Abstract
Many jurisdictions use point‐of‐collection (POC) oral fluid testing devices to identify driving under the influence of cannabis, indexed by the presence of Δ9‐tetrahydrocannabinol (THC), an intoxicating cannabinoid, in oral fluid. Although the use of the non‐intoxicating cannabinoid, cannabidiol (CBD), is not prohibited among drivers, it is unclear whether these devices can reliably distinguish between CBD and THC, which have similar chemical structures. This study determined whether orally administered CBD produces false‐positive tests for THC on standard, POC oral fluid testing devices. In a randomised, double‐blind, crossover design, healthy participants (n = 17) completed four treatment sessions involving the administration of either placebo or 15‐, 300‐ or 1500‐mg pure CBD in a high‐fat dietary supplement. Oral fluid was sampled, and the DrugWipe®‐5S (DW‐5S; 10 ng·ml−1 THC cut‐off) and Drug Test® 5000 (DT5000; 10 ng·mL−1 THC cut‐off) devices administered, at baseline (pretreatment) and ~20‐, ~145‐ and ~185‐min posttreatment. Oral fluid cannabinoid concentrations were measured using ultra‐high performance liquid chromatography–tandem mass spectrometry. Median (interquartile range [IQR]) oral fluid CBD concentrations were highest at ~20 min, quantified as 0.4 (6.0), 15.8 (41.6) and 167 (233) ng·ml−1 on the 15‐, 300‐ and 1500‐mg CBD treatments, respectively. THC, cannabinol and cannabigerol were not detected in any samples. A total of 259 DW‐5S and 256 DT5000 tests were successfully completed, and no THC‐positive tests were observed. Orally administered CBD does not appear to produce false‐positive (or true‐positive) tests for THC on the DW‐5S and DT5000. The likelihood of an individual who is using a CBD (only) oral formulation being falsely accused of DUIC therefore appears low.
Keywords: cannabidiol, cannabis, drug driving, oral fluid, point‐of‐collection testing
CBD did not produce any false‐positive (or true‐positive) tests for THC on the Securetec DrugWipe® 5S or Dräger DrugTest® 5000 when administered orally and in doses of 15, 300 and 1500 mg. This suggests that the oral fluid testing devices used by authorities to identify driving under the influence of cannabis are able to distinguish between THC and CBD, despite their structural similarities.

1. INTRODUCTION
Cannabidiol (CBD) is a non‐intoxicating cannabinoid found in the Cannabis sativa plant. 1 CBD has demonstrated considerable therapeutic potential at oral doses of ~300–1500 mg 2 and is increasingly being prescribed for the treatment of anxiety, epilepsy, pain and other conditions. 3 The use of non‐prescription CBD has also become common in Europe and North America where low‐dose ‘nutraceuticals’ (e.g., oils, capsules, topicals and edibles, often containing ~10–20 mg·ml−1) can be purchased over the counter. 4 Unlike the other major plant‐derived cannabinoid, Δ9‐tetrahydrocannabinol (THC), 5 , 6 CBD does not appear to impair cognitive function or induce intoxication. 6 , 7 Thus, although most jurisdictions worldwide have enacted legislation restricting THC use in conjunction with driving (i.e., driving under the influence of cannabis [DUIC]), 8 CBD use is not typically prohibited among drivers.
Although they are distinct chemical entities with differing pharmacological profiles, CBD is structurally related to THC with the latter being a transannular cyclisation product of the former. 9 THC, however, has a relatively flat and rigid fused tricyclic structure whereas CBD has a bicyclic core with an ‘out‐of‐plane’ geometry for the two rings. 9 Nevertheless, their chemical similarity is such that there may be some risk of CBD being mistakenly identified as‐ (i.e., cross‐reactive with‐) THC on rapid drug tests that use less‐specific structure‐dependent analytical methods (e.g., lateral flow immunoassays). This includes the point‐of‐collection (POC) oral fluid testing devices used by authorities to identify DUIC. 10 , 11
POC oral fluid testing devices are currently used to identify DUIC in a number of countries including Norway, Germany, Belgium and Australia. 12 In Australia, this process is known as mobile drug testing and typically involves an initial test for THC in oral fluid using the Securetec DrugWipe® (DW). If positive, a second test may be performed using the Dräger DrugTest® 5000 (DT5000). If both devices yield positive results, confirmatory analyses are later completed on a stored oral fluid sample using more sophisticated mass spectrometry techniques. Recent studies have shown that although the DW‐5S has high specificity (i.e., can distinguish between THC and placebo) (≥92%), its sensitivity (45%–51%) and accuracy (68%–79%) are poor. 12 , 13 Some, although not all, studies have also found the sensitivity (40%–100% 12 , 13 , 14 ), specificity (71%–100% 13 , 14 , 15 , 16 ) and accuracy (56%–90% 12 , 13 ) of the DT5000 to be poor (ranges include data obtained at a screening cut‐off ≤10 ng·ml−1 and confirmatory cut‐off ≥1 ng·ml−1, only 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 ). Whether these devices, which use lateral flow immunoassay technology, 10 , 11 can effectively distinguish between different cannabinoids with similar molecular structures is, however, unclear.
