Abstract
Oxytosis/ferroptosis is a form of non-apoptotic regulated cell death characterized by glutathione (GSH) depletion and dysregulated production of mitochondrial ROS that results in lethal lipid peroxidation. As the significance of oxytosis/ferroptosis to age-associated human diseases is now beginning to be appreciated, the development of innovative approaches to identify novel therapeutics that target the oxytosis/ferroptosis pathway could not be more timely. Due to their sessile nature, plants are exposed to a variety of stresses that trigger physiological changes similar to those found in oxytosis/ferroptosis. As such, they have evolved a rich array of chemical strategies to deal with those challenging conditions. This review details a drug discovery approach for identifying potent inhibitors of oxytosis/ferroptosis from plants for the treatment of Alzheimer’s disease and related dementias, thereby highlighting the tremendous potential of plant-based research for developing new medicines while simultaneously being a catalyst for sustainability.
Keywords: dementia, drug discovery, lipid peroxidation, oxidative stress, plant secondary metabolites, phenotypic screening
1 |. INTRODUCTION
For thousands of years, continuous trial and error experimentation of nature by humans has identified plants that can be harvested and judiciously prepared for treating a variety of ailments. The consequences of this long-standing interaction between humans and plants, embraced by virtually all traditional cultures around the globe, are so well integrated into our lifestyles today that they can easily go unnoticed. Medicinal plants were not only an essential part of the first forms of medicine, but they also have contributed to the progress of societies and influenced geopolitical decisions that ended up shaping the course of history (Choffnes, 2016).
Some of the oldest and most impressive records describing the medicinal value of plants include remarkable Sumerian clay tablets from 2200 B.C. found in Nippur (Mesopotamia) listing prescriptions using hundreds of different plants, the Egyptian medical text Ebers papyrus that details herbal knowledge written about 1500 B.C., the Indian books called Vedas used in Ayurvedic medicine dating back to 1200 B. C., the distinguished Chinese book on medicinal plants ‘Shen Nong Ben Cao Jing’ (The Divine Farmer’s Materia Medica Classic) (200 A.D.), the ‘Naturalis Historia’ from Pliny the Elder (77 A.D.) and the famous ‘De Materia Medica’ written by the Greek Pedanius Dioscorides (50–70 A. D.) (Gurib-Fakim, 2006; Petrovska, 2012; Van Wyk & Wink, 2019). The latter was the most influential herbal pharmacopeia in European medicine for 1500 years. Importantly, these and other traditional medicine systems are still in practice today, coexisting side by side with modern allopathic medicine, particularly in many rural communities in Africa, Asia and Central and South America, where plant-based medicine is accessible and affordable (Allkin, 2017).
Even though the knowledge of medicinal plants has been largely generated empirically, it has led to the development of major pharmaceuticals that are essential to modern allopathic medicine. It is estimated that nearly half of all approved drugs in the last 30 years are from natural origin or derived from natural compounds (Newman & Cragg, 2016), with a significant contribution from plants (Atanasov et al., 2015; Gurib-Fakim, 2006; Kinghorn et al., 2011; Lautie et al., 2020). Some well-known examples include the natural anti-cancer agents paclitaxel (from Taxus species), vincristine and vinblastine (from Catharanthus roseus) and camptothecin (from Camptotheca acuminata), as well as the antimalarial artemisinin (from Artemisia annua), whose discovery culminated in a Nobel Prize in Physiology or Medicine to Tu Youyou in 2015 (Atanasov et al., 2015). Given that most of the chemical diversity entailed in plant physiology remains uncharted from a therapeutic point of view and that there are over 28,000 plant species known to have medicinal applications (Allkin, 2017), the potential for discovering new remedies from plants to treat human diseases cannot be overstated.
For over a decade, our laboratories have been using natural compounds derived from plants to develop treatments for neurodegenerative diseases, mainly Alzheimer’s disease (AD) (Maher et al., 2020; Prior et al., 2014; Schubert et al., 2018). There are no drugs for AD that prevent or slow down its progression and this is likely due to a lack of therapies that target the primary drivers of the disease. With this in mind and considering that old age is the greatest risk factor for AD and related dementias, we have established a drug discovery approach that relies on cell and animal models of age-associated toxicities that are relevant to AD (Maher et al., 2020; Prior et al., 2014; Schubert et al., 2018). The hypothesis is that by targeting specific pathological aspects of aging in the brain, it is possible to prevent the early events that contribute to the onset of AD. In this context, a unique mechanism of neurodegeneration called oxytosis/ferroptosis has been identified that became central to our search for neuroprotective compounds (Maher et al., 2020).
In this review, an approach designed to identify novel anti-oxytotic/ferroptotic compounds from plants is described. These natural compounds can be further improved through medicinal chemistry to generate neurotherapeutics. The value of the chemical diversity inherent to plants is analysed in light of the meaning of oxytosis/ferroptosis to plant biology. We hope that this discussion presents a new path to carrying out plant-based drug discovery in a sensible manner while effectively driving scientific progress towards the improvement of human health.
2 |. OXYTOSIS/FERROPTOSIS AND ITS RELEVANCE TO DEMENTIA
Although aging is the biggest risk factor for AD, its value for AD drug discovery has been relatively disregarded. Oxytosis/ferroptosis has emerged as an age-associated degenerative mechanism that may play a key role in the disease (Maher et al., 2020). Oxytosis/ferroptosis is a form of non-apoptotic regulated cell death characterized by GSH depletion and dysregulated production of ROS from mitochondria that results in lethal lipid peroxidation (Lewerenz et al., 2018; Maher et al., 2020; Tan et al., 1998, 2001) (Figure 1). At the molecular level, oxytosis/ferroptosis can be triggered by inhibiting cystine uptake via system xc− with either glutamate or erastin, which subsequently depletes intracellular GSH (Lewerenz et al., 2018; Maher et al., 2020; Tan et al., 1998; Tan et al., 2001). System xc− (cystine/glutamate transporter/SL7A11) is a heterodimeric amino acid antiporter that imports cystine into cells with a 1:1 counter-transport of glutamate. Once inside the cells, cystine is reduced to cysteine, which is utilized for GSH synthesis. GSH depletion leads to inhibition of the GSH-dependent enzyme GSH peroxidase 4 (GPX4) and activation of lipoxygenases (LOXs). GPX4 can also be directly inhibited by the chemical RSL3. In both cases, ROS and lipid peroxides are generated, which potentiate intracellular calcium influx through store-operated calcium (Ca2+) channels and cell death. Iron plays an important role in oxytosis/ferroptosis, as iron chelators prevent oxytosis/ferroptosis-mediated cell death (Dixon et al., 2012; Maher et al., 2020). It is thought that iron can not only generate ROS via the Fenton reaction but also promote the activation of the non-heme iron-containing LOX enzymes (Chen et al., 2020).
FIGURE 1.
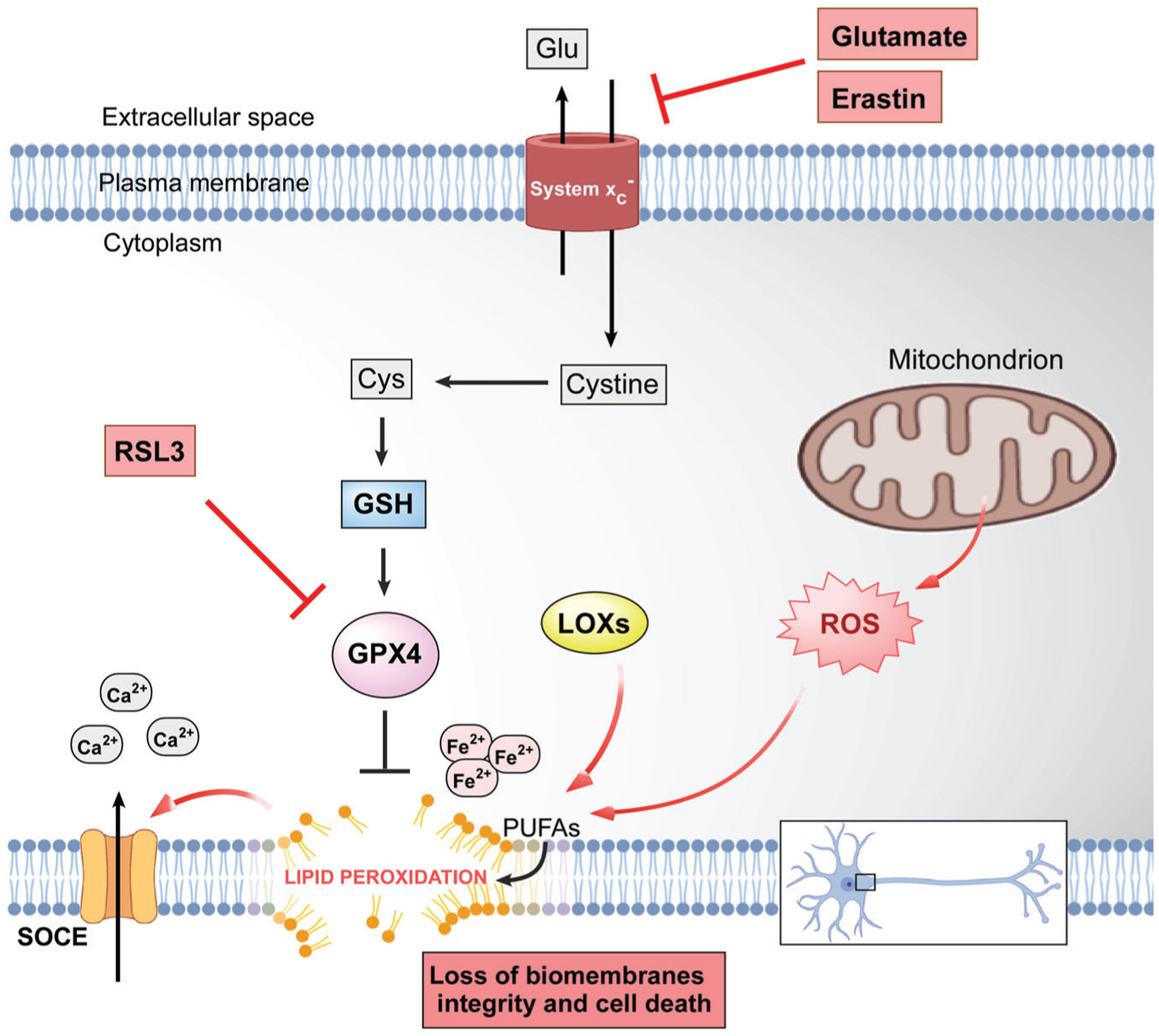
The molecular cascade of oxytosis/ferroptosis cell death programme. Ca2+, calcium; Cys, cysteine; Fe2+, iron; Glu, glutamate; GPX4, GSH peroxidase 4; GSH, GSH; LOXs, lipoxygenases; PUFAs, polyunsaturated fatty acids; SOCE, store-operated calcium entry
There is strong evidence demonstrating that these processes are also seen with aging. Brain total GSH and/or reduced GSH levels decrease with age in humans and are accelerated in several CNS diseases including AD (Ansari & Scheff, 2010; Ballatori et al., 2009; Currais & Maher, 2013; Emir et al., 2011; Mandal et al., 2015). The loss of GSH is associated with impairments in cognitive function, microglial activation and endothelial dysfunction, all of which are pathological features of AD (Currais & Maher, 2013; Tan et al., 2001). Moreover, multiple studies have shown decreases in blood GSH levels with age in humans (Droge et al., 2006; Giustarini et al., 2006; Maher, 2005; Sekhar et al., 2011), which is exacerbated in mild cognitive impairment and AD (Bermejo et al., 2008; Rae & Williams, 2017).
