Abstract
Purpose
To determine the risk of patients with an early diagnosis of heritable retinoblastoma being diagnosed with TRb (or pineoblastoma) asynchronously in a later stage and its effect on screening.
Methods
We updated the search (PubMed and Embase) for published literature as performed by our research group in 2014 and 2019. Trilateral retinoblastoma (TRb) patients were eligible for inclusion if identifiable as unique and the age at which TRb was diagnosed was available. The search yielded 97 new studies. Three new studies and eight new patients were included. Combined with 189 patients from the previous meta‐analysis, the database included 197 patients. The main outcome was the percentage of asynchronous TRb in patients diagnosed before and after preset age thresholds of 6 and 12 months of age at retinoblastoma diagnosis.
Results
Seventy‐nine per cent of patients with pineoblastoma are diagnosed with retinoblastoma before the age of 12 months. However, baseline MRI screening at time of retinoblastoma diagnosis fails to detect the later diagnosed pineal TRb in 89% of patients. We modelled that an additional MRI performed at the age of 29 months picks up 53% of pineoblastomas in an asymptomatic phase. The detection rate increased to 72%, 87% and 92%, respectively, with 2, 3 and 4 additional MRIs.
Conclusions
An MRI of the brain in heritable retinoblastoma before the age of 12 months misses most pineoblastomas, while retinoblastomas are diagnosed most often before the age of 12 months. Optimally timed additional MRI scans of the brain can increase the asymptomatic detection rate of pineoblastoma.
Keywords: retinoblastoma, magnetic resonance imaging, MRI, trilateral retinoblastoma, PNET, pineoblastoma, pineal gland
Introduction
Unilateral or bilateral heritable retinoblastoma associated with midline intracranial neuroectodermal tumour is known as trilateral retinoblastoma (TRb) (Soliman et al., 2017a). Survival from TRb has increased considerably in the last decades from practically nil to about 50% (de Jong et al. 2014). Early‐detected small TRb has a more favourable prognosis and, when diagnosed at the same time as the eye tumour, is typically in the supra‐ and parasellar region (de Jong et al. 2014). The more common pineal location of TRb accounts for three quarters of all patients. Pineal TRb or pineoblastoma has an incidence of about 3% in patients with heritable retinoblastoma and often is diagnosed later, up to the age of 40 months (de Jong et al. 2015; de Jong et al. 2020).
The Canadian retinoblastoma management guidelines (Canadian‐Retinoblastoma‐Society 2009) recommended initial magnetic resonance imaging (MRI) of the brain at diagnosis only, and state ‘Children with germline RB1 mutations are predisposed to TRb. However, considering its rarity, repeated screening for TRb by MRI of the head and orbits after the first negative MRI is not practical in Canada today’. Baseline MRI is performed for staging the ocular tumours and generally includes imaging of the pineal and sellar area. Because of our recent experience of two children with retinoblastoma and an unremarkable pineal gland at baseline in which asynchronous pineal TRb developed, we explored the possibilities of detecting asymptomatic TRb.
We updated our database of TRb (de Jong et al. 2014; de Jong et al. 2020) with new published cases and performed a meta‐analysis, in an effort to explain the apparently preferential occurrence in these two patients of asynchronous pineal TRb when retinoblastoma was diagnosed at a relatively young age with an initially normal MRI. We analysed and modelled the possible implications of our findings for TRb screening.
Methods
We performed this study according to the EQUATOR (enhancing the quality and transparency of health research) reporting guidelines, including meta‐analysis of observational studies in epidemiology (MOOSE), a proposal for reporting (Stroup et al. 2000). This study adhered to the Declaration of Helsinki. The ethics committee (METc VUmc) approved this study with a waiver of informed consent.
Data sources and study selection
For this study, we updated the search for English, Dutch and German literature for patients with TRb as performed for the 2014 and the 2019 meta‐analyses by De Jong et al. (de Jong et al. 2014; de Jong et al. 2020) with a new search (PubMed and Embase) performed on 13 April 2020 (Appendix A, constructed by MCJ with 10 years of experience in conducting systematic reviews and meta‐analyses). Only published original articles were qualified. Reference lists of eligible articles were also checked for additional articles. We ensured sensitivity of the search strategy by including only terms that describe the target disease (Appendix A). Newly published articles since the previous update and the 2 cases we present in this article (Figs 1 and 2) were included. Two authors (MCJ and RWJ) selected and included studies independently in a similar fashion as in the previous meta‐analyses (de Jong et al. 2014; de Jong et al. 2020). Discrepancies were resolved by consensus.