The aim of the current study was to determine whether acute, oral CBD treatment at doses of 15, 300 and 1500 mg produces false‐positive tests for THC on two standard, POC oral fluid testing devices: the DW‐5S and DT5000. The study was conducted within another randomised controlled trial (RCT) investigating the effects of CBD on driving performance. 20
2. METHODS
The protocol of the parent RCT has been published elsewhere 20 and registered on the Australia and New Zealand Clinical Trials Registry (ACTRN12619001552178). The current article summarises the substudy only. This investigation was approved by the University of Sydney's Human Research Ethics Committee (2019/474).
2.1. Participant population
Participants were healthy males and females aged between 18 and 65 years who had not used cannabis in the last 3 months. Individuals were informed of the study requirements and risks before providing written informed consent. The full eligibility criteria and details of the recruitment and screening processes are published elsewhere. 20
2.2. Study design
Participants completed four treatment sessions involving the oral administration of either placebo, or 15‐, 300‐ or 1500‐mg CBD. Treatment order was randomised as described elsewhere 20 and double‐blind. Sessions were separated by a washout period ≥7 days. Individuals were instructed to avoid using illicit drugs (including cannabis) throughout their involvement and to adhere to strict standardisation procedures (e.g., restricting alcohol and caffeine consumption) prior to each treatment session (see McCartney et al. 20 for further details).
2.3. Experimental procedures
Participants arrived at the laboratory between 07:00 am and 09:00 am on the morning of each session and verbally acknowledged compliance to the standardisation procedures; they also completed a urine drug screen (DrugCheck® NxStep Onsite Urine Test Cup) to verify abstinence from illicit drugs.
The DW‐5S and DT5000 POC oral fluid testing devices were administered at baseline (pretreatment) and on three occasions thereafter: ~20‐, 145‐ and 185‐min posttreatment. Separate oral fluid samples were also collected at these time points (as described below). The order of sample collection was (1) DW‐5S; (2) DT5000; and (3) oral fluid. To maintain the blind, neither the participant nor researchers with whom they had direct contact were informed of the test results or of the measured oral fluid cannabinoid concentrations until the study had concluded.
2.4. Study treatments
The investigational product (GD Cann®–C; GD Pharma Pty Ltd, Norwood, Australia) was an oral formulation of synthetic CBD (100 mg·ml−1) dissolved in medium chain triglyceride (MCT) oil. It was administered in different volumes providing either 15, 300 or 1500 mg of CBD. Each dose was made up to a total equivalent volume of 15.0 ml via the addition of placebo (MCT) oil and administered (orally) in a high‐fat supplement (100 ml; 50 g fat) (Calogen®, Nutricia, Macquarie Park, Australia) alongside a standardised breakfast. Neither treatment contained any other minor cannabinoids or terpenes/terpenoids. The intent of the high‐fat supplement was to increase CBD's bioavailability in the parent RCT. 21 , 22
2.5. Data collection
2.5.1. Oral fluid cannabinoid concentrations
Oral fluid (~1 ml) was collected using the Quantisal® Collection Device (Immunalysis Corporation, Pomona, CA, USA). Participants placed the cellulose collection pad under their tongues for between ~2 and 10 min until the indicator turned completely blue (all turned blue within 10 min); the pad was then transferred into the plastic tube containing the preservative buffer and stored at +4°C until analysis. Oral fluid THC and CBD concentrations appear to be ‘stable’ (i.e., remain within 20% of baseline levels) under these conditions for at least 90 days, 23 and most samples (81%) were analysed within this timeframe (range: 3–120 days).