In addition, lipid peroxidation has been linked to AD and is likely to be an early feature in the disease, as post-mortem brain samples from subjects with mild cognitive impairment already show increased levels of lipid peroxidation and by-products of lipid peroxidation in plasma, urine and CSF are elevated throughout the progression of the disease (Bradley et al., 2010; Bradley-Whitman & Lovell, 2015; Gaschler & Stockwell, 2017; Sultana et al., 2013). Several LOXs have also been implicated in AD (Joshi et al., 2015; Joshi & Pratico, 2014; Kuhn et al., 2015; Singh & Rao, 2019). 12/15-LOX is up-regulated in the brain regions that are most vulnerable to AD early in the course of the disease (Joshi et al., 2015) and 5-LOX expression increases with age in the brain in both mice and humans, and this is exacerbated in AD (Joshi & Pratico, 2014).
In general, free polyunsaturated fatty acids as well as lipids containing esterified polyunsaturated fatty acids appear to be particularly susceptible to peroxidation and are required for the execution of oxytosis/ferroptosis by triggering a free radical chain reaction that becomes lethal (Conrad et al., 2018; Maher et al., 2020; Yang et al., 2016). The reaction is initiated with the abstraction of a hydrogen (H) atom from an oxidizable polyunsaturated fatty acid, generating an alkyl radical (L•) that reacts with O2 to produce a peroxyl radical (LOO•). A chain reaction is established when the peroxyl radicals further abstract hydrogen from oxidizable polyunsaturated fatty acids to yield lipid hydroperoxides and new alkyl radicals (Farmer & Mueller, 2013; Han et al., 2020). As a result, lipids are degraded and the integrity, fluidity, permeability and function of the biomembranes may be compromised (Alche, 2019; El-Beltagi & Mohamed, 2013; Farmer & Mueller, 2013). In addition, lipid peroxides frequently decompose to generate a variety of short-chain reactive carbonyl species that can further damage biomolecules such as proteins and DNA in a second phase of lipid peroxidation (Alche, 2019; Farmer & Mueller, 2013). Some of these reactive carbonyl species include malondialdehyde and 4-hydroxynonenal, which have been implicated in a variety of human disorders including cancer, aging, cardiovascular diseases and neurodegenerative diseases (El-Beltagi & Mohamed, 2013; Maher et al., 2020).
A key aspect of oxytosis/ferroptosis is mitochondrial dysfunction and associated ROS production (Lewerenz et al., 2018; Maher et al., 2020), which is one of the hallmarks of aging (Lopez-Otin et al., 2013) that has been directly implicated in AD (Currais, 2015). A decline in cerebral energy metabolism precedes the pathology and symptoms of AD and is more severe than that observed in normal aging (Costantini et al., 2008; Cunnane et al., 2011; Currais, 2015; Yin et al., 2014). Most of the energy derived from glucose oxidation is produced in mitochondria and multiple mitochondrial-dependent functions are impaired during aging and AD. Evidence that mitochondrial dysfunction occurs early and acts causally in AD pathogenesis includes declines in electron transport chain activity and respiration, increases in mitochondrial-derived oxidative stress, significant changes in the mitochondrial metabolite landscape, oxidative damage to mitochondrial DNA (mtDNA), RNA, lipid and protein species and an accumulation of mtDNA mutations (Currais, 2015; Lin & Beal, 2006; Onyango et al., 2016; Swerdlow & Khan, 2004; Yin et al., 2016).
It is becoming increasingly recognized that disruption in biometal homeostasis is an important feature of the onset and progression of AD (Hagemeier et al., 2012; Hare et al., 2013). As the most abundant transition metal in the brain, iron participates in neurotransmitter synthesis, myelination and mitochondrial function (Hare et al., 2013). However, it is thought that the accumulation of iron that has been observed in the brain with aging and in neurodegenerative diseases contributes to the neuropathology by increasing oxidative stress through Fenton chemistry (Hagemeier et al., 2012), in a process similar to that found during oxytosis/ferroptosis. As such, multiple iron chelators have been tested in preclinical models of AD (Hare et al., 2013).
Importantly, a large body of evidence shows that features of oxytosis/ferroptosis are also found in other neurological disorders including Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis (ALS) (Lewerenz et al., 2018; Li et al., 2020; Maher et al., 2020; Ren et al., 2020). Decreases in GSH along with iron accumulation and lipid peroxidation have been reported in the regions of the nervous system that are preferentially affected in all of these diseases (Hagemeier et al., 2012; Hare et al., 2013; Li et al., 2020; Reed, 2011; Ren et al., 2020).
Despite all these observations, the oxytosis/ferroptosis cell death pathway stands as a unique mechanism of neurodegeneration whose therapeutic potential for dementia remains largely unexplored. It should be noted that although mammalian cultured cells die rapidly following the induction of oxytosis/ferroptosis, this might not be the case in vivo (Maher et al., 2020). Oxytosis/ferroptosis follows a series of steps that, in vivo, could take place over an extended time period thereby leading to a slow degeneration of basic neuronal functions prior to any cell death. In the context of aging, this could translate into oxytosis/ferroptosis taking place as a chronic process rather than an acute event and its inhibition being relevant throughout the course of disease progression and not only at its point of initiation (Maher et al., 2020). As such, we have been using inducers of oxytosis/ferroptosis in nerve cell culture as a screening model to identify novel neuroprotective compounds, which are then tested in animal models. This approach has provided drug candidates that are not only protective in transgenic mouse models of AD but also reduce age-associated cognitive decline in SAMP8 mice, a model of age-associated dementia (Ates et al., 2020; Currais et al., 2015, 2018, 2019; Currais, Prior, et al., 2014; Goldberg et al., 2018; Prior et al., 2013, 2014; Schubert et al., 2018). Therefore, drug candidates that are specifically developed on the basis of being anti-oxytotic/ferroptotic have the potential for treating AD and other age-related neurological diseases.
It is worth noting that features of oxytosis/ferroptosis have also been observed in heart, liver, vascular and kidney diseases (Han et al., 2020; Li et al., 2020). These features have been described in rodent models of ischaemia/reperfusion injury in the heart (Fang et al., 2019; Gao et al., 2015) and liver (Friedmann Angeli et al., 2014), where inhibitors of oxytosis/ferroptosis were very protective. Modulation of the oxytosis/ferroptosis molecular pathway also affects the outcome in models of acute kidney injury (Friedmann Angeli et al., 2014; Martin-Sanchez et al., 2017), liver fibrosis (Wang et al., 2019) and haemolytic disorders (NaveenKumar et al., 2018). The value of oxytosis/ferroptosis inhibitors may thus extend well beyond diseases of the nervous system.
3 |. PLANTS AS A SOURCE OF ANTI-OXYTOTIC/FERROPTOTIC COMPOUNDS
Given the therapeutic potential of anti-oxytotic/ferroptotic compounds, the ensuing challenge is to develop these molecules. Although a large proportion of the natural compounds that have been studied as therapeutic leads in the last decades are of plant origin (Kinghorn et al., 2011; Liang et al., 2020), whether plants are more likely to generate therapeutic compounds than other living organisms is unclear. However, two key observations support the idea that plants may not only be a highly favourable biological source for the identification of novel anti-oxytotic/ferroptotic compounds, but that they also present significant advantages when compared with libraries of synthetic compounds. One is the rich metabolic diversity in plants and the other is the evidence for oxytosis/ferroptosis-related processes in plants (Figure 2). The implications of these observations for AD drug discovery are discussed below.
FIGURE 2.
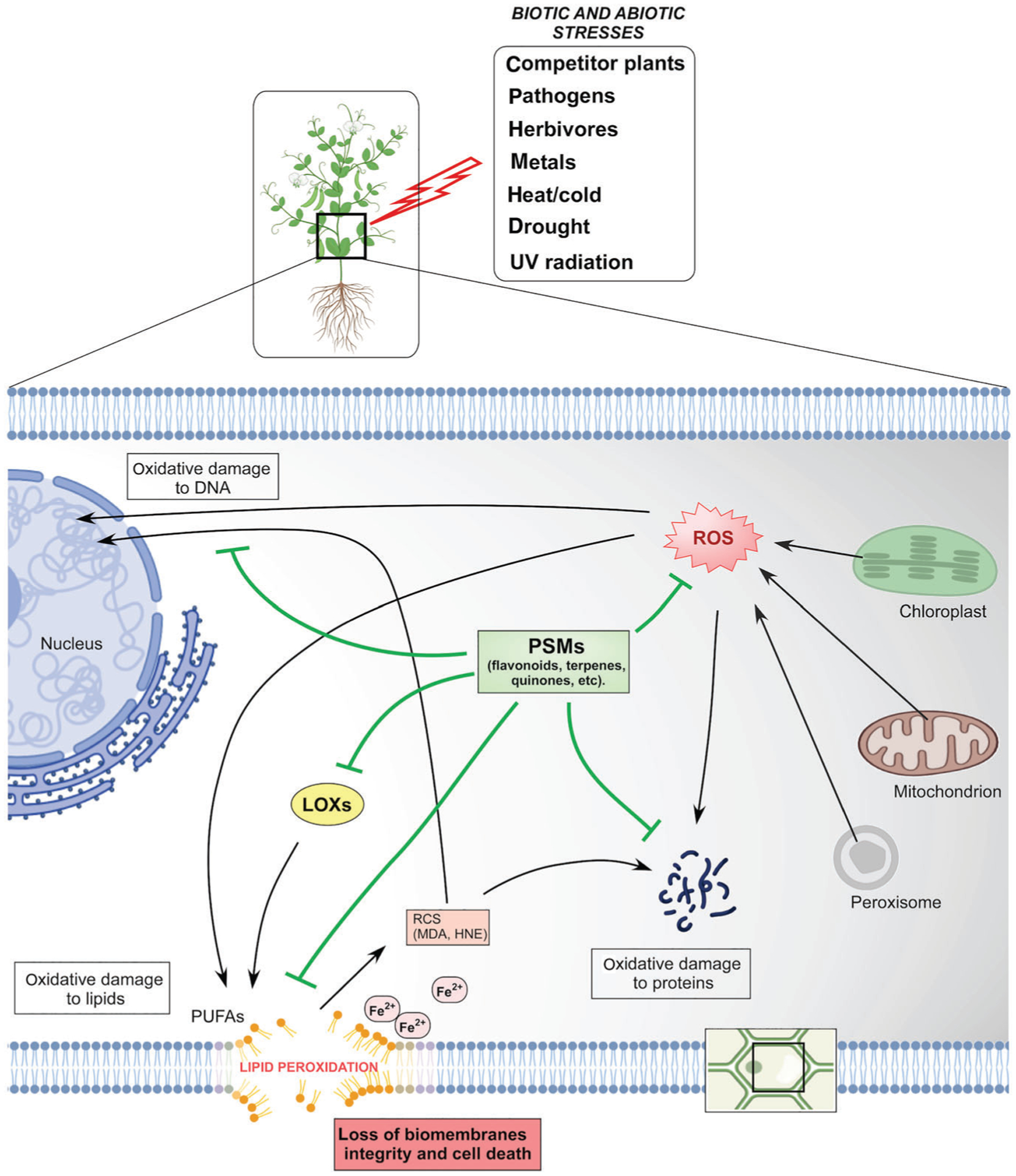
Lipid peroxidation in plants. Fe2+, iron; HNE, 4-hydroxynonenal; LOXs, lipoxygenases; MDA, malondialdehyde; PSMs, plant secondary metabolites; PUFAs, polyunsaturated fatty acids; RCS, reactive carbonyl species
3.1 |. The plant chemical portfolio
The first argument supporting the use of plants in drug discovery stems from the extensive structural diversity of the wide range of pharmacophores that plants produce (Harvey et al., 2015). Although perhaps being ‘Earth’s most accomplished chemists’ (Schuman et al., 2016), the reason for this chemical diversity in plants may be explained by their sessile nature and thus their inability to physically escape the different threats that they face (Knudsen et al., 2018). As such, plant biosynthetic metabolic systems may have evolved to produce a plethora of phytochemicals that increases the chances of their survival upon unfavourable conditions (Knudsen et al., 2018). Phytochemicals can be either primary or secondary metabolites. Plant primary metabolites (are involved in processes such as glycolysis, Krebs cycle, photosynthesis and associated pathways, which are required for basic life functions, including cell division/growth, development and reproduction. Plant primary metabolites are similar to those of non-plant genera. On the other hand, plant secondary metabolites (PSMs) are not involved in basic functions but rather produced as a means of coping with abiotic stresses (e.g. UV radiation, drought, heat and soil toxicities) as well as mediating interactions with antagonists (e.g. herbivores, pathogens and neighbouring plants) and mutualists (e.g. fungi, bacteria and pollinators) (Kessler & Kalske, 2018). PSMs are generated using building blocks and biosynthetic enzymes derived from primary metabolism and are the hub of the chemical diversity found in plants. It is estimated that the plant kingdom has between 200,000 and 1,000,000 different metabolites, with a single species containing over 5000 metabolites (Lautie et al., 2020; Wang, Alseekh, et al., 2019). The vast majority of these compounds are the result of one or more structural modifications of a common backbone (Wang, Alseekh, et al., 2019). Some of these core structures are nitrogen-containing compounds, phenylpropanoids, benzenoids, phenolics and terpenes, which can undergo various modifications such as glycosylation, acylation, methylation, hydroxylation, geranylation and prenylation (Wang, Alseekh, et al., 2019), so that the end result is a vast number of possible different PSMs.