Fig. 1.
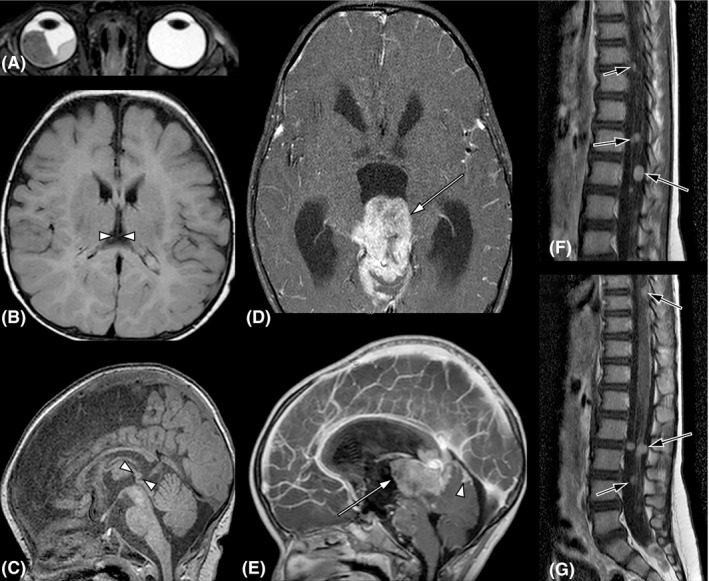
Magnetic resonance images (MRI) of Case 1. A 3.5‐month‐old child presented with bilateral retinoblastoma (cT2a and cT1a by 8th edition TNM) (Mallipatna et al. 2017). Baseline MRI performed on the day of diagnosis of bilateral retinoblastoma. Note a large (cT2a) tumour (14 × 14 × 9 mm) in the right eye (A, T2‐weighted image), while the tumours in the left eye were small (cT1a) and not visible. The midline brain MRI showed a normal 5 × 4 mm pineal gland (B and C, between arrowheads, T1‐weighted non‐contrast‐enhanced images) and no suprasellar mass. The right eye was enucleated and revealed no high‐risk histopathologic features (Sastre et al. 2009). Tumours in the left eye were controlled focally with OCT‐guided laser (Soliman et al. 2017b, 2018). Systemic chemotherapy was not required. The child was examined under anaesthesia (EUA) every 3–4 weeks for 8 months, when the follow‐up intervals were extended to 6 weeks for 6 months then to 8 weeks for 6 months. Twenty months from diagnosis at the age of 23 months, the routine EUA was postponed because of recent vomiting, suspected to be related to a viral infection. Intermittent vomiting persisted despite medical therapy, and after 10 days, the child was found unconscious and brought to emergency care. An urgent MRI of the brain revealed a large pineoblastoma showed a large enhancing pineal mass (37 × 30 × 26 mm) (D and E, arrow, T1‐weighted contrast‐enhanced images) invading the midbrain tectum and tegmentum anteriorly, the superior cerebellum posteriorly, and extending into the aqueduct and the third ventricle superiorly, resulting in acute triventricular hydrocephalus. Tumour deposits cranially cover the cerebellum (E, arrowhead). Multiple nodular drop metastases cover the brainstem, and numerous enhancing nodular metastases were observed along the spinal cord and cauda equina nerve roots (F and G, arrows, T1‐weighted contrast‐enhanced images). The child died two days later.
Fig. 2.
