 (5.9‐15.1) | 3.88 (±1.10) | MD | MD | 1.20 (±0.74) | 4, 8, 12 |
| Pigniczki (2019) 13 | OS | 22 | 8 | 6 (27.3) | 43.1 (±15.2) | 27.1 (±4.4) | UC 22 (100) | 7.9 (±6.5) | NA |
E1: 5 (22.7) E2: 10 (45.5) E3: 7 (31.8) |
7.4 (2.5‐12.3) | 4.82 (±0.70) | MD | MD | 1.27 (±0.55) | 8 | |
| Motobayashi (2018) 27 | OS | 31 | 52 | 12 (39) | 40 (±15) | NA | UC 31 (100) | 6 (±5.5) | NA | MD | 2.6 (0.9‐6.7) | 3.75 (±0.75) | MD | MD | MD | 2, 13, 52 | |
| Anti‐TNFα agents | Schulte (2018) 29 | OS | 35 | 26 | 19 (54.3) | 37.2 (±14.8) | 24.9 (±4.1) |
CD 22 (62.9) UC 13 (37.1) |
4.5 (±6.5) | NA | MD | 4.6 (1.7‐9.8) | 4.75 (±0.81) | 1.38 (±0.49) | 2.69 (±0.61) | 1.52 (±0.69) | 6, 26 |
| Miranda‐Bautista (2014) 14 | OS | 128 | 156 | 64 (50) | 43.6 (±12.8) | 23.9 (±4.6) |
CD 92 (71.9) UC 36 (28.1) |
MD | NA |
L2: 9 (9.7) L3: 53 (58.1) L4: 13 (13.8) E2: 14 (40.6) E3: 16 (43.8) |
1.4 (0.8‐2.5) | 4.68 (±1.10) | 1.37 (±0.45) | 2.62 (±1.84) | 3.28 (±1.92) | 52, 156 | |
| Balint (2013) 28 | OS | 25 | 26 | 11 (45.8) | 39.2 (±11.9) | MD | UC 25 (100) | 9.2 (±7.8) | 25 (100) | MD | MD | 5.17 (±1.41) | MD | MD | 1.36 (±0.67) | 42 | |
| Motobayashi (2018) 29 | OS | 21 | 52 | 9 (43) | 45 (±16) | NA | UC 21 (100) | 8 (±5.3) | NA | MD | 2.6 (0.9‐6.7) | 3.59 (±1.22) | MD | MD | MD | 2, 13, 52 | |
| Tofacitinib | Honap (2020) 24 | OS | 93 | 8 | 56 (60) | 41.7 (±16.8) | MD | UC 93 (100) | 9.1 (±9.0) | 38 (41) | MD | 4 (2‐13.3) | 4.49 (±1.20) | 1.58 (±0.60) | 2.48 (±0.89) | MD | 8 |
| Biemans (2019) 25 | OS | 45 | 12 | 27 (58.7) | 43.3 (±14.3) | 25.0 (±3.80) | UC 45 (100) | 12.2 (±10.4) | 21 (45.7) |
E1: 9 (21.4) E2: 10 (23.8) E3: 23 (54.8) |
5.2 (2.0‐12.9) | 4.65 (±1.03) | 1.42 (±0.48) | 2.80 (±0.85) | 1.52 (±0.75) | 12 | |
| OCTAVE phase 2 (2012) 26 | RCT | 146 | 8 | 83 (56.8) | 41.3 (±14.1) | 25.5 (±4.4) | 146 (100) | 9.3 (±7.7) | 53 (36.3) |
E1: 47 (32.2) E2: 41 (28.1) E3: 51 (34.9) |
6.1 (2.0‐16.5) | 4.92 (±1.00) | 1.42 (±0.43) | 2.90 (±0.76) | 1.30 (±0.68) | 8 | |
| OCTAVE phase 3 Induction 1 (2017) 10 | RCT | 467 | 8 | 277 (58.2) | 41.1 (±13.5) | 24.7 (±5.0) | 467 (100) | 8.3 (±7.1) | 214 (45.0) |
E1: 67 (15.7) E2: 149 (34.8) E3: 108: (49.3) |
4.7 (1.9‐12.0) | 4.73 (±0.96) | 1.51 (±0.48) | 2.66 (±0.74) | 1.22 (±0.59) | 8 | |
| OCTAVE phase 3 Induction 2 (2017) 10 | RCT | 429 | 8 | 259 (60.4) | 41.0 (±13.5) | 25.1 (±5.0) | 429 (100) | 7.9 (±6.9) | 198 (46.2) |
E1: 69 (16.1) E2: 151 (35.2) E3: 214 (49.9) |
5.0 (2.1‐11.8) | 4.65 (±1.01) | 1.47 (±0.45) | 2.61 (±0.80) | 1.27 (±0.61) | 8 | |
| Filgotinib | FITZROY phase 2 (2016) 30 | RCT | 130 | 20 | 59 (45) | 37.4 (±11.6) | 23.8 (±4.3) | CD 130 (100) | 8.8 (±8.5) | 65 (50) |
L1: 24 (18) L2: 29 (22) L3: 77 (59) |
8.2 (3.1‐17.0) | 4.57 (±0.94) | 1.46 (±0.49) | 2.46 (±0.80) | 1.43 (±0.70) | 10, 20 |
| Cyclosporine | Balint (2013) 28 | OS | 72 | 26 | 33 (45.8) | 40.3 (±13.7) | 23.6 (5.1) | UC 72 (100) | 13.5 (±9.8) | 72 (100) | MD | MD | 4.45 (±1.23) | MD | MD | 1.44 (±0.66) | 42 |
Categorical variables are described as number (%); continuous variables are expressed in mean (SD), except CRP and lipid parameters described in median (IQR).
