Abstract
In the past decade, a large volume of peer‐reviewed papers has examined the potential impacts of oil and gas resource extraction in the Canadian oil sands (OS). A large proportion focuses on terrestrial biology: wildlife, birds, and vegetation. We provide a qualitative synthesis of the condition of the environment in the oil sands region (OSR) from 2009 to 2020 to identify gaps and progress cumulative effects assessments. Our objectives were to (1) qualitatively synthesize and critically review knowledge from the OSR; (2) identify consistent trends and generalizable conclusions; and (3) pinpoint gaps in need of greater monitoring or research effort. We visualize knowledge and terrestrial monitoring foci by allocating papers to a conceptual model for the OS. Despite a recent increase in publications, focus has remained concentrated on a few key stressors, especially landscape disturbance, and a few taxa of interest. Stressor and response monitoring is well represented, but direct monitoring of pathways (linkages between stressors and responses) is limited. Important knowledge gaps include understanding effects at multiple spatial scales, mammal health effects monitoring, focused monitoring of local resources important to Indigenous communities, and geospatial coverage and availability, including higher attribute resolution in human footprint, comprehensive land cover mapping, and up‐to‐date LiDAR coverage. Causal attribution based on spatial proximity to operations or spatial orientation of monitoring in the region is common but may be limited in the strength of inference that it provides. Integr Environ Assess Manag 2022;18:388–406. © 2021 The Authors. Integrated Environmental Assessment and Management published by Wiley Periodicals LLC on behalf of Society of Environmental Toxicology & Chemistry (SETAC).
Keywords: Athabasca, Biodiversity, Canada, Cumulative effects, Environmental monitoring
KEY POINTS
We provide a qualitative synthesis of the condition of the environment in the Canadian oil sands region (OSR) in northeastern Alberta from 2009 to 2020 to identify gaps and progress cumulative effects assessments.
Despite a recent increase in publications, focus has remained concentrated on a few key stressors and a few taxa of interest, for which monitoring is well represented, though direct monitoring of pathways (linkages between stressors and responses) is limited.
Important gaps include a lack of understanding of effects at multiple spatial scales, a lack of focused monitoring of local resources important to Indigenous communities, and geospatial data resolution and availability.
Causal attribution based on spatial proximity to oil sands operations or spatial orientation of monitoring in the OSR is common but may be limited in the strength of inference that it provides.
INTRODUCTION
The Canadian oil sands region (OSR) in the western Nearctic boreal forest holds almost a third of the world's hydrocarbon deposits and is a major driver of global economies and geopolitics (Bayoumi & Mühleisen, 2006; Giesy et al., 2010). However, this economic boon, as with all industrial development, comes at an environmental cost. Better understanding the residual and existing environmental impacts of oil sands (OS) development has been a priority for various ambient monitoring and research programs, including those run by independent researchers as well as government agencies, such as through the Oil Sands Monitoring Program (OSM), run jointly by the Governments of Canada and Alberta (Dubé et al., 2021), as well as through Indigenous community‐based monitoring (ICBM) programs.
Oil sands industrial operations necessitate a variety of facilities and developments on the landscape. Open‐pit surface mining of shallow bitumen—the viscous hydrocarbon mixture extracted from OS—involves removing large tracts of topsoil and overburden, effectively removing all biomass from an area. Conversely, in situ operations—bitumen extraction from drilled wells—generate narrow forest cut lines (seismic lines) for exploration and cleared well‐drilling pads. Both types of extraction necessitate large networks of roads, above‐ and below‐ground pipelines, and industrial upgrading facilities used to refine bitumen. Although major developments (surface mines, well pads, bitumen upgrading facilities) sit on land leased to private companies, the monitoring of which falls under regulatory guidelines, other OS‐related footprints (e.g., roads, seismic lines, pipelines) and regional impacts (e.g., contaminant deposition) exist or occur off‐lease, falling within the purview of ambient environmental monitoring programs (Dubé et al., 2021). Environmental monitoring within the OSR (Figure 1) beyond lease boundaries is important as, particularly for terrestrial ecology, many potential changes are likely to be observed at larger landscape scales, in regions both close to and far from OS facilities and on‐lease operations. Further, scientific inconsistencies in regulatory processes such as environmental impact assessments have been recently identified (Campbell et al., 2020), emphasizing the need for third‐party monitoring of the larger condition of the environment in the OSR.
Figure 1.
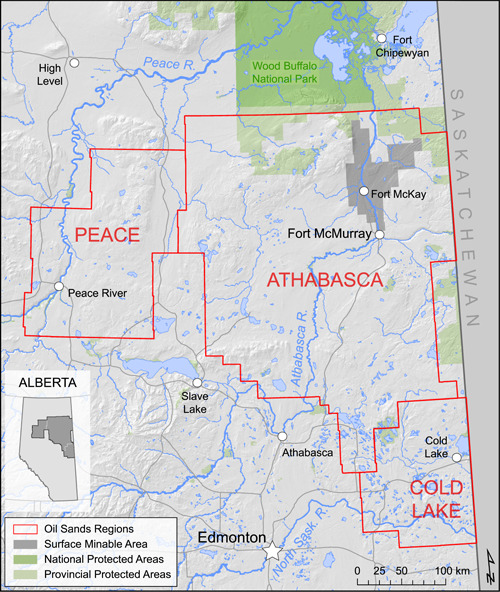
Map of the Canadian oil sands region in northeastern Alberta, Canada, identifying the three main OS regions (Peace, Athabasca, and Cold Lake) as well as urban centers, major hydrological features, the surface minable OS area (in dark gray), and protected areas (in green)
Substantial monitoring and research efforts in the OSR have resulted in a large body of peer‐reviewed literature examining the potential environmental impacts of oil and gas resource extraction. Monitoring priorities in the OSR must be guided by a conceptual understanding of the biotic and abiotic systems in the boreal forest of northern Alberta and hypotheses describing how industrial development may influence these systems. Oil sands‐related publications can be organized into discrete but overlapping theme areas of air, water, and land, and generally consider a wide breadth of anthropogenic and natural stressors, the associated responses to those stressors, and the pathways connecting them. Although reviews are available on specific topics such as seismic lines (Dabros et al., 2018) or more general forest disturbance (Venier et al., 2014), the information in these publications has not been synthesized to examine the condition of the environment in the OSR, inhibiting the progression of larger cumulative effects assessments. Ambient terrestrial monitoring in the OSR includes assessments of potential effects of industrial activities on a variety of taxa, including those of social and cultural importance, species of concern, and ecological resources of value to local communities.
Here, we present a synthetic review of the last decade of peer‐reviewed literature focused on monitoring the potential terrestrial biological effects of OS industrial development, with the objectives being to (1) synthesize knowledge from the OSR via a synthesis of relevant published literature; (2) identify consistent trends in the focus and effort within the reviewed literature; and (3) pinpoint knowledge gaps and opportunities for greater monitoring or research effort. To represent known or suspected environmental relationships, we implement a conceptual modeling approach—a box and line diagram to visualize environmental monitoring components (e.g., stressors, pathways, responses; Box 1) and the links between them. Conceptual models are fundamental tools to inform monitoring foci within environmental monitoring and cumulative effects assessment. We outline major conclusions from the literature, organized by key pressures, and include notable gaps and opportunities. We identify overarching challenges, such as issues of scale. We close with a discussion of specific monitoring and research opportunities in the OSR offered by emerging geospatial methods, which represent a substantial focus in the methodological literature.
Box 1: Conceptual model terminology.
Pressure: a natural or anthropogenic activity that generates one or more stressors.
Stressor: an action or change known or suspected to influence the environment.
Pathway: a functional mechanism that causes or facilitates a manifested response to a stressor in a given indicator.
Response: a change in the function, behavior, or occurrence of any biotic or abiotic component of the environment on which a stressor is acting.
METHODS
Our qualitative synthetic review followed advice derived from systematic reviews (Haddaway et al., 2015; Xiao & Watson, 2019) in that we searched for papers using defined terms and rigorously adhered to delineated criteria for exclusion, and then subjected the result to reviews by the body of authors. Note, however, that this is not a systematic review; we did not use a vote‐counting approach (Berger‐Tal et al., 2019; Xiao & Watson, 2019) or strictly constrain our content to the search criteria, which would overlook publications lacking target keywords but providing critical context. Allowing flexibility in inclusion provides the opportunity to pursue additional relevant literature missed in the original searches. Further, though gray (all non‐peer‐reviewed) literature can hold important information for reviews (Haddaway et al., 2015), we excluded it; such a review would be a massive undertaking worth its own effort. We instead focused on peer‐reviewed literature to begin the knowledge synthesis process for the OSR using the most scientifically credible information available and acknowledge that this subjects us to publication bias (Westgate & Lindenmayer, 2017).
In our synthetic review, we considered all peer‐reviewed papers published from 2009 to 2020 that fulfilled three criteria: (1) covered a terrestrial topic or focal indicator; (2) collected data inclusive of or specific to the OSR; and (3) were relevant to OS industrial operations. Initial searches for literature were performed using general OS‐related search terms within Scopus and Google Scholar. We also consulted reference lists from these publications for literature missed in initial searches. We read all selected papers and recorded predetermined details for each study including monitoring location and dates, focal taxa or topic, stressors, pathways, effects, key findings, and any environmental change reported (Appendix S1). We summarize these findings in this article, with more detailed summaries and discussion provided in Appendix S2. Much has been written about biological monitoring (Ims & Yoccoz, 2017; Yoccoz et al., 2001) and much debate exists around the best approaches, including some approaches adopted within the OSM (Haughland et al., 2010; Lindenmayer & Likens, 2009). Although a rigorous, systematic review that generates new conclusions (like a meta‐analysis) will be of value for the future of research and monitoring in the OSR, the state of the science does not yet lend itself to this meta‐analytical approach; hence, our focus was to critically synthesize what is known, so that future efforts may target what remains unknown.
We excluded papers based solely on three designated, predelineated criteria (Haddaway et al., 2015): (1) laboratory‐only studies; (2) field research specific to land reclamation, recovery, or remediation; and (3) methods development research. All others were retained and exclusion was checked by all authors, lending confidence that we avoided entraining unconscious bias (Haddaway et al., 2015). Reclamation and recovery of disturbed land is an important ecological and economic activity in the OSR (e.g., Caners & Lieffers, 2014; Filicetti et al., 2019; Z. Zhang, 2019) and should be the subject of a dedicated future review. We do not consider the exclusion of reclamation literature as a risk to undermine our conclusions, as our scope is limited to impacts of OS operations, not the reversal of those impacts. It is noteworthy that we did retain monitoring papers with data collection within reclaimed habitats when studies offered knowledge applicable to the larger boreal forest ecosystem. We also retained papers that included the recovery status of features (e.g., amount of regrowth on disturbance features), an environmental covariate explaining variance in terrestrial species responses (e.g., Tattersall et al., 2020a).
Further, our scope was restricted to terrestrial biology. Wetland environments combine qualities of both terrestrial and aquatic systems, and so do not fall squarely into either this review or others (Arciszewski et al., 2021). We divided wetland literature between the terrestrial and surface water themes: terrestrial taxa (e.g., birds, vegetation, etc.) were included even if they were wetland habitat specialists, whereas aquatic taxa (including amphibians) were included in the accompanying surface water review (Arciszewski et al., 2021). A recent review of wetland literature from the OSR has also been completed by Ficken et al. (2021), separate from this Special Series. Artificial divisions of holistic ecosystems (e.g., into terrestrial, aquatic, and air themes) compromise interpretability to some extent, but they are pragmatic; they reflect the thematic divisions of current monitoring efforts in the OSR and parallel natural divisions in expertise among scientists and authors. In effect, the thematic breakdown of this Special Series is largely a product of the structure of the OSM Program, more than an independent decision among all the Special Series authors.
Our review criteria resulted in a final selection of 85 peer‐reviewed papers (Appendix S1), though we cite many others in this review as part of contextual, methods, or other related discussions. A cross‐theme synthesis and complete bibliography of all papers reviewed for water, air, terrestrial biology, and ICBM are provided in the cross‐theme synthesis paper within this Special Series (Roberts et al., 2021a).
RESULTS AND DISCUSSION
We organized key research findings by major stressors (e.g., landscape disturbance and contaminants), as well as specific methodological approaches and challenges. Extended results and discussion, including additional background and references, are provided in Appendix S2. To visualize the effort from the reviewed literature, we allocated the reviewed papers to a terrestrial biological conceptual model for the OSR, where the individual papers supported the inclusion of a model component (i.e., pressure, stressor, pathway, or response) (Figure 2).
Figure 2.