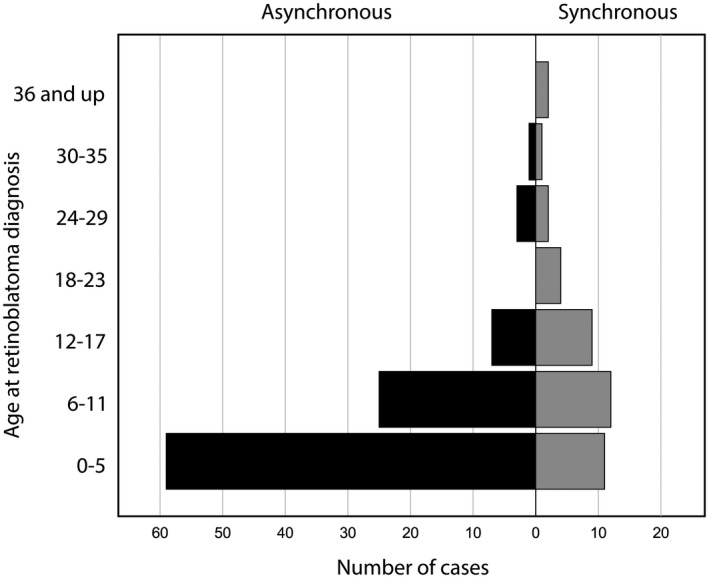
Number of cases with synchronous versus asynchronous (true and surrogate) asymptomatic pineoblastoma by age at diagnosis of retinoblastoma.
Data extraction
Two authors (MCJ and RWJ) extracted data independently to update the entire TRb database (Appendix B) as in the previous studies (de Jong et al. 2014; de Jong et al. 2020). Patients were included only if identifiable as unique and the age at which TRb was diagnosed was available. Overlap between patients was identified using all available data in included studies (such as age at diagnosis, gender and hospital where patient was treated); if uncertainty remained, only one was included. Discrepancies were resolved by consensus.
Data synthesis
The primary objective was to compare the percentage of asynchronous TRb before and after a threshold of 6 and 12 months of age at which retinoblastoma was diagnosed. We defined a synchronous pineal TRb as one diagnosed within (≤) 3 months of retinoblastoma.
As a secondary objective, we calculated relative risk (RR) of developing a pineoblastoma for early (<12 months: the exposed group) versus later diagnosis of retinoblastoma (≥12 months: the unexposed group) using the incidence numbers of bilateral retinoblastoma for the total exposed and unexposed of two published cohorts. To estimate the incidence of bilateral retinoblastoma (as a surrogate for heritable retinoblastoma) by age, we used data from the United Kingdom (MacCarthy et al. 2009) and the United States (Broaddus et al. 2009). We first attempted to compare the percentage of familial cases in each population, because familial cases are screened since birth, and generally, their retinoblastoma is diagnosed earlier than in non‐familial cases, and this can result in a bias.
For further analyses, to increase the relatively low number of asymptomatic pineal TRb patients in the database, we calculated a surrogate age at diagnosis of asymptomatic pineal TRb by subtracting the average lead time of 12 months from the age at diagnosis of symptomatic pineal TRb (de Jong et al. 2020). If the difference between retinoblastoma and pineoblastoma diagnoses was less than 12 months, we assumed that pineoblastoma would be diagnosed at the same age as retinoblastoma, because an asymptomatic diagnosis of pineoblastoma is unlikely before retinoblastoma diagnosis. To visualize how many patients could potentially be diagnosed on a baseline MRI (synchronous TRbs), we plotted the age at diagnosis of the asymptomatic and surrogate asymptomatic pineoblastoma cases against the age at retinoblastoma diagnosis.
We constructed a model to determine the optimal timing of additional MRI scans of the brain after baseline MRI. We included all asynchronous asymptomatic and surrogate asymptomatic pineal TRb cases. For each month, an additional MRI is performed to determine in which ‘state’ each case would fall: (1) MRI detects no pineoblastoma (i.e. the age at diagnosis TRb is more than 3 months in the future), (2) MRI detects early asymptomatic pineoblastoma (i.e. if the age at diagnosis of TRb ranges from 3 months in the future to 6 months in the past), (3) MRI detects late asymptomatic (i.e. if the age at TRb diagnosis is 6–12 months in the past), and (4) patients presents with symptoms before MRI (i.e. if the age at TRb diagnosis is 12 months or more in the past). In case more than 1 additional MRI scan is performed, the ‘state’ of each case was assigned to the month where the 1st additional MRI scan is performed. We assume that on average 3 months before the age at diagnosis of asymptomatic pineoblastoma, an MRI scan might already be able to diagnose the disease. We made this assumption because the estimated lead time of 12 months between diagnosis of asymptomatic and symptomatic pineoblastoma is not the period between when the tumour starts developing and the onset of symptoms, and probably also not between the earliest possible moment that it might be diagnosed with MRI and the onset of symptoms. The size of reported asymptomatic pineal TRb diagnosed since 1995 was n = 22 (IQR: from 11 to 16 mm; median: 13 mm); therefore, especially the larger tumours might be detectable earlier than they were diagnosed (de Jong et al. 2020). Sensitivity analysis was performed to test this assumption.
