Abstract
Objectives
To describe characteristics and compare clinical outcomes including falls, fractures, infections, and neuropsychiatric symptoms (NPS) among long‐term care residents with dementia with and without agitation.
Methods
A cross‐sectional secondary analysis of administrative healthcare data was conducted whereby residents with dementia residing in a long‐term care facility for ≥12 months were identified from the AnalytiCare LLC database (10/2010–06/2014) and were classified into mutually exclusive cohorts (Agitation Cohort or No‐Agitation Cohort) based on available agitation‐related symptoms. Entropy balancing was used to balance demographic and clinical characteristics between the two cohorts. The impact of agitation on clinical outcomes was compared between balanced cohorts using weighted logistic regression models.
Results
The study included 6,265 long‐term care residents with dementia among whom, 3,313 were included in the Agitation Cohort and 2,952 in the No‐Agitation Cohort. Prior to balancing, residents in the Agitation Cohort had greater dementia‐related cognitive impairment and clinical manifestations compared to the No‐Agitation Cohort. After balancing, residents with and without agitation, respectively, received a median of five and four distinct types of medications (including antipsychotics). Further, compared to residents without agitation, those with agitation were significantly more likely to have a recorded fall (OR = 1.58), fracture (OR = 1.29), infection (OR = 1.18), and other NPS (OR = 2.11).
Conclusions
Agitation in long‐term care residents with dementia was associated with numerically higher medication use and an increased likelihood of experiencing falls, fractures, infections, and additional NPS compared to residents without agitation, highlighting the unmet need for effective management of agitation symptoms in this population.
Keywords: agitation, dementia, falls, fractures, infections, long‐term care, medication use, neuropsychiatric symptoms
Key points
Agitation symptoms in long‐term care residents with dementia can be disabling and lead to a loss of independent function
This study suggests that agitation is associated with higher use of polypharmacy, including antipsychotics, and an increased likelihood of falls, fractures, infections as well as other neuropsychiatric symptoms
Together, these outcomes present a major burden not only to the patient, but also to the long‐term care facilities, caregivers, and healthcare system
Findings of this study highlight the complexity of care for long‐term care residents with dementia and the current unmet need for effective management of agitation symptoms in this setting
1. INTRODUCTION
The burden of dementia in the United States (US) has increased due to the progressively aging population, and is now estimated to affect nearly 5.7 million people. 1 Dementia is a general term used to describe disabling declines in cognition, emotion, and behavior, with Alzheimer's disease (AD) being the most common cause in older adults. Furthermore, neuropsychiatric symptoms (NPS), including behavioral disturbances, associated with dementia can affect a substantial proportion of these patients, with estimates ranging between 78%–90%. 2 , 3 , 4 NPS can be generally categorized into four main groups: (1) mood disorders (e.g., anxiety, apathy, depression, and euphoria); (2) sleep disorders (e.g., insomnia, hypersomnia, and night‐day reversal); (3) psychotic symptoms (e.g., delusions and hallucinations); and (4) agitation (e.g., pacing, wandering, sexual disinhibition, and aggression). 5 , 6 The most common NPS in patients with dementia are reported to be mood disorders, sleep disorders, and agitation. 4
Agitation is among the most distressing and persistent NPS associated with dementia, 7 , 8 and has been shown to increase the risk of injury, including both self‐injury and injury to others, as well as mortality. 9 , 10 , 11 Agitation may be influenced by several factors, including the resident's environment, 12 staff‐resident interactions, 8 , 13 an inability to appropriately interpret and respond to pain due to dementia, or the presence of comorbid psychiatric conditions such as anxiety or depression. 10 , 14 , 15 Estimates of the prevalence of agitation vary greatly, ranging from 28%–60% in long‐term care facilities, 7 , 16 , 17 , 18 and 24–67% in community‐based dwellings. 19 , 20 This wide variability may have been due in part to the absence of an established definition of agitation until 2015 when the International Psychogeriatric Association (IPA) developed a consensus definition of agitation in patients with cognitive disorders. 21 Agitation, which is defined as excessive motor activity, verbal or physical aggression, and associated emotional distress, can be highly disruptive, resulting in excess disability not otherwise attributable to other psychiatric or medical disorders. 21 Moreover, agitation in patients with dementia is associated with more rapid decline in cognitive function, which can impede daily activities and lead to a loss of independent function. 15 , 18 , 22 Altogether, the various distressing symptoms associated with agitation often have negative ramifications on the patients' environment, raising concerns about a deterioration in their quality of life and placing a high burden on their family and caregivers, which has been found to consequently increase their likelihood of transfer to long‐term care facilities. 3 , 21
Although several studies have investigated outcomes associated with residing in long‐term care settings, they have mostly focused on the significant costs of residing in these facilities, 23 and the relationship between the patients' unmet needs and their cognitive and functional status. 24 , 25 , 26 To date, few studies have examined clinical outcomes and medication use associated with agitation as a unique condition in individuals with dementia residing in long‐term care facilities. Understanding patient outcomes and treatments as they relate to agitation is imperative for effective disease management. The objectives of this study were to describe characteristics and compare clinical outcomes including falls, fractures, infections (e.g., urinary tract infections), and other NPS in long‐term care residents with dementia with and without agitation.
2. MATERIALS AND METHODS
2.1. Data source
Long‐term care facility data from the AnalytiCare LLC (AnalytiCare) database were analyzed. AnalytiCare is a Health Insurance Portability and Accountability Act (HIPAA) compliant longitudinal database which captures information on residents living in long‐term care institutions. Data include linked resident information, pharmacy prescriptions, and the Minimum Data Set (MDS). The MDS is collected as part of the federally mandated process for clinical assessment of all residents in participating Medicare and Medicaid certified long‐term care facilities. MDS assessments are required for residents on admission to the facility, periodically (e.g., quarterly review assessment, significant change in status), and on discharge. 27
The current analyses used AnalytiCare data from October 2010 to June 2014, which included information on approximately 30,000 residents with dementia.