Several mechanisms that contribute to PSM diversity have been identified (Kessler & Kalske, 2018):- (1) PSMs are derived from chemically simple precursors from primary metabolism, allowing for a large number of possible combinations, (2) biosynthetic genes are organized in large families with multiple genes encoding enzymes of a similar type, expanding the range of produced metabolites from a common precursor, (3) some biosynthetic enzymes can produce different products out of the same precursor, (4) modifying enzymes are also organized in large families and can use multiple substrates and (5) expression of biosynthetic genes takes place in a spatial, temporal and organ-specific manner.
In the past decades, the major pharmaceutical companies shifted their interest from natural products to new technologies that generate large chemical libraries that can be tested in high throughput screening directed at specific molecular targets (Prior et al., 2014). Several limitations to natural compound-based drug discovery were pointed out: incompatibility of several natural compound classes with some high throughput screening reagents and instrumentation (high hydrophobicity, viscosity and fluorescence for example); the possibility of generating nonspecific off-target hits; and the low chances of funding for clinical trials due to patentability issues (Atanasov et al., 2015; Harvey et al., 2015; Lautie et al., 2020; Prior et al., 2014). However, these large screening collections have fallen short on their objectives (Atanasov et al., 2015), as it is becoming clear that ‘diversity within biologically relevant chemical space is more important than library size’ (Harvey et al., 2015). Testing as many chemically diverse compounds as possible is key to identifying new drugs (Lautie et al., 2020), in particular when this diversity may have evolved to serve biological functions (Atanasov et al., 2015). Natural compounds often have a wide range of biological activities as well as better ‘druglikeness’ that fits with Lipinski’s ‘rule-of-five’ than molecules produced by combinatorial chemistry (Atanasov et al., 2015; Kingston, 2011). In some cases, synthetic compound libraries are now being sought to mimic the chemical properties of natural products (Harvey et al., 2015). Therefore, although the chemical diversity inherent to plants may have contributed to their evolutionary success in the face of harsh conditions, it also offers appealing opportunities for drug discovery.
3.2 |. Oxytosis/ferroptosis and redox regulation in plants
Plants are exposed to stresses that share features of oxytosis/ferroptosis and they have evolved PSMs to protect themselves from these stresses. Therefore, the plant chemical portfolio may have evolved in a biological context that is relevant to human disease and once identified, these PSMs can be harnessed to develop new medicines.
Evidence for oxytosis/ferroptosis in plants can be best recognized when studying lipid redox homeostasis. As with animals, the peroxidation of polyunsaturated fatty acids in plants can occur both enzymatically and non-enzymatically (Farmer & Mueller, 2013; Skorzynska-Polit, 2007). Both processes can take place simultaneously and influence each other. Enzymatic lipid peroxidation is catalysed by LOXs and multiple isoforms of plant LOXs have been identified that differ in substrate/product specificity, tissue localization and temporal expression (Skorzynska-Polit, 2007). Plant LOXs mainly belong to the two major families 9-LOX and 13-LOX, depending on whether the dioxygenation of the polyunsaturated fatty acid hydrocarbon backbone takes place at carbon 9 or 13, respectively (Skorzynska-Polit, 2007; Viswanath et al., 2020). Plant LOXs are involved not only in basic physiological processes such as growth, development, seed germination and fruit ripening but also in responses to biotic and abiotic stresses (Viswanath et al., 2020). It is likely that an exacerbated activation of LOXs upon stress turns into a detrimental outcome to cells, similar to the inflammatory process in humans. In fact, LOX activation is closely associated with senescence in plants, along with an increased production of ROS (Skorzynska-Polit, 2007; Viswanath et al., 2020). Cell senescence in humans is a hallmark of aging that also manifests as a pro-inflammatory phenotype (Currais, 2015). Furthermore, it has been shown that heat stress in Arabidopsis thaliana can actually induce an oxytosis/ferroptosis-like cell death that is characterized by ROS accumulation, GSH depletion, polyunsaturated fatty acid oxidation and abnormal mitochondria and that can be prevented with inhibitors of oxytosis/ferroptosis (Distefano et al., 2017). The role of oxytosis/ferroptosis in plant cell death is supported by the observations that either blocking LOX activity chemically or genetically or overexpressing plant glutathione peroxidase (GPX) enzymes protect against multiple abiotic stresses (Conrad et al., 2018).
In non-enzymatic lipid peroxidation, the reaction is triggered by ROS and transition metal ions. Although chloroplasts (via photosystems I and II) and peroxisomes are the main source of ROS during the daytime, at night, ROS are predominantly generated in the mitochondria (from the electron transport chain) (Xie et al., 2019). Increases in oxidative stress (and carbonyl stress) can take place when plants are exposed to a variety of environmental factors such as high salinity, drought, metal toxicity, extreme temperature, air pollutants, UV radiation, pesticides and pathogen infection (El-Beltagi & Mohamed, 2013; Xie et al., 2019). It is widely acknowledged that, at least to some extent, the ROS generated participate in molecular signalling pathways that are essential for the cellular functioning. However, an aggravated oxidative response can perturb the redox balance in cells and become lethal. In plants, the cost of generating energy through the photosynthetic and respiratory electron systems results in an enormous load of free radicals that are generated as a by-product. As such, plants have developed complex network systems of redox regulation, which rely on enzymatic and non-enzymatic mechanisms that operate throughout different cellular compartments to maintain intracellular ROS pools at low levels. Major enzymatic systems involved in redox regulation in plants include ascorbate peroxidase (APX), catalase (CAT), dehydroascorbate reductase (DHAR), GSH peroxidase (GPX), GSH reductase (GR), glutaredoxin (GRX), GSH S-transferase (GST), guaiacol peroxidase (GP), monodehydroascorbate reductase (MDAR), NADH-thioredoxin reductase (NTR), peroxiredoxin (PRX), SOD and thioredoxin (TRX) (Noctor et al., 2018; Xie et al., 2019). Non-enzymatic antioxidant defences in plants include multiple compounds of low MW, of which ascorbate and GSH are the most prominent because they are used by the enzymatic systems in redox-cycling reactions (Noctor et al., 2018). Additional natural antioxidants include pigments, like carotenoids and other PSMs, flavonoids for example (Noctor et al., 2018).
Importantly, experiments have shown that both short-term and long-term exposure to the metals iron, cadmium, copper, zinc, mercury and lead may not only increase non-enzymatic lipid peroxidation via Fenton chemistry but also activate LOXs (Skorzynska-Polit, 2007). Because multiple transition metals have the potential to exacerbate oxytosis/ferroptosis (Maher, 2018), the sessile nature of plants and their dependence on soil for nutrient exchange exposes them to stresses that overlap with those found in oxytosis/ferroptosis. It is thus conceivable to think that many PSMs have evolved to acquire metal chelator properties. This idea is supported by the fact that chelation is a key strategy in metal-tolerance in plants (Anjum et al., 2015). Chelators or iron are of particular interest because they can provide protection against oxytosis/ferroptosis.
As mentioned above, in vivo, oxytosis/ferroptosis can manifest itself over a lengthy time period before cells die, thereby offering a window for therapeutic intervention. This is why PSMs that regulate cellular ROS and lipid peroxidation may be particularly useful for developing therapeutics that target oxytosis/ferroptosis in AD.
4 |. IDENTIFYING ANTI-OXYTOTIC/FERROPTOTIC COMPOUNDS FROM PLANTS
Although there are libraries of pure natural compounds that are commercially available and can be tested readily for identifying natural inhibitors of oxytosis/ferroptosis, the best way to take advantage of the rich chemical diversity in plants is to work directly from plants. This approach offers some challenges including missing out on low abundant compounds in extracts; interference and synergistic actions of multiple compounds present in an extract (fractionated extracts may not retain activity); cost and complexity in identifying active compounds from extracts; possible low yield of an active compound in a plant species renders structure determination and other studies more complicated; seasonal variability in chemical composition; complexity in determining the mechanism of action of natural products (particularly if it is an extract); difficulty in accessing some plant species; time consuming nature of the preparation of plant material for analysis (Atanasov et al., 2015; Harvey et al., 2015; Lautie et al., 2020). However, most of these limitations can be dealt with and it is argued here that the benefits largely outweigh the drawbacks. Various methods to screen plant extracts and identify biologically active compounds have been used. A step-wise approach to identifying novel anti-oxytotic/ferroptotic neuroprotective compounds from plants is described below based on our own experience in successfully developing AD drug candidates as well as on the published literature (Atanasov et al., 2015; Azmir et al., 2013; Currais et al., 2018, 2019; Currais, Chiruta, et al., 2014; Currais, Prior, et al., 2014; Fischer et al., 2019; Maher, Fischer, et al., 2020) (Figure 3) (for more details, please see an excellent review from Atanasov et al., 2015).
FIGURE 3.
