Abstract
Background
About one‐tenth of patients with difficult‐to‐treat chronic rhinosinusitis with nasal polyps (CRSwNP) have comorbid non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease (NSAID‐ERD). Dupilumab, a fully human monoclonal antibody that blocks the shared interleukin (IL)‐4/IL‐13 receptor component, is an approved add‐on treatment in severe CRSwNP. This post hoc analysis evaluated dupilumab efficacy and safety in patients with CRSwNP with/without NSAID‐ERD.
Methods
Data were pooled from the phase 3 SINUS‐24 and SINUS‐52 studies in adults with uncontrolled severe CRSwNP who received dupilumab 300 mg or placebo every 2 weeks. CRSwNP, nasal airflow, lung function, and asthma control outcomes at Week 24 were evaluated, and treatment–subgroup interactions were assessed for patients with and without NSAID‐ERD.
Results
Of 724 patients, 204 (28.2%) had a diagnosis of NSAID‐ERD. At Week 24, least squares mean treatment differences demonstrated significant improvements in nasal polyp score, nasal congestion (NC), Lund–Mackay computed tomography, 22‐item Sinonasal Outcome Test (SNOT‐22), Total Symptom Score (TSS), rhinosinusitis severity visual analog scale, peak nasal inspiratory flow (PNIF), six‐item Asthma Control Questionnaire score, and improvement in smell with dupilumab versus placebo (all p < .0001) in patients with NSAID‐ERD. Treatment comparisons demonstrated significantly greater improvements with dupilumab in patients with versus without NSAID‐ERD for NC (p = .0044), SNOT‐22 (p = .0313), TSS (p = .0425), and PNIF (p = .0123).
Conclusions
In patients with uncontrolled severe CRSwNP, dupilumab significantly improved objective measures and patient‐reported symptoms to a greater extent in the presence of comorbid NSAID‐ERD than without. Dupilumab was well tolerated in patients with/without NSAID‐ERD.
Keywords: chronic rhinosinusitis with nasal polyps, dupilumab, IL‐13, IL‐4, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease
In patients pooled from the SINUS‐24 (NCT02912468) and SINUS‐52 (NCT02898454) studies, patients with CRSwNP and comorbid NSAID‐ERD represent a severe and difficult‐to‐treat population. Improvements in disease control, lung function, and symptom burden were observed with dupilumab treatment versus placebo, irrespective of NSAID‐ERD status. The dupilumab treatment effect was greater in NSAID‐ERD patients for NC, SNOT‐22 total score, TSS, and PNIF.
Abbreviations: ACQ‐6, 6‐item asthma control questionnaire; CRSwNP, chronic rhinosinusitis with nasal polyps; FEV1, forced expiratory volume in 1 second; ITT, intent‐to‐treat; LMK‐CT, Lund–Mackay score by computed tomography; NC, nasal congestion; NPS, nasal polyps score; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease; PNIF, peak nasal inspiratory flow; SNOT‐22, 22‐item sinonasal outcome test; TSS, total symptom score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale

Abbreviations
- ACQ‐6
six‐item Asthma Control Questionnaire
- AE
adverse event
- CRSwNP
chronic rhinosinusitis with nasal polyps
- CI
confidence interval
- ECP
eosinophil cationic protein
- FEV1
forced expiratory volume in 1 second
- HR
hazard ratio
- HRQoL
health‐related quality of life
- IgE
immunoglobulin E
- IL
interleukin
- ITT
intent‐to‐treat
- LMK‐CT
Lund–Mackay computed tomography
- LS
least squares
- LTE4
leukotriene E4
- MFNS
mometasone furoate nasal spray
- NC
nasal congestion
- NP
nasal polyp
- NPS
nasal polyp score
- NSAID‐ERD
non‐steroidal anti‐inflammatory drug‐exacerbated disease
- PNIF
peak nasal inspiratory flow
- q2/4w
every 2/4 weeks
- SCS
systemic corticosteroids
- SNOT‐22
22‐item Sinonasal Outcome Test
- TARC
thymus and activation‐regulated chemokine
- TEAE
treatment‐emergent adverse event
- Th2
T helper 2
- TSS
total symptom score
- UPSIT
University of Pennsylvania Smell Identification Test
- VAS
visual analog scale
1. INTRODUCTION
Chronic rhinosinusitis with nasal polyps (CRSwNP) is an inflammatory disease of the nasal passages and paranasal sinuses characterized by two or more symptoms, one of which should be either rhinorrhea or nasal congestion/obstruction, with/without facial pain/pressure or loss of sense of smell for at least 12 weeks. 1 , 2 The disease is associated with a high symptom burden and poor health‐related quality of life (HRQoL). 1 , 2 , 3 , 4 A subgroup of patients suffer from uncontrolled severe CRSwNP, characterized by the polyp mass, symptoms, and lack of control by the standard of care. 5 The pathophysiology of CRSwNP is characterized predominantly by type 2 inflammation with interleukin (IL)‐4, IL‐13, and IL‐5 as prominent cytokines, and tissue infiltration by eosinophils, lymphocytes, basophils, and mast cells. 1 , 6 , 7 , 8 , 9
Approximately 10% of patients with CRSwNP have a diagnosis of non‐steroidal anti‐inflammatory drug‐exacerbated respiratory disease (NSAID‐ERD). 10 NSAID‐ERD—also known as aspirin‐exacerbated respiratory disease or Samter's triad—is a type 2 immune‐mediated clinical syndrome associated with aspirin/non‐steroidal anti‐inflammatory drug (NSAID) sensitivity, asthma, and CRSwNP. 11 , 12 , 13 The syndrome encompasses two distinct phases consisting of a chronic baseline airway inflammation that presents as asthma and recurrent nasal polyps (NPs), and an acute hypersensitivity reaction triggered by NSAIDs. Onset and severity of symptoms are dose related and characterized by upper and/or lower airway symptoms, which can vary from mild rhinorrhea, wheezing, and shortness of breath, to severe laryngospasm and bronchospasm. 13 , 14 Although these respiratory reactions are the defining feature of the syndrome, the initial inflammatory process begins and continues independently of exposure to NSAIDs. Both airway inflammation and the clinical reactions to NSAIDs are characterized by a potent type 2 immune response with the activation of effector cells, including mast cells, eosinophils, and basophils, and derangements in arachidonic acid metabolism. Lipid mediators, including prostaglandins and leukotrienes, which are central to the pathogenesis of NSAID‐ERD, are potent innate lymphoid cell 2 activators, which rapidly and robustly induce the production of T helper 2 (Th2) cytokines upon upregulation. 11 , 12
Patients with CRSwNP and NSAID‐ERD have more severe sinus disease and worse HRQoL than those without NSAID‐ERD. 13 , 15 , 16 The current standards of care for CRSwNP with NSAID‐ERD include corticosteroid use, with sinonasal surgery for patients who are inadequate responders. 13 However, reoccurrences of polyps are common. Aspirin desensitization post‐sinonasal surgery has also been utilized as part of a multifaceted treatment approach. 17 However, aspirin desensitization is not appropriate for all patients due to the risk of exacerbation of underlying asthma and side effects, including laryngeal spasm and cutaneous and gastrointestinal toxicity. 18 , 19 , 20 Thus, an alternative management approach that reduces systemic corticosteroids (SCS) or NP surgery need is desirable.