Abbreviations: BMI, body mass index; CD, Crohn's disease; CRP, C‐reactive protein; HDL‐c, high density lipoprotein cholesterol; LDL‐c, low density lipoprotein cholesterol; MD, missing data; MMX, Multi Matrix®; n, number of patients; OS, observational study; RCT, randomised controlled trials; UC, ulcerative colitis.
3.2.1. Treatment regimens
Three studies investigated lipid effects of corticosteroids (n = 73), four of anti‐TNFα agents (n = 207), five of tofacitinib (n = 1257), one of filgotinib (n = 128) and one of cyclosporine (n = 72). The treatment regimen of corticosteroids consisted of (a) methylprednisolone weekly dose intravenous bolus‐administered vs conventional orally taken daily (both arms 12‐week tapering regimen starting from 450 mg/wk) and (b) next‐generation budesonide‐MMX orally (8‐week regimen of 9 mg stable dose). In the third study, the specification of type and dose of corticosteroids was not disclosed. 9 , 27 , 29 Anti‐TNFα agents used included infliximab only, 9 , 28 either infliximab or adalimumab (n = 104 and n = 10, respectively), 14 and unspecified anti‐TNFα agents. 29 Two studies reported concomitant use of steroids at baseline. 28 , 29 No data on dosing or interval of anti‐TNFα agents were available. 14 Three tofacitinib studies included were randomised clinical trials 10 , 26 and two were real‐life cohorts. 24 , 25 In these studies different treatment induction doses were used (range 0.5‐15 mg), but maintenance regimens were similar in all studies (5 mg or 10 mg twice daily). 10 , 24 , 25 , 26 In the tofacitinib cohorts, concomitant steroid use at baseline varied from 36.3% to 50%.
3.2.2. Lipid levels before and after therapy
Eight studies reported lipid changes after induction and five studies after maintenance treatment. Data on modification of lipid levels were available for total cholesterol (11 studies), HDL‐c (8 studies), LDL‐c (8 studies) and triglycerides (7 studies).
3.3. Quality and risk of bias
Details of the quality assessment are shown in Tables S3 and S4. Two observational cohort studies featured a control group, namely healthy controls 13 and other chronic inflammatory conditions. 9 Two studies were of high quality (≥7 stars allocated) 9 , 13 and six studies of moderate quality, either due to no selection of a non‐exposed cohort, 14 , 24 , 25 , 27 , 28 , 29 insufficient length of follow‐up (<4 weeks) 29 and/or incomplete assessment of lipid levels during follow‐up (<80%). 25 , 29 The randomised controlled trials had a low risk of bias. 10 , 26 , 30 Test for publication bias by funnel plot was not performed since less than 10 studies per outcome were included in the meta‐analysis, thus the power of the test was too low to distinguish change from real asymmetry.
3.4. Results of meta‐analysis
3.4.1. Lipid changes in different IBD drug classes
Results of meta‐analyses on drug‐induced lipid changes are shown in Figures 2, 3, 4, 5 and Table 2.
FIGURE 2.
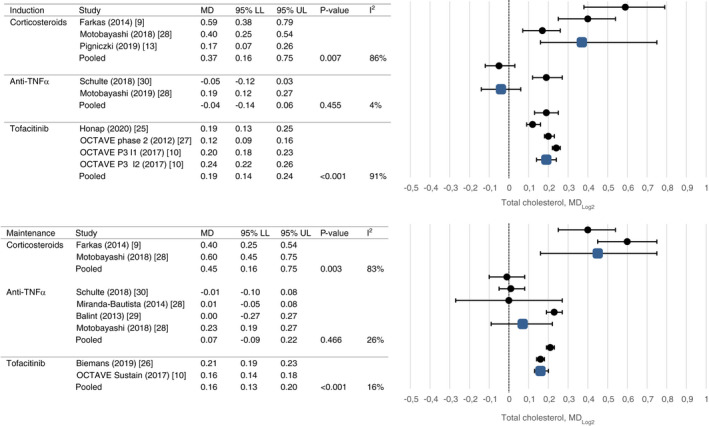
Mean change in total cholesterol after induction and maintenance therapy
FIGURE 3.
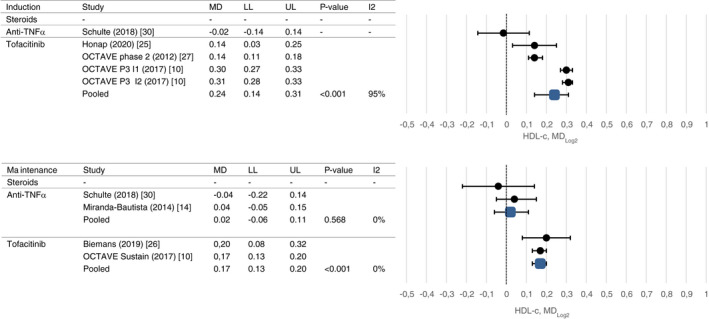
Mean change in HDL‐c after induction and maintenance therapy
FIGURE 4.