2.2. Study design, sample selection, and cohort balancing
The analyses for this study were conducted based on a cross‐sectional design (Figure 1). Residents with a dementia diagnosis were selected for this study if they resided in a long‐term care facility for at least 12 continuous months, had at least two MDS 3.0 assessments during this period, and had known information on agitation symptoms. Residents with dementia were classified into two mutually exclusive cohorts: the Agitation Cohort and the No‐Agitation Cohort based on whether or not they had an indicator of agitation at any time following their first observed diagnosis of dementia. An indicator of agitation was defined as a score of one or greater on at least one of the following MDS collected items: physical behavioral symptoms directed toward others, verbal behavioral symptoms directed toward others, other behavioral symptoms not directed toward others, rejection of care, wandering that impacts the resident and/or others, and being short‐tempered or easily annoyed. The behaviors used to define agitation were selected based on their alignment to dimensions of agitation as defined by the IPA 21 and the availability of information in MDS assessments.
FIGURE 1.
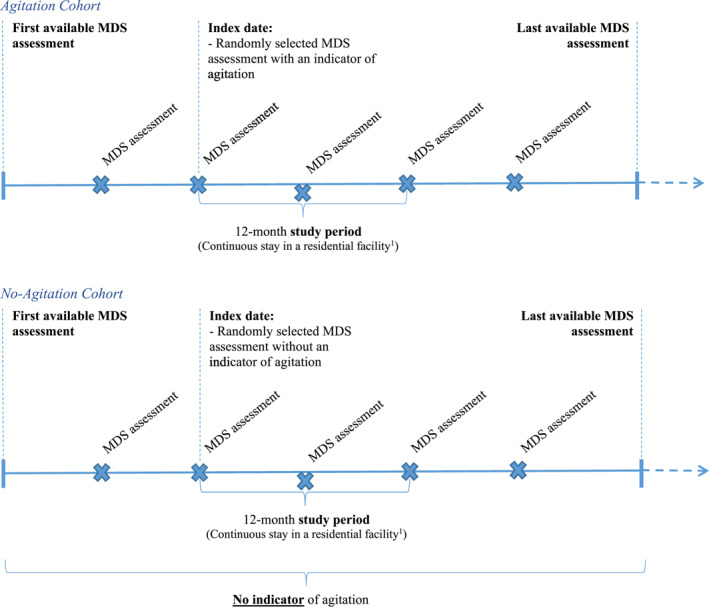
Study design. A period of continuous stay was defined as an ongoing residential stay with no discharge lasting longer than 14 days. Considering residents may have short discharge episodes for non‐medical reasons, a short discharge episode of 14 days or less was not considered the end of a continuous stay. *MDS assessments are indicated for illustrative purposes only
For each resident in the Agitation Cohort, the index date was randomly selected among all MDS assessments at which an indicator of agitation was recorded. This approach was used in order to capture a representative portrait of the residents' journey in a long‐term care facility. Since outcomes are expected to vary over time based on changes in resident characteristics and disease severity, a random index date allows outcomes to be captured that are representative of the overall population of residents with agitation in a real‐world long‐term care setting. For each resident in the No‐Agitation Cohort, the index date was randomly selected among all MDS assessments. For both cohorts, the study period was defined as the 12‐month period of continuous stay in a long‐term care facility following the index date.
2.3. Measures, outcomes, and statistical analyses
Entropy balancing, an analytical technique that has been used to achieve covariate balance in numerous observational studies with binary conditions, 28 , 29 , 30 was applied to reweight demographic and clinical characteristics of individuals included in the two cohorts. Residents in the No‐Agitation Cohort were reweighted so that the distribution of specified covariates had the exact same moments (mean and standard deviation [SD]) as the distribution of covariates for residents in the Agitation Cohort. Characteristics used to reweight the two cohorts included demographics (i.e., age, gender, race/ethnicity, language, marital status, year of index date, dementia due to Alzheimer's disease), cognitive characteristics (i.e., Brief Interview for Mental Status [BIMS] score, short‐term memory problem, long‐term memory problem, resident recall), and psychiatric disorders (i.e., psychotic disorders other than schizophrenia, schizophrenia, manic depression, and post‐traumatic stress disorder) measured on the index date.
Descriptive statistics were used to summarize characteristics of individuals in the Agitation and No‐Agitation Cohorts, separately, before and after reweighting. The mean, SD, and median were reported for continuous variables, and the frequency count and percentages were reported for categorical variables. In addition, standardized differences (Sdiff) between the two cohorts were reported.
Medication use including antipsychotic (AP) medication use, use of other medications, and number of distinct medication types as well as outcomes including infections (i.e., urinary tract infections), falls, fractures, and other NPS (i.e., depression, anxiety, delusions, and hallucinations) were measured during the 12‐months study period for the balanced Agitation and No‐Agitation Cohorts.
The impact of agitation on infections (i.e., urinary tract infections), falls, fractures, and other NPS was estimated using weighted logistic regression models, where the dependent variable was the outcome of interest and the independent variable was a dummy variable for the Agitation Cohort. Results were reported as odds ratios (OR) with 95% confidence intervals (CIs) and p‐values.
3. RESULTS
3.1. Resident characteristics
A total of 6,265 residents met the eligibility criteria for inclusion in the current study (Figure 2), of whom 3,313 (52.9%) were classified in the Agitation Cohort and 2,952 (47.1%) were classified in the No‐Agitation Cohort. Residents in the Agitation Cohort had on average 1.5 ± 0.9 distinct agitation symptoms on the index date (Table 1). The most frequent symptoms related to agitation were rejection of care (46.2%), followed by verbal abuse (30.1%), socially inappropriate behavior (28.2%), physical abuse (21.7%), being short‐tempered or easily annoyed (19.3%), and wandering that impacts the resident and/or others (5.3%).
FIGURE 2.