Dupilumab is a fully human VelocImmune®‐derived monoclonal antibody that blocks the shared receptor component for IL‐4 and IL‐13, which are key and central drivers of type 2 inflammation in multiple diseases. 21 , 22 , 23 In the randomized, double‐blind, 24‐week SINUS‐24 (NCT02912468) and 52‐week SINUS‐52 (NCT02898454) phase 3 trials in patients with uncontrolled severe CRSwNP, dupilumab significantly improved endoscopic, radiologic, and clinical outcomes, including sense of smell, versus placebo, and was generally well tolerated. 24 In a systematic review, dupilumab treatment after 24 weeks improved disease‐specific and general HRQoL versus placebo in patients with uncontrolled severe CRSwNP. 25 Dupilumab is approved by the United States Food and Drug Administration 26 and the European Medicines Agency 27 as an add‐on treatment in adult patients with inadequately controlled CRSwNP.
In pre‐specified subgroup analyses of patients with CRSwNP with comorbid NSAID‐ERD from SINUS‐24 and SINUS‐52, dupilumab showed significant improvements in nasal polyp score (NPS), nasal congestion (NC) score, and Lund–Mackay computed tomography (LMK‐CT) score at Week 24, compared with placebo (all p < .0001). 24 The aim of this study was to analyze the efficacy and safety of dupilumab versus placebo across a range of outcome measures in patients with CRSwNP with and without comorbid NSAID‐ERD in the pooled SINUS‐24 and SINUS‐52 studies.
2. METHODS
2.1. Study design
SINUS‐24 and SINUS‐52 were two multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, phase 3 studies of dupilumab in patients with uncontrolled severe CRSwNP. The study design, randomization process, and methodology for SINUS‐24 and SINUS‐52 have been reported previously. 24 In both studies, randomization was stratified by asthma or NSAID‐ERD disease status at screening, previous surgery at screening, and country.
2.2. Participants
The eligibility criteria were identical for both studies and previously published (see Table S1). 24 Briefly, adults with bilateral NPs and symptoms of chronic rhinosinusitis despite intranasal corticosteroid therapy, who had received SCS in the preceding 2 years (or had a medical contraindication or intolerance to SCS) or previous sinonasal surgery were included. A pre‐specified enrollment goal was to have at least 50% of patients with asthma, NSAID‐ERD, or both, and 50% of patients having had previous polyp surgery. Written informed consent was provided by all patients in the SINUS‐24 and SINUS‐52 trials. 24
To ascertain a diagnosis of NSAID‐ERD, a two‐question survey based on a diagnostic algorithm endorsed by an expert panel 13 was administered during the medical/surgery history interview. The two questions were as follows: Have you ever had respiratory, nasal, and/or bronchial symptoms following the intake of aspirin or/and NSAID? and While having a positive clinical history of NSAID‐ERD, have you ever undergone an aspirin provocation test, either nasal, bronchial, or oral? An affirmative response to either question was considered to indicate a clinical diagnosis of NSAID‐ERD.
Details of study sites have been previously published. 24
2.3. Interventions
Patients were randomly assigned (1:1) to receive subcutaneous dupilumab 300 mg or placebo every 2 weeks (q2w) for 24 weeks in SINUS‐24, and (1:1:1) to receive dupilumab 300 mg q2w for 52 weeks, dupilumab q2w for 24 weeks and then every 4 weeks (q4w) for the remaining 28 weeks, or placebo q2w for 52 weeks in SINUS‐52. Patients received 100 mg of mometasone furoate nasal spray (MFNS) in each nostril twice daily throughout the trial starting from 4 weeks prior to randomization. Patients were permitted to use saline nasal lavage as needed, systemic antibiotics, short‐course SCS, or to undergo sinonasal surgery during treatment and follow‐up.
2.4. Outcome measures
The coprimary endpoints in the SINUS‐24 and SINUS‐52 studies were change from baseline in NPS and NC, at Week 24. 24 In this post hoc analysis, changes from baseline at Week 24 in NPS, patient‐assessed symptom severity score for NC (NC score 0–3; 0 = no symptoms, 1 = mild, 2 = moderate, and 3 = severe), LMK‐CT score, 22‐item Sinonasal Outcome Test (SNOT‐22) score, patient‐reported Total Symptom Score (TSS; a composite severity score consisting of the sum of daily symptoms of NC, loss of smell, and anterior or posterior rhinorrhea), and visual analog scale (VAS) for rhinosinusitis severity were analyzed. Changes in smell were determined using University of Pennsylvania Smell Identification Test (UPSIT) score, proportion of patients who were anosmic (UPSIT score ≤18), loss of smell score, and SNOT‐22 smell/taste item scores. The need for rescue intervention was assessed as time to first SCS use or need for NP surgery over 52 weeks of the treatment period. In patients with concomitant asthma, nasal obstruction, lung function, and asthma control were assessed by peak nasal inspiratory flow (PNIF), forced expiratory volume in 1 second (FEV1), and six‐item Asthma Control Questionnaire (ACQ‐6) score, respectively.
Eosinophil count, serum total immunoglobulin E (IgE), thymus and activation‐regulated chemokine (TARC; also known as C‐C motif chemokine 17), periostin, and plasma eotaxin‐3 (also known as C‐C motif chemokine 26) concentrations were assessed in blood samples taken at baseline and Week 24. Eosinophil cationic protein (ECP), eotaxin‐3, and total IgE were assessed in nasal secretion samples taken at baseline and Week 24, and urinary leukotriene E4 (LTE4) was measured in samples taken at baseline and Week 24. Serum periostin, serum TARC, eotaxin‐3 in both blood and nasal secretion samples, and total IgE and ECP in nasal secretion samples were also assessed at Week 24 in the SINUS‐52 population.
Safety measures included assessments of vital signs, physical examination, clinical laboratory assessment, 12‐lead electrocardiogram findings, and incidence of treatment‐emergent adverse events (TEAEs).
2.5. Sample size, randomization, and masking
The sample size calculation, randomization process, and masking procedures for the SINUS‐24 and SINUS‐52 studies have been described previously. 24
2.6. Statistical methods
Analyses of the efficacy endpoints (NPS, NC score, LMK‐CT score, SNOT‐22 score, TSS, rhinosinusitis VAS), nasal airflow (PNIF), lung function (FEV1), and asthma control (ACQ‐6) used the same imputed dataset using worst observation carried forward and multiple imputation methods, as done for the primary analysis. 24 Further details on statistical methods are given in the Supplementary Materials.
3. RESULTS
3.1. Patient characteristics
Of the 724 patients included in the pooled intent‐to‐treat population, 204 (28.2%) had a clinical diagnosis consistent with NSAID‐ERD (Table S2). Of these, 203 patients had a history of intolerance to aspirin and/or NSAIDs, and 30 patients had undergone an aspirin provocation test, of which 26 had a positive test (Table S2). There were more female patients (p < .0001) among those with NSAID‐ERD than among those without NSAID‐ERD. Patients with NSAID‐ERD had more severe CRSwNP than those without, as reflected by the significant differences in baseline disease characteristics shown between the two groups (Table 1). A greater proportion of patients with NSAID‐ERD had undergone prior sinonasal surgery compared with those without NSAID‐ERD (78% vs. 58%, respectively; p < .001). Patients with NSAID‐ERD had greater sinus opacification (higher LMK‐CT score), worse sense of smell (lower UPSIT score, greater percentage of patients with anosmia, higher daily loss of smell score, and higher SNOT‐22 smell/taste item score), and higher TSS than those without NSAID‐ERD (all p < .05). The type 2 inflammatory biomarkers including blood eosinophils, serum periostin, plasma eotaxin‐3, nasal secretion eotaxin‐3, and urinary LTE4 were higher in patients with NSAID‐ERD compared with those without NSAID‐ERD (all p < .05). There was no significant difference in serum total IgE. In the subgroup of patients with CRSwNP with comorbid asthma (n = 428), patients with NSAID‐ERD (n = 181) had poorer lung function (FEV1) compared with patients without NSAID‐ERD (n = 247) (Table 1).