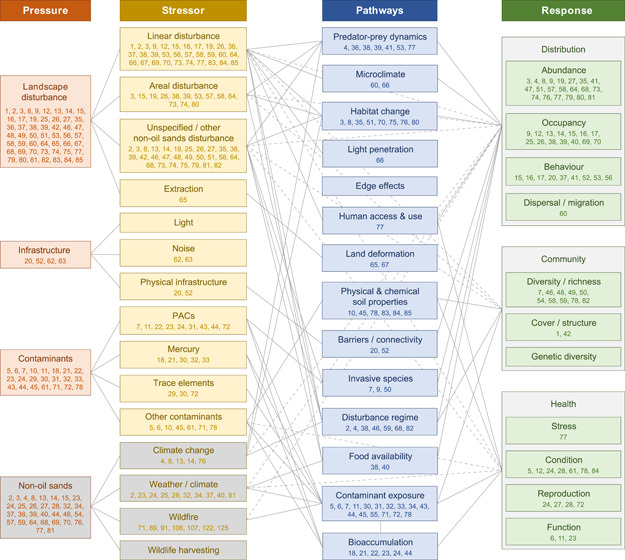
Terrestrial biological conceptual model for the oil sands region, showing pressures, stressors, pathways, and responses. For reference, papers included in our review that demonstrated evidence for that model component are shown in the respective boxes by a number corresponding to the review tables (Appendix S1) and numbered bibliography (Appendix S3). Boxes are connected by gray lines if we found evidence of their connection in the reviewed literature (i.e., if a paper appears in multiple boxes, they are connected). Dashed lines represent stressor–response relationships in the literature that did not have a defined pathway
Key pressures
Landscape disturbance
Landscape disturbance—removal of mature forest or wetland and creation of an anthropogenic feature—is arguably the most widespread stressor in the OSR, with roughly 13.8% of the land base qualifying as “disturbed” habitat (2012 data) in the form of agriculture (7.4%, largely concentrated in the Cold Lake and Peace River OS regions), forestry (2.9%), and energy exploration and extraction (2.2%) (Alberta Biodiversity Monitoring Institute [ABMI], 2014) (Figure 3). Publications addressing landscape disturbance were the dominant focus of the literature from the OSR that we reviewed (Figure 2). This focus may be a result of landscape disturbance being highly visible, directly quantifiable (both on the ground and geospatially), and discrete in its distribution on the landscape.
Figure 3.
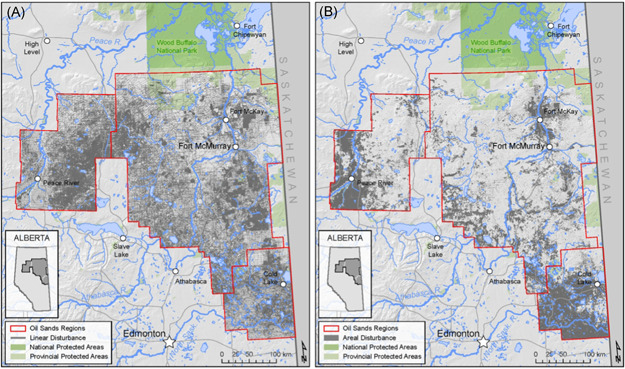
Extent of landscape disturbance in the oil sands region of northeastern Alberta (ABMI, 2017), showing the extent of human footprint in the area as (A) mapped linear disturbance features and (B) mapped polygonal disturbance features
It can be challenging to separate terrestrial impacts of OS‐specific landscape disturbances (e.g., 3D seismic lines common to in situ development, surface mines, OS roads) from non‐OS landscape disturbances (e.g., agriculture, forestry, urban development, conventional oil and gas, non‐OS roads). For example, very few papers that considered landscape disturbance as a stressor specifically investigated responses to features identifiable as OS related. Individual disturbance features may be attributable to a specific industry when classified one by one in focused monitoring studies, but the more typical approach of utilizing geospatial human footprint layers (e.g., Burton et al., 2014) complicates attribution to specific industries (especially OS vs. conventional oil and gas).
Habitat alteration from OS development can be divided into two categories, each with different associated environmental responses: (1) linear disturbance (narrow and long clearings, such as seismic lines and roads) and (2) polygonal disturbance (more contiguous clearings, such as well pads, agricultural clearing, and forestry cut blocks). Although linear disturbances cover less total area at the local scale relative to polygonal disturbances, they can extend across vast distances and are ubiquitous within the OSR ( ≥1 km/km2 in most of the OSR, Figure 3), making them the main source of forest edges (Dabros et al., 2018). They also naturally regenerate slowly enough to remain on the landscape for decades, and features with ongoing human access (e.g., roads, seismic lines accessible to off‐highway vehicles; OHVs) may persist on the landscape indefinitely (Finnegan et al., 2019; Pigeon et al., 2016; Van Rensen et al., 2015).
Linear disturbances affect biota in two main ways. First, they create extensive forest edges and associated edge effects. These ecological changes—including those in behavior, abundance, and community—manifest where two distinct habitats meet (e.g., forest and clearings, old and young growth forest) (Murcia, 1995). Polygonal disturbances, while larger and contiguous, produce fewer forest edges and habitat transitions per unit area. Therefore, when buffering effects around disturbances are considered, the ubiquitous linear features that segment the OSR landscape may represent a much larger area of disturbance than what is directly reported (ABMI, 2014) (Figure 3).
Second, linear features create networks of interconnected low‐vegetation corridors that facilitate movement for some species such as wolf (Canis lupus) (Dickie et al., 2017; Latham, Latham, Boyce, & Boutin, 2011). This has indirect impacts on other species by increasing predator–prey encounters (Mckenzie et al., 2012) and wolf hunting efficiency (Dickie et al., 2017), which consequently leads to avoidance of these features by prey species (Latham, Latham, Boyce, & Boutin, 2011). Such altered predator–prey interactions have, in combination with changes in deer (Odocoileus virginianus) habitats and populations (Dawe et al., 2014; Dawe & Boutin, 2016; Fisher et al., 2020; Fisher & Burton, 2021), been implicated in the decline of woodland caribou (Rangifer tarandus) in the OSR (S. Boutin et al., 2012).
Knowledge of wolf–caribou predator–prey dynamics is unique among mammal monitoring in its completeness and consistency. Findings among most mammal monitoring studies are generally more variable and often contradictory (Appendices S2 and S4). This may be an artifact of different sampling methodologies, different spatial or temporal scales of measurement, or simply due to large uncertainties associated with quantifying wildlife responses across large multistressor areas (i.e., science is hard). In fact, a central consistency within the literature is that mammal responses to anthropogenic landscape features and other changes in forest seral stage in the OSR are complex and idiosyncratic across species (Fisher & Burton, 2018; Wittische et al., 2021).
While landbird species also respond to landscape disturbance idiosyncratically, they tend to do so more predictably, in alignment with their habitat preferences (Mahon et al., 2019). Generally speaking, in the boreal forest, landbird species distribution and communities are related to a combination of climate, forest structure, and human disturbance, to varying degrees of importance depending on the specific species guild (J. Zhang et al., 2013). Species preferring open or early seral habitats are generally found in greater abundance on disturbed sites, while the most negatively affected species are those commonly associated with older forest stages or shrublands, as well as habitat generalists (Bayne et al., 2016; Foster et al., 2017); this is a consistent pattern across the larger boreal forest (see review by Schieck & Song, 2006). Future projections of industrial development trends in the OSR suggest that old growth habitat specialists may be at risk of population decline through the next century (Mahon et al., 2014).
Further, cumulative effects on boreal birds may be underestimated, as additive and interactive effects may have a larger influence on bird abundances and communities than previously recognized. For many species, habitat and either single or multiple disturbance variables interact when accounting for species abundances, suggesting complex functional relationships between landbirds and their environments, including anthropogenic disturbance (Mahon et al., 2019). The implication of this complexity, likely extending beyond birds to other taxa, highlights the necessity of considering not just individual responses to specific stressors but also cumulative effects resulting from the multistressor OSR environment (Roberts et al., 2021a). Most landbirds are also migratory and the boreal is their breeding habitat, so disturbance effects in the rest of their ranges may compound those of OS activity.
Most vegetation monitoring papers focus on richness or other community‐level responses at various scales. Species‐level responses to various footprint types, and the pathways by which those responses are aggravated, are poorly understood by comparison. Responses may be complex (e.g., interactions between abiotic factors like light and biotic factors like pollinator movements), and evidence from the boreal region outside the OSR suggests idiosyncratic variation among species, as has been seen with other taxa (Dabros et al., 2018). Broadly, the majority of vegetation studies in the OSR have focused on linear features, including conventional (wider) seismic lines and 3D (narrower, denser) seismic lines. Roads are also unique as a “hard” linear feature in the OSR that alters the hydrology of forested peatlands, thus affecting canopy and understory species and communities (Miller et al., 2015; Saraswati et al., 2020).
The physical and chemical properties of soils are changed in disturbance features, representing an important pathway for vegetation community change. This specifically includes changes in soil chemistry resulting from changes in organic cover and decomposition rates (S. J. Davidson, Goud, et al., 2020), in soil water from winter road use (Strack et al., 2018), in soil temperature (Finnegan et al., 2019), and in microtopographic simplification (Stevenson et al., 2019). A small body of literature on terrestrial invertebrates from the larger boreal region often considers the effects of forest harvesting in addition to those of conventional oil and gas or OS effects specifically. Exceptions to this include monitoring responses to energy‐related disturbances of a few native butterflies (Riva et al., 2018a, 2018b) and nonnative earthworm species (e.g., Cameron et al., 2007), though most knowledge on the latter comes from pre‐2010 publications and from boreal habitats outside the OSR specifically.
Idiosyncratic species responses to landscape disturbance are a common thread across taxa in monitoring studies from the OSR, and mammal monitoring work has demonstrated that consideration of a complexity of covariates may be required when assessing the impacts of landscape disturbance. For example, various levels of seismic line regeneration, snow cover, human use, line density, and line width can differentially alter the magnitude and direction of occupancy rates for different species (Tattersall et al., 2020a), and the effect of these covariates likely changes at different temporal and spatial scales (Beirne et al., 2021; Tattersall et al., 2020b). Consequently, while species‐level focused monitoring may be useful for indicator or focal species, this approach may be too resource‐intensive for widespread application. Instead, OSR monitoring would benefit from multispecies monitoring using tools that measure responses of multiple species simultaneously, such as remote cameras for mammals (Burton et al., 2015) and autonomous recording units for birds and amphibians (Shonfield & Bayne, 2017a), or for other taxa community‐level indicators (e.g., richness, diversity) that can assess biodiversity and ecosystem health. Such multitaxa approaches would be further strengthened by an increase in integrated monitoring designs with colocated and coincidental data collection across known stressor gradients.
Finally, from a conservation perspective, it is spurious to interpret idiosyncratic species responses to disturbance as a zero‐sum game in which losses in one species are offset by gains in another. The decline in the abundance of some species in parallel with increases in others will result in a net loss of diversity, and potentially ecosystem function, over time and, if disturbance continues under consistent trends, local extirpation of some species, likely those favoring intact late‐stage forests, may be the result.
Landscape disturbance: gaps and opportunities
The complexity of the boreal environment and the multistressor nature of the OSR introduce challenges when determining responses of terrestrial biology to landscape disturbance. Species and taxa (e.g., birds, mammals, insects, and vegetation) respond idiosyncratically and differences in observed responses may depend, for example, on the type and nature of disturbances, the habitat preferences of the individual species, or the spatial scale of measurement (Fisher & Burton, 2018; Toews et al., 2018; Venier et al., 2014). High‐latitude boreal systems have a well‐known low diversity and a lack of functional redundancy (Cooke et al., 2019; Hillebrand, 2004), so a spectrum of unique responses is entirely expected.
Still, there are opportunities to refine monitoring approaches to bring more consistency to and comparability between observations. We observed few consistent monitoring approaches or designs among taxa, with research instead tailored to targeted questions. This yields important specific knowledge but undermines multitaxa syntheses and inhibits understanding of whole‐ecosystem connections (such as between mammals, birds, and plants, as in trophic cascades) (Terborgh & Estes, 2010). Standardizing monitoring designs across taxa, colocating and integrating monitoring across the region at nested spatial and temporal scales, using sample sizes derived from power analyses, and encouraging consistency in field methods would generate more consistent results and stronger inference with respect to species responses to landscape disturbance.
Current gaps in disturbance research and monitoring exist with respect to species (e.g., mustelids, shrubs and forbs, invertebrates), methods (e.g., combining data sets, issues of scale), and ecological mechanisms generating observed patterns (i.e., pathways) (Figure 2). Causal attribution is limited by the complexity of attributing specific species responses to specifically OSR‐generated disturbances—with the exception of 3D seismic lines, which are exclusive to OS operations. Enhanced geospatial human footprint layers with precise industrial attribution of anthropogenic features would bridge this gap, as would a design that captures the critical importance of scale when it comes to landscape disturbance response. Future monitoring must consider terrestrial biological responses at multiple scales, as these are often different and sometimes contrasting.
One of the most notable geospatial gaps in the OSR is that the regional human footprint data coverage, often implemented for landscape‐scale analyses, does not include narrow 3D seismic lines, largely associated with in situ OS development (ABMI, 2017). Attribution of disturbance features and ecological response at fine scales is critical for accurately modeling responses and informing mitigation. Data enrichment efforts, such as adding greater attribute resolution to geospatial footprint layers, would help address this need. Even with such data improvements, however, the multistressor quality of the landscape in the OSR, where many habitat disturbances overlap in time and space, will continue to undermine causal attribution. To address this challenge, more causally inferential experimental designs are required, such as pre‐ and postconstruction monitoring to establish before–after stressor–response data. Geospatial approaches may offer support in this respect. For example, long‐term remote‐sensing data such as decades‐long satellite imagery records can be used to develop time series of visible landscape changes (e.g., Chowdhury et al., 2017).