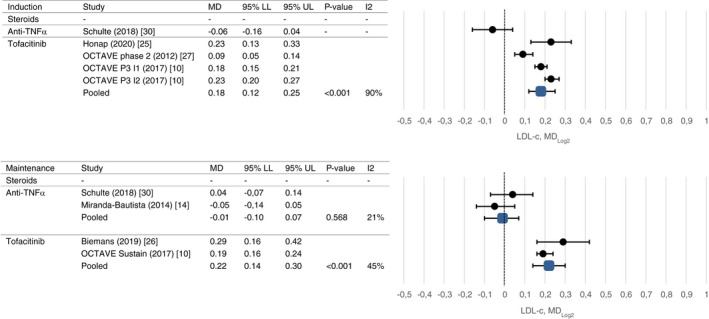
Mean change in LDL‐c after induction and maintenance therapy
FIGURE 5.
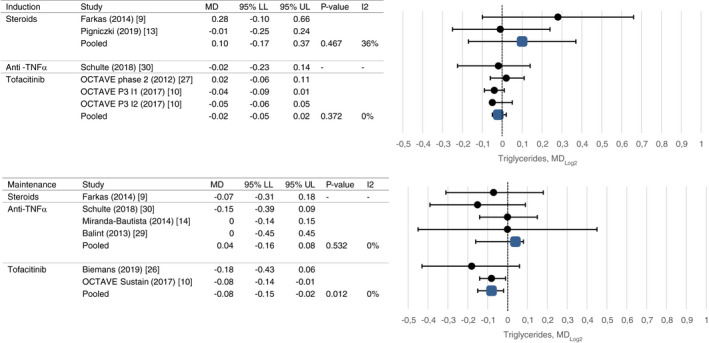
Mean change in triglycerides after induction and maintenance therapy.
Abbreviations: Forest plots visualizing effect sizes of drug classes on lipid profiles, expressed as pooled mean difference on the Log2 scale. LL, lower limit; MD, mean difference; UL, upper limit
TABLE 2.
Lipid changes from baseline to follow‐up on linear scale (in mmol/L)
| Drug | Lipid parameter | Treatment duration | Mean baseline | Change, mmol/L |
95% CI LL |
95% CI UL |
|---|---|---|---|---|---|---|
| Corticosteroids | Total cholesterol | Induction | 4.27 | 1.25 | 0.31 | 2.39 |
| Maintenance | 3.56 | 1.29 | 0.40 | 2.38 | ||
| HDL‐c | Induction | — | — | — | — | |
| Maintenance | — | — | — | — | ||
| LDL‐c | Induction | — | — | — | — | |
| Maintenance | — | — | — | — | ||
| Triglycerides | Induction | 1.16 | 0.08 | −0.13 | 0.34 | |
| Maintenance | — | — | — | — | ||
| Anti‐TNFα agents | Total cholesterol | Induction | 4.65 | −0.12 | −0.41 | 0.20 |
| Maintenance | 4.64 | 0.08 | −0.14 | 0.32 | ||
| HDL‐c | Induction | — | — | — | — | |
| Maintenance | 1.30 | 0.02 | −0.05 | 0.10 | ||
| LDL‐c | Induction | — | — | — | — | |
| Maintenance | 2.57 | −0.02 | −0.17 | 0.13 | ||
| Triglycerides | Induction | — | — | — | — | |
| Maintenance | 0.91 | −0.02 | −0.09 | 0.05 | ||
| Tofacitinib | Total cholesterol | Induction | 4.62 | 0.65 | 0.47 | 0.84 |
| Maintenance | 4.60 | 0.55 | 0.43 | 0.68 | ||
| HDL‐c | Induction | 1.42 | 0.24 | 0.15 | 0.34 | |
| Maintenance | 1.49 | 0.18 | 0.15 | 0.22 | ||
| LDL‐c | Induction | 2.56 | 0.35 | 0.21 | 0.48 | |
| Maintenance | 2.60 | 0.42 | 0.26 | 0.60 | ||
| Triglycerides | Induction | 1.14 | −0.01 | −0.01 | −0.04 | |
| Maintenance | 1.13 | −0.06 | −0.11 | −0.01 |
Abbreviations: LL, lower limit; UL, upper limit.
For corticosteroids, significant changes in total cholesterol levels were observed after induction (mean change 1.19 mmol/L, CI 0.52 and 2.59, P = 0.007) and maintenance therapy up to 14 weeks (mean change 1.33 mmol/L, CI95 0.28 and 2.26, P = 0.003) with high rates of heterogeneity (I2 86% and 83%, respectively). No significant changes in triglyceride levels were observed after short‐term treatment. None of the studies assessed HDL‐c or LDL‐c changes.
Use of anti‐TNFα agents was not associated with total cholesterol changes after induction therapy and maintenance therapy (mean change −0.11 mmol/L, CI −0.26 and 0.36, P = 0.455 and mean change 0.08 mmol/L, CI −0.07 and 0.39, P = 0.466, respectively), nor long‐term changes in HDL‐c, LDL‐c or triglyceride levels were observed. Only one study assessed short‐term changes in HDL‐c, LDL‐c and triglycerides [30143407]. Levels of heterogeneity were low in all analyses.