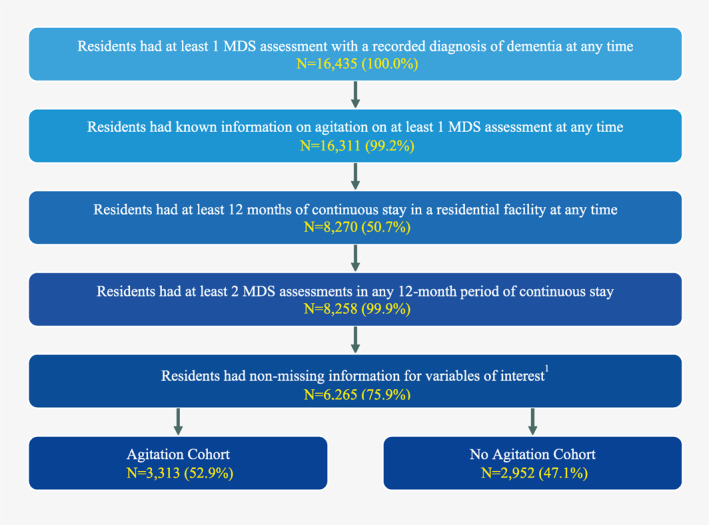
Sample selection. Variables of interest were gender, race, and an indicator of agitation. MDS, Minimum Data Set version 3.0
TABLE 1.
Resident characteristics
| Original cohort | Balanced cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Resident characteristics a | Agitation cohort (reference) | No‐agitation cohort | No‐agitation cohort | |||||
| Number of residents, N | N = 3,313 | N = 2,952 | Standardized difference | N = 2,952 | Standardized difference | |||
| As of the index date | ||||||||
| Age, mean ± SD [median] | 78.2 ± 10.0 [80.0] | 79.4 ± 9.7 [81.0] | 0.12 | 78.2 ± 10.0 [80.0] | 0.00 | |||
| Female, N (%) | 2,243 | (67.7%) | 2,105 | (71.3%) | 0.08 | 1,999 | (67.7%) | 0.00 |
| Race/Ethnicity, N (%) | ||||||||
| White, not of Hispanic origin | 2,150 | (64.9%) | 1,816 | (61.5%) | 0.07 | 1,916 | (64.9%) | 0.00 |
| Black, not of Hispanic origin | 560 | (16.9%) | 633 | (21.4%) | 0.12 | 499 | (16.9%) | 0.00 |
| Hispanic | 550 | (16.6%) | 445 | (15.1%) | 0.04 | 490 | (16.6%) | 0.00 |
| Asian/Pacific Islander | 39 | (1.2%) | 54 | (1.8%) | 0.05 | 35 | (1.2%) | 0.00 |
| American Indian/Alaskan | 14 | (0.4%) | 4 | (0.1%) | 0.05 | 12 | (0.4%) | 0.00 |
| Marital status, N (%) | ||||||||
| Widowed | 1,534 | (46.3%) | 1,517 | (51.4%) | 0.10 | 1,367 | (46.3%) | 0.00 |
| Married | 759 | (22.9%) | 617 | (20.9%) | 0.05 | 676 | (22.9%) | 0.00 |
| Divorced | 420 | (12.7%) | 289 | (9.8%) | 0.09 | 374 | (12.7%) | 0.00 |
| Never married | 337 | (10.2%) | 322 | (10.9%) | 0.02 | 300 | (10.2%) | 0.00 |
| Separated | 263 | (7.9%) | 207 | (7.0%) | 0.04 | 234 | (7.9%) | 0.00 |
| State of residence (5 most frequent), N (%) | ||||||||
| Texas | 1,363 | (41.1%) | 960 | (32.5%) | 0.18 | 1,055 | (35.7%) | 0.11 |
| North Carolina | 582 | (17.6%) | 561 | (19.0%) | 0.04 | 546 | (18.5%) | 0.02 |
| Colorado | 417 | (12.6%) | 164 | (5.6%) | 0.25 | 155 | (5.3%) | 0.26 |
| Michigan | 212 | (6.4%) | 226 | (7.7%) | 0.05 | 202 | (6.8%) | 0.02 |
| Maryland | 156 | (4.7%) | 241 | (8.2%) | 0.14 | 230 | (7.8%) | 0.13 |
| Alzheimer's disease b , N (%) | 2,107 | (63.6%) | 1,670 | (56.6%) | 0.14 | 1,877 | (63.6%) | 0.00 |
| Cognitive and clinical characteristics, N (%) | ||||||||
| Brief Interview for Mental Status c score, mean ± SD [median] | 10.1 ± 3.5 [10.0] | 10.7 ± 3.3 [11.0] | 0.16 | 10.1 ± 3.4 [10.0] | 0.01 | |||
| Individuals with missing values | 2,367 | (71.4%) | 1,653 | (56.0%) | 0.33 | 2,111 | (71.5%) | 0.00 |
| Psychiatric/mood disorder d | 2,410 | (72.7%) | 1,683 | (57.0%) | 0.33 | 1,881 | (63.7%) | 0.19 |
| Depression | 1,747 | (52.7%) | 1,395 | (47.3%) | 0.11 | 1,434 | (48.6%) | 0.08 |
| Anxiety disorder | 990 | (29.9%) | 478 | (16.2%) | 0.33 | 523 | (17.7%) | 0.29 |
| Dependence in daily decision‐making e | 2,349 | (70.9%) | 1,635 | (55.4%) | 0.33 | 2,091 | (70.8%) | 0.00 |
| Signs and symptoms of delirium | ||||||||
| Inattention | 1,062 | (32.1%) | 295 | (10.0%) | 0.56 | 373 | (12.6%) | 0.48 |
| Disorganized thinking | 978 | (29.5%) | 238 | (8.1%) | 0.57 | 303 | (10.3%) | 0.50 |
| Altered level of consciousness | 150 | (4.5%) | 73 | (2.5%) | 0.11 | 92 | (3.1%) | 0.07 |
| Psychomotor retardation | 149 | (4.5%) | 71 | (2.4%) | 0.11 | 93 | (3.1%) | 0.07 |
| Behavioral symptoms f , N (%) | ||||||||
| Number of distinct behavioral symptoms (indicators of agitation), mean ± SD [median] | 1.5 ± 0.9 [1.0] | – | – | – | – | |||
| Rejection of care | 1,529 | (46.2%) | – | – | – | – | ||
| Verbal abuse | 997 | (30.1%) | – | – | – | – | ||
| Socially inappropriate behavior | 934 | (28.2%) | – | – | – | – | ||
| Physical abuse | 719 | (21.7%) | – | – | – | – | ||
| Short‐tempered or easily annoyed | 641 | (19.3%) | – | – | – | – | ||
| Wandering that impacts the resident and/or others g | 174 | (5.3%) | – | – | – | – | ||
| Other behavioral symptoms | ||||||||
| Delusion | 290 | (8.8%) | 43 | (1.5%) | 0.34 | 47 | (1.6%) | 0.33 |
| Hallucinations | 99 | (3.0%) | 14 | (0.5%) | 0.19 | 18 | (0.6%) | 0.18 |
| During the study period | ||||||||
| Functional status and activities of daily living, N (%) | ||||||||
| Extensive or total dependence in any ADL h | 3,263 | (98.5%) | 2,888 | (97.8%) | 0.05 | 2,893 | (98.0%) | 0.04 |
| Bathing | 3,255 | (98.2%) | 2,876 | (97.4%) | 0.06 | 2,876 | (97.4%) | 0.06 |
| Personal hygiene | 3,035 | (91.6%) | 2,573 | (87.2%) | 0.14 | 2,621 | (88.8%) | 0.09 |
| Dressing | 2,933 | (88.5%) | 2,511 | (85.1%) | 0.10 | 2,564 | (86.9%) | 0.05 |
| Toilet use | 2,878 | (86.9%) | 2,477 | (83.9%) | 0.08 | 2,517 | (85.3%) | 0.05 |
Abbreviations: N, number; SD, standard deviation; MDS, Minimum Data Set; ICD‐9‐CM, International Classification of Diseases, Ninth Revision.