The comprehensive identification and characterization of landscape disturbance, including narrow 3D seismic features, within geospatial human footprint data sets will facilitate local‐ and landscape‐scale analyses of both responses and pathways related to landscape disturbance. Such data could also enable investigation into other potential pathways that are underrepresented or absent from the literature with respect to vegetation responses, including local changes in hydrology, microtopography, physical removal or disturbance effects such as soil compaction, or changes to larger mesoclimate patterns from larger‐scale disturbance patterns. Polygonal features and their edge effects on plants, while assumed to be similar to linear disturbance effects, are less well understood and less often monitored.
While causal attribution is critical for the regulation and management of OS operations, the attributes required to attribute cause may not be the same as those required to refine ecological response analyses and models. For example, the ability to differentiate a linear disturbance as either a pipeline or a wide seismic line is likely less important in a wildlife distribution model than the ability to determine the width, vegetation cover, or other ecologically relevant variables of the feature itself. In many respects, the original human use of the feature is secondary to the ecological character of the feature, which can also be determined largely by human tertiary uses, such as OHV use of seismic lines that inhibits regeneration (Pigeon et al., 2016; Van Rensen et al., 2015). Moving beyond disturbance attribution to disturbance characteristic and use may produce stronger explanatory models where attributes are useful explanatory variables (e.g., Tattersall et al., 2020a) or serve to eliminate covariates as drivers of occupancy and abundance when they are not. Although the regeneration of disturbance features was beyond the scope of this review, it is an extensive and valuable body of knowledge that could provide relevant guidance in this context.
Oil sands disturbance features are unique. The most conspicuous polygonal features are, of course, active OS surface mines. While biodiversity on these sites is effectively zero, they represent a comparatively small area of actual landscape disturbance (<1% of Canadian OS deposits in 2018). The more ubiquitous and widespread OS‐related landscape disturbances are networks of roads, above‐ground pipes, wellsites, and seismic lines, and there is ample evidence to conclude that these features affect species distributions, and that they do so additively or synergistically with a variety of other anthropogenic landscape disturbances from a breadth of industries and activities (and, of course, climate change). Consequently, any causal attribution to OS‐specific disturbance is extremely difficult. Idiosyncratic responses to disturbance between species, scales, and methodologies highlight the need for monitoring at multiple spatial extents and with consistent protocols whose assumptions and limitations are well understood. To evaluate a more comprehensive suite of species, especially among the smaller mammals, additional monitoring protocols may need to be considered. A larger, integrated experimental design across multiple research monitoring partners would be advantageous.
Contaminants
Contaminant research in the OSR can be broken down into three main components: (1) deposition of contaminants; (2) uptake by wildlife; and (3) effects of tissue burdens on species. While many studies quantify atmospheric deposition using biological organisms as indicators (Arciszewski et al., 2021; Horb et al., forthcoming), a few also investigate the biological or ecological responses to those depositions. This latter effort largely lies within the terrestrial effects monitoring literature, as well as being a notable focus of ICBM programs in the OSR (Beausoleil et al., 2021).
Plants, soils, and wildlife represent the three ecological endpoints of contaminant deposition, and they are monitored within the OSR largely via two projects: (1) the Forest Health Monitoring Program, implemented by the Wood Buffalo Environmental Association (WBEA), and (2) wildlife toxicology monitoring, implemented by Environment and Climate Change Canada (ECCC). Local community collaborations and Traditional Knowledge drive a large portion of the faunal species selection for the latter. While some monitoring has been opportunistic, to a certain extent, as with ICBM of harvested wildlife (Thomas et al., 2021), other monitoring has been initiated in response to specific concerns from traditional land users, such as on unintended impacts of rodenticides (Thomas et al., 2017a). Perceived changes in sentinel or keystone species as early indicators have also triggered monitoring for health hazards to the larger ecological system (Beausoleil et al., 2021; Cruz‐Martinez & Smits, 2013).
While uncertainty remains around the pathways that move contaminants into and through the terrestrial biological system in the OSR, some basic vectors are well understood. In very general terms, contaminants transported in the air are deposited on the landscape, either (1) onto bare or snow‐covered ground, where they may infiltrate soils and be absorbed by plant roots; (2) into surface water, either via direct deposition or via snowmelt, where they may be detected within aquatic organisms (Arciszewski et al., 2021); or (3) directly onto plants and animals, where they may be absorbed into tissue. The bioaccumulation or simple trophic movement of contaminants through the food chain is also a well‐known phenomenon. Although this review does not address aquatic biology directly (covered in Arciszewski et al., 2021), aquatic toxicology issues are relevant to terrestrial organisms that feed on aquatic organisms and may therefore bioaccumulate contaminants in this way (Wallace et al., 2020).
Organisms within the OSR are being exposed to a variety of contaminants—some petrogenic in nature and some pyrogenic, some naturally occurring, and some anthropogenic (Figure 2). Despite the challenge of complex environments, several studies have directly observed the accumulation of petrogenic or other anthropogenic contaminants in plant and animal tissues and linked these accumulations to proximity to OS operations. This has been observed for a variety of chemical constituents in plants (e.g., C. Boutin & Carpenter, 2017; Laxton et al., 2010; Proemse et al., 2016), for polycyclic aromatic hydrocarbons (PACs) in tree swallow (Tachycineta bicolor) nestlings (Cruz‐Martinez et al., 2015; Fernie, Marteinson, Chen, et al., 2018; Fernie et al., 2019), and for mercury in colonial waterbird eggs (Dolgova et al., 2018; Hebert, 2019; Hebert et al., 2011, 2013). Similar patterns in larger mammals have been observed (e.g., Lundin et al., 2015) but less frequently. Temporal alignment of contaminant accumulations with extreme weather events such as heavy precipitation and flooding has been observed in colonial waterbird eggs (Hebert, 2019), potentially reflecting a bioaccumulation via aquatic food sources when river sediment is mobilized, and with precipitation and temperature patterns for tree swallow nestlings (Fernie et al., 2019; Godwin et al., 2019). Projected future expansion of OS developments, combined with projected increases in extreme weather events under climate change, may increase contaminant loads.
Chemical changes to soils from contaminants and resulting effects on plant health and communities are the focus of the Forest Health Monitoring Program—a decade‐long integrated monitoring effort that simultaneously measures atmospheric deposition and terrestrial effects (soils and vegetation) at acid‐sensitive jack pine (Pinus banksiana) sites through OSR (C. J. Davidson, Foster, et al., 2020; Foster et al., 2019). Founded on concerns of soil acidification in forest stands, the scope of this monitoring has expanded to include a range of depositional stressors and ecological responses. To date, there is little evidence of acidification of soils or associated effects on understory plant communities in jack pine stands (MacKenzie & Dietrich, 2020; Watmough et al., 2019) and no adverse forest health responses due to acidifying deposition, potentially a result of coincidental and neutralizing base cation deposition. However, data suggest that changes in understory species productivity and communities are occurring, likely driven by fertilization from nitrogen and sulfur deposition (Bartels et al., 2019; C. J. Davidson, Foster, et al., 2020; Watmough et al., 2019).
Contaminants: gaps and opportunities
Multiple gaps and opportunities were identified during this review. Among the gaps was inconsistency of linking observed tissue or body contaminant accumulations in monitored fauna. Changes in functional or health responses of organisms, while observed under certain environmental conditions, are inconsistent and seldom directly linked to chemical stressors (see the review by Wallace et al., 2020). Additionally, despite evidence of exposures, very few effects on populations or the health of organisms such as mammals have been confirmed (Appendix S2). A recent exception to this was an observed change in baculum bone strength in PAC‐exposed otters within the OSR, suggesting a change in organism condition with increased PAC burdens (Thomas et al., 2021). However, evidence of reproductive declines in otters was lacking and spatial relationships with OS operations were unclear. Similarly, a large body of contaminant‐related literature has been produced for tree swallow nestlings (Appendix S2), and while contaminant burdens in tissues and corresponding functional changes (e.g., thyroid function) have been observed, how these factors may manifest in survival and reproduction responses are not obvious. Evidence suggests that health responses depend not only on tissue burdens but also on a variety of other environmental variables, making effects measurable only in years of extreme weather when cold and/or wet conditions prevail (Fernie, Marteinson, Soos, et al., 2018; Godwin et al., 2019).
Many studies rely on spatial gradients or site orientations to attribute cause. Experimental designs that use spatial proximity with OS operations as a causal proxy, as is the case in the aforementioned otter and tree‐swallow monitoring, may be limited in their ability to pinpoint sources of petrogenic PACs and other contaminants. Naturally exposed bituminous geology is common in the area around OS development, and patterns of atmospheric deposition of contaminants may not be as straightforward as assumed in many monitoring designs (Horb et al., forthcoming). Additionally, large mammals such as wolf, caribou, and moose (Alces alces) have large home ranges, making source apportionment conclusions difficult to draw. Direct tissue sampling of smaller mammals may be better suited to clarify these patterns, as smaller home ranges can more appropriately pinpoint exposure. Even for stationary organisms such as plants, the underlying spatial structure of environmental covariates can introduce confounding patterns in results. For example, jack pine growth rates in response to nitrogen and sulfur deposition appear to be correlated with proximity to OS operations as point sources but may equally or more likely be driven by existing patterns of forest stand ages or natural gradients of growing conditions, which favor sites closer to the Athabasca River, where OS operations are also concentrated (Bartels et al., 2019; C. J. Davidson, Foster, et al., 2020). Increased integration of wildlife toxicology research with larger aerial contaminant and deposition monitoring programs would be advantageous, as would leveraging geospatial data sets that catalog development over time or real‐time industrial activity.
Despite these challenges, there may be times when the strategy of ascribing anthropogenic source apportionment in terrestrial receptors based on spatial proximity to development can provide some level of inference. However, this would be limited to when sources and pathways are well understood and when the spatial arrangement of sites is relevant in the context of known and confirmed stressor or deposition patterns around industrial activity (Horb et al., forthcoming). However, for focused monitoring efforts intended to investigate cause where knowledge—particularly of pathways—is limited, carefully designed studies with experimental controls should be preferred (to the extent possible in a natural environment). To support these efforts, scientists also require reliable and timely data on the location and activity levels of different industrial facilities or even different areas within individual facilities, such as OS surface mines (e.g., Landis et al., 2019). For example, in studies that measure local air contaminants directly, exposure and uptake may not correlate linearly with geographic proximity to OS facilities or lease centroids but rather may be linked to proximity to active mining locations, amount of road traffic, etc. (e.g., Fernie, Marteinson, Chen, et al., 2018).
ICBM programs represent ongoing local‐scale contaminant monitoring opportunities, with Indigenous hunters and trappers in the OSR providing samples of semi‐aquatic mammals for toxicological analysis for mammal health monitoring (e.g., Thomas et al., 2021) and ongoing methodological developments in mammal toxicology (Eccles, Littlewood, et al., 2019; Eccles et al., 2020). Monitoring plant contaminants, specifically the deposition of contaminants onto food, medicinal, and culturally important plants, is also conducted within ICBM programs and there is extensive Traditional Knowledge on the topic, covered in the ICBM review in this special series (Beausoleil et al., 2021). Plant contaminant knowledge is less extensive within the Western science peer‐reviewed literature, and Indigenous communities have assisted in identifying priority focal species or groups. Logistical sampling limitations, such as the inability to access remote regions or the efficiency of using roads and trails for site access (Kolowski & Forrester, 2017; Sólymos et al., 2020), can also spatially bias monitoring data. Integration of ICBM programs and collaboration between Western scientists and holders of local and Indigenous Knowledge represents an opportunity to address this limitation (Beausoleil et al., 2021), as is demonstrated by recent ICBM collaborations with local hunters and trappers (e.g., Thomas et al., 2021).
Established thresholds for evaluating toxicological endpoints, such as tissue burdens, are neither widely available nor widely used in the OS literature. The near complete absence of well‐established thresholds or limits‐of‐change for contaminant burdens in terrestrial organisms, other than those based on bioaccumulation in aquatic prey (Canadian Council of Ministers of the Environment [CCME], 1999), may hinder adaptive monitoring programs that rely on specific triggers to guide monitoring effort (Lindenmayer & Likens, 2009) and encourage the development and/or application of inconsistent criteria, potentially limiting the straightforward consolidation of conclusions, further stalling the development of robust and rigorous integrated monitoring programs (Arciszewski et al., 2021, forthcoming).
Infrastructure
Physical infrastructure related to industrial development in the OSR, such as worker camps and large facilities, received limited attention in the reviewed literature relative to both landscape disturbance and contaminants. This may be a result of a lack of specific data for these features, as they are often grouped in with other anthropogenic features in the oft‐used human footprint index. The notable exception to this is above‐ground pipelines (35–50 cm diameter pipes mounted on support racks within a cleared right‐of‐way), noise from industrial facilities, and aquatic tailings ponds in the context of whooping crane stopovers. Light pollution and its potential effects on wildlife received no attention in the published literature from the OSR in the last decade.