Oral fluid CBD, THC, cannabinol (CBN) and cannabigerol (CBG) concentrations were measured using ultra‐high performance liquid chromatography–tandem mass spectrometry (UHPLC–MS/MS). Briefly, cannabinoids were extracted from 400‐μl Quantisal‐buffered oral fluid in triplicate via supported liquid extraction (Biotage Isolute 400‐μl capacity 96 well plates), using 700‐μl dichloromethane and 900‐μl methyl tert‐butyl ether sequentially as extraction solvents. Extracted samples were dried under a gentle stream of nitrogen and reconstituted in a 1:1 mixture of 0.1% formic acid in water and acetonitrile for UHPLC–MS/MS analysis.
Cannabinoids were identified and quantified on a Shimadzu Nexera UHPLC equipped with an Agilent Zorbax XDB‐C18 analytical column (2.1 × 50 mm, 3.5‐μm particle size), coupled with a Shimadzu 8040 triple‐quadrupole mass spectrometer operated in positive electrospray ionisation mode with multiple reaction monitoring. Gradient elution was performed using a mixture of (A) 0.1% formic acid in water and (B) acetonitrile, at a total flow rate of 0.6 ml/min and a column oven temperature of 50°C. Mobile phase composition was initially set 50% B where it was held for 1.5 min, before increasing to 75% B at 2.75 min and held until 4.75 min, followed by a final increase to 95% B at 5.25 min before re‐equilibration at 50% B for a total run‐time of 6 min. Accuracy, precision, linearity and sensitivity were assessed using Food and Drug Administration (FDA) guidelines for bioanalytical method validation. 24 Each analyte was quantified against a 7‐point standard curve with deuterated internal standards (d3‐THC and d3‐CBD) used to account for matrix effects. Low, medium, and high‐concentration quality control (QC) samples were added to each plate to ensure sufficient accuracy (100 ± 15%) and precision (relative standard deviation <15%). Positive analyte identification required a retention time match to analytical reference material together with correct ion transitions (one quantifier and one qualifier transition per analyte). Further analytical parameters, including limits of detection and lower limits of quantification are provided in Table S1.
2.5.2. Securetec DrugWipe® 5S (DW‐5S) and Dräger DrugTest® 5000 (DT5000)
The Securetec DrugWipe® 5S (DW‐5S) detects cannabis (THC at concentrations >10 ng·ml−1), opiates, cocaine and (meth)amphetamines. Participants were instructed to circle the inside of their mouths with their tongue three times before gently wiping the collection pad from the centre‐middle of their tongue to the tip. The pad was then attached to the device and the test performed as per the manufacturer's instructions.
The Dräger DrugTest® 5000 (DT5000) detects cannabis, opiates, (meth)amphetamines, methadone, ketamine and benzodiazepines. The limit of detection can be set to 5, 10 or 25 ng·ml−1 THC; this study used a mid‐range limit (10 ng·ml−1) comparable with that of the DW‐5S as the authorities have not published the cut‐offs used during mobile drug testing in Australia. Participants were instructed to move the collection pad around the inside of their mouths for between for ~1–4 min until the indicator turned blue. The pad was then inserted into the device and the test performed as per the manufacturer's instructions. Both of these devices generated qualitative results (i.e., positive, negative and invalid).
2.6. Data analysis
The results of the DW‐5S and DT5000 drug tests were classified as true positive (a positive drug test confirmed by a UHLC–MS/MS THC concentration less than or equal to the limit of quantification [LOQ]), true negative (a negative drug test confirmed by a UHLC–MS/MS THC concentration <LOQ), false positive (a positive drug test accompanied by a UHLC–MS/MS THC concentration <LOQ) or false negative (a negative drug test accompanied by a UHLC–MS/MS THC concentration ≥LOQ) based on the UHLC–MS/MS quantified oral fluid THC concentrations and a LOQ of 0.25 ng·ml−1 THC. Data are presented as median (interquartile range [IQR]) unless otherwise stated.