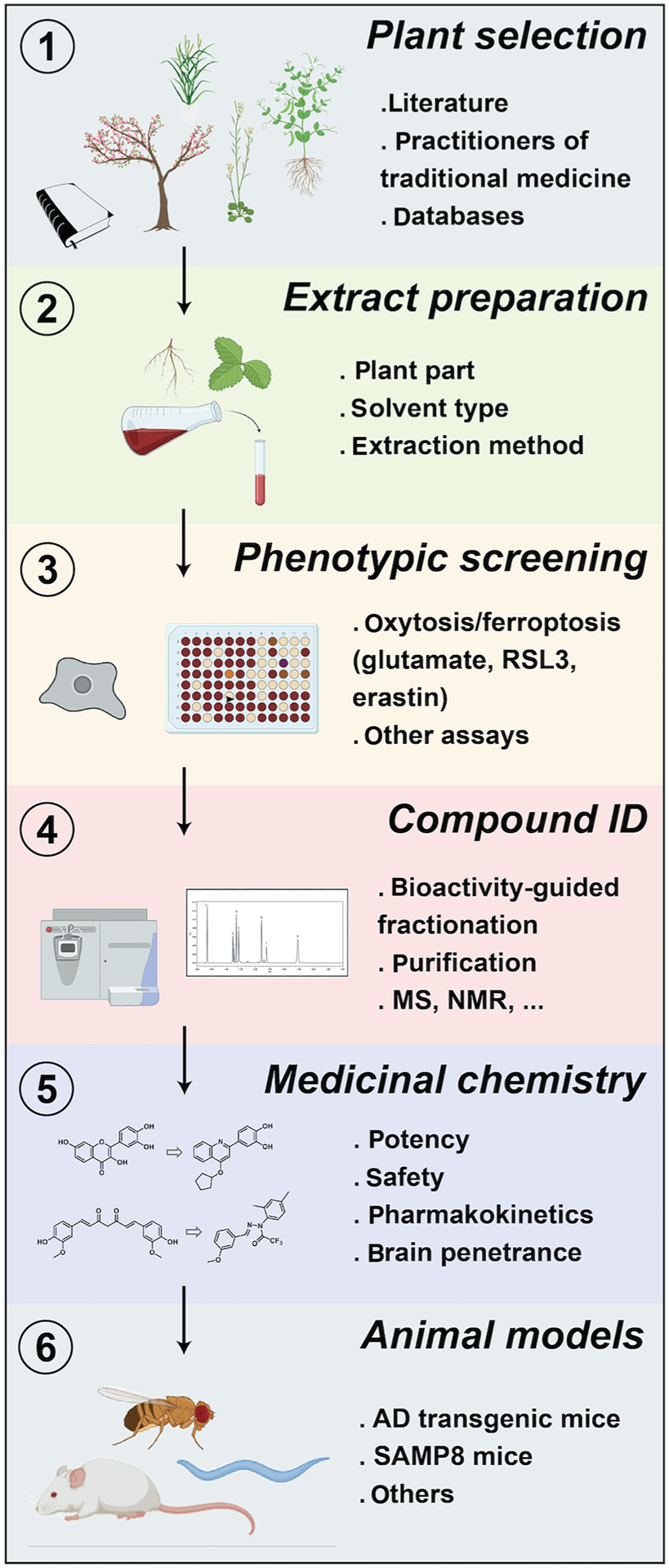
Drug discovery pipeline for identifying anti-oxytotic/ferroptotic compounds from plants
4.1 |. Step 1: Plant selection
An unbiased approach to selecting plants without a previous pharmacological record can be carried out. However, to have a reasonable number of plant candidates that can be realistically managed demands a rational approach and choosing plants with available ethnopharmacological knowledge is a good starting point (Atanasov et al., 2015). Ethnopharmacological information can be obtained from various sources such as documented literature on medicinal plants (manuals, books and articles), practitioners of traditional medicine and databases (Atanasov et al., 2015; Buenz et al., 2018).
The value of herbal medicines for neurodegenerative diseases may not be immediately recognized, as it could be argued that historically these diseases were not a major health concern given the shorter lifespan and the lack of proper diagnoses. However, traditional herbal medicines have been used for a long time in many places in the world to treat dementia-related symptoms (Perry & Howes, 2011). In addition, a number of ailments for which medicinal herbs have been identified are of relevance for neurodegenerative diseases, including memory problems, headaches, vision decay, neuropathies, different types of pain, paralysis and movement disorders. Given that old age is the major risk factor for most neurodegenerative diseases, it is also worth considering herbal medicines that are used to treat maladies associated with aging such as arthritis, frailty, heart and kidney problems, inflammation and rheumatism.
The plant material can be obtained from publicly or commercially available collections (Figure 4). Another alternative is to work in collaboration with qualified entities such as research groups or botanists that have access to the plants of interest. We have recently shown that herbarium collections are also an excellent source of plants and that extracts can be prepared from small amounts of specimen material in a non-invasive manner (Maher, Fischer, et al., 2020). For initial screening purposes, the amount required for most assays is, in fact, minimal. The choice of the plant parts to be studied will depend on the traditional medicine usage and often varies between the whole plant, leaves, stems, flowers, bark, seeds or roots.
FIGURE 4.
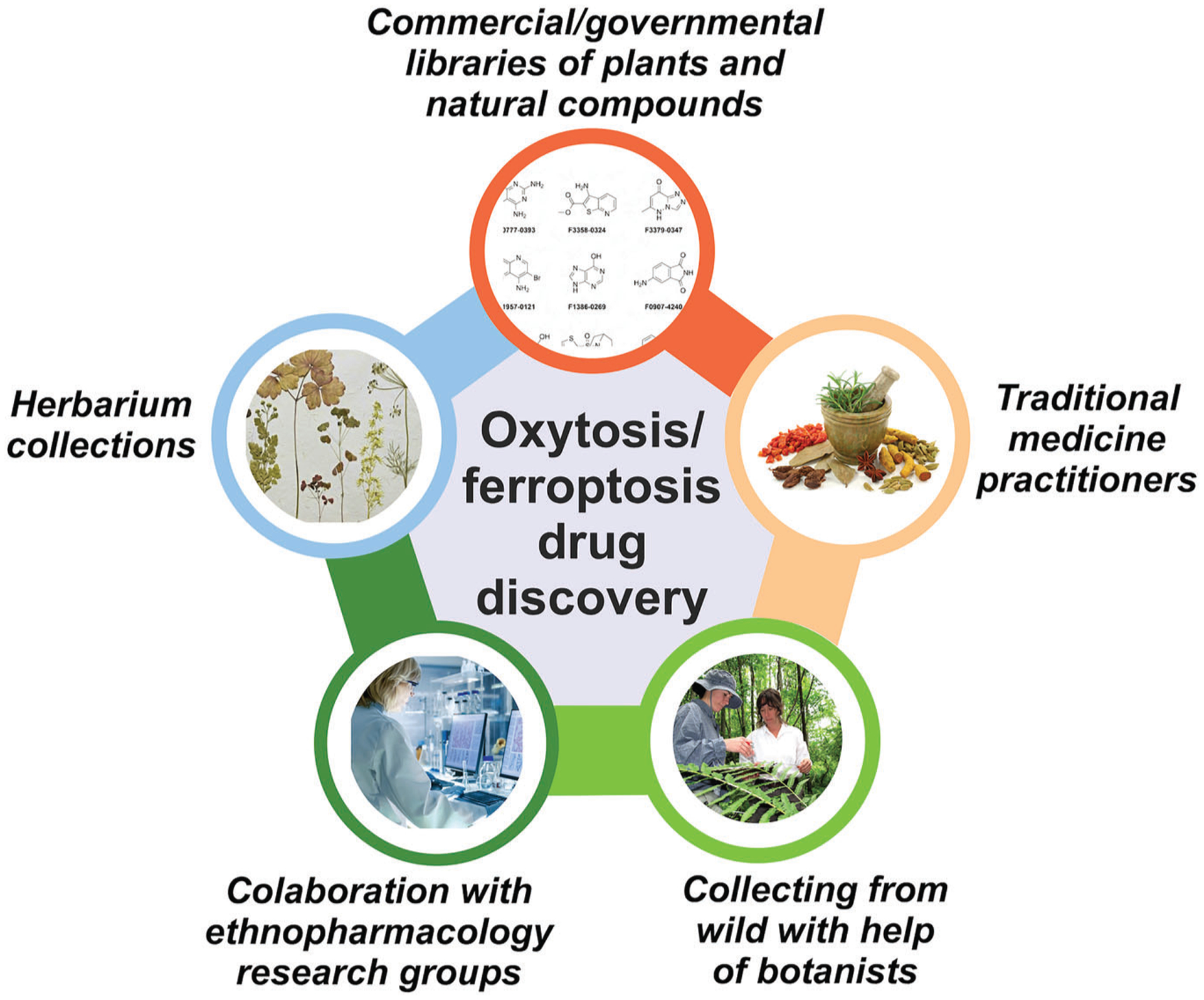
Sources of plants and natural compounds for the identification of anti-oxytotic/ferroptotic compounds. When appropriate, plant-based research should comply with the the United Nations Convention on Biological Diversity (CBD) and Nagoya protocol
4.2 |. Step 2: Extract preparation
Once the material is collected, it is dried and ground in order to facilitate the extraction, which most commonly involves a solvent (alternatives include distillation, pressing and sublimation) (Zhang et al., 2018). The extraction method chosen will determine the qualitative and quantitative chemical composition of the final extract, thereby affecting its biological activity (Azmir et al., 2013). As such, the type of compounds desired, their amount and the technology and resources available are determinant in deciding the appropriate approach. In addition, when studying plants used in traditional medicine, an extraction method that more closely resembles the traditional method of the original herbal preparation may be desired. This will allow a better comparison between any biological activity identified in the subsequent assays with the traditional knowledge of the plant.
Of the several factors that affect the extraction process, including the plant part, temperature, pressure and duration, the type of solvent (polarity) is the most crucial in determining the chemical composition of the extract (Azmir et al., 2013). Thus, solvents will be more efficient at extracting compounds of similar polarity. Common solvents used can be polar (e.g. water and ethanol), intermediate polar (e.g. acetone and dichloromethane) or non-polar (e.g. ether and chloroform) (Abubakar & Haque, 2020). Because most traditional uses of medicinal plants are associated with the preparation of polar compounds via infusions or decoctions (and sometimes tinctures), water and ethanol are good choices when studying plants used in traditional medicine. It also happens that these are the most easily accessible, safe and cheap solvents available. Ethanol has the additional advantage of making it easier to concentrate or dry the extracted compound mixture (Abubakar & Haque, 2020). A more comprehensive approach may involve the sequential use of solvents of increasing polarities (Abubakar & Haque, 2020; Lautie et al., 2020).
Conventional extraction methods include maceration, infusion, decoction, percolation, Soxhlet extraction and hydrodistillation, which have been described in detail in the literature (Abubakar & Haque, 2020; Azmir et al., 2013; Zhang et al., 2018). Other less conventional methods (e.g. supercritical fluid extraction, ultrasound assisted extraction and microwave assisted extraction) may offer additional advantages, such as higher yields and faster procedures, but require specialized equipment (Abubakar & Haque, 2020; Azmir et al., 2013; Zhang et al., 2018).
Once the extract is prepared, it should be concentrated in order to be tested in the biological assays. Dried solid extracts can be resuspended in a small volume of a biological-compatible solvent such as DMSO, polyethylene glycol, ethanol or an aqueous buffer.