Entropy balancing was used to reweight residents so that resident characteristics on the index date were similar between residents with and without agitation. Weights were normalized so that the sum of weights was equal to the number of residents in each cohort.
Alzheimer's disease was defined as an indicator of Alzheimer's disease recorded on an MDS assessment or the ICD‐9‐CM 331.0 recorded on an MDS assessment.
Brief Interview for Mental Status (BIMS) scores range from 0 to 15, where a score between 0 and 7 indicates severe impairment; a score between 8 and 12 indicates moderate impairment; and a score between 13 and 15 indicates the individual is cognitively intact. BIMS scores were assessed among residents that could be understood by staff. Residents that were rarely/never understood by staff had missing values for the BIMS score. For these residents, cognitive impairment was assessed by staff using indicators such as dependence in daily decision making.
Psychiatric/mood disorder was defined as an indicator of depression, anxiety disorder, psychotic disorder (other than schizophrenia), schizophrenia, manic depression, or post‐traumatic stress disorder on an MDS assessment.
Dependence in daily decision‐making was defined as modified independence (i.e., some difficulty in new situations only), moderate impairment (i.e., poor decisions and cues or supervision were required), or severe impairment (i.e., never or rarely made decisions) in decisions regarding tasks of daily life.
Based on AnalytiCare LLC recommendations and exploratory assessment of MDS data, missing values for binary variables (i.e., 0 or 1) have been reported as a value of 0. Therefore, no distinction between the absence of a condition and unknown information on a condition could be made.
Wandering that impacts the resident and/or others was defined as wandering that placed the resident at significant risk of getting to a potentially dangerous place (e.g., stairs, outside of the facility) or significantly intruded on the privacy or activities of others.
Extensive or total dependence in an activity of daily living was indicated if the resident required extensive assistance (i.e., staff provided weight‐bearing support) at least 3 times in the 7‐day period prior to MDS assessment or the resident required complete staff assistance every time the activity was performed in the 7‐day period prior to MDS assessment.
The unbalanced resident demographics and clinical characteristics are first reported as follows (Table 1). The median age of the Agitation and No‐Agitation Cohorts was 80.0 and 81.0, respectively, and the majority of both cohorts were female (67.7% and 71.3%, respectively) and had a diagnosis of AD (63.6% and 56.6%, respectively).
Additionally, residents in the Agitation Cohort had greater dementia‐related cognitive impairment and associated clinical manifestations compared to the No‐Agitation Cohort (Table 1). Signs and symptoms of delirium were numerically higher among residents in the Agitation versus No‐Agitation Cohort, and included inattention (32.1% vs. 10.0%, Sdiff = 0.56), disorganized thinking (29.5% vs. 8.1%, Sdiff = 0.57), altered level of consciousness (4.5% vs. 2.5%, Sdiff = 0.11), and psychomotor retardation (4.5% vs. 2.4%, Sdiff = 0.11). Psychiatric and mood disorders were also more frequently reported in the Agitation Cohort (72.7%) compared to the No‐Agitation Cohort (57.0%, Sdiff = 0.33). Furthermore, 70.9% of the Agitation Cohort had some degree of dependence in daily decision‐making compared to 55.4% in the No‐Agitation Cohort (Sdiff = 0.33). Extensive or total dependence in any activities of daily living (ADL) was reported for nearly all residents in both the Agitation and No‐Agitation Cohorts (98.5% and 97.8%, respectively), and included extensive or total dependence in bathing (98.2% and 97.4%, respectively) and personal hygiene (91.6% and 87.2%, respectively).
As detailed above, balancing techniques were used to distinguish the effects of agitation independently of resident characteristics. After applying entropy balancing, the Agitation and No‐Agitation Cohorts had similar demographic, clinical, and cognitive characteristics (i.e., Sdiff <0.20). However, some differences remained between characteristics that are correlated with agitation, and were therefore not used for balancing, including anxiety disorder (29.9% and 17.7% in the Agitation and No‐Agitation Cohorts, respectively, Sdiff = 0.29), inattention (32.1% and 12.6%, respectively, Sdiff = 0.48), disorganized thinking (29.5% and 10.3%, respectively, Sdiff = 0.50), and delusions (8.8% and 1.6%, respectively, Sdiff = 0.33) (Table 1).
3.2. Medication use
During the 12‐months study period, residents with agitation used a median of 5.0 distinct types of medications compared to 4.0 distinct types of medications among those without agitation (Figure 3). The most common medications used in the Agitation and No‐Agitation Cohorts included antidepressants (67.4% vs. 57.4%, Sdiff = 0.21), followed by APs (55.9% vs. 38.4%, Sdiff = 0.36), antidementia drugs (52.1% vs. 50.2%, Sdiff = 0.04), antianxiety drugs (45.6% vs. 28.3%, Sdiff = 0.36), narcotics (43.9% vs. 39.1%, Sdiff = 0.10), and antiepileptic drugs (42.7% vs. 31.6%, Sdiff = 0.23).