After induction treatment with tofacitinib, total cholesterol was increased (mean change 0.66 mmol/L, CI 0.42 and 0.79, P < 0.001), as was HDL‐c (mean change 0.25 mmol/L, CI 0.10 and 0.30, P < 0.001) and LDL‐c (mean change 0.35 mmol/L, CI 0.20 and 0.47, P < 0.001). Similar changes were observed after maintenance treatment (mean change 0.55 mmol/L, CI 0.43 and 0.68, P < 0.001; mean change 0.18 mmol/L, CI 0.14 and 0.21, P < 0.001; mean change 0.42 mmol/L, CI 0.28 and 0.62, P < 0.001). Triglyceride levels decreased after induction and maintenance treatment which reached statistical significance only after maintenance treatment (mean change −0.06 mmol/L, CI −0.10 and −0.01, P = 0.012). High rates of heterogeneity were present between studies evaluating the short‐term effect of tofacitinib on lipid profiles (I2 90%‐95%).
In filgotinib, increases in total cholesterol, HDL‐c, LDL‐c and triglycerides were observed after induction treatment (mean change +0.39 mmol/L [SD ±0.87], P < 0.001; mean change +0.24 mmol/L [SD ±0.40], P < 0.001; mean change +0.15 mmol/L [SD ±0.68], P = 0.199; and mean change +0.007 mmol/L [SD ±0.59], P = 0.877, respectively) and maintenance treatment (mean change +0.28 mmol/L [SD ±0.85], P = 0.003; mean change +0.05 mmol/L [SD ±0.45], P = 0.085; mean change +0.16 mmol/L [SD ±0.63], P = 0.024; and mean change +0.16 mmol/L [SD ±1.10], P = 0.313, respectively).
Only one study assessed the effect of cyclosporine on lipid levels. No short term data were available. After a median treatment duration of 9.4 months with cyclosporine, a significant difference in total cholesterol and triglycerides were reported (mean change +1.62 mmol/L [SD ±1.38]; and mean change +0.73 mmol/L [SD ±1.73], respectively).
3.4.2. Effect of patient and disease characteristics on drug‐induced lipid changes
Data of 1211 patients from 6 studies 9 , 10 , 13 , 24 , 26 , 27 were available for analyses of the influence of sex, age, BMI, CRP, IBD subtype, disease duration, disease extent and concomitant corticosteroid use at baseline on drug‐induced changes in total cholesterol. Neither of these factors had a meaningful influence on the mean change in total cholesterol between baseline and 10 weeks follow‐up (Table 3; compare Models 1 and 2). Reliable estimates of any treatment modifying effect could be obtained in 1135 patients using tofacitinib. The degree of the tofacitinib‐induced increase in total cholesterol was significantly related to age, but not with the other factors (Table 3; Models 3). An increase was seen even in the youngest patients (18 years: +0.39 mmol/L). A reverse relationship with CRP changes was observed for total cholesterol, HDL‐c and LDL‐c but not triglyceride changes, both in the total population (R −0.204, R −0.182, R −0.101, P < 0.001 and R 0.016, P = 0.293, respectively) as for all individual treatments.
TABLE 3.
Mean changes in total cholesterol values in relation to IBD treatment
| Baseline | Follow‐up | Change | P for comparison with tofacitinib | |
|---|---|---|---|---|
| Model 1—Unadjusted effects | ||||
| Corticosteroids | 3.95 | 4.84 | 0.89 | <0.001 |
| Anti‐TNFα agents | 4.61 | 4.49 | −0.12 | <0.001 |
| Tofacitinib | 4.69 | 5.25 | 0.55 | |
| Model 2—Effects adjusted for sex, age, BMI, CRP, IBD subtype, disease duration, disease extent and concomitant corticosteroid use at baseline | ||||
| Corticosteroids a | 4.28 | 5.25 | 0.96 | <0.001 |
| Anti‐TNFα agents a | 4.95 | 4.75 | −0.20 | <0.001 |
| Tofacitinib a | 4.92 | 5.50 | 0.58 | |
| Baseline | Follow‐up | Change | P for interaction | |
|---|---|---|---|---|
| Models 3—Effects of tofacitinib treatment in clinically relevant strata | ||||
| Sex | ||||
| Man | 4.79 | 5.32 | 0.54 | 0.298 |
| Woman | 4.61 | 5.18 | 0.56 | |
| Age, years | ||||
| 18 | 4.33 | 4.72 | 0.39 | 0.001 |
| 41.3 | 4.68 | 5.23 | 0.55 | |
| 65 | 5.06 | 5.80 | 0.74 | |
| CRP, mg/L | ||||
| 0.3 | 4.70 | 5.29 | 0.59 | 0.079 |
| 3.4 | 4.68 | 5.25 | 0.57 | |
| 15 | 4.60 | 5.13 | 0.53 | |
| BMI, kg/m2 | ||||
| 18 | 4.61 | 5.11 | 0.50 | 0.149 |
| 24.3 | 4.74 | 5.29 | 0.56 | |
| 30 | 4.85 | 5.46 | 0.61 | |
| Disease duration, years | ||||
| 1 | 4.51 | 5.04 | 0.53 | 0.766 |
| 9 | 4.69 | 5.25 | 0.56 | |
| 17 | 4.88 | 5.46 | 0.59 | |
| Disease extent | ||||
| Proctitis | 4.89 | 5.36 | 0.46 | 0.061 |
| Left‐sided | 4.87 | 5.40 | 0.54 | 0.307 |
| Pancolitis | 4.61 | 5.25 | 0.64 | <0.001 |
| Concomitant corticosteroid use at baseline | ||||
| No | 4.52 | 5.08 | 0.55 | 0.489 |
| Yes | 4.89 | 5.51 | 0.63 | |
Mean for patients with “average” sex, age, BMI, CRP, disease duration, disease extent and concomitant steroid use at baseline.