3. RESULTS
3.1. Participants
Nineteen participants were initially randomised in this study. However, one was unable to complete all four treatment sessions within the 60‐day (drug expiration) period due a suspension on face‐to‐face research during the SARS‐CoV‐2 pandemic and another had detectable levels of 11‐COOH‐THC in plasma at baseline indicating that she had not abstained from cannabis as instructed (data reported elsewhere 25 ). Both individuals were removed from the final sample. The characteristics of the 17 remaining participants are summarised in Table 1.
TABLE 1.
Participant characteristics
| Characteristic | Participants (n = 17) |
|---|---|
| Sex (M/F) (n) | 10/7 |
| Age (years) | 27.9 (7.0) |
| Weight (kg) | 67.4 (23.0) |
| Body mass index (kg·m2) | 22.0 (4.3) |
| Lifetime cannabis exposures (n) | |
| ≤10 uses | 6 |
| >10 uses | 10 |
| No use | 1 |
| Time since last cannabis use (n) | |
| 3–6 months | 3 |
| 6–12 months | 5 |
| 1–2 years | 3 |
| 2–4 years | 2 |
| >4 years | 3 |
| Lifetime CBD exposures (n) | |
| ≤10 uses | 1 |
| >10 uses | 2 |
| No use | 14 |
| Time since last CBD use (n) | |
| 3–6 months | 0 |
| 6–12 months | 2 |
| 1–2 years | 1 |
| 2–4 years | 0 |
| >4 years | 0 |
Note: Values are n, Median (IQR) and frequency (n) as appropriate.
Abbreviations: CBD, cannabidiol; F, females; M, males.
3.2. Oral fluid cannabinoid concentrations
Oral fluid cannabinoid concentrations were determined for 240 samples (60 treatment sessions); the remaining 32 samples (obtained over eight treatment sessions: two each on the 1500‐ and 300‐mg CBD treatments, three on the 15‐mg CBD treatment and one on the placebo) were excluded because QC samples in those (two) batches failed to meet accuracy requirements; that is, the measured CBD concentration of the QC differed from the normative value by >15% (and there was insufficient sample remaining for reanalysis).
Oral fluid CBD concentrations are presented in Table 2 and Figure 1; CBD was not detected in oral fluid on the placebo trial or at baseline (pretreatment). THC, CBN and CBG were not detected in any samples, including those that did not meet accuracy requirements. Median (IQR) oral fluid CBD concentrations were highest ~20‐min posttreatment, quantified as <LOQ, 0.4 (6.0), 15.8 (41.6) and 167 (233) ng·ml−1 on the placebo and the 15‐, 300‐ and 1500‐mg CBD treatments, respectively.
TABLE 2.
Oral fluid CBD concentrations at baseline and ~20‐, ~145‐ and ~185‐min posttreatment on the placebo and the 15‐, 300‐ and 1500‐mg CBD treatments
| Oral fluid CBD concentrations (ng·ml−1) | ||||
|---|---|---|---|---|
| Placebo | 15‐mg CBD | 300‐mg CBD | 1500‐mg CBD | |
| Baseline | <LOQ | <LOQ | <LOQ | <LOQ |
| +20 min | <LOQ | 0.4 (6.0) | 15.8 (41.6) | 167 (233) |
| +145 min | <LOQ | <LOQ (1.6) | 5.4 (10.2) | 77.0 (112) |
| +185 min | <LOQ | <LOQ (0.6) | 1.4 (8.1) | 15.1 (68.7) |
Note: All values are median (IQR).
Abbreviations: CBD, cannabidiol; LOQ, limit of quantification.
FIGURE 1.