FIGURE 3.
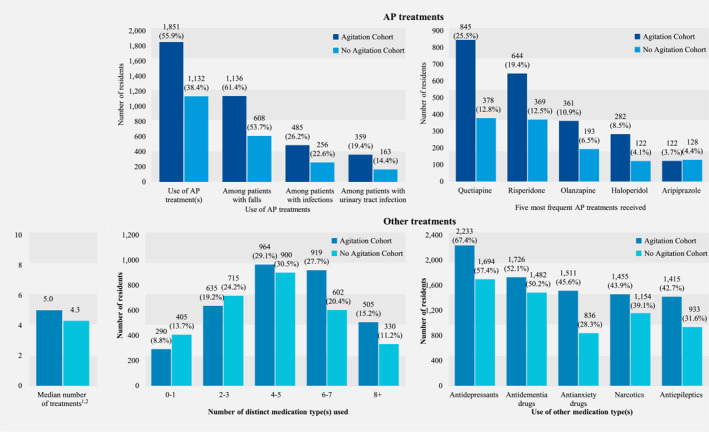
Treatment patterns–balanced cohorts. Treatment types were based on GPI classification and are mutually exclusive. The number of distinct medication types is based on the following medication classes: APs, antidepressants, antidementia drugs, antianxiety drugs, narcotics, antiepileptics, antihypertensives, lipid‐lowering drugs, antidiabetics, antihistamines, anticoagulants, antiplatelet drugs, pain control drugs, anti‐Parkinson drugs, hypnotics, antiemetic drugs, stimulants, and anticholinergics. AP, antipsychotic
3.3. Outcomes
Findings showed significant associations between agitation and several clinical outcomes (Figure 4). Specifically, compared to residents without agitation, residents with agitation were 1.58 times more likely to have a recorded fall (56.9% vs. 45.5%, p < 0.001) and 1.29 times more likely to have a recorded fracture (6.6% vs. 5.2%, p = 0.04). Residents with agitation were also 1.18 times more likely to have a recorded infection (25.1% vs. 22.1%, p = 0.01), particularly a urinary tract infection (18.3% vs. 14.5%, p < 0.001) compared to residents without agitation. In addition, other NPS were 2.11 times more prevalent among residents with agitation compared to those without agitation (82.4% vs. 68.9%, p < 0.001).
FIGURE 4.
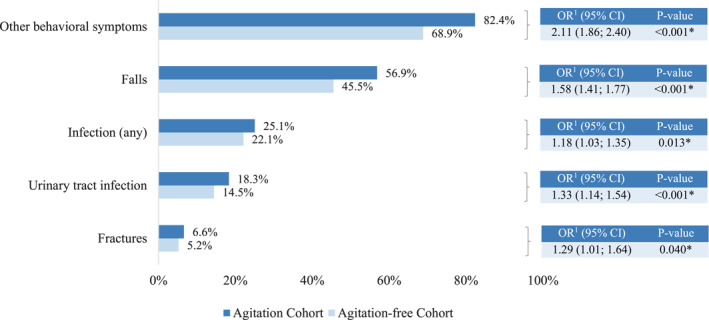
Impact of agitation on study outcomes − balanced cohorts. Odds ratios were estimated using weighted logistic regression models. An odds ratio >1 indicates that residents with agitation are more likely to experience the outcome compared to residents without agitation. Other behavioral symptoms include depression, anxiety, delusion, and hallucinations. OR, odds ratio; CI, confidence interval; * Significant at the 5% level
4. DISCUSSION
Residents with dementia living in long‐term care facilities comprise frail, elderly individuals, many of whom are also affected by delirium, other psychiatric and mood disorders, and a high level of dependency. The results of this study indicate that a proportion of residents with dementia living in these settings are further affected by a host of debilitating agitation symptoms, such as verbal abuse, physical abuse, and socially inappropriate behavior. In addition to the burden caused by these symptoms, agitation was found to be associated with polypharmacy and an increased risk of falls, fractures, infections, notably urinary tract infections, and other NPS.
This study highlights the high number of residents in long‐term care facilities who are treated with multiple classes of medications, which are more often prescribed among residents with agitation. Residents with agitation were more than two times more likely to exhibit other NPS such as anxiety, depression, delusions, or hallucinations. These findings are consistent with recent literature that demonstrated significantly greater use of multiple medications among patients with dementia and NPS compared to those without. 5 Existing meta‐analyses and systematic reviews have also indicated evidence of a high use of conventional and atypical AP medications in this population which could be used to address NPS, including agitation, 31 , 32 or other psychiatric disorders and NPS that may be correlated with agitation. 14 , 33 , 34 , 35 , 36 The high rates of polypharmacy, which increase the risk of adverse drug reactions and drug‐drug interactions, 37 , 38 together with the high use of APs, particularly among residents with agitation, demonstrate the challenging nature of managing agitation symptoms in this elderly population.