4.3 |. Step 3: Screening in cell-based disease models
The screening for biological activity should be simple enough to allow the testing of a large number of extracts. It is essential that the biological assays chosen fit with a strong hypothesis, given that a large proportion of the clinical trials fail due to preclinical research carried out using inappropriate disease models (Atanasov et al., 2015). This is of particular importance for AD, where a huge investment has not generated a successful treatment so far (Götz et al., 2018; Neff, 2019; Prior et al., 2014). The assays for preclinical development can range from simple assays using purified proteins, cell-based target-oriented assays, cell-based phenotypic assays, various tests with tissues and organs, to disease animal models at later stages of the process (Atanasov et al., 2015). Our laboratories have introduced phenotypic screening in AD drug discovery that relies on cell-based models of old age-associated toxicities (Prior et al., 2014). Phenotypic screening is a forward pharmacology approach that uses cellular (or animal) disease models to identify compounds that modify the phenotype in such a way as to generate a positive outcome relative to the disease (Prior et al., 2014). It is also the classical way of developing medicines that prioritizes treating the disease to understanding mechanisms of action. Because our focus is on oxytosis/ferroptosis, we use a phenotypic screening assay that exposes cultured nerve cells to chemical inducers of oxytosis/ferroptosis (glutamate, erastin and RSL3) (Maher et al., 2020). We have shown that this assay is not only good at identifying natural inhibitors of oxytosis/ferroptosis but can also be used to screen extracts from plants with ethnopharmacological value and identify the underlying active agents (Currais, Chiruta, et al., 2014; Fischer et al., 2019; Maher, Fischer, et al., 2020). The assay also has the advantages of being simple, quick, of relatively low cost and high throughput, which significantly minimizes the unnecessary killing of animals. The most protective compounds identified can undergo further testing using additional assays that mimic other toxicities relevant to AD, such as microglial inflammation, energy deprivation, Aβ toxicity and neurotrophic support withdrawal (Prior et al., 2014). Recently, the AD field has seen a surge in new models using induced pluripotent stem cells from patients as well as simpler organisms, such as worms (Caenorhabditis elegans), flies (Drosophila melanogaster) and zebrafish (Danio rerio). Future studies should look into incorporating these biological models into the screening process.
4.4 |. Step 4: Identification of active compounds
It is possible that the protection afforded by a plant extract is due to a combination of different compounds, perhaps even acting synergistically. Although clinical testing in humans of herbal products or fractionated extracts is feasible, it presents several caveats when compared with testing pure compounds. The standardization of the chemical composition in an herbal extract, crucial to allow a reliable clinical trial, is a challenging step given the seasonal and geographical variation in plant chemicals. It also makes the task of identifying a therapeutic mechanism of action very unlikely, because the extract will contain different compounds each with a different target. In addition, having a single biologically active compound allows the optimization of its chemical and biological properties, while improving its intellectual property value. The latter is fundamental to attract the financial support required to cover the often expensive human clinical trials, such as those for AD. Finally, once a synthetic process is established to produce a compound in the laboratory, there is no longer a need to harvest plants. This is an important sustainable concern because most of the medicinal plants currently used in the world are harvested from the wild (Atanasov et al., 2015). Therefore, if given the choice, using an approach that singles out biologically active compounds is certainly preferable to extracts.
There are several strategies available to identify compounds from plant extracts (Atanasov et al., 2015). Although time-consuming, a bioactivity-guided fractionation is probably the most efficient. It involves consecutive fractionation cycles coupled with biological screening (against oxytosis-ferroptosis) and chemical profiling analysis (e.g. mass spectrometry; MS) of the protective extracts, that eventually end up with one pure active compound (Atanasov et al., 2015). The exact chemical identity of the candidate molecule(s) in each biologically active peak may directly lead to the active component if the chemical constituents of the extract have been characterized and reported in the literature. If not, the mass value and the MS/MS pattern can be used to search online databases of natural product structures (Atanasov et al., 2015; Buenz et al., 2018). Fortunately, the constantly improving online databases of phytochemical MW and mass fragmentation signatures have dramatically improved the identification of compounds in plant extracts. Compounds that are commercially available can be purchased and tested in the oxytosis/ferroptosis screen to confirm the identification. If the compounds are not commercially available, the compound must be purified from the extracts and then tested for protection against oxytosis/ferroptosis. The fractionation and compound purification are commonly achieved by a combination of chromatographic techniques, such as paper chromatography (PC), TLC, gas chromatography (GC) and HPLC (Abubakar & Haque, 2020; Atanasov et al., 2015). In all cases, confirmation of the structure should be done by NMR spectroscopy, which does not require a very large amount of compound.
As mentioned above, a potential combined effect of multiple compounds in an extract may be lost in this process. Low abundance active compounds may also be missed. However, these are acceptable caveats given that a positive hit in the screening can thus lead to the identification of a compound that is very potent. The chances of success increase with the number of plant candidates tested. We have shown that this approach produces satisfying results. Examples include, the identification of the alkaloid voacamine from Voacanga africana, when testing a small number of African medicinal plants (Currais, Chiruta, et al., 2014), and of the flavanone sterubin from Eriodictyon californicum, from a library of 400 plants with identified pharmacological uses (Fischer et al., 2019).
Other strategies for compound identification include metabolic profiling (correlation of qualitative and quantitative metabolite analysis with bioactivity), direct phytochemical isolation (direct isolation and identification of compounds), synergy-directed fractionation (similar to bioactivity-guided fractionation but with a focus on synergies) and metabolism-directed approach (identification of bioactive metabolites that may be generated as a consequence of metabolic transformation in the body or by gut microorganisms) (Atanasov et al., 2015).
Although for the initial screening only a small amount of plant material is usually used, if a natural compound turns out to be promising, larger amounts will likely be required. Sustainable harvesting should be encouraged and alternative sources of supply should be sought, which could include plant tissue culture, heterologous biosynthesis (reconstitution of the biosynthetic pathway in another organism), total chemical synthesis or semi-synthesis (from isolated precursors that are more abundant in nature) (Atanasov et al., 2015).
4.5 |. Step 5: Medicinal chemical optimization
The candidate natural inhibitor of oxytosis/ferroptosis identified may be further improved chemically and biologically via medicinal chemistry. The goal is to generate compounds with lower EC50s against oxytosis/ferroptosis (more potent), that are safe, present optimal pharmacokinetics and have good brain penetrance. As a guideline, successful CNS drugs should have MW ≤ 400, CLogP ≤ 5, tPSA ≤ 90, HBD ≤ 3 and HBA ≤ 7 for best penetration of the blood–brain barrier (BBB) (Hitchcock & Pennington, 2006; Pajouhesh & Lenz, 2005; Prior et al., 2014).
Preliminary pharmacokinetics analysis can be done by assaying blood and (perfused) brain distribution at multiple timepoints up to a few days following both gavage and intravenous administration of the compound in mice or rats. Plasma and brain concentrations are determined by LC/MS/MS. For absorption, distribution, metabolism and excretion (ADME) studies, a variety of assays can be performed including cytochrome P450 assays and the Caco-2 permeability assay, which is an established in vitro model to screen for oral absorption and to evaluate the mechanism of transport. Compound toxicity can be evaluated by the Ames test (mutagenicity), the hERG assay (cardiac safety) and the micronucleus test (chromosomal stability). The goal is to improve the drug-like properties of a compound prior to testing in animal models of AD.
In the case of herbal products based on crude or fractionated extracts, the poor solubility of its constituents often results in low bioavailability and subsequently decreased therapeutic action (Mukherjee et al., 2015). Although for pure compounds these challenges can be overcome by improving the pharmacokinetics properties through medicinal chemistry, for herbal products, the solution may rely on the use of auxiliary delivery systems, such as lipid-based carriers (Mukherjee et al., 2015).
4.6 |. Step 6: Testing in animal disease models
The selection of animal models to study age-associated dementia has always been a challenge and is often cited as one of the chief difficulties for AD-related drug development (Götz et al., 2018; Neff, 2019; Prior et al., 2014). Because the main hallmarks of AD include the aggregation of Aβ in the form of extracellular plaques and the intracellular formation of neurofibrillary tangles of the microtubule-associated protein tau (MAPT) in brain neurons, the majority of the models for AD have been developed focusing on these two features. However, patients with AD also develop other pathological features associated with old age, such as vascular and immune abnormalities, which are now thought to play a major role in the disease. The reality is that no model mimics all of the complexity that characterizes AD pathology in humans. To account for the weaknesses of each individual model, a combination of models can be used that includes both transgenic and non-transgenic models to investigate basic molecular pathological mechanisms and to test therapeutic candidates.
The most widely used models for AD rely on the expression of mutations that are found in human familial AD (FAD), including the amyloid precursor protein (APP) and presenilins (PSEN1 and PSEN2) (Götz et al., 2018). These models are good to address the amyloid hypothesis because they develop cognitive dysfunction associated with strong Aβ plaque deposition. However, FAD only accounts for less than 5% of the total population of AD patients (Götz et al., 2018; Neff, 2019) and may be distinct from the much more prevalent, old age-associated sporadic form of AD. Aging is the greatest risk factor for sporadic AD. Therefore, in addition to transgenic AD models, we and others have been working with the SAMP8 non-transgenic mouse model of accelerated aging. The SAMP8 mice have been proposed as a good model for sporadic AD (Pallàs, 2012), as they exhibit an age-related deterioration in learning and memory and develop a number of brain alterations similar to those found in AD, including increased oxidative stress, inflammation, vascular impairment, gliosis, Aβ deposition and tau hyperphosphorylation (Currais et al., 2015; Pallàs, 2012; Pallas et al., 2008; Takeda, 2009).
A candidate oxytosis/ferroptosis inhibitor is tested in these models for its effects on multiple behaviours reporting on changes in anxiety levels and memory function, as well as its ability to prevent pathological biochemical and histological changes. These data can be complemented using multiple omic technologies (transcriptomics, proteomics and metabolomics) that detect large-scale molecular changes in the brain to quantify and compare in detail the effects of a compound in the animals (can also be applied to cell-based models) (Ates et al., 2020; Currais et al., 2015, 2019, 2020; Huang et al., 2020). The sensitivity of the instrumentation underlying these omic approaches has evolved so much in the past decades that it is now becoming possible to study transcripts, proteins and metabolites in single cells. This will allow an in-depth understanding of diseases and effects of drug candidates on specific cell type populations within a tissue.
Our two most neuroprotective AD drug candidates, CMS121 and J147, are the result of medicinal chemistry optimization of the natural compounds fisetin and curcumin, respectively (Chen et al., 2011; Chiruta et al., 2012). Both CMS121 and J147 were initially identified as inhibitors of oxytosis/ferroptosis in HT22 nerve cells. CMS121 and J147 reduced cognitive decline as well as metabolic and transcriptional markers of aging in the brain when administered to transgenic AD and SAMP8 mice (Ates et al., 2020; Chen et al., 2011; Currais et al., 2015, 2019). Importantly, these effects strongly correlated with the preservation of mitochondrial homeostasis and were associated with activation of the AMPK/ACC1 pathway (Currais et al., 2019). J147, whose target is the mitochondrial ATP synthase (Goldberg et al., 2018), is now in a Phase I clinical trial for AD (NCT03838185). CMS121 recently received Investigational New Drug (IND) approval and it works as an inhibitor of fatty acid synthase (Ates et al., 2020). Further research on J147 and CMS121 has recently been reviewed in Maher et al. (2020).
The protective effects of J147 and CMS121 in transgenic AD and SAMP8 mice further indicate that oxytosis/ferroptosis plays a role in these models. In fact, increases in markers of brain lipid peroxidation, protein oxidation and changes in Ca2+ homeostasis have been reported in both models as well as in human AD (Ates et al., 2020; Butterfield et al., 2001; Goldberg et al., 2020; Poon et al., 2004; Takeda, 2009).