We used IBM SPSS Statistics (version 26) and MedCalc (version 19.2) for statistical analyses. Microsoft Excel (2016 version) was used for modelling. Fisher’s exact test was used to compare groups.
Risk of bias and study quality
We updated the assessment risk of bias and methodological quality of each newly included article with the checklist proposed by Murad et al. (2018) scored independently by two authors (MCJ and RWJ). Discrepancies were resolved by consensus.
Results
Inclusion of new studies and patients
The updated search gave 3 new article inclusions (Abdelbaki et al. 2020; Farouk Sait et al. 2020; Qureshi et al. 2020) all from the same institution, combined with the two patients presented in Figure 1 and Appendix C. This resulted in the inclusion of 8 new patients and updated information on 4 already included patients (see Appendices B and D). The database included a total of 197 patients.
Prevalence of asynchronous pineoblastoma
The median age at diagnosis of asymptomatic pineoblastoma was 16 months (interquartile range [IQR] 10–32) and 20 months (IQR 12–30) when also including the surrogate asymptomatic patients. Conversely, the median age at diagnosis of asymptomatic non‐pineal trilateral retinoblastoma is 10 months (IQR 6–24), similar to symptomatic non‐pineal trilateral retinoblastoma at 8 months (IQR 4–17).
Of 136 pineoblastomas, 27 (20%) were synchronous, whereas the remaining 109 (80%) developed asynchronous pineoblastoma (Table 1). Stratifying for age of retinoblastoma diagnosis (<6 months versus ≥6 months) showed that of the 70 cases diagnosed <6 months of age, only 5 (7%) had synchronous pineoblastoma, while of the 66 cases diagnosed ≥6 months, 22 (34%) had synchronous pineoblastoma (p = 0.0002). Moving the cut‐off to 12 months showed a similar pattern: of the 107 cases diagnosed with retinoblastoma <12 months of age, 12 (11%) had synchronous pineoblastoma, while of the 29 cases diagnosed ≥12 months of age, 15 (52%) had synchronous pineoblastoma (p < 0.0001; Table 1).
Table 1.
Frequency of synchronous (concurrent) versus asynchronous (metachronous) pineoblastoma.
| Pineoblastoma with retinoblastoma diagnosed at | Synchronous (≤3 months) | Asynchronous (>3 months) | Total | p‐value* |
|---|---|---|---|---|
| <6 months | 5 (7%) | 65 (93%) | 70 | 0.0002 |
| ≥6 months | 22 (33%) | 44 (67%) | 66 | |
| <12 months | 12 (11%) | 95 (89%) | 107 | <0.0001 |
| ≥12 months | 15 (52%) | 14 (48%) | 29 | |
| Total | 27 (20%) | 109 (80%) | 136 |
Dx = diagnosis, Rb = retinoblastoma.
Fisher’s exact test (2‐sided).
Even after subtracting 1 year from the age at diagnosis of pineoblastoma – the estimated lead‐time bias from an asymptomatic to a symptomatic TRb – most patients would have not been diagnosed with a pineoblastoma at baseline (see Figure 2 and Appendix E).
Of 136 pineoblastoma patients, 107 (79%) were diagnosed with retinoblastoma before the age of 12 months versus 29 (21%) after (Table 1). Restricting to familial retinoblastoma of the 49 pineoblastomas, 46 (94%) was diagnosed with retinoblastoma before the age of 12 months versus only 3 (6%) after.