Noise from industrial operations and facilities has been studied and was not found to influence occupancy or detection of three different owl species (Shonfield & Bayne, 2017b) or the abundance, movement, or body mass of mice and voles (Shonfield & Bayne, 2019). However, some pre‐2009 studies on songbirds in the boreal forest documented declines in species abundance as a result of industrial noise exposure (see the review by Venier et al., 2014).
Stopover location monitoring within the OSR for the endangered whooping crane (Grus americana) is undertaken by the Canadian Wildlife Service. The whooping crane migration corridor passes through the OSR on the way to or from northern breeding grounds in the Wood Buffalo National Park and overwintering areas in the southern United States. Stopover patterns in the OSR are important as the selection of landing sites by birds on industrial water features such as tailings ponds, while low risk as direct mortality events (Pearse et al., 2018), has the potential to expose birds to OS‐related contaminants. While publications specific to whooping crane in the OSR are limited, there is extensive reporting on range‐wide migration patterns (e.g., Pearse et al., 2019).
Some limited research indicates that above‐ground pipes do not restrict the movement of deer and wolves due to the animals' willingness to cross underneath pipes, even when clearance is very low (Dunne & Quinn, 2009). For larger ungulates such as moose and caribou, however, they can represent a movement barrier, though moose are largely unimpeded when pipes have high clearances underneath (>140 cm) or where equipped with purpose‐built crossing structures (Dunne & Quinn, 2009). Models based on remote camera data suggest that caribou remain impeded even where above‐ground pipe crossing structures exist—caribou movement patterns change and home range sizes decrease nonlinearly as landscapes contain more infrastructure and become less permeable (Muhly et al., 2015). Although studies did not investigate the effects of altered movement or range sizes on species survival or reproduction, given the ubiquity of above‐ground pipes due to in situ developments and the likelihood that they will increase in density and coverage in the future, they should be considered an important stressor of in situ development, particularly for larger mammals such as moose and caribou. Careful management of construction standards (e.g., including areas of higher clearance and crossing structures) can mitigate these responses for some species. For caribou, potential nonlinear relationships suggest that even small increases in habitat permeability may result in facilitated movement and increased home range sizes (Muhly et al., 2015), though the demographic implications of these benefits are not clear.
Climate change
Global climate change has well‐established causal links to anthropogenic greenhouse gas production in general, and carbon‐intensive industries in particular, including OS production (Environment and Climate Change Canada, 2018; Horb et al., forthcoming). However, while there are some limited examples of climate change‐related investigations from the last decade specific to the OSR (e.g., Campos‐Ruiz et al., 2018), none consider change in the context of OS industrial activity or cumulative effects. For the most part, climate change is considered in the context of larger habitat change studies, such as changes in mammal distributions (Barber et al., 2018; Dawe et al., 2014; Dawe & Boutin, 2016; Fisher et al., 2020) and boreal bird communities (Cadieux et al., 2020). In contrast to climate change as a pressure, studies also linked microtopographic simplification and altered soil water depth from landscape disturbance pressures to greenhouse gas emissions via methane and carbon release (Lovitt et al., 2018; Strack et al., 2018), presenting an important opportunity for cross‐theme integration with atmospheric emissions monitoring.
Further opportunities for leveraging long‐term observations to inform environmental change in response to climate change exist via ICBM programs or collaborations between Indigenous Knowledge holders and Western scientists. Local community histories and Indigenous Knowledge often include long periods of record of local environmental conditions, such as water levels and floods or phenology and distribution of culturally important plants (Beausoleil et al., 2021).
Novel geospatial methods and newly available remotely sensed data sets represent an emerging opportunity—both within and beyond climate change applications—to investigate long‐temporal‐scale landscape changes. In one such example, researchers used Google Earth Engine and the historical archive of Landsat satellite imagery to develop time‐series data of wetland inundation in the Peace‐Athabasca Delta, linking hydrologic alteration to a corresponding decrease in the abundance of muskrat—a semiaquatic rodent that relies on ephemeral water bodies as habitat (Ward & Gorelick, 2018).
Issues of scale
Ecological systems operate on a continuum of spatial and temporal scales (Wiens, 1989), and thus scales of measurement and analysis are influential methodological variables in monitoring. Small‐scale processes may or may not translate into large‐scale patterns and vice versa (Allen & Holling, 2002; Fisher et al., 2011; Levin, 1992; O'Neill et al., 1996), necessitating the monitoring of indicators at multiple spatial scales. This is recognized, for example, in measured changes in populations, where effects may appear magnified or minimized as the spatial scale of monitoring or analysis is decreased or increased, respectively. For this reason, we must exercise caution when generalizing to larger or smaller time and space scales from smaller‐ or larger‐scale monitoring.
Space
Terrestrial biological stressors and responses in the OSR manifest at nearly every spatial scale, from the local (e.g., anticoagulant rodenticides around facilities; Thomas et al., 2017b), to regional (e.g., mammal responses to seismic line density; Tigner et al., 2015), to global (e.g., boreal bird responses to climate change; Stralberg et al., 2015). A basic ecological premise is that the extent to which disturbance affects taxa or alters communities is dependent on the spatial and temporal scales of measurement as well as the spatial and temporal scales (and duration and/or intensity) of the disturbance.
There are examples of the same stressor producing different terrestrial biological responses when considered at different spatial and/or temporal scales. For example, the magnitude and direction of large mammal abundance response to human landscape disturbance vary both by species and by scale (Toews et al., 2018). Conversely, similar terrestrial biological responses may result from different stressors operating simultaneously at different spatial and temporal scales. For example, species occupancy may change simultaneously at the local scale due to edge effects from disturbances, at the landscape scale by overall density of disturbances, and at the global scale due to climate change. Unravelling these patterns represents a key challenge within terrestrial monitoring.
Increasingly vexing for terrestrial biological researchers, the spatial grain or extent of field measurement or data analysis of stressors and responses can influence conclusions. For example, for bird abundance responses to landscape disturbance, both the magnitude and the associated uncertainty of measured responses tend to decrease with larger sampling areas (Bayne et al., 2016). Similarly, the importance of predictors in bird habitat models also varies with the spatial grain of data layers (J. Zhang et al., 2013). When modeling large mammal abundance, Toews et al. (2018) found variations in model selection and support across different spatial grains for different species but consistency among the relative magnitude and direction of responses. Likewise, Fisher and Burton (2018) observed that the spatial scale at which OS features best explained mammal distribution changed among species. Spatial scale is thus a key part of understanding OS impacts and should continue to be a focus.
Another consideration for landscape‐ and regional‐scale analyses is the size of the OSR itself, nearly 500 km latitudinally, and therefore containing many ecologically relevant spatial environmental gradients. While these include some commonly considered gradients, such as climate, photoperiod, and geology, other gradients may be more clandestinely important to distributions of terrestrial biota, and thus potentially mischaracterized in explanatory models or other analyses. For example, the distance to agricultural development (in the south), where species abundances are markedly different, may yield different community compositions concordantly across latitude. Also potentially confounded by other north–south gradients are the impacts of the OS surface mines, which are concentrated in the northern latitudes of the OSR.
Such spatial complexities have implications not just for scientific inference or even management recommendations but also for the usefulness of monitoring data and knowledge to people in local communities. Indigenous communities in the OSR may utilize resources in areas much smaller (e.g., a single berry patch) than what Western science might consider a local monitoring program, consequently limiting the utility of monitoring knowledge for local communities practicing traditional lifestyles. Such “super‐local” monitoring may occur through ICBM or other Indigenous‐led programs and may be reported in ICBM reports or gray literature (Beausoleil et al., 2021; Fisher et al., 2021). In this respect, this may represent a gap in the Western science literature more so than a gap in the monitoring knowledge itself. Though opportunities for integrated analysis or integrated interpretation of monitoring data may be currently limited, improvements in data cataloguing and publicly accessible data systems could provide additional analytical opportunities.
Spatial structure within the environment
Environmental data of all kinds are typically fraught with underlying spatial autocorrelation (Legendre, 1993) and monitoring data from the OSR are no exception, as this is a landscape where both natural and anthropogenic stressors are structured in space (Figures 1 and 3). For example, along the south‐to‐north gradient of the Athabasca River, environmental covariates such as natural geology can affect water chemistry, elevation can affect climate and thus community composition, and even latitude itself can affect day length and thus productivity (Rose & Lyon, 2013). This is exemplified in jack pine growth in the OSR, where differences in growth are correlated with proximity to OS operations but are also likely due to environmental site differences—also structured in space but unrelated to industrial development and associated deposition patterns from OS facilities (Bartels et al., 2019; C. J. Davidson, Foster, et al., 2020).
Nonindependent data collection—for example, collection of multiple samples from the same site, same colony, or even simply too close together in a structured landscape—results in statistical pseudoreplication when samples are treated as independent observations, increasing the potential for Type 1 errors (false positives). Thoughtful analytical approaches and careful statistical analysis can address such sampling issues, even while simultaneously integrating data sets, via range standardization, spatial averaging, and aggregation of observations (Eccles, Pauli, et al., 2019). Logistic and economic challenges of fieldwork in the OSR notwithstanding, comprehensive monitoring along known gradients (stressors and otherwise) is critical if strong causal inferences are to be made.
Time
Spatial topics were more prevalent in the peer‐reviewed publications than temporal topics, despite both being necessary to guide sampling and monitoring methodologies and to inform our understanding of ecological processes. Terrestrial biological monitoring has unique temporal considerations such as multiyear population cycles (O'Donoghue et al., 1997) that must be considered when investigating change or trends over longer periods if reliable inferences are to be made (Barraquand et al., 2017). However, temporal‐scale issues have received less direct attention in the OS terrestrial literature, with very few papers directly addressing the influence of temporal sampling variability or breadth, such as considerations of site revisit frequency or analytical period of record (but see Fisher et al., 2020; J. Zhang et al., 2014). Temporal considerations and periods of record represent an opportunity to integrate Traditional Knowledge from local Indigenous communities, as many have extended time scales of environmental observations. For example, populations of bison in the Wood Buffalo region have long been monitored by local communities in the region (Beausoleil et al., 2021).
With a number of long‐term and consistent‐protocol terrestrial biological sampling projects currently operating in the OSR, the data resources to perform such investigations exist but thus far remain underutilized for this task. This could reflect simply the extended period required for long‐term data to identify meaningful change in the context of high degrees of natural variation, or could reflect something academically more (but environmentally less) problematic, such as a publication bias against negative results (i.e., most monitored changes are non‐significant). Ergo, such investigations may more often appear in the gray literature (e.g., ABMI, 2018; Bayne et al., 2017) and so would fall beyond the scope of this review.
Geospatial methods
Geospatial methods—encapsulating geographic information systems (GIS) and remote sensing—are quickly emerging as important methodological tools to assess stressors and associated ecological responses in the OSR. In the past decade, a variety of monitoring and analytical opportunities have been identified and applied in the OSR, largely either for data generation (i.e., production of geospatial data, such as complete coverages of stressor surfaces, potentially for use as covariates in empirical models) or analysis and modeling (e.g., response modeling to geospatial surfaces such as human footprint). See Appendix S2 for a more comprehensive coverage of the recent geospatial literature.
Increases in the past decade in computing power, open‐source GIS, image processing, and statistics software, and availability of remotely sensed data have dramatically increased the quantity and utility of geospatial data products. Many of these products have been used to inform environmental monitoring in the OS, including large‐scale landcover data (e.g., Castilla et al., 2014; Latifovic & Pouliot, 2014; Pouliot & Latifovic, 2016) and disturbance mapping and the development of human footprint inventories (ABMI, 2017). While these data layers are becoming essential environmental science tools, they can be expensive and labor‐intensive to create and may lack required feature resolution for many analyses. They also require comprehensive validation and ground truthing if they are to be trustworthy reporters on landscape condition.
Human footprint and other landcover data, including habitat classifications, are used extensively to support the development of species models for the OSR. Nearly every wildlife modeling study within the OSR, including spatially explicit analyses of field‐collected data, relies on geospatial data sets, geospatial analytical tools, or both. Typically, geospatial data are incorporated into empirical or statistical models as habitat or resource selection predictor variables, with a GIS often used to determine landcover or human footprint classes (i.e., anthropogenic landscape disturbance) at or around species observation locations or monitoring sites. Such studies use models to explain species occurrence (e.g., Fisher & Burton, 2018; Latham, Latham, Boyce, 2011; Shonfield & Bayne, 2017b), abundance (e.g., Ball et al., 2016; Mahon et al., 2016; Toews et al., 2018), movement (e.g., Latham, Latham, Latham, Boyce, & Boutin, 2011; Whitman et al., 2017), predation (e.g., Neilson & Boutin, 2017), reproduction (e.g., Fisher & Burton, 2021), or contaminant burdens (e.g., Thomas et al., 2017b).