TABLE 1.
Baseline demographic and disease characteristics in patients with CRSwNP with and without NSAID‐ERD
| Characteristics/Parameters | Patients with CRSwNP with NSAID‐ERD (n = 204) | Patients with CRSwNP without NSAID‐ERD (n = 520) | p‐value NSAID‐ERD versus non‐NSAID‐ERD a |
|---|---|---|---|
| Age, mean (SD), years | 50.30 (12.87) | 51.82 (12.79) | .1523 |
| Female gender, n (%) | 116 (56.9) | 171 (32.9) | < .0001 |
| Time since first NP diagnosis, mean (SD), years | 12.85 (9.20) | 10.28 (9.45) | .001 |
| Patients with previous surgery for NP, n (%) | 159 (77.9) | 300 (57.7) | < .0001 |
| Patients with SCS use during the previous 2 years, n (%) | 148 (72.5) | 390 (75.0) | .4971 |
| Bilateral endoscopic NPS, b mean (SD), range 0–8 | 5.92 (1.32) | 5.98 (1.22) | .5405 |
| Daily NC score, b mean (SD), range 0–3 | 2.43 (0.58) | 2.39 (0.58) | .3792 |
| LMK‐CT score, b mean (SD), range 0–24 | 19.33 (3.83) | 17.99 (4.09) | < .0001 |
| SNOT−22 total score, b mean (SD), range 0–110 | 52.86 (19.63) | 50.19 (21.02) | .1208 |
| TSS, b mean (SD), range 0–9 | 7.33 (1.35) | 7.09 (1.45) | .0452 |
| Rhinosinusitis severity (VAS), b mean (SD), range 0–10 | 7.99 (2.09) | 7.83 (2.07) | .3563 |
| UPSIT score, b mean (SD), range 0–40 | 11.88 (6.47) | 14.81 (8.67) | < .0001 |
| Anosmia (UPSIT score ≤18), c n (%) | 178 (88.6) | 373 (73.3) | < .0001 |
| Daily loss of smell score, b mean (SD), range 0–3 | 2.83 (0.43) | 2.70 (0.56) | .0009 |
| SNOT−22 smell/taste item, b mean (SD) | 4.48 (1.04) | 4.19 (1.20) | .0017 |
| Patients with comorbid asthma, n (%) | 181 (88.7) | 247 (47.5) | < .0001 |
| PNIF, L/min, mean (SD) | 84.86 (54.75) | 87.94 (56.62) | .5067 |
| FEV1, L, mean (SD) d | 2.56 (0.86) | 2.65 (0.90) | .2928 |
| ACQ‐6 score, mean (SD), d range 0–6 | 1.56 (1.12) | 1.62 (1.10) | .6149 |
| Total IgE, IU/mL, median (95% CI) |
127.00 (108.00, 163.00) |
115.50 (99.00, 133.00) |
.1701 |
| Serum TARC, pg/mL, median (95% CI) |
280.00 (262.00, 299.00) |
297.50 (277.00, 312.00) |
.5541 |
| Serum periostin, ng/mL, median (95% CI) |
117.50 (110.00, 127.00) |
99.00 (94.60, 104.00) |
< .0001 |
| Eotaxin‐3, pg/mL, median (95% CI) |
69.25 (62.70, 77.30) |
57.30 (53.70, 60.80) |
.0093 |
| Blood eosinophils, Giga/L, median (95% CI) |
0.36 (0.33, 0.43) |
0.34 (0.31, 0.37) |
.0277 |
| Urinary LTE4, pg/mL, median (95% CI) |
218.00 (178.00, 286.00) |
85.00 (70.40, 94.50) |
< .0001 |
| Nasal eotaxin‐3, e pg/mL, median (95% CI) |
43.40 (13.70, 93.20) |
16.50 (9.52, 22.80) |
.0095 |
| Nasal IgE, e IU/mL, median (95% CI) |
9.00 (5.00, 26.00) |
5.00 (4.00, 8.00) |
.0632 |
| Nasal ECP, e ng/mL, median (95% CI) |
35.00 (24.00, 118.00) |
25.00 (11.00, 40.00) |
.0705 |
Abbreviations: ACQ‐6, six‐item Asthma Control Questionnaire; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; IgE, immunoglobulin E; LMK‐CT, Lund–Mackay computed tomography; LTE4, leukotriene E4; NC, nasal congestion; NP, nasal polyp; NPS, nasal polyp score; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; PNIF, peak nasal inspiratory flow; SCS, systemic corticosteroids; SD, standard deviation; SNOT‐22, 22‐item Sinonasal Outcome Test; TARC, thymus and activation‐regulated chemokine; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
Nominal p‐values for comparing patients with and without NSAID‐ERD based on t test for equal variance for quantitative parameters (t test for unequal variances in cases where homogeneity of variances was not met) and chi‐square test for qualitative parameters. Wilcoxon rank‐sum test was used to compare non‐parametric data for the biomarkers.
Higher mean scores indicate more severe disease, except for UPSIT score, where lower scores indicate more severe disease.
Analyzed in patients who completed an UPSIT smell test at baseline. CRSwNP with NSAID‐ERD, n = 201; CRSwNP without NSAID‐ERD, n = 509.
Analyzed in patients with asthma with NSAID‐ERD (n = 181) and without NSAID‐ERD (n = 247).
Nasal secretion biomarkers were assessed only in patients from SINUS‐52. CRSwNP with NSAID‐ERD, n = 120; CRSwNP without NSAID‐ERD, n = 328.
3.2. Changes in disease control and symptom burden
Dupilumab treatment, compared with placebo, demonstrated meaningful improvements in assessed outcome measures of CRSwNP in both patients with and without NSAID‐ERD at Week 24 (nominal p < .0001; Figure 1). Improvement versus placebo was evident from the first assessment time point for each endpoint.
FIGURE 1.