The percentage of familial retinoblastoma in patients with bilateral retinoblastoma in the British cohort (MacCarthy et al. 2009) was 35% (205/581), similar to the percentage in our pineoblastoma database with 38% (39/104) of bilateral retinoblastoma patients (p = 0.66). When also including unilateral cases from our database, the percentage of familial cases was also quite similar with 36% (49/136) compared with the British cohort (p = 0.92). The percentage of familial patients in the American cohort (Broaddus et al. 2009) was not reported. The RR of being diagnosed with asynchronous pineoblastoma was 3.3–3.7 for patients diagnosed with retinoblastoma at <12 months versus ≥12 months of age. Restricting the analysis to patients with bilateral retinoblastoma resulted in an RR of 3.1–3.5 (Table 2).
Table 2.
Relative risk of developing a pineoblastoma when retinoblastoma is diagnosed <12 months of age versus ≥12 months of age.
| Source | Rb Dx <12 months | Rb Dx ≥12 months |
|---|---|---|
| Incidence of bilateral Rb | ||
| United Kingdom (1963–2002) 1 | 376 (A + B)*x | 205 (C + D)*x |
| SEER registry of the United States (1983–2004) 2 | 118 (A + B)*x | 58 (C + D)*x |
| Patients with pineal TRb | ||
| With uni‐ or bilateral Rb 3 | 107 (A) | 29 (C) |
| With bilateral Rb 3 | 84 (A) | 20 (C) |
| Only patients with asynchronous pineal TRb | ||
| With uni‐ or bilateral Rb 3 | 95 (A) | 14 (C) |
| With bilateral Rb 3 | 76 (A) | 12 (C) |
| Only patients with symptomatic pineal TRb | ||
| With uni‐ or bilateral Rb 3 | 58 (A) | 12 (C) |
| With bilateral Rb 3 | 50 (A) | 9 (C) |
| Relative risk* (95% CI) | p‐value | |
|---|---|---|
| Patients with synchronous and asynchronous pineal TRb | ||
| United Kingdom (1963–2002) 1 + all pTRb patients 3 | 2.0 (1.3–3.1) | 0.0020 |
| SEER registry of the United States (1983–2004) 2 + all pTRb patients | 1.8 (1.1–3.0) | 0.024 |
| United Kingdom (1963–2002) 1 + pTRb with only bilateral Rb 3 | 2.3 (1.4–3.8) | 0.0017 |
| SEER registry of the United States (1983–2004) 2 + pTRb with only bilateral Rb | 2.1 (1.2–3.7) | 0.014 |
| Only patients with asynchronous pineal TRb | ||
| United Kingdom (1963–2002) 1 + asynchronous pTRb patients 3 | 3.7 (2.1–6.6) | <0.0001 |
| SEER registry of the United States (1983–2004) 2 + asynchronous pTRb patients 3 | 3.3 (1.8–6.3) | 0.0002 |
| United Kingdom (1963–2002) 1 + asynchronous pTRb with only bilateral Rb | 3.5 (1.8–6.4) | 0.0001 |
| SEER registry of the United States (1983–2004) 2 + asynchronous pTRb with only bilateral Rb 3 | 3.1 (1.6–6.2) | 0.0012 |
| Only patients with symptomatic pineal TRb | ||
| United Kingdom (1963–2002) 1 + symptomatic pTRb patients 3 | 2.6 (1.4–5.0) | 0.0032 |
| SEER registry of the United States (1983–2004) 2 + symptomatic pTRb patients 3 | 2.4 (1.4–4.8) | 0.015 |
| United Kingdom (1963–2002) 1 + symptomatic pTRb with only bilateral Rb 3 | 3.0 (1.5–6.3) | 0.0029 |
| SEER registry of the United States (1983–2004) 2 + symptomatic pTRb with only bilateral Rb 3 | 2.7 (1.3–5.9) | 0.011 |
Rb = retinoblastoma, TRb = trilateral retinoblastoma, Dx = diagnosis, 95% CI = 95% confidence interval
A = number with a positive outcome (pineoblastoma) in patients diagnosed with Rb <12 months (exposed),
B = number with a positive outcome (pineoblastoma) in patients diagnosed with Rb ≥12 months (unexposed),
C = number with a negative outcome (no pineoblastoma) in patients diagnosed with Rb <12 months (exposed),
D = number with a negative outcome (no pineoblastoma) in patients diagnosed with Rb ≥12 months (unexposed),
x represents an unknown cohort‐specific constant to account for the difference in the size of the cohorts.