There has been substantial progress on landscape disturbance mapping and the development of inventories of human footprint, including several wall‐to‐wall (i.e., gapless spatial coverages) human footprint inventories created by the ABMI (Figure 3) for the entire province of Alberta at regular time intervals since 2010 (ABMI, 2017). These data have high accuracy and have been created for multiple years, allowing for critical temporal matching of environmental monitoring and anthropogenic landscape features. However, these data are assembled in a cumulative‐only framework and, as such, do not track regeneration or recovery of human footprint features postdisturbance (i.e., features are constantly added but never removed). While some features may be considered permanent vegetation alteration (e.g., roads, agriculture), many others are likely to regenerate, at least partially, after the initial disturbance has ceased (e.g., forestry cut blocks, some seismic lines). The current cumulative footprint inventory approach may result in an overestimation of landscape disturbance (failing to account for regeneration and succession), potentially biasing model conclusions. There exist geospatial tools capable of assessing vegetation regeneration and recovery on some disturbance features (e.g., Abib et al., 2019; Chasmer et al., 2018; Van Rensen et al., 2015), and widening the application of these techniques to assess regeneration and recovery on all human footprint would help address this shortcoming. This, in combination with increased attribute resolution of human footprint layers (e.g., specific industry attribution of features), could permit more confident causal attribution within the OSR.
In addition to mapping and monitoring human footprint development, geospatial science can assess vegetation disturbance more broadly through the development of disturbance indices, which are sensitive to vegetation disturbance due to fire, insect infestation, and changes in moisture conditions (Coops et al., 2009). Such approaches typically assess regional‐scale changes using coarse resolution satellite imagery, and as such are unable to resolve fine‐scale human footprint features (Coops et al., 2009). However, regional‐scale vegetation disturbance monitoring (e.g., Guo et al., 2017) could complement smaller‐scale human footprint and land use change monitoring and provide regional context and information on natural variability and regional‐scale stressors.
Some limitations of geospatial approaches for wildlife data analysis and modeling are similar to other environmental monitoring challenges. Geospatial data are not immune to spatial biases, including inconsistencies in effects across scales of measurement, so spatial and temporal resolutions and breadths must be carefully considered. Direct comparison between model outputs is also difficult, as the source of geospatial data used to create the same predictor variables is inconsistent between studies and the quality of input data is rarely assessed. More problematic for ecological understanding, the choice of geospatial data used to parameterize models may affect subsequent interpretations of species' habitat characterizations when landcover classifications differ systematically between layers. For example, Hedley et al. (2020) compared habitat predictions for yellow rail (Coturnicops noveboracensis) based on four different geospatial landcover layers, finding that the characterization of preferred habitat varied between several different wetland classes and wet coniferous forest.
Further, limited availability of landcover or other geospatial data within the OSR can result in temporal differences between the data used as predictor variables and the observed biological monitoring response data, undermining the recognition of causal linkages. To fill data gaps, there may be opportunities to work in collaboration with local Indigenous communities, who have a long history of monitoring local environmental conditions, including landcover and land change, especially vegetation change (Beausoleil et al., 2021).
CONCLUSIONS
We structured this review based on major anthropogenic terrestrial biological stressors in the OSR, but it is important to recognize that no stressor, natural or anthropogenic, acts in isolation. The OSR is a multistressor landscape, with terrestrial biota simultaneously subject to habitat alteration, human activity and infrastructure, chemical contaminants from both natural and anthropogenic sources, natural disturbance regimes, and climate change. The interconnectedness of model components, specifically the convergence of multiple pathways into single responses, within the terrestrial conceptual model (Figure 2) exemplifies this challenge and helps visualize the accumulated environmental state as it is captured in the peer‐reviewed literature.
Despite widespread recognition of this complexity, very few research papers from the past decade have incorporated multiple stressors (multivariate modeling papers being the notable exception) and even fewer directly addressed cumulative effects (excluding analyses of human footprint impacts across sectors). The pathway—or mechanistic processes—through which species respond to many stressors, especially landscape disturbance, remain largely unaddressed. The exception to this is the pathway of contaminant loading of wildlife tissue, where many transport and uptake vectors are known and the general process of trophic bioaccumulation is understood. With respect to cumulative effects, knowledge is limited and it has been suggested that species‐level data are largely insufficient to attempt cumulative effects assessments in the boreal forest in general (Venier et al., 2014). The situation may not be quite so bleak if innovative approaches are implemented that (1) integrate multiple data and knowledge sources and (2) address cumulative effects questions with geospatial or other computational/statistical tools that make the most of limited or disparate data.
The complexity of the ecological system, and correlations of the multiple natural and anthropogenic stressors in the OSR also make direct linking of terrestrial biological responses to specific OS operations extremely difficult. For stressors such as contaminant exposure or tissue toxicology, a key challenge lies in differentiating responses to natural versus anthropogenic exposures as well as between OS‐related and other anthropogenic sources (e.g., nearby upstream urban development, background global industrialization). For stressors such as habitat alteration and landscape disturbance, the challenge is not in quantifying the impact or source attribution of development, which is more straightforward, but rather in building cohesive conceptual understanding from a complicated mix of interacting species and stressors.
Simple data limitations, such as the lack of attribution of landscape disturbances to specific industries, also hinder causal investigation. Geospatial approaches are powerful tools that can address these and other analytical or data shortcomings, but they are not a magic solution for every monitoring or knowledge gap. Truly integrated monitoring involves temporally concurrent and spatially coincidental monitoring of multiple indicators (stressors and responses), preferably at multiple spatial scales. An integrated monitoring program must be more than integrated interpretation of results from disparate and independently designed projects. The Forest Health Monitoring Program for jack pine acidification is a good example of integrated monitoring across theme areas, as it measures contaminant deposition coincidentally with biotic responses in an ecologically sensitive indicator. Similarly, the tree swallow contaminant work sought to connect deposition and contaminant burdens with population effects. Monitoring on multiple scales (e.g., measuring local reproductive effects in combination with regional population effects) would also facilitate stronger causal inferences for larger‐scale indicator metrics such as populations.
Although geospatial science has been widely used as a tool for terrestrial monitoring within the OSR, both opportunities and challenges remain. Critically, geospatial science can produce several of the foundational data sets that are required for subsequent analyses including the development of statistical models and quantifying stressor–response relationships (e.g., Ball et al., 2016; Fisher & Burton, 2018; Latham, Latham, Boyce, & Boutin, 2011). Several studies have demonstrated the applicability of remote sensing to map and monitor land cover, land use, and vegetation health over regional scales (e.g., Castilla et al., 2014; Guo et al., 2017; Pouliot & Latifovic, 2016), and the development of annual, consistent, wall‐to‐wall (i.e., complete spatial coverage) data products would allow for change detection monitoring and serve as foundational data in species response models. Producing such foundational data would allow for species response models to be constructed at appropriate spatial extents (Toews et al., 2017), allow for the temporal alignment of predictor and response variables, and facilitate comparison between model outputs.
Over the past decade, the scientific community has generated a great deal of knowledge about the responses of terrestrial biota to various stressors in the OSR. While there remain critical knowledge gaps, terrestrial ecologists and monitoring professionals have a heavy toolbox, and continued development of both field and desktop methodologies will present numerous opportunities going forward. A key lesson from well‐understood terrestrial biological responses, encapsulated by the complicated caribou story, is that the development of knowledge within complex ecological systems takes not only time and resources but also productive collaboration among scientists and other knowledge holders, local communities, industry, and regulators. Integration between monitoring theme areas (e.g., air, water, land, and biodiversity) is challenging but essential for comprehensive reporting on the state of the environment in the Alberta OS.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Monique G. Dubé conceived the work. David R. Roberts, Danielle Beausoleil, Roderick O. Hazewinkel, Diogo Sayanda, and Faye Wyatt developed the methods and assisted with the review. David R. Roberts performed the main review and led manuscript writing, with Erin M. Bayne, Jacqueline Dennett, and Jason T. Fisher contributing content according to their subject matter expertise. All authors reviewed the final manuscript.
Supporting information
This article includes online‐only Supporting Information.
Appendix 1: Compiled tables of all reviewed literature.
Appendix 2: Extended results and discussion.
Appendix 3: Numbered bibliography of reviewed literature, sorted by conceptual model column and box.
Appendix 4: Summary table of mammal responses to landscape disturbance in the OSR.
ACKNOWLEDGMENT
The authors acknowledge Tim Arciszewski, Dave Hervieux, Erin Horb, Brian Kopach, Kelly Munkittrick, Simon Slater, Phil Thomas, and Greg Wentworth for helpful comments on this manuscript, and also Ashley Mahaffey for substantial contributions to the review. This work was funded under the Oil Sands Monitoring Program (OSM) but does not necessarily reflect the position of OSM.
This article is part of the special series “A Decade of Research and Monitoring in the Oil Sands Region of Alberta, Canada.” The series documents the history of monitoring in the region and critically reviews a synthesis of monitoring results published within key environmental theme areas to identify patterns of consistent responses or effects; significant gaps in knowledge; and recommendations for improved monitoring, assessment, and management of the region.
DATA AVAILABILITY STATEMENT
No new data were generated by this literature review.
REFERENCES
- Abib, T. H. , Chasmer, L. , Hopkinson, C. , Mahoney, C. , & Rodriguez, L. C. E. (2019). Seismic line impacts on proximal boreal forest and wetland environments in Alberta. Science of the Total Environment, 658, 1601–1613. 10.1016/j.scitotenv.2018.12.244 [DOI] [PubMed] [Google Scholar]
- Alberta Biodiversity Monitoring Institute (ABMI) . (2014). The status of biodiversity in the oil sands region of Alberta: Preliminary assessment. Retrieved August 23, 2019, from: https://abmi.ca/home/publications/1-50/40 [Google Scholar]
- Alberta Biodiversity Monitoring Institute (ABMI) . (2017). ABMI human footprint inventory: Wall‐to‐wall human footprint inventory. Retrieved August 18, 2020, from: https://ftp-public.abmi.ca/GISData/HumanFootprint/2017/HFI2017v1_Metadata.pdf [Google Scholar]