In addition, this study finds that residents with agitation had higher odds of sustaining a fall or a fracture compared to those without agitation. Although the incidence of falls is known to increase with advancing age, 39 and there are a variety of risk factors that can predispose older adults to falls (e.g., sensory decline, cardiovascular disease, gait imbalance, cognitive impairment), 40 , 41 agitation has been previously shown to be an important risk factor. 42 A study from 1990 found that residents of long‐term care facilities who experienced a fall manifested more agitated behaviors (e.g., pacing, general restlessness, hitting, and kicking) compared to those who did not experience a fall. 43 This increased risk of falls among residents with agitation may be due to exhaustion resulting from agitated behaviors, which may not be adequately treated, or sedation from medications such as APs, anxiolytics, anticonvulsants, and narcotic‐analgesics. 44 , 45 , 46 , 47 Falls may lead to injuries, including fractures, particularly in frail individuals, and indeed, the current study also found that the risk of fractures was higher among residents who experienced agitation. The high prevalence of fall‐related injuries is further associated with substantial healthcare costs, and older adults often experience further decline in functional status and report a reduced quality of life subsequent to a fall‐related injury. 48 , 49
The current findings also suggest that residents with agitation have higher odds of experiencing an infection, particularly a urinary tract infection. This may be due in part to the profound cognitive deficits and functional impairments that can render these individuals less active and less communicative about their health, thereby increasing their risk of infections. 50 In nursing home residents, urinary tract infections are the most commonly diagnosed infections, which may also be due to reduced hygiene associated with urinary and fecal incontinence in this population. 51 , 52 , 53 Studies have also shown that incontinence may lead to an increased risk of urinary tract infections in residents with agitation who may be more likely to refuse care from staff. 54 Notably, in this study, rejection of care was the most frequently observed symptom of agitation. The increased risk of infections among long‐term care residents with dementia and agitation, combined with their limited ability to complain about typical genitourinary symptoms and susceptibility to antimicrobial‐resistant bacteria, poses additional challenges in clinical assessment and treatment of these residents. 51 , 52
The outcomes experienced by residents with agitation present a major burden not only to the individual, but also to the long‐term care facilities and professional caregivers. Residents with high ADL‐dependency are often unable to identify, communicate, and respond to their own needs, further complicating their care. 15 Agitated behaviors such as general restlessness and pacing, which are common, can also be distressing to staff and other residents, while verbally and physically abusive behaviors may necessitate intervention from long‐term care personnel. 55 Additionally, clinical outcomes associated with agitation in long‐term care settings may place a substantial burden on the healthcare system as these sequelae may be associated with significant healthcare resource utilization and costs. Indeed, recent estimates indicate nearly $50 billion in expenditures attributable to falls among all older adults, 56 as well as up to $2 billion for hospitalizations due to infections in the long‐term care setting, in the US each year. 57 The need for effective interventions to manage agitation in LTC residents and mitigate these negative outcomes requires further resources for pertinent staff training (for example, to more quickly identify and respond to unmet needs, such as pain or dehydration, which may prompt agitated behaviors) and approaches that prioritize patient‐centric care. 8 In addition, the residents environment, 12 staff‐resident interactions, 8 , 13 and interventions such as cognitive training, physical exercise, and multisensory stimulation 58 may contribute to effective management of agitation symptoms in LTC residents with dementia.
This study further elucidates the burden of agitation among residents with dementia in long‐term care settings and emphasizes the complexity of care for these residents, which is underscored by the fact that there are no approved medications for the management of agitation by the US Food and Drug Administration (FDA). Furthermore, the use of available medications has been associated with adverse side effects among the elderly population with dementia. 59 Nevertheless, higher medication use, particularly AP use, has been documented in the literature. This suggests an urgent need for the development of interventions, both non‐pharmacologic as well as pharmacologic, which target agitation in residents with dementia in order to manage the condition and improve the individuals' quality of life while minimizing harmful effects. Since the needs of residents are often multiple and complex in these care settings, 24 , 60 appropriate interventions for agitation may also help to alleviate the demands placed on the long‐term care facilities and staff and the healthcare system at large.
Results of this study should be interpreted in light of certain limitations. First, no validated agitation scale (e.g., Cohen‐Mansfield Agitation Inventory [CMAI], Pittsburgh Agitation Scale [PAS]) was available in the AnalytiCare database to identify residents with agitation. Therefore, the indicators used to define agitation were selected from available MDS items concerning resident behavior based on their alignment to dimensions of agitation as defined by the IPA. Second, the present study evaluated the impact of agitation at any severity on outcomes of interest, thus, future studies are warranted to better understand the impact of agitation by severity level on outcomes. Third, although many important factors such as age, gender, race, cognitive characteristics, and psychiatric disorders were balanced between cohorts, residual confounding due to unobserved characteristics may remain. Fourth, a 12‐months study period was selected in order to capture outcomes of interest, including falls and fractures, which may not be captured accurately using a shorter study period. However, when studying residents in long‐term care facilities, requiring longer observation periods may induce survival bias. That is, residents who remain in a long‐term care facility for at least 12 months may be different from residents who remain in a long‐term care facility for less than 12 months due to death or discharge. Fifth, this study is subject to limitations inherent in analyses of real‐world data such as occasional coding errors and inaccurate or missing data on prescriptions or diagnoses. However, potential inaccuracies are assumed to be randomly distributed across cohorts. Sixth, the most recent MDS data available in the AnalytiCare database at the time of study completion included data through June 2014, and therefore, results of this study reflect outcomes in long‐term care facilities from 2010 through 2014. Finally, the AnalytiCare database includes data from skilled nursing facilities with at least 50 beds, and does not provide facility‐level information. As such, outcomes in this population may differ from what would be expected among residents of small long‐term care facilities, and findings may not be generalizable to the overall long‐term care population in the US. Further studies are therefore necessary to assess the impact of agitation among residents with dementia residing in various types of care facilities in order to assess the impact of these facility‐level factors and adjust for potential within‐facility effects.
The results of this study indicate that agitation among residents with dementia in long‐term care facilities was associated with higher likelihood of additional NPS, higher medication use, particularly AP use, and a higher risk of falls, fractures, and infections. These findings highlight the unmet need in this population for effective management of agitation as a strategy to reduce complications in care and improve the well‐being of long‐term care residents with dementia and agitation.
CONFLICT OF INTEREST DISCLOSURE
Patrick Gagnon‐Sanschagrin, Martin Cloutier, Mikhaïl Davidson, Elizabeth Serra, and Annie Guérin are employees of Analysis Group, Inc., a consulting company that has received consultancy fees from Otsuka Pharmaceutical Development and Commercialization, Inc., to conduct this study. Myrlene Sanon Aigbogun was an employee of Otsuka Pharmaceutical Development and Commercialization, Inc. at the time the study was conducted. Ross A. Baker is an employee of Otsuka Pharmaceutical Development and Commercialization, Inc. Christy R. Houle is an employee of Lundbeck Pharmaceuticals.
ETHICS APPROVAL STATEMENT
The AnalytiCare database is Health Insurance Portability and Accountability Act (HIPAA) compliant. A full review by an institutional review board (IRB) was not necessary as this study was a retrospective database analysis; an IRB exemption letter was received.
ACKNOWLEDGEMENTS
This study was funded by Otsuka Pharmaceutical Development and Commercialization Inc. and Lundbeck Pharmaceuticals. Medical writing assistance was provided by Loraine Georgy, PhD, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development and Commercialization Inc.