4. DISCUSSION
In this meta‐analysis of aggregated and individual patient data, we demonstrated significant and persistent lipid changes in IBD patients starting corticosteroids and tofacitinib independent of treatment regimens. In addition, tofacitinib increased HDL‐c and LDL‐c and reduced triglyceride levels significantly both after induction and maintenance therapy. The effect of anti‐TNFα agents on total cholesterol changes was neutral. Lipid data of other agents used in IBD were limited or unavailable. Elevation of total cholesterol levels was modified by age and inversely correlated with CRP levels.
As expected, patients treated with corticosteroids showed the most prominent increase in total cholesterol levels after induction treatment up to 14 weeks. Baseline total cholesterol levels were significantly lower in the corticosteroids group, which might reflect the severity of underlying disease since corticosteroids are mostly indicated in acute episodes to induce disease remission. Moreover, 30%‐50% of patients in the anti‐TNFα and tofacitinib group used concomitant corticosteroids at baseline. Clinical trials of tofacitinib in rheumatoid arthritis, psoriasis and psoriatic arthritis patients showed similar lipid changes in the first 3 months of treatment. 31 , 32 , 33 Interestingly, in these conditions anti‐TNFα agents were associated with a relative increase in total cholesterol, HDL‐c and triglyceride levels as well. 34 , 35 Therefore effect on lipids of immune‐modulating drugs might be partly disease‐specific.
In agreement with other studies, we found a reverse relationship between CRP levels and total cholesterol, HDL‐c and LDL‐c, that supports the hypothesis that suppression of inflammation partially explains lipid increases. However, the variable degree and pattern of the observed lipid changes between drug classes suggests a drug‐specific mechanism of action as well. Corticosteroids influence lipid metabolism via hormonal, enzymatic and receptor pathways. Lipids increase directly or secondarily to corticosteroid‐induced metabolic effects including down‐regulation of LDL‐c receptors, insulin resistance and inhibition of lipolysis. 36 Lipid increasing effects were even present in the topically‐acting budesonide with low systemic availability. 27 , 37 The relationship between the janus kinase pathway and lipid metabolism is unclear. A mechanism of action study in RA patients demonstrated that tofacitinib‐induced lipid increases are driven by decreased cholesterol ester catabolism and improved anti‐atherogenic HDL‐c function. 38 To our knowledge, evidence on the pathomechanisms of TNFα inhibition on the lipid metabolism is limited. TNFα is suggested to promote hepatic lipogenesis, induce lipolysis and regulate cholesterol metabolism and other adipocyte‐derived adipokines. 39 In IBD, anti‐TNFα agents did not influence small dense LDL‐c levels despite significant improvement of disease activity. 9 Moreover, anti‐TNFα administration is associated with beneficial cardiovascular effects such as improvement of insulin sensitivity, endothelial function and coronary plaque stability. 40 , 41
Our study showed that women have more unfavourable lipid profiles as compared to men. Sex differences in lipid profiles of IBD patients have not been described before. In the general population, premenopausal women tend to have higher HDL‐c levels and lower LDL‐c and triglyceride levels. Likewise, age and BMI were positively correlated with total cholesterol levels. Ageing is known to be associated with lipid disorders, however greater lipid‐modifying effect of IBD treatment with increasing age was not reported before. A direct relationship between increasing BMI and total cholesterol changes was not found. Therefore, it is unlikely that total cholesterol changes are partially explained by weight gain, which is a known adverse effect of corticosteroids and also described in tofacitinib and anti‐TNFα agents. 12 , 19 The role of improvement in general condition and nutritional state due to mucosal healing has not been clarified.
Substantial gaps in our understanding invite for further study. The complex interplay between inflammatory cells and lipid metabolism remains to be elucidated. Kinetic studies of lipid metabolism would be useful to differentiate between catabolic causes (dominant pathways) and anabolic causes (use of anti‐inflammatory agents) of drug‐induced lipid increases. To further understand the link between lipids and cardiovascular risk in IBD, lipidomics and proteomics of LDL‐c and HDL‐c (eg levels, composition and function of lipoprotein subfractions not detectable using standard laboratory tests) might reveal potential atherogenicity. Especially of interest in IBD is the influence of local intestinal inflammation on lipid metabolism and homeostasis. Moreover, clinical studies might address the lipid‐modulating effect of other drug classes, including immunomodulators and newer biologic agents. Proper assessment of inflammatory burden (clinical disease activity scores, faecal calprotectin and endoscopic findings) might add to current findings. To confirm a potential cardiovascular effect of these treatment modalities, trials with specific cardiovascular endpoints are needed. The clinical use of lipid measurements in IBD requires further development.