This combination of animal models and cell-based assays with solid in vivo pharmacology is key to identifying new therapeutics for AD. It is important to note that AD is a heterogeneous disease and it is likely that multiple drugs will be required to treat all patients, similar to what has turned out to be the case in the cancer field. In addition, most drug candidates do not make it out of clinical trials, so it is critical to have multiple candidates in the pipeline if we are to have any chance of success in treating this disease. The main goal is to identify potent inhibitors of oxytosis/ferroptosis that are safe, present good pharmacokinetics and brain penetrance and are highly protective in animal models of AD.
There are other animal models of AD that are currently being developed but have not been sufficiently studied. Some include transgenic mice that model genetic variations associated with sporadic AD, such as those found in the APOE and TREM2 genes (MODEL-AD consortium) (King, 2018; Neff, 2019). It will be interesting to test any novel inhibitors of oxytosis/ferroptosis in these new models once their pathologies have been sufficiently well characterized.
5 |. RESEARCH WITH RESPECT
When bioprospecting plants there are immediate concerns that must be evaluated. Major risks to loss of natural product materials include excessive harvesting (two thirds of medicinal plants are collected from the wild) and environmental decay caused by humans (Atanasov et al., 2015; Buenz et al., 2018; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 2019). Therefore, a sensible scientific approach takes advantage of natural diversity in a sustainable and respectful manner. In fact, if done properly, the opportunities offered by plants and the related ethnopharmacology are immense to both humans and nature, as the potential to generate new medicines only adds value and urgency to the necessity of preserving existing natural resources. Any future, viable research should thus comply with the legal framework that recognizes the importance of biodiversity for the health of the people and planet while ensuring its long-term use for the benefit of all (Diversity, S.o.t.C.o.B, 2000; Diversity, S. o.t.C.o.B, 2011; Duke & Parsons, 2018; Nations, U, 1992).
The World Health Organization (WHO) has developed guidelines to facilitate the integration of traditional medicine into national health systems and the promotion of scientific research by providing international standards, technical guidelines and methodologies for products, practices and practitioners (WHO Traditional Medicine Strategy 2014–2023). However, only the United Nations Convention on Biological Diversity (Nations, U, 1992), signed in 1992, first introduced legal considerations on the accessibility and use of biodiversity (defined as ‘genetic resources’) as well as on the sharing of benefits and patentability issues within and across national borders. The United Nations Convention on Biological Diversity was implemented with three main objectives: (1) the conservation of biodiversity; (2) sustainable use of the components of biodiversity; and (3) sharing the benefits arising from the commercial and other utilization of genetic resources in a fair and equitable way (Diversity, S.o.t.C.o. B, 2000).
The Addis Ababa Principles and Guidelines for the Sustainable use of Biodiversity (2004) provide a practical framework for assisting governments, indigenous and local communities, resource managers, the private sector and other stakeholders, on how to ensure that their uses of biological diversity will not lead to its long-term decline (Diversity, S.o.t.C.o.B, 2004).
In 2010, the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the United Nations Convention on Biological Diversity was adopted to further advance the implementation of the United Nations Convention on Biological Diversity’s third objective, by providing a strong basis for greater legal certainty and transparency for both providers and users of genetic resources (Diversity, S.o.t.C.o.B, 2011). In particular, the Nagoya Protocol looks to strengthen the ability of indigenous and local communities to benefit monetarily or non-monetarily from the use of their traditional knowledge, innovations and practices associated with genetic resources (Diversity, S.o.t.C.o.B, 2011). Given that most of the world’s biodiversity is found in developing countries, such biodiversity and associated knowledge and practices must be respected, preserved and maintained (Diversity, S.o.t.C.o.B, 2000). Some examples of monetary benefits include access fees for samples, payment of royalties, funding for research and sharing of intellectual property rights. Non-monetary benefits can consist of sharing of data/publications, research collaborations, educational and training programmes, capacity-building initiatives, access to scientific information and research directed towards priority needs (Diversity, S.o. t.C.o.B, 2011).
The signatories of the the United Nations Convention on Biological Diversity and Nagoya Protocol are legally bound to the obligations defined in the texts. In practice, these policies can be executed by obtaining Prior Informed Consent (PIC) of the country (or partner entity) in which the genetic resource is located before accessing the resource and negotiating and agreeing on the terms and conditions of access and use of this resource through the establishment of Mutually Agreed Terms (MAT) (Diversity, S.o.t.C.o.B, 2000; Diversity, S.o.t.C.o.B, 2011; Duke & Parsons, 2018). An agreement should include the sharing of benefits arising from the use of the resource with the provider as a prerequisite for access to the genetic resource and its use. Conversely, countries (or partner entities), when acting as providers of genetic resources, should deliver fair and non-arbitrary rules and procedures for access to their genetic resources. A very useful set of practical recommendations on carrying out research under the Nagoya Protocol has been put forward by Duke and Parsons (Duke & Parsons, 2018).
More recently, the United Nations, Education, Scientific and Cultural Organization (UNESCO) has described a vision for Open Science that includes a series of recommendations on how to make scientific research and data accessible to all based on transparency, sharing and collaboration (UNESCO, 2020). The Open Science movement encourages science to accelerate the achievement of sustainable, scientific solutions for global challenges, by promoting equal opportunities for all, bridging the science, technology and innovation gaps between and within countries and fulfilling the human right to science.
International legislation should embolden, rather than prevent responsible research that can contribute to the United Nation’s (UN) Sustainable Development Goals (SDGs) (Antonelli et al., 2019). If carried out responsibly, plant-based drug discovery can contribute to the conservation of biodiversity, sustainable use of its components and their development as well as human well-being (Diversity, S.o.t.C.o.B, 2011).
6 |. CONCLUSIONS
There are no disease-modifying therapies for AD, in part due to the need for new drug targets identified from an understanding of the upstream events driving the disease. The amyloid hypothesis has provided only a few drug targets for AD treatment and has not resulted in a disease-modifying drug. Even though it is still possible that this hypothesis will lead to a successful drug, the identification of new therapeutic targets is one of the most important challenges in the field. Oxytosis/ferroptosis is a unique degenerative mechanism that is strongly associated with aging and is likely to be a primary contributor to AD pathology.
Plants display a great diversity of biochemicals that have evolved to deal with physiological stresses, some of which are relevant to human medicine. As in oxytosis/ferroptosis, high levels of ROS and lipid peroxides are generated as by-products of plant metabolism. Therefore, plants represent an excellent source of anti-oxytotic/ferroptotic compounds. The step-wise drug discovery approach described here was designed to accelerate the advancement of novel potent inhibitors of oxytosis/ferroptosis to target early pathology in AD. Although the discussion was focused on plants, it is worth mentioning the value of other organisms to provide ethnopharmacological leads, namely fungi (Antonelli et al., 2019; Buenz et al., 2018).
The main innovations of this approach include (1) the use of small amounts of plant material for screening in cell-based models of oxytosis/ferroptosis, allowing the use of new and rich sources of material (such as herbarium collections), (2) the combined use of animal models of accelerated aging and AD, leading to an understanding of the effects of the compounds not only on classical AD pathology but importantly on the upstream events in AD that are associated with aging, (3) the use of a comprehensive battery of technical tools to assess the mechanistic effects of drug candidates on the animal models, including not just cognitive tests coupled with standard biochemical and immunohistochemical analyses, but also multiple, large-data omic technologies (transcriptomics, proteomics and metabolomics), (4) the compilation of an extensive library of compounds that are potent inhibitors of oxytosis/ferroptosis and that can be further chemically optimized and screened for other therapeutic properties in the future and (5) the identification of compounds that are potent inhibitors of oxytosis/ferroptosis, likely safe, present good pharmacological properties and are effective at reducing the cognitive and physiological deficits seen in animal models of the disease.
Drug candidates developed in this way will be in an excellent position to initiate the IND studies required for clinical trials. Importantly, different organs and tissues in the body share molecular commonalities that are associated with the aging process and it is becoming clearer that oxytosis/ferroptosis can play a role in other human diseases (Han et al., 2020). As such, the value of oxytosis/ferroptosis may go well beyond AD and the research discussed here could have important implications to drug discovery programmes for other diseases of old age.
In summary, because it was first described over 30 years ago, the oxytosis/ferroptosis cell death pathway is gaining tremendous momentum as its relevance to human disease is being brought to light. The drug discovery process described herein has been optimized to efficiently take advantage of the natural chemical richness existing in plants and identify novel, potent inhibitors of oxytosis/ferroptosis. Therefore, the loss of biodiversity due to an unsustainable use of the planet’s natural resources also signifies a loss of medical opportunities for mankind. It is argued that a sensible path to plant-based drug discovery can not only hasten the development of new medicines to treat human diseases but also simultaneously be a catalyst for sustainable progress.
6.1 |. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Fabbro, et al., 2019; Alexander, Kelly, et al., 2019).
ACKNOWLEDGEMENTS
We would like to thank Dr Alexandra Marques for help with comments and suggestions. This work was supported by the Shiley-Marcos Alzheimer’s Disease Research Center at University of California San Diego (A.C.), an Innovation Award from the Salk Institute (A.C.), the Shiley Foundation (D.S.C.), the Paul F. Glenn Center for Biology of Aging Research at the Salk Institute (Z.L.) and the National Institutes of Health grant R01AG069206 (P.M.).
Funding information
National Institutes of Health, Grant/Award Number: R01AG069206; Salk Institute; Shiley-Marcos Alzheimer’s Disease Research Center at University of California San Diego; Paul F. Glenn Center for Biology of Aging Research; Shiley Foundation; Innovation Award from the Salk Institute
Abbreviations:
- AD
Alzheimer’s disease
- PSMs
plant secondary metabolites
- LOXs
lipoxygenases
Footnotes
CONFLICT OF INTERESTS
The Salk Institute holds the patents for J147 and CMS121. The authors declare that there are no other conflicts of interest.