Fillit H, Aigbogun MS, Gagnon‐Sanschagrin P, et al. Impact of agitation in long‐term care residents with dementia in the United States. Int J Geriatr Psychiatry. 2021;36(12):1959‐1969. 10.1002/gps.5604
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from AnalytiCare LLC. Restrictions apply to the availability of these data, which were used under license for this study. Therefore, the data used in the study cannot be shared.
REFERENCES
- 1. Alzheimer's Association . Alzheimer's disease facts and figures. Alzheimer's Dementia. 2018;14(3):367‐429. [Google Scholar]
- 2. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim care companion J Clin psychiatry. 2001;3(3):93‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Müller‐Spahn F. Behavioral disturbances in dementia. Dialogues Clin Neurosci. 2003;5(1):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanon Aigbogun S, Jones E, Husbands J, et al. Behavioral symptoms and disturbances in dementia: a highly prevalent condition. AAIC. 2019;2019. [Google Scholar]
- 5. Aigbogun MS, Stellhorn R, Hartry A, Baker RA, Fillit H. Treatment patterns and burden of behavioral disturbances in patients with dementia in the United States: a claims database analysis. BMC Neurol. 2019;19(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desai AK, Schwartz L, Grossberg GT. Behavioral disturbance in dementia. Curr Psychiatry Rep. 2012;14(4):298‐309. [DOI] [PubMed] [Google Scholar]
- 7. Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer's disease. Int Psychogeriatr. 2010;22(3):346‐372. [DOI] [PubMed] [Google Scholar]
- 8. Ballard C, Corbett A. Agitation and aggression in people with Alzheimer's disease. Curr Opin Psychiatr. 2013;26(3):252‐259. [DOI] [PubMed] [Google Scholar]
- 9. Hoe J, Hancock G, Livingston G, Woods B, Challis D, Orrell M. Changes in the quality of life of people with dementia living in care homes. Alzheimer Dis Assoc Disord. 2009;23(3):285‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ahn H, Horgas A. Disruptive behaviors in nursing home residents with dementia: management approaches. J Clin Outcome Manag. 2013;20(12):566‐576. [Google Scholar]
- 11. Cohen‐Mansfield J, Marx MS, Lipson S, Werner P. Predictors of mortality in nursing home residents. J Clin Epidemiol. 1999;52(4):273‐280. [DOI] [PubMed] [Google Scholar]
- 12. McManus M, McClenaghan C. Hearing, Sound and the Acoustic Environment for People with Dementia. Dementia Services Development Centre, University of Stirling; 2010. [Google Scholar]
- 13. Gaugler JE, Yu F, Davila HW, Shippee T. Alzheimer’s disease and nursing homes. Health Aff. 2014;33(4):650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Twelftree H, Qazi A. Relationship between anxiety and agitation in dementia. Aging Ment Health. 2006;10(4):362‐367. [DOI] [PubMed] [Google Scholar]
- 15. Livingston G, Barber J, Marston L, et al. Prevalence of and associations with agitation in residents with dementia living in care homes: MARQUE cross‐sectional study. BJPsych open. 2017;3(4):171‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kverno KS, Rabins PV, Blass DM, Hicks KL, Black BS. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs. 2008;34(12):8‐14 quiz 16‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Margallo‐Lana M, Swann A, O'Brien J, et al. Prevalence and pharmacological management of behavioural and psychological symptoms amongst dementia sufferers living in care environments. Int J Geriatr Psychiatr. 2001;16(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 18. Zuidema S, Koopmans R, Verhey F. Prevalence and predictors of neuropsychiatric symptoms in cognitively impaired nursing home patients. J Geriatr Psychiatr Neurol. 2007;20(1):41‐49. [DOI] [PubMed] [Google Scholar]
- 19. Tractenberg RE, Weiner MF, Thal LJ. Estimating the prevalence of agitation in community‐dwelling persons with Alzheimer's disease. J neuropsychiatry Clin Neurosci. 2002;14(1):11‐18. [DOI] [PubMed] [Google Scholar]
- 20. Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the cache county study on memory in aging. Am J Psychiatry. 2000;157(5):708‐714. [DOI] [PubMed] [Google Scholar]
- 21. Cummings J, Mintzer J, Brodaty H, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27(1):7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen‐Mansfield J. Agitated behavior and cognitive functioning in nursing home residents. Clin Gerontol. 1988;7(3‐4):11‐22. [Google Scholar]
- 23. Feder J, Komisar HL, Niefeld M. Long‐term care in the United States: an overview. Health Aff. 2000;19(3):40‐56. [DOI] [PubMed] [Google Scholar]
- 24. Ferreira AR, Dias CC, Fernandes L. Needs in nursing homes and their relation with cognitive and functional decline, behavioral and psychological symptoms. Front Aging Neurosci. 2016;8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scocco P, Rapattoni M, Fantoni G. Nursing home institutionalization: a source of eustress or distress for the elderly? Int J Geriatr Psychiatr. 2006;21(3):281‐287. [DOI] [PubMed] [Google Scholar]
- 26. Gonzalez‐Colaco Harmand M, Meillon C, Rullier L, et al. Cognitive decline after entering a nursing home: a 22‐year follow‐up study of institutionalized and noninstitutionalized elderly people. J Am Med Dir Assoc. 2014;15(7):504‐508. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Medicare & Medicaid Services . Minimum Data Set 3.0 Public Reports; 2012. https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Computer‐Data‐and‐Systems/Minimum‐Data‐Set‐3‐0‐Public‐Reports/index.html Accessed Accessed in 07/19/2018. [Google Scholar]
- 28. Hainmueller J. Entropy balancing for causal effects: a multivariate reweighting method to produce balanced samples in observational studies. Polit Anal. 2012;20(1):25‐46. [Google Scholar]
- 29. Brettschneider C, Bleibler F, Hiller TS, et al. The allocation of resources in the care for patients with panic disorder in Germany: an excess cost analysis informing policy and science. Cost Eff Resour Alloc. 2019;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heider D, Matschinger H, Meid AD, et al. The impact of potentially inappropriate medication on the development of health care costs and its moderation by the number of prescribed substances. Results of a retrospective matched cohort study. PLoS One. 2018;13(7):e0198004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta‐analysis of randomized, placebo‐controlled trials. Am J Geriatr Psychiatr. 2006;14(3):191‐210. [DOI] [PubMed] [Google Scholar]