MacCarthy et al. (2009).
Broaddus et al. (2009).
DeJong et al. (2014)
Because the relative risk can be expressed as a ratio in which the unknown constant × cancels out, only the ratio of the exposed to the unexposed groups from the published incidence data remains in the formula:
A model for optimal timing of additional MRI examinations after the baseline MRI
One additional MRI performed at the age of 29 months is able to pick up 53% (95% confidence interval [CI] 41%–65%) of pineoblastomas in an asymptomatic phase and 35% (95% CI 24%–47%) of pineoblastoma in an early asymptomatic phase (Appendix F).
The optimal detection rate of asymptomatic pineoblastoma increases to 72% (95% CI 60%–82%), 87% (95% CI 77%–93%) and 92% (95% CI 83%–97%), respectively, for a series of 2, 3 and 4 four additional MRI scans at 20, 19 and 18 months of age for the first additional MRI scan (Appendix F); the subsequent scans are all performed with 9‐month intervals, and this does not include the baseline MRI scan. Restricting to only early asymptomatic pineoblastoma, the optimal detection rates were 57% (95% CI 45%–69%), 80% (95% CI 69%–88%) and 89% (95% CI 80%–95%), respectively, for a series of 2, 3 and 4 four additional MRI scans at 19, 15 and 15 months of age for the first additional MRI scan (Appendix F).
Sensitivity analysis shows a decrease in the optimal detection rate to 25% (95% CI 16%–37%), which was at 29 months of age, when we define early asymptomatic detection as 0 months before to 6 months after asymptomatic pineoblastoma diagnosis, and it shows an increase in the optimal detection rate to 44% (95% CI 33%–56%) at 23 months of age when early asymptomatic detection is defined as 6 months before to 6 months after asymptomatic pineoblastoma diagnosis (Appendix G).
Risk of bias and study quality
In line with previous results, the new inclusions did not fulfil the first criterion in the quality checklist (Appendix H), indicating that they likely reported patients who were interesting and did not necessarily present the entire experience the authors had with TRb.
Discussion
Seventy‐nine per cent of pineoblastomas were diagnosed in children who were diagnosed with retinoblastoma <12 months of age. However, children with early‐diagnosed retinoblastoma <12 months of age were most often diagnosed with asynchronous pineoblastoma (89% of cases) versus in 48% of children diagnosed with retinoblastoma >12 months of age. The RR of pineoblastoma to be asynchronous is three times as high when a retinoblastoma is diagnosed at <12 months of age rather than later.
The majority of pineoblastomas thus seem to develop asynchronously and relatively late compared with the retinoblastoma, even considering lead‐time bias, so that they often cannot be detected concurrently with a retinoblastoma diagnosed at a young age. Moreover, a large majority of retinoblastoma patients who develop pineoblastoma would not be detected by the baseline MRI. However, we do still strongly recommend performing imaging of the brain at the time of the baseline MRI for retinoblastoma to detect the small percentage of synchronous pineoblastomas and the often‐synchronous non‐pineal trilateral retinoblastomas. Furthermore, a baseline pineal gland scan is useful for future MR imaging comparisons.
We presented a model showing the potential benefit of 1 or more optimally timed additional MRI scans performed after the baseline MRI scan in terms of detection level of asymptomatic pineoblastoma. We modelled that a single additional MRI at the age of 29 months of age can increase the detection rate of an asymptomatic pineoblastoma to up to 53%. The detection rate of 2, 3 and 4 additional MRI scans increases to 72%, 87% and 92%, respectively. An issue with performing additional MRI scans is that there will be unclear results requiring follow‐up MRI examinations (Qureshi et al. 2020).
Qureshi et al. (2020) showed that 202 MRI scans are required to detect one patient with TRb. From our database of TRb patients (de Jong et al. 2020), we had similarly estimated that a screening programme for heritable retinoblastoma patients would require 311 scans to detect one TRb, and 776 scans to save a life (de Jong et al. 2020). Even then, our second case (Fig. 2) would not have been selected for screening because the tumour was classified as non‐heritable (unilateral, no family history, no detectable RB1 mutation in blood).