- Alberta Biodiversity Monitoring Institute (ABMI) . (2018). ABMI 10‐year science and program review. Retrieved August 23, 2019, from: https://abmi10years.ca [Google Scholar]
- Allen, C. R. , & Holling, C. S. (2002). Cross‐scale structure and scale breaks in ecosystems and other complex systems. Ecosystems, 5(4), 315–318. 10.1007/s10021-001-0075-3 [DOI] [Google Scholar]
- Arciszewski, T. J. , Hazewinkel, R. R. O. , Ussery, E. J. , & Dubé, M. G. (2021). A critical review of the status of lakes and rivers from Canada's oil sands region. Integrated Environmental Assessment and Management, 18(2), 361–387. 10.1002/ieam.4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciszewski, T. J. , Roberts, D. R. , Munkittrick, K. R. , & Scrimgeour, G. J. (2021). Challenges and benefits of approaches used to integrate regional monitoring programs. Frontiers in Environmental Science, 9, 666698. 10.3389/fenvs.2021.666698 [DOI] [Google Scholar]
- Ball, J. R. , Sólymos, P. , Schmiegelow, F. K. A. , Hache, S. , Schieck, J. , & Bayne, E. (2016). Regional habitat needs of a nationally listed species, Canada Warbler (Cardellina canadensis), in Alberta, Canada. Avian Conservation and Ecology, 11(2), 10. 10.5751/ACE-00916-110210 [DOI] [Google Scholar]
- Barber, Q. E. , Parisien, M. A. , Whitman, E. , Stralberg, D. , Johnson, C. J. , St‐Laurent, M. H. , DeLancey, E. R. , Price, D. T. , Arseneault, D. , Wang, X. , & Flannigan, M. D. (2018). Potential impacts of climate change on the habitat of boreal woodland caribou. Ecosphere, 9(10), 800. 10.1002/ecs2.2472 [DOI] [Google Scholar]
- Barraquand, F. , Louca, S. , Abbott, K. C. , Cobbold, C. A. , Cordoleani, F. , DeAngelis, D. L. , Elderd, B. D. , Fox, J. W. , Greenwood, P. , Hilker, F. M. , Murray, D. L. , Stieha, C. R. , Taylor, R. A. , Vitense, K. , Wolkowicz, G. , & Tyson, R. C. (2017). Moving forward in circles: challenges and opportunities in modelling population cycles. Ecology Letters, 20(8), 1074–1092. 10.1111/ele.12789 [DOI] [PubMed] [Google Scholar]
- Bartels, S. F. , Gendreau‐Berthiaume, B. , & Macdonald, S. E. (2019). The impact of atmospheric acid deposition on tree growth and forest understory vegetation in the Athabasca oil sands region. Science of the Total Environment, 696, 133877. 10.1016/j.scitotenv.2019.133877 [DOI] [PubMed] [Google Scholar]
- Bayne, E. , Knaggs, M. , & Solymos, P. (2017). How to most effectively use autonomous recording units when data are processed by human listeners. Alberta Biodiversity Monitoring Institute, Bioacoustic Unit. http://bioacoustic.abmi.ca/ [Google Scholar]
- Bayne, E. , Leston, L. , Mahon, C. L. , Sólymos, P. , Machtans, C. , Lankau, H. , Ball, J. R. , Van Wilgenburg, S. L. , Cumming, S. G. , Fontaine, T. , Schmiegelow, F. K. A. , & Song, S. J. (2016). Boreal bird abundance estimates within different energy sector disturbances vary with point count radius. Condor, 118(2), 376–390. 10.1650/CONDOR-15-126.1 [DOI] [Google Scholar]
- Bayoumi, T. , & Mühleisen, M. (2006). Energy, the Exchange Rate, and the Economy: Macroeconomic Benefits of Canada's Oil Sands Production (IMF Working Papers. 06(70)), p. 1. 10.5089/9781451863307.001 [DOI]
- Beausoleil, D. L. , Munkittrick, K. R. , Dubé, M. G. , & Wyatt, F. (2021). Essential components and pathways for developing Indigenous community‐based monitoring: Examples from the Canadian oil sands region. Integrated Environmental Assessment and Management, 18(2), 407-427. 10.1002/ieam.4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirne, C. , Sun, C. , Tattersall, E. R. , Fisher, J. T. , & Burton, A. C. (2021). Multispecies modelling reveals potential for habitat restoration to re‐establish boreal vertebrate community dynamics. Journal of Applied Ecology. 10.1111/1365-2664.14020 [DOI] [Google Scholar]
- Berger‐Tal, O. , Greggor, A. L. , Macura, B. , Adams, C. A. , Blumenthal, A. , Bouskila, A. , Candolin, U. , Doran, C. , Fernández‐Juricic, E. , Gotanda, K. M. , Price, C. , Putman, B. J. , Segoli, M. , Snijders, L. , Wong, B. B. M. , & Blumstein, D. T. (2019). Systematic reviews and maps as tools for applying behavioral ecology to management and policy. Behavioral Ecology, 30(1), 1–8. 10.1093/beheco/ary130 [DOI] [Google Scholar]
- Boutin, C. , & Carpenter, D. J. (2017). Assessment of wetland/upland vegetation communities and evaluation of soil‐plant contamination by polycyclic aromatic hydrocarbons and trace metals in regions near oil sands mining in Alberta. Science of the Total Environment, 576, 829–839. 10.1016/j.scitotenv.2016.10.062 [DOI] [PubMed] [Google Scholar]
- Boutin, S. , Boyce, M. S. , Hebblewhite, M. , Hervieux, D. , Knopff, K. H. , Latham, M. C. , Latham, A. D. M. , Nagy, J. , Seip, D. , & Serrouya, R. (2012). Why are caribou declining in the oil sands? Frontiers in Ecology and the Environment, 10(2), 65–67. 10.1890/12.WB.005 [DOI] [Google Scholar]
- Burton, A. C. , Huggard, D. , Bayne, E. , Schieck, J. , Sólymos, P. , Muhly, T. , Farr, D. , & Boutin, S. (2014). A framework for adaptive monitoring of the cumulative effects of human footprint on biodiversity. Environmental Monitoring and Assessment, 186(6), 3605–3617. 10.1007/s10661-014-3643-7 [DOI] [PubMed] [Google Scholar]
- Burton, A. C. , Neilson, E. , Moreira, D. , Ladle, A. , Steenweg, R. , Fisher, J. T. , Bayne, E. , & Boutin, S. (2015). Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. Journal of Applied Ecology, 52(3), 675–685. 10.1111/1365-2664.12432 [DOI] [Google Scholar]
- Cadieux, P. , Boulanger, Y. , Cyr, D. , Taylor, A. R. , Price, D. T. , Sólymos, P. , Stralberg, D. , Chen, H. Y. H. , Brecka, A. , & Tremblay, J. A. (2020). Projected effects of climate change on boreal bird community accentuated by anthropogenic disturbances in western boreal forest, Canada. Diversity and Distributions, 26(6), 668–682. 10.1111/ddi.13057 [DOI] [Google Scholar]
- Cameron, E. K. , Bayne, E. M. , & Clapperton, M. J. (2007). Human‐facilitated invasion of exotic earthworms into northern boreal forests. Ecoscience, 14(4), 482–490. 10.2980/1195-6860(2007)14[482:HIOEEI]2.0.CO;2 [DOI] [Google Scholar]
- Campbell, M. A. , Kopach, B. , Komers, P. E. , & Ford, A. T. (2020). Quantifying the impacts of oil sands development on wildlife: perspectives from impact assessments. Environmental Reviews, 28(2), 129–137. 10.1139/er-2018-0118 [DOI] [Google Scholar]
- Campos‐Ruiz, R. , Parisien, M. A. , & Flannigan, M. D. (2018). Temporal patterns of wildfire activity in areas of contrasting human influence in the Canadian boreal forest. Forests, 9(4), 159. 10.3390/f9040159 [DOI] [Google Scholar]
- Canadian Council of Ministers of the Environment (CCME) . (1999). Canadian tissue residue guidelines for the protection of wildlife consumers of aquatic biota: DDT (total). In: Canadian environmental quality guidelines. Canadian Council of Ministers of the Environment, Winnipeg.
- Caners, R. T. , & Lieffers, V. J. (2014). Divergent pathways of successional recovery for in situ oil sands exploration drilling pads on wooded moderate‐rich fens in Alberta, Canada. Restoration Ecology, 22(5), 657–667. 10.1111/rec.12123 [DOI] [Google Scholar]
- Castilla, G. , Hird, J. , Hall, R. J. , Schieck, J. , & McDermid, G. J. (2014). Completion and updating of a Landsat‐based land cover polygon layer for Alberta, Canada. Canadian Journal of Remote Sensing, 40(2), 92–109. 10.1080/07038992.2014.933073 [DOI] [Google Scholar]
- Chasmer, L. , Baker, T. , Carey, S. K. , Straker, J. , Strilesky, S. , & Petrone, R. (2018). Monitoring ecosystem reclamation recovery using optical remote sensing: Comparison with field measurements and eddy covariance. Science of the Total Environment, 642, 436–446. 10.1016/j.scitotenv.2018.06.039 [DOI] [PubMed] [Google Scholar]
- Chowdhury, S. , Chao, D. K. , Shipman, T. C. , & Wulder, M. A. (2017). Utilization of Landsat data to quantify land‐use and land‐cover changes related to oil and gas activities in west‐central Alberta from 2005 to 2013. GIScience and Remote Sensing, 54(5), 700–720. 10.1080/15481603.2017.1317453 [DOI] [Google Scholar]
- Cooke, R. S. C. , Bates, A. E. , & Eigenbrod, F. (2019). Global trade‐offs of functional redundancy and functional dispersion for birds and mammals. Global Ecology and Biogeography, 28(4), 484–495. 10.1111/geb.12869 [DOI] [Google Scholar]
- Coops, N. C. , Wulder, M. A. , & Iwanicka, D. (2009). Large area monitoring with a MODIS‐based Disturbance Index (DI) sensitive to annual and seasonal variations. Remote Sensing of Environment, 113(6), 1250–1261. 10.1016/j.rse.2009.02.015 [DOI] [Google Scholar]
- Cruz‐Martinez, L. , Fernie, K. J. , Soos, C. , Harner, T. , Getachew, F. , & Smits, J. E. G. (2015). Detoxification, endocrine, and immune responses of tree swallow nestlings naturally exposed to air contaminants from the Alberta oil sands. Science of the Total Environment, 502, 8–15. 10.1016/j.scitotenv.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Cruz‐Martinez, L. , & Smits, J. E. G. (2013). Potential to use animals as monitors of ecosystem health in the oil sands region—July 2013 update. Oil Sands Research and Information Network, University of Alberta, School of Energy and the Environment.
- Dabros, A. , Pyper, M. , & Castilla, G. (2018). Seismic lines in the boreal and arctic ecosystems of North America: Environmental impacts, challenges, and opportunities. Environmental Reviews, 26(2), 214–229. 10.1139/er-2017-0080 [DOI] [Google Scholar]
- Davidson, C. J. , Foster, K. R. , & Tanna, R. N. (2020). Forest health effects due to atmospheric deposition: Findings from long‐term forest health monitoring in the Athabasca oil sands region. Science of the Total Environment, 699, 134277. 10.1016/j.scitotenv.2019.134277 [DOI] [PubMed] [Google Scholar]
- Davidson, S. J. , Goud, E. M. , Franklin, C. , Nielsen, S. E. , & Strack, M. (2020). Seismic line disturbance alters soil physical and chemical properties across boreal forest and peatland soils. Frontiers in Earth Science, 8, 281. 10.3389/feart.2020.00281 [DOI] [Google Scholar]
- Dawe, K. L. , Bayne, E. M. , & Boutin, S. (2014). Influence of climate and human land use on the distribution of white‐tailed deer (Odocoileus virginianus) in the western boreal forest. Canadian Journal of Zoology, 92(4), 353–363. 10.1139/cjz-2013-0262 [DOI] [Google Scholar]
- Dawe, K. L. , & Boutin, S. (2016). Climate change is the primary driver of white‐tailed deer (Odocoileus virginianus) range expansion at the northern extent of its range; land use is secondary. Ecology and Evolution, 6(18), 6435–6451. 10.1002/ece3.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie, M. , Serrouya, R. , McNay, R. S. , & Boutin, S. (2017). Faster and farther: Wolf movement on linear features and implications for hunting behaviour. Journal of Applied Ecology, 54(1), 253–263. 10.1111/1365-2664.12732 [DOI] [Google Scholar]
- Dolgova, S. , Popp, B. N. , Courtoreille, K. , Espie, R. H. M. , Maclean, B. , McMaster, M. , Straka, J. R. , Tetreault, G. R. , Wilkie, S. , & Hebert, C. E. (2018). Spatial trends in a biomagnifying contaminant: Application of amino acid compound‐specific stable nitrogen isotope analysis to the interpretation of bird mercury levels. Environmental Toxicology and Chemistry, 37(5), 1466–1475. 10.1002/etc.4113 [DOI] [PubMed] [Google Scholar]
- Dubé, M. G. , Dunlop, J. M. , Davidson, C. J. , Beausoleil, D. L. , Hazewinkel, R. R. O. , & Wyatt, F. (2021). History, overview and governance of environmental monitoring in the oil sands region of Alberta, Canada. Integrated Environmental Assessment and Management, 18(2), 319-332. 10.1002/ieam.4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne, B. M. , & Quinn, M. S. (2009). Effectiveness of above‐ground pipeline mitigation for moose (Alces alces) and other large mammals. Biological Conservation, 142(2), 332–343. 10.1016/j.biocon.2008.10.029 [DOI] [Google Scholar]
- Eccles, K. M. , Littlewood, E. S. , Thomas, P. J. , & Chan, H. M. (2019). Distribution of organic and inorganic mercury across the pelts of Canadian river otter (Lontra canadensis). Scientific Reports, 9(1), 1–11. 10.1038/s41598-019-39893-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, K. M. , Pauli, B. D. , & Chan, H. M. (2019). The use of geographic information systems for spatial ecological risk assessments: An example from the Athabasca oil sands area in Canada. Environmental Toxicology and Chemistry, 38(12), 2797–2810. 10.1002/etc.4577 [DOI] [PubMed] [Google Scholar]
- Eccles, K. M. , Thomas, P. J. , & Chan, H. M. (2020). Relationships between mercury concentrations in fur and stomach contents of river otter (Lontra canadensis) and mink (Neovison vison) in northern Alberta Canada and their applications as proxies for environmental factors determining mercury bioavailability. Environmental Research, 181, 108961. 10.1016/j.envres.2019.108961 [DOI] [PubMed] [Google Scholar]