The strength of our study is that this is the first comprehensive systematic review and meta‐analysis assessing the effect of different classes of IBD therapy on lipid levels. Moreover, we had access to individual patient data of 8 out of 11 studies. Some limitations should be taken into consideration. First, the majority of observational studies lacked a control group (non‐exposed IBD patients or healthy controls). As a consequence, correction for natural variations could not be made, illustrated by the modest increase in lipids in patients randomised to placebo in the OCTAVE trials. 19 Secondly, in most studies (9/11) the use of lipid‐lowering medication was not mentioned. As a result, we cannot eliminate an effect of drug interaction or in‐between intervention (eg start of statins) for hyperlipidaemia. Miranda‐Bautista et al showed that normolipidaemic patients were more prone to lipid changes as compared to hyperlipidaemic patients. 14 Since previous studies showed that the number of IBD patients using statins is very low, the missing data on statin use have probably not affected our results. 42 Thirdly, insufficient data were available to draw firm conclusions regarding changes in HDL‐c, LDL‐c and TG levels. Subsequent calculation of atherogenic indices (eg total cholesterol/HDL‐c or LDL‐c/HDL‐c ratio) might be better predictors of cardiovascular risk. 7 Moreover, a type 2 error might be introduced for variables only assessed in small cohorts, such as the effect IBD subtype on lipid profiles. Lastly, high levels of heterogeneity were found between studies. For corticosteroids, a possible explanation could be the variety of modes of administration as well as different doses used in studies. In the tofacitinib studies, both real‐life cohorts and pivotal trials were included, which differ not only in sample size but also in clinical characteristics, for example only 30% of patients qualify for enrolment in these studies. 43 Heterogeneity was lowest among anti‐TNFα studies. Nevertheless, selection bias might be introduced since several authors not taking part in our meta‐analysis support the hypothesis of a lipid increasing effect of anti‐TNFα agents in IBD. 12 , 44 , 45 In line with this, lack of data from other registered drug therapies such as immunomodulators (eg thiopurines and methotrexate) and modern biologics (eg ustekinumab and vedolizumab) might as well be the consequence of reporting bias rather than unavailability of data.
Currently, it is unknown if dyslipidaemia associated with IBD treatment plays a role in cardiovascular risk in IBD. Whether a drug‐induced increase in lipid levels is clinically important, needs to be determined on individual patient level and depends on total cardiovascular risk, taking into consideration not only lipid levels but also other risk factors, such as age, sex, smoking habit and blood pressure. Evidence from genetic, epidemiological and clinical studies suggest that each mmol/L reduction in LDL‐c is associated with up to 52% reduction in cardiovascular risk. 46 Effects depend on the duration of exposure to LDL‐c, implicating that long‐term exposure to even marginal increases in LDL‐c can eventually lead to substantial cardiovascular risk increase. From a clinical point of view, the net effect of improving inflammatory burden but increasing cholesterol levels on cardiovascular risk remains undetermined. Disease remission, biologic therapy and intestinal surgery have been associated with reduced incidence of cardiovascular events. 47 , 48 However, the majority of patients continue to experience disease flares, persistent disease activity and short‐term corticosteroid exposure during their disease course, all associated with an increased risk of myocardial infarction, stroke and heart failure. 49 , 50 It is as yet uncertain if in IBD patients cholesterol is an independent risk factor of cardiovascular disease due to fluctuating patterns of inflammation. Nevertheless, given the elevated cardiovascular risk in IBD patients, assessing and treating cardiovascular risk factors regardless of inflammation is essential for cardiovascular risk prevention. Previous studies suggest the use of other methods of risk assessment, such as evaluation of carotid intima‐media thickness by ultrasonography. 5 The development of IBD‐specific cardiovascular risk assessment tools might help to identify patients who will benefit from intervention.
In conclusion, this systematic review and meta‐analysis provide evidence of an association between drug therapy and lipid changes in patients with IBD. Our results support routine evaluation of lipid profiles in patients with active disease initiating corticosteroids and JAK‐STAT inhibitors in daily practice.
AUTHORSHIP
Guarantor of the article: A.C. de Vries.
Authors' contributions: JS and EB: analysis. JS, JR, EB, JW, AV: study design and manuscript preparation. LM, ML, KF, NK, VB shared individual patient data. All authors approved the final version of the manuscript and agree with publication in AP&T.
PATIENT CONSENT FOR PUBLICATION
Not required.
Supporting information
Table S1‐S4
ACKNOWLEDGEMENTS
We would like to thank the following contributors: biomedical information specialist W.M. Bramer for literature search; R.W.M. Pauwels for title/abstract screening and quality scoring; A. Balint, M. Motobayashi and Galapagos NV, Belgium (NCT02048618) for sharing aggregated data. This publication is based on research using data from Pfizer (NCT00787202, NCT01465763, NCT01458951, NCT01458574) that has been made available through Vivli, Inc Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Declaration of personal interests: J.A.M. Sleutjes: nothing to declare. J.E. Roeters van Lennep: nothing to declare. E. Boersma: nothing to declare. L.A. Menchen: received unrestricted grants from MSD and Abbvie, and served as an advisory board member and/or speaker for MSD, Abbvie, Pfizer, Janssen, Takeda, Biogen, Sandoz, Dr Falk‐Pharma, FAES, Ferring, General Electric and Medtronic. M. Laudes: nothing to declare. K. Farkas: received speaker's honoraria from AbbVie, Janssen, Ferring, Takeda and Goodwill Pharma, and served as an advisory board member of Takeda. T. Molnár: received speaker's honoraria from AbbVie, Egis, Goodwill Pharma, Takeda, Pfizer and Teva, and served as an advisory board member of Takeda. N.A. Kennedy: has received honoraria from Janssen, Falk, Allergan, Pharmacosmos and Takeda for unrelated topics and is an associate editor of Alimentary Pharmacology & Therapeutics. M.J. Pierik: has served on an advisory boards, or as speaker or consultant for Abbvie, Janssen‐Cilag, MSD, Takeda, Ferring, Dr Falk and Sandoz and has received unrestricted grants from Janssen‐Cilag, Abbvie and Takeda outside the submitted work. C.J. van der Woude: served as an advisory board member of Celltrion, Takeda and Abbvie. A.C. de Vries: served as an advisory board member of Jansen, Abbvie and Takeda.