REFERENCES
- Abubakar AR, & Haque M (2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy & Bioallied Sciences, 12(1), 1–10. 10.4103/jpbs.JPBS_175_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alche JD (2019). A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biology, 23, 101136. 10.1016/j.redox.2019.101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–S396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). The concise guide to pharmacology 2019/20: Transporters. British Journal of Pharmacology, 176(Suppl 1), S397–S493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allkin B (2017). Useful plants - medicines: At least 28,187 plant species are currently recorded as being of medicinal use. In Willis KJ (Ed.), State of the World’s Plants 2017. London (UK). [PubMed] [Google Scholar]
- Anjum NA, Hasanuzzaman M, Hossain MA, Thangavel P, Roychoudhury A, Gill SS, Rodrigo MA, Adam V, Fujita M, Kizek R, & Duarte AC (2015). Jacks of metal/metalloid chelation trade in plants-an overview. Frontiers in Plant Science, 6, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, & Scheff SW (2010). Oxidative stress in the progression of Alzheimer disease in the frontal cortex. Journal of Neuropathology and Experimental Neurology, 69(2), 155–167. 10.1097/NEN.0b013e3181cb5af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli A, Smith RJ, & Simmonds MSJ (2019). Unlocking the properties of plants and fungi for sustainable development. Nat Plants, 5(11), 1100–1102. 10.1038/s41477-019-0554-1 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH, Rollinger JM, Schuster D, Breuss JM, Bochkov V, Mihovilovic MD, Kopp B, Bauer R, Dirsch VM, & Stuppner H (2015). Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances, 33(8), 1582–1614. 10.1016/j.biotechadv.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ates G, Goldberg J, Currais A, & Maher P (2020). CMS121, a fatty acid synthase inhibitor, protects against excess lipid peroxidation and inflammation and alleviates cognitive loss in a transgenic mouse model of Alzheimer’s disease. Redox Biology, 36, 101648. 10.1016/j.redox.2020.101648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, & Omar AKM (2013). Techniques for extraction of bioactive compounds from plant materials: A review. Journal of Food Engineering, 117(4), 426–436. 10.1016/j.jfoodeng.2013.01.014 [DOI] [Google Scholar]
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, & Hammond CL (2009). Glutathione dysregulation and the etiology and progression of human diseases. Biological Chemistry, 390(3), 191–214. 10.1515/BC.2009.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo P, Martín-Aragón S, Benedí J, Susín C, Felici E, Gil P, Manuel Ribera J, & Villar ÁM (2008). Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. Free Radical Research, 42(2), 162–170. 10.1080/10715760701861373 [DOI] [PubMed] [Google Scholar]
- Bradley MA, Markesbery WR, & Lovell MA (2010). Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radical Biology & Medicine, 48(12), 1570–1576. 10.1016/j.freeradbiomed.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley-Whitman MA, & Lovell MA (2015). Biomarkers of lipid peroxidation in Alzheimer disease (AD): An update. Archives of Toxicology, 89(7), 1035–1044. 10.1007/s00204-015-1517-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenz EJ, Verpoorte R, & Bauer BA (2018). The ethnopharmacologic contribution to bioprospecting natural products. Annual Review of Pharmacology and Toxicology, 58, 509–530. 10.1146/annurev-pharmtox-010617-052703 [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, & Castegna A (2001). Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid beta-peptide. Trends in Molecular Medicine, 7(12), 548–554. 10.1016/S1471-4914(01)02173-6 [DOI] [PubMed] [Google Scholar]
- Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, & Schubert D (2011). A novel neurotrophic drug for cognitive enhancement and Alzheimer’s disease. PLoS ONE, 6(12), e27865. 10.1371/journal.pone.0027865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yu C, Kang R, & Tang D (2020). Iron metabolism in ferroptosis. Frontiers in Cell and Development Biology, 8, 590226. 10.3389/fcell.2020.590226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiruta C, Schubert D, Dargusch R, & Maher P (2012). Chemical modification of the multitarget neuroprotective compound fisetin. Journal of Medicinal Chemistry, 55(1), 378–389. 10.1021/jm2012563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choffnes D (2016). Nature’s pharmacopeia: A world of medicinal plants. New York: Columbia University Press. xiii, 332 pages. 10.7312/chof16660 [DOI] [Google Scholar]
- Conrad M, Kagan VE, Bayir H, Pagnussat GC, Head B, Traber MG, & Stockwell BR (2018). Regulation of lipid peroxidation and ferroptosis in diverse species. Genes & Development, 32(9–10), 602–619. 10.1101/gad.314674.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini LC, Barr LJ, Vogel JL, & Henderson ST (2008). Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neuroscience, 9(Suppl 2), S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M’, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, & Rapoport SI (2011). Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition, 27(1), 3–20. 10.1016/j.nut.2010.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A (2015). Ageing and inflammation—A central role for mitochondria in brain health and disease. Ageing Research Reviews, 21, 30–42. 10.1016/j.arr.2015.02.001 [DOI] [PubMed] [Google Scholar]
- Currais A, Chiruta C, Goujon-Svrzic M, Costa G, Santos T, Batista MT, Paiva J, Céu Madureira M, & Maher P (2014). Screening and identification of neuroprotective compounds relevant to Alzheimers disease from medicinal plants of Sao Tome and Principe. Journal of Ethnopharmacology, 155(1), 830–840. 10.1016/j.jep.2014.06.046 [DOI] [PubMed] [Google Scholar]
- Currais A, Farrokhi C, Dargusch R, Armando A, Quehenberger O, Schubert D, & Maher P (2018). Fisetin reduces the impact of aging on behavior and physiology in the rapidly aging SAMP8 mouse. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 73(3), 299–307. 10.1093/gerona/glx104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Goldberg J, Farrokhi C, Chang M, Prior M, Dargusch R, Daugherty D, Armando A, Quehenberger O, Maher P, & Schubert D (2015). A comprehensive multiomics approach toward understanding the relationship between aging and dementia. Aging (Albany NY), 7(11), 937–955. 10.18632/aging.100838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Huang L, Goldberg J, Petrascheck M, Ates G, Pinto-Duarte A, Shokhirev MN, Schubert D, & Maher P (2019). Elevating acetyl-CoA levels reduces aspects of brain aging. eLife, 8. 10.7554/eLife.47866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, Huang L, Petrascheck M, Maher P, & Schubert D (2020). A chemical biology approach to identifying molecular pathways associated with aging. GeroScience, 43, 353–365. 10.1007/s11357-020-00238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currais A, & Maher P (2013). Functional consequences of age-dependent changes in glutathione status in the brain. Antioxidants & Redox Signaling, 19(8), 813–822. 10.1089/ars.2012.4996 [DOI] [PubMed] [Google Scholar]
- Currais A, Prior M, Dargusch R, Armando A, Ehren J, Schubert D, Quehenberger O, & Maher P (2014). Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell, 13(2), 379–390. 10.1111/acel.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano AM, Martin MV, Cordoba JP, Bellido AM, D’Ippolito S, Colman SL, Soto D, Rold an JA, Bartoli CG, Zabaleta EJ, & Fiol DF (2017). Heat stress induces ferroptosis-like cell death in plants. The Journal of Cell Biology, 216(2), 463–476. 10.1083/jcb.201605110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diversity, S.o.t.C.o.B. (2000). Sustainable life on Earth: How the convention on biological diversity promotes nature and human well-being.
- Diversity, S.o.t.C.o.B. (2004). Addis Ababa principles and guidelines for the sustainable use of biodiversity. Secretariat of the Convention on Biological Diversity. [Google Scholar]
- Diversity, S.o.t.C.o.B. (2011). Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B III, & Stockwell BR (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell, 149(5), 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W, Kinscherf R, Hildebrandt W, & Schmitt T (2006). The deficit in low molecular weight thiols as a target for antiageing therapy. Current Drug Targets, 7(11), 1505–1512. 10.2174/1389450110607011505 [DOI] [PubMed] [Google Scholar]
- Duke CS, & Parsons JP (2018). Do you conduct international research? What you need to know about access, benefit-sharing, and the Nagoya protocol. The Bulletin of the Ecological Society of America, 99(2), 257–258. 10.1002/bes2.1399 [DOI] [Google Scholar]
- El-Beltagi HS, & Mohamed HI (2013). Reactive oxygen species, lipid peroxidation and antioxidative defense mechanism. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 41(1), 44–57. 10.15835/nbha4118929 [DOI] [Google Scholar]
- Emir UE, Raatz S, McPherson S, Hodges JS, Torkelson C, Tawfik P, White T, & Terpstra M (2011). Noninvasive quantification of ascorbate and glutathione concentration in the elderly human brain. NMR in Biomedicine, 24(7), 888–894. 10.1002/nbm.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, & Wang F (2019). Ferroptosis as a target for protection against cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America, 116(7), 2672–2680. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, & Mueller MJ (2013). ROS-mediated lipid peroxidation and RES-activated signaling. Annual Review of Plant Biology, 64, 429–450. 10.1146/annurev-arplant-050312-120132 [DOI] [PubMed] [Google Scholar]
- Fischer W, Currais A, Liang Z, Pinto A, & Maher P (2019). Old age-associated phenotypic screening for Alzheimer’s disease drug candidates identifies sterubin as a potent neuroprotective compound from yerba santa. Redox Biology, 21, 101089. 10.1016/j.redox.2018.101089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, … Conrad M (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nature Cell Biology, 16(12), 1180–1191. 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Monian P, Quadri N, Ramasamy R, & Jiang X (2015). Glutaminolysis and transferrin regulate ferroptosis. Molecular Cell, 59 (2), 298–308. 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschler MM, & Stockwell BR (2017). Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications, 482(3), 419–425. 10.1016/j.bbrc.2016.10.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Lorenzini S, Milzani A, & Rossi R (2006). Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. The Journals of Gerontology. Series a, Biological Sciences and Medical Sciences, 61(10), 1030–1038. 10.1093/gerona/61.10.1030 [DOI] [PubMed] [Google Scholar]
- Goldberg J, Currais A, Ates G, Huang L, Shokhirev M, Maher P, & Schubert D (2020). Targeting of intracellular Ca2+ stores as a therapeutic strategy against age-related neurotoxicities. NPJ Aging and Mechanisms of Disease, 6, 10. 10.1038/s41514-020-00048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Molto PB, & Cuezva JM (2018). The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell, 17(2), e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz J, Bodea L-G, & Goedert M (2018). Rodent models for Alzheimer disease. Nature Reviews Neuroscience, 19(10), 583–598. 10.1038/s41583-018-0054-8 [DOI] [PubMed] [Google Scholar]
- Gurib-Fakim A (2006). Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molecular Aspects of Medicine, 27(1), 1–93. 10.1016/j.mam.2005.07.008 [DOI] [PubMed] [Google Scholar]
- Hagemeier J, Geurts JJ, & Zivadinov R (2012). Brain iron accumulation in aging and neurodegenerative disorders. Expert Review of Neuro-therapeutics, 12(12), 1467–1480. 10.1586/ern.12.128 [DOI] [PubMed] [Google Scholar]
- Han C, Liu Y, Dai R, Ismail N, Su W, & Li B (2020). Ferroptosis and its potential role in human diseases. Frontiers in Pharmacology, 11, 239. 10.3389/fphar.2020.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare D, Ayton S, Bush AI, & Lei P (2013). A delicate balance: Iron metabolism and diseases of the brain. Frontiers in Aging Neuroscience, 5, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AL, Edrada-Ebel R, & Quinn RJ (2015). The re-emergence of natural products for drug discovery in the genomics era. Nature Reviews. Drug Discovery, 14(2), 111–129. 10.1038/nrd4510 [DOI] [PubMed] [Google Scholar]
- Hitchcock SA, & Pennington LD (2006). Structure-brain exposure relationships. Journal of Medicinal Chemistry, 49(26), 7559–7583. 10.1021/jm060642i [DOI] [PubMed] [Google Scholar]