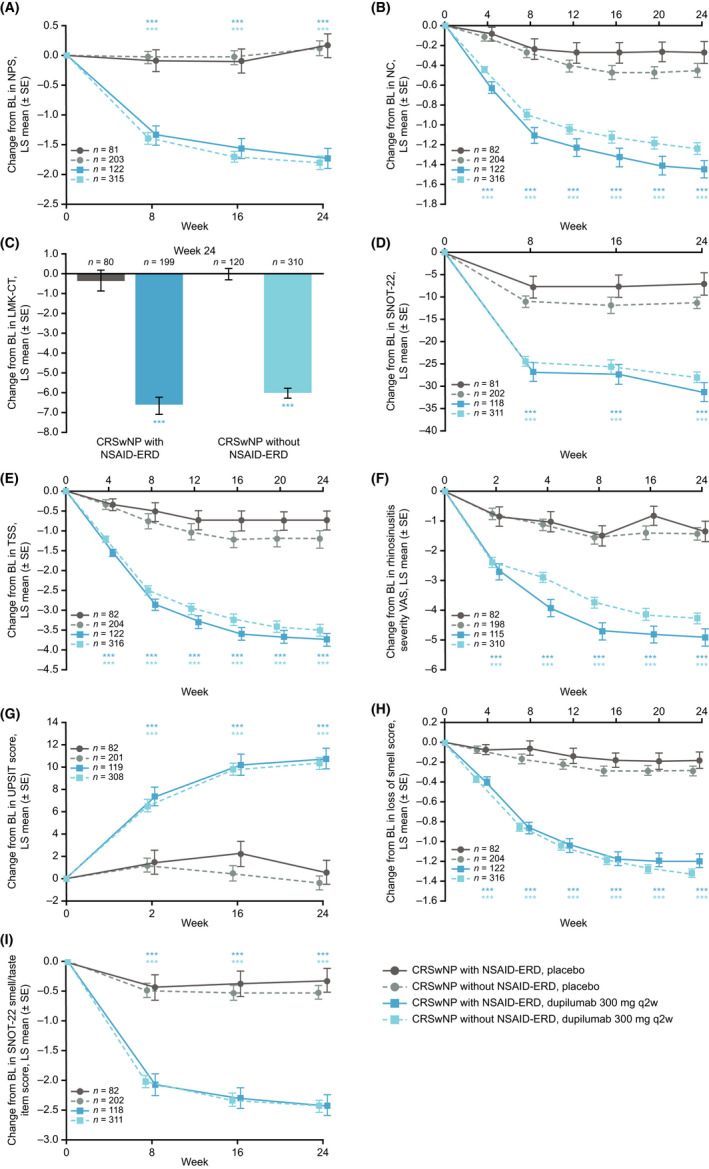
Changes from baseline to Week 24 in CRSwNP disease control and symptom burden in patients with CRSwNP with and without NSAID‐ERD, as assessed by (A) NPS, (B) patient‐assessed symptom severity score for NC or obstruction, (C) LMK‐CT score, (D) SNOT‐22 score, (E) patient‐reported TSS, (F) VAS for rhinosinusitis, (G) UPSIT score, (H) loss of smell score, and (I) SNOT‐22 smell/taste item score. ***Nominal p < .0001 versus placebo. Abbreviations: BL, baseline; CRSwNP, chronic rhinosinusitis with nasal polyps; LMK‐CT, Lund–Mackay computed tomography; LS, least squares; NPS, nasal polyp score; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; q2w, every 2 weeks; SE, standard error; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
When compared, improvement versus placebo for NPS, LMK‐CT score, rhinosinusitis VAS, UPSIT, loss of smell score, and SNOT‐22 decrease in smell/taste scores were similar in patients with or without NSAID‐ERD status (Table 2). Improvements in NC score, TSS, and SNOT‐22 total score were greater with dupilumab in patients with NSAID‐ERD than in patients without NSAID‐ERD (all nominal p < .05 for interaction; Table 2).
TABLE 2.
Treatment effect comparisons on CRSwNP disease control, airway function, and sense of smell at Week 24 in patients with CRSwNP with and without NSAID‐ERD (SINUS‐24 and SINUS‐52 pooled)
|
Characteristics/ Parameters |
Patients with CRSwNP with NSAID‐ERD | Patients with CRSwNP without NSAID‐ERD | Overall interaction p‐value in patients with CRSwNP with/without NSAID‐ERD b |
|---|---|---|---|
| LS mean difference versus placebo (95% CI), p‐value versus placebo a | LS mean difference versus placebo (95% CI), p‐value versus placebo a | ||
| NPS score, range 0–8 |
–1.89 (–2.30, –1.49) < .0001 |
–1.92 (–2.20, –1.64) < .0001 |
.9786 |
| NC score, range 0–3 |
–1.17 (–1.39, –0.95) < .0001 |
–0.77 (–0.92, –0.63) < .0001 |
.0044 |
| LMK‐CT score, range 0–24 |
–6.33 (–7.39, –5.27) < .0001 |
–6.04 (–6.69, –5.40) < .0001 |
.5637 |
| SNOT‐22 score, range 0–110 |
–24.35 (–29.54, –19.17) < .0001 |
–16.80 (–19.91, –13.69) < .0001 |
.0313 |
| TSS, range 0–9 |
–3.01 (–3.53, –2.49) < .0001 |
–2.32 (–2.70, –1.95) < .0001 |
.0425 |
| Rhinosinusitis (VAS), range 0–10 |
–3.59 (–4.30, –2.87) < .0001 |
–2.84 (–3.32, –2.37) < .0001 |
.2354 |
| UPSIT score, range 0–40 |
10.17 (7.95, 12.39) < .0001 |
10.69 (9.32, 12.07) < .0001 |
.8548 |
| Daily loss of smell score, range 0–3 |
–1.01 (–1.23, –0.79) < .0001 |
–1.05 (–1.20, –0.89) < .0001 |
.9338 |
| SNOT‐22 decreased smell/taste score |
–2.12 (–2.54, –1.70) < .0001 |
–1.92 (–2.18, –1.66) < .0001 |
.5352 |
| FEV1,c L |
0.26 (0.15, 0.36) < .0001 |
0.19 (0.08, 0.30) .001 |
.3434 |
| ACQ‐6 score,c range 0–6 |
–0.97 (–1.22, –0.72) < .0001 |
–0.72 (–0.92, –0.52) < .0001 |
.1630 |
| PNIF, L/min |
52.05 (39.71, 64.40) < .0001 |
33.11 (25.48, 40.74) < .0001 |
.0123 |
Abbreviations: ACQ‐6, six‐item Asthma Control Questionnaire; ANCOVA, analysis of covariance; CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; FEV1, forced expiratory volume in 1 second; LMK‐CT, Lund–Mackay computed tomography; LS, least squares; MI, multiple imputation; NC, nasal congestion; NPS, nasal polyp score; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; PNIF, peak nasal inspiratory flow; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale; WOCF, worst observation carried forward.
Each of the imputed complete data was analyzed by fitting an ANCOVA model with change from baseline at the corresponding visit as the response variable, and the corresponding baseline value, treatment group, prior surgery history, region, and study indicator (SINUS‐52 = 0 and SINUS‐24 = 1) as covariates. Analysis was based on the same imputed dataset using WOCF/MI from primary analysis of the endpoint in each of the two studies (SINUS‐52 and SINUS‐24).
Each of the imputed complete data was analyzed by fitting an ANCOVA model with the corresponding baseline value, treatment group, NSAID‐ERD status, prior surgery history, region, NSAID‐ERD status‐by‐treatment interaction, and study indicator (SINUS‐52 = 0 and SINUS‐24 = 1) as covariates.
Analyzed in patients with asthma only.
As loss of smell is a cardinal symptom of CRSwNP, the impact of dupilumab on sense of smell was further examined. Among patients in the NSAID‐ERD subgroup, the proportion of anosmic patients (UPSIT ≤18) at baseline was similar in the dupilumab and placebo arms. At Week 24, significantly greater proportions of dupilumab‐treated patients improved sense of smell (UPSIT >18) versus placebo‐treated patients (60.2% vs. 11.0%, respectively; p < .0001) (Table S3). Among patients with CRSwNP without NSAID‐ERD, 76.7% of dupilumab‐treated patients versus 28.0% of placebo‐treated patients improved UPSIT >18 at Week 24 (Table S3). Among patients with anosmia at baseline, the proportions of patients who regained some sense of smell (UPSIT >18) at Week 24 were comparable in patients with and without NSAID‐ERD (35.4% vs. 44.0%, respectively; p = .1133) (Table S4).
TABLE 3.