- 32. Ringman JM, Schneider L. Treatment options for agitation in dementia. Curr Treat Options Neurol. 2019;21(7):30. [DOI] [PubMed] [Google Scholar]
- 33. Bartels SJ, Horn SD, Smout RJ, et al. Agitation and depression in frail nursing home elderly patients with dementia: treatment characteristics and service use. Am J Geriatr Psychiatr. 2003;11(2):231–238. [PubMed] [Google Scholar]
- 34. Rockwell E, Jackson E, Vilke G, Jeste DV. A study of delusions in a large cohort of Alzheimer's disease patients. Am J Geriatr Psychiatr. 1994;2(2):157‐164. [DOI] [PubMed] [Google Scholar]
- 35. Ikeda M, Shigenobu K, Fukuhara R, et al. Delusions of Japanese patients with Alzheimer's disease. Int J Geriatr Psychiatr. 2003;18(6):527‐532. [DOI] [PubMed] [Google Scholar]
- 36. Cooper JK, Mungas D, Verma M, Weiler PG. Psychotic symtoms in Alzheimer's disease. Int J Geriatr Psychiatr. 1991;6:721‐726. [Google Scholar]
- 37. Caterina P, Antonello D, Chiara G, et al. Pharmacokinetic drug‐drug interaction and their implication in clinical management. J Res Med Sci. 2013;18:600‐609. [PMC free article] [PubMed] [Google Scholar]
- 38. Prybys K, Melville K, Hanna J, Gee A, Chyka P. Polypharmacy in the eldery: clinical challenges in emergency practice: Part 1: overview, etiology, and drug interactions. Emerg Med Rep. 2002;23:145‐153. [Google Scholar]
- 39. Centers for Disease Control and Prevention . Home and Recreational Safety. Important Facts about Falls; 2017. https://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html Accessed September 23, 2019. [Google Scholar]
- 40. National Institute on Aging . Prevent Falls and Fractures; 2017. https://www.nia.nih.gov/health/prevent‐falls‐and‐fractures Accessed September 24, 2019. [Google Scholar]
- 41. Vassallo M, Mallela SK, Williams A, Kwan J, Allen S, Sharma JC. Fall risk factors in elderly patients with cognitive impairment on rehabilitation wards. Geriatr Gerontol Int. 2009;9(1):41‐46. [DOI] [PubMed] [Google Scholar]
- 42. Agency for Healthcare Research and Quality . Preventing Falls in Hospitals; 2018. https://www.ahrq.gov/professionals/systems/hospital/fallpxtoolkit/index.html Accessed Septemeber 24, 2019. [Google Scholar]
- 43. Marx MS, Cohen‐Mansfield J, Werner P. Agitation and falls in institutionalized elderly persons. J Appl Gerontol. 1990;9(1):106‐117. [DOI] [PubMed] [Google Scholar]
- 44. Perttila NM, Ohman H, Strandberg TE, et al. How do community‐dwelling persons with alzheimer disease fall? Falls in the FINALEX study. Dement Geriatr Cogn Dis Extra. 2017;7(2):195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tapiainen V, Lavikainen P, Koponen M, et al. The risk of head injuries associated with antipsychotic use among persons with Alzheimer's disease. J Am Geriatr Soc. 2020;68(3):595‐602. [DOI] [PubMed] [Google Scholar]
- 46. Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system‐active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50(10):1629‐1637. [DOI] [PubMed] [Google Scholar]
- 47. Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporos Int. 2006;17(6):807‐816. [DOI] [PubMed] [Google Scholar]
- 48. Stel VS, Smit JH, Pluijm SM, Lips P. Consequences of falling in older men and women and risk factors for health service use and functional decline. Age Ageing. 2004;33(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 49. Hartholt KA, van Beeck EF, Polinder S, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long‐term reduced quality of life. J Trauma. 2011;71(3):748‐753. [DOI] [PubMed] [Google Scholar]
- 50. Mitchell SL, Shaffer ML, Loeb MB, et al. Infection management and multidrug‐resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med. 2014;174(10):1660‐1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. D'Agata E, Loeb MB, Mitchell SL. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc. 2013;61(1):62‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang L, Lansing B, Symons K, et al. Infection rate and colonization with antibiotic‐resistant organisms in skilled nursing facility residents with indwelling devices. Eur J Clin Microbiol Infect Dis. 2012;31(8):1797‐1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Musa MK, Saga S, Blekken LE, Harris R, Goodman C, Norton C. The prevalence, incidence, and correlates of fecal incontinence among older people residing in care homes: a systematic review. J Am Med Dir Assoc. 2019;20(8):956‐962. [DOI] [PubMed] [Google Scholar]
- 54. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. [DOI] [PubMed] [Google Scholar]
- 55. Cohen‐Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77‐M84. [DOI] [PubMed] [Google Scholar]
- 56. Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66(4):693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cohen CC, Choi YJ, Stone PW. Costs of infection prevention practices in long‐term care settings: a systematic review. Nurs Econ. 2016;34(1):16‐24. [PMC free article] [PubMed] [Google Scholar]
- 58. Millán‐Calenti JC, Lorenzo‐López L, Alonso‐Búa B, De Labra C, González‐Abraldes I, Maseda A. Optimal nonpharmacological management of agitation in Alzheimer’s disease: challenges and solutions. Clin interventions Aging. 2016;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zdanys KF, Carvalho AF, Tampi RR, Steffens DC. The treatment of behavioral and psychological symptoms of dementia: weighing benefits and risks. Curr Alzheimer Res. 2016;13(10):1124‐1133. [DOI] [PubMed] [Google Scholar]
- 60. Martin MD, Hancock GA, Richardson B, et al. An evaluation of needs in elderly continuing‐care settings. Int Psychogeriatr. 2002;14(4):379‐388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from AnalytiCare LLC. Restrictions apply to the availability of these data, which were used under license for this study. Therefore, the data used in the study cannot be shared.