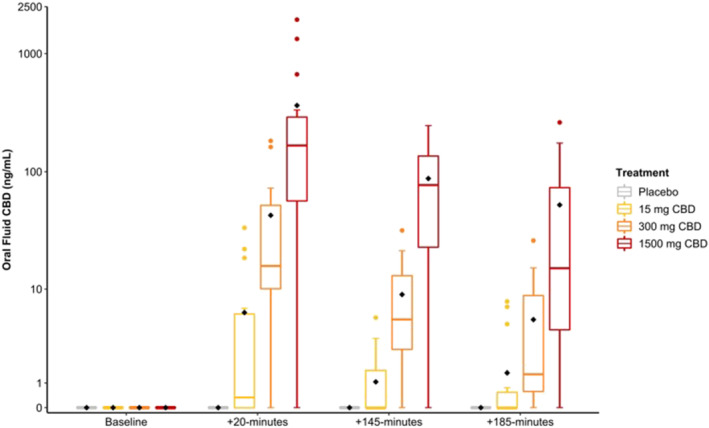
Oral fluid cannabidiol (CBD) concentrations at baseline and ~20‐, ~145‐ and ~185‐min posttreatment. Grey: placebo; yellow: 15‐mg CBD; orange: 300‐mg CBD; and red: 1500‐mg CBD. The black diamond represents the mean value [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Securetec DrugWipe® 5S (DW‐5S)
There were 259 DW‐5S tests successfully completed (Table 3): 61 at baseline, 51 post‐placebo, 48 post‐15‐mg CBD, 48 post‐300‐mg CBD and 51 post‐1500‐mg CBD. The remaining 13 tests could not be completed as there were insufficient DW‐5S devices (awaiting shipment). No positive tests for THC (or any other substances) were observed (100% true negative).
TABLE 3.
Total number of Securetec DrugWipe® 5S (DW‐5S) and Dräger DrugTest® 5000 (DT5000) drug tests performed under each treatment at each time point
| Placebo | 15‐mg CBD | 300‐mg CBD | 1500‐mg CBD | |
|---|---|---|---|---|
| DW‐5S | ||||
| Baseline | 16 | 16 | 15 | 14 |
| +20 min | 17 | 16 | 16 | 17 |
| +145 min | 17 | 16 | 16 | 17 |
| +185 min | 17 | 16 | 16 | 17 |
| DT5000 | ||||
| Baseline | 16 | 16 | 17 | 16 |
| +20 min | 15 | 16 | 17 | 16 |
| +145 min | 15 | 16 | 16 | 16 |
| +185 min | 15 | 16 | 17 | 16 |
Note: All values are n. The DW‐5S and DT5000 were administered at baseline (pretreatment) and on three occasions thereafter, ~20‐, ~145‐ and ~185‐min posttreatment.
Abbreviation: CBD, cannabidiol.
3.4. Dräger DrugTest® 5000 (DT5000)
There were 256 DT5000 tests successfully completed (Table 3): 65 at baseline, 45 postplacebo, 48 post‐15‐mg CBD, 50 post‐300‐mg CBD and 48 post‐1500‐mg CBD. Fifteen tests could not be completed due to technical difficulties (broken down DT5000 device); the remaining test produced an invalid result. No positive tests for THC (or any other substances) were observed (100% true negative).
4. DISCUSSION
CBD did not produce any false‐positive (or true‐positive) tests for THC on the DW‐5S or DT5000 when administered orally at doses of 15, 300 and 1500 mg in the current investigation. This suggests that the POC oral fluid testing devices used by authorities to identify DUIC are able to distinguish between THC and CBD, despite their structural similarities.
Mobile drug testing is a major concern for medicinal cannabis patients in Australia: individuals using legal medicinal cannabis products are not exempt from roadside testing and will be charged if THC is detected in their oral fluid. 26 Around one‐quarter of medicinal cannabis patients in Australia currently use CBD‐dominant products 3 and may be anxious about the prospect of returning a THC‐positive test, despite being on a low‐THC or THC‐free product. The current results therefore provide some reassurance that THC‐positive roadside tests are unlikely to occur when CBD‐only products are used.
Although no previous studies appear to have determined whether POC oral fluid testing devices can distinguish between different cannabinoids, some relevant, albeit non‐peer reviewed, data should be noted. First, Dräger states that the DT5000 is sensitive to CBD—but only at high (likely unattainable) concentrations (i.e., ≥90 000 ng·ml−1). 10 A second report states that the DW‐5S is not sensitive to CBD at concentrations ≤10 000 ng·ml−1. 27 These reports should be interpreted with caution as the methods used to evaluate the DT5000 are not described, and the study of the DW‐5S did not involve human participants (i.e., oral fluid samples were collected, ‘spiked’ with varying amounts of CBD and then applied to the device). However, the current results appear to substantiate their claims indicating that CBD does not produce false‐positive tests for THC at physiologically relevant concentrations.