Summary of safety over 24 weeks among patients with CRSwNP with and without NSAID‐ERD
| Patients with CRSwNP with NSAID‐ERD a | Patients with CRSwNP without NSAID‐ERD a | |||
|---|---|---|---|---|
| Patients, n (%) |
Placebo (n = 82) |
Dupilumab 300 mg q2w (n = 121) |
Placebo (n = 200) |
Dupilumab 300 mg q2w (n = 319) |
| Any TEAE | 65 (79.3) | 85 (70.2) | 143 (71.5) | 220 (69.0) |
| Any serious TEAE | 5 (6.1) | 6 (5.0) | 11 (5.5) | 9 (2.8) |
| Any TEAE leading to death | 0 | 0 | 0 | 0 |
| Any TEAE leading to permanent treatment discontinuation | 4 (4.9) | 4 (3.3) | 11 (5.5) | 7 (2.2) |
| TEAEs occurring in ≥5% of patients (MedDRA PT), n (%) | ||||
| Nasopharyngitis | 12 (14.6) | 19 (15.7) | 29 (14.5) | 36 (11.3) |
| Upper respiratory tract infection | 5 (6.1) | 2 (1.7) | NA | NA |
| Headache | 10 (12.2) | 8 (6.6) | 14 (7.0) | 24 (7.5) |
| Asthma | 10 (12.2) | 5 (4.1) | 10 (5.0) | 2 (0.6) |
| Nasal polyps | 12 (14.6) | 4 (3.3) | 21 (10.5) | 8 (2.5) |
| Arthralgia | 0 | 8 (6.6) | NA | NA |
| Acute sinusitis | NA | NA | 11 (5.5) | 5 (1.6) |
| Epistaxis | NA | NA | 16 (8.0) | 21 (6.6) |
| Injection‐site erythema | 9 (11.0) | 13 (10.7) | 13 (6.5) | 15 (4.7) |
Abbreviations: CRSwNP, chronic rhinosinusitis with nasal polyps; MedDRA, Medical Dictionary for Regulatory Activities; NA, not available; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; PT, Preferred Term; q2w, every 2 weeks; TEAE, treatment‐emergent adverse event.
One patient in each patient group (with NSAID‐ERD, without NSAID‐ERD) was randomized but not treated.
Fewer patients required rescue with SCS or sinonasal surgery with dupilumab than with placebo, irrespective of NSAID‐ERD status. In patients with NSAID‐ERD, 15/122 (12.3%) in the dupilumab group and 33/82 (40.2%) in the placebo group required rescue with SCS (hazard ratio [HR] 0.219; 95% confidence interval [CI] 0.116, 0.412; nominal p < .0001). In patients without NSAID‐ERD, SCS rescue was used for 26/316 dupilumab‐treated patients (8.2%) versus 55/204 placebo‐treated patients (27.0%) (HR 0.281; 95% CI 0.175, 0.450; nominal p < .0001) (Figure 2A). In patients with NSAID‐ERD, rescue NP surgery was performed in 2/122 patients (1.6%) in the dupilumab group and 9/82 patients (11.0%) in the placebo group (HR 0.204; 95% CI 0.044, 0.948; nominal p < .05). In patients without NSAID‐ERD, rescue NP surgery was performed in 3/316 patients (0.9%) in the dupilumab group and 13/204 patients (6.4%) in the placebo group (HR 0.162; 95% CI 0.046, 0.573; nominal p < .005) (Figure 2B).
FIGURE 2.
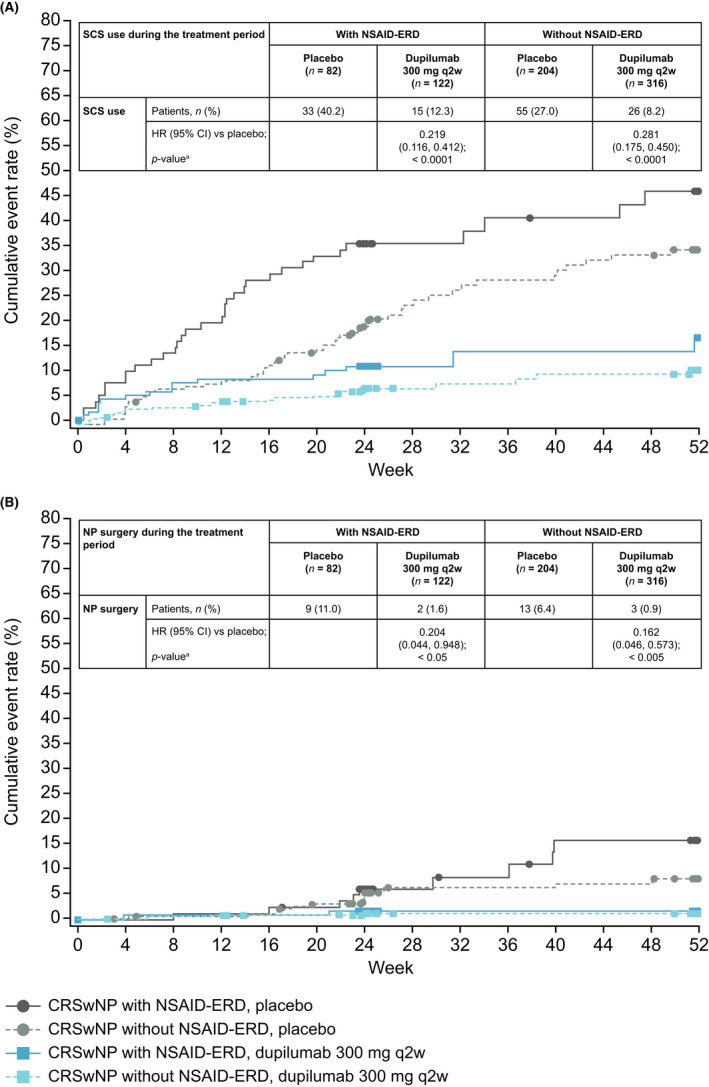
Kaplan–Meier analysis of time to first (A) SCS use and (B) NP surgery in patients with CRSwNP with and without NSAID‐ERD. Abbreviations: CI, confidence interval; CRSwNP, chronic rhinosinusitis with nasal polyps; HR, hazard ratio; NP, nasal polyp; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; q2w, every 2 weeks; SCS, systemic corticosteriods.
3.3. Changes in airway function and asthma control
Nasal airflow as assessed by PNIF was significantly improved by dupilumab treatment versus placebo in patients with CRSwNP with and without NSAID‐ERD at all time points up to Week 24 (nominal p < .0001 for all time points; Figure 3A). Treatment effect with dupilumab was greater in patients with NSAID‐ERD than those without NSAID‐ERD (overall interaction p = .0123) (Table 2).
FIGURE 3.
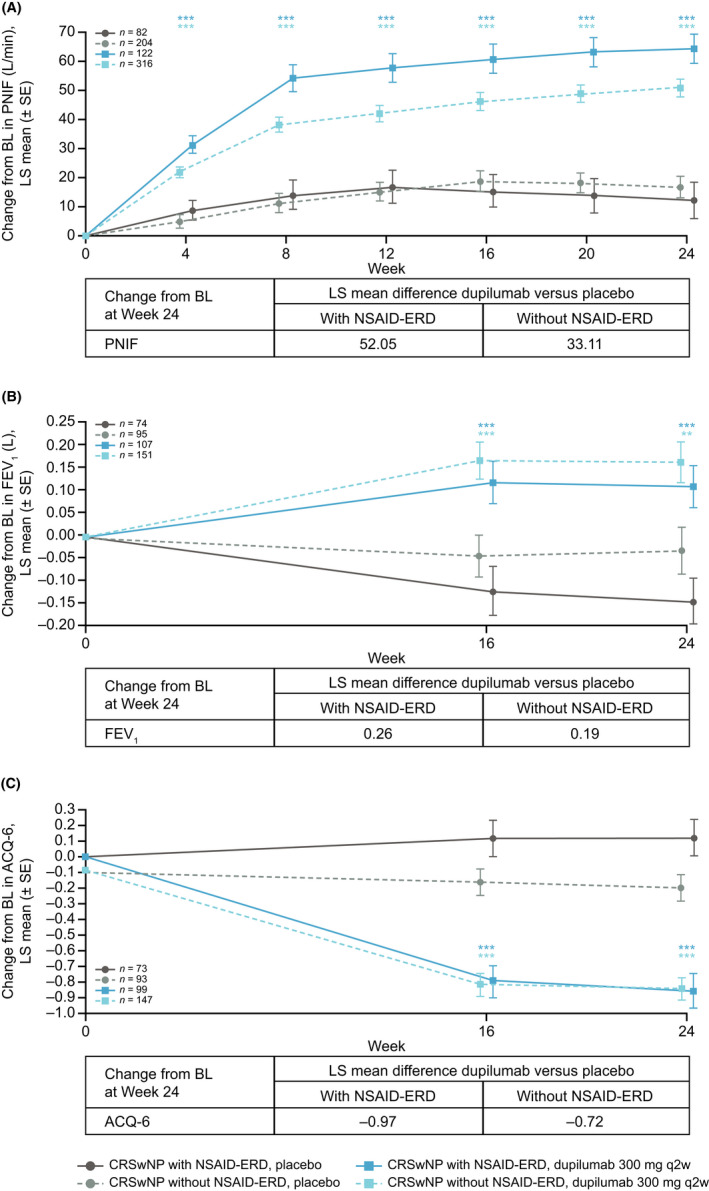
Changes from baseline to Week 24 in airway function and asthma control in patients with CRSwNP with and without NSAID‐ERD, as assessed by (A) PNIF, (B) FEV1, and (C) ACQ‐6 score. Asthma‐specific endpoints (FEV1 and ACQ‐6) were measured only in patients with a recorded history of asthma. **Nominal p =.001, ***nominal p < .0001 versus placebo. Abbreviations: ACQ‐6, six‐item Asthma Control Questionnaire; BL, baseline; CRSwNP, chronic rhinosinusitis with nasal polyps; FEV1, forced expiratory volume in 1 second; LS, least squares; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; PNIF, peak nasal inspiratory flow; q2w, every 2 weeks; SE, standard error.