Declaration of funding interests: This study was not funded by any party.
Sleutjes JAM, Roeters van Lennep JE, Boersma E, et al. Systematic review with meta‐analysis: effect of inflammatory bowel disease therapy on lipid levels. Aliment Pharmacol Ther. 2021;54:999–1012. 10.1111/apt.16580
Jasmijn A. M. Sleutjes and Jeanine E. Roeters van Lennep shared first authorship.
C. Janneke van der Woude and Annemarie C. de Vries shared last authorship.
As part of AP&T's peer‐review process, a technical check of this meta‐analysis was performed by Dr Y Yuan. The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
Funding information
There has been no financial support for this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from Pfizer (through Vivli Inc) and Gilead/Galapagos. Restrictions apply to the availability of these data, which were used under license for this study. The data that support the findings of this study are available from separate authors upon reasonable request.
REFERENCES
- 1. Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fumery M, Xiaocang C, Dauchet L, Gower‐Rousseau C, Peyrin‐Biroulet L, Colombel JF. Thromboembolic events and cardiovascular mortality in inflammatory bowel diseases: a meta‐analysis of observational studies. J Crohns Colitis. 2014;8:469‐479. [DOI] [PubMed] [Google Scholar]
- 3. Sun HH, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta‐analysis. Eur J Prev Cardiol. 2018;25:1623‐1631. [DOI] [PubMed] [Google Scholar]
- 4. Aarestrup J, Jess T, Kobylecki CJ, Nordestgaard BG, Allin KH. Cardiovascular risk profile among patients with inflammatory bowel disease: a population‐based study of more than 100 000 individuals. J Crohns Colitis. 2019;13:319‐323. [DOI] [PubMed] [Google Scholar]
- 5. Biondi RB, Salmazo PS, Bazan SGZ, Hueb JC, de Paiva SAR, Sassaki LY. Cardiovascular risk in individuals with inflammatory bowel disease. Clin Exp Gastroenterol. 2020;13:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agouridis AP, Elisaf M, Milionis HJ. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann Gastroenterol. 2011;24:181‐187. [PMC free article] [PubMed] [Google Scholar]
- 7. Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513‐523. [DOI] [PubMed] [Google Scholar]
- 8. Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. Anawalt B, Boyce A, Endotext [Internet]; South Dartmouth (MA): MDText.com, Inc.; 2000. https://www.ncbi.nlm.nih.gov/books/NBK326741/ [Google Scholar]
- 9. Schulte DM, Paulsen K, Türk K, et al. Small dense LDL cholesterol in human subjects with different chronic inflammatory diseases. Nutr Metab Cardiovasc Dis. 2018;28:1100‐1105. [DOI] [PubMed] [Google Scholar]
- 10. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723‐1736. [DOI] [PubMed] [Google Scholar]
- 11. Sands BE, Paulsen K, Türk K, et al. Lipid profiles in patients with ulcerative colitis receiving tofacitinib‐implications for cardiovascular risk and patient management. Inflamm Bowel Dis. 2021;27:797‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koutroubakis IE, Oustamanolakis P, Malliaraki N, et al. Effects of tumor necrosis factor alpha inhibition with infliximab on lipid levels and insulin resistance in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:283‐288. [DOI] [PubMed] [Google Scholar]
- 13. Farkas K, Bálint A, Valkusz Z, et al. Bolus administration of steroid therapy is more favorable than the conventional use in preventing decrease of bone density and the increase of body fat percentage in patients with inflammatory bowel disease. J Crohns Colitis. 2014;8:992‐997. [DOI] [PubMed] [Google Scholar]
- 14. Miranda‐Bautista J, de Gracia‐Fernández C, López‐Ibáñez M, et al. Lipid profile in inflammatory bowel disease patients on anti‐TNFalpha therapy. Dig Dis Sci. 2015;60:2130‐2135. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2; Chichester (UK): John Wiley & Sons, Ltd.; 2021. www.training.cochrane.org/handbook [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses; 2013. [Google Scholar]
- 19. Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:123‐132.e3. [DOI] [PubMed] [Google Scholar]
- 20. Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605‐613. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 23. Vangeneugden T, Laenen A, Geys H, Renard D, Molenberghs G. Applying linear mixed models to estimate reliability in clinical trial data with repeated measurements. Control Clin Trials. 2004;25:13‐30. [DOI] [PubMed] [Google Scholar]
- 24. Honap S, Chee D, Chapman TP, et al. Real‐world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. 2020;14:1385‐1393. [DOI] [PubMed] [Google Scholar]
- 25. Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: results of the prospective Dutch Initiative on Crohn and Colitis (ICC) registry. Aliment Pharmacol Ther. 2020;51:880‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616‐624. [DOI] [PubMed] [Google Scholar]
- 27. Pigniczki D, Szanto K, Rutka M, et al. Effects and safety of a colon‐long absorbing budesonide product in patients with mild to moderate ulcerative colitis. J Crohns Colitis. 2020;14:S393‐S394. [Google Scholar]