There are several caveats to this research. First, it is important to recognise that the current investigational product comprised of CBD in MCT oil, only. Some ‘full spectrum’ prescription medications and over‐the‐counter nutraceutical products also contain small amounts of THC (e.g., ~2.0 mg per 100 mg of CBD) and therefore have the potential to elicit true‐positive tests on POC oral fluid testing devices. The only study that appears to have measured oral fluid THC concentrations after a ‘low’, oral dose of THC (i.e., <10 mg) found these rarely exceeded 10 ng·ml−1 at a dose of 5 mg (Cmax = 6.0 [0.8–12.7] ng·ml−1; mean [range]). 28 Oral CBD formulations containing small amounts of THC therefore appear unlikely to elicit (true‐)positive tests on POC oral fluid testing devices. That said, the same investigation 28 also observed low oral fluid THC concentrations at a dose of 15 mg (Cmax = 1.6 [0.7–20.5] ng·ml−1)—much lower than those reported with 10 mg of THC (oral) in other studies (i.e., Cmax > 100 ng·ml−1). 29 , 30 Further research is therefore required to confirm the likelihood of these formulations eliciting true‐positive tests for THC on POC oral fluid testing devices.
Second, it is important to recognise that the current route of administration (i.e., oral ingestion) may produce lower oral fluid CBD concentrations than other methods; namely, inhalation. Indeed, a Cmax of 506 [15–2935] ng·ml−1; ~3‐fold higher than the highest concentration observed in the current study (20‐min post‐1500‐mg CBD: 167 [0–1942] ng·ml−1), has previously been observed following vaporisation of CBD (13.75 mg). 12 Some research also suggests that smoking and vaporisation produce higher oral fluid THC concentrations than oral ingestion. 31 Individuals inhaling relatively low doses of CBD (e.g., one ‘light cannabis’ cigarette may contain ~60‐mg CBD 32 ) could therefore achieve higher oral fluid CBD concentrations than observed in the current study—and it is unclear whether these have greater potential to elicit false‐positive tests for THC on POC oral fluid testing devices.
Third, it is possible that the high‐fat supplement (in which the CBD was administered; see Section 2.4 ‘Study treatments’) reduced the amount of contact between CBD and the oral mucosa in the current study. Oral fluid CBD concentrations might therefore have been lower than otherwise observed if CBD had been administered in a small volume of MCT oil, only. Indeed, CBD's presence in oral fluid is believed to result from contamination of the oral cavity during administration (rather than the transfer from blood). 33 That said, it is difficult to predict whether the supplement affected oral fluid CBD concentrations as few studies appear to have measured these after oral CBD administration (i.e., providing reference data).
One final observation to note is that no oral fluid samples collected in the current study contained detectable levels of THC, CBN or CBG. Indeed, although a small amount of in vitro research suggests that CBD can undergo conversion to THC with prolonged exposure to simulated gastric fluid (e.g., 3–20 h), 34 , 35 several in vivo studies have failed to detect THC in human plasma, 36 , 37 the blood of rodents and minipigs 38 , 39 and the small intestine and stomach of minipigs 38 following CBD treatment (sometimes at high doses, e.g., 700 mg·day−1 for 30 days 36 ). The current data add to this body of evidence providing further confirmation that CBD is unlikely to convert to THC, CBN or CBG in oral fluid.
5. CONCLUSION
This study suggests that orally administered CBD is unlikely to elicit false‐positive (or true‐positive) tests for THC on the DW‐5S or DT5000 POC oral fluid testing devices. The likelihood of an individual who is using a CBD (only) oral formulation being falsely accused of DUIC therefore appears low. However, further research is required to determine whether inhaled CBD and CBD formulations containing small amounts of THC have the potential to elicit positive tests.
Supporting information
Table S1. Quantification parameters for UHPLC–MS/MS analysis of cannabinoids in saliva
ACKNOWLEDGEMENTS
The authors would like to thank Zoe Schrire, Sarah Abelev, Jun Zhi Teh, and Isobel Lavender for their assistance with day‐to‐day trial activities. We are grateful to Barry and Joy Lambert and family for their ongoing support of the research activities of the Lambert Initiative for Cannabinoid Therapeutics.