In patients with CRSwNP with comorbid asthma, dupilumab treatment resulted in greater improvements in lung function (assessed by FEV1) and asthma control (assessed by ACQ‐6) than placebo, irrespective of NSAID‐ERD status (nominal p ≤ .001 for all time points in both subgroups; Figure 3B and 3C). Treatment effect with dupilumab at Week 24 did not differ in patients with asthma with or without NSAID‐ERD for FEV1 (overall interaction p = .3434) or ACQ‐6 (overall interaction p = .1630) (Table 2).
3.4. Changes in biomarkers
There were more pronounced decreases from baseline to Week 24 in the following biomarkers in dupilumab‐treated patients with NSAID‐ERD than in those without NSAID‐ERD: urinary LTE4 (median percentage change –76.2% vs. –37.2%); nasal secretion total IgE (–70.8% vs. –18.3% [SINUS‐52]); and nasal ECP (–70.6% vs. –35.3% [SINUS‐52]) (Tables S5 and S6).
Significantly (p < .01) greater reductions in serum total IgE, TARC and periostin, plasma eotaxin‐3, nasal fluid eotaxin‐3, and urinary LTE4 were observed with dupilumab treatment compared with placebo in patients with or without NSAID‐ERD. There were no significant differences in the change from baseline in blood eosinophils or nasal ECP at Week 24 between dupilumab‐treated patients versus placebo‐treated patients, irrespective of NSAID‐ERD status (Tables S5 and S6).
3.5. Safety
Dupilumab was well tolerated among patients with CRSwNP with or without NSAID‐ERD (Table 3). In patients with CRSwNP and NSAID‐ERD, adverse events (AEs) were reported in 70.2% and 79.3% of dupilumab‐treated and placebo‐treated patients, respectively. The most commonly reported TEAEs (>5%) among patients with CRSwNP and NSAID‐ERD were nasopharyngitis, injection‐site erythema, headache, and arthralgia. In patients without NSAID‐ERD, AEs were reported in 69.0% and 71.5% of dupilumab‐ and placebo‐treated patients, respectively, over the same period, and nasopharyngitis, headache, and epistaxis were the most commonly reported TEAEs (Table 3). There were no deaths. Serious AEs occurred in ≤5.0% of patients with or without NSAID‐ERD who received dupilumab—an incidence that was lower than that in the placebo group.
4. DISCUSSION
This analysis of pooled data from the SINUS‐24 and SINUS‐52 trials demonstrated that patients with CRSwNP with comorbid NSAID‐ERD had more severe disease at baseline compared with patients without NSAID‐ERD, including worse LMK‐CT, TSS, UPSIT, and daily loss of smell scores, more prior surgeries, and higher levels of some biomarkers of type 2 inflammation than those without NSAID‐ERD. Higher percentages of patients with NSAID‐ERD were anosmic, had comorbid asthma, and had reduced lung function (FEV1). These findings substantiate those of other studies which reported that patients with CRSwNP with NSAID‐ERD generally have more severe and difficult‐to‐treat disease than those who are NSAID‐ERD tolerant. 13 , 15 , 16 This analysis demonstrated that dupilumab treatment resulted in clinically relevant improvements in patient‐reported symptoms and objective measures in patients with CRSwNP and comorbid NSAID‐ERD. The improvements in NPS, computed tomorgraphy sinus opacification scores, rhinosinusitis symptoms, and sense of smell in both patients with and without NSAID‐ERD were rapid, evident at the first assessment time points (Weeks 2–4), and sustained over the study duration. Dupilumab also improved lung function and asthma control in patients with CRSwNP with NSAID‐ERD and asthma.
Although statistically significant treatment–subgroup interactions were observed for NC score, SNOT‐22 score, TSS, and PNIF outcomes in patients with CRSwNP and NSAID‐ERD compared with those without NSAID‐ERD, the treatment effect was statistically and clinically meaningful in either subgroup. It is possible that as the patients with NSAID‐ERD had more severe disease, they were more likely to perceive improvement in symptoms. Although mean improvements in NPS were not different in patients with and without NSAID‐ERD, the magnitude of increase in PNIF was greater in patients with NSAID‐ERD. This finding may be related to a greater improvement in NC in patients with NSAID‐ERD compared with those without NSAID‐ERD. Less congested and less edematous nasal passages thereby may have been translated into a better PNIF in patients with NSAID‐ERD. These results suggest that there is a benefit for the addition of dupilumab for patients with CRSwNP and NSAID‐ERD. The magnitude of treatment effects across both subgroups was generally aligned with the overall primary analysis in patients with CRSwNP. 24 Dupilumab was generally well tolerated in CRSwNP, irrespective of the presence of comorbid NSAID‐ERD.
This analysis has some limitations, which may impact the interpretation of its findings. In this post hoc analysis, endpoints were not adjusted for multiplicity. In addition, there are several interesting research questions that this study was unable to address. While the effect of dupilumab on upper and lower airway inflammation and function is clear from these data, acute exacerbation following exposure to NSAIDs—a component of the syndrome—was not assessed. It is also unknown from the findings of this study if dupilumab modulates leukotriene receptor expression or its effect on prostaglandin (PG) D2 production. PGD2 is a potent driver in NSAID‐ERD as a major recruiter of Th2 cells, eosinophils, and basophils. 28 Further clinical studies are warranted to investigate the effects of dupilumab on these aspects of the disease. Even though the diagnosis of NSAID‐ERD was based on a two‐item questionnaire established by an expert panel, is consistent with current approaches for diagnosing NSAID‐ERD, 2 , 13 and was administered by healthcare professionals, it is possible that some patients may not have understood the question about “respiratory” and “bronchial” symptoms and may have been miscategorized.
In this randomized controlled trial, patients with CRSwNP and NSAID‐ERD required frequent courses of SCS and were more likely to undergo repeated surgeries. 13 There is a need for interventions for uncontrolled severe CRSwNP that can reduce the need for SCS and NP surgery. 13 Dupilumab treatment was associated with substantial SCS‐sparing benefits, reducing the use of SCS by 78.1% and 71.9% among those with and without NSAID‐ERD, respectively. Similarly, for patients receiving dupilumab, the risk of NP surgery was reduced by 79.6% and 83.8%, respectively.