- 28. Bálint A, Farkas K, Szűcs M, et al. Long‐term increase in serum cholesterol levels in ulcerative colitis patients treated with cyclosporine: an underdiagnosed side effect frequently associated with other drug‐related complications. Scand J Gastroenterol. 2014;49:59‐65. [DOI] [PubMed] [Google Scholar]
- 29. Motobayashi M, Matsuoka K, Takenaka K, et al. Predictors of mucosal healing during induction therapy in patients with acute moderate‐to‐severe ulcerative colitis. J Gastroenterol Hepatol. 2019;34:1004‐1010. [DOI] [PubMed] [Google Scholar]
- 30. Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate‐to‐severe Crohn's disease treated with filgotinib (the FITZROY study): results from a phase 2, double‐blind, randomised, placebo‐controlled trial. Lancet. 2017;389:266‐275. [DOI] [PubMed] [Google Scholar]
- 31. Charles‐Schoeman C, Wicker P, Gonzalez‐Gay MA, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. 2016;46:261‐271. [DOI] [PubMed] [Google Scholar]
- 32. Wolk R, Armstrong EJ, Hansen PR, et al. Effect of tofacitinib on lipid levels and lipid‐related parameters in patients with moderate to severe psoriasis. J Clin Lipidol. 2017;11:1243‐1256. [DOI] [PubMed] [Google Scholar]
- 33. Gladman DD, Charles‐Schoeman C, McInnes IB, et al. Changes in lipid levels and incidence of cardiovascular events following tofacitinib treatment in patients with psoriatic arthritis: a pooled analysis across phase III and long‐term extension studies. Arthritis Care Res. 2019;71:1387‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta‐analysis. Ann Rheum Dis. 2012;71:862‐868. [DOI] [PubMed] [Google Scholar]
- 35. Hassan S, Milman U, Feld J, et al. Effects of anti‐TNF‐α treatment on lipid profile in rheumatic diseases: an analytical cohort study. Arthritis Res Ther. 2016;18:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gregg RE. The role and mechanism of glucocorticoids on the modulation of lipid and lipoprotein metabolism. Steinmetz A, Schneider J, Kaffarnik H, Hormones in Lipoprotein Metabolism. Marburg, Germany: Springer; 1993:169‐173. [Google Scholar]
- 37. O'Donnell S, O'Morain CA. Therapeutic benefits of budesonide in gastroenterology. Ther Adv Chronic Dis. 2010;1:177‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Charles‐Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 2015;67:616‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X, Xun K, Chen L, Wang Y. TNF‐alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27:407‐416. [DOI] [PubMed] [Google Scholar]
- 40. Paschou SA, Kothonas F, Lafkas A, et al. Effect of anti‐TNF therapy on insulin sensitivity in nonobese, nondiabetic patients with inflammatory bowel disease. Int J Endocrinol. 2018;2018:6712901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schinzari F, Armuzzi A, De Pascalis B, et al. Tumor necrosis factor‐alpha antagonism improves endothelial dysfunction in patients with Crohn's disease. Clin Pharmacol Ther. 2008;83:70‐76. [DOI] [PubMed] [Google Scholar]
- 42. Emanuel G, Charlton J, Ashworth M, Gulliford MC, Dregan A. Cardiovascular risk assessment and treatment in chronic inflammatory disorders in primary care. Heart. 2016;102:1957‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002‐1007; quiz e78. [DOI] [PubMed] [Google Scholar]
- 44. Konstantopoulos P, Papamichael K, Archavlis E, et al. P401. Long term effect of anti‐TNFα agents on the lipidemic profile of IBD patients. J Crohns Colitis. 2012;6:S169. [Google Scholar]
- 45. Kalkan Ç, Karakaya F, Törüner M, Çetinkaya H, Soykan I. Anti‐TNF‐α agents and serum lipids in inflammatory bowel diseases. Clin Res Hepatol Gastroenterol. 2016;40:e46‐e47. [DOI] [PubMed] [Google Scholar]
- 46. Ference BA, Ginsberg HN, et al. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459‐2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kirchgesner J, Nyboe Andersen N, Carrat F, Jess T, Beaugerie L; BERENICE study group . Risk of acute arterial events associated with treatment of inflammatory bowel diseases: nationwide French cohort study. Gut. 2020;69:852‐858. [DOI] [PubMed] [Google Scholar]
- 48. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689‐694. [DOI] [PubMed] [Google Scholar]
- 49. Kristensen SL, Ahlehoff O, Lindhardsen J, et al. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardiovascular death–a Danish nationwide cohort study. PLoS One. 2013;8:e56944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aniwan S, Pardi DS, Tremaine WJ, Loftus EV Jr. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1607‐1615.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4
Data Availability Statement
The data that support the findings of this study are available from Pfizer (through Vivli Inc) and Gilead/Galapagos. Restrictions apply to the availability of these data, which were used under license for this study. The data that support the findings of this study are available from separate authors upon reasonable request.