Regular follow‐up screening for asynchronous TRb would be expensive and would represent a significant load on the healthcare system with a very small chance of changing outcomes meaningfully. Indeed, most centres omit screening for TRb after the baseline scan, unless evidence of an abnormal pineal gland (e.g. a cystic one) is detected at baseline (Popovic et al. 2007; de Jong et al. 2016; Galluzzi et al. 2016; Sirin et al. 2016). Within our ERIC group, we are currently prospectively researching the usefulness of selective follow screening after the baseline MRI, which is often performed before the age of 12 months, but maybe the follow‐up of pineal glands that raised suspicion is too early, and at that time, they could simply not yet be precursors of a pineoblastoma. For the familial patient with a known RB1 mutation, ideally diagnosed before or at birth (Soliman et al. 2016), no ‘baseline’ MRI is performed for retinoblastoma, which is screened for by retinal examination. This raises the question when the first MRI in this patient population should be performed. Perhaps one additional optimally timed MRI scan when the child is older and supplemented with follow‐up scans of only suspicious pineal glands might substantially increase the detection level above that of just one additional MRI scan. Such a screening and follow‐up programme should be evaluated in an international prospective multicentre study to shed light on its performance to detect pineoblastoma in an early stage and its effect on outcome.
Limitations
The calculations should be interpreted with care, because the data are compiled from numerous publications and cohorts and therefore likely are subject to heterogeneity. The model includes some assumptions that could alter the detection rate of performing the additional MRI scans. First, we needed to include the ‘surrogate asymptomatic’ patients to increase the sample size; second, the model assumes that the patients in our database are a fair representation of the pineoblastoma population; third, we assumed perfect accuracy of MRI to detect pineoblastoma (which might not be true for small early tumours); fourth, additional follow‐up of suspicious glands with MRI might underestimate the model detection rate; and finally, the model assumes independence between the age of diagnosis of retinoblastoma and TRb, for which there seems to be some evidence (de Jong et al. 2020).
In our previous meta‐analysis, we graded the level of evidence as low according to the GRADE system; similarly, this score also applies to the data used in this meta‐analysis (Guyatt et al. 2008).
Conclusions
The majority of retinoblastoma patients who develop pineoblastoma cannot be diagnosed with pineoblastoma on the baseline MRI at the time of retinoblastoma diagnosis, especially if retinoblastoma is diagnosed before 12 months of age. One or more optimally timed additional MRI scans at an older age might increase the detection rate of pineoblastoma in these patients.
Supporting information
Appendix A. Pubmed and Embase searches.
Appendix B. Flow chart of article and patient inclusion.
Appendix C. Case information and magnetic resonance images (MRI) of Case 2.
Appendix D. Patient characteristics.
Appendix E. Age at diagnosis of pineoblastoma versus age at diagnosis of retinoblastoma.
Appendix F. Model of the detection rate of pineoblastoma when performing an additional MRI scan.
Appendix G. Sensitivity analysis of the definition of an early asymptomatic diagnosis.
Appendix H. Risk of bias and study quality.