Urinary LTE4 is a marker of more severe CRSwNP, 29 levels of which are reduced after endoscopic sinus surgery. 30 As found in other studies, baseline urinary LTE4 levels were significantly greater in patients with NSAID‐ERD than in patients without NSAID‐ERD, with greater reductions from baseline observed with dupilumab treatment in patients with NSAID‐ERD versus those without NSAID‐ERD. The greater reduction in patients with NSAID‐ERD might be accounted for by differences in the contribution of IL‐4Rα signaling in these patients. IL‐4Rα signaling inhibits PG E2 production, resulting in increased constitutive production of cysteinyl leukotrienes and increased excretion of LTE4 in the urine. By inhibiting IL‐4/IL‐13, dupilumab lowers production of LTE4, as reflected by a greater decrease in urinary LTE4 in patients with NSAID‐ERD than without. 12 This more dramatic reduction in urinary LTE4 levels might explain the differences in the magnitude of clinical response observed for some endpoints; however, the exact mechanisms remain unexplained. A small study of 10 patients in clinical practice has also demonstrated a reduction in urinary LTE4 levels with dupilumab in patients with uncontrolled CRSwNP and comorbid NSAID‐ERD. 31 Reductions in type 2 biomarkers both in systemic circulation (total IgE, TARC, eotaxin‐3, and periostin) and locally in nasal secretions (eotaxin‐3) observed in these studies were consistent with the mode of action of dupilumab and with previous dupilumab studies in asthma and atopic dermatitis. 8
Dupilumab was generally well tolerated in patients with CRSwNP with and without NSAID‐ERD, with nasopharyngitis being the most common TEAE. The safety data were consistent with those of the primary analysis and studies of dupilumab in other patient populations, 24 , 32 , 33 , 34 although patients with CRSwNP and NSAID‐ERD who received dupilumab had an increased incidence of arthralgia compared with patients without NSAID‐ERD (6.6% vs. 0%, respectively). There have been other reports of arthralgia experienced in patients receiving dupilumab outside of clinical trials. 35 , 36 The exact mechanisms of these AEs are unclear. Another adverse drug reaction previously observed with dupilumab in patients with uncontrolled asthma is increased levels of eosinophils; these increases were transient and resolved to baseline levels at 1 year after starting treatment. 34 In the current study, increases in eosinophils were not observed in the subgroup with no clinical diagnosis of NSAID‐ERD, but a median increase of 40% from baseline was reported among the subgroup of patients with NSAID‐ERD at Week 24. The exact reasons for such a differential response between the groups are unclear. However, it is important to note that the median percentage increase in eosinophil counts observed in the dupilumab‐treated group was statistically not different from that of the placebo‐treated group, irrespective of NSAID‐ERD status, and eosinophil levels returned to baseline by Week 52 in patients with and without NSAID‐ERD (data not shown). Increase in blood eosinophil levels is consistent with the hypothesis that by IL‐4 and IL‐13 blockade, dupilumab suppresses the production of eotaxin‐3 and vascular cell adhesion molecule (VCAM)‐1, and inhibition of trafficking of eosinophils from blood into tissues, but not the production and release of eosinophils from bone marrow, thereby resulting in the observed transient increase in circulating eosinophils — a finding consistent with other clinical studies of dupilumab. 24 , 34
5. CONCLUSIONS
Patients with CRSwNP and comorbid NSAID‐ERD are the most severe and difficult‐to‐treat subset of patients with CRSwNP, with high rates of NP recurrence and high disease burden. Dupilumab improved symptoms, endoscopic and radiologic outcomes, HRQoL, and airway function, and suppressed underlying type 2 inflammation compared with placebo in patients with CRSwNP and NSAID‐ERD. A greater improvement was observed with dupilumab for some outcomes in patients with comorbid NSAID‐ERD compared with those without. Dupilumab was generally well tolerated in patients with CRSwNP with and without NSAID‐ERD. These observations suggest that even greater consideration should be given to the use of dupilumab as a treatment option in the population of patients with CRSwNP and NSAID‐ERD.
CONFLICTS OF INTEREST
J. Mullol reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study and grants, personal fees, and other from ALK‐Abelló, AstraZeneca, Genentech, GlaxoSmithKline, Glenmark, Menarini, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mylan‐Meda Pharmaceuticals (Viatris), Novartis, Proctor & Gamble, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, UCB Pharma, and Uriach Group, outside the submitted work.
T.M. Laidlaw reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study and other from AstraZeneca, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, Inc., and Sanofi‐Aventis, outside the submitted work.
C. Bachert reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study and personal fees and other from ALK, AstraZeneca, GlaxoSmithKline, Mylan, Novartis, Sanofi, and Stallergenes Greer, outside the submitted work.
L.P. Mannent reports personal fees, non‐financial support, and other from Sanofi; and non‐financial support from Regeneron Pharmaceuticals, Inc., during the conduct of the study.
G.W. Canonica reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study and personal fees and other from ALK, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, HAL Allergy, Menarini, Mundipharma, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi, Stallergenes Greer, and Uriach Group, outside the submitted work.
J.K. Han reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study; and other from Sanofi, outside the submitted work.
J.F. Maspero reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study; grants and personal fees from Novartis; and personal fees from AstraZeneca, GlaxoSmithKline, Menarini, Sanofi, Teva, and Uriach Group, outside the submitted work.
C. Picado reports non‐financial support from Regeneron Pharmaceuticals, Inc. and Sanofi, during the conduct of the study; grants, personal fees, and other from Novartis; and personal fees from AstraZeneca, GlaxoSmithKline and Menarini, outside the submitted work.
N. Daizadeh reports personal fees and non‐financial support from Sanofi; and non‐financial support from Regeneron Pharmaceuticals, Inc., during the conduct of the study.
B. Ortiz reports personal fees, non‐financial support, and other from Regeneron Pharmaceuticals, Inc.; and non‐financial support from Sanofi, during the conduct of the study.
Y. Li reports personal fees, non‐financial support, and other from Sanofi; and non‐financial support from Regeneron Pharmaceuticals, Inc., during the conduct of the study.
M. Ruddy reports personal fees, non‐financial support, and other from Regeneron Pharmaceuticals, Inc.; and non‐financial support from Sanofi, during the conduct of the study.
E. Laws reports personal fees, non‐financial support, and other from Sanofi; and non‐financial support from Regeneron Pharmaceuticals, Inc., during the conduct of the study.
N. Amin reports personal fees, non‐financial support, and other from Regeneron Pharmaceuticals, Inc.; and non‐financial support from Sanofi, during the conduct of the study.
AUTHOR CONTRIBUTIONS
Joaquim Mullol, Claus Bachert, and Leda P. Mannent conceived and designed the study, performed experiments, and analyzed data. Tanya M. Laidlaw, G. Walter Canonica, Joseph K. Han, and Jorge F. Maspero performed experiments and analyzed data. Nikhil Amin conceived and designed the study and analyzed data. Cesar Picado, Nadia Daizadeh, Benjamin Ortiz, Yongtao Li, Marcella Ruddy, and Elizabeth Laws performed data analysis. All authors revised and approved the final version.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
Research sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. ClinicalTrials.gov Identifiers: NCT02912468 (SINUS‐24) and NCT02898454 (SINUS‐52). Full trial protocols for SINUS‐24 and SINUS‐52 available on ClinicalTrials.gov. Medical writing/editorial assistance provided by Peter Tran, PhD, of Adelphi Group, funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Mullol J, Laidlaw TM, Bachert C, et al. Efficacy and safety of dupilumab in patients with uncontrolled severe chronic rhinosinusitis with nasal polyps and a clinical diagnosis of NSAID‐ERD: Results from two randomized placebo‐controlled phase 3 trials. Allergy.2022;77:1231–1244. 10.1111/all.15067
Data Availability Statement
Qualified researchers may request access to data and related study documents. Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial patients. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.