- Environment and Climate Change Canada . (2018). 2019 National inventory report 1990–2017: Greenhouse gas sources and sinks in Canada. Canada's submission to the United Nations Framework Convention on Climate Change. Environment and Climate Change Canada, Government of Canada. https://www.canada.ca/en/environment-climate-change/services/climate-change/greenhouse-gas-emissions/inventory.html
- Fernie, K. J. , Marteinson, S. C. , Chen, D. , Eng, A. , Harner, T. , Smits, J. E. G. , & Soos, C. (2018). Elevated exposure, uptake and accumulation of polycyclic aromatic hydrocarbons by nestling tree swallows (Tachycineta bicolor) through multiple exposure routes in active mining‐related areas of the Athabasca oil sands region. Science of the Total Environment, 624, 250–261. 10.1016/j.scitotenv.2017.12.123 [DOI] [PubMed] [Google Scholar]
- Fernie, K. J. , Marteinson, S. C. , Chen, D. , Palace, V. , Peters, L. , Soos, C. , & Smits, J. E. G. (2019). Changes in thyroid function of nestling tree swallows (Tachycineta bicolor) in relation to polycyclic aromatic compounds and other environmental stressors in the Athabasca oil sands region. Environmental Research, 169, 464–475. 10.1016/j.envres.2018.11.031 [DOI] [PubMed] [Google Scholar]
- Fernie, K. J. , Marteinson, S. C. , Soos, C. , Chen, D. , Cruz‐Martinez, L. , & Smits, J. E. G. (2018). Reproductive and developmental changes in tree swallows (Tachycineta bicolor) are influenced by multiple stressors, including polycyclic aromatic compounds, in the Athabasca oil sands. Environmental Pollution, 238, 931–941. 10.1016/j.envpol.2018.03.074 [DOI] [PubMed] [Google Scholar]
- Ficken, C. D. , Connnor, S. J. , Rooney, R. C. , & Cobbaert, D. (2021). Drivers, pressures, and wetland state responses to inform long‐term oil sands wetland monitoring program objectives. Wetlands Ecology and Management. 10.1007/s11273-021-09828-2 [DOI] [Google Scholar]
- Filicetti, A. T. , Cody, M. , & Nielsen, S. E. (2019). Caribou conservation: Restoring trees on seismic lines in Alberta, Canada. Forests. 10(2), 185. 10.3390/f10020185. http://www.mdpi.com/1999-4907/10/2/185 [DOI] [Google Scholar]
- Finnegan, L. , Pigeon, K. E. , & MacNearney, D. (2019). Predicting patterns of vegetation recovery on seismic lines: Informing restoration based on understory species composition and growth. Forest Ecology and Management, 446, 175–192. 10.1016/j.foreco.2019.05.026 [DOI] [Google Scholar]
- Fisher, J. T. , Anholt, B. , & Volpe, J. P. (2011). Body mass explains characteristic scales of habitat selection in terrestrial mammals. Ecology and Evolution, 1(4), 517–528. 10.1002/ece3.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, J. T. , & Burton, A. C. (2018). Wildlife winners and losers in an oil sands landscape. Frontiers in Ecology and the Environment, 16(6), 323–328. 10.1002/fee.1807 [DOI] [Google Scholar]
- Fisher, J. T. , & Burton, A. C. (2021). Spatial structure of reproductive success infers mechanisms of ungulate invasion in nearctic boreal landscapes. Ecology and Evolution, 11(2), 900–911. 10.1002/ece3.7103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, J. T. , Burton, A. C. , Nolan, L. , & Roy, L. (2020). Influences of landscape change and winter severity on invasive ungulate persistence in the nearctic boreal forest. Scientific Reports, 10(1), 1–11. 10.1038/s41598-020-65385-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, J. T. , Grey, F. , Anderson, N. , Sawan, J. , Anderson, N. , Chai, S.‐L. , Nolan, L. , Underwood, A. , Amerongen Maddison, J. , Fuller, H. W. , & Frey, S. (2021). Indigenous‐led camera‐trap research on traditional territories informs conservation decisions for resource extraction. FACETS, 6, 1266–1284. 10.1139/facets-2020-0087 [DOI] [Google Scholar]
- Foster, K. R. , Davidson, C. , Tanna, R. N. , & Spink, D. (2019). Introduction to the virtual special issue monitoring ecological responses to air quality and atmospheric deposition in the Athabasca Oil Sands region the Wood Buffalo Environmental Association's Forest health monitoring program. Science of the Total Environment, 686, 345–359. 10.1016/j.scitotenv.2019.05.353 [DOI] [PubMed] [Google Scholar]
- Foster, K. R. , Godwin, C. M. , Pyle, P. , & Saracco, J. F. (2017). Reclamation and habitat‐disturbance effects on landbird abundance and productivity indices in the oil sands region of northeastern Alberta, Canada. Restoration Ecology, 25(4), 532–538. 10.1111/rec.12478 [DOI] [Google Scholar]
- Giesy, J. P. , Anderson, J. C. , & Wiseman, S. B. (2010). Alberta oil sands development. Proceedings of the National Academy of Sciences of the United States of America, 107(3), 951–952. 10.1073/pnas.0912880107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin, C. M. , Barclay, R. M. R. , & Smits, J. E. G. (2019). Tree swallow (Tachycineta bicolor) nest success and nestling growth near oil sands mining operations in northeastern Alberta, Canada. Canadian Journal of Zoology, 97(6), 547–557. 10.1139/cjz-2018-0247 [DOI] [Google Scholar]
- Guo, X. , Coops, N. C. , Tompalski, P. , Nielsen, S. E. , Bater, C. W. , & John Stadt, J. (2017). Regional mapping of vegetation structure for biodiversity monitoring using airborne LiDAR data. Ecological Informatics, 38, 50–61. 10.1016/j.ecoinf.2017.01.005 [DOI] [Google Scholar]
- Haddaway, N. R. , Woodcock, P. , Macura, B. , & Collins, A. (2015). Making literature reviews more reliable through application of lessons from systematic reviews. Conservation Biology, 29, 1596–1605. 10.1111/cobi.12541 [DOI] [PubMed] [Google Scholar]
- Haughland, D. L. , Hero, J.‐M. , Schieck, J. , Castley, J. G. , Boutin, S. , Sólymos, P. , Lawson, B. E. , Holloway, G. , & Magnusson, W. E. (2010). Planning forwards: Biodiversity research and monitoring systems for better management. Trends in Ecology & Evolution, 25(4), 199–200. 10.1016/j.tree.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Hebert, C. E. (2019). The river runs through it: The Athabasca River delivers mercury to aquatic birds breeding far downstream. PLoS One, 14(4), 1–19. 10.1371/journal.pone.0206192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert, C. E. , Campbell, D. , Kindopp, R. , Macmillan, S. , Martin, P. , Neugebauer, E. , Patterson, L. , & Shatford, J. (2013). Mercury trends in colonial waterbird eggs downstream of the oil sands region of Alberta, Canada. Environmental Science and Technology, 47(20), 11785–11792. 10.1021/es402542w [DOI] [PubMed] [Google Scholar]
- Hebert, C. E. , Weseloh, D. V. C. , Macmillan, S. , Campbell, D. , & Nordstrom, W. (2011). Metals and polycyclic aromatic hydrocarbons in colonial waterbird eggs from Lake Athabasca and the Peace‐Athabasca Delta, Canada. Environmental Toxicology and Chemistry, 30(5), 1178–1183. 10.1002/etc.489 [DOI] [PubMed] [Google Scholar]
- Hedley, R. W. , McLeod, L. J. T. , Yip, D. A. , Farr, D. , Knaga, P. , Drake, K. L. , & Bayne, E. (2020). Modeling the occurrence of the yellow rail (Coturnicops noveboracensis) in the context of ongoing resource development in the oil sands region of Alberta. Avian Conservation and Ecology, 15(1), 1–14. 10.5751/ACE-01538-150110 [DOI] [Google Scholar]
- Hillebrand, H. (2004). On the generality of the latitudinal diversity gradient. The American Naturalist, 163(2), 192–211. 10.1086/381004 [DOI] [PubMed] [Google Scholar]
- Horb, E. C. , Wentworth, G. R. , Makar, P. A. , Liggio, J. , Hayden, K. , Boutzis, E. I. , Beausoleil, D. L. , Hazewinkel, R. O. , Mahaffey, A. C. , Sayanda, D. , Wyatt, F. , & Dubé, M. G. (2021). A decadal synthesis of atmospheric emissions, ambient air quality, and deposition in the oil sands region. Integrated Environmental Assessment and Management, 18(2), 333-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ims, R. A. , & Yoccoz, N. G. (2017). Ecosystem‐based monitoring in the age of rapid climate change and new technologies. Current Opinion in Environmental Sustainability, 29, 170–176. 10.1016/j.cosust.2018.01.003 [DOI] [Google Scholar]
- Kolowski, J. M. , & Forrester, T. D. (2017). Camera trap placement and the potential for bias due to trails and other features. PLoS One, 12(10), 1–20. 10.1371/journal.pone.0186679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, M. S. , Studabaker, W. B. , Patrick Pancras, J. , Graney, J. R. , Puckett, K. , White, E. M. , & Edgerton, E. S. (2019). Source apportionment of an epiphytic lichen biomonitor to elucidate the sources and spatial distribution of polycyclic aromatic hydrocarbons in the Athabasca oil sands region, Alberta, Canada. Science of the Total Environment, 654, 1241–1257. 10.1016/j.scitotenv.2018.11.131 [DOI] [PubMed] [Google Scholar]
- Latham, A. D. M. , Latham, M. C. , & Boyce, M. S. (2011). Habitat selection and spatial relationships of black bears (Ursus americanus) with woodland caribou (Rangifer tarandus caribou) in northeastern Alberta. Canadian Journal of Zoology, 89(4), 267–276. 10.1139/z10-115 [DOI] [Google Scholar]
- Latham, A. D. M. , Latham, M. C. , Boyce, M. S. , & Boutin, S. (2011). Movement responses by wolves to industrial linear features and their effect on woodland caribou in northeastern alberta. Ecological Applications, 21(8), 2854–2865. 10.1890/11-0666.1 [DOI] [Google Scholar]
- Latifovic, R. , & Pouliot, D. (2014). Monitoring cumulative long‐term vegetation changes over the Athabasca oil sands region. IEEE Journal of Selected Topics in Applied Earth Observations and Remote Sensing, 7(8), 3380–3392. 10.1109/JSTARS.2014.2321058 [DOI] [Google Scholar]
- Laxton, D. L. , Watmough, S. A. , Aherne, J. , & Straker, J. (2010). An assessment of nitrogen saturation in Pinus banksiana plots in the Athabasca oil sands region, Alberta. Journal of Limnology, 69(Suppl. 1), 171–180. 10.3274/JL10-69-S1-17 [DOI] [Google Scholar]
- Legendre, P. (1993). Spatial autocorrelation: Trouble or new paradigm? Ecology, 74(6), 1659–1673. 10.2307/1939924 [DOI] [Google Scholar]
- Levin, S. A. (1992). The problem of pattern and scale in ecology. Ecology, 73(6), 1943–1967. 10.2307/1941447 [DOI] [Google Scholar]
- Lindenmayer, D. B. , & Likens, G. E. (2009). Adaptive monitoring: A new paradigm for long‐term research and monitoring. Trends in Ecology and Evolution, 24(9), 482–486. 10.1016/j.tree.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Lovitt, J. , Rahman, M. M. , Saraswati, S. , McDermid, G. J. , Strack, M. , & Xu, B. (2018). UAV remote sensing can reveal the effects of low‐impact seismic lines on surface morphology, hydrology, and methane (CH4) release in a boreal treed bog. Journal of Geophysical Research: Biogeosciences, 123(3), 1117–1129. 10.1002/2017JG004232 [DOI] [Google Scholar]
- Lundin, J. I. , Riffell, J. A. , & Wasser, S. K. (2015). Polycyclic aromatic hydrocarbons in caribou, moose, and wolf scat samples from three areas of the Alberta oil sands. Environmental Pollution, 206, 527–534. 10.1016/j.envpol.2015.07.035 [DOI] [PubMed] [Google Scholar]
- MacKenzie, M. D. , & Dietrich, S. T. (2020). Atmospheric sulfur and nitrogen deposition in the Athabasca oil sands region is correlated with foliar nutrient levels and soil chemical properties. Science of the Total Environment, 711, 134737. 10.1016/j.scitotenv.2019.134737 [DOI] [PubMed] [Google Scholar]
- Mahon, C. L. , Bayne, E. M. , Sólymos, P. , Matsuoka, S. M. , Carlson, M. , Dzus, E. , Schmiegelow, F. K. A. , & Song, S. J. (2014). Does expected future landscape condition support proposed population objectives for boreal birds? Forest Ecology and Management, 312, 28–39. 10.1016/j.foreco.2013.10.025 [DOI] [Google Scholar]
- Mahon, C. L. , Holloway, G. , Sólymos, P. , Cumming, S. G. , Bayne, E. M. , Schmiegelow, F. K. A. , & Song, S. J. (2016). Community structure and niche characteristics of upland and lowland western boreal birds at multiple spatial scales. Forest Ecology and Management, 361, 99–116. 10.1016/j.foreco.2015.11.007 [DOI] [Google Scholar]
- Mahon, C. L. , Holloway, G. L. , Bayne, E. M. , & Toms, J. D. (2019). Additive and interactive cumulative effects on boreal landbirds: winners and losers in a multi‐stressor landscape. Ecological Applications, 29(5), e01895. 10.1002/eap.1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie, H. W. , Merrill, E. H. , Spiteri, R. J. , & Lewis, M. A. (2012). How linear features alter predator movement and the functional response. Interface Focus, 2(2), 205–216. 10.1098/rsfs.2011.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. A. , Benscoter, B. W. , & Turetsky, M. R. (2015). The effect of long‐term drying associated with experimental drainage and road construction on vegetation composition and productivity in boreal fens. Wetlands Ecology and Management, 23(5), 845–854. 10.1007/s11273-015-9423-5 [DOI] [Google Scholar]