- Huang L, McClatchy DB, Maher P, Liang Z, Diedrich JK, Soriano-Castell D, Goldberg J, Shokhirev M, Yates JR III, Schubert D, & Currais A (2020). Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death. Cell Death & Disease, 11(10), 828. 10.1038/s41419-020-03020-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. (2019). In Brondizio ES, Settele J, Díaz S, & Ngo HT (Eds.), Global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services. Bonn, Germany. [Google Scholar]
- Joshi YB, Giannopoulos PF, & Pratico D (2015). The 12/15--lipoxygenase as an emerging therapeutic target for Alzheimer’s disease. Trends in Pharmacological Sciences, 36(3), 181–186. 10.1016/j.tips.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi YB, & Pratico D (2014). The 5-lipoxygenase pathway: Oxidative and inflammatory contributions to the Alzheimer’s disease phenotype. Frontiers in Cellular Neuroscience, 8, 436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, & Kalske A (2018). Plant secondary metabolite diversity and species interactions. Annual Review of Ecology, Evolution, and Systematics, 49, 115–138. 10.1146/annurev-ecolsys-110617-062406 [DOI] [Google Scholar]
- King A (2018). The search for better animal models of Alzheimer’s disease. Nature, 559(7715), S13–S15. 10.1038/d41586-018-05722-9 [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Pan L, Fletcher JN, & Chai H (2011). The relevance of higher plants in lead compound discovery programs. Journal of Natural Products, 74(6), 1539–1555. 10.1021/np200391c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston DG (2011). Modern natural products drug discovery and its relevance to biodiversity conservation. Journal of Natural Products, 74(3), 496–511. 10.1021/np100550t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen C, Gallage NJ, Hansen CC, Møller BL, & Laursen T (2018). Dynamic metabolic solutions to the sessile life style of plants. Natural Product Reports, 35(11), 1140–1155. 10.1039/C8NP00037A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, Banthiya S, & van Leyen K (2015). Mammalian lipoxygenases and their biological relevance. Biochimica et Biophysica Acta, 1851(4), 308–330. 10.1016/j.bbalip.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautie E, Russo O, Ducrot P, & Boutin JA (2020). Unraveling plant natural chemical diversity for drug discovery purposes. Frontiers in Pharmacology, 11, 397. 10.3389/fphar.2020.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Ates G, Methner A, Conrad M, & Maher P (2018). Oxytosis/ferroptosis-(re-) emerging roles for oxidative stress-dependent non-apoptotic cell death in diseases of the central nervous system. Frontiers in Neuroscience, 12, 214. 10.3389/fnins.2018.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, & Wang G (2020). Ferroptosis: Past, present and future. Cell Death & Disease, 11 (2), 88. 10.1038/s41419-020-2298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Currais A, Soriano-Castell D, Schubert D, & Maher P (2020). Natural products targeting mitochondria: Emerging therapeutics for age-associated neurological disorders. Pharmacology & Therapeutics, 107749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, & Beal MF (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443(7113), 787–795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, & Kroemer G (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P (2005). The effects of stress and aging on glutathione metabolism. Ageing Research Reviews, 4(2), 288–314. 10.1016/j.arr.2005.02.005 [DOI] [PubMed] [Google Scholar]
- Maher P (2018). Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radical Biology & Medicine, 115, 92–104. 10.1016/j.freeradbiomed.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Maher P, Currais A, & Schubert D (2020). Using the Oxytosis/Ferroptosis pathway to understand and treat age-associated neurodegenerative diseases. Cell Chemical Biology, 27(12), 1456–1471. 10.1016/j.chembiol.2020.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher P, Fischer W, Liang Z, Soriano-Castell D, Pinto AFM, Rebman J, & Currais A (2020). The value of herbarium collections to the discovery of novel treatments for Alzheimer’s disease, a case made with the genus eriodictyon. Frontiers in Pharmacology, 11, 208. 10.3389/fphar.2020.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal PK, Saharan S, Tripathi M, & Murari G (2015). Brain glutathione levels—A novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biological Psychiatry, 78(10), 702–710. 10.1016/j.biopsych.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, Ruiz Ortega M, Egido J, Linkermann A, Ortiz A, & Sanz AB (2017). Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. Journal of the American Society of Nephrology, 28(1), 218–229. 10.1681/ASN.2015121376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Harwansh RK, & Bhattacharyya S (2015). Chapter 10—Bioavailability of herbal products: Approach toward improved pharmacokinetics. In Mukherjee PK (Ed.), Evidence-based validation of herbal medicine (pp. 217–245). Boston: Elsevier. [Google Scholar]
- Nations, U. (1992). Convention on biological diversity.
- NaveenKumar SK, SharathBabu BN, Hemshekhar M, Kemparaju K, Girish KS, & Mugesh G (2018). The role of reactive oxygen species and ferroptosis in heme-mediated activation of human platelets. ACS Chemical Biology, 13(8), 1996–2002. 10.1021/acschembio.8b00458 [DOI] [PubMed] [Google Scholar]
- Neff EP (2019). Animal models of Alzheimer’s disease embrace diversity. Lab Animal (NY), 48(9), 255–259. 10.1038/s41684-019-0377-8 [DOI] [PubMed] [Google Scholar]
- Newman DJ, & Cragg GM (2016). Natural products as sources of new drugs from 1981 to 2014. Journal of Natural Products, 79(3), 629–661. 10.1021/acs.jnatprod.5b01055 [DOI] [PubMed] [Google Scholar]
- Noctor G, Reichheld JP, & Foyer CH (2018). ROS-related redox regulation and signaling in plants. Seminars in Cell & Developmental Biology, 80, 3–12. 10.1016/j.semcdb.2017.07.013 [DOI] [PubMed] [Google Scholar]
- Onyango IG, Dennis J, & Khan SM (2016). Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging and Disease, 7(2), 201–214. 10.14336/AD.2015.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajouhesh H, & Lenz GR (2005). Medicinal chemical properties of successful central nervous system drugs. NeuroRx, 2(4), 541–553. 10.1602/neurorx.2.4.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas M, Camins A, Smith MA, Perry G, Lee HG, & Casadesus G (2008). From aging to Alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). Journal of Alzheimer’s Disease, 15(4), 615–624. 10.3233/JAD-2008-15408 [DOI] [PubMed] [Google Scholar]
- Pallàs M (2012). Senescence-accelerated mice P8: A tool to study brain aging and Alzheimer’s disease in a mouse model. ISRN Cell Biology, 2012, 917167. [Google Scholar]
- Perry E, & Howes MJ (2011). Medicinal plants and dementia therapy: Herbal hopes for brain aging? CNS Neuroscience & Therapeutics, 17(6), 683–698. 10.1111/j.1755-5949.2010.00202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovska BB (2012). Historical review of medicinal plants’ usage. Pharmacognosy Reviews, 6(11), 1–5. 10.4103/0973-7847.95849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, Calabrese V, & Butterfield DA (2004). Antisense directed at the Aβ region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Research, 1018(1), 86–96. 10.1016/j.brainres.2004.05.048 [DOI] [PubMed] [Google Scholar]
- Prior M, Chiruta C, Currais A, Goldberg J, Ramsey J, Dargusch R, Maher PA, & Schubert D (2014). Back to the future with phenotypic screening. ACS Chemical Neuroscience, 5(7), 503–513. 10.1021/cn500051h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior M, Dargusch R, Ehren JL, Chiruta C, & Schubert D (2013). The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer’s disease mice. Alzheimer’s Research & Therapy, 5(3), 25. 10.1186/alzrt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD, & Williams SR (2017). Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Analytical Biochemistry, 529, 127–143. 10.1016/j.ab.2016.12.022 [DOI] [PubMed] [Google Scholar]
- Reed TT (2011). Lipid peroxidation and neurodegenerative disease. Free Radical Biology & Medicine, 51(7), 1302–1319. 10.1016/j.freeradbiomed.2011.06.027 [DOI] [PubMed] [Google Scholar]
- Ren JX, Sun X, Yan XL, Guo ZN, & Yang Y (2020). Ferroptosis in neurological diseases. Frontiers in Cellular Neuroscience, 14, 218. 10.3389/fncel.2020.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Currais A, Goldberg J, Finley K, Petrascheck M, & Maher P (2018). Geroneuroprotectors: Effective geroprotectors for the brain. Trends in Pharmacological Sciences, 39(12), 1004–1007. 10.1016/j.tips.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman MC, van Dam NM, Beran F, & Harpole WS (2016). How does plant chemical diversity contribute to biodiversity at higher trophic levels? Current Opinion in Insect Science, 14, 46–55. 10.1016/j.cois.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Sekhar RV, Patel SG, Guthikonda AP, Reid M, Balasubramanyam A, Taffet GE, & Jahoor F (2011). Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. The American Journal of Clinical Nutrition, 94(3), 847–853. 10.3945/ajcn.110.003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, & Rao GN (2019). Emerging role of 12/15-lipoxygenase (ALOX15) in human pathologies. Progress in Lipid Research, 73, 28–45. 10.1016/j.plipres.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorzynska-Polit E (2007). Lipid peroxidation in plant cells, its physiological role and changes under heavy metal stress. Acta Societatis Botanicorum Poloniae, 76(1), 49–54. [Google Scholar]
- Sultana R, Perluigi M, & Butterfield DA (2013). Lipid peroxidation triggers neurodegeneration: A redox proteomics view into the Alzheimer disease brain. Free Radical Biology & Medicine, 62, 157–169. 10.1016/j.freeradbiomed.2012.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, & Khan SM (2004). A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Medical Hypotheses, 63(1), 8–20. 10.1016/j.mehy.2003.12.045 [DOI] [PubMed] [Google Scholar]
- Takeda T (2009). Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochemical Research, 34(4), 639–659. 10.1007/s11064-009-9922-y [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, & Schubert D (1998). The regulation of reactive oxygen species production during programmed cell death. The Journal of Cell Biology, 141(6), 1423–1432. 10.1083/jcb.141.6.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Schubert D, & Maher P (2001). Oxytosis: A novel form of programmed cell death. Current Topics in Medicinal Chemistry, 1(6), 497–506. 10.2174/1568026013394741 [DOI] [PubMed] [Google Scholar]
- UNESCO. (2020). First draft of the UNESCO Recommendation on Open Science.
- Van Wyk BE, & Wink M (2019). Medicinal plants of the world: An illustrated scientific guide to important medicinal plants and their uses (2nd ed.). CABI. [Google Scholar]
- Viswanath KK, Varakumar P, Pamuru RR, Basha SJ, Mehta S, & Rao AD (2020). Plant lipoxygenases and their role in plant physiology. Journal of Plant Biology, 63, 83–95. 10.1007/s12374-020-09241-x [DOI] [Google Scholar]
- Wang L, Zhang Z, Li M, Wang F, Jia Y, Zhang F, Shao J, Chen A, & Zheng S (2019). P53-dependent induction of ferroptosis is required for artemether to alleviate carbon tetrachloride-induced liver fibrosis and hepatic stellate cell activation. IUBMB Life, 71(1), 45–56. 10.1002/iub.1895 [DOI] [PubMed] [Google Scholar]
- Wang S, Alseekh S, Fernie AR, & Luo J (2019). The structure and function of major plant metabolite modifications. Molecular Plant, 12 (7), 899–919. 10.1016/j.molp.2019.06.001 [DOI] [PubMed] [Google Scholar]
- Xie X, He Z, Chen N, Tang Z, Wang Q, & Cai Y (2019). The roles of environmental factors in regulation of oxidative stress in plant. BioMed Research International, 2019, 9732325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, & Stockwell BR (2016). Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proceedings of the National Academy of Sciences of the United States of America, 113(34), E4966–E4975. 10.1073/pnas.1603244113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Boveris A, & Cadenas E (2014). Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxidants & Redox Signaling, 20(2), 353–371. 10.1089/ars.2012.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Sancheti H, Liu Z, & Cadenas E (2016). Mitochondrial function in ageing: Coordination with signalling and transcriptional pathways. The Journal of Physiology, 594(8), 2025–2042. 10.1113/JP270541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q-W, Lin L-G, & Ye W-C (2018). Techniques for extraction and isolation of natural products: A comprehensive review. Chinese Medicine, 13(1), 20. 10.1186/s13020-018-0177-x [DOI] [PMC free article] [PubMed] [Google Scholar]