McCartney D, Kevin RC, Suraev AS, et al. Orally administered cannabidiol does not produce false‐positive tests for Δ9‐tetrahydrocannabinol on the Securetec DrugWipe® 5S or Dräger DrugTest® 5000. Drug Test Anal. 2022;14(1):137-143. doi: 10.1002/dta.3153
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. Phytocannabinoids. 2017;103:1‐36. [DOI] [PubMed] [Google Scholar]
- 2. Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O'Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85(9):1888‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arnold JC, Nation T, McGregor IS. Prescribing medicinal cannabis. Aust Prescr. 2020;43(5):152‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGregor IS, Cairns EA, Abelev S, et al. Access to cannabidiol without a prescription: a cross‐country comparison and analysis. Int J Drug Policy. 2020;85:102935. [DOI] [PubMed] [Google Scholar]
- 5. Arkell TR, Lintzeris N, Kevin RC, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)‐induced impairment of driving and cognition. Psychopharmacology. 2019;236(9):2713‐2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arkell TR, Vinckenbosch F, Kevin RC, Theunissen EL, McGregor IS, Ramaekers JG. Effect of cannabidiol and Δ9‐tetrahydrocannabinol on driving performance: a randomized clinical trial. JAMA. 2020;324(21):2177‐2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spindle TR, Cone EJ, Goffi E, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD‐dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211:107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong K, Brady JE, Li G. Establishing legal limits for driving under the influence of marijuana. Inj Epidemiol. 2014;1(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banister SD, Arnold JC, Connor M, Glass M, McGregor IS. Dark classics in chemical neuroscience: Δ9‐tetrahydrocannabinol. ACS Chem Neurosci. 2019;10(5):2160‐2175. [DOI] [PubMed] [Google Scholar]
- 10. Dräger . Dräger DrugTest 5000 STK IVD manual. Available from: https://www.ucd.ie/mbrs/t4media/Drager%20DrugTest%205000%20STK%20IVD%20Manual.pdf
- 11. Securetec . Rapid drug tests—how do they work? Available from: https://www.securetec.net/en/rapid-drug-tests-how-do-they-work/
- 12. Arkell TR, Kevin RC, Stuart J, et al. Detection of Δ9 THC in oral fluid following vaporized cannabis with varied cannabidiol (CBD) content: an evaluation of two point‐of‐collection testing devices. Drug Test Anal. 2019;11(10):1486‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wille SM et al. Evaluation of on‐site oral fluid screening using Drugwipe‐5+®, RapidSTAT® and DrugTest 5000® for the detection of drugs of abuse in drivers. Forensic Sci Int. 2010;198(1–3):2‐6. [DOI] [PubMed] [Google Scholar]
- 14. Bosker W et al. A placebo‐controlled study to assess Standardized Field Sobriety Tests performance during alcohol and cannabis intoxication in heavy cannabis users and accuracy of point of collection testing devices for detecting THC in oral fluid. Psychopharmacology. 2012;223(4):439‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Desrosiers NA, Lee D, Schwope DM, et al. On‐site test for cannabinoids in oral fluid. Clin Chem. 2012;58(10):1418‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desrosiers NA, Milman G, Mendu DR, et al. Cannabinoids in oral fluid by on‐site immunoassay and by GC‐MS using two different oral fluid collection devices. Anal Bioanal Chem. 2014;406(17):4117‐4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gjerde H, Clausen GB, Andreassen E, Furuhaugen H. Evaluation of Dräger DrugTest 5000 in a naturalistic setting. J Anal Toxicol. 2018;42(4):248‐254. [DOI] [PubMed] [Google Scholar]
- 18. Blencowe T, Pehrsson A, Lillsunde P, et al. An analytical evaluation of eight on‐site oral fluid drug screening devices using laboratory confirmation results from oral fluid. Forensic Sci Int. 2011;208(1–3):173‐179. [DOI] [PubMed] [Google Scholar]
- 19. Swortwood MJ, Newmeyer MN, Abulseoud OA, et al. On‐site oral fluid Δ9‐tetrahydrocannabinol (THC) screening after controlled smoked, vaporized, and oral cannabis administration. Forensic Toxicology. 2017;35(1):133‐145. [Google Scholar]
- 20. McCartney D, Benson MJ, Suraev AS, et al. The effect of cannabidiol on simulated car driving performance: a randomised, double‐blind, placebo‐controlled, crossover, dose‐ranging clinical trial protocol. Hum Psychopharmacol Clin Exp. 2020;35(5):e2749. [DOI] [PubMed] [Google Scholar]
- 21. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birnbaum AK, Karanam A, Marino SE, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60(8):1586‐1592. [DOI] [PubMed] [Google Scholar]
- 23. Scheidweiler KB, Andersson M, Swortwood MJ, Sempio C, Huestis MA. Long‐term stability of cannabinoids in oral fluid after controlled cannabis administration. Drug Test Anal. 2017;9(1):143‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration . Bioanalytical method validation guidance for industry. US Department of Health and Human Services, 2018; 1‐41.