References
- Abdelbaki MS, Abu‐Arja MH, Davidson TB et al. (2020): Pineoblastoma in children less than six years of age: The Head Start I, II, and III experience. Pediatr Blood Cancer 67: e28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broaddus E, Topham A & Singh AD (2009): Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol 93: 21–23. [DOI] [PubMed] [Google Scholar]
- Canadian‐Retinoblastoma‐Society (2009): National Retinoblastoma Strategy Canadian Guidelines for Care: Strategie therapeutique du retinoblastome guide clinique canadien. Can J Ophthalmol 44(Suppl 2): S1–88. [DOI] [PubMed] [Google Scholar]
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Kivelä T & Moll AC (2014): Trilateral retinoblastoma: a systematic review and meta‐analysis. Lancet Oncol 15: 1157–1167. [DOI] [PubMed] [Google Scholar]
- de Jong MC, Kors WA, de Graaf P, Castelijns JA, Moll AC & Kivelä T (2015): The incidence of trilateral retinoblastoma: a systematic review and meta‐analysis. Am J Ophthalmol 160: 1116‐1126 e1115. [DOI] [PubMed] [Google Scholar]
- de Jong MC, Kors WA, Moll AC et al. (2020): Screening for pineal trilateral retinoblastoma revisited: a meta‐analysis. Ophthalmology 127: 601–607. [DOI] [PubMed] [Google Scholar]
- de Jong MC, Moll AC, Goricke S, van der Valk P, Kors WA, Castelijns JA & de Graaf P (2016): From a suspicious cystic pineal gland to pineoblastoma in a patient with familial unilateral retinoblastoma. Ophthalmic Genet 37: 116–118. [DOI] [PubMed] [Google Scholar]
- Farouk Sait S, Haque S, Karimi S et al. (2020): A potential role for apparent diffusion coefficient in the diagnosis of trilateral retinoblastoma. J Pediatr Hematol Oncol 42: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi P, de Jong MC, Sirin S et al. (2016): MRI‐based assessment of the pineal gland in a large population of children aged 0–5 years and comparison with pineoblastoma: part I, the solid gland. Neuroradiology 58: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P & Schünemann HJ & GW Group (2008): GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCarthy A, Birch JM, Draper GJ et al. (2009): Retinoblastoma in Great Britain 1963–2002. Br J Ophthalmol 93: 33–37. [DOI] [PubMed] [Google Scholar]
- Mallipatna ACGB, Gallie BL & Chéves‐Barrios, P et al. (2017): Retinoblastoma. In: Amin MB ed. AJCC Staging Manual. 8th Edn, New York: Springer. 827–839. [Google Scholar]
- Murad MH, Sultan S, Haffar S & Bazerbachi F (2018): Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 23: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic MB, Diezi M, Kuchler H, Abouzeid H, Maeder P, Balmer A & Munier FL (2007): Trilateral retinoblastoma with suprasellar tumor and associated pineal cyst. J Pediatr Hematol Oncol 29: 53–56. [DOI] [PubMed] [Google Scholar]
- Qureshi S, Francis JH, Haque SS, Dunkel IJ, Souweidane MM, Friedman DN & Abramson DH (2020): Magnetic resonance imaging screening for trilateral retinoblastoma: the memorial sloan kettering cancer center experience 2006–2016. Ophthalmol Retina 4: 327–335. [DOI] [PubMed] [Google Scholar]
- Sastre X, Chantada GL, Doz F et al. (2009): Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med 133: 1199–1202. [DOI] [PubMed] [Google Scholar]
- Sirin S, de Jong MC, Galluzzi P et al. (2016): MRI‐based assessment of the pineal gland in a large population of children aged 0–5 years and comparison with pineoblastoma: part II, the cystic gland. Neuroradiology 58: 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman SE, Dimaras H, Khetan V, Gardiner JA, Chan HS, Heon E & Gallie BL (2016): Prenatal versus postnatal screening for familial retinoblastoma. Ophthalmology 123: 2610–2617. [DOI] [PubMed] [Google Scholar]
- Soliman S, Kletke S, Roelofs K, VandenHoven C, McKeen L & Gallie B (2018): Precision laser therapy for retinoblastoma. Expert Rev Ophthalmol 13: 149–159. [Google Scholar]
- Soliman SE, Racher H, Zhang C, MacDonald H & Gallie BL (2017a): Genetics and molecular diagnostics in retinoblastoma–an update. Asia Pac J Ophthalmol (Phila) 6: 197–207. [DOI] [PubMed] [Google Scholar]
- Soliman SE, VandenHoven C, MacKeen LD, Heon E & Gallie BL (2017b): Optical coherence tomography‐guided decisions in retinoblastoma management. Ophthalmology 124: 859–872. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC et al. (2000): Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Pubmed and Embase searches.
Appendix B. Flow chart of article and patient inclusion.
Appendix C. Case information and magnetic resonance images (MRI) of Case 2.
Appendix D. Patient characteristics.
Appendix E. Age at diagnosis of pineoblastoma versus age at diagnosis of retinoblastoma.
Appendix F. Model of the detection rate of pineoblastoma when performing an additional MRI scan.
Appendix G. Sensitivity analysis of the definition of an early asymptomatic diagnosis.
Appendix H. Risk of bias and study quality.