- Muhly, T. , Serrouya, R. , Neilson, E. , Li, H. , & Boutin, S. (2015). Influence of in‐situ oil sands development on caribou (Rangifer tarandus) movement. PLoS One, 10(9), e0136933. 10.1371/journal.pone.0136933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murcia, C. (1995). Edge effects in fragmented forests: Implications for conservation. Trends in Ecology & Evolution, 10(2), 58–62. 10.1016/S0169-5347(00)88977-6 [DOI] [PubMed]
- Neilson, E. W. , & Boutin, S. (2017). Human disturbance alters the predation rate of moose in the Athabasca oil sands. Ecosphere, 8(8), e01913. 10.1002/ecs2.1913 [DOI] [Google Scholar]
- O'Donoghue, M. , Boutin, S. , Krebs, C. J. , & Hofer, E. J. (1997). Numerical responses of coyotes and lynx to the snowshoe hare cycle. Oikos, 80(1), 150. 10.2307/3546526 [DOI] [Google Scholar]
- O'Neill, R. V. , Hunsaker, C. T. , Timmins, S. P. , Jackson, B. L. , Jones, K. B. , Riitters, K. H. , & Wickham, J. D. (1996). Scale problems in reporting landscape pattern at the regional scale. Landscape Ecology, 11(3), 169–180. 10.1007/BF02447515 [DOI] [Google Scholar]
- Pearse, A. T. , Brandt, D. A. , Hartup, B. K. , & Bidwell, M. T. (2018). Mortality in Aransas‐Wood Buffalo whooping cranes: Timing, location, and causes. In Nyhus P. J., French J. B. Jr., Converse S. J., Austin J. E., & Delap J. H. (Eds.), Whooping cranes: Biology and conservation: Biodiversity of the world: Conservation from genes to landscapes (pp. 125–138). Academic Press. [Google Scholar]
- Pearse, A. T. , Metzger, K. L. , Brandt, D. A. , Bidwell, M. T. , Harner, M. J. , Baasch, D. M. , & Harrell, W. (2019). Heterogeneity in migration strategies of whooping cranes. Condor, 122(1), 1–15. 10.1093/condor/duz056 [DOI] [Google Scholar]
- Pigeon, K. E. , Anderson, M. , MacNearney, D. , Cranston, J. , Stenhouse, G. , & Finnegan, L. (2016). Toward the restoration of caribou habitat: Understanding factors associated with human motorized use of legacy seismic lines. Environmental Management, 58(5), 821–832. 10.1007/s00267-016-0763-6 [DOI] [PubMed] [Google Scholar]
- Pouliot, D. , & Latifovic, R. (2016). Land change attribution based on Landsat time series and integration of ancillary disturbance data in the Athabasca oil sands region of Canada. GIScience and Remote Sensing, 53(3), 382–401. 10.1080/15481603.2015.1137112 [DOI] [Google Scholar]
- Proemse, B. C. , Maynard, D. G. , & Mayer, B. (2016). Foliage chemistry of Pinus banksiana in the Athabasca oil sands region, Alberta, Canada. Forests, 7(12), 312–325. 10.3390/f7120312 [DOI] [Google Scholar]
- Riva, F. , Acorn, J. H. , & Nielsen, S. E. (2018a). Distribution of cranberry blue butterflies (Agriades optilete) and their responses to forest disturbance from in situ oil sands and wildfires. Diversity, 10(4), 112. 10.3390/d10040112. http://www.mdpi.com/1424-2818/10/4/112 [DOI] [Google Scholar]
- Riva, F. , Acorn, J. H. , & Nielsen, S. E. (2018b). Localized disturbances from oil sands developments increase butterfly diversity and abundance in Alberta's boreal forests. Biological Conservation, 217, 173–180. 10.1016/j.biocon.2017.10.022 [DOI] [Google Scholar]
- Roberts, D. R. , Hazewinkel, R. R. O. , Arciszewski, T. J. , Beausoleil, D. L. , Davidson, C. J. , Horb, E. C. , Sayanda, D. , Wentworth, G. R. , Wyatt, F. , & Dubé, M. G. (2021a). An integrated knowledge synthesis of regional ambient monitoring in Canada's oil sands. Integrated Environmental Assessment and Management, 18(2), 428-441. 10.1002/ieam.4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, A. P. , & Lyon, B. E. (2013). Day length, reproductive effort, and the avian latitudinal clutch size gradient. Ecology, 94(6), 1327–1337. 10.1890/12-0953.1 [DOI] [PubMed] [Google Scholar]
- Saraswati, S. , Petrone, R. M. , Rahman, M. M. , McDermid, G. J. , Xu, B. , & Strack, M. (2020). Hydrological effects of resource‐access road crossings on boreal forested peatlands. Journal of Hydrology, 584, 124748. 10.1016/j.jhydrol.2020.124748 [DOI] [Google Scholar]
- Schieck, J. , & Song, S. J. (2006). Changes in bird communities throughout succession following fire and harvest in boreal forests of western North America: Literature review and meta‐analyses. Canadian Journal of Forest Research, 36(5), 1299–1318. 10.1139/X06-017 [DOI] [Google Scholar]
- Shonfield, J. , & Bayne, E. M. (2017a). Autonomous recording units in avian ecological research: Current use and future applications. Avian Conservation and Ecology, 12(1), 14. 10.5751/ACE-00974-120114 [DOI] [Google Scholar]
- Shonfield, J. , & Bayne, E. M. (2017b). The effect of industrial noise on owl occupancy in the boreal forest at multiple spatial scales. Avian Conservation and Ecology, 12(2), art13. 10.5751/ACE-01042-120213. http://www.ace-eco.org/vol12/iss2/art13/ [DOI] [Google Scholar]
- Shonfield, J. , & Bayne, E. M. (2019). Effects of industrial disturbance on abundance and activity of small mammals. Canadian Journal of Zoology, 97(11), 1013–1020. 10.1139/cjz-2019-0098 [DOI] [Google Scholar]
- Sólymos, P. , Toms, J. D. , Matsuoka, S. M. , Cumming, S. G. , Barker, N. K. S. , Thogmartin, W. E. , Stralberg, D. , Crosby, A. D. , Dénes, F. V. , Haché, S. , Mahon, C. L. , Schmiegelow, F. K. A. , & Bayne, E. M. (2020). Lessons learned from comparing spatially explicit models and the Partners in Flight approach to estimate population sizes of boreal birds in Alberta, Canada. Condor, 122(2), 1–21. 10.1093/condor/duaa007 [DOI] [Google Scholar]
- Stevenson, C. J. , Filicetti, A. T. , & Nielsen, S. E. (2019). High precision altimeter demonstrates simplification and depression of microtopography on seismic lines in treed peatlands. Forests, 10(4), 295. 10.3390/f10040295 [DOI] [Google Scholar]
- Strack, M. , Softa, D. , Bird, M. , & Xu, B. (2018). Impact of winter roads on boreal peatland carbon exchange. Global Change Biology, 24(1), e201–e212. 10.1111/gcb.13844 [DOI] [PubMed] [Google Scholar]
- Stralberg, D. , Matsuoka, S. M. , Hamann, A. , Bayne, E. M. , Sólymos, P. , Schmiegelow, F. K. A. , Wang, X. , Cumming, S. G. , & Song, S. J. (2015). Projecting boreal bird responses to climate change: The signal exceeds the noise. Ecological Applications, 25(1), 52–69. 10.1890/13-2289.1 [DOI] [PubMed] [Google Scholar]
- Tattersall, E. R. , Burgar, J. M. , Fisher, J. T. , & Burton, A. C. (2020a). Mammal seismic line use varies with restoration: Applying habitat restoration to species at risk conservation in a working landscape. Biological Conservation, 241, 108295. 10.1016/j.biocon.2019.108295 [DOI] [Google Scholar]
- Tattersall, E. R. , Burgar, J. M. , Fisher, J. T. , & Burton, A. C. (2020b). Boreal predator co‐occurrences reveal shared use of seismic lines in a working landscape. Ecology and Evolution, 10(3), 1678–1691. 10.1002/ece3.6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terborgh, J. & Estes, J. A. , (Eds). (2010). Trophic cascades: Predators, prey and the changing dynamics of nature. Island Press. [Google Scholar]
- Thomas, P. J. , Eccles, K. M. , & Mundy, L. J. (2017a). Spatial modelling of non‐target exposure to anticoagulant rodenticides can inform mitigation options in two boreal predators inhabiting areas with intensive oil and gas development. Biological Conservation, 212, 111–119. 10.1016/j.biocon.2017.06.005 [DOI] [Google Scholar]
- Thomas, P. J. , Eccles, K. M. , & Mundy, L. J. (2017b). Spatial modelling of non‐target exposure to anticoagulant rodenticides can inform mitigation options in two boreal predators inhabiting areas with intensive oil and gas development. Biological Conservation, 212, 111–119. 10.1016/j.biocon.2017.06.005 [DOI] [Google Scholar]
- Thomas, P. J. , Newell, E. E. , Eccles, K. , Holloway, A. C. , Idowu, I. , Xia, Z. , Hassan, E. , Tomy, G. , & Quenneville, C. (2021). Co‐exposures to trace elements and polycyclic aromatic compounds (PACs) impacts North American river otter (Lontra canadensis) baculum. Chemosphere, 265, 128920. 10.1016/j.chemosphere.2020.128920 [DOI] [PubMed] [Google Scholar]
- Tigner, J. , Bayne, E. M. , & Boutin, S. (2015). American marten respond to seismic lines in northern Canada at two spatial scales. PLoS One, 10(3), e0118720. 10.1371/journal.pone.0118720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews, M. , Juanes, F. , & Burton, A. C. (2017). Mammal responses to human footprint vary with spatial extent but not with spatial grain. Ecosphere, 8(3), e01735. 10.1002/ecs2.1735 [DOI] [Google Scholar]
- Toews, M. , Juanes, F. , & Burton, A. C. (2018). Mammal responses to the human footprint vary across species and stressors. Journal of Environmental Management, 217, 690–699. 10.1016/j.jenvman.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Van Rensen, C. K. , Nielsen, S. E. , White, B. , Vinge, T. , & Lieffers, V. J. (2015). Natural regeneration of forest vegetation on legacy seismic lines in boreal habitats in Alberta's oil sands region. Biological Conservation, 184, 127–135. 10.1016/j.biocon.2015.01.020 [DOI] [Google Scholar]
- Venier, L. A. , Thompson, I. D. , Fleming, R. , Malcolm, J. , Aubin, I. , Trofymow, J. A. , Langor, D. , Sturrock, R. , Patry, C. , Outerbridge, R. O. , Holmes, S. B. , Haeussler, S. , De Grandpré, L. , Chen, H. Y. H. , Bayne, E. , Arsenault, A. , & Brandt, J. P. (2014). Effects of natural resource development on the terrestrial biodiversity of Canadian boreal forests. Environmental Reviews, 22(4), 457–490. 10.1139/er-2013-0075 [DOI] [Google Scholar]
- Wallace, S. J. , de Solla, S. R. , Head, J. A. , Hodson, P. V. , Parrott, J. L. , Thomas, P. J. , Berthiaume, A. , & Langlois, V. S. (2020). Polycyclic aromatic compounds (PACs) in the Canadian environment: Exposure and effects on wildlife. Environmental Pollution, 265, 114863. 10.1016/j.envpol.2020.114863 [DOI] [PubMed] [Google Scholar]
- Ward, E. M. , & Gorelick, S. M. (2018). Drying drives decline in muskrat population in the Peace‐Athabasca Delta, Canada. Environmental Research Letters, 13(12), 124026. 10.1088/1748-9326/aaf0ec [DOI] [Google Scholar]
- Watmough, S. A. , Bird, A. , McDonough, A. , & Grimm, E. (2019). Forest fertilization associated with oil sands emissions. Ecosystems, 22(1), 1–14. 10.1007/s10021-018-0249-x [DOI] [Google Scholar]
- Westgate, M. J. , & Lindenmayer, D. B. (2017). The difficulties of systematic reviews. Conservation Biology, 31(5), 1002–1007. 10.1111/cobi.12890 [DOI] [PubMed] [Google Scholar]
- Whitman, E. , Parisien, M. A. , Price, D. T. , St‐Laurent, M. H. , Johnson, C. J. , Delancey, E. R. , Arseneault, D. , & Flannigan, M. D. (2017). A framework for modeling habitat quality in disturbance‐prone areas demonstrated with woodland caribou and wildfire. Ecosphere, 8(4), e01787. 10.1002/ecs2.1787 [DOI] [Google Scholar]
- Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology, 3(4), 385. 10.2307/2389612 [DOI] [Google Scholar]
- Wittische, J. , Heckbert, S. , James, P. M. A. , Burton, A. C. , & Fisher, J. T. (2021). Community‐level modelling of boreal forest mammal distribution in an oil sands landscape. Science of the Total Environment, 755, 142500. 10.1016/j.scitotenv.2020.142500 [DOI] [PubMed] [Google Scholar]
- Xiao, Y. , & Watson, M. (2019). Guidance on conducting a systematic literature review. Journal of Planning Education and Research, 39(1), 93–112. 10.1177/0739456X17723971 [DOI] [Google Scholar]
- Yoccoz, N. G. , Nichols, J. D. , & Boulinier, T. (2001). Monitoring of biological diversity in space and time. Trends in Ecology & Evolution, 16(8), 446–453. 10.1016/S0169-5347(01)02205-4 [DOI] [Google Scholar]
- Zhang, J. , Kissling, W. D. , & He, F. (2013). Local forest structure, climate and human disturbance determine regional distribution of boreal bird species richness in Alberta, Canada. Journal of Biogeography, 40(6), 1131–1142. 10.1111/jbi.12063 [DOI] [Google Scholar]
- Zhang, J. , Nielsen, S. E. , Grainger, T. N. , Kohler, M. , Chipchar, T. , & Farr, D. R. (2014). Sampling plant diversity and rarity at landscape scales: Importance of sampling time in species detectability. PLoS One, 9(4), e95334. 10.1371/journal.pone.0095334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. (2019). Economic incentives for land reclamation: Evidence from the oilsands industry in Alberta [MSc]. University of Alberta. https://era.library.ualberta.ca/items/897ca5ba-6ecb-428b-8b3b-0d1bfe027991
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supporting Information.
Appendix 1: Compiled tables of all reviewed literature.
Appendix 2: Extended results and discussion.
Appendix 3: Numbered bibliography of reviewed literature, sorted by conceptual model column and box.
Appendix 4: Summary table of mammal responses to landscape disturbance in the OSR.
Data Availability Statement
No new data were generated by this literature review.