- 25. McCartney D, Suraev AS, Doohan PT, et al. Effects of cannabidiol (CBD) on simulated driving and cognitive performance: a dose‐ranging randomised controlled trial. Manuscript in development. [DOI] [PMC free article] [PubMed]
- 26. Perkins D, Brophy H, McGregor IS, et al. Medicinal cannabis and driving: the intersection of health and road safety policy. Int J Drug Policy. 2021;97:103307. [DOI] [PubMed] [Google Scholar]
- 27. Mohr ALA, Salas JR, Logan BK. Final report: laboratory‐based evaluation of the DrugWipe 5S performance.Available from: https://www.clinihealth.co.za/Pack_Inserts/Securetec/DrugWipe5S%20Evaluation_WipeAlyser_190724.pdf
- 28. Lee D, Karschner EL, Milman G, Barnes AJ, Goodwin RS, Huestis MA. Can oral fluid cannabinoid testing monitor medication compliance and/or cannabis smoking during oral THC and oromucosal Sativex administration? Drug Alcohol Depend. 2013;130(1–3):68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandrey R, Herrmann ES, Mitchell JM, et al. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol. 2017;41(2):83‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spindle TR, Cone EJ, Herrmann ES, et al. Pharmacokinetics of cannabis brownies: a controlled examination of Δ9‐tetrahydrocannabinol and metabolites in blood and oral fluid of healthy adult males and females. J Anal Toxicol. 2020;44(7):661‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Swortwood MJ, Newmeyer MN, Andersson M, Abulseoud OA, Scheidweiler KB, Huestis MA. Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal. 2017;9(6):905‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pacifici R, Pichini S, Pellegrini M, et al. THC and CBD concentrations in blood, oral fluid and urine following a single and repeated administration of “light cannabis”. Clin Chem Lab Med. 2020;58(5):682‐689. [DOI] [PubMed] [Google Scholar]
- 33. Lee D, Huestis MA. Current knowledge on cannabinoids in oral fluid. Drug Test Anal. 2014;6(1–2):88‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merrick J, Lane B, Sebree T, Yaksh T, O'Neill C, Banks SL. Identification of psychoactive degradants of cannabidiol in simulated gastric and physiological fluid. Cannabis Cannabinoid Res. 2016;1(1):102‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watanabe K, Itokawa Y, Yamaori S, et al. Conversion of cannabidiol to Δ9‐tetrahydrocannabinol and related cannabinoids in artificial gastric juice, and their pharmacological effects in mice. Forens Toxicol. 2007;25(1):16‐21. [Google Scholar]
- 36. Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high‐dose repeated daily oral administration in humans. Pharmacol Biochem Behav. 1991;40(3):517‐522. [DOI] [PubMed] [Google Scholar]
- 37. Crippa JAS, Zuardi AW, Hallak JEC, et al. Oral cannabidiol does not convert to Δ8‐THC or Δ9‐THC in humans: a pharmacokinetic study in healthy subjects. Cannabis Cannabinoid Res. 2020;5(1):89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wray L, Stott C, Jones N, Wright S. Cannabidiol does not convert to Δ9‐tetrahydrocannabinol in an in vivo animal model. Cannabis Cannabinoid Res. 2017;2(1):282‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palazzoli F, Citti C, Licata M, et al. Development of a simple and sensitive liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) method for the determination of cannabidiol (CBD), Δ9‐tetrahydrocannabinol (THC) and its metabolites in rat whole blood after oral administration of a single high dose of CBD. J Pharm Biomed Anal. 2018;150:25‐32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quantification parameters for UHPLC–MS/MS analysis of cannabinoids in saliva
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