REFERENCES
- 1. Bachert C, Marple B, Schlosser RJ, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers 2020;6:86. [DOI] [PubMed] [Google Scholar]
- 2. Fokkens WJ, Lund VJ, Hopkins C, et al. Epos 2020: European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020;58:1‐464. [DOI] [PubMed] [Google Scholar]
- 3. Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract 2016;4:565‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klonaris D, Doulaptsi M, Karatzanis A, Velegrakis S, Milioni A, Prokopakis E. Assessing quality of life and burden of disease in chronic rhinosinusitis: A review. Rhinology Online 2019;2:6‐13. [Google Scholar]
- 5. Bachert C, Han JK, Wagenmann M, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J Allergy Clin Immunol 2021;147:29‐36. [DOI] [PubMed] [Google Scholar]
- 6. Boztepe F, Ural A, Paludetti G, De Corso E. Pathophysiology of chronic rhinosinusitis with nasal polyps. In: Cingi C, Bayar Muluk N, eds. All Around The Nose. Springer; 2020: pp 333‐337. [Google Scholar]
- 7. Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin‐exacerbated respiratory disease. Am J Respir Crit Care Med 2015;192:682‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro‐inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy 2019;74:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Workman AD, Kohanski MA, Cohen NA. Biomarkers in chronic rhinosinusitis with nasal polyps. Immunol Allergy Clin North Am 2018;38:679‐692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin‐exacerbated respiratory disease among asthmatic patients: A meta‐analysis of the literature. J Allergy Clin Immunol 2015;135:676‐681.e671. [DOI] [PubMed] [Google Scholar]
- 11. White AA, Doherty TA. Role of group 2 innate lymphocytes in aspirin‐exacerbated respiratory disease pathogenesis. Am J Rhinol Allergy 2018;32:7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinke JW, Payne SC, Borish L. Eosinophils and mast cells in aspirin‐exacerbated respiratory disease. Immunol Allergy Clin North Am 2016;36:719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowalski ML, Agache I, Bavbek S, et al. Diagnosis and management of NSAID‐exacerbated respiratory disease (N‐ERD)‐A EAACI position paper. Allergy 2019;74:28‐39. [DOI] [PubMed] [Google Scholar]
- 14. Laidlaw TM, Boyce JA. Aspirin‐exacerbated respiratory disease–new prime suspects. N Engl J Med 2016;374:484‐488. [DOI] [PubMed] [Google Scholar]
- 15. Schneider S, Campion NJ, Villazala‐Merino S, et al. Associations between the quality of life and nasal polyp size in patients suffering from chronic rhinosinusitis without nasal polyps, with nasal polyps or aspirin‐exacerbated respiratory disease. J Clin Med 2020;9(4):925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens WW, Peters AT, Hirsch AG, et al. Clinical characteristics of patients with chronic rhinosinusitis with nasal polyps, asthma, and aspirin‐exacerbated respiratory disease. J Allergy Clin Immunol Pract 2017;5:1061‐1070.e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryan L, Segarra D, Tabor M, Parasher A. Systematic review of outcomes for endoscopic sinus surgery and subsequent aspirin desensitization in aspirin‐exacerbated respiratory disease. World J Otorhinolaryngol Head Neck Surg 2020;6:220‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams AN, Simon RA, Woessner KM, Stevenson DD. The relationship between historical aspirin‐induced asthma and severity of asthma induced during oral aspirin challenges. J Allergy Clin Immunol 2007;120:273‐277. [DOI] [PubMed] [Google Scholar]
- 19. Levy JM, Smith TL. Is aspirin desensitization indicated for the treatment recalcitrant chronic rhinosinusitis with nasal polyposis in aspirin‐exacerbated respiratory disease? Laryngoscope 2017;127:776‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stevens WW, Jerschow E, Baptist AP, et al. The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin‐exacerbated respiratory disease: A Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2021;147:827‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macdonald LE, Karow M, Stevens S, et al. Precise and in situ genetic humanization of 6 Mb of mouse immunoglobulin genes. Proc Natl Acad Sci U S A 2014;111:5147‐5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci 2014;111:5153‐5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL‐4/IL‐13 pathway in atopic diseases. Expert Rev Clin Immunol 2017;13:425‐437. [DOI] [PubMed] [Google Scholar]
- 24. Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS‐24 and LIBERTY NP SINUS‐52): Results from two multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group phase 3 trials. Lancet 2019;394:1638‐1650. [DOI] [PubMed] [Google Scholar]
- 25. Chong LY, Piromchai P, Sharp S, et al. Biologics for chronic rhinosinusitis. Cochrane Database Syst Rev 2020;29(2):CD013513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DUPIXENT® (dupilumab). Prescribing information, Regeneron Pharmaceuticals. Revised June 2020. Accessed November 24, 2020. https://www.regeneron.com/sites/default/files/Dupixent_FPI.pdf
- 27.DUPIXENT® (dupilumab). Summary of product characteristics, Regeneron Pharmaceuticals. Revised June 2020. Accessed November 24, 2020. https://www.ema.europa.eu/en/documents/product‐information/dupixent‐epar‐product‐information_en.pdf
- 28. Kariyawasam HH, James LK, Gane SB. Dupilumab: Clinical efficacy of blocking IL‐4/IL‐13 signalling in chronic rhinosinusitis with nasal polyps. Drug Des Devel Ther 2020;14:1757‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choby G, O'Brien EK, Smith A, et al. Elevated urine leukotriene E4 is associated with worse objective markers in nasal polyposis patients. Laryngoscope 2020;131(5):961‐966. doi: 10.1002/lary.29137. [DOI] [PubMed] [Google Scholar]
- 30. Jerschow E, Edin ML, Chi Y, et al. Sinus surgery is associated with a decrease in aspirin‐induced reaction severity in patients with aspirin exacerbated respiratory disease. J Allergy Clin Immunol Pract 2019;7:1580‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mustafa SS, Vadamalai K, Scott B, Ramsey A. Dupilumab as add‐on therapy for chronic rhinosinusitis with nasal polyposis in aspirin exacerbated respiratory disease. Am J Rhinol Allergy 2021;35(3):399‐407. doi: 10.1177/1945892420961969. [DOI] [PubMed] [Google Scholar]
- 32. Hirano I, Dellon ES, Hamilton JD, et al. Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 2020;158:111‐122.e110. [DOI] [PubMed] [Google Scholar]
- 33. Rabe KF, Nair P, Brusselle G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med 2018;378:2475‐2485. [DOI] [PubMed] [Google Scholar]
- 34. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate‐to‐severe uncontrolled asthma. N Engl J Med 2018;378:2486‐2496. [DOI] [PubMed] [Google Scholar]
- 35. de Wijs LEM, van der Waa JD, de Jong PHP, Hijnen DJ. Acute arthritis and arthralgia as an adverse drug reaction to dupilumab. Clin Exp Dermatol 2020;45:262‐263. [DOI] [PubMed] [Google Scholar]
- 36. Willsmore ZN, Woolf RT, Hughes C, et al. Development of inflammatory arthritis and enthesitis in patients on dupilumab: A case series. Br J Dermatol 2019;181:1068‐1070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Qualified researchers may request access to data and related study documents. Patient‐level data will be anonymized, and study documents will be redacted to protect the privacy of trial patients. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com.


